Note: Descriptions are shown in the official language in which they were submitted.
I
CA 2789214 2017-04-18
1
COMBINATION OF THEOBROMINE WITH A DECONGESTANT AND ITS USE
FOR THE TREATMENT OF COUGH
Field of the Invention
This invention relates to a drug combination, its composition and its use
in therapy, particularly in the therapy of cough.
Background of the Invention
Cough is a protective reflex. Persistent cough can be distressing. Over-
the-counter remedies are available but their effectiveness is doubtful.
W098/42322 discloses the use of theobromine for the treatment of
cough, to be given orally.
Usmani, OS., Belvisi, M.G., Patel, H.J., Crispino, N., Birrell, M.A.,
Korbonits, M., Korbonits, D., Barnes, P.J., Theobromine Inhibits Sensory Nerve
Activation and Cough, FASEB J. 19(2): 231-233 (2005), discloses that
theobromine inhibits sensory nerve action and cough. Data are provided,
showing effects following oral dosing in citric-acid induced cough in the
guinea
pig, and in the capsaicin cough challenge in humans, and following bathing of
isolated guinea pig vagus nerve preparations.
The decongestant pseudoephedrine has been to shown to have very
limited efficacy in the citric acid induced cough model in guinea-pigs
(Minamizawa K., Goto H., Ohi Y., Shimada Y., Terasawa K., and Haji A., Effect
of d-Pseudoephedrine on Cough Reflex and Its Mode of Action in Guinea Pigs,
J. Pharmacol. Sci. 102: 136-142 (2006)). However, most of the literature fails
to
demonstrate that pseudoephedrine has an antitussive effect. A number of
papers describe effects on "cough and cold" (which has little meaning in the
medical field), but none describes or even examines direct antitussive effect.
Summary of the Invention
The invention is based at least in part on data showing a synergistic
antitussive effect for theobromine combined with the decongestant
pseudoephedrine, in a citric acid-induced cough model. The data show that
when theobromine is combined with pseudoephedrine, the effect is surprisingly
potent and greater than the sum of the individual drugs, revealing that the
combination has a substantially improved effect. This is particularly
surprising
given that it is doubtful that pseudoephedrine has an antitussive effect at
all.
CA 2789214 2017-04-18
1A
Consequently, a considerably reduced dose of both drugs can be given
for an equivalent effect for each individual drug, so reducing side-effects
and
drug burden.
Therefore, according to a first aspect of, the present invention, an agent
comprises theobromine and a decongestant, as a combined preparation for
simultaneous, sequential or separate use in therapy.
CA 02784214 2012-06-13
WO 2011/073646
PCT/GB2010/052085
2
According to a second aspect, a pharmaceutical composition comprises
theobromine and a decongestant.
It is believed that this synergistic relationship will be exhibited by all
decongestants. Without wishing to be bound by theory, this may be due to the
structural similarity of the members of the decongestant class of drugs.
Description of the Drawing
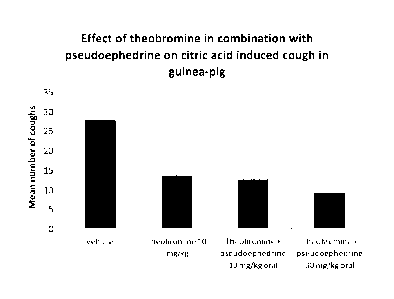
Figure 1 shows the effect of theobromine, and of a combination of
theobromine and pseudoephedrine, on citric acid-induced cough in guinea-pig.
Description of the Invention
As used herein, the term "decongestant" is a defined class of drugs,
which is well known to the skilled person. Preferably, the decongestant is a
a-adrenergic receptor agonist. Any suitable form of the decongestant agent may
be chosen. These include salts, prodrugs and active metabolites.
As used herein, the treatment of cough means any therapy that reduces
the number and/or the severity of cough. Preferably, it means a reduction in
the
number of coughs, i.e. a direct antitussive effect that reduces the body's
urge to
cough. Therefore, according to a preferred embodiment of the invention, an
agent comprises theobromine and a decongestant, for use as an antitussive
pharmaceutical composition. An agent of the invention is useful as an
antitussive in the control of cough. Preferably, it is used in the control of
non-
productive cough.
The decongestant may be used in an amount that is already known for its
use, although combination according to this invention means that a reduced
dose may be effective. The dose of the decongestant that is administered with
the theobromine will of course depend on the usual factors, including its
potency,
but is preferably at least 0.1, e.g. at least 5, and may be up to 50
mg/kg/day.
Preferably, the decongestant is dosed in a range of 0.1 to 30 mg/kg/day.
Any suitable form of theobromine can be chosen. These include salts,
prodrugs and active metabolites. Theobromine may also be in the form of cocoa
or chocolate. Suitable dose ranges for theobromine are known in the art and
will
depend on the usual factors (age etc); although the synergistic effect of the
combination means that the effective dose may be reduced.
CA 02784214 2012-06-13
WO 2011/073646
PCT/GB2010/052085
3
A combination according to the invention may be provided in a single
formulation or in separate formulations, for combined, simultaneous or
sequential administration.
This decongestant is preferably chosen from the following drugs:
ephedrine, levmetamfetamine, naphazoline, oxymetazoline, phenylephrine,
phenylpropanolamine, propylhexedrine, pseudoephedrine, synephrine and
tetrahydrozoline. More preferably, the decongestant is pseudoephedrine.
The compounds of the invention may be administered by any available
route, such as via the oral, inhaled, intranasal, sublingual, intravenous,
rectal
and vaginal routes.
The compounds of the invention are preferably as combinations to be
administered orally, for example as tables, troches, lozenges, aqueous or oily
suspensions, dispersible powders or granules. Preferred
pharmaceutical
compositions of the invention are tablets and capsules. Liquid dispersions for
oral administration may be syrups, emulsions and suspensions. More
preferably, the pharmaceutical composition of the combination is a pressed
tablet or capsule with conventional excipients, examples of which are given
below.
Compositions intended for oral use may be prepared according to any
method known to the art for the manufacture of pharmaceutical compositions,
and such compositions may contain one or more agents selected from the group
consisting of sweetening agents, flavouring agents, colouring agents and
preserving agents in order to provide pharmaceutically elegant and palatable
preparations. Tablets contain the combined active ingredients in admixture
with
non-toxic pharmaceutically acceptable excipients which are suitable for the
manufacture of tablets. These excipients may be, for example, inert diluents,
such as calcium carbonate, sodium carbonate, lactose, calcium phosphate or
sodium phosphate; granulating and disintegrating agents, for example corn
starch or alginic acid; binding agents, for example starch gelatin, acacia,
microcrystalline cellulose or polyvinyl pyrrolidone; and lubricating agents,
for
example magnesium stearate, stearic acid or talc. The tablets may be uncoated
or they may be coated by known techniques to delay disintegration and
absorption in the gastrointestinal tract and thereby provide a sustained
action
CA 02784214 2012-06-13
WO 2011/073646
PCT/GB2010/052085
4
over a longer period. For example, a time delay material such as glyceryl
monostearate or glyceryl distearate may be employed.
Aqueous suspensions contain the combined active materials in admixture
with excipients suitable for the manufacture of aqueous suspensions. Such
excipients are suspending agents, for example sodium carboxymethylcellulose,
methylcellulose, hydroxypropylmethylcellulose, sodium alginate, polyvinyl
pyrrolidone, gum tragacanth and gum acacia; dispersing or wetting agents may
be a naturally occurring phosphatide, for example lecithin, or condensation
products of an alkylene oxide with fatty acids, for example polyoxyethylene
stearate, or condensation products of ethylene oxide with long-chain aliphatic
alcohols, for example heptadecaethyleneoxycetanol, or condensation products
of ethylene oxide with partial esters derived from fatty acids, for example
polyoxyethylene sorbitan monooleate. The aqueous suspensions may also
contain one or more preservatives, for example ethyl or n-propyl p-
hydroxybenzoate, one or more colouring agents, one or more flavouring agents,
and one or more sweetening agents, such as sucrose or saccharin.
Oily suspensions may be formulated by suspending the active ingredient
in a vegetable oil, for example arachis oil, olive oil, sesame oil or coconut
oil,
polyoxyethylene hydrogenated castor oil, fatty acids such as oleic acid, or in
a
mineral oil such as liquid paraffin or in other surfactants or detergents. The
oily
suspensions may contain a thickening agent, for example beeswax, hard
paraffin or cetyl alcohol. Sweetening agents, such as those set forth above,
and
flavouring agents may be added to provide a palatable oral preparation. These
compositions may be preserved by the addition of an antioxidant such as
ascorbic acid.
Dispersible powders and granules suitable for preparation of an aqueous
suspension by the addition of water provide the combined active ingredients in
admixture with a dispersing or wetting agent, suspending agent and one or more
preservatives. Suitable sweetening, flavouring and colouring agents may also
be present.
The combined pharmaceutical compositions of the invention may also be
in the form of oil-in-water emulsions. The oily phase may be a vegetable oil,
for
example olive oil or arachis oil, or a mineral oil, for example liquid
paraffin, or
CA 02784214 2012-06-13
WO 2011/073646
PCT/GB2010/052085
mixtures of these. Suitable emulsifying agents may be naturally occurring
gums,
for example gum acacia or gum tragacanth, naturally occurring phosphatides,
for
example soya bean, lecithin, and esters or partial esters derived from fatty
acids
and hexitol anhydrides, for example sorbitan monooleate and condensation
5 products of the said partial esters with ethylene oxide, for example
polyoxyethylene sorbitan monooleate. The emulsions may also contain
sweetening and flavouring agents.
Syrups and elixirs may be formulated with sweetening agents, for
example glycerol, propylene glycol, sorbitol or sucrose. Such formulations may
also contain a demulcent, a preservative, flavouring and colouring agents.
Suspensions and emulsions may contain a carrier, for example a natural
gum, agar, sodium alginate, pectin, methylcellulose, carboxymethylcellulose,
or
polyvinyl alcohol.
Combined compositions according to the invention may be produced
using conventional formulation techniques. In particular, spray-drying may be
used to produce microparticles comprising the active agent dispersed or
suspended within a material that provides the controlled release properties.
The process of milling, for example jet milling, may also be used to
formulate the therapeutic composition. This applies particularly to particles
intended for administration by inhalation. The manufacture of fine particles
by
milling can be achieved using conventional techniques. The term "milling" is
used herein to refer to any mechanical process which applies sufficient force
to
the particles of active material to break or grind the particles down into
fine
particles. Various milling devices and conditions are suitable for use in the
production of the compositions of the invention.
The selection of appropriate milling conditions, for example, intensity of
milling and duration, to provide the required degree of force, will be within
the
ability of the skilled person. Ball milling is a preferred method.
Alternatively, a
high pressure homogeniser may be used, in which a fluid containing the
particles
is forced through a valve at high pressure, producing conditions of high shear
and turbulence. Shear forces on the particles, impacts between the particles
and machine surfaces or other particles, and cavitation due to acceleration of
the
fluid, may all contribute to the fracture of the particles.
CA 02784214 2012-06-13
WO 2011/073646
PCT/GB2010/052085
6
Suitable homogenisers include the EmulsiFlex high pressure
homogeniser, the Niro Soavi high pressure homogeniser and the Microfluidics
Microfluidiser. The milling process can be used to provide the microparticles
with mass median aerodynamic diameters as specified above. If hygroscopic,
the active agent may be milled with a hydrophobic material, as stated above.
If it is required, the microparticles produced by the milling step can then
be formulated with an additional excipient. This may be achieved by a spray-
drying process, e.g. co-spray-drying. In this embodiment, the particles are
suspended in a solvent and co-spray-dried with a solution or suspension of the
additional excipient. Preferred additional excipients include polysaccharides.
Additional pharmaceutically effective excipients may also be used.
Compositions of the combination intended for inhaled, topical, intranasal,
sublingual, intravenous, rectal and vaginal use may be prepared according to
any method known to the art for the manufacture of pharmaceutical
compositions.
Therapy according to the invention may be conducted in generally known
manner, depending on various factors, such as the sex, age or condition of the
patient, and the existence or otherwise of one or more concomitant therapies.
The patient population may be important.
The present invention is based at least in part on the following study.
Study
A study was designed to investigate the antitussive activity of
theobromine in combination with two different doses of pseudoephedrine, on
citric acid-induced cough in conscious guinea pigs.
Test Doses
1 -vehicle control
2 - theobromine (10 mg/kg. p.o.)
3 - theobromine (10 mg/kg, p.o.) + pseudoephedrine (10 mg/kg. p.o.)
4 - theobromine (10 mg/kg, p.o.) + pseudoephedrine (30 mg/kg. p.o.)
Method
Male Dunkin Hartley guinea pigs (400-500 g, supplied by Harlan UK Ltd)
were used throughout the study.
CA 02784214 2012-06-13
WO 2011/073646
PCT/GB2010/052085
7
Thirty guinea pigs were randomly allocated to one of the four treatment
groups according to the blinding code. The blinding code was not revealed to
the investigator until coughs from all of the animals had been tallied.
Guinea pigs were dosed via oral gavage (dose volume 2 mL/kg) with
theobromine dosed 2 hours prior to citric acid exposure. Pseudoephedrine was
dosed 30 min prior to citric acid exposure and vehicle control animals were
dosed both at 2 hours and 30 minutes prior to citric acid exposure.
Individual guinea pigs were placed in an exposure chamber with airflow
of 2 L/min at t -10 min prior to citric acid exposure to acclimatise. At t = 0
min,
cough responses were induced by exposure to citric acid aerosol (1 M)
generated by an ultrasonic nebuliser at a nebulisation rate of 0.6 mL/min for
10
min.
Coughs were counted throughout the 10 min citric acid exposure and for
a further 5 min post exposure.
Results
The results show that pre-treatment with theobromine ( 10 mg/kg, p.o.)
caused a significant reduction to the number of citric acid induced coughs (13
3) (see Figure 1), as well as to the onset to the first cough (156 15s)
(data not
shown). In combination with pseudoephedrine (10 mg/kg and 30 mg/kg), the
inhibitory response of theobromine on the citric acid-induced tussive
activity, was
potentiated with the highest dose, both in respect of total number of coughs
(9
2 c.f. 13 3) (Figure 1) and onset time to the first cough (170 15s c.f.
156
15s) (data not shown).