Note: Descriptions are shown in the official language in which they were submitted.
CA 02793481 2012-09-17
DEVICE AND METHOD FOR DELIVERING MEDICINE INTO THE TYMPANIC
CAVITY, WITH SLIDING ASSIST
Inventors: Lev Rosenblum, Christian Pfeffer, George B. Kenney
Assignee: EntraTympanic, L.L.C.
CROSS REFERENCE OF RELATED APPLICATIONS
[0001] This application claims priority to and fully incorporates by reference
U.S.
Provisional Patent Application No. 61/314,018 and International Patent
Application
PCT/US2010/052569.
FIELD OF INVENTION
[0002] This invention relates, generally, to otologic devices and methods of
treatments
of various ear-related disorders, and more specifically to delivering medicine
into the
tympanic cavity.
BACKGROUND OF THE INVENTION
[0003] The device and method in the present invention relate to delivery of
medicine to
the middle and/or inner ear and evacuation of fluid, if any, located in the
tympanic cavity.
The device and the method can be used, for example, for treatment and/or
prevention of
various ear-related ailments, such as acute otitis media.
[0004] It is frequently desirable to deliver various types of medicine into
the tympanic
cavity. Such medicine can be directed at treating ailments of the middle as
well as the inner
ear. For example, medically effective amounts of antibiotic and/or anti-
inflammatory drug(s)
can be delivered through the tympanic membrane to treat middle ear infections.
Currently,
delivery of the drugs info the tympanic cavity is usually done when the
tympanic membrane
has ruptured or the patient has a previously-inserted tube in the membrane,
that is, non-
surgical delivery of medicine to the tympanic cavity is usually done only when
there is an
existing perforation in the tympanic membrane, through which the medicine is
delivered. The
majority of the patients, however, do not have an existing perforation through
which
medicine can be delivered; consequently, such procedure is not available to
them.
[0005] Alternatively, a physician can use a syringe to inject medicine through
the
tympanic membrane. However, this procedure can be dangerous for several
reasons. First, the
1
CA 02793481 2012-09-17
tympanic cavity houses a variety of vulnerable structures, such as the
malleus, incus, stapes,
facial nerve, and in some cases carotid artery. An accidental contact with any
of these
structures can result in adverse effects that range from pain and severe
bleeding (in case of a
punctured carotid artery or branches of the internal jugular vein) to
permanent disability, such
as hearing loss.
[0006] Any incisions and/or perforations of the tympanic membrane in the
posterosuperior and anterosuperior quadrants are highly discouraged because
the most
vulnerable structures located in the tympanic cavity are positioned
proximately behind these
two quadrants. Consequently, incisions and/or perforations of the tympanic
membrane are
usually performed in the posteroinferior and anteroinferior quadrants.
Further, incisions
and/or perforations made in the posteroinferior and anteroinferior quadrants
must also be
done with extreme care, and accidental penetration more than a minimal depth
beyond the
normal physiological position of the tympanic membrane can cause severe
injuries. Because
the physician must insert the needle in a tiny area and with minimal
penetration, the margin
for error is very small. Consequently, incisions and/or penetrations of the
tympanic
membrane in children are usually performed under general anesthesia, to avoid
accidental
over-penetration or an unwanted penetration in a wrong location (for example,
a perforation
in the posterosuperior quadrant or contact with the ear canal) as a result of
the child's
inability to remain stationary during the procedure.
[0007] This invention offers a novel way of safely delivering desired amounts
of
medicine into, as well as aspirating fluid from, the tympanic cavity. The
invention allows
evacuation of fluid from the tympanic cavity, delivery of medicine into the
tympanic cavity,
and/or biopsy of the tympanic membrane by making a minute perforation in the
membrane.
Furthermore, the invention allows the procedure to be performed quickly,
safely and without
general anesthesia by limiting the depth and location of the penetration on
the tympanic
membrane. The invention also allows for safe removal of fluid accumulated in
the tympanic
cavity and subsequent analysis of the fluid. Such analysis, for example, may
include a test for
the presence of bacteria and a determination of the type of bacteria present.
Consequently, the
invention will reduce the need for systemic treatment of ailments related to
middle and inner
ear in patients who do not have a perforated tympanic membrane, especially in
children.
2
CA 02793481 2012-09-17
[0008] One of the major deficiencies of the current devices is the potential
to make
contact with crucial physiological structures behind the membrane, injuring
the patient. This
potential for injury is amplified in young patients. Although an adult patient
is likely to
comply with a request to remain stationary while the physician injects him
with a four-inch
needle, a child is likely to ignore such request.
SUMMARY OF THE INVENTION
[0009] This invention is directed at delivering medicine to the middle and
inner ear as
well as evacuating fluid, if any, located in the tympanic cavity. This device
overcomes the
deficiencies of prior devices by eliminating the potential for penetrating
into the tympanic
cavity beyond the normal physiological position of the tympanic membrane. By
doing so, this
device presents a novel and safe way for delivering medicine into and/or
removing fluid from
the tympanic cavity. Instead of penetrating the tympanic membrane by plunging
a needle
through the membrane and into the tympanic cavity, the present invention
allows to flex the
membrane toward a movable body and keep the movable body in contact with the
tympanic
membrane after at least one piercing element punctures the membrane. Because
no piercing
element enters the tympanic cavity beyond the normal physiological position of
the tympanic
membrane, there is no risk of contact with any of the structures in the
tympanic cavity. The
invention also facilitates one or more fluid-holding chamber in the movable
body of the
device, which may hold substance that would be injected into and/or fluid that
would be
evacuated from the tympanic cavity. Multiple piercing elements can be used.
For example,
one hollow piercing element can be used to inject the medicine into the
tympanic cavity and
another to evacuate the fluid from the tympanic cavity. Alternatively, a
double-walled
piercing element (for example, a double-lumen needle) can also be used.
Additionally, a
hollow piercing element can be used to obtain a biopsy of the tympanic
membrane tissue,
which can be later analyzed. After the evacuation of fluid, the injection of
medicine, and/or
the biopsy are completed, the tympanic membrane is released and will return to
its normal
physiological position. Subsequently, the device is removed from the patient's
ear canal.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] Fig. 1 shows the list of elements.
[0011] Fig. 2 shows the front view of the device.
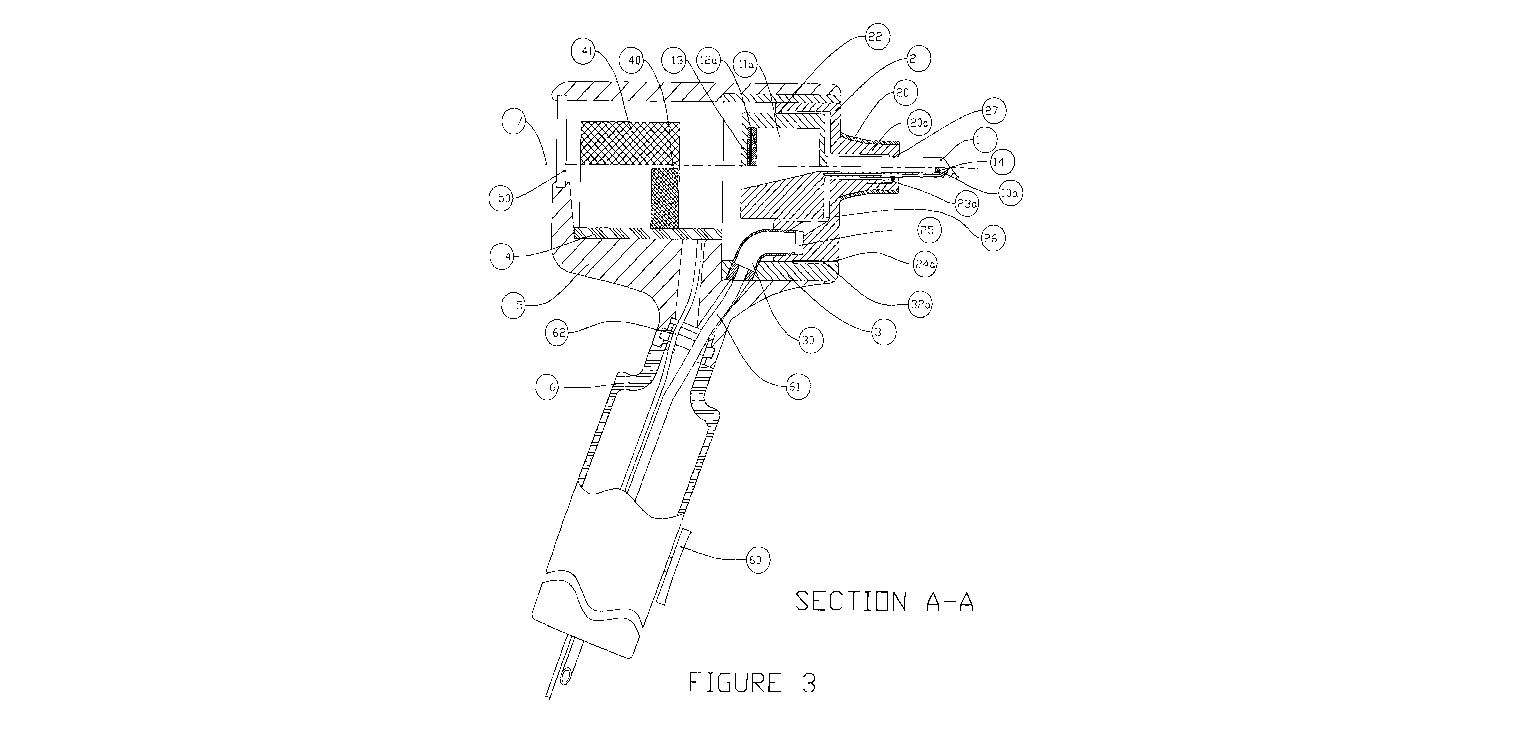
[0012] Fig. 3 shows the Section A-A of the device in a fully-extended
position.
[0013] Fig. 4 shows the Section B-B of the device in a fully-extended
position.
3
CA 02793481 2012-09-17
[0014] Fig. 5 shows the Section A-A of the device in a fully-retracted
position, inserted
into the ear canal.
[0015] Fig. 6 shows the side view of the device in a fully-extended position,
inserted into
the ear canal.
[0016] Fig. 7 shows a flowchart of the operations of the device.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0017] Below is the description of the preferred embodiments of this
invention. It is
recognized, however, that other embodiments would be obvious to those skilled
in the art.
[0018] The front view of the device assembly is shown in Fig. 2 shows the
locations of
section views A-A and B-B, displayed in Figures 3 and 4, respectively. In the
preferred
embodiment, the device is comprised of the following elements, shown in Figs.
2, 3, and 4:
movable body 1, stationary body 2, housing insert 3, printed circuit board 4,
housing 5, and
handle 6. In the preferred embodiment, an insert 3 is used mainly for ease of
assembly of the
device; it is affixed in the housing 5 with flat head screws 99. The housing 5
and handle 6 are
made from 6061-T6 aluminum, and the housing insert 3 is made from Teflon PTFE
Grade
860 (polytetrafluoroethylene). However, many other materials are suitable for
the housing 5,
handle 6, housing insert 3 as well as other components of the devices, which
are known or
would be obvious to those skilled in the art. Further, other configurations,
which would not
change the basic functionality of the device, for example a device without the
handle 6,
would be obvious to those skilled in the art.
[0019] The movable body 1 is disposed within the stationary body 2, and the
pair is
inserted into the housing insert 3, as shown in Figs. 2, 3, and 4. The
stationary body is held in
place using a stopper ball 33 and stopper spring 34 combinations, as shown in
Fig. 4. There
are many other methods of securing the movable body 1 and stationary body 2
pair in the
device, which are known to those skilled in the art. In the preferred
embodiment, the movable
body 1 and stationary body 2 are designed to be disposable and are removable
from the
housing 5. However, other configurations of the device--for example, where the
housing 5
and the stationary body 2 would be a single element--are known or would be
obvious to those
skilled in the art.
4
CA 02793481 2012-09-17
[0020] As shown in Figs. 2, 3, and 4, in the preferred embodiment, the movable
body 1
comprises: the large section of the movable body la, the small section of the
movable body
lb, the distal surface of the movable body lc, and the proximal surface of the
movable body
Id. The large section of the movable body la and the small section of the
movable body lb
are essentially cylindrical. However, other suitable shapes can be used and
would be obvious
to those skilled in the art. Further, an injection chamber lla; evacuation
chamber llb;
piercing element 10a connected to the injection chamber lla; and piercing
element l0b
connected to the evacuation chamber 11 b are disposed in the movable body 1.
[0021] As shown in Figs. 2, 3 and 4, in the preferred embodiment, the distal
surface of
the movable body lc is disposed approximately in a plane oriented at a
compound angle with
respect to the x-axis 7, such that when the device is inserted into the ear,
the distal surface of
the movable body 1 c can be aligned to be approximately parallel to the
tympanic membrane.
That is, the distal surface of the movable body 1 c is disposed approximately
in a plane that is
at an angle with respect to the plane formed by the x-axis 7 and y-axis 8, and
at an angle with
respect to the plane formed by x-axis 7 and z-axis 9, as shown in Figs. 2, 3,
and 4. It is
recognized that the distal surface of the movable body lc can be disposed at
various angles
with respect to planes formed by x-axis 7 and y-axis 8, x-axis 7 and z-axis 9,
and y-axis 8 and
z-axis 9. For example, the distal surface of the movable body I c can be
approximately
disposed in a plane normal to the x-axis 7, which would be parallel to a plane
formed by the
y-axis 8 and z-axis 9. Furthermore, the distal surface of the movable body lc
can have
various shapes; for example, it can approximate the shape of the tympanic
membrane.
[0022] One or more piercing element can be disposed in the movable body; and
some or
all of the piercing elements can be either solid or hollow. Further, some or
all of the piercing
elements can be connected to a chamber capable of holding substance or can be
standalone
(unconnected). In the preferred embodiment, two hollow piercing elements 10a
and 10b,
which are connected to two chambers l la and 11b, respectively, are used, as
shown in Figs. 2
and 4. Because the hollow piercing element(s) are capable of holding fluid
inside the hollow
portion of the shaft, the element(s) can also serve as fluid-holding
chamber(s). After the
tympanic membrane is punctured, a substance (if any) located in the injection
chamber 11 a
can be delivered through the piercing element 10a into the tympanic cavity.
Simultaneously,
any fluid located in the tympanic cavity can be evacuated through the piercing
element 10b,
into the evacuation chamber l lb, as shown in Figs. 2, 3 and 4, which is
connected to the
CA 02793481 2012-09-17
evacuation chamber 1 lb. A biopsy of the tympanic membrane can also be
obtained from the
evacuation chamber or a piercing element designed for biopsy can be used.
[0023] In the preferred embodiment, the movable body 1 is made from medical
grade
polypropylene, and the piercing elements 10a and 10b are fixed in place with a
light curing
adhesive, such as DYMAX MD 1162-M. There are many other materials for and
methods
of manufacturing the same, which are known to those skilled in the art.
[0024] In the preferred embodiment, the viewing opening 18 and front lens 14
are
disposed in the movable body, as shown in Fig. 2, 3, and 4. The user can see
the tympanic
membrane through the viewing opening 18 before advancing the movable body 1
and
piercing the membrane. The viewing opening improves the safety of the device
by allowing
the user to view the tympanic membrane while the movable body is advanced
toward the
membrane. Other mechanisms known to those skilled in the art can also be used
to view the
tympanic membrane. For example, an endoscope or other video visualization
devices can be
used. In the preferred embodiment, the front lens 14 is disposed in the small
section of the
movable body lb part of the viewing opening 18, and in some embodiments it may
be
unnecessary all together. However, the lens can be disposed anywhere along the
viewing
opening 18. Further, the lens may be a magnifying lens. There are many
suitable materials
that the lens 14 can be made from. For example, the lens can be made from a
clear plastic
such as Lexan . To illuminate the ear canal and the tympanic membrane, an LED
40 is used,
as shown in Figs. 3 and 4. A magnifying lens 50 is disposed in the housing 5,
as shown in
Figs. 3 and 4. The magnifying lens 50 improves visibility of the tympanic
membrane during
the use of the device.
[0025] As shown in Figs. 2, 3, and 4, in the preferred embodiment, the
stationary body 2,
comprises: large section of the stationary body 2a, small section of the
stationary body 2b,
distal surface of the stationary body 2c, and proximal surface of the
stationary body 2d. The
movable body 1 is disposed within the stationary body 2 and is free to move
axially toward
the tympanic membrane (inward) and away from the tympanic membrane (outward).
In the
preferred embodiment, the movable body 1 is induced to move inward when a
slight vacuum
is created in the ear canal. The pressure difference created by the slight
vacuum in the ear
canal creates a force on the movable body 1 that equals to the vacuum x area
of the cross-
6
CA 02793481 2012-09-17
section of the large section of the movable body ld. This force pushes the
movable body
inward, toward the tympanic membrane.
[0026] In the preferred embodiment, the selector panel 45 is disposed on the
side of the
housing 5, as shown in Fig. 6. The device is turned on by depressing the power
button 46.
After the device is turned on, the user may select the type of movable body
that has been
inserted into the device. If the injection chamber l la, shown in Fig. 4, is
empty, the select
button for empty cartridge 48 is depressed. The injection chamber 11a may also
contain a
substance, for example an antibiotic-steroid otic suspension such as Ciprodex
. If the
injection chamber 1 la contains a substance, the select button for filled-
chamber cartridge 47
should be depressed.
[0027] The small section of the stationary body 2b is inserted into the ear
canal of the
patient and a slight vacuum seal is created between the ear canal tissue and
small section of
the stationary body 2b, as shown in Fig. 5. The small section of the
stationary body 2b is
approximately cylindrical. However, other suitable shapes, which would be
obvious to those
skilled in the art, can be used. In the preferred embodiment, the outer
compressible layer of
the stationary body 20 helps facilitate the vacuum seal between the stationary
body 2 and the
ear canal. When the small section of the stationary body 2b is inserted into
the ear canal, the
outer compressible layer 20 deforms and takes the shape of the ear canal,
thereby creating an
airtight seal. Underlying the outer compressible layer 20 is the inner rigid
layer 20a. The
outer compressible layer 20 can be made from any suitable material known to
those skilled in
the art. In the preferred embodiment, the outer compressible layer 20 is made
from silicone,
which is overmolded over the inner rigid layer of the stationary body 20a. A
compressible
layer is not required to create a sufficiently airtight seal between the ear
canal and the
stationary body 2. After the small section of the stationary body 2b is
inserted into the ear
canal and the vacuum seal is created between the ear canal and the stationary
body 2, a small
amount of air is withdrawn from the ear canal to create the necessary vacuum.
The air is
withdrawn through the vacuum line 61, shown in Fig. 3, which is connected to
the vacuum
fitting 30. The vacuum fitting 30 is connected to the vacuum inlet 25 of the
stationary body 2.
After the vacuum valve 60 is opened, air flows out of the ear canal through
the vacuum ports
26a, shown in Figs. 2 and 4, and subsequently through the vacuum channels 26
in stationary
body 2, thereby creating the necessary vacuum in the ear canal. Vacuum line 61
is connected
to a commercially available vacuum pump. Various manual, mechanical, or
7
CA 02793481 2012-09-17
electromechanical mechanisms for generating necessary vacuum may be used as
standalone
units that are connected to the device or may be incorporated into the device.
[0028] The back seal 22, shown in Figs. 3 and 4, maintains the pressure
difference
between the distal surface of the movable body lc and the proximal surface of
the movable
body 1 d, by preventing air from entering into the ear canal. There are many
ways to make a
functionally-equivalent seal, which are known to those skilled in the art. For
example, a
rubber gasket or an o-ring can be used in either the movable body 1 or the
stationary body 2
to create an airtight seal. Because the required vacuum is very low, in the
preferred
embodiment, the seal is not completely airtight; rather, the seal acts as a
temporary barrier
that impedes the air from entering through the back seal 22. Consequently, in
the preferred
embodiment the seal is created by having a sliding fit, with about 0.03mm per
side clearance
between the movable body 1 and the back seal 22. The small clearance also
allows the back
seal 22 to function as a guide, approximately maintaining the motion of the
movable body 1
in a predetermined path. Furthermore, because the back seal 22 allows some air
to flow
through, it serves as a safety mechanism, limiting the maximum vacuum in the
ear cavity as
well as the force on the movable body 1. The allowable clearance between the
seal and the
movable body depends on the size of the movable body 1, types of materials
used for the
stationary body 2 and the movable body 1, surface roughness, and the vacuum
used to
advance the movable body. In the preferred embodiment, the area of the cross-
section of the
large section of the movable body 2a is approximately 500mm2, and the vacuum
used is
about 40-70 Torr. The movable body 1 is made from medical grade polypropylene,
and the
stationary body 2 is made from Teflon PFA Grade 445 HP (perfluoroalkoxy
copolymer).
There are many suitable materials that can be used for the movable body 1 and
stationary
body 2, which would be obvious to those skilled in the art. The vacuum range
can be
increased or decreased depending on the area of the cross-section of the
largest portion of the
movable body. However, higher levels of vacuum may be painful for the patient
and,
depending on the pressure in the tympanic cavity, may rupture the tympanic
membrane. In
the preferred embodiment, the force that is applied to the sliding body is
less than ION.
Although greater force may be used, it might result in some additional
discomfort to the
patient.
[0029] Many mechanisms, known to those skilled in the art, can be used to
advance the
movable body toward the tympanic membrane such as electrical, mechanical,
hydraulic,
8
CA 02793481 2012-09-17
pneumatic, or their various combinations. For example, linear actuators, screw
mechanisms,
electromechanical and magnetic linear actuators, hydraulic or pneumatic
actuators, as well as
many other mechanisms known to those skilled in the art. The movable body 1
can also be
moved manually inward and/or outward. The vacuum seal between the stationary
body 2 and
the ear canal may be unnecessary, depending on the method chosen for advancing
the
movable body 1 and whether vacuum is used in the ear canal to deflect the
tympanic
membrane toward the movable body 1.
[0030] The front guide 27, shown on Fig. 3, ensures that the movable body 1
moves
approximately along a predetermined path. The front guide 27 does not have to
function as a
seal, although such functionality can be added without changing the operation
of the device.
Consequently, in the preferred embodiment, the clearance between the small
section of the
movable body lb and the front guide 27 is about 0.08mm per side. However, it
would be
obvious to those skilled in the art to choose a different suitable clearance.
[0031] In the preferred embodiment, the piercing elements 10a and 10b are made
from
316 Stainless Steel. However, piercing elements can be made from a variety of
suitable
materials known to those skilled in the art, including nonmetallic materials.
As shown in
Figs. 2 and 3, the piercing elements 10a and 10b glide over the piercing
element contacts 23a
and 23b respectively. The piercing element contacts 23a and 23b are disposed
in the
stationary body 2. Wires connect the piercing element contacts 23a and 23b to
the stationary
body contacts 24a and 24b respectively. When the stationary body 2 is inserted
into the insert
3, the stationary body contacts 24a and 24b make connection with the insert
contacts 32a and
32b respectively. Wires connect insert contacts 32a and 32b to the ohmmeter
module 42,
which is imbedded in the printed circuit board 4. The main power supply line
62 delivers the
required power to the circuit board. The CPU 43 controls the sequence of
operations. When
the piercing elements contact the tympanic membrane, the measured resistance
between the
two piercing elements will change. This change will be detected by the
ohmmeter module 42.
At a predetermined time interval, such as 0.5 seconds after the change in
resistance has been
detected, electromagnetic clamps 31, shown in Fig. 4, are activated and move
forward to
clamp around the movable body 1. The electromagnetic clamps 31 prevent the
movable body
1 from further motion in either inward or outward direction. The compression
layer of
electromagnetic clamp 3 1 a is disposed on the parts of the electromagnetic
clamps 31 that
come into contact with the large section of the movable body la. The
compression layer
9
CA 02793481 2012-09-17
deforms around the movable body, to reduce deformation of the large section of
the movable
body la. The electromagnetic clamps provide the device with an additional
safety measure
and also aid in preventing movement of the movable body 1 if/when a substance
is injected
from and/or evacuated into the chambers 11 a and/ or 1 lb respectively. Other
mechanisms
known to those skilled in the art can also be used to both detect the final
position of the
movable body 1 and/or to fixate the movable body once it has reached the final
position. For
example, a pressure sensor can be used to detect contact of the movable body 1
with the
tympanic membrane. Furthermore, in some embodiments, the injection/evacuation
mechanism may be disposed within the movable body. Consequently, during the
injection/evacuation operation, the movable body will not experience external
forces from the
injection/evacuation mechanism, and the electromagnetic clamps 31 would not be
required to
hold the movable body stationary during the injection/evacuation operation.
After the
procedure is completed, the speaker 44 can be used to signal the end of the
process.
[0032] If the resistance between the piercing elements 10a and 10b changes
again, after
the piercing elements came into contact with the tympanic membrane, it is
because the
piercing elements came into contact with fluid in the tympanic cavity.
Consequently, the
device can also be used to detect the presence or absence of fluid in the
tympanic cavity.
[0033] In the preferred embodiment, after the piercing elements 10a and 10b
have
penetrated the tympanic membrane, the substance (if any) located in the
injection chamber
11a can be injected into the tympanic cavity, while the fluid located in the
tympanic cavity (if
any) can be evacuated. The injection chamber plunger 12a is disposed in the
retracted
position-at the proximal end of the chamber, away from the point of connection
to the
piercing element 10a; and the evacuation chamber plunger 12b is disposed in
the fully-
extended position-at the distal end of the chamber, closest to the connection
point to the
piercing element 10b. Further, the injection chamber is connected to the
evacuation chamber
with the vent channel 15. When the injection chamber plunger 12a moves
forward, toward
the distal end of the chamber, the air is drawn out of the evacuation chamber
l lb. The
reduction in air volume in the evacuation chamber 1 lb pulls the evacuation
chamber plunger
12b backward, away from the distal end of the chamber. As the evacuation
chamber plunger
12b moves backward, it creates a slight vacuum, which draws fluid from the
tympanic cavity
through the piercing element 10b and into the evacuation chamber llb.
Consequently,
injection of a substance into and evacuation of the fluid from the tympanic
cavity can be are
CA 02793481 2012-09-17
performed essentially simultaneously. There are other methods, known to those
skilled in the
art, which can be used to synchronize the injection and evacuation functions
of the device.
Furthermore, injection and evacuation need not be performed simultaneously and
can be done
sequentially. Also, only one of the functions, either injection or evacuation,
can be performed
independently, without performing the other function.
[0034] In the preferred embodiment, a magnetizable insert 13 is imbedded in
the
injection chamber plunger 12a, as shown in Fig. 3. The injection chamber
plunger 12a is
advanced forward via application of a magnetic field, which is created by the
electromagnet
41, shown in Fig. 4. As discussed in paragraph [0033], the movement of the
injection
chamber plunger 12a pulls back the evacuation chamber plunger 12b. If the
injection
chamber 11 a does not contain any substance, and the device is used only for
evacuation of
fluid, the same function can be used, that is, an injection chamber plunger
12a can be pushed
forward to pull back the evacuation chamber plunger 12b to evacuate the fluid
from the
tympanic cavity.
[0035] Many other mechanisms can be used to advance the injection chamber
plunger
12a forward and/or move the evacuation chamber plunger 12b backward. For
example, linear
actuators, various screw mechanism, electromechanical and magnetic linear
actuators,
hydraulic or pneumatic actuators, as well as many other mechanisms known to
those skilled
in the art can be used. Furthermore, the injection chamber plunger 12a and the
evacuation
chamber plunger 12b can be moved manually.
[0036] After injection and/or evacuation function is completed, the device can
be
removed from the patient's ear canal. In the preferred embodiment, the device
sounds a long
beep at the end of the injection/evacuation process. The end of the process is
determined by
the time elapsed from the commencement of the injection function. In the
preferred
embodiment, the time-delay between the commencement and the long beep,
indicating the
end of the process, is 10 seconds. There are many other ways, known to those
skilled in the
art, to determine whether the injection/evacuation has been completed. For
example, a sensor
can be used to determine the position of either the injection chamber plunger
or evacuation
chamber plunger. Also, there are many ways that the end of the procedure can
be signaled to
the user.
11
CA 02793481 2012-09-17
[00371 Fig. 7 is an example of a sequence of operations of the device in the
preferred
embodiment. Not all of the operations in the sequence shown in Fig. 7 are
essential to the
invention. Likewise, a different sequence of the operations, which would not
alter the essence
of the invention, would be obvious to those skilled in the art. First, the
device is activated
(powered up), the user selects the type of cartridge that is inserted into the
device, and the
small section of the stationary body 2b is inserted into the patient's ear.
Then, the user locates
the tympanic membrane by looking through the viewing opening 18. Once the
tympanic
membrane is located, the user opens the vacuum valve 60. After the vacuum
valve 60 is
opened, the air flows out of the ear canal creating a slight vacuum. The
slight vacuum creates
a pressure difference between the distal surface of the movable body I c and
the proximal
surface of the movable body l d, which is exposed to the ambient pressure.
This pressure
difference applies a force that equals to the area of the cross-section of the
large section of the
movable body la x pressure difference; the force advances the movable body
inward, toward
the tympanic membrane. The user can maintain visual contact with the tympanic
membrane
while the movable body I advances inward. Subsequently, the movable body 1
makes contact
with the tympanic membrane, and the piercing elements 10a and 10b penetrate
the
membrane. As the piercing elements make contact with the tympanic membrane,
the
ohmmeter module 42 detects the change in resistance between the piercing
elements and after
a time-delay of 0.5 seconds, electromagnetic clamps are activated and grip
around the
movable body 1. If the selection for an empty cartridge was made, the
resistance between
piercing elements is checked again. If the resistance is different than at the
time of contact
with the tympanic membrane, that indicates that there is fluid in the tympanic
cavity, and the
device beeps twice to notify the user of the presence of fluid. Subsequently,
the
injection/evacuation function is activated. If the resistance between the
piercing elements did
not change after the initial contact of the piercing elements with the
tympanic membrane, this
indicates that there is no fluid in the tympanic cavity, at least at the level
reachable by the
piercing elements. Subsequently, the injection/evacuation process will not be
commenced,
and a long beep will sound, signaling to the user that the procedure has been
completed. If the
user has preselected a cartridge that includes a substance for injection, the
process of testing
for presence of fluid can be bypassed, and injection/evacuation function is
commenced after
the electromagnetic clamps are engaged. Upon completion of the
injection/evacuation
function a long beep will sound, signaling the completion of the procedure.
12