Note: Descriptions are shown in the official language in which they were submitted.
CA 02789790 2012-09-06
PROCESS AND APPARATUS FOR GASIFICATION OF REFUSE
FIELD OF THE INVENTION
The present invention relates to synthetic fuel gas production from low cost
carbonaceous
materials and waste products such as biomass, municipal solid waste, plastic
and rubber
residues, wastewater treatment sludge, pulp and paper liquors, heavy petroleum
residues.
More specifically, the present invention relates to an apparatus and method
for the
production of a synthetic gas sufficiently clean to comply with current
environmental
regulations.
BACKGROUND OF THE INVENTION
Synthetic gas has various uses such as:
= fuel for gas burners/boilers for hot water or steam generation
= fuel for internal combustion engines (Diesel, Otto, Stirling)
= fuel for gas turbines to generate electricity
= feedstock for chemical synthesis
feedstock for the production of hydrogen-rich gas by shifting the CO and
reforming any
hydrocarbons present in the synthetic gas.
Gasification is a known technique for converting carbonaceous materials to
valuable
combustible synthetic gas. In a gasification reactor, a fraction of the
feedstock is oxidized
thereby generating high temperatures inside the reactor (exothermic reaction).
The
remainder of the feedstock decomposes at the high temperatures generated by
the
oxidation reactions generating hot combustible synthetic gas and small amounts
of char
(endothermic reaction). The reactor is constantly fed with carbonaceous
material and
oxygen-containing gas to keep the exothermic reaction going so as to maintain
a high
temperature inside the reactor. Typically, the oxygen supply is about 25-30%
of
stochiometric values for total combustion. The partial oxidation regime
present in the
reactor renders the process self-sufficient in energy. Additional reaction
processes taking
place concurrently with thermal decomposition in the gasification reactor are
steam
reforming, the Boudouard reaction and the shift conversion reaction. The
extent of these
reactions determines, to a large degree, the composition of the synthetic gas
produced.
CA 02789790 2012-09-06
2
At steady-state, a typical gasification reactor operates at temperatures above
700 C and
pressures above 101 kPa.
Current gasification technology improvements focus on gasification reactor
design and gas
conditioning operations prior to its final use.
U.S. Patent 4,968,325 discloses a biomass gasification process and plant
design. This process
is commercially known as BIOSYN (trademark). The process uses a fluid bed
gasification
reactor containing fine sand fluidized by finely dispersed bubbles, introduced
through a
conveniently placed grid at the bottom of the reactor, of an oxygen-containing
gas.
Technical viability of this process has been demonstrated for wood residues at
capacities as
high as 10 tones/h. Moreover, the synthetic gas resulting from wood residue
gasification is
not sufficiently "clean" to be used as a fuel in modern energy conversion
devices. What is
meant by "clean" is a synthetic gas free of harmful contaminants such as tar
and particulate
material including chemically aggressive species as defined by current
environmental
regulations.
U.S. Patent 4,448,588 relates to a synthetic gas conditioning process. What is
meant by
"conditioning" is the treatment of the synthetic gas prior to its use as a
fuel or other
intended use. The process shows the use of the carbon-rich solids, i.e. char,
resulting from
gasification to remove organic vapors from the synthetic gas.
There is still a need for a process and apparatus that overcomes the drawbacks
of the prior
art, more specifically, there is a need for a versatile and commercially
valuable process and
apparatus for gasification of various sources of refuse and industrial by-
products and
wastes.
There is also a need for synthetic gas conditioning steps and related
apparatus for purifying
the synthetic gas and minimizing the final effluents to be disposed of.
The scope of applicability of the present invention will become apparent from
the detailed
description given hereinafter. It should be understood, however, that this
detailed
description, while indicating preferred embodiments of the
CA 02789790 2012-09-06
WO 00/69994 PCT/CAOO/00552
3
invention, is given by way of illustration only, since various changes and
modifications
within the spirit and scope of the invention will become apparent to those
skilled in the art.
SUMMARY OF THE INVENTION
In general terms, the present invention provides for improvements relating to
a gasification
reaction vessel for converting carbonaceous feedstock to synthetic gas_ The
reaction vessel
being of the type having a bottom portion with a grid for retaining a bed of
fluidizable and
heat-retaining particulate material. The latter is most preferably silica sand
of average
diameter of 200 to 700 ,um. The bed is fluidized by a stream of oxygen-
containing gas fed
through the grid tuyeres, the bottom portion also being adapted to receive,
laterally, a feed
of carbonaceous material to be gasified, the reaction vessel further having a
top portion for
recovering and evacuating the synthetic gas produced in the bottom portion.
The
improvements generally consisting of: a top portion of the reaction vessel
having an
enlarged average internal cross-sectional area compared to the average cross-
sectional area
of the bottom portion so as to facilitate disengagement and removal of said
synthetic gas
from the bed of particulate material.
The present invention also provides an improved gasification process using a
reaction
vessel containing a fluidized bed for receiving a carbonaceous material
feedstock for
gasification and a fluidizing gas, the improvement in the gasification process
consisting of:
providing as the fluidization gas an oxygen-enriched air containing up to 50%,
preferably
- 40%, oxygen to said reaction vessel (percentages based on volume).
More specifically, the present invention relates to a process for the
gasification of a biomass
25 material, the process comprising:
a) selecting a biomass material derived from forest, agricultural or urban
wastes, having a
given moisture content;
b) pre-drying the biomass material to obtain a pre-dried biomass material
having a moisture
content below about 20% in weight;
30 c) converting the pre-dried biomass material to a synthetic gas in a
gasification reaction
vessel, the vessel having a lower section with a grid retaining a bed of
granular material
fluidized by a stream of gas fed through the grid, the lower section receiving
the pre-
CA 02789790 2012-09-06
3a
dried biomass material to be gasified, and an upper-section for recovering and
evacuating
the synthetic gas produced in the lower section, the upper-section having an
enlarged
internal cross-sectional area compared to the lower section, and wherein a
height of the
bed at rest is comprised between 1.5 and 2 times an internal cross-section of
the lower-
section, the process also comprising providing to the feedstock a stream of
oxygen-enriched
air having an oxygen content comprised between 30% and 42% vol. oxygen.
The present invention also relates to a process for the gasification and
conditioning of a
carbonaceous material feedstock, the process comprising: achieving
gasification of a feedstock
of carbonaceous material in a reaction vessel comprising a fluidized
gasification bed of
particulate material, a feed port for feeding the carbonaceous material and a
grid bearing
tuyeres for introducing a fluidization gas to the gasification bed, the
improvements in the
gasification process consisting of: selecting as the carbonaceous material
feedstock a forest,
agricultural or urban waste biomass material, providing as the fluidization
gas an oxygen-
enriched air containing about 25 to about 40% oxygen to the reaction vessel,
and conducting
the gasification at temperatures ranging between about 680 and about 765 C and
submitting
the resulting synthetic gas to a conditioning process for removing impurities
and pollutants, the
improvement in the conditioning process consisting of: subjecting the
synthetic gas to a
sequential treatment in at least one cyclone to remove particulate material
therefrom, followed
by treatment in at least one counter-current wet-scrubber tower to cool the
gas and solubilize
or entrain further pollutants, followed by treatment in at least one venturi
scrubber to remove
further pollutants, followed by treatment in at least one demister to remove
liquid droplets.
The present invention also provides for an improved conditioning process for
the hot
synthetic gas exiting the reaction vessel. The conditioning process provides a
clean, cold
synthetic gas ready for use in various applications such as a fuel for boiler
furnaces, internal
combustion engines, gas turbines, etc. The process improvements consisting of:
subjecting
the synthetic gas to a sequential treatment in at least one cyclone to remove
particulate
material therefrom, followed by a treatment in at least one counter-current
wet-scrubber
tower to cool said gas and solubilize or entrain further pollutants, followed
by a treatment
in at least one venturi scrubber to remove further pollutants, followed by
treatment in a at
least one demister to remove residual liquid droplets. Optional additional
CA 02789790 2012-09-06
4
conditioning treatments are also provided. Recycling steps are also described
and are aimed
at revealing means to increase energy efficiency and minimize by-product
generation.
According to a first aspect, the invention relates to a process for the
gasification of
carbonaceous material to yield a synthetic gas and the conditioning of the
synthetic gas, the
process comprising:
= feeding the carbonaceous material into a reaction vessel containing a
fluidized
gasification bed of particulate material;
= introducing an oxygen-enriched fluidizing gas into the reaction vessel;
= conducting the gasification at a temperature between 680 and 765 C, thereby
producing the synthetic gas;
= feeding the synthetic gas into at least one cyclone to yield a synthetic gas
that is
substantially free of particulate material; and
= submitting the synthetic gas substantially free of particulate material to a
three-stage
wet-scrubbing treatment to yield a clean cold gas, or to a hot-gas
conditioning treatment to
yield a hot gas.
According to a second aspect, the invention relates to an apparatus for the
gasification of
carbonaceous material to yield a synthetic gas, comprising a reaction vessel
having an upper
section and a lower section, wherein:
= the upper section has an internal cross-section that is larger than an
internal cross-
section of the lower section;
= the lower section comprises means for introducing particulate material,
means for
introducing fluidized gas, means for feeding the carbonaceous material, and
means for
removing coarse solid materials; and
= the upper section has means for removing the synthetic gas.
According to a third aspect, the invention relates to a plant system for the
gasification of
carbonaceous material to yield a synthetic gas and the conditioning of the
synthetic gas, the
system comprising:
= an apparatus for the gasification of the carbonaceous material, comprising a
reaction
vessel having an upper section and a lower section, wherein:
CA 02789790 2012-09-06
the upper section has an internal cross-section that is larger than an
internal cross-
section of the lower section,
the lower section comprises means for introducing particulate material, means
for
introducing fluidized gas, means for feeding the carbonaceous material, and
means for
5 removing coarse solid materials, and
the upper section has means for removing the synthetic gas;
= at least one cyclone attached to the upper section of the reaction vessel;
= at least one water spray column attached to the at least one cyclone;
= at least one counter-current venture scrubber attached to the at least one
water spray
column; and
= a cyclonic separator.
According to a fourth aspect, the invention relates to a plant system for the
gasification of
carbonaceous material to yield a synthetic gas and the conditioning of the
synthetic gas, the
system comprising:
= an apparatus for the gasification of the carbonaceous material, comprising a
reaction
vessel having an upper section and a lower section, wherein:
the upper section has an internal cross-section that is larger than an
internal cross-
section of the lower section,
the lower section comprises means for introducing particulate material, means
for
introducing fluidized gas, means for feeding the carbonaceous material, and
means for
removing coarse solid materials, and
the upper section has means for removing the synthetic gas;
= a mobile granular filter; and
= a multitubular fixed-bed catalytic reforming reactor.
According to a fifth aspect, the invention relates to a plant system for the
gasification of
carbonaceous material to yield a synthetic gas and the conditioning of the
synthetic gas, the
system comprising:
= an apparatus for the gasification of the carbonaceous material, comprising a
reaction
vessel having an upper section and a lower section, wherein:
CA 02789790 2012-09-06
6
the upper section has an internal cross-section that is larger than an
internal cross-
section of the lower section,
the lower section comprises means for introducing a fluidized gasified
particulate
material, means for introducing a fluidized gas, means for feeding the
carbonaceous
material, and means for removing coarse solid materials, and
the upper section has means for removing the synthetic gas;
= at least one cyclone attached to the upper section of the reaction vessel;
= at least one water spray column attached to the at least one cyclone;
= at least one counter-current venturi scrubber attached to the at least one
water spray
column;
= a cyclonic separator;
= a mobile granular filter; and
= a multitubular fixed-bed tar catalytic reforming reactor.
According to a sixth aspect, the invention relates to a process for the
conditioning of
synthetic gas obtained from gasification of carbonaceous material, the process
comprising:
= feeding the synthetic gas into at least one cyclone to yield a synthetic gas
that is
substantially free of particulate material; and
= submitting the synthetic gas substantially free of particulate material to a
three-stage
wet-scrubbing treatment to yield a clean cold gas, or to a hot-gas
conditioning treatment to
yield a hot gas.
BRIEF DESCRIPTION OF THE FIGURES
= Figure 1 represents the gasifier, in accordance with the invention;
= Figure 2 is a schematic representation of the process of the present
invention;
= Figure 3 shows the cooling tower of the gas scrubbing system of this
invention;
Figures 4.1-4.5 present the composition of the producer gas as function of the
oxygen
content of the gasifying agent;
= Figure 4.6 gives the producer gas flow rate as function of the oxygen
content of the
gasifying agent;
0 Figure 4.7 gives the gasification temperature as function of the oxygen
content of the
CA 02789790 2012-09-06
7
gasifying agent;
= Figure 4.8 presents the higher heating value (HHV) of the gas as function of
the gasifying
agent;
= Figure 4.9 presents the cold gas efficiency as function of the percentage of
oxygen
content of the gasifying agent;
= Figures 5.1-5.5 present the composition of the producer gas as function of
the oxygen
content of the gasifying agent;
= Figure 5.6 gives the producer gas flow rate as function of the oxygen
content of the
gasifying agent;
Figure 5.7 gives the gasification temperature as function of the oxygen
content of the
gasifying agent;
= Figure 5.8 presents the HHV of the gas as function of the gasifying agent;
= Figure 5.9 presents the cold gas efficiency as function of the oxygen
content of the
gasifying agent;
Figure 5.10 presents the tar by-product generation as function of the oxygen
content of
the gasifying agent.
DETAILED DESCRIPTION OF THE INVENTION
This invention will be described herein below, by referring to specific
embodiments and
appended Figures.
The invention proposes a process to convert organic-rich wastes into a clean
synthetic gas
using an atmospheric or pressurized bubbling fluid-bed gasification reactor.
The gasification
is followed by gas conditioning which will yield cold clean gas for use as a
fuel in boilers,
internal combustion engines, etc.
The present invention can advantageously be used to convert various forms of
refuse rich in
carbonaceous material. For example, wood residue, refuse derived fuel RDF
from
municipal solid waste, plastic and rubber residues, wastewater treatment
sludge as well as
residues from various industrial operations such as pulp and paper black
liquors, petroleum
heavy residues altogether with other non dangerous carbonaceous materials are
examples
of gasifiable residual streams.
CA 02789790 2012-09-06
8
These carbonaceous feedstocks may be predried or preheated in order to
increase the
efficiency of the overall process. Typically their moisture content does not
exceed 20 wt% of
the feedstock.
During gasification various chemical reactions take place. These chemical
reactions are
responsible for the conversion of the feedstock into 'synthetic gas'. The non-
condensable
portion of the synthetic gas is a mixture of CO, H2, CO2, N2, H2O and light
hydrocarbons (HC)
of up to 7 carbon atoms. The relative concentration of each constituent
depends on the
feedstock but it is a strong function of the gasification agent (percentage of
oxygen in the
oxygen-containing gas fed to the gasification reactor), the ratio of
gasification agent to
feedstock, and the extent of reactions taking place in the freeboard of the
reactor. The
synthetic gas entrains small particles, known as particulate matter, and
contains low
amounts of higher molecular weight hydrocarbons in the gaseous phase, known in
the
literature as tar. The particles come from two sources: the inorganic matter
present in the
feedstock and hydrocarbons condensation reactions responsible also for the
formation of tar
present in the synthetic gas. The latter reactions lower the carbon conversion
of the process
thus decreasing its total energetic efficiency. Appropriate conditions are
used as function of
the feedstock to minimize the condensation reactions.
In accordance with the present invention, the hot gas exiting the gasification
reactor, after
passing through a cyclone system (typically one or two cyclones) is sent to a
wet scrubbing
apparatus, generally multi-step, for gas cooling and conditioning. Generally
speaking, the
purpose of the wet scrubbing is to remove the particulate and the condensable
species
present in the synthetic gas.
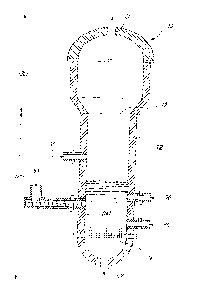
Referring to FIG. 1, the gasification reactor of the present invention is
illustrated. In its
preferred embodiment, gasifier (10) is a cylindrical reaction vessel
internally lined with
appropriate insulation and refractory material layers, generally referred to
as insulation (12).
Gasifier (10) may operate either under atmospheric or above atmospheric
pressure. The
lower section (18) of gasifier (10) is equipped with a channel-onlet (17) for
entry of an
oxidizing gas, and an oxygen-rich gas distribution grid (14). Above the grid
(14) there is a
section known as the fluidization bed (16) which is advantageously filled with
silica sand or
CA 02789790 2012-09-06
9
alumina sand. However, other granular material, for example magnesia, chromia,
etc., are
also acceptable. The granular material is introduced into the gasifier (10)
via inlet (52).
Advantageously, the mean size of the granular material used in the bed will
vary between
200 and 700 m. One skilled in the art will readily appreciate that the final
choice will
depend on carbonaceous feedstock reactivity, oxygen content fed to the
gasification reactor,
operation pressure and reactor geometry (defining the fluid-dynamics and the
residence-
time distributions).
Preferably, the height of bed at rest (16) is between 1.5 and 2 times the
internal cross-
sectional area of the gasifier (10). Gasifier (10) is preferably cylindrical.
However, one skilled
in the art will readily appreciate that gasifier (10) may be conical,
pyramidal, square or
rectangular or other suitable shapes. In all cases, the lower section (18)
extending to an
upper section (20) at a boundary (19) is designed to be filled almost entirely
by the
expanded fluid bed (16) during gasification. The upper section, i.e. the
freeboard (20) will
advantageously have an enlarged internal cross-sectional area when compared to
the lower
section (18), in the case of a cylindrical gasifier (10), it will have up to
about 1.5 times the
cross-sectional area of the lower section (18). In the case of conical or
pyramidal gasifier
(10), it would be installed with the wider portion on top. The larger cross-
sectional area at
the top favors an appropriate disengagement of the gas from the fluidized bed
of granular
solids and thus results in a shorter vessel than a reactor having an identical
diameter in both
the fluidization and the freeboard sections for the same levels of
disengagement.
Once again, one skilled in the art will appreciate that the relative heights
of the two sections
(18) and (20) of the gasifier (10) will depend on feedstock physico-chemical
properties and
several other parameters among which gasification fluid-dynamics and kinetics
as well as the
desired synthetic gas composition.
Coarse solids not entrained by the synthetic gas exiting the gasifier (10)
remain in the fluid
bed and can be removed continuously from an outlet (50) at the bottom of the
gasifier (10).
The separation of the coarse solids from the bed material (i.e. silica sand or
alumina) is
achieved naturally during fluidization due to density differences. In general,
particulate
matter of low inorganic content material has lower density than sand and tends
to stay at
CA 02789790 2012-09-06
the top of the expanded bed. This leads to higher attrition rates which
gradually decrease
the size of these particles and facilitate the 'washing' of these solids off
the fluid bed. If high
density inorganic material accompanies the feedstock it may be removed from
the bottom
of the bed through appropriately designed exit ports.
5 The total residence time of the synthetic gas evolved in gasifier (10) is
about 10 to 20
seconds, usually half of it in the lower section (18) of the reactor.
Advantageously, the ratio
of lower section (18) height to top section (20) height is between 2:1 and
3:1.
The gasifier (10) advantageously operates at gas velocities ranging between 5
and 20 times
the minimum fluidization velocity (as defined in standard fluidization
engineering literature).
10 Overall the gasification is "autothermal" meaning that once at steady
state, gasifier (10)
requires essentially no external heating or cooling. For the start-up,
gasifier (10) will require
preheating. This is preferably done with a gas burner to reach a temperature
of about 500 C.
It is to be understood that steady state temperature inside gasifier (10) is
set by a
combination of the following parameters:
reactor geometry;
= operation pressure;
= insulation and refractory lining thermal properties;
= fluid-bed material and size;
= feedstock nature, physico-chemical properties (composition, humidity, size)
and input
rate;
= oxygen-containing gas composition, velocity and flow rate.
Due to the complexity of the numerous reactions taking place during
gasification it is
observed that for the same reactive mixture, different operating parameters
can lead to
different synthetic gas compositions. This means that some steady states are
in fact
metastable and slight parameter fluctuations can lead to other steady states.
The feedstock is introduced into gasifier (10) via an inlet (54) which
comprises water cooler
(not illustrated) and screw feeder. Tar may be injected with the gasifier (10)
via an inlet (56)
which comprises a valve and a nozzle (not illustrated).
CA 02789790 2012-09-06
11
One key and surprising feature of the present invention is the discovery that
the use of an
oxygen-enriched fluidizing gas vastly improves the properties of the resulting
synthetic gas.
More specifically, it was discovered that using oxygen-enriched air, of about
30 to 50%vol of
oxygen, preferably 30 - 40 % vol. oxygen as a fluidizing agent greatly
improves the key
gasification parameters: cold gas efficiency, lower tar production and higher
calorific values
of the synthetic gas. Supporting data is found in Figures 4.1 to 5.10 and in
Tables 1 to 7
herein below.
CA 02789790 2012-09-06
12 Lr) N O M O~ v
>>Q ` O r O r 0 0 M CO M O N
00 > O r N M M N r r
O N o
.~ 0 O to M M'1: O O N c HM r r u1 r\
QJ O o 00
r' r M r O
4~ O Q) CO m M ul M N 00
N r
0
N V
0
oO Q0 co 0,r, u', Co C M N. N N.
rn
O o ~1 r M C)
r-, co M N O
O II
S ~ V
of
N -Q
to
4.
o y
N o N
u CO
O 0 co 1-0 N d CO O .- r r N N
W o rl Ln N O M Lcno N^ ~D r v, I'D II o
Z N ~ O E
E0
L
W
m i O
Q C
M QD E M V
V Z E tU o o p -p O C
z C Z v O v j +' d
4J X Q) N > U E
U N O p~ -p > > > > > O v_ O
z O p n N o o CJ v, V
v N
+ Q
N N N u N U to
U ro o = Z O p V 0
= o LO = V V= c N
V F Q L c c
Z3 (1)
~ ~ Q Q CC
Q Q Q Q Q Q Q Q Q m m m m m m N M
M d Lt1 ~O r~ CO dl N M v,
C
Lr)
CA 02789790 2012-09-06
13
V rv o
CL O M m O O N Lt1 M 0) I, N r-I
Li.. 0 1~ K O co O M Ln %D N
~~ Ln N ct N
-19
~
LL. O Lon M N ,,., Ln Ln N oo v; 0) CO
y c 'D "' M '- , CO M
y
R
W
o
Q Lt.. O O Ln -'T Ln N m CO =- '-0 O m
N O
GG o 1~ 1-1 CT r 2 p CO O N N O
v MO ~
.` v ~ V
1
0
O
V V o 4)
000 p M O
W o N .- M p M M r- '- .V)- cn
Lr)
Z F - cC
~ O
E
N p
L.
-I
co w rt/
n n C
~. O Q) Q) ^ .~ .~ V L
E _ u _ \ -Q ~ -0 -0 -a = c = v -0
E\ u Zs E no -d~- - 0
Z c ~i It z -a > j a
z ZZ O Q) a) 0 Ln V) c ea c ` y'
v~ Q) > O v E ~ ~- N
2 co
~+< ` c`a u O = Z NO O V O W C7 v;
= U V N = V U= Q v
L C C
c:
Q)
Q Q Q Q Q Q Q Cf~ co (~ C0 OD 00 r- N M
N M Ln z r-I CO m '- N M Ln tD
G/1
a
Ln
CA 02789790 2012-09-06
14
U
-a 0
O 0 M , n co O Ln c, O co N 1, Ln
O c' cn Ln O O N CO rr-I -- r4 cn -o rj V
U
I
") o
o co ~0 N ^ V M Co c N N
j Ln N O M cal ~p
O
U
0 o
O N O IT co O~ ~O O M 7 p, cy
N Cr,
U N M O r- M .-
N
Ljr)
W ~ v O
W
W > R
W >
ti
-
W. jp
X
O
W E O
W N O a
to -c a cu
W N r~ s V
0] U Z -C N o p 0 rn
Z > c
U U V1 " - - > > > >
Z O L QJ ru a)
o 0 0 0 v i
=va+Q~ o-ZOU0 NVvVi
o m- = U c
V F- o
U vi c
- =- v
QQQQQ Q , mmmmm m 1:l 1 N M It u~ ~p N. CO C N M 'T Ln t0
vi
CA 02789790 2012-09-06
U L
U) N Ln m ko CO co
Ln Ln C6 C6
Z N N N
U
to 1-. vi M N co O m O
V = O C6 co Z-~
J ~.
CC
cC 0 N O v rn ,n M
in Ql O
X Q Z co rn rn
z
w Z p O O M
L
O V O N M M
W co z cc U') Ol
M W '
O O
Z) ru
o _~ a O a) O
LL
Q) U O a4
ti v Co ~ o ~.u ~Lj a =o -c D
a) a) z E Ew
LL. - O QJ O a M O op O
W L u L u
v c x
af V-- W W
Q Q Q [.n CD CD
N M N M
CA 02789790 2012-09-06
16
U)
N
Lary
lL n N M Ln O O O
Q O
CO Ln
Ln
z
O
V M
Cn tl to M O " ^ CO U-) N Ln "T M M U-)
N Z N V' t" t" O i i O N
p II
Z
Q ¾ ~
ck:
W LLJ :3
Q 0 N\ O^ M I~ O O
OULn,r;ov co co o M
:tom Z
UP)
L u N
c c ~.
J Q V O o c
O V) Ln M M tty O o) - N E
Lr) r (~! Ln O i i O M Lry O
m, C O U
t
Q Q Z
Q Q O O z V O Q
Q Q U V
w W >-=v c v
Q U o
C
u" O N T M O cc co Ln o u'y N ~t 00
C) u
Ln c) Ln U-)
(? Z olio m't
in V ux ~-o
W L44
'n L)
I~ ---
tb c O
Q W O +~ c p--
I-- _ OCCJ M N O M n =_ to
N N X- L E N > E tz O
N Z Z O D U to Z= (v O O O r'
tLc i Y vi N
c0
O
a, m [s p a~ aJ p
LLI
c c o
c m
Flo
Q Q Q Q Q Q m m m m m co m m U U Q ..
N M Ln 'O '- N M ct Lr) '-O n co N
Q m U
'C
C
C1
v
J
try
CA 02789790 2012-09-06
17
Ln
N M t~ O co 01 N V M ul co cc) r co
O O Ln
m 00
cy M Ln co cV Ln M +
CV -~ 0 0 0 0 0 0
Z N 7 N `- N P- ' _ r' r O
ON O"i N t-, N Ln 0 O
M
co U') rn N O M + CO CO m M O
co O O C C
Z co
O
CD "o
C14 t, 1 14 CO u-, c~
t/) M t~ M op r c0 U) + Ln O p O a0 ,0 O O ttJ O
W Z co N M Lr) N r N Ln r dl r O
V
Z 0
Q rn r 0
m p V p r t. N zt N p et ~D O r) O cal C
(Cl (U
O N r + In r4
114
to C-
N t" N^ N V' p 0 0 0 a I 0 0 110 Q Z M N
Y M
O
V - et O co O M O 'T Lr) M N rt7 rU O
Q N ~D "D N + m 'T 0 V) II N N U') r N p M r r ~D co N r D O N
0 Z
W
W
LL co M
< '7' IT p N N 1.0 'I LJ- p O 00
N I- M i\ R N r ~D O
V) Q C n,, - O r,4 N N ,D M l0 Ln ,.0 O CO Q) p r r O 0 O -
Z ~ O ~ pip
w cc c:)
C 3J
Q0 O Lr~ p p Lr,) U-) 'IT N M N M O "~ C fl Lr) Z N r Ol M N N N ,0 06 Ql N
L; c)
O O
N
co
p_ N O N Ln dt t, -zt Lin N u; 1
J 0 N C? Ln O N OO
LL Z co Ln M Ln N O Ln al
Q O r
L N ~
n L \ rn ___
M i O
D b0 M U Q t0 _ _
C
L- E Z E D Z o
aQ E L3) -6b -o > u U Q7 > > > > > > N O
X Z O L N` o o Q X U 0-0 E_0 ] _ o_O p v ~V
Z v D V U Z O + Q N N O O N S ~J V cn
N '- o= o V Z=U O v v
~o U F- m c c
Q m
QQQQ Q Q Q Q Q Q mmmmm m m mmm
r N M J Ln ii N. c0 a) O r '-- N M 7' Ln LD N. co CT)
vi III -
tZ
Ln
CA 02789790 2012-09-06
18
a~ a4 ,l, 00
N 1 ._ C Ln ....
N c.j Q N 00 co O O
Q N _Q 1~ O O O O, ,l co
N 1~O O
O 1 u 4
vVi O
L
a) no
O cy .~ C CJ N
0 U)
Z
cn O
O b c O
d' O it y O " 00
Ln N r O M
Z _O Q `\
V O
Ln a) do
wi c:
n m m M O ~v ca o
Q O L T O
y W
of c a) oo cn
N Q> t _C M
Q L O ``~ N Ln M cC
1 110 O >
V Q N N C C O N td
O Ln
V b z O
G) Ln
cffl~
F-- a,
N a) oD co m
cr, O
m LE
I- C14 CD
Z3 0 Z) co m
V n O
to J co [mow e O O
CG r b d N M M N O
6 CD I-
p \.D o0 Ln d, O
0
a) a0 O ct3 c~
CO w c a~ U
N N - v1 O - O w
Q -Q O N Ln t- O M OA vu
z . O L ~- ~-- - It tt~ b0
C u O C
v~ O
c o
=-
~ ~ ~ ~ ~ rn v~ cri
o
o M U > E
N N O O N 5 = Z O
Z b4
O U x=uz
LLJ o
Q Q Q Q Q Q Q m m tSS -O
J Q m
CA 02789790 2012-09-06
19
The remaining apparatus and process steps relate to gas conditioning of the
synthetic
gas evolved from gasifier (10). These conditioning steps will now be described
in greater
detail.
Referring now to FIG. 2, the synthetic gas exiting through a channel-outlet
(21) of
gasifier (10) shown in FIG. 1 passes through cyclones (22) and (24) connected
in series.
The number of cyclones require will depend on the cleaning strategy. In this
preferred
embodiment, cyclones (22) and (24) will generally retain between 90 and 95% of
the
total amount of particles entrained by the synthetic gas as fly ash. Modern
cyclones are
indeed able to cut out of the synthetic gas stream 99% of particles with mean
particle
size higher than 10 pm. This means that the remaining particles in the
synthetic gas,
estimated between 1000 and 2000 ppm/v, have a mean particle size of less than
10 pm.
The particles removed through cyclone (22) are split into two streams : C9 and
C10. C9
is the solids purge of the gasification process while C10 is recycled back to
the gasifier
to increase the carbon conversion of the system. The ratio of these two
streams (or
expressed differently the recycle ratio) is a function of the carbon content
of the particles
removed by cyclone (22). These particles may eventually be mixed with any
solids
purged directly from the gasifier.
In an alternate embodiment, stream C9 may be partially directed to water
treatment
column (26) for use as an absorbent as described herein below. Indeed, the
carbon in
the particles has specific surface area and adsorption features resembling
those of
activated carbon. The proposed process utilizes this carbon-containing
particle (also
known as ash) together with activated carbon to treat, through adsorption, the
wastewater purge. Upon saturation of the carbon by adsorption, part of the
solids are
recycled back to the gasifier to increase the carbon conversion while
decreasing the
amount of solids to be disposed of.
Thus the synthetic gas exiting cyclones (22) and (24) is subject to further
conditioning
prior to its use as fuel. At this point there are two main conditioning
options:
= Wet scrubbing as described in the present patent application if clean cold
gas is
desired (i.e. the case for use in burners/boilers or internal combustion
engines); and
CA 02789790 2012-09-06
= Hot gas conditioning if hot gas is required (i.e. the case for use in gas
turbines or in
integrated gasifier combined cycled: IGCC).
The hot gas conditioning module consists of a mobile granular filter and a
multitubular
fixed-bed tar catalytic reforming reactor. The mobile granular filter and the
catalysts are
5 proprietary inventions of the applicants and described in separate patents
and/or
applications.
Particles removed by cyclone (24) are fed to water treatment system (26) as
shown by
stream C36. These particles are characterized by sufficient surface area to be
used as
10 adsorbent. Nevertheless, additional amounts of activated carbon, stream
C37, are
necessary for compliance with environmental regulations for disposal of
treated water
effluent, stream C38. As will be apparent from the following description,
water treatment
system (26) is used to treat the water used to scrub remaining pollutants from
the synthesis
gas exiting cyclone (24), stream C7.
To remove the remaining particles from the synthetic gas, stream C7, and to
condense and
remove the tarry products, which are in the vapor state at temperatures higher
than 400 C,
the process includes the use of a three stage wet (water) scrubbing module
comprising
water spray column (28), venturi scrubber (30) and cyclonic separator (32).
The synthetic gas stream C7 enters water spray column (28). Column (28) is
illustrated in
greater detail in Fig.3. Column (28) is a water-spray column preferably
counter-current,
cylindrical and with a conical bottom. Column (28) is used as a first stage
scrubber for (a)
cooling the synthetic gas down to about 90 C, b) removing about half of all
remaining
particles (targeting those comprised between 2 and 10 pm) and c) condensing
all tarry
compounds having boiling points higher than 100 C (excluding some volatile
organic
compounds (VOCs)).
Referring to FIG.3, water stream W1 which is the scrubbing/quenching solution,
enters from
the top of column (28), under pressure, and is dispersed using screw-shaped
nozzles (34)
producting wide angle sprays (36). W2 represents the new synthetic gas. W3
represents the
wastewater. And W4 represents the treated synthetic gas.
CA 02789790 2012-09-06
WO 00/69994 PCT/CAOO/00552
21
The pH of the scrubbing/quenching solution is adjusted in accordance with the
carbonaceous feedstock and the pollutants contained in the synthetic gas.
There may be
present: acid gases (HCI, HCN, SOX, N,,Oy), alkaline gases such as ammonia as
well as
volatile metal and salts. Acid gases are removed through alkaline scrubbing.
Ammonia is
removed through acidification of the scrubbing water. Volatile metals and
salts are
removed through quenching. Alkaline and acidic scrubbing produce
environmentally
neutral rejects. Condensed metals are subdivided into two categories : 1)
alkali and alkaline
earth metals giving non polluting salts and 2) heavy metals under their
elemental form or as
oxides if present in the gasified feedstock . The latter, being essentially
insoluble in water,
as shown in metal distribution studies, precipitate and can be recovered and
removed from
the process , streams C19 and C28.
Returning to FIG. 2, contaminated water stream C15 exists column (28) and is
routed to
heat exchanger (38) for further cooling to about 30 C. From there, stream C17
enters
decanter/skimmer tank (40) to remove precipitated material. In tank (40), the
heavier
inorganics (and the organics sticking to the inorganics organics) decant and
can be
removed. Meanwhile the lighter organics float at the water surface and are
removed by
skimming.
The decanting and floating matter altogether with some entrained wastewater
are pumped
out , streams C18a, C18b, C19 and, after being mixed with other residual
streams (defined
later) or even separately, are sent back to gasifier (10). A purge, stream
C32, equal to about
10% of the recirculating matter, is removed for final disposal to ensure the
stability of the
system.
The scrubbed synthetic gas, stream C16, still containing micronic particles,
water and tar
droplets, VOCs and some very volatile metals (if present in the initial solid
feedstock of the
gasifier) enters the second stage scrubbing process, namely venturi scrubber
(30).
Venturi (30) is preferably designed to operate at gas velocity range of 80-
100m/s and gives a
total pressure drop between 0.07 and 0.15 atm. These conditions give a near
micronic
dispersion of the water stream entering the throat of the cyclone. This
provides an
CA 02789790 2012-09-06
22
appropriate removal of the remaining particles with an efficiency of near 99%
for particles
of size as low as 0.01 pm. Ignoring the humidity of the synthetic gas, in this
venturi
scrubber, the ratio gas/water used is about 1 to 1 w/w. During the contact
time (usually of
less than 2s) the synthetic gas is cooled to about 35-40 C without significant
temperature
increase of the water stream.
From venturi (30), the synthetic gas enters the third and last stage of the
scrubbing module,
namely cyclonic separator (32). The water leaving the bottom of the gas-liquid
cyclonic
separator, stream C24 is fed into a second decanting/skimming tank (42) much
like tank
(40). Tank (42) is of significantly smaller size due to the lesser amounts of
water involved
in this recycle loop. Thus, streams C27 and C28 of Fig. 2 are pumped out to
join the
analogous streams C18 and C19 of tank (40). The cyclonic separator is a high
velocity
design unit able to remove droplets of mean size 5 um with an efficiency of
about 80%.
This means that the global separation efficiency is higher than 95%.
Nevertheless smaller
droplets are still present in the exiting gas stream (Fig. 2, stream C23) and
require further
removal.
Filter (44) is a high efficiency demister for completing droplet removal.
Commercially
available viscous filters as well as corrugated plate coalescers can be used
to accomplish
this task. Filter (44) can efficiently remove residual aromatics present in
minute quantities
(ppm or ppb). Removal is generally performed with a bed of activated carbon
which also
removes residual organic vapors. Once saturated the spent activated carbon may
be
recycled back to gasifier (10).
The use of a dehumidification unit (46) depends on specifications imposed by
the synthetic
gas end-use devices. The synthetic gas leaving the filter/demister will have
an average
temperature of about 25 C and will be saturated with water. This means that it
will contain
between 2 and 3%w/w of water. Usually this level of humidity is not
prohibitive for final
synthetic gas use in commercial burners/boilers, internal combustion engines
and gas
turbines. Dehumidification units (46) work by adsorption, for example by
passing over a
bed of alumina, and can reduce the moisture content of the gas to the desired
levels.
CA 02789790 2012-09-06
WO 00/69994 PCT/CAOO/00552
23
EXAMPLES OF GASIFICATION PERFORMANCE
Description of the unit
The runs have taken place in a Process Development Unit (or pilot plant). The
gasification
reactor is a 4 m high refractory-lined cylinder having 60 cm as outside
diameter in the
lower section, expanded to 74 cm in the upper section. The inner diameters are
30 cm
and 45 cm, respectively.
Fluidizing gas (air or oxygen-enriched air) enters the reactor through a
distribution grid
formed by an assembly of 9 tuyeres. The grid, of proprietary design, also
serves as support
for the sand bed.
The gasifier is fed from a 1.3 m3 live-bottom hopper by a system consisting of
a transfer
screw, a chute and an injection screw which can be located in either one of
two ports
situated at 48 cm or 29 cm above the grid.
Air (or oxygen-enriched air) is supplied by a compressor. The main flow (about
80%)
enters the reactor through the grid. The rest (20%) is directed to the feeding
system to
prevent back flow of gases from the reactor to the hopper. The sand (SiO2) bed
height (at
rest) has been varied between 60 and 45 cm. The average diameter of the sand
particles is
0.5 mm (2.6 kg/1 as density). Fluidizing velocities are comprised between 0.3
and 0.75
m/s. The biomass solids in the bed are estimated at less than 10% of the total
bed solids.
The volumetric bed capacity is 1.12 tones of biomass (mbed3 = h).
The gas produced exits the gasifier and passes through two cyclones in series
and the wet
scrubbing module described in the present patent application. A bypass system
permits to
either send the entire gas produced to a granular filter and then to the
flare, or go directly
to the flare. Char and ash are collected in two reservoirs connected to the
cyclones as well
as in the filter media used.
CA 02789790 2012-09-06
24
The installation is equipped with more than 40 thermocouples and 20 pressure
transducers.
Air and producer gas flows are measured by thermal dispersion and orifice flow
meters,
respectively.
The waste feed rate is monitored by a system of 3 load cells. The feed rate is
adjusted by
the rotation of the screws at the bottom of the hopper.
All measured variables are recorded by a Hewlett Packard data acquisition
system taking
samples at 10s intervals.
Feedstocks that have been successfully gasified
(1) Woody biomass;
(2) Forest and agricultural residues;
(3) Biological treatment sludge;
(4) Refuse Derived Fuel (RDF) from urban wastes (MSW);
(5) Rubber residues containing 5-15% KEVLAR residues;
(6) Granular polyethylene and polypropylene residues.
Analytical strategies
Sampling trains, designed following EPA's and Canadian standard methods, each
consisting
of a particulate material filter, a tar-water condenser, a demister and a
drying/absorber
allow collection and analysis of the gases.
When hot gas conditioning is applied three isokinetic samplers are used to
determine the
particulate, tar,VOCs and water contents in the off gases before and after gas
conditioning.
Each isokinetic gas sampling probe has a heated filter, a water/tarNOCs
condensing heat
exchanger/collector, a gas impact demister, a drierite gas dryer, a regulated
pump, a dry
gas flow meter and an appropriate orifice/manometer arrangement insuring
isokinetic
conditions.
When wet scrubbing is applied two isokinetic sampling trains are sufficient to
measure the
efficiency of the gas conditioning. An HP 5890 gas chromatograph analyses dry,
tar free
CA 02789790 2012-09-06
gases by separation of components on Porapak Q and Molecular Sieve 13X columns
in
series followed by TC detection. The sampling combined with the appropriate
analysis
methods allows to:
5 = Calculate the gross composition of the stack gas (the H2O, H2, CO, C02,
N2, and 02
content) using Gas Chromatography (combination of Porapak Q & Molecular sieve
column packings) as well as the particulate matter load of the stack gas.
= Detect and quantify toxic and acid components via colorimetric methods
(Matheson-
Kitagawa's Kits) in the stack gas (i.e. SOx, NOx, HCI) .
10 = Identify and quantify the main organic components dissolved in the
scrubbing water by
means of GC/MS analysis.
= Measure the inorganic anions retained by scrubbing water using ion
chromatography.
= Identify and quantify the distribution of metals in 'the output streams of
the gasifier and
its modules using X-rays fluorescence, AES/ICP, SEM and AA analyses.
Other analyses have been used in order to gather additional information as
follows:
= Calorimetry for the different feedstocks tested.
= TGA for the study of the thermal stability/instability of the different
feedstocks tested.
= TOC, COD and BOD5 analyses for the scrubbing water before and after
treatment
= BET analysis for the porosity of the carbon-rich gasification ashes used for
the adsorption
tests.
= GC/MS analysis for tar and halogenated hydrocarbons.
EXAMPLE 1
Results with residual wood as gasification feedstock
The four (4) runs reported herein below have the following common
parameters/variables:
= Stoichiometric (or equivalence) ratio: A= 30% (refers to the stoichiometric
amount of
oxygen needed for complete combustion of the carbon and hydrogen fed to the
gasifier),
= Mean Solids feed rate: 35 kg/h;
CA 02789790 2012-09-06
26
= Mean Solids humidity: 12%w/w;
= Mean Solids size: 1cm;
= Oxygen content of the fluidizing air was 20.9; 30; 40; and 50%vol. for the
four runs
respectively.
The results obtained are presented as follows:
= Figures 4.1-4.5 present the composition of the producer gas as function of
the oxygen
content of the gasifying agent. We can conclude that as oxygen content in the
fluidizing gas increases the inert gases N2 and Ar are decreasing while all
other gases
coming from organic matter gasification increase.
= Figure 4.6 presents the synthetic gas flow rate as function of the oxygen
content of the
gasifying agent. As expected, the gas flow rate is decreasing. It is
preferable for the
commercialization of the technology to have flow rates as low as possible in
order to
decrease the cost associated with gas conditioning, piping and handling in
general.
Moreover, for the same gasification reactor, lower gasifying agent and
producer gas
flow rates lead to lower linear velocity profiles and higher residence times
in the fluid-
bed gasification vessel. This in turn leads to lower particle entrainment
(carry-over) and
allows heavy tar gasification and CO shift reactions to proceed at higher
conversion
rates.
= Figure 4.7 presents the gasification temperature as function of the oxygen
content of the
gasifying agent. As the available heat is transferred to a smaller gas flow
rate the
temperature increases nearly linearly with the oxygen content.
= Figure 4.8 presents the HHV of the synthetic gas as function of the
gasifying agent. It is
also an increasing function. Values between 9 and 12 MJ/Nm3 are obtained. Such
values classify the synthetic gas as a medium calorific value gas and render
its use in
end-use devices easier and more efficient than low calorific value producer
gas.
CA 02789790 2012-09-06
27
EXAMPLE 2
Results with RDF as gasification feedstock
The runs reported herein below have the following common parameters/variables:
= Stoichiometric (or equivalence) ratio: A = 3001o;
= Mean Solids feed rate: 35 kg/h;
= Mean Solids humidity: 12%w/w;
= Mean Solids size: 1cm;
= Oxygen content of the fluidizing air was 20.9; 30; and 40%v/v for the three
runs
respectively.
The following observations can be made from the results:
= Figures 5.1-5.5 present the composition of the producer gas as a function of
the oxygen
content of the gasifying agent. We can also conclude, as in the wood case,
that as
oxygen content increases in the fluidizing gas the inert gases N2 and Ar are
decreasing
while all other gases coming from organic matter gasification increase.
= Figure 5.6 gives the producer gas flow rate as a function of the oxygen
content of the
gasifying agent. The trend and the comments are the same as in the wood case.
= Figure 5.7 gives the gasification temperature as function of the oxygen
content of the
gasifying agent. The trend is the same as in the wood case. Here we observed
however
a levelling-off behavior around 35% of oxygen content. As the RDF calorific
value is
lower than that of wood it is probable that the balance between heat
production over
heat transfer and losses has attained its equilibrium faster than in the case
of wood
gasification.
= Figure 5.8 presents the HHV of the gas as function of the gasifying agent.
It is also an
increasing function. As in the case of wood gasification, values between 9 and
12
M)/Nm3 are measured.
The scope of the claims should not be limited by the preferred embodiments set
forth in the
examples, but should be given broadest interpretation consistent with the
description as a
whole.