Note: Descriptions are shown in the official language in which they were submitted.
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
UTILIZATION OF WASTE HEAT USING FIBER SORBENT SYSTEM AND
METHOD OF USING SAME
FIELD OF THE DISCLOSED SUBJECT MATTER
[0001] The disclosed subject matter relates to a fiber sorbent system, and
particularly
a sorbent system for rapid heat transfer capable of being heated and cooled
rapidly.
BACKGROUND OF THE DISCLOSED SUBJECT MATTER
[0002] Chemical processing operations, including petroleum refining and
chemical
processing operations, are energy intensive. It is often necessary to conduct
these
operations at high temperatures using high temperature heat sources including
but not
limited to steam and other hot streams present in refining and petrochemical
processing
facilities. After the steam and other hot streams have performed their
intended functions,
there remains "waste" or unutilized energy that can be further utilized.
Refineries and
petrochemical facilities typically utilize only about 70% of the input energy
needed to
conduct processing of crude oil to products.
[0003] In an effort to increase efficiency, it is desirable to recover and
utilize the
waste or unutilized heat. One method described in U.S. Patent No. 5,823,003 to
Rosser
et al. attempts to make use of waste heat and apply such heat to an adsorbent
material in
order to release an adsorbed gas at a higher pressure, which in turn can be
used in a
refrigeration cycle that contains an expansion valve. U.S. Patent No.
5,823,003, the
entirety of which is incorporated herein, describes the use of a zeolite-water
combination
for a sorption refrigeration system.
[0004] Current methods to obtain refrigeration and work from sorbent materials
in
chemical process applications have limitations. For example, the temperature
swings
(AT) provided by lower grade heat sources, such as waste heat, are less than
that which
would be provided using primary heat sources. Such limitations render the
recovery of
useful energy from waste heat economically unsustainable, or impractical.
-1-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0005] Accordingly, there remains a need to improve unutilized heat recovery
efforts
(e.g., waste heat recovery) and render such efforts more cost-effective by
maximizing
output from the temperature swings (AT) provided by lower grade sources. There
is a
need to provide sorption systems with improved heat transfer rate which are
capable of
being heated and cooled rapidly, thus rendering sorption systems driven by
lower grade
heat sources more economically sustainable.
SUMMARY OF THE DISCLOSED SUBJECT MATTER
[0006] The purpose and advantages of the disclosed subject matter will be set
forth
in and apparent from the description that follows, as well as will be learned
by practice
of the disclosed subject matter. Additional advantages of the disclosed
subject matter
will be realized and attained by the methods and systems particularly pointed
out in the
written description and claims hereof, as well as from the appended drawings.
[0007] To achieve these and other advantages and in accordance with the
purpose of
the disclosed subject matter, as embodied and broadly described, the disclosed
subject
matter includes a hollow fiber sorbent system and particularly a sorption
system capable
of being heated and cooled rapidly.
[0008] In accordance with one aspect of the present invention, a fiber
sorption
system is provided. The system includes at least one vessel, a working fluid,
at least one
thermal fluid and at least one hollow fiber located within the at least one
vessel. Each
hollow fiber includes a sorbent material and binder material that together
form an
elongated body. The elongated body has a hollow interior and an inner surface
adjacent
the hollow interior. One of the inner surface and the outer surface has a
coating layer
formed thereon. The coating layer being impermeable to both the working fluid
and the
thermal fluid.
[0009] The coating layer may be formed from a material selected from the group
consisting of poly(vinyl chloride), poly(vinylidene chloride), poly(vinyl
floride),
poly(vinylidene floride), ethylene vinyl alcohol copolymer, poly vinyl
alcohol,
polyamides, polyethylene (preferably high density), polypropylene (preferably
high
density), polyesters, polyimides, polyacrylonitrile, polysulfone,
polyurethane,
combinations thereof and derivatives thereof.
-2-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0010] In accordance with one aspect of the present invention, the coating
layer is
formed on the inner surface. The thermal fluid passes the hollow interior, but
does not
pass through the coating layer to the sorbent material. The thermal fluid may
include a
heating fluid and a cooling fluid. The working fluid may include carbon
dioxide. The
carbon dioxide may be supplied from a process stream within a petrochemical or
chemical processing operation. The working fluid is in fluid communication
with the
outer surface of the hollow fiber.
[0011] In accordance with another aspect of the present invention, the coating
layer
is formed on the outer surface. The working fluid passing through the hollow
interior
such that it is capable of being adsorbed and desorbed by the sorbent material
in the
elongated body.
[0012] In accordance with another aspect of the present invention, a fiber
sorption
system is disclosed comprising at least one vessel, a working fluid, at least
one thermal
fluid and at least one fiber located within the at least one vessel. Each
fiber includes a
sorbent material and binder material forming an elongated body having an outer
surface.
The working fluid flows past the outer surface and is capable of being
adsorbed and
desorbed by the sorbent material. The thermal fluid may flow past the outer
surface and
is capable of transferring heat without wetting the fiber surface. Thermal
fluid contact
angle with the fiber surface is more than 90 degrees. The fiber may further
include an
outer coating on the outer surface. The outer coating is permeable to the
working fluid
such that working fluid may pass through the outer coating for adsorption and
desorption
by the sorbent material. The outer coating is impermeable to the thermal
fluid, whereby
the thermal fluid is prevented from passing through the outer coating to the
sorbent
material. The outer coating may be formed from an organometallic compound.
[0013] In accordance with another aspect of the disclosed subject matter, a
fiber
sorption system is disclosed comprising at least one hollow fiber including an
inner
coating generally impermeable to a thermal fluid (i.e. heating fluid or a
cooling fluid) as
well as working fluid. The inner coating defines a channel adapted to receive
a supply of
the thermal fluid (e.g., steam). The hollow fiber further includes an outer
surface that is
permeable to a working fluid. A chamber is defined by and between the outer
surface
-3-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
and the inner coating, with a sorbent material contained within the chamber.
The fiber
sorption system further comprises a supply of the working fluid (e.g., carbon
dioxide) in
fluid communication with the outer surface of hollow fiber. Additionally, the
inner
coating can be, for example, poly(vinyl chloride), poly(vinylidene chloride),
poly(vinyl
floride), Poly(vinylidene floride), Ethylene vinyl alcohol copolymer, poly
vinyl alcohol,
polyamides, polyethylene (preferably high density), polypropylene (preferably
high
density), polyesters, polyimides, polyacrylonitrile, polysulfone,
polyurethane, etc. - their
combinations or derivatives.
[0014] The disclosed subject matter also includes a method of creating work
from a
pressurized working fluid that includes providing a vessel containing a fiber
sorption
system as disclosed herein, and introducing a supply of the working fluid to
an exterior
surface of the outer coating; introducing the thermal fluid (e.g., heating
fluid) to the inner
channel to obtain a pressurized working fluid; and directing the pressurized
working
fluid to a work component. The work component can be an expansion valve to
provide
refrigeration, or a turboexpander to provide electricity.
[0015] In accordance with another aspect of the disclosed subject matter, a
fiber
sorption system is disclosed that includes at least one hollow fiber including
an inner
surface that is permeable to a working fluid, with the inner surface defining
a channel
adapted to receive a supply of the working fluid (e.g., carbon dioxide). The
hollow fiber
further includes an outer coating that is impermeable to the thermal fluid and
working
fluid, wherein the outer coating defines a chamber between the outer coating
and the
inner surface , with a sorbent material contained within the chamber. The
fiber sorption
system further includes a supply of the working fluid in fluid communication
with the
inner surface. Additionally, the outer coating can be, for example, poly(vinyl
chloride),
poly(vinylidene chloride), poly(vinyl floride), Poly(vinylidene floride),
Ethylene vinyl
alcohol copolymer, poly vinyl alcohol, polyamides, polyethylene (preferably
high
density), polypropylene (preferably high density), polyesters, polyimides,
polyacrylonitrile, polysulfone, polyurethane, etc. - their combinations or
derivatives.
[0016] The disclosed subject matter also includes a method of creating a
pressurized
working fluid that includes providing a vessel containing a fiber absorption
system as
disclosed herein, and introducing a supply of the working fluid to the
channel;
-4-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
introducing the heating fluid to an exterior surface of the chamber to obtain
a pressurized
working fluid; and directing the pressurized working fluid to a work
component. The
work component can be an expansion valve to provide refrigeration, or a
turboexpander
to provide electricity.
[0017] In an exemplary embodiment, the channel and the chamber are each
circular
in cross-section and concentric with each other wherein the cross-section of
the channel
is about 50 microns to about 400 microns in diameter. Additionally, the linear
distance
from an interior surface of the outer membrane to an exterior surface of the
inner
membrane is from about 50 to about 400 microns. The sorbent material is a
zeolite,
such as zeolite 13X, and is about 10% to about 95% of the total weight of the
chamber.
[0018] The fiber sorption system disclosed herein is suitable for use in
applications
in which the carbon dioxide is obtained from a process stream within a
petrochemical or
chemical processing operation, such as a combustion operation.
[0019] It is to be understood that both the foregoing general description and
the
following detailed description are exemplary and are intended to provide
further
explanation of the disclosed subject matter claimed.
[0020] The accompanying drawings, which are incorporated in and constitute
part of
this specification, are included to illustrate and provide a further
understanding of the
method and system of the disclosed subject matter. Together with the
description, the
drawings serve to explain the principles of the disclosed subject matter.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] Figure 1 is a schematic representation of a conventional adsorption
system.
[0022] Figure 2 is a graphical illustration depicting the adsorptive
properties of a
working fluid in accordance with the disclosed subject matter.
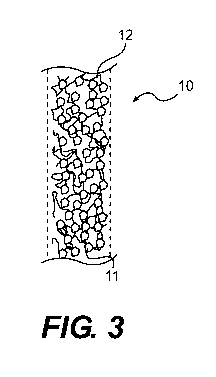
[0023] Figure 3 is a sectional view of an uncoated fiber for use in the fiber
sorbent
system according to an embodiment of the present invention.
-5-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0024] Figure 4 is a sectional view of a coated fiber for use in the fiber
sorbent
system according to another embodiment of the present invention.
[0025] Figure 5 is a cross-sectional view of a hollow fiber for use in the
fiber sorbent
system according to another embodiment of the present invention.
[0026] Figure 6 is a cross-sectional view of another hollow fiber for use in
the fiber
sorbent system according to a yet another embodiment of the present invention.
[0027] Figure 7 is a cross-section view of yet another hollow fiber for use in
the fiber
sorbent system according to the present invention.
DETAILED DESCRIPTION OF THE DISCLOSED SUBJECT MATTER
[0028] The presently disclosed subject matter will now be described in greater
detail
in connection with the Figures and the following terms.
[0029] As used herein, the term "sorbent material" refers to a material that
reversibly
binds to a working fluid. Sorbent materials include, but are not limited to,
adsorbents.
[0030] As used herein, the term "working fluid" refers to a liquid or gas that
can
reversibly bind to the sorbent material, either in a chemical or physical
sense. When the
working fluid is introduced to an expansion valve, it can also be referred to
as a
refrigerant.
[0031] As used herein, the term "driver device" refers to a turbine, shaft or
other
mechanism driven by a working fluid to generate electricity or work.
[0032] As used herein, the term "vessel" refers to a container suitable for
containing
the fibers and a thermal fluid under suitable conditions to permit sorption
and desorption.
[0033] As used herein, the term "thermal fluid" refers to a liquid or gas
capable of
introducing a temperature change to the sorbent material. Thermal fluid can be
a heating
fluid or a cooling fluid.
[0034] As used herein, the term "unutilized heat" or "unutilized heat source"
refers
to the residual or remaining heat (e.g., steam) following the processing
operation after
-6-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
the heat sources has been used for its primary purpose in the refining or
petrochemical
processing operation. One example of an unutilized heat source is "waste
heat." For
example, the unutilized heat or unutilized heat source can be a heat source
that is no
longer used in refining and/or petrochemical processing operation and would
traditionally be discarded. The unutilized heat can be provided as an
unutilized heat
stream. For example, but not limitation, unutilized heat can include steam
that was
employed in a heat exchanger used in petroleum and petrochemical processing.
[0035] Reference will now be made to various aspects and embodiments of the
disclosed subject matter in view of the definitions above. Reference to the
methods will
be made in conjunction with, and understood from, the systems disclosed
herein.
[0036] For the purpose background and not admission of prior art, an
adsorption
system 1000 is shown in Figure 1. The system 1000 is disclosed in US Patent
Application No. 12/603,243 entitled "System Using Unutilized Heat For Cooling
and/or
Power Generation". The disclosure of which is hereby incorporated in its
entirety. An
adsorption bed (110) is provided, that contains tubes packed with adsorbents
(e.g.,
MOFs/ZIFs/Zeolites/Carbon). The adsorption bed is adapted to receive either a
feed of
waste heat (120) or cold water (130). During an adsorption stroke, the
adsorption bed is
provided with a feed of cold water and the adsorbents adsorb working fluid
(e.g., C02) at
a lower temperature, T3, and lower pressure, P2. The cold water supply is then
valved
off, and a feed of waste heat is then fed to the adsorption bed to heat the
adsorbent bed to
Ti (>T2) to release adsorbed working fluid. The heating increases the pressure
of the
released working fluid P1 (>P2). Thus the adsorbent acts as a compressor, and
conventional devices, e.g., pumps, are not required to drive the cycle.
[0037] The pressurized working fluid can be introduced to a turboexpander
(140) to
generate electricity. Downstream of the turboexpander, working fluid is now at
a lower
pressure, P2 and lower temperature, T2. The thermodynamic conditions are such
that the
working fluid is in an at least a partially condensed phase. After exiting the
turboexpander, the condensed working fluid is fed to an evaporator (150) to
chill a given
process stream in the refinery, which in turn increases the temperature of the
working
fluid to T3. The working fluid is again introduced to adsorption bed and the
process is
repeated.
-7-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0038] The adsorption system shown in Figure 1 is equipped with a second
adsorption bed (160), also adapted to receive a feed of either waste heat
(170) or cold
water (180). Having two adsorption beds in parallel allows one adsorption bed
to be
regenerated (adsorption stroke) while the other adsorption bed is in
desorption mode.
Other details regarding sorption systems can be found in U.S. Patent
Application No.
12/603,243, which is hereby incorporated by reference in its entirety.
[0039] However, conventional designs have certain disadvantages. For example,
the
indirect heating and cooling of the adsorbent results in a slower heat
transfer rate and
longer temperature swing cycle times. Consequently, this design requires
bigger beds
and/or multiple beds which increases the cost of the adsorption system and the
infrastructure footprint. Additionally, such prior art systems can be
ineffective and/or
cost prohibitive for use with low grade waste heat, i.e., temperature below
300 F.
[0040] One aspect of the disclosed subject matter is directed to a replacement
for the
conventional adsorption beds. Particularly, a fiber sorption system and method
is
provided for creating a pressurized working fluid comprising at least one
hollow fiber.
The hollow fiber can be constructed with an inner coating generally
impermeable to a
thermal fluid and working fluid, and defining a channel adapted to receive a
supply of
the thermal fluid. The hollow fiber also includes an outer surface generally
posing no
resistance to working fluid that defines a chamber between the outer surface
and the
inner coating. A sorbent material is contained within the chamber between the
inner
coating and outer surface. In this configuration, a supply of working fluid is
introduced
to an exterior surface of the fiber, and the thermal fluid, e.g., heating
fluid, is introduced
to the channel to obtain a pressurized working fluid from the sorbent
material.
[0041] Alternatively, the disclosed subject matter provides a fiber sorption
system
and method for creating a pressurized working fluid wherein the hollow fiber
is
constructed with an inner surface posing no resistance to working fluid
permeation, and
defining a channel adapted to receive a supply of the working fluid. The
hollow fiber
also includes an outer coating generally impermeable to a thermal fluid (e.g.,
a heating
fluid) and working fluid to define a chamber between the outer coating and the
inner
surface. A sorbent material is contained within the chamber between the inner
surface
and outer coating. In this configuration, a supply of working fluid is
introduced to the
-8-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
inner channel of the fiber, and the thermal fluid is introduced to the
exterior surface of
the chamber to obtain a pressurized working fluid from the sorbent material.
[0042] The system and methods of the disclosed subject matter utilize the
adsorptive
properties of the selected sorbent, such as MPFs/ZIFs/Zeolites, or the like,
with respect
to the working fluids such as C02, or the like. A schematic representation of
these
adsorptive relationship is illustrated in Figure 2. Particularly, an increase
in temperature
reduces the amount of CO2 uptake. Further, an increase in pressure reduces the
CO2
uptake.
[0043] For purpose of illustration and not limitation, reference is now made
to
several representative embodiments of the present invention.
[0044] Fig. 3 discloses an uncoated fiber 10 for use in a sorbent system in
accordance with aspects of the present invention. The fiber 10 includes an
adsorbent 11
and a binder 12. In accordance with an aspect of the present invention, the
fiber 10 is
made from an adsorbent 11 and a binder 12 whose capacity and rate of
adsorption and
desorption of working fluid is not affected by the presence of thermal fluid.
With such
an arrangement, the fiber 10 is permeable to both the working fluid and the
thermal fluid
does not wet the fiber surface. Suitable adsorbents are described in greater
detail below.
The binder 12 or binding agent may be an inorganic material (including but not
limited
to clay and silica resin) or a polymeric material (including but not limited
to polyimide,
polyamide, polyvinylalcohol, and cellulosic). Other binder materials are
considered to
be well within the scope of the present invention provided such binder
materials do not
adversely impact the capacity and rate of adsorption and desorption of the
working fluid
on the adsorbent 11.
[0045] In accordance with an aspect of the present invention utilizing a fiber
10, the
sorption system includes a plurality of fibers 10 housed or otherwise
contained within a
vessel (e.g., adsorption beds 110 and 160). The working fluid and the thermal
fluid are
capable of mixing within the vessel. While the present invention is being
described in
connection with the system 1000 illustrated in Fig. 1, the present invention
is not
intended to be so limited; rather, it is contemplated that the fibers 10 may
be utilized in
any sorption system permitting the mixing of the working fluid and the thermal
fluid.
-9-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0046] Fig. 4 discloses a coated fiber 20 for use in a sorbent system in
accordance
with aspects of the present invention. The fiber 20 includes an adsorbent 21,
a binder 22
and an outer coating 23. The outer coating 23 is permeable to the working
fluid, but is
impermeable to the thermal fluid. With such an arrangement, the selection of
the
adsorbent 21 and the binder 22 is not limited to those materials whose
capacity and rate
of adsorption and desorption of working fluid is not affected by the presence
of thermal
fluid.
[0047] The outer coating 23 is preferably an organometallic compound. The
metallo
component of the organometallic compounds is from Groups 4-15 based on the
IUPAC
format for the Periodic Table having Groups 1-18, preferably Group 14,more
preferably
silicon and tin, especially silicon. The organo components of the
organometallic
compounds are hydrocarbyl groups having from 1 to 30 carbon atoms, preferably
from 1
to 20 carbon atoms, more preferably 1-10 carbon atoms. The hydrocarbyl group
may be
aliphatic or aromatic groups which aliphatic or aromatic groups may be
substituted with
functional groups such as oxygen, halogen, hydroxy and the like. Preferred
hydrocarbyl
groups include methyl, ethyl, methoxy, ethoxy and phenyl. Preferred
organometallic
compounds include alkoxysilanes, silanes, silazanes and phenyl siloxanes.
Especially
preferred compounds include alkoxysilanes having from 1 to 4 alkoxy groups,
especially
tetraalkoxy compounds such as tetraethoxy-silane, dialkoxysilanes having from
1 to 6
alkoxy groups, especially hexamethyl-disiloxane.
[0048] The outer coating 23 of the organometallic material on the fiber 20
should
have a high water contact angle, higher than 90 degrees, preferably higher
than 110
degrees. The outer coating 23 may not cover the entire outer surface of the
fiber 20. In
accordance with the present invention, the outer coating 23 should cover from
greater
than 25% of the outer surface of the fiber 20 to 100% of the surface,
preferably from 50
to 100%,more preferably from 80 to 100%. The amount of the outer surface
covered is
most preferably 100% or as close to 100% as possible.
[0049] In accordance with an aspect of the present invention utilizing a fiber
20, the
sorption system includes a plurality of fibers 20 housed or otherwise
contained within a
vessel (e.g., adsorption beds 110 and 160). The working fluid and the thermal
fluid are
capable of mixing within the vessel. The outer coating 23 prevents the thermal
fluid
-10-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
from passing through the fiber 20 into the interior of the fiber 20 to the
adsorbent 21 and
the binder 22. While the present invention is being described in connection
with the
system 1000 illustrated in Fig. 1, the present invention is not intended to be
so limited;
rather, it is contemplated that the fibers 20 may be utilized in any sorption
system
permitting the mixing of the working fluid and the thermal fluid, which
prevents the
passage of the thermal fluid into the fiber 20.
[0050] Fig. 5 discloses a hollow fiber 30 for use in a sorbent system in
accordance
with aspects of the present invention. The hollow fiber 30 includes an
adsorbent 31, a
binder 32, and an inner coating 33. The hollow fiber 30 contains a hollow
interior 34,
which extends the length of the fiber 30. The hollow interior 34 is configured
to permit
the thermal fluid to flow therein. The inner coating 33 separates the hollow
interior 34
from the adsorbent 31 and binder 32. The inner coating 33 is impermeable to
both the
working fluid and the thermal fluid. With such an arrangement, the selection
of the
adsorbent 31 and the binder 32 is not limited to those materials whose
capacity and rate
of adsorption and desorption of working fluid is not affected by the presence
of thermal
fluid. The thermal fluid will not pass from the hollow interior 34 into the
interior of the
fiber 30. The working fluid is adsorbed into the adsorbent through the
exterior of the
fiber 30.
[0051] The inner coating 33 can be, for example, poly(vinyl chloride),
poly(vinylidene chloride), poly(vinyl floride), poly(vinylidene floride),
ethylene vinyl
alcohol copolymer, poly vinyl alcohol, polyamides, polyethylene (preferably
high
density), polypropylene (preferably high density), polyesters, polyimides,
polyacrylonitrile, polysulfone, polyurethane, etc., their combinations and
derivatives
thereof.
[0052] In accordance with the present invention utilizing a fiber 30, the
sorption
system includes a plurality of fibers 30 housed or otherwise contained within
a vessel
(e.g., adsorption beds 110 and 160). The thermal fluid flows through the
hollow interiors
34 of the fibers 30. The thermal fluid provides the necessary heat transfer to
permit the
adsorption and desorption of the working fluid into the adsorbent 31. The
working fluid
is capable of passing from the fiber 30 into the interior of the vessel
without mixing with
the thermal fluid. While the present invention is being described in
connection with the
-11-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
system 1000 illustrated in Fig. 1, the present invention is not intended to be
so limited;
rather, it is contemplated that the fibers 30 may be utilized in any sorption
system, which
prevents the mixing of the working fluid and the thermal fluid.
[0053] Fig. 6 discloses a hollow fiber 40 for use in a sorbent system in
accordance
with aspects of the present invention. The hollow fiber 40 includes an
adsorbent 41, a
binder 42, and an outer coating 43. The hollow fiber 40 contains a hollow
interior 44,
which extends the length of the fiber 40. The hollow interior 44 is configured
to permit
the working fluid to flow therein. The working fluid can pass from the hollow
interior
44 into the adsorbent 41 and binder 42. The outer coating 43 is impermeable to
both the
working fluid and the thermal fluid. With such an arrangement, the selection
of the
adsorbent 41 and the binder 42 is not limited to those materials whose
capacity and rate
of adsorption and desorption of working fluid is not affected by the presence
of thermal
fluid. The thermal fluid will not pass into the fiber 40.
[0054] The outer coating 43 can be, for example, poly(vinyl chloride),
poly(vinylidene chloride), poly(vinyl floride), poly(vinylidene floride),
ethylene vinyl
alcohol copolymer, poly vinyl alcohol, polyamides, polyethylene (preferably
high
density), polypropylene (preferably high density), polyesters, poly imides,
polyacrylonitril, polysulfone, polyurethane, etc. - their combinations and
derivatives
thereof.
[0055] Fig. 7 depicts a representative embodiment of the fiber sorption system
in
which at least one hollow fiber 50 is provided with sorbents contained
therein.
Generally, however, the sorption system includes a plurality of fibers housed
or
otherwise contained within a vessel. In this non-limiting embodiment, the
channel 51 is
adapted to receive steam (heating fluid) and water (cooling fluid). The
channel 51 is
defined by an impermeable inner coating 52, such as polyacrylonitrile (PAN). A
chamber 53 is defined between the inner coating 51 and an outer coating 54 and
is
packed with sorbent particles 55, such as zeolite 13X or mesoporous silica
with adhered
amines. The chamber also includes polymer support materials 56 to assist in
maintaining
the structural integrity of the hollow fiber.
-12-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0056] The hollow fibers 56 can be formed in a tubular configuration and
include an
inner coating 51 and an outer coating 54 defining a chamber 53 there between.
In a
preferred embodiment, the chamber 53 extends along a length which is
coextensive with
the inner and outer coating and contains the sorbent material (e.g., zeolite
13X). This
maximizes the amount of sorbent material which can be disposed within the
chamber.
Preferably, the sorbent material is disposed within the chamber in an uniform
concentration or density along the length of the hollow fiber. The inner
coating defines a
channel or bore within each hollow fiber. The channel extends the entire
length of the
hollow fiber and is adapted to receive a supply fluid for direct contact with
the inner
coating. Depending on the embodiment of the hollow fiber sorption system, as
described
further below, the fluid received within the channel can be either a working
fluid, or a
thermal fluid (e.g., heating/cooling fluid).
[0057] In one embodiment, the inner coating is generally impermeable to a
thermal
fluid, and the outer coating, which is generally permeable to a working fluid,
defines a
chamber between the outer coating and the inner coating. In this
configuration, a supply
of working fluid is introduced to an exterior surface of the outer coating,
and the thermal
fluid (e.g., heating fluid) is introduced within the channel to obtain a
pressurized working
fluid from the sorbent material. Alternatively, the inner coating can be
generally
permeable to a working fluid, and the outer coating can be generally
impermeable to a
thermal fluid. In this configuration, a supply of working fluid is introduced
within the
inner channel of the fiber, and the thermal fluid (e.g., heating fluid) is
introduced to the
exterior surface of the chamber to obtain a pressurized working fluid from the
sorbent
material.
[0058] In an exemplary embodiment, the hollow fibers of approximately 100
micron
inner diameter, and 100 micron chamber thickness. This configuration allows
for dense
packing of sorbents within the sorption bed. Fibers of this scale are
advantageous in that
the temperature of the sorption bed can be altered from hot to cold within
seconds.
Further, such a frequency of temperature swing allows for the size and
footprint of the
sorption system to be minimized. The channel and the chamber of each hollow
fiber
preferably circular in cross-section and oriented with a concentric
configuration. For
-13-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
example, the channel is substantially circular and from about 50 microns to
about 400
microns in diameter. Additionally, the linear chamber thickness can be from
about 50 to
about 400 microns.
[0059] In accordance with another aspect of the disclosed subject matter, a
plurality
of fibers can be arranged in a bundle similar to a shell and tube heat
exchanger. The
plurality of fibers can be aligned in a generally parallel arrangement.
Alternatively, the
plurality of fibers can be oriented at an angle with respect to each other.
The fibers can
be disposed with portions of adjacent fibers in contact with each other, or
provided with
a uniform space disposed therebetween over the entire length of the fibers. In
an
exemplary embodiment, with the outer surface posing no resistance to a working
fluid
and an inner coating impermeable to a thermal and working fluids, the shell
side can be
in communication with a working fluid (e.g., CO2) and the bore side can be in
communication with heating medium (e.g., steam) or cooling medium.
[0060] In a preferred embodiment, waste heat (e.g., low grade waste heat) is
used as
a heating fluid to drive the sorption system. In some applications of the
disclosed subject
matter, the heating is provided by waste heat from a chemical processing or
petrochemical refining operation. In one embodiment, the unutilized heat
ranges from
about 343K to about 573K, or more preferably from about 363K to about 523K.
[0061] While the working fluid is, for purposes of simplicity, largely
described in the
context of CO2, other working fluids can be employed. In one embodiment, the
working
fluid is a gas and is selected from carbon dioxide, methane, ethane, propane,
butane,
ammonia, chlorofluorocarbons (e.g., FreonTM), other refrigerants, or other
suitable fluids.
Similarly, the sorbent material is largely described in the context of zeolite
13X, but is
not limited thereto. In one embodiment, the sorbent material is selected from
zeolites,
silicagel, carbon, activated carbon, metal organic frameworks (MOFs), and
zeolitic
imidazolate frameworks (ZIFs). In one embodiment the working fluid is carbon
dioxide
and/or the sorbent material is a zeolite. In one embodiment the working fluid
is carbon
dioxide and the zeolite is a zeolite X, preferably a zeolite 13X.
-14-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
Sorbent Materials
[0062] As noted above, and as used in this application, the term "sorbent
material"
refers to a material that reversibly binds the working fluid, in a chemical or
physical
sense. Sorbent materials include adsorbents.
[0063] Sorbent materials that can be used in embodiments of the disclosed
subject
matter include, but are not limited to, metal-organic framework-based (MOF-
based)
sorbents, zeolitic imidazole framework (ZIF) sorbent materials, zeolites and
carbon.
[0064] MOF-based sorbents include, but are not limited to, MOF-based sorbents
with a plurality of metal, metal oxide, metal cluster or metal oxide cluster
building units.
As disclosed in International Published Application No. WO 2007/111738, which
is
hereby incorporated by reference in its entirety, the metal can be selected
from the
transition metals in the periodic table, and beryllium. Exemplary metals
include zinc
(Zn), cadmium (Cd), mercury (Hg), and beryllium (Be). The metal building units
can be
linked by organic compounds to form a porous structure, where the organic
compounds
for linking the adjacent metal building units can include 1,3,5-
benzenetribenzoate
(BTB); 1,4-benzenedicarboxylate (BDC); cyclobutyl 1,4-benzenedicarboxylate (CB
BDC); 2-amino 1,4 benzenedicarboxylate (H2N BDC); tetrahydropyrene 2,7-
dicarboxylate (HPDC); terphenyl dicarboxylate (TPDC); 2,6 naphthalene
dicarboxylate
(2,6-NDC); pyrene 2,7-dicarboxylate (PDC); biphenyl dicarboxylate (BDC); or
any
dicarboxylate having phenyl compounds.
[0065] Specific materials MOF-based sorbent materials include: MOF-177, a
material having a general formula of Zn4O(1, 3, 5-benzenetribenzoate)2; MOF-5,
also
known as IRMOF-I, a material having a general formula of Zn40(1,4-
benzenedicarboxylate)3; IRMOF-6, a material having a general formula of
Zn40(cyclobutyl 1,4-benzenedicarboxylate); IRMOF-3, a material having a
general
formula of Zn4O(2-amino 1,4 benzenedicarboxylate)3; and IRMOF-11, a material
having
a general formula of Zn40(terphenyl dicarboxylate)3 or Zn40(tetrahydropyrene
2,7-
dicarboxylate)3; and IRMOF-8, a material having a general formula of Zn4O(2,6
naphthalene dicarboxylate)3.
-15-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0066] Exemplary zeolitic imidazole framework (ZIF) sorbent materials include,
but
are not limited to, ZIF-68, ZIF-60, ZIF-70, ZIF-95, ZIF-100 developed at the
University
of California at Los Angeles and generally discussed in Nature 453, 207-211 (8
May
2008), hereby incorporated by reference in its entirety.
[0067] Zeolite adsorbent materials include, but are not limited to,
aluminosilicates
that are represented by the formula M2/õO'Al2O3'ySiO2'wH2O, where y is 2 or
greater, M
is the charge balancing cation, such as sodium, potassium, magnesium and
calcium, N is
the cation valence, and w represents the moles of water contained in the
zeolitic voids.
Examples of zeolites that can be included in the methods and systems of the
present
application include natural and synthetic zeolites.
[0068] Natural zeolites include, but are not limited to, chabazite (CAS
Registry No.
12251-32-0; typical formula Ca2[(AlO2)4(SiO2)8]'13H2O), mordenite (CAS
Registry No.
12173-98-7; typical formula Na8[(AlO2)8(SiO2)40]24H2O), erionite (CAS Registry
No.
12150-42-8; typical formula (Ca, Mg, Nat, K2)4.5[(AlO2)9(SiO2)27]27H2O),
faujasite
(CAS Registry No. 12173-28-3, typical formula (Ca, Mg, Nat,
K2)29.5[(A102)59(SiO2)133]235H20), clinoptilolite (CAS Registry No. 12321-85-
6, typical
formula Na6[(A102)6(SiO2)30]24H20) and phillipsite (typical formula: (0.5Ca,
Na,
K)3 [(A1O2)3(SiO2)5]-6H2O).
[0069] Synthetic zeolites include, but are not limited to, zeolite A (typical
formula:
Na12[(A102)12(SiO2)12]27H20), zeolite X (CAS Registry No. 68989-23-1; typical
formula: Na86[AlO2)86(SiO2)106]264H2O), zeolite Y (typical formula:
Na56[(A1O2)56(SiO2)136]250H2O), zeolite L (typical formula:
K9[(AlO2)9(SiO2)27]22H2O), zeolite omega (typical formula:
Na6.8TMA1.6[A1O2)8(SiO2)28].21H2O, where TMA is tetramethylammonium) and ZSM-5
(typical formula: (Na, TPA)3[(AlO2)3(SiO2)93]-16H2O, where TPA is
tetrapropylammonium).
[0070] Zeolites that can be used in the embodiments of the present application
also
include the zeolites disclosed in the Encyclopedia of Chemical Technology by
Kirk-
Othmer, Volume 16, Fourth Edition, under the heading "Molecular Sieves," which
is
hereby incorporated by reference in its entirety.
-16-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
[0071] Synthetic zeolite sorbent materials are commercially available, such as
under
the Sylosiv brand from W.R. Grace and Co. (Columbia, Md.) and from Chengdu
Beyond Chemical (Sichuan, P.R. China). For example, Sylosiv A10 is one
commercially available zeolite 13 X product.
Uses of the Fiber Sorbent Systems of the Present Application
[0072] The adsorbent systems of the present application can be used in various
applications provided the setting allows for the presence of a vessel that
contains a
sorbent material, a supply of working fluid, a heat supply and means to
effectively direct
the desorbed working fluid to an expansion device to provide refrigeration or
a driver
device to provide electricity or work. For example, the desorbed gas may be
directed to
a Joule-Thompson expansion valve, to provide refrigeration. Alternatively, the
desorbed
working fluid can be directed to a turbine to provide electricity or to a
shaft to provide
work. The sorption systems described herein may be used to provide chilling,
power and
chilling in combination with power.
[0073] Possible applications for sorption systems of the present application
include
residential (for generating air conditioning in the summer and a heat pump in
the winter),
vehicular (where the on-board air conditioning utilizes exhaust heat) and
industrial
(refining and chemical plants).
[0074] In a preferred embodiment of the present application, the adsorbent
system is
used within a chemical or petrochemical refining plant, and the desorbed
working fluid is
used to provide refrigeration to aid in other process areas, particularly
areas that rely on
temperature differences to separate components of a mixture. For example, the
refrigeration can be used to recover liquefied petroleum gas (LPG, C3+) from
flue gases
going up a stack, or the refrigeration can be used to operate condensers to
improve the
effectiveness of vacuum distillation columns, particularly in the summer
months.
[0075] By proper selection of the adsorbent and working fluid, the sorbent
system
can make effective use of lower grade heat than previously provided by
sorption systems
in the prior art. For example, in one embodiment of the present application,
the heat
supply is "unutilized heat" which has a temperature of from about 70 C to
about 300 C,
more preferably from about 90 C to about 250 C. In accordance with the
present
-17-
CA 02795060 2012-09-28
WO 2011/123600 PCT/US2011/030664
invention, it is contemplated that the adsorbent and working fluid may be
selected
utilizing the pressure index disclosed in US Patent Application No. 12/603,243
entitled
"System Using Unutilized Heat For Cooling and/or Power Generation". The
disclosure
of which is hereby incorporated in its entirety. By proper selection of
thermal fluid and
coating material the negative effect of capillary action should be kept
minimal. By using
appropriate surfactant and additives in thermal fluid / coating material to
reduce
interfacial tension between the thermal fluid and the coating, e.g., for
water, detergent
and the like and for triethylene glycol, stearic acid and the like.
[0076] This representative embodiment is provided for exemplary purposes;
neither
the application nor the invention is limited to the specific embodiments
discussed above,
or elsewhere in the application.
[0077] The disclosed subject matter is not to be limited in scope by the
specific
embodiments described herein. Indeed, various modifications of the invention
in
addition to those described herein will become apparent to those skilled in
the art from
the foregoing description and the accompanying Figures. Such modifications are
intended to fall within the scope of the appended claims.
[0078] It is further to be understood that all values are approximate, and are
provided
for description.
[0079] Patents, patent applications, publications, product descriptions, and
protocols
are cited throughout this application, the disclosures of each of which is
incorporated
herein by reference in its entirety for all purposes.
-18-