Note: Descriptions are shown in the official language in which they were submitted.
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
HETEROCYCLYL COMPOUNDS AS HISTAMINE 113 RECEPTOR LIGANDS
Field of Invention
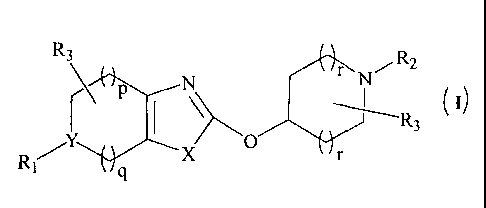
The present invention relates to heterocyclyl compounds of formula (I) and
their
pharmaceutically acceptable salts, its process of preparation and compositions
containing them,
for the treatment of various disorders that are related to Histamine H3
receptors.
R3 RrN
p
(I)
I \
R R3
Background of the Invention
Histamine H3 receptor is a G-protein coupled receptor (GPCR) and one out of
the four
receptors of Histamine family. Histamine H3 receptor is identified in 1983 and
its cloning and
characterization were done in 1999. Histamine H3 receptor is expressed to a
larger extent in
central nervous system and lesser extent in the peripheral nervous system.
Literature evidence suggests that Histamine H3 receptors can be used in
treatment of
cognitive disorders (British Journal of Pharmacology, 2008, 154(6), 1166-
1181), sleep
disorders (Trends in Pharmacology Science, 2004, 25(12), 618-625), obesity
(Molecular
Interventions, 2006, 6 (2), 77-88) and pain (Pain, 2008, 138(1), 61-69).
Patents / Patent publications US4695575, EP0151826, W02010026113,
W02004022060 and W02003004480 disclosed series of compounds as ligands at
Histamine
H3 receptors. ,While some Histamine H3 receptor ligands have been disclosed,
no compound
till date is launched in market in this area of research, and there still
exists a need and scope to
discover new drugs with novel chemical structures for treatment of disorders
affected by
Histamine H3 receptors.
Summary of the Invention
The present invention relates to novel Histamine H3 receptor ligands of the
formula (I);
R3
R2
(I)
I ).\
R3
R 1/Y X
wherein,
- 1 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
R5 R5
RI is -C(0)-R4, -S(0)2-R4,¨N¨ C(0)-R4,¨N¨ S(0)2-R4 substituted or
unsubstituted
cycloalkyl, aryl or heteroaryl; wherein substituents, may be one or more and
are independently
selected from hydrogen, hydroxy, halogen, alkyl, alkoxy, haloalkyl or
haloalkoxy;
R4 is substituted or unsubstituted alkyl, cycloalkyl, cycloalkylalkyl, aryl,
heteroaryl,
heterocyclyl or heterocyclylallcyl; wherein substituents, may be one or more
and are
independently selected from hydrogen, hydroxy, halogen, alkyl, alkoxy,
haloalkyl or
haloalkoxy;
R5 is hydrogen, alkyl or cycloalkyl;
at each occurrence, R3 is independently selected from hydrogen, halo, alkyl or
alkoxy;
R2 is hydrogen, substituted or unsubstituted alkyl or cycloalkyl; wherein
substituents,
may be one or more and are independently selected from hydrogen, hydroxy,
halogen, alkyl,
alkoxy, haloalkyl or haloalkoxy;
X is S, N or 0;
Y is C or N;
"p" is an integer ranging from 0 to 2;
"q" is an integer ranging from 0 to 2;
"r" is an integer ranging from 0 to 1; or a pharmaceutically acceptable salt
thereof.
The present invention relates to use of a therapeutically effective amount of
compound
of formula (I), to manufacture a medicament in the treatment of various
disorders that are
related to Histamine H3 receptors.
Specifically, the compounds of this invention are useful in the treatment of
various
disorders such as cognitive disorders, sleep disorders, obesity and pain.
In another aspect, the invention relates to pharmaceutical compositions
containing a
therapeutically effective amount of at least one compound of formula (I), and
pharmaceutically
acceptable salts thereof, in admixture with pharmaceutical acceptable
excipient.
In still another aspect, the invention relates to methods for using compounds
of formula
(1).
Inyet another aspect, the invention further relates to the process for
preparing
compounds of formula (I) and their pharmaceutically acceptable salts.
Representative compounds of the present invention include those specified
below and
their pharmaceutically acceptable salts. The present invention should not be
construed to be
limited to them:
1124 I -Cyclobutyl-piperidin-4-yloxy)-6,7-d ihydro-4H-thiazolo[5,4-c]pyrid in-
5-y1]-propan-1-
one tartrate;
2
CA 02812970 2014-04-14
[2-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,7,8-tetrahydro-thiazolo[5,4-d]azepin-6-
y1]-
cyclopropyl-methanone tartrate;
N42-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,6,7-tetrahydro-benzothiazol-6 -y1]-
propionamide;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y11-cyclopropyl-
methanone tartrate;
Cyclobutyl-[2-(1-cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1]-
methanone tartrate;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-(2-fluoro-
pheny1)-methanone tartrate;
1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-3-methyl-
butan-1-one tartrate;
Cyclobutyl-[2-(1-isopropyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1]-
methanone;
Cyclopropyl-[2-(1-isopropyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1]-
methanone tartrate;
Cyclopropyl-[2-(1-cyclopropyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-
y1]-methanone tartrate;
Cyclobutyl-[2-(1-cyclopropyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1]-
methanone tartrate;
1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-2-
morpholin-4-yl-ethanone tartrate;
[4-(5-Cyclobuty1-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-2-yloxy)-piperidin-l-
y1]-cyclopropyl-
methanone tartrate;
[3-(5-Cyclobuty1-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-2-yloxy)-piperidin-l-
y1]-cyclopropyl-
methanone tartrate;
[2-(1-Cyclobutyl-piperidin-3-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y11-cyclopropyl-
methanone tartrate;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
yll-pyridin-4-yl-
methanone tai __ ti ate;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-(4-methoxy-
pheny1)-methanone tartrate;
1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-2-
piperidin-1-yl-ethanone tartrate;
1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-2-
cyclopropyl-ethanone;
2-(1-Cyclobutyl-piperidin-4-yloxy)-5-(2-fluoro-benzenesulfony1)-,6,7-dihydro-
4H-
thiazolo[5,4-c]pyridine tartrate;
- 3 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
2-(1-Cyclobutyl-piperidin-4-yloxy)-5-methanesulfonyl-,6,7-dihydro-4H-
thiazolo[5,4-
c]pyridine;
1-[2-(1-Cyclobutyl-piperid in-4-yloxy)-6,7-d ihydro-4H-thiazolo[5,4-c]pyridin-
5-y1]-2-methyl-
propan-l-one tartrate;
2-(1-Cyclobutyl-piperidin-4-yloxy)-5-(2-trifluoromethyl-pyridin-5-y1)-,6,7-
dihydro-4H-
thiazolo[5,4-c]pyridine;
Cyclopropy112-( I -isobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1]-
methanone tartrate;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-411-thiazolo[5,4-c]pyridin-5-
y1]-(2-
tri fluoromethyl-pyridin-5-y1)-methanone;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y11-pyridin-3-yl-
methanone tartrate;
2-(1-Cyclobutyl-piperidin-4-yloxy)-5-pyridin-3-y1-,6,7-dihydro-4H-thiazolo[5,4-
c]pyridine
tartrate;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-(tetrahydro-
pyran-4-y1)-methanone;
[2-(1-Cyclobutyl-piperid in-4-yloxy)-6,7-dihyd ro-4H-thiazolo[5,4-c] pyrid in-
5-y11-morphol in-4-
yl-methanone tartrate;
[2-(1-Cyclobutyl-piperid ihydro-
4H-thiazolo[5,4-c]pyridin-5-y1]-piperidin-1-
yl-methanone hydrochloride;
6-[2-(1-Cyclpbutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1]-
nicotinamide;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y1J-cyclopentyl-
methanone tartrate;
[2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-5H-thiazolo[5,4-b]pyridin-4-
y1]-cyclopropyl-
methanone tartrate;
Cyclopropyl-[2-(1-isopropyl-piperidin-4-yloxy)-4,5,7,8-tetrahydro-thiazolo[5,4-
d]azepin-6-y11-
methanone tartrate;
[2-(1-Cyclobutyl-piperidi n-4-yloxy)-6,7-d ihyd ro-5H-thiazolo[5,4-b]pyrid in-
4-y1J-cyclopropyl-
methanone;
1-[2-(1-Cyclobutyl-pi perid in-4-yloxy)-6,7-d ihydro-5H-th iazolo[5,4-b]pyrid
n-4-y1Fpropan-1-
one;
Cyclobutyl-[2-(1-cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-5H-thiazolo[5,4-
b]pyridin-4-y1]-
methanone;
N42-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,6,7-tetrahydro-benzothiazol-7-y1]-
propionam ide;
1-[2-(1-Cyclobutyl-pi perid ihydro-
4H-oxazolo[5,4-c] pyrid in-5-yli-propan-1-
one;
- 4 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
142-(1-Cyclobutyl-piperidin-4-yloxy)-4,6-dthydro-pyrrolo[4,3-d]oxazol-5-y11-
propan-1-one;
N-[2-( I -Cyclobutyl-piperid in-4-y loxy)-5,6-dihydro-4H-cyclopentaoxazol-5-
y1]-propionamide;
N-[2-( I -Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentathiazol-5-y1}-
propionamide;
I -[2-(1-Cyclobutyl-piperidin-4-yloxy)-4,6-dihydro-pyrrolo[4,3-d]thiazol-5-y1}-
propan- I -one;
I -[2-( 1 -Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-pyrrolo[3,2-d]thiazol-4-
y11-propan- I -one;
N-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentathiazol-6-y1]-
propionamide;
N-[2-( I -Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentaoxazol-6-y1]-
propionamide;
1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-pyrrolo[3,2-d]oxazol-4-
y1Wpropan- 1-one;
1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-4,6-dihydro-pyrrolo[4,3-d]oxazol-5-y1]-
propan-1-one;
N-[2-( I -Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentaoxazol-5-y1]-
propionamide;
1-[2-(1-Cyclobutyl-pi peridin-4-yloxy)-4,6,7,8-tetrahydro-th iazolo[5,4-
c]azepin-5-y1}-propan-1-
one;
I -[2-( I -Cyclobutyl-piperidin-4-yloxy)-4-methy1-6,7-dihydroL4H-thiazolo[5,4-
c]pyridin-5-y1]-
propan-l-one;
142-(1-Cyclobutyl-piperidin-4-yloxy)-7-fluoro-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1}-
propan- I -one;
1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-7-methy1-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1}-
propan- 1-one;
1-[2-(1-Cyclobutyl-pi peridin-4-yloxy)-6-methy1-6,7-d ihydro-4H-thiazolo[5,4-
c]pyridin-5-y11-
propan- 1-one;
1-[2-(1-Cyclobuty1-3-methy I-pi peridin-4-yloxy)-6,7-d ihydro-4H-thiazolo[5,4-
c]pyridin-5-y1F
propan- 1-one;
1-[2-( I -Cyclobuty1-3-fluoro-piperid in-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1}-
propan- 1-one;
1-Cyclobuty1-4-(5-propiony1-4,5,6,7-tetrahydro-thiazolo[5,4-c]pyridin-2-yloxy)-
piperidine-3-
carbonitri le;
1-[2-(1-Cyclobutyl-azepan-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-y1]-
propan- I -
one;
1-[2-(1-Cyclobutyl-azepan-4-yloxy)-4,5,7,8-tetrahydro-thiazolo[5,4-d]azepin-6-
y1J-propan- 1-
one;
142-(1-Cyclobutyl-pyrrol idin-3-yloxy)-4,5,7,8-tetrahydro-thiazolo[5,4-
d]azepin-6-y1Fpropan-
I -one;
1-(2-(1-Cyclobutyl-pyrrol id i n-3-yloxy)-4,6-d ihydro-pyrrolo[4,3-d]thiazol-5-
y11-propan- I -one;
1-[2-(1-Cyclobutyl-azepan-4-yloxy)-4,6-dihydro-pyrrolo[4,3-d]thiazol-5-
y1Fpropan-l-one;
1-124 I -(2-Hydroxy-ethyp-piperid in-4-yloxy]-6,7-d ihydro-4H-thiazolo[5,4-
c]pyrid in-5-y' } -
propan-l-one;
- 5 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
1-[2-(1-Ethoxymethyl-piperidin-4-yloxy)-6,7-d ihyd ro-4H-thiazolo[5,4-c]pyrid
in-5-y1]-propan-
1-one;
1-{2-[1-(2,2,2-Trifluoro-ethyl)-piperidin-4-y loxy]-6,7-d ihydro-4H-th azolo
[5,4-c]pyrid in-5-
yl )-propan-l-one;
1-[2-(1-Cyclobutyl-azetidin-3-yloxy)-6,7-d ihydro-4H-th iazolo[5,4-c]pyrid n-5-
y1]-propan-1-
one; and their pharmaceutically acceptable salts.
Detailed Description of the Invention
Unless otherwise stated, the following terms used in the specification and
claims have
the meanings given below:
The term "halogen" means fluorine, chlorine, bromine or iodine.
The term "alkyl" means straight chain or branched hydrocarbon radical
consisting
solely of carbon and hydrogen atoms, containing no unsaturation, having from
one to eight
carbon atoms, and which is attached to the rest of the molecule by a single
bond. Exemplary
"alkyl" groups include methyl, ethyl, n-propyl, iso-propyl and the like.
The term "alkoxy" means an alkyl group attached via an oxygen linkage to the
rest of
the molecule. Exemplary "alkoxy" groups include methoxy, ethoxy, propyloxy,
iso-propyloxy
and the like.
The term "haloalkyl" means straight or branched chain alkyl radicals
containing one to
three carbon atoms. Exemplary "haloalkyl" groups include fluoromethyl,
difluoromethyl,
trifluoromethyl, trifluoroethyl, fluoroethyl, difluoroethyl and the like.
The term "haloalkoxy" means straight or branched chain alkoxy radicals
containing
one to three carbon atoms. Exemplary "haloalkoxy" groups include
fluoromethoxy,
difluoromethoxy, trifluoromethoxy, trifluoroethoxy, fluoroethoxy,
difluoroethoxy and the like.
The term "cycloalkyl" means non-aromatic mono cyclic ring of 3 to 8 carbon
atoms.
Exemplary "cycloalkyl" groups include cyclopropyl, cyclobutyl, cyclopentyl and
the like.
The term "cycloalkylalkyl" means cycloalkyl ring radical directly bonded to an
alkyl
group.
The term "aryl" means any functional group or substituent derived from a
simple
aromatic ring, Exemplary "aryl" groups include phenyl, naphthyl and the like.
The term "heteroaryl" means organic compounds that contain a ring structure
containing atoms in addition to carbon such as sulfur, oxygen or nitrogen, as
part of the ring,
these additional atoms may be repeated more than once in ring. These rings may
be either
simple aromatic rings. Exemplary "heteroaryl" groups include pyridine,
pyrimidine,
benzofuranyl, benzothiophene, furyl, dioxalanyl, pyrrolyl, oxazolyl, pyridyl,
pyridazinyl,
pyrimidinyl, pyrazinyl, quinolinyl, indolyl and the like.
The term "heterocycly1" means non-aromatic mono cyclic ring of 2 to 7 carbon
atoms,
whose ring structures include 1 to 3 heteroatoms, these additional atoms may
be repeated more
- 6 -
CA 02812970 2013-06-20
than once in ring. Exemplary "heterocycly1" groups include pyrrolidinyl,
piperidinyl,
piperazinyl, morpholinyl and the like.
The term "heterocyclylalkyl" means heterocyclyl ring radical directly bonded
to an
alkyl group.
The terms "treating", "treat" or "treatment" embrace all the meanings such as
preventative, prophylactic and palliative.
The phrase "pharmaceutically acceptable salts" indicates that the substance or
composition must be compatible chemically and/or toxicologically, with the
other ingredients
comprising a formulation, the mammal being treated therewith.
The phrase "therapeutically effective amount" is defined as 'an amount of a
compound
of the present invention that (i) treats or prevents the particular disease,
condition or disorder
(ii) attenuates, ameliorates or eliminates one or more symptoms of the
particular disease,
condition or disorder (iii) prevents or delays the onset of one or more
symptoms of the
particular disease, condition or disorder described herein.
Commercial reagents were utilized without further purification. Room
temperature
refers to 25 - 40 C. Unless otherwise stated, all mass spectra were carried
out using ES!
conditions. 'H-NMR spectra were recorded at 400 MHz on a BrukerTM instrument.
Deuterated
chloroform (99.8 % D), methanol or dimethylsulfoxide was used as solvent. TMS
was used as
internal reference standard. Chemical shift values are expressed in parts per
million (6) values.
The following abbreviations are used for the multiplicity for the NMR signals:
s=singlet,
bs=broad singlet, d=doublet, t=triplet, q=quartet, qui=quintet, h=heptet,
dd=double doublet,
dt=double triplet, tt=triplet of triplets, m=multiplet. Chromatography refers
to column
chromatography performed using 100 - 200 mesh silica gel and executed under
nitrogen
pressure (flash chromatography) conditions.
The compounds nomenclature is generated by using Chem Draw UltraTM 7Ø
Pharmaceutical compositions
In order to use the compounds of formula (I) in therapy, they will normally be
formulated into a pharmaceutical composition in accordance with standard
pharmaceutical
practice.
The pharmaceutical compositions of the present invention may be formulated in
a
conventional manner using one or more pharmaceutically acceptable excipient.
The
pharmaceutically acceptable excipient is carrier or diluent. Thus, the active
compounds of the
invention may be formulated for oral, intranasal or parenteral (e.g.,
intravenous, intramuscular
or subcutaneous). Such pharmaceutical compositions and processes for preparing
same are well
known in the art. (See, e.g., Remington: The Science and Practice of Pharmacy
(D.B. Troy,
Editor, 21st Edition, Lippincott, Williams & Wilkins, 2006).
- 7 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
The dose of the active compounds can vary depending on factors such as the
route of
administration, age and weight of patient, nature and severity of the disease
to be treated and
similar factors. Therefore, any reference herein to a pharmacologically
effective amount of the
compounds of general formula (I) refers to the aforementioned factors.
Method of Preparation
The compounds of formula (I) can be prepared by Scheme I as shown below.
R3
R2
I \ __ Br
H R3
X
0 (1) (2)
R3
R2
x
R3
0
0 (3)
R3
N R2
+ R1 ¨Cl
R3
H
X 0
(5)
(4)
=
R3
RrN 2
(I)
RI X
Scheme 1
In above Scheme 1, p is 1; q is 1; r is 1; Y is N; X is S; RI is -C(0)-R4, -
S(0)2-R4,
substituted or unsubstituted cycloallcyl, aryl or heteroaryl, and all other
symbols are as defined
above.
The compound of formula (1) is coupled with compound of formula (2) to form
compound of formula (3). The compound of formula (3) is deprotected to form
compound of
formula (4). The compound of formula (4) is coupled with compound of formula
(5) to form
compound of formula (1).
- 8 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
In the first step of the above preparation, the compound of formula (1) is
coupled with
compound of formula (2) to form compound formula (3). This reaction is
preferably carried out
in solvent such as tetrahydrofuran, toluene, ethyl acetate, dichloromethane,
triethylamine,
dimethylformamide, and the like or a mixture thereof and preferably by using
tetrahydrofuran.
The reaction may be affected in the presence of a base such as sodium hydride,
sodium
carbonate, potassium carbonate, sodium bicarbonate, sodium hydroxide or
mixtures thereof and
preferably by using sodium hydride. The reaction is carried out at room
temperature. The
duration of the reaction may range from 4 to 8 hours, preferably from a period
of 5 to 7 hours.
In the second step of the above preparation, the compound of formula (3) is
deprotected
to form compound of formula (4). This reaction is preferably carried out in
solvent such as
tetrahydrofuran, toluene, ethyl acetate, dichloromethane, acetonitrile, 1,4-
dioxone,
dimethylformamide, and the like or a mixture thereof and preferably by using
dichloromethane.
The reaction may be affected in the presence of a acid such as trifluoroacetic
acid, sulfuric acid,
acetic acid, perchloric acid, hydrochloric acid, and the like or a mixture
thereof and preferable
by using trifluoroacetic acid. The reaction is carried out at temperature of
60 C to 85 C and
preferably temperature in range of 65 C to 75 C. The duration of the
reaction may range from
2 to 6 hours, preferably from a period of 3 to 5 hours.
In the third step of the above preparation, the compound of formula (4) is
coupled with
compound of formula (5) to form compound of formula (I). This reaction is
preferably carried
out in solvent such as dichloromethane, tetrahydrofuran, toluene, ethyl
acetate,
dimethylformamide, and the like or a mixture thereof and preferably by using
dichloromethane.
The reaction may be affected in the presence of a base such as triethylamine,
potassium
carbonate, diisopropylethylamine and pyridine and preferable by using
triethylamine. The
reaction is carried out at room temperature. The duration of the reaction may
range from 15
minutes to 45 minutes, preferably from a period of 25 minutes to 35minutes.
The compounds of formula (1), formula (2) and formula (5) may be commercially
available or can be prepared by conventional methods or by modification, using
known process.
The compounds of formula (I) can also be prepared by using Scheme II as shown
below
R3 R3 R3
0
4- R1 ¨CI \r
Br
k-rcl
(6) (5) (7) (8)
- 9 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
R
R3 3
R2
I _____________________________________________________ Br r N
I > __ NH2 R3
RI X
R r19( X
(9) (10) (2)
R3 \r N
r R2 - (I)
Scheme II
In above Scheme II, p is 1; q is 2; r is 1; Y is N; X is S; RI is -C(0)-R4,
substituted or unsubstituted cycloalkyl, aryl or heteroaryl, and all other
symbols are as defined
above.
The compound of formula (6) is coupled with compound of formula (5) to form
compound of formula (7). The compound of formula (7) is brominated to form
compound of
formula (8). The compound of formula (8) is cyclized to form compound of
formula (9). The
compound of formula (9) is diazotized to form compound of formula (10). The
compound of
formula (10) is coupled with compound of formula (2) to form compound of
formula (I).
In the first step of the above preparation, the compound of formula (6) is
coupled with
compound of formula (5) to form compound of formula (7). This reaction is
preferably carried
out in solvent such as tetrahydrofuran, toluene, ethyl acetate,
dichloromethane,
dimethylformamide, and the like or a mixture thereof and preferably by using
dichloromethane.
The reaction may be affected in the presence of a base such as triethylamine,
potassium
carbonate, diisopropylethylamine and pyridine and preferable by using
triethylamine.The
reaction is carried out at room temperature. The duration of the reaction may
range from 15
minutes to 45 minutes, preferably from a period of 25 minutes to 35minutes.
In the second step of the above preparation, the compound of formula (7) is
brominated
to form compound of formula (8). The reaction may be affected in the presence
of an acid such
as sulfuric acid, acetic acid, perchloric acid, hydrochloric acid, and the
like or a mixture thereof
and preferable by using acetic acid. The reaction may be affected in the
presence of a
bromating agent such as bromine, copper (II) bromide, hydrobromic acid, N-
bromosuccinimide, pyridine hydrobromide perbromide, tetrabromomethane, and the
like or a
mixture thereof and preferably by using bromine.The reaction is carried out at
room
-10-
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
temperature. The duration of the reaction may range from 16 hours to 20 hours
preferably from
a period of 17 hours to 19 hours.
In the third step of the above preparation, the compound of formula (8) is
cyclized to
form compound of formula (9). This reaction is preferably carried out in
solvent such as
isopropyl alcohol, tetrahydrofuran, toluene, ethyl acetate, dichloromethane,
triethylamine,
dimethylformamide, and the like or a mixture thereof and preferably by using
isopropyl
alcohol. The reaction may be affected in the presence of urea or thiourea. The
reaction is
carried out at temperature of 60 C to 85 C and preferably temperature in
range of 65 C to 75
C. The duration of the reaction may range from 15 minutes to 45 minutes,
preferably from a
period of 25 minutes to 35minutes.
In the fourth step of the above preparation, the compound of formula (9) is
diazotized
to form compound of formula (10). This reaction is preferably carried out in
solvent such as
acetonitrile, tetrahydrofuran, isopropyl alcohol, toluene, ethyl acetate,
dichloromethane,
triethylamine, dimethylformamide, and the like or a mixture thereof and
preferably by using
acetonitrile. The reaction may be affected in the presence of a brominating
agent such as copper
(II) bromide, hydrobromic acid, N-bromosuccinimide, pyridine hydrobromide
perbromide,
tetrabromomethane, and the like or a mixture thereof and preferably by using
copper (10
bromide. The reaction may be affected in the presence of alkyl nitrites and
preferably by using
tert-butyl nitrite. The reaction is carried out at room temperature. The
duration of the reaction
may range from 15 minutes to 45 minutes, preferably from a period of 25
minutes to
35minutes.
In the fifth step of the above preparation, the compound of formula (10) is
coupled with
compound of formula (2) to form compound of formula (1). This reaction is
preferably carried
out in solvent such as tetrahydrofuran, toluene, ethyl acetate,
dichloromethane, triethylamine,
dimethylformamide, and the like or a mixture thereof and preferably by using
tetrahydrofuran.
The reaction may be affected in the presence of a base such as sodium hydride,
sodium
carbonate, potassium carbonate, sodium bicarbonate, sodium hydroxide, or
mixtures thereof
and preferably by using sodium hydride. The reaction may be affected in the
presence of urea
or thiourea. The reaction is carried out at temperature of 60 C to 85 C and
preferably
temperature in range of 65 C to 75 C. The duration of the reaction may range
from 12 to 18
hours, preferably from a period of 14 to 16 hours.
The compounds of formula (2), formula (5) and formula (6) may be commercially
available or can be prepared by conventional methods or by modification, using
known process.
The compounds of formula (1) can also be prepared by using Scheme III as shown
below
- 11 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
R3
I ) ___________________________________ Br + R1¨C1
H2N X
(11) (5)
R3
R2
I, __________ Br
H
R11 X 0
( 12) ( 2)
R3
R2
(I)
I I >\
R3
R1/Y(,) X
Scheme III
R5
In above Scheme III, p is 1; q is 1; r is 1; Y is C; Xis S; RI is¨N¨ C(0)-114;
and all
other symbols are as defined above.
The compound of formula (11) is coupled with compound of formula (5) to form
compound of formula (12). The compound of formula (12) is coupled with
compound of
formula (2) to form compound of formula (I).
In the first step of the above preparation, the compound of formula (11) is
coupled with
compound of formula (5) to form compound of formula (12). This reaction is
preferably carried
out in solvent such as tetrahydrofuran, toluene, ethyl acetate,
dichloromethane,
dimethylformamide, and the like or a mixture thereof and preferably by using
dicloromethane.
The reaction may be affected in the presence of a base such as triethylamine,
potassium
carbonate, diisopropylethylamine and pyridine and preferable by using
triethylamine. The
reaction is carried out at room temperature. The duration of the reaction may
range from 15
minutes to 45 minutes, preferably from a period of 25 minutes to 35minutes.
In the second step of the above preparation, the compound of formula (12) is
coupled
with compound of formula (2) to form compound formula (I). This reaction is
preferably
carried out in solvent such as tetrahydrofuran, toluene, ethyl acetate,
dichloromethane,
triethylamine, dimethylformamide, and the like or a mixture thereof and
preferably by using
dimethylformamide. The reaction may be affected in the presence of a base such
as sodium
- 12 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
hydride, sodium carbonate, potassium carbonate, sodium bicarbonate, sodium
hydroxide or
mixtures thereof and preferably by using sodium hydride. The reaction is
carried out at room
temperature. The duration of the reaction may range from 45 to 51 hours,
preferably from a
period of 47 to 49 hours.
The compounds of formula (2), formula (5) and formula (11) may be commercially
available or can be prepared by conventional methods or by modification, using
known process.
If necessary, any one or more than one of the following steps can be carried
out,
i) converting a compound of the formula (I) into another compound of the
formula (I) or
ii) forming a pharmaceutically acceptable salt.
Process (i) may be performed by further chemical modifications using well
known
reactions such as oxidation, reduction, protection, deprotection,
rearrangement reaction,
halogenation, hydroxylation, alkylation, alkylthiolation, demethylation, 0-
allcylation, 0-
acylation, N-allcylation, N-allcenylation, N-acylation, N-cyanation, N-
sulfonylation, coupling
reaction using transition metals and the like.
In process (ii) pharmaceutically acceptable salts may be prepared
conventionally by
reaction with the appropriate acid or acid derivative.
Suitable pharmaceutically acceptable salts will be apparent to those skilled
in the art
and include those described in J. Phami. Sci., 1977, 66, 1-19, such as acid
addition salts formed
with inorganic acids e. g. hydrochloric, hydrobromic, sulfuric, nitric or
phosphoric acid and
organic acids e. g. succinic, maleic, acetic, fumaric, citric, malic,
tartaric, benzoic, p-toluic, p-
toluenesulfonic, methanesulfonic or naphthalenesulfonic acid.
The pharmaceutically acceptable salts forming a part of this invention may be
prepared
by treating the compound of formula (I) with 1-6 equivalents of a base such as
sodium hydride,
sodium methoxide, sodium ethoxide, sodium hydroxide, potassium t-butoxide,
calcium
hydroxide, calcium acetate, calcium chloride, magnesium hydroxide, magnesium
chloride and
the like. Solvents such as water, acetone, ether, THF, methanol, ethanol, t-
butanol, dioxane,
isopropanol, isopropyl ether or mixtures thereof may be used.
Examples
The novel compounds of the present invention were prepared according to the
following experimental procedures, using appropriate materials and appropriate
conditions.
Preparation 1: Preparation of 2-Bromo-6,7-dihydro-4H-thiazolo15,4-cipyridine-5-
carboxylic acid tert-butyl ester
Step (i): Preparation of 3-Bromo-4-oxo-piperidine-1-carboxylic acid tert-butyl
ester.
A solution of 4-0xo-piperidin- 1 -carboxylic acid tert-butyl ester (10 grams,
50 mmol)
and aluminium chloride (0.67 grams, 5 mmol) in tetrahydrofuran (30 mL) and
diethyl ether (30
mL) was cooled to 0 C, and then treated with bromine (2.6 mL, 50 mmol) over a
period of 30
minutes. Stirred the reaction mass for 24 hours at 0 - 5 C. After completion
of reaction, the
- 13 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
obtain solids were filtered and the mother liquor was concentrated under
vacuum. The obtain
crude was triturated with diethyl ether and solids were filtered and dried
under vacuum to
obtain the title compound (10 grams).
11-1 - NMR (8 ppm): 1.51 (9H, s), 2.42 -2.48 (1H, m), 3.04 (1H, m), 3.59 -
3.74 (2H, m), 3.97
(2H, m), 4.33 (1H, m);
Mass (m/z): 278 (M+H)+, 280 (M+31-1)+.
Step (ii): Preparation of 2-Amino-6,7-dihydro-4H-thiazoloI5,4-c]pyridine-5-
carboxylic
acid tert - butyl ester
A suspension of 3-Bromo-4-oxo-piperidine- 1-carboxylic acid tert-butyl ester
(10
grams, 35 mmol, obtained in above step) and thiourea (3.28 grams, 42 mmol) in
isopropanol
(100 mL) was refluxed for 1 hour. After completion of reaction, reaction mass
was
concentrated and resulted crude was triturated with diethyl ether (50 mL),
solids were filtered
and dried under vacuum to obtain the title compound (10 grams).
11-1 - NMR (8 ppm): 1.39 (9H, s), 2.52 (2H, m), 3.56 - 3.59 (2H, t), 4.30 (2H,
s), 7.10 (2H, bs);
Mass (m/z): 256 (M+H)+.
Step (iii): Preparation of 2-Bromo-6,7-dihydro-4H-thiazolo15,4-c1pyridine-5-
carboxylic
acid tent- butyl ester
A solution of 2-A mino-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-carboxylic
acid tert-
butyl ester (10 grams, 40 mmol, obtained in above step) and copper (II)
bromide (9.6 grams, 43
mmol) in acetonitrile (50 mL) was cooled to 0 C. Tert - butyl nitrite (5.1
mL, 43 mmol) was
added drop wise over a period of 30 minutes at 0 C. Stirred the reaction mass
for 30 minutes
and the reaction mass was quenched with 6N hydrochloric acid solution. Product
was extracted
with ethyl acetate (3 x 100 mL), combined organics were washed with water
followed by brine
and dried over anhydrous sodium sulphate. Organic volatiles were evaporated
under vacuum.
The residue obtained was purified by flash chromatography (ethyl acetate / n-
hexane, 0.5 / 9.5)
to obtain the title compound (3.0 grams).
'H - NMR (8 ppm): 1.49 (9H, s), 2.85 (2H, m), 3.72 (2H, m), 4.56 (2H, s);
Mass (m/z): 319.3 (M+H)+, 321.3 (M+H)+.
Preparation 2: Preparation of (2-Bromo-4,5,6,7-tetrahydro-benzothiazol-6-y1)-
carbamic
acid tert -butyl ester
Step (i): Preparation of (3-Bromo-4-oxo-cyclohexyl)-carbamic acid tert-butyl
ester.
A solution of (4-0xo-cyclohexyl)-carbamic acid tert-butyl ester (10 grams, 46
mmol)
and aluminum chloride (0.25 grams, 2 mmol) in tetrahydrofuran (30 mL) and
diethyl ether (30
mL) was cooled to 0 C, then treated with bromine (2.4 mL, 46 mmol) over a
period of 30
minutes. The reaction mass was stirred for 24 hours at 0 - 5 C. After
completion of reaction,
-14-
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
the obtained solids were filtered and lower filtrate was concentrated under
vacuum. The
obtained crude was triturated with diethyl ether and the resulted solids were
filtered and dried
under vacuum to obtain the title compound (9.0 grams).
Mass (m/z): 292.3 (M+H)+, 294.3 (M+3H)+.
Step (ii): Preparation of 2-Amino-4,5,6,7-tetrahydro-benzothiazol-6-y1)-
carbamic acid
tert-butyl ester
A suspension of 3-Bromo-4-oxo-piperidine-1 -carboxylic acid tert-butyl ester
(9 grams,
31 mmol, obtained in above step) and thiourea (2.4 grams, 31 mmol) in
isopropanol (100 mL)
was refluxed for 1 hour. After completion of reaction, reaction mass was
concentrated and
resulted crude was triturated with diethyl ether (50 mL), solids were filtered
and dried under
vacuum to obtain the title compound (9 grams).
IF1 - NMR ((5 ppm): 1.38 (9H, s), 1.61 - 1.71 (1H, m), 1.84- 1.86 (1H, m),
2.29 - 2.35 (1H, m),
2.53 - 2.57 (2H, m), 2.71 - 2.76 (1H, m), 3.71 - 3.76 (1H, m), 9.11 (2H, s),
12.86 - 12.93 (1H,
bs);
Mass (rn/z): 2703 (M+H)+.
Step (iii): Preparation of (2-Bromo-4,5,6,7-tetrahydro-benzothiazol-6-y1)-
carbamic acid
tert-butyl ester
A solution of 2-Amino-4,5,6,7-tetrahydro-benzothiazol-6-y1)-carbamic acid tert-
butyl
ester (9 grams, 33 mmol, obtained in above step) and coper (II) bromide (8.3
grams, 37 mmol)
in acetonitrile (70 mL) was cooled to 0 C. The resulting mass was treated
with tert - butyl
nitrite (4.5 mL, 37 mmol) over a period of 30 minutes at 0 C. The reaction
mass was stirred for
minutes and quenched with 6N hydrochloric acid solution. Product was extracted
with ethyl
acetate (3 x 100 mL), combined organics were washed with water followed by
brine and dried
over anhydrous sodium sulphate. Organic volatiles were evaporated under
vacuum. The
25 residue was purified by flash chromatography (ethylacetate / n-hexane,
0.5 / 9.5) to obtain the
title compound (23 grams).
'H - NMR ((5 ppm): 1.45 (9H, s), 1.89- 1.93 (1H, m), 2.03 -2.07 (1H, m), 2.59 -
2.63 (1H, m),
2.85 - 2.91 (1H, m),3.09 -3.13 (1H, m), 4.05- 4.09 (1H, m), 4.63- 4.66 (1H,
m), 12.36 - 12.42
(1H, bs);
30 Mass (m/z): 333.1 (M+H)+, 335.3 (M+3H)+.
Example 1: Preparation of 1-12-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-
thiazolo15,4 - clpyridin-5-yll-propan-l-one tartrate
Step (i): Preparation of 2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-
thiazolo15,4-
clpyridine- 5-carboxylic acid tert-butyl ester
1-Cyclobutyl-piperidin-4-ol (1.6 grams, 10 mmol) in tetrahydrofuran (20 mL)
was
treated with cooled and stirred suspension of sodium hydride (0.9 grams, 18
mmol) in
tetrahydrofuran (20 mL) slowly over a period of 30 minutes; the reaction
mixture was stirred
- 15-
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
for 1 hour. A solution of 2-Bromo-6,7-dihydro-4H-thiazolo[5,4-c]pyridine-5-
carboxylic acid
tert-butyl ester (3 grams, 9 mmol, obtained in preparation 1) in
tetrahydrofuran (30 mL) was
added drop wise over a period of 15 minutes and refluxed the reaction for 6
hours. Reaction
mass was quenched with ice cold water and the product was extracted with ethyl
acetate (3 x 50
mL). Combined organics were washed with water followed by brine and dried over
anhydrous
sodium sulphate. Organic volatiles were evaporated under vacuum. The residue
was purified by
flash chromatography (ethylacetate / n-hexane, 1 / 1) to obtain the title
compound (2.0 grams).
IF1 - NMR (8 ppm): 1.48 (9H, s), 1.65 - 1.72 (2H, m), 1.85 - 1.92 (4H, m),
2.01 -2.07 (4H, m),
2.18 - 2.19 (2H, m), 2.57 (2H, m), 2.62 - 2.66 (2H, m), 2.71 -2.75 (1H, m),
3.70 (2H, m), 4.43
(21-1, m), 4.93 (I H, m);
Mass (m/z): 394.2 (WHY..
Step (ii): Preparation of 2-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,6,7-
tetrahydro-
thiazolo[5,4-0 pyridine
A solution of 2-(1-Cyclobutyl-piperidin-4-yloxy)-6,7- dihydro-4H-thiazolo[5,4-
c]pyridine-5-carboxylic acid tert-butyl ester (2.0 grams, 5 mmol, obtained in
above step) in
dichloromethane (30 mL) was treated with trifluroaceticacid (5.0 mL, 50 mmol),
at 0 C.
Reaction mass was stirred for 4 hours. After completion of reaction, the
reaction mass was
quenched into ice cold water and adjust pH to 10, by using 40% aqueous sodium
hydroxide
= solution. The product was extracted with dichloromethane (3 x 50 mL),
combined organics
were washed with water followed by brine and dried over anhydrous sodium
sulphate. Organic
volatiles were evaporated under vacuum to obtain the title compound (1.3
grams).
'H - NMR (8 ppm): 1.68- 1.74 (2H, m), 1.85- 1.93 (4H, m), 2.06(4H, m), 2.19
(2H, m), 2.60
- 2.61 (4H, m), 2.73 -2.80 (1H, m), 2.90 - 3.10 (1H, m), 3.13 -3.16 (2H, m),
3.85 (2H, s), 4.90
-4.93 (1H, m);
Mass (m/z): 294.2 (M+H)+.
Step (iii): Preparation of 1-12-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-
4H-
thiazolo15,4- cIpyridin-5-y11-propan-1-one
A solution of 2-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,6,7-tetrahydro-thiazolo
[5,4-
c]pyridine (1.3 grams, 4 mmol, obtained in above step) and triethylamine (1.9
mL, 13 mmol) in
dichloromethane (30 mL) was cooled to 0 C. Propionylchloride (0.4 mL, 5 mmol)
in
dichloromethane (5 mL) was added drop wise over a period of 15 minutes and
stirred the
reaction for 30 minutes. Reaction mass was poured onto ice cold water and the
product was
extracted with ethyl acetate (3 x 50 mL). Combined organics were washed with
water followed
by brine and dried over anhydrous sodium sulphate. Organic volatiles were
evaporated under
vacuum. The residue was purified by flash chromatography (methanol /
chloroform, 2 / 98) to
obtain the title compound (1.0 gram).
- 16 -
CA 02812970 2014-04-14
1H - NMR (8 ppm): 1.17 - 1.21 (3H, m), 1.65 - 1.72 (5H, m), 1.87 - 1.91 (4H,
m), 2.01 - 2.07
(4H, m), 2.22 (1H, m), 2.38 - 2.45 (2H, m), 2.45 (1H, m), 2.68 - 2.76 (3H, m),
3.72 - 3.74 (1H,
m), 4.47 - 4.62 (2H, m), 4.92 - 4.94 (1H, m).
Mass (m/z): 350.4 (M+H)+.
Step (iv): Preparation of 1-p-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-
thiazolo[5,4- clpyridin-5-y1]-propan-1-one tartrate
A solution of 1-[2-(1 -Cyclobutyl-p iperi din-4-yloxy)-6,7-dihydro-
4H-thiazo lo [5,4-
c]pyridin-5-yli-propan- 1-one (0.8 grams, 2.3 mmol, obtained in above step) in
methanol (10
mL) was treated with L(+)-Tartaric acid (0.34 grams, 2.3 mmol) at 0 C.
Stirred the reaction
mass for about 1 hour and the solvent was evaporated under vacuum to dryness.
The solids
were washed with diethyl ether and dried under vacuum to obtain the title
compound (1.1
grams).
11-1 - NMR (8 ppm): 1.12 - 1.20 (3H, m), 1.82 - 1.87 (2H, m), 2.16 - 2.32 (7H,
m), 2.45 - 2.55
(2H, m), 2.63 - 2.66 (3H, m), 2.72 (1H, m), 3.20 (2H, m), 3.47 - 3.50 (1H, m),
3.66 - 3.70 (1H,
m), 3.81 -3.88 (2H, m), 4.45 (2H, s), 4.60 (2H, s), 5.18 (5H, m);
Mass (m/z): 350.4 (M+H)+.
Example 2: Preparation of 12-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,7,8-
tetrahydro-
thiazolo[5,4-d]azepin- 6-yll-cyclopropyl-methanone tartrate
Step (i): Preparation of 1-Cyclopropanecarbonyl-azepan-4-one
A solution of Azepan-4-one (1.75 grams, 15.3 mmol) and triethylamine (6.45 ml,
3.1
mmol) in dichloromethane (15 mL) was cooled to 0 C. Cyclopropionylchloride
(0.17 mL, 1.8
mmol) in dichloromethane (2 mL) was added and stirred the reaction for 30
minutes. Reaction
mass was poured onto ice cold water and the product was extracted with
dichloromethane (3
x15 mL). The combined organics were washed with water followed by brine and
dried over
anhydrous sodium sulphate. Organic volatiles were evaporated under vacuum to
obtain the title
compound (2.7 grams).
11-1 - NMR (8 ppm): 0.66 - 0.69 (4H, m), 1.56 - 1.57 (111, m), 1.71 - 1.75
(1H, m), 1.87 - 1.89
(1H, m), 2.45 - 2.49 (1H, m), 2.59 - 2.62 (1H, m), 3.57 - 3.91 (4H, m);
Mass (m/z): 182 (M+H)+.
Step (ii): Preparation of 5-Bromo-1-cyclopropanecarbonyl-azepan-4-one.
A solution of 1-Cyclopropanecarbonyl-azepan-4-one (2.7grams, 14.9 mmol,
obtained
in above step) in acetic acid (30 mL) was cooled to 10 C and treated with
bromine (0.71 mL,
14.9 mmol) over a period of 15 minutes. The resulting slurry was stirred for
18 hours under
nitrogen atmosphere. After completion of reaction, mass was concentrated to
dryness to obtain
the title compound (3.87 grams).
Mass (m/z): 260, 262 (M+H)+.
- 17 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
Step (iii): Preparation of (2-Amino-4,5,7,8-tetrahydro-thiazolo15,4-djazepin-6-
yI)-
cyclopropyl- methanone
A suspension of 5-Bromo-1-cyclopropanecarbonyl-azepan-4-one (3.87 grams, 14.8
mmol, obtained in above step) and thiourea (1.13 grams, 14.8 mmol) in
isopropanol (40 mL)
was refluxed for 6 hours. After completion of reaction, reaction mass was
concentrated and the
residue obtained was purified by flash chromatography (methanol / chloroform,
3 / 97) to
obtain the title compound (0.4 grams).
'H - NMR (5 ppm): 0.66 - 0.73 (4H, m), 1.95 (1H, m), 2.66 - 2.69 (2H, m), 2.75
- 2.76 (2H, m),
3.55 - 3.62 (2H, m), 3.79- 3.86 (2H, m), 6.61 (2H, bs);
Mass (m/z): 238 (M+H)+.
Step (iv): Preparation of (2-Bromo-4,5,7,8-tetrahydro-thiazoloI5,4-d]azepin-6-
y1)-
cyclopropyl- methanone
A solution of (2-Amino-4,5,7,8-tetrahydro-thiazolo[5,4-d]azepin-6-y1)-
cyclopropyl-
methanone (0.4 grams, 1.68 mmol, obtained in above step) and copper (II)
bromide (0.37
grams, 1.68 mmol) in acetonitrile (40 mL) was cooled to 0 C. Tertiary butyl
nitrite (0.2 mL,
1.68 mmol) was added drop wise over a period of 10 minutes at 0 C. The
reaction mass was
stirred for 30 minutes and the reaction mass was quenched with 3N hydrochloric
acid solution.
The product was extracted with ethyl acetate (3 x 15 mL) and the combined
organics were
washed with water followed by brine and dried over sodium sulphate. Organic
volatiles were
evaporated under vacuum. The residue, thus obtained, was purified by flash
chromatography
(ethylacetate / n-hexane, 7 / 3) to obtain the title compound (0.053 grams).
'H - NMR (5 ppm): 0.71 - 0.75 (4H, m), 1.97 (1H, m), 2.86- 2.92 (2H, m), 2.99 -
3.08 (2H, m),
3.61 - 3.86 (2H, m), 3.86 - 3.90 (2H, m);
Mass (m/z): 301 (M+H)+.
Step (v): Preparation of 12-(4-Cyclobutylpiperidin-4y1-oxy)-4,5,7,8-tetrahydro-
thiazolo15,4-dJazepin-6-yll-cyclopropyl-methanone
1-Cyclobutyl-piperidin-4-ol (0.04 grams, 0.26 mmol) in tetrahydrofuran (3 mL)
was
treated with cooled and stirred suspension of sodium hydride (0.021 grams,
0.51 nunol) in
tetrahydrofuran (8 mL) slowly over the period of 5 minutes and the reaction
mixture was stirred
for 2 hours at room temperature. A solution of (2-Bromo-4,5,7,8-tetrahydro-
thiazolo[5,4-
d]azepin-6-y1)-cyclopropyl-methanone (0.053 grams, 0.17 mmol, obtained in
above step) in
tetrahydrofuran (3 mL ) was added drop wise over a period of 5 minutes and
refluxed for 15
hours. Reaction mass was quenched onto ice cold water and the product was
extracted with
ethyl acetate (3 x 10 mL). The combined organics were washed with water
followed by brine
and dried over anhydrous sodium sulphate. Organic volatiles were evaporated
under vacuum.
The residue obtained was purified by flash chromatography (methanol /
chloroform 3 / 97) to
obtain the title compound (0.05 grams).
- 18-
CA 02812970 2014-04-14
- NMR (8 ppm): 0.84 - 0.86 (4H, m), 0.91 (1H, m), 1.73 - 1.76 (2H, m), 1.86 -
1.93 (4H,
m), 2.07 - 2.08 (5H, m), 2.63 (2H, m), 2.80 - 2.84 (2H, m), 2.93 - 2.99 (3H,
m), 3.74 - 3.79 (2H,
m), 3.94 -4.00 (2H, m).
Mass (m/z): 376.4 (M+H)+.
Step (vi): Preparation of 12-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,7,8-
tetrahydro-
thiazolo [5,4-d] azepin-6-yll -cyclop ropy l-metha none tartrate
A solution of [2-(4-Cyclobutyl-piperidin-4-yloxy)-4,5,7,8-tetrahydro-
thiazolo[5,4-
d]azepin-6-y1]-cyclopropyl-methanone (0.078 grams, 0.208 mmol, obtained in
above step) in
methanol (5 mL) was treated with L(+)-Tartaric acid (0.031 grams, 0.208 mmol)
at 0 C.
Stirred the reaction mass for about 1 hour and the solvent was evaporated
under vacuum to
dryness. The solids were washed with diethyl ether and dried under vacuum to
obtain the title
compound (0.1 gram).
IFI - NMR (8 ppm): 0.84 - 0.99 (4H, m), 1.83 - 1.90 (2H, m), 2.00 - 2.02 (1H,
m), 2.18 - 2.23
(5H, m), 2.32 -2.41 (2H, m), 2.82 -2.87 (2H, m), 2.95 -2.99 (2H, m), 3.13 -
3.20 (5H, m), 3.62
- 3.66 (1H, m), 3.74 - 3.79 (2H, m), 3.95 - 3.99 (2H, m), 4.43 (2H, s), 5.13
(1H, s).
Mass (m/z): 376.4 (M+H)+.
Example 3: Preparation of N-12-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,6,7-
tetrahydro-
benzothiazol-6 -yll-propionamide
Step (i): Preparation of 2-Bromo-4,5,6,7-tetrahydro-benzothiazol-6-ylamine
A solution of (2-Bromo-4,5,6,7-tetrahydro-benzothiazol-6-y1)-carbamic acid
tert-butyl
ester (0.50 grams, 1.5 mmol, obtained in preparation 2) in dichloromethane (30
mL) was
treated with trifluroaceticacid (1.1 mL, 15 mmol) at 0 C. Reaction mass was
stirred for 4
hours. After completion of the reaction, the mass was quenched with ice cold
water and
adjusted pH to 10, by using 40% aqueous sodium hydroxide solution. The product
was
extracted with dichloromethane (3 x 50 mL) and the combined organics were
washed with
water followed by brine and dried over anhydrous sodium sulphate. The organic
volatiles were
evaporated under vacuum to obtain the title compound (0.36 grams).
Mass (m/z): 233.0 (M+H)+, 235.0 (M+3H) .
Step (ii): Preparation of N-(2-Bromo-4,5,6,7-tetrahydro-benzothiazol-6-yl)-
propionamide
A solution of 2-Bromo-4,5,6,7-tetrahydro-benzothiazol-6-ylamine (0.36 grams,
1.5
mmol, obtained in above step) and triethylamine (0.43 mL, mmol) in
dichloromethane (15 mL)
was cooled to 0 C. Propionylchloride (0.17 mL, 1.8 mmol) in dichloromethane
(2 mL) were
added and stirred the reaction mass for 30 minutes. After completion of
reaction, the mass was
poured onto ice cold water and the product was extracted with ethyl acetate (3
x 15 mL).
Thecombined organics were washed with water followed by brine, dried over
anhydrous
sodium sulphate and the organic volatiles were evaporated under vacuum. The
residue was
- 19 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
purified by flash chromatography (methanol / chloroform, 2 / 98) to obtain the
title compound
(0.4 grams).
IF1 - NMR (5 ppm): 0.81 -0.85 (1H, m), 1.14 - 1.18 (3H, m), 1.20- 1.28 (2H,
m), 1.90 - 1.96
(1H, m), 2.02 -2.05 (1H, m), 2.18 -2.23 (21-1, m), 2.57 - 2.63 (1H, m), 2.86-
2.91 (1H, m), 3.10
- 3.15 (1H, m);
Mass (m/z): 289.2 (M+H)+, 291.2 (M+31-1)+.
Step (iii): Preparation of N-12-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,6,7-
tetrahydro-
benzothiazol-6 - yll-propionamide
1-Cyclobutyl-piperidin-4-ol (0.25 grams, 1.6 =not) in N,N-dimethyl formamide
(5
mL) was treated with cooled and stirred suspension of sodium hydride (0.1
grams, 2.08 mmol)
in N,N-dimethyl formamide (10 mL) slowly over the period of 30 minutes and the
reaction
mixture was further stirred for 1 hour. A solution of N-2-Bromo-4,5,6,7-
tetrahydro-
benzothiazol-6-y1)-propionamide (0.4 grams, 1.3 rnmol, obtained in above step)
in N,N-
dimethyl formamide (5 rnL ) was added drop wise over a period of 10 minutes
and stirred the
resulting mass for 48 hours. After completion of reaction, the mass was
quenched onto ice cold
water and the product was extracted with ethyl acetate (3 x 15 mL). The
combined organics
were washed with water followed by brine, dried over anhydrous sodium
sulphate, and the
organic volatiles were evaporated under vacuum. The residue was purified by
flash
chromatography (ethylacetate / n-hexane, 1 / 1) to obtain the title compound
(0.068 grams).
'H - NMR (5 ppm): 0.81 -0.88 (2H, m), 1.14 - 1.,18 (3H, m), 1.65 - 1.70 (2H,
m), 1.89 - 1.91
(5H, m), 2.03 -2.05 (5H, m), 2.17 -2.23 (3H, m), 2.49 - 2.75 (4H, m), 2.96-
3.01 (2H, m), 4.35
-4.39 (1H, m), 4.90- 3.96 (1H, m), 5.50- 5.53 (1H, m);
Mass (m/z): 364.3 (M+1-1)+.
Examples 4 - 34:
The compounds of Examples 4 - 34 were prepared by following the procedures as
described in Examples 1 to 3, with some non-critical variations
4. [2-(1-Cyclobutyl-piperidin- 'H - NMR (5 ppm): 0.86 - 0.90 (5H, m), 1.27-
1.29 (2H, m), 1.82 -4-yloxy)-6,7-dihydro-41-1- 1.90 (2H, m), 2.01 - 2.11
(1H, m), 2.25 - 2.31 (7H, m), 2.64 (1H, m),
thiazolo[5,4-c]pyridin-5-y1[- 2.77 (1H, m), 3.20 (3H, bs), 3.65 - 3.69 (1H,
m), 3.88 (1H, m), 4.04
cyclopropyl-methanone - 4.06 (1H, m), 4.44 (2H, m), 4.60 (1H, s),
5.19 (1H, m);
tartrate Mass (m/z): 362.2 (M+1-ff.
5. Cyclobutyl-[2-(1- NMR (5 ppm): 1.27 - 1.38 (2H, m), 1.81 - 1.88 (3H,
m), 2.00 -
cyclobutyl-piperidin-4- 2.10 (1H, m), 2.20 - 2.33 (11H, m), 2.68 (1H,
m), 3.19 (3H, m), 3.33
yloxy)-6,7-dihydro-4H- -3.36 (1H, m), 3.52 (1H, m),'3.67 -3.73 (2H,
m),3.86 (1H, in), 4.44
thiazolo[5,4-clpyridin-5- (2H, s), 4.47 (1H, s), 4.58 (1H, s), 5.18 (1H,
m);
yn-methanone tartrate Mass (m/z): 376.3 (M+H)+.
- 20 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
6. [2-(1-Cyclobutyl-piperidin- 11-1 - NMR (8 ppm): 1.83 - 1.91 (2H, m),
2.16 (2H, s), 2.22 - 2.25
4-yloxy)-6,7-dihydro-4H- (5H, m), 2.33 (3H, m), 2.78 (1H, m), 3.13 -3.20 (4H,
m), 3.65 -3.70
thiazolo[5,4-c]pyridin-5- (2H, m), 4.08 (11-1, m), 4.43 - 4.45 (3H, m),
5.19 (1H, bs), 7.23 -
y1]-(2-fluoro-phenyl)- 7.34 (2H, m), 7.40- 7.43 (1H, m), 7.53 (1H, m);
methanone tartrate Mass (rn/z): 416.3 (M+H)+.
7. 1-[2-(1-Cyclobutyl- 'H - NMR (5 ppm): 0.94 - 1.00 (7H, m), 1.85 -
1.91 (2H, m), 2.05 -
piperidin-4-yloxy)-6,7- 2.16 (7H, m), 2.21 - 2.38 (4H, m), 2.64 - 2.72
(2H, m), 3.18 (4H,
dihydro-4H-thiazolo[5,4- m), 3.63 - 3.67 (1H, m), 3.83 - 3.90 (2H, m), 4.44
(2H, 4.61 (1H,
c]pyridin-5-yI]-3-methyl- s), 5.18 (1H, m);
butan-1 -one tartrate Mass (m/z): 378.2 (M-41)+.
8. Cyclobutyl-[2-(1-isopropyl- 11-1 - NMR (5 ppm): 1.43 (7H, m), 1.87 -
1.89 (I H, m), 1.97 - 2.04
piperidin-4-yloxy)-6,7- (I H, m), 2.19 - 2.24 (2H, m), 2.33 - 2.41 (4H,
m), 2.62 (2H, bs),
dihydro-4H-thiazolo[5,4- 2.76 (1H, m), 3.07 (2H, m), 3.25 -3.26 (3H, m),
3.43 -3.50 (1H, m),
c]pyridin-5-y11-methanone 3.62 - 3.65 (3H, m), 4.61 (1H, s), 5.23 (1H, m);
Mass (rn/z): 364.2 (M+H)+.
9. Cyclopropyl-[2-(1- 114 - NMR (5 ppm): 0.86 -0.90 (4H, m), 1.27 - 1.39
(7H, m), 1.98 -
isopropyl-piperidin-4- 2.08 (2H, m), 2.30 (3H, m), 2.64 - 2.77 (2H, m),
3.13 (2H, m), 3.48
yloxy)76,7-dihydro-4H- (2H, m), 3.52 - 3.57 (1H, m), 3.88 (1H, m), 4.05
(1H, m), 4.45 (2H,
thiazolo[5,4-c]pyridin-5- s), 4.68 (1H, s), 5.19(111, m);
yll-methanone tartrate Mass (tn/z): 350.4 (M+H)+.
10. Cyclopropyl-[2-(1- 'H - NMR (5 ppm): 0.79 - 0.91 (8H, m), 1.99 -
2.18 (5H, m), 2.39
cyclopropyl-piperidin-4- (1H, bs), 2.64 -2.77 (3H, m), 3.12 (2H, bs), 3.28
(2H, s), 3.88 (1H,
yloxy)-6,7-dihydro-4H- bs), 4.05 (1H, m), 4.45 (2H, s), 4.60 (1H, s),
5.08 (1H, bs);
thiazolo[5,4-c]pyridin-5- Mass (m/z): 348.2 (M4-1-1)+.
yI]-methanone tartrate
11. Cyclobutyl-[2-(1- 'H - NMR (5 ppm): 0.76 -0.77 (3H, m), 0.87 -0.92
(2H, m), 1.80 -
cyclopropyl-piperidin-4- 1.87 (2H, m), 2.02 - 2.03 (3H, m), 2.21 - 2.33
(6H, m), 2.63 - 2.67
yloxy)-6,7-dihydro-4H- (2H, m), 3.05 (1H, m), 3.21 -3.23 (2H, m), 3.43 -
3.52 (2H, m), 3.71
tliiazolo[5,4-c]pyridin-5- -3.73 (1H, m), 3.85 -3.88 (1H, m), 4.43 (211,
s), 4.58 (111, s), 5.05
yI]-methanone tartrate (1H, m);
Mass (m/z): 362.2 (M+H)+.
- 21 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
12. 1-[2(1-Cyclobutyl- 11-1 - NMR (5 ppm): 1.27 - 1.31 (IH, m), 1.81 -
1.89 (2H, m), 2.21 -
piperidin-4-yloxy)-6,7- 2.32 (8H, m), 2.52 -2.56 (4H, m), 2.65 (1H, m),
2.78 (1H, m), 3.13 -
dihydro-4H-thiazolo[5,4- 3.20 (4H, m), 3.39 (1H, s), 3.60 - 3.64 (2H, m),
3.70 - 3.72 (3H, m),
c]pyridin-5-y1]-2- 3.87 - 3.90 (2H, m), 4.41 (2H, s), 4.60 (1H, s), 4.71
(1H, s), 5.17
morpholin-4-yl-ethanone (1H, bs);
tartrate Mass (m/z): 421.4 (M+H)+.
13. [4-(5-Cyclobutyl-,6,7- 114 - NMR (6 ppm): 0.80 - 0.88 (4H, m), 1.27 -
1.29 (2H, d), L38
dihydro-4H-thiazolo[5,4- (IH, s), 1.85 - 1.90 (3H, m), 1.99 (2H, m), 2.17 -
2.20 (3H, m), 2.31
c]pyridin-2-yloxy)- (3H, m), 2.89 - 2.90 (2H, m), 3.24 - 3.28 (3H, m), 3.35
(1H, s), 3.62
piperidin-1-y1]- -3.64 (2H, m), 4.01 (2H, s), 5.16 - 5.19 (IH, m);
cyclopropyl-methanone Mass (m/z): 362.3 (M+14)+.
tartrate
14. [3-(5-Cyclobutyl-,6,7- 'H - NMR (6 ppm): 0.60 - 0.79 (1H, m), 0.81 -
0.91 (4H, m), 1.16 -
dihydro-4H-thiazolo[5,4- 1.29 (1H, m), 1.56 (IH, m), 1.78 (1H, m), 1.89 -
2.06 (4H, m), 2.29
c]pyridin-2-yloxy)- (2H, m), 2.41 - 2.43 (2H, m), 2.98 (2H, m), 3.13 (IH,
m), 3.60 (IH,
piperidin-1-yI]- m), 3.70 (IH, m), 3.90 - 3.91 (2H, m), 4.09 (2H,
m), 4.35 - 4.50 (1H,
cyclopropyl-methanone m), 4.54 (21-1, s), 4.95 - 4.96 (IH, m);
tartrate Mass (m/z): 362.3 (M+H)+.
15. [2-(1-Cyclobutyl-piperidin- 'H - NMR (5 ppm): 0.81 -0.91 (4H, m), 1.80 -
1.87 (3H, m), 1.98 -3-yloxy)-6,7-dihydro-4H- 2.10 (5H, m), 2.24 - 2.28
(3H, m), 2.61 (1H, m), 2.78 (1H, m), 2.80
thiazolo[5,4-c]pyridin-5- (1H, m), 3.20- 3.21 (2H, m), 3.53 (1H, m), 3.88
(1H, bs), 4.05 (IH,
yll-cyclopropyl-methanone m), 4.43 (2H, s), 4.61 (1H, s), 4.84 - 4.85 (2H, m),
5.27 (1H, bs);
tartrate Mass (m/z): 362.3 (M+H)+.
16. [2-(1-Cyclobutyl-piperidin- 'H - NMR (5 ppm): 1.79 - 1.88 (2H, m), 2.30
- 2.32 (8H, m), 2.71
4-yloxy)-6,7-dihydro-4H- (IH, m), 2.79 (1H, s), 3.18- 3.23 (4H, m), 3.62 -
3.67 (2H, m), 3.67
thiazolo[5,4-c]pyridin-5- (1H, m), 4.44 (3H, s), 4.46 (IH, s), 5.20 (1H,
bs), 7.48 - 7.51 (2H,
yI]-pyridin-4-yl-methanone m), 8.69 (2H, m);
tartrate Mass (m/z): 399.6 (M+H)+.
17. [2-(1-Cyclobutyl-piperidin- 'H - NMR (5 ppm): 1.27- 1.29 (IH, m), 1.81 -
1.90 (2H, m), 2.24 -4-yloxy)-6,7-dihydro-4H- 2.31 (8H, m), 2.74 (2H, m),
3.13 - 3.17 (3H, m), 3.62 - 3.66 (IH, m),
thiazolo[5,4-c]pyridin-5- 3.82 - 3.83 (1H, m), 3.85 (3H, s), 3.90 - 4.10
(1H, bs), 4.43 (2H, s),
yI]-(4-methoxy-phenyl)- 4.67 (2H, m), 5.18 (IH, m), 7.01 - 7.03 (2H, d),
7.43 - 7.45 (2H, d);
methanone tartrate Mass (m/z): 428.3 (M+H)+.
- 22 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
18. 1-[2-(1-Cyclobutyl- 114 - NMR (8 ppm): 1.66(2H, bs), 1.83 - 1.87
(6H, m), 2.16 (2H, m),
piperidin-4-yloxy)-6,7- 2.22 - 2.26 (5H, m), 2.68 - 2.78 (3H, m), .3.09 -
3.16 (8H, m), 3.49 -
dihydro-4H-thiazolo[5,4- 3.51 (1H, m), 3.75 (IH, m), 3.91 (1H, m), 4.08 -
4.14 (2H, d), 4.40
c]pyridin-5-yI]-2-piperidin- (2H, s), 4.55 (1H, s), 4.65 (1H, s), 5.49 (1H,
s);
I-yl-ethanone tartrate Mass (m/z): 419.5 (M+H)+.
19. 1-[2-(1-Cyclobutyl- - NMR (8 ppm): 0.17 - 0.21 (2H, m), 0.57 - 0.60
(2H, m), 1.09
piperidin-4-yloxy)-6,7- (1H, m), 1.26 - 1.29 (2H, m), 1.63 - 1.72 (4H, m),
1.85 - 1.90 (4H,
dihydro-4H-thiazolo[5,4- m), 2.03 - 2.05 (4H, m), 2.20 (2H, bs), 2.32 -
2.38 (2H, m), 2.57
clpyridin-5-y1]-2- (2H, bs), 2.71 - 2.73 (2H, m), 3.72 - 3.75 (IH, m),
4.92 (1H, m);
cyclopropyl-ethanone Mass (m/z): 376.3 (M+H)+.
20. 2-0 -Cyclobutyl-piperidin- 'H - NMR (8 ppm): 1.83 - 1.91 (2H, m), 2.21 -
2.25 (6H, m), 2.30 -4-yloxy)-5-(2-fluoro- 2.34 (2H, m), 2.60 -2.62 (2H, m),
3.31 -3.18 (3H, m), 3.64 - 3.69
benzenesulfony1)-,6,7- (3H, m), 4.40 (2H, s), 4.45 (3H, s), 5.1 (1H, m),
7.27- 7.31 (1H, m),
dihydro-4H-thiazolo[5,4- 7.35 - 7.39 (1H, m), 7.65 - 7.68 (1H, m), 7.90 -
7.94 (1H, m);
c]pyridine tartrate Mass (m/z): 452.2 (M+H)+.
21. 2-(I -Cyclobutyl-piperidin- 'H - NMR (8 ppm): 1.42 (1H, s), 1.66- 1.73
(2H, m), 1.88 (4H, m),
4-yloxy)-5- 2.04 - 2.05 (4H, m), 2.20 (2H, m), 2.59 (1H, m),
2.74 - 2.79 (3H,
methanesulfonyl-,6,7- m), 2.86 (3H, s), 3.64 - 3.67 (2H, t), 4.38 (2H,
s), 4.92 - 4.97(1H,
dihydro-4H-thiazolo[5,4- m);
clpyridine Mass (m/z): 372.3 (M+H)+.
22. I -[2-(1-Cyclobutyl- 'H - NMR (8 ppm): 1.08- 1.10 (6H, m), 1.13 -
1.18 (4H, m), 1.81 -
piperidin-4-yloxy)-6,7- 1.89 (21-1, m), 2.20 - 2.29 (7H, m), 2.64 - 2.66
(1H, m), 2.73 (1H,
dihydro-4H-thiazolo[5,4- m), 3.13 (3H, m), 3.35 (1H, s), 3.58 - 3.62 (1H,
t), 3.87 - 3.89 (1H,
cipyridin-5-y1]-2-methyl- m), 4.41 (2H, s), 4.60 (1H, s), 4.66 (1H, s);
propan- 1 -one tartrate Mass (m/z): 364.2 (M+H)+.
23. 2-(I-Cyclobutyl-piperidin- 111 - NMR (8 ppm): 1.56 - 1.77 (7H, m), 1.95
- 1.98 (4H, s), 2.04
4-yloxy)-5-(2- (2H, m), 2.48 - 2.49 (1H, m), 2.68 - 2.69 (3H, m),
3.79 - 3.82 (2H,
trifluoromethyl-pyridin-5- m), 4.49 (2H, s), 4.82 - 4.84 (1H, m), 7.49 -
7.52 (1H,.dd), 7.63 -
y1)-,6,7-dihydro-4H- 7.65 (11-1, d),8.48 - 8.49 (1H, d);
thiazolo[5,4-c]pyridine Mass (m/z): 439.2 (M+H)+.
24. Cyclopropyl-[2-(1-isobutyl- 'H - NMR (8 ppm): 0.86 - 0.90 (4H, m), 1.05-
1.07 (6H, d), 1.29
piperidin-4-yloxy)-6,7- (1H, m), 1.90 (1H, m), 2.10 (11-1, m), 2.30 (3H,
m), 2.77 (2H, m),
dihydro-4H-thiazolo[5,4- 2.99 - 3.01 (2H, d), 3.10 (1H, m), 3.48 (2H, m),
3.88 - 4.07 (2H, m),
clpyridin-5-y1]-methanone 4.46 (2H, s), 4.60 (1H, s), 4.84 -4.86 (2H, m), 5.18
(1H, m);
tartrate Mass (m/z): 363.5 (M+H)+.
- 23 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
25. [2-(1-Cyclobutyl-piperidin- 'H - NMR (5 ppm): 1.62 - 1.67 (4H, m), 1.69
- 1.72 (31-L, m), 1.93
4-yloxy)-6,7-dihydro-4H- (4H, m), 2.07 (3H, m), 2.28 (1H, m), 2.61 - 2.62 (1H,
m),2.74 - 2.84
thiazolo[5,4-c]pyridin-5- (211, m), 3.50 (1H, s), 3.68 (1H, m), 4.79 (1H,
bs), 4.98 (1H, m),
y1]-(2-trifluoromethyl- 7.79- 7.81 (1H, d), 7.98 - 8.00 (1H, d), 8.82 (1H,
bs);
PYridin-5-y1)-methanone Mass (m/z): 467.3 (M+H)+.
26. [2-(1-Cyclobutyl-piperidin- 'H - NMR (5 ppm): 1.79 - 1.88 (2H, m), 2.16-
2.20 (2H, m), 2.26 -4-yloxy)-6,7-dihydro-4H- 2.31 (4H, m), 2.74 - 2.79
(2H, m), 3.13 - 3.19 (4H, m), 3.35 (2H, s),
thiazolo[5,4-c]pyridin-5- 3.62 - 3.68 (I H, m), 3.72 (1H, bs), 4.07 (1H,
m), 4.43 (2H, s), 4.55
yI]-pyridin-3-yl-methanone (1H, bs), 4.78 (1H, s), 5.19 (1H, bs), 7.56 (1H,
m), 7.95 - 7.97 (1H,
tartrate d), 8.67 (2H, s);
Mass (m/z): 398.5 (M+H)+.
27. 2-(I-Cyclobutyl-piperidin- 'H - NMR (5 ppm):
1.83 - 1.88 (2H, m), 2.21 - 2.32 (7H, m), 2.65 -4-yloxy)-5-pyridin-3-yl-
2.75 (2H, m), 3.13 - 3.25 (5H, m), 3.60 - 3.62 (2H, m), 3.73 - 3.76
,6,7-dihydro-4H- (2H, t), 4.38 (2H, s), 7.29 - 7.32 (1H, dd , J=
4.62, 8.42 Hz), 7.48 -
thiazolo[5,4-c]pyridine 7.50(1H, d, J= 8.07 Hz), 7.97 - 7.98 (1H, d , J=
4.21 Hz), 8.29 (1H,
tartrate s);
Mass (m/z): 371.3 (M+H)+.
28. [2-(I-Cyclobutyl-piperidin- - NMR (5 ppm): 1.13 - 1.19 (I H, t),
1.22 (1H, s), 1.54- 1.74 (9H,
4-yloxy)-6,7-dihydro-4H- m), 1.88 (1H, s), 1.93 - 1.95 (5H, m), 2.64 - 2.67
(3H, m), 3.32 -
thiazolo[5,4-c]pyridin-5- 3.42 (41-I, m), 3.73 - 3.84 (4H, m), 4.48 (I H,
s), 4.62 (IH, s), 4.71 -
y1]-(tetrahydro-pyran-4-y1)- 4.90 (1H, m);
methanone Mass (m/z): 406.3 (M+1-1)+.
29. [2-(I-Cyclobutyl-piperidin- 'H - NMR (5 ppm): 1.84 - 1.98 (3H, m), 2.16
-2.32 (9H, m), 2.70 -4-yloxy)-6,7-dihydro-4H- 2.71 (2H, t), 3.13 - 3.25
(6H, m), 3.56- 3.61 (3H, 0, 3.67 - 3.69 (4H,
thiazolo[5,4-c]pyridin-5- t), 4.33 (2H, s), 4.42 (21-I, s), 5.12 - 5.22
(1H, m);
yli-morpholin-4-yl- Mass (m/z): 407.3 (M+1-1)+.
methanone tartrate
30. [2-(1-Cyclobutyl-piperidin- - NMR (5 ppm): 1.59 -
1.65 (6H, m), 1.85 - 1.88 (2H, m), 2.22 -4-yloxy)-6,7Ldihydro-4H- 2.27 (4H,
t), 2.29- 2.33 (2H, t), 2.69 - 2.72 (2H, t), 3.13 - 3.17 (3H,
thiazolo[5,4-c]pyridin-5- m), 3.28 - 3.35 (5H, m), 3.52 = 3.55 (3H, t),
3.65 -3.75 (1H, m), 4.29
yli-piperidin-l-yl- (2H, s), 4.43 (2H, s), 5.12 - 5.22 (1H, m);
methanone hydrochloride Mass (m/z): 405.4 (M+H)+.
- 24 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
31. 6-[2-(1 -Cyclobutyl- - NMR (8 ppm): 1.82 - 1.99 (2H, m), 2.25 - 2.33
(8H, m), 2.74 -
piperidin-4-yloxy)-6,7- 2.76 (2H, m), 3.14 - 3.25 (4H, m), 3.62 - 3.66 (1H,
m), 4.03 - 4.06
dihydro-4H-thiazolo[5,4- (2H, t), 4.43 (2H, s), 4.73 (211, s), 4.99 - 5.16
(1H, m), 6.91 - 6.93
c]pyridin-5-y1J- (1H, d, J= 9.03 Hz), 8.01 - 8.03 (IH, dd , J= 2.40
,9.00 Hz), 8.65 -
nicotinamide 8.66 (1H, d , J= 2.28 Hz);
Mass (m/z): 414.3 (M+H)+.
32. [2-(1-Cyclobutyl-piperidin- 11-1 - NMR (8 ppm): 1.27 - 1.29 (2H, m),
1.38 (1H, s), 1.62 - 1.64
4-yloxy)-6,7-dihydro-4H- (2H, m), 1.71 - 1.74(4H, m), 1.78- 1.90 (4H, m),
2.21 - 2.25 (4H,
thiazolo[5,4-c]pyridin-5- m), 2.30 - 2.34 (2H, m), 2.60 (11-1, m), 2.72
(1H, m), 3.13 -3.18 (4H,
ylj-cyclopentyl-methanone m), 3.66 (1H, m), 3.66 - 3.91 (2H, m), 4.44 (2H, s),
4.60 -4.66 (2H,
tartrate d), 5.18 (1H, m);
Mass (m/z): 390.5 (WH).
33. [2-(1-Cyclobutyl-piperidin- - NMR (8 ppm):
0.86 - 0.90 (5H, m), 1.27 - 1.29 (2H, m), 1.82 -4-yloxy)-6,7-dihydro-5H-
1.90 (2H, m), 2.01 - 2.11 (1H, m), 2.25 - 2.31 (7H, m), 2.64 (1H, m),
thiazolo[5,4-b]pyridin-4- 2.77 (1H, m), 3.20(3H, bs), 3.65 - 3.69 (1H, m),
3.88 (1H, m), 4.04 -
yli-cyclopropyl-methanone 4.06 (1H, m), 4.44 (2H, m), 4.60 (1H, s), 5.19 (1H,
m);
tartrate Mass (m/z): 362.2 (M+H)+.
34. Cyclopropyl-[2-(1- 11-1 - NMR (8 ppm): 0.84 -0.91 (4H, m), 1.27-
1.29 (1H, m), 1.37 -
isopropyl-piperidin-4- 1.41 (7H, m), 2.00 - 2.02 (1H, m), 2.21 - 2.31 (4H,
m), 2.81 - 2.88
yloxy)-4,5,7,8-tetrahydro- (2H, m), 2.96 - 3.01 (2H, m), 3.51 - 3.61 (2H,
m),3.72 - 3.79 (2H,
thiazolo[5,4-d]azepin-6-yI]- m), 3.93 -3.99 (2H, m), 4.40 -4.55 (3H, m), 5.13
(1H, s);
methanone tartrate Mass (m/z): 364 (M+H)+.
Examples 35- 66:
The person skilled in the art can prepare the compounds of Examples 35 - 66 by
following the procedures described above.
35. [2-(1-Cyclobuty 1-piperidin-4-yloxy)-6,7-d ihydro-5H-th iazolo[5,4-
b]pyrid in-4-y1j-cyclopropy I- ¨
methanone
36. 142-(1-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-5H-thiazolo[5,4-b]pyridin-
4-y1J-propan-l-one
37. Cyclobutyl-[2-(1-cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-5H-thiazolo[5,4-
b]pyridin-4-y1J-
methanone
38. N-[2-(1-Cyclobutyl-piperidin-4-yloxy)-4,5,6,7-tetrahydro-benzothiazol-7-
yl]-propionamide
39. 17[2-(I-Cyclobutyl-piperidin-4-yloxy)-6,7-dihydro-4H-oxazolo[5,4-c]pyridin-
5-y1J-propan-l-one
40. 1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-4,6-dihydro-pyrrolo[4,3-d]oxazol-5-
y1Fpropan-l-one
41. N-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentaoxazol-5-
y1]-propionamide
42. N-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentathiazol-5-
y1]-propionamide
- 25 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
43. 1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-4,6-dihydro-pyrrolo[4,3-d]thiazol-5-
yl]-propan-l-one
- 44. 1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-pyrrolo[3,2-
d]thiazol-4-y1]-propan-1-one
45. N12-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentathiazol-6-
y1]-propionamide
46. N-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-4H-cyclopentaoxazo1-6-
y1]-propionamide
47. 1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dihydro-pyrrolo[3,2-d]oxazol-4-
y1Wpropan-l-one
48. 142-(1-Cyclobutyl-piperidin-4-yloxy)-4,6-dihydro-pyrrolo[4,3-d]oxazol-5-
y1]-propan-1-one
49. N-[2-(1-Cyclobutyl-piperidin-4-yloxy)-5,6-dthydro-4H-cyclopentaoxazol-5-
y1J-propionamide
50. 1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-4,6,7,8-tetrahydro-thiazolo[5,4-
c]azepin-5-y1J-propan-l-one
51. 1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-4-rnethyl-6,7-dihydro-4H-th
iazolo[5,4-c]pyridin-5-y
propan-1-one
52. 1-[2-(1-Cyclobutyl-piperidin-4-yloxy)-7-fluoro-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1J-
propan-l-one
53. 142-(1-Cyclobutyl-piperidin-4-yloxy)-7-methy1-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y11-
propan-1-one
54. 142-(1-Cyclobutyl-piperidin-4-yloxy)-6-methy1-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y11-
propan-l-one
55. 1-[2-(1-Cyclobuty1-3-methyl-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y11-
. propan-l-one
56. 1-[2-(1-Cyclobuty1-3-fluoro-piperidin-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-
c]pyridin-5-y1]-
propan-1-one
57. 1-Cyclobuty1-4-(5-propiony1-4,5,6,7-tetrahydro-thiazolo[5,4-c]pyridin-2-
yloxy)-piperidine-3-
carbonitrile
58. 1-[2-(1-Cyclobutyl-azepan-4-yloxy)-6,7-dihydro-4H-thiazolo[5,4-c]pyridin-5-
y11-propan-1-one
59. 1-[2-(1-Cyclobutyl-azepan-4-yloxy)-4,5,7,8-tetrahydro-thiazolo[5,4-
djazepin-6-y1]-propan-1-one
60. 1-[2-(1-Cyclobutyl-pyrrol idi n-3-y loxy)-4,5,7,8-tetrahydro-
thiazolo[5,4-d]azepin-6-y1]-propan-1-
one
61. 1-[2-(1-Cyclobutyl-pyrro I idin-3 -y loxy)-4,6-dihyd ro-pyrrolo[4,3-
d]thiazol-5-y1J-propan-1-one
62. 1-[2-(1-Cyclobutyl-azepan-4-yloxy)-4,6-dihydro-pyrrolo[4,3-d]thiazol-5-yli-
propan-1-one
63. 1- { 2-[1-(2-Hydroxy-ethyp-piperidial-4-yloxy]-6,7-dihydro-4H-
thiazolo[5,4-cipyridin-5-y1)-
propan-1-one
64. 1-[2-(1-Ethoxymethyl-piperid in-4-yloxy)-6,7-d hydro-4H-thiazolo[5,4-
c]pyridin-5-y1]-propan-1-
one
65. 1- { 2-[1-(2,2,2-Trifluoro-ethyl)-piperidin-4-yloxy]-6,7-dihydro-4H-th
iazolo[5,4-c]pyridin-5-y1) -
propan-l-one
66. 1-[2-(1-Cyclobutyl-azetid in-3-y loxy)-6,7-dihyd ro-4 H-th iazolo[5,4-
c]pyrid n-5-yI]-propan-l-one
- 26 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
Biological Assays
Example 67: Binding and functional assays for human or rat histamine 113
receptor
Compounds can be evaluated according to the following procedures.
Materials and Methods:
Receptor source: Rat brain frontal cortex or recombinant human cDNA expressed
in
CHO cells ,
Radioligand: [3H] R-a-methylhistamine
Final ligand concentration - [3.0 nM]
Non-specific determinant: R-a-methylhistamine (100 uM)
Reference compound: R-a-methylhistamine
Positive control: R-a-methylhistamine
Incubation conditions:
Increasing concentrations of test compounds or standard were incubated with
membrane receptors and radioligand in 5 mM MgC12 and 50 mM TRIS-HC1 (pH 7.4)
for 60
minutes at room temperature. The reaction was terminated by rapid vacuum
filtration onto the
glass fiber filters. Radioactivity trapped onto the filters was determined and
compared to the
control values in order to ascertain any interactions of the test compound(s)
with either cloned
human or rat receptor binding site.
Example Number K (nM)
1. 3.83
2. 2.0
3. 26.67
4. 7.2
5. 3.3
6. 13.4
7. 10.5
8. 7.69
9. 9.94
10. 5.43
11. 11.98
12. 8.44
14. 23.6
16. 33.55
17. 21.2
18. 6.96
19. 12.28
- 27 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
20. 7.4
21. 22.6
22. 9.5
23. 0.3
25. 35.33
26. 32.54
27. 0.75
28. 38.96
29. 11.3
30. 4.19
31. 1.62
32. 4.6
33. 15.45
34. 15.6
Literature Reference: Millipore data sheet.
Example 68: Rodent Pharmacokinetic Study
Male Wistar rats (230 - 280 grams) obtained from NIN (National Institute of
Nutrition,
Hyderabad, India) were used as an experimental animal. Three animals were
housed in each
cage. Animals were kept fasted over night and maintained on a 12 hours
light/dark cycle.
Three rats were dosed New chemical entity (NCE) orally (3 or 10 mg/kg) and
intravenously (1
or 5 mg/kg) on day 0 and day 2.
At each time point blood was collected by jugular vein. Blood was stored at 2 -
8 C
until analysis. The concentrations of the NCE compound in blood were
determined using LC-
MS/MS method. Schedule time points: Pre dose 0.08, 0.25,0.5, 1, 2, 4, 6, 8 and
24 hours after
dosing (n=3). The NCE compounds were quantified in blood by partially
validated LC-MS/MS
method using acetonitrile precipitation technique. NCE compounds were
quantified in the
calibration range of 1-2000 ng/mL in blood. Study samples were analyzed using
calibration
samples in the batch and quality control samples spread across the batch.
Phannacokinetic parameters were calculated by non-compartmental model using
software WinNonlin version 5Ø1.
Exa Strain Dos Vehicle Route of cr.\T AUC, T112
Bioavaila
mple / e administrati (ng/mL) (h) (ng:hr/mL) (h) bility
Num Gende (mg on (%)
her r /kg)
1. Wistar 1 Water intravenous 436 118 0.08 356 61
0.98 1 0.13 49 5
/Male 0.00
- 28 -
CA 02812970 2013-02-21
WO 2012/029070 PCT/1N2010/000740
3 Water Per Oral 329 23 0.42 524 51
0.95 0.07
0.14
4. Wistar 1 Water Intravenous 320 62 0.08
348 7 1.25 1 0.11 39 1 8
/Male 0.00
3 Water Per Oral 199 48 0.67 407 89
1.67 0.21
0.29
5. Wistar 1 Water Intravenous 287 1 58 0.08
155 38 0.43 0.10 25 1
/Male 0.00
3 Water Per Oral 101 37 0.42 117 34 0.57
0.10
0.14
12. Wistar 1 Water Intravenous 237 38 0.08
334 39 2.86 0.82 43 17
/Male 0.00
3 Water Per Oral 151 18 0.83 417 120
2.85 1 0.38
0.29
22. Wistar 1 Water Intravenous 205 47 0.08
110 15 0.5 0.05 65 24
/Male 0.00
3 Water Per Oral 189 56 0.42 206 56
0.54 0.11
0.14
28. Wistar 1 Water Intravenous 370 25 0.08
282 23 0.67 0.06 82 38
/Male 0.00
3 Water Per Oral 286 40 0.83 701 328
0.95 0.28
0.29
Example 69: Rodent Brain Penetration Study.
Male Wister rats (230 - 280 grams) obtained from NIN (National Institute of
Nutrition,
Hyderabad, India) was used as experimental animals. Three animals were housed
in each cage.
Animals were given water and food ad libitum throughout the experiment, and
maintained on a
12 hours light/dark cycle.
New chemical entity (NCE) was dissolved in suitable vehicle and administered
orally
(3 or 10 mg/kg). Around Tõ,. (i.e, 0.5 hour, 1.0 hour and 2.0 hours) animals
were sacrificed.
Blood and brain tissue were collected and brain was homogenized to yield 20 %
w/v. Blood
was stored at 2 - 8 C and brain homogenate was frozen at -20 C until
analysis. The
concentrations of NCE in blood and brain were quantified using LC-MS/MS
method.
The NCE was quantified in blood and brain homogenate by partially validated LC-
MS/MS method using acetonitrile precipitation technique. NCE compounds were
quantified in
the calibration range of 1-500 ng/mL in blood and brain homogenate. Study
samples were
analyzed using calibration samples in the batch and quality control samples
spread across the
batch. Extents of brain-blood ratio were calculated (Cb/Cp).
Example Strain/ Dose Vehicle Route of
Brain Penetration
Number Gender (mg/kg) administration
Ratio (Cb/Cp)
- 29 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
1. Wistar/Male 1 Water Intravenous 1.97 0.24
3 Water Per Oral
4. Wistar/Male 1
Water Intravenous 2.17 0.17
3 Water Per Oral
5. Wistar/Male I
Water Intravenous 3.08 0.45
3 Water Per Oral
22. Wistar/Male 1 Water Intravenous 2.10 0.13
3 Water Per Oral
Example 70: Object Recognition Task Model
The cognition enhancing properties of compounds of this invention were
estimated by
using this model.
Male Wister rats (230 - 280 grams) obtained from N. 1. N. (National Institute
of
Nutrition, Hyderabad, India) was used as experimental animals. Four animals
were housed in
each cage. Animals were kept on 20 % food deprivation before one day and given
water ad
libitum throughout the experiment and maintained on a 12 hours light/dark
cycle. Also the rats
were habituated to individual arenas for 1 hour in the absence of any objects.
One group of 12 rats received vehicle (1 mL/Kg) orally and another set of
animals
received compound of the formula (I) either orally or i.p., before one hour of
the familiar (T1)
and choice trial (T2).
The experiment was carried out in a 50 x 50 x 50 cm open field made up of
acrylic. In
the familiarization phase, (T1), the rats were placed individually in the open
field for 3 minutes,
in wgich two identical objects (plastic bottles, 12.5 cm height x 5.5 cm
diameter) covered in
yellow masking tape alone (al and a2) were positioned in two adjacent corners,
10 cms from
. the walls. After 24 hours of the (T1) trial for long-term memory test, the
same rats were placed
in the same arena as they were placed in T1 trial. Choice phase (12) rats were
allowed to
explore the open field for 3 minutes in presence of one familiar object (a3)
and one novel object
(b) (Amber color glass bottle, 12 cm high and 5 cm in diameter). Familiar
objects presented
similar textures, colors and sizes. During the T1 and 12 trial, explorations
of each object
(defined as sniffing, licking, chewing or having moving vibrissae whilst
directing the nose
towards the object at a distance of less than 1 cm) were recorded separately
by stopwatch.
Sitting on an object was not regarded as exploratory activity, however, it was
rarely observed.
T1 is the total time spent exploring the familiar objects (al + a2).
T2 is the total time spent exploring the familiar object and novel object (a3
+b).
The object recognition test was performed as described by Ennaceur, A.,
Delacour, J.,
1988, A new one-trial test for neurobiological studies of memory in rats -
Behavioural data,
- 30 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
Behav. Brain Res., 31, 47-59.
Example Dose mg/kg, p.o. Exploration time mean S.E.M (sec)
Inference
Number Familiar object Novel object
1. 3 mg/kg 7.8 1.80 23.95 3.15 Active
4. 10 mg/kg 7.8 1.36
20.32 2.22 Active
5. 3 mg/kg 7.15 0.91
11.44 1.58 Active
28. 0.3 mg/kg 6.12 1.22 11.44 1.69 Active
Example 71: Morris Water Maze
The cognition-enhancing properties of compounds of this invention were
estimated by
using this model.
The water maze apparatus consisted of a circular pool (1.8 m diameter, 0.6 m
high)
constructed in black Perspex (TSE systems, Germany) filled with water (24 2
C ) and
positioned underneath a wide-angled video camera to track animal. The 10 cm2
perspex
platform, lying 1 cm below the water surface, was placed in the centre of one
of the four
imaginary quadrants, which remained constant for all rats. The black Perspex
used in the
construction of the maze and platform offered no intrarnaze cues to guide
escape behavior. By
contrast, the training room offered several strong extramaze visual cues to
aid the formation of
the spatial map necessary for escape learning. An automated tracking system,
[Videomot 2
(5.51), TSE systems, Germany] was employed. This program analyzes video images
acquired
via a digital camera and an image acquisition boards that determined path
length, swim speed
and the number of entries and duration of swim time spent in each quadrant of
the water maze.
Example Number Reversal of Scopolamine Induced
amnesia
I. 10 mg/kg, p.o.
4. 10 mg/kg, p.o.
5. 3 mg/kg, p.o.
12. 20 mg/kg, p.o.
28. 10 mg/kg, p.o.
Example 72: Inhibition of food intake
The anti-obesity properties of compounds of this invention were estimated
using this
model
The experiment consisted of 6 days. The rats were adapted to the 18 hours
fasting and 6
hours feeding pattern. The animals were housed in a group of three in the
cages provided with
the fasting grills and was fasted for 18 hours. After 18 hours fasting the
rats were separated and
- 31 -
CA 02812970 2013-02-21
WO 2012/029070
PCT/1N2010/000740
placed individually in the cage. Weighed amount of feed was provided to rats
for 6 hours and
the feed intake at 1 hour, 2 hours, 4 hours and 6 hours was measured
Again the rats were regrouped and fasted for 18 hours. The above procedure was
followed for 5 days. The average cumulative food intake by the rats on the
last 3 days was
calculated. Animals were randomized on the basis of their previous three days
food intake. On
the day of experiment the rats were orally treated test compounds or vehicle.
After 60 minutes,
the feed was provided to the rats and the food intake at I hour, 2 hours, 4
hours and 6 hours was
measured. The food intake by the rats treated with test compound was compared
with the
vehicle treated group by using Unpaired Student's t test.
Example Number Inhibition of food intake
4. 20 mg/kg, p.o.
15
25
35
- 32 -