Note: Descriptions are shown in the official language in which they were submitted.
CA 02794448 2012-11-02
- 1 -
SYSTEM AND METHOD FOR DELIVERING A SUBSTANCE TO A
BODY CAVITY
[0001]
FIELD OF THE INVENTION
[0002] The present invention relates to a system and method for delivering
a
substance to a body cavity. More particularly, the present invention relates
to a
system and method for delivering a substance to a body cavity in conjunction
with a
minimally invasive operative procedure or for therapeutic treatment unrelated
to a
surgical procedure.
BACKGROUND
[0003] Among problems that physicians have encountered during diagnostic or
surgical procedures, using both "open" techniques, and minimally invasive
(laparoscopic) surgical techniques, are numerous post procedural
complications.
These complications can consist of, but are not limited to, post operative
pain,
infections, tissue adhesions, and tumor formation. Numerous products, such as
medications and associated delivery systems, addressing these issues exist on
the
market to improve the surgical or invasive experience and patient outcomes.
Among
these products are suction and irrigation wands that are used for flushing
tissue sites
with sterile water or saline and removing blood. There are medications, which
are
spread over exposed organs, to coat or provide a barrier between tissue and
organs for
prevention of adhesions. These materials may be in gel form, sheet form, spray
(liquid) form, or aerosol foun to coat organs or tissues, or to provide thin
layer
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 2 -
deposition to the organs in the operative site. Some of these materials may be
used in
both open and minimally invasive surgical techniques.
[0004] The problems with these materials, and their application as related
to
laparoscopy, are their inability to be used easily and effectively in a
minimally
invasive laparoseopic environment. Among the difficulties associated with
spraying
of liquids, is the pooling and lack of containment of the fluids used with
irrigation and
aspiration wands. It is also difficult to cover large areas (greater than
several square
centimeters), and do so without using much more medicament than is necessary.
This
contributes to the cost of excessive medication, and adding to the cost and
time of the
surgery.
[0005] Materials used in sheet form are not practical to apply to the
organs when
using laparoscopic minimally invasive techniques, due to the difficulty in
getting the
material through standard trocars, and then spreading the material out over
the
affected area, and keeping it in place once positioned. The liquid spray
technique has
many of the same problems as the irrigation approach. These devices normally
force
a liquid through a cannula like device under pressure. The introduction of
additional
fluid into the body cavity can cause increases in pressure and do not include
a means
for pressure relief. Without a means for directing the spray, it is difficult
to control
where the medication is deposited, and in what amount. Also, the precise
disposition
of the medication as to amount and location is difficult to control.
[0006] Compound materials are sometimes mixed prior to being aerosolized by
a
hand held syringe device, and then by applying an air stream to the mixed
medication
as it is being dispensed, to create an aerosolized stream that is used to
"paint" the
organs. This method also ignores the problem of the creation of additional
pressure in
the organ with no relief mechanism. Creating an aerosol "cloud" contends with
the
problem of how to effectively coat all the surfaces required, but also
introduces the
problem of increasing abdominal pressures uncontrollably inside an insufflated
body
cavity or organ, such as the peritoneum.
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 3 -
[0007] All of the above methodologies, while focused on applying substances
in
different physical forms for the purpose of treating or coating tissues and/or
organs,
have not been optimized for use in the laparoscopic, minimally invasive
environment.
The term "substance", as used in this specification, includes, without
limitation, a
liquid, powder or gas, or any combination thereof.
BRIEF SUMMARY
[0008] In order to address the deficiencies in the prior art, a system and
method
for providing a substance to a body cavity is discussed below. According to a
first
aspect of the invention, a system is provided that will allow the application
of a
substance, such as an aerosolized medicament to a distended body cavity that
will
allow for the efficient, safe, and effective application of any number of
substances,
such as aerosolized liquids, which can be used for pain management
(analgesics),
infection prevention (prophylactic antibiotics), tissue adhesion (any number
of
formulations can be used including naturally occurring lubricious medications
such as
hyaluronic acid, or any number of other medicaments such as heparin, glycerin
or
glycol medications, or even humidity), and tumor prevention (using targeted or
prophylactic chemotherapy drugs or methods). A pressure relief or maintenance
device controllably keeps the pressure within a desired range, compensating
for the
introduction of substances into the body cavity that can build up unwanted
pressure.
A central controller in communication with a substance introducing device and
an
insufflator may coordinate all of the parameters of pressure, flow,
temperature and so
on.
[00091 According to another aspect of this invention, a method for
providing
continued or postoperative application of a substance, by re-instituting an
environment in the patient in which subsequent applications of medication may
be
administered, is disclosed. The method includes providing a patient with a
port or
other device that will fasten to the outer abdomen wall and the interior
abdomen wall
to provide a passage into a body cavity of a patient. A supply of insufflation
gas is
CA 02794448 2012-11-02
WO 2005/035035
PCMJS2004/033024
- 4 -
provided through the port and a substance is introduced, for example in
aerosol form,
into the body cavity through the port. In one embodiment the substance is
introduced
via a nebulizing catheter. In one embodiment, the method may relate to a
therapeutic
treatment for cancerous tumors and the substance supplied to the body cavity
may be
chemotherapy medication. In another embodiment, the method may be related to
post-operative pain or infection treatment, such as the application of
analgesic or
antibiotic substances, respectively.
[0010] In yet a further aspect of this invention a method and system are
disclosed
that improve upon typical methods of applying medications to an insufflated
organ by
controlling and coordinating the requirements of pressure maintenance and
relief
within the organ, coordinating the application of the aerosolized medicament
within
the patient (including the amount, rate of application, timing of the
administration of
the medicament, and control of the direction or formation of an optimized
aerosolized
laparoscopic medicated environment), maintaining proper distention for
visualization
and operative manipulation of instruments, and providing feedback (visual and
or
audible) on the information or data required for controlling the operative,
diagnostic,
or post operative treatment of a patient.
[0011] Further aspects and advantages of the invention are discussed below
in
conjunction with the preferred embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] FIG. 1 illustrates an embodiment of fluid connections in a system
for
laparoscopic delivery of aerosolized medication according to one embodiment of
the
=
present invention.
[0013] FIG. 2 is a perspective view of a nebulizing catheter suitable for
use in the
system of FIG. 1.
[0014] FIG. 3 is an alternative embodiment of the nebulizing catheter of
FIG. 2.
[0015] FIG. 4 is an alternative embodiment of the system of FIG. 1.
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 5 -
[0016] FIG. 5 is a schematic view of an embodiment of control connections
of the
system of FIG. 1.
[0017] FIG. 6 is a block diagram of a regulated liquid and gas dispensing
controller suitable for use in the system of FIG. 1.
[0018] FIG. 7 is a block diagram of an alternative embodiment of the
regulated
liquid and gas dispensing controller of FIG. 6 having a fluid mixing chamber
for
dispensing and mixing multiple fluids.
[0019] FIG. 8 is a block diagram of a second alternative embodiment of the
regulated liquid and gas dispensing controller of FIG. 6 having a y-tube for
dispensing and mixing multiple fluids.
[0020] FIG. 9 is a block diagram of a third alternative embodiment of the
regulated liquid and gas dispensing controller of FIG. 6 having a gas mixing
chamber
for providing a mixed insufflation gas.
[0021] FIG. 10 is a block diagram of a disposable catheter, syringe and
tubing set
attached to the regulated liquid and gas dispensing controller of FIG. 6.
[0022] FIG. 11 is a perspective view of a syringe pump having a receiving
slot for
a disposable syringe/catheter/tubeset.
DETAILED DESCRIPTION OF THE PRESENTLY PREFERRED
EMBODIMENTS
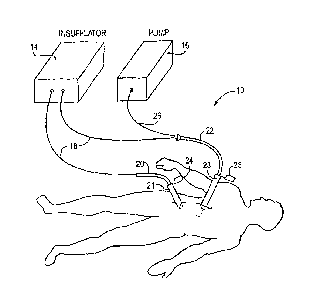
[0023] Referring to FIG. 1, an embodiment of a system 10 for delivery of a
substance to a body cavity, is shown connected to a patient 12. The system 10
includes an insufflator 14 for providing a supply of insufflation gas to the
patient 12.
The system also includes a pump 16 configured to controllably supply a
medicament
to the patient 12. The insufflator 14 connects to gas delivery lines 18 and
then to one
or more catheters 20, 22. The insufflator 14 may include an integrated gas
temperature control mechanism or may be combined with one or more in-line gas
heaters to control the temperature of gas supplied for insufflation and/or
nebulization.
A first catheter is an insufflation catheter 20 sized for cooperating with a
trocar 21 or
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 6 -
other standard catheter insertion needle so that the insufflation catheter may
be
directed into the peritoneum or other specific location in the patient 12. A
pressure
relief control valve 24 is positioned along the supply of insufflation gas,
for example
on the catheter 20 or trocar 21, so that pressure in the peritoneum or other
target
location in the patient will be monitored and adjusted to maintain a desired
level.
[0024] An aerosolization gas supply, preferably separately controllable
from the
general insufflation gas sent through the gas delivery line 18 to the
nebulization
catheter 22, is also supplied by the insufflator 14. This aerosolization gas
supply is
directed through a gas line 18 connected to a nebulization catheter 22
inserted into the
peritoneum through another trocar 23 or other suitable needle. Although the
system
may operate with a single pressure relief control valve positioned anywhere
along
the components making up the insufflation gas supply chain, a separate and
independently controllable pressure relief valve 25 may be positioned on the
nebulization gas supply, such as at the trocar 23 for the nebulization
catheter 22. The
nebulization catheter receives a medicament in fluid form from fluid supply
line 26
connected with the pump 16. The gas provided to the nebulizing catheter is
mixed
with a fluid medicament supplied by the pump 16 and generates a nebulized
medicament for deposit on specific organs, on the peritoneum cavity wall and
other
locations within the patient 12. The system of FIG. 1 is shown with only the
basic
fluid and gas lines for clarity. A central controller, described in greater
detail below,
coordinates the actions of the insufflator, pump, and pressure relief control
valve(s) so
that any of the system parameters, such as pressure, gas or fluid flow rate,
temperature and so on, may be managed. Although a nebulizing catheter is
shown,
any of a number of other devices for introducing a substance into a body
cavity may
also be used. For example, the nebulizing catheter 22 may be replaced by a
suction
irrigation wand to infuse the body cavity with a substance.
[0025] The insufflator 14 may be any of a number of insufflators, such as
the
OMNIFLATOR Model 6620 available from Northgate Technologies, Inc. of Elgin,
CA 02794448 2012-11-02
- 7 -
Illinois. Examples of suitable insufflators are described in U.S. Pat. No.
6,299,592
and U.S. Patent No, 7,654,975 and
the entirety of each of these references
may be referred to . The insufflator may include a
pressurized source of
insufflation gas. Examples of insufflation gases include, but are not limited
to, carbon
dioxide, nitrous oxide, argon, or helium. The insufflation gas is typically
reduced in
pressure by the instifflator to approximately 45 to 55 millitneters of inert-
Lily (also
know as a "push" pressure), although the pressure may be changed depending on
the
insufflator in use and any regulations that may be in force. While the push
pressure
may be ni the range of 45-55 millimeters of mercury, the actual pressure
maintained
in the peritoneum or other body cavity is preferably less than 25-30 mm of
mercury
and, in the case of many laparoscopic surgeries, most preferably in the range
of 12
mm of mercury.
[0026] The pump 16 maybe a peristaltic pump, syringe pump,
hydraulic (air over
liquid) pump or any other mechanism capable of controlling the dispensing of
medication. Controllable pump parameters may include the rate and volume, as
well
as the timing, of delivery. It is contemplated that continuous and periodic
pumping
may be desired. Delayed pumping of medication, such as the transpout of
medication
to the nebulization catheter 22 at predetermined times for predetermined
intervals is
also contemplated. In one embodiment, the pump may include a heating mechanism
to heat the fluid to a controlled temperature prior to delivery to the fluid
line 26 and
nebulizing catheter 22.
[0027] The gas and fluid lines 18,26 may be constructed from
disposable
polyvinyl chloride tubes, although in other embodiments any suitable materials
may
be used. For example, the tubing may be made of a silicone material that is
reusable.
The diameters of the tubes may be varied depending on flow rate requirements
and
any regulations that are in force. Also, the inner diameter of each of the
tubes may be
= different from each other. A filter (not shown) may be located in each of
the tubes
used for the gas lines 18 to provide a particulate barrier. In one embodiment,
the filter
CA 02794448 2012-11-02
- 8 -
may be a glass-fiber hydrophobic filter that provides a particulate barrier of
approximately 0.2 microns and operates at a ninety-nine percent rate of
efficiency. In
other embodiments any number of commonly used filters, with different
filtering
capabilities, may also be used.
[0028] The pressure relief valves (PRV's) 24, 25
associated with the insufflation
and aerosolization gas supplies, respectively, maybe located within the gas
supply
lines 18 or the catheters 20, 22. In other embodiments the valves 24, 25 may
each be
a discrete valve such as commonly available from Pneutronics, a division of
Parker
Hannifin Corporation of Cleveland, Ohio. Any of a number of types of valves
may
be used. For example, the valve may be operated electrically, pneumatically,
or
hydraulically. In other embodiments, the valve may be a mechanical pressure
relief
valve preset to relieve pressure once a preset maximum has been reached. For
example, when the pressure of the insufflation gas reaches a preset pressure,
a spring
operated valve opens and provides pressure relief. Preferably, the valve is
operated
by a signal generated by a controller associated with the electronics of the
insufflator.
An example of such a controller is contained within the control circuitry of
the
Nordigate OMNIFLATOR 6620 insufflator, and an example of such a valve is a
pinch valve. The signal is generated via feedback due to the monitoring of
flow
restriction or back pressure sensed by a central controller 130 (See FIG. 5).
The
monitoring of the pressure of the insufflation gas is accomplished via a
pressure
transducer (not shown) in the controller 130 that monitors the pressure.
= 100291 The nebulizing catheter 22 preferably includes a
combination of at least
one fluid lumen and at least one gas lumen oriented to mix the gas and fluid
to
generate an aerosol mist inside the peritoneum. Any of a number of nebulizing
catheters may be utilized, such as those described in U.S. Patent No.
5,964,223,
issued October 12, 1999 and entitled "Nebulizing Catheter and Methods of Use
and
Manufacture", the entirety of which may be referred to. Some
= examples of nebulizing catheters are shown in FIGS, 2-4.
CA 02794448 2012-11-02
WO 2005/035035
PCT/IIS2004/033024
- 9 -
[0030] FIG. 2 shows a nebulization catheter 30 with a distal end that can
be
located inside of a peritoneum via a trocar. The nebulization catheter 30 has
a coaxial
tubular arrangement with an outer tube 32 surrounding an inner tube 34 so that
a fluid
delivered from a distal liquid orifice 36 of the inner tube 34 is nebulized by
the flow
of a pressurized gas delivered in a distal direction from the annular region
between
the inner and outer tubes at the distal orifice 38 of the outer tube 32. In
addition,
another lumen 40 extends through the shaft of the nebulization catheter 30.
This
additional lumen 40 connects to a distal tubular extension 42. The tubular
extension
42 extends distally of the distal end of the nebulization catheter 30. A
distal end 44 of
the distal tubular extension 42 curves back on itself so that a distal orifice
46 of the
tubular extension 42 is oriented in a proximal direction back at the orifices
36 and 38
of the inner and outer tubes.
[0031] The additional lumen 40 also carries a pressurized gas which is
directed in
a proximal direction by the orifice 46 against the direction of the aerosol
plume
generated by the gas and liquid exiting the orifices 36 and 38. The gas from
the
additional lumen 40 presents a counterflow to the gas from these orifices
thereby
slowing down the velocity of the particles generated from these orifices. In a
preferred embodiment, the distal tubular extension 42 may be folined of a
suitable
material such as stainless steel needle stock.
[0032] FIG. 3 shows another embodiment of a nebulizing catheter 50 that
incorporates a counterflow arrangement. Like the embodiment described above,
in
this embodiment the nebulizing catheter 50 may be positioned in a trocar. The
nebulization catheter 50 has a distal section 52 that curves back on itself.
The
nebulization catheter 50 has distal orifices 54 and 56 that generate a plume
of
nebulized particles in a reverse, i.e. proximal, direction. Also located in
the
nebulization catheter 50 is another lumen 58 for carrying a pressurized gas.
The
additional lumen 58 has a distal orifice 60 oriented in a distal direction.
The distal
orifice 60 of the additional lumen 58 is aligned with respect to the distal
orifices 52
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 10 -
and 54 of the nebulization catheter 50 so that the flow of gas from the
additional
lumen 58 slows down the velocity of the nebulization plume generated from the
nebulization catheter 50. The aerosol plume generated by the nebulization
catheter
reverses direction and is delivered to the peritoneum carried by the flow of
gas from
the additional lumen 58.
=
[0033] In another embodiment of a nebulization catheter arrangement, the
catheter
may include three lumens, two gas and one liquid, where the second of the two
liquid
lumens is utilized to sense pressure and/or provide pressure relief to the
body cavity.
[0034] Referring to FIG. 4, an alternative embodiment of the system 100 is
shown. In this embodiment, the system 100 provides both the insufflation gas
and the
aerosolization gas through a single gas line 118 that is routed through the
nebulization
catheter 122 via the trocar 123 or other needle inserted into the patient 112.
A
combined insufflator/pump 114 provides both the insufflation gas and the fluid
through the nebulizing catheter 122. The fluid is provided along a fluid line
126 that
may pass through an optional heating sleeve 127 controlled by a heater
controller 128
to warm the fluid to a desired temperature. In an alternative embodiment, the
fluid
heating mechanism may be integral with the pump or provided by an in-line
heater.
In another embodiment, where the pump is a syringe pump for controlling fluid
discharge from a removable syringe, heat may be supplied to the fluid using
syringe
heater tape available from Watlow Electric Manufacturing Co. of St. Louis, MO.
The
heater may be controlled through a central controller at the combination
insufflator/pump 114. The temperature of the fluid is preferably adjusted such
that
heat loss in the remaining path to the body cavity is accounted for so the
fluid is
within the desired temperature range as it enters the body cavity. A relief
valve
mechanism 125 is provided to control the gas pressure so that the gas pressure
in the
peritoneum or other body cavity is maintained at a desired level. The pressure
relief
valve 125 may be integrated with the trocar or may be a separate relief valve
mechanism positioned along the gas line 118 or in the insufflator. As
illustrated in
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
-11 -
FIG. 4, the system 100 may include combined or separate gas and fluid sources.
Additionally, the system may work through a single trocar 123 or through
separate
trocars as is illustrated in the embodiment of FIG. 1.
[0035] As shown in FIG. 5, the system 10 of FIG. 1, is preferably
controlled by a
central controller 130 which may be integral with, or separate from, the
insufflator 14.
The insufflator may also include a display 132 for simultaneously or
selectively
displaying one or more of the parameters managed by the central controller
130.
Preferably, the central controller 130 is in communication with each of the
components of the system, whether integrated with the insufflator 14 or
discrete.
Thus, the central controller 130 may monitor and adjust the temperature and
humidification control of the insufflation and catheter gas via the gas
controller 134,
the operation of the pump 16 providing medication to the catheter and the
controller
136 connected with the pressure relief valve or valves on the insufflation gas
supply
and/or the catheter gas supply. One or more of the controllers 130, 134, 136
and the
display 132 may be integrally foimed with, or independent of, the insufflator
14. The
display may be provided with one or more standard interface buttons, or a
touch
screen capability. Any of a number of communication protocols and foimats may
be
used between the central controller 130 and any of the integrated or discrete
controllers.
[0036] A more detailed diagram of an embodiment of a regulated liquid and
gas
dispensing controller 150 incorporating a syringe pump, independent CPU and
optional active pressure relief mechanism as shown in FIG. 6. The controller
150
combines insufflator and pump controller tasks. In one embodiment, the
controller is
preferably configured in a high pressure, low flow arrangement that differs
from the
typical low pressure, high flow arrangement of insufflatorS generally.
Insufflation gas
from a high pressure gas source, such as pressurized bottled gas, is connected
at the
gas inputs 152. A high pressure manifold 154 regulates the pressure from the
initial
high pressure source, in which gas can be at a pressure in the range of 2000
p.s.i., and
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 12 -
reduces the supply pressure through a high pressure regulator 156. In one
embodiment, the high pressure regulator 156 reduces the received gas pressure
to
approximately 150 p.s.i. Any of a number of types of high pressure regulators
may be
used.
[0037] The pressure
of insufflation gas supplied to a patient generally needs to be
at a lower pressure and so the gas from high pressure manifold at, for
example,
150 p.s.i. is then processed through a low pressure manifold 158. The low
pressure
manifold includes a low pressure regulator 160 configured to further reduce
the gas
pressures. In this example, the gas pressure is reduced from 150 p.s.i. to 100
p.s.i.
This pressure translates to a flow rate of 2-3 liters per minute actually
introduced to
the body cavity due. The pressures discussed above are merely presented as
examples
and the various pressure settings in the high and low pressure manifolds may
be user
adjustable, or may be preset at the manufacturer with no manual settings
necessary, at
any of a number of pressures. The low pressure manifold also includes a
passive
pressure relief valve (PRV) 162 set to mechanically release pressure above a
predetermined threshold which, in this example, is 0.9 pounds per square inch
gauge
(p.s.i.g.). An electrically controllable output valve 164 meters the gas
output sent on
to a catheter. Pressure monitor lines connect a central processor (CPU) 166 to
the low
pressure manifold via high pressure sensors 168. When used in an insufflator
arrangement, at least one of a passive pressure relief valve 24 (See FIG. 1)
at the
patient may be used to control the pressure introduced to the patient, or the
optional
active pressure controller 194, described in more detail below, may be
utilized. The
syringe pump motor controller 170 is also controlled by the CPU to meter the
amount
of fluid provided to a patient.
[0038] An actuator
192 may be connected with the controller 150 to initiate one or
more actions by the controller 150. For example, the actuator 192 may send a
signal
to the CPU 166 that will start or stop the production of insufflation gas, the
dispensing
of fluids or other activities. In one embodiment, the actuator 192 may be a
foot pedal
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 13 -
or some other foul' of actuator that allows a medical practitioner to keep
both hands
free. Push buttons, levers, touch-screens or any of a number of actuation
input means
are also contemplated.
[0039] An optional portion of the regulated liquid and gas dispensing
controller is
an active pressure controller 194 that, in addition to the mechanical, passive
pressure
relief valve 162, can provide a mechanism for limiting pressure supplied to
the
patient. Although optional, the active pressure controller 194 can provide
more
precise pressure control by taking a pressure measurement supplied from a
sensor via
an external pressure sense line 196 at the patient's body and allowing the CPU
166 to
actively regulate the pressure. Pressure data may be provided to the CPU 166
by way
of low pressure sensors 198. The active pressure controller can reduce the
pressure
supplied to the patient through one or more active pressure relief valves
electrically
controllable by the CPU.
[0040] Some operative and post-operative therapies may require a mixture of
more than one fluid. The fluid mixture can be achieved through a number of
minor
modifications. One embodiment of a regulated liquid and gas dispensing
controller 150 with multiple fluid sources is illustrated in FIG. 7. In the
embodiment
of FIG. 7, a mixture of fluids is provided by a configuration of the regulated
liquid
and gas dispensing controller 151 that utilizes a fluid mixing chamber 172 to
mix
different fluids provided by separate syringes 174, 176. The motor controller
170
interprets instructions from the CPU 166 to activate the separate motors 178,
180
linked to push plates 182, 184 to engage the respective syringes 174, 176.
[0041] Upon a signal from the CPU 166 and motor controller 170, each motor
178, 180 will move its push plate a certain metered distance and cause the
syringe to
eject a measured amount of fluid into the fluid mixing chamber 172. Each motor
178,
180 may be instructed to move the same or different amount depending on the
desired
mixture of fluids. Check valves 186, 188 may be included on the input ports of
the
fluid mixing chamber as added protection against back flow into the same or
different
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 14 -
syringe. in order to provide sufficient pressure to eject the mixture of fluid
from the
fluid mixing chamber, such as a 20 p.s.i. or other low pressure regulator,
supply of
gas from the low pressure manifold 159 is taken after the low pressure
regulator 160
and further processed through a mixing chamber pressure regulator 190 down to,
in
this example, 20 p.s.i. The gas is then transmitted to the fluid mixing
chamber to
propel the mixed fluid to the catheter for nebulization in a body cavity, for
topical
application or other application. Using this embodiment, the different fluids
can be
administered in combination or consecutively, where a single fluid is sent
through,
and evacuated from, the mixing chamber before the next fluid is dispensed.
[0042] Another embodiment of a controller 202 configured for fluid mixture
is
shown in FIG. 8. In this embodiment, all the same components as in FIG. 7 are
identified with the same reference numbers. The embodiment of FIG. 8 differs
from
that of FIG. 7 in that a passive y-tube 204 replaces the fluid mixing chamber
172 and
fluid mixing chamber regulator 190 of FIG. 7. Thus, the mixing of fluids and
delivery of the fluid from the syringes 174, 176 to the catheter takes place
using the
force of the push plates 178, 180 on the syringes. The different fluids may be
combined in the y-tube by simultaneously dispensing the fluids from the
syringes.
Alternatively, the fluids may be dispensed consecutively or at widely spaced
time
intervals depending on the application.
[0043] In addition to providing configurations of a controller for
providing a
single type of fluid, or multiple types of fluids, embodiments of the present
invention
include configurations and methods for accommodating multiple different gases.
In
one embodiment, shown in FIG. 9, a modified high pressure manifold 208 and
mixing
chamber 210 in a controller 212 may be used to replace the high pressure
manifold
154 of FIGS. 6 and 7. The remaining components of the controller 212 in FIG. 8
identical to those in FIGS. 6 and 7 retain the same reference numerals for
clarity.
Using the controller 212 of FIG. 8, a mixture of different insufflation gases
214, 216,
218 are processed in respective high pressure regulators 220, 222, 224 to
bring their
CA 02794448 2012-11-02
= .
- 15 -
pressures down to a lower pressure, 100 p.s.i. in this example, more easily
managed
by the mixing chamber 210. The mixing chamber, an example of which is
disclosed
in U.S. Patent No. 7,654,975
incorporated above, combines substantially
even amounts of the gases into a mixture that is then processed through the
low
pressure manifold 158 as previously described. Examples of applications for
mixed
gas instifflation include the prevention Of acidosis through the addition of
oxygen to
the hasufflation gas, the reduction of post-operative pain through the
addition of
helium or oxygen, and other such applications.
[0044] In another embodiment, the fluid pump assembly of
the regulated liquid
and gas dispensing controller, which includes the motor controller 170, motor
178,
and push plate 182, may be adapted to work with a disposable catheter, syringe
and
tubing set 226. As shown in FIG. 10, the set allows for increased isolation of
any
fluid from contact with the rest of the controller 228. This is achieved by
including a
direct syringe 230 to tube 232 to catheter 234 connection rather than a
separate, fixed
syringe holder that encloses a syringe on the interior of the holder and
attaches a tube
to the outside of the syringe holder where fluid contacts a conduit built into
the holder
between the syringe and catheter or tube. To provide further isolation from
contamination, the tube 236 or other conduit from the gas outlet of the low
pressure
manifold to the catheter is also preferably part of the set 226. FIG. 11
discloses a
perspective view of a syringe pump having a receiving slot for a disposable
syringe/catheter/tubeset. The catheter may be preassembled as attached to the
syringe
and replaceably insertable into the syringe pump, or the catheter may be
separated
from the syringe and still directly attached to the syringe without any
intervening,
non-disposable lumen. The syringe pump may be in communication with a remote
processor or contain its own processor for managing operation of the syringe
pump.
A gas input port for the catheter may be integrated into the syringe pump
assembly.
[0045] Utilizing the integrated system or separate system
components described
above, a method of providing a substance, such as a nebulized medication, to a
body
CA 02794448 2012-11-02
- 16 -
= cavity during a minimally invasive procedure is now described. Although a
= laparoscopic procedure is specifically identified below, the applications
of medication
using this system can include administration of nebulized substances onto or
into
specific organs and lumens in the body, as well as topical applications. In
many
normal laparoscopic procedures, such as for gall bladders, hernias, bowl
resections
=.:- and ete., a patient is placed hi the prone position and sedated. A
verres4ype needle is
placed in the patient to transport gas to the patient and this verres needle
is connected
to the insufflator to pump up the peritoneum. One suitable verres needle or,
more
generally, insertion device is disclosed in U.S. Application Ser. No.
09/841,125, filed
April 24, 2001 and Published on December 5, 2002 as Pub. No. US 2002/0183715,
the entirety of which may be referred to.
The verres needle may then
be removed and a trocar inserted through the needle hole already made, while
maintaining a supply gas in the cavity. Using the opening provided by the
trocar, an
endoscope is inserted so that a physician may see inside the body. At this
point,
several other smaller trocars may be inserted into the body for instruments to
be used
as needed for the particular procedure. Utilizing the system described above,
the
insufflatien gas is preferably heated and humidified, and an appropriate
medicament
= treatment is applied. For example, to avoi.d adhesion problems which may
often
occur in laparoscopic procedures, an aerosol can be provided via the
aerosolization
catheter to cover the exposed organs and wall of the abdomen. This anti-
adhesion
treatment may be repeated multiple times during a surgical procedure and be
preprogrammed into the central controller 130 of the system. During the
procedure,
the parameters relating to the delivery of gas and fluid may be displayed and
individually controlled. The parameters may include humidity, temperature, pH,
volume, rate, pressure, and duration of any of the fluid or gas being injected
into the
patient. The pH may be adjusted by, for example, the introduction of acid or
buffer
solutions to the fluid. While any of a number of catheters may be used with
the
various embodiments of the regulated liquid and gas dispensing controller to
apply a
CA 02794448 2012-11-02
. =,
- 17 -
medication, or supply both the insufflation gas and a medication, two suitable
catheters are disclosed in U.S. Pat. Nos. 6,379,373, issued April 30, 2002,
and
6,165,201, issued December 26, 2000. The disclosure of both of these U.S.
patents
which may be referred to.
[0046] With the system and method described above, a
physician may apply an
aerosolized medicament to a distended body cavity that will allow for
efficient, safe
and effective application of any number of potentially aerosolized liquids
which can
be used for pain and management (analgesics), infection prevention
(prophylactic
antibiotics), tissue adhesion (any number of formulations can be used
including
naturally occurring lubricious medications such as hylauronic acid, or any
number of
other medicaments such as heparin, glycerin or glycol medications, or even
humidity), and tumor prevention (using targeted or prophylactic chemotherapy
drugs
or methods) or to control bleeding or blood clotting. Although laparoscopic
procedures arc specifically discussed above, the systems and methods disclosed
herein are contemplated for use in any endoscopic or other minimally invasive
procedure.
[0047] With reference to targeted or prophylactic
chemotherapy, according to
another aspect of this invention, the system may be used for general
continued, and
post-operative applications of a substance by re-instituting an environment in
the
patient in which subsequent applications of the substance, such as an
aerosolized
medication, may be administered. This may be accomplished by leaving a port
device in the patient after a surgical procedure, or by surgically placing a
port in the
patient in preparation of a non-surgical treatment regimen. The port may be
any
device capable of providing a sanitary access point to a body cavity, where
the device
is a resealable mechanism that attaches to the exterior of the abdomen and the
interior
wall of the abdomen. One example of a suitable port is an enteral feeding tube
port.
The port permits the device for applying a substance to the body cavity, in
this
instance a nebulizing catheter, and the remainder of the system 10 of FIG. 1
to be
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 18 -
reconnected to the patient at a later time to apply the substance or other
treatment. In
one embodiment, the substance may be an analgesic to assist with post-
operative pain.
In another embodiment, the substance may be an antibiotic for application
sometime
after surgery to combat infection that may arise.
[0048] Alternatively, independently of any post-operative pain or
infection, a
patient may be provided with such a port for the purpose of allowing an
effective
chemotherapy treatment. In this situation, a patient would be provided with
the port
so that the organ or organs affected by a cancer may be directly treated with
aerosol
treatment customized for that particular patient or tumor. In either
situation, post-
operative reentry or chemotherapy application, treatment may be accomplished
without an endoscope. In some embodiments, an endoscope may be used to allow a
medical professional to properly apply the aerosol to the desired region and
so that a
distal end of a nebulizing catheter may be oriented to provide optimal aerosol
placement. During the re-entry into the peritoneum, the pressure relief valve
or
valves (active or passive) are used to maintain a safe cavity pressure. By
maintaining
proper pressure within the peritoneum, any additional pressure introduced by
the gas
used in the aerosolization of the medicine, or pressure from the introduction
of fluids
or other substances from outside the body cavity may be accounted for.
[0049] As discussed above, a method and apparatus for creating a medicated
atmosphere in an organ or body cavity has been disclosed. The method permits a
creation of an aerosol cloud allowing for the deposition of a substance
comprising a
medicament on all or a selected number of interior surfaces. The apparatus
comprises
an aerosolization catheter that can be manipulated during use, a device for
the
introduction of the aerosolization catheter, a medication delivery system
linked to a
control means for the control of rate, amount, and time of delivery of the
medication,
a system for providing pressure control of a gas to the catheter which is also
controlled as to pressure, timing and rate of gas flow, a means for monitoring
and
relieving the pressure of the gas, alone or in conjunction with an
insufflator, and a
CA 02794448 2012-11-02
WO 2005/035035
PCT/US2004/033024
- 19 -
means of integrating the control of the various gas and fluid supplies for
complete
system control. Additionally, means for reentering the peritoneum or organ
post-
operatively to recreate a medicated environment for a post-operative treatment
is
disclosed. The insufflation and nebulization may both be perfoinied through a
single
gas lumen in a catheter, or multiple gas lumens, using the same regulated
liquid and
gas dispensing controller.
[0050] It is intended that the foregoing detailed description be regarded
as
illustrative rather than limiting, and that it be understood that the
following claims,
including all equivalents, are intended to define the scope of this invention.