Note: Descriptions are shown in the official language in which they were submitted.
CA 02822801 2013-08-02
64166-43D1
METHODS, SYSTEMS AND DEVICES FOR CARDIAC VALVE REPAIR
This is a divisional of Canadian National Phase Patent Application Serial No.
2,597,066
filed on February 7, 2006.
BACKGROUND
[0001] The present invention relates generally to medical methods, devices,
= and systems. In particular, the present invention relates to methods,
devices, and
systems for the endovascuiar or minimally invasive surgical repair of the
atrioventricular valves of the heart, particularly the mitral valve.
[0002] Mitral valve regurgitation Is characterized by retrograde flow from the
left ventricle of .a heart through an incompetent mitral valve into the left
atrium.
During a normal cycle of heart contraction (systole), the mitral valve acts as
a check
valve to prevent flow of oxygenated blood back Into the left atrium. In this
way, the
oxygenated blood is pumped into the aorta through the aortic valve.
Regurgitation of =
=
the valve can significantly decrease the pumping efficiency of the heart,
placing the
patient at risk of severe, progressive heart failure.
[0003] Mital valve regurgitation can result from a number of different
mechanical defects in the mitral valve. The valve leaflets, the valve chordae
which
connect the leaflets to the papillary muscles, or the papillary muscles
themselves
may be damaged or otherwise dysfunctional. Commonly, the valve annulus may be
damaged, dilated, or weakened limiting the ability of the mital valve to close
adequately against the high pressures of the left ventricle. In some cases the
mitral
valve leaflets detach from the chordae tendinae, the structure that tethers
them to
the ventricular wail so that they are positioned to coapt or close against the
other
valve leaflet during systole. In this case, the leaflet "flails or billows
into the left
atrium during systole instead of coapting or sealing against the neighboring
leaflet
1
CA 02822801 2013-08-02
64166-43D1
alloWihgbli56dItom tife ventricle to surge into the left atrium -during
systole. In
addition, rhitrat valve disease can include functional mitrel valve disease
which is
usually characterized by the failure of the mital valve leaflets to-coapt due
to an
enlarged ventricle, or other impediment to the leaflets rising up far enough
toward
each other to close the gap or seal against each other during systole.
100041 The most common treatments for Mita( valve regurgitation rely on
valve replacement or strengthening of the valve anntilus by implanting a
mechanical
Support ring or other structure. The latter is generally referred to as valve
= annuloplasty. A recent technique for mitrel valve repair which relies on
suturing .
adjacent segments of the opposed valve leaflets together is referred to as the
"bow-
tie" or "edge-to-edge" technique; While all these techniques can be very
effective,
they usually rely on open heart siargery where the patient's chest is opened,
typically
via a stemotomy, and the patient placed on cardiopulmonary bypass. The need to
both open the chest and place the patient on bypass is traumatic and has
associated.
=
morbidity.
SUMMARY
[0005] . For the foregoing reasons, it would be desirable to provide
alternative and additional methods, devices, and systems forperforming the
repair of
mitral and other cardiac valVes, inclUding the tricUtpid Valve; Whithit-the
other
- atrioventricular valve. In some embodiments of the present
invention, methods and
devices may be deployed directly into the heart chambers via a trans-thoracic
approach, utilizing a small incision in the chest wall, or the placement of a
cannula or
a port. In other embodiments, such methods, devices, and systems may not
require
open chest access and be capable of being performed endovascularly, i.e.,
using
devices which are advanced to the heart from a point in the patient's
vasculature
2
CA 02822801 2013-08-02
64166-43D1
remote from the heart. In some.embodiments, the methods, devices, and systems
should not require that the heart be bypassed, although the methods, devices,
and
systems should be useful with patients who are bypassed and/or whose heart may
be
temporarily stopped by drugs or other techniques.
=
[0005a] According to one aspect of the present invention, there is provided
a
system for treating a heart, comprising: a first anchor that attaches to a
first location
of a wall of a left ventricle of the, heart; a second anchor that attaches to
a second
location of the heart, wherein the second location is located opposite the
first location;
and a mechanism that moves the first anchor and the second anchor toward one
another to cause the first location and second location to move toward one
another
so as to re-shape at least one of the mitral valve annulus or the left
ventricle in a
manner that reduces backflow through the mitral valve, wherein the mechanism
comprises a tether that interconnects the first and second anchors.
= [0005b] According to another aspect of the present
invention, there is provided
a system for treating a heart, comprising: a steerable delivery device adapted
to be
percutaneously introduced into the heart; a valve removably coupled to the
steerable
delivery device, wherein the valve is adapted for placement in a pulmonary
vein of
the heart such that the valve regulates blood flow into the left ventricle of
the heart
when positioned in the pulmonary vein.
[0005c] According to another aspect of the present invention, there is
provided
a device for treating a heart, comprising: a steerable delivery device adapted
to be
percutaneously introduced into the heart; a first wedge-shaped device
removably
coupled to the steerable delivery device, wherein the first wedge-shaped
device has
a contact surface adapted to be positioned adjacent a first mitral valve
leaflet of the
heart.
=
[0006] In another aspect, there is disclosed a method of treating a heart,
comprising
attaching a first device to a first location of a wall of a left ventricle of
the heart
attaching a second device to a second location of the heart, wherein the
second
3
CA 02822801 2013-08-02
64166-43D1
location is located opposite the first location; and moving the first device
and the
second device toward one another to cause the first location and second
location to
move toward one another so as to re-shape at least one of the mitral valve
annulus or
the left ventricle in a manner that reduces backfiow through the mitral valve.
[0007] In another aspect, there is disclosed a method of treating a heart,
comprising
coupling at least one valve to a steerable delivery device; percutaneously
introducing
the valve into the heart using the steerable delivery device; and placing the
valve in a
pulmonary vein of the heart, wherein the valve regulates blood flow into the
left
* ventricle of the heart.
[0008] In another aspect, there is disclosed a method of treating a heart,
comprising
coupling a first wedge-shaped device to a steerable delivery device, wherein
the first
device has a contact surface adapted to be positioned adjacent a first mitral
valve
leaflet of the heart; percutaneously introducing the first device into the
heart using the
steerable delivery device; and securing the first device to the
3a
CA 02822801 2013-08-02
64166-43D1
head sudh filet' the first mitral valve leaflet iscositioned adjacent the
contact surface
of the device.
[0009] In another aspect, there is disclosed a device for
treating heart
disease comprising a prosthetic comprising a wedge having a length that is
about
= equal to a length of a line of coaptation of a mitral valve and a depth
sufficient to
=
- prevent prolapse of a mitral valve when the wedge is placed atop an annulus
of the
mitral valve along the line of coaptation; and One or More anchors protruding
from
the wedge for coupling the wedge to the annulus of the mitre! valve..
[0010] Other features and advantages should be apparent from
the
= following description of various embodiments, which illustrate, by way of
example,
the principles of the disclosure.
=
BRIEF DESCRIPTION OF THE DRAWINGS
=
[0011] Figure IA is a schematic illustration of the left
ventricle of a heart =
=
showing blood flow during systole with arrows.
[0012] Figure 1B shows a cross-sectional view of the heart
wherein a
flexible stent is positioned at or near the mitre! valve.
[0013] Figure 2A shows a cross-sectional view of the heart
showing one or
- more magnets positioned around the_annulus_of the mitral valve.
. [0014] Figure 2B shows an annular band with magnets that can
be
positioned on the mitral valve annulus.
[0015] Figure 3 shows a cross-sectional view of the heart
identifying
locations for placement of valves.
[0016] Figure 4 show a cross-sectional view of the heart
with a pair of flaps
mounted at or near the mitral valve.
4
CA 02822801 2013-08-02
64166-43D1
.[0017] figure 5A shows a schematic side view of the mitral valve leaflets
with a flaizt positioned immediately below each leaflet.
10018] Figure 5B shows a downward view of the mitral valve with a pair of.
exemplary flaps superimposed over the leaflets.
[0019] Figure 5C shows a pair of mitral valve leaflet flaps having
Complementary shapes.
[0020] Figure 6A shows a cress-sectional view of the heart with a
membrane ring positioned at the mitrel valve annulus.
=
=
= = 100211
Figure -6B shows a schematic view of the membrane ring, which . =
- includes an annular ring on which is mounted a membrane.
100221 Figure 7A shows a cross-sectional view of a heart with a bladder
.V device positioned partially within the left ventricle and partially
within the left atrium.
. [0023] Figure 7B shows a schematic side view of the mitral valve leaflets
failing to coapt.
[0024] Figure 7-C shows a schematic.side view of a the mitral valve
leaflets
with a bladder positioned between the leaflets.
[0025] Figure 7D shows a plan view of the mitral valve with the leaflets in
an abnormal closure state such that a gap is present between the leaflets.
[0026] Figure 8 shows a cross-sectional view of the heart wherein a one-
ay Nial-Ve-&iiZWIS fdtédihthè leff atrium.
.[0027] Figufe .9A shOWs a prosthetic ring that is sized to fit within a
mitral
valve.
[0028] 'Figure 9B shows another embodiment of a prosthetic ring wherein a
one-way valve is positioned inside the ring.
[0029] Figure 10 shows a prosthetic with one or more tongues or
flaps that
are configured to be positioned adjacent the flaps of the mitral valve
=
CA 02822801 2013-08-02
64166-43D1
[00301 figure 11A shows an exemplary embodiment-of one or
more clips - =
that are positioned on free edges of the leaflets.
[0031] Figure 11B shows pair of leaflets with a magnetic
clip attached to the
underside of each leaflet.
[0032] Figure 11C shows the leaflets coapted-as a result
of the magnetic
=
attraction between the magnetic clips.
= [0033] Figure 11D shows a pair of leaflets with a single clip
attached to one=
of the leaflets.
= [0034] figure 12 shows a schematic, cross-
sectional view of the heart with
a wedge positioned below at least one of the leaflets of the mitral valve.
. .
[0035] Figure 13A shows an artificial Chordae tendon.
= [0036] Figures 13B and 13C show attachment devices for attaching
the
artificial chordae tendon to a heart Wall.
[0037] Figure 14 shows a cross-sectional view of the
heart with a first and
second anchor attached to a wall of the heart.
[0038] Figure 15 shows a catheter that has been
introduced into the heart.
[0039] Figure 16 shows a schematic view of a papillary
muscle with a ring
positioned over the muscle.
[0040] Figure 17 shows a cross-sectional view of the
heart with one or
more magnets attached to a wall of the left ventricle.
=
100411 Figure 18A shows another embodiment of a procedure
wherein
magnets are implanted in the heart to geometrically reshape the annulus or the
left
ventricle.
[0042] Figure 1813 shows the heart wherein tethered
magnets are implanted
in various locations to geometrically reshape the annulus or the left
ventricle.
6
CA 02822801 2013-08-02
64166-43D1
[0043] Figure 18C shows the heart wherein magnets are implanted in
various locations to geometrically reshape the annulus or the left ventricle."
.=
[0044] Figure 19 shows another embodiment of a procedure wherein
magnets are implanted in the heart to geometrically reshape the annulus or the
left
ventricle. =
[0045] Figure 20 shows a cross-sectional view of the left ventricle
with a
tether positioned therein.
. .
[0046] Figure 21 shows a cross-sectional view of the left ventricle
with a
delivery catheter positioned therein.
=
[0047] Figure 22 shows a cross-sectional view of the left
ventricle with the
delivery catheter penetrating a wall of the left ventricle.
=
=[00481 . Figure 23"shows a cross-sectional.view -of the left ventricle with
the
=
delivery catheter delivering a patch to the wall of the left ventricle. =
[0049] Figure 24 shows a cross-sectional view of the 'left
ventricle with the =
delivery penetrating delivering a second patch.
[0050] Figure 25 shows a cross-sectional view of the left
ventricle-with two
tethers attached together at opposite ends from the patches mounted in the
heart.
[0051] Figure 26 shows a cross-sectional view of the left
ventricle with a
needle or delivery catheter passed transthoracicaly into the left ventricle LV
to deliver
a patch to the exterior of the ventricular wall.
- [0052]= Figure 27 shows a schematic, cross-sectional view of the left
ventricle in a healthy state with the mitral valve closed.
[0053] Figure 28 shows the left ventricle in a dysfunctional
state.
[0054] Figure 29 shows the left ventricle with a biasing member
mounted
between the papillary muscles.
7
CA 02822801 2013-08-02
64166-43D1
[0055] Figure 30 shows the left ventricle with a suture mounted
between = =
the papillary muscles. . .
[0056] Figure 31 shows the left ventricle with a snare
positioned around the
-chordae at or near the location where the chordae attach with the papillary
muscles.
[0057] Figure 32 shows a leaflet grasping device that is
configured to grasp
= arid secure the leaflets of the mitral valve.
= [0058] Figures 33A-33C show the leaflet grasping device grasping
leaflets
of the mitre! valve.
[00591 Figure 34 shows the left ventricle with a needle being
advanced from
- the leftatrium into the left ventricle via the leaflet grasping
device.
[0060] Figure 35 shows the left ventricle with sutures holding
the papillary
muscles in a desired position.
[0061] Figure 36 shows a croas-sectionalview of the heart with
one or
more clips. clipped to each of the papillary muscles.
[0062] Figure 37 shows a cross-sectional view of the heart with
tethered
dips attached to opposed walls of the left ventricle.
DETAILED DESCRIPTION
[0063] The present invention provides methods, systems, and
devices for
the endovascular repair of cardiac valves, particularly the atrioventricular
valves
which inhibit back flow of blood from a heart ventricle during contraction
(systole),
most particularly the mitral valve between the left atrium and the left
ventricle. By
"endovascular," it is meant that the procedure(s) of the present invention are
performed with interventional tools, guides and supporting catheters and other
equipment introduced to the heart chambers from the patient's arterial or
venous
vasculature remote from the heart. The interventional tools and other
equipment may
8
CA 02822801 2013-08-02
64166-43D1
be introduced percutaneously, i.e., through an access sheath,:or maybe
introduced - . .=
via a surgical cut down, and then advanced from the remote access-site through
the
vasculature until they reach the heart. Thus, the procedures of the present
invention
will generally not require penetrations made directly through the exterior
heart =
. muscle, i.e., myocardium, although there May be some instances
wherepenetratiohs
will bemade interior to the heart, e.g., through the interatrial septum to
provide fora
desired access route.
= .
[00641 While the procedures of the present invention will usually
be
percutaneous and intravascular, many of the tools will find use in minimally
invasive
and open surgical procedures as well that includes a surgical incision or port
access . .
through the heart wall. In particular, the tools for capturing the valve
leaflets prior to
attachment can find use in virtually any type of procedure for modifying
cardiac valve
function.
[0065] The atrioventricular valves are located at the junctions of
theatria
and their respective ventricles. The atrioventricular valve between the right
atrium
and the right ventricle has three valve leaflets (cusps) and is referred to as
the
tricuspid or right atrioventricular valve. The atrioventricular valve between
the left
atrium and the left ventricle is a bicuspid valve having only two leaflets -
{cusps) and is =
generally referred to as the mita! valve. In both cases, the valve leaflets
are
connected to the base of the atrial chamber in a region referred to as the
valve
annulus, and the valve leaflets extend generally downwardly from the annulus
into
the associated ventricle. In this way, the valve leaflets open during diastole
when the
heart atria fill with blood, allowing the blood to pass into the ventricle.
9
CA 02822801 2013-08-02
64166-43D1
=
100661
During systole, however, the valveteeflets are pushed togetherand
closed to prevent back flow of blood into the atria. The lower ends of the
valve
leaflets are connected through tendon-like tissue structures calledihe
chordae,
which in turn are connected at their lower ends to the papillary muscles.
Interventions according to the present invention may he directed at any one of
the
leaflets, chordae, annulus, or papillary muscles, or combinations thereof. It
will be.
the general purpose of suchinterventionsto modify the manner in Which the
valve
leaflets coapt or close during systole so that back flow or regurgitationts
minimized
=
or prevented.
_
[0067] The
left ventricle LV of a normal heart H in systole Is illustrated In.
Figure 1A. The left ventricle LV is contracting and blood flows outwardly
through the
" tricuspid (aortic) valve AV in the direction of the arrows. Beck floW of
blood or
"regurgitation* through the mitral valve MV is prevented since the mitral
valve is
configured as a "check valve" which prevents back flow when pressure in the
left
ventricle is higher than that in the left atrium LA. The mitral valve IVIV
comprises a
pair of leaflets having free edges FE which meet evenly to close, as
illustrated in
Figure 1A. The opposite ends of the leaflets LE are attached to the
surrounding
heart structure along an annular region referred to as the annulus AN.. The
free
edges FE of the leaflets LF are secured to the lower portions of the left
ventricle LV
_ .
through chordae tendineae CT (referred to hereinafter as the chordae) which
include
plurality of branching tendons secured over the lower surfaces of each of the
valve
leaflets LF. The chordae CT in turn, are attached to the papillary muscles PM
which
extend upwardly from the lower portions of the left ventricle and
interventricular
septum IVS.
CA 02822801 2013-08-02
64166-43D1
=
[00883 While the procedureS of the present Invention .will be most
useful'
with the atrioventricular valves, at least some of the tools described
hereinafter may
be useful in the repair of other cardiac valves, such as peripheral valves or
valves on
the venous side of the cardiac circulation, or the aortic valve.
[0069] The methpds of the present invention can comprise accessing
a
patient's vaSculature at a location remote from the heart, advancing an
interventional
=
toe! through the vasculature to a ventricle and/or atrium, and engaging the
tool
against a fissile structure which forms or supports the atrioventricular
valve. By
engaging the toot against the tissue structure, the tissueistructure is
modified In a
manner that reduces valve leakage or regurgitation during ventricular systole-
. The
tissue structure May be any of one or more of the group consisting of the
valve =
leaflets, chbrdae, the valve annulus, and the papillary muscles, etrial_wall,
ventricular
=
wall or adjacent structures. Optionally, the interventiOnal tool will be
oriented relative
to the atrioventricular valve and/or tissue structure prior to engaging the
tool against
the tissue structure. The interventional tool may beself-orienting (e.g., pre-
shaped) -
or may include active mechanisms to steer, adjust, or otherwise position the
tool.
[0070] Alternatively; orientation of the interventional tool may
be
accomplished in whole or in part using a separate guide catheter, where the
guide
catheter may be pre-shaped and/or include active steering or other positioning
means such as those devices set forth in United States Patent Application
'Serial
Numbers 10/441,753 filed May 19, 2003, 10/441,508 filed May 19, 2003 and
10/441,687 filed May 19, 2003.
In all cases, it will usually be desirable to confirm the position prior to
engaging the valve leaflets or other tissue structures. Such orienting step
may
comprise positioning the tool relative to a line of coaptation in the
atrioventricular
11
CA 02822801 2013-08-02
64166-43D1
valve, e.g., engaging positioning elements in the valve commissures and
confirming
the desired location using a variety of imaging means such as magnetic
resonant
imaging (NIRO, intracardiac echocardiography (ICE), transesophageal echo
(TEE),
fluoroscopy, endoscopy, intravascular ultrasound (IV-US) and the like.
[0071] In some embodiments, heart disease in general, and
valve repair in
== particular, are treated by targeting the pacing of the heartbeat. In one
embodiment,
=
heart disease is treated by introducing one or more pacing leads into a heart
. chamber. The pacing leads are placed in contact with a heart muscle
and are in =
- electrical communication with a power source. The power source
provides paced
= electrical stimuli to the heart muscle. The electrical stimuli are
provided during or
= immediately after systole to extend systolic contraction of the heart,
thereby
= extending the range of systole during each heartbeat. This extension of
systole
extends the amount of time in which the heart muscle tightens when it would
otherwise be relaxing, when there is most mitral regurgitation in diseased
mitral
valves.
[0072] Other embodiments are directed to annuloplasty to
treat heart
disease in general and valve repair in particular. In one embodiment, shown
generally in Figure 1B, a stent is used to treat the mitral valve. Figure 1B
shows a
- -cross-sectional view-of the a flexible stent 100 is positioned
at or near
the mitre! valve MV. The stent 100 is annular and is sized and shaped to be
positioned on the annulus of the mitral valve. The stent 100 can transition
between a
collapsed state of reduced size and an expanded state of enlarged size
relative to
the collapsed state.
12
CA 02822801 2013-08-02
64166-43D1
f0073] = The flexible stent 100 can be percutaneously introduced into an
individual's heart while being biased toward the collapsed state. The stent is
advanced partially through the annulus of the mital valve so that it is
coaxially
positioned within the annulus, as shown in figure 1B. The stent 100 is then
secured
to the annulus such that the stent exerts an inward force on the annulus
thereby
causing the annulus to resist dilation during diastole of the heart.
100741 - In yet another embodiment, a device is disclosed for treating the
mitral valve. The device can be a stent, such as the stent 100, that-is sized
to fit
coaxially within anannulus of a mitral valve. The stent includes a hollow
frame. The
frame can be annular such that it has a cross-sectional diameter that is sized
such
that an outer surface of the frame is in continuous coaxial contact with the
annulus.
The frame also includes one or more anchors protruding from it for securing
the stent
to the annulus. The anchors can be prongs, barbs, protrusions, or any
structure =
adapted to secure the stent to the annulus. The stent is flexible between an
expanded configuration and a contracted configuration and is biased toward the
contracted configuration so that it exerts an inward force on the annulus.
[0075] In one embodiment, the stent 100 is delivered using a
delivery
catheter 10 that is advanced from the inferior vena cave 1VC into the right
atrium RA.
Once the catheter 10 reaches the anterior side of the interatrial septum IAS,
a
needle 12 may be advanced so that it penetrates through the septum at the
fossa
ovalis FO or the foramen ovale into the left atrium LA. At this point, a
delivery device
can be exchanged for the needle and the delivery device used to deliver the
stent
100. The catheter 10 can also approach the heart in other manners.
13
CA 02822801 2013-08-02
64166-43D1
T00761
Figure 2A shows a cross-sectional view of the heart-showing one or
more magnets 210 positioned around the annulus of the mitral valve MV. A
corresponding method of treating heart disease involves the use of magnets.
The ,
method includes percutaneously introducing at least a first magnet 205 into an
individual's heart and securing it to the mitral valve-MV annulus. At least a
second
magnet 210 is perqutaneously introduced into the heart and advanced so that it
is .
. within a magnetic field of the first magnet. The second Magnet is secured to
the
= heart. The polarity of one of the two magnets is then cyclically changed
in
=
=
synchronization with the heart beat so that the magnets attract and repel each
other
-
in synchronization with the heart beat. The first magnet therefore moves in
relation
-
to the second magnet and exerts an inward closing force on the mitral valve
during
=
systole. The magnets 210 can be positioned on an annular band 215 (shown in.
=
Figure 28) that is sized and shaped to be implanted on the annulus of the
mitre!
valve. The band 215 can be, for example, a stent.
[0077]
In one embodiment, the magnets 210 or the annular band 215 are
delivered using a delivery catheter 10 that is advanced from the inferior vena
cave
IVC into the right atrium RA, as described above with reference to Figure 1..
Any of
the devices described herein can be percutaneously delivered into the heart by
coupling the device to a delivery device, such as a steerable delivery-
catheter.
_
_ = - ---- [0078] = In yet another embodiment involving magnets,
two or more magnets
are percutaneously introduced into an individual's coronary sinus such that
they
attract or repel each other to reshape the coronary sinus and an underlying
mitral
valve annulus.
14
CA 02822801 2013-08-02
64166-43D1
= =
=
100791 ' Other embodiments involve various prosthetics for treating heart
disease in general and defective or diseased mitral Valves in particular. = In
one -
embodiment, a method of treatment InCludes placing one or More one4ay valves
in .
one or more pulmonary veins of an individual either near the ostium of the
vein or at
some point along the length of the PV. Valves that may. be used, for example
may .
be stentless valves. such as designs similar to the TORONT-0-SPVD (Stentless
. Porcine Valve) valve, mechanical or tissue heart valves or
percutaneous heart
Valves as are known in the art provided they are sized appropriately to fit
within the
= lumen of the pulmonary vein, as shown In Figure 3. In figUre 3, the
locations in the
left atrium LA where valves can be positioned In pulmonary vein orifices are
= represented by an "X". In addition, certain venous valve devices and
techniques
may be employed such as those described in United States Patent, 6,299,637 and
=
=
6,585,761, and United States Patent Applications 20040215339 and 20050273160.
A Valve.
prosthesis for placement in the ostla of the pulmonary vein from the left
atrium may
be in the range of 6-20mm in diameter. Placement of individual valves in the
pulmonary vein ostia (where the pulmonary veins open or take off from the left
atrium) may be achieved by obtaining trans septet access to the left atrium
with a
stearable catheter, positioning a guidewire through the catheter and into the
targeted
pulmonary vein, and deploying a valve delivery catheter over the guidewire and
=
deploying the valve out of the delivery catheter. The valve may be formed of a
deformable material, such as stainless steel, or Of a self-expanding material
such as
NITI, and include tissue leaflets or leaflets formed of a synthetic material,
such as is
known in the art. A line of +++++ symbols in figure 3 represents a mld-atrial
location
above the mital valve where a single valve can be positioned as disclosed
later in
this specification.
=
CA 02822801 2013-08-02
64166-43D1
100501 The,folloWing references
describe devices such as steerable catheters) and methods for
delivering interventional devices to a target location within a body: United
States
Patent Application Serial Numbers 10/441,753 flied May 19, 2003, 10/441,508
filed
May 19, 2003 and 10/441,687 filed May 19, 2003.
[0081] Figure 4 show a cross-sectional view of the heart with a pair of
flaps
'mounted at or near the mitre! valve. Figure 5A shows a schematic side view of
the
mital valve leaflets LF with a flap 300 positioned Immediately beloweach
leaflet.
The flap 300 can be contoured so as to conform at least approximately to the
shape
of a leaflet, or the flap 300 can be straight as shown In Figure 4. Figure 5B
shows a
downward view of the mitral valve with a pair of exemplary flaps superimposed
over
the leaflets LF. As shown in Figure 5C, the flaps can have complementary
shapes
with a first flap having a protrusion that mates with a corresponding recess
in a
second flap. "
[0082] In corresponding method of treatment, shown in Figures 4
and 5C, a
first flap 300 with an attachment end 305 and a free end 310 is provided. The
attachment end 305 of the first flap 300 is secured to the inside wall of the
ventricle
below the mitral valve. A second flap 315 with an attachment end 320 and a
free
end 330 is provided and is also secured to the inside wall of the ventricle
below the
mitral valve. The first and second flaps 300, 315 are oriented so that they
face each
other and the free ends 310, 330 are biased toward each other and approximate
against each other during systole. This system provides a redundant valving
system
to assist the function of the native mitral valve. .
16
CA 02822801 2013-08-02
64166-43D1 =
[0083] In other embodiments, devices and methods that involve
prosthetic
discs are disclosed. For example, Figure 6A shows a cross-sectional view of
the -
heart with a membrane ring 610 positioned at the mitrel valve annulus. Figure
6B -
shows a schematic view of The membrane ring 610, which includes an annular
ring
on which is mounted a membrane. The membrane includes a series of perforations
= 615 extending through the membrane surface. One or more anchor devices,
such
= as prongs, can be located on the ring for securing the ring to the mitre!
valve.
=[0084] In one embodiment, a device for treating heart disease in general
= and defective or diseased mital valves in particular includes a disc
having a ring, a
membrane Stretched across an opening of the ring, and one or more anchors for
.
.
=
=
securing the disc to an annulus of a mitre! valve. The disc is sized to cover
the
annulus of-the mitral valve, and the Membrane includes one or more
perforations
that permit one way fluid flow through the disc. Methods of treatment using
the
device are also provided.
[0085] In other embodiments, devices and methods that involve
fluid-filled
bladders are disclosed. Figure 7A shows a cross-sectional view of a heart with
a
bladder device positioned partially within the left ventricle and partially
within the left
atrium. A device for treating heart disease in general and defective or
diseased
mitral valves in particular includes a fluid-filled bladder 600. The bladder
600 is
placed across the mitral valve between the left atrium and the left ventricle.
Upon
compression of the left ventricle, the volume of the bladder is expanded on
the left
atrial-side of the heart, providing a baffle or sealing volume to which the
leaflets of
the mital valve coact. The bladder may also act as a blocking device in the
case of
flail of a leaflet, blocking said flailing leaflet from billowing into the
left atrium causing
regurgitation. The bladder also includes one or more anchors for securing the
17
CA 02822801 2013-08-02
=
64166-43D1
bladder to an annulus of a mitral valve, or may be formed on a cage or other
=
infrastructure to position it within the line of coaptation of the mitral
valve.
[0086] A bladder can also be used to treat functional mitral valve disease.
As mentioned, functional mitral valve disease is usually characterized by the
failure
of the mitral valve leaflets to coapt due to an enlarged ventricle, or other
impediment
to the leaflets rising up far enough toward each other to close the gap or se.
al against
each other during systole. Figure 76 shows a schematic side view of the mitral
valve
leaflets LF failing to coapt such that regurgitation can occur (as represented
by the
arrow RF.) With reference to Figure 7C, a baffle or bladder 630 is positioned
=
between the leaflets LF along the line of coaptation of the leaflets. The
bladder630
provides a surface against which at least a portion of the leaflets LF can
seal-
=
against. The bladder 630 thus serves as a coaptation device for the leaflets.
The
bladder can be attached to various locations adjacent to or on the mitral
valve.
Figure 7D shows a plan view of the mitral valve with the leaflets LF in an
abnormal
closure state such that a gap G is present between the leaflets. In.one
embodiment,
the bladder is attached or anchored to the mitral valve at opposite edges E of
the
gap G.
[0087] Methods of treatment using the bladder include providing the
bladder and inserting it through an annulus of a mitral valve such that the
bladder is
coaxially positioned through the mitral valve. An atrial portion of the
bladder extends
into the left atrium, and a ventricular portion of the bladder extends into
the left
ventricle. A mid portion of the bladder may be secured to the annulus of the
mitral
valve such that the mid portion remains stationery while the atrial and
ventricular
portions expand and contract passively between the atrium and ventricle based
on
pressure differentials during systole and diastole.
18
CA 02822801 2013-08-02
64166-43D1
10088] -Figure 8 shows a cross-sectional view of the heart wherein a one- .
=
way valve device 700 is located in the left atrium. The valve device is
represented
schematically in Figure 8. A4orresponding method of treating heart disease
includes introducing a one-way valve device 700 into the left atrium of an
individual's
= heart proximal the mitral valve. The valve device 700 is configured to
permit fluid
flow in one direction while preventing fluid flow in an opposite. direction.
.The valve
device can have various structures. For example, the device can comprise a
valve
that is. mounted on.a stent that issized to be positioned in the left atrium_
Valves that
= may be used, for example may be stentless valves such as the TORONTO SPV0
=
(Sientless Porcine Valve) valve, mechanical or tissue heart valves or
peroutaneous
heart valves as are known in the art. The outer wall of the one-way valve
device is
sealed to the inner wall of the atrium so that a fluid-tight seal is formed
between the.
= outer wall of the one-way valve device and the inner wall of the left
atrium. In this
regard, the valve device can include a seal member that is configured to seal
to the
inner wall of the atrium.
[00891 Another embodiment involves a prosthetic for treating
heart disease
in general and defective or diseased mitral valves in particular. Figure 9A
shows a
prosthetic ring 800 that is sized to fit within a mitral valve annulus The
ring includes
one or more anchors 805 that extend around the periphery of the ring 800. In
. _
addition, one or more struts 810 struts extend across the diameter of the
ring, and
can be made of a material that includes nitinol or magnetic wires for
selectively
adjusting the shape of the ring. The struts can also be instrumental in
baffling mitral
valve leaflet "flair. Figure 9B shows another embodiment of a prosthetic ring
807
wherein a one-way valve 815 is positioned inside the ring such that blood flow
BF
19
CA 02822801 2013-08-02
64166-43D1
can flow through the valve in only one direction. The valve can be
manufactured of
various materials, such as silicone.
100901 Figure 10 shows a prosthetic with one or more tongues or flaps
that
are configured to be positioned adjacent the flaps of the mitral valve. The
prosthetic
includes a ring 900 sized to fit within a mitral valve annulus. At least two
tongues
=
910 project from the ring 900 in a caudal direction when the ring is implanted
into a
heart of an individual. The ring is flexible between an expanded configuration
and a=
=
contracted configuration and is biased toward the contracted configuration.
One or
more anchors 920 protrude from the flexible ring for coupling the ring
coaxially to the
annulus such that the contracted configuration of the ring exerts an inward
force to
= the annulus. Alternatively, or in addition, the two tongues can each have
a length
=
sufficient to prevent prolapse of a mitral valve when the ring is placed atop
the
leaflets of the mitral valve. In a further embodiment the tongue elements may
be
attached at a central point.
100911 In yet another embodiment, a prosthetic for treating heart
disease in
general and a defective or diseased mitral valve in particular includes a
wedge. The
wedge has a length that is about equal to a length of the line of coaptation
of a mitral
valve. The wedge has a depth sufficient to prevent prolapse of a mitral valve
when
the wedge is placed atop an annulus of the mitral valve along the line of
coaptation,
and may provide a point of coaptation for each leaflet. One or more anchors
protrude from the wedge for coupling the wedge to the annulus of the mitral
valve.
Methods of treatment using the wedge are also disclosed. The methods include
inserting the wedge into an individual's heart, placing the wedge lengthwise
along
the line of coaptation of the mitral valve. The wedge is then secured to an
annulus of
CA 02822801 2013-08-02
64166-43D1
the Mitral valve along the line of coaptation. The wedge maybe positioned also
just
=
under one segment of the leaflet (likely P2 in the case of functional MR).
[0092] In yet another embodiment, a device-for treating
heart disease
includes a clip for attachment to a free end of a heart valve leaflet. -Figure
11A
shows an exemplary embodiment of one or more clips 1101 that are positioned on
=
= free edges of the leaflets LF. Each of the clips 1101 has a shape that
prevents flail
of the leaflet by catching against an underside of an opposing leaflet.
Methods of - =
- treatment using the clip are also disclosed. The methods include
introducing the clip
- into an individual's heart and attaching the clip to a free end
of a heart valve leaflet
opposite the free end of an opposing leaflet of the heart valve so that the
clip catches
to the underside of the opposing leaflet during systole. In a further
embodiment, a
clip may be placed on both leaflets such that the clips meet orcatch when the
=
leaflets are in proximity. The clips may attach momentarily during systole,
and then
detach during diastole, or may clip permanently resulting in a double orifice
mitrel.
valve anatomy. The clips of this embodiment may include a magnetic element, or
= one may be magnetic and the other of a metal material attracted to said
electromagnetic field of the magnetic clip.
[0093] In the case of magnetic clips, the clip elements
may be placed on
. . _ .
the underside of the leaflets Kg. not hecessarily on.the free edge of the
leaflet),
-provided that the magnetic field of the clip is sufficient to attract the
opposing
magnetic or metal clip element. This is further described with reference to
'Figure
11B, which shows pair of leaflets LF with a clip 1101 attached to the
underside of
each leaflet. At least one of the clips is magnetic, while the other clip is
of an
opposite magnetic polarity than the first clip or of a metal attracted to the
magnetic
field of the first clip. The magnetic field is sufficiently strong such that
the clips 1101
21
CA 02822801 2013-08-02
64166-43D1
can attach to one another either momentarily or permanently to coapt the
leaflets, as =
shown in Figure 11 C.
[0094] In another embodiment, shown in figure 11D,. a single clip
1101 is
attached to one of the leaflets. The dip 1101 is sufficiently long to increase
the = "
likelihood that the clip 1101 will coapt with the opposite leaflet.
=
[0095] In yet another embodiment, a device for treating heart
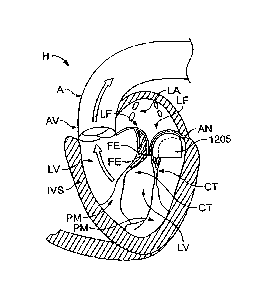
disease
includes a wedge for placement under a heart valve leaflet. figure 12 *Ars a
schematic, cross-sectional view of the heart with a wedge 1205 positioned
below at
least one of the leaflets of the mitral valve. The wedge 1205 can be
positioned
=
below one or both of the leaflets. The wedge 1205 is sized to fit under the
valve
leaflet and caudal the annulus of the heart valve. The wedge 1205 can have a
-
shape that is contoured so as to provide support toa lower surface of the
leaflet. (In
Figure 12, the left atrium is labeled LA and the left ventricle is labeled
LV.) An
anchor is attached to the wedge for coupling the wedge to a wall of the heart
chamber adjacent the heart valve. The wedge forms a fixed backstop against the
bottom side of the heart valve leaflet, thereby providing a location for the
leaflet to
coapt against, andior providing support or "pushing up" a restricted leaflet.
[0096] Other embodiments are directed to altering the size, shape,
chemistry, stiffness, or other physical attributes of heart valve leaflets. In
one
embodiment in particular, a method of treating heart disease includes
obtaining
access to a heart valve leaflet and injecting a stiffening agent into the
leaflet to stiffen
the leaflet and minimize flail.
[0097] Other
embodiments are directed to the chordae that connect heart
valve leaflets to the inner walls of the heart. In one embodiment in
particular, a
22
CA 02822801 2013-08-02
64166-43D1
method of -treating heart disease includes obtaining access to a heart valve
chord = = =
and cutting it mechanically or with energy such as a .laser, or -by heating
the chordae
to elongate them, thereby allowing the previously restricted leaflet to be
less
restricted so that it can coapt with the opposing leaflet.
[0098] . In another embodiment directed to the chordae that connect heart
valve leaflets to the inner walls of the heart, a cam-shaped ring is
disclosed. The
cam-shaped ring is sized to fit within a left ventricle of a heart. The ring
forms a hole
that is sized to receive two or more chordae tendineae. The ring is formed by
= =
= connecting two detachable ends of the ring.
[0099] Methods of treatment using the cam-shaped ring are also
disclosed.
One method in particular includes introducing the ring into a left.ventridle
of a heart.
One or more chordae tendineae. are then surrounded by the ring, and the.
twoends.
of the ring are then attached to form a closed ring around the chordae
tendineae.
The ring is then rotated such that one or more of the chordae tendineae are
shifted
away from their initial orientation by the rotation of the cam-shaped ring.
The ring
may then be fixed in the rotated or tightened position_
= [01001 An embodiment directed at the chordae of heart valve
leaflets is now
described. Figure 13A shows a device that can be used to alter a chordae. A
= method includes obtaining access to a chordae tendinea (chord) within an
individual's heart chamber. The chordae is then cut at a point along its
length so that
a length of the chorda tendinea is freed from the heart chamber leaving behind
a
length of chorda tendinea having a free end and an end attached to an edge of
a
heart valve.
23
CA 02822801 2013-08-02
64166-43D1
10101) With reference to Figure 13A, a synthetic chord -
1005 of greater -
. length than the free length of chordae is introduced into the heartzhamber.
One-end
of the synthetic chordae 1005 is connected to a wall 1305 of the heart chamber
or-to.
a muscle attached to the wall of the heart chamber. Another end of the
synthetic
chord is attached to the free end of the chorda tendinea or to the leaflet.
[0102] In this regard, the. end of the chord 1005 that is
attached the wall= =
1305 can have any of a variety of devices that facilitate such attachment.
'Figures =
- 13B and 13C show enlarged views of attachment devices-contained
within box 13 of =
Figure 13A. The attachment devices can be used to attach the chord 1005 to the
wal11305. In Figure 13B, the attachment device 1310 is an enlarged ball having
a
distal trocar for penetrating the wall 1305. In Figure 13C, the attachment
device
= 1310 is a hook that is configured to penetrate through the wall 1305. It
should be =
=
appreciated that the attachment device 1310 can have other structures and it
not
limited to the structures shown in Figures 138 and 13C. In variations of these
embodiments, it may be advantageous to adjust the length of the chordae
(synthetic,
or modified), determine the therapeutic effect of the shortening or
lengthening, and
then fix the chordae at the most efficacious location.
[0103] Other embodiments are directed to atrial or
ventricular remodeling to
alter the shape of an atrium or ventricle. Figure 14 shows a cross-sectional
view of
the heart with a first and second anchor attached to a wall of the heart. The
system
includes a first anchor 1410a having a screw portion 141-5 forscrewing into a
wall of
the heart and a connector portion. The connector portion is rotatable around
an axis
of rotation. The first anchor includes a power source to power rotation of the
connector portion and a receiver for receiving telemetric signals from an
external
controller for controlling the rotation of the connector portion. The system
includes a
24
CA 02822801 2013-08-02
64166-43D1
=
second anchor 1441013 having a screw portion 1415b for screwing into a wall of
the-
heart and a connector-portion. Also included is a tether 1420 having two
freeends. . = =
One of the free ends is coupled to the connector portion of the first anchor,
and the =
other free end is coupled to the connector portion of the second anchor. An
external
controller is also included. The external controller has a telemetric
transmitter for
communicating with the receiver and controls the rotation of the connector
portion.
Alternatively, the anchors may be placed with a torqueable catheter. .
[0164] In another embodiment, a method of altering a
geometry of a heart = = =
includes Introducing a first coupler into a heart chamber. The first coupler
has an = - =
anchor portion and a connector portion. The connector portion is rotatable
around . .
=
an axis of rotation and, is connected to a power source to power rotation Pf
the -
connector portion. The power source is in communication With a telemetric-
Signal
receiver. The first coupler is secured to the wall of the heart chamber by
anchoring
= the anchor portion to the wall: A second coupler is introduced into the
heart'
chamber. The second coupler includes an anchor portion and a connector
portion.
The second coupler is secured to the wall of the heart chamber by anchoring
the
anchor portion to the wall at a distance from the first coupler.
[0105] A tensile member is introduced into the heart
chamber. One end of - =
the tensile member is connected to the connector portion of the first-coupler,
and
another end of the tensile member is connected to the connector portion of the
second coupler. The distance between the first and second couplers is adjusted
by - -
transmitting a telemetric signal to the receiver, thus causing the connector
portion to.
rotate around the axis of rotation and threading the tensile member around the
=
connector portion to reduce the distance between the first and second
couplers.
CA 02822801 2013-08-02
64166-43D1
[01061 In another embodiment, a system for altering the geometry of
a=
heart chamberfacludes a planar tensile member having substantially inelastic
material. At least two anchors are included for anchoring the planar tensile
member
to an inner wall of a heart chamber. The planar tensile member is
substantially
shorter in length than a left ventricle of a heart so that when the planar
tensile
member is anchored in a caudal direction along a length Of the left ventricle
a tensile
force exerted by the planar tensile member between the two anchors -prevents
the
left ventricle from dilating caudally. = . = .
[01071 In another embodiment, a method for altering the geometry of
a _ =
heart includes providing a tensile member having a substantially inelastic
material. .
The tensile member is substantially shorter in length than a left ventricle of
a heart.
The tensile member=fs inserted into the left ventricle of the heart and a
proximal end =
of the tensile member is anchored to the left ventricle adjacent the Mitral
valve. A
= distal end of the tensile member is anchored to the left ventricle caudal
the proximal
end so that a tensile force exerted by the tensile memberbetwaen the two
anchors
prevents the left ventricle from dilating caudally.
. .
[0108] Other embodiments are directed to strengthening or reshaping
the
left ventricle of the heart. In one embodiment in particular, a method of
reinforcing:
the left ventricle includes injecting a strengthening agent into a wall of the
left
ventricle in an enlarged region of the ventricle, as shown in Figure 15:
Figure 15
shows a catheter 1510 that has been introduced into the heart. The Catheter
1510.
has an internal lumen through which the strengthening agent 1512 can be
injected.
A proximal end of the catheter is connected to a source of the strengthening
agent
and a distal end of the catheter is configured to release the strengthening
agent. As
=
26
CA 02822801 2013-08-02
64166-43D1
shown in Figure 15, the distal end of the catheter is-positioned at or near a
wall of =
the heart and the strengthening agent t512 is injected into the wall of the
heart.
[0109] In another embodiment, a method is directed to altering the
geometry of a heart. The method includes injecting a polymerizing agent into a
pericardial space adjacent a left ventricle, thereby exerting a medial
(inward) force
.
.
against the left ventricle.
=
[0110] In yet another embodiment, a method of altering the geometry of a
heart includes inserting a balloon into a pericardial space adjacent to a left
ventricle
-
. .
of the heart, or extend into the pericardium of the heart. The balloon is
inflated by
injecting it with a fluid, and it exerts a medial force against the left
ventricle upon
inflation. In certain embodiments, .the balloon can be inflated at the time of
=
implantation, or at a later time. If inflated at a later time, the balloon
would be .self-
sealing, and may be inflated by accessing the balloon with a needle placed
through
the chest wall.
[01111 Other embodiments are directed to adjusting the length or
orientation of papillary muscles. Figure 16 shows a schematic view of the
heart
showing the papillary muscles PM. With reference to Figure 16, a method of
treating heart disease includes inserting an anchor, cuff or sleeve 1205 into
the left
ventricle of an individual's heart, and sliding a cuff or sleeve around a
papillary
muscle P. The size of the cuff or sleeve is reduced so that the cuff or sleeve
squeezes the papillary muscle. As the size of the cuff or sleeve is reduced,
the
=
papillary muscle stretches and increased in length.
[0112] In yet another embodiment, a method of treating heart
disease
includes obtaining access to a papillary muscle in a left ventricle of the
heart. The
27
CA 02822801 2013-08-02
=
64166-43D1
papillary muscle is cut and reattached at-a new location on an Inner wail of
the . : =
ventricle closer to the mitral valve.
[0113] Additional embodiments that-employ magnets in the heart
are now
described with reference to Figures 17-19, which show cross-sectional views of
the .= .
= heart. With reference to Figure 17, in oneembodiment one or more magnets
1705
are implanted or otherwise attached to a waft 1710 of the left ventricle LV. -
One or
more other magnets 1715 are implanted or otherwise attached to a wall 1720-ef
the
right ventricle. The magnets 1705 and 1715 are attached to the walls 1710 and
_
1720 such that they assert an attractive magnetic force (as represented by the
arrows 1725 in Figure 17) toward each other. The magnetic force 1725 -assists
in .
remodeling of the left ventricle during pumping of the heart. That is, the
Magnets =
=
1705 and 1715 are urged toward one another (thereby also urging the Walla
1710 =
and 1720 toward one another) to re-shape either the annulus AN or the left
ventricle
= LV. The. annulus or the left ventricle LV are re-shaped in a manner that
reduceaar
eliminates backflow through the mitre! valve MV. It should be appreciated that
a
similar procedure can be performed on the right ventricle RV and associated
valves.
[0114] Figure 18A shows another embodiment of a procedure
wherein
magnets are implanted in the heart to geometrically reshape the annulus or the
left
ventricle. One or more magnets 1705-are implanted or otherwise attached to a
first
" --Walt1710a of the left ventricle LV. One or more magnets 1705 are also
implanted or -
otherwise attached to a second, opposed wall 1710b of the left ventricle. The
magnets on the opposed walls 1710a, 171-0b exert an attractive magnetic force
toward one another to draw the wails 1710a, 1710b toward one another and re-
shape the left ventricle LV or the annulus AN.
=
28
CA 02822801 2013-08-02
64166-43D1
[0115] Another embodiment of a procedure uses magnets-to anchor
tethers
within the heart at various locations to optimize the shape of cardiac
structures to
improve cardiac function. The tethers are placed to either reshape the cardiac
structure or to prevent dilatation of the structure over time. The tethers
must be
securely anchored to the heart structures. A method of anchoring which-enables
tethering in various positions and directions within the cardiac structures is
important
for the clinician to optimize cardiac reshaping base' d on each individual
patient
anatomy and disease state. A method of anchoring which is atraumatio is also
desirable.
[0116] Figure
18B shows a side view of the heart with sets of magnets A,
Al, B, and B1 positioned to various locations of the heart or to anatomical
structures
adjacent the heart. In one embodiment, at least one magnet A is placed on the
interventricular septum within the right ventricle RV. At least one magnet Al
is
. placed within the left ventricle LV opposite magnet A. The magnetic force
between A
and Al maintains the position of the magnets. The magnets may be enclosed in
materials that will promote tissue in-growth and healing to the
interventricular septum
to ensure stability of location and to eliminate the need for long term anti-
coagulation. Additionally, the enclosure material which is flexible and can
delivered in a low profile can be significantly larger in size than the
magnets to
increase the surface area of contact with the heart wall which will increase
the
tension that can ultimately be placed on the anchor over time.
= [0117]
A second set of magnets B and B1 are then delivered to another
location selected within or adjacent to the heart. The set of magnets NM are
attached to the set of magnets B/B1 using at least one tether 1805, as shown
in
Figure 18B. The tether 1805 can be attached to either or both of the magnets
A/A1
29
=
CA 02822801 2013-08-02
64166-43D1
=
=
at one end and to either of both of the magnets-B/B1 at an opposite end.
When.the
set of magnets B/B1 are tethered under tension to the set of magnets A/A1, a .
change in the shape of the cardiac structure results to improve-cardiac
function.
Figure 18B shows magnet B positioned in the LV and-81 positioned in a blood
=
vessel BV adjacent to the heart. The magnetic force between B and B1 maintains
the location of B and B1. Magnets B and Bt are delivered on or within
materials and =
structures which promote healing and increase the amount of tension that can
be- - - -
placed on the anchor over time. For example, magnet 81 can be delivered on a
=
stent which is of a length, diameter and material which will heal within the
BV to
= provide sufficient resistance to forces placed on it by the tethers.
[1is] The tethers may be pre-attached to the magnets A and B1 or
they . .
- may be attached after A and B1 have been positioned. The tether length
may be
shortened and/or adjusted after placement of the anchors. Alternatively the
final = =
tether length may be pre-selected-based on the patient's cardiac structure
geometry
and the effect the clinician desires. Placing sets of magnets in this method,
enables
anchoring of tethers within the heart in various positions and angles which
provides
increased flexibility and variation for clinicians to select optimal re-
shaping of the
cardiac structures based on specific patient characteristic.
[0119) Examples which demonstrate the flexibility of this approach
include
placing anchors at the annulus and at the apex of the heart and tethered to
shorten
the length of the LV; anchors can be placed in the around the annulus and
tethered
to change the shape of the annulus. More specifically, one or more sets of
magnets
can be placed in the RA and LA at the level of the mitral valve annulus on the
= =
anterior side of the annulus) and one or more sets of magnets can be placed in
the
LA and LV on opposite sides of the annulus on the posterior portion of the
annulus.
CA 02822801 2013-08-02
64166-43D1
The posterior-sets of magnets can then be tetheted to ttte anterior sets of
magnets to
change the shape of the annulus. Alternatively, the magnet anchors Can be
plac.ed=
at the level of the annulus in the LA and in a BV adjacent to the heart at the
level Of =
the annulus and these then tethered to the anterior annulus magnet anchor :
=
described above.
= =
=
- [0120] The magnets A and At-can also be a single magnet
that.extends
= through the intententricular septum. Moreover, only one of the magnets A
or Al'
need be implanted. One or more Magnets B and/or B2 are located opposite the
location of the magnet(s) A and/or Al. The magnet(s) B is located within the
left =
ventricle opposite the magnets A/A1, such as on the left ventricular wall. The
=
magnet B1 is Iodated on an anatomical structure adjacent the heart, t uch'at
on a
==
=
blood vessel BV.
- [0121] In another eMbodiment shown in Figure 18C, the
magnets A, Al,
-
and B1 ,-or combinations thereof, are implanted in the heart without tethers.
The
magnets A, Al, B, and B1 can be positioned in various combinations so as to
exert
magnetic attractions to one another to re-shape the left ventricle or the
mitral valve
annulus. For example; the magnets A and B can be implanted such that they
exert
an attractive magnetic force relative to one another. The magnets A and B2 can
alternately be implanted. Other possible combinations are the magnets Al and B
or
the magnets Al and B2. The magnets can be implanted without tethers such that
art = =
attractive magnetic force F causes the magnets and the attached region of the
heart
=
to move toward one another to re-shape the heart. Alternately, the magnets can
be
attached to one another with tethers.
31
CA 02822801 2013-08-02
64166-43D1
[0122] In yet .another embodiment, one or more magnets 1705 are
implanted in the walls 1710 of the left ventricle LV and/or the right
ventricle RV, as
shown in Figure 19. The magnets 1705 are positioned in opposed locations on
the
walls 1710 and one or more tethers 1905 attach opposed pairs of magnets 1705
to
one another. One or more of the tethers 1905 extend through the
interventricular
septum to connect a first magnet disposed In the left ventricle and a second
magnet
disposed in the right ventricle. In certain embodiments, magnet elements=do
not
= include tethers, but rely on the magnetic attraction to each other to
remodel the
tissue between them. For example, a magnetic element May be placed on .either
side of the interventricular septum, or one element within the septum. Maher
. .
magnetic element may be placed on or within the opposite left ventricular
wall, or in
=
an adjacent vessel on the left ventricular wall. The electromagnetic-field of
elements can then interact to cause a remodeling of the left ventricle
toastist with
ventricular function.
[0123] The tethers 1905 can be elastic so to exert an attractive
force
.
between the attached magnets 1705 and re-shape the left ventricle LV or
annulus
AN. Alternately, or in combination with elastic tethers, the tethers 1905 can
be
shortened in length after placement to thereby pull the walls of the left
ventricle LV
=
toward one another and re-shape the left ventricle LV or the annulus AN. In
combination with the force provided by the tethers 1905, the magnets 1705exert
an
attractive magnetic force toward one another to assist in pulling the heart
walls
toward each other.
[0124] It should be appreciated that one or more magnets can be
positioned in other locations of the heart or adjacent anatomical structures
for re-
shaping of the heart. For example, one or more magnets can be positioned
around
32
CA 02822801 2013-08-02
64166-43D1 =
the annulus AN or can be positioned in the coronary sinus in such a manner
that the
magnets exert attractive forces toward one another to cause Fe-shaping of a
desired
portion of the heart.
[0125] In another embodiment, cardiac re-shaping is achieved through
percutaneous placement of one or more tethers that are cinched or anchored in
the
walls of the left ventricle LV. The tethers provide tension between the walls
of the
left ventricle to reshape the left ventricle LV in a desired manner. Figure 20
shows a
cross-sectional view of the left ventricle LV with a tether 2010 positioned
therein.
The tether 2010 has a first end anchOred to a first wall of the left ventricle
LV and a
second end anchored to an opposed wall of the left ventricle LV. The tether
2010 is
tensioned to pull the walls toward one another (as represented by the phantom
lines
2012 in Figure 20) and re-shape the left ventricle LV. It should=be
appreciated that
the phantom lines 2012 in Figure 20 are merely representative of the geometric
re-
shaping. The left ventricle LV can be re-shaped in various manners and the
amount
of re-shaping can vary depending on the tension applied to the tether 2010 and
the
location of attachment to the walls of the left ventricle LV. The tether may
be
inelastic or somewhat elastic.
[0126] The tether 2010 can be anchored or otherwise attached to the walls
in various manners. In an exemplary embodiment, a patch 2015 (shown in
Figure 20) of material is positioned on an exterior surface of the ventricular
wall and
is attached to one end of the tether 2010. A similar patch can also be
positioned on
the opposed wall and attached to the opposite end of the tether.
[0127] With reference to Figure 21, the patch is delivered to a desired
location using a catheter 2105 having a sharpened distal end 21 10 that is
positioned
33
CA 02822801 2013-08-02
64166-43D1 =
within the left ventricle LV. The catheter 2105 can be delivered to the left
ventricle= =
LV in various manners, including'trans-aortically (via the aorta), trans-
septally (by
piercing the interventricular septum), and trans-atrially (via the left
atrium)pursuart
to well-known methods. As shown in Figure 22, the sharpened distal end 2110=
.
pierces the ventricular wall such that the distal end 2110 is positioned
exterior to the
ventricular wall. The catheter 2105= has an internal delivery lumen having an
opening
at the distal end 2110. The patch 2015 is configured to be transported in a
contracted state through the delivery lumen and delivered out of the opening
at the
distal end 2110, where the patch 2015 expands into an expanded state at the
exterior of the ventricular wall to seal against the exterior of the left
ventritular wall.
[0128] When
positioned at the exterior of the ventricular wall, the patch =
2015 is configured to act as a reservoir that receives a fluid material that
can be
delivered to the patch via the delivery lumen of the catheter 2105. The fluid
material- - -
has a first viscous state of sufficient fluidity such that the material can
flow through
the delivery lumen of the catheter 2105 and out of the distal end 2110 to the
location - =
of the patch 2015. The fluid material changes to a Second viscous state when
positioned exterior to the ventricular wall at the patch 2015. The second
viscous
state is of greater viscosity (i.e., more resistant to flow) than the first
viscous state
such that the fluid material provides support and a level of rigidity to the
patch 2015
and to the left ventricular wall. The fluid material can change to the second
viscous
state after a predetermined time period, after contact with the patch, or when
the
patch is completely filled. A catalyst can be injected into the fluid material-
to cause it
to change to the second viscous state.
[0129] As shown in Figure 23, the catheter 2105 can then be disengaged
from the patch 2015 such that the patch 2015 is disposed exterior to the
ventricular
34
CA 02822801 2013-08-02
64166-43D1
. _
wall. The patch 2015 can be firmly attached to the ventricular wall-(such as
using an .
= adhesive) to minimize wear or friction between the patch and the
ventrictilar wall.
Next, an -end of the tether 2010 is attached to the patch 2015. The -
cathetet2105
=
can be used to deliver the tether 2010 to the patch 2015 or, alternately, a
zecond
catheter can be used. In one embodiment, the tether 2015 is already positioned
in a
delivery lumen of the catheter 2010 while the patch-2015 is being delivered.
:The
=
catheter 2010 is then pulled back while the end of the tether-2015 remains
attached
to the patch 2015 to thereby let the tether 2010 out from the catheter2010, as
shown
=
in Figure 23.
[0130] With reference now to
Figure 24, a second patch 2415 is deployed :
=.
in or exterior to an opposed ventricular wall in a manner similar to that
desCribed
above. The opposite end of the tether 2010 is then attached to the second
anchor
=
2415 such that the tether 2010 extends between the two anchors, as shown in
=
Figure 20. Alternately, as shown in figure 24, a -second tether2420 is
attached ate
first end to the second anchor 2415. As shown in Figure 25, the two tethers
2010
and 2420 can then be attached together at opposite ends from the patches, such
as
= =
by using a clip 2510, to form a single attachment -tether between the patches
2015
and 2415. The tether 2510 can be twisted or adjusted within the clip to
tension the =
resulting attachment tether between the patches 2415 and 2015 and pull the
ventricular walls toward one another via the tether. Once properly tensioned,
the
tether can be clipped or clamped to maintain its position.
[0131] In another embodiment, shown in Figure 26, a needle 2610 or
delivery catheter is passed trans-thoracically into the left ventricle LV to
deliver a
patch 2615 to the exterior of the ventricular wall, as described above. A
Sealing
means, such as a sealing balloon, can be used to seal one or more puncture
holes in
CA 02822801 2013-08-02
64166-43D1
the Wall of the left ventricle caused by the needle 26 during delivery of the
patch = =
2615. Visualization means, such as fluoroscopy,can be used to visualize proper
placement of the needle 2610. A-second patch is attached to an opposed wall to
=
form a tether attachment between the walls,-as shown in figure 20. The .tether
is=
then tensioned to pull the walls together and re-shape the left ventricle or
annulus of
the mitrel valve in a desired manner.
[0132] In other embodiments, described with reference to 'Figures
27-31,
cardiac re-shaping is achieved by manipulation of the papillary muscles.
figure 27
shows a schematic, cross-sectional view of the left ventricle LV in a healthy
state
with the mitral valve closed. The valve chordae CH connect the leaflets LP of
the
mitral valve to the papillary muscles PM. The papillary muscles PM and the and
=
chordae CH are positioned such that at least a portion of the leaflets IF
contact one
another when the mitral valve is in the closed state, resulting in functional
coapkition =
=
of the leaflets.
[0133] Figure 28 shows the left ventricle LV in a dysfunctional
state. The
valve chordae CH or the papillary muscles PM are damaged or otherwise
dysfunctional such that the leaflets =LF do not properly coapt (contact one
another).
The dysfunction can be manifested by excess tension in the chordae CH such
that a
gap is located between the leaflets LF, or in some cases one leaflet may
function at
a different level from the other (e.g. lower (prolapse) or higher (flail))
thereby limiting
the ability of the mital valve to close resulting in mitral regurgitation. The
dysfunctional left ventricle LV and in some cases leaflet prolapse orflail,
can be
treated by manipulating papillary Muscles PM to adjust the position of the
leaflets LF.
In one embodiment, the papillary muscles PM are repositioned toward one
another
to reduce the distance between the papillary muscles PM.
36
CA 02822801 2013-08-02
64166-43D1
-[0134] In an embodiment described with reference-to 'Figure 29,
a biasing -.=
member, such as a rod of adjustable length, or a spring 2910, -is mounted
between
the papillary muscles PM with a first-end of the spring-2910 attathed to a
first
papillary muscle and a second end of the spring 2910 attached to a second
papillary
muscle: The spring 2910 has a pre-load such that the spring 2910-provides a
biasing force (represented by the arrows 2915 in figure 29) that pulls the
papillary =
muscles PM toward one another. Such a spring may be covered with polyester
.
fabric or other coating to promote ingrowth into the muscle tissue and
minimkethe
=
potential for clot formation. The repositioning of the papillary muscles PM re-
shapes
= the left ventricle andlor changes the distance that the leaflets need to
move on the
= chordae CH such that the leaflets LF contact one another to close the
rhitral valve.
= The tension provided by the spring 2910 can be varied or different
springs can be
=
used to achieve a proper repositioning of the papillary muscles PM. The
tension May
be modified at the time of the procedure or during a subsequent procedure if
it is
determined that additional coaptation is required.
=
[01351 in another embodiment, described with reference to-figure
30, a
suture 3010 is mounted between the papillary muscles PM with a first end of
the
suture 3010 attached to a first papillary muscle and-a Second end of the
suture 3010
attached to a second papillary muscle. The suture 3010 can be attached to the
=
papillary muscles in various manners. For example, an attachment device 3015,
such as an anchor, cuff or sleeve, can be positioned around or-partially
around -each.
of the papillary muscles. The ends of the suture 3010 are attached to The
attachment devices 3015 to secure the suture 3010 to the suture to the-
papillary
muscles.
37
CA 02822801 2013-08-02
64166-43D1
=
{0136] The suture 3010 is tensioned such that it provides
a force that-pulls- = .
the papillary muscles PM toward one another. The suture 3010 can be tensioned,
-
for example,- by twisting the suture 30t0 to reduce Its the overall length and
thereby
reduce the distance between the papillary muscles PM, and fixing=the suture
with a
. crimping element or other stay element. The. amount of twisting
or=shortening can be
=
varied-to vary the tension provided by the suture 3010. In addition, o
crimping
member may be used to fix the sutures once a desired tension between the
muscles
is reached. Exemplary crimping members are described in international-Patent -
Application Number-PCT1US03/06149.
=
As in the previous embodiment, the repositioning .of the papillary: =
=
muscles PM re-shapes the, left ventricle and/or changes the tension .on.the
chordae =
CH such that the leaflets LF contact one another to close the Mita! Valve.
duffs-or.
sleeves may be placed around the papillary muscles PM to sUchoOthote
preViously-
-
=
'described, to affect the repositioning.
[0137] With reference now to Figure 31., the-papillary
muscles PM can also
- =
be repositioned by snaring the papillary muscles. A snare 3110 comprised of a
looped strand of material is positioned around the chordae CH at or near the
location
where the chordae attach with the papillary muscles PM. The-snare 3110 is
=
tightened to draw the papillary muscles PM toward one another and re-shape the
left
ventricle and/or changes the distance that the leaflets need to travel during
'systole
such that the leaflets LF contact one another to close the mitratvalve.
[0138] In yet another embodiment, shown in Figure 36, one
or more clips
3610 are clipped to each of the papillary muscles PM. The structure of the
clips
3610 can vary. A tether 3615 attaches the clips 3610 to one another.. The
tether
3615 is cinched to shorten the length of the tether 3615 and pull the
papillary
38
CA 02822801 2013-08-02
64166-43D1
muscles PM toward one another and re-shape the left ventricle and/or.changes
the
distance that the leaflets need to travel during systole such that the
leaflets LF
contact one another to close the mita! valve.
[0139] In yet another embodiment, shown in Figure 37, one or
more clips
3610 are clipped to opposed walls of the left ventricle LV. The clips 3610 can
be
= delivered to the left ventricle using a delivery catheter 2105. A tether
attaches the
= clips to one another. The tether is cinched to shorten the length of the
tether and
pull the ventricular walls toward one another and re-shape the left ventricle
and/or
changes the distance that the leaflets need to travel during systole such that
the
leaflets LF contact one another to close the mitre! valve.
=
[01401 In all embodiments, once the papillary muscles are
fixed or
repositioned, it may be advantageous to further treat the area by selectively
elongating or shortening the chordae tendinae to achieve further optimal valve
function. In addition, a mitral valve clip may be deployed to augment the
desired
valve function, either before papillary or chordal manipulation, or after, if
the desired
leaflet coaptation is not achieved with one particular approach.
[0141] As discussed above with reference to Figure 28, a
dysfunctional left
ventricle can be manifested by excess tension in the chordae CH such that a
gap is
positioned between the valve leaflets LF. It can be desirable to eliminate or
relieve
the excess tension by cutting the chordae CH, and/or cutting the chordae and
replacing them with artificial chordae. Prior to cutting the chordae, it can
be
desirable to evaluate the placement of the artificial chordae to confirm that
implantation of the chordae will indeed provide the desired clinical result.
This
process is now described with reference to Figures 32-35.
39
CA 02822801 2013-08-02
64166-43D1
[0142] 'Figure 32 shows a leaflet grasping device 1100 that Is
configured to
grasp and secure the leaflets of the mitral valve. The device 11'00 and
corresponding methods of use are described in more detail in U.S. Patent
Application Serial No. 10/635,776, entitled "Methods and Apparatus For Cardiac
=
Valve Repair", which is incorporated hereinby reference in Its entirety.
Additional
= = leaflet grasping devices are described in U.S. Patent Application
Serial
- No.
10/441,508, filed May 19,2003, U.S. Patent No. 6,269,819, issued August 7,=
.
2001, and U.S.. Patent No. 6,461,366, issued October 8., 2002.
[0143] Referring to Figure 32, the device 1100 is comprised of a
catheter
- shaft 1102 having a distal end 1104 and a proximal end 1106. The catheter
shaft= = .
1102 Is comprised of, among others, a cendbit 1108, a-coaxial outer sheath
1110, a
central lumen 1111 through which a double-jaw grasper 1113 may be. inserted,
and a
central guidewire lumen 1105. The catheter shaft 1102 can have additional
lumens
for the passage of one or more needles, as described more fully below.
[0144] Toward the distal end 1104, an optional pair of
stabilizers 1112 are
fixedly mounted on the outer sheath 1110 at their proximal end 1114 and
fixedly
attached to extenders 1116 at their distal end 1118. The stabilizers 1112 are
shown
in an outwardly bowed position, however they may be inwardly collapsed by
either
extending the extenders 1116 or retracting the outer sheath 1110. Bowing may
be
achieved by the reverse process.
[0145] The double-jaw grasper 1113 is comprised of two
articulating jaw
arms 1120 which may be opened and closed against the-central shaft 1122
(movement depicted by arrows) either independently or in tandem. The grasper
CA 02822801 2013-08-02
64166-43D1
1113 is shown in the open position inTigure 32. Re surfaces of thelPw arms.
1120
and central shaft 1122 may be toothed, as shown, or may have differing surface
=
textures for varying degrees of friction. The jaw arms 1120 each includes
needle
passageway 1121 comprised of a cutout or a slot that extends at least
partially along -
the length of each jaw arm 1120. As described in more detail below, the needle
passageway provides a location where a needle can pais through the jaw arm
1120
during manipulation of the papillary muscle.
=
=
[0146] The above described components may be manipulated and
controlled by a handle 1126-connected to the proximal end 1106 of the.catheter
shaft .
1102, as shown in fig. 86. the handle 1026 permits independent control of the
components described above. =
[0147] Referring to Figures 33A-C, the device 1100 may be used at
least
temporarily grasp and restrain the valve leaflets LF of the mitral valve MV.
The
double-jaw grasper 1113 extends through the valve such that the leaflets Ll,
L2 are
grasped from below. Thus, the device 1100 is termed "atrial-ventricular."
[0148] Referring to -Figure 33A, the atrial device 1100 may be
stabilized
against the mitral valve MV. The stabilizers 1112 may be positioned on the
superior
surface of the valve leaflets LF1, LF2 at a 90 degree angle to the line of
coaptation.
The grasper 1113 may be advanced in its closed position from the conduit 1108
between the leaflets LF1, LF2 until the jaw arms 1120 are fully below the
leaflets in
the ventricle. At this point, the grasper 1113 may be opened and retracted so
that
the jaw arms 1120 engage the inferior surface of the leaflets LF1, LF2. In
this -
manner, the leaflets are secured -between the stabilizers 1112 and the jaw am-
is
1120.
41
CA 02822801 2013-08-02
64166-43D1
[01491
Referring to Figure 33B, the .grasper 1113 will gradually close, =
drawing the leaflets LF-1, LF2 together while maintaining a secure hold on the
leaflets
between the jaw arms 1120 and the stabilizers 1112. This may be accomplished
by
number of methods. For example, the stabilizers 1112 may be gradually
collapsed
by either extending the extenders 1116 or retracting the outer sheath 1110. As
the
.
stabilizers 1112 collapse, the jaw arms 1120 may collapse due to spring
loading to
= gradually close the grasper 1113. Alternatively, the jaw arms 1120 may be
acttiated
to close against the central shaft 1122 applying force to the stabilizers 1112
causing
them to collapse. .In either case, such action allows the stabilizers 1112 to
.
. .
. .
simultaneously vertically retract and withdraw from-the leaflets as the
leaflets are
= = clamped between the jaw arms 1120 and the central shaft 1122. In
this manner, the
leaflets are effectively "transferred" to the grasper 1113. Referring to
Figure 33C,
=
once the collapsed stabilizers 1112 are completely withdrawn, the leaflets
Lf1,.LF2
are held in Vertical opposition by the grasper 1113 in a more natural
coaptation
geometry.
[0150]
With reference now to Figure 34, a needle 3410 is advanced from
the left atrium into the left ventricle. The needle 3410 can be passed through
a
lumen in the device 1100 or it can be passed external to the deviee 1100. In
any
event, the needle 3410 passes through a leaflet LF and into a papillary muscle
PM.
= As mentioned, the jaw arms 1120 have needle passageways 1121 (shown in
figure
32) that permit passage of the needle through the jaw arms 1120.
[0151] The needle 3410 is attached to a suture 3415 that
extends distally
through the device 1100. The suture 3415 is then anchored to the papillary
muscle
PM such that the suture 3415 provides an attachment for holding, pulling, or
otherwise manipulating the papillary muscle PM. The tension in the suture 3415
can
42
CA 02822801 2013-08-02
=
64166-43D1
. =
be adjusted to re-position the .papillary muscle PM such that the leaflets LF
contact
one another to close-the mitre{ valve. The same process-can be performed with
the
.other papillary muscle.
=
(0162] With the sutures 3415 holding the papillary. muscles PM in a desired
position, as shown in Figure 35, the chordde CH may. be cut. The--sutures
3415= = - =
function as artificial chordae that retain the leaflets LF and papillary-
muscles PM in a
desired orientation. = -
= . .
=
- = .p153] A fixation device such as a clip can then be
attached to-the leaflets
. 'using methods and-device described in U.S. Patent Application Serial No:
= =
10/635,776, filed Auglist 5, 2003, U.S. Patent Application Serial No.
101441,508, filed
May 1.9, 2003;U:8..Patent No. 6,269,819, issued August 7,2001, and U.S. Patent
No. 6,461,366, issued October 8,2002.
The sutures 3415 can be attached to the clip 3510 or directly=to.the
leaflets LF. It should be appreciated that any' quantityof sutures 3415 can be
used
as artificial chordae between the leaflets and the papillary muscles. It
should be
appreciated that the leaflet clip can also be used In conjunction with
cutting,
elongating, or shortening of the chordae pursuant to the methods described -
above.
= (0154) Prior-to permanently placing the chordae or
clips, the result-can be
previewed on ultrasound (TEE, ICE, echocardiography), to determine if the
-appropriate valve coaptaticn IS restored. In addition., It Is within the
sco:pe of the
= present invention to implant a initial valve clip in addition to
performed papillary
muscle approximation or chordal Implantation.
[0155) Although embodiments of various methods and devices
are
described herein in detail with reference to certain versions, it should be
appreciated
43
CA 02822801 2013-08-02
64166-43D1
that other versions, embodiments, methods of use, and tombinations thereof are
also possible. Therefore the scope of the appended dalm8 shoUld=not ioe .
limited to the description of the embodiments contained herein.
=
=
== = =
. .
. .
=
= .
=
=
=
=
=
-
. .
=
. .
.
=
= =
=
=
=
=
=
= =
=
44