Note: Descriptions are shown in the official language in which they were submitted.
CA 02787724 2012-07-20
COLLECTOR AND FROTHING AGENT FOR FLOTATION BASED ON ORGANIC
RESIDUES FOR THE RECOVERY OF METALS FROM MINERALS BY FROTH
FLOTATION, COLLECTOR AND FROTHING AGENT RECOVERY PROCESS
AND FOAMING FLOTATION PROCESS THAT USES THE COLLECTOR AND
FROTHING AGENT
WORK SPECIFICATIONS
Field of the Invention
The present invention relates to collector and foaming agents based on organic
waste, useful
in froth flotation processes to recover commercially valuable metals from
either sulfide
minerals (copper, zinc, lead, iron, molybdenum, etc.) or non-sulfide ores
(gold, etc.). It
consists of multifunctional flotation agents used as collector and foaming
agents based on
organic waste that are derived from aerobic or anaerobic treatment or
decomposition
processes, or from just a fraction of them (extract). It also involves the
production process
and use of such collector and foaming agents in a froth flotation process to
recover metals of
commercial value from minerals. These processes result in the creation of
tailings whose
composition is suitable for an environmental remediation treatment.
Background
The current scenario has imposed resource, operation and exogenous challenges
on the
mining industry, particularly on associated metallurgical processes. In the
case of resources,
there has been not only a continuous decrease of mineral grades, and therefore
a sustained
increase in liabilities and environmental waste, but also the creation of new
mineralogical
associations. From an operational standpoint, the most important, the urgent
need to reduce
energy costs and water consumption. In the case of exogenous challenges,
increasingly
demanding and rigorous environmental policies have been imposed. Also, new
standards of
product quality requirements have been implemented, and in recent years, there
has been a
strong impact on production costs associated with supplies used in both mining
and
metallurgical operations.
It becomes more urgent than ever the need to address these challenges
efficiently and
effectively. This invention aims to address some of these challenges,
particularly with regard
to improving the mineral concentration stage through the development of
unique,
bifunctional, effective, inexpensive and very competitive flotation reagents.
It also improves
the efficiency of flotation and generates tailings that favor a positive
environmental
management to implement technologies such as phytoremediation.
1
CA 02787724 2012-07-20
The froth flotation process is the most use in the beneficiation of either
sulfide minerals
(copper, zinc, lead, iron, molybdenum, etc.) or non-sulfide ores (gold, etc.)
containing metals
of value. The process makes it possible to separate commercially valuable
metals from the
gangue and /or to separate valuable metals from each other, from minerals
previously
subjected to crushing and grinding stages.
In the case of sulfide mineral subjected to froth flotation, different
chemical compounds of
specific action, such as frothers, collectors and modifiers are used.
Collectors are organic
compounds of relatively short carbonic chain and without foaming capacity.
After the
injection of air into the mineral pulp under stirring, a foam composed of an
aqueous solution
of the finely ground mineral containing a foaming agent (e.g., pine oil,
cresylic acid, ROH
alcohols as methyl isobutyl , carbonyl, polyglycols and 2 ethyl hexanol) is
formed. An
important advantage of the separation by froth flotation is its substantially
lower cost than
other beneficiation processes, such as gravitational and centrifugal
concentrations, among
others.
During flotation, one or more reagents called collectors or promoters are
added, which make
the selective transformation of a lyophilic surface (hydrophilic in the case
of the use of water)
into a lyophobic surface (hydrophobic in the case of the use of water)
possible in minerals
containing the valuable metal to be obtained as final product. From a
scientific standpoint, it
was found that the separation of a mineral species from another by flotation
depends on the
wettability of its surfaces in water, which is determined by the net balance
of the interfacial
energies, i.e. the variation of free energy per unit area among different
phases: solid, liquid
and gas. Various reagents as collectors in froth flotation processes for
recovery of
commercial valuable metals have been suggested and used, the most common are
xanthates
(xanthates, xanthate esters), carbamates (dithiocarbamates, thiocarbamates),
mercaptans ,
mercaptobenzo-thiazole and the organic derivatives of phosphoric or phosphorus
acid
(dithiophosphates, thiophosphates, dialkyldithiophosphate acid).
One of the problems associated with these collectors is that lower pyrite and
pyrrhotine
depressions are obtained at a pH lower than 11. Also, experience has shown
that as the pH
decreases, the collector ability of these reagents also decreases, reducing
significantly the
feasibility of using them in slightly alkaline, neutral or acidic pulps.
During manufacturing
operations, the pH adjustment is performed by adding lime, alkali and
hydroxide metal
oxides, among others. The inclusion of an inorganic base is widely used to
achieve desired
pH values. After controlling the pH of the pulp at levels of 8.0 and higher,
frequently around
11, the collector performance improves.
Other relevant aspects related to collectors on the market is their high cost,
their specificity is
very sensitive and interfered by other species present in the pulp and not
very efficient in
pyrite/pyrrhotite depression. Also, foaming agents must be added, which are
costly, making
the costs of the whole process to increase due to the large amount of mineral
that is processed
in the mining sector.
2
CA 02787724 2012-07-20
In operational terms, the application of flotation aims to obtain a range of
copper concentrate
of 25% - 30% (w/w, dry basis), from low grade minerals (0.5 - 2% Cu).
Currently, the
concentration operation by flotation in the copper industry reaches a copper
recovery range
between 80% and 85%, and in some cases, optimal values close to 90%. However,
the above
is obtained at very high operational costs.
Currently, flotation reagents used are formed by recalcitrant chemicals, which
have a
negative environmental impact.
In that sense, having flotation reagents of lower cost and less environmental
impact, like
organic waste either from treatment processes or aerobic or anaerobic
decomposition, or just
a fraction of them, such as those generated from household wastewater
purification processes
(biosolids) and/or livestock production systems (slurry, manure) would have a
significant
economic impact on the mining industry, and would simultaneously solve
environmental and
social problems related to its current operational management. The treatment
of household
wastewater, using activated sludge, generates a significant amount of organic
waste or
biosolids on landfills and monorrellenos, with a significant cost not only for
water companies
but also for users of the system. It is important then to identify
alternatives for recovery of
organic soils, which due to their massive nature should relate to other big
industrial
processes, such as large-scale mining industry.
In the state-of-art there is a proposal to use treatment plant wastewater
effluents as process
water for froth flotation of minerals without altering their effectiveness, as
disclosed in U.S.
Patent 4,028,235 in 1976. The document mentions that the effluent must be
conditioned with
polyglycerol or with a physical treatment of clarification, sedimentation
and/or aeration to
obtain water of adequate quality and to not adversely affect the froth
flotation. It is also
mentioned that the conditioning of the effluent with polyglycerol is
essential, critical and
cheaper in the process than other suggested alternatives. The direct use of
the effluent without
polyglycerol has negative effects on the froth flotation process, as the
gangue floats instead of
being depressed, producing a concentrate of lesser quality. Although the
mechanism of action
of polyglycerol in the effluent is unknown, an effluent addition between 3 and
10 parts per
million (ppm) is suggested. Larger amounts of polyglycerol do not benefit the
effluent.
Additionally, the document mentions that the froth flotation process, based on
the use of
polyglycerol-conditioned effluents, requires the addition of foaming and
collectors
conventionally used in the rougher stage of flotation. As shown, the solution
proposed in this
document is the use of effluents of treatment wastewater plants conditioned
with
polyglycerol. It is not intended to replace all or part of the foams
conventionally used in froth
flotation processes, but it is intended to be used as an alternative source of
water in mining
process in places where the chances of access to natural water sources are
limited.
Definitions
3
CA 02787724 2012-07-20
In the present specification, the term "multifunctional flotation agent" means
an agent that
can have both, collector and foaming functions. Also, the term "foaming and
collector agent"
refers to a single multifunctional agent that includes both collector and
foaming functions.
In the context of the present invention, the term "organic matter" refers to
biosolids from
wastewater treatment plants, sewage organic sludge from biogas production
systems, water-
soluble organic matter from compost or other biologically similar organic
waste treated or
stabilized under aerobic and/or anaerobic conditions, industrial sludge from
treatments of
industrial organic liquid waste, organic matter from vegetable peat, manure or
a combination
of two or more of any of them, or just a fraction of them.
The term "fulvic acids" refers to organic compounds present in the organic
material (i.e.
biosolids, manure) which are obtained by basic extraction and do not
precipitate at low pH.
The term "humic acids" refers to organic compounds present in the organic
material (i.e.
biosolids, manure) which are obtained by basic extraction and precipitate at
low pH.
The term "liquid extract from multifunctional flotation agent" is understood
as a liquid agent
that may have collector and foaming function. It is a liquid extract obtained
from the
processing of organic waste derived from treatment processes or aerobic
decomposition, such
as biosolids and/or manure.
Technical Problem
There is a need to generate frothing and collector agents for flotation of
either sulfide
minerals (copper, zinc, lead, iron, molybdenum, etc.) or non-sulfide (gold,
among others.)
with low environmental impact, low cost, multifunctional agents (simultaneous
collector and
foaming functions) and efficient concentration and separation of multiple
valued metals from
minerals subjected to flotation, operating at wide ranges of pH in the
recovery processes of
commercially valuable metals by froth flotation.
Technical Solution
The present invention provides multifunctional flotation agents, with both,
collector and
foaming functions based on organic waste derived from treatment processes or
aerobic or
anaerobic decomposition, or from just a fraction of them, available for
foaming flotation
processes for the recovery of valued metals from minerals. Also, it provides a
manufacturing
process of these agents and how to use them in mineral flotation processes.
Advantageous Effects
The main advantage of the present invention over the current state of
technology is the lower
cost and lower environmental impact of this foaming and collector agent versus
the current
chemical collector and foaming reagents. Also, it has a better selectivity for
the recovery of
commercially valuable metals from minerals and a wider range of applications.
4
CA 02787724 2014-12-02
=
These collector and foaming agents have the advantage of having a much more
competitive
cost than collectors and foaming agents on the market. Additionally, due to
their organic
origin, they are harmless to human health, environment and subsequent
metallurgical
processes, as they are biodegradable. The latter attribute is particularly
important in terms
of job stress and health, as collector and foaming chemical agents in the
market are toxic
and flammable organic compounds and are stored in tailings deposits after
their use.
Also, this invention increases the value to organic waste derived from
treatment processes
or from aerobic or anaerobic decomposition, or just a fraction of them. For
example, water
companies could increase the value of biosolids generated by their plants of
household
wastewater treatment, and the livestock industry could increase the value of
its organic
waste (manure, slurry).
This invention provides an environmentally safe and valued view for massive
waste, which
has traditionally had a very negative social perception. Simultaneously, the
total or partial
replacement of existing reagents from organic waste flotation or just a
fraction of them
would eliminate the environmental hazards associated with the existing
chemical flotation
reagents.
In accordance with an aspect of the present invention, there is provided a
collector and
foaming agent for froth flotation processes in the recovery of commercially
valuable metals
from sulfide minerals or non-sulfide ores, the collector and foaming agent
comprising
organic waste derived from treatment processes, aerobic decomposition,
anaerobic
decomposition, or a fraction thereof.
In accordance with another aspect of the present invention, there is provided
a collector and
foaming agent for froth flotation processes in the recovery of commercially
valuable metals
from sulfide minerals or non-sulfide ores, the collector and foaming agent
comprising
organic waste derived from treatment processes, aerobic decomposition,
anaerobic
decomposition, or a fraction thereof, wherein the organic waste is derived
from treatment
processes or selected aerobic or anaerobic decomposition of biosolids and/or
manure and/or
humic substances and wherein the biosolids comprise between 35% and 98%
organic matter
on a dry basis.
In accordance with another aspect of the present invention, there is provided
a production
process for the foaming and collector agent as described above, the process
comprising:
(a) collecting organic waste from generating sources of organic matter from
treatment processes, aerobic decomposition, anaerobic decomposition, or a
fraction thereof
and determining properties selected from the group consisting of organic
matter content and
humic substances content;
(b) conditioning of the collected material from (a) by the following steps:
(i) dehydrating the collected material to a moisture content less than or
equal to
75%; and
(ii) reduction of size and separation of the dried material by milling and
sieving
to obtain material with a size less than or equal to 10 millimeters (mm); and
(c) compacting the obtained material from (ii) to form pellets or briquettes.
-
CA 02787724 2014-12-02
In accordance with another aspect of the present invention, there is provided
a froth
flotation process for the recovery of commercially valuable metals from
sulfide minerals or
non-sulfide ores, the process comprising the steps of:
- reducing the size of sulfide minerals or non-sulfide ores to a particle
size below
400 microns by first, second and third crushing, and afterwards, semi-
autogenous or
conventional grinding;
- conditioning of the mineral ground into a pulp by mixing the ground mineral,
water to
obtain a mineral pulp with a range from 5% to 20% of solids weight, pH
modifiers, strong
bases, and the collector and foaming agent as described above;
- receiving the conditioned pulp in a flotation device and adding water to
obtain a
pulp with a range of 20% to 50% of solids weight;
- stirring to keep the material in suspension, aerating such pulp with a
stream
between 5 and 200 cubic meters per minute for a period of time 2 to 20
minutes, and
concentrating the commercially valued metal in a foam and depressing a
flotation tail; and
- collecting the foam rich in the commercially valued metal as a metal
concentrate.
In accordance with another aspect of the present invention, there is provided
a use of the
collector and foaming agent as described above, in froth flotation of sulfide
ores or non-
sulfide.
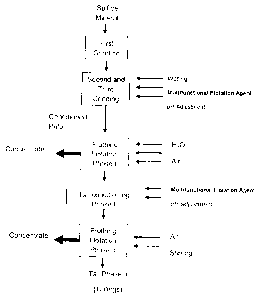
Brief Description of the Figures
Figure 1: It shows the stages of froth flotation process for the recovery of
commercially
valuable metals.
Figure 2: It illustrates the variation of surface tension at pH 7 and 10, at
different
concentrations of humic substances (HS), biosolids (BS) and methyl isobutyl
carbinol
(MIBC): (A) shows the results obtained for a total concentration of foaming
agent (HS, BS,
MIBC), (B) illustrates the results obtained for a concentration of foaming
agent corrected by
the fraction of seditnented material. Both graphs show the average values (n>
4); the error
bars are within the symbols.
Figure 3: It shows the quantification of the hydrophobic fractions of the
copper sulfide
mineral (M), chalcopyrite (CPY) and pyrite (Py) for a dose of HS, BS, goat
manure and
RQCI obtained for the experimental condition of 100% water (surface tension of
72.1 mN
m'). Average values (n> 4) and the error bars are shown.
Figure 4: It illustrates the kinetics of froth flotation obtained from
industrial chemical
reagent dosage (collector + frother), biosolids (BS) and humic substances
(HS), (A)
illustrates the results obtained with respect to the copper grade and (B)
shows the results
obtained with respect to iron grade.
Detailed Description of the Invention
The present invention consists of a multifunctional flotation agent with
collector and
foaming functions used in froth flotation processes for the recovery of
commercially
valuable metals either from sulfide ores (copper, zinc, lead, iron,
molybdenum, etc.) or non-
sulfide minerals
_
5a
CA 02787724 2012-07-20
(gold, etc.), which are organic waste derived from treatment processes or
aerobic or anaerobic
decomposition, or from just a fraction of them (extract).
The multifunctional flotation agent, or "collector and foaming agent" is
organic waste derived
from treatment processes or aerobic or anaerobic decomposition, such as
biosolids and/or
manure. Results of physical and chemical analysis obtained from literature for
biosolids and
manures are shown in Table 1. The percentages are given on a dry basis.
Table 1: Ranges for the chemical composition and physical characteristics of
biosolids and
manures obtained from literature.
Parameter Range
Organic matter (%) 35 ¨ 98
Total N (%) 1.2 ¨ 8.0
Total P (%) 0.2 ¨ 3.0
Total C (%) 20.0 ¨ 70.0
Humic and fulvic acids (%) 1.0 ¨ 25.0
Proteins (%) 5.0 -25.0
Sugars (%) 0.1 ¨5.0
Ethereal extract (%) 0.2 ¨ 0.5
Apparent Density (g/mL) 0.2 ¨ 0.8
131-1 6.0 ¨ 8.5
Electrical conductivity (mS/cm) 4 ¨ 15
The production process of the multifunctional flotation agent of this
invention consists of the
following stages:
1. To collect the organic matter of biosolids and/or manure from the
generating sources and
select according to the physical and chemical properties listed in Table 1.
2. To condition some of the following operations, depending on their origin
and mode of
application:
a. To dehydrate until reaching a moisture content less than or equal to 75%
and more
generally to a moisture content less than or equal to 20%.
b. To reduce size and separate, for example through grinding and sieving to
values less than
or equal to 10 millimeters (mm).
c. To compact in the form of pellets or briquettes, among other options.
3. Packaging of the product.
The product obtained in number 2 can be subjected to a liquid aqueous
extraction process
using acids and/or strong bases to maintain the same characteristics mentioned
for the
multifunctional flotation agent (foaming and collector agent) of this
invention.
6
CA 02787724 2012-07-20
The liquid extraction process of the multifunctional flotation agent (foaming
and collector
agent) consists of the following steps:
1. Take the product at the end of step 2 as described above.
2. Perform an extraction that considers some of the following alternative
methods, depending
on its origin and mode of application:
a. Extract using an acid-base process that considers a pH reduction between 1
and 2 with a
strong acid such as HC1, H2SO4, H3PO4, at room temperature. Adjust the volume
of the
solution with acid until obtaining a ratio between 1:5 and 1:10 organic waste:
acidic solution
(mass: volume), dry basis. Stir the suspension for a period of time less than
or equal to 10
hours. Separate and reserve the supernatant of the solid fraction. Adjust the
pH of the solid
fraction to neutrality using a strong base such as KOH, NaOH, etc, at room
temperature.
Adjust the volume of the solution with a base to obtain a ratio between 1:5
and 1:10, solid
fraction: basic solution (mass:volume). Stir the suspension for a period of
time less than or
equal to 10 hours. Separate and reserve the second supernatant from the second
solid fraction.
Mix the supernatants of the first and second stages as described above to
obtain the extract.
b. Extract using water as aqueous extractant considering to adjust the volume
of the solution
with water in a range between 1:5 and 1:10, organic residue: water
(volume:volume) in dry
basis, at ambient conditions. Stir the suspension for a period of time less
than or equal to 10
hours. Separate and reserve the supernatant (extract) of the solid fraction.
3. Bottle the extract.
Description of a froth flotation method in mining
There is a competitive and alternative process of froth flotation for the
recovery of
commercially valuable metals from either sulfide or non-sulfide minerals,
which uses the
multifunctional flotation agent (foaming and collector agent) of the invention
as an
alternative and highly competitive element compared to collector and foaming
agents used
until this invention (Figure 1).
The froth flotation process for the recovery of commercially valuable metals
from sulfide or
non-sulfide minerals according to the present invention consists of the
following steps:
1. Reducing the size of sulfide or non-sulfide minerals to a particle size
below 400 microns.
This includes the first, second and third grinding stages, and the subsequent
semi-autogenous
and conventional grinding;
2. Conditioning of the mineral ground into a pulp by mixing:
a. the ground mineral;
b. water to obtain a mineral pulp with a range from 5% to 20% of solid weight;
7
,
CA 02787724 2012-07-20
c. PH modifiers such as lime, strong bases such as KOH, NaOH, etc.
d. collector and foaming agent, it is generally added in amounts less than or
equal to 30% of
the mineral weight and preferably between 5% and 20%;
3. Placing the conditioned pulp in a flotation device, then water is added to
obtain a pulp with
a range of 20% to 50% solid weight, preferably between 30% and 40%;
4. Stirring to keep the material in suspension, preferably at a speed in a
range of 40 to 500
rpm, ideally from 70 to 90 rpm, airing then with a stream of 5 to 200 cubic
meters per minute
for a period of 2 to 20 minutes, concentrating the valued metal in the foam
and comprising a
flotation tail.
5. Collecting such foam rich in metal as a concentrate of the metal.
In addition, the froth flotation process for the recovery of commercially
valued metals
according to the present invention consists of the additional steps:
6. Transferring such flotation tail to a second flotation equipment to collect
a second
commercially valued metal;
7. Conditioning the flotation tail using:
a. liquid extract from the multifunctional flotation agent (foaming and
collector agent).
Generally, a foaming and collector agent amount less than or equal to 45% of
the mineral
weight are added, more preferably between 2% and 30%;
b. PH modifiers such as lime, strong bases such as KOH, NaOH, etc.;
8. Subjecting the conditioned tail to a second froth flotation while stirring
to keep the material
in suspension, at a speed range of 70 to 90 rpm and aeration at 15 - 200 cubic
meters per
minute for a period of 2 to 20 minutes, concentrating the second metal as a
foam and
depressing the gangue;
9. Collecting the foam rich in the second metal.
10. Clearing the tail (tailings) for final destination. The tailings are
discarded in tailings
deposits built specifically for this purpose, following the procedures and
methods used for
each plant tailings.
The froth flotation process, according to the present invention, is suitable
to benefit sulfide
minerals (copper, zinc, lead, iron, molybdenum, etc.) or non-sulfide ones
(gold, etc.), and also
commercially valuable metals present in tails of the first processing phase,
out of two, of
flotation froth. For example, copper can be benefited from minerals such as
chalcopyrite
(CuFeS2) and mixtures of minerals (chalcocite, Cu2S, covellite, CuS, bornite,
Cu5FeS4, etc.).
Normally, copper sulfide ores contain pyrite (FeS2) and other metal sulfides
that are also
benefited.
8
CA 02787724 2012-07-20
From this point forward, the description will be applied to benefit and
recover copper from
sulfide ores, as an example. To not limit the invention, however, this
description also applies
to other sulfide and non- sulfide ores of commercially valuable metals, such
as galena (PbS)
and spheralite (ZnS), among others. The process of the present invention has
proven to be
suited to benefit copper sulfide ores, minerals such as copper sulfide type
associated with
Pyrite, like the typical association CuFeS2/ FeS2.
In step 2 and optionally in step 6, the multifunctional flotation agent is
added. It has collector
and foaming functions, and the amount will depend on various factors such as
the physical
and chemical properties, speciation, particle size distribution, mineral grade
and release rate,
among others.
In a first stage of froth flotation, most commercially valuable iron content
can be recovered in
the foam as iron concentrate and commercially valued copper minerals can be
depressed (e.g.
chalcopyrite) as well as other commercially valuable metal sulfides in the
pulp (molybdenum,
silver, etc..).
In the first stage of flotation, between 5% and 25% of the mineral weight
collector and
foaming agent is added. The pulp is agitated and aerated for a period of time
that maximizes
the recovery of iron. The specific period of time will depend on the physical,
chemical
speciation, particle size distribution, rate of liberation and grade
properties among others; the
time needed to float a certain mineral can be estimated according to the
efficiency and
production plans of the concentrator plant. Typically, the flotation is
conducted for a period
between 2 and 20 minutes and more preferably for a period between 5 and 15
minutes.
Once the first phase of flotation is over, the iron concentrate is collected
and the tail is
subjected to the second phase of froth flotation. The tail then undergoes a
second phase of
froth flotation to recover most of the commercially valuable copper content in
the foam
(copper concentrate) and depress minerals without commercial value and the
gangue that
remains in the lower phase (tailings).
In the second stage of flotation, between 2% and 30% of the liquid extract
mineral weight of
the collector and foaming agent is added. The tail is agitated and aerated for
a period of time
that maximizes the recovery of copper. The specific period of time depends on
the physical,
chemical speciation, particle size distribution, rate release and grade
properties, among
others; the time needed to float a certain mineral can be estimated according
to the production
goals and efficiency of the concentrator. Typically, the flotation is
conducted for a period
between 2 and 20 minutes, and preferably, for a period between 5 and 15
minutes.
Once the second flotation stage for the desired time period has been
completed, the copper
concentrate is collected and the tailing or new tail is removed and discarded.
The tailings are
discarded in tailings deposits built for this purpose, according to the
procedures established in
each tailings plant. A fraction of organic waste used as foaming and collector
agents in the
froth flotation process of this invention is retained in the generated
tailings, leaving them in a
better condition for subsequent environmental remediation processes.
9
CA 02787724 2012-07-20
Both the first and second flotation stages (stages I and II), the
multifunctional flotation agent
(foaming and collector agent) of the present invention can be supplemented
with one or more
of the traditionally used frothers and/or collectors in a specific operation
of froth flotation of
sulfide or non-sulfide minerals; the amount of frother and/or collector added
will depend on
the desired characteristics and the critical variables of the process, which
are determined by
the specificities and peculiarities of each mineral concentration process.
The use of such auxiliary and traditional collector and/or foaming agents in
combination with
the multifunctional flotation agent (foaming and collector agent) of this
invention often
results in higher recoveries and consequently a better efficiency in the stage
of mineral iron
and/or copper concentration. In the case of collectors, any of the market
collectors, such as
compounds containing anionic and cationic polar groups (e.g. fatty acids,
xanthates, xanthate
esters, dithiocarbamates, mercaptans, thioureas and tionocarbamatos), can be
used with new
collectors shown in phases I and II of this invention (Figure 1). Also, a wide
variety of
foaming agents have been successfully used in the flotation of minerals from
sulfide ores,
such as alcohols dihydrocarbonated of low molecular weight (for example methyl
isobutyl
carbinol, MIBC, polyglycol, pine oils, polyglycol monoesters and alcohol
ethoxylates, etc.).
Any of them can be used in a complementary and synergistic way in the process
of this
invention.
While this invention may use a single flotation equipment, both in froth
flotation Phase I and
Phase II (Figure 1), it is preferred to use a multiple system of flotation
devices in both phases,
as this allows a better recovery of commercially valuable metals due to higher-
contacting
time of the flotation reagents with minerals and the possibility of adding
additional amounts
of collectors or auxiliary chemicals when they are required.
The froth flotation process of the present invention provides a better quality
copper
concentrate due to the lowest content of iron minerals, increasing its
commercial value for
sale in either the domestic or international market. However, the copper
concentrate obtained
by the present invention maintains an adequate iron content to the
requirements of the
smelting stage, in the case of those processes using Teniente converter
furnaces.
APPLICATION EXAMPLE
These application examples used a multifunctional flotation agent, with
collector and
foaming functions based on sanitary sludge (biosolids) and a foaming and
collector agent
based on humic substances whose characteristics are given in the following
Tables:
Table 2: General physical and chemical characteristics of biosolids
Parameters Value
CA 02787724 2012-07-20
Total Solids (%) 76.9
Organic matter (%)* 55.0
pH in water 7.5
Electrical conductivity (mS cm-I) 7.80
Density (g mL-1) 0.71
Total Ca (mg kg-I)* 20,313
Total Cu (mg kg')* 407
Total Zn (mg kg')* 1,222
Total Fe (mg kg')* 17,382
Total N (g kg-1)* 41.3
Total P (mg kg')* 21,210
Total SO4-2 (mg kg-5* 1,000
Total C (%) 31.7
Total N (%) 4.4
Fulvic Acids (%) 3.0
Humic Acids (%) 7.8
* Dry basis
Table 3: General chemical characteristics of Humic Substances
Parameter Value
C (%) 44.67
H(%) 5.87
N(%) 4.88
11
CA 02787724 2012-07-20
(%) 43.9
S(%) ND
P(%) ND
Total Acidity (mol/Kg) 12.3
COOH (mol/Kg) 4.1
Phenolic OH (mol/Kg) 8.2
Ash (%) 0.58
ND: Not determined
Unless otherwise indicated, all parts and percentages are based on dry weight.
The copper ore
used in this example consists primarily of chalcopyrite-pyrite, with an
average content of
0.74% copper and 4.50% iron and a particle size less than or equal to 400
microns.
Example 1: Foaming power: Measurement of surface tension
The surface tension measurements were performed on a Kriiss K8 tensiometer
using the Du
Nouy method at a room temperature of 18 C. Solutions of biosolids (BS),
humic substances
(HS) and methyl isobutyl carbinol (MIBC) were prepared with deionized
ultrafiltered water,
with a resistivity of 18 MS-2-cm (equivalent to 5.55 x 10-2 S cm -I electrical
conductivity), and
a surface tension of 72.1 mN The
concentrations tested for BS were 0, 1, 10, 25, 50 and
100 g L-I; for HS, 0,0.1, 1,5, 10 and 25 g LI and for MIBC, 0,0.1; 0.5, 1,
2.5, 5 and 7.5 g
I. The tested concentrations expressed in grams per liter of humic substances
are equivalent
to BS and HS. A pH adjustment for each BS, HS and MIBC solution, at pH 7 and
10 was
subsequently carried out, adding small aliquots of NaOH and 0.1 M HC1
solution. The
samples were measured at least four times for the various concentrations
tested. The results
obtained are shown in Figure 2.
Results showed that HS, BS and MIBC have a surfactant activity in the whole
concentration
range measured. The surface tension of the HS is pH dependent; showing that at
pH 10 is
more surfactant than at pH 7. A similar behavior showed BS and MIBC. Figure 2A
shows
that BS and MIBC are able to change the surface tension, determining that a
concentration of
100 g of
BS, the surface tension is 40 mN rn-I, while MIBC obtains a similar surface
tension with a concentration of 7.5 g L
Figure 2B shows that when correcting HS and BS concentrations for the
sedimented fraction
of these substances, biosolids have a behavior similar to MIBC. BS dosages
lower than 4 g
are shown to be more surfactant at both pH tested, compared to MIBC, and
therefore, they
have better foaming properties.
12
= CA 02787724 2012-07-20
Example 2: Foamability Measurement and foam stability.
Foaming tests were performed using the Bikerman method. This method determines
the
dynamic generation of foam, E and the static stability, T. In each trial, 20
mL of solution
according to the following foaming concentrations of methyl isobutyl carbinol
(MIBC),
humic substances (HS) and biosolids (BS): 0.1, 1, 5 and 10 g L1 were used. The
samples
were prepared with double distilled water, adjusting the initial pH of the
solutions with small
solution aliquots of NaOH and 0.1 M HC1 to reach pH 7 and 10, agitating and
homogenizing
the samples for 10 minutes at 200 rpm. All trials were performed in duplicate
and at room
temperature.
The dynamic foam generation is produced continuously by injection of
atmospheric air. To
do so, a dry air compressor was used with four air flows 1, 2, 3 and 4 L min-
I. The injected air
passed through an air flow meter (Gilmont Instruments, Inc., USA) and then
through Pyrex
glass filter of porosity grade 2, with an average diameter between 40 and 100
jinn. The test
sample (20 mL of solution) was inside the filter. The air passed through the
liquid in a
column, and for each flow of air injected, the height of the foam at steady
state was
determined. The inaccuracy in measuring the foam height at steady state was
1 cm,
depending on the type and concentration of foaming and air flow used. Also,
the static
stability of the foam was quantified T, which corresponds to the total time
until total decrease
of the foam produced, once the gas flow is turned off. The results are shown
in Table 4.
labia 4: Bikerman parameters for Humic Substances (SH), Biosolids (BS) and
Metil-lsobutil-Carbinol
(MIBC).
c (s) t1 (s) DS T2 (s) DS t3 (s) DS
t4 (s) DS
pH pH pH pH
pH
Conc.
Reagent 7 10 7 10 7 10 7 10
7 10
(g/1.)*
HS 0.9 0.8 0.4 0.1 0.4 0.1 0.5 0.1 0.5
0.1 0.6 0.1 0.6 0.1 0.8 0.1 0.8 0.1
MIBC 2.1 1.7 4.4 0.1 3.6 0.1 4.1 0.2 4.3
0,2 5.1 0,2 4.7 0.1 4.6 0,2 6.9 0.7
0.1
13.5 15.4
BS 0.5 0.9 4.8 0.3 2.0 0.3 7.8 0.3
1.8 0.3 0.8 2.7 0.5 0.9 2.1 + 0.1
11.6 10.7
16.2
HS 1 1.2 0.9 4.1 0.6 7.9 1.0 2.1 0.3
0.8 2.1 0.2 2.1 1.9 0.8
1.0 MIBC 2.1 1.6 5.6 0.4 8.2 0.2 7.2 0.2 9.6
0.3 6.1 0.1 8.1 0.3 4.8 0.1 5.6 0.3
12.0 18.3
BS 2.2 1.5 5.6 0.6 3.2 0.2 6.9 0.6
3.5 0.5 1.3 3.9 0.5 1.9 3.3 0.4
13
CA 02787724 2012-07-20
16.9 27.2 17.7
HS 2.1 2.3 1.3 37.9 3.8 7.2 0.3 1.4 7.3
1.0 24.1 1.3 5.5 0.6 1.0
5.0 MIBC 1.9 3.1 9.9 0.4 9.7 0.3 6.6 0.1 9.1
0.3 5.9 0.1 7.4 0.2 6.3 0.3 9.2 0.1
13.4 18.6 10.3 19.9 31.7 12.6
BS 2.3 1.3 0.5 24.4 4.1 1.8 2.3 2.1
11.1 2.1 3.4 3.6
99.9 81.8 80.0 65.4 32.0 20.5
SH 1.1 1.8 5.4 120.0 4.0 8.5 2.0 3.1
40.3 5.0 1.6 2.7
11.4
10.0
MIBC 4.2 3.9 7.5 0.2 6.4 0.1 8.3 0.2
7.7 0.2 9.4 0.1 8.9 0.2 0.5 9.3 0.2
44.4 53.0 10.1 49.7 54.4 18.6
BS 3.8 3.2 3.1 24.2 1.2 3.6 0.3 2.9
11.3 0.3 3.7 1.1
* The Humic substance (HS) and Biosolids (BS) concentration are expressed in
HS grams per liter of
solution.
Table 4 above shows that for all concentrations and pH tested, HS, MIBC and BS
can
generate foam. For HS and BS, the pH has an effect on the volume of foam
generated. In all
cases, the foam volume presents a linear dependence on the gas flow.
HS, BS and MIBC show a positive relationship between concentration and the
generation and
static stability of the foam. Concentrations of 0.1 and 1 g L-I of HS, BS and
MIBK have
T values that increase depending on the airflow, but at concentrations of 5
and 10 g Li of HS
and BS, the relationship is opposite, showing that for a specific
concentration, when
increasing the air flow, T decreases drastically. By increasing the air flow,
the foam is more
unstable, promoting coalescence of the bubbles produced. Also, BS show
Bikerman
parameters (c and T) of similar magnitude to those obtained for MIBC, for
both, the
concentrations and airflows tested.
Example 3: Collector Power: Film flotation Tests
The "film flotation" technique determines the hydrophilic and hydrophobic
fractions of a
mineral and/or mineral species exposed to different mixtures of water:
alcohol. Humic
substances (HS), biosolids (BS) and goat manure (GM) were added in a dosage of
1.5% of
humic substances (w/w, dry basis), while the industrial chemical collector
reagents (ICCR)
were used in the following dosages: dialkyl dithiophosphate potassium (Lib-K),
16 g ton-I;
isobutyl xanthate, sodium 5 g ton-I; mercaptan (P-3), 11 g ton-I. Mineral
samples (copper
sulfide mineral, chalcopyrite, and pyrite) were conditioned by the addition of
collector
reagents (SH, BS, GM and ICCR) for a period between 10 and 20 minutes.
Afterwards, the
pH was adjusted with HC1 and/or NaOH, and each experimental condition was
agitated on a
shaker for 3 hours at 25 C. In each trial, a particle size between 75 and
106 microns was
14
CA 02787724 2012-07-20
used. Depending on the wettability characteristics of the solid in each sample
and at a given
surface tension of the mixture water:alcohol, the hydrophilic fraction was
recovered, dried
and weighed, and using mass difference, the hydrophobic fraction was
quantified. The results
for the experimental condition of 100% water are seen in Figure 3.
Figure 3 shows that the natural buoyancy, without addition of reagents, of the
copper sulfide
mineral and mineralogical species, such as chalcopyrite and pyrite, is low
(around 10%). The
use of ICCR changes the natural buoyancy of the copper sulfide mineral and
mineralogical
species, making chalcopyrite and pyrite float 40%. ICCR make such
mineralogical species to
float in a non-selectively way, increasing the natural hydrophobicity of both
mineralogical
species. The HS increased the natural buoyancy of copper sulfide ore and/or
mineralogical
species in 15%. BS and GM show a better affinity with pyrite compared to
chalcopyrite. BS
makes pyrite to float in a 42%, while GM results in 37.5% of this mineral
species to float.
Now regarding chalcopyrite, BS reaches 21% and GM 25%. Therefore, BS and GM
behaved
similarly regarding the sulfide mineral, chalcopyrite and pyrite tested,
showing more
selectiveness for pyrite. At the same time, BS and GM change the natural
buoyancy of copper
sulfide mineral, making it possible to float 36% and 26% of the mineral,
respectively.
Example 4: Denver Cell Froth Flotation Test
In the Denver cell tests, a copper sulfide mineral with a particle size
between 30 and 300
microns (greater at 400 mesh and lower at 50 mesh) was used. A solid
concentration of 30%
was used; the pulp was stirred at 1100 rpm while maintaining a pH between 10
and 11, at
room temperature. PH adjustment was made with lime and/or NaOH. Tests with
industrial
chemical reagents were used in the following dosage: 300 g ton' lime; 250 2.5
g ton-I
DowFroth; 25 g ton-I methyl isobutyl carbinol; 16 g ton-1 dialkyl
dithiophosphate potassium
(Lib-K); 5 g ton' isobutyl xanthate, sodium; 11 g ton-I mercaptan (P-3).
Biosolids (BS) and
humic substances (HS) were used as frothing and collector agents in a dosage
of 1.5% of
humic substances (w/w dry basis). For all experimental conditions tested a
conditioning time
of 10 minutes was used. The experimental procedure considers the opening of
the air
injection valve of the cell to form a froth phase in the pulp, which is
extracted from the
surface of the froth using the rotating paddle and the following times: 1 -3
minutes, 3-6
minutes 6-10 minutes 10-14 minutes 14-18 minutes. At such times, concentrate
samples are
collected, filtered, dried and chemically analyzed via atomic absorption
method.
The experimental conditions tested in Denver cell are described in the
following table:
N Experimental Condition
Mineral Collector and Frothing Agents pH Adjustment
1 Copper Sulfide Mineral ICCR + ICFR Cal
2 Copper Sulfide Mineral ICCR+ ICFR NaOH
3 Copper Sulfide Mineral Biosolids (BS 1, typel) NaOH
4 Copper Sulfide Mineral Biosolids (BS 2, type 2) NaOH
Copper sulfide Mineral Humic Substances (HS 1, type 1) NaOH
6 Copper Sulfide Mineral Humic Substances (HS 2, type 2) NaOH
CA 02787724 2012-07-20
Type 1 and type 2 biosolids (BS 1 and BS 2) refer to biosolids samples from
the same
household wastewater treatment plant; BS 1 was generated at least 2 years
before BS 2.
Type 1 and type 2 Humic substances (HS 1 and HS 2) refer to the same material
tested in two
different runs (repetitions).
ICCR= Industrial chemical collector reagent (dialkyl dithiophosphate
potassium, sodium
isobutyl xanthate, mercaptan)
ICFR = Industrial chemical froth reagent (DowFroth, methyl isobutyl carbinol)
Concentrated copper and iron grade results are shown in Figure 4. The results
prove that BS
can recover a concentrate with a copper grade lower than that obtained with HS
and ICCR +
ICFR. However, BS produces a concentrate with an iron grade similar to that
obtained with
HS and ICCR + ICFR. Figure 4B shows that BS can recover a concentrate with a
high iron
grade. Also, the extract of the collector and foaming reagent, i.e., humic
substances shows in
Figure 4A that such reagent recovers a copper concentrate with a higher grade
during the first
minutes of flotation, compared to the copper concentrate grade copper using
ICCR +
ICFR. As it seems evident from the examples, biosolids are effective frothers
and collectors
of iron in froth flotation systems, while humic substances are effective
copper collectors in
the froth flotation systems at levels comparable with standard flotation
reagents used.
The present invention has been explained (pictured) in relation to some of its
possibilities, but
it must be understood that these examples and specific information given are
not intended to
limit the spirit or field of the claimed invention.
16