Note: Descriptions are shown in the official language in which they were submitted.
CA 02808179 2013-02-28
-1-
DESCRIPTION
PROMOTER FOR INTRODUCING A GENE INTO A LYMPHOCYTE OR BLOOD
CELL AND APPLICATION THEREOF
TECHNICAL FIELD
[0001]
The present invention is related to a promoter for
introducing a gene into lymphocytes or blood cells, and the
application thereof.
BACKGROUND ART
[0002]
There has been a demand for the establishment
of a technique for gene therapy on lymphoid cells in order
to treat various diseases targeting lymphoid cells, e.g.,
human immunodeficiency virus (HIV) infection. However, no
satisfactory vector system for introducing a desired gene
into lymphoid cells has been developed.
[0003]
Herpesvirus (HHV) is a generic term referring
to viruses of the family Herpesviridae. Both human
herpesvirus 6 and 7 (HHV-6 and HHV-7) are double-stranded
DNA viruses of the subfamily p Herpesviridae of the family
Herpesviridae, which are responsible for exanthem subitum.
(Yamanishi K. et al., "Identification of human herpesvirus
6 as a casual agent for exanthem subitum", Lancet 1988; i:
1065-1067 and Tanaka X. et al., "Human herupesvirus 7:
Another casual agent for roseola (exanthem subitum)", J.
pediatr., 1994; 125: 1-5) HHV-6 includes two strains, HHV-
CA 02808179 2013-02-28
- 2 -
6A and HHV-6B. HHV-6 causes a viral infectious disease
which often occurs during infancy and induces sudden high
fever and exanthema before and after the reduction of fever.
Its prognosis is generally good. HHV-7 infection tends to
occur later than HHV-6 infection (Tanaka K. et al.,
"Seroepidemiological study of human herpesvirus-6 and -7 in
children of different ages and detection of those two
viruses in throat swabs by polymerase chain reaction",
Journal of Medical Virology, 1996; 48: 88-94). Therefore,
exanthem subitum caused by HHV-7 is clinically experienced
as second exanthem subitum. A seroepidemiological study of
HHV-6 and HHV-7 demonstrated that most children become
positive for antibodies for HHV-6 and HHV-7 before the age
of two or three. It has been reported that the inapparent
infection rate is 20 to 40.
[0004]
HHV-7 is a herpesvirus which was newly found by
Frenkel et al. in 1990 when a cytopathic effect occurred
during culturing of CDC T lymphoid cells of a healthy
person's peripheral blood (Frankel N. et al., "Isolation of
a new herpesvirus from human CD4+ T cells", ProNAS USA, 87:
749-752, ProNAS USA, 87: 749-752, 1990). The virus was
isolated from mononuclear cells of human peripheral blood.
Both HHV-6 and -7 are CDC T lymphoid cell tropic viruses.
HHV-7 infects the cell via a CD4 receptor on the cell.
HHV-7 can grow only in human T lymphoid cells. Therefore.
HHV-7 is a virus which can be used for gene modification of
human T lymphoid cells.
[0005]
The HHV-7 genome is double-stranded DNA of
about 145 kbp. The whole base sequence has been determined
= by Nicholas et al. It is known that at least 101 genes are
CA 02808179 2013-02-28
- 3 -
present on the genome (John N. et al., Journal of Virology,
Sep. 1996, 5975 to 5989).
[0006]
However, with respect to these HHVs, no detailed
analysis has been conducted so far regarding the promoter
activity thereof. Moreover, what is lymphoid cell specific
for the viruses was due to the interaction with receptors
in the cells, and the life cycle in which the viruses can
only be propagated in a human T-lymphocytes.
[0007]
In addition, it is believed that these viruses,
particularly HHV-7 virus, have no adverse effect on healthy
persons. If a gene containing an antigenic determinant of
various viruses (e.g., mumps) is incorporated into the
viral genome of HHV-7 and is expressed in HHV-7, HHV-7 is
considered to be useful as a vaccine. However, when HHV-7
is used as a vaccine, it is not preferable that the
genotype is changed as the virus is subcultured, in terms
of quality control and quality assurance. Therefore, when
the recombinant virus is used as a vaccine, it is necessary
to stably supply a virus derived from a single recombinant
genotype virus. For this purpose, a technique for
producing a HHV-7 recombinant virus having a single
genotype has been desired.
[0008]
In addition, the mutual relationship between
the HIV infection of a T lymphoid cell strain SupT1 cell
and a T lymphoid cell tropic human herpesvirus (HHV-6A
(U1102 strain), HHV-7 (MRK, MS0 strains)) has been studied.
The HHV-7 strain, which is bound by a CD4 receptor of cells,
exhibits satisfactory growth in SupT1 cells. However,
infection could not been established for SupTl/HIV cells.
CA 02808179 2013-02-28
- 4 -
In contrast, it has been recognized that the HHV-6A strain
infects HIV-persistent infection SupT1 (SupT1 /HIV) cells
and exhibits clear CPE (Masao Yamada et al., "HIV
Jizokukansen SupT1 Saibo heno HHV-6 oyobi -7
Choufukukannsen no Kokoromi (Attempt for HHV-6 and -7
Superinfection to HIV Persistent Infection Sup-Ti Cell)",
Title No. 122, Titles and Abstracts of the 7th Annual
Meeting of the Japanese Society for AIDS Research, 1993,
Tokyo).
[0009]
An ideal HIV vaccine can provide perfect and
long-term protection from all types of HIV. On the other
hand, conventional inactivated HIV vaccines have advantages
and disadvantages, some of which will be described below.
A method for producing a recombinant vaccine employs common
techniques. However, since it is difficult to maintain
immunogenicity (since immunogenicity is low), high
antigenic load and frequent inoculation of an adjuvant are
required. Safety is the greatest concern. A subunit
vaccine containing either a native or recombinant subunit
may be safe. However, such a subunit vaccine requires high
antigen load and frequent vaccination with adjuvant,
because of the use of a subunit and the low immunogenicity.
Moreover, safety is the most important issue. Furthermore,
subunit vaccines comprising either a native or a
recombinant subunit may be safe, however, they are
subjected to limitation due to low selectivity and low
immunogenicity of the subunit, thereby they allow
development of usable vaccines for treating an immune
responsible cell such as HIV vaccines and the like.
CA 02808179 2013-02-28
-5--
[non-patent literature 1]Yamanishl K et al.,
"Identification of human herpesvirus 6 as a casual agent
for exanthem subitum."Lancet 1988;i: pp. 1065-1067
[non-patent literature 2]Tanaka K et al., "Human
herpesvirus 7: Another casual agent for roseola (exanthem
subitum)"J pediatr.1994;125:pp.1-5
[non-patent literature 3]Tanaka-Taya K et
. al.,"Seroepidemiological study of human herpesvirus-6 and -
7 in children of different ages and detection of those two
viruses in throat swabs by polymerase chain reaction"
Journal of Medical Virology. 1996;48: pp.88-94
[non-patent literature 4] Frankel N et al.õ"Isolation of
a new
herpesvirus from human CD44-111 cells. "ProNAS USA 87:749-
752,Pr0NAS USA 87:749-752,1990
[non-patent literature 5]John N. et al. ,,Journal of
Virology, Sep.1996, pp. 5975-5989
[non-patent literature6] Masao Yamada et al., "HIV
Jizokukansen SupT1 Saibo heno HHV-6 oyobi -7
Choufukukanusen no Kokoromi (Attempt for HHV-6 and -7
Superinfection to HIV Persistent Infection Sup-Ti Cell)",
Title No. 122, Titles and Abstracts of the 7th Annual
Meeting of the Japanese Society for AIDS Research, 1993,
Tokyo
DISCLOSURE OF THE INVENTION
PROBLEM TO BE SOLVED BY THE INVENTION
[0010]
It is an object of the present invention to provide a
promoter which induces gene expression in an immune system
CA 02808179 2013-02-28
- 6 -
cell or blood cell such as lymphoid cells, in a selective
and potent manner.
MEANS FOR SOLVING THE PROBLEM
[0011]
The above mentioned problems have been solved by the
present invention by discovering that MIE promoter of HHV6,
MIE promoter of HHV67,m and U95 promoter of HHV7
surprisingly induce specific expression in an immune
responsible cell such as T lymphoid cell, or hemocyto-
lineage cells.
[0012]
In development of DNA vaccines, which are an
attractive new technology, potent expression promoters are
essential. To date, human cytomegalovirus (HCMV) immediate
early (IE) promoter is widely used in DNA vaccines. This
is due to the fact that HCMV IE promoters are believed to
exhibit potent activity in a variety of cells in general.
However, it is reported that the expression efficiency
thereof is low in lymphoid lineage cells, and the
phenomenon of inactivation due to methylation and the like
is observed. Moreover, there are problems associated with
a variety of restrictions which inhibit realization of the
application of the HHV IE promoter on DNA vaccines.
[0013]
HCMV is known to have a limited number of cells which
it can infect, but is known to infect fibroblast cells and
blood endothelial cells and the like. On the other hand,
HHV-6 infects human infants and causes exanthema subitum or
roseola infantum, and it is known that it proliferates well
in human lymphocytes, in particular, T cells. The present
CA 02808179 2013-02-28
- 7 -
inventors have elucidated in the present invention that
major immediate early gene '(MIE) of HHV-6, which belongs
to the same B virus subgenus of the human herpesvirus genus,
the same as HCMV, exhibits strong promoter activity. The
present inventors have also elucidated that the MIE gene
promoter is available for DNA vaccines. HHV-7 is also a
CD44 T lymphocyte directed virus, and the promoter thereof
can be used to develop DNA vaccines, for example, to
prevent or treat a disease related to CD4+ T lymphocytes.
The present inventors have elucidated in the present
invention the utility of HHV 7 MIE promoter and HHV 7 U95
promoter, and thus also elucidated that these promoters can
be used for DNA vaccines.
[0014]
Therefore, the present invention provides the
following:
[0015]
(1) An MIE promoter of HHV6B.
[0016]
(2) The promoter according to item 1, which comprises
at least eight contiguous nucleotides of the sequence set
forth in SEQ ID NO: 1.
[0017]
(3) The promoter according to item 1, which comprises
=25 at least the R3 region of the sequence set forth in SEQ ID
NO: 1 or a functional variant thereof.
[0018]
(4) The promoter according to item 1, which compriies
at least the sequence of -574 to -427 (SEQ ID NO: 13) from
the transcription initiation point of the SEQ ID NO: 1.
[00191
CA 02808179 2013-02-28
-8-
(5) The promoter according to item 1, which comprises
at least the sequence of -1051 to -427 (SEQ ID NO: 14) from
the transcription initiation point of SEQ ID NO: 1.
[0020]
(6) The promoter according to item 1, which comprises
a motif of NF-KB and a motif of AP-1.
[0021]
(7) The promoter according to item 1, which comprises
the sequence set forth in SEQ ID NO: 1.
[0022]
(8) The promoter according to item 1, wherein the promoter
comprises: (a) a polynucleotide having the base sequence
set forth in SEQ ID NO: 1, or the base sequence
corresponding thereto or a fragment sequence thereof;
(b) a polynucleotide of an allelic variant of the base
sequence set forth in SEQ ID NO: 1 or the base sequence
corresponding thereto or a fragment sequence thereof;
(c) a polynucleotide which hybridizes a polynucleotide of
any of (a) or (b) and has a biological activity thereof; or
(d) a polynucleotide which consists of the base sequence of
any of (a) to (c) or a complement sequence thereof with at
least 70 identity, and has a biological activity thereof.
[0023]
(9) The promoter according to item 1, which is at least 10
contiguous nucleotides in length.
[0024]
(10) The promoter according to item 8, wherein the
biological activity is the promoter activity.
[0025]
(11) A nucleic acid construct comprising the promoter
according to item 1.
[0026]
CA 02808179 2013-02-28
-9-
(12) The nucleic acid construct according to Item 11, which
comprises a sequence encoding a foreign gene which is not
related to the promoter but is operatively linked to the
sequence of the promoter.
[0027]
(13) The nucleic acid construct according to item 12,
wherein the foreign gene encodes an RNAi molecule, a drug,
a recessive gene to be deleted, or a selective marker.
[0028]
(14) The nucleic acid construct according to item 13,
wherein the selective marker allows selection in a medium
of a host in which the nucleic acid construct is introduced.
[0029]
(15) The nucleic acid construct according to item 13,
wherein the selective marker allows visual selection in a
host in which the nucleic acid construct is introduced.
[0030]
(16) The nucleic acid construct according to item 13,
wherein the selective marker comprises hypoxanthine guanine
phosphoribosyl transferase (hprt) or a fluorescent marker
selected from the group consisting of green fluorescent
protein (GFP), cyan fluorescent. protein (CFP), yellow
fluorescent protein (YFP) and red fluorescent protein
(dsRed).
[0031]
(17) The nucleic acid construct according to item 13,
wherein the selective marker does not substantially exhibit
toxicity against the host in which the nucleic acid
construct is introduced.
[0032]
(18) The nucleic acid construct according to item 13,
wherein the recessive gene to be deleted is selected from
CA 02808179 2013-02-28
-10-
the group consisting of ADA gene, PNP gene, y c chain gene,
TAP gene, MHC II gene, X-linked WASP, CD40 ligand, PI3K-
like gene and DNA helicase.
[0033]
(19) The nucleic acid construct according to item 13,
wherein the drug is selected from the group consisting of a
cytokine, a chemokine, a growth factor, a protein hormone,
and a peptide hormone (e.g. interferon (IFN)-a, IFN-y,
interleukin [IL]-2, IL-12, granulocyte colony stimulating
factor [G-CSF], granulocyte macrophage colony stimulating
factor [GM-CSF]).
[0034]
(20) The nucleic acid construct according to item 12,
wherein the promoter induces specific expression of the
foreign gene in a hemocyto-lineage cell, in particular, in
a T cell.
[0035]
(21) An expression vector comprising the nucleic acid
construct according to item 11.
[0036]
(22) A cell comprising the nucleic acid construct according
to item 11.
[0037]
(23) The cell according to item 22, wherein the cell is
heterogenous to the promoter sequence.
[0038]
(24) A tissue comprising the nucleic acid construct
according to item 11.
[0039]
(25) An organ comprising the nucleic acid construct
according to item 11.
[0040]
CA 02808179 2013-02-28
-11-
(26) An organism comprising the nucleic acid construct
according to item 11.
[0041/
(27) A pharmaceutical composition comprising the promoter
according to item 1 and a sequence encoding an antigen.
[0042]
(28) The pharmaceutical composition according to item 27,
which is a DNA vaccine.
[0043]
(29) A pharmaceutical composition for treating a disease,
disorder or condition in which a lymphocyte-specific
treatment is desired, which comprises the promoter
according to item 1, and a nucleic acid sequence for the
treatment.
[0044]
(30) The pharmaceutical composition according to item 29,
wherein the nucleic acid sequence for the treatment
comprises a sequence selected from the group consisting of
those encoding cytokines, chemokines, growth factors,
protein hormones, peptide hormones, ribozymes and RNAis
(HIV-1 gp41;
AATAAGACAGGGCTTGGAAAGACACTTTCCAAGCCCTGTCT;PATTTTT(SEQ ID NO:
33)/HIV-1 tat:
AAGCATCCAGGAAGTCAGCCTACAAGGCTGACTTCCTGGATGCTTTTT(SEQ ID NO:
34)/HTLV-1 tax:
GAACATTGGTGAGGAAGGCACAGCCTTCCTCACCAATGTTCTTTTT(SEQ ID NO:
35)).
[0045]
(31) A method for expressing a protein in a lymphocyte =
specific manner, comprising the steps of:
CA 02808179 2013-02-28
-12-
A) preparing a nucleic acid construct in which the
promoter according to item 1 is operatively linked to a
nucleic acid sequence encoding the protein; and
- B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0046]
(32) A kit for expressing a protein in a lymphocyte
specific manner, comprising:
A) a nucleic acid construct in which the promoter
according to item 1 is operatively linked to a nucleic acid
sequence encoding the protein; and
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0047]
(33) A kit for expressing a protein in a lymphocyte
specific manner, comprising:
A) the promoter according to item 1; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0048]
(34) A. method for treating or preventing a disease,
disorder or condition which requires the expression of a
protein in a lymphocyte specific manner, comprising the
steps of:
A) producing a nucleic acid construct in which the
promoter according to item 1 is linked to a nucleic acid
sequence encoding the protein; and
CA 02808179 2013-02-28
-13-
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0049]
(35) A kit for treating or preventing a disease, disorder
or condition which requires the expression of a protein in
a lymphocyte specific manner, comprising:
A) a nucleic acid construct in which the promoter
according to item 1 is linked to a nucleic acid sequence
encoding the protein; and
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0050]
(36) A kit for treating or preventing a disease, disorder
or condition which requires the expression of a protein in
a lymphocyte specific manner, comprising:
A) the promoter according to item 1; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0051]
(37) A method for producing a protein, comprising the steps
of:
A) preparing a nucleic acid construct in which the
promoter according to item 1 is linked to a nucleic acid
sequence encoding the protein; and
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0052]
(38) A kit for producing a protein, comprising:
CA 02808179 2013-02-28
-14-
A) a nucleic acid construct in which the promoter
according to item 1 is linked to a nucleic acid sequence
encoding the protein; and
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0053]
(39) A kit for producing a protein, comprising:
A) the promoter according to item 1; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0054]
(40) Use of the promoter according to item 1, for
manufacture of a pharmaceutical composition for treating or
preventing a disease, disorder or condition which requires
the expression of a protein in a lymphocyte specific manner.
[0055]
(41) An MIE promoter of HHV7.
[0056]
(42) The promoter according to item 41, which comprises at
least eight contiguous nucleotides of the sequence set
forth in SEQ ID NO: 2.
[0057]
(43) The promoter according to item 41, which comprises at
least the R2 region of the sequence set forth in SEQ ID NO:
2 or a functional variant thereof.
[0058]
(44) The promoter according to item 41, which comprises at
least the sequence of +22 to -233 of the SEQ ID NO: 2.
[0059]
CA 02808179 2013-02-28
-15-
(45) The promoter according to item 41, which comprises at
least the sequence of +22 to -388 of the SEQ ID NO: 2.
[0060]
(46) The promoter according to item 41, which comprises a
motif of NF-KB present in the R2 region.
[0061]
(47) The promoter according to item 41, which comprises the
sequence set forth in SEQ ID NO: 15.
[0062]
(48) The promoter according to item 41, wherein the
promoter comprises:
(a) a polynucleotide having the base sequence set forth in
SEQ ID NO. 2, or a base sequence corresponding thereto or a
fragment sequence thereof;
(b) a polynucleotide of an allelic variant of the base
sequence set forth in SEQ ID NO. 2 or the base sequence
corresponding thereto or a fragment sequence thereof;
(c) a polynucleotide which hybridizes a polynucleotide of
any of (a) or (b) and has a biological activity thereof; or
(d) a polynucleotide which consists of the base sequence of
any of (a) to (c) or a complement sequence thereof with at
least 70 % identity, and has a biological activity thereof.
[0063]
(49) The promoter according to item 41, which is at least
10 contiguous nucleotides in length.
[0064]
(50) The promoter according to item 48, wherein the
biological activity is the promoter activity.
[0065]
(51) A nucleic acid construct comprising the promoter
according to item 41.
[0066]
CA 02808179 2013-02-28
-16-
(52) The nucleic acid construct according to Item 51, which
comprises a sequence encoding a foreign gene which is not
related to the promoter but is operatively linked to the
sequence of the promoter.
[0067]
(53) The nucleic acid construct according to item 52,
wherein the foreign gene encodes an RNAi molecule, a drug,
a recessive gene to be deleted, or a selective marker.
[0068]
(54) The nucleic acid construct according to item 53,
wherein the selective marker allows selection in a medium
of a host in which the nucleic acid construct is introduced.
[0069]
(55) The nucleic acid construct according to item 53,
wherein the selective marker allows visual selection in a
host in which the nucleic acid construct is introduced.
[0070]
(56) The nucleic acid construct according to item 53,
wherein the selective marker comprises hypoxanthine guanine
phosphoribosyl transferase (hprt) or a fluorescent marker
selected from the group consisting of green fluorescent
protein (GFP), cyan fluorescent protein (CFP), yellow
fluorescent protein (YFP) and red fluorescent protein
(dsRed).
[0071]
(57) The nucleic acid construct according to item 53,
wherein the selective marker does not substantially exhibit
toxicity against the host in which the nucleic acid
construct is introduced.
[0072]
(58) The nucleic acid construct according to item 53,
wherein the recessive gene to be deleted is selected from
CA 02808179 2013-02-28
-17-
the group consisting of ADA gene, PNP gene, y c chain gene,
TAP gene, MHC II gene, X-linked WASP, CD40 ligand, PI3K-
like gene and DNA helicase.
[0073]
(59) The nucleic acid construct according to item 53,
wherein the drug is selected from the group consisting of a
cytokine, a chemokine, a growth factor, a protein hormone,
and a peptide hormone (IFN-a, IFN-y, IL-2, IL-12, G-CSF,
GM-CSF).
[0074]
(60) The nucleic acid construct according to item 52,
wherein the promoter induces specific expression of the
foreign gene in a hemocyto-lineage cell, in particular, in
a T cell.
[0075]
(61) An expression vector comprising the nucleic acid
construct according to item 51.
[0076]
(62) A cell comprising the nucleic acid construct according
to item 51.
[0077]
(63) The cell according to item 62, wherein the cell is
heterogenous to the promoter sequence.
[0078]
(64) A tissue comprising the nucleic acid construct
according to item 51.
[0079]
(65) An organ comprising the nucleic acid construct
according to item 51.
[0080]
(66) An organism comprising the nucleic acid construct
according to item 51.
CA 02808179 2013-02-28
-18-
[0081]
(67) A pharmaceutical composition comprising the promoter
according to item 41 and a sequence encoding an antigen.
[0082]
(68) The pharmaceutical composition according to item 67,
which is a DNA vaccine.
[0083]
(69) A pharmaceutical composition for treating a disease,
disorder or condition in which a lymphocyte-specific
treatment is desired, which comprises the promoter
according to item 41, and a nucleic acid sequence for the
treatment.
[0084]
(70) The pharmaceutical composition according to item 69,
wherein the nucleic acid sequence for the treatment
comprises a sequence selected from the group consisting of
those encoding cytokines, chemokines, growth factors,
protein hormones, peptide hormones, ribozymes and RNAis
(HIV-1 gp41:
AATAAGACAGGGCTTGGAAAGACACTTTCCAAGCCCTGTCTTATTTTT(SEQ ID NO:
33)/HIV-1 tat:
AAGCATCCAGGAAGTCAGCCTACAAGGCTGACTTCCTGGATGCTTTTT(SEQ ID NO:
34)/HTLV-1 tax:
GAACATTGGTGAGGAAGGCACAGCCTTCCTCACCAATGTTCTTTTT(SEQ ID NO:
35)).
[0085]
(71) A method for expressing a protein in a lymphocyte
specific manner, comprising the steps of:
A) preparing a nucleic acid construct in which the
promoter according to item 41 is operatively linked to a
nucleic acid sequence encoding the protein; and
CA 02808179 2013-02-28
-19-
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0086]
(72) A kit for expressing a protein in a lymphocyte
specific manner, comprising:
A) a nucleic acid construct in which the promoter
according to item 41 is operatively linked to a nucleic
acid sequence encoding the protein; and
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0087]
(73) A kit for expressing a protein in a lymphocyte
specific manner, comprising:
A) the promoter according to item 41; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0088]
(74) A method for treating or preventing a disease,
disorder or condition which requires the expression of a
protein in a lymphocyte specific manner, comprising the
steps of:
A) producing a nucleic acid construct in which the
promoter according to item 41 is linked to a nucleic acid
sequence encoding the protein; and
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0089]
CA 02808179 2013-02-28
-20-
(75) A kit for treating or preventing a disease, disorder
or condition which requires the expression of a protein in
a lymphocyte specific manner, comprising:
A) a nucleic acid construct in which the promoter
according to item 41 is linked to a nucleic acid sequence
encoding the protein; and
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0090]
(76) A kit for treating or preventing a disease, disorder
or condition which requires the expression of a protein in
a lymphocyte specific manner, comprising:
A) the promoter according to item 41; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0091]
(77) A method for producing a protein, comprising the steps
of:
A) preparing a nucleic acid construct in which the
promoter according to item 41 is linked to a nucleic acid
sequence encoding the protein; and
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0092]
(78) A kit for producing a protein, comprising:
A) a nucleic acid construct in which the promoter
according to item 41 is linked to a nucleic acid sequence
encoding the protein; and
CA 02808179 2013-02-28
-21-
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0093]
(79) A kit for producing a protein, comprising:
A) the promoter according to item 41; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0094]
(80) Use of the promoter according to item 41, for
manufacture of a pharmaceutical composition for treating or
preventing a disease, disorder or condition which requires
the expression of a protein in a lymphocyte specific manner.
[0095]
(81) A U95 promoter of HHV7.
[0096]
(82) The promoter according to item 81, which comprises at
least eight contiguous nucleotides of the sequence set
forth in SEQ ID NO: 12.
[D097]
.(83) The promoter according to item 81, which comprises at
least the R2 region of the sequence set forth in SEQ ID NO:
12 or a functional variant thereof.
[0098]
(84) The promoter according to item 81, which comprises at
least the sequence of +16 to -233 of the SEQ ID NO: 12.
[0099]
(85) The promoter according to item 81, which comprises at
least the sequence of +16 to -379 of the SEQ ID NO: 12.
[0100]
CA 02808179 2013-02-28
-22-
(86) The promoter according to item 81, which comprises a
motif of NF-KB present in the R2 region.
[0101]
(87) The promoter according to item 81, which comprises the
sequence set forth in SEQ ID NO: 16.
[0102]
(88) The promoter according to item 81, wherein the
promoter comprises:
(a) a polynucleotide having the base sequence set forth in
SEQ ID NO. 12, or the base sequence corresponding thereto
or a fragment sequence thereof;
(b) a polynucleotide of an allelic variant of the base
sequence set forth in SEQ ID NO. 12 or the base sequence
corresponding thereto or a fragment sequence thereof;
(c) a polynucleotide which hybridizes a polynucleotide of
any of (a) or (b) and has a biological activity thereof; or
(d) a polynucleotide which consists of the base sequence of
any of (a) to (c) or a complement sequence thereof with at
least 70 % identity, and has a biological activity thereof.
[0103]
(89) The promoter according to item 81, which is at least
10 contiguous nucleotides in length.
[01041
(90) The promoter according to item 88, wherein the
biological activity is the promoter activity.
[0105]
(91) A nucleic acid construct comprising the promoter
according to item 81.
(0106]
(92) The nucleic acid construct according to Item 91, which
comprises a sequence encoding a foreign gene which is not
CA 02808179 2013-02-28
-23-
related to the promoter but is operatively linked to the
sequence of the promoter.
[0107]
(93) The nucleic acid construct according to item 92,
wherein the foreign gene encodes an RNAi molecule, a drug,
a recessive gene to be deleted, or a selective marker.
[0108]
(94) The nucleic acid construct according to item 93,
wherein the selective marker allows selection in a medium
of a host in which the nucleic acid construct is introduced.
[0109]
(95) The nucleic acid construct according to item 93,
wherein the selective marker allows visual selection in a
host in which the nucleic acid construct is introduced.
[0110]
(96) The nucleic acid construct according to item 93,
wherein the selective marker comprises hypoxanthine guanine
phosphoribosyl transferase (hprt) or a fluorescent marker
selected from the group consisting of green fluorescent
protein (GFP), cyan fluorescent protein (CFP), yellow
fluorescent protein (YFP) and red fluorescent protein
(dsRed).
[0111]
(97) The nucleic acid construct according to item 93,
wherein the selective marker does not substantially exhibit
toxicity against the host in which the nucleic acid
construct is introduced.
[0112]
(98) The nucleic acid construct according to item 93,
wherein the recessive gene to be deleted is selected from
the group consisting of ADA gene, PNP gene, y c chain gene,
CA 02808179 2013-02-28
- 24 -
TAP gene, MHC II gene, X-linked WASP, CD40 ligand, PI3K-
like gene and DNA helicase.
[0113]
(99) The nucleic acid construct according to item 93,
wherein the drug is selected from the group consisting of a
cytokine, a chemokine, a growth factor; a protein hormone,
and a peptide hormone (IFN-a,IFN-y,IL-2,IL-12,G-CSF,GM-CSF).
[0114]
(100) The nucleic acid construct according to item 92,
wherein the promoter induces specific expression of the
foreign gene in a hemocyto-lineage cell, in particular, in
a T cell.
[0115]
(101) An expression vector comprising the nucleic acid
construct according to item 91.
[0116]
(102) A cell comprising the nucleic acid construct
according to item 91.
[0117]
(103) The cell according to item 102, wherein the cell is
heterogenous to the promoter sequence.
[0118]
(104) A tissue comprising the nucleic acid construct
according to item 91.
[0119]
(105) An organ comprising the nucleic acid construct
according to item 91.
[0120]
(106) An organism comprising the nucleic acid construct
according to item 91.
[0121]
CA 02808179 2013-02-28
-25-
(107) A pharmaceutical composition comprising the promoter
according to item 81 and a sequence encoding an antigen.
[0122]
(108) The pharmaceutical composition according to item 107,
which is a DNA vaccine.
[0123]
(109) A pharmaceutical composition for treating a disease,
disorder or condition in which a lymphocyte-specific
treatment is desired, which comprises the promoter
according to item 81, and a nucleic acid sequence for the
treatment.
[0124]
(110) The pharmaceutical composition according to item 109,
wherein the nucleic acid sequence for the treatment
comprises a sequence selected from the group consisting of
those encoding cytokines, chemokines, growth factors,
protein hormones, peptide hormones, ribozymes and RNAis
(HIV-1 gp41:
AATAAGACAGGGCTTGGAAAGACACTTTCCAAGCCCTGTCTTATTTTT(SEQ ID NO:
33)/HIV-1 tat:
AAGCATCCAGGAAGTCAGCCTACAAGGCTGACTTCCTGGATGCTTTTT(SEQ ID NO:
34)/HTLV-1 tax:
GAACATTGGTGAGGAAGGCACAGCCTTCCTCACCAATGTTCTTTTT(SEQ ID NO:
35)).
[0125]
(111) A method for expressing a protein in a lymphocyte
specific manner, comprising the steps of:
A) preparing a nucleic acid construct in which the
promoter according to item 81 is operatively linked to a
nucleic acid sequence encoding the protein; and
CA 02808179 2013-02-28
- 26 -
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0126]
(112) A kit for expressing a protein in a lymphocyte
specific manner, comprising:
A) a nucleic acid construct in which the promoter
according to item 81 is operatively linked to a nucleic
acid sequence encoding the protein; and
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0127]
(113) A kit for expressing a protein in a lymphocyte
specific manner, comprising:
A) the promoter according to item 81; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0128]
(114) A method for treating or preventing a disease,
disorder or condition which requires the expression of a
protein in a lymphocyte specific manner, comprising the
steps of:
A) producing a nucleic acid construct in which the
promoter according to item 81 is linked to a nucleic acid
sequence encoding the protein; and
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0129]
CA 02808179 2013-02-28
- 27-
(115) A kit for treating or preventing a disease, disorder
or condition which requires the expression of a protein in
a lymphocyte specific manner, comprising:
A) a nucleic acid construct in which the promoter
according to item 81 is linked to a nucleic acid sequence
encoding the protein; and
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0130]
(116) A kit for treating or preventing a disease, disorder
or condition which requires the expression of a protein in
a lymphocyte specific manner, comprising:
A) the promoter according to item 81; and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0131]
(117) A method for producing a protein, comprising the
steps of:
A) preparing a nucleic acid construct in which the
promoter according to item 81 is linked to a nucleic acid
sequence encoding the protein; and
B) placing the nucleic acid construct under a
condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0132]
(118) A kit for producing a protein, comprising:
A) a nucleic acid construct in which the promoter
according to item 81 is linked to a nucleic acid sequence.
encoding the protein; and
CA 02808179 2013-02-28
-28-
B) means for placing the nucleic acid construct under
a condition in which the promoter induces the expression of
the nucleic acid sequence encoding the protein.
[0133]
(119) A kit for producing a.protein, comprising:
A) the promoter according to item 81:: and
B) means for producing a nucleic acid construct in
which the promoter is linked to a nucleic acid sequence
encoding the protein.
[0134]
(120) Use of the promoter according to item 81, for
manufacture of a pharmaceutical composition for treating or
preventing a disease, disorder or condition which requires
the expression of a protein in a lymphocyte specific manner.
[0135]
Hereinafter, preferable embodiments of the
present invention are presented. It should be understood
that those skilled in the art would appropriately practice
the embodiments thereof based on the description of the
present invention in view of the well known and routinely
used technology in the art, and the functions and effects
attained by the present invention should be readily
understood.
EFFECTS OF THE INVENTION
[0136]
The present invention provides promoters which
selectively induce the expression of a protein in a cell of
the immune system such as T lymphocytes. The promoters of
the present invention are used to provide a method and
medicament for effectively preventing or treating
immunological disease such as innate immune deficiency
CA 02808179 2013-02-28
- 29 -
syndrome and the like. The present invention also provides
a technology in order to efficiently conduct gene therapy.
BRIEF DESCRIPTION OF THE DRAWINGS
[0137]
[Figure 1] Figure 1 depicts a comparison of promoter
activities in adhesive cells. The x-axis aligns a variety
of promoters, and the promoter activities of the Vero cell,
the HEL cell, the L929 cell, the 293 cell and the 373 cell
are shown using Log(RLU)/B-gal with logarithmic reference.
[Figure 2] Figure 2 depicts the comparison of promoter
activities in lymphocytes. The x-axis aligns a variety of
promoters, and the promoter activities of the THP-1 cell,
the SupT1 cell and the U937 cell are shown using
Log(RLU)/B-gal with logarithmic reference.
[Figure 31 Figure 3 depicts the promoter activity of the
HHV-6 MIE region in the case of stimulating a cell with TPA
(Vero cell). On the x-axis, a variety of promoters are
aligned, and the activity of a promoter with and without
TPA is shown using Log(RLU)/13-gal in a logarithmic manner.
[Figure 41 Figure 4 depicts promoter activity of the HHV-
6 MIE region (L929 cell) when the cell has been stimulated
with TPO. On the x-axis, a variety of promoters are
aligned, and the promoter activity in the presence or
absence of TPA with respect to the respective promoters is
depicted using Log(RLU)/B-gal in a logarithmic manner.
[Figure 5] Figure 5 depicts illustrations of a variety of
deletion variants in a promoter region of the HHV6B. The
upper panel shows the promoter region, and a variety of
motifs in the promoter regions.
[Figure 6] Figure 6 depicts a measurement system for
promoter activity.
CA 02808179 2013-02-28
-30-
[Figure 7] Figure 7 depicts the -promoter activity
(relative luciferase activity) with illustrations of a
variety of deletion variants in the promoter region in the
HHV6B.
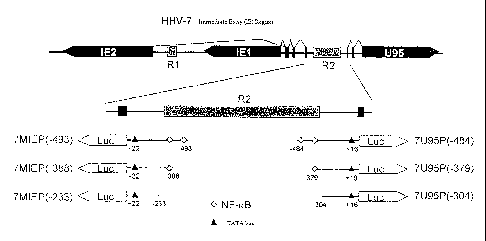
[Figure 8] Figure 8
depicts illustrations of the
immediate early (IE) gene relating to the promoter region
of the HHV7 and the promoter thereof. The left column
shows, from the top,7MIE promoter (-493), 7MIE promoter (-
388), and 7MIE promoter (-233), and the right column shows,
from the top, 7U95 promoter (-484), 7U95 promoter (-379),
and 7U95promoter (-304).
[Figure 9] Figure 9 depicts the activity of the IE
promoter of the HHV7 in a lymphocytic cell line. The upper
left graph shows Jurkat cells, the upper right graph shows
Molt-3 cells, the lower left graph shows SupT1 cells, and
the lower right graph shows SAS-413 cells. Each
graph
shows, from the left, CMVP, 6MIEP, 6U95P, 7MIE(-493), 7U95
and P(-484), respectively.
[Figure 101 Figure 10 depicts the effects of R2 deletion
on promoter activity. The upper
left graph shows Jurkat
cells, the upper right graph shows Molt-3 cells, the'lower
left graph shows SupT1 cells, and the lower right graph
shows SAS-413 cells. The graphs show from the left,7MIE
promoter (-493), 7MIE promoter (-388), 7MIE promoter (-233),
7U95 promoter (-484), 7U95 promoter (-379) and 7U95
promoter (-304).
[Figure 11] Figure 11 depicts the promoter activity in a,
peripheral blood monocytic cell (PBMC). It shows lineage 1,
linage 2 and lineage 3, in the upper left, upper right and
lower panels, respectively.
BRIEF DESCRIPTION OF SEQUENCE LISTING
CA 02808179 2013-02-28
-31-
[0138]
SEQ ID NO: 1 is the sequence of HHV6B MIE promoter.
SEQ ID NO: 2 is the sequence of HHV7 MIE promoter.
= SEQ ID NO: 3 is the sequence of HHV6A MIE promoter.
SEQ ID NO: 4 is the sequence of HHV6B R3 region.
SEQ ID NO: 5 is the sequence of 20u used in Example 1.
SEQ ID NO: 6 is the sequence of 9u used in Example 1.
SEQ ID NO: 7 is the sequence of MIE used in Example 1.
SEQ ID NO: 8 is the sequence of U95 used in Example 1.
SEQ ID NO: 9 is the sequence of CMV used in Example 1.
SEQ ID NO: 10 is the sequence of MIE/3K used in
Example 1.
SEQ ID NO: 11 is the sequence of U95/3K used in
Example 1.
SEQ ID NO: 12 is the sequence of HHV7 U95 promoter
SEQ ID NO: 13 is the sequence of -574 to -427 from
the transcription initiation site of HHV6B MIE.
SEQ ID NO: 14 is the sequence of -1051 to -427 from
the transcription initiation site of HHV6B MIE.
SEQ ID NO: 15 is the sequence of +22 to -493 from the
transcription initiation site of HHV7 MIE.
SEQ ID NO: 16 is the sequence of +16 to -484 from the
transcription initiation site of HHV7 MIE.
SEQ ID NO: 17 is the sequence of 9u-d2-7 used in
Example 1.
SEQ ID NO: 18 is the sequence of 9u-d1-4 used in
Example 1.
SEQ ID NO: 19 is the sequence of 9u-d1-5 used in
Example 1.
SEQ ID NO: 20 is the sequence of 9u-d1-7 used in
Example 1.
CA 02808179 2013-02-28
- 32 -
SEQ ID NO: 21 is the sequence of 9u-d3-7 used in ,
Example 1.
SEQ ID NO: 22 is the sequence of 9u-d5 used in
Example 1.
SEQ ID NO: 23 is the sequence of 9u-d6 used in
Example 1.
SEQ ID NO: 24 is the sequence of 9u-d7 used in
Example 1.
SEQ ID NO: 25 is the sequence of 9u-d8 used in
Example 1.
SEQ ID NO: 26 is the sequence of 7MIEP (-493) used in
Example 2.
SEQ ID NO: 27 is the sequence of 7MIEP (-388) used in
Example 2.
SEQ ID NO: 28 is the sequence of 7MIEP (-233) used in
Example 2.
SEQ ID NO: 29 is the sequence of 7U95P (-484) used in
Example 2.
SEQ ID NO: 30 is the sequence of 7U95P (-379) used in
Example 2.
SEQ ID NO: 31 is the sequence of 7U95P (-304) used in
Example 2.
SEQ ID NO: 32 is the sequence of pGL3 Basic used in
Example 2.
SEQ ID NO: 33 is an example of RNAi of HIV-1 gp41.
SEQ ID NO: 34 is an example of RNAi of HIV-1 tat..
SEQ ID NO: 35 is an example of RNA1 of HIV-1 tax.
=
BEST MODE FOR CARRYING OUT THE INVENTION
Hereinafter, the present invention will be
described. It should be understood throughout the present
CA 02808179 2013-02-28
-33-
specification that articles for singular forms (e.g., "a",
"an", "the", etc. in English, and articles, adjectives, etc.
in other languages) include plural referents unless the
context clearly dictates otherwise. It should be also
understood that the terms as used herein have definitions
typically used in the art unless otherwise mentioned.
Accordingly, unless otherwise defined, all terminology and
technical terms used herein will have the same meanings as
those generally understood by those skilled in the art
belonging to the filed of the present invention. If there
is contradiction, the present specification (including the
definition) takes precedence.
[0140]
(Definition of Terms)
The definitions of terms used herein are
described below.
[0141]
As used herein the term "HHV" refers to a human
herpes virus, of which there are types 1, 2, 3, 4, 5, 6, 7,
8 and the like.
[0142]
As used herein, the term "herpesvirus" includes
all of HHV-6A, HHV-6B, and HHV-7, and both their wild-types
and recombinant types unless otherwise mentioned. As used
herein, the term "HHV-6 (human herpes virus 6)" includes
HHV-6A and HHV-6B, and both their wild-types and
recombinant types unless otherwise mentioned. HHV6 belongs
to the 13 subgenus as cytomegalovirus HHV-5, and HHV6B is a
causative virus of exanthema subitum, and it is said that
most Japanese will have been infected therewith by the age
of two years old. As used herein, the term "HHV-7 (human
herpes virus 7)" refers to any herpes virus belonging to
CA 02808179 2013-02-28
-34-
this type of herpes virus. HHV7 is also said to be a
causative body of exanthema subitum, however, in comparison
to HHV6B, the occurrence thereof is lower, and the age
where the patients are infected is older. As with HHV6,
HHV7 belongs to the B subgenus, and it is also said that it
is believed to infect CDC cells, and thus cause the onset
of pityriasis rosea Gibert, and it is also said that most
Japanese will have been infected therewith by the age of
two years old.
(0143]
As used herein, the term "wild strain" in
relation to herpesvirus refers to a herpesvirus strain
which is not artificially modified and is isolated from
nature. An example of a wild strain includes, but is not
limited to, strain J1.
[0144]
As used herein, the term "wild strain" in
relation to HHV-6A refers to a HHV-6A strain which is not
artificially modified and is isolated from nature. An
example of a wild strain includes, but is not limited to,
strain U1102.
(0145]
As used herein, the term "mutant strain" refers
to a herpesvirus strain which has a mutation due to
mutagenesis, multiple subculturings or the like.
Mutagenesis of a herpesvirus strain may be either random
mutagenesis or site-specific mutagenesis.
[0146]
As used herein, the term "wild strain" in
relation to HHV-6B refers to a HHV-6B strain which is not
artificially modified and is isolated from nature. An
CA 02808179 2013-02-28
-35-
example of a wild strain includes, but is not limited to,
strain HST.
[0147]
The terms "protein",
"polypeptide",
"oligopeptide" and "peptide" as used herein have the same
meaning and refer to an amino acid polymer having any
length.
[0148]
The terms "polynucleotide", "oligonucleotide",
and "nucleic acid" as used herein have the same meaning and
refer to a nucleotide polymer having any length. Unless
otherwise indicated, a particular nucleic acid sequence
also implicitly encompasses conservatively-modified
variants thereof (e.g. degenerate codon substitutions) and
complementary sequences as well as the sequence explicitly
indicated. Specifically, degenerate codon substitutions may
be produced by generating sequences in which the third
position of one or more selected (or all) codons is
substituted with mixed-base and/or deoxyinosine residues
(Batzer et al., Nucleic Acid Res. 19:5081(1991); Ohtsuka et
al., J. Biol. Chem. 260:2605-2608 (1985); Rossolini et al.,
Mol. Cell. Probes 8:91-98(1994)).
[0149]
As used herein, the term "gene" refers to an
element defining a genetic trait. A gene is
typically
arranged in a given sequence on a chromosome. A gene which
defines the primary structure of a protein is called a
structural gene. A gene which regulates the expression of
a structural gene is called a regulatory gene, As used
herein, "gene" may refer to "polynucleotide",
"oligonucleotide", "nucleic acid", and "nucleic acid
molecule" and/or "protein", "polypeptide", "oligopeptide"
CA 02808179 2013-02-28
-36-
and "peptide". As used herein, the term "open reading
frame" or "ORF" in relation to a gene, refers to a reading
frame which is one of three frames obtained by sectioning
the base sequence of a gene at intervals of three bases,
and has a start codon and a certain length curtailed by the
appearance of a stop codon, and has the possibility of
actually coding a protein. The entire base sequence of the
genome of herpesvirus has been determined, identifying at
least 101 genes. Each of the genes is known to have an
open reading frame(ORF).
(0150]
As used herein, the term "RNAi" is an
abbreviation of RNA interference and refers to a phenomenon
where an agent for causing RNAi, such as double-stranded
RNA (also called dsRNA), is introduced into cells and mRNA
homologous thereto is specifically degraded, so that the
synthesis of gene products is suppressed, and techniques
using the phenomenon. As used herein, RNAi may have the
same meaning as that of an agent which causes RNAi.
. 20 [0151]
As used herein, the term "an agent causing
RNAi" refers to any agent capable of causing RNAi. As used
herein, "an agent causing RNAt of a gene" indicates that
the agent causes RNAi relating to the gene and that the
effect of RNAi is successfully achieved (e.g.. suppression
of expression of the gene,.and the like). Examples of such
an agent causing RNAi include, but are not limited to, a
sequence having at least about 70% homology with the
nucleic acid sequence of a target gene or a sequence
hybridizable thereto under stringent conditions, and RNA
containing a double-stranded portion having a length of at
least 10 nucleotides or variants thereof. Here, this agent
CA 02808179 2013-02-28
-37-
may be preferably DNA containing a 3' protruding end, and
more preferably the 3' protruding end has a length of 2 or
more nucleotides (e.g., 2-4 nucleotides in length).
[0152]
Though not wishing to be bound by any theory, a
mechanism which causes RNAi is considered to be as follows.
When a molecule which causes RNAi, such as dsRNA, is
introduced into a cell, an RNaseIII-like nuclease having a
helicase domain (called dicer) cleaves the molecule at
about 20 base pair intervals from the 3' terminus in the
presence of ATP, in the case where the RNA is relatively
long (e.g., 40 or more base pairs). As used herein, the
term "siRNA" is an abbreviation of short interfering RNA
and refers to short double-stranded RNA of 10 or more base
pairs which are artificially chemically synthesized or
biochemically synthesized, synthesized by an organism, or
produced by double-stranded RNA of about 40 or more base
pairs being degraded within the organism. siRNA typically
has a structure comprising 5'-phosphate and 3'-OH, where
the 3' terminus projects by about 2 bases. A
specific
protein is bound to siRNA to form RISC (RNA-induced-
silencing-complex). This complex recognizes and binds to
mRNA having the same sequence as that of siRNA and cleaves
mRNA at the middle of siRNA due to RNaseIII-like enzymatic
activity. It is preferable that the relationship between
the sequence of siRNA and the sequence of mRNA to be
cleaved as a target is a 100% match.
However, base
mutations at a site away from the middle of siRNA do not
completely remove the cleavage activity by RNAi, leaving
partial activity, while base mutations in the middle of
siRNA have a large influence and the mRNA cleavage activity
by RNAI is considerably lowered. By
utilizing such a
CA 02808179 2013-02-28
-38-
characteristic, only mRNA having a mutation can be
specifically degraded. Specifically, siRNA in which the
mutation is provided in the middle thereof is synthesized
and is introduced into a cell. Therefore, in the present
invention, siRNA per se, as well as an agent capable of
producing siRNA (e.g., representatively dsRNA of about 40
or more base pairs) can be used as an agent capable of
= eliciting RNAl.
[0153]
Also, though not wishing to be bound by any
theory, apart from the above-described pathway, the
antisense strand of siRNA binds to mRNA and siRNA functions
as a primer for RNA-dependent RNA polymerase (RdRP), so
that dsRNA is synthesized. This dsRNA is a substrate for a
dicer again, leading to production of new siRNA. It is
intended that such a reaction is amplified. Therefore, in
the present invention, siRNA per se, as well as an agent
capable of producing siRNA are useful. In fact, in insects
and the like, for example, 35 dsRNA molecules can
substantially or completely degrade 1,000 or more copies of
intracellular mRNA, and therefore, it will be understood
that siRNA per se, as well as an agent capable of producing
siRNA, is useful.
[0154]
In the present invention, double-stranded RNA
having a length of about 20 bases (e.g., representatively
about 21 to 23 bases) or less than about 20 bases, called
siRNA, can be used.
Expression of siRNA in cells can
suppress expression of a pathogenic gene targeted by the
siRNA. Therefore, siRNA can be used for the treatment,
prophylaxis, prognosis, and the like of diseases.
[0155]
CA 02808179 2013-02-28
-39-
The siRNA of the present invention may be in
any form as long as it can elicit RNAi.
(0156)
In another embodiment, an agent capable of
causing RNAI may have a short hairpin structure having a
sticky portion at the 3' terminus (shRNA; short hairpin
RNA). As
used herein, the term "shRNA" refers to a
molecule of about 20 or more base pairs in which a single-
stranded RNA partially contains a palindromic base sequence
and forms a double-strand structure therein (i.e., a-
hairpin structure). shRNA can be artificially chemically
synthesized.
Alternatively, shRNA can be produced by
linking sense and antisense strands of a DNA sequence in
reverse directions and synthesizing RNA in vitro with T7
RNA polymerase using the DNA as a template. Though not
wishing to be bound by any theory, it should be understood
that after shRNA is introduced into a cell, the shRNA is
degraded in the cell to a length of about 20 bases (e.g.,
=
representatively 21, 22, 23 bases), and causes RNAi as with
siRNA, leading to the treatment effects of the present
invention. It should be understood that such an effect is
exhibited in a wide range of organisms, such as insects,
plants, animals (including mammals), and the like. Thus.
shRNA elicits RNA1 as with siRNA and therefore can be used
as an effective component of the present invention. shRNA
may preferably have a 3' protruding end. The length of the
double-stranded portion is not particularly limited, but is
preferably about 10 or more nucleotides, and more
preferably about 20 or more nucleotides. Here, the 3'
protruding end may be preferably DNA, more preferably DNA
of at least 2 nucleotides in length, and even more
preferably DNA of 2-4 nucleotides in length.
CA 02808179 2013-02-28
-40-
[0157]
An agent capable of causing RNA1 used in the
present invention may be artificially synthesized
(chemically or biochemically) or naturally occurring.
There is substantially no difference between the two in
terms of the effect of the present invention. A chemically
synthesized agent is preferably purified by liquid
chromatography or the like.
[0158]
An agent capable of causing RNAi used in the
present invention can be produced in vitro. In
this
synthesis system, T7 RNA polymerase and T7 promoter are
used to synthesize antisense and sense RNAs from template
DNA. These RNAs are annealed and thereafter introduced
into a cell. In this case, RNAi is caused via the above-
described mechanism, thereby achieving the effect of the =
present invention. Here, for example, the introduction of
RNA into a cell can be carried out using a calcium
phosphate method.
[0159]
Another example of an agent capable of causing
RNA1 according to the present invention is a single-
stranded nucleic acid hybridizable to mRNA, or all nucleic
acid analogs thereof. Such
agents are useful for the
method and composition of the present invention.
[0160]
As used herein, the term "corresponding" amino
acid or nucleic acid refers to an amino acid or nucleotide
in a given polypeptide or polynucleotide molecule, which
has, or is anticipated to have, a function similar to that
of a predetermined amino acid or nucleotide in a
polypeptide or polynucleotide as a reference for comparison.
CA 02808179 2013-02-28
-41-
For example, in the case of ubiquitin, it refers to an
amino acid contributing in a similar manner to the
catalytic activity and present in a similar location as in
the sequence (for example, glycine at the C-terminus) which
is responsible for lysine. For example, in the case of
nucleic acid sequence, the term refers to a similar portion
which affects a similar function to the particular portion
which it encodes.
01611
As used herein, the term "corresponding" gene
(e.g., a polypeptide or polynucleotide molecule) refers to
a gene in a given species, which has, or is anticipated to
have, a function similar to that of a predetermined gene in
a species as a reference for comparison. When there are
pluralities of genes having such a function, the term
refers to a gene having the same evolutionary origin.
Therefore, a gene corresponding to a given gene may be an
ortholog of the given gene. Therefore, genes corresponding
to those such as herpes virus type 6B and tumor antigen and
the like, can be found in other organisms (for example,
herpes virus type 7). Such a corresponding gene can be
identified by techniques well known in the art. Therefore,
for example, a corresponding gene in a given organism can
be found by searching a sequence database of the organism
(e.g., herpes virus 6B) using the sequence of a reference
gene (e.g., mouse cyclin gene, etc.) as a query sequence.
Alternatively, wet experiments are used for screening a
library to find out the same.
[0162]
As used herein, the term "isolated" means that
naturally accompanying Material is at least reduced, or
preferably substantially or completely eliminated, in
CA 02808179 2013-02-28
-42-
normal circumstances. Therefore, the term "isolated cell"
refers to a cell substantially free from other accompanying
substances (e.g., other cells, proteins, nucleic acids,
etc.) in natural circumstances. The
term "isolated" in
relation to nucleic acids or polypeptides means that, for
example, the nucleic acids or the polypeptides are
substantially free from cellular substances or culture
media when they are produced by recombinant DNA techniques;
or precursory chemical substances or other chemical
substances when they are chemically synthesized.
(0163]
As used herein, the term "purified" biological
agent (e.g., nucleic acids, proteins, and the like) refers
to one from which at least a portion of naturally
accompanying agents has been removed.
Therefore,
ordinarily, the purity of a purified biological agent is
higher than that of the biological agent in a normal state
(i.e., concentrated).
[0164]
As used herein, the terms "purified" and
"isolated" mean that the same type of biological agent is =
present preferably at least 75* by weight, more preferably
at least 85* by weight, even more preferably at least 95%
by weight, and most preferably at least 98% by weight.
[0165]
As used herein, the term "homology" in relation
to a sequence (e.g., a nucleic acid sequence, an amino acid
sequence, etc.) refers to the proportion of identity
between two or more gene sequences. Therefore, the greater
the homology between two given genes, the greater the
identity or similarity between their sequences. Whether or
not two genes have homology is determined by comparing
CA 02808179 2013-02-28
-43-
their sequences directly or by a hybridization method under
stringent conditions. When two gene sequences are directly
compared with each other, these genes have homology if the
DNA sequences of the genes have representatively at least
50% identity, preferably at least 70% identity, more
preferably at least 80%, 90%, 95%, 96%, 97%, 98%, or 99%
identity with each other.
[0166]
As used herein, "polynucleotides hybridizing
under stringent conditions" refers to conditions commonly
used and well known in the art. Such a polynucleotide can
be obtained by conducting colony hybridization, plaque
hybridization, Southern blot hybridization, or the like
using a polynucleotide selected from the polynucleotides of
the present invention. Specifically, a filter on which DNA
derived from a colony or plaque is immobilized is used to
conduct hybridization at 65 C in the presence of 0.7 to
1.0 M NaCl. Thereafter, a 0.1 to 2-fold concentration SSC
(saline-sodium citrate) solution (1-fold concentration SSC
solution is composed of 150 mM sodium chloride and 15 mM
sodium citrate) is used to wash the filter at 65 C.
Polynucleotides identified by this method are referred to
as "polynucleotides hybridizing under stringent conditions".
Hybridization can be conducted in accordance with a method
described in, for example, Molecular Cloning 2nd ed.,
Current Protocols in Molecular Biology, Supplement 1-38,
DNA Cloning 1: Core Techniques, A Practical Approach,
Second Edition, Oxford University Press (1995), and the
like. Here,
sequences hybridizing under stringent
conditions exclude, preferably, sequences containing only A
or T.
"Hybridizable polynucleotide" refers to a
polynucleotide which can hybridize to other polynucleotides
CA 02808179 2013-02-28
- 44 -
under the above-described hybridization conditions.
Specifically, the hybridizable polynucleotide includes at
least a polynucleotide having a homology of at least 60% to
the base sequence of DNA encoding a polypeptide having an
amino acid sequence specifically herein disclosed,
preferably a polynucleotide having a homology of at least
80%, and more preferably a polynucleotide having a homology
of at least 95%.
[0167)
The similarity, identity and homology of amino
acid sequences and base sequences are herein compared using
FASTA with the default parameters.
Alternatively, an
identity search may be conducted, for example, using NCBI's
BLAST 2.2.9 (published May 12, 2004). As used herein, the
value of identity usually refers to the value as a result
of alignment with the BLAST as described above using the
default parameters. If the change of parameters results in
higher values, then the highest value is employed herein as
the value of the identity. When a plurality of regions are
evaluated for identity, the highest value is employed
herein as the value of the identity.
[0168]
As used herein, the term "search" indicates
that a given nucleic acid sequence is utilized to find
other nucleic acid base sequences having a specific
function and/or property either electronically or
biologically, or using other methods.
Examples of an
electronic search include, but are not limited to, BLAST
(Altschul et al., J. Mol. Biol. 215:403-410 (1990)), FASTA
(Pearson & Lipman, Proc. Natl. Acad. Sci., USA 85:2444-2448
(1988)), Smith and Waterman method (Smith and Waterman, J.
Mol. Biol. 147:195-197 (1981)), and Needleman and Wunsch
CA 02808179 2013-02-28
- 45 -
method (Needleman and Wunsch, J. Mol. Biol. 48:443-453
(1970)), and the like.
Examples of a biological search
include, but are not limited to, a macroarray in which
genomic DNA is attached to a nylon membrane or the like or
a microarray (microassay) in which genomic DNA is attached
to a glass plate under stringent hybridization, PCR and in
situ hybridization, and the like. As used herein, it is
intended that promoters used in the present invention
encompass a sequence corresponding to those identified by
such an electronic or biological search.
[0169]
As used herein, the term "expression" of a gene
product, such as a gene, a polynucleotide, a polypeptide,
or the like, indicates that the gene or the like is
affected by a predetermined action in vivo to be changed
into another form.
Preferably, the term "expression"
indicates that genes, polynucleotides, or the like are
transcribed and translated into polypeptides. In one
embodiment of the present invention, genes may be
transcribed into mRNA. More preferably, these polypeptides
may have post-translational processing modifications.
[0170]
As used herein amino acids may be referred to
with the generally known three-letter abbreviation or the
one letter-abbreviation proposed by the IUPAC-IUB
Biochemical Nomenclature Commission. Nucleotides may also
be referred to with the generally known one-letter
abbreviations which are generally accepted.
[0171]
The letter codes are as follows:
Amino Acids:
3-letter single-letter reference
CA 02808179 2013-02-28
- 46 -
Ala A alanine
Cys C cysteine
Asp D aspartic acid
Gill E glutamic acid
Phe F phenylalanine
Gly G glycine
His H histidine
Ile I 'isoleucine
Lys K lysine
Leu L leucine
Met M methionine
Asn N asparagine
Pro P proline
Gin Q glutamine
Arg R arginine
Ser S serine
Thr T threonine
=
Val V valine
Trp W tryptophane
Tyr Y tyrosine
Asx aSparatic acid or asparagine
Glx glutamine or glutamic acid
Xaa unknown or other amino acid
[0172]
Base (Nucleotide)
abbreviation reference
a adenine
guanine
c cytosine
thymine
uracyl
CA 02808179 2013-02-28
- 47 -
r guanine or adenine purine
thymine/uracil or cytosine purimidine
adenin or cytocine amino group
guanine or thymine uracil keto group
s guanin or cytosine
adenine or thymine/uracil
guanine or cytocine or thymine/uracil
adenine or guanine or thymine/uracil
adenine or cytosine or thymine/uracil
v adenine or guanine or cytosine
adenine or guanine or cytosine or
thymine/uracil, unknown or other base
[0173]
As used herein, the term "fragment" with
respect to a polypeptide or polynucleotide refers to a
polypeptide or polynucleotide having a sequence length
ranging from 1 to n-1 with respect to the full length of
the reference polypeptide or polynucleotide (of length n).
The length of the fragment can be appropriately changed
depending on the purpose. For example, in the case of
polypeptides, the lower limit of the length of the fragment
includes 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 40, 50 or
more nucleotides. Lengths represented by integers which
are not herein specified (e.g., 11 and the like) may be
appropriate as a lower limit. For example, in the case of
polynucleotides, the lower limit of the length of the
fragment includes 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 40, 50,
75, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000 or
more nucleotides. Lengths represented by integers which
are not herein specified (e.g., 11 and the like) may be
appropriate as a, lower limit.
[0174]
CA 02808179 2013-02-28
-48-
A polypeptide used in the present invention may have
at least one (for example, one or several or more) amino
acid substitutions, addition and/or deletion in the amino
acid sequence, as long as it has substantially identical
function as the wild type polypeptide.
(01751
. It is well known that if a given amino acid is
substituted with another amino acid having a similar
hydrophobicity index, the resultant protein may still have
a biological function similar to that of the original
protein (e.g., a protein having an equivalent enzymatic
activity). For
such an amino acid substitution, the
hydrophobicity index is preferably within 2, more
preferably within 1, and even more preferably within 0.5.
It is understood in the art that hydrophobicity is
considered in the modification of a protein. As described
in US Patent No. 4,554,101, amino acid residues are given
the following hydrophilicity indices: arginine (+3.0);
lysine (+3.0); aspartic acid (+3.0 1); glutamic acid
(+3.0 1); serine (+0.3); asparagine (+0.2); glutamine
(+0.2); glycine (0); threonine (-0.4); proline (-0.5 1);
alanine (-0.5); histidine (-0.5); cystelne (-1.0);
methionine (-1.3); valine (-1.5); leucine (-1.8);
isoleucine (-1.8); tyrosine (-2.3); phenylalanine (-2.5);
and tryptophan (-3.4). It is understood that an amino acid
may be substituted with another amino acid which has a
similar hydrophilicity index and can still provide a
biological equivalent. For such an amino acid substitution,
the hydrophilicity index is preferably within 2, more
preferably 1, and even more preferably 0.5. A
hydrophilicity index is also useful for modification of an
amino acid sequence of the present invention. As described
CA 02808179 2013-02-28
-49-
in US Patent No. 4,554,101, amino acid residues are given
the following hydrophilicity indices: arginine (+3".0);
lysine (+3.0); aspartic acid (+3.0 1); glutamic acid
(+3.0 1); serine (+0.3); asparagine (+0.2); glutamine
= 5 (+0.2); glycine (0); threonine (-0.4); proline (-0.5 1);
alanine (-0.5); histidine (-0.5); cysteine (-1.0);
methionine (-1.3); valine (-1.5); leucine (-1.8);
isoleucine (-1.8); tyrosine (-2.3); phenylalanine (-2.5);
and tryptophan (-3.4). It is understood that an amino acid
may be substituted with another amino acid which has a
similar hydrophilicity index and can still provide a
biological equivalent. For such an amino acid substitution,
the hydrophilicity index is preferably within 2, more
preferably 1, and even more preferably 0.5.
[0176]
The term "conservative substitution" as used
herein refers to an amino acid substitution in which a
substituted amino acid and a substituting amino acid have
similar hydrophilicity indices or/and hydrophobicity
indices. For example, the conservative substitution is
carried out between amino acids having a hydrophilicity or
hydrophobicity index of within 2, preferably within 1,
and more preferably within 0.5. Examples of conservative
substitution include, but are not limited to, substitutions
within each of the following residue pairs: arginine and
lysine; glutamic acid and aspartic acid; serine and
threonine; glutamine and asparagine; and valine, leucine,
and isoleucine, which are well known to those skilled in
the art.
[0177]
As used herein, the term "variant" refers to a
substance, such as a polypeptide, polynucleotide, or the
CA 02808179 2013-02-28
-50-
like, which differs partially from the original substance.
Examples of such a variant include a substitution variant,
an addition variant, a deletion variant, a truncated
Variant, an allelic variant, and the like. Examples of
such a variant include, but are not limited to, a
nucleotide or polypeptide having one or several
substitutions, additions and/or deletions or a nucleotide
or polypeptide having at least one substitution, addition
and/or deletion. The term "allele" as used herein refers
to a genetic variant located at a locus identical to a
corresponding gene, where the two genes are distinguishable
from each other. Therefore, the term "allelic variant" as
used herein refers to a variant which has an allelic
relationship with a given gene. Such an allelic variant
ordinarily has a sequence the same as or highly similar to
that of the corresponding allele, and ordinarily has almost
the same biological activity, though it rarely has
different biological activity. The term "species homolog"
or "homolog" as used herein refers to one that has an amino
acid or nucleotide homology with a given gene in a given
species (preferably at least 60% homology, more preferably .
at least 80%, at least 85%, at least 90%, and at least 95%
homology). A method for obtaining such a species homolog
is clearly understood from the description of the present
specification. The term "orthologs" (also called
orthologous genes) refers to genes in different species
derived from a common ancestry (due to speciation). For
example, in the case of the hemoglobin gene family having
multigene structure, human and mouse a-hemoglobin genes
are orthologs, while the human a-hemoglobin gene and the
human 13-hemoglobin gene are paralogs (genes arising from
gene duplication). Orthologs are useful for estimation of
CA 02808179 2013-02-28
-51-
molecular phylogenetic trees.
Usually, orthologs in
= different species may have a function similar to that of
the original species. Therefore, orthologs of the present
invention may be useful in the present invention.
[0178]
As used herein the term "functional variant" refers
to a variant which retains a biological activity (in
particular, promoter activity" which the sequence of
standard is responsible for.
[0179]
As used herein, the term "conservative (or
conservatively modified) variant" applies to both amino
acid and nucleic acid sequences. With respect to particular
nucleic acid sequences, conservatively modified variants
refer to those nucleic acids which encode identical or
essentially identical amino acid sequences. Because of the
degeneracy of the genetic code, a large number of
functionally identical nucleic acids encode any given
protein. For example, the codons GCA, GCC, GCG and GCU all
encode the amino acid alanine. Thus, at every position
where an alanine is specified by a codon, the codon can be
altered to any of the corresponding codons described
without altering the encoded polypeptide. Such
nucleic
acid variations are "silent variations" which represent one
species of conservatively modified variation. In a
nucleic acid, a conservative substitution can be confirmed
by measuring promoter activity, for example.
[0180]
In order to prepare functionally equivalent
polypeptides, amino acid additions, deletions, or
modifications can be performed in addition to amino acid
substitutions. Amino acid substitution(s) refers to the
CA 02808179 2013-02-28
-52-
replacement of at least one amino acid of an original
peptide with different amino acids, such as the replacement
of 1 to 10 amino acids, preferably 1 to 5 amino acids, and
more preferably 1 to 3 amino acids with different amino
acids. Amino acid addition(s) refers to the addition of at
least one amino acid to an original peptide chain, such as
= the addition of 1 to 10 amino acids, preferably 1 to 5
amino acids, and more preferably 1 to 3 amino acids to an
original peptide chain. Amino acid deletion(s) refers to
the deletion of at least one amino acid, such as the
= deletion of 1 to 10 amino acids, preferably 1 to 5 amino
acids, and more preferably 1 to 3 amino acids. Amino acid
modification includes, but is not limited to, amidation,
carboxylation, sulfation, halogenation, truncation,
lipidation, alkylation, glycosylation, phosphorylation,
hydroxylation, acylation (e.g., acetylation), and the like.
Amino acids to be substituted or added may be naturally-
occurring or nonnaturally-occurring amino acids, or amino
acid analogs.
Naturally-occurring amino acids are
preferable.
[0181] Nucleic acid embodiment of =
the
polypeptide to be expressed as used herein refers to a
nucleic acid which allows expression of the protein
embodiment of the polypeptide. Such a
nucleic acid
includes one in which a part of the sequence of the nucleic
acid is deleted or is substituted with other base(s), or an
additional nucleic acid sequence is inserted, as long as a
polypeptide expressed by the nucleic acid has substantially
the same activity as that of the naturally-occurring
polypeptide, as described above.
Alternatively, an
additional nucleic acid may be linked to the 5' terminus
and/or 3' terminus of the nucleic acid. The nucleic acid
CA 02808179 2013-02-28
-53-
molecule may include one that is hybridizable to a gene
encoding a polypeptide under stringent conditions and
encodes a polypeptide having substantially the same
function as that of that polypeptide. Such a gene is known
in the art and can be used in the present invention.
[0182]
The above-described nucleic acid can be
obtained by a well-known PCR method, i.e., chemical
synthesis. This method may be combined with, for example,
site-specific mutagenesis, hybridization, or the like.
[0183]
As used herein, the term "substitution,
addition or deletion" for a polypeptide or a polynucleotide
refers to the substitution, addition or deletion of an
amino acid or its substitute, or a nucleotide or its
substitute with respect to the original polypeptide or
polynucleotide. This is achieved by techniques well known
in the art, including a site-specific mutagenesis technique
and the like. A polypeptide or a polynucleotide may have
any number (>0) of substitutions, additions, or deletions.
The number can be as large as a variant having such a
number of substitutions, additions or deletions can
maintain an intended function (e.g., the information
transfer function of hormones and cytokines, etc.). For
example, such a number may be one or several, and
preferably within 20% or 10% of the full length sequence,
or no more than 100, no more than 50, no more than 25, or
the like.
[0184]
(promoter)
As used herein, the term 'promoter (or promoter
sequence)" refers to a base sequence which determines the
CA 02808179 2013-02-28
-54-
initiation site of transcription of a gene and is a DNA
region which directly regulates the frequency of
transcription. Transcription is started by RNA polymerase
binding to a promoter.
Accordingly, as used herein a
portion having the function of a promoter of a gene refers
to "a promoter portion". A promoter region can be deduced
by predicting the protein coding region in a genomic base
sequence using DNA analysis software.
Deduced promoter
regions are usually located upstream of the structural gene
10. although it varies, and is not limited thereto, and may
also be downstream of the structural gene.
[0185]
As used herein, the term "MIE promoter" refers to a
major immediate early promoter, which is a promoter of a
gene which is immediately transcribed by a transcription
factor derived from a host and a virion after viral
infection. The MIE gene may be identified by RT-PCR using
an RNA extracted from an infected cell treated with
cycloheximide.
[0186]
As used herein, the term "U95 promoter" refers to a
promoter of the immediate early gene U95. U95 is also an
immediate early gene, and thus is immediately transcribed
by a transcription factor derived from a host or a virion
after the viral infection.
[0187]
As used herein, the identification method of a
promoter is as follows: some sequences in the vicinity of
the structural gene are screened (for example, using an
expression cassette described in the Examples), and the
sequence having the gene expression promoting activity is
mapped. As such, a sequence having significant promoting
CA 02808179 2013-02-28
-55-
activity may be identified.
Usually, it is located
upstream of the structural gene, but is not limited thereto.
[0188]
As used herein, the term "HHV6B MIE promoter" or "MIE
promoter of HHV6B" refers to any sequence having promoter
activity in SEQ ID NO: 1. Preferably, the promoter has
position -814 to position 0 from the transcription
initiation point in SEQ ID NO: 1. Such a sequence includes,
but is not' limited to SEQ ID NO: 1 or a sequence
corresponding thereto. In the expression control of HHV6B
gene, it is preferable to be located in the region at -574
to -427 from the upstream, and preferably, in the region of
-1051 to -427, and the base sequence thereof includes
sequences set forth in SEQ ID NO: 15, 16 and the like.
Amongst them, it has been elucidated herein that NF-x B and
AP-1 motifs (-603 to -594 from the transcription initiation
point as the origin, corresponds to NF-x B motif, and -488
to -478 and -249 to -239 correspond to the AP-1 motifs.)
may be motifs from experiments of base sequence
substitution. Accordingly,
preferably, the HHV6B MIE
promoter of the present invention comprises: (a) a
polynucleotide having the base sequence set forth in SEQ ID
NO: 1, or the base sequence corresponding thereto or a
fragment sequence thereof; (b) a polynucleotide of an
allelic variant of the base sequence set forth in SEQ ID
NO: 1 or the base sequence corresponding thereto or a
fragment sequence thereof; (c) a polynucleotide which
hybridizes a polynucleotide of any of (a) or (b) and has a
biological activity thereof; or (d) a polynucleotide which
consists of the base sequence of any of (a) to (c) or a
complement sequence thereof with at least 70 % identity,
and has a biological activity thereof.
CA 02808179 2013-02-28
-56-
[0189]
As used herein, the term "HHV7 MIE promoter" or "MIE
promoter of HHV7" refers to any sequence having promoter
activity in SEQ ID NO: 2. Preferably, the promoter has
position -493 to position +22 from the transcription
initiation point in SEQ ID NO: 2. Such a sequence includes,
but is not limited to SEQ ID NO: 2 or a sequence
corresponding thereto. In the expression control of the
HHV7 gene, it is preferable to be located in the region at-
427 from the upstream, and preferably, in the region of -
493, and the base sequence thereof includes sequences set
forth in SEQ ID NO: 2 and the like. Amongst them, it has
been elucidated herein that NF-KB motifs (-464 to -478 and
-359 to -350 from the transcription initiation point as the
origin, corresponds to NF-KB motifs) may be motifs from
experiments of base sequence substitution. Accordingly,
preferably, the HHV7 MIE promoter of the present invention
comprises: (a) a polynucleotide having the base sequence
set forth in SEQ ID NO. 2, or the base sequence
corresponding thereto or a fragment sequence thereof; (b) a
polynucleotide of an allelic variant of the base sequence
set forth in SEQ ID NO. 2 or the base sequence
corresponding thereto or a fragment sequence thereof; (c) a
polynucleotide which hybridizes a polynucleotide of any of
(a) or (b) and has a biological activity thereof; or (d) a
polynucleotide which consists of the base sequence of any
of (a) to (c) or a complement sequence thereof with at
least 70 % identity, and has a biological activity thereof.
[0190]
As used herein, the term "HHV7 U95 promoter" or "U95
promoter of HHV7" refers to any sequence having promoter
activity in SEQ ID NO: 12. Preferably, the promoter has
CA 02808179 2013-02-28
-57-
position -484 to position +16 from the transcription
initiation point in SEQ ID NO: 12. In the
expression
control of the HHV7 gene, it is preferable to be located in
the region at -379 from the upstream, and preferably, in
the region of -484, and the base sequence thereof includes
sequences set forth in SEQ ID NO: 2 and the like. Amongst
them, it has been elucidated herein that NF-x13 motifs (-478
to -469 and -373 to -364 from the transcription initiation
point as the origin, correspond to NF-x13 motifs) may be
motifs from experiments of base sequence substitution.
Accordingly, preferably, the HHV7 U95 promoter of the
present invention comprises: (a) a polynucleotide having
the base sequence set forth in SEQ ID NO. 12, or the base
sequence corresponding thereto or a fragment sequence
thereof; (b) a polynucleotide of an allelic variant of the
base sequence set forth in SEQ ID NO. 12 or the base
sequence corresponding thereto or a fragment sequence
thereof; (c) a polynucleotide which hybridizes a
polynucleotide of any of (a) or (b) and has a biological
activity thereof; or (d) a polynucleotide which consists of
the base sequence of any of (a) to (c) or a complement
sequence thereof with at least 70 t identity, and has a
biological activity thereof.
[0191]
"Constitutive" expression of a gene- by a
promoter of the present invention as used herein refers to
a trait in which expression is found at a substantial but
unchanged amount in any tissue of an organism during any
stage in the course of the growth of the organism.
Specifically, when northern blot analysis is carried out
under conditions similar to those in the examples described
herein, if substantial and similar expression is observed
CA 02808179 2013-02-28
-58-
in the same or corresponding site thereof on any time
points (e.g. two or more time points such as day 5 and day
15), the expression is regarded as being constitutive by
the definition in the present invention.
Constitutive
promoters are believed to play a role in the homeostasis of
organisms in a normal growth environment. These traits can
be determined by extracting RNA from an arbitrary portion
and subjecting the RNA to northern blot analysis to analyze
expression amounts.
[0192]
"Enhancer" may be used .so as to enhance the
expression efficiency of a gene of interest. As such an
enhancer, an enhancer region containing an upstream
sequence within the CaMV35S promoter is preferable. A
plurality of enhancers or a single enhancer may be used, or
no enhancer may be used. A region in a promoter which
enhances the activity of the promoter may also be referred
to as an enhancer.
[0193]
As used herein, "operatively linked" or "operative
link" refers to the fact that the expression (operation) of
a desired sequence is located under the control of a
transcription regulation sequence (e.g. promoter, enhancer
or the like) or a translation regulation sequence. In
order that a promoter is operably linked to a gene, the
promoter is usually located immediately upstream of the
gene, but is not necessarily located in a flanking manner.
[0194]
(Nucleic acid construct)
As used herein, the term "nucleic acid construct" or
"gene cassette" are interchangeably used to refer to a
nucleic acid sequence comprising nucleic acid (for example,
CA 02808179 2013-02-28
-59-
DNA, RNA) encoding a gene, a nucleic acid sequence
comprising a gene promoter operably linked thereto (such
that it can control the expression of the nucleic acid), a
promoter, and optionally a heterologous gene operably
linked thereto (i.e., in frame). It is intended that the
use of this cassette or the construct optionally in
combination with another regulatory element is encompassed
in the present invention. Preferably expression cassettes
or nucleic acid constructs are those which are amenable to
specific restriction enzyme digestion and are feasible for
recovery.
[0195]
When a gene is mentioned herein, the term
"recombinant vector" refers to a vector transferring a
polynucleotide sequence of interest to a target cell. Such
a vector is capable of self-replication or incorporation
into a chromosome of a host cell (e.g., a prokaryotic cell,
yeast, an animal cell, a plant cell, an insect cell, an
individual animal, and an individual plant, etc.), and
contains a promoter at a site suitable for transcription of
a polynucleotide of the present invention. In the present
application, for example, BAC vectors may be used. BAC
vector refers to a plasmid produced based on the F plasmid
of an E.coll, and is capable of propagating and stably
maintaining a DNA fragment of about 300kb or greater in
size, in a bacteria such as E.coli or the like. BAC vector
comprises at least a region essential for replication of
BAC vectors. Such a
region essential for replication
includes, for example, oriS, a replication initiation point
of F plasmid, or a variant thereof.
[0196]
CA 02808179 2013-02-28
-60-
As used herein, "selective marker" refers to a gene
which functions as guidance for selecting a host cell
comprising a nucleic acid construct or a vector. Selective
markers include, but are not limited to: fluorescent
markers, luminescent markers and drug selective markers.
"Fluorescent markers" include, but are not limited to gene
encoding fluorescence proteins such as green fluorescent
protein (GFP), cyan fluorescent protein (CFP), yellow
fluorescent protein (YFP), red fluorescent protein (dsRFP).
"Luminescent markers" include but are not limited to genes
encoding luminescent proteins such as luciferases. "Drug
selective markers" include but are not limited to:
hypoxanthine guanine phosphoribosyl transferase (hprt),
dihydrofolate reductase gene, glutamine synthase gene,
aspartate transaminase, metallothionein (MT), adenosine
aminase (ADA). AMP deaminase (AMPD1,2), xanthine-guanine-
phosphoribosyl transferase, UN? synthase, P-glycoprotein,
asparagine synthase, and ornithine decarboxylase. A
combination of a drug in conjunction with these drug
selective markers including those encoding proteins, for
example: the combination of dihydrofolate reductase (DHFR)
gene and methotrexate (MTX); the combination of glutamine
synthase (GS) gene and methionine sulfoximine (Msx); the
combination of aspartate transaminase (AST) gene and N-
phosphone acetyl-L-aspartate (PALA); the combination of MT
gene and cadmium (Cd2+); the combination of adenosine
deaminase (ADA) gene and adenosine, alanosine, 2'-
deoxycoformycin; the combination of AMP deaminase (AMPD1.2)
gene and adenine, azaserine and coformicin; the combination
of xanthine-guanine-phosphoribosyl transferase gene and
mycophenolic acid; the combination of UMP synthase gene and
6-azauridine, pyrazofuran; the combination of P-
CA 02808179 2013-02-28
-61-
glycoprotein (P-gp, MDR) gene and multi drugs; the
combination of aspartate synthase (AS) gene and B-aspartyl
hydroxamic acid or albizziln; ornithine carboxylase (ODC)
gene and a-difluoromethyl-ornithine
(DFMO) and the like.
[0197]
As used herein, the term "expression vector"'
refers to a nucleic acid sequence comprising a structural
gene and a promoter for regulating expression thereof, and
in addition, various regulatory elements in a state that
allows them to operate within host cells. The regulatory
element may include, preferably, terminators, selectable
markers such as drug-resistance genes (e.g. kanamycin
resistant gene, hygromycin resistant gene and the like),
and enhancers. It is well known in the art that a type of
.expression vector of a living organism such as an animal
and a species of a regulatory element used may vary
depending on the type of host cell used.
[0198]
As used herein, the term "recombinant vector" refers
to a vector transferring a polynucleotide sequence of
interest to a target cell. Such a vector is capable of
self-replication or incorporation into a chromosome in a
host cell (e.g., a prokaryotic cell, yeast, an animal cell,
a plant cell, an insect cell, an individual animal, and an
individual plant, etc.), and contains a promoter at a site
suitable for transcription of a polynucleotide of the
present invention.
[0199]
As used herein, the term "terminator" refers to
a sequence which is located downstream of a protein-
encoding region of a gene and which is involved in the
CA 02808179 2013-02-28
-62-
termination of transcription when DNA is transcribed into
mRNA, and the addition of a poly-A sequence. It is known
that a terminator contributes to the stability of mRNA, and
has an influence on the amount of gene expression.
Terminators include, but are not limited to, a sequence
including AATAAA.
[0200]
As used herein, the term "foreign gene" to a
particular organism refers to a gene which does not
natively exist in the particular organism. Such a foreign
gene may be a gene modified from a gene which naturally
occurs in the particular organism, or a gene which
naturally occurs in an organism that is different from the
particular organism (such as ADA gene), or an artificially
synthesized gene, or a complex thereof such as a fusion.
An organism comprising such a foreign gene may express a
genetic product which is not expressed in nature. For
example, a recessive gene to be deleted (for example, ADA
gene, PNP gene, y c chain gene, TAP gene, MHC II gene, X-
linked WASP, CD40 ligand, PI3K-like gene, DNA helicase) may
be used as a foreign gene.
[0201]
As used herein, the foreign gene may be a gene of a
cytokine. As used herein, the term "cytokine" is defined
as in the broadest sense used in the art, and a
physiologically active substance which is produced from a
cell and acts on the same cell or a different cell.
Cytokines are generally a protein or a polypeptide, and
have a controlling action of immunological response,
regulation of endocrine system, regulation of the nerve
system, antitumor activity, antiviral activity, regulation
of cell proliferation, regulation of cellular
CA 02808179 2013-02-28
- 63 -
=
differentiation and the like. As used herein, cytokines
may exist in a form of protein or nucleic acid, and at the
actual time of action, cytokines usually mean a protein
form. As used herein, the term "growth factor" refers to a
substance which promotes or controls the growth of a cell.
Growth factors may substitute the action of serum
macromolecular substances by addition to a medium in cell
culture or tissue culture. Many growth factors have been
found to function as a regulation factor of a
differentiation state other than growth of a cell.
Cytokines typically include interleukins, chemokines,
hematopoietic factors such as colony stimulation factors,
tumor necrosis factors, interferons.
Growth factors
typically include platelet derived growth factor (PDGF),
epidermal growth factor (EGF), fibroblast growth factor
(FGF), hepatocytic growth factor (HGF), vessel endothelial
growth factor (VEGF), and the like, which show growth
activity.
(0202]
In the present invention, those having homology
with a foreign gene of a native form as described above may
be used as a foreign gene to be expressed. Such foreign
genes having such homology include, but are not limited to:
for example, when conducting comparison using default
parameters of Blast in comparison to a foreign gene of
reference to be compared, nucleic acids having sequences
of identity or similarity of at least about 30 %, at least
about 35 %, at least about 40 %, at least about 30 %, at
least about 45 %, at least about 50 %, at least about 55 %,
at least about 60 %, at least about 65 %, at least about
70 %, at least about 75 t, at least about 80 %, at least
about 85 %, at least about 90 %, at least about 95 %, at
CA 02808179 2013-02-28
- 64 -
=
least about 99 %, or polypeptides having amino acid
sequence of identity or similarity of at least about 30 *,
at least about 35 %, at least about 40 %, at least about
30 %, at least about 45 15, at least about 50 %, at least
about 55 15, at least about 60 %, at least about 65 %, at
least about 70 %, at least about 75 t, at least about 80 I,
at least about 85 %, at least about 90 %, at least about
95 %, at least about 99 %.
[0203]
As used herein, the term "expression" of a gene
product, such as a gene, a polynucleotide, a polypeptide,
or the like, indicates that the gene or the like is
affected by a predetermined action in vivo to be changed
into another form.
Preferably, the term "expression"
indicates that genes, polynucleotides, or the like are
transcribed and translated into polypeptides. In one
embodiment of the present invention, genes may be
transcribed into mRNA. More preferably, these polypeptides
may have post-translational processing modifications.
[0204]
Accordingly, as used herein, "reduction" of
"expression" of a gene, a polynucleotide, a polypeptide or
the like refers to when an agent of the present invention
is subjected to an action, whereby the amount of expression
is significantly reduced compared to that when the agent is
not subjected to an action. Preferably, the reduction of
expression includes a reduction of the level of polypeptide
expression. As used herein, the "increase" of "expression"
of a gene, a polynucleotide, a polypeptide or the like
refers to when an agent of the present invention is
subjected to an action (or an agent relating to gene
expression into a cell, for example, a gene to be expressed
CA 02808179 2013-02-28
-65-
or an agent for regulating the same), whereby the amount of
expression is significantly increased compared to when the
agent is not subjected to an action.
Preferably, the
increase of expression includes an increase in the level of
polypeptide expression. As used
herein, the term
"induction" of "expression" of a gene refers to an increase
in the level of expression of the gene by, acting an agent
on a cell.
Accordingly, the induction of expression
encompasses when the level of expression of the gene is
observed to increase from an observed level of no
expression, to a noticeable level of expression of the gene.
[0205]
As used herein, the term "specifically
express(ing)" of a gene refers to expression in a different
level (preferably in a higher level) in a specific site or
period of time than that of the other site or period of
time. Specific expression may refer to expression in a
certain site (specific site) or may also refer to the
expression including that in another site.
Preferably,
specific expression refers to the expression in the certain
site only.
[0206]
Methods of introducing a recombinant vector are
also achieved by any of the above-mentioned methods for
introducing DNA into a cell, and include for example,
transfection, transduction, transformation and the like,
such as calcium phosphate, liposome methods, DEAE dextran
methods, electroporation methods, particle gun methods
(gene gun), and the like, lipofection, spheroplast Proc.
Natl. Acad. Sci. USA, 84, 1929 (1978)], lithium acetate
method [J. Bacteriol., 153, 163 (1983)], a method described
in Proc. Natl. Acad. Sci. USA, 75, 1929 (1978) and the like.
CA 02808179 2013-02-28
-66-
[0207]
Transitional expression of Cre enzyme, DNA mapping on
the chromosomes and the like, used in a method for removing
a genome or genomic locus used herein or the like are well
5 known in the art as described in "FISH jikken purotokooru =
hito/genomu kaiseki kara senshokutai/idenshi shindan made
(FISH Experimental Protocol: from human/genomic analysis to
chromosomal./genetic diagnosis)" one of "Saibo Kogaku
Bessatsu Jikken Purotokooru siriizu (Cell Engineering,
Special Edition, Experimental Protocol Series), ed.
. Ken'ichl Matsubara, Hiroshi Yoshikawa, Shujunsha (Tokyo)
and the like.
[0208]
As used herein, gene expression (e.g., mRNA
. 15 expression, polypeptide expression) may be "detected" or
"quantified" by an appropriate method, including mRNA
measurement and immunological measurement.
Examples of
molecular biological measurement methods include Northern
blotting methods, dot blotting methods, PCR methods, and
the like. Examples of immunological measurement methods
include ELISA methods, RIA methods, fluorescent antibody
methods, Western blotting methods, immunohistological
staining methods, and the like, where a microtiter plate
may be used. Examples of quantification methods include
ELISA methods, RIA methods, and the like. A gene analysis
method using an array (e.g.,. a DNA array, a protein array,
etc.) may be used. The DNA array is widely reviewed in
Saibo-Kogaku [Cell Engineering], special issue, "DNA
Microarray and Up-to-date PCR Method", edited by Shujun-sha.
The protein array is described in detail in Nat Genet. 2002
Dec; 32 Supp1:526-32. Examples of methods for analyzing
gene expression Include, but are not limited to, RT-PCR
CA 02808179 2013-02-28
-67-
methods, RACE methods, SSCP methods, immunoprecipitation
methods, two-hybrid systems, in vitro translation methods,
and the like in addition to the above-described techniques.
Other analysis methods are described in, for example,
"Genome Analysis Experimental Method, Yusuke Nakamura's
Lab-Manual, edited by Yusuke Nakamura, Yodo-sha (2002), and
the like.
[0209]
As used herein, the term "expression level (or
amount)" refers to the amount of a polypeptide or mRNA
expressed in a subject cell. The term "expression level"
includes the level of protein expression of a polypeptide
evaluated by any appropriate method using an antibody,
including immunological measurement methods (e.g., an ELISA
method, an RIA method, a fluorescent antibody method, a
Western blotting method, an iromunohistological staining
method, and the like, or the mRNA level of expression of a
polypeptide evaluated by any appropriate method, including
molecular biological measurement methods (e.g., a Northern
blotting method, a dot blotting method, a PCR method, and
the like). The term "change in expression level" indicates
that an increase or decrease in the protein or mRNA level
of expression of a polypeptide evaluated by an appropriate
method including the above-described immunological
measurement method or molecular biological measurement
method.
[0210]
As used herein, the terms "transformation",
"transduction" and "transfection" are used interchangeably
unless otherwise mentioned, and refer to introduction of a
nucleic acid into host cells. As a transformation method,
CA 02808179 2013-02-28
-68-
any technique for introducing DNA into host cells can be
used, including various well-known techniques, such as, for
example, the electroporation method, the particle gun
method (gene gun), the calcium phosphate method, and the
like.
[0211]
As used herein, the term "transformant" refers
to the whole or a part of an organism, such as a cell,
which is produced by transformation. Examples of a
transformant include prokaryotic cells, yeast, animal cells,
plant cells, insect cells and the like. Transformants may
be referred to as transformed cells, transformed tissue,
transformed hosts, or the like, depending on the subject.
As used herein, all of the forms are encompassed, however,
a particular form may be specified in a particular context.
[0212]
Examples of prokaryotic cells include
prokaryotic cells of the genera Escherichia, Serratia,
Bacillus, Brevibacterium, Corynebacterium, Microbacterium,
Pseudomonas, and the like, e.g., Escherichia coli XLI-Blue,
Escherichia coli XL2-Blue, Escherichia coli DH1,
Escherichia coil MC1000, Escherichia coil KY3276,
Escherichia coil W1485, Escherichia coil M4109, Escherichia
coli HB101, Escherichia coli No.49, Escherichia coli W3110,
Escherlchia coil NY49, Escherichia coli BL21(DE3),
Escherichia coli BL21(DE3)pLysS, Escherichia coli
HMS174(DE3), Escherichia coli HMS174(DE3)pLysS, Serratia
ficaria, Serratia fonticola, Serratia liquefaciens,
Serratia marcescens, Bacillus subtilis, Bacillus
amyloliquefaciens, Brevibacterium
arnrnmoniagenes,
Brevibacterium immariophilum ATCC14068, Brevibacterium
saccharolyticum ATCC14066, Corynebacterium glutamicum
CA 02808179 2013-02-28
- 69 -
ATCC13032, Corynebacterium glutamicum
ATCC14067,
Corynebacterium glutamicum ATCC13869, Corynebacterium
acetoacidophilum ATCC13870, Microbacterium ammoniaphilum
ATCC15354, Pseudomonas sn.D-0110. and the like.
[0213]
Examples of animal cells include cord blood
mononuclear cells, peripheral blood mononuclear cells, Sup-
Ti cells, and the like.
[0214]
The term "animal" is used herein in its
broadest sense and refers to vertebrates and invertebrates
(e.g., arthropods). Examples of animals include, but are
not limited to, any of the class Mammalia, the class Ayes,
the class Reptilia, the class Amphibia, the class Pisces,
the class Insecta, the class Vermes, and the like,
[0215]
As used herein, the term "tissue" in relation
to organisms refers to an aggregate of cells having
substantially the same function. Therefore, a tissue may
be a part of an organ. Organs usually have cells having
the same function, and may have coexisting cells having
slightly different functions. Therefore, as used herein,
tissues may have various kinds of cells as long as a
certain property is shared by the cells.
[0216]
As used herein, the term "organ" refers to a
structure which has a single independent form and in which
one or more tissues are associated together to perform a
specific function. In plants, examples of organs include,
but are not limited to, callus, root, stem, trunk, leaf,
flower, seed, embryo bud, embryo, fruit, and the like. In
animals, examples of organs include, but are not limited
CA 02808179 2013-02-28
-70-
to, stomach, liver, intestine, pancreas, lung, airway,
nose, heart, artery, vein, lymph node (lymphatic system),
thymus, ovary, eye, ear, tongue, skin, and the like.
[0217]
As .used herein, the term "transgenic" refers to
incorporation of a specific gene into an organism (e.g.,
plants or animals (mice, etc.)) or such an organism having
an incorporated gene.
[0218]
When organisms of the present invention are
animals, the transgenic organisms can be produced by a
microinjection method (a trace amount injection method), a
viral vector method, an embryonic stem (ES) cell method, a
sperm vector method, a chromosome fragment introducing
method (transsomic method), an episome method, or the like.
These transgenic animal producing techniques are well known
in the art.
=
[0219] =
As used herein, the term "screening" refers to
selection of a substance, a host cell, a virus, or the like
having a given specific property of interest from a number
of candidates using a specific operation/evaluation method.
It will be understood that the present invention
encompasses viruses having desired activity obtained by
screening.
[0220]
As used herein, the terms "chip" or "microchip"
are used interchangeably to refer to a micro-integrated
circuit which has versatile functions and constitutes a
portion of a system. Examples of a chip include, but are
not limited to, DNA chips, protein chips, and the like.
[0221]
CA 02808179 2013-02-28
-71-
The herpesvirus promoters of the present
invention can be used as an ingredient of a pharmaceutical
composition for the treatment, prevention, and/or therapy
of lymphatic lineage or hemato-lineage, immune, and
infectious diseases.
[0222]
As used herein, the term "effective amount" in
relation to a drug refers to an amount which causes the
drug to exhibit intended efficacy. As used herein, an
effective amount corresponding to a smallest concentration
may be referred to as a minimum effective amount. Such a
minimum effective amount is well known in the art.
Typically, the minimum effective amount of a drug has been
determined or can be determined as appropriate by those
skilled in the art. The determination of such an effective
amount can be achieved by actual administration, use of an
=
animal model, or the like. The present invention is also
useful for the determination of such an effective amount.
[0223]
As used herein, the term "pharmaceutically
acceptable carrier" refers to a material which is used for
production of a pharmaceutical agent or an agricultural
chemical (e.g., an animal drug), and has no adverse effect
on effective ingredients.
Examples of such a
pharmaceutically acceptable carrier include, but are not
limited to: antioxidants, preservatives, colorants,
flavoring agents, diluents, emulsifiers, suspending agents,
solvents, fillers, bulking agents, buffers, delivery
vehicles, excipients, and/or agricultural or pharmaceutical
adjuvants.
[0224]
CA 02808179 2013-02-28
-72-
The type and amount of a pharmaceutical agent
used in the treatment method of the present invention can
be easily determined by those skilled in the art based on
information obtained by the method of the present invention
(e.g., information relating to a disease) in view of the
purpose of use, the target disease (type, severity, etc.),
the subject's age, size, sex, and case history, the
morphology and type of a site of a subject of
administration, or the like. The frequency of subjecting a
subject (patient) to the monitoring method of the present
invention is also easily determined by those skilled in the
art with respect to the purpose of use, the target disease
(type, severity, etc.), the subject's age, size, sex, and
case history, the progression of the therapy, and the like.
Examples of the frequency of monitoring the state of a
disease include once per day to once per several months
(e.g., once per week to once per month).
Preferably,
monitoring is performed once per week to once per month
with reference to the progression.
[0225]
As used herein, the term "instructions" refers
to a description of the method of the present invention for
a person who performs administration, such as a medical
doctor, a patient, or the like. Instructions state when to
administer a medicament of the present invention, such as
immediately after or before radiation therapy (e.g., within
24 hours, etc.). The
instructions are prepared in
accordance with a format defined by an authority of a
country in which the present invention is practiced (e.g.,
Health, Labor and Welfare Ministry in Japan, Food and Drug
Administration (FDA) in the U.S., and the like), explicitly
describing that the instructions are approved by the
CA 02808179 2013-02-28
-73-
authority. The instructions are so-called package insert
and are typically provided in paper media. The
instructions are not so limited and may be provided in the
form of electronic media (e.g., web sites, electronic mails,
and the like provided on the Internet).
[0226]
In a therapy of the present invention, two or
more pharmaceutical agents may be used as required. When
two or more pharmaceutical agents are used, these agents
may have similar properties or may be derived from similar
origins, or alternatively, may have different properties or
may be derived from different origins. A method of the
present invention can be used to obtain information about
the drug resistance level of a method of administering two
or more pharmaceutical agents.
[0227]
Culturing methods used in the present invention are
described and supported in, for example, "Doubutsu
Baiyosibo Manuaru (Animal Culture Cell Manual), Eeno et al.
eds., Kyoritsu shuppan, 1993.
[0228]
(Methods for producing polypeptides)
The polypeptides of the present invention may
be produced by culturing a transformant derived from a
microorganism or an animal cell possessing a recombinant
vector with a DNA encoding the polypeptide of the present
invention incorporated therein, in a normal culturing
manner, and producing and depositing the polypeptide of the
present invention, and recovering the polypeptide of the
present invention from the culture of the present invention.
[0229]
CA 02808179 2013-02-28
- 74 -
The method for culturing the transformant of
the present invention in a medium may be conducted
according to the normal methods used in the culture of a
host. Culture medium for culturing the transformant
obtained by using a prokaryotic cell such as E. colt and
the like or a eukaryotic cell such as yeast as a host,
include those comprising a carbon source, nitrogen source,
inorganic salts and the like which can be assimilated by
the organism of the present invention, and in which a
transformant can efficiently be cultured, which may be
natural or synthetic. [0230]
As a carbon source, those which can be assimilated by
the microorganism can be used and include, for example,
glucose, fructose, sucrose, sugar or honey containing the
same, starch, starch hydrolysate, organic acids such as
acetic acid and propionic acid, alcohols such as ethanol
and propanol and the like.
[0231]
As a nitrogen source, for example, the following can
be used: ammonia, a variety of ammonium salts of inorganic
or organic acid salt such as ammonium chloride, ammonium
sulfate, ammonium acetate, ammonium phosphate, other
nitrogen containing substance and the like, peptin, meat
extract, yeast extract, corn steep liquid, casein
hydrolysate, soybean powder, soybean powder hydrolysate, a
variety of fermented bacterial bodies, and the digests
thereof and the like.
[0232]
As inorganic salts, the following can be used for
example: potassium primary phosphate, potassium secondary
phosphate, magnesium phosphate, magnesium sulfate, sodium
chloride, ferrous phosphate, manganese sulfate, copper
CA 02808179 2013-02-28
- 75 - =
sulfate, calcium carbonate and the like. Culture will be
conducted under aerobic conditions such as shaking or deep
aerator agitating culture.
[0233]
Culture temperature is preferably from 15-40 degrees
Celsius. The period of time for culture is usually from
five hours to seven days but is not limited thereto. The
pH during the culture is kept from 3.0 to 9Ø The
adjustment of the pH may be conducted by adding inorganic
or organic acid or alkaline solution, urea, calcium
carbonate, ammonia and the like. During
the culture,
antibiotics such as ampicillin or tetracycline or the like
may be added as necessary.
[0234]
When culturing a microorganism which has been
transformed using an expression vector containing an
inducible promoter, the culture medium may be optionally
supplemented with an inducer. For
example, when a
microorganism, which has been transformed using an
expression vector containing a lac promoter, is cultured,
isopropyl-P-D-thlogalactopyranoside or the like may be
added to the culture medium. When a microorganism, which
has been transformed using an expression vector containing
a trp promoter, is cultured, indole acrylic acid or the
like may be added to the culture medium. A cell or an
organ into which a gene has been introduced can be cultured
in a large volume using a jar fermenter. Generally used
medium for culture are used herein such as Murashige and
Skoog (MS) medium, White medium, or these medium
supplemented with auxin, cytokine or plant hormones and the
like.
[0235)
CA 02808179 2013-02-28
- 76 -
For example, when using an animal cell, mediums used
for culturing the cell of the subject invention include,
for example those generally used such as RMPI1640 medium
[The Journal of the American Medical
Association,199,519(1967)], Eagle's MEN
medium
[Science,122,501(1952)] DMEM medium [Virology,8,396(1959)],
199 medium [Proceedings of the Society for the Biological
Medicine,73,1(1950)], or such a culture medium supplemented
with fetal bovine serum or the like.
[0236]
Culture is normally carried out for 1 to
7 days under conditions such as pH 6 to 8, 25 to 40 C, 5%
CO2. An
antibiotic, such as kanamycin, penicillin,
streptomycin, or the like may be optionally added to the
culture medium during cultivation.
A polypeptide of the present invention can be
isolated or purified from a culture of a transformant,
which has been transformed with a nucleic acid sequence
encoding the polypeptide, using an ordinary method for
isolating or purifying enzymes, which are well known and
commonly used in the art. For example, when a polypeptide
of the present invention is secreted outside a transformant
for producing the polypeptide, the culture is subjected to
centrifugation or the like to obtain a soluble fraction. A
purified specimen can be obtained from the soluble fraction
by a technique, such as solvent extraction, salting-
out/desalting with ammonium sulfate or the like,
precipitation with organic solvent, anion exchange
chromatography with a resin (e.g., diethylaminoethyl
(DEAE)-SepharoseTm, DIAIONTM HPA-75 (Mitsubishi Chemical
Corporation), etc.), cation exchange chromatography with a
resin (e.g., S-Sepharose FF (Pharmacia), etc.), hydrophobic
CA 02808179 2013-02-28
-77-
chromatography with a resin (e.g., buthylsepharose,
phenylsepharose, etc.), gel filtration with a molecular
sieve, affinity chromatography, chromatofocusing,
electrophoresis (e.g., isoelectric focusing electrophoresis,
etc.).
[0237]
When the polypeptide of the present invention
has been expressed and formed insoluble bodies within cells,
the cells are harvested, pulverized, and centrifuged. From
the resulting precipitate fraction, the polypeptide of the
present invention is collected using a commonly used method.
The insoluble polypeptide is solubilized using a
polypeptide denaturant. The resulting solubilized solution
is diluted Or dialyzed into a denaturant-free solution or a .
dilute solution, where the concentration of the polypeptide
denaturant is too low to denature the polypeptide. The
polypeptide of the present invention is allowed to form a
normal three-dimensional structure, and the purified
specimen is obtained by isolation and purification as
described above.
[0238]
Purification can be carried out in accordance
with a commonly used protein purification method (J. Evan.
Sadler et al.: Methods in Enzymology, 83, 458).
Alternatively, the polypeptide of the present invention can
be fused with other proteins to produce a fusion protein,
and the fusion protein can be purified using affinity
chromatography using a substance having affinity to the
fusion protein (Akio Yamakawa, Experimental Medicine, 13,
469-474 (1995)). For example, in accordance with a method
described in Lowe et al., Proc. Natl. Acad. Sci., USA, 86,
8227-8231 (1989), Genes Develop., 4, 1288(1990)), a fusion
CA 02808179 2013-02-28
-78-
protein of the polypeptide of the present invention with
protein A is produced, followed by purification with
affinity chromatography using immunoglobulin G.
[0239]
A fusion protein of the polypeptide of the
present invention with a FLAGTM peptide is produced, followed
by purification with affinity chromatography using anti-
FLAG antibodies (Proc. Natl. Acad. Sci., USA, 86,
8227(1989), Genes Develop., 4, 1288 (1990)).
The polypeptide of the present invention can be
purified with affinity chromatography using antibodies
which bind to the polypeptide. The polypeptide of the
present invention can be produced using an in vitro
transcription/translation system in accordance with a known
method (J. Biomolecular NMR, 6, 129-134; Science, 242,
1162-1164; J. Biochem., 110, 166-168 (1991)).
[0241]
The polypeptide of the present invention can
also be produced by a chemical synthesis method, such as
the Fmoc method (fluorenylmethyloxycarbonyl method), the
tBoc method (t-buthyloxycarbonyl method), or the like,
based on the amino acid information thereof. The peptide
can be chemically synthesized using a peptide synthesizer
(manufactured by Advanced ChemTech, Applied Biosystems,
Pharmacia Biotech, Protein Technology Instrument,
Synthecell-Vega, PerSeptive, Shimadzu, or the like).
[0242)
The structure of the purified polypeptide of
the present invention can be produced by methods commonly
used in protein chemistry (see, for example, Hisashi Hirano.
"Protein Structure Analysis for Gene Cloning", published by
Tokyo Kagaku Dojin, 1993). The physiological activity of a
CA 02808179 2013-02-28
- 79 --
polypeptide of the present invention can be measured in
accordance with a known measurement method.
[0243]
(Method for producing variant polypeptides
Deletion, substitution or addition of an amino acid
of the polypeptide of the present invention may be carried
out by site directed mutagenesis, which was well known
prior to the present application. Those with one or more
amino acids deleted, substituted or added may be prepared
in accordance with the methods described in: Molecular
Cloning, A Laboratory Manual, Second Edition, Cold Spring
Harbor Laboratory Press(1989), Current Protocols in
. Molecular Biology, Supplement 1-38,JohnWiley & Sons(1987-
1997) Nucleic Acids Research,10,6487(1982)
Proc.Natl.Acad.Sci.,USA,79,6409(1982), Gene,34,315(1985)
Nucleic Acids Research,13,4431(1985), Proc.Natl.Acad.Sci
USA,82,488(1985) Proc.Natl.Acad.Sci.,USA,81,5662(1984) .
Science,224,1431(1984) PCT W085/00817(1985)
Nature,316,601(1985) and the like.
[0244]
(Gene Therapy)
In certain embodiments, a nucleic acid comprising a
sequence encoding an antibody or a functional derivative
thereof is administered for the purpose of gene therapy for
treating, inhibiting or preventing a disease or disorder
related to abnormal expression and/or activity of a
polypeptide used in the present invention. Gene therapy
refers to a therapy performed by administering a nucleic
acid, which has been expressed or is capable of being
expressed, into subjects. In this embodiment of the
present invention, a nucleic acid produces a protein
CA 02808179 2013-02-28
-80-
encoded thereby and the protein mediates a therapeutic
effect.
[0245]
Any method available in the art for gene
therapy may be used in accordance with the present
invention. Illustrative methods are described below.
[0246]
See the following review articles for gene
therapy: Goldspie]. et al., Clinical Pharmacy 12:488-505
(1993); Wu and Wu, Biotherapy 3:87-95 (1991); Tolstoshev,
Ann. Rev. Pharmacol. Toxicol. 32:573-596 (1993); Mulligan,
Science 260:926-932 (1993); and Morgan and Anderson, Ann.
Rev. Biochem. 62:191-217 (1993); and May, TIBTECH
11(5):155:215(1993).
Generally known recombinant DNA
techniques used for gene therapy are described in Ausubel
et al. (eds.), Current Protocols in Molecular Biology, John
Wiley & Sons, NY (1993); and Kriegler, Gene Transfer and
Expression, A Laboratory Manual, Stockton Press, NY (1990).
(0247]
(Demonstration of therapeutic activity or preventive
activity)
The compounds or pharmaceutical compositions of
the present invention are preferably tested in vitro, and
then In vivo for the desired therapeutic or prophylactic
activity, prior to use in humans. For example, in vitro
assays to demonstrate the therapeutic or prophylactic
utility of a compound or pharmaceutical composition include,
the effect of a compound on a cell line or a patient tissue
sample. The effect of the compound or composition on the
cell line and/or tissue sample can be determined utilizing
techniques known to those skilled in the art (including,
but not limited to, cell lysis assays). In accordance with
CA 02808179 2013-02-28
-81-
the present invention, in vitro assays which can be used to
determine whether administration of a specific compound is
indicated, include in vitro cell culture assays in which a
patient tissue sample is grown in culture, and exposed to
or otherwise administered a compound, and the effect of
such compound upon the tissue sample is observed.
(0248]
(Therapeutic/Prophylactic Administration and
Composition)
The present invention provides methods of
treatment, prevention and prophylaxis by administration to
a subject of an effective amount of a component or
pharmaceutical composition comprising the promoter of the
present invention. In a preferable aspect, the component
comprising a promoter may be substantially purified (for
example, including the state where the effects are reduced,
or a substance causing undesirable side effect is
substantially free). Subjects may preferably be an animal
including but not limited to: cattle, pigs, horses,
chickens, cats, dogs and the like, and preferably primates,
and most preferably humans.
[0249]
When a nucleic acid molecule or polypeptide of
the present invention is used as a medicament, the
medicament may further comprise a pharmaceutically
acceptable carrier. Any
pharmaceutically acceptable
carrier known in the art may be used in the medicament of
the present invention.
(0250]
Examples of a pharmaceutically acceptable
carrier or a suitable formulation material include, but are
not limited to, antioxidants, preservatives, colorants,
CA 02808179 2013-02-28
-82-
flavoring agents, diluents, emulsifiers, suspending agents,
solvents, fillers, bulky agents, buffers, delivery vehicles,
and/or pharmaceutical adjuvants. Typically, a medicament
of the present invention is administered in the form of a
composition comprising a polypeptide or a polynucleotide or
a variant or fragment thereof, or a variant or derivative
thereof, or an agent capable of modulating any of these
substances, with at least one physiologically acceptable
carrier, excipient or diluent. For example, an appropriate
vehicle may be injection solution, physiological solution,
or artificial cerebrospinal fluid, which can be
supplemented with other substances which are commonly used
for compositions for parenteral delivery.
[0251]
Acceptable carriers, excipients or stabilizers
used herein preferably are nontoxic to recipients and are
preferably inert at the dosages and concentrations employed,
and preferably include phosphate, citrate, or other organic
acids; ascorbic acid, a-tocopherol; low molecular weight
polypeptides; proteins (e.g., serum albumin, gelatin, or
immunoglobulins); hydrophilic polymers (e.g.,
polyvinylpyrrolidone); amino acids (e.g., glycine,
glutamine, asparagine, arginine or lysine); monosaccharides,
disaccharides, and other carbohydrates (glucose, mannose,
or dextrins); chelating agents (e.g., EDTA); sugar alcohols
(e.g., mannitol or sorbitol); salt-forming counterions
(e.g., sodium); and/or nonionic surfactants (e.g., Tweee4,
pluronics or polyethylene glycol (PEG)).
[0252]
Examples of appropriate carriers include
neutral buffered saline or saline mixed with serum albumin.
Preferably, the product is formulated as a .1yophilizate
CA 02808179 2013-02-28
-83-
using appropriate excipients (e.g., sucrose). Other
standard carriers, diluents, and excipients may be included
as desired. Other
exemplary compositions comprise Tris
buffer of about pH 7.0-8.5, or acetate buffer of about pH
4.0-5.5, which may further include sorbitol or a suitable
substitute therefor.
[0253]
The medicament of the present invention may be
administered orally or parenterally. Alternatively, the
medicament of the present invention may be administered
intravenously or subcutaneously. When
systemically
administered, the medicament for use in the present
invention may be in the form of a pyrogen-free,
pharmaceutically acceptable aqueous solution. The
preparation of such pharmaceutically acceptable
compositions, with due regard to pH, isotonicity, stability
and the like, is within the skill of the art.
Administration methods may be herein oral, parenteral
administration (e.g., intravenous,
intramuscular,
subcutaneous, intradermal, to mucosa, intrarectal, vaginal,
topical to an affected site, to the skin, etc.). A
prescription for such administration may be provided in any
formulation form. Such a formulation form includes liquid
formulations, injections, sustained preparations, and the
like.
[0254]
The medicament of the present invention may be
prepared for storage by mixing a sugar chain composition
having the desired degree of purity with optional
physiologically acceptable carriers, excipients, or
stabilizers (Japanese Pharmacopeia ver. 14, or a supplement
thereto or the latest version; Remington's Pharmaceutical
CA 02808179 2013-02-28
-84-
Sciences, 18th Edition, A. R. Gennaro, ed., Mack Publishing
Company, 1990; and the like), in the form of lyophilized
cake or aqueous solutions.
[0255]
The type and amount of a pharmaceutical agent
used in the treatment method of the present invention can
be easily determined by those skilled in the art based on
information obtained by the method of the present invention
(e.g., information relating to a disease) in view of the
purpose of use, the target disease (type, severity, etc.),
the subject's age, size, sex, and case history, the
morphology and type of a site of a subject of
administration, or the like. The frequency of subjecting a
subject (patient) to the monitoring method of the present
invention is also easily determined by those skilled in the
art with respect to the purpose of use, the target disease
(type, severity, etc.), the subject's age, size, sex, and
case history, the progression of the therapy, and the like.
Examples of the frequency of monitoring the state of a
disease include once per day to once per several months
(e.g., once per week to once per month).
Preferably,
monitoring is performed once per week to once per month
with reference to the progression.
[0256]
(Immune therapy)
As used herein the term "vaccine" refers to a
composition (for example, suspension or solution)
comprising a usually infectious agent or a portion of an
infectious agent, an agent (for example, gene sequence)
which allows production of such an agent or portion, to
induce an active immune response.
Antigenic portions
constituting vaccines may be a microorganism (such as a
CA 02808179 2013-02-28
-85-
virus or bacteria or the like), a native product purified
from such a microorganism, a synthetic product or
genetically engineered proteins, peptides, polysaccharides
or similar products and nucleic acid molecules comprising a
nucleic acid sequence encoding such proteins. Vaccines
express the effects thereof by causing a neutralizing
antibody.
[0257]
As used herein the term "gene vaccine" refers to a
composition (for example, suspension or solution or the
like) comprising an agent (typically nucleic acid molecule)
which is expressed in the subject to be administered and
whose expressed product has vaccine action.
Typical
genetic vaccines may be nucleic acid molecules comprising
the nucleic acid sequence encoding a gene product having
antigenicity (for example, vectors, plasmids, Naked DNA and
the like).
[0258]
As used herein, immunologic effects of the vaccines
according to the present invention can be confirmed by
. using any method known in the art. Such a method includes,
but is not limited to: for example, CTL precursor cell
frequency analysis, ELISPOT method, tetramer method,
realtime PCR method and the like. As an
exemplary
description for CTL precursor frequency analysis,
peripheral lymphocyte or antigenic peptide and lymphocyte
cultured in the presence of IL-2, were subjected to
limitation dilution, and IL-2 and feeder cells were
cultured under coexistence, and the wells having
propagation were stimulated with vaccines or their
candidates, and the presence or absence of IFN-y production
is measured using ELISA. Herein, positive wells are used
CA 02808179 2013-02-28
-86-
to calculate the frequency of CTL precursor cells according
to the Poisson Analysis, to evaluate efficacy of the
vaccines. As used herein, the number of positive cells is
the number of antigen-specific CTLs and the greater the
number is, the greater the efficacy of the vaccine.
[059]
The present invention may be used as a cancer vaccine.
In such a case cancer antigens may be incorporated as a
foreign gene.
[0260]
As used herein, the term "cancer antigen" refers to
an antigen molecule which will be newly expressed in
association with canceration of a normal cell. Such a
cancer antigen includes, but is not limited to, for
example:
(1) tumor virus derived antigens (for example, T
antigens or the like from DNA type tumor virus such as
adenovlrus, polyoma virus, SV40 and the like). In RNA-type
tumor virus of human or mouse, viral envelope proteins are
expressed on the cellular surface;
(2) tumor specific transplantation antigen (TSTA);
this antigen refers to a target antigen of a cancer cell of
the same lineage, when the cancer cell is rejected as a
result of formation of a specific immune response. Genetic
mutations cause variant proteins in a cancer cell, which
allows expression thereof on the cellular surface of the
cancer cell by association with a molecule of major
histocompatibility (MHC) antigen gene complex as peptide
fragments, as in other intracellular normal proteins.;
(3) tumor associated antigen (TAA): antigens which
exhibit specific expression in association with canceration,
although it is not necessarily specific to the cancer cell.
CA 02808179 2013-02-28
-87-
For example, it corresponds to a-fetoprotein in liver
cancer, carcinoembryonic antigen (CEA) in enteric cancer
and the like. These are proteins which are originally
present only in normal fetuses, and are not found in the
tissues of an adult. However, these proteins are called
oncofetal antigens as re-expression will occur with the
canceration.
[0261]
As used herein, any form of cancer antigen may be
used, and in particular, a form of carcinoma-related
antigen is preferably used. This is because it will be
expressed on the surface of a cancer cell upon association
with MHC.
[0262]
As used herein, the term "adjuvant" refers to a
substance which increases, or otherwise alters, immune
response when mixed with immunogen administered thereinto.
Adjuvants are classified in view of minerals, bacteria,
plants, synthetic, or products of a host, for example.
[0263]
As used herein, the term "pathogen" refers to an
organism or agent which allows onset of a disease or a
disorder to a host.
[0264]
As used herein, the terms "prophylaxis",
"prophylactic" "prevention" and "prevent" refer to a
treatment of a disease or a disorder, in which such a
disease or disorder should not be caused prior to the
actual onset thereof.
[0265]
As used herein, the terms "therapy",
"treatment" and "treat" refer to a treatment in which in
CA 02808179 2013-02-28
- 88 -
the case where such occurs, deterioration of such a disease
or disorder is prevented, preferably, at least maintaining
the status quo, more preferably, alleviation further more
preferably, cleared.
[0266]
The vaccines of the present invention are
= preferably tested in vitro, and then in vivo for the
desired therapeutic or prophylactic activity, prior to use
in humans. For example, in vitro assays to demonstrate the
therapeutic or prophylactic utility of vaccines according
to the present invention include testing the effect of a
vaccine on a cell line or a patient tissue sample. The
effect of the vaccines on the cell line and/or tissue
sample can be determined utilizing techniques known to
those of skill in the art (for example, immunological assay
such as ELISA). In vivo tests include but are not limited
to: for example, a method for testing whether a
neutralizing antibody is raised.
[0267]
As used herein the term "patient" or "subject"
refers to an organism to which the treatment or composition
of the present invention is applied.
Preferably, the
patient may be a human.
[02681
The present invention provides methods of
treatment, inhibition and prophylaxis by administration to
a subject of an effective amount of a gene vaccine of the
present invention. In a preferred aspect, the compound is
substantially purified (e. g., substantially free from
substances that limit its effect or produce undesired side-
effects).
[0269]
CA 02808179 2013-02-28
-89-
As used herein, the term "administer" means
that the polypeptides, polynucleotides or the like of the
present invention or pharmaceutical. compositions containing
them are incorporated into the cells, tissue or body of an
organism either alone or in combination with other
therapeutic agents.
Combinations may be administered
either concomitantly (e.g., as an admixture), separately
but simultaneously or concurrently; or sequentially. This
includes presentations in which the combined agents are
administered together as a therapeutic mixture, and also
procedures in which the combined agents are administered
separately but simultaneously (e.g., as through separate or
the same mucosa into the same individual). "Combination"
administration further includes the separate administration
of one of the compounds or agents given first, followed by
the second.
[0270]
Administration of vaccines according to the present
invention may be conducted in any manner, and preferably it
.is advantageous to use a needleless syringe. This is
because it can administer without causing undue load to a
patient.
[0271]
As used herein the term "needleless syringe" refers
to a medical device which transfers a drug solution into
the skin by moving a piston by gas pressure or elasticity
of an elastic member, thereby administering a drug
component into subcutaneous or preferably into the cell's
subcutaneous site.
[0272]
Specifically, for example, Shimajet"4 (manufactured
by Shimadzu, inc.), Medi-Jector Visionm (manufactured by
CA 02808179 2013-02-28
-90-
Elitemedica), PenJeem (manufactured by PenJet), which are
commercially available. Gene gun (particle gun) refers to
a medical and experimental device which allows in vivo gene
introduction by accelerating high density particles such as
gold or tungsten coated with DNA using gas pressure of
helium or the like.
Advantageous effects of gene guns
include effective intracellular, introduction of a low
amount of DNA, and stable results have been obtained with
different operators.
[0273]
Specifically, for example, Helios Gene Gun from Bio-
Rad, USA is commercially available.
[0274]
As used herein, the term "instructions" refers
to a description of the method of the present invention for
a person who performs administration, such as a medical
doctor, a patient, or the like. Instructions state when to
administer a medicament of the present invention, such as
immediately after or before radiation therapy (e.g., within
24 hours, etc.). The
instructions are prepared in
accordance with a format defined by an authority of a
country in which the present invention is practiced (e.g.,
Health, Labor and Welfare Ministry in Japan, Food and Drug
Administration (FDA) in the U.S., and the like), explicitly
describing that the instructions are approved by the
authority. The instructions are so-called package insert
and are typically provided in paper media. The
instructions are not so limited and may be provided in the
form of electronic media (e.g., web sites, electronic mails,
and the like provided on the Internet).
[02751
CA 02808179 2013-02-28
-91-
The judgment of termination of treatment or prevention with
a method of the present invention may be supported by the
result of an antibody raised using a commercially available
assay or device.
[0276]
The present invention also provides a
pharmaceutical package or kit comprising containers loaded
With one or more pharmaceutical compositions according to
the present invention. A notice in a form defined by a
government agency which regulates the production, use or
sale of pharmaceutical products or biological products may
be arbitrarily attached to such a container, representing
the approval of the government agency relating to
production, use or sale with respect to administration to
humans.
[0277]
(General techniques used herein)
Techniques used herein are within the technical
scope of the present invention unless otherwise specified.
These techniques are commonly used in the fields of sugar
chain science, fluidics, microfabrication, organic
chemistry, biochemistry, genetic engineering, molecular
biology, microbiology, genetics, and their relevant fields.
The techniques are sufficiently well described in documents
described below and other documents mentioned herein.
[0278]
Microfabrication is described in, for example,
Campbell, S.A, (1996), The Science and Engineering of
Microelectronic Fabrication, Oxford University Press; Zaut,
P.V. (1996), Micromicroarray Fabrication: a Practical Guide
to Semiconductor Processing, Semiconductor Services; Madou,
M.J. (1997), Fundamentals of Microfabrication, CRC1 5
CA 02808179 2013-02-28
- 92 -
Press; Rai-Choudhury, P. (1997). Handbook of
Micro lithography, Micromachining &
Microfabrication:
Microlithography.
[0279]
Molecular biology techniques, biochemistry
techniques, and microbiology techniques used herein are
well known and commonly used In the art, and are described
in, for example, Maniatis, T. et al. (1989), Molecular
Cloning: A Laboratory Manual, Cold Spring Harbor and its
3rd Ed. (2001); Ausubel, F.M. et al: ads, Current Protocols
in Molecular Biology, John Wiley & Sons Inc., NY, 10158
(2000); Innis, M.A. (1990), PCR Protocols: A Guide to
Methods and Applications, Academic Press; Innis, M.A. at al.
(1995), PCR Strategies, Academic Press; Sninsky, J.J. at al.
(1999), PCR Applications: Protocols for Functional Genomics,
Academic Press; Gait, M.
.1. (1985), Oligonucleotide
Synthesis: A Practical Approach, IRL Press; Gait, M.J.
(1990), Oligonucleotide Synthesis: A Practical Approach,
IRL Press; Eckstein, F. (1991), Oligonucleotides and
Analogues: A Practical Approac, IRL Press; Adams, R.L. at
al. (1992), The Biochemistry of the Nucleic Acids, Chapman
& Hall; Shabarava, Z. et al. (1994), Advanced Organic
Chemistry of Nucleic Acids, Weinheim; Blackburn, G.M. at al.
(1996), Nucleic Acids in Chemistry and Biology. Oxford
University Press; Hermanson, G.T. (1996), Bioconjugate
Techniques, Academic Press; Method in Enzymology 230, 242,
247, Academic Press, 1994; Special issue, Jikken Igaku
(Experimental Medicine) 'Idenshi Donyu & Hatsugenkaiseki
Jikkenho (Experimental Method for Gene introduction &
Expression Analysis)", Yodo-sha, 1997; and the like.
CA 02808179 2013-02-28
- 93-
[0280]
(Description of preferred embodiments)
Hereinafter, the present invention will be
described by way of embodiments.
Embodiments described
below are provided only for illustrative purposes.
Accordingly, the scope of the present invention is not
limited by the embodiments except as by the appended claims.
It will be clearly appreciated by those skilled in the art
that variations and modifications can be made without
departing from the scope of the present invention with
reference to the specification.
[0281]
In an aspect, the present invention provides MIE
promoters of HHV (including HHV6A and HHV6B, in particular
HHV6B) and HHV7, and/or U95 promoter of HHV7. In
particular, it has been discovered that MIE promoter of
HHV6B, MIE promoter of HHV7, and U95 promoter of HHV7 are
surprisingly enhanced in selectivity to lymphocytes in
comparison to IE promoters of HCMV. In
particular,
adhesive cells (293 cells, Vero cells and the like) only
showed one hundredth the activity of that of HCMV IE
promoter, whereas in lymphoid cells such as SupT1, U937 and
the like, a several fold increase in expression efficiency
has been obtained. Such a high level of selectivity or
specificity elucidated that it can be applied to the
development of a pharmaceutical which is targeted to DNA
vaccines, gene therapy, in particular, to lymphocytes.
Moreover, in an expression system in vivo, since activities
are diminished even in the case of CMV promoters which have
potent activity, due to the action of methylase, it is
CA 02808179 2013-02-28
-94-
understood that the promoter of the present invention may
be used to secure expression amount in vivo in blood cells
or lymphocyte cells. In genetic diseases, gene therapy of
cancer, retroviruses are generally used, however, LTR
activity is not so potent, as a promoter, the introduction
of the promoter of the present invention upstream of the
gene to be expressed allows potent expression in blood cell
lineage cells. The present invention is also useful in
gene therapy targeting blood cell diseases such as leukemia
and the like. Furthermore, RNAi is used as a method of
knocking out gene expression, and the promoter of the
present invention is used as a promoter for hair-pin type
RNA expression vectors, allowing more efficient effects of
inhibition of expression in the blood cell lineage.
Macrophages or dendritic cells or the like are purified
from native peripheral blood using flow cytometry, and
these cells are transfected with plasmids constructed so as
to express cancer specific antigen or tumor necrosis factor
(TNF) or the like under the control of the promoter of the
present invention, and reintroduced to the original body
after confirmation of expression of cancer antigen, thereby
practicing the gene therapy of cancer as a result of
efficient activation of tumor antigen specific CTL via
Glass I-HLA.
[0282]
In one embodiment, the promoters of the present
invention may have a length of at least 8 contiguous
nucleotides.
Preferably, the promoter of the present
invention includes at least the R3 region or the functional
variant thereof, amongst the sequence set forth in SEQ ID
NO: 1. More
preferably, the promoter of the present
invention includes at least the sequence of -574 to -427
CA 02808179 2013-02-28
- 95 -
from the transcription initiation point of the SEQ ID NO:
1; more preferably, at least the sequence of -1051 to -427
from the transcription initiation point of the SEQ ID NO: 1.
This is because it is predicted that these regions have
regions having enhancer activity.
[0283]
In one embodiment, the promoter of the present
invention comprises NF-KB and AP-1 motives.
[0284]
In a preferable embodiment, the promoter of the
present invention comprises a sequence set forth in SEQ ID
= NO: 1, and more preferably consists essentially of the
sequence set forth in SEQ ID NO: 1.
[0285]
In one embodiment, the promoter of the present
invention comprises: (a) a polynucleotide having the base
sequence set forth in SEQ ID NO: 1, or the base sequence
corresponding thereto or a fragment sequence thereof; (b) a
polynucleotide of an allelic variant of the base sequence
set forth in SEQ ID NO: 1 or the base sequence
= corresponding thereto or a fragment sequence thereof; (c) a
polynucleotide which hybridizes a polynucleotide of any of
(a) or (b) and has a biological activity thereof; or (d) a
polynucleotide which consists of the base sequence of any
of (a) to (c) or a complement sequence thereof with at
least 70 % identity, and has a biological activity thereof.
As used herein, the biological activity may be promoter
and/or enhancer activities but is not limited thereto.
Promoter and enhancer activities may be measured using well
known technology in the art, and such a technology is
described herein and exemplified in the Examples.
[0286]
CA 02808179 2013-02-28
-96-
In one preferred embodiment, the number of
substitutions, additions and deletions described in (a)
through (d) above may be limited to, for example,
preferably 50 or less, 40 or less, 30 or less, 20 or less,
15 or less, 10 or less, 9 or less, 8 or less, 7 or less, 6
or less, 5 or less, 4 or less, 3 or less, or 2 or less.
The number of substitutions, additions and deletions is
preferably small, but may be large as long as the
biological activity is maintained (preferably, the activity
is similar to or substantially the same as that of HEV6B
MIE promoter).
[0287]
In a preferred embodiment, the identity to any
one of the polynucleotides described in (a) to (d) above or
a complementary sequence thereof may be at least about 80%,
more preferably at least about 90%, even more preferably at
least about 98%, and most preferably at least about 99%.
[0288]
In a preferred embodiment, the nucleic acid
molecule of the present invention may have a length of at
least 8 contiguous nucleotides. The appropriate nucleotide
length of the nucleic acid molecule of the present
invention may vary depending on the purpose of use of the
present invention. More preferably, the nucleic acid
molecule of the present invention may have a length of at
least 10 contiguous nucleotides, even more preferably at
. . . _
least 15 contiguous nucleotides, and still even more
preferably at least 20 contiguous nucleotides. These lower
limits of the nucleotide length may be present between the
above-specified numbers (e.g., 9, 11, 12, 13, 14, 16, and
the like) or above the above-specified numbers (e.g., 21,
22, ¨ 30, and the like). The upper limit of the length of
CA 02808179 2013-02-28
-97- - =
the polypeptide of the present invention is not limited as
long as it can be used for the intended purpose (e.g.
promoter). Stringency may be high, or intermediate or low,
and the level of stringency may be appropriately determined
according to the circumstances.
[0289]
In a different embodiment, the promoter according to
the present invention may have a length of at least 8
contiguous nucleotides. Preferably, the promoter of the
present invention includes at least the R2 region or the
functional variant thereof, amongst the sequence set forth
in SEQ ID NO; 2. More preferably, the promoter of the
present invention includes at least the sequence of -388 to
+22 from the transcription initiation point of the SEQ ID
NO: 2; more preferably, at least the sequence of -493 to
+22 from the transcription initiation point of the SEQ ID
NO: 2.
This is because it is predicted that these regions
contain regions having enhancer activity.
[0290]
In one embodiment, the promoter according to the
present invention includes NF-KB motifs (-464 to -478 and -
359 to -350 in SEQ ID NO: 2).
[0291]
=
In a preferable embodiment, the promoter of the
present invention comprises, the sequence set forth in SEQ
ID NO: 2, and more preferably, consists essentially of the
sequence set forth in SEQ ID NO; 2.
[0292]
In one embodiment, the promoter of the present
invention comprises: (a) a polynucleotide having the base
sequence set forth in SEQ ID NO: 2, or the base sequence
corresponding thereto or a fragment sequence thereof; (b) a
CA 02808179 2013-02-28
-98-
polynucleotide of an allelic variant of the base sequence
set forth in SEQ ID NO: 2 or the base sequence
corresponding thereto or a fragment sequence thereof; (c) a
polynucleotide which hybridizes a polynucleotide of any of
(a) or (b) and has a biological activity thereof; or (d) a
polynucleotide which consists of the base sequence of any
of (a) to (c) or a complement sequence thereof with at
least 70 % identity, and has a biological activity thereof.
As used herein, the biological activity may be promoter
and/or enhancer activities but is not limited thereto.
Promoter and enhancer activities may be measured using well
known technology in the art, and such a technology is
described herein and exemplified in the Examples.
[0293]
In one preferred embodiment, the number of
substitutions, additions and deletions described in (a)
through (d) above may be limited to, for example,
preferably 50 or less, 40 or less', 30 or less, 20 or less,
15 or less, 10 or less, 9 or less, 8 or less, 7 or less, 6
or less, 5 or less, 4 or less, 3 or less, or 2 or less.
The number of substitutions, additions and deletions is
preferably small, but may be large as long as the
biological activity is maintained (preferably, the activity
is similar to or substantially the same as that of HHV7 MIE
promoter).
[0294]
In a preferred embodiment, the identity to any
one of the polynucleotides described in (a) to (d) above or
a complementary sequence thereof may be at least about 80%,
more preferably at least about 90%, even more preferably at
least about 98%, and most preferably at least about 99%.
[0295]
CA 02808179 2013-02-28
- 99 -
In a preferred embodiment, the nucleic acid
molecule of the present invention may have a length of at
least 8 contiguous nucleotides. The appropriate nucleotide
length of the nucleic acid molecule of the present
invention may vary depending on the purpose of use of the
present invention. More
preferably, the nucleic acid
molecule of the present invention may have a length of at
least 10 contiguous nucleotides, even more preferably at
least 15 contiguous nucleotides, and still even more
preferably at least 20 contiguous nucleotides. These lower
limits of the nucleotide length may be present between the
above-specified numbers (e.g., 9, 11, 12, 13, 14, 16, and
the like) or above the above-specified numbers (e.g., 21,
22, ¨ 30, and the like). The upper limit of the length of
the polypeptide of the present invention is not limited as
long as it can be used for the intended purpose (e.g.
promoter). Stringency may be high, or intermediate or low,
and the level of stringency may be appropriately determined
according to the circumstances.
[0296]
In another embodiment, the promoter of the present
invention may have a length of at least 8 contiguous
nucleotides.
Preferably, the promoter of the present
invention includes at least the R2 region or the functional
variant thereof, amongst the sequence set forth in SEQ ID
NO; 12. More
preferably, the promoter of the present
invention includes at least the sequence of -379 to +16
from the transcription initiation point of the SEQ ID NO:
12; more preferably, at least the sequence of -484 to +16
from the transcription initiation point of the SEQ ID NO:
12. This is because it is predicted that these regions
containing regions having enhancer activity.
CA 02808179 2013-02-28
-100-
[0297]
In one embodiment, the promoter according to the
present invention includes NF-KB motifs (-478 to -469 and -
373 to -364 in SEQ ID NO: 12).
[0298]
In a preferable embodiment, the promoter of the
present invention comprises, the sequence set forth in SEQ
ID NO: 12, and more preferably, consists essentially of the
sequence set forth in SEQ ID NO; 12.
[0299]
In one embodiment, the promoter of the present
invention comprises: (a) a polynucleotide having the base
sequence set forth in SEQ ID NO: 12, or the base sequence
corresponding thereto or a fragment sequence thereof; (b) a
polynucleotide of an allelic variant of the base sequence
set forth in SEQ ID NO: 12 or the base sequence
corresponding thereto or a fragment sequence thereof; (c) a
polynucleotide which hybridizes a polynucleotide of any of
(a) or (b) and has a biological activity thereof; or (d) a
polynucleotide which consists of the base sequence of any
of (a) to (c) or a complement sequence thereof with at
least 70 % identity, and has a biological activity thereof.
As used herein, the biological activity may be promoter
and/or enhancer activities but is not limited thereto.
Promoter and enhancer activities may be measured using well
known technology in the art, and such a technology is
described herein and exemplified in the Examples.
[0300] In one preferred embodiment, the number
of substitutions, additions and deletions described in (a)
through (d) above may be limited to, for example,
preferably 50 or less, 40 or less, 30 or less, 20 or less,
15 or less, 10 or less, 9 or less, 8 or less, 7 or less, 6
CA 02808179 2013-02-28
-101-
or less, 5 or less, 4 or less, 3 or less, or 2 or less.
The number of substitutions, additions and deletions is
preferably small, but may be large as long as the
biological activity is maintained (preferably, the activity
is similar to or substantially the same as that of the HHV7
U95 promoter).
[0301]
In a preferred embodiment, the identity to any
one of the polynucleotides described in (a) to (d) above or
a complementary sequence thereof may be at least about 80%,
more preferably at least about 90%, even more preferably at
least about 98%, and most preferably at least about 99%.
[0302]
In =a preferred embodiment, the nucleic acid
molecule of the present invention may have a length of at
least 8 contiguous nucleotides. The appropriate nucleotide
length of the nucleic acid molecule of the present
invention may vary depending on the purpose of use of the
present invention. More
preferably, the nucleic acid
molecule of the present invention may have a length of at
least 10 contiguous nucleotides, even more preferably at
least 15 contiguous nucleotides, and still even more
preferably at least 20 contiguous nucleotides. These lower
limits of the nucleotide length may be present between the
above-specified numbers (e.g., 9, 11, 12, 13, 14, 16, and
the like) or above the above-specified numbers (e.g., 21,
22, ¨ 30, and the like). The upper limit of the length of
the polypeptide of the present invention is not limited as
long as it can be used for the intended purpose (e.g.
promoter). Stringency may be high, or intermediate or low,
and the level of stringency may be appropriately determined
according to the circumstances.
CA 02808179 2013-02-28
-102-
[0303]
In another aspect, the present invention provides a
nucleic acid construct comprising a promoter of the present
invention (MIE promoter of HHV6, MIE promoter of HHV7, 1395
promoter of HHV7 and the like). Such a
nucleic acid
construct has a property of inducing expression in a
lymphocyte specific manner, and the utility thereof is high,
and exhibits unexpectedly high selectivity in comparison to
human cytomegalovirus (HCMV) IE promoter.
[0304]
Accordingly, in one embodiment, the nucleic
acid construct of the present invention comprises a
sequence encoding a foreign gene having a different origin
than the promoter of the present invention, with a sequence
of the present invention operably linked thereto.
[0305]
Such a foreign gene includes, but is not limited to,
for example, those encoding an RNAi molecule, drug
resistance, a recessive gene to be deleted, a selective
marker and the like.
[0306]
Preferably, selective markers used in the present
invention are those allowing selection in a medium for the
host into which the nucleic acid construct is introduced,
. and for example, these selective markers may be those
allowing visible selection in the host into which the
nucleic acid construct is introduced, and exemplifies
hypoxanthine guanine transferase (hprt) or a fluorescent
marker selected from the group consisting of green
fluorescent protein (GFP), cyan fluorescent protein (CFP),
yellow fluorescent protein (IF?) and red fluorescent
protein (dsRed) and the like.
CA 02808179 2013-02-28
-103-
[0307]
Preferably, selective markers included in the nucleic
acid construct of the present invention are advantageously
.those substantially exhibiting no toxicity against the host
into which the nucleic acid construct is introduced
according to the present invention. This is because, when
using the present invention for the purpose of therapy or
prevention, adverse effects should be preferably none.
[0308]
Those to be included in the nucleic acid
construct according to the present invention include for
example, a recessive gene to be deleted. As used herein, a
recessive gene to be deleted refers to any recessive gene
which exhibits diseased condition when deleted, and
includes, but is not limited to, for example: ADA gene
(which is related to severe combined immunodeficiency
(SCID)), PNP gene (severe combined immunodeficiency (SCID)),
y c chain gene (which is related to severe combined
immunodeficiency (SCID)). TAP gene (which is related to MHC
I deficiency), MHC II gene (which is related to MHC II
deficiency), X-linked WASP (which is related to Wiskott-
Aldrich syndrome), CD40 ligand (which is related to X-
linked high IgM syndrome), PI3K-like gene (which is related
to granuloma telangiectaticum) and DNA helicase (which is
related to Bloom's syndrome), and the like.
[0309]
In a preferable embodiment, drugs to be included in
the nucleic acid construct of the present invention may be
proteineous agents such as a cytokine, a chemokine, a
growth factor, a protein hormone, and a peptide hormone
such as IFN-a, IFN-y, IL-2, IL-12, G-CSF, GM-CSF and the
like.
CA 02808179 2013-02-28
-104-
[0310]
In one embodiment, in the nucleic acid construct of
the present invention the promoter induces specific
expression of the foreign gene in a hemocyto-lineage cell,
in particular, in a T cell.
[0311]
In another aspect, the present invention provides an
expression vector comprising the nucleic acid construct
according to the present invention. Such
an expression
vector may include elements essential to expression, which
may not exist in the nucleic acid construct of the present
invention, for example, terminator,' enhancer sequences, in
an operably linked manner, which allow expression in the
host.
[0312]
In another preferable embodiment, selective markers
may be immortalizing genes (for example bc1-2).
Alternatively, selective markers may be hypoxanthine
guanine phosphoribosyl transferase (hprt), a gene encoding
a toxic product, a toxic gene product depending on a
condition in combination with a suicide substrate (for
example, herpes simplex virus thymidine kinase (HSV-TK) in
combination with acyclovir.
[0313]
In another aspect, the present invention provides a
cell comprising the nucleic acid construct according to the
present invention. Such a
cell, in the case of a
lymphocyte, promotes the expression of a protein encoding a
foreign gene.
[0314]
Preferably, it may be advantageous that the cell of
the present invention is heterogenous to the promoter
CA 02808179 2013-02-28
-105-
sequence of the present invention. It is
one of the
surprising effects to have promoter activity even if the
cell is heterogenous. A method for introducing a nucleic
acid into a cell used in the present invention are well
known in the art, and described in detail hereinabove.
Alternatively, such a cell may be identified by screening a
cell comprising the nucleic acid molecule in a sample
comprising the same. The cell comprising the nucleic acid
molecule according to the present invention may preferably
be in an undifferentiated state. The cells expressing the
nucleic acid molecule of the present invention is usually
in a state of undifferentiation. Accordingly, *a cell into
which such a nucleic acid molecule has been introduced so
as to be expressed in a controllable manner, may be
controlled with respect to the undifferentiated state.
Alternatively, such a cell may be used to produce a large
amount of the nucleic acid according to the present
invention. Such production methods are well known in the
art and are described in the literature described herein.
[0315]
In another aspect, the present invention
provides a tissue comprising the nucleic acid construct
according to the present invention. Such a nucleic acid
sequence is preferably operably linked to a control
sequence. Such an
organ may be an animal tissue, or a
tissue of a different organism such as a plant.
Alternatively, such a tissue is used to produce a nucleic
acid molecule according to the present invention in a large
amount. Such a production method is well known in the art,
and described in the reference described herein.
[0316]
CA 02808179 2013-02-28
-106-
In another aspect, the present invention
provides an organ comprising the nucleic acid construct
according to the present invention. Such a nucleic acid
sequence is preferably operably linked to a control
sequence. Such an
organ may be an animal organ, or an
organ of a different organism such as a plant.
Alternatively, such an organ is used to product a nucleic
acid molecule according to the present invention in a large
amount. Such a production method is well known in the art,
and described in the reference described herein.
[0317]
In another aspect, the present invention
provides an organism comprising the nucleic acid construct
according to the present invention. Such an organism is
used to product a nucleic acid molecule according to the
present invention in a large amount. Such a production
method is well known in the art, and described in the
reference described, herein.
= [0318]
In another aspect, the present invention
provides a pharmaceutical composition comprising the
promoter according to the present invention. As
used
herein, antigen used may be any proteins desired to raise
immune response in a host. Such antigens include, but are
not limited to, for example, cancer antigen and the like.
Accordingly, the pharmaceutical composition according to
the present invention may preferably be DNA vaccine.
[0319]
In another aspect, the present invention provides a
pharmaceutical composition for treating a disease, disorder
or condition in which a lymphocyte-specific treatment is
desired, which comprises the promoter according to the
CA 02808179 2013-02-28
- 107 -
present invention, and a nucleic acid sequence for the
treatment. As
used herein, the target of the
pharmaceutical composition may appropriately be any
diseases, disorders, conditions and the like desired to
have lymphocyte specific treatment, and are exemplified by
acquired immunodeficiency syndromes.
Acquired
immunodeficiency syndromes include, severe combined
immunodeficiency (SCID), MHC I deficiency, MHC II
deficiency, Wiskott-Aldrich syndrome, X-linked high IgH
syndrome, granuloma telangiectaticum, Bloom's syndrome and
the like. Although not wishing to be bound by theory,
acquired immunodeficiency syndrome is caused by some
deficiency in a recessive gene (which is herein also called
a recessive gene to be deleted). It is thus possible to
carry out somatic gene therapy in which this gene to be
deleted is introduced to bone marrow cells taken from a
patient then the cells are reintroduced into the patient.
In this regard, the HHV6B MIE promoter of the present
invention is likely employed to increase the gene
expression efficiency in a cell differentiated into T cell
or macrophage and the like. Introduction of such a gene
construct is, for example, possible by using retrovirus and
the like.
[0320]
In a preferable embodiment, the nucleic acid
sequences for the treatment include a sequence selected
from the group consisting of those encoding cytokines,
chemokines, growth factors, protein hormones, peptide
hormones, ribozymes and siRNA (HIV-1 gp41:
AATAAGACAGGGCTTGGAAAGACACTTTCCAAGCCCTGTCTTATTTTT(SEQ ID NO:
33)/HIV-1 tat:
AAGCATCCAGGAAGTCAGCCTACAAGGCTGACTTCCTGGATGCTTTTT(SEQ ID NO:
CA 02808179 2013-02-28
- 108 -
34)/HTLV-1
tax:
GAACATTGGTGAGGAAGGCACAGCCTTCCTCACCAATGTTCTTTTT(SEQ ID NO:
35)).
[0321]
In another aspect, the present invention provides a
method for expressing a protein in a lymphocyte specific
manner, comprising the steps of: A) preparing a nucleic
acid construct in which the promoter according to the
present invention is operatively linked to a nucleic acid
sequence encoding the protein; and B) placing the nucleic
acid construct under a condition in which the promoter.
induces the expression of the nucleic acid sequence
encoding the protein.
[0322]
In another aspect, the present invention provides a
kit for expressing a protein in a lymphocyte specific
manner, comprising: A) a nucleic acid construct in which
the promoter according to the present invention is
operatively linked to a nucleic acid sequence encoding the
. 20 protein; and B) means for placing the nucleic acid
construct under a condition in which the promoter induces
the expression of the nucleic acid sequence encoding the
protein.
[0323]
In another aspect, the present invention further
provides a kit for expressing a protein in a lymphocyte
specific manner, comprising: A) the promoter according to
the present invention; and B) means for producing a nucleic
acid construct in which the promoter is linked to a nucleic
acid sequence encoding the protein.
[0324]
CA 02808179 2013-02-28
- 109 -
In another aspect, the present invention further
provides a method for treating or preventing a disease,
disorder or condition which requires the expression of a
protein in a lymphocyte specific manner, comprising the
steps of: A) producing a nucleic acid construct in which
the promoter according to the present invention is linked
to a nucleic acid sequence encoding the protein; and B)
placing the nucleic acid construct under a condition in
which the promoter induces the expression of the nucleic
acid sequence encoding the protein.
[0325]
In another aspect, the present invention further
provides a kit for treating or preventing a disease,
disorder or condition which requires the expression of a
protein in a lymphocyte specific manner, comprising: A) a
nucleic acid construct in which the promoter according to
the present invention is linked to a nucleic acid sequence
encoding the protein; and B) means for placing the nucleic
acid construct under a condition in which the promoter
induces the expression of the nucleic acid sequence
encoding the protein.
(0326]
In another aspect, the present invention further
provides a kit for treating or preventing a disease,
disorder or condition which requires the expression of a
protein in a lymphocyte specific manner, comprising: A) the
promoter according to the present invention; and B) means
for producing a nucleic acid construct in which' the
promoter is linked to a nucleic acid sequence encoding the
protein.
[0327]
CA 02808179 2013-02-28
-110- OBK008
In another aspect, the present invention further
provides a method for producing a protein, comprising the
steps of: A) preparing a nucleic acid construct in which
the promoter according to the present invention is linked
to a nucleic acid sequence encoding the protein; and B)
placing the nucleic acid construct under a condition in
which the promoter induces the expression of the nucleic
acid sequence encoding the protein.
[0328]
In another aspect, the present invention further
provides a kit for producing a protein, comprising: A) a
nucleic acid construct in which the promoter according to
the present invention is linked to a nucleic acid sequence
encoding the protein; and B) means for placing the nucleic
acid construct under a condition in which the promoter
induces the expression of the nucleic acid sequence
encoding the protein.
[0329]
In another aspect, the present invention further
provides a kit for producing a protein, comprising: A) the
promoter according to the present invention; and B) means
for producing a nucleic acid construct in which the
promoter is linked to a nucleic acid sequence encoding the
protein.
[0330]
In another aspect, the present invention further
provides use of the promoter according to the present
invention, for manufacture of a pharmaceutical composition
for treating or preventing a disease, disorder or condition
which requires the expression of a protein in a lymphocyte
specific manner.
[0331]
CA 02808179 2013-02-28
-111-
[0332]
The preferred embodiments of the present
invention have been heretofore described for a better
understanding of the present invention. Hereinafter, the
present invention will be described by way of examples.
Examples described below are provided only for illustrative
purposes. Accordingly, the scope of the present invention
is not limited except as by the appended claims.
EXAMPLES
[0333]
Handling of animals used in the following Examples
are in accordance with the provisions set forth in Osaka
University.
[0334]
(EXAMPLE 1: Search for HHV6B promoters and
Development of DNA vaccines)
With respect to promoters of immediate early protein
of HHV-6 (9U.2017,141E.U95.MIE/3K.U95/3K, which are different
in size), the activity thereof was compared to that of
cytomegalovirus (CMV) promoter. With respect to methods,
the respective promoter regions were inserted upstream of
the luciferase gene of pGL3-Basic Vector (Promega), which
were transfected with the respective cells to compare the
activity thereof using luciferase activity as reference.
Hereinafter, the details of materials and methods are
described.
[0335]
(Materials and Methods)
CA 02808179 2013-02-28
-112-
(Outline)
The promoter region of MIE gene of HHV-6B (about
1.2kbp) was cloned, which was linked to an outer membrane
glycoprotein of Japan encephalitis virus Beijing-1 strain
cDNA downstream thereof to construct the plasmid p9u/JEVenv.
Green fluorescence protein expression plasmid pEGFP-N1 used
was commercially available (available from Clontech).
[0336]
It was constructed using a plasmid (pcDNA3.1/JEVenv)
as reference in which JEVenv was linked downstream of HCMV-
IE promoter of pcDNA3.1Zeo+ vector. Green
fluorescence
protein expression plasmid pEGFP-N1 used was commercially
available (available from BD Biosciences).
Furthermore,
luciferase expression plasmid used herein was that which
has already been constructed (pGL3-Basic; available from
Promega).
[0337]
These plasmids were introduced to the following
cells: 293 cell (derived from human kidney), Vero
cell(derived from simian kidney), SupT1 cell (derived from
human T lymphocyte), U937 cell (derived from human
monocyte) and the like (these cells are available from
American Type Culture Collection (ATCC), RIKEN Cell Bank,
Gene banks and the like). The expression of outer membrane
glycoprotein in a cell was studied using indirect
fluorescence antibody method using anti-JEV polyclonal
antibody, and Western blot with cell extract thereof.
[0338]
HHV-6MIE promoter region was inserted upstream of
firefly luciferase gene of luciferase vector pGL3-Basic
(Promega) to form p9u, which was used to prepare truncated
CA 02808179 2013-02-28
-113-
mutants by removing bases by Mung Bean Exonuclease from
upstream of MIE promoter.
[0339]
Vero cell was transfected with these truncated
mutants and Renilla luciferase expression plasmid for
transfection efficiency correction (phRL-SV40) by the
lipofection method. Cells were collected 24 hours after
the transfection, and cell lysis solution was added thereto.
Thereafter, luminescent level was measured in the firefly
luciferase and Renilla luciferase in the lysate. In order
to correct the efficiency of transfection, the luminescence
level of the firefly luciferase was divided by that of
Renilla luciferase.
[0340]
1) Cells
The following eight types of cell lines were used for
promoter activity measurement.
[0341]
(1)Vero cell (derived from simian kidney)
(2)HEL cell (derived from human embryonic fibroblast cell)
(3)L929 cell (derived from murine fibroblast cell)
(4)293 cell (derived from human kidney)
(5)U373 cell (derived from human glioma)
(6)THP-1 cell (derived from human monocyte)
(7)SupT1 cell (derived from human T cell)
(8)U937 cell (derived from human monocyte)
(These cells are available from American Type Culture
Collection (ATCC)).
[0342]
2) Plasmids for the measurement of promoter activity
In order to measure promoter activity, pGL3-Basic
(Promega) having firefly luciferase gene was used. This
CA 02808179 2013-02-28
-114-
plasmid has no promoter sequence or enhancer sequence
derived from eukaryotic cells, a variety of base sequences
are introduced upstream of the luciferase gene, and the
amount of luciferase expressed is measured to allow
measurement of the promoter activity of the inserted
sequence.
[0343]
3) Promoter sequence with pGL3-Basic incorporated therein
For measurement, as described below, HHV-6MIE
promoter region, the promoter region of U95 gene, an
immediate early gene of HHV-6 and HCMV MIE promoter region
as commercially available expression vectors were used.
[0344]
HHV-6 promoter region was used after proliferating by PCR
and having inserted into pGL3-Basic.
[0345]
(1) 20u [one with HHV-6MIE promoter region
(1393814-
140624:1243bp) inserted thereinto] (SEQ ID NO: 5)
(2) 9u [one with HHV-6MIE promoter region
(1393814-
140427:1046bp) inserted thereinto] (SEQ ID NO: 6)
(3) MIE [one with HHV-6MIE promoter region (139457 4--
140211:7
54bp) inserted thereinto] (SEQ ID NO: 7)
(4) U95 [one with HHV-6 U95 gene promoter region (141823-4
142578:756bp) inserted thereinto] (SEQ ID NO: 8)
(5) CMV [one with HCMV MIE promoter excised from
commercially available expression vector (pcDNA3.1)
inserted thereinto:750bp] (SEQ ID NO: 9)
(6) MIE/3K [one with HHV-6MIE promoter region (1394434'-
142578:3136bp) inserted thereinto] (SEQ ID NO: 10)
(7) U95/3K [one with HHV-6MIE promoter region (139443-4
142578:3136bp) inserted thereinto] (SEQ ID NO: 11), as a
CA 02808179 2013-02-28
- 115 -
control, intact pGL3-Basic with no base sequence inserted
was used.
[0346]
Furthermore, a variety of deletion variants were
produced. These schematic figures are shown in Figure 5.
As variants, the following products were prepared as shown
in Figure 5.
[0347]
(1)9u: -1051 to +1 (SEQ ID NO: 5)
(2)9u-d2-7: -814 to +1 (SEQ ID NO: 17)
(3)9u-d1-4: -574 to +1 (SEQ ID NO: 18)
(4)9u-d1-5: -427 to +1 (SEQ ID NO: 19)
(5)9u-d1-7: -350 to +1 (SEQ ID NO: 20)
(6)9u-d3-7: -276 to +1 (SEQ ID NO: 21)
(7)9u-d5: -240 to +1 (SEQ ID NO: 22)
(8)9u-d6: -212 to +1 (SEQ ID NO: 23)
(9)9u-d7: -116 to +1 (SEQ ID NO: 24)
(10)9u-d8: -77 to +1 (SEQ ID NO: 25),
[0348]
4) Transfection of a cell with a plasmid
Transfection was conducted with Lipofection method
using SuperFecem (QIAGEN).
[0349]
In order to correct transfection efficiency,
expression plasmids of 8-galactosidase (pCH110, Pharmacia)
were simultaneously introduced to a cell, and 8-
galactosidase activity was measured. pCH110 expresses 8-
galactosidase under control of the early promoter of SV40.
[0350]
pGL3construct (8111) and pCH110 (0.2121) were mixed together
and Superfect reagent (8111) was added thereto to conduct
transfection.
CA 02808179 2013-02-28
- 116 -
( 035 11
5) Measurement of luciferase activity
Luciferase activity was measured using Luciferase
Assay System (Promega).
[0352]
pGL3construct and pCH110 were cotransfected, and 48
hours later, the cells were recovered. After twice washing
with PBS, it was dissolved into 150 g L of cell lysis
solution. One hundred gL of luciferase substrate solution
was added to the cell lysis solution supernatant (20 #L),
and thirty seconds later, luminescence was measured with a
luminometer.
[0353]
6) Measurement of B-galactosidase activity
B-galactosidase activity was measured using B-gal
reporter system (Clontech). To twenty # L of cell lysis
solution prepared in a similar manner as in the luciferase
activity measurement was added 100 g L of luminescent
substrate solution, and luminescence was measured after one
hour using a luminometer.
[0354]
7) Measurement of promoter activity under conditions
where cells were activated with TPA
The plasmids were transfected with Vero cells and
L929 cells, and 24 hours later, the cultures were conducted
in the presence and absence of TPA (25ng/m1) for an
additional 24 hours. Thereafter, the cells were collected,
and measured for the activity of luciferase and B-
galactosidase.
[0355]
(Results)
1) Promoter activity of the HHV-6 MIE region:
CA 02808179 2013-02-28
-117-
Promoter activity of HHV-6 MIE region and promoter
activity of HCMV MIE showed different behaviour in
endothelial adhesive cells and lymphocyte cells.
[0356]
(1) Comparison of promoter activities in adhesive
cells (Figure 1)
The promoter sequence of HHV-6MIE had weaker activity
than HCMV in adhesive cells, with some promoter activity.
The promoter of U95, a HHV-6 immediate early gene, showed
little activity. On the other hand, HCMV MIE promoter
showed about 10 to 50 fold more activity than that of HHV-6
MIE promoter in adhesive cells. In
particular, in HEL
cells and U373 cells, HCMV proliferation competent cells,
it showed potent activity.
[0357]
With respect to the promoter activity of the HHV-6
MIE region, those having the promoter region from 0.7kb to
1.2 kb in length showed substantially the same activity,
but reduction in the activity was recognized in the
sequence áf 3kb.
[0358]
(2) Comparison of promoter activities in lymphocyte
cells (Figure 2)
In lymphocyte cells which are proliferation competent
cells of HHV-6, the HHV-6 MIE region showed about ten times
higher promoter activity than HCMV. In
particular, it
showed potent activity in THP-1 and U937 which are cell
lines of monocytic macrophages. HCMV MIE promoter did not
exhibit so strong activity in lymphocytes.
[0359]
The promoter activity of the HHV-6 MIE region
increased the activity thereof in accordance with the
CA 02808179 2013-02-28
-118-
length from 0.7 kb to 1.2 kb in the promoter region,
however, the length of 3 kb reduced its promoter activity.
[0360]
2) Promoter activity of the HHV-6 MIE region when
stimulated by a cell with TPA (Figures 3 and 4)
Vero cells were stimulated with 12-0-tetradecanoyl
phorbol 13-acetate (TPA) to measure the promoter activity
of HHV-6 MIE, and all promoter activities were increased,
and showed substantially the same level as that of HCMV MIE
promoter (Figure 3).
[0361]
However, in L929 cells, no increase in promoter
activity was observed upon cell activation with TPA
stimulation (Figure 4). This is believed to be due to the
difference in reactivity of TPA on cell type.
[0362]
In Vero cells, it is believed that TPA increased the
HHV-6 MIE promoter activity by inducing a large amount of a
variety of transcriptional activation factors. That is,
the maximum activity of HHV-6 MIE promoter is as much as
HCMV MIE promoter. Therefore, the promoter of the present
invention has been demonstrated with respect to its
specificity and selectivity.
[0363]
As such, in the present invention, adhesive cells
such as Vero cells, HEL cells, L929 cells, 293 cells, U373
cells, CMV promoters showed ten times more potent activity
than that of HHV-6 (Figure 1). However, in cells derived
from human lymphocytes such as THP-1 cells, SupT1 cells,
U938 cells, several times as much activity as that of CMV
promoter was observed in HHV-6 promoter, and it was also
CA 02808179 2013-02-28
-119-
observed that the more truncated, the more potent activity
was found.
[0364]
Promising promoters from HHV-6 have been confirmed,
and from these results, it is understood that these
promoters can be applied to DNA vaccines (mumps vaccines)
and are extremely promising.
[0365]
HCMV IE promoter was used as a control to compare and
study the HHV-6B MIE promoter activity which has been
cloned by the present inventors, in a luciferase expression
system. As a result, in adhesive cells such as 293 cells,
Vero cells and the like, HHV-6MIE promoter only showed
about one tenth as much activity as that of HCMHE promoter.
However, in lymphocyte cells such as SupT1, and U937 and
the like, it was found that several times greater
expression efficiency was obtained. Conducting an assay on
the expression of the outer membrane glycoprotein of JEV by
using p9u/JEVenv linked to JEV cDNA downstream of the
subject promoter, no expression of the JEV protein was
detected in any adhesive cells or flowing lymphocytes after
48 hours of transfection.
[0366]
On the other hand, in pCDNA3.1/JEVenv using the IE
promoter of HCMV, the expression of JEV protein was
confirmed.
[0367]
Moreover, in JEV infected Vero cells, which were used
as a positive control, outer membrane glycoprotein was
readily detected. In order
to analyze the cause,
transfection efficiency was confirmed using GFP protein
expression plasmids. As a
result, the introduction
CA 02808179 2013-02-28
-120-
efficiency in SupT1 cell was as low as 0.1% or less,
however, adhesive 293 cells and Vero cells had a higher
introduction efficiency of 45 % and 20 %, respectively.
HHV-6 MIE promoter cloned, showed a several times higher
expression activity than HCMV MIE promoter in lymphocyte
cells. However, expressed gene was not detected with its
activity when it was converted to outer membrane
glycoprotein of JEV.
[0368]
It is of interest that the HHV-6 MIE promoter cloned
herein showed several times higher expression activity than
that of the HCMV-IE promoter in lymphocytes. However, it
was unpredictable that when the gene to be expressed had
been converted to outer membrane glycoprotein of JEV from
the reporter gene, no activity was detected. Therefore,
the cause thereof was analyzed as to whether expressed JEV
protein acted in a feedback manner, and thus the promoter
activity was inhibited in an adverse manner, or that
alternatively the expressed antigen is unstable in these
cells:
[0369]
The present Example is summarized as follows:
[0370]
1) HHV-6 MIE promoter showed about ten times higher
activity than HCMV MIE promoter in lymphocyte cells, in
particular, monocyte/macrophage cells.
[0371]
2) In epithelial adhesive cells, HHV6 MIE promoter
activity was about one tenth of that of HCMV MIE promoter.
[0372]
3) HHV-6 MIE promoter is suggested to exhibit
substantially the same activity as HCMV MIE promoter under
CA 02808179 2013-02-28
-121-
conditions where a large amount of a variety of
transcriptional factors was induced.
[0373]
As described above, in the present Example, those
which were inserted about 12kbp (6MIEP) upstream of the
major immediate early (MIE) gene of HFIV-6B and about 700 bp
upstream of U95 gene (6U95) upstream of the luciferase gene
of pGL3Basic vector (Promega) were used. In comparison to
the conventional promoters, in order to study the
possibility of the application of these IE promoters to DNA
vaccines, comparison with human cytomegalovirus '(HCMV) IE
enhancer-promoter (CMVP) in activity were conducted using
blood cell lineage cells. In the
present Example,
immediate early (IE) promoter encoded by human herpes virus
6B (HHV-6B) was demonstrated to have extremely high
activity in blood cell lineage cells.
[0374]
4) Furthermore, as depicted in Figure 7, it was shown
that activities in the respective fragments were
investigated, and at least -572 to -427 and in particular -
1051 to -427 upstream of the initiation point have promoter
activity with preferable enhancer activity. The site of -
417 to +1 appears to be necessary for promoter activity,
and the enhancer activity appears to be necessary to secure
specificity. The
portions responsible for enhancer
activity are elucidated to have NF-K B and AP-1 motifs.
Therefore, it appears that it is important to have these
motifs in order to have specificity in lymphocytes.
[0375]
(Example 2: MIE and U95 promoters of HHV-7)
Next, experiments relating to promoters from HHV-7
were conducted.
CA 02808179 2013-02-28
- 122 -
[ 0376 ]
The activity of two immediate early promoters of
HHV-7 (7MIEP.7U95P) were compared with the activity of
cytomegalovirus (CMV) promoter and HHV-6 IE promoter (9U
and U95). Methods of comparison are as follows: the
respective promoter regions were inserted upstream of the
luciferase gene of pGL3-Basic Vector (Promega), which were
transfected with the respective cells to compare the
activity thereof using luciferase activity as reference.
In order to study the effects of the R2 region present
upstream of the respective promoters, a variety of deletion
variants have been prepared to measure promoter activity. .
[0377]
(Outline)
As a reporter plasmid, about 500 bp from the
respective MIE and u95 genes of HHV-7 (7MIEP and 7U95P)
were inserted upstream of luciferase gene of pGL3Basic
vector (Promega) and used in the present Example.
[0378]
A reporter plasmid was introduced to T cell lines
(Jurkat.Molt-3,SupT-1), and bone marrow cell line (SAS-413)
with lipofection methods, and to peripheral blood monocytic
cells (PBMC) with electroporation, and luciferase activity
was measured. As a result, in comparison with HCMV MIE
promoter, HHV-7 MIE promoter and HHV-7 U95 promoter showed
several times higher activity than HCMV MIE promoter in T
cell lines, and in SAS-413 cells, HCMV MIE promoter has
more than ten times higher activity. In the experiment
where introduction was made to three lots of PBMC, HHV-7
MIE promoter and HHV-7 U95 promoter showed low activity.
In comparison with HHV-6 IE promoters (9U and U95), both
promoters of HHV-7 showed lower activity in any cell
CA 02808179 2013-02-28
-123-
species. Further, in the experiments with the deletion
mutants of the respective HHV7 MIE promoter and HHV7 U95
promoter, it was shown that although there is some
difference from cell type to cell type, R2 is responsible
for major enhancer activity against both promoter's
activity. In the present Example, it was demonstrated that
immediate early (IE) promoter encoded by human herpes virus
7 (HHV-7) has extremely high activity in blood cell lineage
cells.
[0379]
Hereinafter, materials and methods are described in
detail.
[0380]
(Materials and Methods)
1) Cells
The following five types of cells were used for
measuring promoter activity.
=
[0381]
(1) Jurkat cell (derived from human T cell)
(2) Molt-3 cell (derived from human T cell)
(3) SupT1 cell (derived from human T cell)
(4) SAS-413 cell (derived from human bone marrow cell)
(5) peripheral blood monocytic cells (PBMC)
2) plasmids for measuring promoter activity
pGL3 Basic (Promega) having Firefly luciferase gene
was used for measuring promoter activity.
[0382]
3) Promoter sequences inserted into pGL3 Basic
HHV-7 MIE gene promoter region (7MIEP) and HHV-7 U95
gene promoter region (U95P) were amplified to about 500bp
CA 02808179 2013-02-28
-124-
by PCR, and deletion mutants were prepared for each. These
are schematically illustrated in Figure 8.
[0383]
(1) 7MIEP(-493) [one inserted with upstream 493pb to
downstream 22bp from the transcription initiation point of
HHV-7 MIE gene] (SEQ ID NO: 26)
(2) 7MIEP(-388) [one inserted with upstream 388pb to
downstream 22bp from the transcription initiation point of
HHV-7 MIE gene] (SEQ ID NO: 27)
(3) 7MIEP(-233) [one inserted with upstream 233pb to
downstream 22bp from the transcription initiation point of
HHV-7 MIE gene] (SEQ ID NO: 28)
(4) 7U95P(-484) [one inserted with upstream 484pb to
=
downstream 16bp from the transcription initiation point of
HHV-7 1395 gene] (SEQ ID NO: 29)
(5) 7U95P(-379) [one inserted with upstream 379pb to
downstream 16bp from the transcription initiation point of
HHV-7 1395 gene] (SEQ ID NO: 30)
(6) 7U95P(-304) [one inserted with upstream 304pb to
. 20 downstream 16bp from the transcription initiation point of
HHV-7 U95 gene] (SEQ ID NO: 31)
pGL3 Basic without promoter sequence has been used
as a control.
[0384]
4) Transfection of cell with plasmids
Transfection was conducted regarding Jurkat cell,
Molt-3 cell, SupT1 cell, and SAS-413 cell with lipofection
using Lipofectamine 2000 (Invitrogen), and regarding PBMC,
using electroporation with Nucleofectorn4(amaxa).
[0385]
In order to correct transfection efficiency,
expression plasmids of Renilla luciferase (pRL-TK, Promega)
CA 02808179 2013-02-28
-125-
were simultaneously introduced to a cell, and Renilla
luciferase activity was measured. pRL-TK expresses Renilla
luciferase under control of herpes simplex virus thymidine
kinase (TK) promoter.
[0386]
pGL3 reporter (1.2 jig) and pRL-TK (50 ng) were mixed
together and 2111 of Lipofectamine 2000 were added thereto
to conduct transfection.
[0387]
5) Measurement of luciferase
For the measurement of luciferase activity, Dual-
Luciferase Reporter Assay System (Promega) was used.
[0388]
The cells were collected 16 hours after the
transfection, and were lysed in cell lysis solution (100p1).
To Five pl of supernatant of cell lysis solution, firefly
luciferase substrate solution (25p1) was added, - and
immediately thereafter, luminescence was measured using a
luminometer. Next, to the sample after the measurement,
Renilla luciferase substrate solution (25p1) was added and
immediately thereafter, luminescence was measured using a
luminometer.
[0389]
(Results)
1) Activity of promoter region of HHV-7 MIE
As a result of experiments using four types of cell
lines, when compared with the activity of CMV promoter,
7MIEP(-493) showed about 6-7 times higher activity in Molt-
3 cell and SuptT1 cell, similar activity in Jurkat cells,
and about 1/11 activity in SAS-413 cells. Moreover, when
comparing with HHV-6 IE promoters (9U and U95), in all cell
types, 7MIEP(-493) showed lower activity (Figure 9).
CA 02808179 2013-02-28
-126-
[0390]
As a result of experiments using three lots of PBMC,
the activity of 7MIEP (-493) was similar or slightly lower
than CMV promoter and 9U, and similar or slightly higher
than HHV-6 U95 (Figure 10).
[0391]
2) Activity of HHV-7 U95 promoter region
As a result of experiments using four types of cell
lines, when compared with the activity of CMV promoter,
7U95P(-484) showed about 2.5 times higher activity in
Jurkat cells, four time in Molt-3 cells, twenty times in
SupT cells, however, about 1/8 activity in SAS-413 cells.
Moreover, when comparing with HHV-6 IE promoters (9U and
U95), U95P(-484) showed slightly higher activity in SupT1
than U95, however, was about 1/2 of that of 9U, and showed
lower activity in other cells (Figure 9).
[0392]
In an experiment where three lots of PBMC were used,
7U95P(-494) showed only about 1/2 to 1/4 as much promoter
activity as that of the others. (Figure 10).
[0393]
3) Effects of the R2 region on promoter activity
It was elucidated as a result of an experiment where
the respective deletion mutants of 7MIEP and 7U95P were
introduced into four types of cell lines, that it depends
.on the type of cell whether the promoter activity is
lowered by the deletion of R2. Specifically, 7MIEP showed
no effects with the R2 deletion in Jurkat cells, but
reduced its activity by about 1/5 to 1/2 in other cell
lines. Moreover, 7U95P showed no effects by R2 deletion in
SAS-413 cells, but reduced its activity by about 1/7 to 1/2
in other cell lines. (Figure 11).
CA 02808179 2013-02-28
-127-
[0394]
(Summary)
The present Examples are summarized as follows:
[0395]
1) 7MIEP(-493) and 7U95P(-494) both generally showed
more potent activity than CMV promoter in T cell lines, but
showed lower activity in the SAS-413 cell, which is a bone
marrow cell line. In PBMC, 7MIEP0(-493) showed
substantially the same activity as CMV promoter, and
7U95P(-494) showed lower activity than CMV promoter.
[0396]
2) In comparison with HHV-6 IE promoters, all cell
types showed higher activity in two types of IE promoters
of HHV-6 (9U and U95) than 7MIEP(-493) and 7U95(-494).
[0397]
3) It was shown that the R2 region functions as an
enhancer against 7MIEP and 7U95P in a number of cells.
Transcriptional factors binding to the R2 region are
unidentified, but in view of the fact that the R3 region of
HHV-6 functions as an enhancer of the U95 promoter by
binding NF-KB, it is highly likely that the NF-KB binding
motifs present in a repetitive manner in the R2 region may
be responsible for enhancer activity of the R2 region
(Figure 8).
[0398]
(Example 3: Construction of specific deletion
system)
Knocking out of gene expression in blood cell
lineage cells is conducted using an IE promoter and the
RNAi method. IE promoters are advantageous for analysis
since they are expressed in blood cell lineage cells in a
large amount.
CA 02808179 2013-02-28
-128-
[0399]
1) Preparation of cells (in the case of macrophages)
Healthy human peripheral blood is obtained and
separated and purified by density gradient using Ficoll174/
Hypaque. The PBMCs are cultured in a AIM V serum medium
(Life Technologies) supplemented with M-CSF (R&D systems,
100U/m1). The medium is exchanged every three days, and
macrophages at Day 6 or 7 are used for experiments.
[0400]
2) Preparation of siRNA expression retrovirus vector
In order to express hair-pin type RNA, a synthetic
oligo-DNA comprising "a sense strand target sequence", "a
loop sequence", "an antisense strand target sequence" and
"a terminator sequence" is prepared. Such a sense strand
target sequence, loop sequence, antisense target sequence,
terminator sequence may be made using well known technology
in the art. Those skilled in the art can readily
understand that when actually using these, an appropriate
sequence may be employed depending on the actual situation.
[0401]
The above-mentioned DNA is incorporated into a
plasmid vector in which the oligo-DNA is linked downstream
of the IE sequence, and of gag, pol and env which are
necessary for replication of a retrovirus, and which
comprises NeoR gene making use of restriction enzyme
sequences and the like. Plasmid vector produced (10111) is
added to 100111 of competent cells and transformation is
conducted and cultured for 16 hours at 37 degrees Celsius
after plating into LBAmp plate. Colonies obtained by the
transformation are cultured on LBApm liquid medium at 37
degrees Celsius for 16 hours, and plasmids are extracted
CA 02808179 2013-02-28
-129-
and purified using conventional methods from the culture
solution.
[0402]
Retrovirus packaging cells expressing gag, pol and
env are plated on a disc with a 10 cm diameter, and
transfection reagent is opened to transfect the plasmid
(1011g). 24-48
hours later, the cells are subjected to
limitation dilution into G418 containing medium (50011g/m1)
and passaged.
[0403]
Every three to four days, G418 medium is exchanged
and cultured for about two weeks in total. Colonies are
collected and at the time where growth is found at a
confluent level on a six-well plate, the medium is changed
to a G418 free medium and the supernatant is collected 24
hours later. The cells will be stocked.
[0404]
Retrovirus vectors included in the supernatant are
subjected to limitation dilution, and infected into NIH/3T3
cells, and colonies grown are counted to calculate the
infection value.
[0405]
3) Gene introduction experiments using retrovirus
vectors
Retrovirus vectors are infected with blood cell
lineage cells such as macrophages prepared in 1).
Immediately after washing, it was plated to form 0.5-2.5 x
104 cells/cm2 on a plate. Twenty
four hours after the
infection, the medium is exchanged with G418 containing
medium, and every three to four days, medium is exchanged.
About two weeks later, gene introduced cells are obtained.
CA 02808179 2013-02-28
-130-
The cells are used to confirm the expression level of the
desired knocked out gene.
[0406]
These experiments are conducted to actually confirm
that after gene introduction, lymphocyte specific
expression of a foreign gene can be knocked out with the
promoter of the present invention.
[0407]
(Example 4: Specific Expression)
Instead of the RNAi of Example 3, a nucleic acid
molecule encoding a gene (for example, cytokines such as
TGF 8) desired for expression is introduced.
[0408]
As a result, by conducting similar experiments as in
Example 3, after gene introduction, it is confirmed that
the promoter according to the present invention actually
induces the lymphocyte specific foreign gene expression.
[0409]
As described above, the present invention is
illustrated by way of the preferred embodiments. However,
it will be understood that the scope of the present
invention should be interpreted only by the accompanying
claims. The scope of the claims should not be limited by the
preferred embodiments set forth in the examples, but should
be given the broadest interpretation consistent with the des-
cription as a whole.
[Industrial Applicability]
CA 02808179 2013-02-28
-131-
[0410]
The present invention provides promoters which
selectively induce the expression of protein in an immune
responsible cells such as T lymphocytes. The promoters of
the present invention are useful in method and medicaments
for effectively preventing or treating immune diseases such
as acquired immunodeficiency syndromes and the like. The
present invention is also useful in the technologies for
efficiently conducting gene therapy.