Note: Descriptions are shown in the official language in which they were submitted.
CA 02819340 2016-11-09
CHROMIUM-FREE CONVERSION COATING
FIELD
This disclosure relates generally to the field of coatings and corrosion
control on
metals, and in particular to a chromium-free conversion coating for aircraft
applications.
BACKGROUND
Corrosion is defined as the chemical or electrochemical reaction between a
material,
usually a metal, and its environment that produces a deterioration of the
material and its
properties.
Corrosive attack begins on the surface of the metal. The corrosion process
involves
two chemical changes. The metal that is attacked or oxidized undergoes an
anodic change,
with the corrosive agent being reduced and undergoing a cathodic change. The
tendency of
most metals to corrode creates one of the major problems in the maintenance of
aircraft,
particularly in areas where adverse environmental or weather conditions exist.
Chromium-based anti-corrosive systems containing hexavalent Chromium compounds
have proven to be an extremely useful and versatile group of chemistries that
are extensively
used in aircraft metal treatment processes. They impart many beneficial and
essential anti-
corrosive characteristics to metallic substrates on which they are applied and
have been used
extensively for the pre-treatment of metals before coating, adhesive bonding
and surface
finishing.
Chemically, Chromium-based anti-corrosive systems have involved the
combination(s)
of hexavalent Chromium (i.e., Cr03, Cr042-, Cr2072-) and Hydrofluoric Acid
(HF) in the case of
aluminum and its alloys. The Hydrofluoric Acid removes oxide film from the
surface of the
metallic substrate (i.e., Aluminum) and the hexavalent Chromium reacts with
the exposed
metal and a trivalent Chromium Oxide precipitates. Using Aluminum as an
example:
Cr2072 + 2AI + 2H+ -> Cr203 = H20 + A1203
Chromium Oxide such as that produced according to the above reaction is quite
useful
in anti-corrosive applications. It is quite stable in alkaline environments,
it is water repellant
1
CA 02819340 2013-06-17
(hydrophobic) and may act as a barrier coating towards water. Finally, it
exhibits a "self-
healing effect" ¨ that is, residual hexavalent Chromium present in the coating
may react with
damaged areas of the coating ¨ thereby producing more trivalent Chromium Oxide
at
= damaged sites and therefore "healing" itself.
Consequently, Chromium-based, and in particular hexavalent Chromium-based
systems have been extensively used in the aircraft industry because they have
proven to be:
highly effective at preventing corrosion and as an adhesion promoter for
organic coatings and
adhesives; particularly resilient as the application/treatment process
exhibits a low sensitivity
towards variation in process conditions; extremely effective on most/all
Aluminum alloys; and
ensure considerable quality control characteristics as a skilled worker may
tell the amount of
Chromium on the surface of a substrate by mere inspection (color) of the
coating.
Concern about Chromium ¨ and in particular hexavalent Chromium ¨ in the
environment has generated a need to replace Chromium-based systems. Hexavalent
Chromium salts are classified as hazardous substances (toxic, sensitizing and
carcinogenic)
consequently they are environmentally and toxicologically undesirable. The
European
Parliament has published directives requiring the elimination of hexavalent
Chromium such as
directive 2002/95/EC for electrical and electronic equipment and directive
2000/53/EC for
automotive sector. Therefore "environmentally friendly", commercially
acceptable alternative
to Chromium-based systems are highly desirable.
Prior art attempts to provide Chromium-free coatings have met with limited
success.
For example, R.J.Racicot and S.C.Yang describe and compare the corrosion
resistance
performance of a polyaniline based conductive polymer coating versus a
chromate conversion
coating on two aluminum alloys in a paper entitled "CORROSION PROTECTION
COMPARISON OF A CHROMATE CONVERSION COATING TO A NOVEL CONDUCTIVE
POLYMER COATING ON ALUMINUM ALLOYS", which was presented at CORROSION 97,
paper 531, pp.531/1-531/7, Houston, Tx., 1997. As disclosed by the authors,
the double
strand polyaniline exhibited limited corrosion protection for aluminum alloys
AA2024-T3 and
AA7075-T6 in salt-spray and salt and acid immersion tests.
The double strand polyaniline employed is a molecular complex of two polymers,
polyaniline and a second polyanion. The two linear polymers are bonded non-
covalently in a
side-by-side fashion to form a stable molecular complex. As noted by the
authors, the
2
CA 02819340 2013-06-17
advantages to such double strand complexes is: 1) that the conductive state of
the polymer is
very stable; 2) with proper choice of the polymeric dopant, the conductive
polymer may be
dispersed in solvents and used as a coating material; and 3) the polymeric
dopant provides
sites for functionalization to achieve good adhesion to metal surfaces.
-
I.Paloumpa, A.Yfantis, P.Hoffmann, Y.Burkov, D.Yfantis and D.Schmeiber
describe, in
-
a paper entitled MECHANISMS TO INHIBIT CORROSION OF Al ALLOYS BY POLYMERIC
CONVERSION COATINGS, which appeared in Surface and Coatings Technology,180-
181,
pp.308-312, 2004, describe an polypyrrole-based coating which can be formed on
an
aluminum surface from an aqueous polypyrrole (PPY) chemisorbed on titanium an
zinc oxides
and exhibits advanced corrosion resistance.
U.S. Patent No. 5,342,456 to Doaln on August 30, 1994 describes a PROCESS FOR
COATING METAL SURFACES TO PROTECT AGAINST CORROSION wherein a chromium
free conversion coating can be formed on metals ¨ particularly galvanized
steel, by dry-in-
place aqueous acidic liquids. The liquid comprises a component of anions,
particularly at least
four fluorine atoms and at least one atom from a group consisting of titanium,
zirconium,
hafnium, silicon, and boron and optionally, one or more oxygen atoms.
Additional cations from
the group consisting of cobalt, magnesium, manganese, zinc, nickel, tin,
zirconium, iron,
aluminum and copper, a sufficient free acid to give a pH in the range of 0.5
to 5.0 and
optionally a compound that will form an organic resinous film upon drying in
place.
A CORROSION RESISTANT ALUMINUM ARTICLE COATED WITH EMERALDINE
BASE POLYANILINE, was described in a United States Patent No. 5,928,795 which
issued to
Spellane et al on July 27, 1999. The polyaniline used as the coating was a
well-known
emeraldine base form and is easily formed by the oxidative polymerization of
aniline in excess
hydrochloric acid by ammonium persulfate followed by treatment with ammonium
hydroxide.
United States Patent No. 5,980,723 which issued to Runge-Marchese et al on
Nov. 9,
1999, describes an ELECTROCHEMICAL DEPOSITION OF A COMPOSITE POLYMER
METAL OXIDE, which is a process for forming polymer films through
electrochemical
techniques utilizing electrolytes which include conductive polymer. The
resulting polymer films
described are electrically conductive and corrosion and wear resistant.
Example polymer films
included polyaminobenzine (polyaniline).
3
CA 02819340 2013-06-17
An aqueous liquid surface treatment composition having a pH value not more
than 6.5
and containing phosphoric acid ions, condensed phosphoric acid ions, an
oxidizing agent and
a water-soluble polymer was described in United States Patent No. 6,153,022
which issued to
Yoshida on Nov. 28, 2000. The patentee therein reports that such coating
rapidly forms on the
surface of a metal, a conversion coating that has good corrosion resistance
and adhesion to
subsequently applied organic coatings such as paint and is less easily damaged
by
mechanical stresses than prior art conversion coatings.
ELECTROACTIVE POLYMER COATINGS FOR CORROSION CONTROL were
described in United States Patent No. 6,150,032, which issued to Yang et al on
November 21,
2000. In that patent, the patentees describe an anti-corrosive polymeric
complex which
comprises a plurality of double-stranded molecular complexes including
conductive polymer
and a strand of a copolymer. The strands of the polymeric complex are non-
covalently
bonded to each other along the contour of the strands to form a side-by-side,
twisted, double-
stranded configuration.
United States Patent No. 6,328,874, issued to Kinlen et al on December 11,
2001 for
ANODICALLY FORMED INTRINSICALLY CONDUCTIVE POLYMER-ALUMINUM OXIDE
COMPOSITE AS A COATING ON ALUMINUM, describes a method for forming a coating
on
aluminum by contacting the aluminum with water, at least one multifunctional
polymeric
organic acid, a monomer of an intrinsically conductive polymer (ICP) and
polymerizing the ICP
monomer and forming aluminum oxide by imposing an electrical potential between
the
aluminum surface as an anode and a cathode. The intrinsically conductive
polymer salt and
aluminum oxide coating that is formed resists corrosion and is resistant to de-
doping during
immersion in hot water.
A NONCHROMATE RUST PREVENTIVE AGENT FOR ALUMINUM, METHOD OF
RUST PREVENTION AND RUST-PREVENTIVE ALUMINUM PRODUCTS was described in
United States Patent No. 6,419,731 which issued to lnbe et al on July 16,2002.
The
patentees therein describe a nonchromate rust preventive agent for aluminum
that comprises
a zirconium compound, a fluoride ion, a water soluble resin and an aluminum
salt.
Sako et al, in United States Patent No. 6,736,908 entitled "COMPOSITION AN
PROCESS FOR TREATING METAL SURFACES AND RESULTING ARTICLE", which issued
on May 18, 2004, describes a metal treating composition comprising at least a
specific type of
4
CA 02819340 2013-06-17
dissolved and/or dispersed organic resin, a dissolved vanadium compound in
which the
valence of the vanadium is from 3 to 5, and a dissolved compound that contains
at least one of
the metals Zr, Ti, Mo, W, Mn, and Ce. According to the patentees, the
treatment provides
metal surfaces with superior corrosion resistance, alkali resistance, and
fingerprint resistance.
=
Advantageously, their composition contains no Chromium.
United States Patent No. 6,758,916 for COMPOSITION AND PROCESS FOR
TREATING METALS issued to David McCormick on July 6, 2004 describes a chromium-
free
conversion coating at least equivalent in corrosion protective quality to
conventional chromate
conversions that can be formed on metals, particularly cold rolled steel, by
dry-in-place
aqueous acidic liquid. The liquid has a pH value between 0.5 and 5.0 and
comprises
"fluorometallate" anions consisting of at least four fluorine atoms; at least
one atom of an
element selected from the group consisting of titanium, zirconium, hafnium,
silicon, aluminum,
and boron, and optionally, one or more of ionizable hydrogen atoms and oxygen
atoms; a
component of divalent or tetravalent cations of elements selected from the
group consisting of
cobalt, magnesium, manganese, zinc, nickel, tin, copper, zirconium, iron, and
strontium - in
very precise relative proportions.
Despite the developments of the prior art, the corrosion resistance imparted
by non-
chromate type treatments is invariably less than that provided by chromate
type methods and
agents and has not satisfied practical needs ¨ particularly those in the
aircraft industries. One
object of present invention therefore is to provide a chromium-free coating
which, despite
being chromium-free, is capable of providing corrosion protection equivalent
to or superior
than a chromium-type coating, or at least to provide a commercially viable
alternative to known
coatings.
Wim J. Van Ooij et al. described, in a paper entitled "Modified silane
coatings as an
alternative to chromates for corrosion protection of aluminum alloys", which
was published in
Silanes and other coupling agents Vol. 3, pp 135-159, Ed. K.L.Mittal, 2004,
bis-(3-
triethoxysilylpropyl)tetrasulfide, bis-(trimethoxysilylpropyl)amine and
vinyltriacetoxysilane
based treatments applied onto AA2024-T3 alloys which also incorporated cerium
nitrate,
tolytriazole and benzotriazole corrosion inhibitors and silica nanoparticles.
Self-healing effects
.. were reported for some of the treatments as well as good paint adhesion
performance.
5
CA 02819340 2013-06-17
Wim J. Van Ooij et al. described, in a paper entitled "Overview: The Potential
of silanes
for chromate replacement in metal finishing industries" which was published in
Silicon
Chemistry Volume 3, Numbers 1-2 (2006), that bis-(3-triethoxysilylpropyl)
tetrasulfide treated
7075-16 panels did not exhibit any sign of corrosion after 336 hours of salt
spray exposure.
It is known that alcohol-based silanes offer a higher corrosion resistance to
the water-
based silane systems as the higher alcohol content removes more water from the
film upon
drying and the silanol groups can react more easily to form a cross-linked and
denser film.
Also, the water-soluble silanes remain more hydrophilic even after drying, so
they allow higher
ingress of water than the solvent-based silanes. It is therefore important
that these low-VOC
water-based silanes systems are further modified to increase their corrosion
inhibition
efficiency. In the publication "Effects of addition of corrosion inhibitors to
silane films on the
performance of AA2024-T3 in a 0.5M NaCl solution" by Wim J. Van Ooij et al.,
which was
published in Progress in Organic Coatings 53 (2005) 153-168, corrosion
inhibitors (tolytriazole,
benzotriazole and inorganic cerium salts) were added to silane films and their
corrosion
properties studied in 0.5 M NaCI solution. The water-based silane solutions
were prepared by
mixing Bis[3-(trimethoxysilyppropyl]amine and Vinyltriacetoxysilane in 2:1 and
4:1 parts by
volume and about 5% of this mixture was hydrolyzed with 95% of DI water.
Silane films when
loaded with organic or inorganic inhibitors provided improved corrosion
resistance. A scratch
cell test confirmed that the cerium salts were also potential inhibitors for
adding self-healing
capabilities for silane films.
The publication "A comparative study on the corrosion resistance of AA2024-T3
substrates pre-treated with different silane solutions" by A.M. Cabral et al.
that was published
in Progress in organic coatings 54 (2005) 322-331, reported a comparative
study of AA-2024-
13 pre-treated with three different silane solutions (1, 2-Bis(Triethoxysily1)
Ethane, bis-(3-
triethoxysilylpropyl)tetrasulfide and y-mercaptopropyltrimethoxysilane). The
silane treated
samples were further treated with y-aminopropyltrimethoxy silane prior to
paint them with a
polyurethane enamel. The AC impedance results showed that silane films
provided protection
to the substrate. For a short time, the performance was even better than that
conferred by the
chromate reference treatment.
J.B. Bajat et al. described, in a paper entitled "Corrosion stability of epoxy
coatings on
aluminum pretreated by vinyltriethoxysilane", which was published in Corrosion
Science 50
(2008) 2078-2084, the electrochemical and transport properties and adhesion of
epoxy
6
CA 02819340 2013-06-17
coatings electrodeposited on aluminum 99.7 pretreated by vinyltriethoxyslane
(VTES). It was
concluded that 5% solution for 10 minutes provided enhanced adhesion and also
improved the
corrosion stability of a protective system VI-ES/epoxy coating. The same
authors reported, in a
publication entitled "Corrosion protection of aluminium pretreated by
vinyltriethoxysilane in
sodium chloride solution", which was published in Corrosion Science 52 (2010)
1060-1069,
EIS and potential-time measurements of VTES films deposited on A199.5%
substrates in 3%
NaCI medium exposure. It was shown that the concentration of VTES had a great
influence on
the corrosion behavior and morphology of the VTES films while curing time
exhibited smaller
influence of the VTES film properties.
B. Naderi Zand et al. described, in a paper entitled "Corrosion and adhesion
study of
polyurethane coating on silane pretreated aluminium", which was published in
Surface &
Coatings Technology 203 (2009) 1677-1681, the effect of the silane
pretreatment's pH on the
adhesion strength and on the corrosion protection of subsequent polyurethane
(PU) coating on
aluminum alloy substrate. The practical adhesion of the coating on the
substrate was
measured in dry, wet and recovered states via pull-off method for desmutted,
chromated and
vinyltrimethoxysilane (\fTMS) pretreated M1050 aluminum alloy. VTMS resulted
in good
adhesion performance in dry, wet and recovered states at pH < isoelectric
point (IEP).
Corrosion protection of PU coating was studied with EIS and salt spray in the
presence of
silane layer. At pH < IEP protective performance was considerably higher and
comparable with
that of chromated specimens.
F. Brusciotti et al. described, in a paper entitled "Characterization of thin
water-based
silane pre-treatments on aluminium with the incorporation of nano-dispersed
Ce02 particles",
which was published in Surface and Coatings Technology 205 (2010) 603-613,
novel thin films
of water-based 1, 2-bis(Triethoxysilyl)ethane (BTSE) with the incorporation of
nano-dispersed
Ce02 particles for improved barrier properties. EIS investigations pointed out
a better
performance for the coatings where the Ce02particles were nano-dispersed and
uniformly
distributed in the layer.
U.S. Pat. No. 6,071,566 which was issued to Kevon Brown et al., relates to a
method
that comprises applying a solution containing one or more vinyl silanes with
or more multi-silyl-
functional silanes for treating a metal substrate providing corrosion
resistance. The method is
particularly suitable for use in zinc coated surfaces. The particular
preferred vinyl silane is
7
CA 02819340 2016-11-09
vinyltriethoxysilane (VS) and the preferred multi-functional silane is 1, 2-
bis(triethoxysilyl)ethane (BTSE).
Accordingly, the present disclosure addresses the disadvantages associated
with the
prior art, and seeks a chromium-free coating with improved adhesion
properties, or at least to
provide a commercially viable alternative to known coatings.
SUMMARY
In the following passages different aspects/embodiments are defined in more
detail.
Each aspect/embodiment so defined may be combined with any other
aspect/embodiment or
aspects/embodiments unless clearly indicated to the contrary. In particular,
any feature
indicated as being preferred or advantageous may be combined with any other
feature or
features indicated as being preferred or advantageous.
In one embodiment, there is provided a process for treatment of metallic
surfaces. The
process includes the steps of pretreating the metallic surfaces, and coating
the metallic
surfaces with a conversion coating by contacting the metallic surfaces with a
conducting
polymer dispersion consisting of a conducting polymer selected from the group
consisting of
polyaniline (PANI), polyethylenedioxythiophene (PEDOT) and polypyrrole (PPY),
and one or
more silanes selected from the group consisting of (3-
Glycidoxypropyl)trimethoxysilane
(GPMS), 1,2-Bis(trimethoxysilyl)ethane (TMSE), 1, 2-Bis(Triethoxysily1) Ethane
(BTSE), and
Vinyltriacetoxysilane (VTAS), or combinations of two or more thereof. The
conducting polymer
dispersion further consists of inorganic metallic salts selected from at least
one of
molybdenum, magnesium, zirconium, titanium, vanadium, cerium, hafnium,
silicon, aluminum,
boron, cobalt and zinc in concentrations of the inorganic metallic salts
between 2.0 g/L (grams
per liter) and 20 g/L (grams per liter) and a pH value of between 1 and 6Ø
The process
further comprises the step of drying the metallic surfaces.
In another embodiment, there is provided a process for treatment of metallic
surfaces.
The process includes the steps of pretreating the metallic surfaces, and
coating the metallic
surfaces with a conversion coating by contacting the metallic surfaces with a
conducting
polymer dispersion consisting of a conducting polymer, and one or more silanes
selected from
the group consisting of (3-Glycidoxypropyl)trimethoxysilane (GPMS), 1,2-
Bis(trimethoxysilyl)ethane (TMSE), 1, 2-Bis(Triethoxysily1) Ethane (BTSE), and
8
CA 02819340 2016-11-09
Vinyltriacetoxysilane (VTAS), or combinations of two or more thereof. The
conducting polymer
dispersion further consists of inorganic metallic salts selected from at least
one of
molybdenum, magnesium, zirconium, titanium, vanadium, cerium, hafnium,
silicon, aluminum,
boron, cobalt and zinc in concentrations of the inorganic metallic salts
between 2.0 g/L (grams
per liter) and 20 g/L (grams per liter) and a pH value of between 1 and 6Ø
The process
further includes the step of drying the metallic surfaces.
In another embodiment, there is provided a process for treatment of metallic
surfaces.
The process includes the steps of pre-treating the surfaces, coating the
surfaces with a
conversion coating by contacting them with a conducting polymer dispersion
containing one or
more silanes selected from (3-Glycidoxypropyl)trimethoxysilane (GPMS), 1,2-
Bis(trimethoxysily1) ethane (TMSE), 1, 2-Bis(Triethoxysily1) Ethane (BTSE),
Bis[3-
(trimethoxysilyl)propyl]amine (BAS) and Vinyltriacetoxysilane (VTAS), or
combinations of two
or more thereof, and inorganic metallic salts selected from at least one of
Molybdenum,
Magnesium, Zirconium, Titanium, Vanadium, Cerium, Hafnium, Silicon, Aluminum,
Boron,
Cobalt and Zinc in concentrations of the metal salt between 2.0 and 20 g/L and
a pH value of
between 1 and 6.0, wherein the inorganic metal salt is or comprises a salt of
Zirconium, and
the concentration of inorganic metallic salts of Zirconium are
produced/adjusted with K2ZrF6.
The process further includes the step of drying the surfaces, wherein said
conducting polymer
is one selected from the group consisting of: Polyaniline (PANI),
Polyethylenedioxythiophene
(PEDOT) and Polypyrrole (PPY).
In another embodiment, there is provided a conversion coating for the
treatment of
metallic surfaces. The coating includes a conducting polymer dispersion
containing one or
more silanes, and an inorganic metallic salt of at least one of Molybdenum,
Magnesium,
Zirconium, Titanium, Vanadium, Cerium, Hafnium, Silicon, Aluminum, Boron,
Cobalt and Zinc.
The concentration of the inorganic metallic salt is between 2.0 and 20 g/L and
the pH of the
coating is between 1 and 6Ø The inorganic metal salt is or comprises a salt
of Zirconium, and
the concentration of inorganic metallic salts of Zirconium are
produced/adjusted with K2ZrF6.
The conducting polymer is one selected from the group consisting of
Polyaniline (PANI),
Polyethylenedioxythiophene (PEDOT) and Polypyrrole (PPY), and wherein the one
or more
silanes are selected from (3-Glycidoxypropyl) trimethoxysilane (GPMS), 1,2-
Bis(trimethoxysily1) ethane (TMSE), 1, 2-Bis(Triethoxysily1) Ethane (BTSE),
Bis[3-
9
CA 02819340 2016-11-09
(trimethoxysilyppropyliamine (BAS) and Vinyltriacetoxysilane (VTAS), or
combinations of two
or more thereof.
Chemical conversion coatings must perform a dual function: to improve the
corrosion
resistance of the substrate alloy and to promote a good adhesion of the
subsequent organic
coatings. Additionally, these coatings can be used in parts where surface
contact electrical
resistance is also a requirement (the maximum electrical resistance values
allowed by the
MIL-DTL-81706-B standard are 5000pD/square inch before the salt spray exposure
test and
10000p0/square inch after 168h of salt spray exposure test under an applied
electrode
pressure of 200 pound per square inch (psi). Individual readings not greater
than 20% in
excess of the specified maximums shall be acceptable, provided that the
average of all
readings does not exceed the specified maximum resistance).
New processes and compositions for Cr-free conversion coatings with good
corrosion
resistance were invented by the authors and the patent application US
2010/0009083 was
submitted. The conversion coatings described in the abovementioned patent
application
showed very good corrosion performance. However, the present inventors have
now
discovered that they can improve the adhesion of these coatings to subsequent
organic
coatings without jeopardizing the corrosion protection.
Indeed, the inclusion of a silane in the coating of US2010/0009083 has been
found to
provide an improvement. The conducting polymer dispersion described in the
patent
comprises at least one silane compound apart from the other bath components
(conducting
polymer and inorganic salts). The silane compounds may enhance the adhesion
performance
so the coatings comply with the requirements of aeronautical applications
while the corrosion
protection as described in the examples is maintained.
Moreover, the new conversion coatings obtained with the conducting polymer
dispersions comprising at least one silane compound may offer low surface
contact electrical
resistance, compliant with the requirements for aeronautical applications.
The process and composition has now been improved in an attempt to enhance the
adhesion of the subsequent organic coatings, while maintaining the corrosion
protection of the
substrate. Additionally, the coatings obtained with these processes based on
compositions
CA 02819340 2016-11-09
comprising at least one silane compound may have low contact electrical
surface resistance,
compliant with the requirements for aeronautical applications.
The challenge has been to optimize the chemical conversion process and the
coating
composition to enhance the adhesion and reduce the electrical resistance
without jeopardizing
the corrosion protection.
In the process of silane treatment of metals, two main reactions occur on the
basis of
the number of silanols (SiOH) after the silane molecules are adsorbed onto the
metal surface.
On the one hand, the condensation between silanols (SiOH) from the silane
solution and the
metal hydroxyls (Me0H) from the metal surface hydroxides forms metallo-
siloxane bonds
(Me0Si). On the other hand, the condensation among the excess SiOH groups
adsorbed on
the metals forms a siloxane (SiOSi) film on the top of the coating.
A medium may be used for silane solution preparation; silanes may be
classified in
water-based or solvent-based silanes. It may be desirable to use water-based
silane systems
with nil or very little alcohol content, due to VOC restrictions. The one or
more silanes may be
water soluble.
The one or more silanes may be present in an amount of from 0.01 v%. ¨ 1.0 v%
(v/v)
of the polymer dispersion, more preferably from 0.1 v% to 0.5v%. With this
range of silane
content the beneficial effects of the silane may be observed, possibly without
compromising
the corrosion resistance.
The one or more silanes are of the formula
YSiX(3_a)Za
wherein,
X are independently selected hydrolysable groups,
Y is non-hydrolysable and includes a functional group,
Z is independently selected from H or alkyl, and
a is 0, 1 or 2.
By hydrolysable group it is meant that the group is susceptible to
nucleophilic attack to
cleave the group from the silicon atom. X are independently selected
hydrolysable groups. It is
preferred that the hydrolysable groups are selected from methoxy (OCH3) or
ethoxy (0021-15).
11
CA 02819340 2016-11-09
These groups may allow for good adhesion of the compound to nucleophilic sites
on the metal
and/or further coatings.
By non-hydrolysable it is meant that the group is not susceptible to
nucleophilic attack
to cleave the group from the silicon atom. Preferably the Y moiety is joined
to the silicon atom
by a silicon-carbon bond.
Y includes a functional group. That is, Y includes a group capable of reacting
to link the
silicon atom to a further coating, a further silane or to the metal substrate.
The functional group
is preferably selected from vinyl (-CH=CH2), amino (-NH2), epoxy or mercapto (-
SH)). The
functional group may consist of this functional group, but preferably the
functional group is
linked to the silicon atom by an alkyl chain, preferably a lower alkyl (C1-C6)
chain, an
alkylether or an alkylamine. Thus, for example, Y may preferably be: -(CH2)n-
NH2; -(CH2)n-SH,
or -(CH2)n- HC=CH2, and n is preferably 0-6, preferably 1-6, more preferably 0-
3.
In one embodiment the functional group may be a further silicon-containing
moiety.
Thus, Y may be ¨RSiX3.aZa, where X, Z and a are as defined above. R may be an
alkyl, an
ether or an alkylamine. For example, R is an ether, such as CH2OCH2 or R is an
amine, such
as (CH2)3NH(CH2)3. According to their chemical structures, silane molecules
are divided into
two major categories, mono-silanes and bis-silanes. Bis-type silanes have two
silicon atoms in
their molecule whereas mono-silanes have only one, with a general formula of
X3Si-R-SiX3.
Preferably the silane is symmetrical in this way for ease of synthesis and to
minimize
production costs. The main difference between mono and bis-silanes is that the
number of
hydrolysable X groups is double in a bis-silane molecule than in a mono-silane
molecule.
Thus, it is reported that bis-silanes offer stronger interfacial adhesion
(with the substrate) and
denser films leading to better corrosion performance compared to mono-silanes,
especially in
unpainted state.
By "alkyl" is meant a straight or branched chain saturated cyclic (i.e.,
cycloalkyl) or
acyclic hydrocarbon group of from 1 to 12 carbons, unless otherwise specified.
Exemplary
alkyl groups include C1-C8, C1-C6, C1-C4, 02-C7, C3-C12, and C3-C6 alkyl.
Specific
examples include methyl, ethyl, 1-propyl, 2-propyl, 2-methyl-1-propyl, 1-
butyl, 2-butyl, and the
like. Unless otherwise noted, alkyl groups, used in any context herein, may
optionally be
substituted with halogen, amino or sulfyl groups, or may include one or more
heteroatoms in
the alkyl chain, such as oxygen (an ether) or nitrogen (an amine).
12
CA 02819340 2016-11-09
Z may be independently selected from H or alkyl. That is, Z may be a non-
hydrolysable
group that does not include a functional group for linking the silane to a
further coating, a
further silane or to the metal substrate. In particular, it is preferred that
a is 0.
The one or more silanes may be selected from (3-Glycidoxypropyl)
trimethoxysilane
(GPMS), 1,2-Bis(trimethoxysilyl)ethane (TMSE), 1, 2-Bis(Triethoxysily1) Ethane
(BTSE), Bis[3-
(trimethoxysily0propyl]amine (BAS) and Vinyltriacetoxysilane (VTAS), or
combinations of two
or more thereof.
The conducting polymer may be one selected from the group consisting of:
Polyanaline
(PANI), Polyethylenedioxythiophene (PEDOT) and Polypyrrole (PPY).
The coating step may be applied to the surfaces by spraying, or immersion. If
the
coating step is immersion, preferably it is for a period of substantially 2
minutes in the
treatment bath. The drying step may be performed at substantially room
temperature.
The pretreatment step may serve to prepare the surface of the metal for the
coating.
Accordingly, the pretreatment step at least involves the cleaning of the
surface. The
pretreatment step may further comprise the steps of:
degreasing the surfaces;
cleaning the surfaces; and
deoxidizing the surfaces.
The method may further comprise the steps of: rinsing the surfaces after
cleaning; and
rinsing the surfaces after deoxidizing; and not-rinsing the surfaces after
coating.
The inorganic metal salt may or may comprise a salt of Zirconium, the
concentration of
inorganic metallic salts of Zirconium are produced/adjusted with K2ZrF6 and
the pH is adjusted
with H2ZrF6 and/or NH4OH.
The metallic sheets may be ones selected from the group consisting of
aluminum,
copper, iron, or alloys thereof, and may be selected from the group consisting
of 2024-T3 and
7075-T6.
The inorganic salts may be present in a concentration of 2.0 to 8.0 g/L and/or
wherein
the contacting step is carried out at a pH of 2 to 5.
13
=
According to a second aspect, there is provided a conversion coating for the
treatment
of metallic surfaces, said coating comprising one or more silanes, and a
conducting polymer
dispersion containing an inorganic metallic salt of at least one of
Molybdenum, Magnesium,
Zirconium, Titanium, Vanadium, Cerium, Hafnium, Silicon, Aluminum, Boron,
Cobalt and Zinc,
wherein the concentration of the inorganic metallic salt is between 2.0 and 20
g/L and the pH
of the coating is between 1 and 6Ø
The one or more silanes may be as described above and as discussed herein.
The conducting polymer may be one selected from the group consisting of:
Polyanaline
(PANI), Polyethylenedioxythiophene (PEDOT) and Polypyrrole (PPY), and/or
wherein the one
or more silanes are selected from (3-Glycidoxypropyl)trimethoxysilane (GPMS),
1,2-
Bis(trimethoxysilyl)ethane (TMSE), 1, 2-Bis(Triethoxysily1) Ethane (BTSE),
Bis[3-
(trimethoxysilyl)propyl]amine (BAS) and Vinyltriacetoxysilane (VTAS), or
combinations of two
or more thereof.
Various embodiments of the claimed invention relate to a process for treatment
of a
metallic surface, said process comprising the steps of: coating the surface
with a conversion
coating by contacting it with a conducting polymer dispersion containing: a
conducting polymer
selected from the group consisting of: Polyaniline (PAN1),
Polyethylenedioxythiophene
(PEDOT) and Polypyrrole (PPY); one or more silanes selected from (3-
Glycidoxypropyl)trimethoxysilane (GPMS), 1,2-Bis(trimethoxysily1) ethane
(TMSE), 1, 2-
Bis(Triethoxysily1) Ethane (BTSE), Bis[3-(trimethoxysily0propyl]amine (BAS)
and
Vinyltriacetoxysilane (VTAS), or combinations of two or more thereof; and one
or more
inorganic metallic salts comprising a salt of zirconium in concentrations of
the metal salt
between 2.0 and 20 g/L and a pH value of between 1 and 6.0, wherein the
concentration the
salt of Zirconium is adjusted with K2ZrF6; and drying the surface.
These and other features and advantages of the present invention will become
apparent with reference to the attached drawing and detailed description.
13a
CA 2819340 2018-06-18
BRIEF DESCRIPTION OF THE DRAWINGS
A more complete understanding of the present disclosure may be realized by
reference
to the accompanying drawing and tables in which:
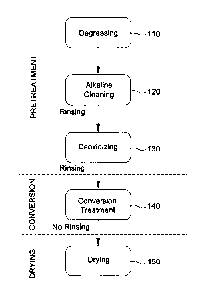
FIG. 1 is a process flow chart depicting the steps associated with our
chromium-free
conversion coating.
DETAILED DESCRIPTION
TABLE 1 shows the properties of several conducting polymers according to data
provided by suppliers of the polymers;
TABLE 2 shows the experimental conditions for PEDOT/Zr for both tested alloys;
TABLE 3 shows the experimental conditions for PPY/Zr for both tested alloys;
TABLE 4 shows the measured corrosion of alloys treated with PEDOT/Zr; and
TABLE 5 shows the measured corrosion of selected alloys treated with PPY/Zr.
TABLE 6 is a table that shows the molecular and structural formula of several
silanes
used in the present disclosure.
TABLE 7 is a table that shows the experimental conditions for PEDOT/Zr/silane
for
both tested alloys according to the disclosure.
TABLE 8 is a table that shows the experimental conditions for PPY/Zr/silane
for both
tested alloys according to the disclosure.
TABLE 9 is a table that shows the measured corrosion, adhesion and surface
contact
electrical resistance of alloys treated with PEDOT/Zr according to the
disclosure.
TABLE 10 is a table that shows the measured corrosion, adhesion and surface
contact
electrical resistance of alloys treated with PPY/Zr according to the
disclosure.
As can be appreciated by those skilled in the art, chemical conversion surface
treatments/coatings generally involve the process of immersion or other
contact of a metal
(i.e., Aluminum and/or Alloys of Aluminum) with an active bath or spray that ¨
through a redox
reaction at the metallic surface or chemical deposition at the metallic
surface due to
physicochemical changes in the treatment bath ¨ form a superficial adhered
protective
coating. Such conversion coatings typically exhibit quite low solubility and ¨
in the case of
Aluminum ¨ a thickness of approximately 20nm to 1mm, depending upon the
process
parameters and the alloy treated, while the substrate thickness lost is quite
small or minimal.
13b
CA 2819340 2018-06-18
The color of the resulting conversion coating obtained depends upon the base
material
and the bath/spray parameters.
Advantageously, the conversion coating described herein may be prepared in a
single-
step immersion process. Accordingly, parts, i.e., panels, to be coated are
bathed in a
conducting polymer dispersion in which different inorganic salts and silanes
along with other
additives that affect the bath and/or resulting coating, i.e., bath dispersion
agents, wetting
agents, or polymeric film formation agents.
FIG 1 depicts an overview of the steps of the process. More particularly, the
process
includes three general phases namely, pretreatment, conversion, and drying.
And while our
discussion herein is concerned primarily with Aluminum and certain specific
alloys of
Aluminum, our invention is not so limited. In particular, different metal
compositions and alloys
as well as additional applications, i.e., automotive, industrial, etc., would
benefit from our
inventive method and resulting coating as well.
Returning now to FIG 1, it may be observed that Pretreatment begins by
degreasing
the panels to be coated (Block 110). Degreasing may be performed using any of
a variety of
known detergent solutions and/or organic solvents. Additionally, such
degreasing ¨ like all of
the process steps ¨ may be performed by spray application or bath/immersion or
a mixture of
the two techniques.
Once the panel(s) to be coated is degreased, it is then cleaned/washed with an
alkali
solution (Block 120). Such solutions are commercially available under various
trade names
i.e., TURCO (4215NCLT), and this alkali.cleaning/washing is advantageously
performed for
approximately 10 minutes at a modest elevated temperature, i.e., 50 C. After
14
CA 2819340 2018-06-18
CA 02819340 2016-11-09
cleaning/washing, the panel is rinsed with water and then deoxidized (Block
130), with, for
example TURCO Smut Go NC for approximately 5 minutes at ambient temperature(s)
and
then rinsed. Other pickling or desmutting steps may be used depending on the
treated
substrate material and surface material or thickness to be removed.
As can be now appreciated, the process employs commercially available
pretreatment
steps. Such pretreatment is compatible with a variety of alloys and their
application is widely
understood.
In an exemplary embodiment, conversion treatment (Block 140) includes
immersion of
aluminum alloy panels, in a bath for a period of time followed by direct (no
rinse) drying (Block
150) of the treated panels. Generally, the conversion treatment bath is
prepared by an initial
stirring of a conducting, polymeric dispersion. The polymer dispersion(s) used
may be
commercially available water-based ones and exhibit satisfactory
formulation(s) including solid
content, pH, and dispersive additives. Consequently, only a minimal amount of
stirring may
be required for these commercial dispersions. Of possible further advantage,
the conversion
treatment in the bath is only a 2 minutes process.
Such conducting polymeric dispersions include Polyanaline (PANI),
Polyethylenedioxythiophene (PEDOT) and Polypyrrole (PRY) among others. The
particular
conducting polymeric dispersions used in our examples and their physical
properties are
shown in Table 1. While the discussion herein to those conducting polymeric
dispersions
exhibiting superior performance in our experiments, it should be noted that a
number of
dispersions may be suitable ¨ depending upon the particular application
requirements. More
specifically, dispersions of polyphenylene, polyphenylene vinylene,
polyethylenesulfide and
derivatives of all the mentioned conducting polymers may produce satisfactory
results.
In addition, other polymeric components such as acrylics, polyurethanes,
epoxies,
amino resins, phenolics, vinylics, polyesters, etc., may be added to enhance
particular
characteristics of the coating.
Returning now to the method, after stirring the conducting polymeric
dispersion (and
any polymeric components), a quantity of inorganic salt(s) or mixtures thereof
are added to the
conducting polymeric dispersion and subsequently mixed
15
CA 02819340 2013-06-17
until the added salts are suitably dissolved. Example salts include the
inorganic salts of
Molybdenum, Manganese, Zirconium and Titanium. More particularly, Sodium
Molybdate,
Potassium Permanganate, Potassium Hexafluorozirconate and Potassium
Hexaflorotitanate
have been used with success. Final concentrations of the added salts in the
bath solution(s)
may vary over a wide range, i.e., 2 ¨20 g/L.
After the inorganic salt(s) or mixtures thereof are added to the conducting
polymeric
dispersion and subsequently mixed until the added salts are suitably
dissolved, a quantity of
silane(s) or mixtures thereof are added to the conducting polymeric/salt(s)
dispersion and
subsequently mixed until the added silanes are suitably dissolved. The
particular silanes used
in the examples and their molecular and structural formula are shown in table
6. (3-
Glycidoxypropyl)trimethoxysilane (GPMS), 1,2-Bis(trimethoxysilyl)ethane
(TMSE), 1, 2-
Bis(Triethoxysily1) Ethane (BTSE), Bis[3-(trimethoxysilyl)propyl]amine (BAS)
and
Vinyltriacetoxysilane (VTAS) have been used with success. The final
concentrations of the
added salts in the bath solution(s) may vary over a range, e.g., % 0.01 vol.
(% v/v) ¨ % 1.0 vol.
(% v/v).
Finally, the polymeric dispersion/inorganic salt /silane solution is
subsequently pH
adjusted using alkaline compounds such as ammonia or phosphate or acidic
compounds,
including hexaflorozirconic acid and fluorhydric acid.
EXPERIMENTAL / RESULTS
A number of samples of two particular aluminum alloys, namely 2024T3 and
7075T6
alloys were subjected to our inventive Chromium-free conversion process and
evaluated.
Those showing superior characteristics in salt spray fog corrosion tests
(SSFCT) were
obtained using PPY and PEDOT in combination with Hexaflorozirconate. The
particular
experimental conditions are shown in Table 2, Table 3 for PEDOT/Zr, PPY/Zr,
based
compositions and Table 4, and Table 5 show the obtained results respectively.
For all of the
samples shown in these Tables, the drying conditions were substantially room
temperature
and pressure, for a period of time of at least 24 hours.
More specifically, Table 2 shows the experimental conditions for PEDOT/Zr. In
this
set, the [Zr] concentration was effected by varying the amounts of K2ZrF6 and
the pH was
adjusted with H2ZrF6 and/or NH4OH.
16
Table 3 shows the experimental conditions used for a PPY/Zr set of samples. In
this
particular set, the [Zr] concentration was effected by varying the amounts of
K2ZrF6 and the pH
was adjusted with H2ZrF6 and/or NH4OH.
Turning now to Table 4, there it shows the corrosion resistance for our
inventive
PEDOT/Zr conversion coating on both 2024-T3 and 7075-T6 aluminum alloys. The
results
obtained were after 168 hours of salt spray fog corrosion testing (SSFCT) and
the hexavalent
chromium based commercial ALODINE 1200STM exhibits the best corrosion
performance,
with a corrosion score of 10Ø The corrosion score values go from 0 for the
worst corrosion
performance to 10 for best corrosion performance.
Similarly, Table 5 shows the corrosion resistance for our inventive PPY/Zr
coating on
the 2024-T3 and 7075-T6 alloys as well as the ALODINE 1200S TM treated alloys.
A number of samples of two particular aluminum alloys, namely 2024T3 and
7075T6
alloys, were subjected to the polymeric dispersion/inorganic salt /silane
chromium-free
conversion process and evaluated. Those showing superior characteristics in
salt spray fog
corrosion tests (SSFCT) were obtained using PPY and PEDOT in combination with
hexaflorozirconate and GPMS, TMSE and BTSE silanes ¨ added either alone or in
combination. Those showing superior characteristics in the scribed wet tape
paint adhesion
tests of a subsequently applied organic coating were obtained using PPY and
PEDOT in
combination with hexaflorozirconate and GPMS, TMSE, BTSE, BAS and VTAS silanes
¨
added either alone or in combination ¨. Some of the proposed treatments
provided combined
superior characteristics in salt spray fog corrosion tests and in the scribed
wet tape paint
adhesion tests of a subsequently applied organic coating. Additionally, some
of those
treatments providing combined superior characteristics in salt spray fog
corrosion tests and in
wet tape paint adhesion tests of a subsequently applied organic coating also
provided superior
characteristics in surface contact electrical resistance measurements. The
particular
experimental conditions are shown in the tables of TABLES 8 and 9 for
PEDOT/Zr/silane,
PPY/Zr/silane, based compositions and the tables of TABLES 10 and 11 show the
obtained
results, respectively. For all of the samples shown in these tables of TABLES
8-11, the drying
conditions were substantially room temperature and pressure, for a period of
time of at least
24 hours.
17
CA 2819340 2018-06-18
CA 02819340 2013-06-17
More specifically, the table of TABLE 8 shows the experimental conditions for
PEDOT/Zr/silane. In this set, the [Zr] (zirconium) concentration was effected
by varying the
amounts of K2ZrF6 (potassium hexafluorozircoante) and the pH was adjusted with
H2ZrF6
(fluorozirconic acid) and/or NH4OH (ammonium hydroxide).
The table of TABLE 9 shows the experimental conditions for a PPY/Zr/silane set
of
samples. In this particular set, the [Zr] (zirconium) concentration was
effected by varying the
amounts of K2ZrF6 (potassium hexafluorozirconate) and the pH was adjusted with
H2ZrF6
(fluorozirconic acid) and/or NH4OH (ammonium hydroxide).
Turning now to the table of TABLE 10, there it shows the corrosion resistance
for the
PEDOT/Zr/silane conversion coating on both 2024-13 and 7075-16 aluminum
alloys. The
results obtained were after 168 hours of salt spray fog corrosion testing
(SSFCT) and the
hexavalent chromium based commercial ALODINE 1200S exhibited the best
corrosion
performance, with a corrosion score of 10Ø The corrosion score values go
from 0 (zero) for
the worst corrosion performance to 10 (ten) for best corrosion performance.
The table in
TABLE 8 also shows the adhesion performance of a subsequently applied organic
coating on
both 2024-T3 and 7075-T6 aluminum alloys. The paint adhesion performance was
measured
according to a wet tape paint adhesion test. Once dried (after 14 days air
curing), the
corresponding conversion coated panels were painted with an epoxy primer
according to the
MIL-PRF-85582 standard. The epoxy primer used was a water-reducible epoxy
primer system
made of 10PW20-4 base and ECW-104 hardener according to MIL-PRF-85582 Type 1
Class
2, provided by Akzo Nobel Aerospace Coatings, By. Two parallel, 2 inch long
scratches, 3/4 to
1 inch apart through the coating and to the substrate were made on the panels.
The parallel
scratches were joined with two intersecting lines, or an "X" pattern. The
primed and scribed
panels were immersed in deionised water during 24 hours, prior to carrying out
the wet paint
adhesion tests. Within 2 minutes after removing test panels from water
adhesive tape was
applied and pressed against the test surface with firm hand pressure and then
removed. The
hexavalent chromium based commercial ALOD1NE 1200S exhibited the best pant
adhesion
performance, with an adhesion score of 10ØThe adhesion test score values go
from 0 (zero)
for the worst adhesion performance (total detachment of the primer) to 10
(ten) for best
adhesion performance (no detachment of the primer). The table in TABLE 8 also
shows the
surface contact electrical resistance for the PEDOT/Zr/silane conversion
coating on 2024-T3,
7075-T6 and 6061-16 aluminum alloys. The surface contact electrical resistance
of the
18
CA 02819340 2016-11-09
coatings was measured as described in the MIL-DTL-81706-B standard. The
applied load
shall be within one percent of the calculated 200psi applied pressure. The
contacting
electrodes were copper or silver-plated copper with a finish not rougher than
that obtained by
the use of 000 metallographic abrasive paper. The electrodes were flat enough
so that when
the load was applied without a specimen between them, light is not visible
through the
contacting surface. The area of the upper electrode was one square inch (25
square mm) and
the area of the lower electrodes was larger. The maximum electrical resistance
values allowed
by aeronautical standards for 6061 T6 alloy are of 5000p)/square inch (5
mO/square inch)
before salt spray exposure test. The hexavalent chromium based commercial
ALODINE
1200S exhibited the lowest surface contact electrical resistance for the 2024-
T3 and 7075-T6
alloys. The values for the PEDOT/Zr/silane treatments were also well below the
5 mn/square
inch.
Similarly, the table of TABLE11 shows the corrosion resistance, the paint
adhesion
performance and the surface contact electrical resistance measurements for the
PPY/Zr/silane
coatings on the 2024-13, 7075-16 and 6061-T6 alloys as well as the ALODINE
1200S treated
alloys.
At this point, it should be noted that in addition to the Zr salts used in
these exemplary
tests, other salts ¨ either alone or in combination ¨ may produce satisfactory
results as well.
In particular, salts of Vanadium, Cerium, Hafnium, Silicon, Aluminum, Boron,
Cobalt,
Magnesium, and Zinc may be employed. Additionally, other bath components such
as pH
adjusting compounds, solvents, non-aqueous dispersion media, other silanes,
dispersing
agents, surfactants and coalescing solvents may be used to provide various
degrees of
coating effectiveness. Further, while the method and resulting coating(s) have
been
described in the context of immersion bath(s), it is understood that
alternative coating, i.e.,
spray coating may be used as well. Lastly, other metallic substrates, such as
steel, aluminum,
copper, and/or iron and/or their alloys, may benefit from the method and
coating(s) described
herein.
19
CA 02819340 2013-06-17
A
Material
PAM PEDOT PPY
Property
-
Polymer Polyanifine Polyethylenedloxythlopherie Polypyrrole
Solid
6.0% 1.2 - 1.4% 6.0%
Content
Dilution
Water Water Water
With
pH 2.3 1.6 -2.5 3 or less
Conductivity p 1 - 2
Up To 10 0.01 -0.001
(Slen)) pews Cast Film Cast Film
Surface
Resistivity 10E4 10E8 10E4 - 10E8
(Ohm)
1/18C08tty 18 Pas 60 100 mPas NA.
Supplier Pantpol Bayer Eeonyx
Trade Name Panlpol W Baytron P Eeonomer 7000
TABLE 1
PEDOT/Zr
Experiment (PEDOT] (%) [Zr] (g/L) = pH "
PEDOT 1 0.43 8 2
PEDOT 2 0.86 6 3.6
,
PEDOT 3 0.43 2 2
PEDOT 4 1.30 2 2
PEDOT 5 043 a
PEDOT 6 1.30 2 6
PEDOT 7 1.30 8 2
PEOOI6 0.86 5 3.5
P60019 0.43 2 6
PEDOT 10 0.88 5 3.6
P600111 1.30 8
PEDOT 12 0.43 5 3.5
P600113 0.88 2 3.5
PEDOT 14 0.86 5 2
TABLE 2
Experimental Conditions for PEDOT / Zr
atzd on. 0K2Z11s1 911.; "pH Adjusted with H27.1Fs andlor 5ti4OH
CA 02819340 2013-06-17
PPY / Zr
Experiment IPEDOT] (%) [Zr) (g/L) * pH **
PPY 1 8 2 2
PPY 2 2 8 2
PPY 3 6 8 5
PPY 4 2 2 2
PPY 5 4 5 3.5
PPY6 4 5 3.5
PPYT 2 2 5
PPY 8 4 6 3.6
PPY 9 6 8 2
PPY 10 2 8 5
PPY 11 6 2 5
PPY 12 2 5 3.5
PPY 13 4 2 3.5
PPY 14 4 5 2
TABLE 3
Experimental Conditions for PPY / Zr
.rzjg1IZpHAcmt.dMthH2Zv1artWor N}14011
Corrosion Score
EXPERIMENT
2024 T3 707616
PEDOT 1 3.0 3.0
PEDOT 2 3.6 8.5
PEDOT 3 1.0 3.5
PEDOT 4 1.5 3.7
PEDOT 5 4.0 9.0
PEDOTG 2.0 8.6
PEDOT 7 2.0 3.5
PEDOT 8 3.5 8.2
P5001 9 3.5 9.0
P5001 10 4.0 4.5
P5001 11 8.0 2.7
P5001 12 9.5 9.5
PEDOT 13 2.6 5.5
PEDOT 14 2.6 2.0
ALODINE 12008 10 10
TABLE 4
Experimental Results for PEDOT / Zr
21
CA 02819340 2013-06-17
Corrosion Score
EXPERIMENT
2024T3 7075 T6
PPY 1 1.0 1.50
PPY 2 1.0 1.75 .
PPY 3 4.0 5.0
PPY 4 1.0 1.75
PPY 5 5.0 6.5
PPY 6 4.0 5.5
PPY 7 4.0 4.5
PRY 8 4.0 5.25 .
PPY 9 0.75 1.75
PPY 10 4.76 8.0
PPY 11 2.5 6.0 -
PPY 12 4.0 5.0
,
PPY 13 3.0 4.0
PPY 14 0.5 1.6
ALODINE 1200s 10 10
TABLE 5
Experimental Results for PPY / Zr
22
CA 02819340 2013-06-17
TABLE 6
SHORT
= COMPOUND MOLECULAR FORMULA ESTRUCTURAL FORMULA
NAME
(3- OcH3
1
Glycidoxypropyl) GPMS C9H2005S1
trimethoxysilane 0CH3 0
1,2-Bis OC H3
9CH36._ocH
(trimethoxysily1) TMSE Ca F122 06S i2
ethane CSCH3 0CH3
H3C..===-,0
1,2-
H3c,......,o-Si--., 9cH3
r
Bis(Triethoxysily1) BTSE [-CH2Si(0C2H5)312 0 \-li-
0,,,-CH3
0.,
Ethane
cH3 1
cH3
' ocH3 ycH,
Bis[3-
(trimethoxysily1) BAS [(C1130)3Si(CH2)3]2NH
I 14
propyllamine &M ocHa
a
Vinyltriacetoxy
VTAS (0,1-13C0-2)3Sirs--,. .1-1=--CH2
......<-1i-0
silane
a
TABLE 7 - Experimental conditions for PEDOT/Zr/Silane
,
PEDOT / Zr / Silane
Experiment [PEDOT] r/o) [Zr] (g/L)* [Silane] (% vol.) pH**
PEDOT 15 0.43 5 0.10 GPMS 3.5 .
PEDOT 16 0.43 , 5 0.25 GPMS 3.5
PEDOT 17 0.15 5 0.10 GPMS 3.5
PEDOT 18 0.15 5 0.06 TMSE 3.5
* [Zr] g/L = [K2ZrF6] g/L
** pH adjusted with H2ZrF6 and/or NH4OH
23
,
CA 02819340 2013-06-17
TABLE 8 - Experimental conditions for PPY/Zr/Silane
PPY / Zr / Silane
Experiment [PPY] (YO) [Zr] (g/L)* [Silane] (% vol.) pH**
PPY 15 0.25 5 0.10 GPMS 3.5
PPY 16 0.25 5 0.10 GPMS 3.0
PPY 17 0.25 5 0.05 GPMS 3.0
PPY 18 0.10 5 0.10 GPMS 3.5
PPY 19 0.50 5 0.10 GPMS 3.5
PPY 20 0.15 5 0.10 GPMS 3.5
PPY 21 0.25 5 0.25 GPMS 3.5
PPY 22 0.50 5 0.25 GPMS 3.5
PPY 23 0.25 5 0.06 EASE 3.5
PPY 24 0.25 5 0.06 TMSE 5.0
PPY 25 0.25 5 0.15 TMSE 3.5
PPY 26 0.25 5 0.15 TMSE 5.0
PPY 27 2.0 8 0.06 TMSE 5.0
PPY 28 0.25 5 0.10 GPMS + 0.09 TMSE 3.5
PPY 29 0.25 8 0.06 TMSE 3.5
PPY 30 0.25 8 0.15 TMSE 3.5
PPY 31 0.25 8 0.10 GPMS + 0.09 TMSE 3.5
PPY 32 0.25 5 0.08 BTSE 3.5
PPY 33 0.25 5 0.08 BTSE 5.0
PPY 34 0.25 5 0.21 BTSE 3.5
PPY 35 0.25 5 0.21 BTSE 5.0
PPY 36 0.25 5 0.10 GPMS + 0.13 BTSE 3.5
PPY 37 0.25 8 0.08 BTSE 3.5
PPY 38 0.25 5 0.10 GPMS + 0.25 3.0
(BAS:VTAS 4:1 vol.)
PPY 39 0.25 5 0.25 BAS:VTAS 4:1 vol. 3.0
PPY 40 0.25 5 0.10 GPMS + 0.05 TMSE 3.0
PPY 41 0.25 5 0.10 GPMS + 0.10 TMSE 3.0
PPY 42 0.25 5 0.10 GPMS + 0.15 TMSE 3.0
PPY 43 0.25 5 0.10 BTSE 3.0
PPY 44 0.25 5 0.25 BTSE 3.0
PPY 45 0.25 5 0.25 BTSE 2.5
PPY 46 0.25 5 0.05 GPMS + 0.15 TMSE 3.0
PPY 47 0.25 5 0.10 GPMS + 0.20 TMSE 3.0
* [Zr] g/L = [K2ZrF6] g/L
** pH adjusted with H2ZrF6 and/or NH4OH
24
,
CA 02819340 2013-06-17
- TABLE 9 - Experimental results for PEDOT/Zr/Silane
EXPERIMENT CORROSION ADHESION SCORE SURFACE CONTACT ELECTRICAL
SCORE RESISTANCE (nnohm/sq inch)
2024 T; 7075 T6 2024 T3 7075 16 2024 T3 7075 T6 6061 T6
PEDOT 12 9.5 9.5 0 0 2.21 1.29 0.23
PEDOT 15 4.0 7.8 1.60 2.15 - - -
PEDOT 16 4.0 7.5 8.65 7.80 - - -
PEDOT 17 6.4 9.4 9.70 9.70 - - -
PEDOT 18 - 8.0 - 9.65 - - -
Alodine 1200S 10.0 10.0 10.0 10.0 0,71 0,86 X
,
,
CA 02819340 2013-06-17
TABLE 10 - Experimental results for PPY/Zr/Silane
EXPERIMENT CORROSION SCORE ADHESION SCORE SURFACE CONTACT ELECTRICAL
RESISTANCE (nohm/sq inch)
2024 T3 7075 T6 2024 T3 7075 T6 2024 T3 7075 T6 6061 T6
. PPY 10 4.75 8.0 0 0 1.21 2.06 0.42
PPY 15 - 9.0 - 9.8 - - 0.69
PPY 16 7.2 8.7 9.75 9.8 - - -
PPY 17 - 9.3 - 9.8 - - -
PPY 18 5.0 8.2 9.8 9.8 - - -
PPY 19 6.2 8.5 4.05 8.05 - - -
PPY 20 7.0 8.0 9.8 9.8 - - -
PPY 21 4.6 7.6 9.75 9.8 - - -
PPY 22 5.3 7.3 5.1 8.5 - - -
PPY 23 _ 9.0 - 9.7 - - -
PPY 24 _ 9.5 - 9.45 - - -
PPY 25 - 9.3 - 9.6 - - -
PPY 26 _ 8.5 _ 9.5 _ _ _
PPY 27 _ 8.5 - 7.05 - - -
PPY 28 _ 9.2 - 9.8 - - -
PPY 29 . 8.8 - 9.75 - - -
PPY 30 - 8.5 - 9.75 - - -
PPY 31 _ 8.7 _ 9.75 _ _ -
PPY 32 _ 8.4 - 9.75 - - -
PPY 33 _ 9.0 - 9.7 - - -
PPY 34 _ 8.5 - 9.7 - - -
PPY 35 _ 8.6 - 9.55 - - -
PPY 36 _ 8.0 - 9.7 - - -
PPY 37 _ 9.0 - 9.75 - - -
PPY 38 _ <9.3 - 9.8 - - -
PPY 39 _ <9.3 _ 9.8 _ _ -
PPY 40 _ <9.3 _ 9.8 _ . _
PPY 41 _ <9.3 _ 9.8 - - -
PPY 42 _ 9.3 _ 9.75 _ - -
PPY 43 _ <9.3 - 9.8 - - -
PPY 44 .. <9.3 _ 9.8 - - -
PPY 45 _ <9.3 - 8.65 _ - -
PPY 46 - <9.3 - 9.8 - - -
PPY 47 - <9.3 - 9.8 - - -
Alodine 1200 10.0 10.0 10.0 10.0 0,71 0,86 X
26
,
CA 02819340 2016-11-09
While we have discussed and described embodiments using some specific
examples,
those skilled in the art will recognize that the teachings herein are not so
limited. More
specifically, it is understood that the method and coating may be used in
virtually any
application requiring corrosion protection, and/or adhesion of subsequently
applied organic
coating(s) and/or low electrical surface contact resistance and in particular
those applications
concerned with the problems associated with hexavalent Chromium. Accordingly,
it is
understood that the method and coating may be applicable to any automotive,
marine,
construction, industrial, or household use in addition to aeronautical
applications.
27