Note: Descriptions are shown in the official language in which they were submitted.
CA 02799548 2012-11-15
DESCRIPTION
PROCESS FOR PRODUCING GRANULAR METAL
TECHNICAL FIELD
[0001]
The present invention relates to a process for producing granular metal by
feeding agglomerates configured by a raw material mixture containing a metal
oxide
and a carbonaceous reducing agent onto a hearth, and by heating the same
thereon to
reduce and to melt the metal oxide in the raw material mixture.
[0002]
Mainly described herein is the process for producing granular metallic iron,
in
which the present invention is utilized most effectively. However, the present
invention is not limited to the above but can be effectively utilized also to
a case of
heating and reducing chromium-containing ore or nickel-containing ore, for
example, to
produce ferrochromium, ferronickel, or the like. Moreover, the term "granular"
in the
present invention does not necessarily mean a perfectly spherical shape, but
also
includes elliptical and ovoidal shapes, as well as any shapes obtained by
slightly
flattening these shapes, and the like.
BACKGROUND ART
[0003]
There has been developed a direct reduced iron manufacturing method for
obtaining granular metallic iron from agglomerates configured by a raw
material
mixture including an iron oxide-containing material such as iron ore or iron
oxide, and a
carbonaceous reducing agent. In this iron producing process, the agglomerates
are
1
CA 02799548 2012-11-15
charged onto a hearth of a heating furnace and then heated in the furnace by
the gas heat
transfer with use of a heating burner or by radiation heat to reduce the iron
contained in
the agglomerates by the carbonaceous reducing agent. Subsequently, the reduced
iron
obtained by said heating step is carburized, melted, and then coalesced in the
form of
granules while being separated from sub-generated slag, and the granules are
cooled and
solidified to obtain granular metallic iron.
[0004]
The above iron producing process does not require a large scale facility such
as
a blast furnace and has high flexibility with regard to resources, for
example, because of
no need to use coke, and therefore, in recent years, this process has widely
been studied
for practical use. However, this iron producing process still has many
problems to be
solved in order to be applied on an industrial scale, including the stability
of operation,
safety, economic efficiency, quality of the granular metallic iron (i.e., a
final product),
and productivity. In view of these problems, the applicant of the present
invention
previously proposed a method disclosed in Patent Document 1. In this method,
upon
heating and reducing formed products containing a carbonaceous reducing agent
and
iron oxide to produce metallic iron, suppressed as much as possible are the
amount of
the carbonaceous reducing agent consumed and the thermal energy necessary for
the
heating and reducing process so as to efficiently reduce the iron oxide at
lower cost on a
commercial scale. This document discloses an example in which iron ore, a
carbonaceous material, and a binder are blended together to produce granular
pellets
having the average diameter of 17 mm, and the pellets are heated and reduced
to
produce metallic iron.
PRIOR ART DOCUMENT
2
CA 02799548 2012-11-15
PATENT DOCUMENT
[0005]
Patent Document 1: Japanese Unexamined Patent Publication No. H 11-241111
SUMMARY OF THE INVENTION
PROBLEM TO BE SOLVED BY THE INVENTION
[0006]
According to above Patent Document 1, the carbonaceous reducing agent is
blended at an amount in consideration of the stoichiometric amount required to
the
reduction of iron oxide and the solution C content into the metallic iron to
be generated,
and the heating temperature is appropriately controlled in consideration of
the melting
point of the metallic iron upon the solution of C. Thus, heating and reducing
the iron
oxide as well as the separation from slag by melting the iron oxide can be
effectively
progressed by using the carbonaceous reducing agent of the minimum amount
required
at the heating temperature as low as possible. As a result, there was
established a
process for producing metallic iron more economically and highly practically
on an
industrial scale. However, what is required is to further increase the amount
of
granular metallic iron produced per unit area of the effective hearth per unit
time, in
order to improve the productivity of the granular metallic iron.
[0007]
The present invention was made in consideration of the above circumstances,
and an object thereof is to provide a technique that further improves the
process for
producing granular metal by heating agglomerates containing a metal oxide and
a
carbonaceous reducing agent, and reducing and melting the metal oxide included
in the
agglomerates.
3
CA 02799548 2012-11-15
SOLUTIONS TO THE PROBLEMS
[0008]
A process for producing granular metal, according to the present invention is
characterized by comprising the steps of:
feeding agglomerates containing a metal oxide and a carbonaceous reducing
agent onto a hearth of a moving hearth-type reduction melting furnace;
heating the agglomerates to reduce and to melt the metal oxide;
cooling the granular metal obtained by said heating step; and
discharging the cooled granular metal out of the furnace to recover the same,
wherein the agglomerates having an average diameter of not smaller than 17.5
mm are fed onto the hearth when the agglomerates are heated at a spread
density of not
lower than 0.5 on the hearth.
[0009]
It is preferable that a carbonaceous material is spread on the hearth and then
the
agglomerates are fed on the carbonaceous material to form a single layer.
[0010]
Iron oxide or steelmaking dust is, for example, used as the metal oxide.
A rotary hearth furnace is, for example, used as the moving hearth-type
reduction melting furnace.
It is preferable that the moving hearth-type reduction melting furnace
comprises a upstream area having a temperature controlled to be from 1300 C to
1450 C and a downstream area having a temperature controlled to be from 1400 C
to
1550 C.
And it is preferable that the downstream area is set to have a temperature
4
CA 02799548 2012-11-15
higher than that of the upstream area in the moving hearth-type reduction
melting
furnace.
EFFECT OF THE INVENTION
[0011]
In the present invention, the average diameter of the agglomerates fed onto
the
hearth and the spread density of the agglomerates heated on the hearth are
appropriately
controlled, which improves the productivity of the granular metal.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012]
FIG 1 is a plan view schematically showing agglomerates spread on a hearth.
FIG. 2 includes pictures in substitution for drawings, which show states where
agglomerates having the average diameter of 18.2 mm are spread.
FIG 3 is a graph indicating the relationship between the distance "r" of
adjacent agglomerates and the projected area ratio or spread density.
FIG. 4 is a graph indicating the relationship between the spread density and
the
amount of agglomerates fed to a furnace.
FIG 5 is a graph indicating the relationship between an average diameter (Dp)
of a test material (i.e., agglomerates) and reaction time.
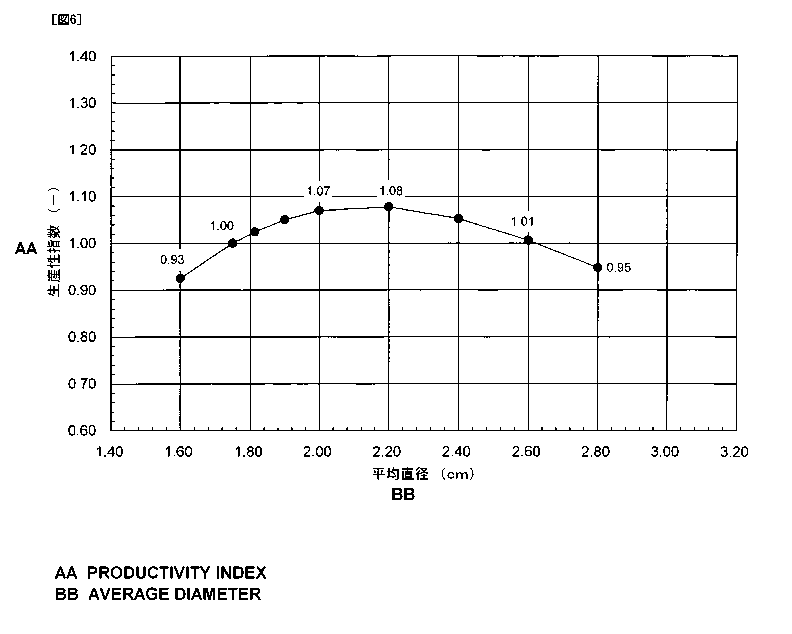
FIG 6 is a graph indicating the relationship between the average diameter of
agglomerates and the productivity index in a case where granular metallic iron
is
produced from the agglomerates spread at a constant density.
FIG. 7 is a graph indicating the relationship between the average diameter of
agglomerates and the productivity index when granular metallic iron is
produced from
5
CA 02799548 2012-11-15
the agglomerates (i.e., a test material) apart from each other at the constant
distance "r"
in the hearth.
MODE FOR CARRYING OUT THE INVENTION
[0013]
The inventor of the present application conducted diligent investigations to
improve the process for producing granular metal by feeding onto a hearth of a
moving
hearth-type reduction melting furnace and heating thereon agglomerates
containing a
metal oxide and a carbonaceous reducing agent to reduce and to melt the metal
oxide
included in the agglomerates. The inventor finally found out that the
productivity of
the granular metal can be improved by:
(1) preparing the agglomerates so as to have an average diameter of not
smaller
than 17.5 mm; and
(2) heating the agglomerates that are spread on the hearth at the spread
density of
not lower than 0.5,
to achieve the present invention. The details of the achievement of the
present
invention are described below.
[0014]
In the above Patent Document, when metallic iron is produced by heating and
reducing formed products containing a carbonaceous reducing agent and iron
oxide,
pellets (i.e., agglomerates) having an average diameter of 17 mm are used as
the formed
products. The reason why the agglomerates having an average diameter of 17 mm
are
used has been thought to be that agglomerates of a larger size will require
longer time to
transfer heat to the agglomerates on the hearth in the furnace, resulting in a
longer
reaction time and therefore the deterioration in the productivity of granular
metallic
6
CA 02799548 2012-11-15
iron.
[0015]
However, the inventor of the present application investigated in more detail
on
the relationship between the size of the agglomerates and the productivity to
find a new
fact that the productivity of granular metal can be better improved with use
of
agglomerates having an average diameter of not smaller than 17.5 mm. This new
finding is described with reference to FIG. 7.
[0016]
FIG. 7 is a graph referred to in an example to be described later, indicating
the
relationship between the average diameter of agglomerates and the productivity
index.
In FIG 7, the productivity index is a relative value to the productivity that
is set to 1.00
in a case where granular metallic iron is produced with use of agglomerates
having the
average diameter of 17.5 mm (i.e., 1.75 cm). This productivity represents a
quantity of
granular metallic iron produced per unit area of the effective hearth per unit
time (to be
detailed later).
[0017]
As apparent from FIG 7, the productivity index is larger and the productivity
of granular metallic iron is improved by using agglomerates having an average
diameter
of not smaller than 17.5 mm (more specifically, an average diameter from 17.5
to 32.0
mm) in comparison to the case of using agglomerates having the average
diameter of
16.0 mm (i.e., 1.60 cm).
[0018]
FIG 7 indicates a result of re-evaluation (i.e., simulation), on the basis of
the
results of various experiments, of the relationship in the cases where the
distance "r"
between the adjacent agglomerates on the hearth is kept constant (in other
words, when
7
CA 02799548 2012-11-15
the agglomerates are spread on the hearth at different spread density). The
spread
density is the density of filled agglomerates that are spread per unit area of
the effective
hearth, and can be calculated from the projected area of the agglomerates on
the hearth
(to be detailed later). FIG 7 indicates the result of re-evaluation on the
basis of the
result indicated in FIG 5. As seen from the relationship between the average
diameter
and the reaction time indicated in FIG. 5, each of the actual measurement
values is
slightly varied. Therefore, there was applied the normalization of the
relationship
between by the approximation thereof with a curve that is used in the re-
evaluation.
This is one of the approaches of scientific analyses.
[0019]
The most important factors in the evaluation of the productivity of granular
metal are the reaction time and the yield rate (in other words, the product
recovery rate).
Accordingly, these properties are particularly normalized in accordance with
the
experimental data to conduct the re-evaluation. It is noted that the apparent
density of
agglomerates is another important factor that influences the productivity.
However, it
is preliminarily evaluated that agglomerates having a diameter from 16.0 to
32.0 mm,
for example, have small variations in the apparent density as long as the
agglomerates
are prepared by using an identical agglomeration method, and that the apparent
density
can be therefore regarded as being substantially constant in the comprehensive
evaluation. According to FIG 7, as will be referred to in the example to be
described
later, the spread density of agglomerates is increased as the average diameter
of the
agglomerates is larger (see Table 6 below). Therefore, it is understood from
FIG 7 that
the productivity of granular metallic iron can be improved by appropriately
controlling
the spread density, as well as by the control of the average diameter of
agglomerates.
Consequently, the present invention clarifies that the productivity of
granular metallic
8
CA 02799548 2012-11-15
iron can be improved by the control of the spread density as well as the
average
diameter of agglomerates.
[0020]
Described in detail below is the producing method according to the present
invention.
[0021]
Prepared in the present invention are agglomerates having an average diameter
of not smaller than 17.5 mm.
[0022]
The agglomerates are prepared by agglomerating a mixture containing a metal
oxide and a carbonaceous reducing agent. The metal oxide may be an iron
oxide-containing material, chromium-containing ore, nickel-containing ore, or
the like.
In particular, what can be used as the iron oxide-containing material is iron
ore, iron
sand, steelmaking dust, nonferrous smelting residue, steelmaking waste, or the
like.
The carbonaceous reducing agent may be a carbon-containing material such as
coal or
coke.
[0023]
The mixture may be blended with an additional component such as a binder, an
MgO-containing material, or a CaO-containing material. The binder may be a
polysaccharide (e.g., starch such as flour). The MgO-containing material may
be
powdered MgO, those extracted from natural ore, seawater, or the like,
magnesium
carbonate (i.e., MgCO3), or the like. The CaO-containing material may be
quicklime
(i.e., CaO), limestone (i.e., composed mostly of CaC03), or the like.
[0024]
The agglomerates are prepared to have an average diameter of not smaller than
9
CA 02799548 2012-11-15
17.5 mm. If the average diameter of the agglomerates is smaller, the time
required to
the heat transfer in the furnace is shortened in general, which also shorten
the reaction
time. However, when the average diameter of the agglomerates is small, it is
difficult
to spread the agglomerates evenly on the carbonaceous material laid on the
hearth.
Moreover, the particle diameter and unit mass of granular metal are inevitably
decreased,
which granular metal is obtained by heating the agglomerates. Such small
granular
metal obtained by said heating step needs to be handled with special care,
which results
in the difficulty in feeding the granular metal into a finery such as an
electric furnace or
a converter. Furthermore, the small granular metal is not preferable in view
of the
melting property. Therefore, the present invention uses agglomerates having an
average diameter of not smaller than 17.5 mm. The average diameter of the
agglomerates is preferably not smaller than 18.5 mm, more preferably not
smaller than
19.5 mm, and further preferably not smaller than 20 mm. There is no particular
upper
limit to the average diameter of agglomerates. Nevertheless, such agglomerates
having an average diameter of more than 32 mm require too much time for the
heat
transfer in the furnace, resulting in longer reaction time and deterioration
in productivity.
In addition, the larger average diameter of agglomerates tends to deteriorate
the
granulation efficiency. Therefore, the agglomerates are preferably prepared to
have an
average diameter of not more than 31 mm. The average diameter of the
agglomerates
is more preferably not more than 28 mm.
[0025]
There is no particular limitation to the shape of the agglomerates, which may
be in the shape of pellets, briquettes, or the like.
[0026]
In order to obtain the diameter of each of the agglomerates, the longer
diameter
CA 02799548 2012-11-15
of the agglomerate and the shorter diameter thereof in the direction
perpendicular to the
longer diameter are measured with use of a vernier caliper, and these longer
and shorter
diameters are averaged [diameter = (longer diameter + shorter diameter)/2].
The
average diameter of the agglomerates is obtained by measuring and averaging
the
diameters of at least 20 particles with use of the vernier caliper. In a case
where the
average diameter of the agglomerates is equal to a mm, the diameters (absolute
values)
of the agglomerates are preferably distributed in the range of a 5 mm.
[0027]
It is important in the present invention to heat agglomerates having an
average
diameter of not smaller than 17.5 mm which are spread on the hearth at the
density of
not lower than 0.5 on the hearth. It has been conventionally recognized that
agglomerates having a larger average diameter deteriorate the productivity.
However,
the present invention has clarified the extremely important fact contradictory
to the
conventional common knowledge, as to be proved in the examples later. That is,
the
productivity is improved in a case where agglomerates having an average
diameter of
not smaller than 17.5 mm are heated at the spread density of not lower than
0.5 on the
hearth. However, if the spread density of agglomerates is lower than 0.5, the
density
of the agglomerates spread per unit area of the effective hearth is too small.
In this
case, the amount of granular metal generated is totally decreased even though
the
particle diameter is increased to be not smaller than 17.5 mm, which leads to
failure in
improving the productivity. Accordingly, agglomerates need to be spread at the
density of not lower than 0.5. The spread density is desirably set to be as
large as
possible, and is preferably not lower than 0.6. There is no particular upper
limit to the
spread density of agglomerates. However, if agglomerates are fed at a spread
density
of more than 0.8, such agglomerates may be laid in two or more layers. In this
case, it
11
CA 02799548 2012-11-15
is difficult to evenly heat the agglomerates, which results in difficulty in
producing
granular iron of high quality. Therefore, the spread density of agglomerates
is
preferably set to have the upper limit of 0.8, and is more preferably not more
than 0.7.
[0028]
The spread density of agglomerates is described in detail below. The spread
density of agglomerates is calculated from the projected area ratio, relative
to the hearth,
of the agglomerates spread on the hearth. Described below is the method of
calculating the spread density with reference to FIG. 1.
[0029]
FIG 1 is a plan view schematically showing agglomerates spread on the hearth.
The projected area ratio of the agglomerates onto the hearth can be calculated
by
equation (1).
Projected area ratio [projected area of all agglomerates on hearth/effective
hearth
area] x 100 ... (1)
[0030]
The agglomerates are assumed to have a perfectly spherical shape, and the
average diameter of the agglomerates and the distance of the adjacent
agglomerates are
expressed by Dp and r, respectively, the projected area ratio of the
agglomerates onto
the hearth can be calculated by the following equation (2):
Projected area ratio (%) = 7c x (Dp)2/4/{(Dp + r) x (Dp + r) x 305/2} x 100
(%) ... (2)
[0031]
12
CA 02799548 2012-11-15
In a case where the distance "r" between the adjacent agglomerates is set to
0,
the projected area ratio has the maximum value and the maximum projected area
ratio
has a constant value (i.e., 90.69%). Assuming that the maximum projected area
ratio
is equal to 1, the present invention defines, as the spread density, a
relative value of the
projected area ratio that is calculated in accordance with equation (2) from
the average
diameter Dp of the agglomerates and the distance "r" between the adjacent
agglomerates.
[0032]
In order to describe the actual cases of the spread density in more detail,
FIG 2
shows states where agglomerates having the average diameter of 18.2 mm are
spread in
containers each in a flat plate shape of approximately 61 cm square.
[0033]
Case (a) in FIG 2 shows an example of filling in a container agglomerates
weighing 9.3 kg per unit area of 1 m2, in which case the spread density was
equal to 0.4.
The theoretical amount of agglomerates filled at the spread density of 0.4
weighs 9.33
kg per unit area of I m2. It is therefore found out that the filled amount and
the spread
density in Case (a) is substantially equal to the theoretical values.
[0034]
Case (b) in FIG 2 shows an example of filling in a container agglomerates
weighing 13.9 kg per unit area of I m2, in which case the spread density was
equal to
0.6. The theoretical amount of agglomerates filled at the spread density of
0.6 weighs
14.0 kg per unit area of 1 m2. It is therefore found out that the filled
amount and the
spread density in Case (b) is substantially equal to the theoretical values.
[0035]
Case (c) in FIG. 2 shows an example of filling in a container agglomerates
13
CA 02799548 2012-11-15
weighing 18.5 kg per unit area of 1 m2, in which case the spread density was
equal to
0.8. The theoretical amount of agglomerates filled at the spread density of
0.8 weighs
18.66 kg per unit area of 1 m2. It is therefore found out that the filled
amount and the
spread density in Case (c) is substantially equal to the theoretical values.
[0036]
Case (d) in FIG. 2 shows an example of filling in a container agglomerates
weighing 23.2 kg per unit area of 1 m2, in which case the spread density was
equal to
1Ø The theoretical amount of agglomerates filled at the spread density of
1.0 weighs
23.33 kg per unit area of 1 m2. It is therefore found out that the filled
amount and the
spread density in Case (d) is substantially equal to the theoretical values.
[0037]
It is quite difficult to spread agglomerates on an actual hearth at the spread
density of 1.0 as shown in Case (d) of FIG. 2. In an actual case where
agglomerates
are fed to a furnace in the amount of the spread density equal to 1.0, there
is caused
another problem such as the charged agglomerates being overlaid with each
other. In
order to feed agglomerates to the furnace so as not to be overlaid with each
other, it was
found out, through the various demonstration experiments, that the upper limit
of the
spread density was preferably set to approximately 0.8, as shown in Case (c)
of FIG. 2.
[0038]
On the other hand, as shown in Case (a) of FIG 2, the spread density equal to
0.4 causes quite a large number of spaces on the hearth, which will extremely
deteriorate the productivity. Thus, the ideal lower limit of the spread
density will be
approximately 0.5, which is an intermediate value of those of Case (a) and
Case (b) in
FIG 2.
[0039]
14
CA 02799548 2012-11-15
FIG 3 indicates the relationship between the distance "r" of adjacent
agglomerates and the projected area ratio or spread density. In FIG 3, the
marks =
indicate the results of projected area ratios, while the marks ^ indicate the
results of
spread densities. As apparent from FIG 3, as the distance "r" between the
adjacent
agglomerates is increased, both the projected area ratio and the spread
density of the
agglomerates are reduced. There is recognized a favorable correlation between
the
projected area ratio and the spread density relative to the distance "r"
between the
adjacent agglomerates.
[0040]
FIG. 4 indicates the relationship between the spread density and the amount of
agglomerates fed to the furnace in a case where the average diameter of the
agglomerates is changed in the range from 14.0 to 32.0 mm. The amount of the
fed
agglomerates is indicated by the mass of the fed agglomerates in the effective
hearth
area.
[0041]
In FIG 4, a straight line connecting a point (A) and a point (B) indicates a
range of the amount of agglomerates fed to the furnace in a case where the
agglomerates
have an average diameter of not smaller than 17.5 mm and are spread at the
density of
0.5. A straight line connecting a point (C) and a point (D) indicates a range
of the
amount of agglomerates fed to the furnace in a case where the agglomerates
have an
average diameter of not smaller than 17.5 mm and are spread at the density of
0.8. As
can be seen from this FIG 4, the average diameter of the agglomerates and the
amount
of agglomerates to be fed to furnace (i.e, the mass of agglomerates to be fed
per
effective hearth area) may be adjusted to control the spread density of the
agglomerates
on the hearth to not lower than 0.5.
CA 02799548 2012-11-15
[0042]
The agglomerates are heated in a moving hearth-type reduction melting furnace
to reduce and to melt a metal oxide in the agglomerates so as to manufacture
granular
metal. The moving hearth-type reduction melting furnace and the heating
condition in
the furnace are not particularly limited in the present invention, and there
can be
adopted a known condition.
[0043]
As the above moving hearth-type reduction melting furnace, there can be used,
for example, a rotary hearth furnace. There is no particular limitation to the
width of
the hearth of the moving hearth-type reduction melting furnace. According to
the
present invention, it is possible to improve the productivity of granular
metal under an
economically advantageous condition even with use of an actual machine having
a
hearth width of not smaller than 4 in.
[0044]
It is preferable to spread the carbonaceous material (hereinafter, also
referred to
as bed material) on the hearth and then to feed the agglomerates on the
carbonaceous
material, so that the agglomerates are fed to form a single layer on the
carbonaceous
material layer. The bed material serves as a carbon resource in a case where
the
carbon included in the agglomerates is not sufficient, and also serves as a
hearth
protective material.
[0045]
Although there is no particular limitation to the thickness of the bed
material,
the thickness is preferably not less than 3 mm. More specifically, in a case
where the
moving hearth-type reduction melting furnace is actually used, the hearth
width will
have several meters. Accordingly, it is difficult to spread evenly the bed
material
16
CA 02799548 2012-11-15
across the width direction and there may be caused variations in thickness
from about 2
to 8 mm. It is preferable to spread the bed material so as to have a thickness
of not less
than 3 mm in order to cause no portion on the hearth not covered with the bed
material.
The thickness of the bed material is more preferably not less than 5 mm, and
further
preferably not less than 10 mm. Because the present invention uses
particularly large
agglomerates, such agglomerates are unlikely to be buried even in the bed
material
having a large thickness, and the reduction efficiency will be hardly
deteriorated.
More specifically, the bed material having a larger thickness is particularly
effective in a
case of using agglomerates that have an average diameter of not less than 20
mm.
There is no particular limitation either to the upper limit of the thickness
of the bed
material. However, if the thickness of the bed material is more than 30 mm,
agglomerates may be buried in the bed material even in the present invention,
which
may inhibit the supply of heat to the agglomerates and thus deteriorate the
reduction
efficiency. As a result, granular metal is likely to be deformed or
deteriorated in
interior quality thereof. Therefore, the thickness of the bed material is
preferably not
more than 30 mm, more preferably not more than 20 mm, and further preferably
not
more than 15 mm.
[0046]
The carbonaceous material used as the bed material can be selected from those
exemplified as the carbonaceous reducing agent. The carbonaceous material
desirably
has a particle diameter of not more than 3.0 mm, for example. If the particle
diameter
of the carbonaceous material is more than 3.0 mm, the molten slag may flow
down
through the spaces in the carbonaceous material to reach the surface of the
hearth and
erode the hearth. The particle diameter of the carbonaceous material is more
preferably not more than 2.0 mm. However, if the proportion of the particles
having a
17
CA 02799548 2012-11-15
diameter of smaller than 0.5 mm is too large in the carbonaceous material, the
agglomerates will be buried in the bed material to lead to the deteriorations
in heating
efficiency as well as in productivity of granular metal, which is not
preferable.
[0047]
The agglomerates are preferably fed onto the hearth so as to form a single
layer
over the bed material that is spread on the hearth. One general idea for the
increase in
the production quantity of granular metallic iron will be increasing the
amount of
agglomerates to be fed to the furnace. In such a case of increasing the amount
of fed
agglomerates, the agglomerates are stacked into two or more layers on the
hearth. In
this case, the upper agglomerates receive sufficient heat from a furnace body
to be
reduced and melted, while sufficient heat is not fed to the lower
agglomerates, which
are likely to cause residual portions not having been reduced. If molten iron
obtained
only from the reduced and melted upper agglomerates is combined with the lower
un-melted and un-reduced iron and the like, it is impossible to obtain
granular metallic
iron of high quality. Therefore, in order to reliably achieve reduction in the
solid state
as well as carburizing and melting inside the furnace as in the present
invention, it is
desirable to feed agglomerates onto the hearth so as to form substantially a
single layer.
[0048]
Upon feeding agglomerates onto the hearth so as to form a single layer, a
pellet
leveler or the like may be used to control the agglomerates to be spread on
the hearth so
that the agglomerates are evenly spread over the effective hearth across the
width
direction thereof before the agglomerates fed to the furnace enter a thermal
reaction
zone.
[0049]
It is possible to apply a common heating condition to the case where the
18
CA 02799548 2012-11-15
agglomerates are heated in a moving hearth-type reduction melting furnace to
reduce
and to melt the metal oxide included in the agglomerates. More specifically,
the
agglomerates are fed onto the hearth, reduced in the solid state at a
predetermined
temperature, and further continuously heated until being melted, so as to
obtain
manufactured slag (i.e., oxide) comprising impurities and granular metallic
iron.
The agglomerates on the hearth receive heat from combustion flames of a
plurality of
burners installed in an upper portion in the furnace (e.g., on a ceiling) or
on a side wall,
or radiation heat from a refractory material in the furnace, which is heated
to a high
temperature. The received heat is transferred from the peripheral portions to
the inner
portions of the agglomerates so as to progress the reduction reaction in the
solid state.
[0050]
In the upstream area in the furnace, the reduction reaction progresses while
the
agglomerates being kept in the solid state. In the downstream area in the
furnace,
microscopic particles of reduced iron in the agglomerates, which have been
already
reduced in the solid state, are carburized and then coalesced to each other in
the process
of being melted, so as to form granular metallic iron while being separated
from the
impurities (i.e., slag components) in the agglomerates.
[0051]
The temperature of the upstream area in the furnace is preferably controlled
to
be at approximately 1300 C to 1450 C so as to cause the iron oxide in the
agglomerates
to be reduced in the solid state. The temperature of the downstream area in
the furnace
is preferably controlled to be at approximately 1400 C to 1550 C so as to
cause the
reduced iron in the agglomerates to be carburized, melted, and coalesced. If
the
furnace is heated to be higher than 1550 C, heat is excessively applied to the
agglomerates to exceed the rate of the heat transferred into the agglomerates.
In this
19
CA 02799548 2012-11-15
case, the agglomerates are partially melted before being completely reduced in
the solid
state. As a result, the reaction progresses rapidly to cause a molten
reduction reaction,
which generates abnormal slag formation.
[0052]
The downstream area in the furnace may be set to a temperature higher than
that in the upstream area in the furnace.
[0053]
In the present invention, the productivity of the case where the agglomerates
are heated to reduce and to melt the metal oxide to produce granular metal is
evaluated
by the production quantity (ton) of the granular metal per unit area (m2) of
the effective
hearth per unit time (time), as expressed by equation (3) below.
Productivity (ton/m2/time) = production quantity of granular metal (granular-
metal
ton/time)/effective hearth area (m2) ... (3)
[0054]
In equation (3), the production quantity of granular metal (granular-metal
ton/time) is expressed by equation (4) below.
Production quantity of granular metal (granular-metal-ton/time) = amount of
agglomerates charged (agglomerates-ton/time) x mass of granular metal produced
from
1 ton of agglomerates (granular-metal-ton/ agglomerates-ton) x product
recovery
rate ... (4)
[0055]
CA 02799548 2012-11-15
In equation (4), the product recovery rate is calculated as a proportion of
granular metallic iron having a diameter of not smaller than 3.35 mm to the
total mass
of the granular metal obtained [mass of granular metallic iron having a
diameter of not
smaller than 3.35 mm/total mass of granular metallic iron x 100].
[0056]
In Experimental Examples 2 and 3 in the examples to be described later, in
order to quantitatively evaluate the effects of the present invention, a test
material (i.e.,
agglomerates) having the average diameter of 17.5 mm is regarded as including
standard agglomerates, and the productivity of each of the agglomerates is
indicated as a
relative value (i.e., productivity index) in a case where the productivity of
the standard
agglomerates is set to 1.00.
[0057]
The present invention will be described in more detail with reference to the
examples. It is noted that the present invention is never limited to the
following
examples but can be of course embodied with appropriate modifications as long
as
being adaptable to the purposes of the above statement and the following
statement.
Such modifications are also included in the technical scope of the present
invention.
Examples
[0058]
Experimental Example 1
Agglomerates were prepared from a raw material mixture containing a metal
oxide and a carbonaceous reducing agent, and the agglomerates were fed onto a
hearth
of a moving hearth-type reduction melting furnace and were heated thereon to
reduce
and to melt the metal oxide in the raw material mixture, so as to produce
granular
21
CA 02799548 2012-11-15
metallic iron.
[0059]
In this case, iron ore having the component compositions listed in Table 1
below was used as the metal oxide, and coal having the component compositions
listed
in Table 2 below was used as the carbonaceous reducing agent, to produce the
agglomerates. More specifically, the mixture containing the iron ore and the
coal was
blended with flour serving as a binder and an auxiliary material such as
limestone or
dolomite, to produce agglomerates (i.e., test materials) in the shapes of
pellets having
different average diameters. The blend compositions (i.e., weight percentages)
of the
test materials are listed in Table 3 below. Further, the longer diameters and
the shorter
diameters of the test materials were measured with use of a vernier caliper to
calculate
the average diameters, which are listed in Table 4 below. Each of the average
diameters of the test materials is obtained by measuring the sizes of 20
particles of each
of the test materials.
[0060]
There are also listed in Table 4 unit mass and an apparent density of each of
the
test materials. The unit mass of each of the test materials is equal to an
average value
obtained by measuring the mass of 20 particles. The apparent density of each
of the
test materials is obtained by immersing the agglomerates in a liquid (i.e.,
mercury) and
measuring buoyant forces thereof.
[0061]
Each of the test materials thus obtained and having the different average
diameters was heated in a small heating furnace on a laboratory scale (i.e.,
the
temperature in the furnace being set to 1450 C) to reduce and to melt the iron
ore
included in the corresponding test material, in order to measure time required
for the
22
CA 02799548 2012-11-15
reaction (i.e., reaction time). The measurement results on the reaction time
are listed
in Table 4 below.
[0062]
FIG. 5 indicates the relationship between the average diameter (Dp) and the
reaction time of the test material. In FIG. 5, a dotted curve shows an
approximated
curve including plotted points, which is expressed by a quadratic of the
average
diameter of the test material. As apparent from FIG. 5, as the average
diameter of the
test material increases, the reaction time is longer.
[0063]
According to the results of Experimental Example 1, the reaction time and the
product recovery rate were normalized to comprehensively evaluate the
productivity of
a case where the distance between the adjacent particles of the test material
is changed
(see Experimental Example 2 to be described later), or of a case where the
spread
density of the test material is changed (see Experimental Example 3 to be
described
later).
[0064]
Table 1
Component composition (mass%)
Iron Total Fe FeO Si02 CaO AIZ03 MgO MnO TiOZ P S
ore
67.73 I 29.40 4.54 0.42 0.21 0.47 0.34 0.07 0.018 0.002
[0065]
Table 2
Component composition (mass%)
Coal Fixed carbon Volatile Ash Total
77.21 16.65 6.14 100
23
CA 02799548 2012-11-15
[0066]
Table 3
Blend composition (mass%)
Test material Iron ore Coal Binder Auxiliary material
71.95 17.01 0.90 11.55
[0067]
Table 4
Average diameter Unit mass Apparent density Reaction time
No.
(mm) (g/Piece) (g/cm3) (min)
1 17.3 6.06 2.23 8.7
2 18.8 7.58 2.19 8.8
3 19.4 8.46 2.21 9.0
4 21.3 11.16 2.21 10.0
5 23.1 14.60 2.27 10.7
6 25.2 18.77 2.24 12.0
7 27.0 22.98 2.23 13.2
[0068]
Experimental Example 2
In Experimental Example 2, test materials, which have average diameters of
16.0 to 28.0 mm (i.e., 1.60 to 2.80 cm) and are spread at a constant density
on a hearth,
were heated in an actual moving hearth-type reduction melting furnace to
produce
granular metallic iron. Comprehensively investigated was how the average
diameter
of the test material influences on the productivity of granular metallic iron
thus
produced.
[0069]
A rotary hearth furnace was used as the moving hearth-type reduction melting
24
CA 02799548 2012-11-15
furnace, and each of the test materials was fed onto the hearth at the spread
density of
0.66 and was heated thereon to reduce and to melt iron ore so as to produce
granular
metallic iron. The temperature of the upstream area in the furnace was set to
1400 C
and the temperature of the downstream area thereof was set to 1470 C. In the
upstream area, the iron ore in the test material is reduced in the solid
state. In the
downstream area, microscopic particles of reduced iron, which are generated
and melted
in the test material, are carburized, melted, and eventually coalesced so as
to separate
molten iron from slag.
[0070]
The spread density of the test material on the hearth was controlled by
regulating the amount of the test material fed to the furnace and the moving
speed (i.e.,
rotating speed) of the hearth. More specifically, the moving speed of the
hearth was
determined such that the iron ore was reduced and melted in the heating zone
under an
atmospheric condition set in accordance with the result of the preliminary
experiment.
The supply amount of the test material was regulated in consideration of this
moving
speed, so that the spread density of the test material on the hearth was
controlled to 0.66.
Table 5 below shows the distance "r" between the adjacent particles of the
test materials
as reference values.
[0071]
The productivity of granular metallic iron produced by reducing and melting
each of the test materials was calculated in accordance with above equation
(3), and the
productivity of each of the test materials was indicated as a relative value
(i.e.,
productivity index), assuming that the productivity of the test material No.
12 (i.e.,
standard agglomerates) has a standard value (i.e., productivity index equal to
1.00).
The productivity indices of the respective test materials are listed in Table
5 below.
CA 02799548 2012-11-15
Further, FIG 6 indicates the relationship between the average diameter and the
productivity index of the test material.
[0072]
As apparent from FIG 6, when the spread density on the hearth is kept
constant,
the productivity can be improved by setting the average diameter of the test
material to
be not smaller than 17.5 mm in comparison to the case of setting the average
diameter
of the test material to 16.0 mm. In other words, the productivity is gradually
improved
as the average diameter of the test material increases, and the productivity
index reaches
the maximum value in the case where the average diameter of the test material
equal to
22.0 mm.
[0073]
However, if the average diameter of the test material is set to be larger than
26.0 mm, the productivity of granular metallic iron tends to be gradually
deteriorated.
The productivity will be deteriorated because the reaction time is longer with
the test
material of a larger size. Accordingly, when the spread density is kept
constant, it is
found that the productivity can be improved by setting the average diameter of
the test
material to the range from 17.5 to 26.0 mm in comparison to the case of using
the test
material having the average diameter of 16.0 mm.
[0074]
Table 5
26
CA 02799548 2012-11-15
Average diameter Distance rSpread density
No. Productivity index
(cm) (cm) (- )
11 1.60 0.37 0.66 0.93
12 1.75 0.37 0.66 1.00
13 1.81 0.42 0.66 1.02
14 1.90 0.44 0.66 1.05
15 2.00 0.46 0.66 1.07
16 2.20 0.50 0.66 1.08
17 2.40 0.55 0.66 1.05
18 2.60 0.60 0.66 1.01
19 2.80 0.64 0.66 0.95
[0075]
Experimental Example 3
In Experimental Example 3, assuming test materials each having an average
diameter of 16.0 to 32.0 mm (i.e., 1.60 to 3.20 cm), adjacent particles of
each of the test
materials being apart from each other at a constant distance "r" (i.e., 0.42
cm) on the
hearth were heated to produce granular metallic iron in an actual moving
hearth-type
reduction melting furnace with the spread densities of the test materials
being changed.
In this manner, investigated was how the spread density of the test material
influenced
on the productivity of granular metallic iron.
[0076]
In the evaluation in this case, a rotary hearth furnace was used as the moving
hearth-type reduction melting furnace, and each of the test materials, which
have the
average diameters listed in Table 6 below and were fed onto the hearth, was
heated to
reduce and to melt iron ore so as to produce granular metallic iron. The
heating
condition in the furnace was set identically with that of Experimental Example
2
described earlier. The spread densities of the test materials on the hearth
are listed in
27
CA 02799548 2012-11-15
Table 6.
[0077]
The productivity of the granular metallic iron produced by reducing and
melting each of the test materials was calculated in accordance with equation
(3) above,
and the productivity of each of the test materials was indicated as a relative
value (i.e.,
productivity index), assuming that the productivity of the test material No.
22 (i.e.,
standard agglomerates) has a standard value (i.e., 1.00). The productivity
indices of
the respective test materials are listed in Table 6 below. Further, FIG. 7
indicates the
relationship between the average diameter and the productivity index of the
test
material.
[0078]
As apparent from Table 6 and FIG. 7 below, in the case where the distance "r"
between the adjacent particles of the test material is kept constant, the
spread density of
the test material on the hearth can be increased by setting the average
diameter of the
test material to be not smaller than 17.5 mm. Further, the productivity of the
granular
metallic iron can be improved by increasing the average diameter of the test
material in
comparison to the case of setting the average diameter of the test material to
16.0 mm.
In other words, the productivity is gradually improved as the average diameter
of the
test material increases, and the productivity index reaches the maximum value
in the
case where the average diameter of the test material is equal to 24.0 mm.
[0079]
However, if the average diameter of the test material is larger than 24.0 mm,
the productivity of the granular metallic iron tends to be gradually
deteriorated. The
productivity will be deteriorated because the reaction time is longer with the
test
material of a larger size. Accordingly, it is found that the productivity can
be
28
CA 02799548 2012-11-15
improved by setting the average diameter of the test material to the range
from 17.5 mm
to 32.0 mm in comparison to the case of using the test material having the
average
diameter of 16.0 mm.
[0080]
Table 6
Average diameter Distance rSpread density
No. Productivity index
(cm) (cm) (-)
21 1.60 0.42 0.63 0.89
22 1.75 0.42 0.65 1.00
23 1.81 0.42 0.66 1.04
24 1.90 0.42 0.67 1.08
25 2.00 0.42 0.69 1.12
26 2.20 0.42 0.71 1.17
27 2.40 0.42 0.73 1.17
28 2.60 0.42 0.74 1.15
29 2.80 0.42 0.76 1.10
30 3.00 0.42 0.77 1.05
31 3.20 0.42 0.78 0.99
[0081]
The following conclusion can be obtained by combining the results of
Experimental Examples 2 and 3. As described in Experimental Example 2, when
using agglomerates having a large average diameter (e.g., agglomerates having
an
average diameter of more than 28.0 mm), the productivity of granular metallic
iron may
be deteriorated at a constant spread density. However, as described in
Experimental
Example 3, if the spread density is increased, the productivity can be
improved even in
the case of using the agglomerates having an average diameter of more than
28.0 mm.
In summary, the productivity can be improved by feeding onto the hearth at a
spread
density of not lower than 0.5 the agglomerates (i.e., test material) having an
average
29
CA 02799548 2012-11-15
diameter of not smaller than 17.5 mm and heating the agglomerates on the
hearth. In
other words, it is possible to productively produce granular metallic iron by
preparing
agglomerates having an average diameter of not smaller than 17.5 mm and
feeding the
agglomerates onto the hearth at a spread density of not lower than 0.5 to heat
the same
in the furnace.
INDUSTRIAL APPLICABILITY
The present invention is applicable to improve the productivity of the
granular
metal.