Note: Descriptions are shown in the official language in which they were submitted.
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
DIESEL FUEL ADDITIVE
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application Serial No.
61/360,880,
filed July 1, 2010, the contents of which are incorporated herein in their
entirety.
BACKGROUND OF THE INVENTION
Field of the Invention
This invention relates to additives for use with diesel fuel, in particular
diesel fuel used in
internal combustion engines.
Description of the Related Art
Diesel fuel has been used for a long period of time, and, when used in
internal
combustion engines, confers many advantages when compared to gasoline.
However, there is
nonetheless room for improving the performance and characteristics of diesel
fuel. Diesel fuel,
when combusted, produces significant pollution, including particulate
emissions. It would be
advantageous to find a means by which these adverse effects can be minimized,
in addition to
improving the efficiency of diesel fuel combustion.
SUMMARY OF THE INVENTION
Some embodiments of the present invention are directed to a new additive for
use with
diesel fuel. When combined with diesel fuel used in internal combustion
engines, including
automobiles, this additive provides many advantages. These advantages include,
but are not
limited to, reducing combustion byproduct emissions, such as carbon dioxide,
sulfur, and other
pollutants, as well as reducing diesel fuel consumption.
Embodiments of the present invention, when used as an additive in diesel fuel,
may be
used in cars, trucks, power generators, and other machines using internal
combustion engines.
The additive is compatible with ordinary fuel systems and does not require any
modification to
an engine before use.
BRIEF DESCRIPTION OF THE DRAWINGS
-1-
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
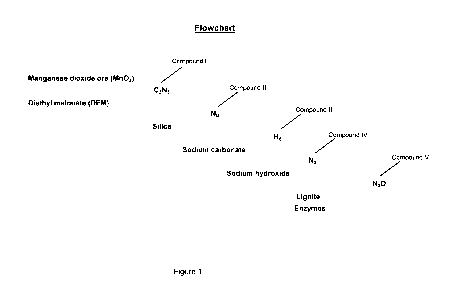
FIG. 1 illustrates a flowchart describing a synthetic procedure for production
of an
embodiment of the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The present invention comprises one or more compounds synthesized using a
multi-step
process. The compound or compounds resulting from this synthetic process,
typically in the
form of a powder or gel, constitute an additive that may then be added to
diesel fuel, including
petrodiesel and biodiesel fuel, although the additive could also be added to
unleaded gasoline or
other types of fuel as well. As will be explained in further detail below,
this additive confers
several benefits and advantages in the combustion of diesel fuel, for example
in an internal
combustion engine, compared to diesel fuel without an additive.
With reference to Figure 1, the process for the synthesis and preparation of a
diesel fuel
additive involves multiple steps and the creation of several precursor
compounds before the final
product is complete. In one embodiment, the ingredients used in the synthetic
procedure can be
added in proportions according to Table 1 below, in which the percentages
indicated are based on
100% of the ingredients added to the diesel fuel additive.
The synthetic procedure involves the use of a manganese compound, preferably
manganese dioxide, and more preferably as manganese dioxide ore which can be
provided in
powder form. The manganese dioxide ore can be obtained from many sources. One
preferred
source is manganese dioxide ore from India, which can provide an ore of 78%
quality or purity.
Variations in the quality or purity of the ore are acceptable, for example,
2%, 5%, 8%, 10%,
12%, 15%., or 20%.. Note that the amount of ore is preferably adjusted to
maintain the
percentage of manganese dioxide according to Table 1. Although the amount of
manganese
dioxide ore is 43% or about 43% in some embodiments,, according to Table 1
below, other
amounts may be appropriate, for example a range between about 42-44%, 40-46%,
35-50%, 25-
50%, or 5-60%.
Diethyl malonate ("DEM") is then added to the ore. The amount of DEM may vary
between an amount just barely sufficient to mix with the ore to approximately
20%, although in
some embodiments, 9%, about 9%, between about 8-10%, 6-12%, or 5-15% is used.
While
mixing, the mixture is heated, in some embodiments only slightly such as 75-
120 F, or to 95 F.
-2-
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
This can be accomplished, for example, in a steam boiler. The mixing is
continued for a
relatively short period of time such as about 25-105 minutes, or about 65
minutes. The resulting
compound is Compound I, which may appear as a light brown or brownish color.
Silica is then added to Compound I, and this may then be mixed for a
relatively short
period of time, for example about 5 to 55 minutes, or about 25 minutes. Other
silicon
compounds, including silicates and silicon, may be used as well. The amount of
silica used can
range in some cases of no more than about20%, or no more than aboutl0%, or
sometimes
between about 5-10%, or around 3.5%. While mixing, the mixture is preferably
heated, such as
from 80 F to 130 F, or to 95 F. After cooling to room temperature such as
between 60 F to
75 F, the resulting mixture forms a neutral colloid, or Compound II. Compound
II usually
presents as a very light brown or sandy color. Compound II is then mixed with
a carbonate, for
example sodium carbonate in powder form, and with or without heating (for
example, at room
temperature) for a short period of time such as 5-60 minutes, such as about 15
minutes, so as to
form Compound III, which may appear red or red-tinted. The sodium carbonate
can be, for
example, in the range of about 7%, between about 5-15%, or no more than about
20%, 15%, or
10%.
Next, a base, such as a strong base such as sodium hydroxide in aqueous
solution, in
some embodiments at a concentration of 48%, 45-50%, 40-60%, or 30-70%, may be
added and
mixed with Compound III. The mixture is preferably mixed for a short period of
time, such as 5-
45 minutes, or 35 minutes, at a relatively low temperature, for example room
temperature such as
between 60 F to 75 F. The percentage of sodium hydroxide may vary, for
example between
1% and 10%, or between 1% and 5%, but is preferably around 2.5%. At this
point, Compound
IV is formed as a powder, and is usually reddish or red-tinted in color.
Lignite powder is then added to Compound IV and blended during a relatively
brief time
interval, for example from 1-15 minutes, or 10 minutes. Although lignite
powder is preferably
used, other hydrocarbon and carbon compounds such as anthracite or other
grades of coal may be
suitable as well. The blending can occur in some embodiments at room
temperature or some
other similarly low temperature, such as between 60 F to 75 F, or less than
about 75 F.
Preferably, the lignite powder is of a higher grade, for example greater than
55%, 60%, 65%,
70%, 75%, or more and may be obtained from India. A grade of 55% 1.2% has
been found to
-3-
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
perform acceptably, although other grades may also be possible. Preferably,
the lignite is black in
color. The weight of lignite added may range between 1-20% of the final
product, such as
between 5-10%, or 3%. Enzymes, such as one, two, or more oxidoreductases,
including
dehydrogenases or oxidases, are then added to the above mixture. The enzymes
preferably
include a mixture of EC 1.18 enzymes (enzymes acting on iron-sulfur proteins
as donors) and EC
1.1 enzymes (enzymes acting on the CH-OH group of donors). The EC codes
correspond to the
classification nomenclature set forth by the Enzyme Commission, now published
by the
International Union of Biochemistry and Molecular Biology at Enzyme
Nomenclature 1992
[Academic Press, San Diego, California, ISBN 0-12-227164-5 (hardback), 0-12-
227165-3
(paperback)] with Supplement 1 (1993), Supplement 2 (1994), Supplement 3
(1995), Supplement
4 (1997) and Supplement 5 (in Eur. J. Biochem. 1994, 223, 1-5; Eur. J.
Biochem. 1995, 232, 1-6;
Eur. J. Biochem. 1996, 237, 1-5; Eur. J. Biochem. 1997, 250; 1-6, and Eur. J.
Biochem. 1999,
264, 610-650; respectively), all of which are hereby incorporated by reference
in their entireties.
EC 1.1 enzymes can include those with NAD or NADP as an acceptor (EC 1.1.1,
e.g., alcohol
dehydrogenase), with a cytochrome as an acceptor (EC 1.1.2, e.g., lactate
dehydrogenase), with
oxygen as an acceptor (EC 1.1.3, e.g., alcohol oxidase), with a disulfide as
an acceptor (EC 1.1.4,
e.g., vitamin-K-epoxide reductase), with a quinine or similar compound as an
acceptor (EC 1.1.5,
e.g., quinoprotein glucose dehydrogenase), or with other acceptors (EC
1.1.99). EC 1.18 enzymes
can include rubredoxin-NAD+ reductase, ferredoxin-NADP+ reductase, ferredoxin-
NAD+
reductase, rubredoxin-NAD(P)+ reductase, or nitrogenases for example. These
enzymes may be
purchased from suppliers such as Advanced Enzyme Technologies Ltd. (Thane,
India) or
Microgenix Specialities Pvt. Ltd. (Gujarat, India). In some embodiments, the
EC 1.18 enzymes
make up 9% or about 9% of the product, and the EC 1.1 enzymes make up 8% or
about 8% of
the product. However, these enzymes may each be used in the range of, for
example, less than
about 25%, 20%, 15%, 12%, or 10%. This mixture is combined together, such as
at a relatively
low temperature such as room temperature, such as between 60 F to 75 F, or
less than about 75
F until thoroughly blended. The mixture typically forms a powder, or Compound
V, which may
appear as a white, off-white, or pale yellow color. At this stage, among
others, the powder may
be used as a diesel fuel additive.
-4-
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
Optionally, a chelator such as diethylene triamine pentaacetic acid ("DTPA"),
and an
polar aprotic solvent such as dimethylformamide ("DMF"), both liquid, are
mixed together in
preferably approximately equal parts. Other chelators that may be used include
ethylenediaminetetraacetic acid ("EDTA"). Other polar aprotic solvents that
may be used
include dimethyl sulfoxide ("DMSO"). As listed in Table I below, the DTPA and
DMF together
preferably form approximately 15% of the final product in equal 7.5%
proportions in one
embodiment; however, these ratios may be varied by reducing either the DTPA or
DMF present
by up to 2%, 3%, 3.5%, 4%, or 5%, as long as the amount of the corresponding
DMF or DTPA is
increased so that the total amount of the two materials equals approximately
15%, although the
total amount could be, for example, between about 12-18%, 10-20%, or 5-25% in
other
embodiments. This DTPA/DMF mixture can then be added to Compound V and mixed
until a
gel forms. This resulting gel is another form of the diesel fuel additive,
which can be used in the
same manner as the powder.
The following Table I lists one non-limiting example of ingredients which may
be used
to create a diesel fuel additive according to the procedure illustrated above.
The percentage
values represent one potential preferred amount of each ingredient by mass
that is added to create
the final product. In the procedure listed above, purity or quality values may
be listed, and the
percentages listed below are based on the use of those ingredients at that
given purity. The
amounts of ingredients can thus be adjusted if the purity of a given
ingredient is different. Other
percentages, or ranges described elsewhere in the specification can also be
utilized depending on
the desired result. The final product could also include amounts of other
compounds, such as a
diluent for example, and the percentages listed below exclude percentages of
those other
compounds.
TABLE I
Name of Products in Diesel Fuel Additive Percentage
Manganese dioxide ore (Mn02) 43%
Diethyl malonate (DEM) 9%
Silica (Si02) 3.5%
-5-
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
Sodium carbonate (powder) (Na2CO3) 7%
Sodium hydroxide (NaOH) 2.5%
Lignite (powder) 3%
Enzymes: EC 1.18 9%
EC 1.1 8%
Diethylene triamine pentaacetic acid (DTPA) 7.5%
Dimethylformamide (DMF) 7.5%
Total Percent 100 %
Without wishing to be bound by theory, the diesel fuel additive produced
according to the
procedure set forth above is believed to function, once mixed with diesel
fuel, by reacting with
sulfur present in the fuel. This forms a first intermediate compound. When
this first
intermediate compound is then mixed with phenolic compounds present in the
fuel, it creates a
second intermediate compound. Subsequently, when the diesel fuel is combusted,
typically in an
internal combustion engine, the presence of this second intermediate compound
makes the diesel
fuel burn more cleanly and with fewer pollutants. Also, the presence of these
intermediate
compounds may provide additional power and reduce fuel consumption.
In order to use the diesel fuel additive, an amount of diesel fuel additive is
added to a tank
of diesel fuel. Only a small amount of diesel fuel additive may need to be
added to obtain
advantageous results. For example, one gram of diesel fuel additive powder per
U.S. gallon of
diesel fuel may be sufficient. Similarly, approximately 1.2 grams of diesel
fuel additive gel per
U.S. gallon of diesel fuel may be sufficient. In other embodiments, no more
than about 10 grams,
9 grams, 8 grams, 7 grams, 6 grams, 5 grams, 4 grams, 3 grams, 2 grams, 1.8
grams, 1.6 grams,
1.4 grams, 1.2 grams, 1 gram, or less of diesel fuel additive gel or powder
per U.S. gallon of
diesel fuel is added to improve the diesel fuel. The diesel fuel additive may
be added as either a
powder (Compound V from the procedure above), or as a gel. Both the powder and
the gel forms
of the product can be provided in a diluent suitable for addition to diesel
fuel.
Below are experimental results which demonstrate the uses and effectiveness of
the diesel
fuel additive described above.
EXAMPLE 1
-6-
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
A 1992 6.2L medium-duty GMC diesel truck was tested by a professional testing
service
(Rod's Truck Repair, Santa Fe Springs, California) using the diesel fuel
additive described
above. The truck had a baseline fuel consumption of 15.1 miles per gallon. To
test the additive,
the additive was mixed with two gallons of Chevron diesel fuel, which was then
added to an
additional 25 gallons of diesel fuel pumped into the truck.
The truck was then operated in typical stop-and-go traffic for a total of 419
miles. At this
point, the fuel was drained from the truck's tank, and a total of 23 gallons
of diesel fuel with
additive was consumed. This yielded a fuel consumption of 18.2 miles per
gallon, corresponding
to a fuel mileage increase of 20.5%. Additionally, emissions were tested.
Nitric oxide emissions
were reduced by 26%, and the exhaust smoke opacity was reduced by 40%.
EXAMPLE 2
A long-term mileage test was conducted by the same testing service above using
a 2005
Volvo tractor, with a baseline diesel fuel consumption of 5.24 miles per
gallon, and a baseline
smoke opacity of 5.35%. The truck was driven over 6439 miles (including
mountainous terrain);
over several tanks of fuel with additive added, the resulting average fuel
consumption was
calculated to be 7.61 miles per gallon. The smoke opacity was measured at
2.02%. This yields a
fuel mileage improvement of 45% and a decrease in opacity of 62%.
EXAMPLE 3
Another test similar to Example 1 above was performed on a 2007 Peterbilt
tractor, which
had a baseline fuel consumption of 5.84 miles per gallon and a baseline smoke
opacity of 10.6.
After usage of the diesel fuel additive, average fuel consumption was
calculated to be 8.88 miles
per gallon, and opacity was calculated at 8.61. Thus, fuel mileage was
improved by 52% and
opacity was reduced by 19%.
Although certain embodiments of the disclosure have been described in detail,
certain
variations and modifications will be apparent to those skilled in the art,
including embodiments
that do not provide all the features and benefits described herein. It will be
understood by those
skilled in the art that the present disclosure extends beyond the specifically
disclosed
-7-
CA 02803896 2012-12-21
WO 2012/003323 PCT/US2011/042604
embodiments to other alternative or additional embodiments and/or uses and
obvious
modifications and equivalents thereof. In addition, while a number of
variations have been
shown and described in varying detail, other modifications, which are within
the scope of the
present disclosure, will be readily apparent to those of skill in the art
based upon this disclosure.
It is also contemplated that various combinations or subcombinations of the
specific features and
aspects of the embodiments may be made and still fall within the scope of the
present disclosure.
Accordingly, it should be understood that various features and aspects of the
disclosed
embodiments can be combined with or substituted for one another in order to
form varying
modes of the present disclosure. Thus, it is intended that the scope of the
present disclosure
herein disclosed should not be limited by the particular disclosed embodiments
described above.
For all of the embodiments described above, the steps of any methods need not
be performed
sequentially.
-8-