Note: Descriptions are shown in the official language in which they were submitted.
CA 02803910 2012-12-24
1
INK COMPOSITION, INKJET RECORDING METHOD AND COLORED BODY
TECHNICAL FIELD
The present invention relates to an ink composition
containing specific two kinds of coloring matters, an ink jet
recording method using the ink composition, and a colored body
colored by the ink composition.
BACKGROUND ART
One of the representative methods for various color
recording methods may be a recording method using an ink jet
printer, that is, an ink jet recording method. This recording
method involves producing small droplets of ink, and attaching
these small droplets to various record-receiving materials
(paper, film, clothes and the like) to perform recording. In
this method, the method is characterized in that since the
recording head and the record-receiving material are not
brought into direct contact, the process is quiet with less
noise generation, and size reduction or an increased speed can
easily be achieved. Therefore, the method has rapidly become
popular in recent years, and an extensive growth in use of the
method is expected.
Conventionally, aqueous inks prepared by dissolving a
water-soluble coloring matter in an aqueous medium have been
used as the inks for fountain pens, felt pens and the like and
as the inks for ink jet recording. In these aqueous inks,
water-soluble organic solvents are generally added so as to
CA 02803910 2012-12-24
2
prevent clogging of the inks at the pen tips or ink discharge
nozzles. Furthermore, these inks are required to have
advantages such as the production of recorded images of
sufficient densities, no occurrence of clogging at the pent
tips or nozzles, satisfactory drying properties on record-
receiving materials, less bleeding, and excellent storage
stability. Also, the water-soluble coloring matters used
therein is required to have high solubility particularly in
water, and high solubility in the water-soluble organic
solvents that are added to the ink. In addition, the images
thus formed are required to have various image fastness
properties such as water resistance, light fastness, gas
fastness and moisture resistance. Furthermore, development of
a coloring matter producing high recorded images is required.
Among these, the above-described term gas fastness is a
resistance to the phenomenon that oxidizing gas such as ozone
gas present in air acts on the coloring matter within a
recording paper and changes the color of a printed image. In
addition to ozone gas, examples of oxidizing gases having this
kind of action include NOx and SOx. However, among these
oxidizing gases, ozone gas is considered as a main causative
substance which accelerates the phenomenon of discoloration
and fading of ink jet recorded images. For this reason, among
the gas fastness, particularly ozone gas fastness tends to be
regarded as most important. In an ink-receiving layer provided
on the surface of exclusive ink jet paper of photographic
image quality, materials such as porous white inorganic
CA 02803910 2012-12-24
3
substances are frequently used for the purpose of speeding up
drying of the ink, and reducing the bleeding at high image
quality. In the images recorded in such a recording paper,
significant discoloration and fading of the recorded image due
to ozone gas is observed. Since the phenomenon of
discoloration and fading due to an oxidizing gas, is
characteristic to ink jet images, an enhancement of ozone gas
fastness has been one of the most important problems to be
solved in the ink jet recording method.
Indicators for deterioration of recorded images to light
are divided broadly into 2 kinds of indicators. One is those
using the degree of decline of print density of recorded
images as an indicator, and the other one is those using the
degree of change of the hues of recorded images as an
indicator. The decline of print density is observed as the
fading of recorded images, and the change of the hues is
observed as the color change of recorded images.
In order to store recorded images in the state of high
quality for a long time, it is required to develop a coloring
matter that is hard to have occurrence of the decline of the
print density and the change of the hues to light, and is
excellent in light fastness.
It is known that a coloring matter producing high
recorded images is used to obtain recorded images having high
quality of further density feeling. Additionally, it is
possible to solve nozzle clogging when printing with an ink
jet printer.
CA 02803910 2012-12-24
4
The nozzle clogging of an ink jet printer is often
derived from the fact that the moisture in the ink in the
vicinity of the nozzles is evaporated earlier than other
solvents or additives, and coloring matters are solidified and
precipitated when the ink comes to have a composition state
where the moisture is less and the solvents or the additives
are more. As a method for solving the nozzle clogging, a
technique of using a coloring matter having high print density
is known. Use of the coloring matter having high print density
makes it possible to lessen the solid content of the coloring
matters in the ink while maintaining conventional print
density. This makes it difficult for the coloring matters to
be precipitated, and also is advantageous in terms of the cost,
and a coloring matter having high print density is desired to
be developed.
In order to expand the field of use in those recording
(printing) methods using ink in the future, it is strongly
desired to enhance light fastness, ozone gas fastness,
moisture resistance and water resistance and to further
enhance color development properties in the ink composition
used in ink jet recording and the colored body colored by the
ink composition.
Inks of various colors have been prepared from various
coloring matters; however, among them, a black ink is an
important ink that is used in both of mono-color and full-
color images. However, it has many difficult points
technically to develop a coloring matter having a neutral hue
CA 02803910 2012-12-24
between the dark color gamut and the light color gamut, and
having high print density, and further having less dependency
of the hue on the light source and exhibiting good black. Thus,
although many researches and developments have been performed,
those having sufficient performances are still few. Thus, it
is generally performed to prepare a black ink by combining
multiple, versatile coloring matters. However, the preparation
of an ink by mixing multiple coloring matters has problems
such as 1) variation of the hues depending on the medium
(record-receiving materials) and 2) particularly, increase of
discoloration by decomposition of the coloring matters by
light or ozone gas, in comparison to adjustment of an ink with
a single coloring matter.
A black ink composition for ink jet to render a printed
matter to have good various durabilities is proposed in, for
example, Patent Documents 1, 2 and 3, and the like. This ink
composition is a greatly improved ink composition having good
image fastness properties of a printed matter. However, use of
these ink compositions as an ink in a single color has not
been applied to a neutral black printed matter, and have not
sufficiently satisfied further demands of the market requiring
high image fastness properties. In addition, Patent Documents
4 and 5 disclose a technique of combining various inks to
obtain a black ink composition. However, there is no disclosed
ink composition satisfying high fastness properties and color
development properties, whereby to satisfy demands of the
market.
CA 02803910 2012-12-24
6
Patent Document 1: Japanese Unexamined Patent Application,
Publication No. 2009-84346
Patent Document 2: PCT International Publication No.
W02009/069279
Patent Document 3: PCT International Publication No.
W02005/097912
Patent Document 4: PCT International Publication No.
W02007/077931
Patent Document 4: Japanese Translation of PCT
International Publication, Publication No. 2009-512737
DISCLOSURE OF THE INVENTION
Problems to be Solved by the Invention
An object of the present invention is to provide an
aqueous black ink composition which exhibits neutral black
with low chroma and no color tone, and produces black recorded
images having high density of printed images.
Means for Solving the Problems
The inventors of the present invention repeatedly
conducted thorough investigations so as to solve such problems
described above, and as a result, the inventors found that an
ink composition containing a coloring matter (I) represented
by a specific formula and a coloring matter (II), which is an
azo compound having Amax in a range of 550 to 660 nm, can
solve the problems described above, and thus completing the
present invention.
CA 02803910 2012-12-24
7
Accordingly, a first aspect of the present invention
provides an ink composition containing at least one kind of
the compound represented by the following formula (1) or a
salt thereof as a coloring matter (I):
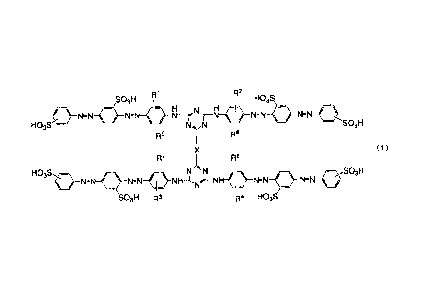
S03H R' R2
-N-N N='" j --r-Nawo
HO3S N TN I SO3H
Rs Re
X (1)
Rr Re
HO3S H_NN-N~ NH-'N~IN N.r--NW<~ N (}-N=N SO3H
SO3H R3 R4 HO3S
in the formula (1),
R1 to R8 each independently represent a hydrogen atom; a
halogen atom; a sulfo group; a carboxy group; a sulfamoyl
group; a carbamoyl group; a Cl-C4 alkyl group; a C1-C4 alkoxy
group; a Cl-C4 alkoxy group substituted with at least one
group selected from the group consisting of a hydroxy group, a
C1-C4 alkoxy group, a hydroxy-Cl-C4 alkoxy group, a sulfo
group, and a carboxy group; a C1-C4 alkylcarbonylamino group;
a C1-C4 alkylcarbonylamino group substituted with a carboxy
group; a ureido group; a mono-Cl-C4 alkylureido group; a di-
C1-C4 alkylureido group; a mono-C1-C4 alkylureido group
substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, and a carboxy
group; a di-C1-C4 alkylureido group substituted with at least
one group selected from the group consisting of a hydroxy
group, a sulfo group, and a carboxy group; a benzoylamino
group; a benzoylamino group having its benzene ring
substituted with at least one group selected from the group
CA 02803910 2012-12-24
8
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group; a
benzenesulfonylamino group; or a phenylsulfonylamino group
having its benzene ring substituted with at least one group
selected from the group consisting of a halogen atom, a Cl-C4
alkyl group, a nitro group, a sulfo group, and a carboxy group;
and
X represents a divalent crosslinking group,
and
an azo compound having Amax in a range of 550 to 660 nm
as a coloring matter (II).
A second aspect of the invention provides the ink
composition according to the first aspect, wherein the
coloring matter (I) is the compound represented by the
following formula (2) or a salt thereof:
so3H R' H RZ Ho
3
-N-N N=N*N-1 N rH N=N
H03S - fff---"' N N ~SOsH
RS T R6
X (2)
R7 I Ra
HO3S N N ~~S03H
-N-N~N=N~NHIN~- NH N=N ~-N=N-~~
SO3H R3 R4 H03S
in the formula (2),
R1 to R8 each independently represent a hydrogen atom; a
halogen atom; a sulfo group; a carboxy group; a sulfamoyl
group; a carbamoyl group; a Cl-C4 alkyl group; a Cl-C4 alkoxy
group; a Cl-C4 alkoxy group substituted with at least one
group selected from the group consisting of a hydroxy group, a
Cl-C4 alkoxy group, a hydroxy-C1-C4 alkoxy group, a sulfo
CA 02803910 2012-12-24
9
group, and a carboxy group; a Cl-C4 alkylcarbonylamino group;
a Cl-C4 alkylcarbonylamino group substituted with a carboxy
group; a ureido group; a mono-C1-C4 alkylureido group; a di-
Cl-C4 alkylureido group; a mono-Cl-C4 alkylureido group
substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, and a carboxy
group; a di-Cl-C4 alkylureido group substituted with at least
one group selected from the group consisting of a hydroxy
group, a sulfo group, and a carboxy group; a benzoylamino
group; a benzoylamino group having its benzene ring
substituted with at least one group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group; a
benzenesulfonylamino group; or a phenylsulfonylamino group
having its benzene ring substituted with at least one group
selected from the group consisting of a halogen atom, a Cl-C4
alkyl group, a nitro group, a sulfo group, and a carboxy group;
and
X represents a divalent crosslinking group.
A third aspect of the invention provides the ink
composition according to the first or second aspect, wherein
in the formula (1) or (2), R1 to R8 each independently
represent a hydrogen atom; a halogen atom; a Cl-C4 alkyl
group; a Cl-C4 alkoxy group; a Cl-C4 alkoxy group substituted
with a sulfo group or a carboxy group; or a Cl-C4
alkylcarbonylamino group.
A fourth aspect of the invention provides the ink
CA 02803910 2012-12-24
composition according to any one of the first to third aspects,
wherein in the formula (1) or (2), X represents a Cl-C8
alkylenediamino group; a C1-C8 alkylenediamino group
substituted with a carboxy group; an N-Cl-C4 alkyl-Cl-C6
alkylenediamino group having its alkyl moiety substituted with
a hydroxy group; an amino-C1-C4 alkoxy-Cl-C4 alkoxy-Cl-C4
alkylamino group; a xylylenediamino group; or a piperazine-
1,4-diyl group.
A fifth aspect of the invention provides the ink
composition according to any one of the first to fourth
aspects, wherein in the formula (1) or (2),
at least one of R1 and R2 is a sulfopropoxy group,
at least one of R3 and R4 is a sulfopropoxy group,
R5 to R8 are a Cl-C4 alkyl group, and
X is a C2-C4 alkylenediamino group; a C2-C6
alkylenediamino group substituted with a carboxy group; an N-
C2-C3 alkyl-C2-C3 alkylenediamino group having its alkyl
moiety substituted with a hydroxy group; an amino-C2-C3 alkoxy
C2-C3 alkoxy C2-C3 alkylamino group; an m- or p-
xylylenediamino group; or a piperazine-l,4-diyl group.
A sixth aspect of the invention provides the ink
composition according to any one of the first to fifth aspects,
wherein the coloring matter (II) is the compound represented
by the following formula (3) or a tautomer thereof or a salt
thereof:
CA 02803910 2012-12-24
11
8105 R1D2
H 101
R'~ \ )-N=N ' N=N /
8107 5 t~ R~oa I N=N \~N (3)
R H03S
(SMn HO D
in the formula (3),
n is 0 or 1,
8101 represents a carboxy group; a Cl-C8 alkoxycarbonyl
group; a Cl-C4 alkyl group which may be substituted with a Cl-
C8 alkoxycarbonyl group or a carboxy group; or a phenyl group
which may be substituted with a hydroxy group, a sulfo group,
or a carboxy group;
R102 to R104 each independently represent a hydrogen atom;
a halogen atom; a hydroxy group; a sulfo group; a carboxy
group; a sulfamoyl group; a carbamoyl group; a Cl-C4 alkyl
group; a Cl-C4 alkoxy group which may be substituted with at
least one group selected from the group consisting of a
hydroxy group, a Cl-C4 alkoxy group, a hydroxy-Cl-C4 alkoxy
group, a sulfo group, and a carboxy group; a mono- or di-Cl-C4
alkylamino group which may be substituted with at least one
group selected from the group consisting of a hydroxy group, a
sulfo group, and a carboxy group; a Cl-C4 alkylcarbonylamino
group which may be substituted with a hydroxy group or a
carboxy group; an N'-Cl-C4 alkylureido group which may be
substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, and a carboxy
group; a phenylamino group having its benzene ring which may
be substituted with at least one group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
CA 02803910 2012-12-24
12
group, a sulfo group, and a carboxy group; a benzoylamino
group having its benzene ring which may be substituted with at
least one group selected from the group consisting of a
halogen atom, a C1-C4 alkyl group, a nitro group, a sulfo
group, and a carboxy group; or a phenylsulfonylamino group
having its benzene ring which may be substituted with at least
one group selected from the group consisting of a halogen atom,
a Cl-C4 alkyl group, a nitro group, a sulfo group, and a
carboxy group;
R105 to R107 each independently represent a hydrogen atom;
a halogen atom; a carboxy group; a sulfo group; a nitro group;
a hydroxy group; a carbamoyl group; a sulfamoyl group; a Cl-C4
alkyl group; a Cl-C4 alkoxy group which may be substituted
with at least one group selected from the group consisting of
a hydroxy group, a Cl-C4 alkoxy group, a sulfo group, and a
carboxy group; a Cl-C4 alkylsulfonyl group which may be
substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, and a carboxy
group; or a phenylsulfonyl group having its benzene ring which
may be substituted with at least one group selected from the
group consisting of a halogen atom, a Cl-C4 alkyl group, a
nitro group, a sulfo group, and a carboxy group; and
the group D represents an optionally substituted phenyl
group or naphthyl group, wherein
in the case where the group D is a substituted phenyl
group, the group D has a substituent selected from the group
consisting of a hydroxy group; a sulfo group; a carboxy group;
CA 02803910 2012-12-24
13
a C1-C4 alkyl group; a Cl-C4 alkoxy group; an amino group; a
mono- or di-C1-C4 alkylamino group; a C1-C4 alkylcarbonylamino
group; a benzoylamino group having its benzene ring which may
be substituted with a halogen atom, a Cl-C4 alkyl group, a
nitro group, a sulfo group, or a carboxy group; and a
phenylsulfonyloxy group having its benzene ring which may be
substituted with a halogen atom, a nitro group, or a C1-C4
alkyl group; or
in the case where the group D is a substituted naphthyl
group, the group D has a substituent selected from the group
consisting of a hydroxy group; a sulfo group; a carboxy group;
a Cl-C4 alkyl group; a C1-C4 alkoxy group; an amino group; a
mono- or di-Cl-C4 alkylamino group; a C1-C4 alkylcarbonylamino
group; a benzoylamino group having its benzene ring which may
be substituted with a halogen atom, a C1-C4 alkyl group, a
nitro group, a sulfo group, or a carboxy group; and a
phenylsulfonyloxy group having its benzene ring which may be
substituted with a halogen atom, a nitro group, or a C1-C4
alkyl group.
A seventh aspect of the invention provides the ink
composition according to the sixth aspect, wherein in the
formula (3),
n is 1,
8101 is a carboxy group or a phenyl group,
R102 is a Cl-C4 alkoxy group substituted with a sulfo
group,
R103 is a hydrogen atom,
CA 02803910 2012-12-24
14
8104 is a Cl-C4 alkyl group,
R' 5 is a hydrogen atom or a sulfo group,
8106 is a hydrogen atom, a halogen atom, a carboxy group,
a sulfo group, a Cl-C4 alkoxy group, or a Cl-C4 alkylsulfonyl
group,
R107 is a hydrogen atom or a sulfo group, and
the group D is a phenyl group or naphthyl group
substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, a carboxy group,
a Cl-C4 alkyl group, and a Cl-C4 alkoxy group;
An eighth aspect of the invention provides the ink
composition according to the sixth aspect, wherein in the
formula (3),
n is 1,
8101 is a carboxy group,
R102 is a Cl-C4 alkoxy group substituted with a sulfo
group,
8103 is a hydrogen atom,
8104 is a Cl-C4 alkyl group,
R105 is a hydrogen atom or a sulfo group,
8106 is a Cl-C4 alkoxy group,
R107 is a hydrogen atom or a sulfo group, and
the group D is a phenyl group or a naphthyl group
substituted with a sulfo group.
A ninth aspect of the invention provides the ink
composition according to any one of the first to eighth
aspects, wherein the ratio of the coloring matter (I) is 10 to
CA 02803910 2012-12-24
406 by mass, and the ratio of the coloring matter (II) is 10
to 80% by mass in the total mass of the coloring matter
contained in the ink composition.
A tenth aspect of the invention provides an ink jet
recording method, including performing recording by using the
ink composition according to any one of the first to ninth
aspects as an ink, discharging droplets of the ink in
accordance with a recording signal, and thereby attaching the
droplets onto a record-receiving material.
An eleventh aspect of the invention provides the ink jet
recording method according to the tenth aspect, wherein the
record-receiving material is a communication sheet.
A twelfth aspect of the invention provides the ink jet
recording method according to the eleventh aspect, wherein the
communication sheet is a sheet having an ink-receiving layer
containing a porous white inorganic substance.
A thirteenth aspect of the invention provides a colored
body colored by means of the ink composition according to any
one of the first to ninth aspects, or
the ink jet recording method according to any one of the
tenth to twelfth aspects.
A fourteenth aspect of the invention provides an ink jet
printer loaded with a container containing the ink composition
according to any one of the first to ninth aspects.
Effects of the Invention
According to the present invention, obtained is an
CA 02803910 2012-12-24
16
aqueous black ink composition exhibiting neutral black with
low chroma and color tone, and producing black recorded images
having high density of printed images.
PREFERRED MODE FOR CARRYING OUT THE INVENTION
Hereinafter, the present invention will be described in
detail.
Meanwhile, in order to avoid complication in the present
specification described below, any of "a compound", "a
tautomer thereof" and "a salt thereof" is simply described as
a "compound". Unless particularly stated otherwise in the
present specification, acid functional groups such as a sulfo
group and a carboxy group are presented in the form of free
acid.
The ink composition of the present invention is an ink
composition containing a coloring matter (I), which is a
compound represented by a specific formula, and a coloring
matter (II), which is an azo compound having Amax in a range
of 550 to 660 nm.
The coloring matter (I) contained in the ink composition
of the present invention will be described.
The coloring matter (I) contained in the ink composition
of the present invention is a compound represented by the
formula (1) . The coloring matter (I) is composed of at least
one kind of a compound represented by the formula (1), and may
be a coloring matter composed of a single compound represented
by the formula (1), or may be a mixture of coloring matters
CA 02803910 2012-12-24
17
composed of multiple compounds. Meanwhile, the compound
represented by the formula (1) is a water-soluble dye.
In the formula (1), examples of the halogen atom for R1
to R8 include a fluorine atom, a chlorine atom, a bromine atom,
and an iodine atom. Among these, a fluorine atom, a chlorine
atom and a bromine atom are preferred, and a chlorine atom is
particularly preferred.
The Cl-C4 alkyl group for R1 to R8 may be a linear or
branched alkyl group, and a linear alkyl group is preferred.
Specific examples include, for example, linear alkyl groups
such as methyl, ethyl, n-propyl and n-butyl; and branched
alkyl groups such as isopropyl, isobutyl, sec-butyl, and t-
butyl. Specific preferred examples include methyl and ethyl,
and methyl is particularly preferred.
The Cl-C4 alkoxy group for R1 to R8 may be a linear or
branched alkoxy group. Specific examples include linear alkoxy
groups such as methoxy, ethoxy, n-propoxy, and n-butoxy; and
branched alkoxy groups such as isopropoxy, isobutoxy, sec-
butoxy, and t-butoxy. Specific preferred examples include
methoxy and ethoxy, and methoxy is particularly preferred.
The Cl-C4 alkoxy group substituted with at least one
group selected from the group consisting of a hydroxy group, a
Cl-C4 alkoxy group, a hydroxy-Cl-C4 alkoxy group, a sulfo
group and a carboxy group for R1 to R8 may be a Cl-C4 alkoxy
group having these substituents on any arbitrary carbon atoms
in the alkoxy group. The number of the substituents is usually
one or two, and preferably one. There are no particular
CA 02803910 2012-12-24
18
limitations on the position of the substituent, but it is
preferable that two or more oxygen atoms do not substitute the
same carbon atom.
Specific examples include hydroxy-Cl-C4 alkoxy groups
such as 2-hydroxyethoxy, 2-hydroxypropoxy, and 3-
hydroxypropoxy; Cl-C4 alkoxy-Cl-C4 alkoxy groups such as
methoxyethoxy, ethoxyethoxy, n-propoxyethoxy, isopropoxyethoxy,
n-butoxyethoxy, methoxypropoxy, ethoxypropoxy, n-
propoxypropoxy, isopropoxybutoxy, and n-propoxybutoxy;
hydroxy-Cl-C4 alkoxy-Cl-C4 alkoxy groups such as 2-
hydroxyethoxyethoxy; carboxy-Cl-C4 alkoxy groups such as
carboxymethoxy, 2-carboxyethoxy, and 3-carboxypropoxy; and
sulfo-Cl-C4 alkoxy groups such as 2-sulfoethoxy, 3-
sulfopropoxy, and 4-sulfopropoxy. Among these, 3-sulfopropoxy
is particularly preferred.
The Cl-C4 alkylcarbonylamino group for R1 to R8 may be a
linear or branched alkylcarbonylamino group, and a linear
alkylcarbonylamino group is preferred. Specific examples
include linear groups such as acetylamino
(methylcarbonylamino), ethylcarbonylamino, propylcarbonylamino,
and butylcarbonylamino; and branched groups such as
isopropylcarbonylamino and t-butylcarbonylamino.
Specific examples of the Cl-C4 alkylcarbonylamino group
substituted with a carboxy group for R1 to R8 include, for
example, carboxy-Cl-C4 alkylcarbonylamino groups such as 2-
carboxyethylcarbonylamino, and 3-carboxypropylcarbonylamino.
The number of substituting carboxy groups is usually one or
CA 02803910 2012-12-24
19
two, and preferably one.
The mono-Cl-C4 alkylureido group for R1 to R8 may be an
alkylureido group having a linear or branched alkyl moiety.
There are no particular limitations on the position of
substitution of the Cl-C4 alkyl, but it is preferable that the
C1-C4 alkyl be substituted at the position of "N'".
Meanwhile, according to the present specification, the
term "mono-Cl-C4 alkylureido group" means a "C1-C4 alkyl-NH-
CO-NH-" group or a "H2N-CO-N(C1-C4 alkyl)-" group. In the
benzene ring to which R1 and R8 are bonded, the nitrogen atom
which is directly bonded to the benzene ring is designated as
"N", and the nitrogen atom which is bonded to the benzene ring
through this nitrogen atom and a carbonyl (CO) group is
designated as "N "'. Therefore, in regard to the position of
substitution of the Cl-C4 alkyl, the former indicates "N'",
and the latter indicates "N".
Specific examples include linear groups such as N'-
ethylureido, N'-propylureido, and N'-butylureido; and branched
groups such as N'-isopropylureido, N'-isobutylureido, and N'-
t-butylureido.
The di-C1-C4 alkylureido group for R1 to R8 may be a
linear or branched alkylureido group. There are no particular
limitations on the position of substitution of the Cl-C4 alkyl,
and those alkylureido groups may have one alkyl each on the
"N" and 'N'" positions, or having two alkyls on the "W"
position, conforming to the position of substitution in the
"mono-C1-C4 alkylureido group", and the latter is preferred.
CA 02803910 2012-12-24
Furthermore, the two Cl-C4 alkyls may be identical with or
different from each other, but it is preferable that the two
alkyls be identical.
Specific examples include linear groups such as N',N'-
dimethylureido, N',N'-diethylureido, N',N'-dipropylureido, and
N',N'-dibutylureido; and branched groups such as N',N'-
diisopropylureido, and N',N'-diisobutylureido.
The mono-C1-C4 alkylureido group substituted with at
least one group selected from the group consisting of a
hydroxy group, a sulfo group and a carboxy group for R1 to R8
may be a mono-C1-C4 alkylureido group having these
substituents on any arbitrary carbon atoms in the alkylureido
group. The number of the substituents is usually one or two,
and preferably one. There are no particular limitations on the
position of substituent, but it is preferable that a nitrogen
atom and a hydroxy group do not substitute the same carbon
atom.
Specific examples include N'-mono(hydroxy-C1-C4
alkyl)ureido groups such as N'-2-hydroxyethylureido and N'-3-
hydroxypropylureido; N'-mono(sulfo-C1-C4 alkyl)ureido groups
such as N'-2-sulfoethylureido and N'-3-sulfopropylureido; and
N'-mono(carboxy-C1-C4 alkyl)ureido groups such as N-
carboxymethylureido, N'-2-carboxyethylureido, N'-3-
carboxypropylureido, and N'-4-carboxybutylureido.
The di-Cl-C4 alkylureido group substituted with at least
one group selected from the group consisting of a hydroxy
group, a sulfo group and a carboxy group for Rl to R8 may be a
CA 02803910 2012-12-24
21
di-Cl-C4 alkylureido group having these substituents on any
arbitrary carbon atoms in the dialkylureido group. The number
of the substituents is usually one or two, and preferably two.
There are no particular limitations on the position of
substituent, but it is preferable that a nitrogen atom and a
hydroxy group do not substitute the same carbon atom.
Furthermore, when the group has plural substituents, the
substituents may be of the same kind or of different kinds,
but it is preferable that the substituents be the same.
Specific examples include N',N'-di(hydroxy-C1-C4
alkyl)ureido groups such as N',N'-di(2-hydroxyethyl)ureido,
N',N'-di(2-hydroxypropyl)ureido, and N',N'-di(3-
hydroxypropyl)ureido; N',N'-di(sulfo-C1-C4 alkyl)ureido groups
such as N',N'-di(3-sulfopropyl)ureido; and N',N'-di(carboxy-
Cl-C4 alkyl)ureido groups such as N',N'-
di(carboxymethyl)ureido.
The benzoylamino group having its benzene ring
substituted with at least one group selected from the group
consisting of a halogen atom (examples include a fluorine atom,
a chlorine atom, a bromine atom and an iodine atom, and a
chlorine atom is particularly preferred), a C1-C4 alkyl group,
a nitro group, a sulfo group and a carboxy group for R1 to R8
may be a benzoylamino group having one to three, and
preferably one or two, of these substituents. When the group
has plural substituents, the substituents may be of the same
kind or of different kinds, but it is preferable that the
substituents be the same.
CA 02803910 2012-12-24
22
Specific examples include halogen atom-substituted
benzoyl amino groups such as 2-chlorobenzoylamino, 4-
chlorobenzoylamino, and 2,4-dichlorobenzoylamino; Cl-C4 alkyl-
substituted benzoylamino groups such as 2-methylbenzoylamino,
3-methylbenzoylamino, and 4-methylbenzoylamino; nitro-
substituted benzoylamino groups such as 2-nitrobenzoylamino,
4-nitrobenzoylamino, and 3,5-dinitrobenzoylamino; sulfo-
substituted benzoylamino groups such as 2-sulfobenzoylamino
and 4-sulfobenzoylamino; and carboxy-substituted benzoylamino
groups such as 2-carboxybenzoylamino, 4-carboxybenzoylamino,
and 3,5-dicarboxybenzoylamino.
The phenylsulfonylamino group having its benzene ring
substituted with at least one group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group and a carboxy group for R1 to R8 may be a
phenylsulfonylamino group having one to three, preferably one
or two, and more preferably one, of these substituents. When
the group has plural substituents, the substituents may be of
the same kind or of different kinds.
Specific examples include halogen atom-substituted
phenylsulfonylamino groups such as 2-chlorophenylsulfonylamino
and 4-chlorophenylsulfonylamino; Cl-C4 alkyl-substituted
phenylsulfonylamino groups such as 2-methylphenylsulfonylamino,
4-methylphenylsulfonylamino, and 4-t-butylphenylsulfonylamino;
nitro-substituted phenylsulfonylamino groups such as 2-
nitrophenylsulfonylamino, 3-nitrophenylsulfonylamino, and 4-
nitrophenylsulfonylamino; sulfo-substituted
CA 02803910 2012-12-24
23
phenylsulfonylamino groups such as 3-sulfophenylsulfonylamino
and 4-sulfophenylsulfonylamino; and carboxy-substituted
phenylsulfonylamino groups such as 3-
carboxyphenylsulfonylamino and 4-carboxyphenylsulfonylamino.
Among the substituents described above, R1 to R8 are each
preferably a hydrogen atom; a halogen atom; a Cl-C4 alkyl
group; a Cl-C4 alkoxy group; a Cl-C4 alkoxy group substituted
with a sulfo group or a carboxy group; or a Cl-C4
alkylcarbonylamino group. Among these, a hydrogen atom, methyl,
ethyl, t-butyl, 2-carboxyethoxy, 3-carboxypropoxy, 2-
sulfoethoxy, 3-sulfopropoxy, and 4-sulfobutoxy are more
preferred. Particularly preferred examples include a hydrogen
atom, methyl, and 3-sulfopropoxy.
In the formula (1), it is preferable that at least one of
R1 to R8 be a C1-C4 alkoxy group substituted with a sulfo group.
Furthermore, it is more preferable that R1 to R4 each
independently represent a hydrogen atom, a C1-C4 alkyl group,
or a Cl-C4 alkoxy group substituted with a sulfo group, while
at least one of R1 to R4 be a C1-C4 alkoxy group substituted
with a sulfo group, and R5 to R8 each independently represent a
hydrogen atom or a Cl-C4 alkyl group.
It is even more preferable that at least one of R1 and R2
be a sulfopropoxy group; at least one of R3 and R4 be a
sulfopropoxy group; and R5 to R8 be C1-C4 alkyl groups.
There are no particular limitations on the positions of
substitution of R1 to R8, but it is preferable that for the
respective benzene rings substituted with these substituents,
CA 02803910 2012-12-24
24
when the position of substitution of the nitrogen atom bonded
to the triazine ring is designated as the 1-position, and the
position of substitution of the azo group is designated as the
4-position, R1 to R4 be substituted at the 2-position, and R5
to R8 be substituted at the 5-position.
In the formula (1), X represents a divalent crosslinking
group.
The crosslinking group is not particularly limited as
long as it is a divalent group to the extent that the compound
represented by the formula (1) exhibits solubility in water.
Here, in regard to the solubility of the compound represented
by the formula (1) in water, it is desirable that the compound
represented by the formula (1) dissolve in 1 liter of water in
an amount of usually 5 g or more, preferably 10 g or more,
more preferably 25 g or more, even more preferably 50 g or
more, and particularly preferably 100 g or more.
Specific examples include a divalent atom (preferably a
divalent heteroatom) such as a nitrogen atom, an oxygen atom
or a sulfur atom; a Cl-C8 alkylenediamino group, a Cl-C8
alkylenedioxy group or a Cl-C8 alkylenedithio group; an N,N'-
hydrazinediyl group; a group in which two alkylamino groups
are substituted at an oxygen atom, such as an
aminoalkoxyalkylamino group; and a group in which an alkylene
oxide chain containing one or more ether bonds is substituted
with one amino group and one alkylamino group at the chain
ends, such as an aminoalkoxyalkoxyalkylamino group.
These divalent crosslinking groups for X may respectively
CA 02803910 2012-12-24
have a group selected from the group consisting of a hydroxy
group, a carboxy group and an alkoxy group as a substituent of
the carbon atom; and an alkyl group which may be substituted
with a hydroxy group or a carboxy group at the alkyl moiety as
a substituent for the nitrogen atom.
The divalent crosslinking group for X is preferably any
one group selected from the group consisting of a Cl-C8
alkylenediamino group; a Cl-C8 alkylenediamino group
substituted with a hydroxy group or a carboxy group; an N-Cl-
C4 alkyl-C1-C6 alkylenediamino group; an N-Cl-C4 alkyl-Cl-C6
alkylenediamino group having its alkyl moiety substituted with
a hydroxy group or a carboxy group; an amino-Cl-C6 alkoxy-Cl-
C6 alkylamino group; amino-Cl-C4 alkoxy-Cl-C4 alkoxy-Cl-C4
alkylamino group; a xylylenediamino group; a piperazine-l,4-
diyl group; a piperazine-l,4-diyl group substituted with a Ci-
C4 alkyl group or a Cl-C4 alkoxy group; and a phenylenediamino
group.
Meanwhile, these divalent crosslinking groups are all
"diamino" groups each having two amino groups. Therefore, for
example, among the "diamino" groups excluding some groups such
as piperazine-l,4-diyl, the present invention includes both
the case where crosslinking is achieved at any one of the
nitrogen atoms (that is, N,N-diyl is obtained), and the case
where crosslinking is achieved at two different nitrogen atoms
(that is, N,N'-diyl is obtained). Among these, the latter case
of obtaining "N,N'-diyl" is particularly preferred.
The Cl-C8 alkylenediamino group for X may be a linear or
CA 02803910 2012-12-24
26
branched alkylenediamino group, and a linear alkylenediamino
group is preferred. The carbon number is usually in the range
of Cl-C8, preferably C2-C8, more preferably C2-C6, and even
more preferably C2-C4.
Specific examples include linear groups such as
ethylenediamino, 1,3-propylenediamino, 1,4-butylenediamino,
1,5-pentylenediamino, 1,6-hexylenediamino, 1,7-
heptylenediamino, and 1,8-octylenediamino; and branched groups
such as 2-methyl-l,3-propylenediamino, 3-methyl-l,4-
butylenediamino, and 4-methyl-1,6-hexylenediamino.
The C1-C8 alkylenediamino group substituted with a
hydroxy group or a carboxy group for X may be a Cl-C8
alkylenediamino group having these substituents on any
arbitrary carbon atoms in the alkylenediamino group. There are
no particular limitations on the number of the substituents,
but the number is preferably one or two. Furthermore, when the
group has plural substituents, the substituents may be of the
same kind or of different kinds, but it is preferable that the
substituents be the same.
Specific examples include hydroxy-substituted Cl-C8
alkylenediamino groups such as 2-hydroxy-l,3-propylenediamino,
2-hydroxy-1,4-butylenediamino, and 3-hydroxy-1,6-
hexylenediamino; and carboxy-substituted C1-C8 alkylenediamino
groups such as 1-carboxyethylenediamino, 1-carboxy-l,3-
propylenediamino, 1-carboxy-l,4-butylenediamino, 1-carboxy-
1,5-pentylenediamino, and 1,5-dicarboxy-l,5-pentylenediamino.
The N-Cl-C4 alkyl-Cl-C6 alkylenediamino group for X means
CA 02803910 2012-12-24
27
that one of the nitrogen atoms of a Cl-C6 alkylenediamino
group has been substituted with a Cl-C4 alkyl group. According
to the present specification, in the diamino group, the
nitrogen atom substituted with a Cl-C4 alkyl group is
indicated as "N", and if necessary, the other nitrogen atom is
indicated as "N'".
The carbon number of the alkylene moiety is usually in
the range of Cl-C6, preferably C2-C4, and particularly
preferably C2 or C3.
The Cl-C4 alkyl group may be a linear or branched alkyl
group, and a linear alkyl group is preferred.
Specific examples include N-linear Cl-C4 alkyl-Cl-C6
alkylenediamino groups such as an N-methylethylenediamino
group, an N-ethylethylenediamino group, an N-
propylethylenediamino group, and N-butylethylenediamino group;
and N-branched Cl-C4 alkyl-Cl-C6 alkylenediamino groups such
as an N-isopropylethylenediamino group, an N-
isobutylethylenediamino group, an N-sec-butylethylenediamino
group, and N-t-butylethylenediamino group.
The N-Cl-C4 alkyl-C1-C6 alkylenediamino group having its
alkyl moiety substituted with a hydroxy group or a carboxy
group for X may be an N-Cl-C4 alkyl-Cl-C6 alkylenediamino
group having these substituents on any arbitrary carbon atoms
of the alkyl moiety of the N-Cl-C4 alkyl group. There are no
particular limitations on the position of substituents, but it
is preferable that a nitrogen atom and a hydroxy group do not
substitute the same carbon atom. The carbon number of the
CA 02803910 2012-12-24
28
alkylene moiety may be in the same range, including preferred
ranges, as that of the N-C1-C4 alkyl-C1-C6 alkylenediamino
group described above. Furthermore, the carbon number of the
alkyl moiety is usually in the range of Cl-C4, preferably C2-
C4, and more preferably C2-C3.
The number of the substituents is usually one or two, and
preferably one. Further, when the group has plural
substituents, the substituents may be of the same kind or of
different kinds, but it is preferable that the substituents be
the same.
Specific examples include N-hydroxy-substituted C1-C4
alkyl-C1-C6 alkylendiamino groups such as an N-(2-
hydroxyethyl) ethylenediamino group, an N-(3-
hydroxypropyl) ethylenediamino group, an N-(2-
hydroxypropyl)ethylenediamino group, and N-(4-
hydroxybutyl) ethylenediamino group; and N-carboxy-substituted
Cl-C4 alkyl-C1-C6 alkylenediamino groups such as an N-
(carboxymethyl)ethylenediamino group, an N-(2-
carboxyethyl)ethylenediamino group, an N-(3-
carboxypropyl) ethylenediamino group, and N-(4-
carboxybutyl) ethylenediamino group.
The amino-Cl-C6 alkoxy C1-C6 alkylamino group for X may
be a linear or branched aminoalkoxyalkylamino group, and a
linear group is preferred. In regard to the range of the
carbon number, usually an amino-C1-C6 alkoxy Cl-C6 alkylamino
group, and preferably, an amino-C2-C4 alkoxy C2-C4 alkylamino
group may be used, and particularly preferably, an amino-C2-C3
CA 02803910 2012-12-24
29
alkoxy C2-C3 alkylamino group may be used. Specific examples
include aminoethoxyethylamino, aminoethoxypropylamino,
aminopropoxypropylamino, aminoethoxypentylamino and the like.
The amino-C1-C4 alkoxy-Cl-C4 alkoxy-Cl-C4 alkylamino
group for X may be a linear or branched
aminoalkoxyalkoxyalkylamino group, and a linear group is
preferred. In regard to the range of the carbon number,
usually an amino-C1-C4 alkoxy-Cl-C4 alkoxy-Cl-C4 alkylamino
group, and preferably, an amino-C2-C4 alkoxy-C2-C4 alkoxy-C2-
C4 alkylamino group may be used, and particularly preferably,
an amino-C2-C3 alkoxy-C2-C3 alkoxy-C2-C3 alkylamino group may
be used.
Specific examples include linear groups such as
aminoethoxyethoxyethylamino, aminoethoxypropoxyethylamino, and
aminoethoxybutoxyethylamino; and branched groups such as
aminoethoxy(2-methylethoxy)ethylamino, and aminioethoxy(2-
methylpropoxy)ethylamino.
The xylylenediamino group for X may be, for example, an
o-xylylenediamino group, an m-xylylenediamino group and a p-
xylylenediamino group, and an m-xylylenediamino group or p-
xylylenediamino group is preferred.
The piperazine-l,4-diyl group substituted with a Cl-C4
alkyl group or a Cl-C4 alkoxy group for X may be a
piperazinediyl group having these substituents on any
arbitrary carbon atoms among the ring-constituting atoms of
the piperazine ring. The number of the substituents is usually
one or two, and preferably one. Furthermore, when the group
CA 02803910 2012-12-24
has plural substituents, the substituents may be of the same
kind or of different kinds, but it is preferable that the
substituents be the same.
Specific examples include a 2-methylpiperazine-l,4-diyl
group, a 2-ethylpiperazine-l,4-diyl group, a 2,5-
dimethylpiperazine-l,4-diyl group, a 2,6-dimethylpiperazine-
1,4-diyl group, a 2,5-diethylpiperazine-l,4-diyl group, and a
2-methyl-5-ethylpiperazine-1,4-diyl group.
The phenylenediamino group for X may be an o-, m- or p-
phenylenediamino group, and an m- or p-phenylenediamino group
is preferred.
Among the substituents described above, X is preferably a
C1-C8 alkylenediamino group; a Cl-C8 alkylenediamino group
substituted with a carboxy group; an N-Cl-C4 alkyl-C1-C6
alkylenediamino group having its alkyl moiety substituted with
a hydroxy group; an aminio-C1-C4 alkoxy-Cl-C4 alkoxy-Cl-C4
alkylamino group; a xylylenediamino group; or a piperazine-
1,4-diyl group.
X is more preferably a C2-C4 alkylenediamino group; a C2-
C6 alkylenediamino group substituted with a carboxy group; an
N-C2-C3 alkyl-C2-C3 alkylenediamino group having its alkyl
moiety substituted with a hydroxy group; an amino-C2-C3
alkoxy-C2-C3 alkoxy-C2-C3 alkylamino group; and an m- or p-
xylylenediamino group; or a piperazine-1,4-diyl group.
X is even more preferably a Cl-C8 alkylenediamino group;
a xylylenediamino group; or a piperazine-l,4-diyl group.
Among these, specific preferred examples include 1,2-
CA 02803910 2012-12-24
31
ethylenediamino; 1,3-propylenediamino; 1,4-butylenediamino; 1-
carboxypentylene-1, 5-diamino; N-2-hydroxyethylethylenediamino;
aminioethoxyethoxyethylamino; m-xylylenediamino; and
piperazine-1,4-diyl.
In the above formula (1), the positions of substitution
of the four sulfo groups whose respective positions of
substitution are not specified, are not particularly limited.
The sulfo group substituted at the benzene ring having one azo
bond may be substituted at the 2-position, 3-position or 4-
position, with respect to the position of substitution of the
azo bond as the 1-position, and is preferably substituted at
the 4-position.
A preferred example of the azo compound of the present
invention represented by the formula (1) is a compound
represented by the above formula (2), and a more preferred
example is a compound represented by the following formula
(4).
.80 H R' R2 HO,S
HO3SN=N~N=N~N *N~N~N N-~ N=N-~ -SO3H
N N
Rs T Re
X (4)
R7 Re
HO3S-O'N-N-(: -N wt~ NHINl--N _ N=N N=N-(D--SO3H
SO3H R3 R4 HO3S
R' to R8 and X in the formulae (2) and (4) have the same
meanings, including specific examples and preferred examples,
as R1 to R8 and X, respectively, as defined for the formula (1)
In regard to R1 to R8 in the formulae (1) , (2) and (4)
described above, the positions of substitution of R1 to R8 in
CA 02803910 2012-12-24
32
the formula (2), and the positions of substitution of the
sulfo groups whose positions of substitution in the formulae
(1) and (2) are not specified, a compound of combinations of
preferred examples is more preferable, and a compound of
combinations of more preferred examples is even more
preferable. The same applies also to combinations of even more
preferred examples, combinations of preferred examples and
more preferred examples, and the like.
There are no particular limitations on the suitable
specific examples of the compound represented by the formula
(1), but some suitable specific examples include the compounds
presented in the following Tables 1 to 22, etc.
In the respective tables, the functional groups such as a
sulfo group and a carboxy group are indicated in the form of
free acid, for convenience.
[Table 1]
CA 02803910 2012-12-24
33
Compound No. Structural formula
--SO3H
SO,H 0 HO3S
HOA J\ N=N O N=N Ny N N\N=N-O-N=N-&SO3H
H3C NYN CH3
HN
HN~
H3C CH3
H03S Q N=N F\_ N=N N-INN N=N N=N SOSH
SO3H 0 H03S
HO3S-
000H
SO3H 0 H035
HO35 \ N=N / \ N=N
-Q- r N Y,Nl N ~_\' N=N -b-N=N 503H
H3C NYN CH3
HN
2
HN
H3C N N CH3
H03S C-N=N N= H~N~ N=N N=N S03H _tI S03H 0 HO3S
HOOC-"
-SO3H
SO3H 0 HO3S
H03S N=N N=N NYN~,N N=N N N SO3H
H3C NYN NHAc
HN
3
HN
AcHN NON CH3
H03S aN=N ~_~ N=N H'~N N=N N=N S03H
SO3H 0 H03S
HO3S_
[Table 2]
CA 02803910 2012-12-24
34
Compound No. Structural formula
OCH2CH3
S03H H3CH2CO H03S
H03S N=N I N=N NYNYN " N=N N=N S03H
N T N
NH
4 ~i
HN
H03S V N=N N=N _H NCH N=N N=N S03H
SO3H OCH2CH3 HO3S
H3CH2CO
H03S-\I ~-SO3H
SOH 0 O HO3S
HO3S &N=N W N O NYNI N 0 N=N N N S03H
H3C NyN CH3
NH
HN
H3C N`lN CH3
H03S N=N \-N=N:\\\ H'-NCH / N=N \ N=N-&S03H
SO3H 0 0 HO3S
H03S-j SO3H
S03H H03S
H03S a N=N N=N N INly N\ N=N N=N S03H
H3C N. N CH3
NH
6 1~
HN
H3C NJIN CH3
H03S-&N=N / \ N=N H~N~H 6 N=N , / N=N S03H
SO3H HO3S
[Table 3]
CA 02803910 2012-12-24
Compound No Structural formula
HO's --I
SO3H 0 H03S
H03S rV N=N \ N=N O N ,NN N=N N=N & S03H
H3C N,,N CH3
NH
7 I~
HN
H3C N"L N CH3
H03S N=N N=N H~N~H / N=N N=N SO3H
SO3H O H03S
~-S03H
H03S-,,~~ /_/-S03H
S03H O 0 H03S
H03S M=N 0 N=N N7N~ N t~ N=N N N S03H
H3C N T N CH3
HN
8
HN
H3C N'~N CH3
N N HNJulH N N N=N 803H
HO3S N=N
-Q 0/
SO3H 0 0 HO33
HO3S- '-SO3H
S03H HO3S
H035 ~_~ N=N N:N NYNYN \/_~ N=N N=N SO3H
H3C N1,N CH3
HN
9
HN
H3C NJJ-N CH3
HO3S a N=N 7 N=N 6 H'N~H ~ N=N ~ N=N & S03H
SO3H HO3S
[Table 4]
CA 02803910 2012-12-24
36
Compound No. Structural formula
HO3S-\--\
SO3H 0 HO3S
OH _--\\_
H03S N=N N=N NyN N/ N=N N=N S03H
H3C NTN CH3
HIM
HN
H3C NIJ~N CH3
HO3S N=N N=N H~N~H ~ N=N Q N=N S03H
SO3H 0 HO3S
-SO3H
S03H H03S
H03S a N=N N=N NYN~,N Q N=N N=N Q S03H
H3C NT N CH3
HN
11 I~
NH
H3C NJIN CH3
H03S N N \ \ N=N HNH N=N N=N S0H
SO3H HO3S
H03S- ,S03H
S03H 0 0 HO3S
H03S ~_~ N=N N N N7NYN O N=N N:N S03H
H3C N,,N CH3
HN
12
NH
H3C N~N CH3
H03S a N=N \ N=N HANH N=N N=N S03H
SO3H 0 0 HODS
HO3S-/--, ~SO3H
[Table 5]
CA 02803910 2012-12-24
37
Compound No. Structural formula
H03S
SO3H ~{ H HO3S
H035-N=N-r-N=N~ `}-NYNI,NN=N N=N 50H
H3C NI.N CH3
HN
13 0
NH
HO3S a WN4 rN-NH3C
irHANkH HN=N N=N 503H
SO3H 0 HO3S
HO3S-/~
S03H OCH3 H03S
H03S \ N=N f\ N=N NYN,,N \ N=N N=N a S03H
H3C NTN CH3
HN'P
14 I~
NH
H3C HNJIN CH3
H03S \ N N \ N=N N ,-N N=N , ~ N=N S03H
SO,H H3C H03S
SO,H HO3S
H03S a N=N / \ N:N NY NI,N N=N NON- j -SO3H
H3C NIr N H3
1 5 HN-
N~H /~
H03S- N=N N3H 3 H'J`N.IH j - H033 ~T'N=N S03H
0
H03S-\__\ -S03H
S03H 00, } HOA
H03S- -N=N N N- - 1 IrN-( }-N=N N N S03H
H3C NTN CH3
HNc
1 6 I~
NH
H3C NIN CH3
H03S \ N=N / \ N=N-(~ irHANJ.H N=N--I U--N=N S03H
S03H 0 0 H03S
H03S -\-S03H
[Table 61
CA 02803910 2012-12-24
38
Compound No. Structural formula
H03S I
S03H 0 HO3S
HO3S / \ N=N / \ N=N /_\ NYNYN \ N=N N=N \ / SO3H
H3C N T N CH3
HNP
17
NH
H3C N"N CH3
HO3S / N=N -e \ N=N \ / H)IINH N=N \ / N=N \~/ S03H
S03H 0 H03S
"-S03H
OCH3 H3CO S03H H03S
H03S /_\ N=N / \ N=N NY NI N N-N \ / S03H
H3C NfN CH3
HN
18 )
HN
H3C N-IN CH3
\ / N=N \ / N-N \ / S0H
H03S / \ N=N / \ N=N \ / H~NN-,\-/'
SO3H OCH3 H3CO HO3S
H03S . S03H
S03H {O 0}-~ r-,,-'03H
HO3S
H03S / \ N=N / \ N=N-( NYNT N-Q rN=N \ /N=N \ /S03H
H3C N T N CH3
1 9 HN)
HN
H3C N I N CH3
H03S / \ N=N N=N- /, H'-N"-H \ / N=N \ / N=N \ /SO3H
SO3H 0 0 HO3S
H03S ~S03H
SO3H HO3S
HO3S / \ N:N / \ N
N:N 13 My N' /\N:N N=N \ /SOH
H3C N1=N CH3
HN
20 )
HN
H3C NJIN CH3
H03S / \ N=N / \ N N \ / H~N~H \ / N=N \ / N=N \ / SOH
SO3H HO3S
[Table 7]
CA 02803910 2012-12-24
39
Compound No. Structural formula
HO3S- __r-SO3H
SO3H 0 O HO3S
H03S N N N N HN NYN N=N N=N-O-SO3H
H3C N T N CH3
21 HN
HNJ
CH
H03S \ N-N \ N=N3 H~N~H N=N N=N & S03H
SO3H HO3S
HO3S- -SO3H
S03H 0 H038
H03S / N N N=N NINA N O N=N N=N--&SO3H
H3C N1-N CH3
2 2 HN"
H3C NIN CH3
HO3S \ N=N N=N ANH N=N N=N SO3H
SO3H 0 HO3S
H03S-j--j
S03H HO3S
HO's-F\-NN N=N NYNI N N=N N=N S03H
H3C N1,N CH3
HN
2 3 HN
H3C N"L N CH3
H038a N=N \' N=N HEN"-H N=N N=N SO3H
SO3H 0 H03S
H03S-/--/
r-I-S03H
S03H H 0 H03S
}-~
H03S ~N=N N=NNyN N-(' }-N=N N N-a303H
H3C NIfN CH3
HN
24
HN
H3C NJ'N CH3
H03S a N=N \ N=N H"3. N.91H N-N- ~N=N a SO3H
SO3H 0 HO3S
H03S-/-
[Table 8]
CA 02803910 2012-12-24
Compound No Structural formula
-S03H
S03H H O H03
H03S / \ N=N / \ N=N \ NY N NN=N \ / N=N \ / S03H
H3C Ny N NHAc
HN
HN
N'~ N
H03S /\ N=N N AcHN
\-0-N-' -N \ /CHN'N \ / N N-&S03H
S03H 0 H03S
H03S~
--S%H
H03S / \N=N / N=N /_\NY NI, N-~ `-N=N \ / N=N \ / S03H
H3C N y N CI
HN
26
HN
CI NON CH3
H035--aN=N--~ TN=N \ / H~NH j N=N \ / N=N-aSO3H
SO3H 0 HO3S
H03S~
SO3H OCH3 H03S
/ \ N=N \ / N=N \ / SO3H
H03S / \ N=N / \ N=N Ny N.
H3C NTN CH3
HN
2 7 HN
H3C N'J'N CH3
H03S- N=N / \ N=N \ /FFIIN- -NCH \ / N=N \ / N=N \ /S03H
SO3H H3C0 HO3S
SOH OCH3 HO3S
H035 / \N=N / \ N N C\ NYNYN / \ N=N \ / N=N \ / S03H
NIr N CH3
28
H
HN
3NjJ-N
H03S / \N=N / \ N=NC / Fi NJ, N~11N~~ ~>-N=N \ / N=N-~SO3H
SO3H H3CO HO3S
[Table 9]
CA 02803910 2012-12-24
41
Compound No Structural formula
S03H OCH2CH3 H03S
H03S N=NN=N NI Nr N=N-b-N=N-C~-SO3H
H3CH2CO NN CH3
::<
29 H3C N~N-(/OCH2CH3
H03S a N=N-f y-N=N HINH-(~ ~j-N=N~N=N S03H
SO3H H3CH2CO HO3S
S03H yyyy~~~'~ H03S
HO3S--N=N--NFN--~ rNYN,,,N \' N=N-b-N=N-aSO3H
H3C7 ' Ny.N CH3
HN
30 `S)
HN
H3C N1N CH3 /
H03S-Q-N=N-,~ \-N=N-~ N-"N''-N N=N- J,D-N=N-a SO3H
- (S03H H03S
SO3H ~--~ HOaS
H03S & N=N N=N-~ YOy NrN-~ N=N- N=N S03H
H3C NTN H3
HN
1
HN H3C Nvl'N CH3
H03S-&N=N-?-~rN,H - j -~1~N~H H=0N3S N=N-Q-S03H
SO -S03H
SO3H y~ O H03S
H035-aN=N / N=N- yNyNq,N-}--~ C y-N=N-~D-N=N-aSgH
H3C N rN CH3
HN
32
HN
H3C NLN CH3
H03S-aN=N-~ IN=N N~ONH WN N=N-aS%H
SO3H 0 FI HO3S
H03S~
[Table 10]
CA 02803910 2012-12-24
42
Compound No. Structural formula
,rS03H
SO3H HH 0 H03S
H%S a N=N N=N NYN~,N N=N -N N-aS%H
H3C NYN CH3
HN
33
NH
H3 NON CH3
H03S ' Y N=N \' N=N-(~ i}-N~NH N=N N=N S%H
SO3H vv--YY0 FI HO3S
HO3S-'
_SO3H
S03H 0 HO3S
H03S N=N N=N N rN~ N N=N N=N & S03H
H3C NYN CI
HN
34
HN
CI NJIN CH3
H03S a N^N a N=N FNI-NH /, WN N=N a S03H
SO3H 0 H03S
H03S-'
r_f-SO3H
SO3H 0 HO3S
H03S N=N /\ N=N NYN N N=N N=N S03H
H3C NYN NHAc
HN
HN
AGHN N'1- N CH3
H03S a N=N N=N N~NKH \/ N=N N=N S03H
SO3H 0 H03S
HO3S-'
[Table 11]
CA 02803910 2012-12-24
43
Compound No Structural formula
~SO3H
SO3H OCH3 0 H03S
H03S / \ N=N / \ N=N / \ NYNTN4/,.\( N=N-b N=N \ / S03H
H3CO N T N CH3
NH
36
HN
H3C N'N OCH3
HO3S /_\ N=N N=N \ / HNH -5:/ N=N N=N \ / SO3H
S03H HCO H03S
HO3S~
H03S 1 r_1-SO3H
SO3H O 0 H03S
HO3S / \ N=N / \ N N / N .NYN / \ N=N \ / N=N \ / S03H
H3C NTN CH3
HNl
3 7 H0 . J
NI
H3C NN CH3
H03S / \ N=N / \ N:N \ / HEN)-H \ / N`N \ / N`N \ / S03H
SO3H 0 0 HO3S
H03S-/--j \-~SO3H
HO3S- ,S03H
SO3H 0 0 H03S
H03S / \ N N / \ N:N / \ NYNly N N=N \ /N=N\ /S03H
H3C N T N CH3
HN
3 8 F13C-N
H3C N"L N CH3
H03S / \ N=N / \ N=N HEN), N N=N \ /N:N \ /SO3H
SO3H 0 0 HO3S
H03SJ ~SO3H
[Table 12]
CA 02803910 2012-12-24
44
Compound No. Structural formula
H03S- -SO3H
S03H 0 0 H03S
H03S / \ N N / \ N=N NYNyN / \ N=N N=N \ / S03H
H3C N1-N CH3
HN
3 9 }N
H3C N'IN CH3
H03S / \ N=N /_\ N=N \ / H~N~'H \ / N=N \ / N=N \ / SO3H
SO3H 0 0 HO3S
HO3S-/ S03H
H03S- -SO3H
SO3H 0 0 HO3S
H03S / \ N=N /_\ N=N / \ NYNYN / \ N=N \ /N=N \ / S03H
H3C N1-N CH3
HN
H02C N
H3C N`IN CH3
H03S / \ N=N / \ N=N H)I- N~11 H N=N \ / N=N \ / S0H
SO3H 0 0 HO3S
H03S-/--/ \-~S03H
H03S -S03H
S03H O 0 H03S
HO's-F\-NN N=N NYNYN /_\ N=N N=N \ / SO3H
H3C N1-N CH3
HN
41
H07C-' N
H3C N-IN CH3
H 0 3 S NN Q\- N H)"NCH N=N \ / N=N \ / S03H
SO3H 0 0 H03S
HO3s--f- \-~S03H
[Table 13]
CA 02803910 2012-12-24
Compound No. Structural formula
HOS- SOH
SO3H ,-~(0 H03S
HOS-O-N=N-O-N=N-{ `}-N YNY-j-~ ( yN=N- , -N=N-aS%H
H3C N,I,N CH3
HN
42 How )
N
H3C N11N CH3
H03S / \ N=N N=N \ N-'Y-M N=N N=N-aS03H
SOH 0 0 HO3
HO3S-/~ SOH
HOS-\
SOH O H ^ HO3S
HOS / N=N / \ N=N / \ NYNI N- rN=N \ / N=N \ /SOH
H3C N T N (CH3
HN
4 3 HO,
N
H3C NI~N CH3
HOS / \ N=N / \ N=N \ / H~N~FI /,,N- N S03H
SO3H O HO3S
"-~SO3H
SO3H HO3S
HOS / \ N=N / \ N=N / \ N. /_\ N=N \ / N=N \ / SOH
H3C N T N CH3
HN
4 4 HO,__ NJ
H3C NIN CH3
HOS / \ N=N N=N \ / H-'--N-5-H \ / N=N \ / N=N \ / SOH
SOH HO3S
HOB OH H
SO3H 0 O HOS
HOS / N=N/ \ N=N / \ NYN~ N / \ N=N \ / N=N \ / SOH
H3C NYN CH3
HN
4 5 HO,/N)
/ \ / \ H3C J' N1 3
HOS N=N N=N \ / N N ' N \ / N=N \ / N=N \ / SOH
S03H O 0 HO3S
HO OH
[Table 141
CA 02803910 2012-12-24
46
Compound No. Structural formula
H03S~ _f-503H
S03H 0 0 HO3S
H03S / \ N=N / \ N=N / \ N7Ny N /_\ N=N N=N \ / S03H
H3C N rN CH3
HN
CO2H
46
HN
H3C NJIN CH3
HO3S / \ N=N / \ N=N \ / H~N~H \ / N N \ / N N \ /S0H
S03H 0 0 HO3S
H03S~ S03H
H03S--,-,
S03H O HO3S
H03S / \ N:N / \ WN / \ N~ NYN / \ N=N \ / N:N \ / S0H
H3C NYN CH3
HN
CO2H
47
HN
H3C NON CH3
H03S / \ N:N / \ N N \ / N~N~H \ / N"N \ / N-N \ / S03H
SO3H O HO3S
\-~SO3H
SO,H HO3S
H03S / \ N:N /_\ N:N N,rN7N / \ N:N \ / N:N \ / S03H
H3C NIfN CH3
HN
CO2H
48
HN
H,
NON _ _
H03S N=N /_\ N:N C_/ H~H \ CH
N=N \ / N N \ / 503H
S03H H03S
[Table 15]
CA 02803910 2012-12-24
47
Compound No. Structural formula
H03S- ,j-S03H
S03H (O 0 H03S
H03SN=N~ N=N-~ } NYNõrN (/ N=N N=N S03H
H3C NYN CH3
HN
CO2H
49
CO2H
HN
H3C NON CH3
H03S / N=N \ N=N ,\ HH N=N N N SO3H
SO3H 0 0 HO3S
H03S- ~SO3H
H03S-
S03H O H03S
H03S N=N N-N NY N7N N=N N=N S03H
H3C NYN CH3
HN
CO2H
CO2H
HN
H3C NON CH3
1-103S a N=N V N=N H)I- NA, NOW N, D/, N=N SO3H
SO3H O HO3S
"-SO3H
SO3H HO3S
H03S f\ N=N N=N ~_~ NyN 1. r \ N=N N^N / SO3H
H3C NYN CH3
HN
CO2H
51
CO2H
HN
H3C NON CH3
H03S N=N N=N H)INH N=N , ~ N=N ,\ ~ SO3H
SO3H HO3S
[Table 16]
CA 02803910 2012-12-24
48
Compound Na. Structural formula
SOH OCH3 H3CO ON S
H038/ \ N=N / \ N=N NYN N N=N3 \ / N N \ / S03H
H3CO N Y N OCH3
HN
CO2H
52
CO2H
HN
H3CO N OCH3
HO3S N=N N=N \ / HNH \ / N=N \ / N-N \ /S03H
SO3H OCH3 H3CO HOSS
HO3S- SO3H
SO3H 0 d-1
HO3S
H03S / \ N=N / \ N=N / \ N1 NYN / \ N=N \ / N-N \ / SO3H
H3C NYN CH3
HN
53
HN
H3C NON CH3
HO3S N=N / \ N=N \ / H~N'H N;N \ / N=N \ / SO3H
SO3H 0 O HO3S
HO3S- SO3H
H03S-\_\
SO3H 0 HO35
H035-aN=N / \ N=N ( } NY NYN / \ N=N \ / N=N \ / S03H
H3C NYN CH3
HN
54
HN
H3C NON CH3
HO3S / \ N=N / \ N=N \ / H--NCH \ / N=N \ / N=N \ /503H
SO3H 0 HO3S
'-BOSH
[Table 17]
CA 02803910 2012-12-24
49
Compound No. Structural formula
H03S~ /-SO3H
S03H O Or HO3S
HO3S / N=N / N=N / N,FN~N / \ N=N / N=N / S03H
H3C NIf N CH3
HN.
0
0
NH
H3C NkN CH3
H03S / N=N / WN / HNN / N=N / N=N / S03H
S03H 0 n 0 HO3S
HO3S-/~ SO3H
HO3S-\__\
S03H O H03S
H03S-aN=N -N=N / NYNlyN / N=N n / N=N-aS03H
H3C N y N CH3
HN_
56 S
O
NH
H3C N1~N CH3
H03S N=N \ N=N-H'~'N~ / N=N / N=N / SO3H
SO3H O HO3S
"-SO3H
r_1-SO3H
SO3H 0 HO3S
H03S / \ N=N / \ N N / N~ NYN / N=N / N=N / S03H
H3C NYN CH3
HN
57 Io
0
NH
H3C NON CH3
H03S / N^N / \' N=N / H~N~M N N / N=N / S03H
SO3H HO3S
[Table 18]
CA 02803910 2012-12-24
Compound No. Structural formula
SOyH OCH3 H03S
H03S WN / N=N \' NYN,rN N=N~-N=N-~j -S03H
H3 NY.N CH3
HN_
0
58 S
0
NH
H3C NON CH3
i
HO3S N=N ~_~ N=N ,\ /, H~NIH N=N-- WN-~SO3H
SO3H H3CO HO3S
HO3S- r_.1-SO3H
S03H O 0 H03S
N=NN=N S03H
H03SN=N N=N NYNN-qH3
H3C NN 5 9 N
N
H3C ~ FI N N 3 _
H03S N=N-~ ' N N- i}-N.4Wk CHNzN , N=N aS03H
SO3H 0 Fi 0 HO3S
H03S~ ~S03H
SO3H y~ HO3S
HO3S N^N / N=N-~ NHYNYN N=N N N S03H
H3c NYN CH3
6 0 N
H03S \ N N \/-N=N H3C
H-11 N~11 N=N , ~ N N-O-SOH
S03H Fi HO3S
H03S-\__\
S03H {0 HO3S
H03S N=N N=N- -NY, \ N=N N=N-aS03H
H3C N T N CH3
6 1 (N)
N
H3C N LN CH3
i
H03S V N=N N=N HEN' N N=N N=N SO3H
SO3H 0 H03S
-SO3H
[Table 19]
CA 02803910 2012-12-24
51
Compound No. Structural formula
H03S- __/-S03H
SO3H 0 H 0 HO3S
H03S / \ WN / \ N=N NYN, N N=N-/ N N / SO,H
H3C N1. N CH3
62 (N
N
H3C N`lN CH3
N
H03S / \ N=N / \ N=N A
HN~H o / N=N\ ~N=N- / -SO3H
SO3H H03S
H038-\, ~S03H
S03H 0 H 0 HO3S
HO3S / \ N N N=N / NYNYN-( } N=N / N=N / SO3H
H3C N T N CH3
63 (NJ
H3C NJ, N CH3
H03S / N=N N N / H--N--H N=N-N-N / S03H
SO3H O HO3S
,-S03H
SO3H HO3S
H H
H03S aN=N / N=N / NY,N r `N=N N=N--SO3H
H3C N1,N ((CH3
C N
6 4 N
H3C N`,N CH3
HO3S / aN=N / \' N=N N--NH NON , / N=N / S03H
S03H 0 HO3S
"SOuH
H03S-,__\ _,f-S03H
SO3H 0 0 HO3S
HO3S / \ N=N4/\ N=N / NYNYN / N=N \ / N=N / S0H
H3C N1'-N CH3
N
H3C N
H3C N N CH3
H03S / a N=N / \ N N / H~NH SO3H
SO3H 0 0 HO3S
H03S-/--i ~S03H
[Table 20]
CA 02803910 2012-12-24
52
Compound No Structural formula
SO3H HO3S
H03S \ N=N ' N-N _~ N~ NYN \ N=N N=N & SO,H
H3C N T N CH3
~N~CH3
66
H3C N
H3C N.LN CH3
i
H03S / \ N-N \ N=N HEN"- N=N / N=N , /, S03H
S03H HO3S
HO3S-,~
SO3H 0 HO3S
H03S \ N=N N=N NYN~ N N=N iO- N=N SOaH
`
H3C N T N t CH3
67 N
H3CN CH3
H3C Nj-N CH3
H03S \ N=N N=N \ H'I" NH N=N N=N-&SO3H
SO3H 0 HO3S
~SO3H
H03S-\__~
S03H 0 HO3S
HO3S N=N I N=N NYNYN N N 1 N=N S03H
H3C N T N CH3
6 8 N CH3
H3CN
H3C N-IN (CH3
H03S \ N=N N=N H~NXN- rN=N , / N=N SO3H
S03H 0??? ~~~ H03S
"-SO3H
~COOH
SO3H O HO3S
H03S a N-N / N=N NY NIT N{ N=N N=N a S03H
H3C N T N CH3
6 9 (N)
NI
H3C N`1N CH3
HO3S a N=N N=N-\ N~Y-N N=N N-N a S03H
S03H 0 HO3S
HOOC-I'
[Table 21]
CA 02803910 2012-12-24
53
Compound N..11 Structural formula
HOB rOH
SOP ,--~0 O H03S
H03S N=N N=N~ y-NYNrN "' N-N N=N-aS03H
HO-/- NYN 0- \-0H
70 (N)
HO ( OHS
HO3S N-N N O\ H~NH-(\ ~r N--(~ , N-N S03H
S03H O FI Y"~ HO3S
H
OH
SO3H OCH3 H03S
HO3S N=N N=N NYNYN N=N-~--N=N 1 S03H
H3C NN CH3
71 (N
H3C
_ N'lN CH
~
H03S N=N N=N M'N'H N=N _NIN S03H
S03H H3CO_ HO3S
1--rSO3H
SO3H 0 HO3S
H03S N=N N=N NYN r -0-WN N=N a S03H
H3C NYN NHAc
7 2 N
AcHN NJLN CH3
H03S N=N N=N 1'NillH N=N N=N SOSH
SO3H 0 H03S
HO3S-/---
-S03H
SO3H H 0 H03S
HO35 \ N=N / \ N=N ~_~ NYNYN N=N N=N SO3H
H3C N T N CI
7 3 N
C /-~
HO3S N=N~ rN=NB H~N~H b /HN N- Q-N=NSOH
-(SO3H 0 H03SY'
HO3S-'
[Table 22]
CA 02803910 2012-12-24
54
Compound No. Structural formula
--COON
SOH H O N=H03SN -
H N O-N-N--SO H
HO,S~~--N=N-~-N=N~N ~. N.
H0C N-fN CH3
7 4 CNJ H3
N
H3C NJ'N CH3
HO,S-~aN=N ".N ~~~H}'N~H-- N-N-J/J-N-N-~-SOyH
S03H 0 HO'S -'
HOOC~
r_rS03H
S03H ~{ O HO3S
HO3S / N=N NN / NYNI,N-N=NLO -N=N-~aS03H
H3C N T N NHAc
7 5 H3CTNXCH3
N
ACHN} NJIN CH3 ~--~
H03S-~aN=N--g-~ y-NzN-G Mj--N-kH-~"N-N-4 ~rN-N~-S03H
SOoH "-{ O n H03S
HO3S~
-SO3H
SO3H ry'-~ H 0}~ HO3S
HO,S N=N N N-~_ oTNI,N-C }-N=N / N=N-~-503H
H3C NN `'iCI
7 6 N
Ci Nd~N CH3
H03S / N=N-t rN=N / H~N~~J / N=N , / N-N SO3H
SO3H O HO,S
H03S-rI
HO3S-,_\ __,'-SO3H
S03H O 0 H03S
HO'S / a N=N / N=N NYNIr N-Q N=N-~-N=N-aS03H
HO3S 0 N N N 0 503H
7 7 H03S N SO3H
~0 NJIN O'
H03S-aN=N / \' N N~~ /j-H16 N~H / N=N / N=N-aS03H
S03H 0 H03
H03Sf `"-SO,H
The coloring matter (II) contained in the ink composition
of the present invention will be described.
There are no particular limitations on the coloring
matter (II) contained in the ink composition of the present
invention as long as it is an azo compound having Amax in a
range of 550 to 660 nm, and may be arbitrarily selected by
means of adjustment of the hue.
Here, the "azo compound having Amax in a range of 550 to
660 nm" means an azo compound which has Amax in a range of 550
CA 02803910 2012-12-24
to 660 nm when the concentration of an aqueous solution of the
coloring matter is adjusted in the measurement of the
absorbance such that the pH of an aqueous solution containing
the azo compound is in a range of 5.5 to 8.0, and the
absorbance at Amax (maximal absorption wavelength) in water is
in a range of 0.5 to 1.5. Furthermore, the azo compound is
further preferably an azo compound having Amax in a range of
580 to 630 nm from the viewpoint of color development
properties. Meanwhile, the azo compound is a general term of
an organic compound in which two organic groups are linked
with an azo group.
As the azo compound having Amax in a range of 550 to 660
nm, the compound represented by the formula (3) is suitably
used from the viewpoint of color development properties.
Here, the compound represented by the formula (3) will be
described.
The compound represented by the formula (3) has a
tautomer, and contemplates isomers represented by the
following formulae (5) to (7) and the like, in addition to the
formula (3), and the like. These tautomers are also
encompassed in the present invention.
Meanwhile, 8101 to R107 in the following formulae (5) to (7)
all have the same meanings as R101 to R107 for the formula (3).
Rios Rioz
OH Rios
Rios 21-
_N N=N
~~ S I -N (5)
R~oa Rica R+os HO S N
3 (SO3F~n p \
CA 02803910 2012-12-24
56
8105 102
\\ N~ _ R o,
R1106
06 N -N
N
Rio? 104 Rion N=N \\N (6)
R HO3S (S034n HO D
R1 os R102
Rios
Rios (/N N=N _Rt0 N H
R~oi S~ Rioa HO S \ \ I N-N N (7)
3 (S03in O \D
In the case where R10'for the formula (3) is a C1-C8
alkoxycarbonyl group, the alkoxycarbonyl group may be any one
of a linear alkoxycarbonyl group, a branched alkoxycarbonyl
group, and an alkoxycarbonyl group having a cyclic structure
in its alkyl moiety, and a linear alkoxycarbonyl group and a
branched alkoxycarbonyl group are preferred. Specific examples
include linear groups such as methoxycarbonyl, ethoxycarbonyl,
n-propoxycarbonyl, n-butoxycarbonyl, n-pentyloxycarbonyl, n-
hexyloxycarbonyl, n-heptyloxycarbonyl and n-octyloxycarbonyl;
branched groups such as isopropoxycarbonyl,
isobutyloxycarbonyl, sec-butoxycarbonyl, tert-butoxycarbonyl,
2,2-dimethylpropoxycarbonyl, isopentyloxycarbonyl, sec-
pentyloxycarbonyl and 2-methylbutyloxycarbonyl; groups having
a cyclic structure in its alkyl moiety such as
cyclopropylmethyloxycarbonyl, cyclobutylmethyloxycarbonyl,
cyclopentyloxycarbonyl and cyclohexyloxycarbonyl; and the like.
The alkoxycarbonyl group is more preferably a linear Ci-
C6 alkoxycarbonyl group, and further preferably a linear Cl-C4
alkoxycarbonyl group. Specific examples are the same as those
corresponding among those described above.
8101 for the formula (3) is a Cl-C4 alkyl group which may
CA 02803910 2012-12-24
57
be substituted with a Cl-C8 alkoxycarbonyl group or a carboxy
group. In the case where the Cl-C4 alkyl group is an
unsubstituted Cl-C4 alkyl group, the alkyl group may be linear
alkyl group or a branched alkyl group, and is preferably a
linear alkyl group.
Specific examples of the Cl-C4 alkyl group include linear
groups such as methyl, ethyl, n-propyl and n-butyl; branched
groups such as isopropyl, isobutyl, sec-butyl and tert-butyl;
and the like. Further, in the case where the Cl-C4 alkyl group
has a substituent, the alkyl group may be the same as those
described above including the preferred ones.
In the case where the substituent is a Ci-C8
alkoxycarbonyl group, the alkoxycarbonyl group may be the same
as the Cl-C8 alkoxycarbonyl group for Rios including the
preferred ones. In the case where Rloi is a Cl-C4 alkyl group
substituted with a Cl-C8 alkoxycarbonyl group, specific
examples of preferred Rio' include methoxycarbonylmethyl,
ethoxycarbonyl ethyl, n-butoxycarbonylmethyl, n-
octyloxycarboxy ethyl and the like.
In the case where R10' is a Cl-C4 alkyl group substituted
with a carboxy group, specific examples of preferred Rio'
include carboxymethyl, 2-carboxy ethyl, 3-carboxypropyl and
the like.
In the case where Rio' for the formula (3) is a phenyl
group which may be substituted with a hydroxy group, a sulfo
group, or a carboxy group, specific examples include an
unsubstituted phenyl group; hydroxy-substituted phenyl groups
CA 02803910 2012-12-24
58
such as 2-hydroxyphenyl and 4-hydroxyphenyl; sulfo-substituted
phenyl groups such as 2-sulfophenyl, 4-sulfophenyl, 2,4-
disulfophenyl and 3,5-disulfophenyl; carboxy-substituted
phenyl groups such as 2-carboxyphenyl, 4-carboxyphenyl and
3,5-dicarboxyphenyl; phenyl groups substituted with multiple
kinds of groups such as 2-hydroxy-5-sulfophenyl; and the like.
Among those described above, R101for the formula (3) is
preferably a carboxy group; a Cl-C4 alkoxycarbonyl group; an
unsubstituted C1-C4 alkyl group; a carboxy group-substituted
Cl-C4 alkyl groups; or an unsubstituted phenyl group.
Specific examples of preferred R101 for the formula (3)
include methyl, ethyl, tert-butyl, carboxymethyl, 3-
carboxypropyl, methoxycarbonylmethyl, carboxy, methoxycarboxy,
ethoxycarboxy, n-octyloxycarboxy, phenyl, 2-hydroxyphenyl and
4-sulfophenylmethyl, more preferably methyl, carboxymethyl,
carboxy, phenyl, further preferably carboxy and phenyl, and
most preferably carboxy.
Examples of the halogen atom for R102 to R104 in the
formula (3) include a fluorine atom, a chlorine atom, a
bromine atom, and an iodine atom. Among these, a fluorine atom,
a chlorine atom, and a bromine atom are preferred, and a
chlorine atom is particularly preferred.
In the case where R102 to R104 for the formula (3) is a C1-
C4 alkyl group, the alkyl group may be a linear alkyl group, a
branched alkyl group, or a cyclic alkyl group, but is
preferably a linear alkyl group, or a branched alkyl group,
and further preferably a linear alkyl group. Specific examples
CA 02803910 2012-12-24
59
include linear groups such as methyl, ethyl, n-propyl and n-
butyl; branched groups such as isopropyl, isobutyl, sec-butyl
and tert-butyl; and the like.
In the case where R102 to 8104 for the formula (3) is an
unsubstituted Cl-C4 alkoxy group, the alkoxy group is
preferably either a linear alkoxy group or a branched alkoxy
group. Specific examples include methoxy, ethoxy, n-propoxy,
isopropoxy, n-butoxy, sec-butoxy, tert-butoxy and the like.
In the case where the alkoxy group is substituted with at
least one group selected from the group consisting of a
hydroxy group, a Cl-C4 alkoxy group, a hydroxy-Cl-C4 alkoxy
group, a sulfo group and a carboxy group, specific examples
include hydroxy-Cl-C4 alkoxy groups such as 2-hydroxyethoxy,
2-hydroxypropoxy and 3-hydroxypropoxy; Cl-C4 alkoxy-Cl-C4
alkoxy groups such as methoxyethoxy, ethoxyethoxy, n-
propoxyethoxy, isopropoxyethoxy, n-butoxyethoxy,
methoxypropoxy, ethoxypropoxy, n-propoxypropoxy,
isopropoxybutoxy and n-propoxybutoxy; hydroxy-C1-C4 alkoxy-Cl-
C4 alkoxy groups such as 2-hydroxyethoxyethoxy; sulfo-Cl-C4
alkoxy groups such as 3-sulfopropoxy and 4-sulfo butoxy;
carboxy-Cl-C4 alkoxy groups such as carboxymethoxy, 2-carboxy
ethoxy and 3-carboxypropoxy; and the like.
In the case where R102 to R104 for the formula (3) is an
unsubstituted mono- or di-C1-C4 alkylamino group, the Cl-C4
alkyl moiety is preferably either a linear one or a branched
one. Specific examples include linear groups such as
methylamino, ethylamino, n-propylamino, isopropylamino, n-
CA 02803910 2012-12-24
butylamino, dimethylamino, diethylamino, di-n-propylamino and
di-n-butylamino; branched groups such as sec-butylamino, tert-
butylamino and diisopropylamino; and the like.
In the case where the mono- or di-Cl-C4 alkylamino group
is substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, and a carboxy
group, specific examples include hydroxy-substituted mono- or
di-C1-C4 alkylamino groups such as 2-hydroxyethylamino, 2-
hydroxypropylamino and 2,2'-dihydroxydiethylamino; sulfo-
substituted mono- or di-Cl-C4 alkylamino groups such as 2-
sulfoethylamino, 3-sulfopropylamino, 4-sulfo butylamino and
3,3'-disulfodipropylamino; carboxy-substituted mono- or di-Cl-
C4 alkylamino groups such as carboxymethylamino, 2-carboxy
ethylamino, 3-carboxypropylamino and 2,2'-dicarboxy
diethylamino; and the like.
In the case where R102 to R104 for the formula (3) are an
unsubstituted C1-C4 alkylcarbonylamino group, the Cl-C4 alkyl
moiety may be either a linear one or a branched one, but is
preferably a linear one. Specific examples include acetylamino,
propanoylamino, butanoylamino and the like.
In the case where the C1-C4 alkylcarbonylamino group is
substituted with a hydroxy group or a carboxy group, specific
examples of the C1-C4 alkylcarbonylamino group include
hydroxy-Cl-C4 alkylcarbonylamino groups such as
hydroxyethanoylamino, 2-hydroxypropanoylamino and 4-
hydroxybutanoylamino; carboxy-Cl-C4 alkylcarbonylamino groups
such as 3-carboxypropanoylamino; and the like.
CA 02803910 2012-12-24
61
In the case where R102 to R104 for the formula (3) are an
N'-C1-C4 alkylureido group, it preferably has a substituent,
rather than being unsubstituted.
In the case where the N'-C1-C4 alkylureido group is
substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, and a carboxy
group, specific examples include N'-hydroxy-C1-C4 alkylureido
groups such as N'-2-hydroxyethylureido and N'-3-
hydroxyethylureido; N'-sulfo Cl-C4 alkylureido groups such as
N'-2-sulfoethylureido and N'-3-sulfopropylureido; N'-carboxy
C1-C4 alkylureido groups such as N'-carboxymethylureido, N'-2-
carboxyethylureido, N'-3-carboxypropylureido and N'-4-
carboxybutylureido; and the like.
In the case where R102 to R104 for the formula (3) are a
phenylamino group having its benzene ring which may be
substituted with at least one group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group, specific examples
include unsubstituted phenylamino groups; halogen atom-
substituted phenylamino groups such as 2-chlorophenylamino, 4-
chlorophenylamino and 2,4-dichlorophenylamino; Cl-C4 alkyl-
substituted phenylamino groups such as 2-methylphenylamino, 4-
methylphenylamino and 4-tert-butylphenylamino; nitro-
substituted phenylamino groups such as 2-nitrophenylamino and
4-nitrophenylamino; sulfo-substituted phenylamino groups such
as 3-sulfophenylamino, 4-sulfophenylamino, 2,4-
disulfophenylamino and 3,5-disulfophenylamino; carboxy-
CA 02803910 2012-12-24
62
substituted phenylamino groups such as 2-carboxyphenylamino,
4-carboxyphenylamino, 2,5-dicarboxyphenylamino and 3,5-
dicarboxyphenylamino; and the like.
In the case where R102 to R104 for the formula (3) are a
substituted phenylamino group, benzoylamino group, or
phenylsulfonylamino group, and the substituent of the benzene
ring contained in the respective groups is a Cl-C4 alkyl group,
the alkyl group may be a linear one or a branched one, or a
cyclic one, but is preferably a linear one or a branched one.
Specific examples include linear groups such as methyl, ethyl,
n-propyl and n-butyl; branched groups such as isopropyl,
isobutyl, sec-butyl and tert-butyl; and the like.
In the case where R102 to R104 for the formula (3) are a
benzoylamino group having its benzene ring which may be
substituted with at least one group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group, specific examples
include unsubstituted benzoylamino groups; halogen atom-
substituted benzoylamino groups such as 2-chlorobenzoylamino,
4-chlorobenzoylamino and 2,4-dichlorophenylamino; Cl-C4 alkyl-
substituted benzoylamino groups such as 2-methylbenzoylamino,
3-methylbenzoylamino and 4-methylbenzoylamino; nitro-
substituted benzoylamino groups such as 2-nitrobenzoylamino,
4-nitrobenzoylamino and 3,5-dinitrobenzoylamino; sulfo-
substituted benzoylamino groups such as 2-sulfo benzoylamino
and 4-sulfo benzoylamino; carboxy-substituted benzoylamino
groups such as 2-carboxy benzoylamino, 4-carboxy benzoylamino
CA 02803910 2012-12-24
63
and 3,5-dicarboxy benzoylamino; and the like.
In the case where R102 to R104 for the formula (3) are a
phenylsulfonylamino group having its benzene ring which may be
substituted with at least one group selected from the group
consisting of a halogen atom, a C1-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group, specific examples
include unsubstituted phenylsulfonylamino groups; halogen
atom-substituted phenylsulfonylamino groups such as 2-
chlorophenylsulfonylamino and 4-chlorophenylsulfonylamino; C1-
C4 alkyl-substituted phenylsulfonylamino groups such as 2-
methylphenylsulfonylamino, 4-methylphenylsulfonylamino and 4-
tert-butylphenylsulfonylamino; nitro-substituted
phenylsulfonylamino groups such as 2-nitrophenylsulfonylamino,
3-nitrophenylsulfonylamino and 4-nitrophenylsulfonylamino;
sulfo-substituted phenylsulfonylamino groups such as 3-
sulfophenylsulfonylamino and 4-sulfophenylsulfonylamino;
carboxy-substituted phenylsulfonylamino groups such as 3-
carboxyphenylsulfonylamino and 4-carboxyphenylsulfonylamino;
and the like.
Among those described above, R102 is preferably a sulfo
group; a Cl-C4 alkoxy group substituted with at least one
group selected from the group consisting of a hydroxy group, a
hydroxy-Cl-C4 alkoxy group, a sulfo group, and a carboxy group;
and more preferably a Cl-C4 alkoxy group substituted with a
sulfo group.
Among those described above, 8103 is particularly
preferably a hydrogen atom.
CA 02803910 2012-12-24
64
Among those described above, 8104 is preferably a Cl-C4
alkyl group; a Cl-C4 alkylcarbonylamino group; and a mono-C1-
C4 alkylureido group substituted with at least one group
selected from the group consisting of a hydroxy group, a sulfo
group, and a carboxy group. 8104 is more preferably a Cl-C4
alkyl group; a Cl-C4 alkylcarbonylamino group; and a mono-C1-
C4 alkylureido group substituted with a sulfo group. R' 4 is
further preferably a Cl-C4 alkyl group.
Examples of the halogen atom for R105 to R1p7 in the
formula (3) include a fluorine atom, a chlorine atom, a
bromine atom, and an iodine atom. Among these, a fluorine atom,
a chlorine atom, and a bromine atom are preferred, and a
chlorine atom is particularly preferred.
In the case where R105 to 8107 for the formula (3) are a
Cl-C4 alkyl group, the alkyl group may be a linear one, a
branched one, or a cyclic one, but is preferably a linear one
or a branched one, and is further preferably a linear one.
Specific examples include linear groups such as methyl, ethyl,
n-propyl and n-butyl; branched groups such as isopropyl,
isobutyl, sec-butyl and tert-butyl; and the like.
In the case where R105 to R107 for the formula (3) are a
Cl-C4 alkoxy group which may be substituted with at least one
group selected from the group consisting of a hydroxy group, a
Cl-C4 alkoxy group, a sulfo group, and a carboxy group, the
substituent may be the same as the Cl-C4 alkoxy group that
corresponds to R102 to R104 described above, including the
preferred ones.
CA 02803910 2012-12-24
In the case where R105 to R107 for the formula (3) is a C1-
C4 alkylsulfonyl group which may be substituted with at least
one group selected from the group consisting of a hydroxy
group, a sulfo group, and a carboxy group, specific examples
include linear or a branched Cl-C4 alkylsulfonyl groups such
as methylsulfonyl, ethylsulfonyl, propylsulfonyl,
isopropylsulfonyl, n-butylsulfonyl, sec-butylsulfonyl and
tert-butylsulfonyl; hydroxy-Cl-C4 alkylsulfonyl groups such as
2-hydroxyethylsulfonyl and 3-hydroxypropylsulfonyl; sulfo-Cl-
C4 alkylsulfonyl groups such as 2-sulfopropylsulfonyl, 3-
sulfopropylsulfonyl and 4-sulfobutylsulfonyl; carboxy-Cl-C4
alkylsulfonyl groups such as carboxymethylsulfonyl, 2-carboxy
ethylsulfonyl and 3-carboxypropylsulfonyl; and the like.
In the case where R105 to R107 for the formula (3) is a
phenylsulfonyl group having its benzene ring which may be
substituted with at least one group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group, specific examples
include unsubstituted phenylsulfonyl groups; halogen atom-
substituted phenylsulfonyl groups such as 2-
chlorophenylsulfonyl and 4-chlorophenylsulfonyl; Cl-C4 alkyl-
substituted phenylsulfonyl groups such as 2-
methylphenylsulfonyl, 4-methylphenylsulfonyl, 2,4-
dimethylphenylsulfonyl and 4-tert-butylphenylsulfonyl; nitro-
substituted phenylsulfonyl groups such as 2-
nitrophenylsulfonyl and 4-nitrophenylsulfonyl; sulfo-
substituted phenylsulfonyl groups such as 3-
CA 02803910 2012-12-24
66
sulfophenylsulfonyl, 4-sulfophenylsulfonyl and 3,5-
disulfophenylsulfonyl; carboxy-substituted phenylsulfonyl
groups such as 2-carboxyphenylsulfonyl, 4-
carboxyphenylsulfonyl and 3,5-dicarboxyphenylsulfonyl; and the
like.
Specific examples of preferred R105 to R107 for the formula
(3) is a hydrogen atom, a halogen atom, carboxy, sulfo, nitro,
methyl, ethyl, methoxy, ethoxy, 2-hydroxyethoxy, 2-sulfoethoxy,
3-sulfopropoxy, 4-sulfobutoxy, carboxymethoxy, 2-carboxy
ethoxy, methylsulfonyl, ethylsulfonyl, tert-butylsulfonyl, 2-
hydroxyethylsulfonyl, 3-sulfopropylsulfonyl, 2-carboxy
ethylsulfonyl, phenylsulfonyl, 4-chlorophenylsulfonyl, 4-
methylphenylsulfonyl, 2,4-dimethylphenylsulfonyl, 4-
nitrophenylsulfonyl, 4-sulfophenylsulfonyl, 2-
carboxyphenylsulfonyl, 4-carboxyphenylsulfonyl and the like.
8105 to R107 for the formula (3) is more preferably a hydrogen
atom, a chlorine atom, carboxy, sulfo, nitro, methyl, methoxy,
methylsulfonyl or 2-carboxyphenylsulfonyl, and further
preferably a hydrogen atom, sulfo or methoxy. At least one of
8105 to R107 is preferably a hydrogen atom.
The position of substitution of preferred R105 to R107 on
the benzothiazole ring is the 4-position or the 5-position for
8105, the 6-position for R106, and the 7-position for R107
Among those described above, R105 is preferably a hydrogen
atom or a sulfo group. Furthermore, among those described
above, R106 is preferably a hydrogen atom, a halogen atom, a
carboxy group, a sulfo group, a Cl-C4 alkoxy group, or a Cl-C4
CA 02803910 2012-12-24
67
alkylsulfonyl group, and more preferably a Cl-C4 alkoxy group.
Furthermore, among those described above, R107 is preferably a
hydrogen atom or a sulfo group.
Examples of particularly preferred combinations of R105 to
R107 include a combination in which R105 is a sulfo group at the
5-position of the substitution position, 8106 is a Cl-C4 alkoxy
group at the 6-position of the substitution position, and R107
is a hydrogen atom; or a combination in which R105 is a
hydrogen atom, R106 is a Cl-C4 alkoxy group at the 6-position
of the substitution position, and R107 is a sulfo group at the
7-position of the substitution position.
n in the formula (3) is preferably 1.
The group D in the formula (3) is a phenyl group or a
naphthyl group. The phenyl group and a naphthyl group may be
substituted, respectively with at least one group selected
from the group consisting of the specific groups described
above.
When the group D in the formula (3) is a phenyl group,
the phenyl group substituted with the specific group described
above will be described. The number of the substituents is
usually one to three, preferably one or two, more preferably
one. When multiple groups are substituted, there are no
particular limitations on the kinds of the substituents, but
the same kind is preferred. Further, there are no particular
limitations on the position of the substituent, but when the
position of the bond with the nitrogen atom of the pyrazolone
ring is designated as the 1-position, it is preferable that
CA 02803910 2012-12-24
68
the substituents are substituted at the 2-position, the 3-
position, and the 5-position, respectively when the number of
the substituents is three; the 2-position and the 4-position,
or the 2-position and the 5-position, or the 3-position and
the 5-position when the number of the substituents is two; and
the 4-position when the number of the substituents is one.
Meanwhile, the position of the substituent on the phenyl
group in each constitution described below is described such
that the position of the bond with the nitrogen atom of the
pyrazolone ring is designated as the 1-position.
Examples of the phenyl group substituted with a hydroxy
group for the group D include 2-hydroxyphenyl, 3-hydroxyphenyl,
4-hydroxyphenyl and the like.
Examples of the phenyl group substituted with a sulfo
group for the group D include 2-sulfophenyl, 4-sulfophenyl,
2,4-disulfophenyl, 2,5-disulfophenyl, 3,5-disulfophenyl and
the like.
Examples of the phenyl group substituted with a carboxy
group for the group D include 4-carboxyphenyl, 3,5-
dicarboxyphenyl and the like.
Examples of the phenyl group substituted with a Cl-C4
alkyl group for the group D include 4-methylphenyl, 3-
methylphenyl and the like. Furthermore, examples of the Cl-C4
alkyl group, which is the substituent, include those described
for the "C1-C4 alkyl group for R102 to R104" described above,
including the preferred ones and the like.
Examples of the phenyl group substituted with a Cl-C4
CA 02803910 2012-12-24
69
alkoxy group for the group D include 4-methoxyphenyl and the
like. Furthermore, examples of the Cl-C4 alkoxy group, which
is the substituent, include those described for the "C1-C4
alkoxy group for R102 to R104" described above, including the
preferred ones and the like.
Examples of the phenyl group substituted with an amino
group for the group D include 2-aminophenyl, 3-aminophenyl, 4-
aminophenyl and the like.
Examples of the phenyl group substituted with a mono-Cl-
C4 alkylamino group for the group D include 4-
methylaminophenyl and the like. Furthermore, examples of the
mono-C1-C4 alkylamino group, which is the substituent, include
those described for the "mono-Cl-C4 alkylamino group for R102
to R104" described above, including the preferred ones and the
like.
Examples of the phenyl group substituted with a di-C1-C4
alkylamino group for the group D include 4-dimethylaminophenyl
and the like. Furthermore, examples of the di-Cl-C4 alkylamino
group, which is the substituent, include those described for
the "di-Cl-C4 alkylamino group for R102 to R104" described above,
including the preferred ones and the like.
Examples of the phenyl group substituted with a Cl-C4
alkylcarbonylamino group for the group D include 4-
acetylaminophenyl and the like. Furthermore, examples of the
Cl-C4 alkylcarbonylamino group, which is the substituent,
include those described for the "C1-C4 alkylcarbonylamino
group for R102 to R10411 described above, including the preferred
CA 02803910 2012-12-24
ones and the like.
Examples of the phenyl group substituted with a
benzoylamino group for the group D include 4-
benzoylaminophenyl and the like.
Examples of the phenyl group substituted with "a
benzoylamino group having its benzene ring substituted with at
least one group selected from the group consisting of a
halogen atom, a C1-C4 alkyl group, a nitro group, a sulfo
group, and a carboxy group" for the group D, include 4-(4-
chlorophenylbenzoylamino)phenyl, 4-(4-
methylphenylbenzoylamino)phenyl, 4-(4-
nitrophenylbenzoylamino)phenyl, 4-(4-
sulfophenylbenzoylamino)phenyl, 4-(4-
carboxyphenylbenzoylamino)phenyl and the like. Furthermore,
examples of the "benzoylamino group having its benzene ring
substituted with at least one group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group", which is the
substituent, include those described for the "benzoylamino
group having its benzene ring substituted with at least one
group selected from the group consisting of a halogen atom, a
Cl-C4 alkyl group, a nitro group, a sulfo group, and a carboxy
group for R102 to R104" described above, including the preferred
ones and the like.
Examples of the phenyl group substituted with the
phenylsulfonyloxy group for the group D include 4-
phenylsulfonyloxyphenyl and the like.
CA 02803910 2012-12-24
71
For the phenyl group substituted with "a
phenylsulfonyloxy group having its benzene ring substituted
with at least one group selected from the group consisting of
a halogen atom, a nitro group, and a Cl-C4 alkyl group" for
the group D, examples of the "phenylsulfonyloxy group having
its benzene ring substituted with at least one group selected
from the group consisting of a halogen atom, a nitro group,
and a Cl-C4 alkyl group" include usually those substituted
with one to three, preferably one or two of these groups. When
multiple groups are substituted, there are no particular
limitations on the kinds of the substituents, but the same
kind is preferred. There are no particular limitations on the
position of the substituent. Specific examples include those
substituted with a halogen atom such as 4-
chlorophenylsulfonyloxy, 2,4-dichlorophenylsulfonyloxy and
3,5-dichlorophenylsulfonyloxy; those substituted with a nitro
group such as 2-nitrophenylsulfonyloxy and 4-
nitrophenylsulfonyloxy; those substituted with a Cl-C4 alkyl
group such as 4-methylphenylsulfonyloxy and 2,4-
dimethylphenylsulfonyloxy; and the like.
Meanwhile, examples of the Ci-C4 alkyl group among these
substituents include those described for the "Cl-C4 alkyl
group for R102 to R104" described above, including the preferred
ones and the like.
Examples of the phenyl group substituted with a
substituted phenylsulfonyloxy group described above in the
group D include 4-(4-methylphenyl)sulfonyloxyphenyl and the
CA 02803910 2012-12-24
72
like.
Examples of the phenyl group substituted with multiple
kinds of the groups for the group D include those substituted
with a hydroxy group and a carboxy group such as 3-hydroxy-4-
carboxyphenyl; those substituted with a hydroxy group, a sulfo
group, and a carboxy group such as 3-carboxy-2-hydroxy-5-
sulfophenyl; and the like.
When the group D in the formula (3) is a naphthyl group,
a naphthyl group substituted with the specific group described
above will be described. The number of the substituents is one
to three. When multiple groups are substituted, there are no
particular limitations on the kinds of the substituents, but
the same kind is preferred. The position of the bond of the
group D with the nitrogen atom of the pyrazolone ring is
preferably the 1-position or the 2-position, that is, 1-
naphthyl or 2-naphthyl is preferred. There are no particular
limitations on the position of the substituent on the naphthyl
group, but those described below are preferred.
Specifically,
[When the group D is a 1-naphthyl group]
(a) When the number of the substituents is one, the 3-, 4-, 5-,
6- and 7-positions.
(b) When the number of the substituents is two, the
combinations of the 3-position and the 4-position, the 3-
position and the 5-position, the 3-position and the 6-position,
the 3-position and the 7-position, the 4-position and the 6-
position, the 4-position and the 7-position, and the 5-
CA 02803910 2012-12-24
73
position and the 7-position.
(c) When the number of the substituents is three, the
combinations of the 3-position, the 4-position and the 6-
position, the 3-position, the 4-position and the 7-position,
the 3-position, the 5-position and the 6-position, the 3-
position, the 5-position and the 7-position, and the 3-
position, the 6-position and the 7-position.
[When the group D is a 2-naphthyl group]
(d) When the number of the substituents is one, the 4-, 5-, 6-,
7-, and 8-positions.
(e) When the number of the substituents is two, the
combinations of the 4-position and the 6-position, the 4-
position and the 7-position, the 4-position and 8-position,
the 5-position and the 6-position, the 5-position and the 7-
position, the 5-position and 8-position, and the 6-position
and 8-position.
(f) When the number of the substituents is three, the
combinations of the 4-position, the 6-position and the 7-
position, the 4-position, the 6-position and 8-position, and
the 4-position, the 7-position and 8-position.
Meanwhile, the position of the bond of the nitrogen atom
of the pyrazolone ring to the naphthyl group, and the position
of the respective substituents on the naphthyl group in each
constitution to be described below are described conforming to
those described above.
Examples of the naphthyl group substituted with a hydroxy
group for the group D include 4-hydroxynaphth-l-yl, 6-
CA 02803910 2012-12-24
74
hydroxynaphth-l-yl, 5-hydroxynaphth-2-yl and the like.
Examples of the naphthyl group substituted with a sulfo
group for the group D include 7-sulfonaphth-l-yl, 5,7-
disulfonaphth-2-yl, 6,8-disulfonaphth-2-yl, 4,8-disulfonaphth-
2-yl, 4,6,8-trisulfonaphth-2-yl, 4,7,8-trisulfonaphth-2-yl and
the like.
Examples of the naphthyl group substituted with a carboxy
group for the group D include 7-carboxy naphth-l-yl and the
like.
Examples of the naphthyl group substituted with a Cl-C4
alkyl group for the group D include 4-methylnaphth-l-yl and
the like. Furthermore, examples of the Cl-C4 alkyl group,
which is the substituent, include those described for the "Cl-
C4 alkyl group for R102 to R104" described above, including the
preferred ones and the like.
Examples of the naphthyl group substituted with a Cl-C4
alkoxy group for the group D include 4-methoxynaphth-1-yl, 6-
methoxynaphth-2-yl and the like. Furthermore, examples of the
Cl-C4 alkoxy group, which is the substituent, include those
described for the "Cl-C4 alkoxy group for R102 to R1 4"
described above, including the preferred ones and the like.
Examples of the naphthyl group substituted with an amino
group for the group D include 4-aminonaphth-l-yl and the like.
Examples of the naphthyl group substituted with a mono-
Cl-C4 alkylamino group for the group D include 4-
methylaminonaphth-l-yl and the like. Furthermore, examples of
the mono-Cl-C4 alkylamino group, which is the substituent,
CA 02803910 2012-12-24
include those described for the "mono-Cl-C4 alkylamino group
for R102 to R104" described above, including the preferred ones
and the like.
Examples of the naphthyl group substituted with a di-Cl-
C4 alkylamino group for the group D include 4-
dimethylaminonaphth-l-yl and the like. Furthermore, examples
of the di-C1-C4 alkylamino group, which is the substituent,
include those described for the "di-C1-C4 alkylamino group for
R102 to 8104" described above, including the preferred ones and
the like.
Examples of the naphthyl group substituted with a C1-C4
alkylcarbonylamino group for the group D include 4-
acetylaminonaphth-l-yl and the like. Furthermore, examples of
the di-Cl-C4 alkylcarbonylamino group, which is the
substituent, include those described for the "Cl-C4
alkylcarbonylamino group for R102 to R104" described above,
including the preferred ones and the like.
Examples of the naphthyl group substituted with "a
benzoylamino group having its benzene ring substituted with a
group selected from the group consisting of a halogen atom, a
Cl-C4 alkyl group, a nitro group, a sulfo group, and a carboxy
group" for the group D include 4-(4-
chlorophenylbenzoylamino)naphtho-l-yl and the like.
Furthermore, examples of the "benzoylamino group having its
benzene ring substituted with a group selected from the group
consisting of a halogen atom, a Cl-C4 alkyl group, a nitro
group, a sulfo group, and a carboxy group", which is the
CA 02803910 2012-12-24
76
substituent, include those described for the "benzoylamino
group having its benzene ring substituted with at least one
group selected from the group consisting of a halogen atom, a
Cl-C4 alkyl group, a nitro group, a sulfo group, and a carboxy
group for R102 to R104" described above, including the preferred
ones and the like.
Examples of the naphthyl group substituted with a
phenylsulfonyloxy group for the group D include 4-
phenylsulfonyloxynaphth-l-yl, 5-phenylsulfonyloxynaphth-2-yl
and the like.
For a naphthyl group substituted with "a
phenylsulfonyloxy group having its benzene ring substituted
with at least one group selected from the group consisting of
a halogen atom, a nitro group, and a Cl-C4 alkyl group" in the
group D, examples of the " phenylsulfonyloxy group having its
benzene ring substituted with at least one group selected from
the group consisting of a halogen atom, a nitro group, and a
Cl-C4 alkyl group" include usually those substituted with one
to three, preferably one or two of these groups. When multiple
groups are substituted, there are no particular limitations on
the kinds of the substituents, but the same kind is preferred.
There are no particular limitations on the position of the
substituent. Specific examples include those substituted with
a halogen atom such as 4-chlorophenylsulfonyloxy, 2,4-
dichlorophenylsulfonyloxy and 3,5-dichlorophenylsulfonyloxy;
those substituted with a nitro group such as 2-
nitrophenylsulfonyloxy and 4-nitrophenylsulfonyloxy; those
CA 02803910 2012-12-24
77
substituted with a Cl-C4 alkyl group such as 4-
methylphenylsulfonyloxy and 2,4-dimethylphenylsulfonyloxy; and
the like.
Meanwhile, among these substituents, examples of the C1-
C4 alkyl group include those described for the "Cl-C4 alkyl
group for R102 to R104" described above, including the preferred
ones and the like.
Examples of the naphthyl group substituted with a
substituted phenylsulfonyloxy group described above in the
group D include 4-(4-methylphenyl)sulfonyloxynaphth-l-yl, 5-
(4-methylphenyl)sulfonyloxynaphth-2-yl and the like.
Among those described above, preferred ones as the group
D are a phenyl group; a phenyl group substituted with at least
one group selected from the group consisting of a hydroxy
group, a sulfo group, a carboxy group, a Cl-C4 alkyl group,
and a Cl-C4 alkoxy group; a naphthyl group; a naphthyl group
substituted with at least one group selected from the group
consisting of a hydroxy group, a sulfo group, a carboxy group,
a Cl-C4 alkyl group, and a Cl-C4 alkoxy group; and the like.
More preferably, the group D is a phenyl group, a phenyl group
substituted with a sulfo group, a phenyl group substituted
with a carboxy group, or a naphthyl group substituted with a
sulfo group, and further preferably a phenyl group substituted
with a sulfo group, or a naphthyl group substituted with a
sulfo group.
A compound of a combination of the preferred ones
described for the substituents of the formula (3) is more
CA 02803910 2012-12-24
78
preferred, and a compound of a combination of the more
preferred ones is further preferred. The same applies also to
combinations of even more preferred examples, combinations of
preferred examples and more preferred examples, and the like.
There are no particular limitations on the suitable
specific examples of the azo compound of the present invention
represented by the formula (3), but some suitable specific
examples include the compounds presented in the following
Tables 23 to 34, etc.
In the respective tables, the functional groups such as a
sulfo group and a carboxy group in each table are indicated in
the form of free acid, for convenience.
[Table 23]
CA 02803910 2012-12-24
79
Compound Structural formula
No.
SO3H
0 OH
N HO C
7 8 2 } N=N3 \/ N=9 I -ry
HOC S HC HOS NN HIV CO2H
S03H OH
CO2H
S03H
q0 OH
i N HO2C
7 9 N'N O"'-NzN
H3COS N:N
S03H H3C H03S O
503F1 OH S03H
H3C SO3H
O OH
N HO C
8 0 &-s N'N N
HO S N=N \ N
3 H3C H03S SO3H OH SOH
N SO3H OH HOZC
I rN=N N N N
8 1 H03S N-N = N
HN HO3S
S03H OH SO3H
H3C
S03H
O- OH
8 2 N=N \/ N=N I N S03H
N
H C HO =N
: 3S N
3 3 S03H OH
S03H
SO3H
0 OH CO2CH3
8 3 I N>-N=N \ / N NN
HO3S S i i N=N \ tj
H3C H03S SO3H OH
SO3H
[Table 24]
CA 02803910 2012-12-24
Compound Structural formula
No
SO3H
OJ
HO3S r N OH HO2C
8 4 N=N / N=N q N
CI S r r N=-\ N
SO3H H3C HO,S
S03H O
OH I '' SO3H
SO3H OH
r S N \ \ HOZC
HO3S ~ I )-N=N
i t N~N-(~ N ~
HN H03S
8 5 HNI~O S03H pH r CH3
S03H
SO3H
OH CH3
}N=N NN
8 6
H3C0rS03H H3C HO3S I r r N=N N Ir rSO3H
SO3H OH
SO3H
SO3H
8 7 HOyS r N i-( OH HOZC
H3CO S r i NN
H3C HO3S N
SO3H OH r S03H
SO3H
N O- OH CH3
8 8 jaj } N-N \ / N=N `J-N OH
HO S S r N'N ~\ N COZH
H3C HO3S 1O
SO3H OH r
803H
SO3H
0 OH
i N HOpC
8 9 3 }-N N / N=N N
HO S S i r N=N ~~
H30 HO3S
SO3H OH SO 3H
[Table 25]
CA 02803910 2012-12-24
81
Compound Structural formula
No.
S03H
S03H 0_1 OH
N HOzC
9 0 I j N=N N-N I N
OzN S i N=N-(' N
H3C H03S
SO3H OH S03H
SO3H
0 OH CO2CH3
9 1 I N>-NtNoN=N N SO H
H3CO2C S \/ N-N \.N 3
H3C HO3S
S03H OH SO3H
rSO3H
S03H OJ OH
N HOZC
92 j N'N N=N N
H3C02S S i N-NN \
H3C HO3S S03H 111OH /
S03H
SO3H
OH
}-N N N=N N S03H
93 H02C N N \ N S03H
H3C H03
S03H OH i
SO3H
SO3H
O OH
HOzC
94 N,>_W \/ N=N I
H02C S i N=N N
H3C H03S
S03H OF S03H
f-S03H
O OH C02H
N
z >-N`N \ / N N - -- N
9 5 HOC a S N'N \
H3C H03
S03H OH COzH
[Table 26]
CA 02803910 2012-12-24
82
Compound Structural formula
No.
/'~S03H
OJ OH
HOC}~`
96 H03S I SrN=N N=N i N=N~ N COH
H3C H03S SO3H 'OOOOH i ~r
COZH
S03H
O-J-- OH
,as rN=N \ / N=N N
9 7 H03S \ S H B i N=N N ~'COxH
3C HO3S S03H OH L iJ
CO2H
S03H
O- OH
N HOZC
9 8 } N=N \/ NN `N
NO3S S t ? N=N N
H3C HO3S SOH OH OCH
3
SO3H
_ OJ OH HOC SO3H
z
1 O 0 3 4 NrN=N \/ N=N ~
HO S S i N=N
H3C HOSS S03H OH i
S03H
SO3H 0-/- OH
NrN HOZC
1 0 1 3 S ~ i i N=N~NN
HO S \
H3C HO3S
SOSH O
OH i
rS03H
HOpC
rsl>-N-N OJ OH
1 0 2 N=N - i N=N
H
H3C HO3S SO3H OH
S03H
[Table 27]
CA 02803910 2012-12-24
83
Compound Structural formula
No.
SO3H
HoZC~ ke~ OH
HO/ I rN'H3 \/ HO S I i N-NN S03H
1 0 3
S03H OH S03H
S03H
rSO3H
OJ OH
N HOZC
S}'N'N P3HO,.)( N`N NNN 503H - ~-
1 0 4 H3co ~
S03H S03H OH I i
S03H
SO3H
rSo3H
H3C 0-/--
J OH
HOZC~~..
H3 -k-N I S03H
I\ i NN
105 HO3S /I srNN\ HN03NS ~T'
S03H OH i S03H
SO3H
N SO5H OH HOC
~ I rN=N \ / N=N I ~ ~ N SOH
S i i N=N \ N
1 0 6 HO3S HN H03S
O S03H OH SO3H
H3 S03H
S03H
O OH
1 0? I Sl'WN \/ N-N I N N S03H
H03S H3C H03S
503H OH S03H
So3H
SO3H
HO3S N OH HOC
\ I
1 0 8 rN:N \ N--N N S03H
S i i N=N N
H3CO
H3 H03S
S03H OH S03H
SO3H
[Table 28]
CA 02803910 2012-12-24
84
Compound Structural formula
No.
SO3H
O-/- OH
H03S~~. N _ ., HO3C
1 0 9 CI 7 S N=N N:N 1 N=N TIC^~'SS03H
S03H H3C HO3S S03H OH ` ~~
T _ SOSH
S03H
$03H OH HO2C
F- N I\ N N N NI` SO3H
3$ 777777
1 1 0 HN~O S03H OH S03H
SO3H
SO3H
S03H
OH
N HOzC
iT-N=NY Y N
1 1 1 H3CO -y S_J!"d~lit-N=N N I SOSH
H3C HOSH S03H OH
S03H
S03H
H03S N OH HOZC}~
l l L HSCO \ I S r N H3C HO's N, N N=N i4 I S03H
S03H OH
SO3H
rS03H
Of OH
N HOzC
I y-N=N NON N SO3H
1 1 3 H03Ss \ i N N1J
H3C H03S S03H OH 1QOL
T _ S03H
SO3H
S03H
O-
N OH H02C
}-N=N NON N
1 1 4 H03S S H C H03S N=N-~\ N S03H
3 S03H OH I
S03H
[Table 29]
CA 02803910 2012-12-24
Compound Structural formula
No.
S03H
O OH
1 1 5 HOzC s H C / Ho" I / N=N N SO3H
3 S03H OHS03H
SO3H
~S03H
OH
H03S N _ O /
}-N-N \ / N=N \ _N SO3H
I- N=N ry lT
1 1 6 H3C0 S H3C HO3S I/
S03H OH _ 503H
SO3H
SO3H SO3H OH
N HOZC
rN=N \ / N=N N SO3H
1 1 7 HO3S S N=N \ ry
HN H03S
~O S03H OH
S03H
H3C SO3H
SO3H
SO3H 0-/- OH
N ~
1 1 8 l N=ry \ / N=N `Y ,N SO3H
CI S / / N=N \ N
H3C H03S SO3H OH /
SO3H
S03H
S03H
~( OOH ~~^
1 1 9 Nr N=N-- il-N.N ~N SO3H
H3CO \ IS03H H3C -'' HO3S / NN \ N
SO3H OH / / S03H
S03H
rSO3H
SO3H 0- OH
HO2C
1 2 0 F1039 I Q l- N N N`N SO3H
H3C HO3S S03H OH
N02
SO3H
[Table 30]
CA 02803910 2012-12-24
86
Compound Structural formula
No
SO3H
SO3H 0-'--- OH CH3
1 2 1 l SYN-N \/ N-N I i N-N \ N SO3H
H03S H3C HO3S
SO3H OH N02
S03H
SO3H S03H OH
\ l N`}-N=N \ / N=N HOzC
N S03H
HOsS S N`N \ N
HN HO3S
1 2 2 HN) O SO3H OM S03H
S03H
SO3H
S03H SO3H OH \ /
N
I Sj N-N \ / N-3 N N \ N
1 2 3 H03S HN _0 HO3S
S03H OH S03H
HN S03H
SO3H
SO3H
SO3H O" OH
HOZCr
1 2 4 H03S 4)01
NH3 \/ HO S N`N N S03H
TTT
SO3H OH
S03H
SO3H
S03H
O OH
N HO'C
1 2 5 H03S & S H3 WN \ N I ~
rN_NS \ N=NN SO3H
SO3H OH i i S03H
SO3H
SO3H
S03H O-/-- OH
HO
1 2 6 NrN=N \/ N=N l\ N SO3H
CI S i N=N N
H3C HO35
S03FI OH i SO3H
SO3H
[Table 31]
CA 02803910 2012-12-24
87
Compound
N Structural formula
No,
SO3H
OH
1 2 7 ~ 1~ -N=N \ / N`N I HOC}~~
H02C S H3C H03S NN 7 % C02H
S03H OH
SO3H
S03H
HOBS N OH H O 1 2 $ lf }-N-N / N:N N
H3C0 S H3C H034 N-N"' N S03H
SO3H OH `SJ
S03H
503H
i I N?-N-N N-N 10~ I HO'
1 2 9 H03S S H3C H03S N-N NS03H
S03H OH
S03H
S03H
N 0 OH HOgC
1 3 0 -N
H03S S H3C H03S N N A COzH
S03H OH
S03H
SO3H
SO3H 0-/- OH
~ ^ HO2C
1 3 1 rN=N \ / N-N -`\ }SN
H C O3 ~ ~ NzNT-N,,,~~COyH
3 S03H OH II
SO3H
SO3H
S03H OH
1 3 2 H3CO2S S i H3C N=N N S03H
H03S
SO3H OH I;
SOuH
[Table 32]
CA 02803910 2012-12-24
88
Compound Structural formula
No.
SO3H
O-'-- OH /
N
1 3 3 : I rN=N \/ N=N I ~N
HOzC S i N=N C02H
H3C H03 S03H OH
SO3H
S03H
SO 3H OJ OH /
1 3 4 I SrNN ":N- I i N='n, SO3H
H3COzS H3C HO3S
SO3H OH d
S03H
SO3H
S03H 0-/- OH
HOzC
1 3 6 NrN-N \ / N'N IN
CI S N:N SO3H
H3C H03S 5O3H OH I i
SO3H
SO3H
SO3H 0-/- OH
Iry HOZC}}--
1 3 6 H03S SrN=N \/ N'N N=N-t\.j.N COzH
H3C H035 S03H lOH i
SO3H
S03H
0-/-- OH
ry HOC
2
1 3 7 N-N \/ N=N I
H3C0 N=NSO3H
S03H H3C HOS 1"
S03H OH i
S03H
SO3H
S03H O' OH
HOzc
1 3 8 I SrN:N N 1 N \ \ N:NL S03H
H03S H3C H03S I
S03H OH
SO3H
[Table 33]
CA 02803910 2012-12-24
89
Compound Structural formula
No.
rS03H
SOuH OJ OH
I Nr-N'N ~ / N:N I `,N
1 3 9 H03S N=N SO3H
H3C H03S 503H OH I
SO3H
rS03H
S03H O- OH
i I
14 )-WN
0 I ~p3S SN=N C02H
H3C HO35
SO3H OH I
S03H
S03H
S03H OJ OH HO2C
1 4 1 HO3S / 15}-N=N / N=NN.NN .. CI
H3C H035 SOuH OH
S03H
S03H
SO3H OJ OH
N
142 Hp35 l ST'N N / N:N I\ i N=N ,~ CI
H3C H03S S03H OH ( i
SO3H
SO3H
SO3H O-f- OH H3C
1 4 3 CI S N=N N-N N SO3H
H3C H03S SO3H OH I i
SO3H
5O3H
/"- N ~-{ OJ OH H3Chh
F44 H035 N N=H3 N:N~\.N SO3H
H03 SO3H OH
i
SO3H
[Table 34]
CA 02803910 2012-12-24
Compound Structural formula
No.
SO3H
O-/-- OH
HO3S / N
1 4 5 I e- N`N N=N I
/ N=N S0 H
H3C0 H3C HO3S S03H 3
OH
SO3H
/- S03H
S03H OJ OH
N HO2C
rN.N \ / N'N 1 N
1 4 6 H3COA S H C HO S N=N S03H
a 3 S03H OH I /
SO3H
-~S03H
SO3H o-/ OH
H ,C
147 rN'N \/ N=N N
H3CO2S S S I / / N=N' SO3H
H3C HO3 503H OH
SO3H
S03H
HO 3S N 0 OH HO2C
148 ) N'N \ / N=N \ \N
C2Hs0 S / / N-N \ N SO3H
H3C HO3S
SO3H OH 11/1
S03H
-- SO3H
O OH
HO2C~
1 4 9 C2H50 I Sr N.N NN I N'N-{~ SO3H
S03H H3C HO3S S03H '11jO'""H
SO3H
SO3H
O-/-- OH
1 S 0 I N? N-N \/ N`N N
H3CO S / N=N \ N SO3H
SO3H H3C H03S 5O3H OH
SO3H
The mixing ratios of the coloring matter (I) and the
coloring matter (II) in the present invention are such that
the ratio of the coloring matter (I) is 10 to 40% by mass, and
the ratio of the coloring matter (II) is 10 to 80% by mass,
and preferably the ratio of the coloring matter (I) is 15 to
35% by mass, and the ratio of the coloring matter (II) is 20
to 80% by mass in the total mass of the coloring matters
contained in the ink composition.
The ink composition of the present invention may contain
CA 02803910 2012-12-24
91
other coloring matters having various colors in addition to
the coloring matters (I) and (II) for the purpose of subtly
adjusting neutral, high quality black hue with low chroma and
no color tone to more desirable hue and the like, to the
extent that it does not harm the effects obtained according to
the present invention.
In this case, coloring matters of other colors such as
black coloring matters having the other hues; yellow (for
example, C.I. Direct Yellow 34, C.I. Direct Yellow 58, C.I.
Direct Yellow 86, C.I. Direct Yellow 132, C.I. Direct Yellow
142, C.I. Direct Yellow 161 and the like); orange (for example,
C.I. Direct Orange 17, C.I. Direct Orange 26, C.I. Direct
Orange 29, C.I. Direct Orange 39, C.I. Direct Orange 49 and
the like); brown; scarlet (for example, C.I. Direct Red 89 and
the like); red (for example, C.I. Direct Red 62, C.I. Direct
Red 75, C.I. Direct Red 79, C.I. Direct Red 80, C.I. Direct
Red 84, C.I. Direct Red 225, C.I. Direct Red 226 and the like),
magenta (for example, C.I. Direct Red 227, C.I. Acid Red 249,
C.I. Acid Red 254 and the like); blue (for example, C.I. Acid
Blue 9, C.I. Acid Blue 83, C.I. Acid Blue 90, C.I. Acid Blue
249, C.I. Direct Blue 86, C.I. Direct Blue 87, C.I. Direct
Blue 199 and the like); violet; navy; cyan; green, black (for
example, C.I. Food Black 2, C.I. Direct Black 19 and the like);
can be incorporated and used singly or as mixtures.
Furthermore, examples of the other coloring matters
described above that can be used include the compounds
described in Japanese Unexamined Patent Application,
CA 02803910 2012-12-24
92
Publication No. 2003-201412, the compounds described in PCT
International Publication No. W02005/054374, the compounds
described in PCT International Publication No. W02005/097912,
the compounds described in PCT International Publication No.
W02006/051850, and the like. For example, examples include the
compounds described in Tables 1 to 3 of Japanese Unexamined
Patent Application, Publication No. 2003-201412, the compounds
described in Tables 2 to 5 of PCT International Publication No.
WO 2005/054374, the compounds described in Tables 2 to 5 of
PCT International Publication No. W02005/097912, the compounds
described in Tables 2 to 4 of PCT International Publication No.
W02006/051850, and the like.
When these coloring matters are contained in the ink
composition of the present invention, it is difficult to
decide the content sweepingly. For the criteria, in the case
where coloring matters having the hues of yellow to red are
used, they are preferably used within a range of the content
ratio of the coloring matter (I) described above, and in the
case where coloring matters having the hues of cyan to black
are used, they are preferably used within a range of the
content ratio of the coloring matter (II) described above.
A salt of the compounds represented by the formula (1) to
the formula (4) or a tautomer thereof is a salt with an
inorganic or organic cation. Among them, specific examples of
the inorganic salts include alkaline metal salts, alkaline
earth metal salts, and ammonium salts. Examples of preferred
inorganic salts include respective salts with lithium, sodium
CA 02803910 2012-12-24
93
and potassium, and ammonium salts. On the other hand, examples
of the salt with organic cations include, for example, but not
limited to, salts with quaternary ammonium represented by the
following formula (8) . Furthermore, free acid, a tautomer
thereof, and various salts thereof may be in a mixture. For
example, any combinations may be used such as a mixture of a
sodium salt and an ammonium salt, a mixture of a free acid and
a sodium salt, and a mixture of lithium salt, a sodium salt,
and an ammonium salt. In some cases, the properties of
respective compounds such as solubility may vary depending on
the type of the salt. Thus, it is possible to obtain a mixture
having properties that suit the purpose by appropriately
selecting the type of the salt as necessary; by changing the
ratios of salts if the system contains plural salts and the
like; or the like.
z
Z4-N'-ZZ (8)
Z3
In the formula (8) , Z1, Z2, Z3 and Z4 each independently
represent a group selected from the group consisting of a
hydrogen atom, an alkyl group, a hydroxyalkyl group, and a
hydroxyalkoxyalkyl group, and at least any one of them
represents a group other than a hydrogen atom.
Specific examples of the alkyl group of Z1, Z2, Z3 and Z4
for the formula (8) include methyl, ethyl, n-propyl, isopropyl,
n-butyl, isobutyl, sec-butyl, t-butyl and the like. Specific
examples of the hydroxyalkyl group include hydroxy-Cl-C4 alkyl
groups such as hydroxymethyl, hydroxyethyl, 3-hydroxypropyl,
CA 02803910 2012-12-24
94
2-hydroxypropyl, 4-hydroxybutyl, 3-hydroxybutyl and 2-
hydroxybutyl. Specific examples of the hydroxyalkoxyalkyl
group include hydroxy-Cl-C4 alkoxy-Cl-C4 alkyl groups such as
hydroxyethoxymethyl, 2-hydroxyethoxyethyl, 3-
hydroxyethoxypropyl, 2-hydroxyethoxypropyl, 4-
hydroxyethoxybutyl, 3-hydroxyethoxybutyl and 2-
hydroxyethoxybutyl. Among these, hydroxyethoxy-Cl-C4 alkyl is
preferred. Particularly preferred examples include a hydrogen
atom; methyl; hydroxy-Cl-C4 alkyl groups such as hydroxymethyl,
hydroxyethyl, 3-hydroxypropyl, 2-hydroxypropyl, 4-hydroxybutyl,
3-hydroxybutyl and 2-hydroxybutyl; and hydroxyethoxy-Cl-C4
alkyl groups such as hydroxyethoxymethyl, 2-hydroxyethoxyethyl,
3-hydroxyethoxypropyl, 2-hydroxyethoxypropyl, 4-
hydroxyethoxybutyl, 3-hydroxyethoxybutyl and 2-
hydroxyethoxybutyl.
Specific examples of the combinations of Z1, Z2, Z3, and
z 4 for preferred compounds of the formula (8) are presented in
the following Table 35.
[Table 35]
Compound No. Z 1 Z2 Z 3 Z
1-1 H CH3 CH3 CH3
........... ............ .......- . . ...........................
1-2 CH3 CH3 CH3 CH3
................................................... .......... ............ 1-
3 H -C2H4OH -C2H40H C2K40H
1-4 CH3 -C2H4OH -C2H4OH -C2H40H
--=-------- -- ----- =. ------------._................--=---.......-=----- ----
--........---.......---
1.5 H -CH2CH(OH)CH3 -CH2CH(OH)CH3 -CH2CH(OH)CH3
----=------=----........ ....-=-------- =-- ------- ----------- -------...... -
--
1-6 CH3 -CH2CH(OH)CH3 -CH2CH(OH)CH3 -cl12CH(OH)CH3
....... ..-------- ...... .......... .-------- --------
1-7 H _ C2H40H H C2H40H
..... ..... . ..............................
1.8CH3 -C2H40H H -CZH4OH
...... ... -...-------==-- ==- -= ................=-......
]-.9 H -CH2CH(OH).CH3 H -CH2CH(OH)CH3
.......
1-10 CH3 -CH2CH(OH)CHi H -Ctt2CH(OH)CHI
.................. .--- ---------=-._.......---. ......... ........... ... --
1.11....._ CH3 -CzH40H .... .CH3 -C2H4OH
..
.. ........... ------ -
1-12 CH3 -CH2CH(OH)CH3 CH3 -CH2CH(OH)CH3
CA 02803910 2012-12-24
A method of synthesizing respective compounds represented
by the formula (1) to the formula (4) will be described.
The compounds represented by the formula (1), the formula
(2), and the formula (4) can be synthesized by, for example, a
method such as described below. Meanwhile, the structural
formulae of the compounds given in each step are expressed in
the form of a free acid, and R' to R8 and X that are
appropriately used in the following formulae (9) to (24) have
the same meanings as Rl to R8 and X, respectively, as defined
for the formula (1), the formula (2), and the formula (4).
First, a compound represented by the following formula (9)
is diazotized by a routine method, and this product and a
compound represented by the following formula (10) are
subjected to a coupling reaction by a routine method.
Thus, a compound represented by the following formula (11)
is obtained. As another synthesis method for the compound
represented by the formula (11), the following method may be
used. Specifically, a compound represented by the following
formula (9) is diazotized by a routine method, and this
product and a methyl--sulfonic acid derivative of aniline are
subjected to a coupling reaction by a routine method and then
to hydrolysis under alkaline conditions. Thus, a compound
represented by the following formula (12) is obtained. The
compound represented by the formula (12) thus obtained is
sulfonated by treating the compound with fuming sulfuric acid
or the like. Thus, a compound represented by the formula (11)
CA 02803910 2012-12-24
96
can be obtained. Furthermore, among the compounds represented
by the formula (11), there are also products available as
commercial products (for example, C.I. Acid yellow 9).
/ \ NH2 (9)
H03S- =
S03H
J_NH2 (10)
SO3H
`~-N=NNH2 (11)
H03S
H%S NUJ -NFIz (12)
Subsequently, the compound represented by the formula
(11) thus obtained is diazotized by a routine method, and then
this product and a compound represented by the following
formula (13) are subjected to a coupling reaction by a routine
method. Thus, a compound represented by the following formula
(14) is obtained.
R
~j NMt (13)
RS
R1
SQ3H
N}N-NON-J/ (14)
HQ3S
R5
On the other hand, the compound represented by the
formula (11) is diazotized by a routine method, and then this
product and a compound represented by the following formula
(15) are subjected to a coupling reaction by a routine method.
CA 02803910 2012-12-24
97
Thus, a compound represented by the following formula (16) is
obtained.
R2
(15)
H2N
-110-1
RB
R2
HO3S
HzN N--N-N (16)
S03H
R6
In the same manner, the compound represented by the
formula (11) is diazotized by a routine method, and then this
product and a compound represented by the following formula
(17) are subjected to a coupling reaction by a routine method.
Thus, a compound represented by the following formula (18) is
obtained.
R7
0"Hz (1 7)
R3
R7
HO3S
N-N- -N-N NH2 (18)
O3H R3
In the same manner, the compound represented by the
following formula (11) is diazotized by a routine method, and
then this product and a compound represented by the following
formula (19) are subjected to a coupling reaction by a routine
method. Thus, a compound represented by the following formula
(20) is obtained.
CA 02803910 2012-12-24
98
Rs
H2N-{? (19)
r
S03 H
/ N N - (20)
H2N ~I} H OS
R4
Then, the compound represented by the formula (14) thus
obtained is subjected to a condensation reaction with a
cyanuric halide, for example, cyanuric chloride, by a routine
method, and thus a compound represented by the following
formula (21) is obtained.
R'
SO3H f
HQSS'IT--F -6-N 1- I ~}- V N N N CI (21)
R5 Y
CI
Subsequently, the compound represented by the formula
(21) thus obtained is subjected to a condensation reaction
with the compound represented by the formula (16) by a routine
method, and thus a compound represented by the following
formula (22) is obtained.
R1 R2
HOgS~-- 3 w I/ N N N- I N N3 -N;V- . ~9H (22)
R5 i~)
NIN RB
CI
In the same manner, the compound represented by the
formula (18) thus obtained is subjected to a condensation
reaction with a cyanuric halide, for example, cyanuric
chloride, by a routine method, and thus a compound represented
by the following formula (23) is obtained.
CA 02803910 2012-12-24
99
Ry
S03H
N=N N N N -Y
H035 R3 TN (23)
Subsequently, the compound represented by the formula
(23) thus obtained is subjected to a condensation reaction
with the compound represented by the formula (20) by a routine
method, and thus a compound represented by the following
formula (24) is obtained.
RV CI Re
N ~ S03H
H N-N NTN-(~, /, H~N"T, WN _WN J (24)
503H R3 R4 H03$
The compound represented by the formula (22), the
compound represented by the formula (24) thus obtained, and a
compound represented by the following formula (25), which
corresponds to the crosslinking group X, are subjected to a
condensation reaction by a routine method. Thus, the compound
represented by the formula (1) can be obtained.
H-X-H (25)
The diazotization of the compound represented by the
formula (9) is carried out by a method that is known per se.
For example, the diazotization is carried out in an inorganic
acid medium at a temperature of, for example, -5 C to 30 C,
and preferably 0 C to 20 C, using a nitrous acid salt, for
example, a nitrous acid alkali metal salt such as sodium
nitrite.
The coupling reaction between a diazotization product of
CA 02803910 2012-12-24
100
a compound represented by the formula (9) and a compound
represented by the formula (10) is also carried out under
reaction conditions that are known per se. For example, it is
advantageous to carry out the reaction in water or an aqueous
organic medium at a temperature of 0 C to 30 C, and preferably
C to 25 C, and at a pH of an acidic to weakly acidic value,
for example, at pH 1 to 6. The diazotization reaction liquid
is acidic, and with the progress of the coupling reaction, the
reaction system is even further acidified. Therefore, it is
preferable to adjust the reaction liquid to the pH value
described above by adding a base. Examples of the base that
can be used include alkali metal hydroxides such as lithium
hydroxide and sodium hydroxide; alkali metal carbonates such
as lithium carbonate, sodium carbonate, and potassium
carbonate; acetates such as sodium acetate; ammonia or organic
amines. The compound represented by the formula (9) and the
compound represented by the formula (10) are used in nearly
stoichiometric amounts.
The diazotization of the compound represented by the
formula (11) is carried out by a method that is known per se.
For example, the diazotization is carried out in an inorganic
acid medium at a temperature of, for example, -5 C to 30 C,
and preferably 0 C to 25 C, using a nitrous acid salt, for
example, a nitric acid alkali metal salt such as sodium
nitrite.
The coupling reaction between a diazotization product of
a compound represented by the formula (11) and a compound
CA 02803910 2012-12-24
101
represented by the formula (13), formula (15), formula (17) or
formula (19) is also carried out under reaction conditions
that are known per se. For example, it is advantageous to
carry out the reaction in water or an aqueous organic medium
at a temperature of 0 C to 30 C, and preferably 5 C to 25 C,
and at a pH of an acidic to weakly acidic value, for example,
at pH 1 to 6. The diazotization reaction liquid is acidic, and
with the progress of the coupling reaction, the reaction
system is even further acidified. Therefore, it is preferable
to adjust the reaction liquid to the pH value described above
by adding a base. As the base, the same compounds as those
described above can be used. The compound represented by the
formula (11) and the compound represented by the formula (13),
the formula (15), the formula (17), or the formula (19) are
used in nearly stoichiometric amounts.
The condensation reaction between a compound represented
by the formula (14) or the formula (18) and a cyanuric halide,
for example, cyanuric chloride is carried out by a method that
is known per se. For example, it is advantageous to carry out
the reaction in water or an aqueous organic medium at a
temperature of 0 C to 30 C, and preferably 5 C to 25 C, and at
a pH of a weakly acidic to neutral value, for example, at pH 3
to 8. With the progress of the reaction, the reaction system
is acidified, and therefore, it is preferable to adjust the
system to the pH value described above by adding a base. As
the base, the same compounds as those described above can be
used. The compound represented by the formula (14) or (18) and
CA 02803910 2012-12-24
102
cyanuric halide are used in nearly stoichiometric amounts.
The condensation reaction between a compound represented
by the formula (16) and a compound represented by the formula
(21), or the condensation reaction between a compound
represented by the formula (20) and a compound represented by
the formula (23) is carried out by a method that is known per
se. For example, it is advantageous to carry out the reaction
in water or an aqueous organic medium at a temperature of 10 C
to 80 C, and preferably 25 C to 70 C, and at a pH of a weakly
acidic to weakly alkaline value, for example, at pH 5 to 9.
The adjustment of the pH value is carried out by adding a base.
As the base, the same compounds as those described above can
be used. The compound represented by the formula (16) and the
compound represented by the formula (21), or the compound
represented by the formula (20) and the compound represented
by the formula (23) are used in nearly stoichiometric amounts.
The condensation reaction of the compound represented by
the formula (22) and the compound represented by the formula
(24) and the compound represented by the formula (25) is
carried out by a method that is known per se. For example, it
is advantageous to carry out the reaction in water or an
aqueous organic medium at a temperature of 50 C to 100 C, and
preferably 60 C to 95 C, and at a pH of a neutral to weakly
alkaline value, for example, at pH 7 to 10. The adjustment of
the pH value is carried out by adding a base. As the base, the
same compounds as those described above can be used. The
compound represented by formula (25) is used in an amount of
CA 02803910 2012-12-24
103
0.4 to 0.6 equivalents, and preferably 0.5 equivalents,
relative to one equivalent of the compound represented by
formula (22) and one equivalent of the compound represented by
formula (24).
The compound represented by the formula (3) can be easily
synthesized by a person having ordinary skill in the art by
appropriately selecting synthetic raw materials in accordance
with the method described in Japanese Unexamined Patent
Application, Publication No. 2009-84346, or a similar method
thereto.
Examples of the method of synthesizing a desired salt of
the compounds represented by the formulae (1) to (4) include a
method of adding, after completion of the final step in the
synthesis reaction for the respective compounds, a desired
inorganic salt or a desired organic cation salt to the
reaction liquid, and salting out; or a method of adding a
mineral acid such as hydrochloric acid to the reaction liquid
to isolate the compounds in the form of free acid from the
reaction liquid, subsequently washing the free acid thus
obtained with water, acidic water, an aqueous organic medium
or the like as necessary, to remove inorganic salts, and then
neutralizing the free acid in an aqueous medium by means of a
desired inorganic or organic base. Through such methods, a
desired salt of the compound can be obtained in the form of a
solid of corresponding salt or a solution thereof. Here, the
term acidic water means, for example, a solution prepared by
dissolving a mineral acid such as sulfuric acid or
CA 02803910 2012-12-24
104
hydrochloric acid, or an organic acid such as acetic acid in
water, and acidifying the water. Further, examples of the
aqueous organic medium include a mixture of water with an
organic substance that is miscible with water, or so-called an
organic solvent that is miscible with water (specific examples
thereof include water-soluble organic solvents and the like
that will be described below), and the like. Examples of the
inorganic salt include alkaline metal salts such as lithium
chloride, sodium chloride and potassium chloride; ammonium
salts such as ammonium chloride and ammonium bromide; and the
like. Examples of the organic cation salt include halide salts
of the quaternary ammonium represented by the formula (8) and
the like. Examples of the inorganic base include alkaline
metal hydroxides such as lithium hydroxide, sodium hydroxide
and potassium hydroxide; ammonium hydroxide (aqueous ammonia);
alkaline metal carbonates such as lithium carbonate, sodium
carbonate and potassium carbonate; and the like. Examples of
the organic base include, but are not limited to, organic
amines such as diethanolamine and triethanolamine; hydroxides
or halides of the quaternary ammonium represented by the
formula (8); and the like.
The ink composition of the present invention will be
described.
The respective reaction liquids after completion of the
final step in the respective synthesis reactions for the
compounds represented by the formulae (1) to (4) can be
directly used in the preparation of the ink composition of the
CA 02803910 2012-12-24
105
present invention. Furthermore, the respective compounds can
be isolated from the reaction liquid by methods such as, for
example, drying, for example, spray-drying the reaction liquid
containing the respective coloring matters individually first;
adding inorganic salts such as sodium chloride, potassium
chloride, calcium chloride and sodium sulfate, and salting out;
adding mineral acids such as hydrochloric acid, sulfuric acid
and nitric acid, and acid-precipitating; acid salting out by
combining the salting out and the acid-precipitation; and the
like, and these respective compounds can be mixed whereby to
prepare an ink composition.
Preferred coloring matters as the coloring matter (I)
contained in the ink composition of the present invention are
the compounds exemplified as the preferred ones in the
respective compounds represented by the formulae (1), (2) and
(4). The same applies also to more preferred coloring matters
and the like. Furthermore, preferred coloring matters as the
coloring matter (II) are similarly the compounds exemplified
as preferred ones in the compound represented by the formula
(3). The same applies also to more preferred coloring matter
and the like. Furthermore, an ink composition containing the
preferred compounds as the coloring matter (I) and the
coloring matter (II) is more preferred, and an ink composition
containing the more preferred compounds is further preferred.
The same applies also to an ink composition containing the
preferred compounds and more preferred compounds, and the like.
Examples of preferred combinations of the coloring matter
CA 02803910 2012-12-24
106
(I) and the coloring matter (II) include combinations of the
compounds represented by No. 59 or No. 61 as described in
Table 18 as the coloring matter (I), and the compound
represented by No. 79 as described in Table 23, the compound
represented by No. 87 as described in Table 24, the compounds
represented by No. 104 or No. 108 as described in Table 27,
the compound represented by No. 128 as described in Table 31,
or the compound represented by No. 137 as described in Table
32 as the coloring matter (II) . The ink composition of the
present invention containing this combination of the coloring
matters is preferred one as the ink composition of the present
invention.
The ratio of the coloring matter (I) is 10 to 40% by mass,
preferably 15 to 35% by mass, and the ratio of the coloring
matter (II) is 20 to 80% by mass in the total mass of the
coloring matters contained in the ink composition of the
present invention.
Furthermore, the sum of the masses of the coloring matter
(I) and the coloring matter (II) contained in the ink
composition of the present invention is usually 0.1 to 20% by
mass, preferably 1 to 10% by mass, and more preferably 2 to 8%
by mass, relative to the total mass of the ink composition.
The ink composition of the present invention is prepared
by using water as a medium, so that the ink composition may
contain a water-soluble organic solvent if necessary, to the
extent that the effect of the present invention is not
impaired. The water-soluble organic solvent is used for the
CA 02803910 2012-12-24
107
purpose of obtaining effects such as the dissolution of dyes,
prevention of drying (maintenance of a wetted state),
adjustment of viscosity, acceleration of penetration,
adjustment of the surface tension, and defoaming in the ink
composition of the present invention, and thus it is
preferable that the water-soluble organic solvent be included
in the ink composition of the present invention.
Examples of the ink preparation agents include known
additives such as a preservative and fungicide, a pH adjusting
agent, a chelating reagent, a rust-preventive agent, an
ultraviolet ray absorbing agent, a water-soluble polymer
compound, a coloring matter solubilizer, a surfactant, and an
oxidation-preventive agent (a fading-preventive agent).
The content of the water-soluble organic solvent is 0% to
60% by mass, and preferably 10% to 50% by mass, relative to
the total mass of the ink composition of the present invention,
and it is desirable to use the ink formulating agents
similarly in an amount of 0% to 20% by mass, and preferably 0%
to 15% by mass. The balance other than the components
described above is water.
The pH of the ink composition of the present invention is
preferably pH 5 to 11, and more preferably pH 7 to 10 for the
purpose of enhancing the storage stability. Furthermore, the
surface tension of the ink composition is preferably 25 to 70
mN/m, and more preferably 25 to 60 mN/m. In addition, the
viscosity of the ink composition is preferably 30 mPa=s or
lower, and more preferably 20 mPa=s or lower. The pH and the
CA 02803910 2012-12-24
108
surface tension of the ink composition of the present
invention can be appropriately adjusted with the pH adjusting
agent and the surfactant as described below.
In the case where the ink composition of the present
invention is used as an ink for ink jet recording, it is
preferable to use ink compositions having smaller contents of
inorganic impurities such as chlorides of metal cations (for
example, sodium chloride) and sulfates (for example, sodium
sulfate) in respective coloring matters (specifically, the
respective compounds represented by the formulae (1) to (4))
contained in the ink composition of the present invention. The
criteria for the content of the inorganic impurities is
generally about 1% or lower by mass, relative to the total
mass of the coloring matters, and the lower limit may be equal
to or lower than the detection limit of the detecting
instrument, that is, 0%. As a method of producing the compound
with less inorganic impurities, for example, a desalting
treatment may be carried out by an ordinary method of using a
reverse osmosis membrane; a method of stirring a dried product
or a wet cake of a coloring matter in a mixed solvent of Cl-C4
alcohol such as methanol and water, and filtering and
isolating the precipitate, and drying the product; a method of
using an ion exchange resin; and the like.
Specific examples of the water-soluble organic solvent
include Cl-C4 alkanols such as methanol, ethanol, propanol,
isopropanol, butanol isobutanol, secondary butanol and
tertiary butanol; carboxylic amides such as N,N-
CA 02803910 2012-12-24
109
dimethylformamide and N,N-dimethylacetamide; lactam such as 2-
pyrrolidone, N-methyl-2-pyrrolidone, N-methylpyrrolidin-2-one;
cyclic ureas such as 1,3-dimethylimidazolidin-2-one and 1,3-
dimethylhexahydropyrimid-2-one; ketones or keto alcohols such
as acetone, methyl ethyl ketone, and 2-methyl-2-hydroxypentan-
4-one; cyclic ethers such as tetrahydrofuran and dioxane;
mono-, oligo- or polyalkylene glycols or thioglycols having a
C2-C6 alkylene unit, such as ethylene glycol, 1,2-propylene
glycol, 1,3-propylene glycol, 1,2-butylene glycol, 1,4-
butylene glycol, 1,6-hexylene glycol, diethylene glycol,
triethylene glycol, tetraethylene glycol, dipropylene glycol,
polyethylene glycol, polypropylene glycol, thiodiglycol and
dithiodiglycol; polyols (triols) such as trimethylolpropane,
glycerin and hexane-1,2,6-triol; Cl-C4 alkyl ethers of
polyhydric alcohols, such as ethylene glycol monomethyl ether,
ethylene glycol monoethyl ether, diethylene glycol monomethyl
ether, diethylene glycol monoethyl ether, diethylene glycol
monobutyl ether (butyl carbitol), triethylene glycol
monomethyl ether, and triethylene glycol monoethyl ether;
lactones such as y-butyrolactone; and sulfoxide such as
dimethyl sulfoxide. These water-soluble organic solvents may
be used alone, or in combination of two kinds or more.
Among these, isopropanol, N-methyl-2-pyrrolidone,
glycerin, butylcarbitol and the like are preferred.
Meanwhile, the water-soluble organic solvent described
above also includes a substance that is solid at normal
temperature such as trimethylolpropane. However, the substance
CA 02803910 2012-12-24
110
and the like exhibits water-solubility as a solid, and further
an aqueous solution containing the substance and the like
exhibits similar properties to those of a water-soluble
organic solvent, and can be used for the same purpose. For
this reason, such solid substance is encompassed in the
category of the water-soluble organic solvents in the present
specification for convenience as long as it can be used for
the same purpose described above.
Specific examples of the fungicide include dehydrosodium
acetate, benzoate sodium, sodium pyridine thione-l-oxide, p-
hydroxybenzoate ethyl ester, 1,2-benzisothiazolin-3-one and a
salt thereof and the like.
Specific examples of the preservative include a compound
of organic sulfur based, organic nitrogen sulfur based,
organic halogen based, haloallyl sulfone based, iodopropargyl
based, haloalkylthio based, nitrile based, pyridine based, 8-
oxyquinoline based, benzothiazole based, isothiazoline based,
dithiol based, pyridineoxide based, nitropropane based,
organic tin based, phenol based, quaternary ammonium salt
based, triazine based, triazine based, anilide based,
adamantane based, dithiocarbamate based, brominated indanone
based, benzylbromoacetate based, inorganic salt based or the
like.
Specific examples of the organic halogen based compound
include, for example, sodium pentachlorophenol. Specific
examples of the pyridineoxide based compound include, for
example, sodium 2-pyridinethiol-l-oxide. Specific examples of
CA 02803910 2012-12-24
111
the isothiazoline based compound include, for example, 1,2-
benzisothiazolin-3-one, 2-n-octyl-4-isothiazolin-3-one, 5-
chloro-2-methyl-4-isothiazolin-3-one, 5-chloro-2-methyl-4-
isothiazolin-3-one magnesiumchloride, 5-chloro-2-methyl-4-
isothiazolin-3-one calciumchloride, 2-methyl-4-isothiazolin-3-
one calciumchloride, and the like. Specific examples of the
other preservative fungicide include anhydrous sodium acetate,
sodium sorbate, sodium benzoate, or (trade name) ProxelRTM GXL
(S) and ProxelRTM XL-2 (S) manufactured by Arch Chemical, Inc.,
and the like.
As used herein, the superscript notation of "RTM" means a
registered trademark.
As the pH adjusting agent, an arbitrary substance can be
used as long as the pH of the ink can be controlled to fall
within the range of, for example, 5 to 11 without bad
influences on the ink prepared. Specific examples thereof
include alkanol amines such as diethanolamine, triethanolamine
and N-methyldiethanolamine; alkaline metal hydroxides such as
lithium hydroxide, sodium hydroxide and potassium hydroxide;
ammonium hydroxide (aqueous ammonia); alkaline metal
carbonates such as lithium carbonate, sodium carbonate, sodium
hydrocarbonate and potassium carbonate; alkaline metal salts
of an organic acid such as sodium silicate and potassium
acetate; inorganic bases such as disodium phosphate;
aminosulfonic acids such as taurine; and the like.
Specific examples of the chelating reagent include
disodium ethylenediamine tetraacetate, sodium nitrilo
CA 02803910 2012-12-24
112
triacetate, sodium hydroxyethylethylenediamine triacetate,
sodium diethylenetriamine pentaacetate, sodium uracil
diacetate and the like.
Specific examples of the rust-preventive agent include
acidic sulfite, sodium thiosulfate, ammonium thioglycolate,
diisopropylammonium nitrite, pentaerythritol tetranitrate,
dicyclohexylammonium nitrite, and the like.
Specific examples of the ultraviolet ray absorbing agent
include those water-soluble such as sulfonated benzophenone
based compounds, benzotriazole based compounds, salicylic acid
based compounds, cinnamic acid based compounds and triazine
based compound.
Specific examples of the water-soluble polymer compound
include polyvinyl alcohols, cellulose derivatives, polyamine,
polyimine and the like.
Specific examples of the coloring matter solubilizer
include -caprolactam, ethylene carbonate, urea and the like.
As the oxidation-preventive agent, various organic based
and metal complex based discoloration-preventive agent can be
used. Specific examples of the fading-preventive agent include
hydroquinones, alkoxy phenols, dialkoxy phenols, phenols,
anilines, amines, indanes, chromanes, alkoxy anilines,
heterocycles and the like.
Specific examples of the surfactant include known
surfactants such as anionic based surfactants, cationic based
surfactants and nonionic based surfactants.
Examples of the anionic surfactant include alkyl sulfonic
CA 02803910 2012-12-24
113
acid salts, alkylcarboxylic acid salts, a-olefinsulfonic acid
salts, polyoxyethylenealkyl ether acetic acid salts, N-
acylamino acid and salts thereof, N-acylmethyltaurine salts,
alkylsulfate polyoxyalkyl ether sulfuric acid salts,
alkylsulfate polyoxyethylenealkyl ether phosphoric acid salts,
rosin acid soap, castor oil sulfate ester salts, lauryl
alcohol sulfate ester salts, alkylphenolic phosphate esters,
alkylated phosphate esters, alkylarylsulfonic acid salts,
diethyl sulfosuccinic acid salts, diethylhexyl sulfosuccinic
acid salts, dioctyl sulfosuccinic acid salts, and the like.
Examples of the cationic surfactant include 2-
vinylpyridine derivatives, poly(4-vinylpyridine) derivatives,
and the like.
Examples of the amphoteric surfactant include
lauryldimethylamino acetate betaine, 2-alkyl-N-carboxymethyl-
N-hydroxyethylimidazolinium betaine, coconut oil fatty acid
amide propyldimethylamino acetate betaine,
polyoctylpolyaminoethylglycine, imidazoline derivatives, and
the like.
Examples of the nonionic surfactant include: ether based
surfactants such as polyoxyethylene nonylphenyl ether,
polyoxyethylene octylphenyl ether, polyoxyethylene dodecyl
phenyl ether, polyoxyethylene oleyl ether, polyoxyethylene
lauryl ether and polyoxyethylene alkyl ether; ester based
surfactants such as polyoxyethylene oleate esters,
polyoxyethylene distearate esters, sorbitan laurate, sorbitan
monostearate, sorbitan monooleate, sorbitan sesquioleate,
CA 02803910 2012-12-24
114
polyoxyethylene monooleate and polyoxyethylene stearate;
acetylene alcohol based surfactants such as 2,4,7,9-
tetramethyl-5-decyne-4,7-diol, 3,6-dimethyl-4-octyne-3,6-diol
and 3,5-dimethyl-l-hexyn-3-ol; and the like. Specific examples
of commercially available products include (trade name)
SurfynolRTM 104, 105, 82 and 465; and OlfineRTM STG, all
manufactured by Nissin Chemical Industry Co., Ltd.
These ink preparation agents are used singly or as
mixtures.
For the preparation of the ink composition of the present
invention, there are no particular limitations on the order of
dissolving respective agents such as additives. The water used
at the time of preparing the ink composition is preferably
water with low impurities, such as ion-exchanged water or
distilled water. Furthermore, if necessary, any contaminants
in the ink composition may be removed by performing precision
filtration using a membrane filter and the like after the
preparation of the ink composition. Particularly, in the case
of using the ink composition of the present invention as an
ink for ink jet recording, it is preferable to perform
precision filtration. The pore size of the filter that is used
to perform precision filtration is usually 1 pm to 0.1 pm, and
preferably 0.8 pm to 0.1 pm.
The ink composition of the present invention is suitable
for use in printing, copying, marking, writing, drawing,
stamping or recording (printing), particularly ink jet
recording. Furthermore, the ink composition of the present
CA 02803910 2012-12-24
115
invention is such that solid precipitation does not easily
occur even against drying in the vicinity of the nozzles of
the recording head of ink jet printers, and for this reason,
blockage of the recording head also does not easily occur.
The ink jet recording method of the present invention
will be described. The ink jet recording method of the present
invention is a method of using the ink composition of the
present invention as an ink and discharging ink droplets of
the ink in response to recording signals, and thus attaching
the ink droplets onto a record-receiving material whereby to
perform recording. The ink nozzles and the like that are used
in the recording are not particularly limited, and can be
appropriately selected in accordance with the purpose.
Examples of the recording method that may be adopted
include known respective systems, for example, a charge
control system which utilizes electrostatic attraction to
discharge an ink; a drop-on-demand system (pressure pulse
system) which utilizes vibration pressure of a piezo device;
an acoustic ink jet system which changes an electric signal to
acoustic beam and irradiates an ink with the acoustic beam,
and utilizes the radiation pressure to discharge the ink; a
thermal inkjet, specifically Bubblejet (registered trademark)
system which forms bubbles by heating an ink, and utilizes
generated pressure; and the like.
Meanwhile, the ink jet recording method also encompasses
a system which injects an ink having low density of coloring
matters (the content of the coloring matters) in the ink,
CA 02803910 2012-12-24
116
which is called a photo ink, in a large number with a small
volume; a system which utilizes multiple inks having different
densities of coloring matters in an ink with a substantially
identical hue to improve the image quality; a system which
utilizes a colorless transparent ink; and the like.
The colored body of the present invention is a colored
substance by means of
a) the ink composition of the present invention according to
any one of the first to ninth aspects described above, or
b) the ink jet recording method of the present invention
according to any one of the tenth to twelfth aspects described
above, and preferably a substance colored by means of the ink
jet recording method of the present invention using the ink
composition of the present invention.
The substance is preferably the following record-
receiving materials.
In regard to the record-receiving material that can be
colored, there are no particular limitations on the material.
Examples include communication sheets such as paper and films;
fabrics or clothes (cellulose, nylon, wool and the like),
leather, and materials for color filters. Among these,
communication sheets are preferred.
Preferred examples of the communication sheets include
surface-treated sheets, more specifically, sheets provided
with an ink-receiving layer on a base material such as a paper,
a synthetic paper or a film. The ink-receiving layer is
provided by, for example, a method of impregnating or coating
CA 02803910 2012-12-24
117
the base material with a cationic polymer; or a method of
coating an inorganic substance capable of absorbing the
coloring matter in the ink, such as porous silica, an alumina
sol or a special ceramic substance, together with a
hydrophilic polymer such as polyvinyl alcohol or
polyvinylpyrrolidone on the surface of the base material.
Those sheets provided with such an ink-receiving layer are
generally referred to as exclusive ink jet paper, exclusive
ink jet film, glossy papers, glossy films, and the like.
Among the communication sheets described above, a sheet
coated with porous white inorganic substances on the surface
particularly has high surface glossiness, and also excellent
water resistance, and thus is particularly suitable for
recording of photographic image quality. However, it is known
that recorded images on the sheet have increase of
discoloration by ozone gas. However, the ink composition of
the present invention is excellent in the ozone gas fastness,
and thus also exerts great effects even when an ink jet is
recorded on such record-receiving materials.
Representative examples of commercial products of the
sheet coated with porous white inorganic substances on the
surface described above include (trade name:) Photo Paper
Glossy Pro (platinum grade), Photo Paper Gloss Gold
manufactured by Canon, Inc.; Photo Paper CrispiaRTM (high
glossy), Photo Paper (glossy), and Photo Matte Paper
manufactured by Seiko Epson Corp.; (trade name:) Advanced
Photo Paper (glossy) manufactured by Hewlett-Packard Japan,
CA 02803910 2012-12-24
118
Ltd.; and (trade name:) Kassai Photo Finish Pro manufactured
by Fujifilm Corporation. However, the applications of the ink
composition of the present invention are not limited to these
exclusive paper and the like.
In addition to the exclusive paper described above,
examples of the record-receiving materials include plain
papers. The plain paper is those provided with the ink-
receiving layer described above. Examples of the commercial
products include plain papers exclusive for ink jet such as
(trade name:) GF-500, Canon Plain Paper White manufactured by
Canon, Inc.; and (trade name:) Two-side Fine Quality Plain
Paper manufactured by Seiko Epson Corp. Furthermore, examples
of those not exclusive for ink jet that may be used include
PPC (Plain Paper Copy) paper and the like.
In recording with the ink jet recording method of the
present invention on a record-receiving material such as a
communication sheet, for example, a container containing the
ink composition described above is loaded at a predetermined
position of an ink jet printer, and recording may be performed
on the record-receiving material with an ordinary recording
method.
The ink jet recording method of the present invention can
use, together with the ink composition of the present
invention, for example, a known ink composition of respective
colors such as a magenta ink, a cyan ink or a yellow ink, and
if necessary, a green ink, a blue ink (or a violet ink) and a
red ink (or an orange ink) in combination.
CA 02803910 2012-12-24
119
The ink compositions of respective colors are filled into
their respective containers, and the respective containers are
loaded onto a predetermined position of the ink jet printer,
similarly to the container containing the ink composition of
the present invention for use in the ink jet recording.
The respective compounds contained as the coloring matter
(I) and the coloring matter (II) in the ink composition of the
present invention, are easily synthesized and cheap.
Furthermore, the respective compounds have high solubility
with respect to an aqueous medium, and also have excellent
water solubility, and thus have satisfactory filterability
through membrane filters in the process of preparing an ink
composition.
The ink composition of the present invention or an ink
prepared from the ink composition has excellent storage
stability and discharge stability as well. Specifically, the
ink composition of the present invention has no solid
precipitation, no change of physical property, no change of
the hue and the like after storage for a long time, and has
satisfactory storage stability.
Furthermore, the ink composition of the present invention
is suitably used for ink jet recording, for writing tools and
the like. The ink composition of the present invention
exhibits neutral black to gray color without color tone in any
case of dark color printing and light color printing
particularly when recorded on exclusive ink jet paper, and has
less change of the hue even when recorded in a different
CA 02803910 2012-12-24
120
medium. Furthermore, the ink composition of the present
invention has very high print (printing) density of recorded
images, and causes no bronzing on the images even when printed
with a high density solution. In addition, the ink composition
of the present invention is also excellent in various fastness
properties such as moisture resistance and water resistance,
particularly light fastness and ozone gas fastness.
Furthermore, the ink composition of the present invention
is excellent in various fastness properties in combination
with another ink composition containing coloring matters of
magenta, cyan, and yellow, and allows full-color ink jet
recording that is excellent in storability. Further, the ink
composition of the present invention can also be positively
used in plain paper.
As described above, the ink composition of the present
invention is very useful as a black ink for ink jet recording.
EXAMPLES
Hereinafter, the present invention will be described more
specifically by way of Examples, but the present invention is
not intended to be limited by the following Examples.
The "parts" and "percent (o)" in the Examples are on a
mass basis, unless particularly stated otherwise. Furthermore,
the respective operations of the various synthesis reactions,
crystallization and the like were all carried out under
stirring. In the case where a desired amount of an intended
compound is not obtained with one time of a synthesis reaction,
CA 02803910 2012-12-24
121
the reaction was repeatedly carried out until the desired
amount was obtained.
Furthermore, in the following respective formula, the
functional groups such as a sulfo group and a carboxy group
are described in the form of a free acid for convenience.
Furthermore, all of the pH values and the reaction
temperatures described in Examples exhibit values measured in
the reaction system.
Furthermore, the maximal absorption wavelengths (Amax) of
the synthesized compounds were measured in an aqueous solution
at pH 5 to 8, and were results as diluted so as to give 0.5 to
1.5 of the absorbance when measured. In addition, in case of a
compound which has change of the absorption wavelength in the
visible light region occurs after the dilution, a value
measured after convergence of the change of the absorption
wavelength under a shield condition was described as the
maximal absorption wavelength.
Synthesis Example 1
(Step 1)
35.7 parts of a monoazo compound represented by the
following formula (26) (C.I. Acid Yellow 9) was added to 200
parts of water, and the monoazo compound was dissolved therein
while the system was adjusted to pH 6 with sodium hydroxide.
Subsequently, 7.2 parts of sodium nitrite was added thereto.
This solution was added dropwise over 30 minutes to 300 parts
of 5% hydrochloric acid maintained at 0 C to 10 C, and then
CA 02803910 2012-12-24
122
the mixture was stirred for one hour at or below 20 C to
perform a diazotization reaction. Thus, a diazo reaction
liquid was prepared.
SO3H
HO3S N-N--(,-( -NHZ (26)
Meanwhile, 10.7 parts of 3-methylaniline, 10.4 parts of
sodium hydrogen bisulfite, and 8.6 parts of a 35% aqueous
formalin solution were added to 260 parts of water, and a
methyl-w-sulfonate derivative was obtained by a routine method.
The aqueous solution of the methyl-w-sulfonate derivative
thus obtained was added to the diazo reaction liquid
previously prepared, and the mixture was allowed to react for
hours at 0 C to 15 C, while the system was adjusted to pH 4
to 5 by adding sodium hydrogen carbonate 100 parts of 35%
hydrochloric acid was added to the reaction liquid, and then
the mixture was allowed to react further for 5 hours at 70 C
to 80 C. Sodium chloride was added to the reaction liquid for
salting-out, and a solid precipitated therefrom was isolated
by filtration. Thus, 120 parts of a compound represented by
the following formula (27) was obtained as a wet cake.
SO3H
H033_aN-N_C -N=N- P--NH2 (27)
H3C
(Step 2)
35.7 parts of the monoazo compound represented by the
above formula (27) (C.I. Acid Yellow 9) was added to 200 parts
of water, and was dissolved therein while the system was
CA 02803910 2012-12-24
123
adjusted to pH 6 with sodium hydroxide. Subsequently, 7.2
parts of sodium nitrite was added thereto. This solution was
added dropwise over 30 minutes to an aqueous solution prepared
by diluting 31.3 parts of 35% hydrochloric acid with 200 parts
of water, while maintaining the system at 0 C to 10 C, and
then the mixture was stirred for one hour at or below 20 C to
perform a diazotization reaction. 0.4 parts of sulfamic acid
was added to the reaction liquid thus obtained, and the
resulting mixture was stirred for 5 minutes. Thus, a diazo
reaction liquid was prepared.
Meanwhile, 24.0 parts of a compound represented by the
following formula (28) obtained by the method described in
Japanese Unexamined Patent Application, Publication No. 2004-
083492, and a 25% aqueous solution of sodium hydroxide were
added to 300 parts of warm water at 40 C to 50 C, and the
mixture was adjusted to pH 5 to 6. Thus, an aqueous solution
was obtained. To this aqueous solution, the diazo reaction
liquid obtained as described above was added dropwise over 30
minutes at 15 C to 25 C. During the dropwise addition, the
system was maintained at pH 5 to 6 by adding an aqueous
solution of sodium carbonate. After the dropwise addition, the
mixture was stirred for 2 hours at the same temperature and at
the same pH, and then the mixture was adjusted to pH 0 to 1 by
adding 35% hydrochloric acid. The liquid thus obtained was
heated to 65 C, and was stirred for 2 hours at the same
temperature. Subsequently, the liquid was cooled to room
temperature, and a solid precipitated therefrom was isolated
CA 02803910 2012-12-24
124
by filtration. Thereby, 130 parts of a wet cake containing a
compound represented by the following formula (29) was
obtained.
H035
NH2 (28)
H3C~
HO3S-'-~
S03H
HO,S-aWN-- -N=N NH, (29)
H3C
(Step 3)
50 parts of the wet cake containing the compound
represented by formula (27) obtained in the (Step 1) was
dissolved in 300 parts of water by adjusting the system to pH
8 to 9 by adding a 25% aqueous solution of sodium hydroxide.
To this solution, 0.48 parts of (trade name:) LeocolRTM TD90
(surfactant, hereinafter simply referred to as "LeocolRTM
TD90") manufactured by Lion Corp. was added, and then 7.3
parts of cyanuric chloride was added thereto at 5 C to 10 C.
After the addition, the mixture was stirred for 6 hours at 5 C
to 10 C while the pH value was maintained at 6 to 7 by adding
an aqueous solution of sodium carbonate.
Meanwhile, 51 parts of the wet cake containing the
compound represented by formula (29) obtained in the (Step 2)
was dissolved in 150 parts of water by adjusting the system to
pH 7 to 8 by adding a 25% aqueous solution of sodium hydroxide.
CA 02803910 2012-12-24
125
Thus, a solution was obtained. This solution was added to the
reaction liquid described above, and then the resulting
mixture was heated to 65 C to 70 C. While the pH value was
maintained at 6 to 7 by adding an aqueous solution of sodium
carbonate, the mixture was stirred for 7 hours. Subsequently,
1.7 parts of piperazine was added thereto, and then the
resulting mixture was heated to 90 C to 95 C. While the pH
value was maintained at 7 to 8 by adding an aqueous solution
of sodium carbonate, the mixture was stirred for 18 hours.
The reaction liquid thus obtained was cooled to 20 C to
30 C, and then salting-out was carried out by adding sodium
chloride. A solid precipitated therefrom was isolated by
filtration, and thus a wet cake was obtained.
This wet cake was dissolved in 600 parts of water. To
this solution, 50 parts of methanol, and then 800 parts of 2-
propanol were added, and the mixture was stirred for 30
minutes. A solid precipitated therefrom was isolated by
filtration, and thereby a wet cake was obtained. The wet cake
thus obtained was dissolved again in 400 parts of water, and
1000 parts of 2-propanol was added thereto. A solid
precipitated therefrom was isolated by filtration and dried.
Thereby, 25.3 parts of an azo compound represented by the
following formula (31) (Amax: 435 nm) was obtained as a sodium
salt.
CA 02803910 2012-12-24
126
-"f--S03H
.(S03H HO3S
~- - l-- -S03H
HOIg .N_ J-N,N N--rN-rA~~N-N!!J
'~! N N
H3C CH3
C) (30)
N
H3C N N CH3
N+I-( -503H
H036 - N-N -IN NH~N' ~--NH dV--~~
s 9 H038
H03S
Synthesis Example 2
65 parts of the wet cake containing the compound
represented by formula (29) obtained in the (Step 2) of
Synthesis Example 1 was dissolved in 250 parts of water by
adjusting the system to pH 7 to 8 by adding a 251 aqueous
solution of sodium hydroxide. To this solution, LeocolRTM TD90
(0.10 parts) was added, and then 3.8 parts of cyanuric
chloride was added thereto at 15 C to 25 C. After the
addition, while the pH value was maintained at 5 to 6 by
adding an aqueous solution of sodium carbonate, the mixture
was stirred for 2 hours at 15 C to 25 C. Subsequently, this
reaction liquid was heated to 60 C to 65 C, and while the pH
value was maintained at 6 to 7 by adding an aqueous solution
of sodium carbonate, the reaction liquid was stirred for 5
hours.
Subsequently, 0.89 parts of piperazine was added thereto,
and then the resulting mixture was heated to 90 C to 95 C.
While the pH value was maintained at 8 to 9 by adding an
aqueous solution of sodium carbonate, the mixture was stirred
for 16 hours.
CA 02803910 2012-12-24
127
The reaction liquid thus obtained was cooled to 20 C to
30 C, and then salting-out was carried out by adding sodium
chloride. A solid precipitated therefrom was isolated by
filtration, and thus a wet cake was obtained. This wet cake
was dissolved in 400 parts of water. To this solution, 50
parts of methanol, and then 800 parts of 2-propanol were
added, and the resulting mixture was stirred for 30 minutes. A
solid precipitated therefrom was isolated by filtration, and
thereby, a wet cake was obtained. The wet cake thus obtained
was dissolved again in 200 parts of water, and 800 parts of 2-
propanol was added thereto. A solid precipitated therefrom was
isolated by filtration and dried. Thus, 13.5 parts of an azo
compound represented by the following formula (31) (Amax: 436
nm) was obtained as a sodium salt.
H03S0 __FS03H
603H H0 3S
H036-~-N-N -N~N-rNrMAN-N -N~1-O---503H
/ ~~--"" N N
H3C H3
CNN)
(3l)
H3C CH3
NN
N-N-4a-603H
HO36 N-N N-N -NH N NI ---
3H 0 H03S
H03S S03H
Synthesis Example 3
(Step 1)
5.0 parts of 2-amino-6-methoxybenzothiazole was slowly
added to 16 parts of 15% fuming sulfuric acid at 15 C to 25 C.
After the addition, the mixture was stirred for 2 hours at the
same temperature. Subsequently, the mixture was added dropwise
CA 02803910 2012-12-24
128
to 60 parts of ice water over about 10 minutes. The
precipitated crystals were taken by filtration and dried. Thus,
6.4 parts of a compound represented by the following formula
(32) was obtained.
H03S r l N -NH2 (32)
H3CO $
(Step 2)
3.2 parts of the compound represented by the formula (32)
obtained in the (Step 1) was suspended in 20 parts of 50%
sulfuric acid. 4.7 parts of 40% nitrosylsulfuric acid was
added dropwise thereto over about 10 minutes at 5 C to 10 C
under stirring, and thereby a diazo suspension was obtained.
Meanwhile, 2.9 parts of a compound represented by the
formula (33) and 0.4 parts of sulfamic acid were added to 30
parts of water, and then the mixture was adjusted to pH 5.0 to
5.5 by adding sodium hydroxide, and thus an aqueous solution
was obtained. To the aqueous solution thus obtained, the diazo
suspension described above was added dropwise over about 10
minutes at a reaction temperature of 20 C to 30 C. After
completion of the dropwise addition, the mixture was stirred
at the same temperature for 2 hours, and adjusted to pH 0.7 to
1.2 by adding sodium hydroxide, and then a solid precipitated
therefrom was taken by filtration. Thus, 11.8 parts of a wet
cake containing a compound of the following formula (34) was
obtained.
Meanwhile, the compound represented by the following
formula (33) was obtained by the method described in Japanese
CA 02803910 2012-12-24
129
Unexamined Patent Application, Publication No. 2004-083492.
_1S43H
OJ
NHp (33)
H3C
SO3H
HO3S ~Ia e-N -N NH2 (34)
H3CO S
H3C
(Step 3)
To 30 parts of water, 2.7 parts of a compound represented
by the following formula (35) was added, and then the mixture
was adjusted to pH 7.5 to 8.0 by adding sodium hydroxide, and
thus an aqueous solution was obtained.
Meanwhile, the wet cake containing the compound
represented by the formula (34) obtained in the (Step 2) was
suspended in 110 parts of water under stirring, and the
mixture was adjusted to pH 6.0 to 6.5 by adding sodium
hydroxide, and thus an aqueous solution was obtained.
To the aqueous solution thus obtained, 2.6 parts of 35%
hydrochloric acid, and then 2.0 parts of an aqueous solution
of 40% sodium nitrite at a reaction temperature of 15 C to
20 C were added dropwise over about 5 minutes. Thus, a diazo
suspension was obtained.
The diazo suspension thus obtained was added dropwise
over 20 minutes to the aqueous solution containing the
compound represented by the formula (37) previously obtained
at a reaction temperature of 20 C to 30 C. At this time, the
reaction system was added with sodium carbonate so as to be
CA 02803910 2012-12-24
130
maintained at 7.0 to 8.0 of the pH value. After completion of
the dropwise addition, the reaction system was stirred at the
same temperature for 2 hours, added with sodium chloride
whereby to carry out salting-out. A solid precipitated
therefrom was taken by filtration. Thus, 16.9 parts of the wet
cake containing a compound represented by the following
formula (36) was obtained.
OH
i
H03S NH2 (35)
SO3H
SO3H
o-~
H03S
S OH
HaCO I N=N / N=N i s (36)
H3C H03S S03H H
(Step 4)
To 30 parts of water, 12.7 parts of 3,5-disulfo aniline,
18.3 parts of 35% hydrochloric acid, and then 9.1 parts of an
aqueous solution of 40% sodium nitrite at a reaction
temperature of 0 C to 5 C were added dropwise over about 5
minutes. Thus, a diazo liquid was obtained. Meanwhile, to
dimethylacetyl succinate, 9 parts of water, and then 2 parts
of ethanol were added and suspended under stirring, and then
the diazo liquid previously obtained was added dropwise
thereto over 15 minutes at a reaction temperature of 10 C to
20 C. After the dropwise addition, the reaction system was
added with sodium acetate so as to be maintained at 7.0 to 8.0
of the pH value. After completion of the dropwise addition,
the reaction system was stirred at the same temperature for 2
CA 02803910 2012-12-24
131
hours. The reaction system was added with sodium hydroxide and
stirred at pH 13.0 to 13.5 and at 15 C to 20 C for 2 hours.
Then, the reaction system was added with 35% hydrochloric acid
stirred at pH 0 to 0.5 and at 5 C to 10 C for 2 hours. The
precipitated crystals were taken by filtration. Thus, 10.9
parts of the compound represented by the following formula (37)
was obtained.
HO2C
n k SOaH
OH q (37)
503H
(Step 5)
To 30 parts of water, 2.5 parts of the compound
represented by the formula (37) obtained in the (Step 4) was
added, and then the mixture was adjusted to pH 7.5 to 8.0 by
adding sodium hydroxide. Thus, an aqueous solution was
obtained.
Meanwhile, under stirring, the total amount of the wet
cake containing the compound represented by the formula (36)
obtained in the (Step 3) was dissolved in 150 parts of water,
and 3.5 parts of 35% hydrochloric acid, and then 1.5 parts of
an aqueous solution of 40% sodium nitrite at a reaction
temperature of 20 C to 25 C were added dropwise thereto over
about 5 minutes. Thus, a diazo liquid was obtained.
The diazo liquid thus obtained was added dropwise to the
aqueous solution containing the compound of the formula (37)
previously obtained, over for 30 minutes at a reaction
temperature of 20 C to 30 C. At this time, the reaction system
CA 02803910 2012-12-24
132
was added with sodium carbonate so as to be maintained at 7.0
to 8.0 of the pH value.
After completion of the dropwise addition, the reaction
system was stirred at the same temperature for 2 hours, and
added with sodium chloride whereby to carry out salting-out. A
solid precipitated therefrom was taken by filtration. Thus,
40.8 parts of a wet cake was obtained. The wet cake thus
obtained was dissolved in 180 parts of water, and added with
250 parts of methanol whereby to be crystallized. A solid
precipitated therefrom was taken by filtration, and thereby a
wet cake was obtained. In addition, the wet cake thus obtained
was dissolved in 180 parts of water, added with 22 parts of
lithium chloride, and added with 200 parts of methanol whereby
to be crystallized. A solid precipitated therefrom was taken
by filtration, and thereby a wet cake was obtained. The wet
cake thus obtained was dissolved again in 80 parts of water,
and added with 200 parts of methanol whereby to be
crystallized. A solid precipitated therefrom was taken by
filtration and dried. Thus, 7.0 parts of the compound
represented by the following formula (38) (Amax: 606.5 nm) was
obtained as a lithium salt.
Meanwhile, this compound is a mixture of the compound
represented by No. 128 as described in Table 31, and the
compound represented by No. 137 as described in Table 32.
CA 02803910 2012-12-24
133
SO3H
H039N OH HOgC
H3C0 S r N=N--~~_M S03H
H3C H03S 1'' (ss)
S03H OH
S03H
Synthesis Example 4
The compound represented by the following formula (39)
was obtained by using 19.2 parts of 2-aminonaphthalene-4,6,8-
trisulfonic acid instead of 12.7 parts of 3,5-disulfo aniline
as a raw material in the synthesis of the compound represented
by the formula (37), and using this as a raw material,
Synthesis Example 2 (Step 5) was performed. Thus, a compound
represented by the following formula (40) (Amax: 607.0 nm) was
obtained.
Meanwhile, this compound is a mixture of the compound
represented by No. 104 and the compound represented by No. 108
as described in Table 27.
HO2C
N so3H
OH (39)
SO3H
S03H
O
H033 ` NrN=N \ N=N OH H02C
N S03H
r N=N
N3G0 S l:L
H3C H03S (40)
503H OH I
SO3H
SO3H
Synthesis Example 5
A compound represented by the following formula (41)
CA 02803910 2012-12-24
134
(hmax: 604.0 nm) was synthesized by the method described in
Examples 2 of Japanese Unexamined Patent Application,
Publication No. 2009-84346. This compound is a mixture of the
compound represented by No. 79 as described in Table 23, and
the compound represented by No. 87 as described in Table 24.
SOH
OH
HO3S rYN HOOC
H3CO S H, H03 S -N N (41)
SO3H HO f
SO3H
Examples 1 to 5 and Comparative Examples 1 to 3
(A) Preparation of ink
The respective components described in the following
Table 36 were mixed. Thus, the ink compositions of the present
invention and the ink compositions for comparison were
obtained, respectively, and then contaminants were separated
by filtration with a 0.45 pm membrane filter. Thus, an ink for
a test was obtained. The ink thus obtained was taken as
Examples 1 to 5.
The inks of Comparative Examples 1 to 3 were obtained by
mixing the respective components described in Table 36,
similarly to Examples 1 to 5. The compound (42) in the table
is the compound represented by the following formula (42)
disclosed in Synthesis Example 2 of PCT International
Publication No. W02008/142989. The synthesis was performed by
the disclosed technique, and preparation for an ink for
Comparative Example was carried out.
CA 02803910 2012-12-24
135
The inks of Comparative Examples 1 to 3 contained 2 kinds
of coloring matters, and were prepared in the same
compositions as those of respective Examples except using the
compound represented by the following formula (42) instead of
using the coloring matter (I) of the present invention
therein.
HO3S-,--\~SO3H
0 0
~~WN_<~~_ b N=N
HOO N YN 000H
(N) (42)
HOOC I~ COOH
N-N - NI H ~N=N_
~D_ "
0
HS J/ ~SO3H
Furthermore, ion-exchanged water was used in the
preparation of the inks in the respective Examples and
Comparative Examples described below. After the preparation of
the respective inks, the system was adjusted to pH 8 to 10
appropriately using an aqueous solution of lithium hydroxide
or hydrochloric acid water only when the pH of the respective
inks was beyond the range of 8 to 10.
[Table 36]
CA 02803910 2012-12-24
136
Comparative
Ink Example
Example
composition -
1 2 3 4 5 1 2 3
Coloring (31) (31) (31) (31) (31) - - -
----------- ---------- ----------- ----------------------- ---------- ---------
--------------
matter (I) 1.0 1.0 1.0 1.0 0.6 - - -
Coloring (38) (40) (41) (41) (41) (38) (40) (41)
------- --- ---------- ----------- ----------------------- --------------------
--------------
matter (II) 4.0 4.0 4.0 4.0 4.0 4.0 4.0 4.0
Other - - - - DY.86 (42) (42) (42)
-------- - - ---------------------- ----------------------- ---------- --------
---------------
coloring
- - - - 0.4 1.0 1.0 1.0
matter
GLY 5 5 5 5 5 5 5 5
Urea 5 5 5 5 5 5 5 5
NMP 4 4 4 4 4 4 4 4
IPA 3 3 3 3 3 3 3 3
BCTL 2 2 2 2 2 2 2 2
EDTA 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1
SURF 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1
Water 75.8 75.8 75.8 75.8 75.8 75.8 75.8 75.8
Total 100 100 100 100 100 100 100 100
Table 36 described above will be described.
The coloring matter (I) or the coloring matter (II) in
the table corresponds to the coloring matter (I) or the
coloring matter (II) contained in the ink composition of the
present invention, respectively. In the field of the other
coloring matters in the table, coloring matters not
corresponding to the coloring matter (I) and the coloring
matter (II) are described. The fields of the respective
CA 02803910 2012-12-24
137
coloring matters are divided into two up and down by the
dotted lines. The numbers in the parentheses described in the
upper field correspond to the numbers of the formulae of the
compounds described in Examples. In the lower field, the
numbers of the parts used are described.
Any numbers described in the fields of the water-soluble
organic solvent, respective additive and the like other than
the field of the coloring matter, describe the numbers of the
parts in the compositions.
Meanwhile, the abbreviations in the table represent the
meanings described below. DY. 86: C.I. Direct Yellow 86
GLY: Glycerin
NMP: N-methyl-2-pyrrolidone
IPA: Isopropanol
BCTL: Butylcarbitol
EDTA'2Na: Ethylenediamine tetraacetate disodium
SURF: Trade name SurfynolRTM manufactured by Nissin Chemical
Industry CO., Ltd.
(B) Ink jet recording
Ink jet print was carried out on Glossy Paper 1, Glossy
Paper 2, and Glossy Paper 3 using the inks obtained in the
respective Examples, and the respective Comparative Examples
described above by means of an ink jet printer, (trade name:)
"PIXUSRTM 1P4500" manufactured by Canon, Inc. Glossy Paper 1
was (trade name:) "Kassai Photo Finish Pr0RTM <high glossy>"
manufactured by Fujifilm Corporation, Glossy Paper 2 was,
CA 02803910 2012-12-24
138
(trade name:) "Photo Paper Glossy Pro (platinum grade RTM),,
manufactured by Canon, Inc. and Glossy Paper 3 was (trade
name:) "Photo Paper Gloss GoldRTM" manufactured by Canon, Inc.
At the time of ink jet recording, image patterns were produced
such that six grades of gradation at densities of 100%, 800,
60%, 40%, 20% and 10% were obtained, and recorded materials of
the gradation from dark black to light black were obtained.
The recorded materials thus obtained were dried at room
temperature for 24 hours or more after the printing, which
were used for the evaluations as the test specimens.
(C) Evaluation of recorded images
The respective test specimens obtained by the procedures
described above were used for the print density test. Any of
the colorimetric determinations of the recorded images in the
evaluation was carried out using a colorimeter, (trade name:)
"SpectroEye" manufactured by GRETAG-MACBETH. Any of the
colorimetric determinations was carried out under the
conditions of a viewing angle of 20 and a light source of D65,
using a density standard of DIN.
(D) Evaluation for print density
Black reflection density, Dk value was measured using the
colorimetric determination system described above in regard to
the 100% density gradation area, the gradation area printed
most heavily in the respective test specimens. The value was
evaluated according to the following criteria. The results of
CA 02803910 2012-12-24
139
the evaluations are presented in Table 37. Meanwhile, the Dk
values thus obtained are such that a larger value means
superior print density.
A: The Dk value is equal to or greater than 2.40.
B: The Dk value is less than 2.40 and equal to or greater
than 2.35.
C: The Dk value is less than 2.35 and equal to or greater
than 2.25.
D: The Dk value is less than 2.25.
[Table 37]
Evaluation result Print density Dk
Ink Glossy paper Glossy paper Glossy paper
No. 1 2 3
1 A A A
2 A A A
Example 3 A A A
4 B A B
A A A
1 C B C
Comparativ
2 D C D
e Example
3 B B C
As shown from the results of Table 37, the inks of the
respective Examples received A or B of the evaluation results,
and the inks of the respective Comparative Examples received B
to D of the evaluation results in all the glossy papers.
CA 02803910 2012-12-24
140
Specifically, it was shown that the print densities of
the obtained recorded images were different apparently when
the inks of the respective Examples using the coloring matter
(I) were compared with the inks of the respective Comparative
Examples not using the coloring matter (I), and that high
color development properties were obtained with use of the
coloring matter (I).
From the results described above, it was revealed that
any ink composition of the present invention containing 2
kinds of coloring matters of specific coloring matter (I) and
coloring matter (II) was excellent in various fastness
properties required in ink jet recorded images, and produced
neutral high quality images with low chroma and no color tone,
and produced high print density of recorded images as black
when compared with a conventional black ink composition.
INDUSTRIAL APPLICABILITY
The ink composition of the present invention is suitable
as a black ink liquid for ink jet recording, for various
recordings such as writing tools, particularly for ink jet
recording.