Note: Descriptions are shown in the official language in which they were submitted.
CA 02801533 2013-01-11
- 1 -
MULTI-BRANCHED N-DOPED CARBON NANOTUBES AND
THE PROCESS FOR MAKING SAME
FIELD OF THE INVENTION
The present relates to vertically aligned multi-branched N-doped (nitrogen-
doped) carbon
nanotubes (CNT) and a process for making them.
BACKGROUND
CNTs have drawn considerable attention from researchers due to their
outstanding electrical
and mechanical properties. Their complex spatial architecture has contributed
to numerous
applications of nanotubes in sensors, fuel cells, batteries, field emission
devices, transistors, and logic
circuits. Continuous requirements of miniaturization and complex nanoscale
devices have generated
an increasing interest in developing novel nanomaterials with complicated
structures. In order to
integrate nanomaterials with different properties into functional systems,
attention is becoming
focused on branched CNTs.
Up to now, several techniques have been employed to produce branched carbon
nanotubes.
Initially, Y-shaped or branched CNTs have been synthesized by arc discharge
and alumina template.
Furthermore, a high-intensity electron beam has been used to join crossed
CNTs. Another approach
used a two-step process to attach catalyst particles to the grown CNTs during
the chemical vapor
deposition (CVD) growth process to initialize and sustain branches formation.
Recently, more
complicated branched CNTs have been reported by using a pyrolysis method, in
which gas flow
fluctuation has been considered the key factor that influences the branch
occurrence. Inter-connected
CNT networks have been prepared by Fu Y. et al. ("Templated Growth of
Covalently Bonded Three-
Dimensional Carbon Nanotube Networks Originated from Graphene" Advanced
Materials Volume 24,
Issue 12, pages 1576-1581, March 22,2012) by CVD on nickel template.
However, previously reported deposition methods have disadvantages of
inconsistent
repeatability and introduction of external templates or additional steps that
make the process complex
and difficult to control. A single-step method to synthesize branched CNTs
with a strong control of
structure and composition is still desirable.
SUMMARY
It is therefore an aim of the present invention to provide a multi-stage multi-
branched N-doped
carbon nanotube and a process for producing it in a single-step controlled
operation.
In accordance with one aspect of the present invention, there is provided a
multibranched N-
doped carbon nanotube comprising: a first-stage stalk having a direction and
comprising a first-stage
base, and a first-stage top opposite to and attached with the first-stage
base, wherein the first-stage
base includes a catalyst inclusion, at least two second-stage bundles, each of
which comprises a
CA 02801533 2013-01-11
- 2 -
second-stage base attached with the first-stage top, and a second-stage top
opposite to and attached
to the second-stage base, and wherein the second-stage bundles branch from the
first-stage stalk in
substantially the direction of the first stage stalk, and a plurality of third-
stage nanotubes each of which
comprises a third-stage base attached with the second-stage top, a third-stage
top opposite to and
attached to the third-stage base, and wherein the third-stage nanotubes branch
from the second-stage
bundles.
In accordance with another aspect of the N-doped carbon nanotube herein
described the
third-stage nanotubes branch from the second-stage bundles in substantially
the same direction as
the first stage-stalk.
In accordance with another aspect of the N-doped carbon nanotube herein
described, the
first-stage stalk has an average diameter of about 145nm to about 450nm.
In accordance with yet another aspect of the N-doped carbon nanotube herein
described, the
first-stage stalk has an average diameter of about 200nm to about 250nm.
In accordance with still another aspect of the N-doped carbon nanotube herein
described, the
first-stage stalk has an average diameter of about 210nm.
In accordance with yet still another aspect of the N-doped carbon nanotube
herein described,
the second-stage bundles has an average diameter of about 25nm to about 60nm.
In accordance with a further aspect of the N-doped carbon nanotube herein
described, the
second-stage bundle has an average diameter of about 40nm.
In accordance with yet a further aspect of the N-doped carbon nanotube herein
described, the
plurality of third-stage nanotubes each has an average diameter of about 5 nm
to about 25nm.
In accordance with still a further aspect of the N-doped carbon nanotube
herein described, the
plurality of third-stage nanotubes each has an average diameter of about 10 nm
to about 20nm.
In accordance with yet still a further aspect of the N-doped carbon nanotube
herein described,
the plurality of third-stage nanotubes is from 10 to 30, branching from the
second-stage bundles.
In accordance with one embodiment of the N-doped carbon nanotube herein
described, the
plurality of third-stage nanotubes is from 20 to 25, branching from the second-
stage bundles.
In accordance with another embodiment of the N-doped carbon nanotube herein
described,
comprising a total length from the first-stage base to the third-stage top of
about 4pm to about 6 pm.
In accordance with another aspect of the present invention, there is provided
a process of
producing vertically aligned multiple-branched nitrogen-doped carbon
nanotubes, comprising the steps
of: providing a temperature controlled deposition chamber adjusted to a
temperature from 675 C and
CA 02801533 2013-01-11
=
=
- 3 -
850 C; providing a liquid having a carbon/nitrogen feedstock and an iron
catalyst at a branching
concentration, providing a carrier gas; providing a substrate in the chamber
onto which the nanotubes
are deposited; injecting a volume of the liquid into the gas to produce a fine
mist in the chamber
oriented towards the substrate for a period of time between 40 and 1 hour,
wherein the liquid injected
pyrolyzes the iron catalyst and the carbon/nitrogen feedstock into active
species that adhere to the
substrate and form the vertically aligned multiple-branched nitrogen-doped
carbon nanotubes.
In accordance with yet another embodiment of the process herein described, the
carbon/nitrogen feedstock is acetonitrile.
In accordance with still another embodiment of the process herein described,
the iron catalyst
is ferrocene.
In accordance with yet still another embodiment of the process herein
described, the
branching concentration of the ferrocene is greater than 0.5 wt% in the
liquid.
In accordance with a further embodiment of the process herein described, the
branching
concentration of the ferrocene is from 2.5 wt% to 3.5 wt% of the liquid.
In accordance with a further embodiment of the process herein described,
injecting the
volume of the liquid into the gas is at a rate of about 0.02m1/min to about
0.06m1/min.
In accordance with yet a further embodiment of the process herein described,
the substrate
comprises a high purity silicon wafer comprising a native oxide layer.
In accordance with still a further embodiment of the process herein described,
the substrate
includes an Al underlayer of an average thickness of about 30nm.
In accordance with yet still a further embodiment of the process herein
described, the period
of time is about 50 minutes.
Further in accordance with another embodiment of the process herein described,
the
temperature of the deposition chamber is from about 700 C to about 800 C.
BRIEF DESCRIPTION OF THE DRAWINGS
Reference will now be made to the accompanying drawings, showing by way of
illustration a
particular embodiment of the present invention and in which:
Fig. 1 is a schematic process flowsheet of a spray pyrolysis chemical vapour
deposition
system for producing N-doped carbon nanotubes according to one embodiment of
the present
invention;
CA 02801533 2013-01-11
- 4 -
Fig 2a is Scanning Electron Micrograph (SEM) image of a well-aligned branched
CNTs array
according to one embodiment of the present invention clearly indicating with
different generations /
stages visible;
Fig. 2b is a further detailed smaller scale SEM image of a well-aligned
branched CNTs of Fig.
2a indicating the positions of the end of the 1st generation/stage, the start
of the second
generation/stage; the end of the second generation/stage and the beginning of
the third
generation/stage of CNTs indicated;
Fig. 3a Transmission Electron Micrograph (TEM) image a branched CNTs according
to one
embodiment of the present invention illustrating from left to right; Y-
junctions on third-generation/stage
of nanotubes; multiple branches formed between the third and second-
generation/stage bundles of
CNTs; multiple branches formed between second-generation/stage bundles of CNTs
and a first
generation/stage stalk nanotube; and the stalk nanotube with catalyst
inclusion at the bottom right
hand corner of the Fig. 3a;
Fig. 3b is a further detailed smaller scale TEM image of a branched CNT
according to another
embodiment of the present invention a top of a CNT and Y-junctions of the
third generation/stage of
nanotubes, and a clear illustration of the multiple stalks of CNT;
Fig. 4a is a Raman spectrograph of a multibranched CNTs according to one
embodiment of
the present invention;
Fig. 4b is an X-ray photoelectron spectroscopic (XPS) survey scan of a
multibranched CNTs
according to one embodiment of the present invention;
Fig. 4c is an (nitrogen) N is XPS spectrum of a multibranched CNTs according
to one
embodiment of the present invention;
Fig. 5a is a SEM of a multibranched CNTs according to one embodiment of the
present
invention produced using 2.5 wt% ferrocene catalyst in solution;
Fig. 5b is a SEM of a multibranched CNTs according to another embodiment of
the present
invention produced using 0.5 wt% ferrocene catalyst in solution; and
Fig. 6 is a schematic diagram (not to scale) of a growth mechanism of the a
branched CNT
according to one embodiment of the present invention.
DETAILED DESCRIPTION OF PARTICULAR EMBODIMENTS
The multi-branched N-doped (nitrogen-doped) carbon nanotubes (CNT) and a
process for
making them will be further described with references to the appended figures
and description.
CA 02801533 2013-01-11
- 5 -
The expression "N-doped carbon nanotube" is defined herein as a carbon
nanotube
comprising a quantity of nitrogen within the structure of the nanotube.
The term "multibranched" nanotube is defined herein as a nanotube that include
a plurality of
branched nanotubes, and that those braches are found at one end of the
nanotube.
The term "hierarchical" nanotube is defined herein as a nanotube that includes
different
generations or stages of development that are oriented in a direction that is
substantially co-linear with
the radial axis of the nanotube or perpendicular to the substrate. This
hierarchical structure is due to
the means by which the nanotube is produced through a series of mechanistic
steps. The
multibranched N-doped carbon nanotubes described herein have at least tvvp and
more preferably
three generations/stages.
The expression "vertically aligned" means that the nanotubes described herein
are produced
to be vertically aligned with adjacent CNTs and are analogous to trees in a
forest.
The "substrate" is defined herein as a material on which the N-doped nanotubes
described
herein are produced.
The expression "first-stage stalk" is defined herein as the portion of the
nanotube that is first
produced, or the "first generation. This first-stage stalk is on and adjacent
with a substrate and
catalyst. The terms "stage" and "generation" are used interchangeable in this
description. The stalk is
oriented in a direction in an axial orientation along its length or is
perpendicular to the substrate. The
"first-stage base" is a lowest or base portion of the first-stage stalk that
has a catalyst "inclusion" that
is associated with the substrate. The first-stage base extends towards a
"first-stage top" opposite the
first-stage base. The term "inclusion" is defined herein to be exactly that, a
portion of the first-stage
base stalk nanotube that includes a higher concentration of catalyst. The
inclusion is visible in the
Scanning Electromicrographs included herein.
The expression "second-stage bundle" is defined herein as a second generation
of nanotubes
and is attached to the first-stage top. At least two second-stage bundles
branching from the first-stage
top. There may be from 2 to 25 second-stage bundles per first-stage stalk, and
preferably from 6 to 20
second-stage bundles per first-stage stalk. The branched bundles are oriented
in substantially the
direction of the stalk. The term "bundle" is also meant to indicate that this
second-stage includes
nanotubes that are organized and coalesce into a larger axially oriented
nanotube stage or unit in a
bundle that is axially combined, and similar to a cylindrically oriented hay
bail. The second-stage
bundle includes a "second-stage base" that is attached to the first-stage top
and is generally
associated with coalesced catalyst particles found at this juncture between
the first and second
stages. The term "second-stage top" is defined as the portion of the second-
stage bundle extending
from and opposite the second-stage base.
CA 02801533 2013-01-11
- 6 -
The term "third-stage nanotubes" are defined herein as nanotubes that are
produced and
found at the second-stage top of the nanotube described herein. The third-
stage nanotubes are
generally similar to convention N-doped nanotubes with the exception that they
are anchored on a
second-stage bundle top and generally extend in a direction aligned with or
substantially that of the
first-stage stalk. There may be from 2 to 25 third-stage nanotube per second-
stage bundle, and
preferably from 6 to 20 third-stage nanotube per second-stage bundle. The
third-stage includes "third-
stage base" and a "third-stage top" opposite and extending from the third-
stage base.
The first-stage stalk, the second-stage bundle and the third-stage nanotubes
all have an
different diameter. The stalk diameter in greater than the bundle diameter
which is greater than the
third-stage nanotube diameter. The average diameter of: the first-stage stalk
nanotube is 145nm to
450nm; the second-stage bundle is 16nm to 65nm; and the third-stage nanotubes
is 2nm to 15nm.
Branches are oriented in substantially the same direction as the stalk
nanotube. The diameter of the
second-stage bundle or aggregation of branches is almost identical to the
diameter of the stalk
nanotube.
The first-stage stalk, the second-stage bundle and the third-stage nanotubes
all have different
lengths. The stalk length is greater than that of the bundle length which is
greater than the third-stage
nanotube length.
The term "branching concentration" is defined herein as an optimal
concentration of catalyst in
wt% in a liquid solution that is injected into the process producing the
multibranched N-doped CNTs as
described herein. The branching concentration induces branch formation of the
CNTs, where a
concentration below the branching concentration produces a nanotube free of
branches. While a
concentration above the branching concentration produces the same number of
branches and
therefore no advantage for the cost of addition catalyst in the liquid
solution.
Referring now to the drawings, Fig. 1 illustrates a process flowsheet of a
spray pyrolysis
chemical vapor deposition (SP-CVD) system 10 for producing N-doped carbon
nanotubes (N-CNT)
according to one embodiment of the present invention. The system is designed
for N-CNT deposition
and includes two sections: a first is a substrate temperature control section
20, and the second section
is a liquid spray section 30.
The temperature control section 20 controls the temperature within the SP-CVD
system 10
from room temperature (20 C) to 1100 C; preferably between 650 C to 850 C and
more preferably
from 700 C to 850 C.
The temperature control section 20 includes: a tube or vessel 22; a heater 25
and substrate
base 27.
The tube or vessel 22, is in a preferred embodiment a quartz tube. The tube or
vessel 22
defines at least an inlet 23 and an outlet 29, opposite the inlet 22. The
inlet 22 is coupled hermetically
with the liquid spray section 30. The heater 25 is generally located on an
outside wall 24 of the vessel
CA 02801533 2013-01-11
-7-
22 between the inlet 23 and the outlet 29. The temperature of the tube or
vessel 22 is raised using the
heater 25 that may be any type of indirect or direct heater capable of raising
the temperature in the
vessel 22 to at least 1100 C. In a preferred embodiment the heater 25 is an
inductive electric heater
or a steam jacket. The heater 25 in a particularly preferred embodiment of the
SP-CVD system 10 is
an electronically controlled electric inductive heater.
The substrate base 27 is located within the tube 22 between the inlet 23 and
the outlet 29 and
in a heated zone 26 within the tube 22 adjacent the heater 25. In a preferred
embodiment the
substrate base 27 is adjacent lower surface of an inner tube wall 28 although
the base 26 may be
placed on any inner surface 28 of the tube including an upper surface of the
inner tube wall 28.
The substrate on which growth of the N-doped CNT occurs is in a preferred
embodiment is a
smooth and flat surface, so as to reduce flow fluctuations around the
substrate. In a preferred
embodiment a silicon wafer of high purity including an native oxide layer
(Si02), that has not been
removed is used as the substrate. In a particularly preferred embodiment the
silicon wafer is a
oriented n-type (1,0,0) silicon (Si) wafer. The substrate may also include an
aluminum (Al) under-layer,
with a thickness in the order of 30 nm. In a particularly preferred embodiment
a further Al layer is
magnetron sputtered on the substrate surface to improve catalyst particle
cluster formation. A possible
substitute for Al on the substrate surface in the growth of N-doped CNTs is
aluminium oxide.
The liquid spray section 30 of the SP-CVD system 10 includes an injection pump
32 that
meters a controlled flow of a liquid 7 from a container (not illustrated) at a
flowrate in a range of
ml/min to 1p1/min. The liquid injected is generally a solution that includes
catalyst dissolved in the
solution or dispersed in suspension. At the pressure side of the pump 32 the
liquid enters a liquid tube
33 upstream of a carrier gas liquid mixer 34. The mixer 34 includes a carrier
gas inlet 38 through
which a carrier gas 5 enters an annular mixer section 35. The liquid tube 33
connects to a liquid mixer
tube 37 via an adapter 36 at the upstream side of the mixer 34. The mixer tube
37 extends through
the annular mixer section 35 towards a venturi 39 that is adjacent an outlet
42 of the mixer 34, to
mixture chamber 40 between the venturi 39 and the outlet 42 ensures that the
solvent 7 and the
carrier gas 5 are well mixed at the low flow rates at a micronozzle 41 and the
outlet 42. The outlet 42
was tested with diameters or 261 and 515 pm.
The pressure and flow of the carrier gas 5 at the venture 39 and inside the
mixture chamber
40 pulverizes the solvent 7 through the micronozzle 42 into a liquid jet 44,
that in a preferred
embodiment takes the form of a cone. The type of micronozzle 41 and shape of
the jet 44 had little
effect on the N-doped CNTs when the jet was localized in the high temperature
of the heated zone 26
of the SP-CVD system 10.
As can be seen in Fig. 1 the outlet 42 of the mixer 34 is within the tube 22.
The outlet 22 need
not be placed within the tube 22 but may be flanged or connected directly to
the tube or vessel 22.
Importantly, for top performance the liquid jet 44 pulverized by the carrier
gas 5 should be created
within the elevated temperature zone 26 produced by the heater 25.
CA 02801533 2013-01-11
- 8 -
Example: A spraying nozzle 41 is designed to continuously pulverize solutions
injected at very
low feed rates of microliters per minute. The system 10 consists of an
electronically controlled
heater/furnace 25 with 300 mm effective heating length, a quartz reactor
deposition tube/chamber 22
(i.d. 25.4 mm). The system 10 delivers a liquid spray at low flow rates and
consists of an injection
pump 32 connected to a copper tube 33 (i.d. 1.5 mm) for carrying the active
liquid solution 7. The
copper tube 33 is sealed inside a carrier gas tube solvent mixer 34 (i.d. 4.2
mm) having an outlet 42
with a capillary spraying nozzle 41 (i.d. 0.292 mm, Sigma Aldrich syringe
needle gauge #24). The
pressure generated inside the carrier gas tube liquid mixer 34 pulverizes, the
solution 7 even at low
flow rates, through the nozzle 41, into the deposition chamber 22, up to the
substrate base 27 surface
that is placed in the middle of the deposition chamber 22. The present
deposition method is
economical, controllable, and can be used continuously for depositions
requiring long time intervals.
Oriented n-type (1, 0, 0) silicon (Si) wafers are used in the Example as a
substrate, that
included a native oxide layer, that is the Si wafer was used without removing
the native oxide layer. An
aluminum (Al) under-layer, with the thickness of 30 nm, was magnetron
sputtered on the Si substrate.
The liquid 7 is an active solution of 0.03 g/m1 concentration ferrocene
(Fe(C5H5)2) dissolved in
acetonitrile (CH3CN) or 0.016mmol/ml. Ferrocene produces metallic iron
particles and acts as the
catalyst during the pyrolysis/synthesis process while the acetonitrile
provides the carbon/nitrogen
feedstock for the multi-branched N-doped CNT described herein. The liquid 7 is
continuously sprayed
into the deposition chamber set and controlled at a temperature of 700 C.
Nitrogen is used as the
carrier gas at a flow rate of 150 sccm (standard cm3). The solution is sprayed
into the deposition
chamber that is at a set temperature and produces a pyrolysis reaction. The
reaction breaks down the
carbon/nitrogen feedstock and catalyst into various active species, producing
multibranched N-doped
carbon nanotubes, comprising at least three stages.
The reaction is maintained for 50 min. Upon completion a total of 3 ml of
solution is fed at a
rate of 0.06 ml/min in the CVD system 10. After the N-doped CNTs growth is
completed, the reactor is
allowed to cool down under nitrogen flow before exposure to air.
The N-doped CNTs samples were characterized by scanning electron microscopy
(SEM -
Hitachi S-4800), transmission electron microscopy (TEM - Philips CM-10) and
Raman spectroscopy
(Raman - Renishaw 785 nm laser excitation). The TEM samples were prepared by
sonicating a small
piece of as-grown nanotubes in ethanol for 10 min and drying a few drops of
suspension on a Cu
micro-grid. The nitrogen amount was determined by X-ray photoelectron
spectroscopy (XPS - Kratos
AXIS Ultra, AlKa).
Results and discussion: The Si substrate from the deposition chamber was
totally covered by
a black carpet-like deposit film upon injection of the pyrolyzed nitrogen
containing liquid. SEM
observation of the product reveals that the film consists of vertical aligned
and multi-branched carbon
nanotubes with an average length of about 5 pm (Fig. 2a and 2b). Along the
length direction of the
nanotubes include (from top to bottom towards the substrate): multiple thin
nanotubes from a third-
CA 02801533 2013-01-11
- 9 -
generation of branches converging into thicker nanotube bundles that are all
(approximately) at the
same height from the substrate. These nanotubes bundles form the second-
generation of branches
and are joined onto a stalk nanotube. The length of the first-stage stalk
nanotubes is approximately
2.5 pm, the stalk is understood as the first generation or first stage of CNT
adjacent the Si substrate.
The outer diameter of stalk nanotubes ranges from 145 nm to 450 nm with an
average of about 210
nm. The stalk nanotubes adhere to the substrate through a catalyst particle
inclusion on the base,
when ferrocene is used the catalyst particle inclusion are iron inclusions (as
illustrated in Fig. 6). The
structure composed by different generation of branches and the stalk nanotubes
forms what can be
defined as the hierarchical multi-branched structure.
Fig. 2a clearly illustrates a Scanning Electron Micrograph of an entire length
of well-aligned
branched CNTs array with different generation of branches visible produced by
the process previously
described. Fig. 2b in a smaller scale SEM that more clearly illustrates the
location of branching from
the first generation/stage to the second and then from the second
generation/stage to the third
generation/stage.
The TEM images of Fig. 3a and 3b, provide insight into the fine structure of
the branched
CNTs that show two typical images of the multi-branched CNT, indicating the
structure transition from
the first-generation of nanotubes to the third generation of the nanotube. The
nanotubes from third-
generation/stage (opposite the stalk) have an average diameter of 15 nm. The
average number of
third¨stage nanotubes grown from a single stalk nanotube may exceeds 20
branches for thick stalk
nanotubes. A feature visible in the TEM micrographs is that some nanotubes
from third¨generation
present an additional and short Y- junction at their tip (Fig. 3b). In Figure
3a and 3b junctions between
the third and second-generation of nanotubes are visible. Most branches are
formed from multi-
terminal junctions where two or more branches from the second-generation
converge into the first
generation stalk nanotube (Fig. 3b). Branches are generally oriented in the
same direction as the stalk
nanotube. The diameter of the nanotubes increased due to an aggregation of
branches is almost the
same size as the diameter of the stalk nanotube. The third generation of
nanotubes features a closed
tip without any visible catalyst particle inclusion. In addition, the product
contains minor amounts of
amorphous carbon or catalyst particles encapsulated in the inner core or
attached to the nanotube
surface. The catalyst particles remain encapsulated at the root of the stalk
nanotubes, likely indicating
that branches are formed by the coalescence of the catalyst particles on the
substrate and the
branched CNTs follow a base growth mode.
Multiple branches are formed from the stalk nanotube approximately at the same
height from
the substrate and the diameter of the aggregation of branches is almost
identical to the diameter of
the stem nanotube. The branched junctions had a similar configuration in that
an average of six
branches with similar diameters, which were oriented in nearly the same
direction, converged into a
larger diameter stem. In comparison with these results, the branched structure
obtained in our study
present large diameter stalk nanotubes, multiple generations of branches, and
a large number of first-
CA 02801533 2013-01-11
- 10 -
generation of CNTs. To the best of our knowledge, it is reported for the first
time that such a large
number of branched CNTs can be generated from a single stalk CNT.
TEM investigation also indicates that branch nanotubes and stalk nanotube
present interlinked
bamboo-like structure and irregular corrugated structure. This disordered
structure was further
characterized by Raman spectroscopy.
First-order Raman spectrum (Fig. 4a) indicates a strong signal around 1578 cm-
1, which is
referred to as the G-band. The G-band corresponds to the optical phonon modes
of E29 symmetry in
graphite and indicates the formation of well graphitized carbon nanotubes. The
D-band at 1349 cm-1
originates from defects that occur in the curved graphene layers and at the
tube ends. The high
intensity of D-band indicates that the tubes present lattice defects and
disorders. Absence of radial
breathing modes in the spectrum is explained by the wide range of tube
diameters present in the
sample, including large diameters, and demonstrates that the CNTs are multi-
walled. The origin of
corrugated structure and high density of defects, observed in TEM and Raman
characterizations, may
be associated with nitrogen incorporation in the branched CNTs during the
nanotube synthesis. The
nitrogen doping concentration was determined from the atomic percentage ratio
of nitrogen and
carbon in the XPS measurements.
Figure 4b shows an XPS survey scan spectrum of the branched CNTs. The peaks of
C is, N
is and 0 is are labeled at 285, 402 and 532 eV, respectively. The oxygen
signal might originate from
oxygen functional groups or the residual air in the nanotubes. Nitrogen can
induce different
configurations of bonding environments in the nanotubes assembly. Usually four
types of nitrogen are
found in CNx: pyridinic, graphitic, pyrrolic and molecular nitrogen. Pyridinic
nitrogen type represents a
nitrogen atom located at the edge or at a defect of the graphene sheet.
Graphitic nitrogen is a nitrogen
atom that substitutes a carbon atom located in the graphene sheet. Pyridinic
and graphitic nitrogen are
both sp2 hybridized. Pyrrolic nitrogen is also substitutional, but is a part
of a five-membered ring and is
sp3 hybridized. Molecular nitrogen can be encapsulated inside the tubes or
exist as intercalated form
between the graphite layers.
The N is XPS spectrum of the branched nanotubes is presented in Figure 4b-d.
The Nis
peak can be deconvoluted into three main component peaks with binding energies
of 398.9 eV (Ni),
401.6 eV (N2), and 403.6 eV (N3). The low-energy N1 peak (I) at 398.9 eV
corresponds to pyridine-
like nitrogen. The pyridine-like sites is considered to be responsible for the
wall roughness and
interlinked morphologies. The peak N2 (II) at 401.6 eV is more dominant and is
attributed to graphitic
nitrogen. The peak N3 (III) at 403.6 eV is attributed to molecular nitrogen.
Molecular nitrogen is
intercalated between the nanotube layers or encapsulated in the central
nanotube hollow and thus it
should have no influence on the structural characteristics of nanotubes. In
addition, a less intensive
peak could be detected at 405.4 eV (N4) and it is assigned to the chemisorbed
nitrogen oxide in the
graphite layers. Pyrrolic nitrogen was not observed in the spectrum. An atomic
nitrogen concentration
of 2.4% was obtained by XPS measurement. Electron microscopy, Raman, and XPS
characterizations
CA 02801533 2013-01-11
- 11 -
indicate that the formation of the multi-stage branched carbon nanotubes
depends greatly on the
employed spray precursors and growth parameters.
Catalyst Concentration: During synthesis experiments of branched CNTs, it has
been found
that the amount of ferrocene can evidently affect the branching behavior of
the nanotube growth. The
optimized concentration used in previous experiments for the branched
structure growth and was 3.0
wt% ferrocene in acetonitrile. No obvious changes were observed for a higher
concentration of
ferrocene (3.5 wt%, not shown). By decreasing the ferrocene concentration to
2.5 wt%, the total length
increased to about 6 pm (Fig. 4a). The diameter of stalk nanotubes decreased
to 150 nm. While the
branched structure is still visible (Fig. 4b), the average number of branches
obtained from a stalk
nanotube decreased to 12 branches. For a further decrease of ferrocene
concentration down to 0.5
wt%, the nanotubes became much longer and thinner, with a total length of 70
pm (Fig. 4c). In this
case the branches almost disappeared (Fig. 4d).
Injection Rate: Injection rates over 0.1 ml/min have been reported for growing
carbon
nanotubes in a spray pyrolysis method. In this study, it has been found that
lower injection rates
favored the formation of branched CNTs. Branched nanotubes with length of 6.2
pm and stalk part of
approximately 3.7 pm were obtained at an injection rate of 0.02 ml/min. The
diameters of the stalk
nanotube ranged from 147 nm to 450 nm. The number of the branches connected to
one stalk
nanotube could reach 18 branches on average. A higher injection rate of 0.06
ml/min, gave shorter
branched nanotubes with the total length of 5.0 pm and a stalk length of about
2.5 pm. At this injection
rate, the diameter of the stalk nanotubes and the number of nanotubes
generated by a stalk remained
almost unchanged based on the cross sectional view of SEM image.
When the injection rate was increased to 0.1 ml/min, an obvious change of the
branch density
is observed. The stalk nanotubes were shorter (1.2 pm) and the diameter
decreased and ranged
between 97 nm and 211 nm. Consequently, the average number of the nanotubes
connected to one
stalk nanotube was reduced to 9 branches. The density of the branches from a
single stalk nanotube
can be adjusted to by varying the liquid injection rate onto the substrate.
Temperature: The optimized temperature for growing multi-stage hierarchical
carbon
nanotubes was 700 C. CNTs could not be produced at a lower temperature of 650
C. By increasing
the temperature to 800 C and 850 C, the CNTs present fewer branches and more
visible catalyst
particle inclusions. With a further increase of the growth temperature to 900
C the branched structure
disappeared and corrugated CNTs were obtained.
Time: To study the formation process of different hierarchies of the multi-
stage branched
CNTs, the reaction was stopped after a growth time of 10, 20, 30, 40 and 60
min. In the reaction
process, After 10 min. growth time and 0.6 ml injected, the nanotubes are thin
with a length of around
100 nm, though some longer leading nanotubes are also formed. At this initial
growth stage no
branches are observed. By increasing the growth time to 20 min., the
continuous feeding of active
solution made the original catalyst particles larger and initiated their
coalescence causing the
CA 02801533 2013-01-11
- 12 -
formation of first-generation of branches. After 30 min. the stalks of the
first-generation of CNTs
elongate and start to converge into a larger stalk tube due to aggregation of
the catalyst particles. With
a further increase of the growth time to 40 min, the stalk nanotubes are
clearly formed and sustain the
first and second- generation of CNTs. By increasing the deposition time to 50
min. or 60 min., the stalk
nanotubes elongate and no further branches are observed. This may be explained
due to the large
diameter, thick walls, and strong adhesion to the substrate of stalk
nanotubes.
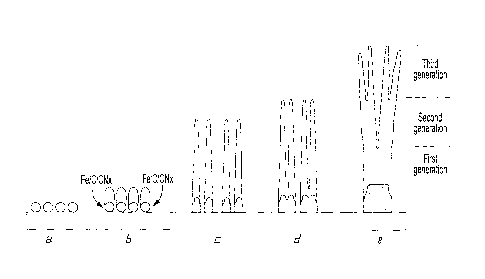
Growth Mechanism : Based on the above SEM and TEM observations a possible
formation
mechanism of branched and multi-stage CNTs is proposed (Fig. 6) including
steps a) to e).
a) The decomposition of acetonitrile/ferrocene droplets by spray pyrolysis
produces catalyst
clusters on the substrate and an atmosphere rich in active species such as
C,Fly, CN, species, N2, and
H2 molecules. Fe clusters adhere to the substrate, start to form
nanoparticles, and react with C,Fly,
CNõ species, and N2 molecules.
b) C and CN, species are absorbed and precipitate on the Fe catalyst particles
to form
individual capped CNTs. The small and reactive metal particles give a strong
interaction with
precipitated species and form a graphene cap that stops the carbon source flux
over the catalyst. The
carbon source can be provided only at the catalyst/substrate interface along
with more catalyst
particles. The Fe particle density and adhesion to the substrate are large
enough to sustain vertically
aligned nanotubes in a base type growth.
c) During the CNT growth, elongation of the catalyst particle increases its
strain energy. This
results in pushing the capped nanotubes away from the substrate and in forming
bamboo or
corrugated structures. This leads to growth of the first-stage CNTs.
d) Injecting the precursors at low rates increases the catalyst size and,
consequently, the
distance between catalyst particles is gradually reduced. At this stage,
multiple adjacent catalyst
particles coalesce due to continuous spraying. After the Fe catalyst particles
become larger, the first-
stage of nanotubes is complete, multi-terminal branches are formed, and stem
CNTs with larger
diameters continue to grow on the substrate. This results in the production of
the first-generation of
branched CNTs.
e) Following a similar process, the second-generation of CNTs is formed which
converge into
a large diameters stalk nanotubes.
By continuing the synthesis process, the stalk nanotubes continue to grow
without being able
to generate further branches due to their large diameters, thick walls, and
strong adhesion to the
substrate. The formation mechanism of the multi-stage hierarchical CNTs can be
attributed to the
coalescence of the catalyst particles on the substrate during the growth
process.
CA 02801533 2013-01-11
- 13 -
This N-doped CNT structure described herein provides several important
characteristics,
particularly: stalk nanotubes having large diameters that may be applicable to
nano-energetic material
enclosures; consistent height (distance) of branch formation occurs from the
level of the substrate,
and the CNTs includes a multiple stages and a plurality of branches. These
properties are likely to be
useful nanoelectronics and energy conversion devices.
The embodiments of the invention described above are intended to be exemplary.
Those
skilled in the art will therefore appreciate that the foregoing description is
illustrative only, and that
various alternate configurations and modifications can be devised without
departing from the spirit of
the present invention. Accordingly, the present invention is intended to
embrace all such alternate
configurations, modifications and variances which fall within the scope of the
appended claims.