Note: Descriptions are shown in the official language in which they were submitted.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
1
TITLE OF THE INVENTION
COMPOSITIONS COMPRISING A RADIOSENSITIZER AND AN ANTI-CANCER AGENT AND METHODS
OF USES THEREOF
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application is a PCT application filed on April 26, 2010 and
published in English under
PCT Article 21(2), which claims benefit of U.S. provisional application serial
No. 61/172,482, filed on April
24, 2009. All documents above are incorporated herein in their entirety by
reference.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR DEVELOPMENT
[0002] N.A.
FIELD OF THE INVENTION
[0003] The present invention relates to compositions comprising a
radiosensitizer and an anti-
cancer agent useful in the treatment of cancer. More specifically, the present
invention is concerned with a
metal radiosensitizer and an anti-cancer agent for increasing the amount of
strand breaks in the DNA of a
cell following radiotherapy.
BACKGROUND OF THE INVENTION
[0004] Cancer is a worldwide problem that afflicts millions of people each
year. As such, finding
new methods of treatments is of vital interest. Both chemotherapy and
radiation therapy are used in the
treatment of cancer. Radiation treatment has become a conventional part of
cancer therapy and is used in
approximately 60% of treatment regimens. The cytotoxic effect of radiation on
cancer cells arises from the
ability of radiation to cause breaks in one or both strands of the DNA
molecules inside the cells. Cells in all
phases of the cell cycle are susceptible to this effect. However, the DNA
damage in cancer cells is more
likely to be lethal because these cells are less capable of repairing their
DNA. The side effects of radiation
are similar to those of chemotherapy and occur for the same reason i.e., the
damage of healthy cells and
tissue. Thus, a shortcoming to radiotherapy is the destruction of normal,
healthy tissue surrounding the
tumor during treatment. Another shortcoming is that after cessation of
treatment, recurrence of the tumor
can and does occur. Recurrence of the tumor has been partly attributed to the
presence of radioresistant
hypoxic cells, and the enhancement of radiation doses to damage the hypoxic
tumor tissue is often
necessary. However, to save normal, healthy tissue, a reduction in the total
radiation dose would be
desirable. Obviously, these two factors are contradictory. Therefore, the use
of certain drugs and chemicals
that preferentially sensitize hypoxic tumor cells to radiation,
radiosensitizers, are employed. Radiosensitizers
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
2
normally are chemical agents that have the capacity to increase the lethal
effects of radiation when
administered in conjunction with radiation and there are a variety of
radiosensitizers that act by more than
one mechanism. Furthermore, in the treatment of cancer with radiation and
chemotherapy, local tumor
control is often improved when radiation is administered synchronously with
the chemotherapeutic agent
(21). This observation has been attributed to a super additive effect on tumor
regression due to a synergistic
interaction between the radiation and the drug. Nevertheless, despite the
above improvements in cancer
treatment, cancer remains difficult to treat and cells still become resistant
to radiation therapy.
[0005] Thus, alternative methods and compositions which will increase the
sensitivity of cancer
cells to radiation therapy, thereby allowing for less exposure to toxic
chemotherapeutic agents and radiation
therapy, reduced side-effects and improved beneficial results, are still
desirable. The present invention
seeks to meet these and other needs.
[0006] The present description refers to a number of documents, the content of
which is
incorporated by reference in their entirety.
SUMMARY OF THE INVENTION
[0007] Accordingly, the present invention generally relates to new
compositions, methods and uses
for increasing the sensitivity of cancer cells to radiation therapy., for
potentiating radiotherapy treatment
and/or for ncreasing the amount of strand breaks in DNA in a cell (e.g., tumor
cell).
[0008] It has been surprisingly discovered that metal radiosensitizers such as
gold nanoparticles
(GNP or AuNp) synergistically increase the anti-cancer activity of anti-cancer
agents and of radiotherapy. It
was established that by combining metal radiosensitizers with anti-cancer
agents in radiotherapy, the
number of DNA double strand breaks (DSB) and/or single strand breaks (SSB)
could be increased up to
ten-fold as compared to radiation alone. Without being limited to any theory,
this synergistic effect could be
due, among other things, to the higher density of low energy electrons and
reactive species around the
metal radiosensitizers and the weakening of the bonds adjacent to the anti-
cancer agent located on or in
close proximity to the DNA backbone.
[0009] Anti-cancer agent cisplatin was chemically linked to pGEM-3Zf plasmid
DNA to produce a
cisplatin-DNA complex. Gold nanoparticles which bind electrostatically to pure
DNA were used as a metal
radiosensitizer and were added to this complex. Dry films of pure plasmid DNA
and DNA-cisplatin, DNA-
gold nanoparticles and DNA-cisplatin-gold nanoparticles complexes were
bombarded by 60 keV electrons.
The yields of single and double strand breaks were measured as a function of
exposure by electrophoresis.
From comparison of yields generated by the different types of films, it was
found that the binding of only one
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
3
gold nanoparticle to a plasmid-cisplatin complex containing 3,197 base pairs
increases by a factor of 4 the
efficiency of chemotherapeutic agent cisplatin to produce double strand breaks
in irradiated DNA.
Furthermore, adding two cisplatin molecules and one gold nanoparticle to DNA
enhances by an order of
magnitude radiation-induced double strand breaks. A number of phenomena could
contribute to this huge
enhancement, including the higher density of low energy electrons and reactive
species around the
radiosensitizers and the weakening of bonds adjacent to cisplatin in the DNA
backbone.
[0010] Radiosensitizer and anti-tumor agent loaded-liposomes were also
administered in vivo to a
rat cancer model.
[0011] Accordingly, in an embodiment, the present invention provides a method
of treating cancer
comprising administering to a subject in need thereof a composition comprising
an effective amount of an
anti-cancer agent and a metal radiosensitizer and treating said patient with
radiotherapy. The administration
of the metal radiosensitizer and anti-cancer agent provides for a synergistic
effect in increasing the amount
of DNA strand breaks in cancer cells when said patient is treated with
radiotherapy.
[0012] In a specific embodiment, the cancer is glioma. In a specific
embodiment when the drug
is cisplatin, the cancer is of a type for which this drug has been approved by
the FDA. Without being so
limited, the cancer is bladder cancer (e.g., that cannot be treated with
surgery or radiotherapy alone),
ovarian cancers (e.g., that has metastasized and not improved with other
drugs, in patients who have
already had surgery or radiotherapy), testicular cancer (e.g., in patients who
have already had surgery or
radiotherapy), squamous cell carcinoma of the head and neck (SCCHN) (e.g.,
locally advanced that cannot
be treated with surgery), cervical cancer (e.g., late-stage that cannot be
treated with surgery or radiotherapy
alone), malignant mesothelioma (e.g., that cannot be treated with surgery),
non-small cell lung cancer
(NSCLC) (e.g., locally advanced, advanced, or metastatic that cannot be
treated with surgery). In addition to
the uses that have been approved by the FDA, cisplatin is sometimes used alone
or with other drugs to treat
other types of cancer.
[0013] In a related aspect, the present invention provides a method of
potentiating radiotherapy
treatment comprising administering to a subject in need thereof an effective
amount of an anti-cancer agent
and of a metal radiosensitizer prior to radiotherapy.
[0014] In another aspect, the present invention provides a method of enhancing
radiosensitivity of a
cell population comprising exposing said cell population to an effective
amount of a metal radiosensitizer
and of an anti-cancer agent.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
4
[0015] In yet another aspect, the present invention provides a method of
increasing the amount of
strand breaks in DNA in a cell comprising: contacting said cell with an
effective amount of an anti-cancer
agent and of a metal radiosensitizer; and submitting said cell to
radiotherapy.
[0016] In a specific embodiment, the radiotherapy comprises ionizing
radiation. In another
embodiment, the ionizing radiation comprises low energy electrons. In another
embodiment, said anti-
cancer agent is a platinum compound or a derivative thereof. In another
embodiment, said platinum
compound is cisplatin, carboplatin, oxaliplatin, a derivative thereof or a
combination thereof. In another
embodiment, said platinum compound is cisplatin. In another embodiment, said
metal radiosensitizer
comprises nanoparticles of an inert metal. In another embodiment, said metal
radiosensitizer comprises
gold nanoparticles. In another embodiment, said gold nanoparticles have an
average diameter of between 1
and 60 nanometres. In another embodiment, said strand breaks are double strand
breaks. In another
embodiment, said anti-cancer agent and said metal radiosensitizer are
administered simultaneously. In
another embodiment, said anti-cancer agent and said metal radiosensitizer are
administered separately.
[0017] In another embodiment, said anti-cancer agent and said metal
radiosensitizer are
encapsulated in liposomes. In another embodiment, a majority of said liposomes
have a diameter of less
than 400 nm. In another embodiment, a majority of said liposomes have a
diameter between about 100 and
about 150 nm. In another embodiment, said liposomes are coated with
Polyethylene glycol (PEG). In
another embodiment, said liposomes preferentially target cancer cells. In
another embodiment, said
liposomes comprise dipalmitoyl phosphatidyl glycerol (DPPG), soy phosphatidyl
choline, cholesterol and
methoxy-polyethylene glycol-distearoyl phosphatidyl-ethanolamine (mPEG2ooo-
DSPE). In another
embodiment, said liposomes comprise dipalmitoylphosphatidylcholine (DPPC), 3[i-
[N-(M,M-
dimethylaminoethane)-carbamoyl]-cholesterol (DC-Choi) and Dioleoyl
Phosphatidylethanolamine (DOPE).
In a further embodiment, said liposomes comprise polyethylene glycol (PEG). In
another embodiment, said
liposomes have a mean diameter of between about 70 nm to 152 nm. In another
embodiment, said
liposomes further comprise a stabilizer. In another embodiment, said
stabilizer is polyacrylamide, polyvinyl,
dextrose, D-glucose and dithiolated diethylenetriaminepentaacetic acid
(DTDTPA).
[0018] In another aspect, the present invention provides radiosensitizing
compositions comprising
an effective amount of an anti-cancer agent, a metal radiosensitizer and a
suitable pharmaceutical carrier.
In an embodiment, the radiosensitizing composition potentiates the amount of
DNA strand breaks in cells
following radiotherapy.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
[0019] In another aspect, the present invention provides the use of an
effective amount of a metal
radiosensitizer and an anti-cancer agent for the treatment of cancer in a
subject in need thereof. The
present invention provides the use of a composition comprising a metal
radiosensitizer and an anti-cancer
agent for the preparation of a medicament for the treatment of cancer.
[0020] In another aspect, the present invention provides the use of an
effective amount of a metal
radiosensitizer and of an anti-cancer agent for (a) enhancing radiosensitivity
of a cell population, or for (b)
potentiating radiotherapy treatment; or (c) increasing the amount of strand
breaks in DNA in a cell during
radiotherapy.
[0021] In another aspect, the present invention provides a use of an effective
amount of a metal
radiosensitizer and of an anti-cancer agent in the manufacture of a medicament
for (a) enhancing
radiosensitivity of a cell population, or for (b) potentiating radiotherapy
treatment; or (c) increasing the
amount of strand breaks in DNA in a cell during radiotherapy.
[0022] In a specific embodiment, said radiotherapy comprises ionizing
radiation. In another specific
embodiment, said ionizing radiation comprises low energy electrons. In another
specific embodiment, said
anti-cancer agent is a platinum compound or a derivative thereof. In another
specific embodiment, said
platinum compound is cisplatin, carboplatin, oxaliplatin, a derivative thereof
or a combination thereof. In
another specific embodiment, said platinum compound is cisplatin. In another
specific embodiment, said
metal radiosensitizer comprises nanoparticles of an inert metal. In another
specific embodiment, said metal
radiosensitizer comprises gold nanoparticles. In another specific embodiment,
said gold nanoparticles have
an average diameter of between 1 and 60 nanometres. In another specific
embodiment, said strand breaks
are double strand breaks. In another specific embodiment, said anti-cancer
agent and said metal
radiosensitizer are adapted for simultaneous administration. In another
specific embodiment, said anti-
cancer agent and said metal radiosensitizer are adapted for separate
administration.
[0023] In another specific embodiment of said uses, said anti-cancer agent and
said metal
radiosensitizer are encapsulated in liposomes. In another specific embodiment,
a majority of said
liposomes have a diameter of less than 400 nm. In another specific embodiment,
a majority of said
liposomes have a diameter of between about 100 nm and about 150 nm. In another
specific embodiment,
said liposomes are coated with polyethylene glycol (PEG). In another specific
embodiment, said liposomes
are adapted to preferentially target cancer cells. In another specific
embodiment, said liposomes comprise
dipalmitoylphosphatidylcholine (DPPC), 3[3-[N-(M,W-dimethylaminoethane)-
carbamoyl]-cholesterol (DC-
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
6
Chol), Dioleoyl Phosphatidylethanolamine (DOPE) and polyethylene glycol (PEG).
In another specific
embodiment, said liposomes comprise dipalmitoyl phosphatidyl glycerol (DPPG),
soy phosphatidyl choline,
cholesterol and methoxy-polyethylene glycol-distearoyl phosphatidyl-
ethanolamine (mPEG2ooo-DSPE). In
another specific embodiment, said liposomes have a mean diameter of between
about 70 nm and about
152 nm. In another specific embodiment, said liposomes further comprise a
stabilizer. In another specific
embodiment, the stabilizer comprises polyacrylamide, polyvinyl, dextrose, D-
glucose or dithiolated
diethylenetriaminepentaacetic acid (DTDTPA).
[0024] In another aspect, the present invention provides a use of a
composition comprising a metal
radiosensitizer and an anti-cancer agent for potentiating radiotherapy
treatment.
[0025] In another aspect, the present invention provides a use of an effective
amount of a metal
radiosensitizer and an anti-cancer agent for increasing the amount of DNA
strand breaks in a cell
population.
[0026] In another embodiment, the present invention relates to the use of an
effective amount of a
metal radiosensitizer and an anti-cancer agent for synergistically increasing
the amount of DNA breaks in a
cell population when treated with ionizing radiation.
[0027] In another aspect, the present invention provides a use of an effective
amount of a metal
radiosensitizer and an anti-cancer agent for enhancing radiosensitivity of a
cell population.
[0028] In another aspect, the present invention concerns a composition
comprising a metal
radiosensitizer and an anti-cancer agent for use in potentiating the effect of
radiotherapy.
[0029] In another aspect, the present invention concerns a pharmaceutical
composition comprising
a metal radiosensitizer and an anti-cancer agent for use in (a) potentiating
the effect of radiotherapy; (b)
enhancing radiosensitivity of a cell population; or (c) increasing the amount
of DNA strand breaks in a cell
population.
[0030] In another aspect, the present invention concerns a pharmaceutical
composition comprising
an effective amount of an anti-cancer agent, a metal radiosensitizer and a
pharmaceutically acceptable
carrier.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
7
[0031] In a specific embodiment of the compositions, said radiotherapy
comprises ionizing
radiation. In another specific embodiment, said ionizing radiation comprises
low energy electrons. In
another specific embodiment, said anti-cancer agent is a platinum compound or
a derivative thereof. In
another specific embodiment, said platinum compound is cisplatin, carboplatin,
oxaliplatin, a derivative
thereof or a combination thereof. In another specific embodiment, said
platinum compound is cisplatin. In
another specific embodiment, said metal radiosensitizer comprises
nanoparticles of an inert metal. In
another specific embodiment, said metal radiosensitizer comprises gold
nanoparticles. In another specific
embodiment, said gold nanoparticles have an average diameter of between 1
nanometres and 60
nanometres. In another specific embodiment, said strand breaks are double
strand breaks. In another
specific embodiment, said anti-cancer agent and said metal radiosensitizer are
adapted for simultaneous
administration. In another specific embodiment, said anti-cancer agent and
said metal radiosensitizer are
adapted for separate administration.
[0032] In another specific embodiment of the compositions, said anti-cancer
agent and said metal
radiosensitizer are encapsulated in liposomes. In another specific embodiment,
In another specific
embodiment, a majority of said liposomes have a diameter of less than 400 nm.
In another specific
embodiment, a majority of said liposomes have a diameter of between about 100
and about 150 nm. In
another specific embodiment, said liposomes are coated with polyethylene
glycol (PEG). In another specific
embodiment, said liposomes are adapted to preferentially target cancer cells.
In another specific
embodiment, said liposomes comprise dipalmitoylphosphatidylcholine (DPPC), 30-
[N-(W,M-
dimethylaminoethane)-carbamoyl]-cholesterol (DC-Chol), Dioleoyl
Phosphatidylethanolamine (DOPE) and
polyethylene glycol (PEG). In another specific embodiment, said liposomes
comprise dipalmitoyl
phosphatidyl glycerol (DPPG), soy phosphatidyl choline, cholesterol and
methoxy-polyethylene glycol-
distearoyl phosphatidyl-ethanolamine (mPEG2ooo-DSPE). In another specific
embodiment, said liposomes
have a mean diameter of between about 70 nm and about 152 nm. In another
specific embodiment, said
liposomes further comprise a stabilizer. In another specific embodiment, said
stabilizer comprises
polyacrylamide, polyvinyl, dextrose, D-glucose or dithiolated
diethylenetriaminepentaacetic acid (DTDTPA).
[0033] In accordance with another aspect of the present invention, there is
provided a
pharmaceutical composition comprising a metal radiosensitizer and an anti-
cancer agent for use in
potentiating the effect of radiotherapy.
[0034] In accordance with another aspect of the present invention, there is
provided a
pharmaceutical composition comprising a metal radiosensitizer and an anti-
cancer agent for use in
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
8
enhancing radiosensitivity of a cell population.
[0035] In accordance with another aspect of the present invention, there is
provided a
pharmaceutical composition comprising a metal radiosensitizer and an anti-
cancer agent for use in
increasing the amount of DNA strand breaks in a cell population.
[0036] In another aspect, the present invention relates to a method of
reducing the toxicity of an
anti-cancer therapy comprising administering, before the radiotherapy
treatment, to a subject in need
thereof an effective amount of a radiosensitizer in combination with
(simultaneously with (separately or
together), or sequentially) an anti-cancer agent.
[0037] In an embodiment, the above-mentioned anti-cancer agent of the present
invention is an
anti-cancer agent which binds to DNA. In another embodiment, the anti-cancer
agent is an alkylating agent.
In a further embodiment, the anti-cancer agent is a platinum compound or
derivative thereof. In yet a further
embodiment, the platinum compound of the present invention is cisplatin,
carboplatin, oxaliplatin, derivatives
thereof or combinations thereof. In an embodiment, the above-mentioned
platinum compound is cisplatin.
[0038] In an embodiment, the above-mentioned metal radiosensitizer comprises
nanoparticles of at
least one inert metal. In an embodiment, the at least one inert metal is gold.
In an embodiment, the
nanoparticles are between about I and about 60 nanometres. "About" as used
herein refers to a difference
of 0.2 or less.
[0039] In an embodiment, in the compositions and methods of the present
invention, the above-
mentioned DNA breaks caused by the synergistic effect of the metal
radiosensitizer and anti-cancer agent
are double strand breaks.
[0040] In a particular embodiment of the present invention, the anti-cancer
agent and metal
radiosensitizer are administered simultaneously. Alternatively, the anti-
cancer agent and metal
radiosensitizer can be administered separately as long as both the agent and
the radiosensitizer are
administered prior to radiotherapy and in such a way that both are present in
the same cells at the time of
irradiation, so as to provide a synergistic effect on the amount of strand
breaks to DNA.
[0041] In another particular embodiment of the present invention, the above-
mentioned anti-cancer
agent and metal radiosensitizer are encapsulated in liposomes.
[0042] In an embodiment, the liposomes are less than 400 nm in size. In
another embodiment, the
liposomes are between 100 and 150 nm in size. In a further embodiment, the
liposomes are coated with
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
9
Polyethylene glycol (PEG). In another embodiment, the liposomes have a mean
diameter of between
about 70 nm and 152 nm size.
[0043] In another specific embodiment, the liposomes comprise
dipalmitoylphosphatidylcholine
(DPPC), 30-[N-(M,M-dimethylaminoethane)-carbamoyl]-cholesterol (DC-Chol),
dioleoyl
Phosphatidylethanolamine (DOPE). In more specific embodiments, the liposomes
comprise PEG. PEG can
be administered alone or attached to another lipids such as but not limited to
DPPC and/or 1,2-distearoyl-
sn-glycero-3-phosphoethanolamine-N (DSPE). and polyethylene glycol PEG.
[0044] In a particular embodiment, the liposomes comprise dipalmitoyl
phosphatidyl glycerol
(DPPG), soy phosphatidyl choline, cholesterol and methoxy-polyethylene glycol-
distearoyl phosphatidyl-
ethanolamine (mPEG2ooo-DSPE). Preferably, the liposomes used in accordance
with the present invention
have a mean diameter of between about 70 nm and 152 nm size and are similar to
the liposomes used for
the LipoplatinTM formulation.
[0045] In an embodiment, the compositions and liposomes of the present
invention preferentially
target cancer cells over healthy cells.
[0046] The present invention also provides a kit or package comprising the
above-mentioned agent
or pharmaceutical compositions. Such kit may further comprises, for example,
instructions for the prevention
and/or treatment of cancer, containers, devices for administering the
agent/composition, etc.
[0047] More specifically, in accordance with the present invention, there is
provided a kit comprising
a metal radiosensitizer and an anti-cancer agent and instructions for use in
(a) increasing the amount of
strand breaks in DNA in a cell; (b) potentiating the effect of radiotherapy;
or (c) enhancing radiosensitivity of
a cell population.
[0048] In a specific embodiment of the kit, said radiosensitizer is gold or
platinum nanoparticles
and said anti-cancer agent is cisplatin, carboplatin, oxaliplatin or a
derivative thereof. In another specific
embodiment, said radiosensitizer is gold nanoparticles and said anti-cancer
agent is cisplatin.
[0049] Other objects, advantages and features of the present invention will
become more apparent
upon reading of the following non-restrictive description of specific
embodiments thereof, given by way of
example only with reference to the accompanying drawings.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
BRIEF DESCRIPTION OF THE DRAWINGS
[0050] In the appended drawings:
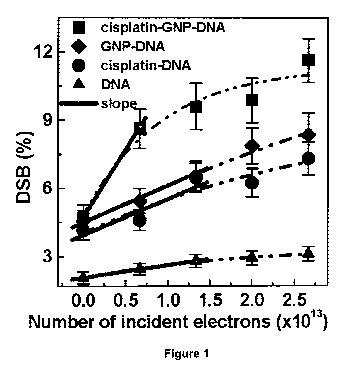
[0051] Figure 1 shows a dose response curve of DSB induced by a 60 keV
electron impact on 2900
nm films of pure DNA (A ), cisplatin-plasmid (=), gold nanoparticles (GNP)-
plasmid (+) and cisplatin-GNP-
plasmid (^) complexes in ratios of 2:1, 1:1, and 2:1:1, respectively. The dash
lines are exponential fits;
[0052] Figure 2 shows the enhancement factor (EF) relative to pure DNA of GNP-
DNA (1:1),
cisplatin-DNA (2:1) and cisplatin-GNP-DNA (2:1:1) complexes for SSB and DSB
induced by 60 keV
electrons;
[0053] Figure 3 shows the enhancement factor (EF) relative to pure DNA of GNP-
DNA (1:10),
cisplatin-DNA (2:1) and cisplatin-GNP-DNA (20:1:10) complexes for SSB and DSB
induced by 60 keV
electrons;
[0054] Figure 4 shows the transmission electron microscopy (TEM) of GNP and
platinum-
compound-loaded liposomes of the fraction lof liposomes eluted in the
SephadexTM column described in
Example 3.
[0055] Figure 5 shows the transmission electron microscopy (TEM) of GNP and
platinum-
compound-loaded liposomes of the fraction 2 of liposomes eluted in the
SephadexTM column described in
Examples 3 and 4; and
[0056] Figure 6 presents the median surviving time obtained for seven animal
groups. The number
of animal for each group is : cisplatin = 4, sham = 6, LipoplatinTM = 8,
LipoxalTM = 12, LipoplatinTM + GK = 11,
LipoxalTM + GK = 9, LipoGold = 3. *GK = combination with Gamma Knife (i.e.
radiation).
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0057] The articles "a," "an" and "the" are used herein to refer to one or to
more than one (i.e., to at
least one) of the grammatical objects of the article.
[0058] The term "including" and "comprising" are used herein to mean, and are
used
interchangeably with, the phrases "including but not limited to" and
"comprising but not limited to".
[0059] The term "such as" is used herein to mean, and is used interchangeably
with, the phrase
"such as but not limited to".
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
11
[0060] As used herein the term "cancer" is intended to include any form of
cancer or tumors. Non-
limiting examples of cancers include brain cancer (e.g., glioma), gastric
cancer, pancreatic cancer, non-
small cell lung cancer, small cell lung cancer, prostate cancer, colon cancer,
non-Hodgkin's lymphoma,
sarcoma, testicular cancer, acute non-lymphocytic leukemia and breast cancer.
[0061] The methods, compositions formulations and uses described herein are
suitable for both
humans and animals, preferably mammals.
[0062] Thus, as used herein, the term "subject" in the context of the present
invention relates to any
mammal including a mouse, rat, pig, monkey and horse. In a specific
embodiment, it refers to a human. A
"subject in need thereof " or a "patient" in the context of the present
invention is intended to include any
subject that will benefit or that is likely to benefit from the compositions
and pharmaceutical compositions of
the present invention. Thus, the "subject" of the present invention is a
subject suffering from any form of
cancer that might benefit from a combination of radiotherapy and chemotherapy
and in particular that could
benefit from an increase in strand breaks of DNA in the cancer cell
population. The subject may suffer from
a cancer of any stage such that it could be an early non invasive cancer or
could be a late stage cancer that
has already progressed to form metastases in the body.
[0063] The terms "treat/treating/treatment" as used herein, refers to
eliciting the desired biological
response, i.e., a therapeutic effect. Hence "treat/treating/treatment" in the
expression "treating cancer" is
meant to refer for example to any partial or complete reduction in tumor size
and/or an arrest or reduction in
tumor growth or a delay in the apparition of resistant hypoxic tumor cells.
The terms
"treat/treating/treatment" further include an increase in cancer cells death
due to an increase in the number
of strand breaks in DNA of tumor cells provided by the synergistic action of
the metal radiosensitizer and
anti-cancer agent of the present invention.
[0064] In addition, the therapeutic effect may comprise an amelioration of one
or more other
symptoms associated with cancer (anaemia, chronic fatigue, nausea, loss of
bone mass, progressive loss of
both fat and skeletal muscle, refractoriness of weight loss to increased
nutritional input, elevated resting
energy expenditure (REE), decreased protein synthesis, altered carbohydrate
metabolism, hyper-
catabolism/increased degradation of muscle via the ATP-ubiquitin-proteasome
pathway of proteolysis and
of adipose tissue via lipolysis, asthenia, etc.) and/or an increased survival
time of the affected subject,
following administration of a pharmaceutical composition of the present
invention.
1. Anti-cancer agents
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
12
[0065] The anti-cancer agents of the present invention comprise anti-cancer
agents that directly
cross-link nucleic acids, specifically DNA and are envisaged to facilitate DNA
damage leading to a
synergistic, antineoplastic combination with the metal radiosensitizer and
radiotherapy (e.g., alkylating
agents). Non-limiting examples of anti-cancer agents include platinum-based
anti-cancer agents which are
thought to function as cell cycle inhibitors by binding to DNA, i.e., acting
as alkylating agents of DNA.
[0066] Non-limiting examples of platinum compounds which may be used in
accordance with the
present invention include cisplatin, carboplatin and oxaliplatin, or
derivatives thereof or combinations
thereof. Non-limiting examples of derivatives include conventional platinum
compounds in which the Pt atom
is replaced by rutherium or palladium. Other examples include: (CPA)2Pt(DOLYM)
and (DACH)Pt(DOLYM)
cisplatin (Choi et al,, Arch. Pharmacol Res. 22(2):151-156, 1999), Cis-
(PtCl2(4,7-H-5-methyl-7-
oxo)1,2,4(triazolo(1,5-a)pyrimidine)2) (Navarro et al., J. Med. Chem.
4i(3):332-338, 1998), (Pt(cis-1,4-
DACH)(trans-C12)(CBDCA)) = MeOH cisplatin (Shamsuddin et al., Inorg. Chem.
36(25):5969-5971, 1997), 4-
pyridoxate diamine hydroxy platinum (Tokunaga et al,, Phann. Sci. 3(7):353-
356, 1997), Pt(II) = Pt(II)
(Pt2(NHCHN(C(CH2)(CH3)))4) (Navarro et al., Inorg. Chem. 35(26):7829-7835,
1996), 254-S cisplatin
analogue (Koga et al., Neurol, Res. 15(3):244- 247, 1996), trans,cis-
(Pt(OAc)212(en)) (Kratochwil et al., J.
Med. Chem. 39(13):2499-2507, 1996), cis- 1,4-diaminocyclohexane cisplatin
analogues (Shamsuddin et al.,
J. Inorg. Biochem. 61 (4):291-301, 1996), 5orientational isomer of cis-
(Pt(NH3)(4-aminoTEMP-O){d(GpG)})
(Dunham & Lippard, J. Am. Chem. Soc. 117(43): 10702- 12, 1995), chelating
diamine-bearing cisplatin
analogues (Koeckerbauer & Bednarski, J. Pharm. Sci. S4(7):819-23, 1995),
(ethylenediamine)platinum(II)
complexes (Pasini et al., J. Chem. Soc, Dalton Trans. 4:579-85, 1995), CI-973
cisplatin analogue (Yang et
al., Int. J. Oncol. 5(3):597-602, 1994), cis-diamminedichloroplatinum(11) and
its analogues cis-1,1-
cyclobutanedicarbosylato(2R)-2-methyl-1,4-butanediam-mineplatinum(H) and cis-
diammine(glycolato)platinum (Claycamp & Zimbrick, J. Inorg. Biochem.,
26(4):257-67, 1986; Fan et al.,
Cancer Res. 48(11):3135-9, 1988; Heiger-Bernays et al., Biochemistry
29(36):8461-6, 1990; Kikkawa et al.,
J. Exp. Clin. Cancer Res. 72(4):233-40, 1993; Murray et al., Biochemistry
37(47): 11812-17, 1992;
Takahashi et al., Cancer Chemother. Pharmacol. 33(1):31-5, 1993), cis-amine-
cyclohexylamine-
dichloroplatinum(II) (Yoshida et al., Biochem. Pharmacol. 48(A):193-9, 1994),
gem-diphosphonate cisplatin
analogues (FR 2683529), (meso-1,2-bis(2,6-dichloro-4-hydroxyplenyl)ethylenedia
mine) dichloroplatinum(II)
(Bednarski et al., J. Med. Chem. 35(23):4479-85, 1992), trans-
diamminedichloroplatinum(II) and cis-
(Pt(NH3)2(N3-cytosine)CI) (Bellon & Lippard, Biophys. Chem. 35(2-3): 179-88,
1990), 3H-cis-1,2-
diaminocyclohexanedichloroplatinum(II) and 3H-cis-1,2-
diaminocyclohexanemalonato-platinum (II) (Oswald
et al., Res. Commun. Chem. Pathol. Pharmacol. 54(l):41-58, 1989),
diaminocarboxylatoplatinum (EPA
296321), aminoalkylaminoanthraquinone-derived cisplatin analogues (Kitov et
al., Eur. J. Med. Chem.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
13
23(4):381-3, 1988), spiroplatin, carboplatin, iproplatin and JM40 platinum
analogues (Schroyen et al., Eur. J.
Cancer Clin. Oncol. 24(8): 1309- 12, 1988), bidentate tertiary diamine-
containing cisplatinum derivatives
(Orbell et al,, Inorg. Chim. Acta 752(2): 125-34, 1988), platinum(li),
platinum(IV) (Liu & Wang, Shandong
Yike DaxueXuebao 2(1):35-41, 1986), cis- diammine(1,1-
cyclobutanedicarboxylato-)platinum(II)
(carboplatin, JM8) and ethylenediamine- malonatoplatinum(II) (JM40) (Begg et
al., Radiother. Oncol. 9(2):
157-65, 1987), JM8 and JM9 cisplatin analogues (Harstrick et al., Int. J.
Androl. 10(1); 139-45, 1987),
(NPr4)2((PtCL4).cis-(PtCI2- (NH2Me)2)) (Brammer et al., J. Chem. Soc, Chem.
Commun. 5:443-5, 1987),
aliphatic tricarboxylic acid platinum complexes (EPA 185225), cis-
dichloro(amino acid)(tert-
butylamine)platinum(II) complexes (Pasini & Bersanetti, Inorg. Chim. Acta
107(A):259-67, 1985),
Polynuclear Platinum BBR3464, BBR3571, and BBR3610 (Billecke C. et al., Neuro-
Oncol 8(3):215-26,
2006).
[0067] It should be understood that combinations of two or more anti-cancer
agents can be used in
accordance with the present invention as long as 1) at least one of the anti-
cancer agents has a synergistic
effect with the metal radiosensitizer and increases the number of strand
breaks in cells DNA after
radiotherapy; or 2)A combination of the at least one anti-cancer agent with
the metal radiosensitizer
enables the anti-cancer agent to be administered at a lower concentration
while achieving a similar
therapeutic effect (e.g., single andbr single strand breaks, survive time,
etc.) after radiotherapy as would a
higher dose of the anti-cancer agent not administered in combination with the
metal radiosensitizer.
2. Radiation therapy
[0068] Radiation therapy (also called radiotherapy, X-ray therapy or
irradiation) comprises the use
of ionizing radiation to kill cancer cells and shrink tumors. Radiation
therapy can be administered externally
via external beam radiotherapy (EBRT) or internally via brachytherapy. Thus,
radiation therapy is the
medical use of ionizing radiation as part of cancer treatment to control
malignant cells. Most common
cancer types can benefit from a combination of radiotherapy and chemotherapy.
Radiotherapy works by
destroying the cancer cells (by damaging their DNA) in the treated area.
[0069] Ionizing radiation consists of subatomic particles or electromagnetic
waves that are
energetic enough to detach electrons from atoms or molecules, ionizing them.
The occurrence of ionization
depends on the energy of the impinging individual particles or waves, and not
on their number. An intense
flood of particles or waves will not cause ionization if these particles or
waves do not carry enough energy to
be ionizing. Roughly speaking, particles or photons with energies above a few
electron volts (eV) are
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
14
ionizing. Electrons, x rays, gamma rays or atomic ions may be used in
radiation therapy to treat malignant
tumors (cancer). An energy in the order of MeVs is usually used in
conventional radiotherapy. This high
energy radiation, after interaction with matter (Compton effect), produces
slow electrons that produce low
energy electrons. For example, one photon of 1 MeV will produces near 40,000
low energy electrons. A
total energy of 60 eV of radiation is believed to represent what is generally
used in conventional
radiotherapy.
[0070] Radiotherapy treatment can cure cancers and can also reduce the chance
of a cancer
coming back after surgery. It may be used to reduce cancer symptoms. Radiation
therapy is commonly
applied to the cancerous tumor. The radiation fields may also include the
draining of lymph nodes if they are
clinically or radiologically involved with the tumor or if there is a
potential risk of subclinical malignant
spread. It is necessary to include a margin of normal tissue around the 'tumor
to allow for uncertainties in
daily set-up and internal tumor motion. These uncertainties can be caused by
internal movement (for
example, respiration and bladder filling) and movement of external skin marks
relative to the tumor position.
To spare normal tissues (such as skin or organs through which radiation must
pass in order to treat the
tumor), shaped radiation beams are conventionally aimed from several angles of
exposure to intersect at
the tumor, providing a much larger absorbed dose there than in the
surrounding, healthy tissue.
[0071] There are different types of radiotherapy machines, which work in
slightly different ways. The
number and duration of the radiotherapy sessions depend on the type of cancer
and where it is located in
the body. A superficial skin cancer may need only a few short treatments,
whereas a cancer deeper in the
body may need more prolonged treatment. Patients usually have external
radiotherapy in small doses -
each dose is called a fraction - usually five days in a row, from Monday to
Friday, so normal tissue has a
chance to recover from the treatment during the weekend. Accordingly, in an
embodiment, the compositions
of the present invention are administered daily prior to radiotherapy. The
time of administration will depend
on the specific formulation and on the time necessary for the anti-cancer
agent and metal radiosensitizer to
reach the target cells. The time of administration will be chosen so as to
provide the optimal concentration of
anti-cancer agent and metal radiosensitizers at the time of irradiation.
3. Radiosensitizers
[0072] Radiosensitizers are drugs that make cancer cells more sensitive to the
effects of radiation
therapy. The radiosensitizers used in the present invention are metal
nanoparticles, preferably inert metal
nanoparticles. The metal radiosensitizers of the present invention, when
irradiated near an anti-cancer
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
agent which is located on or in the proximity of the DNA, cause an increase in
single and double strand
breaks in DNA, a lethal type of damage, as compared to that caused by the anti-
cancer agent alone. Non-
limiting examples of metal radiosensitizers that could be used in accordance
with the present invention
include metals, preferably inert metals such as platinum, gold, iridium,
osmium, palladium, rodium, zinc,
chromium, copper, silver, cobalt, nickel and ruthenium. The greater the atomic
number, the better is the
interaction with radiation. Toxic metals such as mercury and lead have a
useful atomic mass but should not
be used in accordance with the present invention. Other useful metals,
although less preferred because of
their small atomic number, include iron. Preferred inert metals are gold and
platinum. Thus, in a preferred
embodiment, the metal radiosensitizer comprises nanoparticles made of an inert
metal, such that its
administration to a subject does not cause any important immune reaction or
adverse side effect. For
example, gold nanoparticles are preferred because they are known to be inert
in mammals, having long
been used to treat rheumatoid arthritis. Without being tied to any particular
theory, it is believed that when
gold nanoparticles are irradiated near the DNA with an anti-cancer agent,
preferably a platinum anti-cancer
agent, they synergistically cause an increase in DNA double breaks, as
compared to the amount of DNA
double breaks caused by 1) radiation alone; 2) radiation and gold
nanoparticles; or 3) radiation combined
with the anti-cancer agent.
[0073] In a specific embodiment, the composition of the present invention may
comprise additional
elements for increasing biocompatibility of the metal particles for example.
Non-limiting examples of such
elements include elements of the class of halogens such as bromide or iodine.
4. Formulation and Administration
[0074] The pharmaceutical composition may further comprise a pharmaceutically
acceptable carrier
or excipient. As used herein "pharmaceutically acceptable carrier" or
"excipient" includes any and all
solvents, buffers, dispersion media, coatings, antibacterial and antifungal
agents, isotonic and absorption
delaying agents and the like that are physiologically compatible. The carrier
is selected for administration by
the selected route of administration. The use of such media and agents for
pharmaceutically active
substances is well known in the art (Rowe et al., Handbook of pharmaceutical
excipients, 2003, 4th edition,
Pharmaceutical Press, London UK). Except insofar as any conventional medium or
agent is incompatible
with the active compounds (i.e., the metal radiosensitizer and the anti-cancer
agent), use thereof in the
pharmaceutical compositions of the invention is contemplated.
[0075] Non-limiting pharmaceutically suitable materials that may be
incorporated in pharmaceutical
preparations of the present invention include solubilizing/diluting agents,
antioxidants, enteric coatings,
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
16
absorption enhancers, pH adjusting agents and buffers, dispersing agents,
coatings, antibacterial and
antifungal agents, absorption delaying agents, osmolarity adjusters, isotonic
agents, preservative agents,
stabilizers (e.g., radiosensitizer stabilizer and/or anti-cancer stabilizer),
surfactants, thickening agents,
solvents, co-solvents, emollients, coloring agents, wetting agents and
ligands/pilote/targeting molecules.
Methods for preparing appropriate formulations are well known in the art (see
e.g., Hendrickson, 2005).
[0076] Solubilizing agents useful for the present invention encompass
polyoxyethylene-sorbitan-
fatty acid esters, polyoxyethylene fatty acid esters, PEG glyceryl fatty acid
esters, propylene glycol mono- or
di-fatty acid esters, sorbitan fatty acid esters, polyoxyethylene-
polyoxypropylene co-polymers, glycerol
triacetate, monoglycerides, acetylated monoglycerides, polysorbate glycerol
fatty acid esters, acetylated
esters of fatty acids, acacia, carbomer copolymer, carbomer interpolymer,
cholesterol, diethanolamine
aluminium monostearate, carboxy methyl cellulose, sodium desoxycholate, egg
yolk phospholipid,
hydrolyzed gelatin, lecithin, lanolin alcohols, poloxamer, povidone, sodium
dodecyl sulphate, sorbitol, oils
such as vegetable oils or animal oils (see relevant sections of USP-NF). Non-
limiting examples of vegetable
oils include canola, corn, flax seeds, cotton seeds, soybean, walnut, pine
nut, peanut, grape seed,
sunflower, safflower, olive, coconut, palm oil, etc...). Non-limiting examples
of animal oils include fish, seal
oil and castor oil. In more specific embodiments, they include any polysorbate
including polysorbates 20, 21,
40, 60, 61, 65, 80, 81 and 85; BrijTM (polyoxyethyleneglycol alkyl ether
having a polar side of 10 to 100
monomers) and CremophorTM (e.g., CremophorTM EL (derivative of castor oil and
ethylene oxide));
CremophorTM A6 (Polyethylene glycol 260 mono(hexadecyl/octadecyl) ether and 1-
octadecanol) and
CremophorTM A25 (polyethylene glycol 1100 mono(hexadecyl/octadecyl) ether).
[0077] The solubilizers containing polyoxyethylene chains such as
polysorbates, PEG, and Brij TM
are susceptible to formation of peroxides by radicalar reactions catalyzed by
light and oxygen. Non-limiting
examples of solubilizers include PEG400, CremophorTM EL, polysorbate 60 and
polysorbate 80.
[0078] Antioxidants useful for formulations of the present invention include
plant extracts (i.e., fruit,
vegetable and/or leguminous extracts), algae extracts, microorganisms extracts
such as yeast extracts and
their derivatives, ferments, proteolysis hydrolysates, peptides, animal
derivative extracts and synthetic
compounds. More particularly, such ingredients include
ethylbisiminomethylguaiacol manganese chloride;
dipalmitoyl hydroxyproline, dimethylmethoxy chromanol; bioflavonoid hesperidin
olive leaf extract,
ubiquinone, super-oxide dismutase, flavanols, isoflavones, furfuryladenine,
panthenol, lipoic acid,
niacinamide, melatonin, catalase, glutathione, polyphenols, cysteine,
allantoin, kinetin, squalane, grape
seed extract and camellia sinensis extract, ascorbic acid and its derivatives
(e.g., ascorbyl palmitate,
magnesium ascorbyl phosphate, sodium ascorbyl phosphate), vitamin E and its
derivatives (e.g., a-
tocopherol, 6-tocopherol, y-tocopherol, tocopheryl acetate, a hydrophilic
vitamin E analog such as 6-
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
17
hyd roxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (TroloxTM), alpha-
tocopherol acetate,
alpha-tocopheryl polyethylene glycol succinate, alpha-tocopherol palmitate),
butylated hydroxytoluene
(BHT), butylated hydroxyanisole (BHA), hypophosphorous acid, monothioglycerol,
potassium metabisulfite,
propyl gallate, sodium bisulfite, sodium formaldehyde sulfoxylate, sodium
metabisulfite, sodium sulfite,
sodium thiosulfate and sulfur dioxide (see USP-NF).
[0079] The terms "preservative agent" as used herein are meant to refer to any
ingredient capable
of retarding or preventing microbial or chemical spoilage and protecting
against discoloration. Without being
so limited, they include benzalkonium chloride, benzethonium chloride, benzyl
alcohol, butylparaben,
chlorobutanol, chlorocresol, cresol, ethylparaben, methylparaben, myristyl
gamma-picolinium chloride,
phenol, phenoxyethanol, phenylmercuric acetate, phenylmercuric nitrate,
propylparaben and thimerosal.
[0080] The terms "isotonic agent" as used herein are meant to refer to
ingredients capable of
adjusting osmolarity. Without being so limited, they include dibasic sodium
phosphate, sodium bicarbonate,
calcium chloride, potassium chloride, sodium lactate, glycerol, sorbitol,
xylitol, sodium chloride, dextrose, a
Ringer's solution, a lactated Ringer's solution and a mixture of dextrose and
a mixture thereof (see relevant
sections of USP-NF). A lactated Ringer's solution is a solution of recently
boiled distilled water containing 39
mmol/L of sodium ion, 42 mmol/L of chloride ion, 0.6 mmol/L of bicarbonate
ion, 1.4 mmol/L of potassium
ion and 42 mmol/L of calcium ion - the same concentrations as their occurrence
in body fluids. Ingredients
are: NaCl 2.25 g, KCI 0.105 g, CaCl2 0.12 g, NaHCO3 0.05 g.
[0081] The term "solvent" as used herein is meant to refer to ingredients
capable of facilitating the
solubilization of an active ingredient within the formulation. Without being
so limited, it includes water, water-
alcohol solutions, emulsions or suspensions, including saline and buffered
medical parenteral vehicles
including sodium chloride solution, Ringer's dextrose solution, dextrose plus
sodium chloride solution,
Ringer's solution containing lactose and fixed oils. Intravenous vehicles may
include fluid and nutrient
replenishers, electrolyte replenishers such as those based upon Ringer's
dextrose, and the like.
[0082] The term "stabilizer" as used herein is meant to refer to ingredients
that enable a higher
amount of active ingredients (e.g., radiosensitizer and'6r anti-cancer agent)
to be included in delivery
system (e.g., liposomes), or that increase the pharmacokinetic of compositions
of the present invention. For
example, without being so limited, useful radiosensitizer compound (e.g., gold
particles) stabilizers for the
present invention include polyacrylamide, polyvinyl, dextrose, D-glucose and
dithiolated
diethylenetriaminepentaacetic acid (DTDTPA). These stabilizers reduce
aggregation of the gold
nanoparticles into larger particles. Such stabilizers may be used during
production of nanoparticles.
Parenteral formulations
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
18
[0083] In cases where parenteral administration is elected as the route of
administration,
pharmaceutical compositions of the present invention may be provided to
patients in combination with
additional pharmaceutically acceptable sterile aqueous or non-aqueous
solvents, suspensions or emulsions.
Formulations to be used for in vivo administration are preferably sterile.
This is readily accomplished, for
example, by filtration through sterile filtration membranes.
[0084] Pharmaceutically acceptable carriers for parenteral formulations
include sterile aqueous
solutions or dispersions and sterile powders for the extemporaneous
preparation of sterile injectable
solutions or dispersions. Aqueous solvents/carriers include water, water-
alcohol solutions, emulsions or
suspensions, including saline and buffered medical parenteral vehicles
including sodium chloride solution,
Ringer's dextrose solution, dextrose plus sodium chloride solution, Ringer's
solution containing lactose, and
fixed oils. Intravenous vehicles may include fluid and nutrient replenishers,
electrolyte replenishers such as
those based upon Ringer's dextrose, and the like.
[0085] Supplementary active ingredients such as additional anti-cancer agents
or radiosensitizers
can be incorporated into the compositions. "Pharmaceutically or
pharmacologically acceptable" refer to
compositions that do not produce an adverse, allergic or other untoward
reaction when administered to an
animal or a human, as appropriate.
[0086] Solutions of therapeutic compositions can be prepared in water suitably
mixed with a
surfactant, such as hydroxypropylcellulose. Dispersions also can be prepared
in glycerol, liquid polyethylene
glycols, mixtures thereof and in oils. Under ordinary conditions of storage
and use, these preparations
contain a preservative to prevent the growth of microorganisms.
Route of administration
[0087] The therapeutic compositions of the present invention can be
administered in the form of
injectable compositions (e.g., intravenously, intramuscularly, subcutaneously
and intra-articularly), either as
liquid solutions or suspensions; solid forms suitable for solution in, or
suspension in, liquid prior to injection
also may be prepared. These preparations also may be emulsified. Other
pharmaceutically acceptable
carriers include aqueous solutions, non-toxic excipients including salts,
preservatives, buffers and the like.
[0088] Examples of non-aqueous solvents are propylene glycol, polyethylene
glycol, vegetable oil
and injectable organic esters such as ethyloleate. Intravenous vehicles
include fluid and nutrient
replenishers. Preservatives include antimicrobial agents, anti-oxidants,
chelating agents and inert gases.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
19
The pH and exact concentration of the various components of the pharmaceutical
composition are adjusted
according to well-known parameters.
[0089] Pharmaceutical compositions can also be administered by routes such as
orally, nasally,
rectally, topically, intravenously, intramuscularly, subcutaneously,
sublingually, intrathecally,
intraperitoneally, intra-articularly or intradermally. Preferably, the route
of administration is intravenously,
intra-arterially or orally. Preferably, the pharmaceutical compositions are
administered intravenously or intra-
arterially.
[0090] Hence in specific embodiments, when the composition of the present
invention is for oral
administration, the composition is in a tablet, a solution or capsule such as
a soft gel capsule for example. In
other specific embodiments, when the composition of the present invention is
for oral administration, it has
an enteric coating. In other specific embodiments, when the composition of the
present invention is for oral
administration, it is an oil-based syrup.
Dosage
[0091] The compositions and formulations of the present invention are
administered in amounts and
at frequencies sufficient to treat cancer. A subject's progress can be
determined by measuring and
observing changes in the concentration of cancer markers; by measuring the
actual size of the tumor over
time and/or by determining any other relevant clinical markers which are well-
known in the art. The
determination, measurement, and evaluation of such characteristics and markers
associated with clinical
progress are known to those of ordinary skill in the art.
[0092] Any amount of a pharmaceutical composition can be administered to a
subject. The dosages
will depend on many factors including the mode of administration, the type of
metal radiosensitizer and anti-
cancer agent used and the age of the subject.
[0093] As used herein, the terms "effective amount" or "therapeutically
effective amount" are meant
to refer to an amount effective to achieve the desired therapeutic effect or
biological effect while avoiding
adverse side effects. Thus, an effective amount of a metal radiosensitizer of
the present invention is an
amount sufficient to provide a synergistic effect with an anti-cancer agent
following irradiation. Similarly, an
effective amount of an anti-cancer agent of the present invention is an amount
sufficient to provide a
synergistic effect with a metal radiosensitizer following irradiation. This
synergistic effect is characterized by
an increase in the number of strand breaks (double strand and/or single
strand, preferably double strand
breaks) of DNA in cells which are contacted with the radiosensitizer and anti-
cancer agent and which are
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
treated with radiation therapy. Typically and depending on the route of
administration and anti-cancer
agent, the compositions of the present invention comprise a ratio of metal
radiosensitizer: anti-cancer agent
that will allow for an increase in the number of DNA strand breaks in cancer
cells as compared to combined
radiation and chemotherapy, or to combined radiotherapy and metal
radiosensitizer. Generally, the ratio
between the radiosensitizer and anti-cancer agent is between 1:1 and 1:1000;
more particularly between 1:1
and 1:500; between 1:1 and 1:200; between 1:1 and 1:100, 1:1 and 1:50; between
1:1 and 1:20, and
between 1:2 and 1:20. Additional non-limiting examples of ratio that may be
used in accordance with the
present invention include a ratio of radiosensitizer: anti-cancer agent of
1:1, 2:1, 3:1, 4:1, 5:1, 6:1, 7:1, 8:1,
9:1,10:1,1:2,1:3,1:4,1:5,1:6,1:7,1:8,1:9,1:10.
[0094] An effective amount of the therapeutic composition is determined based
on the intended
goal. The term "unit dose" or "dosage" refers to physically discrete units
suitable for use in a subject, each
unit containing a predetermined quantity of the therapeutic composition
calculated to produce the desired
response, discussed above, in association with its administration, i.e., the
appropriate route and treatment
regimen. The quantity to be administered, both according to number of
treatments and unit dose, depends
on the desired effect.
[0095] Precise amounts of the therapeutic composition also depend on the
judgment of the
practitioner and are peculiar to each individual. Factors affecting dose
include physical and clinical state of
the patient, the route of administration and the potency, stability and
toxicity of the particular radiosensitizer
and anti-cancer agent.
[0096] Thus, the dosage will also be adapted by the clinician in accordance
with conventional
factors such as the extent of the disease (e.g., stage of the cancer, type of
cancer, etc...), the particular anti-
cancer agent used and different parameters from the patient (e.g., age, sex,
general health, etc...) and may
depend on whether the subject is also taking other drugs for treating another
disease or condition. For
example, preclinical studies with LipoplatinTM and LipoxalTM were conducted
with 100mg/m2 to 350mg/m2 of
anticancer agent. The body surface area (BSA) is the total surface area of the
human body. The BSA is
used in many measurements in medicine, including the calculation of drug
dosages and the amount of fluids
to be administered. The "normal" BSA is generally taken to be 1.7 m2 but, in
actual fact, the BSA depends
on more than just height and weight. Other influential factors include the age
and gender of the individual.
For example, Average BSA for adult men: 1.9 m2; Average BSA for adult women:
1.6 m2; Average BSA for
children (9 years): 1.07 m2; Average BSA for children (10 years): 1.14 m2;
Average BSA for children (12-13
years): 1.33 m2. The dosage is adapted accordingly.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
21
[0097] Dosages may be provided in either a single or multiple dosage regimen.
It could be
administered, for example, every day for 3, 4, 5, 7, 8, 10 days or more.
Alternatively, it may administered
once a week for 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 weeks or for as many weeks as
the skilled practitioner sees fit.
The radiosensitizer and anti-cancer agent can be administered separately or
together and may be part of a
single formulation or of separate formulations as long as they are
administered prior to radiotherapy and
that their biological concentration is in an effective amount in cells at the
time of irradiation.
LIPOSOMES
[0098] The degree of radiosensitization is directly related to the degree of
metal radiosensitizer
adduction. Hence, a prolonged infusion of the radiosensitizer in free form
would maximize its incorporation.
In addition, the total number of cells that must be sensitized to obtain any
significant effect on the tumor is
also important, as well as the rate of hepatic degradation and elimination.
According to specific
embodiments, the present invention, seeks to optimize the number of sensitized
cells and to reduce hepatic
degradation by encapsulating the anti-cancer agent and metal radiosensitizer
in liposomes.
[0099] "Liposome" is a generic term encompassing a variety of single and
multilamellar lipid
vehicles formed by the generation of enclosed lipid bilayers.
[00100] Liposomes can be filled with drugs and used to deliver drugs for
cancer and other diseases.
Membranes are usually made of phospholipids, which are molecules that have a
head and a tail. The head
is attracted to water, and the tail, which is made of oil (hydrocarbon), is
repelled by water. In nature,
phospholipids are found in stable membranes composed of two layers (a
bilayer). In the presence of water,
the heads are attracted to water and line up to form a surface facing the
water. The tails are repelled by
water, and line up to form a surface away from the water. In a cell, one layer
of heads faces outside of the
cell, attracted to the water in the environment. Another layer of heads faces
inside the cell, attracted by the
water inside the cell. The hydrocarbon tails of one layer face the hydrocarbon
tails of the other layer, and
the combined structure forms a bilayer. When membrane phospholipids are
disrupted, they can reassemble
into tiny spheres/vesicles, smaller than a normal cell, either as bilayers or
monolayers and these vesicles
are called liposomes.
[00101] The lipids in the plasma membrane are chiefly phospholipids like
phosphatidyl ethanolamine
and cholesterol. Phospholipids are amphiphilic with the hydrocarbon tail of
the molecule being hydrophobic
and its polar head hydrophilic. As the plasma membrane faces watery solutions
on both sides, its
phospholipids accommodate this by forming a phospholipid bilayer with the
hydrophobic tails facing each
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
22
other. They can carry a net positive charge, a net negative charge or be
neutral. For example, dicetyl
phosphate can be employed to confer a negative charge on the liposomes, and
stearylamine can be used to
confer a positive charge on the liposomes.
[00102] Further advances in liposome research have been able to allow
liposomes to avoid detection
by the body's immune system, specifically, the cells of the
reticuloendothelial system (RES). These
liposomes are known as "stealth liposomes", and are constructed with
Polyethylene Glycol (PEG) studding
the outside of the membrane. The PEG coating, which is inert in the body,
allows for longer circulatory life
(2-3 days) for the drug delivery mechanism.
[00103] Liposomes are used for drug delivery due to their unique properties.
Dissolved hydrophilic
solutes cannot readily pass through the lipids. Hydrophobic chemicals can be
dissolved into the membrane,
and in this way liposomes can carry both hydrophobic molecules and hydrophilic
molecules. To deliver the
molecules to sites of action, the lipid bilayer can fuse with other bilayers
such as the cell membrane, thus
delivering the liposome contents. By making liposomes in a solution of DNA or
drugs (which would normally
be unable to diffuse through the membrane), they can be (indiscriminately)
delivered past the lipid bilayer.
[00104] Another interesting property of liposomes is their natural ability to
target tumor tissues. The
endothelial walls of all healthy human blood vessels are encapsulated by
endothelial cells that are bound
together by tight junctions. These tight junctions stop any large particles in
the blood from leaking out of the
vessel. Tumor vessels do not contain the same level of seal between cells and
are diagnostically leaky. This
ability is known as the Enhanced Permeability and Retention effect. Liposomes
of certain sizes, typically
less than 400 nm, can rapidly enter tumor sites from the blood, but are kept
in the bloodstream by the
endothelial wall in healthy tissue vasculature. Anti-cancer drugs such as
DoxorubicinTM (Doxil),
CamptothecinTM, DaunorubicinTM (Daunoxome), LipoplatinTM (cisplatin) and
LipoxalTM (oxaliplatin) are
currently being marketed in liposome delivery systems.
[00105] Liposomes can also be designed to deliver drugs in other ways.
Liposomes that contain low
(or high) pH can be constructed such that dissolved aqueous drugs will be
charged in solution (i.e., the pH
is outside the drug's pl range). As the pH naturally neutralizes within the
liposome (protons can pass
through some membranes), the drug will also be neutralized, allowing it to
freely pass through a membrane.
These liposomes work to deliver drug by diffusion rather than by direct cell
fusion. Another strategy for
liposome drug delivery is to target endocytosis events. Liposomes can be made
in a particular size range
that makes them viable targets for natural macrophage phagocytosis. These
liposomes may be digested
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
23
while in the macrophage's phagosome, thus releasing its drug. Liposomes can
also be decorated with
opsonins and ligands to activate endocytosis in other cell types.
[00106] Lipids suitable for use according to the present invention can be
obtained from commercial
sources, For example, dimyristyl phosphatidylcholine ("DMPC") can be obtained
from Sigma Chemical Co.,
dicetyl phosphate ("DCP") is obtained from K & K Laboratories (Plainview,
N.Y.); cholesterol ("Choi") is
obtained from Calbiochem (La Jolla, Calif.); DPPC and DPPC-Peg2000, DC-Chol,
dimyristyl
phosphatidylglycerol ("DMPG ) and other lipids may be obtained from Avanti
Polar Lipids, Inc. (Birmingham,
Ala.). Stock solutions of lipids in chloroform, chloroform/methanol or t-
butanol can be stored at about -20
C. Preferably, chloroform is used as the only solvent since it is more readily
evaporated than methanol.
[00107] Phospholipids from natural sources, such as egg or soybean
phosphatidylcholine, brain
phosphatidic acid, brain or plant phosphatidylinositol, heart cardiolipin and
plant or bacterial
phosphatidylethanolamine are often preferably not used as the primary
phosphatide, i.e., constituting 50%
or more of the total phosphatide composition, because of the instability and
leakiness of the resulting
liposomes.
[00108] Liposomes within the scope of the present invention can be prepared in
accordance with
known laboratory techniques. In one embodiment, liposomes can be prepared by
mixing liposomal lipids in
a solvent, in a container, e.g., a glass or pear-shaped flask. The container
optimally has a volume ten times
greater than the volume of the expected suspension of liposomes. Using a
rotary evaporator, the solvent is
removed at approximately 40 C under negative pressure. The solvent normally is
removed within about 5
min to 2 h, depending on the desired volume of the liposomes. The composition
can be dried further in a
desiccator under vacuum. The dried lipids generally are discarded after about
1 wk because of a tendency
to deteriorate with time.
[00109] Dried lipids can be hydrated at approximately 25-50 mM phospholipid in
sterile, pyrogen-free
water by shaking until all the lipid film is resuspended. The aqueous
liposomes can then be separated into
aliquots, each placed in a vial, lyophilized and sealed under vacuum.
[00110] In the alternative, liposomes can be prepared in accordance with other
known laboratory
procedures: the method of Bangham et al. (1965), the contents of which are
incorporated herein by
reference; the method of Gregoriadis, as described in Drug Carriers In Biology
and Medicine, G. Gregoriadis
(1979), the contents of which are incorporated herein by reference; the method
of Deamer and Uster
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
24
(1983), the contents of which are incorporated by reference; and the reverse-
phase evaporation method as
described by Szoka and Papahadjopoulos (1978). The aforementioned methods
differ in their respective
abilities to entrap aqueous material and their respective aqueous space-to-
lipid ratios.
[00111] Preferred liposomes of the present invention are peggylated and of the
type used in the
LipoplatinTM formulation. Because they have PEG coating, they enter in the
category of stealth liposomes.
This liposomal formulation allows for longer circulatory life (half life of 2-
3 days). Because such liposomal
formulations avoid detection by the body's immune system (specifically the
cells of the reticuloendothelial
system) and have a preferential accumulation to the tumor site, they have a
less toxic effect on healthy
tissues (19).
[00112] Where clinical application of liposomes containing radiosensitizer and
anti-cancer agent is
undertaken, it is desirable to prepare the liposome complex as a
pharmaceutical composition appropriate for
the intended application. Generally, this entails preparing a pharmaceutical
composition that is essentially
free of pyrogens, as well as any other impurities that could be harmful to
humans or animals. One also will
generally desire to employ appropriate buffers to render the complex stable
and allow for uptake by target
cells.
[00113] Thus, aqueous compositions of the present invention can comprise an
effective amount of
the radiosensitizer and anti-cancer agent in a liposome as discussed above,
further dispersed in
pharmaceutically acceptable carriers and/or aqueous media. Such compositions
also are referred to as
innocula.
[00114] The present invention is illustrated in further details by the
following non-limiting examples.
EXAMPLE I
Material & Methods
Sample preparation
[00115] Supercoiled plasmid DNA [pGEM-3Zf(-), 3197 base pairs], GNP and GNP-
DNA and
cisplatin-DNA complexes were prepared and purified as previously described (5,
6). The trace of TE buffer
(Tris-HCI and EDTA) was removed by a SephedexTM G-50 (Pharmacia) column. The
pure DNA was diluted
in distilled and deionized water (dd H20) without any salt. A solution of cis-
diammineplatinum (II) dichloride
(cisplatin, 98% purity, Sigma Aldrich) was mixed with the plasmid DNA solution
to obtain a molar ratio (R) of
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
cisplatin to plasmid molecules of 2:1. The mixture was kept in darkness at 37
C for 48 h forming the DNA-
cisplatin complex. In reacting with DNA, cisplatin loses its chloride ions and
usually binds to the N7 position
of guanine at a GG site, producing about 90% of an interstrand adduct (7).
Thus, the cisplatin adduct to
DNA carries a position charge (2+).
[00116] GNP were synthesized by mixing the 10 ml of 8 mM NaBH4 with 10 ml of 3
mM HAuCI4
(Sigma) (5). The average diameter of GNP was 5 2 nm (i.e., 3-7 mm), as
characterized by TEM. Taking
into account the size distribution and assuming a cubic close packing of the
Au atoms (0.288 nm Dia.), the
number of gold atoms per GNP and the concentration of the GNP solution were
deduced to be 3000 and
0.5 0.2 NM, respectively (8).
[00117] The GNP-DNA and DNA-cisplatin-GNP complexes were prepared by mixing
DNA or the
DNA-cisplatin complex with the GNP solution so as to obtain the molecular
ratios (1:2), (1:1) and (1:2:1),
respectively. These ratios were chosen to remain close to those of clinical
conditions relative to cisplatin
concentration (Zheng Y, Sanche L. Gold nanoparticles enhance DNA damage
induced by anti-cancer drugs
and radiation. Radiat Res. 2009 Jul; 172(1):114-9) while providing an easily
observable enhancement factor
(EF), defined as the yield measured from a given complex divided by that
obtained from pure DNA. With this
preparation, the GNPs were electrostatically bound to DNA (5). Since the gold
nanoparticles are slightly
negatively charged (9), they are expected to be attracted to the positively
charged site (i.e., counter ion of
the phosphate group) within the DNA molecules. Furthermore, the induced
polarization in DNA by the gold
nanoparticle negative charge as well as the charge-dipole interaction add to
the binding potential. When
cisplatin is already bound to DNA, the positive charge on the cisplatin adduct
should also contribute to this
electrostatic interaction. In clinical use, it is not necessary that the metal
be negatively charged to remain in
proximity of DNA.
Electron irradiation
[00118] Five NI aliquots of DNA, cisplatin-DNA, GNP-DNA and DNA-cisplatin-GNP
solutions were
deposited on a 1-mm-thick gold foil (99.99%, Laboratoire MAT). The samples
were dried in a glove box at
ambient temperature, at a relative humidity of 10 %. This procedure produced
films of 2900 nm average
thickness estimated from the density of DNA of 1.7 g cm-3 (10). Analysis of
TEM images of GNP-DNA
showed local binding of the GNP to DNA. Variations in the number of GNP
present within small different
areas of the sample (200x200 nm2) indicated that the sample thickness did not
vary by more than 30%.
Similar observations could be made with films of DNA-cisplatin-GNP complexes.
It is estimated from these
images that the film is sufficiently thick and uniform to absorb sufficient
energy from the electron beam,
while avoiding the effects of secondary electrons emitted from the metal
substrate.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
26
[0001] Afterwards, the samples were transferred to the TEM (H-7100 Hitachi)
chamber, where they
were irradiated or not by the 60 KeV electron beam with a current of 15 pA for
periods varying from 5 to
30s. Data were recorded at five different doses under identical experimental
conditions and each data point
was the average of three experiments. The incident electron fluence of the TEM
was measured with a
radiochromatic dosimetry film as described previously (11). Taking into
account the area of the electron
beam of 4.6 mm2, the incident electron flux was determined to be 2.9 X1013
electrons s-1 cm-2. For the same
fluence, the radiation dose absorbed by the GNP-DNA and DNA-cisplatin-GNP
complexes was larger than
that of pure DNA due to the larger mass absorption coefficient of gold and Pt.
Agarose gel electrophoresis
[00119] Once removed from the UHV chamber, the samples were recovered by
dissolving in 20 pl of
dd H20. The different forms of SSB, DSB and supercoiled DNA were separated by
1% neutral agarose gel
electrophoresis (5, 6). The gels were scanned with a STORM86OTM in the blue
fluorescence mode
(Molecular Dynamics) having an excitation wavelength of 450 nm. The relative
amount of DNA in each form
was quantified with the ImageQuantTM (Molecular Dynamics).
EXAMPLE 2
Synergistic effect of metal nanoparticles and cisplatin
in the production of DSB and SSB on DNA
[00120] Various complexes of DNA were prepared with different molar ratios (R)
of adduct to DNA.
Films of pure DNA and the complexes GNP-DNA (R = 1:1 and 1:10), cisplatin-DNA
(R = 2:1) and cisplatin-
GNP-DNA (R = 2:1:1 and 20:1:10) were exposed to the 60 keV electron beam of a
transmission electron
microscope (TEM). After a given electron fluence, the samples were retrieved
from the TEM and the DNA
damage analyzed by electrophoresis. The dependence of the yields of single and
double strand breaks
(SSB and DSB) as well as the loss of supercoiled DNA were measured as
functions of exposure. Since
most radiation treatments use 1-18 MeV photons, which generate essentially
Compton electrons over a
wide energy range, the action of 60 KeV electrons is considered to represent
the interaction of the high
energy electrons liberated in cancer cells during radiotherapy (4).
[00121] The curves in Fig.1 show the dependence of the yields of DSB on
exposure to 60 keV
electrons of films of pure plasmid DNA and the complexes of cisplatin-plasmid,
GNP-plasmid and cisplatin-
GNP-plasmid for R = 2:1, 1:1, and 2:1:1, respectively. Similar curves were
generated for SSB (data not
shown). If the current density of electrons impinging on the target is defined
as J, an infinitesimal relative
number of damaged products can be expressed as:
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
27
dN/N = aJdt,
[00122] where N is the number of DNA molecules in the sample, or the cross
section corresponding
to the total DNA damage. Integration over time gives
N5(t) = Ns(o) exp(-crJt),
[00123] where N5(o) and Ns are the initial and the final amount of supercoiled
DNA molecules in the
sample, respectively. If this exponential function is expanded into the
Taylor's series around a small time
interval and the first two terms of the expansion are kept, the equation
giving the decrease of supercoiled
DNA becomes
Ns(t) = Nso(1-QJt).
[00124] and the total damage Nd(t) = Ns(o) - Ns(t) - Ns(o) 6Jt.
[00125] Defining Nss, Nds and Nr as the damage and au, ads and Ur as the cross
sections for damage
in the form of SSB, DSB and the remaining lesions, respectively, due to SSB,
DSB and the remaining of the
lesions, respectively, it can be written that
Nd(t) = Nds(t) + Nds(t) + Nr(t) = Ns(o) Jt [ass + Qu + Qr]
[00126] It follows that individual damages to DNA can be expressed as the
initial slope of an
exponential function. The curves drawn through the data points in Fig. 1 were
therefore fitted to an
exponential function. The yields of supercoiled DNA, SSB and DSB per electron
per molecule, expressed as
the percentage of initial DNA in the film, were obtained from the initial
slope of such exponential fits. The
results at zero fluence, obtained under identical conditions, indicate that
the complexes were more fragile
than pure DNA to the manipulations. Since they were recorded from the linear
portion of the dose-response
curves, these yields were generated by the interaction of a single 60 keV
electron. They are given in Table 1
below for different DNA film preparations. Both GNP and cisplatin binding to
DNA increase the production of
SSB and DSB, but the highest yields were obtained with both species bound to
DNA.
Table 1. The yields (Y in 10-15 electron-' molecule-') for the formation of
SSB, DSB and loss of supercoiled
DNA induced by 60 keV electrons in 2900 nm thick films of DNA of different
compositions deposited on a
gold foil. The quoted errors represent the maximum deviations of three
identical measurements.
Samples Y
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
28
SSB DSB Loss of
Supercoiled
pure DNA 3.72 0.3 0.77 0.1 -5.46 t 0.54
GNP:DNA=1:1 8.65 0.9 1.79 0.2 -10.5 1.1
Cisplatin:DNA= 2:1 9.49 0.91 1.95 0.2 -12.3 t 1.2
Cisplatin: GNP:DNA= 11.1 t 1.2 7.68 1.0 -20.1 2.0
2:1:1
GNP:DNA=1:10 5.38 0.56 1.11 0.3 -6.8 1.0
Cisplatin: GNP:DNA= 10.2 1.1 3.93 0.5 -14.9 1.6
20:1:10
[0100] The histograms of Figs. 2 and 3 exhibit the EF defined previously. For
ratios of GNP
to DNA of 1:1 and cisplatin to DNA of 2:1, the EF of SSB lies between 2-2.5
and increases to 3 with
cisplatin-GNP-plasmid in a ratio of 2:1:1. A similar effect is observed for
DSB with the exception that the
binding of GNP and cisplatin to DNA creates a spectacular increase in the EF;
i.e., DSB are increased by an
order of magnitude with respect to pure DNA. As shown in Fig. 3, even when the
GNP-DNA ratio is lowered
to 1:10, the EF for DSB is still as high as 5.1.
[0101] When only one GNP was added to a cisplatin-DNA complex before
irradiation by high
energy electrons, the production of DSB was increased by a factor of 4. Even
when only one GNP was
electrostatically bound to one cisplatin-DNA complex out of 10, the yield of
DSB from the cisplatin-DNA
complex was doubled. Compared to the yield of DSB from pure DNA, these
enhancements translate into
EFs of 9.9 and 5.1, respectively. Since the number of generated additional LEE
is directly proportional to
the number of GNP, this non-linearity between the number of GNP and the EF was
surprising. Such a high
sensitivity of cisplatin-DNA complexes to irradiated GNP cannot be explained
by any changes that may be
induced in DNA by the GNP, since in this case the additional damage would be
directly proportional to the
number of GNP up to R of 1:1. As shown in previous experiments on DNA damage
induced by 60 keV
radiation incident 300 nm films of GNP-DNA complexes, the formation of DSB as
a function of the ratio
GNP/plasmid rose rapidly between 0 and 0.25 and tended to saturate thereafter.
The previous results and
present EF values point to damage caused by the generation of additional
secondary electrons from the
GNP, which can reach many DNA molecules. Since the diameter of the DNA is 2
nm, secondary electrons
of energy lower than 200 eV, which have an effective range of about 10 nm (15,
16), could reach 10 DNA
molecules in the films. For 60 keV electrons, gold has a mass absorption
coefficient approximately 9 times
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
29
larger than that of biological material (17). Additional ions and secondary
electrons are generated by about
the same factor in this metal; the latter can easily escape the GNP and
further damage nearby DNA. The
most probable energy of secondary electrons usually lies around 9-10 eV, with
most secondary electrons
having energies well below 200 eV (18). Thus, the local density of LEE near
DNA was greatly increased
and, as seen from Figs. 2 and 3, the presence of one GNP per plasmid about
doubled the formation of SSB
and DSB.
[00127] When both cisplatin and GNP were bound to DNA, the EF for SSB
increased about
threefold. A strong synergy is observed between GNP and cisplatin in the case
of DSBs, which are
increased by an order of magnitude for R = 2:1:1 (Fig. 2). A number of basic
phenomena could contribute to
this huge enhancement. First, the possibility of two-event processes triggered
by the interaction of a single
60 keV electron with a GNP can be considered. The yield of DSB is expected to
be highly dependent on
two-event processes, such as the damage created by two LEE within the range of
the distance between 10
base pairs (- 4 nm). Furthermore, a SSB/DSB ratio of 4-5 has been observed in
several LEE irradiation
experiments compared to that of 10-20 for photons. As previously mentioned,
GNPs increase the density of
LEE within 10 nm of their site by an average factor of about 9 in the case of
60 keV radiation. Hence, DSB
formation by two LEE interactions is expected to considerably increase within
the distance of 10 base pairs
from a GNP. Following DEA, simultaneous electron transfer and reaction of NH3
or the Pt(l) adduct could
also cause a DSB. The much higher EF obtained by combining cisplatin and GNP
compared to GNP alone
may also partly arise from the energy requirement to produce a DSB. Cisplatin
locally modifies the topology
of DNA (7). The different topology could potentiate DNA damage and raise the
amount of DSB produced
with and without irradiation. Combined with the electron affinity and chemical
reactivity of cisplatin, these
modifications could appreciably lower the energy required to break two
adjacent bonds. The energy
required to break bonds being lower, the probability of breaking bonds will be
higher which leads to a better
efficiency and a better control of tumoral proliferation.
[00128] It has been shown that the binding of only one GNP to a plasmid-
cisplatin complex
containing 3197 base pairs increased by a factor of 4 the efficiency of the
chemotherapeutic agent cisplatin
to produce DSB in DNA irradiated by high energy electrons. Furthermore, the
overall increase in DSB
compared to pure DNA reached an order of magnitude. The present results were
obtained with 6.25 x 10-4
cisplatin molecules per base pair (mol./b.p.). In chemotherapy, with cisplatin
incorporated into a liposome,
concentrations can reach values of 4 x 10.4 cisplatin mol./b.p. in DNA,
assuming a uniform distribution of the
drug in cancer tissues (19). Since cisplatin accumulates preferentially in the
DNA of cancer cells (20), the
present work should therefore easily represent clinical concentrations.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
EXAMPLE 3
Liposomes encapsulated gold nanoparticles and platinum anti-cancer agent
Composition of LipoGoldTM
[00129] Lipids composition in molar ratio:
Dipalmitoylphosphatidylcholine (DPPC)=1.3 : 30-[N-(1',W-dimethylaminoethane)-
carbamoyl]-cholesterol
(DC-Chol) =1 : Dioleoyl Phosphatidylethanolamine (DOPE)=1 : DPPC-
Peg2000=0.033.
Load: Gold nanoparticles (AuNp) solution: size of gold = 5.6nm, concentration
= 5mM
Cisplatin in a concentration of 5mM.
Fabrication
[00130] Lipid layer: Lipids were thawed at room temperature and then mixed
together at 52 C in
chloroform. Chloroform was removed using rotary evaporation (1 h, 52 C, 250
mbar, 120 rpm).
After evaporation of the chloroform, a thin layer of lipids remained on the
inner surface of the round bottom
flask.
[00131] Hydration: The load (AuNp + cisplatin) was diluted 1/30 in a solution
of sterile clinical
dextrose (Dextrose 5%). 1.5 ml of this solution was then used for hydration of
the lipids bilayer with vigorous
agitation.
[00132] Freeze and thaw:
The solution of lipids and AuNp+cisplatin was subjected to 5 freeze-&-thaw
cycles (5min in liquid nitrogen,
5min at 52 C / cycle).
[00133] Purification: The liposomes resulting from the freeze-&-thaw step was
diluted (1/2) and
purified through a column of SephadexTM G-25M and collected in three
fractions. The purification step was
performed to separate the liposomal formulation from the non encapsulated free
AuNp and cisplatin.
Fraction 1 was defined as a mix of solvent and liposomes of big size, Fraction
2 was a liposomal fraction
used for the in vivo experiment in Example 4 below and Fraction 3 consisted of
free AuNp and free cisplatin.
Analysis
[00134] TEM (transmission electron microscopy): TEM images were taken for
Fractions 1 and 2.
As shown in Figures 4 (Fraction 1) and 5 (Fraction 2), there was an artefact
of dispersion probably caused
by the step of drying of the sample before image acquisition. The dispersion
follows a drying pattern of
agglutination probably caused by the viscosity of the solvent (dextrose 5%)
that produced compaction and
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
31
deformation of the liposomes when drying. Nevertheless, the TEM images allowed
the observation of
individual AuNp nanospheres with a mean diameter of 6 nm and the aggregation
of these AuNp with a
diameter of approximately 112.55 nm 40nm (fraction 2). The lipid membrane of
the liposomes is not
visible because no staining agent (molybdene) was used for these TEM
observations. Nevertheless, the
characteristic agglomerations of AuNp suggests that AuNp particles (and
cisplatin) were well entrapped into
liposomes.
[00135] Concentration: The concentration of Au and Pt of the liposomal
formulation for Fractions 1
and 2 was measured by ICP-MS (Inductively coupled plasma mass spectrometer).
Using standards as
reference, the concentration of metal was as follows
Fraction 1: Au = 0.875Ng/ml, Pt = 0.885pg/ml. Encapsulation efficiency = Au
52%, Pt 53%. Fraction 2:
Au = 2.89pg/ml, Pt = 2.4pg/ml
Encapsulation efficiency = Au 173%, Pt 143%. Ratio Au : Cisplatin of about
1:1.
[00136] The high encapsulation efficiency of Fraction 2 is likely due to a
concentration of metal at the
hydration step.
EXAMPLE 4
In vivo administration of liposomes-encapsulated gold nanoparticles and
platinum anti-cancer agent
Fischer rats
[00137] The experimental protocol was approved by the institutional ethical
committee and
conformed to regulations of the Canadian Council on Animal Care. For all
procedures (implantation,
chemotherapy, radiotherapy and euthanasia), male Fischer rats (Charles River
Laboratories) were
anesthetised with an intraperitoneal injection of Ketamin/Xylazin (87/13
mg/ml) at 1 ml/kg and additional
injection were delivered to maintain anaesthesia throughout the procedures
when needed. When
anesthetised, ocular lubricant was applied on the animal's eyes to avoid
drying.
F98 glial cell implantation in Fischer rats brain
[00138] F98 glioma tumors were implanted in Fischer rat brains as described by
Blanchard et al.
(Blanchard J, Mathieu D, Patenaude Y, Fortin D., MR-pathological comparison in
F98-Fischer glioma model
using a human gantry. Can J Neurol Sci. 2006 Feb;33(1):86-91). Briefly, a
midline scalp incision was
performed on anesthetized animals to expose the bregma, and then the animal
head was fixed on a
stereotactic frame (David Kopf Instrument). A burr hole was drilled 3mm to the
right and 1 mm anterior to the
bregma with a 16s-gauge needle. A 25s-gauge needle was inserted to a depth of
6.5mm and then
withdrawn to a target depth of 6mm from the skull interior surface. The 25s-
gauge needle (SGE) was held in
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
32
a microinfusion pump (WPI model UMP3) fixed on the stereotactic frame. The
pump driving unit (WPI model
MICRO4 UMC4) was programmed to inject 1pl/min on 5 min. for a total of 5pl (10
000 cells) in the target
location (the right fontal lobe). Two minutes after F98 glioma cells
implantation, the syringe was slowly
retracted over one minute, the burr hole was filled with bone wax and the
scalp was closed by sutures.
Antibiotic and analgesic ointment was then apposed on the sutures.
Intra carotid chemotherapeutic drug delivery
[00139] Ten days after F98 glioma cells implantation, LipoGoldTM (Fraction 2)
was infused in the
internal carotid artery in a retrograde manner via the right external carotid
as described by Fortin et al.
(Fortin D, Adams R, Gallez A. A blood-brain barrier disruption model
eliminating the hemodynamic effect of
ketamine. Can J Neurol Sci. 2004 May;31(2):248-53.).
[00140] After complete injection, the external carotid was sacrificed and the
neck of the animal was
closed by sutures. Antibiotic and analgesic ointment was then apposed on the
sutures. The efficiency of
LipoGoldTM was compared to other platinum compounds using the same surgical
procedures. To be able to
administer a platinum compound dose that corresponds to a dose equivalent to
that for human treatment,
the first approximation of equivalent dose was established with respect to the
body surface area (BSA), that
is determined to be 0.04 m2 for rats weighting 250g. Platinum doses
administered to rats in this study were
1 ml/rat of oxaliplatin 3 mgtfnl, cisplatin 3 mghnl, LipoplatinTM 3mg'fnl (of
cisplatin), LipoxalTM 3mg4r l (of
oxaliplatin) and LipoGoldTM 0.00289mg/ml. Free platinum compounds were diluted
in 1 ml of 5% dextrose
solution (Baxter, Toronto, Canada). LipoplatinTM and LipoxalTM were used
without dilution at a concentration
of 3 mg platinum / ml. and the solutions of 1 ml of platinum formulation were
injected over 20 min.
Sham animals
[00141] Control animals had the same surgical procedures as animal treated
with platinum drugs.
These animals were implanted with F98 glioma cell line. Ten days after
implantation, these animals received
an intra-arterial injection of 1 ml of clinical dextrose 5% (solvent for
platinum-based drugs) using the same
procedures as other animals treated by platinum-based drugs.
Gamma Knife irradiation
[00142] Twenty four hours after chemotherapeutic treatments (platinum-based
drugs and sham), rats
were anesthetized and positioned in home-made stereotactic frame for use with
the Gamma Knife TM 4C
(ELEKTA) (Charest G, Mathieu D, Lepage M, Fortin D, Paquette B, Sanche L.
Polymer gel in rat skull to
assess the accuracy of a new rat stereotactic device for use with the Gamma
Knife. Acta Neurochir (Wien)
2009 Jun;151(6):677-83; Epub 2009 Apr 18). This stereotactic frame allows
reliable positioning to target
precisely the tumor volume including a minimum surrounding brain parenchyma
volume. The 8-mm
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
33
collimator was used to deliver radiation at predetermined coordinates
targeting the tumor location. The
tumor, that has roughly a diameter of 4 mm (Blanchard J, Mathieu D, Patenaude
Y, Fortin D., MR-
pathological comparison in F98-Fischer glioma model using a human gantry. Can
J Neurol Sci. 2006
Feb;33(1):86-91) and the surrounding brain volume were treated with a maximum
dose of 15 Gy at a dose
rate of approximately 2.8 Gy/min. For rats that received LipoGoldTM, radiation
treatments were given 8h
after chemotherapy. For one rat, radiation was given in one shot of 15 Gy and
two other rats, radiation was
given in two shots of 7.5 Gy separated by 20 min.
End point experiment for surviving time
[00143] Weight, mobility, coordination, loss of self grooming (periocular
secretion accumulation) and
landing ability were evaluated daily. In accordance with the ethical committee
regulations, the survival time
end point of the experiment was complete lethargy (and apathy) of the animals.
At this point, animals were
anesthetised and an intracardiac infusion of paraformaldehyde 4% (PFA) was use
to fix the brain tissue.
The brain was removed by craniotomy to corroborate the presence of tumor and
kept in PFA for future
analysis.
Results of surviving assay
[00144] As was also observed for LipoplatinTM and LipoxalTM, LipoGoldTM did
not produce any
apparent toxicity on the animal treated following drug administration.
Conversely, free cisplatin produced an
apparent high toxicity. Animals that received cisplatin showed complete apathy
shortly after the
administration and died before sham animals, In the same manner, animals
treated with oxaliplatin showed
apparent high toxicity (parastesy, parasteny, lack of capillary blood flow,
jump reflex and apathy) and had a
short life span, similar to sham animals. Life spans for treated animals are
shown in Figure 6.
Conclusion
[00145] Even if the concentration of cisplatin in LipoGoldTM is 1000 time less
than the cisplatin
concentration of LipoplatinTM(i.e. 0.0024 mg'fnl LipoGoldTM vs. 3 mg'fnl), the
effect on the life span is about
the same in both cases (Figure 6). Furthermore, LipoGoldTM did not produce
apparent toxicity in healthy
tissue.
EXAMPLE 5
Determining the synergistic effect of the combination of LipoGoldTM and
radiation
[00146] The synergistic effect of the combination of LipoGoldTM and radiation
will be further
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
34
evaluated by combining different doses of LipoGoIdTM (e.g., 1 pM a 80pM) alone
or combined with radiation
(Charest G, Paquette B, Fortin D, Mathieu D, Sanche L.Concomitant treatment of
F98 glioma cells with new
liposomal platinum compounds and ionizing radiation. J Neurooncol. 2010
Apr;97(2):187-93. Epub 2009
Sep 17). The survival fraction of each modality will be analysed for
Combination Index (CI). These tests will
determine an optimized ratio of AuNp and cisplatin in liposomes to achieve the
best radiosensitive effect.
Briefly, the Cl is calculated using the equation:
(Pt) (IR) (Pt)(IR)
el = (Ptx) + (IRR) (Ptx) (IRx)
[00147] where the denominator, (Ptx) is the concentration of platinum compound
that inhibits
colonies formation at x%, and (lRx) corresponds to the radiation dose which
results in the same x% of
colonies formation inhibition. In the numerator (Pt)(IR) "in combination" also
inhibit the colonies formation at
the equivalent x%. If the sum of these two fractions is equal to 1, additive
effect is indicated. If the Cl value
is smaller than 1, synergism in indicated, and if the Cl value is greater than
1, antagonism is suggested.
EXAMPLE 6
Higher doses of platinum-based compound and gold in LipoGoIdTM
[00148] Non toxic AuNp stabilisers (polyacrylamide, polyvinyl, dextrose, ,D-
glucose, dithiolated
diethylenetriaminepentaacetic acid (DTDTPA)) are mixed with AuNp and
cisplatin. The solution of stabilized
AuNp and cisplatin (0.03mg/ml, 0.3 mg/ml, 3 mg/ml, 4 mg/ml, 5 mg/ml, 6mg/ml,
7mg/ml, 8 mg/ml. 9 mg/ml)
is encapsulated into liposomes.
EXAMPLE 7
Determination of maximum tolerated dose and dose limited toxicity
[00149] The maximum tolerated dose (MTD) and dose limited toxicity (DLT) will
be determined by
classical in vivo pharmacologic assays. The role of toxicology studies in new
anticancer drug development
is essentially threefold: to define a safe starting dose for phase I clinical
trials by determining the maximal
tolerated dose (MTD) in animals; to catalogue the dose-limiting toxicity (DLT)
and other toxicities to alert
clinical investigators to potential problems in clinical studies; and to
retract an agent from further evaluation
due to excessive and unpredictable toxicity. Toxicology studies will be
perform following the
recommandation of the FDA (DeGeorge JJ, Ahn CH, Andrews PA, Brower ME, Giorgio
DW, Goheer MA,
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
Lee-Ham DY, McGuinn WD, Schmidt W, Sun CJ, Tripathi SC. Regulatory
considerations for preclinical
development of anticancer drugs. Cancer Chemother Pharmacol. 1998;41(3):173-
85).
EXAMPLE 8
Determination of long-term stability
[00150] Leakage tests will be performed at different times after production of
LipoGoIdTM. Attt
various time after manufacture, lipogold solution samples will be deposited in
osmotic bag shaving pores of
10pM. This will enable the separation of intact liposomes from those which
will have released their content
i.e. the content will have crossed through the osmotic bag membrane. The
fractions outside the bag and
inside the bag will then be analyzed for their content in platinum compounds
and radiosensitizer (e.g. gold
nanoparticles). Exclusion columns can be used as an alternative to osmotic
bags.
EXAMPLE 9
Determination of drug tumor uptake
[00151] Liposomes preferentially target tumors by means of the Enhanced
Permeability and
Retention (EPR) property of the neovascularisation of the tumor. Different
lipid compositions (i.e. pH
sensitive lipids, cationic lipids) will be used in the liposomes to increase
the drug uptake by the tumor. The
drug uptake will be measured in the tumor, adjacent healthy tissues, different
organs such as kidney, liver
and blood for each LipoGold TM formulation.
[00152] Although the present invention has been described hereinabove by way
of specific
embodiments thereof, it can be modified, without departing from the spirit and
nature of the subject
invention as defined in the appended claims.
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
36
REFERENCES
1. P. J. Eifel, Concurrent chemotherapy and radiation therapy as the standard
of care for cervical
cancer. Nat. Clin. Pract Oncol. 5, 248-255 (2006).
2. Cancer: Principles and Practice of Oncology, edited by V. T. Devita, S.
Hellman and S. A.
Rosenburg, 6th ed. Lippincott Williams and Wilkins, Philadelphia, PA, 2001.
3. V. Brabec, Platinum-based Drugs in cancer therapy, edited by LR. Kelland
and N. Farrell, Humana
Press Inc., Totowa, NJ, 2000.
4. H. E. Johns and J. R. Cunningham, The physics of Radiology, edited by H.
Springfield, Charles C.
Thomas Publisher, U. S. A. 1983.
5. Y. Zheng, D. Hunting, P. Ayotte and L. Sanche, Radiosensitization of DNA by
gold nanoparticles
irradiated with high-energy electrons. Radiat Res. 169, 19-27 (2008).
6. Y. Zheng, D. Hunting, P. Ayotte and L. Sanche, Role of secondary low energy
electrons in the
concomitant chemoradiation therapy of cancer. Phys. Rev. Lett. 100, 198101-4
(2008).
7. R. N. Bose, Biomolecular targets for platinum antitumor drugs. Mini-Rev.
Med. Chem. 2, 103-111
(2002).
8. M. J. Hostetler, J. E. Wingate, C. Zhong, J. E. Harris, R. W. Vache, M. R.
Clark, D. Londono, S. J.
Green, J. Stokes and R. W. Murray, Alkanethiolate gold cluster molecules with
core diameter from 1.5 to 5.2
nm: core and monolayer properties as a function of core size. Langmuir 14, 17-
30 (1998).
9. M. A. Hayat, Colloid Gold, Principles, Methods and Applications. Academic
Press, New York, 1989.
10. G. D. Fashman, Handbook of Biochemistry and Molecular Biology 3rd ed. CRC
Press, Boca Raton,
FL, 1995.
11. Z. Cai, X. Pan, D. Hunting, P. Cloutier, R. Lemay and L. Sanche, Dosimetry
of ultrasoft X-rays (1,56
keV AIK ) using radiochromatic films and color scanners. Phys. Med. Biol. 48,
4111-4124 (2003).
12. J. Berdys, I. Anusiewicz, P. Skurski and J. Simons, Damage to model DNA
fragments from very
low-energy (1< eV) electrons. J. Am. Chem. Soc. 126, 6441-6447 (2004).
13. Kumar and M. D. Sevilla, The role of it6* excited states in electron-
induced DNA strand break
formation: A time-dependent density functional theory study. J. Am. Chem. Soc.
130, 2130-2131 (2008).
14. L. Sanche in "Radiation Induced Molecular Phenomena in Nucleic Acid, A
comprehensive
theoretical and experimental analysis series", Vol. 5, edited by M.K. Shukla
and J. Leszczynski, Springer,
Netherland, 2008.
15. J. Meesungnoen, J.-P. Jay-Gerin, A. Filali-Mouhim and S. Mankhetkorn, Low-
energy electron
penetration range in liquid water. Radiat. Res. 158, 657-660 (2002).
16. B. Boudaiffa, P. Cloutier, D. Hunting, M. A. Huels and L. Sanche, Cross
sections for low-energy
(10-50eV) electron damage to DNA. Radiat. Res., 157, 227-234 (2002).
17. Stopping power and range tables for electrons, Data from NIST website :
http://physics. nist.gov/PhysRefData/StartText/ESTAR. html
18. S. M. Pimblott and J. A. LaVerne, Production of low-energy electrons by
ionizing radiation. Rad.
Phys. Chem. 76, 1244-1249 (2007).
19. T. Boulikas, Molecular mechanisms of cisplatin and its liposomally
encapsulated form, LipoplatinTM,
as a chemotherapy and antiangiogenesis drug. Cancer Therapy 5, 349-376 (2007).
20. N. Kitada, K. Takara, T. Minegaki, C. Itoh, M. Tsujimoto, T. Sakaeda and
T. Yokoyama, Factors
affecting sensitivity to antitumor platinum derivatives of human colorectal
tumor cell lines. Cancer
CA 02792953 2012-09-12
WO 2010/121368 PCT/CA2010/000583
37
Chemother. Pharmacol. 62, 577-584 (2008).
21. Merrill S. Kies, Charles L. Bennett and Everett E. Vokes, Locally advanced
head and neck cancer,
Current Treatment Options in Oncology, vol. 2, No. 1, pp: 7-13.
22. Charest G. et al., Acta Neurochir. 2009 Acta Neurochir (Wien) 2009
Jun;151(6):677-83.