Note: Descriptions are shown in the official language in which they were submitted.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
1
METHOD FOR ENSURING AND MONITORING ELECTROLYZER SAFETY
AND PERFORMANCES
FIELD OF THE INVENTION
[0001]The present invention relates to a method for monitoring and ensuring
electrolyzer safety and performances in a manufacturing process which uses at
least one electrolyzing cell containing a cathode and an anode separated by a
membrane.
[0002] The present invention also relates to a system for monitoring and
ensuring electrolyzer safety and performances in a manufacturing process
performed in a manufacturing unit which uses at least one electrolyzing cell
capable of carrying out the method.
BACKGROUND
[0003] Electrolysis is used to produce higher value chemical in different
areas
of the chemical industry, such as for the production of sodium chlorate,
caustic
soda and chlorine. Usually, the electrolysis takes place in an electrolyzer
comprising an anode wherein oxidation reaction takes place, a cathode
wherein a reduction reaction takes place, these two electrodes being separated
by a an ion exchange membrane.
[0004] An electrolyzer is usually composed of an electrolyzing cell 2
comprising
an anode 3 and cathode 5 (see figure 1). It is at the anode 3 that the
oxidation
takes place and at the cathode 5 that the oxidant is electrochemically
reduced.
Electrons are generated at the anode 3 and flow through an external load to
the
cathode 5. Ions flow between the anode 3 and the cathode 5 in an electrolyte
to
complete the circuit. A thin proton exchange membrane 7 enables the passage
of the ions from the anodic compartment to the cathodic compartment.
[0005] In case of production of chlorine, saturated brine (sodium chloride,
NaCI)
is provided at the anode side of the cell where chloride ions (Cr) are
oxidized to
chlorine (Cl2). At the cathode side of the cell, water is reduced to hydrogen
(H2)
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
2
and hydroxide ions (OH-). The latter combine with the sodium ions (Na'),
migrating through the membrane from the anode side, to form caustic soda
(NaOH).
[0006] The ways the anodes and cathodes are connected differ according to
the technology. The electrodes can be connected in parallel, in series or in a
combination thereof.
[0007] One of the problems associated with the monitoring of electrolyzing
cells
is the extremely hostile conditions in which they operate. This makes data
acquisition difficult and unreliable. It is known, that the single cell
voltage is
without any delay responding to a malfunction of a cell. But the single cell
voltage is also changing during normal operation, for example during a load
change. Known single voltage monitoring systems are not precise and reliable
enough to work as a safety system in a cell room and to cover the high risk
related to the production of chlorine or/and hydrogen.
[0008] It is common, to install as a safety system a balance voltage
monitoring
system, which compares the average voltage of a group of cells with the
average voltage of another group. The method can be unreliable, for example
in the case of a short circuit, the single voltage of the faulty cell is
decreased
while the voltage of the immediate two neighbor cells is increased, which does
not change the overall balance of the electrolyzer. It is also common to
analyze
the product quality to detect a malfunction of a cell. For example a defect
membrane in case of the electrolysis of the sodium chloride may generate an
explosive mixture of hydrogen and chlorine. In most plants one analyzer is
installed after the main chlorine cooler. Therefore, in theory only an
explosion
outside the cell room can be avoided. But in practice also explosions in the
chlorine treatment section happened, because of the response time in minutes
of that analyzer(usually a gas chromatograph or thermal conductivity
detector).
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
3
SUMMARY
[0009] There is described herein a method and a system working together as a
safety system according to SIL 2 of IEC 61511 to detect any malfunction of a
cell and to shut down the cell before a risk occurs.
[0010] There is also described herein a method of monitoring an electrolysis
and determining if individual cells are failing or badly performing.
[0011]To this end, one aspect is to provide a method for monitoring
electrolyzer safety and performances in a manufacturing process which uses at
least one electrolyzing cell containing at least one cathode and at least one
anode separated by a membrane, comprising the step of: determining a safe
single voltage operation range depending of the current and corresponding to
the normally working electrolyzing cell; determining a reference voltage
deviation depending on the time derivation of the current; measuring the
single
voltage over time at the terminals of the electrolyzing cell; determining the
measured single voltage deviation by calculating the time derivative of the
measured single voltage; comparing the measured single voltage of a cell with
the safe single voltage operation range and the measured single voltage
deviation of a cell with the reference voltage deviation and the measured
single
voltage deviation of a cell with the average voltage deviation of a group of
reference cells over time; and stopping the manufacturing process when the
measured single voltage is outside the safe single voltage operation range, or
the difference between the measured single voltage deviation and the
reference voltage deviation is outside a predetermined range, or a single
voltage behavior is different than the average of a group of reference cells.
[0012] The method enables to compare the real voltage measured at the
terminals of the electrolyzing cell as well as its voltage deviation and to
compare these real data to the reference one wherein the electrolyzing cell
works normally, that is to say that no event leading to the spoiling or the
destruction of one electrolyzing cell may occur.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
4
[0013] The normal functioning of the electrolyzing cell is determined by some
conditions such as it's age and/or performance. It was found that the majority
of
events responsible for spoiling and/or destroying an electrolyzing cell
generate
big variations of their voltage within a short time. Therefore, monitoring the
cell's voltage became critical for protecting the safety of the process and
the
plant.
[0014] Causes of spoiling and/or destroying an electrolyzing cell may
comprise:
anode loss of coating, cathode loss of coating, cathode poisoning, electrode
passivation, blockage in electrolyzing cell or liquor circuit, problem with
the
purification, insufficient brine feed, loss of feed caustic flow, membrane
blistering, membrane fooling and membrane piercing.
[0015]Accurate and early detection of any anomaly requires a cell specific
operation range. Therefore, a first step of the method is the analysis and
identification of the normal behavior using polarization curves as shown in
(fig
4). In one embodiment, the limits of safe single voltage operation range are
the
maximum voltage Umax(t) and the minimum voltage Umin(t) depending on the
current I and the time t determined by respectively the following formulae:
Umin(t) = Uo, min + (kmin/A) x I(t),
Umax(t) = Uo, max + (kmax/A) X I(t),
wherein:
I(t) is the current passing through the cell;
Uo, min and Uo, max are comprised respectively between 2.20 V and 2.40 V
and between 2.60 V and 2.80 V;
kmin and kmax are comprised respectively between 0.05 V/kA.m-2 and
0.15 V/kA.m"2 and between 0.15V/kA. M-2 and 0.25 V/kA.m"2; and A is
comprised between 1.5 m2 and 5.4 m2.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
[0016] In one embodiment, the reference voltage deviation is equal to :
wherein k is comprised between 0.10V/kA. M-2 and 0.20 V/kA.m"2 and A is
comprised between 1.5 m2 and 5.4 m2.
5 [0017] In one embodiment, the method is applied to a plurality of
electrolyzing cells mounted in series.
[0018] In one embodiment, an aqueous solution comprising a salt of
chloride is electrolyzed.
[0019] Another aspect described herein is a system for ensuring and
monitoring electrolyzer safety and performances in a manufacturing process
performed in a manufacturing unit which uses at least one electrolyzing cell
capable of carrying out the method, the system comprising: a plurality of
acquisition and transmission units, each of the acquisition and transmission
units are configured to measure the single voltage at the terminals of each
electrolyzing cell over time and to transmit the measured voltage; a treatment
device for collecting the single measured voltage transmitted by each of the
acquisition and transmission units, configured to determine a safe single
voltage operation range depending on the current and corresponding to a
normally working electrolyzing cell; determine a reference single voltage
deviation depending on the time derivation of the current; determine the
measured single voltage deviation by calculating the time derivative of the
measured single voltage; compare the measured single voltage of a cell with
the safe single voltage operation range and the measured single voltage
deviation of a cell with the reference voltage deviation and the measured
single
voltage deviation of a cell with the average voltage deviation of a group of
reference cells over time; and transmit the data to relay means; and a relay
unit
configured for implementing stopping the manufacturing process when the
measured single voltage is outside the safe single voltage operation range or
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
6
the difference between the measured single voltage deviation and the
reference voltage deviation is outside a predetermined range or a single
voltage behavior is different than the average of a group of reference cells,
with
the data issed from the treatment device and for transmitting an order for
stopping the manufacturing process.
[0020] In one embodiment, the treatment device is connected to a server
for receiving and analyzing the data issued from the treatment device.
[0021] In one embodiment, the treatment device is connected to
intermediate device configured to relay and/or format the determined data to
the server.
[0022] In one embodiment, the acquisition and transmission units are
connected to the treatment device by at least one optical fiber.
[0023] There is also described a computer program product comprising
one or more stored sequence of instruction that is accessible to a processor
and which, when executed by the processor, causes the processor to carry out
the steps of the method.
[0024] There is also described a computer readable medium carrying one
or more sequences of instructions of the computer program product.
[0025] In one embodiment, the treatment device comprises means for
implementing the computer readable medium.
BRIEF DESCRIPTION OF THE DRAWINGS
[0026] The invention will be better understood by the following description
and
is illustrated by the following figures:
[0027] figure 1 is a schematic view of an electrolyzer;
[0028] figure 2 is a schematic view of a cell for the electrolysis of sodium
chloride;
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
7
[0029] figure 3 is a schematic diagram of a method in accordance with one
embodiment;
[0030] figure 4 is a polarization curve of one electrolyzing cell;
[0031 ] figure 5 is a schematic view of a system according to an embodiment;
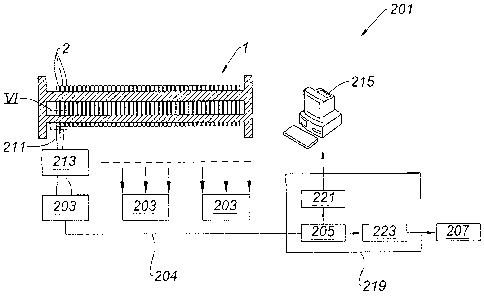
[0032] figure 6 is a view of the section VI of figure 5.
[0033] Elements in the figures are illustrated for simplicity and clarity and
have
not necessarily been drawn to scale. For example, the dimensions of some of
the elements in the figure may be exaggerated relative to other elements to
help improve the understanding of the embodiments.
DETAILED DESCRIPTION
[0034]As indicated in figures 1 and 2, one cell 2 belonging to the
electrolyzer 1
comprises an anode 3 and a cathode 5, with an ion exchange membrane 7
placed therebetween.
[0035] The anode 3 may be made of a titanium substrate with a noble metal
based catalyst. The cathode 5 may be made of a nickel substrate with a noble
metal based catalyst. The membrane 7 may be made of perfluorinated
polymers with substituted carboxylic and sulphonic groups.
[0036] The cell 2 may be filled with an aqueous solution 9 of a saturated
brine
containing sodium chloride at the anode side 5 of the cell where chloride ions
are oxidized to chlorine 10. At the cathode side 5 of the cell, water 13
forming
the electrolyte is reduced to hydrogen 15 and hydroxide ions 17 which are
exctracted from the cell 1. The latter combine with the sodium ions, migrating
through the membrane 7 from the anode side 5, to form caustic soda.
[0037] In another embodiment, a solution of saturated potassium chloride is
used, which results in the formation of caustic potash inside the cathode
compartment 5.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
8
[0038] According to an embodiment, hydrochloric acid is used, which results in
the formation of water inside the cathode compartment 5.
[0039] According to another embodiment, the material of the cathode 5 may be
adapted for consuming oxygen instead of producing hydrogen.
[0040] Surprisingly, it has been found that a cathode and membrane poisoning,
an anode and cathode loss of coating and an electrode passivation start with a
slow voltage increase, which evolves exponentially. If the electrolysis is not
stopped, the electrolyte will boil and membranes or/and hoses will be
destroyed. In the case of a membrane failure, the voltage will drop from
abnormal high to abnormal low because of internal or external short circuits,
or
the production of oxygen instead of chlorine as a consequence of mixed
electrolytes, brine and caustic soda.
[0041] Furthermore, in case of insufficient electrolyte feed, temperature and
concentration control failing the voltage will rise fast and the electrolyte
may
boil. In case of differential pressure out of range, a reverse differential
pressure
tends to lead to a voltage increase as soon as the membrane starts failing,
the
voltage is decreasing. Membrane pinholes, tears and blisters, result in an
abnormally low voltage. Leaking cells present an abnormal low voltage. if the
electrolytes are mixed or abnormally high voltages if a compartment runs dry.
[0042]A short circuit normally affects the cell voltage of three cells. It has
been
found that the cell voltage of the cell in the middle is abnormally low and
the
cell voltages of the neighbors are abnormally high.
[0043] In summary, the voltage of the affected cell increases or/and decreases
significantly within a short time and leaves the allowed operating band, which
is
a function of the actual current.
[0044]As illustrated in figures 2 and 3, the method 101 comprises the step of:
(A) determining a safe single voltage operation range corresponding to the
normally working electrolyzing cell 2; (B) determining a reference voltage
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
9
deviation determined by the time derivative of the current passing through the
cell; (C) measuring the single voltage over time at the terminals 303 and 305
of
the electrolyzing cell 2; (D) determining the measured single voltage
deviation
by calculating the time derivative of the measured single voltage; (E)
comparing
the measured single voltage of a cell with the safe single voltage operation
range and the measured single voltage deviation of a cell with the reference
voltage deviation and the measured single voltage deviation of a cell with the
average voltage deviation of a group of reference cells over time; and (F)
stopping the manufacturing process when the measured single voltage is
outside de safe single voltage operation range or the difference between the
measured single voltage deviation and the reference voltage deviation is
outside a predetermined range or a single voltage behavior is different than
the
average of a group of reference cells.
[0045] The method 101 enables a detection of an abnormal value of the voltage
at the terminal 3 and 5 of at least one of the electrolyzing cell 2 as well as
an
abnormal variation of the latter. Therefore, the method implies monitoring the
cell voltage and current and stopping the manufacturing process if the cell
voltage is outside of the allowed range, before a chlorine 10 and/or hydrogen
15 release or an explosive mixture of chlorine and hydrogen occurs.
[0046] According to one embodiment, the method may be applied to a plurality
of electrolyzing cells, such as two, ten or a hundred. More precisely, step A
of
the method implies the determination of a safe single voltage operation range
in which the electrolyzing cell works normally. The wording "works normally "
correspond to a running of the electrolysis in the electrolyzing cell 2 in
which
there is no risk of explosion or spoiling the electrolyzing cell 2.
[0047] In case of a plurality of electrolyzing cells 2 monitored by a method
101,
the electrolyzing cells 2 may be identical or different. The normal operating
range depends on the performance of the installed membrane, the electrode
gap, the type of catalyst on the anodes and cathodes etc. The definition of
the
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
allowed safe single voltage operation range depends also on the process and
the electrolyzing cell 2 technology supplier.
[0048] The limits of the safe single voltage operation range of step A of the
method may be determined by experiments or, in one embodiment, by
5 calculating the value of the maximum voltage and the minimum voltage of the
electrolyzing cell 2 for each time.
[0049] The calculation may be executed by these formulae:
Umin(t) = UO, min + (kmin/A) x I(t),
Umax(t) = Uo, max + (kmax/A) x I(t),
10 wherein Uo, min, Uo, max, kmin and kmax are determined by the polarization
curve of
the cell (see figure 4).
[0050] In figure 4 is represented a real polarization curve 20 which is
bounded
by two polarization curves 21 and 22. The polarization curve 20 is obtained by
reporting the real voltage (U) at the terminals 303 and 305 of the cell 2
measured at different current levels (I) (see figure 2). The two curves 21 and
22 delimit the normally working of the electrolyzing cell 2. They are
calculated
during step A of the method. The resulting allowed operating range is covering
the normal fluctuations of the parameters UO and k. The method includes a
software module to determine the curves 21 and 22 from historical data.
However, they can be generated also with parameters given by the cell or cell
component suppliers.
[0051 ]The real polarization curve 20 presents a real slope 25 representing
the
resistive effect of the cell 2. This real slope 25 is surrounded by the
minimal 27
and the maximal 29 slopes kmin and kmax of the two theoretical polarization
curves 21 and 22.
[0052] kmin may be included between 0.05 VkA.m2 and 0.15 VkA.m"2, around
0.10 VkA.m"2. kmax may be included between 0.15 VkA.m2 and 0.25 VkA.m2, i.e.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
11
around 0.20 VkA.m"2. Uomin and Uomax of the formula correspond to the
intercept
with the voltage axis of the polarization curve 31 and 33. Uomin may be
comprised between 2.20 V and 2.40 V, i.e. around 2.30 V. Uomax may be
comprised between 2.60 V and 2.80 V, i.e. around 2.70 V.
[0053]A is the total surface of the cell and is typically included between 1.5
m2
and 5.4 m2, i.e. around 3.0 m2. Consequently, the high precision (+/- 1.5 mV)
of
the inventive system enables the earliest possible detection of abnormal
behaviors by monitoring the voltage deviation over time (step E of the
method).
[0054] Step B is performed by determining the reference voltage deviation
determined by the time derivation of the current passing through the cell.
According to one embodiment, the reference voltage deviation is obtained by
the formula:
k x'.
wherein k is the slope 25 of the real polarization curve 20 and comprised
between 0.10 VkA.m-2 and 0.20 VkA.m-2 , around 0.15 VkA.m-2; and A is the
total surface of the cell 2 and typically inlcuded between 1.5 m2 and 5.4 m2,
around 3.0 m2.
[0055] Step C is performed by measuring the voltage over time at the terminals
303 and 305 of one electrolyzing cell or of each of the plurality cells (see
figure
2). According to a variant, the voltage may be measured at regular time range,
such as 1 second or/and 1 minute.
[0056] Step D is performed by determining the measured voltage deviation by
calculating the time derivative of the measured voltage.
[0057] Step E is performed by comparing the measured voltage to the safe
single voltage operation range and the measured voltage deviation to the
reference voltage deviation over time.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
12
[0058] Step F is performed by stopping the manufacturing process when the
measured voltage is outside the safe single voltage operation range or the
difference between the measured voltage deviation and the reference voltage
deviation is outside a predetermined range.
[0059] According to one embodiment, the predetermined range is between 50
mV and 500 mV.
[0060] The steps D, E and F may be carried out by an appropriate computer
program executed by a computer.
[0061] The method 101 presents the feature of being able to detect any
problem occuring during the working of one or a plurality of electrolyzing
cells 2
in a few steps. As indicated above, in case of a plurality of electrolyzing
cells 2,
these latter are mounted in series. The measure voltage is thus the voltage
measured at the terminals 303 and 305 of each electrolyzing cell 2 (see figure
2). The amount of electrolyzing cells 2 mounted in series may lie typically
between 1 and 200 electrolyzing cells 2 per electrolyzer 1.
[0062] The chemical potential required for the reaction to take place may lie
between 2 VDC and 4 VDC. In case, 200 electrolyzing cells 2 are mounted in
serie, the total potential of the electrolyzer 1 from end to end may reach
about
800 VDC. The current required for the electrolysis depends on the surface of
the electrodes 3 and 5 and the desired production rate. For example, the
electrolyzing cell 2 may operate between 2 kA. M-2 and 7 kA. M-2.
[0063] As illustrated in figure 5, the method 101 may be implemented by a
system 201 for monitoring an electrolyzer 1 performance in a manufacturing
process which uses at least one electrolyzing cell 2 as described above, the
system 201 comprising: a plurality of acquisition and transmission units 203,
each of the acquisition and transmission units 203 intended to measure the
single voltage at the terminals 303 and 305 of each electrolyzing cell 2 over
time according to step C and to transmit the measured voltage; a treatment
device 205 for collecting the single measured voltage transmitted by each of
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
13
the acquisition and transmission units 203, intended to implement steps A, B,
D
and E and to transmit a shutdown order to a shutdown system 207; and a
shutdown system 207 connected to the manufacturing unit comprising the
electrolyzer 1, wherein the manufacturing process takes place, intended to
stop
the manufacturing process according to an shutdown order sent by the
treatment device 205.
[0064] The data acquisition and transmission unit 203 is intended to measure
the voltage at the terminal 303 and 305 of each electrolyzing cell 2 over time
according to step C and to transmit the measured voltage. The measurement of
the voltage may be performed by using metal wires 211 connected to the inputs
of the said acquisition unit 203. To increase the accuracy of the measuring
values and the minimization of noise, the wires 211 may be concentrated in a
multicable protected cable. As illustrated by figure 6, the wires 211 may be
bound to the terminals 303 and 305 of an electrolyzing cell 2 by any means
known by the skilled person, such as bolts.
[0065]A TFP (Terminal Fuse Protection) device 213 may be disposed between
the electrolyzing cell(s) 2 and the acquisition and transmission unit 203. The
acquisition and transmission unit 203 may specifiquely contain a hardware
devices capable of acquiring data from one or a plurality of electrolyzing
cells
2, and transmit them to the other units. It may include electronic boards
called
MODA (Module Acquisition) that measure at least one of the voltage at the
terminals 303 and 305 of the electrolyzing cell 2 and may further be adapted
to
measure other variables such as temperatures and gas concentrations
measured by adequate sensors. The MODA contains A/D converters that
convert the analogic signals into digital signals with a defined sampling
rate,
memory buffers, digital filters that eliminate the undesired noise and, in the
core, a microcontroller used to run the acquisition and transmission
procedures. The components of the acquisition units 203 may be contained in a
hermetic box that protects them from the surrounding hostile environment.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
14
[0066] All the data issued from the acquisition and transmission unit 203 are
sent to the treatment device 205, called the SFOCOM (SIL Fiber Optic
Communication Module).
[0067]According to one embodiment, the data is sent via optical fibers 204.
The SFOCOM 205 is a board plugged in a personal computer or terminal. It is
capable of receiving, concentrating and formating the data streams into data
rows to make them recordable by the database and data management unit
215. The SFOCOM 205 may be located in a hermetic enclosure 219, called
ECAM enabling the supply of the power of the treatment device 205. The
treatment device 205 may comprise means for implementing a product of
computer program capable of carried out the method. The SFOCOM 205 is
also connected to a database and data management unit 215, such as a
server, for receiving the data issued from the SFOCOM 205. The server 215 is
equipped with a database to store all the sampled data and events for further
investigation if any abnormal situation will occur.
[0068]An intermediate device 221, called EFOCOM (Ethernet Fiber Optic
Communication Module), may be used for connecting the SFOCOM 205 with
the server 215. The intermediate device 221 is able to relay the data
collected
by the SFOCOM 205 to the server 215. The intermediate device 221 is mainly
used for data transmission purpose and the execution of additional advanced
supervision algorithms if needed. The relay unit 223 is connected to the
treatment device 205. The relay unit 223 is configured for implementing step F
with the data issued from the treatment device and for transmitting an order
for
stopping the manufacturing process. The order delivered by the relay unit 223
is sent to a shutdown unit 207 is capable of stopping the electrolysis by
sending
an order sent by the SFOCOM 205. The shutdown unit 207 may be for
example the central Digital Control System of the plant (DCS) or/and the
control system of the transformer rectifier.
CA 02794737 2012-09-27
WO 2011/130819 PCT/CA2010/000635
[0069] The connection between the SFOCOM 205, the server 215, the relay
unit 223 and, if necessary, the EFOCOM 221, may be made by using optical
fibers.
[0070] The method and system described apply to an electrolysis carried out in
5 any electrolyzer. In particular, it may be also used in a fuel cell.
[0071] A fuel cell is a special type of electrolyzer that is used as a
generator. It
converts the chemical energy of a fuel into electrical energy. A fuel cell is
usually composed of a number of electrolyzing cells 2 each comprising an
anode 3 and cathode 5. It is at the anode 3 that the fuel is electrochemically
10 oxidized and at the cathode 5 that the oxidant is electrochemically
reduced.
Electrons are generated at the anode 3 and flow through an external load to
the
cathode 5. Ions flow between the anode 3 and the cathode 5 in an electrolyte
to
complete the circuit. A thin proton exchange membrane 7 enables the passage
of the ions from the anodic compartment to the cathodic compartment.
15 [0072] There are different fuel cell technologies. The proton exchange
membrane fuel cell (PEMFC) is one of them. The PEMFC is also known as a
solid polymer electrolyte (SPE) fuel cell.
[0073] The embodiments described above are intended to be exemplary only.
In particular, any of the features illustrated in the attached drawings and
described above may be used in various combinations thereof. The scope of
the invention is therefore intended to be limited solely by the scope of the
appended claims.