Note: Descriptions are shown in the official language in which they were submitted.
CA 02820541 2013-06-21
79580-110D
1
CASPASE INHIBITORS AND USES THEREOF
This application is a divisional of Canadian Patent Application
No. 2,557,645, filed February 28, 2005.
It will be understood that any reference to "the present
invention" or the like may refer to the subject matter of this
.
divisional application and/or its parent.
Field of the Invention
[0001] This invention relates to compounds, and
compositions thereof, that are useful as caspase inhibitors.
[0002] This invention also relates to processes for
preparing these compounds.
[0003] This invention further relates to pharmaceutical
compositions comprising said compounds and to the use of the
compounds and compositions thereof for the treatment of
diseases and disorders related to caspase-mediated conditions.
Background of the Invention
[0004] Caspases are a family of cysteine protease enzymes
that are key mediators in inflammation. Caapase-1 (ICE)
processes pre-IL-113 to produce the active form of IL-113 [WO
99/47545]. ICE has also been linked to the conversion of pro-
TGIF to IGIF and/or to the production of IFN-7 [Id.]. Both IL-
_
lp and IFN-y contribute to the pathology associated with
=
inflammatory, infectious, and autoimmune diseases (see, e.g.,
WO 99/47545; J. Invest. Dermatology, 120(1), pp. 164-167
(2003); Br. J. Dermatology, 141, pp. 739-746 (1999); Science,
CA 02820541 2013-06-21
=
W02005/085236 PCT/US2005/006540
-2-
282, pp. 490-493 (1998); Schweiz. Med. Wochenschr, 130, pp.
1656-1661 (2000)1.
[0005] Caspases are also key mediators in the signaling
pathways for apoptosis and cell disassembly [N.A. Thornberry,
Chem. Biol., 5, pp. R97-R103 (19981]. These signaling
pathways vary depending on cell type and stimulus, but all
apoptosis pathways appear to converge at a common effector
pathway leading to proteolysis of key proteins. Caspases are
involved in both the effector phase of the signaling pathway
and further upstream at its initiation. The upstream caspases
involved in initiation events become activated and in turn
activate other caspases that are involved in the later phases
of apoptosis.
[0006] The utility of caspase inhibitors to treat a variety
of mammalian disease states associated with an increase in
cellular apoptosis has been demonstrated using peptidic
caspase inhibitors. For example, in rodent models, caspase
inhibitors have been shown to reduce infarct size and inhibit
cardiomyocyte apoptosis after myocardial infarction, to reduce
lesion volume and neurological deficit resulting from stroke,
to reduce post-traumatic apoptosis and neurological deficit in
traumatic brain injury, to be effective in treating fulminant
liver destruction, and to improve survival after endotoxic
shock [H. Yaoita et al., Circulation, 97, pp. 276-281 (1998);
M. Endres et al., J. Cerebral Blood Flow and Metabolism, 18,
pp. 238-247, (1998); Y. Cheng et al., J. din. Invest., 101,
pp. 1992-1999 (1998); A.G. Yakovlev et al., J. Neurosci., 17,
pp. 7415-7424 (1997); I. Rodriquez et al., J. Exp. Med., 184,
pp. 2067-2072 (1996); Grobmyer et al., Mol. Med., 5, p. 585
(1999)].
[0007] However, due to their peptidic nature, such
inhibitors are typically characterized by undesirable
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-3-
pharmacological properties, such as poor cellular penetration
and cellular activity, poor oral absorption, poor stability
and rapid metabolism [J.J. Plattner and D.W. Norbeck, in Drug
Discovery Technologies, C.R. Clark and W.H. Moos, Eds. (Ellis
Horwood, Chichester, England, 1990), pp. 92-126]. This has
hampered their development into effective drugs. These and
other studies with peptidic caspase inhibitors have
demonstrated that an aspartic acid residue is involved in a
key interaction with the caspase enzyme [K.P. Wilson et al.,
Nature, 370, pp. 270-275 (1994); Lazebnik et al., Nature, 371,
p. 346 (1994)].
[0008] Accordingly, peptidyl and non-peptidyl aspartic acid
compounds are useful as caspase inhibitors.
(0009] A need nevertheless exists for compounds that have
the ability to act as caspase inhibitors, particularly with
selective activity against certain caspases.
Summary of the Invention
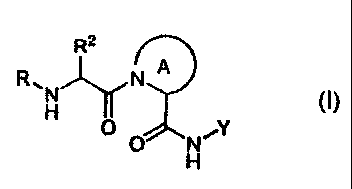
[0010] The present invention provides a compound of formula
R2
R,
0
0
wherein the variables are as defined herein.
[0011] The present invention also provides processes for
preparing these compounds, compositions, pharmaceutical
compositions, and methods of using such compounds and
compositions for inhibiting caspases. These compounds are
particularly useful as selective caspase-1/capase-8
inhibitors.
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-4-
Detailed Description of the Invention
[0012] The present invention provides a compound of foimula
R2
RN
N
0
0
wherein:
OH
5140
Y is 0 or
R '
R is R3C (0) HC (0) , R3S02-, R30C (0) , (R3) 2NC (0)
( R3 ) (H)NC(0), R3C(0)C(0)-, R3-, (R3)2NC(0)C(0),
(R3) (H)NC(0)C(0), or R30C(0)C(0)-;
R1 is H, aliphatic, cycloaliphatic, aryl, heterocyclyl,
heteroaryl, cycloalkyl-aliphatic-, cycloalkenyl-aliphatic-,
aryl-aliphatic-, heterocyclyl-aliphatic-, or heteroaryl-
aliphatic-, wherein any hydrogen atom is optionally and
independently replaced by R8 and any set of two hydrogen atoms
bound to the same atom is optionally and independently
replaced by carbonyl;
Ring A is:
or
uvw
wherein, in each ring, any hydrogen atom is optionally and
independently replaced by R4 and any set of two hydrogen atoms
bound to the same atom is optionally and independently
replaced by carbonyl;
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-5-
R3 is aliphatic, cycloaliphatic, aryl, heterocyclyl,
heteroaryl, cycloaliphatic-aliphatic-, aryl-aliphatic-,
heterocyclyl-aliphatic-, or heteroaryl-aliphatic-; or two R3
groups bound to the same atom form together with that atom a
3-10 membered aromatic or nonaromatic ring; wherein any ring
is optionally fused to an aryl, heteroaryl, cycloalkyl, or
heterocyclyl; wherein up to 3 aliphatic carbon atoms may be
replaced by a group selected from 0, N, S. SO, and SO2,
wherein R3 is substituted with up to 6 substituents
independently selected from 119;
R4 is halogen, -0R9, -NO2, -CN, -CF3, -0CF3, -R9, 1, 2-
methylenedioxy, 1, 2-ethylen.edioxy, -N(R9)2, -SR9, -SOR9, -S02R9,
-SO2N(R9) 2, -S03R9, -C(0)R9, -C (0)C (0)R9, -C (0)C (0)0R9.
-C (0) C (0)N(R9) 2, -C (0) CH2C (0)R9, -C(S)R9, (S)0R9, (0)0R9,
- (0)R9, -C (0)N(R9) 2, -OC (0)N(R9)2,
(S)N(R9) ""' (CH2)0-
2NHC (0)R9, -N(R9)N (R9) COR9, -N(R9)N(R9)C(0)0R9,
-N(R9)N(R9) CON (R9) 2, -N (R9) SO2R9, -N (R9) SO2N (R9) 2, -N(R9)C (0) OR9,
-N (R9) C (0)R9, -N (R9) C (S)R9, -N (R9) C (0)N (R9) 2, -N (R9) C (S)N (R9)2,
-N(COR9)COR9, -N(0R9)R9, -C (=NH)N(R9)2, (0)N(0R9)R9,
-C (=NOR9) R9, -OP (0) (OR9) 2, -13 (0) (R9)2, -P(0) (OR9) 2, or
-P(0) (H) (OR9) ;
R2 is -C(R5)(R6)(R7), aryl, heteroaryl, or C3-7 cycloalkyl;
R5 is H or a C1_6 straight-chained or branched alkyl;
R6 is H or a C1-6 straight-chained or branched alkyl;
R7 is -CF3, -C3_7cycloalkyl, aryl, heteroaryl, heterocycle,
or a C1-6 straight-chained or branched alkyl, wherein each
carbon atom of the alkyl is optionally and independently
substituted with R10;
Or R5 and R7 takentogether with the carbon atom to which
they are attached foLia a 3-10 membered cycloaliphatic;
R8 and RY are each independently halogen, -0R9, -NO2, -CN,
-CF3, -0CF3, -R9, 1,2-methylenedioxy, 1,2-ethylenedioxy,
CA 02820541 2013-06-21
- 79580-110D
= - 6
-N (R9) 2, -SR9, -SOR9, -S02R9, -SO2N (R9) 2, -S03R9 -C (0) R9,
--c (0) C (0) R9, -C (0) C (0) OR9, -C (0) C (0)N (R9) 2, -C (0) CH2C (0) R9,
-C (S) R9, -C (S) OR9, -C (0) OR9, -OC (0) R9, -C (0)N (R9) 21 -OC (0)N (R9)
2r
--c (S) N (R9)2, - (Cl-I2) 0-2NHC (0) R9, -N (R9) N (R9) COR9,
-N (R9)N (R9)C(0) 0R9, -N(R9)N(R9)CON(R9)2, -N(R9)S02R9,
-N(R9)s02N (R9)2, -N(R9)c(0)0R9, -N(R9)C(0)R9, -N(R9)c(s)R9,
-N(R9)C(0)N(R9)2, -N(R9)C(S)N(R9)2, -N(COR9)COR9, -N (OR9) R9,
-C (=NH)N (R9)2, -C (0)N (OR9)R9, -C (=NOR9) R9, -OP(0) (00)2,
-P (0) (R9)2, -P (0) (OR9)2, and -P (0) (H) (0R9) ;
109
R is hydrogen, aliphatic, cycloaliphatic, aryl,
heterocyclyl, heteroaryl, cycloaliphatic-aliphatic-, aryl-
aliphatic-, heterocyclyl-aliphatic-, or heteroaryl-aliphatic-;
wherein any hydrogen atom is optionally and independently
replaced by R8 and any set of two hydrogen atoms bound to the
. same atom is optionally and independently replaced by carbonyl;
RI is halogen, -ORn, -NO2, -CN, -CF3, -0CF3, -Rn, or
-SRn; wherein Ril is C1-4-aliphatic-.
[0012a] In a particular embodiment, the present invention
relates to a compound of formula I:
R2
A
NN/./
0
0
=
wherein:
= CA 02820541 2013-06-21
, 79580-110D
6a
0
0
is
L2-4((1431-1H
or ,te240
0 0¨R1
Y
R is H, C112aliphatic, C3_10cycloaliphatic, C6_10ary1,
5-10 membered heterocyclyl, 5-10 membered heteroaryl,
(C3_10cycloa1ky1)-(C1-12aliphatic)-, cycloalkenyl-
(C1_12aliphatic)-, (C6-10arY1)-(Ci-12aliphatic)-, (5-10 membered
heterocycly1)-(C1-12a1iphatic)-, or (5-10 membered heteroary1)-
(C1_12aliphatic)-, wherein any hydrogen atom is optionally and
independently replaced by R9 and any set of two hydrogen atoms
bound to the same atom is optionally and independently replaced
by.carbonyl;
Ring A is:
Or =AArv ;
wherein, in each ring, any hydrogen atom is
optionally and independently replaced by R4 and any set of two
hydrogen atoms bound to the same atom is optionally and
independently replaced by carbonyl;
= R4 is halogen, -0R9, -NO2, -CN, -CF3, -0CF3, -R9,
1,2-methylenedioxy,.1,2-ethylenedioxy, -N(R9)2, -SR9, -SOR9,
-S02R9, -SO2N (R9) 2/ -S03R9f -C (0) R9, -C (0) C (0) R9, -C (0) C (0) OR9,
-C (0) C (0) N (R9) 2, -C (0) CH2C (0) R9r (S) R9, -C (S) OR9, -C (0) OR9,
-OC (0) R9, -C (0)N (R9) 2, -0C (0)N (R9) 2, -C (S) N (R9) 2,
CA 02820541 2013-06-21
79580-110D
= 6h
- (CH2) 0-2NHC (0) R9, -N (R9) N (R9) COR9, -N (R9) N (R9) C (0) OR9,
-N (R9) N (R9) CON (R9) 2, -N (R9) S02R9, -N (R9) SO2N (R9) 2, -N (R9) C (0)
OR9,
-N (R9) C (0) R9, -N (R9) C (S) R9, -N (R9) C (0) N (R9)2, -N (R9) C (S) N
(R9)2,
-N (00R9) COR9, -N (OR9) R9, -C (=NH) N (R9)2, -C (0) N (OR9) R9,
-C(=NOR9)R9, -OP(0) (OR9) 2, -P(0) (R9)2, -P(0) (OR9)2, or
-P(0) (H) (OR9) ; =
R2 is -C (R6) (R6) (R7) , C6_10aryl, 5-10 membered
heteroaryl, or C3-7 Cycloalkyl;
R6 is H or a C1-6 straight-chained or branched alkyl;
R6 is H or a C1-6 straight-chained or branched alkyl;
= R7 is -CF3, -C3_7cycloalkyl, C6_19ary1, 5-10 membered
heteroaryl, heterocycle, or a C1-6 straight-chained or branched
alkyl, wherein each carbon atom of the alkyl is optionally and
independently substituted with R1-0;
or R5 and R7 taken together with the carbon atom to
which they are attached form a 3-10 membered cycloaliphatic;
and R8'. are each independently halogen, -0R9, -NO2,
-CN, -CF3, -0CF3, -R9, 1, 2-methylenedioxy, 1, 2-ethylenedioxy,
-N (R9) 2, -SR9, -SOR9, -S02R9, -SO2N (R9) 2, -S03R9, -C (0) R9,
-C (0 ) C (0) R9, -C (0) C (0) OR9, -C (0)C (0)N (R9)2, -C (0)CH2C (0) R9, =
-C (S) R9, -C (S) OR9, -C (0) OR9, -OC (0) R9, -C (0)N (R9)2, -0C (0)N (R9)2,
-C (S) N (R9) 2, - (CH2) 0_2NHC (0) R9, -N (R9) N (R9) COR9,
-N (R9)N (R9) C (0) OR9, ;-1\1 (R9)N (R9)CON (R9)2, -N (R9) SO2R9,
-N(R9) SO2N (R9)2, -N (R9) C (0) OR9, -N (R9)C (0)R9, -N (R9) C (S)R9,
-N (R9) C (0)N (R9)2, -N (R9) C (S)N (R9)2, -N(COR9)COR9, -N (OR9) R9,
-C (=NH) N (R9)2, -C (0 ) N (OR9) R9, -C (=NOR9) R9, -OP(0) (OR9)2,
-P (0) (R9.)2, -P (0) (OR9) 2, or -P (0) (H) (OR9) ;
CA 02820541 2013-06-21
79580-110D
6c
R9 is hydrogen, C1-12aliphatic, C3_10cycloaliphatic,
C6,10aryl, 5-10 membered heterocyclyl, 5-10 membered heteroaryl,
(C3,10cycloaliphatic)-(Ci_12aliphatic)-, (C6_10ary1)-
(C1,12aliphatic)-, (5-10 membered heterocycly1)-
(Ci_i2aliphatic)-, or heteroary1-(C1.42aliphatic)-; wherein any
hydrogen atom is optionally and independently replaced by R9
and any set of two hydrogen atoms bound to the same atom is
optionally and independently replaced by carbonyl;
R1 is halogen, -0R11, -NO2, -CN, -CF3, -0CF3, -R11, or
-SR11; and
Ril is C1-1-aliphatic-.
[0013] The present invention also provides a compound of
formula II:
0 R2
A
R3x N(-3
, 0 ,2=NY
0
II
wherein:
0
0
0H
(-1.2(4f6
Y is 0 or 0-W ;
CA 02820541 2013-06-21
W02005/085236 PCT/US2005/006540
-7-
R1 is H, aliphatic, cycloalkyl (e.g., cyclopentyl),
cycloalkenyl, aryl, heterocyclyl, heteroaryl, cycloalkyl-
aliphatic- cycloalkenyl-aliphatic-, aryl-aliphatic-,
heterocyclyl-aliphatic-, or heteroaryl-aliphatic-, wherein any
hydrogen atom is optionally and independently replaced by R8
and any set of two hydrogen atoms bound to the same atom is
optionally and independently replaced by carbonyl;
Ring A is:
Nr?N7 VN`2(
or ww ;
wherein, in each ring, any hydrogen atom is optionally and
independently replaced by R4 and any set of two hydrogen atoms
bound to the same atom is optionally and independently
replaced by carbonyl (or in an alternative embodiment,
carbonyl or (C3-C6) spirocycle; )
R4 is halogen, -00, -NO2, -CN, -CF3, -0CF3, -R9, 1,2-
methyl enedioxy, 1, 2 -ethyl enedioxy, -N(R9) 2, -SR9, -SOR9, -SA4R9,
-SO2N(R9)2, -S03R9, -C(0)R9, -C (0)C (0)R9, -C(0)C(0)0R9,
-C(0)C(0)N(R9)2, -C(0)CH2C(0)R9, -C(S)R9, -C(S)0R9, -C(0)0R9,
(0)R9, -C(0)N(R9)2, -0C(0)N(R9)2, -C(S)N(R9)2, (CH2)0-
2NHC(0)R9, -N(R9)N(R9)COR9, -N(R9)N(R9)C(0)0R9,
-N(R9)N(R9)CON(R9)2, -N(R9) S02R9, -N(R9) SO2N(R9) 2, -N(R9)C(0)0R9,
-N(R9)C(0)R9, -N(R9)C(S)R9, -N(R9)C(0)N(R9)2, -N(R9)C(S)N(R9)2,
-N(COR9)COR9, -N(0R9)R9, -C(=Nii)N(R9)2, -C(0)N(0R9)R9,
-C (=NOR9) R9 , -OP (0) (OR9)2, -P(0) (R9)2, -P(0) (OR9)2, or
-P(0) (H) (OR9);
R2 is -C(R5) (R6) (R7) , aryl, heteroaryl, or -C3_7 cycloalkyl;
R5 is H or a C1-6 straight-chained or branched alkyl;
R6 is H or a C1-6 straight-chained or branched alkyl;
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-8-
R7 is -CF3, -C3_7 cycloalkyl, aryl, heteroaryl,
heterocycle, or a C1-6 straight-chained or branched alkyl,
wherein each carbon atom of the alkyl is optionally and
independently substituted with R10;
(or in an alternative embodiment, R5 and R7 taken together
with the carbon atom to which they are attached form a 3-10
membered cycloaliphatic);
R3 is phenyl, thiophene, or pyridine, wherein each ring is
optionally substituted with up to 5 groups independently
selected from R8', and wherein at least one position on the
phenyl, thiophen.e, or pyridine adjacent to bond x is
substituted by R12, wherein R12 has no more than 5 straight-
chained atoms;
R8 and R8' are each independently halogen, -0R9, -NO2, -CN,
-CF3 -0CF3, -R9, 1, 2 -methylenedioxy, 1, 2-ethylenedioxy,
-N(R9)2,- SR9 - SOR9 -S02R9 -SO2N (R9 ) 2 , -S03R9 , -C (0 ) R9 ,
-C (0 ) C(0)R9, -C (0)C (0)0R9, -C (0)C (0)N(R9) 2, -C (0)CH2C (0) R9F
- (S ) R9, -C(S)0R9, -C (0) OR9, -0C (0)R9, -C (0)N (R9)2, -0C (0)N (R9)2,
-C(S)N (R9) 2, - (CH2) 0-2NHC (0) R9, -N(R9)N(R9)COR9,
-N (R9)N (R9) C (0) OR9, -N(R9)N(R9)CON(R9)2, -N (R9) SO2R9,
-N (R9) SO2N(R9) 2, -N(R9)C(0)0R9, -N (R9) C (0)R9, -N(R9)C(S)R9,
-N(R9)C(0)N(R9)2, -N(R9)C(S)N(R9)2, -N(COR9)COR9, -N(0R9)R9,
-C (=NH)N (R9) 2, -C (0)N (OR9) R9, -C (=NOR9)R9, -OP(0) (OR9)
-P(0) (R9)2? -P(0) (OR9) 2, and -P(0) (H) (OR9);
R9 is hydrogen, aliphatic, cycloalkyl, cycloalkenyl, aryl,
heterocyclyl, heteroaryl, cycloaliphatic-aliphatic-, aryl-
aliphatic-, heterocyclyl-aliphatic-, or heteroaryl-aliphatic--;
(in certain embodiments, any hydrogen atom of R9 is optionally
and independently replaced by R8 and any set of two hydrogen
atoms bound to the same atom is optionally and independently
replaced by carbonyl; provided that if R9 is substituted with a
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-9-
R8, wherein the R8 comprises a R9 substituent, then that R9
substituent is not substituted with R8);
R113 is halogen, -01111, -NO2, -CN, -CF3, -0CF3,
or
-SR11;
is C1..4-aliphatic-; and
Ru is halogen, -0R11, -NO2, -CN, -CF3, -0CF3, -R11, -SR9.
[0014] As used in the definition of R12, IT
atoms" refers to atoms that are linearly bound, regardless of
whether those atoms also have atoms bound in a branched
fashion. According to this definition, an ethyl group and a
trifluoromethoxy group each have three straight-chained atoms,
and a methyl group has two straight-chained atoms. In the
above embodiment, R12 has no more than 5 straight-chained
atoms. In two other embodiments, R12 has no more than 4
straight-chained atoms and no more than 3 straight-chained
atoms. In yet other embodiments, R12 has 2 straight-chained
atoms or 1 atom.
0015] As used herein, a position adjacent to the bond x
refers to a position which is located next to the position at
which x is bound. In an aryl ring, this position is often
called "the ortho position" or, in the case of a phenyl ring,
it may be called "the 2-position". By way of example, in the
structures immediately below, R12 is bound to the phenyl,
thiophene, and pyridine rings at "the position adjacent to
bond x".
R12, R1 2....r/s R12r.)
--
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-10-
Jw
Ru R12 Ru R12
N9
[0016] In one embodiment of this invention, R is R3C(0)-.
[0017] In some embodiments, R3 is optionally substituted
C6_10ary1 or heteroaryl. In other embodiments R3 is optionally
substituted phenyl. In yet other embodiments, R3 is a 8-10
membered optionally substituted heteroaryl (i.e. quinoline,
isoquinoline, or quinazoline) In yet other embodiments, R3 is
an optionally substituted 5-6 membered heteroaryl (i.e.,
pyridyl, pyrimidyl, pyrazinyl, thiophenyl, furanyl,
thiazoly1)
[0018] In some embodiments, R3 is optionally and
independently substituted by 0-5 REv groups.
[0019] In one embodiment, the compound of this invention is
represented by formula II:
0 R2
IC P-0
R3 x N
0
II
wherein:
a) R:3 is phenyl, thiophene, or pyridine;
b) each ring is optionally substituted with up to 5
groups independently selected from Rv; and
c) at least one position on the phenyl, thiophene, or
pyridine adjacent to bond x is substituted by R12,
wherein R12 has no more than 5 straight-chained atoms.
[0020] Another embodiment of this invention provides a
compound wherein Y is:
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/LTS2005/006540
-11-
0
\40
0-R1
[0021] In one embodiment of this invention, RI is
substituted with up to 3 groups selected independently from
carbonyl and R8.
(0022] In another embodiment, RI is C1-12aliphatic or
C3_10cycloalkyl, wherein each RI is optionally substituted with
1-3 groups selected independently from R8. In yet another
embodiment, RI is a straight-chain or branched C1-4 alkyl that
is optionally substituted with 1-3 groups selected
independently from R8.
[0023] In one embodiment, RI is an unsubstituted, straight-
chain or branched C1-4 alkyl (e.g., ethyl, isopropyl, n-propyl,
or n-butyl) . In another embodiment, RI is ethyl.
[0024] In any of these embodiments, R8 is halogen, -ORB,
-CN, -CF3, -0CF3, or -R9. In another embodiment wherein RB is
-R9, that R9 is benzyl.
[0025] In another embodiment, Y is
0
0
[0026] In another embodiment, Ring A is substituted with up
to 3 groups (preferably, 1 group) selected independently from
carbonyl and R4.
(0027] In one embodiment, Ring A is:
Ncõ..N7 VN
or
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-12-
optionally substituted with R4.
[0028] In yet another embodiment, Ring A is:
t242.
õ,NIR
VIAAI
optionally substituted with R4.
[0029] In another form of this embodiment, Ring A is
unsubstituted proline (i.e., R4 is hydrogen).
[0030] In yet another embodiment, Ring A is:
VNN
or -- optionally substituted with R4.
. [0031] In one embodiment, Ring A is optionally
substituted with R4.
[0032] In any of these embodiments, R4 is halogen, -0R9,
-CF3, -0CF3, -R9, or -SR9. In certain embodiments R4 is H.
[0033] In one embodiment, R2 is a C3-4 branched alkyl group.
[0034] In another embodiment, R5 is H or -CH3, R6 is -CH3,
and R7 is -CH3.
[0035] In another embodiment, R12 is -0CF3, -OCH3, -CF3,
-CH3, -CH2CH3, -Cl, or -F.
[0036] In yet another embodiment, R12 is -CF3, -CH3, -Cl, or
-F.
[0037] In yet another embodiment, R12 is -CH3, -Cl, or -F.
[0038] In another embodiment, each Riv, if present, is
independently halogen, -0R9, -NO2, -CN, -CF3, -0CF3, -R9, 1,2-
methylenedioxy, 1,2-ethylenedioxy, -N(R9)2, -SR9, -SOR9, -S02R9,
-SO2N(R9)2, -C(0)R9, -c(0)C(0)N(R9)2, -C(0)N(R9)2, -0C(0)N(R9)2,
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-13-
-(CH2)0_2NHC(0)R9, -N(R9)S02R9, -N(R9)S02N(R9) 2i -N(R9)C(0)0R9,
-N(R9)C(0)R9, Or -N(R9)C(0)N(R9)2.
[0039] In another embodiment, R8' is -NH2, -N(R9) 2,
-N(R9)C(0)R9, -0CF3, -0R9, -CF3, -R9, -SR9, or halo. In this
embodiment, halo is, preferably, Cl or F and R9 is, preferably,
straight or branched C1-4 alkyl.
[00403 According to one embodiment, this invention provides
compounds of foi.mula III:
R2
R,NõLir 0
NT =
0 0 N40
0,R1
III;
wherein the variables are as defined in any of the embodiments
herein.
(00413 In one form of this embodiment, the compound has the
stereochemistry indicated below:
R2 0
R,NATN(;)
0 cd-,N40
0,R1
wherein the variables are as defined in any of the embodiments
herein.
[0042] In other forms of this embodiment, the compound has
the stereochemistry indicated below:
R2 R2
Wk
0 0
R,N,Kr.NOA R,
H
tJ 0 N o ,do
o N
or H
0,R 0,R1
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-14-
wherein the variables are as defined in any of the embodiments
herein.
(0043] According to another embodiment, this invention
provides compound of formula IV:
R2 0
RJ
OH
0
0
IV;
wherein the variables are as defined in any of the embodiments
herein.
[0044] In one form of this embodiment, the compound has the
stereochemistry indicated below:
R2 0
R A
0
0
wherein the variables are as defined in any of the embodiments
herein.
[0045] The embodiments herein may be combined to provide a
compound according to this invention.
[0046] According to one embodiment, the present invention
provides a compound selected from Table 1 below:
Table 1
F.st
\o
0 0 0
0
* iirko *
0
0
0 0 0
0 0 N 0 N
H 0 H 0 H 0
1-1 1-2 1-3
CA 02820541 2013-06-21
WO 2005/085236 PCT/1LTS2005/006540
-15--
HO 0 H2N 0 CI 0
0 0 0
* EX.N'i, .<0 . X- Nii 1.1.(0 =
I)c= Nii
H
0 0CI 0
ON 0 N".. ON
H 0 H 0 H 0
C C C
1-4 1-5 I-6
0 0 0
(Xl(N Nii
&LA. Nii 0
(11'%.7-....%
S H
0 N 0 0
0 N 0 N 0 N
H 0 H 0 H 0
C C C
1-7 I-8 I-9
CI 0
ct ci 0 0 -0
l \---144.N.- Ni ri A.-11-N. N kli
CI
,. ....
0 ,<0 0 0 1q0
0 N 0 N 0 N
H 0 H 0 H 0
I-10 I-11 1-12
i
1 o o ,
1 o
o o
o o
0 * NI H..Nl
O. . (.,..)NT
O N'
0 0
0
. XII? N
0 1q0 0 N H 0)
H 01/
H 0 1-13 1-14 1-15
CI 0 0 CF30
= if c r NI i jeo * 0
X. Ni ir.k * X N? 0
O A 00 0 4 .0
0 N 0 N'',. ON
H 0 H 0 H 0
4s, (`= 4"s.
1-16 1-17 1-18
CF30 CI 0
CI 0 0
* X.N? 0 * N Nli =
H
0 N N?
H
0 .<0
O 0 N 0 N
o rl H 0 H 0
H 0
---C C
i
1-19 1-20 1-21
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
. . -16-
CF30 CI 0 CI 0
0
= vi)(9 eci'lo = hiNI?<0
.)- . * Nr-Nii
H 1q0
0 AL 0 0 0
0' N 0 N 0 N
H 0 H 0 H 0
C__/
1-22 1-23 1-24
0
,, CI o' CI
F3C-`-' . N..rµfi o
= N.Nli 0
H ,10 0 4
0
ON q ON
H 0 H 0
=C
1-25 1-26
CI 0 CI 0
0 0
= villµlii = V.X Ni
0 0 .6
0 N = 0 N =
Ht;i1 H
00
L .c '
1-27 1-28
010
CI 0
CI
0 0
0 = vi .1\11i
=
INXIµI 0
* hl i 0 10) lq.0
0iN 0 N = 0
0 N H".'._ o N
H 0 a
H 0
c_._/ __J ---c
1-29 1-30 1-31
CI 0 CI 0
O
* hi Nii V-o =
0 .,0 H
ON = 0
H ''''..._ o N
oH 0
---C C
1-32 1-33
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
-1 7-
CI 0 4,, CI 0
,0 GI
o * o
Ni 0 ill\lii 4$ * IIX=i,<0 * IX Nii
0 N 0 0 H N 0 N
C CC) H CC)
1-34 1-35 1-36
CI o CI 0 ro
o ci 0
0
HO ..,,,,
o
µ0 * 11- NT o A * NAc Nji
X
H tilli
0 o o 40
o N-4. 0 N
HO H
CC) \ C
1-37 1-38 1-39
C)/
CI 0 0 c)./
HN
o 0 HN o
HN * NNT
* igi Nii 0
0
0 N 0
0 N 0 .<0
0 N
HC o HC 0
\
1-40 1-41 1-42
CI 0 *F 0 F 0 v.
HN
* NN .ii ..<
00 1-N * NN? o $C)
0 0 HN
o
0 N 0 0 N 0 0 N
H /0
C C \
1-43 1-44 1-45
Cl 0 CI 0
>-0 0 CI
* N NT 0
H .0 HO * N=liii.
H ,0 (121
. 0 0
0 N4 o N 0 NI? 4
ON
H o H o
C C H e
1-46 1-47 1-48
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/1152005/006540
,
-18-
0 4., CI 0 µ CI 0
0
0
t\I ll
[IirNiii eC(3,0 0 i * Hi?
4 I-1 N Ilk X.1\1?
0
0
0 0
0 N-1. 0 N 0 N
H C0 H 0
H 0
C
1-49 1-50 1-51
CI
CI 0
0
jc o
1 p 0 et ti.
0
HN *
4 N--zt 0
o
N
0 N 0
.kk,S H 0
H 0
C C
1-52 1-53
S,) S
CI 0 ._ri
H2N o cl 0 0 1 0
/Ili t [X N? jqo to H
NA*110 Nri fito Ali CI N Alf Nri ..0
illel'Ill
0 H 0
0 N 0 ,Nii -I; 0 N
H 0
H 0
C C C
1-54 1-55 1-56
CI 0
I Cl 0 o o
40 H
0 to N;sterii 4.0
H 0 y 4,3
o N
H 0
C C
1-57 1-58
1/0
CH3 0
0
=,,, is XI 0 ci 0
0 N0 110 PI/1\1)c
0 H
0 H 0 --- \ 0 \
1-59 1-60
Cl 0 jcrelf 0 Cl 0 0
fi *
H 40 JL * Nor N7N4
""1/4N 0 N 0
N 0 H o-\
1-61
0
'-\ --A
1-61 1-62
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-19--
F 0
v K A 1101 oril 4
0 IN
1 0 N
H 0
-1
1-63
I-64
CH3 0
F 0
)c, 17 .4:
,,.0 io 4-.)(4.7
N....d00
0 N 0
0.1 0 H zt
..--\
I-65
1-66
CI 0
110 XII ,,e:
0
N .
O H
.5....% ../loi * Iltrorq 4:
0 N
0-,
1-67 1
I-68
0l 0 .
jt, * Ntra 40 filo,. 0 N
1.r.N-4140
1, 0
11 0 .
Il 0 H
== N H 0
- 0
CI 0I .)'.14
H 0-.1
1
1-69 .
I-70
I-I2N 0
0
* IX.ril 4
f-f M
0 0 N
IP 11..)C
.40
CI 0_, . .2,4
0
CI 0 il
0....,
1-71 X
I-72
10 0 nO
I -N Xqt\i-C
0
15,-.1
X
I-73
_
..
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-20-
[0047] According to another embodiment, the present invention
provides a compound of formula II selected from Table 2 below:
Table 2
-0 0
0 CI 0 v.,.
0 0
* I\X NT j(rOH I. N -Y)r N OH 0 H
0 H 0 H 0 H
0 N 0 N 0 N
H 0 H0 H 0
II-1 11-2 11-3
F E
= FX0 0
0 0 0
40 NorNiii 4 OH N
0 HO
* i_IN:: T .0H OH
H H 1111 IFX0
4.
o r\li
Lr()H
0 N
11o
o N
H 0 0 N
H0
11-4 11-5 11-6
o
H2 FF a
N o
o a o
o F o
* NI NT KoHli N3, Nii ,01iii IiiOH
I* to NN
H
0 N 0 N 0 N
H0 H 0 H0
11-7 11-8 11-9
CI 0 CI 0
o
o
* NrNii4 OH a 400 1XKOH 4)0 f)c. N?
H
0 H 0 H 0 o N. OH
,<r H
0 N 0 N Cl
H 0 H 0 H o
II-10 II-11 11-12
CI o o o
0 o i
o
* dc-N? ,OH 419 NN? ,OH NI OH
IW 11 -r TH
CI 0 H o H 0
H o H0 H0
11-13 11-14 11-15
_
CA 02820541 2013-06-21
WO 2005/085236 PCT/1JS2005/006540
-21-
JIG' 0
0
N H0 N H 0 i
0
K H H
0 CAN ON
H 0 H 0 H0
11-16 11-17 11-18
cI 0 0 0 irCF3
-0 0
Ni 11 )cr N? 0 OH Ni
. IX N? i _.OH
0
CI 0 H 0 H
0 N Me0 0 N
H0 H 0 H0
11-19 11-20 11-21
..s
0 0 N Cl 0 CF,
0
. Nri, * OH N N? = vi-c- N 0
i ._.
OH
H 0 õ)H
H H H
, 0 0 N Me0 0 N 0 NI
H 0 H 0 H o
11-22 11-23 11-24
S,>
Ci 0 I NI CF3 0
0
0
* \
[il 0 NT ,..kcOH 40 0 I.
0
r\ii 0 1\ ...kiOH =
0
ri N ., H
0H
H
0 N TN H 0? 0 N
H 0 H 0 H 0
11-25 11-26 11-27
=
CI 0 cF3o CI o
Me0 N 1OH
0
0
* Nr Ni ilk i .<kr OH hl = N?
N.. HF4 . i \X .
0? H
0 N
0 N H 0 0
H 0 H 0 H 0
11-28 11-29 11-30
F 0 CI 0
Me0 0
h1)(0r N F3C. i kr()OH = hi) NII OH
0 H
H
0 N 0 N
H0 H 0
11-31 11-32
CA 02820541 2013-06-21
W02005/085236 PCT/US2005/006540
-22- =
tp.-0 ,C1 c) # CI o 4- i CI
o o 0 , o
iNii_i\iTkrOH * [,iii\fi OH * 6-NT
0 H 0 H 0 0 NH
0 N o N
H 0 rl 0 H o
11-33 11-34 11-35
CI 0 )p. CI 0 y 'Cc
0 CI o
0
* NO1:Lt0H P * rYli :Ii0H
0 H 0 H *
P1).0NTNY(A)H"
g 0 N
0 I
H 0 H 0 H0
11-36 11-37 II-38
Oy
CI 0 0 )1õ,.
HN
o
HO o o
.()H HN 40) 1-11µ114
i(rOH * 0
ilhi ,10H
H 0 Ei
0 N H 04\ 0 0 N 0 N
H 0 H 0 H
11-39 11-40 11-41
or CI o
HN o
o
0 F 0 *
--)1-
0
*HN * N )(roil N
Ficc: fi ,. 1-1 HN = M 0 Isli .Dill
0 H %,.. . H ,,,.
O N 0 0 N 0 0 N
H 0 H 0 H 0
11-42 11-43 11-44
F 0 _. CI 0 )1=õ.. CI 0
O o
HN 40 tnri krol-i ---(0 =11.: NN
?OH
= 0 H LiC)1.1 HO * X
i 0X.nN 0
=-=,, 0 o N H 0
v H 0 . .,OHli
O N 0 H 0
11-45 11-46 11-47
CI 0 ie. CI 0 te.. CI 0
* itnrf J0 11 * Ni-Nii ,0f HN N * .
NII 0
H 0 .oLr!).H
HN H OH
0 N 04)¨ 0 N 0 0 N m
H 0 H 0 H 0
11-48 11-49 11-50
'
CA 02820541 2013-06-21
,
WO 2005/085236
PCT/US2005/006540
-23-
ill
CI 0 4... 0 (0:1 =
CI o CI 0
0 0 0
A.N?
HN 46 t.,1-N'i j(OH 0H
==== 0 H 411 I-410 N? õ NH9
0 0 0 N 0 N - o N
1 H0 H0 H 0
11-51 11-52 11-53
CI 0 CH
3 0 0
0
* X OH NX a
1
,,
.,OH
H , N H
0
oy N H
H0 0
11-54 11-55
0
CI 0 CI 0 v7kIrl
* XI 40H OH
H
)1 * N
0
0 N 0 N
0 H
H
0 0
11-56 11-57
CI 0 ),,ir 0 CI 0 N
OH
1
=-A0 * Ntfor N4OH H 0 * N 0 NI H
N 0 H 0 IA
H 0 0
11-58 11-59
0 F 0 -kir
i)I
l irx.0
F 0
1 ..,(OH ,,0 to 11 OH
0 * IX
H 0 H
0 0 N n NLIr
- H
" 0 0
11-60 11-61
0 0
0 0 jcrl
OH
H 00
tirNtlil ,,(OH
0
li) 1101 N
H
101 H
0
N 0 N
H
H 0 H 0
CI CI
11-62 11-63
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-24-
W.,
0
0 Njcrl
41N40H
,40HH
N 0 0 N 0 H
0
0
/1-64 11-65
0 N?
OH
0 0 m
a " 0
11-66
[0048] In certain embodiments of this invention, the
variable definitions are selected from those depicted in the
compounds of Table 1 and/or Table 2.
[0049] As used herein, a specified number atoms includes
any integer therein. For example, a group having from 1-4
atoms, could have 1, 2, 3, or 4 atoms.
[0050] As used herein, an aliphatic group includes
straight-chained and branched groups having the specified
number of atoms. If the number of atoms is unspecified, the
aliphatic group has from 1 to 12 carbon atoms. As would be
understood, alkenyl and/or alkynyl aliphatic groups have a
minimum of 2 carbon atoms. Preferred aliphatic groups are
alkyl groups (preferably having from 1 to 6 atoms).
[0051] Cycloalkyl and cycloalkenyl groups have between 3
and 10 carbon atoms and are monocyclic or bicyclic, including
linearly fused, bridged, or spirocyclic.
[0052] As used herein, "aromatic group" or "aryl" refers to
a 6-10-membered ring system that contains at least one
aromatic ring. Examples of aromatic rings include phenyl and
naphthyl.
[0053] As used herein a "heteroaryl" refers to ring system
having 5-10 members and 1, 2, or 3 heteroatoms independently
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-25-
selected from N, N(R9), 0, S, SO, and 502., wherein at least
one ring is heteroaromatic (e.g., pyri_dyl, thiophene, or
thiazole).
[0054] As used herein a "heterocycLen refers to ring system
having 3-10 members and 1, 2, or 3 heberoatoms independently
selected from N, N(R9) , 0, S, SO, and .S02, wherein no ring is
aromatic (e.g., piperidine and morphoLine).
[0055] Further examples of heteroar-y1 rings include
2-furanyl, 3-furanyl, N-imidazolyl, 2-imidazolyl,
4-imidazolyl, 5-imidazolyl, benzimidazolyl, 3-isoxazolyl, 4-
isoxazolyl, 5-isoxazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl,
N-pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, 2-pyridyl, 3-pyridyl, 4-
pyridyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl,
pyridazinyl (e.g., 3-pyridazinyl), 2-t_hiazolyl, 4-thiazolyl,
5-thiazolyl, tetrazolyl (e.g., 5-tetra_zoly1) , triazolyl (e.g.,
2-triazoly1 and 5-triazoly1), 2-thieny-1, 3-thienyl,
benzofuryl, benzothiophenyl, indolyl (e.g., 2-indoly1),
pyrazolyl (e.g., 2-pyrazoly1), isothia_zolyl, 1,2,3-
oxadiazolyl, 1,2,5-oxadiazolyl, 1,2,4-oxadiazolyl,
1,2,3-triazolyl, 1,2,3-thiadiazolyl, 1,3,4-thiadiazolyl,
1,2,5-thiadiazolyl, purinyl, pyrazinyL, 1,3,5-triazinyl,
quinolinyl (e.g., 2-quinolinyl, 3-quin_olinyl, 4-quinolinyl),
and isoquinolinyl (e.g., 1-isoquinolin_ya, 3-isoquinolinyl, or
4-isoquinoliny1).
[0056] Further examples of heterocy-clic rings include 3-1H-
benzimidazol-2-one, 3- (1-alkyl) -benziwiidazol-2-one, 2-
tetrahydrofuranyl, 3-tetrahydrofuranyL,
2-tetrahydrothiophenyl, 3-tetrahydrothiophenyl, 2-morpholino,
3-morpholino, 4-morpholino, 2-thiomorpholino, 3-
thiomorpholino, 4-thiomorpholino, 1-py-rrolidinyl, 2-
pyrrolidinyl, 3-pyrrolidinyl, 1-tetrah_ydropiperazinyl, 2-
tetrahydropiperazinyl, 3-tetrahydropiperazinyl, 1-piperidinyl,
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-26-
2-piperidinyl, 3-piperidinyl, 1-pyrazolinyl, 3-pyrazolinyl,
4-pyrazolinyl, 5-pyrazolinyl, 1-piperidinyl, 2-piperidinyl, 3-
piperidinyl, 4-piperidinyl, 2-thiazolidinyl, 3-thiazolidinyl,
4-thiazolidinyl, 1-imidazolidinyl, 2-imidazolidinyl, 4-
imidazolidinyl, 5-imidazolidinyl, indolinyl,
tetrahydroquinolinyl, tetrahydroisoquinolinyl, benzothiolane,
benzodithiane, and 1,3-dihydro-imidazol-2-one.
[00571 Each of the above aliphatic, aryl, cycloaliphatic,
heteroaryl, and heterocyclyl may contain appropriate
substituents (preferably up to 5) independently selected from,
for example, carbonyl and R8. Preferred substituents are
halogen, -0R9, -NO2, -CF3, -0CF3, -R9, oxo, -0R9, -0-benzyl, -0-
phenyl, 1,2-methylenedioxy, 1,2-ethylenedioxy, -N(R9)2,
-C(0)R9, -COOR9 or -CON(R9)2, wherein R9 is defined herein (and
is preferably H, (C1-C6)-alkyl, or (C2-C6)-alkenyl and
alkynyl), with (C1-C6)-alkyl being most preferred). It should
be understood that this definition would include a
perfluorinated alkyl group.
[0058] It will be apparent to one skilled in the art that
certain compounds of this invention may exist in tautomeric
forms or hydrated forms, all such forms of the compounds being
within the scope of the invention. Unless otherwise stated,
structures depicted herein are also meant to include all
stereochemical forms of the structure; i.e., the R and S
configurations for each asymmetric center. Therefore, single
stereochemical isomers as well as enantiomeric and
diastereomeric mixtures of the present compounds are within
the scope of the invention.
[0059] Unless otherwise stated, structures depicted herein
are also meant to include compounds that differ only in the
presence of one or more isotopically enriched atoms. For
example, compounds having the present structures except for
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-27-
the replacement of a hydrogen hpy a deuterium or tritium, or
the replacement of a carbon by a 13C- or 14C-enriched carbon are
within the scope of this invention.
(0060] The compounds of this invention may be obtained by
any method, including general, synthetic methods known to
those skilled in the art for analogous compounds (see e.g., WO
99/47545). For the purposes oE illustration, the following
Schemes for the synthesis of ttie compounds of the present
invention are provided.
[00613 The following abbreviations are used:
EDC is 1- (3-dimethylaminopropyL ) -3-ethylcarbodiimide
HOBt is 1-hydroxybenzotriazole
THF is tetrahydrofuran
TFA is trifluoroacetic acid
DCM is dichloromethane
DMAP is 4-dimethylaminopyridin
DIPEA is diisopropylethylamine
DMF is dimethylfo/mamide
TFA is trifluoroacetic acid
Z is benzyloxycarbonyl
IH NMR is nuclear magnetic resonance
TLC is thin layer chromatography
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-28-
Scheme I. General scheme for the preparation of E and F
poi OPG1
A
PG2 fiA.-2
- 0
OH "-
H2N 0 N -41 0 144
COOH ^ 0-R1
A
R.
R2 (72 0
'TN COOH
o 0
14 0
0 N
n 0 H 0-81
[0062] Scheme I depicts a general route to prepare the
compounds E and F disclosed in this invention. The amino
group of species A, readily obtained from reduction of the a-
carboxylic group of aspartic acid (protected with PG1 as an
ester), is coupled to the carboxylic acid moiety of species B
(N-protected with PG2) to give species C. PG2 and PG2 are
orthogonal protecting groups (i.e., protecting groups where a
protecting group may be selectively removed in the presence of
another protecting group. Ideally, PG1 should be able to be
removed without removing PG2 and-vice versa). Here, the
aspartate part of the molecule is then manipulated in an
oxidation/ketalisation/ deprotection/cyclisation sequence to
give species D. The Ring A portion of D is then
functionalized further to give species E which is part of the
disclosed invention. Deprotection of the ketal gives species
F which represent the other part of the disclosed invention.
[0063] In various embodiments of this invention, PG2 is a
suitable amine protecting group, including but not limited to,
the amine protecting groups described in T.W. Greene & P.G.M
Wutz, "Protective Groups in Organic Synthesis", 3rd Edition,
CA 02820541 2013-06-21
W02005/085236
PCT/11S2005/006540
-29-
John Wiley & Sons, Inc. (1999 and other editions) ("Greene").
A "Z" protecting group (benzyloxycarbonyl) is a particularly
useful N-protecting group for use in connection with this
invention. In compounds wherein PG2is protecting the nitrogen
of a proline, PG2is preferably Z. It should be understood
that modified Z groups ("Z-type protecting groups") employed
in connection with the compounds and processes of this
invention would also fall within the scope of this invention.
For example, Z could be substituted at the CH2 group or the
phenyl group with R8 (preferably halo or C1-6 straight-chained
or branched alkyl) to provide a Z-type protecting group.
[0064] In various embodiments of this invention, PGi is a
suitable carboxylic acid protecting group, including but not
limited to the acid protecting groups described in Greene. In
certain embodiments, PGI is C1-6 straight-chained or branched
alkyl group. A t-butyl group is a particularly useful acid
protecting group for use in connection with this invention.
[0065] In Scheme I, compound A is a modified aspartic acid
residue. In addition to compound A, other modified aspartic
acid residues, including the following, have been reported:
0 OPG1 OPG1
N ,R,
1\1
H2N4 H211 NH2 H2N 0PG4
OPG3
OPG4
wherein, PG3 and PG4 are appropriate protecting groups. These
modified aspartic acids may be prepared by methods known to
skilled practitioners. See, for example, United States Patent
Application Publication US 2002/0042376 (especially page 9,
paragraph [0121] and pages 21-22, paragraph [0250] and the
documents cited at paragraph [0123]) and United States Patent
6,235,899. See also, C. Gros et al. "Stereochemical control
in the preparation of a-amino N-methylthiazolidine Masked
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-30-
Aldehydes used for Peptide Aldehyde Syn.thesi" Tetrahedron,
58, pp. 2673-2680 (2002); K.T. Chapman, "Synthesis of a Potent
Reversible Inhibitor of Interleukin-Z Converting Enzyme"
Bioorg. Med. Chem. Letts., 2, pp. 613-618 (1982); M.D.
Mullican et al. "The Synthesis and Evaluatiora of Peptidyl
Aspartyl Aldehydes as Inhibitors of ICE'" 4, pp. 2359-2364
(1994); M.H. Chen, et al. "An Efficient Steroselective
Synthesis of [3S (1S, 9S) ] -3- [ [ [9- (Benzoylamino) octahydro-6,10-
Dioxo-6H-pyridazino- (1,2-a) (1,2) -Diaz epin-1-y11-carbonyl
amino]-4-oxobutanoic acid, an interleukin converting enzyme
(ICE) Inhibitor" 9, pp. 1587-1592 (1999). Accordingly, Scheme
(and also Scheme III below) could be modified to use these
other aspartic acid residues.
Scheme II. Preparation of Compounds of Formulae I and II
R2
H2N-kstrNii
0 N
H ORI
0 R2 i? R2
0
1 R3.1( N N? (c) R3-11 N
OH
IL
0 0
0 N o N
H oR1 H 0
HN?
o N
H ORI
7
Reagent and conditions: (a) R3COOH, HOBt, DMA.P, EDC, THF; (b)
R3CONHCH(R2)COOH, HOBt, DMAP, EDC, THF; (c) 2M HC1, MeCN.
CA 02820541 2013-06-21
79580-110
-31-
[0066] Scheme II depicts formation of compounds of formula
I and 11, wherein Ring A is unsubstituted proline. Here the
cyclic acetal form of a compound of this invention is depicted
as formula I and the aldehyde form is depicted as formula TI.
Compounds having a Ring A other than unsubstituted proline
could .be substituted in the methods depicted in Scheme I.
[00673 Scheme II depicts the routes utilized to prepare
compounds of formulae I and II. Compounds I can be prepared
from compounds 1 by condensation of the amino group in 1 with
the suitably functionalized carboxylic acid (or derivative).
In this step, standard coupling reagents to form amide bonds
have been depicted; other conditions known in the art to form
amide bonds can also be used.
[0068] As known to skilled practitioners, a carboxylic acid
(-C(0)0H) can be coupled to the amine under appropriate
conditions for coupling amines and carboxylic acids.
Alternatively, in such couplings, a carboxylic acid derivative
(-C(0)X) may be employed instead of the carboxylic acid. It
should be understood that in the context of coupling an amine
and a carboxylic acid derivative, the derivative would
activate the acid to facilitate coupling to an amine.
Appropriate X groups are essentially leaving groups and are
known to skilled practitioners. "March's Advanced Organic
Chemistry", 5th Ed., Ed.: Smith, M.B. and March, J., John
Wiley & Sons, New York: 2001.
[0069] Typical conditions for coupling an amine and an acid
include combining a suitable solvent, a carboxylic acid, a
base, and a peptide-coupling reagent. Examples of suitable
conditions are described in US2002/0042376 and WO 01/81330.
In certain embodiments, the conditions are as described in the
Schemes and Examples herein.
CA 02820541 2013-06-21
W02005/085236 PCT/US2005/006540
-32-
(0070] Examples of appropriate derivatives include, but are
not limited to, compounds of the formula RX wherein X is Cl,
F, OC(=0)R" (R" is aliphatic or aryl), SH, SR, SAr, or SeAr.
In some embodiments R is C(=0). Suitable conditions fox:- using
these appropriate derivatives are known in the art.
Scheme III. Preparation of Compound 1
.eco2tsu (a) zni? co21su (b) (c) CO2iBu
d
H2N
0 NI ---41- 0 N
0 N 0 IR1
H OR1
2 3 4 5
R2
(d) tic) (e) HN? R2 (f) ZHN CD
.* ZHN.X.y0H
0
0 0 N 0 0 N
H OW H OR1 H cDR1
6 7 8 9
R2
0
(g) H2N 141 irko
0
o /4"Iµ
H oW
1
Reagent and conditions: (a) Cbz-Pro-OH, EDC, HOBt, DMAP,
DIPEA, THF; (b) Swern; (c) R1OH, 3A sieves, DCM, Ts0H; (d) TFA,
DCM; (e) H2, Pd(OH)2, Et0Ac, DMF, EtiN; (f) EDC, HOBt, Et3N,
Et0Ac, DMF; (g) H2, Pd/C, Citrate Acid.
[0071] Scheme III
depicts a possible route to prepare
compounds 7 and compounds 1 described in scheme I. Comound
2, readily obtained from reduction of the a-carboxylic group
of aspartic acid, is coupled to N-protected proline (or other
ring, wherein Ring A is other than unsubstituted prolin) to
form 3. Here, the proline is N-protected with a Z
(benzyloxycarbonyl) group. Compounds 3 are then oxidizei into
the aldehydes 4 which are acetalized in situ to give the
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-33-
acetals 5. Acetals can be formed in the presence of R1-0H (or
a suitable acetal forming reagent), a protic acid (for
example, Ts0H), or a Lewis acid, and a suitable solvent.
Examples of suitable acetal forming reagents that form
compounds wherein R1 is to become ethyl can be considered
ethanol equivalents and include, but are not limited to,
triethylorthoformate or a diethylacetal, such as a
(CH3)2C(OCH2CH3)2. Preferably, the solvent is CH2C12, toluene,
or chlorobenzene. Appropriate protic acids include, but are
not limited to, TFA, p-T50H. Appropriate Lewis acids
include, but are not limited to TiC14, MgEr2, and ZnC12.
[0072] In Scheme III, the oxidation of compounds 3 to
compounds 4 is depicted as being done under Swern conditions.
Other oxidation conditions may also be employed to prepare
compounds of this invention. Preferred oxidation conditions
are those that a mild and relatively quick to minimize
epimerization at the acid side chain of the modified aspartic
acid residue. In one embodiment, the oxidation step is a
TEMPO oxidation (see Example I-1, Method C, below). Other
oxidation conditions include a Dess-Martin oxidation and a
tetrapropylammonium perruthenate (TPAP) oxidation.
[0073] Aldehydes 4 may be isolated but are preferably
carried through directly to 5 without isolation. Deprotection
of the tert-butyl ester (in 5) is accompanied by spontaneous
ring cyclization to give a mixture of diastereoisomers which
were separated by column chromatography to give the
enantiomerically pure syn ketals 6 and anti ketals (not
represented in this scheme). The deprotection may be done
under protic acid or Lewis acid conditions in an appropriate
solvent. Appropriate solvents include, but are not limited
to, toluene, chlorobenzene, and DCM. Appropriate protic acids
include, but are not limited to, TFA, p-T50H. Appropriate
CA 02820541 2013-06-21
W02005/085236
PCT/US2005/006540
-34-
Lewis acids include, but are not limited to TiC14, MgBr2, and
ZnC12. For clarity of the scheme, only syn ketals are
represented in the next steps to form compounds 7 and 1 but
the same sequence may be used to form anti ketals. Compounds
6 are submitted to hydrogenolysis and the resulting compounds
7 are reacted with Z-protected aminoacids, using conditions
known in the art to prepare amide bonds, to yield compounds 9.
Compounds 7 may be generated and used in situ. If isolated,
it is preferable to use compounds 7 relatively soon after
generation. Compounds 9 are finally submitted to
hydrogenolysis to give compounds 1, which can be used directly
to prepare compounds I, as depicted in Scheme II.
[0074] Alternatively, compounds 7 can be used to prepare
compounds I, as depicted in Scheme II. In this preparation,
an amino acid residue and the desired N -terminal group is =
prepared in one step (see, Scheme II, reaction (b)).
[0075] As described in connection with Scheme I, aspartic
acid derivatives other than compounds 2 can be employed to
obtain compounds of this invention.
Scheme IV. Preparation of Compounds of Foriuulae III and IV
Fe
0 N
H 0R1
2
R2 R 0
11 R11)SrT4) -(-c2o-
0
o N o
OR1 " 0
44:) na TV
o N
H oW
17
Reagent and conditions: (a) ROH / HOBt / DMAP / EDC/ THF or
Rd 1 / Et3N / DCM; (b) RNHCH(R2)COOH, HOBt, DMAP, EDC, THF;(c)
2M HC1, MeCN.
CA 02820541 2013-06-21
W02005/085236 PCT/US2005/006540
-35-
[0076] Scheme rv depicts formation of compounds of formula
III and IV, wherein Ring A is 2-Aza-bicyclo[2.2.1]-heptane-3-
carboxylic acid. Here the cyclic acetal form of a compound of
this invention is depicted as formula III and the aldehyde
form is depicted as formula IV. Scheme IV depicts the routes
utilized to prepare compounds of formulae III and IV.
Compounds III can be prepared from compounds 11 by
condensation of the amino group in 11 under conditions to
provide the desired R group, such as suitably functionalized
carboxylic acid (or derivative), sulfonic acid (or
derivative), chloroformate or carbamoyl chloride (or
isocyanate), for example, under appropriate reaction
condition. In this step, standard coupling reagents to form
CO-NH bonds have been depicted; other conditions known in the
art to form CO-NH or alkyl-N, or S02-N) bonds can also be used
to provide the desired compound comprising R-N.
Alternatively, compounds I can be prepared from compounds 17
by condensation of the amino group in 17 with the suitably
functionalized carboxylic acid (or derivative), sulfonic acid
(or derivative), chlorofolmate or carbamoyl chloride (or
isocyanate). In this step, standard coupling reagents to form
CO-NH bonds have been depicted; other conditions known in the
art to form CO-NH bonds can also be used.
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-36-
Scheme V. Preparation of Compound 11
= 62.i =
eco2tOH u (a) ZNt? LeCO21Bu (b) ZI(14 021Bu
(c) ZN? CO21Bu
eL ..OH ,c() fy0R1
0 OH 0 0 N
re ow
2 10 13 14 15
R2
(d) zi,C? (e)R2 0
N
40 + ZHN ZHNAy0H (f)),
0
0 0 N
H OR1 H OR1 0 0 N
H 0121
16 17 18 19
R2
(g)
0 = 40
0 N
H
11
Reagent and conditions: (a) EDC, HOBt, DMAP, DIPEA, THF; (b)
Swern; (c) R1OH, 3A sieves, DCM, Ts0H; (d) TFA, DCM; (e) H2,
Pd(OH)2, EtOAC, DMF, Et3N; (f) EDC, HOBt, Et3N, Et0Ac, DMF; (g)
11[2, Pd/C, Citrate Acid.
[0077] Scheme V depicts
a possible route to prepare
compounds 17 and compounds 11 described in scheme III.
Compound 2, readily obtained from reduction of the a-
carboxylic group of aspartic acid, is coupled to N-protected
2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid 10 (prepared as
in Tetrahedron: Asymmetry, 13, 2002, 25-28) to form 13.
Compound 13 is then oxidized into the aldehyde 14 which is
acetalized in situ to give the acetals 15. Deprotection of
the tert-butyl ester is accompanied by spontaneous ring
cyclization to give a mixture of diastereoisomers which were
separated by column chromatography to give the
enantiomerically pure syn ketals 16 and anti ketals (not
represented in this scheme). Alternative Ring A groups are
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-37-
either commercially available, reported in the literature, or
may be prepared according to methods known in the literature.
[0078] For clarity of the scheme, only syn ketals are
represented in the next steps to form compounds 17 and 11 but
the same sequence may be used to form anti ketals. Compounds
16 are submitted to hydrogenolysis and the resulting compounds
17 are reacted with Z-protected aminoacids, using conditions
known in the art to prepare amide bonds, to yield compounds
19.
[0079] Alternatively, compounds 17 can be used to prepare
compounds III, as depicted in Scheme IV. Compounds 19 are
finally submitted to hydrogenolysis to give compounds 11,
which can be used directly to prepare compounds III, as
depicted in Scheme IV.
[0080] The R3COOH used in Scheme II are either commercially
available, reported in the literature, or prepared according
to methods known in the literature. For compound 11-30, 2-
chloro-3-methoxybentoic acid was prepared as in J.Org.Chem,
59, 1994, 2939-2944.
[0081] For compound 11-32, 2-chloro-3-
trifluoromethoxybenzoic acid was prepared from 2-amino-3-
trifluoromethoxybenzoic acid (prepared as in J.Org.Chem, 68,
2003, 4693-4699) using a Sandmeyer replacement of the amino
group by a chloro, according to a method substantially similar
to the one reported in J.Org.Chem, 59, 1994, 2939-2944.
[0082] Accordingly, this invention also provides a process
for preparing a compound of this invention.
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-3 8 -
[ 00831 In one embodiment is provided a process for
preparing a compound of formula I:
R2
o
0
wherein Y is:
0
0,
= R
and the other variables are as defined in any of the
embodiments herein;
comprising reacting a compound of formula 1:
R2
A 0
0
0
0
R
1
wherein the variables are as defined in any of the embodiments
herein; and a compound of formula RX, wherein X is OH or an
appropriate derivative (i.e., leaving group), in the presence
of conditions for coupling an amine and an acid (when X is OH)
or an amine and an appropriate acid derivative (when X is not
OH (i.e., a leaving group; for example, Cl) to provide the
compound of formula I.
[0084] Another embodiment provides a process for preparing
a compound of formula I:
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-39-
R2
RNLr
H
wherein Y is:
'22.246
and the other variables are as defined in any of the
embodiments herein;
comprising reacting a compound of formula 7:
1(3 0
0
0
Rl
wherein the variables are as defined in any of the embodiments
herein, and a compound of formula RNHCH(R2)C(0)X, wherein X is
OH or an appropriate derivative, in the presence of conditions
for coupling an amine and an acid (when X is OH) or an
appropriate acid derivative (when x is not OH; for example, X
is Cl) to provide the compound of formula I.
[0085] Yet
another embodiment of this invention provides a
process for preparing a compound of formula IV:
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-40-
R2
OH
= 0
IV
wherein the variables are as defined in any of the embodiments
herein, comprising reacting a compound of formula I:
R2
R,
0 ,Y
0 N
wherein Y is:
0
\40
0-R1
wherein R and R1 are each independently as defined in any of
the embodiments herein, under hydrolysis conditions, to
provide the compound of formula II. In certain embodiments, R
is R3C(=0) . In yet other embodiments, when A is proline, R is
R3C(=0) . Hydrolysis conditions for converting I to II are well
known to skilled practitioners (see e.g., Greene). Such
conditions include an appropriate solvent (e.g., acetonitrile)
and aqueous acid (e.g., 2M HC1).
[0086] Another
embodiment provides a process for preparing
a compound of formula 6-A:
PG2 ¨N 0
0
0 N
OR1
6-A
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-41-
wherein PG2 is a suitable nitrogen protecting group and R' is
as defined in any of the embodiments herein, comprising
reacting a compound of formula 5-A:
0
PG2¨N"1'PG1
0
Ges'14 ORI
5-A
under suitable ring cyclization conditions, to provide the
compound of formula 6-A. Suitable ring cyclization conditions
include an acid and a suitable solvent; for example, TFA in
DCM.
[0087] Another
embodiment provides a process for preparing
a compound of formula 5-A:
PG2¨N(,) ,PG1
0
0 N
5-A
comprising reacting a compound of formula 4-A:
0
PG2 ¨N 0'PGI
ONThrH
0
4-A
in the presence of R.3.-OH (or a suitable acetal forming
reagent), protic or Lewis acid (for example, Ts0H), and a
suitable solvent to provide the compound of formula 5-A.
[0088] Another embodiment provides a process for preparing
a compound of formula 4-A:
CA 02820541 2013-06-21
..
=
WO 2005/085236
PCT/US2005/006540
-42-
0
PG2 ¨T0
.... ,PG1
H
0 N
H 0
4-A
comprising reacting a compound of formula 3-A:
0
PG2---N A AoõPG'
(:1?
0 N"¨')
H OH
3-A
under suitable oxidation conditions (for example, a Swern
oxidation: Mancuso, A.J.; Swern, D. Synthesis, 1981, 165-185)
to provide the compound of formula 4-A. Preferred oxidation
conditions include a TEMPO oxidation (see Example I-1,
Method C, below).
[00893
Another embodiment provides a process for preparing
a compound of formula 3-A:
0
PG2 ¨Nli2t,õ1õ ,PG1
0
0 N
H
OH
3-A
comprising:
reacting a compound of formula 2:
0
..õ.11,o,PG1
H2N----)
OH
2
with a compound of foLmula 20-A:
_
CA 02820541 2013-06-21
=
W02005/085236
PCMS2005/006540
-43-
PG2-01
0 X
20-A
under conditions for coupling an amine and a carboxylic acid
(when X is OH), or an amine and an appropriate carboxylic acid
(when X is not OH), to provide the compound of formula 3-A.
[0090] Another
embodiment provides a process for preparing
a compound of foLmula 6:
0
pG2_Ny
0
0 N'14
OR"
6
wherein PG2 is a suitable nitrogen protecting group and R1 is
as defined in any of the embodiments herein, comprising
reacting a compound of formula 5:
0
PG2 ¨NO ...,PG1
0
O1
0 N R
OR"
under suitable cyclization conditions, to provide the compound
of formula 6.
[0091] Another
embodiment provides a process for preparing
a compound of formula 5:
0
PG2 -N õ.PG1
0
0 N
OR1
5
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-44-
comprising reacting a compound of formula 4:
0
pG2_1,1,) ,.PG1
0
OteyH
0
4
in the presence of 1R.3--OH (or a suitable acetal forming
reagent), protic or Lewis acid (for example, Ts0H), and a
suitable solvent to provide the compound of formula 5.
Preferably, the solvent is CH2C12, toluene, or chlorobenzene.
[0092] Another
embodiment provides a process for preparing
a compound of formula 4:
0
PG2-N PG1
0
O Nr--yH
0
4
comprising reacting a compound of foLmula 3:
0
PG2-N õ..PG1
0
ON
OH
3
under suitable oxidation conditions (for example a Swern
oxidation) to provide the compound of formula 4. Preferred
oxidation conditions include a TEMPO oxidation (see Example
I-1, Method C, below).
[0093] Another
embodiment provides a process for preparing
a compound of formula 3:
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-45-
0
pG2¨d\ pG,
0
OH
3
comprising:
reacting a compound of formula 2:
0
,PG1
0
OH
2
with a compound of formula 20:
PG2 ¨NO
0 X
under conditions for coupling an amine and a carboxylic acid
(when X is OH), or an amine and an appropriate carboxylic acid
(when X is not OH), to provide the compound of formula 3.
[0094] Another
embodiment provides a process for preparing
a compound of formula 16:
0
PG2
0
ON
OR1
16
wherein PG2 is a suitable nitrogen protecting group and R1 is
as defined in any of the embodiments herein, comprising
reacting a compound of formula 15:
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-46-
.
0
PG2¨N
0
0
= OR1
under suitable cycli.zation conditions, to provide the compound
of formula 16.
[0095] Another
embodiment provides a process for preparing
a. compound of formula 15:
0
PG2-1V,,,. PG
0' I
OR1
0 N
= OR1
comprising reacting a compound of formula 14:
0
PG2 ,PG1
0
0 N
= 0
14
in the presence of R'-OH (or a suitable acetal forming
reagent), protic or Lewis acid (for example, Ts0H), and a
suitable solvent to provide the compound of formula 15.
[0096] Another
embodiment provides a process for preparing
a compound of formula 14:
0
PG2 ¨IT A .13G,
0
N---e
= 0
14
comprising reacting a compound of formula 13:
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-47-
0
,PG1
0
0 N
OH
13
under suitable oxidation conditions (example, a Swern
oxidation) to provide the compound of formula 14.
[0097] Another
embodiment provides a process for preparing
a compound of formula 13:
0
PG2-1\140
, ,pGi
0 N
OH
13
comprising reacting a compound of formula 2 with a compound
of formula 21:
0
0
pG2-N
OH 0 X
2 21
under conditions for coupling an amine and a carboxylic acid
(when X is OH), or an amine and an appropriate carboxylic acid
(when X is not OH), to provide the compound of formula 13.
[0098] Another
embodiment provides a process for preparing
a compound of formula 22:
0
0õ.
,PG1
OR1
H2N
OR1
22
comprising reacting a compound of formula 23:
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-48-
0
/1:A; .,PG1
0
H2N
0
23
in the presence of R.1-0H (or a suitable acetal fotwing
reagent), protic or Lewis acid (for example, Ts0H), and a
suitable solvent to provide the compound of formula 22.
Acetal forming equivalents include, but are not limited to,
triethylorthoformate, a diethylacetal, such as a
(CH3)2C(OCH2CH3)2. Preferably, the solvent is CH2C12, toluene,
or chlorobenzene.
[0099]
Another embodiment provides a process for preparing
a compound of formula 23 comprising reacting a compound of
=
formula 2:
0
0'13G1
H2N
OH
2
under suitable oxidation conditions (example Swern) to provide
the compound of formula 23.
[0100]
Another embodiment provides a process for preparing
a compound of formula 5-A
0
PG2¨N Poi
0'
oNyOR1
OR1
5-A
wherein PG1 is a suitable carboxylic acid protecting group, PG2
is a suitable nitrogen-protecting group, and R1 is as defined
in any one of claims 1 or 5-9, comprising:
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-49-
reacting a compound of formula 20-A:
PG2¨NA
0 X
20-A
with a compound of formula 22
0
= õ.PG1
0
OR"
1-121q
22
under conditions for coupling an amine and a carboxylic acid
(when X is OH), or an amine and an appropriate carboxylic acid
(when X is an appropriate leaving group), to provide the
compound of formula 5-.A.
[0101]
Another embodiment provides a process for preparing
a compound of formula 5:
0
PG2¨NO PG1
0
0 OR1
OR1
comprising reacting a compound of formula 20:
PG2-12
0 X
with a compound of formula 22
CA 02820541 2013-06-21
..,
.
A
'
=
. W02005/085236
PCT/US2005/006540
,
-50 -
0
PG1
0
H2N,..,-..i...0R1
OR1
22
under conditions for coupling an amine and a carboxylic acid
(when X is OH), or an amine and an appropriate carboxylic acid
.
(when X is not OH), to provide the compound of formula 5.
.
(0102]
Another embodiment provides a process for preparing
a compound of formula 5-A:
0
PG2 --1,.... -1:3(11 ,
0
OR1
0 N
H OR1
5-A ,
comprising reacting a compound of formula 21:
PG2.--N
0 X
21
with a compound of formula 22
0
PG.I
0
=
OW
HOs1
OR1
22
under conditions for coupling an amine and a carboxylic acid
(when X is OH), or an amine and an appropriate carboxylic acid
(when X is not OH), to provide the compound of formula 5-A.
,
_
CA 02820541 2013-06-21
=
9580-110
-51-
[0103] In accordance with this invention, the processes may
be used alone or in combination to provide a compound of this
invention.
[0104] Certain specific embodiments of this invention
provide processes for preparing compounds 4 from 3 (in
embodiments where compounds 4 are isolated); 5 from 3 (in
embodiments where compounds 4 is not isolated but carried on
directly, e.g., generated in situ); 5 from 4; and 6 from 5
according to the methods disclosed herein. In a preferred
embodiment, compounds 6 are prepared from. compounds 5;
compounds 5 are prepared from compounds 4 (Whether isolated or
not); and compounds 4 are prepared from 3. Preferably,
compounds 6 are used in the preparation of proline containing
caspase inhibitors. Such proline containing caspase
inhibitors include, but are not limited to, those disclosed in
WO 95/35308, WO 99/47545, W0.01/81330, and WO 01/90063.
For example,
compound IA (and stereoisomers thereof) of WO 01/90063
could be
prepared as disclosed herein (see, e.g., page 13). For the
avoidance of doubt, it should be understood that such proline
containing compounds could be depicted by- formula I except
that Ring A is pyrrolidine -- (i.e. is derived from
proline).
0105] The processes for converting compounds 6 to proline
containing caspase inhibitors are preferably as disclosed
herein. The processes for preparing compounds 3 are also
preferably as disclosed herein. However other processes known
to skilled practitioners could be used to convert compounds 6
to proline containing caspase inhibitors and/or to prepare
compounds 3.
CA 02820541 2013-06-21
&
, WO 2005/085236
PCTMS2005/006540
-52-
(0106] Other embodiments of this invention provide the
compounds of formula 3 to 6, 3-A to 6-A, and 13-16.
(0107] One embodiment of this invention provides the
compounds of formula 4A:
PG2.--i\CD oõPG1
O4 H
H 0
4A.
(0108] Another embodiment of this invention provides the
compounds of formula 4:
0
PG2-0 o./.PG1
0N H
H
, 0
4.
[0109] Another embodiment of this invention provides the
compounds of formula 14:
0
PGr-N ,,11,=0,PG1
14'
=
O N---)(H
H 0
14.
[0110] One embodiment of this invention provides the
compounds of formula 5-A:
0
PG2¨NO. )1.,o'Pal
ONrOR1
H
OR1
5-A.
0111] Another embodiment of this invention provides the
compounds of formula 5:
-
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-53-
0
PG2-N2 cr,PG1
OR1
0 N
OR1
5.
[0112] Another embodiment of this invention provides the
compounds of formula 15:
PG2-1\11 o.,PG1
OR1
0 N
= OR1
15.
[0113] One embodiment of this invention provides the
compounds of formula 3-A:
0
PG2¨N
= OH
3-A.
[0114] Another embodiment of this invention provides the
compounds of formula 3:
0
PG2¨Ny
0
0 N
OH
3
[0115] Another embodiment of this invention provides the
compounds of formula 13:
0
PG2¨N71,A. .PG1
0
0 N
= OH
13.
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-54-
[0116] In all the above embodiments, the variables are as
defined in any of the embodiments herein. In a preferred form
of 3, PG2 is Z and PG1 is C1-6 straight-chained or bran.ched
alkyl group (preferably a t-butyl group), either alone or in
combination.
[0117] As would be realized by skilled practitioners
certain process steps may be accomplished in discrete steps or
in situ. For example, deprotection and subsequent reaction of
an amine may be accomplished by step-wise (by isolating the
amine) or in a one step procedure (without isolating the
amine).
[0118] In certain embodiments, the above processes are
conducted as described herein (e.g., in the schemes, examples,
and accompanying description).
[0119] Compounds such as 3 could be used in processes for
preparing proline containing compounds, such as caspase
inhibitors. Proline containing caspase inhibitors include,
but are not limited to, those disclosed in WO 95/35308, WO
99/47545, WO 01/81330, and WO 01/90063 (which are all
incorporated herein by reference). For example, compound IA
(and stereoisomers thereof) of WO 01/90063 (which are
specifically incoLporated herein by reference) could be
prepared as disclosed herein (see, e.g., page 13).
[0120] The compounds utilized in the compositions and
methods of this invention may also be modified by appending
appropriate functionalities to enhance selective biological
properties. Such modifications are known in the art arid
include those which increase biological penetration into a
given biological system (e.g., blood, lymphatic system,
central nervous system), increase oral availability, increase
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-55-
solubility to allow administration by injection, alter
metabolism and alter rate of excretion.
[0121] For example, a carboxylic acid group in a compound
of this invention may be derivatized as, for example, an
ester. Preferred esters would be those derived from:
a C1-6 straight-chained or branched alkyl, alkenyl,
or alkynyl, wherein the alkyl, alkenyl, or alkynyl is
optionally substituted with C6_10ary1, CF3, Cl, F, OMe, OFt,
OCF3, CN, or NMe2:
a C1-6 cycloalkyl, wherein 1-2 carbon atoms in the
cycloalkyl is optionally replaced with -0- or -NR9-.
[0122] Compounds of this invention having a carbonyl group
may be similarly derivatized as, e.g., an acetal, ketal, oxime
(=NOR9), hydrazine (=NN(R9)2), thioacetal, or thioketal.
[0123] Appropriate derivatives of amines are known in the
art and are also included within the scope of this invention.
[0124] Certain of the above derivatives would include the
protective groups known to skilled practitioners (see, e.g.,
Greene). As would be recognized by a skilled practitioner,
these protective groups may also be employed in the processes
of this invention.
[0123] The compounds of this invention may be assayed for
their ability to inhibit apoptosis, the release of IL-41or
caspase activity directly. Assays for each of the activities
are known in the art. However, as would be recognized by a
skilled practitioner, a prodrug compound of this invention
should be active only in assays where the prodrug moiety would
be cleaved, typically in in vivo assays.
[0126] Assays for caspase activity are described in WO
99/47545.
[0127] According to another embodiment, the present
invention provides a pharmaceutical composition comprising:
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/U52005/006540
-56-
a) a compound of the in-vention., as defined herein,
or a pharmaceutically acceptable salt thereof; and
b) a pharmaceutically acceptable carrier, adjuvant
or vehicle.
[0128] It should be understood that compounds and
pharmaceutically acceptable salts thereof are included within
this invention are. If pharmaceutically acceptable salts of
the compounds of this invention are utilized in these
compositions, those salts are preferably derived from
inorganic or organic acids and bases. Included among such
acid salts are the following: acetate, adipate, alginate,
aspartate, benzoate, benzene sulfonate, bisulfate, butyrate,
citrate, camphorate, camphor sulfonate,
cyclopentanepropionate, diglucon.ate, dodecylsulfate,
ethanesulfonate, fumarate, glucohptanoate, glycerophosphate,
hemisulfate, heptanoate, hexanoat, hydrochloride,
hydrobromide, hydroiodide, 2-hydroxyethanesulfonate, lactate,
maleate, methanesulfonate, 2-n.aphthalenesulfonate, nicotinate,
oxalate, pamoate, pectinate, persulfate, 3-phenyl-propionate,
picrate, pivalate, propionate, succinate, tartrate,
thiocyanate, tosylate and undecanoate. Base salts include
ammonium salts, alkali metal salts, such as sodium and
potassium salts, alkaline earth rrItal salts, such as calcium
and magnesium salts, salts with organic bases, such as
dicyclohexylamine salts, N-methyl--D-glucamine, and salts with
amino acids such as arginine, lysine, and so forth.
[01293 Also, the basic nitrogera-containing groups can be
quaternized with such agents as lower alkyl halides, such as
methyl, ethyl, propyl, and butyl chloride, bromides and
iodides; dialkyl sulfates, such as dimethyl, diethyl, dibutyl
and diamyl sulfates, long chain halides such as decyl, lauryl,
myristyl and stearyl chlorides, bromides and iodides, aralkyl
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-57-
halides, such as benzyl and phenethyl bromides and others.
Water or oil-soluble or dispersible products are thereby
obtained.
[0130] Pharmaceutically acceptable carriers that may be
used in these compositions include, but are not limited to,
ion exchangers, alumina, aluminum stearate, lecithin, serum
proteins, such as human serum albumin, buffer substances such
as phosphates, glycine, sorbic acid, potassium sorbate,
partial glyceride mixtures of saturated vegetable fatty acids,
water, salts or electrolytes, such as protamine sulfate,
disodium hydrogen phosphate, potassium hydrogen phosphate,
sodium chloride, zinc salts, colloidal silica, magnesium
trisilicate, polyvinyl pyrrolidone, cellulose-based
substances, polyethylene glycol, sodium
carboxymethylcellulose, polyacrylates, waxes,
polyethylene-polyoxypropylene-block polymers, polyethylene
glycol and wool fat.
[0131] According to a preferred embodiment, the
compositions of this invention are formulated for
phaLmaceutical administration to a mammal, preferably a human
being.
[0132] Such pharmaceutical compositions of the present
invention may be administered orally, parenterally, by
inhalation spray, topically, rectally, nasally, buccally,
vaginally or via an implanted reservoir. The term
"parenteral" as used herein includes subcutaneous,
intravenous, intramuscular, intra-articular, intra-synovial,
intrasternal, intrathecal, intrahepatic, intralesional and
intracranial injection or infusion techniques. Preferably,
the compositions are administered orally or intravenously.
[01333 Sterile injectable forms of the compositions of this
invention may be aqueous or oleaginous suspension. These
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-58-
suspensions may be formulated according to techniques known in
the art using suitable dispersing or wetting agents and
suspending agents. The sterile injectable preparation may
also be a sterile injectable solution or suspension in a
non-toxic parenterally-acceptable diluent c>r solvent, for
example as a solution in 1,3-butanediol. Among the acceptable
vehicles and solvents that may be employed are water, Ringer's
solution and isotonic sodium chloride solution. In addition,
sterile, fixed oils are conventionally empLoyed as a solvent
or suspending medium. For this purpose, an_y bland fixed oil
may be employed including synthetic mono- or di-glycerides.
Fatty acids, such as oleic acid and its glyceride derivatives
are useful in the preparation of injectabls, as are natural
pharmaceutically-acceptable oils, such as olive oil or castor
oil, especially in their polyoxyethylated versions. These oil
=
solutions or suspensions may also contain a_ long-chain alcohol
diluent or dispersant, such as carboxymethy-1 cellulose or,
similar dispersing agents which are commonly used in the
formulation of pharmaceutically acceptable dosage forms
including emulsions and suspensions. Other commOnly used
surfactants, such as Tweens, Spans and other emulsifying
agents or bioavailability enhancers which are commonly used in
the manufacture of pharmaceutically acceptable solid, liquid,
or other dosage forms may also be used for the purposes of
formulation.
[0134] The pharmaceutical compositions of this invention
may be orally administered in any orally acceptable dosage
form including, but not limited to, capsules, tablets, aqueous
suspensions or solutions. In the case of tablets for oral
use, carriers which are commonly used inclu_de lactose and corn
starch. Lubricating agents, such as magnesium stearate, are
also typically added. For oral administration in a capsule
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-59-
form, useful diluents include lactose and dried corn starch.
When aqueous suspensions are required or oral use, the active
ingredient is combined with emulsifying and suspending agents.
If desired, certain sweetening, flavoring or coloring agents
may also be added.
[0135] Alternatively, the pharmaceutical compositions of
this invention may be administered in the foLm of
suppositories for rectal administration. These can be
prepared by mixing the agent with a suitable non-irritating
excipient which is solid at room temperature but liquid at
rectal temperature and therefore will melt in the rectum to
release the drug., Such materials include cocoa butter,
beeswax and polyethylene glycols.
[0136] The pharmaceutical compositions of this invention
may also be administered topically, especially when the target
of treatment includes areas or organs readily accessible by
topical application, including diseases of the eye, the slan,
or the lower intestinal tract. SuitabLe topical formulations
are readily prepared for each of these areas or organs.
[0137] Topical application for the Lower intestinal tract
can be effected in a rectal suppository formulation (see
above) or in a suitable enema formulation.
Topically-transdermal patches may also be used.
[0138] For topical applications, the pharmaceutical
compositions may be formulated in a sui_table ointment
containing the active component suspenaed or dissolved in one
or more carriers. Carriers for topical_ administration of the
compounds of this invention include, but are not limited to,
mineral oil, liquid petrolatum, white petrolatum, propylene
glycol, polyoxyethylene, polyoxypropylenJe compound,
emulsifying wax and water. Alternatively, the pharmaceutical
compositions can be formulated in a suitable lotion or cream
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-60-
containing the active components suspended or dissolved in one
or more pharmaceutically acceptable carriers. Suitable
carriers include, but are not limited to, mineral oil,
sorbitan monostearate, polysorbate 60, ,cetyl esters wax,
cetearyl alcohol, 2-octyldodecanol, benzyl alcohol and water.
[01393 For ophthalmic use, the pharmaceutical compositions
may be formulated as micronized suspensions in isotonic, pH
adjusted sterile saline, or, preferably, as solutions in
isotonic, pH adjusted sterile saline, either with our without
a preservative such as benzylalkonium chloride.
Alternatively, for ophthalmic uses, the pharmaceutical
compositions may be formulated in an ointment such as
petrolatum. In one embodiment, the compositions are as
formulated in, e.g., U.S. Patent 6,645,994 and/or U.S. Patent
=
6,630,473.
[01403 The pharmaceutical compositions of this invention
may also be administered by nasal aerosol or inhalation. Such
compositions are prepared according to techniques well-known
in the art of pharmaceutical formulation and may be prepared
as solutions in saline, employing benzyl alcohol or other
suitable preservatives, absorption promoters to enhance
bioavailability, fluorocarbons, and/or other conventional
solubilizing or dispersing agents.
[0141] The above-described compounds and compositions are
particularly useful in therapeutic applications relating to an
IL-1 mediated disease, an apoptosis mediated disease, an
inflammatory disease, an autoimmune disease, a destructive
bone disorder, a proliferative disorder, an infectious disease
(e.g., bacterial infections, preferably, eye infections), a
degenerative disease, a disease associated with cell death, an
excess dietary alcohol intake disease, a viral mediated
disease, retinal disorders, uveitis, inflammatory peritonitis,
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
- 61-
osteoarthritis, pancreatitis, asthma, adult respiratory
distress syndrome, glomerulonephritis, rheumatoid arthritis,
systemic lupus erythematosus, scleroderma, chronic
thyroiditis, Grave's disease, autoimmune gastritis, diabetes,
autoimmune hemolytic anemia, autoimmune neutropenia,
thrombocytopenia, chronic active hepatitis, myasthenia
gravis, inflammatory bowel disease, Crohn's disease,
psoriasis, atopic dermatitis, scarring, graft vs. host
disease, organ transplant rejection, organ apoptosis after
burn injury, osteoporosis, leukemias and related disorders,
myelodysplastic syndrome, multiple myeloma-related bone
disorder, acute myelogenous leukemia, chronic myelogenous
leukemia, metastatic melanoma, Kaposi's sarcoma, multiple
myeloma, hemorrhagic shock, sepsis, septic shock, burns,
Shigellosis, Alzheimer's disease, Parkinson's disease,
Huntington's disease, Kennedy's disease, prion disease,
cerebral ischemia, epilepsy, myocardial ischemia, acute and
chronic heart disease, myocardial infarction, congestive heat
failure, atherosclerosis, coronary artery bypass graft, spinal
muscular atrophy, amyotrophic lateral sclerosis, multiple
sclerosis, HIV-related encephalitis, aging, alopecia,
neurological damage due to stroke, ulcerative colitis,
traumatic brain injury, spinal cord injury, hepatitis-B,
hepatitis-C, hepatitis-G, yellow fever, dengue fever, JaPanese
encephalitis, various forms of liver disease, renal disease,
polycystic kidney disease, H. pylori-associated gastric and
duodenal ulcer disease, HIV infection, tuberculosis,
meningitis, toxic epidermal necrolysis, pemphigus, and
autoinflammatory diseases (sometimes referred to as
autoinflammatory fever syndromes) and related syndromes such
as Muckle-Wells Syndrome (MWS), Familial Cold Urticaria (FCU>,
Familial Mediterranean Fever (FMF), Chronic Infantile
CA 02820541 2013-06-21
79580-110
-62-
Neurological Cutaneous and Articular Syndrome (CINCAS), a.k.a.
= Neonatal Onset Multisystem Inflammatory Disease (NOMID),
TNFR1-Associated Periodic Syndrome (TRAPS), and Hyper-IgD
periodic fever Syndrome (HIDS). The compounds and
compositions are also useful in treating complications
associated with coronary artery bypass grafts. The compounds
, and compositions are also useful for decreasing IGIF (also
known as IL-18) or IFN-y production and for inhibiting a caspase-
mediated function' in a patient. The compounds and compositions
are also useful in immunotherapy as a cancer treatment.
[0142] The compounds and compositions may also be used in
methods for preserving cells. These methods would be useful
for preserving organs, particularly those intended for
transplant, or blood products.
[0143] . The compounds of this invention are useful as dual
caspase-1 and capase-8 inhibitors. Without being bound by
theory, the R.2 and R3 groups of the compounds of this invention
appear to be related to this surprising activity. Bridged A
groups of the compounds of this invention, such as
\1-9
or , also appear to be related to this surprising
activity. As such, the compounds and compositions of this
invention are particularly useful in treating or preventing
inflammatory conditions.
[0144] According to another embodiment, the compositions of
this invention may further comprise another therapeutic agent
(i.e., one or more additional agents). Such agents include,
but are not limited to, thrombolytic agents such as tissue
plasminogen activator and streptokinase. When an additional
agent is used, the additional agent may be administered either
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-63-
as a separate dosage form or as part of a single dosage form
with the compounds or compositions of this invention.
[0145] The amount of compound present in the compositions
of this invention should be sufficient to cause a detectable
decrease in the severity of the disease or in caspase activity
and/or cell apoptosis, as measured by any of the assays known
in the art.
[0146] Dosage levels of between about 0.01 and about 50 or
about 100 mg/kg body weight per day, preferably between 0.5
and about 75 mg/kg body weight per day and most preferably
between about 1 and about 25 or about 50 mg/kg body weight per
day of the active ingredient compound are useful in a
mono therapy.
[0147] Typically, a compound or composition of this
invention will be administered from about 1 to about 5 times
per day or alternatively, as a continuous infusion. Such
administration can be used as a chronic or acute therapy. The
amount of active ingredient that may be combined with the
carrier materials to produce a single dosage form will vary'
depending upon the host treated and the particular mode of
administration. A typical preparation will contain from about
5% to about 95% active compound (w/w). Preferably, such
preparations contain from about 20% to about 80% active
compound.
[0148] When the compositions of this invention comprise a
combination of a compound of this invention and one or more
additional therapeutic or prophylactic agents, both the
compound and the additional agent should be present at dosage
levels of between about 10% to about 100%, and more preferably
between about 10% to about 80% of the dosage normally
administered in a monotherapy regime.
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-64-
[0149] Upon improvement of a patient's condition, a
maintenance dose of a compound, composition or combination of
this invention may be administered, if necessary.
Subsequently, the dosage or frequency of administration, or
both, may be reduced, as a function of the symptoms, to a
level at which the improved condition is retained when the
symptoms have been alleviated to the desired level, treatment
should cease. Patients may, however, require intermittent
treatment on a long-term basis upon any recurrence of disease
symptoms.
[0150] As the skilled practitioner will appreciate, lower
or higher doses than those recited above may be required. It
should be understood that a specific dosage and treatment
regimens for any particular patient will depend upon a variety
of factors, including the activity of the specific compound
. employed, the age, body weight, general health, sex, diet,
time of administration, rate of excretion, drug combination,
the severity and course of the particular disease, the
patient's disposition to the disease being treated, and the
judgment of the treating physician. The amount of active
ingredients will also depend upon the particular compound and
other therapeutic agent, if present, in the composition.
L0151] In a preferred embodiment, the invention provides a
method of treating a patient, preferably a mammal, having one
of the aforementioned diseases, comprising the step of
administering to said patient a compound or a pharmaceutically
acceptable composition described above. In this embodiment,
if the patient is also administered another therapeutic agent
or caspase inhibitor, it may be delivered together with the
compound of this invention in a single dosage form, or, as a
separate dosage fault. When administered as a separate dosage
form, the other caspase inhibitor or agent may be administered
CA 02820541 2013-06-21
WO 2005/085236
PCT/HS2005/006540
-65-
prior to, at the same time as, or following administration of
a pharmaceutically acceptable composition comprising a
compound of this invention.
[0152] The compounds of this invention may also be
incorporated into compositions for coating implantable medical
devices, such as prostheses, artificial valves, vascular
grafts, stents and catheters. Accordingly, the present
invention, in another aspect, includes a composition for
coating an implantable device comprising a compound of the
present invention and a carrier suitable for coating said
implantable device. In still another aspect, the present
invention includes an implantable device coated with a
composition comprising a compound of the present invention and
a carrier suitable for coating said implantable device.
[0153] Another aspect of the invention relates to ,
inhibiting caspase activity in a biological sample, which
method comprises contacting said biological sample with a
compound of this invention or a composition comprising said
compound. The term thiological sample", as used herein,
includes, without limitation, cell cultures or extracts
thereof; biopsied material obtained from a mammal or extracts
thereof; and blood, saliva, urine, feces, semen, tears, or
other body fluids or extracts thereof.
[0154] Inhibition of caspase activity in a biological
sample is useful for a variety of purposes that are known to
one of skill in the art. Examples of such purposes include,
but are not limited to, blood transfusion, organ-
transplantation, biological specimen storage, and biological
assays.
[0155] The compounds of this invention are useful in
methods for preserving cells, such as may be needed for an
organ transplant or for preserving blood products. Similar
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-662.
uses for caspase inhibitors have been reported [Schierle et
al., Nature Medicine, 5, 97 (1999)]. The method involves
treating the cells or tissue to be preserved with a solution
comprising the caspase inhibitor. The amount of caspase
inhibitor needed will depend on the effectiveness of the
inhibitor for the given cell type and the length of time
required to preserve the cells from apoptotic cell death.
[01563 Without being bound by theory, applicants' cyclic
acetal compounds are believed to be prodrugs. That is, the
acetal portion is cleaved in vivo to provide a corresponding
acid-aldehyde compound. As would be recognized by a skilled
practitioner, chemical compounds may be metabolized in vivo,
e.g., at a site other than the prodrug cleavage site. Any
such metabolites are included within the scope of this
invention.
(0157] In order that this invention be more fully
understood, the following preparative and testing examples are
set forth. These examples are for the purpose of illustration
only and are not to be construed as limiting the scope of the
invention in any way.
Example I-1
(S,S,S,R)-1-[(2,9)-(3-Methoxy-2-methyl-benzoylamino)-3-methyl-
butyry1}-pyrrolidine-(25)-carboxylic acid [(2R)-ethoxy-5-oxo-
tetrahydro-furan-(3S)-yll-amide
,o
=
ilk r.1(0
0
H
CA 02820541 2013-06-21
.9580-110
-67-
Method A
(S) -3 -Amino -4-hydroxy -butyric acid tert-butyl ester
oH
[0158] A solution of (S)-benzyloxycarbonylamino -4-hydroxy-
butyric acid tart -butyl ester (prepared as described in Michel
etal, Helvetica Chimica Acta 1999, 1960)(0.94g) in ethyl
acetate (15 ml) was hydrogenated over palladium
hydroxide/carbon (20% w/w, 160mg). The catalyst was removed
= TM
via filtration through Celite. Concentration of the filtrate
in vacuo afforded the subtitle compound as a colorless oil
(486mg, 91%); IH NKR (400MHz,CDC13) 8 1.48 (9H, s), 1.95 (3H,
brs), 2.28 (1H, dd), 2.46 (1H, dd), 3.29 (1H, brm), 3.42 (111,
m), 3.60 (IH, m).
Method B
(1S) -2 -((S) -2-tert -Butoxycarbonyl-1 -hydroxymethyl -
ethylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester
0
oirNy.,a,
0 OH
0 N
[0159] To a stirred solution of (S)-3 -Amino -4 -hydroxy-
butyric acid tert-butyl ester (800mg, 4.57mmol) and Z-Pro-OH
(1.14g, 4.57mmol) in THF (30m1) was added 2-
hydroxybenzotriazole hydrate (741mg, 1.2eq,), DMAP (698mg,
1.25eq.), diisopropylethylamine (1.03m1, 1.3eq.) and 1-(3-
dimethylaminopropyl) -3 -ethylcarbodiimide hydrochloride (EDC,
1.05g, 1.2eq.). The resulting mixture was stirred at ambient
temperature for 18 hours then diluted with ethyl acetate. The
mixture was then washed with water, saturated aqueous sodium
bicarbonate solution and brine, dried over magnesium sulfate,
CA 02820541 2013-06-21
W02005/085236
PCT/1JS2005/006540
-68-
filtered and concentrated under reduced pressure. The residue
was purified by flash chromatography (60% ethyl
acetate/petrol) to afford the sub-title compound as a
colorless solid (1.483g, 90%); MS ES (+) 407.3.
Method C
(1S)-2-((S)-2-tert-Butoxycarbony1-1-formyl-ethylcarbamoy1)-
pyrrolidine-l-carboxylic acid benzyl ester
y 0
1.1 .õ(J1Lro,k
0 N
0
[01601 A solution of (1S)-2-((S)-2-tert-Butoxycarbony1-1-
hydroxymethya-ethylcarbamoy1)-pyrrolidine-l-carboxylic acid
benzyl ester (10 g) in DCM (100 ml) was cooled to 0 C under
nitrogen. 2,2,6,6-tetramethylpiperidinyloxy (TEMPO, 38 mg)
was then added followed by trichloroisocyanuric acid (6 g)1
portionwise over 30 minutes. The mixture was stirred at
ambient temperature for 2 hours, then filtered through celite.
The filtrate was washed with water, 1M sodium thiosulfate
solution and water. Drying over magnesium sulfate and
concentration under reduced pressure gave the sub-title
compound as a pale yellow oil (9.92 g, 99%); IH NMR (400MHz,d-6
DMSO) .5 1.38 (9H, d), 1.79-1,86 (3H, m), 2.08-2.23 (1H, m),
2.36-2.51 (1H, 2 x dd), 2.61-2.86 (1H, 2 x dd), 3.88-3.46 (2H,
m), 4.24-4.30 (2H, m), 5.05 (2H, quin), 7.28-7.37 (5H. m),
8.59-8.64 (IH, 2 x d), 9.21 (0.57H, s), 9.37 (0.43H, s).
CA 02820541 2013-06-21
WO 2005/085236
PCT/I1S2005/006540
-69-
Method D
(1S)-2-((S)-1-tert-Butoxycarbonylmethy1-2,2-diethoxy-
ethylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester
y 0
:),J
41111 OyN j
i15
00 N
[0161] To a
solution of (1S)-2-((S)-2-tert-Butoxycarbonyl-
1-formyl-ethylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl
ester (4.98 g) in dichloromethane (70 ml) was added triethyl
orthofoLmate (6.2 mL) and p-toluenesulfonic acid monohydrate
(47 mg). The resulting mixture was stirred at ambient
temperature until no aldehyde remained by TLC. The mixture was
concentrated in vacuo, the re-dissolved in dichloromethane (35
mL). Saturated aqueous sodium bicarbonate solution (35 mL)
was then added and the organic phase removed. This was washed
with water and brine, dried (magnesium sulfate), filtered and
concentrated under reduced pressure. This gave the sub-title
compound as a pale yellow oil (4.85 g, 82%); 11-1 NMR (400MHz,d-6
DMSO) 5 1.04-1.11 (6H, m), 1.35-1.37 (9H, m), 1.73-1.89 (3H,
m), 2.01-2.49 (3H, m), 3.43-3.52 (6H, m), 4.05-4.29 (3H, m),
4.96-5.06 (2H, m), 7.27-7.38 (5H, m), 7.80 (0.5H, d), 7.88
(0.5H, d).
Method E
(1S)-2-((2R, 3S)-2-Ethoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-l-carboxylic acid benzyl ester 6.1
(1S)-2-((2S, 3S)-2-Ethoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.2
Sti 0 0 0= 0õN
8y 0
Y0 40
N ,
H H
6.1 6.2
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-70-
(0162] A solution of (1S)-2-((S)-1-tert-
Butoxycarbonylmethy1-2,2-diethoxy-ethylcarbamoy1)-pyrrolidine-
1-carboxylic acid benzyl ester (4.85 g) in dichloromethane (25
ml) was cooled to 0 C under nitrogen. Trifluoroacetic acid (6
ml) was then added and the mixture stirred at 0 C for 15
minutes, then warmed to ambient temperature and stirred until
the reaction was complete by TLC. The mixture was then diluted
with dichloromethane (90 ml) and saturated aqueous sodium
bicarbonate solution (130 ml) and stirred for 15 minutes. The
organic phase was then removed and washed with 1:1 saturated
aqueous sodium bicarbonate/brine (100 ml), the combined
aqueous washings was re-extracted with DCM (100 ml) and the
combined organic layers dried (magnesium sulfate), filtered
and concentrated under reduced pressure. This afforded the
sub-title compound as a mixture of epimers at the ketal centre
(C2). The epimers were separated on silica gel, eluting with
30% acetone/petrol. Syn-isomer 6.1 (white solid); 111 MIR
(400MHz,d-6 DMSO) 8 1.08-1.17 (311, m), 1.78-2.01 (3H, m),
2.08-2.12 (1H, m), 2.37-2.57 (1H, 2 x dd), 2.61-2.79 (111, 2 x
dd), 3.35-3.51 (211, m), 3.55-3.68 (111, m), 3.71-3.82 (1H, d),
4.20-4.32 (1H, m), 4.52-4.61 (111, m), 4.98-5.11 (2H, m), 5.53-
5.58 (1H, m), 7.24-7.42 (5h, m), 8.25-8.31 (111, m); MS ES +
377.3 (100%), ES - 375.3 (10%); Anti-isomer 6.2 (colorless
oil); 114 NMR (400MEz,d-6 DMSO) 8 1.08-1.19 (311, m), 1.78-1.89
(3H, m), 2.10-2.34 (1H, m), 2.92-3.07 (1H, 2 x dd), 3.36-3.51
(3H, m), 3.62-3.78 (211, m), 4.12-4.21 (2H, m), 4.97-5.12 (311,
m), 7.28-7.40 (5H, m), 8.51-8.58 (111, m); MS ES 377.4
(100%), ES - 375.3 (10%).
(1S)-2-((2R,3S)-2-Methoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.3
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-71-
(1S)-2-((2S,3S)-2-Methoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.4
lel 0
0110
0 0
0 N 0 N
H H
6.3 6.4
[0163] Prepared in a similar manner to that described in
methods A-E, using trimethylorthoformate in step D, to afford
the sub-title compounds as a mixture of epimers 6.3 and 6.4.
The epimers were separated on silica gel eluting with 30% to
40% 2-Eutanone/Petrol to 70% Acetone/Petrol. Syn-isomer 6.3
(viscous colorless oil); 1-11 NMR (400MHz,d-6 DMSO) 6 1.77-1.89
(3H, m), 2.07-2.12 (1H, m), 2.32-2.43 (1H, 2 x d), 2.55-2.61
(1H, 2 x d), 2.71-2.81 (1H, 2 x d), 3.39-3.62 (4H, m), 4.21-
4.30 (1H, m), 4.57-4.64 (1H, m), 5.01-5.09. (2H, m), 5.42-5.47
(1H, m), 7.27-7.42 (5H, m), 8.24-8.31 (1H, m); Anti-isomer 6.4
(white solid); 1H NMR (400MHz,d-6 DMSO) 8 1.79-1.90 (3H, m),
2.09-2.21 (1H, m.), 2.23-41 (1H, 2 x d), 2.91-3.05 (1H, 2 x
dd), 3.35-3.71 (5H, m), 4.09-4.21 (2H, m), 4.98-5.19 (3H, m),
7.28-7.41 (5H, m), 8.51-8.58 (111, m).
(1S)-2-((2R,3S)-2-Isopropoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.5
(1S)-2-((2S,3S)-2-Isopropoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.6
1.1 0
0 0
0 N
H H
6.5 6.6
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-72--
[0164] Prepared in a similar manner to that described in
Methods A-E, using triisopropylorthoformate in step D, to
afford the sub-title compound as a mixture of epimers 6.5 and
6.6. The epimers were separated on silica gel eluting with 30%
to 40% 2-Butanone/Petrol. $yn-isomer 6.5 (colorless gum);
NMR (400MHz,d-6 DMSO) 8 1.07-1.16 (6H, m), 1.81-1.86 (2H, m),
2.37-2.71 (2H, m), 3.35-3.53 (2H, m), 3.86-3.90 (1H, m), 4.18-
4.24 (1H, m), 4.46-4.55 (1H, m), 4.95-5.10 (2H, m), 5.63 (IH,
d), 7.27-7.38 (5H, m), 8.22-8.30 (1H, m); MS ES + 391.3
(100%); Anti-isomer 6.6 (white solid); 1H NMR (400MHz,d-6 DMSO)
8 1.07-1.15 (6H, m), 1.78-1.82 (3H, m), 2.07-2.41 (2H, m),
2.87-3.01 (1H, m), 3.35-3.50 (2H, m), 3.74-3.96 (1H, m), 4.07-
4.18 (2H, m), 4.95-5.11 (2H, m), 5.22 (1H, 2 x s), 7.24-7.39
(5H, m), 8.48-8.53 (1H, m); MS ES + 391.4 (100%).
(1S)-2-((2R,3S)-2-Propoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.7
(1S)-2-((2S,3S)-2-Propoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.8
410 OyNy 0
41:1 0y10 0
:(:50
0 N 0 N =
H H
6.7 6.8
[0165] Prepared in a similar manner to that described in
methods A-E, using tripropylorthoformate in step D, to afford
the sub-title compounds as a mixture of epimers 6.7 and 6.8.
The epimers were separated on silica gel eluting with 30% to
40% 2-Butanone/Petrol. $yn-isomer 6.7 (colorless gum); NMR
(400MHz,d-6 DMSO) 8 0.84-0.93 (3H, m), 1.55 (2H, m), 1.81-1.89
(3H, m), 2.08-2.22 (1H, m), 2.37-2.61 (1H, 2 x dd), 2.71-2.80
(1H, 2 x dd), 3.31-3.53 (2H, m), 3.60-3.69 (1H, m), 4.20-4.29
(IH, m), 4.52-4.61 (IH, m), 4.95-5.11 (2H, m), 5.50 (1H, m),
CA 02820541 2013-06-21
=
=
WO 2005/085236 PCT/1JS2005/006540
-73-
7.27-7.36 (5H, m), 8.27 (1H, m); Anti-isomer 6.8 (colorless
oil); 11.1 NMR (400MHz,d-6 DMSO) 8 0.82-0.90 (3H, m), 1.46-1.57
(2H, m), 1.77-1.89 (3H, m), 2.06-2.41 (1H, m), 2.90-3.05 (1H,
2 x dd), 3.33-3.66 (5H, m), 4.11-4.20 (211, m), 4.94-5.10 (3H,
m), 7.28-7.37 (5H, m), 8.51(1H, m).
(1S)-2-((2R,3S)-2-Butoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-pyrrolidine-1-carboxylic acid benzyl ester 6.9
(15)-2-((2S,3S)-2-Butoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoya)-pyrrolidine-1-carboxylic acid benzyl ester 6.10
8
OyNy 0
0,,Nj:5y 0 )
0
0 N
H 0 N
6.9 6.10
[0166] Prepared in a similar manner to that described in
methods A-E, using tributylorthoformate in step D, to afford
the sub-title compounds as a mixture of epimers 6.9 and 6.10.
The epimers were separated on silica gel eluting with 30% to
40% 2-Butanone/Petrol. Syn-isomer 6.9 (colorless gum); IH NMR
(400MHz,d-6 DMSO) 8 0.86-0.92 (3H, m), 1.28-1.37 (2H, m),
1.45-1.54 (211, m), 1.79-1.88 (3H, m), 2.07-2.21 (111, m), 2.35-
2,78 (2H, m), 3.31-3.54 (2H, m), 3.63-3.70 (1H, m), 4.21-4.29
(1H, m), 4.51-4.61 (111, m), 4.95-5.09 (2H, m), 5.50 (1H, m),
7.27-7.37 (5H, m), 8.25 (IH, m); Anti-isomer 6.10 (colorless
oil); 1H NMR (400MHz,d-6 DMSO) 8 0.85-0.93 (3H, m), 1.26-1.36
(2H, m), 1.44-1.56 (2H, m), 1.77-1.90 (3H, m), 2.08-2.40 (1H,
m), 2.89-3.05 (111, 2 x dd), 3.34-3.70 (5H, m), 4.08-4.19 (2H,
m), 4.95-5.10 (3H, m), 7.28-7.39 (5H, m), 8.53(1H, m).
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/1JS2005/006540
-74-
Method F
{(S)-1-[(1R,3S,4S)-3-((2R,3S)-2-Ethoxy-5-oxo-tetrahydro-furan-
3ylcarbamoy1)-2-pyrrolidine-2-carbonyl]-2,2-dimethyl-propy1)-
carbamic acid benzyl ester
=
0
ANtri\3-
0 H
[0167] To a solution of (1S)-2-((2R, 3S)-2-Ethoxy-5-oxo-
tetrahydro-furan-3-ylcarbamoy1)-pyrrolidine-1-carboxylic acid
benzyl ester 6.1 (4.68g) in ethyl acetate (160m1) and DMF
(25m1) was added triethylamine (2.5g) followed by palladium
hydroxide/carbon (20% w/w, lg). The mixture was stirred under
an atmosphere of hydrogen until no starting material was
present by TLC. The catalyst was removed by filtration through
celite. To the filtrate was added (S)-2-
benzyloxycarbonylamino-3,3-dimethyl-butyric acid (4.93g),
hydroxybenzotriazole hydrate (2.01g) and 1-(3-
dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride (EDC,
2.85g). The resulting mixture was stirred at ambient
temperature overnight. Saturated aqueous sodium bicarbonate
solution (180m1) was then added and the organic phase removed.
This was washed with saturated aqueous ammonium chloride (180
ml), then brine (180m1), dried (magnesium sulfate), filtered
and concentrated under reduced pressure. The crude product was
purified on silica gel, eluting with 40-75% ethyl
acetate/petrol. The sub-title compound was obtained as a white
foam (4.02g, 66%); IH NMR (400MHz, CDC13) 8 0.97 (9H, s), 1.14
(3H, t), 1.79-1.94 (3H, m), 2.02-2.10 (1H, m), 2.44 (1H, dd),
2.75 (1H, dd), 3.52-3.66 (2H, m), 3.70-3.79 (2H, m), 4.22 (IH,
d), 4.38-4.41 (1H, m), 4.48-4.58 (IH, m), 5.03 (2H, q), 5.56
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-75-
(1H, d), 7.26 (1H, d), 7.29-7.40 (5H, m), 8.24 (1H, d); MS ES
+ 490.6 (100%), ES - 488.8 (10%).
Method G
(S, 3,S,R) -1- [ (23) - (3-Methoxy-2-methyl-benzoylamino) -3-methyl-
butyryl] -pyrrolidine- (2,5) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan-(3S)-y1]-amide
,o o
rr)r.NT
0 N
H o,
(0168] To a solution of {(S)-1-[(1R,3S,4S)-3-((2R,3S)-2-
Ethoxy-5-oxo-tetrahydro-furan-3ylcarbamoy1)-2-pyrrolidine-2-
carbonyl] -2 , 2-dimethyl-propyll-carbamic acid benzyl ester
(344mg) in ethyl acetate (20m1)was added palladium
hydroxide/carbon (20% w/w, 74mg). The mixture was stirred
under an atmosphere of hydrogen until no starting material was
present by TLC. The catalyst was removed by filtration through
celite and the filtrate concentrated under reduced pressure to
give the amine as a brown foam (260mg). A portion of this
material (153mg) was dissolved in THF and 3-methoxy-2-methyl
benzoic acid (146mg), diisopropylamine (191111),
hydroxybenzotriazole hydrate (77mg) and 1-(3-
dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride (EDC,
109mg) were added. The resulting mixture was stirred at
ambient temperature for 24 hours then diluted with saturated
aqueous sodium bicarbonate. The organic phase was removed and
washed with saturated aqueous ammonium chloride, then brine,
dried (magnesium sulfate), filtered and concentrated under
reduced pressure. The crude product was purified on silica
gel, eluting with ethyl acetate. This gave the sub-title
compound as a white solid (138mg, 62%); analytical data
summarized in Table 3.
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-76-
[01693 Compounds
of .formula 1-2 to 1-58 have been prepared
by methods substantially similar to those described in Example
I-1.
Example I-2
(S, S, S, R) -1--[ (2S) - (2 -Methoxy-benzoylamino ) -3 -methyl-butyryl ] -
pyrrolidine-(2S)-carboxylic acid [ (2R)-ethoxy-5-oxo-
tetrahydro-furan- (3S) -y1]-amide
\o o
=
o N
H o
Example 1-3
(S,S, S, R) -1- [3-Methyl- (2S) - (2-trifluoromethoxy-benzoylamino) -
butyry1]-pyrrolidine- (2S) -carboxylic acid [ (2R)-ethoxy-5-oxo-
tetra.hydro-furan- (3S) -y1]-amide
F.sf
0 4--
0
[`nriT
0
o N
H o
Example 1-4
(S, S, S, R) -1- [ (2S) - (3-Hydroxy-2-methyl-benzoylamino)-3-methyl-
butyry1]-pyrrolidine- (2S) -carboxylic acid [ (2R)-ethoxy-5-oxo-
tetrahydro-furan- (3 S) -y1]--amide
HO 0
0
=
0
0 N
H0
=
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/1JS2005/006540
-77 -
Example I-5
(S, S, S, R) -1-- [ (2S) - ( 3 -Amino-2 -methyl-benzoylamino) -3 -methyl-
butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -ethoxy-5-oxo-
_
tetrahydro-furan- (35) -yl] -amide
H2N 0
*0 0
0 N
H o
Example I-6
(S, S, S, R) -1- [ (2S)- (2, 6-Dichloro-benzoylamino) -3 -methyl-
butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI
\
a o
o N
H
Example 1-7
(S. S, S, R) -N- { (1S) - [ (2S)- ( (2R) -Ethoxy-5-oxo-tetrahydro- furan-
( 3 S) -ylcarbamoyl ) -pyrrolidine-l-carbonyl ] -2 -methyl-propyl } -2 -
methyl -nico-t inamide
(1-Xl(NNT
H 0 4.0
0 N
II /0
Example I - 8
(S. 5, S, R)-N- (1S) - [ (2S)- ( (2R) -Ethoxy-5 -oxo- tetrahydro- furan-
( 3S) -ylcarba.moyl ) -pyrrolidine-l-carbonyl ] -2 -methyl-propy11-4-
methyl -nicot.inamide
o
Ni? JC?
A
0 N
H
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
- 7 8-
Example I-9
(S, S, S, R) -1- {3-Methyl-- (2S) -[ (3 -methyl - thiophene-2 -carbonyl ) -
amino] -butyryl) -pyrrolidine- (2S) -carboxylic acid [ (2R) -ethoxy-
-oxo- tetrahydro- furan- (3S) -yl] -amide
i no
0
0 N
H CO
Example I-10
(S, S, SIR) -2, 3-Dichloro-N-{ (1S) - [ (2S) - ( (2R) -ethoxy-5-oxo-
tetrahydro- furan- ( 3 S) -ylcarbamoyl ) -pyrrolidine-1- carbonyl ] -2 -
methyl-propyl } -isonicotinamide
Cl
a o
0 0
0 N
H 0
Example I-11
(S, S, S, R) -3 , 5-Dichloro-N-{ (1S) [ (2S) - ( (2R) -ethoxy-5-oxo-
tetrahydro-furan- (35) -ylcarbamoyl ) -pyrrolidine-l-carbonyl] -2 -
methyl -propyl } -ison.icotinamide
a o
N H N
CI 0
H 0
=
CA 02820541 2013-06-21
W02005/085236 PCT/US2005/006540
-79 -
Exampl e I-12
( S, S, S, R) -1- [ (2S) - ( 3 -Methoxy-2-methyl-benzoylamino ) -3, 3 -
dimethyl-butyryl ] -pyrrol i dine- (25) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetra]aydro-furan- (3S) -yl] -amide
-o
= riX.Nli
0 N
H 0
Example I-13
( S, S, S, R) -1-- [ (2S) - ( 3 -Methoxy-2 -methyl-ben.z oylamino ) -3-methyl-
butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -methoxy--5-oxo-
tetrahydro-furan- ( 3S) -yl ] -amide
0
410 Xr"i 0
0
0 N
H -
Example 1-14
(S, S. S, R) -1- [ (2S) - ( 3 -Methoxy-2-methyl-benz oyl amino) -3-methyl -
butyry1]-pyrrolidine- (2S) -carboxylic acid [ (2R) - isopropoxy-5-
oxo - t etrahydro- f uran- (3S) -yl] -amide
0
0
400 NC;IT 0
4.0
0
0 N
H Os(
Example 1-15
( S, S, S, R) -1- [ (2S) - ( 3 -Methoxy-2 -methyl-benz oylamino ) -3-methyl-
butyryl ] -pyrrolidine- (2S) -carboxylic acid (5-oxo- (2R) -propoxy-
5- tetrahydro- furan- (3S) -yl] -amide
0
400 tIC-rji 0
0
0 N
HO)
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-80-
Example I-16
(S, S, S, R) -1- [ (2S) - (2-Ch1oro-benzoylamino) -3 -methyl -butyryl] -
pyrrolidine- (25) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan- (3S) -y1 ] -amide
CI 0
4ji f?Cr"i 0
0
0 N
H 0
Example I-17
(S, S, S, R) -1- [3 , 3 -Dimethyl- (2S) - (2 -methyl-benzoylamin.o ) -
butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan- (3S) -y' :J -amide
0
400 NYC;44i
4.0
0
0 N
o
Example I-18
(S, S. S, R) -1- [3-Methyl-2 (S)- (2-tri fluoromethyl-benzoylamino) -
butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan-3 CS) -y1] -amide
CF30
.<0
0
o N
H 0
Example I-19
(S, S. S, R) -1-- [2 (S) - (2-Ch1oro-benzoylamino) -3,3-dimethyl-
butyryl] -pyrrolidine- (2S) -carboxylic acid [ (2R) -methoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI 0
* N? 0
)q0
0
0 N
H o
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
- 81-
Exampl e 1-20
(S, S, S, R) -1- [3 , 3-Dimethyl- (2S) - ( 2- tri fluoromethyl-
benzoylamin.o ) -butyryl I -pyrrolidine- (2S) -carboxylic acid [ (2R) -
isopropoxy-5-oxo-tetrahydro-furan- (3S) -yl] -amide
CF30
41* [NiT
o N
H 0
Example I-21
(S, S, S, R) -1- [ (2S) - ( 2 -Chloro-benzoylamino ) -3 , 3 -dimethyl-
butyryl] -pyrrolidin.e- (2S) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
Ct o
4## Vd(ji 0
0
0 N
H 0
Example 1-22
(S, S, S, R) -1- [3 , 3 -Dimethyl - (2S) - (2-trifluoromethyl-
benzoylamino) -butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan- (3S) -yl] -amide
CF30
N(r4i
õ<o
0 N
H 0
Example 1-23
(S, S, S,R) -1- [ (2S) - (2-Chloro-benzoylamino) -3, 3 -dimethyl-
butyryl ] -pyrrolidin.e- (2S) -carboxylic acid [5-oxo- ( 2R) -prOPoxY-
tetrahydro-furan- (3S) -yl] -amide
CI 0
ijk 11(r"i 0
0
0 N
H o
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-82--
Example 1-24
(S, S, S, R) - 1- [ (2S) ( 2 -Chloro-benz oylamino) -3, 3 -dimethyl-
butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -butoxy-5-oxo-
tetrahydro-furan.- (3S) -ylj -amide
CI 0
4.0
=
o N
H o
Example I-25
(S, S, S, R) -1- [ (2S) - (2-Chloro-3-tri f luoromethoxy-benzoylamino) -
3 , 3-dimethyl-butyryl] -pyrrolidine- (2S) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan- (3S) -ylj -amide
,.., CI 0
F3C-u* Nri/ 0
N
H 0
Example I-26
(S, S, SIR) -1- [ (2S) - ( 2 -Chloro-ben.zoylamino ) -3 -methyl -butyryl ] -
pyrrolidine- (2S) -carboxylic acid [ 5-oxo- (2R) -propoxy-
tetrahydro- furan- (3S) -yl] -amide
Cl 0
* rci\IT.<0
0
o N
H 0
Example I-27
(S, S. S, S) -1- [ (2S) - (2-Chloro-benzoylamino) -3 -methyl-butyryl ] -
pyrrolidine- (2S) -carboxylic acid [5-oxo- (2S) -propoxy-
tetrahydro-furan- (3S) -yll -amide
CI 0
=
ININT
o N =
H
0
CA 02820541 2013-06-21
=
=
WO 2005/085236 PCT/US2005/006540
-83 -
Example 1-28
(S, S, S, S) -1- [ (2S ) - ( 2 -Chloro-benzoylamino ) -3 -methyl-butyryl] -
pyrrolidine- (2S) -carboxylic acid ( (2S ) -ethoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI
400 dC-ri
o N =
H
S.
Example 1-29
(S, S, S, R) -1- [ (2S) - ( 2 -Chloro-benzoylamino ) -3 -methyl-butyryl ] -
PYrrolidine- (2S) -carboxylic acid (2R) -butoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI o
0
0 N
o
Example 1-30
(S, S, S. S) -1- [ (2S) - ( 2 -Chloro-benzoylaraino ) -3-methyl-butyryl] -
pyrrolidine- (2S) -carboxylic acid [(2S) -butoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI ,D
0
o N
H 120
Example I-31
( S, S, S, R) -1- [ (2S) - (2-Chloro-benzoylamino) -3 -methyl-butyryl ] -
pyrrolidine- (2S) -carboxylic acid (2R) -is opropoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI o
400 dc-ri 0
0 N
H o
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-84--
Example I-32
(S, S. S, S) -I- [ (2S) - (2-Chloro-benzoylamin.o ) -3 -methyl-butyryl] -
pyrrolidine- (2S) -carboxylic acid [ (2S) -isopropoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI o
400 VA-r"i 0
0
0 N
H
0
Example I-33
(S, S, S, R) -1- [ (2S ) - ( 2 -Chloro-3-cyclopropyloxy-benzoylamino ) -
3 , 3-dimethyl-butyryl] -pyrrolidine- (2S) --carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan.- (3S) -yl] -amide
0 CI 0
7- 400 NJCriT
0
0 N
H
Example I-34
(S, S, S, R) -1- [ (2S) - ( 2 -Chloro-3 -methyl -benzoylamino) -3 , 3 -
dimethyl-butyryl ] -pyrrol i dine- (2S) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan- (3S) -yl] -amide
CI
*
4.o
0 N
H 0
Example I-35
(S, S, S, R) -1-- [ (2S) - ( 2 -chl oro-3 -methoxy-benzoylamino ) -3 -methyl-
butyryl ] -pyrrol i dine- (2S) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan- (35) -yl] -amide
CI 0
,0
* [\XN? 0
jqo
0
o N
H 0
=
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-85-
Example 1-36
(S, S, S,R) -1- [ (2S) - (2-Chloro-3-ethyl -benzoyl amino) -3 , 3 -
dimethyl -butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan- (3S) -yl] -amide
CI 0
* NT
0 õ<cs
0 N
H o
Example I-37
(S, S, S, R) -1- [ (2S)- (2-chloro-4-methoxy-benzoylamino) -3-methyl-
butyryl ] -pyrrolidin.e- (2S) -carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan- (3S) -yl] -amide
CI 0 )r..
\O 0
0
0 N
H o
Example 1-38
(S, S, S,R) -1- [ (2S) - (2-Chloro-3-cyclopropylmethyl -
benzoylamin.o) -3 , 3 -dimethyl -butyryl ] -pyrroli dine- (2S) -
carboxylic acid [ (2R) -ethoxy-5-oxo-tetrahydro-furan- (3S) -yl] -
amide
CI 0
r iµN N/ jeo
0
0 14)!
H o
Example I-39
(S, S, S, R) -1- [ (2S ) (2-Chloro-3-hydroxy -benzoylamino) -3 , 3 -
dimethyl-butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -
ethoxy- 5 - oxo- tetrahydro- furan- (3S) -yl] -amide
CA 02820541 2013-06-21
WO 2005/085236
MA182005/006540
-86-
HO CI
if. N? 0
0
0 N
H 0
Example 1-40
(S, S, S, R) -1- [ (2S) - (2-Chloro-4-acetamido-benzoylamino) -3 -
methyl -butyryl ] -pyrrolidine- (2S) -carboxylic acid [ (2R) -ethoxy-
5-oxo-tetrahydro-furan- (3S) -yl] -amide
CI 0
HN
0 0
0 0 N
H 0
Example 1-41
(S, S, S,R) -1- [ (2S) - (2-Chloro-3-acetamido -benzoyl amino) -3 , 3 -
dimethyl-butyry13 -pyrrolidine-, (2S) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan- (3S) -y11 -amide
CI 0
HN
= AcN? 0
4.0
0
0 N
H 0
Example I-42
(S, S, S, R) -1- [ (2S ) - (2-methyl -3 -acetamido -benzoylamino) -3 , 3 -
dimethyl-butyryl ] -pyrrolidine- ( 2S) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan- (3S) -yl] -amide
HN 0
0
* [gi irko
0
0 N*.=
H 0
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-8 7-
ExampL e I-43
(S, S, S, R) -1- [ (2S) - (2-Chloro-4-acetamido -benzoylamino) -3 , 3 -
dimethyl-butyryl ] -pyrrolidine- ( 2S) -carboxylic acid [ (2R) -
ethoxy- 5 -oxo- t etrahydro- f uran- ( 3S) -yl] -amide
CI 0
HN 411 1)C N?
0 0
0 N
H 0
ExampL e I-44
( S, S, S, R) -1- [ (2S) - ( 2 - fluor -4- acetamido -benzoylamino) -3 , 3 -
dimethyl-butyryl ] -pyrrolidine- ( 2S) -carboxylic acid [ (2R) -
ethoxy- 5 -oxo- te trahydro- furan- ( 3S) -yl] -amide
F
HN *
= 0 0
0 N
H
ExampL e 1-45
(S, S, S, R) -1- [ (2S) - (2-fluoro -4- acetamido-benzoylamino) -3 -
methyl-butyryl] -pyrrolidine- (2S ) -carboxylic acid [ (2R) -ethoxy-
-oxo- tetrahydro- furan- (3S) -yl] -amide
F 0
0 4116
HN N?
0 1C
0 N
H 0
ExampL e - 4 6
(S, S, SIR) -1- [ (2S)- (2-chloro -4- isopropyloxy -benzoylamino) -3 -
methyl-butyryl -pyrrolidine- (2S ) -carboxylic acid [ (2R) -eth.oxy-
5 -oxo - tetrahydro- furan- (3S) -yl] -amide
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-88-
C'0
>'0 111 N=NT
0
0 N
H 0
Example 1-47
(S75,S,R)-1-[(2S)-(2-chloro -4-hydroxy -benzoylamino)-3,3-
.
dimethyl-butyry1]-pyrrondine-(2S)-carboxylic acid [(2R)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
CI
HO
O N
H 0
Example 1-48
(S,S,S,R)-1-[(2S)-(2-chloro -4-methoxymethyl -benzoylamino)-
3,3-dimethyl-butyrylj-pyrrolidine-(2S)-carboxylic acid [(2R)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
Cl 0
P 11# 11)N13.
o 0
O N
H 0
Example 1-49
(S,S,S,R)-1-[(2S)-(2-Chloro-4-isobutyrylamido -benzoylamino)-
3,3-dimethyl-butyry1]-pyrrolidine-(2S)-carboxylic acid [(2R)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
Ct 0 4,
()HN
0
O N
H 0
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-89-
Example I-50
(5,S,S,R)-1-[(2S)-(2-Chloro-4-acetamido --benzoylamino)-3-
cyclohexyl]-pyrrolidine-(2S)-carboxylic aacid [(2R)-ethoxy-5-
oxo-tetrahydro-furan-(3S)-y1]-amide
CI 0
FIN=
lqo
0 N
H 0
(
Example 1-51
(S, S, S, R) -1- [ (2S) - (2-Chloro-4-methoxycarloonylamino -
ben.zoylamino) -3 ,3-dimethyl-butyryl] -pyrrolidine- (2S) -
carboxylic acid [(2R)-ethoxy-5-oxo-tetrahlydro-furan-(3S)-y1]-
amide
CI 0
0
NNT
HN H
0 0
0 N
H o
Example I-52
(S, S, S, R) -1-[ (2S ) (2-Chloro-3-phenoxy -b9nzoylarnino) -3,3-
dimethyl-butyry1]-pyrrolidine- (2S)-carboxylic acid [ (2R)-
ethoxy--5-oxo-tetrahydro-furan- (3S)-y1]-armide
CI 0
# 0 *
0
0
0 N
H o
Example 1-53
(S,S,S,R)-1-[(2S)-(2-Chloro-4-thiazolylanaino -benzoylamino)-
3,3-dimethyl-butyry1]-pyrrolidine-(2S)-ceurboxylic acid [(2R)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-yal-amaide
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
-90-
C'0
0
FIN* rIN? jqo
0 N
H 0
Example 1-54
(S,S,S,R)-1-[(2S)-(3-Amino-2-chloro-benzoylamino)-3-methyl-
butyryll-pyrrolidine-(2S)-carboxylic acid [(,2R)-ethoxy-5-oxo-
tetrahydro-furan-(3S)-y1]-amide
Cl 0
H2N 0
NT ti,(0
0
0 1\1-=
H 0
Example 1-55
(S,S,S,R)- 1-[(2S)-(2-Chloro-benzoylamino)-3-thiazol-4-yl-
propiony1]-pyrrolidine-(2S)-carboxylic acid [(2R)-ethoxy-5-
oxo-tetrahydro-furan-(3S)-y1]-amide
CI 0
NAIN
101 H 0 ri
0 N
H 0
Example 1-56
(S,S,S,R)- 1-[(2S)-(3-Methoxy-2-methyl-benzoylamino)-3-
thiazol-4-yl-propiony1]-pyrrolidine-(2S)-carboxylic acid
[(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
EV1
(1 0
410H 40
0 N
H 0
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/ES2005/006540
-91-
Example 1-57
(S, S, S,R)- 1-( (2S) - (2-Chloro-3-methoxy-benzoylamino) -3,3-
dimethyl-butyryl ] -pyrrolidine- (2 S ) -carboxylic acid [ (2R) -
ethoxy-5-oxo-tetrahydro-furan- (3 S ) -y1] -amide
C1 0 0
0 110 )(1
N N?
0
Example 1-58
(S,S,S,R)- 1-[(2S)-(2-Chloro-benzoylamino)-3,3-dimethyl-
butyry1]-piperidine-(2S)-carboxylic acid [(2R)-ethoxy-5-oxo-
tetrahydro-furan-(3S)-y1]-amide
CI 0
Y 0
0 N 0
Example 1-59
2-[(2S)-(3-Methoxy-2-methyl-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic
acid [(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
0i-i3 0 Aj 0
0 N
0 H
METHOD H
(1R,3S,4S)-3((S)-2-tert-Butoxycarbony1-1-hydroxymethyl-
ethylcarbamoy1)-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid
benzyl ester
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
-92-
=
01111 ONJOk
0
0 H
OH =
(0170] To a stirred solution of (S)-3-Amino-4-hyc-Mcoxy-
butyric acid tert-butyl ester (486mg) and (1R,3S,4S)-2-Aza-
bicyclo[2.2.1]heptane-2,3-dicarboxylic acid 2 benzya ester
(prepared as described in Tararov eta/, Tett. Asymm. 2002, 13,
25-28) (767mg) in THF (18m1) was added 2-hydroxybenotriazole
hydrate, (452mg), DMAP (426mg), diisopropylethylamine (63101)
and 1-(3-dimethylaminopropy1)-3-ethylcarbodiimide
hydrochloride (EDC, 641mg). The resulting mixture waz stirred
at ambient temperature for 18 hours then diluted with ethyl
acetate. The mixture was then washed with water, sa-turated
aqueous sodium bicarbonate solution and brine, dried over
magnesium sulfate, filtered and concentrated under 3reduced
pressure. The residue was purified by flash-chromatc:Dgraphy
(60% ethyl acetate/petrol) to afford the sub-title compound as
a colorless oil (1.1g, 91%); 111 NMR (400MHz,d-6 DMSO) 8 1.13-
1.25 (1H, m), 1.30-1.48 (9H, m), 1.49-1.88,(6H, m), 2.20-2.52
(2H, m), 3.09-3.34 (2H, m), 3.64 (IH, d), 4.00-4.16 (2H, brm),
4.80 (1H, m), 4.90-5.15 (2H, m), 7.21-7.41 (5H, m), 7.50-7.75
(1H, m); MS ES (+) 433.37. .
Method I
(1R,3S,4S)-3-((S)-2-tert-Butoxycarbony1-1-formyl-
ethylcarbamoyl) -2-aza-bicyclo[2 .2 .1]heptane-2-carboylic acid
benzyl ester
CA 02820541 2013-06-21
a WO 2005/085236
PCT/US2005/006540
-93-
#
I3V E1
0
[0171] A solution of (1R,3S,4S)-3((S)-2-tert-
Butoxycarbony1-1-hydroxymethyl-ethylcarbamoya)-2-aza-
bicyclo[2.2.1]heptane-2-carboxylic acid benzyl ester (1.1g) in
DCM (10m1) was cooled to 0 C under nitrogen. 2,2,6,6-
tetramethylpiperidinyloxy (TEMPO, 4mg) was then added followed
by trichloroisocyanuric acid (621mg) portionwise over 30
minutes. The mixture was stirred at ambient temperature for 1
hour, then filtered through celite. The filtrate was washed
with water, 1M sodium thiosulfate solution and brine. Drying
over magnesium sulfate and concentration under reduced
pressure gave the sub-title compound as a colorless oil
(698mg, 64%); 111 NMR (400MHz,d-6 DMSO) 8 1.16-1.89 (16H, m),
2.30-2.80 (211, m), 3.68-3.81 (1H, m), 4.19 (1H, brm), 4.39,
(111, m), 4.91-5.16 (2H, m), 7.21-7.43 (5H, m), 8.45 (0.4H, d),
8.60 (0.6, d), 9.19 (0.6H, s), 9.37 (0.4H, s).
Method
(1R,3S,4S)-3-((S)-1-tert-Butoxycarbonylmethy1-2,2-diethoxy-
ethylcarbamoy1)-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid
benzyl ester
ay.74.ok
0
0 H
[0172] To a solution of (1R,3S,4S)-3-((S)-2-tert-
Butoxycarbony1-1-formyl-ethylcarbamoy1)-2-aza-
bicyclo[2.2.1]heptane-2-carboxylic acid benzyl ester (698mg)
in dichloromethane (10m1) was added triethyl orthoformate
(720mg) and p-toluenesulfonic acid monohydrate (6mg). The
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-94-
resulting mixture was stirred at ambient temperature -until no
aldehyde remained by TLC. Saturated aqueous sodium
bicarbonate solution was then added and the organic phase
removed. This was washed with water and brine, dried
(magnesium sulfate), filtered and concentrated under seduced
pressure. This gave the sub-title compound as a pale yellow
oil (635mg, 78%); 1HNMR (400MHz,d-6 DMSO) 8 0.96-1.15 (6H, m),
1.26-1.84 (16H, m), 2.20-2.50 (2H, m), 3.40-3.81 (5H, m),
4.10-4.28 (2H, m), 4.37 (1H, m), 4.88-5.14 (2H, m), 7.20-7.40
(5H, m), 7.65 (0.5H, d), 7.80 (0.5H, d).
Method K
(1R,3S,4S)-3-((2R,3S)-2-Ethoxy-5-oxo-tetrahydro-furan-3-
ylcarbamoy1)-2-aza-bicyclo[2.2.1]heptane-2-carboxylic acid
benzyl ester
1.1 IrN7
0
0 H
[0173] A solution of (1R,35,4S)-3-((S)-1-tert-
Hutoxycarbonylmethy1-2,2-diethoxy-ethylcarbamoy1)-2-aza-
bicyclo[2.2.11heptane-2-carboxylic acid benzyl ester (635mg)
in dichloromethane (3m1) was cooled to 0 C under nitrc,gen.
Trifluoroacetic acid (0.7m1) was then added and the mixture
stirred at 0 C for 15 minutes, then warmed to ambient
temperature and stirred until the reaction was complete by
TLC. The mixture was then diluted with dichloromethane (10m1)
and saturated aqueous sodium bicarbonate solution (14=1.1). The
organic phase was then removed and washed with 1:1 saturated
aqueous sodium bicarbonate/brine (8m1), dried (magnesium
sulfate), filtered and concentrated under reduced pressure.
This afforded the sub-title compound as a mixture of epimers
at the ketal centre. The epimers were separated on silica gel,
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-95-
eluting with 30% 2-butanone/petrol. Syn-isomer (oil) (115mg,
23%); 111 NMR (400MHz,d-6 DMSO) 8 0.80-1.91 (10H, m), 2.35-2.79
(2H, m), 3.56 (1H, m), 3.66-3.80 (2H, m), 4.18 (1H, m), 4.59
(1H, m), 4.94-5.11 (2H, m), 5.53 (1H, d), 7.20-7.40 (5H, m),
8.18 (0.5H, d), 8.27 (0.5H, d); MS ES + 403.31 (100%), ES -
401.37 (15%); Anti-isomer (oil)(103mg, 20%); 111 NMR (400MHz,d-6
DMSO) 8 0.80-1.85 (10H, m), 2.25-2.60 (1H, m), 2.95 (1H, m),
3.42 (1H, m), 3.5-3.75 (2H, m), 4.88-5.15 (3H, m), 7.21-7.40
(5H, m), 8.50 (0.4H, d), 8.59 (0.6H, d).
Method L
{(S)-1-[(1R,3S,4S)-3-((2R,3S)-2-Ethoxy-5-oxo-tetrahydro-furan-
3ylcarbamoy1)-2-aza-bicyclo[2.2.1]heptane-2-carbonyl]-2,2-
dimethyl-propy1}-carbamic acid benzyl ester
iX\T
0
0
0 H 0¨A
[0174] To a solution of (1R,3S,4S)-3-((2R,3S)-2-Ethoxy-5-
oxo-tetrahydro-furan-3-ylcarbamoy1)-2-aza-
bicyclo[2.2.1]heptane-2-carboxylic acid benzyl ester (5g) in
ethyl acetate (160m1) and DMF (25m1) was added triethylamine
(2.5g) followed by palladium hydroxide/carbon (20% w/w, 1g).
The mixture was stirred under an atmosphere of hydrogen until
no starting material was present by TLC. The catalyst was
removed by filtration through celite. To the filtrate was
added (5)-2-benzyloxycarbonylamino-3,3-dimethyl-butyric acid
(4.93g), hydroxybenzotriazole hydrate (2.01g) and 1-(3-
dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride (EDC,
2.85g). The resulting mixture was stirred at ambient
temperature overnight. Saturated aqueous sodium bicarbonate
solution (180m1) was then added and the organic phase removed.
This was washed with saturated aqueous ammonium chloride (180
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-96-
ml), then brine (180m1), dried (magnesium sulfate), filtered
and concentrated under reduced pressure. The crude product was
purified on silica gel, eluting with 40-75% ethyl
acetate/petrol. The sub-title compound was obtained as a white
foam (5.25g, 81%); 1H NMR (400MHz,d-6 DMSO) 8 0.85-1.03 (10H,
m), 1.07-1.20 (311, t), 1.30 (111, m), 1.40 (111, m), 1.50-1.80
(3H, m), 1.93 (IH, m), 2.40-2.50 (111, m), 2.78 (1H, m), 3.60
(1H, m), 3.78 (111, m), 3.89 (1H, s), 4.26 (111, d), 4.52 (2H,
m), 4.96-5.12 (211, m), 5.56 (1H, d), 7.10 (IH, d), 7.24-7.40
(5H, m), 8.27 (IH, d); MS ES 516.93 (100%), ES - 515.05
(100%).
Method M
(1R,3S,4S)-2-[(S)-2-(3-methoxy-2-methylbenzoylamino)-3,3-
dimethyl-butyry1]-2-aza-bicyclo[2.2.1]heptane-3-carboxylic
acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-y1)-amide =
0
=
(110 NIX(1\7NO
0
0 H
[0175] To a solution of C(S)-1-[(1R,3S,45)-3-((2R,3S)-2-
Ethoxy-5-oxo-tetrahydro-furan-3ylcarbamoy1)-2-aza-
bicyclo[2.2.11heptane-2-carbony1]-2,2-dimethyl-propyll-
carbamic acid benzyl ester (370mg) in ethyl acetate (20m1)was
added palladium hydroxide/carbon (20% w/w, 74mg). The mixture
was stirred under an atmosphere of hydrogen until no starting
material was present by TLC. The catalyst was removed by
filtration through celite and the filtrate concentrated under
reduced pressure to give the amine as a brown foam (272mg). A
portion of this material (167mg) was dissolved in THF and 3-
methoxy-2-methyl benzoic acid (146mg), diisopropylamirie
(19101), hydroxybenzotriazole hydrate (77mg) and 1-(3-
dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride (EDC,
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-97-
109mg) were added. The resulting mixture was stirred at
ambient temperature for 24 hours then diluted with saturated
aqueous sodium bicarbonate. The organic phase was removed and
washed with saturated aqueous ammonium chloride, then brine,
dried (magnesium sulfate), filtered and concentrated under
reduced pressure. The crude product was purified on silica
gel, eluting with ethyl acetate. This gave the sub-title
compound as a white solid (121mg, 52%); 111 NMR (400MHz,CDC13) 8
1.10 (9H, s), 1.28 (3H, t), 1.43-1.56 (IH, m), 1.79-1.86 (3H,
m), 1.99 (1H, brd), 2.29 (3H, s), 2.30-2.37 (1H, m), 2.83 (1H,
dd), 3.02 (1H, brs), 3.66-3.74 (1H, m), 3.87 (3H, s), 3.88-
3.94 (111, m), 4.16 (111, brs), 4.54 (1H, brs), 4.66-4.74 (IH,
m), 4.97 (1H, d), 5.46 (1H, d), 6.44 (1H, brd), 6.93 (1H, d),
7.00 (1H, d), 7.22 (IH, t), 7.78 (IH, brd); IR (solid) cm-1
2960, 1791, 1624, 1505, 1438, 1261, 1115, 975;, MS ES + 530; ES
- 528.
[0176]
Compounds of formula 1-60 to 1-73 have been prepared
by methods substantially similar to those described in Example
1-59.
Example 1-60
2-[(2S)-(2-Chloro-benzoylamino)-3,3-dimethyl-butyry1]-2-
(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic acid [(2R)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-y13-amide
oi 0
*I )(I
H 0 0
N46
0 H
X
Example 1-61
2-[(2S)-(4-Acetylamino-2-chloro-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.11heptane-(3S)-carboxylic
acid [(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
CA 02820541 2013-06-21
A WO 2005/085236
PCT/US2005/006540
-98-
CI 0 Icrl
*0 N 0
0 H
Example 1-62
2-1(2S)-(2-Chloro-4-propionylamino-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.11heptane-(35)-carboxylic
acid [(2R)-ethoxy-5-oxo-tetrahydro-furan-(35)-y1]-amide
= ci 0 0
JLN *
N N40
0 H
1
Example 1-63
2-[(2S)-(2-Chloro-3-isobutyrylamino-benzoylamino)-3,3-
dimethyl-butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-
carboxylic acid [(2R)-ethoxy-5-oxo-tetrahydro-furan-(38)-y1]-
amide
))1-H a 0
N PI
0
0 0 N
H
Example 1-64
2-[(2S)-(2-Fluoro-3-methoxy-benzoylamino)-3,3-dimethyl-
butyry1]-2-(18,4R)-aza-bicyclo[2.2.11heptane-(35)-carboxylic
acid [(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
F 0 0
,0 410 1,,X474
Hoomo
Example 1-65
2-[(2S)-(2-Fluoro-3-methoxy-benzoylamino)-3-methyl-butyry1]-2-
(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic acid [(2R)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-99 -
F 0 0
0
xi.14
Example 1-66
2-[(2S)-(3-methoxy-2-methyl-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic
acid [(2S)-ethoxy-5-oxo-tetrahydro-furan-(3S)-yll-amide
0
CH3 :irrq
0 0
*H 0 0
Example 1-67
2-[(2S)-(2-Chloro-benzoylamino)-3,3-dimethyl-butyry1]-2-
(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic acid [(2S)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
U 0 0
* jo
H 0 N
,\
Example 1-70
2-[(2S)-(4-Acetylamino-3-chloro-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.11heptane-(3S)-carboxylic
acid [(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
0
Example 1-69
2-[(2S)-(3-Chloro-4-propionylamino-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic
acid [(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
CA 02820541 2013-06-21
4
=
WO 2005/085236
PCT/US2005/006540
-100-
0 1,:ittri7
0 0 N
1-1 a O.
Example 1-70
2-[(2S)-(isoquinolin-1-ylcarbonylamino)-3,3-dimethyl-butyry1]-
2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic acid
[(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
0
11101.,.
0 0 N
H
Example 1-71
2-[(2S)-(4-Amino-3-ch1oro-benzoylamino)-3,3-dimethyl-butyry1]-
2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic acid ,
=
[(2R)-ethoxy-5-oxo-tetrahydro-furan-(3S)-yl]amide
rill l
H 0 N'Y
H2N 0 H
Example 1-72
2-[(2S)-(4-Amino-3-chloro-benzoylamino)-3-methyl-butyry1]-2-
(1S,4R)-aza-bicyclo[2.2.1]heptane-(35)-carboxylic acid [(2R)-
ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
?Corl
N40
H2N 0 H 0-.1
Example 1-73
2-[(2S)-(isoquinolin-l-ylcarbonylamino)-3,3-dimethyl-butyryll-
2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carboxylic acid
[(2S)-ethoxy-5-oxo-tetrahydro-furan-(3S)-y1]-amide
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-101-
so 0
isti7 0
0 0 N
Table 3. Characterization Data for Selected Compounds of
Formula I (by Compound Number)
Nb. 144-1 1H-NMR
(obs)
(DMSO-d6) 0.94-0.95 (311, m), 0.98-0.99
(311, m), 1.13-1.16 (3H, m), 1.80-2.0
(4H, m), 2.10 (311, s), 2.47-2.51 (211,
m), 2.73 (111, m), 3.34-3.61 (2H, m),
I-1 490.1 3.73-3.77 (2H, m), 3.79 (3H, s), 3.90
(1H, m), 4.39 (111, m), 4.55 (111, m),
5.55 (1H, d), 6.83 (111, d), 6.99 (111,
d), 7.19 (1H, m), 8.27 (111, d), 8.34
(1H, d)
(CDC13) 1.01-1.15 (6H, m), 1.26 (311, t),
1.90-2.29 (5H, m), 2.55-2.59 (1H, m),
2.75-2.83 (1H, m), 3.65-3.98 (311, m),
1-2 476.0 4.04 (3H, s), 4.44-4.49 (1H, m), 4.62-
4.69 (111, m), 4.75-4.80 (111, m), 5.60
(111, d), 7.09 (1H, t), 7.19 (111, d),
7.52 (111, t), 7.97 (111, d)
(CDC13) 1.02-1.10 (611, m), 1.23-1.34
(3H, m), 1.88-2.19 (511, m), 2.32-2.44
(2H, m), 2.81-2.89 (1H, m), 3.66-3.72
1-3 530.0 (2H, m), 3.83-3.98 (211, m), 4.56-4.73
(2H, m), 4.84-4.90 (111, m), 5.46 (111,
d), 7.15 (111, d), 7.35-7.60 (411, m),
7.99 (111, d)
(CDC13) 1.02 (311, d), 1.09 (311, d), 1.29
(3H, t), 1.93-2.19 (411, m), 2.29 (311,
s), 2.39 (2H, dd), 2.84 (111, dd), 3.66-
1-4 476.1 3.71 (2H, m), 3.88-3.95 (2H, m), 4.63
(1H, dd), 4.68-4.74 (111, m), 4.85 (1H,
dd), 5.32 (1H, s), 5.47 (111, d), 6.44
(1H, d), 6.87 (111, d), 6.98-7.00 (111,
m), 7.07-7.12 (111, m), 7.36 (111, d)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-102-
' No. m+1 1114am
(obs)
(CDC13) 0.95-1.10 (6H, m), 1.31 (311, t),
1.93-2.21 (4H, m), 2.25 (311, s), 2.34-
2.41 (211, m), 2.80-2.88 (1H, m), 3.63-
1-5 475.0 3.75 (411, m), 3.87-3.93 (211, m), 4.65-
4.75 (211, m), 4.82-4.88 (1H, m), 5.47
(111, d), 6.43 (IH, d), 6.74 (111, d),
6.81 (1H, d), 7.04 (111, t), 7.40 (1H,
d)
(CDC13) 1.03-1.05 (311, m), 1.09-1.13
(311, m), 1.22-1.30 (311, m), 1.95 (111,
m), 2.14-2.17 (2H, m), 2.44-2.51 (2H,
1-6 514.4 m), 2.79 (111, m), 3.65-3.68 (211, m),
3.86-3.90 (211, m), 4.12 (IH, m), 4.60-
4.61 (211, m), 4.86 (1H, m), 5.47 (111,
m), 6.4 (1H, 2 x d), 7.29-7.37 (3H, m),
7.54 (111, m)
(DMS0--(16) 0.95-1.01 (6H, m), 1.13-1.16
(311, m), 1.80-2.10 (4H, m), 2.45-2.51
(511, m), 2.74 (IH, ra), 3.33-3.59 (2H,
1-7 461.1 m), 3.68 (111, m), 3.95 (111, m), 4.38-
4.44 (211, m), 4.55 (1H, m), 5.55 (111,
m), 7.25 (1H, m), 7.62 (111, m), 8.27
(IH, m), 8.48 (IH, m), 8.63 (111, m)
(CDC13) 1.02 (3H, d), 1.08 (3H, d), 1.28
(311, t), 1.95-2.2 (411, m), 2.4-2.5 (211,
m), 2.55 (3H, s), 2.8-2.9 (1H, m), 3.7-
1-8 461.1 3.8 (211, m), 3.85-3.95 (211, m), 4.7-
4.85 (211, m), 4.9-4.95 (1H, m), 5.55
(IH, d), 6.6-6.65 (111, m), 7.2-7.25
(1H, m), 7.35-7.4 (IH, m), 8.6 (111, d),
- 8.7 (IH, s)
(DmSo-d0 0.93-0.95 (3H, m), 0.99-1.00
(3H, m), 1.13-1.16 (3H, m), 1.80-2.10
(411, m), 2.40 (311, s), 2.40-2.47 (211,
1-9 465.6 m), 2.73 (1H, m), 3.59-3.61 (211, m),
3.73-3.75 (211, m), 4.37-4.43 (2H, m),
4.55 (111, m), 5.53 (111, d), 6.97 (IH,
m), 7.59 (111, m), 7.81 (IH, d), 8.28
(1H, d)
_
=
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-103-
No. N+1 1H-NMR
(obs)
(CDC13) 1.01 (3H, d), 1.25 (3H, d), 1.27
(3H, t), 1.97-2.10 (2H, m), 2.14-2.26
(1H, m) 2.38 (2H, dd), 2.84 (1H, dd),
2-10 515.0 3.67-3.71 (2H, m), 3.81-3.87 (1H, m),
3.90-3.98 (1H, m), 4.58-4.61 (1H, m),
4.65-4.73 (1H, m), 4.86-4.90 (1H, dd),
5.47 (1H, d), 6.78 (1H, d), 7.23 (1H,
d), 7.42 (1H, d), 8.40 (1H, d)
(CDC13) 1.01 (3H, d), 1.14 (3H, d), 1.28
(3H, t), 1.96-2.12 (211, m), 2.17-2.23
(2H, m), 2.38 (2H, dd), 2.83 (1H, dd),
T-11 515.0 3.66-3.71 (211, m), 3.82-3.95 (211, m),
4.59-4.62 (111, m), 4.65-4.71 (111, m),
4.91 (1H, dd), 5.47 (111, d), 6.54 (111,
br dd), 7.21 (111, br dd), 8.57 (2H, s)
(DMSO-d6) 0.9-1.08 (9H, s), 1.12 (3H,
t), 1.75-2.00 (3H, m), 2.00-2.15 (4H,
m), 2.34-2.50 (1H, m), 2.80 (1H, m),
1-12 504.4 3.48-3.91 (7H, m), 4.40 (1H, m), 4.46-
4,70 (211, m), 5.58 (111, d), 7.81 (111,
d), 7.00 (1H, d), 7.19 (111, dd), 8.07
(111, d), 8.27 (1H, d)
(CDC13) 0.98-1.09 (6H, m), 1.90-2.05
(4H, m), 2.35-2.56 (2H, m), 2.70-2.85
(111, m), 3.49+3.55 (3H, 2xs), 3-55-3.67
1-13 476.0 (1H, m), 3.86 (3H, s), 4.00-4.09 (1H,
m), 4.58-4.90 (3H, m), 5.34-5.37 (111,
m), 6.25+6.40 (1H, 2xd), 6.90-7.01 (2H,
m), 7.18-7.25 (111, m), 7.37+7.54 (111,
2xd)
(CDC13) 0.99-1.11 (6H, m), 1.18-1.30
(6H, m), 1.86-2.15 (411, m), 1.28+1.30
(311, 2xs), 2.36-2.86 (311, m), 3.56-3.68
1-14 504.0 (111, m), 3.86 (311, s), 3.87-4.05 (2H,
m), 4.50-4.84 (311, m), 5.55+5.59 (111,
2xd), 6.86-7.01 (2H, m), 7.16-7.23 (111,
m), 7.37+7.54 (1H, 2xd)
(CDC13) 0.85-1.11 (9H, m), 1.55-1.73
(211, m), 1.89-2.20 (4H, m), 2.28+2.29
(3H, 2xs), 2.35-2.55 (211, m), 2.71-2.87
1-15 504.0 (111, m), 3.48-3.76 (311, m), 3.86 (311,
s), 3.98-4.06 (1H, m), 4.52-4.86 (311,
m), 5.44-5.49 (1H, m), 6.24+6.35 (111,
2xd), 6.88-6.99 (2H, m), 7.14-7.21 (1H,
m), 7.41+7.55 (1H, 2xd)
CA 02820541 2013-06-21
WO 2005/085236
PCT/1TS2005/006540
-104-
No. 14+1
(obs)
(CDC13) 1.0-1.15 (6H, m), 1.3-1.4 (3H,
m), 1.9-2.2 (4H, m), 2.4-2.5 (2H, m),
2.8-2.9 (1H, m), 3.7-3.8 (2H, m), 3.9-
1-16 480.5 4.0 (2H, m), 4.65-4.75 (2H, m), 4.88-
4.92 (1H, m), 5.5-5.52 (1H, m), 6.85-
6.9 (1H, m), 7.4-7.55 (1H, m), 7.7-7.75
(1H, m)
(DMSO-d6) 1.05 (9H, s), 1.15 (3H, t),
1.8-2.1 (4H, m), 2.3 (3H, s), 2.4-2.5
1-17 474.6 (1H, m), 2.7-2.8 (1H, m), 3.6-3.9 (4H,
m), 4.4-4.45 (1H, m), 4.5-4.7 (2H, m),
5.55-5.6 (1H, m), 7.2-7.4 (4H, m), 8.1
(1H, d), 8.25 (1H, d)
(DMSO-d6) 0.9-1.0 (6H, m), 1.15 (3H, t),
1.8-2.1 (4H, m), 2.4-2.5 (111, m), 2.7-
1-18 514.5 2.8 (1H, m), 3.6-3.85 (3H, m), 3.9-3.95
(1H4, m), 4.4-4.6 (3H, m), 5.55-5.6 (1H,
m), 7.4-7.45 (1H, m), 7.6-7.8 (3H, m),
8:22 (1H, d), 8.75 (1H, d)
(CDC13) 1.13 (9H, s), 1.90-2.20 (3H, m),
2.35-2.44 (211, m), 2.86 (111, dd), 3.56
(3H, s), 3.72-3.74 (1H, m), 3.90-3.99
1-19 480.5 (111, m), 4.62-4.65 (1H, m), 4.69-4.70
(111, in), 4.90 (111, d), 5.36 (1H, d),
6.94 (111, d), 7.28-7.46 (411, m), 7.71
(1H, dd)
(CDC13) 1.09 (9H, s), 1.27 (6H, m),
1.93-2.14 (3H, m), 2.34-2.42 (2H, m),
2.79-2.83 (111, m), 3.71 (1H, m), 3.90-
1-20 542.5 3.94 (1H, m), 4.01-4.04 (1H, m), 4.62-
4.67 (211, m), 4.88-4.91 (111, m), 5.56
(111, m), 6.46 (111, m), 7.40 (111, m),
7.54-7.62 (3H, m), 7.74 (111, m)
(CDC13) 1.12 (9H, s), 1.29 (311, t),
1.90-2.20 (3H, m), 2.36-2.43 (2H, m),
1-21 494.5 2.85 (1H, dd), 3.67-3.72 (2H, m), 3.90-
3.96 (211, m), 4.62-4.65 (2H, m) 4.91
(1H, d), 5.46 (1H, d), 6.95 (111, d),
7.34-7.46 (411, m), 7.71 (1H, dd)
(CDC13) 1.10 (911, s), 1.29 (3H, t),
1.90-2.20(3H, m), 2.35-2.42 (211, m),
2.84 (1H, dd), 3.68-3.72 (211, m), 3.90-
1-22 528.4 3.95 (2H, m), 4.62-4.80 (2H, m), 4.89
(111, d), 5.47 (111, d), 6.45 (1H, d),
7.43 (111, d), 7.54-7.61 (3H, m), 7.73
(1H, dd)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-105-
No. M+1
LH-NMR
(obs)
(CDC13) 0.95 (3H, t), 1.12 (9H, s),
1.60-1.70 (2H, m), 1.88-2.20 (3H, m),
2.35-2.45 (2H, m), 2.77-2.85 (1H, m),
1-23 508.5 3.53-3.61 (1H, m), 3.65-3.75 (1H, m),
3.76-3.84 (1H, m), 3.88-3.96 (1H, m),
4.60-4.73 (2H, m), 4.91 (11, d), 5.44
(1H, d), 6.96 (1H, d), 7.30-7.50 (4H,
m), 7.73 (1H, d)
(CDC13) 0.86 (3H, t), 1.18 (911, s),
1.21-1.65 (4H, m), 1.85-2.17 (3H, m),
2.36-2.59 (2H, m), 2.68-2.78 (IH, m),
1-24 522.5 3.44-3.54 (1H, m), 3.56-3.72 (211, m),
3.98-4.10 (IH, m), 4.56-4.85 (3H, m),
5.44 (111, d), 6.95-7.02 (1H, m), 7.32-
7,74 (5H, m)
(DMSO-d6) 0.99-1.21 (1211, m), 1.70-2.00
= (3H, m), 2.01-2.17 (111, m), 2.40-2.51
(111, m), 2.70-2.80 (1H, m), 3.50-3.88
1-25 578.3 (411, m), 4.40 (IH, m), 4.55 (1H, m),
4.65 (1H, m), 5.58 (1H, d), 7.36 (111,
m), 7.50 (1H, m), 7.61 (111, m), 8.21
(111, d), 8.70 (111, d)
(CDC13) 0.95 (3H, t), 1.05-1.15 (611, m),
1.55-1.8 (3H, m), 2.0-2.25 (411, m),
2.4-2.5 (IH, m), 2.6-2.9 (2H, m), 3.55-
1-26 494.5 3.8 (3H, m), 3.85-3.95 (1H, m), 4.05-
4,1 (111, m), 4.7-4.85 (211, m), 5.5-5.55
(111, m), 6.85-6.9 (1H, m), 7.4-7.6 (311,
m), 7.7-7.8 (IH, m)
(CDC13) 0.95 (311, t), 1.05-1.15 (6H, m),
1.5-1.7 (311, m), 2.0-2.2 (4H, m), 2.4-
2,6 (211, m), 2.9-3.1 (111, m), 3.4-3.5
1-27 494.5 (111, m), 3.55-3.7 (211, m), 4.0-4.1 (111,
m), 4.35-4.5 (211, m), 4.6-4.75 (111, m),
4.8-4.9 (0.5H, m), 5.35-5.38 (1H, m),
6.85-6.95 (111, m), 7.4-7.55 (3H, m),
7.64-7.8 (1.511, m)
(CDC13) 1.02-1.19 (7H, m), 1.22-1.28
(2H, m), 1.90-2.21 (311, m), 2.32-2.53
(2H, m), 2.95 (111, 2 x dd), 3.44-3.50
1-28 480.3 (1H, m), 3.59-78 (2H, m), 3.83-3.92
(1H, m), 4.02-4.09 (1H, m), 4.29-4.41
(1H, m), 5.34 (IH, 2 x s), 6.88 (IH, 2
x brd d), 7.31-7.42 (411, m), 7.57 (1H,
2 x brd d), 7.70 (IH, 2 x dd)
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-106-
No. M+1 111-11MR.
(obs)
(CDC13) 0.83-0.97 (3H, m), 1.02-1.14
(6H, m), 1.26-1.53 (3H, m), 1.55-1.66
(111, m), 1.91-2.20 (411, m), 2.35-2.61
(211, m), 2.73-2.90 (111, m), 3.54-3.74
1-29 508 (311, m), 3.84-3.90 (0.511, m), 3.99-4.06
(0.5H, m), 4.61-4.75 (2H, m), 4.77-4.93
(0.511, m), 5.45-5.51 (1H, m), 6.87 (1H,
brd), 7.34-7.45 (4H, m), 7.55 (0.5H,
brd), 7.70-7.22 (1H, m)
(400 MHz, CDC13) 0.87-0.97 (3H, m),
0.99-1.16 (6H, m), 1.27-1.40 (211, m),
1.48-1.59 (111, m), 1.91-2.19 (4H, m),
2.30-2.52 (2H, m), 2.90-3.07 (1H, m),
3.39-3.45 (0,511, m), 3.54-3.71 (211, m),
1-30 508 3.78-3.82 (0.5H, m), 3.86-3.92 (0.511,
m), 4.04-4.09 (0.5H, m), 4.31-4.35 (111,
m), 4.39-4.43 (111, m), 4.56-4.59 (0.5H,
m), 4.66-4.68 (1H, m), 4.80-4.86 (0.511,
m), 5.32-5.41 (1H, m), 6.87-6.91 (1H,
m), 7.31-7.45 (4H, m), 7.55-7.76 (2H,
m)
(CDC13) 1.04-1.19 (8H, m), 1.25-1.28
(311, m), 1.92-2.18 (411, m), 2.32-2.43
(1H, m), 2.62-2.87 (211, m), 3.59-3.71
1-31 494.4 (111, m), 3.85-3.95 (1H, m), 4.00-4.05
(1H, m), 4.60-4.67 (311, m), 5.60 (111, 2
x d), 6.88 (1H, brd d), 7.36-7.50 (411,
m), 7.52-7.56 (1H, m), 7.76 (111, 2 x
dd)
(CDC13) 0.87-1.24 (1011, m), 1.88-2.07
(3H, m), 2.13-2.21 (111, m), 2.32-2.54
(2H, m), 2.94 (1H, 2 x dd), 3.57-3.68
(111, m), 3.83-3.87 (1H, m), 4.02-4.09
1-32 494.3 (111, m), 4.27-4.30 (111, m), 4.41 (111,
dd), 4.51-4.69 (1H, m), 5.43 (111, 2 x
s), 6.89 (1h, 2 x brd d), 7.30-7.45
(4H, m), 7.52 (111, 2 x brd d), 7.70
(1H, 2 x dd)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-107-
No. M 1 111-1114R
(obs)
(DMSO) 0.70 (211, in, 0112), 0.89 (211, in,
CH2), 0.95-1.20 (1211, In, CH3, tbutyl),
1.71-2.13 (411, in, 0H2), 2.45 (111, in,
asp CH2), 2.75 (1H, m, asp CH2), 3.35-
1-33 550.5 3.89 (4H, in, 0112, CH), 3.99 (111, m,
CH), 4.37 (1H, in, CH), 4.51 (111, m,
CH), 4.65 (111, in, CH), 5.58 (111, d,
CHO), 6.90 (1H, in, aryl H), 7.35 (111,
in, aryl H), 7.45 (1H, in, aryl H), 8.25
(1H, d, NH), 8.35 (111, d, NH)
DMSO) 0.99-1.21 (1211, m, 0113, tBu),
1.75-2.14 (4H, m, CH2), 2.38 (3H, S.
0113), 2.40-2.51 (111, m, asp CH2), 2.70-
2.82 (111, in, asp CH2), 3.37-3.90 (411,
1-34 508.5 m, 0112, CH), 4.39 (1H, m, CH), 4.55
(1H, in, CH), 4.67 (111, in, CH), 5.58
(1H, d, CH), 7.15 (1H, m, aryl H), 7.28
(1H, in, aryl H), 7.38 (1H, in, aryl H),
8.25 (111, in, NH), 8.38 (111, in, NH)
CDC13 1.00 (3H, d), 1.10 (311, d)', 1.27
(3H, t), 1.90-2.19 (411, m), 2.34-2.45
(2H, m), 2.79-2.87 (1H, M), 3.65-3.71
1-35 510 (211, m), 3.84-4.93 (211, m), 3.92 (3H,
s), 4.56-4.70 (211, m), 4.82-4.88 (111,
m), 4.45 (1H, d), 6.69 (111, d), 6.99
(111, d), 7.16 (111, d), 7.27 (1H, t),
7.37 (1H, d)
(DMSO) 0.95-1.25 (15H, in, tBu, 0113),
1.78-2.13 (411, in, CH2), 2.43 (1H, in,
CH2), 2.65-2.80 (311, m, 0112), 3.50-3.88
1-36 522.5 (411, in, 0112, CH), 4.42 (111, m, CH),
4.58 (1H, in, CH), 4.70 (111, d, CH),
5.58 (1H, d, CH), 7.15 (111, in, aryl H),
7.27 (1H, in, aryl H), 7.38 (111, in, aryl
H), 8.27 (111, d, NH), 8.39 (1H, d, NH)
0D013 1.05-1.12 (6H, m), 1.25-1.3 (311,
m), 1.9-2.2 (2H, m), 2.4-2.5 (2H, m),
2.8-2.9 (111, m), 3.65-3.75 (2H, m),
1-37 510.5 3.85 (3H, s), 3.9-4.0 (1H, m), 4.65-
4.75 (211, m), 4.85-4.9 (1H, m), 6.9-
6.93 (111, m), 6.98 (111, s), 7.05-7.1
(1H, m), 7.4-7.45 (111, m), 7.75-7.8
(1H, d)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-108-
No. 14+1 H-NMR
(ohs)
CDC13 0.38-0.42 (2H, m), 0.63-0.71
(2H, m), 1.11 (911, s), 1.23-1.35 (4H,
m), 1.88-2.20 (3H, m), 2.34-2.45 (2H,
1-38 564 m), 2.76-2.87 (1H, m), 3.66-3.75 (2H,
m), 3.87-3.96 (4H, m), 4.62-4.73 (2H,
m), 4.89 (1H, d), 5.47 (1H, d), 6.80
(1H, d), 7.00 (1H, d), 7.19-7,29 (2H,
m), 7.48 (1H, d)
(DMSO) 1.11 (9H, s), 1.28 (311, t),
1.83-2.22 (3H, m), 2.36-2.43 (2H, m),
2.82-2.87 (111, m), 3.66-3.76 (2H, m),
1-39 510 3.86-3.97 (2H, m), 4.62-4.71 (2H, m),
4.88 (1H, d), 5.45 (1H, d), 6.31 (1H,
s), 6.73 (111, d), 7.05-7.20 (3H, m),
7.38 (1H, d)
(CDC13) 1.06 (611, dd), 1.28-1.31 (4H,
m), 1.91-2.20 (4H, m), 2.23 (3H, s),
2.39 (1H, dd), 2.84 (1H, dd), 3,65-3.72
1-40 537.4 (211, m), 3.86-3.94 (2H, m), 4.61-4.73
(211, m), 4.87 (111, dd), 5.46 (111, dd),
7.00-7.04 (1H, m), 7.22 (111, brd s),
7.38-7.45 (211, m), 7.73 (111, d), 7.80
(1H, brd s)
(DMSO) 0.95-1.20 (1211, in, tBu, CH3),
2.75-2.15 (7H, in, CH2, COCH3), 2.42
(111, m, CH2), 2.77 (111, m, CH2), 3.50-
3.88 (411, in, CH2, CH), 4.37 (1H, In,
1-41 551.5 CH), 4.55 (111, in, CH), 4.67 (1H, d,
CH), 5.58 (1H, d, CH), 7.09 (111, in,
aryl H), 7.32 (1H, in, aryl H), 7.71
(1H, m, aryl H), 8.26 (1H, in, NH), 8.49
(111, m, NH), 9.58 (1H, in, NH)
(DMSO) 0.95-1.20 (12H, m, tBu, CH3),
1.75-2.17 (1011, in, CH3, COCH3, CH2),
2.45 (1H, m, CH2), 2.77 (1H, m, CH2),
1-42 531.6 3.48-3.91 (4H, m, CH2, CH), 4.31-4.70
(3H, m, CH), 5.55 (1H, d, CH), 7.04
(1H, m, aryl H), 7.18 (111, in, aryl H),
7.41 (111, in, aryl H), 8.20 (1H, d, NH),
8.27 (111, d, NH), 9.39 (1H, brs, NH)
CA 02820541 2013-06-21
WO 2005/085236
PCMS2005/006540
-109-
No. 144.1 1H-NR
(obs)
DMSO) 1.04 (9H, s), 1.12-1.17 (311, m),
1.78-1.95 (4H, m), 2.06 (3H, s), 2.45
(111, dd), 2.72 (1H, dd), 3.52-3.81 (411,
1-43 551.4 m), 4.36-4.39 (111, m), 4.47-4.54 (IH,
m), 4.64 (111, d), 5.54 (111, dd), 7.33-
7.35 (111, m), 7.43-7.46 (1H, m), 7.81
(IH, brd s), 8.21-8.25 (211, m), 10.23
(111, brd s)
(DMSO) 1.02 (9H, s), 1.14 (3H, t),
1.78-1.98 (411, m), 2.08 (3H, s), 2.48
(1H, dd), 2.79 (1H, dd), 3.51-3.82 (411,
1-44 535.4 m), 4.36-4.39 (1H, m), 4.49-4.58 (111,
m), 4.71 (1H, d), 5.54 (111, d), 7.31-
7.34 (111, m), 7.65-7.72 (311, m), 8.49
(1H, d), 10.38 (IH, s)
(DMSO) 0.95 (6H, dd), 1.12-1.16 (4H,
m), 1.72-1.97 (4H, m), 2.07 (311, s),
2.48 (111, dd), 2.73 (1H, dd), 3.51-3.62
1-45 521.4 (211, m), 3.71-3.83 (211, m), 4.35-4.38
(111, m), 4.48-4.59 (211, m), 5.53 (1H,
d), 7.29-7.31 (111, m), 7.59-7.67 (211,
m), 8.01-8.05 (111, m), 8.28 (IH, d),
10.35 (111, s)
(CDC13 1.1-1.12 (611, m), 1.3 (3H, m),
1.4 (6H, d), 2.0-2.2 (211, m), 2.4-2.5
(2H, m), 2.8-2.9 (1H, m), 3.7-3.75 (211,
1-46 538.5 m), 3.9-4.0 (IH, m), 4.6-4.75 (3H, m),
4.85-4.95 (IH, m), 6.85-6.9 (111, m),
6.95 (111, s), 7.05-7.1 (IH, m), 7.4-
7.45 (111, m), 7.8 (111, d)
(CDC13) 1.15 (911, m), 1.25 (311, t),
2.0-2.2 (41-1, m), 2.4-2.5 (2H, m), 2.8-
2.9 (111, m), 3.7-3.85 (211, m), 3.9-4.0
1-47 510.5 (111, m), 4.05-4.1 (111, m), 4.7-4.8 (IH,
m), 4.85 (1H, d), 5.5 (IH, m), 6.5 (111,
d), 6.8 (111, s), 7.2 (111, d), 7.4 (1H,
d), 7.55 (111, d)
(CDC13) 1.12 (9H, s), 1.29 (311, t),
1.90-2.20 (3H, m), 2.36-2.43 (211, m),
2.85 (111, m), 3.42 (311, s), 3.68-3.74
1-48 538.5 (2H, m), 3.91-3.95 (211, m), 4.48 (2H,
s), 4.62-4.75 (211, m), 4.90 (111, m),
5.47 (111, m), 7.00 (111, m), 7.31 (111,
m), 7.43-7.54 (2H, m), 7.72 (IH, m)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-110-
No. M+1 la4ata
(obs)
(CDC13) 1.12 (9H, s), 1.28-1.31 (9H,
m), 1.90-2.20 (3H, m), 2.36-2.43 (2H,
m), 2.54 (1H, m), 2.85 (1H, m), 3.68-
1-49 579.5 3.72 (2H, m), 3.91-3.95 (2H, m), 4.62-
4.69 (2H, m), 4.88 (1H, d), 5.47 (1H,
m), 7.14 (1H, d), 7.27 (1H, m), 7.41
(1H, m), 7.50 (1H, d), 7.78 (1H, d),
7.87 (1H, m)
(DMSO) 1.12-1.16 (7H, m), 1.58-1.81
(5H, m), 1.83-1.92 (5H, m), 2.04-2.08
(4H, m), 2.50 (111, ad), 2.75 (1H, dd),
3.57-3.66 (2H, m), 3.72-3.78 (1H, m),
1-50 577-3 3.82-3.91 (1H, m), 4.33-4.36 (1H, m),
4.46 (1H, t), 4.52-4.61 (1H, m), 5.54
(1H, d), 7.32 (1H, d), 7.43 (1H, dd),
7.81 (1H, d), 8.25 (1H, d), 8.47 (1H,
d), 10.22 (1H, s)
(DMSO) 0.98-1.25 (12H, in, tBu, CH3),
1.78-2.14 (4H, in, CH2), 2.44 (1H, m,
CH2), 2.78 (1H, in, CH2), 3.50-3.88 (7H,
1-51 567.4 in, CH3, CH2, CH), 4.38 (1H, in, CH),
4.55 (1H, in, CH), 4.67 (IH, d, CH),
5.58 (1H, d, CH), 7.30-7.42 (2H, in,
aryl H), 7.60 (1H, brs, NH), 8.21 (211,
in, aryl H, NH), 9.99 (1H, brs, NH).
(DMSO) 0.95-1.24 (1211, in, tBu, CH3),
1.70-2.13 (4H, in, C112), 2.44 (111, in,
CH2), 2.75 (111, in, CH2), 3.45-3.90 (4H,
in, CH2, CH), 4.37 (111, m, CH), 4.55
1-52 586.4 (1H, m, CH), 4.70 (1H, d, CH), 5.57
(1H, d, CH), 6.91 (2H, d, aryl H),
7.06-7.19 (3H, in, aryl H), 7.30-7.45
m, aryl H), 8.20 (1H, d, NH), 8.55
(1H, d, NH)
(DMSO) 0.9-1.0 (6H, m), 1.18 (3H, t),
1.8-2.15 (411, m), 2.4-2.5 (1H, m), 2.7-
1-53 578.5 2.8 (1H, m), 3.6-3.85 (411, m), 4.4-4.6
(3H, m), 5.55 (111, d), 7.05 (IH, d),
7.3-7.35 (2H, m), 7.98 (1H, s), 8.3
(1H, d), 8.45 (1H, d), 10.7 (111, S)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-111-
No. 14+1 1H-NMR
(obs)
(DMSO) 0.94-0.98 (6H, in) , 1.13-1.18
(3H, in), 1.80-2.10 (5H, in) , 2.50 (1H,
m) , 2.73 (1H, m), 3.58-3.61 (2H, m),
1-54 495.0 3.74 (1H, m), 3.9 (1H, m) , 4.38-4.41
(2H, in) , 4.60 (1H, rah 5.46 (2H, s),
5.54 (1H, in), 6.48 (111, in), 6.80 (1H,
m), 7.04 (1H, in), 8.27 (1H, d) , 8.40
(1H, d)
(CDC13) 1.25 (3H, t), 1.99-2.01 (3H,
s), 2.30-2.39 (1H, m), 2.68 (1H, dd),
2.79 (1H, dd), 3.21-3.27 (1H, m) , 3.39
1-55 535.0 (1H, dd), 3.47-3.51 (2H, in), 3.65-3.75
(1H, rn) , 3.88-3.94 (111, in) , 4.64-4.68
(1H, in), 4.70-4.78 (1H, in) , 5.56 (1H,
d) , 7.31-7.35 (511, in) , 7.63-7.65 (1H,
in), 8.00 (1H, d), 8.76 (1H, d)
(CDC13 ) 1.25 (311, t) , 2.01-2.03 (3H,
m), 2.25 (311, s), 2.30-2.37 (11i, in) ,
2.65 (111, dd), 2.80 (1H, dd), 3.27-3.41
(2H, in), 3.47 (1H, dd), 3.65-3.79 (211,
1-56 545.0 in), 3.85 (3H, s), 3.86-3.90 (1H, in),
4.64-4.67 (1H, m), 4.71-4.80 (1H, in),
5.18-5.22 (111, In), 5.54 (111, d) , 6.83
(111, d) , 6.90-6.97 (2H, m), 7.19 (1H,
t), 7.24-7.28 (1H, m), 7.90 (1H, d),
8.77 (1H, d)
(CDC13 ) 1.12 (911, s) , 1.31 (3H, t),
1.93-2.20 (311, in), 2.35-2.46 (211, m),
2.79-2 .86 (1H, in), 3.65-3.74 (211, In),
1-57 524.0 3.87-3 .96 (211, in), 3.95 (3H, s) , 4.65-
4.74 (211, m), 4.89 (111, d), 5.47 (1H,
d), 6.76 (1H, d), 7.03 (11-1, d), 7.30
(1H,t) , 7.48 (1H, d)
(CDC13 ) 1.10 (911, s), 1.28 (311, t),
1.43-1.56 (111, in), 1.79-1.86 (311, in),
1.99 (111, brd), 2.29 (3H, s) , 2.30-2.37
(111, in), 2.83 (111, dd), 3.02 (111, brs),
1-58 530.4 3.66-3 .74 (111, in), 3.87 (3H, s) , 3.88-
3.94 (111, in), 4.16 (111, brs), 4.54 (1H,
brs), 4.66-4.74 (1H, in), 4.97 (111, d),
5.46 (111, d), 6.44 (111, brd), 6.93 (111,
d), 7.00 (1H, d), 7.22 (111, t), 7.78
(111, brd)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-112--
No. M+1
1H-1\1111R
(obs)
(CDC13) 1.13 (9H, s), 1.29 (3H, t),
1.76-1.90 (3H, in), 2.00 (1H, brd), 2.35
(111, dd), 2.83 (1H, dd) , 3.66-3.74 (111,
1-59 520.5 m), 3,87-3.94 (1H, in), 4.15 (111, s),
4.54 (1H, brs), 4.62-4.78 (111, m), 4.99
(111, d), 5.46 (111, d) , 6.92 (1H, brd)
7.33-7.46 (3H, in), 7.69 (1H, brdd),
7.77 (1H, brd)
(CDC13) 1.12 (9H, s) , 1.26-1.31 (3H,
m), 1.43-1.45 (111, in), 1.83 (311, brs)
1.99 (1H, brd), 2.06 (1H, m), 2.23 (3H,
s) , 2.34 (111, brdd) , 2.83 (1H, brdd)
1-60 577.5 3.01 (1H, brs), 3.66-3.74 (111, m),
3.87-3.95 (111, m), 4.12-4.19 (111, m),
4.53 (1H, brs) , 4.65-4.76 (1H, rah 4.98
(1H, d), 5.45-5.47 (111, in), 7.08 (1H,
brd) , 7.30 (111, m) , 7.37 (1H, brd) ,
7.73-7.75 (111, in), 7.80-7.82 (211, m)
(CDC13) 1.14 (911, s), 1.22-1.30 (6H,
m), 1.54-1.57 (111, in), 1.77-1.85 (3H,
m), 1.97 (1H, d), 2.30-2.45 (3H, m),
2.75-2.84 (1H, m), 3.00 (1H, s), 3.63-
1-61 591.5 3.72 (1H, in), 3.84-3.93 (1H, in), 4.10-
4.16 (111, in), 4.51 (111, s), 4.64-4.71
(111, in), 4.96 (111, d), 5.45 (1H, d),
7.05 (111, d), 7.26 (1H, s), 7.36 (111,
d), 7.73 (111, d), 7.80 (111, d), 7.82
(1H, s)
(CDC13) .1.15 (911, s), 1.3 (3H, t),
1.35 (611, d) , 1.4-1.55 (3H, in), 1.8-
1.95 (3H, in), 2.0-2.1 (1H, m), 2.3-2.4
(111, in), 2.65-2.75 (111, m), 2.8-2.9
1-62 605.6 (111, in), 3.05 (111, s), 3 .7-3 .8 (1H, m),
3.9-4.0 (1H, in), 4.2 (111, s), 4.55 (111,
s), 4.7-4.8 (1H, m), 5.0 (1H, d), 5.5
(111, d) , 6.6 (111, d) , 7.3-7.45 (211, m),
7.75 (1H, d), 7.85 (111, s), 8.55 (1H,
d)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-113-
No.. 111+1 311-MMR
(obs)
(CDC13) 1.13 (9H, s), 1.31 (3H, t),
1.42-1.48 (1H, m), 1.56 (1H, brs),
1.77-1.83 (3H, m), 1.99 (1H, brd), 2.35
(1H, dd), 2.83 (1E, dd), 3.01 (1H,
1-63 534.4 brs), 3.67-3.76 (111, m), 3.88-3.99 (4H,
m), 4.14 (1H, brs) , 4.52 (IH, brs),
4.65-4.73 (1H, m), 5.00 (1H, dd), 5.47
(1H, d), 7.10-7.21 (2H, m), 7.34-7.39
(1H, m), 7.56-7.61 (1H, m), 7.89 (1H,
d)
(CDC13) 1.03 (3H, d), 1.10 (3H, d),
1.32 (3H, t), 1.50 (1H, m), 1.59 (1H,
m), 1.812-1.84 (31-I, m), 2.0 (1H, m),
2.15 (1H, m), 2.36 (1H, m), 2.83 (1H,
1-64 520.5 m), 3.02 (1H, br s), 3.69 (1H, m),
3.90-3.95 (4H, m), 4.13 (111, br s),
4.40 (111, br s), 4.67 (1H, m), 4.97
(1H, m), 5.47 (1H, d), 7.12-7.21 (2H,
m), 7.28 (1H, m), 7.59 (IH, m), 7.80
(1H, m)
(DMSO) 0.91-2.40 (23H, m), 2.95-3.40
(2H, m), 3.51-3.81 (5H, m), 4.00-4.71
1-65 530-9 (3H, m), 5.29 (IH, m), 6.80 (IH, d),
7.00 (IH, d), 7.19 (1H, t), 7.94 (1H,
d), 8.48 (1H, d)
(DMS ) 0.95-1.20 (12H, m), 1.24-1.40
(2H, m), 1.41-2.40 (6H, m), 3.05 (1H,
1-66 522.8 m), 3.50-3.80 (3H, m), 4.15 (IH, m),
4.60 (IH, m), 4.70 (IH, d), 5.30 (1H,
s), 7.28-7.50 (4H, m), 8.35 (1H, d),
8.48 (1H, d)
(CDC13) 5 1.10 (9H, s), 1.26-1.33 (3H,
m), 1.43-1.45 (1H, m), 1.74-1.83 (2H,
m), 2.01 (1H, brd) , 2.06 (1H, m), 2.30
(3H, s), 2.37 (11-3, brdd), 2.85 (1H,
1_67 577.5 brdd), 2.99 (1H, brs), 3.69-3.76 (1H,
m), 3.89-3.97 (1H, m), 4.11-4.31 (2H,
m), 4.53 (IH, brs) , 4.65-4.76 (1H, m),
4.95 (1H, d), 5.45-5.47 (1H, m), 6.75
(1H, brd), 7.67-7.69 (2H, m), 7.78 (1H,
brs), 7.92 (1H, m) , 8.55 (1H, brd)
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-114-
o. M+1 111¨NISR
(obs)
(CDC13) 1.10 (9H, s), 1.26-1.33 (3H,
m), 1.43-1.45 (1H, in), 1.74-1.83 (2H,
m), 2.01 (1H, brd), 2.06 (1H, m), 2.30
(3H, s), 2.37 (1H, lordd), 2.85 (1H,
1_68 577.5 brdd), 2.99 (1H, brs) , 3.69-3.76 (IH,
m), 3.89-3.97 (1H, in), 4.11-4.31 (211,
m), 4.53 (111, brs), 4.65-4.76 (1H, m),
4.95 (111, d), 5.45-5.47 (1H, m), 6.75
(111, brd), 7.67-7.69 (2H, m), 7.78 (1H,
brs), 7.92 (111, m), 8.55 (1H, brd)
(CDC13) 1.10 (9H, s), 1.26-1.33 (6H,
m), 1.42-1.16 (111, in), 1.55-1.83 (411,
m), 2.01 (111, brd), 2.36 (111, dd), 2.53
(2H, q), 2.83 (1H, cad), 2.99 (111, brs),
1-69 591.5 3.69-3.76 (111, m), 3.89-3.96 (1H, m),
4.11 (111, s), 4.53 (IH, brs), 4.66-4.77
(1H, m), 4.95 (IH, (a), 5.48 (1H, d),
6.76 (111, d), 7.67-7.74 (2H, m), 8.80
(1H, s), 7.90 (111, (a), 8.58 (IH, d)
(CDC13) 1.12 (911, s), 1.23-1.30 (3H,
m), 1.36-1.41 (111, in), 1.73-1.84 (3H,
m), 1.98-2.03 (111, In), 2.33-2.41 (111,
m), 2.75-2.83 (IH, in), 2.96 (1H, brs),
1-70 537.4 3.65-3.73 (1H, m), 3.84-3.93 (111, m),
4.11 (1H, brs), 4.56 (111, s), 4.63-4.71
(111, m), 4.96-4.99 (111, m), 5.43-5.46
(111, m). 7.64-7.72 (2H, m), 7.79-7.87
(3H, m), 8.48-8.52 (111, m), 8.90 (111,
brd), 9.51 (111, d)
(CDC13) 1.09 (911, s), 1.32 (311, t),
1.41-1.71 (5H, m), 1.76-1.87 (3H, m),
2.00 (111, brd), 2.37 (1H, dd), 2.83
(111, dd), 2.98 (IH, brs), 3.68-3.77
1-71 535.6 (111, m), 3.89-3.97 (111, m), 4.11 (111,
s), 4.54 (111, brs), 4.67-4.74 (111, 111),
4.95 (1H, d), 5.48 (111, d), 6.64 (111,
brd), 6.78 (IH, d), 7.54 (111, dd), 7.71
(1H, brd), 7.78 (1H, d)
=
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-115-
No. 14+1 111-111v1R
(obs) _
(CDC13) 1.05 (3H, d), 1.15 (3H, d),
1.35 (3H, t), 1.5-1.6 (1H, m), 1.6-1.7
(1H, m), 1.8-1.9 (2H, s), 2.0-2.05 (1H,
m), 2.15-2.25 (1H, m), 2.35-2.45 (1H,
1-72 521.5 m), 2.8-2.9 (1H, m), 2.95 (1H, s), 3.7-
3.8 (1H, m), 3.9-4.0 (1H, m), 4.1 (1H,
s) , 4.45 (3H, s), 4.7-4.8 (1H, in), 4.9-
4.95 (1H, m), 5.55 (1H, d), 6.7 (111,
d), 6.85 (1H, d), 7.65 (1H, d), 7.75
(1H, d), 7.82 ( 1H, s)
(CDC13 ) 1.14 (9H, s), 1.25 (3H, t),
1.40-1.46 (1H, m), 1.77-1.89 (3H, m),
1.98-2.02 (1H, m), 2.34 (1H, dd), 2.99-
3.05 (1H, m), 3.62-3.69 (1H, m), 3.83-
1-73 537.4 3.91 (1H, m), 4.12 (1H, s), 4.29-4.34
(1H, ra), 4.59 (1H, s), 4.99 (1H, d),
5.38 (1H, s), 7.67-7.76 (2H, m), 7.86
(2H, dd), 8.13 (1H, d), 8.56 (1H, d),
8.96 (1H, d), 9.56 (1H, d)
Example 1I-1
(S, S, S) - (3S)- ( {1- (2S)- ( 3 -Methoxy-2-methyl-benzoylamino) -3-
methyl-butyryll-pyrrolidine- (2S) -c arbonyl )-amino)-4-oxo-
butyric acid
.-o o
* OH
0
0 N
0
[01771 Method I
(S, S. 5, R) -1- ( 2 S) -(3-methoxy-2-methyl-benzoylamino)-3-methyl-
butyry11-pyrrolidine-(2S)-carboxylic acid [ (2R) -ethoxy-5-oxo-
tetrahydro-furan- (3S)-yli-amide (97.6mg, 0.20mmol) was
dissolved in a mixture of 2M HC1 (2m1) and MeCN (2m1). The
reaction mixture was stirred at room temperature for 2.5
hours. The resulting crude mixture was diluted with Et0Ac and
washed with water. The aqueous layer was extracted twice with
Et0Ac. The combined organic extracts were washed with brine,
dried over magnesium sulfate, filtered and concentrated in
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
-116-
vacuo . The residue was co-evaporated with DCM/Petrol to
afford the title compound as a white solid (81.3mg, 88%
yield) .
[01783 Compounds of formula 11-2 to 11-61 have been
prepared by methods substantially similar to those described
in Example 11-1.
Example II-2
(S, S, S) - (3S) - ( {1- [ (2S) - (2-Chloro-benzoylamino) -3-methyl-
butyry1]-pyrrolidine- (25)-carbony1)-amino)-4-oxo-butyric acid
a 0
NT 40H
0 N
0
Example II-3
(S, S, S) - (3S) - ( (1- [3-Methyl- (25) - (2-methyl-benzoylamino ) -
butyry1]-pyrrolidine-(25)-carbonyll-amino)-4-oxo-butyric acid
0
* rcl NT 4
or 0H
0 N
0
Example II-4
(S, S, S) - (35) - ( (1- [ (2S)- ( 2 -methoxy-benzoylamino ) -3 -methyl-
butyryl ] -pyrrolidine- (28) -carbonyl} -amino) -4- oxo-butyri c acid
\o 0 4f
el# N? 4 OH
0 N
0
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
-117 -
Exampl e II-5
(5,3, S) - (3S) - ( {1- [3-Methyl- (2S) - (2 - trifl-uoromethoxy-
benzoylamino ) -butyryl] -pyrrolidine- (2S) -carbonyl} -amino) -4-
oxo-butyri c acid
F F
F)I=0 0
0
* X NT OH
0
0 NK
Example II-6
(S, S, S) - (33)- ( {1- [ (2S) - ( 3 -Hydroxy-2-methyl-benzoylamino ) -3-
methyl-butyryl ] -pyrrol i dine- (23) -carbonyl} -amino) -4-oxo-
butyric acid
HO 0
0
*O J.
OH
0 N
Example II- 7
(3, 3, S) - ( 3 S) - ({l- [ (2S) - (3 -Amino-2-methyl -benzoylamino) -3-
methyl-butyryl ] -pyrrolidine- (2S) -carbonyl} -amino) - 4- oxo-
butyric acid
H2N o
=111
OH
O
0 N
0
Example 11-8
(S,S,S)-(32)-({1-[(2S)-(2,3-Dichloro-benzoylamino)-3-methyl-
butyry1]-pyrrolidine-(2S)-carbony1}-amino)-4-oxo-butyric acid
u 0
ço
hi? KOH
0 N
0
CA 02820541 2013-06-21
WO 2005/085236
PCT/1JS2005/006540
-118-
Example 11-9
(S, S, S) - (3S) - ( {1- [ (2S) - (2-Chloro-3-trifluoromethyl-
benzoylamino) -3 -methyl -butyryl ] -pyrrolidine- (2S) - c arbonyll
amino ) -4- oxo-butyri c acid
F CI 0
fl* N
0
.(rOH
0 H
0 N
0
Example II-10
(S, S, S) - (3S) - (U.- [ ( 2 S) (3-Chloro-2-methyl-benzoylamino) -3 -
methyl -butyryl ] -pyrrolidine- (2S) -carbonyl } -amino) -4-oxo-
butyric acid
ci o 0
= NyoH
o
o
Example II-11
(S, S, S) (3S) - ( {1- [ (2S) - (2, 4-Dichloro-benzoylam no) -3-methyl-
butyryl ] -pyrrol dine- (2S) -carbonyl} -amino) -4-oxo-butyric acid
0
NIT.N?OH
CI
0 N
0
Example 11-12
(s, s, s) - (3s) ( {1- [ (2S) - (2, 5 -Dichloro-benzoylamino ) -3 -methyl-
butyryl ] -pyrrol i dine- ( 2 S) - carbonyl } -amino) -4-oxo-butyric acid
ci
r.N0 H
CI 0 N
0
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-119 -
Example 11-13
(S, S, S) (3S) - ( {1- [ (2S) - (2,6-Dichloro-benzoylamin.o) -3-methyl-
butyryl ] -pyrrolidin.e- (2S) -carbonyl}-amino ) -4-oxo -butyric acid
lit a N:).141 .40H
0 N
0
Example 11-14
(S, S, S) - (3S) - ( {1- [ (2S) - (2,6-Methyl-benzoylamino) -3-methyl-
butyryl ] -pyrrolidine- (2S) -carbonyl ) -amino) -4-oxo-butyric acid
NA)or ,Li(;)OH
0 N
Example 11-15
(S, S, S) - (3S) - [ (1- {3-Methyl- (2S) [ (2-methyl-pyridine-3-
carbonyl ) -amino] -butyryll-pyrrolidine- (2S) -carbonyl ) -amino] -4 -
oxo-butyric acid =
0 4,
OH
0
0 N
Example I1-16
(S, S, 5)- (3S) - [ (1- {3-Methyl- (25)- [ ( 4-methyl-pyridine-3-
carbonyl ) -amino] -butyryl) -pyrrolidine- (28) -carbonyl) -amino] -4-
oxo-butyric acid
61(0 )r
1161(0F1
0
0 N
0
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-120-
Example I1-17
(3,3, S) - (33) - [ (1- {3-Methyl- (2S) - [ (3 -methyl-thiophene-2 -
carbonyl) -amino] -butyryll-pyrrolidine- (2S) -carbonyl) -amino] -4-
oxo-butyric acid
6-1)&iµ)criNT
S H KOH
0
0 N
Example 11-18
(S, S, S) (3S) - [ (1- ( (2.9) - [ (2,3-Dichloro-pyridine-4-carbonyl) -
amino] -3 -methyl-butyryl )-pyrrolidin.e- (23) -carbonyl) -amino] -4-
oxo-butyric acid
a
a
0
NI1 HN)cNi
0
0 N
Example 11-19.
(S, S, S) - (3S) - [ (1-{ (2.5`) - [ (3,5-Dichloro-pyridine-4-carbonyl ) -
amino] -3 -methyl-butyryll -pyrrol id.ine- (2S) -carbonyl) -amino] -4-
oxo-butyric acid
a
01 0
0 N
Example 11-20
(S, S, S) - (3S) - ( {1- [ (2.5') - (3-Methoxy-2-methyl-benzoylamirio) -3,3-
dimethyl-butyryl ] -pyrrolidine- (2S) - carbonyl} -amino) -4- oxo-
butyric acid
_ *OH
0 N
0
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-121-
Example 11-21
(S,S,S)-4-0xo- (3S) - ( {1- [4,4,4-trifluoro- (2S)- (2-methy1-3 -
methww-benzoylamino ) -butyryl] -pyrrolidine- (2S) -carbonyl }-
amino) -butyric acid
-0 o CF
111 NOH
0 N
0
Example 11-22
(S, S, S) - (3S) - ( {1- [ (2S) - (5-Methoxy-2-methyl-benzoylamino) -3
methyl -butyryl ] -pyrrolidine- (2S) - carbonyl} -amino) -4 - oxo-
butyri c acid
=OH
0 N
" o
Example 11-23
(S, S, S) - (3S) - ({l- [ (2S) - ( 3 -Methoxy-2-methyl-ben.zoylamino) -3 -
thiazol-4-yl-propionyl ] -pyrrolidine- (2S) -carbonyl} -amino ) -4-
oxo-butyri c acid
Me0 0
0
0
0 N
0
Example 11-24
(S,S, S)- (3S)- ( {1- [ (2S) - (2-Chloro-benzoylamino) -4,4,4-
tri f luoro-butyryl ] -pyrrolidine- (2S) -carbonyl} -amino) -4-oxo-
butyric acid
CI o CF3
=
N_cor o
OH
0 N
0
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-12 2 -
Example II-25
(S, S, S) - (3S) - (Cl- (2S) - (2-Ch1oro-benzoylamino) -3 -thiazo1-4-y1¨
propionyl ] -pyrrolidine- (2S) -carbonyl) -amino) -4-oxo-butyric
acid
CI o
N 0 KOH
0 N
0
Example I1- 2 6
(S. S, S) ( 3 S ) (C1- [3, 3 -Dimethyl- (2S) - (2-methyl-benzoylamino) -
butyryl] -pyrrolidine- (2S) -carbonyl ) -amino) -4 -oxo-butyric acid
NIc"? jOH
0 N
Example II-27
(S, S, S) - (3S) ({l- [3-Methyl- (2S) - (2-trifluoromethyl-
benzoylamino) -butyryl ] -pyrrolidine- (2S) -carbonyl -amino) -4-
oxo-butyric acid
CF30
OH
0 N
0
Example 11-28
( 5, S, S) - ( 3 S) ( (1- [ ( 2S ) ( 2 -chaoro-benzoylamino ) -3, 3 -dimethyl-
butyryl ] -pyrrolidine- (2S) -carbonyl) -amino) - 4 -oxo-butyric acid
Cl o
= N? 0H
0
0 N
0
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-123 -
Example 11-29
(S, S, S) - (3S) - ( {1- [3,3-Dimethyl- (2S) - (2-trifluoromethyl-
benzoylamino) -butyryl I -pyrrolidine- (2S) -carbonyl} -amino) -4-
oxo-butyric acid
CF30
OH
0
0 N
o
Example 11-30
(S, S, S) - (3S) - ( {1- [ (2S ) - (2-Chloro-3-methoxy-benzoylamino) -3,3-
dimethyl -butyryl -p-yrrolidine- (2S) -carbonyl} -amino) -4- oxo-
butyri c acid
CI 0
Me0
[1-r-Nii.OH
0
0 Nfl
= 0
Example II-31
(S, S, S) - (3S) - ({l- [ (2S) - (2-Fluoro-3-metho3cy-benzoylamino) -3,3 -
dimethyl-butyryl -pyrrolidine- (2S) -carbonyl) -amino) -4-oxo-
butyric acid
F 0
Me0
NNAOil
0
0 Nfl
= 0
Example 11-32
(S, S, S) - (3S) - ( {1- [ (2S) - (2-Chloro-3-trifliaoromethoxy-
benzoylamino) -3,3 -dimethyl-butyryl ) -pyrrolidine- (2S) -
carbonyl} -amino) -4 -oxo-butyri c acid
0 CI
F3C' 410
N N
H CJH
0 AI-1
o N
0
CA 02820541 2013-06-21
=
W02005/085236
PCT/US2005/006540
-124-
Example 11-33
($,S,S)-(3S)-((1-[(2S)-(2-Chloro-3-cyclopropyloxy-
benzoylamino)-3,3-dimethyl-butyry1]-pyrrolidine-(2S)-
carbony1}-amino)-4-oxo-butyric acid
CI 0
VD.H H
0 A
0 N
0
Example 11-34
(S,S,S)-(3S)-({1-[(2S)-(2-Chloro-3-methyl -benzoylamino)-3,3-
dimethyl-butyry1]-pyrrolidine-(2S)-carbony1}-amino)-4-oxo-
butyric acid
CI 0.4,
* =
OH
0 N
= 0
Example 11-35
(S,S,S)-(3S)-({1-[(2S)-(2-chloro-3-methoxy -benzoylamino)-3-
methyl-butyry11-pyrrolidine-(2S)-carbonyll-amino)-4-oxo-
butyric acid
1 0: 4" 0
OH
0
0 N
H 0
Example 11-36
(S,S,S)-(3S)-({1-[(2S)-(2-Chloro-3-ethyl -benzoylamino)-3,3-
dimethyl-butyry1]-pyrrolidine-(2S)-carbony1)7amino)-4-oxo-
butyric acid
CI 0
* [siNNIT kr
OH
0 N
= 0
CA 02820541 2013-06-21
=
WO 2005/085236
PCTATS2005/006540
-125--
Example 11-37
(S, S, S) ( 3 S) - ( {1- [ (2S) - ( 2 -chloro-4-methoxy -benz oylamino) -3-
methyl-butyryll-pyrrolidine- (2S) -carbonyl} -amino ) -4-oxo-
butyric acid
C'0
0
'0* NI? drOH
0 NI
JH
Example 11-38
(S, S, S) - ( 3 3) (C1- [ (2S) - ( 2 -Chl oro- 3 -cyclopropylmathoxy -
benzoylamino ) -3, 3 -dimethyl -butyryl -pyrrolidine- (2S) -
carbonyl} -amino) -4 - oxo -butyric acid
CI 0
0 0
* I OH
0 N
= 0
Example 11-39
(S, S, S) - (3S) - ( (1- [ (2S ) - (2-Chloro-3-hydroxy -ben.oylamino) -3,3 -
dimethyl-butyryl] -pyrrolidine- (25) -carbonyl} -amino) -4- oxo-
butyri c acid
CI 0
HO 0
OH
0 N
= 0
Example 11-40
(S, S,S)- (3S) - ( {I- [ (2S) - (2-chloro-4-acetamido -bnzoylamino) -3-
methyl-butyryl -pyrro 1 i dine- ( 2S) -carbonyl ) -aminc)) -4-oxo-
butyric acid
CI 0
0
HN 400 11-110.1\11i.,OH
0 N
= 0
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/11S2005/006540
-126-
,
Example II-41
(S, S, S) - (35) - ( {1- [ (2S) - (2-Chloro-3-acetamido -benzoylamino)
3,3 -dimethyl-butyryl ] -pyrrolidin.e- (2S) -carbonyl) -amino) -4-oxo-
butyric acid
HN CI 0
0
OH
0
0 N
0
Example 11-42
(S, S, S) - (3S) - ( {1- [ (2S) - (2-methyl -3 -acetamido -benzoylamino) -
3,3 -dimethyl -butyryl ] -pyrrolidine- (2S) - carbonyl} -amino ) -4-oxo-
butyric acid
(21
HN 0
0
VNriii
0 N
H 0
Example 11-43
(S, S, S)- (3S) - ( {1- [ (2S) - (2-chloro -4-acetamido -benzoylamino)
3,3 -dimethyl -butyryl ] -pyrrolidine- (2S) -carbonyl) -amino) -4 - oxo-
butyric acid
CI 0
0
HN 11*
0
ONff
0
Example 11-44
S, S, S) - (3S) - ( {1- [ (2S) - (2- fluor -4-acetamido -benzoylamino) -
3,3 -dimethyl -butyryl -pyrrolidine- (2S) -carbonyl}--amino) -4-oxo-
butyric acid
=
CA 02820541 2013-06-21
WO 2005/085236 PCTTUS2005/006540
-127-
F 0
H N HN
0 H
0 0 N
= 0
Example II-45
(S, S, S) (3S) - ( {1- [ (2S) - (2-fluoro -4-acetamido -ben.zoylamino)
3 -methyl-butyryl ] -pyrrolidine- (2S) -carbonyl} ¨amino) -4- oxo-
butyric acid
F0
0
HN IXN?
0
0 0 N
= 0
Example 11-46
(S,S,S)- (3S) - ( {1- [ (2S) - (2-chloro -4-isopropyloxy -
benzoylamino ) -3 -methyl-butyryl ] -pyrrolidine- (2S) - carbony11-
amino ) -4-oxo-butyric acid
CI \Ir.. .
0
. =
* LrOH =
0
0 N
0
Example 11-47
(S, S, S) - (3S) ( {1- [ (2S) - (2-chloro -4-hydroxy -benzoylamino) -
3,3-dimethyl-butyryl] -pyrrolidine- (2S) -carbonyl} -amino) -4-oxo-
butyric acid
CI
0
HO* N.N?
0
0 N
= 0
Example 11-48
(S, S, S) - (3S) - ( {1- [ (2S) - (2- chloro -4-methoxymethyl -
benzoylamino ) -3, 3 -dimethyl-butyryl] -pyrrolid.ine- (2S) -
carbonyl} -amino) -4- oxo -butyric acid
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
= -128-
CI
41*OHH
0
0 N
0
Example 11-49
(S,S,S)-(3S)-({1-[(2S)-(2-chloro -4-isobutyrylamido -
benzoylamino)-3,3-dimethyl-butyryll-pyrrolidine-(2S)-
carbonyll-amino)-4-oxo-butyric acid
Cl 0
0
HN 410 11\-11,..0H
00 N
0 H
Example 11-50
(S,S,S)-(3S)-({1-[(2S)-(2-chloro -4-acetamido -benzoylamino)-
3-cyclohexyll-pyrrolidine-(2S)-carbonyll-amino)-4-oxo-butyric
acid
CI 0
0
HN
0 OH
0
0 N
0
Example 11-51
(S,S,S)-(3S)-({1-[(2S)-(2-chloro -4-methoxycarbonylamino -
benzoylamino)-3,3-dimethyl-butyry1]-pyrrolidine-(2S)-
carbony1}-amino)-4-oxo-butyric acid
Cl 0 4,
HN410 OrifoH
0
00 ON(
1 0
Example 11-52
(S,S,S)-(3S)-({1-[(2S)-(2-chloro -3-phenoxy -benzoylamino)-
3,3-dimethyl-butyryll-pyrrolidine-(2S)-carbony1)-amino)-4-oxo-
butyric acid
CA 02820541 2013-06-21
WO 2005/085236
PCT/11S2005/006540
-129-
o
CI 0
0 jH
0 N
0
Example 11-53
(S,S,S)-(3S)-((1-[(2S)-(2-chloro -6-amino -benzoylamino)-3-
methyl-butyry1]-pyrrolidine-(2S)-carbony1}-amino)-4-oxo-
butyric acid
CI 0
0
OH
NH2
0 N
0
Example 11-54
(S,S,S)-(3S)-({1-[(2S)-(2-chloro-benzoylamino)-3,3-dimethyl-
butyryli-piperidine-(2S)-carbonyl)-amino)-4-oxo-butyric acid
C'0
OH
0
0 N
Example 1-55
(3S)-(f2-[(2S)-(3-Methoxy-2-methyl-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carbonyll-
amino)-4-oxo-butyric acid
cH3 0
iojCirl 40H
0 0 N
0
Example 11-56
(3S)-((2-[(2S)-(2-Chloro-benzoylamino)-3,3-dimethyl-butyry1]-
2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carbonyl}-amino)-4-
oxo-butyric acid
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
-130-
a 0)Cirl..,
OH
0 0 m
"
Example 11-57
(3S)-({2-[(2S)-(4-Acetylamino-2-chloro-benzoylamino)-3,3-
dimethyl-butyry1)-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-
carbonyl}-amino)-4-oxo-butyric acid
o
%Criµ7 4 F4H
0 H
0
Example 11-58
(3S)-({2-[(2S)-(2-Chloro-4-propionylamino-benzoylamino)-3,3-
dimethyl-butyry11-2-(18,4R)-aza-bicyclo[2.2.1]heptane-(38)-
carbonyl}-amino)-4-oxo-butyric acid
a o
,,it 100 l....OH
0 0 N
" 0
Example 11-59
(3S)-({2-[(2S)-(2-Chloro-3-isobutyrylamino-benzoylamino)-3,3-
dimethyl-butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-
carbonyl)-amino)-4-oxo-butyric acid
H CI 0 jcril
1/0 OH
0 0
" o
Example 11-60
(3S)-((2-[(2S)-(2-Fluoro-3-methoxy-benzoylamino)-313-dimethyl-
butyry11-2-(13,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carbony1)-
amino)-4-oxo-butyric acid
CA 02820541 2013-06-21
=
WO 2005/085236 PCT/US2005/006540
-131-
0 0 N. j(OH
Example 11-61
(3S)-({2-[(2S)-(2-Fluoro-3-methoxy-benzoylamino)-3-methyl-
butyrya]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carbony1)-
amino)-4-oxo-butyric acid
F 0 0
X.Tr47 õ(OH
H 0 0 H
Example 11-62
(3S)-([2-[(2S)-(4-Acetylamino-3-chloro-benzoylamino)-3,3-
dimethyl-butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-
carbony1}-amino)-4-oxo-butyric acid
0 0
XI0HH
0 0 N
" o
a
Example 11-63
(3S)-((2-[(2S)-(3-Chlor0-4-propionylamino-benzoylamino)-3,3-
dimethyl-butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-
carbony1}-amino)-4-oxo-butyric acid
0
jt. X17 40H
0 0 N
" o
a
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-132-
Example 11-64
(3S)-((2-[(2S)-(Isoquinolin-l-ylcarbonylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carbonyll-
amino)-4-oxo-butyric acid
110 N
0
OH
22N
H
Example 11-65
(3S)-(f2-[(2S)-(4-Amino-3-chloro-benzoylamino)-3,3-dimethyl-
butyry1]-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carbony1}-
amino)-4-oxo-butyric acid
OH
H2N 0 0 14
Example 11-66
(3S)-({2-[(2S)-(4-Amino-3-chloro-benzoylamino)-3-methyl-
butyry1)-2-(1S,4R)-aza-bicyclo[2.2.1]heptane-(3S)-carbony1}-
amino)-4-oxo-butyric acid
H2N o 0 N
a N o
[01791 The characterization data for compounds 11-1 to
11-66 is summarized in Table 4 below and includes HPLC, LC/MS
(observed) and IH NMR data. IH NMR data was obtained at 400
MHz, and was found to be consistent with structure.
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
=
-133-
Table 4. Characterization Data for Selected Compounds of
Formula II (According to Compound Number)
114.1
No. 1H-NMR
(obs)
(DMSO-d6) 0.82-0.98 (611, m), 1.89-2.07
(511, m), 2.10 (3H, s), 3.0 (1H, m),
3.63 (111, m), 3.79 (3H, s), 3.88 (1H,
II-1 462.1 m), 4.00 (1H, m), 4.25 (111, m), 4.40-
4.44 (211, m), 5.45 (1H, br s), 6.83
(1H, d), 7.00 (1H, d), 7.19 (111, t),
7.77 (1H, br s), 8.32-8.50 (211, m)
(DMSO-d6) 0.95-0.99 (6H, m), 1.87-2.09
(5H, m), 3.00 (111, m), 3.64 (111, m),
11-2 452.0 3.85 (111, m), 4.04 (1H, m), 4.25 (111,
m), 4.40 (1H, m), 4.47 (1H, m), 5.45
(1H, m), 7.34-7.49 (4H, m), 7.78 (1H,
m), 8.40 (1H, m), 8.64 (111, m)
(DMSO-d6) 0.94-0.99 (GH, m), 1.87-2.09
(5H, m), 2.30 (3H, s), 2.90 (1H, m),
11-3 432.1 3.64 (1H, m), 3.88 (111, m), 4.03 (1H,
m), 4.30 J111, m), 4.44 (1H, m), 5.45
(111, m), 7.19-7.33 (411, m), 7.77 (1H,
br s), 8.35-8.40 (2H, m)
(CD30D) 1.05-1.18 (611, m), 2.00-2.30
(5H, m), 2.52-2.75 (2H, m), 3.66-3.83
(1H, m), 3.92-4.03 (111, m), 4.26-4.35
11-4 448.0 (1H, m), 4.45-4.55 (111, m), 4.61-4.70
(111, m), 4.78-4.85 (1H, m), 7.13 (1H,
t), 7.21 (111, d), 7.57 (111, t), 8.00
(1H, d), 8.69 (1H, d)
(CD30D) 0.98-1.15 (611, m), 1.95-2.26
(5H, m), 2.54-2.76 (211, m), 3.73-3.84
11-5 502.0 (1H, m), 3.99-4.06 (111, m), 4.21-4.32
(1H, m), 4.45-4.53 (111, m), 4.60-4.70
(2H, m), 7.30-4.47 (2H, m), 7.54-7.64
(2H, m)
(DMSO-d6) 0.92-0.98 (611, m), 1.85-2.04
(5H, m), 2.07 (3H, s), 3.00 (1H, m),
3.63 (111, m), 3.87 (111, m), 4.03 (111,
11-6 448.1
ml 4.25 (111, m), 4.41 (111, m), 5.45
(1H, m), 6.68 (111, m), 6.82 (111, m),
7.01 (1H, m), 7.81 (111, m), 8.25 (111,
d), 8.40 (1H, m), 9.5 (1H, m)
CA 02820541 2013-06-21
=
WO 2005/085236
PCT/US2005/006540
-134-
M+1
No. 111-NMR.
(obs)
(CD30D) 1.02-1.18 (6H, m), 1.88-2.28
(5H, m), 2.39 (3H, s), 2.50-2.78 (2H,
11-7 447.0 m), 3.75-3.83 (1H, m), 4.00-4.10 (1H,
m), 4.21-4.32 (1H, m), 4.45-4.52 (111,
m), 4.60-4.65 (2H, m), 7.39-7.54 (3H,
m)
(DMSO-d6) 0.94-0.99 (6H, m), 1.71-2.12
(4H, m), 2.33 (1H, br s), 2.67 (1H, br
s), 2.94-3.07 (IH, m), 3.61-3.69 (1H,
11-8 446.0 m), 3.82-3.87 (111, m), 4.03-4.10 (IH,
m), 4.19-4.28 (1H, m), 4.30-4.43 (2H,
m), 5.42-5.47 (1H, m), 7.28-7.30 (1H,
m), 7.37-7.40 (1H, m), 7.68-7.82 (2H,
m), 8.77 (1H, d)
(DMSO-d6) 0.94-0.99 (6H, m), 1.86-2.09
(5H, m), 3.00 (1H, m), 3.65 (Iii, m),
3.84 (1H, m), 4.05 (1H, m), 4.24 (IH,
11-9 519.9 m), 4.40 (IH, m), 4.51 (111, m), 5.45
(1H, m), 7.57-7.62 (2H, m), 7.77 (1H,
d), 7.90 (1H, m), 8.40 (1H, d), 8.87
(1H, d)
(DMSO-d6) 0.93-0.99 (6H, 2 x d), 1.77-
2.19 (5H, m), 2.29 (3H, s), 2.97 (IH,
br s), 3.62-3.65 (1H, m), 3.85-3.88
II-10 466.0 (IH, m), 4.00-4.32 (2H, br m), 4.41-
4.53 (2H, m), 5.45 (1h, br s), 7.18-
7.27 (2H, m), 7.45-7.50 (1H, m), 7,85
(1h, br d), 8.41 (1H, br d), 8.57 (1H,
d)
(DMSO-d5) 0.82-0.86 (3H, m), 0.93-0.98
(3H, m), 1.87-2.08 (5H, m), 3.00 (1H,
m), 3.64 (111, m), 3.82 (1H, m), 4.10
II-11 485.9 (1H, m), 4.30 (1H, m), 4.45 (1H, m),
4.47 (1H, m), 5.44 (1H, d), 7.37 (IH,
m), 7.47 (1H, m), 7.65 (1H, m), 7.77
(1H, m), 8.40 (1H, m), 8.72 (IF!, m)
(DMSO-d6) 0.94-0.99 (61!, m), 1.91-2.09
(51!, m), 3.00 (1H, m), 3.64 (1H, m),
3.83 (11!, m), 4.03 (11!, m), 4.20 (11!,
11-12 485.9 m), 4.40 (11!, m), 4.47 (IH, m), 5.45
(11!, m), 7.37 (1H, s), 7.50-7.52 (21!,
m), 7.78 (1H, m), 8.44 (11!, m), 8.79
(1H, m)
CA 02820541 2013-06-21
. -
=
WO 2005/085236 PCT/US2005/006540
-135-
14+1
No. 111-10R
(obs)
(DMSO-d0 0.82-0.86 (3H, m), 0.92-0.99
(3H, m), 1.80-1.87 (2H, m), 1.99-2.02
(4H, m), 2.48 (0.5 H, m), 2.95 (0.5 H,
11-13 486.3 m), 3.51 (1H, m), 3.80-4.56 (4H, m),
5.00 and 5.47 (1H, 2 x m), 7.37-7.48
(3H, m), 7.76-8.32 (1H, m), 8.95-9.39
(1H, 3 x dd)
(DMSO-d0 0.93-0.99 (6H, m), 1.80-2.09
(5H, m), 2.17 (6H, d), 2.95 (1H, br s),
3.63-3.65 (1H, m), 3.96-3.99 (1H, m),
11-14 446.0 4.10 (1H, br s), 4.30 (1H, br s), 4.44
(1H, t), 5.48 (1H, br s), 7.00 (2H, d),
7.14 (1H, t), 7.78 (IH, br s), 8.50
(1H, br s), 8.55 (1H, d)
(DMSO-d0 0.91-1.02 (6H, m), 1.80-2.20
(5H, m), 2.66-2.68 (3H, s), 3.00 (1H,
m), 3.62-3.85 (3H, m), 4.10 (1H, m),
11-15 433.1 4.24 (1H, m), 4.51 (1H, m), 5.72 (IH,
m), 7.73-7.76 (2H, m), 8.19 (IH, m),
8.52 (1H, m), 8.75 (1H, d), 8.90 (1H,
m)
(DMSO-d0 0.9-1.05 (6H, m), 1.8-2.2
(6H, m), 2.3-2.4 (111, m), 2.7-2.75 (1H,
m), 2.9-3.0 (1H, m), 3.65-3.75 (1H, m), '
11-16 433.1 3.8_3.9 (111,
) 4.1-4.15 (1H, m), 4.3-
4.4 (1H, m), 4.45-4.65 (111, m), 7.8-7.9
(1H, m), 8.7-8.8 (2H, d), 8.9.8.95 (1H,
(DmS0-d6) 0.83-0.99 (6H, m), 1.80-2.20
(5H, m), 2.40 (3H, s), 3.00 (1H, m),
3.61 (1H, m), 3.81 (IH, m), 4.10 (1H,
11-17 438.0 m), 4.25 (1H, m), 4.42-4.46 (2H, m),
5,44 (1H, br s), 6.97 (1H, m), ,7.34
(1H, m), 7.59 (1H, m), 7.81 (1H, m),
8.49 (1H, m)
(DMSO-d6) 0.92-1.00 (6H, m), 1.75-2.08
(5H, m), 2.30-2.34 (1H, m), 2.99 (IH,
dd), 3.62-3.67 (1H, m), 3.78-3.82 (1H,
11-18 487.0 m), 3.78-3.82 (1H, m), 4.05-4.26 (1H,
m), 4.38-4.54 (2H, m), 5.44-5.72 (1H,
m), 7.37-7.41 (1H, m), 8.41-8.43 (2H,
m), 8.97-9.00 (1H, d)
CA 02820541 2013-06-21
_ .
=
WO 2005/085236
PCT/1JS2005/006540
-136-
No. 111-4ata
(obs)
(DMSO-dd 0.94-1.00 (611, m), 1.77-2.15
(5H, m), 3.02 (111, dd), 3.61-3.70 (111,
11-19 487.0 m), 3.80-3.90 (111, m), 4.03-4.08 (111,
m), 4.52-4.56 (1H, m), 4.95 (2H, br s),
5.45 (111, s), 8.42 (111, d), 8.67 (211,
s), 9.17 (111, d)
(DMSO-dd 0.91-1.11 (9H, m), 1.70-2.14
(7H, m), 2.31 (111, m), 3.01 (1H, m),
11-20 476.4 3.50-3.97 (5H, m), 4.00-4.62 (311, m),
,5.50 (111, m), 6.77 (111, d), 7.00 (111,
d), 7.18 (1H, dd), 7.50-8.50 (311, m)
(DMSO-dd 1.80-2.00 (311, m), 2.11 (411,
overlapping s and m), 2.60-2.80 (2H,
m), 3.64-3.69 (1H, m), 3.80 (3H, s),
11-21 502.1 4.10 (1H, vbrs), 4.30 (1H, vbrs), 5.00
(111, m), 6.86 (1H, d), 7.03 (1H, d),
7.22 (111, t), 8.45 (1H, vbrs), 8.81
(1H, d)
(DMSO-dd 0.93-1.00 (611, m), 1.70-2.15
(511, m), 2.22 (311, s), 2.33 (111, d),
2.99 (111, dd), 3.60-3.65 (211, m), 3.74
11-22 462.4 (3H, s), 4.04-4.08 (1H, m), 4.21-4.27
(1H, m), 4.40-4.58 (2H, m), 5.46 (1H, ,
brd d), 6.78-6.81 (111, m), 6.85-6.91
(1H, m), 7.09-7.14 (111, m), 8.37 (211, 2
x brd d)
(DMSO-dd 1.77-2.19 (511, m), 2.95-3.28
(311, m), 3.60 (1H, brd d), 3.71-3.78
(411, m), 4.10-4.42 (6H, m), 4.97 (1H,
11-23 517.0 brd s), 5.45-72 (1H, m), 6.74 (111, d),
6.97 (111, d), 7.10-7.22 (111, m), 7.44
(111, m), 8.37-8.68 (2H, m), 9.05 (111,
brd s)
(DMSO-dd 1.75-1.98 (311, m), 2.08-2.13
(111, m), 2.64-2.77 (2H, m), 2.99 (0.511,
dd), 3.63-3.73 (2H, m), 4.08 (0.511,
11-24 492.0 brt), 4.20 (0.5H, dd), 4.23-4.49 (3
multiplets, 1H total), 5.00-5.10 (1H,
m), 5.42 (0.5H, s), 7.36-7.52 (411, m),
7.77 (111, m), 8.30 (0.511, d), 9.09 (1H,
d)
=
CA 02820541 2013-06-21
=
WO 2005/085236
PCMJS2005/006540
-137-
M+1
No. 11-1-4vima
(ohs)
(DMSO-d6) 1.79-1.96 (5H, m), 2.94-3.28 -
(3H, m), 3.58 (1H, brd d), 3.73 (1H,
11-25 507.0 brd d), 4.04-4.59 (2H, m), 4.98-5.02 -
(1h, m), 5.54-5.74 (2H, m), 7.26-7.46
(511, m), 8.43 (1H, d), 8.82 (1H, d),
9.39 (1H, brd s)
(DMSO-d6) 1.05 (9H, s), 1.15 (3H, t),
1.8-2.1 (4H, m), 2.3 (3H, s), 2.4-2.5
(1H, m), 2.9-3.0 (1H, m), 3.7-3.75 (1H,
11-26 446.6 m), 3.8-3.85 (1H, m), 4.1-4.15 (0.5H,
m), 4.25-4.3 (1H, m), 4.4-4.5 (0.5H,
m), 4.7-4.75 (1H, m), 5.55-5.6 (1H, m),
7.2-7.4 (4H, m), 7.7-7.75 (1H, m), 8.1-
8,15 (1H, m), 8.35-8.4 (1H, m)
(DMSO-d0 0.95-1.05 (6H, m), 1.8-2.1
(4H, m), 2.4-2.5 (1H, m), 3.0-3.1 (1H,
m), 3.7-3.75 (1H, m), 3.8-3.85 (1H, m),
11-27 486.5 4.1-4.15 (0.5H, m), 4.25-4.3 (1H, m),
4.4-4.5 (0.5H, m), 5.55-5.6 (1H, m),
7.4-7.45 (1H, m), 7.6-7.8 (3H, m), 8.4-
8,45 (1H, m), 8.75-8.8 (1H, m)
(CDC13) 1.11-1.16 (9H, m), 1.94-2.22
(4H, m), 2.38-2.50 (2H, m), 2.77-2.87
11-28 466.1 (1H, m), 3.71-3.79 (1H, m), 3.96-4.06
(1H, m), 4.56-4.67 (2H, m), 4.85-4.91
(1H, m), 6.99-7.02 (1H, .m), 7.28-7.45
(3H, m), 7.60-7.84 (2H, m)
(CDC13) 1.07 (9H, s), 1.85-2.19 (2H,
m), 2.37-2.40 (2H, m), 2.81-3.07 (1H,
11-29 500.2 m), 3.37 (1H, brs), 4.01 (1H, brs),
4.46-4.67 (2H, m), 4.87 (1H, d), 5.73
(1H, brs), 6.68 (1H, brs), 7.38-7.74
(5H, m)
(CD30D) 1.15 (9H, s), 1.85-2.20 (4H,
m), 2.46-2.72 (2H, m), 3.74-3.81 (1H,
11-30 496.2 m), 3.92 (3H, s), 3.93-4.03 (1H, m),
4.20-4.31 (1H, m), 4.45-4.52 (1H, m),
4.60-4.75 (1H, m), 4.83 (1H, s), 7.00
(1H, d), 7.15 (1H, d), 7.33 (1H, t)
(DMSO-d5) 1.05 (9H, s), 1.8-2.1 (4H,
m), 2.4-2.5 (1H, m), 3.75-3.8 (1H, m),
11-31 480.5 3.8-3.85 (1H, m), 3.9 (3H, s), 4.1-4.3
(1H, m), 4.7 (1H, d), 5.3-5.5 (0.5H, hr
s), 7.1-7.3 (3H, m), 7.7-7.8 (1H, m),
8.0-8.1 (1H, m), 8.35-8.45 (1H, m)
CA 02820541 2013-06-21
=
WO 2005/085236 PCTMS2005/006540
-138-
M4-1
No. 111-NNR
(obs)
(DMSO-d0 0.91-1.10 (9H, m), 1.70-2.15
(5H, m), 2.60-3.08 (1H, m), 3.60-3.90
11-32 550.3 (2H, m), 3.98-4.71 (3H, m), 5.40-5.80
(1H, m), 7.30-7.91 (3H, m), 8.30-8.80
(3H, m)
(DMSO) 0.60-0.90 (4H, in, cyclopropyl
CH2), 0.92-1.10 (9H, in, tBu), 1.71-2.21
(5H, in, CH2), 2.65-3.10 (1H, brm, CH2),
11-33 523.3 3.36-3.50 (1H, in, CH), 3.60-4.75 (6H,
in, CH), 6.92 (1H, d, aryl H), 7.36 (1H,
m, aryl H), 7.45 (1H, m, aryl H), 7.65-
8,60 (3H, in, NH, OH)
(DMSO) 0.99-1.10 (9H, in, tBu), 1.70-
2,12 (5H, in, CH2), 2.35 (3H, s, CH3),
2.60-3.08 (1H, in, CH2), 3.58-3.87 (2H,
11-34 480-3 in, CH), 4.00-4.70 (3H, in, CH), 5.38-
5,79 (111, in, CH), 7.12 (1H, d, aryl H),
7.24 (1H, in, aryl H), 7.38 (1H, m, aryl
H), 7.69-8.55 (3H, in, NH, OH)
CD3OD 1.01-1.15 (6H, m), 1.95-2.22
(5H, m), 2.48-2.69 (2H, m), 3.73-3.80
11-35 482 (1H, m), 4.92 (3H, s), 3.99-4.19 (1H,
m), 4.20-4.30 (1H, m), 4.58-4.67 (2H,
m), 7.00 (1H, d), 7.14 (1H, d), 7.31
(1H, t)
(DMSO) 0.94-1.08 (9H,s, tBu), 1.19 (3H,
t, CH3), 1.70-2.40 (5H, in, CH2), 2.60-
3,08 (3H, in, CH2), 3.69 (1H, in, CH),
11-36 494.4 3.81 (1H, In, CH), 4.04-4.71 (3H, in,
CH), 5.40-5.80 (1H, in, CH), 7.14 (1H,
in, aryl H), 7.31 (IH, in, aryl H), 7.39
(1H, m, aryl H), 7.70-8.50 (3H, in, NH,
OH)
(DMSO) 0.9-1.0 (6H, m), 1.85-2.3 (4H,
m), 3.0-3.1 (1H, m), 3.65-3.7 (1H, m),
3.78 (3H, s), 3.8-3.85 (1H, m), 4.1-
11-37 482.5 4.15 (0.5H, m), 4.25-4.3 (0.5H, m),
4.5-4.55 (1H, m), 5.5-5.55 (1H, m),
6.93 (1H, d), 6.98 (1H, s), 7.35 (111,
d), 7.75-7.8 (1H, m), 8.45 (1H, d)
CA 02820541 2013-06-21
WO 2005/085236 PCT/US2005/006540
-139-
M+1
No.
(obs)
(CD3OD ) 0.34-0.40 (2H, m), 0.60-0.67
(2H, m), 1.16 (911, s), 1.25-1.32 (1H,
m), 1.93-2.22 (411, m), 2.50-2.66 (211,
21-38 536 m), 3.74-3.84 (1H, m), 3.91-4.03 (3H,
m), 4.22-4.32 (1H, m), 4.45-4.54 (IH,
m), 4.61-4,69 (111, m), 4.82 (111, d),
6.99 (111, d), 7.12 (111, d), 7.32 (111,
t), 8.40 (1H, d)
(CD3OD ) 1.12 (9H, s), 1.90-2.22 (411,
in), 2.512.70 (211, in), 3.75-3.83 (1H,
1I-39 482 =m), 3.97-4.05 (1H, m), 4.23-4.30 (111,
m), 4.46-4.54 (1H, m), 4.63-4.70 (111,
m), 4.83 (111, d), 6.91 (111, d), 6.99
(111, d), 7.17 (1H,t), 8.36 (1H, d)
(DMSO) 0.93-0.98 (611, m) 1.71-2.09
(1011, m), 2.35-2.45 (211, m), 3.61-3.64
(1H, m), 4.02-4.04 (1H, m), 4.06-4.35 "
11-40 509.3 (211, m), 4.43-4.46 (111, m), 7.33 (111,
d), 7.43-7.46 (1H, m), 7.80 (111, brd
s), 8.28-8.49 (2H, m), 10.25 (111, brd
s)
(DMSO) 0.95-1.08 (911, s, tBu), 1.70-
2,38 (811, in, COCH3, CH2), 2.58-3.08 =
=
(1H, in, CH2), 3.65 (1H, in, CH), 3.82
11-41 523.3 (111, In, CHO), 3.95-4.69 (3H, in, CH),
5.40-5.60 (111, in, CH), 7.09 (1H, in,
aryl H), 7.31 (1H, in, aryl H), 7.64-
8,60 (411, in, aryl H, NH), 9.55 (1H, in,
CH)
(DMSO) 0.91-1.08 (911, s, tBu), 1.70-
2,40 (11H, in, CH3, COCH3, CH2), 2.60-
3,08 (111, m, C112), 3.66 (111, in, CH),
11-42 503.4 3.87 (111, in, CH), 4.00-4.65 (311, in,
CH), 5.40-5.78 (1H, in, CH), 7.04 (111,
in, aryl H), 7.18 (IH, in, aryl H), 7.38
(1H, in, aryl H)--, 7.65-7.88 (111, m, NH),
8.07-8.70 (211, in, NH), 9.34 (111, in, CH)
(DMSO) 1.03 (9H, s), 1.71-2.00 (3H, m),
2.07 (311, s), 2.55-2.73 (111, m), 2.97
(111, dd), 3.60-3.67 (111, m), 3.75-3.82
(1H, m), 3.98-4.04 (1H, m), 4.19-4.24
21-43 523.3 (1H, m), 4.37-4.45 (111, m), 4.63 (111,
d), 5.45 (111, d), 7.33-7.35 (111, m),
7.43-7.45 (111, d), 7.76-7.83 (211, m),
8.25-8.28 (111, m), 8.41-8.58 (1H, m),
10.27 (1H, s)
CA 02820541 2013-06-21
W02005/085236
PCT/US2005/006540
-140-
1441.
No. 1H-NMR
(obs)
(DMSO) 1.01 (9H, 2 x s), 1.72-1.99 (4H,
m), 2.05-2.09 (4H, m), 2.35-2.57 (2H,
m), 2.71-3.00 (1H, brd m), 3.60-3.65
11-44 507.4 (111, m), 3.71-3.80 (1H, m), 4.08-4.37
(211, brd m), 4.70 (111, d), 7.32 (111,
dd), 7.65-7.80 (311, m), 8.33-8.52 (IH,
brd m), 10.37 (111, s)
(DMSO) 0.94 (6H, dd), 1.72-1.99 (10H,
m), 2.36-2.52 (2H, m), 3.57-3.68 (111,
11-45 493.4 m), 3.76-3.88 (111, m), 4.20-4.43 (2H,
m), 4.51-4.55 (111, m), 7.30 (IH, dd),
7.58-7.77 (311, m), 8.00-8.04 (111, m),
10.34 (111, s)
(DMSO) 0.95-1.0 (6H, m), 1.25 (6H, d),
1.85-2.2 (4H, m), 3.0-3.1 (IH, m), 3.9-
11-46 510.5 4.0 (3H, m), 4.2-4.3 (0.511, m), 4.4-4.5
(0.5H, m), 4.7-4.8 (1H, m), 6.9-6.95
(111, d), 6.99 (111, s), 7.3 (1H, d),
8.3-8.4 (1H, m)
(DMSO) 1.05 (9H, m), 1.8-2.1 (411, m),
2.6-2.7 (111, m), 2.9-3.0 (2H, m), 3.6-
3.7 (211, m), 3.8-3.9 (111, m), 4.0-4.1
11-47 482.5 (111, m), 4.2-4.3 (111, m), 4.6-4.65 (1H,
m), 5.5-5.55 (111, m), 6.75-6.85 (2H,
m), 7.35 (1H, d), 7.75 (111, d), 8.0-8.1
(1H, m), 8.35 (1H, m), 10.25 (111, s)
(DMSO) 1.03 (9H, s), 1.80-2.10 (411, m),
3.00 (1H, br s), 3.30 (311, s), 3.66
11-48 510.5 (111, m), 3.81 (111, m), 4.06 (1H, m),
4.25 (1H, m), 4.44 (211, s), 4.65 (1H,
d), 5.46 111, br s), 7.29-7.39 (311, m),
7.77 (IH, br s), 8.43 (111, m)
(DMSO) 1.03 (911, s), 1.09 (3H, m), 1.11
(311, m), 1.79-2.15 (411, m), 2.32 (1H,
m), 2.98 (1H, m), 3.51 (1H, m), 3.79
11-49 551.5 (1H, m), 4.10 (1H, m), 4.23 (111, m),
4.40-4.65 (2H, m), 5.45-5.73 (111, m),
7.35 (111, m), 7.49 (1H, m), 7.76-7.84
(211, m), 8.23-8.60 (2H, m), 10.11 (111,
s)
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-141-
M+1
No. 1H-NMR
(obs)
(DMSO) 0.92-1.19 (4H, m), 1.49-1.90
(911, m), 1.91-1.99 (211, m), 2.06 (411,
brd s), 2.49-2.52 (2H, m), 3.57-3.68'
11-50 493.3 (111, m), 3.80-3.90 (111, m), 4.01-4.28
(2H, m), 4.46 (111, t), 7.32 (1H, d),
7.43 (1H, dd), 7.81 (211, brd s), 8.31-
8.78 (111, m), 8.46 (1H, d), 10.22 (1H,
s)
(DMSO) 0.90-1.07 (9H, s, tBu), 1.70-
2.40 (411, brm, CH2), 2.54-3.07 (1H, in,
CH2), 3.52-3.88 (511, in, CH3, CH), 4.00-
=-51 539.3 4.65 (311, in, CH), 5.40-5.80 (111, in,
CH), 7.30-7.44 (211, m, aryl H), 7.60
(1H, in, aryl H), 7.67 (111, br, NH),
8.10-8.70 (2H, m, NH), 10.00 (111, in,
CH)
(DMSO) 0.91-1.11 (911, s, tBu), 1.70-
2.41 (4H, in, CH2), 2.56-3.09 (111, in,
CH2), 3.60-3.90 (211, in, CH), 4.14-4.72
11-52 558.3 (3H, in, CH), 5.38-5.80 (111, in, CH),
6.98 (211, in, aryl H), 7.07-7.20 (3H, m,
aryl H), 7.31-7.46 (311, m, aryl H),
7.66-8.67 (3H, in, NH, OH)
(DMSO) 0.83-1.04 (6H, m), 1.81-2.08
(5H, m), 3.34-3.63 (111, m), 3.84-3.90
11-53 467 (1H, m), 4.00-4.60 (3H, m), 5.29-5.75
(211, m), 6.53-6.59 (1H, m), 6.70-6.90
(1H, m), 7.20-7.35 (0.5H, m), 7.78
(0.5H. brs), 8.43-8.60 (2H, m)
(DMSO) 0.96 (111, s). 1.03 (911, s),
1.30-1.39 (211, m), 1.68-1.71 (211, m),
1.79-1.82 (111, m), 1.97 (111, brd), 2.11
11-55 502.6 (311, s), 3.79 (311, s), 3.84 (1H, vbrs),
4.09 (111, vbrs), 4.56-4.58 (1H, m),
4.67 (1H, d), 6.81 (111, d), 7.00 (111,
d), 7.19 (1H, t), 7.79 (0.511, vbrs),
7.93 (1H, brd), 8.42 (0.511, vbrs)
(CDC13) 1.08-1.14 (9H, m), 1.85-2.05
(411, m), 2.32-2.45 (111, m), 2.79-2.85
(1H, m), 3.01-3.07 (1H, m), 4.13-4.17
11-56 492.5 (111, m), 4.53-4.70 (111, m), 4.98 (111,
t), 5.70 and 5.81 (1H total , brs and
brd), 6.91-7.00 (1H, m), 7.34-7.44 (311,
m), 7.67-7.75 (111, m)
CA 02820541 2013-06-21
= =
WO 2005/085236
PCT/US2005/006540
142-
No. 1-11-NMR
(obS)
(DMSO) 1.03 (9H, s), 1.31-1.38 (2H, m),
1.62-1.74 (3H, m), 1.98 (1H, brt), 2.07
(3H, s), 2.36 (1H, vbrs), 2.83 (1H,
11-57 549.5 vbrs), 3.84 (1H, brs), 4.17 (1H, vbrs),
4.54-4.57 (111, m), 4.70 (1H, d), 7.34
(1H, d), 7.42-7.45 (1H, m), 8.16 (1H,
t), 8.37 (11-I, brs), 10.23 (1H, s)
(CD30D) 1.17 (9H, s), 1.21 (311, t),
1.41-1.55 (211, m), 1.75-1.90 (311, m),
2.03-2.19 (111, m), 2.37-2,50 (3H, m),
11-58 563.5 2.58-2.78 (2H, m), 3.87-4.02 (111, m),
4.20-4.30 (1H, m), 4.55-4.70 (2H, m),
4.91 (1H, obscured), 7.45 (111, d), 7.51
(111, d), 7.85 (111, s), 8.29 (1H, d)
(DMSO) 1.05 (9H, s), 1.15 (6H, d),
1.35-1.5 (211, m), 1.75-1.9 (311, m),
2.0-2.1 (111, m), 2.3-2.45 (1H, m), 2.7-
11-59 577.5 2.9 (111, m), 4.05-4.15 (111, m), 4.65
(1H, s), 4.7-4.75 (1H, m), 7.15 (111,
d), 7.35 (111, t), 7.7 (111, d), 8.4-8.55
(2H, m), 9.5 (1H, s)
(DMSO)1.03 (9H, s), 1.31-1.38 (211, m),
1.68 (311, m), 2.30-2.33 (2H, m), 2.67
(0.511, brs), 2.99 (0.511, brs), 3.34
11-60 506.5 (0.511, brs), 3.76 (31-1, s), 4.04 (0.5H,
m), 4.58 (111, s), 4.72 (1H, d), 7.09-
7.12 (1H, m), 7.16-7.20 (111, m), 7.26-
7.30 (111, m), 7.78 (0,5H, vbrs), 8.02
(111, brs), 8.42 (0.511, vbrs)
(DMSO) 0.95 (3H, d), 0.10 (3H, d), 1.17
(1H, m), 1.32 (1H, m), 1.64-1.80 (311,
m), 2.00 (111, m), 2.30 (111, br s), 2.67
11-61 492.8 (0.5H, br s), 2.99 (0.511, br s), 3.75
(0.511, br s), 3.85 (311, s), 4.06 (0.511,
m), 4.50-4.55 (211, m), 5.42 (1H, br s),
7.07 (1H, m), 7.17 (1H, m), 7.26 (1H,
m), 7.80 (1H, br s), 8.35 (1H, m)
DMSO) 8 1.04 (9H, s), 1.29-1.34 (2H,
m), 1.59-1.67 (3H, m), 1.91-1.97 (1H,
m), 2.13 (3H, s), 2.96 (111, vbrs), 3.77
(111, vbrs), 4.10 (111, vbrs), 4.72 (111,
11-62 549.5 s), 4.76 (1H, d), 7.80-7.83 (1H, m),
7.88-7.91 (111, m), 8.00-8.02 (1H, m),
8.18-8.24 (12H, m), 8.39 (1H, vbrs),
9.62 (111, s)
CA 02820541 2013-06-21
= WO
2005/085236 PCT/US2005/006540
-143-
N+1 3.
No. H-NNIR
(obs)
(DMSO) 1.05 (9H, s), 1.09 (3H, t),
1.19-1.37 (3H, m), 1.47-1.77 (3H, m),
1.91-1.99 (1H, m), 2.28 (0.5H, brdd),
2.48 (2H, q), 2.63-2.74 (1H, m), 3.01
11-63 563.5 (0.5H, dd), 3.63 (0.5H, s), 3.78-4.37
(2H, total, m), 4.42-4.59 (1H. m), 4.75
(1H, d), 5.42 (0.5H, d), 7.76 (0.5H,
d), 7.80-7.83 (1H, m), 7.87 (1H, d),
8.01 (1H, m), 8.08-8.15 (1H, m), 8.36
(0.5H, d), 9.53 (IH, s)
(DMSO) 1.07 (9H, s), 1.34-1.37 (2H, m),
1.64-1.72 (311, m), 1.95-2.04 (1H, m),
2.31-2.35 (111, m), 2.65-2.70 (111, m),
3.01-3.03 (111, m), 3.99 (0.511, m),
11-64 509.5 4.26-4.28 (0.511, m), 4.68 (1H, s), 4.82
(1H, d), 5.45 (0.511, s), 7.73-7.86 (311,
m), 8.05-8.08 (2H, m), 8.49 (0.511, d),
8.57-8.59 (111, m), 8.69 (0.511, d), 9.15
(1H, d)
(DMSO) 1.02 (9H, s), 1.28-1.34 (211, m),
1.57-1.64 (3H, m), 1.90-1.96 (1H, m),
11-65 507.5 3.72-3.80 (1H, m), 4.50 (1H, brs),
4.72-4.74 (111, m), 5.91 (IH, s), 6.76
(111, d), 7.58-7.61 (1H, m), 7.81-7.83
(1H, m)
11-66 493.5
EXAMPLE III
BIOLOGICAL METHODS
[0180] Compounds of this invention may be tested using the
methods described below. Table 5 lists caspase-1 and caspase-
8 enzyme inhibition data for compounds 11-1-11-25. In the
Table, compounds with a Ki of <10 are assigned category A,
compounds with a Ki of 10-20 are assigned category B, and
compounds with a Ki of 21-30 are assigned category C.
In Vitro Assays
Enzyme Inhibition
[0181] Ki values for test compounds with caspase-1 and
caspase-8 were obtained by the method of Margolin et al. (J.
CA 02820541 2013-06-21
W02005/085236
PCT/US2005/006540
-144-
Biol. Chem., 272 pp. 7223-7228 (1997)). Other caspases may be
assayed similarly (see, e.g., WO 99/47545). Assays were
performed in 10 mM Tris (Sigma Corp, St Louis MO) pH 7.5, 1 mM
Dithiothreitol (DTT, Research Organic INC, Cleveland, OH) and
0.1% CHAPS (Pierce, Rockford IL) at 37 C. For caspase-3, a
solution of 8% glycerol was added to the assay buffer to
improve enzyme stability. A 65 pL aliquot of the assay buffer
and 5 pL aliquot of the appropriate dilutions of inhibitor in
DMSO where pipetted into a 96 well plate, treated with 10 pL
of caspase, than diluted in assay buffer (0.5-40 nM active
protein by active site titration). A control containing DMSO
but no compound was included for each determination. The
plates were then incubated for 15 minutes at 37 C, before
addition of the appropriate substrate (20 pL, final
concentration 1-4 X Km, final assay volume 100 pL) to initiate
the reaction. Reaction rates were measured at 37 C either by
following the time dependant increase in absorbance at 405 nM
(for the pNA substrates) or in fluorescence (Ex 390, Em 460)
(for the AMC substrates). The rates obtained were plotted
against inhibitor concentration and the data fit to the
Morrison tight-binding equation for competitive inhibitors
(Morrison, J. F., Biochem. Biophys. Acta, 185 pp. 269-286
(1969)). The substrates used for the individual assays were
as follows:
[0182] Caspase-1 Suc-YVAD-pNA (Sachem, King of Prussia, PA)
(final concentration in the assay 80 pM);
[0183] Caspase-8 Ac-DEVD-pNA (Sachem, King of Prussia, PA)
(final concentration in assay 80 pM).
Table 5: Caspase-1 (Cl) and caspase-8 (c8) inhibition data.
Ki Cl Ki C8
Compound
(nM) (nM)
II-1 A A
CA 02820541 2013-06-21
. =
WO 2005/085236
PCT/US2005/006540
-145-
11-2 A A
11-3 A A
11-4 A
11-5 A
11-6 A A
11-7 A
11-8 A
11-9 A
11-10 A
11-11 A
11-12 A
11-13
11-14 B A
11-15 A
11-16 A
11-17 A A
11-18 A
11-19 B A
11-20 A A
11-21 A
11-22 A
11-23 A
11-24 A
11-25 A
11-26 A A
11-27 A A
11-28 A A
11-29 A A
11-30 A A
11-31 A A
11-32 A A
CA 02820541 2013-06-21
4 WO 2005/085236
PCT/US2005/006540
-146-
11-33 A A
11-34 A A
11-35 A A
11-36 A A
11-37 A
11-38 A A
11-39 A A
11-40 A
11-41 A A
11-42 A
11-43 A A
11-44 A A
11-45 A A
11-46 A
11-47 A A
11-48 A A
11-49 A A
11-50 A
11-51 A A
11-52 A A
11-53 A
11-55 A A
11-56 A A
11-57 A A
11-58 A A
11-59 A A
11-60 A A
11-61 A A
11-62 A
11-63 A
11-64
CA 02820541 2013-06-21
W02005/085236 PCT/1JS2005/006540
-147-
11-65 A A
11-66 B A
PBMC Cell Assay
IL-1# Assay with a Mixed Population of Human Peripheral Blood
Mononuclear Cells (PBMC) or Enriched Adherent Mononuclear
Cells
(0184] Processing of pre-IL-1P by ICE may be measured in
cell culture using a variety of cell sources. Human PBMC
obtained from healthy donors provides a mixed population of
lymphocyte subtypes and mononuclear cells that produce a
spectrum of interleukins and cytokines in response to many
classes of physiological stimulators. Adherent mononuclear
cells from PBMC provides an enriched source of normal
monocytes for selective studies of cytokine production by
activated cells.
Experimental Procedure:.
[0185] An initial dilution series of test compound in DMS0
or ethanol is prepared, with a subsequent dilution into RPMI-
10% FBS media (containing 2 mM L-glutamine, 10 mM HEPES, 50 U
and 50 ug/ml pen/strep) respectively to yield drugs at 4x the
final test concentration containing 0.4% DMSO or 0.4% ethanol.
The final concentration of DMSO is 0.1% for all drug
dilutions. A concentration titration which brackets the
apparent Ki for a test compound determined in an ICE
inhibition assay is generally used for the primary compound
screen.
[0186] Generally 5-6 compound dilutions are tested and the
cellular component of the assay is performed in duplicate,
with duplicate ELISA determinations on each cell culture
supernatant.
CA 02820541 2013-06-21
A
WO 2005/085236
PCIMS2005/006540
-148-
PBMC Isolation and IL-1 Assay:
[01873 Buffy coat cells isolated from one pint human blood
(yielding 40-45 ml final volume plasma plus cells) are diluted
with media to 80 ml and LeukoPREP separation tubes (Becton
Dickinson) are each overlaid with 10 ml of cell suspension.
After 15 min centrifugation at 1500-1800 xg, the plasma/media
layer is aspirated and then the mononuclear cell layer is
collected with a Pasteur pipette and transferred to a 15 ml
conical centrifuge tube (Corning). Media is added to bring
the volume to 15 ml, gently mix the cells by inversion and
centrifuge at 300 xg for 15 min. The PBMC pellet is
resuspended in a small volume of media, the cells are counted
and adjusted to 6 x 106 cells/ml.
[0188] For the cellular assay, 1.0 ml of the cell
suspension is added to each well of a 24-well flat bottom
tissue culture plate (Corning), 0.5 ml test compound dilution
and 0.5 ml LPS solution (Sigma #L-3012; 20 ng/ml solution
prepared in complete RPMI media; final LPS concentration 5
ng/ml). The 0.5 ml additions of test compound and LPS are
usually sufficient to mix the contents of the wells. Three
control mixtures are run per experiment, with either LPS
alone, solvent vehicle control, and/or additional media to
adjust the final culture volume to 2.0 ml. The cell cultures
are incubated for 16-18 hr at 37 C in the presence of 5% CO2.
[0189] At the end of the incubation period, cells are
harvested and transferred to 15 ml conical centrifuge tubes.
After centrifugation for 10 min at 200 xg, supernatants are
harvested and transferred to 1.5 ml Eppendorf tubes. It may
be noted that the cell pellet may be utilized for a
biochemical evaluation of pre-IL-1P and/or mature IL-113 content
in cytosol extracts by Western blotting or ELISA with pre-IL-
specific antisera.
CA 02820541 2013-06-21
WO 2005/085236
PCT/US2005/006540
-149-
Isolation of Adherent Mononuclear cells:
[0190] PBMC are isolated and prepared as described above.
Media (1.0 ml) is first added to wells followed by 0.5 ml of
the PBMC suspension. After a one hour incubation, plates are
gently shaken and nonadherent cells aspirated from each well.
Wells are then gently washed three times with 1.0 ml of media
and final resuspended in 1.0 ml media. The enrichment for
adherent cells generally yields 2.5-3.0 x 105 cells per well.
The addition of test compounds, LPS, cell incubation
conditions and processing of supernatants proceeds as
described above.
ELISA:
[0191] Quantikine kits (R&D Systems) may be used for the
measurement of mature IL-113. Assays are performed according
to the manufacturer's directions. Mature IL-13 levels of
about 1-3 ng/na in both PBMC and adherent mononuclear cell
positive controls are observed. ELISA assays are perfoLmed on
1:5, 1:10 and 1:20 dilutions of supernatants from LPS-positive
controls to select the optimal dilution for supernatants in
the test panel.
[0192] The inhibitory potency of the compounds can be
represented by an IC50 value, which is the concentration of
inhibitor at which 50% of mature IL-1D is detected in the
supernatant as compared to the positive controls.
[0193] The skilled practitioner realizes that values
obtained in cell assays may depend on multiple factors. The
values may not necessarily represent fine quantitative
results.
[0194] Selected compounds of this invention have been
tested for inhibition of IL-113 release from PBMCs with IC50
values between 300nM and 4pM.
CA 02820541 2013-06-21
4 =
WO 2005/085236
PCT/US2005/006540
-150-
Whole Blood Assay for IL-113 Production
(0195] Whole blood assay IC50 values for compounds of this
invention may be obtained using the method described below:
PuLpose:
[0196] The whole blood assay is a simple method for
measuring the production of IL-if (or other cytokines) and the
activity of potential inhibitors. The complexity of this
assay system, with its full couiplement of lymphoid and
inflammatory cell types, spectrum of plasma proteins and red
blood cells is an ideal in vitro representation of human in
vivo physiologic conditions.
Materials:
=
Pyrogen-free syringes (- 30 cc)
Pyrogen-free sterile vacuum tubes containing lyophilized
Na2EDTA (4.5 mg/10 ml tube)
Human whole blood sample (- 30-50 cc)
1.5 ml Eppendorf tubes
Test compound stock solutions (- 25mM in DMSO or other
solvent)
Endotoxin -free sodium chloride solution (0.9%) and HBSS
Lipopolysaccharide (Sigma; Cat.# L-3012) stock solution at
lmg/m1 in HBSS
IL-10 ELISA Kit (R & D Systems; Cat # DLB50)
TNFot ELISA Kit (R & D Systems; Cat # DTA50)
Water bath or incubator
Whole Blood Assay Experimental Procedure:
[0197] Set incubator or water bath at 30 C. Aliquot
0.25m1 of blood into 1.5 ml eppendorf tubes. Note: be sure
to invert the whole blood sample tubes after every two
aliquots. Differences in replicates may result if the cells
sediment and are not uniformly suspended. Use of a positive
CA 02820541 2013-06-21
W02005/085236
PCT/US2005/006540
-151-
displacement pipette will also minimize differences between
replicate aliquots.
[0198] Prepare drug dilutions in sterile pyrogen-free
saline by serial dilution. A dilution series which brackets
the apparent Ki for a test compound determined in an ICE
inhibition assay is generally used for the primary compound
screen. For extremely hydrophobic compounds, prepare compound
dilutions in fresh plasma obtained from the same blood donor
or in PBS-containing 5% DMSO to enhance solubility.
[0199] Add 25 pl test compound dilution or vehicle control
and gently mix the sample. Then add 5.0 ul UPS solution (250
ng/ml stocked prepared fresh: 5.0 ng/ml final concentration
UPS), and mix again. Incubate the tubes at 30 C in a water
bath for 16-18 hr with occasional mixing. Alternatively, the
' tubes can be placed in a rotator set at 4 rpm for the same
incubation period. This assay should be set up in duplicate
or triplicate with the following controls: negative control -
no UPS; positive control - no test inhibitor; vehicle control
- the highest concentration of DM50 or compound solvent used
in the experiment. Additional saline is added to all control
tubes to normalize volumes for both control and experimental
whole blood test samples.
[0200] After the in_cubation period, whole blood samples are
centrifuged for 10 min.utes at - 2000 Lim in the microfuge,
plasma is transferred to a fresh microfuge tube and
centrifuged at 1000 x g to pellet residual platelets if
necessary. Plasma samples may be stored frozen at -70 C
prior to assay for cytokine levels by ELISA.
ELISA:
[0201] R & D Systems (614 McKinley Place N.E. Minneapolis,
MN 55413) Quantikine kits may be used for measurement of IL-1J3
and TNF-a. The assays are performed according to the
CA 02820541 2013-06-21
, .
9580-110
-152--
manufacturer's directions. IL-113 levels of - 1-5 ng/ml in
positive controls among a range of individuals may be
observed. A 1:200 dilution of plasma for all samples is
usually sufficient for experiments for ELISA results to fall
on the linear range of the ELISA standard curves. It may be
necessary to optimize standard dilutions if you observe
differences in the whole blood assay. Nerad, J.L. et al., J.
Leukocyte Biol., 52, pp. 687-692 (1992).
[0202] Selected compounds of this invention have been
. tested for inhibition of IL-1 release from whole blood with
IC50 values between 1pM and 40pM.
In Vivo Assays
[0203] Compounds of this invention may tested
in in vivo
=
assays such as those described in WO 99/47545.
[0204]
[0205] While we have described a number of embodiments of
this invention, it is apparent that our basic examples may be
altered to provide other embodiments which utilize the
compounds and methods of this invention. Therefore, it will
be appreciated that the scope of this invention is to be
defined by the appended claims rather than by the specific
embodiments that have been represented by way of example
above.