Note: Descriptions are shown in the official language in which they were submitted.
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
TITLE
BIODEGRADATION OF RENEWABLE HYDROCARBON FUEL BLENDS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is related to and claims the benefit of priority of U.S.
Provisional Application No. 61/347,127, filed May 21, 2010, the entirety of
which
is herein incorporated by reference.
FIELD OF THE INVENTION
The invention relates to the field of renewable fuel compositions and the
fate of said compositions in the environment.-
BA BACKGROUND OF THE INVENTION
lsobutanol is attractive as a biofuel molecule suitable for use in gasoline
because it can be produced from renewable feedstocks and has many properties
that potentially make it a more attractive fuel additive than ethanol; it has
a
greater energy density, lower water absorption, better blending ability, and
it can
be used in conventional combustion engines without modification (Durre, 2007õ
Biotech. J. 2:1525-1578). Evaluation of the effect of ethanol on the
environmental fate of gasoline components has demonstrated that in aerobic
systems fuel ethanol is preferentially biodegraded before the benzene,
toluene,
ethylb n ene, and x lene (BTEX) components of gasoline, and that ethanol
degradation in aquifers rapidly consumed dissolved oxygen and other nutrients
(ors uil at al,, 1998, W at. Res. 32-12065-2072; Da Silva and Alvarez, 2002,
Appl. Environ. Microbiol. 70:4720-4726; Capiro et al., 2007, Water Research,
41:
656-664). As a result, the presence of ethanol in gasoline could result in a
lag in
BTEX degradation and ultimately result in enhanced BTEX Plumes (Pourers et
al., 2001, Environ. Sci. Technol. 35: : 24A-30A.). Model simulations by Deeb
et al
(2002, J. Environ. Engin. 128, 868-875.) predicted a 16-34% increase in
benzene
plume lengths in the presence of ethanol, while others have suggested that the
lifespan of benzene plumes may decrease with greater ethanol concentration in
I
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
gasoline if greater microbial biomass is produced by growth on ethanol (Gomez
and Alvarez, 2009, Water Resour. Res, 45).
A study by Mariano and colleagues (2009; Biomass and Bioenergy, 33: 1175-
1131.) used indirect measurements ( ' CO2 evolution and dye reduction) to
evaluate the affect of n-butanol on gasoline biodegradation in aerobic
microcosms containing uncontaminatedsoil, river water, or a combination of
uncontaminated soil and river water. Results of the study suggested that n-
butanol may enhance gasoline degradation in soil, and that the enhancement
factor may be greater than that achieved with ethanol. Results obtained by
Garcia-RÃvero et al, (2097:. J. Environ. Eng. Sci. 6, 339.395.) also suggest
that
addition of n-butanol to hydrocarbon mixtures may enhance the rate of
hydrocarbon aerobic biodegradation, Studies performed in the 1970s used
biological oxygen demand (BOD)-based measurements to evaluate isobutanol
biodegradation (Price et al 1974, J. Water Pollut. Contr. Fed. 46, 63-77, Dias
and
I Alexander, 1971, Appl. licrobiol. 22, 1114--1118), and several studies
(c.f,, Deeb
et al., 2000, Biodegradation, 11171-135; Pruden and Suidan, 2004,
Biodegradation, 15,213-227 Somsamak et at, 2005, Environ. ei. Technol. 39,
103-109; Vainberg et at, 2002, J. Environ. Erg. 128: 842-851) have evaluated
the biodegradation of tert-butyl alcohol (TBA) which is the primary
biodegradation
product of the gasoline oxygenate methyl tert-butyl ether (MTBE) (Hat finger
et
al., 2001, A pl, Environ Microbiol. 67, 5601-5607, Steffan et al., 1997, Appl.
Environ. Microbial. 63:4216-4222).
There is a need to increase renewable components of gasoline and/or
transport fuel compositions without a resulting adverse environmental effect
associated with the environmental fate of said compositions in circumstances
of
environmental contamination..
BRIEF SUMMARY OF THE INVENTION
2
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
The invention provides methods and compositions for improving the
environmental fate of hydrocarbon fuel compositions under circumstances of
environmental release while increasing the renewability of said fuel
compositions.
An aspect of the invention is a method for improving the environmental
fate of hydrocarbon fuel compositions by the inclusion of isobutanol to said
compositions resulting in improved biodegradability of one or more BTEX
compounds of the gasoline. In one aspect, the methods and compositions
provide improved biodegradation under anaerobic conditions. In one aspect, the
methods and compositions provide improved biodegradation under aerobic
tU conditions. In one aspect, the methods and compositions provide improved
biodegradation under nitrate reducing conditions. In one aspect, the inclusion
of
isobutanol in hydrocarbon fuel compositions improves the biodegradation of
benzene.
Another aspect of the invention is a method for improving the
environmental fate of liquid fuel compositions comprising ethanol by the
addition
of isobutanol to said compositions resulting in improved biodegradation of one
or
more BTEX compounds of the gasoline.
Another aspect of the invention is a method to reduce the transport of
ethanol in a soil matrix when said ethanol is a component of a hydrocarbon
fuel
2 composition released into an environmental compartment (e.g. soil,
sediments,
groundwater), said method comprising combining isobutanol with the fuel
composition.
Another aspect of the invention is a method of reducing a BTEX plume
caused by release of a, hydrocarbon composition optionally comprising ethanol
into an environmental compartment, said method comprising adding a suitable
amount of isobutanol wherein said isobutanol acts as a cosolvent for the
hydrocarbon and ethanol components of the hydrocarbon composition, thereby
retarding and/or partially containing the BTEX plume and reducing the
potential
for its leakage into a water table.
Another aspect of the invention is directed to liquid fuel compositions
comprising hydrocarbons, ethanol and isobutanol in an amount sufficient to
3
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
improve the renewability of the hydrocarbon composition without increasing
potential environmental impact of said composition if it were to contaminate
an
environmental compartment, in another aspect the renewability of the fuel
composition is increased and the potential environmental impact is decreased
by
the inclusion of isobutanol, for example. BTEX plume expansion may be
decreased as compared to a BTEX plume expansion of the same composition
without the isobutanol present, particularly under aerobic environmental
conditions.
Also provided herein are methods for improving the environmental fate of
tU a hydrocarbon fuel composition comprising isobutanol in an environmental
compartment under anaerobic conditions, the methods comprising. adding an
electron acceptor to said compartment in an amount sufficient to increase the
rate of biodegradation of one or more BTEX components.
Provided herein are methods for increasing the renewability of a
5 hydrocarbon fuel composition and limiting the impact on an environmental
compartment upon contamination by said hydrocarbon fuel composition,
comprising combining said hydrocarbon fuel composition with a suitable amount
of isobutanol. In embodiments, the hydrocarbon fuel composition further
comprises ethanol. I n embodiments, the ethanol comprises up to about 10% of
2 the fuel composition prior to addition of isobutanol.. In embodiments, the
isobutanol provides for improved biodegradation of at least one of the BTEX
components of the hydrocarbon fuel composition, In embodiments, the
isobutanol provides for improved biodegradation of benzene, In embodiments,
the environmental compartment includes a soil matrix and wherein the
isobutanol
25 reduces the transport of ethanol in a soil matrix. In embodiments, the
isobutanol
impedes expansion of BTEX plume from said composition, In embodiments,
the isobutanol is present in an amount amount suitable for increasing the
biodegradability of the hydrocarbon fuel composition. In embodiments, the
improved biodegradation occurs under aerobic conditions. In embodiments, the
30 improved biodegradation occurs under nitrate-reducing or sulfate-reducing:
conditions.
4
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Also provided are methods of improving the environmental fate of a hydrocarbon
fuel composition comprising isobutanol in an environmental compartment under
anaerobic conditions comprising adding an electron acceptor to said
compartment in an amount sufficient to increase the rate of biodegradation of
one or more BTEX components. In embodiments, the electron acceptor is iron,
sulfate, or nitrate, or a combination thereof. In embodiments, the electron
acceptor is Fe(OH). In embodiments, the electron acceptor is NaNO3. In
embodiments, the electron acceptor is MgS Ã, In embodiments, the one or more
BIEX components comprise toluene, xylene, or benzene. In embodiments, the
IU electron acceptor is nitrate and is added in an amount sufficient to create
nitrate-
reducing conditions. In embodiments, the electron acceptor is sulfate and is
present in an amount sufficient to create sulfate-reducing conditions, In
embodiments, the electron acceptor is nitrate and is present in an amount
sufficient to create nitrate-reducing conditions and wherein toluene
biodegrades
in about the same number of days as isobutanol, In embodiments, the electron
acceptor is sulfate and is present in an amount sufficient to create sulfate-
reducing conditions and wherein toluene biodegrades in about the same number
of days as isobutanol. In embodiments, the electron acceptor is nitrate and is
present in an amount sufficient to create nitrate-reducing conditions and
wherein
2E benzene biodegrades in about the same number of clays as isobutanol. In
embodiments, the electron acceptor is sulfate and is present in an amount
sufficient to create sulfate-reducing conditions and wherein benzene
biodegradation is improved as compared to its biodegradation without sulfate-
reducing conditions. In embodiments, the electron acceptor is sulfate and is
present in an amount sufficient to create sulfate-reducing conditions and
wherein
benzene biodegradation is improved as compared to its biodegradation in the
absence of isobutanol. In embodiments, the electron acceptor is sulfate and is
present in an amount sufficient to create sulfate-reducing conditions and
wherein
benzene biodegradation is improved as compared to its biodegradation in the
presence of ethanol,
5
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Also provided herein are compositions comprising gasoline; isobutanol and at
least one of Fe(OH)3, NaNO3, or Mg 04 Also provided are compositions
comprising gasoline, isobutanol and at least one of Fe(OH)3, Na: O3, KN0 ,,
I HNO Na2S04, Ca S04. MgS04. chelated iron, zero-valent iron, and nano zero-
valent iron
BRIEF DESCRIPTION OF THE DRAWINGS/FIGURES
Figure 1 a shows isobutanol degradation up to 10 days. Error bars
represent 95% confidence intervals..
tU Figure 1 b shows isobutanol degradation up to 50 days.
Figure I c shows ethanol degradation up to 50 days.
Figures 2A, B, C, and D show the impact of isobutanol on biodegradation
of BTEX at high concentrations. (Figure 2A shows benzene,,. 26 shows toluene:
2C shows ethyl benzene; 2D shows total xylenes.) Error bars represent 95%
5 confidence intervals.
Figures 3A, B, C and D compare the impact of isobutanol and ethanol
concentration on BTEX biodegradation at various treatment levels. (Figure 3A
shows benzene; 3B shows toluene; 3C shows ethylbenzene; 3D shows total
xylenes.) Error bars represent 95% confidence intervals.
. Figures 4A, B. C. and D show the impact of isobutanol on biodegradation
of BTEX at lower concentrations. (Figure 4A shows benzene; 4B shows toluene;
4C shows ethylbenzene; 4D shows total xylenes.)Error bars represent 95%
confidence intervals.
Figures 5k, B, C, and D show isobutanol biodegradation - higher
25 concentration -- under various anaerobic reducing conditions. (Figure 5A
shows
Treatment 2-Unaendd, bB shows Treatment 6-Nitrate Reducing; 5C shows
Treatment 9-iron reducing; 5D shows Treatment 12-Sulfate Reducing,)Error bars
represent 95% confidence intervals. The dashed line for Treatment 2 indicates
the time when the microcosms were re-amended with isobutanol.
30 Figures 6A and 6B show ethanol biodegradation in Treatment 4
(Unarnended) and Treatment 14 (Sulfate reducing), respectively. Error bars
6
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
represent 95% confidence intervals. The dashed line for Treatment 4 indicates
the time when the microcosms were re-amended with ethanol after the residual
sulfate in the groundwater was reduced.
Figures 7A, B, C, and D show benzene and toluene biodegradation
(higher concentration) under various anaerobic reducing conditions and in the
presence or absence of isobutanol (IBA). (A and B show benzene and toluene,
respectively, for Treatments 1, 2, 5, and C and 0 show benzene and toluene,
respectively, for Treatments 8, 9, 11, and 12.) Error bars represent 95%
confidence intervals.
Figures 8A, B, C, and D show BTEX biodegradation - lower concentration
under various anaerobic reducing conditions and in the presence and absence
of isobutanol (IBA) or ethanol, (Figure BA shows benzene; 8B shows toluene; 8C
shows ethylben ene~ 8D shows total xylenes.)Erro bars represent 95%
confidence intervals,
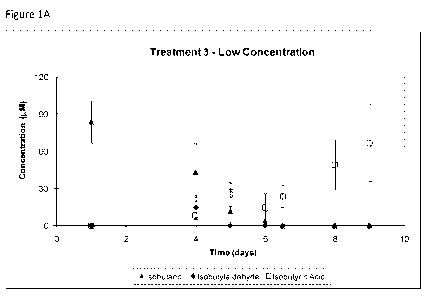
Figures 9 A, B, C, and D show isobutanol biodegradation - lower
concentration. (A shows Treatment 3--Unamended; shows Treatment 7-Nitrate
Reducing; C shows Treatment 10-Iron Reducing; 0 shows Treatment 13-Sulfate
Reducing.) Error bars represent 95% confidence intervals. Supplemental
monitoring showed that the isobutyric acid concentrations had decreased below
2E the analytical detection limit by day 160 for Treatment 3 and by day 48 for
Treatment 13 (data not shown).
Figures 1WA, B, C, and Q show high concentration ethylbenzene and total
xylenes biodegradation under various anaerobic reducing conditions and in the
presence or absence of isobutanol (IBA). (A and B show ethylbenzene and total
25 xylenes, respectively, for Treatments 1, 2, 5, and 6; C and 0 show ethylben
ene
and total xylenes, respectively, for Treatments 8, 9, 11, and 12.) Error bars
represent 95% confidence intervals.
Figures 11 and 12 demonstrate the behavior of isobutanol when 1.3% and
2.6% water are initially added to an E10 gasoline. In Figure 11, increased
levels
30 of isobutanol result in reduced aqueous phase volume during phase
separation.
7
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Figure 12 shows there is less ethanol in the aqueous portion when the
separation
does occur.
Figure 13 depicts the results from microbial analyses,. Error bars
represent 95% confidence intervals. IBA = isobutanol,
DETAILED DESCRIPTION OF THE INVENTION
Unless defined otherwise, alà technical and scientific terms used herein
have the same meaning as commonly understood by one of ordinary skill in the
art to which this invention belongs. In case of conflict, the present
application
including the definitions will control. Also, unless otherwise required by
context,
singular terms shall include pluralities and plural terms shall include the
singular,
All publications, patents and other references mentioned herein are
incorporated
by reference in their entireties for all purposes.
In order to further define this invention, the following terms and definitions
are herein provided
As used herein, qs "comprises," ~y e
y~ {gp," "s," "aVtlin{,g," "contains" ~ynta terms ":containin ,"comprising," "
or any other "includes," variation
'Including,"
thereof, will be understood to imply the inclusion of a stated integer or
group of
integers but not the exclusion of any other integer or group of integers.. For
example, a composition, a mixture, a process, a method, an article, or an
apparatus that comprises a list of elements is not necessarily limited to only
those elements but may include other elements not expressly listed or inherent
to
such composition, mixture, process, method, article, or apparatus. Further,
unless expressly stated to the contrary, "or" refers to an inclusive or and
not to an
exclusive or. For example, a condition A or B Is satisfied by any one of the
following: A is true (or present) and B is false (or not present), A is false
(or not
present) and B is true (or present), and both A and B are true (or present).
As used herein, the term "consists of," or variations such as "consist of or
"consisting of," as used throughout the specification and claims, indicate the
inclusion of any recited integer or group of integers, but that no additional
integer
or group of integers may be added to the specified method, structure, or
composition.
8
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
As used herein, the term "consists essentially of," or variations such as
..consist essentially of or '"consisting essentially of," as used throughout
the
specification and claims, indicate the inclusion of any recited integer or
group of
integers, and the optional inclusion of any recited integer or group of
integers that
do not materially change the basic or novel properties of the specified
method,
structure or composition. See M.P.E.P. :111.03..
Also, the indefinite articles "a" and "an" preceding an element or
component of the invention are intended to be nonrestrictive regarding the
number of instances, i.e., occurrences of the element or component. Therefore
tU "a" or "an" should be read to include one or at least one, and the singular
word
form of the element or component also includes the plural unless the number is
obviously meant to be singular.
The term "invention" or "present invention" as used herein is a non-limiting
term and is not intended to refer to any single embodiment of the particular
invention but encompasses all possible embodiments as described in the
application
As used herein, the term "about" modifying the quantity of an ingredient or
reactant of the invention employed refers to variation in the numerical
quantity
that can occur, for example, through typical measuring and liquid handling
2 procedures used for making concentrate or solutions in the real world,
through
inadvertent error in these procedures; through differences in the manufacture,
source, or purity of the ingredients employed to make the compositions or to
carry out the methods; and the like. The term "about" also encompasses
amounts that differ due to different equilibrium conditions for a composition
resulting from a particular initial mixture. Whether or not modified by the
term
"about'", the claims include equivalents to the quantities, In one embodiment,
the
term "about" means within 10% of the reported numerical value, preferably
within
5% of the reported numerical value.
As described herein, hydrocarbon fuel compositions comprising isobutanol
provide fuel compositions with increased renewability attributable to a
renewable
component that is also biodegradable in the environment. Isobutanol is a
9
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
renewable component which, as shown herein is degraded rapidly when added
to aquifer microcosms. Further, results presented herein demonstrate that not
only is isobutanol 'itself biodegraded, but isobutanol also provides
additional
biodegradation benefits to gasoline under various environmental conditions. As
has been described in the art, isobutanol may be biologically produced by
microorganisms which convert carbon substrates derived from renewable
feedstocks such as biomass into isobutanol. Thus, biologically-produced
isobutanol, when added to fuel compositions provides a valuable mechanism for
introducing renewable components to fuel and, at the same time, provides for
reduced environmental impact of the fuel composition if it were to contaminate
a
given environmental area.
It is well known that the benzene, toluene, ethylbenene, and total xylenes
(BTEX) components of gasoline are undesirable environmental contaminants.
Adding. ethanol to gasoline can increase the renewability of the gasoline but
this
addition may also potentiate the damage resulting from BTEX as ethanol may
result in expansion of BTEX plumes. In contrast, adding isobutanol to gasoline
provides for a mechanism. of increasing renewable component of fuels without
increasing a BTEX plume as compared to adding ethanol as the additive should
the fuel blend be released an environmental area or compartment (e.g, soil,
2E sediment groundwater) Further, as shown herein, the addition of isobutanol
does not impede BTEX biodegradation to the extent as is observed for ethanol
under certain conditions.
"Biodegradation" or "degradation", as used herein, refers to primary
transformation of the compound of interest to a byproduct.
25, "Improving environmental fate" as used herein means reducing the
amount of one: or more components of a hydrocarbon fuel blend in an
environmental compartment, increasing the degradation rate of one or more
components of a hydrocarbon fuel blend in an environmental compartment,
decreasing the size of the environmental compartment contacted by one or more
0 components of a hydrocarbon fuel blend, or a combination thereof.
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
"Environmental compartment" as used herein refers to the area contacted
by a fuel composition and may include, for example, soil, sediment,
groundwater,
or a combination thereof.
"BTEX plume" as used herein refers to a dissolved prase plume,
When used in reference to fuel compositions, "increased renewability"
means an increased portion of the composition was produced from resources
considered to be renewable, such as biomass, as opposed to resources that are
not renewable such as fossil fuels and petroleum.
Provided herein are methods for simultaneously increasing the amount of
IU renewable components in a fuel composition while reducing the environmental
impact of a fuel composition by including isobutanol in the fuel composition.
The
hydrocarbon portion of fuel compositions suitable for the methods disclosed
herein comprise gasoline blend stocks useable for making gaolines for
consumption in internal combustion engines, including but not limited to spark
ignition engines. Gasoline blend stocks include, but are not limited to, blend
stocks for gasolines meeting A. T 4814; EU specification EN228, and blend
stocks for reformulated gasoline. Amounts of isobutanol in hydrocarbon fuel
compositions (by volume) for the methods disclosed herein include amounts of
at
least about 2%, at least about 5%, at least at least about 7%, at least about
10%,
2E or at least about 15%. In some aspects, amounts of isobutanol (by volume)
include amount~s fiord about 2% to about 20%, and amounts from about 10% to
about 16%. It will be appreciated that the amount of isobutanol may be a
function
of the vehicle technology. As such, in embodiments, isobutanol amounts can be
up to about 85% by volume. The isobutanol can be combined with the
hydrocarbon portion of the fuel composition using any methods known in the
art..
In some embodiments, the hydrocarbon fuel composition further
comprises ethanol, and in some embodiments, the ethanol comprises up to
about 10%, up to about 15% up to about 20%, or up to about 50% of the
hydrocarbon composition prior to the addition of isobutanol. In some
0 embodiments, isobutanol is substituted for ethanol in a fuel composition. In
other
11
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
embodiments, isobutarnol is added to a fuel composition which comprises
ethanol.
Methods provided herein include a method of retardation of BTEX plume
expansion from a hydrocarbon fuel composition by including isobutanol in said
composition. The potential environmental impact of a hydrocarbon fuel
composition should a release occur can be assessed by measuring, the size of
BTEX plume in the impacted area, and/or the rate of expansion, and/or the
concentration of the BTEX plume. The size and rate of expansion of the BTEX
plume can be assessed by methods known in the art such as direct sampling of
tU groundwater and methods such as U.S. Environmental Protection Agency (EPA)
method SW84
In some embodiments, degradation of a fuel composition comprising
isobutanol occurs faster than a fuel composition comprising ethanol but no
Ãsobutanol. Degradation of fuel components can be measured in environmental
samples using methods known in the art such as gas chromatography (GC), for
example with U. S. EPA method 8015, or GC-mass spectrometry, for example
with U.S. EPA method 8260. As shown herein, isobutanol degrades at least as
fast as ethanol and, one or more fTE components degrade faster in the
presence of isobutanol than in the presence of ethanol. In some embodiments,
2( isobutanol is degraded with a first order rate constant of at least about
0.081 d-1.
In some embodiments, isobutanol is degraded with a first order rate constant
of
at least about 0.28 d '. In some embodiments, isobutanol is degraded with a
first order rate constant of greater than about 8.874 d "1. In some cases,
fuel
compositions comprising isobutanol have increased rates of BTEX
25 biodegradation as compared to fuel compositions comprising no renewable
component. In some cases, fuel compositions comprising isobutanol have
increased rates of BTEX biodegradation as compared to fuel compositions
comprising ethanol but no isobutanoÃ.
Furthermore, as shown herein (see Figures 11 and 12), incorporating:
0 butanol in gasoline containing ethanol reduces the volume of an aqueous
phase
when phase separation occurs, and limits the weight percent of ethanol
12
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
contained within the aqueous phase. While not wishing to be bound by theory,
it
is believed that butanol may limit the amount of ethanol that leaches into
groundwater by maintaining it in the less water soluble hydrocarbon fraction.
The
reduced transport of ethanol in the soil matrix may thereby retard the
expansion
of BTEX into the water table.
Thus, it will be appreciated, that methods provided herein can
advantageously decrease the time needed to remediate a contaminated site, can
limit the size of BTEX plumes, or can provide both advantages, therefore
improving the environmental fate of a hydrocarbon fuel composition.
tU Methods provided herein can improve the environmental fate of a
hydrocarbon fuel composition comprising isobutanol in an environmental
compartment under anaerobic conditions, In embodiments, the methods
comprise adding an electron acceptor to said compartment in an amount
sufficient to increase the rate of biodegradation of one or more BTEX
components.. Suitable electron acceptors include nitrates, including, but not
limited to NaNO3,, NH4N 3; KN ;, and sulfates, including but not limited to
MgSC4 and aSO4,, Na2SO4, and iron compounds including, but not limited to
Fe(OH)3, chelated iron, zero-alert iron, and nano zero-valent iron. Electron
acceptors may be added to an environmental compartment using methods
including, but not limited to, injection as a slurry, emplacement, injection
through
a monitoring well, gravity injection, and/or pressurized injection, or
combinations
thereof, in amounts sufficient to achieve nitrate-reducing, iron reducing,
and/or
sulfate reducing conditions. The injection of sulfates and/or nitrates and/or
iron
compounds may be used to biostimulate sulfate reducing and/or nitrate reducing
bacteria, if present, to biodegrade BTEX contamination due the release of
isobutanol containing gasoline underground. Such biostimulation may result in
increased bloactivity, population, and or metabolism of the bacteria.
Aerobic Conditions
3t) Comparison of isobutanol and ethanol biodegradation rates using a first-
order approximation shows that some isobutanol treatments resulted in an
13
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
observed first-order rate constant of 0.081 0.0044 d-1 (R2 = 0.80, using
data
from both Treatment 4 and Treatment 5, Example 1), while ethanol treatment
resulted in an observed first-order rate constant of 0.074 0.0023 d"' (R2 =
0.90).
Thus:, isobutanol was biodegraded at a rate greater than that of ethanol in
the
higher concentration treatments. A. rate constant of 0,28 0,054 d"' (R 2 =
0.69)
was attained for isobutanol in the lower concentration treatment (Treatment
3),
which is approximately 3.5 times greater than the biodegradation rate constant
for either isobutanol or ethanol in the higher concentration treatments. The
differences were not due to differences in bacterial growth atone. While not
tU wishing to be bound by theory, it is believed that one explanation for this
discrepancy is that isobutanol biodegradation (and growth of isobutanol -
degrading bacteria) were nutrient limited over the first two weeks of the
study
when most of the isobutanol biodegradation occurred. Nutrient limitations may
not have been as severe for the lower concentration isobutanol treatment,
15 resulting in an increased rate constant for the lower isobutanol treatment.
Alternatively, the discrepancy in isobutanol rate constants may be due to
isobutanol substrate inhibition at elevated concentrations. Substrate
inhibition
was observed during studies of n-butanol biodegradation at concentrations as
low as approximately 3 000 pM (Alagappan and Cowan, 2001, Biotechnol and
2 Boengin, 75: 393-405)_
Results are presented herein showing that ethanol undesireably reduces
the rate of BTE : biodegradation more than isobutanol. Interestingly, benzene
exhibited the most pronounced difference when comparing the effects of
isobutanol and ethanol on BTEX biodegradation, as benzene aerobic
25 biodegradation was approximately 12-times more stowed in the presence of
ethanol than in the presence of isobutanol. Benzene often is the regulatory
driver from groundwater contamination at gasoline-contaminated sites.
Anaerobic Conditions
30 Results presented herein demonstrate that isobutanol is readily
biodegraded under nitrate-reducing,. iron-reducing, sulfate-reducing, and
14
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
methanogenic condition. For the conditions of this study, isobutanol either
did
not slow BTEX biodegradation, or the extent to which isobutanol lowers the
rate
of BTEX biodegradation was less than to the extent to which ethanol slowed
BTEX biodegradation. In some cases, addition of isobutanol enhanced the
observed rates of BTEX biodegradation. Thus, the persistence of isobutanol and
its impact on BTEX biodegradation under anaerobic aquifer conditions are
considered more favorable than that of ethanol,
EXAMPLES
tU EXAMPLE 11 AEROBIC TESTING
Materials and Methods
Soil and Groundwater Samples
Soil and groundwater for laboratory microcosm testing were collected from
within Site 60 at Vandenberg Air Force Base, CA, The site has a history of
5 gasoline contamination, but has undergone an extensive cleanup program,
Collected groundwater was containerized in sterile stainless steel soda kegs
(18.5 L) under nitrogen headspace. Soil located approximately 8 to 12 feet
below ground surface (bgs) was collected using a Geoprobe 66200T with
acetate core sleeves. The core samples in acetate sleeves were capped and
2E sealed in the field to minimize exposure to air, shipped overnight on ice
to the
laboratory, and stored at 4: C,
Soil was removed from the acetate sleeves in an anaerobic chamber (Coy
Laboratory Products, Inc., Grass Lake,. Ml) and the first 10 cm of the core
ends
that may have been exposed to oxygen were discarded, Collected soil consisted
of silty sand with some gravel and larger stones, The soil was passed through
a
0.95 cm sieve, homogenized, and then stored in amber glass jars with Teflon-
lined caps at 40C until microcosm setup was complete, Baseline soil and
groundwater data are presented in Table 1. (NA = Not Analyzed; .õ SVOC (semi
volatile organic compound) detections include 6,00 mg L..-1 phenol and 0,003
mg
30 bis( -eth the yl phthalate; standard units).
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Table 1: Baseline soil and groundwater data
Parameter Groundwater Soil
Total Organic Carbon 22 1,700
Gasoline Range 57 NA
r anics
Total SVOCS 0.011 0.084
Total Iron 490 NA
Dissolved Iron <O 1 NA
Nitrate (as N) 1,5 NA
-------------------------------------- ------- -------- ----- ---------
- ------------------------- ---------------------------------------------------
-----------------------
Sulfate (as p
105 NA
Al alinity (as CaCo,4 391 NA
--------- --------' --------' -------' --------' --------- --------' --------'
--------' --------' --------
Methane 0.005 NA
-l ------------------------------------------------------------ ---------------
--------- -------------------------- . .-----------------------------
----- ------- -------- --------------------------- ----------------------------
- ------
Dissolved Oxygen 0.8 NA
Microcosm Ex eriments
The overall approach for the microcosm experiments was to evaluate the
biodegradation of BTEX (benzene; toluene, ethylben ene, and total xylenes)and!
isobutanol at both "high" and `IoW' concentrations in soil-groundwater
slurries.
Final transformation products were not determined. For comparison, one
treatment was prepared using ethanol instead of isobutanoI. Target BTEX
concentrations for each BTEX compound are listed under the BTEX column in
Table 2 which shows the experimental treatment matrix. Controls were amended
with mercuric chloride and formaldehyde. BTEX and alcohol concentrations
were selected to represent (approximately) potential g cL ndw ter
concentrations
that would be observed within a source area and in the near downgradient
plume. The greater ethanol concentrations relative to the isobutanol
concentrations used in this study were intended to reflect effective
solubilities of
Ãsobu anol and ethanol in groundwater. Ethanol has an aqueous solubility
approximately 10-times that of isobutanol; and the octanol-water partition
coefficient of isobutanol is approximately 1O-tunes that of ethanol
(Organization
for Economic Co-operation and Development, 2004. SIDS Assessment Report
for SIAM 19- Ethanol (CAS No. 64-17-5). Berlin, Germany-, Organization for
Economic Co-operation and Development, 2004. SIDS Assessment Report for
SIAM 19- 1sobutanol (CAS No. 78-83-1), Berlin, Germany). So an ethanol molar
16
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
concentration approximately 3-times greater than isobutanol was conservatively
selected for the testing in this study. The SET I treatments were prepared
within
4 days of sample collection whereas SET 2 treatments were prepared after
approximately 2 months of soil and groundwater storage. The experimental
treatment matrices are shown in Tables 2A and 213,
Table 2A, Experimental Treatment Matrix for SETI
Treatment BTEX (M.M) Isobutanol Ethanol
t.~ (MM)
-------------------------------------------------------- - --------------------
----------------------------------------
--- -------- --------- -
Control 1 1:80138/38/75
--- --- ------- -------- ------- --
Control 2 180/38/38/75 3,400
-------------------------------------------------------- - --------------------
----------------------------------------
- -----------------------------------------------
T 3 15/3,8/18/7,5
Treatment 1 15/3.8/3,8/7.5
Treatment 2 180/38/38/75
Treatment 3 15/3.8/3,8/7.5 68
Treatment 4 180/38/38/75 3,400
Table 2B: Experimental Treatment Matrix for SET2
Treatment BTEX (MM) Isobutanol Ethanol
(M M) (MM)
Control 4 180/38/38/75 11.000
---------------------------
Treatment 5 180/38/38/75 3;400
Treatment 6 180/38/38/7
1.000
5
Microcosms were prepared by placing 40 g of site soil into each of 54
glass serum bottles (approxin-late volume 160 mL each). BTEX and alcohol
(isobutanol or ethanol) were added to the treatment bottles to attain the
target
concentrations shown in Tables 2A and B. Bottles were filled with groundwater
so as to leave 10 mL. of headspace. Controls were amended with mercuric
5 chloride (700 mg/L in bottles) to inhibit microbial activity. Controls were
subsequently amended with formaldehyde (I% v/v in bottles) after 4 days to
limit
17
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
microbial activity. Treatments were prepared with a minimum of 3 and up to 8
replicates each.
The prepared microcosms were incubated at 150C on a rotary shaker
operating at 100 rpm. Headspace in each of the bottles was monitored for BTEX
and oxygen, Aqueous BTEX concentrations were calculated by applying Henry's
Law. Samples of the aqueous phase were analyzed for isobutanol and ethanol:
as well as potential isobutanol degradation products (iso-butylaldehyde and
iso-
butyric acid). The head pace of each bottle was periodically flushed with
oxygen
gas to maintain aerobic conditions in the bottles. The headspace of each
control
tU bottle also was flushed with oxygen to evaluate potential losses of BTEX
due to
the flushing process.
Analytical Methods
Headspace gases were analyzed for BTEX by using a: Varian CP-3900 gas
chromatograph equipped with an FÃD and Rester QSPLOT column (30 m
length, 0.32 mm diameter) with an injector temperature of 260 . and a detector
temperature of 290 C. Headspace oxygen was analyzed by using a Varian CP-
3800 gas chromatograph equipped with a Pulsed Discharge Helium Ionization
Detector (PDHID) and both a CP- Mols ene 5A column and a CP-ParaBond Q
2E column (both 10 m length, 0.32 mm diameter), an injector temperature of
210'C
and a detector temperature of 240 C. Oxygen eluted at 1.20 min. Aqueous
alcohol concentrations (as well as iso-butylaldehyde and iso-butyric acid)
were
measured by first collecting a 130 pL subsample preserved with mercuric
chloride, These samples were analyzed by using a Varian CP-3600 gas
chromatograph equipped with a flame ionization detector (FID) and a Stabilwax
DA column (30 r length, 320 pm diameter), an injector temperature of 230 C,
and a detector temperature of 260 C.
Microbial-,Analyse
s
Samples (2 mL) were collected from microcosms amended with BTEX and
isobutanol at the start, midway (approximately), and end of the experiment to
18
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
evaluate changes in the microbial population over the course of the study.
Samples %, ere serially diluted and plated onto R 2A Agar (BD Difco) and Basal
Salts Medium (B M; Hareland et al.,1975, J. Bacteriot 121; 272-285,) agar
immediately after retrieval as per Method SM9215C. BSM plates were incubated
in sealed containers with either BTEX or isobutanol to select for bacteria
capable
of growth on these substrates. Colony counts were performed manually after 3
days for R 2A agar and after 10 days for selective media. Samples ( -8 ml_)
also
were immediately frozen at -70 C and at the conclusion of the study were
shipped on dry ice to Microbial Insights, Rockford, TN, for CENSUS analysis.
tU CENSUS employed quantitative polymerise chain reaction (qPCR) assays to
quantify total Eubacteria based on enumeration of Eubacterial 165 rRNA gene
copies (for more information on the CENSUS method see Microbial Insights,
2009 microbe, co m/how-census-works. html and
http,/lmicrobe.com/census-applications/anaerobie-btex.htmi; accessed
07.07. 009 and 05,08.Ã 9, respectively).
lsobutanol and Ethanol Degradation
No measurable decreases (>10%) in isobutanol or ethanol, accumulation
of isobutylaldehyde, or accumulation of isobutyric acid were observed in any
of
2 the controls. Isobutanol biodegradation in Treatments 3 and 4 are shown in
Figure 1. In the lower concentration treatment (Treatment 3), isobutanol was
degraded to below the analytical detection limit (3 pM) within 7 days.
Isobutanol
degradation products isobutylaidehyde and `Ãsobutyric acid were first detected
at
4 days of incubation, with isobutylaldehyde subsequently decreasing to below
25 detection within 5 days. Isobutyric acid concentrations increased
initially, but
samples taken after 82 days confirm that iisobutyric acid also was further
degraded in the microcosms (data not shown). The biodegradation products of
isobutyric acid were not determined, but previous studies have shown that
isobutyric acid readily biodegraded Linder aerobic conditions, and that
butyrate is
0 readily transformed to CO2 0under aerobic conditions (Miller, 2001, J. Anim.
S i.
79: 2503-2512; Bonartseva, 2003, AppÃ. Biochem. Biotech. 109. 285-301).
19
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
In the higher concentration treatment (Treatment 4, Fig 1 b,), isobutanol
was degraded from an initial concentration of 3,400 rM to below the analytical
detection limit within 23 days. As isobutanol was degraded, the formation and
subsequent degradation of two isobutanol breakdown products, isobutylaidehyde
and isobutyric acid. were observed, Isobutylaldeh,yde reached a peak
concentration of 900 pM on day 9 and then declined below the analytical
detection limit of 11 pM after 19 days. Isobutyric acid increased to a peak
concentration of 1,750 pM on day 25 and then decreased to 100 pM by day 48,
In the second set of microcosms used for comparing isobutanol and ethanol
(Treatment 5), isobutanol was degraded in a similar timeframe and isobutyric
acid was observed in similar quantities. However, only trace levels of
isobutylaidehyde (78 ISM) were observed.
In ethanol amended microcosm : ethanol concentrations decreased from
11,000 p M to below the analytical detection limit of 22 pM in approximately
40 to
5 45 days (Figure 1c). No decreases in ethanol were observed in the controls,
To
ensure that the observed ethanol degradation was not limited by available
nutrients, nutrients in the form of a modified basal salt media (Hareland et
at,
1975) were added to all treatments on day 22. No measurable increase in the
rate of ethanol (or any other compound) degradation was observed, suggesting
2 that contaminant biodegradation was not limited by nutrient availability
near day
22 in the experimental system.
BTEX Degradation
Microcosms amended with higher concentrations of BTEX, with and
25 without isobutanol, are shown in Figure 2. Results for the lower
concentration
BTEX are provided in Figure 4. Observed lag times and regressed first-order-
effective half lives are in days and shown in Table 3. Effective half-lives
are the
regressed half-life plus the lag time. (IBA = Ãsobutanol, values indicate
95%
confidence intervals.)
30 Table Lag times and regressed effective half-lives T112) _for BTE.
--------------
Treatment Benzene Toluene Ethylbenzene Total lenes
--- ---- ---------------------------------------------
- ------------------------------------------------------------ ------ ---------
----------------------------
L` 1/ Lag --- - 1' --------- ag fag - t. ------------
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
1 (Low 0 2.0 0 1.A 2 2.3-x-0.08 2 2.8
BTEX) 0.33 0.17 0.06 '=
0
2 (High 7.7 5 5.8 5,9 0,11 41STEM} 0.34 0.0
0.33
8
r
---
3 (Low 0 27 0 1,6 1' 1.0 0.10 1 2.0 Ã
BTE. + 0.33 0.18 0.0
IBA)
0
4.1 13.3
0 6:9
4 (High 1 13 1.6 5 6.3
9
BTEX + 0.31 . 57
IBA
6(High 1
2.4 .00 1 2.3 1' 1.9 -0,07 1 4.8
BTEX + 0.10 0.33
IBA)
------------------------------------------------ ---- ----
---- --
-------------- -----------------
.3 1.7 0,05 r
6 (High 1 30 4,3 1
9
1
22 3,6
BTEX + 0.06
Ethanol)
BTEX concentrations in the high concentration controls showed no
observable decreasing trend through approximately 25 days, at which time
decreases in the control concentrations were observed for some compounds (up
to approximately 20%). The controls were subsequently re-amended with
formaldehyde to inhibit microbial activity, of the additional formaldehyde and
to
prevent further decreases in the controls. By 25 days, most of the BTEX
compounds had been degraded, so these losses did not impact evaluation of the
data. No significant (>10%) decreases in the low concentration BTEX controls
were observed, except for the total xylenes where an approximately 25%
its decrease in total xylenes concentration was observed over the 10_day
duration of
this experiment.
Half lives obtained for the BTEX compounds under aerobic conditions
(Table 3) were generally within the range observed by others (see review in
United States Geological Survey, 2006, "Description, properties, and
degradation
of selected volatile organic compounds detected in groundwater---a review of
selected literature. Open File Report 2006-1338). Results for the comparison
microcosms (Treatments 4, 5 and 6) show that ethanol generally exhibited
greater adverse impacts on BTEX biodegradation than isobutanol did (Figure 3
and Table 3), The only exception was ethylbenene; where isobutanol and
fit) ethanol impacted ethylbenzene biodegradation similarly. Moreover, the
addition
21
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
of isobutanol resulted in a small but measurable increase in ethyl benzene and
total xylenes biodegradation in the low concentration treatments (Treatments 1
and 3), possibly due to the fortuitous growth of ethylbenzene and total xylene-
degraders on isobutanol.
The effective half lives for BTEX in Treatment 5 were less than in
Treatment 4 (Table 3) by approximately a factor of two to five. However, the
rate
of isobutanol biodegradation in these two treatments was approximately the
same.
Microbial Characterization.
Results of microbial colony counts and qP R analyses are shown in
Figure 13. These data show that microbial concentrations in the microcosms
increased throughout the duration of the study, Both the microbial colony
counts
and total Eubacteria data show similar trends, although Eubacteria
5 concentrations determined by molecular analysis were greater than the
microbial
colony counts. A discrepancy between aerobic plate counts and total bacterial
by molecular analysis Is not unusual because some bacteria do not grow well on
all agar plates, and the latter method measures culturable, non--culturable,
anaerobic, and aerobic bacteria. The plate count data confirmed that natural
2( bacteria capable of degrading both isobutanol and BTEX were present in the
site
materials. As indicated in the low concentration treatments, !-microbial
growth
became limited when substrate (either BTEX or isobutanol) was depleted.
5,
EXAMPLE 2 -ANAEROBIC TESTING
22
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Materials and Methods
Soil and Groundwater
The soil and groundwater and sample collection and handling procedures
are as described above, Baseline soil and groundwater data are presented in
Table 4. (NA = Not Analyzed; * SVOC detections include 0.008 mg L--1 phenol
and 0.003 mg L 9 kris(-ethylhexyl) phthalate;** standard units)
Fable Groundwater and soil p rameters
Parameter Groundwater (mg/L) Soil (m /kg)
---- ---- ----- ---- --------- ---- --------- --------- -------- ---------- ---
--------- --------- ---------
Total Organic Carbon 22 1,700
----------------------------------------------------------------------------
--- -------------------------------------------------------------- ------ -----
---- --------- --------
soline Range ,' NA
Organics
Total SVOCs 0;011 X <0.084
Total Iron 490 NA
------ ---- -------------------------------------------------------- ----------
---------------------------------------------------------- -- -----------------
-------------------------------------------------.
Dissolved Iron 0_1 NA
----- -------- ------------------------------------- ----
litrate (as -- 1NA
------- - -------- -------- -------- -------- ------ --------- --------- ------
--
Sulfate (as 10 A
- ---- - --------- --------- --------- --------- ---------- -- --------- ------
--- --------- --
Alkalinity (as CaCo3) 391 NA
----------------------------------------------------------------------------
- ----- -------- -------- -------- ------ --------- --------- --------
Methane <0.00
5
NA
pH 7>2** NA
Dissolved Oxygen
8
0, NA
Microcosm Preparation
For the microcosm experiments, the biodegradation of BTEX and
isobutanol in soil-groundwater slurries under conditions ranging from nitrate-
reducing to nethanogen were eraÃuated. BT and lsobuta of were evaluated
at "high" and "low" concentrations (Table 5), Two treatments were prepared
using ethanol instead of isobutanol. Electron acceptor concentrations reflect
the
amount added to the sample and (Table 5) do not include background electron
acceptor concentrations in site ground water. BTEX and alcohol concentrations
23
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
represent potential groundwater concentrations that might be observed within a
source area and in the near downgradient plume.. The greater ethanol
concentrations relative to the isobutanol concentrations used in this study
were
intended to reflect effective solubilities of isobutanol and ethanol in
groundwater.
Ethanol has an aqueous solubility approximately 10-times that of isobutanol,
and
the octanol-water partition coefficient of isobutanol is approximately 10-
times that
of ethanol (Organization for Economic Co-operation and Development, 2004.
SIDS Assessment Report for SIAM 19- Ethanol (CAS No. 64-17-5). Berlin,
Germany: Organization for Economic Co-operation and Development, 2004. 10 SIDS
Assessment Report for SIAM 19- lsobutanol (CAS No. 78-83-1), Berlin,
Germany). Because the rate of ethanol biodegradation was anticipated to be
greater than that of isobutanol, an ethanol molar concentration approximately
3-
times greater than isobutanol was conservatively selected for testing.
All microcosm preparation was performed in an anaerobic chamber.
t 5 Microcosms were prepared by placing 40 g of site soil into 160 mL glass
serum
bottles. BTEX and alcohol were added to the treatment bottles to attain the
target concentrations shown in Table 5, (Target BTEX concentrations for each
BTEX compound are listed under the BTE. column.) Bottles were filled with
groundwater, leaving approximately 2 mL of headspace, Control microcosms
2( were amended with mercuric chloride (700 mg L-1 in bottles) and
formaldehyde
(1% by volume in bottles) to limit microbial activity. Treatments were
prepared
with a minimum of 3 replicates for alcohol and BTEX analyses; one additional
replicate per treatment was used for monitoring electron acceptors and
methane,
The bottles were crimp sealed with Teflon-lined butyl stoppers, and
25 incubated at 1500 on a rotary shaker operating at 100 rpm. If needed,
additional
electron acceptor was added to maintain the desired reducing conditions.
Controls were amended with mercuric chloride and formaldehyde. Amended
electron acceptor concentrations are shown in the last column. Nutrients, in
the
form of a modified basal salt medium (Hareland et al., 1975), were added to
all
30 treatments at 22 days.
24
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Table 5: Experimental Treatment Matrix
Treatment BTEX (1LM) Isobutanol Ethanol (l,ilVI) Electron
gi )
tk ~l Acceptor
Control 1 180/38/38/75
Control 2 180/ 38/38/75 3,400 - -
Contrel 3 1513.8/3.8/7.5 68
--------------------------------------------- - -------------------------------
------------ -------------------------------------------- ---------------------
------------------------------------------------------------------------
entroà 4 15/3,8/3,8/7,5 11,000
--------------------------------
------------------------------ --------- ------- -------- -----
Treatment 1 180138/38/7 Ã
--- --- --- ---- ---- --
--------------------------------
Treetment 2 180/38/38/75 3,400
Treatment 3 1513,8/3,817,5 68
Treatment 4 Ã5/3.8/3.817.5 11;808
Treatment 5 180138/38/75 7,168
(NaNO3)
--------------------------------------------- ----- - -- - ------ - -------- -
--- ------------ - ------------- ---------------------------------------------
---------------------------------------------
----
Treatment 6 1 80/38138/75 3,400 7,100 (NaN r)
Treatment 7 15/3,8/3.817.5 68 7,100
(NaNO3)
Treatment 8 180/3&38!75 - 2,600
(Fe(OH)3)
---- --------------------------------------------- ----------------------------
----------------
-------------------------------------------- ------------- --------------- - --
------------------------------
Treatment 9 180/38138/75 3,406 2:600
Treatment 10 15/3.8/3,817,5 68 2;600
(e(OH) )
Treatment 11 Ã 80138/38/75 3,200
(M S04)
Treatment 12 180/38/38/75 3,400 - 3,20Ã
Treatment 13 15/3.8/3.817.5 68 3,200
Ig0.}
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Treatment 14 15/3,8/3.817.5 11 000 3,200
(MgSO4)
-------------
,naltical Methods
Head; pace gases were analyzedfor BTEX by using a Varian CP-3900
gas chromat graph, (GC) equipped with a flame ionization detector (FID) and a
Restek PLOT column, and for methane by using a equipped with an PID
and a Restek Fit-Alumina columnAqueous concentrations were calculated by
applying Henry's Law.
Aqueous alcohol concentrations is well as potential isobutanol
degradation products Ãso-butylaidehyde and iso-butyric acid) were analyzed by
to using a Varian CP-3800 gas chromatograph, equipped with an P1D and a
tabilwax DA column. Aqueous samples were collected for anions analysis via
ion chromatography (Dionex DX-120, Sunnyvale, CA). Nitrate also was
periodically measured using Quant Nitrate Test strips ( MO Chemicals,
Gibbstown, NJ). Total and dissolved iron were measured using Hach test Kits
5 (Hach, Loveland, O) according to the manufacturer's instructions,
"licrobial AnaÃyses
Microcosm, slurry sub-samples samples (approximately 8 note} were
obtained from Treatments 2, 6, 9, and 12 at the start and at the end of the
experiment to ascertain changes in the microbial population over the course of
20 the study. Samples were immediately frozen at -700C and (at the conclusion
of
the study) were shipped on dry ice to Microbial Insights, Rockford: TN, for
quantitative polyr era e chain reaction (qPCR) of total Eubacteria,
denitrifying
bacteria, iron and sulfate reducing bacteria, and methanogenic bacteria by the
CENSUS""' quantitative P R technique (Microbial Insights,
25 2009http: / w. microbe. com!how-census-worÃks.html and
http=//microbe.comlcensus-applications/anaerobic-btex. htmi;,, accessed
07.07.2009 and 05.08.09, respectively)..
26
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Ãsobutanol and Ethanol ire,. radation
Isobutanol was completely degraded in the high concentration treatments,
and its degradation rates varied under different anaerobic conditions. Figure
5
shows isobutanol and electron acceptor concentrations in the higher
concentration treatments (Treatments 6, 9, and 12). Background sulfate
concentrations are shown for the unarended treatment. No decreases in
isobutanol or ethanol, nor accumulation of isobutylaidehyde or isobutyric
acid,
were observed in the control microcosms. The most rapid isobutanol
biodegradation was observed in the nitrate-amended microcosms. Within 16
days, Ãsobutanol was degraded to below detection limits under nitrate-
reducing:
conditions (Treatment 6). Nitrate was WiÃized concurrently with the isobutanol
degradation, and decreased to non-detect by day 13 before being re-amended
on day 14, No measurable decrease in the background sulfate was observed in
Treatment 6 through 19 days (data not shown).
lsobutanol biodegradation was observed in both the sulfate amended
treatments (Treatment 12) and the unamended microcosms (Treatment 2) where
limited background sulfate existed. To evaluate biodegradation of isobutanol
after depletion of sulfate (i.e., methanogenic conditions) microcosms bottles
for
Treatment 2 were re-spiked with isobutanol (to a final concentration of 3,400
pM)
at 88 days. The additional isobutanol was degraded within approximately 30
days. However, only trace (< 2 pM) levels of methane were observed in
Treatment 2 following depletion of the sulfate, which is similar to the
methane
levels in the controls.
Isobutanol in the iron-amended treatment was biodegraded to below the
analytical detection limit within approximately 80 days. Ferric iron,
monitored
through 44 days, showed concentrations ranging from approximately 18 to 36
pM. However, only relatively low levels of dissolved ferrous iron (up to 36
ply)
were observed. The absence of appreciable ferrous iron accumulation was likely
the result of iron sulfide formation in the microcosm bottles, as a black
precipitate
was observed. In addition, and consistent with the formation of iron sulfides,
background sulfate concentrations decreased in the iron-amended treatment.
27
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Decreases in isobutanol concentrations correlated with decreases in sulfate
levels.
In the lower isobutanol concentration microcosms (Treatments 3, 7, 10
and 13) isobutanol was completely biodegraded within approximately 25 days in
the iron-amended treatment, and approximately 15 days in the unamended,
nitrate-amended and sulfate-amended treatments (Figure 9'. In addition to the
amended electron acceptors, each microcosm contained approximately 100 M
background nitrate levels in the groundwater material, and background nitrate
decreases correlating with isobutanol biodegradation were observed during the
IU incubation. The degradation of one mole of isobutanol theoretically
requires four
moles of nitrate which is assumed to be completely reduced into nitrogen
(McCarty, "Bioengineering issues related to in situ remediation of
contaminated
soils and groundwater Environmental Technology, in : Omen 2 G,S_ Reducing
risks from environmental chemicals through biotechnology, Plenum press, New
5 York, pp. 143-162 1988) Enrichment culture experiments suggested that the
mole ratio of nitrate consumed and benzene degraded was ten, twice the
theoretical number (Burland and Edwards, 1999, Appl Environ Microbiol 65(2).-
529-533). The 199 pM background nitrate could facilitate the biodegradation of
approximately 13 pM isobutanol, 18% of the initial isobutanol in low
concentration
2E treatments. No background sulfate reduction was observed in the low
concentration treatments.
lsobutyric acid and trace levels of isobutylaldehyde were identified as
transient biodegradation intermediates; the subsequent biodegradation of both
of
these compounds was observed in all treatments. Iso-butyric acid accumulated
25 to near (with a factor of approximately 2) stochiornetric quantities, with
the
exception of the high concentration nitrate-amended treatment in which only 5%
accumulation was observed. The limited generation of isobutyric acid in the
high
concentration nitrate treatment is not readily explained.
In ethanol-amended microcosms (Treatments 4 and 14), ethanol was
30 degraded to below the analytical detection limit in approximately 60 days
under
sulfate reducing conditions (Figure 6). Methane was detected in both
Treatments
28
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
4 and 14 (4 and 25 pM, respectively) at day 44, but decreased to non-detect
levels by day 78.,
To evaluate ethanol biodegradation after depletion of sulfate, ethanol was
re-spiked to Treatment 4 bottles at 88 days. Subsequent biodegradation of the
ethanol to below the analytical detection limit occurred within 90 days. This
result is consistent with previous anaerobic ethanol studies documenting
ethanol
biodegradation via fermentation in the absence of sulfate (Laanbroek et at,
1'. 982,
Arch. Microbiol. 188; 178-184),
BTEX Degradation.
to Decreases in BTEX concentrations in the controls were negligible (<15%)
during the course of the experiments. BTEX biodegradation in the higher
concentration treatments are shown in Figures 5 and 7, lower concentration
BTEX results (with comparative ethanol treatments) are presented in Figure 8.
No data are available for the last two time points for Treatment 4 because
BTEX
was inadvertently re-spiked into this treatment.
Toluene biodegradation was observed in all the high concentration
microcosms amended with electron acceptors (Figure 7). When incubated
without alcohols, approximately 38pM toluene was degraded to below detection
fir its within 80 days under nitrate-reducing, iron-reducing and sulfate-
reducing:
conditions, respectively, The presence of isobutanol exhibited slight impacts
on
toluene degradation under nitrate-reducing and sulfate-reducing conditions,.
but
slowed the toluene degradation in the iron amended microcosms. In the
unamended high concentration microcosms (Treatment 2), however no
measurable toluene concentration decreasing trend relative to the controls Was
observed, Background sulfate in Treatment 2 became depleted in the first 80
days, presumably due to the isobutanol biodegradation, and the lack of
electron
acceptors was likely the reason of toluene persistence in the unamended
treatments. In the lower concentration microcosms where sufficient electron
acceptors were present, isobutanol exhibited little impacts on the toluene
biodegradation in both the unamended (limited sulfate) and any of the
29
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
microcosms amended with electron acceptors (Figure 8). Results for
ethylbenzene and total xylenes (Figure 10) were similar to those for toluene,
although biodegradations of ethylbenzene and total xylenes occurred slightly
slower compared to toluene.
In the higher BTEX concentration treatments without isobutanol, no
substantial benzene biodegradation was observed under any anaerobic
conditions throughout 162 days of incubation (Figure 7). However, the presence
of isobutanol appeared to stimulate benzene biodegradation under iron-reducing
and sulfate-reducing conditions, as the benzene concentrations in Treatments 9
IU and 12 started to decrease by the end of the experiment. In the low
concentration treatments under sulfate-reducing conditions, benzene
concentrations decreased to below the analytical detection limit (0.50 pM) in
the
microcosms with isobutariol (Treatment 13), and to approximately 8.88 pM in
the
microcosms with ethanol (Treatment 14) after approximately 300 days of
5 incubation. Benzene biodegradation started before day 120 in Treatment 14,
and
occurred behAfeen 160 and 300 days in Treatment 13.
MicrobialAnalyses
Results of the microbial analyses, shown in Table 6, generally showed an
increase in microbial concentrations during the incubations. (Values are in
cells
2() mL-'- values indicate 95% confidence intervals.)
Table Results of microbial analyses
- - --- - ------------------------------ - ------------------ -- - ------------
------------------- ------------- - - ------------ - ------------- -------- - -
-- - -------------------------------------- -----------------------------------
-------
Treatment Day Total Denitrifying Denitrifying Iron & Methanogens
s Eubacteria bacteria bacteria Sulfate- (mcrA)
(nir) (nirl) reducing
bacteria (c5
protobacteri
Tr. 2- 0 81 7.7x 10+08x 5 7.9 x 1,3 8:4x10 54 4.7 x
Unamended 10 10 10 10-5
132 a,2 4.1 x 1.3 8.5x1.9 1.7x 1.4 0.3 x 3,8 2.9 x
1 19 19' 107 197.
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
Tr. 6- 0 5.6 4.5 x 3.3 5.8 x 1 0 0 9 x 2,6 3.9 x 4.8 3.9 x
Nitrate 10 18" 191 181 18.
reducing. 14 5,9 4.9 x 5,9 8.3 x ; 5. 1 5,8 x a 1.2 x 4.7 3.3 x
19R 1Ã00 196 19' 1Ã3
- ------ ------- ----- ----- -- -----
13 0.5 .x 1.3 0.2x 4.7 x 0.8 1.1 x. 4.6 4.3 x
19 10 19 104 197
Tr. 94 on 0 8.1 : .8 x: 4.6 4.0 1.2 0.6 x 2.0 3.5 x 2.5 1.0 x
reducing 18 106 101 1 190
13 1 1.5x 5. 4.9 x 1.9 0..4x1.7 1.9 x 1.9 1.9 x
1 9 10 107 10' 187
Tr. 12- 0 8.0 0.9x 2.7 3.9x1 3.9 1.5x 2.2 2.4 x 1.1 8.4 x
18 1 g6,
-4
Sulfate 10, lo 10
reducing 132 7.1 7.0 x 1.3 9.8 x 8.7 3.3x 1.1 0.3 x 3.31 1.9 x
1F
1 10-4 10
1-' 101
In the microcosms containing ferric iron (Treatment 9) or sulfate
(Treatments 2, and 12), the population of corresponding iron reducing and
sulfate
reducing bacteria increased 1989-fold, respectively. nitrate was depleted
5 toward the end of the anaerobic incubation in Treatment 6, the methanogen
population increased 100-fold. Slightly lower levels of methanogen biomass
increase were observed in the other treatments.
Increased growth of denitrifying bacteria was not observed in the nitrate
amended treatments. One explanation for this observation is that, although two
different nitrate reductase genes were monitored., the functional genes for
the
denitrifying bacteria in our system were not quantified. Alternately, while
the
activity of the denitrifying bacteria may have been substantial, growth of
denitrifying bacteria may have been low, as observed in n-butanol studies
under
denitrifying conditions by Dubbels et al. (2899, In t, J. Syst. Evo1.
MicrobÃol. 89.
1 1576-1578). Likewise, fermentative bacteria that do not rely on nitrate
reduction
for growth also may be involved in isobutanol degradation under denitrifying
conditions (Laanbroek et al., 1982, Arch.. Microbiol. 133: 178-184),
31
CA 02797555 2012-10-25
WO 2011/146849 PCT/US2011/037360
EXAMPLE 3 - ISOBUTANOL AS A CO-SOLVENT FOR ETHANOL
Scoping water tolerance and phase separation tests were conducted on
isobutanol - ethanol - gasoline blends at 65 IF, Increasing amounts of
isobutanol
were mixed with E10 gasoline and then either 1.3% or 2.6% water were added to
all of the blends. The water needed to be increased to 2,6vol% for some blends
because gasoline blends containing ethanol absorb larger amounts of water and
1.3% water was not always sufficient to induce the formatÃon of separate
tU aqueous and hydrocarbon phases for analysis (I.e,, the 1,3vol% water was
completely absorbed by the higher isobutanol blends). This can be seen in
Figure 11 where 1.3 vol% was sufficient to cause two phases until the amount
of
isobutanol in E10 reached Svol%, but for 13vol% isobutanol in E10,. the level
needed to be increased to 2,6vol% water to induce phase separation.
The data in Figure 12 show that the amount of ethanol extracted into the
aqueous phase decreased with increasing iso-butanol concentration indicating
that iso-butanol acts as a co-solvent for ethanol. Concentrations in both the
hydrocarbon and aqueous phase were determined by GC.
The foregoing description of the specific embodiments will so fully reveal
2E the general nature of the invention that others can, by applying knowledge
within
the skill of the art, readily modify and/or adapt for various applications
such
specific embodiments, without undue experimentation, without departing from
the
general concept of the present invention, Therefore, such adaptations and
modifications are intended to be within the meaning and range of equivalents
of
25 the disclosed embodiments, based on the teaching and guidance presented
herein, It is to be understood that the phraseology or terminology herein is
for
the purpose of description and not of limitation` such that the terminology or
phraseology of the present specification is to be interpreted by the skilled
artisan
in light of the teachings and guidance.
0
32