Note: Descriptions are shown in the official language in which they were submitted.
,
,
CA 02821421 2013-06-12
ELECTRORECOVERY OF GOLD AND SILVER FROM LEACHING
SOLUTIONS BY SIMULTANEOUS CATHODIC AND ANODIC
DEPOSITS
FIELD OF THE INVENTION
The present invention is related to the mining industry for treat-
ment of minerals and materials which contain gold and silver.
Specifically, it is related to a process to recover gold and silver,
from leaching solutions with a simultaneous anodic and cathodic
electrodeposition process, after which the poor solution is recy-
cled back to the leaching stage.
BACKGROUND OF THE INVENTION
The recovery of gold and silver from their minerals has been
performed by various methods; among the most employed are
pyrometallurgical treatments, in which upon the addition of a
considerable amount of energy, part of the mineral is oxidized,
in this manner liberating the precious metals. This great amount
of energy is the principal inconvenience of the process, which in
the end reflects on the operation costs.
On the other hand, the hydrometallurgical methods are charac-
CA 02821421 2013-06-12
2
REPLACEMENT SHEET UNDER ARTICLE 19
terized for their high selectivity and relatively low reagent and
energy costs. Gold and silver has been obtained by one such
method for over 100 years, using cyanide and oxygen as a
complexing agent and an oxidant, respectively. Despite the high
efficiency of this system, the treatment of complex minerals, as
well as environmental restrictions, has encouraged research on
other leaching systems that could compete with cyanide, without
its disadvantages.
Thiosulfate, in the presence of copper, and the combination of
thiourea with formamidine disulfide (Poisot-Diaz, M.E., Gonza-
lez, I. and Lapidus, G.T. (2008), " Effect of Copper, Iron and
Zinc Ions on the Selective Electrodeposition of Doree from Acid-
ic thiourea Solutions", Hydrometallurgy 2008, Eds. C.A. Young,
P.R. Taylor, C.G. Anderson y Y. Choi, Society for Mining, Metal-
lurgy and Exploration, Inc. (SME), Littleton, Colorado, U.S.A.,
ISBN: 978-0-87335-266-6, pp. 843-848 and Alonso-Gomez, A.R.
and Lapidus, G.T. (2008), "Pretreatment for Refractory Gold and
Silver Minerals before Leaching with Ammoniacal Copper Thio-
sulfate", Hydrometallurgy 2008, Eds. C.A. Young, P.R. Taylor,
C.G. Anderson y Y. Choi, Society for Mining, Metallurgy and Ex-
ploration, Inc. (SME), Littleton, Colorado, U.S.A., ISBN: 978-0-
87335-266-6, pp. 817-822.) are two chemical systems that leach
gold and silver from minerals for which cyanidation has proved
to be inefficient. In this same manner, it was shown possible to
= CA 02821421 2013-06-12
3
REPLACEMENT SHEET UNDER ARTICLE 19
recover gold and silver metals in both systems using direct
electrodeposition (A. Alonso. G.T. Lapidus and I. Gonzalez, A
strategy to determine the potential interval for selective silver
electrodeposition from ammoniacal thiosulfate solutions Hydro-
metallurgy, Volume 85, Issues 2-4, March 2007, Pages 144-153);
However, this recovery was accomplished in geometrically com-
plex reactors (F.C. Walsh, C. Ponce de Leon and C.T. Low, The
rotating cylinder electrode (RCE) an its application to the
electrodeposition of metals, Australian Journal of Chemistry, 58,
(4), 246-262 and A. Alonso, G.T. Lapidus and I. Gonzalez, Se-
lective silver electroseparation from ammoniacal thiosulfate so-
lutions using a rotating cylinder electrode reactor (RCE), Hy-
drometallurgy, Volume 92, Issues 3-4, June 2008, Pages 115-
123), with an energy consumption that renders un attractive from
an economic and financial standpoint.
At this point, it is important to mention a characteristic of the
thiourea and thiosulfate systems: both complexing agents can
oxidize at potentials near the reduction potential of silver (Fig-
ures 1 and 2). The diagrams of both ligands with gold are simi-
lar. This originates the formation of a narrow potential region
where Ag(I) and Au(I) ions are soluble and because of this, both
the leaching as well as the electroseparation conditions should
be controlled with precision. This could imply a great disad-
vantage with respect to other systems and has motivated the use
CA 02821421 2013-06-12
4
REPLACEMENT SHEET UNDER ARTICLE 19
of membrane reactors, in order to avoid contact of these solu-
tions with the anode.
OBJECTIVES OF THE INVENTION
One objective of the present invention is to provide a method to
separate gold and silver from thiosulfate or thiourea solutions by
simultaneous anodic and cathodic electrodeposition, increasing
in this manner the velocity of the process. Another is to accom-
plish this with a minimum affectation of the solution composi-
tion, so that it may be recirculated back to the leaching stage.
Yet another is to promote efficient energy use.
Other objectives and advantages that apply the principles and
are derived from the present invention may be apparent from the
study of the following description and diagrams that are included
here for illustrative and not limitative purposes.
BRIEF DESCRIPTION OF THE INVENTION
The present invention is intended to solve the problem of gold
,
=
CA 02821421 2013-06-12
REPLACEMENT SHEET UNDER ARTICLE 19
ans silver separation from thiosulfate and thiourea leaching so-
lutions, providing an improvement over the traditional electro-
chemical reactors now in use. This improvement is characterizes
by a novel process to simultaneously deposit metals in on the
5 anode and cathode in a one compartment reactor, using a com-
mercial copper sheet as the anode and a titanium sheet as the
cathode.
The conditions which permit this technique to operate were cho-
sen from the analysis of Figure 1, where a region of the soluble
complex Ag(S203)23- is observed within the metallic silver stabil-
ity zone. When the potential is decreased below -110 mV, the
Ag(I) species is reduced to Ago, in a typical electrolytic process.
However, the most interesting aspect of this diagram is when the
potential is less negative than -50 mV, where part of the thiosul-
fate oxidizes, destabilizing the soluble complex and forming me-
tallic silver. The present invention takes advantage of this phe-
nomenon and has not been previously reported for this or other
ligands.
The application of the simultaneous anodic-cathodic
electrodeposition of gold and silver allows more efficient use of
the electrical energy in electrochemical reactors of simple ge-
ometry without a membrane; additionally, the separation process
occurs in less time than that required in conventional electro-
chemical reactors.
CA 02821421 2013-06-12
6
REPLACEMENT SHEET UNDER ARTICLE 19
In order to better understand the characteristics of the inven-
tion, the following description is accompanied by diagrams and
figures, which form an integral part of the same and are meant
to be illustrative but not !imitative and are described in the fol-
lowing section.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a Pourbaix-type diagram in which the predominance
zones for the soluble species Ag(S203)23- (thiosulfate-silver
complex) and metallic silver Ago are shown.
Figure 2 is a Pourbaix-type diagram in which the predominance
zones for the soluble species AgTu3+ (thiourea-silver complex)
and metallic silver Ago are shown.
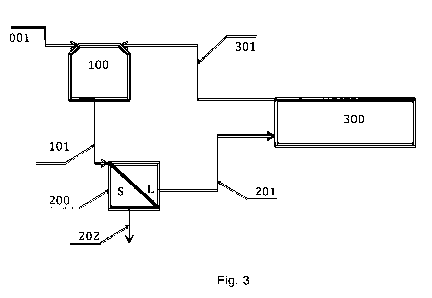
Figure 3 shows a leaching-electrodeposition scheme for obtain-
ing gold and silver which utilizes the present invention.
Figure 4 is a diagram showing a recirculation system which in-
cludes the electrochemical reactor.
CA 02821421 2013-06-12
7
REPLACEMENT SHEET UNDER ARTICLE 19
Figure 5 is a schematic diagram of the electrochemical cell in
which the simultaneous anodic and cathodic deposits are
achieved.
Figure 6 is a graphic representation of the change in silver con-
centration with leaching time.
Figure 7 is a graphic representation of the change in silver con-
centration with electrolysis time where there is simultaneous an-
odic and cathodic electrodeposition.
Figure 8 is a graph that compares the change in silver concen-
tration for leaches 1, 2 and 3 with the same solution.
Figure 9 shows the comparison of the silver concentration during
electrolysis 1, 2 and 3 with the same solution.
Figure 10 shows a comparison of XRD spectra for the anodic de-
posit obtained after the electrolysis and for pure metallic silver.
DETAILED DESCRIPTION OF THE INVENTION
The simultaneous electrodeposition process, referred to in the
present invention, is illustrated in Figure 3.
= A thiosulfate or thiourea solution, rich in gold and silver
ions, originating from the leaching stage (100) and after having
CA 02821421 2013-06-12
8
REPLACEMENT SHEET UNDER ARTICLE 19
been filtered (200), is introduced into the electrochemical reac-
tor (300).
= Once the electrodeposition has finalized, the cathode (312,
Figure 5) and the anode (313, Figure 5) are removed from the
reactor and mechanically abraded to remove the gold and silver
metals. The solution is then recirculated back to the leaching
stage (301).
The electrodeposition is performed in a recirculation scheme,
illustrated in Figure 4, in which the solution is charged to the
reservoir (320) from which it is pumped (330) to the electro-
chemical reactor (310) and then returned by gravity to the reser-
voir.
EXAMPLES
EXAMPLE 1
To better understand the invention, one of the many experiments
is detailed as an example, which employs a system such as that
schematized in Figures 3 to 5. A 60 cm2 (exposed geometrical
area) titanium plate was used as the cathode and a copper plate
with the same exposed area was the anode.
CA 02821421 2013-06-12
9
REPLACEMENT SHEET UNDER ARTICLE 19
As shown in Figure 3, the first stage is gold and silver leaching
from the mineral or concentrate, using a thiosulfate solution, in
this case, whose composition is presented in Table 1. The pH
was adjusted to 10.0 with NH4OH.
Table 1. Composition of the leaching solution
Component Composition (mol/L)
(NH4)2S203 0.2
CuSO4 0.05
EDTA 0.025
(NH4)2HPO4 0.1
The solutions were prepared with reagent grade chemicals using
deionized water (1x1010 MOcm-1). 500 mL of this solution was
placed in contact with 3.75 g of a flotation concentrate, with a
particle size less than 10 pm, containing 21 kg/ton of silver. Af-
ter six hours in continuous agitation, the solution was separated
from the solid by filtration and placed in a reactor such as that
represented in Figures 4 and 5.
During the electrodeposition, a flow of 1.1 L/min was used with a
cell voltage of 100 mV; with this voltage, the potential at the
cathode was -260 mV versus the normal hydrogen electrode
(NHE), which is adequate to obtain a selective silver deposit on
" CA 02821421 2013-06-12
REPLACEMENT SHEET UNDER ARTICLE 19
this electrode
Figure 6 shows a graphic representation of the silver concentra-
tion with respect to the leaching time. A maximum value was at-
5 tamed in 120 minutes, after which time the concentration re-
mained relatively constant.
The change in silver concentration during the electrolysis is
shown in Figure 7. Within the first 15 minutes a sharp descent is
10 observed, which then gradually decreases to values below 10
mg/L. The current registered throughout the experiment was 0.01
A, which together with the cell voltage translates to 0.004 W-h.
Considering that the deposited mass of silver was 0.065 g, the
energy consumption was 0.062 W-h per g of deposited silver.
After finalizing the electrodeposition, the solution was recycled
back to the leaching stage, where it was contacted with fresh
unleached concentrate, under the same conditions as described
previously. The entire procedure was repeated until three full
cycles were completed.
Figure 8 shows a graphic representation of the leaching results
for all three cycles; an increase in the leaching velocity and the
maximum silver concentration may be observed in the second
and third leach, relative to the first, possibly due to the stabili-
CA 02821421 2013-06-12
11
REPLACEMENT SHEET UNDER ARTICLE 19
zation of the equilibria between the thiosulfate and the Cu(II)
and Cu(I) ions.
On the other hand, the second and third electrolyses (the
dashed and dotted lines of Figure 9) show similar tendencies to
that of the first (solid line), only differentiable by the initial val-
ue, which depends on the previous leaching stage. In all three
cases, the values reached below 10 mg/L in approximately 4
hours.
These results clearly show that the thiosulfate solution can be
recirculated after the electrodeposition stage, back to the leach-
ing stage, at least three times without reconditioning or make-
up. Additionally, during the three electrolyses, the current main-
tained a constant value of 0.01 A, conserving the same energy
expenditure as the first cycle. Anode consumption was negligible
after three electrodeposition cycles.
Finally, it is important to mention that X-ray diffraction analysis
of both the anodic and the cathodic deposits showed that they
consisted exclusively of metallic silver. Figure 10 compares the
XRD spectra for the deposit obtained from the anode, at the end
of the electrolysis and the corresponding spectra for pure metal-
lic silver. As can be observed, the anodic deposit corresponds to
metallic silver; indicating that oxidation of thiosulfate is forming
only soluble species, such as tetrathionate, dithionate or even
sulfate, and is not contaminating the deposit.