Note: Descriptions are shown in the official language in which they were submitted.
CA 02811357 2013-03-14
SGL CARBON SE 2010/054W0
07.03.2013
1
Electrolytic cell for extracting aluminium
The invention relates to a cathode for an electrolytic cell for extracting
aluminium by fused-salt electrolysis. The invention further relates to a
process
for extracting aluminium by fused-salt electrolysis.
The Hall-Heroult process is currently used for the industrial extraction of
aluminium from its oxide. This is an electrolytic process in which aluminium
oxide (A1203) is dissolved in molten cryolite (Na3 [AlF6]) and the resulting
mixture acts as a liquid electrolyte in an electrolytic cell. In principal,
the design
of such an electrolytic cell used to carry out the Hall-Heroult process is
depicted
schematically in Figures la to lc, wherein Figure 1 a shows a cross-section
through a traditional cell, while Figure lb shows an external side view of the
cell. Fig. lc shows a perspective view of an electrolytic cell.
Reference symbol 1 denotes a cathode, which may, for example, be made from
graphite, anthracite or a mixture thereof. Alternatively, coke-based
graphitised
cathodes may also be used. The cathode 1 is generally embedded in a
mounting 2 made from steel and/or a fire-resistant material or the like. The
cathode 1 may be made in one piece as well as may be made from individual
cathode blocks. Reference symbol 20 denotes the side walls of the electrolytic
cell, which form a tank along with the cathode. This tank may also be regarded
as the internal lining of the mounting. The side walls may also be made in one
piece as well as may be made from individual blocks.
Over the entire length of the cell, a number of current supply bars 3 are
introduced into the cathode 1, although only a single current supply bar 3 can
be seen in the cross-sectional view in Figure la. It can be seen in Fig. lc
that
two current supply bars, for example, may be provided for each cathode block.
The current supply bars are used to supply the cell with the current required
for
the electrolytic process. There is a plurality of prismatic anodes 4 opposite
the
cathode 1, while two anodes 4 are depicted in Figure la. Fig. 1 c shows a
CA 02811357 2013-03-14
SGL CARBON SE
2010/054W0
07.03.2013
2
detailed configuration of anodes in an electrolytic cell. During the
performance
of the process, the aluminium oxide dissolved in cryolite is reduced to
aluminium wherein the aluminium cations move to the molten aluminium ¨
actually the cathode from an electrochemical point of view ¨ where they accept
electrons by applying a voltage between cathode 1 and anodes 4. Due to the
greater density, aluminium 5 gathers in the liquid phase beneath the molten
mixture 6 of aluminium oxide and cryolite. The oxygen ions are reduced to
oxygen at the anode, said oxygen reacting with the carbon of the anodes.
Reference symbols 7 and 8 are the schematic representations of the negative
and positive poles, respectively, of a voltage source for providing the
voltage
required for the electrolytic process, the value of which lies between around
3.5
and 5 V, for example.
As can be seen in the side view in Figure 1 b, the mounting 2 and therefore
the
entire electrolytic cell traditionally has an elongated form, in which a
plurality of
current supply bars 3 are conducted vertically through the side walls of the
mounting 2. The longitudinal expansion of cells currently in use is typically
between around 8 and 15 m, while the width expansion is around 3 to 4 m. A
cathode, as is shown here in Figure la, is disclosed in EP 1845174, for
example.
The high binding energy between aluminium and oxygen and also the heat and
resistance losses cause a high energy requirement when producing aluminium
by fused-salt electrolysis. The associated energy costs constitute the main
part
of the process costs. Reducing these costs is one of the major problems that
need to be overcome in the field of fused-salt electrolysis. A major factor in
relation to the energy efficiency of a fused-salt electrolytic cell is the
distance
required between the anode and cathode or, more accurately, the distance
between the anode and the molten aluminium. The greater this distance has to
be, the higher is the specific energy requirement of the electrolytic cell.
The distance required between the anode and cathode is primarily determined
,
CA 02811357 2013-03-14
SGL CARBON SE 2010/054W0
07.03.2013
3 ,
by the magnetic field induced by the extremely high current intensities of up
to
approx. 500 kA, by the electromagnetic interactions and by the thereby
resulting wave movements and bulges of the liquid aluminium. As already
explained above, the active cathode is actually the liquid aluminium during
electrolysis. In this case, however, the solid body forming a base plate is
referred to as the cathode.
It is customary for the resulting liquid aluminium to be extracted
discontinuously, once a day for example. This requires the crust that forms on
the top of the electrolyte during the process, i.e. the mixture of cryolite
and
aluminium oxide, to be broken, in order to reach the aluminium. Between two
such removals of aluminium, the level of the same rises in the cathode tank.
Generally, when aluminium is produced roughly 10 ¨ 20 % of the existing liquid
aluminium is removed, while the remainder stays in the cathode tank. The
aluminium level is roughly 15 - 50 cm on average. Due to the forces exerted on
the aluminium through the electromagnetic interaction, bulges and wave
formation are resulting in the molten aluminium. This means that in order to
avoid a short-circuit the anodes must be spaced further away from the surface
of the liquid aluminium than would be the case without the wave formation. For
this reason, it would be desirable for the bulge and wave movement of the
aluminium melt to be kept as low as possible on a continuous basis. Moreover,
the discontinuous extraction of aluminium causes a significant disruption of
the
electrical and thermal equilibrium in the electrolytic cell.
One problem addressed by the invention is therefore to specify an electrolytic
cell used to extract aluminium, in which the level of the aluminium melt can
be
kept constant and can be stabilised. A further problem addressed by the
invention is to specify a fused-salt electrolysis process, in which high
energy
efficiency can be achieved.
This problem is solved according to the invention by an electrolytic cell with
the
features contained in claim 1 and also by a process according to claim 11.
Preferred embodiments are specified in the respective dependent claims.
CA 02811357 2013-03-14
SGL CARBON SE 2010/054W0
07.03.2013
4
In accordance with embodiments of the invention an electrolytic cell used to
extract aluminium from its oxide exhibits a cathode and a surrounding side
wall,
wherein the side wall is provided with a number of passages that open up
outwardly into an overflow trough.
Within the meaning of the invention, the term "cathode" is interpreted quite
generally. It may be, for example (although not exclusively), a so-called
cathode bottom, which is made from a plurality of cathode blocks, so that the
core aspects according to the invention are realised as a whole by this
cathode
bottom in the electrolytic cell according to the invention. However, the term
cathode is also intended to refer to the partial structures forming such a
cathode
bottom, within the meaning of cathode blocks. All features that may contribute
to the invention in connection with a "cathode" do so in the same way in
connection with a "cathode block" or "cathode blocks", without this having to
be
expressly explained below.
When using an electrolytic cell according to the invention to extract
aluminium
by fused-salt electrolysis, it is possible for the resulting aluminium to be
continuously removed, so that the aluminium level in the actual electrolytic
cell
can always be kept at a constant level. The passages advantageously reduce
reflection of the flowing aluminium at the side walls of the electrolytic
cell. The
aforementioned wave formations can thereby be reduced, which is why the
anode or anodes of the electrolytic cell can be moved close to the surface of
the
aluminium, without there being the risk of a short-circuit. However, this also
means that the distance between the anode(s) and cathode can be reduced
and the specific energy requirement for the fused-salt electrolysis process
can
thereby be reduced compared with the state of the art. Through the continuous
removal of the aluminium formed, the operating performance of the cell is
stabilised, as the conditions within the cell do not change, or barely change,
over time.
It is advantageous in an embodiment of the electrolytic cell according to the
CA 02811357 2013-03-14
SGL CARBON SE 2010/054W0
07.03.2013
r 5
invention for a collecting launder to be attached to the outer periphery of
the
overflow trough in such a manner that liquid (i.e. particularly liquid
aluminium)
passing over the upper edge of an outer border of the overflow trough flows
into the collecting launder. This kind of embodiment makes it easier for the
liquid aluminium produced during fused-salt electrolysis to be continuously
removed.
In this embodiment, the aluminium accumulating above the cathode during the
process passes via the passages formed in the surrounding side wall into the
overflow trough. The level of liquid aluminium thereby produced exhibits the
same or virtually the same height in the overflow trough and in the tank of
the
electrolytic cell. If further aluminium is now produced in the electrolytic
cell
during the performance of the fused-salt electrolysis, the level rises in the
cathode tank and also in the overflow trough. If it thereby reaches the upper
edge of the outer border of the overflow trough, any further rise of the level
will
cause aluminium to flow over the border into the collecting launder, from
where
it can be removed. This means that the aluminium level within the cathode tank
never rises beyond a specific level, which is determined by the height of the
upper edge of the outer border of the tank.
At least some of the passages in the surrounding side wall of the electrolytic
cell, particularly all passages, may advantageously be formed at the same
height above the cathode. This makes an even discharge of the liquid
aluminium into the tank easy.
Moreover, it has proved to be particularly favourable for the cathode to be
horizontal. In this way, the filling level of the resulting aluminium at each
point
within the tank is at least virtually the same depth during the performance of
the
aforementioned fused-salt electrolysis and uniform process conditions can
therefore be maintained over the entire cathode surface.
In accordance with an embodiment of the invention the overflow trough exhibits
outer walls, which are provided with a thermal insulation. This thermal
CA 02811357 2013-03-14
SGL CARBON SE 2010/054W0
07.03.2013
6
insulation fulfils the function of preventing the molten aluminium from
cooling
too quickly. In order to be able to maintain the effect of keeping the
aluminium
level constant by means of an overflow structure, which can be achieved with
the electrolytic cell according to the embodiments of the invention, it is
necessary for the aluminium to remain in its liquid state until it has been
removed from the overflow trough or from the collecting launder. However, this
requires a sufficiently high temperature outside the cathode tank too. The
insulation of the overflow; trough and possibly also of the collecting launder
helps to minimise unnecessary heat losses from those components.
In a further advantageous embodiment of the electrolytic cell, the passages
are
formed equidistant to one another in the surrounding side wall. In this way,
the
discharge of liquid aluminium, i.e. of aluminium melt, is largely uniform and
even, in which case the creation of turbulences and the formation of waves
within the cathode tank are largely precluded during the discharge. For the
same reason, it is favourable to limit the number of passages not to a too
small
number. A number of roughly 2 to 5 passages per m length of side wall have
proved to be particularly suitable.
In relation to the size and shape of the passages, these may exhibit a
diameter
of between 3 and 15 cm, for example, if they are circular in cross-section,
which
is advantageous with regard to flow conditions. If a cross-sectional shape
other
than a circle is chosen, the design can correspond to the equivalent diameters
with regard to the upper values. "Equivalent diameter" refers in this case to
any
cross-sectional dimension that produces a cross-sectional area corresponding
to a circular area with this diameter. A rectangular cross-section may also be
advantageous, in which case a width of the cross-section may be greater than
its height.
In accordance with an embodiment of the invention, the surrounding outer wall
of the cathode is disposed rotationally symmetrically around a central column.
In this case the outer wall may exhibit a circular or a regular polygonal form
when viewed from above. In this context, "rotationally symmetrical" means any
CA 02811357 2013-03-14
SGL CARBON SE
2010/054W0
07.03.2013
7
form that can be brought into congruence with the original form when rotated
around the centre at an angle of rotation of under 360 . Examples of such
outer
wall profiles may also be regular polygons. The rotational symmetry produces a
uniform discharge of the aluminium melt over the entire tank area of the
tank-shaped cathode.
The aforementioned central column may be tapered downwards radially
towards the outside. This is particularly advantageous when it comes to
filling
the cathode tank, as will be discussed in greater detail below.
With regard to the versatility of use of the electrolytic cell, it is
advantageous for
the outer side wall of the overflow trough to be height-adjustable. As the
height
of the outer side wall, therefore the height of the upper edge of the outer
border
above the passages, determines the height of the aluminium level within the
cathode tank, a height-adjustable outer side wall enables the aluminium level
to
be varied quickly and easily according to need. A mechanical or motorised
adjusting device may be provided to adjust the height of the side wall.
A process for continuously extracting aluminium from its oxide by fused-salt
electrolysis, in which the electrolytic cell according to the invention can be
used,
comprises the removal of the aluminium produced by fused-salt electrolysis
from the collecting launder, which is fed from an overflow of an overflow
trough,
which is connected by passages to the inside of an electrolytic cell used
during
fused-salt electrolysis below the level of the aluminium produced in the
cathode
tank.
Since the aluminium is therefore discharged from the side of the electrolytic
cell, it can flow into the overflow trough continuously and uniformly, which
means that the level of liquid aluminium is always kept constant in the
cathode
tank. As already mentioned, movements in the liquid or melt can thereby be
largely avoided, so that the surface of the molten aluminium in the cathode
tank
remains smooth and the anodes can therefore also be brought close to the level
of the liquid aluminium, without there being any risk of a short-circuit
between
,1
CA 02811357 2013-03-14
SGL CARBON SE 2010/054 WO
07.03.2013
8
the anodes and cathodes through the aluminium. The energy efficiency of the
aluminium production process can therefore be significantly improved.
Moreover, the continuous discharge means that a more stable method of
operation is guaranteed.
In order to ensure the smoothest possible removal of liquid aluminium, it is
thereby advantageous for the fused-salt electrolysis to be carried out above a
temperature of approx. 750 C, particularly between 930 und 1000 C. A lower
temperature threshold of 750 C ensures that the aluminium melt is still
sufficiently liquid outside the tank of the electrolytic cell to be able to
flow over
the edge of the overflow trough into the collecting launder and to be removed
from there. Where there are suitable conditions, in which the heat loss in the
outer parts of the overflow trough and collecting launder is kept low, it is
prevented to fall below the aforementioned lower temperature limit. However,
because this kind of process is advantageously conducted well above the
aluminium melting point, for technical reasons, adequate flowability is
generally
ensured for the aluminium without further measures being required.
The invention will now be described in greater detail with reference to the
attached drawing using a non-restrictive embodiment. In the drawing:
Fig. la shows a cross section of an electrolytic cell for the extraction of
aluminium oxide according to the state of the art,
Fig. lb shows the electrolytic cell from Fig. la in an external
longitudinal
view,
Fig. lc shows a perspective view, partially in section, of an electrolytic
cell for the extraction of aluminium from aluminium oxide
according to the state of the art,
Fig. 2a shows a cross-sectional view of a section of an electrolytic cell
according to an embodiment of the invention,
CA 02811357 2013-03-14
SGL CARBON SE 2010/054W0
07.03.2013
9
Fig. 2b shows a top view of the electrolytic cell from Fig. 2a; and
Fig. 3 shows a configuration of anodes, which is adapted to the cathode
form according to Fig. 2a and Fig. 2b,
The same reference symbols are used in the figures to refer to the same or
corresponding elements in the different representations.
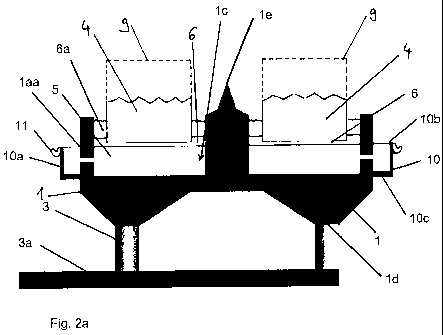
With reference to Figure 2a, an electrolytic cell according to a first
embodiment
is shown in cross-section, which is suitable for use in the extraction of
aluminium from aluminium oxide using the Hall-Heroult process already
described. The electrolytic cell is made up of a cathode 1 and a surrounding
side wall 1a, which is provided with passages 1aa. The side wall 1a along with
the cathode 1 delimits the tank 1c, which gives the electrolytic cell its tank
shape. There are several connections 1d at the lower end of the cathode 1,
which are used to connect to pins 3, which are disposed vertically in the
present
example and are connected to a common current bar 3a.
A central column 1e is formed in the centre of the tank 1c, which exhibits a
tip,
as can be seen in Fig. 2a, i.e. runs downwards in an outward direction. This
shape assists with the introduction of the mixture 6 of aluminium oxide and
possibly cryolite and/or aluminium fluoride, in other words the liquid
electrolytes, into the tank lc of the electrolytic cell. In this case, the
outward
tapering brings about a desired reduction in wave movements in the liquid
aluminium when the electrolytic cell is filled during fused-salt electrolysis.
Different levels of the substances involved in the process are drawn into the
figure: the lowest line shows the level of liquid aluminium 5 that accumulates
close to the cathode 1. The mixture 6 is located above the aluminium 5 and is
upwardly limited by a crust 6a of solidified melt 6, which forms during the
course
of the process.
The anodes 4 used as an opposite pole for the cathode 1 in the fused-salt
CA 02811357 2013-03-14
SGL CARBON SE
2010/054W0
07.03.2013
electrolysis process are contained in the cover 9. As can be seen in the
figure,
the anodes 4 are lowered so far into the tank lc of the electrolytic cell that
from
the upper end they come close to the level of the aluminium 5. This is
possible
because the electrolytic cell is an electrolytic cell according to an
embodiment
of the invention, which exhibits an overflow trough 10 adjacent to the
surrounding side wall 1a, whose outer side wall 10a is limited at the top by
the
upper edge of an outer border 10b and at the bottom by a lower outer wall 10c.
A collecting launder 11 is attached to the outer side wall 10a or formed in
one
piece together with the collecting launder.
When performing the fused-salt electrolysis according to the known
Hall-Heroult process, liquid aluminium 5 is produced at the cathode 1 from the
liquid electrolyte, which settles close to the cathode 1. Since the cathode
tank
1c is connected via the passages 1 aa in the side wall 1a of the electrolytic
cell
below a desired target level for the aluminium 5, the level in the overflow
trough
10 also rises as more aluminium is produced. If the filling level shown in
Figure
2a is reached, according to which the level of the aluminium 5 is at the
height of
the upper edge of the outer border 10b of the overflow trough 10, as the
process continues aluminium 5 flows over the outer border 10b into the
collecting launder 11, from where it can be removed using traditional means.
As a result of this design, the level of aluminium 5 both within the overflow
trough 10 and in the tank 1c of the cathode 1 is limited to a desired target
level,
which is defined by the height of the upper edge of the outer border 10b of
the
overflow trough. So that the target level can be easily altered, it is
favourable for
the outer side wall 10a of the overflow trough 10 to be height-adjustable via
the
lower outer wall 10c. This may, for example, be achieved through an
immersible wall construction (not shown). Furthermore, it is favourable in
relation to the process management for heat losses from the molten aluminium
5 close to the overflow trough 10 and also the collecting launder 11 to be
minimised as far as possible, in order to prevent the melt from starting to
solidify
before it has been removed from the run-off collector 11. This may be
achieved,
for example, by thermal insulation (not shown in the figures) of the outer
walls
,
CA 02811357 2013-03-14
SGL CARBON SE
2010/054 WO
07.03.2013
11
10a, 10c of the overflow trough and possibly also of the collecting launder
11.
Structural measures aimed at keeping the surface of the aluminium 5 within the
overflow trough 10 as small as possible also have the same effect. It is
favourable, therefore, for the overflow trough 10 to be designed with a small
width b, between roughly 50 mm and 100 mm, for example.
Now with reference to Figure 2b, a top view of the electrolytic cell in Figure
2a is
shown. It can be seen that the side wall la of the electrolytic cell exhibits
rotational symmetry here. The shape of the base area of the cathode 1 is that
of
a star with cropped jags. It should be noted that this shape is not obligatory
and
that a traditional rectangular tank lc or another tank shape, for example, is
also
possible for the electrolytic cell according to the invention.
The top view of Figure 2b furthermore shows the central column le, which
exhibits the shape of a regular hexagon in this case. Dotted lines are used to
identify the connections 1d (six of them in this case) for the pins 3 shown in
Figure 2a designed as current supply devices, which run into the cathode 1
perpendicular to the image plane. In the embodiment shown the connections 1d
represent a radial centre around which respective bulges in the side wall la
run.
As likewise indicated by dotted lines, the tank 1c of the electrolytic cell
may be
divided into individual sections if or sectors, which can be regarded as
partial
cells of an electrolytic cell connected to one another. Because the dotted
lines
do not represent genuine barriers, but simply serve as virtual limits to
clarify the
position of the sections if or sectors, only a single section if was defined
in its
limits by these lines in the figure.
Beyond the side wall la of the electrolytic cell, the outer side wall 10a of
the
overflow trough 10 can be seen, which is continuous in the case shown and has
an outer contour that matches the outer contour of the side wall 1a of the
cathode 1. The passages 1aa and the collecting launder 11 are not shown in
Figure 2b.
ri
CA 02811357 2013-03-14
SGL CARBON SE 2010/054 WO
07.03.2013
n 12
With reference to Figure 3, a configuration of anodes 4 is shown from above,
which are designed as part of the section of an electrolytic cell represented
in
Figures 2a and 2b to act as the entire electrolytic cell.
In this case the base areas 4a of the anodes 4 exhibit the shape of elongated,
inward-tapering hexagons (six of them here) in the top view, which are
disposed in a star shape around a common centre Z. This means that the
shape and number of anodes 4 corresponds to the shape and number of
sections If or sectors of the cathode 1 in Figures 2a and 2b. However, it
should
be noted that the size of the sections If does not correspond to those of the
associated anodes 4. Instead, the base area of the anodes 4, which is visible
in
the top view in Figure 3, is smaller than that of the associated section if,
which
means that the anodes 4 can be lowered into the tank 1c of the cathode 1 while
the electrolytic cell is in operation.
With the cathode unit according to the invention and also the process
according
to the invention, the energy efficiency of an electrolytic cell for fused-salt
electrolysis used to extract aluminium can be improved, due to the fact that
the
anodes and cathode can be moved closer together, as the continuous removal
of the aluminium produced means that the filling level of the same in the
cathode tank is low and/or a surface largely free from wave movements can be
achieved. The height of the level can be kept at least largely at a target
position
within the cathode tank, so that in addition to a tracking of the anodes due
to
their consumption during the process, no additional tracking is required. A
high
quality of the aluminium produced and also an optimised temperature control
can thereby be achieved.
In relation to cathode and anode materials, any materials known to the person
skilled in the art and suitable for the electrolysis of aluminium from its
oxide may
be used. Suitable materials are specified in DE 10261745, for example, the
content of which, in this respect, is to be incorporated here by reference.
CA 02811357 2013-03-14
=
SGL CARBON SE
2010/054W0
07.03.2013
13
Reference list
1 Cathode
1a Side wall
1aa Passage
1c (Cathode) tank
1d Connections
1e Central column
If Section
2 Mounting
3 (Current-carrying) pin
3a Current bar
4 Anode
4a Base area
Aluminium
6 Mixture (aluminium oxide, cryolite)
6a Crust of solidified melt 6
7 Negative pole, voltage source
8 Positive pole, voltage source
9 Cover
Overflow trough
10a Outer side wall
10b Outer border
10c Lower outer wall
11 Collecting launder