Note: Descriptions are shown in the official language in which they were submitted.
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
DEAMIDATED BARLEY PROTEINS
Field of the Invention
The present invention is directed to deamidated barley proteins.
Background of the Invention
Deamidation is a chemical modification known to improve solubility and other
functional properties of some food proteins (Malabat et al., 2001). In cereal
proteins,
deamidation is a particularly important modification since up to one-third of
their total amino
acid content is glutamine (Hamada, 1992; Lan et al., 2010). The conversion of
the amide
groups on glutamine side chains into acid groups is believed to improve cereal
protein
solubility as a high content of glutamine residues may cause the aggregation
of the protein
molecules via hydrogen bonding. Deamidation may also partially unfold the
protein and
indirectly lead to protein hydrolysis by cleavage of the peptide bond (Cabra
et al., 2007).
These changes improve the functional properties of wheat, corn, rice and soy
proteins, making
them useful for the food and pharmaceutical industries (Cabra et al., 2007;
Chan and Ma,
1999; Hamada and Marshall, 1989; Li et al., 2009; Li et al., 2010; Matsudomi
et al., 1985;
Paraman et al., 2007; Yong et al., 2006). However, excessive molecular charges
on proteins
and peptide bond cleavage could affect the protein structure and cause
undesirable functional
properties that reduce their utility (Cabra et al., 2007).
In general, systematic research of protein molecular structure and subsequent
functionality (as a function of degree of deamidation values) is limited. More
specifically,
information about protein structure transition at low deamidation levels and
subsequent
functionality is lacking.
As the fourth most widely cultivated cereal in the world after wheat, rice and
corn,
barley is gaining increasing popularity as a part of the human diet because of
the recent health
WO 2012/006731 CA 02805677 2013-01-16PCT/CA2011/000831
claim made about its 13-glucan (FDA, 2005; Yalcin et al., 2008). This soluble
dietary fibre
component of barley is known to reduce both blood cholesterol and the glycemic
index (Kalra
and Jood, 2000; Wood, 2004). Additionally, barley represents a potential
abundant and
affordable source of plant proteins. The overall barley grain protein content
is 8-13% (w/w)
depending on the variety (Pomeranz and Shands, 1974).
Glutelin comprises approximately 35-40% of the total barley grain protein, and
is one
of the main storage proteins of barley (Shewry, 1993). Glutelin exhibits high
surface
hydrophobicity which markedly reduces protein solubility, hindering glutelin
applications.
Hordein is a barley prolamin and comprises approximately 35-55% of the total
barley
grain protein, and is the main storage protein for barley (Shewry, 1993).
Barley hordeins are
divided into four groups based on their electrophoretic mobilities and amino
acid
compositions: the B (30-50 kDa, sulfur-rich) and C (55-80 kDa, sulfur-poor)
hordeins (70-
80% and 10-20% of the hordein fraction, respectively) and the D (80-90 kDa)
and A (15 kDa)
hordeins (less than 5% of the total hordein fraction). The A hordeins are
likely alcohol-
soluble albumins or globulins, or breakdown products of larger hordeins rather
than true
hordeins. C and some B hordeins appear as monomers, while most B and D
hordeins are
linked by inter-chain disulfide bridges (Celus et al., 2006).
Hordein is rich in hydrophobic amino acids (40%), with the highest levels
corresponding to proline, leucine, and valine (Wang et al., 2010). This amino
acid profile
results in high protein surface hydrophobicity, which favors rapid adsorption
at the
hydrophobic interface and then forms a viscoelastic film to stabilize foams
and emulsions
(Wang et al., 2010). However, these features also result in a marked reduction
in water
solubility and a tendency of protein aggregation. Both of these changes hinder
their
functional application, since protein water solubility is critical to impart
other desired and
necessary properties such as emulsifying and foaming functionalities
(Kinsella, 1976).
2
WO 2012/006731 CA 02805677 2013-01-16
PCT/CA2011/000831
Barley endosperm proteins such as glutelin and hordein are typically regarded
as
contaminants by the brewing industry and are precipitated out in the spent
grains for use as
animal feed. Development of extraction and fractionation techniques of barley
proteins and
the subsequent characterization of their functional properties may facilitate
the diversified
opportunities for barley protein fractions in food and non-food applications,
and identify
value-added applications for barley proteins.
Therefore, there is a need in the art for converting barley byproducts into
useful
products.
Summary Of The Invention
The present invention relates to partially deamidated barley proteins having
various
degrees of deamidation. In one embodiment, the deamidated proteins have
improved
functional properties with respect to solubility, and emulsifying and foaming
at acidic and
neutral pHs, compared to those of a non-deamidated protein.
In one aspect, the invention comprises a method of producing a deamidated
protein
from barley, comprising the steps of:
a) extracting protein from barley;
b) treating the extracted protein with an alkaline or acidic solution for a
sufficient
time and temperature for partial deamidation; and
c) recovering the partially deamidated protein from the solution.
In one embodiment, the deamidated protein has a degree of deamidation less
than about 40%,
and preferably less than about 20%, more preferably less than about 10%. In
one
embodiment, the deamidated protein has a degree of deamidation of between
about 0.5% and
5.0%.
In one embodiment, the deamidated protein is glutelin. In one embodiment, in
step
(a), barley endosperm flour is mixed with an alkaline solution adjusted with
sodium3
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
hydroxide. In one embodiment, the ratio of the solution to flour is 10:1
(v/w). In one
embodiment, the mixture is stirred for about half an hour at room temperature.
In one
embodiment, glutelin is precipitated and freeze-dried.
In one embodiment, the deamidated protein is hordein. In one embodiment, in
step
(a), pearled grain flour is mixed with ethanol. In one embodiment, the ratio
of ethanol to flour
is 6:1 (v/w). In one embodiment, the mixture is stirred for about two hours at
about 60 C. In
one embodiment, hordein is precipitated and freeze-dried.
In one embodiment, in step (b), glutelin is treated with an alkaline solution.
In one
embodiment, in step (b), hordein is treated with an alcohol/alkaline solution.
In one
embodiment, in step (b), the reaction temperature is between about 40 C to
about 60 C, and
the time of reaction is between about 10 minutes to about 120 minutes. In one
embodiment,
in step (c), the deamidated protein is recovered in the form of a freeze-dried
protein
concentrate.
In another aspect, the invention comprises deamidated glutelin produced by the
above
method and having a degree of deamidation ranging between about 0.5% to about
40%. In
one embodiment, the degree of deamidation ranges between about 1.0% to about
15%. In one
embodiment, the degree of deamidation ranges between about 1.0% to about 2.5%.
In another aspect, the invention comprises deamidated hordein produced by the
above
method and having a degree of deamidation ranging between about 0.7% to about
40%. In
one embodiment, the degree of deamidation ranges between about 2.4% to about
4.7%.
In another aspect, the invention comprises a food, cosmetic or pharmaceutical
product
comprising the deamidated barley protein produced by the above method.
In yet another aspect, the invention comprises use of deamidated barley
protein
produced by the above method in a food, cosmetic or pharmaceutical product.
4
WO 2012/006731 CA 02805677 2013-01-16PCT/CA2011/000831
Additional aspects and advantages of the present invention will be apparent in
view of
the description, which follows. It should be understood, however, that the
detailed description
and the specific examples, while indicating preferred embodiments of the
invention, are given
by way of illustration only, since various changes and modifications within
the spirit and
scope of the invention will become apparent to those skilled in the art from
this detailed
description.
Brief Description Of The Drawings
The invention will now be described by way of an exemplary embodiment with
reference to the accompanying simplified, diagrammatic, not-to-scale drawings:
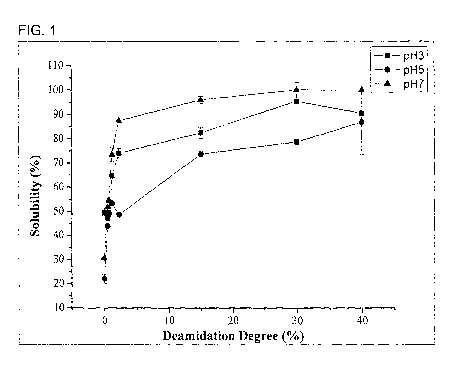
Figure 1 is a graph showing solubility of deamidated glutelin at different pHs
as a
function of DD.
Figure 2 is a graph showing emulsion capacity and stability for deamidated
glutelin.
Figure 3 is a graph showing a time-dependent increase of DD of hordein induced
by
alkaline reaction.
Figure 4 is a graph showing electrophoretic mobilities of deamidated hordeins
at
different pH as a function of DD.
Figure 5 is a graph showing degree of hydrolysis and surface hydrophobicity of
deamidated hordeins as a function of DD.
Figure 6 is a photograph of a SDS-PAGE gel showing deamidated hordeins (a:
unmodified hordein; b: DD 0.7%; c: DD 1.2%; d: DD 4.7%; e: DD 9.8%).
5
WO 2012/006731 CA 02805677 2013-
01-16 PCT/CA2011/000831
Figure 7 shows SEC-HPLC chromatograms of deamidated hordeins (a: unmodified
hordein; b: DD 0.7%; c: DD 1.2%; d: DD 4.7%; e: DD 9.8%; f: DD 17%).
Figure 8 shows FTIR spectra of deamidated hordeins (a: DD 1.2%; b: DD 4.7%; c:
DD 9.8%; d: DD 17%).
Figure 9 is a graph showing solubility of deamidated hordeins at different pHs
as a
function of DD.
Figures 10A-C are graphs showing foaming capacity and stability of deamidated
hordeins at pH 3 (Figure 10A); pH 5 (Figure 10B); and pH 7 (Figure 10C) as a
function of
DD.
Figures 11A-C are graphs showing emulsion centrifugation stability and
emulsion
thermal stability of deamidated hordeins at pH 3 (Figure 11A); pH 5 (Figure
11B); and pH 7
(Figure 11C) as a function of DD.
Detailed Description Of Preferred Embodiments
The present invention relates to partially deamidated barley proteins. When
describing the present invention, all terms not defined herein have their
common art-
recognized meanings. To the extent that the following description is of a
specific
embodiment or a particular use of the invention, it is intended to be
illustrative only, and not
limiting of the claimed invention. The following description is intended to
cover all
alternatives, modifications and equivalents that are included in the spirit
and scope of the
invention, as defined in the appended claims.
The present invention relates to partially deamidated barley proteins having
various
degrees of deamidation (DD), and methods for preparing same. In one
embodiment, the
deamidated barley protein is glutelin. In one embodiment, the deamidated
barley protein is6
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
hordein. As used herein, "hordein" refers to prolamin proteins which may be
extracted from
barley with ethanol, and includes hordeins A, B, C and D.
As used herein, the term "deamidation" refers to a chemical reaction in which
an
amide functional group is removed from an polypeptide. The primary targets of
deamidation
are asparagine and glutamine residues.
In one embodiment, the invention comprises a method of producing a deamidated
protein from barley, comprising the steps of:
a) extracting protein from barley;
b) treating the extracted protein with an alkaline or acidic solution for a
sufficient
time and temperature for deamidation; and
c) recovering deamidated protein from the solution, wherein the deamidated
protein
has improved functional properties with respect to solubility, and emulsifying
and foaming
properties at acidic and neutral pHs compared to those of a non-deamidated
protein.
The deamidated proteins are produced from barley using the methods described
herein. The method generally involves at least the steps of extracting protein
from barley
using an alkaline solution or an alcohol; treating the extracted protein with
an alkaline or
acidic solution for a sufficient time and temperature for deamidation; and
recovering
deamidated protein from the solution. The physicochemical properties of the
resultant
deamidated barley proteins may have functional properties and suitability for
particular
applications such as, for example, as emulsion and foam stabilizers in food,
cosmetic and
pharmaceutical products.
The detailed steps of the process are as follows. Barley is used as the
starting
material. As used herein, the term "barley" means a grass in the genus
Hordeum. Barley
proteins may be extracted using the method described by Wang et al. (2010). In
one
embodiment, the barley protein is extracted using a suitable extractant.
Hordein is soluble in
7
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
alcohol, while glutelin is insoluble in alcohol. Glutelin may be extracted
with an alkaline
solution from the barley fraction which is insoluble in alcohol.
In one embodiment, barley is pearled and milled. Pearling is used to remove
the
grain's outer layers (mainly bran and germ) so that the barley cytoplasmic
proteins (albumin
and globulin) are enriched in the pearling flour, while endosperm proteins
(hordein and
glutelin) are enriched in the pearled grain flour. The pearled grain flour is
treated with an
alcohol. In one embodiment, the alcohol is ethanol, methanol or propane, and
is preferably a
50% - 70% ethanol. In one embodiment, the ratio of alcohol to flour is 6:1
(v/w). In one
embodiment, the solution is stirred for about two hours at about 60 C. After
extraction, the
insoluble solids are separated by centrifugation. The alcoholic supernatant is
collected, and
hordein is isolated by cold precipitation and may be freeze-dried for storage
and future use.
The barley endosperm flour residue which is insoluble in alcohol is mixed with
an
alkaline solution to extract glutelin. In one embodiment, the alkaline
solution is a solution of
NaOH and has a pH of about 10 or higher. In one embodiment, the ratio of the
alkaline
solution to flour is 10:1 (v/w). In one embodiment, the mixture is stirred for
about half an
hour at about room temperature. After extraction, the insoluble solids are
separated by
centrifugation. The supernatants collected from the alkaline extracts are
adjusted to an acidic
pH to precipitate the proteins. Glutelin protein isolates are then obtained by
centrifugation
and may be freeze-dried for storage and future use.
The barley proteins are modified to form deamidated proteins having desirable
physical and chemical properties (Examples 4 and 5). Barley proteins may be
deamidated by
an alkaline or an acidic process. In one embodiment, the alkaline method is
preferred because
it is considered to be more efficient than an acidic method. However, barley
proteins
deamidated by an acidic method or by other methods are considered within the
scope of this
invention.
8
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
In one embodiment, glutelin is deamidated by treatment with an alkaline
solution at an
elevated temperature. In one embodiment, the alkaline solution comprises a
0.1M to about
1.0M sodium hydroxide solution. In one embodiment, the temperature is about 40
C.
In one embodiment, hordein is deamidated by treatment with an alcohol/alkaline
solution at an elevated temperature. In one embodiment, the solution comprises
70% ethanol
with 0.1M to about 1.0M sodium hydroxide. In one embodiment, the temperature
is between
about 40 C to about 60 C.
A greater DD may be achieved with a stronger alkaline solution, longer
reaction time
and/or higher temperatures. Using hordein as an example, Figure 3 shows the DD
obtained as
a function of the reaction time. At 40 C, the DD reached 9.8% after two
hours. Prolonged
time did not further increase the DD. Increasing the temperature to 60 C
significantly
enhanced the reaction rate and the DD reached more than 80% within two hours.
Samples
having DD values in the range of 0.7-9.8% and 17-40% which had been obtained
at 40 C and
60 C respectively, were selected for further study (Table 1).
Table 1.
Sample NaOH Time Temperature DD
(M) (min) ( C) (%)
1 0.5 10 40 0.7
2 0.5 30 40 1.2
3 0.5 40 40 2.4
4 0.5 80 40 4.7
5 0.5 120 40 9.8
6 0.5 20 60 17
7 0.5 30 60 31
8 0.5 40 60 40
One skilled in the art may thus produce deamidated barley proteins with a
desired DD by
varying the strength of the alkaline solution, the reaction time and/or
temperature. In one
9
WO 2012/006731 CA 02805677 2013-01-16PCT/CA2011/000831
embodiment, the reaction temperature is between about 40 C to about 60 C. In
one
embodiment, the time of reaction is between about 10 minutes to about 120
minutes.
After a desired time, the reaction is halted by addition of acid or alkaline,
as the case
may be. In one embodiment, the acid comprises hydrochloric acid. The solution
is dialyzed
against deionized water and the deamidated protein is recovered in the form of
a freeze-dried
protein concentrate.
The physicochemical properties of the resultant deamidated barley proteins may
be
evaluated to assess their suitability for particular applications. Such
properties may vary as a
function of the DD, and may include, but are not limited to, degree of
hydrolysis (Example 6),
solubility (Example 7), foaming properties (Example 8), emulsion properties
(Example 9),
electrophoretic mobility (Example 10), surface hydrophobicity (Example 11),
molecular
weight (Examples 12 and 13), and secondary structure (Example 14). Since
proteins are
amphoteric polyelectrolytes, emulsifying and foaming behaviours are expected
to vary with
pH; thus, the impact of pH on various functional properties, including
emulsifying and
foaming behaviors, may be assessed as described herein.
The following are specific examples of embodiments of the present invention.
These
examples demonstrate exemplary deamidated barley proteins, namely glutelin and
hordein.
These examples are offered by way of illustration and are not intended to
limit the invention
in any manner.
In one embodiment, the invention comprises deamidated glutelins having various
DD
which result in enhanced solubility while maintaining the main glutelin
structures, and
improved emulsifying and foaming properties. In one embodiment, the invention
comprises
deamidated glutelins produced by the above method and having a DD ranging
between about
0.5% to about 40%. In one embodiment, the DD ranges between about 1.0% to
about 15%.
In one embodiment, the DD ranges between about 1.0% to about 2.5%.
10
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
The DD, degree of hydrolysis (HD) and surface hydrophobicity for exemplary
deamidated glutelins are set forth in Table 2:
Table 2.
Sample NaOH Time Temperature Degree of Degree of Surface
(M) (mins) ( C) deamidation hydrolysis hydrophobicity
(%) (%) (cm2/m1)
1 0.1 30 40 0.05 14.51877 132.6
2 0.1 40 40 0.07 16.1535 150
3 0.1 50 40 1.18 16.39774 169.98
4 0.1 80 40 2.23 19.24648 202.88
0.5 40 40 15 21.01847 189.935
6 0.5 90 40 30.0 22.6259 147.27
7 0.5 120 40 40.0 17.28763 149.14
5 Unmodified glutelin shows low solubility (less than 20%) at pH 3-7 and
is soluble in
water only in the presence of high concentrations of alkali (pH 11) due to the
high proportion
of nonpolar amino acid residues and high surface hydrophobicity (data not
shown). However,
the solubility of deamidated glutelin increases significantly at acidic and
neutral pHs (Figure
1). The remarkably improved solubility after deamidation at both acidic and
neutral pHs will
enable a broader range of glutelin usage in various applications.
Unmodified glutelin shows good emulsifying stability at both acidic and
neutral
conditions (data not shown). These data were obtained by dehydrating
unmodified glutelin at
pH 11, followed by adjusting the pH back to acidic and neutral conditions
before evaluating
the emulsifying property. For the present invention, the emulsifying stability
was evaluated
by dispersing deamidated glutelins directly in buffer at different pHs. Figure
2 shows the
emulsion centrifugation capacity and emulsion thermal stability of deamidated
glutelin having
different DD. Deamidated glutelin demonstrates an excellent capacity to
stabilize the
emulsion at a broad range of DD since around 60-65% of formed emulsions
remained even
after heating and centrifugation. Without restriction to any theory, this
favorable property is
likely due to glutelin's unique molecular structure, strong surface
hydrophobic, and tendency
to form aggregates.
11
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
In one embodiment, the invention comprises deamidated hordeins having various
DD
which result in dissociated hordein aggregates. In one embodiment, the
invention comprises
deamidated hordein produced by the above method and having a DD ranging
between about
0.7% to about 40%. In one embodiment, the DD ranges between about 2.4% to
about 4.7%.
Optimum functionalities are obtained in a DD range between about 2.4% to about
4.7%,
where hordein demonstrates improved solubility, and emulsifying and foaming
properties at
both acidic and neutral pHs. A DD greater than about 4.7% results in extensive
protein
hydrolysis and a marked change in protein secondary structure which greatly
influences
functionality.
The electrophoretic mobilities of deamidated hordeins at different pHs were
determined (Figure 4). The zeta-potential of deamidated hordeins in different
pH buffers are
expressed as a function of DD. Limited surface charge (-5 mV) is observed for
hordein with a
DD of 0.7% at pH 5. This value, however, increases to -33 mV at a DD of 31%,
and then
decreases to -17 mV at a DD of 40%. The surface charge of the deamidated
hordein changes
in the same manner at pH 7, but the zeta-potential is generally higher than
that at pH 5,
especially at relatively low or high DD range. Conversely, the protein
molecule surfaces are
slightly positively charged (+ 5 mV) at pH 3 when the DD is 0.7%. With
increasing of the
DD to 4.7%, hordein surface charge decreases to near zero. Without restriction
to a theory,
hordein may have an aggregation structure similar to gliadins. The isoelectric
point (IEP) of
hordein (without deamidation) is between pH 3 and 5. As the DD increases, the
IEP shifts
from about pH 5 to about pH 3 due to the introduction of additional carboxyl
groups on the
protein side chains as a result of deamidation. The IEPs of other proteins
similarly shift to
acidic pH after deamidation (Matsudomi et al., 1985). These data also explain
the increase of
the protein surface charge with DD until 31% at pH 5 and 7. The decrease of
zeta-potential at
a DD of 40% in both pH 5 and 7 buffers is unexpected, and may be attributed to
cleavage of
the small peptides with high density of charge due to significant hydrolysis.
These small
peptides are lost during dialysis, even though very low molecular weight cut-
off (1 kDa)
dialysis tubing is used.
12
WO 2012/006731 CA 02805677 2013-
01-16 PCT/CA2011/000831
Figure 5 shows the HD and surface hydrophobicity of deamidated hordeins. The
HD
increases linearly in proportion to the DD until a DD of 9.8%, after which the
HD value levels
off This result suggests that hordein peptide bond cleavage occurs rapidly
within a DD
ranging between about 0.7% to about 9.8%, and the hydrolysis rate slows after
a DD of 9.8%.
The degree of surface hydrophobicity of the deamidated hordein increases
markedly as the
DD increases to about 4.7%, suggesting that the hydrophobic regions are
progressively
exposed at the molecular surface. A further increase in the DD results in a
significant
decrease in surface hydrophobicity. A DD greater than about 5% thus leads to
protein
unfolding and extensive hydrolysis. Since more polar groups on protein side
chains are
exposed, a decrease in surface hydrophobicity is observed.
Figure 6 shows SDS-PAGE of deamidated hordeins. Three subunits of hordein were
identified with bands at 55-80, 30-50 and less than 15 kDa corresponding to C,
B and A
hordeins, respectively. A weak band at 80-90 kDa corresponding to D hordein is
observed
when ethanol is used as the sole extraction agent (Bilgi et al., 2004). Most
bands remain
visible in SDS-PAGE until a DD of 4.7%, but the band intensity of C and B
hordeins
decreases gradually. After a DD of 4.7%, all bands disappear. The result
indicates that
partial hydrolysis occurs at a DD less than or equal to 4.7%, whereas
extensive hydrolysis
occurs within a DD of between about 5% to about 9.8%, resulting in formation
of peptides
having molecular weights of less than 10 kDa.
The dissociation of large protein aggregates may lead to the formation of
water soluble
peptide aggregates. SEC chromatograms of deamidated hordeins in phosphate
buffer are
shown in Figure 7. Deamidated hordein with a DD of 0.7% contains two main
broad peaks
(peak 1 and peak 2) corresponding to subunits having molecular weights of less
than 15 kDa
and 20-67 kDa, respectively. The former can be assigned to A hordeins, whereas
the latter
could be B and C hordeins together. A small sharp peak (peak 3) was also
observed at 114
kDa, which could be assigned to some aggregated large peptides. This
phenomenon has been
reported for barley proteins, where high molecular weight subunits form a
backbone which
binds low molecular weight subunits through disulfide bridges to form a gel-
like aggregate13
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
(Celus et al., 2006). Increasing the DD to between about 2.4 to about 4.7%
significantly
alters the chromatogram patterns. Peak 2 is markedly sharpened and peak 3
amplitude is
dramatically enhanced. The sharpened peak 2 corresponds to the remaining of
more
hydrolysis-resistant subunits, likely corresponding to C-hordeins since they
are more slowly
degraded than B-hordeins (Celus et al., 2006). The increased peak 3 intensity
can be
attributed to an increased solubility of the large polypeptides due to an
increased net negative
charge by deamidation (Chan et al., 1999). A further increase of DD value
equal or greater
than 9.8% results in the dissociation of the aggregated large peptides as the
peak 3 almost
disappears. An obvious shift of peak 2 to lower molecular weight range is
observed,
indicating that the resistant subunits in hordein start to be hydrolyzed after
a DD of 4.7%.
The degradation may account for the extensive hydrolysis of deamidated
hordeins within a
DD range of between about 5% to about 9.8%.
Secondary structure may be determined by Fourier transform infrared
spectroscopy
(FTIR). Through proper fitting of the amide I band of the original FTIR
spectrum of a
protein, the conformation of the protein (i.e., helix, sheet or turn) can be
obtained. Hordein
having a DD of 0.7% shows several bands in the amide I region (Figure 8). Such
bands
represent protein secondary structures in accordance with previous reports
(Liu et al., 2009;
Mejia etal., 2007; Siu etal., 2002; Wellner etal., 1996; Yong etal., 2006): a-
helices (1652
cm-I), 13-sheets (1617, 1635 and 1683 cm-I), 13-turn (1669 and 1675 cm-I), and
random coils
(1646 cm-I). The band at 1660 cm' could be mainly assigned to the carbonyl
stretching of
the glutamine side chain. The bands at 1683 and 1917 cm -I are believed to be
associated with
the aggregation process. When the DD increases from about 2.4% to about 4.7%,
the
intensity of the bands at both 1683 and 1917-1621 cm-I decreases, suggesting
disassociation
of protein aggregates, probably due to increased repulsions between protein
molecular chains
as a resulted of increase surface charges. Consequently, more hydrophobic
patches on protein
unit surfaces are exposed outside, thus increasing the surface hydrophobicity.
Marked shifts
in the band positions in the wavelength range of 1623-1657 cm-I are observed
with a further
increase of the DD. The absorption corresponding to the glutamine side chain
shifts to 1656
cm-I, reflecting a change of intra- or inter-molecular hydrogen bonds between
glutamine side
14
WO 2012/006731 CA 02805677 2013-
01-16 PCT/CA2011/000831
chains (Weliner et al., 1996). Additionally, the a-helix band shifts to lower
wavelength and
the random coil band (about 1642 cm") intensity increases notably. This
suggests that
marked protein confirmation changes occur following a DD value of 4.7%, likely
associated
with protein partial unfolding as a result of both strong negative charge on
protein molecular
chains and extensive protein hydrolysis.
Unmodified hordein shows low solubility (less than 20%) at pH 3-7 and a
significant
increase in solubility (about 50%) at pH 10 (Wang et al., 2010). Due to the
high proportion of
nonpolar amino acid residues and high surface hydrophobicity, unmodified
hordein is soluble
in water only in the presence of alcohol, high concentrations of urea, high
concentrations of
alkali (pH II), or anionic detergents, similar to other prolamin proteins
(Shukla and Cheryan,
2001). The solubility is relatively low at pH 3 due to shift of the hordein
isoelectric point to
acidic pH. In comparison to unmodified hordein, deamidated hordeins exhibit
improved
solubility at both acidic and neutral pHs (Figure 9). Protein solubility at pH
5 increases from
15% to 75% as the DD increases to 40%. Without restriction to a theory, the
improvement in
solubility within a DD of between about 0.7% to about 4.7% may be attributed
to the
dissociation of hordein aggregates and partial protein hydrolysis. Further
increased solubility
after a DD of 4.7% may be due to protein partial unfolding and extensive
hydrolysis. Such
structural changes lead to the exposure of more charged and polar groups to
the surrounding
water, thus promoting protein-water interaction and an increased solubility
(Chan and Ma,
1999).
Unmodified hordein shows good foaming capacity at both pH 3 and neutral
conditions
(150-160%), but a relatively lower foaming capacity at pH 5 (90%). However,
due to its
inherent poor solubility, unmodified hordein requires dehydration at pH 11
followed by
adjusting pH back to acidic and neutral conditions to enable foaming and
emulsifying
functionalities. This procedure is impractical in commercial food systems.
However,
deamidation significantly improves hordein solubility even within a limited DD
range, thus
allowing functionality testing by dispersing samples at different pH buffers
directly. Hordein
having a DD between about 2.4% to about 4.7% has improved foaming capacity
(FC) at pH 515
WO 2012/006731 CA 02805677 2013-01-16PCT/CA2011/000831
(145%) and pH 7 (190-200%) compared to unmodified hordein (Figures 10A-C).
With
increasing DD, the FC initially increases until a DD between about 2.4% to
about 4.7%, then
decreases at acidic and neutral pHs. A much more rapid decrease in FC is
observed at pH 3
and pH 5 than pH 7. The optical FC values were obtained at a narrow DD range
(about 2.4%
to about 4.7%) where a significant improvement of FC was observed at pH 5
(145%) and pH
7 (190-200%) compared to unmodified hordein. The optical FC obtained at pH 3
is on a same
level as that of the unmodified sample.
Without restriction to a theory, the initial increase of the FC value within a
DD
between about 0.7% to about 4.7% may be due to an increase of the protein
solubility,
enabling diffusion to the air/water surface. The exposed hydrophobic side
chains facilitate
binding of deamidated hordein at hydrophobic air surfaces, and these proteins
may aggregate
via surface hydrophobic patches to form films around bubble surfaces. Although
deamidated
hordeins exhibit good solubility when the DD is greater than about 4.7%, their
surface
hydrophobicity decreases with a further increase of the DD. This decreased
protein surface
hydrophobicity may be one of the major reasons accounting for the decreased FC
values at the
DD range of about 9.8% to about 40%.
The protein surface charge also influences the FC value. A significantly
greater FC is
observed at pH 7 compared to pH 3 and 5 within the optical DD range. Without
restriction to
a theory, this may be related to a greater surface charge on protein molecular
chains at neutral
pH, resulting in a strong repulsion between adjacent bubbles, and preventing
quick foam
coalescence during homogenization process. This greater surface charge could
also explain
the slower decrease in the rate of the FC values at pH 7 after a DD of about
4.7%.
Deamidated hordein shows an increased foaming stability (20% to 50-60%) at
both
pH 5 and 7 when the DD value is raised from about 0.7 to about 4.7%, and then
levels off
after a DD of about 4.6% (Figures 10B-C). Low FS values are observed at a DD
range of
about 0.7 to about 4.7% at pH 3, and this value increased rapidly after a DD
of 4.7% (Figure
10A). 16
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
Without restriction to a theory, the overall stability of foam is related to
the resistance
of the lamella to drain and of the bubbles to collapse. These factors are
dependent on the
rheological and adhesive properties of the interfacial film surrounding the
bubble (German et
al., 1985). Normally high molecular weight proteins exhibit greater film
strength and foam
stability; thus, the aggregated large peptides observed in the SEC
chromatograms at the DD
range of about 2.4 to about 4.7% may have contributed to the increased hordein
foaming
stability at both pH 5 and 7. However, the FS values did not decrease after DD
value of about
4.7%. This is difficult to explain because large peptide aggregates were
dissociated at a DD
equal or greater than 9.8% according to the SEC chromatograms. The FC values
decreased
significantly after a DD of 4.7% at both pH 5 and 7. It is deduced that fewer
protein
molecular chains have suitable molecular structures for foam forming compared
to hordeins
with DD of about 2.4% to about 4.7%. However, once foams were prepared, the
protein
chains with a suitable molecular structure could form continuous, rather rigid
films around
bubbles, which might explain why the ES value remained almost unchanged at a
DD value
equal or greater than about 9.8%. Optical foaming stability is observed near
the protein IEP.
Proteins can adsorb better to the air/water interface at minimum electrostatic
repulsion to form
a rigid film against coalescence (German et al., 1985). However, a very low FS
value (equal
to or less than 9.8%) was observed for deamidated hordeins at a DD range of
0.7-4.7% when
the pH was 3. It is assumed that the deamidated hordeins, including the large
peptide
aggregates, also formed a thick and rigid film at air/water interface at pH 3.
This film may
have a strong tendency to aggregate when the surface charge is low, resulting
in extensive
aggregation of protein films between adjacent gas bubbles, film rupture, and
foam instability.
After increasing the DD to equal or greater than 9.8%, large peptides were
dissociated and the
hordein peptide bonds were cleaved. The hydrolyzed peptides may exhibit fewer
tendencies
to aggregate, forming rigid and viscoelastic films without aggregation near
protein IEP. This
explains a significant increase of the FS value observed with a DD value
increasing from 9.8
to 40% at pH 3.
17
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
Unmodified hordein shows good emulsifying stability at pH 3 and neutral
conditions
(ECS 57-61%, ETS 51%), where low emulsifying property was observed at pH 5
(ECS 31%,
ETS 18%) (Wang et al., 2010). These data were obtained by dehydrating
unmodified hordein
at pH 11 followed by adjusting pH back to acidic and neutral conditions before
evaluating
their foaming property. Emulsifying stability may be evaluated by dispersing
deamidated
hordeins directly in buffer at different pHs. An increase of the ECS is
observed until the DD
of between about 2.4 to about 4.7% at all pHs (Figures 11A-C). After a DD of
about 4.7%,
the ECS value decreases rapidly. The change of the ETS as a function of the
deamidation
degree follows the same trend.
The initial increase of the ECS can be attributed to the increase in
solubility and
exposed hydrophobic side chains, since protein solubility and hydrophobicity
have a strong
correlation with emulsifying properties (Nakai, 1983; Townsend and Nakai,
2006). The
aggregated large peptides observed in the SEC chromatograms may also play a
role in
stabilizing the emulsions. Large peptides can generally form a rigid film at
the oil/water
interface to prevent the close contact of oil droplets, and decrease
flocculation and
coalescence (Agyare et al., 2009; McClements, 1999). A further increase of the
DD equal or
greater than about 9.8% resulted in decreased surface hydrophobicity due to
protein unfolding
to expose the polar side chains, dissociation of the large peptides, and
extensive protein
hydrolysis. Such factors would prevent the formation of a continuous protein
film at the oil-
liquid interface, leading to reduced emulsion stability. Deamidated hordein
demonstrates an
excellent capacity to stabilize the emulsion at a DD of between about 2.4% to
about 4.7%
since approximately 70% of the formed emulsions remained after heating and
centrifugation.
This favorable property is likely due to hordein's unique molecular structure,
strong surface
hydrophobicity, and tendency to form aggregates. The excellent emulsion
thermal stability
may be due to further gelation of the deamidated hordein around the oil
droplets during
thermal treatment to form a reinforced film.
Accordingly, the deamidated barley proteins produced by the methods described
herein may be used for example, as emulsion and foam stabilizers in food,
cosmetic and
18
WO 2012/006731 CA 02805677 2013-01-16PCT/CA2011/000831
pharmaceutical products. In one embodiment, the invention comprises use of
deamidated
barley proteins in a food, beverage, cosmetic or pharmaceutical product. In
one embodiment,
the invention comprises a food, beverage, cosmetic or pharmaceutical product
comprising the
deamidated barley proteins. As examples, the emulsifying property of
deamidated glutelin
and the foaming property of deamidated hordein may support their development
as functional
ingredients in food formulations such as whipped topping, salad dressing, and
processed
meats.
In other embodiments, the invention may comprise products such as
nutraceuticals,
agricultural, personal care, paint, ink, coatings, detergent, soap, or
firefighting compositions,
which comprises a deamidated barley protein.
Exemplary embodiments of the present invention are described in the following
Examples, which are set forth to aid in the understanding of the invention,
and should not be
construed to limit in any way the scope of the invention as defined in the
claims which follow
thereafter.
Example 1 - Materials
Regular barley grains (Falcon) were provided by James Helm, Alberta
Agricultural
and Rural Development, Lacombe, Alberta, Canada. Protein content was 13.2%
(w/w) as
determined by combustion with a nitrogen analyzer (FP-428, Leco Corporation,
St. Joseph,
MI, USA) calibrated with analytical reagent-grade EDTA and a protein
calculation factor of
6.25. Canola oil used for the emulsifying study was purchased locally.
Unstained standard
protein molecule marker for SDS-PAGE was purchased from Bio-RAD (Richmond, CA,
USA). An Ammonia Assay Kit, o-phthaldialdehyde reagent, 1-anilinonaphthalene-8-
sulfonic
acid and standard molecular markers for HPLC analysis (BSA, 67 kDa; ovalbumin,
43 kDa;
lactoglobulin, 35 kDa; cytochrome C, 13.6 kDa; aprotinin, 6.5 kDa and vitamin
B12, 1.4 kDa)
were purchased from Sigma-Aldrich Canada Ltd. (Oakville, ON, Canada). All
other
chemicals were of reagent grade.
19
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
Example 2 - Extraction of hordein
Barley hordein was extracted according to Wang etal. (2010). After pearling
and
milling, barley endosperm flour was dispersed in the 55% ethanol solution at a
solvent-to-
flour ratio of 6:1 (v/w) with stirring for two hours at 60 C. After
extraction, the solids were
separated by centrifuge (8,500 x g for 15 mm at 23 C) (Beckman Coulter
AvantiTM J-E
Centrifuge System, USA). The hordein fraction was isolated from the
supernatant by cold
precipitation at 4 C overnight. The isolated hordein was freeze-dried and
refrigerated at 4
C.
Example 3 - Extraction of glutelin
Barley glutelin was extracted according to Wang et al. (2010). After alcohol
extraction of the hordein fraction, the remaining barley endosperm flour was
dispersed in the
pH 11 alkaline solution adjusted using 0.1 M NaOH solution at a solvent-to-
flour ratio of 10:1
(v/w) with stirring for 0.5 h at room temperature. After extraction, the
insoluble solids were
separated by centrifuge (8,500 x g for 15 min at 23 C) (Beckman Coulter
AvantiTM J-E
Centrifuge System, USA). The supernatants were adjusted to approximately pH 5
with 0.5 M
HC1 to precipitate the proteins. Protein isolates were obtained by
centrifugation at 8,500 x g
for fifteen minutes at room temperature. The protein fractions were freeze-
dried and
refrigerated at 4 C. Protein content of the protein fractions was determined
using a nitrogen
analyzer (FP-428, Leco Corporation, St. Joseph, MI, USA).
Example 4 - Preparation of deamidated barley proteins
Deamidated barley proteins of different DD were prepared according to Yong et
al.
(2004) with modifications. Glutelin (5%, w/v) was suspended in 0.1-0.5 M NaOH
solution at
40 C under constant stirring. Hordein (5% w/v) was suspended in 70% (v/v)
ethanol with
0.5 M NaOH solution at 40 and 60 C. All samples were withdrawn at different
time
intervals (10-120 minutes) to provide a relatively broad range of DD values
(particularly those
within limited DD range of less than or equal to 10%), and neutralized using
0.5 M HC1
before dialysis against deionized water and then freeze dried. The dried
samples were stored
at 4 C until use.
20
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
Example 5 - Determination of the degree of deamidation (DD)
The DD was determined by measurement of the released ammonia after deamidation
using an Ammonia Assay Kit according to the manufacturer's instructions. DD
was
calculated as the ratio of ammonia generated in the modified sample to that of
the completely
deamidated protein. Complete deamidation was achieved by refluxing the sample
with 2 M
HC1 for two hours.
Example 6 - Determination of the degree of hydrolysis (HD)
The HD was assayed by quantifying cleaved peptide bonds using o-
phthaldialdehyde
(OPA) (Paraman et al., 2007). The OPA reagent was prepared by dissolving 7.62
g of
disodium tetraborate decahydrate and 200 mg of SDS in 150 mL of deionized
water, followed
by addition of 160 mg of OPA dissolved in 4 mL of ethanol and 176 mg of 99%
dithiothreitol. The volume of the mixture was adjusted to 200 mL using
deionized water.
OPA reagent (3 ml) was mixed with 400 I.AL of deamidated hordein sample (10
mg/mL) and
the absorbance at 340 nm was measured using a spectrophotometer (Jenway 6505
UVNis
Spectrophotometers, UK). The standard solution was prepared by dissolving 10
mg of serine
into 100 mL of deionized water.
Deamidated glutelin (1.25 mg/mL) was dissolved in 12.5 mM of borate buffer (pH
8.5) plus 2% (w/v) of SDS. This solution (50 L) was mixed with 1 mL of reagent
(50 mL of
0.1 M borate buffer (pH 9.3), 1.25 mL of 20% (w/v) of SDS, 100 mg of N,N-
dimethy1-2-
mercaptoethylammonium chloride, and 40 mg of OPA dissolved in 1 mL of
methanol). The
mixture was left to stand for two minutes before measurement of the absorbance
at 340 nm.
The number of amino groups was determined with reference to the L-leucine
standard curve
(between 0.5 and 5 mM). The HD was calculated as:
HD (%) = [(a - ni) I nT] x 100 (1)
21
WO 2012/006731 CA 02805677 2013-01-16
PCT/CA2011/000831
where nT = the total number of amino groups in original glutelin after total
hydrolysis with 6
M HCI for twenty-four hours; ni = the number of amino groups in glutelin; and
a = the
number of free amino groups measured in the deamidated glutelin.
Example 7 - pH solubility profile
Deamidated barley proteins (125 mg) were dispersed in 25 mL of buffer at pH 3,
5 and
7. The dispersions were mixed for 1 h at room temperature by magnetic stirring
before
centrifuging at 1,200 x g (for hordeins) or 3,000 x g (for glutelins) for 20
min at 4 C. The
supernatants were filtered through a Whatman No. 1 filter paper to obtain
clear filtrates. The
protein concentration in the filtrates was determined by dye assay (Bradford,
1976) with
bovine serum albumin as the standard. The solubility was expressed as a
percentage of the
total protein content of the starting sample.
Example 8 - Foaming properties
Foaming capacity (FC %) and foaming stability (FS %) was determined according
to
Ahmedna et al. (1999) with modifications. Protein samples (0.5%, w/v) were
dispersed in 50
mL of buffer at pH 3, 5 and 7. The solution was mixed for two minutes with a
homogenizer
(PowerGenTM 1000, Fisher Scientific, Fairlawn, NJ, USA) at speed three.
Volumes were
recorded before and after homogenization using a graduated cylinder. The
percentage volume
increase was calculated as:
FC (%) = (Vf2¨ Vfi)/ Vfi x 100 (2)
where Vfi and Vf2 represent the volume of the protein solution and the formed
foams before
and after homogenization. FS (%) was determined as the volume of foam that
remained after
0.5 hours at 23 C expressed as a percentage of the initial foam volume:
FS (%) = Vf2 / Vfi x 100 22 (3)
WO 2012/006731 CA 02805677 2013-01-16
PCT/CA2011/000831
Example 9 - Emulsion properties
The emulsion centrifugation stability (ECS) and emulsion thermal stability
(ETS)
were determined according to Yasumatsu et al. (1972) with modifications.
Barley protein
samples (0.5%, w/v) were dispersed in 50 mL of buffer at pH 3, 5 and 7,
followed by addition
of 50 mL of canola oil. The mixture was homogenized at speed six (for
glutelins) or speed
three (for hordeins) for two minutes to form an emulsion (PowerGenTM 1000,
Fisher
Scientific, Fairlawn, NJ, US). The emulsion was then centrifuged at 1,500 x g
for five
minutes. ECS (%) is calculated by measuring the volume of the emulsion
remaining after
centrifugation (Vet) and before (i.e., total volume or Vet) using a graduated
cylinder and
recorded as:
ECS (%) = VellVetx 100 (4)
The emulsion samples were then heated to 80 C in a water bath for thirty
minutes and cooled
to 23 C. Upon cooling, these tubes were centrifuged at 1500 x g for five
minutes. The
volume of the remaining emulsified fraction (Ve 2) was recorded. ETS (%) is
calculated as:
ETS (%) = (Ve21Ve1)x 100 (5)
Example 10 - Electrophoretic mobilities
The electrophoretic mobilities of the deamidated hordein samples in different
pH
buffers (pH 3 and 5: 0.2 M acetate buffer, pH 7: 0.2 M phosphate buffer) were
measured by
laser Doppler velocimetry using a ZetasizerTM NanoS (model ZEN1600, Malvern
Instruments
Ltd, UK). Electrophoretic mobility (i.e., velocity of a particle within an
electric field) was
related to the zeta potential (g) using the Henry equation (Liu et al., 2009):
2t x x (KM 31/ (6)
23
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
where II = the dispersion viscosity; c = the permittivity; and f (Ka) = a
function related to the
ratio of particle radius (a) and the Debye length (lc). The results are
reported as the average of
at least three measurements. Typical standard deviations were less than 3
mV. The same
buffers were used in following studies.
Example 11 - Surface hydrophobicity
Surface hydrophobicity of the deamidated hordeins in sodium phosphate buffer
(pH 7)
was determined using a fluorescence probe, 1-anilinonaphthalene-8-sulfonic
acid, according
to Kato and Nakai (1980). Fluorescence intensity (Fl) was measured at
wavelengths of 390
nm (excitation) and 470 nm (emission) using a fluorospectrometer (FP-6300,
Jasco, Tokyo,
Japan). The surface hydrophobicity degree (So) was calculated by linear
regression analysis
using the slope of the straight line obtained by plotting FT as a function of
protein
concentration.
Example 12 ¨ Electrophoresis
SDS-PAGE was performed to evaluate hordein subunits in the barley protein
fractions
using a vertical mini-gel system (Mini-ProteinTm Tetra Cell, Bio-Rad,
Hercules, CA, USA).
Deamidated hordein samples were mixed with the loading buffer (0.125 M Tris-
HC1, pH 6.8,
4% SDS (w/v), 20% glycerol (v/v), 0.05% 2-mercaptoethanol (v/v) and 1%
bromophenol blue
(w/v)) and then heated at 100 C for 5 min. After cooling, 18 ptL of sample (5
mg/mL) was
loaded on 5% stacking gel and 12% separating gel and subjected to
electrophoresis at a
constant voltage of 80 V. After electrophoresis, the gels were stained with
0.1% (w/v)
Coomassie Brilliant Blue-R-250 in water-methanol-acetic acid (4:5:1, v/v) for
30 mM and
destained with water-methanol-acetic acid (4:5:1, v/v).
Example 13 - Size Exclusion Chromatography (SEC)
SEC chromatography was performed using a HPLC system (Varian ProStar, USA)
combined with a size exclusion column (SuperdexTm200 10/300 GL, Amersham
Biosciences,
USA). 50 mM phosphate buffer containing 150 mM sodium chloride was used as a
mobile
phase at a flow rate of 0.4 mL/min at 25 0.5 C. 50 ptL of sample solution
was injected into
HPLC system and the protein was monitored at a UV wavelength of 280 nm.
Standard
24
CA 02805677 2013-01-16
WO 2012/006731 PCT/CA2011/000831
molecular markers were used to calculate the weight-average molecular weight
of the
deamidated hordeins.
Example 14 - FTIR spectroscopy
Protein conformation was studied with a Fourier transform infrared (FTIR)
spectroscopy (Varian FTS-7000, US) in the wavenumber range from 400 to 4000 cm-
1 during
128 scans, with 4 cm-1 resolution. 5% deamidated hordein samples were
dissolved in D20
solution. To ensure complete IUD exchange, samples were prepared two days
before and
kept at 4 C prior to infrared measurements. Samples were placed between two
CaF2
windows separated by 25 [tm polyethylene terephthalate film spacer. To study
the amide I
region of the protein, Fourier self-deconvolutions were performed using the
software provided
with the spectrometer. Band narrowing was achieved with a full width at half
maximum of 20
cm-1 and with a resolution enhancement factor of 2.0 cm*
Example 15 - Statistical analysis
All experiments were performed at least in triplicate. Error bars on graphs
represent
standard deviations. Statistical significances of the differences were
determined by Student's
t-test. The level of significance wasp < 0.05.
As will be apparent to those skilled in the art, various modifications,
adaptations and
variations of the foregoing specific disclosure can be made without departing
from the scope
of the invention claimed herein.
References
The following references are incorporated by reference, where permitted, as if
reproduced herein in their entirety. These references are also indicative of
the level of skill in
the art.
Agyare, K.K.; Addo, K. and Xiong, Y.L. (2009) Emulsifying and foaming
properties of
transglutaminase-treated wheat gluten hydrolysate as influenced by pH,
temperature and salt.
Food Hydrocoll. 23:72-81.
25
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
Ahmedna, M.; Prinyawiwatkul, W. and Rao, R.M. (1999) Solubilized wheat protein
isolate:
Functional properties and potential food applications. I Agric. Food Chem.
47:1340-1345.
Bilgi, B. and celik, S. (2004) Solubility and emulsifying properties of barley
protein
concentrate. Eur. Food Res. Technot 218:437-441.
Bradford, M.M. (1976) Rapid and sensitive method for quantization of microgram
quantities
of protein utilizing principle of protein-dye binding. Analyt. Biochem. 72:248-
254.
Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R. and Farres, A. (2007) Effect of
alkaline
deamidation on the structure, surface hydrophobicity, and emulsifying
properties of the Z19 r-
zein. I Agric. Food Chem. 55:439-445.
Celus, I.; Brijs, K. and Delcour, J.A. (2006) The effects of malting and
mashing on barley
protein extractability. I Cereal Sci. 44:203-211.
Chan, W.M. and Ma, C.Y. (1999) Acid modification of proteins from soymilk
residue
(okara). Food Res. Int. 32:119-127.
Chiue, H.; Kusano, T. and Iwami, K. (1997) Antioxidative activity of barley
hordein and its
loss by deamidation. I. Nutr. Sc L Vitaminot 43:145-154.
Food and Drug Administration (2005) Food labeling: health claims; soluble
dietary fiber from
certain foods and coronary heart disease. Final Rule Federal Register Doc.
2004P-0512, filed
12-23-05.
Friedli, G.L. (1996) Interaction of deamidated soluble wheat protein with
other food proteins
and metals. PhD Thesis, University of Surrey, Guildford, UK.
26
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
German, J.B.; O'Neill, T.E. and Kinsella, J.E. (1985) Film forming and foaming
behavior of
food proteins. JAOCS 62:1358-1366.
Hamada, J.S. (1992) Effects of heat and proteolysis on deamidation of food
proteins using
peptidoglutaminase. J Agric. Food Chem. 40:719-723.
Hamada, J.S. and Marshall, W. E. (1989) Preparation and functional properties
of
enzymatically deamidated soy proteins. J Food. Sci. 54:598-601.
Kalra, S. and Jood, S. (2000) Effect of dietary barley 0-glucan on cholesterol
and lipoprotein
fractions in rat. J Cereal Sci. 31:141-145.
Kato, A. and Nakai, S. (1980) Hydrophobicity determined by a fluorescence
probe method
and its correlation with surface properties of proteins. Biochim. Biophys.
Acta. 624:13-20.
Kawase, S.; Murakami, H.; Matsumura, Y. and Mori, T. (2003) Effects of
fragmentation and
deamidation on the antioxidative activity of C hordein..I Oleo Sci. 52:175-
184.
Kinsella, J. E. (1976) Functional properties of proteins in foods: a survey.
CRC Crit. Rev.
Food Sci. Nutr. 7:219-280.
Lan, L.; Zhao, M.; Ren, J.; Zhao, H.; Cui, C. and Hu, X. (2010) Effect of
acetic acid
deamidation-induced modification on functional and nutritional properties and
conformation
of wheat gluten. J. ScL Food Agric. 90:409-417.
Li, D.; Xu, M.; Lundin, L. and Wooster, T.J. (2009) Interfacial properties of
deamidated
wheat protein in relation to its ability to stabilise oil-in-water emulsions.
Food Hydrocoll.
23:2158-2167.
27
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
Li, X.; Liu, Y.; Yi, C.; Cheng, Y.; Zhou, S. and Hua, Y. (2010) Microstructure
and
rheological properties of mixtures of acid-deamidated rice protein and
dextran. J Cereal Sci.
51:7-12.
Liu, G.; Li, J.; Shi, K.; Wang S.; Chen, J.; Liu, Y. and Huang Q. (2009)
Composition,
secondary structure, and self-assembly of oat protein isolate. J. Agric. Food
Chem. 57:4552-
4558.
Liu, S.; Low, N.H. and Nickerson M.T. (2009) Effect of pH, salt, and
biopolymer ratio on the
formation of pea protein isolate-gum arabic complexes. Agric. Food Chem.
57:1521-1526.
Malabat, C.; Sanchez-Vioque, R.; Rabiller, C. and Gu, J. (2001) Emulsifying
and foaming
properties of native and chemically modified peptides from the 2S and 12S
proteins of
rapeseed (Brassica napus L.). JAOCS. 78:235-242.
Matsudomi, N.; Sasaki, T.; Kato, A. and Kobayashi, K. (1985) Conformational
changes and
functional properties of acid-modified soy protein. Agric. Biol Chem. 49:1251-
1256.
McClements, D.J. (1999) Food emulsions: principles, practice, and techniques.
CRC Press:
Boca Raton, FL.
Mejia, C.D.; Mauer, L.J. and Hamaker, B.R. (2007) Similarities and differences
in secondary
structure of viscoelastic polymers of maize a-zein and wheat gluten proteins.
J. Cereal Sci.
45:353-359.
Nakai, S. (1983) Structure-function relationships of food proteins: with an
emphasis on the
importance of protein hydrophobicity. J. Agric. Food Chem. 31:676-683.
28
WO 2012/006731 CA 02805677 2013-01-16
PCT/CA2011/000831
Paraman, I.; Hettiarachchy, N.S. and Schaefer, C. (2007) Glycosylation and
deamidation of
rice endosperm protein for improved solubility and emulsifying properties.
Cereal Chem.
84:593-599.
Pomeranz, Y. and Shands, H.L. (1974) Food uses of barley. Critical Rev. Food
Sci. Nutr.
4:377-394.
Shewry, P.R. (1993) Barley seed proteins. In Barley: Chemistry and Technology;
MacGregor,
A.W. and Bhatty, R.S., eds. American Association of Cereal Chemists Inc.: St.
Paul,
Minnesota, USA, pp. 131-197.
Shukla, R. and Cheryan, M. (2001) Zein: the industrial protein from corn. Ind.
Crop. Prod.
13:171-192.
Siu, N.; Ma, C.; Mock, W. and Mine, Y. (2002) Functional properties of oat
globulin
modified by a calcium-independent microbial transglutaminase. 1 Agric. Food
Chem.
50:2666-2672.
Townsend, A.A. and Nakai, S. (2006) Relationships between hydrophobicity and
foaming
characteristics of food proteins. J. Food Sci. 48:588-594.
Wang, C.; Tian, Z.; Chen, L.; Temelli, F.; Liu, H. and Wang, Y. (2010)
Functionality of
barley proteins extracted and fractionated by alkaline and alcohol methods.
Cereal Chem.
87(6):597-606.
Wellner, N.; Belton, P.S. and Tatham, A.S. (1996) Fourier transform IR
spectroscopic study
of hydration-induced structure changes in the solid state of w-gliadins.
Biochem. 1 319:741-
747.
Wood, P.J. (2004) Relationships between solution properties of cereal P-
glucans and
physiological effects - a review. Trend. Food Sci. Technot 15:313-320.29
WO 2012/006731 CA 02805677 2013-01-16 PCT/CA2011/000831
Yalcin, E.; Celik, S. and Ibanoglu, E. (2008) Foaming properties of barley
protein isolates and
hydrolysates. Eur. Food Res. Technol. 226:967-974.
Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T. and
Ishii, K. (1972)
Studies on function properties of food-grade soybean products. Whipping and
emulsifying
properties of food-grade soybean products. Agr. Biol. Chem. 36:719-727.
Yong, Y.H.; Yamaguchi, S. and Matsumura Y. (2006) Effects of enzymatic
deamidation by
protein-glutaminase on structure and functional properties of wheat gluten. J.
Agric. Food
Chem. 54:6034-6040.
Yong, Y.H.; Yamaguchi, S.; Gu, Y.S.; Mori, T. and Matsumura, Y. (2004) Effects
of
enzymatic deamidation by protein-glutaminase on structure and functional
properties of
alpha-zein. J. Agric. Food Chem. 52:7094-7100.
30