Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 254
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 254
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
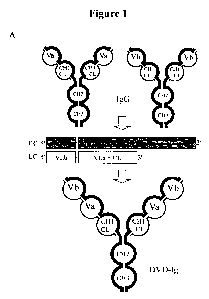
DUAL VARIABLE DOMAIN IlVIMUNOGLOBULINS AND USES THEREOF
Cross Reference to Related Applications
This application is a non-provisional application claiming priority to U.S.
Provisional
Application Serial No. 61/377,117, filed August 26, 2010, the entire content
of which is hereby
incorporated by reference.
Field
Multivalent and multispecific binding proteins, methods of making, and
specifically to
their uses in the, diagnosis, prevention and/or treatment of acute and chronic
inflammatory
diseases, cancer, and other diseases are provided.
Background
Engineered proteins, such as multispecific antibodies capable of binding two
or more
antigens are known in the art. Such multispecific binding proteins can be
generated using cell
fusion, chemical conjugation, or recombinant DNA techniques.
Bispecific antibodies have been produced using quadroma technology (see
Milstein, C.
and A.C. Cuello (1983) Nature 305(5934):537-40) based on the somatic fusion of
two different
hybridoma cell lines expressing murine monoclonal antibodies (mAbs) with the
desired
specificities of the bispecific antibody. Because of the random pairing of two
different
immunoglobulin (Ig) heavy and light chains within the resulting
hybrid¨hybridoma (or
quadroma) cell line, up to ten different Ig species are generated, of which
only one is the
functional bispecific antibody. The presence of mis-paired by-products, and
significantly reduced
production yields, means sophisticated purification procedures are required.
Bispecific antibodies can also be produced by chemical conjugation of two
different
mAbs (see Staerz, U.D., et al. (1985) Nature 314(6012): 628-31). This approach
does not yield
homogeneous preparation. Other approaches have used chemical conjugation of
two different
mAbs or smaller antibody fragments (see Brennan, M., etal. (1985) Science
229(4708): 81-3).
Another method used to produce bispecific antibodies is the coupling of two
parental
antibodies with a hetero-bifunctional crosslinker, but the resulting
bispecific antibodies suffer
from significant molecular heterogeneity because reaction of the crosslinker
with the parental
antibodies is not site-directed. To obtain more homogeneous preparations of
bispecific antibodies
two different Fab fragments have been chemically crosslinked at their hinge
cysteine residues in
1
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
a site-directed manner (see Glennie, M.J., et al. (1987) J. Immunol. 139(7):
2367-75). But this
method results in Fab'2 fragments, not full IgG molecule.
A wide variety of other recombinant bispecific antibody formats have been
developed
(see Kriangkum, J., et al. (2001) Biomol. Eng. 18(2): 31-40). Amongst them
tandem single-chain
Fv molecules and diabodies, and various derivatives thereof, are the most
widely used. Routinely,
construction of these molecules starts from two single-chain Fv (scFv)
fragments that recognize
different antigens (see Economides, A.N., et al. (2003) Nat. Med. 9(1): 47-
52). Tandem scFv
molecules (taFv) represent a straightforward format simply connecting the two
scFv molecules
with an additional peptide linker. The two scFv fragments present in these
tandem scFv
molecules form separate folding entities. Various linkers can be used to
connect the two scFv
fragments and linkers with a length of up to 63 residues (see Nakanishi, K.,
et al. (2001) Ann.
Rev. Immunol. 19: 423-74). Although the parental scFv fragments can normally
be expressed in
soluble form in bacteria, it is, however, often observed that tandem scFv
molecules form
insoluble aggregates in bacteria. Hence, refolding protocols or the use of
mammalian expression
systems are routinely applied to produce soluble tandem scFv molecules. In a
recent study, in
vivo expression by transgenic rabbits and cattle of a tandem scFv directed
against CD28 and a
melanoma-associated proteoglycan was reported (see Gracie, J.A., et al. (1999)
J. Clin. Invest.
104(10): 1393-401). In this construct, the two scFv molecules were connected
by a CHI linker
and serum concentrations of up to 100 mg/L of the bispecific antibody were
found. Various
strategies including variations of the domain order or using middle linkers
with varying length or
flexibility were employed to allow soluble expression in bacteria. A few
studies have now
reported expression of soluble tandem scFv molecules in bacteria (see Leung,
B.P., et al. (2000)
J. Immunol. 164(12): 6495-502; Ito, A., et al. (2003)3. Immunol. 170(9): 4802-
9; Karni, A., et al.
(2002) J. Neuroimmunol. 125(1-2): 134-40) using either a very short Ala3
linker or long
glycine/serine-rich linkers. In another recent study, phage display of a
tandem scFv repertoire
containing randomized middle linkers with a length of 3 or 6 residues was
employed to enrich for
those molecules that are produced in soluble and active form in bacteria. This
approach resulted
in the isolation of a tandem scFv molecule with a 6 amino acid residue linker
(see Arndt, M. and
J. Krauss (2003) Methods Mol. Biol. 207: 305-21). It is unclear whether this
linker sequence
represents a general solution to the soluble expression of tandem scFv
molecules. Nevertheless,
this study demonstrated that phage display of tandem scFv molecules in
combination with
directed mutagenesis is a powerful tool to enrich for these molecules, which
can be expressed in
bacteria in an active form.
2
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Bispecific diabodies (Db) utilize the diabody format for expression. Diabodies
are
produced from scFv fragments by reducing the length of the linker connecting
the VH and VL
domain to approximately 5 residues (see Peipp, M. and T. Valerius (2002)
Biochem. Soc. Trans.
30(4): 507-11). This reduction of linker size facilitates dimerization of two
polypeptide chains by
crossover pairing of the VH and VL domains. Bispecific diabodies are produced
by expressing,
two polypeptide chains with, either the structure VHA-VLB and VHB-VLA (VH-VL
configuration), or VLA-VHB and VLB-VHA (VL-VH configuration) within the same
cell. A
large variety of different bispecific diabodies have been produced in the past
and most of them
are expressed in soluble form in bacteria. However, a recent comparative study
demonstrates that
the orientation of the variable domains can influence expression and formation
of active binding
sites (see Mack, M. et al.(1995) Proc. Natl. Acad. Sci. U S A 92(15): 7021-5).
Nevertheless,
soluble expression in bacteria represents an important advantage over tandem
scFv molecules.
However, since two different polypeptide chains are expressed within a single
cell inactive
homodimers can be produced together with active heterodimers. This
necessitates the
implementation of additional purification steps in order to obtain homogenous
preparations of
bispecific diabodies. One approach to force the generation of bispecific
diabodies is the
production of knob-into-hole diabodies (see Holliger, P., T. Prospero, and G.
Winter (1993) Proc.
Natl. Acad. Sci. U S A 90(14): 6444-8.18). This approach was demonstrated for
a bispecific
diabody directed against HER2 and CD3. A large knob was introduced in the VH
domain by
exchanging Va137 with Phe and Leu45 with Trp and a complementary hole was
produced in the
VL domain by mutating Phe98 to Met and Tyr87 to Ala, either in the anti- HER2
or the anti-CD3
variable domains. By using this approach the production of bispecific
diabodies could be
increased from 72% by the parental diabody to over 90% by the knob-into-hole
diabody.
Importantly, production yields only slightly decrease as a result of these
mutations. However, a
reduction in antigen-binding activity was observed for several constructs.
Thus, this rather
elaborate approach requires the analysis of various constructs in order to
identify those mutations
that produce heterodimeric molecule with unaltered binding activity. In
addition, such approach
requires mutational modification of the immunoglobulin sequence at the
constant region, thus
creating non-native and non-natural form of the antibody sequence, which may
result in increased
immunogenicity, poor in vivo stability, as well as undesirable
pharmacokinetics.
Single-chain diabodies (scDb) represent an alternative strategy for improving
the
formation of bispecific diabody-like molecules (see Holliger, P. and G. Winter
(1997) Cancer
Immunol. Immunother. 45(3-4): 128-30; Wu, A.M., et al. (1996) Immunotechnology
2(1): p. 21-
36). Bispecific single-chain diabodies are produced by connecting the two
diabody-forming
polypeptide chains with an additional middle linker with a length of
approximately 15 amino acid
3
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
residues. Consequently, all molecules with a molecular weight corresponding to
monomeric
single-chain diabodies (50-60 kDa) are bispecific. Several studies have
demonstrated that
bispecific single chain diabodies are expressed in bacteria in soluble and
active form with the
majority of purified molecules present as monomers (see Holliger, P. and G.
Winter (1997)
Cancer Immunol. Immunother. 45(3-4): 128-30; Wu, A.M., et al. (1996)
Immunotechnol. 2(1):
21-36; Pluckthun, A. and P. Pack (1997) Immunotechnol. 3(2): 83-105; Ridgway,
J.B., et al.
(1996) Protein Engin. 9(7): 617-21). Thus, single-chain diabodies combine the
advantages of
tandem scFvs (all monomers are bispecific) and diabodies (soluble expression
in bacteria).
More recently diabodies have been fused to Fe to generate more Ig-like
molecules,
named di-diabodies (see Lu, D., et al. (2004) J. Biol. Chem. 279(4): 2856-65).
In addition,
multivalent antibody construct comprising two Fab repeats in the heavy chain
of an IgG and
capable of binding four antigen molecules has been described (see WO
0177342A1, and Miller,
K, et al. (2003) J. Immunol. 170(9): 4854-61).
There is a need in the art for improved multivalent binding proteins capable
of binding
two or more antigens. U.S. Patent Application Serial No. 11/507,050 provides a
novel family of
binding proteins capable of binding two or more antigens with high affinity,
which are called
dual variable domain immunoglobulins (DVD-IgTm). Novel binding proteins
capable of binding
two or more antigens are also provided.
Summary
Multivalent binding proteins capable of binding two or more antigens are
provided. A
novel family of binding proteins capable of binding two or more antigens with
high affinity are
also provided.
In one embodiment a binding protein comprising a polypeptide chain, wherein
the
polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n, wherein VD I is a first
variable domain,
VD2 is a second variable domain, C is a constant domain, X1 represents an
amino acid or
polypeptide, X2 represents an Fe region and n is 0 or 1 is provided. In an
embodiment the VD1
and VD2 in the binding protein are heavy chain variable domains. In another
embodiment, the
heavy chain variable domain is a murine heavy chain variable domain, a human
heavy chain
variable domain, a CDR grafted heavy chain variable domain, or a humanized
heavy chain
variable domain. In yet another, embodiment VD1 and VD2 are capable of binding
the same
antigen. In another embodiment VD1 and VD2 are capable of binding different
antigens. In still
another embodiment, C is a heavy chain constant domain. For example, X1 is a
linker with the
proviso that X1 is not CHI. For example, X1 is AKTTPKLEEGEFSEAR (SEQ ID NO:
1);
4
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
AKTTPKLEEGEFSEARV (SEQ ID NO: 2); AKTTPKLGG (SEQ ID NO: 3); SAKTTPKLGG
(SEQ ID NO: 4); SAKTTP (SEQ ID NO: 5); RADAAP (SEQ ID NO: 6); RADAAPTVS (SEQ
ID NO: 7); RADAAAAGGPGS (SEQ ID NO: 8); RADAAAA(G4S)4 (SEQ ID NO: 9),
SAKTTPKLEEGEFSEARV (SEQ ID NO: 10); ADAAP (SEQ ID NO: 11); ADAAPTVSIFPP
(SEQ ID NO: 12); TVAAP (SEQ ID NO: 13); TVAAPSVFIFPP (SEQ ID NO: 14); QPKAAP
(SEQ ID NO: 15); QPKAAPSVTLFPP (SEQ ID NO: 16); AKTTPP (SEQ ID NO: 17);
AKTTPPSVTPLAP (SEQ ID NO: 18); AKTTAP (SEQ ID NO: 19); AKTTAPSVYPLAP (SEQ
ID NO: 20); ASTKGP (SEQ ID NO: 21); ASTKGPSVFPLAP (SEQ ID NO: 22),
GGGGSGGGGSGGGGS (SEQ ID NO: 23); GENKVEYAPALMALS (SEQ ID NO: 24);
GPAKELTPLKEAKVS (SEQ ID NO: 25); or GHEAAAVMQVQYPAS (SEQ ID NO: 26) In an
embodiment, X2 is an Fe region. In another embodiment, X2 is a variant Fe
region.
In an embodiment the binding protein disclosed herein comprises a polypeptide
chain,
wherein the polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is
a first
heavy chain variable domain, VD2 is a second heavy chain variable domain, C is
a heavy chain
constant domain, X1 is a linker with the proviso that it is not CH1, and X2 is
an Fe region.
In an embodiment, VD1 and VD2 in the binding protein are light chain variable
domains.
In an embodiment, the light chain variable domain is a murine light chain
variable domain, a
human light chain variable domain, a CDR grafted light chain variable domain,
or a humanized
light chain variable domain. In one embodiment VD1 and VD2 are capable of
binding the same
antigen. In another embodiment VD1 and VD2 are capable of binding different
antigens. In an
embodiment, C is a light chain constant domain. In another embodiment, X1 is a
linker with the
proviso that X1 is not CL. In an embodiment, X1 is AKTTPKLEEGEFSEAR (SEQ ID
NO: 1);
AKTTPKLEEGEFSEARV (SEQ ID NO: 2); AKTTPKLGG (SEQ ID NO: 3); SAKTTPKLGG
(SEQ ID NO: 4); SAKTTP (SEQ ID NO: 5); RADAAP (SEQ ID NO: 6); RADAAPTVS (SEQ
ID NO: 7); RADAAAAGGPGS (SEQ ID NO: 8); RADAAAA(G4S)4 (SEQ ID NO: 9),
SAKTTPKLEEGEFSEARV (SEQ ID NO: 10); ADAAP (SEQ ID NO: 11); ADAAPTVSIFPP
(SEQ ID NO: 12); TVAAP (SEQ ID NO: 13); TVAAPSVFIFPP (SEQ ID NO: 14); QPKAAP
(SEQ ID NO: 15); QPKAAPSVTLFPP (SEQ ID NO: 16); AKTTPP (SEQ ID NO: 17);
AKTTPPSVTPLAP (SEQ ID NO: 18); AKTTAP (SEQ ID NO: 19); AKTTAPSVYPLAP (SEQ
ID NO: 20); ASTKGP (SEQ ID NO: 21); ASTKGPSVFPLAP (SEQ ID NO: 22),
GGGGSGGGGSGGGGS (SEQ ID NO: 23); GENKVEYAPALMALS (SEQ ID NO: 24);
GPAKELTPLKEAKVS (SEQ ID NO: 25); or GHEAAAVMQVQYPAS (SEQ ID NO: 26). In an
embodiment, the binding protein does not comprise X2.
5
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
In an embodiment, both the variable heavy and variable light chain comprise
the same
linker. In another embodiment, the variable heavy and variable light chain
comprise different
linkers. In another embodiment, both the variable heavy and variable light
chain comprise a short
(about 6 amino acids) linker. In another embodiment, both the variable heavy
and variable light
chain comprise a long (greater than 6 amino acids) linker. In another
embodiment, the variable
heavy chain comprises a short linker and the variable light chain comprises a
long linker. In
another embodiment, the variable heavy chain comprises a long linker and the
variable light chain
comprises a short linker.
In an embodiment the binding protein disclosed herein comprises a polypeptide
chain,
wherein said polypeptide chain comprises VD1-(X 1)n-VD2-C-(X2)n, wherein VD1
is a first light
chain variable domain, VD2 is a second light chain variable domain, C is a
light chain constant
domain, X1 is a linker with the proviso that it is not CL, and X2 does not
comprise an Fe region.
In another embodiment a binding protein comprising two polypeptide chains,
wherein
said first polypeptide chain comprises VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a
first heavy
chain variable domain, VD2 is a second heavy chain variable domain, C is a
heavy chain constant
domain, X1 is a linker with the proviso that it is not CH1, and X2 is an Fe
region; and said
second polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is a
first light
chain variable domain, VD2 is a second light chain variable domain, C is a
light chain constant
domain, X1 is a linker (optionally which is not CL), and X2 does not comprise
an Fe region is
provided. In a particular embodiment, the binding protein (i.e., Dual Variable
Domain
immunoglobulin (DVD-Ig)) comprises four polypeptide chains wherein the first
two polypeptide
chains comprises VD1-(Xl)n-VD2-C-(X2)n, respectively wherein VD1 is a first
heavy chain
variable domain, VD2 is a second heavy chain variable domain, C is a heavy
chain constant
domain, X1 is a linker with the proviso that it is not CH1, and X2 is an Fe
region; and the second
two polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n respectively, wherein
VD1 is a first
light chain variable domain, VD2 is a second light chain variable domain, C is
a light chain
constant domain, X1 is a linker (optionally which is not CL), and X2 does not
comprise an Fe
region. Such a binding protein has four antigen binding sites.
In another embodiment the binding proteins disclosed herein are capable of
binding one
or more targets. Accordingly, in some embodiments, the binding proteins
comprise at least two
variable domain sequences (e.g., VD1 and VD2) capable of binding at least two
different targets.
In some embodiments, VD1 and VD2 are independently chosen. Therefore, in some
embodiments, VD1 and VD2 comprise the same SEQ ID NO and, in other
embodiments, VD1
and VD2 comprise different SEQ ID NOS.
6
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
In another embodiment the binding proteins disclosed herein bind one or more
targets. In
an embodiment, the target is a cytokine, a cell surface protein, an enzymes,
or a receptor. In
another embodiment, the binding protein is capable of modulating a biological
function of one or
more targets. In another embodiment, the binding protein is capable of
neutralizing one or more
targets. In one embodiment, the binding protein is capable of binding
cytokines. In some
embodiments, the cytokines are lymphokines, monokines, polypeptide hormones,
receptors, or
tumor markers. In some embodiments, the DVD-Ig is capable of binding two or
more of the
following: Tumor Necrosis Factor (TNF), Nerve Growth Factor (NGF), Sclerostin
(SOST),
Prostaglandin E2 (PGE2), Lysophosphatidic Acid (LPA) (see also Table 2). In a
specific
embodiment the binding protein is capable of binding TNF (seq. 2) and NGF; TNF
(seq. 3) and
NGF; TNF (seq. 4) and NGF; TNF (seq. 5) and NGF; TNF (seq. 6) and NGF; TNF
(seq. 2) and
SOST; TNF (seq. 3) and SOST; TNF (seq. 4) and SOST; TNF (seq. 5) and SOST; TNF
(seq. 6)
and SOST; TNF (seq. 2) and PGE2; TNF (seq. 3) and PGE2; TNF (seq. 4) and PGE2;
TNF (seq.
5) and PGE2; TNF (seq. 6) and PGE2; TNF (seq. 1) and LPA; TNF (seq. 2) and
LPA; TNF (seq.
3) and LPA; TNF (seq. 4) and LPA; TNF (seq. 5) and LPA; or TNF (seq. 6) and
LPA. In an
embodiment, the DVD-Ig contains one or more VH chains that comprise a short
linker and one or
more VL chaims that contain a long linker. In an embodiment, the DVD-Ig
contains one or more
VH chains that comprise a long linker and one or more VL chaims that contain a
short linker. In
an embodiment, the -Ig contains one or more VI-1 chains that comprise a long
linker and one or
more VL chaims that contain a long linker.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 52 or SEQ ID NO. 54;
and a light
chain amino acid sequence of SEQ ID NO. 53 or SEQ ID NO. 55. In an embodiment,
the binding
protein capable of binding TNF (seq. 2) and NGF comprises a heavy chain amino
acid sequence
of SEQ ID NO. 52 and a light chain amino acid sequence of SEQ ID NO: 53. In
another
embodiment, the binding protein capable of binding TNF (seq. 2) and NGF has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 54
and a light
chain amino acid sequence of SEQ ID NO: 55.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 56 or SEQ ID NO. 58;
and a light
chain amino acid sequence of SEQ ID NO. 57 or SEQ ID NO. 59. In an embodiment,
the binding
protein capable of binding TNF (seq. 3) and NGF comprises a heavy chain amino
acid sequence
of SEQ ID NO. 56 and a light chain amino acid sequence of SEQ ID NO: 57. In
another
embodiment, the binding protein capable of binding TNF (seq. 3) and NGF has a
reverse
7
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 58
and a light
chain amino acid sequence of SEQ ID NO: 59.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and NGF
comprises a heavy chain amino acid of SEQ ID NO. 60 or SEQ ID NO.62; and a
light chain
amino acid sequence of SEQ ID NO. 61 or SEQ ID NO.63. In an embodiment, the
binding
protein capable of binding TNF (seq. 4) and NGF comprises a heavy chain amino
acid sequence
of SEQ ID NO. 60 and a light chain amino acid sequence of SEQ ID NO:61. In
another
embodiment, the binding protein capable of binding TNF (seq. 4) and NGF has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 62
and a light
chain amino acid sequence of SEQ ID NO: 63.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 64 or SEQ ID NO. 66;
and a light
chain amino acid sequence of SEQ ID NO. 65 or SEQ ID NO. 67. In an embodiment,
the binding
protein capable of binding TNF (seq. 5) and NGF comprises a heavy chain amino
acid sequence
of SEQ ID NO. 64 and a light chain amino acid sequence of SEQ ID NO: 65. In
another
embodiment, the binding protein capable of binding TNF (seq. 5) and NGF has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 66
and a light
chain amino acid sequence of SEQ ID NO: 67.
Ti an embodiment, the binding protein capable of binding TNF (seq. 6) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 68 or SEQ ID NO. 70;
and a light
chain amino acid sequence of SEQ ID NO. 69 or SEQ ID NO. 71. In an embodiment,
the binding
protein capable of binding TNF (seq. 6) and NGF comprises a heavy chain amino
acid sequence
of SEQ ID NO. 68 and a light chain amino acid sequence of SEQ ID NO: 69. In
another
embodiment, the binding protein capable of binding TNF (seq. 6) and NGF has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 70
and a light
chain amino acid sequence of SEQ ID NO: 71.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 72 or SEQ ID NO. 74;
and a light
chain amino acid sequence of SEQ ID NO. 73 or SEQ ID NO. 75. In an embodiment,
the binding
protein capable of binding TNF (seq. 2) and SOST comprises a heavy chain amino
acid sequence
of SEQ ID NO. 72 and a light chain amino acid sequence of SEQ ID NO: 73. In
another
embodiment, the binding protein capable of binding TNF (seq. 2) and SOST has a
reverse
8
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 74
and a light
chain amino acid sequence of SEQ ID NO: 75.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 76 or SEQ ID NO. 78;
and a light
chain amino acid sequence of SEQ ID NO. 77 or SEQ ID NO. 79. In an embodiment,
the binding
protein capable of binding TNF (seq. 3) and SOST comprises a heavy chain amino
acid sequence
of SEQ ID NO. 76 and a light chain amino acid sequence of SEQ ID NO: 77. In
another
embodiment, the binding protein capable of binding TNF (seq. 3) and SOST has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 78
and a light
chain amino acid sequence of SEQ ID NO: 79.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 80 or SEQ ID NO. 82;
and a light
chain amino acid sequence of SEQ ID NO. 81 or SEQ ID NO. 83. In an embodiment,
the binding
protein capable of binding TNF (seq. 4) and SOST comprises a heavy chain amino
acid sequence
of SEQ ID NO. 80 and a light chain amino acid sequence of SEQ ID NO: 81. In
another
embodiment, the binding protein capable of binding TNF (seq. 4) and SOST has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 82
and a light
chain amino acid sequence of SEQ ID NO: 83.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 84 or SEQ ID NO. 86;
and a light
chain amino acid sequence of SEQ ID NO. 85 or SEQ ID NO. 87. In an embodiment,
the binding
protein capable of binding TNF (seq. 5) and SOST comprises a heavy chain amino
acid sequence
of SEQ ID NO. 84 and a light chain amino acid sequence of SEQ ID NO: 85. In
another
embodiment, the binding protein capable of binding TNF (seq. 5) and SOST has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 86
and a light
chain amino acid sequence of SEQ ID NO: 87.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 88 or SEQ ID NO. 90;
and a light
chain amino acid sequence of SEQ ID NO. 89 or SEQ ID NO. 91. In an embodiment,
the binding
protein capable of binding TNF (seq. 6) and SOST comprises a heavy chain amino
acid sequence
of SEQ ID NO. 88 and a light chain amino acid sequence of SEQ ID NO: 89. hi
another
embodiment, the binding protein capable of binding TNF (seq. 6) and SOST has a
reverse
9
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 90
and a light
chain amino acid sequence of SEQ ID NO: 91.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 92 or SEQ ID NO. 94;
and a light
chain amino acid sequence of SEQ ID NO. 93 or SEQ ID NO. 95. In an embodiment,
the binding
protein capable of binding TNF (seq. 2) and PGE2 comprises a heavy chain amino
acid sequence
of SEQ ID NO. 92 and a light chain amino acid sequence of SEQ ID NO: 93. In
another
embodiment, the binding protein capable of binding TNF (seq. 2) and PGE2 has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 94
and a light
chain amino acid sequence of SEQ ID NO: 95.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 96 or SEQ ID NO. 98;
and a light
chain amino acid sequence of SEQ ID NO. 97 or SEQ ID NO. 99. In an embodiment,
the binding
protein capable of binding TNF (seq. 3) and PGE2 comprises a heavy chain amino
acid sequence
of SEQ ID NO. 96 and a light chain amino acid sequence of SEQ ID NO: 97. In
another
embodiment, the binding protein capable of binding TNF (seq. 3) and PGE2 has a
reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 98
and a light
chain amino acid sequence of SEQ ID NO: 99.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 100 or SEQ ID NO.
102; and a
light chain amino acid sequence of SEQ ID NO. 101 or SEQ ID NO. 103. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 100 and a light chain amino acid sequence of SEQ ID NO:
101. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 102
and a light
chain amino acid sequence of SEQ ID NO: 103.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO 104 or SEQ ID NO.
106; and a
light chain amino acid sequence of SEQ ID NO. 105 or SEQ ID NO. 107. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 104 and a light chain amino acid sequence of SEQ ID NO:
105. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
PGE2 has a reverse
10
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 106
and a light
chain amino acid sequence of SEQ ID NO: 107.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 108 or SEQ ID NO.
110; and a
light chain amino acid sequence of SEQ ID NO. 109 or SEQ ID NO. 111. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 108 and a light chain amino acid sequence of SEQ ID NO:
109. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 110
and a light
chain amino acid sequence of SEQ ID NO: 111.
In an embodiment, the binding protein capable of binding TNF (seq. 1) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 112 or SEQ ID NO.
114; and a
light chain amino acid sequence of SEQ ID NO. 113 or SEQ ID NO. 115. In an
embodiment, the
binding protein capable of binding TNF (seq. 1) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 112 and a light chain amino acid sequence of SEQ ID NO:
113. In
another embodiment, the binding protein capable of binding TNF (seq. 1) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 114
and a light
chain amino acid sequence of SEQ ID NO: 115.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 116 or SEQ ID NO.
118; and a
light chain amino acid sequence of SEQ ID NO. 117 or SEQ ID NO. 119. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 116 and a light chain amino acid sequence of SEQ ID NO:
117. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 118
and a light
chain amino acid sequence of SEQ ID NO: 119.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 120 or SEQ ID NO.
122; and a
light chain amino acid sequence of SEQ ID NO. 121 or SEQ ID NO. 123. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 120 and a light chain amino acid sequence of SEQ ID NO:
121. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
LPA has a reverse
11
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ JD NO. 122
and a light
chain amino acid sequence of SEQ ID NO: 123.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 124 or SEQ ID NO.
126; and a
light chain amino acid sequence of SEQ ID NO. 125 or SEQ ID NO. 127. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 124 and a light chain amino acid sequence of SEQ ID NO:
125. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 126
and a light
chain amino acid sequence of SEQ ID NO: 127.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 128 or SEQ ID NO.
130; and a
light chain amino acid sequence of SEQ ID NO. 129 or SEQ ID NO. 131. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 128 and a light chain amino acid sequence of SEQ ID NO:
129. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 130
and a light
chain amino acid sequence of SEQ ID NO: 131.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 132 or SEQ ID NO.
134; and a
light chain amino acid sequence of SEQ ID NO. 133 or SEQ ID NO. 135. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 132 and a light chain amino acid sequence of SEQ ID NO:
133. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 134
and a light
chain amino acid sequence of SEQ ID NO: 135.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 136 or SEQ ID NO.
138; and a
light chain amino acid sequence of SEQ ID NO. 137 or SEQ ID NO. 139. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 136 and a light chain amino acid sequence of SEQ ID NO:
137. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
NGF has a reverse
12
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 138
and a light
chain amino acid sequence of SEQ ID NO: 139.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 140 or SEQ ID NO.
142; and a
light chain amino acid sequence of SEQ ID NO. 141 or SEQ ID NO. 143. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 140 and a light chain amino acid sequence of SEQ ID NO:
141. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
NGF has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 142
and a light
chain amino acid sequence of SEQ ID NO: 143.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 144 or SEQ ID NO.
146; and a
light chain amino acid sequence of SEQ ID NO. 145 or SEQ ID NO. 147. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 144 and a light chain amino acid sequence of SEQ ID NO:
145. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
NGF has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 146
and a light
chain amino acid sequence of SEQ ID NO: 147.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 148 or SEQ ID NO.
150; and a
light chain amino acid sequence of SEQ ID NO. 149 or SEQ ID NO. 151. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 148 and a light chain amino acid sequence of SEQ ID NO:
149. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
NGF has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 150
and a light
chain amino acid sequence of SEQ ID NO: 151.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 152 or SEQ ID NO.
154; and a
light chain amino acid sequence of SEQ ID NO. 153 or SEQ ID NO. 155. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 152 and a light chain amino acid sequence of SEQ ID NO:
153. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
NGF has a reverse
13
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 154
and a light
chain amino acid sequence of SEQ ID NO: 155.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 156 or SEQ ID NO.
158; and a
light chain Amino acid sequence of SEQ ID NO. 157 or SEQ ID NO. 159. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 156 and a light chain amino acid sequence of SEQ ID NO:
157. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 158 and a
light chain amino acid sequence of SEQ ID NO: 159.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 160 or SEQ ID NO.
162; and a
light chain amino acid sequence of SEQ ID NO. 161 or SEQ ID NO. 163. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 160 and a light chain amino acid sequence of SEQ ID NO:
161. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 162 and a
light chain amino acid sequence of SEQ ID NO: 163.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 164 or SEQ ID NO.
166; and a
light chain amino acid sequence of SEQ ID NO. 165 or SEQ ID NO. 167. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 164 and a light chain amino acid sequence of SEQ ID NO:
165. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 166 and a
light chain amino acid sequence of SEQ ID NO: 167.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 168 or SEQ ID NO.
170; and a
light chain amino acid sequence of SEQ ID NO. 169 or SEQ ID NO. 171. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 168 and a light chain amino acid sequence of SEQ ID NO:
169. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
SOST has a
14
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 170 and a
light chain amino acid sequence of SEQ ID NO: 171.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 172 or SEQ ID NO.
174; and a
light chain amino acid sequence of SEQ ID NO. 173 or SEQ ID NO. 175. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 172 and a light chain amino acid sequence of SEQ ID NO:
173. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 174 and a
light chain amino acid sequence of SEQ ID NO: 175.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 176 or SEQ ID NO.
178; and a
light chain amino acid sequence of SEQ ID NO. 177 or SEQ ID NO. 179. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 176 and a light chain amino acid sequence of SEQ ID NO:
177. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 178
and a light
chain amino acid sequence of SEQ ID NO: 179.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 180 or SEQ ID NO.
182; arid a
light chain amino acid sequence of SEQ ID NO. 181 or SEQ ID NO. 183. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 180 and a light chain amino acid sequence of SEQ ID NO:
181. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 182
and a light
chain amino acid sequence of SEQ ID NO: 183.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 184 or SEQ ID NO.
186; and a
light chain amino acid sequence of SEQ ID NO. 185 or SEQ ID NO. 187. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 184 and a light chain amino acid sequence of SEQ ID NO:
185. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
PGE2 has a reverse
15
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 186
and a light
chain amino acid sequence of SEQ ID NO: 187.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 188 or SEQ ID NO.
190; and a
light chain amino acid sequence of SEQ ID NO. 189 or SEQ ID NO. 191. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 188 and a light chain amino acid sequence of SEQ ID NO:
189. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 190
and a light
chain amino acid sequence of SEQ ID NO: 191.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 192 or SEQ ID NO.
194; and a
light chain amino acid sequence of SEQ ID NO. 193 or SEQ ID NO. 195. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 192 and a light chain amino acid sequence of SEQ ID NO:
193. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 194
and a light
chain amino acid sequence of SEQ ID NO: 195.
In an embodiment, the binding protein capable of binding TNF (seq. 1) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 196 or SEQ ID NO.
198; and a
light chain amino acid sequence of SEQ ID NO. 197 or SEQ ID NO. 199. In an
embodiment, the
binding protein capable of binding TNF (seq. 1) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 196 and a light chain amino acid sequence of SEQ ID NO:
197. In
another embodiment, the binding protein capable of binding TNF (seq. 1) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 198
and a light
chain amino acid sequence of SEQ ID NO: 199.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 200 or SEQ ID NO.
202; and a
light chain amino acid sequence of SEQ ID NO. 201 or SEQ ID NO. 203. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 200 and a light chain amino acid sequence of SEQ ID NO:
201. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
LPA has a reverse
16
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 202
and a light
chain amino acid sequence of SEQ ID NO: 203.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 204 or SEQ ID NO.
206; and a
light chain amino acid sequence of SEQ ID NO. 205 or SEQ ID NO. 207. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 204 and a light chain amino acid sequence of SEQ ID NO:
205. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 206
and a light
chain amino acid sequence of SEQ ID NO: 207.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 208 or SEQ ID NO.
210; and a
light chain amino acid sequence of SEQ ID NO. 209 or SEQ ID NO. 211. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 208 and a light chain amino acid sequence of SEQ ID NO:
209. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 210
and a light
chain amino acid sequence of SEQ ID NO: 211.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 212 or SEQ ID NO.
214; and a
light chain amino acid sequence of SEQ ID NO. 213 or SEQ ID NO. 215. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 212 and a light chain amino acid sequence of SEQ ID NO:
213. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 214
and a light
chain amino acid sequence of SEQ ID NO: 215.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 216 or SEQ ID NO.
218; and a
light chain amino acid sequence of SEQ ID NO. 217 or SEQ ID NO. 219. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 216 and a light chain amino acid sequence of SEQ ID NO:
217. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
LPA has a reverse
17
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 218
and a light
chain amino acid sequence of SEQ ID NO: 219.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 220 or SEQ ID NO.
222; and a
light chain amino acid sequence of SEQ ID NO. 221 or SEQ ID NO. 223. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 220 and a light chain amino acid sequence of SEQ ID NO:
221. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
NGF has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 222
and a light
chain amino acid sequence of SEQ ID NO: 223.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 224 or SEQ ID NO.
226; and a
light chain amino acid sequence of SEQ ID NO. 225 or SEQ ID NO. 227. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 224 and a light chain amino acid sequence of SEQ ID NO:
225. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
NGF has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 226
and a light
chain amino acid sequence of SEQ ID NO: 227.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 228 or SEQ ID NO.
230; and a
light chain amino acid sequence of SEQ ID NO. 229 or SEQ ID NO. 231. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 228 and a light chain amino acid sequence of SEQ ID NO:
229. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
NGF has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 230
and a light
chain amino acid sequence of SEQ ID NO: 231.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 232 or SEQ ID NO.
234; and a
light chain amino acid sequence of SEQ ID NO. 233 or SEQ ID NO. 235. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 232 and a light chain amino acid sequence of SEQ ID NO:
233. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
NGF has a reverse
18
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 234
and a light
chain amino acid sequence of SEQ ID NO: 235.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and NGF
comprises a heavy chain amino acid sequence of SEQ ID NO. 236 or SEQ ID NO.
238; and a
light chain amino acid sequence of SEQ BD NO. 237 or SEQ ID NO. 239. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and NGF comprises a heavy
chain amino acid
sequence of SEQ ID NO. 236 and a light chain amino acid sequence of SEQ ID NO:
237. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
NGF has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 238
and a light
chain amino acid sequence of SEQ ID NO: 239.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 240 or SEQ ID NO.
242; and a
light chain amino acid sequence of SEQ ID NO. 241 or SEQ ID NO. 243. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 240 and a light chain amino acid sequence of SEQ ID NO:
241. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 242 and a
light chain amino acid sequence of SEQ ID NO: 243.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 244 or SEQ ID NO.
246; and a
light chain amino acid sequence of SEQ ID NO. 245 or SEQ ID NO. 247. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 244 and a light chain amino acid sequence of SEQ ID NO:
245. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 246 and a
light chain amino acid sequence of SEQ ID NO: 247.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and
SOSTcomprises a heavy chain amino acid sequence of SEQ ID NO. 248 or SEQ ID
NO. 250; and
a light chain amino acid sequence of SEQ ID NO. 249 or SEQ ID NO. 251. In an
embodiment,
the binding protein capable of binding TNF (seq. 4) and SOST comprises a heavy
chain amino
acid sequence of SEQ ID NO. 248 and a light chain amino acid sequence of SEQ
ID NO: 249. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
SOST has a
19
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 250 and a
light chain amino acid sequence of SEQ ID NO: 251.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 252 or SEQ ID NO.
254; and a
light chain amino acid sequence of SEQ ID NO. 253 or SEQ ID NO. 255. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 252 and a light chain amino acid sequence of SEQ ID NO:
253. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 254 and a
light chain amino acid sequence of SEQ ID NO: 255.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and SOST
comprises a heavy chain amino acid sequence of SEQ ID NO. 256 or SEQ ID NO.
258; and a
light chain amino acid sequence of SEQ ID NO. 257 or SEQ ID NO. 259. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and SOST comprises a heavy
chain amino acid
sequence of SEQ ID NO. 256 and a light chain amino acid sequence of SEQ ID NO:
257. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
SOST has a
reverse orientation and comprises a heavy chain amino acid sequence of SEQ ID
NO. 258 and a
light chain amino acid sequence of SEQ ID NO: 259.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 260 or SEQ ID NO.
262; and a
light chain amino acid sequence of SEQ ID NO. 261 or SEQ ID NO. 263. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 260 and a light chain amino acid sequence of SEQ ID NO:
261. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 262
and a light
chain amino acid sequence of SEQ ID NO: 263.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 264 or SEQ ID NO.
266; and a
light chain amino acid sequence of SEQ ID NO. 265 or SEQ ID NO. 267. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 264 and a light chain amino acid sequence of SEQ ID NO:
265. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
PGE2 has a reverse
20
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 266
and a light
chain amino acid sequence of SEQ ID NO: 267.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 268 or SEQ ID NO.
270; and a
light chain amino acid sequence of SEQ ID NO. 269 or SEQ ID NO. 271. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 268 and a light chain amino acid sequence of SEQ ID NO:
269. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 270
and a light
chain amino acid sequence of SEQ ID NO: 271.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 272 or SEQ ID NO.
274; and a
light chain amino acid sequence of SEQ ID NO. 273 or SEQ ID NO. 275. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 272 and a light chain amino acid sequence of SEQ ID NO:
273. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 274
and a light
chain amino acid sequence of SEQ ID NO: 275.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and PGE2
comprises a heavy chain amino acid sequence of SEQ ID NO. 276 or SEQ ID NO.
278; and a
light chain amino acid sequence of SEQ ID NO. 277 or SEQ ID NO. 279. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and PGE2 comprises a heavy
chain amino acid
sequence of SEQ ID NO. 276 and a light chain amino acid sequence of SEQ ID NO:
277. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
PGE2 has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 278
and a light
chain amino acid sequence of SEQ ID NO: 279.
In an embodiment, the binding protein capable of binding TNF (seq. 1) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 280 or SEQ ID NO.
282; and a
light chain amino acid sequence of SEQ ID NO. 281 or SEQ ID NO. 283. In an
embodiment, the
binding protein capable of binding TNF (seq. 1) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 280 and a light chain amino acid sequence of SEQ ID NO:
281. In
another embodiment, the binding protein capable of binding TNF (seq. 1) and
LPA has a reverse
21
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 282
and a light
chain amino acid sequence of SEQ ID NO: 283.
In an embodiment, the binding protein capable of binding TNF (seq. 2) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 284 or SEQ ID NO.
286; and a
light chain amino acid sequence of SEQ ID NO. 285 or SEQ ID NO. 287. In an
embodiment, the
binding protein capable of binding TNF (seq. 2) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 284 and a light chain amino acid sequence of SEQ ID NO:
285. In
another embodiment, the binding protein capable of binding TNF (seq. 2) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 286
and a light
chain amino acid sequence of SEQ ID NO: 287.
In an embodiment, the binding protein capable of binding TNF (seq. 3) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 288 or SEQ ID NO.
290; and a
light chain amino acid sequence of SEQ ID NO. 289 or SEQ ID NO. 291. In an
embodiment, the
binding protein capable of binding TNF (seq. 3) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 288 and a light chain amino acid sequence of SEQ ID NO:
289. In
another embodiment, the binding protein capable of binding TNF (seq. 3) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 290
and a light
chain amino acid sequence of SEQ ID NO: 291.
In an embodiment, the binding protein capable of binding TNF (seq. 4) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 292 or SEQ ID NO.
294; and a
light chain amino acid sequence of SEQ ID NO. 293 or SEQ ID NO. 295. In an
embodiment, the
binding protein capable of binding TNF (seq. 4) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 292 and a light chain amino acid sequence of SEQ ID NO:
293. In
another embodiment, the binding protein capable of binding TNF (seq. 4) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 294
and a light
chain amino acid sequence of SEQ ID NO: 295.
In an embodiment, the binding protein capable of binding TNF (seq. 5) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 296 or SEQ ID NO.
298; and a
light chain amino acid sequence of SEQ ID NO. 297 or SEQ ID NO. 299. In an
embodiment, the
binding protein capable of binding TNF (seq. 5) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 296 and a light chain amino acid sequence of SEQ ID NO:
297. In
another embodiment, the binding protein capable of binding TNF (seq. 5) and
LPA has a reverse
22
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 298
and a light
chain amino acid sequence of SEQ ID NO: 299.
In an embodiment, the binding protein capable of binding TNF (seq. 6) and LPA
comprises a heavy chain amino acid sequence of SEQ ID NO. 300 or SEQ ID NO.
302; and a
light chain amino acid sequence of SEQ ID NO. 301 or SEQ ID NO. 303. In an
embodiment, the
binding protein capable of binding TNF (seq. 6) and LPA comprises a heavy
chain amino acid
sequence of SEQ ID NO. 300 and a light chain amino acid sequence of SEQ ID NO:
301. In
another embodiment, the binding protein capable of binding TNF (seq. 6) and
LPA has a reverse
orientation and comprises a heavy chain amino acid sequence of SEQ ID NO. 302
and a light
chain amino acid sequence of SEQ ID NO: 303.
In another embodiment, the binding protein comprises a polypeptide chain,
wherein said
polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n, wherein; VD1 is a first
heavy chain
variable domain obtained from a first parent antibody or antigen binding
portion thereof; VD2 is
a second heavy chain variable domain obtained from a second parent antibody or
antigen binding
portion thereof; C is a heavy chain constant domain; (Xl)n is a linker with
the proviso that it is
not CH1, wherein said (XI )n is either present or absent; and (X2)n is an Fc
region, wherein said
(X2)n is either present or absent. In an embodiment, the Fc region is absent
from the binding
protein.
In another embodiment, the binding protein comprises a polypeptide chain,
wherein said
polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n, wherein, VD1 is a first
light chain
variable domain obtained from a first parent antibody or antigen binding
portion thereof; VD2 is
a second light chain variable domain obtained from a second parent antibody or
antigen binding
portion thereof; C is a light chain constant domain; (Xl)n is a linker with
the proviso that it is not
CL, wherein said (Xl)n is either present or absent; and (X2)n does not
comprise an Fe region,
wherein said (X2)n is either present or absent. In an embodiment, (X2)n is
absent from the
binding protein.
In another embodiment the binding protein comprises first and second
polypeptide
chains, wherein said first polypeptide chain comprises a first VD1-(Xl)n-VD2-C-
(X2)n, wherein
VD1 is a first heavy chain variable domain obtained from a first parent
antibody or antigen
binding portion thereof; VD2 is a second heavy chain variable domain obtained
from a second
parent antibody or antigen binding portion thereof; C is a heavy chain
constant domain; (Xl)n is
a linker with the proviso that it is not CH1, wherein said (Xl)n is either
present or absent; and
(X2)n is an Fc region, wherein said (X2)n is either present or absent; and
wherein said second
23
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
polypeptide chain comprises a second VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is a
first light
chain variable domain obtained from a first parent antibody or antigen binding
portion thereof;
VD2 is a second light chain variable domain obtained from a second parent
antibody or antigen
binding portion thereof; C is a light chain constant domain; (Xl)n is a linker
(optionally which is
not CL), wherein said (Xl)n is either present or absent; and (X2)n does not
comprise an Fc
region, wherein said (X2)n is either present or absent. In another embodiment,
the binding protein
comprises two first polypeptide chains and two second polypeptide chains. In
yet another
embodiment, (X2)n is absent from the second polypeptide. In still another
embodiment, the Fc
region, if present in the first polypeptide is a native sequence Fc region or
a variant sequence Fc
region. In still another embodiment, the Fe region is from an IgGl, IgG2,
IgG3, IgG4, IgA, IgM,
IgE, or IgD.
In another embodiment, the binding protein is a DVD-Ig capable of binding two
antigens
comprising four polypeptide chains, wherein, first and third polypeptide
chains comprise VD1-
(Xl)n-VD2-C-(X2)n, wherein,VD1 is a first heavy chain variable domain obtained
from a first
parent antibody or antigen binding portion thereof; VD2 is a second heavy
chain variable domain
obtained from a second parent antibody or antigen binding portion thereof; C
is a heavy chain
constant domain; (Xl)n is a linker with the proviso that it is not CH1,
wherein said (Xl)n is
either present or absent; and (X2)n is an Fc region, wherein said (X2)n is
either present or absent;
and wherein second and fourth polypeptide chains comprise VD1-(X1)n-VD2-C-
(X2)n, wherein
VD1 is a first light chain variable domain obtained from a first parent
antibody or antigen binding
portion thereof; VD2 is a second light chain variable domain obtained from a
second parent
antibody or antigen binding portion thereof; C is a light chain constant
domain; (Xl)n is a linker
(optionally which is not CL), wherein said (Xl)n is either present or absent;
and (X2)n does not
comprise an Fc region, wherein said (X2)n is either present or absent.
A method of making a DVD-Ig binding protein by preselecting the parent
antibodies is
also provided. In an embodiment, the method of making a Dual Variable Domain
Immunoglobulin capable of binding two antigens comprising the steps of a)
obtaining a first
parent antibody or antigen binding portion thereof, capable of binding a first
antigen; b) obtaining
a second parent antibody or antigen binding portion thereof, capable of
binding a second antigen;
c) constructing first and third polypeptide chains comprising VD1-(Xl)n-VD2-C-
(X2)n, wherein,
VD1 is a first heavy chain variable domain obtained from said first parent
antibody or antigen
binding portion thereof; VD2 is a second heavy chain variable domain obtained
from said second
parent antibody or antigen binding portion thereof; C is a heavy chain
constant domain; (Xl)n is
a linker with the proviso that it is not CH I, wherein said (Xl)n is either
present or absent; and
24
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
(X2)n is an Fe region, wherein said (X2)n is either present or absent; d)
constructing second and
fourth polypeptide chains comprising VD1-(Xl)n-VD2-C-(X2)n, wherein, VD1 is a
first light
chain variable domain obtained from said first parent antibody or antigen
binding portion thereof;
VD2 is a second light chain variable domain obtained from said second parent
antibody or
antigen binding thereof; C is a light chain constant domain; (Xl)n is a linker
(optionally which is
not CL), wherein said (Xl)n is either present or absent; and (X2)n does not
comprise an Fe
region, wherein said (X2)n is either present or absent; e) expressing said
first, second, third and
fourth polypeptide chains; such that a Dual Variable Domain Immunoglobulin
capable of binding
said first and said second antigen is generated.
In still another embodiment, a method of generating a Dual Variable Domain
Immunoglobulin capable of binding two antigens with desired properties
comprising the steps of
a) obtaining a first parent antibody or antigen binding portion thereof,
capable of binding a first
antigen and possessing at least one desired property exhibited by the Dual
Variable Domain
Immunoglobulin; b) obtaining a second parent antibody or antigen binding
portion thereof,
capable of binding a second antigen and possessing at least one desired
property exhibited by the
Dual Variable Domain Immunoglobulin; c) constructing first and third
polypeptide chains
comprising VDI-(XI)n-VD2-C-(X2)n, wherein; VD1 is a first heavy chain variable
domain
obtained from said first parent antibody or antigen binding portion thereof;
VD2 is a second
heavy chain variable domain obtained from said second parent antibody or
antigen binding
portion thereof; C is a heavy chain constant domain; (X 1)n is a linker with
the proviso that it is
not CH1, wherein said (Xl)n is either present or absent; and (X2)n is an Fe
region, wherein said
(X2)n is either present or absent; d) constructing second and fourth
polypeptide chains
comprising VD1-(X1)n-VD2-C-(X2)n, wherein; VDI is a first light chain variable
domain
obtained from said first parent antibody or antigen binding portion thereof;
VD2 is a second light
chain variable domain obtained from said second parent antibody or antigen
binding portion
thereof; C is a light chain constant domain; (Xl)n is a linker (optionally
which is not CL),
wherein said (Xl)n is either present or absent; and (X2)n does not comprise an
Fe region,
wherein said (X2)n is either present or absent; e) expressing said first,
second, third and fourth
polypeptide chains; such that a Dual Variable Domain Immunoglobulin capable of
binding said
first and said second antigen with desired properties is generated is
provided.
In one embodiment, the VDI of the first and second polypeptide chains
disclosed herein
are obtained from the same parent antibody or antigen binding portion thereof.
In another
embodiment, the VDI of the first and second polypeptide chains disclosed
herein are obtained
from different parent antibodies or antigen binding portions thereof. In
another embodiment, the
25
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
VD2 of the first and second polypeptide chains disclosed herein are obtained
from the same
parent antibody or antigen binding portion thereof. In another embodiment, the
VD2 of the first
and second polypeptide chains disclosed herein are obtained from different
parent antibodies or
antigen binding portions thereof.
In one embodiment the first parent antibody or antigen binding portion
thereof, and the
second parent antibody or antigen binding portion thereof, are the same
antibody. In another
embodiment the first parent antibody or antigen binding portion thereof, and
the second parent
antibody or antigen binding portion thereof, are different antibodies.
In one embodiment the first parent antibody or antigen binding portion
thereof, binds a
first antigen and the second parent antibody or antigen binding portion
thereof, binds a second
antigen. In a particular embodiment, the first and second antigens are the
same antigen. In another
embodiment, the parent antibodies bind different epitopes on the same antigen.
In another
embodiment the first and second antigens are different antigens. In another
embodiment, the first
parent antibody or antigen binding portion thereof, binds the first antigen
with a potency different
from the potency with which the second parent antibody or antigen binding
portion thereof, binds
the second antigen. In yet another embodiment, the first parent antibody or
antigen binding
portion thereof, binds the first antigen with an affinity different from the
affinity with which the
second parent antibody or antigen binding portion thereof, binds the second
antigen.
In another embodiment the first parent antibody or antigen binding portion
thereof, and
the second parent antibody or antigen binding portion thereof, are a human
antibody, a CDR
grafted antibody, or a humanized antibody. In an embodiment, the antigen
binding portions are
Fab fragments, F(ab')2 fragments, bivalent fragments comprising two Fab
fragments linked by a
disulfide bridge at the hinge region, Fd fragments comprising VH and CH1
domains, Fv
fragments comprising the VL and VH domains of a single arm of an antibody, dAb
fragments,
isolated complementarity determining regions (CDR), single chain antibodies,
or diabodies.
In another embodiment the binding protein possesses at least one desired
property
exhibited by the first parent antibody or antigen binding portion thereof, or
the second parent
antibody or antigen binding portion thereof. Alternatively, the first parent
antibody or antigen
binding portion thereof and the second parent antibody or antigen binding
portion thereof possess
at least one desired property exhibited by the Dual Variable Domain
Immunoglobulin. In an
embodiment, the desired property is selected from one or more antibody
parameters. In another
embodiment, the antibody parameters are antigen specificity, affinity to
antigen, potency,
biological function, epitope recognition, stability, solubility, production
efficiency,
26
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
immunogenicity, pharmacokinetics, bioavailability, tissue cross reactivity, or
orthologous antigen
binding. In an embodiment the binding protein is multivalent. In another
embodiment, the binding
protein is multispecific. The multivalent and or multispecific binding
proteins described herein
have desirable properties particularly from a therapeutic standpoint. For
instance, the multivalent
and or multispecific binding protein may (1) be internalized (and/or
catabolized) faster than a
bivalent antibody by a cell expressing an antigen to which the antibodies
bind; (2) be an agonist
antibody; and/or (3) induce cell death and/or apoptosis of a cell expressing
an antigen which the
multivalent antibody is capable of binding to. The "parent antibody" which
provides at least one
antigen binding specificity of the multivalent and or multispecific binding
proteins may be one
which is internalized (and/or catabolized) by a cell expressing an antigen to
which the antibody
binds; and/or may be an agonist, cell death-inducing, and/or apoptosis-
inducing antibody, and the
multivalent and or multispecific binding protein as described herein may
display improvement(s)
in one or more of these properties. Moreover, the parent antibody may lack any
one or more of
these properties, but may be endowed with them when constructed as a
multivalent binding
protein as described herein.
In another embodiment the binding protein has an on rate constant (Kon) to one
or more
targets of: at least about 102M-1s-1; at least about 103M-15-1; at least about
104M-Is-1; at least about
105M-1s-1; and at least about 106M-1s-1, as measured by surface plasmon
resonance. In an
embodiment, the binding protein has an on rate constant (Kon) to one or more
targets between
102M4s-1 and 103M-1s-1; between 103M-1s-I and 104M-I s-I; between 104M-1s-I
and 105M1 s; or
between 105M-Is-1 and 106M-Is1, as measured by surface plasmon resonance.
In another embodiment the binding protein has an off rate constant (Koff) for
one or
more targets of: at most about 10-3s-1; at most about 10-4s-1; at most about
10-5s-1; and at most
about 10-6s-1, as measured by surface plasmon resonance. In an embodiment, the
binding protein
has an off rate constant (Koff) to one or more targets of 10-3s-1 to 10-4s-1;
of 10-4s-1 to 10-5S-1; or of
10-5s-1 to 10-6s-1, as measured by surface plasmon resonance.
In another embodiment the binding protein has a dissociation constant (KD) to
one or
more targets of: at most about 10-7 M; at most about 10-8 M; at most about 10-
9 M; at most about
10-10 M; at most about 10-" M; at most about 10-12 M; and at most I 0-13 M. In
an embodiment, the
binding protein has a dissociation constant (KD) to its targets of 10-7 M to
10-8 M; of 108 M to 10-
9 M; of leM to 10-10 M; of 10-1 to 10-" M; of 10-" M to 10-12 M; or of 10-12
to M 1013 M.
In another embodiment, the binding protein described herein is a conjugate
further
comprising an agent, wherein said agent is an immunoadhesion molecule, an
imaging agent, a
27
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
therapeutic agent, or a cytotoxic agent. In an embodiment, the imaging agent
is a radiolabel, an
enzyme, a fluorescent label, a luminescent label, a bioluminescent label, a
magnetic label, or
biotin. In another embodiment, the radiolabel is: 3H, 14C, 35s, 90y, 99Tc,
111/ 125/ n, , 131/,177/4 166/10,
or I53Sm. In yet another embodiment, the therapeutic or cytotoxic agent is an
anti-metabolite, an
alkylating agent, an antibiotic, a growth factor, a cytokine, an anti-
angiogenic agent, an anti-
mitotic agent, an anthracycline, toxin, or an apoptotic agent.
In another embodiment, the binding protein described herein is a crystallized
binding
protein and exists as a crystal. In an embodiment, the crystal is a carrier-
free pharmaceutical
controlled release crystal. In yet another embodiment, the crystallized
binding protein has a
greater half life in vivo than the soluble counterpart of said binding
protein. In still another
embodiment, the crystallized binding protein retains biological activity.
In another embodiment, the binding protein described herein is glycosylated.
For
example, the glycosylation is a human glycosylation pattern.
An isolated nucleic acid encoding any one of the binding proteins disclosed
herein is also
provided. A further embodiment provides a vector comprising the isolated
nucleic acid disclosed
herein wherein said vector is pcDNA; pTT (Durocher et al., Nucleic Acids
Research 2002, Vol
30, No.2); pTT3 (pTT with additional multiple cloning site; pEFBOS (Mizushima,
S. and Nagata,
S., (1990) Nucleic acids Research Vol 18, No. 17); pBV; pJV; pcDNA3.1 TOPO;
pEF6 TOPO;
or pBJ. In an embodiment, the vector is a vector disclosed in US Patent
Application Serial No.
61/021,282.
In another aspect a host cell is transformed with the vector disclosed herein.
In an
embodiment, the host cell is a prokaryotic cell. In another embodiment, the
host cell is E.Coli. In
a related embodiment the host cell is a eukaryotic cell. In another
embodiment, the eukaryotic
cell is a protist cell, an animal cell, a plant cell, or a fungal cell. In yet
another embodiment, the
host cell is a mammalian cell including, but not limited to, CHO, COS; NSO,
SP2, PER.C6 or a
fungal cell such as Saccharomyces cerevisiae; or an insect cell such as Sf9.
In an embodiment, two or more DVD-Igs, e.g., with different specificities, are
produced
in a single recombinant host cell. For example, the expression of a mixture of
antibodies has been
called OligoclonicsTM Merus B.V., The Netherlands); U.S. Patent Nos.
7,262,028; 7,429,486.
A method of producing a binding protein disclosed herein comprising culturing
any one
of the host cells also disclosed herein in a culture medium under conditions
sufficient to produce
the binding protein is also provided. In an embodiment, 50%-75% of the binding
protein
28
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
produced by this method is a dual specific tetravalent binding protein. In a
particular
embodiment, 75%-90% of the binding protein produced by this method is a dual
specific
tetravalent binding protein. In a particular embodiment, 90%-95% of the
binding protein
produced is a dual specific tetravalent binding protein.
One embodiment provides a composition for the release of a binding protein
wherein the
composition comprises a formulation that in turn comprises a crystallized
binding protein, as
disclosed herein, and an ingredient, and at least one polymeric carrier. For
example, the
polymeric carrier is: poly (acrylic acid), poly (cyanoacrylates), poly (amino
acids), poly
(anhydrides), poly (depsipeptide), poly (esters), poly (lactic acid), poly
(lactic-co-glycolic acid)
or PLGA, poly (b-hydroxybutryate), poly (caprolactone), poly (dioxanone); poly
(ethylene
glycol), poly ((hydroxypropyl) methacrylamide, poly [(organo)phosphazene],
poly (ortho esters),
poly (vinyl alcohol), poly (vinylpyrrolidone), maleic anhydride- alkyl vinyl
ether copolymers,
pluronic polyols, albumin, alginate, cellulose and cellulose derivatives,
collagen, fibrin, gelatin,
hyaluronic acid, oligosaccharides, glycaminoglycans, sulfated polysaccharides,
blends and
copolymers thereof. For example, in some embodiments the ingredient is
albumin, sucrose,
trehalose, lactitol, gelatin, hydroxypropyl-P- cyclodextrin,
methoxypolyethylene glycol, or
polyethylene glycol. Another embodiment provides a method for treating a
mammal comprising
the step of administering to the mammal an effective amount of the composition
disclosed herein.
A pharmaceutical composition comprising a binding protein, as disclosed herein
and a
pharmaceutically acceptable carrier is also provided. In a further embodiment
the pharmaceutical
composition comprises at least one additional therapeutic agent for treating a
disorder. For
example, the additional agent is: a therapeutic agent, an imaging agent, a
cytotoxic agent, an
angiogenesis inhibitor (including but not limited to an anti-VEGF antibody or
a VEGF-trap), a
kinase inhibitor (including but not limited to a KDR and a TIE-2 inhibitor), a
co-stimulation
molecule blocker (including but not limited to anti-B7.1, anti-B7.2, CTLA4-Ig,
anti-CD20), an
adhesion molecule blocker (including but not limited to an anti-LFA-1
antibody, an anti-E/L
selectin antibody, a small molecule inhibitor), an anti-cytokine antibody or
functional fragment
thereof (including but not limited to an anti-IL-18, an anti-TNF, and an anti-
IL-6/cytokine
receptor antibody), methotrexate, cyclosporin, rapamycin, FK506, a detectable
label or reporter, a
TNF antagonist, an antirheumatic, a muscle relaxant, a narcotic, a non-steroid
anti-inflammatory
drug (NSAID), an analgesic, an anesthetic, a sedative, a local anesthetic, a
neuromuscular
blocker, an antimicrobial, an antipsoriatic, a corticosteriod, an anabolic
steroid, an erythropoietin,
an immunization, an immunoglobulin, an immunosuppressive, a growth hormone, a
hormone
replacement drug, a radiopharmaceutical, an antidepressant, an antipsychotic,
a stimulant, an
29
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
asthma medication, a beta agonist, an inhaled steroid, an epinephrine or
analog, a cytokine, or a
cytokine antagonist.
A method for treating a human subject suffering from a disorder in which the
target, or
targets, capable of being bound by the binding protein disclosed herein is
detrimental, comprising
administering to the human subject a binding protein disclosed herein such
that the activity of the
target, or targets in the human subject is inhibited and one of more symptoms
is alleviated or
treatment is achieved is also provided. For example, the disorder is selected
from the group
comprising arthritis, osteoarthritis, juvenile chronic arthritis, septic
arthritis, Lyme arthritis,
psoriatic arthritis, reactive arthritis, spondyloarthropathy, systemic lupus
erythematosus, Crohn's
disease, ulcerative colitis, inflammatory bowel disease, insulin dependent
diabetes mellitus,
thyroiditis, asthma, allergic diseases, psoriasis, dermatitis scleroderma,
graft versus host disease,
organ transplant rejection, acute or chronic immune disease associated with
organ
transplantation, sarcoidosis, atherosclerosis, disseminated intravascular
coagulation, Kawasaki's
disease, Grave's disease, nephrotic syndrome, chronic fatigue syndrome,
Wegener's
granulomatosis, Henoch-Schoenlein purpurea, microscopic vasculitis of the
kidneys, chronic
active hepatitis, uveitis, septic shock, toxic shock syndrome, sepsis
syndrome, cachexia,
infectious diseases, parasitic diseases, acquired immunodeficiency syndrome,
acute transverse
myelitis, Huntington's chorea, Parkinson's disease, Alzheimer's disease,
stroke, primary biliary
cirrhosis, hemolytic anemia, malignancies, heart failure, myocardial
infarction, Addison's disease,
sporadic polyglandular deficiency type I and polyglandular deficiency type II,
Schmidt's
syndrome, adult (acute) respiratory distress syndrome, alopecia, alopecia
areata, seronegative
arthopathy, arthropathy, Reiter's disease, psoriatic arthropathy, ulcerative
colitic arthropathy,
enteropathic synovitis, chlamydia, yersinia and salmonella associated
arthropathy,
spondyloarthopathy, atheromatous disease/arteriosclerosis, atopic allergy,
autoimmune bullous
disease, pemphigus vulgaris, pemphigus foliaceus, pemphigoid, linear IgA
disease, autoimmune
haemolytic anaemia, Coombs positive haemolytic anaemia, acquired pernicious
anaemia, juvenile
pernicious anaemia, myalgic encephalitis/Royal Free Disease, chronic
mucocutaneous
candidiasis, giant cell arteritis, primary sclerosing hepatitis, cryptogenic
autoimmune hepatitis,
Acquired Immunodeficiency Disease Syndrome, Acquired Immunodeficiency Related
Diseases,
Hepatitis B, Hepatitis C, common varied immunodeficiency (common variable
hypogammaglobulinaemia), dilated cardiomyopathy, female infertility, ovarian
failure, premature
ovarian failure, fibrotic lung disease, cryptogenic fibrosing alveolitis, post-
inflammatory
interstitial lung disease, interstitial pneumonitis, connective tissue disease
associated interstitial
lung disease, mixed connective tissue disease associated lung disease,
systemic sclerosis
associated interstitial lung disease, rheumatoid arthritis associated
interstitial lung disease,
30
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
systemic lupus erythematosus associated lung disease,
dermatomyositis/polymyositis associated
lung disease, Sjogren's disease associated lung disease, ankylosing
spondylitis associated lung
disease, vasculitic diffuse lung disease, haemosiderosis associated lung
disease, drug-induced
interstitial lung disease, fibrosis, radiation fibrosis, bronchiolitis
obliterans, chronic eosinophilic
pneumonia, lymphocytic infiltrative lung disease, postinfectious interstitial
lung disease, gouty
arthritis, autoimmune hepatitis, type-1 autoimmune hepatitis (classical
autoimmune or lupoid
hepatitis), type-2 autoimmune hepatitis (anti-LKM antibody hepatitis),
autoimmune mediated
hypoglycaemia, type B insulin resistance with acanthosis nigricans,
hypoparathyroidism, acute
immune disease associated with organ transplantation, chronic immune disease
associated with
organ transplantation, osteoarthrosis, primary sclerosing cholangitis,
psoriasis type 1, psoriasis
type 2, idiopathic leucopaenia, autoimmune neutropaenia, renal disease NOS,
glomerulonephritides, microscopic vasulitis of the kidneys, lyme disease,
discoid lupus
erythematosus, male infertility idiopathic or NOS, sperm autoimmunity,
multiple sclerosis (all
subtypes), sympathetic ophthalmia, pulmonary hypertension secondary to
connective tissue
disease, Goodpasture's syndrome, pulmonary manifestation of polyarteritis
nodosa, acute
rheumatic fever, rheumatoid spondylitis, Still's disease, systemic sclerosis,
Sjorgren's syndrome,
Takayasu's disease/arteritis, autoimmune thrombocytopaenia, idiopathic
thrombocytopaenia,
autoimmune thyroid disease, hyperthyroidism, goitrous autoimmune
hypothyroidism
(Hashimoto's disease), atrophic autoimmune hypothyroidism, primary myxoedema,
phacogenic
uveitis, primary vasculitis, vitiligo acute liver disease, chronic liver
diseases, alcoholic cirrhosis,
alcohol-induced liver injury, choleosatatis, idiosyncratic liver disease, Drug-
Induced hepatitis,
Non-alcoholic Steatohepatitis, allergy and asthma, group B streptococci (GBS)
infection, mental
disorders (e.g., depression and schizophrenia), Th2 Type and Thl Type mediated
diseases, acute
and chronic pain (different forms of pain), and cancers such as lung, breast,
stomach, bladder,
colon, pancreas, ovarian, prostate and rectal cancer and hematopoietic
malignancies (leukemia
and lymphoma), Abetalipoprotemia, Acrocyanosis, acute and chronic parasitic or
infectious
processes, acute leukemia, acute lymphoblastic leukemia (ALL), acute myeloid
leukemia (AML),
acute or chronic bacterial infection, acute pancreatitis, acute renal failure,
adenocarcinomas,
aerial ectopic beats, AIDS dementia complex, alcohol-induced hepatitis,
allergic conjunctivitis,
allergic contact dermatitis, allergic rhinitis, allograft rejection, alpha-l-
antitrypsin deficiency,
amyotrophic lateral sclerosis, anemia, angina pectoris, anterior horn cell
degeneration, anti cd3
therapy, antiphospholipid syndrome, anti-receptor hypersensitivity reactions,
aortic and
peripheral aneuryisms, aortic dissection, arterial hypertension,
arteriosclerosis, arteriovenous
fistula, ataxia, atrial fibrillation (sustained or paroxysmal), atrial
flutter, atrioventricular block, B
cell lymphoma, bone graft rejection, bone marrow transplant (BMT) rejection,
bundle branch
block, Burkitt's lymphoma, Burns, cardiac arrhythmias, cardiac stun syndrome,
cardiac tumors,
31
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
cardiomyopathy, cardiopulmonary bypass inflammation response, cartilage
transplant rejection,
cerebellar cortical degenerations, cerebellar disorders, chaotic or multifocal
atrial tachycardia,
chemotherapy associated disorders, chronic myelocytic leukemia (CML), chronic
alcoholism,
chronic inflammatory pathologies, chronic lymphocytic leukemia (CLL), chronic
obstructive
pulmonary disease (COPD), chronic salicylate intoxication, colorectal
carcinoma, congestive
heart failure, conjunctivitis, contact dermatitis, cor pulmonale, coronary
artery disease,
Creutzfeldt-Jakob disease, culture negative sepsis, cystic fibrosis, cytokine
therapy associated
disorders, Dementia pugilistica, demyelinating diseases, dengue hemorrhagic
fever, dermatitis,
dermatologic conditions, diabetes, diabetes mellitus, diabetic ateriosclerotic
disease, Diffuse
Lewy body disease, dilated congestive cardiomyopathy, disorders of the basal
ganglia, Down's
Syndrome in middle age, drug- induced movement disorders induced by drugs
which block CNS
dopamine receptors, drug sensitivity, eczema, encephalomyelitis, endocarditis,
endocrinopathy,
epiglottitis, epstein-barr virus infection, erythromelalgia, extrapyramidal
and cerebellar disorders,
familial hematophagocytic lymphohistiocytosis, fetal thymus implant rejection,
Friedreich's
ataxia, functional peripheral arterial disorders, fungal sepsis, gas gangrene,
gastric ulcer,
glomerular nephritis, graft rejection of any organ or tissue, gram negative
sepsis, gram positive
sepsis, granulomas due to intracellular organisms, hairy cell leukemia,
Hallerrorden-Spatz
disease, hashimoto's thyroiditis, hay fever, heart transplant rejection,
hemachromatosis,
hemodialysis, hemolytic uremic syndrome/thrombolytic thrombocytopenic purpura,
hemorrhage,
hepatitis (A), His bundle arrythmias, HIV infection/HIV neuropathy, Hodgkin's
disease,
hyperkinetic movement disorders, hypersensitity reactions, hypersensitivity
pneumonitis,
hypertension, hypokinetic movement disorders, hypothalamic-pituitary-adrenal
axis evaluation,
idiopathic Addison's disease, idiopathic pulmonary fibrosis, antibody mediated
cytotoxicity,
Asthenia, infantile spinal muscular atrophy, inflammation of the aorta,
influenza a, ionizing
radiation exposure, iridocyclitis/uveitis/optic neuritis, ischemia-
reperfusion injury, ischemic
stroke, juvenile rheumatoid arthritis, juvenile spinal muscular atrophy,
Kaposi's sarcoma, kidney
transplant rejection, legionella, leishmaniasis, leprosy, lesions of the
corticospinal system,
lipedema, liver transplant rejection, lymphederma, malaria, malignamt
Lymphoma, malignant
histiocytosis, malignant melanoma, meningitis, meningococcemia,
metabolic/idiopathic diseases,
migraine headache, mitochondria] multi.system disorder, mixed connective
tissue disease,
monoclonal gammopathy, multiple myeloma, multiple systems degenerations
(Mencel Dejerine-
Thomas Shi-Drager and Machado-Joseph), myasthenia gravis, mycobacterium avium
intracellulare, mycobacterium tuberculosis, myelodyplastic syndrome,
myocardial infarction,
myocardial ischemic disorders, nasopharyngeal carcinoma, neonatal chronic lung
disease,
nephritis, nephrosis, neurodegenerative diseases, neurogenic I muscular
atrophies, neutropenic
fever, non- hodgkins lymphoma, occlusion of the abdominal aorta and its
branches, occlusive
32
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
arterial disorders, okt3 therapy, orchitis/epidydimitis, orchitis/vasectomy
reversal procedures,
organomegaly, osteoporosis, pancreas transplant rejection, pancreatic
carcinoma, paraneoplastic
syndrome/hypercalcemia of malignancy, parathyroid transplant rejection, pelvic
inflammatory
disease, perennial rhinitis, pericardial disease, peripheral atherlosclerotic
disease, peripheral
vascular disorders, peritonitis, pernicious anemia, pneumocystis carinii
pneumonia, pneumonia,
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal
gammopathy,
and skin changes syndrome), post perfusion syndrome, post pump syndrome, post-
MI cardiotomy
syndrome, preeclampsia, Progressive supranucleo Palsy, primary pulmonary
hypertension,
radiation therapy, Raynaud's phenomenon and disease, Raynoud's disease,
Refsum's disease,
regular narrow QRS tachycardia, renovascular hypertension, reperfusion injury,
restrictive
cardiomyopathy, sarcomas, scleroderma, senile chorea, Senile Dementia of Lewy
body type,
seronegative arthropathies, shock, sickle cell anemia, skin allograft
rejection, skin changes
syndrome, small bowel transplant rejection, solid tumors, specific arrythmias,
spinal ataxia,
spinocerebellar degenerations, streptococcal myositis, structural lesions of
the cerebellum,
Subacute sclerosing panencephalitis, Syncope, syphilis of the cardiovascular
system, systemic
anaphalaxis, systemic inflammatory response syndrome, systemic onset juvenile
rheumatoid
arthritis, T-cell or FAB ALL, Telangiectasia, thromboangitis obliterans,
thrombocytopenia,
toxicity, transplants, trauma/hemorrhage, type HI hypersensitivity reactions,
type IV
hypersensitivity, unstable angina, uremia, urosepsis, urticaria, valvular
heart diseases, varicose
veins, vasculitis, venous diseases, venous thrombosis, ventricular
fibrillation, viral and fungal
infections, vital encephalitis/aseptic meningitis, vital-associated
hemaphagocytic syndrome,
Wernicke- Korsakoff syndrome, Wilson's disease, xenograft rejection of any
organ or tissue,
acute coronary syndromes, acute idiopathic polyneuritis, acute inflammatory
demyelinating
polyradiculoneuropathy, acute ischemia, adult Still's disease, alopecia
areata, anaphylaxis, anti-
phospholipid antibody syndrome, aplastic anemia, arteriosclerosis, atonic
eczema, atopic
dermatitis, autoimmune dermatitis, autoimmune disorder associated with
streptococcus infection,
autoimmune enteropathy, autoimmune hearing loss, autoimmune
lymphoproliferative syndrome
(ALPS), autoimmune myocarditis, autoimmune premature ovarian failure,
blepharitis,
bronchiectasis, bullous pemphigoid, cardiovascular disease, catastrophic
antiphospholipid
syndrome, celiac disease, cervical spondylosis, chronic ischemia, cicatricial
pemphigoid,
clinically isolated syndrome (cis) with risk for multiple sclerosis,
conjunctivitis, childhood onset
psychiatric disorder, chronic obstructive pulmonary disease (COPD),
dacryocystitis,
dermatomyositis, diabetic retinopathy, diabetes mellitus, disk herniation,
disk prolaps, drug
induced immune hemolytic anemia, endocarditis, endometriosis, endophthalmitis,
episcleritis,
erythema multiforme, erythema multiforme major, gestational pemphigoid,
Guillain-Barre
syndrome (GBS), hay fever, Hughes syndrome, idiopathic Parkinson's disease,
idiopathic
33
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
interstitial pneumonia, IgE-mediated allergy, immune hemolytic anemia,
inclusion body myositis,
infectious ocular inflammatory disease, inflammatory demyelinating disease,
inflammatory heart
disease, inflammatory kidney disease, IPF/LTLP, iritis, keratitis,
keratojuntivitis sicca, Kussmaul
disease or Kussmaul-Meier disease, Landry's paralysis, Langerhan's cell
histiocytosis, livedo
reticularis, macular degeneration, microscopic polyangiitis, morbus bechterev,
motor neuron
disorders, mucous membrane pemphigoid, multiple organ failure, myasthenia
gravis,
myelodysplastic syndrome, myocarditis, nerve root disorders, neuropathy, non-A
non-B hepatitis,
optic neuritis, osteolysis, ovarian cancer, pauciarticular JRA, peripheral
artery occlusive disease
(PAOD), peripheral vascular disease (PVD), peripheral artery, disease (PAD),
phlebitis,
polyarteritis nodosa (or periarteritis nodosa), polychondritis, polymyalgia
rheumatica, poliosis,
polyarticular JRA, polyendocrine deficiency syndrome, polymyositis,
polymyalgia rheumatica
(PMR), post-pump syndrome, primary Parkinsonism, prostate and rectal cancer
and
hematopoietic malignancies (leukemia and lymphoma), prostatitis, pure red cell
aplasia, primary
adrenal insufficiency, recurrent neuromyelitis optica, restenosis, rheumatic
heart disease, sapho
(synovitis, acne, pustulosis, hyperostosis, and osteitis), scleroderma,
secondary amyloidosis,
shock lung, scleritis, sciatica, secondary adrenal insufficiency, silicone
associated connective
tissue disease, sneddon-wilkinson dermatosis, spondilitis ankylosans, Stevens-
Johnson syndrome
(SJS), systemic inflammatory response syndrome, temporal arteritis,
toxoplasmic retinitis, toxic
epidermal necrolysis, transverse myelitis, TRAPS (tumor necrosis factor
receptor, type 1 allergic
reaction, type II diabetes, urticaria, usual interstitial pneumonia (UIP),
vasculitis, vernal
conjunctivitis, viral retinitis, Vogt-Koyanagi-Harada syndrome (VKH syndrome),
wet macular
degeneration, wound healing, yersinia and salmonella associated arthropathy.
In an embodiment, diseases that can be treated or diagnosed with the
compositions and
methods disclosed herein include, but are not limited to, primary and
metastatic cancers,
including carcinomas of breast, colon, rectum, lung, oropharynx, hypopharynx,
esophagus,
stomach, pancreas, liver, gallbladder and bile ducts, small intestine, urinary
tract (including
kidney, bladder and urothelium), female genital tract (including cervix,
uterus, and ovaries as
well as choriocarcinoma and gestational trophoblastic disease), male genital
tract (including
prostate, seminal vesicles, testes and germ cell tumors), endocrine glands
(including the thyroid,
adrenal, and pituitary glands), and skin, as well as hemangiomas, melanomas,
sarcomas
(including those arising from bone and soft tissues as well as Kaposi's
sarcoma), tumors of the
brain, nerves, eyes, and meninges (including astrocytomas, gliomas,
glioblastomas,
retinoblastomas, neuromas, neuroblastomas, Schwannomas, and meningiomas),
solid tumors
arising from hematopoietic malignancies such as leukemias, and lymphomas (both
Hodgkin's and
non-Hodgkin's lymphomas).
34
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
The DVD-Igs disclosed herein may also treat one or more of the following
diseases:
Acute coronary syndromes, Acute Idiopathic Polyneuritis, Acute Inflammatory
Demyelinating
Polyradiculoneuropathy, Acute ischemia, Adult Still's Disease, Alopecia
areata, Anaphylaxis,
Anti-Phospholipid Antibody Syndrome, Aplastic anemia, Arteriosclerosis, Atopic
eczema,
Atopic dermatitis, Autoimmune dermatitis, Autoimmune disorder associated with
Streptococcus
infection, Autoimmune hearingloss, Autoimmune Lymphoproliferative Syndrome
(ALPS),
Autoimmune myocarditis, autoimmune thrombocytopenia (AITP), Blepharitis,
Bronchiectasis,
Bullous pemphigoid, Cardiovascular Disease, Catastrophic Antiphospholipid
Syndrome, Celiac
Disease, Cervical Spondylosis, Chronic ischemia, Cicatricial pemphigoid,
Clinically isolated
Syndrome (CIS) with Risk for Multiple Sclerosis, Conjunctivitis, Childhood
Onset Psychiatric
Disorder, Chronic obstructive pulmonary disease (COPD), Dacryocystitis,
dermatomyositis,
Diabetic retinopathy, Diabetes mellitus, Disk herniation, Disk prolaps, Drug
induced immune
hemolytic anemia, Endocarditis, Endometriosis, endophthalmitisõ Episcleritis,
Erythema
multiforme, erythema multiforme major, Gestational pemphigoid, Guillain-Barre
Syndrome
(GBS), Hay Fever, Hughes Syndrome , Idiopathic Parkinson's Disease, idiopathic
interstitial
pneumonia, IgE-mediated Allergy, Immune hemolytic anemia, Inclusion Body
Myositis,
Infectious ocular inflammatory disease, Inflammatory demyelinating disease,
Inflammatory heart
disease, Inflammatory kidney disease, IPF/UIP, Iritis, Keratitis,
Keratojuntivitis sicca, Kussmaul
disease or Kussmaul-Meier Disease, Landry's Paralysis, Langerhan's Cell
Histiocytosis, Livedo
reticularis, Macular Degeneration, malignancies, Microscopic Polyangiitis,
Morbus Bechterev,
Motor Neuron Disorders, Mucous membrane pemphigoid, Multiple Organ failure,
Myasthenia
Gravis, Myelodysplastic Syndrome, Myocarditis, Nerve Root Disorders,
Neuropathy, Non-A
Non-B Hepatitis, Optic Neuritis, Osteolysis, Ovarian cancer, Paueiarticular
JRA, peripheral
artery occlusive disease (PAOD), peripheral vascular disease (PVD), peripheral
artery disease
(PAD), Phlebitis, Polyarteritis nodosa (or periarteritis nodosa),
Polychondritis, Polymyalgia
Rheumatica, Poliosis, Polyarticular JRA, Polyendocrine Deficiency Syndrome,
Polymyositis,
polymyalgia rheumatica (P1VIR), Post-Pump Syndrome, primary parkinsonism,
prostate and rectal
cancer and hematopoietic malignancies (leukemia and lymphoma), Prostatitis,
Pure red cell
aplasia, Primary Adrenal Insufficiency, Recurrent Neuromyelitis Optica,
Restenosis, Rheumatic
heart disease, SAPHO (synovitis, acne, pustulosis, hyperostosis, and
osteitis), Scleroderma,
Secondary Amyloidosis, Shock lung, Scleritis, Sciatica, Secondary Adrenal
Insufficiency,
Silicone associated connective tissue disease, Sneddon-Wilkinson Dermatosis,
spondilitis
anlcylosans, Stevens-Johnson Syndrome (SJS), Systemic inflammatory response
syndrome,
Temporal arteritis, toxoplasmic retinitis, toxic epidermal necrolysis,
Transverse myelitis, TRAPS
(Tumor Necrosis Factor Receptor, Type 1 allergic reaction, Type II Diabetes,
Urticaria, Usual
35
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
interstitial pneumonia (UIP), Vasculitis, Vernal conjunctivitis, viral
retinitis, Vogt-Koyanagi-
Harada syndrome (VKH syndrome), Wet macular degeneration, and Wound healing.
In an embodiment, the binding proteins disclosed herein or antigen-binding
portions
thereof, are used to treat cancer or in the prevention of metastases from the
tumors described
herein either when used alone or in combination with radiotherapy and/or other
chemotherapeutic
agents.
In another aspect, a method of treating a patient suffering from a disorder
comprising the
step of administering any one of the binding proteins disclosed herein before,
concurrent, or after
the administration of a second agent, as discussed herein is provided. In a
particular embodiment
the second agent is budenoside, epidermal growth factor, corticosteroids,
cyclosporin,
sulfasalazine, aminosalicylates, 6-mercaptopurine, azathioprine,
metronidazole, lipoxygenase
inhibitors, mesalamine, olsalazine, balsalazide, antioxidants, thromboxane
inhibitors, IL-1
receptor antagonists, anti-IL-113 mAbs, anti-IL-6 or IL-6 receptor mAbs,
growth factors, elastase
inhibitors, pyridinyl-imidazole compounds, antibodies or agonists of TNF, LT,
IL-I, IL-2, IL-6,
IL-7, IL-8, IL-12, IL-13, IL-15, IL-16, IL-18, IL-23, EMAP-H, GM-CSF, FGF, and
PDGF,
antibodies of CD2, CD3, CD4, CD8, CD-19, CD25, CD28, CD30, CD40, CD45, CD69,
CD90 or
their ligands, methotrexate, cyclosporin, FK506, rapamycin, mycophenolate
mofetil, leflunomide,
NSAIDs, ibuprofen, corticosteroids, prednisolone, phosphodiesterase
inhibitors, adensosine
agonists, antithrombotic agents, complement inhibitors, adrenergic agents,
IRAK, NIK, IKK, p38,
MAP kinase inhibitors, IL-113 converting enzyme inhibitors, TNFcc converting
enzyme inhibitors,
T-cell signalling inhibitors, metalloproteinase inhibitors, sulfasalazine,
azathioprine, 6-
mercaptopurines, angiotensin converting enzyme inhibitors, soluble cytokine
receptors, soluble
p55 TNF receptor, soluble p75 TNF receptor, sIL-1R1, sIL-1RII, sIL-6R,
antiinflammatory
cytokines, IL-4, IL-10, IL-11, IL-13, or TGF13.
In a particular embodiment the pharmaceutical compositions disclosed herein
are
administered to the patient by at least one mode selected from parenteral,
subcutaneous,
intramuscular, intravenous, intrarticular, intrabronchial, intraabdominal,
intracapsular,
intracartilaginous, intracavitary, intracelial, intracerebellar,
intracerebroventricular, intracolic,
intracervical, intragastric, intrahepatic, intramyocardial, intraosteal,
intrapelvic, intrapericardiac,
intraperitoneal, intrapleural, intraprostatic, intrapulmonary, intrarectal,
intrarenal, intraretinal,
intraspinal, intrasynovial, intrathoracic, intrauterine, intravesical, bolus,
vaginal, rectal, buccal,
sublingual, intranasal, and transdermal.
36
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
In one embodiment, at least one anti-idiotype antibody to at least one binding
protein
disclosed herein is provided. The anti-idiotype antibody includes any protein
or peptide
containing molecule that comprises at least a portion of an imrnunoglobulin
molecule such as, but
not limited to, at least one complementarily determining region (CDR) of a
heavy or light chain
or a ligand binding portion thereof, a heavy chain or light chain variable
region, a heavy chain or
light chain constant region, a framework region, or any portion thereof, that
can be incorporated
into a binding protein disclosed herein.
Brief Description of the Drawings
Figure 1A is a schematic representation of Dual Variable Domain immunoglobulin
(DVD-Ig)
constructs and shows the strategy for generation of a DVD-Ig from two parent
antibodies;
Figure 1B, is a schematic representation of constructs DVD1-Ig, DVD2-Ig, and
two chimeric
mono-specific antibodies from hybridoma clones 2D13.E3 (anti-IL¨la) and
13F5.G5 (anti-
IL-1 p).
Detailed Description
Multivalent and/or multispecific binding proteins capable of binding two or
more
antigens are provided. Specifically, dual variable domain immunoglobulins (DVD-
Ig), and
pharmaceutical compositions thereof, as well as nucleic acids, recombinant
expression vectors
and host cells for making such DVD-Igs are provided. Methods of using the DVD-
Igs provided
herein to detect specific antigens, either in vitro or in vivo are also
provided.
Unless otherwise defined herein, scientific and technical terms used herein
shall have
the meanings that are commonly understood by those of ordinary skill in the
art. The meaning
and scope of the terms should be clear, however, in the event of any latent
ambiguity, definitions
provided herein take precedent over any dictionary or extrinsic definition.
Further, unless
otherwise required by context, singular terms shall include pluralities and
plural terms shall
include the singular. In this application, the use of "or" means "and/or"
unless stated otherwise.
Furthermore, the use of the term "including", as well as other forms, such as
"includes" and
"included", is not limiting. Also, terms such as "element" or "component"
encompass both
elements and components comprising one unit and elements and components that
comprise more
than one subunit unless specifically stated otherwise.
Generally, nomenclatures used in connection with, and techniques of, cell and
tissue
culture, molecular biology, immunology, microbiology, genetics and protein and
nucleic acid
37
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
chemistry and hybridization described herein are those well known and commonly
used in the
art. The methods and techniques provided herein are generally performed
according to
conventional methods well known in the art and as described in various general
and more
specific references that are cited and discussed throughout the present
specification unless
otherwise indicated. Enzymatic reactions and purification techniques are
performed according to
manufacturer's specifications, as commonly accomplished in the art or as
described herein. The
nomenclatures used in connection with, and the laboratory procedures and
techniques of,
analytical chemistry, synthetic organic chemistry, and medicinal and
pharmaceutical chemistry
described herein are those well known and commonly used in the art. Standard
techniques are
used for chemical syntheses, chemical analyses, pharmaceutical preparation,
formulation, and
delivery, and treatment of patients.
Select terms are defined below:
The term "polypeptide" as used herein, refers to any polymeric chain of amino
acids. The
terms "peptide" and "protein" are used interchangeably with the term
polypeptide and also refer
to a polymeric chain of amino acids. The term "polypeptide" encompasses native
or artificial
proteins, protein fragments and polypeptide analogs of a protein sequence. A
polypeptide may be
monomeric or polymeric. Use of "polypeptide" herein is intended to encompass
polypeptide and
fragments and variants (including fragments of variants) thereof, unless
otherwise contradicted
by context. For an antigenic polypeptide, a fragment of polypeptide optionally
contains at least
one contiguous or nonlinear epitope of polypeptide. The precise boundaries of
the at least one
epitope fragment can be confirmed using ordinary skill in the art. The
fragment comprises at least
about 5 contiguous amino acids, such as at least about 10 contiguous amino
acids, at least about
15 contiguous amino acids, or at least about 20 contiguous amino acids. A
variant of polypeptide
is as described herein.
The term "isolated protein" or "isolated polypeptide" is a protein or
polypeptide that by
virtue of its origin or source of derivation is not associated with naturally
associated components
that accompany it in its native state; is substantially free of other proteins
from the same species;
is expressed by a cell from a different species; or does not occur in nature.
Thus, a polypeptide
that is chemically synthesized or synthesized in a cellular system different
from the cell from
which it naturally originates will be "isolated" from its naturally associated
components. A
protein may also be rendered substantially free of naturally associated
components by isolation,
using protein purification techniques well known in the art.
38
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
The term "recovering" as used herein, refers to the process of rendering a
chemical
species such as a polypeptide substantially free of naturally associated
components by isolation,
e.g., using protein purification techniques well known in the art.
"Biological activity" as used herein, refers to any one or more inherent
biological
properties of a molecule (whether present naturally as found in vivo, or
provided or enabled by
recombinant means). Biological properties include but are not limited to
binding receptor;
induction of cell proliferation, inhibiting cell growth, inductions of other
cytokines, induction of
apoptosis, and enzymatic activity. Biological activity also includes activity
of an Ig molecule.
The terms "specific binding" or "specifically binding", as used herein, in
reference to the
interaction of an antibody, a protein, or a peptide with a second chemical
species, mean that the
interaction is dependent upon the presence of a particular structure (e.g., an
antigenic determinant
or epitope) on the chemical species; for example, an antibody recognizes and
binds to a specific
protein structure rather than to proteins generally. If an antibody is
specific for epitope "A", the
presence of a molecule containing epitope A (or free, unlabeled A), in a
reaction containing
labeled "A" and the antibody, will reduce the amount of labeled A bound to the
antibody.
The term "antibody", as used herein, broadly refers to any immunoglobulin (Ig)
molecule
comprised of four polypeptide chains, two heavy (H) chains and two light (L)
chains, or any
functional fragment, mutant, variant, or derivation thereof, which retains the
essential epitope
binding features of an Ig molecule. Such mutant, variant, or derivative
antibody formats are
known in the art. Nonlimiting embodiments of which are discussed below.
In a full-length antibody, each heavy chain is comprised of a heavy chain
variable region
(abbreviated herein as HCVR or VH) and a heavy chain constant region. The
heavy chain
constant region is comprised of three domains, CH1, CH2 and CH3. Each light
chain is
comprised of a light chain variable region (abbreviated herein as LCVR or VL)
and a light chain
constant region. The light chain constant region is comprised of one domain,
CL. The VH and
VL regions can be further subdivided into regions of hypervariability, termed
complementarity
determining regions (CDR), interspersed with regions that are more conserved,
termed
framework regions (FR). Each VH and VL is composed of three CDRs and four FRs,
arranged
from amino-terminus to carboxy-terminus in the following order: FR1, CDR1,
FR2, CDR2, FR3,
CDR3, FR4. Immunoglobulin molecules can be of any type (e.g., IgG, IgE, IgM,
IgD, IgA and
IgY), class (e.g., IgG 1, IgG2, IgG 3, IgG4, IgAl and IgA2) or subclass.
The term "Fe region" is used to define the C-terminal region of an
immunoglobulin
heavy chain, which may be generated by papain digestion of an intact antibody.
The Fe region
39
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
may be a native sequence Fc region or a variant Fc region. The Fc region of an
immunoglobulin
generally comprises two constant domains, a CH2 domain and a CH3 domain, and
optionally
comprises a CH4 domain. Replacements of amino acid residues in the Fc portion
to alter antibody
effector function are known in the art (Winter, et al. US Patent Nos 5,648,260
and 5,624,821).
The Fc portion of an antibody mediates several important effector functions
e.g.,cytokine
induction, ADCC, phagocytosis, complement dependent cytotoxicity (CDC) and
half-life/
clearance rate of antibody and antigen-antibody complexes. In some cases these
effector
functions are desirable for therapeutic antibody but in other cases might be
unnecessary or even
deleterious, depending on the therapeutic objectives. Certain human IgG
isotypes, particularly
IgG1 and IgG3, mediate ADCC and CDC via binding to FcyRs and complement Cl q,
respectively. Neonatal Fc receptors (FcRn) are the critical components
determining the
circulating half-life of antibodies. In still another embodiment at least one
amino acid residue is
replaced in the constant region of the antibody, for example the Fc region of
the antibody, such
that effector functions of the antibody are altered. The dimerization of two
identical heavy chains
of an immunoglobulin is mediated by the dimerization of CH3 domains and is
stabilized by the
disulfide bonds within the hinge region (Huber et al. Nature; 264: 415-20;
Thies et al 1999 J Mol
Biol; 293: 67-79.). Mutation of cysteine residues within the hinge regions to
prevent heavy chain-
heavy chain disulfide bonds will destabilize dimeration of CH3 domains.
Residues responsible
for CH3 dimerization have been identified (Dall'Acqua 1998 Biochemistry 37:
9266-73.).
Therefore, it is possible to generate a monovalent half-Ig. Interestingly,
these monovalent half Ig
molecules have been found in nature for both IgG and IgA subclasses (Seligman
1978 Ann
Immunol 129: 855-70; Biewenga et al 1983 Clin Exp Immunol 51: 395-400). The
stoichiometry
of FcRn: Ig Fc region has been determined to be 2:1 (West et al .2000
Biochemistry 39: 9698-
708), and half Fc is sufficient for mediating FeRn binding (Kim et al 1994 Eur
J Immunol; 24:
542-548.). Mutations to disrupt the dimerization of CH3 domain may not have
greater adverse
effect on its FcRn binding as the residues important for CH3 dimerization are
located on the inner
interface of CH3 b sheet structure, whereas the region responsible for FcRn
binding is located on
the outside interface of CH2-CH3 domains. However the half Ig molecule may
have certain
advantage in tissue penetration due to its smaller size than that of a regular
antibody. In one
embodiment at least one amino acid residue is replaced in the constant region
of the binding
protein, for example the Fc region, such that the dimerization of the heavy
chains is disrupted,
resulting in half DVD Ig molecules. The anti-inflammatory activity of IgG is
completely
dependent on sialylation of the N-linked glycan of the IgG Fc fragment. The
precise glycan
requirements for anti-inflammatory activity has been determined, such that an
appropriate IgG1
Fc fragment can be created, thereby generating a fully recombinant, sialylated
IgG1 Fc with
greatly enhanced potency (Anthony, R.M., et al. (2008) Science 320:373-376).
40
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
The term "antigen-binding portion" of an antibody (or simply "antibody
portion"), as
used herein, refers to one or more fragments of an antibody that retain the
ability to specifically
bind to an antigen. It has been shown that the antigen-binding function of an
antibody can be
performed by fragments of a full-length antibody. Such antibody embodiments
may also be
bispecific, dual specific, or multi-specific formats; specifically binding to
two or more different
antigens. Examples of binding fragments encompassed within the term "antigen-
binding portion"
of an antibody include (i) a Fab fragment, a monovalent fragment comprising
the VL, VH, CL
and CH1 domains; (ii) a F(ab1)2 fragment, a bivalent fragment comprising two
Fab fragments
linked by a disulfide bridge at the hinge region; (iii) a Fd fragment
comprising the VH and CH1
domains; (iv) a Fv fragment comprising the VL and VH domains of a single arm
of an antibody,
(v) a dAb fragment (Ward et al., (1989) Nature 341:544-546, Winter et al., PCT
publication WO
90/05144 Al), which comprises a single variable domain; and (vi) an isolated
complementarity
determining region (CDR). Furthermore, although the two domains of the Fv
fragment, VL and
VH, are coded for by separate genes, they can be joined, using recombinant
methods, by a
synthetic linker that enables them to be made as a single protein chain in
which the VL and VII
regions pair to form monovalent molecules (known as single chain Fv (scFv);
see e.g., Bird et al.
(1988) Science 242:423-426; and Huston et al. (1988) Proc. Natl. Acad. Sci USA
85:5879-5883).
Such single chain antibodies are also intended to be encompassed within the
term "antigen-
binding portion" of an antibody. Other forms of single chain antibodies, such
as diabodies are
also encompassed. Diabodies are bivalent, bispecific antibodies in which VII
and VL domains
are expressed on a single polypeptide chain, but using a linker that is too
short to allow for
pairing between the two domains on the same chain, thereby forcing the domains
to pair with
complementary domains of another chain and creating two antigen binding sites
(see e.g.,
Holliger, P., etal. (1993) Proc. Natl. Acad. Sci. USA 90:6444-6448; Poljak,
R.J., et al. (1994)
Structure 2:1121-1123). Such antibody binding portions are known in the art
(Kontermann and
Dubel eds., Antibody Engineering (2001) Springer-Verlag. New York. 790 pp.
(ISBN 3-540-
41354-5). In addition single chain antibodies also include "linear antibodies"
comprising a pair of
tandem Fv segments (VH-CI-11-V1-1-CH1) which, together with complementary
light chain
polypeptides, form a pair of antigen binding regions (Zapata et al. Protein
Eng. 8(10):1057-1062
(1995); and US Patent No. 5,641,870).
The term "multivalent binding protein" is used throughout this specification
to denote a
binding protein comprising two or more antigen binding sites. In an
embodiment, the multivalent
binding protein is engineered to have the three or more antigen binding sites,
and is generally not
a naturally occurring antibody. The term "multispecific binding protein"
refers to a binding
protein capable of binding two or more related or unrelated targets. Dual
variable domain (DVD)
41
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
binding proteins provided herein comprise two or more antigen binding sites
and are tetravalent
or multivalent binding proteins. In certain embodiments, the binding proteins
are monospecific,
i.e., capable of binding one antigen or multispecific, i.e. capable of binding
two or more antigens.
DVD binding proteins comprising two heavy chain polypeptides and two light
chain polypeptides
are referred to as DVD-Igs. Each half of a DVD-Ig comprises a heavy chain
polypeptide, and a
light chain polypeptide, and two antigen binding sites. Each binding site
comprises a heavy chain
variable domain and a light chain variable domain with a total of 6 CDRs
involved in antigen
binding per antigen binding site.
The term "bispecific antibody", as used herein, refers to full-length
antibodies that are
generated by quadroma technology (see Milstein, C. and A.C. Cuello, Nature,
1983. 305(5934):
p. 537-40), by chemical conjugation of two different monoclonal antibodies
(see Staerz, U.D., et
al., Nature, 1985. 314(6012): p. 628-31), or by knob-into-hole or similar
approaches which
introduces mutations in the Fe region (see Holliger, P., T. Prospero, and G.
Winter, Proc Natl
Acad Sci U S A, 1993. 90(14): p. 6444-8.18), resulting in multiple different
immunoglobulin
species of which only one is the functional bispecific antibody. By molecular
function, a
bispecific antibody binds one antigen (or epitope) on one of its two binding
arms (one pair of
HC/LC), and binds a different antigen (or epitope) on its second arm (a
different pair of HC/LC).
By this definition, a bispecific antibody has two distinct antigen binding
arms (in both specificity
and CDR sequences), and is monovalent for each antigen it binds to.
The term "dual-specific antibody", as used herein, refers to full-length
antibodies that can
bind two different antigens (or epitopes) in each of its two binding arms (a
pair of HC/LC) (see
PCT publication WO 02/02773). Accordingly a dual-specific binding protein has
two identical
antigen binding arms, with identical specificity and identical CDR sequences,
and is bivalent for
each antigen it binds to.
A "functional antigen binding site" of a binding protein is one that is
capable of binding a
target antigen. The antigen binding affinity of the antigen binding site is
not necessarily as strong
as the parent antibody from which the antigen binding site is derived, but the
ability to bind
antigen must be measurable using any one of a variety of methods known for
evaluating antibody
binding to an antigen. Moreover, the antigen binding affinity of each of the
antigen binding sites
of a multivalent antibody herein need not be quantitatively the same.
The term "cytokine" is a generic term for proteins released by one cell
population, which
act on another cell population as intercellular mediators. Examples of such
cytokines are
lymphokines, monokines, and traditional polypeptide hormones. Included among
the cytokines
42
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
are growth hormone such as human growth hormone, N-methionyl human growth
hormone, and
bovine growth hormone; parathyroid hormone; thyroxine; insulin; proinsulin;
relaxin; prorelaxin;
glycoprotein hormones such as follicle stimulating hormone (FSH), thyroid
stimulating hormone
(TSH), and luteinizing hormone (LH); hepatic growth factor; fibroblast growth
factor; prolactin;
placental lactogen; tumor necrosis factor-alpha and - beta; mullerian-
inhibiting substance; mouse
gonadotropin-associated peptide; inhibin; activin; vascular endothelial growth
factor; integrin;
thrombopoietin (TP0); nerve growth factors such as NGF-alpha; platelet-growth
factor; placental
growth factor, transforming growth factors (TGFs) such as TGF- alpha and TGF-
beta; insulin-
like growth factor-1 and -11; erythropoietin (EPO); osteoinductive factors;
interferons such as
interferon-alpha, -beta and -gamma colony stimulating factors (CSFs) such as
macrophage-CSF
(M-CSF); granulocyte macrophage-CSF (GM-CSF); and granulocyte-CSF (G-CSF);
interleukins
(ILs) such as IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-
11, IL-12, IL-13, IL-15,
IL-18, IL-21, IL-22, IL-23, IL-33; a tumor necrosis factor such as INF-alpha
or TNF-beta; and
other polypeptide factors including LIF and kit ligand (KL). As used herein,
the term cytokine
includes proteins from natural sources or from recombinant cell culture and
biologically active
equivalents of the native sequence cytokines.
The term "linker" is used to denote polypeptides comprising two or more amino
acid
residues joined by peptide bonds and are used to link one or more antigen
binding portions. Such
linker polypeptides are well known in the art (see e.g., Holliger, P., et al.
(1993) Proc. Natl.
Acad. ScL USA 90:6444-6448; Poljak, R.J., etal. (1994) Structure 2:1121-1123).
Exemplary
linkers include, but are not limited to, AKTTPKLEEGEFSEAR (SEQ ID NO: 1);
AKTTPKLEEGEFSEARV (SEQ ID NO: 2); AKTTPKLGG (SEQ ID NO: 3); SAKTTPKLGG
(SEQ ID NO: 4); SAKTTP (SEQ ID NO: 5); RADAAP (SEQ ID NO: 6); RADAAPTVS (SEQ
ID NO: 7); RADAAAAGGPGS (SEQ ID NO: 8); RADAAAA(G4S)4 (SEQ ID NO: 9),
SAKTTPKLEEGEFSEARV (SEQ ID NO: 10); ADAAP (SEQ ID NO: 11); ADAAPTVSIFPP
(SEQ ID NO: 12); TVAAP (SEQ ID NO: 13); TVAAPSVFIFPP (SEQ ID NO: 14); QPKAAP
(SEQ ID NO: 15); QPKAAPSVTLFPP (SEQ ID NO: 16); AKTTPP (SEQ ID NO: 17);
AKTTPPSVTPLAP (SEQ ID NO: 18); AKTTAP (SEQ ID NO: 19); AKTTAPSVYPLAP (SEQ
ID NO: 20); ASTKGP (SEQ ID NO: 21); ASTKGPSVFPLAP (SEQ ID NO: 22),
GGGGSGGGGSGGGGS (SEQ ID NO: 23); GENKVEYAPALMALS (SEQ ID NO: 24);
GPAKELTPLKEAKVS (SEQ ID NO: 25); and GHEAAAVMQVQYPAS (SEQ ID NO: 26).
An immunoglobulin constant domain refers to a heavy or light chain constant
domain.
Human IgG heavy chain and light chain constant domain amino acid sequences are
known in the
art.
43
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
The term "monoclonal antibody" or "mAb" as used herein refers to an antibody
obtained
from a population of substantially homogeneous antibodies, i.e., the
individual antibodies
comprising the population are identical except for possible naturally
occurring mutations that
may be present in minor amounts. Monoclonal antibodies are highly specific,
being directed
against a single antigen. Furthermore, in contrast to polyclonal antibody
preparations that
typically include different antibodies directed against different determinants
(epitopes), each
mAb is directed against a single determinant on the antigen. The modifier
"monoclonal" is not to
be construed as requiring production of the antibody by any particular method.
The term "human antibody", as used herein, is intended to include antibodies
having
variable and constant regions derived from human germline immunoglobulin
sequences. The
human antibodies provided herein may include amino acid residues not encoded
by human
germline immunoglobulin sequences (e.g., mutations introduced by random or
site-specific
mutagenesis in vitro or by somatic mutation in vivo), for example in the CDRs
and in particular
CDR3. However, the term "human antibody", as used herein, is not intended to
include antibodies
in which CDR sequences derived from the germline of another mammalian species,
such as a
mouse, have been grafted onto human framework sequences.
The term "recombinant human antibody", as used herein, is intended to include
all human
antibodies that are prepared, expressed, created or isolated by recombinant
means, such as
antibodies expressed using a recombinant expression vector transfected into a
host cell (described
further in Section II C, below), antibodies isolated from a recombinant,
combinatorial human
antibody library (Hoogenboom H.R. (1997) TIB Tech. 15:62-70; Azzazy H., and
Highsmith W.E.
(2002) Clin. Biochem. 35:425-445; Gavilondo J.V., and Larrick J.W. (2002)
BioTechniques
29:128-145; Hoogenboom H., and Chames P. (2000) Immunology Today 21:371-378),
antibodies isolated from an animal (e.g., a mouse) that is transgenic for
human immunoglobulin
genes (see, Taylor, L. D., et al. (1992) Nucl. Acids Res. 20:6287-6295;
Kellermann S-A. and
Green L.L. (2002) Current Opinion in Biotechnology 13:593-597; Little M. et
al. (2000)
Immunology Today 21:364-370) or antibodies prepared, expressed, created or
isolated by any
other means that involves splicing of human immunoglobulin gene sequences to
other DNA
sequences. Such recombinant human antibodies have variable and constant
regions derived from
human germline immunoglobulin sequences. In certain embodiments, however, such
recombinant
human antibodies are subjected to in vitro mutagenesis (or, when an animal
transgenic for human
Ig sequences is used, in vivo somatic mutagenesis) and thus the amino acid
sequences of the VH
and VL regions of the recombinant antibodies are sequences that, while derived
from and related
44
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
to human germline VH and VL sequences, may not naturally exist within the
human antibody
germline repertoire in vivo.
An "affinity matured" antibody is an antibody with one or more alterations in
one or
more CDRs thereof which result an improvement in the affinity of the antibody
for antigen,
compared to a parent antibody which does not possess those alteration(s).
Exemplary affinity
matured antibodies will have nanomolar or even picomolar affinities for the
target antigen.
Affinity matured antibodies are produced by procedures known in the art. Marks
et al.
BidlTechnology 10:779-783 (1992) describes affinity maturation by VII and VL
domain
shuffling. Random mutagenesis of CDR and/or framework residues is described
by: Barbas et al.
Proc Nat. Acad. Sci, USA 91:3809-3813 (1994); Schier et al. Gene 169:147- 155
(1995); Yelton
et al. J. Immunol. 155:1994-2004 (1995); Jackson et al., J. Immunol.
154(7):3310-9 (1995);
Hawkins et al, J. Mol. BioL 226:889-896 (1992) and selective mutation at
selective mutagenesis
positions, contact or hypermutation positions with an activity enhancing amino
acid residue as
described in US patent US 6914128B1.
The term "chimeric antibody" refers to antibodies which comprise heavy and
light chain
variable region sequences from one species and constant region sequences from
another species,
such as antibodies having murine heavy and light chain variable regions linked
to human constant
regions.
The term "CDR-grafted antibody" refers to antibodies which comprise heavy and
light
chain variable region sequences from one species but in which the sequences of
one or more of
the CDR regions of VII and/or VL are replaced with CDR sequences of another
species, such as
antibodies having murine heavy and light chain variable regions in which one
or more of the
murine CDRs (e.g., CDR3) has been replaced with human CDR sequences.
The term "humanized antibody" refers to antibodies which comprise heavy and
light
chain variable region sequences from a non-human species (e.g , a mouse) but
in which at least a
portion of the VH and/or VL sequence has been altered to be more "human-like",
i.e., more
similar to human germline variable sequences. One type of humanized antibody
is a CDR-grafted
antibody, in which human CDR sequences are introduced into non-human VII and
VL sequences
to replace the corresponding nonhuman CDR sequences. Also "humanized
antibody"is an
antibody or a variant, derivative, analog or fragment thereof which
immunospecifically binds to
an antigen of interest and which comprises a framework (FR) region having
substantially the
amino acid sequence of a human antibody and a complementary determining region
(CDR)
having substantially the amino acid sequence of a non-human antibody. As used
herein, the term
45
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
"substantially" in the context of a CDR refers to a CDR having an amino acid
sequence at least
80%, at least 85%, at least 90%, at least 95%, at least 98% or at least 99%
identical to the amino
acid sequence of a non-human antibody CDR. A humanized antibody comprises
substantially all
of at least one, and typically two, variable domains (Fab, Fab', F(ab') 2,
FabC, Fv) in which all or
substantially all of the CDR regions correspond to those of a non-human
immunoglobulin (i.e.,
donor antibody) and all or substantially all of the framework regions are
those of a human
immunoglobulin consensus sequence. In an embodiment, a humanized antibody also
comprises at
least a portion of an immunoglobulin constant region (Fe), typically that of a
human
immunoglobulin. In some embodiments, a humanized antibody contains both the
light chain as
well as at least the variable domain of a heavy chain. The antibody also may
include the CH1,
hinge, CH2, CH3, and CH4 regions of the heavy chain. In some embodiments, a
humanized
antibody only contains a humanized light chain. In some embodiments, a
humanized antibody
only contains a humanized heavy chain. In specific embodiments, a humanized
antibody only
contains a humanized variable domain of a light chain and/or humanized heavy
chain.
The terms "Kabat numbering", "Kabat definitions" and "Kabat labeling" are used
interchangeably herein. These terms, which are recognized in the art, refer to
a system of
numbering amino acid residues which are more variable (i.e. hypervariable)
than other amino
acid residues in the heavy and light chain variable regions of an antibody, or
an antigen binding
portion thereof (Kabat et al. (1971) Ann. NY Acad, Sci. 190:382-391 and,
Kabat, E.A., et al.
(1991) Sequences of Proteins of Immunological Interest, Fifth Edition, U.S.
Department of
Health and Human Services, NIH Publication No. 91-3242). For the heavy chain
variable region,
the hypervariable region ranges from amino acid positions 31 to 35 for CDR1,
amino acid
positions 50 to 65 for CDR2, and amino acid positions 95 to 102 for CDR3. For
the light chain
variable region, the hypervariable region ranges from amino acid positions 24
to 34 for CDR1,
amino acid positions 50 to 56 for CDR2, and amino acid positions 89 to 97 for
CDR3.
As used herein, the term "CDR" refers to the complementarity determining
region within
antibody variable sequences. There are three CDRs in each of the variable
regions of the heavy
chain and the light chain, which are designated CDR1, CDR2 and CDR3, for each
of the variable
regions. The term "CDR set" as used herein refers to a group of three CDRs
that occur in a single
variable region capable of binding the antigen. The exact boundaries of these
CDRs have been
defined differently according to different systems. The system described by
Kabat (Kabat et al.,
Sequences of Proteins of Immunological Interest (National Institutes of
Health, Bethesda, Md.
(1987) and (1991)) not only provides an unambiguous residue numbering system
applicable to
any variable region of an antibody, but also provides precise residue
boundaries defining the
46
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
three CDRs. These CDRs may be referred to as Kabat CDRs. Chothia and coworkers
(Chothia
&Lesk, J. Mol. Biol. 196:901-917 (1987) and Chothia et al., Nature 342:877-883
(1989)) found
that certain sub- portions within Kabat CDRs adopt nearly identical peptide
backbone
conformations, despite having great diversity at the level of amino acid
sequence. These sub-
portions were designated as Li, L2 and L3 or H1, H2 and H3 where the "L" and
the "H"
designates the light chain and the heavy chains regions, respectively. These
regions may be
referred to as Chothia CDRs, which have boundaries that overlap with Kabat
CDRs. Other
boundaries defining CDRs overlapping with the Kabat CDRs have been described
by Padlan
(FASEB J. 9:133-139 (1995)) and MacCallum (J Mol Biol 262(5):732-45 (1996)).
Still other
CDR boundary definitions may not strictly follow one of the herein systems,
but will nonetheless
overlap with the Kabat CDRs, although they may be shortened or lengthened in
light of
prediction or experimental findings that particular residues or groups of
residues or even entire
CDRs do not significantly impact antigen binding. The methods used herein may
utilize CDRs
defined according to any of these systems, although certain embodiments use
Kabat or Chothia
defined CDRs.
As used herein, the term "framework" or "framework sequence" refers to the
remaining
sequences of a variable region minus the CDRs. Because the exact definition of
a CDR sequence
can be determined by different systems, the meaning of a framework sequence is
subject to
correspondingly different interpretations. The six CDRs (CDR-L1, -L2, and -L3
of light chain
and CDR-H1, -H2, and -H3 of heavy chain) also divide the framework regions on
the light chain
and the heavy chain into four sub-regions (FR1, FR2, FR3 and FR4) on each
chain, in which
CDR1 is positioned between FRI and FR2, CDR2 between FR2 and FR3, and CDR3
between
FR3 and FR4. Without specifying the particular sub-regions as FR1, FR2, FR3 or
FR4, a
framework region, as referred by others, represents the combined FR's within
the variable region
of a single, naturally occurring immunoglobulin chain. As used herein, a FR
represents one of the
four sub- regions, and FRs represents two or more of the four sub- regions
constituting a
framework region.
As used herein, the term "germline antibody gene" or "gene fragment" refers to
an
immunoglobulin sequence encoded by non- lymphoid cells that have not undergone
the
maturation process that leads to genetic rearrangement and mutation for
expression of a particular
immunoglobulin. (See, e.g., Shapiro et al., Crit. Rev. Immunol. 22(3): 183-200
(2002);
Marchalonis et al., Adv Exp Med Biol. 484:13-30 (2001)). One of the advantages
provided by
various embodiments provided herein stems from the recognition that germline
antibody genes
are more likely than mature antibody genes to conserve essential amino acid
sequence structures
47
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
characteristic of individuals in the species, hence less likely to be
recognized as from a foreign
source when used therapeutically in that species.
As used herein, the term "neutralizing" refers to counteracting the biological
activity of
an antigen when a binding protein specifically binds the antigen. In an
embodiment, the
neutralizing binding protein binds the cytokine and reduces its biologically
activity by at least
about 20%, 40%, 60%, 80%, 85% or more.
The term "activity" includes activities such as the binding specificity and
affinity of a
binding protein for two or more antigens.
The term "epitope" includes any polypeptide determinant capable of specific
binding to
an immunoglobulin or T-cell receptor. In certain embodiments, epitope
determinants include
chemically active surface groupings of molecules such as amino acids, sugar
side chains,
phosphoryl, or sulfonyl, and, in certain embodiments, may have specific three
dimensional
structural characteristics, and/or specific charge characteristics. An epitope
is a region of an
antigen that is bound by an antibody. In certain embodiments, an antibody is
said to specifically
bind an antigen when it recognizes its target antigen in a complex mixture of
proteins and/or
macromolecules. Antibodies are said to "bind to the same epitope" if the
antibodies cross-
compete (one prevents the binding or modulating effect of the other). In
addition structural
definitions of epitopes (overlapping, similar, identical) are informative, but
functional definitions
are often more relevant as they encompass structural (binding) and functional
(modulation,
competition) parameters.
The term "surface plasmon resonance", as used herein, refers to an optical
phenomenon
that allows for the analysis of real-time biospecifie interactions by
detection of alterations in
protein concentrations within a biosensor matrix, for example using the
BIAcore system
(BIAcore International AB, a GE Healthcare company, Uppsala, Sweden and
Piscataway, NJ).
For further descriptions, see Jonsson, U., et al. (1993) Ann. Biol. Clin.
51:19-26; Jonsson, U., et
aL (1991) Biotechniques 11:620-627; Johnsson, B., eta!, (1995)1 MoL Recognit.
8:125-131; and
Johnnson, B., et al. (1991) Anal. Biochem. 198:268-277.
The term "Kim", as used herein, is intended to refer to the on rate constant
for association
of a binding protein (e.g., an antibody) to the antigen to form the, e.g.,
antibody/antigen complex
as is known in the art. The "Kon" also is known by the terms "association rate
constant", or "ka",
as used interchangeably herein. This value indicating the binding rate of an
antibody to its target
48
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
antigen or the rate of complex formation between an antibody and antigen also
is shown by the
equation below:
Antibody ("Ab") + Antigen ("Ag")-->Ab-Ag.
The term "Koff", as used herein, is intended to refer to the off rate constant
for
dissociation, or "dissociation rate constant", of a binding protein (e.g., an
antibody) from the,
e.g., antibody/antigen complex as is known in the art. This value indicates
the dissociation rate of
an antibody from its target antigen or separation of Ab-Ag complex over time
into free antibody
and antigen as shown by the equation below:
Ab + Ag*--Ab-Ag.
The term "KEI", as used herein, is intended to refer to the "equilibrium
dissociation
constant", and refers to the value obtained in a titration measurement at
equilibrium, or by
dividing the dissociation rate constant (koff) by the association rate
constant (kon). The
association rate constant, the dissociation rate constant and the equilibrium
dissociation constant
are used to represent the binding affinity of an antibody to an antigen.
Methods for determining
association and dissociation rate constants are well known in the art. Using
fluorescence¨based
techniques offers high sensitivity and the ability to examine samples in
physiological buffers at
equilibrium. Other experimental approaches and instruments such as a BIAcore
(biomolecular
interaction analysis) assay can be used (e.g., instrument available from
BIAcore International AB,
a GE Healthcare company, Uppsala, Sweden). Additionally, a KinExA (Kinetic
Exclusion
Assay) assay, available from Sapidyne Instruments (Boise, Idaho) can also be
used.
"Label" and "detectable label" mean a moiety attached to a specific binding
partner,
such as an antibody or an analyte, e.g., to render the reaction between
members of a specific
binding pair, such as an antibody and an analyte, detectable, and the specific
binding partner,
e.g., antibody or analyte, so labeled is referred to as "detectably labeled."
Thus, the term
"labeled binding protein" as used herein, refers to a protein with a label
incorporated that
provides for the identification of the binding protein. In an embodiment, the
label is a detectable
marker that can produce a signal that is detectable by visual or instrumental
means, e.g.,
incorporation of a radiolabeled amino acid or attachment to a polypeptide of
biotinyl moieties
that can be detected by marked avidin (e.g., streptavidin containing a
fluorescent marker or
enzymatic activity that can be detected by optical or colorimetric methods).
Examples of labels
for polypeptides include, but are not limited to, the following: radioisotopes
or radionuclides
(e.g., 3H, 14C, 35S, 90Y, 99Tc, 111In, 1251, 1311, 177Lu,166H-r-ro, or 153Sm);
chromogens, fluorescent labels
49
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
(e.g., FITC, rhodamine, lanthanide phosphors), enzymatic labels (e.g.,
horseradish peroxidase,
luciferase, alkaline phosphatase); themiluminescent markers; biotinyl groups;
predetermined
polypeptide epitopes recognized by a secondary reporter (e.g., leucine zipper
pair sequences,
binding sites for secondary antibodies, metal binding domains, epitope tags);
and magnetic
agents, such as gadolinium chelates. Representative examples of labels
commonly employed for
immunassays include moieties that produce light, e.g., acridinium compounds,
and moieties that
produce fluorescence, e.g., fluorescein. Other labels are described herein. In
this regard, the
moiety itself may not be detectably labeled but may become detectable upon
reaction with yet
another moiety. Use of "detectably labeled" is intended to encompass the
latter type of
detectable labeling.
The term "conjugate" refers to a binding protein, such as an antibody,
chemically linked
to a second chemical moiety, such as a therapeutic or cytotoxic agent. The
term "agent" is used
herein to denote a chemical compound, a mixture of chemical compounds, a
biological
macromolecule, or an extract made from biological materials. In an embodiment,
the therapeutic
or cytotoxic agents include, but are not limited to, pertussis toxin, taxol,
cytochalasin B,
gramicidin D, ethidium bromide, emetine, mitomycin, etoposide, tenoposide,
vincristine,
vinblastine, colchicin, doxorubicin, daunorubicin, dihydroxy anthracin dione,
mitoxantrone,
mithramycin, actinomycin D, 1-dehydrotestosterone, glucocorticoids, procaine,
tetracaine,
lidocaine, propranolol, and puromycin and analogs or homologs thereof. When
employed in the
context of an immunoassay, the conjugate antibody may be a detectably labeled
antibody used as
the detection antibody.
The terms "crystal" and "crystallized" as used herein, refer to a binding
protein (e.g., an
antibody), or antigen binding portion thereof, that exists in the form of a
crystal. Crystals are
one form of the solid state of matter, which is distinct from other forms such
as the amorphous
solid state or the liquid crystalline state. Crystals are composed of regular,
repeating, three-
dimensional arrays of atoms, ions, molecules (e.g., proteins such as
antibodies), or molecular
assemblies (e.g., antigen/antibody complexes). These three-dimensional arrays
are arranged
according to specific mathematical relationships that are well-understood in
the field. The
fundamental unit, or building block, that is repeated in a crystal is called
the asymmetric unit.
Repetition of the asymmetric unit in an arrangement that conforms to a given,
well-defined
crystallographic symmetry provides the "unit cell" of the crystal. Repetition
of the unit cell by
regular translations in all three dimensions provides the crystal. See Giege,
R. and Ducruix, A.
Barrett, Crystallization of Nucleic Acids and Proteins, a Practical Approach,
2nd ea., pp. 20 1-
16, Oxford University Press, New York, New York, (1999)."
50
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
The term "polynucleotide" means a polymeric form of two or more nucleotides,
either
ribonucleotides or deoxvnucleotides or a modified form of either type of
nucleotide. The term
includes single and double stranded forms of DNA.
The term "isolated polynucleotide" shall mean a polynucleotide (e.g., of
genomic, cDNA,
or synthetic origin, or some combination thereof) that, by virtue of its
origin, the "isolated
polynucleotide" is not associated with all or a portion of a polynucleotide
with which the
"isolated polynucleotide" is found in nature; is operably linked to a
polynucleotide that it is not
linked to in nature; or does not occur in nature as part of a larger sequence.
The term "vector", is intended to refer to a nucleic acid molecule capable of
transporting
another nucleic acid to which it has been linked. One type of vector is a
"plasmid", which refers
to a circular double stranded DNA loop into which additional DNA segments may
be ligated.
Another type of vector is a viral vector, wherein additional DNA segments may
be ligated into
the viral genome. Certain vectors are capable of autonomous replication in a
host cell into which
they are introduced (e.g., bacterial vectors having a bacterial origin of
replication and episomal
mammalian vectors). Other vectors (e.g., non-episomal mammalian vectors) can
be integrated
into the genome of a host cell upon introduction into the host cell, and
thereby are replicated
along with the host genome. Moreover, certain vectors are capable of directing
the expression of
genes to which they are operatively linked. Such vectors are referred to
herein as "recombinant
expression vectors" (or simply, "expression vectors"). In general, expression
vectors of utility in
recombinant DNA techniques are often in the form of plasmids. In the present
specification,
"plasmid" and "vector" may be used interchangeably as the plasmid is the most
commonly used
form of vector. However, embodiments include such other forms of expression
vectors, such as
viral vectors (e.g., replication defective retroviruses, adenoviruses and
adeno-associated viruses),
which serve equivalent functions.
The term "operably linked" refers to a juxtaposition wherein the components
described
are in a relationship permitting them to function in their intended manner. A
control sequence
"operably linked" to a coding sequence is ligated in such a way that
expression of the coding
sequence is achieved under conditions compatible with the control sequences.
"Operably linked"
sequences include both expression control sequences that are contiguous with
the gene of interest
and expression control sequences that act in trans or at a distance to control
the gene of interest.
The term "expression control sequence" as used herein refers to polynucleotide
sequences which
are necessary to effect the expression and processing of coding sequences to
which they are
ligated. Expression control sequences include appropriate transcription
initiation, termination,
promoter and enhancer sequences; efficient RNA processing signals such as
splicing and
51
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
polyadenylation signals; sequences that stabilize cytoplasmic mRNA; sequences
that enhance
translation efficiency (i.e., Kozak consensus sequence); sequences that
enhance protein stability;
and when desired, sequences that enhance protein secretion. The nature of such
control sequences
differs depending upon the host organism; in prokaryotes, such control
sequences generally
include promoter, ribosomal binding site, and transcription termination
sequence; in eukaryotes,
generally, such control sequences include promoters and transcription
termination sequence. The
term "control sequences" is intended to include components whose presence is
essential for
expression and processing, and can also include additional components whose
presence is
advantageous, for example, leader sequences and fusion partner sequences.
"Transformation", refers to any process by which exogenous DNA enters a host
cell.
Transformation may occur under natural or artificial conditions using various
methods well
known in the art. Transformation may rely on any known method for the
insertion of foreign
nucleic acid sequences into a prokaryotic or eukaryotic host cell. The method
is selected based on
the host cell being transformed and may include, but is not limited to, viral
infection,
electroporation, lipofection, and particle bombardment. Such "transformed"
cells include stably
transformed cells in which the inserted DNA is capable of replication either
as an autonomously
replicating plasmid or as part of the host chromosome. They also include cells
which transiently
express the inserted DNA or RNA for limited periods of time.
The term "recombinant host cell" (or simply "host cell"), is intended to refer
to a cell into
which exogenous DNA has been introduced. In an embodiment, the host cell
comprises two or
more (e.g., multiple) nucleic acids encoding antibodies, such as the host
cells described in US
Patent No. 7,262,028, for example. Such terms are intended to refer not only
to the particular
subject cell, but also to the progeny of such a cell. Because certain
modifications may occur in
succeeding generations due to either mutation or environmental influences,
such progeny may
not, in fact, be identical to the parent cell, but are still included within
the scope of the term "host
cell" as used herein. In an embodiment, host cells include prokaryotic and
eukaryotic cells
selected from any of the Kingdoms of life. In another embodiment, eukaryotic
cells include
protist, fungal, plant and animal cells. In another embodiment, host cells
include but are not
limited to the prokaryotic cell line E.Coli; mammalian cell lines CHO, HEK
293, COS, NSO, SP2
and PER.C6; the insect cell line Sf9; and the fungal cell Saccharomyces
cerevisiae.
Standard techniques may be used for recombinant DNA, oligonucleotide
synthesis, and
tissue culture and transformation (e.g., electroporation, lipofection).
Enzymatic reactions and
purification techniques may be performed according to manufacturer's
specifications or as
commonly accomplished in the art or as described herein. The foregoing
techniques and
52
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
procedures may be generally performed according to conventional methods well
known in the art
and as described in various general and more specific references that are
cited and discussed
throughout the present specification. See e.g., Sambrook et al. Molecular
Cloning: A Laboratory
Manual (2d ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
(1989)).
"Transgenic organism", as known in the art, refers to an organism having cells
that
contain a transgene, wherein the transgene introduced into the organism (or an
ancestor of the
organism) expresses a polypeptide not naturally expressed in the organism. A
"transgene" is a
DNA construct, which is stably and operably integrated into the genome of a
cell from which a
transgenic organism develops, directing the expression of an encoded gene
product in one or
more cell types or tissues of the transgenic organism.
The term "regulate"and "modulate" are used interchangeably, and, as used
herein, refers
to a change or an alteration in the activity of a molecule of interest (e.g.,
the biological activity of
a cytokine). Modulation may be an increase or a decrease in the magnitude of a
certain activity or
function of the molecule of interest. Exemplary activities and functions of a
molecule include, but
are not limited to, binding characteristics, enzymatic activity, cell receptor
activation, and signal
transduction.
Correspondingly, the term "modulator" is a compound capable of changing or
altering an
activity or function of a molecule of interest (e.g., the biological activity
of a cytokine). For
example, a modulator may cause an increase or decrease in the magnitude of a
certain activity or
function of a molecule compared to the magnitude of the activity or function
observed in the
absence of the modulator. In certain embodiments, a modulator is an inhibitor,
which decreases
the magnitude of at least one activity or function of a molecule. Exemplary
inhibitors include, but
are not limited to, proteins, peptides, antibodies, peptibodies, carbohydrates
or small organic
molecules. Peptibodies are described, e.g., in W001/83525.
The term "agonist", refers to a modulator that, when contacted with a molecule
of
interest, causes an increase in the magnitude of a certain activity or
function of the molecule
compared to the magnitude of the activity or function observed in the absence
of the agonist.
Particular agonists of interest may include, but are not limited to,
polypeptides, nucleic acids,
carbohydrates, or any other molecules that bind to the antigen.
The term "antagonist" or "inhibitor", refer to a modulator that, when
contacted with a
molecule of interest causes a decrease in the magnitude of a certain activity
or function of the
molecule compared to the magnitude of the activity or function observed in the
absence of the
antagonist. Particular antagonists of interest include those that block or
modulate the biological
53
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
or immunological activity of of the antigen. Antagonists and inhibitors of
antigens may include,
but are not limited to, proteins, nucleic acids, carbohydrates, or any other
molecules, which bind
to the antigen.
As used herein, the term "effective amount" refers to the amount of a therapy
which is
sufficient to reduce or ameliorate the severity and/or duration of a disorder
or one or more
symptoms thereof, prevent the advancement of a disorder, cause regression of a
disorder, prevent
the recurrence, development, onset or progression of one or more symptoms
associated with a
disorder, detect a disorder, or enhance or improve the prophylactic or
therapeutic effect(s) of
another therapy (e.g., prophylactic or therapeutic agent).
"Patient" and "subject" may be used interchangeably herein to refer to an
animal, such as
a mammal, including a primate (for example, a human, a monkey, and a
chimpanzee), a non-
primate (for example, a cow, a pig, a camel, a llama, a horse, a goat, a
rabbit, a sheep, a hamster,
a guinea pig, a cat, a dog, a rat, a mouse, a whale), a bird (e.g., a duck or
a goose), and a shark.
Preferably, the patient or subject is a human, such as a human being treated
or assessed for a
disease, disorder or condition, a human at risk for a disease, disorder or
condition, a human
having a disease, disorder or condition, and/or human being treated for a
disease, disorder or
condition.
The term "sample", as used herein, is used in its broadest sense. A
"biological sample",
as used herein, includes, but is not limited to, any quantity of a substance
from a living thing or
formerly living thing. Such living things include, but are not limited to,
humans, mice, rats,
monkeys, dogs, rabbits and other animals. Such substances include, but are not
limited to, blood
(e.g., whole blood), plasma, serum, urine, amniotic fluid, synovial fluid,
endothelial cells,
leukocytes, monocytes, other cells, organs, tissues, bone marrow, lymph nodes
and spleen.
"Component," "components," and "at least one component," refer generally to a
capture
antibody, a detection or conjugate antibody, a control, a calibrator, a series
of calibrators, a
sensitivity panel, a container, a buffer, a diluent, a salt, an enzyme, a co-
factor for an enzyme, a
detection reagent, a pretreatment reagent/solution, a substrate (e.g., as a
solution), a stop solution,
and the like that can be included in a kit for assay of a test sample, such as
a patient urine, serum
or plasma sample, in accordance with the methods described herein and other
methods known in
the art. Thus, in the context of the present disclosure, "at least one
component," "component,"
and "components" can include a polypeptide or other analyte as above, such as
a composition
comprising an analyte such as polypeptide, which is optionally immobilized on
a solid support,
54
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
such as by binding to an anti-analyte (e.g., anti-polypeptide) antibody. Some
components can be
in solution or lyophilized for reconstitution for use in an assay.
"Control" refers to a composition known to not analyte ("negative control") or
to contain
analyte ("positive control"). A positive control can comprise a known
concentration of analyte.
"Control," "positive control," and "calibrator" may be used interchangeably
herein to refer to a
composition comprising a known concentration of analyte. A "positive control"
can be used to
establish assay performance characteristics and is a useful indicator of the
integrity of reagents
(e.g., analytes).
"Predetermined cutoff' and "predetermined level" refer generally to an assay
cutoff
value that is used to assess diagnostic/prognostic/therapeutic efficacy
results by comparing the
assay results against the predetermined cutoff/level, where the predetermined
cutoff/level already
has been linked or associated with various clinical parameters (e.g., severity
of disease,
progression/nonprogression/improvement, etc.). While the present disclosure
may provide
exemplary predetermined levels, it is well-known that cutoff values may vary
depending on the
nature of the immunoassay (e.g., antibodies employed, etc.). It further is
well within the ordinary
skill of one in the art to adapt the disclosure herein for other immunoassays
to obtain
immunoassay-specific cutoff values for those other immunoassays based on this
disclosure.
Whereas the precise value of the predetermined cutoff/level may vary between
assays,
correlations as described herein (if any) should be generally applicable.
"Pretreatment reagent," e.g., lysis, precipitation and/or solubilization
reagent, as used in a
diagnostic assay as described herein is one that lyses any cells and/or
solubilizes any analyte that
is/are present in a test sample. Pretreatment is not necessary for all
samples, as described further
herein. Among other things, solubilizing the analyte (e.g., polypeptide of
interest) may entail
release of the analyte from any endogenous binding proteins present in the
sample. A
pretreatment reagent may be homogeneous (not requiring a separation step) or
heterogeneous
(requiring a separation step). With use of a heterogeneous pretreatment
reagent there is removal
of any precipitated analyte binding proteins from the test sample prior to
proceeding to the next
step of the assay.
"Quality control reagents" in the context of immunoassays and kits described
herein,
include, but are not limited to, calibrators, controls, and sensitivity
panels. A "calibrator" or
"standard" typically is used (e.g., one or more, such as a plurality) in order
to establish
calibration (standard) curves for interpolation of the concentration of an
analyte, such as an
antibody or an analyte. Alternatively, a single calibrator, which is near a
predetermined
55
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
positive/negative cutoff, can be used. Multiple calibrators (i.e., more than
one calibrator or a
varying amount of calibrator(s)) can be used in conjunction so as to comprise
a "sensitivity
panel."
"Risk" refers to the possibility or probability of a particular event
occurring either
presently or at some point in the future. "Risk stratification" refers to an
array of known clinical
risk factors that allows physicians to classify patients into a low, moderate,
high or highest risk of
developing a particular disease, disorder or condition.
"Specific" and "specificity" in the context of an interaction between members
of a
specific binding pair (e.g., an antigen (or fragment thereof) and an antibody
(or antigenically
reactive fragment thereof)) refer to the selective reactivity of the
interaction. The phrase
"specifically binds to" and analogous phrases refer to the ability of
antibodies (or antigenically
reactive fragments thereof) to bind specifically to analyte (or a fragment
thereof) and not bind
specifically to other entities.
"Specific binding partner" is a member of a specific binding pair. A specific
binding pair
comprises two different molecules, which specifically bind to each other
through chemical or
physical means. Therefore, in addition to antigen and antibody specific
binding pairs of common
immunoassays, other specific binding pairs can include biotin and avidin (or
streptavidin),
carbohydrates and lectins, complementary nucleotide sequences, effector and
receptor molecules,
cofactors and enzymes, enzyme inhibitors and enzymes, and the like.
Furthermore, specific
binding pairs can include members that are analogs of the original specific
binding members, for
example, an analyte-analog. Immunoreactive specific binding members include
antigens, antigen
fragments, and antibodies, including monoclonal and polyclonal antibodies as
well as complexes,
fragments, and variants (including fragments of variants) thereof, whether
isolated or
recombinantly produced.
"Variant" as used herein means a polypeptide that differs from a given
polypeptide (e.g.,
IL-18, BNP, NGAL or BTV polypeptide or anti-polypeptide antibody) in amino
acid sequence by
the addition (e.g., insertion), deletion, or conservative substitution of
amino acids, but that retains
the biological activity of the given polypeptide (e.g., a variant IL-18 can
compete with anti-IL-18
antibody for binding to 1L-18). A conservative substitution of an amino acid,
i.e., replacing an
amino acid with a different amino acid of similar properties (e.g.,
hydrophilicity and degree and
distribution of charged regions) is recognized in the art as typically
involving a minor change.
These minor changes can be identified, in part, by considering the hydropathic
index of amino
acids, as understood in the art (see, e.g., Kyte et al., J. Mol. Biol. 157:
105-132 (1982)). The
56
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
hydropathic index of an amino acid is based on a consideration of its
hydrophobicity and charge.
It is known in the art that amino acids of similar hydropathic indexes can be
substituted and still
retain protein function. In one aspect, amino acids having hydropathic indexes
of 2 are
substituted. The hydrophilicity of amino acids also can be used to reveal
substitutions that would
result in proteins retaining biological function. A consideration of the
hydrophilicity of amino
acids in the context of a peptide permits calculation of the greatest local
average hydrophilicity of
that peptide, a useful measure that has been reported to correlate well with
antigenicity and
immunogenicity (see, e.g., U.S. Pat. No. 4,554,101). Substitution of amino
acids having similar
hydrophilicity values can result in peptides retaining biological activity,
for example
immunogenicity, as is understood in the art. In one aspect, substitutions are
performed with
amino acids having hydrophilicity values within 2 of each other. Both the
hydrophobicity index
and the hydrophilicity value of amino acids are influenced by the particular
side chain of that
amino acid. Consistent with that observation, amino acid substitutions that
are compatible with
biological function are understood to depend on the relative similarity of the
amino acids, and
particularly the side chains of those amino acids, as revealed by the
hydrophobicity,
hydrophilicity, charge, size, and other properties. "Variant" also can be used
to describe a
polypeptide or fragment thereof that has been differentially processed, such
as by proteolysis,
phosphorylation, or other post-translational modification, yet retains its
biological activity or
antigen reactivity, e.g., the ability to bind to IL-18. Use of "variant"
herein is intended to
encompass fragments of a variant unless otherwise contradicted by context.
I. Generation of binding proteins
Dual Variable Domain binding proteins capable of binding one or more targets
and
methods of making the same are provided. In an embodiment, the binding protein
comprises a
polypeptide chain, wherein said polypeptide chain comprises VD1-(Xl)n-VD2-C-
(X2)n, wherein
VD1 is a first variable domain, VD2 is a second variable domain, C is a
constant domain, X1
represents an amino acid or polypeptide, X2 represents an Fe region and n is 0
or 1. The binding
proteins can be generated using various techniques. Expression vectors, host
cell and methods of
generating the binding protein are provided.
A. Generation of parent monoclonal antibodies
The variable domains of the binding proteins provided herein can be obtained
from
parent antibodies, including polyclonal and mAbs capable of binding antigens
of interest. These
antibodies may be naturally occurring or may be generated by recombinant
technology.
57
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
MAbs can be prepared using a wide variety of techniques known in the art
including the
use of hybridoma, recombinant, and phage display technologies, or a
combination thereof. For
example, mAbs can be produced using hybridoma techniques including those known
in the art
and taught, for example, in Harlow et al. , Antibodies: A Laboratory Manual,
(Cold Spring
Harbor Laboratory Press, 2nd ed. 1988); Hammerling, et al., in: Monoclonal
Antibodies and T-
Cell Hybridomas 563-681 (Elsevier, N.Y., 1981). The term "monoclonal antibody"
as used herein
is not limited to antibodies produced through hybridoma technology. The term
"monoclonal
antibody" refers to an antibody that is derived from a single clone, including
any eukaryotic,
prokaryotic, or phage clone, and not the method by which it is produced.
Hybridomas are
selected, cloned and further screened for desirable characteristics, including
robust hybridoma
growth, high antibody production and desirable antibody characteristics, as
discussed in Example
lbelow. Hybridomas may be cultured and expanded in vivo in syngeneic animals,
in animals that
lack an immune system, e.g., nude mice, or in cell culture in vitro. Methods
of selecting, cloning
and expanding hybridomas are well known to those of ordinary skill in the art.
In a particular
embodiment, the hybridomas are mouse hybridomas. In another embodiment, the
hybridomas are
produced in a non-human, non-mouse species such as rats, sheep, pigs, goats,
cattle or horses. In
another embodiment, the hybridomas are human hybridomas, in which a human non-
secretory
myeloma is fused with a human cell expressing an antibody capable of binding a
specific antigen.
Recombinant mAbs are also generated from single, isolated lymphocytes using a
procedure referred to in the art as the selected lymphocyte antibody method
(SLAM), as
described in U.S. Patent No. 5,627,052, PCT Publication WO 92/02551 and
Babcock, J.S. etal.
(1996) Proc. Natl. Acad. Sci. USA 93:7843-7848. In this method, single cells
secreting antibodies
of interest, e.g., lymphocytes derived from an immunized animal, are
identified, and, heavy- and
light-chain variable region cDNAs are rescued from the cells by reverse
transcriptase-PCR and
these variable regions can then be expressed, in the context of appropriate
immunoglobulin
constant regions (e.g., human constant regions), in mammalian host cells, such
as COS or CHO
cells. The host cells transfected with the amplified immunoglobulin sequences,
derived from in
vivo selected lymphocytes, can then undergo further analysis and selection in
vitro, for example
by panning the transfected cells to isolate cells expressing antibodies to the
antigen of interest.
The amplified immunoglobulin sequences further can be manipulated in vitro,
such as by in vitro
affinity maturation methods such as those described in PCT Publication WO
97/29131 and PCT
Publication WO 00/56772.
Monoclonal antibodies are also produced by immunizing a non-human animal
comprising some, or all, of the human immunoglobulin locus with an antigen of
interest. In an
58
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
embodiment, the non-human animal is a XENOMOUSE transgenic mouse, an
engineered
mouse strain that comprises large fragments of the human immunoglobulin loci
and is deficient
in mouse antibody production. See, e.g., Green et al. Nature Genetics 7:13-
21(1994) and
United States Patents Nos. 5,916,771, 5,939,598, 5,985,615, 5,998,209,
6,075,181, 6,091,001,
6,114,598 and 6,130,364. See also WO 91/10741, published July 25,1991, WO
94/02602,
published February 3, 1994, WO 96/34096 and WO 96/33735, both published
October 31,
1996, WO 98/16654, published April 23, 1998, WO 98/24893, published June 11,
1998, WO
98/50433, published November 12, 1998, WO 99/45031, published September 10,
1999, WO
99/53049, published October 21, 1999, WO 00 09560, published February 24, 2000
and WO
00/037504, published June 29, 2000. The XENOMOUSE transgenic mouse produces an
adult-
like human repertoire of fully human antibodies, and generates antigen-
specific human
monoclonal antibodies. The XENOMOUSE transgenic mouse contains approximately
80% of
the human antibody repertoire through introduction of megabase sized, germline
configuration
YAC fragments of the human heavy chain loci and x light chain loci. See Mendez
et al., Nature
Genetics 15:146-156 (1997), Green and Jakobovits J. Exp. Med. 188:483-495
(1998).
In vitro methods also can be used to make the parent antibodies, wherein an
antibody
library is screened to identify an antibody having the desired binding
specificity. Methods for
such screening of recombinant antibody libraries are well known in the art and
include methods
described in, for example, Ladner et al. U.S. Patent No. 5,223,409; Kang et
al. PCT Publication
No. WO 92/18619; Dower et al. PCT Publication No. WO 91/17271; Winter etal.
PCT
Publication No. WO 92/20791; Markland et al. PCT Publication No. WO 92/15679;
Breitling et
al. PCT Publication No. WO 93/01288; McCafferty et al. PCT Publication No. WO
92/01047;
Garrard et al. PCT Publication No. WO 92/09690; Fuchs et al. (1991)
Bio/Technology 9:1370-
1372; Hay etal. (1992) Hum Antibod Hybridomas 3:81-85; Huse etal. (1989)
Science 246:1275-
1281; McCafferty et al., Nature (1990) 348:552-554; Griffiths etal. (1993)
EMBO J12:725-734;
Hawkins etal. (1992)J MoI Biol 226:889-896; Clackson etal. (1991) Nature
352:624-628; Gram
etal. (1992) PNAS 89:3576-3580; Garrad etal. (1991) Bio/Technology 9:1373-
1377;
Hoogenboom et al. (1991) Nue Acid Res 19:4133-4137; and Barbas et al. (1991)
PNAS 88:7978-
7982, US patent application publication 20030186374, and PCT Publication No.
WO 97/29131.
Parent antibodies can also be generated using various phage display methods
known in
the art. In phage display methods, functional antibody domains are displayed
on the surface of
phage particles that carry the polynucleotide sequences encoding them. In a
particular, such
phage can be utilized to display antigen-binding domains expressed from a
repertoire or
combinatorial antibody library (e. g., human or murine). Phage expressing an
antigen binding
59
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
domain that binds the antigen of interest can be selected or identified with
antigen, e.g., using
labeled antigen or antigen bound or captured to a solid surface or bead. Phage
used in these
methods are typically filamentous phage including fd and M13 binding domains
expressed from
phage with Fab, Fv or disulfide stabilized Fv antibody domains recombinantly
fused to either the
phage gene III or gene VIII protein. Examples of phage display methods include
those disclosed
in Brinkman etal., J. Immunol. Methods 182:41-50 (1995); Ames etal., J.
Immunol. Methods
184:177-186 (1995); Kettleborough et al., Eur. J. Immunol. 24:952-958 (1994);
Persic et al.,
Gene 187 9-18 (1997); Burton et al., Advances in Immunology 57:191-280 (1994);
PCT
application No. PCT/GB91/01434; PCT publications WO 90/02809; WO 91/10737; WO
92/01047; WO 92/18619; WO 93/11236; WO 95/15982; WO 95/20401; and U.S. Pat.
Nos.
5,698,426; 5,223,409; 5,403,484; 5,580,717; 5,427,908; 5,750,753; 5,821,047;
5,571,698;
5,427,908; 5,516,637; 5,780, 225; 5,658,727; 5,733,743 and 5,969,108.
As described in the herein references, after phage selection, the antibody
coding regions
from the phage can be isolated and used to generate whole antibodies including
human antibodies
or any other desired antigen binding fragment, and expressed in any desired
host, including
mammalian cells, insect cells, plant cells, yeast, and bacteria, e.g., as
described in detail below.
For example, techniques to recombinantly produce Fab, Fab' and F(ab')2
fragments can also be
employed using methods known in the art such as those disclosed in PCT
publication WO
92/22324; Mullinax et al., BioTechniques 12(6):864-869 (1992); and Sawai et
al., AJRI 34:26-34
(1995); and Better etal., Science 240:1041-1043 (1988). Examples of techniques
which can be
used to produce single-chain Fvs and antibodies include those described in
U.S. Pat. 4,946,778
and 5,258, 498; Huston et al., Methods in Enzymology 203:46-88 (1991); Shu et
aL, PNAS
90:7995-7999 (1993); and Skerra et al., Science 240:1038-1040 (1988).
Alternative to screening of recombinant antibody libraries by phage display,
other
methodologies known in the art for screening large combinatorial libraries can
be applied to the
identification of parent antibodies. One type of alternative expression system
is one in which the
recombinant antibody library is expressed as RNA-protein fusions, as described
in PCT
Publication No. WO 98/31700 by Szostak and Roberts, and in Roberts, R.W. and
Szostak, J.W.
(1997) Proc. Natl. Acad. ScL USA 94:12297-12302. In this system, a covalent
fusion is created
between an mRNA and the peptide or protein that it encodes by in vitro
translation of synthetic
mRNAs that carry puromycin, a peptidyl acceptor antibiotic, at their 3' end.
Thus, a specific
mRNA can be enriched from a complex mixture of mRNAs (e.g., a combinatorial
library) based
on the properties of the encoded peptide or protein, e.g., antibody, or
portion thereof, such as
binding of the antibody, or portion thereof, to the dual specificity antigen.
Nucleic acid sequences
60
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
encoding antibodies, or portions thereof, recovered from screening of such
libraries can be
expressed by recombinant means as described herein (e.g., in mammalian host
cells) and,
moreover, can be subjected to further affinity maturation by either additional
rounds of screening
of mRNA-peptide fusions in which mutations have been introduced into the
originally selected
sequence(s), or by other methods for affinity maturation in vitro of
recombinant antibodies, as
described herein.
In another approach the parent antibodies can also be generated using yeast
display
methods known in the art. In yeast display methods, genetic methods are used
to tether antibody
domains to the yeast cell wall and display them on the surface of yeast. In
particular, such yeast
can be utilized to display antigen-binding domains expressed from a repertoire
or combinatorial
antibody library (e.g., human or murine). Examples of yeast display methods
that can be used to
make the parent antibodies include those disclosed in Wittrup, et al. U.S.
Patent No. 6,699,658.
The antibodies described herein can be further modified to generate CDR
grafted and
humanized parent antibodies. CDR-grafted parent antibodies comprise heavy and
light chain
variable region sequences from a human antibody wherein one or more of the CDR
regions of VH
and/or VL are replaced with CDR sequences of murine antibodies capable of
binding antigen of
interest. A framework sequence from any human antibody may serve as the
template for CDR
grafting. However, straight chain replacement onto such a framework often
leads to some loss of
binding affinity to the antigen. The more homologous a human antibody is to
the original murine
antibody, the less likely the possibility that combining the murine CDRs with
the human
framework will introduce distortions in the CDRs that could reduce affinity.
Therefore, in an
embodiment, the human variable framework that is chosen to replace the murine
variable
framework apart from the CDRs have at least a 65% sequence identity with the
murine antibody
variable region framework. In an embodiment, the human and murine variable
regions apart from
the CDRs have at least 70% sequence identify. In a particular embodiment, that
the human and
murine variable regions apart from the CDRs have at least 75% sequence
identity. In another
embodiment, the human and murine variable regions apart from the CDRs have at
least 80%
sequence identity. Methods for producing such antibodies are known in the art
( see EP 239,400;
PCT publication WO 91/09967; U.S. Pat. Nos. 5,225,539; 5,530,101; and
5,585,089), veneering
or resurfacing (EP 592,106; EP 519,596; Padlan, Molecular Immunology
28(4/5):489-498
(1991); Studnicka et al., Protein Engineering 7(6):805-814 (1994); Roguska et
al., PNAS 91:969-
973 (1994)), and chain shuffling (U.S. Pat. No. 5,565,352); and anti-idiotypic
antibodies.
Humanized antibodies are antibody molecules from non-human species antibody
that
binds the desired antigen having one or more complementarity determining
regions (CDRs) from
61
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
the non-human species and framework regions from a human immunoglobulin
molecule. Known
human Ig sequences are disclosed, e.g., www.ncbi.nlm.nih.gov/entrez-
/query.fcgi;
www.atcc.org/phage/hdb.html; vvww.sciquest.comi; www.abcam.comi;
www.antibodyresource.com/onlinecomp.html;
www.public.iastate.eduLabout.pedro/research_tools.html; www.mgen.uni-
heidelberg.de/SDAT/IT.html; www.whfreeman.com/immunology/CH- 05/kuby05.htin;
wvvw.library.thinkquest.org/12429/Immune/Antibody.html;
www.hhmi.org/grants/lectures/1996/vlab/; www.path.cam.ac.uk/.about.mrc7/m-
ikeimages.html;
www.antibodyresource.com/; mcb.harvard.edu/BioLinks/Immuno-
logy.html.www.immunologylink.comi; pathbox.wustl.edu/.about.hcenter/index.-
html;
www.biotech.ufteduLabout.hc1/; www.pebio.com/pa1340913/340913.html-;
www.nal.usda.gov/awic/pubs/antibody/; www.m.ehime-u.acjp/.about.yasuhito-
/Elisa.html;
www.biodesign.com/table.asp; www.icnet.uldaxp/facs/davies/lin- ks.html;
www.biotech.ufLeduLabout.fccl/protocol.html; wvvw.isac-net.org/sites_geo.html;
aximtl.imt.uni-
marburg.de/.about.rek/AEP- Start.html;
baserv.uci.kun.n1/.about.jraats/linksl.html;
vvww.recab.uni-hd.de/immuno.bme.nwu.edui; www.mrc-cpe.cam.ac.uk/imt-doc/pu-
blic/INTRO.html; www.ibt.unam.mx/virN_mice.html; imgt.cnusc.fr:8104/;
www.biochem.ucl.ac.uk/.about.martin/abs/index.html; antibody.bath.ac.uld;
abgen.cvm.tamu.edu/lab/wwwabgen.html; www.unizh.chLabout.honegger/AHOsem-
inar/Slide0 1 .html; www.cryst.bbk.ac.ukhabout.ubcgO7s/;
www.nimr.mrc.ac.uk/CC/ccaewg/ccaewg.htm; www.path.cam.ac.uk/.about.mrc7/h-
umanisation/TAHHP.html; wvv-w.ibt.unam.mx/vir/structure/stat_aim.html;
www.biosci.missouri.edu/smithgp/index.html; www.cryst.bioc.cam.ac.ukhabo-
utimolina/Web-
pages/Pept/spottech.html; wwvv.jerini.de/fr roducts.htm;
www.patents.ibm.com/ibm.html.Kabat
et al., Sequences of Proteins of Immunological Interest, U.S. Dept. Health
(1983). Such imported
sequences can be used to reduce immunogenicity or reduce, enhance or modify
binding, affinity,
on-rate, off-rate, avidity, specificity, half-life, or any other suitable
characteristic, as known in the
art.
Framework residues in the human framework regions may be substituted with the
corresponding residue from the CDR donor antibody to alter, e.g., improve,
antigen binding.
These framework substitutions are identified by methods well known in the art,
e.g., by modeling
of the interactions of the CDR and framework residues to identify framework
residues important
for antigen binding and sequence comparison to identify unusual framework
residues at particular
positions. (See, e.g., Queen et al., U.S. Pat. No. 5,585,089; Riechmann et
al., Nature 332:323
(1988). Three-dimensional immunoglobulin models are commonly available and are
familiar to
62
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
those skilled in the art. Computer programs are available which illustrate and
display probable
three-dimensional conformational structures of selected candidate
immunoglobulin sequences.
Inspection of these displays permits analysis of the likely role of the
residues in the functioning
of the candidate immunoglobulin sequence, i.e., the analysis of residues that
influence the ability
of the candidate immunoglobulin to bind its antigen. In this way, FR residues
can be selected and
combined from the consensus and import sequences so that the desired antibody
characteristic,
such as increased affinity for the target antigen(s), is achieved. In general,
the CDR residues are
directly and most substantially involved in influencing antigen binding.
Antibodies can be
humanized using a variety of techniques known in the art, such as but not
limited to those
described in Jones et al., Nature 321:522 (1986); Verhoeyen et al., Science
239:1534 (1988)),
Sims et al., J. Immunol. 151: 2296 (1993); Chothia and Lesk, J. Mol. Biol.
196:901 (1987),
Carter et al., Proc. Natl. Acad. Sci. U.S.A. 89:4285 (1992); Presta et al., J.
Immunol. 151:2623
(1993), Padlan, Molecular Immunology 28(4/5):489-498 (1991); Studnicka et al.,
Protein
Engineering 7(6):805-814 (1994); Roguska. et al. , PNAS 91:969-973 (1994); PCT
publication
WO 91/09967, PCT/: US98/16280, US96/18978, US91/09630, US91/05939, US94/01234,
GB89/01334, GB91/01134, GB92/01755; W090/14443, W090/14424, W090/14430, EP
229246, EP 592,106; EP 519,596, EP 239,400, U.S. Pat. Nos. 5,565,332,
5,723,323, 5,976,862,
5,824,514, 5,817,483, 5814476, 5763192, 5723323, 5,766886, 5,714,352,
6,204,023, 6,180,370,
5,693,762, 5,530,101, 5,585,089, 5,225,539; 4,816,567.
B. Criteria for selecting parent monoclonal antibodies
In an embodiment, parent antibodies are selected with at least one or more
properties
desired in the DVD-Ig molecule. In an embodiment, the desired property is
selected from one or
more antibody parameters. In another embodiment, the antibody parameters are
antigen
specificity, affinity to antigen, potency, biological function, epitope
recognition, stability,
solubility, production efficiency, immunogenicity, pharmacokinetics,
bioavailability, tissue cross
reactivity, or orthologous antigen binding.
Bl. Affinity to Antigen
The desired affinity of a therapeutic mAb may depend upon the nature of the
antigen, and
the desired therapeutic end-point. In an embodiment, monoclonal antibodies
have higher
affinities (Kd = 0.01 ¨ 0.50 pM) when blocking a cytokine-cytokine receptor
interaction as such
interaction are usually high affinity interactions (e.g., <pM ¨ <nM ranges).
In such instances, the
mAb affinity for its target should be equal to or better than the affinity of
the cytokine (ligand)
for its receptor. On the other hand, mAb with lesser affinity (> nM range)
could be
63
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
therapeutically effective e.g., in clearing circulating potentially pathogenic
proteins, e.g.,
monoclonal antibodies that bind to, sequester, and clear circulating species
of A-13 amyloid. In
other instances, reducing the affinity of an existing high affinity mAb by
site-directed
mutagenesis or using a mAb with lower affinity for its target could be used to
avoid potential
side-effects e.g.,a high affinity mAb may sequester/neutralize all of its
intended target, thereby
completely depleting/eliminating the function(s) of the targeted protein. In
this scenario, a low
affinity mAb may sequester/neutralize a fraction of the target that may be
responsible for the
disease symptoms (the pathological or over-produced levels), thus allowing a
fraction of the
target to continue to perform its normal physiological function(s). Therefore,
it may be possible
to reduce the Kd to adjust dose and/or reduce side-effects. The affinity of
the parental mAb might
play a role in appropriately targeting cell surface molecules to achieve
desired therapeutic out-
come. For example, if a target is expressed on cancer cells with high density
and on normal cells
with low density, a lower affinity mAb will bind a greater number of targets
on tumor cells than
normal cells, resulting in tumor cell elimination via ADCC or CDC, and
therefore might have
therapeutically desirable effects. Thus selecting a mAb with desired affinity
may be relevant for
both soluble and surface targets.
Signaling through a receptor upon interaction with its ligand may depend upon
the
affinity of the receptor-ligand interaction. Similarly, it is conceivable that
the affinity of a mAb
for a surface receptor could determine the nature of intracellular signaling
and whether the mAb
may deliver an agonist or an antagonist signal. The affinity-based nature of
mAb-mediated
signaling may have an impact of its side-effect profile. Therefore, the
desired affinity and desired
functions of therapeutic monoclonal antibodies need to be determined carefully
by in vitro and in
vivo experimentation.
The desired Kd of a binding protein (e.g., an antibody) may be determined
experimentally depending on the desired therapeutic outcome_ In an embodiment,
parent
antibodies with affinity (Kd) for a particular antigen equal to, or better
than, the desired affinity
of the DVD-Ig for the same antigen are selected. The antigen binding affinity
and kinetics are
assessed by Biacore or another similar technique. In one embodiment, each
parent antibody has a
dissociation constant (Kd) to its antigen of: at most about 1 0-7 M; at most
about 1 0 M; at most
about 10-9 M; at most about 10-1 M; at most about 10-11 M; at most about 10-
12 M; or at most 10-13
M. First parent antibody from which VD1 is obtained and second parent antibody
from which
VD2 is obtained may have similar or different affinity (KD) for the respective
antigen. Each
parent antibody has an on rate constant (Kon) to the antigen of: at least
about 102M-1s-1; at least
about 103M-1s-1; at least about 104M-1s-1; at least about 105M-1s-1; or at
least about 106M-1s-1, as
64
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
measured by surface plasmon resonance. The first parent antibody from which
VD1 is obtained
and the second parent antibody from which VD2 is obtained may have similar or
different on rate
constant (Kon) for the respective antigen. In one embodiment, each parent
antibody has an off
rate constant (Koff) to the antigen of: at most about 10-3s-1; at most about
104s-1; at most about
10-5s-1; or at most about les-I, as measured by surface plasmon resonance. The
first parent
antibody from which VD1 is obtained and the second parent antibody from which
VD2 is
obtained may have similar or different off rate constants (Koff) for the
respective antigen.
B2. Potency
The desired affinity/potency of parental monoclonal antibodies will depend on
the
desired therapeutic outcome. For example, for receptor-ligand (R-L)
interactions the affinity (kd)
is equal to or better than the R-L kd (pM range). For simple clearance of a
pathologic circulating
protein, the kd could be in low nM range e.g., clearance of various species of
circulating A-13
peptide. In addition, the kd will also depend on whether the target expresses
multiple copies of
the same epitope e.g a mAb targeting conformational epitope in Al3 oligomers.
Where VDI and VD2 bind the same antigen, but distint epitopes, the DVD-Ig will
contain 4 binding sites for the same antigen, thus increasing avidity and
thereby the apparent kd
of the DVD-Ig. In an embodiment, parent antibodies with equal or lower kd than
that desired in
the DVD-Ig are chosen. The affinity considerations of a parental mAb may also
depend upon
whether the DVD-Ig contains four or more identical antigen binding sites (i.e;
a DVD-Ig from a
single mAb). In this case, the apparent kd would be greater than the mAb due
to avidity. Such
DVD-Igs can be employed for cross-linking surface receptor, increase
neutralization potency,
enhance clearance of pathological proteins etc.
In an embodiment parent antibodies with neutralization potency for specific
antigen
equal to or better than the desired neutralization potential of the DVD-Ig for
the same antigen are
selected. The neutralization potency can be assessed by a target-dependent
bioassay where cells
of appropriate type produce a measurable signal (i.e. proliferation or
cytokine production) in
response to target stimulation, and target neutralization by the mAb can
reduce the signal in a
dose-dependent manner.
B3. Biological functions
Monoclonal antibodies can perform potentially several functions. Some of these
functions are listed in Table I. These functions can be assessed by both in
vitro assays (e.g., cell-
based and biochemical assays) and in vivo animal models.
65
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
Table 1: Some Potential Applications For Therapeutic Antibodies
Target (Class) Mechanism of Action (target)
Soluble Neutralization of activity (e.g., a cytokine)
(cytokines,other) Enhance clearance (e.g., A13 oligomers)
Increase half-life (e.g., GLP 1)
Cell Surface Agonist (e.g., GLP1 R; EPO R; etc.)
(Receptors, other) Antagonist (e.g., integrins; etc.)
Cytotoxic (CD 20; etc.)
Protein deposits Enhance clearance/degradation (e.g., A13 plaques, amyloid
deposits)
MAbs with distinct functions described in the examples herein in Table 1 can
be selected
to achieve desired therapeutic outcomes. Two or more selected parent
monoclonal antibodies can
then be used in DVD-Ig format to achieve two distinct functions in a single
DVD-Ig molecule.
For example, a DVD-Ig can be generated by selecting a parent mAb that
neutralizes function of a
specific cytokine, and selecting a parent mAb that enhances clearance of a
pathological protein.
Similarly, we can select two parent monoclonal antibodies that recognize two
different cell
surface receptors, one mAb with an agonist function on one receptor and the
other mAb with an
antagonist function on a different receptor. These two selected monoclonal
antibodies each with a
distinct function can be used to construct a single DVD-Ig molecule that will
possess the two
distinct functions (agonist and antagonist) of the selected monoclonal
antibodies in a single
molecule. Similarly, two antagonistic monoclonal antibodies to cell surface
receptors each
blocking binding of respective receptor ligands (e.g., EGF and IGF) can be
used in a DVD-Ig
format. Conversely, an antagonistic anti-receptor mAb (e.g., anti-EGFR) and a
neutralizing anti-
soluble mediator (e.g., anti-IGF1/2) mAb can be selected to make a DVD-Ig.
B4. Epitope Recognition
Different regions of proteins may perform different functions. For example
specific
regions of a cytokine interact with the cytokine receptor to bring about
receptor activation
whereas other regions of the protein may be required for stabilizing the
cytokine. In this instance
one may select a mAb that binds specifically to the receptor interacting
region(s) on the cytokine
and thereby block cytokine-receptor interaction. In some cases, for example
certain chemokine
receptors that bind multiple ligands, a mAb that binds to the epitope (region
on chemokine
receptor) that interacts with only one ligand can be selected. In other
instances, monoclonal
antibodies can bind to epitopes on a target that are not directly responsible
for physiological
functions of the protein, but binding of a mAb to these regions could either
interfere with
physiological functions (steric hindrance) or alter the conformation of the
protein such that the
66
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
protein cannot function (mAb to receptors with multiple ligand which alter the
receptor
conformation such that none of the ligand can bind). Anti-cytokine monoclonal
antibodies that do
not block binding of the cytokine to its receptor, but block signal
transduction have also been
identified (e.g., 125-211, an anti-11,-18 mAb).
Examples of epitopes and mAb functions include, but are not limited to,
blocking
Receptor-Ligand (R-L) interaction (neutralizing mAb that binds R-interacting
site); steric
hindrance resulting in diminished or no R-binding. An Ab can bind the target
at a site other than a
receptor binding site, but still interferes with receptor binding and
functions of the target by
inducing conformational change and eliminate function (e.g., Xolair), binding
to R but block
signaling (125-211).
In an embodiment, the parental mAb needs to target the appropriate epitope for
maximum
efficacy. Such epitope should be conserved in the DVD-Ig. The binding epitope
of a mAb can be
determined by several approaches, including co-crystallography, limited
proteolysis of mAb-
antigen complex plus mass spectrometric peptide mapping (Legros V. et al 2000
Protein Sci.
9:1002-10), phage displayed peptide libraries (O'Connor KIT et al 2005 J
Immunol Methods.
299:21-35), as well as mutagenesis (Wu C. et al . 2003 J Immunol 170:5571-7).
B5. Physicochemical and pharmaceutical properties
Therapeutic treatment with antibodies often requires administration of high
doses, often
several mg/kg (due to a low potency on a mass basis as a consequence of a
typically large
molecular weight). In order to accommodate patient compliance and to
adequately address
chronic disease therapies and outpatient treatment, subcutaneous (s.c.) or
intramuscular (i.m.)
administration of therapeutic mAbs is desirable. For example, the maximum
desirable volume for
s.c. administration is ¨1.0 mL, and therefore, concentrations of >100 mg/mL
are desirable to limit
the number of injections per dose. In an embodiment, the therapeutic antibody
is administered in
one dose. The development of such formulations is constrained, however, by
protein-protein
interactions (e.g., aggregation, which potentially increases immunogenicity
risks) and by
limitations during processing and delivery (e.g., viscosity). Consequently,
the large quantities
required for clinical efficacy and the associated development constraints
limit full exploitation of
the potential of antibody formulation and s.c. administration in high-dose
regimens. It is apparent
that the physicochemical and pharmaceutical properties of a protein molecule
and the protein
solution are of utmost importance, e.g.,stability, solubility and viscosity
features.
67
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
B5.1. Stability
A "stable" antibody formulation is one in which the antibody therein
essentially retains
its physical stability and/or chemical stability and/or biological activity
upon storage. Stability
can be measured at a selected temperature for a selected time period. In an
embodiment, the
antibody in the formulation is stable at room temperature (about 30 C) or at
40 C for at least 1
month and/or stable at about 2-8 C. for at least 1 year for at least 2 years.
Furthermore, in an
embodiment, the formulation is stable following freezing (to, e.g., -70 C) and
thawing of the
formulation, hereinafter referred to as a "freeze/thaw cycle." In another
example, a "stable"
formulation may be one wherein less than about 10% and less than about 5% of
the protein is
present as an aggregate in the formulation.
A DVD-Ig stable in vitro at various temperatures for an extended time period
is desirable.
One can achieve this by rapid screening of parental mAbs stable in vitro at
elevated temperature,
e.g.,at 40 C for 2-4 weeks, and then assess stability. During storage at 2-8
C, the protein reveals
stability for at least 12 months, e.g., at least 24 months. Stability (% of
monomeric, intact
molecule) can be assessed using various techniques such as cation exchange
chromatography,
size exclusion chromatography, SDS-PAGE, as well as bioactivity testing. For a
more
comprehensive list of analytical techniques that may be employed to analyze
covalent and
conformational modifications see Jones, A. J. S. (1993) Analytical methods for
the assessment of
protein formulations and delivery systems. In: Cleland, J. L.; Langer, R.,
editors. Formulation and
delivery of peptides and proteins, 1 s' edition, Washington, ACS, pg. 22-45;
and Pearlman, R.;
Nguyen, T. H.(1990) Analysis of protein drugs. In: Lee, V. H., editor. Peptide
and protein drug
delivery, 1st edition, New York, Marcel Dekker, Inc., pg. 247-301.
Heterogeneity and aggregate formation: stability of the antibody may be such
that the
formulation may reveal less than about 10%, and, in an embodiment, less than
about 5%, in
another embodiment, less than about 2%, or, in an embodiment, within the range
of 0.5% to 1.5%
or less in the GMP antibody material that is present as aggregate. Size
exclusion chromatography
is a method that is sensitive, reproducible, and very robust in the detection
of protein aggregates.
In addition to low aggregate levels, the antibody must, in an embodiment, be
chemically
stable. Chemical stability may be determined by ion exchange chromatography
(e.g.,cation or
anion exchange chromatography), hydrophobic interaction chromatography, or
other methods
such as isoelectric focusing or capillary electrophoresis. For instance,
chemical stability of the
antibody may be such that after storage of at least 12 months at 2-8 C the
peak representing
unmodified antibody in a cation exchange chromatography may increase not more
than 20%, in
68
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
an embodiment, not more than 10%, or, in another embodiment, not more than 5%
as compared
to the antibody solution prior to storage testing.
In an embodiment, the parent antibodies display structural integrity; correct
disulfide
bond formation, and correct folding: Chemical instability due to changes in
secondary or tertiary
structure of an antibody may impact antibody activity. For instance, stability
as indicated by
activity of the antibody may be such that after storage of at least 12 months
at 2-8 C the activity
of the antibody may decrease not more than 50%, in an embodiment not more than
30%, or even
not more than 10%, or in an embodiment not more than 5% or 1% as compared to
the antibody
solution prior to storage testing. Suitable antigen-binding assays can be
employed to determine
antibody activity.
B5.2. Solubility
The "solubility" of a mAb correlates with the production of correctly folded,
monomeric
IgG. The solubility of the IgG may therefore be assessed by HPLC. For example,
soluble
(monomeric) IgG will give rise to a single peak on the HPLC chromatograph,
whereas insoluble
(e.g., multimeric and aggregated) will give rise to a plurality of peaks. A
person skilled in the art
will therefore be able to detect an increase or decrease in solubility of an
IgG using routine HPLC
techniques. For a more comprehensive list of analytical techniques that may be
employed to
analyze solubility (see Jones, A. G. Dep. Chem. Biochem. Eng., Univ. Coll.
London, London,
UK. Editor(s): Shamlou, P. Ayazi. Process. Solid-Liq. Suspensions (1993), 93-
117. Publisher:
Butterworth-Heinemann, Oxford, UK and Pearlman, Rodney; Nguyen, Tue H,
Advances in
Parenteral Sciences (1990), 4 (Pept. Protein Drug Delivery), 247-301).
Solubility of a therapeutic
mAb is critical for formulating to high concentration often required for
adequate dosing. As
outlined herein, solubilities of >100 mg/mL may be required to accommodate
efficient antibody
dosing. For instance, antibody solubility may be not less than about 5 mg/mL
in early research
phase, in an embodiment not less than about 25 mg/mL in advanced process
science stages, or in
an embodiment not less than about 100 mg/mL, or in an embodiment not less than
about 150
mg/mL. It is obvious to a person skilled in the art that the intrinsic
properties of a protein
molecule are important the physico-chemical properties of the protein
solution, e.g.,stability,
solubility, viscosity. However, a person skilled in the art will appreciate
that a broad variety of
excipients exist that may be used as additives to beneficially impact the
characteristics of the
final protein formulation. These excipients may include: (i) liquid solvents,
cosolvents (e.g.,
alcohols such as ethanol); (ii) buffering agents (e.g., phosphate, acetate,
citrate, amino acid
buffers); (iii) sugars or sugar alcohols (e.g., sucrose, trehalose, fructose,
raffinose, mannitol,
sorbitol, dextrans); (iv) surfactants (e.g., polysorbate 20, 40, 60, 80,
poloxamers); (v) isotonicity
69
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
modifiers (e.g., salts such as NaC1, sugars, sugar alcohols); and (vi) others
(e.g., preservatives,
chelating agents, antioxidants, chelating substances (e.g., EDTA),
biodegradable polymers,
carrier molecules (e.g., HSA, PEGs)
Viscosity is a parameter of high importance with regard to antibody
manufacture and
antibody processing (e.g., diafiltration/ultrafiltration), fill-finish
processes (pumping aspects,
filtration aspects) and delivery aspects (syringeability, sophisticated device
delivery). Low
viscosities enable the liquid solution of the antibody having a higher
concentration. This enables
the same dose may be administered in smaller volumes. Small injection volumes
inhere the
advantage of lower pain on injection sensations, and the solutions not
necessarily have to be
isotonic to reduce pain on injection in the patient. The viscosity of the
antibody solution may be
such that at shear rates of 100 (1/s) antibody solution viscosity is below 200
mPa s, in an
embodiment below 125 mPa s, in another embodiment below 70 mPa s, and in yet
another
embodiment below 25 mPa s or even below 10 mPa s.
B 5.3. Production efficiency
The generation of a DVD-Ig that is efficiently expressed in mammalian cells,
such as
Chinese hamster ovary cells (CHO), will in an embodiment require two parental
monoclonal
antibodies which are themselves expressed efficiently in mammalian cells. The
production yield
from a stable mammalian line (i.e., CHO) should be above about 0.5g/L, in an
embodiment above
about lg/L, and in another embodiment in the range of about 2 to about 5 g/L
or more
(Kipriyanov SM, Little M. 1999 Mol Biotechnol. 12:173-201; Carroll S, Al-
Rubeai M. 2004
Expert Opin Biol Ther. 4:1821-9).
Production of antibodies and Ig fusion proteins in mammalian cells is
influenced by
several factors. Engineering of the expression vector via incorporation of
strong promoters,
enhancers and selection markers can maximize transcription of the gene of
interest from an
integrated vector copy. The identification of vector integration sites that
are permissive for high
levels of gene transcription can augment protein expression from a vector
(Wurm et al, 2004,
Nature Biotechnology, 2004, Vol/Iss/Pg. 22/11(1393-1398)). Furthermore, levels
of production
are affected by the ratio of antibody heavy and light chains and various steps
in the process of
protein assembly and secretion (Jiang et al. 2006, Biotechnology Progress, Jan-
Feb 2006, vol. 22,
no. 1, p. 313-8).
70
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
B 6. Immunogenicity
Administration of a therapeutic mAb may results in certain incidence of an
immune
response (i.e., the formation of endogenous antibodies directed against the
therapeutic mAb).
Potential elements that might induce immunogenicity should be analyzed during
selection of the
parental monoclonal antibodies, and steps to reduce such risk can be taken to
optimize the
parental monoclonal antibodies prior to DVD-Ig construction. Mouse-derived
antibodies have
been found to be highly immunogenic in patients. The generation of chimeric
antibodies
comprised of mouse variable and human constant regions presents a logical next
step to reduce
the immunogenicity of therapeutic antibodies (Morrison and Schlom, 1990).
Alternatively,
immunogenicity can be reduced by transferring murine CDR sequences into a
human antibody
framework (reshaping/CDR grafting/humanization), as described for a
therapeutic antibody by
Riechmann et al., 1988. Another method is referred to as "resurfacing" or
"veneering", starting
with the rodent variable light and heavy domains, only surface-accessible
framework amino acids
are altered to human ones, while the CDR and buried amino acids remain from
the parental
rodent antibody (Roguska et al., 1996). In another type of humanization,
instead of grafting the
entire CDRs, one technique grafts only the "specificity-determining regions"
(SDRs), defined as
the subset of CDR residues that are involved in binding of the antibody to its
target (Kashmiri et
al., 2005). This necessitates identification of the SDRs either through
analysis of available three-
dimensional structures of antibody-target complexes or mutational analysis of
the antibody CDR
residues to determine which interact with the target. Alternatively, fully
human antibodies may
have reduced immunogenicity compared to murine, chimeric or humanized
antibodies.
Another approach to reduce the immunogenicity of therapeutic antibodies is the
elimination of certain specific sequences that are predicted to be
immunogenic. In one approach,
after a first generation biologic has been tested in humans and found to be
unacceptably
immunogenic, the B-cell epitopes can be mapped and then altered to avoid
immune detection.
Another approach uses methods to predict and remove potential T-cell epitopes.
Computational
methods have been developed to scan and to identify the peptide sequences of
biologic
therapeutics with the potential to bind to MHC proteins (Desmet et al., 2005).
Alternatively a
human dendritic cell-based method can be used to identify CD4+ T-cell epitopes
in potential
protein allergens (Stickler et al., 2005; S.L. Morrison and J. Schlom,
Important Adv. Oncol.
(1990), pp. 3-18; Riechmann, L., Clark, M., Waldmann, H. and Winter, G.
"Reshaping human
antibodies for therapy." Nature (1988) 332: 323-327; Roguska-M-A, Pedersen-J-
T, Henry-A-H,
Searle-S-M, Roja-C-M, Avery-B, Hoffee-M, Cook-S, Lambert-J-M, Blattler-W-A,
Rees-A-R,
Guild-B-C. A comparison of two murine mAbs humanized by CDR-grafting and
variable domain
71
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
resurfacing.Protein engineering, {Protein-Eng}, 1996, vol. 9, p. 895-904;
Kashmiri-Syed-V-S,
De-Pascalis-Roberto, Gonzales-Noreen-R, Schlom-Jeffrey. SDR grafting--a new
approach to
antibody humanization. Methods (San Diego Calif.), {Methods}, May 2005, vol.
36, no. 1, p. 25-
34; Desmet-Johan, Meersseman-Geert, Boutonnet-Nathalie, Pletinckx-Jurgen, De-
Clercq-
Krista, Debulpaep-Maja, Braeckman-Tessa, Lasters-Ignace. Anchor profiles of
HLA-specific
peptides: analysis by a novel affinity scoring method and experimental
validation. Proteins, 2005,
vol. 58, p. 53-69; Stickler-M-M, Estell-D-A, Harding-F-A. CD4+ T-cell epitope
determination
using unexposed human donor peripheral blood mononuclear cells. Journal of
immunotherapy
2000, vol. 23, p. 654-60.)
B 7. In vivo efficacy
To generate a DVD-Ig molecule with desired in vivo efficacy, it is important
to generate
and select mAbs with similarly desired in vivo efficacy when given in
combination. However, in
some instances the DVD-Ig may exhibit in vivo efficacy that cannot be achieved
with the
combination of two separate mAbs. For instance, a DVD-Ig may bring two targets
in close
proximity leading to an activity that cannot be achieved with the combination
of two separate
mAbs. Additional desirable biological functions are described herein in
section B 3. Parent
antibodies with characteristics desirable in the DVD-Ig molecule may be
selected based on
factors such as pharmacokinetic t 1/2; tissue distribution; soluble versus
cell surface targets; and
target concentration- soluble/density ¨surface.
B 8. In vivo tissue distribution
To generate a DVD-Ig molecule with desired in vivo tissue distribution, in an
embodiment parent mAbs with similar desired in vivo tissue distribution
profile must be selected.
Alternatively, based on the mechanism of the dual-specific targeting strategy,
it may at other
times not be required to select parent mAbs with the similarly desired in vivo
tissue distribution
when given in combination. For instance, in the case of a DVD-Ig in which one
binding
component targets the DVD-Ig to a specific site thereby bringing the second
binding component
to the same target site. For example, one binding specificity of a DVD-Ig
could target pancreas
(islet cells) and the other specificity could bring GLP I to the pancreas to
induce insulin.
B 9. Isotype
To generate a DVD-Ig molecule with desired properties including, but not
limited to,
Isotype, Effector functions and the circulating half-life, in an embodiment
parent mAbs with
appropriate Fc-effector functions depending on the therapeutic utility and the
desired therapeutic
72
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
end-point are selected. There are five main heavy-chain classes or isotypes
some of which have
several sub-types and these determine the effector functions of an antibody
molecule. These
effector functions reside in the hinge region, CH2 and CH3 domains of the
antibody molecule.
However, residues in other parts of an antibody molecule may have effects on
effector functions
as well. The hinge region Fc-effector functions include: (i) antibody-
dependent cellular
cytotoxicity, (ii) complement (Cl q) binding, activation and complement-
dependent cytotoxicity
(CDC), (iii) phagocytosis/clearance of antigen-antibody complexes, and (iv)
cytokine release in
some instances. These Fc-effector functions of an antibody molecule are
mediated through the
interaction of the Fe-region with a set of class-specific cell surface
receptors. Antibodies of the
IgG1 isotype are most active while IgG2 and IgG4 having minimal or no effector
functions. The
effector functions of the IgG antibodies are mediated through interactions
with three structurally
homologous cellular Fe receptor types (and sub-types) (FcgR1, FcgRII and
FcgRIII). These
effector functions of an IgG I can be eliminated by mutating specific amino
acid residues in the
lower hinge region (e.g.,L234A, L235A) that are required for FcgR and Clq
binding. Amino acid
residues in the Fe region, in particular the CH2-CH3 domains, also determine
the circulating half-
life of the antibody molecule. This Fe function is mediated through the
binding of the Fe-region
to the neonatal Fe receptor (FcRn) which is responsible for recycling of
antibody molecules from
the acidic lysosomes back to the general circulation.
Whether a mAb should have an active or an inactive isotype will depend on the
desired
therapeutic end-point for an antibody. Some examples of usage of isotypes and
desired
therapeutic outcome are listed below:
a) If the desired end-point is functional neutralization of a soluble cytokine
then an inactive
isotype may be used;
b) If the desired out-come is clearance of a pathological protein an active
isotype may be
used;
c) If the desired out-come is clearance of protein aggregates an active
isotype may be used;
d) If the desired outcome is to antagonize a surface receptor an inactive
isotype is used
(Tysabri, IgG4; OKT3, mutated IgG1);
e) If the desired outcome is to eliminate target cells an active isotype is
used (Herceptin,
IgG I (and with enhanced effector functions); and
73
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
f) If the desired outcome is to clear proteins from circulation without
entering the CNS an
IgM isotype may be used (e.g.,clearing circulating Ab peptide species).
The Fc effector functions of a parental mAb can be determined by various in
vitro methods well
known in the art.
As discussed, the selection of isotype, and thereby the effector functions
will depend
upon the desired therapeutic end-point. In cases where simple neutralization
of a circulating
target is desired, for example blocking receptor-ligand interactions, the
effector functions may
not be required. In such instances isotypes or mutations in the Fc-region of
an antibody that
eliminate effector functions are desirable. In other instances where
elimination of target cells is
the therapeutic end-point, for example elimination of tumor cells, isotypes or
mutations or de-
fucosylation in the Fc-region that enhance effector functions are desirable
(Presta GL, Adv. Drug
Delivery Rev. 58:640-656, 2006; Satoh M., Iida S., Shitara K. Expert Opinion
Biol. Ther. 6:1161-
1173, 2006). Similarly, depending up on the therapeutic utility, the
circulating half-life of an
antibody molecule can be reduced/prolonged by modulating antibody-FcRn
interactions by
introducing specific mutations in the Fc region (Dall'Acqua WF, Kiener PA, Wu
H. J. Biol.
Chem. 281:23514-23524, 2006; Petkova SB., Akilesh S., Sproule TJ. et al.
Internat. Immunol.
18:1759-1769, 2006; Vaccaro C., Bawdon R., Wanjie S et al. PNAS 103:18709-
18714, 2007).
The published information on the various residues that influence the different
effector
functions of a normal therapeutic mAb may need to be confirmed for DVD-Ig. It
may be possible
that in a DVD-Ig format additional (different) Fe-region residues, other than
those identified for
the modulation of monoclonal antibody effector functions, may be important.
Overall, the decision as to which Fc-effector functions (isotype) will be
critical in the
final DVD-Ig format will depend up on the disease indication, therapeutic
target, desired
therapeutic end-point and safety considerations. Listed below are exemplary
appropriate heavy
chain and light chain constant regions including, but not limited to:
o IgG1 ¨ allotype: Glmz
o IgG1 mutant ¨ A234, A235
o IgG2 ¨ allotype: G2m(n-)
o Kappa ¨ Km3
o Lambda
74
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Fc Receptor and Clq Studies: The possibility of unwanted antibody-dependent
cell-
mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) by
antibody
complexing to any overexpressed target on cell membranes can be abrogated by
the (for example,
L234A, L23 5A) hinge-region mutations. These substituted amino acids, present
in the IgG1 hinge
region of mAb, are expected to result in diminished binding of mAb to human Fc
receptors (but
not FcRn), as FcgR binding is thought to occur within overlapping sites on the
IgG1 hinge region.
This feature of mAb may lead to an improved safety profile over antibodies
containing a wild-
type IgG. Binding of mAb to human Fc receptors can be determined by flow
cytometry
experiments using cell lines (e.g.,THP-1, K562) and an engineered CHO cell
line that expresses
FcgRIlb (or other FcgRs). Compared to IgG1 control monoclonal antibodies, mAb
show reduced
binding to FcgRI and FcgRlIa whereas binding to FcgRilb is unaffected. The
binding and
activation of Clq by antigen/IgG immune complexes triggers the classical
complement cascade
with consequent inflammatory and/or immunoregulatory responses. The Clq
binding site on IgGs
has been localized to residues within the IgG hinge region. Clq binding to
increasing
concentrations of mAb was assessed by Clq ELISA. The results demonstrate that
mAb is unable
to bind to Clq, as expected when compared to the binding of a wildtype control
IgG 1. Overall,
the L234A, L235A hinge region mutation abolishes binding of mAb to FcgRI,
FcgRlia and Clq
but does not impact the interaction of mAb with FcgRIIb. This data suggests
that in vivo, mAb
with mutant Fc will interact normally with the inhibitory FcgRlIb but will
likely fail to interact
with the activating FcgRI and FcgRlIa receptors or Clq.
Human FcRn binding: The neonatal receptor (FcRn) is responsible for transport
of IgG
across the placenta and to control the catabolic half-life of the IgG
molecules. It might be
desirable to increase the terminal half-life of an antibody to improve
efficacy, to reduce the dose
or frequency of administration, or to improve localization to the target.
Alternatively, it might be
advantageous to do the converse that is, to decrease the terminal half-life of
an antibody to reduce
whole body exposure or to improve the target-to-non-target binding ratios.
Tailoring the
interaction between IgG and its salvage receptor, FcRn, offers a way to
increase or decrease the
terminal half-life of IgG. Proteins in the circulation, including IgG, are
taken up in the fluid phase
through micropinocytosis by certain cells, such as those of the vascular
endothelia. IgG can bind
FeRn in endosomes under slightly acidic conditions (pH 6.0-6.5) and can
recycle to the cell
surface, where it is released under almost neutral conditions (pH 7.0-7.4).
Mapping of the Fe-
region-binding site on FcRn80, 16, 17 showed that two histidine residues that
are conserved
across species, His310 and His435, are responsible for the pH dependence of
this interaction.
Using phage-display technology, a mouse Fc-region mutation that increases
binding to FcRn and
extends the half-life of mouse IgG was identified (see Victor, G. et al.;
Nature Biotechnology
75
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
(1997), 15(7), 637-640). Fe-region mutations that increase the binding
affinity of human IgG for
FeRn at pH 6.0, but not at pH 7.4, have also been identified (see Dall'Acqua
William F, et al.,
Journal of Immunology (2002), 169(9), 5171-80). Moreover, in one case, a
similar pH-dependent
increase in binding (up to 27-fold) was also observed for rhesus FeRn, and
this resulted in a
twofold increase in serum half-life in rhesus monkeys compared with the parent
IgG (see Hinton,
Paul R. et al., Journal of Biological Chemistry (2004), 279(8), 6213-6216).
These findings
indicate that it is feasible to extend the plasma half-life of antibody
therapeutics by tailoring the
interaction of the Fe region with FcRn. Conversely, Fc-region mutations that
attenuate interaction
with FeRn can reduce antibody half-life.
B.10 Pharmacokinetics (PK)
To generate a DVD-Ig molecule with desired pharmacokinetic profile, in an
embodiment
parent mAbs with the similarly desired pharmacokinetic profile are selected.
One consideration is
that immunogenic response to monoclonal antibodies (i.e., HAHA, human anti-
human antibody
response; HACA, human anti-chimeric antibody response) further complicates the
pharmacokinetics of these therapeutic agents. In an embodiment, monoclonal
antibodies with
minimal or no immunogenicity are used for constructing DVD-Ig molecules such
that the
resulting DVD-Igs will also have minimal or no immunogenicity. Some of the
factors that
determine the PK of a mAb include, but are not limited to, Intrinsic
properties of the mAb (VH
amino acid sequence); immunogenicity; FeRn binding and Fe functions.
The PK profile of selected parental monoclonal antibodies can be easily
determined in
rodents as the PK profile in rodents correlates well with (or closely
predicts) the PK profile of
monoclonal antibodies in cynomolgus monkey and humans. The PK profile is
determined as
described in Example section 1.2.2.3.A.
After the parental monoclonal antibodies with desired PK characteristics (and
other
desired functional properties as discussed herein) are selected, the DVD-Ig is
constructed. As the
DVD-Ig molecules contain two antigen-binding domains from two parental
monoclonal
antibodies, the PK properties of the DVD-Ig are assessed as well. Therefore,
while determining
the PK properties of the DVD-Ig, PK assays may be employed that determine the
PK profile
based on functionality of both antigen-binding domains derived from the 2
parent monoclonal
antibodies. The PK profile of a DVD-Ig can be determined as described in
Example 1.2.2.3.A.
Additional factors that may impact the PK profile of DVD-Ig include the
antigen-binding domain
(CDR) orientation; Linker size; and Fe / FeRn interactions. PK characteristics
of parent
76
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
antibodies can be evaluated by assessing the following parameters: absorption,
distribution,
metabolism and excretion.
Absorption: To date, administration of therapeutic monoclonal antibodies is
via
parenteral routes (e.g., intravenous [IV], subcutaneous [SC], or intramuscular
[IM]). Absorption
of a mAb into the systemic circulation following either SC or IM
administration from the
interstitial space is primarily through the lymphatic pathway. Saturable,
presystemic, proteolytic
degradation may result in variable absolute bioavailability following
extravascular
administration. Usually, increases in absolute bioavailability with increasing
doses of monoclonal
antibodies may be observed due to saturated proteolytic capacity at higher
doses. The absorption
process for a mAb is usually quite slow as the lymph fluid drains slowly into
the vascular system,
and the duration of absorption may occur over hours to several days. The
absolute bioavailability
of monoclonal antibodies following SC administration generally ranges from 50%
to 100%. In
the case of a transport-mediating structure at the blood-brain barrier
targeted by the DVD-Ig
construct, circulation times in plasma may be reduced due to enhanced trans-
cellular transport at
the blood brain barrier (BBB) into the CNS compartment, where the DVD-Ig is
liberated to
enable interaction via its second antigen recognition site.
Distribution: Following IV administration, monoclonal antibodies usually
follow a
biphasic serum (or plasma) concentration-time profile, beginning with a rapid
distribution phase,
followed by a slow elimination phase. In general, a biexponential
pharmacokinetic model best
describes this kind of pharmacokinetic profile. The volume of distribution in
the central
compartment (Vc) for a mAb is usually equal to or slightly larger than the
plasma volume (2-3
liters). A distinct biphasic pattern in serum (plasma) concentration versus
time profile may not be
apparent with other parenteral routes of administration, such as IM or SC,
because the
distribution phase of the serum (plasma) concentration-time curve is masked by
the long
absorption portion. Many factors, including physicochemical properties, site-
specific and target-
oriented receptor mediated uptake, binding capacity of tissue, and mAb dose
can influence
biodistribution of a mAb. Some of these factors can contribute to nonlinearity
in biodistribution
for a mAb.
Metabolism and Excretion: Due to the molecular size, intact monoclonal
antibodies are
not excreted into the urine via kidney. They are primarily inactivated by
metabolism (e.g.,
catabolism). For IgG-based therapeutic monoclonal antibodies, half-lives
typically ranges from
hours or 1-2 days to over 20 days. The elimination of a mAb can be affected by
many factors,
including, but not limited to, affinity for the FeRn receptor, immunogenicity
of the mAb, the
77
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
degree of glycosylation of the mAb, the susceptibility for the mAb to
proteolysis, and receptor-
mediated elimination.
B.1.1 Tissue cross-reactivity pattern on human and tox species
Identical staining pattern suggests that potential human toxicity can be
evaluated in tox
species. Tox species are those animal in which unrelated toxicity is studied.
The individual antibodies are selected to meet two criteria. (1) Tissue
staining
appropriate for the known expression of the antibody target. (2) Similar
staining pattern between
human and tox species tissues from the same organ.
Criterion 1: Immunizations and/or antibody selections typically employ
recombinant or
synthesized antigens (proteins, carbohydrates or other molecules). Binding to
the natural
counterpart and counterscreen against unrelated antigens are often part of the
screening funnel for
therapeutic antibodies. However, screening against a multitude of antigens is
often unpractical.
Therefore tissue cross-reactivity studies with human tissues from all major
organs serve to rule
out unwanted binding of the antibody to any unrelated antigens.
Criterion 2: Comparative tissue cross reactivity studies with human and tox
species
tissues (cynomolgus monkey, dog, possibly rodents and others, the same 36 or
37 tissues are
being tested as in the human study) help to validate the selection of a tox
species. In the typical
tissue cross-reactivity studies on frozen tissues sections therapeutic
antibodies may demonstrate
the expected binding to the known antigen and/or to a lesser degree binding to
tissues based
either on low level interactions (unspecific binding, low level binding to
similar antigens, low
level charge based interactions, etc.). In any case the most relevant
toxicology animal species is
the one with the highest degree of coincidence of binding to human and animal
tissue.
Tissue cross reactivity studies follow the appropriate regulatory guidelines
including EC
CPMP Guideline III/5271/94 "Production and quality control of mAbs" and the
1997 US
FDA/CBER "Points to Consider in the Manufacture and Testing of Monoclonal
Antibody
Products for Human Use". Cryosections (5 i.im) of human tissues obtained at
autopsy or biopsy
were fixed and dried on object glass. The peroxidase staining of tissue
sections was performed,
using the avidin-biotin system. FDA's Guidance "Points to Consider in the
Manufacture and
Testing of Monoclonal Antibody Products for Human Use". Relevant references
include Clarke J
2004, Boon L. 2002a, Boon L 2002b, Ryan A 1999.
78
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Tissue cross reactivity studies are often done in two stages, with the first
stage including
cryosections of 32 tissues (typically: Adrenal Gland, Gastrointestinal Tract,
Prostate, Bladder,
Heart, Skeletal Muscle, Blood Cells, Kidney, Skin, Bone Marrow, Liver, Spinal
Cord, Breast,
Lung, Spleen, Cerebellum, Lymph Node, Testes, Cerebral Cortex, Ovary, Thymus,
Colon,
Pancreas, Thyroid, Endothelium, Parathyroid, Ureter, Eye, Pituitary, Uterus,
Fallopian Tube and
Placenta) from one human donor. In the second phase a full cross reactivity
study is performed
with up to 38 tissues (including adrenal, blood, blood vessel, bone marrow,
cerebellum,
cerebrum, cervix, esophagus, eye, heart, kidney, large intestine, liver, lung,
lymph node, breast
mammary gland, ovary, oviduct, pancreas, parathyroid, peripheral nerve,
pituitary, placenta,
prostate, salivary gland, skin, small intestine, spinal cord, spleen, stomach,
striated muscle, testis,
thymus, thyroid, tonsil, ureter, urinary bladder, and uterus) from 3 unrelated
adults. Studies are
done typically at minimally two dose levels.
The therapeutic antibody (i.e., test article) and isotype matched control
antibody may be
biotinylated for avidin-biotin complex (ABC) detection; other detection
methods may include
tertiary antibody detection for a FITC (or otherwise) labeled test article, or
precomplexing with a
labeled anti-human IgG for an unlabeled test article.
Briefly, cryosections (about 5 um) of human tissues obtained at autopsy or
biopsy are
fixed and dried on object glass. The peroxidase staining of tissue sections is
performed, using the
avidin-biotin system. First (in case of a precomplexing detection system), the
test article is
incubated with the secondary biotinylated anti-human IgG and developed into
immune complex.
The immune complex at the final concentrations of 2 and 10 ug/mL of test
article is added onto
tissue sections on object glass and then the tissue sections were reacted for
30 minutes with a
avidin-biotin-peroxidase kit. Subsequently, DAB (3,3'-diaminobenzidine), a
substrate for the
peroxidase reaction, was applied for 4 minutes for tissue staining. Antigen-
Sepharose beads are
used as positive control tissue sections.
Any specific staining is judged to be either an expected (e.g.,consistent with
antigen
expression) or unexpected reactivity based upon known expression of the target
antigen in
question. Any staining judged specific is scored for intensity and frequency.
Antigen or serum
competion or blocking studies can assist further in determining whether
observed staining is
specific or nonspecific.
If two selected antibodies are found to meet the selction criteria ¨
appropriate tissue
staining, matching staining between human and toxicology animal specific
tissue ¨ they can be
selected for DVD-Ig generation.
79
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
The tissue cross reactivity study has to be repeated with the final DVD-Ig
construct, but
while these studies follow the same protocol as outline herein, they are more
complex to evaluate
because any binding can come from any of the two parent antibodies, and any
unexplained
binding needs to be confirmed with complex antigen competition studies.
It is readily apparent that the complex undertaking of tissue crossreactivity
studies with a
multispecific molecule like a DVD-Ig is greatly simplified if the two parental
antibodies are
selected for (1) lack of unexpected tissue cross reactivity findings and (2)
for appropriate
similarity of tissue cross reactivity findings between the corresponding human
and toxicology
animal species tissues.
B.12 Specificity and selectivity
To generate a DVD-Ig molecule with desired specificity and selectivity, one
needs to
generate and select parent mAbs with the similarly desired specificity and
selectivity profile.
Binding studies for specificity and selectivity with a DVD-Ig can be complex
due to the
four or more binding sites, two each for each antigen. Briefly, binding
studies using ELISA,
BIAcore. KinExA or other interaction studies with a DVD-Ig need to monitor the
binding of one,
two or more antigens to the DVD-Ig molecule. While BIAcore technology can
resolve the
sequential, independent binding of multiple antigens, more traditional methods
including ELISA
or more modern techniques like KinExA cannot. Therefore careful
characterization of each
parent antibody is critical. After each individual antibody has been
characterized for specificity,
confirmation of specificity retention of the individual binding sites in the
DVD-Ig molecule is
greatly simplified.
It is readily apparent that the complex undertaking of determining the
specificity of a
DVD-Ig is greatly simplified if the two parental antibodies are selected for
specificity prior to
being combined into a DVD-Ig.
Antigen¨antibody interaction studies can take many forms, including many
classical
protein protein interaction studies, including ELISA (Enzyme linked
immunosorbent assay),
Mass spectrometry, chemical cross linking, SEC with light scattering,
equilibrium dialysis, gel
permeation, ultrafiltration, gel chromatography, large-zone analytical SEC,
micropreparative
ultracentrigugation (sedimentation equilibrium), spectroscopic methods,
titration
microcalorimetry, sedimentation equilibrium (in analytical ultracentrifuge),
sedimentation
velocity (in analytical centrifuge), surface plasmon resonance (including
BIAcore). Relevant
references include "Current Protocols in Protein Science", John E. Coligan,
Ben M. Dunn, David
80
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
W. Speicher, Paul T, Wingfield (eds.) Volume 3, chapters 19 and 20, published
by John Wiley &
Sons Inc., and references included therein and "Current Protocols in
Immunology", John E.
Coligan, Barbara E. Bierer, David H. Margulies, Ethan M. Shevach, Warren
Strober (eds.)
published by John Wiley & Sons Inc and relevant references included therein.
Cytokine Release in Whole Blood: The interaction of mAb with human blood cells
can
be investigated by a cytokine release assay (Wing, M. G. Therapeutic
Immunology (1995), 2(4),
183-190; "Current Protocols in Pharmacology", S.J. Enna, Michael Williams,
John W. Ferkany,
Terry Kenakin, Paul Moser, (eds.) published by John Wiley & Sons Inc;
Madhusudan, S. Clinical
Cancer Research (2004), 10(19), 6528-6534; Cox, J. Methods (2006), 38(4), 274-
282; Choi, I.
European Journal of Immunology (2001), 31(1), 94-106). Briefly, various
concentrations of mAb
are incubated with human whole blood for 24 hours. The concentration tested
should cover a
wide range including final concentrations mimicking typical blood levels in
patients (including
but not limited to 100 ng/ml ¨ 100 g/m1). Following the incubation,
supernatants and cell lysates
were analyzed for the presence of IL-1Ra, TNF-a, IL-lb, IL-6 and IL-8.
Cytokine concentration
profiles generated for mAb were compared to profiles produced by a negative
human IgG control
and a positive LPS or PHA control. The cytokine profile displayed by mAb from
both cell
supernatants and cell lysates was comparable to control human IgG. In an
embodiment, the
monoclonal antibody does not interact with human blood cells to spontaneously
release
inflammatory cytokines.
Cytokine release studies for a DVD-Ig are complex due to the four or more
binding sites,
two each for each antigen. Briefly, cytokine release studies as described
herein measure the effect
of the whole DVD-Ig molecule on whole blood or other cell systems, but can
resolve which
portion of the molecule causes cytokine release. Once cytokine release has
been detected, the
purity of the DVD-Ig preparation has to be ascertained, because some co-
purifying cellular
components can cause cytokine release on their own. If purity is not the
issue, fragmentation of
DVD-Ig (including but not limited to removal of Fc portion, separation of
binding sites etc.),
binding site mutagenesis or other methods may need to be employed to
deconvolute any
observations. It is readily apparent that this complex undertaking is greatly
simplified if the two
parental antibodies are selected for lack of cytokine release prior to being
combined into a DVD-
Ig.
11.13 Cross reactivity to other species for toxicological studies
In an embodiment, the individual antibodies selected with sufficient cross-
reactivity to
appropriate tox species, for example, cynomolgus monkey. Parental antibodies
need to bind to
81
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
orthologous species target (i.e. cynomolgus monkey) and elicit appropriate
response (modulation,
neutralization, activation). In an embodiment, the cross-reactivity
(affinity/potency) to
orthologous species target should be within 10-fold of the human target. In
practice, the parental
antibodies are evaluated for multiple species, including mouse, rat, dog,
monkey (and other non-
human primates), as well as disease model species (i.e. sheep for asthma
model). The acceptable
cross-reactivity to tox species from the perantal monoclonal antibodies allows
future toxicology
studies of DVD-Ig-Ig in the same species. For that reason, the two parental
monoclonal
antibodies should have acceptable cross-reactivity for a common tox species
therefore allowing
toxicology studies of DVD-Ig in the same species.
Parent mAbs may be selected from various mAbs capable of binding specific
targets and
well known in the art. These include, but are not limited to anti-INF antibody
(US Patent No.
6,258,562), anti-IL-12 and/or anti-IL-12p40 antibody (US Patent No.
6,914,128); anti-IL-18
antibody (US 2005/0147610 Al), anti-CS, anti-CBL, anti-CD147, anti-gp120, anti-
VLA-4, anti-
CD1 I a, anti-CD18, anti-VEGF, anti-CD4OL, anti CD-40 (e.g., see W02007124299)
anti-Id, anti-
ICAM-1, anti-CXCL13, anti-CD2, anti-EGFR, anti-TGF-beta 2, anti-HGF, anti-
cMet, anti DLL-
4, anti-NPR1, anti-PLGF, anti-ErbB3, anti-E-selectin, anti-Fact VII, anti-
Her2/neu, anti-F gp,
anti-CD11/18, anti-CD14, anti-ICAM-3, anti-RON, anti CD-19, anti-CD80 (e.g.,
see
W02003039486, anti-CD4, anti-CD3, anti-CD23, anti-beta2-integrin, anti-
alpha4beta7, anti-
CD52, anti-HLA DR, anti-CD22 (e.g., see US Patent NO: 5,789,554), anti-CD20,
anti-MIF, anti-
CD64 (FcR), anti-TCR alpha beta, anti-CD2, anti-Hep B, anti-CA 125, anti-
EpCAM, anti-gp120,
anti-CMV, anti-gpIIbIIIa, anti-IgE, anti-CD25, anti-CD33, anti-HLA, anti-
IGF1,2, anti IGFR,
anti-VNRintegrin, anti-IL-Ialpha, anti-IL-lbeta, anti-IL-1 receptor, anti-1L-2
receptor, anti-IL-4,
anti-IL-4 receptor, anti-1L5, anti-IL-5 receptor, anti-IL-6, anti- IL-6R,
RANKL, NGF, DKK,
alphaVbeta3, IL-17A, anti-IL-8, anti-IL-9, anti-IL-13, anti-IL-13 receptor,
anti-IL-17, and anti-IL-
23; IL-23p19; (see Presta LG. 2005 Selection, design, and engineering of
therapeutic antibodies J
Allergy Clin Immunol. 116:731-6 and
http://www.path.cam.ac.uk/mrc7/humanisation/antibodies.html).
Parent mAbs may also be selected from various therapeutic antibodies approved
for use,
in clinical trials, or in development for clinical use. Such therapeutic
antibodies include, but are
not limited to, rituximab (Rituxan , IDEC/Genentech/Roche) (see for example U.
S. Pat. No.
5,736,137), a chimeric anti-CD20 antibody approved to treat Non-Hodgkin's
lymphoma;
HuMax-CD20, an anti-CD20 currently being developed by Genmab, an anti-CD20
antibody
described in U.S. Pat. No. 5, 500,362, AME-133 (Applied Molecular Evolution),
hA20
(Immunomedics, Inc.), HumaLYM (Intracel), and PR070769 (PCT/US2003/040426,
entitled
82
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
"Immunoglobulin Variants and Uses Thereof'), trastuzumab (Herceptin ,
Genentech) (see for
example U.S. Pat. No. 5,677,171), a humanized anti- Her2/neu antibody approved
to treat breast
cancer; pertuzumab (rhuMab-2C4, Omnitarge), currently being developed by
Genentech; an
anti-Her2 antibody described in U.S. Pat. No. 4,753,894; cetuximab (Erbitux ,
Imclone) (U.S.
Pat. No. 4,943,533; PCT WO 96/40210), a chimeric anti-EGFR antibody in
clinical trials for a
variety of cancers; ABX-EGF (U.S. Pat. No. 6,235,883), currently being
developed by Abgenix-
Immunex-Amgen; HuMax- EGFr (U.S. Ser. No. 10/172,317), currently being
developed by
Genmab; 425, EMD55900, EMD62000, and EMD72000 (Merck KGaA) (U.S. Pat. No.
5,558,864; Murthy etal. 1987, Arch Biochem Biophys. 252(2):549-60; Rodeck et
al., 1987, J
Cell Biochem. 35(4):315-20; Kettleborough et al., 1991, Protein Eng. 4(7):773-
83); ICR62
(Institute of Cancer Research) (PCT WO 95/20045; Modjtahedi et al., 1993, J.
Cell Biophys.
1993, 22(1-3):129-46; Modjtahedi etal., 1993, Br J Cancer. 1993, 67(2):247-53;
Modjtahedi et
al, 1996, Br J Cancer, 73(2):228-35; Modjtahedi et al, 2003, Int J Cancer,
105(2):273-80);
TheraCIM hR3 (YM Biosciences, Canada and Centro de Immunologia Molecular, Cuba
(U.S.
Pat. No. 5,891,996; U.S. Pat. No. 6,506, 883; Mateo et al, 1997,
Immunotechnology, 3(1):71-
81); mAb-806 (Ludwig Institue for Cancer Research, Memorial Sloan-Kettering)
(Jungbluth et
al. 2003, Proc Natl Acad Sci USA. 100(2):639-44); KSB-102 (KS Biomedix); MR1-1
(WAX,
National Cancer Institute) (PCT WO 0162931A2); and SC100 (Scancell) (PCT WO
01/88138);
alemtuzumab (Campath , Millenium), a humanized mAb currently approved for
treatment of B-
cell chronic lymphocytic leukemia; muromonab-CD3 (Orthoclone OKT30), an anti-
CD3
antibody developed by Ortho Biotech/Johnson & Johnson, ibritumomab tiuxetan
(Zevaline), an
anti-CD20 antibody developed by IDEC/Schering AG, gemtuzumab ozogamicin
(Mylotarge),
an anti-CD33 (p67 protein) antibody developed by Celltech/Wyeth, alefacept
(Amevive0), an
anti-LFA-3 Fc fusion developed by Biogen), abciximab (ReoProe), developed by
Centocor/Lilly, basiliximab (Simulecte), developed by Novartis, palivizumab
(Synagise),
developed by Medimmune, infliximab (Remicade0), an anti-TNFalpha antibody
developed by
Centocor, adalimumab (Humira ), an anti-TNFalpha antibody developed by Abbott,
Humicade , an anti-TNFalpha antibody developed by Celltech, golimumab (CNTO-
148), a
fully human TNF antibody developed by Centocor, etanercept (Enbrel ), an p75
TNF receptor
Fc fusion developed by Immunex/Amgen, lenercept, an p55TNF receptor Fc fusion
previously
developed by Roche, ABX-CBL, an anti-CD147 antibody being developed by
Abgenix, ABX-
IL8, an anti-1L8 antibody being developed by Abgenix, ABX-MA1, an anti-MUC18
antibody
being developed by Abgenix, Pemtumomab (R1549, 90Y-muHMFG1), an anti-MUC I in
development by Antisoma, Therex (R1550), an anti-MUC1 antibody being developed
by
Antisoma, AngioMab (AS1405), being developed by Antisoma, HuBC-1, being
developed by
Antisoma, Thioplatin (AS1407) being developed by Antisoma, Antegren
(natalizumab), an
83
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
anti-alpha-4-beta-1 (VLA-4) and alpha-4-beta-7 antibody being developed by
Biogen, VLA-1
mAb, an anti-VLA-1 integrin antibody being developed by Biogen, LTBR mAb, an
anti-
lymphotoxin beta receptor (LTBR) antibody being developed by Biogen, CAT-152,
an anti-
TGF-I32 antibody being developed by Cambridge Antibody Technology, ABT 874
(J695), an
anti- IL-12 p40 antibody being developed by Abbott, CAT-192, an anti-TGFf31
antibody being
developed by Cambridge Antibody Technology and Genzyme, CAT-213, an anti-
Eotaxinl
antibody being developed by Cambridge Antibody Technology, LymphoStat-B8 an
anti-Blys
antibody being developed by Cambridge Antibody Technology and Human Genome
Sciences
Inc., TRAIL-R1mAb, an anti-TRAIL-R1 antibody being developed by Cambridge
Antibody
Technology and Human Genome Sciences, Inc. , Avastin bevacizumab, rhuMAb-
VEGF), an
anti-VEGF antibody being developed by Genentech, an anti-HER receptor family
antibody
being developed by Genentech, Anti-Tissue Factor (ATF), an anti-Tissue Factor
antibody being
developed by Genentech, Xolair (Omalizumab), an anti-IgE antibody being
developed by
Genentech, Raptiva (Efalizumab), an anti- CD1 1 a antibody being developed by
Genentech and
Xoma, MLN-02 Antibody (formerly LDP-02), being developed by Genentech and
Millenium
Pharmaceuticals, HuMax CD4, an anti-CD4 antibody being developed by Genmab,
HuMax-
IL15, an anti-1L15 antibody being developed by Genmab and Amgen, HuMax-Inflam,
being
developed by Genmab and Medarex, HuMax-Cancer, an anti-Heparanase I antibody
being
developed by Genmab and Medarex and Oxford GcoSciences, HuMax-Lymphoma, being
developed by Genmab and Amgen, HuMax-TAC, being developed by Genmab, IDEC-131,
and
anti-CD4OL antibody being developed by IDEC Pharmaceuticals, IDEC-151
(Clenoliximab), an
anti- CD4 antibody being developed by IDEC Pharmaceuticals, IDEC-114, an anti-
CD80
antibody being developed by IDEC Pharmaceuticals, IDEC-152, an anti- CD23
being developed
by IDEC Pharmaceuticals, anti-macrophage migration factor (MIF) antibodies
being developed
by IDEC Pharmaceuticals, BEC2, an anti-idiotypic antibody being developed by
Imclone, IMC-
1C11, an anti-KDR antibody being developed by Imclone, DC101, an anti-flk-1
antibody being
developed by Imclone, anti-VE cadherin antibodies being developed by Imclone,
CEA-Cide
(labetuzumab), an anti-carcinoembryonic antigen (CEA) antibody being developed
by
Immunomedics, LymphoCidee (Epratuzumab), an anti-CD22 antibody being developed
by
Immunomedics, AFP-Cide, being developed by Immunomedics, MyelomaCide, being
developed
by Immunomedics, LkoCide, being developed by Immunomedics, ProstaCide, being
developed
by Immunomedics, MDX-010, an anti-CTLA4 antibody being developed by Medarex,
MDX-
060, an anti-CD30 antibody being developed by Medarex, MDX-070 being developed
by
Medarex, MDX-018 being developed by Medarex, Osidem (IDM-1), and anti-Her2
antibody
being developed by Medarex and Immuno-Designed Molecules, HuMax -CD4, an anti-
CD4
antibody being developed by Medarex and Genmab, HuMax-IL15, an anti-IL15
antibody being
84
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
developed by Medarex and Genmab, CNTO 148, an anti-TNFa antibody being
developed by
Medarex and Centocor/J&J, CNTO 1275, an anti-cytokine antibody being developed
by
Centocor/J&J, MOR101 and MOR102, anti-intercellular adhesion molecule-1 (ICAM-
1)
(CD54) antibodies being developed by MorphoSys, MOR201, an anti-fibroblast
growth factor
receptor 3 (FGFR-3) antibody being developed by MorphoSys, Nuvion
(visilizumab), an anti-
CD3 antibody being developed by Protein Design Labs, HuZAFO, an anti-gamma
interferon
antibody being developed by Protein Design Labs, Anti-cc 5131 Integrin, being
developed by
Protein Design Labs, anti-IL-12, being developed by Protein Design Labs, ING-
1, an anti-Ep-
CAM antibody being developed by Xoma, Xolair (Omalizumab) a humanized anti-
IgE
antibody developed by Genentech and Novartis, and MLN01, an anti-Beta2
integrin antibody
being developed by Xoma. In another embodiment, the therapeutics include
KRN330 (Kirin);
huA33 antibody (A33, Ludwig Institute for Cancer Research); CNTO 95 (alpha V
integrins,
Centocor); MEDI-522 (alpha Vi33 integrin, Medimmune); volociximab (alpha V131
integrin,
Biogen/PDL); Human mAb 216 (B cell glycosolated epitope, NCI); BiTE MT103
(bispecific
CD19 x CD3, Medimmune); 4G7xH22 (Bispecific BcellxFcgammaRl, Medarex/Merck
KGa);
rM28 (Bispecific CD28 x MAPG, US Patent No. EP1444268); MDX447 (EMD 82633)
(Bispecific CD64 x EGFR, Medarex); Catumaxomab (removab) (Bispecific EpCAM x
anti-
CD3, Trion/Fres); Ertumaxomab (bispecific HER2/CD3, Fresenius Biotech);
oregovomab
(OvaRex) (CA-125, ViRexx); Rencarex (WX G250) (carbonic anhydrase IX, Wilex);
CNTO
888 (CCL2, Centocor); TRC105 (CD105 (endoglin), Tracon); BMS-663513 (CD137
agonist,
Brystol Myers Squibb); MDX-1342 (CD19, Medarex); Siplizumab (MEDI-507) (CD2,
Medimmune); Ofatumumab (Humax-CD20) (CD20, Genmab); Rituximab (Rituxan) (CD20,
Genentech); veltuzumab ( hA20) (CD20, Immunomedics); Epratuzumab (CD22,
Amgen);
lumiliximab (IDEC 152) (CD23, Biogen); muromonab-CD3 (CD3, Ortho); HuM291 (CD3
fc
receptor, PDL Biopharma); HeFi-1, CD30, NCI); MDX-060 (CD30, Medarex); MDX-
1401
(CD30, Medarex); SGN-30 (CD30, Seattle Genentics); SGN-33 (Lintuzumab) (CD33,
Seattle
Genentics); Zanolimumab (HuMax-CD4) (CD4, Genmab); HCD122 (CD40, Novartis);
SGN-40
(CD40, Seattle Genentics); Campathlh (Alemtuzumab) (CD52, Genzyme); MDX-1411
(CD70,
Medarex); hLL1 (EPB-1) (CD74.38, Immunomedics); Galiximab (IDEC-144) (CD80,
Biogen);
MT293 (TRC093/D93) (cleaved collagen, Tracon); HuLuc63 (CS1, PDL Pharma);
ipilimumab
(MDX-010) (CTLA4, Brystol Myers Squibb); Tremelimumab (Ticilimumab, CP-675,2)
(CTLA4, Pfizer); HGS-ETR1 (Mapatumumab) (DR4 TRAIL-R1 agonist, Human Genome
Science /Glaxo Smith Kline); AMG-655 (DR5, Amgen); Apomab (DRS, Genentech); CS-
1008
(DR5, Daiichi Sankyo); HGS-ETR2 (lexatumumab) (DR5 TRAIL-R2 agonist, FIGS);
Cetuximab
(Erbitux) (EGFR, Imclone); IMC-11F8, (EGFR, Imclone); Nimotuzumab (EGFR, YM
Bio);
Panitumumab (Vectabix) (EGFR, Amgen); Zalutumumab (HuMaxEGFr) (EGFR, Genmab);
85
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
CDX-110 (EGFRvIII, AVANT Immunotherapeutics); adecatumumab (MT201) (Epcam ,
Merck); edrecolomab (Panorex, 17-1A) (Epcam , Glaxo/Centocor); MORAb-003
(folate
receptor a, Morphotech); KW-2871 (ganglioside GD3, Kyowa); MORAb-009 (GP-9,
Morphotech); CDX-1307 (MDX-1307) (hCGb, Celldex); Trastuzumab (Herceptin)
(HERZ,
Celldex); Pertuzumab (rhuMAb 2C4) (HER2 (DI), Genentech); apolizumab (HLA-DR
beta
chain, PDL Pharma); AMG-479 (IGF-1R, Amgen); anti-IGF-1R R1507 (IGF1-R,
Roche); CP
751871 (IGF1-R, Pfizer); IMC-Al2 (IGF1-R, Imclone); BIIB022 (IGF-1R , Biogen);
Mik-beta-1
(IL-2Rb (CD122), Hoffman LaRoche); CNTO 328 (IL6, Centocor); Anti-KIR (1-7F9)
(Killer
cell Ig-like Receptor (KIR), Novo); Hu3S193 (Lewis (y), Wyeth, Ludwig
Institute of Cancer
Research); hCBE-11 (LTBR, Biogen); HuHMFG1 (MUC1, Antisoma/NCI); RAV12 (N-
linked
carbohydrate epitope, Raven); CAL (parathyroid hormone-related protein (PTH-
rP), University
of California); CT-011 (PDI, CureTech); MDX-1106 (ono-4538) (PD1,
Medarex/Ono); MAb
CT-011 (PD1, Curetech); IMC-3G3 (PDGFRa, Imclone); bavituximab
(phosphatidylserine,
Peregrine); huJ591 (PSMA, Cornell Research Foundation); muJ591 (PSMA, Cornell
Research
Foundation); GC1008 (TGFb (pan) inhibitor (IgG4), Genzyme); Infliximab
(Remicade) (TNFa,
Centocor); A27.15 (transferrin receptor, Salk Institute, INSERN WO
2005/111082); E2.3
(transferrin receptor, Salk Institute); Bevacizumab (Avastin) (VEGF,
Genentech); HuMV833
(VEGF, Tsukuba Research Lab-WO/2000/034337, University of Texas); IMC-18F1
(VEGFR1,
Imclone); IMC-1121 (VEGFR2, Imclone).
C. Construction of binding protein molecules
The dual variable domain immunoglobulin (DVD-Ig) molecule is designed such
that two
different light chain variable domains (VL) from the two different parent
monoclonal antibodies
are linked in tandem directly or via a short linker by recombinant DNA
techniques, followed by
the light chain constant domain. Similarly, the heavy chain comprises two
different heavy chain
variable domains (VH) linked in tandem, followed by the constant domain CH1
and Fe region
(Figure 1A).
The variable domains can be obtained using recombinant DNA techniques from a
parent
antibody generated by any one of the methods described herein. In an
embodiment, the variable
domain is a murine heavy or light chain variable domain. In another
embodiment, the variable
domain is a CDR grafted or a humanized variable heavy or light chain domain.
In an
embodiment, the variable domain is a human heavy or light chain variable
domain.
In one embodiment the first and second variable domains are linked directly to
each other
using recombinant DNA techniques. In another embodiment the variable domains
are linked via a
86
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
linker sequence. In an embodiment, two variable domains are linked. Three or
more variable
domains may also be linked directly or via a linker sequence. The variable
domains may bind the
same antigen or may bind different antigens. DVD-Ig molecules provided herein
may include one
immunoglobulin variable domain and one non- immunoglobulin variable domain
such as ligand
binding domain of a receptor, active domain of an enzyme. DVD-Ig molecules may
also comprise
2 or more non-Ig domains.
The linker sequence may be a single amino acid or a polypeptide sequence. In
an
embodiment, the linker sequences are AKTTPKLEEGEF SEAR (SEQ ID NO: 1);
AKTTPKLEEGEFSEARV (SEQ ID NO: 2); AKTTPKLGG (SEQ ID NO: 3); SAKTTPKLGG
(SEQ ID NO: 4); SAKTTP (SEQ ID NO: 5); RADAAP (SEQ ID NO: 6); RADAAPTVS (SEQ
ID NO: 7); RADAAAAGGPGS (SEQ ID NO: 8); RADAAAA(G4S)4 (SEQ ID NO: 9),
SAKTTPKLEEGEFSEARV (SEQ ID NO: 10); ADAAP (SEQ ID NO: 11); ADAAPTVSIFPP
(SEQ ID NO: 12); TVAAP (SEQ ID NO: 13); TVAAPSVFIFPP (SEQ ID NO: 14); QPKAAP
(SEQ ID NO: 15); QPKAAPSVTLFPP (SEQ ID NO: 16); AKTTPP (SEQ ID NO: 17);
AKTTPPSVTPLAP (SEQ ID NO: 18); AKTTAP (SEQ ID NO: 19); AKTTAPSVYPLAP (SEQ
ID NO: 20); ASTKGP (SEQ ID NO: 21); ASTKGPSVFPLAP (SEQ ID NO: 22),
GGGGSGGGGSGGGGS (SEQ ID NO: 23); GENKVEYAPALMALS (SEQ ID NO: 24);
GPAKELTPLKEAKVS (SEQ ID NO: 25); or GHEAAAVMQVQYPAS (SEQ ID NO: 26). The
choice of linker sequences is based on crystal structure analysis of several
Fab molecules. There
is a natural flexible linkage between the variable domain and the CH1/CL
constant domain in Fab
or antibody molecular structure. This natural linkage comprises approximately
10-12 amino acid
residues, contributed by 4-6 residues from C-terminus of V domain and 4-6
residues from the N-
terminus of CL/CH1 domain. DVD Igs provided herein were generated using N-
terminal 5-6
amino acid residues, or 11-12 amino acid residues, of CL or CHI as linker in
light chain and
heavy chain of DVD-Ig, respectively. The N-terminal residues of CL or CH1
domains,
particularly the first 5-6 amino acid residues, adopt a loop conformation
without strong secondary
structures, therefore can act as flexible linkers between the two variable
domains. The N-terminal
residues of CL or CHI domains are natural extension of the variable domains,
as they are part of
the Ig sequences, therefore minimize to a large extent any immunogenicity
potentially arising
from the linkers and junctions.
Other linker sequences may include any sequence of any length of CL/CH1 domain
but
not all residues of CL/CH1 domain; for example the first 5-12 amino acid
residues of the
CL/CH1 domains; the light chain linkers can be from CI( or CA.,; and the heavy
chain linkers can
be derived from CH1 of any isotypes, including Cyl, Cy2, Cy3, C74, Cal, Ca2,
C6, Ca, and CIL
87
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
Linker sequences may also be derived from other proteins such as Ig-like
proteins, (e.g.TCR,
FcR, KIR); G/S based sequences (e.g., 04S (SEQ ID NO: 27) or G4S repeats);
hinge region-
derived sequences; and other natural sequences from other proteins.
In an embodiment a constant domain is linked to the two linked variable
domains using
recombinant DNA techniques. In an embodiment, sequence comprising linked heavy
chain
variable domains, is linked to a heavy chain constant domain and sequence
comprising linked
light chain variable domains is linked to a light chain constant domain. In an
embodiment, the
constant domains are human heavy chain constant domain and human light chain
constant
domain respectively. In an embodiment, the heavy chain is further linked to an
Fc region. The Fc
region may be a native sequence Fc region, or a variant Fe region. In another
embodiment, the Fe
region is a human Fc region. In another embodiment the Fc region includes Fe
region from IgGl,
IgG2, IgG3, IgG4, IgA, IgM, IgE, or IgD.
In another embodiment two heavy chain polypeptides and two light chain
polypeptides
are combined to form a DVD-Ig molecule. Table 2 lists amino acid sequences of
VI-1 and VL
regions of exemplary antibodies for targets useful for treating disease, e.g.,
for treating cancer. In
an embodiment, the DVD Ig comprises at least two of the VII and/or VL regions
listed in Table
2, in any orientation. In some embodiments, VD1 and VD2 are independently
chosen. Therefore, in some
embodiments, VD1 and VD2 comprise the same SEQ ID NO and, in other
embodiments, VD1 and VD2
comprise different SEQ ID NOS.
The VH and VL domain sequences provided below comprise complementary
determining region
(CDR) and framework sequences that are either known in the art or readily
discernable using methods
known in the art. In some embodiments, one or more of these CDR and/or
framework sequences are
replaced, without loss of function, by other CDR and/or framework sequences
from binding proteins that are
known in the art to bind to the same antigen.
Table 2: List of Amino Acid Sequences of VII and VL regions of Antibodies for
Generating
DVD-Igs
SEQ ID ABT Protein
Sequence
No. Unique ID Region
123456789012345678901234567890123456
EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQA
28 AB017VH VII TNF
PGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNAKNSLY
(seq. 1) LQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVS
29 VL TNF
DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKP
AB017VL ( 1)
CKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDVATYYCQRYNRAPYTFGQGTKVEIKR
88
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
SEQ ID ABT Protein Sequence
No. Unique ID Region
123456789012345678901234567890123456
QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLNWIRQP
PGKGLEWIGIIWGDGTTDYNSAVKSRVTISKDTSKNQFSL
30 ABO2OVH VH NGF
KLSSVTAADTAVYYCARGGYWYATSYYFDYWGQGTLVTVS
DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNWYQQKP
31 ABO2OVL VL NGF GKAPKLLIYYTSRFHSGVPSRFSGSGSGTDFTFTISSLQP
EDIATYYCQQEHTLPYTFGQGTKLEIKR
EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMSWVKQS
32 AB022VH VH SOST HGKSLEWIGDINPYSGETTYNQKFKGTATLTVDKSSSIAY
MEIRGLTSEDSAVYYCARDDYDASPFAYWGQGTLVTVSA
DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNWFQQKP
33 AB022VH VL SOST GKAPKLLIYGSSNLEDGVPSRFSGSRYGTDFTLTISSLED
EDLATYFCLQHSYLPYTFGGGTKLEIKR
EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLGWVRQA
34 AB048VH VH PGE2 PGQGLEWMGDIYPGYDYTHYNEKFKDRVTLTTDTSTSTAY
MELRSLRSDDTAVYYCARSDGSSTYWGQGTLVTVSS
DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGNTYLEW
35 AB048VL VL PGE2 YLQKPGQSPQLLIYKVSNRFSGVPDRFSGSGSGTDFTLKI
SRVEAEDVGVYYCFQVSHVPYTFGGGTKVEIKR
QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVNWVRQP
VH TNF
36 AB213VH PGKGLEWLGMIWGDGSTDYDSTLKSRLSISKDNSKSQIFL
(seq. 2)
KMNSLQTDDTARYYCAREWHHGPVAYWGQGTLVTVSA
DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAWYQQKP
VL T NF
37 AB213VL GQSPKLLIYWASTRHTGVPDRFTGSGSVTDFTLTIHNLQA
(seq. 2)
EDLALYYCQQHYSTPFTFGSGTKLEIKR
EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMNWVRQS
VH TNF
38 AB214VHPEKGLEWVAEIRSKSINSATHYAESVKGRFTISRDDSKSA
(seq. 3) VYLQMTDLRTEDTGVYYCSRNYYGSTYDYWGQGTTLTVSS
VL TNF
39 AB214VL 21StR2======:MKTS
(seq. 3)
EDIADYYCQESHSWPFTFGSGTNLEVKR
QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMHWVRQA
VH TNF PGNGLEWVAFMSYDGSNKYAKDSVKGRFTISRDNSKNTLY
40 AB215VH
(seq. 4) LQMNSLRAEDTAVYYCARDRGIAAGGNYYYYGMDVWGQGT
TVTVSS
EIVLTQSPATLSLSPGFRATLSCRASQSVYSYLAWYQQKP
VL TNF
41 AB215VL GQAPRLLIYDASNRATGIPARFSGSGSGTDFTLTISSLEP
(seq. 4)
EDFAVYYCQQRSNWPPFTFGPGTKVDIKR
QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIEWIKQR
PGQGLEWIGLINPGSDYTNYNENFKGKATLTADKSSSTAY
42 A3216V1-I VH LA
MHLSSLTSEDSAVYFCARRFGYYGSGNYFDYWGQGTTLTV
SS
DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGNTYLHW
43 AB216VL VL LPA YLQKPGQSPKLLIYKVSNLFSGVPDRFSGSGSGTDFTLKI
SRVEAEDLGVYFCSQSTHFPFTFGTGTKLEIKR
QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMHWVRQA
VH TNF PGKGLEWVAVIWSDGSIKYYADSVKGRFTISRDNSKNTLY
44 AB217VH
(seq. 5) LQMNSLRAEDTAVYYCAREVESAMGGFYYNGMDVWGQGTT
VTVSS
DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGWYQQKP
VL TNFGKAPKRLIYAASTLQSGVPSRFSGSGSGTEFIFTISSLQP
45 AB217VL
(seq. 5)
EDFASYYCLQHKSYPLTFGGGTKVEIKR
EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMSWVRQA
VH TNF
46 AB218VH PGKGLEWVSVIYSGDRTYYADSVKGRFTISRDNSKNTLYL
(seq. 6)
QMNSLRAEDTAVYYCARGEGGFDYWGQGTLVTVSS
EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAWYQQKP
VL TNF
47 AB218VL GQAPRLLIHGASIRATGLPARFSGSGSGTEFTLTISSLQS
(seq. 6)
EDFAVYYCQQYNYWWTFGQGTKVEIKR
89
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Detailed description of specific DVD-Ig molecules capable of binding specific
targets,
and methods of making the same, is provided in the Examples section below.
D. Production of binding proteins
Binding proteins provided herein may be produced by any of a number of
techniques
known in the art. For example, expression from host cells, wherein expression
vector(s) encoding
the heavy and light chains is (are) transfected into a host cell by standard
techniques. The various
forms of the term "transfection" are intended to encompass a wide variety of
techniques
commonly used for the introduction of exogenous DNA into a prokaryotic or
eukaryotic host cell,
e.g., electroporation, calcium-phosphate precipitation, DEAE-dextran
transfection and the like.
Although it is possible to express the binding proteins provided herein in
either prokaryotic or
eukaryotic host cells, binding proteins are expressed in eukaryotic cells, for
example, mammalian
host cells, because such eukaryotic cells (and in particular mammalian cells)
are more likely than
prokaryotic cells to assemble and secrete a properly folded and
immunologically active binding
protein.
Exemplary mammalian host cells for expressing the recombinant binding proteins
include Chinese Hamster Ovary (CHO cells) (including dhfr- CHO cells,
described in Urlaub and
Chasin, (1980) Proc. Nall. Acad. Sci. USA 77:4216-4220, used with a DHFR
selectable marker,
e.g., as described in R.J. Kaufman and P.A. Sharp (1982) Mol. Biol. 159:601-
621), NSO myeloma
cells, COS cells, SP2 and PER.C6 cells. When recombinant expression vectors
encoding binding
proteins are introduced into mammalian host cells, the binding proteins are
produced by culturing
the host cells for a period of time sufficient to allow for expression of the
binding proteins in the
host cells or secretion of the binding proteins into the culture medium in
which the host cells are
grown. Binding proteins can be recovered from the culture medium using
standard protein
purification methods.
In an exemplary system for recombinant expression, a recombinant expression
vector
encoding both the heavy chain and the light chain is introduced into dhfr- CHO
cells by calcium
phosphate-mediated transfection. Within the recombinant expression vector, the
heavy and light
chain genes are each operatively linked to CMV enhancer/AdMLP promoter
regulatory elements
to drive high levels of transcription of the genes. The recombinant expression
vector also carries
a DHFR gene, which allows for selection of CHO cells that have been
transfected with the vector
using methotrexate selection/amplification. The selected transformant host
cells are cultured to
allow for expression of the heavy and light chains and intact binding protein
is recovered from
the culture medium. Standard molecular biology techniques are used to prepare
the recombinant
90
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
expression vector, transfect the host cells, select for transformants, culture
the host cells and
recover the binding protein from the culture medium. Still further a method of
synthesizing a
binding protein by culturing a host cell in a suitable culture medium until a
binding protein is
synthesized is provided. The method can further comprise isolating the binding
protein from the
culture medium.
An important feature of DVD-Ig is that it can be produced and purified in a
similar way
as a conventional antibody. The production of DVD-Ig results in a homogeneous,
single major
product with desired dual-specific activity, without any sequence modification
of the constant
region or chemical modifications of any kind. Other previously described
methods to generate
"bi-specific", "multi-specific", and "multi-specific multivalent" full length
binding proteins do
not lead to a single primary product but instead lead to the intracellular or
secreted production of
a mixture of assembled inactive, mono-specific, multi-specific, multivalent,
full length binding
proteins, and multivalent full length binding proteins with combination of
different binding sites.
As an example, based on the design described by Miller and Presta (PCT
publication
W02001/077342(A1), there are 16 possible combinations of heavy and light
chains.
Consequently only 6.25% of protein is likely to be in the desired active form,
and not as a single
major product or single primary product compared to the other 15 possible
combinations.
Separation of the desired, fully active forms of the protein from inactive and
partially active
forms of the protein using standard chromatography techniques, typically used
in large scale
manufacturing, is yet to be demonstrated.
Surprisingly the design of the "dual-specific multivalent full length binding
proteins"
leads to a dual variable domain light chain and a dual variable domain heavy
chain which
assemble primarily to the desired "dual-specific multivalent full length
binding proteins".
At least 50%, at least 75% and at least 90% of the assembled, and expressed
dual
variable domain immunoglobulin molecules are the desired dual-specific
tetravalent protein. This
embodiment particularly enhances commercial utility. Therefore, a method to
express a dual
variable domain light chain and a dual variable domain heavy chain in a single
cell leading to a
single primary product of a "dual-specific tetravalent full length binding
protein" is provided.
Methods of expressing a dual variable domain light chain and a dual variable
domain
heavy chain in a single cell leading to a "primary product" of a "dual-
specific tetravalent full
length binding protein", where the "primary product" is more than 50% of all
assembled protein,
comprising a dual variable domain light chain and a dual variable domain heavy
chain are
provided.
91
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Methods of expressing a dual variable domain light chain and a dual variable
domain
heavy chain in a single cell leading to a single "primary product" of a "dual-
specific tetravalent
full length binding protein", where the "primary product" is more than 75% of
all assembled
protein, comprising a dual variable domain light chain and a dual variable
domain heavy chain
are provided.
Methods of expressing a dual variable domain light chain and a dual variable
domain
heavy chain in a single cell leading to a single "primary product" of a "dual-
specific tetravalent
full length binding protein", where the "primary product" is more than 90% of
all assembled
protein, comprising a dual variable domain light chain and a dual variable
domain heavy chain
are provided.
II. Derivatized binding proteins
One embodiment provides a labeled binding protein wherein the binding protein
is
derivatized or linked to another functional molecule (e.g., another peptide or
protein). For
example, a labeled binding protein can be derived by functionally linking a
binding protein
provided herein (by chemical coupling, genetic fusion, noncovalent association
or otherwise) to
one or more other molecular entities, such as another antibody (e.g., a
bispecific antibody or a
diabody), a detectable agent, a cytotoxic agent, a pharmaceutical agent,
and/or a protein or
peptide that can mediate association of the binding protein with another
molecule (such as a
streptavidin core region or a polyhistidine tag).
Useful detectable agents with which a binding protein provided herein may be
derivatized include fluorescent compounds. Exemplary fluorescent detectable
agents include
fluorescein, fluorescein isothiocyanate, rhodamine, 5-dimethylamine-l-
napthalenesulfonyl
chloride, phycoerythrin and the like. A binding protein may also be
derivatized with detectable
enzymes, such as alkaline phosphatase, horseradish peroxidase, glucose oxidase
and the like.
When a binding protein is derivatized with a detectable enzyme, it is detected
by adding
additional reagents that the enzyme uses to produce a detectable reaction
product. For example,
when the detectable agent horseradish peroxidase is present, the addition of
hydrogen peroxide
and diaminobenzidine leads to a colored reaction product, which is detectable,
a binding protein
may also be derivatized with biotin, and detected through indirect measurement
of avidin or
streptavidin binding.
Another embodiment provides a crystallized binding protein and formulations
and
compositions comprising such crystals. In one embodiment the crystallized
binding protein has a
92
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
greater half-life in vivo than the soluble counterpart of the binding protein.
In another
embodiment the binding protein retains biological activity after
crystallization.
Crystallized binding proteins may be produced according to methods known in
the art
and as disclosed in WO 02072636.
Another embodiment provides a glycosylated binding protein wherein the binding
protein
or antigen-binding portion thereof comprises one or more carbohydrate
residues. Nascent in vivo
protein production may undergo further processing, known as post-translational
modification. In
particular, sugar (glycosyl) residues may be added enzymatically, a process
known as
glycosylation. The resulting proteins bearing covalently linked
oligosaccharide side chains are
known as glycosylated proteins or glycoproteins. Antibodies are glycoproteins
with one or more
carbohydrate residues in the Fc domain, as well as the variable domain.
Carbohydrate residues in
the Fc domain have important effect on the effector function of the Fc domain,
with minimal
effect on antigen binding or half-life of the antibody (R. Jefferis,
Biotechnol. Frog. 21 (2005), pp.
11-16). In contrast, glycosylation of the variable domain may have an effect
on the antigen
binding activity of the antibody. Glycosylation in the variable domain may
have a negative effect
on antibody binding affinity, likely due to steric hindrance (Co, M.S., et
al., Mol. Immunol.
(1993) 30:1361- 1367), or result in increased affinity for the antigen
(Wallick, S.C., et al., Exp.
Med. (1988) 168:1099-1109; Wright, A., et al., EMBO J. (1991) 10:2717 2723).
One aspect is directed to generating glycosylation site mutants in which the 0-
or N-
linked glycosylation site of the binding protein has been mutated. One skilled
in the art can
generate such mutants using standard well-known technologies. Glycosylation
site mutants that
retain the biological activity but have increased or decreased binding
activity are other
embodiments.
In still another embodiment, the glycosylation of the binding protein or
antigen-binding
portion thereof is modified. For example, an aglycoslated binding protein can
be made (i.e., the
binding protein lacks glycosylation). Glycosylation can be altered to, for
example, increase the
affinity of the binding protein for antigen. Such carbohydrate modifications
can be accomplished
by, for example, altering one or more sites of glycosylation within the
binding protein sequence.
For example, one or more amino acid substitutions can be made that result in
elimination of one
or more variable region glycosylation sites to thereby eliminate glycosylation
at that site. Such
aglycosylation may increase the affinity of the binding protein for antigen.
Such an approach is
described in further detail in PCT Publication W02003016466A2, and U.S. Pat.
Nos. 5,714,350
and 6,350,861.
93
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Additionally or alternatively, a modified binding protein can be made that has
an altered
type of glycosylation, such as a hypofucosylated binding protein having
reduced amounts of
filcosyl residues (see Kanda, Yutaka et al., Journal of Biotechnology (2007),
130(3), 300-310) or
binding protein having increased bisecting GlcNAc structures. Such altered
glycosylation
patterns have been demonstrated to increase the ADCC ability of antibodies.
Such carbohydrate
modifications can be accomplished by, for example, expressing the binding
proteins in a host cell
with altered glycosylation machinery. Cells with altered glycosylation
machinery have been
described in the art and can be used as host cells in which to express the
recombinant binding
proteins to thereby produce binding proteins with altered glycosylation. See,
for example,
Shields, R. L. etal. (2002) J. Biol. Chem. 277:26733-26740; Umana et al.
(1999) Nat. Biotech.
17:176-1, as well as, European Patent No: EP 1,176,195; PCT Publications WO
03/035835; WO
99/54342 80.
Protein glycosylation depends on the amino acid sequence of the protein of
interest, as
well as the host cell in which the protein is expressed. Different organisms
may produce different
glycosylation enzymes (eg., glycosyltransferases and glycosidases), and have
different substrates
(nucleotide sugars) available. Due to such factors, protein glycosylation
pattern, and composition
of glycosyl residues, may differ depending on the host system in which the
particular protein is
expressed. Glycosyl residues may include, but are not limited to, glucose,
galactose, mannose,
fucose, n-acetylglucosamine and sialic acid. In an embodiment, the
glycosylated binding protein
comprises glycosyl residues such that the glycosylation pattern is human.
It is known to those skilled in the art that differing protein glycosylation
may result in
differing protein characteristics. For instance, the efficacy of a therapeutic
protein produced in a
microorganism host, such as yeast, and glycosylated utilizing the yeast
endogenous pathway may
be reduced compared to that of the same protein expressed in a mammalian cell,
such as a CHO
cell line. Such glycoproteins may also be immunogenic in humans and show
reduced half-life in
vivo after administration. Specific receptors in humans and other animals may
recognize specific
glycosyl residues and promote the rapid clearance of the protein from the
bloodstream. Other
adverse effects may include changes in protein folding, solubility,
susceptibility to proteases,
trafficking, transport, compartmentalization, secretion, recognition by other
proteins or factors,
antigenicity, or allergenicity. Accordingly, a practitioner may choose a
therapeutic protein with a
specific composition and pattern of glycosylation, for example glycosylation
composition and
pattern identical, or at least similar, to that produced in human cells or in
the species-specific
cells of the intended subject animal.
94
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Expressing glycosylated proteins different from that of a host cell may be
achieved by
genetically modifying the host cell to express heterologous glycosylation
enzymes. Using
techniques known in the art a practitioner may generate binding proteins or
antigen-binding
portions thereof exhibiting human protein glycosylation. For example, yeast
strains have been
genetically modified to express non-naturally occurring glycosylation enzymes
such that
glycosylated proteins (glycoproteins) produced in these yeast strains exhibit
protein glycosylation
identical to that of animal cells, especially human cells (U.S patent
applications 20040018590
and 20020137134 and PCT publication W02005100584 A2).
In addition to the binding proteins, certain embodiments are also directed to
anti-
idiotypic (anti-Id) antibodies specific for such binding proteins. An anti-Id
antibody is an
antibody, which recognizes unique determinants generally associated with the
antigen-binding
region of another antibody. The anti-1d can be prepared by immunizing an
animal with the
binding protein or a CDR containing region thereof. The immunized animal will
recognize, and
respond to the idiotypic determinants of the immunizing binding protein and
produce an anti-Id
antibody. It is readily apparent that it may be easier to generate anti-
idiotypic antibodies to the
two or more parent antibodies incorporated into a DVD-Ig molecule; and confirm
binding studies
by methods well recognized in the art (e.g., BlAcore, ELISA) to verify that
anti-idiotypic
antibodies specific for the idiotype of each parent antibody also recognize
the idiotype (e.g.,
antigen binding site) in the context of the DVD-Ig. The anti-idiotypic
antibodies specific for each
of the two or more antigen binding sites of a DVD-Ig provide ideal reagents to
measure DVD-Ig
concentrations of a human DVD-Ig in patrient serum; DVD-Ig concentration
assays can be
established using a "sandwich assay ELISA format" with an antibody to a first
antigen binding
regions coated on the solid phase (e.g., BIAcore chip, ELISA plate etc.),
rinsed with rinsing
buffer, incubation with the serum sample, another rinsing step and ultimately
incubation with
another anti-idiotypic antibody to the another antigen binding site, itself
labeled with an enzyme
for quantitation of the binding reaction. In an embodiment, for a DVD-Ig with
more than two
different binding sites, anti-idiotypic antibodies to the two outermost
binding sites (most distal
and proximal from the constant region) will not only help in determining the
DVD-Ig
concentration in human serum but also document the integrity of the molecule
in vivo. Each anti-
Id antibody may also be used as an "immunogen" to induce an immune response in
yet another
animal, producing a so-called anti-anti-Id antibody.
Further, it will be appreciated by one skilled in the art that a protein of
interest may be
expressed using a library of host cells genetically engineered to express
various glycosylation
enzymes, such that member host cells of the library produce the protein of
interest with variant
95
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
glycosylation patterns. A practitioner may then select and isolate the protein
of interest with
particular novel glycosylation patterns. In an embodiment, the protein having
a particularly
selected novel glycosylation pattern exhibits improved or altered biological
properties.
Ill. Uses of DVD-Ig
Given their ability to bind to two or more antigens the binding proteins
provided herein
can be used to detect the antigens (e.g., in a biological sample, such as
serum or plasma), using a
conventional immunoassay, such as an enzyme linked immunosorbent assays
(ELISA), an
radioimmunoassay (RIA) or tissue immunohistochemistry. The DVD-Ig is directly
or indirectly
labeled with a detectable substance to facilitate detection of the bound or
unbound DVD-Ig.
Suitable detectable substances include various enzymes, prosthetic groups,
fluorescent materials,
luminescent materials and radioactive materials. Examples of suitable enzymes
include
horseradish peroxidase, alkaline phosphatase,13-galactosidase, or
acetylcholinesterase; examples
of suitable prosthetic group complexes include streptavidin/biotin and
avidin/biotin; examples of
suitable fluorescent materials include umbelliferone, fluorescein, fluorescein
isothiocyanate,
rhodamine, dichlorotriazinylamine fluorescein, dansyl chloride or
phycoerythrin; an example of a
luminescent material includes luminol; and examples of suitable radioactive
material include 311.
14c 35s, 90y, 99Te, 1251, 131/, 177Lu, 166llo,or 153Sm.
In an embodiment, the binding proteins are capable of neutralizing the
activity of the
antigens both in vitro and in vivo. Accordingly, such DVD-Igs can be used to
inhibit antigen
activity, e.g., in a cell culture containing the antigens, in human subjects
or in other mammalian
subjects having the antigens with which a binding protein cross-reacts. In
another embodiment, a
method for reducing antigen activity in a subject suffering from a disease or
disorder in which the
antigen activity is detrimental is provided. In some embodiments, the binding
proteins can be
administered to a human subject for therapeutic purposes.
As used herein, the term "a disorder in which antigen activity is detrimental"
is intended
to include diseases and other disorders in which the presence of the antigen
in a subject suffering
from the disorder has been shown to be or is suspected of being either
responsible for the
pathophysiology of the disorder or a factor that contributes to a worsening of
the disorder.
Accordingly, a disorder in which antigen activity is detrimental is a disorder
in which reduction
of antigen activity is expected to alleviate the symptoms and/or progression
of the disorder. Such
disorders may be evidenced, for example, by an increase in the concentration
of the antigen in a
biological fluid of a subject suffering from the disorder (e.g., an increase
in the concentration of
antigen in serum, plasma, synovial fluid, etc. of the subject). Non-limiting
examples of disorders
96
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
that can be treated with the binding proteins provided herein include those
disorders discussed
below and in the section pertaining to pharmaceutical compositions.
The DVD-Igs provided herein may bind one antigen or multiple antigens. Such
antigens
include, but are not limited to, the targets listed in the following
databases, which databases are
incorporated herein by reference. These target databases include those
listings:
Therapeutic targets (http://xin.cz3.nus.edu.sg/group/cjttd/ttd.asp);
Cytokines and cytokine receptors (http://www.cytokinewebfacts.com/,
http://wvvw.copewitheytokines.de/cope.cgi, and
http://cmbi.bjmu.edu.cn/cmbidatakgf/CGF_Database/cytokine.medic.kumamoto-
u.ac.jp/CFC/indexR.html);
Chemokines (http://cytokine.medic.kumamoto-u.ac.jp/CFC/CK/Chemokine.html);
Chemokine receptors and GPCRs (http://csp.medic.kumamoto-
u.ac.jp/CSP/Receptor.html,
http://www.gper.org/7tm/);
Olfactory Receptors (http://senselab.med.yale.edu/senselab/ORDB/default.asp);
Receptors (http://www.iuphar-db.org/iuphar-rd/list/index.htm);
Cancer targets (http://cged.hgc.jp/cgi-bin/input.cgi);
Secreted proteins as potential antibody targets (http://spd.cbi.pku.edu.cn/);
Protein kinases (http://spd.cbi.pku.edu.cn/), and
Human CD markers
(http://content.labvelocity.com/tools/6/1226/CD_table_final_locked.pdf) and
(Zola H, 2005 CD molecules 2005: human cell differentiation molecules Blood,
106:3123-6).
DVD-Igs are useful as therapeutic agents to simultaneously block two different
targets to
enhance efficacy/safety and/or increase patient coverage. Such targets may
include soluble
targets (TNF) and cell surface receptor targets (VEGFR and EGFR). It can also
be used to induce
redirected cytotoxicity between tumor cells and T cells (Her2 and CD3) for
cancer therapy, or
between autoreactive cell and effector cells for autoimmune disease or
transplantation, or
between any target cell and effector cell to eliminate disease-causing cells
in any given disease.
97
WO 2012/027570 CA 02809433 2013-02-
22 PCT/US2011/049147
In addition, DVD-Ig can be used to trigger receptor clustering and activation
when it is
designed to target two different epitopes on the same receptor. This may have
benefit in making
agonistic and antagonistic anti-GPCR therapeutics. In this case, DVD-Ig can be
used to target two
different epitopes (including epitopes on both the loop regions and the
extracellular domain) on
one cell for clustering/signaling (two cell surface molecules) or signaling
(on one molecule).
Similarly, a DVD-Ig molecule can be designed to triger CTLA-4 ligation, and a
negative signal
by targeting two different epitopes (or 2 copies of the same epitope) of CTLA-
4 extracellular
domain, leading to down regulation of the immune response. CTLA-4 is a
clinically validated
target for therapeutic treatment of a number of immunological disorders. CTLA-
4/B7 interactions
negatively regulate T cell activation by attenuating cell cycle progression,
IL-2 production, and
proliferation of T cells following activation, and CTLA-4 (CD152) engagement
can down-
regulate T cell activation and promote the induction of immune tolerance.
However, the strategy
of attenuating T cell activation by agonistic antibody engagement of CTLA-4
has been
unsuccessful since CTLA-4 activation requires ligation. The molecular
interaction of CTLA-4/B7
is in "skewed zipper" arrays, as demonstrated by crystal structural analysis
(Stamper 2001 Nature
410:608). However none of the currently available CTLA-4 binding reagents have
ligation
'properties, including anti-CTLA-4 mAbs. There have been several attempts to
address this issue.
In one case, a cell member-bound single chain antibody was generated, and
significantly inhibited
allogeneic rejection in mice (Hwang 2002 JI 169:633). In a separate case,
artificial APC surface-
linked single-chain antibody to CTLA-4 was generated and demonstrated to
attenuate T cell
responses (Griffin 2000 JI 164:4433). In both cases, CTLA-4 ligation was
achieved by closely
localized member-bound antibodies in artificial systems. While these
experiments provide proof-
of-concept for immune down-regulation by triggering CTLA-4 negative signaling,
the reagents
used in these reports are not suitable for therapeutic use. To this end, CTLA-
4 ligation may be
achieved by using a DVD-Ig molecule, which target two different epitopes (or 2
copies of the
same epitope) of CTLA-4 extracellular domain. The rationale is that the
distance spanning two
binding sites of an IgG, approximately 150-170A, is too large for active
ligation of CTLA-4 (30-
50 A between 2 CTLA-4 homodimer). However the distance between the two binding
sites on
CTLA-4.DVD-Ig (one arm) is much shorter, also in the range of 30-50 A,
allowing proper ligation of
Similarly, DVD-Ig can target two different members of a cell surface receptor
complex
(e.g.,IL-12R alpha and beta). Furthermore, DVD-Ig can target CR1 and a soluble
protein/pathogen to drive rapid clearance of the target soluble
protein/pathogen.
98
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Additionally, DVD-Igs provided herein can be employed for tissue-specific
delivery
(target a tissue marker and a disease mediator for enhanced local PK thus
higher efficacy and/or
lower toxicity), including intracellular delivery (targeting an internalizing
receptor and a
intracellular molecule), delivering to inside brain (targeting transferrin
receptor and a CNS
disease mediator for crossing the blood-brain barrier). DVD-Ig can also serve
as a carrier protein
to deliver an antigen to a specific location via binding to a non-neutralizing
epitope of that
antigen and also to increase the half-life of the antigen. Furthermore, DVD-Ig
can be designed to
either be physically linked to medical devices implanted into patients or
target these medical
devices (see Burke, Sandra E.; Kuntz, Richard E.; Schwartz, Lewis B.,
Zotarolimus eluting
stents. Advanced Drug Delivery Reviews (2006), 58(3), 437-446; Surface
coatings for biological
activation and functionalization of medical devices, Hildebrand, H. F.;
Blanchemain, N.; Mayer,
G.; Chai, F.; Lefebvre, M.; Boschin, F., Surface and Coatings Technology
(2006), 200(22-23),
6318-6324; Drug/ device combinations for local drug therapies and infection
prophylaxis, Wu,
Peng; Grainger, David W., Biomaterials (2006), 27(11), 2450-2467; Mediation of
the cytokine
network in the implantation of orthopedic devices., Marques, A. P.; Hunt, J.
A.; Reis, Rui L.,
Biodegradable Systems in Tissue Engineering and Regenerative Medicine (2005),
377-397).
Briefly, directing appropriate types of cell to the site of medical implant
may promote healing and
restoring normal tissue function. Alternatively, inhibition of mediators
(including but not limited
to cytokines), released upon device implantation by a DVD-Ig coupled to or
target to a device is
also provided. For example, Stents have been used for years in interventional
cardiology to clear
blocked arteries and to improve the flow of blood to the heart muscle.
However, traditional bare
metal stents have been known to cause restenosis (re-narrowing of the artery
in a treated area) in
some patients and can lead to blood clots. Recently, an anti-CD34 antibody
coated stent has been
described which reduced restenosis and prevents blood clots from occurring by
capturing
endothelial progenitor cells (EPC) circulating throughout the blood.
Endothelial cells are cells
that line blood vessels, allowing blood to flow smoothly. The EPCs adhere to
the hard surface of
the stent forming a smooth layer that not only promotes healing but prevents
restenosis and blood
clots, complications previously associated with the use of stents (Aoji et al.
2005 J Am Coll
Cardiol. 45(10):1574-9). In addition to improving outcomes for patients
requiring stents, there are
also implications for patients requiring cardiovascular bypass surgery. For
example, a prosthetic
vascular conduit (artificial artery) coated with anti-EPC antibodies would
eliminate the need to
use arteries from patients legs or arms for bypass surgery grafts. This would
reduce surgery and
anesthesia times, which in turn will reduce coronary surgery deaths. DVD-Ig
are designed in such
a way that it binds to a cell surface marker (such as CD34) as well as a
protein (or an epitope of
any kind, including but not limited to proteins, lipids and polysaccharides)
that has been coated
on the implanted device to facilitate the cell recruitment. Such approaches
can also be applied to
99
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
other medical implants in general. Alternatively, DVD-Igs can be coated on
medical devices and
upon implantation and releasing all DVD-Igs from the device (or any other need
which may
require additional fresh DVD-Ig, including aging and denaturation of the
already loaded DVD-Ig)
the device could be reloaded by systemic administration of fresh DVD-Ig to the
patient, where
the DVD-Ig is designed to binds to a target of interest (a cytokine, a cell
surface marker (such as
CD34) etc.) with one set of binding sites and to a target coated on the device
(including a protein,
an epitope of any kind, including but not limited to lipids, polysaccharides
and polymers ) with
the other. This technology has the advantage of extending the usefulness of
coated implants.
A. Use of DVD-Igs in various diseases
DVD-Ig molecules provided herein are also useful as therapeutic molecules to
treat
various diseases. Such molecules may bind one or more targets involved in a
specific disease.
Examples of such targets in various diseases are described below.
Al. Human Autoimmune and Inflammatory Response
Many proteins have been implicated in general autoimmune and inflammatory
responses,
including C5, CCL1 (1-309), CCL11 (eotaxin), CCL13 (mcp-4), CCL15 (MIP-1d),
CCL16 (HCC-
4), CCL17 (TARC), CCL18 (PARC), CCL19, CCL2 (mcp-1), CCL20 (MIP-3a), CCL21
(MIP-2),
CCL23 (MPIF-1), CCL24 (MPIF-2 / eotaxin-2), CCL25 (TECK), CCL26, CCL3 (MIP-
1a), CCL4
(MIP-1b), CCL5 (RANTES), CCL7 (mcp-3), CCL8 (mcp-2), CXCL1, CXCL10 (IP-10),
CXCL11 (I-TAC / IP-9), CXCLI2 (SDF1), CXCL13, CXCL14, CXCL2, CXCL3, CXCL5
(ENA-78 / LIX), CXCL6 (GCP-2), CXCL9, IL13, IL8, CCL13 (mcp-4), CCR1, CCR2,
CCR3,
CCR4, CCR5, CCR6, CCR7, CCR8, CCR9, CX3CR1, IL8RA, XCR1 (CCXCR1), IFNA2, IL10,
IL13, IL17C, IL1A, IL1B, IL1F10, IL1F5, IL1F6, IL1F7, IL1F8, IL1F9,1L22, 1L5,
1L8, IL9,
LTA, LTB, MIF, SCYE1 (endothelial Monocyte-activating cytokine), SPP1, TNF,
TNFSF5,
IFNA2, ILlORA, ILlORB, IL13, IL13RA1, IL5RA, IL9, IL9R, ABCF1, BCL6, C3, C4A,
CEBPB, CRP, ICEBERG, IL1R1, IL1RN, IL8RB, LTB4R, TOLLIP, FADD, IRAK1, IRAK2,
MYD88, NCK2, TNFAIF'3, TRADD, TRAF1, TRAF2, TRAF3, TRAF4, TRAF5, TRAF6,
ACVR1, ACVR1B, ACVR2, ACVR2B, ACVRL1, CD28, CD3E, CD3G, CD3Z, CD69, CD80,
CD86, CNR1, CTLA4, CYSLTR1, FCER1A, FCER2, FCGR3A, GPR44, HAVCR2, OPRD1,
P2RX7, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, BLR1, CCL1,
CCL2,
CCL3, CCL4, CCL5, CCL7, CCL8, CCL11, CCL13, CCL15, CCL16, CCL17, CCL18, CCL19,
CCL20, CCL21, CCL22, CCL23, CCL24, CCL25, CCR1, CCR2, CCR3, CCR4, CCR5, CCR6,
CCR7, CCR8, CCR9, CX3CL1, CX3CR1, CXCLI, CXCL2, CXCL3, CXCL5, CXCL6,
CXCL10, CXCL11, CXCL12, CXCL13, CXCR4, GPR2, SCYE1, SDF2, XCL1, XCL2, XCR1,
100
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
AMH, AMFIR2, BMPRIA, BMPR1B, BMPR2, Cl9orf10 (IL27w), CER1, CSF1, CSF2, CSF3,
DKFZp451J0118, FGF2, GFI1, IFNA1, IFNBI, IFNG, IGF I, 1L1A, IL1B, ILIR1,
IL1R2, IL2,
IL2RA, IL2RB, IL2RG, IL3, IL4, IL4R, ILS, ILSRA, IL6, IL6R, FL6ST, IL7, 1L8,
IL8RA,
IL8RB, IL9, IL9R, IL10, ILI ORA, ILlORB, IL 11, IL11RA, IL12A, IL12B, 1L12RB1,
IL12RB2,
IL13, IL13RA1, IL13RA2, IL15, IL15RA, IL16, IL17, IL17R, IL18,1L18R1, IL19,
IL20,
KITLG, LEP, LTA, LTB, LTB4R, LTB4R2, LTBR, MIF, NPPB, PDGFB, TBX21, TDGF I,
TGFA, TGFB I, TGFB1I1, TGFB2, TGFB3, TGFBI, TGFBR1, TGFBR2, TGFBR3, THIL, TNF,
TNFRSFIA, TNFRSF1B, TNFRSF7, TNFRSF8, TNFRSF9, TNFRSF11A, TNFRSF21,
TNFSF4, TNFSF5, TNFSF6, TNFSF I 1, VEGF, ZFPM2, and RNF110 (ZNF144). In one
aspect,
DVD-Igs capable of binding one or more of the targets listed herein are
provided.
DVD Igs capable of binding the following pairs of targets to treat
inflammatory disease
are contemplated: TNF and IL-17A; TNF and RANKL; TNF and VEGF; TNF and SOST
(seq.
I); TNF and DKK; TNF and alphaVbeta3; TNF and NGF; TNF and IL-23p19; TNF and
IL-6;
TNF and SOST (seq. 2); TNF and IL-6R; TNF and CD-20; TNF and LPA; TNF and
PGE2; IgE
and IL-13 (seq. 1); IL-13 (seq. 1) and IL23p19; IgE and IL-4; IgE and IL-9
(seq. 1); IgE and IL-9
(seq. 2); IgE and IL-13 (seq. 2); IL-13 (seq. 1) and IL-9 (seq. 1); IL-13
(seq. 1) and IL-4; IL-13
(seq. I) and IL-9 (seq. 2); IL-13 (seq. 2) and IL-9 (seq. 1); IL-13 (seq. 2)
and IL-4; IL-13 (seq. 2)
and IL-23p19; IL-13 (seq. 2) and IL-9 (seq. 2); IL-6R and VEGF; IL-6R and IL-
17A; IL-6R and
RANKL; IL-17A and IL-lbeta (seq. 1); IL-lbeta (seq. 1) and RANKL; IL-lbeta
(seq. 1) and
VEGF; RANKL and CD-20; IL-lalpha and IL-lbeta (seq. 1); IL-lalpha and IL-lbeta
(seq. 2) (see
Examples 2.1 to 2.40).
A2. Asthma
Allergic asthma is characterized by the presence of eosinophilia, goblet cell
metaplasia,
epithelial cell alterations, airway hyperreactivity (AHR), and Th2 and Thl
cytokine expression,
as well as elevated serum IgE levels. It is now widely accepted that airway
inflammation is the
key factor underlying the pathogenesis of asthma, involving a complex
interplay of inflammatory
cells such as T cells, B cells, eosinophils, mast cells and macrophages, and
of their secreted
mediators including cytokines and chemokines. Corticosteroids are the most
important anti-
inflammatory treatment for asthma today, however their mechanism of action is
non-specific and
safety concerns exist, especially in the juvenile patient population. The
development of more
specific and targeted therapies is therefore warranted. There is increasing
evidence that IL-13 in
mice mimics many of the features of asthma, including AHR, mucus
hypersecretion and airway
fibrosis, independently of eosinophilic inflammation (Finotto et al.,
International Immunology
(2005), 17(8), 993-1007; Padilla et al., Journal of Immunology (2005),
174(12), 8097-8105).
101
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
IL-13 has been implicated as having a pivotal role in causing pathological
responses
associated with asthma. The development of anti-IL-13 mAb therapy to reduce
the effects of IL-
13 in the lung is an exciting new approach that offers considerable promise as
a novel treatment
for asthma. However other mediators of differential immunological pathways are
also involved in
asthma pathogenesis, and blocking these mediators, in addition to IL-13, may
offer additional
therapeutic benefit. Such target pairs include, but are not limited to, IL-13
and a pro-
inflammatory cytokine, such as tumor necrosis factor-a (TNF-a). TNF-a may
amplify the
inflammatory response in asthma and may be linked to disease severity
(McDonnell, et al.,
Progress in Respiratory Research (2001), 31(New Drugs for Asthma, Allergy and
COPD), 247-
250.). This suggests that blocking both IL-13 and TNF-a may have beneficial
effects, particularly
in severe airway disease. In another embodiment the DVD-Ig binds the targets
IL-13 and TNFoc
and is used for treating asthma.
Animal models such as OVA-induced asthma mouse model, where both inflammation
and AHR can be assessed, are known in the art and may be used to determine the
ability of
various DVD-Ig molecules to treat asthma. Animal models for studying asthma
are disclosed in
Coffman, et al., Journal of Experimental Medicine (2005), 201(12), 1875-1879;
Lloyd, et al.,
Advances in Immunology (2001), 77, 263-295; Boyce et al., Journal of
Experimental Medicine
(2005), 201(12), 1869-1873; and Snibson, et al., Journal of the British
Society for Allergy and
Clinical Immunology (2005), 35(2), 146-52. In addition to routine safety
assessments of these
target pairs specific tests for the degree of immunosuppression may be
warranted and helpful in
selecting the best target pairs (see Luster et al., Toxicology (1994), 92(1-
3), 229-43; Descotes, et
al., Developments in biological standardization (1992), 77 99-102; Hart et
al., Journal of Allergy
and Clinical Immunology (2001), 108(2), 250-257).
Based on the rationale disclosed herein and using the same evaluation model
for efficacy
and safety other pairs of targets that DVD-Ig molecules can bind and be useful
to treat asthma
may be determined. In an embodiment, such targets include, but are not limited
to, IL-13 and IL-
1 beta, since IL-lbeta is also implicated in inflammatory response in asthma;
IL-13 and cytokines
and chemokines that are involved in inflammation, such as IL-13 and IL-9; IL-
13 and IL-4; IL-13
and IL-5; IL-13 and IL-25; IL-13 and TARC; IL-13 and MDC; IL-13 and MIF; IL-13
and TGF-P;
IL-13 and LHR agonist; IL-13 and CL25; IL-13 and SPRR2a; IL-13 and SPRR2b; and
IL-13 and
ADAMS. DVD-Igs capable of binding one or more targets involved in asthma are
also provided.
In some embodiments the targets are CSF1 (MCSF), CSF2 (GM-CSF), CSF3 (GCSF),
FGF2,
IFNA1, IFNB1, IFNG, histamine and histamine receptors, ILIA, IL1B, IL2, IL3,
IL4, IL5, IL6,
IL7, IL8, IL9, IL10, IL11, IL12A, IL12B, IL13, IL14, IL15, IL16, IL17, IL18,
IL19, KITLG,
102
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
PDGFB, IL2RA, IL4R, IL5RA, IL8RA, IL8RB, IL12RBI, IL12RB2, IL13RA1, IL13RA2,
IL18R1, TSLP, CCL1, CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, CCL13, CCL17, CCL18,
CCL19, CCL20, CCL22, CCL24,CX3CL I, CXCL1, CXCL2, CXCL3, XCL1, CCR2, CCR3,
CCR4, CCR5, CCR6, CCR7, CCR8, CX3CR1, GPR2, XCR1, FOS, GATA3, JAK1, JAK3,
STAT6, TBX21, TGFBI, TNF, TNFSF6, YY1, CYSLTR1, FCER1A, FCER2, LTB4R, TB4R2,
LTBR, or Chitinase.
A3. Rheumatoid arthritis
Rheumatoid arthritis (RA), a systemic disease, is characterized by a chronic
inflammatory reaction in the synovium of joints and is associated with
degeneration of cartilage
and erosion of juxta-articular bone. Many pro-inflammatory cytokines including
TNF,
chemokines, and growth factors are expressed in diseased joints. Systemic
administration of anti-
TNF antibody or sTNFR fusion protein to mouse models of RA was shown to be
anti-
inflammatory and joint protective. Clinical investigations in which the
activcity of TNF in RA
patients was blocked with intravenously administered infliximab (Harriman G,
Harper LK,
Schaible TF. 1999 Summary of clinical trials in rheumatoid arthritis using
infliximab, an anti-
TNFalpha treatment. Ann Rheum Dis 58 Suppl 1:161-4), a chimeric anti-TNF mAb,
has provided
evidence that TNF regulates IL-6, IL-8, MCP-1, and VEGF production,
recruitment of immune
and inflammatory cells into joints, angiogenesis, and reduction of blood
levels of matrix
metalloproteinases-1 and -3. A better understanding of the inflammatory
pathway in rheumatoid
arthritis has led to identification of other therapeutic targets involved in
rheumatoid arthritis.
Promising treatments such as interleukin-6 antagonists (IL-6 receptor antibody
MRA, developed
by Chugai, Roche (see Nishimoto, Norihiro et al., Arthritis & Rheumatism
(2004), 50(6), 1761-
1769), CTLA4Ig (abatacept, Genovese Mc et al 2005 Abatacept for rheumatoid
arthritis
refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 353:1114-
23.), and anti-B cell
therapy (rituximab, Okamoto H, Kamatani N. 2004 Rituximab for rheumatoid
arthritis. N Engl J
Med. 351:1909) have already been tested in randomized controlled trials over
the past year. Other
cytokines have been identified and have been shown to be of benefit in animal
models, including
interleukin-15 (therapeutic antibody HuMax-IL _15, AMG 714 see Baslund, Bo et
al., Arthritis &
Rheumatism (2005), 52(9), 2686-2692), interleukin-17, and interleukin-18, and
clinical trials of
these agents are currently under way. Dual-specific antibody therapy,
combining anti-TNF and
another mediator, has great potential in enhancing clinical efficacy and/or
patient coverage. For
example, blocking both TNF and VEGF can potentially eradicate inflammation and
angiogenesis,
both of which are involved in pathophysiology of RA. Blocking other pairs of
targets involved in
RA including, but not limited to, TNF and IL-18; TNF and IL-12; TNF and IL-23;
TNF and IL-
103
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
lbeta; TNF and MIF; TNF and IL-17; and TNF and IL-15 with specific DVD Igs is
also
contemplated. In addition to routine safety assessments of these target pairs,
specific tests for the
degree of immunosuppress ion may be warranted and helpful in selecting the
best target pairs (see
Luster et al., Toxicology (1994), 92(1-3), 229-43; Descotes, et al.,
Developments in biological
standardization (1992), 77 99-102; Hart et al., Journal of Allergy and
Clinical Immunology
(2001), 108(2), 250-257). Whether a DVD Ig molecule will be useful for the
treatment of
rheumatoid arthritis can be assessed using pre-clinical animal RA models such
as the collagen-
induced arthritis mouse model. Other useful models are also well known in the
art (see Brand
DD., Comp Med. (2005) 55(2):114-22). Based on the cross-reactivity of the
parental antibodies
for human and mouse othologues (e.g.,reactivity for human and mouse TNF, human
and mouse
IL-15 etc.) validation studies in the mouse CIA model may be conducted with
"matched surrogate
antibody" derived DVD-Ig molecules; briefly, a DVD-Ig based on two (or more)
mouse target
specific antibodies may be matched to the extent possible to the
characteristics of the parental
human or humanized antibodies used for human DVD-Ig construction (similar
affinity, similar
neutralization potency, similar half-life etc.).
A4. SLE
The immunopathogenic hallmark of SLE is the polyclonal B cell activation,
which leads
to hyperglobulinemia, autoantibody production and immune complex formation.
The
fundamental abnormality appears to be the failure of T cells to suppress the
forbidden B cell
clones due to generalized T cell dysregulation. In addition, B and T-cell
interaction is facilitated
by several cytokines such as IL-10 as well as co-stimulatory molecules such as
CD40 and
CD4OL, B7 and CD28 and CTLA-4, which initiate the second signal. These
interactions together
with impaired phagocytic clearance of immune complexes and apoptotic material,
perpetuate the
immune response with resultant tissue injury. The following targets may be
involved in SLE and
can potentially be used for DVD-Ig approach for therapeutic intervention: B
cell targeted
therapies: CD-20, CD-22, CD-19, CD28, CD4, CD80, HLA-DRA, IL10, IL2, IL4,
TNFRSF5,
TNFRSF6, TNFSF5, TNFSF6, BLR1, HDAC4, HDAC5, HDAC7A, HDAC9, ICOSL, IGBP1,
MS4A1, RGS1, SLA2, CD81, IFNB1, ILI 0, TNFRSF5, TNFRSF7, TNFSF5, AICDA, BLNK,
GALNAC4S-6ST, HDAC4, HDAC5, HDAC7A, HDAC9, IL10, IL11, 1L4, INHA, INHBA,
KLF6, TNFRSF7, CD28, CD38, CD69, CD80, CD83, CD86, DPP4, FCER2, IL2RA,
TNFRSF8,
INFSF7, CD24, CD37, CD40, CD72, CD74, CD79A, CD79B, CR2, IL1R2, ITGA2, ITGA3,
MS4A1, ST6GAL1, CD1C, CHST10, HLA-A, HLA-DRA, and NT5E.; co-stimulatory
signals:
CTLA4 or B7.1/B7.2; inhibition of B cell survival: BlyS, BAFF; Complement
inactivation: C5;
Cytokine modulation: the key principle is that the net biologic response in
any tissue is the result
104
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
of a balance between local levels of proinflammatory or anti-inflammatory
cytokines (see
Sfikakis PP et al 2005 Curr Opin Rheumatol 17:550-7). SLE is considered to be
a Th-2 driven
disease with documented elevations in serum IL-4, IL-6, IL-10. In some
embodiments the DVD
Igs are capable of binding IL-4, IL-6, IL-10, IFN-a, or TNF-a. Combination of
targets discussed
herein will enhance therapeutic efficacy for SLE which can be tested in a
number of lupus
preclinical models (see Peng SL (2004) Methods Mol Med.;102:227-72). Based on
the cross-
reactivity of the parental antibodies for human and mouse othologues
(e.g.,reactivity for human
and mouse CD20, human and mouse Interferon alpha etc.) validation studies in a
mouse lupus
model may be conducted with "matched surrogate antibody" derived DVD-Ig
molecules; briefly,
a DVD-Ig based two (or more) mouse target specific antibodies may be matched
to the extent
possible to the characteristics of the parental human or humanized antibodies
used for human
DVD-Ig construction (similar affinity, similar neutralization potency, similar
half-life etc.).
A5. Multiple sclerosis
Multiple sclerosis (MS) is a complex human autoimmune-type disease with a
predominantly unknown etiology. Immunologic destruction of myelin basic
protein (MBP)
throughout the nervous system is the major pathology of multiple sclerosis. MS
is a disease of
complex pathologies, which involves infiltration by CD4+ and CD8+ T cells and
of response
within the central nervous system. Expression in the CNS of cytokines,
reactive nitrogen species
and costimulator molecules have all been described in MS. Of major
consideration are
immunological mechanisms that contribute to the development of autoimmunity.
In particular,
antigen expression, cytokine and leukocyte interactions, and regulatory T-
cells, which help
balance/modulate other T-cells such as Thl and Th2 cells, are important areas
for therapeutic
target identification.
IL-12 is a proinflammatory cytokine that is produced by APC and promotes
differentiation of Thl effector cells. IL-12 is produced in the developing
lesions of patients with
MS as well as in EAE-affected animals. Previously it was shown that
interference in IL-12
pathways effectively prevents EAE in rodents, and that in vivo neutralization
of IL-12p40 using a
anti-IL-12 mAb has beneficial effects in the myelin-induced EAE model in
common marmosets.
TWEAK is a member of the TNF family, constitutively expressed in the central
nervous
system (CNS), with pro-inflammatory, proliferative or apoptotic effects
depending upon cell
types. Its receptor, Fn14, is expressed in CNS by endothelial cells, reactive
astrocytes and
neurons. TWEAK and Fn14 mRNA expression increased in spinal cord during
experimental
autoimmune encephalomyelitis (EAE). Anti-TWEAK antibody treatment in myelin
105
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
oligodendrocyte glycoprotein (MUG) induced EAE in C57BL/6 mice resulted in a
reduction of
disease severity and leukocyte infiltration when mice were treated after the
priming phase.
One embodiment pertains to DVD Ig molecules capable of binding one or more,
for
example two, targets. In some embodiments, the targets are IL-12, TWEAK, IL-
23, CXCL13,
CD40, CD4OL, IL-18, VEGF, VLA-4, TNF, CD45RB, CD200, IFNgamma, GM-CSF, FGF,
C5,
CD52, or CCR2. An embodiment includes a dual-specific anti-IL-12/TWEAK DVD Ig
as a
therapeutic agent beneficial for the treatment of MS.
Several animal models for assessing the usefulness of the DVD-Ig molecules to
treat MS
are known in the art (see Steinman L, et al., (2005) Trends Immunol.
26(11):565-71; Lublin FD.,
et al., (1985) Springer Semin Immunopathol.8(3):197-208; Genain CP, et al.,
(1997) J Mol Med.
75(3):187-97; Tuohy VK, etal., (1999) J Exp Med. 189(7):1033-42; Owens T, et
al., (1995)
Neurol Clin.13(1):51-73; and 't Hart BA, et al., (2005) J Immunol 175(7):4761-
8. Based on the
cross-reactivity of the parental antibodies for human and animal species
othologues
(e.g.,reactivity for human and mouse IL-12, human and mouse TWEAK etc.)
validation studies in
the mouse EAE model may be conducted with "matched surrogate antibody" derived
DVD-Ig
molecules; briefly, a DVD-Ig based on to (or more) mouse target specific
antibodies may be
matched to the extent possible to the characteristics of the parental human or
humanized
antibodies used for human DVD-Ig construction (similar affinity, similar
neutralization potency,
similar half-life etc.). The same concept applies to animal models in other
non-rodent species,
where a "matched surrogate antibody" derived DVD-Ig would be selected for the
anticipated
pharmacology and possibly safety studies. In addition to routine safety
assessments of these
target pairs specific tests for the degree of immunosuppression may be
warranted and helpful in
selecting the best target pairs (see Luster et al., Toxicology (1994), 92(1-
3), 229-43; Descotes, et
al., Developments in biological standardization (1992), 77 99-102; Jones R.
2000 Rovelizumab
(ICOS Corp). IDrugs.3(4):442-6).
A6. Sepsis
The pathophysiology of sepsis is initiated by the outer membrane components of
both
gram-negative organisms (lipopolysaccharide [LPS], lipid A, endotoxin) and
gram-positive
organisms (lipoteichoic acid, peptidoglycan). These outer membrane components
are able to bind
to the CD14 receptor on the surface of monocytes. By virtue of the recently
described toll-like
receptors, a signal is then transmitted to the cell, leading to the eventual
production of the
proinflammatory cytokines tumor necrosis factor-alpha (TNF-alpha) and
interleukin-1 (IL-1).
Overwhelming inflammatory and immune responses are essential features of
septic shock and
106
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
play a central part in the pathogenesis of tissue damage, multiple organ
failure, and death induced
by sepsis. Cytokines, especially tumor necrosis factor (TNF) and interleukin
(IL-1), have been
shown to be critical mediators of septic shock. These cytokines have a direct
toxic effect on
tissues; they also activate phospholipase A2. These and other effects lead to
increased
concentrations of platelet-activating factor, promotion of nitric oxide
synthase activity, promotion
of tissue infiltration by neutrophils, and promotion of neutrophil activity.
The treatment of sepsis and septic shock remains a clinical conundrum, and
recent
prospective trials with biological response modifiers (i.e. anti-TNF, anti-
MIF) aimed at the
inflammatory response have shown only modest clinical benefit. Recently,
interest has shifted
toward therapies aimed at reversing the accompanying periods of immune
suppression. Studies in
experimental animals and critically ill patients have demonstrated that
increased apoptosis of
lymphoid organs and some parenchymal tissues contribute to this immune
suppression, anergy,
and organ system dysfunction. During sepsis syndromes, lymphocyte apoptosis
can be triggered
by the absence of IL-2 or by the release of glucocorticoids, granzymes, or the
so-called 'death'
cytokines: tumor necrosis factor alpha or Fas ligand. Apoptosis proceeds via
auto-activation of
cytosolic and/or mitochondrial caspases, which can be influenced by the pro-
and anti-apoptotic
members of the Bc1-2 family. In experimental animals, not only can treatment
with inhibitors of
apoptosis prevent lymphoid cell apoptosis; it may also improve outcome.
Although clinical trials
with anti-apoptotic agents remain distant due in large part to technical
difficulties associated with
their administration and tissue targeting, inhibition of lymphocyte apoptosis
represents an
attractive therapeutic target for the septic patient. Likewise, a dual-
specific agent targeting both
inflammatory mediator and a apoptotic mediator, may have added benefit. One
embodiment
pertains to DVD Igs capable of binding one or more targets involved in sepsis,
in an embodiment
two targets. In some embodiments, the targets are TNF, IL-1, MIF, IL-6, IL-8,
IL-18, IL-12, IL-
23, FasL, LPS, Toll-like receptors, TLR-4, tissue factor, MIP-2, ADORA2A,
CASP1, CASP4,
IL-10, IL-1B, NFKB1, PROC, TNFRSF1A, CSF3, CCR3, IL1RN, MIF, NFKB1, PTAFR,
TLR2,
TLR4, GPR44, IftvIOX1, midkine, IRAK1, NFKB2, SERPINA1, SERPINE I, or TREMI.
The
efficacy of such DVD Igs for sepsis can be assessed in preclinical animal
models known in the art
(see Buras JA, et al.,(2005) Nat Rev Drug Discov. 4(10):854-65 and Calandra T,
et al., (2000)
Nat Med. 6(2):164-70).
107
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
A7. Neurological disorders
A7.1. Neurodegenerative Diseases
Neurodegenerative diseases are either chronic in which case they are usually
age-
dependent or acute (e.g., stroke, traumatic brain injury, spinal cord injury,
etc.). They are
characterized by progressive loss of neuronal functions (neuronal cell death,
demyelination), loss
of mobility and loss of memory. Emerging knowledge of the mechanisms
underlying chronic
neurodegenerative diseases (e.g., Alzheimer's disease disease) show a complex
etiology and a
variety of factors have been recognized to contribute to their development and
progression
e.g.,age, glycemic status, amyloid production and multimerization,
accumulation of advanced
glycation-end products (AGE) which bind to their receptor RAGE (receptor for
AGE), increased
brain oxidative stress, decreased cerebral blood flow, neuroinflammation
including release of
inflammatory cytokines and chemokines, neuronal dysfunction and microglial
activation. Thus
these chronic neurodegenerative diseases represent a complex interaction
between multiple cell
types and mediators. Treatment strategies for such diseases are limited and
mostly constitute
either blocking inflammatory processes with non-specific anti-inflammatory
agents (e.g.,
corticosteroids, COX inhibitors) or agents to prevent neuron loss and/or
synaptic functions.
These treatments fail to stop disease progression. Recent studies suggest that
more targeted
therapies such as antibodies to soluble A-b peptide (including the A-b
oligomeric forms) can not
only help stop disease progression but may help maintain memory as well. These
preliminary
observations suggest that specific therapies targeting more than one disease
mediator (e.g.,A-b
and a pro-inflammatory cytokine such as TNF) may provide even better
therapeutic efficacy for
chronic neurodegenerative diseases than observed with targeting a single
disease mechanism
(e.g.,soluble A-b alone) (see C.E. Shepherd, et al, Neurobiol Aging. 2005 Oct
24; Nelson RB.,
Curr Pharm Des. 2005;11:3335; William L. Klein.; Neurochem Int. 2002 ;41:345;
Michelle C
Janelsins, etal., J Neuroinflammation. 2005 ;2:23; Soloman B., Curr Alzheimer
Res. 2004;1:149;
Igor Klyubin, et al., Nat Med. 2005;11:556-61; Arancio 0, et al., EMBO Journal
(2004) 1-10;
Bornemann KD, et al., Am J Pathol. 2001;158:63; Deane R, etal., Nat Med.
2003;9:907-13; and
Eliezer Masliah, et al., Neuron. 2005;46:857).
The DVD-Ig molecules provided herein can bind one or more targets involved in
Chronic
neurodegenerative diseases such as Alzheimers. Such targets include, but are
not limited to, any
mediator, soluble or cell surface, implicated in AD pathogenesis, e.g., AGE
(S100 A,
amphoterin), pro-inflammatory cytokines (e.g., IL-1), chemokines (e.g., MCP
1), molecules that
inhibit nerve regeneration (e.g., Nogo, RGM A), molecules that enhance neurite
growth
(neurotrophins) and molecules that can mediate transport at the blood brain
barrier (e.g.,
108
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
transferrin receptor, insulin receptor or RAGE). The efficacy of DVD-Ig
molecules can be
validated in pre-clinical animal models such as the transgenic mice that over-
express amyloid
precursor protein or RAGE and develop Alzheimer's disease-like symptoms. In
addition, DVD-
Ig molecules can be constructed and tested for efficacy in the animal models
and the best
therapeutic DVD-Ig can be selected for testing in human patients. DVD-Ig
molecules can also be
employed for treatment of other neurodegenerative diseases such as Parkinson's
disease. Alpha-
Synuclein is involved in Parkinson's pathology. A DVD-Ig capable of targeting
alpha-synuclein
and inflammatory mediators such as INF, IL-1, MCP-1 can prove effective
therapy for
Parkinson's disease and are also contemplated.
A7.2 Neuronal Regeneration and Spinal Cord Injury
Despite an increase in knowledge of the pathologic mechanisms, spinal cord
injury (SCI)
is still a devastating condition and represents a medical indication
characterized by a high
medical need. Most spinal cord injuries are contusion or compression injuries
and the primary
injury is usually followed by secondary injury mechanisms (inflammatory
mediators e.g.,
cytokines and chemokines) that worsen the initial injury and result in
significant enlargement of
the lesion area, sometimes more than 10-fold. These primary and secondary
mechanisms in SCI
are very similar to those in brain injury caused by other means e.g., stroke.
No satisfying
treatment exists and high dose bolus injection of methylprednisolone (MP) is
the only used
therapy within a narrow time window of 8 h post injury. This treatment,
however, is only
intended to prevent secondary injury without causing any significant
functional recovery. It is
heavily critisized for the lack of unequivocal efficacy and severe adverse
effects, like
immunosuppression with subsequent infections and severe histopathological
muscle alterations.
No other drugs, biologics or small molecules, stimulating the endogenous
regenerative potential
are approved, but promising treatment principles and drug candidates have
shown efficacy in
animal models of SCI in recent years. To a large extent the lack of functional
recovery in human
SCI is caused by factors inhibiting neurite growth, at lesion sites, in scar
tissue, in myelin as well
as on injury-associated cells. Such factors are the myelin-associated proteins
NogoA, 0Mgp and
MAG, RGM A, the scar-associated CSPG (Chondroitin Sulfate Proteoglycans) and
inhibitory
factors on reactive astrocytes (some semaphorins and ephrins). However, at the
lesion site not
only growth inhibitory molecules are found but also neurite growth stimulating
factors like
neurotrophins, laminin, L 1 and others. This ensemble of neurite growth
inhibitory and growth
promoting molecules may explain that blocking single factors, like NogoA or
RGM A, resulted in
significant functional recovery in rodent SCI models, because a reduction of
the inhibitory
influences could shift the balance from growth inhibition to growth promotion.
However,
109
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
recoveries observed with blocking a single neurite outgrowth inhibitory
molecule were not
complete. To achieve faster and more pronounced recoveries either blocking two
neurite
outgrowth inhibitory molecules, e.g., Nogo and RGM A, or blocking an neurite
outgrowth
inhibitory molecule and enhancing functions of a neurite outgrowth enhancing
molecule, e.g.,
Nogo and neurotrophins, or blocking a neurite outgrowth inhibitory moleclule,
e.g.,Nogo and a
pro-inflammatory molecule e.g.,TNF, may be desirable (see McGee AW, et al.
(2003) Trends
Neurosci. 26:193; Marco Domeniconi, et al. (2005) J. Neurol. Sci. 233:43;
Milan Makwanal, et
al. (2005) FEBS J. 272:2628; Barry J. Dickson (2002) Science 298:1959; Felicia
Yu Hsuan Teng,
et at. (2005) J. Neurosci. Res. 79:273; Tara Kamezis, et at. (2004) Nature
Neuroscience 7:736;
Gang Xu, et al. (2004) J. Neurochem. 91:1018).
In one aspect, DVD-Igs capable of binding target pairs such as NgR and RGM A;
NogoA
and RGM A; MAG and RGM A; OMGp and RGM A; RGM A and RGM B; CSPGs and RGM
A; aggrecan, midkine, neurocan, versican, phosphacan, Te38 and TNF-a; AB
globulomer-specific
antibodies combined with antibodies promoting dendrite & axon sprouting are
provided. Dendrite
pathology is a very early sign of AD and it is known that NOGO A restricts
dendrite growth. One
can combine such type of ab with any of the SCI-candidate (myelin-proteins)
Ab. Other DVD-Ig
targets may include any combination of NgR-p75, NgR-Troy, NgR-Nogo66 (Nogo),
NgR-Lingo,
Lingo-Troy, Lingo-p75, MAG or Omgp. Additionally, targets may also include any
mediator,
soluble or cell surface, implicated in inhibition of neurite e.g Nogo, Ompg,
MAG, RGM A,
semaphorins, ephrins, soluble A-b, pro-inflammatory cytokines (e.g., IL-1),
chemokines (e.g.,
MIP la), molecules that inhibit nerve regeneration. The efficacy of anti-nogo
/ anti-RGM A or
similar DVD-Ig molecules can be validated in pre-clinical animal models of
spinal cord injury. In
addition, these DVD-Ig molecules can be constructed and tested for efficacy in
the animal models
and the best therapeutic DVD-Ig can be selected for testing in human patients.
In addition, DVD-
Ig molecules can be constructed that target two distinct ligand binding sites
on a single receptor
e.g., Nogo receptor which binds three ligand Nogo, Ompg, and MAG and RAGE that
binds A-b
and S100 A. Furthermore, neurite outgrowth inihibitors e.g., nogo and nogo
receptor, also play a
role in preventing nerve regeneration in immunological diseases like multiple
sclerosis. Inhibition
of nogo-nogo receptor interaction has been shown to enhance recovery in animal
models of
multiple sclerosis. Therefore, DVD-Ig molecules that can block the function of
one immune
mediator eg a cytokine like IL-12 and a neurite outgrowth inhibitor molecule
eg nogo or RGM
may offer faster and greater efficacy than blocking either an immune or an
neurite outgrowth
inhibitor molecule alone.
110
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
In general, antibodies do not cross the blood brain barrier (BBB) in an
efficient and
relevant manner. However, in certain neurologic diseases, e.g., stroke,
traumatic brain injury,
multiple sclerosis, etc., the BBB may be compromised and allows for increased
penetration of
DVD-Igs and antibodies into the brain. In other neurological conditions, where
BBB leakage is
not occuring, one may employ the targeting of endogenous transport systems,
including carrier-
mediated transporters such as glucose and amino acid carriers and receptor-
mediated
transcytosis-mediating cell structures/receptors at the vascular endothelium
of the BBB, thus
enabling trans-BBB transport of the DVD-Ig. Structures at the BBB enabling
such transport
include but are not limited to the insulin receptor, transferrin receptor, LRP
and RAGE. In
addition, strategies enable the use of DVD-Igs also as shuttles to transport
potential drugs into the
CNS including low molecular weight drugs, nanoparticles and nucleic acids
(Coloma MJ, et al.
(2000) Pharm Res. 17(3):266-74; Boado RJ, et al. (2007) Bioconjug. Chem.
18(2):447-55).
A8. Oncological disorders
Monoclonal antibody therapy has emerged as an important therapeutic modality
for
cancer (von Mehren, M., et al. (2003) Annu. Rev. Med. 54:343-69). Antibodies
may exert
antitumor effects by inducing apoptosis, redirected cytotoxicity, interfering
with ligand-receptor
interactions, or preventing the expression of proteins that are critical to
the neoplastic phenotype.
In addition, antibodies can target components of the tumor microenvironment,
perturbing vital
structures such as the formation of tumor-associated vasculature. Antibodies
can also target
receptors whose ligands are growth factors, such as the epidermal growth
factor receptor. The
antibody thus inhibits natural ligands that stimulate cell growth from binding
to targeted tumor
cells. Alternatively, antibodies may induce an anti-idiotype network,
complement-mediated
cytotoxicity, or antibody-dependent cellular cytotoxicity (ADCC). The use of
dual-specific
binding proteins that target two separate tumor mediators will likely give
additional benefit
compared to a mono-specific therapy.
In another embodiment, the DVD Ig is capable of binding VEGF and
phosphatidylserine;
VEGF and ErbB3; VEGF and PLGF; VEGF and ROB04; VEGF and BSG2; VEGF and CDCP1;
VEGF and ANPEP; VEGF and c-MET; HER-2 and ERB3; HER-2 and BSG2; HER-2 and
CDCP1; HER-2 and ANPEP; EGFR and CD64; EGFR and BSG2; EGFR and CDCP1; EGFR
and ANPEP; IGF IR and PDGFR; IGF1R and VEGF; IGF1R and CD20; CD20 and CD74;
CD20
and CD30; CD20 and DR4; CD20 and VEGFR2; CD20 and CD52; CD20 and CD4; HGF and
c-
MET; HGF and NRP1; HGF and phosphatidylserine; ErbB3 and IGF1R; ErbB3 and
IGF1,2; c-
Met and Her-2; c-Met and NRP1; c-Met and IGF1R; IGF1,2 and PDGFR; IGF1,2 and
CD20;
IGF1,2 and IGF1R; IGF2 and EGFR; IGF2 and HER2; IGF2 and CD20; IGF2 and VEGF;
IGF2
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
and IGF1R; IGF1 and IGF2; PDGFRa and VEGFR2; PDGFRa and PLGF; PDGFRa and VEGF;
PDGFRa and c-Met; PDGFRa and EGFR; PDGFRb and VEGFR2; PDGFRb and c-Met;
PDGFRb and EGFR; RON and c-Met; RON and MTSP1; RON and MSP; RON and CDCP1;
VGFR1 and PLGF; VGFR1 and RON; VGFR1 and EGFR; VEGFR2 and PLGF; VEGFR2 and
NRP1; VEGFR2 and RON; VEGFR2 and DLL4; VEGFR2 and EGFR; VEGFR2 and ROB04;
VEGFR2 and CD55; LPA and S1P; EPHI32 and RON; CTLA4 and VEGF; CD3 and EPCAM;
CD40 and IL6; CD40 and IGF; CD40 and CD56; CD40 and CD70; CD40 and VEGFR1;
CD40
and DR5; CD40 and DR4; CD40 and APRIL; CD40 and BCMA; CD40 and RANKL; CD28 and
MAPG; CD80 and CD40; CD80 and CD30; CD80 and CD33; CD80 and CD74; CD80 and
CD2;
CD80 and CD3; CD80 and CD19; CD80 and CD4; CD80 and CD52; CD80 and VEGF; CD80
and DR5; CD80 and VEGFR2; CD22 and CD20; CD22 and CD80; CD22 and CD40; CD22
and
CD23; CD22 and CD33; CD22 and CD74; CD22 and CD19; CD22 and DR5; CD22 and DR4;
CD22 and VEGF; CD22 and CD52; CD30 and CD20; CD30 and CD22; CD30 and CD23;
CD30
and CD40; CD30 and VEGF; CD30 and CD74; CD30 and CD19; CD30 and DR5; CD30 and
DR4; CD30 and VEGFR2; CD30 and CD52; CD30 and CD4; CD138 and RANKL; CD33 and
FTL3; CD33 and VEGF; CD33 and VEGFR2; CD33 and CD44; CD33 and DR4; CD33 and
DR5; DR4 and CD137; DR4 and IGF1,2; DR4 and IGF1R; DR4 and DR5; DR5 and CD40;
DR5
and CD137; DR5 and CD20; DR5 and EGFR; DRS and IGF1,2; DRS and IGFR, DR5 and
HER-
2, and EGFR and DLL4. Other target combinations include one or more members of
the
EGF/erb-2/erb-3 family. Other targets (one or more) involved in oncological
diseases that DVD
Igs may bind include, but are not limited to: CD52, CD20, CD19, CD3, CD4, CD8,
BMP6,
IL12A, ILIA, IL1B, IL2, IL24, INHA, TNF, TNFSF10, BMP6, EGF, FGFI, FGF10, FGFI
I,
FGF12, FGFI3, FGF14, FGF16, FGF17, FGF18, FGF19, FGF2, FGF20, FGF21, FGF22,
FGF23,
FGF3, FGF4, FGF5, FGF6, FGF7, FGF8, FGF9, GRP, IGF1, IGF2, IL12A, ILIA, IL1B,
IL2,
INHA, TGFA, TGFB1, TGFB2, TGFB3, VEGF, CDK2, FGF10, FGF18, FGF2, FGF4, FGF7,
IGF1R, IL2, BCL2, CD164, CDKN1A, CDKN1B, CDKN1C, CDKN2A, CDKN2B, CDKN2C,
CDKN3, GNRH1, IGFBP6, ILIA, IL1B, ODZ1, PAWR, PLG, TGFB111, AR, BRCA1, CDK3,
CDK4, CDK5, CDK6, CDK7, CDK9, E2F1, EGFR, EN01, ERBB2, ESR1, ESR2, IGFBP3,
IGFBP6, IL2, INSL4, MYC, NOX5, NR6A1, PAP, PCNA, PRKCQ, PRI(D1, PRL, TP53,
FGF22, FGF23, FGF9, IGFBP3, IL2, INH_A, KLK6, TP53, CHGB, GNRI-11, IGF I,
IGF2, INHA,
INSL3, INSL4, PRL, KLK6, SHBG, NR1D1, NR1H3, NR1I3, NR2F6, NR4A3, ESR1, ESR2,
NROB1, NROB2, NR1D2, NR1H2, NR1H4, NR1I2, NR2C1, NR2C2, NR2E1, NR2E3, NR2F1,
NR2F2, NR3C1, NR3C2, NR4A1, NR4A2, NR5A1, NR5A2, NR6A1, PGR, RARB, FGF1,
FGF2, FGF6, KLK3, KRT1, APOC I , BRCA1, CHGA, CHGB, CLU, COL 1A1, COL6A I ,
EGF,
ERBB2, ERK8, FGF1, FGF I 0, FGF11, FGF13, FGF I 4, FGF16, FGF17, FGF18, FGF2,
FGF20,
FGF21, FGF22, FGF23, FGF3, FGF4, FGF5, FGF6, FGF7, FGF8, FGF9, GNRHI, IGF I,
IGF2,
112
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
IGFBP3, IGFBP6, IL12A, ILIA, ILIB, IL2, IL24, INHA, INSL3, INSL4, KLK10,
KLK12,
KLK13, KLK14, KLK15, KLK3, KLK4, KLK5, KLK6, KLK9, MMP2, MMP9, MSMB, NTN4,
ODZ1, PAP, PLAU, PRL, PSAP, SERPINA3, SHBG, TGFA, TIMP3, CD44, CDH1, CDH10,
CDH19, CDH20, CDH7, CDH9, CDHI, CDH10, CDH13, CDH18, CDH19, CDH20, CDH7,
CDH8, CDH9, ROB02, CD44, ILK, ITGA I, APC, CD164, COL6A1, MTSS1, PAP, TGFB1I1,
AGR2, AIGI, AKAPI, AKAP2, CANTI, CAVI, CDH12, CLDN3, CLN3, CYB5, CYC I,
DAB2IP, DES, DNCL1, ELAC2, EN02, EN03, FASN, Fill 2584, FLJ25530, GAGEB1,
GAGEC1, GGT1, GSTP1, HIP1, HUMCYT2A, IL29, K6HF, KAII, KRT2A, MIB1, PART1,
PATE, PCA3, PIAS2, PIK3CG, PPID, PR1, PSCA, SLC2A2, SLC33A I, SLC43A1, STEAP,
STEAP2, TPM1, TPM2, TRPC6, ANGPT1, ANGPT2, ANPEP, ECGF1, EREG, FGFI, FGF2,
FIGF, FLT, JAG1, KDR, LAMAS, NRP1, NRP2, PGF, PLXDCI, STAB1, VEGF, VEGFC,
ANGPTL3, BAIl, COL4A3, IL8, LAMAS, NRP I, NRP2, STAB1, ANGPTL4, PECAM1, PF4,
PROK2, SERPINF1, TNFAIP2, CCL11, CCL2, CXCL1, CXCL10, CXCL3, CXCL5, CXCL6,
CXCL9, IFNA1, IFNB1, IFNG, IL 1B, IL6, MDK, EDG1, EFNA1, EFNA3, EFNB2, EGF,
EPHB4, FGFR3, HGF, IGFI, ITGB3, PDGFA, TEK, TGFA, TGFB1, TGFB2, TGFBR1, CCL2,
CDH5, COL18A1, EDG1, ENG, ITGAV, ITGB3, THBS1, THBS2, BAD, BAG1, BCL2,
= CCNA1, CCNA2, CCND1, CCNE1, CCNE2, CDH1 (E-cadherin), CDKN1B (p27Kipl),
CDKN2A (p16INK4a), COL6A1, CTNNB1 (b-eaten in), CTSB (cathepsin B), ERBB2 (Her-
2),
ESR1, ESR2, F3 (TF), FOSL1 (FRA-1), GATA3, GSN (Gelsolin), IGFBP2, IL2RA, IL6,
IL6R,
IL6ST (glycoprotein 130), ITGA6 (a6 integrin), JUN, KLK5, KRT19, MAP2K7 (c-
Jun), MKI67
(Ki-67), NGFB (NGF), NGFR, NME1 (NM23A), PGR, PLAU (uPA), PTEN, SERPINB5
(maspin), SERPINE1 (PAI-1), TGFA, THBS1 (thrombospondin-1), TIE (Tie-I),
TNFRSF6 (Fas),
TNFSF6 (FasL), TOP2A (topoisomerase Ea), TP53, AZGP1 (zinc-a-glycoprotein),
BPAGI
(plectin), CDKN1A (p21Wapl/Cipl), CLDN7 (claudin-7), CLU (clusterin), ERBB2
(Her-2),
FGF1, FLRT1 (fibronectin), GABRP (GABAa), GNAS1, ID2, ITGA6 (a6 integrin),
ITGB4 (b 4
integrin), KLF5 (GC Box BP), KRT19 (Keratin 19), KRTHB6 (hair-specific type II
keratin),
MACMARCKS, MT3 (metallothionectin-III), MUC1 (mucin), PTGS2 (COX-2), RAC2
(p21Rac2), S100A2, SCGB1D2 (lipophilin B), SCGB2A1 (mammaglobin 2), SCGB2A2
(mammaglobin 1), SPRR1B (Sprl), THBS1, THBS2, THBS4, and TNFAIP2 (B94), RON, c-
Met,
CD64, DLL4, PLGF, CTLA4, phophatidylserine, ROB04, CD80, CD22, CD40, CD23,
CD28,
CD80, CD55, CD38, CD70, CD74, CD30, CD138, CD56, CD33, CD2, CD137, DR4, DR5,
RANKL, VEGFR2, PDGFR, VEGFR1, MTSP1, MSP, EPHB2, EPHAl, EPHA2, EpCAM,
PGE2, NKG2D, LPA, SIP, APRIL, BCMA, MAPG, FLT3, PDGFR alpha, PDGFR beta, RORI,
PSMA, PSCA, SCD1, or CD59.
113
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
IV. Pharmaceutical Compositions
Pharmaceutical compositions comprising a binding protein disclosed herein and
a
pharmaceutically acceptable carrier are also provided. The pharmaceutical
compositions
comprising binding proteins disclosed herein are for use in, but not limited
to, diagnosing,
detecting, or monitoring a disorder, in preventing, treating, managing, or
ameliorating of a
disorder or one or more symptoms thereof, and/or in research. In a specific
embodiment, a
composition comprises one or more binding proteins disclosed herein. In
another embodiment,
the pharmaceutical composition comprises one or more binding proteins and one
or more
prophylactic or therapeutic agents other than binding proteins provided herein
for treating a
disorder. In an embodiment, the prophylactic or therapeutic agents are known
to be useful for or
having been or currently being used in the prevention, treatment, management,
or amelioration of
a disorder or one or more symptoms thereof. In accordance with these
embodiments, the
composition may further comprise of a carrier, diluent or excipient.
The binding proteins provided herein can be incorporated into pharmaceutical
compositions suitable for administration to a subject. Typically, the
pharmaceutical composition
comprises a binding protein and a pharmaceutically acceptable carrier. As used
herein,
"pharmaceutically acceptable carrier" includes any and all solvents,
dispersion media, coatings,
antibacterial and antifungal agents, isotonic and absorption delaying agents,
and the like that are
physiologically compatible. Examples of pharmaceutically acceptable carriers
include one or
more of water, saline, phosphate buffered saline, dextrose, glycerol, ethanol
and the like, as well
as combinations thereof. In some embodiments, isotonic agents, for example,
sugars,
polyalcohols such as mannitol, sorbitol, or sodium chloride, are included in
the composition.
Pharmaceutically acceptable carriers may further comprise minor amounts of
auxiliary substances
such as wetting or emulsifying agents, preservatives or buffers, which enhance
the shelf life or
effectiveness of the binding protein or antigen-biding portion.
Various delivery systems are known and can be used to administer one or more
binding
proteins provided herein or the combination of one or more binding proteins
and a prophylactic
agent or therapeutic agent useful for preventing, managing, treating, or
ameliorating a disorder or
one or more symptoms thereof, e.g., encapsulation in liposomes,
microparticles, microcapsules,
recombinant cells capable of expressing the binding protein or antigen-binding
fragment,
receptor-mediated endocytosis (see, e. g., Wu and Wu, J. Biol. Chem. 262:4429-
4432 (1987)),
construction of a nucleic acid as part of a retroviral or other vector, etc.
Methods of administering
a prophylactic or therapeutic agents include, but are not limited to,
parenteral administration
(e.g., intradermal, intramuscular, intraperitoneal, intravenous and
subcutaneous) , epidurala
114
WO 2012/027570
CA 02809433 2013-02-22
PCT/US2011/049147
administration, intratumoral administration, and mucosa] adminsitration (e.g.,
intranasal and oral
routes). In addition, pulmonary administration can be employed, e.g., by use
of an inhaler or
nebulizer, and formulation with an aerosolizing agent. See, e.g., U.S. Pat.
Nos. 6,019,968;
5,985,320; 5,985,309; 5,934,272; 5,874,064; 5,855,913; 5,290,540; and
4,880,078; and PCT
Publication Nos. WO 92/19244; WO 97/32572; WO 97/44013; WO 98/31346; and WO
99/66903. In one embodiment, a binding protein, combination therapy, or a
composition
disclosed herein is administered using Alkermes AIR pulmonary drug delivery
technology
(Alkermes, Inc., Cambridge, Mass.). In a specific embodiment, prophylactic or
therapeutic agents
disclosed herein are administered intramuscularly, intravenously,
intratumorally, orally,
intranasally, pulmonary, or subcutaneously. The prophylactic or therapeutic
agents may be
administered by any convenient route, for example by infusion or bolus
injection, by absorption
through epithelial or mucocutaneous linings (e.g., oral mucosa, rectal and
intestinal mucosa, etc.)
and may be administered together with other biologically active agents.
Administration can be
systemic or local.In an embodiment, specific binding of antibody-coupled
carbon nanotubes (CNTs) to
tumor cells in vitro, followed by their highly specific ablation with near-
infrared (NIR) light can
be used to target tumor cells. For example, biotinylated polar lipids can be
used to prepare stable,
biocompatible, noncytotoxic CNT dispersions that are then attached to one or
two different
neutralite avidin-derivatized DVD-Igs directed against one or more tumor
antigens (e.g., CD22)
(Chakravarty, P. et al. (2008) Proc. Natl. Acad. Sci. USA 105:8697-8702.
In a specific embodiment, it may be desirable to administer the prophylactic
or
therapeutic agents provided herein locally to the area in need of treatment;
this may be achieved
by, for example, and not by way of limitation, local infusion, by injection,
or by means of an
implant, said implant being of a porous or non-porous material, including
membranes and
matrices, such as sialastic membranes, polymers, fibrous matrices (e.g.,
Tissue10), or collagen
matrices. In one embodiment, an effective amount of one or more binding
proteins provided
herein is administered locally to the affected area to a subject to prevent,
treat, manage, and/or
ameliorate a disorder or a symptom thereof. In another embodiment, an
effective amount of one
or more binding proteins provided herein is administered locally to the
affected area in
combination with an effective amount of one or more therapies (e. g., one or
more prophylactic or
therapeutic agents) other than a binding protein provided herein to prevent,
treat, manage, and/or
ameliorate a disorder or one or more symptoms thereof.
In another embodiment, the prophylactic or therapeutic agent can be delivered
in a
controlled release or sustained release system. In one embodiment, a pump may
be used to
1 15
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
achieve controlled or sustained release (see Langer, supra; Sefton, 1987, CRC
Crit. Ref. Biomed.
Eng. 14:20; Buchwald et al., 1980, Surgery 88:507; Saudek et al., 1989, N.
Engl. J. Med.
321:574). In another embodiment, polymeric materials can be used to achieve
controlled or
sustained release of the therapies provided herein (see e.g., Medical
Applications of Controlled
Release, Langer and Wise (eds.), CRC Pres., Boca Raton, Fla. (1974);
Controlled Drug
Bioavailability, Drug Product Design and Performance, Smolen and Ball (eds.),
Wiley, New
York (1984); Ranger and Peppas, 1983, J., Macromol. Sci. Rev. Macromol. Chem.
23:61; see
also Levy et al., 1985, Science 228:190; During et al., 1989, Ann. Neurol.
25:351; Howard et al.,
1989, J. Neurosurg. 7 1:105); U.S. Pat. No. 5,679,377; U.S. Pat. No.
5,916,597; U. S. Pat. No.
5,912,015; U.S. Pat. No. 5,989,463; U.S. Pat. No. 5,128,326; PCT Publication
No. WO 99/15154;
and PCT Publication No. WO 99/20253. Examples of polymers used in sustained
release
formulations include, but are not limited to, poly(2-hydroxy ethyl
methacrylate), poly(methyl
methacrylate), poly(acrylic acid), poly(ethylene-co-vinyl acetate),
poly(methacrylic acid),
polyglycolides (PLG), polyanhydrides, poly(N- vinyl pyrrolidone), poly(vinyl
alcohol),
polyacrylamide, poly(ethylene glycol), polylactides (PLA), poly(lactide-co-
glycolides) (PLGA),
and polyorthoesters. In an embodiment, the polymer used in a sustained release
formulation is
inert, free of leachable impurities, stable on storage, sterile, and
biodegradable. In yet another
embodiment, a controlled or sustained release system can be placed in
proximity of the
prophylactic or therapeutic target, thus requiring only a fraction of the
systemic dose (see, e.g.,
Goodson, in Medical Applications of Controlled Release, supra, vol. 2, pp. 115-
138 (1984)).
Controlled release systems are discussed in the review by Langer (1990,
Science
249:1527-1533). Any technique known to one of skill in the art can be used to
produce sustained
release formulations comprising one or more therapeutic agents provided
herein. See, e.g., U. S.
Pat. No. 4,526, 938, PCT publication WO 91/05548, PCT publication WO 96/20698,
Ning et al.,
1996, "Intratumoral Radioimmunotheraphy of a Human Colon Cancer Xenograft
Using a
Sustained-Release Gel," Radiotherapy &Oncology 39:179-189, Song et al., 1995,
"Antibody
Mediated Lung Targeting of Long- Circulating Emulsions," PDA Journal of
Pharmaceutical
Science &Technology 50:372-397, Cleek et al., 1997, "Biodegradable Polymeric
Carriers for a
bFGF Antibody for Cardiovascular Application," Pro. Intl. Symp. Control. Rel.
Bioact. Mater.
24:853-854, and Lam et al., 1997, "Microencapsulation of Recombinant Humanized
Monoclonal
Antibody for Local Delivery," Proc. Infl. Symp. Control Rel. Bioact. Mater.
24:759- 760.
In a specific embodiment, where the composition is a nucleic acid encoding a
prophylactic or therapeutic agent, the nucleic acid can be administered in
vivo to promote
expression of its encoded prophylactic or therapeutic agent, by constructing
it as part of an
116
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
appropriate nucleic acid expression vector and administering it so that it
becomes intracellular,
e.g., by use of a retroviral vector (see U. S. Pat. No. 4,980,286), or by
direct injection, or by use
of microparticle bombardment (e.g., a gene gun; Biolistic, Dupont), or coating
with lipids or cell-
surface receptors or transfecting agents, or by administering it in linkage to
a homeobox-like
peptide which is known to enter the nucleus (see, e.g., Joliot et al., 1991,
Proc. Natl. Acad. Sci.
USA 88:1864-1868). Alternatively, a nucleic acid can be introduced
intracellularly and
incorporated within host cell DNA for expression by homologous recombination.
A pharmaceutical composition provided herein is formulated to be compatible
with its
intended route of administration. Examples of routes of administration
include, but are not
limited to, parenteral, e.g., intravenous, intradermal, subcutaneous, oral,
intranasal (e.g.,
inhalation), transdermal (e.g., topical), transmucosal, and rectal
administration. In a specific
embodiment, the composition is formulated in accordance with routine
procedures as a
pharmaceutical composition adapted for intravenous, subcutaneous,
intramuscular, oral,
intranasal, or topical administration to human beings. Typically, compositions
for intravenous
administration are solutions in sterile isotonic aqueous buffer. Where
necessary, the composition
may also include a solubilizing agent and a local anesthetic such as
lignocamne to ease pain at the
site of the injection.
If the compositions provided herein are to be administered topically, the
compositions
can be formulated in the form of an ointment, cream, transdermal patch,
lotion, gel, shampoo,
spray, aerosol, solution, emulsion, or other form well-known to one of skill
in the art. See, e.g.,
Remington's Pharmaceutical Sciences and Introduction to Pharmaceutical Dosage
Forms, 19th
ed., Mack Pub. Co., Easton, Pa. (1995). In an embodiment, for non- sprayable
topical dosage
forms, viscous to semi-solid or solid forms comprising a carrier or one or
more excipients
compatible with topical application and having a dynamic viscosity greater
than water are
employed. Suitable formulations include, without limitation, solutions,
suspensions, emulsions,
creams, ointments, powders, liniments, salves, and the like, which are, if
desired, sterilized or
mixed with auxiliary agents (e.g., preservatives, stabilizers, wetting agents,
buffers, or salts) for
influencing various properties, such as, for example, osmotic pressure. Other
suitable topical
dosage forms include sprayable aerosol preparations wherein the active
ingredient, in an
embodiment, in combination with a solid or liquid inert carrier, is packaged
in a mixture with a
pressurized volatile (e.g., a gaseous propellant, such as freon) or in a
squeeze bottle. Moisturizers
or humectants can also be added to pharmaceutical compositions and dosage
forms if desired.
Examples of such additional ingredients are well-known in the art.
117
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
If the method provided herein comprises intranasal administration of a
composition, the
composition can be formulated in an aerosol form, spray, mist or in the form
of drops. In
particular, prophylactic or therapeutic agents can be conveniently delivered
in the form of an
aerosol spray presentation from pressurized packs or a nebuliser, with the use
of a suitable
propellant (e.g., dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane,
carbon dioxide or other suitable gas). In the case of a pressurized aerosol
the dosage unit may be
determined by providing a valve to deliver a metered amount. Capsules and
cartridges (composed
of, e.g., gelatin) for use in an inhaler or insufflator may be formulated
containing a powder mix of
the compound and a suitable powder base such as lactose or starch.
If the method comprises oral administration, compositions can be formulated
orally in the
form of tablets, capsules, cachets, gelcaps, solutions, suspensions, and the
like. Tablets or
capsules can be prepared by conventional means with pharmaceutically
acceptable excipients
such as binding agents (e.g., pregelatinised maize starch,
polyvinylpyrrolidone, or hydroxypropyl
methylcellulose); fillers (e.g., lactose, microcrystalline cellulose, or
calcium hydrogen phosphate)
; lubricants (e.g., magnesium stearate, talc, or silica); disintegrants (e.g.,
potato starch or sodium
starch glycolate) ; or wetting agents (e.g., sodium lauryl sulphate). The
tablets may be coated by
methods well-known in the art. Liquid preparations for oral administration may
take the form of,
but not limited to, solutions, syrups or suspensions, or they may be presented
as a dry product for
constitution with water or other suitable vehicle before use. Such liquid
preparations may be
prepared by conventional means with pharmaceutically acceptable additives such
as suspending
agents (e.g., sorbitol syrup, cellulose derivatives, or hydrogenated edible
fats); emulsifying agents
(e.g., lecithin or acacia); non-aqueous vehicles (e.g., almond oil, oily
esters, ethyl alcohol, or
fractionated vegetable oils); and preservatives (e.g., methyl or propyl-p-
hydroxybenzoates or
sorbic acid). The preparations may also contain buffer salts, flavoring,
coloring, and sweetening
agents as appropriate. Preparations for oral administration may be suitably
formulated for slow
release, controlled release, or sustained release of a prophylactic or
therapeutic agent(s).
The method provided herein may comprise pulmonary administration, e.g., by use
of an
inhaler or nebulizer, of a composition formulated with an aerosolizing agent.
See, e.g., U.S. Pat.
Nos. 6,019,968; 5,985,320; 5,985,309; 5,934,272; 5,874,064; 5,855,913;
5,290,540; and
4,880,078; and PCT Publication Nos. WO 92/19244; WO 97/32572; WO 97/44013; WO
98/31346; and WO 99/66903. In a specific embodiment, a binding protein,
combination therapy,
and/or composition provided herein is administered using Alkermes AIR
pulmonary drug
delivery technology (Alkermes, Inc., Cambridge, Mass.).
118
WO 2012/027570
CA 02809433 2013-02-22
PCT/US2011/049147
The method provided herein may comprise administration of a composition
formulated
= for parenteral administration by injection (e. g., by bolus
injection or continuous infusion).
Formulations for injection may be presented in unit dosage form (e.g., in
ampoules or in multi-
dose containers) with an added preservative. The compositions may take such
forms as
suspensions, solutions or emulsions in oily or aqueous vehicles, and may
contain formulatory
agents such as suspending, stabilizing and/or dispersing agents.
Alternatively, the active
ingredient may be in powder form for constitution with a suitable vehicle
(e.g., sterile pyrogen-
free water) before use.
The methods may additionally comprise of administration of compositions
formulated as
depot preparations. Such long acting formulations may be administered by
implantation (e.g.,
subcutaneously or intramuscularly) or by intramuscular injection. Thus, for
example, the
compositions may be formulated with suitable polymeric or hydrophobic
materials (e.g., as an
emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble
derivatives (e.g., as
a sparingly soluble salt).In some embodiments, the methods encompass
administration of compositions
formulated as neutral or salt forms. Pharmaceutically acceptable salts include
those formed with
anions such as those derived from hydrochloric, phosphoric, acetic, oxalic,
tartaric acids, etc.,
and those formed with cations such as those derived from sodium, potassium,
ammonium,
calcium, ferric hydroxides, isopropylamine, triethylamine, 2- ethylamino
ethanol, histidine,
procaine, etc.
Generally, the ingredients of compositions are supplied either separately or
mixed
together in unit dosage form, for example, as a dry lyophilized powder or
water free concentrate
in a hermetically sealed container such as an ampoule or sachette indicating
the quantity of active
agent. Where the mode of administration is infusion, composition can be
dispensed with an
infusion bottle containing sterile pharmaceutical grade water or saline. Where
the mode of
administration is by injection, an ampoule of sterile water for injection or
saline can be provided
so that the ingredients may be mixed prior to administration.
In some embodiments, one or more of the prophylactic or therapeutic agents, or
pharmaceutical compositions provided herein is packaged in a hermetically
sealed container such
as an ampoule or sachette indicating the quantity of the agent. In one
embodiment, one or more of
the prophylactic or therapeutic agents, or pharmaceutical compositions
provided herein is
supplied as a dry sterilized lyophilized powder or water free concentrate in a
hermetically sealed
container and can be reconstituted (e.g., with water or saline) to the
appropriate concentration for
119
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
administration to a subject. In an embodiment, one or more of the prophylactic
or therapeutic
agents or pharmaceutical compositions provided herein is supplied as a dry
sterile lyophilized
powder in a hermetically sealed container at a unit dosage of at least 5 mg,
at least 10 mg, at least
15 mg, at least 25 mg, at least 35 mg, at least 45 mg, at least 50 mg, at
least 75 mg, or at least 100
mg. The lyophilized prophylactic or therapeutic agents or pharmaceutical
compositions provided
herein should be stored at between 2 C. and 8 C. in its original container
and the prophylactic
or therapeutic agents, or pharmaceutical compositions provided herein should
be administered
within 1 week, e.g., within 5 days, within 72 hours, within 48 hours, within
24 hours, within 12
hours, within 6 hours, within 5 hours, within 3 hours, or within 1 hour after
being reconstituted.
In an alternative embodiment, one or more of the prophylactic or therapeutic
agents or
pharmaceutical compositions provided herein are supplied in liquid form in a
hermetically sealed
container indicating the quantity and concentration of the agent. In an
embodiment, the liquid
form of the administered composition is supplied in a hermetically sealed
container at least 0.25
mg/ml, at least 0.5 mg/ml, at least 1 mg/ml, at least 2.5 mg/ml, at least 5
mg/ml, at least 8 mg/ml,
at least 10 mg/ml, at least 15 mg/kg, at least 25 mg/ml, at least 50 mg/ml, at
least 75 mg/ml or at
least 100 mg/ml. The liquid form should be stored at between 2 C. and 8 C.
in its original
container.
The binding proteins provided herein can be incorporated into a pharmaceutical
composition suitable for parenteral administration. In an embodiment, the
binding protein or
antigen-binding portions will be prepared as an injectable solution containing
0.1-250 mg/ml
binding protein. The injectable solution can be composed of either a liquid or
lyophilized dosage
form in a flint or amber vial, ampule or pre-filled syringe. The buffer can be
L-histidine (1-50
mM), optimally 5-10mM, at pH 5.0 to 7.0 (optimally pH 6.0). Other suitable
buffers include but
are not limited to, sodium succinate, sodium citrate, sodium phosphate or
potassium phosphate.
Sodium chloride can be used to modify the toxicity of the solution at a
concentration of 0-300
mM (optimally 150 mM for a liquid dosage form). Cryoprotectants can be
included for a
lyophilized dosage form, principally 0-10% sucrose (optimally 0.5-1.0%). Other
suitable
cryoprotectants include trehalose and lactose. Bulking agents can be included
for a lyophilized
dosage form, principally 1-10% mannitol (optimally 2-4%). Stabilizers can be
used in both liquid
and lyophilized dosage forms, principally 1-50 mM L-Methionine (optimally 5-10
mM). Other
suitable bulking agents include glycine, arginine, can be included as 0-0.05%
polysorbate-80
(optimally 0.005-0.01%). Additional surfactants include but are not limited to
polysorbate 20 and
BRIJ surfactants. In some embodiments, the pharmaceutical composition
comprising the binding
proteins provided herein is prepared as an injectable solution for parenteral
administration, can
further comprise an agent useful as an adjuvant, such as those used to
increase the absorption, or
120
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
dispersion of a therapeutic protein (e.g., antibody). A particularly useful
adjuvant is
hyaluronidase, such as Hylenex (recombinant human hyaluronidase). Addition of
hyaluronidase
in the injectable solution improves human bioavailability following parenteral
administration,
particularly subcutaneous administration. It also allows for greater injection
site volumes (i.e.
greater than 1 ml) with less pain and discomfort, and minimum incidence of
injection site
reactions. (see W02004078140, and US2006104968).
The compositions provided herein may be in a variety of forms. These include,
for
example, liquid, semi-solid and solid dosage forms, such as liquid solutions
(e.g., injectable and
infusible solutions), dispersions or suspensions, tablets, pills, powders,
liposomes and
suppositories. The form chosen depends on the intended mode of administration
and therapeutic
application. Typical compositions are in the form of injectable or infusible
solutions, such as
compositions similar to those used for passive immunization of humans with
other antibodies.
The chosen mode of administration is parenteral (e.g., intravenous,
subcutaneous, intraperitoneal,
intramuscular). In an embodiment, the binding protein is administered by
intravenous infusion or
injection. In another embodiment, the binding protein is administered by
intramuscular or
subcutaneous injection.
Therapeutic compositions typically must be sterile and stable under the
conditions of
manufacture and storage. The composition can be formulated as a solution,
microemulsion,
dispersion, liposome, or other ordered structure suitable to high drug
concentration. Sterile
injectable solutions can be prepared by incorporating the active compound
(i.e., antibody or
antibody portion) in the required amount in an appropriate solvent with one or
a combination of
ingredients enumerated herein, as required, followed by filtered
sterilization. Generally, dispersions
are prepared by incorporating the active compound into a sterile vehicle that
contains a basic
dispersion medium and the required other ingredients from those enumerated
herein. In the case of
sterile, lyophilized powders for the preparation of sterile injectable
solutions, the methods of
preparation are vacuum drying and spray-drying that yields a powder of the
active ingredient plus
any additional desired ingredient from a previously sterile-filtered solution
thereof. The proper
fluidity of a solution can be maintained, for example, by the use of a coating
such as lecithin, by the
maintenance of the required particle size in the case of dispersion and by the
use of surfactants.
Prolonged absorption of injectable compositions can be brought about by
including, in the
composition, an agent that delays absorption, for example, monostearate salts
and gelatin.
The binding proteins provided herein can be administered by a variety of
methods known
in the art, although for many therapeutic applications, in an embodiment, the
route/mode of
administration is subcutaneous injection, intravenous injection or infusion.
As will be appreciated
121
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
by the skilled artisan, the route and/or mode of administration will vary
depending upon the desired
results. In certain embodiments, the active compound may be prepared with a
carrier that will
protect the compound against rapid release, such as a controlled release
formulation, including
implants, transdermal patches, and microencapsulated delivery systems.
Biodegradable,
biocompatible polymers can be used, such as ethylene vinyl acetate,
polyanhydrides, polyglycolic
acid, collagen, polyorthoesters, and polylactic acid. Many methods for the
preparation of such
formulations are patented or generally known to those skilled in the art. See,
e.g., Sustained and
Controlled Release Drug Delivery Systems, J.R. Robinson, ed., Marcel Dekker,
Inc., New York,
1978.
In certain embodiments, a binding protein provided herein may be orally
administered,
for example, with an inert diluent or an assimilable edible carrier. The
compound (and other
ingredients, if desired) may also be enclosed in a hard or soft shell gelatin
capsule, compressed
into tablets, or incorporated directly into the subject's diet. For oral
therapeutic administration,
the compounds may be incorporated with excipients and used in the form of
ingestible tablets,
buccal tablets, troches, capsules, elixirs, suspensions, syrups, wafers, and
the like. To administer
a compound provided herein by other than parenteral administration, it may be
necessary to coat
the compound with, or co-administer the compound with, a material to prevent
its inactivation.
Supplementary active compounds can also be incorporated into the compositions.
In
certain embodiments, a binding protein provided herein is coformulated with
and/or
coadministered with one or more additional therapeutic agents that are useful
for treating
disorders with binding protein provided herein. For example, a binding protein
provided herein
may be coformulated and/or coadministered with one or more additional
antibodies that bind
other targets (e.g., antibodies that bind other cytokines or that bind cell
surface molecules).
Furthermore, one or more binding proteins provided herein may be used in
combination with two
or more of the foregoing therapeutic agents. Such combination therapies may
advantageously
utilize lower dosages of the administered therapeutic agents, thus avoiding
possible toxicities or
complications associated with the various monotherapies.
In certain embodiments, a binding protein is linked to a half-life extending
vehicle
known in the art. Such vehicles include, but are not limited to, the Fc
domain, polyethylene
glycol, and dextran. Such vehicles are described, e.g., in U.S. Application
Serial No. 09/428,082
and published PCT Application No. WO 99/25044.
In a specific embodiment, nucleic acid sequences encoding a binding protein
provided
herein or another prophylactic or therapeutic agent are administered to treat,
prevent, manage, or
122
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
ameliorate a disorder or one or more symptoms thereof by way of gene therapy.
Gene therapy
refers to therapy performed by the administration to a subject of an expressed
or expressible
nucleic acid. In this embodiment, the nucleic acids produce their encoded
binding protein or
prophylactic or therapeutic agent that mediates a prophylactic or therapeutic
effect.
Any of the methods for gene therapy available in the art can be used. For
general reviews
of the methods of gene therapy, see Goldspiel et al., 1993, Clinical Pharmacy
12:488-505; Wu
and Wu, 1991, Biotherapy 3:87-95; Tolstoshev, 1993, Ann. Rev. Pharmacol.
Toxicol. 32:573-
596; Mulligan, Science 260:926- 932 (1993); and Morgan and Anderson, 1993,
Ann. Rev.
Biochem. 62:191-217; May, 1993, TIBTECH 11(5):155-215. Methods commonly known
in the
art of recombinant DNA technology which can be used are described in Ausubel
et al. (eds.),
Current Protocols in Molecular Biology, John Wiley &Sons, NY (1993); and
Kriegler, Gene
Transfer and Expression, A Laboratory Manual, Stockton Press, NY (1990).
Detailed description
of various methods of gene therapy are disclosed in US20050042664 Al.
The binding proteins provided herein are useful in treating various diseases
wherein the
targets that are recognized by the binding proteins are detrimental. Such
diseases include, but are
not limited to, rheumatoid arthritis, osteoarthritis, juvenile chronic
arthritis, septic arthritis, Lyme
arthritis, psoriatic arthritis, reactive arthritis, spondyloarthropathy,
systemic lupus erythematosus,
Crohn's disease, ulcerative colitis, inflammatory bowel disease, insulin
dependent diabetes
mellitus, thyroiditis, asthma, allergic diseases, psoriasis, dermatitis
scleroderma, graft versus host
disease, organ transplant rejection, acute or chronic immune disease
associated with organ
transplantation, sarcoidosis, atherosclerosis, disseminated intravascular
coagulation, Kawasaki's
disease, Grave's disease, nephrotic syndrome, chronic fatigue syndrome,
Wegener's
granulomatosis, Henoch-Schoenlein purpurea, microscopic vasculitis of the
kidneys, chronic
active hepatitis, uveitis, septic shock, toxic shock syndrome, sepsis
syndrome, cachexia,
infectious diseases, parasitic diseases, acquired immunodeficiency syndrome,
acute transverse
myelitis, Huntington's chorea, Parkinson's disease, Alzheimer's disease,
stroke, primary biliary
cirrhosis, hemolytic anemia, malignancies, heart failure, myocardial
infarction, Addison's disease,
sporadic, polyglandular deficiency type I and polyglandular deficiency type
II, Schmidt's
syndrome, adult (acute) respiratory distress syndrome, alopecia, alopecia
areata, seronegative
arthopathy, arthropathy, Reiter's disease, psoriatic arthropathy, ulcerative
colitic arthropathy,
enteropathic synovitis, chlamydia, yersinia and salmonella associated
arthropathy,
spondyloarthopathy, atheromatous disease/arteriosclerosis, atopic allergy,
autoimmune bullous
disease, pemphigus vulgaris, pemphigus foliaceus, pemphigoid, linear IgA
disease, autoimmune
haemolytic anaemia, Coombs positive haemolytic anaemia, acquired pernicious
anaemia, juvenile
123
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
pernicious anaemia, myalgic encephalitis/Royal Free Disease, chronic
mucocutaneous
candidiasis, giant cell arteritis, primary sclerosing hepatitis, cryptogenic
autoimmune hepatitis,
Acquired Immunodeficiency Disease Syndrome, Acquired Immunodeficiency Related
Diseases,
Hepatitis B, Hepatitis C, common varied immunodeficiency (common variable
hypogammaglobulinaemia), dilated cardiomyopathy, female infertility, ovarian
failure, premature
ovarian failure, fibrotic lung disease, cryptogenic fibrosing alveolitis, post-
inflammatory
interstitial lung disease, interstitial pneumonitis, connective tissue disease
associated interstitial
lung disease, mixed connective tissue disease associated lung disease,
systemic sclerosis
associated interstitial lung disease, rheumatoid arthritis associated
interstitial lung disease,
systemic lupus erythematosus associated lung disease,
dermatomyositis/polymyositis associated
lung disease, Sjogren's disease associated lung disease, ankylosing
spondylitis associated lung
disease, vasculitic diffuse lung disease, haemosiderosis associated lung
disease, drug-induced
interstitial lung disease, fibrosis, radiation fibrosis, bronchiolitis
obliterans, chronic eosinophilic
pneumonia, lymphocytic infiltrative lung disease, postinfectious interstitial
lung disease, gouty
arthritis, autoimmune hepatitis, type-1 autoimmune hepatitis (classical
autoimmune or lupoid
hepatitis), type-2 autoimmune hepatitis (anti-LKM antibody hepatitis),
autoimmune mediated
hypoglycaemia, type B insulin resistance with acanthosis nigricans,
hypoparathyroidism, acute
immune disease associated with organ transplantation, chronic immune disease
associated with
organ transplantation, osteoarthrosis, primary sclerosing cholangitis,
psoriasis type 1, psoriasis
type 2, idiopathic leucopaenia, autoimmune neutropaenia, renal disease NOS,
glomerulonephritides, microscopic vasulitis of the kidneys, lyme disease,
discoid lupus
erythematosus, male infertility idiopathic or NOS, sperm autoimmunity,
multiple sclerosis (all
subtypes), sympathetic ophthalmia, pulmonary hypertension secondary to
connective tissue
disease, Goodpasture's syndrome, pulmonary manifestation of polyarteritis
nodosa, acute
rheumatic fever, rheumatoid spondylitis, Still's disease, systemic sclerosis,
Sjorgren's syndrome,
Takayasu's disease/arteritis, autoimmune thrombocytopaenia, idiopathic
thrombocytopaenia,
autoimmune thyroid disease, hyperthyroidism, goitrous autoimmune
hypothyroidism
(Hashimoto's disease), atrophic autoimmune hypothyroidism, primary myxoedema,
phacogenic
uveitis, primary vasculitis, vitiligo acute liver disease, chronic liver
diseases, alcoholic cirrhosis,
alcohol-induced liver injury, choleosatatis, idiosyncratic liver disease, Drug-
Induced hepatitis,
Non-alcoholic Steatohepatitis, allergy and asthma, group B streptococci (GBS)
infection, mental
disorders (e.g., depression and schizophrenia), Th2 Type and Thl Type mediated
diseases, acute
and chronic pain (different forms of pain), and cancers such as lung, breast,
stomach, bladder,
colon, pancreas, ovarian, prostate and rectal cancer and hematopoietic
malignancies (leukemia
and lymphoma), Abetalipoprotemia, Acrocyanosis, acute and chronic parasitic or
infectious
processes, acute leukemia, acute lymphoblastic leukemia (ALL), acute myeloid
leukemia (AML),
124
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
acute or chronic bacterial infection, acute pancreatitis, acute renal failure,
adenocarcinomas,
aerial ectopic beats, AIDS dementia complex, alcohol-induced hepatitis,
allergic conjunctivitis,
allergic contact dermatitis, allergic rhinitis, allograft rejection, alpha-l-
antitrypsin deficiency,
amyotrophic lateral sclerosis, anemia, angina pectoris, anterior horn cell
degeneration, anti cd3
therapy, antiphospholipid syndrome, anti-receptor hypersensitivity reactions,
aordic and
peripheral aneuryisms, aortic dissection, arterial hypertension,
arteriosclerosis, arteriovenous
fistula, ataxia, atrial fibrillation (sustained or paroxysmal), atrial
flutter, atrioventricular block, B
cell lymphoma, bone graft rejection, bone marrow transplant (BMT) rejection,
bundle branch
block, Burkitt's lymphoma, Burns, cardiac arrhythmias, cardiac stun syndrome,
cardiac tumors,
cardiomyopathy, cardiopulmonary bypass inflammation response, cartilage
transplant rejection,
cerebellar cortical degenerations, cerebellar disorders, chaotic or multifocal
atrial tachycardia,
chemotherapy associated disorders, chromic myelocytic leukemia (CML), chronic
alcoholism,
chronic inflammatory pathologies, chronic lymphocytic leukemia (CLL), chronic
obstructive
pulmonary disease (COPD), chronic salicylate intoxication, colorectal
carcinoma, congestive
heart failure, conjunctivitis, contact dermatitis, cor pulmonale, coronary
artery disease,
Creutzfeldt-Jakob disease, culture negative sepsis, cystic fibrosis, cytokine
therapy associated
disorders, Dementia pugilistica, demyelinating diseases, dengue hemorrhagic
fever, dermatitis,
dermatologic conditions, diabetes, diabetes mellitus, diabetic ateriosclerotic
disease, Diffuse
Lewy body disease, dilated congestive cardiomyopathy, disorders of the basal
ganglia, Down's
Syndrome in middle age, drug- induced movement disorders induced by drugs
which block CNS
dopamine receptors, drug sensitivity, eczema, encephalomyelitis, endocarditis,
endocrinopathy,
epiglottitis, epstein-barr virus infection, erythromelalgia, extrapyramidal
and cerebellar disorders,
familial hematophagocytic lymphohistiocytosis, fetal thymus implant rejection,
Friedreich's
ataxia, functional peripheral arterial disorders, fungal sepsis, gas gangrene,
gastric ulcer,
glomerular nephritis, graft rejection of any organ or tissue, gram negative
sepsis, gram positive
sepsis, granulomas due to intracellular organisms, hairy cell leukemia,
Hallerrorden-Spatz
disease, hashimoto's thyroiditis, hay fever, heart transplant rejection,
hemachromatosis,
hemodialysis, hemolytic uremic syndrome/thrombolytic thrombocytopenic purpura,
hemorrhage,
hepatitis (A), His bundle arrythmias, HIV infection/HIV neuropathy, Hodgkin's
disease,
hyperkinetic movement disorders, hypersensitity reactions, hypersensitivity
pneumonitis,
hypertension, hypokinetic movement disorders, hypothalamic-pituitary-adrenal
axis evaluation,
idiopathic Addison's disease, idiopathic pulmonary fibrosis, antibody mediated
cytotoxicity,
Asthenia, infantile spinal muscular atrophy, inflammation of the aorta,
influenza a, ionizing
radiation exposure, iridocyclitis/uveitis/optic neuritis, ischemia-
reperfusion injury, ischemic
stroke, juvenile rheumatoid arthritis, juvenile spinal muscular atrophy,
Kaposi's sarcoma, kidney
transplant rejection, legionella, leishmaniasis, leprosy, lesions of the
corticospinal system,
125
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
lipedema, liver transplant rejection, lymphederma, malaria, malignamt
Lymphoma, malignant
histiocytosis, malignant melanoma, meningitis, meningococcemia,
metabolic/idiopathic, migraine
headache, mitochondrial multi.system disorder, mixed connective tissue
disease, monoclonal
gammopathy, multiple myeloma, multiple systems degenerations (Mencel Dejerine-
Thomas Shi-
Drager and Machado-Joseph), myasthenia gravis, mycobacterium avium
intracellulare,
mycobacterium tuberculosis, myelodyplastic syndrome, myocardial infarction,
myocardial
ischemic disorders, nasopharyngeal carcinoma, neonatal chronic lung disease,
nephritis,
nephrosis, neurodegenerative diseases, neurogenic I muscular atrophies ,
neutropenic fever, non-
hodgkins lymphoma, occlusion of the abdominal aorta and its branches,
occulsive arterial
disorders, okt3 therapy, orchitis/epidydimitis, orchitis/vasectomy reversal
procedures,
organomegaly, osteoporosis, pancreas transplant rejection, pancreatic
carcinoma, paraneoplastic
syndrome/hypercalcemia of malignancy, parathyroid transplant rejection, pelvic
inflammatory
disease, perennial rhinitis, pericardial disease, peripheral atherlosclerotic
disease, peripheral
vascular disorders, peritonitis, pernicious anemia, pneumocystis carinii
pneumonia, pneumonia,
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal
gammopathy,
and skin changes syndrome), post perfusion syndrome, post pump syndrome, post-
MI cardiotomy
syndrome, preeclampsia, Progressive supranucleo Palsy, primary pulmonary
hypertension,
radiation therapy, Raynaud's phenomenon and disease, Raynoud's disease,
Refsum's disease,
regular narrow QRS tachycardia, renovascular hypertension, reperfusion injury,
restrictive
cardiomyopathy, sarcomas, scleroderma, senile chorea, Senile Dementia of Lewy
body type,
seronegative arthropathies, shock, sickle cell anemia, skin allograft
rejection, skin changes
syndrome, small bowel transplant rejection, solid tumors, specific arrythmias,
spinal ataxia,
spinocerebellar degenerations, streptococcal myositis, structural lesions of
the cerebellum,
Subacute sclerosing panencephalitis, Syncope, syphilis of the cardiovascular
system, systemic
anaphalaxis, systemic inflammatory response syndrome, systemic onset juvenile
rheumatoid
arthritis, T-cell or FAB ALL, Telangiectasia, thromboangitis obliterans,
thrombocytopenia,
toxicity, transplants, trauma/hemorrhage, type III hypersensitivity reactions,
type IV
hypersensitivity, unstable angina, uremia, urosepsis, urticaria, valvular
heart diseases, varicose
veins, vasculitis, venous diseases, venous thrombosis, ventricular
fibrillation, viral and fungal
infections, vital encephalitis/aseptic meningitis, vital-associated
hemaphagocytic syndrome,
Wernicke- Korsakoff syndrome, Wilson's disease, xenograft rejection of any
organ or tissue. (see
Perin et al. PCT publication No. W02002097048A2, Leonard et al., PCT
publication No.
W09524918 Al, and Salfeld et al., PCT publication No. W000/56772A1).
The DVD-Igs provided herein may also treat one or more of the following
diseases:
Acute coronary syndromes, Acute Idiopathic Polyneuritis, Acute Inflammatory
Demyelinating
126
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Polyradiculoneuropathy, Acute ischemia, Adult Still's Disease, Alopecia
areata, Anaphylaxis,
Anti-Phospholipid Antibody Syndrome, Aplastic anemia, Arteriosclerosis, Atopic
eczema,
Atopic dermatitis, Autoimmune dermatitis, Autoimmune disorder associated with
Streptococcus
infection, Autoimmune hearingloss, Autoimmune Lymphoproliferative Syndrome
(ALPS),
Autoimmune myocarditis, autoimmune thrombocytopenia (AITP), Blepharitis,
Bronchiectasis,
Bullous pemphigoid, Cardiovascular Disease, Catastrophic Antiphospholipid
Syndrome, Celiac
Disease, Cervical Spondylosis, Chronic ischemia, Cicatricial pemphigoid,
Clinically isolated
Syndrome (CIS) with Risk for Multiple Sclerosis, Conjunctivitis, Childhood
Onset Psychiatric
Disorder, Chronic obstructive pulmonary disease (COPD), Dacryocystitis,
dermatomyositis,
Diabetic retinopathy, Diabetes mellitus, Disk herniation, Disk prolaps, Drug
induced immune
hemolytic anemia, Endocarditis, Endometriosis, endophthalmitisõ Episcleritis,
Erythema
multiforme, erythema multiforme major, Gestational pemphigoid, GuiHain-Barre
Syndrome
(GBS), Hay Fever, Hughes Syndrome , Idiopathic Parkinson's Disease, idiopathic
interstitial
pneumonia, IgE-mediated Allergy, Immune hemolytic anemia, Inclusion Body
Myositis,
Infectious ocular inflammatory disease, Inflammatory demyelinating disease,
Inflammatory heart
disease, Inflammatory kidney disease, IPF/UIP, Iritis, Keratitis,
Keratojuntivitis sicca, Kussmaul
disease or Kussmaul-Meier Disease, Landry's Paralysis, Langerhan's Cell
Histiocytosis, Lived
reticularis, Macular Degeneration, malignancies, Microscopic Polyangiitis,
Morbus Bechterev,
Motor Neuron Disorders, Mucous membrane pemphigoid, Multiple Organ failure,
Myasthenia
Gravis, Myelodysplastic Syndrome, Myocarditis, Nerve Root Disorders,
Neuropathy, Non-A
Non-B Hepatitis, Optic Neuritis, Osteolysis, Ovarian cancer, Pauciarticular
JRA, peripheral
artery occlusive disease (PAOD), peripheral vascular disease (PVD), peripheral
artery disease
(PAD), Phlebitis, Polyarteritis nodosa (or periarteritis nodosa),
Polychondritis, Polymyalgia
Rheumatica, Poliosis, Polyarticular JRA, Polyendocrine Deficiency Syndrome,
Polymyositis,
polymyalgia rheumatica (PMR), Post-Pump Syndrome, primary parkinsonism,
prostate and rectal
cancer and hematopoietic malignancies (leukemia and lymphoma), Prostatitis,
Pure red cell
aplasia, Primary Adrenal Insufficiency, Recurrent Neuromyelitis Optica,
Restenosis, Rheumatic
heart disease, SAPHO (synovitis, acne, pustulosis, hyperostosis, and
osteitis), Scleroderma,
Secondary Amyloidosis, Shock lung, Scleritis, Sciatica, Secondary Adrenal
Insufficiency,
Silicone associated connective tissue disease, Sneddon-Wilkinson Dermatosis,
spondilitis
anicylosans, Stevens-Johnson Syndrome (SJS), Systemic inflammatory response
syndrome,
Temporal arteritis, toxoplasmic retinitis, toxic epidermal necrolysis,
Transverse myelitis, TRAPS
(Tumor Necrosis Factor Receptor, Type 1 allergic reaction, Type II Diabetes,
Urticaria, Usual
interstitial pneumonia (UIP), Vasculitis, Vernal conjunctivitis, viral
retinitis, Vogt-Koyanagi-
Harada syndrome (VKH syndrome), Wet macular degeneration, and Wound healing.
127
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
The binding proteins provided herein can be used to treat humans suffering
from
autoimmune diseases, in particular those associated with inflammation,
including, rheumatoid
arthritis, spondylitis, allergy, autoimmune diabetes, autoimmune uveitis. In
an embodiment, the
binding proteins or antigen-binding portions thereof, are used to treat
rheumatoid arthritis,
Crohn's disease, multiple sclerosis, insulin dependent diabetes mellitus and
psoriasis.
In an embodiment, diseases that can be treated or diagnosed with the
compositions and
methods provided herein include, but are not limited to, primary and
metastatic cancers,
including carcinomas of breast, colon, rectum, lung, oropharynx, hypopharynx,
esophagus,
stomach, pancreas, liver, gallbladder and bile ducts, small intestine, urinary
tract (including
kidney, bladder and urothelium), female genital tract (including cervix,
uterus, and ovaries as
well as choriocarcinoma and gestational trophoblastic disease), male genital
tract (including
prostate, seminal vesicles, testes and germ cell tumors), endocrine glands
(including the thyroid,
adrenal, and pituitary glands), and skin, as well as hemangiomas, melanomas,
sarcomas
(including those arising from bone and soft tissues as well as Kaposi's
sarcoma), tumors of the
brain, nerves, eyes, and meninges (including astrocytomas, gliomas,
glioblastomas,
retinoblastomas, neuromas, neuroblastomas, Schwannomas, and meningiomas),
solid tumors
arising from hematopoietic malignancies such as leukemias, and lymphomas (both
Hodgkin's and
non-Hodgkin's lymphomas).
In an embodiment, the binding proteins provided herein or antigen-binding
portions
thereof, are used to treat cancer or in the prevention of metastases from the
tumors described
herein either when used alone or in combination with radiotherapy and/or other
chemotherapeutic
agents.
The binding proteins provided herein, or antigen binding portions thereof, may
be
combined with agents that include but are not limited to, antineoplastic
agents, radiotherapy,
chemotherapy such as DNA alkylating agents, cisplatin, carboplatin, anti-
tubulin agents,
paclitaxel, docetaxel, taxol, doxorubicin, gemcitabine, gemzar,
anthracyclines, adriamycin,
topoisomerase I inhibitors, topoisomerase II inhibitors, 5-fluorouracil (5-
FU), leucovorin,
irinotecan, receptor tyrosine kinase inhibitors (e.g., erlotinib, gefitinib),
COX-2 inhibitors (e.g.,
celecoxib), kinase inhibitors, and siRNAs.
A binding proteins provided herein also can be administered with one or more
additional
therapeutic agents useful in the treatment of various diseases.
A binding protein provided herein can be used alone or in combination to treat
such
diseases. It should be understood that the binding proteins can be used alone
or in combination
128
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
with an additional agent, e.g., a therapeutic agent, said additional agent
being selected by the
skilled artisan for its intended purpose. For example, the additional agent
can be a therapeutic
agent art-recognized as being useful to treat the disease or condition being
treated by the binding
proteins disclosed herein. The additional agent also can be an agent that
imparts a beneficial
attribute to the therapeutic composition e.g., an agent which effects the
viscosity of the
composition.
It should further be understood that the combinations provided herein are
those
combinations useful for their intended purpose. The agents set forth below are
illustrative for
purposes and not intended to be limited. The combinations provided herein can
be the binding
proteins and at least one additional agent selected from the lists below. The
combinations can
also include more than one additional agent, e.g., two or three additional
agents if the
combination is such that the formed composition can perform its intended
function.
Combinations to treat autoimmune and inflammatory diseases are non-steroidal
anti-
inflammatory drug(s) also referred to as NSAIDS which include drugs like
ibuprofen. Other
combinations are corticosteroids including prednisolone; the well known side-
effects of steroid
use can be reduced or even eliminated by tapering the steroid dose required
when treating
patients in combination with the DVD Igs provided herein. Non-limiting
examples of therapeutic
agents for rheumatoid arthritis with which a binding protein provided herein
can be combined
include the following: cytokine suppressive anti-inflammatory drug(s)
(CSAIDs); antibodies to or
antagonists of other human cytokines or growth factors, for example, TNF, LT,
IL-1, IL-2, IL-3,
IL-4, IL-5, IL-6, IL-7, IL-8, IL-15, IL-16, IL-18, IL-21, IL-23, interferons,
EMAP-II, GM-CSF,
FGF, and PDGF. Binding proteins provided herein, or antigen binding portions
thereof, can be
combined with antibodies to cell surface molecules such as CD2, CD3, CD4, CD8,
CD25, CD28,
CD30, CD40, CD45, CD69, CD80 (B7.1), CD86 (B7.2), CD90, CTLA or their ligands
including
CD154 (gp39 or CD4OL).
Combinations of therapeutic agents may interfere at different points in the
autoimmune
and subsequent inflammatory cascade; examples include TNF antagonists like
chimeric,
humanized or human TNF antibodies, Adalimumab, (PCT Publication No. WO
97/29131), CA2
(RemicadeTm), CDP 571, and soluble p55 or p75 TNF receptors, derivatives,
thereof,
(p75TNFR1gG (EnbrelTM) or p55TNFR1gG (Lenercept), and also TNFcc converting
enzyme
(TACE) inhibitors; similarly IL-1 inhibitors (Interleukin-1 -converting enzyme
inhibitors, IL-1RA
etc.) may be effective for the same reason. Other combinations include
Interleukin 11. Yet
another combination include key players of the autoimmune response which may
act parallel to,
dependent on or in concert with IL-12 function; especially are IL-18
antagonists including IL-18
129
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
antibodies or soluble IL-18 receptors, or IL-18 binding proteins. It has been
shown that IL-12 arid
LL-18 have overlapping but distinct functions and a combination of antagonists
to both may be
most effective. Yet another combination are non-depleting anti-CD4 inhibitors.
Yet other
combinations include antagonists of the co-stimulatory pathway CD80 (B7.1) or
CD86 (B7.2)
including antibodies, soluble receptors or antagonistic ligands.
The binding proteins provided herein may also be combined with agents, such as
methotrexate, 6-MP, azathioprine sulphasalazine, mesalazine, olsalazine
chloroquinine/hydroxychloroquine, pencillamine, aurothiomalate (intramuscular
and oral),
azathioprine, cochicine, corticosteroids (oral, inhaled and local injection),
beta-2 adrenoreceptor
agonists (salbutamol, terbutaline, salmeteral), xanthines (theophylline,
aminophylline),
cromoglycate, nedocromil, ketotifen, ipratropium and oxitropium, cyclosporin,
FK506,
rapamycin, mycophenolate mofetil, leflunomide, NSAIDs, for example, ibuprofen,
corticosteroids such as prednisolone, phosphodiesterase inhibitors, adensosine
agonists,
antithrombotic agents, complement inhibitors, adrenergic agents, agents which
interfere with
signalling by proinflammatory cytokines such as TNF-a or IL-1 (e.g.,IRAK, NIK,
IKK , p38 or
MAP kinase inhibitors), IL-113 converting enzyme inhibitors, TNFa converting
enzyme (TACE)
inhibitors, T-cell signalling inhibitors such as kinase inhibitors,
metalloproteinase inhibitors,
sulfasalazine, azathioprine, 6-mercaptopurines, angiotensin converting enzyme
inhibitors, soluble
cytokine receptors and derivatives thereof (e.g.,soluble p55 or p75 TNF
receptors and the
derivatives p75TNFRIgG (Enbreirm and p55TNFRIgG (Lenercept)), sIL-1RI, sIL-
1RII, sIL-6R),
antiinflammatory cytokines (e.g.,IL-4, IL-10, IL-11, IL-13 and TGFP),
celecoxib, folic acid,
hydroxychloroquine sulfate, rofecoxib, etanercept, infliximab, naproxen,
valdecoxib,
sulfasalazine, methylprednisolone, meloxicam, methylprednisolone acetate, gold
sodium
thiomalate, aspirin, triamcinolone acetonide, propoxyphene napsylate/apap,
folate, nabumetone,
diclofenac, piroxicam, etodolac, diclofenac sodium, oxaprozin, oxycodone hcl,
hydrocodone
bitartrate/apap, diclofenac sodium/misoprostol, fentanyl, anakinra, human
recombinant, tramadol
hcl, salsalate, sulindac, cyanocobalamin/fa/pyridoxine, acetaminophen,
alendronate sodium,
prednisolone, morphine sulfate, lidocaine hydrochloride, indomethacin,
glucosamine
sulf/chondroitin, amitriptyline hcl, sulfadiazine, oxycodone
hcl/acetaminophen, olopatadine hcl,
misoprostol, naproxen sodium, omeprazole, cyclophosphamide, rituximab, IL-1
TRAP, MRA,
CTLA4-IG, IL-18 BP, anti-IL-18, Anti-IL15, BIRB-796, SC10-469, VX-702, AMG-
548, VX-
740, Roflumilast, IC-485, CDC-801, and Mesopram. Combinations include
methotrexate or
leflunomide and in moderate or severe rheumatoid arthritis cases,
cyclosporine.
130
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Nonlimiting additional agents which can also be used in combination with a
binding
protein to treat rheumatoid arthritis include, but are not limited to, the
following: non-steroidal
anti-inflammatory drug(s) (NSAlDs); cytokine suppressive anti-inflammatory
drug(s) (CSAIDs);
CDP-571/BAY-10-3356 (humanized anti-TNFa antibody; Celltech/Bayer);
eA2/infliximab
(chimeric anti-TNFa antibody; Centocor); 75 kdTNFR-IgG/etanercept (75 1(13 TNF
receptor-IgG
fusion protein; Immunex; see e.g., Arthritis & Rheumatism (1994) Vol. 37,
S295; J. Invest. Med.
(1996) Vol. 44, 235A); 55 kdTNF-IgG (55 kl) TNF receptor-IgG fusion protein;
Hoffmann-
LaRoche); IDEC-CE9.1/SB 210396 (non-depleting primatized anti-CD4 antibody;
IDEC/SmithKline; see e.g., Arthritis & Rheumatism (1995) Vol. 38, S185); DAB
486-IL-2 and/or
DAB 389-IL-2 (IL-2 fusion proteins; Seragen; see e.g., Arthritis & Rheumatism
(1993) Vol. 36,
1223); Anti-Tac (humanized anti-IL-2Ra; Protein Design Labs/Roche); IL-4 (anti-
inflammatory
cytokine; DNAX/Schering); IL-10 (SCH 52000; recombinant IL-10, anti-
inflammatory cytokine;
DNAX/Schering); IL-4; IL-10 and/or IL-4 agonists (e.g., agonist antibodies);
IL-1RA (IL-1
receptor antagonist; Synergen/Amgen); anakinra (Kineret /Amgen); TNF-bp/s-TNF
(soluble
TNF binding protein; see e.g., Arthritis & Rheumatism (1996) Vol. 39, No. 9
(supplement), S284;
Amer. J Physiol. - Heart and Circulatory Physiology (1995) Vol. 268, pp. 37-
42); R973401
(phosphodiesterase Type IV inhibitor; see e.g., Arthritis & Rheumatism (1996)
Vol. 39, No. 9
(supplement), S282); MK-966 (COX-2 Inhibitor; see e.g., Arthritis & Rheumatism
(1996) Vol.
39, No. 9 (supplement), S81); Iloprost (see e.g., Arthritis & Rheumatism
(1996) Vol. 39, No. 9
(supplement), S82); methotrexate; thalidomide (see e.g., Arthritis &
Rheumatism (1996) Vol. 39,
No. 9 (supplement), S282) and thalidomide-related drugs (e.g., Celgen);
leflunomide (anti-
inflammatory and cytokine inhibitor; see e.g., Arthritis & Rheumatism (1996)
Vol. 39, No. 9
(supplement), S131; Inflammation Research (1996) Vol. 45, pp. 103-107);
tranexamic acid
(inhibitor of plasminogen activation; see e.g., Arthritis & Rheumatism (1996)
Vol. 39, No. 9
(supplement), S284); T-614 (cytokine inhibitor; see e.g., Arthritis &
Rheumatism (1996) Vol. 39,
No. 9 (supplement), S282); prostaglandin El (see e.g., Arthritis & Rheumatism
(1996) Vol. 39,
No. 9 (supplement), S282); Tenidap (non-steroidal anti-inflammatory drug; see
e.g., Arthritis &
Rheumatism (1996) Vol. 39, No. 9 (supplement), S280); Naproxen (non-steroidal
anti-
inflammatory drug; see e.g., Neuro Report (1996) Vol. 7, pp. 1209-1213);
Meloxicam (non-
steroidal anti-inflammatory drug); Ibuprofen (non-steroidal anti-inflammatory
drug); Piroxicam
(non-steroidal anti-inflammatory drug); Diclofenac (non-steroidal anti-
inflammatory drug);
Indomethacin (non-steroidal anti-inflammatory drug); Sulfasalazine (see e.g.,
Arthritis &
Rheumatism (1996) Vol. 39, No. 9 (supplement), S281); Azathioprine (see e.g.,
Arthritis &
Rheumatism (1996) Vol. 39, No. 9 (supplement), S281); ICE inhibitor (inhibitor
of the enzyme
interleukin-113 converting enzyme); zap-70 and/or lek inhibitor (inhibitor of
the tyrosine kinase
zap-70 or lck); VEGF inhibitor and/or VEGF-R inhibitor (inhibitors of vascular
endothelial cell
131
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
growth factor or vascular endothelial cell growth factor receptor; inhibitors
of angiogenesis);
corticosteroid anti-inflammatory drugs (e.g., SB203580); TNF-convertase
inhibitors; anti-IL-12
antibodies; anti-IL-18 antibodies; interleukin-11 (see e.g., Arthritis &
Rheumatism (1996) Vol.
39, No. 9 (supplement), S296); interleukin-13 (see e.g., Arthritis &
Rheumatism (1996) Vol. 39,
No. 9 (supplement), S308); interleukin -17 inhibitors (see e.g., Arthritis &
Rheumatism (1996)
Vol. 39, No. 9 (supplement), SI20); gold; penicillamine; chloroquine;
chlorambucil;
hydroxychloroquine; cyclosporine; cyclophosphamide; total lymphoid
irradiation; anti-thymocyte
globulin; anti-CD4 antibodies; CD5-toxins; orally-administered peptides and
collagen; lobenzarit
disodium; Cytokine Regulating Agents (CRAs) HP228 and HP466 (Houghten
Pharmaceuticals,
Inc.); ICAM-1 antisense phosphorothioate oligo-deoxynucleotides (ISIS 2302;
Isis
Pharmaceuticals, Inc.); soluble complement receptor 1 (TP10; T Cell Sciences,
Inc.); prednisone;
orgotein; glycosaminoglycan polysulphate; minocycline; anti-IL2R antibodies;
marine and
botanical lipids (fish and plant seed fatty acids; see e.g., DeLuca et al.
(1995) Rheum. Dis.
North Am. 21:759-777); auranofin; phenylbutazone; meclofenamic acid;
flufenamic acid;
intravenous immune globulin; zileuton; azaribine; mycophenolic acid (RS-
61443); tacrolimus
(FK-506); sirolimus (rapamycin); amiprilose (therafectin); cladribine (2-
chlorodeoxyadenosine);
methotrexate; bel-2 inhibitors (see Bruncko, Milan et al., Journal of
Medicinal Chemistry (2007),
50(4), 641-662); antivirals and immune modulating agents.
In one embodiment, the binding protein or antigen-binding portion thereof, is
administered in combination with one of the following agents for the treatment
of rheumatoid
arthritis: small molecule inhibitor of KDR, small molecule inhibitor of Tie-2;
methotrexate;
prednisone; celecoxib; folic acid; hydroxychloroquine sulfate; rofecoxib;
etanercept; infliximab;
leflunomide; naproxen; valdecoxib; sulfasalazine; methylprednisolone;
ibuprofen; meloxicam;
methylprednisolone acetate; gold sodium thiomalate; aspirin; azathioprine;
triamcinolone
acetonide; propxyphene napsylate/apap; folate; nabumetone; diclofenac;
piroxicam; etodolac;
diclofenac sodium; oxaprozin; oxycodone hcl; hydrocodone bitartrate/apap;
diclofenac
sodium/misoprostol; fentanyl; anakinra, human recombinant; tramadol hcl;
salsalate; sulindac;
cyanocobalamin/fa/pyridoxine; acetaminophen; alendronate sodium; prednisolone;
morphine
sulfate; lidocaine hydrochloride; indomethacin; glucosamine
sulfate/chondroitin; cyclosporine;
amitriptyline hcl; sulfadiazine; oxycodone hcl/acetaminophen; olopatadine hcl;
misoprostol;
naproxen sodium; omeprazole; mycophenolate mofetil; cyclophosphamide;
rituximab; IL-1
TRAP; MRA; CTLA4-IG; IL-18 BP; IL-12/23; anti-IL 18; anti-IL 15; BIRB-796;
SC10-469; VX-
702; AMG-548; VX-740; Roflumilast; IC-485; CDC-801; and mesopram.
132
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Non-limiting examples of therapeutic agents for inflammatory bowel disease
with which
a binding protein provided herein can be combined include the following:
budenoside; epidermal
growth factor; corticosteroids; cyclosporin, sulfasalazine; aminosalicylates;
6-mercaptopurine;
azathioprine; metronidazole; lipoxygenase inhibitors; mesalamine; olsalazine;
balsalazide;
antioxidants; thromboxane inhibitors; IL-1 receptor antagonists; anti-IL-l3
mAbs; anti-IL-6
mAbs; growth factors; elastase inhibitors; pyridinyl-imidazole compounds;
antibodies to or
antagonists of other human cytokines or growth factors, for example, TNF, LT,
IL-1, IL-2, IL-6,
IL-7, IL-8, IL-15, IL-16, IL-17, IL-18, EMAP-II, GM-CSF, FGF, and PDGF.
Binding proteins
provided herein, or antigen binding portions thereof, can be combined with
antibodies to cell
surface molecules such as CD2, CD3, CD4, CD8, CD25, CD28, CD30, CD40, CD45,
CD69,
CD90 or their ligands. The binding proteins provided herein, or antigen
binding portions thereof,
may also be combined with agents, such as methotrexate, cyclosporin, FK506,
rapamycin,
mycophenolate mofetil, leflunomide, NSAIDs, for example, ibuprofen,
corticosteroids such as
prednisolone, phosphodiesterase inhibitors, adenosine agonists, antithrombotic
agents,
complement inhibitors, adrenergic agents, agents which interfere with
signalling by
proinflammatory cytokines such as TNFoc or IL-1 (e.g., IRAK, NIK, IKK, p38 or
MAP kinase
inhibitors), IL-l3 converting enzyme inhibitors, TNFcc converting enzyme
inhibitors, T-cell
signalling inhibitors such as kinase inhibitors, metalloproteinase inhibitors,
sulfasalazine,
azathioprine, 6-mercaptopurines, angiotensin converting enzyme inhibitors,
soluble cytokine
receptors and derivatives thereof (e.g.,soluble p55 or p75 TNF receptors, sIL-
1RI, sIL-IRII, sIL-
6R) and antiinflammatory cytokines (e.g., IL-4, IL-10, IL-11, IL-13 and TGFf3)
and bc1-2
inhibitors.
Examples of therapeutic agents for Crohn's disease in which a binding protein
can be
combined include the following: TNF antagonists, for example, anti-TNF
antibodies,
Adalimumab (PCT Publication No. WO 97/29131; HUMIRA), CA2 (REMICADE), CDP 571,
TNFR-Ig constructs, (p75TNFRIgG (ENBREL) and p55TNFRIgG (LENERCEPT))
inhibitors
and PDE4 inhibitors. Binding proteins provided herein, or antigen binding
portions thereof, can
be combined with corticosteroids, for example, budenoside and dexamethasone.
Binding proteins
provided herein or antigen binding portions thereof, may also be combined with
agents such as
sulfasalazine, 5-aminosalicylic acid and olsalazine, and agents which
interfere with synthesis or
action of proinflammatory cytokines such as IL-1, for example, IL-113
converting enzyme
inhibitors and IL-lra. Binding proteins provided herein or antigen binding
portion thereof may
also be used with T cell signaling inhibitors, for example, tyrosine kinase
inhibitors 6-
mercaptopurines. Binding proteins provided herein, or antigen binding portions
thereof, can be
combined with IL-11. Binding proteins provided herein, or antigen binding
portions thereof, can
133
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
be combined with mesalamine, prednisone, azathioprine, mercaptopurine,
infliximab,
methylprednisolone sodium succinate, diphenoxylate/atrop sulfate, loperamide
hydrochloride,
methotrexate, omeprazole, folate, ciprofloxacin/dextrose-water, hydrocodone
bitartrate/apap,
tetracycline hydrochloride, fluocinonide, metronidazole, thimerosal/boric
acid,
cholestyramine/sucrose, ciprofloxacin hydrochloride, hyoscyamine sulfate,
meperidine
hydrochloride, midazolam hydrochloride, oxycodone hcl/acetaminophen,
promethazine
hydrochloride, sodium phosphate, sulfamethoxazole/trimethoprim, celecoxib,
polycarbophil,
propoxyphene napsylate, hydrocortisone, multivitamins, balsalazide disodium,
codeine
phosphate/apap, colesevelam hcl, cyanocobalamin, folic acid, levofloxacin,
methylprednisolone,
natalizumab and interferon-gamma
Non-limiting examples of therapeutic agents for multiple sclerosis with which
binding
proteins provided herein can be combined include the following:
corticosteroids; prednisolone;
methylprednisolone; azathioprine; cyclophosphamide; cyclosporine;
methotrexate; 4-
aminopyridine; tizanidine; interferon-13la (AVONEX; Biogen); interferon-13 lb
(BETASERON;
Chiron/Berlex); interferon a-n3) (Interferon Sciences/Fujimoto), interferon-a
(Alfa
Wassermanna&J), interferon 131A-IF (Serono/Inhale Therapeutics), Peginterferon
a 2b
(Enzon/Schering-Plough), Copolymer 1 (Cop-1; COPAXONE; Teva Pharmaceutical
Industries,
Inc.); hyperbaric oxygen; intravenous immunoglobulin; clabribine; antibodies
to or antagonists of
other human cytokines or growth factors and their receptors, for example, TNF,
LT, IL-1, IL-2,
IL-6, IL-7, IL-8, IL-23, IL-15, IL-16, IL-18, EMAP-II, GM-CSF, FGF, and PDGF.
Binding
proteins provided herein can be combined with antibodies to cell surface
molecules such as CD2,
CD3, CD4, CD8, CD19, CD20, CD25, CD28, CD30, CD40, CD45, CD69, CD80, CD86,
CD90
or their ligands. Binding proteins provided herein, may also be combined with
agents, such as
methotrexate, cyclosporine, FK506, rapamycin, mycophenolate mofetil,
leflunomide, NSAIDs,
for example, ibuprofen, corticosteroids such as prednisolone,
phosphodiesterase inhibitors,
adensosine agonists, antithrombotic agents, complement inhibitors, adrenergic
agents, agents
which interfere with signalling by proinflammatory cytokines such as TNFa or
IL-1 (e.g., IRAK,
NIK, IKK, p38 or MAP kinase inhibitors), IL-10 converting enzyme inhibitors,
TACE inhibitors,
T-cell signaling inhibitors such as kinase inhibitors, metalloproteinase
inhibitors, sulfasalazine,
azathioprine, 6-mercaptopurines, angiotensin converting enzyme inhibitors,
soluble cytokine
receptors and derivatives thereof (e.g., soluble p55 or p75 TNF receptors, sIL-
1RI, sIL-1RII, sIL-
6R), antiinflammatory cytokines (e.g., IL-4, IL-10, IL-13 and TGFP) and bc1-2
inhibitors.
Examples of therapeutic agents for multiple sclerosis in which binding
proteins provided
herein can be combined tinclude interferon-P, for example, IFNI3la and IFNP
lb; copaxone,
134
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
corticosteroids, caspase inhibitors, for example inhibitors of caspase-1, IL-1
inhibitors, TNF
inhibitors, and antibodies to CD40 ligand and CD80.
The binding proteins provided herein, may also be combined with agents, such
as
alemtuzumab, dronabinol, Unimed, daclizumab, mitoxantrone, xaliproden
hydrochloride,
fampridine, glatiramer acetate, natalizumab, sinnabidol, a-immunokine NNS03,
ABR-215062,
AnergiX.MS, chemokine receptor antagonists, BBR-2778, calagualine, CPI-1189,
LEM
(liposome encapsulated mitoxantrone), THC.CBD (cannabinoid agonist) MBP-8298,
mesopram
(PDE4 inhibitor), MNA-715, anti-IL-6 receptor antibody, neurovax, pirfenidone
allotrap 1258
(RDP-1258), sTNF-R1, talampanel, teriflunomide,TGF-beta2, tiplimotide, VLA-4
antagonists
(for example, TR-14035, VLA4 Ultrahaler, Antegran-ELAN/Biogen), interferon
gamma
antagonists, 1L-4 agonists.
Non-limiting examples of therapeutic agents for Angina with which binding
proteins
provided herein can be combined include the following: aspirin, nitroglycerin,
isosorbide
mononitrate, metoprolol succinate, atenolol, metoprolol tartrate, amlodipine
besylate, diltiazem
hydrochloride, isosorbide dinitrate, clopidogrel bisulfate, nifedipine,
atorvastatin calcium,
potassium chloride, furosemide, simvastatin, verapamil hcl, digoxin,
propranolol hydrochloride,
carvedilol, lisinopril, spironolactone, hydrochlorothiazide, enalapril
maleate, nadolol, ramipril,
enoxaparin sodium, heparin sodium, valsartan, sotalol hydrochloride,
fenofibrate, ezetimibe,
bumetanide, losartan potassium, lisinopril/hydrochlorothiazide, felodipine,
captopril, bisoprolol
fumarate.
Non-limiting examples of therapeutic agents for Ankylosing Spondylitis with
which
binding proteins provided herein can be combined include the following:
ibuprofen, diclofenac
and misoprostol, naproxen, meloxicam, indomethacin, diclofenac, celecoxib,
rofecoxib,
Sulfasalazine, Methotrexate, azathioprine, minocyclin, prednisone, etanercept,
infliximab.
Non-limiting examples of therapeutic agents for Asthma with which binding
proteins
provided herein can be combined include the following: albuterol,
salmeterol/fluticasone,
montelukast sodium, fluticasone propionate, budesonide, prednisone, salmeterol
xinafoate,
levalbuterol hcl, albuterol sulfate/ipratropium, prednisolone sodium
phosphate, triamcinolone
acetonide, beclomethasone dipropionate, ipratropium bromide, azithromycin,
pirbuterol acetate,
prednisolone, theophylline anhydrous, methylprednisolone sodium succinate,
clarithromycin,
zafirlukast, formoterol fumarate, influenza virus vaccine, methylprednisolone,
amoxicillin
trihydrate, flunisolide, allergy injection, cromolyn sodium, fexofenadine
hydrochloride,
flunisolide/menthol, amoxicillin/clavulanate, levofloxacin, inhaler assist
device, guaifenesin,
135
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
dexamethasone sodium phosphate, moxifloxacin hcl, doxycycline hyclate,
guaifenesin/d-
methorphan, p-ephedrine/cod/chlorphenir, gatifloxacin, cetirizine
hydrochloride, mometasone
furoate, salmeterol xinafoate, benzonatate, cephalexin,
pe/hydrocodone/chlorphenir, cetirizine
hcl/pseudoephed, phenylephrine/cod/promethazine, codeine/promethazine,
cefprozil,
dexamethasone, guaifenesin/pseudoephedrine, chlorpheniramine/hydrocodone,
nedocromil
sodium, terbutaline sulfate, epinephrine, methylprednisolone, metaproterenol
sulfate.
Non-limiting examples of therapeutic agents for COPD with which binding
proteins
provided herein can be combined include the following: albuterol
sulfate/ipratropium,
ipratropium bromide, salmeterol/fluticasone, albuterol, salmeterol xinafoate,
fluticasone
propionate, prednisone, theophylline anhydrous, methylprednisolone sodium
succinate,
montelukast sodium, budesonide, formoterol fumarate, triamcinolone acetonide,
levofloxacin,
guaifenesin, azithromycin, beclomethasone dipropionate, levalbuterol hcl,
flunisolide, ceftriaxone
sodium, amoxicillin trihydrate, gatifloxacin, zafirlukast,
amoxicillin/clavulanate,
flunisolide/menthol, chlorpheniramine/hydrocodone, metaproterenol sulfate,
methylprednisolone,
mometasone furoate, p-ephedrine/cod/chlorphenir, pirbuterol acetate, p-
ephedrine/loratadine,
terbutaline sulfate, tiotropium bromide, (R,R)-formoterol, TgAAT, Cilomilast,
Roflumilast.
Non-limiting examples of therapeutic agents for HCV with which binding
proteins
provided herein can be combined include the following: Interferon-alpha-2a,
Interferon-alpha-2b,
Interferon-alpha con 1, Interferon-alpha-nl, Pegylated interferon-alpha-2a,
Pegylated interferon-
alpha-2b, ribavirin, Peginterferon alfa-2b + ribavirin, Ursodeoxycholic Acid,
Glycyrrhizic Acid,
Thymalfasin, Maxamine, VX-497 and any compounds that are used to treat HCV
through
intervention with the following targets: HCV polymerase, HCV protease, HCV
helicase, HCV
IRES (internal ribosome entry site).
Non-limiting examples of therapeutic agents for Idiopathic Pulmonary Fibrosis
with
which binding proteins provided herein can be combined include the following:
prednisone,
azathioprine, albuterol, colchicine, albuterol sulfate, digoxin, gamma
interferon,
methylprednisolone sod succ, lorazepam, furosemide, lisinopril, nitroglycerin,
spironolactone,
cyclophosphamide, ipratropium bromide, actinomyein d, alteplase, fluticasone
propionate,
levofloxacin, metaproterenol sulfate, morphine sulfate, oxycodone hcl,
potassium chloride,
triamcinolone acetonide, tacrolimus anhydrous, calcium, interferon-alpha,
methotrexate,
mycophenolate mofetil, Interferon-gamma-113.
Non-limiting examples of therapeutic agents for Myocardial Infarction with
which
binding proteins provided herein can be combined include the following:
aspirin, nitroglycerin,
136
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
metoprolol tartrate, enoxaparin sodium, heparin sodium, clopidogrel bisulfate,
carvedilol,
atenolol, morphine sulfate, metoprolol succinate, warfarin sodium, lisinopril,
isosorbide
mononitrate, digoxin, furosemide, simvastatin, ramipril, tenecteplase,
enalapril maleate,
torsemide, retavase, losartan potassium, quinapril hcl/mag carb, bumetanide,
alteplase,
enalaprilat, amiodarone hydrochloride, tirofiban hcl m-hydrate, diltiazem
hydrochloride,
captopril, irbesartan, valsartan, propranolol hydrochloride, fosinopril
sodium, lidocaine
hydrochloride, eptifibatide, cefazolin sodium, atropine sulfate, aminocaproic
acid,
spironolactone, interferon, sotalol hydrochloride, potassium chloride,
docusate sodium,
dobutamine hcl, alprazolam, pravastatin sodium, atorvastatin calcium,
midazolam hydrochloride,
meperidine hydrochloride, isosorbide din itrate, epinephrine, dopamine
hydrochloride,
bivalirudin, rosuvastatin, ezetimibe/simvastatin, avasimibe, cariporide.
Non-limiting examples of therapeutic agents for Psoriasis with which binding
proteins
provided herein can be combined include the following: small molecule
inhibitor of KDR, small
molecule inhibitor of Tie-2, calcipotriene, clobetasol propionate,
triamcinolone acetonide,
halobetasol propionate, tazarotene, methotrexate, fluocinonide, betamethasone
diprop augmented,
fluocinolone acetonide, acitretin, tar shampoo, betamethasone valerate,
mometasone furoate,
ketoconazole, pramoxine/fluocinolone, hydrocortisone valerate,
flurandrenolide, urea,
betamethasone, clobetasol propionate/emoll, fluticasone propionate,
azithromycin,
hydrocortisone, moisturizing formula, folic acid, desonide, pimecrolimus, coal
tar, diflorasone
diacetate, etanercept folate, lactic acid, methoxsalen, hc/bismuth
subgal/znoxJresor,
methylprednisolone acetate, prednisone, sunscreen, halcinonide, salicylic
acid, anthralin,
clocortolone pivalate, coal extract, coal tar/salicylic acid, coal
tar/salicylic acid/sulfur,
desoximetasone, diazepam, emollient, fluocinonide/emollient, mineral
oil/castor oil/na lact,
mineral oil/peanut oil, petroleum/isopropyl myristate, psoralen, salicylic
acid, soap/tribromsalan,
thimerosal/boric acid, celecoxib, infliximab, cyclosporine, alefacept,
efalizumab, tacrolimus,
pimecrolimus, PUVA, UVB, sulfasalazine.
Non-limiting examples of therapeutic agents for Psoriatic Arthritis with which
binding
proteins provided herein can be combined include the following: methotrexate,
etanercept,
rofecoxib, celecoxib, folic acid, sulfasalazine, naproxen, leflunomide,
methylprednisolone
acetate, indomethacin, hydroxychloroquine sulfate, prednisone, sulindac,
betamethasone diprop
augmented, infliximab, methotrexate, folate, triamcinolone acetonide,
diclofenac,
dimethylsulfoxide, piroxicam, diclofenac sodium, ketoprofen, meloxicam,
methylprednisolone,
nabumetone, tolmetin sodium, calcipotriene, cyclosporine, diclofenac
sodium/misoprostol,
fluocinonide, glucosamine sulfate, gold sodium thiomalate, hydrocodone
bitartrate/apap,
137
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
ibuprofen, risedronate sodium, sulfadiazine, thioguanine, valdecoxib,
alefacept, efalizamab and
bc1-2 inhibitors.
Non-limiting examples of therapeutic agents for Restenosis with which binding
proteins
provided herein can be combined include the following: sirolimus, paclitaxel,
everolimus,
tacrolimus, Zotarolimus, acetaminophen.
Non-limiting examples of therapeutic agents for Sciatica with which binding
proteins
provided herein can be combined include the following: hydrocodone
bitartrate/apap, rofecoxib,
cyclobenzaprine hcl, methylprednisolone, naproxen, ibuprofen, oxycodone
hcl/acetaminophen,
celecoxib, valdecoxib, methylprednisolone acetate, prednisone, codeine
phosphate/apap,
tramadol hcl/acetaminophen, metaxalone, meloxicam, methocarbamol, lidocaine
hydrochloride,
diclofenac sodium, gabapentin, dexamethasone, carisoprodol, ketorolac
tromethamine,
indomethacin, acetaminophen, diazepam, nabumetone, oxycodone hcl, tizanidine
hcl, diclofenac
sodium/misoprostol, propoxyphene napsylate/apap, asa/oxycod/oxycodone ter,
ibuprofen/hydrocodone bit, tramadol hcl, etodolac, propoxyphene hcl,
amitriptyline hcl,
carisoprodol/codeine phos/asa, morphine sulfate, multivitamins, naproxen
sodium, orphenadrine
citrate, temazepam.
Examples of therapeutic agents for SLE (Lupus) in which binding proteins
provided
herein can be combined include the following: NSAIDS, for example, diclofenac,
naproxen,
ibuprofen, piroxicam, indomethacin; COX2 inhibitors, for example, Celecoxib,
rofecoxib,
valdecoxib; anti-malarials, for example, hydroxychloroquine; Steroids, for
example, prednisone,
prednisolone, budenoside, dexamethasone; Cytotoxics, for example,
azathioprine,
cyclophosphamide, mycophenolate mofetil, methotrexate; inhibitors of PDE4 or
purine synthesis
inhibitor, for example Cellcept. Binding proteins provided herein, may also be
combined with
agents such as sulfasalazine, 5-aminosalicylic acid, olsalazine, Imuran and
agents which interfere
with synthesis, production or action of proinflammatory cytokines such as IL-
1, for example,
caspase inhibitors like IL-I 0 converting enzyme inhibitors and IL-lra.
Binding proteins provided
herein may also be used with T cell signaling inhibitors, for example,
tyrosine kinase inhibitors;
or molecules that target T cell activation molecules, for example, CTLA-4-IgG
or anti-B7 family
antibodies, anti-PD-1 family antibodies. Binding proteins provided herein, can
be combined with
IL-11 or anti-cytokine antibodies, for example, fonotolizumab (anti-IFNg
antibody), or anti-
receptor receptor antibodies, for example, anti-IL-6 receptor antibody and
antibodies to B-cell
surface molecules. Binding proteins provided herein or antigen binding portion
thereof may also
be used with LJP 394 (abetimus), agents that deplete or inactivate B-cells,
for example,
Rituximab (anti-CD20 antibody), lymphostat-B (anti-BlyS antibody), TNF
antagonists, for
138
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
example, anti-TNF antibodies, Adalimumab (PCT Publication No. WO 97/29131;
HUMIRA),
CA2 (REMICADE), CDP 571, TNFR-Ig constructs, (p75TNFRIgG (ENBREL) and
p55TNFRIgG (LENERCEPT)) and bc1-2 inhibitors, because bc1-2 overexpression in
transgenic
mice has been demonstrated to cause a lupus like phenotype (see Marquina,
Regina et al., Journal
of Immunology (2004), 172(11), 7177-7185), therefore inhibition is expected to
have therapeutic
effects.
The pharmaceutical compositions provided herein may include a "therapeutically
effective amount" or a "prophylactically effective amount" of a binding
protein provided herein.
A "therapeutically effective amount" refers to an amount effective, at dosages
and for periods of
time necessary, to achieve the desired therapeutic result. A therapeutically
effective amount of
the binding protein may be determined by a person skilled in the art and may
vary according to
factors such as the disease state, age, sex, and weight of the individual, and
the ability of the
binding protein to elicit a desired response in the individual. A
therapeutically effective amount is
also one in which any toxic or detrimental effects of the binding protein or
antigen-binding
portion, are outweighed by the therapeutically beneficial effects. A
"prophylactically effective
amount" refers to an amount effective, at dosages and for periods of time
necessary, to achieve
the desired prophylactic result. Typically, since a prophylactic dose is used
in subjects prior to or
at an earlier stage of disease, the prophylactically effective amount will be
less than the
therapeutically effective amount.
Dosage regimens may be adjusted to provide the optimum desired response (e.g.,
a
therapeutic or prophylactic response). For example, a single bolus may be
administered, several
divided doses may be administered over time or the dose may be proportionally
reduced or
increased as indicated by the exigencies of the therapeutic situation. It is
especially advantageous
to formulate parenteral compositions in dosage unit form for ease of
administration and
uniformity of dosage. Dosage unit form as used herein refers to physically
discrete units suited as
unitary dosages for the mammalian subjects to be treated; each unit containing
a predetermined
quantity of active compound calculated to produce the desired therapeutic
effect in association
with the required pharmaceutical carrier. The specification for the dosage
unit forms provided
herein are dictated by and directly dependent on (a) the unique
characteristics of the active
compound and the particular therapeutic or prophylactic effect to be achieved,
and (b) the
limitations inherent in the art of compounding such an active compound for the
treatment of
sensitivity in individuals.
An exemplary, non-limiting range for a therapeutically or prophylactically
effective
amount of a binding protein provided herein is 0.1-20 mg/kg, for example, 1-10
mg/kg. It is to be
139
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
noted that dosage values may vary with the type and severity of the condition
to be alleviated. It
is to be further understood that for any particular subject, specific dosage
regimens should be
adjusted over time according to the individual need and the professional
judgment of the person
administering or supervising the administration of the compositions, and that
dosage ranges set
forth herein are exemplary only and are not intended to limit the scope or
practice of the claimed
composition.
V. Diagnostics
The disclosure herein also provides diagnostic applications. This is further
elucidated
below.
A. Method of Assay
The present disclosure also provides a method for determining the presence,
amount or
concentration of an analyte (or a fragment thereof) in a test sample using at
least one DVD-Ig as
described herein. Any suitable assay as is known in the art can be used in the
method. Examples
include, but are not limited to, immunoassay, such as sandwich immunoassay
(e.g., monoclonal,
polyclonal and/or DVD-Ig sandwich immunoassays or any variation thereof (e.g.,
monoclonal/DVD-Ig, DVD-Ig/polyclonal, etc.), including radioisotope detection
(radioimmunoassay (RIA)) and enzyme detection (enzyme immunoassay (EIA) or
enzyme-linked
immunosorbent assay (ELISA) (e.g., Quantikine ELISA assays, R&D Systems,
Minneapolis,
MN))), competitive inhibition immunoassay (e.g., forward and reverse),
fluorescence polarization
immunoassay (FPIA), enzyme multiplied immunoassay technique (EMIT),
bioluminescence
resonance energy transfer (BRET), and homogeneous chemiluminescent assay, etc.
In a SELDI-
based immunoassay, a capture reagent that specifically binds an analyte (or a
fragment thereof) of
interest is attached to the surface of a mass spectrometry probe, such as a
pre-activated protein
chip array. The analyte (or a fragment thereof) is then specifically captured
on the biochip, and
the captured analyte (or a fragment thereof) is detected by mass spectrometry.
Alternatively, the
analyte (or a fragment thereof) can be eluted from the capture reagent and
detected by traditional
MALDI (matrix-assisted laser desorption/ionization) or by SELDI. A
chemiluminescent
microparticle immunoassay, in particular one employing the ARCHITECT
automated analyzer
(Abbott Laboratories, Abbott Park, IL), is an example of a preferred
immunoassay.
Methods well-known in the art for collecting, handling and processing urine,
blood,
serum and plasma, and other body fluids, are used in the practice of the
present disclosure, for
instance, when a DVD-Ig as described herein is employed as an immunodiagnostic
reagent and/or
in an analyte immunoassay kit. The test sample can comprise further moieties
in addition to the
140
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
analyte of interest, such as antibodies, antigens, haptens, hormones, drugs,
enzymes, receptors,
proteins, peptides, polypeptides, oligonucleotides and/or polynucleotides. For
example, the
sample can be a whole blood sample obtained from a subject. It can be
necessary or desired that a
test sample, particularly whole blood, be treated prior to immunoassay as
described herein, e.g.,
with a pretreatment reagent. Even in cases where pretreatment is not necessary
(e.g., most urine
samples), pretreatment optionally can be done (e.g., as part of a regimen on a
commercial
platform).
The pretreatment reagent can be any reagent appropriate for use with the
immunoassay
and kits provided herein. The pretreatment optionally comprises: (a) one or
more solvents (e.g.,
methanol and ethylene glycol) and optionally, salt, (b) one or more solvents
and salt, and
optionally, detergent, (c) detergent, or (d) detergent and salt. Pretreatment
reagents are known in
the art, and such pretreatment can be employed, e.g., as used for assays on
Abbott TDx,
AxSYM , and ARCHITECT analyzers (Abbott Laboratories, Abbott Park, IL), as
described in
the literature (see, e.g., Yatscoff et al., Abbott TDx Monoclonal Antibody
Assay Evaluated for
Measuring Cyclosporine in Whole Blood, Clin. Chem. 36: 1969-1973 (1990), and
Wallemacq et
al., Evaluation of the New AxSYM Cyclosporine Assay: Comparison with TDx
Monoclonal
Whole Blood and EMIT Cyclosporine Assays, Clin. Chem. 45: 432-435 (1999)),
and/or as
commercially available. Additionally, pretreatment can be done as described in
Abbott's U.S.
Pat. No. 5,135,875, European Pat. Pub. No. 0 471 293, U.S Provisional Pat.
App. 60/878,017,
filed December 29, 2006, and U.S. Pat. App. Pub. No. 2008/0020401
(incorporated by reference
in its entirety for its teachings regarding pretreatment). The pretreatment
reagent can be a
heterogeneous agent or a homogeneous agent.
With use of a heterogeneous pretreatment reagent, the pretreatment reagent
precipitates
analyte binding protein (e.g., protein that can bind to an analyte or a
fragment thereof) present in
the sample. Such a pretreatment step comprises removing any analyte binding
protein by
separating from the precipitated analyte binding protein the supernatant of
the mixture formed by
addition of the pretreatment agent to sample. In such an assay, the
supernatant of the mixture
absent any binding protein is used in the assay, proceeding directly to the
capture step.
With use of a homogeneous pretreatment reagent there is no such separation
step. The
entire mixture of test sample and pretreatment reagent are contacted with a
labeled specific
binding partner for analyte (or a fragment thereof), such as a labeled anti-
analyte antibody (or an
antigenically reactive fragment thereof). The pretreatment reagent employed
for such an assay
typically is diluted in the pretreated test sample mixture, either before or
during capture by the
first specific binding partner. Despite such dilution, a certain amount of the
pretreatment reagent
141
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
is still present (or remains) in the test sample mixture during capture.
According to certain
embodiments, the labeled specific binding partner can be a DVD-Ig (or a
fragment, a variant, or a
fragment of a variant thereof).
In a heterogeneous format, after the test sample is obtained from a subject, a
first mixture
is prepared. The mixture contains the test sample being assessed for an
analyte (or a fragment
thereof) and a first specific binding partner, wherein the first specific
binding partner and any
analyte contained in the test sample form a first specific binding partner-
analyte complex.
Preferably, the first specific binding.partner is an anti-analyte antibody or
a fragment thereof. The
first specific binding partner can be a DVD-Ig (or a fragment, a variant, or a
fragment of a variant
thereof) as described herein. The order in which the test sample and the first
specific binding
partner are added to form the mixture is not critical. Preferably, the first
specific binding partner
is immobilized on a solid phase. The solid phase used in the immunoassay (for
the first specific
binding partner and, optionally, the second specific binding partner) can be
any solid phase
known in the art, such as, but not limited to, a magnetic particle, a bead, a
test tube, a microtiter
plate, a cuvette, a membrane, a scaffolding molecule, a film, a filter paper,
a disc and a chip.
After the mixture containing the first specific binding partner-analyte
complex is formed,
any unbound analyte is removed from the complex using any technique known in
the art. For
example, the unbound analyte can be removed by washing. Desirably, however,
the first specific
binding partner is present in excess of any analyte present in the test
sample, such that all analyte
that is present in the test sample is bound by the first specific binding
partner.
After any unbound analyte is removed, a second specific binding partner is
added to the
mixture to form a first specific binding partner-analyte-second specific
binding partner complex.
The second specific binding partner is preferably an anti-analyte antibody
that binds to an epitope
on analyte that differs from the epitope on analyte bound by the first
specific binding partner.
Moreover, also preferably, the second specific binding partner is labeled with
or contains a
detectable label as described above. The second specific binding partner can
be a DVD-Ig (or a
fragment, a variant, or a fragment of a variant thereof) as described herein.
Any suitable detectable label as is known in the art can be used. For example,
the
detectable label can be a radioactive label (such as 3H, 1251, 35S, 14C, 32P,
and 33P), an
enzymatic label (such as horseradish peroxidase, alkaline peroxidase, glucose
6-phosphate
dehydrogenase, and the like), a chemiluminescent label (such as acridinium
esters, thioesters, or
sulfonamides; luminol, isoluminol, phenanthridinium esters, and the like), a
fluorescent label
(such as fluorescein (e.g., 5-fluorescein, 6-carboxyfluorescein, 3'6-
carboxyfluorescein, 5(6)-
142
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
carboxyfluorescein, 6-hexachloro-fluorescein, 6-tetrachlorofluorescein,
fluorescein
isothiocyanate, and the like)), rhodamine, phycobiliproteins, R-phycoerythrin,
quantum dots (e.g.,
zinc sulfide-capped cadmium selenide), a thermometric label, or an immuno-
polymerase chain
reaction label. An introduction to labels, labeling procedures and detection
of labels is found in
Polak and Van Noorden, Introduction to Immunocytochemistry, 2nd ed., Springer
Verlag, N.Y.
(1997), and in Haugland, Handbook of Fluorescent Probes and Research Chemicals
(1996),
which is a combined handbook and catalogue published by Molecular Probes,
Inc., Eugene,
Oregon. A fluorescent label can be used in FPIA (see, e.g., U.S. Patent Nos.
5,593,896,
5,573,904, 5,496,925, 5,359,093, and 5,352,803). An acridinium compound can be
used as a
detectable label in a homogeneous or heterogeneous chemiluminescent assay
(see, e.g.,
Adamczyk et al., Bioorg. Med. Chem. Lett. 16: 1324-1328 (2006); Adamczyk et
al., Bioorg. Med.
Chem. Lett. 4: 2313-2317 (2004); Adamczyk et al., Biorg. Med. Chem. Lett. 14:
3917-3921
(2004); and Adamczyk et al., Org. Lett. 5: 3779-3782 (2003)).
A preferred acridinium compound is an acridinium-9-carboxamide. Methods for
preparing acridinium 9-carboxamides are described in Mattingly, J. Biolumin.
Chemilumin. 6:
107-114 (1991); Adamczyk et al., J. Org. Chem. 63: 5636-5639 (1998); Adamczyk
et al.,
Tetrahedron 55: 10899-10914 (1999); Adamczyk et al., Org. Lett. 1: 779-781
(1999); Adamczyk
et al., Bioconjugate Chem. 11: 714-724 (2000); Mattingly et al., In
Luminescence Biotechnology:
Instruments and Applications; Dyke, K. V. Ed.; CRC Press: Boca Raton, pp. 77-
105 (2002);
Adamczyk et al., Org. Lett. 5: 3779-3782 (2003); and U.S. Pat. Nos. 5,468,646,
5,543,524 and
5,783,699. Another preferred acridinium compound is an acridinium-9-
carboxylate aryl ester. An
example of an acridinium-9-carboxylate aryl ester is 10-methy1-9-
(phenoxycarbonyl)acridinium
fluorosulfonate (available from Cayman Chemical, Ann Arbor, MI). Methods for
preparing
acridinium 9-carboxylate aryl esters are described in McCapra et al.,
Photochem. Photobiol. 4:
1111-21 (1965); Razavi et al., Luminescence 15: 245-249 (2000); Razavi et al.,
Luminescence
15: 239-244 (2000); and U.S. Patent No. 5,241,070. Further details regarding
acridinium-9-
carboxylate aryl ester and its use are set forth in US 2008-0248493.
Chemiluminescent assays (e.g., using acridinium as described above or other
chemiluminescent agents) can be performed in accordance with the methods
described in
Adamczyk et al., Anal. Chim. Acta 579(1): 61-67 (2006). While any suitable
assay format can be
used, a microplate chemiluminometer (Mithras LB-940, Berthold Technologies
U.S.A., LLC,
Oak Ridge, TN) enables the assay of multiple samples of small volumes rapidly.
The order in which the test sample and the specific binding partner(s) are
added to form
the mixture for chemiluminescent assay is not critical. If the first specific
binding partner is
143
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
detectably labeled with a chemiluminescent agent such as an acridinium
compound, detectably
labeled first specific binding partner-analyte complexes form. Alternatively,
if a second specific
binding partner is used and the second specific binding partner is detectably
labeled with a
chemiluminescent agent such as an acridinium compound, detectably labeled
first specific
binding partner-analyte-second specific binding partner complexes form. Any
unbound specific
binding partner, whether labeled or unlabeled, can be removed from the mixture
using any
technique known in the art, such as washing.
Hydrogen peroxide can be generated in situ in the mixture or provided or
supplied to the
mixture (e.g., the source of the hydrogen peroxide being one or more buffers
or other solutions
that are known to contain hydrogen peroxide) before, simultaneously with, or
after the addition of
an above-described acridinium compound. Hydrogen peroxide can be generated in
situ in a
number of ways such as would be apparent to one skilled in the art.
Upon the simultaneous or subsequent addition of at least one basic solution to
the
sample, a detectable signal, namely, a chemiluminescent signal, indicative of
the presence of
analyte is generated. The basic solution contains at least one base and has a
pH greater than or
equal to 10, preferably, greater than or equal to 12. Examples of basic
solutions include, but are
not limited to, sodium hydroxide, potassium hydroxide, calcium hydroxide,
ammonium
hydroxide, magnesium hydroxide, sodium carbonate, sodium bicarbonate, calcium
hydroxide,
calcium carbonate, and calcium bicarbonate. The amount of basic solution added
to the sample
depends on the concentration of the basic solution. Based on the concentration
of the basic
solution used, one skilled in the art can easily determine the amount of basic
solution to add to
the sample.
The chemiluminescent signal that is generated can be detected using routine
techniques
known to those skilled in the art. Based on the intensity of the signal
generated, the amount of
analyte in the sample can be quantified. Specifically, the amount of analyte
in the sample is
proportional to the intensity of the signal generated. The amount of analyte
present can be
quantified by comparing the amount of light generated to a standard curve for
analyte or by
comparison to a reference standard. The standard curve can be generated using
serial dilutions or
solutions of known concentrations of analyte by mass spectroscopy, gravimetric
methods, and
other techniques known in the art. While the above is described with emphasis
on use of an
acridinium compound as the chemi luminescent agent, one of ordinary skill in
the art can readily
adapt this description for use of other chemiluminescent agents.
144
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Analyte immunoassays generally can be conducted using any format known in the
art,
such as, but not limited to, a sandwich format. Specifically, in one
immunoassay format, at least
two binding proteins are employed to separate and quantify analyte, such as
human analyte, or a
fragment thereof in a sample. More specifically, the at least two binding
proteins bind to different
epitopes on an analyte (or a fragment thereof) forming an immune complex,
which is referred to
as a "sandwich." Generally, in the immunoassays one or more binding proteins
can be used to
capture the analyte (or a fragment thereof) in the test sample (these binding
proteins are
frequently referred to as a "capture" antibody or "capture" antibodies) and
one or more binding
proteins can be used to bind a detectable (namely, quantifiable) label to the
sandwich (these
binding proteins are frequently referred to as the "detection antibody," the
"detection antibodies,"
the "conjugate," or the "conjugates"). Thus, in the context of a sandwich
immunoassay format, a
DVD-Ig (or a fragment, a variant, or a fragment of a variant thereof) as
described herein can be
used as a capture antibody, a detection antibody, or both. For example, one
DVD-Ig having a
domain that can bind a first epitope on an analyte (or a fragment thereof) can
be used as a capture
antibody and/or another DVD-Ig having a domain that can bind a second epitope
on an analyte
(or a fragment thereof) can be used as a detection antibody. In this regard, a
DVD-Ig having a
first domain that can bind a first epitope on an analyte (or a fragment
thereof) and a second
domain that can bind a second epitope on an analyte (or a fragment thereof)
can be used as a
capture antibody and/or a detection antibody. Alternatively, one DVD-Ig having
a first domain
that can bind an epitope on a first analyte (or a fragment thereof) and a
second domain that can
bind an epitope on a second analyte (or a fragment thereof) can be used as a
capture antibody
and/or a detection antibody to detect, and optionally quantify, two or more
analytes. In the event
that an analyte can be present in a sample in more than one form, such as a
monomeric form and
a dimeric/multimeric form, which can be homomeric or beteromeric, one DVD-Ig
having a
domain that can bind an epitope that is only exposed on the monomeric form and
another DVD-Ig
having a domain that can bind an epitope on a different part of a
dimeric/multimeric form can be
used as capture antibodies and/or detection antibodies, thereby enabling the
detection, and
optional quantification, of different forms of a given analyte. Furthermore,
employing DVD-Igs
with differential affinities within a single DVD-Ig and/or between DVD-Igs can
provide an
avidity advantage. In the context of immunoassays as described herein, it
generally may be
helpful or desired to incorporate one or more linkers within the structure of
a DVD-Ig. When
present, optimally the linker should be of sufficient length and structural
flexibility to enable
binding of an epitope by the inner domains as well as binding of another
epitope by the outer
domains. In this regard, if a DVD-Ig can bind two different analytes and one
analyte is larger than
the other, desirably the larger analyte is bound by the outer domains.
145
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Generally speaking, a sample being tested for (for example, suspected of
containing)
analyte (or a fragment thereof) can be contacted with at least one capture
antibody (or antibodies)
and at least one detection antibody (which can be a second detection antibody
or a third detection
antibody or even a successively numbered antibody, e.g., as where the capture
and/or detection
antibody comprise multiple antibodies) either simultaneously or sequentially
and in any order.
For example, the test sample can be first contacted with at least one capture
antibody and then
(sequentially) with at least one detection antibody. Alternatively, the test
sample can be first
contacted with at least one detection antibody and then (sequentially) with at
least one capture
antibody. In yet another alternative, the test sample can be contacted
simultaneously with a
capture antibody and a detection antibody.
In the sandwich assay format, a sample suspected of containing analyte (or a
fragment
thereof) is first brought into contact with at least one first capture
antibody under conditions that
allow the formation of a first antibody/analyte complex. If more than one
capture antibody is
used, a first capture antibody/analyte complex comprising two or more capture
antibodies is
formed. In a sandwich assay, the antibodies, i.e., preferably, the at least
one capture antibody, are
used in molar excess amounts of the maximum amount of analyte (or a fragment
thereof)
expected in the test sample. For example, from about 5 ug to about 1 mg of
antibody per mL of
buffer (e.g., microparticle coating buffer) can be used.
Competitive inhibition immunoassays, which are often used to measure small
analytes
because binding by only one binding protein is required, comprise sequential
and classic formats.
In a sequential competitive inhibition immunoassay a capture antibody to an
analyte of interest is
coated onto a well of a microtiter plate or other solid support. When the
sample containing the
analyte of interest is added to the well, the analyte of interest binds to the
capture antibody. After
washing, a known amount of labeled (e.g., biotin or horseradish peroxidase
(IIRP)) analyte is
added to the well. A substrate for an enzymatic label is necessary to generate
a signal. An
example of a suitable substrate for HRP is 3,3',5,5'-tetramethylbenzidine
(TMB). After washing,
the signal generated by the labeled analyte is measured and is inversely
proportional to the
amount of analyte in the sample. In a classic competitive inhibition
immunoassay an antibody to
an analyte of interest is coated onto a solid support (e.g., a well of a
microtiter plate). However,
unlike the sequential competitive inhibition immunoassay, the sample and the
labeled analyte are
added to the well at the same time. Any analyte in the sample competes with
labeled analyte for
binding to the capture antibody. After washing, the signal generated by the
labeled analyte is
measured and is inversely proportional to the amount of analyte in the sample.
146
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Optionally, prior to contacting the test sample with the at least one capture
antibody (for
example, the first capture antibody), the at least one capture antibody can be
bound to a solid
support, which facilitates the separation of the first antibody/analyte (or a
fragment thereof)
complex from the test sample. The substrate to which the capture antibody is
bound can be any
suitable solid support or solid phase that facilitates separation of the
capture antibody-analyte
complex from the sample.
Examples include a well of a plate, such as a microtiter plate, a test tube, a
porous gel
(e.g., silica gel, agarose, dextran, or gelatin), a polymeric film (e.g.,
polyacrylamide), beads (e.g.,
polystyrene beads or magnetic beads), a strip of a filter/membrane (e.g.,
nitrocellulose or nylon),
microparticles (e.g., latex particles, magnetizable microparticles (e.g.,
microparticles having
ferric oxide or chromium oxide cores and homo- or hetero-polymeric coats and
radii of about 1-
10 microns). The substrate can comprise a suitable porous material with a
suitable surface
affinity to bind antigens and sufficient porosity to allow access by detection
antibodies. A
microporous material is generally preferred, although a gelatinous material in
a hydrated state can
be used. Such porous substrates are preferably in the form of sheets having a
thickness of about
0.01 to about 0.5 mm, preferably about 0.1 mm. While the pore size may vary
quite a bit,
preferably the pore size is from about 0.025 to about 15 microns, more
preferably from about
0.15 to about 15 microns. The surface of such substrates can be activated by
chemical processes
that cause covalent linkage of an antibody to the substrate. Irreversible
binding, generally by
adsorption through hydrophobic forces, of the antigen or the antibody to the
substrate results;
alternatively, a chemical coupling agent or other means can be used to bind
covalently the
antibody to the substrate, provided that such binding does not interfere with
the ability of the
antibody to bind to analyte. Alternatively, the antibody can be bound with
microparticles, which
have been previously coated with streptavidin (e.g., DYNAL Magnetic Beads,
Invitrogen,
Carlsbad, CA) or biotin (e.g., using Power-BindTM-SA-MP streptavidin-coated
microparticles
(Seradyn, Indianapolis, IN)) or anti-species-specific monoclonal antibodies.
If necessary, the
substrate can be derivatized to allow reactivity with various functional
groups on the antibody.
Such derivatization requires the use of certain coupling agents, examples of
which include, but
are not limited to, maleic anhydride, N-hydroxysuccinimide, and 1-ethy1-3-(3-
dimethylaminopropyl) carbodiimide. If desired, one or more capture reagents,
such as antibodies
(or fragments thereof), each of which is specific for analyte(s) can be
attached to solid phases in
different physical or addressable locations (e.g., such as in a biochip
configuration (see, e.g., U.S.
Pat. No. 6,225,047; Intl Pat. App. Pub. No. WO 99/51773; U.S. Pat. No.
6,329,209; Int'l Pat.
App. Pub. No. WO 00/56934, and 'U.S. Pat. No. 5,242,828). If the capture
reagent is attached to a
mass spectrometry probe as the solid support, the amount of analyte bound to
the probe can be
147
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
detected by laser desorption ionization mass spectrometry. Alternatively, a
single column can be
packed with different beads, which are derivatized with the one or more
capture reagents, thereby
capturing the analyte in a single place (see, antibody-derivatized, bead-based
technologies, e.g.,
the xMAP technology of Luminex (Austin, TX)).
After the test sample being assayed for analyte (or a fragment thereof) is
brought into
contact with the at least one capture antibody (for example, the first capture
antibody), the
mixture is incubated in order to allow for the formation of a first antibody
(or multiple antibody)-
analyte (or a fragment thereof) complex. The incubation can be carried out at
a pH of from about
4.5 to about 10.0, at a temperature of from about 2 C to about 45 C, and for a
period from at least
about one (1) minute to about eighteen (18) hours, preferably from about 1 to
about 24 minutes,
most preferably for about 4 to about 18 minutes. The immunoassay described
herein can be
conducted in one step (meaning the test sample, at least one capture antibody
and at least one
detection antibody are all added sequentially or simultaneously to a reaction
vessel) or in more
than one step, such as two steps, three steps, etc.
After formation of the (first or multiple) capture antibody/analyte (or a
fragment thereof)
complex, the complex is then contacted with at least one detection antibody
under conditions
which allow for the formation of a (first or multiple) capture
antibody/analyte (or a fragment
thereof)/second detection antibody complex). While captioned for clarity as
the "second"
antibody (e.g., second detection antibody), in fact, where multiple antibodies
are used for capture
and/or detection, the at least one detection antibody can be the second,
third, fourth, etc.
antibodies used in the immunoassay. If the capture antibody/analyte (or a
fragment thereof)
complex is contacted with more than one detection antibody, then a (first or
multiple) capture
antibody/analyte (or a fragment thereof)/(multiple) detection antibody complex
is formed. As
with the capture antibody (e.g., the first capture antibody), when the at
least one (e.g., second and
any subsequent) detection antibody is brought into contact with the capture
antibody/analyte (or a
fragment thereof) complex, a period of incubation under conditions similar to
those described
above is required for the formation of the (first or multiple) capture
antibody/analyte (or a
fragment thereof)/(second or multiple) detection antibody complex. Preferably,
at least one
detection antibody contains a detectable label. The detectable label can be
bound to the at least
one detection antibody (e.g., the second detection antibody) prior to,
simultaneously with, or after
the formation of the (first or multiple) capture antibody/analyte (or a
fragment thereof)/(second or
multiple) detection antibody complex. Any detectable label known in the art
can be used (see
discussion above, including of the Polak and Van Noorden (1997) and Haugland
(1996)
references).
148,
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
The detectable label can be bound to the antibodies either directly or through
a coupling
agent. An example of a coupling agent that can be used is EDAC (1-ethy1-3-(3-
dimethylaminopropyl) carbodiimide, hydrochloride), which is commercially
available from
Sigma-Aldrich, St. Louis, MO. Other coupling agents that can be used are known
in the art.
Methods for binding a detectable label to an antibody are known in the art.
Additionally, many
detectable labels can be purchased or synthesized that already contain end
groups that facilitate
the coupling of the detectable label to the antibody, such as CPSP-Acridinium
Ester (i.e., 94N-
tosyl-N-(3-carboxypropyl)]-10-(3-sulfopropyl)acridinium carboxamide) or SPSP-
Acridinium
Ester (i.e., N10-(3-sulfopropy1)-N-(3-sulfopropy1)-acridinium-9-carboxamide).
The (first or multiple) capture antibody/analyte/(second or multiple)
detection antibody
complex can be, but does not have to be, separated from the remainder of the
test sample prior to
quantification of the label. For example, if the at least one capture antibody
(e.g., the first capture
antibody) is bound to a solid support, such as a well or a bead, separation
can be accomplished by
removing the fluid (of the test sample) from contact with the solid support.
Alternatively, if the at
least first capture antibody is bound to a solid support, it can be
simultaneously contacted with
the analyte-containing sample and the at least one second detection antibody
to form a first
(multiple) antibody/analyte/second (multiple) antibody complex, followed by
removal of the fluid
(test sample) from contact with the solid support. If the at least one first
capture antibody is not
bound to a solid support, then the (first or multiple) capture
antibody/analyte/(second or multiple)
detection antibody complex does not have to be removed from the test sample
for quantification
of the amount of the label.
After formation of the labeled capture antibody/analyte/detection antibody
complex (e.g.,
the first capture antibody/analyte/second detection antibody complex), the
amount of label in the
complex is quantified using techniques known in the art. For example, if an
enzymatic label is
used, the labeled complex is reacted with a substrate for the label that gives
a quantifiable
reaction such as the development of color. If the label is a radioactive
label, the label is quantified
using appropriate means, such as a scintillation counter. If the label is a
fluorescent label, the
label is quantified by stimulating the label with a light of one color (which
is known as the
"excitation wavelength") and detecting another color (which is known as the
"emission
wavelength") that is emitted by the label in response to the stimulation. If
the label is a
chemiluminescent label, the label is quantified by detecting the light emitted
either visually or by
using luminometers, x-ray film, high speed photographic film, a CCD camera,
etc. Once the
amount of the label in the complex has been quantified, the concentration of
analyte or a
fragment thereof in the test sample is determined by appropriate means, such
as by use of a
149
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
standard curve that has been generated using serial dilutions of analyte or a
fragment thereof of
known concentration. Other than using serial dilutions of analyte or a
fragment thereof, the
standard curve can be generated gravimetrically, by mass spectroscopy and by
other techniques
known in the art.
In a chemiluminescent microparticle assay employing the ARCHITECT analyzer,
the
conjugate diluent pH should be about 6.0 +/- 0.2, the microparticle coating
buffer should be
maintained at about room temperature (i.e., at from about 17 to about 27 8C),
the microparticle
coating buffer pH should be about 6.5 +/- 0.2, and the microparticle diluent
pH should be about
7.8 +/- 0.2. Solids preferably are less than about 0.2%, such as less than
about 0.15%, less than
about 0.14%, less than about 0.13%, less than about 0.12%, or less than about
0.11%, such as
about 0.10%.
FPIAs are based on competitive binding immunoassay principles. A fluorescently
labeled
compound, when excited by a linearly polarized light, will emit fluorescence
having a degree of
polarization inversely proportional to its rate of rotation. When a
fluorescently labeled tracer-
antibody complex is excited by a linearly polarized light, the emitted light
remains highly
polarized because the fluorophore is constrained from rotating between the
time light is absorbed
and the time light is emitted. When a "free" tracer compound (i.e., a compound
that is not bound
to an antibody) is excited by linearly polarized light, its rotation is much
faster than the
corresponding tracer-antibody conjugate produced in a competitive binding
immunoassay. FPIAs
are advantageous over RIAs inasmuch as there are no radioactive substances
requiring special
handling and disposal. In addition, FPIAs are homogeneous assays that can be
easily and rapidly
performed.
In view of the above, a method of determining the presence, amount, or
concentration of
analyte (or a fragment thereof) in a test sample is provided. The method
comprises assaying the
test sample for an analyte (or a fragment thereof) by an assay (i) employing
(i') at least one of an
antibody, a fragment of an antibody that can bind to an analyte, a variant of
an antibody that can
bind to an analyte, a fragment of a variant of an antibody that can bind to an
analyte, and a DVD-
Ig (or a fragment, a variant, or a fragment of a variant thereof) that can
bind to an analyte, and
(ii') at least one detectable label and (ii) comprising comparing a signal
generated by the
detectable label as a direct or indirect indication of the presence, amount or
concentration of
analyte (or a fragment thereof) in the test sample to a signal generated as a
direct or indirect
indication of the presence, amount or concentration of analyte (or a fragment
thereof) in a control
or calibrator. The calibrator is optionally part of a series of calibrators,
in which each of the
calibrators differs from the other calibrators by the concentration of
analyte.
150
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
The method can comprise (i) contacting the test sample with at least one first
specific
binding partner for analyte (or a fragment thereof) comprising an antibody, a
fragment of an
antibody that can bind to an analyte, a variant of an antibody that can bind
to an analyte, a
fragment of a variant of an antibody that can bind to an analyte, or a DVD-Ig
(or a fragment, a
variant, or a fragment of a variant thereof) that can bind to an analyte so as
to form a first specific
binding partner/analyte (or fragment thereof) complex, (ii) contacting the
first specific binding
partner/analyte (or fragment thereof) complex with at least one second
specific binding partner
for analyte (or fragment thereof) comprising a detectably labeled anti-analyte
antibody, a
detectably labeled fragment of an anti-analyte antibody that can bind to
analyte, a detectably
labeled variant of an anti-analyte antibody that can bind to analyte, a
detectably labeled fragment
of a variant of an anti-analyte antibody that can bind to analyte, or a
detectably labeled DVD-Ig
(or a fragment, a variant, or a fragment of a variant thereof) so as to form a
first specific binding
partner/analyte (or fragment thereof)/second specific binding partner complex,
and (iii)
determining the presence, amount or concentration of analyte in the test
sample by detecting or
measuring the signal generated by the detectable label in the first specific
binding partner/analyte
(or fragment thereof)/second specific binding partner complex formed in (ii).
A method in which
at least one first specific binding partner for analyte (or a fragment
thereof) and/or at least one
second specific binding partner for analyte (or a fragment thereof) is a DVD-
Ig (or a fragment, a
variant, or a fragment of a variant thereof) as described herein can be
preferred.
Alternatively, the method can comprise contacting the test sample with at
least one first
specific binding partner for analyte (or a fragment thereof) comprising an
antibody, a fragment of
an antibody that can bind to an analyte, a variant of an antibody that can
bind to an analyte, a
fragment of a variant of an antibody that can bind to an analyte, or a DVD-Ig
(or a fragment, a
variant, or a fragment of a variant thereof) and simultaneously or
sequentially, in either order,
contacting the test sample with at least one second specific binding partner,
which can compete
with analyte (or a fragment thereof) for binding to the at least one first
specific binding partner
and which is a detectably labeled analyte, a detectably labeled fragment of
analyte that can bind
to the first specific binding partner, a detectably labeled variant of analyte
that can bind to the
first specific binding partner, or a detectably labeled fragment of a variant
of analyte that can
bind to the first specific binding partner. Any analyte (or a fragment
thereof) present in the test
sample and the at least one second specific binding partner compete with each
other to form a
first specific binding partner/analyte (or fragment thereof) complex and a
first specific binding
partner/second specific binding partner complex, respectively. The method
further comprises
determining the presence, amount or concentration of analyte in the test
sample by detecting or
measuring the signal generated by the detectable label in the first specific
binding partner/second
151
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
specific binding partner complex formed in (ii), wherein the signal generated
by the detectable
label in the first specific binding partner/second specific binding partner
complex is inversely
proportional to the amount or concentration of analyte in the test sample.
The above methods can further comprise diagnosing, prognosticating, or
assessing the
efficacy of a therapeutic/prophylactic treatment of a patient from whom the
test sample was
obtained. If the method further comprises assessing the efficacy of a
therapeutic/prophylactic
treatment of the patient from whom the test sample was obtained, the method
optionally further
comprises modifying the therapeutic/prophylactic treatment of the patient as
needed to improve
efficacy. The method can be adapted for use in an automated system or a semi-
automated system.
With regard to the methods of assay (and kit therefor), it may be possible to
employ
commercially available anti-analyte antibodies or methods for production of
anti-analyte as
described in the literature. Commercial supplies of various antibodies
include, but are not limited
to, Santa Cruz Biotechnology Inc. (Santa Cruz, CA), GenWay Biotech, Inc. (San
Diego, CA), and
R&D Systems (RDS; Minneapolis, MN).
Generally, a predetermined level can be employed as a benchmark against which
to
assess results obtained upon assaying a test sample for analyte or a fragment
thereof, e.g., for
detecting disease or risk of disease. Generally, in making such a comparison,
the predetermined
level is obtained by running a particular assay a sufficient number of times
and under appropriate
conditions such that a linkage or association of analyte presence, amount or
concentration with a
particular stage or endpoint of a disease, disorder or condition or with
particular clinical indicia
can be made. Typically, the predetermined level is obtained with assays of
reference subjects (or
populations of subjects). The analyte measured can include fragments thereof,
degradation
products thereof, and/or enzymatic cleavage products thereof.
In particular, with respect to a predetermined level as employed for
monitoring disease
progression and/or treatment, the amount or concentration of analyte or a
fragment thereof may
be "unchanged," "favorable" (or "favorably altered"), or "unfavorable" (or
"unfavorably
altered"). "Elevated" or "increased" refers to an amount or a concentration in
a test sample that is
higher than a typical or normal level or range (e.g., predetermined level), or
is higher than another
reference level or range (e.g., earlier or baseline sample). The term
"lowered" or "reduced" refers
to an amount or a concentration in a test sample that is lower than a typical
or normal level or
range (e.g., predetermined level), or is lower than another reference level or
range (e.g., earlier or
baseline sample). The term "altered" refers to an amount or a concentration in
a sample that is
152
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
altered (increased or decreased) over a typical or normal level or range
(e.g., predetermined
level), or over another reference level or range (e.g., earlier or baseline
sample).
The typical or normal level or range for analyte is defined in accordance with
standard
practice. Because the levels of analyte in some instances will be very low, a
so-called altered
level or alteration can be considered to have occurred when there is any net
change as compared
to the typical or normal level or range, or reference level or range, that
cannot be explained by
experimental error or sample variation. Thus, the level measured in a
particular sample will be
compared with the level or range of levels determined in similar samples from
a so-called normal
subject. In this context, a "normal subject" is an individual with no
detectable disease, for
example, and a "normal" (sometimes termed "control") patient or population
is/are one(s) that
exhibit(s) no detectable disease, respectively, for example. Furthermore,
given that analyte is not
routinely found at a high level in the majority of the human population, a
"normal subject" can be
considered an individual with no substantial detectable increased or elevated
amount or
concentration of analyte, and a "normal" (sometimes termed "control") patient
or population
is/are one(s) that exhibit(s) no substantial detectable increased or elevated
amount or
concentration of analyte. An "apparently normal subject" is one in which
analyte has not yet been
or currently is being assessed. The level of an analyte is said to be
"elevated" when the analyte is
normally undetectable (e.g., the normal level is zero, or within a range of
from about 25 to about
75 percentiles of normal populations), but is detected in a test sample, as
well as when the analyte
is present in the test sample at a higher than normal level. Thus, inter alia,
the disclosure provides
a method of screening for a subject having, or at risk of having, a particular
disease, disorder, or
condition. The method of assay can also involve the assay of other markers and
the like.
Accordingly, the methods described herein also can be used to determine
whether or not
a subject has or is at risk of developing a given disease, disorder or
condition. Specifically, such a
method can comprise the steps of:
(a) determining the concentration or amount in a test sample from a subject of
analyte
(or a fragment thereof) (e.g., using the methods described herein, or methods
known in the art);
and
(b) comparing the concentration or amount of analyte (or a fragment thereof)
determined
in step (a) with a predetermined level, wherein, if the concentration or
amount of analyte
determined in step (a) is favorable with respect to a predetermined level,
then the subject is
determined not to have or be at risk for a given disease, disorder or
condition. However, if the
concentration or amount of analyte determined in step (a) is unfavorable with
respect to the
153
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
predetermined level, then the subject is determined to have or be at risk for
a given disease,
disorder or condition.
Additionally, provided herein is method of monitoring the progression of
disease in a
subject. Optimally the method comprising the steps of:
(a) determining the concentration or amount in a test sample from a subject of
analyte;
(b) determining the concentration or amount in a later test sample from the
subject of
analyte; and
(c) comparing the concentration or amount of analyte as determined in step (b)
with the
concentration or amount of analyte determined in step (a), wherein if the
concentration or amount
determined in step (b) is unchanged or is unfavorable when compared to the
concentration or
amount of analyte determined in step (a), then the disease in the subject is
determined to have
continued, progressed or worsened. By comparison, if the concentration or
amount of analyte as
determined in step (b) is favorable when compared to the concentration or
amount of analyte as
determined in step (a), then the disease in the subject is determined to have
discontinued,
regressed or improved.
Optionally, the method further comprises comparing the concentration or amount
of
analyte as determined in step (b), for example, with a predetermined level.
Further, optionally the
method comprises treating the subject with one or more pharmaceutical
compositions for a period
of time if the comparison shows that the concentration or amount of analyte as
determined in step
(b), for example, is unfavorably altered with respect to the predetermined
level.
Still further, the methods can be used to monitor treatment in a subject
receiving
treatment with one or more pharmaceutical compositions. Specifically, such
methods involve
providing a first test sample from a subject before the subject has been
administered one or more
pharmaceutical compositions. Next, the concentration or amount in a first test
sample from a
subject of analyte is determined (e.g., using the methods described herein or
as known in the art).
After the concentration or amount of analyte is determined, optionally the
concentration or
amount of analyte is then compared with a predetermined level. If the
concentration or amount of
analyte as determined in the first test sample is lower than the predetermined
level, then the
subject is not treated with one or more pharmaceutical compositions. However,
if the
concentration or amount of analyte as determined in the first test sample is
higher than the
predetermined level, then the subject is treated with one or more
pharmaceutical compositions for
a period of time. The period of time that the subject is treated with the one
or more
154
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
pharmaceutical compositions can be determined by one skilled in the art (for
example, the period
of time can be from about seven (7) days to about two years, preferably from
about fourteen (14)
days to about one (1) year).
During the course of treatment with the one or more pharmaceutical
compositions,
second and subsequent test samples are then obtained from the subject. The
number of test
samples and the time in which said test samples are obtained from the subject
are not critical. For
example, a second test sample could be obtained seven (7) days after the
subject is first
administered the one or more pharmaceutical compositions, a third test sample
could be obtained
two (2) weeks after the subject is first administered the one or more
pharmaceutical
compositions, a fourth test sample could be obtained three (3) weeks after the
subject is first
administered the one or more pharmaceutical compositions, a fifth test sample
could be obtained
four (4) weeks after the subject is first administered the one or more
pharmaceutical
compositions, etc.
After each second or subsequent test sample is obtained from the subject, the
concentration or amount of analyte is determined in the second or subsequent
test sample is
determined (e.g., using the methods described herein or as known in the art).
The concentration
or amount of analyte as determined in each of the second and subsequent test
samples is then
compared with the concentration or amount of analyte as determined in the
first test sample (e.g.,
the test sample that was originally optionally compared to the predetermined
level). If the
concentration or amount of analyte as determined in step (c) is favorable when
compared to the
concentration or amount of analyte as determined in step (a), then the disease
in the subject is
determined to have discontinued, regressed or improved, and the subject should
continue to be
administered the one or pharmaceutical compositions of step (b). However, if
the concentration
or amount determined in step (c) is unchanged or is unfavorable when compared
to the
concentration or amount of analyte as determined in step (a), then the disease
in the subject is
determined to have continued, progressed or worsened, and the subject should
be treated with a
higher concentration of the one or more pharmaceutical compositions
administered to the subject
in step (b) or the subject should be treated with one or more pharmaceutical
compositions that are
different from the one or more pharmaceutical compositions administered to the
subject in step
(b). Specifically, the subject can be treated with one or more pharmaceutical
compositions that
are different from the one or more pharmaceutical compositions that the
subject had previously
received to decrease or lower said subject's analyte level.
Generally, for assays in which repeat testing may be done (e.g., monitoring
disease
progression and/or response to treatment), a second or subsequent test sample
is obtained at a
155
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
period in time after the first test sample has been obtained from the subject.
Specifically, a
second test sample from the subject can be obtained minutes, hours, days,
weeks or years after
the first test sample has been obtained from the subject. For example, the
second test sample can
be obtained from the subject at a time period of about 1 minute, about 5
minutes, about 10
minutes, about 15 minutes, about 30 minutes, about 45 minutes, about 60
minutes, about 2 hours,
about 3 hours, about 4 hours, about 5 hours, about 6 hours, about 7 hours,
about 8 hours, about 9
hours, about 10 hours, about 11 hours, about 12 hours, about 13 hours, about
14 hours, about 15
hours, about 16 hours, about 17 hours, about 18 hours, about 19 hours, about
20 hours, about 21
hours, about 22 hours, about 23 hours, about 24 hours, about 2 days, about 3
days, about 4 days,
about 5 days, about 6 days, about 7 days, about 2 weeks, about 3 weeks, about
4 weeks, about 5
weeks, about 6 weeks, about 7 weeks, about 8 weeks, about 9 weeks, about 10
weeks, about 11
weeks, about 12 weeks, about 13 weeks, about 14 weeks, about 15 weeks, about
16 weeks, about
17 weeks, about 18 weeks, about 19 weeks, about 20 weeks, about 21 weeks,
about 22 weeks,
about 23 weeks, about 24 weeks, about 25 weeks, about 26 weeks, about 27
weeks, about 28
weeks, about 29 weeks, about 30 weeks, about 31 weeks, about 32 weeks, about
33 weeks, about
34 weeks, about 35 weeks, about 36 weeks, about 37 weeks, about 38 weeks,
about 39 weeks,
about 40 weeks, about 41 weeks, about 42 weeks, about 43 weeks, about 44
weeks, about 45
weeks, about 46 weeks, about 47 weeks, about 48 weeks, about 49 weeks, about
50 weeks, about
51 weeks , about 52 weeks, about 1.5 years, about 2 years, about 2.5 years,
about 3.0 years, about
3.5 years, about 4.0 years, about 4.5 years, about 5.0 years, about 5.5.
years, about 6.0 years,
about 6.5 years, about 7.0 years, about 7.5 years, about 8.0 years, about 8.5
years, about 9.0
years, about 9.5 years or about 10.0 years after the first test sample from
the subject is obtained.
When used to monitor disease progression, the above assay can be used to
monitor the
progression of disease in subjects suffering from acute conditions. Acute
conditions, also known
as critical care conditions, refer to acute, life-threatening diseases or
other critical medical
conditions involving, for example, the cardiovascular system or excretory
system. Typically,
critical care conditions refer to those conditions requiring acute medical
intervention in a
hospital-based setting (including, but not limited to, the emergency room,
intensive care unit,
trauma center, or other emergent care setting) or administration by a
paramedic or other field-
based medical personnel. For critical care conditions, repeat monitoring is
generally done within
a shorter time frame, namely, minutes, hours or days (e.g., about 1 minute,
about 5 minutes, about
10 minutes, about 15 minutes, about 30 minutes, about 45 minutes, about 60
minutes, about 2
hours, about 3 hours, about 4 hours, about 5 hours, about 6 hours, about 7
hours, about 8 hours,
about 9 hours, about 10 hours, about 11 hours, about 12 hours, about 13 hours,
about 14 hours,
about 15 hours, about 16 hours, about 17 hours, about 18 hours, about 19
hours, about 20 hours,
156
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
about 21 hours, about 22 hours, about 23 hours, about 24 hours, about 2 days,
about 3 days, about
4 days, about 5 days, about 6 days or about 7 days), and the initial assay
likewise is generally
done within a shorter timeframe, e.g., about minutes, hours or days of the
onset of the disease or
condition.
The assays also can be used to monitor the progression of disease in subjects
suffering
from chronic or non-acute conditions. Non-critical care or, non-acute
conditions, refers to
conditions other than acute, life-threatening disease or other critical
medical conditions
involving, for example, the cardiovascular system and/or excretory system.
Typically, non-acute
conditions include those of longer-term or chronic duration. For non-acute
conditions, repeat
monitoring generally is done with a longer timeframe, e.g., hours, days,
weeks, months or years
(e.g., about 1 hour, about 2 hours, about 3 hours, about 4 hours, about 5
hours, about 6 hours,
about 7 hours, about 8 hours, about 9 hours, about 10 hours, about 11 hours,
about 12 hours,
about 13 hours, about 14 hours, about 15 hours, about 16 hours, about 17
hours, about 18 hours,
about 19 hours, about 20 hours, about 21 hours, about 22 hours, about 23
hours, about 24 hours,
about 2 days, about 3 days, about 4 days, about 5 days, about 6 days, about 7
days, about 2
weeks, about 3 weeks, about 4 weeks, about 5 weeks, about 6 weeks, about 7
weeks, about 8
weeks, about 9 weeks, about 10 weeks, about 11 weeks, about 12 weeks, about 13
weeks, about
14 weeks, about 15 weeks, about 16 weeks, about 17 weeks, about 18 weeks,
about 19 weeks,
about 20 weeks, about 21 weeks, about 22 weeks, about 23 weeks, about 24
weeks, about 25
weeks, about 26 weeks, about 27 weeks, about 28 weeks, about 29 weeks, about
30 weeks, about
31 weeks, about 32 weeks, about 33 weeks, about 34 weeks, about 35 weeks,
about 36 weeks,
about 37 weeks, about 38 weeks, about 39 weeks, about 40 weeks, about 41
weeks, about 42
weeks, about 43 weeks, about 44 weeks, about 45 weeks, about 46 weeks, about
47 weeks, about
48 weeks, about 49 weeks, about 50 weeks, about 51 weeks , about 52 weeks,
about 1.5 years,
about 2 years, about 2.5 years, about 3.0 years, about 3.5 years, about 4.0
years, about 4.5 years,
about 5.0 years, about 5.5. years, about 6.0 years, about 6.5 years, about 7.0
years, about 7.5
years, about 8.0 years, about 8.5 years, about 9.0 years, about 9.5 years or
about 10.0 years), and
the initial assay likewise generally is done within a longer time frame, e.g.,
about hours, days,
months or years of the onset of the disease or condition.
Furthermore, the above assays can be performed using a first test sample
obtained from a
subject where the first test sample is obtained from one source, such as
urine, serum or plasma.
Optionally, the above assays can then be repeated using a second test sample
obtained from the
subject where the second test sample is obtained from another source. For
example, if the first
test sample was obtained from urine, the second test sample can be obtained
from serum or
157
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
plasma. The results obtained from the assays using the first test sample and
the second test
sample can be compared. The comparison can be used to assess the status of a
disease or
condition in the subject.
Moreover, the present disclosure also relates to methods of determining
whether a
subject predisposed to or suffering from a given disease, disorder or
condition will benefit from
treatment. In particular, the disclosure relates to analyte companion
diagnostic methods and
products. Thus, the method of "monitoring the treatment of disease in a
subject" as described
herein further optimally also can encompass selecting or identifying
candidates for therapy.
Thus, in particular embodiments, the disclosure also provides a method of
determining
whether a subject having, or at risk for, a given disease, disorder or
condition is a candidate for
therapy. Generally, the subject is one who has experienced some symptom of a
given disease,
disorder or condition or who has actually been diagnosed as having, or being
at risk for, a given
disease, disorder or condition, and/or who demonstrates an unfavorable
concentration or amount
of analyte or a fragment thereof, as described herein.
The method optionally comprises an assay as described herein, where analyte is
assessed
before and following treatment of a subject with one or more pharmaceutical
compositions (e.g.,
particularly with a pharmaceutical related to a mechanism of action involving
analyte), with
immunosuppressive therapy, or by immunoabsorption therapy, or where analyte is
assessed
following such treatment and the concentration or the amount of analyte is
compared against a
predetermined level. An unfavorable concentration of amount of analyte
observed following
treatment confirms that the subject will not benefit from receiving further or
continued treatment,
whereas a favorable concentration or amount of analyte observed following
treatment confirms
that the subject will benefit from receiving further or continued treatment.
This confirmation
assists with management of clinical studies, and provision of improved patient
care.
It goes without saying that, while certain embodiments herein are advantageous
when
employed to assess a given disease, disorder or condition as discussed herein,
the assays and kits
can be employed to assess analyte in other diseases, disorders and conditions.
The method of
assay can also involve the assay of other markers and the like.
The method of assay also can be used to identify a compound that ameliorates a
given
disease, disorder or condition. For example, a cell that expresses analyte can
be contacted with a
candidate compound. The level of expression of analyte in the cell contacted
with the compound
can be compared to that in a control cell using the method of assay described
herein.
158
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
B. Kit
A kit for assaying a test sample for the presence, amount or concentration of
an analyte
(or a fragment thereof) in a test sample is also provided. The kit comprises
at least one
component for assaying the test sample for the analyte (or a fragment thereof)
and instructions for
assaying the test sample for the analyte (or a fragment thereof). The at least
one component for
assaying the test sample for the analyte (or a fragment thereof) can include a
composition
comprising an anti-analyte DVD-Ig (or a fragment, a variant, or a fragment of
a variant thereof),
which is optionally immobilized on a solid phase.
The kit can comprise at least one component for assaying the test sample for
an analyte
by immunoassay, e.g., chemiluminescent microparticle immunoassay, and
instructions for
assaying the test sample for an analyte by immunoassay, e.g., chemiluminescent
microparticle
immunoassay. For example, the kit can comprise at least one specific binding
partner for an
analyte, such as an anti-analyte, monoclonal/polyclonal antibody (or a
fragment thereof that can
bind to the analyte, a variant thereof that can bind to the analyte, or a
fragment of a variant that
can bind to the analyte) or an anti-analyte DVD-Ig (or a fragment, a variant,
or a fragment of a
variant thereof), either of which can be detectably labeled. Alternatively or
additionally, the kit
can comprise detectably labeled analyte (or a fragment thereof that can bind
to an anti-analyte,
monoclonal/polyclonal antibody or an anti-analyte DVD-Ig (or a fragment, a
variant, or a
fragment of a variant thereof)), which can compete with any analyte in a test
sample for binding
to an anti-analyte, monoclonal/polyclonal antibody (or a fragment thereof that
can bind to the
analyte, a variant thereof that can bind to the analyte, or a fragment of a
variant that can bind to
the analyte) or an anti-analyte DVD-1g (or a fragment, a variant, or a
fragment of a variant
thereof), either of which can be immobilized on a solid support. The kit can
comprise a calibrator
or control, e.g., isolated or purified analyte. The kit can comprise at least
one container (e.g.,
tube, microtiter plates or strips, which can be already coated with a first
specific binding partner,
for example) for conducting the assay, and/or a buffer, such as an assay
buffer or a wash buffer,
either one of which can be provided as a concentrated solution, a substrate
solution for the
detectable label (e.g., an enzymatic label), or a stop solution. Preferably,
the kit comprises all
components, i.e., reagents, standards, buffers, diluents, etc., which are
necessary to perform the
assay. The instructions can be in paper form or computer-readable form, such
as a disk, CD,
DVD, or the like.
Any antibodies, such as an anti-analyte antibody or an anti-analyte DVD-Ig, or
tracer can
incorporate a detectable label as described herein, such as a fluorophore, a
radioactive moiety, an
enzyme, a biotin/avidin label, a chromophore, a chemiluminescent label, or the
like, or the kit can
159
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
include reagents for carrying out detectable labeling. The antibodies,
calibrators and/or controls
can be provided in separate containers or pre-dispensed into an appropriate
assay format, for
example, into microtiter plates.
Optionally, the kit includes quality control components (for example,
sensitivity panels,
calibrators, and positive controls). Preparation of quality control reagents
is well-known in the art
and is described on insert sheets for a variety of immunodiagnostic products.
Sensitivity panel
members optionally are used to establish assay performance characteristics,
and further
optionally are useful indicators of the integrity of the immunoassay kit
reagents, and the
standardization of assays.
The kit can also optionally include other reagents required to conduct a
diagnostic assay
or facilitate quality control evaluations, such as buffers, salts, enzymes,
enzyme co-factors,
enzyme substrates, detection reagents, and the like. Other components, such as
buffers and
solutions for the isolation and/or treatment of a test sample (e.g.,
pretreatment reagents), also can
be included in the kit. The kit can additionally include one or more other
controls. One or more of
the components of the kit can be lyophilized, in which case the kit can
further comprise reagents
suitable for the reconstitution of the lyophilized components.
The various components of the kit optionally are provided in suitable
containers as
necessary, e.g., a microtiter plate. The kit can further include containers
for holding or storing a
sample (e.g., a container or cartridge for a urine sample). Where appropriate,
the kit optionally
also can contain reaction vessels, mixing vessels, and other components that
facilitate the
preparation of reagents or the test sample. The kit can also include one or
more instruments for
assisting with obtaining a test sample, such as a syringe, pipette, forceps,
measured spoon, or the
like.
If the detectable label is at least one acridinium compound, the kit can
comprise at least
one acridinium-9-carboxamide, at least one acridinium-9-carboxylate aryl
ester, or any
combination thereof. If the detectable label is at least one acridinium
compound, the kit also can
comprise a source of hydrogen peroxide, such as a buffer, a solution, and/or
at least one basic
solution. If desired, the kit can contain a solid phase, such as a magnetic
particle, bead, test tube,
microtiter plate, cuvette, membrane, scaffolding molecule, film, filter paper,
disc or chip.
C. Adaptation of Kit and Method
The kit (or components thereof), as well as the method of determining the
presence,
amount or concentration of an analyte in a test sample by an assay, such as an
immunoassay as
160
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
described herein, can be adapted for use in a variety of automated and semi-
automated systems
(including those wherein the solid phase comprises a microparticle), as
described, e.g., in U.S.
Patent Nos. 5,089,424 and 5,006,309, and as commercially marketed, e.g., by
Abbott
Laboratories (Abbott Park, IL) as ARCHITECTS.
Some of the differences between an automated or semi-automated system as
compared to
a non-automated system (e.g., ELISA) include the substrate to which the first
specific binding
partner (e.g., an anti-analyte, monoclonal/polyclonal antibody (or a fragment
thereof, a variant
thereof, or a fragment of a variant thereof) or an anti-analyte DVD-Ig (or a
fragment thereof, a
variant thereof, or a fragment of a variant thereof) is attached; either way,
sandwich formation
and analyte reactivity can be impacted), and the length and timing of the
capture, detection and/or
any optional wash steps. Whereas a non-automated format, such as an ELISA, may
require a
relatively longer incubation time with sample and capture reagent (e.g., about
2 hours), an
automated or semi-automated format (e.g., ARCHITECT , Abbott Laboratories) may
have a
relatively shorter incubation time (e.g., approximately 18 minutes for
ARCHITECTS). Similarly,
whereas a non-automated format, such as an ELISA, may incubate a detection
antibody, such as
the conjugate reagent, for a relatively longer incubation time (e.g., about 2
hours), an automated
or semi-automated format (e.g., ARCHITECTS) may have a relatively shorter
incubation time
(e.g., approximately 4 minutes for the ARCHITECTS).
Other platforms available from Abbott Laboratories include, but are not
limited to,
AxSYMS, IMx (see, e.g., U.S. Pat. No. 5,294,404), PRISMS, EIA (bead), and
QuantumTM H,
as well as other platforms. Additionally, the assays, kits and kit components
can be employed in
other formats, for example, on electrochemical or other hand-held or point-of-
care assay systems.
The present disclosure is, for example, applicable to the commercial Abbott
Point of Care (i-
STATC, Abbott Laboratories) electrochemical immunoassay system that performs
sandwich
immunoassays. Immunosensors and their methods of manufacture and operation in
single-use test
devices are described, for example in, U.S. Patent No. 5,063,081, U.S. Pat.
App. Pub. No.
2003/0170881, U.S. Pat. App. Pub. No. 2004/0018577, U.S. Pat. App. Pub. No.
2005/0054078,
and U.S. Pat. App. Pub. No. 2006/0160164.
In particular, with regard to the adaptation of an analyte assay to the I-STAT
system,
the following configuration is preferred. A microfabricated silicon chip is
manufactured with a
pair of gold amperometric working electrodes and a silver-silver chloride
reference electrode. On
one of the working electrodes, polystyrene beads (0.2 mm diameter) with
immobilized anti-
analyte, monoclonal/polyclonal antibody (or a fragment thereof, a variant
thereof, or a fragment
of a variant thereof) or anti-analyte DVD-Ig (or a fragment thereof, a variant
thereof, or a
161
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
fragment of a variant thereof), are adhered to a polymer coating of patterned
polyvinyl alcohol
over the electrode. This chip is assembled into an I-STATO cartridge with a
fluidics format
suitable for immunoassay. On a portion of the wall of the sample-holding
chamber of the
cartridge there is a layer comprising a specific binding partner for an
analyte, such as an anti-
analyte, monoclonal/polyclonal antibody (or a fragment thereof, a variant
thereof, or a fragment
of a variant thereof that can bind the analyte) or an anti-analyte DVD-Ig (or
a fragment thereof, a
variant thereof, or a fragment of a variant thereof that can bind the
analyte), either of which can
be detectably labeled. Within the fluid pouch of the cartridge is an aqueous
reagent that includes
p-aminophenol phosphate.
In operation, a sample suspected of containing an analyte is added to the
holding chamber
of the test cartridge, and the cartridge is inserted into the I-STAT reader.
After the specific
binding partner for an analyte has dissolved into the sample, a pump element
within the cartridge
forces the sample into a conduit containing the chip. Here it is oscillated to
promote formation of
the sandwich. In the penultimate step of the assay, fluid is forced out of the
pouch and into the
conduit to wash the sample off the chip and into a waste chamber. In the final
step of the assay,
the alkaline phosphatase label reacts with p-aminophenol phosphate to cleave
the phosphate
group and permit the liberated p-arninophenol to be electrochemically oxidized
at the working
electrode. Based on the measured current, the reader is able to calculate the
amount of analyte in
the sample by means of an embedded algorithm and factory-determined
calibration curve.
It further goes without saying that the methods and kits as described herein
necessarily
encompass other reagents and methods for carrying out the immunoassay. For
instance,
encompassed are various buffers such as are known in the art and/or which can
be readily
prepared or optimized to be employed, e.g., for washing, as a conjugate
diluent, microparticle
diluent, and/or as a calibrator diluent. An exemplary conjugate diluent is
ARCHITECT
conjugate diluent employed in certain kits (Abbott Laboratories, Abbott Park,
IL) and containing
2-(N-morpholino)ethanesulfonic acid (MES), a salt, a protein blocker, an
antimicrobial agent, and
a detergent. An exemplary calibrator diluent is ARCHITECTS human calibrator
diluent
employed in certain kits (Abbott Laboratories, Abbott Park, IL), which
comprises a buffer
containing MES, other salt, a protein blocker, and an antimicrobial agent.
Additionally, as
described in U.S. Patent Application No. 61/142,048 filed December 31, 2008,
improved signal
generation may be obtained, e.g., in an I-Stat cartridge format, using a
nucleic acid sequence
linked to the signal antibody as a signal amplifier.
162
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
EXEMPLIFICATION
Example 1: Design, Construction, and Analysis of a DVD-Ig
Example 1.1: Assays Used to Identify and Characterize Parent Antibodies and
DVD-Ig
The following assays were used throughout the Examples to identify and
characterize
parent antibodies and DVD-Ig, unless otherwise stated.
Example 1.1.1: Assays Used To Determine Binding and Affinity of Parent
Antibodies and
DVD-Ig for Their Target Antigen(s)
Example 1.1.1A: Direct Bind ELISA
Enzyme Linked Immunosorbent Assays to screen for antibodies that bind a
desired target
antigen were performed as follows. High bind ELISA plates (Corning Costar #
3369, Acton, MA)
were coated with 100A/well of 10 g/m1 of desired target antigen (R&D Systems,
Minneapolis,
MN) or desired target antigen extra-cellular domain / FC fusion protein (R&D
Systems,
Minneapolis, MN) or monoclonal mouse anti-polyHistidine antibody (R&D Systems
# MAB050,
Minneapolis, MN) in phosphate buffered saline (10X PBS, Abbott Bioresearch
Center, Media
Prep# MPS-073, Worcester, MA) overnight at 4 C. Plates were washed four times
with PBS
containing 0.02% Tween 20. Plates were blocked by the addition of 300 uL/well
blocking
solution (non-fat dry milk powder, various retail suppliers, diluted to 2% in
PBS) for 1/2 hour at
room temperature. Plates were washed four times after blocking with PBS
containing 0.02%
Tween 20.
Alternatively, one hundred microliters per well of 10 fig/m1 of Histidine
(His) tagged
desired target antigen (R&D Systems, Minneapolis, MN) was added to ELISA
plates coated with
monoclonal mouse anti-polyHistidine antibody as described above and incubated
for 1 hour at
room temperature. Wells were washed four times with PBS containing 0.02% Tween
20.
One hundred microliters of antibody or DVD-Ig preparations diluted in blocking
solution
as described above was added to the desired target antigen plate or desired
target antigen / FC
fusion plate or the anti-polyHistidine antibody / His tagged desired target
antigen plate prepared
as described above and incubated for 1 hour at room temperature. Wells were
washed four times
with PBS containing 0.02% Tween 20.
One hundred microliters of lOng/mL goat anti-human IgG ¨FC specific HRP
conjugated
antibody (Southern Biotech # 2040-05, Birmingham, AL) was added to each well
of the desired
target antigen plate or anti-polyHistidine antibody / Histidine tagged desired
target antigen plate.
163
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Alternatively, one hundred microliters of 10 ng/mL goat anti-human IgG ¨kappa
light chain
specific HRP conjugated antibody (Southern Biotech # 2060-05 Birmingham, AL)
was added to
each well of the desired target antigen / FC fusion plate and incubated for 1
hour at room
temperature. Plates were washed 4 times with PBS containing 0.02% Tween 20.
One hundred microliters of enhanced TMB solution (Neogen Corp. #308177, K
Blue,
Lexington, KY ) was added to each well and incubated for 10 minutes at room
temperature. The
reaction was stopped by the addition of 50 !..LL 1N sulphuric acid. Plates
were read
spectrophotometrically at a wavelength of 450 nm.
Table 3 contains a list of the antigens used in the Direct Bind Assay.
Table 4 contains the binding data expressed as EC50 in nM for those antibodies
and
DVD-Ig constructs tested in the Direct Bind ELISA assay.
In the Direct Bind ELISA, binding was sometimes not observed, probably because
the
antibody binding site on the target antigen was either "masked" or the antigen
is "distorted" when
coated to the plastic surface. The inability of a DVD-Ig to bind its target
may also be due to steric
limitation imposed on DVD-Ig by the Direct Bind ELISA format. The parent
antibodies and
DVD-Igs that did not bind in the Direct Bind ELISA format bound to target
antigen in other
ELISA formats, such as FACS, Biacore or bioassay. Non-binding of a DVD-Ig was
also restored
by adjusting the linker length between the two variable domains of the DVD-Ig,
as shown
previously.
Example 1.1.1.B: Capture ELISA
ELISA plates (Nunc, MaxiSorp, Rochester, NY) are incubated overnight at 4 C
with anti-
human Fc antibody (5 g/ml in PBS, Jackson Immunoreseareh, West Grove, PA).
Plates are
washed three times in washing buffer (PBS containing 0.05% Tween 20), and
blocked for 1 hour
at 25 C in blocking buffer (PBS containing 1% BSA). Wells are washed three
times, and serial
dilutions of each antibody or DVD-Ig in PBS containing 0.1% BSA are added to
the wells and
incubated at 25 C for 1 hour. The wells are washed three times, and
biotinylated antigen (2nM) is
added to the plates and incubated for 1 hour at 25 C. The wells are washed
three times and
incubated for 1 hour at 25 C with streptavidin-HRP (KPL #474-3000,
Gaithersburg, MD). The
wells are washed three times, and 100 p. 1 of ULTRA-TMB ELISA (Pierce,
Rockford, IL) is
added per well. Following color development the reaction is stopped with 1N
HCL and
absorbance at 450nM is measured.
164
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 1.1.1.C: Affinity Determination Using BIACORE Technology
Table 3: Reagent Used in Biacore Analyses
Assay Antigen Vendor Designation Vendor Catalog #
R&D
NGF Recombinant Human I3-NGF systems 256-GF
Recombinant Human TNF- R&D
TNFa a/TNESF1A systems 210-TA
R&D
SOST Recombinant Human SOST systems 1406-ST
ECD = Extracellular Domain
/FC = antigen/IgG FC domain fusion protein
BIACORE Methods:
The BIACORE assay (Biacore, Inc, Piscataway, NJ) determines the affinity of
antibodies
or DVD-Ig with kinetic measurements of on-rate and off-rate constants. Binding
of antibodies or
DVD-Ig to a target antigen (for example, a purified recombinant target
antigen) is determined by
surface plasmon resonance-based measurements with a Biacore 1000 or 3000
instrument
(Biacore AB, Uppsala, Sweden) using running 1113S-EP (10 mM HEPES [pH 7.4],
150 mM
NaC1, 3 mM EDTA, and 0.005% surfactant P20) at 25 C. All chemicals are
obtained from
Biacore AB (Uppsala, Sweden) or otherwise from a different source as
described in the text.
For example, approximately 5000 RU of goat anti-mouse IgG, (Fcy), fragment
specific polyclonal
antibody (Pierce Biotechnology Inc, Rockford, IL) diluted in 10 mM sodium
acetate (pH 4.5) is
directly immobilized across a CM5 research grade biosensor chip using a
standard amine
coupling kit according to manufacturer's instructions and procedures at 25
tig/ml. Unreacted
moieties on the biosensor surface are blocked with ethanolamine. Modified
carboxymethyl
dextran surface in flowcell 2 and 4 is used as a reaction surface. Unmodified
carboxymethyl
dextran without goat anti-mouse IgG in flow cell 1 and 3 is used as the
reference surface. For
kinetic analysis, rate equations derived from the 1:1 Langmuir binding model
are fitted
simultaneously to association and dissociation phases of all eight injections
(using global fit
analysis) with the use of Biaevaluation 4Ø1 software. Purified antibodies or
DVD-Ig are diluted
in HEPES-buffered saline for capture across goat anti-mouse IgG specific
reaction surfaces.
Antibodies or DVD-Ig to be captured as a ligand (25 jig/m1) are injected over
reaction matrices at
a flow rate of 5 til/min. The association and dissociation rate constants, kon
(M-1s-1) and kw (s-1)
are determined under a continuous flow rate of 25 1..tl/min. Rate constants
are derived by making
kinetic binding measurements at different antigen concentrations ranging from
10 ¨ 200 nM. The
equilibrium dissociation constant (M) of the reaction between antibodies or
DVD-Igs and the
target antigen is then calculated from the kinetic rate constants by the
following formula: KD =
165
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
koffikon. Binding is recorded as a function of time and kinetic rate constants
are calculated. In this
assay, on-rates as fast as 106M-ls-1 and off-rates as slow as 10-6 s-1 can be
measured.
Table 4: BIACORE Analysis of Parental Antibodies and DVD Constructs
N-Terminal C-Terminal kon koff KD
Variable Variable
Parent Antibody Domain Domain
or DVD-Ig ID (VD) (VD) (M-ls-1) (s-1) (M)
AB213 TNF (seq 2) 3.23E+06 1.08E-04 3.35E-11
AB022 SOST 9.68E+06 3.96E-04 4.08E-11
DVD1454 SOST 1.00E+07 8.60E-04 8.40E-11
DVD1454 TNF (seq 2) 2.30E+05 4.90E-05 2.10E-10
AB213 TNF (seq 2) 3.23E+06 1.08E-04 3.35E-11
AB020 NGF 6.06E+05 4.08E-06 6.73E-12
DVD1460 NGF 2.30E+06 2.00E-06 8.50E-13
DVD1460 TNF (seq 2) 1.40E+05 3.60E-05 2.60E-10
AB213 TNF (seq 2) 3.23E+06 1.08E-04 3.35E-11
DVD1466 LPA I TNF (seq 2) 1.60E+05 6.30E-05 3.90E-10
AB017 TNF (seq 1) 1.61E+06 1.29E-04 7.99E-11
DVD1471 TNF (seq 1) LPA 2.20E+06 3.80E-05 1.80E-11
DVD1472 LPA TNF (seq 1) 2.90E+05 1.60E-04 5.50E-10
AB217 TNF (seq 5) 1.85E+06 5.04E-05 2.72E-11
DVD1473 TNF (seq 5) PGE2 2.60E+06 4.40E-05 1.70E-11
AB217 TNF (seq 5) 1.85E+06 5.04E-05 2.72E-11
AB022 SOST 9.68E+06 3.96E-04 , 4.08E-11
DVD1475 TNF (seq 5) 2.70E+06 3.60E-05 1.30E-11
DVD1475 SOST 8.10E+05 7.30E-04 9.00E-10
AB217 TNF (seq 5) 1.85E+06 , 5.04E-05 2.72E-11
AB020 NGF 6.06E+05 4.08E-06 6.73E-12
DVD1477 TNF (seq 5) 2.50E+06 3.80E-05 1.50E-11
DVD1477 NGF 1.60E+05 <1.0E-06 <6.3E-12
DVD1478 NGF 2.40E+06 6.00E-06 2.50E-12
DVD1478 TNF (seq 5) 1.50E+05 1.50E-05 1.00E-10
AB217 TNF (seq 5) , 1.85E+06 5.04E-05 2.72E-11
DVD1479 TNF (seq 5) LPA 2.40E+06 4.10E-05 1.70E-11
DVD1480 LPA TNF (seq 5) 1.30E+05 3.40E-05 2.60E-10
AB218 TNF (seq 6) , 1.79E+06 5.15E-05 2.88E-11
DVD1481 TNF (seq 6) PGE2 2.60E+06 2.40E-05 8.90E-12
DVD1482 PGE2 TNF (seq 6) 1.60E+05 1.50E-05 9.20E-11
AB218 TNF (seq 6) 1.79E+06 5.15E-05 2.88E-11
AB022 SOST 9.68E+06 3.96E-04 4.08E-11
DVD1483 TNF (seq 6) 2.80E+06 3.30E-05 1.20E-11
DVD1483 SOST 1.10E+06 8.30E-04 7.80E-10
AB218 TNF (seq 6) 1.79E+06 5.15E-05 2.88E-11
AB020 NGF 6.06E+05 4.08E-06 6.73E-12
DVD1485 TNF (seq 6) 2.10E+06 3.60E-05 1.70E-11
DVD1485 NGF 1.00E+05 <1.0E-06 <1.0E-11
AB218 TNF (seq 6) 1.79E+06 5.15E-05 2.88E-11
DVD1487 TNF (seq 6) LPA 1.80E+06 2.60E-05 1.40E-11
166
,
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
N-Terminal C-Terminal kon koff Kr.
Variable Variable
Parent Antibody Domain Domain
or DVD-Ig ID (VD) (VD) (M-is-1) (s-1) (M)
DVD1488 LPA TNF (seq 6) 2.30E+05 1.90E-05 840E-11
AB213 TNF (seq 2) 3.23E+06 1.08E-04 3.35E-11
DVD1490 PGE2 TNF (seq 2) 5.00E+05 6.10E-05 1.2E-10
AB213 TNF (seq 2) 3.23E+06 , 1.08E-04 3.35E-11
AB022 SOST 9.68E+06 3.96E-04 4.08E-11
DVD1495 TNF (seq 2) 2.50E+06 5.40E-05 2.10E-11
DVD1495 SOST , 4.40E+04 9.50E-04 2.20E-08
AB213 TNF (seq 2) 3.23E+06 1.08E-04 3.35E-11
AB020 NGF 6.06E+05 4.08E-06 6.73E-12
DVD1502 NGF 2.50E+06 <1.0E-06 <4.0E-13
DVD1502 TNF (seq 2) 1.80E+05 5.50E-05 3.00E-10
AB213 TNF (seq 2) , 3.23E+06 , 1.08E-04 3.35E-11
DVD1508 LPA TNF (seq 2) 2.50E+05 5.00E-05 2.00E-10
AB017 TNF (seq 1) 1.61E+06 1.29E-04 7.99E-11
DVD1514 LPA TNF (seq 1) 3.30E+05 6.70E-05 2.00E-10
AB217 TNF (seq 5) 1.85E+06 5.04E-05 2.72E-11
DVD1515 TNF (seq 5) PGE2 3.10E+06 3.70E-05 1.20E-11
AB217 TNF (seq 5) 1.85E+06 5.04E-05 2.72E-11
AB022 SOST 9.68E+06 3.96E-04 4.08E-11
DVD1517 TNF (seq 5) 2.60E+06 4.70E-05 1.80E-11
DVD1517 SOST 8.90E+04 5.00E-04 , 5.50E-09
AB217 TNF (seq 5) 1.85E+06 5.04E-05 2.72E-11
AB020 NGF 6.06E+05 4.08E-06 6.73E-12
DVD1519 TNF (seq 5) 2.10E+06 4.20E-05 2.00E-11
DVD1519 NGF 1.10E+05 <1.0E-06 <1.0E-11
DVD1520 NGF 2/0E+06 9.30E-06 3.40E-12
DVD1520 TNF (seq 5) 1.10E+06 4.40E-06 3.80E-12
' AB218 TNF (seq 6) 1.79E+06 5.15E-05 2.88E-11
DVD1523 TNF (seq 6) PGE2 2.20E+06 2.00E-05 9.10E-12
DVD1524 PGE2 TNF (seq 6) 1.00E+05 1.70E-06 1.70E-11
AB218 TNF (seq 6) 1.79E+06 5.15E-05 2.88E-11
AB022 SOST 9.68E+06 3.96E-04 4.08E-11
DVD1525 TNF (seq 6) 2.00E+06 3.00E-05 1.50E-11
DVD1525 SOST 9.10E+04 8.90E-04 9.80E-09
AB218 TNF (seq 6) 1.79E+06 5.15E-05 2.88E-11
AB020 NGF 6.06E+05 4.08E-06 6.73E-12
DVD1527 TNF (seq 6) 2.30E-F06 4.00E-05 1.70E-11
DVD1527 NGF 1.00E+05 <1.0E-06 <1.0E-11
= DVD1528 NGF 2.40E+06 7.30E-06 3.00E-12
DVD1528 TNF (seq 6) 1.70E+05 9.40E-06 5.60E-11
AB218 TNF (seq 6) 1.79E-F06 5.15E-05 2.88E-11
DVD1529 TNF (seq 6) LPA 2.50E+06 2.60E-05 1.00E-11
DVD1530 LPA TNF (seq 6) 8.70E+04 1.60E-06 1.90E-11
167
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 1.1.2: Assays Used To Determine the Functional Activity Of Parent
Antibodies And DVD-Ig
Example 1.1.2.A: Cytokine Bioassay
The ability of an anti-cytokine or an anti-growth factor parent antibody or
DVD-Ig
containing anti-cytokine or anti-growth factor sequences to inhibit or
neutralize a target cytokine
or growth factor bioactivity is analyzed by determining the inhibitory
potential of the antibody or
DVD-Ig. For example, the ability of an anti-IL-4 antibody to inhibit IL-4
mediated IgE
production may be used. For example, human naive B cells are isolated from
peripheral blood,
respectively, buffy coats by Ficoll-paque density centrifugation, followed by
magnetic separation
with MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany) specific for
human sIgD
FITC labeled goat F(ab)2 antibodies followed by anti-FITC MACS beads.
Magnetically sorted
naive B cells are adjusted to 3 x 105 cells per ml in XV15 and plated out in
100 piper well of 96-
well plates in a 6 x 6 array in the center of the plate, surrounded by PBS
filled wells during the 10
days of culture at 37 C in the presence of 5% CO2. One plate each is prepared
per antibody to be
tested, comprising 3 wells each of un-induced and induced controls and
quintuplicate repeats of
antibody titrations starting at 7pg/m1 and running in 3-fold dilution down to
29 ng/ml final
concentrations added in 500 four times concentrated pre-dilution. To induce
IgE production,
rhIL-4 at 20 ng/ml plus anti-CD40 monoclonal antibody (Novartis, Basel,
Switzerland) at 0.5
pg/m1 final concentrations in 50 1 each are added to each well, and IgE
concentrations are
determined at the end of the culture period by a standard sandwich ELISA
method.
Example 1.1.2.B: Cytokine Release Assay
The ability of a parent antibody or DVD-Ig to cause cytokine release is
analyzed.
Peripheral blood is withdrawn from three healthy donors by venipuncture into
heparized
vacutainer tubes. Whole blood is diluted 1:5 with RPMI-1640 medium and placed
in 24-well
tissue culture plates at 0.5 mL per well. The anti-cytokine antibodies (e.g.,
anti-IL-4) are diluted
into RPMI-1640 and placed in the plates at 0.5 mL/well to give final
concentrations of 200, 100,
50, 10, and 1 pg/mL. The final dilution of whole blood in the culture plates
is 1:10. LPS and PHA
are added to separate wells at 2 g/mL and 5 g/mL final concentration as a
positive control for
cytokine release. Polyclonal human IgG is used as negative control antibody.
The experiment is
performed in duplicate. Plates are incubated at 37 C at 5% CO2. Twenty-four
hours later the
contents of the wells are transferred into test tubes and spun for 5 minutes
at 1200 rpm. Cell-free
supernatants are collected and frozen for cytokine assays. Cells left over on
the plates and in the
tubes are lysed with 0.5 mL of lysis solution, and placed at ¨20 C and thawed.
0.5 mL of medium
is added (to bring the volume to the same level as the cell-free supernatant
samples) and the cell
preparations are collected and frozen for cytokine assays. Cell-free
supernatants and cell lysates
168
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
are assayed for cytokine levels by ELISA, for example, for levels of IL-8, IL-
6, IL-113, IL-1 RA, or
TNF-a.
Example 1.1.2.C: Cytokine Cross-Reactivity Study
The ability of an anti-cytokine parent antibody or DVD-Ig directed to a
cytokine(s) of
interest to cross react with other cytokines is analyzed. Parent antibodies or
DVD-Ig are
immobilized on a Biacore biosensor matrix. An anti-human Fe mAb is covalently
linked via free
amine groups to the dextran matrix by first activating carboxyl groups on the
matrix with 100mM
N-hydroxysuccinimide (NHS) and 400mM N-Ethyl-N'-(3-dimethylaminopropy1)-
carbodiimide
hydrochloride (EDC). Approximately 501.1L of each antibody or DVD-Ig
preparation at a
concentration of 25Itg/mL, diluted in sodium acetate, pH 4.5, is injected
across the activated
biosensor and free amines on the protein are bound directly to the activated
carboxyl groups.
Typically, 5000 Resonance Units (RU's) are immobilized. Unreacted matrix EDC-
esters are
deactivated by an injection of 1 M ethanolamine. A second flow cell is
prepared as a reference
standard by immobilizing human IgGl/K using the standard amine coupling kit.
SPR
measurements are performed using the CM biosensor chip. All antigens to be
analyzed on the
biosensor surface are diluted in FIBS-EP running buffer containing 0.01% P20.
To examine the cytokine binding specificity, excess cytokine of interest
(100nM, e.g.,
soluble recombinant human) is injected across the anti-cytokine parent
antibody or DVD-Ig
immobilized biosensor surface (5 minute contact time). Before injection of the
cytokine of
interest and immediately afterward, HBS-EP buffer alone flows through each
flow cell. The net
difference in the signals between the baseline and the point corresponding to
approximately 30
seconds after completion of cytokine injection are taken to represent the
final binding value.
Again, the response is measured in Resonance Units. Biosensor matrices are
regenerated using
10mM }ICI before injection of the next sample where a binding event is
observed, otherwise
running buffer was injected over the matrices. Human cytokines (e.g., IL-la,
IL-10, IL-2, IL-3,
IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-16,
IL-17, IL-18, IL-19,
= IL-20, IL-22, IL-23, IL-27, TNF-a, TNF43, and IFN-y, for example) are
also simultaneously
injected over the immobilized mouse IgGI/K reference surface to record any
nonspecific binding
background. By preparing a reference and reaction surface, Biacore can
automatically subtract
the reference surface data from the reaction surface data in order to
eliminate the majority of the
refractive index change and injection noise. Thus, it is possible to ascertain
the true binding
response attributed to an anti-cytokine antibody or DVD-Ig binding reaction.
When a cytokine of interest is injected across immobilized anti-cytokine
antibody,
significant binding is observed. 10mM HCI regeneration completely removes all
non-covalently
169
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
associated proteins. Examination of the sensorgram shows that immobilized anti-
cytokine
antibody or DVD-Ig binding to soluble cytokine is strong and robust. After
confirming the
expected result with the cytokine of interest, the panel of remaining
recombinant human
cytokines is tested, for each antibody or DVD-Ig separately. The amount of
anti-cytokine
antibody or DVD-Ig bound or unbound cytokine for each injection cycle is
recorded. The results
from three independent experiments are used to determine the specificity
profile of each antibody
or DVD-Ig. Antibodies or DVD-Ig with the expected binding to the cytokine of
interest and no
binding to any other cytokine are selected.
Example 1.1.2.D: Tissue Cross Reactivity
Tissue cross reactivity studies are done in three stages, with the first stage
including
cryosections of 32 tissues, second stage including up to 38 tissues, and the
3rd stage including
additional tissues from 3 unrelated adults as described below. Studies are
done typically at two
dose levels.
Stage 1: Cryosections (about 5 gm) of human tissues (32 tissues (typically:
Adrenal
Gland, Gastrointestinal Tract, Prostate, Bladder, Heart, Skeletal Muscle,
Blood Cells, Kidney,
Skin, Bone Marrow, Liver, Spinal Cord, Breast, Lung, Spleen, Cerebellum, Lymph
Node, Testes,
Cerebral Cortex, Ovary, Thymus, Colon, Pancreas, Thyroid, Endothelium,
Parathyroid, Ureter,
Eye, Pituitary, Uterus, Fallopian Tube and Placenta) from one human donor
obtained at autopsy
or biopsy) are fixed and dried on object glass. The peroxidase staining of
tissue sections is
performed, using the avidin-biotin system.
Stage 2: Cryosections (about 5 gm) of human tissues 38 tissues (including
adrenal,
blood, blood vessel, bone marrow, cerebellum, cerebrum, cervix, esophagus,
eye, heart, kidney,
large intestine, liver, lung, lymph node, breast mammary gland, ovary,
oviduct, pancreas,
parathyroid, peripheral nerve, pituitary, placenta, prostate, salivary gland,
skin, small intestine,
spinal cord, spleen, stomach, striated muscle, testis, thymus, thyroid,
tonsil, ureter, urinary
bladder, and uterus) from 3 unrelated adults obtained at autopsy or biopsy)
are fixed and dried on
object glass. The peroxidase staining of tissue sections is performed, using
the avidin-biotin
system.
Stage 3: Cryosections (about 5 gm) of cynomolgus monkey tissues (38 tissues
(including
adrenal, blood, blood vessel, bone marrow, cerebellum, cerebrum, cervix,
esophagus, eye, heart,
kidney, large intestine, liver, lung, lymph node, breast mammary gland, ovary,
oviduct, pancreas,
parathyroid, peripheral nerve, pituitary, placenta, prostate, salivary gland,
skin, small intestine,
spinal cord, spleen, stomach, striated muscle, testis, thymus, thyroid,
tonsil, ureter, urinary
170
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
bladder, and uterus) from 3 unrelated adult monkeys obtained at autopsy or
biopsy) are fixed and
dried on object glass. The peroxidase staining of tissue sections is
performed, using the avidin-
biotin system.
The antibody or DVD-Ig is incubated with the secondary biotinylated anti-human
IgG
and developed into immune complex. The immune complex at the final
concentrations of 2 and
Ag/mL of antibody or DVD-Ig is added onto tissue sections on object glass and
then the tissue
sections are reacted for 30 minutes with a avidin-biotin-peroxidase kit.
Subsequently, DAB (3,3'-
diaminobenzidine), a substrate for the peroxidase reaction, is applied for 4
minutes for tissue
staining. Antigen-Sepharose beads are used as positive control tissue
sections. Target antigen and
10 human serum blocking studies serve as additional controls. The immune
complex at the final
concentrations of 2 and 10 ,g/mL of antibody or DVD-Ig is pre-incubated with
target antigen
(final concentration of 100 gimp or human serum (final concentration 10%) for
30 minutes, and
then added onto the tissue sections on object glass and then the tissue
sections are reacted for 30
minutes with a avidin-biotin-peroxidase kit. Subsequently, DAB (3,3'-
diaminobenzidine), a
substrate for the peroxidase reaction, is applied for 4 minutes for tissue
staining.
Any specific staining is judged to be either an expected (e.g., consistent
with antigen
expression) or unexpected reactivity based upon known expression of the target
antigen in
question. Any staining judged specific is scored for intensity and frequency.
The tissue staining
between stage 2 (human tissue) and stage 3 (cynomolgus monkey tissue) is
either judged to be
similar or different.
Example 1.1.2.D: Neutralization of huTNFa Neutralization of huTNFa
L929 cells were grown to a semi-confluent density and harvested using 0.05%
tryspin
(Gibco#25300). The cells were washed with PBS, counted and resuspended at 1E6
cells/mL in
assay media containing 4 Ag/mL actinomycin D. The cells were seeded in a 96-
well plate
(Costar#3599) at a volume of 50 AL and 5E4 cells/well. The DVD-IgTM and
control IgG were
diluted to a 4x concentration in assay media and serial 1:3 dilutions were
prepared. The huTNFct
was diluted to 400 pg/mL in assay media. An antibody sample (200 AL) was added
to the
huTNFa. (200 AL) in a 1:2 dilution scheme and allowed to incubate for 0.5 hour
at room
temperature.
The DVD-IgTM / huTNFcc solution was added to the plated cells at 100 AL for a
final
concentration of 100 pg/mL huTNFa and 25 nM ¨ 0.00014 nM DVD-IgTM. The plates
were
incubated for 20 hours at 37 C, 5 % CO2. To quantitate viability, 100 AL was
removed from the
wells and 10 AL of WST-1 reagent (Roche cat# 11644807001) was added. Plates
were incubated
under assay conditions for 3.5 hours, centrifuged at 500 xg and 75 AL
supernatant transferred to
171
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
an ELISA plate (Costar cat#3369). The plates were read at OD 420-600 nm on a
Spectromax 190
ELISA plate reader. An average EC50 from several assays is included in Table
5.
Table 5: HuTNFa Neutralization Assay With huTNFa Parent Antibody and DVD-Ig
Constructs
Parent N-terminal C-terminal
Antibody Variable Variable N-terminal VD C-terminal VD
or DVD-Ig Domain Domain TNFa Neutralization TNFa NeutralizationAssay
ID (VD) (VD) Assay EC50 nM EC50 nM
AB017 TNF (seq 1) 0.0461
AB213 TNF (seq 2) 0.124
AB217 TNF (seq 5) 0.0762
AB218 TNF (seq 6) 0.045
DVD1448 PGE2 TNF (seq 2) - 1.388
DVD1454 SOST TNF (seq 2) - 0.951
DVD1466 LPA TNF (seq 2) - 29.92
DVD1471 TNF (seq 1) LPA 0.0153 -
DVD1472 LPA TNF (seq 1) - 0.732
DVD1473 TNF (seq 5) PGE2 0.011 -
DVD1474 PGE2 TNF (seq 5) - 0.273
DVD1475 TNF (seq 5) SOST 0.002 -
DVD1476 SOST TNF (seq 5) - 0.139
DVD1477 TNF (seq 5) NGF 0.009 -
DVD1478 NGF TNF (seq 5) - 0.329
DVD1479 TNF (seq 5) LPA 0.012 -
DVD1480 LPA TNF (seq 5) - 0.183
DVD1481 TNF (seq 6) PGE2 0.011 -
DVD1482 PGE2 TNF (seq 6) 1.453
DVD1483 TNF (seq 6) SOST 0.012
DVD1484 SOST TNF (seq 6) - 0.219
DVD1485 TNF (seq 6) NGF 0.011 -
DVD1486 NGF TNF (seq 6) - 0.363
DVD1487 TNF (seq 6) LPA 0.007 -
DVD1488 LPA TNF (seq 6) - 0.894
DVD1490 PGE2 TNF (seq 2) - 0.283
DVD1495 TNF (seq 2) SOST 0.0038 -
DVD1496 SOST TNF (seq 2) - 0.085
DVD1501 TNF (seq 2) NGF 0.1952 -
DVD1502 NGF TNF (seq 2) - 0.4596
DVD1507 TNF (seq 2) LPA 0.1327 -
DVD1508 LPA TNF (seq 2) - 0.234
DVD1514 LPA TNF (seq 1) - 0.407
DVD1516 PGE2 TNF (seq 5) - 8.709
DVD1517 TNF (seq 5) SOST 0.0012 -
DVD1518 SOST TNF (seq 5) - 0.89
DVD1519 TNF (seq 5) NGF 0.0079 -
DVD1520 NGF TNF (seq 5)- 7.21
172
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Parent N-terminal C-terminal
Antibody Variable Variable N-terminal VD C-terminal VD
or DVD-Ig Domain Domain TNFa Neutralization TNFa NeutralizationAssay
ID (VD) (VD) Assay EC50 nM EC50 nM
DVD1522 LPA TNF (seq 5) 4.179
DVD1524 PGE2 TNF (seq 6) 0.963
DVD1525 TNF (seq 6) SOST 0.0029
DVD1526 SOST TNF (seq 6) 0.059
DVD1527 TNF (seq 6) NGF 0.0029
DVD1528 NGF TNF (seq 6) 0.197
DVD1530 LPA TNF (seq 6) 0.571
All DVD-Ig proteins containing VDs from AB017, AB213, AB217, and AB218 in
either
the N-terminal or C-terminal position showed neutralization in the TNFa
inhbition assay.
Example 1.1.2.E: Inhibition of NGF in TF-1 Cell Proliferation bioassay
TF-1 cells were cultured in RPMI 1640 (Invitrogen) +10% Fetal Bovine Serum
(Hyclone) +L-glutamine (Invitrogen) + rhu GM-CSF (R&D Systems). TF-1 cells
were serum
starved for 24 hours in RPMI 1640 + L-glutamine at 1 x 105 cells per mL and
incubated overnight
at 37 C, 5% CO2. The day of the experiment, TF- 1 cells were plated in opaque
walled 96-well
plates at 2.5 x 104 cells per well in a 100 IL volume + assay media (RPMI-
1640 +L-glutamine +
4% FBS) The cells were stimulated by adding NGF/DVD-Ig or antibody thereto.
The DVD-IgTm
and control IgG were diluted to a 4x concentration in assay media and serial
1:5 dilutions were
performed. The huNGF was diluted to 8 ng/mL in assay media. The DVD-IgTm (50
I) and
huNGF (50 L) solutions were added to the plate for a final concentration of 2
nWmL huNGF
and 25 nM ¨ 0.000003 nM DVD-IgTm. The plates were incubated for 72 hours at 37
C, 5 % CO2.
To quantitate viability, a Cell Titer Glo kit (Promega cat# TB288) was used
(100 p.I of solution
added to each well following manufacturer's instructions). The plates were
read by measuring
luminescence on a Spectromax 190 ELISA plate reader. An average EC50 from
several assays is
included in Table 6.
Table 6: NGF Inhibition Assay With NGF Parent Antibodies and DVD-Ig Constructs
N-Terminal C-Terminal
Parent Variable Variable N-Terminal VD C-Terminal VD
Antibody or Domain Domain NGF Inhibition NGF Inhibition
DVD-Ig ID (VD) (VD) Assay EC50 nM Assay EC50 nM
AB020 NGF 0.003
DVD1460 NGF TNF (seq 2) 1.695
DVD1477 TNF (seq 5) NGF 0.038
DVD1478 NGF TNF (seq 5) 0.019
173
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
N-Terminal C-Terminal
Parent Variable Variable N-Terminal VD C-Terminal VD
Antibody or Domain Domain NGF Inhibition NGF Inhibition
DVD-Ig ID (VD) (VD) Assay EC50 nM Assay EC50 nM
DVD1485 TNF (seq 6) NGF 0.053
DVD1486 NGF TNF (seq 6) 0.076
DVD1501 TNF (seq 2) NGF ND
DVD1502 NGF TNF (seq 2) 0.028
DVD1519 TNF (seq 5) NGF 1.946
DVD1520 NGF TNF (seq 5) 0.025
DVD1527 TNF (seq 6) NGF 0.350
DVD1528 NGF TNF (seq 6) 0.018
All DVD-Ig proteins containing VDs from AB020 in either the N-terminal or C-
terminal
position showed neutralization in the NGF inhbition assay.
Example 1.2.2.F: Inhibition of Sclerostin Activity in the Wnt-1/Luciferase
Double Stable
HEK Clone #14
HEK 293A cells were stably transfected with TopFlash plasmid (TCF reporter
plasmid,
Cell Signaling catalog #21-170, lot# 26217) and infected with Wnt-1 lentivirus
(Origene Cat#
SC303644), resulting in clones that co-express luciferase and Wnt-1. One
double stable clone (
#14) has been maintained in culture medium: DMEM (Invitrogen Cat#11965-092)
with 10%
Qualified FBS (Invitrogen Cat#26140-079), Pen-Strep (Invitrogen Cat#15140-
122), L-glutamine
(Invitrogen Cat#25030-081 2mM final), Sodium Pyruvate (Invitrogen Cat#11360-
070 final
1mM) and 5R/m1Puromycin (Invivogen Cat#ant-pr-1) in T75 flasks until 80-90%
confluent on
day of assay. Assay is performed in assay medium: culture medium without
puromycin. Human
Sclerostin (Abbott PR-1261069 Lot#1769536 1.06mg/mL) was aliquoted into 100
and stored
frozen at -80 C. On day 1, clone #14 cells were plated at 10,000 cells per
well in 50u1 assay
medium in black-sided, clear bottomed tissue culture treated 96 well plates
(Costar #3603) and
incubated at 37 C overnight (20-24 hours). On day 2, the Sclerostin stock is
diluted to 200nM
(4X) in the assay medium. Anti-Sclerostin antibodies were diluted to 4X
(typically 600nM) in
assay medium. Media was removed and replaced with 50p1/well of fresh assay
medium. Cells
were next incubated with 25 1 of Sclerostin at 200nM (4X) for 1 hour. Anti-
Sclerostin antibodies
(4X cone, 25111) were then added to cells and plates were incubated overnight
at 37 C (20-24
hours). The final volume was 100 I. On day 3, cells were washed once with 2000
of PBS (RT).
A Promega Luciferase Kit fiE1501 was used for cell lysis and Luciferase read
out. Briefly, 5X
cell lysis reagent (Promega, cat #E153A) was diluted with milliQ water to IX
and 20111 was
added to each well. To ensure a complete lysis, plate was rotated 500rpm for
20 minutes. 100111
of Luciferase assay reagent (1 vial cat #E151A substrate + 10m1 cat #E152A
assay buffer) was
174
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
added to each well. The plate were read on a TopCount machine (Program:
Luciferase 96, Assay
17: 1 sec/well read). Table 7 represents those antibodies or DVD-Igs that were
able to inhibit
SOST at 150 nM.
Table 7: SOST Inhibition Assay With SOST Parent Antibodies and DVD-Ig
Constructs
Parent N-Terminal C-Terminal
Antibody Variable Variable N-Terminal VD
or DVD- Domain Domain SOST Inhibition C-Terminal VD
Ig ID (VD) (VD) Assay SOST Inhibition Assay
AB022 SOST
DVD145
4 TNF (seq 4) SOST
DVD147 SOST
5 TNF (seq 5)
DVD147 SOST
6 TNF (seq 5)
DVD148 SOST
3 TNF (seq 6) +-
DVD148 SOST
4 TNF (seq 6)
DVD149 SOST
5 TNF (seq 2)
DVD149 SOST
6 TNF (seq 2)
DVD151 SOST
7 TNF (seq 5)
DVD151 SOST
8 TNF (seq 5) +
DVD152 SOST
6 TNF (seq 6)
All DVD-Igs containing VDs from AB022 in either the N-terminal or C-terminal
position
showed neutralization in the SOST inhbition assay.
Example 1.1.2.G: Growth Inhibitory Effect of a Tumor Receptor Monoclonal
Antibody or
DVD-Igs In Vitro
Tumor receptor monoclonal antibodies or DVD-Igs diluted in D-PBS-BSA
(Dulbecco's
phosphate buffered saline with 0.1%BSA) 201AL are added to human tumor cells
at final
concentrations of 0.01 j.tg/mL-100 pg/mL in 180uL. The plates are incubated at
37 C in a
humidified, 5% CO2 atmosphere for 3 days. The number of live cells in each
well is quantified
using MTS reagents according to the manufacturer's instructions (Promega,
Madison, WI) to
determine the percent of tumor growth inhibition. Wells without antibody
treatment are used as
controls of 0% inhibition whereas wells without cells are considered to show
100% inhibition.
175
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 1.1.2.H: Tumoricidal Effect of A Parent or DVD-Ig Antibody In Vitro
Parent antibodies or DVD-Ig that bind to target antigens on tumor cells may be
analyzed
for tumoricidal activity. Briefly, parent antibodies or DVD-Ig are diluted in
D-PBS-BSA
(Dulbecco's phosphate buffered saline with 0.1%BSA) and added to human tumor
cells at final
concentrations of 0.01 pg/mL to 100 pg/mL 2001AL. The plates are incubated at
37 C in a
humidified, 5% CO2 atmosphere for 3 days. The number of live cells in each
well is quantified
using MTS reagents according to the manufacturer's instructions (Promega,
Madison, WI) to
determine the percent of tumor growth inhibition. Wells without antibody
treatment are used as
controls of 0% inhibition whereas wells without cells were considered to show
100% inhibition.
For assessment of apoptosis, caspase-3 activation is determined by the
following
protocol: antibody-treated cells in 96 well plates are lysed in 120 IA of lx
lysis buffer (1.67mM
Hepes, pH 7.4, 7mM KCI, 0.83mM MgCl2, 0.11mM EDTA, 0.11mM EGTA, 0.57% CHAPS,
1mM DTT, lx protease inhibitor cocktail tablet; EDTA-free; Roche
Pharmaceuticals, Nutley, NJ)
at room temperature with shaking for 20 minutes. After cell lysis, 80 id of a
caspase-3 reaction
buffer (48mM Hepes, pH 7.5, 252mM sucrose, 0.1% CHAPS, 4mM DTT, and 20 uM Ac-
DEVD-
AMC substrate; Biomol Research Labs, Inc., Plymouth Meeting, PA) is added and
the plates are
incubated for 2 hours at 37 C. The plates are read on a 1420 VICTOR Multilabel
Counter (Perkin
Elmer Life Sciences, Downers Grove, IL) using the following settings:
excitation= 360/40,
emission= 460/40. An increase of fluorescence units from antibody-treated
cells relative to the
isotype antibody control-treated cells is indicative of apoptosis.
Example 1.1.2.1: Inhibition of Cell Proliferation by Parent Antibody and DVD-
Ig
Constructs
U87-MG human glioma tumor cells are plated at 2,000 cells/well in 100 1 in 96-
well
dishes in RPMI medium supplemented with 5% fetal bovine serum, and incubated
at 37 C, 5%
CO2 overnight. The following day the cells are treated with serial dilutions
of antibody or DVD-
Igs (0.013 nM to 133 nM dose range), and incubated at 37 C in a humidified,
5% CO2
atmosphere for 5 days. Cell survival/proliferation is measured indirectly by
assessing ATP levels
using an ATPlite kit (Perkin Elmer, Waltham, MA) according to the
manufacturer's instructions.
Example 1.1.2.J: VEGF Parent Antibody and DVD-Ig Constructs Prevent VEGF165
Interaction with VEGFR1
ELISA plates (Nunc, MaxiSorp, Rochester, NY) are incubated overnight at 4 C
with100
IA PBS containing recombinant VEGFR1 extra-cellular domain-Fe fusion protein
(54g/ml, R&D
176
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
systems, Minneapolis, MN). Plates are washed three times in washing buffer
(PBS containing
0.05% Tween 20), and blocked for 1 hour at 25 C in blocking buffer (PBS
containing 1% BSA).
Serial dilutions of each antibody/DVD-Ig in PBS containing 0.1% BSA are
incubated with 50[11
of 2nM biotinylated VEGF for 1 hour at 25 C. The antibody/DVD-Ig-biotinylated
VEGF
mixtures (100 1) are then added to the VEGFR1-Fc coated wells and incubated at
25 C for 10
minutes. The wells are washed three times, and then incubated for 1 hour at 25
C with 100 1 of
streptavidin-HRF' (KPL #474-3000, Gaithersburg, MD). The wells are washed
three times, and
100 1 of ULTRA-TMB ELISA (Pierce, Rockford, IL) are added per well. Following
color
development the reaction is stopped with 1N HCL and absorbance at 450nM is
measured.
Example 1.1.2.K: Inhibition of Receptor Phosphorylation by Parent Antibodies
or DVD-Ig
Constructs In Vitro
Human carcinoma cells are plated in 96-well plates at 40,000 cells/well in
180g1 serum-
free medium (DMEM+ 0.1% BSA), and incubated overnight at 37 C, 5% CO2. Costar
EIA plates
(Lowell, MA) are coated with 100 l/well of receptor capture Ab (4pg/m1 final
concentration),
and incubated overnight at room temperature while shaking. The following day,
receptor
antibody-coated ELISA plates are washed (three times with PBST =0.05% Tween 20
in PBS, pH
7.2 - 7.4), and 200 I blocking solution is added (1% BSA, 0.05% NaN3 in PBS,
pH 7.2 - 7.4.) to
block for 2 hours at room temperature on a rocker. Human tumor cells are co-
incubated with
antibodies or DVD-Igs and ligand. Monoclonal antibodies or DVD-Igs diluted in
D-PBS-BSA
(Dulbecco's phosphate buffered saline with 0.1%BSA) are added to human
carcinoma cells at
final concentrations of 0.01 g/mL-100 pg/mL. Growth factors are
simultaneously added to the
cells at concentrations of 1-10Ong/mL (2001tL), and cells are incubated at 37
C in a humidified,
5% CO2 atmosphere for 1 hour. Cells are lysed in 120 1/well of cold cell
extraction buffer (10
mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 1 mM sodium
orthovanadate, 1% Triton X-100, 10% Glycerol, 0.1% SDS, and protease inhibitor
cocktail), and
incubated at 4 C for 20 minutes with shaking. Cell lysates (100 1) are added
to the ELISA plate,
and incubated overnight at 4 C with gentle shaking. The following day, ELISA
plates are washed,
and 100 p1/well of pTyr-HRP detection Ab is added (p-IGF1R ELISA kit, R&D
System #
DYC1770, Minneapolis, MN), and plates are incubated for 2 hours at 25 C in the
dark. Plates are
developed to determine phosphorylation per the manufacturer's instructions.
177
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Example 1.1.2.L: Inhibition Of VEGFR2 (KDR) Phosphorylation By 'VEGF Parent
Antibody And DVD-Ig Constructs
NIH3T3 cells expressing human VEGFR2 (KDR) are plated at 20,000 cells/well
(1000
in 96-well plates in DMEM supplemented with 10% FBS. The following day, the
cells are
washed twice with DMEM and serum-starved for three hours in DMEM without FBS.
Anti-
VEGF parent antibody or DVD-Igs (at final concentrations of 67 nM, 6.7 nM and
0.67 nM)
diluted in DMEM with 0.1%BSA are pre-incubated with recombinant human VEGF165
(50ng/m1)
for 1 hour at 25 C. These antibody/DVD-Ig and VEGF mixtures are then added to
the cells, and
the plates are incubated at 37 C in a humidified, 5% CO2 atmosphere for 10
minutes. Cells are
washed twice with ice cold PBS and lysed by addition of 100p1/ well of Cell
Lysis Buffer (Cell
Signaling, Boston, MA) supplemented with 0.1% NP40. Duplicate samples are
pooled and 170R1
is added to wells of ELISA plates previously coated with anti-VEGFR2 antibody
(R&D systems,
AF357, Minneapolis, MN) and incubated at 25 C with gentle shaking for two
hours. The wells
are washed five times with washing buffer (PBS containing 0.05% Tween 20), and
incubated
with 50 1 of of 1:2000 dilution of biotinylated anti-phosphotyrosine antibody
(4G10; Millipore,
Billerica, MA) for 1 hour at 25 C. The wells are washed five times with PBS
containing 0.05%
Tween 20, and then incubated for 1 hour at 25 C with streptavidin-HRP (KPL
#474-3000,
Gaithersburg, MD). The wells are washed three times with streptavidin-HRP (KPL
#474-3000,
Gaithersburg, MD)). The wells are washed three times with PBS containing 0.05%
Tween 20,
and 100121 of ULTRA-TMB ELISA (Pierce, Rockford, IL) are added per well.
Following color
development the reaction is stopped with 1N HCL and absorbance at 450nM was
measured.
Example 1.1.2.M: Efficacy Of A DVD-Ig On The Growth Of Human Carcinoma
Subcutaneous Flank Xenografts
A-431 human epidermoid carcinoma cells are grown in vitro to 99% viability,
85%
confluence in tissue culture flasks. SCID female mice (Charles Rivers Labs,
Wilmington, MA) at
19-25 grams are injected subcutaneously into the right flank with 1 x 106
human tumor cells (1:1
matrigel) on study day 0. Administration (IP, QD, 3x/ week) of human IgG
control or DVD-Ig
was-initiated after mice are size matched into groups of mice with mean tumor
volumes of
approximately 200 to 320 mm3. The tumors are measured twice a week starting on
approximately
day 10 post tumor cell injection.
178
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 1.1.2.N: Binding of Monoclonal Antibodies to the Surface of Human
Tumor Cell
Lines as Assessed by Flow Cytometry
Stable cell lines overexpressing a cell-surface antigen of interest or human
tumor cell
lines were harvested from tissue culture flasks and resuspended in phosphate
buffered saline
(PBS) containing 5% fetal bovine serum (PBS/PBS). Prior to staining, human
tumor cells were
incubated on ice with (100111) human IgG at 5pg/m1 in PBS/FCS. 1-5 x105 cells
were incubated
with antibody or DVD-Ig (2 p.g/mL) in PBS/PBS for 30-60 minutes on ice. Cells
were washed
twice and 100111 of F(ab')2 goat anti human IgG, Fey- phycoerythrin (1:200
dilution in PBS)
(Jackson ImmunoResearch, West Grove, PA, Cat.#109-116-170) was added. After 30
minutes
incubation on ice, cells were washed twice and resuspended in PBS/PBS.
Fluorescence was
measured using a Becton Dickinson FACSCalibur (Becton Dickinson, San Jose,
CA).
Example 1.1.2.0: Binding of Parent Receptor Antibody and DVD-Ig Constructs to
the
Surface of Human Tumor Cell Lines as Assessed by Flow Cytometry
Stable cell lines overexpressing cell-surface receptors or human tumor cell
lines are
harvested from tissue culture flasks and resuspended in Dulbecco's phosphate
buffered saline
(DPBS) containing 1% fetal calf serum (DPBS/FCS). 1-5 x105 cells are incubated
with 100pL
antibodies or DVD-Igs (lOug/mL) in DPBS/FCS for 30-60 minutes on ice. Cells
are washed
twice and 50pI of goat anti-human IgG-phycoerythrin (1:50 dilution in
DPBS/BSA) (Southern
Biotech Associates, Birmingham, AL cat#2040-09) is added. After 30-45 minutes
incubation on
ice, cells are washed twice and resuspended in 125uL/well 1% formaldehyde in
DPBS/FCS.
Fluorescence was measured using a Becton Dickinson LSRII (Becton Dickinson,
San Jose, CA).
Example 1.2: Generation Of Parent Monoclonal Antibodies to a Human Antigen of
Interest
Parent mouse mAbs able to bind to and neutralize a human antigen of interest
and a
variant thereof are obtained as follows:
Example 1.2.A: Immunization Of Mice With a Human Antigen of Interest
Twenty micrograms of recombinant purified human antigen (e.g., IGF1,2) mixed
with
complete Freund's adjuvant or Immunoeasy adjuvant (Qiagen, Valencia, CA) is
injected
subcutaneously into five 6-8 week-old Balb/C, five C57B/6 mice, and five AJ
mice on Day 1. On
days 24, 38, and 49, twenty micrograms of recombinant purified human antigen
variant mixed
with incomplete Freund's adjuvant or Immunoeasy adjuvant is injected
subcutaneously into the
179
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
same mice. On day 84 or day 112 or day 144, mice are injected intravenously
with li.tg
recombinant purified human antigen of interest.
Example 1.2.B: Generation of a Hybridoma
Splenocytes obtained from the immunized mice described in Example 1.2.A are
fused
with SP2/0-Ag-14 cells at a ratio of 5:1 according to the established method
described in Kohler,
G. and Milstein (1975) Nature, 256:495 to generate hybridomas. Fusion products
are plated in
selection media containing azaserine and hypoxanthine in 96-well plates at a
density of 2.5x106
spleen cells per well. Seven to ten days post fusion, macroscopic hybridoma
colonies are
observed. Supernatant from each well containing hybridoma colonies is tested
by ELISA for the
presence of antibody to the antigen of interest (as described in Example
1.1.1.A). Supernatants
displaying antigen-specific activity are then tested for activity (as
described in the assays of
Example 1.1.2), for example, the ability to neutralize the antigen of interest
in a bioassay such as
that described in Example 1.1.2.1).
Example 1.2.C: Identification And Characterization Of Parent Monoclonal
Antibodies to a
Human Target Antigen of Interest
Example 1.2.C.1: Analyzing Parent Monoclonal Antibody Neutralizing Activity
Hybridoma supernatants are assayed for the presence of parent antibodies that
bind an
antigen of interest, generated according to Examples 1.2.A and 1.2.B, and are
also capable of
binding a variant of the antigen of interest ("antigen variant"). Supernatants
with antibodies
positive in both assays are then tested for their antigen neutralization
potency, for example, in the
cytokine bioassay of Example 1.1.2.1. The hybridomas producing antibodies with
IC50 values in
the bioassay less than 1000pM, in an embodiment, less than 100pM are scaled up
and cloned by
limiting dilution. Hybridoma cells are expanded into media containing 10% low
IgG fetal bovine
serum (Hyclone #SH30151, Logan, UT). On average, 250 mL of each hybridoma
supernatant
(derived from a clonal population) is harvested, concentrated and purified by
protein A affinity
chromatography, as described in Harlow, E. and Lane, D. 1988 "Antibodies: A
Laboratory
Manual". The ability of purified mAbs to inhibit the activity of its target
antigen is determined,
for example, using the cytokine bioassay as described in Example 1.1.2.1.
180
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 1.2.C.2: Analyzing Parent Monoclonal Antibody Cross-Reactivity To
Cynomolgus
Target Antigen Of Interest
To determine whether the selected mAbs described herein recognize cynomolgus
antigen
of interest, BIACORE analysis is conducted as described herein (Example
1.1.1.G) using
recombinant cynomolgus target antigen. In addition, neutralization potencies
of mAbs against
recombinant cynomolgus antigen of interest may also be measured in the
cytokine bioassay
(Example 1.1.2.1). MAbs with good cyno cross-reactivity (in an embodiment,
within 5-fold of
reactivity for human antigen) are selected for future characterization.
Example 1.2.D: Determination Of The Amino Acid Sequence Of The Variable Region
For
Each Murine Anti-Human Monoclonal Antibody
Isolation of the cDNAs, expression and characterization of the recombinant
anti-human
mouse mAbs is conducted as follows. For each amino acid sequence
determination,
approximately 1 x 106hybridoma cells are isolated by centrifugation and
processed to isolate total
RNA with Trizol (Gibco BRL/Invitrogen, Carlsbad, CA.) following manufacturer's
instructions.
Total RNA is subjected to first strand DNA synthesis using the SuperScript
First-Strand
Synthesis System (Invitrogen, Carlsbad, CA) per the manufacturer's
instructions. Oligo(dT) is
used to prime first-strand synthesis to select for poly(A)+ RNA. The first-
strand cDNA product is
then amplified by PCR with primers designed for amplification of murine
immunoglobulin
variable regions (Ig-Primer Sets, Novagen, Madison, WI). PCR products are
resolved on an
agarose gel, excised, purified, and then subcloned with the TOPO Cloning kit
into pCR2.1-TOPO
vector (Invitrogen, Carlsbad, CA) and transformed into TOP10 chemically
competent E. coli
(Invitrogen, Carlsbad, CA). Colony PCR is performed on the transformants to
identify clones
containing insert. Plasmid DNA is isolated from clones containing insert using
a QIAprep
Miniprep kit (Qiagen, Valencia, CA). Inserts in the plasmids are sequenced on
both strands to
determine the variable heavy or variable light chain DNA sequences using MI3
forward and M13
reverse primers (Fermentas Life Sciences, Hanover MD). Variable heavy and
variable light chain
sequences of the mAbs are identified. In an embodiment, the selection criteria
for a panel of lead
mAbs for next step development (humanization) inchtdes the following:
= The antibody does not contain any N-linked glycosylation sites (NXS), except
from the
standard one in CH2
= The antibody does not contain any extra cysteines in addition to the normal
cysteines in
every antibody
181
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
= The antibody sequence is aligned with the closest human germline sequences
for VH and
VL and any unusual amino acids should be checked for occurrence in other
natural
human antibodies
= N-terminal Glutamine (Q) is changed to Glutamic acid (E) if it does not
affect the
activity of the antibody. This will reduce heterogeneity due to cyclization of
Q
= Efficient signal sequence cleavage is confirmed by Mass Spectrophotometry.
This can be
done with COS cell or 293 cell material
= The protein sequence is checked for the risk of deamidation of Asn that
could result in
loss of activity
= The antibody has a low level of aggregation
= The antibody has solubility >5-10 mg/ml (in research phase); >25 mg/ml
= The antibody has a normal size (5-6 nm) by Dynamic Light Scattering (DLS)
= The antibody has a low charge heterogeneity
= The antibody lacks cytokine release (see Example 1.1.2.B)
= The antibody has specificity for the intended cytokine (see Example 1.1.2.C)
= The antibody lacks unexpected tissue cross reactivity (see Example 1.1.2.D)
= The antibody has similarity between human and cynomolgus tissue cross
reactivity (see
Example 1.1.2.D)
Example 1.2.2: Recombinant Humanized Parent Antibodies
Example 1.2.2.1: Construction And Expression Of Recombinant Chimeric Anti
Human
Parent Antibodies
The DNA encoding the heavy chain constant region of murine anti-human parent
mAbs
is replaced by a cDNA fragment encoding the human IgG1 constant region
containing 2 hinge-
region amino acid mutations by homologous recombination in bacteria. These
mutations are a
leucine to alanine change at position 234 (EU numbering) and a leucine to
alanine change at
position 235 (Lund et al., 1991, J. Immunol., 147:2657). The light chain
constant region of each
of these antibodies is replaced by a human kappa constant region. Full-length
chimeric antibodies
are transiently expressed in COS cells by co-transfection of chimeric heavy
and light chain
cDNAs ligated into the pBOS expression plasmid (Mizushima and Nagata, Nucleic
Acids
Research 1990, Vol 18, pg 5322). Cell supernatants containing recombinant
chimeric antibody
are purified by Protein A Sepharose chromatography and bound antibody is
eluted by addition of
acid buffer. Antibodies are neutralized and dialyzed into PBS.
The heavy chain cDNA encoding a chimeric mAb is co-transfected with its
chimeric light
chain cDNA (both ligated in the pBOS vector) into COS cells. Cell supernatant
containing
182
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
recombinant chimeric antibody is purified by Protein A Sepharose
chromatography and bound
antibody is eluted by addition of acid buffer. Antibodies are neutralized and
dialyzed into PBS.
The purified chimeric anti-human parent mAbs are then tested for their ability
to bind (by
Biacore) and for functional activity, e.g., to inhibit the cytokine induced
production of IgE as
described in Examples 1.1.1.G and 1.1.2.B. Chimeric mAbs that maintain the
activity of the
parent hybridoma mAbs are selected for future development.
Example 1.2.2.2: Construction And Expression Of Humanized Anti Human Parent
Antibodies
Example 1.2.2.2.A: Selection Of Human Antibody Frameworks
Each murine variable heavy and variable light chain gene sequence is
separately aligned
against 44 human immunoglobulin germline variable heavy chain or 46 germline
variable light
chain sequences (derived from NCBI Ig Blast website at
http://www.ncbi.nlm.nih.gov/igblast/retrieveig.html.) using Vector NTI
software.
Humanization is based on amino acid sequence homology, CDR cluster analysis,
frequency of use among expressed human antibodies, and available information
on the crystal
structures of human antibodies. Taking into account possible effects on
antibody binding, VH-
VL pairing, and other factors, murine residues are mutated to human residues
where murine and
human framework residues are different, with a few exceptions. Additional
humanization
strategies are designed based on an analysis of human germline antibody
sequences, or a
subgroup thereof, that possessed a high degree of homology, i.e., sequence
similarity, to the
actual amino acid sequence of the murine antibody variable regions.
Homology modeling is used to identify residues unique to the murine antibody
sequences
that are predicted to be critical to the structure of the antibody combining
site, the CDRs.
Homology modeling is a computational method whereby approximate three
dimensional
coordinates are generated for a protein. The source of initial coordinates and
guidance for their
further refinement is a second protein, the reference protein, for which the
three dimensional
coordinates are known and the sequence of which is related to the sequence of
the first protein.
The relationship among the sequences of the two proteins is used to generate a
correspondence
between the reference protein and the protein for which coordinates are
desired, the target
protein. The primary sequences of the reference and target proteins are
aligned with coordinates
of identical portions of the two proteins transferred directly from the
reference protein to the
target protein. Coordinates for mismatched portions of the two proteins, e.g.,
from residue
183
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
mutations, insertions, or deletions, are constructed from generic structural
templates and energy
refined to insure consistency with the already transferred model coordinates.
This computational
protein structure may be further refined or employed directly in modeling
studies. The quality of
the model structure is determined by the accuracy of the contention that the
reference and target
proteins are related and the precision with which the sequence alignment is
constructed.
For the murine mAbs, a combination of BLAST searching and visual inspection is
used
to identify suitable reference structures. Sequence identity of 25% between
the reference and
target amino acid sequences is considered the minimum necessary to attempt a
homology
modeling exercise. Sequence alignments are constructed manually and model
coordinates are
generated with the program Jackal (see Petrey, D. et al. (2003) Proteins 53
(Suppl. 6): 430-435).
The primary sequences of the murine and human framework regions of the
selected
antibodies share significant identity. Residue positions that differ are
candidates for inclusion of
the murine residue in the humanized sequence in order to retain the observed
binding potency of
the murine antibody. A list of framework residues that differ between the
human and murine
sequences is constructed manually. Table 8 shows the framework sequences
chosen for this
study.
Table 8: Sequence Of Human IgG Heavy Chain Constant Domain And Light Chain
Constant Domain
Protein SEQ Sequence
ID NO
12345678901234567890123456789012345678901
Wild type hIgGI 48 ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSW
constant region NSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSV
FLEPPKPKDTLMISRTPEVICVVVDVSHEDPEVKFNWYVDG
VEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCK
VSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQV
SLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSF
FLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSP
GK
Mutant hIgG1 constant 49 ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSW
region NSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYI
CNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGGPSV
FLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDG
VEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCK
VSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQV
SLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSF
FLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSP
GK
Ig kappa constant 50 TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWK
region VDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHK
VYACEVTHQGLSSPVTKSFNRGEC
Ig Lambda 51 QPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTVAW
constant region KADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHR
SYSCQVTHEGSTVEKTVAPTECS
184
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
The likelihood that a given framework residue would impact the binding
properties of the
antibody depends on its proximity to the CDR residues. Therefore, using the
model structures, the
residues that differ between the murine and human sequences are ranked
according to their
distance from any atom in the CDRs. Those residues that fell within 4.5 A of
any CDR atom are
identified as most important and are recommended to be candidates for
retention of the murine
residue in the humanized antibody (i.e., back mutation).
In silico constructed humanized antibodies are constructed using
oligonucleotides. For
each variable region cDNA, 6 oligonucleotides of 60-80 nucleotides each are
designed to overlap
each other by 20 nucleotides at the 5' and/or 3' end of each oligonucleotide.
In an annealing
reaction, all 6 oligonulceotides are combined, boiled, and annealed in the
presence of dNTPs.
DNA polymerase I, Large (Klenow) fragment (New England Biolabs #M0210,
Beverley, MA.) is
added to fill-in the approximately 40bp gaps between the overlapping
oligonucleotides. PCR is
performed to amplify the entire variable region gene using two outermost
primers containing
overhanging sequences complementary to the multiple cloning site in a modified
pBOS vector
(Mizushima, S. and Nagata, S. (1990) Nucleic Acids Res. 18: 17). The PCR
products derived
from each cDNA assembly are separated on an agarose gel and the band
corresponding to the
predicted variable region cDNA size is excised and purified. The variable
heavy region is
inserted in-frame onto a cDNA fragment encoding the human IgG1 constant region
containing 2
hinge-region amino acid mutations by homologous recombination in bacteria.
These mutations
are a leucine to alanine change at position 234 (EU numbering) and a leucine
to alanine change at
position 235 (Lund et al. (1991) J. Immunol. 147:2657). The variable light
chain region is
inserted in-frame with the human kappa constant region by homologous
recombination. Bacterial
colonies are isolated and plasmid DNA extracted. cDNA inserts are sequenced in
their entirety.
Correct humanized heavy and light chains corresponding to each antibody are co-
transfected into
COS cells to transiently produce full-length humanized anti-human antibodies.
Cell supernatants
containing recombinant chimeric antibody are purified by Protein A Sepharose
chromatography
and bound antibody is eluted by addition of acid buffer. Antibodies are
neutralized and dialyzed
into PBS.
Example 1.2.2.3: Characterization Of Humanized Antibodies
The ability of purified humanized antibodies to inhibit a functional activity
is
determined, e.g., using the cytokine bioassay as described in Examples 1.1
.2.A. The binding
affinities of the humanized antibodies to recombinant human antigen are
determined using
surface plasmon resonance (BiacoreO) measurement as described in Example
1.1.1.B. The ICso
values from the bioassays and the affinity of the humanized antibodies are
ranked. The
185
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
humanized mAbs that fully maintain the activity of the parent hybridoma mAbs
are selected as
candidates for future development. The top 2-3 most favorable humanized mAbs
are further
characterized.
Example 1.2.2.3.A: Pharmacokinetic Analysis Of Humanized Antibodies
Pharmacokinetic studies are carried out in Sprague-Dawley rats and cynomolgus
monkeys. Male and female rats and cynomolgus monkeys are dosed intravenously
or
subcutaneously with a single dose of 4mg/kg mAb and samples are analyzed using
antigen
capture ELISA, and pharmacokinetic parameters are determined by
noncompartmental analysis.
Briefly, ELISA plates are coated with goat anti-biotin antibody (5 mg/ml, 4 C,
overnight),
blocked with Superblock (Pierce), and incubated with biotinylated human
antigen at 50 ng/ml in
10% Superblock TTBS at room temperature for 2 hours. Serum samples are
serially diluted
(0.5% serum, 10% Superblock in TTBS) and incubated on the plate for 30 minutes
at room
temperature. Detection is carried out with HRP-labeled goat anti human
antibody and
concentrations are determined with the help of standard curves using the four
parameter logistic
fit. Values for the pharmacokinetic parameters are determined by non-
compartmental model
using WinNonlin software (Pharsight Corporation, Mountain View, CA). Humanized
mAbs with
good pharrnacokinetics profile (T1/2 is 8-13 days or better, with low
clearance and excellent
bioavailability 50-100%) are selected.
Example 1.2.2.3.B: Physicochemical And In Vitro Stability Analysis Of
Humanized
Monoclonal Antibodies
Size exclusion chromatography
Antibodies are diluted to 2.5 mg/mL with water and 20 mL is analyzed on a
Shimadzu
HPLC system using a TSK gel G3000 SWXL column (Tosoh Bioscience, cat# k5539-
05k).
Samples are eluted from the column with 211 mM sodium sulfate, 92 mM sodium
phosphate, pH
7.0, at a flow rate of 0.3 mL/minutes. The HPLC system operating conditions
are the following:
Mobile phase: 211 mM Na2SO4, 92 mM Na2HPO4*7H20, pH 7.0
Gradient: Isocratic
Flow rate: 0.3 mL/minute
Detector wavelength: 280 nm
Autosampler cooler temp: 4 C
Column oven temperature: Ambient
Run time: 50 minutes
186
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Table 9 contains purity data of parent antibodies and DVD-Ig constructs
expressed as
percent monomer (unaggegated protein of the expected molecular weight) as
determined by the
above protocol.
Table 9: Purity of Parent Antibodies and DVD-Ig Constructs as Determined by
Size
Exclusion Chromatography
Parent Antibody N-Terminal C-Terminal % Monomer (purity)
or DVD-Ig ID Variable Variable
Domain Domain
(VD) (VD)
AB017 TNF (seq 1) 97.5
AB020 NGF 88.2
AB022 SOST 93.2
AB048 PGE2 100
AB213 TNF (seq 2) 100
AB214 TNF (seq 3) 100
AB215 TNF (seq 4) 99.9
AB216 LPA 100
AB217 TNF (seq 5) 100
AB218 TNF (seq 6) 100
DVD1448 PGE2 TNF (seq 2) 76.4
DVD1454 SOST TNF (seq 2) 88.8
DVD1460 NGF TNF (seq 2) 87.6
DVD1466 LPA TNF (seq 2) 100
DVD1471 TNF (seq 1) LPA 90.5
DVD1472 LPA TNF (seq 1) 96.8
DVD1473 TNF (seq 5) PGE2 94.4
DVD1474 PGE2 TNF (seq 5) 67.9
DVD1475 TNF (seq 5) SOST 91.6
DVD1476 SOST TNF (seq 5) 76.7
DVD1477 TNF (seq 5) NGF 96.7
DVD1478 NGF TNF (seq 5) 89.2
DVD1479 TNF (seq 5) LPA 95.3
DVD1480 LPA TNF (seq 5) 100
DVD1481 TNF (seq 6) PGE2 97.5
DVD1482 PGE2 TNF (seq 6) 80.7
DVD1483 TNF (seq 6) SOST 90.8
DVD1484 SOST TNF (seq 6) 76
DVD1485 TNF (seq 6) NGF 89.9
DVD1486 NGF TNF (seq 6) 92.2
DVD1487 TNF (seq 6) LPA 90.9
DVD1488 LPA TNF (seq 6) 100
DVD1490 PGE2 TNF (seq 2) 97.2
DVD1495 TNF (seq 2) SOST 98.8
DVD1496 SOST TNF (seq 2) 72.8
DVD1502 NGF TNF (seq 2) 96.2
DVD1508 LPA TNF (seq 2) 100
DVD1513 TNF (seq I) LPA 73.9
187
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
Parent Antibody N-Terminal C-Terminal
A Monomer (purity)
or DVD-Ig ID Variable
Variable
Domain Domain
DVD1514 LPA (VD)
TNF (seq 1) (VD)
96.3
DVD1515 TNF (seq 5) PGE2
97.2
DVD1517 TNF (seq 5) SOST
95.4
DVD1518 SOST
TNF (seq 5)
78.4
DVD1519 TNF (seq 5) NGF
100
DVD1520 NGF
TNF (seq 5)
95.2
DVD1523 TNF (seq 6) PGE2
97.5
DVD1524 PGE2
TNF (seq 6)
98.1
DVD1525 TNF (seq 6) SOST
96.5
DVD1526 SOST
TNF (seq 6)
70.5
DVD1527 TNF (seq 6) NGF
98.2
DVD1528 NGF
TNF (seq 6)
93.3
DVD1529 TNF (seq 6) LPA
81.3
DVD1530 LPA
TNF (seq 6)
100
SDS-PAGE
Antibodies are analyzed by sodium dodecyl sulfate - polyacrylamide gel
electrophoresis
(SDS-PAGE) under both reducing and non-reducing conditions. Adalimumab lot
AFP04C is used
as a control. For reducing conditions, the samples are mixed 1:1 with 2X tris
glycine SDS-PAGE
sample buffer (Invitrogen, cat# LC2676, lot# 1323208) with 100 mM DTT, and
heated at 60 C
for 30 minutes. For non-reducing conditions, the samples are mixed 1:1 with
sample buffer and
heated at 100 C for 5 minutes. The reduced samples (10 mg per lane) are loaded
on a 12% pre-
cast tris-glycine gel (Invitrogen, cat# EC6005box, lot# 6111021), and the non-
reduced samples
(10 mg per lane) are loaded on an 8%-16% pre-cast tris-glycine gel
(Invitrogen, cat# EC6045box,
lot# 6111021). SeeBlue Plus 2 (Invitrogen, cat#LC5925, lot# 1351542) is used
as a molecular
weight marker. The gels are run in a XCell SureLock mini cell gel box
(Invitrogen, cat# E10001)
and the proteins are separated by first applying a voltage of 75 to stack the
samples in the gel,
followed by a constant voltage of 125 until the dye front reached the bottom
of the gel. The
running buffer used is 1X tris glycine SDS buffer, prepared from a 10X tris
glycine SDS buffer
(ABC, MPS-79-080106)). The gels are stained overnight with colloidal blue
stain (Invitrogen
cat# 46-7015, 46-7016) and destained with Milli-Q water until the background
is clear. The
stained gels are then scanned using an Epson Expression scanner (model 1680,
S/N
DASX003641).
Sedimentation Velocity Analysis
Antibodies are loaded into the sample chamber of each of three standard two-
sector
carbon epon centerpieces. These centerpieces have a 1.2 cm optical path length
and are built with
188
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
sapphire windows. PBS is used for a reference buffer and each chamber
contained 140 pt. All
samples are examined simultaneously using a 4-hole (AN-60Ti) rotor in a
Beckman ProteomeLab
XL-I analytical ultracentrifuge (serial # PLI 06C01).
Run conditions are programmed and centrifuge control is performed using
ProteomeLab
(v5.6). The samples and rotor are allowed to thermally equilibrate for one
hour prior to analysis
(20.0 0.1 C). Confirmation of proper cell loading is performed at 3000 rpm
and a single scan is
recorded for each cell. The sedimentation velocity conditions are the
following:
Sample Cell Volume: 420 mL
Reference Cell Volume: 420 mL
Temperature: 20 C
Rotor Speed: 35,000 rpm
Time: 8:00 hours
UV Wavelength: 280 nm
Radial Step Size: 0.003 cm
Data Collection: One data point per step without signal averaging.
Total Number of Scans: 100
LC-MS molecular weight measurement of intact antibodies
Molecular weight of intact antibodies are analyzed by LC-MS. Each antibody is
diluted
to approximately 1 mg/mL with water. An 1100 HPLC (Agilent) system with a
protein microtrap
(Michrom Bioresources, Inc, cat# 004/25109/03) is used to desalt and introduce
5 mg of the
sample into an API Qstar pulsar i mass spectrometer (Applied Biosystems). A
short gradient is
used to elute the samples. The gradient is run with mobile phase A (0.08% FA,
0.02% TFA in
HPLC water) and mobile phase B (0.08% FA and 0.02% TFA in acetonitrile) at a
flow rate of 50
mL/minute. The mass spectrometer is operated at 4.5 kvolts spray voltage with
a scan range from
2000 to 3500 mass to charge ratio.
LC-MS molecular weight measurement of antibody light and heavy chains
Molecular weight measurement of antibody light chain (LC), heavy chain (HC)
and
deglycosylated HC are analyzed by LC-MS. Aantibody is diluted to 1 mg/mL with
water and the
sample is reduced to LC and HC with a final concentration of 10 mM DTT for 30
minutes at
37 C. To deglycosylate the antibody, 100 mg of the antibody is incubated with
2 mL of PNGase
F, 5 mL of 10% N-octylglucoside in a total volume of 100 mL overnight at 37
C. After
deglycosylation the sample is reduced with a final concentration of 10 mM DTT
for 30 minutes
at 37 C. An Agilent 1100 HPLC system with a C4 column (Vydac, cat# 214TP5115,
S/N
189
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
060206537204069) is used to desalt and introduce the sample (5 mg) into an API
Qstar pulsar i
mass spectrometer (Applied Biosystems). A short gradient is used to elute the
sample. The
gradient is run with mobile phase A (0.08% FA, 0.02% TFA in HPLC water) and
mobile phase B
(0.08% FA and 0.02% TFA in acetonitrile) at a flow rate of 50 mL/minute. The
mass
spectrometer is operated at 4.5 kvolts spray voltage with a scan range from
800 to 3500 mass to
charge ratio.
Peptide mapping
Antibody is denatured for 15 minutes at room temperature with a final
concentration of 6
M guanidine hydrochloride in 75 mM ammonium bicarbonate. The denatured samples
are
reduced with a final concentration of 10 mM DTT at 37 C for 60 minutes,
followed by alkylation
with 50 mM iodoacetic acid (IAA) in the dark at 37 C for 30 minutes. Following
alkylation, the
sample is dialyzed overnight against four liters of 10 mM ammonium bicarbonate
at 4 C. The
dialyzed sample is diluted to 1 mg/mL with 10 mM ammonium bicarbonate, pH 7.8
and 100 mg
of antibody is either digested with trypsin (Promega, cat# V5111) or Lys-C
(Roche, cat # 11 047
825 001) at a 1:20 (w/w) trypsin/Lys-C:antibody ratio at 37 C for 4 hrs.
Digests are quenched
with 1 mL of 1 N HC1. For peptide mapping with mass spectrometer detection, 40
mL of the
digests are separated by reverse phase high performance liquid chromatography
(RPHPLC) on a
C18 column (Vydac, cat# 218TP51, S/N NE9606 10.3.5) with an Agilent 1100 HPLC
system.
The peptide separation is run with a gradient using mobile phase A (0.02% TFA
and 0.08% FA in
HPLC grade water) and mobile phase B (0.02% TFA and 0.08% FA in acetonitrile)
at a flow rate
of 50 mL/minutes. The API QSTAR Pulsar i mass spectromer is operated in
positive mode at 4.5
kvolts spray voltage and a scan range from 800 to 2500 mass to charge ratio.
Disulfide Bond Mapping
To denature the antibody, 100 mL of the antibody is mixed with 300 mL of 8 M
guanidine HC1 in 100 mM ammonium bicarbonate. The pH is checked to ensure that
it is between
7 and 8 and the samples are denatured for 15 minutes at room temperature in a
final concentration
of 6 M guanidine HC1. A portion of the denatured sample (100 mL) is diluted to
600 mL with
Milli-Q water to give a final guanidine-HCI concentration of 1 M. The sample
(220 mg) is
digested with either trypsin (Promega, cat # V5111, lot# 22265901) or Lys-C
(Roche, cat#
11047825001, lot# 12808000) at a 1:50 trypsin or 1:50 Lys-C: antibody (w/w)
ratios (4.4 mg
enzyme: 220 mg sample) at 37 C for approximately 16 hours. An additional 5 mg
of trypsin or
Lys-C is added to the samples and digestion is allowed to proceed for an
additional 2 hours at
37 C. Digestions are stopped by adding 1 mL of TFA to each sample. Digested
samples are
190
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
separated by RPHPLC using a C18 column (Vydac, cat# 218TP51 SIN NE020630-4-1A)
on an
Agilent HPLC system. The separation is run with the same gradient used for
peptide mapping
using mobile phase A (0.02% TFA and 0.08% FA in HPLC grade water) and mobile
phase B
(0.02% TFA and 0.08% FA in acetonitrile) at a flow rate of 50 mL/minute. The
HPLC operating
conditions are the same as those used for peptide mapping. The API QSTAR
Pulsar i mass
spectromer is operated in positive mode at 4.5 kvolts spray voltage and a scan
range from 800 to
2500 mass-to-charge ratio. Disulfide bonds are assigned by matching the
observed MWs of
peptides with the predicted MWs of tryptic or Lys-C peptides linked by
disulfide bonds.
Free sulihydryl determination
The method used to quantify free cysteines in an antibody is based on the
reaction of
Ellman's reagent, 5,50- dithio-bis (2-nitrobenzoic acid) (DTNB), with
sulfhydryl groups (SH)
which gives rise to a characteristic chromophoric product, 5-thio-(2-
nitrobenzoic acid) (TNB).
The reaction is illustrated in the formula:
DTNB RSH RS-TNB + TNB- + H+
The absorbance of the TNB- is measured at 412 nm using a Cary 50
spectrophotometer.
An absorbance curve is plotted using dilutions of 2 mercaptoethanol (b-ME) as
the free SH
standard and the concentrations of the free sulfhydryl groups in the protein
are determined from
absorbance at 412 nm of the sample.
The b-ME standard stock is prepared by a serial dilution of 14.2 M b-ME with
HPLC
grade water to a final concentration of 0.142 mM. Then standards in triplicate
for each
concentration are prepared. Antibody is concentrated to 10 mg/mL using an
amicon ultra 10,000
MWCO centrifugal filter (Millipore, cat# UFC801096, lot# L3KN5251) and the
buffer is
changed to the formulation buffer used for adalimumab (5.57 mM sodium
phosphate monobasic,
8.69 mM sodium phosphate dibasic, 106.69 mM NaC1, 1.07 mM sodium citrate, 6.45
mM citric
acid, 66.68 triM mannitol, pH 5.2, 0.1% (w/v) Tween). The samples are mixed on
a shaker at
room temperature for 20 minutes. Then 180 mL of 100 mM Tris buffer, pH 8.1 is
added to each
sample and standard followed by the addition of 300 mL of 2 mM DTNB in 10 mM
phosphate
buffer, pH 8.1. After thorough mixing, the samples and standards are measured
for absorption at
412 nm on a Cary 50 spectrophotometer. The standard curve is obtained by
plotting the amount
of free SH and 0D4.12 nm of the b-ME standards. Free SH content of samples are
calculated based
on this curve after subtraction of the blank.
191
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Weak Cation Exchange Chromatography
Antibody is diluted to 1 mg/mL with 10 mM sodium phosphate, pH 6Ø Charge
heterogeneity is analyzed using a Shimadzu HPLC system with a WCX-10 ProPac
analytical
column (Dionex, cat# 054993, SIN 02722). The samples are loaded on the column
in 80% mobile
phase A (10 mM sodium phosphate, pH 6.0) and 20% mobile phase B (10 mM sodium
phosphate, 500 mM NaCl, pH 6.0) and eluted at a flow rate of 1.0 mL/minute.
Oligosaccharide Profiling
Oligosaccharides released after PNGase F treatment of antibody are derivatized
with 2-
aminobenzamide (2-AB) labeling reagent. The fluorescent-labeled
oligosaccharides are separated
by normal phase high performance liquid chromatography (NPHPLC) and the
different forms of
oligosaccharides are characterized based on retention time comparison with
known standards.
The antibody is first digested with PNGaseF to cleave N-linked
oligosaccharides from
the Fe portion of the heavy chain. The antibody (200 mg) is placed in a 500 mL
Eppendorf tube
along with 2 mL PNGase F and 3 mL of 10% N-octylglucoside. Phosphate buffered
saline is
added to bring the final volume to 60 mL. The sample is incubated overnight at
37 C in an
Eppendorf thermomixer set at 700 RPM. Adalimumab lot AFP04C is also digested
with PNGase
F as a control.
After PNGase F treatment, the samples are incubated at 95 C for 5 minutes in
an
Eppendorf thermomixer set at 750 RPM to precipitate out the proteins, then the
samples are
placed in an Eppendorf centrifuge for 2 minutes at 10,000 RPM to spin down the
precipitated
proteins. The supematent containing the oligosaccharides are transferred to a
500 mL Eppendorf
tube and dried in a speed-vac at 65 C.
The oligosaccharides are labeled with 2AB using a 2AB labeling kit purchased
from
Prozyme (cat# GKK-404, lot# 132026). The labeling reagent is prepared
according to the
manufacturer's instructions. Acetic acid (150 mL, provided in kit) is added to
the DMSO vial
(provided in kit) and mixed by pipeting the solution up and down several
times. The acetic
acid/DMSO mixture (100 mL) is transferred to a vial of 2-AB dye (just prior to
use) and mixed
until the dye is fully dissolved. The dye solution is then added to a vial of
reductant (provided in
kit) and mixed well (labeling reagent). The labeling reagent (5 mL) is added
to each dried
oligosaccharide sample vial, and mixed thoroughly. The reaction vials are
placed in an Eppendorf
thermomixer set at 65 C and 700-800 RPM for 2 hours of reaction.
192
WO 2012/027570 CA 02809433 2013-02-22PCT/US2011/049147
After the labeling reaction, the excess fluorescent dye is removed using
GlycoClean S
Cartridges from Prozyme (cat# GKI-4726). Prior to adding the samples, the
cartridges are washed
with 1 mL of milli-Q water followed with 5 ishes of 1 mL 30% acetic acid
solution. Just prior to
adding the samples, 1 mL of acetonitrile (Burdick and Jackson, cat # AH015-4)
is added to the
cartridges.
After all of the acetonitrile passed through the cartridge, the sample is
spotted onto the
center of the freshly washed disc and allowed to adsorb onto the disc for 10
minutes. The disc is
washed with 1 mL of acetonitrile followed by five ishes of 1 mL of 96%
acetonitrile. The
cartridges are placed over a 1.5 mL Eppendorf tube and the 2-AB labeled
oligosaccharides are
eluted with 3 ishes (400 mL each ish) of milli Q water.
The oligosaccharides are separated using a Glycosep N HPLC (cat# GKI-4728)
column
connected to a Shimadzu HPLC system. The Shimadzu HPLC system consisted of a
system
controller, degasser, binary pumps, autosampler with a sample cooler, and a
fluorescent detector.
Stability at Elevated Temperatures
The buffer of antibody is either 5.57 mM sodium phosphate monobasic, 8.69 mM
sodium
phosphate dibasic, 106.69 mM NaCI, 1.07 mM sodium citrate, 6.45 mM citric
acid, 66.68 mM
mannitol, 0.1% (w/v) Tween, pH 5.2; or 10mM histidine, 10 mM methionine, 4%
mannitol, pH
5.9 using Amicon ultra centrifugal filters. The final concentration of the
antibodies is adjusted to
2 mg/mL with the appropriate buffers. The antibody solutions are then filter
sterized and 0.25 mL
aliquots are prepared under sterile conditions. The aliquots are left at
either -80 C, 5 C, 25 C, or
40 C for I, 2 or 3 weeks. At the end of the incubation period, the samples are
analyzed by size
exclusion chromatography and SDS-PAGE.
The stability samples are analyzed by SDS-PAGE under both reducing and non-
reducing
conditions. The procedure used is the same as described herein. The gels are
stained overnight
with colloidal blue stain (Invitrogen cat# 46-7015, 46-7016) and destained
with Milli-Q water
until the background is clear. The stained gels are then scanned using an
Epson Expression
scanner (model 1680, S/N DASX003641). To obtain more sensitivity, the same
gels are silver
stained using silver staining kit (Owl Scientific) and the recommended
procedures given by the
manufacturer is used.
Dynamic Scanning Fluorimetry
The DVD-Igs were dialysed in 10mM citrate 10mM phosphate buffer, pH 6.0 to get
a
final concentration of 1 mg/ml. Triplicates were run for each DVD-Ig. For each
sample, 27 ftl of
193
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
the DVD was added in a well of a 96 well plate and mixed with 3 pl of 4X
diluted SYPRO
Orange dye (Invitrogen). The dye was supplied in DMSO at a concentration of
5000X and was
diluted to the working concentration of 4X in water. The plate was centrifuged
for 30 seconds to
ensure that both the dye and the protein settled to the bottom of the wells
and complete mixing
was ensured by gentle aspiration by a pipette tip. The plate was then sealed
with an adhesive
film.
A real time PCR (Applied Biosciences, 7500 Series) was used to measure the
change in
fluorescence intensities with temperature. The plate was heated from 25 C to
95 C at a
temperature ramp rate of approximately 0.5 C/minute and emission fluorescence
was collected
using TAMRA filter. The data was exported to Microsoft Excel and plotted as
temperature vs
fluorescence for each DVD-Ig. Onset of melting was noted as the temperature
where the
thermogram rises above the baseline fluorescence. SYPRO Orange is a
hydrophobic dye and
preferentially binds to the exposed hydrophobic residues in an unfolded
protein molecule. Hence
the onset of unfolding temperature, as measured by an increase in fluorescence
is an indication of
the thermal stability of the DVD-Ig. The unfolding temperature for the DVD-Igs
can be found in
Table 10.
Table 10: Thermal Stability of Parent Antibodies and CDR-grafted DVD-Ig
Constructs as
Determined by Dynamic Scanning Fluorimetry
Parent Antibody or N-terminal Variable C-terminal Variable
Onset of melting
DVD-Ig ID Domain (VD) Domain (VD) (rank)
(deg C)
DVD1244 TNF (seq 1) LPA 51.3
DVD1248 TNF (seq 5) SOST 54.0
DVD1249 SOST TNF (seq 5) 54.3
DVDI256 TNF (seq 6) SOST 56
DVD1257 SOST TNF (seq 6) 57
DVD1258 TNF (seq 6) NGF 59.5
DVD1259 NGF TNF (seq 6) 51.7
DVD1261 LPA TNF (seq 6) 54.7
DVD1475 TNF (seq 5) SOST 49.0
DVD1476 SOST TNF (seq 5) 53.0
DVD1483 TNF (seq 6) SOST 52.7
DVD1484 SOST TNF (seq 6) 49.3
DVD1485 TNF (seq 6) NGF 54.7
DVD1488 LPA TNF (seq 6) 52.3
DVD1513 TNF (seq 1) LPA 50.7
DVD1518 SOST TNF (seq 5) 52.7
DVD1525 TNF (seq 6) SOST 55.3
DVD1526 SOST TNF (seq 6) 52.7
DVD1527 TNF (seq 6) NGF 55.7
194
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
I DVD1528 I NGF I TNF (seq 6) I 473 I
Most DVD-Igs showed an unfolding temperature >50. This DVD-Ig profile is
similar to
that observed for parent antibodies.
Solubility determination
DVD-Ig candidates were dialyzed in 15mM His, pH 6.0 followed by concentrating
them
up to 50 1 in centricons with a 30K cutoff. Solubility was visually confirmed
by absence of
precipitation after storage at 4 C and quantitatively determined by UV
absorbance measurement
at 280nm.
Table 11: Solubility of DVD-Ig Constructs
Parent N-terminal C-terminal
Antibody or Variable Domain Variable Domain
DVD-Ig ID (VD) (VD) Solubility(rank) 15mM His, pH 6
Appearance mg/ml
DVD1244 TNF (seq 1) LPA clear >62
DVD1248 TNF (seq 5) SOST clear >103
DVD1249 SOST TNF (seq 5) clear >115
DVD1256 TNF (seq 6) SOST clear >95
DVD1257 SOST TNF (seq 6) clear >80
DVD1258 TNF (seq 6) NGF clear >125
DVD1259 NGF TNF (seq 6) clear >92
DVD1261 LPA TNF (seq 6) clear >52
DVD1475 TNF (seq 5) SOST ppt 84
DVD1476 SOST TNF (seq 5) clear >78
DVD1483 TNF (seq 6) SOST clear >97
DVD1484 SOST TNF (seq 6) clear >78
DVD1485 TNF (seq 6) NGF ppt 26
DVD1488 LPA TNF (seq 6) clear >27
DVD1513 TNF (seq 1) LPA clear >81
DVD1518 SOST TNF (seq 5) clear >21
DVD1525 TNF (seq 6) SOST clear >107
DVD1526 SOST TNF (seq 6) ppt 32
DVD1527 TNF (seq 6) NGF ppt 51
DVD1528 NGF TNF (seq 6) ppt 59
Most DVD-Igs showed clear appearance and could be concentrated to greater than
25
mg/ml. This DVD-Ig profile is similar to that observed for parent antibodies.
Example 1.2.2.3.C: Efficacy Of A Humanized Monoclonal Antibody By Itself Or In
Combination With Chemotherapy On The Growth Of Human Carcinoma Xenografts
Human cancer cells are grown in vitro to 99% viability, 85% confluence in
tissue culture
flasks. SCID female or male mice (Charles Rivers Labs) at 19-25 grams, are ear
tagged and
195
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
shaved. Mice are then inoculated subcutaneously into the right flank with 0.2
ml of 2 x 106
human tumor cells (1:1 matrigel) on study day 0. Administration (IP, Q3D/
week) of vehicle
(PBS), humanized antibody, and/or chemotherapy is initiated after mice are
size matched into
separate cages of mice with mean tumor volumes of approximately 150 to 200
mm3. The tumors
are measured by a pair of calipers twice a week starting on approximately day
10 post inoculation
and the tumor volumes calculated according to the formula V = L x W2/2 (V:
volume, mm3; L:
length, mm; W: width, mm). Reduction in tumor volume is seen in animals
treated with mAb
alone or in combination with chemotherapy relative to tumors in animals that
received only
vehicle or an isotype control mAb.
Example 1.2.2.3.D: FACS Based Redirected Cytotoxicity (rCTL) Assay
Human CD3+ T cells were isolated from previously frozen isolated peripheral
blood
mononuclear cells (PBMC) by a negative selection enrichment column (R&D
Systems,
Minneapolis, MN; Cat.#HTCC-525). T cells were stimulated for 4 days in flasks
(vent cap,
Corning, Acton, MA) coated with 10pg/mL anti-CD3 (OKT-3, eBioscience, Inc.,
San Diego, CA)
and 2pg/mL anti-CD28 (CD28.2, eBioscience, Inc., San Diego, CA) in D-PBS
(Invitrogen,
Carlsbad, CA) and cultured in 30U/mL IL-2 (Roche) in complete RPMI 1640 media
(Invitrogen,
Carlsbad, CA) with L-glutamine, 55mM 13-ME, Pen/Strep, 10% FBS). T cells were
then rested
overnight in 30U/mL IL-2 before using in assay. DoHH2 or Raj i target cells
were labeled with
PKH26 (Sigma-Aldrich, St. Louis, MO) according to manufacturer's instructions.
RPMI 1640
media (no phenol, Invitrogen, Carlsbad, CA) containing L-glutamine and 10% FBS
(Hyclone,
Logan, UT) was used throughout the rCTL assay. (See Dreier et al. (2002) Int J
Cancer 100:690).
Effector T cells (E) and targets (T) were plated at a final cell concentration
of 105 and 104
cells/well in 96-well plates (Costar #3799, Acton, MA), respectively to give
an E:T ratio of 10:1.
DVD-Ig molecules were diluted to obtain concentration-dependent titration
curves. After an
overnight incubation cells are pelleted and washed with D-PBS once before
resuspending in
FACS buffer containing 0.1% BSA (Invitrogen, Carlsbad, CA), 0.1% sodium azide
and 0.5pg/mL
propidium iodide (BD) in D-PBS. FACS data was collected on a FACS Canto II
machine (Becton
Dickinson, San Jose, CA) and analyzed in Flowjo (Treestar). The percent live
targets in the
DVD-Ig treated samples divided by the percent total targets (control, no
treatment) was
calculated to determine percent specific lysis. IC5Os were calculated in Prism
(Graphpad).
A CD3 / CD20 DVD-Ig was tested for redirected toxicity and showed in vitro
tumor
killing with an IC50 = 325pM. The sequence of this CD3 / CD20 DVD-Ig was
disclosed in US
Patent Application Serial No. 20070071675.
196
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 1.4: Generation of a DVD-Ig
DVD-Ig molecules capable of binding two antigens are constructed using two
parent
monoclonal antibodies, one against human antigen A, and the other against
human antigen B,
selected as described herein.
Example 1.4.1: Generation Of A DVD-Ig Having Two Linker Lengths
A constant region containing p.1 Fc with mutations at 234, and 235 to
eliminate
ADCC/CDC effector functions is used. Four different anti-A/B DVD-Ig constructs
are generated:
2 with short linker and 2 with long linker, each in two different domain
orientations: VA-VB-C
and VB-VA-C (see Table 11). The linker sequences, derived from the N-terminal
sequence of
human Cl/Ck or CHI domain, are as follows:
For DVDAB constructs:
light chain (if anti-A has X):Short linker: QPKAAP (SEQ ID NO: 15); Long
linker:
QPKAAPSVTLFPP (SEQ ID NO: 16)
light chain (if anti-A has x):Short linker: TVAAP (SEQ ID NO: 13); Long
linker:
TVAAPSVFIFPP (SEQ ID NO: 14)
heavy chain (y1): Short linker: ASTKGP (SEQ ID NO: 21); Long linker:
ASTKGPSVFPLAP (SEQ ID NO: 22)
For DVDBA constructs:
light chain (if anti-B has X):Short linker: QPKAAP (SEQ ID NO: 15); Long
linker:
QPKAAPSVTLFPP (SEQ ID NO: 16)
light chain (if anti-B has k):Short linker: TVAAP (SEQ ID NO: 13); Long
linker:
TVAAPSVFIFPP (SEQ ID NO: 14)
heavy chain (y1): Short linker: ASTKGP (SEQ ID NO: 21); Long linker:
ASTKGPSVFPLAP (SEQ ID NO: 22)
Heavy and light chain constructs are subcloned into the pBOS expression
vector, and
expressed in COS cells, followed by purification by Protein A chromatography.
The purified
materials are subjected to SDS-PAGE and SEC analysis.
Table 12 describes the heavy chain and light chain constructs used to express
each anti-
A/B DVD-Ig protein.
197
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Table 12: Anti-A/B DVD-Ig Constructs
DVD-Ig protein Heavy chain construct Light chain construct
DVDABSL DVDABHC-SL DVDABLC-SL
DVDABLL DVDABHC-LL DVDABLC-LL
DVDBASL DVDBAHC-SL DVDBALC-SL
DVDBALL DVDBAHC-LL DVDBALC-LL
Example 1.4.2: Molecular cloning of DNA constructs for DVDABSL and DVDABLL
To generate heavy chain constructs DVDABHC-LL and DVDABHC-SL, VII domain of
A antibody is PCR amplified using specific primers (3' primers contain
short/long liner sequence
for SL/LL constructs, respectively); meanwhile VH domain of B antibody is
amplified using
specific primers (5' primers contains short/long liner sequence for SL/LL
constructs,
respectively). Both PCR reactions are performed according to standard PCR
techniques and
procedures. The two PCR products are gel-purified, and used together as
overlapping template
for the subsequent overlapping PCR reaction. The overlapping PCR products are
subcloned into
Srf I and Sal I double digested pBOS-hC71,z non-a mammalian expression vector
(Abbott) by
using standard homologous recombination approach.
To generate light chain constructs DVDABLC-LL and DVDABLC-SL, VL domain of A
antibody is PCR amplified using specific primers (3' primers contain
short/long liner sequence
for SL/LL constructs, respectively); meanwhile VL domain of B antibody is
amplified using
specific primers (5' primers contains short/long liner sequence for SL/LL
constructs,
respectively). Both PCR reactions are performed according to standard PCR
techniques and
procedures. The two PCR products are gel-purified, and used together as
overlapping template
for the subsequent overlapping PCR reaction using standard PCR conditions. The
overlapping
PCR products are subcloned into Srf I and Not I double digested pBOS-hCk
mammalian
expression vector (Abbott) by using standard homologous recombination
approach. Similar
approach has been used to generate DVDBASL and DVDBALL as described below:
Example 1.4.3: Molecular cloning of DNA constructs for DVDBASL and D'VDBALL
To generate heavy chain constructs DVDBAHC-LL and DVDBAHC-SL, VII domain of
antibody B is PCR amplified using specific primers (3' primers contain
short/long liner sequence
198
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
for SL/LL constructs, respectively); meanwhile VH domain of antibody A is
amplified using
specific primers (5' primers contains short/long liner sequence for SL/LL
constructs,
respectively). Both PCR reactions are performed according to standard PCR
techniques and
procedures. The two PCR products are gel-purified, and used together as
overlapping template
for the subsequent overlapping PCR reaction using standard PCR conditions. The
overlapping
PCR products are subcloned into Srf I and Sal I double digested pBOS-hCyl,z
non-a mammalian
expression vector (Abbott) by using standard homologous recombination
approach.
To generate light chain constructs DVDBALC-LL and DVDBALC-SL, VL domain of
antibody B is PCR amplified using specific primers (3' primers contain
short/long liner sequence
for SL/LL constructs, respectively); meanwhile VL domain of antibody A is
amplified using
specific primers (5' primers contains short/long liner sequence for SL/LL
constructs,
respectively). Both PCR reactions are performed according to standard PCR
techniques and
procedures. The two PCR products are gel-purified, and used together as
overlapping template
for the subsequent overlapping PCR reaction using standard PCR conditions. The
overlapping
PCR products are subcloned into Srf I and Not I double digested pBOS-hCk
mammalian
expression vector (Abbott) by using standard homologous recombination
approach.
Example 1.4.4: Construction and Expression of Additional DVD-Ig
Example 1.4.4.1: Preparation of DVD-Ig vector constructs
Parent antibody amino acid sequences for specific antibodies, which recognize
specific
antigens or epitopes thereof, for incorporation into a DVD-Ig can be obtained
by preparation of
hybridomas as described above or can be obtained by sequencing known antibody
proteins or
nucleic acids. In addition, known sequences can be obtained from the
literature. The sequences
can be used to synthesize nucleic acids using standard DNA synthesis or
amplification
technologies and assembling the desired antibody fragments into expression
vectors, using
standard recombinant DNA technology, for expression in cells.
For example, nucleic acid codons were determined from amino acids sequences
and
oligonucleotide DNA was synthesized by Blue Heron Biotechnology, Inc.
(vvww.blueheronbio.com) Bothell, WA USA. The oligonucleotides were assembled
into 300-
2,000 base pair double-stranded DNA fragments, cloned into a plasmid vector
and sequence-
verified. Cloned fragments were assembled using an enzymatic process to yield
the complete
gene and subcloned into an expression vector. (See 7,306,914; 7,297,541;
7,279,159; 7,150,969;
20080115243; 20080102475; 20080081379; 20080075690; 20080063780; 20080050506;
20080038777; 20080022422; 20070289033; 20070287170; 20070254338; 20070243194;
199
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
20070225227; 20070207171; 20070150976; 20070135620; 20070128190; 20070104722;
20070092484; 20070037196; 20070028321; 20060172404; 20060162026; 20060153791;
20030215458; 20030157643).
A group of pHybE vectors (US Patent Application Serial No. 61/021,282) were
used for
parental antibody and DVD-Ig cloning. V1, derived from pJP183; pHybE-
hCgl,z,non-a V2, was
used for cloning of antibody and DVD heavy chains with a wildtype constant
region. V2, derived
from pJP191; pHybE-hCk V2, was used for cloning of antibody and DVD light
chains with a
kappa constant region. V3, derived from pJP192; pHybE-hC1 V2, was used for
cloning of
antibody and DVDs light chains with a lambda constant region. V4, built with a
lambda signal
peptide and a kappa constant region, was used for cloning of DVD light chains
with a lambda-
kappa hybrid V domain. V5, built with a kappa signal peptide and a lambda
constant region, was
used for cloning of DVD light chains with a kappa-lambda hybrid V domain. V7,
derived from
pJP183; pHybE-hCgl,z,non-a V2, was used for cloning of antibody and DVD heavy
chains with
a (234,235 AA) mutant constant region.
Referring to Table 13, a number of vectors were used in the cloning of the
parent
antibodies and DVD-Ig VII and VL chains.
Table 13: Vectors Used to Clone Parent Antibodies and DVD-Igs
ID Heavy chain vector Light chain vector
DVD1447 V1 V2
DVD1448 V1 V2
DVD1449 V1 V2
DVD1450 V1 V2
DVD1451 V1 V2
DVD1452 V1 V2
DVD1453 V1 V2
DVD1454 V1 V2
DVD1455 V1 V2
DVD1456 V1 V2
DVD1457 V1 V2
DVD1468 V1 V2
DVD1459 V1 V2
DVD1460 V1 V2
DVD1461 V1 V2
DVD1462 V1 V2
DVD1463 V1 V2
DVD1464 V1 V2
DVD1465 V1 V2
DVD1466 VI V2
DVD1467 V1 V2
200
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
ID Heavy chain vector Light chain vector
DVD1468 V1 V2
DVD1469 V1 V2
,DVD1470 V1 V2
DVD1471 V1 V2
DVD1472 V1 V2
DVD1473 V1 V2
DVD1474 V1 V2
DVD1475 V1 V2
DVD1476 V1 V2
DVD1477 V1 V2
DVD1478 V1 V2
DVD1479 V1 V2
DVD1480 V1 V2
DVD1481 V1 V2
DVD1482 V1 V2
DVD1483 V1 V2
DVD1484 V1 V2
DVD1485 V1 V2
DVD1486 V1 V2
DVD1487 V1 V2
DVD1488 V1 V2
DVD1489 V1 V2
DVD1490 V1 V2
DVD1491 V1 V2
DVD1492 V1 V2
DVD1493 V1 V2
DVD1494 V1 V2
DVD1495 VI V2
DVD1496 VI V2
DVD1497 V1 V2
DVD1498 VI V2
DVD1499 V1 V2
DVD1500 V1 V2
DVD1501 V1 V2
DVD1502 V1 V2
DVD1503 VI V2
DVD1504 VI V2
DVD1505 V1 V2
DVD1506 V1 V2
DVD1507 V1 V2
DVD1508 V1 V2
DVD1509 V1 V2
DVD1510 V1 V2
DVD1511 V1 V2
DVD1512 V1 V2
DVD1513 V1 V2
201
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
ID Heavy chain vector Light chain vector
DVD1514 V1 V2
DVD1515 V1 V2
DVD1516 V1 V2
DVD1517 VI V2
DVD1518 VI V2
DVD1519 VI V2
DVD1520 VI V2
DVD1521 V1 V2
DVD1522 V1 V2
DVD1523 V1 V2
DVD1524 V1 V2
DVD1525 V1 V2
DVD1526 V1 V2
DVD1527 V1 V2
DVD1528 V1 V2
DVD1529 V1 V2
DVD1530 V1 V2
DVD1531 V1 V2
DVD1532 VI V2
DVD1533 VI V2
DVD1534 VI V2
DVD1535 VI V2
DVD1536 V1 V2
DVD1537 V1 V2
DVD1538 V1 V2
DVD1539 V1 V2
DVD1540 V1 V2
DVD1541 VI V2
DVD1542 V1 V2
DVD1543 VI V2
DVD1544 V1 V2
DVD1545 V1 V2
DVD1546 V1 V2
DVD1547 V1 V2
DVD1548 V1 V2
DVD1549 V1 V2
DVD1550 V1 V2
DVD1551 V1 V2
DVD1552 VI V2
DVD1553 V1 V2
DVD1554 V1 V2
DVD1555 V1 V2
DVD1556 V1 V2
DVD1557 V1 V2
DVD1558 V1 V2
DVD1559 V1 V2
202
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
ED Heavy chain vector Light chain vector
DVD1560 V1 V2
DVD1561 VI V2
DVD1562 V1 V2
DVD1563 VI V2
DVD1564 VI V2
DVD1565 VI V2
DVD1566 V1 V2
DVD1567 VI V2
DVD1568 VI V2
DVD1569 VI V2
DVD1570 VI V2
DVD1571 VI V2
DVD1572 VI V2
Example 1.4.4.2: Transfection And Expression In 293 Cells
Expression of the reference antibodies and DVD-Igs was accomplished by
transiently
cotransfecting 11EK293 (EBNA) cells with plasmids containing the corresponding
light-chain
(LC) and heavy-chain (HC) nucleic acids. HEK293 (EBNA) cells were propagated
in Freestyle
293 media (Invitrogen, Carlsbad CA) at a 0.5L-scale in flasks (2L Corning Cat#
431198) shaking
in a CO2 incubator (8% CO2, 125 RPM, 37 C). When the cultures reached a
density of 1x106
cells/ml, cells were transfected with transfection complex. Transfection
complex was prepared by
first mixing 150pg LC-plasmid and 1001tg HC-plasmid together in 25ml of
Freestyle media,
followed by the addition of 500u1 PEI stock solution [stock solution: 1 mg/ml
(pH 7.0) Linear
25kDa PEI, Polysciences Cat# 23966]. The transfection complex was mixed by
inversion and
allowed to incubate at room temperature for 10 minutes prior to being added to
the cell culture.
Following transfection, cultures continued to be grown in the CO2 incubator
(8% CO2, 125 RPM,
37 C). Twenty-four hours after transfection, the culture was supplemented with
25ml of a 10%
Tryptone Ni solution (Organo Technie, La Courneuve France Cat# 19553). Nine
days after
transfection, cells were removed from the cultures by centrifugation (16,000
g, 10 minutes), and
the retained supernatant was sterile filtered (Millipore HV Durapore Stericup,
0.45um) and
placed at 4 C until initiation of the purification step.
Each antibody or DVD-Ig was individually purified using a disposable lml
packed
column (packed by Orochem Technologies) containing MabSelect SuRe resin (GE
Healthcare).
Columns were pre-equilibriated in PBS and then loaded with the harvested 0.55L
samples
overnight (15 hours) at 1 ml/minute with the flow-through being recirculated
back into the feed
container. Following the loading step, columns were washed with 20m1 PBS and
protein was
eluted by feeding elution buffer [50mM Citric acid pH 3.5] at 4 ml/min and
collecting fractions
(1 ml) in tubes already containing 0.2ml of 1.5M Tris pH 8.2 (bringing the
final pH to
203
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
approximately 6.0). Fractions containing antibody were pooled based on the
chromatograms and
dialyzed into the final storage buffer [10mM citric acid, 10mM Na2HPO4, pH
6.01. Following
dialysis, samples were filtered through a 0.22um Steriflip (Millipore) and the
protein
concentration was determined by absorbance [Hewlett Packard 8453 diode array
spectrophotometer]. SDS-PAGE analysis was performed on analytical samples
(both reduced and
non-reduced) to assess final purity, verify the presence of appropriately
sized heavy- and light-
chain bands, and confirm the absence of significant amounts of free (e.g.,
uncomplexed) light
chain (in the non-reduced samples).
Table 14 contains the yield data for parent antibodies or DVD-Ig constructs
expressed as
milligrams per liter in 293 cells.
Table 14: Transient Expression in Yields of Parent Antibodies and DVD-Ig
Constructs in
293 Cells
Parent Antibody N-terminal C-terminal Expression Yield (mg/L)
or DVD-Ig ID Variable Variable
Domain Domain
(VD) (VD)
DVD1447 TNF (seq 2) PGE2 0.014
DVD1448 PGE2 TNF (seq 2) 1.06
DVD1453 TNF (seq 2) SOST 0.05
DVD1454 SOST TNF (seq 2) 0.184
DVD1459 TNF (seq 2) NGF 0
DVD1460 NGF TNF (seq 2) 0.24
DVD1465 TNF (seq 2) LPA 0
DVD1466 LPA TNF (seq 2) 0
DVD1471 TNF (seq 1) LPA 0.34
DVD1472 LPA TNF (seq 1) 0.66
DVD1473 TNF (seq 5) PGE2 0.14
DVD1474 PGE2 TNF (seq 5) 2
DVD1475 TNF (seq 5) SOST 21.4
DVD1476 SOST TNF (seq 5) 18.2
DVD1477 TNF (seq 5) NGF 3.6
DVD1478 NGF TNF (seq 5) 1.32
DVD1479 TNF (seq 5) LPA 1.6
DVD1480 LPA TNF (seq 5) 6
DVD1481 TNF (seq 6) PGE2 3
DVD1482 PGE2 TNF (seq 6) 5.6
DVD1483 TNF (seq 6) SOST 21.8
DVD1484 SOST TNF (seq 6) 19.8
DVD1485 TNF (seq 6) NGF 8.4
DVD1486 NGF TNF (seq 6) 2.1
DVD1487 TNF (seq 6) LPA 2.6
204
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Parent Antibody N-terminal C-terminal Expression Yield (mg/L)
or DVD-Ig ID Variable Variable
Domain Domain
(VD) (VD)
DVD1488 LPA TNF (seq 6) 8.6
DVD1489 TNF (seq 2) PGE2 0
DVD1490 PGE2 TNF (seq 2) 0.6
DVD1495 TNF (seq 2) SOST 2.2
DVD1496 SOST TNF (seq 2) 5
DVD1501 TNF (seq 2) NGF 0.056
DVD1502 NGF TNF (seq 2) 2.4
DVD1507 TNF (seq 2) LPA 0.05
DVD1508 LPA TNF (seq 2) 3.28
DVD1513 TNF (seq 1) LPA 17.72
DVD1514 LPA TNF (seq 1) 0.8
DVD1515 TNF (seq 5) PGE2 1
DVD1516 PGE2 TNF (seq 5) 4.98
DVD1517 TNF (seq 5) SOST 3.54
DVD1518 SOST TNF (seq 5) 8.78
DVD1519 TNF (seq 5) NGF 0.4
DVD1520 NGF TNF (seq 5) 0.86
DVD1521 TNF (seq 5) LPA 0
DVD1522 LPA TNF (seq 5) 1.34
DVD1523 TNF (seq 6) PGE2 2
DVD1524 PGE2 TNF (seq 6) 2.56
DVD1525 TNF (seq 6) SOST 23.8
DVD1526 SOST TNF (seq 6) 14.6
DVD1527 TNF (seq 6) NGF 11
DVD1528 NGF TNF (seq 6) 11.6
DVD1529 TNF (seq 6) LPA 5.2
DVD1530 LPA TNF (seq 6) 5.2
Example 1.4.5: Characterization and Lead Selection of A/B DVD-Igs
The binding affinities of anti-A/B DVD-Igs are analyzed on Biacore against
both protein
A and protein B. The tetravalent property of the DVD-Ig is examined by
multiple binding studies
on Biacore. Meanwhile, the neutralization potency of the DVD-Igs for protein A
and protein B
are assessed by bioassays, respectively, as described herein. The DVD-Ig
molecules that best
retain the affinity and potency of the original parent mAbs are selected for
in-depth
physicochemical and bio-analytical (rat PK) characterizations as described
herein for each mAb.
Based on the collection of analyses, the final lead DVD-Ig is advanced into
CHO stable cell line
development, and the CHO-derived material is employed in stability,
pharmacokinetic and
efficacy studies in cynomolgus monkey, and preformulation activities.
205
WO 2012/027570 CA 02809433 2013-02-22 PCT/US2011/049147
Example 2: Generation and Characterization of Dual Variable Domain
Immunoglobulins
(DVD-Ig)
Dual variable domain immunoglobulins (DVD-Ig) using parent antibodies with
known
amino acid sequences were generated by synthesizing polynucleotide fragments
encoding DVD-
Ig variable heavy and DVD-Ig variable light chain sequences and cloning the
fragments into a
pHybC-D2 vector according to Example 1.4.4.1. The DVD-Ig contructs were cloned
into and
expressed in 293 cells as described in Example 1,4.4.2. The DVD-Ig protein was
purified
according to standard methods. Functional characteristics were determined
according to the
methods described in Example 1.1.1 and 1.1.2 as indicated. DVD-Ig VII and VL
chains for the
DVD-Igs disclosed herein are provided below.
206
CA 02809433 2013-02-22
WO 2012/027570
PCT/US2011/049147
Example 2.1: Generation of TNF (seq. 2) and NGF DVD-Igs with Linker Set 2
Table 15
DVD Outer
Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name
Name 12345678901234567890123456789012345
NO
52 DVD1459H AB213VH
ABO2OVH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSAASTKGPQVQLQESGPGLVKPSET
LSLTCTVSGFSLIGYDLNWIRQPPGKGLEWIGIIW
GDGTTDYNSAVKSRVTISKDTSKNQFSLKLSSVTA
ADTAVYYCARGGYWYATSYYFDYWGQGTLVTVSS
53 DVD1459L AB213VL ABO2OVL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTFGSGTKLE
IKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQSISNNLNWYQQKPGKAPKLLIYYTSRFH
SGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQ
54 DVD1460H ABO2OVH
AB213VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
EHTLPYTFGQGTKLEIKR
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPQVQLKESGPGLVA
PSQSLSITCTVSGFSLTDYGVNWVRQPPGKGLEWL
GMIWGDGSTDYDSTLKSRLSISKDNSKSQIFLKMN
SLQTDDTARYYCAREWHHGPVAYWGQGTLVTVSA
55 DVD1460L ABO2OVL
AB213VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPDIVMTQSHKFMSTTVGDRVS
ITCKASQAVSSAVAWYQQKPGQSPKLLIYWASTRH
TGVPDRFTGSGSVTDFTLTIHNLQAEDLALYYCQQ
HYSTPFTFGSGTKLEIKR
207
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.2: Generation of TNF (seq. 3) and NGF DVD-Igs with Linker Set 2
Table 16
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
56 DVD1461H AB214VH ABO2OVH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPQVQLQESGPGLVKP
SETLSLTCTVSGFSLIGYDLNWIRQPPGKGLEWIG
IIWGDGTTDYNSAVKSRVTISKDTSKNQFSLKLSS
VTAADTAVYYCARGGYWYATSYYFDYWGQGTLVTV
SS
57 DVD1461L AB214VL ABO2OVL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQSISNNLNWYQQKPGKAPKLLIYYTSRFH
SGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQ
EHTLPYTFGQGTKLEIKR
58 DVD1462H ABO2OVH AB214V1-I QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPEVKLEESGGGLVQ
PGGSMKLSCVASGFIFSNHWMNWVRQSPEKGLEWV
AEIRSKSINSATHYAESVKGRFTISRDDSKSAVYL
QMTDLRTEDTGVYYCSRNYYGSTYDYWGQGTTLTV
SS
59 DVD1462L ABO2OVL AB214VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPDILLTQSPAILSVSPGERVS
FSCRASQFVGSSIHWYQQRTNGSPRLLIKYASESM
SGIPSRFSGSGSGTDFTLSINTVESEDIADYYCQE
SHSWPFTFGSGTNLEVKR
208
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.23: Generation of TNF (seq. 4) and NGF DVD-Igs with Linker Set 2
Table 17
DVD Outer Inner Sequence
SE" Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
60 DVD1463H AB215VH ABO2OVH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPQVQLQESG
PGLVKPSETLSLTCTVSGFSLIGYDLNWIRQPPGK
GLEWIGIIWGDGTTDYNSAVKSRVTISKDTSKNQF
SLKLSSVTAADTAVYYCARGGYWYATSYYFDYWGQ
GTLVTVSS
61 DVD1463L AB215VL ABO2OVL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRV
TITCRASQSISNNLNWYQQKPGKAPKLLIYYTSRF
HSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQ
CIEHTLPYTFGQGTKLEIKR
62 DVD1464H ABO2OVH AB215VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPQVQLVESGGGVVQ
PGRSLRLSCAASGFTFSSYAMHWVRQAPGNGLEWV
AFMSYDGSNKYAKDSVKGRFTISRDNSKNTLYLQM
NSLRAEDTAVYYCARDRGIAAGGNYYYYGMDVWGQ
GTTVTVSS
63 DVD1464L ABO2OVL AB215VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPEIVLTQSPATLSLSPGERAT
LSCRASQSVYSYLAWYQQKPGQAPRLLIYDASNRA
TGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQ
RSNWPPFTFGPGTKVDIKR
209
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.4: Generation of TNF (seq. 5) and NGF DVD-Igs with Linker Set 2
Table 18
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
64 DVD1477H AB217VH ABO2OVH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPQVQLQESGP
GLVKPSETLSLTCTVSGESLIGYDLNWIROPPGKG
LEWIGIIWGDGTTDYNSAVKSRVTISKDTSKNQFS
LKLSSVTAADTAVYYCARGGYWYATSYYFDYWGQG
TLVTVSS
65 DVD1477L AB217VL ABO2OVL DIQMTQSFSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQSISNNLNWYQQKPGKAPELLIYYTSRFH
SGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQ
EHTLPYTFGQGTKLEIKR
66 DVD1478H ABO2OVH AB217VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPQVQLVESGGGVVQ
PGRSLRLSCAASGFTFSSYDMHWVRQAPGKGLEWV
AVIWSDGSIKYYADSVKGRFTISRDNSKNTLYLQM
NSLRAEDTAVYYCAREVESAMGGFYYNGMDVWGQG
TTVTVSS
67 DVD1478L ABO2OVL AB217VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQGIRIDLGWYQQKPGKAPKRLIYAASTLQ
SGVPSRFSGSGSGTEFIFTISSLQPEDFASYYCLQ
HKSYPLTFGGGTKVEIKR
210
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 23: Generation of TNF (seq. 6) and NGF-Igs with Linker Set 2
Table 19
DVD Outer Inner Sequence
SEQVariable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
68 DVD1485H AB218VH ABO2OVH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPQVQLQESGPGLVKPSETLS
LTCTVSGFSLIGYDLNWIRQPPGKGLEWIGIIWGD
GTTDYNSAVKSRVTISKDTSKNQFSLKLSSVTAAD
TAVYYCARGGYWYATSYYFDYWGQGTLVTVSS
69 DVD1485L AB218VL ABO2OVL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVTI
TCRASQSISNNLNWYQQKPGKAPKLLIYYTSRFHS
GVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQE
HTLPYTFGQGTKLEIKR
70 DVD1486H ABO2OVH AB218VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPEVQLVESGGGLIQ
PGGSLRLSCAASGFTVSRNYMSWVRQAPGKGLEWV
SVIYSGDRTYYADSVKGRFTISRDNSKNTLYLQMN
SLRAEDTAVYYCARGEGGFDYWGQGTLVTVSS
71 DVD1486L ABO2OVL AB218VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPEIVMTQSPATLSVSPGERAT
LSCRASQSVSSNLAWYQQKPGQAPRLLIHGASIRA
TGLPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQ
YNYWWTFGQGTKVEIKR
211
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.6: Generation of TNF (seq. 2) and SOST DVD-Igs with Linker Set 2
Table 20
DVD Outer Inner Sequence
SE ^ Variable Variable Variable
'4 Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
72 DVD1453H AB213VH AB022VH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSAASTKGPEVQLQQSGPELVTPGAS
VKISCKASGYTFTDHYMSWVKQSHGKSLEWIGDIN
PYSGETTYNQKFKGTATLTVDKSSSIAYMEIRGLT
SEDSAVYYCARDDYDASPFAYWGQGTLVTVSA
73 DVD1453L AB213VL AB022VL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTFGSGTKLE
IKRTVAAPSVFIFPPDVQMIQSPSSLSASLGDIVT
MTCQASQGTSINLNWFQQKPGKAPKLLIYGSSNLE
DGVPSRFSGSRYGTDFTLTISSLEDEDLATYFCLQ
HSYLPYTFGGGTKLEIKR
74 DVD1454H AB022VH AB213VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPQVQLKESGPGLVAPS
QSLSITCTVSGFSLTDYGVNWVRQPPGKGLEWLGM
IWGDGSTDYDSTLKSRLSISKDNSKSQIFLKMNSL
QTDDTARYYCAREWHHGPVAYWGQGTLVTVSA
75 DVD1454L AB022VL AB213VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPSVFIFPPDIVMTQSHKFMSTTVGDRVS
ITCKASQAVSSAVAWYQQKPGQSPKLLIYWASTRH
TGVPDRFTGSGSVTDFTLTIHNLQAEDLALYYCQQ
HYSTPFTFGSGTKLEIKR
212
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.7: Generation of TNF (seq. 3) and SOST DVD-Igs with Linker Set 2
Table 21
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
76 DVD1455H AB214VH AB022VH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPEVQLQQSGPELVTP
GASVKISCKASGYTFTDHYMSWVKQSHGKSLEWIG
DINPYSGETTYNQKFKGTATLTVDKSSSIAYMEIR
GLTSEDSAVYYCARDDYDASPFAYWGQGTLVTVSA
77 DVD1455L AB214VL AB022VL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPSVFIFPPDVQMIQSPSSLSASLGDIVT
MTCQASQGTSINLNWFQQKPGKAPKLLIYGSSNLE
DGVPSRFSGSRYGTDFTLTISSLEDEDLATYFCLQ
HSYLPYTEGGGTKLEIKR
78 DVD1456H AB022VH AB214VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPEVKLEESGGGLVQPG
GSMKLSCVASGFIFSNHWMNWVRQSPEKGLEWVAE
IRSKSINSATHYAESVKGRFTISRDDSKSAVYLQM
TDLRTEDTGVYYCSRNYYGSTYDYWGQGTTLTVSS
79 DVD1456L AB022VL AB214VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPSVFIFPPDILLTQSPAILSVSPGERVS
FSCRASQFVGSSIHWYQQRTNGSPRLLIKYASESM
SGIPSRFSGSGSGTDFTLSINTVESEDIADYYCQE
SHSWPFTFGSGTNLEVKR
213
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.8: Generation of TISTF(seq. 4) and SOST DVD-Igs with Linker Set 2
Table 22
DVD Outer Inner Sequence
SEQ Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
80 DVD1457H AB215VH AB022VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPEVQLQQSG
PELVTPGASVKISCKASGYTFTDHYMSWVKQSHGK
SLEWIGDINPYSGETTYNQKFKGTATLTVDKSSSI
AYMEIRGLTSEDSAVYYCARDDYDASPFAYWGQGT
LVTVSA
81 DVD1457L AB215VL AB022VL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPSVFIFPPDVQMIQSPSSLSASLGDIV
TMTCQASQGTSINLNWFQQKPGKAPKLLIYGSSNL
EDGVPSRFSGSRYGTDFTLTISSLEDEDLATYFCL
QHSYLPYTFGGGTKLEIKR
82 DVD1458H AB022VH AB215VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPQVQLVESGGGVVQPG
RSLRLSCAASGFTFSSYAMHWVRQAPGNGLEWVAF
MSYDGSNKYAKDSVKGRFTISRDNSKNTLYLQMNS
LRAEDTAVYYCARDRGIAAGGNYYYYGMDVWGQGT
TVTVSS
83 DVD1458L AB022VL AB215VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPSVFIFPPEIVLTQSPATLSLSPGERAT
LSCRASQSVYSYLAWYQQKPGQAPRLLIYDASNRA
TGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQ
RSNWPPFTFGPGTKVDIKR
214
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.9: Generation of TNF (seq. 5) and SOST DVD-Igs with Linker Set 2
Table 23
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
84 DVD1475H AB217VH AB022VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPEVQLQQSGP
ELVTPGASVKISCKASGYTFTDHYMSWVKQSHGKS
LEWIGDINPYSGETTYNQKFKGTATLTVDKSSSIA
YMEIRGLTSEDSAVYYCARDDYDASPFAYWGQGTL
VTVSA
85 DVD1475L AB217VL AB022VL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPSVFIFPPDVQMIQSPSSLSASLGDIVT
MTCQASQGTSINLNWFQQKPGKAPKLLIYGSSNLE
DGVPSRFSGSRYGTDFTLTISSLEDEDLATYFCLQ
HSYLPYTFGGGTKLEIKR
86 DVD1476H AB022VE AB217VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPQVQLVESGGGVVQPG
RSLRLSCAASGFTFSSYDMHWVRQAPGKGLEWVAV
IWSDGSIKYYADSVKGRFTISRDNSKNTLYLQMNS
LRAEDTAVYYCAREVESAMGGFYYNGMDVWGQGTT
VTVSS
87 DVD1476L AB022VL AB217VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQGIRIDLGWYQQKPGKAPKRLIYAASTLQ
SGVPSRFSGSGSGTEFIFTISSLQPEDFASYYCLQ
HKSYPLTFGGGTKVEIKR
215
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.10: Generation of TNF (seq. 6) and SOST DVD-Igs with Linker Set 2
Table 24
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
88 DVD1483H AB218VH AB022VH EVQLVESGGGLIUGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPEVQLQQSGPELVTPGASVK
ISCKASGYTFTDHYMSWVKQSHGKSLEWIGDINPY
SGETTYNQKFKGTATLTVDKSSSIAYMEIRGLTSE
DSAVYYCARDDYDASPFAYWGQGTLVTVSA
89 DVD1483L AB218VL AB022VL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPSVFIFPPDVQMIQSPSSLSASLGDIVTM
TCQASQGTSINLNWFQQKPGKAPKLLIYGSSNLED
GVPSRFSGSRYGTDFTLTISSLEDEDLATYFCLQH
SYLPYTFGGGTKLEIKR
90 DVD1484H AB022VH AB218VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPEVQLVESGGGLIQPG
GSLRLSCAASGFTVSRNYMSWVRQAPGKGLEWVSV
IYSGDRTYYADSVKGRFTISRDNSKNTLYLQMNSL
RAEDTAVYYCARGEGGFDYWGQGTLVTVSS
91 DVD1484L AB022VL AB218VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPSVFIFPPEIVMTQSPATLSVSPGERAT
LSCRASQSVSSNLAWYQQKPGQAPRLLIHGASIKA
TGLPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQ
YNYWWTFGQGTKVEIKR
216
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.11: Generation of TNF (seq. 2) and PGE2 with Linker Set 2
Table 25
DVD Outer Inner Sequence
Variable Variable Variable
SEQ
Domain Domain Domain
ID Name Name Name 1234567890123456789012345678901234
NO
92 DVD1447H AB213VH AB048VH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGV
NWVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLS
ISKDNSKSQIFLKMNSLQTDDTARYYCAREWHHG
PVAYWGQGTLVTVSAASTKGPEVQLVQSGAEVKK
PGASVKVSCKASGYTFTKYWLGWVRQAPGQGLEW
MGDIYPGYDYTHYNEKFKDRVTLTTDTSTSTAYM
ELRSLRSDDTAVYYCARSDGSSTYWGQGTLVTVS
93 DVD1447L AB213VL AB048VL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVA
WYQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSV
TDFTLTIHNLQAEDLALYYCQQHYSTPFTFGSGT
KLEIKRTVAAPSVFIFPPDVLMTQTPLSLPVTPG
EPASISCTSSQNIVHSNGNTYLEWYLQKPGQSPQ
LLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVE
AEDVGVYYCFQVSHVPYTFGGGTKVEIKR
94 DVD1448H AB048VH AB213VH EVQLVQSGAEVKKPGASVEVSCKASGYTFTEYWL
GWVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRV
TLTTDTSTSTAYMELRSLRSDDTAVYYCARSDGS
STYWGQGTLVTVSSASTKGPQVQLKESGPGLVAP
SQSLSITCTVSGFSLTDYGVNWVRQPPGKGLEWL
GMIWGDGSTDYDSTLKSRLSISKDNSKSQIFLKM
NSLQTDDTARYYCAREWHHGPVAYWGQGTLVTVS
A
95 DVD1448L AB048VL AB213VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNG
NTYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFS
GSGSGTDFTLKISRVEAEDVGVYYCFQVSHVPYT
FGGGTKVEIKRTVAAPSVFIFPPDIVMTQSHKFM
STTVGDRVSITCKASQAVSSAVAWYQQKPGQSPK
LLIYWASTRHTGVPDRFTGSGSVTDFTLTIHNLQ
AEDLALYYCQQHYSTPFTFGSGTKLEIKR
217
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.12: Generation of TNF (seq. 3) and PGE2 DVD-Igs with Linker Set 2
Table 26
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
96 DVD1449H AB214VH AB048VH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPEVQLVQSGAEVKKP
GASVKVSCKASGYTETKYWLGWVRQAPGQGLEWMG
DIYPGYDYTHYNEKFKDRVTLTTDTSTSTAYMELR
SLRSDDTAVYYCARSDGSSTYWGQGTLVTVSS
97 DVD1449L AB214VL AB048VL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPSVFIFPPDVLMTQTPLSLPVTPGEPAS
ISCTSSQNIVHSNGNTYLEWYLQKPGQSPQLLIYK
VSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGV
YYCFQVSHVPYTFGGGTKVEIKR
98 DVD1450H AB048VH AB214VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTY
WGQGTLVTVSSASTKGPEVKLEESGGGLVQPGGSM
KLSCVASGFIFSNHWMNWVRQSPEKGLEWVAEIRS
KSINSATHYAESVKGRFTISRDDSKSAVYLQMTDL
RTEDTGVYYCSRNYYGSTYDYWGQGTTLTVSS
99 DVD1450L AB048VL AB214VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPORFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTEGG
GTKVEIKRTVAAPSVFIFPPDILLTQSPAILSVSP
GERVSFSCRASQFVGSSIHWYQQRTNGSPRLLIKY
ASESMSGIPSRFSGSGSGTDFTLSINTVESEDIAD
YYCQESHSWPFTEGSGTNLEVKR
218
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.13: Generation of TN!? (seq. 4) and PGE2 DVD-Igs with Linker Set 2
Table 27
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
100 DVD1451H AB215VH AB048VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPEVQLVQSG
AEVKKPGASVKVSCKASGYTFTKYWLGWVRQAPGQ
GLEWMGDIYPGYDYTHYNEKFKDRVTLTTDTSTST
AYMELRSLRSDDTAVYYCARSDGSSTYWGQGTLVT
VSS
101 DVD1451L AB215VL AB048VL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPSVFIFPPDVLMTQTPLSLPVTPGEPA
SISCTSSQNIVHSNGNTYLEWYLQKPGQSPQLLIY
KVSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVG
VYYCFQVSHVPYTFGGGTKVEIKR
102 DVD1452H AB048VH AB215VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTY
WGQGTLVTVSSASTKGPQVQLVESGGGVVQPGRSL
RLSCAASGFTESSYAMHWVRQAPGNGLEWVAFMSY
DGSNKYAKDSVKGRFTISRDNSKNTLYLQMNSLRA
EDTAVYYCARDRGIAAGGNYYYYGMDVWGQGTTVT
VSS
103 DV1J1452L AB048VL AB215VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTFGG
GTKVETKRTVAAPSVFIFPPEIVLTQSPATLSLSP
GERATLSCRASQSVYSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAV
YYCQQRSNWPPFTFGPGTKVDIKR
219
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.14: Generation of TNF (seq. 5) and PGE2 DVD-Igs with Linker Set 2
Table 28
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
104 DVD1473H AB217VH AB048VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPEVQLVQSGA
EVKKPGASVKVSCKASGYTFTKYWLGWVRQAPGQG
LEWMGDIYPGYDYTHYNEKFKDRVTLTTDTSTSTA
YMELRSLRSDDTAVYYCARSDGSSTYWGQGTLVTV
SS
105 DVD1473L AB217VL AB048VL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPSVFIFPPDVLMTQTPLSLPVTPGEPAS
ISCTSSQNIVHSNGNTYLEWYLQKPGQSPQLLIYK
VSNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGV
YYCFQVSHVPYTFGGGTKVEIKR
106 DVD1474H AB048VH AB217VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTY
WGQGTLVTVSSASTKGPQVQLVESGGGVVQPGRSL
RLSCAASGFTFSSYDMHWVRQAPGKGLEWVAVIWS
DGSIKYYADSVKGRFTISRDNSKNTLYLQMNSLRA
EDTAVYYCAREVESAMGGFYYNGMDVWGQGTTVTV
SS
107 DVD1474L AB048VL AB217VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTFGG
GTKVEIKRTVAAPSVFIFPPDIQMTQSPSSLSASV
GDRVTITCRASQGIRIDLGWYQQKPGKAPKRLIYA
ASTLQSGVPSRFSGSGSGTEFIFTISSLQPEDFAS
YYCLQHKSYPLTFGGGTKVEIKR
220 =
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.15: Generation of TNF (seq. 6) and PGE2 DVD-Igs with Linker Set 2
Table 29
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
108 DVD1481H AB218VH A3048VH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPEVQLVQSGAEVKKPGASVK
VSCKASGYTFTKYWLGWVRQAPGQGLEWMGDIYPG
YDYTHYNEKFKDRVTLTTDTSTSTAYMELRSLRSD
DTAVYYCARSDGSSTYWGQGTLVTVSS
109 DVD1481L AB218VL AB048VL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPSVFIFPPDVLMTQTPLSLPVTPGEPASI
SCTSSQNIVHSNGNTYLEWYLQKPGQSPQLLIYKV
SNRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVY
YCFQVSHVPYTFGGGTKVEIKR
110 DVD1482H AB048VH AB218VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTY
WGQGTLVTVSSASTKGPEVQLVESGGGLIQPGGSL
RLSCAASGFTVSRNYMSWVRQAPGKGLEWVSVIYS
GDRTYYADSVKGRFTISRDNSKNTLYLQMNSLRAE
DTAVYYCARGEGGFDYWGQGTLVTVSS
111 DVD1482L AB048VL AB218VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTFGG
GTKVEIKRTVAAPSVFIFPPEIVMTQSPATLSVSP
GERATLSCRASQSVSSNLAWYQQKPGQAPRLLIHG
ASIRATGLPARFSGSGSGTEFTLTISSLQSEDFAV
YYCQQYNYWWTFGQGTKVEIKR
221
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.16: Generation of TNF (seq. 2) and LPA DVD-Igs with Linker Set 2
Table 30
DVD Outer Inner Sequence
SE Variable Variable Variable
Q Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
112 DVD1465H AB213VH AB216VH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSLAISTKGPQVQLQQSGAELVRPGTS
VKVSCKASGYGFINYLIEWIKQRPGQGLEWIGLIN
PGSDYTNYNENFKGKATLTADKSSSTAYMHLSSLT
SEDSAVYFCARRFGYYGSGNYFDYWGQGTTLTVSS
113 DVD1465L AB213VL AB216VL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTEGSGTKLE
IKRTVAAPSVFIFPPDVVMTQTPLSLPVSLGDQAS
ISCTSGQSLVHINGNTYLHWYLQKPGQSPKLLIYK
VSNLFSGVPDRFSGSGSGTDFTLKISRVEAEDLGV
YFCSQSTHFPFTFGTGTKLEIKR
114 DVD1466H AB216VH AB213VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPQVQLKESGPGLV
APSQSLSITCTVSGFSLTDYGVNWVRQPPGKGLEW
LGMIWGDGSTDYDSTLKSRLSISKDNSKSQIFLKM
NSLQTDDTARYYCAREWHHGPVAYWGQGTLVTVSA
115 DVD1466L AB216VL AB213VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPSVFIFPPDIVMTQSHKFMSTTV
GDRVSITCKASQAVSSAVAWYQQKPGQSPKLLIYW
ASTRHTGVPDRFTGSGSVTDFTLTIHNLQAEDLAL
YYCQQHYSTPFTFGSGTKLEIKR
222
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.17: Generation of TNF (seq. 3) and LPA DVD-Igs with Linker Set 2
Table 31
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
116 DVD1467H AB214VH AB216VH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPQVQLQQSGAELVRP
GTSVKVSCKASGYGFINYLIEWIKQRPGQGLEWIG
LINPGSDYTNYNENFKGKATLTADKSSSTAYMHLS
SLTSEDSAVYFCARRFGYYGSGNYFDYWGQGTTLT
VSS
117 DVD1467L AB214VL AB216VL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPSVFIFPPDVVMTQTPLSLPVSLGDQAS
ISCTSGQSLVHINGNTYLHWYLQKPGQSPKLLIYK
VSNLFSGVPDRFSGSGSGTDFTLKISRVEAEDLGV
YFCSQSTHFPFTFGTGTKLEIKR
118 DVD1468H AB216VH 1B214VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPEVKLEESGGGLV
QPGGSMKLSCVASGFIFSNHWMNWVRQSPEKGLEW
VAEIRSKSINSATHYAESVKGRFTISRDDSKSAVY
LQMTDLRTEDTGVYYCSRNYYGSTYDYWGQGTTLT
VSS
119 DVD1468L AB216VL AB214VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPSVFIFPPDILLTQSPAILSVSP
GERVSFSCRASQFVGSSIHWYQQRTNGSPRLLIKY
ASESMSGIPSRFSGSGSGTDFTLSINTVESEDIAD
YYCQESHSWPFTFGSGTNLEVKR
223
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.18: Generation of TNF (seq. 4) and LPA DVD-Igs with Linker Set 2
Table 32
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
120 DVD1469H AB215VH AB216VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPQVQLQQSG
AELVRPGTSVKVSCKASGYGFINYLIEWIKQRPGQ
GLEWIGLINPGSDYTNYNENFKGKATLTADKSSST
AYMHLSSLTSEDSAVYFCARRFGYYGSGNYFDYWG
QGTTLTVSS
121 DVD1469L AB215VL AB216VL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPSVFIFPPDVVMTQTPLSLPVSLGDQA
SISCTSGQSLVHINGNTYLHWYLQKPGQSPKLLIY
KVSNLFSGVPDRFSGSGSGTDFTLKISRVEAEDLG
VYFCSQSTHFPFTFGTGTKLEIKR
122 DVD1470H AB216VH AB215VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPQVQLVESGGGVV
QPGRSLRLSCAASGFTFSSYAMHWVRQAPGNGLEW
VAFMSYDGSNKYAKDSVKGRFTISRDNSKNTLYLQ
MNSLRAEDTAVYYCARDRGIAAGGNYYYYGMDVWG
QGTTVTVSS
123 DVD1470L AB216VL AB215VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPSVFIFPPEIVLTQSPATLSLSP
GERATLSCRASQSVYSYLAWYQQKPGQAPRLLIYD
ASNRATGIPARFSGSGSGTDFTLTISSLEPEDFAV
YYCQQRSNWPPFTFGPGTKVDIKR
224
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.19: Generation of TNF (seq. 1) and LPA DVD-Igs with Linker Set 2
Table 33
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
124 DVD1471H AB017VH AB216VH EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMH
WVRQAPGKGLEWVSAITWNSGHIDYADSVEGRFTI
SRDNAKNSLYLQMNSLRAEDTAVYYCAKVSYLSTA
SSLDYWGQGTLVTVSSASTKGPQVQLQQSGAELVR
PGTSVKVSCKASGYGFINYLIEWIKQRPGQGLEWI
GLINPGSDYTNYNENFKGKATLTADKSSSTAYMHL
SSLTSEDSAVYFCARRFGYYGSGNYFDYWGQGTTL
TVSS
125 DVD1471L AB017VL AB216VL DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAW
YQQKPGKAPKLLIYAASTLQSGVPSRFSGSGSGTD
FTLTISSLQPEDVATYYCQRYNRAPYTFGQGTKVE
IKRTVAAPSVFIFPPDVVMTQTPLSLPVSLGDQAS
ISCTSGQSLVHINGNTYLHWYLQKPGQSPKLLIYK
VSNLFSGVPDRFSGSGSGTDFTLKISRVEAEDLGV
YFCSQSTHFPFTFGTGTKLEIKR
126 DVD1472H AB216VH AB017VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPEVQLVESGGGLV
QPGRSLRLSCAASGFTFDDYAMHWVRQAPGKGLEW
VSAITWNSGHIDYADSVEGRFTISRDNAKNSLYLQ
MNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLV
TVSS
127 DVD1472L AB216VL AB017VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPSVFIFPPDIQMTQSPSSLSASV
GDRVTITCRASQGIRNYLAWYQQKPGKAPKLLIYA
ASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDVAT
YYCQRYNRAPYTFGQGTKVEIKR
225
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.20: Generation of TNF (seq. 5) and LPA DVD-Igs with Linker Set 2
Table 34
DVD Outer Inner Sequence
SE Variable Variable Variable
Q Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
128 DVD1479H AB217VH AB216VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPQVQLQQSGA
ELVRPGTSVKVSCKASGYGFINYLIEWIKQRPGQG
LEWIGLINPGSDYTNYNENFKGKATLTADKSSSTA
YMHLSSLTSEDSAVYFCARRFGYYGSGNYFDYWGQ
GTTLTVSS
129 DVD1479L AB217VL AB216VL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPSVFIFPPDVVMTQTPLSLPVSLGDQAS
ISCTSGQSLVHINGNTYLHWYLQKPGQSPKLLIYK
VSNLFSGVPDRFSGSGSGTDFTLKISRVEAEDLGV
YFCSQSTHFPFTFGTGTKLEIKR
130 DVD1480H AB216VH AB217VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPQVQLVESGGGVV
QPGRSLRLSCAASGFTFSSYDMHWVRQAPGKGLEW
VAVIWSDGSIKYYADSVKGRFTISRDNSKNTLYLQ
MNSLRAEDTAVYYCAREVESAMGGFYYNGMDVWGQ
GTTVTVSS
131 DVD1480L AB216VL AB217VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPSVFIFPPDIQMTQSPSSLSASV
GDRVTITCRASQGIRIDLGWYQQKPGKAPKRLIYA
ASTLQSGVPSRFSGSGSGTEFIFTISSLQPEDFAS
YYCLQHKSYPLTFGGGTKVEIKR
226
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.21: Generation of TNF (seq. 6) and LPA DVD-Igs with Linker Set 2
Table 35
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
132 DVD1487H AB218VH AB216VH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPQVQLQQSGAELVRPGTSVK
VSCKASGYGFINYLIEWIKQRPGQGLEWIGLINPG
SDYTNYNENFKGKATLTADKSSSTAYMHLSSLTSE
DSAVYFCARRFGYYGSGNYFDYWGQGTTLTVSS
133 DVD1487L AB218VL AB216VL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPSVFIFPPDVVMTQTPLSLPVSLGDQASI
SCTSGQSLVHINGNTYLHWYLQKPGQSPKLLIYKV
SNLFSGVPDRFSGSGSGTDFTLKISRVEAEDLGVY
FCSQSTHFPFTFGTGTKLEIKR
134 DVD1488H AB216VH AB218VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPEVQLVESGGGLI
QPGGSLRLSCAASGFTVSRNYMSWVRQAPGKGLEW
VSVIYSGDRTYYADSVKGRFTISRDNSKNTLYLQM
NSLRAEDTAVYYCARGEGGFDYWGQGTLVTVSS
135 DVD1488L AB216VL AB218VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLOPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPSVFIFPPEIVMTQSPATLSVSP
GERATLSCRASQSVSSNLAWYQQKPGQAPRLLIHG
ASIRATGLPARFSGSGSGTEFTLTISSLQSEDFAV
YYCQQYNYWWTFGQGTEVEIKR
227
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.22: Generation of TNF (seq. 2) and NGF DVD-Igs with Linker Set 3
Table 36
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
136 DVD1501H AB213VH ABO2OVH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSAASTKGPSVPPLAPQVQLQESGPG
LVKPSETLSLTCTVSGFSLIGYDLNWIRQPPGKGL
EWIGIIWGDGTTDYNSAVKSRVTISKDTSKNQFSL
KLSSVTAADTAVYYCARGGYWYATSYYFDYWGQGT
LVTVSS
137 DVD1501L AB213VL ABO2OVL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTFGSGTKLE
IKRTVAAPDIQMTQSPSSLSASVGDRVTITCRASQ
SISNNLNWYQQKPGKAPKLLIYYTSRFHSGVPSRF
SGSGSGTDFTFTISSLQPEDIATYYCQQEHTLPYT
FGQGTKLEIKR
138 DVD1502H ABO2OVH AB213VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPQVQLKE
SGPGLVAPSQSLSITCTVSGFSLTDYGVNWVRQPP
GKGLEWLGMIWGDGSTDYDSTLKSRLSISKDNSKS
QIFLKMNSLQTDDTARYYCAREWHHGPVAYWGQGT
LVTVSA
139 DVD1502L ABO2OVL AB213VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPDIVMTQSHKFMSTTVGDRVSITCKASQ
AVSSAVAWYQQKPGQSPKLLIYWASTRHTGVPDRF
TGSGSVTDFTLTIHNLQAEDLALYYCQQHYSTPFT
FGSGTKLEIKR
228
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.23: Generation of TNF (seq. 3) and NGF DVD-Igs with Linker Set 3
Table 37
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
140 DVD1503H AB214VH ABO2OVH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPSVFPLAPQVQLQES
GPGLVKPSETLSLTCTVSGFSLIGYDLNWIRQPPG
KGLEWIGIIWGDGTTDYNSAVKSRVTISKDTSKNQ
FSLKLSSVTAADTAVYYCARGGYWYATSYYFDYWG
QGTLVTVSS
141 DVD1503L AB214VL ABO2OVL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPDIQMTQSPSSLSASVGDRVTITCRASQ
SISNNLNWYQQKPGKAPKLLIYYTSRFHSGVPSRF
SGSGSGTDFTFTISSLQPEDIATYYCQQEHTLPYT
FGQGTKLEIKR
142 DVD1504H ABO2OVH AB214VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPEVKLEE
SGGGLVQPGGSMKLSCVASGFIFSNHWMNWVRQSP
EKGLEWVAEIRSKSINSATHYAESVKGRFTISRDD
SKSAVYLQMTDLRTEDTGVYYCSRNYYGSTYDYWG
QGTTLTVSS
143 DVD1504L ABO2OVL AB214VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPDILLTQSPAILSVSPGERVSFSCRASQ
FVGSSIHWYQQRTNGSPRLLIKYASESMSGIPSRF
SGSGSGTDFTLSINTVESEDIADYYCQESHSWPFT
FGSGTNLEVKR
229
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.24: Generation of TNF (seq. 4) and NGF DVD-Igs with Linker Set 3
Table 38
DVD Outer Inner Sequence
SEQ Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
144 DVD1505H AB215VH ABO2OVH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPSVFPLAPQ
VQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLNW
IRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTISK
DTSKNQFSLKLSSVTAADTAVYYCARGGYWYATSY
YFDYWGQGTLVTVSS
145 DVD1505L AB215VL ABO2OVL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPDIQMTQSPSSLSASVGDRVTITCRAS
QSISNNLNWYQQKPGKAPKLLIYYTSRFHSGVPSR
FSGSGSGTDFTFTISSLQPEDIATYYCQQEHTLPY
TFGQGTKLEIKR
146 DVD1506H ABO2OVH AB215VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPQVQLVE
SGGGVVQPGRSLRLSCAASGFTFSSYAMHWVRQAP
GNGLEWVAFMSYDGSNKYAKDSVKGRFTISRDNSK
NTLYLQMNSLRAEDTAVYYCARDRGIAAGGNYYYY
GMDVWGQGTTVTVSS
147 DVD1506L ABO2OVL AB215VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPEIVLTQSPATLSLSPGERATLSCRASQ
SVYSYLAWYQQKPGQAPRLLIYDASNRATGIPARF
SGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPPF
TFGPGTKVDIKR
230
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.25: Generation of TNF (seq. 5) and NGF DVD-Igs with Linker Set 3
Table 39
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
148 DVD1519H AB217VH ABO2OVH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPSVFPLAPQV
QLQESGPGLVKPSETLSLTCTVSGFSLIGYDLNWI
RQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTISKD
TSKNQFSLKLSSVTAADTAVYYCARGGYWYATSYY
FDYWGQGTLVTVSS
149 DVD1519L AB217VL ABO2OVL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPDIQMTQSPSSLSASVGDRVTITCRASQ
SISNNLNWYQQKPGKAPKLLIYYTSRFHSGVPSRF
SGSGSGTDFTFTISSLQPEDIATYYCQQEHTLPYT
FGQGTKLEIKR
150 DVD1520H ABO2OVH AB217VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPQVQLVE
SGGGVVQPGRSLRLSCAASGFTFSSYDMHWVRQAP
GKGLEWVAVIWSDGSIKYYADSVKGRFTISRDNSK
NTLYLQMNSLRAEDTAVYYCAREVESAMGGFYYNG
MDVWGQGTTVTVSS
151 DVD1520L ABO2OVL AB217VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
TKRTVAAPDIQMTQSPSSLSASVGDRVTITCRASQ
GIRIDLGWYQQKPGKAPKRLIYAASTLQSGVPSRF
SGSGSGTEFIFTISSLQPEDFASYYCLQHKSYPLT
FGGGTKVEIKR
231
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.26: Generation of TNF (seq. 6) and NGF-Igs with Linker Set 3
Table 40
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
152 DVD1527H AB218VH ABO2OVH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPSVFPLAPQVQLQESGPGLV
KPSETLSLTCTVSGFSLIGYDLNWIRQPPGKGLEW
IGIIWGDGTTDYNSAVKSRVTISKDTSKNQFSLKL
SSVTAADTAVYYCARGGYWYATSYYFDYWGQGTLV
TVSS
153 DVD1527L AB218VL ABO2OVL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPDIQMTQSPSSLSASVGDRVTITCRASQS
ISNNLNWYQQKPGKAPKLLIYYTSRFHSGVPSRFS
GSGSGTDFTFTISSLQPEDIATYYCQQEHTLPYTF
GQGTKLEIKR
154 DVD1528H ABO2OVH AB218VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPEVQLVE
SGGGLIQPGGSLRLSCAASGFTVSRNYMSWVRQAP
GKGLEWVSVIYSGDRTYYADSVKGRFTISRDNSKN
TLYLQMNSLRAEDTAVYYCARGEGGFDYWGQGTLV
TVSS
155 DVD1528L ABO2OVL AB218VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPEIVMTQSPATLSVSPGERATLSCRASQ
SVSSNLAWYQQKPGQAPRLLIHGASIRATGLPARF
SGSGSGTEFTLTISSLQSEDFAVYYCQQYNYWWTF
GQGTKVEIKR
232
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.27: Generation of TNF (seq. 2) and SOST DVD-Igs with Linker Set 3
Table 41
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
156 DVD1495H AB213VH 7B022VH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSAASTKGPSVFPLAPEVQLQQSGPE
LVTPGASVKISCKASGYTFTDHYMSWVKQSHGKSL
EWIGDINPYSGETTYNQKFKGTATLTVDKSSSTAY
MEIRGLTSEDSAVYYCARDDYDASPFAYWGQGTLV
TVSA
157 DVD1495L AB213VL AB022VL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTFGSGTKLE
IKRTVAAPDVQMIQSPSSLSASLGDIVTMTCQASQ
GTSINLNWFQQKPGKAPKLLIYGSSNLEDGVPSRF
SGSRYGTDFTLTISSLEDEDLATYFCLQHSYLPYT
FGGGTKLEIKR
158 DVD1496H AB022VH AB213VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPgVFPLAPQVQLKESG
PGLVAPSQSLSITCTVSGFSLTDYGVNWVRQPPGK
GLEWLGMIWGDGSTDYDSTLKSRLSISKDNSKSQI
FLKMNSLQTDDTARYYCAREWHHGPVAYWGQGTLV
TVSA
159 DVD1496L AB022VL AB213VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPDIVMTQSHKFMSTTVGDRVSITCKASQ
AVSSAVAWYQQKPGQSPKLLIYWASTRHTGVPDRF
TGSGSVTDFTLTIHNLQAEDLALYYCQQHYSTPFT
FGSGTKLEIKR
233
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.28: Generation of TNF (seq. 3) and SOST DVD-Igs with Linker Set 3
Table 42
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
160 DVD1497H AB214VH AB022VH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPSVFPLAPEVQLQQS
GPELVTPGASVKISCKASGYTFTDHYMSWVKQSHG
KSLEWIGDINPYSGETTYNQKFKGTATLTVDKSSS
IAYMEIRGLTSEDSAVYYCARDDYDASPFAYWGQG
TLVTVSA
161 DVD1497L AB214VL AB022VL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPDVQMIQSPSSLSASLGDIVTMTCQASQ
GTSINLNWFQQKPGKAPKLLIYGSSNLEDGVPSRF
SGSRYGTDFTLTISSLEDEDLATYFCLQHSYLPYT
FGGGTKLEIKR
162 DVD1498H AB022VH AB214VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPSVFPLAPEVKLEESG
GGLVQPGGSMKLSCVASGFIFSNHWMNWVRQSPEK
GLEWVAEIRSKSINSATHYAESVKGRFTISRDDSK
SAVYLQMTDLRTEDTGVYYCSRNYYGSTYDYWGQG
TTLTVSS
163 DVD1498L AB022VL AB214VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPDILLTQSPAILSVSPGERVSFSCRASQ
FVGSSIHWYQQRTNGSPRLLIKYASESMSGIPSRF
SGSGSGTDFTLSINTVESEDIADYYCQESHSWPFT
FGSGTNLEVKR
234
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.29: Generation of TNF(seq. 4) and SOST DVD-Igs with Linker Set 3
Table 43
DVD Outer Inner Sequence
SEQ Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
164 DVD1499H AB215VH AB022VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPSVFPLAPE
VQLQQSGPELVTPGASVKISCKASGYTFTDHYMSW
VKQSHGKSLEWIGDINPYSGETTYNOKFEGTATLT
VDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASPF
AYWGQGTLVTVSA
165 DVD1499L AB215VL AB022VL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPDVQMIQSPSSLSASLGDIVTMTCQAS
QGTSINLNWFQQKPGKAPKLLIYGSSNLEDGVPSR
FSGSRYGTDFTLTISSLEDEDLATYFCLQHSYLPY
TFGGGTKLEIKR
166 DVD1500H AB022VH AB215VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPSVFPLAPQVQLVESG
GGVVQPGRSLRLSCAASGFTFSSYAMHWVRQAPGN
GLEWVAFMSYDGSNKYAKDSVKGRFTISRDNSKNT
LYLQMNSLRAEDTAVYYCARDRGIAAGGNYYYYGM
DVWGQGTTVTVSS
167 DVD1500L AB022VL AB215VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPEIVLTQSPATLSLSPGERATLSCRASQ
SVYSYLAWYQQKPGQAPRLLIYDASNRATGIPARF
SGSGSGTDFTLTISSLEPEDFAVYYCQQRSNWPPF
TFGPGTKVDIKR
235
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.30: Generation of T1SIF (seq. 5) and SOST DVD-Igs with Linker Set 3
Table 44
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
168 DVD1517H AB217VH AB022VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPSVFPLAPEV
QLQQSGPELVTPGASVKISCKASGYTFTDHYMSWV
KQSHGKSLEWIGDINPYSGETTYNQKFKGTAILTV
DKSSSIAYMEIRGLTSEDSAVYYCARDDYDASPFA
YWGQGTLVTVSA
169 DVD1517L AB217VL AB022VL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPDVQMIQSPSSLSASLGDIVTMTCQASQ
GTSINLNWFQQKPGKAPKLLIYGSSNLEDGVPSRF
SGSRYGTDFTLTISSLEDEDLATYFCLQHSYLPYT
FGGGTKLEIKR
170 DVD1518H AB022VH AB217VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPSVFPLAPQVQLVESG
GGVVQPGRSLRLSCAASGFTFSSYDMHWVRQAPGK
GLEWVAVIWSDGSIKYYADSVKGRFTISRDNSKNT
LYLQMNSLRAEDTAVYYCAREVESAMGGFYYNGMD
VWGQGTTVTVSS
171 DVD1518L AB022VL AB217VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTEGGGIKLE
IKRTVAAPDIQMTQSPSSLSASVGDRVTITCRASQ
GIRIDLGWYQQKPGKAPKRLIYAASTLQSGVPSRF
SGSGSGTEFIFTISSLQPEDFASYYCLQHKSYPLT
FGGGTKVEIKR
236
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.31: Generation of TNF (seq. 6) and SOST DVD-Igs with Linker Set 3
Table 45
DVD Outer Inner Sequence
SEQ Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
172 DVD1525H AB218VH AB022VH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPSVFPLAPEVQLQQSGPELV
TPGASVKISCKASGYTFTDHYMSWVKQSHGKSLEW
IGDINPYSGETTYNQKFKGTATLTVDKSSSIAYME
IRGLTSEDSAVYYCARDDYDASPFAYWGQGTLVTV
SA
173 DVD1525L AB218VL AB022VL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIRGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPDVQMIQSPSSLSASLGDIVTMTCQASQG
TSINLNWFQQKPGKAPKLLIYGSSNLEDGVPSRFS
GSRYGTDFTLTISSLEDEDLATYFCLQHSYLPYTF
GGGTKLEIKR
174 DVD1526H AB022VH AB218VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPSVFPLAPEVQLVESG
GGLIQPGGSLRLSCAASGFTVSRNYMSWVRQAPGK
GLEWVSVIYSGDRTYYADSVKGRFTISRDNSKNTL
YLQMNSLRAEDTAVYYCARGEGGFDYWGQGTLVTV
SS
175 DVD1526L AB022VL AB218VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPEIVMTQSPATLSVSPGERATLSCRASQ
SVSSNLAWYQQKPGQAPRLLIHGASIRATGLPARF
SGSGSGTEFTLTISSLQSEDFAVYYCQQYNYWWTF
GQGTKVEIKR
237
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.32: Generation of TNF (seq. 2) and PGE2 with Linker Set 3
Table 46
DVD Outer Inner Sequence
SEQ Variable Variable Variable
Domain Domain Domain
ID Name Name Name 1234567890123456769012345678901234
NO
176 DVD1489H AB213VH AB048VH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGV
NWVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLS
ISKDNSKSQIFLKMNSLQTDDTARYYCAREWHHG
PVAYWGQGTLVTVSAASTKGPSVFPLAPEVQLVQ
SGAEVKKPGASVKVSCKASGYTFTKYWLGWVRQA
PGQGLEWMGDIYPGYDYTHYNEKFKDRVTLTTDT
STSTAYMELRSLRSDDTAVYYCARSDGSSTYWGQ
GTLVTVSS
177 DVD1489L AB213VL AB048VL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVA
WYQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSV
TDFTLTIHNLQAEDLALYYCQQHYSTPFTFGSGT
KLEIKRTVAAPDVLMTQTPLSLPVTPGEPASISC
TSSQNIVHSNGNTYLEWYLQKPGQSPQLLIYKVS
NRFSGVPDRFSGSGSGTDFTLKISRVEAEDVGVY
YCFQVSHVPYTFGGGTKVEIKR
178 DVD1490H AB048VH AB213VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWL
GWVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRV
TLTTDTSTSTAYMELRSLRSDDTAVYYCARSDGS
STYWGQGTLVTVSSASTKGPSVFPLAPQVQLKES
GPGLVAPSQSLSITCTVSGFSLTDYGVNWVRQPP
GKGLEWLGMIWGDGSTDYDSTLKSRLSISKDNSK
SQIFLKMNSLQTDDTARYYCAREWHHGPVAYWGQ
GTLVTVSA
179 DVD1490L AB048VL AB213VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNG
NTYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFS
GSGSGTDFTLKISRVEAEDVGVYYCFQVSHVPYT
FGGGTKVEIKRTVAAPDIVMTQSHKFMSTTVGDR
VSITCKASQAVSSAVAWYQQKPGQSPKLLIYWAS
TRHTGVPDRFTGSGSVTDFTLTIHNLQAEDLALY
YCQQHYSTPFTFGSGTKLEIKR
238
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.33: Generation of TNF (seq. 3) and PGE2 DVD-Igs with Linker Set 3
Table 47
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
180 DVD1491H AB214VH AB048VH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPSVFPLAPEVQLVQS
GAEVKKPGASVKVSCKASGYTFTKYWLGWVRQAPG
QGLEWMGDIYPGYDYTHYNEKFKDRVTLTTDTSTS
TAYMELRSLRSDDTAVYYCARSDGSSTYWGQGTLV
TVSS
181 DVD1491L AB214VL AB048VL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPDVLMTQTPLSLPVTPGEPASISCTSSQ
NIVHSNGNTYLEWYLQKPGQSPQLLIYKVSNRFSG
VPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQVS
HVPYTFGGGTKVEIKR
182 DVD1492H AB048VH AB214VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFEDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTY
WGQGTLVTVSSASTKGPSVFPLAPEVKLEESGGGL
VQPGGSMKLSCVASGFIFSNHWMNWVRQSPEKGLE
WVAEIRSKSINSATHYAESVKGRFTISRDDSKSAV
YLQMTDLRTEDTGVYYCSRNYYGSTYDYWGQGTTL
TVSS
183 DVD1492L AB048VL AB214VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTFGG
GTKVEIKRIVAAPDILLTQSPAILSVSPGERVSFS
CRASQFVGSSIHWYQQRTNGSPRLLIKYASESMSG
IPSRFSGSGSGTDFTLSINTVESEDIADYYCQESH
SWPFTFGSGTNLEVKR
239
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.34: Generation of TNF (seq. 4) and PGE2 DVD-Igs with Linker Set 3
Table 48
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
184 DVD1493H AB215VH AB048VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPSVFPLAPE
VQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLGW
VRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTLT
TDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTYW
GQGTLVTVSS
185 DVD1493L AB215VL AB048VL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPDVLMTQTPLSLPVTPGEPASISCTSS
QNIVHSNGNTYLEWYLQKPGQSPQLLIYKVSNRFS
GVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQV
SHVPYTFGGGTKVEIKR
186 DVD1494H AB048VH AB215VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGS STY
WGQGTLVTVSSASTKGPSVFPLAPQVQLVESGGGV
VQPGRSLRLSCAASGFTFSSYAMHWVRQAPGNGLE
WVAFMSYDGSNKYAKDSVKGRFTISRDNSKNTLYL
QMNSLRAEDTAVYYCARDRGIAAGGNYYYYGMDVW
GQGTTVTVSS
187 DVD1494L AB048VL AB215VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTFGG
GTKVEIKRTVAAPEIVLTQSPATLSLSPGERATLS
CRASQSVYSYLAWYQQKPGQAPRLLIYDASNRATG
IPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRS
NWPPFTFGPGTKVDIKR
240
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.35: Generation of TINE (seq. 5) and PGE2 DVD-Igs with Linker Set 3
Table 49
DVD Outer Inner Sequence
SE" Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
188 DVD1515H AB217VH AB048VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPSVFPLAPEV
QLVQSGAEVKKPGASVKVSCKASGYTFTKYWLGWV
RQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTLTT
DTSTSTAYMELRSLRSDDTAVYYCARSDGSSTYWG
QGTLVTVSS
189 DVD1515L AB217VL AB048VL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPDVLMTQTPLSLPVTPGEPASISCTSSQ
NIVHSNGNTYLEWYLQKPGQSPQLLIYKVSNRFSG
VPDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQVS
HVPYTFGGGTKVEIKR
190 DVD1516H AB048VH AB217VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTY
WGQGTLVTVSSASTKGPSVFPLAPQVQLVESGGGV
VQPGRSLRLSCAASGFTFSSYDMHWVRQAPGKGLE
WVAVIWSDGSIKYYADSVKGRFTISRDNSKNTLYL
QMNSLRAEDTAVYYCAREVESAMGGFYYNGMDVWG
QGTTVTVSS
191 DVD1516L AB048VL AB217VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTFGG
GTKVEIKRTVAAPDIQMTQSPSSLSASVGDRVTIT
CRASQGIRIDLGWYQQKPGKAPKRLIYAASTLQSG
VPSRFSGSGSGTEFIFTISSLQPEDFASYYCLQHK
SYPLTFGGGTKVEIKR
241
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.36: Generation of TNF (seq. 6) and PGE2 DVD-Igs with Linker Set 3
Table 50
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
192 DVD1523H AB218VH AB048VH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPSVFPLAPEVQLVQSGAEVK
KPGASVKVSCKASGYTFTKYWLGWVRQAPGQGLEW
MGDIYPGYDYTHYNEKFKDRVTLTTDTSTSTAYME
LRSLRSDDTAVYYCARSDGSSTYWGQGTLVTVSS
193 DVD1523L AB218VL AB048VL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPDVLMTQTPLSLPVTPGEPASISCTSSQN
IVHSNGNTYLEWYLQKPGQSPQLLIYKVSNRFSGV
PDRFSGSGSGTDFTLKISRVEAEDVGVYYCFQVSH
VPYTFGGGTKVEIKR
194 DVD1524H AB048VH AB218VH EVQLVQSGAEVKKPGASVKVSCKASGYTFTKYWLG
WVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTL
TTDTSTSTAYMELRSLRSDDTAVYYCARSDGSSTY
WGQGTLVTVSSASTKGPSVFPLAPEVQLVESGGGL
IQPGGSLRLSCAASGFTVSRNYMSWVRQAPGKGLE
WVSVIYSGDRTYYADSVKGRFTISRDNSKNTLYLQ
MNSLRAEDTAVYYCARGEGGFDYWGQGTLVTVSS
195 DVD1524L AB048VL AB218VL DVLMTQTPLSLPVTPGEPASISCTSSQNIVHSNGN
TYLEWYLQKPGQSPQLLIYKVSNRFSGVPDRFSGS
GSGTDFTLKISRVEAEDVGVYYCFQVSHVPYTFGG
GTKVEIKRTVAAPEIVMTQSPATLSVSPGERATLS
CRASQSVSSNLAWYQQKPGQAPRLLIHGASIRATG
LPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQYN
YWWTFGQGTKVEIKR
242
=
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.37: Generation of TNF (seq. 2) and LPA DVD-Igs with Linker Set 3
Table 51
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
196 DVD1507H AB213VH AB216VH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN =
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSAASTKGPSVFPLAPQVQLQQSGAE
LVRPGTSVKVSCKASGYGFINYLIEWIKQRPGQGL
EWIGLINPGSDYTNYNENFKGKATLTADKSSSTAY
MHLSSLTSEDSAVYFCARRFGYYGSGNYFDYWGQG
TTLTVSS
197 DVD1507L AB213VL AB216VL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTFGSGTKLE
IKRTVAAPDVVMTQTPLSLPVSLGDQASISCTSGQ
SLVHINGNTYLHWYLQKPGQSPKLLIYKVSNLFSG
VPDRFSGSGSGTDFTLKISRVEAEDLGVYFCSQST
HFPFTFGTGTKLEIKR
198 DVD1508H AB216VH AB213VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPSVFPLAPQVQLK
ESGPGLVAPSQSLSITCTVSGFSLTDYGVNWVRQP
PGKGLEWLGMIWGDGSTDYDSTLKSRLSISKDNSK
SQIFLKMNSLQTDDTARYYCAREWHHGPVAYWGQG
TLVTVSA
199 DVD1508L AB216VL AB213VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPDIVMTQSHKFMSTTVGDRVSIT
CKASQAVSSAVAWYQQKPGQSPKLLIYWASTRHTG
VPDRFIGSGSVTDFTLTIHNLQAEDLALYYCQQHY
STPFTFGSGTKLEIKR
243
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.38: Generation of TNF (seq. 3) and LPA D'VD-Igs with Linker Set 3
Table 52
DVD Outer Inner Sequence
SEQ Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
200 DVD1509H AB214VH AB216VH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPSVFPLAPQVQLQQS
GAELVRPGTSVKVSCKASGYGFINYLIEWIKQRPG
QGLEWIGLINPGSDYTNYNENFKGKATLTADKSSS
TAYMHLSSLTSEDSAVYFCARRFGYYGSGNYFDYW
GQGTTLTVSS
201 DVD1509L AB214VL AB216VL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPDVVMTQTPLSLPVSLGDQASISCTSGQ
SLVHINGNTYLHWYLQKPGQSPKLLIYKVSNLFSG
VPDRFSGSGSGTDFTLKISRVEAEDLGVYFCSQST
HFPFTFGTGTKLEIKR
202 DVD1510H AB216VH AB214VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPSVFPLAPEVKLE
ESGGGLVQPGGSMKLSCVASGFIFSNHWMNWVRQS
PEKGLEWVAEIRSKSINSATHYAESVKGRFTISRD
DSKSAVYLQMTDLRTEDTGVYYCSRNYYGSTYDYW
GQGTTLTVSS
203 DVD1510L AB216VL AB214VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLETKRTVAAPDILLTQSPAILSVSPGERVSFS
CRASQFVGSSIHWYQQRTNGSPRLLIKYASESMSG
IPSRFSGSGSGTDFTLSINTVESEDIADYYCQESH
,SWPFTFGSGTNLEVKR
244
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 239: Generation of TNF (seq. 4) and LPA DVD-Igs with Linker Set 3
Table 53
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
204 DVD1511H AB215VH AB216VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPSVFPLAPQ
VQLQQSGAELVRPGTSVKVSCKASGYGFINYLIEW
IKQRPGQGLEWIGLINPGSDYTNYNENFKGKATLT
ADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGSG
NYFDYWGQGTTLTVSS
205 DVD1511L AB215VL AB216VL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLTYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPDVVMTQTPLSLPVSLGDQASISCTSG
QSLVHINGNTYLHWYLQKPGQSPKLLIYKVSNLFS
GVPDRFSGSGSGTDFTLKISRVEAEDLGVYFCSQS
THFPFTFGTGTKLEIKR
206 DVD1512H AB216VH AB215VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPSVFPLAPQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSSYAMHWVRQA
PGNGLEWVAFMSYDGSNKYAKDSVKGRFTISRDNS
KNTLYLQMNSLRAEDTAVYYCARDRGIAAGGNYYY
YGMDVWGQGTTVTVSS
207 DVD1512L AB216VL AB215VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPEIVLTQSPATLSLSPGERATLS
CRASQSVYSYLAWYQQKPGQAPRLLIYDASNRATG
IPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQRS
NWPPFTFGPGTKVDIKR
245
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.40: Generation of TINT (seq. 1) and LPA DVD-Igs with Linker Set 3
Table 54
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
208 DVD1513H AB017VH AB216VH EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMH
WVRQAPGKGLEWVSAITWNSGHIDYADSVEGRFTI
SRDNAKNSLYLQMNSLRAEDTAVYYCAKVSYLSTA
SSLDYWGQGTLVTVSSASTKGPSVFPLAPQVQLQQ
SGAELVRPGTSVKVSCKASGYGFINYLIEWIKQRP
GQGLEWIGLINPGSDYTNYNENFKGKATLTADKSS
STAYMHLSSLTSEDSAVYFCARRFGYYGSGNYFDY
WGQGTTLTVSS
209 DVD1513L AB017VL AB216VL DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAW
YQQKPGKAPKLLIYAASTLQSGVPSRFSGSGSGTD
FTLTISSLQPEDVATYYCQRYNRAPYTFGQGTKVE
IKRTVAAPDVVMTQTPLSLPVSLGDQASISCTSGQ
SLVHINGNTYLHWYLQKPGQSPKLLIYKVSNLFSG
VPDRFSGSGSGTDFTLKISRVEAEDLGVYFCSQST
HFPFTFGTGTKLEIKR
210 DVD1514H AB216VH AB017VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPSVFPLAPEVQLV
ESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQA
PGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNA
KNSLYLQMNSLRAEDTAVYYCAKVSYLSTASSLDY
WGQGTLVTVSS
211 DVD1514L AB216VL AB017VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPDIQMTQSPSSLSASVGDRVTIT
CRASQGIRNYLAWYQQKPGKAPKLLIYAASTLQSG
VPSRFSGSGSGTDFTLTISSLQPEDVATYYCQRYN
RAPYTFGQGTKVEIKR
246
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.41: Generation of TNF (seq. 5) and LPA DVD-Igs with Linker Set 3
Table 55
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
212 DVD1521H AB217VH AB216VH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPSVFPLAPQV
QLQQSGAELVRPGTSVKVSCKASGYGFINYLIEWI
KQRPGQGLEWIGLINPGSDYTNYNENFKGKATLTA
DKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGSGN
YFDYWGQGTTLTVSS
213 DVD1521L AB217VL AB216VL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPDVVMTQTPLSLPVSLGDQASISCTSGQ
SLVHINGNTYLHWYLQKPGQSPKLLIYKVSNLFSG
VPDRFSGSGSGTDFTLKISRVEAEDLGVYFCSQST
HFPFTFGTGTKLEIKR
214 DVD1522H AB216VH AB217VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPSVFPLAPQVQLV
ESGGGVVQPGRSLRLSCAASGFTFSSYDMHWVRQA
PGKGLEWVAVIWSDGSIKYYADSVKGRFTISRDNS
KNTLYLQMNSLRAEDTAVYYCAREVESAMGGFYYN
GMDVWGQGTTVTVSS
215 DVD1522L AB216VL A3217VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLUPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPDIQMTQSPSSLSASVGDRVTIT
CRASQGIRIDLGWYQQKPGKAPKRLIYAASTLQSG
VPSRFSGSGSGTEFIFTISSLQPEDFASYYCLQHK
SYPLTFGGGTKVEIKR
247
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.42: Generation of TNF (seq. 6) and LPA DVD-Igs with Linker Set 3
Table 56
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
216 DVD1529H AB218VH AB216VH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPSVFPLAPQVQLQQSGAELV
RPGTSVKVSCKASGYGFINYLIEWIKQRPGQGLEW
IGLINPGSDYTNYNENFKGKATLTADKSSSTAYMH
LSSLTSEDSAVYFCARRFGYYGSGNYFDYWGQGTT
LTVSS
217 DVD1529L AB218VL AB216VL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPDVVMTQTPLSLPVSLGDQASISCTSGQS
LVHINGNTYLHWYLQKPGQSPKLLIYKVSNLFSGV
PDRFSGSGSGTDFTLKISRVEAEDLGVYFCSQSTH
FPFTFGTGTKLEIKR
218 DVD1530H AB216VH AB218VH QVQLQQSGAELVRPGTSVKVSCKASGYGFINYLIE
WIKQRPGQGLEWIGLINPGSDYTNYNENFKGKATL
TADKSSSTAYMHLSSLTSEDSAVYFCARRFGYYGS
GNYFDYWGQGTTLTVSSASTKGPSVFPLAPEVQLV
ESGGGLIQPGGSLRLSCAASGFTVSRNYMSWVRQA
PGKGLEWVSVIYSGDRTYYADSVKGRFTISRDNSK
NTLYLQMNSLRAEDTAVYYCARGEGGFDYWGQGTL
VTVSS
219 DVD1530L AB216VL A5218VL DVVMTQTPLSLPVSLGDQASISCTSGQSLVHINGN
TYLHWYLQKPGQSPKLLIYKVSNLFSGVPDRFSGS
GSGTDFTLKISRVEAEDLGVYFCSQSTHFPFTFGT
GTKLEIKRTVAAPEIVMTQSPATLSVSPGERATLS
CRASQSVSSNLAWYQQKPGQAPRLLIHGASIRATG
LPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQYN
YWWTFGQGTKVEIKR
248
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.43: Generation of TNF (seq. 2) and NGF DVD-Igs with Linker Set 4
Table 57
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
220 DVD1543H AB213VH ABO2OVH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSAASTKGPSVFPLAPQVQLQESGPG
LVKPSETLSLTCTVSGFSLIGYDLNWIRQPPGKGL
EWIGIIWGDGTTDYNSAVKSRVTISKDTSKNQFSL
KLSSVTAADTAVYYCARGGYWYATSYYFDYWGQGT
LVTVSS
221 DVD1543L AB213VL ABO2OVL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTFGSGTKLE
IKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQSISNNLNWYQQKPGKAPKLLIYYTSRFH
SGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQ
EHTLPYTFGQGTKLEIKR
222 DVD1544H ABO2OVH AB213VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPQVQLKE
SGPGLVAPSQSLSITCTVSGFSLTDYGVNWVRQPP
GKGLEWLGMIWGDGSTDYDSTLKSRLSISKDNSKS
QIFLKMNSLQTDDTARYYCAREWHHGPVAYWGQGT
LVTVSA
223 DVD1544L ABO2OVL AB213VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPDIVMTQSHKFMSTTVGDRVS
ITCKASQAVSSAVAWYQQKPGQSPKLLIYWASTRH
TGVPDRFTGSGSVTDFTLTIHNLQAEDLALYYCQQ
HYSTPFTFGSGTKLEIKR
249
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.44: Generation of TNF (seq. 3) and NGF DVD-Igs with Linker Set 4
Table 58
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
224 DVD1545H AB214VH ABO2OVH EVKLEESGGGLVQPGGSMKLSCVASGFIFSNHWMN
WVRQSPEKGLEWVAEIRSKSINSATHYAESVKGRF
TISRDDSKSAVYLQMTDLRTEDTGVYYCSRNYYGS
TYDYWGQGTTLTVSSASTKGPSVFPLAPQVQLQES
GPGLVKPSETLSLTCTVSGFSLIGYDLNWIRQPPG
KGLEWIGIIWGDGTTDYNSAVKSRVTISKDTSKNQ
FSLKLSSVTAADTAVYYCARGGYWYATSYYFDYWG
QGTLVTVSS
225 DV1J1545L AB214VL ABO2OVL DILLTQSPAILSVSPGERVSFSCRASQFVGSSIHW
YQQRTNGSPRLLIKYASESMSGIPSRFSGSGSGTD
FTLSINTVESEDIADYYCQESHSWPFTFGSGTNLE
VKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQSISNNLNWYQQKPGKAPKLLIYYTSRFH
SGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQ
EHTLPYTFGQGTKLEIKR
226 DVD1546H ABO2OVH A5214V1-I QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPEVKLEE
SGGGLVQPGGSMKLSCVASGFIFSNHWMNWVRQSP
EKGLEWVAEIRSKSINSATHYAESVKGRFTISRDD
SKSAVYLQMTDLRTEDTGVYYCSRNYYGSTYDYWG
QGTTLTVSS
227 DVD1546L ABO2OVL AB214VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPDILLTQSPAILSVSPGERVS
FSCRASQFVGSSIHWYQQRTNGSPRLLIKYASESM
SGIPSRFSGSGSGTDFTLSINTVESEDIADYYCQE
SHSWPFTFGSGTNLEVKR
250
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.45: Generation of TNF (seq. 4) and NGF DVD-Igs with Linker Set 4
Table 59
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
228 DVD1547H AB215VH ABO2OVH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYAMH
WVRQAPGNGLEWVAFMSYDGSNKYAKDSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCARDRGIAAG
GNYYYYGMDVWGQGTTVTVSSASTKGPSVFPLAPQ
VQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLNW
IRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTISK
DTSKNQFSLKLSSVTAADTAVYYCARGGYWYATSY
YFDYWGQGTLVTVSS
229 DVD1547L AB215VL ABO2OVL EIVLTQSPATLSLSPGERATLSCRASQSVYSYLAW
YQQKPGQAPRLLIYDASNRATGIPARFSGSGSGTD
FTLTISSLEPEDFAVYYCQQRSNWPPFTFGPGTKV
DIKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRV
TITCRASQSISNNLNWYQQKPGKAPKLLIYYTSRF
HSGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQ
QEHTLPYTFGQGTKLEIKR
230 DVD1548H ABO2OVH AB215VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPQVQLVE
SGGGVVQPGRSLRLSCAASGFTFSSYAMHWVRQAP
GNGLEWVAFMSYDGSNKYAKDSVKGRFTISRDNSK
NTLYLQMNSLRAEDTAVYYCARDRGIAAGGNYYYY
GMDVWGQGTTVTVSS
231 DVD1548L ABO2OVL AB215VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPEIVLTQSPATLSLSPGERAT
LSCRASQSVYSYLAWYQQKPGQAPRLLIYDASNRA
TGIPARFSGSGSGTDFTLTISSLEPEDFAVYYCQQ
RSNWPPFTFGPGTKVDIKR
251
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.46: Generation of TNF (seq. 5) and NGF DVD-Igs with Linker Set 4
Table 60
DVD Outer Inner Sequence
SE Q Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
232 DVD1561H AB217VH ABC2OVH QVQLVESGGGVVQPGRSLRLSCAASGFTFSSYDMH
WVRQAPGKGLEWVAVIWSDGSIKYYADSVKGRFTI
SRDNSKNTLYLQMNSLRAEDTAVYYCAREVESAMG
GFYYNGMDVWGQGTTVTVSSASTKGPSVFPLAPQV
QLQESGPGLVKPSETLSLTCTVSGFSLIGYDLNWI
RQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTISKD
TSKNQFSLKLSSVTAADTAVYYCARGGYWYATSYY
FDYWGQGTLVTVSS
233 DVD1561L AB217VL ABO2OVL DIQMTQSPSSLSASVGDRVTITCRASQGIRIDLGW
YQQKPGKAPKRLIYAASTLQSGVPSRFSGSGSGTE
FIFTISSLQPEDFASYYCLQHKSYPLTFGGGTKVE
IKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQSISNNLNWYQQKPGKAPKLLIYYTSRFH
SGVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQ
EHTLPYTFGQGTKLEIKR
234 DVD1562H ABO2OVH AB217VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPQVQLVE
SGGGVVQPGRSLRLSCAASGFTFSSYDMHWVRQAP
GKGLEWVAVIWSDGSIKYYADSVKGRFTISRDNSK
NTLYLQMNSLRAEDTAVYYCAREVESAMGGFYYNG
MDVWGQGTTVTVSS
235 DVD1562L ABO2OVL AB217VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVT
ITCRASQGIRIDLGWYQQKPGKAPKRLIYAASTLQ
SGVPSRFSGSGSGTEFIFTISSLQPEDFASYYCLQ
HKSYPLTFGGGTKVEIKR
252
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.47: Generation of TNF (seq. 6) and NGF-Igs with Linker Set 4
Table 61
DVD Outer Inner Sequence
SE Variable Variable Variable
Q
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
236 DVD1569H AB218VH ABO2OVH EVQLVESGGGLIQPGGSLRLSCAASGFTVSRNYMS
WVRQAPGKGLEWVSVIYSGDRTYYADSVKGRFTIS
RDNSKNTLYLQMNSLRAEDTAVYYCARGEGGFDYW
GQGTLVTVSSASTKGPSVFPLAPQVQLQESGPGLV
KPSETLSLTCTVSGFSLIGYDLNWIRQPPGKGLEW
IGIIWGDGTTDYNSAVKSRVTISKDTSKNQFSLKL
SSVTAADTAVYYCARGGYWYATSYYFDYWGQGTLV
TVSS
237 DVD1569L AB218VL ABO2OVL EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAW
YQQKPGQAPRLLIHGASIRATGLPARFSGSGSGTE
FTLTISSLQSEDFAVYYCQQYNYWWTFGQGTKVEI
KRTVAAPSVFIFPPDIQMTQSPSSLSASVGDRVTI
TCRASQSISNNLNWYQQKPGKAPKLLIYYTSRFHS
GVPSRFSGSGSGTDFTFTISSLQPEDIATYYCQQE
HTLPYTFGQGTKLEIKR
238 DVD1570H ABO2OVH AB218VH QVQLQESGPGLVKPSETLSLTCTVSGFSLIGYDLN
WIRQPPGKGLEWIGIIWGDGTTDYNSAVKSRVTIS
KDTSKNQFSLKLSSVTAADTAVYYCARGGYWYATS
YYFDYWGQGTLVTVSSASTKGPSVFPLAPEVQLVE
SGGGLIQPGGSLRLSCAASGFTVSRNYMSWVRQAP
GKGLEWVSVIYSGDRTYYADSVKGRFTISRDNSKN
TLYLQMNSLRAEDTAVYYCARGEGGFDYWGQGTLV
TVSS
239 DVD1570L ABO2OVL AB218VL DIQMTQSPSSLSASVGDRVTITCRASQSISNNLNW
YQQKPGKAPKLLIYYTSRFHSGVPSRFSGSGSGTD
FTFTISSLQPEDIATYYCQQEHTLPYTFGQGTKLE
IKRTVAAPSVFIFPPEIVMTQSPATLSVSPGERAT
LSCRASQSVSSNLAWYQQKPGQAPRLLIHGASIRA
TGLPARFSGSGSGTEFTLTISSLQSEDFAVYYCQQ
YNYWWTFGQGTKVEIKR
253
CA 02809433 2013-02-22
WO 2012/027570 PCT/US2011/049147
Example 2.48: Generation of TNF (seq. 2) and SOST DVD-Igs with Linker Set 4
Table 62
DVD Outer Inner Sequence
SEQ Variable Variable Variable
Domain Domain Domain
ID Name Name Name 12345678901234567890123456789012345
NO
240 DVD1537H AB213VH 7B022VH QVQLKESGPGLVAPSQSLSITCTVSGFSLTDYGVN
WVRQPPGKGLEWLGMIWGDGSTDYDSTLKSRLSIS
KDNSKSQIFLKMNSLQTDDTARYYCAREWHHGPVA
YWGQGTLVTVSAASTKGPSVFPLAPEVQLQQSGPE
LVTPGASVKISCKASGYTFTDHYMSWVKQSHGKSL
EWIGDINPYSGETTYNQKFKGTATLTVDKSSSIAY
MEIRGLTSEDSAVYYCARDDYDASPFAYWGQGTLV
TVSA
241 DVD1537L AB213VL 1B022VL DIVMTQSHKFMSTTVGDRVSITCKASQAVSSAVAW
YQQKPGQSPKLLIYWASTRHTGVPDRFTGSGSVTD
FTLTIHNLQAEDLALYYCQQHYSTPFTFGSGTKLE
IKRTVAAPSVFIFPPDVQMIQSPSSLSASLGDIVT
MTCQASQGTSINLNWFQQKPGKAPKLLIYGSSNLE
DGVPSRFSGSRYGTDFTLTISSLEDEDLATYFCLQ
HSYLPYTFGGGTKLEIKR
242 DVD1538H AB022VH AB213VH EVQLQQSGPELVTPGASVKISCKASGYTFTDHYMS
WVKQSHGKSLEWIGDINPYSGETTYNQKFKGTATL
TVDKSSSIAYMEIRGLTSEDSAVYYCARDDYDASP
FAYWGQGTLVTVSAASTKGPSVFPLAPQVQLKESG
PGLVAPSQSLSITCTVSGFSLTDYGVNWVRQPPGK
GLEWLGMIWGDGSTDYDSTLKSRLSISKDNSKSQI
FLKMNSLQTDDTARYYCAREWHHGPVAYWGQGTLV
TVSA
243 DVD1538L AB022VL AB213VL DVQMIQSPSSLSASLGDIVTMTCQASQGTSINLNW
FQQKPGKAPKLLIYGSSNLEDGVPSRFSGSRYGTD
FTLTISSLEDEDLATYFCLQHSYLPYTFGGGTKLE
IKRTVAAPSVFIFPPDIVMTQSHKFMSTTVGDRVS
ITCKASQAVSSAVAWYQQKPGQSPKLLIYWASTRH
TGVPDRFTGSGSVTDFTLTIHNLQAEDLALYYCQQ
HYSTPFTFGSGTKLEIKR
254
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 254
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 254
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: