Note: Descriptions are shown in the official language in which they were submitted.
CA 02821996 2013-06-17
mr/EP2010/007901/PCT-II June 07,
2013/Wue
R & M Consulting and Trading
GmbH & Co. KG
Richard-Wagner-Strasse 12
D-71638 Ludwigsburg
(Partial) apparatus for preventing incontinence with a fixing device to be
immovably implanted in body tissue
The present invention concerns an apparatus for preventing incontinence and a
part of this apparatus which then interacts with a second part and features a
fixing
device for immovable implantation in body tissue. The part of the apparatus
which is to prevent incontinence features a pipe-shaped body with an initial
longi-
tudinal element inside. Connected to the pipe-shaped body is a tube-like
retaining
element which features a second oblong guide element inside. This ends in an
opening in an axial direction on its opposing side to the pipe-like body. The
tube-
like retaining element is formed as a reversible stretchable and compressible
metal
grille which features a polymer covering, together with which it is kept air-
and
liquid-tight at the opening on the one hand and in the vicinity of the
transition to
the pipe-like body on the other.
The term incontinence basically means the inability to retain something. In
terms
of the present invention this can, for example, be aconuresis. Such an
involuntary
urine loss is definitely a frequently occurring illness which can have various
causes. The general term aconuresis is therefore just the generic term for
very
different types of illness which can be differentiated and defined by means of
their
CA 02821996 2013-06-17
2
causes. In addition to incontinence caused by stress and pressure, motoric and
sensoric urge incontinence are also known. These should in turn be
distinguished
from obstructive overflow incontinence and functional overflow incontinence,
supra-spinal and spinal reflex incontinence or extraurethral incontinence as
further
causes of incontinence.
With regard to stress incontinence, an apparatus for controlling bladder
function
manufactured by the Uromedica company has become known which basically
consists of two small implantable balloons. These are implanted under the skin
next to the bladder during a short surgical procedure. The expansion of the
bal-
loons should protect against involuntary urination by compressing the urinary
bladder. The natural passing of water is thus not inhibited as the balloon
size is set
so that for the purposes of urination, normal bladder pressure is sufficient
to cause
the bladder to be emptied. It should still be possible for a doctor to modify
the fill
quantity of the two balloons after implantation.
An apparatus of this kind has become known through WO-A-98/56311. WO-A-
98/56311 describes an expandable apparatus for preventing aconuresis in which
a
pipe-shaped guide or conduit is connected to a balloon whose circumference can
be modified and which is therefore adjustable. The pipe-shaped conduit
penetrates
the balloon axially in such a manner that the conduit extends into and
projects out
of the balloon. The respective joints between the conduit and the balloon are
sealed liquid-tight, for example with silicone. Instead of sealing with
silicone or a
comparable chemical or polymeric adhesive, ultrasonic welding is also
disclosed
as a possible sealing technique.
An initial passage which runs through the pipe-shaped conduit in an oblong
direc-
tion ends within the balloon and opens out inside the same. This passage
serves to
fill the balloon, for example with a liquid, and thus expand it according to
re-
quirements, or to withdraw fluid and thus reduce the size of the balloon.
CA 02821996 2013-06-17
3
A further passage may be provided which serves to insert the apparatus in the
vi-
cinity of the urethra in a human body. This passage accordingly ends with an
opening at the end area of the conduit which extends out of the balloon and
into
the body. This passage's second opening in the conduit is located in the area
be-
tween the balloon and its proximal end.
For one thing, chemical compounds are specified as materials for the balloon
which are themselves capable of forming a seal with the pipe-shaped conduit in
the areas of contact. Compounds such as cross-linked silicone gel,
polyvinylpyr-
rolidone and karaya gum are named. In addition to the embodiment of the ex-
pandable apparatus with an initial passage with which the balloon can be
filled or
emptied as already explained, WO-A-98/56311 also elucidates that the balloon
wall can also be pierced with a coreless hollow needle and filled or emptied
in this
manner.
A biologically compatible non-elastically restorable elastomer or an
appropriate
polymer mixture of polyurethane, polymers such as polyethylene,
polytetralluoro-
ethylene (PTFE), polystyrene or polyetheretherketone (PEEK) are named as fur-
ther materials for the balloon.
The balloon wall can additionally feature reinforcing structures. With this in
mind, the balloon is double-walled and the reinforcing structure is situated
in be-
tween the walls. It can consist of fibres made of polyester, nylon,
polypropylene,
polytetrafluoroethylene (like TEFLON) or other polymers which feature a high
degree of hardness or a high hardness modulus. The fibres may form a net which
is woven into a supporting structure which is arranged between the walls of
the
balloon. In one embodiment, the fibres can be less elastic than the wall
itself.
The woven support structure then features a loose fibre weave to permit
extension
or diminution of the balloon walls.
This having been said, the fibres of the reinforcing structure can also be
mainly
non-elastic and woven, whereby the fibres are then knotted to also permit
expan-
sion or contraction of the balloon walls.
CA 02821996 2013-06-17
4
The reinforcing structure also has the additional task of holding back
particles let
into the balloon which can be used to expand it in place of a fluid so that
they
cannot leak out into the vicinity of the balloon. The fibres woven into the
support
structure can be equipped with a waterproof ripstop function. This means that
drops which may form when the hollow needle enters the balloon for filling or
emptying can also be absorbed.
With regard to the particles which can be used instead of or together with a
liquid
to fill the balloon, WO-A-98/56311 cites various examples of particles which
by
nature of their size can be injected into the balloon by means of a hollow
needle in
order to then enlarge their diameter within the balloon so that the balloon is
ex-
panded. One example of such particles features a core with numerous arms point-
ing away from it. These particles pass through the hollow needle in a
compressed
state and expand after entering the balloon. Other such particles can feature
a
pipe-shaped, elongated structure in order to pass through the hollow needle.
The
elongated structure then subsequently prevents the particles from flowing back
through the needle or the opening in the balloon which forms when the hollow
needle is withdrawn.
Further embodiments of particles can consist of hydrophilic material such as
polyvinylpyrrolidone, polyethylene glycol, carboxymethyl cellulose or
hyaluronic
acid which expands inside the balloon.
PCT/EP2010/003757 has made a further apparatus for preventing incontinence
known with which instead of the balloon from the previously described state of
technology, a tube-like retainer element in the form of a reversible
expandable and
compressible metal grille is deployed.
This metal grille features a polymer covering and is kept fluid- and
watertight on a
pipe-shaped body in the form of a pipe-shaped, flexible tube. The metal grille
may
feature a diamond pattern whereby the diamonds in a compressed state are
formed
as slits which preferably feature an extension of a pipe-shaped retaining
element
connected to the pipe-shaped body in the longitudinal direction of the
flexible
tube.
CA 02821996 2013-06-17
This pipe-shaped tube serves mainly to bring the retaining element designated
as a
metal grille to the location in the urethra from which the bladder function
can be
effectively and practically controlled.
The pipe-shaped, flexible tube is formed as a fabric-reinforced and/or fabric-
5 braided tube, whereby a single or multiple layer of fabric may be
present. The
pipe-shaped, flexible tube can also feature metallic reinforcement.
The pipe-shaped body in the form of a pipe-shaped, flexible tube can be made
of
at least one biologically compatible thermoplastic. The material is then
preferably
selected from polyolefines, polyvinylchloride, polyvinylidenfluoride,
polytetra-
fluoroethylene and/or Viton. This tube and the tube-like retaining element
with
the metal grille then merge.
The pipe-shaped shrink tube can also feature metallic reinforcement. This
metallic
reinforcement and also the reversible expandable and compressible metal grille
of
the tube-like retaining element are then preferably formed as a sandwich
between
two plastic layers.
In addition, the pipe-shaped body can feature an anti-microbial silver coating
or a
coating of diamond-like carbon in one of its embodiments.
Unlike WO-A-98/56311, the apparatus for preventing incontinence disclosed in
this PCT registration is not filled with a fluid or any other expandable
polymeric
material but with air at atmospheric pressure in the vicinity of the tube-like
retain-
ing element.
The pipe-shaped body features an initial guide element in the form of a
flexible
shaft approximately in its centre with spacers which retain the shaft in the
pipe-
shaped body. In the vicinity of its distal end, this flexible shaft ends in an
actuator
which for its own part features a control positioned distally at the end to
permit
manual adjustment of the apparatus or adjustment by means of a motor drive.
It is of quintessential importance for the success of an apparatus for
preventing
incontinence that the two parts of the apparatus originally precisely
implanted in
the vicinity of the urethra do not subsequently wander and thus cast doubt on
the
reliability of the apparatus.
CA 02821996 2013-06-17
6
According to PCT/EP2010/003757, fixation of the pipe-shaped body and thus of
the entire apparatus at the implantation site is made possible in that this
pipe-
shaped body features fixing elements in the form of protrusions such as
pimples,
hooks and/or scale-like protrusions which protrude outwards on its surface.
These
can be formed on the pipe-shaped body at regular intervals, for example in
rows,
or also at irregular intervals, whereby they are generally formed with it in
one
piece.
Such an arrangement of protrusions of the aforementioned type on the outer cir-
cumference of the pipe-shaped body can indeed fulfil the desired retaining
func-
tion and thus contribute to keeping the implant immovably at the desired
position
after the surgical procedure, but it has disadvantages during the implanting
itself.
The reason for this is that due to the protrusions, at least part of the
mouldings
such as pimples or hooks, the pipe-shaped body has a larger circumference
which
should be avoided if at all possible during a minimally invasive procedure.
This having been said, with WO-A-98/56311 an expandable inter-vertebral spacer
has been disclosed in a completely different technical area, namely that of
verte-
bral column implants. Such inter-vertebral spacers are mainly required in case
of a
slipped disc if parts of the spinal disc obtrude into the vertebral canal,
i.e. the
space in which the spinal cord lies. This process causes considerable pain.
Treat-
ment is either conservative or, in serious cases, by means of a surgical
procedure.
The spinal disc is partly or completely removed in the process and a spacer is
then
introduced between the adjacent spinal discs into which bone grows in order to
join both discs to one another in this manner. This leads to spinal stiffening
at the
site of the procedure. It was a major aim of the aforementioned state of
technol-
ogy to provide an inter-vertebral spacer which requires the minimum surgery
pos-
sible and thus itself features as small a diameter as possible when implanted.
For this reason, WO-A-98/56311 proposed an inter-vertebral spacer with an ini-
tial, smaller diameter which can be inserted in the area between the two
affected
discs and then expanded to a significantly larger diameter. This larger
diameter
CA 02821996 2013-06-17
7
can overlap the initial diameter by three to five times or even more. In this
way,
optimum filling of the inter-vertebral area is achieved by means of the radial
ex-
pansion of the disclosed spacer in accordance with the aforementioned state of
technology without, however, requiring a correspondingly major surgical proce-
dure.
This is achieved by the aforementioned state of technology in that a small
axial
tube is provided which features a surface and a proximal and a distal end. The
surface of the tube exhibits several slits which define at least two axially
displaced
extensions in such a way that the extensions extend out of the surface and
create a
geometry of an expanded spacer when the tube is compressed axially. These axi-
ally displaced extensions comprise at least three or four extensions which
extend
correspondingly from the tube in three or four different directions.
Applied to the inter-vertebral area, such an axial tube means that it is first
inserted
into this inter-vertebral area and then significantly compressed in length,
whereby
extensions expand laterally in the aforementioned three or four different
directions
which significantly increase the circumference of the tube and thus fill the
inter-
vertebral area without a correspondingly large wound being caused by the
surgical
procedure.
Based on this state of technology, the task of the present invention is
therefore to
provide an apparatus for preventing incontinence which features the benefits
of
the apparatus as they are possible with PCT/EP2010/003757 and which simulta-
neously permit an immovable placement of both parts of the apparatus with the
least possible side-effects and with a minimum of invasiveness.
This task is solved according to the present invention by an apparatus for
prevent-
ing incontinence which consists of two parts and with which at least one part
of
the apparatus features a retaining device for immovable implantation in body
tis-
sue, with a pipe-shaped body and an initial oblong guide element inside,
whereby
a tube-like retaining element is connected to the pipe-shaped body which
features
a second oblong guide element inside which ends in an axial direction on its
op-
CA 02821996 2013-06-17
8
posing side to the pipe-like body, and whereby the tube-like retaining element
is
formed as a reversible stretchable and compressible metal grille which
features a
polymer covering together with which it is kept air- and liquid-tight at the
opening
on the one hand and in the vicinity of the transition to the pipe-like body on
the
other. According the present invention, at least this single part of the
apparatus for
preventing incontinence is characterised in that the first oblong guide
element
defines areas with axially displaced protrusions, whereby in a non-extended
state
the protrusions are formed as slits which extend out of the surface of the
guide
element in an axial direction to form the finished extensions when the first
guide
element is compressed.
This particularly takes into account the fact that the requirements made of
the
outward-facing surface of the (partial) apparatus for preventing incontinence
and
the requirements of the precise fit of the implant can fundamentally conflict
with
one another. Whilst the aforementioned surface should be as smooth as possible
to
prevent irritation to the surrounding tissue, firm anchoring in the tissue is
neces-
sary on the other hand to prevent the apparatus from slipping out of place.
This
problem can be counteracted by means of the surface modification provided for
according to the present invention through the extensions which are provided
not
on but inside the pipe-shaped body of the first oblong guide element.
In this way, the first guide element can fulfil its function of stabilising
the pip-
shaped body and simultaneously of keeping the apparatus fixed in place in a
very
tissue-protective manner.
According to a preferred embodiment, the slits in the first oblong guide
element
run mainly parallel to the axis of the first guide element. In a compressed
state,
the protrusions which extend outside project approximately at right angles to
the
axis.
It is especially preferred that the first guide element, including the
protrusions
formed upon it, is formed of a nickel-titanium alloy with a shape memory
effect.
CA 02821996 2013-06-17
9
Such a nickel-titanium alloy with a shape memory effect is, for example, com-
mercially available under the brand name of Nitinol .
The first oblong guide element is preferably formed as a hollow body. If a rod
adapted to the inside diameter of the hollow body which features a pre-defined
curve is for its part adapted to the anatomy of the implantation site and is
then
inserted into the first guide element according to a further embodiment of the
relevant part according to the present invention of an apparatus for
preventing
incontinence, this curve can be transferred to the first guide element and
thus to
the pipe-shaped body.
The axially displaced protrusions can be displaced at an angle of
approximately
900 to one another. The protrusions then extend outwards very distinctively.
The axially displaced protrusions can also be displaced at an angle of approxi-
mately 45 to one another. In this way, the surface features a more pronounced
fine structure which can be more tissue-protective and therefore preferred for
cer-
tain indications during implantation. This kind of tissue-protective formation
of
the protrusions can, however, have a diminishing effect on local anchorage.
The
selection of the surface structure and thus one of the two aforementioned em-
bodiments of displacement, 90 or 45 to one another, must therefore be
weighed
up carefully.
The protrusions which extend out of the surface of the first guide element in
an
axial direction when the first guide element is compressed form shoulders and
form an angle of a to one another. This is preferably variably adjustable.
This can easily be achieved by compressing the first guide element more or
less
strongly. The angle a formed will be correspondingly more or less sharp.
Influence can also be exerted on the surface structure of the first guide
element in
this way. A less sharp angle a causes a less strong manifestation of the
surface
structure and vice versa.
CA 02821996 2013-06-17
The fact that the pipe-shaped body is preferably formed as a pipe-shaped
flexible
tube made of at least one biologically compatible plastic or polymer, whereby
its
material is selected from polyurethane, polyetherblockamides, polyamides,
latex,
polyvinyl chloride and/or silicone means that it can reproduce the structure
5 formed by the external protrusions and simultaneously prevent a possible
injury
hazard for the surrounding tissue due to the structure of the extensions.
With regard to the material, the polyetherblockamides (PEBA), which are avail-
able commercially under the brand name of PEBAX are worthy of special men-
10 tion. As thermoplastic elastomers, they are characterised by a lower
density com-
pared to other thermoplastic elastomers such as polyurethanes. In addition,
they
have outstanding mechanical and dynamic properties. They therefore demonstrate
outstanding elasticity impact and fatigue resistance.
Alternatively, the pipe-shaped body can be formed as a so-called shrink tube
of at
least one biologically compatible thermoplastic. The structure formed by the
ex-
ternal protrusions can then also be well formed and a possible injury hazard
for
surrounding tissue simultaneously avoided due to the structure of the
extensions.
Above all, thermoplastics such as polyolefines, polyvinyl chloride (PVC),
polyvi-
nylidenfluoride (PVDF), polytetrafluoroethylene (PTFE) and/or Viton are worthy
of consideration. Viton is the designation for a plastic which is available
com-
mercially from the DuPont company. If such a shrink tube is used, the pipe-
shaped body and the tube-like retaining element can be joined practically seam-
lessly to the metal grille. No specific connection such as by means of a
marker
tape or a clamp connection needs to be provided for.
If the pipe-shaped body in the shrink tube material is additionally formed
accord-
ing to the so-called braiding process as a fabric tube with a metal lining,
for ex-
ample platinum interlacing, the fine metal wires can merge with the tube-like
re-
taining element and thus end in the pipe-shaped retaining element. Due to
this, the
metal grille is additionally beneficially reinforced in the area of the
transition from
CA 02821996 2013-06-17
11
the pipe-shaped body to the tube-like retaining element. Shrink tubes are
available
commercially in various embodiments. There is a choice of thin, medium and
thick-walled tubes.
If the pipe-shaped body is formed of fabric-reinforced and/or fabric-braided
flexi-
ble tubing and features a metallic reinforcement, it is also possible to form
the
metallic reinforcement as well as the reversible extendable and compressible
metal parts of the tube-like retaining element as a sandwich between two
layers of
plastic. The metallic reinforcement and the metal grille can thereby merge.
The pipe-shaped flexible tube is preferably additionally provided with a hydro-
philic coating. This hydrophilic coating increases the sliding properties of
the tube
surface which makes implantation easier on the one hand and contributes on the
other to increasing the wear comfort of the implanted apparatus.
The pipe-shaped body may additionally feature an anti-microbial silver coating
or
a coating of diamond-like carbon. These coatings are wafer-thin and
respectively
serve to minimise colonisation by germs.
The pipe-shaped body can also be formed as a flexible fabric-reinforced and/or
fabric-braided tube, whereby the fabric lining consists of one single or
multiple
layers.
Such fabric-reinforced and/or fabric-braided flexible tubes can be preferred
in
order to achieve improved transmission of energy. Mention should be made here
of silicone tubes with a monofilament polyester braided lining. A peroxide-
braided, monofilament polyester fabric can also be used. Other plastics such
as
PEBY are suitable instead of polyester. The fabric braiding can be a single or
a
multiple braided layer.
The tube-like retaining element filled with air at normal or atmospheric
pressure is
especially preferred. Many problems connected with the provision of an
apparatus
CA 02821996 2013-06-17
12
for preventing incontinence which provides a retaining element which can be
filled with fluid can thereby be very simply solved. The pressure which the
appa-
ratus according to the invention, and the respective part of the apparatus
according
to the invention, is to exert on the urethra as per the anatomic circumstances
of the
patient in order to effectively prevent their incontinence is adjusted by
extending
or contracting the tube-like retaining element. This expanding or contracting
has
the effect of a spring in practice.
The metal grille of the tube-like retaining element can be formed as a diamond
pattern, whereby in their compressed state the diamonds are formed as slits
which
feature an extension in the longitudinal direction of the flexible tube. The
slits,
and thus the diamonds of the metal grille, are especially preferably of
different
sizes. The stress loading on the grille material with regard to the re-
adjustable and
actively adjustable form of embodiment is thereby significantly reduced and
the
durability of the material is optimised as regards long-term use.
If the part of the apparatus according to the present invention is adjustable,
the
first oblong guide element ends on its distally opposite side in an actuator
and
projects above this for the purposes of manual adjustment or for adjustment
via
connection to a drive motor.
As an alternative to manual adjustment without a motor it is equally possible
to
perform adjustment controlled by a magnet. This type of adjustment offers the
advantage that it can be performed from outside via the skin without surgical
in-
tervention.
If the part of the apparatus according to the present invention is actively
adjust-
able, the first oblong guide element can be formed as a toothpick which is
held
inside the pipe-shaped body by the projections and can be moved mechanically
by
hand or motor-driven via a lifting mechanism.
CA 02821996 2013-06-17
13
The actively adjustable apparatus can be coupled with a movement, inclination,
pressure and/or volume sensor and controlled by at least one of these sensors.
With sensor types deployable according to the present invention, the main
exter-
nal and internal factors for monitoring and control are made accessible which
can
influence the correct use or the functional efficiency of the apparatus for
prevent-
ing incontinence. Both external factors such as barometric pressure and also
inter-
nal factors such as those which are physiologically founded can, for example,
be
queried via the volume sensor.
All the named sensor types are already known within the field of medical
technol-
ogy and are deployed in various applications. It is advantageous that they are
at-
tached externally and therefore place no further strain on the implantation
site.
Since the air column formed in the tube-like retaining element is at
atmospheric
pressure it is correspondingly exposed to fluctuations in air pressure. More
ex-
treme conditions such as air pressure whilst flying or at altitudes of at
least 2000
metres can by all means cause a change. The monitoring of the apparatus accord-
ing to the present invention or the respective part of the invention makes
allow-
ances for this. In the process, either all the aforementioned sensors can be
de-
ployed together or alternatively at least one or two of them selectively as
befits the
purpose.
The previously described embodiments of the apparatus according to the present
invention, including the aforementioned variants, respectively concern a part
of
the apparatus for preventing incontinence. The finished apparatus features two
identical or different parts of the parts of the apparatus introduced here.
These are
respectively implanted adjacent to the patient's urethra.
Each of the embodiments of the apparatus according to the present (partial)
inven-
tion described can now be implanted in a human body in such a way that it is
in
duplicate or combined with another of the parts of the apparatus already de-
scribed.
CA 02821996 2013-06-17
14
The finished apparatus for preventing incontinence can thus be constructed in
dif-
ferent ways. One option is that two parts of the re-adjustable and/or actively
re-
adjustable variant as described in detail above are implanted adjacent to the
pa-
tient's urethra and thus form the finished apparatus.
Another possibility is that one part of the apparatus concerns the re-
adjustable
and/or actively re-adjustable variant whilst the other part is selected in the
form of
the non-re-adjustable variant and that the two different parts are implanted
adja-
cent to the patient's urethra to form the finished apparatus.
It is, of course, also possible that the finished apparatus features two parts
of a
non-re-adjustable variant of the apparatus according to the present invention.
The following is intended to describe the invention in more detail by means of
examples of embodiments and the enclosed drawing.
Shown in the drawing are:
Fig. 1 a schematic cross-section view of an apparatus for preventing in-
continence in a first embodiment, with a first oblong guide element
which features protrusions in an expanded state,
Fig. 2 a schematic cross-section and incomplete view of the first
oblong
guide element with slits,
Fig. 3a a schematic perspective detail view of two opposing
externally pro-
truding protrusions with a spacer,
Fig. 3b a schematic cross-section detail view of two opposing externally
protruding protrusions with a spacer,
CA 02821996 2013-06-17
Fig. 4 a schematic cross-section detail view of an apparatus for
preventing
incontinence in a slightly curved embodiment and with additional
re-adjustment,
5 Fig. 5 a schematic cross-section view of the metal grille in non-
expanded
form as a detail view as per Cut-out 1 of the adjacently depicted
complete apparatus of the first embodiment example,
Fig. 6 a schematic, cross-section detail view of the apparatus
according to
10 the present invention which depicts the connection of the flexible
tube to the intersection with the cage,
Fig. 7 a schematic cross-section view of two adjustable (partial)
appara-
tuses according to the present invention after transplantation into a
15 body lumen,
Fig. 8a a schematic cross-section and incompletely depicted view of an
apparatus for preventing incontinence in a third, actively adjustable
embodiment which displays the actuator as an adjustment mecha-
nism,
Fig. 8b a schematic cross-section detail view of the apparatus
according to
the present invention as in 8a which displays the connection of the
flexible tube to the intersection with the cage,
Fig. 8c a schematic cross-section view of the apparatus according to
the
present invention with protrusions which are displaced axially at an
angle of 45 to one another, and
Fig. 9 a perspective three-dimensional partial view of the first oblong
guide element with protrusions which are displaced relative to one
another by an angle of 45 in an axial direction.
CA 02821996 2013-06-17
16
1. Embodiment: adjustable apparatus
Fig. 1 shows an apparatus for preventing incontinence designated with the
refer-
ence number 1 and one part of such an apparatus 1 which then in an implanted
state is supplemented with a corresponding second part of apparatus 1; both
these
parts of apparatus 1 are then placed on each side of the urethra. For the sake
of
simplification, however, the one part of apparatus 1, which in fact represents
just
one part of the ready-to-use or prepared apparatus 1, is designated as the
entire
apparatus 1.
In its basic construction and functionality apparatus 1, as described
hereinafter,
corresponds to the same apparatus 1 as in the first embodiment of
PCT/EP2010/003757, to which reference is basically made in full. The basic
func-
tionality of apparatus 1 in this first embodiment is, however, hereinafter
described
again, in order to subsequently explain the main modifications made to the
inven-
tion.
Apparatus 1 correspondingly features a pipe-shaped body 3 which is formed as a
flexible tube. Pipe-shaped body 3 is formed of a biologically compatible
plastic or
general polymer so that it can be implanted in a human body. A polyether block
amide (PEBA) which is commercially available under the name of PEBAX ,
latex, polyvinylchloride (PVC) and silicone have proven to be equally suitable
during preliminary tests. It should therefore be clear to someone practised in
the
art that these plastics and polymers represent examples of usable materials
and
that this should not be regarded as limiting. With regard to latex, it should
be
noted that in addition to natural rubber, synthetic rubbers are also suitable.
The same materials which were used in the first embodiment as per
PCT/EP2010/003757 were tested in preliminary tests. What is new is that a sili-
cone tube without further reinforcement was tested. A silicone tube in
accordance
with PCT/EP2010/003757 which featured a monofilament braided polyester lin-
CA 02821996 2013-06-17
17
ing was then used. In a further preliminary test, the same tube was used but
with a
platinum-braided embodiment. It was possible to achieve a hardness of approxi-
mately 70% Shore A with it.
A further preliminary test was performed with a peroxide-braided, monofilament
polyester fabric as reinforcement for the silicone tube.
The reinforcement by the fabric lining and the platinum braiding had the
purpose
as per PCT/EP2010/003757 of making possible optimum transmission of energy.
This no longer appeared to be a major focus for reasons still to be explained.
However, since it is to be expected that the reinforcing fabric lining and the
braid-
ing can contribute to the longevity and resilience of apparatus 1 according to
the
present invention they were also tested as described. For this, it was
necessary to
test the elasticity of the aforementioned reinforcing materials.
All tests were completed with good results as regards the elasticity,
durability and
resilience of the tubes used. It was, however, ascertained that a silicone
tube can
also be used with the apparatus according to the present invention used here,
in
particular the novel, modified design of the pipe-shaped body 3 which still
has to
be explained in detail in the following. This is of great benefit for cost
reasons.
Unlike PCT/EP2010/003757, a different method is suggested to make the flexible
tube which forms pipe-shaped body 3 keep its shape following implantation in
body tissue. According to the present invention it is thus possible to anchor
the
pipe-shaped body 3 and therefore the entire apparatus firmly in place. Such
local
fixation means that the apparatus does not move away from the site of implanta-
tion, either in the long-term or on account of short-term impacts.
In order to achieve such a firm anchorage, pipe-shaped body 3 features a first
ob-
long guide element 5, which due to the protrusions 7 formed upon it also
simulta-
neously acts as a fixing device. This first oblong guide element 5 in the form
of
the fixing device traverses practically the entire length of pipe-shaped body
3's
flexible tube.
In the present embodiment the first oblong guide element 5, including its
protru-
sions 7, is formed of a nickel-titanium alloy which features super-elastic
proper-
CA 02821996 2013-06-17
18
ties and a low elasticity modulus. For this type of alloy and its use as part
of the
first guide element 5 according to the present invention, its elasticity and
the ex-
ploitation of the form memory effect are decisive. This serves to give the
protru-
sions 7 on the first guide element 5 their form by the use of a force and to
thus
adjust them appropriately. This is explained thoroughly further below.
The first oblong guide element 5 ends on its distally opposite side, i.e. with
an
intended implantation at end of the pipe-shaped body 3 facing away from the re-
spective centre of the body in an actuator 9. Its function is also explained
below,
i.e. later, and not here, for reasons of clarity.
At its proximal end, i.e. the end facing the body of the implant wearer, the
first
oblong guide element 5 ends in a coupling 11. This is an elastic coupling 11
made
of an elastomer. The coupling 11 is beige elastic. It serves as a connecting
element
to a threaded rod 13 which is retained at the proximal end of the pipe-shaped
body
3 by means of a threaded nut 15. The transition from the pipe-shaped body 3 to
a
cage altogether designated with the number 17 also takes place approximately
at
this location; this cage is formed in such a way that it can effectively
prevent in-
continence in a patient in conjunction with the pipe-shaped body 3. Threaded
rod
13 passes through cage 17, which features air at normal pressure, and ends in
a
blanking plug 19 which seals cage 17 airtight and fluid-tight and
simultaneously
serves as the axial bearing for threaded rod 13.
After the main features of the basic design of apparatus 1 have been explained
in
this manner, the innovative design of the first oblong guide element 5 in
relation
to PCT/EP2010/003757 is to be examined in more detail.
Pipe-shaped body 3 features the aforementioned protrusions 7 so that both
parts of
the apparatus for preventing incontinence remain at the location where they
were
implanted and do not, for example, wander within the body tissue due to the
vio-
lent movements of the implant wearer. Their formation on the first oblong
guide
element 5 are described in more detail below.
CA 02821996 2013-06-17
19
The first oblong guide element 5 which shows protrusions 7 in a non-expanded
state is depicted in Figs. 2a and 2b. The first oblong guide element is a
hollow
body which here initially features slits 23 at defined locations and at
defined dis-
tances along its surface. For adequate fixing of the oblong guide element 5
and
thus the entire apparatus 1 it is of significance that areas with different
arrange-
ments of the slits 23a, 23b, etc. alternate which results in the different
orientation
of the protrusions. This alternation of the slit arrangements 23a, 23b is to
be un-
derstood in that the slits 23 arranged in area 23a are displaced in an axial
direction
relative to the slits 23 arranged in area 23b. For the fixation of apparatus 1
it is
sufficient if a total of three protrusions 7, but preferably four protrusions
7, are
formed in the areas with different arrangements of the slits 23a and 23b. This
means that both areas 23a and 23b altogether show slits in such a manner that
three, preferably four protrusions are formed.
If the two areas 23a and 23b feature a total of four protrusions, this means
that as
per this example of the embodiment that the protrusions 7 in the first area
23a are
displaced 90 in an axial direction in relation to the protrusions 7 in the
second
area 23b. As can be seen from Figs. 23a and 23b, areas 23a and 23b alternate
along the length of the first oblong guide element 5 insofar as 5 slits 23a
are pro-
vided on the first oblong guide element and 9 protrusions are to be formed
accord-
ingly.
In order to form protrusions 7 emanating from slits 23, the first oblong guide
ele-
ment is compressed in an axial direction. The protrusions 7 predefined by the
formation of slits 9 but not yet protruding from the surface of the pipe-
shaped first
guide element 5 thus point or fold outwards and result in the completely
erected
protrusions 7.
In order to space these protrusions 7 in such a way that suitable anchoring
for the
apparatus 7 is formed in the respective body in which the apparatus is
implanted,
partial areas 25 are provided which feature no slit and additionally spacers
25a by
means of which the angle a at which the protrusions 7 protrude from the
surface
of the first oblong guide element 5 can be adjusted. Fig. 3a shows a
perspective
detail view of two opposing and externally protruding protrusions 7 for which
CA 02821996 2013-06-17
spacer 25a increases the clearance between the shoulders of a particular
protrusion
7 and thus the angle formed between the shoulders. Fig. 3b shows the same view
but in cross-section so that here also the aforementioned angle a can be
specified.
Whilst the spacer 25a defines the clearance between two shoulders of one
particu-
5 lar protrusion, the distance from the slit arrangement of the first area
23a to the slit
arrangement of the second area 23h by means of the sub-areas without slits 25.
The distance between the slit arrangements of the first area 23a and the
second
area 23b is thereby selected in such a way that well-defined, distinguishable,
pim-
ple-like protrusions can form for the purposes of fixing the apparatus for
prevent-
10 ing incontinence 1. In this way a pimple-like, curved surface structure
forms along
the first oblong guide element which guarantees a firm seat for the apparatus
at
the implantation site. In the process, the protrusions 7 are tissue-
protectively ex-
panded outside as rounded-off wedges.
15 Since the material from which the first oblong guide element 5 is formed
is a form
of memory nickel and titanium alloy as available commercially under the brand
name of Nitinol , and since special conditions therefore exist for material
proc-
essing, these will be elucidated here in more detail. Nitinol features a
nickel
content of some 50% and is up to 8% pseudo-elastically malleable.
For the processing of such a nickel-titanium form memory alloy it has proved
to
be especially propitious to cut the slits 23 in the first pipe-shaped oblong
guide
element by means of laser technology. Amongst available laser techniques, spe-
cial mention should in turn be made of cold material processing with which cut-
ting with femtosecond laser pulses can be performed. Such a so-called cold
mate-
rial processing is possible with the StarFemto laser from ROFIN-BAASEL La-
sertech GmbH & Co. KG, Starnberg, Germany. In addition, reference is made to
the well-known technology of laser etching with which slits 23 can also be cut
in
Nitinol .
On the basis of the selected material for a form memory alloy for the pipe-
like
first oblong guide element 5 it is possible to provide a shape curved in the
axis
CA 02821996 2013-06-17
21
which makes it possible to take account of the local anatomical circumstances
in
the area of the urethra, i.e. the implantation site. Two variants must be
differenti-
ated. On the one hand, the first oblong guide element 5 can be formed to the
de-
sired curved shape before implantation in that the slits 23 for the
protrusions 7
feature an unequal length which effect a curve on expansion. The first oblong
guide element 5 can, however, be formed into the desired curved shape in its
ex-
panded state by means of a flexible rod which is preformed to the desired
curve.
This is even possible after implantation, since the first oblong guide element
5 is
formed as a hollow body and the rod can then be bent and formed outside and
then inserted.
Further experiments were performed with slits 23 for the protrusions 7 of
unequal
lengths. Areas 23a and 23b were provided with slits 23 which were cut or
etched
to unequal lengths on opposing sides so that the protrusions 7 extend in a
different
manner on compression, whereby one protrusion 7 is longer than the other oppos-
ing protrusion 7.
Fig. 4 shows an apparatus according to the present invention in a slightly
curved
form which additionally illustrates re-adjustment in situ, i.e. after
implantation.
This is to be dealt with in more detail below.
Re-adjustment of the apparatus 1 according to the present invention can be ex-
plained by means of a comparison of Figures 1 and 4. Fig. 1 and Fig. 4
basically
show one and the same piece of apparatus 1.
The effect of a force still to be described in detail exerted on actuator 9 is
shown
which moves the first oblong guide element 5 and pulls the cage 17 slightly
apart
in the process. The cage 17 is given a more elongated form compared to Fig. 1.
If
already implanted, this means that the pressure on the urethra, in the
immediate
vicinity of which the implant is located, is reduced. The effect exploited
here can
be approximately compared to a spring effect.
This illustrates a significant difference between the effect caused by
adjusting the
balloon fitted as a retaining element in the state of technology compared to
adjust-
CA 02821996 2013-06-17
22
ing the cage according to the present invention. Whilst the balloon according
to
the state of technology is basically filled with a liquid and is, for example,
filled
with more fluid or a comparable medium by means of a hollow needle in order to
enlarge it, or fluid is removed by means of the hollow needle if a reduction
in size
is necessary, adjustment according to the present invention is performed by
modi-
fying an air column. The form of this air column is modified in that a force
is ap-
plied to the actuator 9 which acts on the first oblong guide element 5, moves
it
together with the coupling 11 and thus further elongates the cage 17 ¨ as seen
in
Fig. 4. A space or play 27 is created between the coupling 11 and the threaded
nut
15.
This means that a force is exerted on the first guide element 5 via the
actuator 9
which has the effect of moving the first guide element 5, which in turn acts
on the
cage 17 via the coupling 11 in such a way that it is extended. From comparing
Figures 1 and 4 it is clear that the cage 17 in Fig. 4 features a more
elongated form
than was the case in Fig. 1.
Even if the cage 17, as it is used here, does not differ from the embodiment
in
PCT/EP2010/003757, so that reference can be made to the embodiments therein
to the fullest extent, it is dealt with again in more detail below.
The cage 17 is formed of a fine metal grille 29 shown in Fig. 5 as it has, for
ex-
ample, recently become known and is used for catheter procedures with metal
grille stents in the area of the carotid. The metal grille 29 is thereby
formed as a
diamond pattern, as it is to feature capacity for a necessary extension if the
cage
17 is expanded. This means that due to the form of the diamond pattern, use
can
be made of the as such non-expandable but extremely robust material for an em-
bodiment in which expansion ¨ the diamonds are then extended ¨ and contraction
¨ by reducing the diagonal distances within the diamonds ¨ is made possible.
The
metal material is thus made flexible in this way.
CA 02821996 2013-06-17
23
Fig. 5 shows a sample cut-out of the cage 17 in non-expanded form with the
entire
apparatus 1 in non-expanded form shown once again adjacent to it. This
depiction
shows that the diamond-shaped grille structure is contracted together to slits
which extend along the length of the flexible tube 3. Experiments have shown
that
the effects of stress on the material can be optimised still further if the
slits and
thus the diamonds of the metal grille 29 are of differing sizes.
The metal grille 29 of the cage 17 is additionally coated air- and liquid-
tight with
plastic. In the embodiment in question this is achieved by a PTFE sleeve 31
which
can be seen in Fig. 6 being pulled over the metal grille 29 and held in place
by a
marker tape attached to both sides of the sleeve and a clamp connection 33,
shown
in more detail in Fig. 6. The liquid-tight seal can, however, also be achieved
by
means of a silicone coating or a coating of the metal cage 17 with a
comparable
plastic.
The apparatus for preventing incontinence 1 according to the present invention
as
shown in Figs. 1 and 4 and described in more detail here represents a re-
adjustable
apparatus 1 according to the present invention. Re-adjustment can be performed
in
different ways. In the simplest case, the first oblong guide element 5 is
moved
back and forth manually so that the coupling 11 is moved and the cage 17 ex-
panded or contracted.
In a further embodiment, the part of the apparatus 1 described here features a
mo-
tor drive. The motor itself is not shown in Fig. 1.
Since the actuator 11 is a part of the apparatus for preventing incontinence
accord-
ing to the present invention which differs from the embodiments disclosed in
PCT/EP2010/003757 this is to be explained in more detail below.
The flexible shaft inserted into the flexible tube of the apparatus for
preventing
incontinence as per PCT/EP2010/003757 ensures by means of a rotary movement
that a force is exerted on the threaded rod situated inside the cage by means
of a
CA 02821996 2013-06-17
24
coupling at the opposing distal end. This can then be expanded or contracted
lengthways.
In contrast, the first oblong guide element 5 is not turned according to the
present
invention but performs a linear movement in an axial direction. To achieve
this,
the actuator 11 has a different design compared to PCT/EP2010/003757.
The first oblong guide element 5 is fed through the actuator 11 at its distal
end so
that it projects with a section 3 in a distal position, i.e. on the outside.
In the area
in which the actuator 11 is fed through, the first oblong guide element
features no
slits 23 which form the protrusions 7. In this area it is, however, fitted
with oblong
noses 37 which are formed on the surface and diametrically opposed to one an-
other in such a way that they engage with almost complementary recesses 39
which are worked into the actuator 11. These recesses 39 are only insofar non-
complementary to the noses 37 as these protrude in their length when regarded
from an axial direction. Space to move or play 41 is thus created in an axial
direc-
tion which generally permits the limited adjustment movements which can be
necessary for the precise adjustment of the cage 17. It is unimportant whether
this
adjustment of the cage 17 is done manually or by means of a motor coupled to
the
section 35 which guarantees precise fine adjustment.
The section 35 of the first oblong guide element protruding past the actuator
11 in
a distal position is fitted with a thread 43 on which a nut 45 is arranged.
The first
oblong guide element 5 is held in place by the coupling 11 and this nut 45 at-
tached to the actuator 9.
If the necessity of adjusting or re-adjusting the apparatus according to the
present
invention consists of compressing cage 17 more strongly the nut 45 must first
be
loosened slightly. The first oblong guide element 5 is inserted further into
the
flexible tube which is made possible by the play 41 in the recesses 39 on the
ac-
tuator 9. This movement is transferred via the coupling 11 to the cage 17. The
only limited play 41 in the recesses 39 on the actuator 9 simultaneously
serves to
ensure that unintentional stronger compression over and above what is
acceptable
is prevented. The nut 45 must possibly be slightly retightened after the cage
17
CA 02821996 2013-06-17
has been finally adjusted. This adjustment is possible manually and by means
of a
motor.
If the cage 17 is to be stretched, the first oblong guide element 5 is
carefully re-
5 moved from the actuator. Here also, the only limited play 41 in the
recesses 39 on
the actuator 9 prevents expansion of the cage 17 over and above what is accept-
able. In this case, the nut 45 has slight clearance after complete adjustment
of the
cage 17 and must be tightened accordingly. This adjustment is also possible
manually or motor-driven.
10 A closing cap 47 is fitted on to the distal end of the apparatus 1 and
thus secures it
additionally. If necessary, the locking cap 47 features a socket 49 in its lid
on the
inside for the intervention of the distal end of the first oblong guide
element 5
including the nut 45.
15 Apart from this arrangement of the actuator 9 and its interaction with
the distal
end of the first oblong guide element 5 which has been explained here as an ex-
ample, further designs are possible which guarantee secure adjustment of the
ap-
paratus.
Implantation of the apparatus according to the present invention in a body
lumen in an initial variant
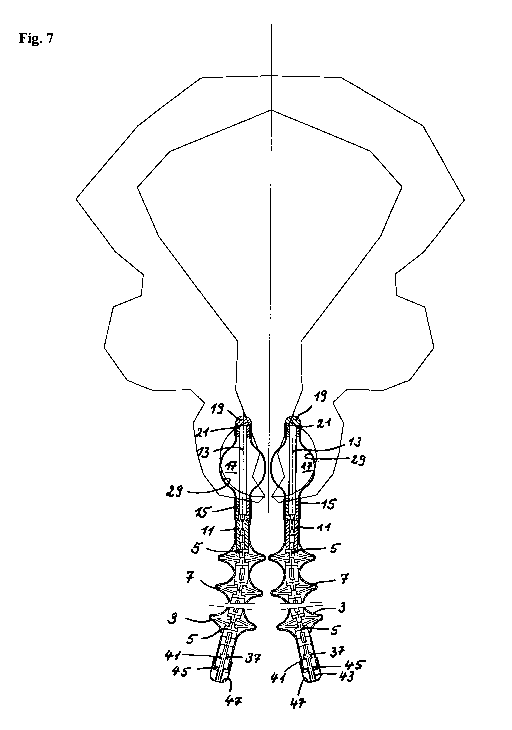
Fig. 7 shows the implantation of the adjustable apparatus for preventing
inconti-
nence 1 according to the present invention in a body lumen. Two parts of the
ap-
paratus 1 are implanted opposite one another in such a manner that they hold
the
urethra of a patient suffering from incontinence centrally between them. The
cage
17 in the embodiment described in the first embodiment above is filled with
air.
Just minimally invasive surgery is required to implant the two parts of
apparatus
1.
CA 02821996 2013-06-17
26
If the two parts of the apparatus are, for example, used in a male patient
following
a prostatectomy they are implanted directly in the vicinity of the operated
urethra.
If the two parts of the apparatus are, for example, used in a female patient
follow-
ing a hysterectomy, they are implanted directly in the vicinity of the
bladder.
The circumference of the cages 17 is chosen so that perfectly normal urination
with normal bladder pressure is neither impaired nor partly or completely
disabled
when the parts of the apparatus 17 are in their final, i.e. adjusted position.
The air
columns in both cages 17 and their position next to the urethra are adjusted
re-
spectively by means of the first guide element 5 proximally connected to the
cou-
pling 11 so that only indiscriminate urination, such as in the case of stress
inconti-
nence, is prevented. This can occur if the patient coughs, sneezes or has to
make
other comparable jerky movements. Lifting weights can also cause stress
inconti-
nence.
The cages 17 are precisely adjusted by means of the two different adjustment
processes already described above, i.e. either manually or motor-driven. In
both
cases, the coupling 11 is moved back and forth over the first oblong guide
element
5 as required and the cage 17 is expanded or contracted in a very simple
manner.
Depending on the embodiment of the first oblong guide element 5, this movement
may also cause the protrusions 7 in relation to angle a, which form their
shoul-
ders, to extend somewhat as angle a is enlarged or compress somewhat if angle
a
becomes sharper so that they protrude further outwards. The movements required
to adjust the size and extension of the cage 17 are in fact very small. The
same
applies to modifications of angle a of the shoulders of the protrusions 7.
For manual operation, the nut 45 offers a good contact surface for a spanner
of the
correct size. The nut 45 can be inter alia a hexagonal or rectangular nut. A
univer-
sal spanner can be used.
In case of motor-driven operation, a motor is connected to the apparatus 1 in
the
vicinity of the actuator 9 via the thread 43 of section 29, i.e. the distal
end of the
first oblong guide element protruding from the actuator 9. The first oblong
guide
CA 02821996 2013-06-17
27
element is then moved backward or forward as required with the coupling 11 by
the motor and the cage 21 is extended or compressed in a simple manner as al-
ready described above. The first oblong guide element 5 is then driven
directly by
means of the motor.
Magnetic valves which interact with an electro-magnet, cantilevers or piezo-
actuator elements have all proved equally suitable for precise fine
adjustment,
whereby the latter are especially sensitive and permit especially good fine
adjust-
ment.
The procedure for adjusting both parts of the apparatus according to the
present
invention is as follows: the parts are inserted in a minimally invasive
surgical pro-
cedure and the wound initially left open for at least one day, which is not a
prob-
lem in terms of minimally invasive surgery. The connection for the motor is im-
planted at the same time if required.
After both parts of the apparatus 1 have been reliably adjusted the nut 45 can
be
firmly aligned, the motor connection removed if necessary, the end cap 47
fitted
and the wound closed.
2. Embodiment: actively operable, adjustable apparatus
Figures 8a to 8c show a further embodiment of the apparatus for preventing in-
continence according to the present invention which is and remains actively
oper-
able after implantation. These are the same components as in the first
embodiment
but with reference numbers offset by 100. The apparatus according to the
present
invention is accordingly designated the number 101.
The apparatus 101 again features a pipe-shaped, flexible tube 103 which is
formed
of a biologically compatible plastic or general polymer so that it can be
implanted
in the human body. Reference is made to the first embodiment in this respect.
CA 02821996 2013-06-17
28
In order to ensure it retains its shape, the flexible tube 103 features the
first oblong
guide element 105 on which the protrusions 107 are formed in the manner
already
described. The first oblong guide element 105 is formed as a toothpick and
ends at
the distal end, i.e. the opposite end of the flexible tube 103 to the centre
of the
respective body in case of implantation in the actuator 109, which is formed
as a
an adjustment device in this embodiment of the present invention which the pa-
tient in the vicinity of whose urethra the apparatus is implanted or the
doctor treat-
ing the patient can operate post-operatively to re-adjust the apparatus.
The toothpick ends at its proximal end, i.e. the end facing towards the body,
via
the coupling 11 in the threaded rod 13, which runs through the cage 17 and is
re-
tained at the proximal end of the flexible tube 3 by means of a threaded nut
15 as
shown in detail in Fig. 6.
The transition from the flexible tube 3 to the cage 17 has already been
described
in detail in the first embodiment to which reference is briefly made here. The
seal-
ing plug 19 closes the cage 17 air- and liquid-tight again and at the same
time
serves as an axial bearing 21 for the threaded rod.
In this embodiment, the plastic used to additionally cover the metal grille 29
of
the cage 17 to make it air- and liquid-tight as described in more detail in
embodi-
ment 1 is a PTFE sleeve 31, which is pulled over the metal grille 29 and held
in
place by a marker tape attached to both sides of the sleeve and a clamp
connection
33. The air- and liquid-tight seal can, however, also be achieved by means of
sili-
cone coating, for example, or a coating of the metal cage 17 with a comparable
plastic.
The actuator 109 formed as an adjustment device for the apparatus for
preventing
incontinence 101 according to the present invention is described in more
detail in
the following as it is shown in Figures 8a ¨ 8c.
The actuator 109 formed as an active adjustment device is retained with its
proximal end in the flexible tube at the distal end of the oblong guide
element
103. Marker tape attached to the outer circumference of the tube and a clamp
con-
CA 02821996 2013-06-17
29
nection 133 serves to retain the adjustment device. The aforementioned
proximal
end of the actuator 109 forms a guide 150 which accommodates the distal end,
i.e.
the end furthest from the body of the first oblong guide element 105 formed as
a
toothpick and thus acts as its bearing.
The actuator 109 according to this embodiment also features an outer sleeve
151
which acts as a connection sleeve from the guide 150 with a piston 153 and is
screwed to the guide 150 with screws 155, normally using 0-rings. The pre-
tensioning of the cage 117 can be controlled axially via the screws 155.
The piston 153 is connected to a fastener 159 by means of a thread 157 which
accommodates the toothpick 105 in the vicinity of its distal end.
A generous amount of play A is provided between the guide 150 and the fastener
159 which is bridged by return springs 161 and defines the path of the
possible
piston stroke.
If the piston 153 moves towards the cage, an end stop 163 inside the outer
sleeve
151 limits the piston stroke in this direction. A pin inserted into the recess
desig-
nated with the reference number 165 is provided to limit the piston stroke in
the
other direction and interacts with a corresponding protrusion 167 on the outer
cir-
cumference of the piston 153 in that the pin forms an end stop for the
protrusion
167. This prevents the guide 150 for the toothpick 105 in the actuator 109
which
is connected to the piston 153 by a threaded connection from being pulled out
of
the outer sleeve 151 completely. Furthermore, sealing elements 171 ensure
suffi-
cient segregation of the inside of the actuator 109 from the outside.
A groove-shaped recess 169 is provided on the distal limit of the guide 150
con-
nected to the piston 153 and thus accessible from outside which serves as an
ad-
justing screw in order to be able to influence the pre-tensioning of the cage
manu-
ally by means of a screwdriver.
Alternatively, with this embodiment a motor can be connected to the apparatus
101 by means of this distal area of the actuator 109 instead of manual post-
operative adjustment by the patient themselves or by the doctor treating them.
CA 02821996 2013-06-17
The air column formed in the cage 117 which features the customary air
pressure
in this further, already described embodiment is subject to air pressure
fluctua-
tions. Longer test series have shown that these air pressure fluctuations
normally
5 only produce minor changes to the air column in the cage. Extreme or more
ex-
treme conditions such as air pressure changes when flying or at altitudes
above
2000 metres can definitely cause a change.
Movement, inclination and/or pressure sensors have been additionally combined
10 with the apparatus according to the present invention. All three sensor
types men-
tioned are already known in the field of medical technology and are used in
vari-
ous applications. Their benefit is that they are attached externally and
therefore do
not place further strain on the implantation site.
Optional placement of the sensors as required was also included in the
preliminary
15 tests. The use of just a pressure sensor has proven sufficient when
flying in accor-
dance with the preliminary tests. Under normal use it can be very convenient
to
use an additional movement sensor which, for example, takes into account the
patient's resting phases and prevents re-adjustment in this time in which no
sig-
nals are transmitted to the pressure sensor. The coupling of the sensors with
one
20 another is possible for a specialist in the art of current control
engineering so that
it does not need to be specifically mentioned in detail here.
Fully automatic re-adjustment is possible accordingly. Steps should in fact be
taken to provide this so that it is not the patient who acts as an untrained
operator
25 of the apparatus but an automatic device which finely adjusts via the
motor and
the adjusting screw 169. Regular checks by the attending doctor then round off
the
adjustment in a medically acceptable manner.
With the (partial) apparatus for preventing incontinence mentioned so far in
one
30 of its variants, the protrusions formed on the first oblong guide
element were dis-
placed relative to one another by an angle of approximately 90 respectively.
CA 02821996 2013-06-17
31
In contrast, Fig. 9 illustrates a first guide element on which the protrusions
are
formed in such a way that they are displaced at an angle of approximately 45
to
one another respectively.
Both variants, an axial displacement of the protrusions to one another at an
angle
of 45 or 90 , both have their advantages depending on the anamnesis and the
nature of the implantation site, so it should be weighed up as to whether a
more
pronounced extrusion of the protrusions or maximum tissue protection is to be
achieved.