Note: Descriptions are shown in the official language in which they were submitted.
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
DENTAL LASER-EMITTING DEVICE AND METHODS
PRIORITY INFORMATION
[000.1] The present application claims priority to U.S. Provisional
Application
No. 61/417,685 filed on November 29, 2010.
TECHNICAL FIELD
[0001] This invention relates to the field of medical lasers and,
in particular,
lasers used in the provision of dental treatment of hard tissue and soft
tissue, including
gingival tissue, skin, muscle, connective tissue, bone, tooth enamel, and
tooth dentin.
BACKGROUND AND SUMMARY
[0002] During dental procedures, it may be necessary to utilize
various
surgical techniques on hard tissue and soft tissue in treatment areas in and
around the oral
cavity. Such techniques may include the cutting and/or removal of either soft
or hard tissue.
In the past, various traditional surgical tools, such as scalpels, have been
utilized to
accomplish these techniques. In addition, medicines and antibiotics have been
utilized for
control of pain, as well as a preventive measure to avoid infection.
[0003] In the late 1950's, the high speed air rotor was developed
for the
removal of dental hard tissue, including enamel, dentin and dental caries. The
high speed air
rotor offered faster removal of hard tissue while also being more comfortable
for the patient
and easier to use for the dentist, compared to available electric belt drive
dental drills. While
offering advantages, the high speed air rotor was found to create excessive
heat and high
frequency vibration which was injurious to the vital tissues in the tooth; and
a water spray or
water misting system was developed in parallel with the high speed air rotor.
The water
spray or water mist was directed toward the operative site while the air rotor
was spinning
and a burr was in contact with tooth structure, thus safely cooling the tooth
structure and
dampening the injurious high frequency vibration.
[0004] Later, mid-infrared lasers became available for the removal
of dental
hard tissues by means of ablation. These lasers also used a water spray or
water mist for
cooling of the tooth structures and as a medium which absorbed the mid-
infrared wavelength
energy emitted by the lasers, thus enhanced the ablation of the dental hard
tissues.
- 1 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 2 -
[0005] Laser-emitting devices are beginning to achieve
increased popularity
as tools to perform the above-described functions. Such laser-emitting devices
may be used
to cut and cauterize skin, including treatment areas on or around the lips and
gums, and high
power laser-emitting devices may be used to ablate bone, tooth dentin and
tooth enamel.
Laser-emitting devices may further be used in the debridement,
denaturalization and
sterilization of root canal surfaces. There are many benefits to using a laser-
emitting device
over traditional methods of performing these operations, including a
significant reduction in
the post-operative healing time, improved control over bleeding due to the
simultaneous
cauterization of the soft-tissue at the time of cutting, the opportunity to
provide less-invasive
treatments by making smaller and more precise cuts, the ability to treat with
less anesthesia
and possibly no anesthesia, the ability to gain access to and effectively
treat otherwise
inaccessible areas (e.g., sterilization and debridement of necrotic tissue,
such as within
periodontal pockets), and promotion of a potentially better surface for
subsequent bonding
procedures due to the lessened need to chemically etch tooth surfaces after
drilling.
[0006] While there may be significant benefits
associated with the use of a
laser-emitting device to perform the above-mentioned treatments, there are
also significant
challenges. Dental lasers have taken considerable time to find adoption within
the
community of dental practitioners for a variety of reasons, including cost,
the learning curve
required to effectively use such devices, complicated setup parameters,
difficulty in diagnosis
of malfunctioning equipment, limited treatment applications for earlier
designs, and
institutionalized treatment methods that stayed relatively static for nearly a
century, to name
just a few. While cost tends to decline as a technology matures, other factors
can be
significantly mitigated through improvements in the design of the laser-
emitting devices,
including those described herein.
[0007] In one exemplary embodiment of the present
invention, a laser-
emitting device is described which comprises a housing, a power supply,. two
or more laser
light sources, a controller configured to modulate one or more of the laser
light sources; a
memory operatively coupled to the controller to store device settings; a
connection used to
operatively couple a smart device to the controller, a handpiece for applying
laser light to the
area of treatment, an airless misting unit to apply a fine water mist to the
area of treatment,
and an articulated arm operatively coupling the laser light source to the
handpiece.
- 2 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 3 -
BRIEF DESCRIPTION OF THE DRAWINGS
[0008] The detailed description particularly refers to the accompanying
figures, in which:
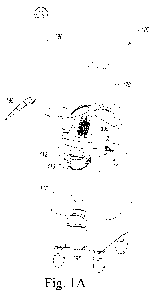
[0009] FIG. lA is an isometric view of an exemplary embodiment of the
dental laser-emitting device described herein;
[0010] FIG. 1B is a detail view of an exemplary embodiment of a
secondary
visual display described herein;
[0011] FIG. 2 is a front elevation view of an exemplary embodiment of
the
dental laser-emitting device described herein;
[0012] FIG. 3 is a side elevation view of an exemplary embodiment of the
dental laser-emitting device described herein;
[0013] FIG. 4 is a top elevation view of an exemplary embodiment of the
dental laser-emitting device described herein;
[0014] FIG. 5 is a rear elevation view of an exemplary embodiment of the
dental laser-emitting device described herein;
[0015] FIG. 6 is an schematic depicting the components of an exemplary
embodiment of the dental laser-emitting device described herein;
[0016] FIG. 7 is a detailed view of the laser light subsystem of an
exemplary
embodiment of the dental laser-emitting device described herein;
[0017] FIG. 8 is a diagram depicting the components of the airless
misting
unit described herein;
[0018] FIG. 9 is a line drawing depicting an exemplary embodiment of the
user interface for the dental laser-emitting device described herein;
[0019] FIG. 10 is a flow chart depicting one exemplary embodiment of a
method wherein the controller responds to user input;
[0020] FIG. 11 is a flow chart depicting one exemplary embodiment of a
method wherein a diagnostic program is executed.
DETAILED DESCRIPTION
[0021] Referring now to FIGS. 1-5, laser-emitting device 100
includes a
housing 110; a power supply 120; laser subsystem 130; a controller 140
configured to
modulate one or more of the laser light sources; a memory 150 operatively
coupled to the
controller to store device settings; a connection 160 used to operatively
couple a smart device
- 3 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
-4-
170 to the controller; a handpiece 180 for applying laser light to the area of
treatment, an
airless misting unit 200 to irrigate the area of treatment; an articulated arm
190 operatively
coupling laser subsystem 130 to handpiece 180; and a user interface 300
located on the
exterior of housing 110 or, alternatively, on smart device 170 operatively
coupled to
controller 140 through connection 160 and that allows an operator to modify
the operational
parameters of the laser subsystem 130 and/or airless misting unit 200. In the
illustrative
embodiment, housing 110 includes a front handle 112 and a rear handle 114.
[0022] An exemplary embodiment of the laser subsystem 130 is
depicted in
FIG. 7. Laser subsystem 130 includes a laser source 131 producing a visible
aiming beam
132, and at least two therapeutic laser light sources 133a, 133b...133n and
emitting laser
beams 134a, 134b... 34n, wherein one or more of the therapeutic laser light
sources may be
designed to operate at a lower power for procedures conducted on soft tissue,
such as skin or
gum tissue, and one or more of the other therapeutic laser light sources may
be designed to
operate at a higher power for procedures on hard tissue, such as tooth enamel,
tooth dentin, or
bone. In one such exemplary embodiment, laser light source 133a is a
Neodymium/YAG or
semiconductor diode laser having a power range adjustable between about 0 to
about 15
Watts, such as from about 0.1 to about 15 Watts, and used for soft-tissue
applications and
laser light source 133b is an Erbium/YAG diode-pumped solid-state laser or a
flashlamp-
pumped solid-state laser used for hard-tissue applications. Laser beams 134
are collected in
optical coupler 135 whereby any of laser beams 132 and 134 emit from a single
location and
exit laser subsystem 130 as beam 136.
[0023] In one exemplary embodiment, power supply 120 includes
insulated-
gate bipolar transistors, which may allow an operator or technician to set a
variable pulse
width for therapeutic laser light source 133a and/or therapeutic laser light
source 133b, in
order to modify the power yield as a function of time. For example, power
supply 120 can be
configured to provide high-power peaks of shorter duration to improve
performance during
hard-tissue ablation procedures. As a further example, power supply 120 can be
configured
with high repetition rates and medium duration pulses to cause cavitation
within root canals
to remove softer tissue and sterilize the interior of the canal. As another
example, power
supply 120 can be configured to provide longer duration power of lower peaks
to improve
comfort, consistency and/or quality of soft-tissue cutting and cauterizing
procedures. In the
illustrative embodiment, laser-emitting device 100 also includes foot pedal
195 that is
operatively coupled to controller 140 using a wireless communication link.
While foot pedal
- 4 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
-5-
195 uses a wireless link in the illustrative embodiment, it may also be
operatively coupled to
controller 140 using a wired connection.
[0024] By utilizing multiple therapeutic laser light sources,
such as 133a,
133b.. .133n, a wide variety of dental procedures may be performed on both
soft-tissue and
hard tissue. The list of soft-tissue procedures includes, but is not limited
to, gingival
troughing for crown impressions, gingivectomy and gingivoplasty, gingival
incision and
excision, soft-tissue crown lengthening, hemostatis and coagulation,
excisional and incisional
biopsies, exposure of unerupted teeth, fibroma removal, frenectomy and
frenotomy, implant
recovery, incision and drainage of abcess, leukoplakia, pulpotomy as an
adjunct to root canal
therapy, operculectomy, oral papilectomies, reduction of gingival hypertrophy,
treatment of
canker sores, herpetic and aphthous ulcers of the oral mucosa, and
vestibuloplasty.
Additional periodontal procedures include sulcular debridement, including
removal of
diseased, infected, inflamed and necrosed soft-tisuse in the periodontal
pocket to improve
clinical indices including gingival index, gingival bleeding index, probe
depth, attachment
loss and tooth mobility; laser soft-tissue curettage, laser removal of
diseased, infected,
inflamed and nectrotic soft-tissue within the periodontal pocket; removal of
highly-inflamed
edematous tissue affected by bacterial penetration of the pocket lining and
junctional
epithelium. The list of hard-tissue procedures includes, but is not limited
to, laser drilling,
bone ablation, tooth enamel and/or dentin ablation, and the desensitization of
nerves within
the tooth pulp by firing low power laser pulses through the relatively
translucent tooth enamel
and dentin. In addition, the use of laser light sources 133a and 133b allows
laser-assisted
whitening/bleaching of teeth and bio-stimulation of both hard-tissue and soft-
tissue, as
desired.
[0025] In one exemplary embodiment, as depicted in FIG. 8,
airless misting
unit 200 described above includes a water source 210, a reservoir 215, a high-
pressure pump
220, a supply line 230, and an atomizing nozzle 240. Optionally, a check valve
250 may also
be included to restrict the flow of water from nozzle 240 when airless misting
unit 200 is not
in operation. Atomizing nozzle 240 is designed to cause a fine mist of water
to be ejected
and mixed with the air present outside the nozzle when high-pressure pump 220
is activated
by controller 140. By pressurizing the water in supply line 230 to from about
2 Bars to about
Bars of pressure (from about 30 psi to about 150 psi), such as from about 4
Bars to about
10 Bars or from about 4 Bars to about 8 Bars, air need not be introduced into
supply line 230
to create the mist. In one exemplary embodiment, atomizing nozzle 240 includes
orifices of
- 5 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 6 -
between about 200 microns and about 500 microns and is manufactured by a laser
drilling
process which allows the airless mist generated by the unit to be optimized to
provide
efficient misting of the treatment area.
[0026] Conventionally, water spray or water mist for both the high
speed air
rotor handpieces and lasers was generated by combining liquid water and
pressurized air.
The liquid water and pressurized air were typically mixed in close proximity
to a misting or
spray orifice and fine particles of water were generated by the rapid
expansion of the
pressurized air as it escaped from the orifice. While effective for creating a
water mist, the
conventional technology necessitates two pressurized conduits, at least two
meters in length,
connected to the dental handpiece, and considerable expense and complexity
associated with
regulating the pressure to the liquid water and pressurized air. Furthermore,
the requisite
pressures were generated by pumps internal to the dental device or by
connection to the
pressurized air supply within a dental office.
[0027] In addition to the expense of regulating the air and water
pressures
within the dental unit or laser, operator error among dental office personnel
could cause the
air connection to the dental unit to be connected to a water supply in the
dental operatory,
with very damaging results.
[0028] Furthermore, dental offices were known to frequently have
contaminated compressed air supplies due to water condensation during the
compression
process. The condensed water may be held in the compressed air tanks of a
dental office for
weeks or months and could become a breeding ground for bacteria, mold and
other forms of
contamination. Spraying contaminated water and air into open operative sites
is a known
source of infection and disease in the dental profession.
[0029] The airless misting system disclosed herein eliminates much
of the
complexity, expense, contamination risk and infection risk by producing a fine
water mist or
spray without the addition of compressed air. The use of a single, small high
pressure water
pump and a removable and cleanable water container allows the airless misting
and improves
the ease of operation of the laser system and also improve its safety.
[0030] As broadly disclosed herein, the airless mist is referred
to water
without any air added to it by way of addition of compressed air to the water.
However, one
of ordinary skill in the art will understand that any suitable liquid, without
the addition of a=
compressed gas, may be used. One example of such a suitable liquid may be a
medicament
liquid. Any suitably liquid may be used so long as it is capable of cooling
the treatment area
- 6 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 7 -
and focusing the laser beam emitted by the disclosed device and also does not
include any
compressed or pressurized gas, such as air.
[0031] Referring now to FIG. 9, an exemplary embodiment
of user interface
300 allows an operator of laser-emitting device 100 to quickly and easily
select appropriate
device settings. For example, an operator could select from an array of pre-
programmed
combinations of laser energy and pulse frequency by pressing a button or icon
on user
interface 300 that is associated with either the soft-tissue laser light
source or the hard-tissue
laser light source. In one such exemplary embodiment, each user-selectable
button or icon
causes the controller to set the laser energy and pulse frequency to a pre-
determined setting
stored in controller memory 150.
[0032] In the illustrative embodiment of FIG. 9, user
interface 300 includes a
bank 310 of user-selected buttons or icons 316 associated with pre-set
parameters for the
hard-tissue laser and a bank 320 of user-selected buttons or icons 326
associated with pre-set
parameters for the soft-tissue laser. In the illustrative embodiment depicted
in FIG. 9, bank
310 includes an icon 312 that indicates that it relates to the hard tissue
laser operations, and
bank 320 includes an icon 314 that it relates to the soft tissue laser
operations. As further
depicted in the illustrative embodiment of FIG. 9, bank 310 of user interface
300 includes
five user-selectable buttons or icons 316a-e and bank 320 includes five user-
selectable
buttons or icons 326a-e.
[0033] In this illustrative embodiment, user interface
300 also provides
additional buttons or icons and each button or icon may have its own
corresponding indicator,
such as an LED or similar device. Referring to FIG. 9, the following
additional buttons/icons
and indicators are depicted: on/off button or icon 330, up arrow button or
icon 340, down
arrow button or icon 350, "function" button or icon 360 with "function"
indicator 362, light
button or icon 370 with light indicator 372, sound button or icon 380 with
sound indicator
382, and standby button or icon 390 with standby indicator 392. On/off button
or icon 330
powers on or powers off laser-emitting device 100. Light button or icon 370
and sound
button or icon 380 may be used to toggle one or more sound and visual
indicators,
respectively. Standby button or icon 390 places laser-emitting device 100 into
or out of
standby mode. Up arrow button or icon 340 and down arrow button or icon 350
allow a user
to manually adjust the power settings from the pre-set parameters associated
therewith.
Furthermore, while bank 310 and bank 320 are each shown to include five
buttons or icons in
the illustrative embodiment, the number of buttons or icons associated with
each bank is not
- 7 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 8 -
limited thereto, but may encompass fewer or more buttons or icons, as
necessary. In yet
further embodiments (not pictured), the user interface may include an optional
bank of
buttons or icons directed to the control of endodontic procedures, such as
preparing a tooth
for and conducting a root canal. As one of ordinary skill in the art will
understand, such
additional buttons or icons for endodontic procedures may be placed on the
user interface by
any suitable method.
[0034] In the illustrative embodiment, bank 310 of the hard-
tissue controls
includes user-selectable button or icon 316a depicting a rabbit indicative of
a "speed" setting;
button or icon 316b depicting a "smiley face" indicative of a "comfort"
setting; button or icon
316c depicting scissors indicative of a hard-tissue cutting or ablation
setting; button or icon
316d depicting a set of wavy lines indicative of a "desensitization,"
"decontamination," or
curettage setting; and button or icon 316e depicting a bone indicative of an
osseous setting
for ablating bone. In one such exemplary embodiment, the pre-set parameters
associated
with each button or icon of bank 310 indicates to controller 140 that the
airless misting unit
200 should operate during operation of the hard-tissue laser. In the
illustrative embodiment,
indicators 318a-318e each corresponds to a user-selectable button or icon 316
to indicate the
currently selected setting. In the illustrative embodiment show, indicators
318a-318e are
depicted as light-emitting diodes that illuminate when each corresponding
button or icon
316a-316e, respectively, is selected. For example, when button or icon 316a is
selected by
the user, indicator 318a changes to indicate the selection of that selection.
While indicators
318 are depicted in FIG. 9 as light-emitting diodes (LEDs), they could also be
elements of a
Liquid Crystal Display (LCD), Organic Light Emitting Diodes (OLEDs) or other
type of
indicator capable of indicating information about the status of laser-emitting
device 100. In
another exemplary embodiment, indicators 318a-e are configurable icons on a
touch-screen.
[0035] Similarly, in the illustrative embodiment, bank 320
includes five user-
selected buttons or icons associated with pre-set parameters for the soft-
tissue laser. As in the
previous example, button or icon 326a depicting a rabbit indicates a "speed"
setting for the
soft-tissue laser; button or icon 326b depicting a smiling face indicates a
"comfort" setting;
button or icon 326c depicting a probe entering between a tooth and gum
indicates a soft-
tissue cutting or curettage setting; button or icon 326d depicting a set of
wavy lines indicates
a "desensitization" or "decontamination" or "curettage" setting; and button or
icon 326e
depicting lines emitting from a surface indicates a "tooth bleaching" or "bio-
stimulation"
setting. In one such exemplary embodiment, the pre-set parameters indicate to
controller 140
- 8 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 9 -
that the airless misting unit 200 should not operate during operation of the
soft-tissue laser.
Furthermore, button or icon 326d could indicate to controller 140 that one set
of laser
parameters including pulse frequency and laser energy should be set, or button
or icon 326d
could be programmed to cycle through three or more different settings having
different pulse
frequencies and laser energy, but providing settings that are effective in one
or more of the
desensitization, decontamination or curettage procedures.
[0036] In the illustrative embodiment, indicators 328 each
correspond to a
user-selectable button or icon 326 to indicate the currently selected setting.
In the illustrative
embodiment shown, indicators 328 are depicted as light-emitting diodes that
illuminate when
each corresponding button or icon 326, respectively, is selected. For example,
when button
or icon 326a is selected by the user, indicator 328a changes to indicate the
selection of the
related pre-set laser parameters. While indicators 328 are depicted in FIG. 9
as light-emitting
diodes (LEDs), they could also be elements of a Liquid Crystal Display (LCD),
Organic
Light Emitting Diodes (OLEDs) or other type of indicator capable of indicating
information
about the status of laser-emitting device 100. In another exemplary
embodiment, indicators
328 are configurable icons on a touch-screen.
[0037] Visual display 400 indicates desired information about
the status of at
least one of laser light sources 133a. For example, in one such exemplary
embodiment,
visual display 400 indicates the operating power of therapeutic laser 133a
corresponding to a
selected setting when a button or icon from bank 310 has been selected, and
visual display
400 indicates the operating power of therapeutic laser 133b corresponding to a
selected
setting when a button or icon from bank 320 has been selected. Other
parameters may be
shown on visual display 400, including pulse width, pulse frequency, or
another laser
parameter of interest to the operator. While visual display 400 is depicted in
FIG. 9 as a
multi-segment light-emitting diode (LED) display, it is not limited thereto.
Visual display
400 could also be a Liquid Crystal Display (LCD), Organic Light Emitting Diode
(OLED) or
other type of display capable of indicating information about the status of at
least one of laser
light sources 133a. In another exemplary embodiment, visual display 400 is
comprised of
configurable icons on a touch-screen.
[0038] In another exemplary embodiment, a secondary visual
display 410, as
depicted in FIG. 1B, provides a visual indicator of a general status of the
laser subsystem
130. The illustrative embodiment includes three cold-cathode tubes, wherein
controller 140
causes a red cold-cathode tube 420 to illuminate to indicate that the laser-
emitting device 100
- 9 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 10 -
is in soft-tissue mode, controller 140 causes a green cold-cathode tube 430 to
illuminate to
indicate that laser-emitting device 100 is in hard-tissue mode, and controller
140 causes a
yellow cold-cathode tube 440 to illuminate to indicate that laser-emitting
device 100 is in
standby mode. Secondary visual display 410 provides a quick visual indication
of the status
of laser-emitting device 100 when an operator may by further away from the
system or may
not be able to see the other visual indicators. While red-, green- and yellow-
colored cold-
cathode tubes are used as secondary visual display 410 in this exemplary
embodiment, other
types and colors of light sources may be used, such as LEDs and OLEDs, or any
other light-
emitting devices of any color. In yet another exemplary embodiment, secondary
visual
display 410 is comprised of configurable icons or graphics on a touch-screen.
[0039] As described in the exemplary embodiment above, each
button or icon
in bank 310 and bank 320 may be configured to correspond to one or more pulse
frequency/laser energy pre-set parameters. Moreover, in one exemplary
embodiment, in
addition to adjusting the laser parameters to the pre-set parameters in FIG.
10, controller 140
is configured to also engage airless misting unit 200 when one of the hard-
tissue laser settings
of bank 310 is selected, and controller 140 is configured to disengage airless
misting unit 200
when one of the soft-tissue laser settings of bank 320 is selected.
[0040] Furthermore, while reference is made to an operator
utilizing bank 310
and bank 320 of buttons or icons to select pre-set parameters for the laser-
emitting device
100, an operator may also make selections on smart device 170 through buttons
or icons. In
one exemplary embodiment, the screen of smart device 170 mimics user interface
300 to
provide a second method of selecting an operating mode of laser-emitting
device 100.
[0041] In addition, smart device 170 may provide alternate
methods of
selecting an operating mode of laser-emitting device 100. In one such
exemplary
embodiment, smart device 170 is configured to use speech recognition to detect
a verbal
command of an operator and communicate with controller 140 to select the
applicable pre-set
parameters. For example, smart device 170 may listen for the operator to speak
verbal
commands, such as "soft tissue speed" or "hard tissue comfort," in response to
which smart
device 170 would communicate the selection to controller 140 which would make
the
corresponding selection of pulse frequency and laser energy and would update
user interface
300, visual display 400, and secondary visual display 410. In addition, smart
device 170
could also be configured to respond with synthesized speech output to provide
an auditory
- 10-
WO 2012/074918 CA 02806864 2013-01-28PCT/US2011/062217
- 11 -
confirmation of the selected operating mode of laser-emitting device 100,
regardless of
whether the selection was made by voice or through the user interface.
[0042] Additional functionality is provided by smart device 170. In one
exemplary embodiment, smart device 170 not only communicates with controller
140, but is
also designed to communicate with other systems apart from laser-emitting
device 100. A
variety of applications exist for such two-way communication. For example, a
diagnostic
program designed to run on smart device 170 could diagnose laser system 100
based upon
operating parameters and/or usage data and transmit that information back to
the
manufacturer of laser-emitting device 100, or to a third-party service
company, to assist in
troubleshooting and repair of a malfunctioning unit.
[0043] In another exemplary embodiment, smart device 170 would receive
software and/or firmware updates from the manufacturer and upgrade laser-
emitting device
100. In yet another exemplary embodiment, smart device 170 could calibrate one
or more of
the lasers 132 and/or 133 utilizing two-way communication between the
manufacturer and
laser-emitting device 100. For example, the manufacturer could initiate an
upgrade to the
laser system 100 through communication with smart device 170 to program power
supply
120 to operate at a different pulse width profile based either on new data
available to the
manufacturer or at the request of the user of laser-emitting device 100.
[0044] In yet another exemplary embodiment, an operator of smart device
170
could initiate a chat, email communication, or online help resource to receive
support. In yet
another exemplary embodiment, an operator of smart device 170 could order
accessories,
consumables, new products or upgrade to a newer version of laser-emitting
device 100.
[0045] Although, in the illustrative embodiment, smart device 170 is
described and depicted as an Apple iPadTM, it is not limited thereto. For
example, smart
device 170 could take the form of any brand of cellular telephone including,
but not limited
to, an Apple brand iPhoneTM cellular telephone, DroidTM cellular telephone or
BlackberryTM
cellular telephone. Smart device 170 could also be a tablet computer (or
tablet-like
computer) of any screen size and capable of being operatively coupled to laser-
emitting
device 100 via a wired or wireless connection.
[0046] Further, while in one exemplary embodiment smart device 170 is
described as having wireless communication capability compatible with an WEE
802.11
standard ("WiFi" or "WiFi Direct"), any wireless communication standard is
considered
within the scope of the present invention. Other examples of wireless
communication
-11 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 12 -
capability include, but are not limited to, CDMA, W-CDMA, GSM, 3G or 4G, or
WiMAX
communication protocols, or any other appropriate wireless communication
protocol.
[0047] Similarly, although the exemplary embodiment depicted in
FIGS. 1-6
illustrates smart device 170 as physically connected to connection 160 in a
"docked
configuration," the invention is not necessarily limited to that connection
type and could also
be connected via a cable (not shown) or a wireless connection, such as IEEE
802.11 WiFi,
WiFi Direct, Bluetooth, WiMAX, or any other appropriate wireless communication
protocol.
[0048] Referring now to FIG. 10, a method of operating a dental
laser-
emitting device is described. After the method begins in step 610, laser-
emitting device
detects whether a user has interacted with the user interface to select an
operating mode in
step 620. Upon detection of user input, in step 630 the controller retrieves
the laser
parameters associated with the selected operating mode. One of the parameters
includes
whether airless misting should be administered, which the method determines in
step 640. If
the laser parameters for a certain operating mode require airless misting, the
airless misting
unit is engaged in step 650. Either when the airless misting is determined to
not be required
in step 640 or after the airless misting unit is engaged in step 650, the
controller sets the laser
energy in step 660 to match the selected parameters retrieved in step 630.
Similarly, in
step670, the controller sets the laser pulse frequency to match the parameters
retrieved in
step630. Upon setting the laser energy and pulse frequency, the controller
energizes the laser
in step 680 and the routine ends in step 690.
[0049] Referring now to FIG. 11, a method of performing remote
diagnostics
and/or telemetry of a dental laser-emitting device is described. After the
routine begins in
step 710, the controller or smart device polls the laser device in step 720 to
record operating
parameters, such as pulse energy, pulse frequency, pulse width, number of
flash-lamp pulses
fired, number of laser pulses fired, hours of laser operation in standby mode,
hours of laser
operation in ready mode, hours of laser operations in operational mode (laser
actually firing),
coolant temperature, laser head temperature, air temperature within the
device, or any other
measurable parameter of interest. If the parameters are in a specified range,
as determined in
step 730, the diagnostic and/or telemetry routine ends in step 820. However,
should the
parameters retrieved in step 720 be outside of the specified range, the smart
device initiates
communications with the device manufacturer or a third-party service company
in step 740.
In one exemplary embodiment, the communications between the smart device is
initiated
through a wireless connection to the intemet, such as through an IEEE 802.11
standards-
- 12-
WO 2012/074918 CA 02806864 2013-01-28 PCT/US2011/062217
- 13 -
based wireless protocol. Another method of connection may also be used,
including
Bluetooth, CDMA, GMA, 3G, 4G or any suitable method for initiating a
connection to the
manufacturer or third-party service company.
[0050] After the connection is established, the smart device sends
the data
polled in step 720 to the manufacturer in step 750. A web-enabled server
associated with the
manufacturer reads the data provided through the communication channel and
compares it to
that stored in a troubleshooting database in step 760. If the data provided
does not match a
condition found in the troubleshooting database, in step 770 the web-enabled
server initiates a
technician review. This can be done in a variety of ways, including by sending
an email
message to a technician, creating an entry in a service database, sending a
text message to a
computer or cellular device, or any other known method of sending a message
between a
web-enabled server and a user, after which the diagnostic and/or telemetry
routine ends in
step 820. While a web-enabled server is described in the illustrative
embodiment, a similar
device capable of communication and assessment of the polled data may also be
used.
[0051] However, should the data provided in step to the web-enabled
server in
step 750 match a condition found in the troubleshooting database, the web-
enabled server in
step 790 transmits a message back to the smart device. Such message may be
sent through
the same communications method as the original message sent from the smart
device to the
web-enabled server. In addition, other communications could be sent in step
790. In one
exemplary embodiment, an email message is transmitted to a distribution list
associated with
the web-enabled server or similar device. In another exemplary embodiment, an
automated
phone call is placed to a telephone number or numbers associated with the web-
enabled
server. In yet another exemplary embodiment, a technician receives a message
to contact the
operator registered to the dental laser-emitting device to discuss the
detected condition.
[0052] In another exemplary embodiment, the data polled in step 720
is used
to facilitate routine, preventative and/or predictive maintenance. For
example, the
communication described in step 790 may include instructions to replace the
flash-lamp after
a certain number of pulses is reached, to alert the user to change a filter
after a certain number
of hours of standby, ready, or operational time has passed. While these
examples are
provided for illustrative purposes, any routine, preventative, or predictive
maintenance may
be initiated based upon the data polled in step 720, and it is not limited to
the examples
provided.
- 1 3 -
WO 2012/074918 CA 02806864 2013-01-28
PCT/US2011/062217
- 14 -
[0053] In certain instances, it may be desirable to shut
down the dental laser-
emitting device when parameters vary outside of a normal range. In the
illustrative
embodiment, the diagnostic method determines in step 800 that the dental laser-
emitting
device should be shut down for safety reasons. Once that determination is
made, a remote
shutdown is initiated in step 810 by sending a command from the web-enabled
server to the
smart device. Once the command is received by the smart device, the diagnostic
program
ends in step 820 and the dental laser-emitting device is shut down. In one
exemplary
embodiment, other activities are triggered by the remote system shutdown, such
as the
initiation of a service call for the malfunctioning dental laser-emitting
device. Said remote
diagnostics within the smart device may provide redundancy and back-up to the
safeguards
and "watchdog" routines within the laser operating software. Should an error
condition be
detected, the smart device is capable of overriding the control of the laser
and shutting the
system down ¨ thus providing greater safety for the operator and the patient.
[0054] Although the invention has been described in detail
with reference to
certain illustrated exemplary embodiments, variations and modifications exist
within the
scope and spirit of the invention.
-14-