Note: Descriptions are shown in the official language in which they were submitted.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
AEROSOL GENERATOR
The present invention relates to an aerosol generator and in
particular to an aerosol generator for liquid aerosols with a
large liquid reservoir volume which contains a similar volume
fraction of liquid and gas. More particularly, the present
invention relates to an aerosol generator which contains a
liquid with a relatively high viscosity in a liquid reservoir
accommodating the liquid to be emitted in form of the
aerosol.
Aerosol generators are mainly used for industrial,
laboratorial, and/or medical application, as well as in the
field of consumer products but are not limited thereto.
Especially the generation of efficient, reproducible and
constant aerosol output for greater liquid volumes is
currently insufficient realized. In all applications which
desire a constant output or dose over the complete aerosol
generation process and reproducible during every application
an optimized aerosol generator are needed.
It has been known that an initial negative pressure (also
referred to as starting negative pressure) increases the
efficiency and total output rate (TOR) of such aerosol
generators (preferable a vibrating mesh or vibrating membrane
nebulizer) as taught for example by WO 97/29851 or US
6,983,747 B2. It might turn out in dose finding studies that
relatively high amount of compound need to be delivered to a
user. Yet, some liquids (e.g. medical substances or
compounds) may not be administered at high concentration for
different reasons, high concentration can be related to
disadvantageous physico-chemical properties for the
nebulization, a compound might not be solvable in high
concentrations or more general the liquid containing the
compound might not be able to carry high concentrations of
the compound (i.e. solution, suspension or liposomal drug
formulation for inhalation therapy). Thus, in the
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
2
administration a relatively large volume of liquid needs to
be emitted in form of the aerosol. The liquid may contain
substance or compounds for example medical liquids, active
substances, drugs or further compounds, such as for
therapeutic, analytic and/or diagnostic applications. In
standard aerosol generators, such as those mentioned in the
above documents and although the efficiency and output rate
is already increased in those aerosol generators because of
the initial negative pressure, a relatively large period of
time is required for emitting the entire liquid containing
the compound in the form of an aerosol.
Such a long period of time, however, is perceived negative
and uncomfortable by a user which can lead to a lower
acceptance of the application (e.g. medical aerosol therapy,
compromised patient compliance, potentially reduced efficacy
of the medical aerosol therapy).
Accordingly, the present invention aims to improve the known
aerosol generators in this regard and to provide an aerosol
generator that enables the emission of even large amounts of
liquid in the form of an aerosol in a shorter period of time
This objective is resolved by an aerosol generator having the
features as defined in claim 1. Embodiments of the present
invention are defined in the dependent claims.
The present invention is based on the finding of the present
inventors that emitting the liquid in the form of an aerosol
while the negative pressure is maintained in a relatively
narrow predetermined range, i.e. consistent value the total
output rate may be increased significantly resulting in a
much shorter period of time required for emitting the liquid.
The present invention suggests maintaining the negative
pressure in a most efficient and simple way by providing a
gas (preferably air) cushion within the liquid reservoir
(above the liquid) that acts as a buffer or damper and is
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
3
sufficiently large to maintain the negative pressure in the
intended and optimized range. Hence, no substantial or
complicated modifications of the existing aerosol generator
are required. Hence, the solution of the present invention
leads to an enormous advantage and is easily implementable.
The inventors have found that the counterforce to the
emission of the liquid through the openings of the membrane
needs to be within an optimal range to maintain a high
efficiency output rate. A minimum negative pressure is
required to prevent the liquid from penetrating through the
openings (holes or ducts) which negatively impacts the
aerosol generation process. A high counterforce prevents the
liquid from passing through the openings. Hence, if the
negative pressure increases, which occur during drainage of
the medication reservoir from an air tight reservoir, are
reduced or even prevented, such increase in the counterforce
may be prevented. Thus, the output efficiency may be
increased or maintained constant leading to a shortened
period of time for the nebulization of the liquid (aerosol
generation).
Further, it has been shown that the optimum range for the
negative pressure depends on the physical and chemical
characteristics of the liquid to be nebulized, such as for
example the viscosity (Newtonian fluid or non-Newtonian fluid
like thixotrope), surface tension, density, kind of fluid
(solution or suspension), solubility, or size of particles in
the liquid (suspension) Further the optimum range for the
negative pressure may be influenced by the surrounding
influencing variables, like ambient air or transportation gas
parameters as for example humidity, temperature, pressure.
Furthermore, the efficiency depends on the design of the
aerosol generation element and the fluid mechanics device
setup. The aerosol generation element (vibrating mesh or
membrane) efficiency is dependent on mesh or membrane
geometry, number of openings, opening arrangements,
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
4
oscillation, excitation frequency, bending mode vibration,
maximum deflexion, power supply and control unit. The fluid
mechanic device setup influences the aerosol deposition in
the device by impaction (flow pattern, turbulences) and
sedimentation. The third theoretical factor the Brownian
molecular movement is in this particle range normally less
important. Therefore the flow pattern of the aerosol/gas flow
throw the device is highly relevant. In particular for
optimized fluid dynamic design of the device a large aerosol
mixing chamber with special inlet and outlet valves with an
optimized mouthpiece are preferable. (Reference is made to
EP 1 227 856 B1 which is incorporated by reference in its
entirety.)
Accordingly, the present invention suggests an aerosol
generator, in particular for aerosols, having a liquid
reservoir defining in a sealed and/or closed state a volume
VR configured to hold an initial volume (starting volume) of
liquid VL. The sealed state is that state in which the
reservoir defines a volume VR immediately before use of the
aerosol generator, i.e. oscillation of the membrane. The
sealed state may be a state in which the reservoir is sealed
with substantially atmospheric pressure within the reservoir.
This state is in the following referred to as V. The
sealed state may as well be a state in which after sealing of
the reservoir the initial volume Vizi of the reservoir (e.g.
this may be with a lid being placed upon the opening of the
reservoir but not being threaded onto the reservoir so as to
create the negative pressure (see later)) is increased. Such
state is in the following referred to as V. The initial
volume of liquid VL is the volume that corresponds to a
predetermined dose of a compound to be administered in one or
more applications (single or multi therapy sessions) of
aerosol generation. This initial volume of the compound may
be packaged for example in a plurality of single containers
or ampoules (blister, vial, vessel, tank) each containing
only one dose (the initial volume of liquid). Alternatively,
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
the single containers or ampoules may each contain a certain
amount of the drug substance (e.g. liquid, powder or
lyophilisate) and this drug is to be mixed with a
predetermined volume of a solvent, wherein the predetermined
volume of the solvent and the drug contained in the single
container upon mixing together form the initial volume of the
liquid. In a further alternative, the compound may be
provided in a package together with a measuring device to be
used for filling the desired dose (initial volume of liquid
VIJ into the liquid reservoir. This measuring device may be
used to measure the drug itself and/or a solvent in order to
enable a user to fill the initial volume of liquid into the
liquid reservoir. For this purpose, the liquid reservoir may
be a reservoir that may be opened and sealed in order to be
filled with the liquid. Alternatively, the liquid reservoir
may as well be formed by an ampoule that already contains the
initial volume of liquid and which is to be inserted into the
aerosol generator. Such an ampoule is e.g. described in
WO 2007/020073 Al, the content of which is hereby
incorporated in its entirety by reference. The ampoule will
also be used for Examples of compounds and active substances
that can be used together with the present invention are
contained in the non-exhaustive list below.
In one embodiment, the aerosol is a medical and/or
pharmaceutical aerosol for the delivery of an active
compound. An active compound is a natural, biotechnology-
derived or synthetic compound or mixture of compounds useful
for the diagnosis, prevention, management, or treatment of a
disease, condition, or symptom of an animal, in particular a
human. Other terms which may be used as synonyms of active
compound include, for example, active ingredient, active
pharmaceutical ingredient, drug substance, drug, and the
like.
The active compound comprised in the aerosol used for the
method of the invention may be a drug substance which is
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
6
useful for the prevention, management, diagnoses, or
treatment of any disease, symptom, or condition affecting the
upper or lower respiratory system (tract), including e.g.
mouth, nose, the sinuses and/or the osteomeatal complex, ear,
eustachian tube, throat, trachea, airways, lungs , main
bronchi's, intermediate bronchus, and alveoli's. The method
of the invention achieves a highly efficient deposition of
the active compound in the wished area of the upper or lower
respiratory system. Thus, it may be advantageously used for
the prevention, management, diagnoses, or treatment of the
above diseases, symptoms or conditions. In addition, the
present method may also be used to deliver active compounds
to the systemic circulation or to the brain for prevention,
management, or treatment of any systemic or brain disease,
symptom, or condition.
The aerosol generator of the present invention further
comprises a membrane having a plurality of openings and
communicating with the liquid reservoir so that the liquid is
fed from the liquid reservoir at one side of the membrane.
The membrane is configured to be oscillatable which may as
one example be achieved by a piezoelectric actuator. When
oscillating the membrane, the liquid is transported through
the openings, whereby the liquid is emitted in the form of an
aerosol on the other side of the membrane. Regarding the
configuration of such membranes, the skilled person is
referred to EP 0 615 470 El which is incorporated by
reference in its entirety.
Moreover, the aerosol generator has a negative pressure
generating device cooperating with the liquid reservoir so to
increase the volume VR of the liquid reservoir in the sealed
state of the liquid reservoir to VRN before the membrane is
oscillated (that is before starting administration or use).
Such a negative pressure generating device may be formed as
disclosed in US 6,983,747 32, which is incorporated by
reference in its entirety. Alternatively, the negative
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
7
pressure generating device may as well be configured as
disclosed in WO 2007/020073 Al, which is incorporated by
reference in its entirety.
The present inventors have found that the higher the ratio of
the initial volume of liquid to the increased volume Vmq of
the liquid reservoir, the higher the increase of the negative
pressure during emission of the liquid in form of the
aerosol. This may at a certain level of the negative
pressure rapidly decrease the total output rate and,
therefore, increase the aerosol generation time. In order to
prevent such a rapid increase of the negative pressure within
the reservoir during aerosol generation in a most effective
and simple manner, the inventors found that an increased
volume VRN of the liquid reservoir configured so as to
contain a gas cushion of more than 8 ml of gas at an initial
volume of liquid VL of at least 4 ml provides for decreased
aerosol generation time upon complete emission of the liquid
in the liquid reservoir. "Complete emission" in this context
means, that once the aerosol generator is started with an
initial volume of liquid, the liquid is emitted in one
application (therapy session) upon completion of the aerosol
generation process. This does not exclude, that a certain
amount of liquid remains within the liquid reservoir. In
particular, in some cases and depending on the orientation of
the membrane, it is conceivable that when a certain minimum
volume of liquid within the liquid reservoir is reached, the
aerosol generator stops operation because the membrane is not
entirely covered by the liquid in the liquid reservoir. Such
case will as well be considered as "complete emission",
although some liquid remains in the reservoir. In addition,
it is to be understood that the volume of gas has to be
considered at an initial state because being compared to the
initial volume of the liquid. That is, the volume of gas
within the liquid reservoir has to be considered before the
membrane is oscillated and the compound or liquid within the
liquid reservoir is administered, that is before use of the
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
8
aerosol generator. The gas (air) cushion of the present
invention is even advantageous compared to a negative
pressure valve or a pressure limiting membrane as disclosed
in NO 2007/020073 Al. The latter may encompass problems
regarding a reproducible manufacture and, hence, will he more
expensive. A negative pressure valve is disadvantageous
regarding the hysteresis of the valve and from the view point
of hygiene and sterilization. Further, the suggestion of the
present invention is advantageous because all mechanical and
electro-technical parts of the existing product may be
maintained only changing the configuration (volume) of the
liquid reservoir to provide for the inventive air/gas
cushion.
As previously indicated, the present invention is
particularly advantageous, if large amounts of liquid are to
be emitted in form of the aerosol and, hence, to liquid
reservoirs configured to accommodate an initial volume of
liquid VL of at least 6 ml, preferably at least 8 ml with a
gas cushion of at least 8 ml.
Further, the inventors have found that the larger the gas
cushion, the more constant the negative pressure during the
aerosol generation. Accordingly, it is preferred that the
volume VR (VRA or Vrni) of the liquid reservoir is configured
to contain more than 11.5 ml, preferably more than 14.5 ml
and even more preferred more than 16.5 ml of a gas before the
membrane is oscillated, that is before use.
Furthermore, an advantageous relationship between the initial
volume of liquid VL and the volume VR (VRA or VRN) of the
liquid reservoir has been observed. The higher the ratio of
the initial liquid within the liquid reservoir to the volume
VR of the liquid reservoir, the higher the increase of the
negative pressure during aerosol generation. Hence, the
effects of the present invention may best be achieved if the
ratio of the volume of the liquid reservoir VR to the volume
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
9
VA of gas in the liquid reservoir is less than 2, preferably
less than 1.8 and most preferably 1.6 at an initial volume of
the liquid VL of at least 6 ml and most preferred at least 8
ml. This ratio as a matter of course applies at an initial
stage at atmospheric pressure (VRA) or after the initial
negative pressure has been generated MN) and before the
membrane is oscillated.
Moreover and according to one embodiment of the present
invention, the aerosol generator has a liquid reservoir that
contains at least 4 ml, preferably at least 6 ml and more
preferably at least 8 ml of liquid. This may for example
apply if the liquid reservoir is formed by an ampoule or
after a user has poured the liquid into the reservoir just
before use.
In addition, it has been found that the efficiency of the
aerosol generator decreases at a negative pressure of
350 mbar. Hence, the increased volume of the liquid
reservoir VRIT is preferably set so that the negative pressure
is maintained in a range between 50 mbar and 400 mbar,
preferably 50 mbar and 350 mbar upon complete emission of the
liquid within the liquid reservoir by the membrane. Further,
it has been found that in case the liquid within the liquid
reservoir is connected to the one side of the membrane
without a negative pressure or even with a slight
overpressure the liquid enters the openings of the membrane
before oscillation. As a result, droplets may form at the
other side of the membrane. These droplets adhering to the
membrane may have a negative influence on the transient
oscillation of the membrane. As a result, the aerosol
generation is delayed or may even not be started. For this
reason, the lower border has been selected at a negative
pressure of 100 mbar in particular for higher viscous
liquids.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
Further and in view of the above, it is preferred that the
initial negative pressure before the membrane is oscillated
resides between 50 and 350 mbar, preferably 100 and 200 mbar
and even most preferred between 100 and 150 mbar.
In addition, the optimum negative pressure inter alia depends
on the physical and chemical characteristics of the liquid to
be emitted in the form of an aerosol. It has been found that
particularly with liquids having a higher viscosity of at
least 1.5 mPa x s such as for example glycerol 17% in a
saline solution (i.e. 1.5% NaC1 solution), the efficiency
within a negative pressure range of 100 mbar to 350 mbar is
nearly constant, whereas the efficiency is clearly decreased
below and above these values. Accordingly, the present
invention is preferably implemented with such highly viscous
liquids.
Furthermore and according to one embodiment, the aerosol
generator, in particular the compartment which contains the
liquid, comprises a calibration mark indicating the initial
volume VL of the liquid to be filled into the liquid
reservoir.
As previously indicated and in accordance with one
embodiment, the liquid reservoir may have an opening and the
aerosol generator further comprises sealing element arranged
on that opening to seal the liquid reservoir interfacing the
aerosol generator, wherein the negative pressure generating
means comprises a slidable element connected to the sealing
element in such a way that a movement of the slidable element
effects movement of at least one section of the sealing
element, whereby the initial volume VR1 of the liquid
reservoir is increased to VRN to generate the initial
negative pressure. Regarding details of this embodiment, the
skilled person is referred to US 6,983,747 B2, which is
incorporated by reference in its entirety.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
11
Preferably, a rotary element connected to the slidable
element is provided such that rotation of the rotary element
effects a substantially linear movement of the slidable
element.
Alternatively, the liquid reservoir is formed by an ampoule
as previously mentioned which is to be inserted into a
housing of the aerosol generator and to be pierced open for
connection to the one side of the membrane. In this context,
the skilled person is referred to WO 2007/020072 Al, which is
incorporated by reference in its entirety.
Further embodiments, features and advantages of the present
invention which may each or together be implemented together
with one or more of the above features will become apparent
from the following description of a preferred embodiment.
This description makes reference to the accompanying
drawings, in which
Figure 1 shows an aerosol generator in which the present
invention may be implemented;
Figure 2 shows the aerosol generator shown in Figure 1 in an
enlarged representation;
Figure 3 shows a graph explaining the relationship between
the time period of aerosol generation upon complete emission
of the liquid within the liquid reservoir and the initial gas
cushion within the liquid reservoir;
Figure 4 shows a graph explaining the relationship between
the negative pressure and the time of aerosol generation
until complete emission of the liquid from the liquid
reservoir;
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
12
Figure 5 is a graph showing a relationship between the
aerosol generation efficiency (proportional to liquid output
rate or total output rate) and the negative pressure; and
Figure 6 is a graph showing the relationship between the
period of time for aerosol generation upon complete emission
of the liquid and the ratio between the increased volume VRN
of the liquid reservoir and the initial volume of liquid
within the liquid reservoir.
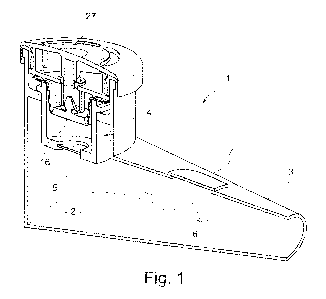
FIG. 1 shows a therapeutic aerosol device 1 with a nebulizing
chamber 2, a mouthpiece 3 and a membrane aerosol generator 4
whose oscillating membrane is marked 5 in FIG. 1. The
oscillating membrane may, for example, be brought to
oscillation by annular piezo elements (not shown), examples
of which are described inter alia in WO 97/29851 Al. When in
use, the liquid is located on one side of the oscillating
membrane 5, see top of FIG. 1, and this liquid is then
transported through openings in the oscillating membrane 5
and emitted on the other side of the oscillating membrane 5,
see bottom of FIG. 1, as an aerosol into the nebulizing
chamber 2. The patient is able to breathe in the aerosol
present in the nebulizing chamber 2 at the mouthpiece 3. So
that the patient does not have to remove or to put down the
therapeutic device from his mouth after inhaling the aerosol,
the mouthpiece 3 has an opening 6 sealed by an elastic valve
element 7 (exhalation valve). If the patient exhales into the
mouthpiece 3 and hence into the nebulizing chamber 2, the
elastic valve element 7 opens so that the exhaled air is able
to escape from the interior of the therapeutic aerosol. On
inhalation, ambient air flows through the nebulizing
chamber 2. The nebulizing chamber 2 has an opening sealed
(not shown) by a further elastic valve element (inhalation
valve). If the patient inhales through the mouthpiece 3 and
sucks from the nebulizing chamber 2, the elastic valve
element opens so that the ambient air is able to enter into
the nebulizing chamber and mixed with the aerosol and leaf
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
13
the interior of the nebulizing chamber 2 to be inhaled. This
will be described in more detail in US 6,962,151, which is
incorporated by reference in its entirety.
Firstly, however, there follows a description of the
structure of the aerosol generator according to the invention
with reference to FIG. 2.
The aerosol generator according to FIG. 2 described here as
an example comprises a cylindrical storage vessel 10 to
supply a liquid that is fed to the membrane 5. As shown in
FIG. 2, the oscillating membrane 5 may be arranged in an end
wall 12 of the cylindrical liquid reservoir 10 to ensure that
the liquid poured into the liquid reservoir comes into direct
contact with the membrane 5 when the aerosol generator
according to the invention is held in the position shown in
FIG. 1. However, other methods may also be used to feed the
liquid to the oscillating membrane without any change being
necessary to the design of the device according to the
invention for the generation of a negative pressure in the
liquid reservoir. However, due to the compact design of the
aerosol generator according to FIGS. 1 and 2, this embodiment
is particularly advantageous.
On the side facing the end wall 12, the cylindrical liquid
container 10 is open. The opening is used to pour the liquid
into the liquid reservoir 10. Slightly below the opening on
the external surface 13 of the peripheral wall 14 there is a
projection 15 which serves as a support when the liquid
container is inserted in an appropriately embodied opening in
a housing 35.
The open end of the liquid container 10 is closed by a
flexible sealing element 16. The sealing element 16 lies on
the end of the peripheral wall 14 of the liquid container 10
and extends in a pot-shaped way into the interior of the
liquid container 10 whereby a conically running wall section
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
14
17 is formed in the sealing element 16 and closed off by a
flat wall section 18 of the sealing element 16. As will be
explained again below, forces act via the flat wall section
18 on the sealing element 16 and so the flat wall section 18
is preferably thicker than the other sections of the sealing
element 16. On the perimeter of the flat wall section 18,
there is a distance to the conical wall section 17 so that
the conical wall section 17 may be folded when the flat wall
section 18 is moved upwards, relative to the representation
in FIG. 2.
On the side of the flat wall section 18 facing away from the
interior of the liquid container, there is a projection
comprising a truncated cone section 19 and a cylindrical
section 20. This design enables the projection to be
introduced and latched into an opening adapted to match the
cylindrical section since the flexible material of the
sealing element 16 permits the deformation of the truncated
cone section 19.
According to the invention, the aerosol generator 4 comprises
a slidable sleeve 21 equipped with an opening of this type
which is substantially a hollow cylinder open on one side.
The opening for the attachment of the sealing element 16 is
embodied in an end wall of the slidable sleeve 21. When the
truncated cone 19 has latched into place, the end wall of the
slidable sleeve 21 containing the opening lies on the flat
sealing element wall section 18. The latching of the
truncated cone 19 into the slidable sleeve enables forces to
be transmitted from the slidable sleeve 21 onto the flat wall
section 18 of the sealing element 16 so that the sealing
section 18 follows the movements of the slidable sleeve 21 in
the direction of the central longitudinal axis of the liquid
container 10.
In a generalized form, the slidable sleeve 21 may be seen as
a slidable element, which may, for example, also be
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
implemented as a slidable rod which may be stuck-on or
inserted in a drill hole. Characteristic of the slidable
element 21 is the fact that it may be used to apply a
substantially linearly directed force onto the flat wall
element 18 of the sealing element 16. Overall, the decisive
factor for the mode of operation of the aerosol generator
according to the invention is the fact that a slidable
element transmits a linear movement onto the sealing element
so that an increase in volume occurs within the liquid
reservoir 10. Since the liquid reservoir 10 is otherwise gas-
tight, this causes a negative pressure to be generated in the
liquid reservoir 10.
The sealing element 16 and the slidable element 21 may be
produced in one piece, i.e. in one operation, but from
different materials. The production technology for this is
available so that a one-piece handlable component for the
aerosol generator according to the invention is created which
may be produced in a fully automatic production step.
The slidable sleeve 21 is open on the end facing the drill
hole for the truncated cone but at least two preferably
diametrically opposite lugs 22 and 23 protrude radially into
the interior of the slidable sleeve 21. A collar 24
encircling the slidable sleeve extends radially outwards.
While the collar 24 is used as a support for the slidable
sleeve 21 in the position shown in FIG. 2, the projections 22
and 23 protruding into the interior of the slidable sleeve 21
are used to absorb the forces acting on the slidable sleeve
21 in particular parallel to the central longitudinal axis.
According to the invention, these forces are generated by
means of two spiral grooves 25 which are located on the
outside of the peripheral wall of a rotary sleeve 26
The device according to the invention may also be implemented
with one of the projections 22 or 23 and one groove 25.
However, preference should be given to a uniformly
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
16
distributed arrangement of two or more projections and a
corresponding number of grooves.
The rotary sleeve 26 is also a cylinder open on one side
whereby the open end is arranged in the slidable sleeve 21
and is hence facing the truncated cone 19 enabling the
truncated cone 19 to penetrate the rotary sleeve 26. In
addition, the rotary sleeve 26 is arranged in the slidable
sleeve 21 in such a way that the projections 22 and 23 lie in
the spiral grooves 25. The inclination of the spiral groove
25 is designed so that, when the rotary sleeve 26 is rotated
in relation to the slidable sleeve 21, the projections 22 and
23 slide along the spiral grooves 25 causing a force directed
parallel to the central longitudinal axis to be exerted on
the sliding projections 22 and 23 and hence on the slidable
sleeve 21. This force displaces the slidable sleeve 21 in the
direction of the central longitudinal axis so that the
sealing element 16 which is latched into the slidable
sleeve's drill hole by means of the truncated cone is also
substantially displaced parallel to the central longitudinal
axis.
The displacement of the sealing element 16 in the direction
of the central longitudinal axis of the liquid container 10
generates a negative pressure in the liquid container 10,
determined inter alia by the distance by which the slidable
sleeve 21 is displaced in the direction of the central
longitudinal axis. The displacement causes the initial volume
VR1 of the gas-tight liquid container 10 to increase to the
volume Viuq and thereby a negative pressure to be generated.
This displacement is in turn defined by the design of the
spiral grooves 25 in the rotary sleeve 26. In this way, the
aerosol generator according to the invention ensures that the
negative pressure in the liquid reservoir 10 may be generated
in the relevant areas by means of simple structural measures.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
17
To ensure that the forces to be applied to generate the
negative pressure when handling the device remain low, the
rotary sleeve 26 is embodied in one piece with a handle 27
whose size is selected to enable the user to rotate the
handle 27, and hence the rotary sleeve 26, manually without
great effort. The handle 27 substantially has the shape of a
flat cylinder or truncated cone which is open on one side so
that a peripheral gripping area 28 is formed on the external
periphery of the handle 27 which is touched by the user's
hand to turn the handle 27. Due to the design of the spiral
grooves 25 and the overall comparatively short distance to be
travelled by the slidable sleeve 21 in the longitudinal
direction to generate a sufficient negative pressure, it is
only necessary to turn the handle 27 and hence the rotary
sleeve 26 through a comparatively small angle. In preferred
embodiments, this angle of rotation lies within a range from
450 to 3600. This embodiment makes a significant contribution
to the ease of handling of the device according to the
invention and an aerosol generator or therapeutic aerosol
equipped therewith.
In order to create a unit which may be operated simply and
uniformly from the slidable sleeve 21 and the rotary sleeve
26 including the handle 27, the example of an embodiment of
the aerosol generator described here has a bearing sleeve 29
for bearing the slidable sleeve 21, which substantially
comprises a flat cylinder open on one side. The diameter of
the peripheral wall 30 of the bearing sleeve 29 is smaller
than the internal diameter of the handle 27 and, in the
example of an embodiment described, is aligned on the
internal diameter of a cylindrical latching ring 31 which is
provided concentrically to the gripping area 28 of the handle
27 but with a smaller diameter on the side of the handle 27
on which the rotary sleeve 26 is also arranged. Embodied on
the side of the cylindrical latching ring 31 facing the
rotary sleeve is a peripheral latching edge 32 which may be
brought into engagement with latching lugs 33 situated at
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
18
intervals on the peripheral wall 30 of the bearing sleeve 29.
This enables the handle 27 to be located on the bearing
sleeve 29 whereby, as shown in FIG. 2, the handle 27 is
placed on the open end of the bearing sleeve 29 and the
latching edge 32 is interlatched with the latching lugs 33.
To hold the slidable sleeve 21, an opening is provided in the
centre of the sealed end of the bearing sleeve 29 in which
the slidable sleeve 21 is arranged, as may be identified in
FIG. 2. The collar 24 of the slidable sleeve 21 lies in the
position shown in FIG. 2 on the surface of the end wall of
the bearing sleeve 29 facing the handle. Extending into the
bearing opening are two diametrically opposite projections 51
and 52, which protrude into two longitudinal grooves 53 and
54 on the peripheral surface of the slidable sleeve 21. The
longitudinal grooves 53 and 54 run parallel to the
longitudinal axis of the slidable sleeve 21. The guide
projections 51 and 52 and the longitudinal grooves 53 and 54
provide anti-rotation locking for the slidable sleeve 21 so
that the rotational movement of the rotary sleeve 26 results
not in rotation but in the linear displacement of the
slidable sleeve 21. As is evident from FIG. 2, this ensures
that the slidable sleeve 21 is held in the combination of the
handle 27 and the bearing sleeve 29 in an axially
displaceable way but locked against rotation. If the handle
27 is now rotated in relation to the bearing sleeve 29, the
rotary sleeve 26 also rotates in relation to the slidable
sleeve 21 whereby the sliding projections 22 and 23 move
along the spiral grooves 25. This causes the slidable sleeve
21 to be displaced in an axial direction in the opening of
the bearing sleeve 29.
It is possible to dispense with the guide projections 51 and
52 in the bearing opening and the longitudinal grooves 53 and
54 in the slidable sleeve 21 if the design of the truncated
cone 19 and the cylinder sections 20 of the sealing elements
16 and the large-area support for the slidable sleeve 21
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
19
holding the truncated cone on the flat sealing element
section 18 achieves anti-rotation locking of the slidable
sleeve 21 by means of friction. For this, the sealing element
16 has to be fixed so it is unable to rotate in relation to
the bearing sleeve 29.
Provided on the surface of the sealed end of the bearing
sleeve 19 facing away from the handle is an annular first
sealing lip 34 concentric to the opening holding the slidable
sleeve. The diameter of the first sealing lip 34 corresponds
to the diameter of the peripheral wall 14 of the liquid
container 10. As may be identified from FIG. 2, this ensures
that the first sealing lip 34 presses the sealing element 16
on the end of the peripheral wall against the liquid
reservoir 10 in such a way that the liquid reservoir 10 is
sealed. In addition, the first sealing lip 34 may also fix
the sealing element 16 so that it is unable to rotate in
relation to the liquid reservoir 10 and the bearing sleeve
29. Due to the materials normally used for the sealing
element on the one hand and the other components of the
device according to the invention on the other, no excessive
force needs to be applied in order to ensure that the
aforesaid components of the device according to the invention
are unable to rotate in relation to each other.
With the advantageous example of an embodiment described
here, the forces required are generated at least to some
extent by means of an interaction between the handle 27 and
the housing 35 in which the liquid reservoir is embodied as
one piece or in which the liquid reservoir 10 is inserted as
shown in FIG. 2. In this case, the liquid reservoir 10
inserted in the casing with the peripheral projection 15 lies
at intervals on a support 36 in the housing 35 which extends
radially into the interior of the housing 35. This enables
the liquid reservoir 10 to be easily removed from the housing
35 for purposes of cleaning. Since support is only provided
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
at intervals, openings are provided for ambient air when the
patient inhales, as is described in more detail below.
Partially identifiable only in FIG. 2 is the rotary lock,
which is implemented by means of the handle 27 on the one
hand and the housing 35 on the other. Only shown are the
locking projections 62 and 63 on the housing 35. However,
there are no special requirements with regard to the design
of the rotary lock as far as the device according to
invention is concerned for the generation of the negative
pressure in the liquid reservoir 10.
According to an embodiment of the present invention, the
liquid reservoir 10 is configured to have a volume VRN of at
least 12 ml, preferably at least 16 ml and most preferred at
least 20 ml so that when for example an amount of 8 ml of
liquid to be emitted in the form of an aerosol is contained
in (filled or poured into) the liquid reservoir 10, an air
cushion of 8 ml is provided. That is, the ratio of the
increased volume Vmq to the initial volume of liquid VL within
the liquid reservoir 10 is at least 2.0 and the ratio between
the volume VA of a gas and VL of the liquid is at least 1Ø
Yet, it has been shown that a liquid reservoir having an
increased volume VIRN of around 15.5 ml is more efficient,
that a reservoir of around 19.5 ml is even more efficient and
that a reservoir of around 22.5 ml even improves over such
reservoirs. That is, it is preferred that the ratio between
VRN and VI, is at least 2.0, more preferred at least 2.4 and
most preferred at least 2.8, the ratio between VA and VL
preferably being at least 1.0, more preferred at least 1.4
and most preferred at least 1.8. That is the volume of the
air cushion is preferably at least 6 ml, more preferred at
least 11 ml and most preferred at least 14 ml.
The ratio of the increased volume VRN to the initial volume
of liquid VL is at least 2Ø Theoretically an unlimited
enlargement of the increased volume VRN of the liquid
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
21
reservoir 10 will result in a nearly stable negative pressure
range. To held an embodiment of the present invention
practicable handy the optimum of the ratio of the increased
volume VRN to the initial volume of liquid VL is within the
range between 2.0 and 4.0 and is preferably between 2.4 and
3.2. Two Examples of the optimum ratio ranges (VRN / VL) for
different initial volume of liquid VL between 4 ml and 8 ml
will be given in the below tables.
VL VRN ratio (V RN VL )
4 ml 8.0 - 16.0 2.0 - 4.0
ml 10.0 - 20.0 2.0 - 4.0
6 ml 12.0 - 24.0 2.0 - 4.0
8 ml 16.0 - 32.0 2.0 - 4.0
VRN ratio (V RN VL )
4 ml 9.5 - 12.8 2.4 - 3.2
5 ml 12,0 - 16,0 2.4 - 3.2
6 ml 14.5 - 19.2 2.4 - 3.2
8 ml 19.5 - 25.6 2.4 - 3.2
The following Figures 3 to 6 show graphs that represent
experimental data proving the effects and advantages of the
present invention.
In these examples, the aerosol generator was an
investigational eflow (nearly standard) of Pan i Pharma GmbH,
Germany. The eflow generator has been altered in regard of
the volume VR of the liquid reservoir. A first aerosol
generator had an initial volume of the liquid reservoir VR1
of 13 ml (A), a second one of 17 ml (B), a third one of 22 ml
(C) and a fourth one of 20 ml (D). That is the increased
volume VRN of the first one had 15.5 ml, the second one
19.5 ml and the third 24.5 ml. It had been measured the time
needed from starting the aerosol generator until complete
emission, that is termination of the operation of the
generator. Further, 8 ml of the liquid were poured into the
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
22
liquid reservoir 10. As shown in Figure 3 an air cushion of
8 ml results to an aerosol generation time period upon
complete emission of 8 ml of the liquid in the liquid
reservoir of between 16 and 14 minutes. An air cushion of 12
ml, however, already decreases the aerosol generation time to
a range between 13 and approximately 12 minutes. The air
cushion of 17 ml further decreases the aerosol generation
time to an amount between 12 and 10.
Further, the first (A) and third (C) version of the above
aerosol generator had been used together with 8 ml of liquid
(i.e. liposomal amikacin). Further, an initial negative
pressure of equal to or less than 50 mbar had been generated
within the liquid reservoir. In addition, the negative
pressure had been measured during the aerosol generation and
is shown over the aerosol generation time in Figure 4. That
is, Figure 4 shows experimental data comparing the negative
pressure range during the aerosol generation time for (C) a
liquid reservoir having an increase volume VRN of 24.5 ml and
(A) one having an increased volume VRN of 15.5 ml with the
initial amount of liquid V. being 8 ml and the initial
negative pressure being about 50 mbar. This graph clearly
shows that a larger air cushion prevents the negative
pressure from increasing above a critical value of 300 mbar.
Further, an experiment has been performed with a nearly
standard eFlow of PARI Pharma GmbH and the aerosol generator
efficiency (proportional to liquid output rate or total
output rate) had been measured in dependency of different
negative pressures. A liquid (i.e. liposomal amikacin)
having a viscosity in the range of 14.5 to 5.5 mPa x s at
sheer forces between 1.1 and 7.4 Pa (thixotrope) has been
used in the experiment. As shown in Figure 5 the efficiency
is optimum in a negative pressure range between 150 mbar to
300 mbar. As may be derived from Figure 5, the efficiency
decreases at a negative pressure below approximately 150 mbar
and at a negative pressure of above 300 mbar.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
23
Furthermore, the same liquid as in Figure 5 has been used in
four different aerosol generators based on the nearly
standard eFlow, wherein the first A) one is a modified eFlow
with an increased volume VRN of the liquid reservoir of 19.5
ml and filled with 8 ml of the liquid. The liquid was that
also used with respect to Figure 5.
The second one had a reservoir with an increased volume VRN
of 16 ml filled with 8 ml of the mentioned liquid, the third
C) one had an increased volume VRR of 24.5 ml, filled with 8
ml of the mentioned liquid and the fourth one had an
increased volume VRN of the liquid reservoir of 22.5 ml
filled with 8 ml of the liquid. Figure 6 represents
experimental data of these four aerosol generators filled
with 8 ml of the substance and shows the aerosol generation
time upon complete emission of the liquid within the liquid
reservoir in relation to the ratio of the increased volume
VRN of the liquid reservoir to the initial volume of liquid VL
in the liquid reservoir before use. This graph clearly
indicates that with the modified aerosol generator device (A)
an aerosol generation time of approximately 16 minutes was
required, whereas the aerosol generation time decreases with
an increased ratio between the increased volume VRN and the
initial volume of liquid and the aerosol generation time
could be reduced by approximately 4 minutes with the third
device version (C) the aerosol generator to below 12 minutes.
In view of the above, it has been proven that a larger air
cushion enables to operate the aerosol generator for a longer
time in a most efficient negative pressure range so that the
total aerosol generation time may significantly be reduced.
Hence, even large amounts of liquid such as 8 ml may be
nebulized (emitted in form of aerosol) in a period of time
below 12 minutes.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
24
The present invention of an aerosol generator can be used for
different liquids, for example for applications in the
medical, pharmaceutical, diagnostic and/or analytic fields
(e.g. human and veterinary aerosol therapies with drugs,
substances or active compounds) as well as for agriculture,
humidification, fragrance, hairspray, pyrotechnic, warfare
agent, combustion engine, extinguishing, lubrication,
adhesive, filtering, cooling, painting, printing, varnishing,
coating processes, technologies and systems. Further examples
are in the field of cell culture, pollen, herbal, medical,
chemical, physical, biological, meteorological, pesticide,
fungicide, biocide, toxic, environment, and exposition
aerosol applications.
Among the active compounds which may be useful for serving
one of the purposes named previously and that may be used
together with the present invention, are, for example,
substances selected from the group consisting of anti-
inflammatory compounds, anti-infective agents, antiseptics,
prostaglandins, endothelin receptor agonists,
phosphodiesterase inhibitors, beta-2-sympathicomimetica,
decongestants, vasoconstrictors, anticholinergics,
immunomodulators, mucolytics, anti-allergic drugs,
antihistaminica, mast-cell stabilizing agents, tumor growth
inhibitory agents, wound healing agents, local anaesthetics,
antioxidants, oligonucleotides, peptides, proteins, vaccines,
vitamins, plant extracts, phosharimidon, vasoactive
intestinal peptide, serotonin receptor antagonists, and
heparins, glucocorticoids, anti-allergic drugs, antioxidants,
vitamins, leucotriene antagonists, anti-infective agents,
antibiotics, antifungals, antivirals, mucolytics,
decongestants, antiseptics, cytostatics, immunomodulators,
vaccines, wound healing agents, local anaesthetics,
oligonucleotides, peptides, proteins and plant extracts.
Such compound may be used in the form of a suspension, a
solution, in a liposome form, etc.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
Examples of potentially useful anti-inflammatory compounds
are glucocorticoids and non-steroidal anti-inflammatory
agents such as betamethasone, beclomethasone, budesonide,
ciclesonide, dexamethasone, desoxymethasone, fluoconolone
acetonide, flucinonide, flunisolide, fluticasone,
icomethasone, rofleponide, triamcinolone acetonide,
fluocortin butyl, hydrocortisone, hydroxycortisone-17-
butyrate, prednicarbate, 6-methylprednisolone aceponate,
mometasone furoate, dehydroepiandrosterone-sulfate (DHEAS),
elastane, prostaglandin, leukotriene, bradykinin antagonists,
non-steroidal anti-inflammatory drugs (NSAIDs), such as
ibuprofen including any pharmaceutically acceptable salts,
esters, isomers, stereoisomers, diastereomers, epimers,
solvates or other hydrates, prodrugs, derivatives, or any
other chemical or physical forms of active compounds
comprising the respective active moieties.
Examples of anti-infective agents, whose class or therapeutic
category is herein understood as comprising compounds which
are effective against bacterial, fungal, and viral
infections, i.e. encompassing the classes of antimicrobials,
antibiotics, antifungals, antiseptics, and antivirals, are
- penicillins, including benzylpenicillins (penicillin-G-
sodium, clemizone penicillin, benzathine penicillin G),
phenoxypenicillins (penicillin V. propicillin),
aminobenzylpenicillins (ampicillin, amoxycillin,
bacampicillin), acylaminopenicillins (azlocillin,
mezlocillin, piperacillin, apalcillin), carboxypenicillins
(carbenicillin, ticarcillin, temocillin), isoxazolyl
penicillins (oxacillin, cloxacillin, dicloxacillin,
flucloxacillin), and amiidine penicillins (mecillinam);
- cephalosporins, including cefazolins (cefazolin,
cefazedone); cefuroximes (cerufoxim, cefamdole, cefotiam),
cefoxitins (cefoxitin, cefotetan, latamoxef, flomoxef),
cefotaximes (cefotaxime, ceftriaxone, ceftizoxime,
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
26
cefmenoxime), ceftazidimes (ceftazidime, cefpirome,
cefepime), cefalexins (cefalexin, cefaclor, cefadroxil,
cefradine, loracarbef, cefprozil), and cefiximes (cefixime,
cefpodoxim proxetile, cefuroxime axetil, cefetamet pivoxil,
cefotiam hexetil), loracarbef, cefepim, clavulanic acid /
amoxicillin, Ceftobiprole;
- synergists, including beta-lactamase inhibitors, such as
clavulanic acid, sulbactam, and tazobactam;
- carbapenems, including imipenem, cilastin, meropenem,
doripenem, tebipenem, ertapenem, ritipenam, and biapenem;
- monobactams, including aztreonam;
- aminoglycosides, such as apramycin, gentamicin,
amikacin, isepamicin, arbekacin, tobramycin, netilmicin,
spectinomycin, streptomycin, capreomycin, neomycin,
paromoycin, and kanamycin;
- macrolides, including erythromycin, clarythromycin,
roxithromycin, azithromycin, dithromycin, josamycin,
spiramycin and telithromycin;
- gyrase inhibitors or fluroquinolones, including
ciprofloxacin, gatifloxacin, norfloxacin, ofloxacin,
levofloxacin, perfloxacin, lomefloxacin, fleroxacin,
garenoxacin, clinafloxacin, sitafloxacin, prulifloxacin,
olamufloxacin, caderofloxacin, gemifloxacin, balofloxacin,
trovafloxacin, and moxifloxacin;
- tetracyclins, including tetracyclin, oxytetracyclin,
rolitetracyclin, minocyclin, doxycycline, tigecycline and
aminocycline;
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
27
- glycopeptides, inlcuding vancomycin, teicoplanin,
ristocetin, avoparcin, oritavancin, ramoplanin, and peptide
4;
- polypeptides, including plectasin, dalbavancin,
daptomycin, oritavancin, ramoplanin, dalbavancin, telavancin,
bacitracin, tyrothricin, neomycin, kanamycin, mupirocin,
paromomycin, polymyxin B and colistin;
- sulfonamides, including sulfadiazine, sulfamethoxazole,
sulfalene, co-trimoxazole, co-trimetrol, co-trimoxazine, and
co-tetraxazine;
- azoles, including clotrimazole, oxiconazole, miconazole,
ketoconazole, itraconazole, fluconazole, metronidazole,
tinidazole, bifonazol, ravuconazol, posaconazol,
voriconazole, and ornidazole and other antifungals including
flucytosin, griseofluvin, tonoftal, naftif in, terbinaf in,
amorolf in, ciclopiroxolamin, echinocandins, such as
micafungin, caspofungin, anidulafungin;
- nitrofurans, including nitrofurantoin and
nitrofuranzone;
- polyenes, including amphotericin B, natamycin, nystatin,
flucocytosine;
- other antibiotics, including tithromycin, lincomycin,
clindamycin, oxazolindiones (linzezolids), ranbezolid,
streptogramine A-i-B, pristinamycin aA+B, Virginiamycin A-i-B,
dalfopristin /qiunupristin (Synercid), chloramphenicol,
ethambutol, pyrazinamid, terizidon, dapson, prothionamid,
fosfomycin, fucidinic acid, rifampicin, isoniazid,
cycloserine, terizidone, ansamycin, lysostaphin, iclaprim,
mirocin B17, clerocidin, filgrastim, and pentamidine;
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
28
- antivirals, including aciclovir, ganciclovir, birivudin,
valaciclovir, zidovudine, didanosin, thiacytidin, stavudin,
lamivudin, zalcitabin, ribavirin, nevirapirin, delaviridin,
trifluridin, ritonavir, saquinavir, indinavir, foscarnet,
amantadin, podophyllotoxin, vidarabine, tromantadine, and
proteinase inhibitors, Si RNA-based drugs;
- antiseptics, including acridine derivatives, iodine-
povidone, benzoates, rivanol, chlorhexidine, quarternary
ammonium compounds, cetrimides, biphenylol, clorofene, and
octenidine;
- plant extracts or ingredients, such as plant extracts
from chamomile, hamamelis, echinacea, calendula, thymian,
papain, pelargonium, pine trees, essential oils, myrtol,
pinen, limonen, cineole, thymol, mental, camphor, tannin,
alpha-hederin, bisabolol, lycopodin, vitapherole;
- wound healing compounds including dexpantenol,
allantoin, vitamins, hyaluronic acid, alpha-antitrypsin,
anorganic and organic zinc salts/compounds, salts of bismuth
and selen
interferones (alpha, beta, gamma), tumor necrosis
factors, cytokines, interleukines;
- immunmodulators including methotrexat, azathioprine,
cyclosporine, tacrolimus, sirolimus, rapamycin, mofetil;
mofetil-mycophenolate.
- cytostatics and metastasis inhibitors;
- alkylants, such as nimustine, melphanlane, carmustine,
lomustine, cyclophosphosphamide, ifosfamide, trofosfamide,
chlorambucil, busulfane, treosulfane, prednimustine,
thiotepa;
CA 02818064 2013-05-15
WO 2012/069531
PCT/EP2011/070799
29
- antimetabolites, e.g. cytarabine, fluorouracil,
methotrexate, mercaptopurine, tioguanine;
- alkaloids, such as vinblastine, vincristine, vindesine;
- antibiotics, such as alcarubicine, bleomycine,
dactinomycine, daunorubicine, doxorubicine, epirubicine,
idarubicine, mitomycine, plicamycine;
- complexes of transition group elements (e.g. Ti, Zr, V,
Nb, Ta, Mo, W, Pt) such as carboplatinum, cis-platinum and
metallocene compounds such as titanocendichloride;
- amsacrine, dacarbazine, estramustine, etoposide,
beraprost, hydroxycarbamide, mitoxanthrone, procarbazine,
temiposide;
- paclitaxel, iressa, zactima, poly-ADP-ribose-polymerase
(PRAP) enzyme inhibitors, banoxantrone, gemcitabine,
pemetrexed, bevacizumab, ranibizumab.
Examples of potentially useful mucolytics are DNase, P2Y2-
agonists (denufosol), drugs affecting chloride and sodium
permeation, such as N-(3,5-Diamino-6-chloropyrazine-2-
carbony)-N'-{4-[4-(2,3-dihydroxypropoxy)-
phenyl]butyl}guanidine methanesulfonate (PARION 552-02),
heparinoids, guaifenesin, acetylcysteine, carbocysteine,
ambroxol, bromhexine, tyloxapol, lecithins, myrtol, and
recombinant surfactant proteins.
Examples of potentially useful vasoconstrictors and
decongestants which may be useful to reduce the swelling of
the mucosa are phenylephrine, naphazoline, tramazoline,
tetryzoline, oxymetazoline, fenoxazoline, xylometazoline,
epinephrine, isoprenaline, hexoprenaline, and ephedrine.
CA 02818064 2013-05-15
WO 2012/069531 PCT/EP2011/070799
Examples of potentially useful local anaesthetic agents
include benzocaine, tetracaine, procaine, lidocaine and
bupivacaine.
Examples of potentially useful antiallergic agents include
the afore-mentioned glucocorticoids, cromolyn sodium,
nedocromil, cetrizin, loratidin, montelukast, roflumilast,
ziluton, omalizumab, heparinoids and other antihistamins,
including azelastine, cetirizin, desloratadin, ebastin,
fexofenadin, levocetirizin, loratadin. This list, however, is
not exhaustive.