Note: Descriptions are shown in the official language in which they were submitted.
81662851
-1-
TREATMENT OF INFLAMMATORY DISORDERS
RELATED APPLICATIONS
This application claims benefit under 35 U.S.C. 119(e) to U.S. Provisional
Application No. 61/351505, filed June 4, 2010, entitled "TREATMENT OF
INFLAMMATORY DISORDERS".
GOVERNMENT SUPPORT
This invention was made in part with government support from the United States
of America
under grant numbers AR 048114 and Al 065858 from the National Institutes of
Health. The
United States Government has certain rights in the invention.
FIELD OF THE INVENTION
This invention relates to methods and compositions for the treatment of
inflammatory
disorders, and more specifically non-joint inflammatory disorders. The methods
involve
administering a cadherin-11 antagonist to a subject to modulate cadherin-11
function,
particularly in fibroblasts.
BACKGROUND OF THE INVENTION
The adhesive interactions between cells and between cells and the
extracellular matrix
may be in involved in a wide variety of processes including, for example,
modulation of the
immune system, regulation of developmental processes and tumor progression and
metastasis.
These interactions are mediated by adhesion molecules which transduce
information from the
extracellular to the intracellular matrix.
Four families of adhesion molecules which mediate these interactions have been
identified: the integrins, the cadherins, the selectins, and immunoglobulin-
related molecules.
In general, adhesion molecules are transmembrane proteins which contain an
extracellular
domain for interacting with an extracellular matrix or cellular component, a
transmembrane
domain spanning the cell membrane and a cytoplasmic domain for interacting
with one or
more cytoskeletal or cytoplasmic components.
Date Recue/Received Date 2020-04-07
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
_9_
The cadherins play an important role in the establishment and maintenance of
intercellular connections between cells of the same type (reviewed in Geiger
B. et al. (1992)
Annual Review of Cell Biology 8:307; Kemler R. (1993) Trends in
Gastroenterology 9:317;
Takeichi M. (1990) Annual Review of Biochem. 59:237; Takeichi M. (1991)
Science
251:1451). Cadherins are a superfamily of structurally related molecules that
function in
Ca+2-dependent homophilic adhesion. Cadherins are expressed on cells that form
solid tissues,
and are responsible for segregating and sorting cells during embryogenesis,
establishing cell
polarity, and maintaining tissue morphology. Structurally, cadherins are
single chain
polypeptides that are synthesized as precursors and cleaved during post-
translational
processing. They have large extracellular regions made up of 5 homologous
domains, a single
transmembrane segment and a cytoplasmic tail.
The cadherins are synthesized as precursors that are cleaved during post-
translational
processing. The mature cadherins are single chain molecules which include a
relatively large
extracellular domain (typically divided into five sections or "ectodomains"),
a single
transmembrane region and a cytoplasmic tail. Among the classical cadherins
(i.e., P-
(placenta), E- (epithelial), and N- (neural) cadherin), the cytoplasmic domain
contains the
highest degree of homology. The high degree of homology observed for the
cytoplasmic
domain reportedly is a reflection of the association of cadherins with a group
of intracellular
proteins, called catenins, that stabilize cadherin active conformation (Kemler
R. (1993)
Trends in Gastroenterology 9:317). It is generally believed that sequences in
the extracellular
domain are necessary to mediate homophilic (i.e., cadherin-to-cadherin)
binding. A review of
the literature indicates that research directed to understanding cadherin-
mediated adhesion has
focussed on efforts to elucidate the mechanism underlying cadherin-mediated
homophilic cell
adhesion. Little attention has been directed to understanding what, if any,
role is played by
cadherins in heterophilic adhesion. While it has been known for some time that
integrins and
other adhesion molecules function in immune system modulation, e.g., by
playing a role in
the adhesion of peripheral lymphocytes to endothelium and in horning to lymph
nodes,
relatively little is known regarding the mechanism by which lymphocytes home
and
transmigrate through the vascular endothelium to specifically target certain
tissue locations.
The most highly conserved sequence shared by cadherins lies within the
cytoplasmic
domain. It is this region that mediates interaction with the cytoplasmic
catenins proteins
(Hirano S. et al. Cell 70:293-301, 1992). The presence of the cytoplasmic
domain is essential
to functioning of the cadherin as deletions in this region abolish catenin
binding as well as
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-3-
cell-to-cell adhesion (Hulsken J, et al. J Cell Biol 127:1375-80, 1994) . The
catenins (a, 102
Ma; 13, 88-93 kna, and 7, 80-83 kDa) begin to associate with cadherins almost
immediately
upon biosynthesis in a stable manner that is not disrupted in TX-100 detergent
(Takeichi M.
Curr Opin Cell Biol 7:619-27, 1995) and are thought to mediate anchorage of
cadherins to the
cytoskeleton (Yap AS. et al. Annu Rev Cell Dev Biol 13:11946, 1997).
Functionally,
a-catenin is necessary for cadherin mediated homophilic adhesion. Tumor cells
expressing
E-cadherin at the cell surface, but lacking a-catenin expression, fail to form
cell-to-cell
contacts unless a-catenin expression is restored through transfection (Chen H.
et al. J Cell Sci
1141345-56, 1997; and Knudsen KA, et al. J Cell Biol 130:67-77, 1995). 13-
catenin is
homologous to the Drosophila segment polarity protein armadillo as well as the
cadherin
associated protein plakoglobin. Plakoglobin, also termed 7-catenin, interacts
more weakly
with the cadherin/catenin complex, and is not always seen in cadherin
precipitates.
A number of newly identified cadherin cDNA clones have been isolated using
consensus oligonucleotides corresponding to cytoplasmic domain sequences that
are highly
conserved among cadherins and PCR cloning (Suzuki S. et al. Cell Reg 2:261-70,
1991).
Although cadherin function classically involves homophilic cell-to-cell
adhesion (i.e.,
E-cadherin binds E-cadherin typically on another cell of the same type),
however, murine E-
cadherin expressed on epidermal keratinocytes also mediates adhesion to E-
cadherin of
Langerhans cells (Tang A. et al. Nature 361:82-5, 1993). Another counter-
receptor for
E-cadherin, namely the integrin aE137 which is expressed on intraepithelial T
cells (Cepek KL.
et al. Nature 372:190-3, 1994 and U.S. Patent No. 5,610,281), has also been
identified. This
represents an example of heterophilic binding of E-cadherin to the aE[37
integrin
counter-receptor.
SUMMARY OF "1'HE INVENTION
The invention provides methods and compositions for the treatment (including
prevention) of inflammatory disorders, and particularly inflammatory disorders
that are not
inflammatory joint disorders (referred to herein as non-joint inflammatory
disorders, or
NJID). The inflammatory disorder may be an autoimmune disorder although it is
not so
limited. Disorders to be treated according to the invention therefore include
but are not
limited to inflammatory bowel disease (e.g., colitis, including severe
colitis, ulcerative colitis,
Crohn's disease), psoriasis, Graves opthalmopathy, various types of
vasculitis, eczema such
as atopic dermatitis, multiple sclerosis, atrial myxoma, inflammation
associated with solid
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-4-
organ transplantation (various types of tissues), glomerulonephritis,
interstitial nephritis,
peritoneal inflammation and diverticulitis (and scarring) post surgical, post
performation
and/or post infection, pleural inflammation (and scarring) post surgical
and/or post trauma
such as blood in pleural space and post infection, as well as inflammation
associated with
burns (which also can lead to scarring).
Although not intending to be bound by any particular theory, it is postulated
that
cadherin-11 expression in fibroblasts can be targeted in order to reduce,
among other things,
the production of cytokines and growth factors, particularly those associated
with
inflammation from such fibroblasts. The target fibroblasts upregulate cadherin-
1 1 expression
to and thus can be targeted by modulating, preferably antagonizing,
cadherin-1 1 function. While
inflammation is known to involve lymphocytes, and particularly activated
lymphocytes,
fibroblasts are not classically considered inflammatory cells. The antagonists
of the invention
can interfere with the ability of fibroblasts to make cytokines such as IL-6,
chemokines such
as MCP-1 and IL-8, prostaglandins such as PGE2, as well as other inflammatory
factors. In
some instances, the cadherin-1 1 antagonists inhibit partially or completely
Th2 and/or Thl 7
immune responses (e.g., by reducing the production and/or secretion of '1112
and/or Thl 7
cytokines such as IL-4, IL-13 and IL-17). In some instances, the cadherin-1 1
antagonists
inhibit partially or completely eosinophil responses (e.g., by reducing the
levels of eosinophils
recruited to a site of inflammation or by reducing the production and/or
secretion of
chemokines such as eotaxin including eotaxin-1). Again, although not intending
to be bound
by any particular mechanism, in part, it is the ability of the antagonists of
the invention to
block production of cytokines, chemokines and/or lipid mediators that renders
them useful in
inhibiting fibroblast inflammatory function and thus the ensuing inflammation,
in some
aspects and embodiments of the invention.
According to one aspect on the invention, a method is provided for treating a
subject
having an inflammatory disorder that is not a inflammatory joint disorder
(i.e., the disorder is
a non-joint inflammatory disorder or a MID). The method involves administering
to a
subject in need of such treatment a therapeutically effective amount of a
cadherin-1 1
antagonist. A cadherin-11 antagonist is an agent that interferes with a
cadherin-1 1 function.
Such interference may lead to partial or complete inhibition of cadherin-1 1
function. For the
purposes of the invention, an important cadherin-11 function is growth factor
or cytokine
production, preferably from fibroblasts associated with NJID. It is to be
understood, however,
that a cadherin-1 1 antagonist may interfere with other cadherin-1 1
functions, including but
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-S-
not limited to cell-cell adhesion, cell-extracellular matrix adhesion, and
apoptosis. In
preferred embodiments, the subject is a human. Preferably, the subjects do not
have an
inflammatory joint disorder, such as arthritis. In some embodiments, the
subject does not
have abnormal cadherin-11 mediated adhesion occurring in the liver or the
brain. Cadherin-
11 mediated adhesion is the binding of cadherin-11 to its counter-receptor and
such counter-
receptor may be another cadherin-11 or another molecule altogether.
In one embodiment, the NJID is an autoimmune disorder. In one embodiment, the
NJID is inflammatory bowel disease, such as colitis and including severe
colitis, ulcerative
colitis, or Crohn's disease. In one embodiment, the NJID is psoriasis. In one
embodiment,
to the NJID is Graves opthalmopathy. In one embodiment, the NJID is
vasculitis (i.e.,
inflammation of the blood vessels). In one embodiment, the NJID is eczema. In
one
embodiment, the NJID is atopic dermatitis. In one embodiment, the NJID is
multiple
sclerosis. In one embodiment, the NJID is atrial myxoma. In one embodiment,
the NJID is
inflammation resulting from solid organ transplantation. In one embodiment,
the NJID is
glomerulonephritis. In one embodiment, the NJID is interstitial nephritis. In
one
embodiment, the NJID is inflammation associated with a burn or other skin
lesion. In one
embodiment, the NJID is peritoneal inflammation and diverticulitis that occurs
post-surgery,
post-performation, or post-infection. In one embodiment, the NJID is pleural
inflammation
that occurs post-surgery, post-trauma, or post-infection.
In another aspect, the invention provides a method for preventing fibrosis in
a subject
by administering to a subject at risk of developing fibrosis a cadherin-11
antagonist. Again,
although not intending to be bound by any particular mechanism, it is believed
that in certain
instances reduction or inhibition of an inflammatory reaction can prevent
subsequent fibrosis.
Accordingly, in some instances the subject manifests no symptoms of fibrosis
(e.g.. scarring).
The cadherin-11 antagonist may be administered systemically, including for
example
intravenously. In some embodiments, the cadherin-11 may be administered via a
stent located
in a blood vessel in a subject. This embodiment is particularly suited for the
treatment of
vasculitis. The stent may be located near the heart in order to treat atrial
myxoma. In some
embodiments, the cadherin-11 antagonist is administered locally including to
the gut, the skin,
or the eyes. The cadherin-11 antagonist may also be administered to a subject
at the site of
tissue or organ transplantation. The tissue or organ may be perfused with the
cadherin-11
antagonist, or the cavity into which the tissue or organ will be placed can be
washed with a
81662851
- 6 -
cadherin-11 antagonist. Alternatively or additionally, the cadherin-11
antagonist may be
administered systemically as well in transplant recipients.
In still other embodiments, the antagonist can be administered orally,
topically, via
a pulmonary route of administration, nasally, transdermally, subcutaneously or
intravenously.
In some instances, the cadherin-11 antagonist is administered by a systemic
route such as a
subcutaneous or intravenous route regardless of the nature of the inflammatory
disorder.
In one embodiment, the cadherin-11 antagonist is a cadherin-11 binding
peptide. In
one embodiment, the cadherin-11 binding peptide is an anti-cadherin-11
antibody or an
antigen-binding antibody fragment. In one embodiment, the cadherin-11 binding
peptide is a
cadherin-11 fusion protein. In one embodiment, the cadherin-11 binding peptide
comprises
full length cadherin or a fragment thereof.
In one embodiment, the cadherin-11 antagonist is a cadherin-11 nucleic acid
antagonist. In one embodiment, the cadherin-11 nucleic acid antagonist is a
cadherin-11
siRNA. In one embodiment, the cadherin-11 nucleic acid antagonist is a
cadherin-11
ribozyme. In one embodiment, the cadherin-11 nucleic acid antagonist is a
cadherin-11
antiscnsc molecule. In one embodiment, eadherin-11 nucleic acid antagonist is
a nucleic acid
encoding full length cadherin-11 or a fragment thereof. In one embodiment,
cadherin-11
nucleic acid antagonist is an aptamer.
In one embodiment, the cadherin-11 antagonist is a small molecule.
In some embodiments, the cadherin-11 antagonists are antibodies while in other
embodiments they are not antibodies. Antibodies may be monoclonal antibodies,
polyclonal
antibodies, chimeric antibodies including humanized antibodies, camelids,
single chain
antibodies, and the like.
The invention also provides, in another aspect, a pharmaceutical composition
(i.e., a
pharmaceutical preparation) comprising an effective amount of a cadherin-11
antagonist in a
pharmaceutically acceptable carrier or formulated as an implant such as a
stent. The invention
CA 2805270 2017-08-23
81662851
- 6a -
also provides methods for making such compositions by placing the cadherin-11
antagonist in
a pharmaceutically acceptable carrier. The pharmaceutical composition
preparation may
contain one or more cadherin-11 antagonists and, optionally, other therapeutic
agents which
are useful in the treatment of the particular disorder being treated.
The invention also provides, in another aspect, use, for treating a subject
having a
non-joint inflammatory disorder, of a therapeutically effective amount of a
cadherin-11
antagonist, wherein the non-joint inflammatory disorder is inflammatory bowel
disease,
psoriasis, atopic dermatitis, inflammation associated with burns of the skin,
Graves
ophthalmopathy, vasculitis, inflammation associated with solid organ
transplantation, multiple
sclerosis, glomerulonephritis, interstitial nephritis, peritoneal inflammation
or diverticulitis, or
pleural inflammation, and wherein the cadherin-11 antagonist is an anti-
cadherin-11 antibody
or an antigen-binding fragment thereof or wherein the cadherin-11 antagonist
is a nucleic acid
that binds to either cadherin-11 or a nucleic acid that encodes cadherin-11.
These and other aspects of the invention, as well as various advantages and
utilities,
.. will be more apparent with reference to the detailed description of the
preferred embodiments
and to the accompanying drawings.
Date Recue/Received Date 2020-04-07
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-7-
BRIEF DESCRIPTION OF THE DRAWINGS
The Examples refer to and include a brief description of various figures. It
is to be
understood that the drawings or figures are illustrative only and are not
required for the
enablement of the inventions disclosed herein.
FIG. 1. A schematic of the structure of the human cadherin-11-Fc fusion
protein. The
sequence of the extracellular juxtamembrane region of wild-type cadherin-11
and the
alterations resulting from fusion with the human Fe region are shown. Regions
corresponding
to the Pc portion are shown in bold.
FIG. 2. Nucleotide sequence of human cadherin-11 (SEQ ID NO:1).
FIG. 3. Amino acid sequence of human cadherin-11 (SEQ ID NO:2).
FIG. 4. The design of anti-cadherin-11 monoclonal antibody (mAb) treatment of
a
mouse model of allergic skin inflammation.
FIG. 5. Anti-caderin-11 mAb treatment suppressed skin inflammation. (A) H & E
.. staining of sections from SAL- and OVA-sensitized skin. (B) Epidermal and
dermal
thickness was significantly decreased by anti-cad-11 mAb treatment compared to
the
untreated group. (C) Eosinophils but not CD4+ T cells were significantly less
recruited into
the inflamed skin.
FIG. 6. Th2 type cytokines were down-regulated in sensitized skin of the anti-
cad-11
.. mAb treated group. Total RNA isolated from sensitized skin was analyzed for
(A) Th2 type
cytokines (IL-4 and IL-13), IL-17, and IFN-y, and (B) eotaxin-1, a chemokine
for recruiting
eosinophils.
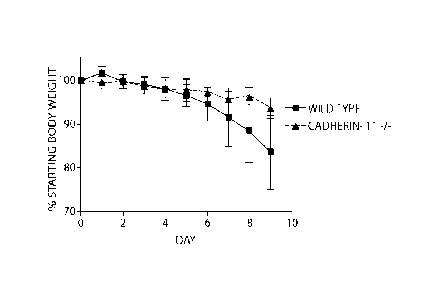
FIG. 7. Cadherin-11-deficient mice are protected from colitis in the dextran
sodium
sulfate model of inflammatory bowel disease, as evidenced by effects on body
weight (A),
and colon length (B).
DETAILED DESCRIPTION OF THE INVENTION
Cadherin-11 is a transmembrane molecule that, inter alia, mediates binding of
cells to
each other through interaction with itself or its counter-receptors. Like
other cadherins,
cadherin-11 is proposed to mediate, among other things, adhesion of like cells
to each other as
well as adhesion of cells of different lineages to each other. The human and
mouse cadherin-
11 genes have been isolated and sequenced previously (Suzuki S. et al. Cell
Reg 2:261-70,
CA 02805270 2013-01-14
WO 2011/153397
PCT/ES2011/039002
-8-
1991). See also, Genbank Accession No. NM_001797, (SEQ ID NO: 1 and SEQ ID NO:
2)
for the human cadherin-11 cDNA and predicted amino acid sequences,
respectively.
The invention is based at least in part on ability of cadherin-11 to modulate
factor or
cytokine secretion by fibroblasts in certain inflammatory disorders. The
invention embraces
the use of cadherin-11 antagonists in the treatment of certain inflammatory
disorders that do
not involve inflammation in the joints of subject.
The methods involve administering to a subject a cadherin-11 antagonist.
Cadherin-
11 antagonists are able to interfere with, including inhibit partially or
completely (1) cell
proliferation, (2) secretion of molecules such as, but not limited to, IL-6,
MCP and TNF-
alpha, (3) apoptosis, (4) migration and/or (5) attachment, of cadherin-11
expressing cells, and
particularly cadherin-11 expressing fibroblasts that exist or are at least
associated with
inflammation. In important embodiments, such antagonists lead to a decrease in
the
production and/or secretion of molecules such as, but not limited to,
cytokines such as IL-4,
IL-6, IL-13, IL-15, IL-17, IL-23, type I IFNs, IL- lbeta and TNF-alpha, growth
factors such as
GM-CSF, VEGF, TOT-beta, PDGF and SCF, chemokines such as CXCL-1, -5, -6, -8, -
9, -10,
-11, -12, -13, CCL-2, -3, -5, eotaxins such as eotaxin-1 (CCL11), and CX3CL1,
bioactive
lipids such as prostaglandins and leukotrienes, and degradative enzymes such
as MMPs and
cathepsins. In some instances, the antagonists inhibit or reduce a preexisting
Th2 immune
response. In some instances, the antagonists inhibit or reduce a preexisting
Th17 immune
response. In some instances, the antagonists inhibit or reduce Th2 and Th17
immune
responses, In some instances, the antagonists reduce eosinophil numbers at a
site of
inflammation. The antagonists can also prevent or down-regulate the expression
(or
overexpression) of cell surface receptors on the fibroblast surface such as
CD40, VCAM-1,
ICAM-1, TLRs, integrins, and cytokine and chemokine receptors.
In one aspect, the invention is directed to a method for treating a subject
having a non-
joint inflammatory disorder. As used herein, a non-joint inflammatory disorder
is an
inflammatory disorder that does not occur or involve the joints. Inflammatory
disorders are
typically characterized by the presence of activated immune cells and/or
increased levels of
inflammatory markers such as a cytokine milieu that stimulates immune cells
such as
macrophages, neutrophils and other granulocytes, T cells and NK cells.
The inflammatory disorders to be treated according to the invention may be
autoimmune disorders. An autoimmune disorder is one in which the body's immune
system
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-9-
reacts against one or more body tissues and thus attacks the tissue as it
would a foreign
antigen or pathogen.
The inflammatory disorder to be treated according to the invention may be
inflammatory bowel disease (e.g., colitis such as severe colitis, ulcerative
colitis, Crohn's
disease), psoriasis, Graves opthalmopathy, vasculitis (i.e., inflammation of
the blood vessels),
eczema such as atopic dermatitis, multiple sclerosis, atrial myxoma,
inflammation resulting
from solid organ transplantation (e.g., transplantation of heart, liver,
kidney, skin, lung, eye,
and the like), glomerulonephritis, interstitial nephritis, inflammation
associated with a burn or
other skin lesion, peritoneal inflammation and diverticulitis that occurs post-
surgery, post-
to performation, or post-infection, or pleural inflammation that occurs
post-surgery, post-trauma,
or post-infection.
The inflammatory disorders to be treated by the methods described herein
exclude
acute and chronic synovitis, arthritis including psoriatic arthritis,
rheumatoid arthritis, arthritis
associated with ankylosing spondylitis, arthritis associated with systemic
lupus
erythrematosus, and juvenile chronic arthritis, chronic I.yme disease, and
Reiter's syndrome.
The antagonist may be administered systemically or locally depending upon
whether
the disorder being treated is systemic or localized.
The cadherin-11 antagonist is administered to the subject in a therapeutically
effective
amount. A therapeutically effective amount is a dosage of the cadherin-11
antagonist
sufficient to provide a medically desirable result. In the treatment methods
of the invention,
the therapeutically effective amount of the cadherin-11 antagonist may be that
amount which
is sufficient to reduce inflammation (or the level of inflammatory markers
such as but not
limited to particular cell types such as eosinophils or particular cytokines
or chemokines such
as IL-4, IL-13, IL-17 or an eotaxin such as eotaxin-1) at a particular
location or systemically
(depending on the disorder), or to alleviate pain or other symptoms associated
with the
inflammatory disorder. As used herein, treatment embraces the use of the
cadherin-11
antagonist in subjects that have the inflammatory disorder as well as in
subjects at risk of
developing the disorder.
In still other embodiments, the therapeutically effective amount is the amount
that
prevents or delays the onset of fibrosis in a subject. Fibrosis refers to the
development of
excess fibrous connective tissue in an organ or tissue. Fibrosis can occur in
a variety of
tissues or organs. Fibrotic conditions include lung fibrosis, hepatic fibrosis
(e.g., associated
with alcohol consumption, viral hepatitis, and/or schistosomiasis),
hypertrophic scars, keloids,
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-10-
burns, Peyronie's disease, Dupuytren's contractures, myelofibrosis, pancreatic
fibrosis, post-
myocardial infarction cardiac fibrosis, kidney/renal fibrosis associated with
diabetes, post-
inflammatory renal fibrosis, and drug-induced fibrosis (e.g., resulting from
chemotherapy
and/or radiation exposure). In some embodiments, the subject does not have a
fibrosis. In
still other embodiments, the subject does not have fibrosis nor does it have
an inflammatory
joint disorder.
A subject, as used herein, refers to any mammal susceptible to having or
presently
having any of the afore-mentioned non-joint inflammatory disorder. The
subjects may be
human and non-human subjects. Non-human subjects include but are not limited
to
companion animals (e.g., dogs and cats), agricultural or competitive animals
(e.g., cows,
horses, etc.). Preferably, the subject is one having one of these non-joint
inflammatory
disorders. In other embodiments, the subject is at risk of developing
fibrosis, and may or may
not yet have an inflammatory disorder that precedes fibrosis. In certain
embodiments, the
subject does not have abnormal calherin-1 1 mediated adhesion in the brain
and/or liver.
Cadherin-11 Antagonists:
As used herein, the term antagonist refers to any protein, polypeptide,
peptide,
peptidomimetic, glycoprotein, antibody, antibody fragment, carbohydrate,
nucleic acid,
organic molecule, inorganic molecule, large molecule, or small molecule that
blocks, inhibits,
reduces or neutralizes the function, activity and/or expression of another
molecule. As used
herein, a cadherin-11 antagonist is an agent that blocks, inhibits, reduces or
neutralizes the
function, activity and/or expression of cadherin-11. As described above,
cadherin-11 is
involved in cell attachment, interaction and/or migration. Cadherin-11 is
known to bind to
itself in what is referred to as homophilic or homotypic binding. The cadherin-
11 antagonists
may interfere with cadherin-11 homotypic binding or heterotypic binding (i.e.,
binding of
cadherin-11 to a counter-receptor that is not cadherin-11). The cadherin-11
antagonist may
interfere with cadherin-11 function by reducing the amount of cadherin-11 that
is expressed
by a cell or by interacting with cadherin-1 1 (or its counter-receptor)
thereby preventing
interaction of cadherin-11 with its target. Accordingly, the cadherin-11
antagonist may
interfere, in whole or in part, with the transcription of cadherin-1 1 or with
the translation of
cadherin-11 (thereby interfering with cadherin-1 1 expression), or it may
interfere with the
ability of cadherin-1 1 to bind to another cadherin-1 1 or to another cadherin-
11 counter-
receptor. The cadherin-11 antagonist may reduce cadherin-11 function or
activity by about
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-11-
10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90% or 100%, relative to a control
such as
PBS. It will be understood that the cadherin-11 antagonist may be used in an
amount that
reduces cadherin-11 function or activity by about these amounts. It will
further be understood
that some cadherin-11 antagonists are preferably used in vitro while others
are more suitable
.. for the in vivo methods provided herein.
Some cadherin-11 antagonists bind to the extracellular domain of cadherin-11,
some
bind to particular regions of the extracellular domain of cadherin-11. As
discussed herein, the
cadherin-11 extracellular domain is comprised of five (5) subdomains each
approximately
about 110 amino acids in size. (See, for example, U.S. Patent No. 7589074 and
Yagi et al.
m .. Genes and Development, 14:1169-1180, 2000.) The invention contemplates
the use of
cadherin-11 antagonists that bind to cadherin-11 EC1 or to a fragment of
cadherin-11 EC1
(e.g., a fragment that comprises about the first 33 through to the first 37
amino acids of EC1),
or to a fragment of cadherin-11 that comprises EC1 (or the first 33-37 amino
acids of EC1).
In some embodiments, the antagonist binds to a region of EC1 having an amino
acid sequence
.. of GWVWN QFFVI EEYTG PDPVI, VGRI,H SDIDS GDGN (SEQ ID NO:3, the first 34
amino acids of EC1). Alternatively or additionally, the antagonist may
comprise some or all
of this amino acid sequence.
The cadherin-11 antagonist may be a peptide or protein, or it may be a nucleic
acid,
or it may be a organic or inorganic small molecule. The antagonists may be
naturally
occurring or non-naturally occurring. They may be isolated from a naturally
occurring source
or they may be synthesized in vitro.
The cadherin-11 antagonists may be conjugated to another agent such as an
imaging
agent or a cytotoxic agent. Imaging agents may be used to visualize cadherin-
11 expression
in vitro (e.g., for immunohistochemical analysis) or in vivo (e.g., for body
imaging).
.. Examples include radionuclides, contrast agents, and particulates routinely
used in medical
imaging. Cytotoxic agents are agents that are toxic to cells. Examples include
chemotherapeutic agents, toxins, and the like. The use of these agents
conjugated to a
cadherin-11 antagonist will target such agents to fibroblasts associated with
inflammatory
disorders. In these instances, therapeutic benefit may be provided by a
combination of the
cadherin-11 antagonist which interferes with the ability of cadherin-11 to
secrete
inflammatory factors such as but not limited to IL-6 and the cytotoxic agent
which is directly
toxic to the fibroblast.
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-12-
Cadherin-11 Binding Peptides:
Cadherin-11 antagonists that are peptide or protein in nature include (1) a
full length
cadherin-11 protein, (2) a fragment of the full length protein, wherein the
fragment comprises
the transmembrane domain of cadherin-11 or a fragment of the extracellular
domain including
for example a fragment comprising or consisting of EC1 (e.g., a fragment that
comprises EC1,
a fragment that comprises EC1 and EC2, a fragment that comprises EC1-EC3, a
fragment that
comprises EC1-EC4, a fragment that comprises EC1-EC5, a fragment that
comprises EC1 and
EC3, a fragment that comprises EC1 and EC4, a fragment that comprises EC1 and
EC5), (3) a
fragment of the full length protein, wherein the fragment comprises one or
more of cadherin-
to 11 extracellular subdomains (e.g., EC1, EC2, EC3, EC4, or EC5 of the 5
extracellular
subdomains of cadherin-11, or any combination thereof), (4) fusion proteins
that comprise full
length cadherin-11 or a fragment thereof, and (5) antibodies and fragments
thereof. In
important embodiments, the cadherin-11 antagonist binds to and/or comprises
the EC1
domain of cadherin-11 or a fragment thereof (such as SEQ ID NO:3 provided
herein).
Cadherin-11 antagonists that are peptide or protein in nature preferably will
bind
preferentially (or selectively) to cadherin-11. Preferential (or selective)
binding to cadherin-
11 means that the peptide or protein binds with greater affinity to cadherin-
11 than to another
protein. In some instances, the peptide or protein binds to cadherin-11 with
an affinity that is
about 2-fold more, about 3-fold more, about 4-fold more, about 5-fold more,
about 10-fold
.. more, about 25-fold more, about 50-fold more, about 100-fold more, about
1000-fold more, or
more than its affinity for a protein that is not cadherin-11 or for any other
moiety. Such
differences in affinity are preferably manifest under physiological conditions
as occur in vivo.
In some embodiments, the cadherin-11 binding peptides bind to EC1 of cadherin-
11, and
optionally to the first 33-37 amino acids, including the first 33, first 34,
first 35, first 36,or
first 37 amino acids of EC1 of cadherin-11, as shown in SEQ Ill NO:2 provided
herein.
Binding to this region of cadherin-11 can be determined through competitive
binding assays
using other binding agents known to bind to this region of cadherin-11 such as
those
described in W02009/089062. The afore-mentioned antagonists are collectively
referred to
as cadherin-11 binding peptides. Cadherin-11 binding peptides may be harvested
and isolated
from naturally occurring sources or they may be synthesized and screened for
their ability to
bind to cadherin-11.
8 1 66285 1
-13-
As used herein with respect to pr.'ptides and proteins, the term "isolated"
means
separated from its native environment f!I sufficiently pure form so that it
can be manipulated
or used for any one of the purposes of the invention.
Binding peptides can also be derived from sources other than antibody
technology,
For example, binding peptides can be provided by degenerate peptide libraries
which can be
readily prepared in solution, in immobilized form, as bacterial flagella
peptide display
libraries or as phage display libraries. Combinatorial libraries also can be
synthesized of
peptides containing one or more amino acids. Libraries can also be made that
are comprised
of peptides and non-peptide synthetic moieties.
Cadherin-11, or a fragment thereof, also can be used to isolate other cadherin-
11
binding peptides or partners. Isolation of binding partners may be performed
according to
well-known methods. For example, cadherin-11 or a fragment thereof (e.g., an
extracellular
fragment) can be attached to a substrate, and then a putative cadherin-11
binding peptide may
be applied to the substrate. If a cadherin-11 binding peptide is present, it
will bind to the
substrate-bound cadherin-11, and it can then be isolated and further analyzed.
Full-Length Cadherin-11 and Cadherin-11 Fragments:
Based on the known nucleotide and amino acid sequence of cadherin-11, suitable
fragments of cadherin-11 may be identified and generated using conventional
technology,
Reference may be made to U.S. Patent Nos. 5597725, 5639634, 5646250, 6787136,
6946768,
7488478, and 7589074, and PCT Patent Publication Nos, WO 93/21302 and
W02009/089062, the teachings of which relating to cadherin-11 nucleotide and
amino acid
sequences and fragments are incorporated by reference herein.
Examples of suitable fragments include those that consist of or comprise amino
acids
1-40, 1-39, 1-38, 1-37, 1-36, 1-35, 1-34, 1-33, 1-32, 1-31 or 1-30 of cadherin
ECI or those
that consist of or comprise amino acids 15-34, 15-35, 15-36, 15-37, 15-38, 15-
39, or 15-40 of
cadherin ECI. The first 40 amino acids of EC1 are underlined and the first 35
amino acids of
Ed 1 are bolded in SEQ ID NO:2 as provided herein. Examples of suitable
fragments are also
provided in W02009/089062 (represented by the amino acid sequences of SEQ ID
NOs: 3,
10, 12, and 13, and also described in US 2009/025320O).
Other fragments may comprise amino acids 1-160, or 1- 259, or 1-269 of SEQ ID
NO:2,
and p t ion al 1 y they may lack amino acids 1-53 of SEQ ID NO:2 which
represents the leader
and pro-region of human cadherin-11,
Date Recue/Received Date 2020-04-07
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-14-
Cadherin-11 binding peptides may also be variants of full-length cadherin-11
or
cadherin-11 fragments. Such variants may differ from cadherin-11 amino acid
sequence by a
degree. For example, variants may be about 80%, about 85%, about 90%, about
91%, about
92%, about 93%, about 94%, about 95%, about 96%, about 97%, about 98%, or
about 99%
identical to full length cadherin-11 or to a cadherin-11 fragment. Variants
may comprise a
cadherin-11 fragment and additional flanking constituents at the amino and/or
carboxy end of
the fragment. Such constituents may be amino acid in nature. In all instances,
the variants
hind to cadherin-11 and interfere with cadherin-11 function or activity.
Cadherin-11 binding peptides may be at least 10, 20, 30, 40, 50, 60, 70, 80,
90, 100,
110, amino acids in length or longer. For example, they may be about or at
least 220, 330,
440, 550 amino acids in length.
In some important embodiments, the cadherin-11 antagonist is a functionally
equivalent peptide analog of cadherin-11. As used herein, the term
functionally equivalent
peptide analog refers to a peptide analog that is capable of inhibiting the
binding of cadherin-
11 to, for example, itself. Functionally equivalent peptide analogs of
cadherin-11 are
identified, for example, using in vitro adhesion assays that measure the
ability of the peptide
analog to inhibit cadherin-11-mediated adhesion either between cells
expressing cadherin-11
or between isolated cadherin-11 proteins, or some combination thereof.
Accordingly,
exemplary functionally equivalent peptide analogs of cadherin-11 include
analogs of full
length cadherin-11 or a cadherin-11 fragment that for example comprises
conservative amino
acid substitutions relative to the wild-type sequence.
Still other cadherin-11 binding peptides are provided in PCT Published
Application
Nos. W099/57149, W02004/048411, and W02009/089062, the specific teachings of
which
relating to cadherin-11 binding peptides and antagonists are incorporated by
reference herein.
Cadherin-11 Fusion Proteins:
The cadherin-11 binding peptide can be a fusion protein. A fusion protein, as
used
herein, is a protein that contains peptide regions from at least two different
proteins. For
example, a cadherin-11 fusion protein contains amino acid sequence from
cadherin-11 and at
least one non-cadherin-11 protein. Such fusion proteins can be formed by
fusing, usually at
the nucleotide level, coding sequence from cadherin-11 to coding sequence from
a non-
cadherin-11 protein. Examples of cadherin-11 fusion proteins include cadherin-
11 GST
fusion protein, cadherin-11 Fc fusion protein, cadherin-11 beta-galactosidase
fusion protein,
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-15-
cadherin-11 poly-His fusion protein, and cadherin-11 GFP fusion protein. Fe
fusion proteins
may comprise regions of the Ig constant domain, including without limitation
the hinge
region, the CH1 domain. the CH2 domain, and/or C113 domain, optionally
conjugated to the
cadherin-11 fragment via the hinge domain. The Fe portion may derive from
human
antibodies or non-human antibodies. The antibodies may be IgG1 or IgG2,
although they are
not so limited. Methods of making Fe fusion proteins are known in the art and
are described
at least in EP0464533.
In some embodiments, the cadherin-11 fusion proteins comprise the entire
extracellular domain of cadherin-11. In some embodiments, the cadherin-11
fusion protein
to comprises one or more extracellular subdomains of cadherin-11, such as
EC1. Examples
include fusion proteins comprising EC1, EC1/2, EC1-3, EC1-4, EC1/3, EC1/4, and
EC1/5, or
fragments of EC1. In important embodiments, the fusion protein binds to the
EC1 domain of
cadherin-11. Examples of cadherin-11 fusion proteins include cadherin-11-EC1-
Fc fusion
protein (comprising the EC1 domain of cadherin-11), cadherin-11-EC1/2-Fc
fusion protein
(comprising the EC1 and EC2 domains of cadherin-11), and cadherin-11-EC1-5-Fc
fusion
protein (comprising the EC1, EC2, EC3, EC4, and EC5 domains of cadherin-11).
Some
fusion proteins may comprise the first 40, first 39, first 38, first 37, first
36, first 35, or first 34
amino acids of the EC1 domain of cadherin-11, as described in WO 2009/089062.
Methods of synthesis of cadherin-11 fusion proteins can be found at least in
U.S.
Patent Nos. 5597725, 5639634, 5646250, 6787136, 6946768, 7488478, and 7589074
and
PCT Patent Publication No. WO 93/21302 and W02009/089062 (see for example SEQ
ID
NOs: 6 and 7, the nucleotide and amino acid sequences of a human cadherin-11-
EC1-hIgG2-
Fe fusion protein), the teachings of which relating to cadherin-11 fusion
proteins are
incorporated by reference herein.
Cadherin-11 Antibodies and Antibody Fragments:
Cadherin-11 antagonists that are cadherin-11 binding peptides may be
antibodies or
antigen-binding antibody fragments. The antibodies may be monoclonal
antibodies or
polyclonal antibodies. They may be chimeric antibodies including humanized
antibodies.
They may be four chain antibodies comprised of two heavy and two light chains,
or they may
be two chain antibodies such as those comprised of two heavy chains (such as
camelid
antibodies) or those comprised of a single heavy chain linked to a single
light chain (such as a
single chain Evs). They can be of any type (e.g., IgG. IgE, IgM, IgD, IgA and
IgY), class
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-16-
(e.g., IgGl, IgG2, IgG3, IgG4, IgAl and IgA2) or subclass. As discussed below,
these various
antibody forms can be prepared according to conventional methodology. The
antibodies and
antibody fragments may be naturally occurring or non-naturally occurring
including for
example recombinantly produced antibodies and fragments.
Significantly, as is well-known in the art, only a small portion of an
antibody
molecule, the paratope, is involved in the binding of the antibody to its
epitope (see, in
general, Clark, W.R. (1986) The Experimental Foundations of Modern Immunology
Wiley &
Sons, Inc., New York; Roitt, I. (1991) Essential Immunology, 7th Ed.,
Blackwell Scientific
Publications, Oxford). The pFc' and Fe regions, for example, are effectors of
the complement
to cascade but are not involved in antigen binding. An antibody from which
the pFc region has
been enzymatically cleaved, or which has been produced without the pFc'
region, designated
an F(ab')2 fragment, retains both of the antigen binding sites of an intact
antibody. Similarly,
an antibody from which the Fc region has been enzymatically cleaved, or which
has been
produced without the Fc region, designated an Fab fragment, retains one of the
antigen
binding sites of an intact antibody molecule. Proceeding further, Fab
fragments consist of a
covalently bound antibody light chain and a portion of the antibody heavy
chain denoted Ed.
The Fd fragments are the major determinant of antibody specificity (a single
Fd fragment may
be associated with up to ten different light chains without altering antibody
specificity) and Fd
fragments retain epitope-binding ability in isolation.
The terms Fab, Fab', Fc, Fd, pFc', F(ab'),), Fv, and dAb are employed with
either
standard immunological meanings [Klein, Immunology (John Wiley, New York, NY,
1982);
Clark, W.R. (1986) The Experimental Foundations of Modern Immunology (Wiley &
Sons,
Inc., New York); Roitt, I. (1991) Essential Immunology, 7th Ed., (Blackwell
Scientific
Publications, Oxford)]. Well-known functionally active antibody fragments
include but are
not limited to E(ab')2, Fab, Ev and Ed fragments of antibodies. These
fragments which lack
the Fc fragment of intact antibody, clear more rapidly from the circulation,
and may have less
non-specific tissue binding than an intact antibody (Wahl et al., J. Nucl.
Med. 24:316-325
(1983)). For example, single-chain antibodies can be constructed in accordance
with the
methods described in U.S. Patent No. 4,946,778 to Ladner et al. Such single-
chain antibodies
include the variable regions of the light and heavy chains joined by a
flexible linker moiety.
Methods for obtaining a single domain antibody ("FT) which comprises an
isolated variable
heavy chain single domain, also have been reported (see, for example, Ward et
al., Nature
341:644-646 (1989), disclosing a method of screening to identify an antibody
heavy chain
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-17-
variable region (VH single domain antibody) with sufficient affinity for its
target epitope to
bind thereto in isolated form). Methods for making recombinant Fv fragments
based on
known antibody heavy chain and light chain variable region sequences are known
in the art
and have been described, e.g., Moore et al., US Patent No. 4,462,334. Other
references
describing the use and generation of antibody fragments include e.g., Fab
fragments (Tijssen,
Practice and Theory of Enzyme Immunoassays (Elsevieer, Amsterdam, 1985)), Fv
fragments
(IIochman et al., Biochemistry 12: 1130 (1973); Sharon et al., Biochemistry
15: 1591 (1976);
Ehrileh et al., U.S. Patent No. 4,355,023) and portions of antibody molecules
(Audilore-
Hargreaves, U.S. patent No. 4,470,925). Thus, those skilled in the art may
construct antibody
fragments from various portions of intact antibodies without destroying the
specificity of the
antibodies.
Within the antigen-binding portion of an antibody, as is well-known in the
art, there
are complementarity determining regions (CDRs), which directly interact with
the epitope of
the antigen, and framework regions (FRs), which maintain the tertiary
structure of the
paratope (see, in general, Clark, 1986; Roitt, 1991). In both the heavy chain
Fd fragment and
the light chain of IgG immunoglobulins, there are four framework regions (FR1
through FR4)
separated respectively by three complementarity determining regions (CDR1
through CDR3).
The CDRs, and in particular the CDR3 regions, and more particularly the heavy
chain CDR3,
are largely responsible for antibody specificity.
It is now well-established in the art that the non-CDR regions of a mammalian
antibody may be replaced with similar regions of conspecific or heterospecific
antibodies
while retaining the epitopic specificity of the original antibody. This is
most clearly
manifested in the development and use of "humanized" antibodies in which non-
human CDRs
are covalently joined to human FR and/or Fc/pFc' regions to produce a
functional antibody.
Thus, for example, PCT International Publication No. WO 92/04381 and published
European
Patent Application No. EP 0239400 teach the production and use of humanized
murine
antibodies in which at least a portion of the murine FR regions have been
replaced by FR
regions of human origin. Such antibodies, including fragments of intact
antibodies with
antigen-binding ability, are often referred to as "chimeric" antibodies. There
are entities in
the United States which will synthesize humanized antibodies from specific
murine antibody
regions commercially, such as Protein Design Labs (Mountain View California),
Abgenix,
and Medarex.
81662851
-18-
Thus, as will be apparent to one of ordinary skill in the art, the present
invention also
provides for 14(abl),, Fab, Ev and Ed fragments; chimeric antibodies in which
the Fe and/or FR
and/or CDR1 'and/or CDR2 and/or light chain CDR3 regions have been replaced by
homologous human or non-human sequences; chimeric F(ab'), fragment antibodies
in which
the FR and/or CDR1 and/or CDR2 and/or light chain CDR3 regions have been
replaced by
homologous human or non-human sequences; chimeric Fab fragment antibodies in
which the
FR and/or CDR1 and/or CDR2 and/or light chain CDR3 regions have been replaced
by
homologous human or non-human sequences; and chimeric Fd fragment antibodies
it which
the FR and/or CDR1 and/or CDR2 regions have been replaced by homologous human
or non-
to human sequences. The present invention also includes single chain
antibodies.
In addition, human monoclonal antibodies may be made by any of the methods
known
in the art, such as those disclosed in US Patent No. 5,567,610, issued to
Borrebaeck et al., US
Patent No. 565,354, issued to Ostberg, US Patent No. 5,571,893, issued to
Baker et al,
Kozber, J. Inununol. 133: 3001 (1984), Brodeur, et al., Monoclonal Antibody
Production
Techniques and Applications, p. 51-63 (Marcel Dekker, Inc, new York, 1987),
and Boemer et
al., J. Innnunol., 147: 86-95 (1991). In addition to the conventional methods
for preparing
human monoclonal antibodies, such antibodies may also be prepared by
immunizing
transgenic animals that are capable of producing human antibodies (e.g.,
Jakobovits et at.,
PNAS USA, 90: 2551 (1993), Jakobovits et al., Nature, 362: 255-258 (1993),
Bruggennann et
al., Year in Mumma, 7:33 (1993) and US Patent No. 5,569,825 issued to
Lonberg).
Exemplary cadherin-11 antibodies and methods for making such antibodies are
described in U.S. Patent Nos. 5597725, 5639634, 5646250, 6787136, 6946768,
7488478, and
7589074, and PCT Patent Publication No. WO 93/21302 and W02009!089062.
Examples of
cadherin- II antibodies include 23C6, 13C2, 27E3, 5182 (commercially available
from
.. Lifespan Science), 1-11M1 antibody (cadherin-11 PX.1. specific antibody
produced by
hybridoma Ill Ml having Alla: Accession No, PTA-9699), 1:114 antibody
(cadherin-11 11111
specific antibody produced by hybridoma 1:11.4 having ATCC Accession No, pTA-
970.1),
8M5096/1A6 (commercially available from Acris Antibodies (imb11), 283416
(commercially
available from R&D Systems), and MAB2014 (commercially available from
Millipore.).
130 Examples of cadherin-11. antibody fragments include the Fab fragment of
antibodies 23C6,
l3C2, 27E3, 51'82, Ill M I antibody, 1114 antibody, 13M5096/1A6, 283416, and
MAB2014.
The antibodies or antibody fragments may comprise one or more CDRs from known.
CA 2805270 2017-08-23
81662851
-19-
antibodies such as the II1M1 or 1114 antibodies, as described in US
2009/0253200.
Antibodies and antibody fragments that bind to the EC1 domain of cadherin-11
are
described in US 2009/0253200 and W02009/089062.
Cadherin-11 antibodies may also be bispecific or bifunctional antibodies
capable of
binding to two different epitopes by virtue of their different antigen-binding
sites.
Still other cadherin-11 antibodies are camelid antibodies as described in PCT
Publication No. WO 94/04678 and U.S. Patent Publication No. 20080124324, and
their
In derivatives in the form of camelid nanobodies as in U.S. Patent No,
5759808. Camelid
antibodies and camelid nanobodies are commercially available from sources such
as Ablynx
(Belgium). It is to be understood that the cadherin-11 camelid antibodies can
be humanized
in a manner similar to that described herein for other antibody types.
In some embodiments, the antagonists do not include the antibodies disclosed
in U.S.
Patent 5,597,725 and in PCT application PCT/US93/03681 (WO/93/21302). Thus, in
certain
related embodiments, the agents of the invention (10 not embrace the
monoclonal antibodies
produced by the hybriclomas designated 30Q8A (HB11316), 30Q4H (HB11317), 45A5G
(HB
11318), 30S2F (HB11319), 45C6A (1-IB 11320), 30T11G (1-1B 11324), 64G11F JIB
11527).
Codherin-1 1 Nucleic Acid Antagonists:
A cadherin-11 antagonist may also be a nucleic acid. These antagonists include
nucleic acids that (1) encode a cadherin-11 polypeptide or a fragment thereof;
(2) are
cadherin-11 antisense molecules which inhibit the transcription or translation
of the foregoing
nucleic acid molecules; (3) are caclherin-11 inhibitory RNA (RNAi or siRNA);
(4) are
cadherin-11 ribozymes; (5) aptamers that are nucleic acid in nature but bind
to the cadherin-
11 as would binding peptides thereby interfering with the binding of eadherin-
11 to another
cadherin-11 or to another cadherin-11 counter-receptor. In sonic embodiments,
a cadherin- 11
antagonist that is a nucleic acid (1) hybridizes under stringent conditions to
a nucleic acid
having a sequence of SEQ ID NO: 1, and (2) codes for a caclherin-11
polypeptide or a
fragment thereof that is capable of binding specifically to cadherin-11.
CA 2805270 2017-08-23
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-20-
Cadherin-11 Encoding Nucleic Acids:
Cadherin-11 antagonists include nucleic acids that encode cadherin-11 and
fragments
of cadherin-11. The cadherin-11 full length nucleotide sequence is provided as
SEQ ID
NO:l. Nucleic acids comprising a nucleotide sequence of SEQ ID NO:1 may be
used as
antagonists, as an example. The cadherin-11 antagonists of the invention also
include
homologs and alleles of a nucleic acid molecule comprising a sequence of SEQ
ID NO: 1.
The cadherin-11 nucleic acid antagonists, may encode polypeptides which are
soluble
cadherin-11 polypeptides, membrane-bound polypeptides, or cadherin-11
fragments such as
fragments that consist of or comprise EC1 or a fragment thereof (e.g., the
first 33-37 amino
to acids of Ed). The soluble cadherin-11 polypeptides lack a transmembrane
domain and,
optimally, contain further amino acids which render the polypeptide soluble
(e.g., fusion
proteins, containing all or part of cadherin-11, which inhibit the binding of
cadherin-11 to
another cadherin-11). Cadherin-11 fragments which are membrane-bound (or
membrane
associated) preferably contain a transmembrane domain. Cadherin-11 nucleic
acid
antagonists further embrace nucleic acid molecules which code for a cadherin-
11 protein
having the amino acid sequence of SEQ ID NO: 2 (or SEQ ID NO:3, for example),
but which
may differ from the sequence of SEQ ID NO: 1 due to the degeneracy of the
genetic code.
Certain cadherin-11 nucleic acid antagonists can be identified by conventional
techniques, e.g., by identifying nucleic acid sequences which code for
cadherin-11 and which
hybridize to a nucleic acid molecule having the sequence of SEQ ID NO: 1 under
stringent
conditions. The term "stringent conditions," as used herein, refers to
parameters with which
the art is familiar. More specifically, stringent conditions, as used herein,
refer to
hybridization at 65C in hybridization buffer (3.5 x SSC, 0.02 % formanaide,
0.02 % polyvinyl
pyrolidone, 0.02 % bovine serum albumin, 2.5 mM NaH11304 (pH 7), 0.5 % SDS, 2
mM
ED'I'A). SSC is 0.15 M sodium chloride/0.15 M sodium citrate, pH 7; SDS is
sodium dodecyl
sulphate; and EDTA is ethylenediaminetetraacetic acid. After hybridization,
the membrane to
which the DNA is transferred is washed at 2x SSC at room temperature and then
at 0.1x
SSC/0.1x SDS at 65C.
There are other conditions, reagents, and so forth which can be used, which
result in a
similar degree of stringency. The skilled artisan will be familiar with such
conditions and,
thus, they are not given here. It will be understood, however, that the
skilled artisan will be
able to manipulate the conditions in a manner to permit the clear
identification of homologs
and alleles of the nucleic acid molecules of the invention. The skilled
artisan also is familiar
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-21-
with the methodology for screening cells and libraries for the expression of
further nucleic
acids molecules which can be isolated and sequenced. In screening for cadheri
n-11 sequences
for example, a Southern blot may be performed using the foregoing conditions,
together with
a radioactive probe. After washing the membrane to which the DNA is finally
transferred, the
membrane can be placed against x-ray film to detect the radioactive signal.
In general, cadherin-11 homologs and alleles typically will share at least 70%
nucleotide identity with SEQ. ID. NO: 1; and in some instances, will share at
least 75%
nucleotide identity; and in still other instances, will share at least 80%
nucleotide identity.
Watson-Crick complements of the foregoing nucleic acids are also embraced by
the invention.
The preferred cadherin-11 homologs have at least 85% sequence homology to SEQ.
ID. NO:
1. More preferably the cadherin-11 homologs have at least 90% and most
preferably at least
95% sequence homology to SEQ. ID. NO: 1. The homology can be calculated using
various,
publicly available software tools developed by NCBI (Bethesda, Maryland) that
can be
obtained through the internet. Exemplary tools include the BLAST system
available at the
NCRI website. Pairwi se and ClustalW alignments (13LOSUM30 matrix setting) as
well as
Kyte-Doolittle hydropathic analysis can be obtained using the Mac Vector
sequence analysis
software (Oxford Molecular Group).
The invention also includes degenerate nucleic acids which include alternative
codons
to those present in the naturally occurring nucleic acid that encodes, for
example, the human
.. cadherin-11 polypeptide. As is well known in the art, and as an example,
serine residues are
encoded by the codons TCA, AGT, TCC, TCG, TCT and AGC. Each of the six codons
is
equivalent for the purposes of encoding a serine residue. Thus, it will be
apparent to one of
ordinary skill in the art that any of the serine-encoding nucleotide codons
may be employed to
direct the protein synthesis apparatus, in vitro or in vivo, to incorporate a
serine residue.
Similarly, nucleotide sequence triplets which encode other amino acid residues
include, but
are not limited to, CCA, CCC, CCG and CCT (proline codons); CGA, CGC, CGG,
CGT,
AGA and AGG (arginine codons); ACA, ACC, ACG and ACT (threonine codons); AAC
and
AAT (asparagine codons); and ATA, ATC and ATT (isoleucine codons). Other amino
acid
residues may be encoded similarly by multiple nucleotide sequences.
As used herein with respect to nucleic acids, the term "isolated" means: (i)
amplified
in vitro by, for example, polymerase chain reaction (PCR); (ii) recombinantly
produced by
cloning; (iii) purified, as by cleavage and gel separation; or (iv)
synthesized by, for example,
chemical synthesis. An isolated nucleic acid is one which is readily
manipulable by
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-22-
recombinant DNA techniques well known in the art. Thus, a nucleotide sequence
contained
in a vector in which 5' and 3' restriction sites are known or for which
polymerase chain
reaction (PCR) primer sequences have been disclosed is considered isolated but
a nucleic acid
sequence existing in its native state in its natural host is not. An isolated
nucleic acid may be
.. substantially purified, but need not be. For example, a nucleic acid that
is isolated within a
cloning or expression vector is not pure in that it may comprise only a tiny
percentage of the
material in the cell in which it resides. Such a nucleic acid is isolated,
however, as the term is
used herein because it is readily manipulable by standard techniques known to
those of
ordinary skill in the art.
The cadherin-11 nucleic acid antagonist, in one embodiment, is operably linked
to a
gene expression sequence which directs the expression of the cadherin-11
nucleic acid
antagonist within a cell such as a eukaryotic cell. The "gene expression
sequence" is any
regulatory nucleotide sequence, such as a promoter sequence or promoter-
enhancer
combination which facilitates the efficient transcription and translation of
the cadherin-11
nucleic acid antagonist to which it is operably linked. The gene expression
sequence may, for
example, be a mammalian or viral promoter, such as a constitutive or inducible
promoter.
Constitutive mammalian promoters include, but are not limited to, the
promoters for the
following genes: hypoxanthine phosphoribosyl transferase (HPTR), adenosine
deaminase,
pyruvate kinase, beta-actin promoter and other constitutive promoters.
Exemplary viral
promoters which function constitutively in eukaryotic cells include, for
example, promoters
from the simian virus, papilloma virus, adenovirus, human immunodeficiency
virus (HIV),
Rous sarcoma virus, cytomegalovirus, the long terminal repeats (LTR) of
moloney leukemia
virus and other retroviruses, and the thymidine kinase promoter of herpes
simplex virus.
Other constitutive promoters are known to those of ordinary skill in the art.
The promoters
useful as gene expression sequences of the invention also include inducible
promoters.
Inducible promoters are expressed in the presence of an inducing agent. For
example, the
metallothionein promoter is induced to promote transcription and translation
in the presence
of certain metal ions. Other inducible promoters are known to those of
ordinary skill in the
art.
In general, the gene expression sequence shall include, as necessary, 5' non-
transcribing and 5' non-translating sequences involved with the initiation of
transcription and
translation, respectively, such as a TATA box, capping sequence, CAAT
sequence, and the
like. Especially, such 5' non-transcribing sequences will include a promoter
region which
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-23-
includes a promoter sequence for transcriptional control of the operably
joined cadherin-11
nucleic acid antagonist. The gene expression sequences optionally includes
enhancer
sequences or upstream activator sequences as desired.
Cadherin-11 nucleic acid antagonist may be used in both in vivo and in vitro
methods.
Nucleic acid molecules of the invention may be introduced into a cell in
vitro, followed by the
transfer of the cell to the site of fibrosis. The cell into which the nucleic
acid molecule is
introduced may be harvested from the site of fibrosis (e.g., a fibroblast) or
it may be a cell
which is not normally present at the site of inflammation. A sequence which
permits
expression of the nucleic acid in a particular tissue (or cell), such as for
example the lung, is
one which is selectively transcriptionally active in the tissue (or cell) and
thereby causes the
expression of the nucleic acid in the tissue (or cell). Those of ordinary
skill in the art will be
able to easily identify alternative promoters that are capable of expressing
such a nucleic acid
molecule in lung tissue, liver tissue, renal tissue, and the like, as
mentioned herein.
Alternatively, a cell transduced with the cadherin-11 nucleic acid antagonist
may be cultured
in vitro in order to produce a cadherin-11 protein antagonist or it may be
used in in vitro
screening assays. For example, the gene expression sequence may be used to
express
cadherin-11 in a cell which does not inherently express cadherin-11.
The nucleic acid molecule sequences of the invention and the gene expression
sequence are said to be "operably linked" when they are covalently linked in
such a way as to
place the transcription and/or translation of the nucleic acid antagonist
(e.g., a cadherin-11
coding sequence) under the influence or control of the gene expression
sequence. If it is
desired that nucleic acid molecule be translated into a functional protein,
two DNA sequences
are said to be operably linked if induction of a promoter in the 5' gene
expression sequence
results in the transcription of the nucleic acid molecule and if the nature of
the linkage
between the two DNA sequences does not (1) result in the introduction of a
frame-shift
mutation, (2) interfere with the ability of the promoter region to direct the
transcription of the
nucleic acid molecule, or (3) interfere with the ability of the corresponding
RNA transcript to
be translated into a polypeptide. Thus, a gene expression sequence would be
operably linked
to a nucleic acid molecule if the gene expression sequence were capable of
effecting
transcription of that nucleic acid molecule such that the resulting transcript
might be
translated into the desired polypeptide.
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-24-
Cadherin-11
The invention contemplates the use of RNA interference agents such as siRNA
and
shRNA as cadherin-11 antagonists. siRNA are RNA molecules capable of causing
interference and thus post-transcriptional silencing of specific genes in
cells, including
mammalian cells. siRNA comprise a double stranded region that is typically
about 5-50 base
pairs, more typically 10-40 base pairs, and even more typically 15-30 base
pairs in length.
The siRNA may be 20-50, 25-50 or 30-40 base pairs in length. These siRNA may
be digested
by the RNase III Dicer to yield smaller siRNA in the range of 19-28 base
pairs, including 19
base pairs, 21 base pairs, 23 base pairs, 25 base pairs, and 27 base pairs in
length. It is known
that siRNA in this size range can be incorporated into and acted upon by the
enzyme complex
called RNA-Induced Silencing Complex (RISC), with a net result of target RNA
degradation
and/or inhibition of any protein translation therefrom. In a similar manner,
double-stranded
RNAs with other regulatory functions such as microRNAs (miRNA) can also be
used.
Reference can be made to Bass, Nature 411: 428-29 (2001); Elbashir et al.,
Nature 411: 494-
98 (2001); Fire et al., Nature 391: 806-11 (1998); WO 01/75164, and US Patents
6506559,
7056704, 7078196, 7432250, for greater detail on siRNA as well as methods of
making
siRNA. siRNA to cadherin-11 are commercially available from sources such as
Dhammcon.
siRNA forms such as the R- and L-form will have overhangs on one or both ends.
As
.. discussed herein, an R-form siRNA has a 3' overhang on its antisense
strand. It may be
blunted on its other end and/or it may have a 3' overhang on its other end,
including an
overhang comprising DNA residues. Alternatively, an L-form siRNA has a 3'
overhang on
its sense strand. It may be blunted on its other end and/or it may have a 3'
overhang on its
other end, including an overhang comprising DNA residues.
siRNA may be comprised of ribonucleotides or a combination of ribonucleotides
and
deoxyribonucleotides, including in some instances modified versions of one or
both. For
example, ribonucleotides containing a non-naturally occurring base (instead of
a naturally
occurring base) such as uridines and/or cytidines modified at the 5-position,
e.g. 5-(2-
amino)propyl uridine, 5-bromo uridine, or adenosines and/or guanosines
modified at the 8-
position, e.g. 8-bromo guanosine, or deaza nucleotides, e.g. 7-deaza-
adenosine, or 0- and N-
alkylated nucleotides, e.g. N6-methyl adenosine can be incorporated into the
siRNA. As
another example, sugar-modified ribonucleotides having a 2' OH-group replaced
by a group
selected from II. OR, R, halo, SII, SR, NII7, NIIR, NR1 or CN, wherein R is C1-
C6 alkyl,
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-25-
alkenyl or alkynyl and halo is F, Cl, Br or I. As yet another example, the
backbone may be
modified to comprise modified backbone linkages such as but not limited to
phosphorothioates. The siRNA may comprise modifications at the base, sugar
and/or
backbone, including a variety of such modifications.
Thus, siRNA molecules can be provided as and/or derived from one or more forms
including, e.g., as one or more isolated small-interfering RNA (siRNA) double
stranded
duplexes, as longer double-stranded RNA (dsRNA), or as siRNA or dsRNA
transcribed from
a transcriptional cassette in a DNA plasmid. The siRNA molecules may have
overhangs (e.g.,
3' or 5' overhangs as described in Elbashir et al., Genes Dev., 15:188(2001)
or Nykanen et al.,
Cell, 107:309 (2001)), or may lack overhangs (i.e., have blunt ends). The
person of ordinary
skill in the art will appreciate and understand how such starting sources may
be modified in
order to arrive at the R- and L-forms described herein.
siRNA are targeted to genes in vivo or in vitro if all or part of the
nucleotide sequence
of their duplex (or double stranded) is complementary to a nucleotide sequence
of the targeted
gene, such as cadherin-1 1. siRNA made be synthesized based upon known (or
predicted)
nucleotide sequences of nucleic acids that encode proteins or other gene
products. The
sequence may be complementary to a translated or untranslated sequence in the
target. The
degree of complementarity between the siRNA and the target may be 100% or less
than
100%, provided that sufficient identity exists to a target to mediate target-
specific silencing.
The art is familiar with efficacious siRNA that are less than 100%
complementary to their
target.
The level of silencing or interference may be measured in any number of ways,
including quantitation of inRNA species and/or protein species. In some
instances, mRNA
quantitation is preferred particularly where the protein is intracellular or
otherwise difficult to
observe and/or assay. naRNA levels may be measured using RI-PCR or RACE, as an
example. Protein levels may be measured using immunohistochemical staining.
mRNA or
protein levels may be reduced by 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%,
95%,
99%, or even 100%. Depending on the application, partial reduction (i.e., less
than 100%
may be sufficient) as compared to the level in the absence of the exogenously
applied siRNA.
In some embodiments, the level is reduced by 80% or more than 80% as compared
to a
control that has not been exposed to exogenously applied siRNA.
Cadherin-11 Ribozymes:
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-26-
A cadherin-11 ribozyme is an enzymatic RNA molecule capable of catalyzing the
specific cleavage of cadherin-11 RNA. The cadherin-11 ribozyme binds to
cadherin-11 RNA
in a sequence specific manner (i.e., via sequence specific hybridization), and
this is followed
by endonucleolytic cleavage of the cadherin-11 RNA. Examples of ribozymes
include
engineered hairpin or hammerhead motif ribozymes. Ribozyme sequences
complementary to
a target such as cadherin-11 can be identified by scanning the target for
ribozyme cleavage
sites (e.g.. GUA, GUU, and GUC), and then generating a sequence having about
15-20
ribonucleotides spanning the cleavage site.
Cadherin-11 Antisense:
The cadherin-11 nucleic acid antagonist may be an antisense molecule (or
oligonucleotide). Antisense oligonucleotides that selectively bind to a
nucleic acid molecule
encoding a cadherin-11 polypeptide, or a fragment thereof, to decrease
cadherin-11 activity or
function are embraced by the present invention. As used herein, the term
"antisense
oligonucleotide- or "antisense" describes an oligonucleotide that is an
oligoribonucleotide,
oligodeoxyribonucleotide, modified oligoribonucleotide, or modified
oligodeoxyribonucleotide which hybridizes under physiological conditions to
DNA
comprising a particular gene or to an mRNA transcript of that gene and,
thereby, inhibits the
transcription of that gene and/or the translation of that mRNA. The antisense
molecules are
designed so as to interfere with transcription or translation of a target gene
upon hybridization
with the target gene or transcript. Those skilled in the art will recognize
that the exact length
of the antisense oligonucleotide and its degree of complementarity with its
target will depend
upon the specific target selected, including the sequence of the target and
the particular bases
which comprise that sequence. It is preferred that the antisense
oligonucleotide be
constructed and arranged so as to bind selectively with the target under
physiological
conditions, i.e., to hybridize substantially more to the target sequence than
to any other
sequence in the target cell under physiological conditions. Based upon SEQ ID
NO:1 or upon
allelic or homologous genomic and/or cDNA sequences, one of skill in the art
can easily
choose and synthesize any of a number of appropriate antisense molecules for
use in
accordance with the present invention. In order to be sufficiently selective
and potent for
inhibition, such antisense oligonucleotides should comprise at least 10 and,
more preferably,
at least 15 consecutive bases which are complementary to the target, although
in certain cases
modified oligonucleotides as short as 7 bases in length have been used
successfully as
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-27-
antisense oligonucleotides (Wagner et al., Nat. Med. 1(11):1116-1118, 1995).
Most
preferably, the anti sense oligonucleotides comprise a complementary sequence
of 20-30
bases.
Although oligonucleotides may be chosen which are antisense to any region of
the
gene or mRNA transcripts, in preferred embodiments the antisense
oligonucleotides
correspond to N-terminal or 5' upstream sites such as translation initiation,
transcription
initiation or promoter sites. In addition, 3'-untranslated regions may be
targeted by antisense
oligonucleotides. Targeting to mRNA splicing sites has also been used in the
art but may be
less preferred if alternative mRNA splicing occurs. In addition, the antisense
is targeted,
to preferably, to sites in which mRNA secondary structure is not expected
(see, e.g., Sainio et
al.. Cell Mol. Neurobiol. 14(5):439-457, 1994) and at which proteins are not
expected to bind.
Finally, although SEQ ID NO:1 discloses a cDNA sequence, one of ordinary skill
in the art
may easily derive the genomic DNA corresponding to this sequence. Thus, the
present
invention also provides for antisense oligonucleotides which are complementary
to the
genomic DNA corresponding to SEQ ID NO:l. Similarly, antisense to allelic or
homologous
cadherin-11 or alternatively, cadherin-11 counter-receptor cDNAs and genomic
DNAs are
enabled without undue experimentation.
In one set of embodiments, the antisense oligonucleotides of the invention may
be
composed of "natural" deoxyribonucleotides, ribonucleotides, or any
combination thereof.
That is, the 5' end of one native nucleotide and the 3' end of another native
nucleotide may be
covalently linked, as in natural systems, via a phosphodiester internucleoside
linkage. These
oligonucleotides may be prepared by art recognized methods which may be
carried out
manually or by an automated synthesizer. They also may be produced
recombinantly by
vectors.
In preferred embodiments, however, the antisense oligonucleotides of the
invention
also may include "modified" oligonucleotides. That is, the oligonucleotides
may be modified
in a number of ways which do not prevent them from hybridizing to their target
but which
enhance their stability or targeting or which otherwise enhance their
therapeutic effectiveness.
The term "modified oligonucleotide" as used herein describes an
oligonucleotide in
which (1) at least two of its nucleotides are covalently linked via a
synthetic internucleoside
linkage (i.e., a linkage other than a phosphodiester linkage between the 5'
end of one
nucleotide and the 3' end of another nucleotide) and/or (2) a chemical group
not nomially
associated with nucleic acids has been covalently attached to the
oligonucleotide. Preferred
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-28-
synthetic intemucleoside linkages are phosphorothioates, alkylphosphonates,
phosphorodithioates, phosphate esters, alkylphosphonothioates,
phosphoramidates,
carbamates, carbonates, phosphate triesters, acetamidates, carboxymethyl
esters and peptides.
The term "modified oligonucleotide" also encompasses oligonucleotides with a
covalently modified base and/or sugar. For example, modified oligonucleotides
include
oligonucleotides having backbone sugars which are covalently attached to low
molecular
weight organic groups other than a hydroxyl group at the 3' position and other
than a
phosphate group at the 5' position. Thus modified oligonucleotides may include
a 2'-0-
alkylated ribose group. In addition, modified oligonucleotides may include
sugars such as
arabinose instead of ribose.
As used herein with respect to polypeptides, the term "isolated" means
separated from
its native environment in sufficiently pure form so that it can be manipulated
or used for any
one of the purposes of the invention. Thus, isolated means sufficiently pure
to be used (i) to
raise and/or isolate antibodies, (ii) as a reagent in an assay, or (iii) for
sequencing, etc.
As used herein with respect to nucleic acids, the term "isolated" means: (i)
amplified
in vitro by, for example, polymerase chain reaction (PCR); (ii) recombinantly
produced by
cloning; (iii) purified, as by cleavage and gel separation; or (iv)
synthesized by, for example,
chemical synthesis. An isolated nucleic acid is one which is readily
manipulable by
recombinant DNA techniques well known in the art. Thus, a nucleotide sequence
contained
in a vector in which 5' and 3' restriction sites are known or for which
polymerase chain
reaction (PCR) primer sequences have been disclosed is considered isolated but
a nucleic acid
sequence existing in its native state in its natural host is not. An isolated
nucleic acid may be
substantially purified, but need not be. For example, a nucleic acid that is
isolated within a
cloning or expression vector is not pure in that it may comprise only a tiny
percentage of the
material in the cell in which it resides. Such a nucleic acid is isolated,
however, as the term is
used herein because it is readily manipulable by standard techniques known to
those of
ordinary skill in the art.
In general, the antagonists of the invention are divided into two classes:
biological
vectors and chemical/physical vectors. Biological vectors are useful for
delivery/uptake of
nucleic acids to/by a target cell. Biological vectors include, but are not
limited to, plasmids,
phagemids, viruses, other vehicles derived from viral or bacterial sources
that have been
manipulated by the insertion or incorporation of the nucleic acid sequences of
the invention,
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-29-
and additional nucleic acid fragments (e.g., enhancers, promoters) which can
be attached to
the nucleic acid sequences of the invention. Viral vectors are a preferred
type of biological
vector and include, but are not limited to, nucleic acid sequences from the
following viruses:
adenovirus; adeno-associated virus; retrovirus, such as moloney murine
leukemia virus;
harvey murine sarcoma virus; murine mammary tumor virus; rouse sarcoma virus;
SV40-type
viruses; polyoma viruses; Epstein-Barr viruses; papilloma viruses; herpes
virus; vaccinia
virus; polio virus; and RNA virus such as a retrovirus. One can readily employ
other vectors
not named but known in the art.
In addition to the biological vectors, chemical/physical vectors are useful
for
delivery/uptake of nucleic acids or polypeptides to/by a target cell. As used
herein, a
"chemical/physical vector" refers to a natural or synthetic molecule, other
than those derived
from bacteriological or viral sources, capable of delivering the cadherin-11
antagonist to a
cell.
A preferred chemical/physical vector of the invention is a colloidal
dispersion system.
Colloidal dispersion systems include lipid-based systems including oil-in-
water emulsions,
micelles, mixed micelles, and liposomes. A preferred colloidal system of the
invention is a
liposome. Liposomes are artificial membrane vessels which are useful as a
delivery vector in
vivo or in vitro. It has been shown that large unilamellar vessels (LUV),
which range in size
from 0.2 - 4.0 p.M can encapsulate large macromolecules. RNA, DNA, and intact
virions can
be encapsulated within the aqueous interior and be delivered to cells in a
biologically active
form (Fraley, et al., Trends Biochem. Sci., v. 6, p. 77 (1981)). In order for
a liposome to be an
efficient gene transfer vector, one or more of the following characteristics
should be present:
(1) encapsulation of the gene of interest at high efficiency with retention of
biological
activity; (2) preferential and substantial binding to a target cell in
comparison to non-target
cells; (3) delivery of the aqueous contents of the vesicle to the target cell
cytoplasm at high
efficiency; and (4) accurate and effective expression of genetic information.
Liposomes may be targeted to a particular tissue, by coupling the liposome to
a
specific ligand such as a monoclonal antibody, sugar, glycolipid, or protein
specific for the
particular tissue or cell type. Additionally, the vector may be coupled to a
nuclear targeting
peptide, which will direct the cadherin-11 modulating nucleic acid molecule to
the nucleus of
the host cell.
Liposomes are commercially available from Gibco BRL, for example, as
LIPOFECTINTm and LIPOFECTACETm, which are formed of cationic lipids such as
N41-(2,
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-30-
3 dioleyloxy)-propylj-N, N, N-trimethylammonium chloride (DOTMA) and dimethyl
dioetadecylammonium bromide (DDAB). Methods for making liposomes are well
known in
the art and have been described in many publications. Liposomes also have been
reviewed by
Gregoriadis, G. in Trends in Biotechnology, V. 3, p. 235-241 (1985).
The invention contemplates administration of a cadherin-11 antagonist to a
subject as
well as ex vivo contact of cells from the subject, particularly fibroblasts
associated with the
inflammatory disorder, with the cadherin-11 antagonist ex vivo, followed by re-
introduction
of the antagonist into the subject.
The invention further provides a pharmaceutical composition (i.e., a
pharmaceutical
preparation) for modulating a cadherin-11 function in a subject. The
composition includes a
pharmaceutically acceptable carrier and a cadherin-11 antagonist.
The pharmaceutical preparations, as described above, are administered in
effective
amounts. For therapeutic applications, it is generally that amount sufficient
to achieve a
medically desirable result. In general, a therapeutically effective amount is
that amount
necessary to delay the onset of, inhibit the progression of, or halt
altogether the particular
condition being treated. As an example, the effective amount is generally that
amount which
serves to alleviate the symptoms (e.g., pain, inflammation, etc.) of the
disorders described
herein. The effective amount will depend upon the mode of administration, the
particular
condition being treated and the desired outcome. It will also depend upon the
stage of the
condition, the severity of the condition, the age and physical condition of
the subject being
treated, the nature of concurrent therapy, if any, the duration of the
treatment, the specific
route of administration and like factors within the knowledge and expertise of
the medical
practitioner. For prophylactic applications, it is that amount sufficient to
delay the onset of,
inhibit the progression of, or halt altogether the particular condition being
prevented, and may
be measured by the amount required to prevent the onset of symptoms.
Generally, doses of active compounds of the present invention would be from
about
0.01 mg/kg per day to 1000 mg/kg per day, preferably from about 0.1 mg/kg to
200 mg/kg
and most preferably from about 0.2 mg/kg to about 20 mg/kg, in one or more
dose
administrations daily, for one or more days. It is expected that doses ranging
from 1-500
mg/kg, and preferably doses ranging from 1-100 mg/kg, and even more preferably
doses
ranging from 1-50 mg/kg, will be suitable. The preferred amount can be
determined by one
of ordinary skill in the art in accordance with standard practice for
determining optimum
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-31-
dosage levels of the agent. It is generally preferred that a maximum dose of a
cadherin-11
antagonist that is the highest safe dose according to sound medical judgment
be used.
The cadherin-11 antagonists of the invention can be administered to a subject
in need
of such treatment in combination with concurrent therapy for treating an
inflammatory
disorder. The concurrent therapy may be invasive, or may involve drug therapy.
The drug
therapies are administered in amounts which are effective to achieve the
physiological goals
(e.g., to reduce inflammation), in combination with, for example, the cadherin-
11 antagonist
of the invention. Thus, it is contemplated that the drug therapies may be
administered in
amounts which are not capable of preventing or reducing the physiological
consequences of
an inflammatory disorder when the drug therapies are administered alone but
which are
capable of reducing the consequences when administered in combination with the
cadherin-11
antagonist of the invention.
The cadherin-11 antagonist may be administered alone or in combination with
the
above-described drug therapies as part of a pharmaceutical composition. Such a
pharmaceutical composition may include the cadherin-1 1 antagonist in
combination with any
standard physiologically and/or pharmaceutically acceptable carriers which are
known in the
art. The compositions should be sterile and contain a therapeutically
effective amount of the
cadherin-11 antagonist in a unit of weight or volume suitable for
administration to a patient.
The term "pharmaceutically-acceptable carrier" as used herein means one or
more
compatible solid or liquid filler, diluents or encapsulating substances which
are suitable for
administration into a human or other animal. The term "pharmaceutically
acceptable" means a
non-toxic material that does not interfere with the effectiveness of the
biological activity of
the active ingredients. Pharmaceutically acceptable further means a non-toxic
material that is
compatible with a biological system such as a cell, cell culture, tissue, or
organism. The term
"carrier" denotes an organic or inorganic ingredient, natural or synthetic,
with which the
active ingredient is combined to facilitate the application. The
characteristics of the carrier
will depend on the route of administration. The components of the
pharmaceutical
compositions also are capable of being commingled with the agents of the
present invention,
and with each other, in a manner such that there is no interaction which would
substantially
impair the desired pharmaceutical efficacy. The pharmaceutically acceptable
carrier must be
sterile for in vivo administration. Physiologically and pharmaceutically
acceptable carriers
include diluents, fillers, salts, buffers, stabilizers, solubilizers, and
other materials which are
well known in the art.
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-32-
Compositions suitable for parenteral administration conveniently comprise a
sterile
aqueous preparation of the cadherin-1 1 antagonists, which is preferably
isotonic with the
blood of the recipient. This aqueous preparation may be formulated according
to known
methods using suitable dispersing or wetting agents and suspending agents. The
sterile
injectable preparation also may be a sterile injectable solution or suspension
in a non-toxic
parenterally-acceptable diluent or solvent, for example, as a solution in 1,3-
butane diol.
Among the acceptable vehicles and solvents that may be employed are water,
Ringer's
solution, and isotonic sodium chloride solution. In addition, sterile, fixed
oils are
conventionally employed as a solvent or suspending medium. For this purpose,
any bland
m fixed oil may be employed including synthetic mono- or di-glycerides. In
addition, fatty acids
such as oleic acid may be used in the preparation of injectables. Carrier
formulations suitable
for oral, subcutaneous, intravenous, intramuscular, etc. administrations can
be found in
Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, PA.
A variety of administration routes are available. The particular mode selected
will
depend, of course, upon the particular drug selected, the severity of the
condition being
treated, and the dosage required for therapeutic efficacy. The methods of the
invention,
generally speaking, may be practiced using any mode of administration that is
medically
acceptable, meaning any mode that produces effective levels of the active
compounds without
causing clinically unacceptable adverse effects. Such modes of administration
include oral,
rectal, topical, nasal, interdermal, or parenteral routes. The term
"parenteral" includes
subcutaneous, intravenous, intramuscular, or infusion. Intravenous or
intramuscular routes
are not particularly suitable for long-term therapy and prophylaxis. They
could, however, be
preferred in emergency situations. Oral administration will be preferred for
prophylactic
treatment because of the convenience to the patient as well as the dosing
schedule. It will be
.. understood that the route of administration may also depend in some
instances on the
condition being treated. For example, if the condition is topical (e.g.,
atopic dermatitis or
eczema), then the antagonists may be applied topically, intradermally or
subcutaneously.
Topical administration may be achieved through the use of pads, gauzes,
bandages,
compression garments, creams, lotions, sprays, emollients, and the like, all
of which comprise
.. the antagonist of interest.
The pharmaceutical compositions may conveniently be presented in unit dosage
form
and may be prepared by any of the methods well-known in the art of pharmacy.
All methods
include the step of bringing the cadherin-11 antagonists into association with
a carrier which
81662851
constitutes one or more accessory ingredients. In general, the compositions
are prepared by
uniformly and intimately bringing the cadherin-11 antagonists into association
with a liquid
carrier, a finely divided solid carrier, or both, and then, if necessary,
shaping the product.
Compositions suitable for oral administration may be presented as discrete
units, such as
capsules, tablets, lozenges, each containing a predetermined amount of the
cadherin-fl
antagonists. Other compositions include suspensions in aqueous liquids or non-
aqueous
liquids such as a syrup, elixir or an emulsion.
In one particular embodiment, the preferred vehicle for delivery of the
cadherin-11
antagonist of the invention is a biocompatible microparticle or implant that
is suitable for
to implantation into or in the vicinity of an afflicted tissue or organ in
the recipient. Exemplary
bioerodible implants that are useful in accordance with this method are
described in PCT
International application no. PCT/US/03307 (Publication No. WO 95/24929,
entitled
"Polymeric Gene Delivery System"). PCT/US/0307 describes a biocompatible,
preferably
biodegradable polymeric matrix. for containing an exogenous gene under the
control of an
appropriate promoter. The polymeric matrix is used to achieve sustained
release of the .
exogenous gene in the subject. In accordance with the instant invention, the
eaclherin-11
antagonists described herein are encapsulated or dispersed within the
biocompatible,
preferably biodegradable polymeric matrix disclosed in PCT/US/03307.
(Publication No. WO 95/24929). The polymeric
matrix preferably is in the form of a microparticle such as a microsphere
(wherein, for
example, the cadherin-11 antagonist is dispersed throughout a solid polymeric
matrix) or a
microeapsule (wherein, for example, the cadhefin-11 antagonist is stored in
the core of a
polymeric shell). Other forms of the polymeric matrix for containing the
cadherin-11
antagonist include films, coatings, gels, implants, and stents. The size and
composition of the
polymeric matrix device is selected to result in favorable release kinetics in
the tissue into
which the matrix device is implanted. The size of the polymeric matrix devise
is further
selected according to the method of delivery which is to be used, typically
injection into a
tissue, or administration of a suspension by aerosol into the nasal and/or
pulmonary areas.
The polymeric matrix composition can be selected to have both favorable
degradation rates
and also to be formed of a material which is bioadhesive, to further increase
the effectiveness
of transfer when the devise is administered to a particular location in the
body. The matrix
composition also can be selected not to degrade, but rather, to release by
diffusion over an
extended period of time.
CA 2805270 2017-08-23
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-34-
Both non-biodegradable and biodegradable polymeric matrices can be used to
deliver
the cadherin-11 antagonists. Biodegradable matrices are preferred. Such
polymers may be
natural or synthetic polymers. Synthetic polymers are preferred. The polymer
is selected
based on the period of time over which release is desired, generally in the
order of a few hours
to a year or longer. Typically, release over a period ranging from between a
few hours and
three to twelve months is most desirable. The polymer optionally is in the
form of a hydrogel
that can absorb up to about 90% of its weight in water and further, optionally
is cross-linked
with multi-valent ions or other polymers.
In general, the cadherin-11 antagonists of the invention are delivered using
the
bioerodible implant by way of diffusion, or more preferably, by degradation of
the polymeric
matrix. Exemplary synthetic polymers which can be used to form the
biodegradable delivery
system include: polyamides, polycarbonates, polyalkylenes, polyalkylene
glycols,
polyalkylene oxides, polyalkylene terepthalates, polyvinyl alcohols, polyvinyl
ethers,
polyvinyl esters, poly-vinyl halides, polyvinylpyrrolidone, polyglycolides,
polysiloxanes,
polyurethanes and co-polymers thereof, alkyl cellulose, hydroxyalkyl
celluloses, cellulose
ethers, cellulose esters, nitro celluloses, polymers of acrylic and
methacrylic esters, methyl
cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxy-propyl methyl
cellulose,
hydroxybutyl methyl cellulose, cellulose acetate, cellulose propionate,
cellulose acetate
butyrate, cellulose acetate phthalate, carboxylethyl cellulose, cellulose
triacetate, cellulose
sulphate sodium salt, poly(methyl methacrylate), poly(ethyl methacrylate),
poly(butylmethacrylate), poly(isobutyl methacrylate), poly(hexylmethacrylate),
poly(isodecyl
methacrylate), poly(lauryl methacrylate), poly(phenyl methacrylate),
poly(methyl acrylate),
poly(isopiropyl acrylate), poly(isobutyl acrylate), poly(ociadecyl acrylate),
polyethylene,
polypropylene, poly(ethylene glycol), poly(ethylene oxide), poly(ethylene
terephthalate),
poly(vinyl alcohols), polyvinyl acetate, poly vinyl chloride, polystyrene and
polyvinylpyrrolidone.
Examples of biodegradable polymers include synthetic polymers such as polymers
of
lactic acid and glycolic acid, polyanhydrides, poly(ortho)esters,
polyurethanes, poly(butic
acid), poly(valeric acid), and poly(lactide-co-caprolactone), and natural
polymers such as
alginate and other polysaccharides including dextran and cellulose, collagen,
chemical
derivatives thereof (substitutions, additions of chemical groups, for example,
alkyl, alkylene,
hydroxylations, oxidations, and other modifications routinely made by those
skilled in the
art), albumin and other hydrophilic proteins, zein and other prolamines and
hydrophobic
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-35-
proteins, copolymers and mixtures thereof. In general, these materials degrade
either by
enzymatic hydrolysis or exposure to water in vivo, by surface or bulk erosion.
Bioadhesive polymers of particular interest include bioerodible hydrogels
(described
by H.S. Sawhney, C.P. Pathak and J.A. Hubell in Macromolecules, 1993, 26, 581-
587, the
teachings of which are incorporated herein), polyhyaluronic acids, casein,
gelatin, glutin,
polyanhydrides, polyacrylic acid, alginate, chitosan, poly(methyl
methacrylates), poly(ethyl
methacrylates), poly(butylmethacrylate), poly(isobutyl methacrylate),
poly(hexylmethacrylate), poly(isodecyl methacrylate), poly(lauryl
methacrylate), poly(phenyl
methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl
acrylate), and
poly(octadecyl acrylate).
Examples of non-biodegradable polymers include ethylene vinyl acetate,
poly(meth)acrylic acid, polyamides, copolymers and mixtures thereof.
Other delivery systems can include time-release, delayed release or sustained
release
delivery systems. Such systems can avoid repeated administrations of the
cadherin-11
antagonists described above, increasing convenience to the subject and the
physician. Many
types of release delivery systems are available and known to those of ordinary
skill in the art.
They include the above-described polymeric systems, as well as polymer base
systems such
as poly(lactide-glycolide), copolyoxalates, polycaprolactones,
polyesteramides,
polyorthoesters, polyhydroxybutyric acid, and polyanhydrides. Microcapsules of
the
foregoing polymers containing drugs are described in, for example, U.S. Patent
5,075,109.
Delivery systems also include non-polymer systems that are: lipids including
sterols such as
cholesterol, cholesterol esters and fatty acids or neutral fats such as mono-
di- and tri-
glycerides; hydrogel release systems; silastic systems; peptide based systems;
wax coatings;
compressed tablets using conventional binders and excipients; partially fused
implants: and
the like. Specific examples include, but are not limited to: (a) erosional
systems in which the
cadherin-11 antagonist is contained in a form within a matrix such as those
described in U.S.
Patent Nos. 4,452,775, 4,675,189 and 5,736,152 and (b) diffusional systems in
which an
active component permeates at a controlled rate from a polymer such as
described in U.S.
Patent Nos. 3,854,480, 5,133,974 and 5,407,686. In addition, pump-based
hardware delivery
systems can be used, some of which are adapted for implantation.
Use of a long-term sustained release implant may be particularly suitable for
treatment
of chronic conditions. Long-term release, are used herein, means that the
implant is
constructed and arranged to delivery therapeutic levels of the active
ingredient for at least 30
81662851
-36-
days, and preferably 60 days. Long-term sustained release implants are well-
known to those
of ordinary skill in the art and include some of the release systems described
above.
The cadherin-11 antagonists can also be used, for example, to target a toxin
(e.g.,
ricin) or a detectable agent (e.g., a radiolabel, a fluorescent label, an
enzyme label) to cells
which express cadherin-11 counter-receptors or cadherin-11. Methods for
coupling such
toxins and/or agents to proteins and/or antibodies for in vivo and in vitro
applications are
disclosed in, for example, Killen and Lindstrom (1984), "Specific killing of
lymphocytes that
cause experimental Autoimmune Myestenia Gravis by toxin-acetylcholine receptor
conjugates", J. 1mmun. 133:1335; Jansen, F.K., etal. (1982), "Immunotoxins:
Hybrid
molecules combining high specificity and potent cytotoxicity", Immunolog. Rev.
62:185-216.
See also U.S. Patent Nos. 3,652,761; 4,478,946 and 4,554,088.
The invention will be more fully understood by reference to the following
examples.
.. These examples, however, are merely intended to illustrate the embodiments
of the invention
and are not to be construed to limit the scope of the invention. It is also to
be understood that
the reference figures are illustrative only and are not essential to the
enablement of the
claimed invention.
EXAMPLES
Example I: Treatment of atopic dermatitis by anti-cadherin-11 monoclonal
antibody.
To determine whether cadherin-11 modulates skin inflammation, a mouse model of
atopic dermatitis (AD) was used. In this model, skin inflammation displays
many features of
human All or eczema which is a chronically relapsing inflammatory skin
disorder. A
hallmark of AD is dry itchy skin. Scratching causes skin injury and worsens
the disease
symptoms. Therefore, the model mimics skin irritation by first shaving the
back of the mouse
and then applying and removing tegaderm tape several times from the same
shaved area.
Mice are then epicutaneously (EC) sensitized with saline (SAL) or ovalbumin
(OVA) (100
jig/100 of saline) soaked gauze which is applied to the tape-stripped area at
days 0; 3, 22,
.. 25, 43, and 46. The gauze is placed on the back skin for one week periods
as shown in FIG.
4. Chronic skin inflammation develops by repeating the procedure a total of
three times at
two weeks intervals (FIG. 4).
CA 2805270 2017-08-23
CA 02805270 2013-01-14
WO 2011/153397 PCT/US2011/039002
-37-
To determine the effect of blocking cadherin-11 on this skin inflammation
model, we
treated mice with anti-cadherin-11 (cad-11) monoclonal antibody (mAb) during
the third
cycle as shown in FIG. 4. mAb was i.p. injected at days 43, 45, and 47. Mice
were sacrificed
and examined at day 50. For comparison, after the last tape adhesion and
removal, skin
tissues were collected to compare the immune responses against OVA between
untreated and
anti-cadherin-11 mAb treated mice.
Anti-cadherin-11 mAb treatment significantly reduced epidermal and dermal
thickness
in H&E staining of sections from control (saline, SAL) or OVA- sensitized skin
(FIGs. 5A
and 5B). Although the total number of CD4+ T cells recruited the lesions was
not
significantly changed in anti-cadherin-11 mAb treated mice, eosinophil
recruitment was
significantly decreased in anti-cadherin-11 mAb-treated mice compared to
untreated mice
(FIG. SC). Eosinophils are another key effector cell type. Consistent with
this, the level of
eotaxin (a key chemokine for eosinophils) was reduced in OVA-sensitized skin
from anti-
cadherin-11 mAb treated mice.
Since the pathology of this mouse model of AD is usually dominated by Th2
and/or
Th17 immune responses, we determined if anti-cadherin-11 mAb treatment
modifies the
immune responses in the affected skin. We detected Th2 type cytokines (IL-4
and IL-13),
Th17 type cytokine (IL-17), and Th1 type cytokine (IFN-7) by quantitative real
time PCR in
the saline (SAL)- or ovalbumin (OVA)- sensitized skin from control or anti-
cadherin-11
.. mAb-treated mice. Strikingly, there was significantly less production of
both IL-4 and IL-17
in the affected skin from anti-cadherin-11 mAb treated mice compared to
control mice (FIG.
6A). The production of IL-13 also showed a similar pattern as IL-4 or IL-17
(FIG. 6A). In
contrast, the level of IFN-7 (Thl type cytokine) was not changed by anti-
cadherin-11 mAb
treatment (FIG. 6A). Thus, anti-cadherin-11 mAb treatment blocked the Th2
and/or Th17-
immune responses against antigen in the sensitized skin.
These in vivo data indicate that anti-cadherin-11 mAb treatment reduces local
allergic
skin inflammation.
Example 2: Cadherin-11 deficient mice are protected from colitis in an
experimentally
.. induced model of inflammatory bowel disease.
The role of cadherin-11 in inflammatory bowel disease was analyzed using a
dextran
sodium sulfate induced inflammatory bowel disease mouse model. Wildtype and
cadherin-
11-deficient (cad-114-) mice ingested 2% dextran sodium sulfate in their
drinking water ad
CA 02805270 2013-01-14
WO 2011/153397
PCT/US2011/039002
-3 8-
libitum for days 0 through 6 (11,6 mice per group). The development of colitis
was followed
by the severity of weight loss over time, expressed as a change from starting
body weight
(HG. 7A). Cadherin-111- mice do not lose weight compared to wildtype mice
indicating that
colitis is less severe in cad-11-i- mice. In addition, on day 9, mice were
sacrificed to measure
colon length (FIG. 7B). Shorter colon lengths correlate with more severe
colitis. The longer
length in cad-111- mice reflects less colonic disease. These results show that
cadherin-11
deficient mice to not develop severe colitis compared to wild type mice in
this model system.
Cadherin-11 inhibition, for example via anti-cadherin-11 antibodies, is
expected to also
interfere with the development (or maintenance) of inflammatory bowel diseases
such as
colitis.
EQUIVALENTS
While several inventive embodiments have been described and illustrated
herein, those
of ordinary skill in the art will readily envision a variety of other means
and/or structures for
performing the function and/or obtaining the results and/or one or more of the
advantages
described herein, and each of such variations and/or modifications is deemed
to be within the
scope of the inventive embodiments described herein. More generally, those
skilled in the art
will readily appreciate that all parameters, dimensions, materials, and
configurations
described herein are meant to be exemplary and that the actual parameters,
dimensions,
materials, and/or configurations will depend upon the specific application or
applications for
which the inventive teachings is/are used. Those skilled in the art will
recognize, or be able to
ascertain using no more than routine experimentation, many equivalents to the
specific
inventive embodiments described herein. It is, therefore, to be understood
that the foregoing
embodiments are presented by way of example only and that, within the scope of
the
appended claims and equivalents thereto, inventive embodiments may be
practiced otherwise
than as specifically described and claimed. Inventive embodiments of the
present disclosure
are directed to each individual feature, system, article, material, kit,
and/or method described
herein. In addition, any combination of two or more such features, systems,
articles,
materials, kits, and/or methods, if such features, systems, articles,
materials, kits, and/or
methods are not mutually inconsistent, is included within the inventive scope
of the present
disclosure.
81662851
-39-
All definitions, as defined and used herein, should be understood to control
over
dictionary definitions and/or ordinary meanings of the defined terms.
The indefinite articles "a" and "an," as used herein in the specification and
in the
claims, unless clearly indicated to the contrary, should be understood to mean
"at least one."
The phrase "and/or," as used herein in the specification and in the claims,
should be
ID understood to mean "either or both" of the elements so conjoined, i.e.,
elements that are
conjunctively present in some cases and disjunctively present in other cases.
Multiple
elements listed with "and/or" should be construed in the same fashion, i.e.,
"one or more- of
the elements so conjoined. Other elements may optionally be present other than
the elements
specifically identified by the "and/or" clause, whether related or unrelated
to those elements
specifically identified. Thus, as a non-limiting example, a reference to "A
and/or B", when
used in conjunction with open-ended language such as "comprising" can refer,
in one
embodiment, to A only (optionally including elements other than B); in another
embodiment,
to B only (optionally including elements other than A); in yet another
embodiment, to both A
and B (optionally including other elements); etc.
As used herein in the specification and in the claims, "or" should be
understood to
have the same meaning as "and/or" as defined above. For example, when
separating items in
a list, "or" or "and/or" shall be interpreted as being inclusive, i.e., the
inclusion of at least one,
but also including more than one, of a number or list of elements, and,
optionally, additional
unlisted items. Only terms clearly indicated to the contrary, such as "only
one of' or "exactly
one of," or, when used in the claims, "consisting of," will refer to the
inclusion of exactly one
element of a number or list of elements. In general, the term "or" as used
herein shall only be
interpreted as indicating exclusive alternatives (i.e. "one or the other but
not both") when
preceded by terms of exclusivity, such as "either," "one of," "only one of,-
or "exactly one
of." "Consisting essentially of," when used in the claims, shall have its
ordinary meaning as
used in the field of patent law.
As used herein in the specification and in the claims, the phrase "at least
one," in
reference to a list of one or more elements, should be understood to mean at
least one element
selected from any one or more of the elements in the list of elements, but not
necessarily
CA 2805270 2017-08-23
CA 02805270 2013-01-14
-40-
including at least one of each and every element specifically listed within
the list of elements
and not excluding any combinations of elements in the list of elements. This
definition also
allows that elements may optionally be present other than the elements
specifically identified
within the list of elements to which the phrase "at least one" refers, whether
related or
unrelated to those elements specifically identified. Thus, as a non-limiting
example, "at least
one of A and B" (or, equivalently, "at least one of A or B," or, equivalently
"at least one of A
and/or B") can refer, in one embodiment, to at least one, optionally including
more than one,
A, with no B present (and optionally including elements other than B); in
another
embodiment, to at least one, optionally including more than one, B, with no A
present (and
optionally including elements other than A); in yet another embodiment, to at
least one,
optionally including more than one, A, and at least one, optionally including
more than one, B
(and optionally including other elements); etc.
IL should also be understood that, unless clearly indicated to the contrary,
in any
methods claimed herein that include more than one step or act, the order of
the steps or acts of
the method is not necessarily limited to the order in which the steps or acts
of the method are
recited.
In the claims, as well as in the specification above, all transitional phrases
such as
"comprising," "including," "carrying," "having," "containing," "involving,"
"holding,"
"composed of," and the like are to be understood to be open-ended, i.e., to
mean including but
not limited to. Only the transitional phrases "consisting of" and "consisting
essentially of'
shall be closed or semi-closed transitional phrases, respectively, as set
forth in the United
States Patent Office Manual of Patent Examining Procedures, Section 2111.03.