Note: Descriptions are shown in the official language in which they were submitted.
CA 02785897 2013-09-16
METATHESIS CATALYST AND PROCESS FOR USE THEREOF
FIELD OF THE INVENTION
[0003] This invention relates to olefin metathesis, more particularly,
metathesis catalyst
compounds and processes for the use thereof.
BACKGROUND OF THE INVENTION
[0004] The cross-metathesis of two reactant olefins, where each reactant
olefin comprises
at least one unsaturation site, to produce new olefins which are different
from the reactant
olefins is of significant commercial importance. The cross-metathesis reaction
is usually
catalyzed by one or more catalytic metals, usually one or more transition
metals.
[0005] One such commercially significant application is the cross-
metathesis of ethylene
and internal olefins to produce alpha-olefins, which is generally referred to
as ethenolysis. In
particular, the cross-metathesis of ethylene and an internal olefin to produce
linear alpha-
olefins (LA0s) is of particular commercial significance. LAOs are useful as
monomers or
comonomers in certain (co)polyiners (polyalphaolefins or PA0s) and/or as
intermediates in
the production of epoxides, amines, oxo alcohols, synthetic lubricants,
synthetic fatty acids
and alkylated aromatics. Olefins Conversion TechnologyTm, based upon the
Phillips Triolefin
Process, is an example of an ethenolysis reaction converting ethylene and 2-
butene into
propylene. These processes use heterogeneous catalysts, such as tungsten and
rhenium
oxides, which have not proven effective for internal olefins containing
functional groups such
as cis-methyl oleate, a fatty acid methyl ester.
- 1 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0006] Methods for the production of polyalpha-olefins are typically
multi-step processes
that often create unwanted by-products and waste of reactants and energy. Full
range linear
alpha-olefins plants are petroleum-based, are inefficient and result in
mixtures of
oligomerization products that typically yield Schulz-Flory distributions
producing large
quantities of undesirable materials. In recent years there have been new
technologies
implemented to produce "on purpose" linear alpha-olefins such 1-hexene and 1-
octene
through chromium-based selective ethylene trimerization or tetramerization
catalysts.
Alternatively, 1-octene has been produced via the telomerization of butadiene
and methanol.
Similar strategies are not currently available for the production of 1-decene.
[0007] 1-Decene is a co-product typically produced in the cross-metathesis
of ethylene
and methyl oleate. Alkyl oleates are fatty acid esters that can be major
components in
biodiesel produced by the transesterification of alcohol and vegetable oils.
Vegetable oils
containing at least one site of unsaturation include canola, soybean, palm,
peanut, mustard,
sunflower, tung, tall, perilla, grapeseed, rapeseed, linseed, safflower,
pumpkin corn and many
other oils extracted from plant seeds. Alkyl erucates similarly are fatty acid
esters that can be
major components in biodiesel. Useful biodiesel compositions are those which
typically have
high concentrations of oleate and erucate esters. These fatty acid esters
preferably have one
site of unsaturation such that cross-metathesis with ethylene yields 1-decene
as a co-product.
[0008] Biodiesel is a fuel prepared from renewable sources, such as
plant oils or animal
fats. To produce biodiesel, triacylglycerides ("TAG"), the major compound in
plant oils and
animal fats, are converted to fatty acid alkyl esters ("FAAE," i.e.,
biodiesel) and glycerol via
reaction with an alcohol in the presence of a base, acid, or enzyme catalyst.
Biodiesel fuel
can be used in diesel engines, either alone or in a blend with petroleum-based
diesel, or can
be further modified to produce other chemical products.
[0009] Cross-metathesis catalysts reported thus far for the ethenolysis of
methyl oleate
are typically ruthenium-based catalysts bearing phosphine or carbene ligands.
Dow
researchers in 2004 achieved catalysts turnovers of approximately 15,000 using
the 1st
generation Grubb '5 catalyst, bis(tricyclohexylphosphine)benzylidene
ruthenium(IV)
dichloride, (Organometallics 2004, 23, 2027). Researchers at Materia, Inc.
have reported
turnover numbers up to 35,000 using a ruthenium catalyst containing a cyclic
alkyl amino
carbene ligand, (WO 2008/010961). These turnovers were obtained with a
catalyst
reportedly too expensive for industrial consideration due to high costs
associated with the
catalysts being derived from a low yielding synthesis (See Final Technical
Report entitled
- 2 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
"Platform Chemicals from an Oilseed Biorefinery" grant number DE-FG36-
04G014016
awarded by the Department of Energy). Additionally, the introduction of
chelating
isopropoxybenzylidene ligands has led to ruthenium catalysts with improved
activities for
metathesis reactions (J. Am. Chem. Soc. 1999, 121, 791). However, these
ruthenium
alkylidene catalysts are usually prepared by the reaction of ruthenium species
with diazo
compounds. The concerns associated with industrial scale reactions comprising
diazo
compounds have led to increased efforts to prepare ruthenium alkylidenes via
alternate
synthetic routes, such as using terminal alkynes or propargyl alcohols.
[0010]
The synthesis of RuC12(PCy3)2(3-phenylindenylene) has proven useful in
providing an easy route to ruthenium alkylidenes which avoids costly diazo
preparations
(Platinum Metals Rev. 2005, 49, 33). Also, Furstner et at. have prepared (N,N'-
bis(mesityl)imidazol-2-ylidene)RuC12(3-phenylindenylene). However these
types of
complexes have not proven effective in ethenolysis reactions.
[0011]
In order to obtain an economically viable process for 1-decene production via
the
cross-metathesis of ethylene and biodiesel (such as animal or vegetable oils),
higher activity
catalysts must be discovered. Thus there is a need for higher activity
processes that produce
desired products and co-products in commercially desirable ratios.
[0012]
There remains a need for catalysts which demonstrate high activity and
selectivity
in metathesis cross-reactions, including ethenolysis, which are capable of
being synthesized
by both mild and affordable synthetic routes. The instant invention's
metathesis catalyst
compounds provide both a mild and commercially economical and an "atom-
economical"
route to desirable olefins, in particular alpha-olefins, which in turn may be
useful in the
preparation of PAOs. More particularly, instant invention's metathesis
catalyst compounds
demonstrate improved activity and selectivity towards ethenolysis products in
ethylene cross-
metathesis reactions.
SUMMARY OF THE INVENTION
[0013]
This invention relates to a metathesis catalyst compound represented by the
formula:
- 3 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
X L G*
\ /
/ = R1
R---L1
0
G G
G
wherein M is a Group 8 metal; X and Xl are anionic ligands; L is a neutral two
electron
donor; Ll is N, 0, P, or S, preferably N or 0; G* is selected from the group
consisting of
hydrogen, a C1 to C30 hydrocarbyl, and a C1 to C30 substituted hydrocarbyl; R
is a Ci to C30
hydrocarbyl or a C1 to C30 substituted hydrocarbyl; Rl is selected from the
group consisting
of hydrogen, a Ci to C30 hydrocarbyl, and a Ci to C30 substituted hydrocarbyl;
and G is
independently selected from the group consisting of hydrogen, halogen, Ci to
C30
hydrocarbyls and C1 to C30 substituted hydrocarbyls.
[0014] This invention also relates to a process to produce alpha olefin
(preferably 1-
decene) comprising contacting the metathesis catalyst described above with an
olefin
(preferably ethylene), and one or more triacylglycerides such as fatty acid
esters (preferably
fatty acid methyl esters, preferably methyl oleate).
[0015] In a preferred embodiment, this relates to a process to produce
alpha olefin
(preferably 1-decene) comprising contacting the metathesis catalyst described
above with an
olefin (preferably ethylene), and one or more triacylglycerides such as fatty
acid esters
(preferably fatty acid methyl esters, preferably methyl oleate) derived from
biodiesel.
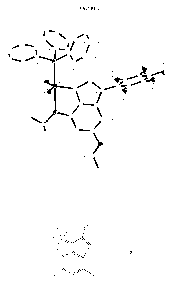
BRIEF DESCRIPTION OF THE FIGURES
[0016] Figure 1 is a representation of the molecular structure of
(PPh3)C12Ru(3-
pentafluoropheny1-6,8-diisopropoxyinden-1-ylidene) (J) drawn with 30% thermal
ellipsoids.
DETAILED DESCRIPTION
[0017] The present invention comprises a novel metathesis catalyst
compound useful for
the cross-metathesis of olefins, and processes for the use thereof. More
particularly, the
present invention comprises a novel metathesis catalyst compound which
comprises a
chelating indenylene group. Even more particularly, the present invention
comprises a novel
- 4 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
metathesis catalyst compound which demonstrates improved activity and
selectivity towards
ethenolysis products in ethylene cross-metathesis reactions.
[0018] This invention also relates to a process comprising contacting a
feed oil or
derivative thereof (and optional alkene) with an olefin metathesis catalyst
under conditions
which yield an alpha-olefin. Typically the feed oil is esterified or
transesterified with an
alcohol prior to contacting with the olefin metathesis catalyst.
[0019] This invention also relates to a process comprising contacting a
triacylglyceride or
a derivative thereof with an optional alkene (such as ethylene) and an olefin
metathesis
catalyst under conditions which yield an alpha-olefin, typically yielding a
linear alpha-olefin
(such asl-decene, 1-heptene, and/or 1-butene) and an ester or acid
functionalized olefin.
[0020] This invention further relates to a process for producing alpha-
olefins (preferably
linear alpha-olefins) comprising contacting a triacylglyceride with an alcohol
(such as
methanol) to produce a fatty acid alkyl ester and thereafter contacting the
fatty acid alkyl
ester with an olefin metathesis catalyst (and optional alkene, such as
ethylene) under
conditions which yield an alpha-olefin (preferably a linear alpha-olefin,
preferably 1-decene,
1-heptene, and/or 1-butene) and an ester or acid functionalized olefin.
[0021] This invention further relates to a process for producing alpha-
olefins (preferably
linear alpha-olefins) comprising contacting a triacylglyceride with water and
or an alkaline
reactant (such as sodium hydroxide) to produce a fatty acid and thereafter
contacting the fatty
acid with an olefin metathesis catalyst (and optional alkene, such as
ethylene) under
conditions which yield an alpha-olefin (preferably a linear alpha-olefin,
preferably 1-decene,
1-heptene, and/or 1-butene) and an ester or acid functionalized olefin.
[0022] This invention further relates to contacting unsaturated fatty
acid with an alkene
(such as ethylene) in the presence of an olefin metathesis catalyst under
conditions which
yield an alpha-olefin (preferably a linear alpha-olefin, preferably 1-decene,
1-heptene, and/or
1-butene) and an ester or acid functionalized olefin.
[0023] This invention further relates to contacting an unsaturated fatty
acid ester with an
alkene (such as ethylene) in the presence of an olefin metathesis catalyst
under conditions
which yield an alpha-olefin (preferably a linear alpha-olefin, preferably 1-
decene, 1-heptene,
and/or 1-butene) and an ester or acid functionalized olefin.
[0024] This invention further relates to contacting an unsaturated fatty
acid alkyl ester
with an alkene (such as ethylene) in the presence of an olefin metathesis
catalyst under
- 5 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
conditions which yield an alpha-olefin (preferably a linear alpha-olefin,
preferably 1-decene,
1-heptene, and/or 1-butene) and an ester or acid functionalized olefin.
[0025] This invention also relates to a process to produce alpha olefin
(preferably linear
alpha olefin, preferably 1-decene, 1-heptene and or 1-butene) comprising
contacting a
metathesis catalyst with an alkene (preferably ethylene), and one or more
fatty acid esters
(preferably fatty acid methyl esters, preferably methyl oleate).
[0026] In a preferred embodiment, this relates to a process to produce
alpha olefin
(preferably linear alpha olefin, preferably 1-decene, 1-heptene and or 1-
butene) comprising
contacting a metathesis catalyst with an alkene (preferably ethylene), and one
or more fatty
acid esters (preferably fatty acid methyl esters, preferably methyl oleate)
derived from
biodiesel.
[0027] In a preferred embodiment, the olefin metathesis catalysts
described herein may
be combined directly with feed oils, triacylglycerides, biodiesel, fatty
acids, fatty acid esters
and/or fatty acid alkyl esters to produce alpha-olefins, preferably linear
alpha olefins,
preferably C4 to C24 alpha-olefins, preferably linear alpha-olefins, such as 1-
decene, 1-
heptene and or 1-butene.
[0028] In a preferred embodiment, a mixture of one or more biodiesels,
triacylglycerides,
fatty acids, fatty acid esters and/or fatty acid alkyl esters is used to
produce alpha-olefins,
preferably linear alpha olefins, preferably C4 to C24 alpha-olefins,
preferably C4 to C24 linear
alpha-olefins. In a preferred embodiment a mixture of alpha olefins,
preferably linear alpha
olefins, preferably 1-decene, 1-heptene and or 1-butene is produced.
Process
[0029] In a preferred embodiment, the metathesis catalysts described
herein may be
combined directly with feed oils, seed oils, biodiesel, triacylglycerides,
fatty acids, fatty acid
esters and/or fatty acid alkyl esters ("feed materials") to produce alpha-
olefins, preferably
linear alpha olefins, preferably C4 to C24 alpha-olefins, preferably C4 to C24
linear alpha-
olefins, such as preferably 1-decene, 1-heptene and or 1-butene.
[0030] Typically, the molar ratio of alkene to unsaturated feed material
(such as
unsaturated fatty acid or fatty acid ester) is greater than about 0.8/1.0,
preferably greater than
about 0.9/1Ø Typically, the molar ratio of alkene to feed material (such as
unsaturated fatty
acid or fatty acid ester) is less than about 3.0/1.0, preferably less than
about 2.0/1Ø
Depending upon the specific reagents, other molar ratios may also be suitable.
With ethylene,
for example, a significantly higher molar ratio can be used, because the self-
metathesis of
- 6 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
ethylene produces only ethylene again; no undesirable co-product olefins are
formed.
Accordingly, the molar ratio of ethylene to feed material (such as unsaturated
fatty acid or
fatty acid ester) may range from greater than about 0.8/1 to typically less
than about 20/1.
[0031] The quantity of metathesis catalyst that is employed in the
process of this
invention is any quantity that provides for an operable metathesis reaction.
Preferably, the
ratio of moles of feed material (preferably fatty acid ester and or fatty acid
alkyl ester) to
moles of metathesis catalyst is typically greater than about 10:1, preferably
greater than about
100:1, preferably greater than about 1000:1, preferably greater than about
10,000:1,
preferably greater than about 25,000:1, preferably greater than about
50,000:1, preferably
greater than about 100,000:1. Alternately, the molar ratio of feed material
(preferably fatty
acid ester and or fatty acid alkyl ester) to metathesis catalyst is typically
less than about
10,000,000:1, preferably less than about 1,000,000:1, and more preferably less
than about
500,000:1.
[0032] The contacting time of the reagents and catalyst in a batch
reactor can be any
duration, provided that the desired olefin metathesis products are obtained.
Generally, the
contacting time in a reactor is greater than about 5 minutes, and preferably
greater than about
10 minutes. Generally, the contacting time in a reactor is less than about 25
hours, preferably
less than about 15 hours, and more preferably less than about 10 hours.
[0033] In a preferred embodiment, the reactants (for example, metathesis
catalyst; feed
materials; optional alkene, optional alcohol, optional water, etc.) are
combined in a reaction
vessel at a temperature of 20 to 300 C (preferably 20 to 200 C, preferably 30
to 100 C,
preferably 40 to 60 C) and an alkene (such as ethylene) at a pressure of 0.1
to 1000 psi (0.7
kPa to 6.9 MPa) (preferably 20 to 400 psi (0.14 MPa to 2.8MPa), preferably 50
to 250 psi
(0.34 MPa to 1.7MPa)), if the alkene is present, for a residence time of 0.5
seconds to 48
hours (preferably 0.25 to 5 hours, preferably 30 minutes to 2 hours).
[0034] In certain embodiments, where the alkene is a gaseous olefin, the
olefin pressure
is greater than about 5 psig (34.5 kPa), preferably greater than about 10 psig
(68.9 kPa), and
more preferably greater than about 45 psig (310 kPa). When a diluent is used
with the
gaseous alkene, the aforementioned pressure ranges may also be suitably
employed as the
total pressure of olefin and diluent. Likewise, when a liquid alkene is
employed and the
process is conducted under an inert gaseous atmosphere, then the
aforementioned pressure
ranges may be suitably employed for the inert gas pressure.
- 7 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0035] In a preferred embodiment, from about 0.005 nmoles to about 500
nmoles,
preferably from about 0.1 to about 250 nmoles, and most preferably from about
1 to about 50
nmoles of the metathesis catalyst are charged to the reactor per 3 mmoles of
feed material
(such as triacylglycerides, biodiesel, fatty acids, fatty acid esters and/or
fatty acid alkyl esters
or mixtures thereof, preferably fatty acid esters) charged.
[0036] In a preferred embodiment, the alkene and an unsaturated fatty
acid ester or
unsaturated fatty acid are co-metathesized to form first and second product
olefins, preferably
a reduced chain first product alpha-olefin and a second product reduced chain
terminal ester
or acid-functionalized alpha-olefin. As a preferred example, the metathesis of
methyloleate
with ethylene will yield co-metathesis products of 1-decene and methyl-9-
decenoate. Both
products are alpha-olefins; the decenoate also terminates in an ester moiety
at the opposite
end of the chain from the carbon-carbon double bond. In addition to the
desired products, the
methyloleate may self-metathesize to produce small amounts of 9-octadecene, a
less desirable
product, and dimethy1-9-octadecene-1,18-dioate, CH30C(0) (CH2)7CH=CH(CH2)
7C(0)0CH3, a second less desirable product.
[0037] In the process of this invention, the conversion of feed material
(preferably fatty
acid ester and or fatty acid alkyl ester) can vary widely depending upon the
specific reagent
olefins, the specific catalyst, and specific process conditions employed. For
the purpose of
this invention, "conversion" is defined as the mole percentage of feed
material that is
converted or reacted to the cross-metathesis alpha-olefin products. Typically,
the conversion
of feed material (preferably fatty acid ester and or fatty acid alkyl ester)
is greater than about
50 mole percent, preferably greater than about 60 mole percent, and more
preferably greater
than about 70 mole percent.
[0038] In the process of this invention, the yields of first product
olefin and ester or acid-
functionalized second product olefin can also vary depending upon the specific
reagent
olefins, catalyst, and process conditions employed. For the purposes of this
invention "yield"
will be defined as the mole percentage of cross-metathesis alpha-olefin
product olefin formed
relative to the initial moles of feed material (such as fatty acid ester and
or fatty acid alkyl
ester) in the feed. Typically, the yield of alpha-olefin will be greater than
about 35 mole
percent, preferably greater than about 50 mole percent. Typically, the yield
of ester or acid-
functionalized alpha-olefin will be greater than about 35 mole percent,
preferably greater than
about 50 mole percent.
- 8 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0039] In a preferred embodiment, the process is typically a solution
process, although it
may be a bulk or high pressure process. Homogeneous processes are preferred.
(A
homogeneous process is defined to be a process where at least 90 wt% of the
product is
soluble in the reaction media.) A bulk homogeneous process is particularly
preferred. (A
bulk process is defined to be a process where reactant concentration in all
feeds to the reactor
is 70 volume % or more.) Alternately no solvent or diluent is present or added
in the reaction
medium, (except for the small amounts used as the carrier for the catalyst or
other additives,
or amounts typically found with the reactants; e.g., propane in propylene).
[0040] Suitable diluents/solvents for the process include non-
coordinating, inert liquids.
Examples include straight and branched-chain hydrocarbons such as isobutane,
butane,
pentane, isopentane, hexanes, isohexane, heptane, octane, dodecane, and
mixtures thereof;
cyclic and alicyclic hydrocarbons such as cyclohexane, cycloheptane,
methylcyclohexane,
methylcycloheptane, and mixtures thereof such as can be found commercially
(IsoparTm);
perhalogenated hydrocarbons such as perfluorinated C4_10 alkanes,
chlorobenzene, and
aromatic and alkylsubstituted aromatic compounds such as benzene, toluene,
mesitylene, and
xylene. Suitable diluents/solvents also include aromatic hydrocarbons, such as
toluene or
xylenes, and chlorinated solvents, such as dichloromethane. In a preferred
embodiment, the
feed concentration for the process is 60 volume % solvent or less, preferably
40 volume % or
less, preferably 20 volume % or less.
[0041] The process may be batch, semi-batch or continuous. As used herein,
the term
continuous means a system that operates without interruption or cessation. For
example, a
continuous process to produce a metathesis product would be one where the
reactants are
continually introduced into one or more reactors and cross-metathesis alpha-
olefin product is
continually withdrawn.
[0042] Useful reaction vessels include reactors (including continuous
stirred tank reactors,
batch reactors, reactive extruder, pipe or pump.
[0043] The processes may be conducted in either glass lined, stainless
steel or similar
type reaction equipment. Useful reaction vessels include reactors (including
continuous
stirred tank reactors, batch reactors, reactive extruder, pipe or pump,
continuous flow fixed
bed reactors, slurry reactors, fluidized bed reactors, and catalytic
distillation reactors). The
reaction zone may be fitted with one or more internal and/or external heat
exchanger(s) in
order to control undue temperature fluctuations, or to prevent "runaway"
reaction
temperatures.
- 9 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0044] If the process is conducted in a continuous flow reactor, then
the weight hourly
space velocity, given in units of grams feed material (preferably fatty acid
ester and or fatty
acid alkyl ester) per gram catalyst per hour (If), will determine the relative
quantities of feed
material to catalyst employed, as well as the residence time in the reactor of
the unsaturated
starting compound. In a flow reactor, the weight hourly space velocity of the
unsaturated
feed material (preferably fatty acid ester and or fatty acid alkyl ester) is
typically greater than
about 0.04 g feed material (preferably fatty acid ester and or fatty acid
alkyl ester) per g
catalyst per hour (If), and preferably greater than about 0.1 h-1. In a flow
reactor, the weight
hourly space velocity of the feed material (preferably fatty acid ester and or
fatty acid alkyl
ester) is typically less than about 100 If', and preferably less than about 20
h-1.
[0045] In certain embodiments, reactions utilizing the catalytic
complexes of the present
invention can be run in a biphasic mixture of solvents, in an emulsion or
suspension, or in a
lipid vesicle or bilayer.
[0046] The feed material is typically provided as a liquid phase,
preferably neat. In
particular embodiments, the feed material is provided in a liquid phase,
preferably neat; while
the alkene is provided as a gas that is dissolved in the liquid phase. In
certain embodiments,
feed material is an unsaturated fatty acid ester or unsaturated fatty acid and
is provided in a
liquid phase, preferably neat; while the alkene is a gaseous alpha-olefin,
such as for example,
ethylene, which is dissolved in the liquid phase.
[0047] Generally, the feed material is an unsaturated fatty acid ester or
unsaturated fatty
acid and is provided as a liquid at the process temperature, and it is
generally preferred to be
used neat, that is, without a diluent or solvent. The use of a solvent usually
increases recycle
requirements and increases costs. Optionally, however, if desired, a solvent
can be employed
with the alkene and/or feed material. A solvent may be desirable, for
instance, where liquid
feed material and alkene are not entirely miscible, and both then can be
solubilized in a
suitable solvent.
[0048] In a preferred embodiment, the productivity of the process is at
least 200 g of
linear alpha-olefin (such as decene-1) per mmol of catalyst per hour,
preferably at least 5000
g/mmol/hour, preferably at least 10,000 g/mmol/hr, preferably at least 300,000
g/mmol/hr.
For the purposes of this invention, "productivity" is defined to be the amount
in grams of
linear alpha-olefin produced per mmol of catalyst introduced into the reactor,
per hour.
[0049] For the purposes of this invention, selectivity is a measure of
conversion of alkene
and feed material to the cross-metathesis alpha-olefin product, and is defined
by the mole
- 10 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
percentage of product olefin formed relative to the initial moles of alkene or
feed material. In
a preferred embodiment, the selectivity of the process is at least 20 wt%
linear alpha-olefin,
based upon the weight to the material exiting the reactor, preferably at least
25%, preferably
at least 30%, preferably at least 35%.
[0050] For the purpose of this invention, catalyst turnover number (TON) is
a measure of
how active the catalyst compound is and is defined as the number of moles of
cross-
metathesis alpha-olefin product formed per mole of catalyst compound. In a
preferred
embodiment, the (TON), of the process is at least 10,000, preferably at least
50,000,
preferably at least 100,000, preferably at least 1,000,000.
[0051] In a preferred embodiment, the alpha olefin yield (when converting
unsaturated
fatty acids, unsaturated fatty acid esters, unsaturated fatty acid alkyl
esters or mixtures
thereof), defined as the mole percentage of cross metathesis alpha olefin
product formed per
mole of unsaturated fatty acids, unsaturated fatty acid esters, unsaturated
fatty acid alkyl
esters or mixtures thereof introduced into the reactor, is 30% or more,
preferably 40% or
more, preferably 45% or more, preferably 50% or more, preferably 55% or more,
preferably
60% or more.
[0052] In a preferred embodiment, the yield for reactions (when
converting
triacylglycerides as represented in the formula below), is defined as the
moles of alpha olefin
formed divided by (the moles of unsaturated Ra + moles of unsaturated Rb +
moles of
unsaturated Rc) introduced into the reactor is 30% or more, preferably 40% or
more,
preferably 45% or more, preferably 50% or more, preferably 55% or more,
preferably 60% or
more,
0
II
H2C ¨ 0 ¨ C¨ Ra
0
II
HC ¨ 0 ¨ C¨ Rb
1 0
II
H2C ¨ 0 ¨ C¨ Re
where Ra, Rb and Rc each, independently, represent a saturated or unsaturated
hydrocarbon
chain (preferably Ra, Rip and Rc each, independently, are a C12 to C28 alkyl
or alkene,
preferably C16 to C22 alkyl or alkene).
- 11 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
Alkenes
[0053] Besides the feed materials, the metathesis process of this
invention may use an
alkene as a reactant. The term "alkene" shall mean an organic compound
containing at least
one carbon-carbon double bond. Alkenes useful herein typically have less than
about 10
carbon atoms. The alkene may have one carbon-carbon unsaturated bond, or
alternatively,
two or more carbon-carbon unsaturated bonds. Since the metathesis reaction can
occur at any
double bond, alkenes having more than one double bond will produce more
metathesis
products. Accordingly, in some embodiments, it is preferred to employ an
alkene having
only one carbon-carbon double bond. The double bond may be, without
limitation, a
terminal double bond or an internal double bond. The alkene may also be
substituted at any
position along the carbon chain with one or more substituents, provided that
the one or more
substituents are essentially inert with respect to the metathesis process.
Suitable substituents
include, without limitation, alkyl, preferably C1-6 alkyl; cycloalkyl,
preferably C3_6 cycloalkyl;
as well as hydroxy, ether, keto, aldehyde, and halogen functionalities. Non-
limiting
examples of suitable alkenes include ethylene, propylene, butene, butadiene,
pentene, hexene,
the various isomers thereof, as well as higher homologues thereof Preferably,
the alkene is a
C2_8 alkene. More preferably the alkene is a C2_6 alkene, even more preferably
a C2_4 alkene,
and most preferably ethylene.
[0054] Useful alkenes include those represented by the formula: R*-HC=CH-
R*,
wherein each R* is independently, hydrogen or a Ci to C20 hydrocarbyl,
preferably hydrogen
or a C1 to C6 hydrocarbyl, preferably hydrogen, methyl, ethyl, propyl or
butyl, more
preferably R* is hydrogen. In a preferred embodiment, both R* are the same,
preferably both
R*are hydrogen. Ethylene, propylene, butene, pentene, hexene, octene and
nonene
(preferably ethylene) are alkenes useful herein.
[0055] For purposes of this invention and the claims thereto, the term
lower olefin means
an alkene represented by the formula: R*-HC=CH-R*, wherein each R* is
independently,
hydrogen or a C1 to C6 hydrocarbyl, preferably hydrogen or a Ci to C3
hydrocarbyl,
preferably hydrogen, methyl, ethyl, propyl or butyl, more preferably R* is
hydrogen. In a
preferred embodiment, both R* are the same, preferably both R*are hydrogen.
Ethylene,
propylene, butene, pentene, hexene, and octene (preferably ethylene) are lower
olefins useful
herein.
- 12 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
Triacylglycerides
[0056] Triacylglycerides (TAGs), also called triglycerides, are a
naturally occurring ester
of three fatty acids and glycerol that is the chief constituent of natural
fats and oils. The three
fatty acids can be all different, all the same, or only two the same, they can
be saturated or
unsaturated fatty acids, and the saturated fatty acids may have one or
multiple unsaturations.
Chain lengths of the fatty acids in naturally occurring triacylglycerides can
be of varying
lengths but 16, 18 and 20 carbons are the most common. Natural fatty acids
found in plants
and animals are typically composed only of even numbers of carbon atoms due to
the way
they are bio-synthesized. Most natural fats contain a complex mixture of
individual
triglycerides and because of this, they melt over a broad range of
temperatures.
[0057] Biodiesel is a mono-alkyl ester derived from the processing of
vegetable or animal
oils and alcohols. The processing is typically carried out by an
esterification reaction
mechanism, and typically is performed in an excess of alcohol to maximize
conversion.
Esterification can refer to direct esterification, such as between a free
fatty acid and an
alcohol, as well as transesterification, such as between an ester and an
alcohol. While
vegetable oil and alcohols are commonly employed as reactants in
esterification reactions, a
fatty acid source such as free fatty acids, soaps, esters, glycerides (mono-,
di- tri-),
phospholipids, lysophospholipids, or amides and a monohydric alcohol source,
such as an
alcohol or an ester, can be esterified. In addition, various combinations of
these reagents can
be employed in an esterification reaction.
[0058] Vegetable and animal oils include triglycerides and neutral fats,
such as
triacylglyderides, the main energy storage form of fat in animals and plants.
These typically
have the chemical structure:
0
II
H2C ¨ 0 ¨ C¨ Ra
0
1 1
HC ¨ 0 ¨ C¨ Rb
1 0
1 1
H2 C ¨ 0 ¨ C¨ Re
where Ra, Rb and Rc each, independently, represent a saturated or non-
saturated hydrocarbon
chain (preferably Ra, Rip and Rc each, independently, are a C12 to C28 alkyl
or alkene,
preferably C16 to C22 alkyl or alkene). Different vegetable oils have
different fatty acid
- 13 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
profiles, with the same or different fatty acids occurring on a single
glycerol. For example,
an oil can have linoleic, oleic, and stearic acids attached to the same
glycerol, with each of Ra,
Rb and Rc representing one of these three fatty acids. In another example,
there can be two
oleic acids and one stearic acid attached to the same glycerol, each of Ra,
Rip and Rc
representing one of these fatty acids. A particularly useful triglyceride
consists of three fatty
acids (e.g., saturated fatty acids of general structure of CH3(CH2)õCOOH,
wherein n is
typically an integer of from 4 to 28 or higher) attached to a glycerol
(C3H5(OH)3) backbone
by ester linkages. In the esterification process, vegetable oils and short
chain alcohols are
reacted to form mono-alkyl esters of the fatty acid and glycerol (also
referred to as glycerin).
When the alcohol used is methanol (CH3OH), a methyl ester is created with the
general form
CH3(CH2)õCOOCH3 for saturated fatty acids. Typically, but not always, the
length of the
carbon backbone chain is from 12 to 24 carbon atoms.
[0059] The esterification process can be catalyzed or non-catalyzed.
Catalyzed processes
are categorized into chemical and enzyme based processes. Chemical catalytic
methods can
employ acid and/or base catalyst mechanisms. The catalysts can be homogeneous
and/or
heterogeneous catalysts. Homogeneous catalysts are typically liquid phase
mixtures, whereas
heterogeneous catalysts are solid phase catalysts mixed with the liquid phase
reactants, oils
and alcohols.
[0060] The fatty acid rich material useful in the processes described
herein can be derived
from plant, animal, microbial, or other sources (feed oil). Preferred feed
oils include
vegetable oils such as corn, soy, rapeseed, canola, sunflower, palm and other
oils that are
readily available; however, any vegetable oil or animal fat can be employed.
Raw or
unrefined oil can be used in certain embodiments; however, filtered and
refined oils are
typically preferred. Use of degummed and filtered feedstock minimizes the
potential for
emulsification and blockage in the reactors. Feedstock with high water content
can be dried
before basic catalyst processing. Feedstock with high free fatty acid content
can be passed
through an esterification process to reduce the free fatty acid content before
the process of
esterification to convert fatty acid glycerides to monoalkyl esters. The
reduction of free fatty
acids and the conversion of fatty acid glycerides can also in the same
processing step.
Feedstock containing other organic compounds (such as hexane, heptane,
isohexane, etc.) can
typically be processed without significant modifications to the reactor. Other
materials
containing fatty acid glycerides or other fatty acid esters can also be
employed, including
phospholipids, lysophospholipids, and fatty acid wax esters. The fatty acid
rich material
- 14 -
CA 02785897 2012-06-28
WO 2011/100022
PCT/US2010/059703
useful in the processes described herein typically includes a mixture of fatty
acids. For
example, the fatty acid profiles of several useful feedstocks are shown in
Table 1. The feed
oil used as feedstock can also include a mixture of fatty acid glycerides from
different
sources. The free fatty acid content of useful vegetable oils is preferably
about 0.1 wt% or
less when employed in a basic homogeneous catalyst esterification reaction.
Higher levels
can be utilized as well, and levels up to about 3 wt%, or even as high as 15
wt% or more can
typically be tolerated.
TABLE 1
Fatty Acid Profile of Several Typical Feed Oils
Fatty Acid Palm Oil Soy Oil High Oleic
(a.k.a. Hi Oleic) Yellow Grease
Rapeseed
0 wt% 0 wt% 0 wt% 0
wt%
C6:0 0 wt% 0 wt% 0 wt% 0
wt%
C8:0 0 wt% 0 wt% 0 wt% 0
wt%
C10:0 0 wt% 0 wt% 0 wt% 0
wt%
C12:0 0 wt% 0 wt% 0 wt% 0
wt%
C14:0 1 wt% 0 wt% 0 wt% 2
wt%
C16:0 44 wt% 7 wt% 4 wt% 23
wt%
C18:0 5 wt% 5 wt% 1 wt% 13
wt%
C18:1 39 wt% 28 wt% 60 wt% 44
wt%
C18:2 10 wt% 53 wt% 21 wt% 7
wt%
C18:3 0 wt% 0 wt% 13 wt% 1
wt%
C20:0 0 wt% 0 wt% 0 wt% 0
wt%
C22:1 0 wt% 0 wt% 0 wt% 0
wt%
Misc. 1 wt% 8 wt% 0 wt% 9
wt%
Total 100 wt% 100 wt% 100 wt%
100 wt%
Alcohol (also referred to as Alkanols)
[0061]
The alcohol used herein can be any monohydric, dihydric, or polyhydric alcohol
that is capable of condensing with the feed material (such as the unsaturated
fatty acid) to
form the corresponding unsaturated ester (such as the fatty acid ester).
Typically, the alcohol
contains at least one carbon atom. Typically, the alcohol contains less than
about 20 carbon
atoms, preferably less than about 12 carbon atoms, and more preferably less
than about 8
carbon atoms. The carbon atoms may be arranged in a straight-chain or branched
structure,
- 15 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
and may be substituted with a variety of substituents, such as those
previously disclosed
hereinabove in connection with the fatty acid, including the aforementioned
alkyl, cycloalkyl,
monocyclic aromatic, arylalkyl, alkylaryl, hydroxyl, halogen, ether, ester,
aldehyde and keto
substituents. Preferably, the alcohol is a straight-chain or branched C1_12
alkanol. A preferred
alcohol is the trihydric alcohol glycerol, the fatty acid esters of which are
known as
"glycerides." Other preferred alcohols include methanol and ethanol.
[0062] Preferably, the alcohol employed in the esterification and/or
transesterification
reactions is preferably a low molecular weight monohydric alcohol such as
methanol, ethanol,
1-propanol, 2-propanol, 1-butanol, 2-butanol, or t-butanol. The alcohol is
preferably
anhydrous; however, a small amount of water in the alcohol may be present
(e.g., less than
about 2 wt%, preferably less than about 1 wt%, and most preferably less than
about 0.5 wt%;
however in certain embodiments higher amounts can be tolerated). Acid
esterification
reactions are more tolerant of the presence of small amounts of water in the
alcohol than are
basic transesterification reactions. While specific monohydric alcohols are
discussed herein
with reference to certain embodiments and examples, the preferred embodiments
are not
limited to such specific monohydric alcohols. Other suitable monohydric
alcohols can also
be employed in the preferred embodiments.
Transesterification /Esterification Reactions
[0063] The conversion of TAGs to fatty acid alkyl esters ("FAAE")
through
transesterification of the TAG typically involves forming a reactant stream,
which includes
TAG (e.g., at least about 75 wt%), alkanol (e.g., about 5 wt% to 20 wt%), a
transesterification
catalyst (e.g., about 0.05 wt% to 1 wt%), and optionally, glycerol (typically
up to about 10
wt%). Suitable alkanols may include C 1 -C6 alkanols and commonly may include
methanol,
ethanol, or mixtures thereof Suitable transesterification catalysts may
include alkali metal
alkoxides having from 1 to 6 carbon atoms and commonly may include alkali
metal
methoxide, such as sodium methoxide and/or potassium methoxide. The basic
catalyst is
desirably selected such that the alkali metal alkoxide may suitably contain an
alkoxide group
which is the counterpart of the alkanol employed in the reaction stream (e.g.,
a combination
of methanol and an alkali metal methoxide such as sodium methoxide and/or
potassium
methoxide). The reactant stream may suitably include about 0.05 wt% to 0.3 wt%
sodium
methoxide, at least about 75 wt% triacylglyceride, about 1 wt% to 7 wt%
glycerol, and at
least about 10 wt% methanol. In some embodiments, the reactant stream may
desirably
- 16 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
include about 0.05 wt% to 0.25 wt% sodium methoxide, at least about 75 wt%
triacylglyceride, about 2 wt% to 5 wt% glycerol, and about 10 wt% to 15 wt%
methanol.
[0064]
The rate and extent of reaction for esterification of the fatty acid
glycerides or
other fatty acid derivates with monohydric alcohol in the presence of a
catalyst depends upon
factors including but not limited to the concentration of the reagents, the
concentration and
type of catalyst, and the temperature and pressure conditions, and time of
reaction. The
reaction generally proceeds at temperatures above about 50 C, preferably at
temperatures
above 65 C; however, the catalyst selected or the amount of catalyst employed
can affect this
temperature to some extent. Higher temperatures generally result in faster
reaction rates.
However, the use of very high temperatures, such as those in excess of about
300 C, or even
those in excess of 250 C, can lead to increased generation of side products,
which can be
undesirable as their presence can increase downstream purification costs.
Higher
temperatures can be advantageously employed, however, e.g., in situations
where the side
products do not present an issue.
[0065] The reaction temperature can be achieved by preheating one or more
of the feed
materials or by heating a mixture of the feed materials. Heating can be
achieved using
apparatus known in the art e.g., heat exchangers, jacketed vessels, submerged
coils, and the
like. While specific temperatures and methods of obtaining the specific
temperatures are
discussed herein with reference to certain embodiments and examples, the
preferred
embodiments are not limited to such specific temperatures and methods of
obtaining the
specific temperatures. Other temperatures and methods of obtaining
temperatures can also be
employed in the preferred embodiments.
[0066]
The amount of alcohol employed in the reaction is preferably in excess of the
amount of fatty acid present on a molar basis. The fatty acid can be free or
combined, such
as to alcohol, glycol or glycerol, with up to three fatty acid moieties being
attached to a
glycerol. Additional amounts of alcohol above stoichiometric provide the
advantage of
assisting in driving the equilibrium of the reaction to produce more of the
fatty acid ester
product. However, greater excesses of alcohol can result in greater processing
costs and
larger capital investment for the larger volumes of reagents employed in the
process, as well
as greater energy costs associated with recovering, purifying, and recycling
this excess
alcohol. Accordingly, it is generally preferred to employ an amount of alcohol
yielding a
molar ratio of alcohol to fatty acid of from about 15:1 to about 1:1
(stoichiometric), and more
preferably from about 4:1 to about 2:1; however, the process can operate over
a much wider
- 17 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
range of alcohol to fatty acid ratios, with nonreacted materials subjected to
recycling or other
processing steps. Generally, lower relative levels of alcohol to fatty acid
result in decreased
yield and higher relative levels of alcohol levels to fatty acid result in
increased capital and
operating expense. Some instances of operation at ratios of alcohol to fatty
acid over a wider
range include when first starting up the process or shutting down the process,
when balancing
the throughput of the reactor to other processing steps or other processing
facilities, such as
one that produces alcohol or utilizes a side stream, or when process upsets
occur. When a
molar ratio of 2:1 methanol to fatty acid is employed and a sodium hydroxide
concentration
of about 0.5 wt% of the total reaction mixture is employed, the ratio of
sodium hydroxide to
methanol is about 2 wt% entering the reactor and about 4 wt% at the exit
because about half
of the alcohol is consumed in the esterification reaction.
[0067] Similarly, higher amounts of catalyst generally result in faster
reactions. However,
higher amounts of catalyst can lead to higher downstream separation costs and
a different
profile of side reaction products. The amount of homogeneous catalyst is
preferably from
about 0.2 wt% to about 1.0 wt% of the reaction mixture when the catalyst is
sodium
hydroxide; at typical concentration of 0.5 wt% when a 2:1 molar ratio of
methanol to fatty
acid is used; however, in certain embodiments higher or lower amounts can be
employed.
The amount of catalyst employed can also vary depending upon the nature of the
catalyst,
feed materials, operating conditions, and other factors. Specifically, the
temperature, pressure,
free fatty acid content of the feed, and degree of mixing can change the
amount of catalyst
preferably employed. While specific catalyst amounts are discussed herein with
reference to
certain embodiments and examples, the preferred embodiments are not limited to
such
specific catalyst amounts. Other suitable catalyst amounts can also be
employed in the
preferred embodiments.
[0068] The esterification reaction can be performed batchwise, such as in a
stirred tank,
or it can be performed continuously, such as in a continuous stirred tank
reactor (CSTR) or a
plug flow reactor (PFR). When operated in continuous mode, a series of
continuous reactors
(including CSTRs, PFRs, or combinations thereof) can advantageously operate in
series.
Alternatively, batch reactors can be arranged in parallel and/or series.
[0069] When the reactor is operated in a continuous fashion, one or more of
the feed
materials is preferably metered into the process. Various techniques for
metering can be
employed (e.g., metering pumps, positive displacement pumps, control valves,
flow meters,
and the like). While specific types of reactors are discussed herein with
reference to certain
- 18 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
embodiments and examples, the preferred embodiments are not limited to such
specific
reactors. Other suitable types of reactors can also be employed in the
preferred embodiments.
Fatty Acids and Fatty Acid Esters
[0070] Fatty acids are carboxylic acids with a saturated or unsaturated
aliphatic tails that
are found naturally in many different fats and oils. Any unsaturated fatty
acid can be suitably
employed in the process of this invention, provided that the unsaturated fatty
acid can be
metathesized in the manner disclosed herein. An unsaturated fatty acid
comprises a long
carbon chain containing at least one carbon-carbon double bond and terminating
in a
carboxylic acid group. Typically, the unsaturated fatty acid will contain
greater than about 8
carbon atoms, preferably greater than about 10 carbon atoms, and more
preferably greater
than about 12 carbon atoms. Typically, the unsaturated fatty acid will contain
less than about
50 carbon atoms, preferably less than about 35 carbon atoms, and more
preferably less than
about 25 carbon atoms. At least one carbon-carbon double bond is present along
the carbon
chain, this double bond usually occurring about the middle of the chain, but
not necessarily.
The carbon-carbon double bond may also occur at any other internal location
along the chain.
A terminal carbon-carbon double bond, at the opposite end of the carbon chain
relative to the
terminal carboxylic acid group, is also suitably employed, although terminal
carbon-carbon
double bonds occur less commonly in fatty acids. Unsaturated fatty acids
containing the
terminal carboxylic acid functionality and two or more carbon-carbon double
bonds may also
be suitably employed in the process of this invention. Since metathesis can
occur at any of
the carbon-carbon double bonds, a fatty acid having more than one double bond
may produce
a variety of metathesis products. The unsaturated fatty acid may be straight
or branched and
substituted along the fatty acid chain with one or more substituents, provided
that the one or
more substituents are substantially inert with respect to the metathesis
process. Non-limiting
examples of suitable substituents include alkyl moieties, preferably Ci_io
alkyl moieties,
including, for example, methyl, ethyl, propyl, butyl, and the like; cycloalkyl
moieties,
preferably C4_8 cycloalkyl moieties, including for example, cyclopentyl and
cyclohexyl;
monocyclic aromatic moieties, preferably C6 aromatic moieties, that is,
phenyl; arylalkyl
moieties, preferably C7_16 arylalkyl moieties, including, for example, benzyl;
and alkylaryl
moieties, preferably C716 alkylaryl moieties, including, for example, tolyl,
ethylphenyl, xylyl,
and the like; as well as hydroxyl, ether, keto, aldehyde, and halide,
preferably chloro and
bromo, functionalities.
- 19 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0071]
Non-limiting examples of suitable unsaturated fatty acids include 3-hexenoic
(hydrosorbic), trans-2-heptenoic, 2-octenoic, 2-nonenoic, cis-and trans-4-
decenoic, 9-
decenoic (caproleic), 10-undecenoic (undecylenic), trans-3-dodecenoic
(linderic), tridecenoic,
cis-9-tetradeceonic (myristoleic), pentadecenoic, cis-9-hexadecenoic (cis-9-
palmitoelic),
trans-9-hexadecenoic (trans-9-palmitoleic), 9-heptadecenoic, cis-6-
octadecenoic
(petroselinic), trans-6-octadecenoic (petroselaidic), cis-9-octadecenoic
(oleic), trans-9-
o ctade cenoic (elaidic), cis-11-o ctadecenoic, trans-11-o ctadecenoic
(vaccenic), cis-5-
eicosenoic, cis-9-eicosenoic (gadoleic), cis-11-docosenoic (cetoleic), cis-13-
docosenoic
(erucic), trans-13 -do co senoic (brassidic), cis-15 -tetraco s enoic
(selacholeic), cis-17-
hexacosenoic (ximenic), and cis-21-triacontenoic (lumequeic) acids, as well as
2,4-
hexadienoic (sorbic), cis-9-cis-12-octadecadienoic
(lino leic), cis-9-cis-12-cis-15-
octadecatrienoic (linolenic), eleostearic, 12-hydroxy-cis-9-octadecenoic
(ricinoleic), and like
acids. Oleic acid is most preferred. Unsaturated fatty acids can be obtained
commercially or
synthesized by saponifying fatty acid esters, this method being known to those
in the art.
[0072]
Fatty acid esters are formed by condensation of a fatty acid and an alcohol.
Fatty
acid alkyl esters are fatty acids where the hydrogen of the -OH of the acid
group is replaced
by a hydrocarbyl group, typically a C1 to Cm alkyl group, preferably a Ci to
Cm alkyl.
[0073]
Fatty acid alkyl esters are fatty acids where the hydrogen of the -OH of the
acid
group is replaced by an alkyl group. Fatty acid alkyl esters useful herein are
typically
represented by the formula: RA-C(0)-0-R*, where RA is a Ci to C100 hydrocarbyl
group,
preferably a C6 to C22 group, preferably a C6 to C14 1-alkene group, and R* is
an alkyl group,
preferably a C1 to Cm alkyl group, preferably methyl, ethyl, butyl, pentyl and
hexyl.
Preferred fatty acid alkyl esters useful herein are typically represented by
the formula: RA-
CH2=CH2-RA-C(0)-0-R*, where each RA is, independently a Ci to Cioo alkyl
group,
preferably a C6 to C20, preferably a C8 to C14 alkyl group, preferably a C9
group and R* is an
alkyl group, preferably a C1 to Cm alkyl group, preferably methyl, ethyl,
butyl, pentyl and
hexyl. Particularly preferred fatty acid alkyl esters useful herein are
represented by the
formula:
CH3-(CH2)n-C=C-(CH2)m-C(0)-0-R*,
where and R* is an alkyl group, preferably a Cl to C20 alkyl group, preferably
methyl, ethyl,
butyl pentyl and hexyl, m and n are, independently 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 11, 12, 13, 14,
15 or 16, preferably 5,7,9 or 11, preferably 7.
- 20 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0074] Fatty acid methyl esters are fatty acids where the hydrogen of
the -OH of the acid
group is replaced by methyl group. Fatty acid methyl esters useful herein are
typically
represented by the formula: RA-C(0)-0-CH3, where RA is a Ci to Cioo
hydrocarbyl group,
preferably a C6 to C22 group, preferably a C6 to C14 1-alkene group. Preferred
fatty acid
methyl esters useful herein are typically represented by the formula: RA-
CH2=CH2-RA-C(0)-
0-CH3, where each RA is, independently a C1 to C100 alkyl group, preferably a
C6 to C205
preferably a C8 to C14 alkyl group, preferably a C9 group. Particularly
preferred fatty acid
methyl esters useful herein are represented by the formula: CH3-(CH2)n-C=C-
(CH2)m-C(0)-
0-CH3, where m and n are, independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12,
13, 14, 15 or 16,
preferably 5, 7,9 or 11, preferably 7.
[0075] Preferred fatty acid methyl esters include methyl palmitoleate,
methyl oleate,
methyl gadoleate, methyl erucate, methyl linoleate, methyl linolenate, methyl
soyate, and
mixtures of methyl esters derived from soybean oil, beef tallow, tall oil,
animal fats, waste
oils/greases, rapeseed oil, algae oil, Canola oil, palm oil, Jathropa oil,
high-oleic soybean oil
(e.g., 75 mole% or more, preferably 85 mole% or more, preferably 90 mole% or
more), high-
oleic safflower oil (e.g., 75 mole% or more, preferably 85 mole% or more,
preferably 90
mole% or more), high-oleic sunflower oil (e.g., 75 mole% or more, preferably
85 mole% or
more, preferably 90 mole% or more), and other plant or animal derived sources
containing
fatty acids.
[0076] A preferred source of fatty acid methyl esters for use herein
includes TAG's and
biodiesel sources. As described above, biodiesel refers to a transesterified
vegetable oil or
animal fat based diesel fuel containing long-chain alkyl (typically methyl,
propyl, or ethyl)
esters. Biodiesel is typically made by chemically reacting lipids (such as
vegetable oil) with
an alcohol. Biodiesel, TAG's and derivatives thereof may be used in the
processes described
herein. Likewise, preferred fatty acid methyl esters useful herein may be
obtained by
reacting canola oil, corn oil, soybean oil, beef tallow, tall oil, animal
fats, waste oils/greases,
rapeseed oil, algae oil, Canola oil, palm oil, Jathropa oil, high-oleic
soybean oil, high-oleic
safflower oil, high-oleic sunflower oil or mixtures of animal and/or vegetable
fats and oils
with one or more alcohols (as described above), preferably methanol.
[0077] Vegetable oils useful herein preferably contain at least one site of
unsaturation and
include, but are not limited to, canola, soybean, palm, peanut, mustard,
sunflower, tung, tall,
perilla, grapeseed, rapeseed, linseed, safflower, pumpkin corn and other oils
extracted from
plant seeds.
-21 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0078] For purposes of this invention and the claims thereto the term
"feed oil" refers to
one or more plant, animal or microbial oils, including, but not limited to,
canola oil, corn oil,
soybean oil, fish oil, beef tallow, tall oil, animal fats, waste oils/greases,
rapeseed oil, algae
oil, peanut oil, mustard oil, sunflower oil, tung oil, perilla oil, grapeseed
oil, linseed oil,
safflower oil, pumpkin oil, palm oil, Jathropa oil, high-oleic soybean oil,
high-oleic safflower
oil, high-oleic sunflower oil, mixtures of animal and/or vegetable fats and
oils, castor bean oil,
dehydrated castor bean oil, cucumber oil, poppyseed oil, flaxseed oil,
lesquerella oil, walnut
oil, cottonseed oil, meadowfoam, tuna oil, and sesame oils.
[0079] In a preferred embodiment, a combination of oils is used herein.
Preferred
combinations include two (three or four) or more of tall oil, palm oil,
tallow, waste grease,
rapeseed oil, canola oil, soy oil and algae oil. Alternate useful combinations
include two
(three or four) or more of soy oil, canola oil, rapeseed oil, algae oil, and
tallow.
[0080] In certain embodiments processed oils, such as blown oils, are
the source of fatty
acids useful herein. While vegetable oils are preferred sources of fatty acids
for practicing
disclosed embodiments of the present process, fatty acids also are available
from animal fats
including, without limitation, lard and fish oils, such as sardine oil and
herring oil, and the
like. As noted above, in certain embodiments a desired fatty acid or fatty
acid precursor is
produced by a plant or animal found in nature. However, particular fatty acids
or fatty acid
precursors are advantageously available from genetically modified organisms,
such as
genetically modified plants, particularly genetically modified algae. Such
genetically
modified organisms are designed to produce a desired fatty acid or fatty acid
precursor
biosynthetically or to produce increased amounts of such compounds.
[0081] Alkyl oleates and alkyl erucates are fatty acid esters that are
often major
components in biodiesel produced by the transesterification of alcohol and
vegetable oils
(preferably the alkyls are a C1 to C30 alkyl group, alternately a Ci to C20
alkyl group).
Biodiesel compositions that are particularly useful in this invention are
those which have high
concentrations of alkyl oleate and alkyl erucate esters. These fatty acid
esters preferably have
one site of unsaturation such that cross-metathesis with ethylene yields 1-
decene as the
coproduct. Biodiesel compositions that are particularly useful are those
produced from
vegetable oils such as canola, rapeseed oil, palm oil, and other high oleate
oil, high erucate
oils. Particularly preferred vegetable oils include those having at least 50%
(on a molar
basis) combined oleic and erucic fatty acid chains of all fatty acid chains,
preferably 60%,
preferably 70%, preferably 80%, preferably 90%.
- 22 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[0082]
In another embodiment, useful fatty acid ester containing mixtures include
those
having at least 50% (on a molar basis) alkyl oleate fatty acid esters,
preferably 60% of alkyl
oleate fatty acid esters, preferably 70% of alkyl oleate fatty acid esters,
preferably 80% of
alkyl oleate fatty acid esters, preferably 90% of alkyl oleate fatty acid
esters.
[0083] In another embodiment, useful fatty acid ester containing mixtures
include those
having at least 50% (on a molar basis) alkyl erucate fatty acid esters,
preferably 60% of alkyl
erucate fatty acid esters, preferably 70% of alkyl erucate fatty acid esters,
preferably 80% of
alkyl erucate fatty acid esters, preferably 90% of alkyl erucate fatty acid
esters.
[0084]
In another embodiment, useful fatty acid ester containing mixtures include
those
having at least 50% (on a molar basis) combined oleic and erucic fatty acid
esters of all fatty
acid ester chains, preferably 60%, preferably 70%, preferably 80%, preferably
90%.
Isomerization
[0085]
In another embodiment, the feed material is first isomerized, then combined
with a
metathesis catalyst as described herein. For example, the processes disclosed
herein may
comprise providing a feed material (typically a fatty acid or fatty acid
derivative),
isomerizing a site of unsaturation in the feed material (typically a fatty
acid or fatty acid
derivative) to produce an isomerized feed material (typically a fatty acid or
fatty acid
derivative), and then contacting the isomerized material with an alkene in the
presence of a
metathesis catalyst. The isomerized material can be produced by isomerization
with or
without subsequent esterification or transesterification. Isomerization can be
catalyzed by
known biochemical or chemical techniques. For example, an isomerase enzyme,
such as a
linoleate isomerase, can be used to isomerize linoleic acid from the cis 9,
cis 12 isomer to the
cis 9, trans 11 isomer.
This isomerization process is stereospecific; however,
nonstereospecific processes can be used because both cis and trans isomers are
suitable for
metathesis. For example, an alternative process employs a chemical
isomerization catalyst,
such as an acidic or basic catalyst, can be used to isomerize an unsaturated
feed material
(typically a fatty acid or fatty acid derivative) having a site of
unsaturation at one location in
the molecule into an isomerized, feed material (typically a fatty acid or
fatty acid derivative)
having a site of unsaturation at a different location in the molecule. Metal
or organometallic
catalysts also can be used to isomerize an unsaturated feed material
(typically a fatty acid or
fatty acid derivative). For example, nickel catalysts are known to catalyze
positional
isomerization of unsaturated sites in fatty acid derivatives.
Similarly, esterification,
transesterification, reduction, oxidation and/or other modifications of the
starting compound
- 23 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
or products, such as a fatty acid or fatty acid derivative, can be catalyzed
by biochemical or
chemical techniques. For example, a fatty acid or fatty acid derivative can be
modified by a
lipase, esterase, reductase or other enzyme before or after isomerization. In
another
embodiment the isomerization described above may be practiced with any
triacylglycerides,
biodiesel, fatty acids, fatty acid esters and/or fatty acid alkyl esters
described herein, typically
before contacting with the metathesis catalyst.
Metathesis Catalyst Compounds
[0086] In a preferred embodiment, the metathesis catalyst compound is
represented by
the Formula (I):
X L G*
\ /
X1-----NA
/ . R1
R-----1-1
0
G G
G Formula (I)
where:
M is a Group 8 metal, preferably Ru or Os, preferably Ru;
X and Xl are, independently, any anionic ligand, preferably a halide
(preferably Cl), an
alkoxide, aryloxide, or an alkyl sulfonate, or X and Xl may be joined to form
a dianionic
group and may form single ring of up to 30 non-hydrogen atoms or a
multinuclear ring
system of up to 30 non-hydrogen atoms;
L is a neutral two electron donor, preferably a phosphine or an N-heterocyclic
carbene or a
cyclic alkyl amino carbene;
Ll is a heteroatom selected from the group consisting of N, 0, P, or S,
preferably N or 0;
L and X may be joined to form a multidentate monoanionic group and may form
single ring
of up to 30 non-hydrogen atoms or a multinuclear ring system of up to 30 non-
hydrogen
atoms;
R is a C1 to C30 hydrocarbyl or a C1 to C30 substituted hydrocarbyl;
- 24 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
G* is selected from the group consisting of hydrogen, a Ci to C30 hydrocarbyl,
and a C1 to
C30 substituted hydrocarbyl, preferably an alkyl or substituted alkyl or
hydrogen, preferably
fluorinated alkyls or hydrogen;
Rl is selected from the group consisting of hydrogen, a Ci to C30 hydrocarbyl,
and a Ci to C30
substituted hydrocarbyl, preferably methoxy- substituted phenyl, preferably
3,5- substituted
phenyl, preferably 3,5-dimethoxyphenyl; and
each G is, independently, selected from the group consisting of hydrogen,
halogen, a C1 to
C30 hydrocarbyl, and a Ci to C30 substituted hydrocarbyl hydrogen, (preferably
a Ci to C30
alkyl or a substituted C1 to C30 alkyl, or a C5 to C30 aryl or a substituted
C5 to C30 aryl).
[0087] For purposes of this invention and claims thereto, a "Group 8 metal"
is an element
from Group 8 of the Periodic Table, as referenced by the IUPAC in Nomenclature
of
Inorganic Chemistry: Recommendations 1990, G.J. Leigh, Editor, Blackwell
Scientific
Publications, 1990.
[0088] For purposes of this invention and claims thereto a substituted
hydrocarbyl is a
radical made of carbon and hydrogen where at least one hydrogen is replaced by
a
heteroatom. For purposes of this invention and claims thereto a substituted
alkyl or aryl
group is a radical made of carbon and hydrogen where at least one hydrogen is
replaced by a
heteroatom or a linear, branched, or cyclic substituted or unsubstituted
hydrocarbyl group
having 1 to 30 carbon atoms.
[0089] For purposes of this invention and claims thereto, "alkoxides"
include those where
the alkyl group is a C1 to C10 hydrocarbyl. The alkyl group may be straight
chain or branched.
Preferred alkoxides include a C1 to C10 alkyl group, preferably methyl, ethyl,
propyl, butyl, or
isopropyl. Preferred alkoxides include those where the alkyl group is a
phenol, substituted
phenol (where the phenol may be substituted with up to 1, 2, 3, 4 or 5 C1 to
C12 hydrocarbyl
groups) or a C1 to Cio hydrocarbyl, preferably a Ci to Ci0 alkyl group,
preferably methyl,
ethyl, propyl, butyl, or phenyl.
[0090] Preferred alkyl sulfonates are represented by the Formula (II):
R2
0 = S-0-
I I
0 Formula (II)
- 25 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
where R2 is a C1 to C30 hydrocarbyl group, fluoro-substituted carbyl group,
chloro-substituted
carbyl group, aryl group, or substituted aryl group, preferably a C1 to C12
alkyl or aryl group,
preferably trifluoromethyl, methyl, phenyl, para-methyl-phenyl.
[0091]
For purposes of this invention and claims thereto, "aryloxides" include those
where the aryl group is a phenol or naphthalene, or substituted phenol or
substituted
naphthalene, where the phenol or naphthalene may be substituted with one or
more
substituents. (Substituted meaning that a hydrogen group is replaced by a
heteroatom or by a
linear, branched, or cyclic hydrocarbyl group having 1 to 30 carbon atoms.)
Suitable
substituents are independently selected and may comprise halogen, C1 to C12
hydrocarbyl
groups, substituted C1 to C12 hydrocarbyl groups, preferably halogen,
trifluoromethyl, amino,
alkyl, alkoxy, alkylcarbonyl, cyano, carbamoyl, alkoxycarbamoyl,
methylendioxy, carboxyl,
alkoxycarbonyl, aminocarbonyl, alkyaminocarbonyl, dialkylaminocarbonyl,
hydroxy, nitro
and the like, more preferably phenyl, chlorophenyl, trifluoromethylphenyl,
chlorofluorophenyl, aminophenyl, methylcarbonylphenyl,
methoxyphenyl,
methylendioxyphenyl, 1-naphthyl and 2-naphthyl.
[0092]
For purposes of this invention and claims thereto, "phosphines" may be
represented by the formula PR3, wherein R is independently selected from the
group
comprising hydrogen, Ci to C12 hydrocarbyl groups, substituted C1 to C12
hydrocarbyl groups,
and halides.
[0093] For purposes of this invention and claims thereto, "N-heterocyclic
carbenes"
(NHCs) are represented by the Formula (III):
R4
/
N
/ =
Q A )
=
N
\
R4
Formula (III)
wherein the ring A is a 4-, 5-, 6-, or 7-membered ring, and Q is a linking
group comprising
from one to four linked vertex atoms selected from the group comprising C, 0,
N, B, Al, P, S
and Si with available valences optionally occupied by hydrogen, oxo or R-
substituents,
wherein R is independently selected from the group comprising C 1 to Ci2
hydrocarbyl groups,
substituted C1 to C12 hydrocarbyl groups, and halides, and each R4 is
independently a
- 26 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
hydrocarbyl group or substituted hydrocarbyl group having 1 to 40 carbon
atoms, preferably
methyl, ethyl, propyl, butyl (including isobutyl and n-butyl), pentyl,
cyclopentyl, hexyl,
cyclohexyl, octyl, cyclooctyl, nonyl, decyl, cyclodecyl, dodecyl,
cyclododecyl, mesityl,
adamantyl, phenyl, benzyl, tolulyl, chlorophenyl, phenol, or substituted
phenol.
R4 R4
R5 / N R5 /
=
I )6 N
=
NN
R5 R5
\ a \
Formula (IV) or Formula (V)
where
each R4 is independently a hydrocarbyl group or substituted hydrocarbyl group
having 1 to 40
carbon atoms, preferably methyl, ethyl, propyl, butyl (including isobutyl and
n-butyl), pentyl,
cyclopentyl, hexyl, cyclohexyl, octyl, cyclooctyl, nonyl, decyl, cyclodecyl,
dodecyl,
cyclododecyl, mesityl, adamantyl, phenyl, benzyl, tolulyl, chlorophenyl,
phenol, substituted
phenol, or CH2C(CH3)3; and
each R5 is independently a hydrogen, a halogen, a Ci to C12 hydrocarbyl group,
or a C1 to C12
substituted hydrocarbyl group, preferably hydrogen, bromine, chlorine, methyl,
ethyl, propyl,
butyl, or aryl.
[0095] In other useful embodiments, one of the N groups bound to the
carbene in
Formulae (IV) or (V) is replaced with another heteroatom, preferably S, 0 or
P, preferably an
S heteroatom. Other useful N-heterocyclic carbenes include the compounds
described in
Hermann, W. A. Chem. Eur. J. 1996, 2, 772 and 1627; Enders, D. et al., Angew.
Chem. Int.
Ed. 1995, 34, 1021; Alder R. W., Angew. Chem. Int. Ed. 1996, 35, 1121; and
Bertrand, G. et
al., Chem. Rev. 2000, 100, 39.
[0096] For purposes of this invention and claims thereto, "cyclic alkyl
amino carbenes"
(CAACs) are represented by the Formula (VI):
-27 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
R4
/
N
e
R4
Formula (VI)
wherein the ring A is a 4-, 5-, 6-, or 7-membered ring, and Q is a linking
group comprising
from one to four linked vertex atoms selected from the group comprising C, 0,
N, B, Al, P, S
and Si with available valences optionally occupied by hydrogen, oxo or R-
substituents,
wherein R is independently selected from the group comprising C1 to C12
hydrocarbyl groups,
substituted Ci to C12 hydrocarbyl groups, and halides, and each R4 is
independently a
hydrocarbyl group or substituted hydrocarbyl group having 1 to 40 carbon
atoms, preferably
methyl, ethyl, propyl, butyl (including isobutyl and n-butyl), pentyl,
cyclopentyl, hexyl,
cyclohexyl, octyl, cyclooctyl, nonyl, decyl, cyclodecyl, dodecyl,
cyclododecyl, mesityl,
adamantyl, phenyl, benzyl, tolulyl, chlorophenyl, phenol, or substituted
phenol.
[0097] Some particularly useful CAACs include:
C C
= = = =
[0098] Other useful CAACs include the compounds described in U.S.
7,312,331 and
Bertrand et al, Angew. Chem. Int. Ed. 2005, 44, 7236-7239.
[0099] Some preferred metathesis catalyst compounds include:
- 28 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
/
Cr + C +
Ck I F F
R
Ru
CI' # * 0 F CI' #u * 11,
0 lp
0 ilp F
F 0---
........./0 /0
\
P
PCy3 Cy3
CI, 1 0' CI, 1
R F F
alu * 0 CI' #u Olt 1.= F
0---
0 . r() = F
F
/0 ........./0
\
/ ____________________ \
0, NN N *
C
Ck / F F
Ru
CI' # * 41* F
0 illpe F F
,......,/0
\
[00100] Although the catalyst compounds herein are described with respect to
olefin cross-
metathesis, one of skill in the art will appreciate that the catalyst
compounds of this invention
may be suitable for any metathesis reaction, including but not limited to,
ring-closing
metathesis, enyne metathesis, acyclic diene metathesis, and so on.
[00101] In certain embodiments, the catalyst compound employed in the process
of this
invention may be bound to or deposited on a solid catalyst support. The solid
catalyst
support will render the catalyst compound heterogeneous, which will simplify
catalyst
recovery. In addition, the catalyst support may increase catalyst strength and
attrition
resistance. Suitable catalyst supports include, without limitation, silicas,
aluminas, silica-
aluminas, aluminosilicates, including zeolites and other crystalline
porousaluminosilicates; as
- 29 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
well as titanias, zirconia, magnesium oxide, carbon, and cross-linked,
reticular polymeric
resins, such as functionalized cross-linked polystyrenes, e.g., chloromethyl-
functionalized
cross-linked polystyrenes. The catalyst compound may be deposited onto the
support by any
method known to those skilled in the art, including, for example,
impregnation, ion-exchange,
deposition-precipitation, and vapor deposition. Alternatively, the catalyst
compound may be
chemically bound to the support via one or more covalent chemical bonds, for
example, the
catalyst compound may be immobilized by one or more covalent bonds with one or
more of
substituents of the indenylene ligand.
[00102] If a catalyst support is used, the catalyst compound may be loaded
onto the
catalyst support in any amount, provided that the metathesis process of this
invention
proceeds to the desired metathesis products. Generally, the catalyst compound
is loaded onto
the support in an amount that is greater than about 0.01 wt% of the Group 8
metal, and
preferably greater than about 0.05 wt% of the Group 8 metal, based on the
total weight of the
catalyst compound plus support. Generally, the catalyst compound is loaded
onto the support
in an amount that is less than about 20 wt% of the Group 8 metal, and
preferably less than
about 10 wt% of the Group 8 metal, based on the total weight of the catalyst
compound and
support.
Synthesis of Metathesis Catalyst Compounds
[00103] The catalyst compounds described herein may be synthesized by any
methods
known to those skilled in the art.
[00104] Representative methods of synthesizing the Group 8 catalyst compound
of the
type described herein include, for example, treating a solution of the ligand
complex in a
suitable solvent, such as THF, with a reactant complex of a Group 8 metal,
such as dichloro-
bis-(triphenylphosphine)ruthenium (II) and acetyl chloride. The mixture may be
heated, for
example to reflux, for a time period appropriate to yield the desired
chelating indenylene
catalyst compound. Typically, removal of the volatiles affords the Group 8
chelating
indenylene catalyst compound, which may optionally be purified by suitable
chromatographical methods, as known in the art.
[00105] A phosphine ligand, such as tricyclohexylphosphine may be added
thereafter, if
desired. The reaction conditions typically include mixing the Group 8 reactant
catalyst
compound and the preferred phosphine ligand in a suitable solvent, such as
benzene, for a
time sufficient to effectuate the phosphine ligand exchange, at a suitable
temperature
typically ambient. Copper (I) chloride is then added in excess and removal of
the volatiles
- 30 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
from resultant slurry typically affords the Group 8 chelating indenylene
catalyst compound
comprising the more preferred phosphine ligand.
[00106] While the present invention describes a variety of transition metal
complexes
useful in catalyzing metathesis reactions, it should be noted that such
complexes may be
formed in situ. Accordingly, additional ligands may be added to a reaction
solution as
separate compounds, or may be complexed to the metal center to form a metal-
ligand
complex prior to introduction to the reaction.
Alpha-Olefin Products of the Metathesis Reaction
[00107] In a preferred embodiment, the processes described herein produce an
alpha olefin,
preferably a linear alpha-olefin, which contains at least one more carbon than
the alkene used
in the reaction to make the alpha-olefin.
[00108] In another embodiment, the processes described herein produce a blend
of an
alpha olefin and an ester-functionalized alpha olefin. Generally a mixture of
non-ester-
containing alpha olefins will be produced due to the presence of mono-, di-,
and tri-
unsubstituted fatty acid chains. The major alpha olefin products are typically
1-decene, 1-
heptene, and 1-butene. The major ester-containing alpha olefin product is
typically methyl 9-
decenoate.
[00109] In a preferred embodiment, the alpha olefin produced herein is 1-
decene.
Typically the co-product of 1-decene is an ester.
[00110] In a preferred embodiment, the major alpha olefin produced herein is 1-
decene.
Typically the coproduct of 1-decene is an ester.
[00111] In a preferred embodiment, ethylene and methyl oleate are combined
with the
metathesis catalysts described herein (such as
triphenylphosphinedichlorideruthenium(3-(3,5-
dimethoxypheny1)-6,8-dimethoxyinden-1-ylidene);
triphenylphosphinedichlorideruthenium(3-pentafluoropheny1-6,8-
diisopropoxyinden-1-
ylidene); and/or tricyclohexylphosphinedichlorideruthenium (3 -p
entafluoropheny1-6,8-
diisopropoxyinden- 1 -ylidene)) to produce 1-decene and methyl 9-decenoate.
[00112] Separation of the 1-olefin (such as the 1-decene) from the ester may
be by means
typically known in the art such as distillation or filtration.
[00113] The linear alpha-olefin cross-metathesis product (such as 1-decene or
a mixture of
C8, C10, C12 linear alpha olefins) is then separated from any esters present
and preferably used
to make poly-alpha-olefins(PA05). Specifically, PAOs may be produced by
the
polymerization of olefin feed in the presence of a catalyst such as A1C13,
BF3, or BF3
-31 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
complexes. Processes for the production of PAOs are disclosed, for example, in
the
following patents: U.S. Patents 3,149,178; 3,382,291; 3,742,082; 3,769,363;
3,780,128;
4,172,855; and 4,956,122, which are fully incorporated by reference. PAOs are
also
discussed in Will, J.G. Lubrication Fundamentals, Marcel Dekker: New York,
1980.
Certain high viscosity index PAO's may also be conveniently made by the
polymerization of
an alpha-olefin in the presence of a polymerization catalyst such as Friedel-
Crafts catalysts.
These include, for example, aluminum trichloride, boron trifluoride, aluminum
trichloride or
boron trifluoride promoted with water, with alcohols such as ethanol,
propanol, or butanol,
with carboxylic acids, or with esters such as ethyl acetate or ethyl
propionate or ether such as
diethyl ether, diisopropyl ether, etc., see for example, the methods disclosed
by U.S. Patents
4,149,178; 3,382,29; 3,742,082; 3,769,363 (Brennan); 3,876,720; 4,239,930;
4,367,352;
4,413,156; 4,434,408; 4,910,355; 4,956,122; 5,068,487; 4,827,073; 4,827,064;
4,967,032; 4,926,004; and 4,914,254. PAO's can also be made using various
metallocene
catalyst systems. Examples include US Patents 6,706,828; 5,688,887; 6,043,401;
6,548,724;
5,087,788; 6,414,090; 6,414,091; 4,704,491; 6,133,209; 6,713,438; WO 96/23751;
WO
03/020856; and EP 0 613 873.
[00114] PAOs are often used as additives and base stocks for lubricants, among
other
things. Additional information on the use of PAO's in the formulations of full
synthetic,
semi-synthetic or part synthetic lubricant or functional fluids can be found
in "Synthetic
Lubricants and High-Performance Functional Fluids", 2nd Ed. L. Rudnick, etc.
Marcel
Dekker, Inc., N.Y. (1999). Additional information on additives used in product
formulation
can be found in "Lubricants and Lubrications, Ed. By T. Mang and W. Dresel, by
Wiley-
VCH GmbH, Weinheim 2001.
[00115] In another embodiment this invention relates to:
1. A metathesis catalyst compound represented by the formula:
- 32 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
X L G*
\ /
X1----m
/ = R1
R---L1
0
G G
G
wherein M is a Group 8 metal; X and Xl are anionic ligands; L is a neutral two
electron
donor; Ll is N, 0, P, or S, preferably N or 0; R is a Ci to C30 hydrocarbyl or
a C1 to C30
substituted hydrocarbyl; G* is selected from the group consisting of hydrogen,
a Ci to C30
hydrocarbyl, and a C1 to C30 substituted hydrocarbyl; Rl is selected from the
group consisting
of hydrogen, a Ci to C30 hydrocarbyl, and a Ci to C30 substituted hydrocarbyl;
and G is
independently selected from the group consisting of hydrogen, halogen, Ci to
C30
hydrocarbyls and C1 to C30 substituted hydrocarbyls, preferably the compound
comprises one
or more of: triphenylphosphinedichlorideruthenium(3-(3,5-dimethoxypheny1)- 6,8-
dimethoxyinden-l-ylidene); triphenylphosphinedichlorideruthenium(3-
pentafluoropheny1-
6,8-diisopropoxyinden-1-ylidene); tricyclohexylphosphinedichlorideruthenium (3-
pentafluoropheny1-6,8-diisopropoxyinden-1-ylidene); or mixtures thereof
2. The catalyst compound of paragraph 1, wherein M is Ru.
3. The catalyst compound of paragraph 1 or 2, wherein X and Xl are,
independently, a
halogen, an alkoxide, aryloxide, or an alkyl sulfonate.
4. The catalyst compound of any of paragraphs 1 to 3, wherein at least of X
and Xl is
chloride, preferably both X and Xl are chloride.
5. The catalyst compound of any of paragraphs 1 to 4, wherein Ll is N or 0.
6. The catalyst compound of any of paragraphs 1 to 5, wherein L is selected
from the
group consisting of a phosphine, an N-heterocyclic carbene, and a cyclic alkyl
amino carbene.
7. The catalyst compound of any of paragraphs 1 to 6, wherein G* is
selected from the
group consisting of hydrogen, an alkyl, and substituted alkyl.
8. The catalyst compound of any of paragraphs 1 to 7, wherein each G is
independently,
a C1 to C30 substituted or unsubstituted alkyl, or a substituted or
unsubstituted C4 to C30 aryl.
- 33 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
9. The catalyst compound of any of paragraphs 1 to 8, wherein Rl is a
methoxy
substituted phenyl.
10. The catalyst compound of any of paragraphs 1 to 9, wherein L and X are
joined to
form a multidentate monoanionic group or a dianionic group and may form a
single ring of
up to 30 non-hydrogen atoms or a multinuclear ring system of up to 30 non-
hydrogen atoms.
11. A process to produce alpha-olefin comprising contacting a feed material
(such as a
feed oil) with the catalyst compound of any of paragraphs 1 to 10.
12. The process of paragraph 11, wherein the feed material is selected from
the group
consisting of canola oil, corn oil, soybean oil, rapeseed oil, algae oil,
peanut oil, mustard oil,
sunflower oil, tung oil, perilla oil, grapeseed oil, linseed oil, safflower
oil, pumpkin oil, palm
oil, Jathropa oil, high-oleic soybean oil, high-oleic safflower oil, high-
oleic sunflower oil,
mixtures of animal and vegetable fats and oils, castor bean oil, dehydrated
castor bean oil,
cucumber oil, poppyseed oil, flaxseed oil, lesquerella oil, walnut oil,
cottonseed oil,
meadowfoam, tuna oil, sesame oils and mixtures thereof.
13. The process of paragraph 11, wherein the feed material is selected from
the group
consisting of palm oil and algae oil.
14. A process to produce alpha-olefin comprising contacting a
triacylglyceride with an
alkene and the catalyst compound of any of paragraphs 1 to 10, preferably
wherein the alpha
olefin produced has at least one more carbon atom than the alkene.
15. The process of paragraph 14, wherein the triacylglyceride is contacted
with alcohol
and converted to a fatty acid ester or fatty acid alkyl ester prior to
contacting with the catalyst
compound of any of paragraphs 1 to 10.
16. The process of paragraph 14, wherein the triacylglyceride is contacted
with water or
an alkaline reagent and converted to a fatty acid prior to contacting with the
catalyst
compound of any of paragraphs 1 to 10.
17. A process to produce alpha-olefin comprising contacting an unsaturated
fatty acid
with an alkene and the catalyst compound of any of paragraphs 1 to 10,
preferably wherein
the alpha olefin produced has at least one more carbon atom than the alkene.
18. A process to produce alpha-olefin comprising contacting a
triacylglyceride with the
catalyst compound of any of paragraphs 1 to 10, preferably wherein the alpha
olefin produced
has at least one more carbon atom than the alkene.
19. A process to produce alpha-olefin comprising contacting an unsaturated
fatty acid
ester and or unsaturated fatty acid alkyl ester with an alkene and the
catalyst compound of
- 34 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
any of paragraphs 1 to 10, preferably wherein the alpha olefin produced has at
least one more
carbon atom than the alkene.
20. The process of any of paragraphs 11 to 19, wherein the alpha olefin
is a linear alpha-
olefin having 4 to 24 carbon atoms.
21. The process of any of paragraphs 11 to 20, wherein the alkene is
ethylene, propylene,
butene, hexene or octene.
22. The process of any of paragraphs 19 to 21, where the fatty acid ester
is a fatty acid
methyl ester.
23. The process of any of paragraphs 14 to 22, wherein the
triacylglyceride, fatty acid,
fatty acid alkyl ester, fatty acid ester is derived from biodiesel.
24. The process of any of paragraphs 11 to 23, wherein the alpha-olefin is
butene-1,
decene-1 and or heptene-1.
25. The process of any of paragraphs 11 to 24, wherein the productivity of
the process is
at least 200 g of linear alpha-olefin per mmol of catalyst per hour.
26. The process of any of paragraphs 11 to 25, wherein the selectivity of
the process is at
least 20 wt% linear alpha-olefin, based upon the weight to the material
exiting the reactor.
27. The process of any of paragraphs 11 to 26, wherein the turnover number,
defined as
the moles of alpha olefin formed per mol of catalyst, of the process is at
least 10,000.
28. The process of any of paragraphs 11 to 27, wherein the yield, when
converting
unsaturated fatty acids, unsaturated fatty acid esters, unsaturated fatty acid
alkyl esters or
mixtures thereof, is 30% or more, said yield being defined as the moles of
alpha olefin
formed per mol of unsaturated fatty acids, unsaturated fatty acid esters,
unsaturated fatty acid
alkyl esters or mixtures thereof introduced into the reactor.
29. The process of any of paragraphs 11 to 27, wherein the yield, when
converting TAGs
as represented in the formula below, is 30% or more, said yield being defined
as the moles of
alpha olefin formed divided by (the moles of unsaturated Ra + moles of
unsaturated Rb +
moles of unsaturated Rc) introduced into the reactor,
0
II
H2C ¨ 0 ¨ C¨ Ra
0
ii
HC ¨ 0 ¨ C¨ Rb
1 0
ii
H2C ¨ 0 ¨ C¨ Re
- 35 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
where Ra, Rb and Rc each, independently, represent a saturated or unsaturated
hydrocarbon
chain.
30. The process of paragraph 28, wherein the yield is 60% or more.
31. A process to produce C4 to C24 linear alpha-olefin comprising
contacting a feed
material with an alkene selected from the group consisting of ethylene,
propylene butene,
pentene, hexene, heptene, octene, nonene and mixtures thereof and a metathesis
catalyst
compound of any of paragraphs 1 to 10, wherein the feed material is a
triacylglyceride, fatty
acid, fatty acid alkyl ester, and/or fatty acid ester derived from seed oil.
32. The process of paragraph 31, wherein the alkene is ethylene, the alpha
olefin is 1-
butene, 1-heptene and or -decene, and the feed material is a fatty acid methyl
ester, and/or
fatty acid ester.
Experimental Section
[00116] For purposes of this invention and the claims thereto, Et is ethyl, Me
is methyl, Ph
is phenyl, Cy is cyclohexyl, THF is tetrahydrofuran, AcC1 is acetyl chloride,
DMF is
dimethylformamide, and TLC is thin layer chromatography.
[00117] Typical dry-box procedures for synthesis of air-sensitive compounds
were
followed including using dried glassware (90 C, 4 hours) and anhydrous
solvents purchased
from Sigma Aldrich (St. Louis, MO) which were further dried over 3 A sieves.
All reagents
were purchased from Sigma-Aldrich, unless otherwise noted. 1H5
u and 3113 spectra were
recorded on Bruker 250 and 500 spectrometers. IR data was recorded on Bruker
Tensor 27
FT-IR spectrometer. Yields of metathesis product and catalyst turnover numbers
were
calculated from data recorded on an Agilent 6890 GC spectrometer as shown
below.
[00118] Typically, a sample of the metathesis product will be taken and
analyzed by GC.
An internal standard, usually tetradecane, is used to derive the amount of
metathesis product
that is obtained. The amount of metathesis product is calculated from the area
under the
desired peak on the GC trace, relative to the internal standard.
[00119] Yield is reported as a percentage and defined as 100 x [micromoles of
metathesis
products obtained by GC]/[micromoles of feed material weighed into reactor].
Selectivity is
reported as a percentage and is defined as 100 x [area under the peak of
desired metathesis
products]/[sum of peak areas of cross-metathesis and the homometathesis
products]. Catalyst
turnovers for production of the metathesis products is defined as the
[micromoles of
metathesis product]/([micromoles of catalyst].
- 36 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[00120] In a particular embodiment, the metathesis of methyl oleate with
ethylene will
yield co-metathesis products of 1-decene and methyl-9-decenoate. In addition
to the desired
products, the methyl oleate may homometathesize to produce small amounts of 9-
octadecene,
a less desirable product, and 1,18-dimethy1-9-octadecenedioate, a second less
desirable
product. Yield is defined as 100 x [micromoles of ethenolysis products
obtained from the
GC]/[micromoles of methyl oleate weighed into reactor]. 1-decene selectivity
is shown as a
percentage and is defined as 100 x [GC peak area of 1-decene & methyl-9-
decenoate]/[sum
of GC peak areas of 1-decene, methyl-9-decenoate, and the homometathesis
products, 9-
octadecene, and 1,18-dimethy1-9-octadecenedioate]. Catalyst turnovers for
production of the
1-decene is defined as the [micromoles of 1-decene obtained from the
GC]/([micromoles of
catalyst].
Examples
[00121] Synthetic protocols for representative alkylidene ligands and the
corresponding
ruthenium alkylidene complexes are as follows. Other alkylidene ligands and
their respective
metal complexes may be derived analogously.
Example 1: Synthesis of (PPh3)C12Ru(3-3,5-dimethoxypheny1-6,8-dimethoxyinden-1-
ylidene)
[00122] Bis(3,5-dimethoxyphenyl)methanol (A): 3,5-Dimethoxybenzaldehyde (5.0
g, 30
mmol) was dissolved in 150 mL THF in a 500 mL round bottom flask. 3,5-
Dimethoxyphenyl
magnesium chloride (1 M in THF, 45 mL) was added slowly. The reaction was
heated at
40 C for 4 hours, then quenched with saturated ammonium chloride. The mixture
was
extracted with 3 portions of ether and the combined organic layers washed with
brine, dried
over anhydrous Mg504, then concentrated to a crude pale yellow solid which was
carried
forward to the next step.: 1H NMR (250 MHz, C6D6): 6 3.29 (d, J = 5.0 Hz,
12H), 6.46 (m,
2H), 6.56 (m, 1H), 6.76 (m, 2H), 7.04 (t, J = 8.2 Hz, 1H).
[00123] Bis(3,5-dimethoxyphenyl)methanone (B): Pyridinium chlorochromate (PCC)
(12.9 g, 30 mmol) was suspended in 30 mL dichloromethane in a 200 mL round
bottom flask.
Crude bis(3,5-dimethoxyphenyl)methanol from above (compound A) was suspended
in 30
mL dichloromethane then added to the chromate suspension. The dark solution
was allowed
to stir at ambient temperature for 18 hours then diluted with ether. After
decantation, the
organic solution was washed twice with 1N NaOH, twice with 10% HC1, saturated
NaHCO3,
and then with brine. It was dried over anhydrous Mg504, filtered and
concentrated to give a
brownish yellow solid. The brownish yellow solid was purified by column
chromatography
-37 -
CA 02785897 2012-06-28
WO 2011/100022
PCT/US2010/059703
using 50% acetone/hexane as eluent giving the product as a yellow solid in 63%
yield over 2
steps: IR (cm-1): 2960, 2938, 2834, 1660, 1592, 1456, 1425, 1349, 1304, 1205,
1157, 1066,
744; 1H NMR (250 MHz, C6D6): 6 3.21 (s, 12H), 6.67 (t, J = 2.2 Hz, 2H), 7.21
(d, J = 2.5 Hz,
4H); 13C NMR (63 MHz, C6D6): 54.9 (4C), 105.3 (2C), 108.0 (4C), 140.2 (2C),
161.1 (4C),
195.2.
[00124] 1,1-Bis(3,5-dimethoxyphenyl)prop-2-yn-1-ol (C): In a 100 mL
flask, bis(3,5-
dimethoxy-phenyl)methanone (compound B, 1.2 g, 3.9 mmol) was dissolved in 20
mL
diethyl ether. Approximately 5 mL THF was added to help solvate the ketone
followed by
the slow addition of ethynylmagnesium bromide (0.5 M in THF, 12 mL). The
reaction was
monitored by TLC and upon consumption of starting material, 2N HC1 was added
to the flask.
The mixture was extracted 3 times with ethyl acetate and the combined organic
layers were
washed with brine, dried over anhydrous MgSO4, filtered, and concentrated to
give a yellow
oil. Purification was achieved with column chromatography using a gradient of
30% to 50%
acetone/hexane. The product was obtained as a pale yellow oil in 73% yield: Rf
0.14 (30:70
acetone/hexane); IR (cm-1): 3441, 3280, 2940, 2837, 1598, 1460, 1289, 1205,
1156, 1053,
834, 748, 689; 1H NMR (250 MHz, C6D6): 6 2.38 (s, 1H), 2.94 (br s, 1H), 3.27
(s, 12H), 6.42
(t, J = 2.5 Hz, 2H), 3.99 (d, J = 2.5 Hz, 4H); 13C NMR (63 MHz, C6D6): 54.8
(4C), 74.5, 75.3,
86.8, 100.1 (2C), 104.9 (4C), 147.6 (2C), 161.2 (4C).
Scheme 1: Synthesis of 1,1-bis(3,5-dimethoxyphenyl)prop-2-yn-1-ol (C)
OH
0 Me0+ _ s is OMe
1
Me0 Me0 MgCI PCC
,,..
CH2Cl2, 63%
OMe OMe
OMe OMe A
0 \\ OH
Me0 0 0 OMe ¨ MgBr Me0 is is OMe
ether, 73%
OMe OMe OMe OMe
B C
[00125] (PPh3)C12Ru(3-3,5-dimethoxypheny1-6,8-dimethoxyinden-1-ylidene) (D):
Acetyl chloride (5 - 10 1) was added to a solution of (PPh3)3RuC12 (336 mg,
0.35 mmol) and
1,1 di(3,5-dimethoxy)phenyl 2-propyn-1-ol (compound C, 172 mg, 0.525 mmol) in
6 mL
THF. The propynol was added as a 0.2 M solution in THF. The solution was
allowed to
reflux for 18 hours, after which the reaction flask was placed under high
vacuum to remove
the solvent. Isopropanol (12 mL) was added to the reaction flask and the
purple material was
- 38 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
removed from the walls by intense stirring overnight. The resulting suspension
was filtered
and washed with 5 mL of isopropanol followed by two pentane washes (5 mL
each). Any
remaining solvent was removed from the red-brown powder in vacuo at 60 C,
yielding 240
mg (92%). The product was characterized by NMR spectra (1H, 13C, and 31P). The
results
are as below:
[00126] 1H NMR (250 MHz, CD2C12, 30 C): 6= 7.4 (bt, 11H), 6.0-7.0 (m,
6H), 4.57 (s,
0.5H), 3.74-4.0 (m, 6H, R-OCH3 x 2), 3.64 (s, 6H, R-OCH3 x 2). 13C NMR (500
MHz,
CD2C12, 30 C): 6= 289.7 (d, Jpc = 100 Hz). 31P NMR (250 MHz, CD2C12, 30 C): 6=
54 ppm.
Scheme 2: Synthesis of (PPh )C12Ru(3 -3,5 -dimethoxypheny1-6,8-dimethoxyinden-
1-ylidene)
(D)
HO
Me0 OMe CI PPh3
\/
AcCI, THF CI-11-Ru
OMe OMe 18h, reflux Me0 *
OMe
OMe
CI
Ru¨P(Ph3)3
Cl,
Example 2: Synthesis of (PPh3)021th(3-pentatluorophenyl-6,8-diisopropoxyinden-
1-
ylidene) (J)
[00127] Isopropyl 3,5-diisopropoxybenzoate (E): In a 1 L round bottom flask,
3,5-
dihydroxybenzoic acid (10g, 64 mmol), potassium carbonate (42 g, 260 mmol) and
cesium
carbonate (30 g, 92 mmol) were dissolved in 300 mL dimethylformamide. After
stirring at
ambient temperature for approximately 20 min, 2-iodopropane (43 g, 256 mmol)
was added.
The reaction was allowed to stir overnight, then quenched with water and
extracted with three
portions of ethyl acetate. The combined organic layers were washed twice with
both water
and brine, then dried (Mg504), filtered and concentrated to a yellow oil: Rf
0.48 (30:70
acetone/hexane); IR (cm-1): 2978, 2935, 1715, 1593, 1449, 1372, 1296, 1234,
1183, 1112,
1038, 769; 1H NMR (250 MHz, C6D6): 6 1.07 (dd, J = 6.7, 12.0 Hz, 18H), 4.20
(qn, J = 6.2
Hz, 2H), 5.22 (qn, J = 6.2 Hz, 1H), 6.75 (t, J = 2.5 Hz, 1H), 7.56 (d, J = 2.5
Hz, 2H); 13C
NMR (63 MHz, C6D6): 23.7 (2C), 23.8 (4C), 70.2, 71.8 (2C), 110.9, 111.1 (2C),
135.5, 161.6
(2C), 167.9.
- 39 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
[00128] 3,5-diisopropoxybenzoic acid (F): Crude isopropoxybenzoate from above
(compound E) was dissolved in 200 mL THF/H20 (1:1) in a 500 mL flask. Excess
lithium
hydroxide (10 g) was added and the reaction refluxed for over 48 hours. The
mixture was
cooled, acidified with HC1 to pH 2, then extracted with several portions of
diethyl ether. The
organic layers were washed with brine, dried over MgSO4, and concentrated to a
white solid
in 55% yield over 2 steps: IR (cm-1): 3064, 2978, 2933, 2639, 1693, 1594,
1300, 1158, 1114,
1040, 767; 1H NMR (250 MHz, CD30D): 6 1.30 (dd, J = 2.3, 5.9 Hz, 12H), 4.59
(qn, J = 6.2
Hz, 2H), 6.61 (t, J = 2.3 Hz, 1H), 7.10 (d, J = 2.5 Hz, 2H); 13C NMR (63 MHz,
CD30D): 22.2
(4C), 71.2 (2C), 109.8, 109.9, 133.8, 160.3 (2C), 169.7.
[00129] 3,5-diisopropoxy-N-methoxy-N-methylbenzamide (G): Diisopropoxybenzoic
acid (compound F, 10g, 41 mmol) was dissolved in benzene (100 mL) in a 500 mL
round
bottom flask. Thionyl chloride (12.2 mL, 168 mmol) was added and the reaction
heated at
reflux for 1 hour. The mixture was then cooled to room temperature and
concentrated under
reduced pressure. The resulting residue was redissolved in dichloromethane and
concentrated
again to give 3,5-diisopropoxybenzoyl chloride. In a separate 200 mL flask,
N,0-
dimethylhydroxylamine-HC1 (4.0 g, 42 mmol) was suspended in 80 mL
dichloromethane at
0 C. Triethylamine (12.4 mL, 88 mmol) was added slowly, followed by crude 3,5-
diisopropoxybenzoyl chloride. The reaction flask was allowed to warm to
ambient
temperature and stirred overnight. The reaction was quenched with water and
extracted with
three portions of dichloromethane. The combined organic layers were washed
with brine,
dried over MgSO4, filtered and concentrated under reduced pressure.
Purification of the
resulting brown oil by column chromatography (30% acetone/hexane) gave the
Weinreb
amide (compound G) as a yellow oil in 60% yield from 3,5-dihydroxybenzoic
acid: Rf 0.33
(30:70 acetone/hexane); IR (cm-1): 2977, 1647, 1590, 1441, 1374, 1184, 1155,
1113, 1037,
964; 1H NMR (250 MHz, C6D6): 6 1.06 (dd, J = 2.5, 5.9 Hz, 12H), 3.00 (s, 3H),
3.05 (s, 3H),
4.19 (qn, J = 6.2 Hz, 2H), 6.69 (t, J = 2.5 Hz, 1H), 7.10 (d, J = 2.5 Hz, 2H);
13C NMR (63
MHz, C6D6): 21.9 (4C), 33.4, 60.4, 69.8 (2C), 106.6, 108.1 (2C), 137.0, 159.3
(2C), 169.8.
[00130] 3,5-diisopropoxyphenylperfluorophenylmethanone (H): In a 200 mL round
bottom flask, the Weinreb amide (compound G, 1 g, 3.5 mmol) was dissolved in
ether and
cooled. Pentafluorophenylmagnesium bromide (0.5 M in THF, 8.52 mL) was added
slowly
and the reaction stirred under ambient conditions overnight. The mixture was
quenched with
saturated ammonium chloride and extracted with three portions of ether.
Combined organic
layers were washed with brine, dried over anhydrous MgSO4, filtered and
concentrated to
- 40 -
CA 02785897 2012-06-28
WO 2011/100022
PCT/US2010/059703
give a dark brown oil which upon column chromatography (40% acetone/hexane)
crystallized
to the desired ketone in 46% yield: Rf 0.60 (30:70 acetone/hexane); IR (cm-1):
2980, 1682,
1588, 1501, 1320, 1185, 1160, 1113, 991, 770; 1H NMR (250 MHz, C6D6): 6 1.02
(d, J = 5.0
Hz, 12 H), 4.10 (qn, J = 7.5 Hz, 2H), 6.66 (t, J = 2.2 Hz, 1H), 7.13 (d, J =
2.2 Hz, 2H); 13C
NMR (63 MHz, C6D6): 21.6 (4C), 70.2 (2C), 109.1 (2C), 109.8, 138.5, 160.1
(2C), 184.9.
[00131] 1-(3,5-diisopropoxypheny1)-1-perfluorophenylprop-2-yn-1-ol (I):
The above
methanone (compound H, 3.2 g, 8.2 mmol) was dissolved in 40 mL ether in a 100
mL round
bottom flask. Ethynylmagnesium bromide (0.5 M in THF, 24.6 mL) was added
slowly and
the reaction stirred overnight. The reaction was quenched with saturated
ammonium chloride
and extracted with three portions of ether. Combined organic layers were
washed with brine,
dried over anhydrous MgSO4, filtered and concentrated. The resulting oil was
purified by
column chromatography (40% acetone/hexane) and gave the desired propargyl
alcohol as a
dark brown oil in 47% yield: Rf 0.15 (40:60 acetone/hexane); IR (cm-1): 3423,
3309, 2979,
1595, 1524, 1492, 1115, 985; 1FINMR (250 MHz, C6D6): 6 1.10 (d, J = 7.5 Hz,
12H), 2.29 (s,
1H), 2.67 (s, 1H), 4.26 (qn, J = 6.7 Hz, 2H), 6.54 (t, J = 2.3 Hz, 1H), 7.11
(d, J = 2.5 Hz, 2H);
13C NMR (63 MHz, C6D6): 21.9 (4C), 69.8 (2C), 71.9, 75.7, 83.7, 103.6, 105.8
(2C), 145.2,
159.8 (2C).
Scheme 3: Synthesis of 143,5 -diisopropoxypheny1)-1-p erfluorophenylprop-2-yn-
1-ol (I)
o 0 1 0
10
HO H 1) SOCI3, benzene 2-iodopropane \C) 401
o LOH \r 401 OH K2CO3, DMF THF, H20 2) Me0MeNH-HCI,
CH2Cl2, 60%
OH 01
E (:)(
F
0 y 0 F
I
\\ OH F
--.T.0 so y
m:OMe pentafluorophenyl- 0 F
magnesium bromide 101 Si MgBr 0 la
F
ether, 46% F F ether, 47% IW
4W
C) 0 F F
G H )0
F F
1
[00132] (PPh3)C12Ru(3-pentafluorophenyl-6,8-diisopropoxyinden-1-ylidene) (J):
A
100 mL flask was charged with 1-(3,5 diisopropoxyphenyl),1-(pentafluoropheny1)-
2-propyn-
1-ol (compound H, 503 mg, 1.2 mmol). THF (47 mL) was then added followed by
Ru(PPh3)3C12 (1.17 g, 1.2 mmol) and acetyl chloride (AcC1) (86 1AL in 0.86 mL
THF). The
reaction was refluxed for 1.5 hours after which all solvent was removed under
a stream of N2.
The residue was suspended in 45 mL isopropanol with vigorous stirring at 40 C
for 1 hour.
The resulting suspension was filtered and washed thrice with isopropanol (20
mL each time)
-41 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
and dried in vacuo. The crude material was loaded onto a flash column
dissolved in 50%
hexane/dichloromethane, and eluted with 100% dichloromethane. The solvent was
removed
in vacuo yielding 240 mg (23%) of the desired compound. Additional crude
material was
eluted with 1% and 2% Me0H in dichloromethane. This material contained PPh3
and an
unidentified decomposition product observed at 28.6 ppm in the 31P spectrum.
The product
was characterized by NMR spectra (1H, 13C, and 31P). The results are as below:
[00133] 1H NMR (500 MHz, CD2C12, 30 C): 6= 6.0-7.0 (m, 15H), 6.62(s, 1H),
6.56 (d, J=
1 Hz, 1H), 6.50 (d, J= 1.5 Hz, 1H), 5.17 (sept d, J= 2, 6 Hz, 1H), 4.61(sept,
J= 6Hz , 1H),
1.75(d, J= 6Hz, 6H), 1.36(d, J= 6Hz, 6H); 19F NMR (250 MHz, CD2C12, 30 C): 6= -
137.39
(d, J= 17.5Hz , 2F), -154.66 (t, J= 22.5Hz, 1F), -162.6 (dt, J= 6.5, 22.5Hz ,
2F); 31P NMR
(250 MHz, CD2C12, 30 C): 6= 63 ppm.
Scheme 4: Synthesis of (PPIII)C12Ru(3-pentafluoropheny1-6,8-diisopropoxyinden-
1-ylidene)
fil
HO /F
PPh3 F F
CI, 1
O, ,F Ru
F F AcCI, THF 0 lip
_______________________________________________ x F F
F
/ I 18 h, reflux
.........(0
+ \ J
CI.
Ru¨(PPh3)3
CI'
Example 3: Synthesis ofPC 3 ChRypy,_Itafluoro hen 1-6 8-cliiso ro ox inclen-1-
ylidene) (K)
[00134] (PCy3)C12Ru(3-pentafluoropheny1-6,8-diisopropoxyinden-1-ylidene) (K):
A
10 mL vial was charged with (PPh3)C12Ru(3-pentafluoropheny1-6,8-
diisopropoxyinden-1-
ylidene) (compound J, 0.40 grams). Benzene (2 mLs) was then added followed by
tricyclohexylphosphine (0.13 grams). The reaction was allowed to sit
overnight. Excess
Cu(I)C1 was added, approximately 0.50 grams. The resulting slurry was dried
under vacuum,
and pentane was used to extract the product (0.038 g) from the solids. The
product was
characterized by NMR spectra (1H, 13C, and 31P). The results are as below: 1H
NMR (250
MHz, CD2C12, 30 C): 6= 7.35 (s, 1H), 6.64(s, 1H), 6.38 (s, 1H), 4.62 (sept ,
1H), 4.26(sept,
1H), 1.72(d, 6H), 1.36(d, 6H), 1.5-2.4 (m, 33H); 19F NMR (250 MHz, CD2C12, 30
C): 6= -
137.34 (d, J= 17.5Hz , 2F), -154.4 (t, J= 22.5Hz, 1F), -161.6 (dt, J= 6.5,
22.5Hz , 2F); 31P
NMR (250 MHz, CD2C12, 30 C): 6= 68 ppm.
- 42 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
Scheme 5: Synthesis of (PCy3)C12Ru(3-pentafluoropheny1-6,8-diisopropoxyinden-1-
ylidene)
(K)
F
PPh3 F F F
PCy3
Ck I Ck I
C1'1,7u * F ____ PCy3, C6H6
CI'7u * F
F F Cu(I)CI 0
F F
X-ray Crysta11o2raphy
[00135] X-ray quality crystals of these ruthenium complexes may be grown by
dissolving
the crude material in a minimal amount of a solvent such as dichloromethane
and then adding
an excess of another solvent of differing polarity, for example, isopropanol
or hexanes. This
solution is then allowed to evaporate at ambient temperature, usually under a
nitrogen
atmosphere, to yield crystals of the desired ruthenium complex. The crystals
are usually
removed from the solvent by using a glass frit. Any solid isolated from the
filtrate usually
contains impure crystals.
[00136] For example, X-ray quality crystals of compound J, above, were grown
by
dissolving the crude material in a minimal amount of dichloromethane and
adding a tenfold
excess of isopropanol. This solution was allowed to partially evaporate
overnight at ambient
temperature under a N2 atmosphere to yield X-ray quality crystals.
[00137] Solid-state structure of Compound J[(PPh3)C12Ru(3-pentafluoropheny1-
6,8-
diisopropoxyinden-1-ylidene)] as determined by single-crystal x-ray
diffraction
Key data and collection parameters: RuC39H32PC1202F5, FW 830.62, red-brown
irregular, 0.6
x 0.3 x 0.06 mm, orthorhombic, a = 13.268(1) A, b = 20.385(2) A, c = 27.051(3)
A, V =
7316(1) A3, Pbca (#61), Z = 8, dcalc = 1.508, MU = 6.77 cm-1, No. obs = 8342,
No. variables =
452, R1 (I> 2a(I)) = 0.115, wR2 (all reflections) = 0.1826, GOF = 1.137, peak
= 0.61, hole =
-0.49, max shift/error = 0.001.
- 43 -
CA 02785897 2012-06-28
WO 2011/100022
PCT/US2010/059703
Atomic coordinates and Biso/Bo.
Atom x Y z Beq
Ru(1) -0.08116(5) 0.18381(3) 0.63755(2) 2.456(14)
C1(1) -0.17357(15) 0.11104(11) 0.58978(8) 3.63(4)
C1(2) 0.00689(16) 0.20266(13) 0.70936(8) 4.36(5)
P(1) 0.05766(14) 0.17048(10) 0.59166(8) 2.65(3)
F(1) -0.1092(4) 0.4978(2)
0.6239(2) 5.44(12)
F(2) -0.0824(4) 0.6051(2)
0.5668(2) 5.95(13)
F(3) -0.1069(4) 0.5980(2)
0.4677(2) 6.48(15)
F(4) -0.1419(4) 0.4794(2)
0.4239(2) 5.97(13)
F(5) -0.1611(4) 0.3709(2) 0.48075(19) 5.65(12)
0(1) -0.2244(3) 0.2022(2) 0.68818(18) 2.93(10)
0(2) -0.4318(5) 0.4008(3) 0.7040(2) 5.67(16)
C(1) 0.0825(5) 0.0832(4)
0.5833(3) 3.15(15)
C(2) 0.0763(7) 0.0422(4)
0.6239(3) 5.2(2)
C(3) 0.0899(9) -0.0244(5)
0.6186(5) 7.2(3)
C(4) 0.1106(8) -0.0518(5)
0.5723(6) 7.0(3)
C(5) 0.1178(7) -0.0110(5)
0.5325(4) 5.3(2)
C(6) 0.1036(5) 0.0560(4)
0.5370(3) 3.91(18)
C(7) 0.1722(6) 0.2041(4)
0.6194(2) 3.38(17)
C(8) 0.2554(6) 0.1642(4)
0.6318(3) 4.30(19)
C(9) 0.3388(6) 0.1911(7)
0.6542(3) 6.1(2)
C(10) 0.3427(8) 0.2556(7)
0.6661(3) 6.9(3)
C(11) 0.2631(8) 0.2957(6)
0.6531(3) 6.5(2)
C(12) 0.1787(6) 0.2691(5)
0.6304(3) 5.1(2)
C(13) 0.0577(6) 0.2029(3)
0.5293(2) 2.97(15)
C(14) 0.1445(6) 0.2284(4)
0.5075(3) 3.73(18)
C(15) 0.1441(7) 0.2494(4)
0.4586(3) 4.7(2)
C(16) 0.0564(8) 0.2462(4)
0.4322(3) 4.8(2)
C(17) -0.0298(7) 0.2228(4)
0.4530(3) 4.4(2)
C(18) -0.0304(6) 0.2011(4)
0.5013(3) 3.49(17)
C(19) -0.1154(5) 0.2667(3)
0.6164(2) 2.51(14)
C(20) -0.0863(5) 0.3180(3)
0.5809(2) 2.84(14)
- 44 -
CA 02785897 2012-06-28
WO 2011/100022 PCT/US2010/059703
C(21) -0.1466(5) 0.3714(3)
0.5859(2) 2.64(14)
C(22) -0.2217(5) 0.3587(3)
0.6258(2) 2.93(15)
C(23) -0.2006(5) 0.2955(3)
0.6420(2) 2.36(13)
C(24) -0.2988(5) 0.3940(4)
0.6473(2) 3.40(17)
C(25) -0.3536(6) 0.3631(4)
0.6861(3) 3.70(18)
C(26) -0.3333(6) 0.2997(4)
0.7013(3) 3.29(16)
C(27) -0.2563(6) 0.2657(3)
0.6786(2) 2.79(14)
C(28) -0.1398(5) 0.4311(4)
0.5552(2) 2.84(15)
C(29) -0.1208(6) 0.4924(4)
0.5747(3) 3.60(17)
C(30) -0.1081(6) 0.5485(4)
0.5462(4) 4.4(2)
C(31) -0.1181(6) 0.5442(4)
0.4959(4) 4.5(2)
C(32) -0.1360(6) 0.4848(4)
0.4747(3) 3.82(18)
C(33) -0.1448(6) 0.4293(4)
0.5033(3) 3.47(17)
C(34) -0.3990(11) 0.4325(9)
0.7869(5) 13.4(6)
C(35) -0.4645(9) 0.3920(5)
0.7544(4) 6.0(2)
C(36) -0.5695(9) 0.4154(7)
0.7577(4) 10.2(4)
C(37) -0.2186(7) 0.1001(4)
0.7306(3) 4.6(2)
C(38) -0.2857(5) 0.1582(4)
0.7192(3) 3.23(16)
C(39) -0.3794(6) 0.1380(4)
0.6904(3) 4.29(19)
Where Beg = 8/3 7c2(U11(aa*)2 + U22(bb*)2 + U33(cc*)2 +
2U12(aa*bb*)cos y + 2U13(aa*cc*)cos 13 + 2U23(bb*cc*)cos a).
Cross metathesis reactions
[00138] Representative experimental protocols for cross metathesis reactions
are presented
in the examples below.
Example 4: Ethylenolysis of methyl oleate with ethylene using compound D
[triphenylphosphineruthenium(3-(3,5-dimethoxypheny1)-5,7-dimethoxy-
indenylidene)].
[00139] In a 120 mL bottle, triphenylphosphineruthenium(3-(3,5-
dimethoxypheny1)-5,7-
dimethoxy-indenylidene) (compound D, 5.0 mg, 6.57 gmol) was combined with 100
mL
dichloromethane to make a stock solution. Some of this ruthenium catalyst
compound stock
solution (3.8 mL, 250 nmol) was added to a 20 mL scintillation vial along with
1 equivalent
of tricyclohexylphosphine (250 nmol, added as a solution in dichloromethane).
Tetradecane
(0.152 g) was then added as a standard for gas chromatography analysis. The
contents of the
vial were transferred to a 100 mL Fisher-Porter vessel equipped with a
stirring bar which was
- 45 -
CA 02785897 2013-09-16
then sealed and charged with ethylene (150 psi). The bottle was then placed in
an oil bath
heated to 40 C for 2 hours. The bottle was depressurized, opened and a few
drops (¨ 0.1 mi.)
of ethyl vinyl ether were added prior to analysis. 1-Decene and methyl-9-
decenoate yields
corresponded to 1800 turnovers of decene per equivalent of ruthenium.
Example 5: Ethylenolysis of Methyl Oleate using Compound K, (PCvi)C12Ru(3-
pentafluorophenyl-6,13-dlisopropoxyinden-1-vlidene)
1001401 The ethylenotysis of methyl oleate was used as a test to determine the
activity of
(PCy3)C12Ru(3-pentalluorophenyl-6,8-di isopropoxyinden-l-ylidene). A catalyst
compound
stock solution (0.1379 mM) was made by dissolving the catalyst compound in
anhydrous
dichloromethane. Methyl oleate (0.87 g, 1.0 m1,), catalyst compound stock
solution (0.906 g),
dichloromethane (4.12 g), and tetradeeane (0.152 g) as an internal standard
were placed in a
Fisher-Porter bottle equipped with a stir bar. The vessel was then filled with
ethylene to 150
psig and placed in an oil bath heated to 40 C for 3 hours. The vessel was then
depressurized
and 5 drops ethyl vinyl ether added to stop the reaction. A sample was
analyzed by gas
chromatography. The cross-metathesis reaction yielded 18.5% 1-decene and
methy1-9-
decenoate with 99% selectivity 1-Decene and methyl-9-decenoate yields
corresponded to
430Q turnovers of decene per equivalent of ruthenium.
[00141] As is apparent from the foregoing general description and the
specific
embodiments, while forms of the invention have been illustrated and described,
various
modifications may be made. Accordingly, it is not intended that the invention
be limited
thereby. The scope of the claims should not be limited by particular
embodiments set forth
herein, but should be construed in a manner consistent with the specification
as a whole.
-46 -