Note: Descriptions are shown in the official language in which they were submitted.
CA 02798880 2012-11-08
1
METHOD FOR PRODUCING ORGANIC AND ORGANO-MINERAL FERTILISERS
WITH HIGH CARBON CONCENTRATION USING PHYSICAL AND BIOLOGICAL
PROCESS
Field of the Invention
The present invention relates to a process for the production of an
agricultural
starting material, more specifically a process for the production of organic
and
.organomineral fertilisers based on the carbon concentration of the organic
material in
natura and correlating such concentration with the fractions of natural
minerals and / or
processed minerals, promoting a high physical interaction of the materials
involved and
enabling and biologically enriching said compound.
Background of the Invention
The goal of putting soil products consisting of the sum of mineral NPK, Ca,
Mg,
S and the like, forming at least the 16 essential elements, with high
concentration of
carbon, is to offer to the plant an environment that is alive and active,
where the
relation soil-plant comprises both quantity and quality of nutrients, ie, to
form a solution
rich in the said soil, thus feeding the plant with a wealth of mineral and
biological
nutrients.
In Australia, for example, there has been concern over the high salinity
caused
by inorganic nutrients, mainly salts extremely soluble in acidic pHs, which do
acidify the
soil and cause loss of the structure thereof and microbiological life
(Shaxson, IF,
"integrated production and protection of watershed", in: XXI Brazilian
Congress of Soil
Science, "The social responsibility of soil science", Campinas, SBCS, 1988, p.
263-
272). The compaction of the soil is another problem that causes reduction in
productivity due primarily to the loss of soil organic carbon. A decrease of
one percent
(1%) of organic carbon in soil is more aggressive than the degradation caused
by
erosion.
We know at least 20 reasons involving the three conceptual aspects - physical,
chemical and biological - to achieve high levels of production with high
quality
products. The reasons for the importance of the concentration of organic
matter in the
soil, according to Albrecht, WA, "Loss of soil organic matter and its
restoration," pgs.
347-360, Soils and Men, 1938, Yearbook of Agr., U.S. Govt. Printing Office,
Washington, DC, Cole, CV, Williams, J., Shaffer, M. and Hanson, J. "Organic
matter
and nutrients as components of models of agricultural production systems" in:
Follett,
RF, Stewart, JWB and Cole, CV (eds), "Soil fertility and organic matter as
critical
CA 02798880 2012-11-08
2
components of production system", p. 147-166, 1987, SSSA Spec. Publ. 19, ASA
and
SSSA, Madison, WI; Doran, JW and Smith, MS, "Managing the use of organic
matter
and soil nutrients and fertilisers, pgs. 53-72, in: Follett, RF, Stewart, JWB
and Cole, CV
(eds), "Soil fertility and organic matter as critical components of production
system", p.
147-166, 1987, SSSA Spec. Publ. 19, ASA and SSSA, Madison, WI, are as follows:
a. Do not allow soil erosion;
b. Provide nutrients to the soil;
c. Protect soil against pH changes;
d. Retaining water in the soil;
e. Increasing the cation exchange capacity, protecting the soil against anions
with nutrients, which promotes the loss thereof;
f. Reduce soil compaction;
g. Store nutrients in the soil without losing them from one to another crop;
h. Reduce heating of the soil;
i. Conditioning a more porous soil, even when slightly wet;
j. Allow more reactions with minerals in the soil;
k. To give more soil health and, therefore, reduce plant diseases;
1. Giving the soil better aeration and higher permeability;
m. Protect soil reactions of heavy metals existing in the same toxicity and
salt;
n. Be a mechanism for storing the additional atmospheric C02;
o. Offer production yields due to the potentiation of the factors described;
p. Promote the restoration of soil microbial, inhibiting the toxic substances;
q. Promote plant growth outside an acidic environment;
r. Allow receipt of other microorganisms that recycle nutrients in the soil;
s. Promote soil formation, and
t. Offer biochemical combinations that enrich the soil the fruit, as the brix
thereof.
There are already many studies showing the value of soil organic matter, as in
Darst, BC and Murphy, LS, "Organic matter in soil: an integral ingredient in
the
production of crops," 1989, Better Crops 74 (1): 4-5 , and KIEHL, EJ,
"Organomineral
fertilisers", Piracicaba, Ceres Agricultural Publishing, 1993, p. 1-6, which
properly refer
to these benefits. The organic matter acts as an energy source for
microorganisms and
other soil organisms and is crucial to chemical and biological and physical
health of the
soil.
CA 02798880 2012-11-08
3
The organic matter added to the soil in the presence of other essential
nutrients
to plants in suitable proportions relative to one another, such as calcium,
sulfur,
nitrogen and other nutrients, are synergistic in nature, with studies showing
that one
can induce microbial populations by reacting the same with carbon to form
complex
molecules of humus and other organic fractions, as in Doran, JW and Smith, DM,
"Management of organic matter and use of soil nutrients and fertilisers, pgs.
53-72 in:
Follett, RF, Stewart, JWB and Cole, CV (eds), "Soil fertility and organic
matter as
critical components of production system", pgs. 147-166, 1987, SSSA Spec.
Publ. 19,
ASA and SSSA, Madison, WI; Hallam. and J. Bartholomew, WV, "Influence of rate
of
addition of plant residues in accelerating the decomposition of organic matter
soils",
Soil Sci Soc. J., 1953, vol. 17th, pgs. 365-368.
The soil organic matter along with biochemical reactions with the humic mass
formed (humic acids) are important factors in the process of soil formation.
The degree
of mineralization of soil organic matter is a subject not discussed, thus
leading to the
issue of adding organic matter to the soil via conventional process, this
decomposing
before application to soil and soil in coming to that stage already
stabilized.
Thus, it has been shown that such decomposition will occur until the stage
wherein the carbon / nitrogen ratio is below 18:1, seeking ever lower levels,
sometimes
reaching up to 5:1, or an organic material very low-carbon. In summary, there
has been
little understanding as regards biological action of humic acids, however, in
recent
years, since it can add that these reactions also result in the development of
soil
profiles, and nutritional quality thereof (Joffe, JS, "Pedology", Rutgers
Univ. Press, New
Brunswick, NJ, 1936, and Konovoa, LKJ, "Routes of degradation in the oxidation
of
carbon aromatic heterocyclic systems," Dept. of Chemistry, Wright State
University
Dayton, Ohio 45431, August 22, 1969). Humic acids should promote continued
biochemical reactions in the soil, leaving all the energy of these reactions
in yourself,
not detaching and said energy pouring into the atmosphere. All humic acids in
soil is
grouped genetic code and the biological processes of the microorganisms.
It is known today that the richest soils above the tropics, usually contains
2200-
5600 kg of nitrogen / ha. This amount is lower and for soils below the tropics
it is even
lower. However, if attention is given to this important subject by analyzing
the
advantage of adding 2% nitrogen via addition of organic matter will be
observed that
the microorganisms mineralize the nutrients of organic matter provide for the
use of
these plant, it is possible to retain soil 100-250 kg of nitrogen / ha / year.
What is not
CA 02798880 2012-11-08
4
known is that while one is losing 990 to 2470 kg of carbon. This represents
1540-3740
kg of precious organic matter being lost. This procedure is being overused and
practiced by many farmers who adopt organic and conventional crops and that
use
waste from other cultures (such as direct planting), leading to decomposition
of organic
matter and loss of nitrogen and carbon, which in turn impoverishes said
organic matter.
We can observe that the nature works to provide the plant residues into the
plant itself,
because it means that the only carbon source is air and it also provides
nitrogen in
70% of the composition. Nitrogen is converted into the plant 2 for NH3. This
reaction
(N2 + 6H + 6e" -+ 2NH3, in the presence of Fe and Mo) is one of the important
reactions occurring in soil, parallel to the reactions of photosynthesis. The
researcher
Brady (Brady, CN and eil, RR, "The nature and properties of soils", 13th ed.,
Prentice
Hall, Upper Saddle River, 2002, p. 960) adds that when the nitrogen is in
excess, the
ratio of carbon and other nutrients is compromised, or such biological
nitrogen shall not
be used and will be lost.
Genetic Engineering of specific microorganisms and organic matter in soils
shows the need to increase levels of organic matter in the soil. Organic waste
from
domestic waste can help replenish the lost mass of carbon in soil and detached
in the
atmosphere, according to Wallace, A., "The soil organic matter should be
restored to
as close to the originals," Commun. Soil Sci. Annual Plant., Vol. 25, pgs. 29-
35).
It is estimated that from 3.5 to 5.4 tons / hectare of crop residues or
agricultural
industries can be annually returned to the soil. This volume is replaced each
year, is
significant and could keep the soil in good balance with good levels of
organic matter,
according to Follett, RF, Stewart, JWB and Cole, CV (eds), "Soil fertility and
organic
matter as critical components of production system", p. 147-166, 1987, SSSA
Spec.
Publ. 19, ASA and SSSA, Madison, WI. The 5.4 tons of organic waste accounts
for
approximately one ton of carbon. With the 25% efficiency in decomposition
processes,
it is estimated that one quarter of the carbon is lost.
If the crop residues were reinserted into the soil, with the continuous
increase of
productivity of these areas, the quality that would reach the ground power
would have
high yields, meaning a rate of 8.8 tons of organic matter / hectare and other
100-204
kg of carbon would be retained. This would add between 890 and 1480 kg of
carbon
retained / hectare per harvest.
The process of composting organic matter is technically improper (Wallace, A.,
"The soil organic matter should be restored to as close to the originals,"
Commun. Soil
CA 02798880 2012-11-08
Sci. Annual Plant., Vol. 25, p. 29-35 ), it increases the concentration of CO2
in the air, is
costly and can work and apply ground 15-30 times and volumes studied admitted
to
different cultures, thus causing 'disaffection and infeasibility of using this
process in
extensive crops obtaining yields similar or higher to those obtained with
conventional
fertilisers.
A complete fertilizer is a balanced fertilizer with adequate levels of
minerals,
organic matter and crude increments that allow high biological activity of
organic acids.
The importance of having a product that has not been composted all the effects
already mentioned biologically, counting and being in combination with other
nutrients
to carry out actions that nature promotes recycling nutrients. (Wallace, A.
and Wallace,
GA, "A possible flaw in the new rule's 1993 Agency of Environmental Protection
on
deposits due to the interaction of heavy metals," Comm. Soil Sci. Annual
Plant., Vol.
25, pp. 129-135, 1994).
Upon interpreting the carbon losses, one can add the wrong ways to use and
even the decomposition process, which deplete the soil.
Besides the need to have a substance richer in carbon, the nitrogen element
also has high value, acting on the biological system of organic matter,
allowing the
organic and biochemical reactions. Thus, it is important to point out that it
is not
enough to consider the organic matter present in the compositions, it is also
necessary
that it is living and active. The involvement of nitrogen, and the fraction
existing in the
organic mass, plays an important role in the fraction of organic material as
well as in
the formulated product, ie in the balance of minerals and organics.
Nitrogen is an element that participates in all metabolic reactions of the
plant,
thus having great importance. It is present in the composition of the most
important
biomolecules such as ATP, NADH, NADPH, chlorophyll, proteins and several
enzymes. 0 nitrogen has been intensively studied in order to maximize the
efficiency of
their use. To this end, we have sought to reduce the loss of nitrogen in the
soil, as well
as improving the absorption and metabolization of said element inside the
plant.
The efficiency of the use of nitrogen to the soil refers to the recovery
degree of
this element by plants, whereas the losses which usually occur. Typically,
less than
50% of the nitrogen applied as fertilizer is utilized by the crops. The losses
in the soil
are due to the numerous processes in which nitrogen is subject. Nitrogen is
lost mainly
by nitrate leaching, volatilization of ammonia emission and N20 and other
nitrogen
oxides, according Anghinoni, I., "Nitrogen in the states of Rio Grande do Sul
and Santa
CA 02798880 2012-11-08
6
Catarina", 1986, at: Santana, MBM, "Nitrogen in Brazil," CEPLAC / SBCS,
Ilheus,
1986, Chapter I, p. 1-18.
The absorption and assimilation of nitrogen by the plant are multivariate
processes that are integrated with the overall plant metabolism. The
multivariate
metabolism of nitrogen complicates the identification of specific metabolic
steps that
are most limiting for increased productivity. The source of nitrogen
assimilation and
location (air or ground) may be important, especially in growth conditions in
which
energy availability is limiting. The care that the nitrogen be present in
certain
proportions and in certain moments, as the cycles of the plants, help in
adding organic
matter nitrogen via medium to high nitrogen concentration and / or addition of
organic
compounds with high concentration of this element such as amino acids,
allowing
better balance of the carbon: nitrogen, searching for relationships 10:1 to
30:1.
It can be said that in addition to nutrients (N, P, K, Ca, Mg, S) and micro
nutrients (Zn, B, Mn, Cu, Fe, Mo, Co, Si, and so on) essential to plant
development
complements the use of these biological agents that act in different ways on
the ground
and even the plant. They may be called regulators, stimulants or biological
activators.
A mixture of two or more plant growth regulators other substances (amino
acids,
nutrients and vitamins) is designated as biostimulant as Castro, PR CE,
Vieira, EL,
"plant growth regulators and biostimulants in maize," in: Fancelli, AL,
Dourado Neto, D.
(Ed.), "Corn: strategies for high productivity," Esalq / USP / LPV,
Piracicaba, 2003, p.
99-115. The use of these substances increases in importance as it increases
the
genetic potential of crops and when aimed at obtaining high yields and
improving the
quality of the picked product.
The identification and cloning of genes for transporters of high affinity
plant shall
assist in breeding programs to obtain plants more efficient at absorbing
nitrogen in a
wide range of availability of this nutrient in the soil.
The addition of amino acids allows more nutrients lead to the formulation,
either
alive and active, allowing the reactions of organic matter are more
accelerated and
also helping to organic acids present in the form of organic matter-rich
compounds to
the soil solution, which will be absorbed by the plant.
The role of amino acids is important and has been studied very little
nutrition in
the soil (root). It can be said that the mechanism by which the level of amino
acids in
the phloem of the root regulates the absorption and assimilation of nitrogen
by the
plant was suggested by Imsande, J., Touraine, BN, "Demand and regulation of
nitrate
CA 02798880 2012-11-08
7
uptake," Plant Physiology, Lancaster, Vol 105, pgs. 3-7, 1994. It is based on
the
realization that, during the fast vegetative growth rates are high nitrate
reduction and
synthesis of amino acid in the leaves. There is even used most of the amino
acids for
the synthesis of chlorophyll, rubisco and other proteins and, thus, is the low
level of
amino acids in the phloem that enters the roots, which leads us to admit that
this can
and should be part of system for plant nutrition. Moreover, during the
reproductive
stage decreases the rate of reduction of nitrate; in parallel, depending on
the
remobilisation of nitrogen leaf inflorescence development, increases the amino
acid
export from leaves, enriched with these compounds, the phloem which enters the
roots. The proposed mechanism suggested that these amino acids lead to a
decrease
in the rate of absorption of NO3. The action of amino acids on the absorption
of
nitrogen is not yet known. Probably, the high levels of amino acids in roots
inhibit the
action of transporters in the membrane of NO3 and synthesis of the enzyme
nitrate
reductase (Lea, PJ, "Nitrogen Metabolism", in: Lea, PJ, and Leegood, RC,
"Biochemistry of and Plant Molecular Biology ", John Wiley and Sons,
Chichester,
1993, Chapter 7, pgs. 155-180, and Lea PJ," Metabolism of nitrogen primer ",
In: Dey,
PM and Harborne, JB," Biochemistry plant ", Academic, 1997, Chapter 7, pgs.
273-
313).
During the development cycle of the crop, these organic compounds depending
on their composition, concentration and proportion of substances, stimulate
plant
growth by increasing cell division, cell elongation and cell differentiation
and thereby
increase the absorption capacity nutrients and water, reflecting directly in
development
(seed germination, growth and development, flowering, fruiting, senescence)
and crop
productivity (Castro, PR CE, Vieira, EL, "bioregulators and biostimulants in
maize," in :
Fancelli, AL, Golden Neto, D. (Ed.), "Corn: strategies for high productivity,"
Esalq / USP
/ LPV, Piracicaba, 2003, p. 99-115). The action of biological activators,
besides the
various functions, have interaction with plant nutrition, increasing
efficiency in the
uptake, transport and assimilation of nutrients. The nature of non polar
organic
compounds enhance the ability of movement of substances through membranes,
which are non polar, according to the constitution thereof (proteins and
phospholipids).
The patent literature also reports some processes for obtaining fertilisers
containing nitrogen, phosphorous and potassium. However, there are no reports
of
processes to enrich the soil with carbon.
CA 02798880 2012-11-08
8
The Brazilian patent application PI 8303056-5 describes a process for
obtaining
organic fertilizer simple and organomineral fertilizer that, after treatment,
stabilization
and degradation of manure, it is pelletized, dried and milled. The resulting
product can
be supplemented with humus lignite, yielding, after granulation, a granular
organic
fertilizer simple. Said resulting product can still receive other raw
materials in addition
to various mineral humus lignite, yielding, after granulation, a organomineral
fertilizer.
After granulation of any of the two types of fertilizer, these are packaged in
plastic
bags, completing the industrial production cycle.
More specifically, we can see that at a certain stage of that process manure
is
supplemented with organic matter, nitrogen, phosphorus, potassium, calcium,
sulfur,
magnesium and micronutrients, whose main sources used are: humus lignite,
potassium nitrate, nitrate ammonium sulfate, ammonium chloride, ammonium
phosphate, urea, superphosphate, DAP, MAP, potassium chloride, among others.
However, the said patent application PI 8303056-5 does not exploit
specifically the
importance of using biological agents (humic and fulvic acids) that are added
for further
biochemical reactions in the soil. Moreover, said patent application does not
provide
strict control of the main relationship between the nutrients, such as N / C,
C / P, K I C,
AMF / C and AHF / minerals. This control is crucial for running the process
proposed
by the present invention.
The Brazilian patent PI 8600757-2 relates to a process for producing
fertilizer
from a wide series of organic residues urban, industrial or agricultural. Said
process
has a low energy cost, can be applied in small, medium or large scale and
produces a
fertilizer completely free of pathogens.
However, the process proposed by this invention does not provide reaction
steps acidic and / or alkaline in order to treat the initial organic mass and
eliminate
pathogens, there is a curing step in the process of the present invention, and
the
patent document PI 8600757-2 does not comprise a stage of activation and
enhancement of biological mass processed using AHF and MFA, as proposed in the
present invention.
The Brazilian patent PI 0704583-2 discloses a process that aims to take the
carnallite as the source of the potassium and magnesium in chemical fertilizer
granulation. The carnallite (KCI .MgCI2.6H2O) is added to a granulator drum-
type rotary
plate, "pug-mill", among others, along with DAP, ammonia and other
conventional
fertilisers to form magnesium phosphate and ammonium double (NH4.MgPO4.nH2O),
CA 02798880 2012-11-08
9
ammonium chloride and potassium chloride. Therefore, in addition to allowing
the use
of carnallite, extremely hygroscopic characteristic, the process allows the
obtainment of
phosphate dual magnesium and ammonium. In this double salt, the phosphorus is
in a
slow release form, which means that the plant may gradually absorb it, thus
reducing
the losses in the soil. Such a process provides a further embodiment of the
invention
where the carnallite is replaced by a solution of magnesium chloride. In this
case, one
obtains a fertilizer rich in magnesium, where the phosphorous is in a form of
slow
release to plants. Furthermore, an embodiment is shown where DAP is replaced
by
ammonia and phosphoric acid.
It is important to note that the patent in question refers more specifically
to the
enrichment of fertilizer in terms of nitrogen, potassium and phosphorus, and
does not
concern the carbon content available. Moreover, no mention is made regarding
the use
of biological agents to compose fertilizer formulation.
The Brazilian patent application PI 0606043-9 refers to a fertilizer that has,
in its
composition, in the form of protein nitrogen (protein vegetable and animal)
assayed in
equilibrium, which leads to a rapid and sustained beneficial effect on crops
and in the
soil. The said fertilizer composition is deodorized by biological additives
enzymatic
catalysts, both of which improve digestion and processing of organic materials
and
facilitate integration with the mineral components.
The said patent document provides the use of biological agents, to promote
biochemical reactions in the soil. There is also some concern controlling the
carbon
content in order to enrich the soil with this essential nutrient. However,
said patent
application PI 0606043-9 does not provide the same concentration ranges of the
main
nutrients, all of which are below that obtained by the process object of the
present
search. Furthermore, the order in reference does not describe the process for
obtaining
the fertilizer being restricted only to the composition.
As mentioned above, there is an increasing need for regeneration and increase
soil fertility, replacing thereto its main mineral nutrients and carbon, in
order to replace
the conventional fertilisers.
Object of the Invention
It is the object of the present invention to provide a process for the
production of
organic and organomineral fertilisers based on the carbon concentration of the
organic
material in nature and relating said concentration with fractions of natural
minerals and
CA 02798880 2012-11-08
/ or minerals processed by promoting a high physical interaction the materials
involved
and enabling and enriching biological compound.
It is further object of this invention to describe these fertilisers, using
the
process described herein.
Description of the Figures
The present invention will be described based on the attached figures,
wherein:
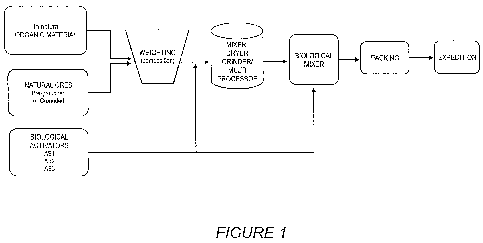
- Figure 1 shows a schematic diagram, which illustrates the production of
powdered or grounded organic fertilizer;
- Figure 2 shows a schematic diagram, which illustrates the production
organomineral fertilizer granulates, and
- Figure 3 shows a schematic diagram, which illustrates the production of
powdered or grounded organic fertilizer.
Detailed Description of the Invention
= Organic Fertiliser
The organic fertilisers of the present invention are formulated with 100%
natural
raw materials, organic materials of different origins and natural mineral
without
chemical processing, just physical.
The compositions of organic fertilizer of the present invention consider the
percentage of organic matter where it must reach the minimum percentage of 15%
carbon, 1% nitrogen, the maximum carbon: nitrogen ration (C:N) of 18:1 and
cation
exchange capacity: total organic carbon (CTC:C) ratio of 20:1. The remaining
guarantees may be declared as the results of the composition, wherein each raw
material has its value of macro and micronutrients needed for plant
development.
The combined organic raw materials in nature and minerals takes place in
conditions where these interactions are intense, caused by the reduction of
the entire
mass of the particles that make up the formulation, in the same instant. The
intense
mixing, drying of the sensitive mass and particle size reduction occurs in
traditional
equipment such operations conventionally used for fertilizer and mining
industries are
on the market which are capable of promoting these functions (blenders, dryers
and
mills) or by a Multiprocessor mill equipment which represents the set of
equipment
described, properly processing the heavy weight, making them unique, uniform,
low
humidity and with a particle size which may vary from microgranulate (particle
size of
0.5 to 2.5 mm) to powder (particle size 0.1 to 0, 5 mm).
CA 02798880 2012-11-08
11
The raw materials most commonly used in the formulations of organic
fertilisers
are:
a) Organic raw materials:
- mammon pie;
- Cotton pie;
- Sunflower pie;
- Pie from cane sugar plant filter;
- Sugar cane bagasse;
- Cotton waste;
- Bark of coffee;
- Manure from livestock;
- Manure chicken (hen);
- chicken bed;
- Manure from pigs;
- Rumen of cattle;
- Blood of cattle, and
- Wood ash.
b) mineral raw materials (natural, in situ - physical process only):
- natural fhosphates;
- Reactive phosphates, natural;
- Feldspar;
- Limestones;
- Gypsum;
- Sulphur;
- Vermiculite;
- Marble powder;
- Minerals weathered, and
- Basalt.
The preparation of the formulations of fertilisers by the process NPK +C for
manufacturing of organic fertilisers has technical premises in order to act to
provide the
nutrients present in natural minerals and organic matter present in natura,
allowing the
biological-chemical formulation can meet the agronomical demand.
The basic assumptions are: the concentrations of organic matter (O.M.%) in the
composition, i.e., "compostable" (% C) and natural minerals; chemicals that
possess
CA 02798880 2012-11-08
12
these minerals in their composition and their cargoes ionic (+) and (-), and
the
concentration of biological agents and relations with the carbon and soil
minerals, thus
observing chemical and biochemical reactions that form the formulated bulk.
The stoichiometric balance of said formulations - carbon versus natural
minerals - is the product of the mass of elements such as nitrogen, which is
active
energy of organic molecules, and other nutrients (+) and (-) present in the
dough and
that sometimes in practice, were also studied in the field of private jobs in
various crops
without knowledge about the process used to obtain the product by the farmer,
with
very satisfactory results in productivity and quality (brix grade). These
established
relationships are presented below.
Nutrient Relations (Macronutrients and Micronutrients) / Carbon - "Minimum
Limits":
Nitrogen: 0.9% N /% C
Phosphorus: 1,16% P /% C
Potassium: 1.35% K / C%
Micronutrients: 1.0% Mic / C%
The organic matter in nature to be processed so that the carbon has minimal
losses, which occur in process NPK + C, from stoichiometric balance mass union
of
said organic matter with other plant nutrition elements, such as mineral and
the
processing equipment (mixer, dryer and traditional mill or windmill
multiprocessor) that
interact intimately mass. Next, the mass that is biologically active and
enriched
(biological agents, AHF and AMF), allowing an average decomposition of the
same, or
is processed in the set of equipment (mixer, dryer and mill), promoting
interactions
between tiny particles transformed the equipment. All this charge goes to the
ground
and there complements the cycle in a stage of decomposition and carbon
biologically
involved, lossless, reacting with nutrients, making them available to plants
and not
allowing their losses with agents that retain these nutrients by ionic
reactions (eg, iron
and aluminum), forming insoluble complexes, or by leaching.
Biological agents, AHF - "humic and fulvic acids" (called AB1) and AMF -
"Amino Acid fish or vegetables" (called AB2), added in steps after processing
the
formulated for intensive mixing, drying and large particle size reduction
(less 35 mesh
to 200 mesh), or mills in multiprocessors, and during the granulation process
will act as
follows:
CA 02798880 2012-11-08
13
1. AHF - "humic and fulvic acids" (AB1): The presence of fulvic acid has an
action biominerals ion forming organic complexes, which retain the cations and
anions
of mineral raw materials present in the composition of the organic fertilizer
of the
present invention. The action of these complexes biominerals prevents the
elements
nutrients available to the soil solution and consequently, the plant, remain
loose soil,
because in the presence of strong ions can be sequestered and / or complexed,
forming insoluble complexes and thus rendering nutrients unavailable to follow
the
plan. Thus, the presence of fulvic acid in the amount and ratio of ions
present in the
mass of soluble or solubilized mineral allows them not to be lost to the
ground. The
macro and micro nutrients added to soil fertilization or even existing in this
indispensable to plants, has your participation in mass values allowed on the
upper
middle (as tests on experimental and commercial areas) require corresponding
mass
of humic reactions for the formation of biominerals, taking into account the
ionic charge
of these elements.
AHF-Minerals ratio 0.70 mL AHF / kg Macro and Micronutrients.
Organic matter in the most advanced stage of decomposition, observed by the
carbon: nitrogen less than 18, so these organic acids (humic, fulvic and
maleic). Thus,
organic matter richer in carbon form more desirable these acids, thereby
allowing the
chelation of ions present in needed nutrients to all plants.
The formulations should thus take into account the concentration of carbon
from
one or more organic materials, preferably those that may arise from animal
sources
(animal waste) and plant sources (residues of agroindustries and cultures) and
are
mixed as seen in Kiehl, EJ, "50 Questions and Answers about Organic Compound",
Sao Paulo, PMSP / ESALQ, 1979, p.9, 1. 17, wherein the waste has:
Plant: High Concentration Carbon -> 60% C
Low Capacity Decomposition - 60 to 120 days
Animals: Low Concentration Carbon - <50% C
High Decomposition Capacity - 30 to 60 days.
The addition of the formulated product AHF, in the presence of medium to high
concentration of organic matter (carbon) and medium to low concentration of
humic
acids formed at medium to low stage of decomposition of the organic aims to
help
reactions, catalyzing the decomposition and hence chelating the soluble salts
present
in the formulated mixture.
CA 02798880 2012-11-08
14
The amount of AHF added is sufficient to the chelation process (formation of
compounds biominerals) in the established relationship, leaving them being
formed by
the decomposition of organic matter over and formulated enriching the end
product, to
help further biological activities.
2. AMF - "Amino acids from fish and vegetables" (AB2): These have the
purpose of playing a dual function in the formulation, namely:
a. They enable the organic matter that is in mid stage of decomposition and
suffered physical intervention to reduce humidity and the number of
microorganisms
(by high temperature and the extraction of the water contained in the organic
matter).
This agent resets the biological nutrient and carbon to nitrogen (amino acid
composition) of organic matter, providing conditions to help in the process of
decomposition of organic matter in the formulated product. Thus, replacing the
carbon
lost during the process of mixing, drying and grinding mill or a
multiprocessor,
activating intensely organic matter formulated (biologically) and enriching
the
properties and interactions of organic matter in the soil allowed to recover
unavailable
nutrients in the soil for the plant, making them available.
b. The presence of phosphates in the form of natural mineral forces that act
intensively these organic matter, attacking them with the organic acids formed
and
added, rendering biominerals organic compounds available to the plant.
The reactions of attack of organic acids with any mineral is a common process
in the soil or even when mixed with these organic matter, such as with rock
phosphates
(phosphorous) where microorganisms play the role of breaking the ore,
reactions that
occur medium to long term (30 to 120 days) depending on the quality of organic
matter
and carbon concentration, thereby increments the biological process when one
has the
presence of amino acids, charged nitrogen agents.
AMF - Carbon ratio: 2.6 mL AMF / C%
AMF - Natural Phosphate ratio: 0.65 mL AMF / C%
In the compositions with the presence of natural phosphates, the sum of the
volume used for the carbon with the volume is used for the natural phosphate,
in order
of relative importance, since these act in both situations (the purposes
described
above).
Equivalence of consumption: 42% for carbon
58% for rock phosphates.
CA 02798880 2012-11-08
It should be added that amino acids are great enhancers for the soil solution,
being also used in plant nutrition and thereby enriching the fertilizer
product.
The nitrogen of these amino acids, in its organic form, participates in the
reactions of formation of biominerals compounds and biochemical compounds in
plants, which are carried by the xylem (input channel system of the plant),
favoring the
process of ATP production, translated by improved the reactions of synthesis
of
chlorophyll and therefore the increase in glucose content (Taiz, L. and
Zeiger, E.,
"Vegetable Physiology", Sao Paulo, New Haven, 2004, p. 719).
The formulations will go to the ground with part of their ongoing reactions
and
mineral nutrients and organic foods available to the plant and throughout the
hours and
days following organic reactions continue: organic acids present in the
formulation and
added continue attacking minerals and gradually providing the remaining
percentage of
nutrients to the plant. The glycine, a component of the precursor amino acid
and for the
synthesis of chlorophyll, is an important metal chelator, which in turn are
important as
micronutrients to plants.
There are strong interactions of ionic character in the soil solution, which
is
formed and is present in areas near the root. Said solution down the channels
of the
phloem, reaches the root and reacts with the organic and inorganic compounds
forming complexes biochemical enrich it. Then, the solution rises to the plant
by the
xylem to leaves carrying nutrients for composing the preparation of
photosynthesis and
fruits.
These reactions and behaviors are already seen in nature in forests and other
biomes with fall leaves and plant debris in the soil, which being in decay -
(formation of
organic acids) are attacking the minerals in the soil, making them available
to plants.
Thus, the process of formulating search act similarly, but with greater speed
and
intensity in reactions with the addition of biological agents, which shall
enhance the
biological activity of natural organic matter, solubilizing nutrients of
natural minerals
and mineralizing, i.e. , converting to organic minerals, the nutrients in
organic matter.
The formulation (mass) is processed biologically activated increasing the
biological activity of organic matter, and enriching this whole mass in the
post-
processing in the mixer, dryer and mill or a mill multiprocessor. As stated
earlier, these
elements act forming organic biochemical reactions which generate rich
molecules
that, in the soil solution, to follow the plant to aid in the metabolism of
this, responding
to further development and improvement of fruits, both quantitatively and
qualitatively.
CA 02798880 2012-11-08
16
The organic acids formed in the stage of decomposition in which organic matter
is processed (raw material) also will go to the grinder, where it will
continue the process
of "decomposition" to reach a favorable environment, such as humidity (plant
in the
rainy period) and temperature, allowing reaction rates ultra rapid (minutes or
hours)
because of the small particles that have been transformed nutrients during
physico-
chemical and physical processing, and also received additives with the purpose
of
being biologically activated after processing, wherein part of the
microorganisms is lost,
and wherein mass enrichment with organic elements important for soil and plant
is
conducted.
Thus, the organic fertilizer obtained via said process of NPK + C is the
result of
both a physical and biological processing of a formulation that aims to
increase
chemical and biological interactions, promoting high-speed (kinetic,
biochemical) of
formation of biochemical compounds, and aiming not lose the high amounts of
carbon
typically lost in conventional processes of decomposition of organic matter
(compost),
which deplete the organic matter in 40% to 70% relative to the mass of carbon
present
and primarily responsible for the formation of organic acids, and hence of
biological
reactions.
It is recommended to leave the organic fertilizer product, which is an organic-
mineral product, stored for 72 hours before being shipped.
A plant for the production of said organic fertilizer comprises:
- Shredder knives used to grind organic matter (a)
- Rotating sieve to organic matter;
- Weighing hopper (bin) for preparing the composition;
- Conveyor belt I used to transfer the heavy material to the mill or blender;
- Knife Mill;
- Helical mixer;
- Rotary drum dryer;
- Hammer mill;
- Mill multiprocessor (b)
- I Mixer (biological activator) used to make the application of biological
agents;
- Bucket elevator used to transfer the processed formulated to silos or
bagging
"Big Bag";
- Bin reception made for packing;
- Bin reception formulated to condition the "Big Bag" and
CA 02798880 2012-11-08
17
- Bin flow formulated to the "Big Bag".
(a) Equipment not used when using the grinder multiprocessor.
(b) The mills multiprocessors replace the entire mixer, dryer and hammer mill.
A better understanding of said plant production of the organic fertilizer for
the
present invention can be obtained from Figure 1.
- Organomineral fertilisers
The organomineral fertilizer of the invention is formulated with organic
matter
from various sources and inorganic, natural and / or chemically processed
minerals.
The organomineral fertilizer compositions of the present invention, in terms
of
primary macronutrient, must consider the percentage of organic matter (carbon)
at
least 8% and the sum of NPK nutrients (macronutrients) at least 10%. Relative
to side
macronutrients (Ca, Mg and S), must reach the minimum sum of 5%. Regarding
micronutrients, is required a sum of at least 4%. Other guarantees may be
declared as
the results of the composition, where each raw material has value of macro and
micronutrients needed for plant development.
The combined organic raw materials in natura as well as natural and / or
processed minerals occurs in conditions where these interactions are intense
and
promoted by reduction of the entire mass of the particles that make up the
formulation,
in the same instant. The intense mixing, drying and particle size reduction
occurs in
high traditional equipment such operations conventionally used in the
fertilizer industry
and mining existing on the market, which promote these functions (blenders,
dryers
and mills) or through a mill multiprocessor which represents the set of
equipment
described above and is responsible to properly handle the heavy weight, making
them
unique, uniform, low humidity and particle sizes which can vary from granules
(mesh
size 2 to 4mm) to a powder (particle size 0.1 0.5 mm).
The raw materials most commonly used formulations are:
a) Organic raw materials:
a) Organic raw materials:
- Castor pie;
- Cotton pie;
- Sunflower pie;
- Pie from cane sugar plant filter;
- Sugar cane bagasse;
- Cotton waste;
CA 02798880 2012-11-08
18
- Bark of coffee;
- Manure from livestock;
- Manure from chicken (hen);
- chicken bed;
- Manure from pigs;
- Rumen of cattle;
- Blood of cattle, and
- Wood ash.
b) Mineral raw materials:
b.1- Natural Minerals:
- Natural phosphates;
- Reactive natural phosphates;
- Feldspar;
- Limestones;
- Gypsum;
- Sulphur;
- Vermiculite;
- Powder of marble;
- Weathered minerals, and
- Basalt.
b.2- Processed Minerals:
- Urea;
- Ammonium sulphate;
- Ammonium nitrate;
- Single superphosphate;
- Triple superphosphate;
- Monoammonium phosphate (MAP);
- Diammonium phosphate (DAP);
- Potassium chloride, and
- Potassium nitrate.
The preparation of fertilizer formulations by the NPK + C process of the
present
invention does comprise technical aspects for the fabrication of organomineral
fertilizers, which have to act in order to provide the nutrients presented in
terms of
CA 02798880 2012-11-08
19
natural minerals, processed minerals (product of chemical reactions) and
organic
matter ( fresh) , thus enabling said composition to meet agronomical
requirements.
The basic assumptions are: the concentrations of organic matter (OM%) in the
composition, ie "compostable" organic carbon (% CO), natural and / or
processed
minerals; chemicals that possess these minerals in their composition and their
ionic
charges (+) and (-), as well as the concentration of biological agents and
relations with
the carbon and soil minerals, thus observing chemical and biochemical
reactions that
shall occur in the formulated bulk.
The stoichiometric balance of such formulations - carbon versus natural and /
or
processed mineral - is the result of the mass of elements such as nitrogen,
which is
active energy of organic molecules, and other nutrients (+) and (-) in the
mass, that
upon gathering minerals processed assumed as soluble salts, have high activity
and
dissociation in short time and with high physical interactions, causing
instant reactions.
These compositions, in a practical way, yet without reaching high levels of
processing
with reduced physical, were also studied in private works in various field
crops such as
grains (corn and soybeans) and fruit (melons, bananas, etc..) without
knowledge about
the process used to obtain the product of the farmer, with satisfactory
results in
productivity and quality (degree brix). These established relationships are
presented
below.
Nutrient Relations (Macronutrients and Micronutrients) / Carbon - "Minimum
Limits":
Nitrogen: 0.9% N /% C
Phosphorus: 1.16% P / C%
Potassium: 1.35% K / C%
Micronutrients: 1.0% Mic / C%
Minimum threshold established by carbon organic matter (OM): 2.1 kg OM / kg
soluble salts
The formulation (mass) is processed and biologically activated increasing the
biological activity of organic matter, thus enriching this whole mass in a
step after
processing in terms of physical and physicochemical aspects as well as in the
granulation step. These biological elements act as mentioned above, forming
biochemical reactions, retaining soluble nutrients present in the formulation
and
achieving rich molecules which, in the soil solution to follow the plant
aiding in
CA 02798880 2012-11-08
metabolism thereof, responding to further development and improvement of the
fruits,
both quantitatively and qualitatively.
The biological agents, AHF and MFA, are added in steps after processing the
formulated composition in the mixer assembly, drier and mill for high
reduction particle
size (menso of 35 mesh to 200 mesh) or in the multiprocessor mill, and during
the
granulation process will act as follows:
1. AHF - "humic and fulvic acids" (AB1): The action of these organic acids
relies
in the composition thereof. The presence of fulvic acid has an ionic action
forming
biomineral organic complexes, which retain the cations and anions of the
mineral raw
materials present in the composition of the organomineral fertilizer of the
invention. The
action of these biomineral complexes prevents the elements nutrients available
to the
soil solution and consequently, the plant, remain loose in the ground, since
the heavy
ions eventually presented can be sequestered and / or complexed, forming
insoluble
complexes thus preventing the nutrients from reaching the normal pathway.
Thus, the
presence of fulvic acid in ratio amounts of these ions present in the mass of
soluble or
solubilized mineral the allows it to retain and prevent losses to the ground.
The macro
and micro nutrients added to soil fertilization or even existing in this
indispensable to
plants, has your participation in mass values allowed on the upper middle (as
tests on
experimental and commercial areas) require corresponding mass of humic
reactions
for the formation of biominerals, taking into account the ionic charge of
these elements.
Relationship AHF-Minerals: 0.70 mL AHF / kg Macro and Micronutrients.
The organic matter in the most advanced stage of decomposition, observed by
the carbon: nitrogen ration being less than 18, does form these organic acids
(humic
and fulvic maleic). Thus, organic matter richer in carbon form is more
desirable in terms
of these acids, thereby allowing the chelation of ions present and that are of
great
value for all plants.
The formulations should thus take into account the concentration of carbon
from
one or more organic materials, preferably those that may arise from animal
sources
(animal waste) and plant sources (residues of agroindustries and cultures) and
are
mixed as seen in iehl, EJ, "50 Questions and Answers about Organic Compound",
Sao
Paulo, PMSP / ESALQ, 1979, p.9, 1. 17, where waste has:
Plant: High Concentration Carbon -> 60% C
Low Decomposition Capacity - 60 to 120 days
Animals: Low Concentration Carbon - <50% C
CA 02798880 2012-11-08
21
High Decomposition Capacity - 30 to 60 days
The addition of AHF in the formulated product in the presence of medium to
high concentration of organic matter (carbon) and medium to low concentration
of
humic acid formed by medium to low stage of decomposition organic matter,
intended
to help the reaction by catalyzing the decomposition and hence chelating the
soluble
salts present in the formulated mixture.
The amount of HFA added is sufficient to chelation process (formation of
compounds biominerals) in the established relationship, leaving them being
formed by
the decomposition of organic matter over and formulated enriching the end
product,
which will furthermore the biological activity s soil.
2. AMF - "Amino acids in fish and vegetables" (AB2): These play a double role
in the formulation, thus providing a dual function, namely:
a. They enable the organic matter that is in mid stage of decomposition and
suffered physical intervention to reduce humidity and the number of
microorganisms
(by high temperature and the extraction of the water contained in the organic
matter).
This agent resets the biological nutrient and carbon to nitrogen (amino acid
composition) of organic matter, providing conditions to assist in the process
of
decomposition of organic matter in the formulated product. Thus, replacing the
carbon
lost during the process of mixing, drying and grinding mill or a
multiprocessor,
activating intensely the formulated organic matter (biologically) and also the
properties
and enriching 'interactions of organic matter in the soil allowed recovery
unavailable
nutrients in the soil for the plant, making them available.
b. The presence of phosphates in the form of natural mineral forces the
organic
matter on acting intensively therein, attacking them with the organic acids
formed as
well as the added ones, rendering biomineral organic compounds available to
the
plant.
The reactions of attack of organic acids with any mineral is a common process
in the soil or even when mixed with these organic matter, such as with rock
phosphates
(phosphorous), wherein the microorganisms play the role of breaking the ore,
reactions
occurring in a medium to long term (30 to 120 days) depending on the quality
of the
organic matter and carbon concentration, and thus it increments the biological
process
when it is the presence of charged amino acids comprising nitrogen.
Carbon - AMF Ratio: 2.6 mL / C%
AMF - Natural Phosphate Ratio: 0.65 mL MPA / C%
CA 02798880 2012-11-08
22
In the compositions the presence of natural phosphates takes up the sum of the
volume used for the carbon with the volume used for the natural phosphate, in
order of
relative importance, since these act in both situations (the purposes
described above).
Equivalence of consumption: 42% for carbon
58% for natural phosphates
It should be noted again that the amino acids are large enhancers of soil
solution, and are also used in plant nutrition and thus enrich the fertilizer
product.
As mentioned earlier, the nitrogen of these amino acids, in organic form,
participates in the formation reactions of biomineral compounds and
biochemical
compounds in plants, which are carried by the xylem (input channel of the
system
plant), favoring the production processes ATP, translated by improved
synthesis
reactions of chlorophyll and therefore the increase in the glucose content
(Taiz, L. and
Zeiger, E., Vegetable Physiology ", Sao Paulo, New Haven, 2004, p. 719).
The formulations will go to the ground with part of their ongoing reactions
and
nutrients available to the plant and, over the following days, organic
reactions continue
with active microorganisms and organic acids present, and added generated
(AHF) in
the formulation, which will continue attacking minerals since they find
favorable
environment such as moisture (plant in the rainy season) and temperature,
allowing
reaction rates ultra rapid (minutes or hours) because of the small particles
in which
were transformed nutrients in physical and physico-chemical processing,
thereby
providing the remaining percentage of nutrients to the plant. These humic
organic acids
also act chemically and retain the cations of the mineral nutrients presented
in
chemically processed matter (soluble salts), forming biochemicals and not
allowing
them to become free and resulting in the retention thereof by the soil strong
anions,
such as iron and aluminum in the case of phosphorus as well as the volatilized
and
washed away such as nitrogen and potassium. Thus, no losses occur and the
efficiency of retention of soluble nutrients added to soil, which must reach
the plant, is
above 95%.
There are strong interactions of ionic character in the soil solution, which
is
formed and is present in areas near the root. Said solution goes down the
channels of
the phloem, reaches the root and reacts with the organic and inorganic
compounds
forming biochemical complexes that enrich it. Then, the solution rises to the
plant by
the xylem to leaves carrying nutrients for composing the preparation of
photosynthesis
and fruits.
CA 02798880 2012-11-08
23
These reactions and behaviors are already seen in nature in forests and other
biomes with fall leaves and plant debris in the soil, that upon the
decomposition thereof
(formation of organic acids) are attacking the minerals in the soil, making
them
available to plants. Thus, the formulation process seeks to act in the same
way, but
with greater speed and intensity of reactions, with the addition of biological
agents,
which shall enhance the biological activity of natural organic matter,
solubilizing
nutrients from natural minerals and nutrients from retaining processed
minerals
(chemical soluble salts) and reducing nutrients to tiny particles, which allow
better
chemical and biological interactions.
Thus, the biofertilizer obtained via the NP + C process is the result of the
processing of physical and biological formulation that aims to increase
chemical and
biological interactions, promoting high-speed (kinetic biochemical) formation
of
biochemical compounds, and does not lose the high amounts of carbon normally
lost in
conventional processes of decomposition of organic matter (compost), which
deplete
the organic matter 40% to 70% relative to the mass of carbon present, the
principal
cause the formation of organic acids and hence of biological reactions.
It is recommended to let the biofertilizer product stored for 72 hours before
the
shipment thereof.
A plant for the production of said organomineral fertilizer comprises:
- Shredder knives used to grind organic matter(s);
- Rotary sieve rotary for organic matter (a)
- Weighing hopper (bin) for preparing the composition;
- Conveyor belt I used to transfer the heavy material to the mill or blender;
- Knife Mill;
- Helical mixer;
- Rotary drum dryer;
- Hammer mill;
- Mill multiprocessor (b)
- Mixer No.] (biological activator) used to make the application of biological
agents;
- Bucket elevator No. I, used to transfer the processed granulesto the
granulator(c) ;
- Granulator used to process the powder formulated into granules with addition
of water and biological element( );
CA 02798880 2012-11-08
24
- Rotary dryer used to dry the formed pellets(c);
- Vibrating sieve used for sorting the formed beads(c);
- Mill used to grind the thick granules, that were disregarded and should
return
to the process(c) ;
- Conveyor belt No. II, used to transfer the fine granules and ground for
return
to process(c)
- Bucket elevator No. II, used to transfer the processed formulated to bins or
bagging such as a "Big Bag";
- Bin reception for the packing of the formulated product;
- Bin reception to condition the formulation in a "Big Bag" and
- Bin flow for the formulation in a "Big Bag".
(a) Equipment not used when using the grinder multiprocessor.
(b) The mills multiprocessors replace the entire mixer, dryer and hammer mill.
(c) Complementary equipment for a manufacturing plant for organomineral
fertilizer pellets.
The others are the equipment for a manufacturing plant for powdered or
pulverized fertilisers.
A better understanding of this production plant organomineral fertiliser of
the
present invention can be obtained from Figures 2 and 3, which illustrate two
different
arrangements of said production process, namely: production of granular
fertilizer
(Figure 2) and production of powdered or pulverized fertilisers (Figure 3).
The examples presented below are intended merely to illustrate the invention
and facilitate the understanding thereof, having no limiting character.
Examples
Assays were performed to compare the efficiency of conventional fertilisers
with
organomineral fertilizer of the invention in soybean.
For the conduction of these trials, there were chosen areas where the soil had
low levels of phosphorus and medium texture. We used a variety of soy early so
as to
assess the planting of winter maize (residual effect of sources of P205).
Therefore, we
evaluated the effects of three main treatments, according to the present
invention, with
different sources and proportions of P205 and with three doses of P205 using
soybean
as test plant, according to Table 1, attached. Furthermore, the table shows
the effects
of treatment with a conventional formulation and the effects of treatment with
a blank
formulation (control).
CA 02798880 2012-11-08
Table 2 shows the concentrations of KC1 used in treatments 10, 11 and 12,
previously shown in Table 1 for a conventional formulation.
The quantities of P2O5 used were based on the total concentration of sources
of
P2O5, being exemplified in Table 3 attached.
It is important to note that the minimum quantity of formulation for each
treatment is 300 Kg.
The experimental design used in these trials was randomized blocks, consisting
of thirteen treatments in four replications for a total of fifty-two
installments.
Each plot consisted of ten lines spaced 0.5 meters in length by 50 meters,
with
a total area of 250 square meters for each plot. The experiment showed a total
area of
19,000 square meters. Were harvested 10 square meters, only the 4 central
lines 5
meters of each plot, considering three boundary lines for each side of the
plot.
The installation of the experiment was conducted with its demarcation and
subsequent collection of soil samples at 0-20 cm and 20-40 cm for the
characterization
of the experimental area. The conduction of the experimental area followed the
same
standards adopted for commercial areas.
Parameters evaluated during the experiment were:
- Average productivity of grains (kg / ha);
- Dry matter (part of the assessment area of 10 plants in R2/R5 stage);
- Foliar nutrients (season foliar diagnosis: early flowering - RI);
- Average weight of 1000 grains (harvest) and
- Chemical analysis of soil (routine + P + P Mehlich resin).
The results obtained using phosphate, MAP and a combination of the two
products are shown in Table 3, in the annex, as well as the results obtained
using
conventional fertilisers.
Upon analyzing the results mentioned above, we can see that the dosage of 90
kg / ha (equivalent to 45 kg / ha of biofertilizer) was best applied to the
conditions of
fertility in the soil was, and in that the dosage product with 100% rock
phosphate had
the same performance as the conventional product. Moreover, we observe that,
in
different dosages tested, organomineral fertilisers obtained results similar
to
conventional fertilisers, but with 50% less than P2O5 in these treatments.
The doses of P2O5 in treatments with organomineral fertilizer are presented in
the attached tables as well as the dosages of conventional fertilisers,
however they do
represent 50% of P2O5 applied, since the compositions are:
CA 02798880 2012-11-08
26
- Organomineral fertilizers used: NPK 02.10.10;
- Conventional fertilizer used: NPK 02-20-20.
CA 02798880 2012-11-08
U)
O
a)
0 0 0 0 0 0 0 0 0 0 0 0
O CD O CD CA cq CD m CD O 04 O
N
a.
0
------------
O
O O
co
O O r
0 C C
cu CT
m O
O 0 0
-0 + + via~CD
0
CO/) Y Y
o + + 0
O a a. U, Y
cv .2
a.
0 + +
N ca
cm - E
cu m
O L ~ O
,ti
+ Q Q O
0 O N
Q* 0-3: 0
Y
C Y co,
a. _ _
Q -20 N CC) C N 0 N (D +
+ >"-, > O 00~ Lr)
c) Q)
= ~O
a
U O O O >
~ O ~ N
: e- U)
0 1 1 1 C I CO O
O 2
D O N U) O <
Q ~
Q)
O O O
Z N
c _ _ Y Y Y
-NM~t!)CDI~ ppCpONM + + Z p
m U U
H _c ,c
c c ~ =~
c
c a) N cn a)
N M L 3 N E N
N O O co J N N
Icu
0 CO I--
CA 02798880 2012-11-08
O CO N 0) CO N- LO C) N 00 CD LO 0) r- CD 00 0 O CD
O O 0 CD U) CC) "t - U) CD N- N M CO - V- 0) M M 00 M N
O M rt U) 4 U) 00 N-- O CA CD CA P- m r- 6 c C '- M
N Nl- 00 0) M CO U*) O CA O M M M M N r N M It
N N r ~- c- N N N N ~ ~ N ~ ~ N N N~ N
N ~t N M N M M N N N M N N N M M N N co N N
C>J N N O CD O O o0 N M O N N 00 O M O CD O Vt 00
N M N M N M M N N N M N N N M N N M N N
CT
Y C N- N co LC) CO U-) - 0 O N UCD N 0) 0) 0)
) O O O N O M O 0') It
Lim r- CA 0 00 00 I-- c- ~ ~ 0 0 N CA I~ 0 m m
E
O) N N- - 0') I - CD M C'') CO - O - M CA Cn CA `q- 0
U)MU)'[tM(DLO U)N-N't N- MLnMCDMNCDO
N r r r c- ' r r N-
0
co MNCACAMCAr- M~NMON077CA
Uf~CDCp0000N`COCAN-C00000C0 CDN- 000DI- f- V -
U - 7CDM7 Mq~ OCA
N CD 7 C.LU 00 N M M lq- "t 00 CD ~ 0 V- M ~ M 0
N N N M CO M M It N M M N C'7 N CM M M M N M
U) N ~ t N~ N ~ "t T7 00 00 LC) M O Cfl ti CA 00 7 U) (05 CC O
M co M M M M m M co M M co co M M M M
MCD MOCO LO NONCD NU)Ln (`NNN~CnNM
M M M M co co M M M co co co co N N M M N M M C`')
O
Y fB N M 00 -T M 00 M CA CA CA N- N- CA CO N 7 N- 'd
U U) Lo ct U) 't LC) U) Ln 't LC) Ln "t "t U. to LL7 d' LO d' U)
to
cc 000(0N00000 I-t O NNN V. N 0CU
N
00 - 0 N- 0 0 O) O N- 0 0 0 m r- c0 (A O N- I- O)
L t- ~- N N ~ N N N
~- ~- N ~-- N
CQ
O 000)OM[tMMMNCAMN0CDNOM
U-
N N T- N N N N N N N N =- N N N N N N N N N
M U) M M C~ ti 0A - M CO N~ M O N- 7 M U) Nl
O ZonCpm00)N-OON~66M000C)OOLC C C0O
N CY) CY) M IT M M S lq- M M M M 'ct m C`') M V-
a. O N
(II M LO
O
CA M N- 0 M N N- M M ti O N M 0 M M N Cn N,-
U cu r- ,t q- v- m CO N- N - O 0 CO LC) N N U) 00 CD C3) LO
O N N O ( M M N- LC) CD (0 U) N 0 N C!) N Cn (0 00 O
O Cn F-- L() LC) CA M 0 N- N CD N- CX) N CD - CD 0 0 CA U) CD U) 0
L, N N r N N N N N N N M N N N N N N N N N N
0 ~
CL.0 -NC'') NM V'~ NM'- NM q - NM
0
co (1)
(1) 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 O CO CD (.DCD0) 0)CA0) NNNN000OCOCOO0CnCnN
0
O r Q
O
OOU ZZZZZZZZZZZZQQQQQQQQQ
co LL LL LL LL LL. LL LL LL LL LL LL LL
M 0 ~t
0 ~ 0
-0 -0 0 O N M ct Cn CD N- 00 0 0 N M U) O ti CO CA O N
H
CA 02798880 2012-11-08
00 ti M CD r ti O O CO 07 0) M 0) D) 0) N-
M CO M It C) O M LO CD N I- CD 00 co
. . . . .
M Ln 6 6 O N O 4 O N- C) t` r-: 06 In 4 6
00 co I- '[t O0 CD CD P- C) M CD N` N- OD LO r 0) Nl-
~- r ~- ~- r r r N r N r r r N r r
N N N N N N CO M I' 'I- N N . N N N N
. . . . .
00 CD O O 00 00 CC) O N N N N N 00 CO 00 IT
NNNN N N r co CO CO N N CO CO N N N N
d 00 co CD CD 00 It co 0 N O 00 co U*) N r Or N O O
OD O r r M 00 r N- N- r r r () 00 r r N- 0)
N 00 N V-- CO 00 00 00 LO N It O N N N 'ct CD CA
M co M N- N CO N CD CO LO LO N- T- CO N- LO 00
r r r r r r r r r r r r r r r r r N
00 N- CA r N- CO N N ~t M M O tt r N r r CA
N~ 06 r t` (fl N O Ld) N- N- 00 4 ti C6 CD CD CD
M 't co N CO O CO CO 0) r N- 0) r M r r N
. . . . . . .
r CA CD O N r CL7 N O CD 00 CD O CO r` CD N
M N N N CO CO CO CO CO d' CO CO CO 't co N N CO
O CD CD CD M O N N LC) N- O Cb 00 ti CD CD
. . . . . . .
M MMM CO CO 'a M CO CO 't?' CO CO CO CO CO CO
N- U) Lf) O O N N N N- C7) N-. . . . 0) LC) CO O U? CA U7
. .
N CO M M CO CO CO CO N N N CO co CO M CO N CO
M N~ C) N N- r r t. LO M CD CD Lf) C) C7) r
. . . . . . . .
U) 'ct V' rt Lo Lf) Lf) Lf) ~' O LC) lf) 'd' L() Lo
o N CD 00 It N CD OD CD 't CD It CD It 00 CD co 0
C6 ti CD 00 Cn 6 O CA CA Cp 0) O O N N- O ti 00
T- r r r r r N r r r r N N r r N r r
CA M C) N T7 C7) O CO O CO C7) M CO M C) (I0 O O
N N N r N N N N r N N N r r N N
N~ N 0~ N IC M N N T7 O C) 0) 0) C) O U? U?
. . . . .
CO N N- N CD CA N N LO LC) N- CD ti N- N- Lf) CD CD
M't c") 'V' CO M v It M M lq- M CO CO V- CO CO
CD
v O N N U) d' N N N M U) Ct N M Lf) . .
. . . . .
N LO N N- O It O CO U) CA N- CO LO 0) O 00 CO CO
N- O N lq- CD CD 0) co r 00 CA LC) N CO 0) LO 0 O
00 N- LO N O r N - I-t d' OD r (D U) N CD N r
NNNN N N N N N N r CO co N N N N N
N M d' r N CO d' r N CO d r N CO V- r N CO
O O O O O O O O O O C) O O O 0 O O O
N N N O CD O O O O O O N N N N O co CD
r r r r r r r
a a a < < < < < < < < < < < < Z Z Z
QQQ22a.2a-2 212112i n.2EL 2[L 2CL 2i [L 2110>0>0
LL LL LL LL Nl LL LL LL LL LL LL LL U U U
N CO 11, LO CD N- 00 0) C) r N CO v U') co N- 00 0)
N N N N N N N N co CO CO CO CO co CO CO CO CO
CA 02798880 2012-11-08
r- C14 04 CD N O O 1- 0) CO N W N co r- rl- C)
O CD CO CO CY) 0) CD N co OCO 0) co N00 CA CO N- CO
T N N N N T N N N O O O ~
N N N N N CO N CO CO O CO N CO
4 00 co CO 4 -t CO O O 4 CO CO N
N N N N N T N CO CO T CO N e-
T N N It CO -0) O CO _ N CO 00 O
T T T T T 0) 0) T T CC) 00 0)
C) 0) T CO C) co T O N CO N
CO N ti ti T CO CO CO 0) 0) ti O CO
.T T T T T T T T T T T
CO CO T N T T C7 CO CO T r-
O CO CO 00 CO 6 N- N- CO 4 CO CD
T T 00 T 0) T N V- N- C Y)
d' O T 00 r- 00 00 CO O O
CO CO CO N CO N N CO CY) CO co CO CO
C) O) O T T I- CO r- O O CO CO 0)
CO CO CO CO CO CO ct CO CO CO
CO O 0 N co O 0) r- CO N O O 0)
co co CO CO CO') CO N N M CO CO M CO
CO O) O Cn OX CO ll~ V It T CO
U') - CD N t CO It It CO CO CO CO CO CO
CD N 00 CO CD 00 N N d' ~h O CD C0
O CO o m r` C) N- r- CO CO t r-
T T N T T T T T T T T T
0) N O CO O) CO T N d C) r- CO
T N N N T T N N N T T T T
CO O CO d' CO C) CO V N O O r- N
cn CO C) CO C) r-, CO 0 N CO CO CD N
CO rt CO CO CO CO
d' 114- "T It CD 00 r- CO CO C R r- r- C) c D O CO N
O T CD r- CO CO T C t co CO r- O
O O N O N N N- r- N CO CO N ti
00 (0 CO CO CO O C) co O C) CO CO CO
T N N N N N N N CO T T T T
N co "T N CO 't T N CO V-
C) CD O CD O ON ON ON
T N N T
0) C) 0) CY) N O O O O
z z z z z z z z z O O O o
>O>0>O>O>O>0>0>O>O> c- c- c-
U U U U U U U U U 0 0 0 0
u L) L) 0
O T N cO It CO CO N. 00 O O T N
~t v ~r ~t v ~r v CO CO CO