Note: Descriptions are shown in the official language in which they were submitted.
CA 02785673 2012-06-26
SPECIFICATION
POLYAMIDE COMPOUND
TECHNICAL FIELD
[0001]
The present invention relates to a polyamide compound (including a polyamide
resin and a polyamide oligomer) that exhibits an oxygen absorbing capability.
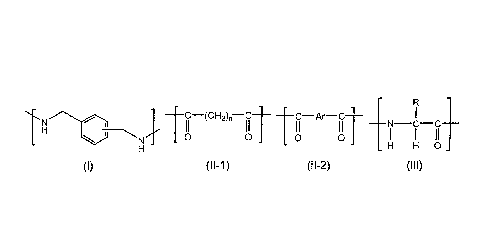
BACKGROUND ART
[0002]
Metallic cans, glass bottles, containers and molded articles formed of a
thermoplastic resin, and the like have been used as packing materials for
medical drugs,
beverages, foods chemical products and the like. Among these, containers and
molded
articles formed of a thermoplastic resin are superior in lightweight property,
moldability,
packaging productivity, such as heat sealing property, and cost, and thus have
been used
in the largest quantity. In general, however, containers and molded articles
formed of a
thermoplastic resin undergo oxygen permeation in nonnegligible extent through
a
container wall, which causes a problem in storageability of contents, although
they are
excellent as a packaging material.
[0003]
For preventing oxygen permeation from the outside of the container, the
container or molded article of a thermoplastic resin is formed to have a
container wall
having a multilayer structure, in which at least one layer thereof is an
oxygen barrier layer,
such as poly-m-xylylene adipamide (which is hereinafter referred to as "N-
MXD6"), an
ethylene-vinyl alcohol copolymer, polyacrylonitrile and an aluminum foil.
However,
the container fails to prevent not only invasion of a slight amount of oxygen
from the
outside of the container, but also deterioration of the contents of the
container that are
sensitive to oxygen, such as beer, with oxygen remaining in the container.
[0004]
An oxygen absorbent has been steadily used for removing oxygen in a container.
For example, Patent Documents 1 and 2 disclose an oxygen absorbing multilayer
material
and an oxygen absorbing film containing an oxygen absorbent, such as iron
powder,
dispersed in a resin. Patent Document 3 discloses an oxygen scavenging barrier
for
packaging that absorbs oxygen inside and outside a container, in which the
oxygen
1
CA 02785673 2012-06-26
,
scavenging barrier contains a polymer material, such as polyamide, to which a
metallic
catalyst, such as cobalt, is added. Patent Document 4 discloses a product
containing an
oxygen removing layer containing an ethylenic unsaturated compound, such as
polybutadiene, and a transition metal catalyst, such as cobalt, and an oxygen
barrier layer,
5 such as polyamide.
CITATION LIST
PATENT LI1ERAT'URE
[0005]
10 [Patent Document 1] JP-A-2-72851
[Patent Document 2] JP-A-4-90848
[Patent Document 3] Japanese Patent No. 2,991,437
[Patent Document 4] JP-A-5-115776
15 SUMMARY OF INVENTION
TECHNICAL PROBLEM
[0006]
An oxygen absorbing multilayer material and an oxygen absorbing film
containing an oxygen absorbent, such as iron powder, dispersed in a resin are
opaque
20 since the resin is colored with the oxygen absorbent, such as iron
powder, and thus has
restrictions in usage, i.e., they may not be used in a field of packaging
requiring
transparency.
An oxygen scavenging resin composition containing a transition metal, such as
cobalt, has an advantage that the composition may be applied to a packaging
container
25 requiring transparency, but is not favorable therefor since the resin
composition is colored
with the transition metal catalyst. In the resin composition, furthermore, the
resin is
oxidized through absorption of oxygen by the transition metal catalyst.
Specifically, it
is considered that the oxidation may occur through such reaction as generation
of a
radical due to withdrawal of a hydrogen atom of a methylene chain adjacent to
an arylene
30 group of the polyamide resin by the transition metal atom, generation of
a peroxy radical
caused by addition of an oxygen molecule to the radical, and withdrawal of a
hydrogen
atom by the perov radical. The resin is oxidized by oxygen absorption through
the
aforementioned mechanism, which results in such problems as generation of
offensive
odor in a content of a container due to decomposition products, and
deterioration of the
35 color, the strength and the like of the container due to
oxidative degradation of the resin.
2
CA 02785673 2012-06-26
[0007]
The problem to be solved by the present invention is to provide a polyamide
compound that exhibits a sufficient oxygen absorbing capability without a
metal
contained, generates no offensive odor, and has considerably good
transparency.
SOLUTION TO PROBLEMS
[0008]
The present invention provides the following polyamide compound.
A polyamide compound containing: from 25 to 50% by mol of a diamine unit,
which contains an aromatic diamine unit represented by the following general
formula (I),
in an amount of 50% by mol or more; from 25 to 50% by mol of a dicarboxylic
acid unit,
which contains a linear aliphatic dicarboxylic acid unit represented by the
following
general formula (II-1) and/or an aromatic dicarboxylic acid unit represented
by the
following general formula (II-2), in an amount in total of 50% by mol or more;
and from
0.1 to 50% by mol of a constitutional unit represented by the following
general formula
(III):
[0009]
C-(CH2)n-C C-Ar-C
N C C
H H 0
(I) (l1-1) (11-2) (III)
[0010]
wherein, in the general formula (II-1), n represents an integer of from 2 to
18, in the
general formula (II-2), Ar represents an arylene group, and in the general
formula (III), R
represents a substituted or unsubstituted alkyl group or a substituted or
unsubstituted aryl
group.
ADVANTAGEOUS EFFECTS OF INVENTION
[0011]
The polyamide compound of the present invention is excellent in oxygen
absorbing capability. Accordingly, for example, the polyamide compound of the
present
invention may be charged in a sachet or the like and favorably used as an
oxygen
absorbent. More preferred examples of the usage of the polyamide compound of
the
present invention include a usage as a packaging material or a packaging
container. A
packaging material or a packaging container using the polyamide compound of
the
3
CA 02785673 2012-06-26
present invention exhibits a sufficient oxygen absorbing capability without a
metal
contained, generates no offensive odor, and has considerably good
transparency, thereby
storing a content in good order.
BRIEF DESCRIPTION OF DRAWINGS
[0012]
Fig. 1 is a 1H-NMIt chart of the polyamide compound 101 produced in Example
101.
Fig. 2 is a 'I-I-NMR chart of the polyamide compound 201 produced in Example
201.
DESCRIPTION OF EMBODIMENTS
[0013]
1. Polyamide Compound
The polyamide compound of the present invention contains: from 25 to 50% by
mol of a diamine unit, which contains an aromatic diamine unit represented by
the
following general formula (I), in an amount of 50% by mol or more; from 25 to
50% by
mol of a dicarboxylic acid unit, which contains a linear aliphatic
dicarboxylic acid unit
represented by the following general formula (II-1) and/or an aromatic
dicarboxylic acid
unit represented by the following general formula (II-2), in an amount in
total of 50% by
mol or more; and from 0.1 to 50% by mol of a tertiary hydrogen-containing
carboxylic
acid unit (preferably a constitutional unit represented by the following
general formula
(111)):
[0014]
r-C
N C C
TAC-A I II
H H H 0
(1) (11-1) (11-2) (111)
[0015]
wherein, in the general formula (II-1), n represents an integer of from 2 to
18, in the
general formula (II-2), Ar represents an arylene group, and in the general
formula (III), R
represents a substituted or unsubstituted alkyl group or a substituted or
unsubstituted aryl
group.
The total content of the diamine unit, the dicarboxylic acid unit and the
tertiary
hydrogen-containing carboxylic acid unit does not exceed 100% by mol. The
4
CA 02785673 2012-06-26
polyamide compound of the present invention may further contain a
constitutional unit
other than those mentioned above in such a range that does not impair the
advantages of
the present invention.
[0016]
The polyamide compound of the present invention includes a polyamide resin
and a polyamide oligomer.
The "polyamide resin" of the present invention means the polyamide compound
of the present invention that has a relative viscosity of 1.8 or more. The
polyamide resin
is a material that can be molded solely and can be processed to a packaging
material and
a packaging container. The polyamide resin of the present invention may
contain,
depending on necessity, another resin and additive added and mixed thereto,
and a
polyamide composition obtained in such a manner may be molded. The polyamide
resin of the present invention exhibits sufficient oxygen absorbing capability
without a
metal contained, generates no offensive odor, and has considerably good
transparency.
[0017]
The "polyamide oligomer" of the present invention means the polyamide
compound of the present invention that has a relative viscosity of less than
1.8. The
polyamide oligomer is a material that cannot generally be molded solely. In
general, an
oligomer often means a polymer having a number average molecular weight of
1,000 or
less, but the polyamide oligomer of the present invention includes not only
the ordinary
oligomer, but also a polymer having a number average molecular weight of less
than
10,000.
[0018]
The polyamide oligomer of the present invention may be favorably charged in a
sachet or the like and used as an oxygen absorbent. The polyamide oligomer of
the
present invention may be favorably used as a resin raw material or a resin
additive. In
the case where the polyamide oligomer of the present invention is used as a
resin raw
material, the polyamide oligomer may be copolymerized with another resin
material,
thereby providing a copolymer resin, and the copolymer resin may be molded
into a
packaging material or a packaging container. In the case where the polyamide
oligomer
of the present invention is used as a resin additive, a resin composition
obtained by
adding the polyamide oligomer to a resin may be molded into a packaging
material or a
packaging container. In this case, a sufficient oxygen absorbing capability
may be
obtained without deterioration of the transparency and the mechanical strength
of the
resin. The copolymer resin and the resin composition obtained by using the
polyamide
5
CA 02785673 2012-06-26
oligomer of the present invention exhibit a sufficient oxygen absorbing
capability without
a metal contained, and generate no offensive odor.
[0019]
In the polyamide compound of the present invention, the content of the
tertiary
hydrogen-containing carboxylic acid unit is from 0.1 to 50% by mol. When the
content
of the tertiary hydrogen-containing carboxylic acid unit is less than 0.1% by
mol, a
sufficient oxygen absorbing capability is not exhibited. When the content of
the tertiary
hydrogen-containing carboxylic acid unit exceeds 50% by mol, the properties of
the
polyamide compound, such as the gas barrier property and the mechanical
property, are
deteriorated since the amount of tertiary hydrogen contained is too large, and
particularly
in the case where the tertiary hydrogen-containing carboxylic acid is an amino
acid, not
only the heat resistance becomes insufficient due to a continuous peptide
chain, but also a
ring structure formed of a dimer of the amino acid is produced and inhibits
the
polymerization. The content of the tertiary hydrogen-containing carboxylic
acid unit is
preferably 0.2% by mol or more, and more preferably 1% by mol or more, and is
preferably 40% by mol or less, and more preferably 30% by mol or less, from
the
standpoint of the oxygen absorbing capability and the properties of the
polyamide
compound.
[0020]
In the polyamide compound of the present invention, the content of the diamine
unit is from 25 to 50% by mol, and from the standpoint of the oxygen absorbing
capability and the properties of the polymer, is preferably from 30 to 50% by
mol. In
the polyamide compound of the present invention, similarly, the content of the
dicarboxylic acid unit is from 25 to 50% by mol, and preferably from 30 to 50%
by mol.
The ratio of the contents of the diamine unit and the dicarboxylic acid unit
is
preferably approximately the same amounts, and more preferably the content of
the
dicarboxylic acid unit is 2% by mol of the content of the diamine unit, from
the
standpoint of the polymerization reaction. When the content of the
dicarboxylic acid
unit deviates from the range of 2% by mol of the content of the diamine unit,
it is
difficult to increase the polymerization degree of the polyamide compound, and
a long
period of time is required for increasing the polymerization degree, which may
cause
thermal degradation.
[0021]
1-1. Diamine Unit
The diamine unit in the polyamide compound of the present invention contains
6
CA 02785673 2012-06-26
an aromatic diamine unit represented by the general formula (I) in an amount
of 50% by
mol or more based on the diamine units, from the standpoint of imparting
excellent gas
barrier property to the polyamide compound, enhancing the transparency and the
color
tone, and improvement of the moldability, and the content thereof is
preferably 70% by
mol or more, more preferably 80% by mol or more, and further preferably 90% by
mol or
more, and is preferably 100% by mol or less.
[0022]
Examples of the compound capable of constituting the aromatic diamine unit
represented by the general formula (I) include o-xylylenediamine, m-
xylylenediamine
and p-xylylenediamine. These compounds may be used solely or as a combination
of
two or more kinds thereof.
[0023]
The diamine unit in the polyamide compound of the present invention preferably
contains a m-xylylenediamine unit in an amount of 50% by mol or more, from the
standpoint of exhibiting the excellent gas barrier property, and improving the
moldability
as a versatile thermoplastic resin, and the content thereof is preferably 70%
by mol or
more, more preferably 80% by mol or more, and further preferably 90% by mol or
more,
and is preferably 100% by mol or less.
[0024]
Examples of the compound capable of constituting other diamine units than the
aromatic diamine unit represented by the general formula (I) include an
aromatic diamine,
such as p-phenylenediamine, an aliphatic diamine, such as 2-methyl-1,5-
pentanediamine
and 1-amino-3-aminomethy1-3,5,5-trimethylcyclohexane, and a polyether diamine
having
an ether bond represented by Jeffamine and Elastamine (both trade names),
produced by
Huntsman International LLC, but are not limited thereto. These compounds may
be
used solely or as a combination of two or more kinds thereof.
[0025]
1-2. Dicarboxylic Acid Unit
The dicarboxylic acid unit in the polyamide compound of the present invention
contains a linear aliphatic dicarboxylic acid unit represented by the general
formula (II-1)
and/or an aromatic dicarboxylic acid unit represented by the general formula
(II-2), in an
amount in total of 50% by mol or more based on the dicarboxylic acid units,
from the
standpoint of the reactivity upon polymerization, and the crystallinity and
the moldability
of the polyamide compound, and the content thereof is preferably from 70% by
mol or
more, more preferably 80% by mol or more, and further preferably 90% by mol or
more,
7
CA 02785673 2012-06-26
and is preferably 100% by mol or less.
[0026]
Examples of the compound capable of constituting other dicarboxylic acid units
than the dicarboxylic acid unit represented by the general formula (II-1) or
(II-2) include
dicarboxylic acids such as oxalic acid, malonic acid, fumaric acid, maleic
acid, 1,3-
benzenediacetic acid and 1,4-benzenediacetic acid, but are not limited
thereto.
[0027]
In the dicarboxylic acid unit in the polyamide compound of the present
invention,
the content ratio of the linear aliphatic dicarboxylic acid unit and the
aromatic
dicarboxylic acid unit (linear aliphatic dicarboxylic acid unit / aromatic
dicarboxylic acid
unit) is not particularly limited and may be appropriately determined
depending on the
purposes. For example, in the case where the glass transition temperature of
the
polyamide compound is to be increased, and the crystallinity of the polyamide
compound
is to be decreased, the ratio (linear aliphatic dicarboxylic acid unit /
aromatic dicarboxylic
acid unit) is preferably from 0/100 to 60/40, more preferably from 0/100 to
40/60, and
further preferably from 0/100 to 30/70, with the total of both the units being
100. In the
case where the glass transition temperature of the polyamide compound is to be
decreased for imparting flexibility to the polyamide compound, the ratio
(linear aliphatic
dicarboxylic acid unit / aromatic dicarboxylic acid unit) is preferably from
40/60 to 100/0,
more preferably from 60/40 to 100/0, and further preferably from 70/30 to
100/0, with the
total of both the units being 100.
[0028]
1-2-1. Linear Aliphatic Dicarboxylic Acid Unit
The polyamide compound of the present invention preferably contains the linear
aliphatic dicarboxylic acid unit represented by the general formula (II-1) for
imparting a
suitable glass transition temperature and crystallinity to the polyamide
compound, and for
imparting thereto flexibility that is required for a packaging material and a
packaging
container.
In the general formula (II-1), n represents an integer of from 2 to 18,
preferably
from 3 to 16, more preferably from 4 to 12, and further preferably from 4 to
8.
Examples of the compound capable of constituting the linear aliphatic
dicarboxylic acid unit represented by the general formula (II-1) include
succinic acid,
glutaric acid, adipic acid, pimelic acid, suberic acid, azelaic acid, sebacic
acid, 1,10-
decanedicarboxylic acid, 1,11-undecanedicarboxylic acid and 1,12-
dodecanedicarboxylic
acid, but are not limited thereto. These compounds may be used solely or as a
8
CA 02785673 2012-06-26
combination of two or more kinds thereof.
[0029]
The kind of the linear aliphatic dicarboxylic acid unit represented by the
general
formula (II-1) may be appropriately determined depending on the purposes. The
linear
aliphatic dicarboxylic acid unit in the polyamide compound of the present
invention
preferably contains at least one selected from the group consisting of an
adipic acid unit,
a sebacic acid unit and a 1,12-dodecanedicarboxylic acid unit, in an amount in
total of
50% by mol or more in the linear aliphatic dicarboxylic acid unit from the
standpoint of
imparting excellent gas barrier property to the polyamide compound, and
maintaining the
heat resistance of a packaging material and a packaging container after
thermal
sterilization, and the content thereof is more preferably 70% by mol or more,
further
preferably 80% by mol or more, and particularly preferably 90% by mol or more,
and is
preferably 100% by mol or less.
[0030]
The linear aliphatic dicarboxylic acid unit in the polyamide compound of the
present invention preferably contains an adipic acid unit in an amount of 50%
by mol or
more based on the linear aliphatic dicarboxylic acid units from the standpoint
of the gas
barrier property and the suitable thermal properties, such as the glass
transition
temperature and the melting point, of the polyamide compound. The linear
aliphatic
dicarboxylic acid unit in the polyamide compound of the present invention
preferably
contains a sebacic acid unit in an amount of 50% by mol or more based on the
linear
aliphatic dicarboxylic acid units from the standpoint of imparting suitable
gas barrier
property and molding suitability to the polyamide compound, and in the case
where the
polyamide compound is applied to such a purpose that requires low water
absorbing
property, weather resistance and heat resistance, the linear aliphatic
dicarboxylic acid unit
preferably contains a 1,12-dodecanedicarboxylic acid unit in an amount of 50%
by mol or
more based on the linear aliphatic dicarboxylic acid units.
[0031]
1-2-2. Aromatic Dicarboxylic Acid Unit
The polyamide compound of the present invention preferably contains the
aromatic dicarboxylic acid unit represented by the general formula (II-2) for
imparting
further gas barrier property to the polyamide compound, and for facilitating
the
moldability of a packaging material and a packaging container.
In the general formula (11-2), Ar represents an arylene group. The arylene
group is preferably an arylene group having from 6 to 30 carbon atoms, and
more
9
CA 02785673 2012-06-26
preferably an arylene group having from 6 to 15 carbon atoms, and examples
thereof
include a phenylene group and a naphthylene group.
Examples of the compound capable of constituting the aromatic dicarboxylic
acid unit represented by the general formula (II-2) include terephthalic acid,
isophthalic
acid and 2,6-naphthalenedicarboxylic acid, but are not limited thereto. These
compounds may be used solely or as a combination of two or more kinds thereof.
[0032]
The kind of the aromatic dicarboxylic acid unit represented by the general
formula (II-2) may be appropriately determined depending on the purposes. The
aromatic dicarboxylic acid unit in the polyamide compound of the present
invention
preferably contains at least one selected from the group consisting of an
isophthalic acid
unit, a terephthalic acid unit and a 2,6-naphthalenedicarboxylic acid unit, in
an amount in
total of 50% by mol or more based on the aromatic dicarboxylic acid units, and
the
content thereof is more preferably 70% by mol or more, further preferably 80%
by mol or
more, and particularly preferably 90% by mol or more, and is preferably 100%
by mol or
less. Among these units, isophthalic acid and/or terephthalic acid are
preferably
contained in the aromatic dicarboxylic acid unit. The content ratio of the
isophthalic
acid unit and the terephthalic acid unit (isophthalic acid unit / terephthalic
acid unit) is
not particularly limited and may be determined depending on the purposes. For
example, from the standpoint of providing a suitable glass transition
temperature and
decreasing the crystallinity, the ratio is preferably from 0/100 to 100/0,
more preferably
from 0/100 to 60/40, further preferably from 0/100 to 40/60, and still further
preferably
from 0/100 to 30/70, with the total of both the units being 100.
[0033]
1-3. Tertiary Hydrogen-containing Carboxylic Acid Unit
The tertiary hydrogen-containing carboxylic acid unit in the present invention
has at least one each of an amino group and a carboxyl group, or has two or
more of
carboxyl groups, from the standpoint of the polymerization of the polyamide
compound.
Specific examples thereof include constitutional units represented by any one
of the
following general formulae (III), (IV) and (V):
[0034]
CA 02785673 2012-06-26
R1
R2
I
_______ N C C _______________ N A', C A2 C ______ C A3 C C
I II II II I II
H HO H H 0 0 HO
(III) (IV) (V)
[0035]
wherein, in the general formulae (III) to (V), R, RI and R2 each represent a
substituent,
and Al to A3 each represent a single bond or a divalent linking group,
provided that the
case where both Al and A2 are single bonds in the general formula (IV) is
excluded.
[0036]
The polyamide compound of the present invention contains the tertiary
hydrogen-containing carboxylic acid unit. Owing to the tertiary hydrogen-
containing
carboxylic acid unit contained as a copolymerization component, the polyamide
compound of the present invention exhibits an excellent oxygen absorbing
capability
without a transition metal contained.
[0037]
The mechanism where the polyamide compound containing the tertiary
hydrogen-containing carboxylic acid unit exhibits a good oxygen absorbing
capability in
the present invention has not yet been clarified, but may be expected as
follows. A
compound capable of constituting the tertiary hydrogen-containing carboxylic
acid unit
has an electron withdrawing group and an electron donating group, both of
which are
bonded on the same carbon atom, and it is thus considered that a very stable
radical is
formed through a phenomenon referred to as the captodative effect where the
unpaired
electron present on the carbon atom is energetically stabilized. Specifically,
the
carboxyl group, which is an electron withdrawing group, makes electron
deficient (5+)
the adjacent carbon having the tertiary hydrogen bonded thereto, and thus the
tertiary
hydrogen also becomes electron deficient (5+), and is dissociated as a proton,
thereby
forming a radical. When oxygen and water are present therewith, it is
considered that
oxygen is reacted with the radical, and an oxygen absorbing capability is
exhibited. It
has been found that higher reactivity is obtained in an environment with a
higher
humidity and a higher temperature.
[0038]
In the general formulae (III) to (V), R, R1 and R2 each represent a
substituent.
Examples of the substituent represented by R, RI and R2 include a halogen atom
(such as
a chlorine atom, a bromine atom and an iodine atom), an alkyl group (such as a
linear,
11
CA 02785673 2012-06-26
branched or cyclic alkyl group having from 1 to 15 carbon atoms, and
preferably from 1
to 6 carbon atoms, e.g., a methyl group, an ethyl group, a n-propyl group, an
isopropyl
group, a t-butyl group, a n-octyl group, a 2-ethylhexyl group, a cyclopropyl
group and a
cyclopentyl group), an alkenyl group (such as a linear, branched or cyclic
alkenyl group
having from 2 to 10 carbon atoms, and preferably from 2 to 6 carbon atoms,
e.g., a vinyl
group and an allyl group), an allcynyl group (such as an alkynyl group having
from 2 to
carbon atoms, and preferably from 2 to 6 carbon atoms, e.g., an ethynyl group
and a
propargyl group), an aryl group (such as an aryl group having from 6 to 16
carbon atoms,
and preferably from 6 to 10 carbon atoms, e.g., a phenyl group and a naphthyl
group), a
10 heterocyclic group (such as a monovalent group having from 1 to 12
carbon atoms, and
preferably from 2 to 6 carbon atoms, which is obtained by removing one
hydrogen atom
from a 5-membered or 6-membered aromatic or nonaromatic heterocyclic compound,
e.g.,
a 1-pyrazoly1 group, a 1-imidazoly1 group and a 2-furyl group), a cyano group,
a
hydroxyl group, a nitro group, an alkov group (such as a linear, branched or
cyclic
alkoxy group having from 1 to 10 carbon atoms, and preferably from 1 to 6
carbon atoms,
e.g., a methoxy group and an ethoxy group), an arylov group (such as an
aryloxy group
having from 6 to 12 carbon atoms, and preferably from 6 to 8 carbon atoms,
e.g., a
phenoxy group), an acyl group (such as a formyl group, an alkylcarbonyl group
having
from 2 to 10 carbon atoms, and preferably from 2 to 6 carbon atoms, and an
arylcarbonyl
group having from 7 to 12 carbon atoms, and preferably from 7 to 9 carbon
atoms, e.g.,
an acetyl group, a pivaloyl group and a benzoyl group), an amino group (such
as an
amino group, an alkylamino group having from 1 to 10 carbon atoms, and
preferably
from 1 to 6 carbon atoms, an anilino group having from 6 to 12 carbon atoms,
and
preferably from 6 to 8 carbon atoms, and a heterocyclic amino group having
from 1 to 12
carbon atoms, and preferably from 2 to 6 carbon atoms, e.g., an amino group, a
methylamino group and an anilino group), a mercapto group, an alkylthio group
(such as
an alkylthio group having from 1 to 10 carbon atoms, and preferably from 1 to
6 carbon
atoms, e.g., a methylthio group and an ethylthio group), an arylthio group
(such as an
arylthio group having from 6 to 12 carbon atoms, and preferably from 6 to 8
carbon
atoms, e.g., a phenylthio group), a heterocyclic thio group (such as a
heterocyclic thio
group having from 2 to 10 carbon atoms, and preferably from 1 to 6 carbon
atoms, e.g., a
2-benzothiazolylthio group), and an imide group (such as an imide group having
from 2
to 10 carbon atoms, and preferably from 4 to 8 carbon atoms, e.g., an N-
succinimide
group and an N-phthalimide group).
[0039]
12
CA 02785673 2012-06-26
=
Among these functional groups, those having a hydrogen atom may be further
substituted by the aforementioned groups, and examples thereof include an
alkyl group
substituted with a hydroxyl group (such as a hydroxyethyl group), an alkyl
group
substituted with an alkoxy group (such as a methoxyethyl group), an alkyl
group
substituted with an aryl group (such as a benzyl group), an aryl group
substituted with an
alkyl group (such as a p-tolyl group) and an aryloxy group substituted with an
alkyl
group (such as a 2-methylphenoxy group), but are not limited thereto.
In the case where the functional group is further substituted, the number of
carbon atoms mentioned above does not contain the number of carbon atoms of
the
further substituent. For example, a benzyl group is considered as an alkyl
group having
one carbon atom substituted with a phenyl group, but is not considered as an
alkyl group
having 7 carbon atoms substituted with a phenyl group. The numbers of carbon
atoms
described hereinbelow are to be similarly understood unless otherwise
indicated.
[0040]
In the general formulae (IV) and (V), A1 to A3 each represent a single bond or
a
divalent linking group. In the general formula (IV), the case where both Al
and A2 are
single bonds is excluded. Examples of the divalent linking group include a
linear,
branched or cyclic alkylene group (such as an alkylene group having from 1 to
12 carbon
atoms, and preferably from 1 to 4 carbon atoms, e.g., a methylene group and an
ethylene
group), an aralkylene group (such as an arallcylene group having from 7 to 30
carbon
atoms, and preferably from 7 to 13 carbon atoms, e.g., a benzylidene group)
and an
arylene group (such as an arylene group having from 6 to 30 carbon atoms, and
preferably from 6 to 15 carbon atoms, e.g., a phenylene group). These groups
may
further have a substituent, and examples of the substituent include those
exemplified as
the functional groups for R, le and R2. Examples thereof include an arylene
group
substituted with an alkyl group (such as a xylylene group), but are not
limited thereto.
[0041]
The polyamide compound of the present invention preferably contains at least
one kind of the constitutional units represented by any one of the general
formulae (III),
(IV) and (V). Among these, a carboxylic acid unit having tertiary hydrogen on
an a-
carbon (a carbon atom that is adjacent to the carboxyl group) is preferred,
and a
constitutional unit represented by the general formula (III) is particularly
preferred from
the standpoint of the availability of the raw material and the enhancement of
the oxygen
absorbing capability.
[0042]
13
CA 02785673 2012-06-26
The substituent R in the general formula (III) has been described above, and
among them, a substituted or unsubstituted alkyl group and a substituted or
unsubstituted
aryl group are preferred, a substituted or unsubstituted alkyl group having
from 1 to 6
carbon atoms and a substituted or unsubstituted aryl group having from 6 to 10
carbon
atoms are more preferred, and a substituted or unsubstituted alkyl group
having from 1 to
4 carbon atoms and a substituted or unsubstituted phenyl group are
particularly preferred.
Preferred examples of R include a methyl group, an ethyl group, a n-propyl
group, an isopropyl group, a n-butyl group, a t-butyl group, a 1-methylpropyl
group, a 2-
methylpropyl group, a hydroxymethyl group, a 1-hydroxyethyl group, a
mercaptomethyl
group, a methylsulfanylethyl group, a phenyl group, a naphthyl group, a benzyl
group
and a 4-hydroxybenzyl group, but are not limited thereto. Among these, a
methyl group,
an ethyl group, a 2-methylpropyl group and a benzyl group are more preferred.
[0043]
Examples of the compound capable of constituting the constitutional unit
represented by the general formula (III) include a-amino acids such as
alanine, 2-
aminobutyric acid, valine, norvaline, leucine, norleucine, tert-leucine,
isoleucine, serine,
threonine, cysteine, methionine, 2-phenylglycine, phenylalanine, tyrosine,
histidine,
tryptophan and proline, but are not limited thereto.
Examples of the compound capable of constituting the constitutional unit
represented by the general formula (IV) include 13-amino acids such as 3-
aminobutyric
acid, and examples of the compound capable of constituting the constitutional
unit
represented by the general formula (V) include dicarboxylic acids such as
methylmalonic
acid, methylsuccinic acid, malic acid and tartaric acid, but are not limited
thereto.
These compounds may be a D-isomer, an L-isomer or a racemic substance, and
may also be an allo-isomer. These compounds may be used solely or as a
combination
of two or more kinds thereof
[0044]
Among these, an a-amino acid having tertiary hydrogen on an a-carbon is
particularly preferred from the standpoint of the availability of the raw
material, the
enhancement of the oxygen absorbing capability and the like. In the a-amino
acid,
alanine is most preferred from the standpoint of the availability, the low
cost, the easiness
of polymerization, and the low yellowness index (YI) of the polymer. Alanine
has a
relatively low molecular weight and thus exhibits a high polymerization ratio
per 1 g of
the polyamide compound of the present invention, and therefore, alanine
provides a good
oxygen absorbing capability per 1 g of the polyamide compound.
14
CA 02785673 2012-06-26
[0045]
The purity of the compound capable of constituting the tertiary hydrogen-
containing carboxylic acid unit is preferably 95% or more, more preferably
98.5% or
more, and further preferably 99% or more, from the standpoint of the influence
on
polymerization such as delay of the polymerization rate, and the influence on
the product
quality such as the yellowness index of the polymer. The amount of a sulfate
ion and an
ammonium ion contained as impurities is preferably 500 ppm or less, more
preferably
200 ppm or less, and further preferably 50 ppm or less.
[0046]
1-4. co-Aminocarboxylic Acid Unit
The polyamide compound of the present invention may further contain an co-
aminocarboxylic acid unit represented by the following general formula (A), in
addition
to the diamine unit, the dicarboxylic acid unit and the tertiary hydrogen-
containing
carboxylic acid unit, in the case where the polyamide compound requires
flexibility or the
like.
[0047]
0
(A)
wherein, in the general formula (A), p represents an integer of from 2 to 18.
The content of the co-aminocarboxylic acid unit is preferably from 0.1 to
49.9%
by mol, more preferably from 3 to 40% by mol, and further preferably from 5 to
35% by
mol, based on the total constitutional units of the polyamide compound. The
total
content of the diamine unit, the dicarboxylic acid unit, the tertiary hydrogen-
containing
carboxylic acid unit and the 0-aminocarboxylic acid unit does not exceed 100%
by mol.
In the general formula (A), p represents an integer of from 2 to 18,
preferably
from 3 to 16, more preferably from 4 to 14, and further preferably from 5 to
12.
[0048]
Examples of the compound capable of constituting the co-aminocarboxylic acid
unit represented by the following general formula (A) include an 0-
aminocarboxylic acid
having from 5 to 19 carbon atoms and a lactam having from 5 to 19 carbon
atoms.
Examples of the co-aminocarboxylic acid having from 5 to 19 carbon atoms
include 6-
aminohexanoic acid and 12-aminododecanoic acid, and examples of the lactam
having
from 5 to 19 carbon atoms include s-caprolactam and laurolactam, but are not
limited
CA 02785673 2012-06-26
thereto. These compounds may be used solely or as a combination of two or more
kinds
thereof.
[0049]
The co-aminocarboxylic acid unit preferably contains a 6-aminohexanoic acid
unit and/or a 12-aminododecanoic acid unit in an amount in total of 50% by mol
or more
based on the co-aminocarboxylic acid units, and the content thereof is more
preferably
70% by mol or more, more preferably 80% by mol or more, and further preferably
90%
by mol or more, and is preferably 100% by mol or less.
[0050]
1-5. Polymerization Degree of Polyamide Compound
With respect to the polymerization degree of the polyamide compound of the
present invention, a relative viscosity is used. The polyamide compound of the
present
invention preferably has a relative viscosity of from 1.01 to 4.2.
In the case where the polyamide compound of the present invention is a
polyamide resin, the relative viscosity thereof is preferably from 1.8 to 4.2,
more
preferably from 1.9 to 4.0, and further preferably from 2.0 to 3.8, from the
standpoint of
the appearance and the molding processability of the molded article. The
relative
viscosity thereof is not limited to the range in the case where the polyamide
resin of the
present invention is used as an additive or a modifier of another
thermoplastic resin.
In the case where the polyamide compound of the present invention is a
polyamide oligomer, the relative viscosity thereof is preferably 1.01 or more
and less than
1.8, more preferably from 1.1 to 1.75, further preferably from 1.2 to 1.65,
and particularly
preferably from 1.3 to 1.6, from the standpoint of the handle ability,
reactivity and heat
stability.
The relative viscosity referred herein is a ratio of the fall time (t) of the
polyamide compound measured by dissolving 1 g of the polyamide compound in 100
inL
of 96% sulfuric acid and measuring the resulting solution with a Cannon-Fenske
viscometer at 25 C, and the fall time (to) of 96% sulfuric acid measured in
the same
manner, and shown by the following expression.
Relative viscosity = t/to
[0051]
1-6. Terminal Amino Group Concentration
The oxygen absorption rate of the polyamide compound and the oxidative
degradation of the polyamide compound due to oxygen absorption may be
controlled by
16
CA 02785673 2012-06-26
changing the terminal amino group concentration of the polyamide compound. In
the
case where the polyamide compound is a polyamide resin, the terminal amino
group
concentration is preferably in a range of from 5 to 150 eq/106 g, more
preferably from 10
to 100 eq/106 g, and further preferably from 15 to 80 eq/106 g, from the
standpoint of the
balance between the oxygen absorption rate and the oxidative degradation.
[0052]
2. Production Method of Polyamide Compound
The polyamide compound of the present invention may be produced by
polycondensation of a diamine component capable of constituting the diamine
unit, a
dicaroxylic acid component capable of constituting the dicarboxylic acid unit,
a tertiary
hydrogen-containing carboxylic acid component capable of constituting the
tertiary
hydrogen-containing carboxylic acid component, and depending on necessity, an
co-
aminocarboxylic acid component capable of constituting the w-aminocarboxylic
acid unit.
The polymerization degree thereof may be controlled by adjusting the
polycondensation
conditions and the like. As a molecular weight controlling agent, a small
amount of a
monoamine and a monocarboxylic acid may be added upon polycondensation.
Furthermore, for providing an intended polymerization degree by suppressing
the
polycondensation reaction, the ratio (molar ratio) of the diamine component
and the
carboxylic acid component constituting the polyamide compound may be deviated
from 1.
[0053]
Examples of the polycondensation method of the polyamide compound of the
present invention include a reactive extrusion method, a pressurized salt
method, an
atmospheric dropping method and a pressurized dropping method, but are not
limited
thereto. The reaction temperature is preferably as low as possible, and
thereby the
polyamide compound may be prevented from suffering yellowing or gelation, and
the
polyamide compound having stable properties may be obtained.
[0054]
2-1. Reactive Extrusion Method
In the reactive extrusion method, a polyamide prepared from the diamine
component and the dicarboxylic acid component (i.e., a polyamide corresponding
to a
precursor of the polyamide compound of the present invention) or a polyamide
prepared
from the diamine component, the dicarboxylic acid component and the 03-
aminocarboxylic acid component (i.e., a polyamide corresponding to a precursor
of the
polyamide compound of the present invention) is reacted with the tertiary
hydrogen-
containing carboxylic acid component by melt-kneading in an extruder. In this
method,
17
CA 02785673 2012-06-26
the tertiary hydrogen-containing carboxylic acid component is incorporated
into the
skeleton of the polyamide through amide exchange reaction, and for performing
the
reaction sufficiently, it is preferred to use a screw suitable for the
reactive extrusion and a
twin screw extruder having a large L/D ratio. This method is convenient and
suitable
for producing the polyamide compound that contains a small amount of the
tertiary
hydrogen-containing carboxylic acid unit.
[0055]
2-2. Pressurized Salt Method
In the pressurized salt method, a nylon salt as a raw material is subjected to
melt
polycondensation under increased pressure. Specifically, a nylon salt aqueous
solution
containing the diamine component, the dicarboxylic acid component, the
tertiary
hydrogen-containing carboxylic acid component, and depending on necessity the
co-
aminocarboxylic acid component is prepared, and then the aqueous solution is
concentrated and then subjected to polycondensation by increasing the
temperature
thereof under increased pressure while removing condensation water. While
returning
the inside of the reaction vessel gradually to the atmospheric pressure, the
temperature is
increased to a temperature higher by approximately 10 C than the melting point
of the
polyamide compound and maintained, and then while the pressure is decreased
gradually
to 0.02 MPaQ the temperature is maintained for continuously performing the
polycondensation. After reaching the agitation torque to a constant value, the
inside of
the reaction vessel is pressurized with nitrogen to approximately 0.3 MPaQ
thereby
recovering the polyamide compound.
The pressurized salt method is useful in the case where a volatile component
is
used as a monomer, and is a preferred polycondensation method in the case
where the
copolymerization ratio of the tertiary hydrogen-containing carboxylic acid
component is
large. The method is particularly preferred for producing the polyamide resin
(A)
containing the tertiary hydrogen-containing carboxylic acid unit in an amount
of 15% by
mol or more based on the total constitutional units of the polyamide compound.
The use
of the pressurized salt method prevents the tertiary hydrogen-containing
carboxylic acid
component from evaporating, and furthermore prevents the tertiary hydrogen-
containing
carboxylic acid component from undergoing polycondensation solely, and thus
the
polycondensation reaction can be smoothly performed, thereby providing the
polyamide
compound having excellent properties.
[0056]
2-3. Atmospheric Dropping Method
18
CA 02785673 2012-06-26
In the atmospheric dropping method, the diamine component is continuously
added dropwise to a mixture obtained by melting under heat the dicarboxylic
acid
component, the tertiary hydrogen-containing carboxylic acid component, and
depending
on necessity, the co-aminocarboxylic acid component, thereby performing the
polycondensation while removing condensation water. The polycondensation
reaction
is performed under heating the reaction system, thereby preventing the
reaction
temperature from becoming lower than the melting point of the polyamide
compound
produced.
As compared to the pressurized salt method, the atmospheric dropping method
provides a larger yield per batch because no water for dissolving the salt is
used, and
undergoes less decrease in reaction rate because vaporization and condensation
of the raw
material components, thereby shortening the process time.
[0057]
2-4. Pressurized Dropping Method
In the pressurized dropping method, the dicarboxylic acid component, the
tertiary hydrogen-containing carboxylic acid component, and depending on
necessity, the
co-aminocarboxylic acid component are charged in a polycondensation vessel,
and the
components are melt-mixed by agitation to prepare a mixture. Subsequently,
while
pressurizing the inside of the vessel to approximately from 0.3 to 0.4 MPaQ
the diamine
component is continuously added dropwise to the mixture, thereby performing
the
polycondensation while removing condensation water. At this time, the
polycondensation reaction is performed under heating the reaction system,
thereby
preventing the reaction temperature from becoming lower than the melting point
of the
polyamide compound produced. After reaching the prescribed molar ratio, the
dropwise
addition of the diamine component is terminated. Then, while returning the
inside of
the vessel gradually to the atmospheric pressure, the temperature is increased
to a
temperature higher by approximately 10 C than the melting point of the
polyamide
compound and maintained, and then while the pressure is decreased gradually to
0.02
MPaCc the temperature is maintained for continuously performing the
polycondensation.
After reaching the agitation torque to a constant value, the inside of the
vessel is
pressurized with nitrogen to approximately 0.3 MPaQ thereby recovering the
polyamide
compound.
As similar to the pressurized salt method, the pressurized dropping method is
useful in the case where a volatile component is used as a monomer, and is a
preferred
polycondensation method in the case where the copolymerization ratio of the
tertiary
19
CA 02785673 2012-06-26
hydrogen-containing carboxylic acid component is large. The method is
particularly
preferred for producing the polyamide compound containing the tertiary
hydrogen-
containing carboxylic acid unit in an amount of 15% by mol or more based on
the total
constitutional units of the polyamide compound. The use of the pressurized
dropping
method prevents the tertiary hydrogen-containing carboxylic acid component
from
evaporating, and furthermore prevents the tertiary hydrogen-containing
carboxylic acid
component from undergoing polycondensation solely, and thus the
polycondensation
reaction can be smoothly performed, thereby providing the polyamide compound
having
excellent properties. Moreover, as compared to the pressurized salt method,
the
pressurized dropping method provides a larger yield per batch because no water
for
dissolving the salt is used, and can shorten the reaction time as similar to
the atmospheric
dropping method, thereby providing the polyamide compound with a low
yellowness
index through prevention of gelation or the like.
[0058]
2-5. Step of increasing Polymerization Degree
The polyamide compound thus produced by the aforementioned
polycondensation methods may be used as it is, or may further be subjected to
a step of
further increasing the polymerization degree. Examples of the step of further
increasing
the polymerization degree include reactive extrusion in an extruder and solid
phase
polymerization. Preferred examples of a heating device used for solid phase
polymerization include a continuous heating and drying device, a rotation drum
heating
device which is referred to as a tumble dryer, a conical dryer and a rotary
dryer, and a
conical heating device having a rotary blade inside the device which is
referred to as a
Nauta mixer, but are not limited thereto, and known methods and devices may be
used.
Particularly in the case where the polyamide compound is subjected to solid
phase
polymerization, the rotation drum heating device is preferred among the above
devices
since the system can be sealed, and thereby the polycondensation can be
performed in a
state where oxygen causing coloration is removed.
[0059]
2-6. Phosphorus Atom-containing Compound and Alkali Metal Compound
In the polycondensation of the polyamide compound of the present invention, a
phosphorus atom-containing compound is preferably added from the standpoint of
acceleration of the amidation reaction.
Examples of the phosphorus atom-containing compound include a phosphinic
acid compound, such as dimethylphosphinic acid and phenylmethylphosphinic
acid; a
CA 02785673 2012-06-26
hypophosphorous acid compound, such as hypophosphorous acid, sodium
hypophosphite,
potassium hypophosphite, lithium hypophosphite, magnesium hypophosphite,
calcium
hypophosphite and ethyl hypophosphite; a phosphonic acid compound, such as
phosphonic acid, sodium phosphonate, potassium phosphonate, lithium
phosphonate,
potassium phosphonate, magnesium phosphonate, calcium phosphonate,
phenylphosphonic acid, ethylphosphonic acid, sodium phenylphosphonate,
potassium
phenylphosphonate, lithium phenylphosphonate, diethyl phenylphosphonate,
sodium
ethylphosphonate and potassium ethylphosphonate; a phosphonous acid compound,
such
as phosphonous acid, sodium phosphonite, lithium phosphonite, potassium
phosphonite,
magnesium phosphonite, calcium phosphonite, phenylphosphonous acid, sodium
phenylphosphonite, potassium phenylphosphonite, lithium phenylphosphonite and
ethyl
phenylphosphonite; and a phosphorous acid compound, such as phosphorous acid,
sodium hydrogen phosphite, sodium phosphite, lithium phosphite, potassium
phosphite,
magnesium phosphite, calcium phosphite, triethyl phosphite, triphenyl
phosphite and
pyrophosphorous acid.
Among these, a metal salt of hypophosphorous acid, such as sodium
hypophosphite, potassium hypophosphite and lithium hypophosphite, is
preferably used
since it greatly accelerates the amidation reaction and is excellent in
prevention of
coloration, and sodium hypophosphite is particularly preferred. The phosphorus
atom-
containing compound that can be used in the present invention is not limited
to these
compounds.
The amount of the phosphorus atom-containing compound added is preferably
from 0.1 to 1,000 ppm, more preferably from 1 to 600 ppm, and further
preferably from 5
to 400 ppm, in terms of phosphorus atom concentration in the polyamide
compound.
When the amount thereof is 0.1 ppm or more, the polyamide compound is hard to
be
colored during the polymerization, thereby providing high transparency. When
the
amount thereof is 1,000 ppm or less, the polyamide compound is hard to be
gelled, and
fish eyes, which are considered to be caused by the phosphorus atom containing
compound, can be suppressed from being contained in a molded article, thereby
providing a molded article with a good appearance.
[0060]
In the polycondensation system of the polyamide compound, an alkali metal
compound is preferably added in combination with the phosphorus atom-
containing
compound. For preventing the polyamide compound from being colored during the
polycondensation, the phosphorus atom-containing compound is necessarily
present in a
21
CA 02785673 2012-06-26
sufficient amount, but it may cause gelation of the polyamide compound in some
cases,
and therefore, an alkali metal compound is preferably used in combination
therewith for
controlling the amidation reaction rate.
Preferred examples of the alkali metal compound include an alkali metal
hydroxide, an alkali metal acetate salt, an alkali metal carbonate salt and an
alkali metal
alkoxide. Specific examples of the alkali metal compound capable of being used
in the
present invention include lithium hydroxide, sodium hydroxide, potassium
hydroxide,
rubidium hydroxide, cesium hydroxide, lithium acetate, sodium acetate,
potassium
acetate, rubidium acetate, cesium acetate, sodium methoxide, sodium ethoxide,
sodium
propoxide, sodium butoxide, potassium methoxide, lithium methoxide and sodium
carbonate. The alkali metal compound may be used without limitation to these
compounds. The ratio of the phosphorus atom-containing compound and the alkali
metal compound, phosphorus atom-containing compound / alkali metal compound,
is
preferably from 1.0/0.05 to 1.0/1.5, more preferably from 1.0/0.1 to 1.0/1.2,
and further
preferably from 1.0/0.2 to 1.0/1.1, from the standpoint of controlling the
polymerization
rate and lowering the yellowness index.
[0061]
3. Polyamide Composition
To the polyamide compound of the present invention, additives may be added
depending on the required purposes and capabilities, such as a lubricant, a
crystallization
nucleating agent, a whitening preventing agent, a matting agent, a heat
resistant stabilizer,
a weather resistant stabilizer, an ultraviolet ray absorbent, a plasticizer, a
flame retardant,
an antistatic agent, a coloration preventing agent, an antioxidant and an
impact resistance
improving agent, thereby forming a polyamide composition. The additives may be
added depending on necessity in such a range that does not impair the
advantages of the
present invention. The polyamide compound of the present invention may also be
mixed with various kinds of resins depending on the required purposes and
capabilities,
thereby forming a polyamide composition. In the polyamide composition, the
polyamide resin or the polyamide oligomer may be reacted with the additives or
the
resins added.
[0062]
The polyamide compound of the present invention and the additive may be
mixed by a known method, and a dry mixing method is preferably employed since
it may
be performed at low cost and without thermal history. For example, the
polyamide
compound and the additive are placed in a tumbler, which is then rotated for
mixing them.
22
CA 02785673 2012-06-26
In the present invention, such a mixing method may be employed that a viscous
liquid as
a spreading agent is attached to the polyamide compound for preventing
classification of
the polyamide compound and the additive after dry mixing, and then the
additive is added
and mixed therewith. Examples of the spreading agent include a surfactant, but
are not
limited thereto, and known ones may be used.
[0063]
3-1. Whitening Preventing Agent
In the polyamide composition containing the polyamide compound of the
present invention, a diamide compound and/or a diester compound is preferably
added to
the polyamide compound for preventing whitening after subjecting to a
hydrothermal
treatment or after elapse of a prolonged period of time. The diamide compound
and/or
the diester compound are effective for prevention of whitening due to
deposition of the
oligomer. The diamide compound and the diester compound may be used solely or
as a
combination thereof.
[0064]
Preferred examples of the diamide compound include a diamide compound
obtained with an aliphatic dicarboxylic acid having from 8 to 30 carbon atoms
and a
diamine having from 2 to 10 carbon atoms. The whitening prevention effect is
expected
with an aliphatic dicarboxylic acid having 8 or more carbon atoms and a
diamine having
2 or more carbon atoms. The diamide compound may be favorably dispersed
uniformly
in the resin composition with an aliphatic dicarboxylic acid having 30 or less
carbon
atoms and a diamine having 10 or less carbon atoms. The aliphatic dicarboxylic
acid
may have a side chain and a double bond, but a linear saturated aliphatic
dicarboxylic
acid is preferred. The diamide compound may be used solely with one kind or as
a
combination of two or more kinds thereof.
[0065]
Examples of the aliphatic dicarboxylic acid include stearic acid (C18),
eicosanoic acid (C20), behenic acid (C22), montanic acid (C28) and
triacontanoic acid
(C30). Examples of the diamine include ethylenediamine, butylenediamine,
hexanediamine, xylylenediamine and bis(aminomethyl)cyclohexane. The diamide
compound that is obtained by combining these compounds is preferred.
A diamide compound obtained with an aliphatic dicarboxylic acid having from 8
to 30 carbon atoms and a diamine mainly containing ethylene diamine, and a
diamine
compound obtained with an aliphatic dicarboxylic acid mainly containing
montanic acid
and a diamine having from 2 to 10 carbon atoms are preferred, and a diamine
compound
23
CA 02785673 2012-06-26
obtained with an aliphatic dicarboxylic acid mainly containing stearic acid
and a diamine
mainly containing ethylenediamine is particularly preferred.
[0066]
Preferred examples of the diester compound include a diester compound
obtained with an aliphatic dicarboxylic acid having from 8 to 30 carbon atoms
and a diol
having from 2 to 10 carbon atoms. The whitening prevention effect is expected
with an
aliphatic dicarboxylic acid having 8 or more carbon atoms and a diamine having
2 or
more carbon atoms. The diester compound may be favorably dispersed uniformly
in the
resin composition with an aliphatic dicarboxylic acid having 30 or less carbon
atoms and
a diol having 10 or less carbon atoms. The aliphatic dicarboxylic acid may
have a side
chain and a double bond, but a linear saturated aliphatic dicarboxylic acid is
preferred.
The diester compound may be used solely with one kind or as a combination of
two or
more kinds thereof.
[0067]
Examples of the aliphatic dicarboxylic acid include stearic acid (C18),
eicosanoic acid (C20), behenic acid (C22), montanic acid (C28) and
triacontanoic acid
(C30). Examples of the diol component of the diester compound used in the
present
invention include ethylene glycol, propanediol, butanediol, hexanediol,
vlylene glycol
and cyclohexanedimethanol. The diester compound that is obtained by combining
these
compounds is preferred.
A diester compound obtained with an aliphatic dicarboxylic acid mainly
containing montanic acid and a diol mainly containing ethylene glycol and/or
1,3-
butanediol is particularly preferred.
[0068]
The amount of the diamide compound and/or the diester compound added may
be from 0.005 to 0.5 parts by mass, preferably from 0.05 to 0.5 parts by mass,
and
particularly preferably from 0.12 to 0.5 parts by mass, per 100 parts by mass
of the
polyamide compound. When these compounds are added in an amount of 0.005 parts
by mass or more per 100 parts by mass of the polyamide compound, and a
crystallization
nucleating agent is used in combination, a synergistic effect of whitening
prevention may
be expected. When the amount is 0.5 parts by mass or less per 100 parts by
mass of the
polyamide compound, a molded article obtained by molding the polyamide
compound of
the present invention may have a clouding point maintained at a low level.
[0069]
3-2. Crystallization Nucleating Agent
24
CA 02785673 2012-06-26
To the polyamide composition containing the polyamide compound of the
present invention, a crystallization nucleating agent is preferably added from
the
standpoint of improvement of transparency. The crystallization nucleating
agent is
effective not only to improvement of transparency, but also to whitening
caused by
crystallization after subjecting to a hydrothermal treatment or after elapse
of a prolonged
period of time, and the addition of the crystallization nucleating agent to
the polyamide
compound may suppress the spherulite size to 1/2 or less of the wavelength of
visible
light. The combination use of the diamide compound and/or the diester compound
and
the crystallization nucleating agent provides, owing to the synergistic effect
thereof, such
whitening prevention that is far greater than that expected from the whitening
prevention
effects obtained from each of them.
[0070]
Examples of the crystallization nucleating agent of inorganic series include
ones
that are ordinarily used in a thermoplastic resins, for example, a glass
filler (such as glass
fibers, pulverized glass fibers (milled fibers), glass flakes and glass
beads), a calcium
silicate filler (such as wollastonite), mica, talc (such as powder talc and
granular talc
containing rosin as a binder), kaolin, potassium titanate whiskers, boron
nitride, clay,
such as a layered silicate, nanofillers, and carbon fibers, and two or more
kinds thereof
may be used in combination. The inorganic crystallization nucleating agent
preferably
has a maximum diameter of from 0.01 to 5 pm. In particular, powder talc having
a
particle diameter of 3.0 m or less is preferred, powder talc having a
particle diameter of
approximately from 1.5 to 3.0 pm is more preferred, and powder talc having a
particle
diameter of 2.0 pm or less is particularly preferred. Granular talc obtained
by adding
rosin as a binder to the powder talc is particularly preferred since it is
dispersed in good
condition in the polyamide composition. Preferred examples of the
crystallization
nucleating agent of organic series include capsules with a two-molecule
membrane in
microlevel size to nanolevel size containing the crystallization nucleating
agent, a
bis(benzylidene) sorbitol transparent crystallization nucleating agent, a
phosphorus
transparent crystallization nucleating agent, and a gelation agent, such as a
rosin amide
gelation agent, and a bis(benzylidene) sorbitol crystallization nucleating
agent is
particularly preferred.
[0071]
The amount of the crystallization nucleating agent added is preferably from
0.005 to 2.0 parts by mass, and particularly preferably from 0.01 to 1.5 parts
by mass, per
100 parts by mass of the polyamide compound. By adding at least one
crystallization
CA 02785673 2012-06-26
nucleating agent is added to the polyamide compound in combination with the
diamide
compound and/or the diester compound, a synergistic effect of whitening
prevention may
be obtained. In particular, the inorganic crystallization nucleating agent,
such as talc, is
preferably added in an amount of from 0.05 to 1.5 parts by mass per 100 parts
by mass of
the polyamide compound, and the organic crystallization nucleating agent, such
as a
bis(benzylidene) sorbitol crystallization nucleating agent, is preferably
added in an
amount of from 0.01 to 0.5 parts by mass per 100 parts by mass of the
polyamide
compound.
[0072]
The bis(benzylidene) sorbitol crystallization nucleating agent is one selected
from bis(benzylidene) sorbitol and a bis(alkylbenzylidene) sorbitol, which is
a
condensation product formed through acetalization reaction between sorbitol
and
benzaldehyde or an alkyl-substituted benzaldehyde (i.e., a diacetal compound),
and can
be favorably prepared according to various synthesis methods known in this
field of art.
The alkyl herein may be linear or cyclic and may be saturated or unsaturated.
According to the general synthesis method, reaction between 1 mol of D-
sorbitol and
approximately 2 mol of aldehyde in the presence of an acid catalyst is
employed. The
reaction temperature varies widely depending on the characteristics (such as
the melting
point) of the aldehyde used as the starting material of the reaction. The
reaction
medium may be an aqueous medium or a non-aqueous medium. One preferred example
of the method for preparing the diacetal is disclosed in U.S. Patent No.
3,721,682. The
disclosure therein is limited to a benzylidene sorbitol, but a
bis(alkylbenzylidene) sorbitol
used in the present invention may also be favorably produced by the method
disclosed
therein.
[0073]
Specific examples of the bis(benzylidene) sorbitol crystallization nucleating
agent (i.e., the diacetal compound) include bis(p-methylbenzylidene) sorbitol,
bis(p-
ethylbenzylidene) sorbitol, bis(n-propylbenzylidene) sorbitol, bis(p-
isopropylbenzylidene) sorbitol, bis(p-isobutylbenzylidene) sorbitol, bis(2,4-
dimethylbenzylidene) sorbitol, bis(3,4-dimethylbenzylidene) sorbitol,
bis(2,4,5-
trimethylbenzylidene) sorbitol, bis(2,4,6-trimethylbenzylidene) sorbitol and
bis(4-
biphenylbenzylidene) sorbitol.
[0074]
Examples of the alkyl-substituted benzaldehyde that is preferred for preparing
the bis(benzylidene) sorbitol crystallization nucleating agent include p-
26
CA 02785673 2012-06-26
methylbenzaldehyde, n-propylbenzaldehyde, p-isopropylbenzaldehyde, 2,4-
dimethylbenzaldehyde, 3,4-dimethylbenzaldehyde, 2,4,5-trimethylbenzaldehyde,
2,4,6-
trimethylbenzaldehyde and 4-biphenylbenzaldehyde.
[0075]
By adding a crystallization nucleating agent, such as talc, mica and clay, to
the
polyamide compound, the crystallization rate is accelerated by twice or more
as
compared to the polyamide compound having no crystallization nucleating agent
added.
There may be no problem in the purpose of injection molding requiring a high
molding
cycle, but when the crystallization rate is too large in producing a stretched
film or a deep
drawn cup molded from a sheet, the film or sheet may not be stretched due to
crystallization, and the moldability is considerably deteriorated due to
breakage and
uneven elongation. However, the bis(benzylidene) sorbitol crystallization
nucleating
agent does not accelerate the crystallization rate even upon adding to the
polyamide
compound, and thus is preferred upon using as a stretched film or a deep drawn
cup
molded from a sheet.
[0076]
It has also been found that the bis(benzylidene) sorbitol crystallization
nucleating agent not only prevents whitening, but also enhances the oxygen
barrier
property upon adding to the polyamide compound. The use of the
bis(benzylidene)
sorbitol (A) crystallization nucleating agent is particularly preferred since
both whitening
prevention and enhancement of the oxygen barrier property are obtained.
[0077]
The polyamide composition containing the polyamide compound of the present
invention that contains a layered silicate may be used as a gas barrier layer,
and can
enhance not only the oxygen barrier property of the molded article, but also
the barrier
property to other gases, such as carbon dioxide gas, thereof
[0078]
The layered silicate is a di-octahedral or tri-octahedral layered silicate
having a
charge density of from 0.25 to 0.6, examples of the di-octahedral one include
montmorillonite and beidellite, and examples of the tri-octahedral one include
hectorite
and saponite. Among these, montmorillonite is preferred.
[0079]
The layered silicate is preferably made in contact with an organic swelling
agent,
such as a polymer compound and an organic compound, in advance, thereby
expanding
the layers of the layered silicate. Preferred examples of the organic swelling
agent
27
CA 02785673 2012-06-26
include a tertiary ammonium salt, and a tertiary ammonium salt haying at least
one alkyl
or alkenyl group having 12 or more carbon atoms is preferably used.
[0080]
Specific examples of the organic swelling agent include a trimethyl alkyl
ammonium salt, such as a trimethyl dodecyl ammonium salt, a trimethyl
tetradecyl
ammonium salt, a trimethyl hexadecyl ammonium salt, a trimethyl octadecyl
ammonium
salt and a trimethyl eicosyl ammonium salt; a trimethyl alkenyl ammonium salt,
such as a
trimethyl octadecenyl ammonium salt and a trimethyl octadecadienyl ammonium
salt; a
triethyl alkyl ammonium salt, such as a triethyl dodecyl ammonium salt, a
triethyl
tetradecyl ammonium salt, a triethyl hexadecyl ammonium salt and a triethyl
octadecyl
ammonium salt; a tributyl alkyl ammonium salt, such as a tributyl dodecyl
ammonium
salt, a tributyl tetradecyl ammonium salt, a tributyl hexadecyl ammonium salt
and a
tributyl octadecyl ammonium salt; a dimethyl dialkyl ammonium salt, such as a
dimethyl
didodecyl ammonium salt, a dimethyl ditetradecyl ammonium salt, a dimethyl
dihexadecyl ammonium salt, a dimethyl dioctadecyl ammonium salt and a dimethyl
ditallow ammonium salt; a dimethyl dialkenyl ammonium salt, such as a dimethyl
dioctadecenyl ammonium salt and a dimethyl dioctadecadienyl ammonium salt; a
diethyl
dialkyl ammonium salt, such as a diethyl didodecyl ammonium salt, a diethyl
ditetradecyl
ammonium salt, a diethyl dihexadecyl ammonium salt and a diethyl dioctadecyl
ammonium salt; a dibutyl dialkyl ammonium salt, such as a dibutyl didodecyl
ammonium
salt, a dibutyl ditetradecyl ammonium salt, a dibutyl dihexadecyl ammonium
salt and a
dibutyl dioctadecyl ammonium salt; a methyl benzyl dialkyl ammonium salt, such
as a
methyl benzyl dihexadecyl ammonium salt; a dibenzyl dialkyl ammonium salt,
such as
dibenzyl dihexadecyl ammonium salt; a trialkyl methyl ammonium salt, such as a
tridecyl
methyl ammonium salt, a tritetradecyl methyl ammonium salt and a trioctadecyl
methyl
ammonium salt; a trialkyl ethyl ammonium salt, such as a tridodecyl ethyl
ammonium
salt; a trialkyl butyl ammonium salt, such as a tridodecyl butyl ammonium
salt; and an co-
amino acid, such as 4-amino-n-butyric acid, 6-amino-n-caproic acid, 8-
aminocaprylic
acid, 10-aminodecanoic acid, 12-aminododecanoic acid, 14-aminotetradecanoic
acid, 16-
aminohexadecanoic acid and 18-aminooctadecanoic acid. An ammonium salt
containing a hydroxyl group and/or an ether group may also be used as the
organic
swelling agent, and particularly a tertiary ammonium salt containing at least
one alkylene
glycol residual group, such as a methyl dialkyl (PAG) ammonium salt, an ethyl
dialkyl
(PAG) ammonium salt, a butyl dialkyl (PAG) ammonium salt, a dimethyl bis(PAG)
ammonium salt, a diethyl bis(PAG) ammonium salt, a dibutyl bis(PAG) ammonium
salt,
28
CA 02785673 2012-06-26
a methyl alkyl bis(PAG) ammonium salt, an ethyl alkyl bis(PAG) ammonium salt,
a butyl
alkyl bis(PAG) ammonium salt, a methyl tri(PAG) ammonium salt, an ethyl
tri(PAG)
ammonium salt, a butyl tri(PAG) ammonium salt and a tetra(PAG) ammonium salt
(wherein the alkyl means an alkyl group having 12 or more carbon atoms, such
as
dodecyl, tetradecyl, hexadecyl, octadecyl and eicosyl, and PAG means a
polyalkylene
glycol residual group, and preferably a polyethylene glycol residual group or
a
polypropylene glycol residual group each having 20 or less carbon atoms), may
also be
used as the organic swelling agent. Among these, a trimethyl dodecyl ammonium
salt, a
trimethyl tetradecyl ammonium salt, a trimethyl hexadecyl ammonium salt, a
trimethyl
octadecyl ammonium salt, a dimethyl didodecyl ammonium salt, a dimethyl
ditetradecyl
ammonium salt, a dimethyl dihexadecyl ammonium salt, a dimethyl dioctadecyl
ammonium salt and a dimethyl ditallow ammonium salt are preferred. The organic
swelling agent may be used solely or as a mixture of plural kinds thereof
[0081]
In the present invention, the polyamide compound containing from 0.5 to 8
parts
by mass of the layered silicate having been treated with the organic swelling
agent added
per 100 parts by mass of the polyamide compound is preferably used, and the
amount of
the layered silicate is more preferably from 1 to 6 parts by mass, and more
preferably
from 2 to 5 parts by mass. When the amount of the layered silicate added is
less than
0.5 parts by mass, the improvement in gas barrier property may be unfavorably
small.
When the amount thereof exceeds 8 parts by mass, the gas barrier layer may be
turbid,
thereby impairing the transparency of the container unfavorably.
[0082]
In the polyamide composition, the layered silicate is preferably dispersed
uniformly without local aggregation. The uniform dispersion referred herein
means that
the layered silicate is separated into flat plates in the polyamide, and 50%
or more of the
flat plates have an interlayer distance of 5 nm or more. The interlayer
distance referred
herein means the distance between barycenters of the flat plates. When the
distance is
larger, a better dispersed state is obtained, which results in improvement of
the
appearance, such as the transparency, and enhancement of the gas barrier
property to
oxygen, carbon dioxide and the like.
[0083]
3-3. Gelation Preventing and Fish Eye Preventing Agent
In the polyamide composition containing the polyamide compound of the
present invention, at least one kind of a carboxylate salt compound selected
from sodium
29
CA 02785673 2012-06-26
acetate, calcium acetate, magnesium acetate, calcium stearate, magnesium
stearate,
sodium stearate, and derivatives thereof is preferably added to the polyamide
compound.
Examples of the derivatives herein include a metal 12-hydroxystearate salt,
such as
calcium 12-hydroxystearate, magnesium 12-hydroxystearate and sodium 12-
hydroxystearate. The addition of the carboxylate salt compound prevents
gelation of the
polyamide compound, which occur during the molding process, and suppresses
fish eyes
in a molded article thereby enhancing the moldability.
[0084]
The amount of the carboxylate salt compound added is preferably from 400 to
10,000 ppm, more preferably from 800 to 5,000 ppm, and further preferably from
1,000
to 3,000 ppm, in terms of concentration in the polyamide composition. When the
amount thereof is 400 ppm or more, the polyamide compound is suppressed from
suffering thermal degradation, thereby preventing gelation. When the amount is
10,000
ppm or less, the polyamide composition may not cause molding failure and may
not
suffer coloration or whitening. When the carboxylate salt compound, which is a
basic
substance, is present in the molten polyamide compound, it is expected that
the
degradation of the polyamide compound caused by heat is delayed, and thereby
formation of gel, which is a final denaturation product, is suppressed.
The carboxylate salt compounds mentioned above are excellent in handleability,
and among these, a metal stearate salt is preferred since it is inexpensive,
has a function
of a lubricant, and stabilizes the molding process. The form of the
carboxylate salt
compound is not particularly limited. The compound in the form of powder with
a
smaller particle diameter is preferred for dry mixing since it can be
uniformly dispersed
in the polyamide composition, and the particle diameter thereof is preferably
0.2 mm or
less.
[0085]
3-4. Antioxidant
The polyamide composition containing the polyamide compound of the present
invention preferably contains an antioxidant from the standpoint of
controlling the
oxygen absorbing capability and suppressing deterioration of the mechanical
properties.
Examples of the antioxidant include a copper antioxidant, a hindered phenol
antioxidant,
a hindered amine antioxidant, a phosphorus antioxidant and a thio antioxidant,
and
among these, a hindered phenol antioxidant and a phosphorus antioxidant are
preferred.
[0086]
Specific examples of the hindered phenol antioxidant include triethylene
glycol
CA 02785673 2012-06-26
bis[3-(3-t-buty1-5-methy1-4-hydroxyphenyl) propionate, 4,4'-butylidene bis(3-
methy1-6-t-
butylphenol), 1,6-hexanediol bis[3-(3,5-di-t-butyl-4-hydroxyphenyl)
propionate, 2,4-
bis(n-octylthio)-6-(4-hydroxy-3,5-di-t-butylanilino)-1,3,5-triazine,
pentaerythrityl
tetralcis[3-(3,5-di-t-buty1-4-hydroxyphenyl) propionate], 2,2-thiodiethylene
bis[3-(3,5-di-
t-butyl-4-hydroxyphenyl) propionate], octadecyl 3-(3,5-di-t-buty1-4-
hydroxyphenyl)
propionate, 2,2-thiobis(4-methyl-6-1-butylphenol), N,N'-hexamethylene bis(3,5-
di-t-
buty1-4-hydroxy-hydroxycinnamide), 3,5-di-t-buty1-4-hydroxybenzylphosphonate
diethyl
ester, 1,3,5-trimethy1-2,4,6-tris(3,5-di-buty1-4-hydroxybenzypbenzene, ethyl
calcium
bis(3,5-di-t-buty1-4-hydroxybenzylsulfonate, tris(3,5-di-t-buty1-4-
hydroxybenzyl)
isocyanurate, 2,6-di-t-butyl-p-cresol, butylated hydroxyanisole, 2,6-di-t-
buty1-4-
ethylphenol, stearyl 13-(3,5-di-t-buty1-4-hydroxyphenyl) propionate, 2,2'-
methylene bis(4-
methy1-6-t-butylphenol), 2,2'-methylene bis(4-ethyl-6-t-butylphenol), 4,4'-
thiobis(3-
methy1-6-t-butylphenol), octylated diphenylamine, 2,4-bis[(octylthio)methy1]-0-
cresol,
isoocty1-3-(3,5-di-t-buty1-4-hydroxyphenyl) propionate, 4,4'-butylidene bis(3-
methy1-6-t-
butylphenol, 3,9-bis[1,1-dimethy1-2413-(3-t-buty1-4-hydroxy-
5-methylphenyl)propionyloxy]ethyl]-2,4,8,10-tetraoxaspiro[5.5]undecane, 1,1,3-
tris(2-
methy1-4-hydroxy-5-t-butylphenyl)butane, 1,3,5-trimethy1-2,4,6-tris(3,5-di-t-
buty1-4-
hydroxybenzyl)benzene, bis[3,3'-bis(4'-hydroxy-3'-t-butylphenyl)butyric acid]
glycol
ester, 1,3,5-tris(3',5'-di-t-buty1-4'-hydroxybenzy1)-sec-triazin-2,4,6-
(1H,3H,5H)trione
and d-cc-tocopherol. These compounds may be used solely or as a mixture
thereof
Specific examples of the commercially available product of the hindered phenol
compound include Irganox 1010 and Irganox 1098 (both trade names), produced by
BASF AG.
[0087]
Specific examples of the phosphorus antioxidant include organic phosphorus
compounds, such as triphenyl phosphite, trioctadecyl phosphite, tridecyl
phosphite,
trinonylphenyl phosphite, diphenylisodecyl phosphite, bis(2,6-di-tert-buty1-4-
methylphenyl) pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl)
pentaerythritol
diphosphite, tris(2,4-di-tert-butylphenyl) phosphite, distearyl
pentaerythritol diphosphite,
tetra(tridecy1-4,4'-isopropylidenediphenyl diphosphite and 2,2-methylene
bis(4,6-di-tert-
butylphenypoctyl phosphite. These compounds may be used solely or as a mixture
thereof
[0088]
The content of the antioxidant in the polyamide composition is not
particularly
limited in such a range that does not impair the capabilities of the
composition, and is
31
CA 02785673 2012-06-26
preferably from 0.001 to 3 parts by mass, and more preferably from 0.01 to 1
parts by
mass, per 100 parts by mass of the polyamide compound of the present
invention, from
the standpoint of controlling the oxygen absorbing capability and suppressing
deterioration of the mechanical properties.
[0089]
3-5. Impact Resistance Improving Agent
To the polyamide composition containing the polyamide compound of the
present invention, an impact resistance improving agent may be added for
improving the
impact resistance and the pinhole resistance and the flexibility of the film.
Examples of
the impact resistance improving agent added include polyolefin, a polyamide
elastomer, a
hydrogenated product of a styrene-butadiene copolymer resin, an ionomer, an
ethylene-
ethyl acrylate copolymer resin, a maleic anhydride-modified product of an
ethylene-ethyl
acrylate copolymer resin, an ethylene-methacrylic acid copolymer resin, nylon
6, 66, 12,
nylon 12, a nylon 12 elastomer, an ethylene-propylene copolymer elastomer and
a
polyester elastomer. The amount of the impact resistance improving agent added
is
preferably from 1 to 10% by mass, further preferably from 1 to 5% by mass, and
particularly preferably from 2 to 3% by mass. When the addition amount is too
large,
the transparency and the gas barrier property may be decreased. When the
addition
amount is too small, the impact resistance and the pinhole resistance and the
flexibility of
the film may not be largely improved.
[0090]
4. Purposes of Polyamide Compound
The polyamide compound of the present invention may be applied to any
purpose that requires an oxygen barrier property or an oxygen absorbing
capability. For
example, the polyamide compound of the present invention solely may be charged
in a
sachet or the like and used as an oxygen absorbent.
Representative examples of the applications of the polyamide compound of the
present invention include a molded article, such as a packaging material and a
packaging
container, but are not limited thereto. The polyamide compound of the present
invention may be used by processing into at least a part of a molded article.
For
example, the polyamide compound of the present invention may be used as at
least a part
of a packaging material in the form of film or sheet, and may be used as at
least a part of
a packaging container, such as a bottle, a tray, a cup, a tube, a flat bag and
various
pouches, e.g., a standing pouch. The layer formed of the polyamide compound or
the
polyamide composition of the present invention is not particularly limited,
and is
32
CA 02785673 2012-06-26
preferably 1 p.m or more.
[0091]
The production method of the molded article, such as a packaging material and
a
packaging container is not particularly limited, and an arbitrary method may
be employed.
For example, for molding a packaging material in the form of film or sheet and
a
packaging material in the form of tube, they may be produced by melting the
polyamide
compound or the polyamide composition by passing through a T-die, a circular
die or the
like, and extruding the molten material from an extruder attached to the
device. The
molded article in the form of film obtained by the aforementioned method may
be
stretched to prepare a stretched film. A packaging container in the form of
bottle may
be produced in such a manner that the molten polyamide compound or polyamide
material is injected from an injection molding machine into a metal mold to
produce a
preform, which is then subjected to blow stretching under heating to a
stretching
temperature.
A container in the form of tray, cup or the like may be produced by a method
of
injecting the molten polyamide compound or polyamide material from an
injection
molding machine into a metal mold, or a method of molding the packaging
material in
the form or sheet by such a molding method as vacuum molding or pneumatic
molding.
The production method of the packaging material and the packaging container is
not
limited to the aforementioned methods, but they may be produced by various
methods.
[0092]
The packaging material and the packaging container produced with the
polyamide compound of the present invention are suitable for packaging and
storing
various articles. For example, they may package and store various articles,
for example,
beverages, seasonings, grain crops, liquid and solid processed foods that are
necessarily
charged aseptically or sterilized by heating, chemical agents, liquid
household materials,
medical drugs, semiconductor integrated circuit devices, and electronic
devices.
EXAMPLES
[0093]
The present invention will be described in more detail with reference to
examples below, but the present invention is not limited to the examples.
In the following examples,
poly-m-xylylene adipamide is referred to as "N-MXD6",
poly-m-xylylene sebacamide is referred to as "N-MXD10",
33
CA 02785673 2012-06-26
poly-m-xylylene dodecanamide is referred to as "N-MXD12",
isophthalic acid-copolymerized poly-m-xylylene adipamide is referred to as "N-
NLICD6r,
isophthalic acid-copolymerized poly-m-xylylene sebacamide is referred to as
"N-MXD10r,
isophthalic acid-copolymerized poly-m-xylylene dodecanamide is referred to as
"N-MXD121",
terephthalic acid-copolymerized poly-m-xylylene adipamide is referred to as "N-
M>CD6T",
2,6-naphthalenedicarboxylic acid-copolymerized poly-m-xylylene adipamide is
referred to as "N-MXD6N",
s-caprolactam-copolymerized poly-m-xylylene adipamide is referred to as "N-
MKD6,6",
s-caprolactam-copolymerized poly-m-xylylene sebacamide is referred to as "N-
MXD10,6",
s-caprolactam-copolymerized poly-m-xylylene dodecanamide is referred to as
"N-MXD12,6", and
laurolactam-copolymerized poly-m-xylylene adipamide is referred to as "N-
MXD6,12".
[0094]
The polyamide compounds obtained in Examples and Comparative Examples
were measured for the component composition, the relative viscosity, the
number average
molecular weight, the glass transition temperature and the melting point in
the following
manners. The measurements of the oxygen absorbing rate, the oxygen absorbing
amount, the oxygen permeability coefficient and the haze, the sensory test,
and the tensile
test were performed in the following manners.
[0095]
(1) Component Composition
The component composition of the copolymer was quantitatively determined
with 11-1-NMR (400 MHz, JNM-AL400, a trade name, produced by JEOL, Ltd.,
measurement mode: NON(1H)). Specifically, a 5% by mass solution of the
polyamide
compound was prepared with formic acid-d as a solvent, and subjected to the 1H-
NMR
measurement.
[0096]
(2) Relative Viscosity
34
CA 02785673 2012-06-26
0.2 g of a pellet sample was precisely weighed and dissolved in 100 mL of 96%
sulfuric acid at 20 to 30 C under stirring. After completely dissolved, 5 mL
of the
solution was quickly placed in a Cannon-Fenske viscometer, which was then
allowed to
stand in a thermostat chamber at 25 C for 10 minutes, and then the fall time
(t) was
measured. The fall time (to) of 96% sulfuric acid was measured in the same
manner.
The relative viscosity was calculated from t and to according to the following
expression.
Relative viscosity = t/to
[0097]
(3) Number Average Molecular Weight (Mn)
The polyamide compound was precisely weighed and dissolved in a solution of
phenol/ethanol = 4/1 by volume at 20 to 30 C under stirring. After completely
dissolved, under stirring, the inner wall of the container was washed out with
5 mL of
methanol, and the solution was subjected to neutralization titration with a
0.01 mol/L
hydrochloric acid aqueous solution, thereby measuring the terminal amino group
concentration [NEI2].
The polyamide compound was precisely weighed and dissolved in benzyl
alcohol at 160 to 180 C under stirring under a nitrogen stream. After
completely
dissolved, the solution was cooled to 80 C or less under a nitrogen stream.
Under
stirring, the inner wall of the container was washed out with 10 mL of
methanol, and the
solution was subjected to neutralization titration with a 0.01 mol/L sodium
hydroxide
aqueous solution, thereby measuring the terminal carboxyl group concentration
[COOH].
The number average molecular weight was obtained from the terminal amino
group concentration [NH2] and the terminal carboxyl group concentration [COOH]
thus
obtained, according to the following expression.
Number average molecular weight = 2 / ([NH2] + [COOH])
wherein [NH2] represents the terminal amino group concentration (eq/g), and
[COOH]
represents the terminal carboxyl group concentration (eq/g).
[0098]
(4) Glass Transition Temperature and Melting Point
DSC measurement (differential scanning calorimeter measurement) was
performed with a differential scanning calorimeter (DSC-60, a trade name,
produced by
Shimadzu Corporation) at a temperature increasing rate of 10 C/min under a
nitrogen
CA 02785673 2012-06-26
stream, thereby measuring the glass transition temperature (Tg) and the
melting point
(Tm).
For reference, the melting points of N-NaD6, N-NaD10 and N-NaD12 that
were not added or copolymerized with another component were 237 C, 192 C and
187 C,
respectively. The melting point of N-M>CD6I that was not added or
copolymerized with
another component was 229 C.
[0099]
(5) Oxygen Absorbing Rate and Oxygen Absorbing Amount
2 g of a powder specimen, which was obtained by finely pulverizing pellets or
powder of the polyamide compound with a pulverizer, was packed with chartula,
and
charged in a bag formed of an aluminum laminated film sealed on three edges
thereof
having a dimension of 25 cm x 18 cm along with cotton impregnated with 10 mL
of water,
and the bag was sealed to make an air amount inside the bag of 400 mL. The
humidity
in the bag was 100%RH (relative humidity). After storing at 40 C for 28 days,
the
oxygen concentration inside the bag was measured with an oxygen concentration
meter
(LC-700F, a trade name, produced by Toray Engineering Co., Ltd.), and the
oxygen
absorbing amount (cc/g) was calculated from the oxygen concentration. A larger
value
thereof is preferred since it means an excellent oxygen absorbing capability.
In Examples 101 to 113 and 116 to 121 and Comparative Examples 101 to 107,
a test piece, which was obtained by cutting out from a film specimen having a
thickness
of approximately 100 Jim into 400 cm2, was placed in the bag, which was stored
at 40 C
for 28 days, as similar to the above operation. Thereafter, the oxygen
concentration
inside the bag was measured with an oxygen concentration meter (LC-700F, a
trade name,
produced by Toray Engineering Co., Ltd.). The oxygen absorbing amount per 1 m2
of
the film specimen was calculated from the oxygen concentration, and the oxygen
absorbing amount per one day was obtained in terms of oxygen absorbing rate
(cc/(m2-day)). A larger value thereof is preferred since it means an excellent
oxygen
absorbing capability.
[0100]
(6) Oxygen Permeability Coefficient
The oxygen permeability of a film having a thickness of 100 1..tm at 23 C and
60%RH by using an oxygen permeability measuring apparatus (Model OX-TRAN
2/21SH, produced by Mocon, Inc.) according to ASTM D3985, and was converted to
the
oxygen permeability coefficient (cc=mm/(m2-day=atm)). A smaller value thereof
is
preferred since it means a small oxygen permeation amount.
36
CA 02785673 2012-06-26
The oxygen permeability coefficients of N-IVDCD6, N-MXD10 and N-MXD12
that were not added or copolymerized with another component were 0.08
cc.mm/(m2.day.atm), 1.6 cc.mm/(m2.day.atm) and 2.9 cc-mm/(m2-day-atm),
respectively.
[0101]
(7) Sensory Test Evaluation
A test piece, which was obtained by cutting out from a film specimen into 400
CM2 , or 2 g of a powder specimen, which was obtained by finely pulverizing
pellets or
powder of the polyamide compound with a pulverizer, was packed with chartula,
and
charged in a bag formed of an aluminum laminated film sealed on three edges
thereof (an
odorless grade sealant was used for sealing the innermost layer) having a
dimension of 25
cm x 18 cm along with cotton impregnated with 10 mL of water, and the bag was
sealed
to make an air amount inside the bag of 400 mL. The humidity in the bag was
100%RH.
After storing at 40 C for 28 days, the air inside the bag was taken out with a
syringe and
evaluated for odor by ten subjects. As the control, a bag containing no film
specimen or
powder specimen was prepared. The sensory test evaluation was performed
according
to the following standard, and an average value of the ten subjects was
calculated. A
smaller value thereof is preferred since it means a less amount of odor.
Evaluation Standard
0: no odor perceived (control)
1: slight difference from control perceived
2: difference from control perceived
3: significant difference from control perceived
[0102]
(8) Tensile Test
A film specimen having a thickness of approximately 100 um was stored in a
thermostat chamber at 40 C and 100%RH for 28 days for absorbing oxygen and
then
subjected to humidity conditioning at 23 C and 50%RH for one week. The film
specimen was cut into a dimension of 10 mm in width and 100 mm in length and
subjected to a tensile test at a tensile rate of 50 mm/min with a tensile
tester (Strograph
V1-C, a trade name, produced by Toyo Seiki Seisalcu-sho, Ltd.) for measuring
the load at
breakage of the film, and the tensile breaking strength was obtained according
to the
following expression.
37
CA 02785673 2012-06-26
Tensile breaking strength (MPa) = (load on breakage (N)) / (cross sectional
area of film
specimen (mm2))
The tensile breaking strength obtained by the test was used as an index of
retention of the mechanical properties. For comparison, a film specimen that
was not
stored (not subjected to oxygen absorption) was subjected to humidity
conditioning at
23 C and 50%RH for one week, and then subjected to the same tensile test. A
larger
value thereof after oxygen absorption is preferred since it means less
deterioration of the
resin.
[0103]
(9) Yellowness Index (YI)
The yellowness index of pellets of the polyamide compound was measured with
a colormeter (Model ZE2000, produced by Nippon Denshoku Industries Co., Ltd.)
[0104]
(10) Haze
A film specimen having a thickness of approximately 100 um was measured
with a haze value measuring apparatus (Model COH-300A, produced by Nippon
Denshoku Industries Co., Ltd.) according to ES K7105, and the measured value
was
converted to a haze per 100 um. A smaller value thereof is preferred since it
means less
coloration.
[0105]
Example 101
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 13,000 g (88.96 mol) of precisely weighed
adipic acid
(produced by Asahi Kasei Chemicals Corporation), 880.56 g (9.88 mol) of DL-
alanine
(produced by Musashino Chemical Laboratory, Ltd.), 11.7 g (0.11 mol) of sodium
hypophosphite and 6.06 g (0.074 mol) of sodium acetate were placed, and after
sufficiently replacing with nitrogen, the system was heated to 170 C under a
small
amount of a nitrogen stream under stirring. 12,075.4 g (88.66 mol) of m-
vlylenediamine (produced by Mitsubishi Gas Chemical Co., Inc.) was added
dropwise
thereto under stirring, and the system was continuously heated while removing
condensation water formed. After completing the dropwise addition of m-
xylylenediamine, the reaction was continued at an inner temperature of 260 C
for 40
38
CA 02785673 2012-06-26
minutes. Thereafter, the system was pressurized with nitrogen, and the polymer
was
taken out from the strand die and pelletized, thereby providing approximately
23 kg of a
polyamide compound.
[0106]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound was charged in a tumble dryer with a jacket, equipped
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
150 C, the pressure inside the system was decreased to 1 ton or less. While
further
increasing the temperature, the temperature of the pellets was increased to
200 C over
approximately 70 minutes, and then maintained at 200 C for 30 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing DL-alanine-
copolymerized N-
MXD6 (polyamide compound 101).
[0107]
Fig. 1 shows a 111-NMit chart of the polyamide compound 101. In Fig. 1, the
absorption peak around 1.5 to 1.7 ppm is an absorption peak al derived from
hydrogen of
the methylene group that is not adjacent to the carbonyl group of adipic acid,
and an
absorption peak bl derived from hydrogen of the methyl group of DL-alanine,
and the
absorption peaks al and bl appear overlapping. Fig. 1 shows the integrated
intensity of
the absorption peak al and the absorption peak bl added. The absorption peak
around
2.5 ppm is an absorption peak cl derived from hydrogen of the methylene group
that is
adjacent to the carbonyl group of adipic acid. The absorption peak around 7.0
to 7.47
ppm is an absorption peak dl derived from hydrogen of the aromatic ring of m-
xylylenediamine (1\IXDA).
[0108]
The amount of the DL-alanine unit in the polyamide compound is calculated
from the integrated intensities of the peaks according to the following
expression.
39
CA 02785673 2012-06-26
Amount of DL-alanine unit in polyamide compound (% by mol)
(al+bl-c1)/3
[(al+bl-c1)/3]+(c1/4)+(d1/4) x 100
[0109]
According to the calculation, it was identified that the polyamide compound
101
contained the DL-alanine unit in an amount of approximately 5.3% by mol (5.8%
by mol
on calculation). Accordingly, it was identified that the polyamide compound
101 had a
ratio MXDA unit/adipic acid unit/DL-alanine unit = 47.3/47.4/5.3 (% by mol).
In Examples and Comparative Examples below, the identification of the
component compositions of the polyamide compounds thus produced was performed
in
the same manner.
[0110]
(Production of Non-stretched Film of Polyamide Compound)
Pellets of the polyamide compound 101 were formed into a film with a single
screw extruder having a diameter of 25 mm at an extrusion temperature of 260
C, a screw
rotation number of 60 rpm and a withdrawing rate of 1.2 m/min, thereby
producing a
non-stretched film having a width of 200 mm and a thickness of from 95 to 105
pm.
[0111]
Example 102
D-alanine-copolymerized N-MXD6 (polyamide compound 102) and a non-
stretched film thereof were produced in the same manner as in Example 101
except that
the a-amino acid was changed to D-alanine (produced by Musashino Chemical
Laboratory, Ltd.).
[0112]
Example 103
D-alanine-copolymerized N-MXD6 (polyamide compound 103) and a non-
stretched film thereof were produced in the same manner as in Example 101
except that
the a-amino acid was changed to L-alanine (produced by Sinogel Amino Acid Co.,
Ltd.).
[0113]
Example 104
DL-2-aminobutyric acid-copolymerized N-MXD6 (polyamide compound 104)
and a non-stretched film thereof were produced in the same manner as in
Example 101
except that the a-amino acid was changed to DL-2-aminobutyric acid (produced
by Japan
Finechem Co., Inc. purified product), and the addition amount thereof was
changed to
CA 02785673 2012-06-26
make a content thereof in the polyamide compound of 2.6% by mol.
[0114]
Example 105
DL-2-aminobutyric acid-copolymerized N-MXD6 (polyamide compound 105)
and a non-stretched film thereof were produced in the same manner as in
Example 104
except that the addition amount of DL-2-aminobutyric acid was changed to make
a
content thereof in the polyamide compound of 5.3% by mol.
[0115]
Example 106
DL-2-aminobutyric acid-copolymerized N-MXD6 (polyamide compound 106)
and a non-stretched film thereof were produced in the same manner as in
Example 104
except that the addition amount of DL-2-aminobutyric acid was changed to make
a
content thereof in the polyamide compound of 11.1% by mol.
[0116]
Example 107
DL-2-aminobutyric acid-copolymerized N-1VIXD6 (polyamide compound 107)
and a non-stretched film thereof were produced in the same manner as in
Example 104
except that the addition amount of DL-2-aminobutyric acid was changed to make
a
content thereof in the polyamide compound of 25.0% by mol.
[0117]
Example 108
DL-valine-copolymerized N-MXD6 (polyamide compound 108) and a non-
stretched film thereof were produced in the same manner as in Example 104
except that
the a-amino acid was changed to DL-valine (produced by Sinogel Amino Acid Co.,
Ltd.).
[0118]
Example 109
DL-leucine-copolymerized N-MXD6 (polyamide compound 109) and a non-
stretched film thereof were produced in the same manner as in Example 104
except that
the a-amino acid was changed to DL-leucine (produced by Ningbo Haishuo Bio-
technology Co., Ltd.).
[0119]
Example 110
DL-tert-leucine-copolymerized N-MXD6 (polyamide compound 110) and a non-
stretched film thereof were produced in the same manner as in Example 104
except that
the a-amino acid was changed to DL-tert-leucine (produced by Japan Finechem
Co., Inc.
41
CA 02785673 2012-06-26
purified product).
[0120]
Example 111
DL-phenylalanine-copolymerized N-IVIXD6 (polyamide compound 111) and a
non-stretched film thereof were produced in the same manner as in Example 104
except
that the a-amino acid was changed to DL-phenylalanine (produced by Sinogel
Amino
Acid Co., Ltd.).
[0121]
Example 112
DL-alanine-copolymerized N-MXD10 (polyamide compound 112) and a non-
stretched film thereof were produced in the same manner as in Example 101
except that
adipic acid was changed to sebacic acid (produced by Itoh Oil Chemicals Co.,
Ltd.), and
the addition amount of DL-alanine was changed to make a content thereof in the
polyamide compound of 2.6% by mol.
[0122]
Example 113
DL-alanine-copolymerized N-MXD12 (polyamide compound 113) and a non-
stretched film thereof were produced in the same manner as in Example 112
except that
adipic acid was changed to dodecanedioic acid (produced by Ube Industries,
Ltd.).
[0123]
Example 114
DL-alanine-copolymerized N-MXD6 (polyamide compound 114) was produced
in the same manner as in Example 101 except that the solid phase
polymerization was not
performed. The molecular weight thereof was not sufficiently increased on
polymerization, and thus the measurement of oxygen permeability coefficient
and haze
and the tensile test for a non-stretched film were not performed.
[0124]
Example 115
DL-alanine-copolymerized N-M>036 (polyamide compound 115) was produced
in the same manner as in Example 101 except that on the melt polymerization
the final
temperature after completing the dropwise addition of m-xylylenediamine was
changed
to 230 C, and the solid phase polymerization was not performed. The molecular
weight
thereof was not sufficiently increased on polymerization, and thus the
measurement of
oxygen permeability coefficient and haze and the tensile test for a non-
stretched film
were not performed.
42
CA 02785673 2012-06-26
[0125]
Example 116
DL-alanine-copolymerized N-11.DCD6 (polyamide compound 116) and a non-
stretched film thereof were produced in the same manner as in Example 101
except that
the retention time after increasing the temperature to 200 C in the solid
phase
polymerization was changed to 150 minutes.
[0126]
Example 117
DL-alanine-copolymerized N-MXD6 (polyamide compound 117) and a non-
stretched film thereof were produced in the same manner as in Example 101
except that
on the melt polymerization the final temperature after completing the dropwise
addition
of m-xylylenediamine was changed to 240 C, and the temperature on the solid
phase
polymerization was changed to 180 C.
[0127]
Example 118
(Melt Polymerization of Polyamide Compound by Pressurized Salt Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 13,000 g (88.96 mol) of precisely weighed
adipic acid
(produced by Asahi Kasei Chemicals Corporation), 880.56 g (9.88 mol) of DL-
alanine
(produced by Musashino Chemical Laboratory, Ltd.), 12,075.4 g (88.66 mol) of m-
xylylenediamine (produced by Mitsubishi Gas Chemical Co., Inc.), 10,000 g of
distilled
water, 11.7 g (0.11 mol) of sodium hypophosphite and 6.06 g (0.074 mol) of
sodium
acetate were placed, and after sufficiently replacing with nitrogen, a nylon
salt was
prepared under an increased pressure of 0.2 MPa. Thereafter, the temperature
of the
system was increased under stirring, and at the time when the inner pressure
reached 1.0
MPa, the pressure of 1.0 MPa was maintained, and the system was heated to 190
C, while
withdrawing water. After discharging water in an amount of 90% of the
theoretical
amount, the reaction was continued at an inner temperature of 230 C for 30
minutes.
Thereafter, the system was depressurized to 600 mmHg, and after confirming
sufficient
increase of the torque, the system was pressurized with nitrogen, and the
polymer was
taken out from the strand die and pelletized, thereby providing approximately
23 kg of a
polyamide compound.
[0128]
(Solid Phase Polymerization of Polyamide Compound)
43
CA 02785673 2012-06-26
The polyamide compound was charged in a tumble dryer with a jacket, equipped
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
150 C, the pressure inside the system was decreased to 1 ton or less. While
further
increasing the temperature, the temperature of the pellets was increased to
180 C over
approximately 60 minutes, and then maintained at 180 C for 60 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing DL-alanine-
copolymerized N-
MXD6 (polyamide compound 118).
[0129]
Example 119
DL-alanine-copolymerized N-MXD6 (polyamide compound 119) and a non-
stretched film thereof were produced in the same manner as in Example 118
except that
the addition amount of DL-alanine was changed to make a content thereof in the
polyamide compound of 11.1% by mol.
[0130]
Example 120
(Melt Polymerization of Polyamide Compound by Pressurized Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 13,000 g (88.96 mol) of precisely weighed
adipic acid
(produced by Asahi Kasei Chemicals Corporation), 880.56 g (9.88 mol) of DL-
alanine
(produced by Musashino Chemical Laboratory, Ltd.), 11.7 g (0.11 mol) of sodium
hypophosphite and 6.06 g (0.074 mol) of sodium acetate were placed, and after
sufficiently replacing with nitrogen, the system was melted by heating to 170
C under
increased pressure of 0.2 MPa under stirring. Thereafter, after adding
12,075.4 g (88.66
mol) of m-xylylenediamine (produced by Mitsubishi Gas Chemical Co., Inc.)
dropwise
thereto, the system was heated under stirring, and at a the time when the
inner pressure
reached 0.4 MPa, the pressure of 0.4 MPa was maintained, the system was heated
to
230 C, and the reaction was continued for 30 minutes while with drawing water.
Thereafter, the system was depressurized to 600 mmHg, and after confirming
sufficient
44
CA 02785673 2012-06-26
increase of the torque, the system was pressurized with nitrogen, and the
polymer was
taken out from the strand die and pelletized, thereby providing approximately
23 kg of a
polyamide compound.
[0131]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound was charged in a tumble dryer with a jacket, equipped
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
150 C, the pressure inside the system was decreased to 1 ton or less. While
further
increasing the temperature, the temperature of the pellets was increased to
180 C over
approximately 60 minutes, and then maintained at 180 C for 60 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing DL-alanine-
copolymerized N-
MXD6 (polyamide compound 120).
[0132]
Example 121
DL-alanine-copolymerized N-MXD6 (polyamide compound 121) and a non-
stretched film thereof were produced in the same manner as in Example 120
except that
the addition amount of DL-alanine was changed to make a content thereof in the
polyamide compound of 11.1% by mol.
[0133]
Comparative Example 101
Glycine-copolymerized N-MXD6 (polyamide compound 122) and a non-
stretched film thereof were produced in the same manner as in Example 104
except that
the a-amino acid was changed to glycine (produced by Tokyo Chemical Industry
Co.,
Ltd.) having secondary hydrogen on the a-position.
[0134]
Comparative Example 102
2-Aminoisobutylic acid-copolymerized N-MXD6 (polyamide compound 123)
and a non-stretched film thereof were produced in the same manner as in
Example 104
except that the a-amino acid was changed to 2-aminoisobutylic acid (2-amino-2-
CA 02785673 2012-06-26
methylpropanoic acid, produced by Japan Finechem Co., Inc., purified product)
having
no hydrogen on the a-position.
[0135]
Comparative Example 103
To N-1VDCD6 (S6007, a trade name, produced by Mitsubishi Gas Chemical Co.,
Inc.), which was polyamide formed of m-xylylenediamine and adipic acid, cobalt
stearate
was added to make a cobalt content in the resin composition of 400 ppm, and
dry-mixed.
The resulting mixture was formed into a film with a twin screw extruder having
a
diameter of 30 mm at an extrusion temperature of 260 C, a screw rotation
number of 60
rpm, a feed screw rotation number of 12 rpm and a withdrawing rate of 1.8
m/min,
thereby producing a non-stretched film having a width of 300 mm and a
thickness of
from 95 to 105 pm.
[0136]
Comparative Example 104
To 100 parts by mass of N-MXD6 (S6007, a trade name, produced by Mitsubishi
Gas Chemical Co., Inc.), which was polyamide formed of m-xylylenediamine and
adipic
acid, 5 parts by mass of maleic acid-modified polybutadiene (PB) (M-2000-20, a
trade
name, produced by Nippon Petrochemicals Co., Ltd.) and cobalt stearate in an
amount
that made a cobalt content in the resin composition of 400 ppm were added, and
dry-
mixed. The resulting mixture was formed into a film with a twin screw extruder
having
a diameter of 30 mm at an extrusion temperature of 260 C, a screw rotation
number of 60
rpm, a feed screw rotation number of 14 rpm and a withdrawing rate of 2.0
m/min,
thereby producing a non-stretched film having a width of 300 mm and a
thickness of
from 95 to 105 pm.
[0137]
Comparative Example 105
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 14,615 g (100 mol) of precisely weighed
adipic acid
(produced by Asahi Kasei Chemicals Corporation), 12.7 g (0.120 mol) of sodium
hypophosphite and 6.60 g (0.0805 mol) of sodium acetate were placed, and after
sufficiently replacing with nitrogen, the system was heated to 170 C under a
small
amount of a nitrogen stream under stirring. 13,539.2 g (99.4 mol) of m-
xylylenediamine (produced by Mitsubishi Gas Chemical Co., Inc.) was added
dropwise
46
CA 02785673 2012-06-26
thereto under stirring, and the system was continuously heated while removing
condensation water formed. After completing the dropwise addition of m-
xylylenediamine, the reaction was continued at an inner temperature of 260 C
for 40
minutes. Thereafter, the system was pressurized with nitrogen, and the polymer
was
taken out from the strand die and pelletized, thereby providing approximately
25 kg of a
polyamide compound.
[0138]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound was charged in a tumble dryer with a jacket, equipped
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
150 C, the pressure inside the system was decreased to 1 torr or less. While
further
increasing the temperature, the temperature of the pellets was increased to
200 C over
approximately 70 minutes, and then maintained at 200 C for 30 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing N-MXD6 (polyamide
compound 124).
[0139]
(Production of Non-stretched Film of Polyamide Compound)
To 100 parts by mass of the polyamide compound 124, 5 parts by mass of DL-
alanine (produced by Musashino Chemical Laboratory, Ltd.) was added and dry-
mixed.
The resulting mixture, which was prevented from being copolymerized, was
formed into
a film with a small-sized single screw extruder having a diameter of 15 mm at
an
extrusion temperature of 260 C, a screw rotation number of 30 rpm, a feed
screw rotation
number of 14 rpm and a withdrawing rate of 1.0 m/min, thereby producing a non-
stretched film having a width of 110 mm and a thickness of from 95 to 105 p.m.
[0140]
Comparative Example 106
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
47
CA 02785673 2012-06-26
introducing tube and a strand die, 17,000 g (84.1 mol) of precisely weighed
sebacic acid
(produced by Itoh Oil Chemicals Co., Ltd.), 13.1 g (0.124 mol) of sodium
hypophosphite
and 6.81 g (0.0830 mol) of sodium acetate were placed, and after sufficiently
replacing
with nitrogen, the system was heated to 170 C under a small amount of a
nitrogen stream
under stirring. 11,391.0 g (83.6 mol) of m-xylylenediamine (produced by
Mitsubishi
Gas Chemical Co., Inc.) was added dropwise thereto under stirring, and the
system was
continuously heated while removing condensation water formed. After completing
the
dropwise addition of m-xylylenediamine, the reaction was continued at an inner
temperature of 260 C for 40 minutes. Thereafter, the system was pressurized
with
nitrogen, and the polymer was taken out from the strand die and pelletized,
thereby
providing approximately 25 kg of a polyamide compound.
[0141]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound was charged in a tumble dryer with a jacket, equipped
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
150 C, the pressure inside the system was decreased to 1 ton or less. While
further
increasing the temperature, the temperature of the pellets was increased to
170 C over
approximately 15 minutes, and then maintained at 170 C for 240 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing N-MXD10 (polyamide
compound 125).
[0142]
(Production of Non-stretched Film of Polyamide Compound)
To 100 parts by mass of the polyamide compound 125, 5 parts by mass of DL-
alanine (produced by Musashino Chemical Laboratory, Ltd.) was added and dry-
mixed.
The resulting mixture, which was prevented from being copolymerized, was
formed into
a film with a small-sized single screw extruder having a diameter of 15 mm at
an
extrusion temperature of 240 C, a screw rotation number of 30 rpm, a feed
screw rotation
number of 14 rpm and a withdrawing rate of 1.0 m/min, thereby producing a non-
stretched film having a width of 110 mm and a thickness of from 95 to 105 m.
48
CA 02785673 2012-06-26
[0143]
Comparative Example 107
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 17,500 g (76.0 mol) of precisely weighed
dodecanedioic acid (produced by Ube Industries, Ltd.), 12.9 g (0.122 mol) of
sodium
hypophosphite and 6.73 g (0.0820 mol) of sodium acetate were placed, and after
sufficiently replacing with nitrogen, the system was heated to 170 C under a
small
amount of a nitrogen stream under stirring. 10,302.3 g (75.6 mol) of m-
xylylenediamine (produced by Mitsubishi Gas Chemical Co., Inc.) was added
dropwise
thereto under stirring, and the system was continuously heated while removing
condensation water formed. After completing the dropwise addition of m-
xylylenediamine, the reaction was continued at an inner temperature of 260 C
for 40
minutes. Thereafter, the system was pressurized with nitrogen, and the polymer
was
taken out from the strand die and pelletized, thereby providing approximately
24 kg of a
polyamide compound.
[0144]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound was charged in a tumble dryer with a jacket, equipped
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
150 C, the pressure inside the system was decreased to 1 ton or less. While
further
increasing the temperature, the temperature of the pellets was increased to
160 C over
approximately 10 minutes, and then maintained at 160 C for 420 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing N-MXD12 (polyamide
compound 126).
[0145]
(Production of Non-stretched Film of Polyamide Compound)
To 100 parts by mass of the polyamide compound 126, 5 parts by mass of DL-
49
CA 02785673 2012-06-26
alanine (produced by Musashino Chemical Laboratory, Ltd.) was added and dry-
mixed.
The resulting mixture, which was prevented from being copolymerized, was
formed into
a film with a small-sized single screw extruder having a diameter of 15 mm at
an
extrusion temperature of 230 C, a screw rotation number of 30 rpm, a feed
screw rotation
number of 12 rpm and a withdrawing rate of 1.0 m/min, thereby producing a non-
stretched film having a width of 110 mm and a thickness of from 95 to 105 pm.
A
[0146]
Table 1
Oxygen
Tensile breaking strength
Amino acid Oxygen absorbing Oxygen absorbing
rate
Relative Ta T
permeability Sensory test (MPa)
Polyamide content - m YI amount (cc/g) (cc/(m2-day))
Haze
viscosity ( C) ( C)
coefficient of evaluation
(% by mol) 40 C, 28 days 40 C, 28 days
Before oxygen After oxygen
film"
absorption absorption
_
DL-alanine-
Example 101 copolymerized 5.3 2.6 86 225 16
26 110 0.001 1 74 70 1.9
N-MXD6
D-alanine-
Example 102 copolymerized 5.3 2.6 86 225 16
25 109 0.001 1 75 70 2.0
N-MXD6 _
L-alanine-
Example 103 copolymerized 5.3 2.6 86 225 18
25 112 0.001 1 74 69 2.0
N-MXD6
DL-AABA '*1-
(-)
Example 104 copolymerized 2.6 2.6 86 231 13
14 45 0.005 1 80 75 2.2 0
N-MXD6
iv
.
-.1
DL .-AABA*1-
co
Example 105 copolymerized 5.3 2.6 85 224 16
26 70 0.001 1 76 70 2.4 in
0,
N-MXD6
-.1
u.)
DL-AABA*1-
iv
Example 106 copolymerized 11.1 2.2 84 207 18
30 82 0.001 1 74 64 2.6 0
H
N-MXD6
iv
DL-AABA .*1-
01
Example 107 copolymerized 25 2.0 82 N.D. 23 35
101 0.001 1 65 60 2.7 0,
i
N-MXD6
iv
DL-valine-
Example 108 copolymerized 2.6 2.6 86 231 13
14 94 0.001 1 75 70 2.0
N-MXD6
- .
DL-leucine-
Example 109 copolymerized 2.6 2.6 86 231 13
15 86 0.001 1 76 71 2.0
N-MXD6
DL-t-leucine- -
Example 110 copolymerized 2.6 2.6 86 231 13
15 68 0.001 1 77 72 1.9
N-MXD6
**: unit of oxygen permeability coefficient cc-mm/(m2.day=atrn)
*1: DL-AABA: DL-2-aminobutyric acid
N.D.: not detected
51
,
,
[0147]
Table 1 (continued)
Amino acid Oxygen
absorbing Oxygen absorbing rate Oxygen Tensile breaking strength
Relative Tg Tm
permeability Sensory test (MPa)
IPolyamide content Y
amount (cc/g) (cc/(m2.day)) Haze
viscosity ( C) ( C) coefficient
of evaluation Before oxygen After oxygen
(% by mol) 40 C, 28 days 40 C, 28 days
film"
absorption absorption
DL-Phe*2-
Example 111 copolymerized 2.6 2.6 86 231 19 12
90 0.001 1 79 73 2.0
N-MXD6 _
DL-alanine-
Example 112 copolymerized 2.6 2.6 61 186 13 13
34 0.2 1 37 32 2.0
N-MXD10
n
-
DL-alanine-
Example 113 copolymerized 2.6 2.6 57 184 11 10
26 0.7 1 30 25 2.0 0
iv
N-MXD12
DL-alanine- _
- co
co
Example 114 copolymerized 5.3 1.7 86 225 16
23 - 1 - - 0,
N-MXD6
-.1
u.)
DL-alanine-
iv
Example 115 copolymerized 5.3 1.3 86 225 9 23
- - 1 _ - - 0
N-MXD6
H
I\)-
i
DL-alanine-
Example 116 copolymerized 5.3 3.4 86 225 20 27
105 0.001 1 83 79 1.8 0
0,
1
N-MXD6
"_
DL-alanine-
0,
Example 117 copolymerized 5.3 2.5 86 225 8 25
110 0.001 1 88 77 1.9
N-MXD6 _
,
DL-alanine-
Example 118 copolymerized 5.3 2.6 86 225 6 27
97 0.001 1 75 69 2.0
N-MXD6
DL-alanine-
Example 119 copolymerized 11.1 2.3 85 207 9
27 118 0.001 1 77 68 1.7
N-MXD6
DL-alanine-
Example 120 copolymerized 5.3 2.6 86 225 3 28
98 0.001 1 74 67 1.8
N-MXD6
,
DL-alanine-
Example 121 copolymerized 11.1 2.3 86 207 8
28 120 0.001 1 76 68 1.8
N-MXD6
**: unit of oxygen permeability coefficient: ccmm/(m2.clay=atm)
*2: DL-Phe: DL-phenylalanine
52
,
[0148]
Table 1 (continued)
Oxygen
Tensile breaking strength
Amino acid Oxygen absorbing Oxygen absorbing
rate
Relative Ta T
permeability Sensory test (MPa)
Polyamide content . 0- m
YI amount (cc/g) (cc/(m2.day)) ¨ Haze
viscosity ( C) ( C) coefficient of
evaluation Before oxygen After oxygen
(% by mol) 40 C, 28 days 40 C, 28 days
film' absorption absorption
Comparative Glycine-
Example 101
copolymerized 2.6 2.6 86 231 11 1 5 0.08 1
82 80 1.8
N-MXD6
Al IP-
Comparative
Example 102 copolymerizedv 2' 6 2.6 86 231 12
0 0 0.08 1 79 76 2.0
N-MXD6
Comparative N-MXD6 0 2.6 87 237 -1 20 38 0.001 2
83 0 1.9
Example 103 (Co mixed)*4
N-MXD6
Comparative
(Co + PB 0 2.6 87 237 5 42 77
0.001 3 78 18 2.5
0
Example 104 mixed),
0
N-MXD6
iv
Comparative
-.1
(DL-alanine 0 2.6 87 237 24 1 0 0.09 1
83 83 1.9 co
Example 105 mixed)
.6
Ui
1:71
N-MXD10
-.1
CA
Comparative
(DL-alanine 0 2.6 51 192 21 0 0 1.6 1
39 39 2.0
Example 106 mixed),
iv
0
H
N-MXD10
iv
Comparative
i
(DL-alanine 0 2.6 54 187 20 0 0 2.9 1
32 32 1.9 0
Example 107 mixed),
0,
,
I\)
**: unit of oxygen permeability coefficient: cc-mm/(m2-day=atm)
0,
*3: AIB: 2-aminoisobutyric acid
*4: 400 ppm of Co mixed
*5: 400 ppm of Co and 5 parts by mass of maleic acid-modified polybutadiene
mixed
*6: 5 parts by mass of DL-alanine mixed
53
CA 02785673 2012-06-26
[0149]
When a polyamide compound formed of repeating units of a single lactam or an
a,o-aminocarboxylic acid monomer or a polyamide compound formed of repeating
units
of a diamine and a dicarboxylic acid is copolymerized with another monomer
component,
there are generally observed decrease of the melting point and change of the
glass
transition temperature. In Examples 101 to 121 and Comparative Examples 101
and
102, a single melting peak is observed, and the melting point is decreased.
Thus, it is
found that the a-amino acid is copolymerized. In Comparative Examples 103 to
107,
on the other hand, the melting point is not decreased, and thus it is found
that no other
component is copolymerized.
[0150]
The polyamide compound that is copolymerized with an a-amino acid having no
tertiary hydrogen is insufficient in oxygen absorbing capability (Comparative
Examples
101 and 102). The ordinary polyamide composition having a cobalt compound
mixed
therein exhibits an oxygen absorbing capability and a good oxygen permeability
coefficient, but has bluish color due to the addition of the cobalt compound
although it
has good transparency, and causes a large amount of odor especially in one
using
polybutadiene. Furthermore, the film after oxygen absorption is deteriorated
to fail to
maintain the shape thereof, and thus is not necessarily favorable in the
purpose of
packaging containers (Comparative Examples 103 and 104). The polyamide
composition that is only mixed with an a-amino acid having tertiary hydrogen,
but is not
copolymerized therewith exhibits no oxygen absorbing capability (Comparative
Examples 105 to 107).
On the other hand, the polyamide compound that is copolymerized with an a-
amino acid having tertiary hydrogen exhibits a sufficient oxygen absorbing
capability
without a metal contained, and generates no offensive odor (Examples 101 to
121). In
the case of the film specimen, in particular, the film has a good oxygen
permeability
coefficient, has good transparency, and maintains the mechanical properties
after oxygen
absorption (Examples 101 to 113 and 116 to 121).
It is found from the comparison among Examples 101 to 103 that the optical
isomers of the a-amino acid do not affect the oxygen absorbing capability. It
is found
from the comparison among Examples 104 to 107 that the increase of the
copolymerization ratio of the a-amino acid enhances the oxygen absorbing
capability.
Furthermore, it is found from the comparison among Examples 101 and 114 to 116
that
the relative viscosity does not affect the oxygen absorbing capability.
Moreover, it is
54
CA 02785673 2012-06-26
found that the YI can be decreased in Examples 115 and 117, in which the melt
polymerization temperature is decreased as compared to Examples 101 to 114. It
is
found that the YI can be decreased also in Examples 118 and 119 where the poly-
amide
compound is polymerized by a pressurized salt method and in Examples 120 and
121
where the polyamide compound is polymerized by a pressurized dropping method.
[0151]
Examples 122 to 131
(Biaxially Stretched Film)
Non-stretched films having a thickness of approximately 250 m were obtained
in the same manner as in Example 101 by using pellets of the polyamide
compounds 101,
104 to 107, and 117 to 121 obtained in Examples 101, 104 to 107, and 117 to
121. The
films were each stretched in MD 4 times and in TD 4 times at a stretching
temperature of
130 C with a biaxial stretching machine (tenter method), produced by Toyo
Seiki
Seisaku-sho, Ltd., and heat-set at 200 C for 30 seconds, thereby providing a
biaxially
stretched film having a thickness of approximately 15 pm. The number of times
of
breakage on continuous production of 20 sheets of the biaxially stretched film
was
designated as an index of moldability. The biaxially stretched film was also
measured
for the initial oxygen permeability coefficient, the period of time where the
initial oxygen
permeability coefficient was completely maintained, and the haze of the film.
The
results are shown in Table 2.
CA 02785673 2012-06-26
[0152]
Table 2
II Number of time of Initial oxygen I Period of I
Content of yrneizabon I
breakage on
method permeability maintaining
initial
Polyamide amino acid (polymerization production 20
coefficient of oxygen permeability
temperature) sheets of b Haze
iaxiallyfllm coefficient (day)
stretched films
DL-alanine- atmospheric
Example 122 copolymerized 5.3 dropping method 5 0.05
26 1.5
N-MXD6 (260 C)
atmospheric
Example 123 copolymerized 2.6 dropping method 3 0.08
15 1.7
N-MXD6 (260 C)
A1131- atmospheric
Example 124 copolymerized 5.3 dropping method 4 0.05
24 1.8
N-MXD6 (260 C)
atmospheric
Example 125 copolymerized 11.1 dropping method 4 0.05
56 1.4
N-MXD6 (260 C)
atmospheric
Example 126 copolymerized 25 dropping method 6 0.03
67 1.4
N-MXD6 (260 C)
DL-alanine- atmospheric
Example 127 copolymerized 5.3 dropping method 3 0.05
26 1.5
N-MXD6 (230 C)
DL-alanine- pressurized salt
Example 128 copolymerized 5.3 method 1 0.05 26
1.4
N-MXD6 (230 C)
DL-alanine- pressurized salt
Example 129 copolymerized 11.1 method 1 0.05 54
1.3
N-MXD6 (230 C)
DL-alanine- . pressurized
Example 130 copolymerized 5.3 dropping method 0 0.05
26 1.3
N-MXD6 (230 C)
DL-alanine- pressurized
Example 131 copolymerized 11.1 dropping method 1 0.05
56 1.4
N-MXD6 (230 C)
**: unit of oxygen permeability coefficient cc-rnm/(m2.day.atm)
*3: AIB: 2-aminoisobutyric acid
[0153]
It is found from Table 2 that in Examples 128 and 129 where polymerization is
performed by a pressurized salt method and Examples 130 and 131 where
polymerization
is performed by a pressurized dropping method, fish eyes and gelation, which
may cause
breakage of films upon biaxial stretching, are less formed, thereby providing
products
with stable properties.
[0154]
Example 201
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 13,000 g (88.9 mol) of precisely weighed
adipic acid
56
CA 02785673 2012-06-26
(produced by Asahi Kasei Chemicals Corporation), 777.8 g (4.7 mol) of high
purity
isophthalic acid (A.G. International Chemical Co., Inc.), 1,472.1 g (16.52
mol) of DL-
alanine (produced by Musashino Chemical Laboratory, Ltd.) as an a-amino acid,
12.57 g
(0.119 mol) of sodium hypophosphite and 6.52 g (0.0795 mol) of sodium acetate
were
placed, and after sufficiently replacing with nitrogen, the system was heated
to 170 C
under a small amount of a nitrogen stream under stirring. 12,710 g (93.3 mol)
of m-
xylylenediamine (MXDA, produced by Mitsubishi Gas Chemical Co., Inc.) was
added
dropwise thereto under stirring, and the system was continuously heated while
removing
condensation water formed. After completing the dropwise addition of m-
xylylenediamine, the reaction was continued at an inner temperature of 260 C
for 40
minutes. Thereafter, the system was pressurized with nitrogen, and the polymer
was
taken out from the strand die and pelletized, thereby providing approximately
24.3 kg of
a polyamide compound.
[0155]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound was charged in a tumble dryer with a jacket, equipped
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
150 C, the pressure inside the system was decreased to 1 ton or less. While
further
increasing the temperature, the temperature of the pellets was increased to
200 C over
approximately 70 minutes, and then maintained at 200 C for 30 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing DL-alanine-
copolymerized N-
MXD6I (polyamide compound 201).
[0156]
Fig. 2 shows a 11-I-NMR chart of the polyamide compound 201. In Fig. 2, the
absorption peak around 1.5 to 1.7 ppm is an absorption peak a2 derived from
hydrogen of
the methylene group that is not adjacent to the carbonyl group of adipic acid,
and an
absorption peak b2 derived from hydrogen of the methyl group of DL-alanine,
and the
absorption peaks al and b2 appear overlapping. Fig. 2 shows the integrated
intensity of
the absorption peak a2 and the absorption peak b2 added. The absorption peak
around
57
CA 02785673 2012-06-26
2.5 ppm is an absorption peak c2 derived from hydrogen of the methylene group
that is
adjacent to the carbonyl group of adipic acid. The absorption peak around 4.5
ppm is an
absorption peak d2 derived from hydrogen of the benzylmethylene group of m-
xylylenediamine that is adjacent to adipic acid, and the absorption peak
around 4.7 ppm
is an absorption peak e2 derived from hydrogen of the benzylmethylene group of
m-
xylylenediamine that is adjacent to isophthalic acid. Fig. 2 shows the
integrated
intensity of the absorption peak d2 and the absorption peak e2 added. The
absorption
peak around 7.4 ppm is an absorption peak fl derived from hydrogen of the
benzene
rings of m-xylylenediamine and isophthalic acid.
[0157]
The amount of the DL-alanine unit in the polyamide compound is calculated
from the integrated intensities of the peaks according to the following
expression.
Amount of DL-alanine unit in polyamide compound (% by mol)
(a2+b2-c2)/3
X 100
[(a2+b2-c2)/3]+(c2/4)+(f2/4)
The amount of the isophthalic acid unit in the polyamide compound is
calculated
according to the following expression.
Amount of isophthalic acid unit in polyamide compound (% by mol)
(d2+e2-c2)/4
X 100
[(a2+b2-c2)13]+(c2/4)+(f2/4)
[0158]
According to the calculation, it was identified that the polyamide compound
201
contained the DL-alanine unit in an amount of approximately 8.1% by mol (9.1%
by mol
on calculation) and the isophthalic acid unit in an amount of approximately
2.3% by mol
(2.0% by mol on calculation). Accordingly, it was identified that the
polyamide
compound 201 had a ratio MXDA unit/adipic acid unit/isophthalic acid unit/DL-
alanine
unit = 43.7/45.9/2.3/8.1 (% by mol).
In Examples and Comparative Examples below, the identification of the
component compositions of the polyamide compounds thus produced was performed
in
the same manner.
[0159]
(Production of Non-stretched Film of Polyamide Compound)
58
CA 02785673 2012-06-26
Pellets of the polyamide compound 201 were formed into a film with a single
screw extruder having a diameter of 25 mm at an extrusion temperature of 260
C, a screw
rotation number of 60 rpm and a withdrawing rate of 1.2 rrilmin, thereby
producing a
non-stretched film having a width of 200 mm and a thickness of from 95 to 105
Jim.
[0160]
Example 202
DL-2-aminobutyric acid-copolymerized N-MXD6I (polyamide compound 202,
MXDA unit/adipic acid unit/isophthalic acid unit/DL-aminobutyric acid unit =-
48.6/46.4/2.4/2.6 (% by mol)) and a non-stretched film thereof were produced
in the
same manner as in Example 201 except that the a-amino acid was changed to DL-2-
aminobutyric acid (DL-AABA, produced by Japan Finechem Co., Inc. purified
product),
and the addition amount thereof was changed to make a content thereof in the
polyamide
compound of 2.6% by mol.
[0161]
Example 203
DL-valine-copolymerized N-MXD6I (polyamide compound 203, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-valine unit = 48.6/46.4/2.4/2.6
(% by mol))
and a non-stretched film thereof were produced in the same manner as in
Example 202
except that the a-amino acid was changed to DL-valine (produced by Sinogel
Amino
Acid Co., Ltd.).
[0162]
Example 204
DL-leucine-copolymerized N-MXD6I (polyamide compound 204, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-leucine unit =
48.6/46.4/2.4/2.6 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
202 except that the a-amino acid was changed to DL-leucine (produced by Ningbo
Haishuo Bio-technology Co., Ltd.).
[0163]
Example 205
DL-phenylalanine-copolymerized N-MXD6I (polyamide compound 205,
MXDA unit/adipic acid unit/isophthalic acid unit/DL-phenylalanine unit =
48.6/46.4/2.4/2.6 (% by mol)) and a non-stretched film thereof were produced
in the
same manner as in Example 202 except that the a-amino acid was changed to DL-
phenylalanine (DL-Phe, produced by Sinogel Amino Acid Co., Ltd.).
[0164]
59
CA 02785673 2012-06-26
Example 206
DL-alanine-copolymerized N-MXD6T (polyamide compound 206, MXDA
unit/adipic acid unit/terephthalic acid unit/DL-alanine unit =
48.6/46.4/2.4/2.6 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
201 except that the addition amount of DL-alanine was changed to make a
content
thereof in the polyamide compound of 2.6% by mol, and high purity isophthalic
acid was
changed to high purity terephthalic acid (PTA, produced by Mitsubishi Gas
Chemical Co.,
Inc.).
[0165]
Example 207
DL-alanine-copolymerized N-MXD6N (polyamide compound 207, IVDCDA
unit/adipic acid unit/NDCA unit/DL-alanine unit = 48.6/46.4/2.4/2.6 (% by
mol)) and a
non-stretched film thereof were produced in the same manner as in Example 206
except
that high purity isophthalic acid was changed to 2,6-naphthalenedicarboxylic
acid
(NDCA, produced by Mitsubishi Gas Chemical Co., Inc.).
[0166]
Example 208
DL-alanine-copolymerized N-MXD6I (polyamide compound 208, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
48.6/46.4/2.4/2.6 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
201 except that the addition amount of DL-alanine was changed to make a
content
thereof in the polyamide compound of 2.6% by mol.
[0167]
Example 209
DL-alanine-copolymerized N-1VDM6I (polyamide compound 209, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
48.6/43.9/4.9/2.6 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
208 except that the addition amount of high purity isophthalic acid was
changed to make
a content thereof in the polyamide compound of 4.9% by mol.
[0168]
Example 210
DL-alanine-copolymerized N-MXD6I (polyamide compound 210, IVDCDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
48.6/41.5/7.3/2.6 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
208 except that the addition amount of high purity isophthalic acid was
changed to make
CA 02785673 2012-06-26
a content thereof in the polyamide compound of 7.3% by mol.
[0169]
Example 211
DL-alanine-copolymerized N-MXD6I (polyamide compound 211, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
48.6/34.2/14.6/2.6 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
208 except that the addition amount of high purity isophthalic acid was
changed to make
a content thereof in the polyamide compound of 14.6% by mol.
[0170]
Example 212
DL-alanine-copolymerized N-MXD6I (polyamide compound 212, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
45.9/41.4/4.6/8.1 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
201 except that the addition amount of high purity isophthalic acid was
changed to make
a content thereof in the polyamide compound of 4.6% by mol.
[0171]
Example 213
DL-alanine-copolymerized N-MXD6I (polyamide compound 213, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
45.9/39.1/6.9/8.1 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
201 except that the addition amount of high purity isophthalic acid was
changed to make
a content thereof in the polyamide compound of 6.9% by mol.
[0172]
Example 214
DL-alanine-copolymerized N-MXD6I (polyamide compound 214, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
45.9/32.2/13.8/8.1 (% by
mol)) was produced in the same manner as in Example 201 except that the
addition
amount of high purity isophthalic acid was changed to make a content thereof
in the
polyamide compound of 13.8% by mol. The molecular weight thereof was not
sufficiently increased on polymerization, and thus the measurement of oxygen
permeability coefficient and haze and the tensile test for a non-stretched
film were not
performed.
[0173]
Example 215
DL-alanine-copolymerized N-MXD6I (polyamide compound 215, MXDA
61
CA 02785673 2012-06-26
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
41.1/39.1/2.1/17.7 (% by
mol)) was produced in the same manner as in Example 201 except that the
addition
amount of DL-alanine was changed to make a content thereof in the polyamide
compound of 17.7% by mol. The molecular weight thereof was not sufficiently
increased on polymerization, and thus the measurement of oxygen permeability
coefficient and haze and the tensile test for a non-stretched film were not
performed.
[0174]
Example 216
DL-alanine-copolymerized N-MXD6I (polyamide compound 216, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
41.1/37.1/4.1/17.7 (% by
mol)) was produced in the same manner as in Example 215 except that the
addition
amount of high purity isophthalic acid was changed to make a content thereof
in the
polyamide compound of 4.1% by mol. The molecular weight thereof was not
sufficiently increased on polymerization, and thus the measurement of oxygen
permeability coefficient and haze and the tensile test for a non-stretched
film were not
performed.
[0175]
Example 217
DL-alanine-copolymerized N-MXD6I (polyamide compound 217, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
41.1/35.0/6.2/17.7 (% by
mol)) was produced in the same manner as in Example 215 except that the
addition
amount of high purity isophthalic acid was changed to make a content thereof
in the
polyamide compound of 6.2% by mol. The molecular weight thereof was not
sufficiently increased on polymerization, and thus the measurement of oxygen
permeability coefficient and haze and the tensile test for a non-stretched
film were not
performed.
[0176]
Example 218
DL-alanine-copolymerized N-M,CD10I (polyamide compound 218, lvDCDA
unit/sebacic acid unit/isophthalic acid unit/DL-alanine unit =
48.6/46.4/2.4/2.6 (% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
208 except that adipic acid was changed to sebacic acid (produced by Itoh Oil
Chemicals
Co., Ltd.).
[0177]
Example 219
62
CA 02785673 2012-06-26
DL-alanine-copolymerized N-MXD12I (polyamide compound 219, MXDA
unit/dodecanedioic acid unit/isophthalic acid unit/DL-alanine unit =
48.6/46.4/2.4/2.6 (%
by mol)) and a non-stretched film thereof were produced in the same manner as
in
Example 208 except that adipic acid was changed to dodecanedioic acid
(produced by
Ube Industries, Ltd.).
[0178]
Example 220
DL-alanine-copolymerized N-MXD6I (polyamide compound 220, MXDA
unit/adipic acid unit/isophthalic acid unit/DL-alanine unit =
34.1/18.9/15.4/31.6 (% by
mol)) was produced in the same manner as in Example 201 except that the
addition
amount of DL-alanine was changed to make a content thereof in the polyamide
compound of 31.6% by mol. The molecular weight thereof was not sufficiently
increased on polymerization, and thus the measurement of oxygen permeability
coefficient and haze, sensory test and the tensile test for a non-stretched
film were not
performed.
[0179]
Comparative Example 201
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 13,000 g (88.9 mol) of precisely weighed
adipic acid
(produced by Asahi Kasei Chemicals Corporation), 777.9 g (4.68 mol) of high
purity
isophthalic acid (produced by A.G International Chemical Co., Inc.), 11.96 g
(0.113 mol)
of sodium hypophosphite and 6.21 g (0.0756 mol) of sodium acetate were placed,
and
after sufficiently replacing with nitrogen, the system was heated to 170 C
under a small
amount of a nitrogen stream under stirring. 12,710 g (93.4 mol) of m-
xylylenediamine
(produced by Mitsubishi Gas Chemical Co., Inc.) was added dropwise thereto
under
stirring, and the system was continuously heated while removing condensation
water
formed. After completing the dropwise addition of m-xylylenediamine, the
reaction
was continued at an inner temperature of 260 C for 40 minutes. Thereafter, the
system
was pressurized with nitrogen, and the polymer was taken out from the strand
die and
pelletized, thereby providing approximately 23.2 kg of a polyamide compound.
[0180]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound was charged in a tumble dryer with a jacket, equipped
63
CA 02785673 2012-06-26
with a nitrogen introducing tube, a vacuum line, a vacuum pump and a
thermocouple for
measuring the inner temperature, and while rotating the tumble dryer at a
constant rate,
the inside of the tumble dryer was sufficiently replaced with nitrogen gas
having a purity
of 99% by volume or more, and the tumble dryer was heated under a stream of
the
nitrogen gas, thereby increasing the temperature of the pellets to 150 C over
approximately 150 minutes. At the time when the temperature of the pellets
reached
. 150 C, the pressure inside the system was decreased to 1 torr or less. While
further
increasing the temperature, the temperature of the pellets was increased to
200 C over
approximately 70 minutes, and then maintained at 200 C for 30 minutes.
Nitrogen gas
having a purity of 99% by volume or more was introduced into the system, and
the
tumble dryer was cooled while rotating, thereby providing N-MXD6I (polyamide
compound 221, MXDA unit/adipic acid unit/isophthalic acid unit = 49.9/47.6/2.5
(% by
mol)).
[0181]
(Production of Non-stretched Film of Polyamide Compound)
Pellets of the polyamide compound 221 were formed into a film with a single
screw extruder having a diameter of 25 mm at an extrusion temperature of 260
C, a screw
rotation number of 60 rpm and a withdrawing rate of 1.2 m/min, thereby
producing a
non-stretched film having a width of 200 mm and a thickness of from 95 to 105
um.
[0182]
Comparative Example 202
N-MXD10I (polyamide compound 222, MXDA unit/sebacic acid
unit/isophthalic acid unit = 49.9/47.6/2.5 (% by mol)) and a non-stretched
film thereof
were produced in the same manner as in Comparative Example 201 except that
adipic
acid was changed to sebacic acid (produced by Itoh Oil Chemicals Co., Ltd.).
[0183]
Comparative Example 203
N-MXD12I (polyamide compound 223, MXDA unit/dodecanedioic acid
unit/isophthalic acid unit = 49.9/47.6/2.5 (% by mol)) and a non-stretched
film thereof
were produced in the same manner as in Comparative Example 201 except that
adipic
acid was changed to dodecanedioic acid (produced by Ube Industries, Ltd.).
[0184]
Comparative Example 204
Glycine-copolymerized N-MXD6I (polyamide compound 224, MXDA
unit/adipic acid unit/isophthalic acid unit/glycine unit = 45.9/43.7/2.3/8.1
(% by mol))
64
CA 02785673 2012-06-26
and a non-stretched film thereof were produced in the same manner as in
Example 201
except that the a-amino acid was changed to glycine (produced by Tokyo
Chemical
Industry Co., Ltd. reagent) having secondary hydrogen on the a-position.
[0185]
Comparative Example 205
2-Aminoisobutylic acid-copolymerized N-MXD6I (polyamide compound 225,
MXDA unit/adipic acid unit/isophthalic acid unit/A113 unit = 45.9/43.7/2.3/8.1
(% by
mol)) and a non-stretched film thereof were produced in the same manner as in
Example
201 except that the a-amino acid was changed to 2-aminoisobutylic acid (2-
amino-2-
methylpropanoic acid, A113, produced by Japan Finechem Co., Inc., purified
product)
having no hydrogen on the a-position.
[0186]
Comparative Example 206
To the polyamide compound 221, cobalt stearate was added to make a cobalt
content in the resin composition of 400 ppm, and dry-mixed. The resulting
mixture was
formed into a film with a twin screw extruder having a diameter of 30 mm at an
extrusion
temperature of 260 C, a screw rotation number of 60 rpm, a feed screw rotation
number
of 12 rpm and a withdrawing rate of 1.8 m/min, thereby producing a non-
stretched film
having a width of 200 mm and a thickness of from 95 to 105 pm.
[0187]
Comparative Example 207
To 100 parts by mass of the polyamide compound 221, 5 parts by mass of maleic
acid-modified polybutadiene (PB) (M-2000-20, a trade name, produced by Nippon
Petrochemicals Co., Ltd.) and cobalt stearate in an amount that made a cobalt
content in
the resin composition of 400 ppm were added, and dry-mixed. The resulting
mixture
was formed into a film with a twin screw extruder having a diameter of 30 mm
at an
extrusion temperature of 260 C, a screw rotation number of 60 rpm, a feed
screw rotation
number of 14 rpm and a withdrawing rate of 2.0 rn/min, thereby producing a
non-stretched film having a width of 200 mm and a thickness of from 95 to 105
m.
[0188]
Comparative Example 208
To 100 parts by mass of the polyamide compound 221, DL-alanine (produced by
Musashino Chemical Laboratory, Ltd.) was added to make a content thereof in
the resin
composition of 5 parts by mass, and dry-mixed. The resulting mixture, which
was
prevented from being copolymerized, was formed into a film with a small-sized
single
CA 02785673 2012-06-26
screw extruder having a diameter of 15 mm at an extrusion temperature of 240
C, a screw
rotation number of 30 rpm, a feed screw rotation number of 14 rpm and a
withdrawing
rate of 1.0 nvirnin, thereby producing a non-stretched film having a width of
110 mm and
a thickness of from 95 to 105 pm.
66
[0189]
Table 3
Aromatic Oxygen
Tensile breaking strength
Amino acid Oxygen absorbing
dicarboxyfic acid Relative Tg Tm permeability
Sensory test (MPa)
Polyamide content amount (cc/g)
Haze
copolymerization viscosity ( C) ( C) Coefficient of
evaluation Before oxygen After oxygen
(% by mol) ratio (% by mol) 40 C, 28 days
film- absorption absorption
DL-alanine-
Example copolymerized 8.1 2.3 2.0 90 N.D. 18 0.001 1 79
68 1.9
201 N-MXD6I _
DL-AABA*1-
Example copolymerized 2.6 2.4 2.6 90 225 10 0.003 1 79
71 1.2
202 N-MXD6I
DL-valine-
Example copolymerized 2.6 2.4 2.6 90 224 8 0.003 1 77
69 1.1
203 N-MXD6I
DL-leucine-
Exam
204 le copolymerized 2.6 2.4 2.2 90 226 8 0.003
1 76 68 1.1 n
N-MXD6I
DL-Phe*2-
'
Example copolymerized 2.6 2.4 2.6 91 225 9 0.002 1 79
72 1.9 0
iv
20 N-MXD6I
.-.3
co
DL-alanine-
co
Exam
o)
206. le copolymerized 2.6 2.4 2.4 90 225 9 0.002
1 81 73 1.5 .-.3
N-MXD6I u.)
,
DL-alanine- -
"
Example
0
copolymerized 2.6 2.4 2.3 94 220 9 0.002 1 84
74 2.1 H
207 N-MXD6I
I\)
1
DL-alanine-
0
Example
208 copolymerized 2.6 2.4 2.6 90 225 12 0.001
1 79 68 1.5 1
N-MXD6I
I\)
DL-alanine-
Example copolymerized 2.6 4.9 2.6 94 221 9 0.001 1 80
73 1.3
209 N-MXD6I .
DL-alanine-
Example
210 copolymerized 2.6 7.3 2.4 98 N.D. 8 0.003
1 71 60 1.2
N-MXD6I .
DL-alanine-
Example copolymerized 2.6 14.6 2.2 110 N.D. 8 0.004
1 70 62 1.0
211 N-MXD6I
**: unit of oxygen permeability coefficient cc.mm/(m2.clay=atm)
*1: DL-AABA: DL-2-aminobutyric acid
*2: DL-Phe: DL-phenylalanine
N.D.: not detected
67
[0190]
Table 3 (continued)
Aromatic Oxygen
Tensile breaking strength
Amino acid Oxygen absorbing
dicarboxyfic acid Relative Tg Tm permeability
Sensory test (MPa)
Polyamide content amount (cc/g)
Haze
(% by mol) copolymerization viscosity ( C) ( C) 40 C, 28 days
coefficient of evaluation Before oxygen After oxygen
ratio (% by mol) film-
absorption absorption
_
DL-alanine-
Example copolymerized 8.1 4.6 2.1 94 N.D. 19 0.001
1 80 70 1.8
212 N-MXD6I .
DL-alanine-
Example copolymerized 8.1 6.9 1.9 98 N.D. 18 0.001
1 76 63 2.3
213
N-MXD6I0
. -
DL-alanine-
Example copolymerized 8.1 13.8 1.6 110 N
- 1 .D. 17 0
214
- . _ iv
N-MXD6I.-
..3
co
DL-alanine-
co
Example copolymerized 17.7 2.1 1.5 90 N.D.
36 - 1 0,
215
- - - .-.3
N-MXD6I
u.)
. ,
DL-alanine-
iv
Example copolymerized 17.7 4.1 1.5
94 N.D. 36 1 0
216 -
- - - H
N-MXD6I
iv
DL-alanine-
Example
0
copolymerized 17.7 6.2 1.5 98 N.D. 36 -
1 - - _ 0,
217
i
N-MXD6I
1\)
- . _
DL-alanine-
0,
Example
218 copolymerized 2.6 2.4 2.3 63 185 10
0.8 1 40 32 2.2
N-MXD101
DL-alanine-
Example
219 copolymerized 2.6 2.4 2.3 56 182 12
1.7 1 35 28 1.5
N-MXD12I .
DL-alanine-
Example copolymerized 31.6 15.4 1.2 N.D. N.D. 83 - =
- - . _
220 N-MXD6I _
**: unit of oxygen permeability coefficient cc=mm/(m2=day=atm)
N.D.: not detected
68
_
,
[0191]
Table 3 (continued)
Amino acid
Aromatic Oxygen
Tensile breaking strength
Oxygen absorbing
dicarboxvlic acid Relative Ta Tm permeability Sensory
test (MPa)
Polyamide content copolymerization. =ty
( ') c ) amount (cc/g) Haze
viscosity C C coefficient of evaluation
(% by mol)40 C, 28 days Before oxygen After oxygen
ratio (% by mol) film" absorption absorption
-
Comparative
Example N-MXD6I 0 2.5 2.6 91 229 0 0.07
1 82 80 1.8
201 _
Comparative
Example MXD101 0 2.5 2.6 64 187 0 1.9
1 247 246 1.3
202
Comparative
Example MXD121 0 2.5 2.6 57 189 0 2.8
1 250 250 1.2
203 .. -
Comparative glycine-
Example copolymerized 8.1 2.3 2.5 90 N.D. 2 0.07 1
83 80 1.8 0
204 N-MXD6I
Comparative AlB*3-
0
Example copolymerized 8.1 2.3 2.6 90 N.
.-
D. 0 0.08 1 79 77 1.7 iv
.]
205 N-MXD6I
co
, _ .
co
Comparative
0,
Example , N-MXD61 0 2.5 2.6 91 229 12 0.001
2 83 0 1.9 .-.]
u.)
206 (Co mixedr
.
iv
Comparative N-MXD6I
0
Example (Co + PB 0 2.5 2.6 91 229 17 0.001
3 78 18 2.5 H
IV
207 mixedr
i
. -
0
Comparative N-MXD6I
0,
Example (DL-alanine 0 2.5 2.6 91 229 0 0.07
1 83 83 1.9 i
1\)
208 mixedr
0,
**: unit of oxygen permeability coefficient: cc.mm/(m2-day=atm)
*3: AIB: 2-aminoisobutyric acid
*4: 400 ppm of Co mixed
*5: 400 ppm of Co and 5 parts by mass of maleic acid-modified polybutadiene
mixed
*6: 5 parts by mass of DL-alanine mixed
N.D.: not detected
69
CA 02785673 2012-06-26
[0192]
The polyamide compound that is produced by copolymerizing only an aromatic
dicarboxylic acid is insufficient in oxygen absorbing capability (Comparative
Examples
201 to 203). The polyamide compound that is copolymerized with an a-amino acid
having no tertiary hydrogen is similarly insufficient in oxygen absorbing
capability
(Comparative Examples 204 and 205). The ordinary polyamide composition having
a
cobalt compound mixed therein exhibits an oxygen absorbing capability and a
good
oxygen permeability coefficient, but has bluish color due to the addition of
the cobalt
compound although it has good transparency, and causes a large amount of odor
especially in one using polybutadiene. Furthermore, the film after oxygen
absorption is
deteriorated to fail to maintain the shape thereof, and thus is not
necessarily favorable in
the purpose of packaging containers (Comparative Examples 206 and 207). The
polyamide composition that is only mixed with an a-amino acid having tertiary
hydrogen,
but is not copolymerized therewith exhibits no oxygen absorbing capability
(Comparative
Example 208).
On the other hand, the polyamide compound that is copolymerized with an a-
amino acid having tertiary hydrogen and aromatic dicarboxylic acid exhibits a
sufficient
oxygen absorbing capability without a metal contained, and generates no
offensive odor
(Examples 201 to 220). In the case of the film specimen, in particular, the
film has a
good oxygen permeability coefficient, has good transparency, and maintains the
mechanical properties after oxygen absorption (Examples 201 to 213, 218 and
219).
The polyamide compound of Example 220 exhibits a considerably good oxygen
absorbing capability although the molecular weight thereof is not increased.
Examples 218 and 219 using sebacic acid or dodecanedioic acid as the linear
aliphatic dicarboxylic acid component have a lower oxygen absorbing capability
than
Examples using adipic acid, but in comparison to N-MXD10I (Comparative Example
202) and N-MXD12I (Comparative Example 203) having no a-amino acid having
tertiary hydrogen copolymerized therewith, have an excellent oxygen absorbing
capability.
[0193]
Example 301
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 13,000 g (89.0 mol) of precisely weighed
adipic acid
CA 02785673 2012-06-26
(produced by Asahi Kasei Chemicals Corporation), 880.56 g (9.88 mol) of DL-
alanine
(produced by Musashino Chemical Laboratory, Ltd.), 4,314.10 g (38.1 mol) of s-
caprolactam (produced by Ube Industries, Ltd.), 13.75 g (0.13 mol) of sodium
hypophosphite and 7.13 g (0.087 mol) of sodium acetate were placed, and after
sufficiently replacing with nitrogen, the system was heated to 170 C under a
small
amount of a nitrogen stream under stirring. 12,433 g (88.7 mol) of m-
xylylenediamine
(produced by Mitsubishi Gas Chemical Co., Inc.) was added dropwise thereto
under
stirring, and the system was continuously heated while removing condensation
water
formed. After completing the dropwise addition of m-xylylenediamine, the
reaction
was continued at an inner temperature of 260 C for 40 minutes. Thereafter, the
system
was pressurized with nitrogen, and the polymer was taken out from the strand
die and
pelletized, thereby providing approximately 26 kg of a polyamide compound.
[0194]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound and 0.5% by mass of water were charged in a tumble
dryer with a jacket, equipped with a nitrogen introducing tube, a vacuum line,
a vacuum
pump and a thermocouple for measuring the inner temperature, and while
rotating the
tumble dryer at a constant rate, the inside of the tumble dryer was
sufficiently replaced
with nitrogen gas having a purity of 99% by volume or more, and the tumble
dryer was
heated under a stream of the nitrogen gas, thereby increasing the temperature
of the
pellets to 150 C over approximately 150 minutes. At the time when the
temperature of
the pellets reached 150 C, the pressure inside the system was decreased to 1
ton or less.
While further increasing the temperature, the temperature of the pellets was
increased to
180 C over approximately 70 minutes, and then maintained at 180 C for 30
minutes.
Nitrogen gas having a purity of 99% by volume or more was introduced into the
system,
and the tumble dryer was cooled while rotating, thereby providing DL-alanine-
copolymerized N-MXD6,6 (polyamide compound 301, MADA unit/adipic acid unit/DL-
alanine unit/c-caprolactam unit = 39.3/39.4/4.4/16.9 (% by mol)).
[0195]
Example 302
D-alanine-copolymerized N-MXD6,6 (polyamide compound 302, MXDA
unit/adipic acid unit/D-alanine unit/6-caprolactam unit = 39.3/39.4/4.4/16.9
(% by mol))
was produced in the same manner as in Example 301 except that DL-alanine was
changed to D-alanine.
[0196]
71
CA 02785673 2012-06-26
Example 303
L-alanine-copolymerized N-MXD6,6 (polyamide compound 303, MXDA
unit/adipic acid unia-alanine unitle-caprolactam unit = 39.3/39.4/4.4/16.9 (%
by mol))
was produced in the same manner as in Example 301 except that DL-alanine was
changed to L-alanine.
[0197]
Example 304
DL-alanine-copolymerized N-MXD6,6 (polyamide compound 304, MXDA
unit/adipic acid unit/DL-alanine unit/s-caprolactam unit = 40.8/40.9/0.8/17.5
(% by mol))
was produced in the same manner as in Example 301 except that the addition
amount of
DL-alanine was changed to make a content thereof in the polyamide compound of
0.8%
by mol, and the addition amount of s-caprolactam was changed to make a content
thereof
in the polyamide compound of 17.5% by mol.
[0198]
Example 305
DL-alanine-copolymerized N-MXD6,6 (polyamide compound 305, MXDA
unit/adipic acid unit/DL-alanine unit/s-caprolactam unit = 34.8/35.2/15.0/15.0
(% by
mol)) was produced in the same manner as in Example 301 except that the
addition
amount of DL-alanine was changed to make a content thereof in the polyamide
compound of 15.0% by mol, and the addition amount of s-caprolactam was changed
to
make a content thereof in the polyamide compound of 15.0% by mol.
[0199]
Example 306
DL-alanine-copolymerized N-MXD6,6 (polyamide compound 306, MXDA
unit/adipic acid unit/DL-alanine unit/s-caprolactam unit = 28.9/29.3/29.3/12.5
(% by
mol)) was produced in the same manner as in Example 301 except that the
addition
amount of DL-alanine was changed to make a content thereof in the polyamide
compound of 29.2% by mol, and the addition amount of s-caprolactam was changed
to
make a content thereof in the polyamide compound of 12.5% by mol.
[0200]
Example 307
DL-alanine-copolymerized N-MXD6,6 (polyamide compound 307, MXDA
unit/adipic acid unit/DL-alanine unit/s-caprolactam unit = 20.7/21.1/49.2/9.0
(% by mol))
was produced in the same manner as in Example 301 except that the addition
amount of
DL-alanine was changed to make a content thereof in the polyamide compound of
49.2%
72
CA 02785673 2012-06-26
by mol, and the addition amount of s-caprolactam was changed to make a content
thereof
in the polyamide compound of 9.0% by mol.
[0201]
Example 308
DL-alanine-copolymerized N-MXD6,6 (polyamide compound 308, MXDA
unit/adipic acid unit/DL-alanine unit/s-caprolactam unit = 32.0/32.2/3.6/32.2
(% by mol))
was produced in the same manner as in Example 301 except that the addition
amount of
DL-alanine was changed to make a content thereof in the polyamide compound of
3.6%
by mol, and the addition amount of s-caprolactam was changed to make a content
thereof
in the polyamide compound of 32.2% by mol.
[0202]
Example 309
DL-alanine-copolymerized N-MXD6,6 (polyamide compound 309, MXDA
unit/adipic acid unit/DL-alanine unit/s-caprolactam unit = 44.9/45.1/5.0/5.0
(% by mol))
was produced in the same manner as in Example 301 except that the addition
amount of
DL-alanine was changed to make a content thereof in the polyamide compound of
5.0%
by mol, and the addition amount of s-caprolactam was changed to make a content
thereof
in the polyamide compound of 5.0% by mol.
[0203]
Example 310
DL-alanine-copolymerized N-MXD6,12 (polyamide compound 310, MXDA
unit/adipic acid unit/DL-alanine unit/laurolactam unit = 39.3/39.4/4.4/16.9 (%
by mol))
was produced in the same manner as in Example 301 except that s-caprolactam
was
changed to laurolactam (produced by Ube Industries, Ltd.).
[0204]
Example 311
DL-2-aminobutyric acid-copolymerized N-M>CD6,6 (polyamide compound 311,
MXDA unit/adipic acid unit/DL-AABA unit/s-caprolactam unit =
39.3/39.4/4.4/16.9 (%
by mol)) was produced in the same manner as in Example 301 except that the a-
amino
acid was changed to DL-2-aminobutyric acid (DL-AABA, produced by Japan
Finechem
Co., Inc., purified product).
[0205]
Example 312
DL-leucine-copolymerized N-MXD6,6 (polyamide compound 312, MXDA
unit/adipic acid unit/DL-leucine unit/s-caprolactam unit = 39.3/39.4/4.4/16.9
(% by mol))
73
CA 02785673 2012-06-26
was produced in the same manner as in Example 301 except that the a-amino acid
was
changed to DL-leucine (produced by Ningbo Haishuo Bio-technology Co., Ltd.).
[0206]
Example 313
DL-phenylalanine-copolymerized N-1VIXD6,6 (polyamide compound 313,
MXDA unit/adipic acid unit/DL-phenylalanine unit/s-caprolactam unit =
39.3/39.4/4.4/16.9 (% by mol)) was produced in the same manner as in Example
301
except that the a-amino acid was changed to DL-phenylalanine (DL-Phe, produced
by
Sinogel Amino Acid Co., Ltd.).
[0207]
Example 314
DL-alanine-copolymerized N-MXD10,6 (polyamide compound 314, IVIXDA
unit/sebacic acid unit/DL-alanine unit/s-caprolactam unit = 39.3/39.4/4.4/16.9
(% by
mol)) was produced in the same manner as in Example 301 except that adipic
acid was
changed to sebacic acid (produced by Itoh Oil Chemicals Co., Ltd.).
[0208]
Example 315
DL-alanine-copolymerized N-MXD12,6 (polyamide compound 315, MXDA
unit/dodecanedioic acid unit/DL-alanine unit/s-caprolactam unit =
39.3/39.4/4.4/16.9 (%
by mol)) was produced in the same manner as in Example 301 except that adipic
acid was
changed to dodecanedioic acid (produced by Ube Industries, Ltd.).
[0209]
Comparative Example 301
(Melt Polymerization of Polyamide Compound by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, 13,000 g (89.0 mol) of precisely weighed
adipic acid
(produced by Asahi Kasei Chemicals Corporation), 4,314.1 g (38.1 mol) of s-
caprolactam
(produced by Ube Industries, Ltd.), 13.4 g (0.13 mol) of sodium hypophosphite
and 6.9 g
(0.084 mol) of sodium acetate were placed, and after sufficiently replacing
with nitrogen,
the system was heated to 170 C under a small amount of a nitrogen stream under
stirring.
12,437 g (88.7 mol) of m-xylylenediamine (MXDA) (produced by Mitsubishi Gas
Chemical Co., Inc.) was added dropwise thereto under stirring, and the system
was
continuously heated while removing condensation water formed. After completing
the
dropwise addition of 1,3-bis(aminomethyl)cyclohexane, the reaction was
continued at an
74
CA 02785673 2012-06-26
inner temperature of 260 C for 40 minutes. Thereafter, the system was
pressurized with
nitrogen, and the polymer was taken out from the strand die and pelletized,
thereby
providing approximately 26 kg of a polyamide compound.
[0210]
(Solid Phase Polymerization of Polyamide Compound)
The polyamide compound and 0.5% by mass of water were charged in a tumble
dryer with a jacket, equipped with a nitrogen introducing tube, a vacuum line,
a vacuum
pump and a thermocouple for measuring the inner temperature, and while
rotating the
tumble dryer at a constant rate, the inside of the tumble dryer was
sufficiently replaced
with nitrogen gas having a purity of 99% by volume or more, and the tumble
dryer was
heated under a stream of the nitrogen gas, thereby increasing the temperature
of the
pellets to 150 C over approximately 150 minutes. At the time when the
temperature of
the pellets reached 150 C, the pressure inside the system was decreased to 1
ton or less.
While further increasing the temperature, the temperature of the pellets was
increased to
180 C over approximately 70 minutes, and then maintained at 180 C for 30
minutes.
Nitrogen gas having a purity of 99% by volume or more was introduced into the
system,
and the tumble dryer was cooled while rotating, thereby providing N-MXD6,6
(polyamide compound 316, MXDA unit/adipic acid unit/s-caprolactam unit =
41.1/41.2/17.7 (% by mol)).
[0211]
Comparative Example 302
Glycine-copolymerized N-M3036,6 (polyamide compound 317, MXDA
unit/adipic acid unit/glycine unit/s-caprolactam unit = 39.3/39.4/4.4/16.9 (%
by mol))
was produced in the same manner as in Example 301 except that the DL-alanine
was
changed to glycine (produced by Tokyo Chemical Industry Co., Ltd.) having
secondary
hydrogen on the a-position.
[0212]
Comparative Example 303
2-Aminoisobutylic acid-copolymerized N-MXD6,6 (polyamide compound 318,
MXDA unit/adipic acid unit/AIB unit/s-caprolactam unit = 39.3/39.4/4.4/16.9 (%
by
mol)) was produced in the same manner as in Example 301 except that DL-alanine
was
changed to 2-aminoisobutylic acid (2-amino-2-methylpropanoic acid, AIB,
produced by
Japan Finechem Co., Inc., purified product) having no hydrogen on the a-
position.
[0213]
Comparative Example 304
CA 02785673 2012-06-26
N-MND6,12 (polyamide compound 319, MXDA unit/adipic acid
unit/laurolactam unit = 41.1/41.2/17.7 (% by mol)) was produced in the same
manner as
in Comparative Example 301 except that s-caprolactam was changed to
laurolactam
(produced by Ube Industries, Ltd.).
[0214]
Comparative Example 305
N-MXD10,6 (polyamide compound 320, MXDA unit/sebacic acid unit/s-
caprolactam unit = 41.1/41.2/17.7 (% by mol)) was produced in the same manner
as in
Comparative Example 301 except that adipic acid was changed to sebacic acid
(produced
by Itoh Oil Chemicals Co., Ltd.).
[0215]
Comparative Example 306
N-MXD12,6 (polyamide compound 321, MXDA unit/ dodecanedioic acid
unit/s-caprolactam unit = 41.1/41.2/17.7 (% by mol)) was produced in the same
manner
as in Comparative Example 301 except that adipic acid was changed to
dodecanedioic
acid (produced by Ube Industries, Ltd.).
[0216]
Comparative Example 307
To the polyamide compound 316 obtained in Comparative Example 301, DL-
alanine (produced by Musashino Chemical Laboratory, Ltd.) was added and dry-
mixed,
thereby providing a mixture of the polyamide compound and DL-alanine (content
of DL-
alanine in the mixture: 5% by mass). The resulting mixture, which was
prevented from
being copolymerized, was extruded with a small-sized single screw extruder
having a
diameter of 15 mm at an extrusion temperature of 240 C, a screw rotation
number of 30
rpm and a feed screw rotation number of 14 rpm, thereby producing pellets of a
DL-
alanine-containing N-MXD6,6.
[0217]
76
4 CA 02785673 2012-06-26
Table 4
d Aminocarboxylic Oxygen
Amino aci
acid Relative Tg Tm
absorbing
Polyamide content
M by mol) copolymerization viscosity 1 C) ( C) , amount (cc/g)
ratio (% by mol) , 40 C,
28 days
DL-alanine-
Example 301 4.4 16.9 2.1 75 197 8
copolymerized N-MXD6,6
D-alanine-
Example 302 4.4 16.9 2.1 75 197 8
copolymerized N-MXD6,6
L-alanine-
Example 303 4.4 16.9 2.1 75 197 8
copolymerized N-MXD6,6 ,
DL-alanine-
Example 304 0.8 17.5 2.1 76 200 3
copolymerized N-MXD6,6
DL-alanine-
Example 305 15.0 15.0 1.9 75 N.D. 23
copolymerized N-MXD6,6
Example 306 DL-.alanine- 29.3 12.5 1.8 75 N.D. 45
copolymerized N-MXD6,6
_
DL-alanine-
Example 307 49.2 9.0 1.6 75 N.D. 67
copolymerized N-MXD6,6 ,
DL-alanine-
Example 308 3'6 32.2 1.8 62 N.D. 8
copolymerized N-MXD6,6
Example 309 DL-alanine- 5.0 5.0 2.1 81 228 9
copolymerized N-MXD6,6
N.D.: not detected
[0218]
Table 4 (continued)
Aminocarboxylic Oxygen
Amino acid acid Relative Tg Tm absorbing
Polyamide content
(% by mbil copolymeration viscosity ( C) ( C) amount (cc/g)
, ratio (% by mol) 40 C, 28 days
DL-alanine-
Example 310 4.4 16.9 2.1 69 N.D. 6
copolymerized N-MXD6,12 .
DL-AABA*1-
Example 311 4.4 16.9 2.1 75 197 8
copolymerized N-MXD6,6
DL-leucine-
Example 312 4.4 16.9 2.1 75 197 8
copolymerized N-MXD6,6
DL-Phe'2-
Example 313 4.4 16.9 2.1 75 197 9
copolymerized N-MXD6,6
DL-alanine-
Example 314 4.4 16.9 2.1 77 N.D. 10
copolymerized N-MXD10,6
Example 315 DL-alanine- 4.4 16.9 2.1 73 N.D. 11
copolymerized N-MXD12,6 I
*1: DL-AABA: DL-2-aminobutyric acid
*2: DL-Phe: DL-phenylalanine
N.D.: not detected
[0219]
77
CA 02785673 2012-06-26
Table 4 (continued)
Aminocarboxylic Oxygen
Amino acid
Poyamide content acid Relative Tg Tm
absorbing
(,,A by moi) copolymerization ebry mizati000n viscosity ( C) ( C)
4arrijrcun2t8 days
Comparative N-MXD6,6 0 17.7 2.1 75 203 0
Example 301
Comparative glycine- 44 16.9 2.1 75 197 0
Example 302 copolymerized N-MXD6,6 .
Comparative AI B- 44 16.9 2.1 75 196 0
Example 303 copolymerized N-MXD6,6
Comparative N-MXD6,12 0 17.7 2.1 69 N.D. 0
Example 304
Comparative N-MXD10,6 0 17.7 2.1 77 201 0
Example 305 .
Comparative N-MXD12,6 0 17.7 2.1 73 N.D. 0
Example 306
Comparative N-MXD6,6 0 17.7 2.1 75 200 1
Example 307 (DL-alanine mixed)7
*3: AIB: 2-aminoisobutyric acid
*7: mixture of N-MXD6,6 and DL-alanine (DL-alanine content: 5% by mass)
N.D.: not detected
[0220]
The polyamide compound that is copolymerized with an a-amino acid having no
tertiary hydrogen is insufficient in oxygen absorbing capability (Comparative
Examples
302 and 303). The polyamide compound that is not copolymerized with an a-amino
acid having tertiary hydrogen and the polyamide composition that is only mixed
with an
a-amino acid having tertiary hydrogen, but is not copolymerized therewith
exhibits no
oxygen absorbing capability (Comparative Examples 301, 304 to 306 and 307).
On the other hand, the polyamide compound that is copolymerized with an a-
amino acid having tertiary hydrogen exhibits a sufficient oxygen absorbing
capability
without a metal contained (Examples 301 to 315).
[0221]
Examples 401 to 416 and Comparative Examples 401 to 407
(Melt Polymerization of Polyamide Oligomer by Atmospheric Dropping Method)
In a reaction vessel having an inner capacity of 50 L, equipped with a
stirrer, a
partial condenser, a total condenser, a thermometer, a dropping funnel, a
nitrogen
introducing tube and a strand die, an aliphatic dicarboxylic acid, an aromatic
dicarboxylic
acid, an a-amino acid, an co-aminocarboxylic acid, sodium hypophosphite and
sodium
acetate of the kinds and the amounts shown in Table 5 were charged, and after
sufficiently replacing with nitrogen, the system was heated to 170 C under a
small
amount of a nitrogen stream under stirring. m-xylylenediamine as an aromatic
diamine
in the amounts shown in Table 5 was added dropwise thereto under stirring, and
the
system was continuously heated while removing condensation water formed. After
completing the dropwise addition of the diamine, the reaction was continued at
an inner
78
CA 02785673 2012-06-26
temperature of 240 C for 40 to 60 minutes while taking care of increase of the
torque.
Thereafter, the system was pressurized with nitrogen, and the polyamide
oligomer was
taken out from the strand die. One that was taken out in the form of strand
and was
pelletized, thereby providing a polyamide oligomer in the form of pellets. One
that was
not taken out in the form of strand due to the low molecular weight and was
separately
pulverized with a pulverizer, thereby providing a pulverized product of a
polyamide
oligomer.
79
[0222]
Table 5
Example
401 402 403 404 405 406
407 408 409 410 411 412
Aromatic diamine MXDA g 12,500 12,500 12,500 12,500
12,500 12,500 12,500 12,000 11,500 9,500 10,000 10,000
mol 91.8 91.8 91.8 91.8 91.8
91.8 91.8 88.1 84.4 69.8 73.4 73.4
adipic acid g 13,827 13,827 13,827 14,422
14,903 13,827 13,274 13,274 12,721 10,509
mol 94.6 94.6 94.6 98.6 102.0
94.6 90.8 90.8 87.1 71.9
Aliphatic g
14,850
dicarboxylic acid sebacic acid
mol 73.4
dodecanedioic acid g
16,909 n
mol
73.4
isophthalic acid g
0
I.)
mol
co
Aromatic
in
terephthalic acid g
c7,
dicarboxylic acid mol
-.3
2,6-naphthalene- g
u.)
dicarboxylic acid mol
O)
DL-alanine g 430 909 2,044 2,044
2,044 3,364 5,015 9,321 1,635 1,635 H
IV
mol 4.8 10.2 22.9 22.9
22.9 37.8 56.3 104.6 18.4 18.4 1
0
DL-2-amino butyric g 2,366
c7,
i
acid mol 22.94
I.)
Amino acid
,
DL-phenylalanine g 3,790.2
c7
mol 22.94
glycine g
mol
co-Aminocarboxylic 6-aminohexanoic g
acid acid mol
sodium g 12.1 12.3 12.8 13.3 13.7 13.0
13.7 12.9 13.6 12.9 12.1 13.1
hypophosphite mmol 114.5 116.4 120.8 125.6 129.6
122.3 129.2 121.4 123.2 121.8 113.9 123.8
Additive
sodium acetate 6.6 6.7 6.9 7.2 7.4 7.0
7.4 7.0 7.1 7.0 6.5 7.1
--g
mmol 80.2 85.0 84.5 87.9 90.7
85.6 90.5 85.0 86.3 85.3 79.7 j 86.7
Specified molar ratio - - 0.970 0.970 0.970
0.930 0.900 -UM- ,'-- rTh"-=-o--Trr" 1.000 1.000
aromatic diamine % by mol - 48.0 46:7 43.8 - 43.0- 42.4 -
43.8 44.7 40.7 ' 37.1 . 28.3 ' 44.41 44.4
aliphatic dicarboxylic % by mol 49.5 48.1 45.2 46.2 47.0
45.2 44.2 41.9 38.2 29.2 44.4 44.4
Charged acid
monomer aromatic dicarboxylic % by mol
composition __ acid
amino acid % by mol 2.5 5.2 11.0 10.8 10.6
11.0 11.1 17.4 24.7 42.5 11.2 11.2
aminocarboxylic acid % by mol
Table 5 (continued)
Exam. le
401 402 403 404 __ 405 406
407 408 409 410 411 412
Amino acid content %b mol 2.5 5.1 10.8 10.8 10.5 11.0
11.0 17.1 24.5 42.1 11.0 11.1
Relative viscosity 1.53 1.51 1.18 1.31 1.04 1.59
1.60 1.57 1.53 1.45 1.41 1.28
Number average molecular weight 8,200 7,870 3,200 5,120 1,230
8,950 9,130 8,790 8,100 7,040 6,500 4,570
Melting =oint C 231 225 207 207 200 208
207 N.D. N.D. N.D. N.D. N.D.
Oxygen absorbing amount
cc/g 5 9 18 17 19 16 15 30
37 65 11 13
(40 C, 100%RH, 28 days)
* N.D.: not detected
0
1.)
co
1.)
0
1.)
0
1.)
81
[0223]
Table 5 (continued)
Example
Comparative Example
413 414 415 416 401 402 403
404 405 406 407
Aromatic diamine MXDA g 12,000 12,000 12,500 11,184
12,500 12,500 12,500 10,000 10,000 12,000 12,000
mol 88.1 88.1 91.8 82.1 91.8 91.8
91.8 73.4 73.4 88.1 82.1
g 11,266 ' 11,266 11,736
12,000 13,827 - 13,829 13,829 11,588 11,184
adipic acid
nnol 77.1 77.1 80.3 82.1 94.6 94.6
94.6 79.3 82.1
_
Aliphatic 9 14,850
sebacic acid
dicarboxylic acid mol
73.4
-= 9
dodecanedioic acid .
16,909 n
mol
73.4
'I:3
isophthalic acid g 1,830 . -
1,463.8
co
mol 11.0
8.8 in
.
c7,
Aromatic g _________ 1,830
terephthalic acid
u.)
dicarboxylic acid mol 11.0
I.)
2,6-naphthalene- g
2,480 o
H
dicarboxylic acid mol
11.5 I.)
,
1
g 1,962 1,962 1,962
1,829 0
DL-alanine - .
___________________________________________________________ c7,
mol 22.0 22.0 22.0
20.5 1
I.)
DL-2-amino butyric g
c7,
acid mol
Amino acid
DL-phenylalanine g
mol
g 1,722 2,366
glycine
-
mol - 22.9 22.9
_
03-Aminocarboxylic 6-aminohexanoic g
1,197 1,197
acid acid mol 9.1
9.1
sodium 9 12.1 12.1 12.9 11.7 12.0 12.6
13.0 11.4 12.5 11.2 10.9
hypophosphite mmol 114.0 114.0 121.5 , 110.0 112.9 119.2
122.3 107.5 117.5 106.1 103.0
Additive
sodium acetate
g 6.5 6.5 7.0 6.3 6.5 6.8
7.0 6.2 6.7 6.1 5.9 mmol 79.8 79.8 85.1 77.0 79.0
83.4 85.6 75.3 82.3 74.3 _ 72.1 -
Specified molar ratio 1.000 1.000 1.000 1.000 0.970
0.970 0.970 1.000 1.000 1.000 1.000
aromatic diamine % by mol 44.4 44.4 44.6 42.4
49.2 43.8 43.8 50.0 5(10 50.0 47.4
aliphatic dicarboxylic 47.4
% by mol 38.9 38.9 39.1 42.4 50.8 45.2 45.2 50.0
50.0 45.0
Charged acid
monomer aromatic dicarboxylic
% by mol 5.6 5.6 5.6 5.0
composition
acid .
amino acid % by mol 11.1 11.1 10.7 10.5
11.0 11.0
aminocarboxylic acid % by mol 4.7 - 5.2
-
_______________________________________________________________________________
_________________
82
Table 5 (continued)
Example
Comparative Example
413 414 415 416 401J 402 403
404 405 406 407
Amino acid content % by mol 10.8 11.0 10.5 10.3 0.0
10.7 10.6 0.0 0.0 0.0 6.0
Relative viscosity 1.60 - 1.50 1.49 1.43 1.37 1.46 1.29
1.56 1.55 1.57 1.54
Number average molecular weight 9,100 7,700 7,540 6,750 5,950
7,230 4,820 8,520 8,490 8,790 8,320
Melting point C N.D. N.D. N.D. 210 237 207
207 192 187 230 227
Oxygen absorbing amount
cc/g 14 15 7 9 0 0 0 0
0 0 0
(40 C, 100%RH, 28 days)
* N.D.: not detected
0
1.)
CO
Ul
0
0
83
CA 02785673 2012-06-26
[0224]
As clear from the results in Table 5, the polyamide oligomer that is not
polymerized with an a-amino acid having tertiary hydrogen (Comparative
Examples 401
and 404 to 407) and the polyamide oligomer that is copolymerized with an amino
acid
having no tertiary hydrogen (Comparative Examples 402 and 403) exhibit no
oxygen
absorbing capability. The polyamide oligomer that is copolymerized with an a-
amino
acid having tertiary hydrogen (Examples 401 to 416) exhibits a sufficient
oxygen
absorbing capability without a metal contained. Accordingly, the polyamide
compound
of the present invention may be used as an oxygen absorbent.
INDUSTRIAL APPLICABILITY
[0225]
The polyamide compound of the present invention is excellent in oxygen
absorbing capability. By applying the polyamide compound of the present
invention to
a packaging material and a packaging container, such a packaging material and
a
packaging container are provided that exhibit a sufficient oxygen absorbing
capability
without a metal contained, generate no offensive odor, and have considerably
good
transparency, thereby storing a content in good conditions.
84