Note: Descriptions are shown in the official language in which they were submitted.
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
1
TITLE OF THE INVENTION
METHOD FOR THE PREVENTION AND TREATMENT OF DIASTOLIC
DYSFUNCTION EMPLOYING AN APOLIPOPROTEINAI (APOA1) MIMETIC
PEPTIDE/PHOSPHOLIPID COMPLEX
This application claims priority from US Provisional Patent Applications
Serial
Number 61/202,051 filed January 23, 2009 and 61/202,191 filed February 5,
2009.
FIELD OF THE INVENTION
[001] The present invention relates to the general field of medical methods
and
compounds and is particularly concerned with a method and compound for the
prevention and treatment of diastolic dysfunction.
BACKGROUND OF THE INVENTION
[002] Diastolic dysfunction is a condition caused by an abnormal filling of
the heart
during diastole. This condition can cause heart failure, pulmonary edema and
many other incapacitating and possibly life-threatening consequences. There is
no effective and side-effect free treatment suitable for all patients for this
condition.
Hence, there exists a need for a new treatment of diastolic dysfunction.
[003] An object of the present invention is therefore to provide a novel
treatment of
diastolic dysfunction.
SUMMARY OF THE INVENTION
[004] In a broad aspect, the invention provides a method for preventing of
treating
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
2
a diastolic dysfunction in a subject, the method comprising administering to a
subject in need of preventing or treating a diastolic dysfunction a
therapeutically
effective amount of a reverse lipid transport agonist to prevent or treat said
diastolic dysfunction. For example, the lipid transport agonist is a reverse
cholesterol transport agonist.
[005] In some embodiments of the invention, the diastolic dysfunction is a
ventricular diastolic dysfunction, a left ventricular diastolic dysfunction,
or any other
diastolic dysfunction.
[006] In some embodiments of the invention, the reverse lipid transport
agonist is
selected from the group consisting of: an HDL, a peptide with HDL-like
physiological effects, a peptide with HDL-like physiological effects complexed
to a
lipid, an HDL-mimetic agent, a CETP modulator, an SRB1 modulator, an
LXR/RXR agonist, an ABCA1 agonist, a PPAR agonist and an Apolipoprotein A-I
(APOA1) mimetic peptide/phospholipid complex.
[007] In this latter case, administering the APOA1 mimetic
peptide/phospholipid
complex may include injecting the APOA1 mimetic peptide/phospholipid complex
in the subject. Examples of dosages in this case are of from about 1 fag to
about
10 g per kg body weight of the subject, about 1 mg to about .5 g per kg body
weight of the subject, and about 25 mg per kg body weight of the subject.
[008] For example, the APOA1 mimetic peptide has the sequence of SEQ ID NO:
1 found herein below, and the APOA1 mimetic peptide may be complexed with
egg sphingomyelin and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC).
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
3
[009] In some embodiments, the subject is a mammal, for example a human.
[0010] A method of controlling a diastolic dysfunction in a subject, the
method
comprising providing, in a subject in need of controlling a diastolic
dysfunction, an
increased amount of reverse cholesterol transport for controlling the
diastolic
dysfunction..
[0011] In yet another broad aspect, the invention provides a method for
preventing
or reversing diastolic dysfunction, the method comprising administering to a
patient in need thereof a reverse lipid transport agonist.
[0012] In yet another broad aspect, the invention provides a method for
controlling
a diastolic dysfunction in a subject, the method comprising administering to a
subject in need thereof a therapeutically effective amount of a reverse lipid
transport agonist.
[0013] For example, controlling the diastolic dysfunction may include reducing
a
rate of progression of the diastolic dysfunction, or reversing, at least in
part, the
diastolic dysfunction.
[0014] In yet another broad aspect, the invention provides the use of a
reverse
lipid transport agonist for controlling diastolic dysfunction in a subject.
[0015] In yet another broad aspect, the invention provides the use of a
reverse
lipid transport agonist for the manufacture of a pharmaceutical composition of
matter for controlling diastolic dysfunction in a subject.
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
4
[0016] In yet another broad aspect, the invention provides a method of
preventing
or treating a diastolic dysfunction in a subject, the method comprising
administering, to a subject in need of preventing or treating a diastolic
dysfunction,
a therapeutically effective amount of a an Apolipoprotein A-I (APOA1) mimetic
peptide/phospholipid complex to prevent or treat said diastolic dysfunction.
[0017] In a variant, the method comprises the administration of a
therapeutically
effective amount of a compound, referred to hereinafter as compound A, that
mimics biologic properties of Apolipoprotein A-I (APOA1). Compound A and other
suitable compounds are described in US Patent Nos. 6,287,590, issued Sep. 11,
2001, and 6,506,799, issued Jan. 14, 2003, which are hereby incorporated by
reference in their entirety. Indeed, it is believed that in view of current
knowledge
regarding the action of the compounds and molecules described in these
Patents,
results similar to those presented herein are obtainable with these compounds
and
molecules.
[0018] An experimental study was performed to determine if APOA1
mimetic peptide infusions could induce reduction of diastolic dysfunction, and
more
specifically, left ventricular diastolic dysfunction.
[0019] Experimental approach: Twelve New-Zealand White male rabbits
received a cholesterol-enriched diet and vitamin D2 until significant aortic
valve
stenosis was detected by echocardiography. The enriched diet was then stopped
to mimic cholesterol-lowering therapy and animals were randomized to receive
saline (control group, n=6) or an APOA1 mimetic peptide (treated group, n=6),
3
times per week for 2 weeks. Before sacrifice, left ventricular diastolic
dysfunction
was studied using transthoracic echocardiography and classified either as
normal,
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
mild, moderate or severe dysfunction based on established criteria.
[0020] Results: At the end of the treatment, left ventricular diastolic
filling patterns
were distributed differently among groups (P=0.018). Left ventricular
diastolic
dysfunction (DD) was attenuated by APOA1 mimetic peptide infusions (33.3% of
5 normal DD and 66.6% of mild DD vs. 66.6% of mild DD and 33.3% of severe DD
for control rabbits).
[0021] Conclusions and implications: Infusions of APOA1 mimetic peptide lead
to reduction of left ventricular diastolic dysfunction in a
hypercholesterolemic rabbit
model. Treatment of diastolic dysfunction is a new application for HDL-based
therapies.
[0022] Other objects, advantages and features of the present invention will
become more apparent upon reading of the following non-restrictive description
of
preferred embodiments thereof, given by way of example only with reference to
the accompanying drawing.
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] In the appended drawings:
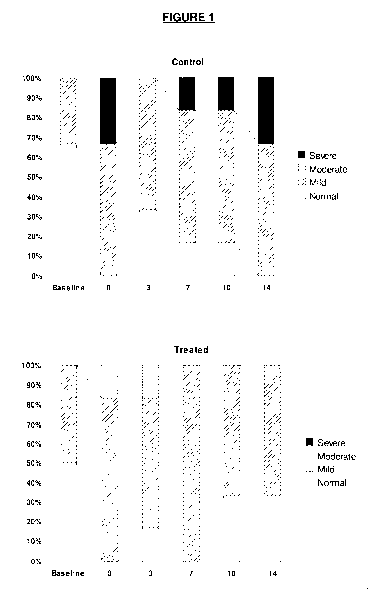
[0024] The only Figure illustrates the effect of the proposed treatment by
comparing the distribution of diastolic dysfunction severity in control (upper
panel)
and treated (in lower panel) subjects as a function of time.
DETAILED DESCRIPTION
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
6
[0025] In this document:
[0026] The term "treating" or "treatment" of a state, disease, disorder or
condition
includes:
[0027] (1) preventing or delaying the appearance of clinical
symptoms of the state, disease, disorder or condition developing in a subject
that
may be afflicted with or predisposed to the state, disease, disorder or
condition but
does not yet experience or display clinical or subclinical symptoms of the
state,
disease, disorder or condition;
[0028] (2) inhibiting the state, disease, disorder or condition, i.e.,
arresting or reducing the development of the state, disease, disorder or
condition
or at least one clinical or subclinical symptom thereof; or
[0029] (3) relieving the state, disease, disorder or condition, i.e.,
causing regression of the state, disease, disorder or condition or at least
one of its
clinical or subclinical symptoms.
[0030] The benefit to a subject receiving treatment is either statistically
significant
or at least perceptible to the subject or to the physician.
[0031] The term "subject" includes mammals (especially humans) and other
animals, such as domestic animals (e.g., household pets including cats and
dogs)
and non-domestic animals (such as wildlife).
[0032] A "therapeutically effective amount" means the amount of a compound
that,
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
7
when administered to a subject for treating a state, disease, disorder or
condition,
is sufficient to effect such treatment. The "therapeutically effective amount"
will
vary depending on the compound, the state, disease, disorder or condition and
its
severity and the age, weight, physical condition and responsiveness of the
subject
receiving treatment.
[0033] Pharmaceutical Compositions
[0034] The pharmaceutical composition of the present invention comprises at
least
one compound of the present invention and a pharmaceutically acceptable
excipient (such as a pharmaceutically acceptable carrier or diluent).
Preferably,
the pharmaceutical composition comprises a therapeutically effective amount of
the compound(s) of the present invention. The compound of the present
invention
may be associated with a pharmaceutically acceptable excipient (such as a
carrier
or a diluent) or be diluted by a carrier, or enclosed within a carrier which
can be in
the form of a capsule, sachet, paper or other container.
[0035] Examples of suitable carriers include, but are not limited to, water,
salt
solutions, alcohols, polyethylene glycols, polyhydroxyethoxylated castor oil,
peanut
oil, olive oil, gelatin, lactose, terra alba, sucrose, dextrin, magnesium
carbonate,
sugar, cyclodextrin, amylose, magnesium stearate, talc, gelatin, agar, pectin,
acacia, stearic acid or lower alkyl ethers of cellulose, silicic acid, fatty
acids, fatty
acid amines, fatty acid monoglycerides and diglycerides, pentaerythritol fatty
acid
esters, polyoxyethylene, hydroxymethylcelIulose and polyvinylpyrrolidone.
[0036] The carrier or diluent may include a sustained release material, such
as
glyceryl monostearate or glyceryl distearate, alone or mixed with a wax.
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
8
[0037] The pharmaceutical composition may also include one or more
pharmaceutically acceptable auxiliary agents, wetting agents, emulsifying
agents,
suspending agents, preserving agents, salts for influencing osmotic pressure,
buffers, sweetening agents, flavoring agents, colorants, or any combination of
the
foregoing. The pharmaceutical composition of the invention may be formulated
so
as to provide quick, sustained, or delayed release of the active ingredient
after
administration to the subject by employing procedures known in the art.
[0038] The pharmaceutical compositions of the present invention may be
prepared
by conventional techniques, e.g., as described in Remington: The Science and
Practice of Pharmacy, 20th Ed., 2003 (Lippincott Williams & Wilkins). For
example, the active compound can be mixed with a carrier, or diluted by a
carrier,
or enclosed within a carrier, which may be in the form of an ampoule, capsule,
sachet, paper, or other container. When the carrier serves as a diluent, it
may be a
solid, semi-solid, or liquid material that acts as a vehicle, excipient, or
medium for
the active compound. The active compound can be adsorbed on a granular solid
container, for example, in a sachet.
[0039] The pharmaceutical compositions may be in conventional forms, for
example, capsules, tablets, aerosols, solutions, suspensions or products for
topical application.
[0040] The route of administration may be any route which effectively
transports
the active compound of the invention to the appropriate or desired site of
action.
Suitable routes of administration include, but are not limited to, oral,
nasal,
pulmonary, buccal, subdermal, intradermal, transdermal, parenteral, rectal,
depot,
subcutaneous, intravenous, intraurethral, intramuscular, intranasal,
ophthalmic
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
9
(such as with an ophthalmic solution) or topical (such as with a topical
ointment).
[0041] The experiments described herein below show that a strategy directed to
increasing the efficiency of the reverse lipid transport mechanism through the
use
of a suitable compound is a possible approach in the treatment and prevention
of
diastolic dysfunction.
[0042] The compound used to test this hypothesis, hereinafter referred to as
Compound A, is a lipoprotein that mimics biologic properties of apolipoprotein
A-I
(APOA1). This type of compound, an APOA1 mimetic or agonist, is described in
further detail in U.S. Patent No. 6,376,464 titled "Lipid complexes of APO A-1
agonist compounds," issued to Dasseux et al. on April 23, 2002. This document
is
hereby incorporated by reference in its entirety.
[0043] Briefly, these compounds include peptides, or analogues thereof, which
are
capable of forming amphipathic alpha-helices in the presence of lipids and
which
mimic the activity of APOA1. They are therefore referred-to as APOA1 agonists.
The agonists have as their main feature a "core" peptide composed of 15 to 29
amino acid residues, preferably 22 amino acid residues, or an analogue thereof
wherein at least one amide linkage in the peptide is replaced with a
substituted
amide, an isostere of an amide or an amide mimetic.
[0044] These APOA1 agonists are based, in part, on the discovery that altering
certain amino acid residues in the primary sequence of the 22-mer consensus
sequence disclosed in Venkatachalapathi et al., 1991, Mol. Conformation and
Biol.
Interactions, Indian Acad. Sci. B: 585-596 (PVLDEFREKLNEELEALKQKLK;
hereinafter "Segrest's consensus 22-mer" or "consensus 22-mer") that were
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
thought to be critical for activity, yields synthetic peptides which exhibit
activities
that approach, or in some embodiments even exceed, the activity of native
APOA1. It was discovered that replacing three charged amino acid residues in
Segrest's consensus 22-mer peptide (Glu-5, Lys-9 and Glu-13) with a
hydrophobic
5 Leu residue provides peptides that mimic the structural and functional
properties of
APOA1 to a degree that was unprecedented in the art.
[0045] Based on their known biological activity and structures, it is believed
that
other compounds, such as the compounds presented in the above-mentioned U.S.
Patent No. 6,376,464, will show effects similar to Compound A.
10 [0046] In addition, while the reverse lipid transport agonist function of
Compound
A, and of other related compounds, is hypothesized to be related to
improvements
in the treatment and prevention of diastolic dysfunction, other aspects of
Compound A are also hypothesized to play a role in the beneficial effects of
Compound A and related compounds. For example, the effect of the APOA1
mimetic could be through other functions of HDL-related therapies, such as
decreased inflammation or improved endothelial function. For example, the
phospholipid composition of the mimetic may have its importance in the anti-
inflammatory action.
[0047] Example
[0048] Methods
[0049] Animals and experiments
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
11
[0050] Animal care and procedures complied with the Canadian Council on Animal
Care guidelines and were approved by the institutional ethics committee for
animal
research.
[0051] Twelve male New-Zealand White rabbits (2.7-3.0 kg, aged 12-13 weeks)
were fed with a 0.5% cholesterol-enriched diet (Harlan, Indianapolis, Indiana,
USA) plus vitamin D2 (50000 IU per day; Sigma, Markham, Canada) in the
drinking water until significant AVS, as defined by a >_10% decrease of aortic
valve
area or of the transvalvular velocities ratio (V1/V2), could be detected by
echocardiography (as described in Busseuil D, Shi Y, Mecteau M, Brand G,
Kernaleguen AE, Thorin E, Latour JG, Rheaume E, Tardif JC (2008). Regression
of aortic valve stenosis by ApoA-I mimetic peptide infusions in rabbits. Brit
J
Pharm 154(4):765-73, the contents of which are hereby incorporated by
reference
in its entirety).
[0052] The animals then returned to a standard diet (without vitamin D2) to
mimic
cholesterol-lowering therapy and were randomly assigned to receive either
saline
(control group, n=6) or the APOA1 mimetic peptide (treated group, n=6).
Rabbits
were given injections through the marginal ear vein of saline or of the APOA1
mimetic peptide (25 mg/kg) complexed with phospholipids, 3 times per week for
2
weeks. Echocardiograms were performed serially (see Echocardiography
Methods), including every 3 to 4 days throughout the randomized treatment
period. Two days after their last infusion, the animals underwent a final
echocardiogram and were sacrificed.
[0053] APOA I mimetic peptide
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
12
[0054] The APOA1 mimetic peptide (Compound A) of sequence: H-Pro-Val-Leu-
Asp-Leu-Phe-Arg-Glu-Leu-Leu-Asn-Glu-Leu-Leu-Glu-Ala-Leu-Lys-Gln-Lys-Leu-
Lys-OH (SEQ ID NO 1) was synthesized by Polypeptide Laboratories (Torrance,
CA, USA), and purity assessed by high performance liquid chromatography and
mass spectral analysis was greater than 98%. The peptide was complexed with
egg sphingomyelin and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (Avanti
Polar
Lipids. Alabaster, AL, USA) in a 1:1:1 weight ratio by mixing the components
in
saline and performing multiple heating and cooling cycles until the solution
appeared perfectly clear. Fresh solution was prepared every week under sterile
conditions and kept at 4 C.
[0055] Echocardiography
[0056] Transthoracic echocardiographic studies were performed at baseline, on
a
weekly basis starting at 8 weeks of hypercholesterolemic diet until
significant AVS
developed, and then after 4, 7, 10 and 14 days of APOA1 mimetic peptide or
saline
control treatments. Studies were carried out with a phased-array probe 1 OS
(4.5
11.5 Megahertz) and a Vivid 7 Dimension system (GE Healthcare Ultrasound,
Horten, Norway). Intra-muscular injections of ketamine (45 mg/kg) and
midazolam
(0.75 mg/kg) were used for sedation.
[0057] Left ventricular (LV) M-mode spectrum was obtained in parasternal long-
axis view to measure LV diameters at both end cardiac diastole (LVDd) and
systole (LVDs). LV fractional shortening was calculated as (LVDd - LVDs) /
LVDd
x 100%. Teicholz method was employed to calculate LV volumes and LV ejection
fraction (EF). Pulsed wave Doppler was used to evaluate transmitral flow (TMF)
and pulmonary venous flow (PVF) in apical 4-chamber view. TMF was used to
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
13
measure the peak velocities during early filling (E) and atrial filling (A)
and to
calculate the E/A ratio. PVF was used to measure the systolic flow (S),
diastolic
flow (D) and reversed atrial flow (Ar). LV basal lateral peak systolic
velocities (Sm)
and mitral annulus velocities during early filling (Em) and atrial filling
(Am) were
derived by tissue Doppler imaging (TDI). The time intervals from the end of Am
to
the beginning of Em (b), and from the beginning to the end of Sm (a) were also
measured on lateral wall TDI.
[0058] Left ventricular diastolic dysfunction (LVDD) was classified according
to
published criteria (Khouri et al., 2004). To further evaluate LVDD, left
atrium (LA)
M-mode spectrum was obtained in parasternal long-axis view at the aortic valve
level and LA dimensions were measured in both end cardiac diastole and
systole.
LA fractional shortening was calculated as (systolic dimension - diastolic
dimension) / systolic dimension x 100%. The average of 3 consecutive cardiac
cycles was used for each measurement.
[0059] All echocardiographic imaging and measurements were performed
throughout the protocol by the same experienced investigator blinded to
randomized treatment assignment.
[0060] Statistical analyses
[0061] Diastolic dysfunction classification was compared across groups using
chi-
square test. All analyses were done with SAS version 9.1 (SAS Institute Inc.,
Cary,
NC, USA) and conducted at the 0.05 significance level.
[0062] Results
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
14
[0063] With Reference to the only Figure, the distribution of the pattern of
DD
classification evolved differently in the control and treated groups. Whereas
severe
DD appeared in some control animals after 7 days of treatment, no moderate nor
severe DD could be detected in treated animals.
[0064] At the end of the treatment, LV diastolic filling patterns were
distributed
differently among groups (P=0.018). Left ventricular diastolic dysfunction
(DD) was
attenuated by APOA1 mimetic peptide infusions (33.3% of normal DD and 66.6%
of mild DD vs. 66.6% of mild DD and 33.3% of severe DD for control rabbits).
[0065] Conclusion
[0066] Infusions of an APOA1 mimetic peptide lead to reduction of left
ventricular
diastolic dysfunction in a hypercholesterolemic rabbit model. Treatment of
diastolic
dysfunction may represent a new application for HDL-based therapies.
[0067] This example suggests that similar results are obtainable in humans
using
any suitable HDL-based therapy, such as for example one or more infusion(s) or
bolus(es) of HDL or peptide (with or without lipids) with HDL-like effects,
orally
administered HDL-mimetic agents, and/or the administration of cholesteryl
ester
transfer protein (CETP) modulators, or scavenger receptor class B, member 1
(SRB1) modulators or liver X receptor (LXR)/retinoid X receptor (RXR)
agonists, or
ATP-binding cassette transporter-1 (ABCA1) agonists, or peroxisome
proliferator-
activated receptor (PPAR) agonists, among others.
[0068] While the experiments described herein concerned the regulation of
diastolic dysfunction, one of ordinary skilled in the art will readily
appreciate that
CA 02788223 2012-07-26
WO 2010/083611 PCT/CA2010/000108
these experiments may be predictive of biological effects in humans or other
mammals and/or may serve as models for use of the present invention in humans
or other mammals for any other similar cardiac dysfunction.
[0069]Although the present invention has been described hereinabove by way of
5 preferred embodiments thereof, it can be modified, without departing from
the
spirit and nature of the subject invention as defined in the appended claims.