Note: Descriptions are shown in the official language in which they were submitted.
CA 02794968 2012-09-27
= 1 ,
WO 2011/122785 PCT/1CR2011/001998
Description
Title of Invention: MAGNESIUM-BASED ALLOY FOR HIGH
TEMPERATURE AND MANUFACTURING METHOD THEREOF
Technical Field
[1] The present invention relates to a magnesium-based alloy for high
temperature and a
manufacturing method thereof.
Background Art
[2] Magnesium .with a specific gravity of 1.7 is not only the lightest element
among
commercially available metals, but its specific strength and specific
stiffness are also
superior to those of iron and aluminum. In addition, excellent mechanical
properties
can be obtained when manufacturing magnesium products by a die casting
process.
Therefore, magnesium is currently being applied to various fields, such as
portable
electronic components, aircrafts and sporting goods, etc., with mainly
focusing on the
field of automobile components. When magnesium alloys are applied to the au-
tomobile components, 30% of a weight reduction can be achieved.
[3] Typical magnesium alloys among the currently available commercial
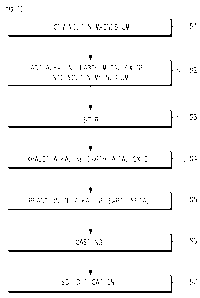
magnesium
alloys for die casting applications are magnesium (Mg)-aluminum (Al) based
alloys
such as AZ91D, AM50 and AM60. These magnesium alloys are low priced and have
good castability as compared to other alloys for the die casting applications.
Par-
ticularly, high strength can be obtained by forming a f3-Mgj,A1u phase during
solidi-
fication at room temperature. However, while automobile and aircraft
components are
generally used in a high temperature environment of 150-200 C, poor thermal
stability
of the 0 phase deteriorates creep resistance of these magnesium alloys. As a
result,
there is a disadvantage that these magnesium alloys are not appropriate for
applying to
the foregoing products used in the high temperature environment.
[4] Many efforts have been made to develop and optimize magnesium alloys for
high
temperature since the 1990's. Magnesium alloys for high temperature are
largely
classified into magnesium alloys for die casting applications and magnesium
alloys for
sand casting applications, which depend on alloy compositions and
manufacturing
methods caused by differences in use temperatures of target components. A
proper
characteristic required for the magnesium alloy for high temperature is
castability that
is appropriate for die casting, and corrosion and oxidation resistances are
also required.
In addition, when considering competitiveness against steel and aluminum, de-
velopment of magnesium alloys excluding high-priced additive elements is
required in
terms of cost.
[5] When examining conventionally developed magnesium alloys for high
temperature
RECTIFIED SHEET (RULE 91) ISA/KR
2
WO 2011/122785 PCT/KR2011/001998
based on the above requirements, magnesium alloys having high addition ratios
of rare
earth elements (RE) are disadvantageous in an aspect of cost. On the other
hand, when
adding alkaline earth metals (e.g., calcium (Ca) and strontium (Sr)) into
magnesium
alloys, there is a problem that the magnesium alloys have poor castability
such as
decrease in melt fluidity, hot tear cracks, and die soldering.
Disclosure of Invention
Technical Problem
[6] The present invention provides a magnesium-based alloy for high
temperature and a
manufacturing method thereof, in which an oxide form of Ca (a widely known
alloying
element for magnesium), i.e., calcium oxide (CaO) is added into a molten
magnesium
to reduce the CaO, the reduced Ca from the CaO reacts with Mg or Al to form a
phase,
and formation of a thermally unstable (3-Mg17A112 phase can be suppressed so
that high-
temperature strength and deformation resistance can be improved.
[7] The present invention also provides a magnesium-based alloy for high
temperature
and a manufacturing method thereof, in which an alkaline earth metal oxide,
i.e., CaO
is added into the magnesium alloy such that ductility and strength can be
improved at
the same time by improving internal soundness of casting such as reduction of
oxides,
inclusions and pores or the like. In Mg alloys, use of each Mg alloy is
generally de-
termined according to the temperature of an environment where products are
used. The
use environment temperature is often classified into 90 C, 120 C and 150 C,
etc. The
present invention also provides a magnesium-based alloy for high temperature
which
can be used at high temperatures of 120 C or more and 175 C or more
including a
temperature of 90 C or more.
[8] The object of the present invention is not limited to the aforesaid, and
other objects
not described herein will be clearly understood by those skilled in the art
from de-
scriptions below.
Solution to Problem
[9] In accordance with an exemplary embodiment of the present invention, a
method of
manufacturing a magnesium-based alloy for high temperature includes: melting
magnesium (Mg) or magnesium alloy into a liquid phase; adding 0.5% to 4.0% by
weight of calcium oxide (CaO) onto a surface of a melt in which the magnesium
or
magnesium alloy is melted; exhausting the CaO to allow the CaO not to at least
sub-
stantially remain in the magnesium or magnesium alloy through a surface
reduction
reaction between the melt and the CaO; and forming a compound by reacting at
least a
portion of calcium (Ca) produced by the surface reduction reaction in the
magnesium
or magnesium alloy.
[10] Specifically, the method may include adding the CaO 1.4 times the weight
of a final
CA 02794968 2012-09-27
3
WO 2011/122785 PCT/KR2011/001998
Ca target composition onto the surface of the melt in which the magnesium or
the
magnesium alloy is melted.
[11] The CaO may be added in the range of 1.0 -3.5 wt%. The Ca may be produced
in the
range of 0.8 -2.4 wt%.
[12] A final composition of the Mg alloy may include 6.0-8.0 wt% of aluminum
(Al),
0.1-0.3 wt% of manganese (Mn), 0.2-0.3 wt% of strontium (Sr), less than 0.04
wt% of
zinc (Zn), less than 0.9 wt% of tin (Sn), and a balance being Mg.
[13] The compound formed may include at least one of MgzCa, Al2Ca and (Mg,
Al)2Ca.
[14] In accordance with another exemplary embodiment of the present invention,
a
magnesium-based alloy for high temperature is characterized by that the
magnesium-
based alloy is manufactured by adding 0.5% to 4.0% by weight of CaO into a
molten
magnesium or magnesium alloy, and partially or wholly exhausting the CaO
through a
surface reduction reaction of the CaO, wherein the magnesium-based alloy
contains a
compound formed through combination of Ca with Mg or other alloying elements
in
the magnesium-based alloy to thereby have larger high-temperature mechanical
properties as compared to a Mg alloy having the same composition manufactured
by
directly adding Ca.
[15] The high-temperature mechanical properties may be high-temperature yield
strength
or high-temperature tensile strength.
[16] The CaO may be added in the range of 1.0 -3.5 wt%. The Ca may be produced
in the
range of 0.8 -2.4 wt%.
[17] A final composition of the Mg alloy may include 6.0-8.0 wt% of Al, 0.1-
0.3 wt% of
Mn, 0.2-0.3 wt% of Sr, less than 0.04 wt% of Zn, less than 0.9 wt% of Sn, and
a
balance being Mg.
[18] The compound formed may include at least one of MgzCa, Al2Ca and (Mg,
Al)2Ca.
[19] In accordance with still another exemplary embodiment of the present
invention, a
magnesium-based alloy for high temperature is characterized by that the
magnesium-
based alloy is manufactured by adding 0.5% to 4.0% by weight of CaO into a
molten
magnesium or magnesium alloy, and partially or wholly exhausting through a
reduction reaction of the CaO, wherein the magnesium-based alloy contains a
compound formed through combination of Ca with Mg or other alloying elements
in
the magnesium-based alloy to thereby have lower high-temperature elongation
and
high-temperature creep strain as compared to a Mg alloy having the same
composition
manufactured by directly adding Ca.
[20] The CaO may be added in the range of 1.0 -3.5 wt%. The Ca may be produced
in the
range of 0.8 -2.4 wt%.
[21] A final composition of the Mg alloy may include 6.0-8.0 wt% of aluminum
(Al),
0.1-0.3 wt% of manganese (Mn), 0.2-0.3 wt% of strontium (Sr), less than 0.04
wt% of
CA 02794968 2012-09-27
4
WO 2011/122785 PCT/KR2011/001998
zinc (Zn), less than 0.9 wt% of tin (Sn), and a balance being Mg.
[22] The compound formed may include at least one of Mg2Ca, Al2Ca and (Mg,
Al)2Ca.
[23] In accordance with yet another exemplary embodiment of the present
invention, A
magnesium-based alloy for high temperature is characterized by that the Mg-
based
alloy is manufactured through addition of CaO into a molten magnesium or
magnesium alloy and a surface reduction reaction of the CaO, wherein strength
and
elongation of room-temperature mechanical properties are increased at the same
time
as compared to a Mg alloy having the same composition manufactured by directly
adding Ca.
Advantageous Effects of Invention
[24] As described above, according to the present invention, when CaO is added
to a com-
mercially available magnesium alloy, microstructure of the magnesium alloy
becomes
finer, and Al2Ca phases or the like are formed. Also, formation of a thermally
unstable
3-Mg17A112 phase is suppressed, and casting defects are greatly reduced. As a
result,
yield strength and tensile strength of the magnesium alloy at high temperature
are
increased, and an abrupt increase in elongation at high temperature is also
suppressed
unlike in typical magnesium alloys.
[25] Also, high-temperature creep strain is reduced by suppressing deformation
at high
temperature. Therefore, high-temperature creep resistance is increased.
Brief Description of Drawings
[26] Exemplary embodiments can be understood in more detail from the following
de-
scription taken in conjunction with the accompanying drawings, in which:
[27] FIG. 1 is a flowchart illustrating a manufacturing method of a magnesium-
based
alloy according to the present invention;
[28] FIG. 2 is a flowchart illustrating dissociation of an alkaline earth
metal oxide (CaO)
added into molten magnesium according to the present invention;
[29] FIG. 3 is a schematic view exemplarily showing dissociation of an
alkaline earth
metal oxide (CaO) through stirring of an upper layer portion of molten
magnesium
according to the present invention;
[30] FIG. 4a is a micrograph of a commercially available MR1153 alloy, and
FIG. 4b is a
micrograph of an Eco-MR1153 alloy manufactured using CaO according to the
present
invention;
[31] FIGS. 5a to 5d are transmission electron microscope (TEM) micrographs of
a
magnesium alloy manufactured by a manufacturing method of a magnesium alloy
according to the present invention;
[32] FIG. 6 is a graph showing yield strength measured at 150 C of magnesium
alloys
manufactured with varying a CaO content according to the present invention;
CA 02794968 2012-09-27
5
WO 2011/122785 PCT/KR2011/001998
[33] FIG. 7 is a graph showing tensile strength measured at 150 C of
magnesium alloys
manufactured with varying a CaO content according to the present invention;
[34] FIG. 8 is a graph showing elongation measured at 150 C of magnesium
alloys manu-
factured with varying a CaO content according to the present invention;
[35] FIG. 9 is a graph showing room-temperature mechanical properties of
MRI153 and
MR1230 (Eco-MR1153 and Eco-MR1230) manufactured using CaO, compared to those
of MR1153 and MR1230 Mg alloys manufactured using Ca;
[36] FIG. 10 is a graph showing high-temperature (150 C) mechanical
properties between
MR1153 manufactured using CaO and MR1153 using Ca;
[37] FIG. 11 is a graph comparing yield strength at room and high temperature
between
an MR1153 (Eco-MR1153) magnesium alloy in which a composition is adjusted by
adding CaO according to the present invention, and an MR1153 alloy in which a
com-
position is adjusted by adding Ca according to a comparative example;
[38] FIG. 12 is a graph comparing tensile strength at room and high
temperature between
an MR1153 (Eco-MR1153) alloy in which a composition is adjusted by adding CaO
according to the present invention, and an MR1153 magnesium alloy in which a
com-
position is adjusted by adding Ca according to a comparative example;
[39] FIG. 13 is a graph comparing elongation at room and high temperature
between an
MR1153 (Eco-MR1153) alloy in which a composition is adjusted by adding CaO
according to the present invention, and an MR1153 magnesium alloy in which a
com-
position is adjusted by adding Ca according to a comparative example;
[40] FIG. 14 is a graph comparing creep strain (200 hr, 50 MPa and 150 C)
between an
MR1153 (Eco-MR1153) alloy in which a composition is adjusted by adding CaO
according to the present invention, and an MR1153 magnesium alloy in which a
com-
position is adjusted by adding Ca according to a comparative example; and
[41] FIG. 15 is a graph comparing creep strain (200 hr, 70 MPa and 175 C)
between an
MR1153 (Eco-MR1153) alloy in which a composition is adjusted by adding CaO
according to the present invention, and an MR1153 magnesium alloy in which a
com-
position is adjusted by adding Ca according to a comparative example.
Best Mode for Carrying out the Invention
[42] Preferred embodiments of the present invention will be described below in
more
detail with reference to the accompanying drawings. In every possible case,
like
reference numerals are used for referring to the same or similar elements in
the de-
scription and drawings. Moreover, detailed descriptions related to well-known
functions or configurations will be ruled out in order not to unnecessarily
obscure
subject matters of the present invention.
[43] In the present invention, a manufacturing method of a new alloy by adding
CaO into
CA 02794968 2012-09-27
6
WO 2011/122785 PCT/KR2011/001998
molten magnesium and an alloy thereof are used to solve problems arising when
calcium is added to magnesium and overcome limitations of physical properties.
[44] FIG. 1 is a flowchart illustrating a manufacturing method of a magnesium-
based
alloy according to the present invention. As shown in FIG. 1, the
manufacturing
method of the magnesium-based alloy according to the present invention
includes the
steps of: forming a magnesium-based melt (Si); adding an alkaline earth metal
oxide
(CaO in the present invention) (S2); stirring the magnesium-based melt (S3);
ex-
hausting the alkaline earth metal oxide (S4); allowing the alkaline earth
metal (Ca in
the present invention) to react with the magnesium-based melt (S5); casting
(S6); and
solidifying (S7). Although step S4 of exhausting the alkaline earth metal
oxide and
step S5 of allowing the alkaline earth metal to react with the magnesium-based
melt
are divided into the separate steps for convenience of description, two steps
S4 and S5
occur almost at the same time. That is, when supplying of the alkaline earth
metal
starts in step 4, step S5 is initiated
[45] In step Si of forming the magnesium-based melt, magnesium or a magnesium
alloy
are put into a crucible, and heated at a temperature ranging from 400 C to
800 C
under a protective gas atmosphere. Then, the magnesium alloy in the crucible
is melted
to form the magnesium-based melt.
[46] Melting Temperature of Magnesium or Magnesium Alloy
[47] In the present invention, temperature for melting magnesium or a
magnesium alloy
means the melting temperature of pure magnesium and the melting temperature of
the
magnesium alloy. The melting temperatures may be different depending on alloy
type.
For a sufficient reaction, CaO is added in the state where magnesium or the
magnesium alloy is completely melted. A temperature at which a solid phase is
suf-
ficiently melted to exist in a complete liquid phase is enough for the melting
tem-
perature of magnesium or the magnesium alloy. However, in the present
invention,
work is necessary to maintain a molten magnesium in the temperature range with
sufficient margin by considering the fact that the temperature of the molten
magnesium
is decreased due to the addition of CaO.
[48] Herein, when the temperature is less than 400 C, the molten magnesium
alloy is
difficult to be formed. On the contrary, when the temperature is more than 800
C,
there is a risk that the magnesium-based melt may be ignited. A molten
magnesium is
generally formed at a temperature of 600 C or more, whereas a molten
magnesium
alloy may be formed at a temperature ranging from 400 C or more to 600 C or
less. In
general, many cases in metallurgy show that a melting point decreases as
alloying
proceeds.
[49] When the melting temperature is increased too high, vaporization of
liquid metal
may occur. Also, magnesium easily ignites due to its own characteristic so
that the
CA 02794968 2012-09-27
7
WO 2011/122785 PCT/KR2011/001998
molten magnesium may be lost and an adverse effect may be exerted on final
physical
properties.
[50] The magnesium used in step Si of forming the magnesium-based melt may be
any
one selected from pure magnesium, a magnesium alloy, and equivalents thereof.
Also,
the magnesium alloy may be any one selected from AZ91D, AM20, AM30, AM50,
AM60, AZ31, AS41, AS31, AS21X, AE42, AE44, AX51, AX52, AJ50X, AJ52X,
AJ62X, MRI153, MR1230, AM-HP2, magnesium-Al, magnesium-Al-Re, magnesium-
Al-Sn, magnesium-Zn-Sn, magnesium-Si, magnesium-Zn-Y, and equivalents thereof;
however, the present invention is not limited thereto. Any magnesium alloy
that is
generally available in industries may be used.
[51] In step S2 of adding the alkaline earth metal oxide, CaO in the form of
powder is
added into the molten magnesium. Here, it is preferable that CaO is in the
powder form
for accelerating a reaction with the magnesium alloy.
[52] Powder Form of CaO
[53] Any form of CaO may be input for the reaction. Desirably, CaO may be
added in a
powder state so as to increase a surface area for efficient reaction..
However, if the
additive is too fine, that is, less than 0.1 um in size, the additive is
liable to be scattered
by vaporized magnesium or hot wind, thereby making it difficult to input the
additive
into a furnace. Further, the additives are agglomerated each other, and thus
clustered
while not being easily mixed with liquid molten metal. On the contrary, if the
powder
is too coarse, it is undesirable because a total surface area is not
increased. It is
preferable that an ideal particle size should not exceed 500 ,um. More
preferably, the
particle size may be 200,um or less.
[54] In order to prevent powder phases from being scattered, it is possible to
input CaO in
the form of pellet that is agglomerated from the powder form.
[55] Added Alkaline Earth Metal Oxide (Calcium Oxide)
[56] In the present invention, CaO is used as an alkaline earth metal oxide
added into the
molten magnesium. In addition, any one selected from strontium oxide (SrO),
beryllium oxide (BeO), magnesium oxide (MgO), and equivalents thereof may be
used
as the alkaline earth metal oxide.
[57] The alkaline earth metal oxide, which is used in step S2 of adding the
alkaline earth
metal oxide, may be generally added in the range of 0.001 wt% to 30 wt%.
[58] An input amount of the alkaline earth meal oxide is determined by a final
target alloy
composition. That is, an amount of CaO may be determined by performing a back-
calculation according to a desired amount of Ca to be alloyed into a magnesium
alloy.
Since physical properties of the magnesium alloy deviate from its original
physical
properties when the amount of Ca, which is indirectly alloyed into the
magnesium
alloy from the CaO, exceeds 21.4 wt% (30 wt% in the case of CaO), it is
preferable
CA 02794968 2012-09-27
8
WO 2011/122785 PCT/KR2011/001998
that the input amount of CaO should be adjusted to 30 wt% or less.
[591 According to the magnesium alloy for high temperature and the
manufacturing
method thereof according to the present invention, the input amount of the
alkaline
earth metal oxide is in the range of 0.5 wt% to 4.0 wt%. Excellent high-
temperature
mechanical properties could be obtained when the input amount of the alkaline
earth
metal oxide was 4.0 wt% or less. Improvement of the above properties was not
relatively large when the input amount was less than 0.5 wt%. More preferably,
the
composition is in the range of 1.0 wt% to 3.5 wt%. Herein, the excellent high-
temperature mechanical properties mean relatively high yield strength and
tensile
strength at high temperature, and relatively low elongation and creep strain.
at high
temperature.
[601 In the present invention, it is more preferable that the input amount of
calcium oxide
(CaO) should be adjusted such that calcium formed by reduction of CaO is
included in
the range of 0.8 wt% to 2.4 wt% in the final magnesium alloy.
[611 In step S3, the molten magnesium is stirred for 1 second to 60 minutes
per 0.1 wt%
of the added CaO.
[621 Here, if the stirring time is less than 1 second per 0.lwt%, the CaO is
not mixed with
the molten magnesium sufficiently; and, if the stirring time is more than 60
minutes
per 0.lwt%, the stirring time of the molten magnesium may be unnecessarily
lengthened. In general, the stirring time depends on the volume of the molten
magnesium and the input amount of CaO.
[631 The oxide powders of a required amount may be input at once. However, to
ac-
celerate the reaction and reduce agglomeration possibility, it is preferable
that the
oxide powders be re-input after a predetermined time elapses from a first
input time, or
the oxide powders are grouped into several batches of appropriate amounts and
the
batches are input in sequence.
[641 Stirring Method and Conditions
[651 It is preferable to stir the molten magnesium for the efficient reaction
between the
magnesium or magnesium alloy and the calcium oxide in the present invention.
The
stirring may be generally performed by generating an electromagnetic field
using a
device capable of applying electromagnetic fields around the furnace holding
the
molten magnesium, thus enabling the convection of the molten magnesium to be
induced. Also, artificial stirring (mechanical stirring) may be performed on
the molten
magnesium from the outside. In the case of mechanical stirring, the stirring
may be
performed in such a manner that the inputted CaO powders are not agglomerated.
The
ultimate purpose of the stirring in the present invention is to induce the
reduction
reaction between the molten magnesium and added powders properly.
[661 The stirring time may vary with the temperature of a molten metal and the
state
CA 02794968 2012-09-27
9
WO 2011/122785 PCT/KR2011/001998
(pre-heating state or the like) of powders added. Preferably, the stirring may
continue
to be performed in principle until the powders are not observed on the surface
of the
molten magnesium. Since the powders are lower in specific gravity than the
molten
magnesium so that they float on the molten magnesium in a steady state, it can
be in-
directly determined that the powders and the molten magnesium sufficiently
react
when the powders are not observed on the molten magnesium any longer. Herein,
the
term 'sufficiently react' means that all of the CaO powders substantially
react with the
molten magnesium and are exhausted.
[67] Although the CaO powders are not observed on the molten magnesium,
possibilities
of existing in the molten magnesium may not be excluded. Therefore, the CaO
powders that do not float yet should be observed for a predetermined holding
time after
the stirring time, and the holding time may be necessary to complete the
reaction of the
CaO powders that did not react with the molten magnesium yet.
[68] Stirring Time
[69] The stirring is effective when it is performed at the same time with the
input of the
oxide powders. In addition, the stirring may start after the oxide powders
receive heat
from the molten magnesium and reach a predetermined temperature or higher,
which
enables acceleration of the reaction. The stirring continues to be performed
until the
added oxide powders are not observed on the surface of the molten magnesium.
After
the calcium oxide is completely exhausted through the reaction, the stirring
is finished.
[70] Surface Reaction
[71] In general, when Ca and Sr of the alkaline earth metals are directly
added into the
molten magnesium, reactions occur as Ca and Sr sink into the molten magnesium
having low specific gravity. Therefore, alloying may be completed by simply
stirring
the molten magnesium to help dissolution of Ca.
[72] On the other hand, when the calcium oxide is input into the molten
magnesium, the
calcium oxide does not sink into the molten magnesium but float on the surface
of the
molten magnesium due to a difference in specific gravity.
[73] In the case of typical metal alloying, it is in general that reactions
are forced to occur
in a molten metal by inducing an active reaction by convection or stirring of
the molten
metal and alloying metal elements. However, in the present invention, when the
reaction was induced actively, the oxide inputted into the molten magnesium
hardly
reacted and left in the final material so that physical properties were
deteriorated or it
acted as the cause of defects. That is, when the reaction was induced inside
the molten
magnesium instead of on the surface of the molten magnesium, there were
relatively
more cases where the calcium oxide remained in the final molten magnesium
rather
than reacted on the surface of the molten magnesium.
[74] Therefore, in the present invention, it is important to create a reaction
environment
CA 02794968 2012-09-27
10
WO 2011/122785 PCT/KR2011/001998
where the oxide reacts on the surface rather than inside the molten magnesium.
To this
end, it is important not to forcibly stir the oxide floating on the surface of
the molten
magnesium into the molten magnesium. It is important to uniformly spread the
calcium
oxide on the molten magnesium surface exposed to air. More preferably, it is
important
to supply the oxide in such a way as to coat the entire surface of the molten
magnesium
with the oxide.
[75] Reaction occurred better in the case of stirring the molten magnesium,
and also
reaction occurred better when the stirring is performed at an outer surface
(surface of
an upper layer portion) rather than inside the molten magnesium. That is, the
molten
magnesium reacted better with the oxide powders exposed to air at the outer
surface
(surface of an upper layer portion) thereof. However, results were not
satisfactory
under a state of vacuum or ambient gas. For sufficient reaction, it is
necessary to
induce the surface reaction through stirring of the upper layer portion.
Herein, the term
'sufficiently react means that all of the additive powders substantially react
with the
molten magnesium and are exhausted. In the present invention, the stirring
inducing
the foregoing surface reaction is denoted as surface stirring. That is, Ca,
which is
produced by a reduction reaction (surface reduction reaction) of the CaO added
onto
the surface of the molten Mg, acts as an alloying element of Mg or Mg alloys.
[76] In Table 1 below, after adding 5 wt%, 10 wt% and 15 wt% of calcium oxide
having a
particle size of 70,um into a molten AM60B magnesium alloy, respectively,
residual
amounts of the calcium oxide in the magnesium alloy according to stirring
methods
were measured. The stirring methods used herein were the stirring of the upper
layer
portion of molten magnesium alloy, the stirring of the inside of the molten
magnesium
alloy, and the rest method was no stirring. According to various stirring
conditions,
when comparing the case of the stirring of only the upper layer portion with
the cases
of no stirring and the stirring of the inside of the molten magnesium alloy,
the smallest
residual amount of the calcium oxide was observed in the case of the stirring
of only
the upper layer portion, that is, the final residual amounts of the calcium
oxide were
0.001 wt%, 0.002 wt% and 0.005 wt% as the calcium oxide was added 5 wt%, 10
wt%
and 15 wt%, respectively. That is, it can be understood that when the upper
layer
portion of molten magnesium alloy is stirred to allow CaO to react at the
outer surface
of the molten magnesium, most of CaO is decomposed into Ca. That is, Ca was
added
into the alloy by inducing the reduction reaction through further addition of
CaO into
the commercially available AM60B alloy.
[77] Table 1
CA 02794968 2012-09-27
11
WO 2011/122785 PCT/KR2011/001998
[Table 1]
Addition of 5 Addition of Addition of
wt%ofCaO lOwt%of 15wt%of
CaO CaO
Residual No stirring 4.5 wt% CaO 8.7 wt% CaO 13.5 wt%
amount of CaO
CaO in the Stirring the inside of the 1.2 wt% CaO 3.1 wt% CaO 5.8 wt% CaO
alloy molten magnesium alloy
Stirring the upper layer 0.001 wt% 0.002 wt% 0.005 wt%
portion of the molten CaO CaO CaO
magnesium alloy (present
invention)
[78] An oxygen component of the calcium oxide is substantially removed above
the
surface of the molten magnesium alloy by the stirring the upper layer portion
of molten
magnesium alloy. It is desirable that the stirring is performed at an upper
layer portion
of which a depth is 20% of a total depth of the molten magnesium from the
surface. If
the depth is beyond 20%, the surface reaction according to a preferred example
of the
present invention is rarely generated. More preferably, the stirring may be
performed
in an upper layer portion of which a depth is 10% of the total depth of the
molten
magnesium from the surface. The substantially floating calcium oxide is
induced to be
positioned in an upper layer portion of which a depth is 10% of an actual
depth of the
molten magnesium, thereby minimizing the turbulence of the molten magnesium.
[79] In step S4 of exhausting the alkaline earth metal oxide, through the
reaction between
the molten magnesium and the added calcium oxide, the calcium oxide is
completely
exhausted so as not to remain in the magnesium alloy at least partially or
substantially.
It is preferable that all the calcium oxide inputted in the present invention
is exhausted
by a sufficient reaction. However, even if some portions do not react and
remain in the
alloy, it is also effective if these do not largely affect physical
properties.
[80] Herein, the exhausting of calcium oxide includes removing an oxygen
component
from the alkaline earth metal oxide. The oxygen component is removed in the
form of
oxygen gas (02) or in the form of dross or sludge through combination with
magnesium or alloying components in the molten magnesium. The oxygen component
is substantially removed out from the top surface of the molten magnesium by
stirring
the upper layer portion of the molten magnesium.
[81] FIG. 3 is a schematic view exemplarily showing dissociation of calcium
oxide
through stirring of an upper layer portion of molten magnesium according to
the
CA 02794968 2012-09-27
12
WO 2011/122785 PCT/KR2011/001998
present invention.
[82] In step S5 of allowing the alkaline earth metal to react with the molten
magnesium,
calcium produced by the exhaustion of the calcium oxide reacts with the molten
magnesium alloy so as not to at least partially or substantially remain in the
magnesium alloy. This means that the calcium produced by the exhaustion is
compounded with at least one of magnesium, aluminum, and other alloying
elements
(components) in the magnesium alloy, and is thus not left remaining
substantially.
Here, a compound refers to an intermetallic compound obtained through bonding
between metals.
[83] In the end, the added calcium oxide is partially or substantially
exhausted by
removing the oxygen component through the reaction with the magnesium alloy,
i.e.,
the molten magnesium alloy, and the produced calcium makes a compound with at
least one of magnesium in the magnesium alloy, aluminum, and other alloying
elements in the molten magnesium alloy. Therefore, the formed calcium will not
remain at least partially or substantially in the magnesium alloy.
[84] In step 5 of exhausting the alkaline earth metal oxide, there occur many
flint flashes
during the reduction reaction of the alkaline earth metal oxide on the surface
of the
molten magnesium. The flint flashes may be used as an index for confirming
whether
the reduction reaction is completed or not. In the case of terminating the
reaction by
tapping the molten magnesium while the flint flashes are being generated, the
alkaline
earth metal oxide added may not be fully exhausted. That is, the tapping of
the molten
magnesium is performed after the flint flashes, which can be used as an index
for in-
directly measuring the reduction reaction, disappear.
[85] Processes described until now are illustrated in FIGS. 1 and 2. FIG. 2 is
a flowchart
illustrating dissociation of calcium oxide used to be added into a molten
magnesium
according to the present invention.
[86] In the casting step S6, casting is performed by putting the molten
magnesium into a
mold at room temperature or in a pre-heating state. Herein, the mold may
include any
one selected from a metallic mold, a ceramic mold, a graphite mold, and
equivalents
thereof. Also, the casting method may include gravity casting, continuous
casting, and
equivalent methods thereof.
[87] In the solidifying step S7, the mold is cooled down to room temperature,
and
thereafter, the magnesium alloy (e.g., magnesium alloy ingot) is taken out
from the
mold.
[88] The magnesium-based alloy formed by the above-described manufacturing
method
may have hardness (HRF) of 40 to 80. However, the hardness value may change
widely depending on processing methods and heat treatment or the like, and
thus the
magnesium-based alloy according to the present invention is not limited to
thereto.
CA 02794968 2012-09-27
13
WO 2011/122785 PCT/KR2011/001998
[89] In pure molten magnesium, magnesium in the molten magnesium reacts with
alkaline
earth metal to thereby form a magnesium (alkaline earth metal) compound. In
the
present invention, when the alkaline earth metal oxide is CaO, MgzCa is
formed.
Oxygen constituting CaO is discharged out of the molten magnesium in the form
of
oxygen gas (02), or combines with Mg to be MgO and is then discharged in the
form
of dross (see Reaction Formula 1 below).
[90] Reaction Formula 1
[91] Pure Mg + CaO -> Mg (Matrix) + MgzCa ... [02 produced + MgO dross
produced]
[92] In a molten magnesium alloy, magnesium in the molten magnesium alloy
reacts with
alkaline earth metal to thereby form a magnesium (alkaline earth metal)
compound or
an aluminum (alkaline earth metal) compound. Also, an alloying element reacts
with
alkaline earth metal to form a compound together with magnesium or aluminum.
In the
present invention, when the alkaline earth metal oxide is CaO, MgzCa, A12Ca,
or (Mg,
Al, other alloying element)2Ca is formed. Oxygen constituting CaO is
discharged out
of the molten magnesium in the form of oxygen gas (02) as in the pure Mg case,
or
combines with Mg to be MgO, which is discharged in the form of dross (see
Reaction
Formula 2 below).
[93] Reaction Formula 2
[94] Mg Alloy + CaO -> Mg Alloy (Matrix) + {MgzCa + Al2Ca + (Mg, Al, other
alloying
element)2Ca} ... [Oz produced + MgO dross produced]
[95] As described above, the present invention makes it possible to
manufacture a
magnesium alloy economically when compared to related art methods of manu-
facturing a magnesium alloy. An alkaline earth metal (e.g., Ca) is relatively
a high-
priced alloying element as compared to an alkaline earth metal oxide (e.g.,
CaO), and
thus it acts as a main factor of increasing the price of magnesium alloys.
Also, alloying
is relatively easy by adding alkaline earth metal oxide into magnesium or
magnesium
alloy instead of adding alkaline earth metal. On the other hand, alloying
effects equal
to or greater than the case of directly adding alkaline earth metal (e.g., Ca)
can be
achieved by adding the chemically stable alkaline earth metal oxide (e.g.,
CaO).. That
is, Ca, which is produced by the reduction reaction of the CaO added into the
molten
Mg, acts as an alloying element of Mg or the Mg alloy.
[96] Also, dissolution of the alkaline earth metal in the magnesium alloy
occurs in a
certain amount when the alkaline earth metal is directly input into magnesium
or the
magnesium alloy. On the other hand, in the case of applying technology of the
present
invention, dissolution is absent or extremely small during the addition of the
alkaline
earth metal oxide (CaO) when comparing degree of the dissolution with the case
of
directly adding the alkaline earth metal. It was confirmed that an
intermetallic
compound including an Al2Ca phase forms much easier when Ca is indirectly
added
CA 02794968 2012-09-27
14
WO 2011/122785 PCT/KR2011/001998
through CaO as compared to the case of directly adding Ca. Therefore, in order
to
improve physical properties of the magnesium alloy, addition of more than a
certain
fraction of the alkaline earth metal is required. On the other hand, in the
case of manu-
facturing the magnesium alloy by adding the alkaline earth metal oxide, it can
be
observed that the physical properties are more improved than the case of
directly
adding Ca due to the fact that a considerable amount of alkaline earth metal
produced
from the alkaline earth metal oxide forms intermetallic compounds with Mg or
Al
(e.g., Mg2Ca or Al2Ca). It was confirmed that 95% or more of the intermetallic
compounds including Al2Ca forms at grain boundaries and the rest of less than
5%
forms in the grains.
[97] FIG. 4a is a micrograph of a commercially available MRI153 magnesium
alloy, and
FIG. 4b is a micrograph of an Eco-MRI 153 alloy manufactured according to the
present invention. Herein, the Eco-MRI 153 alloy denotes a magnesium alloy in
which
CaO is added instead of Ca for obtaining the Ca content equivalent to the com-
mercially available MR1153 magnesium alloy and the corresponding Ca content is
alloyed into the magnesium alloy using the reduction reaction. The meaning of
'CaO
addition' in the present invention implies that the reduction reaction process
is
undergone after the addition of the CaO.
[98] For an embodiment of a magnesium-based alloy for high temperature, the
final Ca
content was formed to 0.98 wt% using the reduction reaction by adding the CaO
into
the molten magnesium or the magnesium alloy. Then, an alloy having an
equivalent
composition to the commercially available MRI153 magnesium alloy was manu-
factured by adjusting other alloying compositions including 7.95 wt% of
aluminum
(Al), 0.20 wt% of manganese (Mn), 0.27 wt% of strontium (Sr), less than 0.01
wt% of
zinc (Zn),and less than 0.01 wt% of tin (Sn).
[99] Herein, the composition of the commercially available MRI153 magnesium
alloy
includes 7.95 wt% of Al, 0.98 wt% of Ca, 0.20 wt% of Mn, 0.27 wt% of Sr, less
than
0.01 wt% of Zn, and less than 0.01 wt% of Sn. A comparative example was manu-
factured to have the MRI153 alloy composition by directly adding Ca.
[100] Comparing with FIGS. 4a and 4b, it can be observed that the MRI153
magnesium
alloy (Eco-MRI153) manufactured by the CaO addition has a finer microstructure
than
the commercially available MRI153 magnesium alloy manufactured through the
direct
addition of Ca and also, casting defects almost do not exist.
[101] For another embodiment, the final Ca content is formed to 2.25 wt% using
the
reduction reaction by adding the CaO into the molten magnesium or the
magnesium
alloy. An alloy (Eco-MR1230) having an equivalent composition to the
commercially
available MR1230 magnesium alloy was manufactured by adjusting other alloying
compositions including 6.45 wt% of Al, 0.27 wt% of Mn, 0.25 wt% of Sr, less
than
CA 02794968 2012-09-27
15
WO 2011/122785 PCT/KR2011/001998
0.01 wt% of Zn, and less than 0.84 wt% of Sn.
[102] Herein, the composition of the commercially available MR1230 magnesium
alloy
includes 6.45 wt% of Al, 2.25 wt% of Ca, 0.27 wt% of Mn, 0.25 wt% of Sr, less
than
0.01 wt% of Zn, less than 0.84 wt% of Sn, and a balance being Mg. A
comparative
example was manufactured to have the MR1230 alloy composition by directly
adding
Ca.
[103] In the two MR1230 alloys (the Eco-MR1230 and the commercial MR1230), it
can
also be understood that the Eco-MR1230 has a finer microstructure than the com-
mercially available MR1230 magnesium alloy and casting defects almost do not
exist
like in the above embodiment.
[104] The final composition of the Mg alloy in the present invention may be
adjusted
within the range including upper and lower limits of the respective alloying
elements
of the commercially available MRI153 and MR1230 magnesium alloys. For example,
in the case of Al, an embodiment is possible in the range of 6.0-8.0 wt%
including the
lower and upper limits of 6.45 wt% and 7.95 wt%, respectively. That is, an em-
bodiment is possible in the ranges including 6.0-8.0 wt% of Al, 0.8-2.4 wt% of
Ca,
0.1-0.3 wt% of Mn, 0.2-0.3 wt% of Sr, less than 0.04 wt% of Zn, and less than
0.9
wt% of Sn. For the embodiment, an added amount of CaO in the present invention
is
adjusted such that the reduced Ca may be included in the ranges of 0.8 wt% to
2.4 wt%
of the final Mg alloy. That is, the added amount of CaO may be adjusted to
1.12-3.36
wt% which is 1.4 times of the amount of Ca.
[105] The total amount of CaO will be added 1.4 times the weight of a final Ca
target com-
position under the assumption that all CaO are reduced into Ca. Herein, for
alloying
the target amount of Ca using the CaO, the added amount of CaO in the molten
magnesium alloy is 1.4 times to 1.7 times the weight of the final Ca target
com-
position. By considering the amount that may not react with the molten
magnesium
alloy and mix with dross on the surface of the molten magnesium alloy, the
amount of
CaO may be added 1.4 times to 1.7 times the weight of the final Ca target
composition.
[106] FIGS. 5a to 5d show compositional analysis of transmission electron
microscope
(TEM) micrographs of the magnesium alloy manufactured by adding 1.8 wt% of CaO
into a AZ61 magnesium alloy by the manufacturing method of the magnesium alloy
according to the present invention. FIGS. 5a, 5b and 5c show that magnesium,
aluminum and calcium components are detected, respectively. As shown in the mi-
crographs, it can be understood that aluminum and calcium are detected in the
same
phase. This implies that Ca is dissociated from the CaO added in the molten
magnesium and combines with aluminum in the molten magnesium to form a
compound.
[107] The following Table 2 presents quantitative data on the composition of
the above
CA 02794968 2012-09-27
16
WO 2011/122785 PCT/KR2011/001998
phase. The compound was formed with Al and Ca, and from a quantitative compo-
sitional analysis of the phase, it can be understood that an Al2Ca phase was
formed.
High-temperature properties of the magnesium alloy are improved by grain
boundary
strengthening due to the formation of the Al2Ca phase and suppressing
formation of a
thermally unstable (3-Mg17A112 phase. The reason is considered due to the
Al2Ca
phases, which are uniformly distributed and formed due to the CaO addition, or
other
formed phases (e.g., Mg2Ca and (Mg, Al, other alloying element)2Ca).
[108] Table 2
[Table 2]
wt% at%
Al 68.73 76.55
Ca 31.27 23.45
Total 100 100
[109] FIG. 6 is a graph showing yield strength (TYS) when adding calcium oxide
in a
magnesium alloy. In the experimental conditions at this time, tensile tests
were
performed on tensile specimens at a rate of 1 mm/min after holding for 30
minutes at
150 C.
[110] In an exemplary embodiment, the experiments were performed by adding 0.5
wt% to
3.8 wt% of CaO into an AM60B magnesium alloy. For the experiments, Ca was
added
into the alloy by inducing the reduction reaction caused by additionally
adding the
CaO into the commercial AM60B alloy.
[111] The yield strength was in the range of 140 MPa to 145 MPa when 0.9 wt%
of the
calcium oxide was added into the magnesium alloy, and the yield strength was
150
MPa when 1.4 wt% of the calcium oxide was added into the magnesium alloy. When
3.5 wt% of calcium oxide was added into the magnesium alloy, the yield
strength was
also 150 MPa.
[112] The yield strength according to the added amount (wt%) of CaO is
presented in
Table 3 below.
[113] Table 3
CA 02794968 2012-09-27
17
WO 2011/122785 PCT/KR2011/001998
[Table 3]
Alloy Added amount of CaO Yield strength [MPa]
Magnesium alloy(AM60B) 0.5-0.9 wt% 141-143
1.0-1.4 wt% 146-151
1.5-1.9 wt% 147-152
2.0-2.5 wt% 150-155
2.6-3.2 wt% 150
3.3-3.8 wt% 150-152
[114] In Table 3, the yield strength, which is capable of being used at a high
temperature of
90 C, is obtained at 0.5-0.9 wt% of the CaO, and a high-temperature
characteristic,
which is appropriate for a temperature of 150 C or more, is obtained at more
than the
above content of the CaO. That is, it can be understood that the yield
strength is
relatively better at high temperature when 1.0-3.5 wt% of the calcium oxide is
added
into the magnesium alloy.
[115] FIG. 7 is a graph showing tensile strength (UTS) when adding the calcium
oxide in
the magnesium alloy. In the experimental conditions at this time, tensile
tests are
performed on tensile specimens at a rate of 1 mm/min after holding for 30
minutes at
150 C.
[116] In an exemplary embodiment, the experiments were performed by adding the
CaO in
the range of 0.5 wt% to 3.8 wt% into an AM60B magnesium alloy. For the ex-
periments, Ca was added into the alloy by inducing the reduction reaction
caused by
additionally adding the CaO into the commercial AM60B alloy.
[117] The tensile strength was 225 MPa when 0.9 wt% of the calcium oxide was
added into
the magnesium alloy, and the tensile strength was 239 MPa when 1.4 wt% of the
calcium oxide was added into the magnesium alloy. When 3.5 wt% of the calcium
oxide was added into the magnesium alloy, the tensile strength was 232 MPa.
[118] The tensile strength according to the added amount (wt%) of CaO is
presented in
Table 4 below.
[119] Table 4
CA 02794968 2012-09-27
18
WO 2011/122785 PCT/KR2011/001998
[Table 4]
Alloy Added amount of CaO Tensile strength [MPa]
Magnesium alloy(AM60B) 0.5-0.9 wt% 222-224
1.0-1.4 wt% 225-230
1.5-1.9 wt% 232-238
2.0-2.5 wt% 234-239
2.6-3.2 wt% 232
3.3-3.8 wt% 230-232
[120] In Table 4, the tensile strength, which is capable of being used at a
high temperature
of 90 i E, is obtained at 0.5-0.9 wt% of the CaO, and a high-temperature
characteristic,
which is appropriate for a temperature of 150 i E or more, is obtained at more
than the
above content of the CaO. That is, it can be understood that the tensile
strengths are
relatively better at high temperature when 1.0-3.5 wt% of the calcium oxide is
added
into the magnesium alloy.
[121] FIG. 8 is a graph showing elongation when adding the calcium oxide in
the
magnesium alloy. In the experimental conditions at this time, tensile tests
were
performed on tensile specimens at a rate of 1 mm/min after holding for 30
minutes at
150 ;E.
[122] In an exemplary embodiment, the experiments were performed by adding the
CaO in
the range of 0.5 wt% to 3.8 wt% into an AM60B magnesium alloy. For the ex-
periments, Ca was added into the alloy by inducing the reduction reaction
caused by
additionally adding the CaO into the commercial AM60B alloy.
[123] As shown in FIG. 8, the elongation obtained was in the range of 13% to
14% when
0.9 wt% of the calcium oxide was added into the magnesium alloy, and the
elongation
obtained was in the range of 14% to 15% when 1.4 wt% of the calcium oxide was
added into the magnesium alloy. When 3.5 wt% of the calcium oxide was added
into
the magnesium alloy, the elongation was 14%.
[124] The elongation depending on the CaO wt% is presented in the following
Table 5.
[125] Table 5
CA 02794968 2012-09-27
19
WO 2011/122785 PCT/KR2011/001998
[Table 5]
Alloy Added amount of CaO Elongation [%]
Magnesium alloy(AM60B) 0.5-0.9 wt% 13-14
1.0-1.4 wt% 14-15
1.5-1.9 wt% 15
2.0-2.5 wt% 14-15
2.6-3.2 wt% 15
3.3-3.8 wt% 14-15
[126] FIG. 9 is a graph comparing room-temperature mechanical properties
between Mg
alloys having compositions of the Eco-MR1153 and the Eco-MR1230 manufactured
using CaO and Mg alloys having compositions of the MR1153 and the MR1230 manu-
factured using Ca.
[127] As shown in FIG. 9, it was found that the magnesium-based alloy for high
tem-
perature (the Eco-MRI153 and the Eco-MRI230) according to the present
invention
exhibit superior yield strength (YS), tensile strength (UTS) and elongation to
the
MR1153 and the MR1230 even at room temperature. That is, the Eco-MR1153 and
the
Eco-MR1230 have better room-temperature mechanical properties than the MR1153
and the MR1230 manufactured using Ca.
[128] FIG. 10 is a graph comparing high-temperature mechanical properties of
Mg alloys
between the MR1153 alloy manufactured using CaO and the MR1153 alloy using Ca.
[129] As shown in FIG. 10, it was found that the magnesium-based alloy (the
Eco-
MRI153) according to the present invention exhibit superior yield strength and
tensile
strength to the MR1153 even at high temperature (150 C). In the case of high-
temperature elongation, the Eco-MR1153 of the present invention was smaller
than the
MRI153. It can be understood that changes in the elongation are small at high
tem-
perature so that the magnesium-based alloy according to the present invention
has
stable mechanical properties even for temperature changes. That is, the
magnesium-
based alloy manufactured using the CaO according to the present invention has
good
elongation as well as good yield strength and tensile strength even at high
temperature.
[130] FIG. 11 is a graph comparing yield strength at room and high temperature
between
an Eco-MR1153 magnesium alloy in which a Ca composition is indirectly adjusted
by
adding CaO and an MR1153 magnesium alloy in which a composition is adjusted by
directly adding Ca. It can be understood that in the case of the Eco-MRI153,
high-
temperature yield strength is increased 8% as compared to the MRI153.
[131] FIG. 12 is a graph comparing tensile strength at room and high
temperature between
CA 02794968 2012-09-27
20
WO 2011/122785 PCT/KR2011/001998
an Eco-MR1153 magnesium alloy in which a Ca composition is indirectly adjusted
by
adding CaO and an MR1153 magnesium alloy in which a composition is adjusted by
adding Ca. It can be understood that the Eco-MR1153 manufactured by adding the
CaO
has higher yield and tensile strengths at room and high temperature (150 C)
than the
MR1153 having the same composition manufactured by directly adding the Ca. It
can
be understood that in the case of the Eco-MRI153, high-temperature tensile
strength is
increased 8% as compared to the MRI153. Particularly, in the case of the high-
temperature tensile strength in FIG. 11, a remarkable improvement may be
confirmed
in the Eco-MRI153 adjusting the composition with the CaO according to the
present
invention.
[132] FIG. 13 is a graph comparing elongation at room and high temperature
between an
Eco-MRI153 magnesium alloy in which a Ca composition is indirectly adjusted by
adding CaO and an MRI153 magnesium alloy in which a composition is adjusted by
adding Ca.
[133] In the case of the elongation at room temperature, elongation of the Eco-
MRI153
manufactured by adding the CaO was higher than that of the MRI153 having the
same
composition manufactured by directly adding the Ca. On the other hand, at high
tem-
perature, the elongation of the Eco-MR1153 manufactured by adding the CaO was
lower than the case of directly adding the Ca. It can be understood that in
the case of
the Eco-MRI153, high-temperature elongation is decreased 42% as compared to
the
MRI153. Particularly, the high-temperature elongation at 150 C was remarkably
low
in the Eco-MR1153 adjusting the composition by adding the CaO. That is,
changes in
the elongation depending on the temperature were smaller in the Eco-MR1153
manu-
factured by adding the CaO than the MR1153 manufactured by directly adding the
Ca.
[134] FIG. 14 is a graph comparing creep strain (200 hr, 50 MPa and 150 C)
between an
Eco-MR1153 magnesium alloy in which a composition is indirectly adjusted by
adding
CaO according to the present invention, and an MR1153 magnesium alloy in which
a
composition is adjusted by adding Ca according to a comparative example.
[135] Creep resistance was better in the Eco-MRI153 alloy manufactured by
adding the
CaO than the commercial MRI153 alloy manufactured by adding the Ca. That is,
creep
strain (elongation) was smaller in the Eco-MR153 alloy.
[136] FIG. 15 is a graph comparing creep strain (200 hr, 70 MPa and 175 C)
between an
MR1153 (Eco-MR1153) alloy in which a composition is adjusted by adding CaO
according to the present invention, and an MR1153 magnesium alloy in which a
com-
position is adjusted by adding Ca according to a comparative example.
[137] Creep resistance at the high temperature was better in the Eco-MR1230
alloy manu-
factured by adding the CaO than the commercial MR1230 alloy manufactured by
adding the Ca. That is, creep strain was smaller in the Eco-MR230 alloy.
CA 02794968 2012-09-27
21
WO 2011/122785 PCT/KR2011/001998
[1381 As described above, when CaO is added into the commercial magnesium
alloy in the
present invention, it is consequently possible to alloy Ca indirectly.
Therefore, the
results show that high-temperature physical properties of the magnesium alloy
are
improved. Microstructure of the magnesium alloy manufactured by the CaO
addition
becomes finer, and Mg2Ca, Al2Ca or (Mg, Al)2Ca phases are formed uniformly.
Formation of a thermally unstable (3-Mg17A112 phase is suppressed, and casting
defects
are remarkably reduced. As a result, yield strength and tensile strength of
the
magnesium alloy at high temperature are increased. In the case of elongation,
rapid
increase in the elongation at high temperature is suppressed unlike typical
magnesium
alloys. That is, high-temperature elongation and creep strain are decreased,
and
therefore, high-temperature creep strength is increased.
[1391 While this invention has been particularly shown and described with
reference to
preferred embodiments thereof, it will be understood by those skilled in the
art that
various changes in form and details may be made therein without departing from
the
spirit and scope of the invention as defined by the appended claims.
Therefore, the
scope of the invention is defined not by the detailed description of the
invention but by
the appended claims, and all differences within the scope will be construed as
being
included in the present invention.
[1401
CA 02794968 2012-09-27