Note: Descriptions are shown in the official language in which they were submitted.
81771080
-1-
METHOD FOR RECYCLING ORGANIC WASTE MATERIAL
The invention relates to a method for recycling organic
waste material containing oxides of phosphorus and
metal oxides, in particular for recycling sewage sludge.
Recycling organic waste material, in particular sewage
sludge, dry sludge, sewage sludge ash and animal meal,
is an important aspect of environmental technology;
because of the complex composition of the waste material
involved, recycling is particularly difficult if, in
addition to largely harmless natural substances, the
waste material also contains environmental toxine such
as heavy metals, halogens, pesticides, herbicides,
antibiotics, carcinogenic and mutagenic contaminants,
chlorinated hydrocarbons, polychlorinated biphenyls,
hormones and endocrines. Along with the heavy metals
copper, zinc and cadmium, sewage sludge also contains
various oxides of calcium, silicon and aluminium, and in
particular the presence of iron oxides renders the
extraction of pure phosphorus impossible since
phosphorus binds with iron to form iron phosphide and
thus cannot be obtained in its pure form when iron is
present. The quantity of the various metals and heavy
metals usually prevents sewage sludge from being used
directly as a fertilizer, and so sewage sludge has to be
dumped in large quantities. However, dumping
sewage
sludge means that the recyclable materials contained
therein, in particular phosphorus, which is flot just of
interest to the fertilizer industry, cannot be put to
use.
Thus, the aim of the present invention is to extract
phosphorus or its derivatives in as pure a form as
possible as a recyclable material from organic waste
material and in particular from sewage sludge, and to
separate out the metals it contains.
CA 2817124 2018-01-12
81771080
-2 -
According to one embodiment of the present invention, the waste
material of the aforementioned type is mixed with chienne carriers
and then heat treated at an air ratio cf 0.85 5 I 5 1.6
and partially oxidized, the metal chlorides formed are
drawn off and recovered and the fraction remaining after
the metal chlorides have been drawn off undergoes a
reduction in order to obtain gaseous elemental phosphorus,
salol elemental phosphorous is drawn off and recovered.
The fact that the waste material to be processed is
mixed with chloride carriers means that .in the
subsequent oxidation step, which is carried out at
raised temperatures, the metals are transformed into
metal chlorides which are volatile and thus can be drawn
off with the gas phase. In accordance with the
invention, the remaining fraction then undergoes a
reduction, whereupon pure phosphorus is obtained from
the oxides of phosphorus. Thus, using the method of the
invention, the aforementioned metals and metal oxides
and in particular the mon oxides are séparated out as
metal chlorides before the phosphorus is extracted in
its elemental form by reduction.
Preferably, the heat treatment is carried out 'at
temperatures of 1300 C - 1600 C.
In a preferred implementation, the method of the
invention is further refined by the tact that in
addition, lime is added to the waste material. In the
method of the present invention, lime forms liquid
molten slag with the inorganic combustion products; the
quantity of lime to be added should be adapted to the
Si02 content of the combustion products; in particular,
a ratio of 0.85 5 CaO/Si02 5 1.3 should be established.
The slag which is produced can be used to good effect in
the cernent industry.
CA 2817124 2018-01-12
CA 02817124 2013-05-07
- 3 -
In order to obtain high efficiency in the oxidation
step, the waste material is advantageously introduced
into the oxidation step in a finely divided state. In
accordance with a preferred embodiment of the present
invention, the method is advantageously further refined
in that for the purposes of oxidation, the waste
material mixed with the chloride carriers is forced into
a combustion chamber with a carrier gas via a cyclone,
whereby preferably, an oxygen-containing gas, in
particular hot air, is used as the carrier gas. As an
example, a method of this type can be carried out with
the device of WO 03/070651 Al, which device has already
been used successfully for melting dust. In that
device, the cyclone is upstream of the actual combustion
chamber and the material or the waste material is blown
in tangentially with the carrier gas and set in a rotary
motion so that the waste material is introduced into the
downstream combustion chamber with a preferred spin.
Upon entering the combustion chamber, rotation of the
flow can be maintained for as long as possible so that a
relatively long contact time with the flame is ensured
over a relatively short axial length in the combustion
chamber, and thus the reaction time which is available
for oxidation is relatively long.
A particularly advantageous synergistic effect exists
with other branches of industry and areas of the waste
industry if, in a preferred embodiment of the present
invention, the method is further refined such that the
chloride carriers are selected from the group consisting
of chloride-containing polymers, alkali chlorides,
alkaline-earth chlorides, cernent kiln dust and steel
mill dust. Chloride-containing polymers are available,
for example, in the form of PVC, and cernent kiln dust
and steel mill dust are considered to be problematic
waste material in the respective branches of the
industry, and so these materials are readily and cheaply
CA 02817124 2013-05-07
- 4 -
available for carrying out the method of the invention
and can be put to good use.
In accordance with the present invention, after the
oxidation step and drawing off the metal chlorides
formed in the oxidation step, a step for reduction of
the remaining fraction, which contains the phosphorus,
is carried out; in this case, preferably, the fraction
remaining after drawing off the metal oxides is reduced
using an at least partially inductively heated column
with lump coke and/or graphite and the elemental
phosphorus which is liberated is drawn off. At the same
time, the reduction gas formed (CO, H2) is drawn off.
Following phosphorus condensation, this gas can be used
as a reduction or combustion gas, for example as an
energy supply for the heat treatment of the waste
material. Reduction on an at
least partially
inductively heated column is known, for example, from
WO 2006/079132 Al; that document also discloses an
appropriate device for carrying out a reduction step.
Using an at least partially inductively heated coke
column means that exceedingly high temperatures can be
obtained, whereupon a reducing atmosphere can be set up
since the carbon of the column is flot in thermal
equilibrium with combustion products and in particular
flot with CO2. In contrast to the method described in WO
2006/079132 Al, when using an inductively heated column
the recyclable material is flot contained in the melt,
but is drawn off with the reaction gas and, in a
preferred embodiment of the present invention, can be
obtained as white phosphorus by quenching.
In a preferred embodiment of the present invention, in
the reduction step, the redox potential of the column is
adjusted by blowing in gases and the temperature of the
column is adjusted by adjusting the electrical power and
by blowing in gases. In this manner, the
redox
CA 02817124 2016-05-04
32272-1
- 5 -
potential and the temperature can be adjusted such that only the
desired phosphorus is reduced and other oxides remain in the
residual fraction.
The method of the invention is employed to separate the metals
or metal oxides from phosphorus prior to the reduction step, in
order to prevent the uncontrolled formation of iron phosphide.
When, however, the phosphorus contained in the waste material
has been produced in its pure form using the method of the
invention, the production of pure iron phosphide may in fact be
desired, since the oxidation of iron phosphide produces iron
phosphate which is a valuable starting material for the
manufacture of lithium iron phosphate. Lithium iron phosphate
itself is rapidly gaining importance as a cathode in lithium ion
batteries. The method of the invention is thus preferably
further refined in that the residual fraction after drawing off
the metal chloride is mixed with iron or iron oxide. The
lithium required may be obtained from clinker kiln dust, for
example.
In a further aspect of the invention, an alternative, simplified
procedure is provided in which the cited waste material and lime
are placed on an inductively heated coke and/or graphite bed.
The resulting exhaust gas then undergoes fractionating cooling:
at 400-600 C, the chloride-containing contaminants precipitate
out and can be separated by means of a cyclone or hot gas
separator. Further cooling, for example in a water quench,
results in the condensation of phosphorus. The remaining gas
can be used as a combustion and/or reaction gas after optional
drying.
CA 02817124 2016-05-04
32272-1
- 5a -
According to one aspect of the present invention, there is
provided a method for recycling organic waste material
containing oxides of phosphorus and metal oxides comprising
iron oxides in which the waste material is mixed with chlorine
5 carriers and then heat treated at an air ratio of 0.85 X --
1.6 and at least partially oxidized, and the metal chlorides
formed are drawn off and recovered and the fraction remaining
after the metal chlorides have been drawn off undergoes a
reduction in order to obtain elemental phosphorus.
According to another aspect of the present invention, there is
provided a method for recycling organic waste material
containing oxides of phosphorus and metal oxides comprising
iron oxides in which the waste material is mixed with chlorine
carriers and then heat treated at an air ratio of 0.85 X _<
1.6 and at least partially oxidized, and the metal chlorides
formed are drawn off and recovered and the fraction remaining
after the metal chlorides have been drawn off is supplemented
with iron or iron oxide in order to produce iron phosphide.
The invention will now be described in more detail with the aid
of an exemplary embodiment illustrated in a diagrammatic manner
in the drawings. In the drawings,
CA 02817124 2015-07-31
25861-123
- 6 -
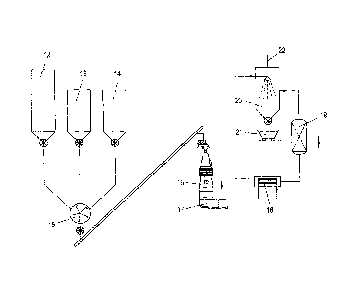
Figure 1 shows a device for carrying out the method of
an embodiment of the invention. Figure 2 shows a block
diagram of a simplified procedure. Figure 3 shows a reduction.
device for carrying out the simplified method.
In Figure 1, the reference numeral 1 indicates a device
for carrying out the method of an embodiment of the invention.
The waste material is supplemented with chloride carriers and/or
lime and sent to the infeed station 2. The reference
numeral 3 indicates a dosing device, for example in the
form of a conveying screw; it is used to convey the
materials into the interior of a combustion chamber 4.
A ring une 5 which opens into the combustion chamber 4
at appropriate locations can be used to set an
appropriate air ratio of air, 02 and, if appropriate,
gaseous chloride carriers. Downstream of the combustion
chamber is a cyclone or droplet separator 6 in which the
melt with the partially oxidized phosphorus is separated
from the product gas. The product gas or reduction gas,
which contains CO2, CO, H20 and H2 in addition to the
metal chlorides, is drawn off via the discharge une 7.
The melt, if appropriate supplemented with 02 and carbon
carriers, is sent to a coke and/or graphite bed 8. The
coke and/or graphite bed 8 is inductively heated by
means of the cou l 9. Reduction to elemental phosphorus
occurs in the bed 8; the phosphorus is drawn off via the
opening 10 and can then be condensed and obtained as
white phosphorus. The residual molten slag collects in
a tundish 11; after tapping, it can, if appropriate,
= be further processed into iron phosphide and iron
phosphate.
In Figure 2, it can be seen that in a simplified
procedure, the waste material along with lime carriers
and coal dust from respective hoppers 12, 13 and 14 are
placed in a mixer 15. From the mixer, the mixture is
placed sent to an infeed device of a reduction reactor
CA 02817124 2013-05-07
-7-
16. In the reduction reactor 16, the metal oxides are
reduced and pig iron and cernent slag can be tapped off
at the tundish 17. The hot gases are
drawn off at
temperatures of approximately 1600 C and cooled to about
400 C in a heat exchanger 18. Heavy metals, alkalis and
halogens can now be separated in an appropriate
separator 19 and the remaining gas, which also contains
phosphorus in addition to CO and H2, is subjected to a
water quench 20, where the phosphorus condenses out and
can be collected in an appropriate receptacle 21 as
white phosphorus. The residual
combustion gas or
product gas is withdrawn via the flue 22.
Figure 3 shows parts of the reduction device in more
detail. The reference
numeral 23 indicates an infeed
device via which the starting materials are supplied to
a coke and/or graphite bed 24 in as even a manner as
possible. The bed 24 rests on a lining 25 formed from
refractory material and is heated inductively by means
of the cou l 26. In addition to the discharge for the
molten iron and the cernent slag, the tundish 17 has a
flue 27 for the product gases which then undergo
fractionating condensation.