Note: Descriptions are shown in the official language in which they were submitted.
CA 02807859 2013-02-21
BHC 13 1 005
- 1 -
FORMS OF METHYL {4,6-DIAMINO-2-11-(2-FLUOROBENZYL)-1H-PYRAZOL013,4-
B1PYRMINO-3-YLWYRIMIIHNO-5-YLIMETHYL CARBAMATE
This present invention relates to forms of methyl {4,6-diamino-241-(2-
fluorobenzy1)-1H-
pyrazolo[3,4-b]pyridino-3-yl]pyrimidino-5-yllmethylcarbamate of formula (I):
0
HC
'r¨'- C1-13
H2 N\
N H2
N
1110
WO 03/095451 discloses the compound of formula (I), and further describes that
this and other
compounds disclosed therein are stimulators of soluble guanylate cyclase, and
may therefore be
used as agents for the prophylaxis and/or treatment of cardiovascular
disorders.
WO 03/095451 describes the preparation of the compound of the formula (I).
However, there are
a number of disadvantages associated with the process disclosed in WO
03/095451, as discussed in
WO 2011/064171. WO 2011/064171 thus discloses an alternative process for
preparing a
compound of the formula (I).
In the process of WO 2011/064171, a compound of the formula (VI) is provided:
CA 02807859 2013-02-21
BHC 13 1 005
- 2 -
0
Hs ,Me
0
/ \ NH2
N
(VI)
The compound of the formula (VI) is reacted in a manner known per se, for
example in accordance
with one of the descriptions in WO 03/0945451 or ChemMedChem 2009, 4, 853-865,
with a
methylating agent Me-X to give a crude product which contains high amounts of
the compound of
the formula (I).
0 0
Hs /I
-Me H3C,
0 n' Me
/ \ NH2
Me-X
N
N ;/--1`1' base
N
=
(VI) (I) (crude product)
The methylating agent Me-X used is methyl iodide, dimethyl sulphate, methyl
toluenesulphonate,
etc., and methyl iodide or dimethyl sulphate is preferred.
The purification of the crude product of the formula (I) for use as
pharmaceutically active
compound is carried out via the compound methyl {4,6-diamino-241-(2-
fluorobenzy1)-1H-
pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}methylcarbamate sulphinyldimethane
(1:1), i.e. a
compound of the formula (II) as isolated intermediate or generated in a
mixture.
CA 02807859 2013-02,-21
BHC 13 1 005
- 3 -
0
H3C,
N C113
H2N 0
' - NE12
I I
N S,
H3Cõ CH3
I N
N N
=
For the purification, initially, a mixture is formed which contains high
amounts of the compound
of the formula (II) as intermediate.
0 0
H3C, H3C,
H2N 0 c H2N
I I
H3CCH3 )r-c¨NH2 0
I I
N'1\11
(I) (crude product) (II)
To this end, the crude product of the formula (I) is dissolved in DMSO
(dimethylsulfoxide,
sulphinyldimethane or (methylsulfinyOmethane which are different names for the
same
compound). To form a solution, the mixture is heated to 40-120 C, preferably
50-100 C. To form
a pharmaceutically acceptable product of the formula (I), the solution has to
be filtered, and the
filtration is carried out hot, the temperatures are 40-120 C, preferably 50-
100 C.
After the filtration, a pharmaceutically acceptable solvent, preferably the
same solvent as above, is
added to the hot filtrate. This results in a crystallization of the product of
the formula (II).
Prior to the isolation of the solid which contains high amounts of the
compound of the formula (II),
to bring the precipitation to completion, the mixture is cooled to a
temperature range of 0-35 C,
preferably to an ambient temperature of, for example, 20-30 C.
CA 02807859 2013-02-21
BHC 13 1 005
- 4 -
For pharmaceutical use, the DMSO has to be removed from the product of the
formula (II) or the
mixture comprising high amounts of the compound of the formula (II).
0 0
H3C, C H3C,
11
N
113
H2N H2N
boiling in a solvent
N
N "
(I) (pure product)
To this end, the product of the formula (II) or the isolated mixture
comprising high amounts of the
product of the formula (II) is boiled in a pharmaceutically acceptable solvent
from the class of the
ketones, ethers, esters or alcohols. Examples of such solvents which may be
mentioned are:
methanol, ethanol, isopropanol, 1-butanol, 2-butanol, ethyl acetate, isopropyl
acetate or propyl
acetate, butyl acetate, tert-butyl methyl ether, diisopropyl ether, acetone,
methyl ethyl ketone,
methyl isobutyl ketone, etc. Preference is given to ethanol, isopropanol,
ethyl acetate, isopropyl
acetate, butyl acetate, methyl ethyl ketone, methyl isobutyl ketone. It is
also possible to use
mixtures of these solvents. Particular preference is given to ethyl acetate or
a mixture of ethyl
acetate with ethanol.
Boiling takes place at reflux of the solvent in question or, if appropriate,
at slightly elevated
pressure. The temperature is 50-150 C, preferably 80-120 C. The solid obtained
is then filtered.
The present invention relates to new forms of methyl {4,6-diamino-241-(2-
fluorobenzy1)-1H-
pyrazolo[3,4-b]pyridino-3-yl]pyrimidino-5-yl}metinylcarbamate of formula (I).
Surprisingly it has been found that the compound of formula (I) crystallizes
in two modifications with
melting points at 268 C (Modification I) and 250 C (Modification II). In
this context modifications
and polymorphs have the same meaning. In addition, three pseudo-polymorphs, a
mono-DMSO
solvate, a sesqui-DMSO solvate, a 1/4-ethyl acetate solvate and the amorphous
form have been
found. The amorphous form can exist at room temperature, but crystallizes very
quickly. All together
¨modifications or polymorphs, pseudo-polymorphs and amorphous forms ¨ are
different forms of the
compound of formula (I) according to the present invention.
CA 02807859 2013-02-21
BHC 13 1 005
- 5 -
Aspects of some embodiments of the present invention which may be beneficial
in the present
pharmaceutical field may include stability (e.g. pressure stability, chemical
stability, storage
stability), compatibility over other ingredients, purity, solubility
(thermodynamically, kinetically),
crystallization properties, properties regarding isolation during the chemical
synthesis and
bioavailability of the forms of the compound of formula (I).
The compound of the formula (I) in the Modification I is the thermodynamically
stable form between
0 C and 80 C.
Two of the solvates occur during synthesis, the mono-DMSO solvate and the 1/4-
ethyl acetate solvate
of the compound of the formula (I). The compound of the formula (I) in the
Modification II can form
from the solvates after solvent release, e.g. during drying at 80 C.
Embodiments of the present invention are not only each single form the
compound of the formula (I)
which are Modification I, Modification II, mono-DMSO solvate, sesqui-DMSO
solvate and 1/4-ethyl
acetate solvate of the compound of the formula (I) but also mixtures
comprising two, three, four or
five forms of the aforementioned.
A pharmaceutical composition according to the present invention comprises
preferably only one of
the forms selected from the group comprising Modification I, Modification II,
mono-DMSO solvate,
sesqui-DMSO solvate and 1/4-ethyl acetate solvate of the compound of the
formula (I) mainly and no
significant fractions of another form of the compound of the formula (I), for
example of another
modification or pseudopolymorph of the compound of the formula (I). More
preferably the
pharmaceutical composition contains more than 90 percent by weight, most
preferably more than 95
percent by weight, and up to 100 percent, of the compound of the formula (I)
in one of the
aforementioned forms related to the total amount of all forms of the compound
of the formula (I)
present in the composition.
Preference is given to a pharmaceutical composition comprising the compound of
the formula (I) in
the Modification I mainly and no significant fractions of another form of the
compound of the
formula (I), for example of another modification or pseudopolymorph of the
compound of the
formula (I). The pharmaceutical composition preferably contains more than 90
percent by weight,
more preferably more than 95 percent by weight, and up to 100 percent of the
compound of the
formula (I) in the Modification I related to the total amount of all forms of
the compound of the
formula (I) present in the composition.
Further preference is given to a pharmaceutical composition comprising the
compound of the formula
(I) in the Modification II mainly and no significant fractions of another form
of the compound of the
CA 02807859 2013-02721
BHC 13 1 005
=
- 6 -
formula (I), for example of another modification or pseudopolymorph of the
compound of the
formula (I). The pharmaceutical composition preferably contains more than 90
percent by weight,
more preferably more than 95 percent by weight, and up to 100 percent of the
compound of the
formula (I) in the Modification II related to the total amount of all forms of
the compound of the
formula (I) present in the composition.
Further preference is given to a pharmaceutical composition comprising the
mono-DMSO solvate of
the compound of the formula (I) mainly and no significant fractions of another
form of the compound
of the formula (I), for example of another modification or pseudopolymorph of
the compound of the
formula (I). The pharmaceutical composition preferably contains more than 90
percent by weight,
more preferably more than 95 percent by weight, and up to 100 percent of the
mono-DMSO solvate
of the compound of the formula (I) related to the total amount of all forms of
the compound of the
formula (I) present in the composition.
Further preference is given to a pharmaceutical composition comprising the
sesqui-DMSO solvate of
the compound of the formula (I) mainly and no significant fractions of another
form of the compound
of the formula (I), for example of another modification or pseudopolymorph of
the compound of the
formula (I). The pharmaceutical composition preferably contains more than 90
percent by weight,
more preferably more than 95 percent by weight, and up to 100 percent of the
sesqui-DMSO solvate
of the compound of the formula (I) related to the total amount of all forms of
the compound of the
formula (I) present in the composition.
Further preference is given to a pharmaceutical composition comprising the 1/4-
ethyl acetate solvate
of the compound of the formula (I) mainly and no significant fractions of
another form of the
compound of the formula (I), for example of another modification or
pseudopolymorph of the
compound of the formula (I). The pharmaceutical composition preferably
contains more than 90
percent by weight, more preferably more than 95 percent by weight, and up to
100 percent of the 1/4-
ethyl acetate solvate of the compound of the formula (I) related to the total
amount of all forms of the
compound of the formula (I) present in the composition.
The forms of the compound of the formula (I) of the present invention are used
alone or together as a
mixture in high purity in pharmaceutical formulations. As mixtures a
combination of the compound
of formula (I) in the Modification I and the compound of formula (I) in the
Modification II, a
combination of the compound of formula (I) in the Modification I and the mono-
DMSO solvate of
the compound of formula (I), a combination of the compound of formula (I) in
the Modification I and
the sesqui-DMSO solvate of the compound of formula (I), a combination of the
compound of formula
(I) in the Modification I and the 1/4-ethyl acetate solvate of the compound of
formula (I), a
combination of the compound of formula (I) in the Modification I and the mono-
DMSO solvate of
CA 02807859 2013-02:21
BHC 13 1 005
- 7 -
the compound of formula (I) and the 1/4-ethyl acetate solvate of the compound
of formula (I), or a
combination of the compound of formula (I) in the Modification I and the
sesqui-DMSO solvate of
the compound of formula (I) and the 1/4-ethyl acetate solvate of the compound
of formula (I)
optionally with no other form of the compound of the formula (I) are
preferred.
The different forms of the compound of formula (I) can be distinguished by X-
ray powder diffraction,
differential scanning calorimetry (DSC), IR-, Raman-, NIR-, FIR- and 13C-solid-
state-NMR-
spectroscopy:
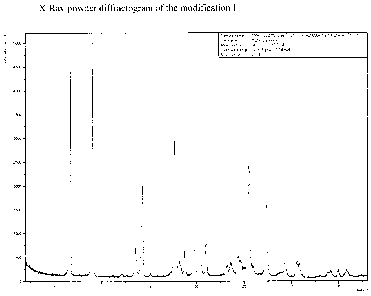
Figure 1: X-Ray powder diffractogram of the modification I
Figure 2: DSC- and TGA-Thermogram of modification I
Figure 3: IR-Spectrum (ATR) of modification I
Figure 4: X-Ray powder diffractogram of the 1/4-ethyl acetate solvate
Figure 5: DSC- and TGA-Thermogram of the 1/4-ethyl acetate solvate
Figure 6: IR-Spectrum (ATR) of the 1/4-ethyl acetate solvate
Figure 7: X-Ray powder diffractogram of the mono-DMSO solvate
Figure 8: DSC- and TGA-Thermogram of the mono-DMSO solvate
Figure 9: 1R-Spectrum (ATR) of the mono-DMSO solvate
Figure 10: X-Ray powder diffractogram of the sesqui-DMSO solvate
Figure 11: DSC- and TGA-Thermogram of the sesqui-DMSO solvate
Figure 12: IR-Spectrum (ATR) of the sesqui-DMSO solvate
Figure 13: X-Ray powder diffractogram of modification II
Figure 14: DSC- and TGA-Thermogram of modification II
Figure 15: IR-Spectrum (ATR) of modification II
Figure 16: X-Ray powder diffractogram of the amorphous form
Figure 17: DSC- and TGA-Thermogram of the amorphous form
CA 02807859 2013-02721
BHC 13 1 005
- 8 -
Figure 18: LR-Spectrum of the amorphous form
The compound of formula (I) in the Modification I can be characterized
unambiguously by a X-
Ray powder diffractogram comprising peak maxima of the 2 Theta angle of 6.7,
9.1, 14.3, 14.4,
17.8, 19.8, 20.2, 24.8, 25.6, 27.3.
The compound of formula (I) in the Modification II can be characterized
unambiguously by a X-
Ray powder diffractogram comprising peak maxima of the 2 Theta angle of 11.2,
12.6, 12.7, 13.9,
15.2, 17.3, 22.5, 22.8, 25.0, 25.5.
The mono-DMSO solvate of the compound of formula (I) can be characterized
unambiguously by
a X-Ray powder diffractogram comprising peak maxima of the 2 Theta angle of
9.0, 10.8, 11.1,
11.2, 13.0, 15.5, 15.9, 16.0, 20.7, 25.6.
The sesqui-DMSO solvate of the compound of formula (I) can be characterized
unambiguously by
a X-Ray powder diffractogram comprising peak maxima of the 2 Theta angle of
8.3, 8.4, 13.7, 13.9,
15.7, 17.2, 18.4, 19.6, 21.4, 24.9.
The 1/4-Ethylacetate solvate of the compound of formula (I) can be
characterized unambiguously by
a X-Ray powder diffractogram comprising peak maxima of the 2 Theta angle of
6.7, 8.3, 8.7, 12.9,
14.2, 17.8, 19.3, 24.0, 25.1, 26.7.
The compound of formula (I) in the Modification I can be characterized
unambiguously by an IR-
spectrogram comprising peak maxima of the 2 Theta angle of 3454, 3360, 3273,
3103, 1688, 1622,
1559, 1284, 1193, 989, 777.
The compound of formula (I) in the Modification II can be characterized
unambiguously by an IR-
spectrogram comprising peak maxima of the 2 Theta angle of 3498, 3382, 3269,
3104, 1704, 1622,
1586, 1563, 1326, 1288, 1106.
The mono-DMSO solvate of the compound of formula (I) can be characterized
unambiguously by
an 1R-spectrogram fractogram comprising peak maxima of the 2 Theta angle of
3401, 3361, 3295,
3168, 1702, 1626, 1560, 1333, 1286, 1042, 751.
The sesqui-DMSO solvate of the compound of formula (I) can be characterized
unambiguously by
an IR-spectrogram comprising peak maxima of the 2 Theta angle of 3407, 3361,
3300, 3190, 1698,
1629, 1558, 1293, 1043, 770, 757.
CA 02807859 2013-02-21
BHC 13 1 005
- 9 -
The 1/4-Ethylacetate solvate of the compound of formula (I) can be
characterized unambiguously by
an IR-spectrogram comprising peak maxima of the 2 Theta angle of 3363, 3275,
1732, 1702, 1619,
1560, 1457, 1246, 899, 810, 771.
The compounds according to the invention may bring about vessel relaxation and
inhibition of
thrombocyte aggregation and lead to a lowering of blood pressure and to an
increase in coronary
blood flow. These effects are due to direct stimulation of soluble guanylate
cyclase and an increase
in intracellular cGMP. Moreover, the compounds according to the invention may
intensify the
action of substances that raise the cGMP level, for example EDRF (endothelium-
derived relaxing
factor), NO donors, protoporphyrin IX, arachidonic acid or phenylhydrazine
derivatives.
The weight data in the tests and examples which follow are, unless stated
otherwise, percentages by
weight; parts are parts by weight. Solvent ratios, dilution ratios and
concentration data of liquid/liquid
solutions are based on each case on the volume.
CA 02807859 2013-02.-21
,
BHC 13 1 005
- 10 -
Working examples
DSC thermograms were recorded using Differential Scanning Calorimeters (model
DSC7, Pyris-1 or
Diamond) from Perkin-Elmer. The measurements were performed with a heating
rate of 20 Kminl
using non-gastight aluminium pans. Flow gas was nitrogen. There was no sample
preparation.
TGA thermograms were recorded using thermobalances (model TGA7 and Pyris 1)
from Perkin-
Elmer.. The measurements were performed with a heating rate of 10 Kmin-1 using
open platinum
pans. Flow gas was nitrogen. There was no sample preparation.
X-Ray diffraction patterns were recorded at room temperature using XRD
¨diffractometers X'Pert
PRO (PANalytical) and STOE STADI-P (radiation Cu K alpha 1, wavelength 1.5406
A). There was
no sample preparation.
Raman spectra were recorded at room temperature using FT-Raman-
spectrophotometers (model RFS
100 and MultiRam) from Bruker. Resolution was 2 cm-'. Measurements were
perfomed in glass vials
or aluminium discs. There was no sample preparation.
1R-ATR-spectra were recorded at room temperature using a FT-IR-
spectrophotometer one with
universal diamond ATR device from Perkin-Elmer. Resolution was 4 cm-1. There
was no sample
preparation.
Example 1
Preparation of purified methyl 4,6-diamino-2-[1-(2-fluorobenzy1)-1H-
pyrazolo[3,4-
b]pyridin-3-y11-5-pyrimidinyhmethyl)carbamate (I) in its modification I
The entire amount of the product of the formula (II) prepared in the Example 6
of
WO 2011/064171 was stirred in 135 ml of ethyl acetate at reflux (about 78 C)
for 1 h and
cooled to about 25 C. The solid was filtered off with suction, washed with a
total of 36 ml
of ethyl acetate and dried under reduced pressure. The weight was 7.6 g or
93.8% of theory.
The content of the product was markedly above 98% by weight (HPLC). As
solvent, ethyl
acetate was present in an amount of about 0.2%. The DMSO content was below
0.1%.
CA 02807859 2013-02-21
BHC 13 1 005
- 11 -
Example 2
Preparation and analytical characterization of methyl {4,6-diamino-2-[1-(2-
fluorobenzy1)-1H-pyrazolo[3,4-13]pyridin-3-yll pyrimidin-5-yl}methylcarbamate
sulphinyldimethane (compound according to formula (I) as mono-DMSO solvate)
14.8 g of a crude product of the formula (I) were dissolved in 28.9 g of DMSO
and 11.85 g
of ethyl acetate at about 94 C. 1.5 g of activated carbon Norit A-Supra and a
further
11.85 g of ethyl acetate were then added, the mixture was stirred at reflux
(88-90 C) for 1 h
and the hot mixture was then filtered to remove the activated carbon. The
solid, some of
which had already precipitated, was re-dissolved by warming to about 78 C, and
the
solution was then allowed to cool slowly. The precipitated solid was filtered
off with
suction at RT, washed three times with in each case 50 ml of ethyl acetate and
dried in a
drying cabinet at 30 C for 18 h. This gave 9.2 g or 52.5% of theory of a
slightly yellowish
crystal powder of the compound of the formula (II).
HPLC: 99.90 area% (without taking the DMSO into account)
DMSO (GC): 14.7% by weight
1H-NMR (400 MHz in DMF-d7):
d = 2.59 (s, about 6H, 2 CH3 at DMSO), 3.13 (s, 3H, N-CH3), 3.58 + 3.67 (two
s, 3H,
hindered rotation at 0-CH3), 5.91 (s ,2H, -CH2-), 6.53 (s, 4H, 2 -NH2), 7.05-
7.40 (m, 5H, 4
aromatic H at the o-fluorobenzyl substituent and 1H at the pyrido ring meta to
the pyrido
nitrogen), 8.60 (dd, 11, at the pyrido ring ortho to the pyrido nitrogen),
9.12 (dd, 1H, at the
pyrido ring para to the pyrido nitrogen).
Elemental analysis:
found C: 52.2% calculated C: 52.79%
H: 4.9% H: 5.03%
N: 22.7% N: 22.39%
CA 02807859 2013-02-21
BHC 13 1 005
- 12 -
Example 3
Preparation of methyl {4,6-diamino-2-11-(2-fluorobenzy1)-1H-pyrazolo13,4-
131pyridin-
3-ylipyrimidin-5-y1}methylcarbamate of formula (I) in its Modification II
0.5 g of the compound according to formula (I) as mono DMSO sovlate was
tempered for 2
days at 80 C.
Example 4
Preparation of methyl {4,6-diamino-241-(2-fluorobenzy1)-1H-pyrazolo13,4-
b]pyridin-
3-yl]pyrimidin-5-yl}methylcarbamate of formula (I) as sesqui-DMSO solvate
160 mg of the compound according to formula (I) in its amorphous form were
suspended in
2 ml Ethylacetat:DMSO (1:1). The suspension was stirred in a sealed container
for three
weeks at room temperature. The residue was filtered and dried at room
temperature.
Example 5
Preparation of methyl {4,6-diamino-241-(2-fluorobenzy1)-1H-pyrazolo[3,4-
131pyridin-
3-yllpyrimidin-5-ylImethylcarbamate of formula (I) in its amorphous form.
0.5 g of the compound according to formula (I) in its Modification (I) were
ground in a
swing mill for 30 mm with a vibration of 30 swings per second.
Example 6
Preparation of methyl {4,6-diamino-241-(2-fluorobenzy1)-1H-pyrazolo[3,4-
b]pyridin-
3-yl]pyrimidin-5-yl}methylcarbamate of formula (I) as Y4 ethyl acetate solvate
9.6 g of compound according to formula (I) was stirred in 135 ml of ethyl
acetate at reflux (about
78 C) for 1 h and cooled to about 25 C. The solid was filtered off with
suction, washed with a
total of 36 ml of ethyl acetate and dried under reduced pressure. The weight
was 7.6 g or 93.8% of
theory. The content of the product was markedly above 98% by weight (HPLC). As
solvent, ethyl
acetate was present in an amount of about 0.2%. The DMSO content was below
0.1%.
In this reaction, an ethyl acetate containing solid polymorph (1/4-ethyl
acetate-solvate) may be
formed and isolated.
CA 02807859 2013-02-21
BHC 13 1 005
- 13 -
Tab. 1: lR bands of the different crystalline forms
IR Bands [cm-1]
Mono-DMS0- Sesqui-DMS0- 1/4-Ethylacetate-
Mod. I Mod. II
Solvate Solvate Solvate
3508 3498 3401 3508 3506
3454 3382 3361 3407 3459
3360 3269 3295 3361 3363
3273 3104 3168 3300 3275
3103 2951 2950 3190 3116
2956 1704 1702 2956 2954
1688 1622 1645 1698 1732
1622 1586 1626 1629 1702
1559 1563 1578 1558 1619
1489 1491 1560 1485 1560
1483 1482 1490 1456 1484
1457 1457 1456 1447 1457
1437 1436 1446 1425 1438
1425 1419 1426 1416 1417
1417 1390 1415 1370 1366
1366 1362 1370 1333 1333
1333 1327 1335 1293 1282
1326 1288 1286 1227 1246
1284 1231 1276 1192 1229
1227 1189 1228 1169 1191
1193 1176 1193 1142 1167
1167 1164 1171 1111 1140
1140 1140 1142 1094 1094
1094 1106 1112 1063 1032
1034 1035 1092 1043 1008
1008 1007 1042 992 991
989 990 1015 950 935
935 943 993 901 899
899 896 947 840 840
840 861 901 811 810
810 842 845 784 771
CA 02807859 2013-02-21
BHC 13 1 005
- 14 -
IR Bands [cm-1] [cont.]
Mono-DMS0- Sesqui-DMS0- 1/4-Ethylacetate-
Mod. I Mod. II
Solvate Solvate Solvate
799 809 810 770 753
777 796 784 757 700
771 769 775 698 637
756 631 751 667 592
700 590 692 572 572
594 573 622 512 512
512 559 593
512
Tab. 2: 10 Major Peaks of 1R bands of the different crystalline forms
IR Major Bands [emi]
Mono-DMS0- Sesqui-DMS0- 1/4-Ethylacetate-
Mod. I Mod. H
Solvate Solvate Solvate
3454 3498 3401 3407 3363
3360 3382 3361 3361 3275
3273 3269 3295 3300 1732
3103 3104 3168 3190 1702
1688 1704 1702 1698 1619
1622 1622 1626 1629 1560
1559 1586 1560 1558 1457
1284 1563 1333 1293 1246
1193 1326 1286 1043 899
989 1288 1042 770 810
777 1106 751 757 771
CA 02807859 2013-02-21
BHC 13 1 005
- 15 -
Tab. 3: X-Ray powder diffractogram of the different crystalline forms
Reflexes [ Position 2Th.]
Mono-DMS0- Sesqui-DMS0- 1/4-Ethylacetate-
Mod. I Mod. II
Solvate Solvate Solvate
6.7 7.6 5.5 6.2 6.7
9.1 8.3 6.2 6.7 7.2
13.7 10.3 6.7 7.3 8.3
13.8 11.2 7.5 8.3 8.7
14.3 12.6 8.4 8.4 9.1
14.4 12.7 9.0 9.0 10.9
17.8 13.9 10.3 10.1 12.9
18.4 14.3 10.8 11.1 13.3
18.7 15.2 11.1 12.4 13.7
18.9 16.6 11.2 12.8 13.8
19.8 17.3 12.4 13.7 14.2
20.2 17.6 12.8 13.9 14.5
21.0 18.2 13.0 14.3 15.4
21.2 20.0 13.4 15.1 16.4
23.3 20.3 13.7 15.7 17.5
23.7 21.8 13.9 16.1 17.8
24.3 22.5 14.2 16.8 18.6
24.8 22.8 14.3 17.2 18.8
25.6 24.9 15.1 17.2 19.3
26.1 25.0 15.5 17.7 19.8
27.3 25.5 15.7 18.4 20.2
27.9 26.2 15.9 18.8 20.3
29.1 26.8 16.0 19.1 20.5
29.4 27.5 16.5 19.1 21.0
30.5 28.1 16.8 19.6 22.8
31.0 28.8 17.2 20.7 23.2
31.3 29.4 17.7 21.1 23.6
33.2 30.0 18.4 21.4 24.0
34.0 30.5 18.7 21.9 24.4
34.2 32.3 19.1 22.3 24.7
34.9 34.0 19.6 22.6 25.1
CA 02807859 2013-02-21
BHC 13 1 005
- 16 -
Reflexes [ Position 2T11.] [cont.]
Mono-DMS0- Sesqui-DMS0- Vi-Ethylacetate-
Mod. I Mod. II
Solvate Solvate Solvate
36.1 34.4 20.2 22.8 25.6
37.5 35.0 20.7 23.4 26.3
35.7 21.1 23.8 26.7
36.5 21.4 24.6 27.1
21.8 24.9 27.4
22.2 25.4 27.6
22.4 25.6 28.2
22.6 25.9 28.6
23.0 26.7 29.1
23.5 26.8 29.7
23.9 27.3 30.0
24.3 27.9 30.7
24.6 28.4 31.3
24.8 28.8 31.8
25.6 29.4 32.7
26.0 29.6 33.1
26.3 30.5 33.5
26.7 31.5 35.2
27.2 31.7 35.9
28.8 32.1 37.6
29.3 32.5
29.8 33.1
30.5 34.0
30.8 35.0
31.4 35.9
32.0 37.2
32.3
34.0
34.9
35.7
36.4
CA 02807859 2013-02-21
BHC 13 1 005
- 17 -
Tab. 4: 10 Major Reflexes of X-Ray powder diffractogram of the different
crystalline forms
Major Reflexes [ Position '2Th.]
Mono-DMS0- Sesqui-DMS0- 1/4-Ethylacetate-
Mod. I Mod. II
Solvate Solvate Solvate
6.7 11.2 9.0 8.3 6.7
9.1 12.6 10.8 8.4 8.3
14.3 12.7 11.1 13.7 8.7
14.4 13.9 11.2 13.9 12.9
17.8 15.2 13.0 15.7 14.2
19.8 17.3 15.5 17.2 17.8
20.2 22.5 15.9 18.4 19.3
24.8 22.8 16.0 19.6 24.0
25.6 25.0 20.7 21.4 25.1
27.3 25.5 25.6 24.9 26.7