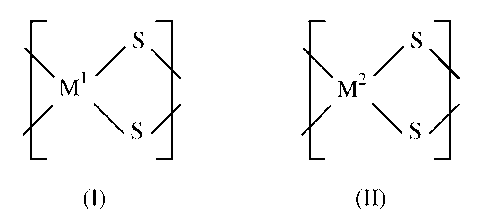Note: Descriptions are shown in the official language in which they were submitted.
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
PROCESS FOR TREATING A HYDROCARBON-CONTAINING FEED
Field of the Invention
The present invention is directed to a process for treating a hydrocarbon-
containing feedstock.
Background of the Invention
Increasingly, resources such as heavy crude oils, bitumen, tar sands, shale
oils,
and hydrocarbons derived from liquefying coal are being utilized as
hydrocarbon
sources due to decreasing availability of easily accessed light sweet crude
oil
reservoirs. These resources are disadvantaged relative to light sweet crude
oils,
containing significant amounts of heavy hydrocarbon fractions such as residue
and
asphaltenes, and often containing significant amounts of sulfur, nitrogen,
metals,
and/or naphthenic acids. The disadvantaged crudes typically require a
considerable
amount of upgrading, for example by cracking and by hydrotreating, in order to
obtain more valuable hydrocarbon products. Upgrading by cracking, either
thermal
cracking, hydrocracking and/or catalytic cracking, is also effective to
partially convert
heavy hydrocarbon fractions such as atmospheric or vacuum residues derived
from
refining a crude oil or hydrocarbons derived from liquefying coal into
lighter, more
valuable hydrocarbons.
Numerous processes have been developed to crack and treat disadvantaged
crude oils and heavy hydrocarbon fractions to recover lighter hydrocarbons and
to
reduce metals, sulfur, nitrogen, and acidity of the hydrocarbon-containing
material.
For example, a hydrocarbon-containing feedstock may be cracked and
hydrotreated
by passing the hydrocarbon-containing feedstock over a catalyst located in a
fixed bed
catalyst reactor in the presence of hydrogen at a temperature effective to
crack heavy
hydrocarbons in the feedstock and/or to reduce the sulfur content, nitrogen
content,
metals content, and/or the acidity of the feedstock. Another commonly used
method
to crack and/or hydrotreat a hydrocarbon-containing feedstock is to disperse a
catalyst
in the feedstock and pass the feedstock and catalyst together with hydrogen
through a
slurry-bed, or fluid-bed, reactor operated at a temperature effective to crack
heavy
hydrocarbons in the feedstock and/or to reduce the sulfur content, nitrogen
content,
metals content, and/or the acidity of the feedstock. Examples of such slurry-
bed or
fluid-bed reactors include ebullating-bed reactors, plug-flow reactors, and
bubble-
column reactors.
1
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
Coke formation, however, is a particular problem in processes for cracking a
hydrocarbon-containing feedstock having a relatively large amount of heavy
hydrocarbons such as residue and asphaltenes. Substantial amounts of coke are
formed in the current processes for cracking heavy hydrocarbon-containing
feedstocks, limiting the yield of lighter molecular weight hydrocarbons that
can be
recovered and decreasing the efficiency of the cracking process by limiting
the extent
of hydrocarbon conversion that can be effected per cracking step in the
process, for
example, by deactivating the catalysts used in the process.
Cracking heavy hydrocarbons involves breaking bonds of the hydrocarbons,
particularly carbon-carbon bonds, thereby forming two hydrocarbon radicals for
each
carbon-carbon bond that is cracked in a hydrocarbon molecule. Numerous
reaction
paths are available to the cracked hydrocarbon radicals, the most important
being: 1)
reaction with a hydrogen donor to form a stable hydrocarbon molecule that is
smaller
in terms of molecular weight than the original hydrocarbon from which it was
derived; and 2) reaction with another hydrocarbon or another hydrocarbon
radical to
form a hydrocarbon molecule larger in terms of molecular weight than the
cracked
hydrocarbon radical-a process called annealation. The first reaction is
desired, it
produces hydrocarbons of lower molecular weight than the heavy hydrocarbons
contained in the feedstock- and preferably produces naphtha, distillate, or
gas oil
hydrocarbons. The second reaction is undesired and leads to the production of
coke
as the reactive hydrocarbon radical combines with another hydrocarbon or
hydrocarbon radical. Furthermore, the second reaction is autocatalytic since
the
growing coke particles are more reactive with the cracked hydrocarbon radicals
than
the hydrocarbon feedstock. Hydrocarbon-containing feedstocks having a
relatively
high concentration of heavy hydrocarbon molecules therein are particularly
susceptible to coking due to the presence of a large quantity of high
molecular weight
hydrocarbons in the feedstock with which cracked hydrocarbon radicals may
combine
to form proto-coke or coke. As a result, cracking processes of heavy
hydrocarbon-
containing feedstocks have been limited by coke formation induced by the
cracking
reaction itself.
Numerous catalysts have been developed for use in processes for cracking
disadvantaged hydrocarbon feedstocks, however, such catalysts have not
eliminated
problems associated with coking, and catalyst activity may be significantly
reduced
over time by accumulation of coke on the catalyst. Catalysts used in fixed
catalyst
2
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
bed reactors typically contain a Group VIB and/or Group VIII metal supported
on a
carrier formed of alumina, silica, or alumina-silica. The carrier is generally
selected
to possess acidic properties that catalytically facilitate cracking by
promoting the
formation of radical carbo-cation hydrocarbon species from cracked
hydrocarbons.
Fixed bed cracking catalysts are also generally porous and highly adsorptive,
where
the pores and pore size distribution of the catalysts are determined by the
carrier on
which active metals are placed. The pores and pore size distribution of such
catalysts
markedly affect the activity, selectivity, and the cracking reaction rate. The
active
Group VIB and/or Group VIII metals of the catalyst facilitate hydrogenation of
the
cracked hydrocarbon radicals. Such catalysts are commonly sulfided to activate
the
catalyst, either before contacting the catalyst with a disadvantaged
hydrocarbon feed
or in situ with the disadvantaged hydrocarbon feed.
Processes that utilize fixed bed catalysts to crack a heavy hydrocarbon-
containing material suffer significantly from catalyst aging due to coke
deposition on
the catalyst over time. As noted above, coke and proto-coke formation occurs
in
cracking a hydrocarbon-containing material, and is particularly problematic
when the
hydrocarbon-containing material is a heavy hydrocarbon-containing material,
for
example, containing at least 20 wt.% pitch, residue, or asphaltenes. The coke
that is
formed in the cracking process deposits on the catalyst progressively over
time,
plugging the catalyst pores and covering the surface of the catalyst. The
coked
catalyst loses its catalytic activity and, ultimately, must be replaced.
Furthermore, the
cracking process must be conducted at relatively low cracking temperatures to
prevent
rapid deactivation of the catalyst by annealation leading to coke deposition.
Slurry catalyst processes have been utilized to address the problem of
catalyst
aging by coke deposition in the course of cracking a hydrocarbon-containing
feedstock. Slurry catalyst particles are selected to be dispersible in the
hydrocarbon-
containing feedstock or in vaporized hydrocarbon-containing feedstock so the
slurry
catalysts circulate with the hydrocarbon-containing feedstock in the course of
cracking the feedstock. The feedstock and the catalyst move together through
the
cracking reactor and are separated upon exiting the cracking reactor. Coke
formed
during the cracking reaction is separated from the feedstock, and any coke
deposited
on the catalyst may be removed from the catalyst by regenerating the catalyst.
The
regenerated catalyst may then be recirculated with fresh hydrocarbon-
containing
feedstock through the cracking reactor. The process, therefore, is not
affected by
3
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
catalyst aging since fresh catalyst may be continually added into the cracking
reactor,
and catalyst upon which coke has been deposited may be continually
regenerated.
Other slurry catalysts have been used in slurry cracking processes for the
purpose of seeding the formation of coke. Very small particle slurry catalysts
may be
dispersed in a hydrocarbon-containing feedstock for the purpose of providing a
plethora of small sites upon which coke may deposit in the course of the
cracking
process. This inhibits the formation of large coke particles since the coke
may be
dispersed throughout the hydrocarbon-containing feedstock on the small
catalyst
particles.
U.S. Patent No. 4,557,821 provides a slurry catalyst formed of dispersed
particles of highly active molybdenum disulfide useful for cracking a
hydrocarbon-
containing feedstock. The slurry catalyst exists as a substantially
homogeneous
dispersion of small particles in oil, where the catalyst's activity is
dependent on the
smallness of the particle size and resultant relatively large surface area
rather than its
pore characteristics. The catalyst does not have a porous support, e.g. a
silica,
alumina, or silica-alumina carrier, but is formed substantially only of
molybdenum
sulfides and molybdenum oxy-sulfides.
Although presently known slurry catalysts and slurry cracking processes
utilizing such catalysts do not suffer the catalyst aging problems of fixed
bed catalysts
and fixed bed catalyst processes in cracking a heavy hydrocarbon-containing
feedstock, coking is still a significant problem. Coking limits the yield of
lighter
molecular weight hydrocarbons that can be recovered from the cracking process
since
a portion of the hydrocarbons in the hydrocarbon-containing feedstock are
converted
to coke rather than to the desired lighter molecular weight hydrocarbons.
Coking also
decreases the efficiency of the cracking process by limiting the extent of
hydrocarbon
conversion that can be effected per cracking step in the process, even in a
slurry
process, since the hydrocarbon-containing feedstock and the catalyst must be
periodically removed from the cracking process to separate developing coke
particles
to prevent excessive coking. The slurry catalysts may actually increase
coking, for
example, the slurry catalyst disclosed in U.S. Patent No. 4,557,821 is
described as "a
very active coking catalyst", and a process is disclosed therein for using
such a slurry
catalyst that requires the use of exacting, slow heating steps to avoid
massive coking.
Improved processes for cracking heavy hydrocarbon-containing feedstocks are
desirable, particularly those in which coke formation is significantly
reduced.
4
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
Summary of the Invention
In one aspect, the present invention describes a process for hydrocracking a
hydrocarbon-containing feedstock, comprising:
mixing, at a temperature selected from 375 C to 500 C and a total pressure
selected from 6.9 MPa to 27.5 MPa,
a) a hydrocarbon-containing feedstock containing at least 20 wt.%
hydrocarbons having a boiling point of greater than 538 C as determined in
accordance with ASTM Method D5307;
b) hydrogen;
c) hydrogen sulfide, where the hydrogen sulfide is provided for mixing at a
mole ratio of hydrogen sulfide to hydrogen of from 0.5:9.5 to 1:1, where the
hydrogen and hydrogen sulfide are provided for mixing such that the
combined hydrogen and hydrogen sulfide partial pressures provide at least
60% of the total pressure; and
d) a catalyst comprising a material comprised of a first metal and a second
metal where the first metal is selected from the group consisting of Cu, Fe,
Ni,
Co, Bi, Ag, Mn, Zn, Sn, Ru, La, Ce, Pr, Sm, Eu, Yb, Lu, Dy, Pb, and Sb,
where the second metal is selected from the group consisting of Mo, W, V, Sn,
and Sb, where the second metal is different from the first metal, and wherein
the material is comprised of at least three linked chain elements, the chain
elements comprising a first chain element including the first metal and having
a structure according to formula (I) and a second chain element including the
second metal and having a structure according to formula (II)
S 2S
M M
S S
(I) (II)
where M1 is the first metal where M2 is the second metal
where the material contains at least one first chain element and at least one
second chain element and where chain elements in the material are linked by
bonds between the two sulfur atoms of a chain element and the metal of an
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
adjacent chain element, where the hydrocarbon-containing feedstock, catalyst,
hydrogen sulfide, and hydrogen form a mixture upon mixing;
while mixing the mixture at the selected temperature and selected total
pressure, separating a vapor comprising a hydrocarbon-containing product
from the mixture, where the hydrocarbon-containing product is comprised of
one or more hydrocarbon compounds that are liquid at STP.
Brief Description of the Drawings
Fig. 1 is a schematic of a system useful for practicing the process of the
present
invention.
Fig. 2 is a schematic of a system useful for practicing the process of the
present
invention including a reactor having three zones.
Fig. 3 is a plot of hydrocracking reaction rates relative to hydrogen sulfide
present in
the reaction.
Fig. 4 is a graph of the carbon content of the products of a hydrocracking
process
conducted in accordance with the present invention.
Detailed Description of the Invention
The present invention is directed to a process for cracking a hydrocarbon-
containing feedstock containing at least 20 wt.% heavy hydrocarbons utilizing
a
thiometallate catalyst including a material comprised of a first metal and a
second
metal and sulfur, where the first metal is selected from the group consisting
of copper
(Cu), iron (Fe), nickel (Ni), cobalt (Co), bismuth (Bi), silver (Ag),
manganese (Mn),
zinc (Zn), tin (Sn), ruthenium (Ru), lanthanum (La), praseodymium (Pr),
samarium
(Sm), europium (Eu), ytterbium (Yb), lutetium (Lu), dysprosium (Dy), lead
(Pb), and
antimony (Sb), and where the second metal is selected from the group
consisting of
molybdenum (Mo), tungsten (W), vanadium (V), tin (Sn), and antimony (Sb),
where
the second metal is not the same as the first metal. The catalyst may have a
structure
in which the catalyst material is comprised of at least three linked chain
elements, the
chain elements comprising a first chain element including the first metal and
having a
structure according to formula (I) and a second chain element including the
second
metal and having a structure according to formula (II)
6
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
i S 2 S
M M
\S S
(I) (II)
where M1 is the first metal, where M2 is the second metal, where the catalyst
material
is comprised of at least one first chain element and at least one second chain
element,
and where chain elements in the material are linked by bonds between the two
sulfur
atoms of a chain element and the metal of an adjacent chain element. The
material of
the catalyst may be a polythiometallate polymer formed of repeating monomeric
units
having the structure (III):
r ~S\ 2 / 'S\
M M
\S/ \S/ x
(III)
where M1 is the first metal, where M2 is the second metal, and where x is at
least two.
The material of the catalyst may be tetrathiometallate material comprised of
alternating M1S4 and M2S4 tetrahedral formations located adjacent to each
other,
where M1 is the first metal and M2 is the second metal as described above, and
where
the metal of each tetrahedral formation is bonded to at least two sulfur atoms
that are
also bonded to the metal of an adjacent tetrahedral formation. The
tetrathiometallate
material may have a polymeric structure wherein a portion of the first metal
is located
within interstices or holes in the polymeric structure, where the portion of
the first
metal located within interstices or holes in the polymeric structure is not
bonded with
a sulfur atom or second metal atom included in the polymeric structure.
The process of the present invention is effective to crack a heavy hydrocarbon-
containing feedstock while producing little, if any, coke, and resulting in a
hydrocarbon-containing product that contains most of the atomic carbon from
the
heavy hydrocarbon-containing feedstock and that contains little, if any,
hydrocarbons
that have a boiling point above 538 C. The catalyst utilized in the process in
combination with a relatively hydrogen sulfide-rich mixture of hydrogen and
7
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
hydrogen sulfide is particularly effective at selectively directing reactions
occurring in
the cracking process to avoid and/or inhibit coke formation.
Although not intending the present invention to be limited thereby, it is
believed that the catalyst is a highly effective catalyst for use in cracking
a heavy
hydrocarbon-containing material due, at least in part, to: 1) the ability of
the catalyst
to donate or share electrons with hydrocarbons based on the molecular
structure of the
catalyst (i.e. to reduce the hydrocarbon so the hydrocarbon forms a radical
anion); and
2) the surface area of the catalyst available to interact with hydrocarbons
and/or
hydrocarbon radicals in the absence of any porous alumina, alumina-silica, or
silica
based carrier or support.
It is believed that the hydrocarbons of a hydrocarbon-containing feedstock are
cracked in the process of the present invention by a Lewis base mediated
reaction,
wherein the catalyst facilitates a reduction at the site of the hydrocarbon
where the
hydrocarbon is cracked, forming two hydrocarbon radical anions from the
initial
hydrocarbon. Radical anions are most stable when present on a primary carbon
atom,
therefore, formation of primary hydrocarbon radical anions may be
energetically
favored when a hydrocarbon is cracked, or the cracked hydrocarbon may
rearrange to
form the more energetically favored primary radical anion. Should the primary
radical anion react with another hydrocarbon to form a larger hydrocarbon, the
reaction will result in the formation of a secondary carbon-carbon bond that
is
susceptible to being cracked again. However, since hydrocarbon radical anions
are
relatively stable they are likely to be hydrogenated by hydrogen present in
the
reaction mixture rather than react with another hydrocarbon in an annealtion
reaction,
and significant hydrocarbon radical anion-hydrocarbon reactions are unlikely.
As a
result, little coke is formed by agglomeration of cracked hydrocarbons.
Conventional hydrocracking catalysts utilize an active hydrogenation metal,
for example a Group VIII metal such as nickel, on a support having Lewis acid
properties, for example, silica, alumina-silica, or alumina supports. It is
believed that
cracking heavy hydrocarbons in the presence of a Lewis acid catalyst results
in the
formation of cracked hydrocarbon radical cations rather than hydrocarbon
radical
anions. Radical cations are most stable when present on a tertiary carbon
atom,
therefore, cracking may be energetically directed to the formation of tertiary
hydrocarbon radical cations, or, most likely, the cracked hydrocarbon may
rearrange
to form the more energetically favored tertiary radical cation. Hydrocarbon
radical
8
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
cations are unstable relative to hydrocarbon radical anions, and may react
rapidly with
other hydrocarbons. Should the tertiary radical cation react with another
hydrocarbon
to form a larger hydrocarbon, the reaction may result in the formation of a
carbon-
carbon bond that is not susceptible to being cracked again. As a result, coke
is formed
by agglomeration of the cracked hydrocarbons.
Again, not intending the present invention to be limited thereby, it is
believed
that the catalyst utilized in the process of the present invention is
particularly effective
for use in cracking a heavy hydrocarbon-containing material due, in part, to
the
molecular structure of the catalyst, which facilitates donation or sharing of
electrons
from the catalyst to a hydrocarbon or a hydrocarbon anion radical. The sulfur
atoms
linking the first and second metals in the catalyst may facilitate the
electron
donating/sharing activity of the catalyst, acting to enable charge transfer
from the first
metal to the second metal or from the second metal to the first metal across
the
molecular orbitals of the sulfur atoms, as well as potentially acting to
directly share
electrons from the sulfur atoms in the catalyst with the hydrocarbon or
hydrocarbon
anion radical. The sulfur atoms may further facilitate donation/sharing of
electrons
from the catalyst to a hydrocarbon or hydrocarbon radical by charge
stabilization of
the catalyst as the catalyst donates/shares electrons with a hydrocarbon or
hydrocarbon radical. It is believed that the structure of the catalyst is
particularly
effective in facilitating donation or sharing of electrons from the catalyst
to a
hydrocarbon or hydrocarbon radical when the catalyst has a polythiometallate
polymeric structure such as set forth in formula (III) above, particularly
when x is at
least 5, since any charge induced in the catalyst by sharing or donation of
electrons to
the hydrocarbon or hydrocarbon radical may be spread over a large number of
sulfur
atoms and first and second metals that form the polymeric structure of the
catalyst.
Again, not intending the present invention to be limited thereby, it is also
believed that the catalyst utilized in the process of the present invention
may be
particularly effective for use in cracking a heavy hydrocarbon-containing
material
since the molecular structure of the catalyst may have sulfided electron-rich
metals
incorporated therein while inhibiting reduction of such electron-rich metals
to a zero-
oxidation state. As discussed above, it is believed that use of a catalyst
having the
ability to donate or share electrons with hydrocarbons and/or hydrocarbon
anion
radicals may facilitate cracking the hydrocarbons without attendant production
of
coke or proto-coke. The catalytic material containing sulfided electron-rich
metals
9
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
utilized in the process of the present invention, therefore, facilitates
hydrocarbon
cracking without formation of coke or proto-coke. However, use of sulfided
electron-
rich metals is typically avoided in hydrocarbon cracking processes since the
metal of
typical electron-rich metal compounds may be easily desulfided and reduced to
its
zero-oxidation state in the presence of hydrogen, and zero-oxidation state
electron-
rich metals catalyze the production of coke in a cracking process. For
example,
copper sulfide is an electron-rich metal that is not typically utilized in
cracking
processes due to its propensity to catalyze coke formation.
The molecular structure of the catalyst utilized in the process of the present
invention, however, enables the use of an electron-rich metal such as copper
or
bismuth in a process for cracking a heavy hydrocarbon-containing material,
where
electron-rich metals such as copper or bismuth are preferred for use as the
first metal
in the catalyst. The electron-rich metal may be bound in the catalyst by two
sulfur
atoms, inhibiting or preventing the reduction of the electron-rich metal to
its zero-
oxidation state, and thereby inhibiting or preventing the formation of coke by
the
zero-oxidation state electron-rich metal. Inclusion of an electron-rich metal,
particularly copper, in the catalyst utilized in the process of the present
invention
promotes the electron donation/sharing characteristics of the catalyst by
increasing the
electron density of the catalyst available to be donated or shared with a
hydrocarbon
or hydrocarbon anion radical.
Again, not intending the present invention to be limited thereby, it is also
believed that the catalyst utilized in the process of the present invention is
particularly
effective for use in cracking a heavy hydrocarbon-containing material due, in
part, to
the physical structure of the catalyst, which facilitates contact of the
catalyst with a
hydrocarbon or a hydrocarbon anion radical. The catalyst does not include a
porous
alumina, alumina-silica, or silica carrier or support material yet may have
substantial
surface area available for contact with the hydrocarbon-containing feedstock,
particularly relative to other "bulk metal" catalytic materials that include
little or no
alumina, alumina-silica, or silica as a carrier or support material. It is
believed that at
least a portion of the catalyst may have a tetrahedral molecular structure and
that the
tetrahedral molecular structure causes the physical structure of the catalyst
to have
significant porosity and pore volume relative to typical non-supported
catalysts
(which may have an octahedral molecular structure with a plate-like physical
structure). The surface area of the present catalyst that is available for
contact with a
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
hydrocarbon-containing feedstock may be relatively large due to the porosity
of the
catalyst. The catalyst may have a surface area, a pore size distribution, a
pore
volume, and porosity comparable to a catalyst having active metals deposited
on an
alumina, alumina-silica, or silica based carrier. Since the surface area of
the catalyst
may be substantially or totally comprised of the active catalytic metals and
sulfur,
rather than islands of active metals deposited on a carrier or support, the
catalyst may
have very high catalytic activity due its large surface area that is
substantially
comprised of the catalytically active metals and sulfur.
Although the process of the invention is not to be limited thereby, it is also
believed that the hydrogen sulfide acts as a further catalyst in the cracking
of
hydrocarbons in the hydrocarbon-containing feedstock in the presence of
hydrogen
and the catalyst comprised of the first metal, second metal, and sulfur, and
inhibits
coke formation under cracking conditions. Use of sufficient hydrogen sulfide
in the
process permits the process to be effected at a mixing zone temperature of at
least at
least 430 C or at least 450 C with little or no increase in coke formation
relative to
cracking conducted at lower temperatures since hydrogen sulfide, in sufficient
quantity, inhibits coke formation. The rate of the process, therefore, may be
greatly
increased with the use of significant quantities of hydrogen sulfide in the
hydrocracking step of the process since the rate of reaction in the
hydrocracking step
of the process increases significantly relative to temperature.
Hydrogen sulfide and hydrogen each may act as a hydrogen atom donor to
hydrogenate a cracked hydrocarbon anion radical to produce a stable
hydrocarbon
having a smaller molecular weight than the hydrocarbon from which the
hydrocarbon
anion radical was derived. It is believed that hydrogen, however, may only act
to
donate a hydrogen atom to a cracked hydrocarbon anion radical at or near a
metal-
containing catalyst surface. It is further believed that hydrogen sulfide,
however, may
act to provide a hydrogen atom to a cracked hydrocarbon anion radical
significantly
further from a metal-containing catalyst surface, and, after donation of a
hydrogen
atom, may accept a hydrogen atom from hydrogen near the surface of the
catalyst.
The hydrogen sulfide, therefore, may act as an atomic hydrogen shuttle to
provide
atomic hydrogen to a cracked hydrocarbon anion radical at a distance from the
thiometallate catalyst.
Hydrogen sulfide also reacts much more rapidly to hydrogenate a cracked
hydrocarbon anion radical than hydrogen since the reaction of hydrogen sulfide
with a
11
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
cracked hydrocarbon anion radical to hydrogenate the radical is substantially
more
energetically favored than hydrogenation of the cracked hydrocarbon anion
radical by
hydrogen. Hydrogen sulfide may inhibit annealation of cracked hydrocarbon
anion
radicals by rapidly reacting with the cracked hydrocarbon anion radicals
before the
cracked hydrocarbon anion radicals react with another hydrocarbon.
Furthermore, the thiol group remaining after hydrogen sulfide has provided a
hydrogen atom to a cracked hydrocarbon anion radical may be provided to
another
hydrocarbon anion radical, thereby forming a meta-stable thiol-containing
hydrocarbon. This may be described chemically as follows:
1. R-C-C-R + heat+ catalyst _ R-C- + -C-R
(catalyst = thiometallate catalyst)
2. R-C- + H2S + R-CH + -SH
3. C-R + -SH + R-C-SH
4. R-C-SH + H2 R-CH + H2S
The thiol of the meta-stable thiol-containing hydrocarbon may be replaced by a
hydrogen atom from either another hydrogen sulfide molecule or hydrogen, or
may
react intramolecularly to form a thiophene compound as a hydrocarbon-
containing
product. Thus, hydrogen sulfide may open up another reaction pathway for
conversion of a cracked hydrocarbon to its hydrogenated counterpart-enhancing
the
rate of the reaction.
It is believed that hydrogen sulfide also lowers the activation energy
required
to crack hydrocarbons in the hydrocarbon-containing feedstock when utilized in
combination with the catalyst described above, thereby increasing the rate of
the
catalytic hydrocracking reaction in the process of the present invention. The
rate of
the process, in particular the rate that the hydrocarbon-containing feedstock
may be
provided to the reaction or that product may be removed from the reaction,
therefore,
may be greatly increased with the use of sufficient quantities of hydrogen
sulfide. For
example, the rate of the process may be increased by at least 1.5 times, or by
at least 2
times, the rate of the process in the absence of significant quantities of
hydrogen
sulfide.
Certain terms that are used herein are defined as follows:
"Acridinic compound" refers to a hydrocarbon compound including the structure:
12
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
\ N
As used in the present application, an acridinic compound includes any
hydrocarbon
compound containing the above structure, including, naphthenic acridines,
napththenic benzoacridines, and benzoacridines, in addition to acridine.
"Anaerobic conditions" means "conditions in which less than 0.5 vol.% oxygen
as a
gas is present". For example, a process that occurs under anaerobic
conditions, as
used herein, is a process that occurs in the presence of less than 0.5 vol.%
oxygen in a
gaseous form. Anaerobic conditions may be such that no detectable oxygen gas
is
present.
"Aqueous" as used herein is defined as containing more than 50 vol.% water.
For
example, an aqueous solution or aqueous mixture, as used herein, contains more
than
50 vol.% water.
"ASTM" refers to American Standard Testing and Materials.
"Atomic hydrogen percentage" and "atomic carbon percentage" of a hydrocarbon-
containing material-including crude oils, crude products such as syncrudes,
bitumen,
tar sands hydrocarbons, shale oil, crude oil atmospheric residues, crude oil
vacuum
residues, naphtha, kerosene, diesel, VGO, and hydrocarbons derived from
liquefying
coal-are as determined by ASTM Method D529 1.
"API Gravity" refers to API Gravity at 15.5 C, and as determined by ASTM
Method
D6822.
"Benzothiophenic compound" refers to a hydrocarbon compound including the
structure:
S
As used in the present application, a benzothiophenic compound includes any
hydrocarbon compound containing the above structure, including di-
benzothiophenes,
naphthenic-benzothiophenes, napththenic-di-benzothiophenes, benzo-naphtho-
thiophenes, naphthenic-benzo-naphthothiophenes, and dinaphtho-thiophenes, in
addition to benzothiophene.
"BET surface area" refers to a surface area of a material as determined by
ASTM
Method D3663.
13
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
"Blending" as used herein is defined to mean contact of two or more substances
by
intimately admixing the two or more substances.
Boiling range distributions for a hydrocarbon-containing material are as
determined
by ASTM Method D5307.
"Bond" as used herein with reference to atoms in a molecule may refer to a
covalent
bond, a dative bond, or an ionic bond, dependent on the context.
"Carbazolic compound" refers to a hydrocarbon compound including the
structure:
H
N
d--b-
As used in the present application, a carbazolic compound includes any
hydrocarbon
compound containing the above structure, including naphthenic carbazoles,
benzocarbazoles, and napthenic benzocarbazoles, in addition to carbazole.
"Carbon number" refers to the total number of carbon atoms in a molecule.
"Catalyst" refers to a substance that increases the rate of a chemical process
and/or
that modifies the selectivity of a chemical process as between potential
products of
the chemical process, where the substance is not consumed by the process. A
catalyst, as used herein, may increase the rate of a chemical process by
reducing the
activation energy required to effect the chemical process. Alternatively, a
catalyst, as
used herein, may increase the rate of a chemical process by modifying the
selectivity
of the process between potential products of the chemical process, which may
increase the rate of the chemical process by affecting the equilibrium balance
of the
process. Further, a catalyst, as used herein, may not increase the rate of
reactivity of a
chemical process but merely may modify the selectivity of the process as
between
potential products.
"Catalyst acidity by ammonia chemisorption" refers to the acidity of a
catalyst
substrate as measured by volume of ammonia adsorbed by the catalyst substrate
and
subsequently desorbed from the catalyst substrate as determined by ammonia
temperature programmed desorption between a temperature of 120 C and 550 C.
For
clarity, a catalyst that is decomposed in the measurement of acidity by
ammonia
temperature programmed desorption to a temperature of 550 C and/or a catalyst
for
which a measurement of acidity may not be determined by ammonia temperature
14
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
programmed desorption, e.g. a liquid or a gas, is defined for purposes of the
present
invention to have an indefinite acidity as measured by ammonia chemisorption.
Ammonia temperature programmed desorption measurement of the acidity of a
catalyst is effected by placing a catalyst sample that has not been exposed to
oxygen
or moisture in a sample container such as a quartz reactor; transferring the
sample
container containing the sample to a temperature programmed desorption
analyzer
such as a Micrometrics TPD/TPR 2900 analyzer; in the analyzer, raising the
temperature of the sample in helium to 550 C at a rate of 10 C per minute;
cooling the
sample in helium to 120 C; alternately flushing the sample with ammonia for 10
minutes and with helium for 25 minutes a total of 3 times, and subsequently
measuring the amount of ammonia desorbed from the sample in the temperature
range
from 120 C to 550 C while raising the temperature at a rate of 10 C per
minute.
"Coke" is a solid carbonaceous material that is formed primarily of a
hydrocarbonaceous material and that is insoluble in toluene as determined by
ASTM
Method D4072.
"Cracking" as used herein with reference to a hydrocarbon-containing material
refers
to breaking hydrocarbon molecules in the hydrocarbon-containing material into
hydrocarbon fragments, where the hydrocarbon fragments have a lower molecular
weight than the hydrocarbon molecule from which they are derived. Cracking
conducted in the presence of a hydrogen donor may be referred to as
hydrocracking.
Cracking effected by temperature in the absence of a catalyst may be referred
to a
thermal cracking. Cracking may also produce some of the effects of
hydrotreating
such as sulfur reduction, metal reduction, nitrogen reduction, and reduction
of TAN.
"Diesel" refers to hydrocarbons with a boiling range distribution from 260 C
up to
343 C (500 F up to 650 F) at a pressure of 0.101 MPa. Diesel content maybe
determined by the quantity of hydrocarbons having a boiling range of from 260
C to
343 C at a pressure of 0.101 MPa relative to a total quantity of hydrocarbons
as
measured by boiling range distribution in accordance with ASTM Method D5307.
"Dispersible" as used herein with respect to mixing a solid, such as a salt,
in a liquid
is defined to mean that the components that form the solid, upon being mixed
with the
liquid, are retained in the liquid at STP for a period of at least 24 hours
upon cessation
of mixing the solid with the liquid. A solid material is dispersible in a
liquid if the
solid or its components are soluble in the liquid. A solid material is also
dispersible in
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
a liquid if the solid or its components form a colloidal dispersion or a
suspension in
the liquid.
"Distillate" or "middle distillate" refers to hydrocarbons with a boiling
range
distribution from 204 C up to 343 C (400 F up to 650 F) at a pressure of 0.101
MPa.
Distillate content is as determined by ASTM Method D5307. Distillate may
include
diesel and kerosene.
"Hydrogen" as used herein refers to molecular hydrogen unless specified as
atomic
hydrogen.
"Insoluble" as used herein refers to a substance a majority (at least 50 wt.%)
of which
does not dissolve in a liquid upon being mixed with the liquid at a specified
temperature and pressure, where the undissolved portion of the substance can
be
recovered from the liquid by physical means. For example, a fine particulate
material
dispersed in a liquid is insoluble in the liquid if 50 wt.% or more of the
material may
be recovered from the liquid by centrifugation and filtration.
"IP" refers to the Institute of Petroleum, now the Energy Institute of London,
United
Kingdom.
"Iso-paraffins" refer to branched chain saturated hydrocarbons.
"Kerosene" refers to hydrocarbons with a boiling range distribution from 204 C
up to
260 C (400 F up to 500 F) at a pressure of 0.101 MPa. Kerosene content maybe
determined by the quantity of hydrocarbons having a boiling range of from 204
C to
260 C at a pressure of 0.101 MPa relative to a total quantity of hydrocarbons
as
measured by boiling range distribution in accordance with ASTM Method D5307.
"Lewis base" refers to a compound and/or material with the ability to donate
one or
more electrons to another compound.
"Ligand" as used herein is defined as a molecule, compound, atom, or ion
attached to,
or capable of attaching to, a metal ion in a coordination complex.
"Light hydrocarbons" refers to hydrocarbons having carbon numbers in a range
from
1 to 6.
"Mixing" as used herein is defined as contacting two or more substances by
intermingling the two or more substances. Blending, as used herein, is a
subclass of
mixing, where blending requires intimately admixing or intimately
intermingling the
two or more substances, for example into a homogenous dispersion.
16
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
"Monomer" as used herein is defined as a molecular compound or portion of a
molecular compound that may be reactively joined with itself or another
monomer in
repeated linked units to form a polymer.
"Naphtha" refers to hydrocarbon components with a boiling range distribution
from
38 C up to 204 C (100 F up to 400 F) at a pressure of 0.101 MPa. Naphtha
content
may be determined by the quantity of hydrocarbons having a boiling range of
from
38 C to 204 C at a pressure of 0.101 MPa relative to a total quantity of
hydrocarbons
as measured by boiling range distribution in accordance with ASTM Method
D5307.
Content of hydrocarbon components, for example, paraffins, iso-paraffins,
olefins,
naphthenes and aromatics in naphtha are as determined by ASTM Method D6730.
"n-Paraffins" refer to normal (straight chain) saturated hydrocarbons.
"Olefins" refer to hydrocarbon compounds with non-aromatic carbon-carbon
double
bonds. Types of olefins include, but are not limited to, cis, trans, internal,
terminal,
branched, and linear.
When two or more elements are described as "operatively connected", the
elements
are defined to be directly or indirectly connected to allow direct or indirect
fluid flow
between the elements.
"Periodic Table" refers to the Periodic Table as specified by the
International Union
of Pure and Applied Chemistry (IUPAC), November 2003. As used herein, an
element of the Periodic Table of Elements may be referred to by its symbol in
the
Periodic Table. For example, Cu may be used to refer to copper, Ag may be used
to
refer to silver, W may be used to refer to tungsten etc.
"Polyaromatic compounds" refer to compounds that include three or more
aromatic
rings. Examples of polyaromatic compounds include, but are not limited to
anthracene and phenanthrene.
"Polymer" as used herein is defined herein as a compound comprised of
repetitively
linked monomers.
"Pore size distribution" refers a distribution of pore size diameters of a
material as
measured by ASTM Method D4641.
"SCFB" refers to standard cubic feet of gas per barrel of crude feed.
"STP" as used herein refers to Standard Temperature and Pressure, which is 25
C and
0.101 MPa.
17
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
"TAN" refers to a total acid number expressed as millgrams ("mg") of KOH per
gram
("g") of sample. TAN is as determined by ASTM Method D664.
"VGO" refers to hydrocarbons with a boiling range distribution of from 343 C
up to
538 C (650 F up to 1000 F) at 0.101 MPa. VGO content may be determined by the
quantity of hydrocarbons having a boiling range of from 343 C to 538 C at a
pressure
of 0.101 MPa relative to a total quantity of hydrocarbons as measured by
boiling
range distribution in accordance with ASTM Method D5307.
"wppm" as used herein refers to parts per million, by weight.
The present invention is directed to a process for cracking a hydrocarbon-
containing feedstock in which the hydrocarbon-containing feedstock, hydrogen,
hydrogen sulfide, and a catalyst, as defined herein, are mixed at a
temperature of from
375 C to 500 C and a total pressure of from 6.9 MPa to 27.5 MPa (1000 psi to
4000
psi), and a vapor comprising a hydrocarbon-containing product comprising one
or
more hydrocarbon compounds that are liquid at STP is separated from the
mixture.
The hydrogen sulfide is mixed in the mixture of hydrocarbon-containing
feedstock,
hydrogen, hydrogen sulfide, and catalyst in an amount sufficient to decrease
the
activation energy of the hydrocracking reaction-preferably at a mole ratio of
hydrogen sulfide to hydrogen of from 0.5:9.5 to 1:1.
Hydrocarbon-containing feedstock
The hydrocarbon-containing feedstock contains heavy hydrocarbons that are
subject to being cracked in the process. The hydrocarbon-containing feedstock,
therefore, is selected to contain at least 20 wt.% hydrocarbons having a
boiling point
of greater than 538 C. The amount of hydrocarbons having a boiling point of
greater
than 538 C in a hydrocarbon-containing material may be determined in
accordance
with ASTM Method D5307. The hydrocarbon-containing feedstock may be selected
to contain at least 25 wt. %, or at least 30 wt. %, or at least 35 wt. %, or
at least 40
wt.%, or at least 45 wt.%, or at least 50 wt.% hydrocarbons having a boiling
point of
greater than 538 C. The hydrocarbon-containing feedstock may be selected to
contain at least 20 wt.% residue, or at least 25 wt.% residue, or at least 30
wt.%
residue, or at least 35 wt.% residue, or at least 40 wt.% residue, or at least
45 wt.%
residue, or least 50 wt.% residue.
18
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
The hydrocarbon-containing feedstock may contain significant quantities of
lighter hydrocarbons as well as the heavy hydrocarbons. The hydrocarbon-
containing
feedstock may contain at least 30 wt.%, or at least 35 wt.%, or at least 40
wt.%, or at
least 45 wt.%, or at least 50 wt.% of hydrocarbons having a boiling point of
538 C or
less. The amount of hydrocarbons having a boiling point of 538 C or less in a
hydrocarbon-containing material may be determined in accordance with ASTM
Method D5307. The hydrocarbon-containing feedstock may contain at least 20
wt.%,
or at least 25 wt.%, or at least 30 wt.%, or at least 35 wt.%, or at least 40
wt.%, or at
least 45 wt.% of naphtha and distillate. The hydrocarbon-containing feedstock
may
be a crude oil, or may be a topped crude oil.
The hydrocarbon-containing feedstock may also contain quantities of metals
such as vanadium and nickel. The hydrocarbon-containing feedstock may contain
at
least 50 wppm vanadium and at least 20 wppm nickel.
The hydrocarbon-containing feedstock may also contain quantities of sulfur
and nitrogen. The hydrocarbon containing feedstock may contain at least 2 wt.%
sulfur, or at least 3 wt.% sulfur; and the hydrocarbon-containing feedstock
may
contain at least 0.25 wt. % nitrogen, or at least 0.4 wt. % nitrogen.
The hydrocarbon-containing feedstock may also contain appreciable quantities
of naphthenic acids. For example, the hydrocarbon-containing feedstock may
have a
TAN of at least 0.5, or at least 1.0, or at least 2Ø
The hydrocarbon-containing feedstock may have a relatively low API gravity.
The API gravity of the hydrocarbon-containing feedstock may be less than 19,
or less
than 15, or less than 10, or less than 5.
The process of the present invention is particularly applicable to certain
heavy
petroleum and coal derived hydrocarbon-containing feedstocks. The hydrocarbon-
containing feedstock may be a heavy or an extra-heavy crude oil containing
significant quantities of residue or pitch; a topped heavy or topped extra-
heavy crude
oil containing significant quantities of residue or pitch; bitumen;
hydrocarbons
derived from tar sands; shale oil; crude oil atmospheric residues; crude oil
vacuum
residues; asphalts; and hydrocarbons derived from liquefying coal.
Hydrogen
The hydrogen that is mixed with the hydrocarbon-containing feedstock, the
hydrogen sulfide, and the catalyst in the process of the present invention is
derived
19
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
from a hydrogen source. The hydrogen source may be hydrogen gas obtained from
any conventional sources or methods for producing hydrogen gas. In one
embodiment
of the process of the present invention, the hydrogen source may be synthesis
gas.
Hydrogen sulfide
The hydrogen sulfide that is mixed with the hydrocarbon-containing
feedstock, the hydrogen, and the catalyst in the process of the present
invention may
be obtained from any conventional source or method for producing hydrogen
sulfide.
The hydrogen sulfide provided to be mixed with the hydrocarbon-containing
feedstock, hydrogen, and catalyst may be a gas or a liquid.
Catalyst
As described above, the catalyst that is mixed with the hydrocarbon-
containing feedstock and the hydrogen is comprised of a material that is
comprised of
a first metal, a second metal, and sulfur. The first metal of the material of
the catalyst
is a metal selected from the group consisting of copper (Cu), iron (Fe),
nickel (Ni),
cobalt (Co), bismuth (Bi), silver (Ag), manganese (Mn), zinc (Zn), tin (Sn),
ruthenium
(Ru), lanthanum (La), cerium (Ce), praseodymium (Pr), samarium (Sm), europium
(Eu), ytterbium (Yb), lutetium (Lu), dysprosium (Dy), lead (Pb), and antimony
(Sb).
In a preferred embodiment, the first metal is relatively electron-rich,
inexpensive, and
relatively non-toxic, and preferably the first metal is selected to be copper
or iron,
most preferably copper. The second metal of the material of the catalyst is a
metal
selected from the group consisting of molybdenum (Mo), tungsten (W), vanadium
(V), tin (Sn), and antimony (Sb), where the second metal is not the same metal
as the
first metal, and preferably is molybdenum.
The material of the catalyst is comprised of at least three linked chain
elements, where the chain elements are comprised of a first chain element and
a
second chain element. The first chain element includes the first metal and
sulfur and
has a structure according to formula (I) and the second chain element includes
the
second metal and sulfur and has a structure according to formula (II):
S 2 S
M M
S S
(I) (II)
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
where M1 is the first metal and M2 is the second metal. The catalyst material
containing the chain elements contains at least one first chain element and at
least one
second chain element. The chain elements of the material of the catalyst are
linked by
bonds between the two sulfur atoms of a chain element and the metal of an
adjacent
chain element. A chain element of the material of the catalyst may be linked
to one,
or two, or three, or four other chain elements, where each chain element may
be
linked to other chain elements by bonds between the two sulfur atoms of a
chain
element and the metal of an adjacent chain element. In an embodiment of the
invention, at least three linked chain elements of the material of the
catalyst are
sequentially linked in series. At least a portion of the material of the
catalyst
containing the chain elements may be comprised of the first metal and the
second
metal linked by, and bonded to, sulfur atoms according to formula (III):
r /S\ 2 'S\
M M
x
(III)
where M1 is the first metal, M2 is the second metal, and x is at least 2. The
material of
the catalyst may be a polythiometallate polymer, where each monomer of the
polymer
is the structure as shown in formula (III) where x=1, and the
polythiometallate
polymer is the structure as shown in formula (III) where x is at least 5. At
least a
portion of the material of the catalyst may be comprised of the first metal
and second
metal, where the first metal is linked to the second metal by sulfur atoms as
according
to formula (IV) or formula (V):
1 ~S\ 2 /S\ 1
M M M
~S/ \S/
(IV)
21
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
S\ 2 /S\ 1/S\ 2 /S
M M M
S \S/ \S/ \S//
(V)
where M1 is the first metal and where M2 is the second metal.
The material of the catalyst may comprise a third chain element comprised of
sulfur and a third metal selected from the group consisting of Cu, Fe, Bi, Ag,
Mn, Zn,
Ni, Co, Sn, Re, Rh, Pd, Ir, Pt, Ce, La, Pr, Sm, Eu, Yb, Lu, Dy, Pb, Cd, Sb,
and In,
where the third metal is not the same as the first metal or the second metal.
The third
chain element has a structure according to formula (VI):
S IN,
S
(VI)
where M3 is the third metal. If the material of the catalyst contains a third
chain
element, at least a portion of the third chain element of the material of the
catalyst is
linked by bonds between the two sulfur atoms of a chain element and the metal
of an
adjacent chain element.
The catalyst used in the process of the present invention preferably is formed
primarily of the material comprised of the first metal, second metal, and
sulfur, and
the material of the catalyst is formed primarily of the first metal, second
metal, and
sulfur. The first metal, second metal, and sulfur may comprise at least 75
wt.%, or at
least 80 wt.%, or at least 85 wt.%, or at least 90 wt.%, or at least 95 wt.%,
or at least
99 wt.% or 100 wt.% of the material of the catalyst, where the material of the
catalyst
comprises at least 50 wt.% or at least 60 wt.%, or at least 70 wt.%, or at
least 75 wt.%,
or at least 80 wt.%, or at least 90 wt.%, or at least 95 wt.%, or at least 99
wt.% or 100
wt.% of the catalyst. In an embodiment, the catalyst comprises at most 0.1
wt.%, or at
most 0.01 wt.%, or at most 0.001 wt.% of alumina, alumina-silica, or silica,
and,
preferably, the catalyst contains no detectable alumina, alumina-silica, or
silica.
The first metal may be present in the material of the catalyst, and/or in the
catalyst, in an atomic ratio relative to the second metal of at least 1:2. The
atomic
ratio of the first metal to the second metal in the material of the catalyst,
and/or in the
22
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
catalyst, may be greater than 1:2, or at least 2:3, or at least 1:1, or at
least 2:1, or at
least 3:1, or at least 5:1. It is believed that the first metal contributes
significantly to
the catalytic activity of the catalyst in the process of the present invention
when the
first metal is present in the material of the catalyst, and/or in the
catalyst, in an amount
relative to the second metal ranging from slightly less of the first metal to
the second
metal to significantly more of the first metal to the second metal. Therefore,
the first
metal may be incorporated in the material of the catalyst, and/or in the
catalyst, in an
amount, relative to the second metal, such that the atomic ratio of the first
metal to the
second metal ranges from one half to significantly greater than one, such that
the first
metal is not merely a promoter of the second metal in the material of the
catalyst,
and/or in the catalyst.
The catalyst and the material of the catalyst may contain little or no oxygen.
As discussed above, the catalytic activity of the catalyst in the process of
the present
invention is, in part, believed to be due to the availability of electrons
from the
catalyst. Due to its electronegativity, oxygen tends to reduce the
availability of
electrons from the catalyst and the material of the catalyst when it is
present in the
material of the catalyst in appreciable quantities, therefore, the catalyst
preferably
contains little or no oxygen. The catalyst, and the material of the catalyst,
may
comprise at most 0.1 wt.%, or at most 0.05 wt.%, or at most 0.01 wt.% oxygen
as
measured by neutron activation. In a preferred embodiment, oxygen is not
detectable
in the catalyst or in the material of the catalyst.
The catalyst used in the process of the present invention is preferably
substantially non-acidic. The catalyst used in the process of the present
invention
may have an acidity as measured by ammonia chemisorption of at most 200 mol
ammonia per gram of catalyst, or at most 100 mol ammonia per gram of
catalyst, or
at most 50 mol ammonia per gram of catalyst, or at most 25, or at most 10
mol
ammonia per gram of catalyst. The catalyst may have an acidity as measured by
ammonia chemisorption of 0 mol ammonia per gram of catalyst. The catalyst
should be sufficiently non-acidic to avoid catalyzing the formation of coke.
It is
believed that coke formation, in part, is induced by the formation of
hydrocarbon
cation radicals upon cracking a hydrocarbon-which is promoted by catalysts
having
significant acidity. Therefore, it is preferred that the catalyst have little
or no acidity
to avoid selectively directing cracking reactions in a manner that promotes
the
formation of coke.
23
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
The catalyst-when primarily formed of the material of the catalyst, where the
material of the catalyst is primarily formed of the first metal, the second
metal, and
sulfur, and particularly when the first metal, the second metal, and the
sulfur that form
the material of the catalyst are not supported on a carrier or support
material to form
the catalyst-may have a significant degree of porosity, pore volume, and
surface
area. In the absence of a support or a carrier, the catalyst may have a pore
size
distribution, where the pore size distribution has a mean and/or median pore
diameter
of from 50 angstroms to 1000 angstroms, or from 60 angstroms to 350 angstroms.
In
the absence of a support or a carrier, the catalyst may have a pore volume of
at least
0.2 cm3/g, or at least 0.25 cm3/g, or at least 0.3 cm3/g, or at least 0.35
cm3/g, or at
least 0.4 cm3/g. In the absence of a support or a carrier, the catalyst may
have a BET
surface area of at least 50 m2/g, or at least 100 m2, and up to 400 m2/g or up
to 500
m2/g.
The relatively large surface area of the catalyst, particularly relative to
conventional non-supported bulk metal catalysts, is believed to be due, in
part, to the
porosity of the catalyst imparted by at least a portion of the material of the
catalyst
being formed of abutting or adjoining linked tetrahedrally structured atomic
formations of the first metal and sulfur and the second metal and sulfur,
where the
tetrahedrally structured atomic formations may be edge-bonded. Interstices or
holes
that form the pore structure of the catalyst may be present in the material of
the
catalyst as a result of the bonding patterns of the tetrahedral structures.
The catalyst,
therefore, may be highly catalytically active since 1) the catalyst has a
relatively large
surface area; and 2) the surface area of the catalyst is formed substantially,
or entirely,
of the elements that provide catalytic activity.
The catalyst may be a solid particulate substance having a particle size
distribution with a relatively small mean particle size and/or median particle
size,
where the solid catalyst particles preferably are nanometer size particles.
The catalyst
may have a particle size distribution with a median particle size and/or mean
particle
size of at least 50 nm, or at least 75 nm, or up to 5 m, or up to 1 m; or up
to 750
nm, or from 50 nm up to 5 m. The solid particulate catalyst having a particle
size
distribution with a large quantity of small particles, for example having a
mean or
median particle size of up to 1 m, has a large aggregate surface area since
little of the
catalyst material is located within the interior of a particle. The
particulate catalyst
having a particle size distribution with a large quantity of small particles,
therefore,
24
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
may be desirable for use in the process of the present invention to provide a
relatively
high degree catalytic activity due to the surface area of the catalyst
available for
catalytic activity. The catalyst used in the process of the invention may be a
solid
particulate substance preferably having a particle size distribution with a
mean or
median particle size of up to 1 m, preferably having a pore size distribution
with a
mean pore diameter of from 50 angstroms to 300 angstroms, preferably having a
porosity of at least 0.2 cm3/g, and preferably having a BET surface area of at
least 50
m2/g.
The solid particulate catalyst may be insoluble in the hydrocarbon-containing
feed and in a hydrocarbon-depleted feed residuum formed by the process of the
present invention. The solid particulate catalyst having a particle size
distribution of
at least 50 nm may be insoluble in the hydrocarbon-containing feed and the
hydrocarbon-depleted residuum due, in part, to the size of the particles,
which may be
too large to be solvated by the hydrocarbon-containing feed or the residuum.
Use of a
solid particulate catalyst which is insoluble in the hydrocarbon-containing
feed and
the hydrocarbon-depleted residuum may be desirable in the process of the
present
invention so that the catalyst may be separated from the residuum formed by
the
process, and subsequently regenerated for reuse in the process.
The material of the catalyst may contain less than 0.5 wt.% of ligands other
than the sulfur-metal bonded complexes between sulfur and the first metal and
between sulfur and the second metal. Ligands, other than the sulfur-metal
bonded
complexes with the first metal and the second metal, may not be present in
significant
quantities in the catalyst material since they may limit the particle size of
the material
of the catalyst to less than 50 nm, for example, by inhibiting the first metal
and the
second metal from forming sulfur-bridged chains.
Method of preparing the catalyst
The material of the catalyst, and/or the catalyst, utilized in the process of
the
present invention may be prepared by mixing a first salt and a second salt in
an
aqueous mixture under anaerobic conditions at a temperature of from 15 C to
150 C,
and separating a solid from the aqueous mixture to produce the catalyst
material.
The first salt utilized to form the material of the catalyst, and/or the
catalyst,
includes a cationic component comprising a metal in any non-zero oxidation
state
selected from the group consisting of Cu, Fe, Ni, Co, Bi, Ag, Mn, Zn, Sn, Ru,
La, Ce,
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
Pr, Sm, Eu, Yb, Lu, Dy, Pb, and Sb, where the metal of the cationic component
is the
first metal of the material of the catalyst. The cationic component of the
first salt may
consist essentially of a metal selected from the group consisting of Cu, Fe,
Ni, Co, Bi,
Ag, Mn, Zn, Sn, Ru, La, Ce, Pr, Sm, Eu, Yb, Lu, Dy, Pb, and Sb. The cationic
component of the first salt must be capable of bonding with the anionic
component of
the second salt to form the material of the catalyst in the aqueous mixture at
a
temperature of from 15 C to 150 C and under anaerobic conditions.
The first salt also contains an anionic component associated with the cationic
component of the first salt to form the first salt. The anionic component of
the first
salt may be selected from a wide range of counterions to the cationic
component of
the first salt so long as the combined cationic component and the anionic
component
of the first salt form a salt that is dispersible, and preferably soluble, in
the aqueous
mixture in which the first salt and the second salt are mixed, and so long as
the
anionic component of the first salt does not prevent the combination of the
cationic
component of the first salt with the anionic component of the second salt in
the
aqueous mixture to form the material of the catalyst. The anionic component of
the
first salt may be selected from the group consisting of sulfate, chloride,
bromide,
iodide, acetate, acetylacetonate, phosphate, nitrate, perchlorate, oxalate,
citrate, and
tartrate.
Certain compounds are preferred for use as the first salt to form the catalyst
material. In particular, the first salt is preferably selected from the group
consisting of
CuS04, copper acetate, copper acetylacetonate, Ni FeS04, Fe2(SO4)3, iron
acetate,
iron acetylacetonate, NiS04, nickel acetate, nickel acetylacetonate, CoS04,
cobalt
acetate, cobalt acetylacetonate, ZnC12, ZnS04, zinc acetate, zinc
acetylacetonate,
silver acetate, silver acetylacetonate, SnS04, SnC14, tin acetate, tin
acetylacetonate,
MnS04, manganese acetate, manganese acetylacetonate, bismuth acetate, bismuth
acetylacetonate, and hydrates thereof. These materials are generally
commercially
available, or may be prepared from commercially available materials according
to
well-known methods.
The first salt may be contained in an aqueous solution or an aqueous mixture,
where the aqueous solution or aqueous mixture containing the first salt
(hereinafter
the "first aqueous solution") may be mixed with an aqueous solution or an
aqueous
mixture containing the second salt (hereinafter the "second aqueous solution")
in the
26
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
aqueous mixture to form the material of the catalyst. The first salt may be
dispersible,
and most preferably soluble, in the first aqueous solution and is dispersible,
and
preferably soluble, in the aqueous mixture of the first and second salts. The
first
aqueous solution may contain more than 50 vol.% water, or at least 75 vol.%
water, or
at least 90 vol.% water, or at least 95 vol.% water, and may contain more than
0 vol.%
but less than 50 vol.%, or at most 25 vol.%, or at most 10 vol.%, or at most 5
vol.% of
an organic solvent containing from 1 to 5 carbons selected from the group
consisting
of an alcohol, a diol, an aldehyde, a ketone, an amine, an amide, a furan, an
ether,
acetonitrile, and mixtures thereof. The organic solvent present in the first
aqueous
solution, if any, should be selected so that the organic compounds in the
organic
solvent do not inhibit reaction of the cationic component of the first salt
with the
anionic component of the second salt upon forming an aqueous mixture
containing the
first and second salts, e.g., by forming ligands or by reacting with the first
or second
salts or their respective cationic or anionic components. The first aqueous
solution
may contain no organic solvent, and may consist essentially of water,
preferably
deionized water, and the first salt.
The concentration of the first salt in the first aqueous solution may be
selected
to promote formation of the material of the catalyst, and/or the catalyst,
having a
particle size distribution with a small mean and/or median particle size,
where the
particles have a relatively large surface area, upon mixing the first salt and
the second
salt in the aqueous mixture. To promote the formation of a catalyst material
having a
relatively large surface area and having particle size distribution with a
relatively
small mean and/or median particle size, the first aqueous solution may contain
at most
3 moles per liter, or at most 2 moles per liter, or at most 1 mole per liter,
or at most
0.6 moles per liter, or at most 0.2 moles per liter of the first salt.
The second salt utilized to form the catalyst material and/or the catalyst
includes an anionic component that is a tetrathiometallate of molybdenum,
tungsten,
vanadium, tin or antimony. In particular, the second salt may contain an
anionic
component that is selected from the group consisting of MoS42 , WS42 , VS42 ,
SnS44-
and SbS43-
The second salt also contains a cationic component associated with the anionic
component of the second salt to form the second salt. The cationic component
of the
second salt may be selected from an ammonium counterion, and alkali metal and
alkaline earth metal counterions to the tetrathiometallate anionic component
of the
27
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
second salt so long as the combined cationic component and the anionic
component of
the second salt form a salt that is dispersable, and preferably soluble, in
the aqueous
mixture in which the first salt and the second salt are mixed, and so long as
the
cationic component of the second salt does not prevent the combination of the
cationic component of the first salt with the anionic component of the second
salt in
the aqueous mixture to form the catalyst material. The cationic component of
the
second salt may comprise one or more sodium ions, or one or more potassium
ions, or
one or more ammonium ions.
Certain compounds are preferred for use as the second salt used to form the
material of the catalyst and/or the catalyst. In particular, the second salt
is preferably
selected from the group consisting of Na2MoS4, Na2WS4, Na3VS4, K2MoS4, K2WS4,
K3VS4, (NH4)2MOS4, (NH4)2WS4, (NH4)3VS4, Na4SnS4, (NH4)4SnS4, (NH4)3SbS4,
Na3SbS4, and hydrates thereof.
The second salt may be a commercially available tetrathiomolybdate or
tetrathiotungstate salt. For example, the second salt may be ammonium
tetrathiomolybdate, which is commercially available from AAA Molybdenum
Products, Inc. 7233 W. 116 Pl., Broomfield, Colorado, USA 80020, or ammonium
tetrathiotungstate, which is commercially available from Sigma-Aldrich, 3050
Spruce
St., St. Louis, Missouri, USA 63103, or ammonium tetrathiovanadate, which is
commercially available from Chemos GmbH, Germany. .
Alternatively, the second salt may be produced from a commercially available
tetrathiomolybdate or tetrathiotungstate salt. For example, the second salt
may be
produced from ammonium tetrathiomolybdate, ammonium tetrathiotungstate, or
from
ammonium tetrathiovanadate. The second salt may be formed from the
commercially
available ammonium tetrathiometallate salts by exchanging the cationic
ammonium
component of the commercially available salt with a desired alkali or alkaline
earth
cationic component from a separate salt. The exchange of the cationic
components to
form the desired second salt may be effected by mixing the commercially
available
salt and the salt containing the desired cationic component in an aqueous
solution to
form the desired second salt.
A method of forming the second salt is to disperse an ammonium
tetrathiomolybdate, ammonium tetrathiotungstate, or ammonium tetrathiovanadate
salt in an aqueous solution, preferably water, and to disperse an alkali metal
or
alkaline earth metal cationic component donor salt, preferably a carbonate, in
the
28
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
aqueous solution, where the cationic component donor salt is provided in an
amount
relative to the ammonium tetrathiomolybdate, ammonium tetrathiotungstate, or
ammonium tetrathiovanadate salt to provide a stoichiometrially equivalent or
greater
amount of its cation to ammonium of the ammonium tetrathiomolybdate, ammonium
tetrathiotungstate, or ammonium tetrathiovanadate salt. The aqueous solution
may be
heated to a temperature of at least 50 C, or at least 65 C up to 100 C to
evolve
ammonia from the ammonium containing salt and carbon dioxide from the
carbonate
containing salt as gases, and to form the second salt. For example a Na2MoS4
salt
may be prepared for use as the second salt by mixing commercially available
(NH4)2M0S4 and Na2CO3 in water at a temperature of 70 C-80 C for a time period
sufficient to permit evolution of a significant amount, preferably
substantially all, of
ammonia and carbon dioxide gases from the solution, typically from 30 minutes
to 4
hours, and usually about 2 hours.
If the second salt is a sodium tetrathiostannate salt, it may be produced by
dissolving Na2Sn(OH)6 and Na2S in a 1:4 molar ratio in boiling deionized water
(100
g of Na2Sn(OH)6 per 700 ml of water and 250 g of Na2S per 700 ml of water),
stirring
the mixture at 90-100 C for 2-3 hours, adding finely pulverized MgO to the
mixture
at a 2:5 wt. ratio relative to the Na2Sn(OH)6 and continuing stirring the
mixture at 90-
100 C for an additional 2-3 hours, cooling and collecting precipitated
impurities from
the mixture, then concentrating the remaining solution by 50-60 vol.%,
allowing the
concentrated solution to stand, then collecting the Na4SnS4 that crystallizes
from the
concentrated solution. An ammonium tetrathiostannate salt may be produced by
mixing SnS2 with (NH4)2S in a 1:2 mole ratio in liquid ammonia under an inert
gas
(e.g. nitrogen), filtering, and recovering the solid (NH)4SnS4 as a residue.
The second salt may be contained in an aqueous solution (the second aqueous
solution, as noted above), where the second aqueous solution containing the
second
salt may be mixed with the first aqueous solution containing the first salt in
the
aqueous mixture to form the material of the catalyst. The second salt is
preferably
dispersible, and most preferably soluble, in the second aqueous solution and
is
dispersible, and preferably soluble, in the aqueous mixture containing the
first and
second salts. The second aqueous solution contains more than 50 vol.% water,
or at
least 75 vol.% water, or at least 90 vol.% water, or at least 95 vol.% water,
and may
contain more than 0 vol.% but less than 50 vol.%, or at most 25 vol.%, or at
most 10
29
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
vol.%, or at most 5 vol.% of an organic solvent containing from 1 to 5 carbons
and
selected from the group consisting of an alcohol, a diol, an aldehyde, a
ketone, an
amine, an amide, a furan, an ether, acetonitrile, and mixtures thereof. The
organic
solvent present in the second aqueous solution, if any, should be selected so
that the
organic compounds in the organic solvent do not inhibit reaction of the
cationic
component of the first salt with the anionic component of the second salt upon
forming an aqueous mixture containing the first and second salts, e.g., by
forming
ligands or by reacting with the first or second salts or their respective
cationic or
anionic components. Preferably, the second aqueous solution contains no
organic
solvent. Most preferably the second aqueous solution consists essentially of
water,
preferably deionized, and the second salt.
The concentration of the second salt in the second aqueous solution may be
selected to promote formation of the material of the catalyst having a
particle size
distribution with a small mean and/or median particle size and having a
relatively
large surface area per particle upon mixing the first salt and the second salt
in the
aqueous mixture. To promote the formation of a catalyst material having a
particle
size distribution with a relatively small mean and/or median particle size,
the second
aqueous solution may contain at most 0.8 moles per liter, or at most 0.6 moles
per
liter, or at most 0.4 moles per liter, or at most 0.2 moles per liter, or at
most 0.1 moles
per liter of the second salt.
The first and second solutions containing the first and second salts,
respectively, may be mixed in an aqueous mixture to form the material of the
catalyst
and/or the catalyst. The amount of the first salt relative to the amount of
the second
salt provided to the aqueous mixture may be selected so that the atomic ratio
of the
cationic component metal of the first salt to the metal of the anionic
component of the
second salt, either molybdenum or tungsten, is at least 1:2, or at least 2:3,
or at least
1:1, and at most 20:1, or at most 15:1, or at most 10:1.
The aqueous mixture of the first and second salts may be formed by adding the
first aqueous solution containing the first salt and the second aqueous
solution
containing the second salt into an aqueous solution separate from both the
first
aqueous solution and the second aqueous solution. The separate aqueous
solution will
be referred hereafter as the "third aqueous solution". The third aqueous
solution may
contain more than 50 vol.% water, or at least 75 vol.% water, or at least 90
vol.%
water, or at least 95 vol.% water, and may contain more than 0 vol.% but less
than 50
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
vol.%, or at most 25 vol.%, or at most 10 vol.%, or at most 5 vol.% of an
organic
solvent containing from 1 to 5 carbons and selected from the group consisting
of an
alcohol, a diol, an aldehyde, a ketone, an amine, an amide, a furan, an ether,
acetonitrile, and mixtures thereof. The organic solvent present in the third
aqueous
solution, if any, should be selected so that the organic compounds in the
organic
solvent do not inhibit reaction of the cationic component of the first salt
with the
anionic component of the second salt upon forming the aqueous mixture, e.g.,
by
forming ligands or reacting with the cationic component of the first salt or
with the
anionic component of the second salt. Preferably, the third aqueous solution
contains
no organic solvent, and most preferably comprises deionized water.
The aqueous mixture of the first and second salts may be formed by
combining the first aqueous solution containing the first salt and the second
aqueous
solution containing the second salt in the third aqueous solution. The volume
ratio of
the third aqueous solution to the first aqueous solution containing the first
salt may be
from 0.5:1 to 50:1 where the first aqueous solution may contain at most 3, or
at most
2, or at most 1, or at most 0.8, or at most 0.5, or at most 0.3 moles of the
first salt per
liter of the first aqueous solution. Likewise, the volume ratio of the third
aqueous
solution to the second aqueous solution containing the second salt may be from
0.5:1
to 50:1 where the second aqueous solution may contain at most 0.8, or at most
0.4, or
at most 0.2, or at most 0.1 moles of the second salt per liter of the second
aqueous
solution.
The first salt and the second salt may be combined in the aqueous mixture so
that the aqueous mixture containing the first and second salts contains at
most 1.5, or
at most 1.2, or at most 1, or at most 0.8, or at most 0.6 moles of the
combined first and
second salts per liter of the aqueous mixture. The particle size of the
catalyst material
produced by mixing the first and second salts in the aqueous mixture
increases, and
the surface area of the particles decreases, with increasing concentrations of
the salts.
Therefore, to limit the particle sizes in the particle size distribution of
the catalyst
material and to increase the relative surface area of the particles, the
aqueous mixture
may contain at most 0.8 moles of the combined first and second salts per liter
of the
aqueous mixture, more preferably at most 0.6 moles, or at most 0.4 moles, or
at most
0.2 moles of the combined first and second salts per liter of the aqueous
mixture. The
amount of the first salt and the total volume of the aqueous mixture may be
selected to
provide at most 1, or at most 0.8, or at most 0.4 moles of the cationic
component of
31
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
the first salt per liter of the aqueous mixture and the amount of the second
salt and the
total volume of the aqueous mixture may be selected to provide at most 0.4, or
at most
0.2, or at most 0.1, or at most 0.01 moles of the anionic component of the
second salt
per liter of the aqueous mixture.
The rate of addition of the first and second aqueous solutions containing the
first and second salts, respectively, to the aqueous mixture may be controlled
to limit
the instantaneous concentration of the first and second salts in the aqueous
mixture to
produce a catalyst material comprised of relatively small particles having
relatively
large surface area. Limiting the instantaneous concentration of the salts in
the
aqueous mixture may reduce the mean and/or median particle size of the
resulting
catalyst material by limiting the simultaneous availability of large
quantities of the
cationic components of the first salt and large quantities of the anionic
components of
the second salt that may interact to form a catalyst material comprised
primarily of
relatively large particles. The rate of addition of the first and second
solutions to the
aqueous mixture may be controlled to limit the instantaneous concentration of
the first
salt and the second salt in the aqueous mixture to at most 0.05 moles per
liter, or at
most 0.01 moles per liter, or at most 0.001 moles per liter.
The first aqueous solution containing the first salt and the second aqueous
solution containing the second salt may be added to the third aqueous
solution,
preferably simultaneously, at a controlled rate selected to provide a desired
instantaneous concentration of the first salt and the second salt in the
aqueous
mixture. The first aqueous solution containing the first salt and the second
aqueous
solution containing the second salt may be added to the third aqueous solution
at a
controlled rate by adding the first aqueous solution and the second aqueous
solution to
the third aqueous solution in a dropwise manner. The rate that drops of the
first
aqueous solution and the second aqueous solution are added to the third
aqueous
solution may be controlled to limit the instantaneous concentration of the
first salt and
the second salt in the aqueous mixture as desired. The first aqueous solution
containing the first salt and the second aqueous solution containing the
second salt
may be dispersed directly into the third aqueous solution at a flow rate
selected to
provide a desired instantaneous concentration of the first salt and the second
salt. The
first aqueous solution and the second aqueous solution may be dispersed
directly into
the third aqueous solution using conventional means for dispersing one
solution into
another solution at a controlled flow rate. For example, the first aqueous
solution and
32
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
the second aqueous solution may be dispersed into the third aqueous solution
through
separate nozzles located within the third aqueous solution, where the flow of
the first
and second solutions through the nozzles is metered by separate flow metering
devices.
The particle size distribution of the catalyst material produced by mixing the
first salt and the second salt in the aqueous mixture is preferably controlled
by the rate
of addition of the first and second aqueous solutions to the third aqueous
solution, as
described above, so that the median and/or mean particle size of the particle
size
distribution falls within a range of from 50 nm to 5 m. The particle size
distribution
of the catalyst material may be controlled by the rate of addition of the
first and
second aqueous solutions to the third aqueous solution so that the median
and/or mean
particle size of the particle size distribution of the catalyst material may
range from at
least 50 nm up to 5 m, or up to 1 m, or up to 750 nm.
The surface area of the catalyst material particles produced by mixing the
first
and second aqueous solutions in the third aqueous solution is preferably
controlled by
the rate of addition of the first and second aqueous solutions to the third
aqueous
solution, as described above, so that the BET surface area of the catalyst
material
particles may range from 50 m2/g to 500 m2/g. The surface area of the catalyst
material particles may be controlled by the rate of addition of the first and
second
aqueous solutions to the third aqueous solution so that the BET surface area
of the
catalyst material particles is from 100 m2/g to 350 m2/g
The aqueous mixture containing the first salt and the second salt may be
mixed to facilitate interaction and reaction of the cationic component of the
first salt
with the anionic component of the second salt to form the catalyst material.
The
aqueous mixture may be mixed by any conventional means for agitating an
aqueous
solution or an aqueous dispersion, for example by mechanical stirring.
During mixing of the aqueous mixture of the first and second salts, the
temperature of the aqueous mixture is maintained in the range of from 15 C to
150 C,
or from 60 C to 125 C, or from 65 C to 100 C. When the cationic component of
the
second salt is ammonium, the temperature should be maintained in a range from
65 C
to 150 C to evolve ammonia as a gas from the second salt. The temperature of
the
aqueous mixture during mixing may be maintained at less than 100 C so that the
mixing may be conducted without the application of positive pressure necessary
to
33
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
inhibit the water in the aqueous mixture from becoming steam. If the second
salt is a
tetrathiostannate, the temperature of the aqueous mixture may be maintained at
100 C
or less to inhibit the degradation of the second salt into tin disulfides.
Maintaining the temperature of the aqueous mixture in a range of from 50 C
to 150 C may result in production of a catalyst material having a relatively
large
surface area and a substantially reduced median or mean particle size relative
to a
catalyst material produced in the same manner at a lower temperature. It is
believed
that maintaining the temperature in the range of 50 C to 150 C drives the
reaction of
the cationic component of the first salt with the anionic component of the
second salt,
reducing the reaction time and limiting the time available for the resulting
product to
agglomerate prior to precipitation. Maintaining the temperature in a range of
from
50 C to 150 C during the mixing of the first and second salts in the aqueous
mixture
may result in production of a catalyst material having a particle size
distribution with
a median or mean particle size of from 50 nm up to 5 m, or up to 1 m, or up
to 750
nm; and having a BET surface area of from 50 m2/g up to 500 m2/g or from 100
m2/g
to 350 m2/g.
The first and second salts in the aqueous mixture may be mixed under a
pressure of from 0.101 MPa to 10 MPa (1.01 bar to 100 bar). Preferably, the
first and
second salts in the aqueous mixture are mixed at atmospheric pressure,
however, if
the mixing is effected at a temperature greater than 100 C the mixing may be
conducted under positive pressure to inhibit the formation of steam.
During mixing, the aqueous mixture of the first and second salts is maintained
under anaerobic conditions. Maintaining the aqueous mixture under anaerobic
conditions during mixing inhibits the oxidation of the catalyst material or
the anionic
component of the second salt so that the catalyst material produced by the
process
contains little, if any oxygen. The aqueous mixture of the first and second
salts may
be maintained under anaerobic conditions during mixing by conducting the
mixing in
an atmosphere containing little or no oxygen, preferably an inert atmosphere.
The
mixing of the first and second salts in the aqueous mixture may be conducted
under
nitrogen gas, argon gas, and/or steam to maintain anaerobic conditions during
the
mixing. An inert gas, preferably nitrogen gas or steam, may be continuously
injected
into the aqueous mixture during mixing to maintain anaerobic conditions and to
34
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
facilitate mixing of the first and second salts in the aqueous mixture and
displacement
of ammonia gas if the second salt contains an ammonium cation.
The first and second salts may be mixed in the aqueous mixture at a
temperature of from 15 C to 150 C under anaerobic conditions for a period of
time
sufficient to permit the formation of the catalyst material. The first and
second salts
may be mixed in the aqueous mixture for a period of at least 1 hour, or at
least 2
hours, or at least 3 hours, or at least 4 hours, or from 1 hour to 10 hours,
or from 2
hours to 9 hours, or from 3 hours to 8 hours, or from 4 hours to 7 hours to
form the
catalyst material. The first and/or second salt(s) may be added to the aqueous
mixture
over a period of from 30 minutes to 4 hours while mixing the aqueous mixture,
and,
after the entirety of the first and second salts have been mixed into the
aqueous
mixture, the aqueous mixture may be mixed for at least an additional 1 hour,
or 2
hours, or 3 hours or 4 hours, or 5 hours to form the catalyst material.
After completing mixing of the aqueous mixture of the first and second salts,
a
solid is separated from the aqueous mixture to produce the material of the
catalyst.
The solid may be separated from the aqueous mixture by any conventional means
for
separating a solid phase material from a liquid phase material. For example,
the solid
may be separated by allowing the solid to settle from the resulting mixture,
preferably
for a period of from 1 hour to 16 hours, and separating the solid from the
mixture by
vacuum or gravitational filtration or by centrifugation. To enhance recovery
of the
solid, water may be added to the aqueous mixture prior to allowing the solid
to settle.
Water may be added to the aqueous mixture in a volume relative to the volume
of the
aqueous mixture of from 0.1:1 to 0.75:1. Alternatively, but less preferably,
the solid
may be separated from the mixture by centrifugation without first allowing the
solid
to settle and/or without the addition of water. Alternatively, the solid may
be
separated from the mixture by spray drying the mixture.
The material of the catalyst, or catalyst, may be washed subsequent to
separation from the aqueous mixture, if desired. The separated material of the
catalyst, or catalyst, may be contaminated with minor amounts, typically less
than 0.5
wt.%, of the anionic component from the first salt and/or the cationic
component from
the second salt. These minor contaminants may be removed from the separated
material of the catalyst, or catalyst, by washing the separated material with
water.
Substantial volumes of water may be used to wash the separated catalyst
material
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
since the separated catalyst material is insoluble in water, and the yield of
catalyst
material will not be significantly affected by the wash.
Process for cracking a hydrocarbon-containing feedstock
In the process of the present invention, a catalyst as described above, the
hydrocarbon-containing feedstock, hydrogen sulfide, and hydrogen are mixed at
a
temperature selected from 375 C to 500 C and a total pressure selected from
6.9 MPa
to 27.5MPa, where the hydrocarbon-containing feedstock, catalyst, hydrogen
sulfide,
and hydrogen form a mixture upon mixing. The catalyst, hydrocarbon-containing
feedstock, hydrogen sulfide, and hydrogen may be mixed by contact with each
other
in a mixing zone maintained at a temperature of from 375 C to 500 C and a
total
pressure of 6.9 MPa to 27.5 MPa. A hydrocarbon-containing product that
comprises
one or more hydrocarbon compounds that are liquid at STP is separated from the
mixture in the mixing zone.
In an embodiment of the process of the invention, as shown in Fig. 1, the
mixing zone 1 may be in a reactor 3, where the conditions of the reactor 3 may
be
controlled to maintain the temperature and pressure in the mixing zone 1 at
375 C to
500 C and 6.9 MPa to 27.5 MPa, respectively. The hydrocarbon-containing
feedstock may be provided continuously or intermittently from a feed supply 2
to the
mixing zone 1 in the reactor 3 through feed inlet 5. The hydrocarbon-
containing
feedstock may be preheated to a temperature of from 100 C to 350 C by a
heating
element 4, which may be a heat exchanger, prior to being fed to the mixing
zone 1.
Hydrogen may be provided continuously or intermittently to the mixing zone 1
of the
reactor 3 through hydrogen inlet line 7, or, alternatively, may be mixed
together with
the hydrocarbon-containing feedstock, and optionally the catalyst, and
provided to the
mixing zone 1 through the feed inlet 5. Hydrogen sulfide may be provided
continuously or intermittently as a liquid or a gas to the mixing zone 1 of
the reactor 1
through a hydrogen sulfide inlet line 27, or, alternatively, may be mixed with
the
hydrocarbon-containing feedstock and provided to the mixing zone 1 with the
hydrocarbon-containing feedstock through the feed inlet 5, or, alternatively,
may be
mixed with the hydrogen and provided to the mixing zone 1 through hydrogen
inlet
line 7.
The catalyst, as described above, may be located in the mixing zone 1 in the
reactor 3 or may be provided to the mixing zone 1 in the reactor 3 during the
process
36
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
of the present invention. The catalyst may be located in the mixing zone 1 in
a
catalyst bed. Preferably, however, the catalyst is provided to the mixing zone
1
during the process, or, if located in the mixing zone initially, may be
blended with the
hydrocarbon-containing feed, hydrogen sulfide, and hydrogen, and is not
present in a
catalyst bed. The catalyst may be provided to the mixing zone 1 together with
the
hydrocarbon-containing feedstock through feed inlet 5, where the catalyst may
be
dispersed in the hydrocarbon-containing feedstock prior to feeding the mixture
to the
mixing zone 1 through the feed inlet 5. Alternatively, the catalyst may be
provided to
the mixing zone 1 through a catalyst inlet 9, where the catalyst may be mixed
with
sufficient hydrocarbon-containing feedstock or another fluid, for example a
hydrocarbon-containing fluid, to enable the catalyst to be delivered to the
mixing zone
1 through the catalyst inlet 9.
The catalyst is provided to be mixed with the hydrocarbon-containing
feedstock, the hydrogen sulfide, and the hydrogen in the mixing zone 1 in a
sufficient
amount to catalytically crack the hydrocarbon-containing feedstock. The
catalyst may
be provided for mixing with the hydrocarbon-containing feedstock, hydrogen
sulfide,
and hydrogen in an amount of from 0.125 g to 5 g of catalyst per kg of
hydrocarbon-
containing feedstock. Alternatively, the catalyst may be provided for mixing
with the
hydrocarbon-containing feedstock, hydrogen sulfide, and hydrogen in an amount
of
from 0.125 g to 50 g of catalyst per kg of hydrocarbons in the hydrocarbon-
containing
feedstock having a boiling point of at least 538 C at a pressure of 0.101 MPa
as
determined in accordance with ASTM Method D5307.
The hydrocarbon-containing feedstock may be provided to the mixing zone 1
of the reactor 3 at a rate of at least 350 kg/hr per m3 of the mixture volume
within
mixing zone 1 of the reactor 3. The mixture volume is defined herein as the
combined volume of the catalyst(s), the hydrocarbon-depleted feed residuum (as
defined herein), and the hydrocarbon-containing feedstock in the mixing zone
1,
where the hydrocarbon-depleted feed residuum may contribute no volume to the
mixture volume (i.e. at the start of the process before a hydrocarbon-depleted
feed
residuum has been produced in the mixing zone 1), and where the hydrocarbon-
containing feedstock may contribute no volume to the mixture volume (i.e.
after
initiation of the process during a period between intermittent addition of
fresh
hydrocarbon-containing feedstock into the mixing zone 1). The mixture volume
37
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
within the mixing zone 1 may be affected by 1) the rate of addition of the
hydrocarbon-containing feedstock into the mixing zone 1; 2) the rate of
removal of
the vapor from the reactor 3; and 3) the rate at which a bleed stream of the
hydrocarbon-depleted feed residuum, catalyst(s), and hydrocarbon-containing
feedstock is separated from and recycled to the reactor 3, as described in
further detail
below. The hydrocarbon-containing feedstock may be provided to the mixing zone
1
of the reactor 3 at a rate of at least 350, or at least 400, or at least 500,
or at least 600,
or at least 700, or at least 800, or at least 900, or at least 1000 kg/hr per
m3 of the
mixture volume within the mixing zone 1 up to 5000 kg/hr per m3 of the mixture
volume within the mixing zone 1.
Preferably, the mixture volume of the hydrocarbon-containing feedstock, the
hydrocarbon-depleted feed residuum, and the catalyst(s) is maintained within
the
mixing zone within a selected range of the reactor volume by selecting 1) the
rate at
which the hydrocarbon-containing feedstock is provided to the mixing zone 1;
and/or
2) the rate at which a bleed stream is removed from and recycled to the mixing
zone
1; and/or 3) the temperature and pressure within the mixing zone 1 and the
reactor 3
to provide a selected rate of vapor removal from the mixing zone 1 and the
reactor 3.
The combined volume of the hydrocarbon-containing feedstock and the
catalyst(s)
initially provided to the mixing zone 1 at the start of the process define an
initial
mixture volume, and the amount of hydrocarbon-containing feedstock and the
amount
of the catalyst(s) initially provided to the mixing zone 1 may be selected to
provide an
initial mixture volume of from 5% to 97% of the reactor volume, preferably
from
30% to 75% of the reactor volume. The rate at which the hydrocarbon-containing
feedstock is provided to the mixing zone 1 and/or the rate at which a bleed
stream is
removed from and recycled to the mixing zone 1 and/or the rate at which vapor
is
removed from the reactor 3 may be selected to maintain the mixture volume of
the
hydrocarbon-containing feedstock, the hydrocarbon-depleted feed residuum, and
the
catalyst(s) at a level of at least 10%, or at least 25%, or within 90%, or
within 70%, or
within 50% of the initial mixture volume during the process.
The hydrocarbon-containing feedstock may be provided to the mixing zone 1
at such relatively high rates for reacting a feedstock containing relatively
large
quantities of heavy, high molecular weight hydrocarbons due to the inhibition
of coke
formation in the process of the present invention. Conventional processes for
cracking heavy hydrocarbonaceous feedstocks are typically operated at rates on
the
38
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
order of 10 to 300 kg/hr per m3 of reaction volume so that the conventional
cracking
process may be conducted either 1) at sufficiently low temperature to avoid
excessive
coke-make to maximize yield of desirable cracked hydrocarbons; or 2) at higher
temperatures with significant quantities of coke production, where the high
levels of
solids produced impedes operation of the process at a high rate.
Hydrogen may be provided to the mixing zone 1 of the reactor 3 at a rate
sufficient to hydrogenate hydrocarbons cracked in the process. The hydrogen
may be
provided to the mixing zone 1 in a ratio relative to the hydrocarbon-
containing
feedstock provided to the mixing zone 1 of from 1 Nm3/m3 to 16,100 Nm3/m3 (5.6
SCFB to 90160 SCFB), or from 2 Nm3/m3 to 8000 Nm3/m3 (11.2 SCFB to 44800
SCFB), or from 3 Nm3/m3 to 4000 Nm3/m3 (16.8 SCFB to 22400 SCFB), or from 5
Nm3/m3 to 320 Nm3/m3 (28 SCFB to 1792 SCFB). The hydrogen partial pressure in
the mixing zone may be maintained in a pressure range of from 2.1 MPa to 26.1
MPa
or from 5 MPa to 20 MPa, or from 10 MPa to 15 MPa.
Hydrogen sulfide is provided to be mixed with the hydrocarbon-containing
feedstock, hydrogen, and catalyst in an amount effective to lower the
activation
energy of the hydrocracking reaction and to increase the rate of the reaction
relative to
hydrocracking the hydrocarbon-containing feedstock with the catalyst and
hydrogen
at an identical temperature and pressure in the absence of the hydrogen
sulfide. In an
embodiment of the process of the present invention, the hydrogen sulfide
provided to
be mixed with the hydrocarbon-containing feedstock, hydrogen, and the catalyst
may
be provided, in an amount on a mole ratio basis relative to hydrogen provided
to be
mixed with the hydrocarbon-containing feedstock and catalyst, of at least 0.5
mole of
hydrogen sulfide per 9.5 moles hydrogen, where the combined hydrogen sulfide
and
hydrogen partial pressures provide at least 60%, or at least 70%, or at least
80%, or at
least 90%, or at least 95% of the total pressure in the reactor. The hydrogen
sulfide
may be provided in an amount on a mole ratio basis relative to the hydrogen
provided
of at least 1:9, or at least 1.5:8.5, or at least 1:4, or at least 2.5:7.5, or
at least 3:7, or at
least 3.5:6.5, or at least 4:6, up to 1:1, where the combined hydrogen sulfide
and
hydrogen partial pressures are at least 60%, or at least 70%, or at least 80%,
or at least
90%, or at least 95% of the total pressure in the reactor. The hydrogen
sulfide partial
pressure in the reactor may be maintained in a pressure range of from 0.4 MPa
to 13.8
MPa, or from 2 MPa to 10 MPa, or from 3 MPa to 7 MPa.
39
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
The combined partial pressure of the hydrogen sulfide and hydrogen in the
reactor may be maintained to provide at least 60% of the total pressure in the
reactor,
where the hydrogen sulfide partial pressure is maintained at a level of at
least 5% of
the hydrogen partial pressure. Preferably, the combined partial pressure of
the
hydrogen sulfide and hydrogen in the reactor is maintained to provide at least
70%, or
at least 75%, or at least 80%, or at least 90%, or at least 95% of the total
pressure in
the reactor, where the hydrogen sulfide partial pressure is maintained at a
level of at
least 5% of the hydrogen partial pressure. Other gases may be present in the
reactor
in minor amounts that provide a pressure contributing to the total pressure in
the
reactor. For example, a non-condensable gas produced in the vapor along with
the
hydrocarbon-containing product may be separated from the hydrocarbon-
containing
product and recycled back into the mixing zone, where the non-condensable gas
may
comprise hydrocarbon gases such as methane, ethane, and propane as well as
hydrogen sulfide and hydrogen.
The catalyst, the hydrocarbon-containing feedstock, the hydrogen sulfide, and
the hydrogen may be mixed by being blended into an intimate admixture in the
mixing zone 1. The catalyst, hydrocarbon-containing feedstock and the hydrogen
may be blended in the mixing zone 1, for example, by stirring a mixture of the
components, for example by a mechanical stirring device located in the mixing
zone
1. The catalyst, hydrocarbon-containing feedstock, and hydrogen may also be
mixed
in the mixing zone 1 by blending the components prior to providing the
components
to the mixing zone 1 and injecting the blended components into the mixing zone
1
through one or more nozzles which may act as the feed inlet 5. The catalyst,
hydrocarbon-containing feedstock, hydrogen sulfide, and hydrogen may also be
blended in the mixing zone 1 by blending the hydrocarbon-containing feedstock
and
catalyst and injecting the mixture into the mixing zone 1 through one or more
feed
inlet nozzles positioned with respect to the hydrogen inlet line 7 and
hydrogen sulfide
inlet line 27 such that the mixture is blended with hydrogen and hydrogen
sulfide
entering the mixing zone 1 through the hydrogen inlet line 7 and the hydrogen
sulfide
inlet line 27, respectively. Baffles may be included in the reactor 3 in the
mixing zone
1 to facilitate blending the hydrocarbon-containing feedstock, catalyst,
hydrogen
sulfide, and hydrogen. Less preferably, the catalyst is present in the mixing
zone 1 in
a catalyst bed, and the hydrocarbon-containing feedstock, hydrogen sulfide,
hydrogen,
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
and catalyst are mixed by bringing the hydrocarbon-containing feedstock,
hydrogen
sulfide, and hydrogen simultaneously into contact with the catalyst in the
catalyst bed.
The temperature and pressure conditions in the mixing zone 1 are maintained
so that heavy hydrocarbons in the hydrocarbon-containing feedstock may be
cracked.
The temperature in the mixing zone 1 is maintained from 375 C to 500 C.
Preferably, the mixing zone 1 is maintained at a temperature of from 425 C to
500 C,
or from 430 C to 500 C, or from 440 C to 500 C, or from 450 C to 500 C. Higher
temperatures may be preferred in the process of the present invention since 1)
the rate
of conversion of the hydrocarbon-containing feedstock to a hydrocarbon-
containing
product increases with temperature; and 2) the present process inhibits or
prevents the
formation of coke, even at temperatures of 430 C or greater, which typically
occurs
rapidly in conventional cracking processes at temperatures of 430 C or
greater.
Mixing the hydrocarbon-containing feedstock, the catalyst, the hydrogen
sulfide, and hydrogen in the mixing zone 1 at a temperature of from 375 C to
500 C
and a total pressure of from 6.9 MPa to 27.5 MPa produces a vapor comprised of
hydrocarbons that are vaporizable at the temperature and pressure within the
mixing
zone 1. The vapor may be comprised of hydrocarbons present initially in the
hydrocarbon-containing feedstock that vaporize at the temperature and pressure
within the mixing zone 1 and hydrocarbons that are not present initially in
the
hydrocarbon-containing feedstock but are produced by cracking and
hydrogenating
hydrocarbons initially in the hydrocarbon-containing feedstock that were not
vaporizable at the temperature and pressure within the mixing zone 1.
At least a portion of the vapor comprised of hydrocarbons that are vaporizable
at the temperature and pressure within the mixing zone 1 may be continuously
or
intermittently separated from the mixture of hydrocarbon-containing feedstock,
hydrogen, and catalyst since the more volatile vapor physically separates from
the
hydrocarbon-containing feedstock, catalyst, hydrogen, and hydrogen sulfide
mixture.
The vapor may also contain hydrogen gas and hydrogen sulfide gas which also
separate from the mixture.
Separation of the vapor from the mixture leaves a hydrocarbon-depleted feed
residuum from which the hydrocarbons present in the vapor have been removed.
The
hydrocarbon-depleted feed residuum is comprised of hydrocarbons that are
liquid at
the temperature and pressure within the mixing zone 1. The hydrocarbon-
depleted
41
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
feed residuum may also be comprised of solids such as metals freed from
cracked
hydrocarbons and minor amounts of coke. The hydrocarbon-depleted feed residuum
may contain little coke or proto-coke since the process of the present
invention
inhibits the generation of coke. The hydrocarbon-depleted feed residuum may
contain, per metric ton of hydrocarbon-containing feedstock provided to the
mixing
zone 1, at most 10 kg, or less than 5 kg, or at most 1 kg of hydrocarbons
insoluble in
toluene as measured by ASTM Method D4072.
At least a portion of the hydrocarbon-depleted feed residuum is retained in
the
mixing zone 1 while the vapor is separated from the mixing zone 1. The portion
of
the hydrocarbon-depleted feed residuum retained in the mixing zone 1 may be
subject
to further cracking to produce more vapor that may be separated from the
mixing zone
1 and then from the reactor 3 from which the liquid hydrocarbon-containing
product
may be produced by cooling. Hydrocarbon-containing feedstock, hydrogen, and
hydrogen sulfide may be continuously or intermittently provided to the mixing
zone 1
at the rates described above and mixed with the catalyst(s) and the
hydrocarbon-
depleted feed residuum retained in the mixing zone 1 to produce further vapor
comprised of hydrocarbons that are vaporizable at the temperature and pressure
within the mixing zone 1 for separation from the mixing zone 1 and the reactor
3.
At least a portion of the vapor separated from the mixture of the hydrocarbon-
containing feedstock, hydrogen sulfide, hydrogen, and catalyst may be
continuously
or intermittently separated from the mixing zone 1 while retaining the
hydrocarbon-
depleted feed residuum, catalyst, and any fresh hydrocarbon-containing
feedstock in
the mixing zone 1. At least a portion of the vapor separated from the mixing
zone 1
may be continuously or intermittently separated from the reactor 3 through a
reactor
product outlet 11. The reactor 3 is preferably configured and operated so that
substantially only vapors and gases may exit the reactor product outlet 11,
where the
vapor product exiting the reactor 3 comprises at most 5 wt.%, or at most 3
wt.%, or at
most 1 wt.%, or at most 0.5 wt.%, or at most 0.1 wt.%, or at most 0.01 wt.%,
or at
most 0.001 wt.% solids and liquids at the temperature and pressure at which
the vapor
product exits the reactor 3.
A stripping gas may be injected into the reactor 3 over the mixing zone 1 to
facilitate separation of the vapor from the mixing zone 1. The stripping gas
may be
heated to a temperature at or above the temperature within the mixing zone 1
to assist
42
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
in separating the vapor from the mixing zone 1. In an embodiment of the
process, the
stripping gas may be hydrogen gas and/or hydrogen sulfide gas.
As shown in Fig. 2, the reactor 3 may be comprised of a mixing zone 1, a
disengagement zone 21, and a vapor/gas zone 23. The vapor comprised of
hydrocarbons that are vaporizable at the temperature and pressure within the
mixing
zone 1 may separate from the mixture of hydrocarbon-depleted residuum,
catalyst,
and fresh hydrocarbon-containing feed, if any, in mixing zone 1 into the
disengagement zone 21. A stripping gas such as hydrogen may be injected into
the
disengagement zone 21 to facilitate separation of the vapor from the mixing
zone 1.
Some liquids and solids may be entrained by the vapor as it is separated from
the
mixing zone 1 into the disengagement zone 21, so that the disengagement zone
21
contains a mixture of vapor and liquids, and potentially solids. At least a
portion of
the vapor separates from the disengagement zone 21 into the vapor/gas zone 23,
where the vapor separating from the disengagement zone 21 into the vapor/gas
zone
23 contains little or no liquids or solids at the temperature and pressure
within the
vapor/gas zone. At least a portion of the vapor in the vapor/gas zone 23 exits
the
reactor 3 through the reactor product outlet 11.
Referring now to Figs 1 and 2, in the process of the present invention, the
hydrocarbons in the hydrocarbon-containing feed are contacted and mixed with
the
catalyst, hydrogen sulfide, and hydrogen in the mixing zone 1 of the reactor
only as
long as necessary to be vaporized and separated from the mixture, and are
retained in
the reactor 3 only as long as necessary to be vaporized and exit the reactor
product
outlet 11. Low molecular weight hydrocarbons having a low boiling point may be
vaporized almost immediately upon being introduced into the mixing zone 1 when
the
mixing zone 1 is maintained at a temperature of 375 C to 500 C and a total
pressure
of from 6.9 MPa to 27.5 MPa. These hydrocarbons may be separated rapidly from
the
reactor 3. High molecular weight hydrocarbons having a high boiling point, for
example hydrocarbons having a boiling point greater than 538 C at 0.101 MPa,
may
remain in the mixing zone 1 until they are cracked into hydrocarbons having a
boiling
point low enough to be vaporized at the temperature and pressure in the mixing
zone
1 and to exit the reactor 3. The hydrocarbons of the hydrocarbon-containing
feed,
therefore, are contacted and mixed with the catalyst, hydrogen sulfide, and
hydrogen
in the mixing zone 1 of the reactor 3 for a variable time period, depending on
the
43
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
boiling point of the hydrocarbons under the conditions in the mixing zone 1
and the
reactor 3.
The rate of the process of producing the vapor product from the hydrocarbon-
containing feedstock may be adjusted by selection of the temperature and/or
pressure
in the reactor 3, and particularly in the mixing zone 1, within the
temperature range of
375 C-500 C and within the pressure range of 6.9 MPa - 27.5 MPa. Increasing
the
temperature and/or decreasing the pressure in the mixing zone 1 permits the
hydrocarbon-containing feedstock to provided to the reactor 3 at an increased
rate and
the vapor product to be removed from the reactor 3 at an increased rate since
the
hydrocarbons in the hydrocarbon-containing feedstock may experience a
decreased
residence time in the reactor 3 due to higher cracking activity and/or reduced
separation time. Conversely, decreasing the temperature and/or increasing the
pressure in the mixing zone 1 may reduce the rate at which the hydrocarbon-
containing feedstock may be provided to the reactor 3 and the vapor product
may be
removed from the reactor 3 since the hydrocarbons in the hydrocarbon-
containing
feedstock may experience an increased residence time in the reactor 3 due to
lower
cracking activity and/or increased separation time.
As a result of the inhibition and/or prevention of the formation of coke in
the
process, the hydrocarbons in the hydrocarbon-containing feed may be contacted
and
mixed with the catalyst, hydrogen sulfide, and hydrogen in the mixing zone 1
at a
temperature of 375 C to 500 C and a total pressure of 6.9 MPa to 27.5 MPa for
as
long as necessary to be vaporized, or to be cracked, hydrogenated, and
vaporized. It
is believed that high boiling, high molecular weight hydrocarbons may remain
in the
mixing zone 1 in the presence of cracked hydrocarbons since the catalyst
promotes
the formation of hydrocarbon radical anions upon cracking that react with
hydrogen
or hydrogen sulfide to form stable hydrocarbon products rather than
hydrocarbon
radical cations that react with other hydrocarbons to form coke. Coke
formation is
also avoided because the cracked hydrogenated hydrocarbons preferentially exit
the
mixing zone 1 as a vapor rather remaining in the mixing zone 1 to combine with
hydrocarbon radicals in the mixing zone 1 to form coke or proto-coke.
At least a portion of the vapor separated from the mixing zone 1 and separated
from the reactor 3 may be condensed apart from the mixing zone 1 to produce
the
liquid hydrocarbon-containing product. Referring now to Fig. 1, the portion of
the
44
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
vapor separated from the reactor 3 may be provided to a condenser 13 wherein
at least
a portion of the vapor separated from the reactor 3 may be condensed to
produce the
hydrocarbon-containing product that is comprised of hydrocarbons that are a
liquid at
STP. A portion of the vapor separated from the reactor 3 may be passed through
a
heat exchanger 15 to cool the vapor prior to providing the vapor to the
condenser 13.
Condensation of the liquid hydrocarbon-containing product from the vapor
separated from the reactor 3 may also produce a non-condensable gas that may
be
comprised of hydrocarbons having a carbon number from 1 to 6, hydrogen, and
hydrogen sulfide. The condensed hydrocarbon-containing liquid product may be
separated from the non-condensable gas through a condenser liquid product
outlet 17
and stored in a product receiver 18, and the non-condensable gas may be
separated
from the condenser 13 through a non-condensable gas outlet 19. The non-
condensable gas may be passed through an amine or caustic scrubber 20 and
recovered through a gas product outlet 22. Alternatively, the non-condensable
gas
may be recycled into the mixing zone 1 without scrubbing to provide hydrogen
and
hydrogen sulfide to the mixture in the mixing zone 1.
Alternatively, referring now to Fig. 2, the portion of the vapor separated
from
the reactor 3 may be provided to a high pressure separator 12 to separate a
liquid
hydrocarbon-containing product from gases not condensable at the temperature
and
pressure within the high pressure separator 12, and the liquid hydrocarbon-
containing
product collected from the high pressure separator may be provided through
line 16 to
a low pressure separator 14 operated at a pressure less than the high pressure
separator
12 to separate the liquid hydrocarbon-containing product from gases that are
not
condensable at the temperature and pressure at which the low pressure
separator 14 is
operated. The vapor/gas exiting the reactor 3 from the reactor product outlet
11 may
be cooled prior to being provided to the high pressure separator 12 by passing
the
vapor/gas through heat exchanger 15. The condensed hydrocarbon-containing
liquid
product may be separated from the non-condensable gas in the low pressure
separator
through a low pressure separator liquid product outlet 10 and stored in a
product
receiver 18. The non-condensable gas may be separated from the high pressure
separator 12 through a high pressure non-condensable gas outlet 24 and from
the low
pressure separator 14 through a low pressure non-condensable gas outlet 26.
The
non-condensable gas streams may be combined in line 28 and passed through an
amine or caustic scrubber 20 and recovered through a gas product outlet 22.
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
Alternatively, the combined non-condensable gas streams may be recycled into
the
mixing zone 1 without scrubbing to provide hydrogen and hydrogen sulfide to
the
mixture in the mixing zone 1.
The vapor separated from the mixing zone 1 and from the reactor 3 through
the reactor product outlet 11 may contain hydrogen sulfide. The hydrogen
sulfide in
the vapor product may be separated from the hydrocarbon-containing liquid
product
in the condenser 13 (Fig. 1) or in the high and low pressure separators 12 and
14 (Fig.
2), where the hydrogen sulfide may form a portion of the non-condensable gas.
In an
embodiment, the hydrocarbon-containing liquid product may be condensed in the
high and/or low pressure separators 12 and 14 at a temperature of from 60 C to
93 C
(140 F-200 F) so that hydrogen sulfide is separated from the hydrocarbon-
containing
liquid product with the non-condensable gas rather than condensing with the
liquid
hydrocarbon-containing product. The hydrogen sulfide may be separated from the
other components of the non-condensable gas by treatment of the non-
condensable
gas to recover the hydrogen sulfide. For example, the non-condensable gas may
be
scrubbed with an amine solution in the scrubber 20 to separate the hydrogen
sulfide
from the other components of the non-condensable gas. The hydrogen sulfide may
then be recovered and recycled back into the mixing zone 1.
In another embodiment of the invention, the vapor separated from the mixing
zone 1 and from the reactor 3 may be further hydroprocessed without condensing
the
hydrocarbon-containing product. For example, the vapor separated from the
reactor
may be hydrotreated to reduce sulfur, nitrogen, and olefins in the hydrocarbon-
containing product by passing the vapor from the reactor 3 to a
hydroprocessing
reactor, where the vapor may be contacted with a conventional hydroprocessing
catalyst and hydrogen at a temperature of from 260 C to 425 C and a total
pressure of
from 3.4 MPa to 27.5 MPa.
A portion of the hydrocarbon-depleted feed residuum and catalyst(s) may be
separated from the mixing zone to remove solids including metals and
hydrocarbonaceous solids including coke from the hydrocarbon-depleted feed
residuum. Referring now to Figs. 1 and 2, the reactor 3 may include a bleed
stream
outlet 25 for removal of a stream of hydrocarbon-depleted feed resdiuum and
catalyst(s) from the mixing zone 1 and the reactor 3. The bleed stream outlet
25 may
be operatively connected to the mixing zone 1 of the reactor 3.
46
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
A portion of the hydrocarbon-depleted feed residuum and the catalyst(s) may
be removed together from the mixing zone 1 and the reactor 3 through the bleed
stream outlet 25 while the process is proceeding. Solids and the catalyst(s)
may be
separated from a liquid portion of the hydrocarbon-depleted feed residuum in a
solid-
liquid separator 30. The solid-liquid separator 30 may be a filter or a
centrifuge. The
liquid portion of the hydrocarbon-depleted feed residuum may be recycled back
into
the mixing zone 1 via a recycle inlet 32 for further processing or may be
combined
with the hydrocarbon-containing feed and recycled into the mixing zone 1
through the
feed inlet 5.
The process of the present invention may be effected for a substantial period
of time on a continuous or semi-continuous basis, in part because the process
generates little or no coke. The hydrocarbon-containing feedstock, hydrogen,
catalyst, and hydrogen sulfide may be continuously or intermittently provided
to the
mixing zone 1 in the reactor 3 and mixed in the mixing zone 1 at a temperature
of
from 375 C-500 C and a total pressure of from 6.9 MPa - 27.5 MPa for a period
of at
least 40 hours, or at least 100 hours, or at least 250 hours, or at least 500
hours, or at
least 750 hours to generate the vapor comprised of hydrocarbons that are
vaporizable
at the temperature and pressure in the mixing zone 1 and the hydrocarbon-
depleted
residuum, as described above. The vapor may be continuously or intermittently
separated from the mixing zone 1 and the reactor 3 over substantially all of
the time
period that the hydrocarbon-containing feedstock, catalyst, hydrogen, and
hydrogen
sulfide are mixed in the mixing zone 1. Fresh hydrocarbon-containing
feedstock,
hydrogen, and hydrogen sulfide may be blended with the hydrocarbon-depleted
residuum in the mixing zone 1 over the course of the time period of the
reaction as
needed. In a preferred embodiment, fresh hydrocarbon-containing feedstock,
hydrogen, and hydrogen sulfide are provided continuously to the mixing zone 1
over
substantially all of the time period the reaction is effected. Solids may be
removed
from the mixing zone 1 continuously or intermittently over the time period the
process is run by separating a bleed stream of the hydrocarbon-containing feed
residuum from the mixing zone 1 and the reactor 3, removing the solids from
the
bleed stream, and recycling the bleed stream from which the solids have been
removed back into the mixing zone 1 as described above.
47
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
The process of the present invention produces, in part, a hydrocarbon-
containing product that is a liquid at STP. The hydrocarbon-containing product
contains less than 4 wt.%, or at most 3 wt.%, or at most 2 wt.%, or at most 1
wt.%, or
at most 0.5 wt.% of hydrocarbons having a boiling point of greater than 538 C
in
accordance with ASTM Method D5307 and at most 0.5 wt.%, or at most 0.25 wt.%,
or at most 0.1 wt.% coke as determined in accordance with ASTM Method D4072.
Furthermore, the hydrocarbon-containing product contains at least 80%, or at
least
85%, or at least 90%, or at least 95%, or at least 97% of the atomic carbon
present in
the hydrocarbon-containing feedstock. Therefore, when the process of the
present
invention is utilized, most of the hydrocarbons in the hydrocarbon-containing
feedstock may be recovered in the hydrocarbon-containing product that is
liquid at
STP, and little of the hydrocarbons in the hydrocarbon-containing feedstock
are
converted to coke or non-condensable gas.
The liquid hydrocarbon-containing product may contain VGO hydrocarbons,
distillate hydrocarbons, and naphtha hydrocarbons. The liquid hydrocarbon-
containing product may contain, per gram of liquid hydrocarbon-containing
product,
at least 0.05 grams, or at least 0.1 grams of hydrocarbons having a boiling
point from
the initial boiling point of the hydrocarbon-containing product up to 204 C
(400 F).
The liquid hydrocarbon-containing product may also contain, per gram of liquid
hydrocarbon-containing product, at least 0.1 grams, or at least 0.15 grams of
hydrocarbons having a boiling point of from 204 C (400 F) up to 260 C (500 F).
The liquid hydrocarbon-containing product may also contain, per gram of liquid
hydrocarbon-containing product, at least 0.25 grams, or at least 0.3 grams, or
at least
0.35 grams of hydrocarbons having a boiling point of from 260 C (500 F) up to
343 C (650 F). The liquid hydrocarbon-containing product may also contain, per
gram of liquid hydrocarbon-containing product, at least 0.3 grams, or at least
0.35
grams, or at least 0.4, or at least 0.45 grams of hydrocarbons having a
boiling point of
from 343 C (500 F) up to 538 C (1000 F). The relative amounts of hydrocarbons
within each boiling range and the boiling range distribution of the
hydrocarbons may
be determined in accordance with ASTM Method D5307.
The hydrocarbon-containing product produced by the process of the present
invention may contain significant amounts of sulfur. The hydrocarbon-
containing
product may contain, per gram, at least 0.0005 gram of sulfur or at least
0.001 gram of
48
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
sulfur. The sulfur content of the hydrocarbon-containing product may be
determined
in accordance with ASTM Method D4294. At least 40 wt.% of the sulfur may be
contained in hydrocarbon compounds having a carbon number of 17 or less as
determined by two-dimensional GC-GC sulfur chemiluminscence, where at least 60
wt. % of the sulfur in the sulfur-containing hydrocarbon compounds having a
carbon
number of 17 or less may be contained in benzothiophenic compounds as
determined
by GC-GC sulfur chemiluminscence.
The hydrocarbon-containing product produced by the process of the present
invention may contain, per gram, at least 0.0005 gram or at least 0.001 gram
of
nitrogen as determined in accordance with ASTM Method D5762. The hydrocarbon-
containing product may have a relatively low ratio of basic nitrogen compounds
to
other nitrogen containing compounds therein. The nitrogen may be contained in
hydrocarbon compounds, where at least 30 wt.% of the nitrogen in the
hydrocarbon
composition is contained in nitrogen-containing hydrocarbon compounds having a
carbon number of 17 or less and where at least 50 wt.% of the nitrogen-
containing
hydrocarbon compounds having a carbon number of 17 or less are acridinic and
carbazolic compounds. The amount of nitrogen-containing hydrocarbon compounds
having a carbon number of 17 or less relative to the amount of nitrogen in all
nitrogen-containing hydrocarbon compounds in the hydrocarbon-containing
product
and the relative amount of acridinic and carbazolic compounds may be
determined by
nitrogen chemiluminscence two dimensional gas chromatography (GCxGC-NCD).
The hydrocarbon-containing product produced by the process of the present
invention may contain significant quantities of aromatic hydrocarbon
compounds.
The hydrocarbon-containing product may contain, per gram, at least 0.3 gram,
or at
least 0.35 gram, or at least 0.4 gram, or at least 0.45 gram, or at least 0.5
gram of
aromatic hydrocarbon compounds.
The hydrocarbon-containing product of the process of the present invention
may contain relatively few polyaromatic hydrocarbon compounds containing three
or
more aromatic ring structures relative to combined mono-aromatic and di-
aromatic
hydrocarbon compounds. The combined mono-aromatic and di-aromatic
hydrocarbon compounds in the hydrocarbon-containing product may be present in
the
hydrocarbon-containing product in a weight ratio relative to the polyaromatic
hydrocarbon compounds of at least 1.5 : 1.0, or at least 2.0 : 1.0, or at
least 2.5 : 1Ø
The relative amounts of mono-, di- and polyaromatic compounds in the
hydrocarbon-
49
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
containing product may be determined by flame ionization detection-two
dimensional
gas chromatography (GCxGC-FID).
The hydrocarbon-containing product of the process of the present invention
may contain olefins, where a significant amount of the olefins may be alpha
olefins
having a terminal double bond. Olefin content in the hydrocarbon-containing
product
may be determined in accordance with ASTM Method D6730. The hydrocarbon-
containing product may contain, per gram, at least 0.05 grams, or at least 0.1
grams of
alpha olefins. The alpha olefins in the hydrocarbon-containing product may be
present in the hydrocarbon-containing product relative to olefins having an
internal
double bond in a weight ratio of alpha olefins to internal double bond olefins
is at
least 0.7 : 1.0, or at least 0.9:1.0, or at least 1.0:1Ø
The hydrocarbon-containing product of the process of the present invention
may contain paraffins, where a significant amount of the paraffins may be n-
paraffins.
Paraffin content in the hydrocarbon-containing product may be determined in
accordance with ASTM Method D6730. The n-paraffins in the hydrocarbon-
containing product may be present relative to isoparaffins in a weight ratio
of
isoparaffins to n-paraffins of at most 1.4:1.0, or at most 1.0:1Ø
To facilitate a better understanding of the present invention, the following
examples of certain aspects of some embodiments are given. In no way should
the
following examples be read to limit, or define, the scope of the invention.
EXAMPLE 1
A catalyst for use in a process of the present invention containing copper,
molybdenum, and sulfur was produced, where at least a portion of the catalyst
had a
structure according to Formula (VII).
Cu Mo Cu
S S '_~S
(VII)
A 22-liter round-bottom flask was charged with a solution of 1199 grams of
copper sulfate (CuSO4) in 2 liters of water. The copper sulfate solution was
heated to
85 C. 520.6 grams of ammonium tetrathiomolybdate (ATTM) { (NH4)2(M0S4) } in 13
liters of water was injected into the heated copper sulfate solution through
an injection
nozzle over a period of 4 hours while stirring the solution. After the
addition was
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
complete, the solution was stirred for 8 hours at 93 C and then was allowed to
cool
and settle overnight.
Solids were then separated from the slurry. Separation of the solids from the
slurry was accomplished using a centrifuge separator at 12,000 Gauss to give a
red
paste. The separated solids were washed with water until conductivity
measurements
of the effluent were under 100 tSiemens at 33 C. Residual water was then
removed
from the solids by vacuum distillation at 55 C and 29 inches of Hg pressure.
409
grams of catalyst solids were recovered. Semi-quantitative XRF (element,
mass%)
measured: Cu, 16.4; Mo, 35.6; S, 47.7; and less than 0.1 wt.% Fe and Co.
The catalyst solids were particulate having a particle size distribution with
a
mean particle size of 47.4 m as determined by laser diffractometry using a
Mastersizer S made by Malvern Instruments. The BET surface area of the
catalyst
was measured to be 113 m2/g and the catalyst pore volume was measured to be
0.157
cm3/g. The catalyst had a pore size distribution, where the median pore size
diameter
was determined to be 56 angstroms. X-ray diffraction and Raman IR spectroscopy
confirmed that at least a portion of the catalyst had a structure in which
copper, sulfur,
and molybdenum were arranged as shown in Formula (VII) above.
EXAMPLE 2
Bitumen from Peace River, Canada was selected as a hydrocarbon-containing
feedstock for cracking. The Peace River bitumen was analyzed to determine its
composition. The properties of the Peace River bitumen are set forth in Table
1:
51
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
TABLE 1
Property Value
Hydrogen (wt.%) 10.1
Carbon (wt.%) 82
Oxygen (wt.%) 0.62
Nitrogen (wt.%) 0.37
Sulfur (wt.%) 6.69
Nickel (wppm) 70
Vanadium (wppm) 205
Microcarbon residue (wt.%) 12.5
C5 asphaltenes (wt.%) 10.9
Density (g/ml) 1.01
Viscosity at 38 C (cSt) 8357
TAN-E (ASTM D664) (mg KOH/g) 3.91
Boiling Range Distribution
Initial Boiling Point-204 C (400 F)(wt. %) [Naphtha] 0
204 C (400 F) - 260 C (500 F) (wt.%) [Kerosene] 1
260 C (500 F) - 343 C (650 F) (wt.%) [Diesel] 14
343 C (650 F) - 538 C (1000 F) (wt.%) [VGO] 37.5
>538 C (1000 F) (wt.%) [Residue] 47.5
Six samples of the Peace River bitumen were separately hydrocracked by
mixing each bitumen sample with the catalyst prepared in Example 1, hydrogen,
and
hydrogen sulfide. The bitumen samples, catalyst, hydrogen, and hydrogen
sulfide
were mixed at selected temperatures, gas flow rates, hydrogen partial
pressures,
hydrogen sulfide partial pressures, feed uptake rates, and space velocities as
set forth
in Table 2 below. The total pressure of each hydrocracking treatment was
maintained
at 13.1 MPa, where the hydrogen partial pressure of the treatments ranged from
8.8
MPa to 10.2 MPa, and the hydrogen sulfide partial pressure ranged from 2.9 MPa
to
4.3 MPa. The total gas flow rate of each hydrocracking treatment was
maintained at
950 standard liters per hour, where the hydrogen flow rate of the treatements
ranged
from 640-720 standard liters per hour and the hydrogen sulfide flow rate of
the
treatments ranged from 210-310 standard liters per hour. The liquid hourly
space
velocity of the bitumen feed for hydrocracking depended on the reaction rate,
and
ranged from 0.6 to 0.8 hr 1. A target temperature was selected for each
hydrocracking
treatment within the range of 420 C to 450 C. The conditions for each
hydrocracking
treatment of the six samples are shown below in Table 2.
In the hydrocracking treatment of each sample, the Peace River bitumen was
preheated to approximately 105 C-115 C in a 10 gallon feed drum and circulated
52
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
through a closed feed loop system from which the bitumen was fed into a semi-
continuous stirred tank reactor with vapor effluent capability, where the
reactor had
an internal volume capacity of 1000 cm3. The reactor was operated in a
continuous
mode with respect to the bitumen feedstream and the vapor effluent product,
however,
the reactor did not include a bleed stream to remove accumulating metals
and/or
carbonaceous solids. The bitumen feed of each sample was fed to the reactor as
needed to maintain a working volume of feed in the reactor of approximately
475 ml,
where a Berthold single-point source nuclear level detector located outside
the reactor
was used to control the working volume in the reactor. 50 grams of the
catalyst was
mixed with the hydrogen, hydrogen sulfide, and bitumen feed sample in the
reactor
during the course of the hydrocracking treatment. The bitumen feed sample,
hydrogen, hydrogen sulfide, and the catalyst were mixed together in the
reactor by
stirring with an Autoclave Engineers MagneDrive impeller at 1200 rpm.
Vaporized
product exited the reactor, where a liquid product was separated from the
vaporized
product by passing the vaporized product through a high pressure separator
operated
at reaction pressure and 80 C and then through a low pressure separator
operated at
0.17 MPa and 80 C to separate the liquid product from non-condensable gases.
Each
hydrocracking treatment was halted when the quantity of solids accumulating in
the
reactor as a byproduct of the hydrocracking reaction halted the impeller
stirring by
breaking the magnetic coupling of the internal mixer magnet with the external
mixing
magnet.
The hydrocracking conditions and liquid product characteristics for each
sample are shown in Table 2:
53
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
TABLE 2
Sample 1 Sample 2 Sample 3 Sample 4 Sample 5
Catalyst loaded (g) 50 50 50 50 50
Temperature ( C) 428 426 435 454 454
Total pressure (MPa) 13.1 13.1 13.1 13.1 13.1
Gas flow rate (SLPH) 952 952 952 952 949
H2 partial pressure (MPa) 9.4 8.9 9.3 8.8 8.8
H2S partial pressure (MPa) 3.7 4.1 3.8 4.3 4.3
Bitumen feed rate ( ) 250 250 305 400 425
Total liquid in (kg) 36.4 20.6 30.4 17.2 17.8
Total liquid out (kg) 29.9 17.5 24.9 14.7 14.1
Liquid recovery (wt.%) 82.1 85.0 82.0 85.2 79.0
Product density ( cm3) 0.9326 0.9268 0.9284 0.9234 0.9235
Product API Gravity (15.6 C) 20.2 21.2 20.9 21.8 21.7
Product viscosity (cSt)(15.6 C) 24.3 22.1 19.7 10.3 10.4
Product carbon content (wt.%) 84.8 84.8 85.1 85.0 85.4
Product sulfur content (wt.%) 3.4 3.4 3.2 3.3 3.2
Product nitrogen content (wt.%) 0.3 0.3 0.3 0.3 0.3
Boiling point fractions (wt.%--
Simulated Distillation as per
ASTM D5307)
Initial boiling point - 204 C 8.5 9.0 10.5 15.5 16.0
(IBP - 400 F)
204 C - 260 C (400 F - 500 F) 10.5 11.0 11.5 14.5 14.5
260 C - 343 C (500 F - 650 F) 31.0 31.0 29.5 31.0 30.5
343 C - 538 C (650 F -1000 F) 48.5 47.5 47.0 37.5 38.0
538 C+ (1000 F +) 1.5 1.5 1.5 1.5 1.0
The liquid products of samples 1 and 2 were combined and the combined
liquid product was then analyzed by GC-GC sulfur chemiluminesence to determine
the carbon number of sulfur-containing hydrocarbons in the combined liquid
product
of hydrocarbons having a carbon number from 6 to 17 and of hydrocarbons having
a
carbon number of 18 or higher, and to determine the type of sulfur-containing
hydrocarbons contained in the combined liquid product. The results are shown
in
Table 3, where non-benzothiophenes include sulfides, thiols, disulfides,
thiophenes,
arylsulfides, benzonaphthothiophenes, and naphthenic benzonaphthothiophenes,
and
where benzothiophenes include benzothiophene, naphthenic benzothiophenes, di-
benzothiophenes, and naphthenic di-benzothiophenes. Sulfur-containing
hydrocarbons for which a carbon number could not be determined are shown as
having an indeterminate carbon number in Table 3.
54
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
TABLE 3
Non- Benzothiophenic Total % of %
benzothiophenic compounds total benzothiophenic
compounds compounds in
fraction
C6-C17 S-
containing 4554 17213 21767 62.9 79.1
hydrocarbons
(w m S)
C18 and
greater S- 1425 1382 2807 8.1
containing
hydrocarbons
(wppm S)
Indetermine
C-number S- 3835 6194 10029 29.0
containing
hydrocarbons
(wppm S)
As shown in Table 3, the hydrocracking treatment provided a hydrocarbon
composition in which a significant portion of the sulfur in the composition
was
contained in relatively low carbon number hydrocarbons. These low carbon
number
heteroatomic hydrocarbons generally have a low molecular weight relative to
the
sulfur containing hydrocarbons having a carbon number of 18 or greater, and
generally are contained in the naphtha and distillate boiling fractions, not
the high
molecular weight, high boiling residue and asphaltene fractions in which
sulfur-
containing hydrocarbons are more refractory.
The combined liquid product was then analyzed by flame ionization detection-
two dimensional gas chromatography (GCxGC-FID) to determine the monoaromatic,
diaromatic, and polyaromatic hydrocarbon (3 or more aromatic rings) content of
the
combined liquid product. Mono-aromatic compounds included mono-aromatics and
naphthenic mono-aromatics, di-aromatic compounds included di-aromatics and
naphthenic di-aromatics, and polyaromatics included polyaromatic compounds and
naphthenic polyaromatic compounds. The results are shown in Table 4:
TABLE 4
Mono- Di-aromatic Combined Polyaromatic Total
aromatic compounds mono- compounds Aromatic
compounds aromatic and compounds
di-aromatic
compounds
wt. % of
composition 19.1 23.2 42.3 22.2 64.5
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
As shown in Table 4, the hydrocracking treatment provided a hydrocarbon
composition that had a significant quantity of mono-aromatic and di-aromatic
hydrocarbon compounds relative to the polyaromatic hydrocarbon compounds,
where
the weight ratio of the combined mono-aromatic and di-aromatic hydrocarbon
compounds relative to the polyaromatic hydrocarbon compounds was 1.9:1.
EXAMPLE 3
Another catalyst was prepared, where at least a portion of the catalyst had
the
structure as shown in formula (VII) above. 781 grams of ammonium
tetrathiomolybdate was mixed with 636 grams of Na2CO3 in 6 liters of water
while
stirring. The resulting solution was heated to 70 C and then stirred for three
hours to
produce a solution of Na2MoS4. The Na2MoS4 solution was then permitted to cool
overnight. A second solution was prepared by mixing 1498 grams of CuSO4'5H20
in
6 liters of water. The CuSO4 solution was then added to the Na2MoS4 solution
via
pneumatic pump through a 0.02" x 0.5" nozzle while stirring the mixture at
ambient
temperature. The mixture was stirred for two hours, and then the resulting
solids were
separated by centrifuge. 880 grams of solid particulate catalyst was
recovered. The
solids were then washed with water until the effluent from the wash had a
conductivity of 488 S at 33 C. The catalyst solids were particulate and had a
particle
size distribution with a mean particle size of 8.5 m as determined by laser
diffractometry using a Mastersizer S (Malvern Instruments). The BET surface
area of
the catalyst solids was measured to be 29.3 m2/g. Semi-quantitative XRF of the
catalyst solids indicated that the catalyst solids contained 45.867 mass% Cu,
18.587
mass% Mo, and 27.527 mass% S. X-ray diffraction and Raman IR spectroscopy
confirmed that at least a portion of the catalyst had a structure in which
copper,
molybdenum, and sulfur were arranged as shown in formula (VII) above.
EXAMPLE 4
Peace River bitumen having the composition shown in Table 1 above was
hydrocracked in a process in accordance with the present invention using
different
hydrogen sulfide levels to determine the effect of hydrogen sulfide on the
rate of the
hydrocracking reaction. Hydrogen sulfide was provided at 5 mol %, 11.4 mol %,
and
20.1 mol % of the gas fed to the reactor. Hydrogen was provided at 70 mol % of
the
gas fed to the reactor when hydrogen sulfide was provided at 5 mol % (mole
ratio of
1:14, hydrogen sulfide:hydrogen); 68.6 mol % of the gas fed to the reactor
when
hydrogen sulfide was provided at 11.4 mol % (mole ratio of 1:6, hydrogen
56
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
sulfide:hydrogen); and 69.9 mol % of the gas fed to the reactor when hydrogen
sulfide
was provided at 20.1 mol % (mole ratio of 1:3.5, hydrogen sulfide:hydrogen). A
control hydrocracking reaction was also run at 0 mol % hydrogen sulfide and
70.2
mol % hydrogen. Nitrogen was provided as an inert gas in the gas fed to the
reactor
to maintain the total pressure of the reaction at 8.3 MPa, where nitrogen was
provided
as 25 mol % of the gas fed to the reactor when hydrogen sulfide was provided
at 5
mol % of the gas fed to the reactor; as 20 mol % of the gas fed to the reactor
when
hydrogen sulfide was provided at 11.4 mol % of the gas fed to the reactor; as
10 mol
% of the gas fed to the reactor when hydrogen sulfide was provided at 20.1 mol
% of
the gas fed to the reactor; and as 29.8 mol % of the gas fed to the reactor in
the
control. Hydrogen and hydrogen sulfide provided 75% of the total pressure in
the
reaction when hydrogen sulfide was provided at 5 mol % of the gas fed to the
reactor,
and provided 80% of the total pressure when hydrogen sulfide was provided at
11.4
mol % and 20.1 mol % of the gas fed to the reactor.
Four samples of the Peace River bitumen were hydrocracked, one each at the
above specified hydrogen sulfide: hydrogen: nitrogen levels. The hydrocracking
conditions were the same as specified above for Example 2 except that the
catalyst
that was used was the catalyst prepared in Example 3, the total pressure was
maintained at 8.3 MPa, hydrogen sulfide and hydrogen partial pressures
depended on
the amount of each provided to each of the hydrocracking reactions as set
forth above,
the temperature was 430 C for each of the hydrocracking reactions, the gas
flow rate
was maintained at 900 standard liters per hour, and the working volume of feed
in the
reactor was maintained at 500 ml.
The rate of the production of hydrocracked product was measured for each of
the samples. The results are shown in Table 5:
TABLE 5
Time [hrs]>> 5 10 15 20
[mol%] H2S Rate [Kg/h.m3]
0.0% 370 335 300 265
5.0% 403 370 338 305
11.4% 426 394 361 329
20.1% 448 418 387 357
57
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
A graphic depiction of the rate of production of product in each of the
hydrocracking
reactions is shown in Fig. 3.
As shown in Table 5 and Fig. 3, the rate of production of product in the
hydrocracking reactions at constant temperature and pressure increases as the
quantity
of hydrogen sulfide in the reaction mixture increases. It is believed that the
rate will
increase further at each hydrogen sulfide partial pressure, respectively, as
temperature
and total pressure are increased, for example, to 450 C and 13.8 MPa. The rate
of the
reaction is maintained above 350 kg/h-m3 for a sustained period when hydrogen
sulfide is present in an amount relative to hydrogen of at least 1:14 where
the
hydrogen sulfide and hydrogen provide at least 60% of the total pressure in
the
reaction, and is sustained for a longer period as the hydrogen sulfide levels
increase.
EXAMPLE 5
Another catalyst was prepared for use in a hydrocracking process of the
present invention to determine the relative amount of liquid hydrocarbon
product,
coke, non-condensable gas, and hold-up produced by the process. A solution was
prepared by mixing 780 grams of ammonium tetrathiomolybdate and 636 grams of
Na2CO3 in 13.5 liters of deionized water. The solution was heated to 85 C to
generate
Na2MoS4. A separate solution of CuSO4 was prepared by mixing 2994 grams of
CuSO4 in 5 liters of water. The CuSO4 solution was heated to 85 C and added to
the
Na2MoS4 solution through a 0.0625" spray nozzle. The mixed solution was
stirred at
85 C for 2 hours and then at room temperature overnight. Solid catalyst
material was
then separated from the solution by centrifuge. The solid catalyst material
was
washed until the wash effluent had a pH of 7 and conductivity of 488 S at 33
C. The
solid catalyst material was then dried. 548 grams of glossy black catalyst
solids were
recovered.
The catalyst solids were particulate and had a particle size distribution with
a
mean particle size of between 400 and 500 nm as determined by laser
diffractometry
using a Mastersizer S. The BET surface area of the catalyst was measured to be
58 m2/g.
Semi-quantitative XRF indicated that the solid catalyst material contained
37.633 mass %
Cu, 22.231 mass % Mo, 27.734 mass % S, and 0.503 mass % Na. X-ray diffraction
and
and Raman IR spectroscopy confirmed that at least a portion of the catalyst
solids had a
structure in which copper, molybdenum, and sulfur were arranged as shown in
formula
(VII) above.
58
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
EXAMPLE 6
Peace River bitumen having the composition shown in Table 1 above was
hydrocracked in a process in accordance with the present invention using gas
containing 36.5 mol % hydrogen sulfide and 63.7 mol % hydrogen (mole ratio
1:1.75,
hydrogen sulfide:hydrogen) to determine the relative amounts of liquid
hydrocarbon
product, non-compressible gas, and coke produced by the hydrocracking
reaction.
Hydrocracking conditions were the same as set forth in Example 2 except that
the
catalyst that was used in the process was the catalyst prepared in Example 5,
the
hydrogen sulfide partial pressure was 4.78 MPa, the temperature was 420 C, the
gas
flow rate was maintained at 948 standard liters per hour, the working volume
of feed
in the reactor was maintained at 500 ml, and the pressure in the low
temperature
separator was maintained at 1.38 MPa to improve the capture yield of
condensable
vapors.
The yield of liquid hydrocarbon product, non-condensable gas-including
hydrogen, hydrogen sulfide, and hydrocarbons having a carbon number of from 1
to
6, coke, and hold-up were measured and compared with the carbon content of the
feed
provided. Hold-up included residual high molecular weight hydrocarbons that
did not
vaporize as product that were soluble in toluene (so not, by definition, coke)
and
metals. The results are shown in Fig. 4. 93.5% of the carbon content of the
material
produced by the hydrocracking reaction was captured as liquid hydrocarbon
product;
0.1 % of the carbon content was produced as coke, 1.2% of the carbon content
was
produced as non-condensable gas, and 3.1% of the carbon content was produced
as
hold-up, where 97.8 % of the carbon content of the bitumen feed was captured
in the
combined liquid hydrocarbon product, non-condensable gas, coke, and hold-up.
The present invention is well adapted to attain the ends and advantages
mentioned as well as those that are inherent therein. The particular
embodiments
disclosed above are illustrative only, as the present invention may be
modified and
practiced in different but equivalent manners apparent to those skilled in the
art
having the benefit of the teachings herein. Furthermore, no limitations are
intended to
the details of construction or design herein shown, other than as described in
the
claims below. It is therefore evident that the particular illustrative
embodiments
disclosed above may be altered or modified and all such variations are
considered
within the scope and spirit of the present invention. While compositions and
methods
are described in terms of "comprising," "containing," or "including" various
59
CA 02785512 2012-06-22
WO 2011/091202 PCT/US2011/021967
components or steps, the compositions and methods can also "consist
essentially of'
or "consist of the various components and steps. Whenever a numerical range
with a
lower limit and an upper limit is disclosed, any number and any included range
falling
within the range is specifically disclosed. In particular, every range of
values (of the
form, "from a to b," or, equivalently, "from a-b") disclosed herein is to be
understood
to set forth every number and range encompassed within the broader range of
values.
Whenever a numerical range having a specific lower limit only, a specific
upper limit
only, or a specific upper limit and a specific lower limit is disclosed, the
range also
includes any numerical value "about" the specified lower limit and/or the
specified
upper limit. Also, the terms in the claims have their plain, ordinary meaning
unless
otherwise explicitly and clearly defined by the patentee. Moreover, the
indefinite
articles "a" or "an", as used in the claims, are defined herein to mean one or
more
than one of the element that it introduces.