Note: Descriptions are shown in the official language in which they were submitted.
CA 02823376 2013-08-07
53983-7D1
=
METHODS OF PROCESSING BIOMASS'COMYRISING
ELECTRON-BEAM RADIATION
This is a divisional application of co-pending Canadian Patent Application No.
2,667,628,
filed Oct 26, 2007.
It will be understood that any reference to "the present invention" or the
like may refer to
the subject matter of this divisional application and/or its parent.
TECHNICAL FIELD
This invention relatestosprocessing biomass, and products made therefrom.
=
BACKGROUND
Cellulosic and lignocellulosic materials, e.g., in fibrous font', are
produced, processed;
and used in large quantities in a number of applications. Often such materials
are used once,
and then discarded as waste, or are simply considered to be waste materials,
e.g., sewage,
bagasse, Sawdust, and stover.
Various cellulosic and lignocellulosic-materials, their uses, and applications
havebeen
described in U.S. Patent Nos... 7,074,918, 6,448,307, 6,258,876,6,207,729,
5,973,035 and
5,952,105; WO 2006/102543; and U.S. Patent Application Publication No.
2007/0045456.
= SUMMARY
. = Generally, this invention relates to carbohydrate-containing materials
(e.g., biomass
=
- materials or biomass-derived materials), methods of making and
processing such Materials to,
change their structure, and products made from the structurally changed
materials. For
example, many of the methods described herein can provide-cellulosic and/or
lignocellulosic
materials that have a lower molecular weight and/or crystallinity relative to
a native material.
Many of the methods provide materials that can be more readily utilized by a
variety of
. '
1
=
=
CA 02823376 2014-03-21
53983-7D13(S)
microorganisms to produce useful products, such as hydrogen, alcohols (e.g.,
ethanol or
butanol), organic acids (e.g., acetic acid), hydrocarbons, co-products (e.g.,
proteins) or
mixtures of any of these.
According to one aspect, the present invention relates to a method of
processing a biomass feedstock, the method comprising: physically treating a
biomass
feedstock that has been pretreated using electron beam irradiation, wherein
the irradiation is
performed at a dose rate of 1 Mrad/s to 10 Mrad/s.
In one aspect, the invention features methods of changing a molecular
structure
of a biomass feedstock that include preparing the biomass feedstock by
reducing one or more
dimensions of individual pieces of biomass; pretreating the biomass feedstock
using two or
more different pretreatment methods that each change the molecular structure,
in which the
different pretreatment methods are selected from radiation, sonication,
pyrolysis, and
oxidation; and processing the prepared and pretreated biomass feedstock to
produce a product.
In some embodiments, the biomass feedstock is prepared and then pretreated.
The biomass feedstock can also be pretreated and then prepared.
Reducing one or more dimensions of individual pieces of biomass can include,
e.g., shearing, cutting, or grinding.
In some embodiments, the two or more pretreatment methods are applied to the
biomass feedstock at or about the same time.
For example, the two or more pretreatment methods can include radiation and
sonication. For example, the radiation can be in the form of an electron beam.
In specific
embodiments, the electron beam radiation is applied at a total dosage of about
10 MRad and
the sonication is applied at a total energy of more than 5 MJ/m3. Radiation
can precede
sonication, or sonication can precede radiation, or radiation and sonication
can be performed
at or about the same time.
2
CA 02823376 2014-03-21
53983-7D13(S)
For example, the change in molecular structure of the biomass feedstock can
include a change in any one or more of an average molecular weight, average
crystallinity,
surface area, polymerization, porosity, branching, grafting, and domain size
of the biomass.
In some embodiments, the change in molecular structure of the biomass
feedstock includes a
decrease in either one or both of an average molecular weight and average
crystallinity of the
biomass or an increase in either one or both of surface area and porosity of
the biomass.
In another aspect, the invention features methods of making products, such as
combustible fuels, that include providing a material that includes a
carbohydrate produced by
a process that includes pretreating a biomass feedstock with any two or more
of radiation,
2a
CA 02823376 2013-08-07
= WO
2008/073186 PCT/US2007/022719
sonication, pyrolysis, and oxidation; and contacting the material with a
microorganism having
the ability to convert at least a portion, e.g., at least about 1 percent by
weight, of the material
to the product, such as a combustible fuel.
In some embodiments, the two or more different pretreatment methods include
radiation
and sonication, radiation and oxidation, radiation and pyrolization,
sonication and oxidation,
sonication and pyrolization, or oxidation and pyrolization.
For example, any method described herein can further include pretreating.the
biomass
feedstock with steam explosion.
For example, in some embodiments, the process does not include hydrolyzing the
biomass with an acid or a base.
In some embodiments, at least about seventy percent by weight of the biomass
is un-
hydrolyzed, e.g., at least at 95 percent by weight of the biomass has not been
hydrolyzed. In
specific embodiments, substantially none of the biomass has been hydrolyzed.
For example, in some embodiments, at least one pretreatment method is
performed on
biomass in which less than about 25 percent by weight of the biomass is wetted
with a liquid,
such as water. Specifically, in some embodiments, at least one pretreatment
method is
performed on biomass in which substantially none of the biomass is wetted with
a liquid, such
as water.
The biomass can have, e.g., less than about five percent by weight retained
water,
measured at 25 C and at fifty percent relative humidity.
In some embodiments, at least one pretreatment method can be performed on
biomass
in which less than about 25 percent by weight of the biomass is in a swollen
state, the swollen
state being characterized as having a volume of more than about 2.5 percent
higher than an
unswollen state. In other embodiments, the biomass is mixed with or includes a
swelling agent.
For example, in any method described herein, the biomass can be mixed with or
and
include a swelling agent, and the biomass can receives a dose of less than
about 10 Mrad of
radiation.
In some embodiments, one of the pretreatment methods is or includes radiation.
In some embodiments, at least one of the pretreatment methods, e.g.,
radiation, is
performed on the biomass feedstock while the biomass feedstock is exposed to
air.
3
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Pressure can be utilized. For example, at least one of the pretreatment
methods, e.g.,
radiation, can be performed on the biomass under a pressure of greater than
about 2.5
atmospheres, such as greater than 5 or 10 atmospheres.
The process can further include oxidizing, pyrolizing, or steam exploding the
biomass.
Examples of biomass feedstock include paper, paper products, paper waste,
wood,
particle board, sawdust, agricultural waste, sewage, silage, grasses, rice
hulls, bagasse, cotton,
jute, hemp, flax, bamboo, sisal, abaca, straw, corn cobs, corn stover,
switchgrass, alfalfa, hay,
rice hulls, coconut hair, cotton, synthetic celluloses, seaweed, algae, or
mixtures of these.
The biomass can be or can include a natural or a synthetic material.
Examples of fuels include one or more of hydrogen, alcohols, and hydrocarbons:
For
example, the alcohols can be ethanol, n-propanol, isoproanol, n-butanol, or
mixtures of these.
The microorganism can be, e.g., a bacterium or a yeast.
Converting can include fermenting the material to the product, such as the
combustible
fuel.
Irradiation can be, e.g., performed utilizing an ionizing radiation, such as
gamma rays, a
beam of electrons, or ultraviolet C radiation having a wavelength of from
about 100 nm to
about 280 nm.
The ionizing radiation can include electron beam radiation.
For example, the radiation can be applied at a total dose of between about 10
Mrad and
about 150 Mrad, such as at a dose rate of about 0.5 to about 10 Mrad/day, or 1
Mrad/s to about
Mrad/s.
In some embodiments, irradiating includes applying two or more radiation
sources, such
as gamma rays and a beam of electrons.
For example, sonication can be performed at a frequency of between about 15
kHz and
about 25 kHz, such as between about 18 kHz and 22 kHz.
In some embodiments, the biomass includes a first cellulose having a first
number
average molecular weight and the carbohydrate material comprises a second
cellulose having a
second number average molecular weight lower than the first number average
molecular
weight. For example, the second number average molecular weight is lower than
the first
number average molecular weight by more than about one-fold. In some
embodiments, the
4
CA 02823376 2013-08-07
W02008/073186 PCT/US2007/022719
first cellulose has a first crystallinity, and the second cellulose has a
second crystallinity lower
than the first crystallinity. For example, the second crystallinity can be
lower than the first
crystallinity by more than about 10 percent.
In some embodiments, the first cellulose can have a first level of oxidation
and the
second cellulose has a second level of oxidation higher than the first level
of oxidation.
For example, the biomass feedstock can be prepared by shearing a biomass fiber
source
to provide a fibrous material. For example, the shearing can be performed with
a rotary knife
cutter. The fibers of the fibrous material can have, e.g., an average length-
to-diameter ratio of
greater than 5/1. The fibrous material can have, e.g., a BET surface area of
greater than 0.25
m2/g.
In some embodiments, the carbohydrate can include one or more -1,4-linkages
and
having a number average molecular weight between about 3,000 and 50,000.
For example, the pretreated biomass material can further include a buffer,
such as
sodium bicarbonate or ammonium chloride, an electrolyte, such as potassium
chloride or
sodium chloride a growth factor, such as biotin and/or a base pair such as
uracil, a surfactant, a
mineral, or a chelating agent.
In some embodiments, the biomass feedstock is pretreated using any three or
more of
radiation, sonication, pyrolysis, and oxidation, in any order, or at about the
same time.
In another aspect, the invention features methods of making a product, such as
a
combustible fuel that includes providing ,a material that includes a
carbohydrate produced by
pretreating a biomass feedstock with any one or more of radiation, sonication,
oxidation,
pyrolysis, and steam explosion, in which less than about 25 percent by weight
of the biomass is
in a swollen state, the swollen state being characterized as having a volume
of more than about
2.5 percent higher than a nominal, unswollen state; and contacting the
material with a
microorganism having the ability to convert at least a portion, e.g., at least
about 1 percent by
weight, of the material to the product, such as the combustible fuel.
In another aspect, the invention features methods of making a product, such as
a
combustible fuel that include providing a material that includes a
carbohydrate produced by
pretreating a biomass feedstock with any one or more of radiation, sonication,
oxidation,
pyrolysis, and steam explosion, wherein less than about 25 percent by weight
of the biomass is
hydrolyzed subsequent to pretreatment; and contacting the material with a
microorganism
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
having the ability to convert at least a portion of the material, e.g., at
least about 1 percent by
weight, to the product, such as the combustible fuel.
In another aspect, the invention features methods of making a product, such as
a
combustible fuel, that includes providing a material includes a carbohydrate
produced by
pretreating a biomass feedstock with any one or more of radiation, sonication,
oxidation,
pyrolysis, and steam explosion, in which less than about 25 percent by weight
of the biomass is
in contact with a liquid, such as water; and contacting the material with a
microorganism
having the ability to convert at least a portion, e.g., at least about I
percent by weight, of the
material to the product, such as the combustible fuel.
In some embodiments, the methods include selecting two or more different
pretreatment
methods. The example, the two or more different pretreatment methods can
include radiation
and sonication, radiation and oxidation, radiation and pyrolization,
sonication and oxidation,
sonication and pyrolization, or oxidation and pyrolization. Optionally,
pretreating the biomass
can include steam explosion.
In another aspect, the invention features methods of making a product, such as
a
combustible fuel, that include providing a material comprising a carbohydrate
produced by
pretreating a sheared biomass feedstock with any one or more of radiation,
sonication,
oxidation, pyrolysis, and steam explosion; and contacting the material with a
microorganism
having the ability to convert at least a portion, e.g., at least about 1
percent by weight, of the
material to the product, such as the combustible fuel.
For example, the sheared biomass can include discrete fibers having a length-
to-
diameter ratio (L/D) of greater than about 5/1. For example, the biomass can
have internal
fibers, and the biomass has been sheared to an extent that its internal fibers
are substantially
exposed. For example, the biomass has been sheared to an extent that it has a
bulk density of
less than about 0.35 g/cm3.
In another aspect, the invention features biomass feedstock processing systems
tat
include a biomass feedstock preparation module for reducing size of pieces of
biomass; two or
more of the following pretreatment modules: a radiation module arranged to
apply radiation to
the biomass; a sonication module arranged to apply sonic energy to the
biomass; an oxidation
module arranged to oxidize the biomass; a pyrolysis module arranged to apply
heat to the
biomass; and a biomass transportation subsystem to move the biomass to and
between modules
6
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
within the processing system. The two or more pretreatment modules are
arranged in series
and/or are arranged to treat a portion of the biomass at about the same time.
For example, any
system can further include a vessel.
In another aspect, the invention features systems that include a reaction
vessel
containing a pretreated biomass material that includes a carbohydrate. The
pretreated biomass
material is produced by any method described herein.
For example, the vessel can also contains a microorganism in contact with the
material,
the microorganism having the ability to convert at least a portion of the
material to a product,
such as a combustible fuel. For example, the vessel can have a total volume of
greater than
about 1,000 L, such as greater than 5,000 L.
For example, any system can include a radiation module and a sonication
module.
In another aspect, the invention features carbohydrate materials produced by a
process
that includes pretreating a sheared biomass feedstock with any one or more of
radiation,
sonication, oxidation, pyrolysis and steam explosion. For example, pretreating
can be
performed with any two or more of radiation, sonication, oxidation, pyrolysis,
and steam
explosion.
In another aspect, the invention features carbohydrate materials produced by a
process
that includes pretreating a biomass feedstock with any two or more of
radiation, sonication,
oxidation, pyrolysis, and steam explosion.
In another aspect, the invention features compositions that include a
cellulosic or a
lignocellulosic material having a peak maximum molecular weight of less than
about 25,000,
and a crystallinity of less than about 55 percent. For example, the cellulosic
or a lignocellulosic
material can have a porosity of greater than about 72 percent, a BET surface
greater than about
0.75 m2/g, or a bulk density of less than about 0.5 g/cm3. For example, the
composition can
further include an enzyme and/or a microbe.
In another aspect, the invention features compositions that include a
cellulosic or a
lignocellulosic material that includes fibers. The cellulosic or
lignocellulosic material has a
peak maximum molecular weight of less than about 25,000, and a bulk density of
less than
about 0.5 g/cm3.
In another aspect, the invention features methods of making composites, such
as a fiber-
resin composite that include irradiating a fibrous material that includes a
first cellulosic and/or
7
CA 02823376 2013-08-07
= WO
2008/073186 PCT/US2007/022719
lignocellulosic material having a first molecular weight to provide a second
cellulosic and/or
lignocellulosic material having a second molecular weight higher than the
first molecular
weight; and combining the second cellulosic and/or lignocellulosic material
with a material,
such as a resin.
In another aspect, the invention features methods of making composites, such
as a fiber-
resin composite, that include combining a material, such as a resin, with a
fibrous material that
includes a cellulosic and/or lignocellulosic material having a first molecular
weight to provide a
composite that includes the fibrous material and a matrix; and
irradiating the composite to increase molecular weight of the first cellulosic
and/or
lignocellulosic material within the matrix, such as a resin matrix.
In another aspect, the invention features methods of making composites, such
as a fiber-
resin composites that include irradiating a fibrous material that includes a
first cellulosic and/or
lignocellulosic material having a first molecular weight to provide a second
cellulosic and/or
lignocellulosic material having a second molecular weight higher than the
first molecular
weight; combining the second cellulosic and/or lignocellulosic material with a
material, such as
a resin, to provide a composite; and irradiating the composite.
In another aspect, the invention features methods of making irradiated wood
products
that include providing a wood product that includes a first carbohydrate-
containing material
having a first molecular weight; and irradiating the wood product to provide
an irradiated wood
product that includes a second carbohydrate-containing material having a
second molecular
weight higher than the first molecular weight. For example, the methods can
further include
sonicating, e.g., prior to, after, or concurrently with the irradiating.
Methods are disclosed that include providing a first material that includes
cellulose
having a first number average molecular weight; irradiating the first material
to provide a
second material that includes cellulose having a second number average
molecular weight
lower than the first number average molecular weight; and combining the second
material with
a microorganism. The microorganism utilizes, e.g., ferments or otherwise
converts, the second
material, and in some instances the first material, to produce a useful
material, e.g., a
combustible fuel. For example, the combustible fuel can include one or more of
hydrogen, an
alcohol, an organic acid, a hydrocarbon or mixtures of these. A preferred
material is ethanol or
butanol, e.g., n-, sec- or t-butanol.
8
CA 02823376 2013-08-07
= WO 2008/073186
PCT/US2007/022719
In some embodiments, the first material includes a cellulosic or a
lignocellulosic
material. For example, the first material can be or can include paper, paper
products, wood,
wood-related materials, particle board, grasses, rice hulls, bagasse, cotton,
jute, hemp, flax,
bamboo, sisal, abaca, straw, corn cobs, rice hulls, algae, seaweed, coconut
hair, cotton,
synthetic celluloses or mixtures of any of these materials.
In preferred embodiments, the first material is in the form of a fibrous
material that
includes fibers provided by shearing a fiber source. Shearing alone can reduce
the crystallinity
of a fibrous material and can work synergistically with any process technique
that also reduces
crystallinity and/or molecular weight. For example, the shearing can be
performed with a
rotary knife cutter. In some embodiments, the fibrous material has an average
length-to-
diameter ratio of greater than 5/1.
The first and/or second material can have, e.g., a BET surface area of greater
than 0.25
m2/g and/or a porosity of greater than about 25 percent.
In some embodiments, the irradiating is performed with an ionizing radiation,
such as
gamma rays or a beam of electrons.
In preferred embodiments, the microorganism is a bacterium or a fungus, such
as a
yeast.
In some embodiments, the second number average molecular weight is at least
about 25
percent lower than the first number average molecular weight, e.g., 50 percent
lower.
Methods are disclosed that include shearing a fiber source to provide a first
fibrous
material that includes cellulose having a first number average molecular
weight; and irradiating
the first fibrous material to provide a second fibrous material that includes
a cellulose having a
second number average molecular weight lower that the first number average
molecular
weight. Shearing can work synergistically with radiation to reduce
crystallinity and/or
molecular weight.
Such methods may further include combining the second material with a
microorganism.
Methods are disclosed that include irradiating a fiber source, e.g., a paper,
that includes
cellulose having a first number average molecular weight to provide an
irradiated fiber source
that includes cellulose having a second number average molecular weight less
than the first
number average molecular weight; and shearing the irradiated fiber source to
provide a fibrous
9
=
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
material. Relative to the pre-irradiated material, the irradiated material can
be brittle and more
susceptible to "opening up" during the shearing.
Such methods can further include combining the fibrous material with a
microorganism.
Methods are disclosed that include providing a first lignocellulosic material
that
includes cellulose having a first number average molecular weight; and
irradiating the first
lignocellulosic material to provide a second lignocellulosic material that
includes cellulose
having a second number average molecular weight lower than the first number
average
molecular weight.
Such methods can further include combining the second lignocellulosic material
with a
microorganism or removing lignin from the second lignocellulosic material to
provide a de-
lignified material, and then combining the de-lignified material with a
microorganism the
microorganism. For example, the lignin from the processing can be utilized as
a processing aid
for plastics or it can be burned to produce energy.
Methods are disclosed that include providing a first fibrous material that
includes
cellulose having a first number average molecular weight; densifying the first
fibrous material
to provide a densified first fibrous material; and irradiating the densified
first fibrous material
to provide a densified second material that includes cellulose having a second
number average
molecular weight lower than the first number average molecular weight.
Densification, can
work synergistically with irradiation to lower molecular weight and can also
increase
throughput in any processing step described herein.
Such methods can further include combining the second densified fibrous
material with
a microorganism or fiberizing the second densifed fibrous material to provide
a second fibrous
material, and then combining the second fibrous material with a microorganism.
To further aid in the reduction of the molecular weight of the cellulose, an
enzyme, e.g.,
a cellulolytic enzyme and/or a swelling agent, can be utilized with any method
described
herein.
Methods are disclosed that include sonicating a first material that includes
cellulose
having a first number average molecular weight to provide a second material
that includes
cellulose having a second number average molecular weight lower than the first
number
average molecular weight; and combining the second material with a
microorganism. The
microorganism utilizes, e.g., ferments or otherwise converts, the second
material, and in some
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
instances the first material, to produce a useful material, e.g., a
combustible fuel. For example,
the combustible fuel can be or can include hydrogen, an alcohol, an organic
acid, a
hydrocarbon or mixtures of these. A preferred material is ethanol or butanol,
e.g., n-, sec- to t-
butanol.
In some embodiments, the first material includes a cellulosic or a
lignocellulosic
material. For example, the first material can be or can include paper, paper
products, wood,
wood-related materials, particle board, grasses, rice hulls, bagasse, cotton,
jute, hemp, flax,
bamboo, sisal, abaca, straw, corn cobs, rice hulls, algae, seaweed, coconut
hair, cotton,
synthetic celluloses or mixtures of any of these materials.
In preferred embodiments, the first material is in the form of a fibrous
material that
includes fibers provided by shearing a fiber source. Shearing can work
synergistically with
sonication to reduce molecular weight and/or crystallinity. For example, the
shearing can be
performed with a rotary knife cutter. In some embodiments, the fibrous
material has an
average length-to-diameter ratio of greater than 5/1.
The first and/or second material can have, e.g., a BET surface area of greater
than 0.25
m2/g and/or a porosity of greater than about 25 percent.
In some embodiments, the sonicating is performed with sound having a frequency
of
from about 16 kHz to about 100 kHz and/or an intensity of from about 30 W/cm2
to about 600
W/cm2.
In preferred embodiments, the microorganism is a bacterium or a fungus, such
as a
yeast.
In some embodiments, the second number average molecular weight is at least
about 25
percent lower than the first number average molecular weight, e.g., 50 percent
lower.
Methods are disclosed that include shearing a fiber source to provide a first
fibrous
material that includes cellulose having a first number average molecular
weight; and sonicating
the first fibrous material to provide a second fibrous material that includes
a cellulose having a
second number average molecular weight lower that the first number average
molecular
weight.
Such methods may further include combining the second material with a
microorganism.
11
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Methods are disclosed that include sonicating a fiber source, e.g., a paper,
that includes
cellulose having a first number average molecular weight to provide a
sonicated fiber source
that includes cellulose having a second number average molecular weight less
than the first
number average molecular weight; and shearing the sonicated fiber source to
provide a fibrous
material. Relative to the pre-sonicated material, the sonicated material can
be brittle and more
susceptible to "opening up" during the shearing.
Such methods can further include combining the fibrous material with a
microorganism.
Methods are disclosed that include sonicating a first lignocellulosic material
that
includes cellulose having a first number average molecular weight to provide a
second
lignocellulosic material that includes cellulose having a second number
average molecular
weight lower than the first number average molecular weight.
Such methods can further include combining the second lignocellulosic material
with a
microorganism or removing lignin from the second lignocellulosic material to
provide a de-
lignified material, and then combining the de-lignified material with a
microorganism the
microorganism.
Methods are disclosed that include sonicating a first fibrous material that
includes
cellulose having a first number average molecular weight to provide a second
fibrous material
that includes cellulose having a second number average molecular weight lower
than the first
number average molecular weight; and densifying the second fibrous material to
provide a
densified fibrous material.
Such methods can further include combining the densified fibrous material with
a
microorganism or fiberizing the densified fibrous material to provide a third
fibrous material,
and then combining the third fibrous material with a microorganism.
To further aid in the reduction of the molecular weight of the cellulose, an
enzyme, e.g.,
a cellulolytic enzyme, or a chemical, e.g., sodium hypochlorite, an acid, a
base or a swelling
agent, can be utilized with any method described herein. The enzyme and/or
chemical
treatment can occur before, during or after sonication.
Methods are disclosed that include pyrolyzing a first material that includes
cellulose
having a first number average molecular weight to provide a second material
that includes
cellulose having a second number average molecular weight lower than the first
number
average molecular weight; and combining the second material with a
microorganism. The
12
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
microorganism utilizes, e.g., ferments or otherwise converts, the second
material, and in some
instances the first material, to produce a useful material, e.g., a
combustible fuel. For example,
the combustible fuel can include one or more of hydrogen, an alcohol, an
organic acid, a
hydrocarbon or mixtures of these. A preferred material is ethanol or butanol.
In some embodiments, the first material includes a cellulosic or a
lignocellulosic
material. For example, the first material can be or can include paper, paper
products, wood,
wood-related materials, particle board, grasses, rice hulls, bagasse, cotton,
jute, hemp, flax,
bamboo, sisal, abaca, straw, corn cobs, rice hulls, algae, seaweed, coconut
hair, cotton,
synthetic celluloses or mixtures of any of these materials.
In preferred embodiments, the first material is in the form of a fibrous
material that
includes fibers provided by shearing a fiber source. Relatively small cross-
section can often be
pyrolyzed with greater control and efficiency. For example, the shearing can
be performed
with a rotary knife cutter. In some embodiments, the fibrous material has an
average length-to-
diameter ratio of greater than 5/1.
The first and/or second material can have, e.g., a BET surface area of greater
than 0.25
m2/g and/or a porosity of greater than about 25 percent. High surface areas
and/or porosities
can enhance reaction rates, making processes more efficient.
In preferred embodiments, the microorganism is a bacterium or a fungus, such
as a
yeast.
In some embodiments, the second number average molecular weight is at least
about 25
percent lower than the first number average molecular weight, e.g., 50 percent
lower.
Methods are disclosed that include shearing a fiber source to provide a first
fibrous
material that includes cellulose having a first number average molecular
weight; and pyrolyzing
the first fibrous material to provide a second fibrous material that includes
a cellulose having a
second number average molecular weight lower that the first number average
molecular
weight.
Such methods may further include combining the second material with a
microorganism.
Methods that include pyrolyzing a fiber source, e.g., a paper, that includes
cellulose
having a first number average molecular weight to provide a pyrolyzed fiber
source that
includes cellulose having a second number average molecular weight less than
the first number
13
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
average molecular weight; and shearing the pyrolyzed fiber source to provide a
fibrous
material. Relative to the pre-pyrolyzed material, the pyrolyzed material can
be brittle and more
susceptible to "opening up" during the shearing. Shearing pyrolyzed material
can be less
energy intensive and can be more efficient.
Such methods can further include combining the fibrous material with a
microorganism.
Methods are disclosed that include pyrolyzing a first lignocellulosic material
that
includes cellulose having a first number average molecular weight to provide a
second
lignocellulosic material that includes cellulose having a second number
average molecular
weight lower than the first number average molecular weight.
Such methods can further include combining the second lignocellulosic material
with a
microorganism or removing lignin from the second lignocellulosic material to
provide a de-
lignified material, and then combining the de-lignified material with a
microorganism the
microorganism.
Methods are disclosed that include pyrolyzing a first fibrous material that
includes
cellulose having a first number average molecular weight to provide a second
fibrous material
that includes cellulose having a second number average molecular weight lower
than the first
number average molecular weight; and densifying the second fibrous material to
provide a
densified fibrous material.
Such methods can further include combining the densified fibrous material with
a
microorganism or fiberizing the densified fibrous material to provide a third
fibrous material,
and then combining the third fibrous material with a microorganism.
To further aid in the reduction of the molecular weight of the cellulose, an
enzyme, e.g.,
a cellulolytic enzyme, or a chemical, e.g., sodium hypochlorite or an acid or
base, can be
utilized with any method described herein. The enzyme and/or chemical
treatment can occur
before, during or after pyrolyzing.
In any aspect or embodiment described herein, pyrolyzing may include any one
or more
of the following features. The pyrolyzing can include heating the first
material using a resistive
heating member, such as a metal filament or metal ribbon. The heating can
occur by direct
contact between the resistive heating member and the first material. The
pyrolyzing can
include heating the first' material by induction, such as by using a Currie-
Point pyrolyzer. The
pyrolyzing can include heating the first material by the application of
radiation, such as infrared
14
, CA 02823376 2013-08-07
3983-7 .
=
radiation. The radiation can be generated by a laser, such as an infrared
laser. The pyrolyzing
can include heating the first material with a convective heat. The convective
heat can be
=
. generated by a flowing stream. of heated gas. The heated gas can be
maintained at a
temperature of less than about 1200 C, such as less than 1000 C, less than
750 C, less than.
600 C, less than 400 C or even less than 300 C. The heated gas. can be
maintained at a
temperature of greater than about 250 C. The convective heat can be generated
by a hot body
surrounding the first material, such as in a furnace. The pyrolyzing can
include heating the first
. material with steam at a temperature above about 250 'C.
Methods are disclosed that include oxidizing a first material that includes
cellulose
=
having a first number average molecular Weight and having a first oxygen
content to provide a
second material that includes cellulose having a second number average
molecular weight and
having a second oxygen content higher than the first oxygen content The second
material can
be combined with a resin, e.g., a molten thermoplastic resin, to provide a
composite. A higher
level of oxidation, while maintaining molecular weight, can provide composites
with
exceptional mechanical properties, such as improved abrasion resistance,
compression strength,
fracture resistance, impact strength, bending strength, tensile modulus,
flexural modulus and
elongation at break. The second material can also be combined with any solid
and/or liquid.
described herein, or any solid and/or liquid described in any patent or
publication described = =
herein.
= To further improve disperSability, the resin can include a component that
includes
= hydrogen-bonding groups, such as one or more anhydride groups, carboxylic
acid groups,
hydroxyl groups, amide groups, amine groups or mixtures of any of these
groups. In some
preferred embodiments, the component includes a polymer copolymerized with
and/or grafted =
with maleic anhydride. Such materials are available from Dupont under the
tradename
FUSABOND .
= The first material can be or can include, e.g., paper, paper products,
wood, wood-related
materials, particle board, grasses, rice hulls, bagasse, cotton, jute, hemp,
flax, bamboo, sisal,
= abaca, straw, corn cobs, rice hulls, coconut hair, cotton, synthetic
celluloses or mixtures of any
of these materials. Other materials that include cellulose are described
herein. Still other
*materials that include cellulose are described in the patents and
publications that have been
described herein.
CA 02823376 2013-08-07
WO 2008/073186 PCIYUS2007/022719
In some desirable embodiments, the first material is in the form of a fibrous
material
that includes fibers. Such fibrous materials can be, e.g., provided by
shearing a fiber source,
such as by shearing a fiber source using a rotary knife cutter. For example,
to maximize
mechanical properties, it is often desirable that the fibers of the fibrous
material have an
average length-to-diameter ratio of greater than 5/1. For example, to maximize
dispersability,
it is often desirable that the first and/or second material have a BET surface
area of greater than
0.25 m2/g and/or a porosity of greater than about 25 percent.
Oxidation of the materials described herein can be aided by a variety of
techniques,
including pyrolysis. For example, the oxidizing can include pyrolyzing the
first material by
heating the first material using a resistive heating member, such as a metal
filament or metal
ribbon, in a oxidizing environment, such as in the presence of air, an oxygen-
enriched inert gas
(e.g., argon), or oxygen itself. In some preferred methods, the heating occurs
by direct contact
between the resistive heating member and the first material. In other methods,
oxidizing
includes pyrolyzing the first material by heating the first material by
induction, such as by a
Currie-Point pyrolyzer, in an oxidizing environment. In other methods,
oxidizing includes
pyrolyzing the first material by heating the first material by the application
of radiation, such as
infrared radiation, in an oxidizing environment. In one method, the radiation
is generated by an
infrared laser. In still other methods, oxidizing includes pyrolyzing the
first material by heating
the first material with a convective heat in an oxidizing environment: For
example, the
convective heat can be generated by a flowing stream of heated gas. For
example, the heated
gas can be maintained at a temperature of less than about 1200 C, such as
less than 1000 C,
less than 750 C, less than 600 C, less than 400 C or even less than 300 C.
In other methods,
the convective heat is generated by a hot body surrounding the first material.
In yet other
methods, the oxidizing includes pyrolyzing the first material by heating the
first material with
steam at a temperature above about 250 C.
Oxidation of the materials can be aided by still other techniques, including
sonication.
For example, the oxidizing can include sonicating the first material in an
oxidizing
environment. For example, the sonication can be performed while the first
material is
dispersed in an aqueous medium. In some desirable embodiments, the sonication
is performed
using sound having a frequency of from about 12 kHz to about 25 kHz.
16
CA 02823376 2013-08-07 =
53983-7D13
Oxidation of the materials can be performed by still other techniques,
including ionizing
and/or non-ionizing radiation. For exam- ple, the oxidizing can include
irradiating the first
material with gamma rays in an oiddizint environment and/or irradiating the
first material with
a beam of electrons in an oxidizing environment.
In some desirable embodiments for providing composites; the second number
average
molecular weight is not more than fifteen percent lower than the first number
average
molecular weight. In some embodiments for providing composites, the second
number average
molecular weight is substantially the same as the first number average
molecular weight. .
In some desirable embodiments, the second oxygen content is at least about
five percent
higher than the first oxygen content, or even more preferably, twenty percent
higher than the
first oxygen content.
= Methods are disclosed that include shearing a fiber source to provide a
first fibrous
material that includes cellulose having a first number average molecular
weight and having a
first oxygen content. The first fibrous materialis.oxidized to provide a
second fibrous material
that includes cellulose having a second number average molecular weight and
having a second
oxygen content higher than the first oxygen content The second fibrous
material can be
utilized to make composites or it can be Utilized for other applications. For
example, the
second material can be combined with any solid and/or liquid described herein,
or any solid
and/or liquid described in any patent or publication described herein.
If desired, and when making a composite, the methods can fizrther include
combining
the second fibrous material with a resin, such as 4 thermoplastic or
thermosetting resin:
Methods are disclosed that include oxidizing a fiber source that includes
cellulose
having a first number average molecular weight and having a first oxygen
content to provide an
= oxidized fiber source that includes cellulose having a second number
average molecular weight
and having a second oxygen content higher than the first oxygen content. The
oxidized fiber
source is then sheared. to provide an oxidized fibrous material that includes
fibers. The
oxidized fibrous material can be utilized to make composites or it can be
utilized for other
applications. For example, the second material can be combined with any solid
and/or liquid
described herein, or any spud and/or liquid described in any patent
application, patent or
publication described herein.
17
CA 02823376 2013-08-07
,
.:3983-7
=
Methods are disclosed that include oxidizing a first material that includes
cellulose .
having a first number average molecular weight and having a first oxygen
content to provide a
second material that includes cellulose having a second number average
molecular weight and
having a second oxygen content higher than the first oxygen content. The
second fibrous
material is densified to provide a densified fibrous material.
If desired, these methods can further include combining the densified fibrous
material
with a resin. In some preferred embodiment, the methods further include
fiberization of the
densified fibrous material to provide a 'third fibrous material, and then
combining the third
fibrous material with a resin, such as a thermoplastic resin. The densified or
third fibrous
material can be also combined with any solid and/or liquid described herein,
or any solid and/or =
liquid described in any patent or publication described herein.
Methods are disclosed that include converting a first material that includes
cellulose.
having a first number average molecular weight and having a first oxygen
content to a second
material that includes cellulose having a second number average molecular
weight and having a =
second oxygen content higher than the first oxygen content; and combining the
second material
= with a resin to provide a composite material. The first and/or second
material can also be
combined with any solid and/or liquid described herein, or any solid and/or
liquid described in
any patent or publication described herein.
Methods are disclosed that include oxidizing a first material that includes
cellulose
having a first number average molecular weight and having a first oxygen
content to provide a
second material that includes cellulose having a second number average
molecular weight
- lower than the first number average molecular weight and having a
second oxygen content
higher than the first oxygen content; and combining the second material with a
microorganism.
The microorganism can utilize the second material, e.g., by fermentation, to
produce a fuel,
such as hydrogen, alcohols, organic acids and hydrocarbons or mixtures of any
of these fuels.
In some embodiments, the first material is combined as well.
Methods are disclosed that include converting a fast material that includes
cellulose
having a first number average molecular weight and having a first oxygen
content to a second
material that includes cellulose having a second number average molecular
weight lower than
the first number average molecular weight and having a second oxygen content
higher than the
18
CA 02823376 2013-08-07
= WO
2008/073186 PCT/US2007/022719
first oxygen content; and combining the second material with a solid and/or a
liquid, such as a
liquid that includes a microorganism, and/or an enzyme. In some instances, the
first material is
combined as well. In some instances, the microorganism utilizes the second
material to
produce a fuel, such as hydrogen, alcohols, organic acids and hydrocarbons or
mixtures of any
of these fuels. In some embodiments, the first material can also be utilized
by the
microorganism to produce a fuel.
When a microorganism is utilized, it can be a natural microorganism or an
engineered
microorganism. For example, the microorganism can be a bacterium, e.g., a
cellulolytic
bacterium, a fungus, e.g., a yeast, a plant or a protist, e.g., an algae, a
protozoa or a fungus-like
protist, e.g., a slime mold. When the organisms are compatible, mixtures may
be utilized.
Generally, various microorganisms can produce a number of useful products,
such as a fuel, by
operating on, e.g., fermenting the materials. For example, alcohols, organic
acids,
hydrocarbons, hydrogen, proteins or mixtures of any of these materials can be
produced by
fermentation or other processes.
Methods are disclosed that include shearing and steam exploding a fiber source
to form
a fibrous material; and contacting the fibrous material with a microorganism
to produce a
product. Examples of useful products include hydrogen, alcohols, organic
acids, hydrocarbons,
proteins, and combinations thereof. Examples of useful fiber sources include
cellulosic
material, lignocellulosic material, and combinations thereof.
Shearing and steam exploding the fiber source to form the fibrous material may
be
performed in any order. In addition, multiple shearing and/or steam exploding
operations may
be performed in any order. Shearing may be performed, for example, using a
rotary knife
cutter. The fiber source may be cut prior to shearing and/or steam exploding.
For example, in some embodiments, the method includes shearing the fiber
source to
form a sheared fiber source, and then steam exploding the sheared fiber source
to form the
fibrous material. It is also possible to produce fibrous material by
additionally shearing the
steam exploded, sheared fiber source. It is also possible to shear the fiber
source a second time
to produce a second sheared fiber source, which is then steam exploded to
produce the fibrous
material.
Methods are disclosed that include steam exploding the fiber source to form a
steam
exploded fiber source, and then shearing the steam exploded fiber source to
produce the fibrous
19
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
material. It is also possible to produce fibrous material by additionally
steam exploding the
sheared, steam exploded fiber source.
Methods are disclosed that include shearing and steam exploding the fiber
source
concurrently to produce the fibrous material.
In some embodiments, the method may include passing sheared material through
one or
more screens, e.g., a screen having an average opening size of 1.59 mm or less
(0.0625 inch).
Screening separates the material according to size. For example, in one
embodiment, the
method includes: shearing the fiber source to produce a sheared fiber source;
passing the
sheared fiber source through a first screen to produce a screened fiber
source; shearing the
screened fiber source to produce a second sheared fiber source; passing the
second sheared
fiber source through a second screen having an average opening size less than
the first screen to
provide a second screened fiber source; and steam exploding the second
screened fiber source
to produce the fibrous material. The method may further include shearing the
second screened
fiber source to produce a third sheared fiber source, and then steam exploding
the third sheared
fibers source to produce the fibrous material.
It is also possible to shear the fiber source and concurrently pass it through
a screen.
The methods may also further include encapsulating the fibrous material in a
substantially gas impermeable material to remove entrapped gas and densify the
fibrous
material. The substantially gas impermeable material may be soluble in water,
and may be
provided in the form of a bag.
Examples of microorganisms that may be used to produce the useful product
include
bacteria, yeasts, enzymes, or combinations thereof. For example, the
microorganism can be a
bacterium, e.g., a cellulolytic bacterium, a fungus, e.g., a yeast, a plant or
a protist, e.g., an
algae, a protozoa or a fungus-like protist, e.g., a slime mold.
Examples of products that may be produced include mono- and polyfunctional Cl-
C6
alkyl alcohols, mono- and poly-functional carboxylic acids, CI-C6
hydrocarbons, and
combinations thereof. Specific examples of suitable alcohols include methanol,
ethanol,
propanol, isopropanol, butanol, ethylene glycol, propylene glycol, 1,4-butane
diol, glycerin,
and combinations thereof. Specific example of suitable carboxylic acids
include formic acid,
acetic acid, propionic acid, butyric acid, valeric acid, caproic acid,
palmitic acid, stearic acid,
oxalic acid, malonic acid, succinic acid, glutaric acid, oleic acid, linoleic
acid, glycolic acid,
CA 02823376 2013-08-07
= WO
2008/073186 PCT/US2007/022719
lactic acid, 7-hydroxybutyric acid, and combinations thereof. Examples of
suitable
hydrocarbons include methane, ethane, propane, pentane, n-hexane, and
combinations thereof.
Many of these products may be used as fuels.
Examples of microorganisms that may be used to produce useful products include
bacteria, yeasts, or combinations thereof. For example, the microorganism can
be a bacterium,
e.g., a cellulolytic bacterium, a fungus, e.g., a yeast, a plant or a protist,
e.g., an algae, a
protozoa or a fungus-like protist, e.g., a slime mold.
Examples of products that may be produced include mono- and polyfunctional Cl-
C6
alkyl alcohols, mono- and poly-functional carboxylic acids, CI-C6
hydrocarbons, and
combinations thereof. Specific examples of suitable alcohols include methanol,
ethanol,
propanol, isopropanol, butanol, ethylene glycol, propylene glycol, 1,4-butane
diol, glycerin,
and combinations thereof. Specific example of suitable carboxylic acids
include formic acid,
acetic acid, propionic acid, butyric acid, valeric acid, caproic acid,
palmitic acid, stearic acid,
oxalic acid, malonic acid, succinic acid, glutaric acid, oleic acid, linoleic
acid, glycolic acid,
lactic acid, 7-hydroxybutyric acid, and combinations thereof. Examples of
suitable
hydrocarbons include methane, ethane, propane, pentane, n-hexane, and
combinations thereof.
Many of these products may be used as fuels.
The term "fibrous material," as used herein, is a material that includes
numerous loose,
discrete and separable fibers. For example, a fibrous material can be prepared
from a bleached
Kraft paper fiber source by shearing, e.g., with a rotary knife cutter.
The term "screen," as used herein, means a member capable of sieving material
according to size. Examples of screens include a perforated plate, cylinder or
the like, or a wire
mesh or cloth fabric.
The term "pyrolysis," as used herein, means to break bonds in a material by
the
application of heat energy. Pyrolysis can occur while the subject material is
under vacuum, or
immersed in a gaseous material, such as an oxidizing gas, e.g., air or oxygen,
or a reducing gas,
such as hydrogen.
Oxygen content is measured by elemental analysis by pyrolyzing a sample in a
furnace
operating at 1300 C or above.
The terms "biomass" refers to any non-fossilized, i.e., renewable, organic
matter. The
various types of biomass include plant biomass (defined below), animal biomass
(any animal
21
CA 02823376 2013-08-07
WO 2008/073186 PCT/1JS2007/022719
by-product, animal waste, etc.) and municipal waste biomass (residential and
light commercial
refuse with recyclables such as metal and glass removed).
The term "plant biomass" and "lingocellulosic biomass" refer to virtually any
plant-
derived organic matter (woody or non-woody) available for energy on a
sustainable basis.
Plant biomass can include, but is not limited to, agricultural crop wastes and
residues such as
corn stover, wheat straw, rice straw, sugar cane bagasse, and the like. Plant
biomass further
includes, but is not limited to, trees, woody energy crops, wood wastes and
residues such as
softwood forest thinnings, barky wastes, sawdust, paper and pulp industry
waste streams, wood
fiber, and the like. Additionally grass crops, such as switchgrass and the
like have potential to
be produced on a large-scale as another plant biomass source. For urban areas,
the best
potential plant biomass feedstock includes yard waste (e.g., grass clippings,
leaves; tree
clippings, and brush) and vegetable processing waste. "Lignocellulosic
feedstock," is any type
of plant biomass such as, but not limited to, non-woody plant biomass,
cultivated crops, such
as, but not limited to, gasses, for example, but not limited to, C4 grasses,
such as switchgrass,
cord grass, rye grass, miscanthus, reed canary grass, or a combination
thereofi=or sugar
processing residues such as bagasse, or beet pulp, agricultural residues, for
example, soybean
stover, corn stover, rice straw, rice hulls, barley straw, corn cobs, wheat
straw, canola straw,
rice straw, oat straw, oat hulls, corn fiber, recycled wood pulp fiber,
sawdust, hardwood, for
example aspen wood and sawdust, softwood, or a combination thereof. Further,
the
lignocellulosic feedstock may include cellulosic waste material such as, but
not limited to,
newsprint, cardboard, sawdust, and the like.
Lignocellulosic feedstock may include one species of fiber or alternatively,
lignocellulosic feedstock may include a mixture of fibers that originate from
different
lignocellulosic feedstocks. Furthermore, the lignocellulosic feedstock may
comprise fresh
lignocellulosic feedstock, partially dried lignocellulosic feedstock, fully
dried lignocellulosic
feedstock or a combination thereof.
For the purposes of this disclosure, carbohydrates are materials that are
composed
=
entirely of one or more saccharide units or that include one or more
saccharide units.
Carbohydrates can be polymeric (e.g., equal to or greater than 10-mer, 100-
mer, 1,000-mer,
10,000-mer, or 100,000-mer), oligomeric (e.g., equal to or greater than a 4-
mer, 5-mer, 6-mer,
22
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
7-mer, 8-mer, 9-mer or 10-mer), trimeric, dimeric, or monomeric. When the
carbohydrates are
formed of more than a single repeat unit, each repeat unit can be the same or
different.
Examples of polymeric carbohydrates include cellulose, xylan, pectin, and
starch, while
cellobiose and lactose are examples of dimeric carbohydrates. Examples of
monomeric
carbohydrates include glucose and xylose.
Carbohydrates can be part of a supramolecular structure, e.g., covalently
bonded into
the structure. Examples of such materials include lignocellulosic materials,
such as that found
in wood.
A combustible fuel is a material capable of burning in the presence of oxygen.
Examples of combustible fuels include ethanol, n-propanol, n-butanol, hydrogen
and mixtures
of any two or more of these.
Swelling agents as used herein are materials that cause a discemable swelling,
e.g., a
2.5 percent increase in volume over an unswollen state of cellulosic and/or
lignocellulosic
materials, when applied to such materials as a solution, e.g., a water
solution. Examples
include alkaline substances, such as sodium hydroxide, potassium hydroxide,
lithium hydroxide
and ammonium hydroxides, acidifying agents, such as mineral acids (e.g.,
sulfuric acid,
hydrochloric acid and phosphoric acid), salts, such as zinc chloride, calcium
carbonate, sodium
carbonate, benzyltrimethylammonium sulfate, and basic organic amines, such as
ethylene
diamine.
A "sheared material," as used herein, is a material that includes discrete
fibers in which
at least about 50% of the discrete fibers, have a length/diameter (LID) ratio
of at least about 5,
and that has an uncompressed bulk density of less than about 0.6 g/cm3. A
sheared material is
thus different from a material that has been cut, chopped or ground.
Changing a molecular structure of a biomass feedstock, as used herein, means
to change
the chemical bonding arrangement or conformation of the structure. For
example, the change
in the molecular structure can include changing the supramolecular structure
of the material,
oxidation of the material, changing an average molecular weight, changing an
average
crystallinity, changing a surface area, changing a degree of polymerization,
changing a
porosity, changing a degree of branching, grafting on other materials,
changing a crystalline
domain size, or an changing an overall domain size.
23
CA 02823376 2013-08-07
=
S3983-7
.=
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although methods and materials similar or equivalent to those
described herein can
be used in the practice or testing of the present invention, suitable methods
and materials are
described below. In case of conflict, the present specification, including
definitions, will
. control. In addition, the materials, methods, and examples are
illustrative only and not
intended to be limiting.
Other features and advantages of the invention will be apparent from the
following
detailed description, and from the claiins.
DESCRIPTION OF DRAWINGS
FIG. I is a block diagram illustrating conversion of biomass into products and
co-
products.
= FIG. 2 is block diagram illustrating conversion of a fiber source into a
firstand second
fibrous material.
FIG. 3 is a cross-sectional view of a rotary knife cutter.
FIG. 4 is block diagram illustrating conversion of a fiber source into a
first, second and =
third fibrous material.
FIG. 5 is block diagram illustrating densification of a material.
FIG. 6 is a perspective view of a pellet mill.
FIG. 7A is a densified fibrous material in pellet form.
FIG. 7B is a transverse cross-section of a hollow pellet in which a center of
the hollow
is in-line with a center of the pellet.
.
FIG. 7C is a transverse cross-section of a hollow pellet in which a center
of the hollow
= is out of line with the center of the pellet.
FIG. 7D is a transverse cross-section of a tri-lobal pellet.
FIG. 8 is a block diagram illustrating a treatment sequence for processing
feedstock.
FIG. 9 is a perspective, cut-away view of a gamma irradiator.
FIG. 10 is an enlarged perspective view of region R of FIG. 9.
24
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
FIG. 11 is a block diagram illustrating an electron beam irradiation feedstock
pretreatment sequence.
FIG. 12 is a schematic view of a system for sonicating a process stream of
cellulosic
material in a liquid medium.
FIG. 13 is a schematic view of a sonicator having two transducers coupled to a
single
horn.
FIG. 14 is a block diagram illustrating a pyrolytic feedstock pretreatment
system.
FIG. 15 is a cross-sectional side view of a pyrolysis chamber.
FIG. 16 is a cross-sectional side view of a pyrolysis chamber.
FIG. 17 is a cross-sectional side view of a pyrolyzer that includes a heated
filament.
FIG. 18 is a schematic cross-sectional side view of a Curie-Point pyrolyzer.
FIG. 19 is a schematic cross-sectional side view of a furnace pyrolyzer.
FIG. 20 is a schematic cross-sectional top view of a laser pyrolysis
apparatus.
FIG. 21 is a schematic cross-sectional top view of a tungsten filament flash
pyrolyzer.
FIG. 22 is a block diagram illustrating an oxidative feedstock pretreatment
system.
FIG. 23 is block diagram illustrating a general overview of the process of
converting a
fiber source into a product, e.g., ethanol.
FIG. 24 is a cross-sectional view of a steam explosion apparatus.
FIG. 25 is a schematic cross-sectional side view of a hybrid electron
beam/sonication
device.
FIG. 26 is a scanning electron micrograph of a fibrous material produced from
polycoated paper at 25 X magnification. The fibrous material was produced on a
rotary knife
cutter utilizing a screen with 1/8 inch openings.
FIG. 27 is a scanning electron micrograph of a fibrous material produced from
bleached
Kraft board paper at 25 X magnification. The fibrous material was produced on
a rotary knife
cutter utilizing a screen with 1/8 inch openings.
FIG. 28 is a scanning electron micrograph of a fibrous material produced from
bleached
Kraft board paper at 25 X magnification. The fibrous material was twice
sheared on a rotary
knife cutter utilizing a screen with 1/16 inch openings during each shearing.
FIG. 29 is a scanning electron micrograph of a fibrous material produced from
bleached
Kraft board paper at 25 X magnification. The fibrous material was thrice
sheared on a rotary
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
knife cutter. During the first shearing, a 1/8 inch screen was used; during
the second shearing,
a 1/16 inch screen was used, and during the third shearing a 1/32 inch screen
was used.
FIG. 30 is a schematic side view of a sonication apparatus, while FIG. 31 is a
cross-
sectional view through the processing cell of FIG. 30.
FIG. 32 is a scanning electron micrograph at 1000 X magnification of a fibrous
material
produced from shearing switchgrass on a rotary knife cutter, and then passing
the sheared
material through a 1/32 inch screen.
FIGS. 33 and 34 are scanning electron micrographs of the fibrous material of
FIG. 32
after irradiation with 10 Mrad and 100 Mrad gamma rays, respectively, at 1000
X
magnification.
FIG. 35 is a scanning electron micrographs of the fibrous material of FIG. 32
after
irradiation with 10 Mrad and sonication at 1000 X magnification.
FIG. 36 is a scanning electron micrographs of the fibrous material of FIG. 32
after
irradiation with 100 Mrad and sonication at 1000 X magnification.
FIG. 37 is an infrared spectrum of Kraft board paper sheared on a rotary knife
cutter.
FIG. 38 is an infrared spectrum of the Kraft paper of FIG. 47 after
irradiation with 100
Mrad of gamma radiation.
FIG. 39 is a schematic view of a process for biomass conversion.
FIG. 40 is schematic view of another process for biomass conversion.
DETAILED DESCRIPTION
Biomass (e.g., plant biomass, animal biomass, and municipal waste biomass) can
be
processed to produce useful products such as fuels. Systems and processes are
described below
that can use as feedstock materials such as cellulosic and/or lignocellulosic
materials that are
readily available, but can be difficult to process, for example, by
fermentation. Feedstock
materials are first physically prepared for processing, often by size
reduction of raw feedstock
materials. Physically prepared feedstock can be pretreated or processed using
one or more of
radiation, sonication, oxidation, pyrolysis, and steam explosion. The various
pretreatment
systems and methods can be used in combinations of two, three, or even four of
these
technologies.
26
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
In some cases, to provide materials that include a carbohydrate, such as
cellulose, that
can be converted by a microorganism to a number of desirable products, such as
a combustible
fuels (e.g., ethanol, butanol or hydrogen), feedstocks that include one or
more saccharide units
can be treated by any one or more of the processes described herein. Other
products and co-
products that can be produced include, for example, human food, animal feed,
pharmaceuticals,
and nutriceuticals. A number of examples are presented that range from bench
scale
implementations of individual pretreatment methods to large scale biomass
processing plants.
TYPES OF BIOMASS
Generally, any biomass material that is or includes carbohydrates composed
entirely of
one or more saccharide units or that include one or more saccharide units can
be processed by
any of the methods described herein. For example, the biomass material can be
cellulosic or
lignocellulosic materials.
For example, such materials can include paper, paper products, wood, wood-
related
materials, particle board, grasses, rice hulls, bagasse, cotton, jute, hemp,
flax, bamboo, sisal,
abaca, straw, corn cobs, rice hulls, coconut hair, algae, seaweed, cotton,
synthetic celluloses, or
mixtures of any of these.
Fiber sources include cellulosic fiber sources, including paper and paper
products (e.g.,
polycoated paper and Kraft paper), and lignocellulosic fiber sources,
including wood, and
wood-related materials, e.g., particle board. Other suitable fiber sources
include natural fiber
sources, e.g., grasses, rice hulls, bagasse, cotton, jute, hemp, flax, bamboo,
sisal, abaca, straw,
corn cobs, rice hulls, coconut hair; fiber sources high in a-cellulose
content, e.g., cotton; and
synthetic fiber sources, e.g., extruded yam (oriented yarn or un-oriented
yarn). Natural or
synthetic fiber sources can be obtained from virgin scrap textile materials,
e.g., remnants or
they can be post consumer waste, e.g., rags. When paper products are used as
fiber sources,
they can be virgin materials, e.g., scrap virgin materials, or they can be
post-consumer waste.
Aside from virgin raw materials, post-consumer, industrial (e.g., offal), and
processing waste
(e.g., effluent from paper processing) can also be used as fiber sources.
Also, the fiber source
can be obtained or derived from human (e.g., sewage), animal or plant wastes.
Additional fiber
sources have been described in U.S. Patent Nos. 6,448,307, 6,258,876,
6,207,729, 5,973,035
and 5,952,105.
27
CA 02823376 2013-08-07
WO 2008/073186
PCT/US2007/022719
In some embodiments, the carbohydrate is or includes a material having one or
more 0-
1,4-linkages and having a number average molecular weight between about 3,000
and 50,000.
Such a carbohydrate is or includes cellulose (I), which is derived from (3-
g1ucose 1) through
condensation of (3(1--> 4)-glycosidic bonds. This linkage contrasts itself
with that for 0(1-= 4)-
glycosidic bonds present in starch and other carbohydrates.
HO
0
OH
HO
OH
( OH
HO . = OH
0
0 0)-
0
HO OH
OH
=
Blends of any of the above materials may also be used.
SYSTEMS FOR TREATING BIOMASS
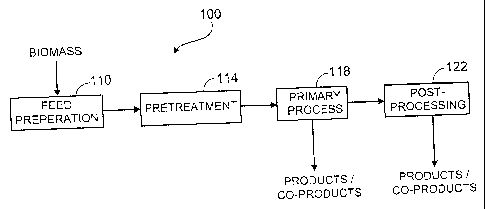
FIG. 1 shows a system 100 for converting biomass, particularly biomass with
significant
cellulosic and lignocellulosic components, into useful products and co-
products. System 100
includes a feed preparation subsystem 110, a pretreatment subsystem 114, a
primary process
subsystem 118, and a post-processing subsystem 122. Feed preparation subsystem
110 receives
biomass in its raw form, physically prepares the biomass for use as feedstock
by downstream
processes (e.g., reduces the size of and homogenizes the biomass), and stores
the biomass both in
its raw and feedstock forms. Biomass feedstock with significant cellulosic and
lignocellulosic
28
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
components can have a high average molecular weight and crystallinity that can
make processing
the feedstock into useful products (e.g., fermenting the feedstock to produce
ethanol) difficult.
Pretreatment subsystem 114 receives feedstock from the feed preparation
subsystem 110
and prepares the feedstock for use in primary production processes by, for
example, reducing the
average molecular weight and crystallinity of the feedstock. Primary process
subsystem 118
receives pretreated feedstock from pretreatment subsystem 114 and produces
useful products (e.g.,
ethanol, other alcohols, pharmaceuticals, and/or food products). In some
cases, the output of
primary process subsystem 118 is directly useful but, in other cases, requires
further processing
provided by post-processing subsystem 122. Post-processing subsystem 122
provides further
processing to product streams from primary process system 118 which require it
(e.g., distillation
and denaturation of ethanol) as well as treatment for waste streams from the
other subsystems. In
some eases, the co-products of subsystems 114, 118, 122 can also be directly
or indirectly useful
as secondary products and/or in increasing the overall efficiency of system
100. For example,
post-processing subsystem 122 can produce treated water to be recycled for use
as process water
in other subsystems and/or can produce burnable waste which can be used as
fuel for boilers
producing steam and/or electricity.
The optimum size for biomass conversion plants is affected by factors
including
economies of scale and the type and availability of biomass used as feedstock.
Increasing plant
size tends to increase economies of scale associated with plant processes.
However, increasing
plant size also tends to increase the costs (e.g., transportation costs) per
unit of feedstock. Studies
analyzing these factors suggest that the appropriate size for biomass
conversion plants can range
from 2000 to 10,000 dried tons of feedstock per day depending at least in part
on the type of
feedstock used. The type of feedstock can also impact plant storage
requirements with plants
designed primarily for processing feedstock whose availability varies
seasonally (e.g., corn
stower) requiring more on- or of-site feedstock storage than plants designed
to process feedstock
whose availability is relatively steady (e.g., waste paper).
FEED PREPARATION
In some cases, methods of processing begin with a physical preparation of the
=
feedstock, e.g., size reduction of raw feedstock materials, such as by
cutting, grinding, shearing
or chopping. In some cases, loose feedstock (e.g., recycled paper or
switchgrass) is prepared
29
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
by shearing or shredding. Screens and/or magnets can be used to remove
oversized or
undesirable objects such as, for example, rocks or nails from the feed stream.
Feed preparation systems can be configured to produce feed streams with
specific
characteristics such as, for example, specific maximum sizes, specific length-
to-width, or
specific surface areas ratios. As a part of feed preparation, the bulk density
of feedstocks can
be controlled (e.g., increased).
Size Reduction
In some embodiments, the material to be processed is in the form of a fibrous
material
that includes fibers provided by shearing a fiber source. For example, the
shearing can be
performed with a rotary knife cutter.
For example, and by reference to FIG 2, a fiber source 210 is sheared, e.g.,
in a rotary
knife cutter, to provide a first fibrous material 212. The first fibrous
material 212 is passed
through a first screen 214 having an average opening size of 1.59 mm or less
(1/16 inch, 0.0625
inch) to provide a second fibrous material 216. If desired, fiber source can
be cut prior to the
shearing, e.g., with a shredder. For example, when a paper is used as the
fiber source, the paper
can be first cut into strips that are, e.g., 1/4- to 1/2-inch wide, using a
shredder, e.g., a counter-
rotating screw shredder, such as those manufactured by Munson (Utica, N.Y.).
As an
alternative to shredding, the paper can be reduced in size by cutting to a
desired size using a
guillotine cutter. For example, the guillotine cutter can be used to cut the
paper into sheets that
are, e.g., 10 inches wide by 12 inches long.
In some embodiments, the shearing of fiber source and the passing of the
resulting first
fibrous material through first screen are performed concurrently. The shearing
and the passing
can also be performed in a batch-type process.
For example, a rotary knife cutter can be used to concurrently shear the fiber
source and
screen the first fibrous material. Referring to FIG 3, a rotary knife cutter
220 includes a hopper
222 that can be loaded with a shredded fiber source 224 prepared by shredding
fiber source.
Shredded fiber source is sheared between stationary blades 230 and rotating
blades 232 to
provide a first fibrous material 240. First fibrous material 240 passes
through screen 242, and
the resulting second fibrous material 244 is captured in bin 250. To aid in
the collection of the
second fibrous material, the bin can have a pressure below nominal atmospheric
pressure, e.g.,
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
at least 10 percent below nominal atmospheric pressure, e.g., at least 25
percent below nominal
atmospheric pressure, at least 50 percent below nominal atmospheric pressure,
or at least 75
percent below nominal atmospheric pressure. In some embodiments, a vacuum
source 252 is
utilized to maintain the bin below nominal atmospheric pressure.
Shearing can be advantageous for "opening up" and "stressing" the fibrous
materials,
making the cellulose of the materials more susceptible to chain scission
and/or reduction of
crystallinity. The open materials can also be more susceptible to oxidation
when irradiated.
The fiber source can be sheared in a dry state, a hydrated state (e.g., having
up to ten
percent by weight absorbed water), or in a wet state, e.g., having between
about 10 percent and
about 75 percent by weight water. The fiber source can even be sheared while
partially or fully
submerged under a liquid, such as water, ethanol, isopropanol.
The fiber source can also be sheared in under a gas (such as a stream or
atmosphere of
gas other than air), e.g., oxygen or nitrogen, or steam.
Other methods of making the fibrous materials include, e.g., stone grinding,
mechanical
ripping or tearing, pin grinding or air attrition milling.
If desired, the fibrous materials can be separated, e.g., continuously or in
batches, into
fractions according to their length, width, density, material type, or some
combination of these
attributes. For example, for forming composites, it is often desirable to have
a relatively
narrow distribution of fiber lengths.
For example, ferrous materials can be separated from any of the fibrous
materials by
passing a fibrous material that includes a ferrous material past a magnet,
e.g., an electromagnet,
and then passing the resulting fibrous material through a series of screens,
each screen having
different sized apertures.
The fibrous materials can also be separated, e.g., by using a high velocity
gas, e.g., air.
In such an approach, the fibrous materials are separated by drawing off
different fractions,
which can be characterized photonically, if desired. Such a separation
apparatus is discussed in
Lindsey et al, U.S. Patent No. 6,883,667.
The fibrous materials can irradiated immediately following their preparation,
or they
can may be dried, e.g., at approximately 105 C for 4-18 hours, so that the
moisture content is,
e.g., less than about 0.5% before use.
31
= CA
02823376 2013-12-13 =
53983-7D13(S)
If desired, lignin can be removed from any of the fibtous materials that
include lignin.
Also,.to aid in the breakdown of the materials that include the cellulose, the
material can be
treated prior to irradiation with heat, a chemical (e.g., mineral acid, base
or a strong oxidizer
=
such as sodium hypochlorite) and/or an enzyme.
= In some embodiments, the average opening size of the first screen is less
than 0.79 mm
(1/32 inch, 0.03125 inch), e.g., less than 0.51 mm (1/50 inch, 0.02000 inch),
less than 0.40 mm =
(1/64 inch, 0.015625 inch), less than 0.23 mm (0.009 inch), less than 0.20 mm
(1/128 inch,
0.0078125 inch), less than 0.18 mm (0.007 inch), less than 0.13 nun (0.005
inch), or even less
than less than 0.10 mm (1/256 inch, 0.00390625 inch). The screen is prepared
by interweaving
. monofilaments having an appropriate diameter to give the
desired opening size. For example,
the monofilaments can be made of a metal, e.g., stainless steel. As the
opening sizes get
smaller, structural demands on the monofilaments may become greater. For
example, for
opening sizes less than 0.40 mm, it can be advantageous to make the screen's
from
monofilaments made from a material other than stainless steel, e.g., titanium,
titanium alloys,
amorphous metals, nickel, tungsten, rhodium, rhenium, ceramics, or glass. In
some
= embodiments, the screen is made from a plate, e.g. a metal plate, having
apertures, e.g., cut .
into the plate using a laser. In some embodiments, the open area of the mesh
is less than 52%,
== e.g., less .than 41%, less than 36%, less than 31%, less than
30%.
In some embodiments, the second fibrous is sheared and passed through the
first screen,
or. a different sized screen. In some embodiments, the second fibrous material
is passed
through.a second screen having an average opening size equal to or less than
that of first
screen. =
= Referring to FIG. 4, a third fibrous material 4220 can be prepared from
the second fibrous
material 216 by shearing the second fibrous material 216 and passing the
resulting material
through a second screen 4222 having an average opening size less than the
first screen 214.
= Generally, the fibers of the fibrous materials can have a relatively
large average length-
to-diameter ratio (e.g., greater than '20-to-1), even if they have been
sheared more than once. In
addition, the fibers of the fibrous materials described herein may have a
relatively narrow
length and/or length-to-diameter ratio distribution.
= As used herein, average fiber widths (i.e., diameters) are those
determined optically by
= randomly selecting approximately 5,000 fibers. Average fiber lengths are
corrected length-
32
=
- = - CA 02823376 2014-03-21
_
53983-7D13(S)
= =
weighted lengths. BET (Brunauer, Emmet and Teller) surface areas are multi-
point surface
= areas, and porosities are those determined by mercury porosimetry.
The average length-to-diaineter ratio of the second fibrous material 216 can
be, e.g.,. '
= greater than 8/1, e.g., greater than 10/1, greater than 15/1, greater
than 20/1, greater than 25/1,
= or greater than 50/1. An=average length of the second fibrous'material
216 can be, e.g.,
between about 0.5 mm and 2.5 mm, e.g., between ab6ut 0.75 mm and 1.0 mm, and
an average
_
. width (i.e., diameter) of the second fibrous material 216 can
be, e.g., between about 5 pm and
50 pm, e.g., between about 10 gm and 30 pm.
= = In some embodiments, a standard deviation of the length of the second
Rbrous material
216 is less than 60 percent of an average length of the second fibrous
material 216, e.g., less than
50 percent of the .average length, less than 40 percent of the average length,
less than 25 percent
= of the average length, less than 10 percent of the average length,
less=than 5 percent of the
average length, or even less than I percent of the average length.
= In some embodiments, a BET surface area of the second fibrous material is
greater than
0.1 m2/g, e.g., greater than 0.25 n12/g, greater than 0.5 m2/g, greater than
1.0 m2fg, greater than
1.5 .m2/g, greater than 1.75 m2/g, greater than 5.0 m2/g, greater than 10
m2/g, greater than 25
m2/g, greater than 35 m2/g, greater than 50m2/g, greater than 60 m2/g, greater
than 75 m2/g, = =
= greater than 100 m2/g, greater than 150 m2/g, greater than 200 m2/g, or
even greater than 250 =
m2/g. A porosity of the second fibrous material 216 can be, e.g., greater than
20 percent, greater
. than 25 percent, greater than 35 percent, greater than 50
percent, greater than 60 percent,
greater than 70 percent, e.g., greater than 80 percent, greater than 85
percent, greater than 90
=
= percent, greater than 92 percent, greater than 94 percent, greater than
95 percent, greater than
97.5 percent, greater than 99 percent, or even greater than 99.5 percent.
=
In some embodiments, a ratio of the average length-to-diameter ratio
of the first fibrous= = =
material to the average length-to-diameter ratio of the second fibrous
material is, e.g., less than
1.5, e.g., less than 1.4, less than 1.25, less than 1.1, less than 1.075, less
than 1.05, less than
= 1.025, or even substantially equal to 1.
In particular embodiments, the second fibrous material is sheared again and
the
resulting fibrous material passed through a second screen having an average
opening size less.
than the first screen to provide a third fibrous material. In such instances,
a ratio of the average
length-to-diameter ratio of the second fibrous material to the average length-
to-diameter ratio
33
=
=
=
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
of the third fibrous material can be, e.g., less than 1.5, e.g., less than
1.4, less than 1.25, or even
Less than 1.1.
In some embodiments, the third fibrous material is passed through a third
screen to
produce a fourth fibrous material. The fourth fibrous material can be, e.g.,
passed through a
fourth screen to produce a fifth material. Similar screening processes can be
repeated as many
times as desired to produce the desired fibrous material having the desired
properties.
Densification
Densified materials can be processed by any of the methods described herein.
A material, e.g., a fibrous material, having a low bulk density can be
densified to a
product having a higher bulk density. For example, a material composition
having a bulk
density of 0.05 g/ cm3 can be densified by sealing the fibrous material in a
relatively gas
impermeable structure, e.g., a bag made of polyethylene or a bag made of
alternating layers of
polyethylene and a nylon, and then evacuating the entrapped gas, e.g., air,
from the structure.
After evacuation of the air from the structure, the fibrous material can have,
e.g., a bulk density
of greater than 0.3 g/cm3, e.g., 0.5 g/cm3, 0.6 g/cm3, 0.7 g/cm3 or more,
e.g., 0.85 g/ cm3. After
densification, the product can processed by any of the methods described
herein, e.g.,
irradiated, e.g., with gamma radiation. This can be advantageous when it is
desirable to
transport the material to another location, e.g., a remote manufacturing
plant, where the fibrous
material composition can be added to a solution, e.g., to produce ethanol.
After piercing the
substantially gas impermeable structure, the densified fibrous material can
revert to nearly its
initial bulk density, e.g., greater than 60 percent of its initial bulk
density, e.g., 70 percent, 80
percent, 85 percent or more, e.g., 95 percent of its initial bulk density. To
reduce static
electricity in the fibrous material, an anti-static agent can be added to the
material.
In some embodiments, the structure, e.g., bag, is formed of a material that
dissolves in a
liquid, such as water. For example, the structure can be forrned from a
polyvinyl alcohol so
that it dissolves when in contact with a water-based system. Such embodiments
allow
densified structures to be added directly to solutions that include a
microorganism, without first
releasing the contents of the structure, e.g., by cutting.
Referring to FIG. 5, a biomass material can be combined with any desired
additives and
a binder, and subsequently densified by application of pressure, e.g., by
passing the material
34
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
through a nip defined between counter-rotating pressure rolls or by passing
the material
through a pellet mill. During the application of pressure, heat can optionally
be applied to aid
in the densification of the fibrous material. The densified material can then
be irradiated.
In some embodiments, the material prior to densification has a bulk density of
less than
0.25 g/cm3, e.g., 0.20 g/cm3, 0.15 g/cm3, 0.10 g/cm3, 0.05 g/cm3 or less,
e.g., 0.025 g/cm3.
Bulk density is determined using ASTM D1895B. Briefly, the method involves
filling a
measuring cylinder of known volume with a sample and obtaining a weight of the
sample. The
bulk density is calculated by dividing the weight of the sample in grams by
the known volume
of the cylinder in cubic centimeters.
The preferred binders include binders that are soluble in water, swollen by
water, or that
has a glass transition temperature of less 25 C, as determined by
differential scanning
calorimetry. By water-soluble binders, we mean binders having a solubility of
at least about
0.05 weight percent in water. By water swellable binders, we mean binders that
increase in
volume by more than 0.5 percent upon exposure to water.
In some embodiments, the binders that are soluble or swollen by water include
a
functional group that is capable of forming a bond, e.g., a hydrogen bond,
with the fibers of the
fibrous material, e.g., cellulosic fibrous material. For example, the
functional group can be a
carboxylic acid group, a carboxylate group, a carbonyl group, e.g., of an
aldehyde or a ketone,
a sulfonic acid group, a sulfonate group, a phosphoric acid group, a phosphate
group, an amide
group, an amine group, a hydroxyl group, e.g., of an alcohol, and combinations
of these groups,
e.g., a carboxylic acid group and a hydroxyl group. Specific monomeric
examples include
glycerin, glyoxal, ascorbic acid, urea, glycine, pentaerythritol, a
monosaccharide or a
disaccharide, citric acid, and tartaric acid. Suitable saccharides include
glucose, sucrose,
lactose, ribose, fructose, mannose, arabinose and erythrose. Polymeric
examples include
polyglycols, polyethylene oxide, polycarboxylic acids, polyamides, polyamines
and
polysulfonic acids polysulfonates. Specific polymeric examples include
polypropylene glycol
(PPG), polyethylene glycol (PEG), polyethylene oxide, e.g., POLY0X41),
copolymers of
ethylene oxide and propylene oxide, polyacrylic acid (PAA), polyacrylamide,
polypeptides,
polyethylenimine, polyvinylpyridine, poly(sodium-4-styrenesulfonate) and
poly(2-acrylamido-
methyl-l-propanesulfonic acid).
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
In some embodiments, the binder includes a polymer that has a glass transition
temperature less 25 C. Examples of such polymers include thermoplastic
elastomers (TPEs).
Examples of TPEs include polyether block amides, such as those available under
the tradename
PEBAX , polyester elastomers, such as those available under the tradename
HYTREL , and
styrenic block copolymers, such as those available under the tradename 'CRATON
. Other
suitable polymers having a glass transition temperature less 25 C include
ethylene vinyl
acetate copolymer (EVA), polyolefins, e.g., polyethylene, polypropylene,
ethylene-propylene
copolymers, and copolymers of ethylene and alpha olefins, e.g., 1-octene, such
as those
available under the tradename ENGAGE . In some embodiments, e.g., when the
material is a
fiberized polycoated paper, the material is densified without the addition of
a separate low glass
transition temperature polymer.
In a particular embodiment, the binder is a lignin, e.g., a natural or
synthetically
modified lignin.
A suitable amount of binder added to the material, calculated on a dry weight
basis, is,
e.g., from about 0.01 percent to about 50 percent, e.g., 0.03 percent, 0.05
percent, 0.1 percent,
0.25 percent, 0.5 percent, 1.0 percent, 5 percent, 10 percent or more, e.g.,
25 percent, based on
a total weight of the densified material. The binder can be added to the
material as a neat, pure
liquid, as a liquid having the binder dissolved therein, as a dry powder of
the binder, or as
pellets of the binder.
The densified fibrous material can be made in a pellet mill. Referring to FIG
6, a pellet
mill 300 has a hopper 301 for holding undensified material 310 that includes a
carbohydrate-
containing materials, such as cellulose. The hopper communicates with an auger
312 that is
driven by variable speed motor 314 so that undensified material can be
transported to a
conditioner 320 that stirs the undensified material with paddles 322 that are
rotated by
conditioner motor 330. Other ingredients, e.g., any of the additives and/or
fillers described
herein, can be added at inlet 332. If desired, heat may be added while the
fibrous material is in
conditioner. After conditioned, the material passes from the conditioner
through a dump chute
340, and to another auger 342. The dump chute, as controlled by actuator 344,
allows for
unobstructed passage of the material from conditioner to auger. Auger is
rotated by motor 346,
and controls the feeding of the fibrous material into die and roller assembly
350. Specifically,
the material is introduced into a hollow, cylindrical die 352, which rotates
about a horizontal
36
CA 02823376 2013-08-07
'1983-7
axis and which has radially extending die holes. Die 352 is rotated about the
axis by motor
360, which includes a horsepower gauge, indicating total power consumed by the
motor.
Densified material 370, e.g., in the form of pellets, drops from chute 372 and
are captured and
processed, such as by irradiation.
The material, after densification, can be conveniently in the form of pellets
or chips
= ..
having a variety of shapes. The pellets can then be irradiated. In some
embodiments, the
pellets or chips are cylindrical in shape, e.g., having a maximum transverse
dimension of, e.g.,
1 mm or more, e.g., 2 mm, 3 mm, 5 mm, 8 mm, 10 mm, 15 mm or more, e.g., 25 mm.
Another
- convenient shape for making composites includes pellets or chips that are
plate-like in form, .
e.g., having a thickness of 1 mm or more, -e.g.,- 2 mm, 3 mm, 5-nun, 8 mrn, 10
mm or more,
e.g., 25 mm; a width of e.g., 5 mm or more, e.g., 10 mm, 15 mm, 25 mm, 30 mm
or more, e.g.,
50 mm; and a length of 5 mm or more, e.g., 10 mm, 15 mm, 25 mm, 30 nun or
more, e.g.; 50
mm.
Referring now FIG 7A-7D, pellets can be made so that they have a hollow
inside. As =
shown, the hollow can be generally in-line with the center of the pellet (FIG
7B), or out of line
with the center of the pellet (FIG 7C). Making the pellet hollow inside can
increase the rate of
- dissolution in a liquid after irradiation.
Referring now to FIG 7D, the pellet can have, e.g., a transverse shape that is
multi-
.
lobal, e.g., tri-lobal as shown, or tetra-lobal, penta-lobal, hexa-lobal or
deca-lobal. Making the
pellets in such transverse shapes can also increase the rate of dissolution in
a solution after
irradiation.
Examples
In one example, half-gallon juice cartons made of un-printed white Kraft board
having a
bulk density of 20 lb/ft3 can be used as a feedstock. Cartons can be folded
flat and then fed into
a shredder to produce a confetti-like material having a width of between 0.1
inch and 0.5 inch,
a length of between 0.25 inch and 1 inch and a thickness equivalent to that of
the starting
material (about 0.075 inch). The confetti-like material can be fed to a ID-
lazy knife cutter which
- shears the confetti-like pieces, tearing the pieces apart and releasing
fibrous material.
In some.cases, multiple shredder-shearer trains can be arranged in series with
output. In
one embodiment, two shredder-shearer trains can be arranged in series with
output from the
37
CA 02823376 2013-08-07
WO 2008/073186 PCT/11S2007/022719
first shearer fed as input to the second shredder. In another embodiment,
three shredder-shearer
trains can be arranged in series with output from the first shearer fed as
input to the second
shredder and output from the second shearer fed as input to the third
shredder. Multiple passes
through shredder-shearer trains are anticipated to increase decrease particle
size and increase
overall surface area within the feedstream.
In another example, fibrous material produced from shredding and shearing
juice
cartons can be treated to increase its bulk density. In some cases, the
fibrous material can be
sprayed with water or a dilute stock solution of POLYOXTM WSR N10
(polyethylene oxide)
prepared in water. The wetted fibrous material can then be processed through a
pellet mill
operating at room temperature. The pellet mill can increase the bulk density
of the feedstream
by more than an order of magnitude.
PRETREATMENT
Physically prepared feedstock can be pretreated for use in primary production
processes
by, for example, reducing the average molecular weight and crystallinity of
the feedstock and/or
increasing the surface area and/or porosity of the feedstock. Pretreatment
processes can include
one or more of irradiation, sonication, oxidation, pyrolysis, and steam
explosion. The various
pretreatment systems and methods can be used in combinations of two, three, or
even four of
these technologies.
Pretreatment Combinations
In some embodiments, biomass can be processed by applying two or more of any
of the
processes described herein, such as two or more of radiation, sonication,
oxidation, pyrolysis,
and steam explosion either with or without prior, intermediate, or subsequent
feedstock
preparation as described herein. The processes can be applied in any order (or
concurrently) to
the biomass, e.g., a cellulosic and/or lignocellulosic material. In other
embodiments, materials
that include a carbohydrate are prepared by applying three, four or more of
any of the processes
described herein (in any order or concurrently). For example, a carbohydrate
can be prepared
by applying radiation, sonication, oxidation, pyrolysis, and, optionally,
steam explosion to a
cellulosic and/or lignocellulosic material (in any order or concurrently). The
provided
carbohydrate-containing material can then be converted by one or more
microorganisms, such
as bacteria, yeast, or mixtures of yeast and bacteria, to a number of
desirable products, as
38
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
described herein. Multiple processes can provide materials that can be more
readily utilized by
a variety of microorganisms because of their lower molecular weight, lower
crystallinity,
and/or enhanced solubility. Multiple processes Can provide synergies and can
reduce overall
energy input required in comparison to any single process.
For example, in some embodiments, feedstocks are provided that include a
carbohydrate that is produced by a process that includes irradiating and
sonicating (in either
order or concurrently) a cellulosic and/or a lignocellulosic material, a
process that includes
irradiating and oxidizing (in either order or concurrently) a cellulosic
and/or a lignocellulosic
material, a process that includes irradiating and pyrolyzing (in either order
or concurrently) a
cellulosic and/or a lignocellulosic material, a process that includes
irradiating and pyrolyzing
(in either order or concurrently) a cellulosic and/or a lignocellulosic
material, or a process that
includes irradiating and steam-exploding (in either order or concurrently) a
cellulosic and/or a
lignocellulosic material. The provided feedstock can then be contacted with a
microorganism
having the ability to convert at least a portion, e.g., at least about 1
percent by weight, of the
feedstock to the product, such as the combustible fuel, as described herein.
In some embodiments, the process does not include hydrolyzing the cellulosic
and/or
lignocellulosic material, such as with an acid or a base, e.g., a mineral
acid, such as
hydrochloric or sulfuric acid.
If desired, some or none of the feedstock can include a hydrolyzed material.
For
example, in some embodiments, at least about seventy percent by weight of the
feedstock is an
unhydrolyzed material, e.g., at least at 95 percent by weight of the feedstock
is an
=hydrolyzed material. In some embodiments, substantially all of the feedstock
is an
unhydrolyzed material.
Any feedstock or any reactor or fermentor charged with a feedstock can include
a
buffer, such as sodium bicarbonate, ammonium chloride or Tris; an electrolyte,
such as
potassium chloride, sodium chloride, or calcium chloride; a growth factor,
such as biotin and/or
a base pair such as uracil or an equivalent thereof; a surfactant, such as
Tween or polyethylene
glycol; a mineral, such as such as calcium, chromium, copper, iodine, iron,
selenium, or zinc;
or a chelating agent, such as ethylene diamine, ethylene diamine tetraacetic
acid (EDTA) (or its
salt form, e.g., sodium or potassium EDTA), or dimercaprol.
39
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
When radiation is utilized, it can be applied to any sample that is dry or
wet, or even
dispersed in a liquid, such as water. For example, irradiation can be
performed on cellulosic
and/or lignocellulosic material in which less than about 25 percent by weight
of the cellulosic
and/or lignocellulosic material has surfaces wetted with a liquid, such as
water. In some
embodiments, irradiating is performed on cellulosic and/or lignocellulosic
material in which
substantially none of the cellulosic and/or lignocellulosic material is wetted
with a liquid, such
as water.
In some embodiments, any processing described herein occurs after the
cellulosic
and/or lignocellulosic material remains dry as acquired or has been dried,
e.g., using heat
and/or reduced pressure. For example, in some embodiments, the cellulosic
and/or
lignocellulosic material has less than about five percent by weight retained
water, measured at
25 C and at fifty percent relative humidity.
If desired, a swelling agent, as defined herein, can be utilized in any
process described
herein. In some embodiments, when a cellulosic and/or lignocellulosic material
is processed
using radiation, less than about 25 percent by weight of the cellulosic and/or
lignocellulosic
material is in a swollen state, the swollen state being characterized as
having a volume of more
than about 2.5 percent higher than an unswollen state, e.g., more than 5.0,
7.5, 10, or 15 percent
higher than the unswollen state. In some embodiments, when radiation is
utilized on a
cellulosic and/or lignocellulosic material, substantially none of the
cellulosic and/or
lignocellulosic material is in a swollen state.
In specific embodiments when radiation is utilized, the cellulosic and/or
lignocellulosic
material includes a swelling agent, and swollen cellulosic and/or
lignocellulosic receives a dose
of less than about 10 Mrad.
When radiation is utilized in any process, it can be applied while the
cellulosic and/or
lignocellulosic is exposed to air, oxygen-enriched air, or even oxygen itself,
or blanketed by an
inert gas such as nitrogen, argon, or helium. When maximum oxidation is
desired, an oxidizing
environment is utilized, such as air or oxygen.
When radiation is utilized, it may be applied to biomass, such as cellulosic
and/or
lignocellulosic material, under a pressure of greater than about 2.5
atmospheres, such as grater
than 5, 10, 15, 20 or even greater than about 50 atmospheres.
CA 02823376 2013-08-07
=
WO 2008/073186 PCT/US2007/022719
In specific embodiments, the process includes irradiating and sonicating and
irradiating
precedes sonicating. In other specific embodiments, sonication precedes
irradiating, or
irradiating and sonicating occur concurrently.
In some embodiments, the process includes irradiating and sonicating (in
either order or
concurrently) and further includes oxidizing, pyrolyzing or steam exploding.
When the process includes radiation, the irradiating can be performed
utilizing an =
ionizing radiation, such as gamma rays, x-rays, energetic ultraviolet
radiation, such as
ultraviolet C radiation having a wavelength of from about 100 nm to about 280
nm, a beam of
particles, such as a beam of electrons, slow neutrons or alpha particles. In
some embodiments,
irradiating includes two or more radiation sources, such as gamma rays and a
beam of
electrons, which can be applied in either order or concurrently.
In specific embodiments, sonicating can performed at a frequency of between
about
15khz and about 25Ichz, such as between about 181chz and 22Ichz utilizing a 1
KW or larger
horn, e.g., a 2, 3, 4, 5, or even a 10 KW horn.
In some embodiments, the cellulosic and/or lignocellulosic material includes a
first
cellulose having a first number average molecular weight and the resulting
carbohydrate
includes a second cellulose having a second number average molecular weight
lower than the
first number average molecular weight. For example, the second number average
molecular
weight is lower than the first number average molecular weight by more than
about twenty-five
percent, e.g., 2x, 3x, 5x, 7x, 10x, 25x, even 100x reduction.
In some embodiments, the first cellulose has a first crystallinity and the
second cellulose
has a second crystallinity lower than the first crystallinity, such as lower
than about two, three,
five, ten, fifteen or twenty-five percent lower.
In some embodiments, the first cellulose has a first level of oxidation and
the second
cellulose has a second level of oxidation higher than the first level of
oxidation, such as two,
three, four, five, ten or even twenty-five percent higher.
In one example of the use of radiation with oxidation as a pretreatment, half-
gallon
juice cartons made of un-printed polycoated white Kraft board having a bulk
density of 20 =
lb/ft3 are used as a feedstock. Cartons are folded flat and then fed into a
sequence of three
shredder-shearer trains arranged in series with output from the first shearer
fed as input to the
second shredder, and output from the second shearer fed as input to the third
shredder. The
41
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
fibrous material produced by the can be sprayed with water and processed
through a pellet mill
operating at room temperature. The densified pellets can be placed in a glass
ampoule which is
sealed under an atmosphere of air. The pellets in the ampoule are irradiated
with gamma
radiation for about 3 hours at a dose rate of about 1 Mrad per hour to provide
an irradiated
material in which the cellulose has a lower molecular weight than the fibrous
Kraft starting
material.
Radiation Treatment
One or more irradiation processing sequences can be used to process raw
feedstock
from a wide variety of different sources to extract useful substances from the
feedstock, and to
provide partially degraded organic material which functions as input to
further processing steps
and/or sequences. Irradiation can reduce the molecular weight and/or
crystallinity of feedstock.
In some embodiments, energy deposited in a material that releases an electron
from its atomic
orbital is used to irradiate the materials. The radiation may be provided by
1) heavy charged
particles, such as alpha particles, 2) electrons, produced, for example, in
beta decay or electron
beam accelerators, or 3) electromagnetic radiation, for example, gamma rays, x
rays, or
ultraviolet rays. In one approach, radiation produced by radioactive
substances can be used to
irradiate the feedstock. In another approach, electromagnetic radiation (e.g.,
produced using
electron beam emitters) can be used to irradiate the feedstock. The doses
applied depend on the
desired effect and the particular feedstock. For example, high doses of
radiation can break
chemical bonds within feedstock components and low doses of radiation can
increase chemical
bonding (e.g., cross-linking) within feedstock components.
Referring to FIG. 8, in one method, a first material 2 that is or includes
cellulose having
a first number average molecular weight (TMNi) is irradiated, e.g., by
treatment with ionizing
radiation (e.g., in the form of gamma radiation, X-ray radiation, 100 nm to
280 nm ultraviolet
(UV) light, a beam of electrons or other charged particles) to provide a
second material 3 that
includes cellulose having a second number average molecular weight (TMN2)
lower than the
first number average molecular weight. The second material (or the first and
second material)
can be combined with a microorganism (e.g., a bacterium or a yeast) that can
utilize the second
and/or first material to produce a fuel 5 that is or includes hydrogen, an
alcohol (e.g., ethanol or
42
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
butanol, such as n-, sec- or t-butanol), an organic acid, a hydrocarbon or
mixtures of any of
these.
Since the second material 3 has cellulose having a reduced molecular weight
relative to
the first material, and in some instances, a reduced crystallinity as well,
the second material is
generally more dispersible, swellable and/or soluble in a solution containing
a microorganism.
These properties make the second material 3 more susceptible to chemical,
enzymatic and/or
biological attack relative to the first material 2, which can greatly improve
the production rate
and/or production level of a desired product, e.g., ethanol. Radiation can
also sterilize the
materials.
In some embodiments, the second number average molecular weight (MN2) is lower
than the first number average molecular weight (TMN1) by more than about 10
percent, e.g., 15,
20, 25, 30, 35, 40, 50 percent, 60 percent, or even more than about 75
percent.
In some instances, the second material has cellulose that has as crystallinity
(TC2) that is
lower than the crystallinity (TC1) of the cellulose of the first material. For
example, (TC2) can
be lower than (1C1) by more than about 10 percent, e.g., 15, 20, 25, 30, 35,
40, or even more
than about 50 percent.
In some embodiments, the starting crystallinity index (prior to irradiation)
is from about
40 to about 87.5 percent, e.g., from about 50 to about 75 percent or from
about 60 to about 70
percent, and the crystallinity index after irradiation is from about 10 to
about 50 percent, e.g.,
from about 15 to about 45 percent or from about 20 to about 40 percent.
However, in some
embodiments, e.g., after extensive irradiation, it is possible to have a
crystallinity index of
lower than 5 percent. In some embodiments, the material after irradiation is
substantially
amorphous.
In some embodiments, the starting number average molecular weight (prior to
irradiation) is from about 200,000 to about 3,200,000, e.g., from about
250,000 to about
1,000,000 or from about 250,000 to about 700,000, and the number average
molecular weight
after irradiation is from about 50,000 to about 200,000, e.g., from about
60,000 to about
150,000 or from about 70,000 to about 125,000. However, in some embodiments,
e.g., after
extensive irradiation, it is possible to have a number average molecular
weight of less than
about 10,000 or even less than about 5,000.
43
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
In some embodiments, the second material can have a level of oxidation (T02)
that is
higher than the level of oxidation (TOO of the first material. A higher level
of oxidation of the
material can aid in its dispersability, swellability and/or solubility,
further enhancing the
materials susceptibility to chemical, enzymatic or biological attack. In some
embodiments, to
increase the level of the oxidation of the second material relative to the
first material, the
irradiation is performed under an oxidizing environment, e.g., under a blanket
of air or oxygen,
producing a second material that is more oxidized than the first material. For
example, the
second material can have more hydroxyl groups, aldehyde groups, ketone groups,
ester groups
or carboxylic acid groups, which can increase its hydrophilicity.
Ionizing Radiation
Each form of radiation ionizes the biomass via particular interactions, as
determined by
the energy of the radiation. Heavy charged particles primarily ionize matter
via Coulomb
scattering; furthermore, these interactions produce energetic electrons that
may further ionize
matter. Alpha particles are identical to the nucleus of a helium atom and are
produced by the
alpha decay of various radioactive nuclei, such as isotopes of bismuth,
polonium, astatine,
radon, francium, radium, several actinides, such as actinium, thorium,
uranium, neptunium,
curium, californium, americium, and plutonium.
Electrons interact via Coulomb scattering and bremssthrahlung radiation
produced by
changes in the velocity of electrons. Electrons may be produced by radioactive
nuclei that
undergo beta decay, such as isotopes of iodine, cesium, technetium, and
iridium. Alternatively,
an electron gun can be used as an electron source via thermiceic emission.
Electromagnetic radiation interacts via three processes: photoelectric
absorption,
Compton scattering, and pair production. The dominating interaction is
determined by the
energy of the incident radiation and the atomic number of the material. The
summation of
interactions contributing to the absorbed radiation in cellulosic material can
be expressed by the
mass absorption coefficient, graphed below as a function of incident energy.
44
CA 02823376 2013-08-07
WO 2008/073186
PCT/US2007/022719
Men absorbtion coefficient for cellulose
le, ,
le
le =
"E I
7
to'
104=
to" to4 to4 toe 10' tot
Incident energy
(MeV)
Electromagnetic radiation is subclassified as gamma rays, x rays, ultraviolet
rays,
infrared rays, microwaves, or radiowaves, depending on its wavelength.
For example, gamma radiation can be employed to irradiate the materials.
Referring to
FIGS. 9 and 10 (an enlarged view of region R), a gamma irradiator 10 includes
gamma
radiation sources 408, e.g., 6oL.,-.0 pellets, a working table 14 for holding
the materials to be
irradiated and storage 16, e.g., made of a plurality iron plates, all of which
are housed in a
concrete containment chamber 20 that includes a maze entranceway 22 beyond a
lead-lined
door 26. Storage 16 includes a plurality of channels 30, e.g., sixteen or more
channels,
allowing the gamma radiation sources to pass through storage on their way
proximate the
working table.
In operation, the sample to be irradiated is placed on.a working table. The
irradiator is
configured to deliver the desired dose rate and monitoring equipment is
connected to an
experimental block 31. The operator then leaves the containment chamber,
passing through the
maze entranceway and through the lead-lined door. The operator mans a control
panel 32,
instructing a computer 33 to lift the radiation sources 12 into working
position using cylinder
36 attached to a hydraulic pump 40.
Gamma radiation has the advantage of a significant penetration depth into a
variety of
material in the sample. Sources of gamma rays include radioactive nuclei, such
as isotopes of
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
cobalt, calcium, technicium, chromium, gallium, indium, iodine, iron, krypton,
samarium,
selenium, sodium, thalium, and xenon.
Sources of x rays include electron beam collision with metal targets, such as
tungsten or
molybdenum or alloys, or compact light sources, such as those produced
commercially by
Lyncean.
Sources for ultraviolet radiation include deuterium or cadmium lamps.
Sources for infrared radiation include sapphire, zinc, or selenide window
ceramic
lamps.
Sources for microwaves include klystions, Slevin type RF sources, or atom beam
sources that employ hydrogen, oxygen, or nitrogen gases.
Electron Beam
In some embodiments, a beam of electrons is used as the radiation source. A
beam of
electrons has the advantages of high dose rates (e.g., 1, 5, or even 10 Mrad
per second), high
throughput, less containment, and less confinement equipment. Electrons can
also be more
efficient at causing chain scission. In addition, electrons having energies of
4-10 MeV can
have a penetration depth of 5 to 30 mm or more, such as 40 mm.
Electron beams can be generated, e.g., by electrostatic generators, cascade
generators,
transformer generators, low energy accelerators with a scanning system, low
energy
accelerators with a linear cathode, linear accelerators, and pulsed
accelerators. Electrons as an
ionizing radiation source can be useful, e.g., for relatively thin piles of
materials, e.g., less than
0.5 inch, e.g., less than 0.4 inch, 0.3 inch, 0.2 inch, or less than 0.1 inch.
In some
embodiments, the energy of each electron of the electron beam is from about
0.3 MeV to about
2.0 MeV (million electron volts), e.g., from about 0.5 MeV to about 1.5 MeV,
or from about
0.7 MeV to about 1.25 MeV.
FIG. 11 shows a process flow diagram 3000 that includes various steps in an
electron
beam irradiation feedstock pretreatment sequence. In first step 3010, a supply
of dry feedstock
is received from a feed source. As discussed above, the dry feedstock from the
feed source
may be pre-processed prior to delivery to the electron beam irradiation
devices. For example,
if the feedstock is derived from plant sources, certain portions of the plant
material may be
removed prior to collection of the plant material and/or before the plant'
material is delivered by
the feedstock transport device. Alternatively, or in addition, as expressed in
optional step 3020,
46
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
the biomass feedstock can be subjected to mechanical processing (e.g., to
reduce the average
length of fibers in the feedstock) prior to delivery to the electron beam
irradiation devices.
In step 3030, the dry feedstock is transferred to a feedstock transport device
(e.g., a
conveyor belt) and is distributed over the cross-sectional width of the
feedstock transport
device approximately uniformly by volume. This can be accomplished, for
example, manually
or by inducing a localized vibration motion at some point in the feedstock
transport device
prior to the electron beam irradiation processing.
In some embodiments, a mixing system introduces a chemical agent 3045 into the
feedstock in an optional process 3040 that produces a slurry. Combining water
with the
processed feedstock in mixing step 3040 creates an aqueous feedstock slurry
that may be
transported through, for example, piping rather than using, for example, a
conveyor belt.
The next step 3050 is a loop that encompasses exposing the feedstock (in dry
or slurry
form) to electron beam radiation via one or more (say, N) electron beam
irradiation devices.
The feedstock slurry is moved through each of the N "showers" of electron
beams at step 3052.
The movement may either be at a continuous speed through and between the
showers, or there
may be a pause through each shower, followed by a sudden movement to the next
shower. A
small slice of the feedstock slurry is exposed to each shower for some
predetermined exposure
time at step 3053.
Electron beam irradiation devices may be procured commercially from Ion Beam
Applications, Louvain-la-Neuve, Belgium or the Titan Corporation, San Diego,
CA. Typical
electron energies can be 1 MeV, 2 MeV, 4.5 MeV, 7.5 MeV, or 10 MeV. Typical
electron
beam irradiation device power can be 1 kW, 5 kW, 10 kW, 20 kW, 50 kW, 100 kW,
250 kW,
or 500 kW. Effectiveness of depolymerization of the feedstock slurry depends
on the electron
energy used and the dose applied, while exposure time depends on the power and
dose.
Typical doses may take values of 1 kGy, 5 kGy, 10 kGy, 20 kGy, 50 kGy, 100
kGy, or 200
kGy.
Tradeoffs in considering electron beam irradiation device power specifications
include
cost to operate, capital costs, depreciation, and device footprint. Tradeoffs
in considering
exposure dose levels of electron beam irradiation would be energy costs and
environment,
safety, and health (ESH) concerns. Tradeoffs in considering electron energies
include energy
47
CA 02823376 2013-08-07
=
.`
WO 2008/073186
PCT/US2007/022719
costs; here, a lower electron energy may be advantageous in encouraging
depolymerization of
certain feedstock slurry (see, for example, Bouchard, et al, Cellulose (2006)
13: 601-610).
It may be advantageous to provide a double-pass of electron beam irradiation
in order to
provide a more effective depolymerization process. For example, the feedstock
transport
device could direct the feedstock (in dry or slurry form) underneath and in a
reverse direction to
its initial transport direction. Double-pass systems can allow thicker
feedstock slurries to be
processed and can provide a more uniform depolymerization through the
thickness of the
feedstock slurry.
The electron beam irradiation device can produce either a fixed beam or a
scanning
beam. A scanning beam may be advantageous with large scan sweep length and
high scan
speeds, as this would effectively replace a large, fixed beam width. Further,
available sweep
widths of 0.5 m, lm, 2 m or more are available.
Once a portion of feedstock slurry has been transported through the N electron
beam
irradiation devices, it may be necessary in some embodiments, as in step 3060,
to mechanically
separate the liquid and solid components of the feedstock slurry. In these
embodiments, a
liquid portion of the feedstock slurry is filtered for residual solid
particles and recycled back to
the slurry preparation step 3040. A solid portion of the feedstock slurry is
then advanced on to
the next processing step 3070 via the feedstock transport device. In other
embodiments, the
feedstock is maintained in slurry form for further processing.
Electromagnetic Radiation
In embodiments in which the irradiating is performed with electromagnetic
radiation,
the electromagnetic radiation can have, e.g., energy per photon (in electron
volts) of greater
than 102 eV, e.g., greater than 103, 104, 105, 106, or even greater than 107
eV. In some
embodiments, the electromagnetic radiation has energy per photon of between
104 and 107, e.g.,
between 105 and 106 eV. The electromagnetic radiation can have a frequency of,
e.g., greater
than 1016 hz, greater than 1017 hz, 1018, 1019, 1020, or even greater than
1021 hz. In some
embodiments, the electromagnetic radiation has a frequency of between 1018 and
1022hz, e.g.,
between 1019 to 1021 hz.
48
CA 02823376 2013-08-07
= .;
= ;1983-7
=
Doses
= In some embodiments, the irradiating (with any radiation source or a
combination of
sources) is performed until the material receives a dose of at least 0.25
Mrad, e.g., at least 1.0
Mrad, at least 2.5 Mrad, at least 5.0 Mrad, or at least 10.0 Mrad. In some
embodiments, the
irradiating is performed until the material receives a dose of between 1.0
Mrad and '6.0 Mrad,
- e.g., between 1.5 Mrad and 4.0 Mrad.
In some embodiments, the irradiating is performed at a dose rate of between
5.0 and
1500.0 kilorads/hour, e.g., between 10.0 and 750.0 kilorads/hour or between
50.0 and 350.0
kilorads/hours.
In some embodiments, two or more radiation sources are used, such as two or
more
ionizing radiations. For example, samples can be treated, in any order, with a
beam of
electrons, followed by gamma radiation and UV light having wavelengths from
about 100 nm
to about 280 nm. In some embodiments, samples are treated with three ionizing
radiation
sources, such as a beam of electrons, gamma radiation, and energetic UV light.
In some embodiments, relatively low doses of radiation can crosslinlc, gall,
or
otherwise increase the molecular weight of a carbohydrate-containing material,
such as a
cellulosic or lignocellulosic material (e.g., cellulose). Such a material
having increased
molecular weight can be useful, e.g., in making a composite, e.g., having
improved mechanical
- properties, such as abrasion resistance, compression strength, fracture
resistance, impact
strength, bending strength, tensile modulus, flexural modulus and elongation
at break. Such a
= material having increased molecular weight can be useful in making a
composition.
For example, a fibrous material that includes a first cellulosic and/or
lignocellulosic
material having a first molecular weight can be irradiated in such a manner as
to provide a
second cellulosic and/or lignocellulosic material having a second molecular
weight higher than -
the first molecular weight. For example, if gamma radiation is utilized as the
radiation source,
a dose of from about 1 Mrad to about 10 Mrad, e.g., from about 1.5 Mrad to
about 7.5 Mrad or
= from about 2.0 Mrad to about 5.0 Mrad; can be applied. After the low dose
of radiation, the
second cellulosic and/or lignocellulosic material can be combined with a resin
and formed into
a composite, e.g., by compression molding, injection molding or extrusion.
Forming
composites is described in WO 2006/102543, and U.S. Patent Nos. 8,074,910; and
7,708,214.
49
CA 02823376 2013-08-07
z.1983-7
=
_ .
Alternatively, a fibrous material that includes a first cellulosic and/or
lignocellulosic
material having a first molecular weight can be combined with a resin to
provide a composite,
and then the composite can be irradiated with a relatively low dose of
radiation so as to provide
= a second cellulosic and/or lignocellulosic material having a second
molecular weight higher
than the first molecular weight. For example, if gamma radiation is utilized
as the radiation
= source, a dose of from about 1 Mrad to about 10 Mrad can be applied.
Using this approach
increases the molecular weight of the material while it is with a resin
matrix. In some
embodiments, the resin is a cross-linkable resin and as such it crosslinks as
the carbohydrate-
== containing material increases in molecular weight, which can
provide a synergistic effect to
provide maximum mechanical properties to the composite. For example, such
composites can
= have excellent low temperature performance, e.g., having a
reducedtendency to break and/or
crack at low temperatures, e.g., temperatures below 0 C, e.g., below -10 C, -
20 C, -40 C, -50
C, -60 C or even below -100 C, and/or excellent performance at high
temperatures, e.g.,
capable of maintaining their advantageous mechanical properties at relatively
high temperature;
e.g., at temperatures above 100 C, e.g., above 125 C, 150 C, 200 C, 250
C, 300 C, 400 C,
or even above 500 C. In addition, such composites can have excellent chemical
resistance, -
e.g., resistance to swelling in a solvent, e.g., a hydrocarbon solvent,
resistance to chemical
attack, e.g., by strong acids, strong bases, strong oxidants (e.g., chlorine
or bleach) or reducing
agents (e.g., active metals such as sodium and potassium).
Alternatively, in another example, a fibrous material that includes a
cellulosic and/or
lignocellulosic material is irradiated and, optionally, treated with acoustic
energy, e.g.,
- ultrasound.
In one example of the use of radiation as a pretreatment, half-gallon juice
cartons made
of un-printed polycoated white Kraft board having, a bulk density of 20 lb/fe
are used as a
feedstock. Cartons are folded flat and then fed into a sequence of three
shredder-shearer trains
arranged in series with output from the first shearer fed as input to the
second shredder, and
output from the second shearer fed as input to the third shredder. The fibrous
material .
produced by the can be sprayed with water and processed through a pellet mill
operating at
room temperature. The densified pellets can be placed in a glass ampoule which
is evacuated
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
=
under high vacuum and then back-filled with argon gas. The ampoule is sealed
under argon.
The pellets in the ampoule are irradiated with gamma radiation for about 3
hours at a dose rate
of about 1 Mrad per hour to provide an irradiated material in which the
cellulose has a lower
molecular weight than the starting material.
Sonication
One or more sonication processing sequences can be used to process raw
feedstock
from a wide variety of different sources to extract useful substances from the
feedstock, and to
provide partially degraded organic material which functions as input to
further processing steps
and/or sequences. Sonication can reduce the molecular weight and/or
crystallinity of feedstock.
Referring again to FIG. 8, in one method, a first material 2 that includes
cellulose
having a first number average molecular weight (IMO is dispersed in a medium,
such as
water, and sonicated and/or otherwise cavitated, to provide a second material
3 that includes.
cellulose having a second number average molecular weight (TMN2) lower than
the first number
average molecular weight. The second material (or the first and second
material in certain
embodiments) can be combined with a microorganism (e.g., a bacterium or a
yeast) that can
utilize the second and/or first material to produce a fuel 5 that is or
includes hydrogen, an
alcohol, an organic acid, a hydrocarbon or mixtures of any of these.
Since the second material has cellulose having a reduced molecular weight
relative to
the first material, and in some instances, a reduced crystallinity as well,
the second material is
generally more dispersible, swellable, and/or soluble in a solution containing
the
microorganism, e.g., at a concentration of greater than 106 microorganisms/mL.
These
properties make the second material 3 more susceptible to chemical, enzymatic,
and/or
microbial attack relative to the first material 2, which can greatly improve
the production rate
and/or production level of a desired product, e.g., ethanol. Sonication can
also sterilize the
materials, but should not be used while the microorganisms are supposed to be
alive.
In some embodiments, the second number average molecular weight (TMN2) is
lower
than the first number average molecular weight NO by more than about 10
percent, e.g., 15,
20, 25, 30, 35, 40, 50 percent, 60 percent, or even more than about 75
percent.
In some instances, the second material has cellulose that has as crystallinity
(1C2) that is
lower than the crystallinity (Tt!) of the cellulose of the first material. For
example, (1C2) can
51
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
be lower than (1CI) by more than about 10 percent, e.g., 15, 20, 25, 30, 35,
40, or even more
than about 50 percent.
In some embodiments, the starting crystallinity index (prior to sonication) is
from about
40 to about 87.5 percent, e.g., from about 50 to about 75 percent or from
about 60 to about 70
percent, and the crystallinity index after sonication is from about 10 to
about 50 percent, e.g.,
from about 15 to about 45 percent or from about 20 to about 40 percent.
However, in certain
embodiments, e.g., after extensive sonication, it is possible to have a
crystallinity index of
lower than 5 percent. In some embodiments, the material after sonication is
substantially
amorphous.
In some embodiments, the starting number average molecular weight (prior to
sonication) is from about 200,000 to about 3,200,000, e.g., from about 250,000
to about
1,000,000 or from about 250,000 to about 700,000, and the number average
molecular weight
after sonication is from about 50,000 to about 200,000, e.g., from about
60,000 to about
150,000 or from about 70,000 to about 125,000. However, in some embodiments,
e.g., after
extensive sonication, it is possible to have a number average molecular weight
of less than
about 10,000 or even less than about 5,000.
In some embodiments, the second material can have a level of oxidation (02)
that is
higher than the level of oxidation (T01) of the first material. A higher level
of oxidation of the
material can aid in its dispersability, swellability and/or solubility,
further enhancing the
materials susceptibility to chemical, enzymatic or microbial attack. In some
embodiments, to
increase the level of the oxidation of the second material relative to the
first material, the
sonication is performed in an oxidizing medium, producing a second material
that is more
oxidized than the first material. For example, the second material can have
more hydroxyl
groups, aldehyde groups, ketone groups, ester groups or carboxylic acid
groups, which can
increase its hydrophilicity.
In some embodiments, the sonication medium is an aqueous medium. If desired,
the
medium can include an oxidant, such as a peroxide (e.g., hydrogen peroxide), a
dispersing
agent and/or a buffer. Examples of dispersing agents include ionic dispersing
agents, e.g.,
sodium lauryl sulfate, and non-ionic dispersing agents, e.g., poly(ethylene
glycol).
52
CA 02823376 2013-08-07
-;
= :3983-7
- In other embodiments, the sonication medium is non-aqueous. For
example, the -
sonication can be performed in a hydrocarbon, e.g., toluene or heptane, an
ether, e.g., diethyl
ether or tetrahydrofuran, or even ifia liquefied gas such as argon, xenon, or
nitrogen..
Without wishing to-be bound by any particular theory, it is believed that
sonication
breaks bonds in the cellulose by creating bubbles in the medium containing the
cellulose, which
grow and then violently collapse; During the collapse of the bubble, which can
take place in .
less than a nanosecond, the implosive force raises the local temperature
within the bubble to
about 5100K (even higher in some instance; see, e.g., Suslick et al., Nature
434, 52-55(2005)) and
generates pressures of from a few hundred atmospheres to over 1000 atmospheres
or more. It
is these high temperatures and pressures that break the bonds. In addition,
without wishing to
be bound by any particular theory, it is believed that reduced crystallinity
arises, at least in part,
. from the extremely high cooling rates during collapse of the bubbles, which
can be greater than
about 10" K/second. The high cooling rates generally do not allow the
cellulose to organiie
and crystallize, resulting in materials that have reduced crystallinity.
Ultrasonic systems and
= sonochemistry are discussed in, e.gõ 011i et al., U.S. Patent No.
5,766,764; Roberts, U.S..
Patent No. 5,828,156; Mason, Chemistry with Ultrasound, Elsevier, Oxford,
(190); Suslick
- (editor), Ultrasound: its Chemical, Physical and .Biological
Effects, VCH, Weinheim, (1988); -
Price, "Current Trends in Sonochemistry" Royal Society of Chemistry,
Cambridge, (1992);
Suslick et al., Ann. Rev. Mater. Sci. 29,295, (1999); Suslick et al., Nature
353, 414 (1991); -
Hiller et al., Phys. Rev. Lett. 69, 1182 (1992); Barber et al., Nature, 352,
414 (1991); Suslick
- et al., J. Am. Chem. Soc., 108, 5641 (1986); Tang et al., Chem. Comm., 2119
(2000); Wang
et al., Advanced Mater., 12, 1137 (2000); Landau et al., J. of Catalysis, 201,
22 (2001); Perkas
et al., Chem. Comm., 988 (2001); Nikitenko et al., Angew. Chem. Inter. Ed.
(December =
2001); Shall et al., J. Phys. Chem B 103, 3358(1999); Avivi et al., J. Amer.
Chem. Soc.
121, 4196(1999); and Avivi et al., J. Amer. Chem. Soc. 122, 4331 (2000).
Sonication Systems -
FIG. 12 shows a general system in which a cellulosic material stream 1210 is
mixed
with a water stream 1212 in a reservoir 1214 to form a process stream 1216. A
first pump 1218 r
draws process stream 1216 from reservoir 1214 and toward a flow cell 1224.
Ultrasonic
transducer 1226 transmits ultrasonic energy into process stream 1216 as the
process stream
53
=
= CA 02823376 2013-08-07
=c1983-7
flows through flow cell 1224. A second pump 1230 draws process stream 1216
from flow cell
1224 and toward subsequent processing.
Reservoir 1214 includes a first intake 1232 and a second intake 1234 in fluid
communication with a volume 1236. A conveyor (not shown) delivers cellulosic
material
=
stream 1210 to reservoir 1214 through first intake 1232. Water stream 1212
enters reservoir
= 1214 through second intake 1234. In some embodiments, water stream 1212
enters volume
=
1236 along a tangent establishing a swirling flow within volume 1236. In
certain
embodiments, cellulosic material stream 1210 and water stream 1212 are
introduced into -
volume 1236 along opposing axes to enhance mixing within the volume.
= FIG. 39 is a schematic view of a process for biomass conversion.
Valve 1238 controls the flow of water stream 1212 through second intake 1232
to
produce a desired ratio of cellulosic material to water (e.g., approximately
10 4 cellulosic
material, weight by volume). -For example, 2000 tons/day of cellulosic
material can be
combined with 1 million to 1.5 million gallons/day, e.g., 1.25 million
gallons/day, of water.
Mixing of cellulosic material and water in reservoir 1214 is controlled by the
size of
volume 1236 and the flow rates of cellulosic material and water into the
volume. In some
embodiments, volume 1236 is sized to create a minimum mixing residence time
for the
cellulosic material and water. For example, when 2000 toms/day of cellulosic
material and 1.25
million gallons/day of water are flowing through reservoir 1214, volume 1236
can be about
32,000 gallons to produce a minimum mixing residence time of about 15 minutes.
Reservoir 121-4 includes a mixer 1240 in fluid communication with volume 1236.
Mixer 1240 agitates the contents of volume 1236 to disperse cellulosic
material throughout the
. water in the volume. For example, mixer 1240 can be a rotating vane
disposed in reservoir
1214. In some embodiments, mixer 1240 disperses the cellulosic material
substantially
= =
uniformly throughout the water.
Reservoir 1214 further includes an exit 1242 in fluid communication with
volume 1236
and process stream 1216. The mixture of cellulosiematerial and water in volume
1236 flows
out of reservoir 1214 via exit 1242. Exit 1242 is arranged near the bottom of
reservoir 1214 to
= allow gravity to pull the mixture of cellulosic material and water out of
reservoir 1214 and into
= process stream 1216.
54
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
First pump 1218 (e.g., any of several recessed impeller vortex pumps made by
Essco
Pumps & Controls, Los Angeles, California) moves the contents of process
stream 1216 toward
flow cell 1224. In some embodiments, first pump 1218 agitates the contents of
process stream
1216 such that the mixture of cellulosic material and water is substantially
uniform at inlet
1220 of flow cell 1224. For example, first pump 1218 agitates process stream
1216 to create a
turbulent flow that persists along the process stream between the first pump
and inlet 1220 of
flow cell 1224.
Flow cell 1224 includes a reactor volume 1244 in fluid communication with
inlet 1220
and outlet 1222. In some embodiments, reactor volume 1244 is a stainless steel
tube capable of
withstanding elevated pressures (e.g., 10 bars). In addition or in the
alternative, reactor volume
1244 includes a rectangular cross section.
Flow cell 1224 further includes a heat exchanger 1246 in thermal communication
with
at least a portion of reactor volume 1244. Cooling fluid 1248 (e.g., water)
flows into heat
exchanger 1246 and absorbs heat generated when process stream 1216 is
sonicated in reactor
volume 1244. In some embodiments, the flow rate of cooling fluid 1248 into
heat exchanger
1246 is controlled to maintain an approximately constant temperature in
reactor volume 1244.
In addition or in the alternative, the temperature of cooling fluid 1248
flowing into heat
exchanger 1246 is controlled to maintain an approximately constant temperature
in reactor
volume 1244. In some embodiments, the temperature of reactor volume 1244 is
maintained at
20 to 50 C, e.g., 25, 30, 35, 40, or 45 C. Additionally or alternatively,
heat transferred to
cooling fluid 1248 from reactor volume 1244 can be used in other parts of the
overall process.
An adapter section 1226 creates fluid communication between reactor volume
1244 and
a booster 1250 coupled (e.g., mechanically coupled using a flange) to
ultrasonic transducer
1226. For example, adapter section 1226 can include a=flange and 0-ring
assembly arranged to =
create a leak tight connection between reactor volume 1244 and booster 1250.
In some
embodiments, ultrasonic transducer 1226 is a high-powered ultrasonic
transducer made by
Hielscher Ultrasonics of Teltow, Germany.
In operation, a generator 1252 delivers electricity to ultrasonic transducer
1252.
Ultrasonic transducer 1226 includes a piezoelectric element that converts the
electrical energy
into sound in the ultrasonic range. In some embodiments, the materials are
sonicated using
sound having a frequency of from about 16 kHz to about 110 kHz, e.g., from
about 18 kHz to
CA 02823376 2013-08-07
3983-7
about 75 kHz or from about 20 kHz to about 40 kHz. (e.g., sound having a
frequency of 20
= kHz to 40 kHz).
The ultrasonic energy is then delivered to the working medium through booster
1248.
The ultrasonic energy traveling through booster 1248 in reactor volume 1244
creates a
series of compressions and rarefactions in process stream 1216 with an
intensity sufficient to
create cavitation in process stream 1216. Cavitation disaggregates the
cellulosic material
dispersed in process stream 1216. Cavitation also produces free radicals in
the water of process =
stream 1216. These free radicals act to further break down the cellulosic
material in process
stream 1216.
In general, 5 to 4000 M.11m3, e.g., 10, 25, 50, 100, 250, 500, 750, 1000,2000,
or 3006
.M.T/m3, of ultrasonic energy is applied to process stream 1216 flowing at a
rate of about 02 m3/s
(about 3200 gallons/min). After exposure to ultrasonic energy in reactor
volume 1244, process
= stream 1216 exits flow cell 1224 through outlet 1222. Second pump 1230
moves process
stream 1216 to subsequent processing (e.g., any of several recessed impeller
vortex pumps
= made by Essco Pumps & Controls, Los Angeles, California).
= While certain embodiments have been described, other embodiments are
possible.
As an example, while process stream 1216 has been described as a single flow
path,
other arrangements are possible. In some embodiments for etample, process
stream 1216
includes multiple parallel flow paths (e.g., flowing at a rate of 10
gallon/min). In addition or in
the alternative, the multiple parallel flow, paths of process stream 1216 flow
into separate flow
= cells and are sonicated in parallel (e.g., using a Plurality of 16 kW
ultrasonic transducers).
As another example, while a single ultrasonic transducer 1226 has been
described as
being coupled to flow cell 1224, other arrangements are possible. In some
embodiments, -a =
plurality of ultrasonic transducers 1226 are arranged in flow cell 1224 (e.g.,
ten ultrasonic .
transducers can be arranged in a flow cell 1224). In some embodiments, the
sound waves
generated by each of the plurality of ultrasonic transducers 1226 are timed
(e.g., synchronized =
out of phase with one another) to enhance the cavitation acting upon process
stream 1216.
As another example, while a single flow cell 1224 has been described, other
arrangements are possible. In some embodiments, second pump 1230 moves process
stream to
=a second flow cell where a second booster and ultrasonic transducer further
sonicate process
stream 1216.
= 56
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
As still another example, while reactor volume 1244 has been described as a
closed
volume, reactor volume 1244 is open to ambient conditions in certain
embodiments. In such
embodiments, sonication pretreatment can be performed substantially
simultaneously with
other pretreatment techniques. For example, ultrasonic energy can be applied
to process stream
1216 in reactor volume 1244 while electron beams are simultaneously introduced
into process
stream 1216.
As another example, while a flow through process has been described, other
arrangements are possible. In some embodiments, sonication can be performed in
a batch
process. For example, a volume can be filled with a 10% (weight by volume)
mixture of
cellulosic material in water and exposed to sound with intensity from about SO
W/cm2 to about
600 W/cm2, e.g., from about 75 W/cm2 to about 300 W/cm2 or from about 95 W/cm2
to about
200 W/cm2. Additionally or alternatively, the mixture in the volume can be
sonicated from
about 1 hour to about 24 hours, e.g., from about 1.5 hours to about 12 hours,
or from about 2
hours to about 10 hours. In certain embodiments, the material is sonicated for
a pre-determined
time, and then allowed to stand for a second pre-determined time before
sonicating again.
Referring now to FIG. 13, in some embodiments, two electroacoustic transducers
are
mechanically coupled to a single horn. As shown, a pair of piezoelectric
transducers 60 and 62
is coupled to a slotted bar horn 64 by respective intermediate coupling horns
70 and 72, the
latter also being known as booster horns. The mechanical vibrations provided
by the
transducers, responsive to high frequency electrical energy applied thereto,
are transmitted to
the respective coupling horns, which may be constructed to provide a
mechanical gain, such as
a ratio of 1 to 1.2. The horns are provided with a respective mounting flange
74 and 76 for
supporting the transducer and horn assembly in a stationary housing.
The vibrations transmitted from the transducers through the coupling or
booster horns
are coupled to the input surface 78 of the horn and are transmitted through
the horn to the
oppositely disposed output surface 80, which, during operation, is in forced
engagement with a
worlcpiece (not shown) to which the vibrations are applied.
The high frequency electrical energy provided by the power supply 82 is fed to
each of
the transducers, electrically connected in parallel, via a balancing
transformer 84 and a
respective series connected capacitor 86 and 90, one capacitor connected in
series with the
electrical connection to each of the transducers. The balancing transformer is
known also as
57
CA 02823376 2013-08-07
1983-7
"balun" standing for "balancing unit." The balancing transformer includes a
magnetic core 92
and a pair of identical windings 94 and 96, also termed the primary winding
and secondary
winding, respectively.
In some embodiments, the transducers include commercially available
piezoelectric
transducers, such as Branson Ultrasonics Corporation models 105 or 502, each
designed for
= operation at 20 kHz and a maximum power rating of 3 kW. The energizing
voltage for
providing maximum motional excursion at the output surface of the transducer
is 930 volt rms. =
The current flow through a transducer may vary between zero and 3.5 ampere
depending on.the
load impedance. At 930 volt rms the output motion is approximately 20 microns.
The
maximum difference in terminal voltage for the same motional amplitude,
therefore, can be 186
volt. Such a voltage difference can give rise to large circulating currents
flowing between the
transducers. The balancing unit 84 assures a balanced condition by providing
equal current
= flow through the transducer's, hence eliminating the possibility of
circulating currents. The
wire size of the windings must be selected for the full load current noted
.above and the
= maximum voltage appearing across a winding input is 93 you
As an alternative to using ultrasonic energy, high-frequency, rotor-stator
devices can be
utilized. This type of device produces high-shear, microcavitation forces
which can
=
=
= disintegrate biomass in contact with such forces. Two commercially
available high-frequency,
rotor-stator dispersion devices are the Supratonlm devices manufactured by
Kremp
Industrieteclunk GmbH and marketed by Dorr-Oliver Deutschland GmbH of
Connecticut, and
the DispaxTm devices manufactured and marketed by &a-Works, Inc. of
Cincinnati, Ohio..
. Operation of such a microcavitation device is discussed in Stuart, U.S.
Patent No. 5,370,999.
=
While ultrasonic transducer 1226 has been described as including one or more
piezoelectric active elements to create ultrasonic energy, other arrangements
are possible. In
some embodiments, ultrasonic transducer 1226 includes active elements made of
other types of .
= magnetostrictive materials (e.g., ferrous metals). Design and operation
of such a high-powered
ultrasonic transducer is discussed in Hansen et al., U.S. Patent No.
6,624,539. In some
embodiments, ultrasonic energy is transferred to process stream 16 through an
electrohydraidic
system.
While ultrasonic transducer 1226 has been described as using the
electromagnetic
= -response of magnetorestrictive materials to produce ultrasonic energy,
other arrangements are
= 58
CA 02823376 2013-08-07
1983-7
possible. In some embodiments, acoustic energy in the form of an intense shock
wave can be
= applied directly to process stream 1216 using an underwater spark. In
some embodiments,
ultrasonic energy is transferred to process stream 1216 through a
therrnohydraulic system. For
example, acoustic waves of high energy density can be produced by applying
power across an
enclosed volume of electrolyte, thereby heating the enclosed volume and
producing a pressure
rise that is subsequently transmitted through a sound propagation medium
(e.g., process stream
1216). Design and operation of such a thermohydraulic transducer is discussed
in Hartmann et
al., U.S. Patent 6,383,152.
Pyrolysis
= One or more pyrolysis processing sequences can be used to process raw
feedstock from
a wide variety of different sources to extract useful substances from the
feedstock, and to'
provide partially degraded organic material which functions as input to
further processing steps
and/or sequences.
Referring again to the general schematic in FIG. 8, a first material 2 that
includes
cellulose having a first number average molecular weight (TMO is pyrolyzed,
e.g., by heating
the first material in a tube furnace, to provide a second material 3 that
includes cellulose having
a second number average molecular weight (T-MN2) lower than the first number
average
molecular weight. The second material (or the first and second material in
certain
embodiments) is/are combined with a microorganism (e.g., a bacterium or a
yeast) that can -
utilize the second and/or first material to produce a fuel 5 that is or
includes hydrogen, an
.
alcohol (e.g., ethanol or butanol, such as n-, sec or t-butanol), an
organic acid, a hydrocarbon or
= mixtures of any of these.'
Since the second material has cellulose having a reduced molecular weight
relative to
the first material, and in some instances, a reduced crystallinity as well,
the second material is
generally more dispersible, swellable and/or soluble in a solution containing
the
= microorganism, e.g., at a concentration of greater than 106
microorganisms/mL. These
properties make the second material 3 more susceptible to chemical, enzymatic
and/or
microbial attack relative to the first material 2, which can greatly improve
the production rate
and/or production level of a desired product, e.g., ethanol. Pyrolysis can
also sterilize the first
and second materials.
59
=
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
In some embodiments, the second number average molecular weight (TMN2) is
lower
than the first number average molecular weight (TMO by more than about 10
percent, e.g., 15,
20, 25, 30, 35, 40, 50 percent, 60 percent, or even more than about 75
percent.
In some instances, the second material has cellulose that has as crystallinity
(1C2) that is
lower than the crystallinity NO of the cellulose of the first material. For
example, (TC2) can
be lower than (1C1) by more than about 10 percent, e.g., 15, 20, 25, 30, 35,
40, or even more
than about 50 percent.
In some embodiments, the starting crystallinity (prior to pyrolysis) is from
about 40 to
about 87.5 percent, e.g., from about 50 to about 75 percent or from about 60
to about 70
percent, and the crystallinity index after pyrolysis is from about 10 to about
50 percent, e.g.,
from about 15 to about 45 percent or from about 20 to about 40 percent.
However, in certain
embodiments, e.g., after extensive pyrolysis, it is possible to have a
crystallinity index of lower
than 5 percent. In some embodiments, the material after pyrolysis is
substantially amorphous.
In some embodiments, the starting number average molecular weight (prior to
pyrolysis) is from about 200,000 to about 3,200,000, e.g., from about 250,000
to about
1,000,000 or from about 250,000 to about 700,000, and the number average
molecular weight
after pyrolysis is from about 50,000 to about 200,000, e.g., from about 60,000
to about 150,000
or from about 70,000 to about 125,000. However, in some embodiments, e.g.,
after extensive
pyrolysis, it is possible to have a number average molecular weight of less
than about 10,000 or
even less than about 5,000.
In some embodiments, the second material can have a level of oxidation (102)
that is
higher than the level of oxidation (T0i) of the first material. A higher level
of oxidation of the
material can aid in its dispersability, swellability and/or solubility,
further enhancing the
materials susceptibility to chemical, enzymatic or microbial attack. In some
embodiments, to
increase the level of the oxidation of the second material relative to the
first material, the
pyrolysis is performed in an oxidizing environment, producing a second
material that is more
oxidized than the first material. For example, the second material can have
more hydroxyl
groups, aldehyde groups, ketone groups, ester groups or carboxylic acid
groups, which can
increase its hydrophilicity.
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
In some embodiments, the pyrolysis of the materials is continuous. In other
embodiments, the material is pyrolyzed for a pre-determined time, and then
allowed to cool for
a second pre-determined time before pyrolyzing again.
Pyrolysis Systems
FIG. 14 shows a process flow diagram 6000 that includes various steps in a
pyrolytic
feedstock pretreatment system.. In first step 6010, a supply of dry feedstock
is received from a
feed source.
As described above, the dry feedstock from the feed source may be pre-
processed prior
to delivery to the pyrolysis chamber. For example, if the feedstock is derived
from plant
sources, certain portions of the plant material may be removed prior to
collection of the plant
material and/or before the plant material is delivered by the feedstock
transport device.
Alternatively, or in addition, the biomass feedstock can be subjected to
mechanical processing
6020 (e.g., to reduce the average length of fibers in the feedstock) prior to
delivery to the
pyrolysis chamber.
Following mechanical processing, the feedstock undergoes a moisture adjustment
step
6030. The nature of the moisture adjustment step depends upon the moisture
content of the
mechanically processed feedstock. Typically, pyrolysis of feedstock occurs
most efficiently
when the moisture content of the feedstock is between about 10% and about 30%
(e.g., between
15% and 25%) by weight of the feedstock. If the moisture content of the
feedstock is larger
than about 40% by weight, the extra thermal load presented by the water
content of the
feedstock increases the energy consumption of subsequent pyrolysis steps.
In some embodiments, if the feedstock has a moisture content which is larger
than about
30% by weight, drier feedstock material 6220 which has a low moisture content
can be blended
in, creating a feedstock. mixture in step 6030 with an average moisture
content that is within the
limits discussed above. In certain embodiments, feedstock with a high moisture
content can
simply be dried by dispersing the feedstock material on a moving conveyor that
cycles the
feedstock through an in-line heating unit. The heating unit evaporates a
portion of the water
present in the feedstock.
In some embodiments, if the feedstock from step 6020 has a moisture content
which is
too low (e.g., lower than about 10% by weight), the mechanically processed
feedstock can be
61
CA 02823376 2013-08-07
WO 2008/073186 PCTMS2007/022719
combined with wetter feedstock material 6230 with a higher moisture content,
such as sewage
sludge. Alternatively, or in addition, water 6240 can be added to the dry
feedstock from step
6020 to increase its moisture content.
In step 6040, the feedstock ¨ now with its moisture content adjusted to fall
within
suitable limits ¨ can be preheated in an optional preheating step 6040.
Preheating step 6040
can be used to increase the temperature of the feedstock to between 75 C and
150 C in
preparation for subsequent pyrolysis of the feedstock. Depending upon the
nature of the
feedstock and the particular design of the pyrolysis chamber, preheating the
feedstock can
ensure that heat distribution within the feedstock remains more uniform during
pyrolysis, and
can reduce the thermal load on the pyrolysis chamber.
The feedstock is then transported to a pyrolysis chamber to undergo pyrolysis
in step
6050. In some embodiments, transport of the feedstock is assisted by adding
one or more
pressurized gases 6210 to the feedstock stream. The gases create a pressure
gradient in a
feedstock transport conduit, propelling the feedstock into the pyrolysis
chamber (and even
through the pyrolysis chamber). In certain embodiments, transport of the
feedstock occurs
mechanically; that is, a transport system that includes a conveyor such as an
auger transports
the feedstock to the pyrolysis chamber.
Other gases 6210 can also be added to the feedstock prior to the pyrolysis
chamber. In
some embodiments, for example, one or more catalyst gases can be added to the
feedstock to
assist decomposition of the feedstock during pyrolysis. In certain
embodiments, one or more
scavenging agents can be added to the feedstock to trap volatile materials
released during
pyrolysis. For example, various sulfur-based compounds such as sulfides can be
liberated
during pyrolysis, and an agent such as hydrogen gas can be added to the
feedstock to cause
desulfurization of the pyrolysis products. Hydrogen combines with sulfides to
form hydrogen
sulfide gas, which can be removed from the pyrolyzed feedstock.
Pyrolysis of the feedstock within the chamber can include heating the
feedstock to
relatively high temperatures to cause partial decomposition of the feedstock.
Typically, the
feedstock is heated to a temperature in a range from 150 C to 1100 C. The
temperature to
which the feedstock is heated depends upon a number of factors, including the
composition of
the feedstock, the feedstock average particle size, the moisture content, and
the desired
62
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
pyrolysis products. For many types of biomass feedstock, for example,
pyrolysis temperatures
between 300 C and 550 'C are used.
The residence time of the feedstock within the pyrolysis chamber generally
depends
upon a number of factors, including the pyrolysis temperature, the composition
of the
feedstock, the feedstock average particle size, the moisture content, and the
desired pyrolysis
products. In some embodiments, feedstock materials are pyrolyzed at a
temperature just above
the decomposition temperature for the material in an inert atmosphere, e.g.,
from about 2 C
above to about 10 C above the decomposition temperature or from about 3 C
above to about 7
C above the decomposition temperature. In such embodiments, the material is
generally kept
at this temperature for greater than 0.5 hours, e.g., greater than 1.0 hours
or greater than about
2.0 hours. In other embodiments, the materials are pyrolyzed at a temperature
well above the
decomposition temperature for the material in an inert atmosphere, e.g., from
about 75 C
above to about 175 C above the decomposition temperature or from about 85 C
above to
about 150 C above the decomposition temperature. In such embodiments, the
material is
generally kept at this temperature for less than 0.5 hour, e.g., less 20
minutes, less than 10
minutes, less than 5 minutes or less than 2 minutes. In still other
embodiments, the materials
are pyrolyzed at an extreme temperature, e.g., from about 200 C above to
about 500 C above
the decomposition temperature of the material in an inert environment or from
about 250 C
above to about 400 C above the decomposition temperature. In such
embodiments, the
material us generally kept at this temperature for less than 1 minute, e.g.,
less than 30 seconds,
less than 15 seconds, less than 10 seconds, less than 5 seconds, less than 1
second or less than
500 ms. Such embodiments are typically referred to as flash pyrolysis.
In some embodiments, the feedstock is heated relatively rapidly to the
selected
pyrolysis temperature within the chamber. For example, the chamber can be
designed to heat
the feedstock at a rate of between 500 'Cis and 11,000 'C/s. Typical heating
rates for biomass-
derived feedstock material are from 500 'Cis to 1000 C/s, for example.
A turbulent flow of feedstock material within the pyrolysis chamber is usually
advantageous, as it ensures relatively efficient heat transfer to the
feedstock material from the
heating sub-system. Turbulent flow can be achieved by blowing the feedstock
material through
the chamber using one or more injected carrier gases 6210, for example. In
general, the carrier
gases are relatively inert towards the feedstock material, even at the high
temperatures in the
63
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
pyrolysis chamber. Exemplary carrier gases include, for example, nitrogen,
argon, methane,
carbon monoxide, and carbon dioxide. Alternatively, or in addition, mechanical
transport
systems such as augers can transport and circulate the feedstock within the
pyrolysis chamber
to create a turbulent feedstock flow.
In some embodiments, pyrolysis of the feedstock occurs substantially in the
absence of
oxygen and other reactive gases. Oxygen can be removed from the pyrolysis
chamber by
periodic purging of the chamber with high pressure nitrogen (e.g., at nitrogen
pressures of 2 bar
or more). Following purging of the chamber, a gas mixture present in the
pyrolysis chamber
(e.g., during pyrolysis of the feedstock) can include less than 4 mole% oxygen
(e.g., less than 1
mole% oxygen, and even less than 0.5 mole% oxygen). The absence of oxygen
ensures that
ignition of the feedstock does not occur at the elevated pyrolysis
temperatures.
In certain embodiments, relatively small amounts of oxygen can be introduced
into the
feedstock and are present during pyrolysis. This technique is referred to as
oxidative pyrolysis.
Typically, oxidative pyrolysis occurs in multiple heating stages. For example,
in a first heating
stage, the feedstock is heated in the presence of oxygen to cause partial
oxidation of the
feedstock. This stage consumes the available oxygen in the pyrolysis chamber.
Then, in
subsequent heating stages, the feedstock temperature is further elevated. With
all of the oxygen
in the chamber consumed, however, feedstock combustion does not occur, and
combustion-free
pyrolytic decomposition of the feedstock (e.g., to generate hydrocarbon
products) occurs. In
general, the process of heating feedstock in the pyrolysis chamber to initiate
decomposition is
endothermic. However, in oxidative pyrolysis, formation of carbon dioxide by
oxidation of the
feedstock is an exothermic process. The heat released from carbon dioxide
formation can assist
further pyrolysis heating stages, thereby lessening the thermal load presented
by the feedstock.
In some embodiments, pyrolysis occurs in an inert environment, such as while
feedstock materials are bathed in argon or nitrogen gas. In certain
embodiments, pyrolysis can
occur in an oxidizing environment, such as in air or argon enriched in air. In
some
embodiments, pyrolysis can take place in a reducing environment, such as while
feedstock
materials are bathed in hydrogen gas. To aid pyrolysis, various chemical
agents, such as
oxidants, reductants, acids or bases can be added to the material prior to or
during pyrolysis.
For example, sulfuric acid can be added, or a peroxide (e.g., benzoyl
peroxide) can be added.
64
CA 02823376 2013-08-07
WO 2008/073186 PCT/1JS2007/022719
As discussed above, a variety of different processing conditions can be used,
depending
upon factors such as the feedstock composition and the desired pyrolysis
products. For
example, for cellulose-containing feedstock material, relatively mild
pyrolysis conditions can
be employed, including flash pyrolysis temperatures between 375 "C and 450 C,
and residence
times of less than 1 second. As another example, for organic solid waste
material such as
sewage sludge, flash pyrolysis temperatures between 500 C and 650 'C are
typically used,
with residence times of between 0.5 and 3 seconds. In general, many of the
pyrolysis process
parameters, including residence time, pyrolysis temperature, feedstock
turbulence, moisture
content, feedstock composition, pyrolysis product composition, and additive
gas composition
can be regulated automatically by a system of regulators and an automated
control system.
Following pyrolysis step 6050, the pyrolysis products undergo a quenching step
6250 to
reduce the temperature of the products prior to further processing. Typically,
quenching step
6250 includes spraying the pyrolysis products with streams of cooling water
6260. The cooling
water also forms a slurry that includes solid, undissolved product material
and various
dissolved products. Also present in the product stream is a mixture that
includes various gases,
including product gases, carrier gases, and other types of process gases.
The product stream is transported via in-line piping to a gas separator that
performs a
gas separation step 6060, in which product gases and other gases are separated
from the slurry
formed by quenching the pyrolysis products. The separated gas mixture is
optionally directed
to a blower 6130, which increases the gas pressure by blowing air into the
mixture. The gas
mixture can be subjected to a filtration step 6140, in which the gas mixture
passes through one
or more filters (e.g., activated charcoal filters) to remove particulates and
other impurities. In a
subsequent step 6150, the filtered gas can be compressed and stored for
further use.
Alternatively, the filtered gas can be subjected to further processing steps
6160. For example,
in some embodiments, the filtered gas can be condensed to separate different
gaseous
compounds within the gas mixture. The different compounds can include, for
example, various
hydrocarbon products (e.g., alcohols, alkanes, allcenes, alkynes, ethers)
produced during
pyrolysis. In certain embodiments, the filtered gas containing a mixture of
hydrocarbon
components can be combined with steam gas 6170 (e.g., a mixture of water vapor
and oxygen)
and subjected to a cracking process to reduce molecular weights of the
hydrocarbon
components.
CA 02823376 2013-08-07
.;3983-7
In some embodiments, the pyrolysis chamber includes heat sources that burn
hydrocarbon gases such as methane, propane, .and/or butane to heat the
feedstock. A portion.
6270 of the separated gases can be recirculated into the pyrolysis chamber for
combustion, to
generate process heat to sustain the pyrolysis process.
In certain einbodiments, the pyrolysis chamber can receive process heat that
can be used
to increase the temperature of feedstock materials. For example, irradiating
feedstock with
= radiation (e.g., gamma radiation, electron beam radiation, or other types
of radiation) can heat
the feedstock materials to relatively high temperatures. The heated feedstock
materials can be
cooled by a heat exchange system that removes some of the excess heat from the
irradiated
feedstock: The heat exchange system can be configured to transport some of the
heat energy to .
the pyrolysis chamber to heat (or pre-heat) feedstock material, thereby
reducing energy cost for
= the pyrolysis process.
=
=
The slurry containing liquid and solid pyrolysis products can undergo an
optional de-
watering step 6070, in which excess water can be removed from the slurry via
processes such
as mechanical pressing and evaporation. The, excess water 6260 can be filtered
and then= -
recirculated for further use in quenching the pyrolysis decomposition products
in step 6250.
The de-watered slurry then undergoes a mechanical separation step 6080, in
which solid
= product material 6110 is separated from liquid product material 6099 by a
series of
. increasingly-fine filters. In step 6100, the liquid product
material 6090 can then be condensed
(e.g., via evaporation) to *remove waste water 6190, and purified by processes
such as
extraction. Extraction can include the addition of one or more organic
solvents 6180, for
example, to separate products such as oils from products such as alcohols.
Suitable organic
solvents include, for example, various hydrocarbons and halo-hydrocarbons. The
purified
liquid products 6200 can then be subjected to further processing steps. Waste
water 6190 can
be filtered if necessary, and recirculated for further use in quenching the
pyrolysis
decomposition products in step 6250.
After separation in step 6080, the solid product material 6110 is optionally
subjected to
a drying step 6120 that can include evaporation of water. Solid material 6110
can then be
stored for later use, or subjected to further processing steps, as
appropriate.
The pyrolysis process parameters discussed above are exemplary. In general,
values of
these parameters can vary widely according to the nature of the feedstock and
the desired
66
CA 02823376 2013-08-07
=
: .
/983-7
. -
- products. Moreover, a wide variety of different pyrolysis
techniques, including using heat
= sources such as hydrocarbon flames and/or furnaces, infrared lasers,
microwave heaters,
induction heaters, resistive heaters, and other heating devices and
configurations can be used.
A wide variety of different pyrolysis chambers can be used to decompose the
feedstock.
. ,
= In some embodiments, for example, pyrolyzing feedstock can include
heating the material
using a resistive heating member, such as a metal filament or metal ribbon.
The heating can
occur by direct contact between the resistive heating member and the material.
=
In certain embodiments, pyrolyzing can include heating the material by
induction, such
as by using a Currie-Point pyrolyzer. In some embodiments, pyrolyzing can
include heating
the material by the application of radiation, such as infrared radiation. The
radiation can be
generated by a laser, such as an infrared laser.
= In certain embodiments, pyrolyzing can include heating the material with
a convective
heat. The convective heat can be generated by a flowing stream of heated gas.
The heated gas
can be maintained at a temperature Of less than about 1200 C, such as less
than 1000 C, less
= than 750 C, less than 600 C, less than 400 C or even less than 300 C.
The heated gas can be
maintained at a temperature of greater than about 250 C. The convective heat
can be
generated by a hot body surrounding the first Material, such as in a furnace.
= In some embodiments, pyrolyzing can include heating the material with
steam at a
temperature above about 250 C.
An embodiment of a pyrolysis chamber is shown in FIG. 15. Chamber 6500
includes.
an insulated chamber wall 6510 with a vent 6600 for exhaust gases, a plurality
of burners 6520 =
that generate heat for the pyrolysis process, a transport duct 6530 for
transporting the feedstock
through chamber 6500, augers 6590 for moving the feedstock through duct 6530
in a turbulent
flow, and a quenching system 6540 that includes an auger 6610 for moving the
pyrolysis
products, water jets 6550 for spraying the pyrolysis products with cooling
water, and a gas-
.
separator for separating gaseous products 6560 from a shiny 6570 containing
solid and liquid
products.
= Another embodiment of a pyrolysis chamber is shown in FIG. 16. Chamber
6700
includes an insulated chamber wall 6710, a feedstock supply duct 6720, a
sloped inner chamber
wall 6730, burners 6740 that generate heat for the pyrolysis process, a vent
6750 for exhaust
gases, and a gas separator 6760 for separating gaseous products 6770 from
liquid and solid
= 67
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
products 6780. Chamber 6700 is configured to rotate in the direction shown by
arrow 6790 to
ensure adequate mixing and turbulent flow of the feedstock within the chamber.
A further embodiment of a pyrolysis chamber is shown in FIG. 17. Filament
pyrolyzer
1712 includes a sample holder 1713 with resistive heating element 1714 in the
form of a wire
winding through the open space defined by the sample holder 1713. Optionally,
the heated
element can be spun about axis 1715 (as indicated by arrow 1716) to tumble the
material that
includes the cellulosic material in sample holder 1713. The space 1718 defined
by enclosure
1719 is maintained at a temperature above room temperature, e.g., 200 to 250
C. In a typical
usage, a carrier gas, e.g., an inert gas, or an oxidizing or reducing gas,
traverses through the
sample holder 1713 while the resistive heating element is rotated and heated
to a desired
temperature, e.g., 325 C. After an appropriate time, e.g., 5 to 10 minutes,
the pyrolyzed
material is emptied from the sample holder. The system shown in FIG. 17 can be
scaled and
made continuous. For example, rather than a wire as the heating member, the
heating member
can be an auger screw. Material can continuously fall into the sample holder,
striking a heated
screw that pyrolizes the material. At the same time, the screw can push the
pyrolyzed material
out of the sample holder to allow for the entry of fresh, unpyrolyzed
material.
Another embodiment of a pyrolysis chamber is shown in FIG. 18, which features
a
Curie-Point pyrolyzer 1820 that includes a sample chamber 1821 housing a
ferromagnetic foil
1822. Surrounding the sample chamber 1821 is an RF coil 1823. The space 1824
defined by
enclosure 1825 is maintained at a temperature above room temperature, e.g.,
200 to 250 C. In
a typical usage, a carrier gas traverses through the sample chamber 1821 while
the foil 1822 is
inductively heated by an applied RF field to pyrolize the material at a
desired temperature.
Yet another embodiment of a pyrolysis chamber is shown in FIG. 19. Furnace
pyrolyzer 130 includes a movable sample holder 131 and a furnace 132. In a
typical usage, the
sample is lowered (as indicated by arrow 137) into a hot zone 135 of furnace
132, while a
carrier gas fills the housing 136 and traverses through the sample holder 131.
The sample is
heated to the desired temperature for a desired time to provide a pyrolyzed
product. The
pyrolyzed product is removed from the pyrolyzer by raising the sample holder
(as indicated by
arrow 134).
In certain embodiments, as shown in FIG. 20, a cellulosic target 140 can be
pyrolyzed
by treating the target, which is housed in a vacuum chamber 141, with laser
light, e.g., light
68
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
having a wavelength of from about 225 nm to about 1500 urn. For example, the
target can be
ablated at 266 nm, using the fourth harmonic of a Nd-YAG laser (Spectra
Physics, GCR170,
San Jose, Calif.). The optical configuration shown allows the nearly
monochromatic light 143
generated by the laser 142 to be directed using mirrors 144 and 145 onto the
target after passing
though a lens 146 in the vacuum chamber 141. = Typically, the pressure in the
vacuum chamber
is maintained at less than about 10-6 mm Hg. In some embodiments, infrared
radiation is used,
e.g., 1.06 micron radiation from a Nd-YAG laser. In such embodiments, a
infrared sensitive
dye can be combined with the cellulosic material to produce a cellulosic
target. The infrared
dye can enhance the heating of the cellulosic material. Laser ablation is
described by Blanchet-
Fincher et al. in U.S. Patent No. 5,942,649.
Referring to FIG. 21, in some embodiments, a cellulosic material can be flash
pyrolyzed
by coating a tungsten filamgnt 150, such as a 5 to 25 mil tungsten filament,
with the desired
cellulosic material while the material is housed in a vacuum chamber 151. To
affect pyrolysis,
current is passed through the filament, which causes a rapid heating of the
filament for a
desired time. Typically, the heating is continued for seconds before allowing
the filament to
cool. In some embodiments, the heating is performed a number of times to
effect the desired
amount of pyrolysis.
In certain embodiments, carbohydrate-containing biomass material can be heated
in an
absence of oxygen in a fluidized bed reactor. If desired, the carbohydrate
containing biomass
can have relatively thin cross-sections, and can include any of the fibrous
materials described
herein, for efficient heat transfer. The material can be heated by thermal
transfer from a hot
metal or ceramic, such as glass beads or sand in the reactor, and the
resulting pyrolysis liquid or
oil can be transported to a central refinery for making combustible fuels or
other useful
products.
Oxidation
One or more oxidative processing sequences can be used to process raw
feedstock from
a wide variety of different sources to extract useful substances from the
feedstock, and to
provide partially degraded organic material which functions as input to
further processing steps
and/or sequences.
69
= CA 02823376 2013-08-07
3983-7
Referring again to FIG. 8, a first material 2 that includes cellulose having a
first number
average molecular weight (TMN1) and having a first oxygen content (T01) is
oxidized, e.g., by
heating the first material in a tube furnace in stream of air or oxygen-
enriched air, to provide a
second material 3 that includes cellulose having a second number average
molecular weight
(TMND and having a Second oxygen content (T02) higher than the first oxygen
content (TO)).
The second material (or the first and second material in certain embodiments)
can be, e.g.,
combined with a resin, such as a molten thermoplastic resin or a
microorganism, to provide a
= composite 4 having desirable mechanical properties, or a fuel 5.
Providing a higher level of
oxidation can improve dispersability of the oxidized material in a resin and
can also improve
the interfacial bond-between the oxidized Material and the resin. Improved
dispersability
= and/or interfacial bonding (in some instances in combination with
maintaining molecular
weight) can provide composites with exceptional mechanical properties, such as
improved
abrasion resistance, compression strength, fracture resistance, impact
strength, bending
strength, tensile modulus, flexural modulus and elongation at break.
Such materials can also be combined with a solid and/or a liquid. For example,
the
liquid can be in the form of a solution and the solid can be particulate in
form. The liquid
= and/or solid can include a microorganism, e.g:, a bacterium, and/or
an enzyme. For example,= =
the bacterium and/or enzyme can Work on the cellulosic or lignocellulosic
material to produce a
fuel, Such as-ethanol, or a coproduct, such as a protein. Fuels and coproducts
are described in
= US Patent No. 7,708,214.
In some embodiments, the second number average molecular weight is not more 97
percent lower than the first number average molecular weight, e.g., not more
than 95 percent,
90, 85, 80, 75, 70, 65,.60, 55, 50, 45, 40, 30, 20, 12.5, 10.0, 7.5, 5.0,4.0,
3.0, 2.5, 2.0 or nOt
= more than 1.0 percent lower than the first number average molecular
weight. The amount of
reduction of molecular weight will depend upon the application. For example,
in some
preferred embodiments that provide composites, the second number average
molecular weight
is substantially the same as the first number average molecular weight In
other applications,
such as making ethanol or another fuel or coproduct, a higher amount of
molecular weight
reduction is generally preferred.
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
For example, in some embodiments that provide a composite, the starting number
average molecular weight (prior to oxidation) is from about 200,000 to about
3,200,000, e.g.,
from about 250,000 to about 1,000,000 or from about 250,000 to about 700,000,
and the
number average molecular weight after oxidation is from about 175,000 to about
3,000,000,
e.g., from about 200,000 to about 750,000 or from about 225,000 to about
600,000.
Resins utilized can be thermosets or thermoplastics. Examples of thermoplastic
resins
include rigid and elastomeric thermoplastics. Rigid thermoplastics include
polyolefins (e.g.,
polyethylene, polypropylene, or polyolefin copolymers), polyesters (e.g.,
polyethylene
terephthalate), polyamides (e.g., nylon 6, 6/12 or 6/10), and
polyethyleneimines. Examples of
elastomeric thermoplastic resins include elastomeric styrenic copolymers
(e.g., styrene-
ethylene-butylene-styrene copolymers), polyamide elastomers (e.g., polyether-
polyamide
copolymers) and ethylene-vinyl acetate copolymer.
In particular embodiments, lignin is utilized, e.g., any lignin that is
generated in any
process described herein.
In some embodiments, the thermoplastic resin has a melt flow rate of between
10 g/10
minutes to 60 g/10 minutes, e.g., between 20 g/10 minutes to 50 g/10 minutes,
or between 30
g/10 minutes to 45 g/10 minutes, as measured using ASTM 1238. In certain
embodiments,
compatible blends of any of the above thermoplastic resins can be used.
In some embodiments, the thermoplastic resin has a polydispersity index (PD!),
i.e., a
ratio of the weight average molecular weight to the number average molecular
weight, of
greater than 1.5, e.g., greater than 2.0, greater than 2.5, greater than 5.0,
greater than 7.5, or
even greater than 10Ø
In specific embodiments, polyolefins or blends of polyolefins are utilized as
the
thermoplastic resin.
Examples of thermosetting resins include natural rubber, butadiene-rubber and
polyurethanes.
In some embodiments in which the materials are used to make a fuel or a
coproduct, the
starting number average molecular weight (prior to oxidation) is from about
200,000 to about
3,200,000, e.g., from about 250,000 to about 1,000,000 or from about 250,000
to about
700,000, and the number average molecular weight after oxidation is from about
50,000 to
about 200,000, e.g., from about 60,000 to about 150,000 or from about 70,000
to about
71
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
125,000. However, in some embodiments, e.g., after extensive oxidation, it is
possible to have
a number average molecular weight of less than about 10,000 or even less than
about 5,000.
In some embodiments, the second oxygen content is at least about five percent
higher
than the first oxygen content, e.g., 7.5 percent higher, 10.0 percent higher,
12.5 percent higher,
15.0 percent higher or 17.5 percent higher. In some preferred embodiments, the
second oxygen
content is at least about 20.0 percent higher than the oxygen content of the
first material.
Oxygen content is measured by elemental analysis by pyrolyzing a sample in a
furnace
operating 1300 C or higher. A suitable elemental analyzer is the LECO CHNS-
932 analyzer
with a VTF-900 high temperature pyrolysis furnace.
In some embodiments, oxidation of first material 200 does not result in a
substantial
change in the crystallinity of the cellulose. However, in some instances,
e.g., after extreme
oxidation, the second material has cellulose that has as crystallinity (1C2)
that is lower than the
crystallinity (TC1) of the cellulose of the first material. For example, (TC2)
can be lower than
(1C1) by more than about 5 percent, e.g., 10, 15, 20, or even 25 percent. This
can be desirable
when optimizing the flexural fatigue properties of the composite is a goal.
For example,
reducing the crystallinity can improve the elongation at break or can enhance
the impact
resistance of a composite. This can also be desirable to enhance solubility of
the materials in a
liquid, such as a liquid that includes a bacterium and/or an enzyme.
In some erribodiments, the starting crystallinity index (prior to oxidation)
is from about
40 to about 87.5 percent, e.g., from about 50 to about 75 percent or from
about 60 to about 70
percent, and the crystallinity index after oxidation is from about 30 to about
75.0 percent, e.g.,
from about 35.0 to about 70.0 percent or from about 37.5 to about 65.0
percent. However, in
certain embodiments, e.g., after extensive oxidation, it is possible to have a
crystallinity index
of lower than 5 percent. In some embodiments, the material after oxidation is
substantially
amorphous.
Without wishing to be bound by any particular theory, it is believed that
oxidation
increases the number of hydrogen-bonding groups on the cellulose, such as
hydroxyl groups,
aldehyde groups, ketone groups carboxylic acid groups or anhydride groups,
which can
increase its dispersability and/or its solubility (e.g., in a liquid). To
further improve
dispersability in a resin, the resin can include a component that includes
hydrogen-bonding
groups, such as one or more anhydride groups, carboxylic acid groups, hydroxyl
groups, amide
72
CA 02823376 2013-08-07
. '1983-7
groups, amine groups or mixtures of any of these groups. In some preferred
embodiments, the
component includes a-polymer copolymerized with and/or grafted With maleic
anhydride. Such
=
materials are available from Dupont under the tradenameFUSABOND . =
. Generally, oxidation of first material 2 occurs in an
oxidizing environment. For
example, the oxidation can be effected or aided by pyrolysis in an oxidizing
environment, such
as in air or argon enriched in air. To aid in the oxidation, various chemical
agents, such as
oxidants, acids or bases can be added to the material prior to or during
oxidation. For example,
a peroxide (e.g., benzpyl peroxide) can be added prior to oxidation.
Oxidation Systems
FIG. 22 shows a process flow diagram 5000 that includes various steps in an
oxidative
feedstock pretreatment system. In first step 5010, a supply of dry feedstock
is received from a =
feed source. The feed source can include, for example, a storage bed or
container that is
connected to an in-line oxidation reactor via a conveyor belt or another
feedstock transport
= device.
As described above, the dry feedstock from the feed source may be pre-
processed prior
to delivery to the oxidation reactor. For example, if the feedstock is derived
from plant
= sources, certain portions of the plant material may be removed prior to
collection of the plant
= material and/or before the plant material is delivered by the feedstock
transport device.
Alternatively, or in addition, the biomass feedstock can be subjected to
mechanical processing =
(e.g., to reduce the average length of fibers in the feedstock) prior to
delivery to the oxidation
reactor.
. . Following mechanical processing 5029, feedstock 5030 is
transported to a mixing
system which introduces water 5150 into the feedstock in a mechanical mixing
process.
Combining water with the processed feedstock in mixing step 5040 creates an
aqueous
feedstock slurry 5050 which can then be treated=with one or more oxidizing
agents. =
Typically, one liter of water is added to the mixture for every 0.02 kg to 1.0
kg of dry
= feedstock. The ratio of feedstock to water in the mixture depends upon
the source of the
feedstock and the specific oxidizing agents used further downstream in the
overall process. For
example, in typical industrial processing sequences for lignocellulosic
biomass, aqueous
73
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
feedstock slurry 5050 includes from about 0.5 kg to about 1.0 kg of dry
biomass per liter of
water.
In some embodiments, one or more fiber-protecting additives 5170 dan also be
added to
the feedstock slurry in feedstock mixing step 5040. Fiber-protecting additives
help to reduce
degradation of certain types of biomass fibers (e.g., cellulose fibers) during
oxidation of the
feedstock. Fiber-protecting additives can be used, for example, if a desired
product from
processing a lignocellulosic feedstock includes cellulose fibers. Exemplary
fiber-protecting
additives include magnesium compounds such as magnesium hydroxide.
Concentrations of
fiber-protecting additives in feedstock slurry 5050 can be from 0.1% to 0.4%
of the dry weight
of the biomass feedstock, for example.
In certain embodiments, aqueous feedstock slurry 5050 can be subjected to an
optional
extraction 5180 with an organic solvent to remove water-insoluble substances
from the slurry.
For example, extraction of slurry 5050 with one or more organic solvents
yields a purified
slurry and an organic waste stream 5210 that includes water-insoluble
materials such as fats,
oils, and other non-polar, hydrocarbon-based substances. Suitable solvents for
performing
extraction of slurry 5050 include various alcohols, hydrocarbons, and halo-
hydrocarbons, for
example.
In some embodiments, aqueous feedstock slurry 5050 can be subjected to an
optional
thermal treatment 5190 to further prepare the feedstock for oxidation. An
example of a thermal
treatment includes heating the feedstock slurry in the presence of pressurized
steam. In fibrous
biomass feedstock, the pressurized steam swells the fibers, exposing a larger
fraction of fiber
surfaces to the aqueous solvent and to oxidizing agents that are introduced in
subsequent
processing steps.
In certain embodiments, aqueous feedstock slurry 5050 can be subjected to an
optional
treatment with basic agents 5200. Treatment with one or more basic agents can
help to
separate lignin from cellulose in lignocellulosic biomass feedstock, thereby
improving
subsequent oxidation of the feedstock. Exemplary basic agents include alkali
and alkaline earth
hydroxides such as sodium hydroxide, potassium hydroxide, and calcium
hydroxide. In
general, a variety of basic agents can be used, typically in concentrations
from about 0.01% to
about 0.5% of the dry weight of the feedstock.
74
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Aqueous feedstock slurry 5050 is transported (e.g., by an in-line piping
system) to a
chamber, which can be an oxidation preprocessing chamber or an oxidation
reactor. In
oxidation preprocessing step 5060, one or more oxidizing agents 5160 are added
to feedstock
slurry 5050 to form an oxidizing medium. In some embodiments, for example,
oxidizing
agents 5160 can include hydrogen peroxide. Hydrogen peroxide can be added to
slurry 5050 as
an aqueous solution, and in proportions ranging from 3% to between 30% and 35%
by weight
of slurry 5050. Hydrogen peroxide has a number of advantages as an oxidizing
agent. For
example, aqueous hydrogen peroxide solution is relatively inexpensive, is
relatively chemically
stable, is not particularly hazardous relative to other oxidizing agents (and
therefore does not
require burdensome handling procedures and expensive safety equipment).
Moreover,
hydrogen peroxide decomposes to form water during oxidation of feedstock, so
that waste
stream cleanup is relatively straightforward and inexpensive.
In certain embodiments, oxidizing agents 5160 can include oxygen (e.g., oxygen
gas)
either alone, or in combination with hydrogen peroxide. Oxygen gas can be
bubbled into slurry
5050 in proportions ranging from 0.5% to 10% by weight of slurry 5050.
Alternatively, or in
addition, oxygen gas can also be introduced into a gaseous phase in
equilibrium with slurry
5050 (e.g., a vapor head above slurry 5050). The oxygen gas can be introduced
into either an
oxidation preprocessing chamber or into an oxidation reactor (or into both),
depending upon the
configuration of the oxidative processing system. Typically, for example, the
partial pressure
of oxygen in the vapor above slurry 5050 is larger than the ambient pressure
of oxygen, and
ranges from 0.5 bar to 35 bar, depending upon the nature of the feedstock.
The oxygen gas can be introduced in pure form, or can be mixed with one or
more
carrier gases. For example, in some embodiments, high-pressure air provides
the oxygen in the
vapor. In certain embodiments, oxygen gas can be supplied continuously to the
vapor phase to
ensure that a concentration of oxygen in the vapor remains within certain
predetermined limits
during processing of the feedstock. In some embodiments, oxygen gas can be
introduced
initially in sufficient concentration to oxidize the feedstock, and then the
feedstock can be
transported to a closed, pressurized vessel (e.g., an oxidation reactor) for
processing.
In certain embodiments, oxidizing agents 5160 can include nascent oxygen
(e.g.,
oxygen radicals). Typically, nascent oxygen is produced as needed in an
oxidation reactor or in
a chamber in fluid communication with an oxidation reactor by one or more
decomposition
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
reactions. For example, in some embodiments, nascent oxygen can be produced
from a
reaction between NO and 02 in a gas mixture or in solution. In certain
embodiments, nascent
oxygen can be produced from decomposition of HOCI in solution. Other methods
by which
nascent oxygen can be produced include via electrochemical generation in
electrolyte solution,
for example.
In general, nascent oxygen is an efficient oxidizing agent due to the
relatively high
reactivity of the oxygen radical. However, nascent oxygen can also be a
relatively selective
oxidizing agent. For example, when lignocellulosic feedstock is treated with
nascent oxygen,
selective oxidation of lignin occurs in preference to the other components of
the feedstock such
as cellulose. As a result, oxidation of feedstock with nascent oxygen provides
a method for
selective removal of the lignin fraction in certain feedstocks. Typically,
nascent oxygen
concentrations of between about 0.5% and 5% of the dry weight of the feedstock
are used to
effect efficient oxidation.
Without wishing to be bound by theory, it is believed that nascent oxygen
reacts with
lignocellulosic feedstock according to at least two different mechanisms. In a
first mechanism,
nascent oxygen undergoes an addition reaction with the lignin, resulting in
partial oxidation of
the lignin, which solubilizes the lignin in aqueous solution. As a result, the
solubilized lignin
can be removed from the rest of the feedstock via washing. In a second
mechanism, nascent
oxygen disrupts butane cross-links and/or opens aromatic rings that are
connected via the
butane cross-links. As a result, solubility of the lignin in aqueous solution
increases, and the
lignin fraction can be separated from the remainder of the feedstock via
washing.
In some embodiments, oxidizing agents 5160 include ozone (03). The use of
ozone can
introduce several chemical handling considerations in the oxidation processing
sequence. If
heated too vigorously, an aqueous solution of ozone can decompose violently,
with potentially
adverse consequences for both human system operators and system equipment.
Accordingly,
ozone is typically generated in a thermally isolated, thick-walled vessel
separate from the
vessel that contains the feedstock slurry, and transported thereto at the
appropriate process
stage.
Without wishing to be bound by theory, it is believed that ozone decomposes
into
oxygen and oxygen radicals, and that the oxygen radicals (e.g., nascent
oxygen) are responsible
for the oxidizing properties of ozone in the manner discussed above. Ozone
typically
76 =
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
preferentially oxidizes the lignin fraction in lignocellulosic materials,
leaving the cellulose
fraction relatively undisturbed.
Conditions for ozone-based oxidation of biomass feedstock generally depend
upon the
nature of the biomass. For example, for cellulosic and/or lignocellulosic
feedstocks, ozone
concentrations of from 0.1 g/m3 to 20 g/m3 of dry feedstock provide for
efficient feedstock
oxidation. Typically, the water content in slurry 5050 is between 10% by
weight and 80% by
weight (e.g., between 40% by weight and 60% by weight). During ozone-based
oxidation, the
temperature of slurry 5050 can be maintained between 0 C and 100 C to avoid
violent
decomposition of the ozone.
In some embodiments, feedstock slurry 5050 can be treated with an aqueous,
alkaline
solution that includes one or more alkali and alkaline earth hydroxides such
as sodium
hydroxide, potassium hydroxide, and calcium hydroxide, and then treated
thereafter with an
ozone-containing gas in an oxidation reactor. This process has been observed
to significantly
increase decomposition of the biomass in slurry 5050. Typically, for example,
a concentration
of hydroxide ions in the alkaline solution is between 0.001% and 10% by weight
of slurry
5050. After the feedstock has been wetted via contact with the alkaline
solution, the ozone-
containing gas is introduced into the oxidation reactor, where it contacts and
oxidizes the
feedstock.
Oxidizing agents 5160 can also include other substances. In some embodiments,
for
example, halogen-based oxidizing agents such as chlorine and oxychlorine
agents (e.g.,
hypochlorite) can be introduced into slurry 5050. In certain embodiments,
nitrogen-containing
oxidizing substances can be introduced into slurry 5050. Exemplary nitrogen-
containing
oxidizing substances include NO and NO2, for example. Nitrogen-containing
agents can also
be combined with oxygen in slurry 5050 to create additional oxidizing agents.
For example,
NO and NO2 both combine with oxygen in slurry 5050 to form nitrate compounds,
which are
effective oxidizing agents for biomass feedstock. Halogen- and nitrogen-based
oxidizing
agents can, in some embodiments, cause bleaching of the biomass feedstock,
depending upon
the nature of the feedstock. The bleaching may be desirable for certain
biomass-derived
products that are extracted in subsequent processing steps.
Other oxidizing agents can include, for example, various peroxyacids,
peroxyacetic
acids, persulfates, percarbonates, permanganates, osmium tetroxide, and
chromium oxides.
77
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
=
=
Following oxidation preprocessing step 5060, feedstock slurry 5050 is oxidized
in step
5070. If oxidizing agents 5160 were added to slurry 5050 in an oxidation
reactor, then
oxidation proceeds in the same reactor. Alternatively, if oxidizing agents
5160 were added to
slurry 5050 in a preprocessing chamber, then slurry 5050 is transported to an
oxidation reactor
via an in-line piping system. Once inside the oxidation reactor, oxidation of
the biomass
feedstock proceeds under a controlled set of environmental conditions.
Typically, for example,
the oxidation reactor is a cylindrical vessel that is closed to the external
environment and
pressurized. Both batch and continuous operation is possible, although
environmental
conditions are typically easier to control in in-line batch processing
operations.
Oxidation of feedstock slurry 5050 typically occurs at elevated temperatures
in the
oxidation reactor. For example, the temperature of slurry 5050 in the
oxidation reactor is
typically maintained above 100 C, in a range from 120 C to 240 C. For many
types of
biomass feedstock, oxidation is particularly efficient if the temperature of
slurry 5050 is
maintained between 150 C and 220 C. Slurry 5050 can be heating using a
variety of thermal
transfer devices. For example, in some embodiments, the oxidation reactor
contacts a heating
bath that includes oil or molten salts. In certain embodiments, a series of
heat exchange pipes
surround and contact the oxidation reactor, and circulation of hot fluid
within the pipes heats
slurry 5050 in the reactor. Other heating devices that can be used to heat
slurry 5050 include
resistive heating elements, induction heaters, and microwave sources, for
example.
The residence time of feedstock slurry 5050 in the oxidation reactor can be
varied as
desired to process the feedstock. Typically, slurry 5050 spends from 1 minute
to 60 minutes
undergoing oxidation in the reactor. For relatively soft biomass material such
as lignocellulosic
matter, the residence time in the oxidation reactor can be from 5 minutes to
30 minutes, for
example, at an oxygen pressure of between 3 and 12 bars in the reactor, and at
a slurry
temperature of between 160 C and 210 C. For other types of feedstock,
however, residence
times in the oxidation reactor can be longer, e.g., as long 48 hours. To
determine appropriate
residence times for slurry 5050 in the oxidation reactor, aliquots of the
slurry can be extracted
from the reactor at specific intervals and analyzed to determine
concentrations of particular
products of interest such as complex saccharides. Information about the
increase in
concentrations of certain products in slurry 5050 as a function of time can be
used to determine
residence times for particular classes of feedstock material.
78
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
In some embodiments, during oxidation of feedstock slurry 5050, adjustment of
the
slurry pH may be performed by introducing one or more chemical agents into the
oxidation
reactor. For example, in certain embodiments, oxidation occurs most
efficiently in a pH range
of about 9-11. To maintain a pH in this range, agents such as alkali and
alkaline earth
hydroxides, carbonates, ammonia, and alkaline buffer solutions can be
introduced into the
oxidation reactor.
Circulation of slurry 5050 during oxidation can be important to ensure
sufficient contact
between oxidizing agents 5160 and the feedstock. Circulation of the slurry can
be achieved
using a variety of techniques. For example, in some embodiments, a mechanical
stirring
apparatus that includes impeller blades or a paddle wheel can be implemented
in the oxidation
reactor. In certain embodiments, the oxidation reactor can be a loop reactor,
in which the
aqueous solvent in which the feedstock is suspended is simultaneously drained
from the bottom
of the reactor and recirculated into the top of the reactor via pumping,
thereby ensuring that the
slurry is continually re-mixed and does not stagnate within the reactor.
After oxidation of the feedstock is complete, the slurry is transported to a
separation
apparatus where a mechanical separation step 5080 occurs. Typically,
mechanical separation
step 5080 includes one or more stages of increasingly-fine filtering of the
slurry to
mechanically separate the solid and liquid constituents.
Liquid phase 5090 is separated from solid phase 5100, and the two phases are
processed
independently thereafter. Solid phase 5100 can optionally undergo a drying
step 5120 in a
drying apparatus, for example. Drying step 5120 can include, for example,
mechanically
dispersing the solid material onto a drying surface, and evaporating water
from solid phase
5100 by gentle heating of the solid material. Following drying step 5120 (or,
alternatively,
without undergoing drying step 5120), solid phase 5100 is transported for
further processing
steps 5140.
Liquid phase 5090 can optionally undergo a drying step 5110 to reduce the
concentration of water in the liquid phase. In some embodiments, for example,
drying step
5110 can include evaporation and/or distillation and/or extraction of water
from liquid phase
5090 by gentle heating of the liquid. Alternatively, or in addition, one or
more chemical drying
agents can be used to remove water from liquid phase 5090. Following drying
step 5110 (or
alternatively, without undergoing drying step 5110), liquid phase 5090 is
transported for further
79
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
processing steps 5130, which can include a variety of chemical and biological
treatment steps
such as chemical and/or enzymatic hydrolysis.
Drying step 5110 creates waste stream 5220, an aqueous solution that can
include
dissolved chemical agents such as acids and bases in relatively low
concentrations. Treatment
of waste stream 5220 can include, for example, pH neutralization with one or
more mineral
acids or bases. Depending upon the concentration of dissolved salts in waste
stream 5220, the
solution may be partially de-ionized (e.g., by passing the waste stream
through an ion exchange
system). Then, the waste stream ¨ which includes primarily water ¨ can be re-
circulated into
the overall process (e.g., as water 5150), diverted to another process, or
discharged.
Typically, for lignocellulosic biomass feedstocks following separation step
5070, liquid
phase 5090 includes a variety of soluble poly- and oligosaccharides, which can
then be
separated and/or reduced to smaller-chain saccharides via further processing
steps. Solid phase
5100 typically includes primarily cellulose, for example, with smaller amounts
of
hemicellulose- and lignin-derived products.
In some embodiments, oxidation can be carried out at elevated temperature in a
reactor
such as a pyrolysis chamber. For example, referring again to FIG. 17,
feedstock materials can
be oxidized in filament pyrolyzer 1712. In a typical usage, an oxidizing
carrier gas, e.g., air or
an air/argon blend, traverses through the sample holder 1713 while the
resistive heating
element is rotated and heated to a desired temperature, e.g., 325 C. After an
appropriate time,
e.g., 5 to 10 minutes, the oxidized material is emptied from the sample
holder. The system
shown in FIG. 2 can be scaled and made continuous. For example, rather than a
wire as the
heating member, the heating member can be an auger screw. Material can
continuously fall
into the sample holder, striking a heated screw that pyrolizes the material.
At the same time,
the screw can push the oxidized material out of the sample holder to allow for
the entry of
fresh, unoxidized material.
Referring again to FIG. 18, feedstock materials can be oxidized in a Curie-
Point
pyrolyzer 1820. In a typical usage, an oxidizing carrier gas traverses through
the sample
chamber 1821 while the foil 1822 is inductively heated by an applied RF field
to oxidize the
material at a desired temperature.
Referring again to FIG. 19, feedstock materials can be oxidized in a furnace
pyrolyzer
130. In a typical usage, the sample is lowered (as indicated by arrow 137)
into a hot zone 135
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
of furnace 132, while an oxidizing carrier gas fills the housing 136 and
traverses through the
sample holder 131. The sample is heated to the desired temperature for a
desired time to
provide an oxidized product. The oxidized product is removed from the
pyrolyzer by raising
the sample holder (as indicated by arrow 134).
Referring again to FIG. 20, feedstock materials can be oxidized by forming a
cellulosic
target 140, along with an oxidant, such as a peroxide, and treating the
target, which is housed in
a vacuum chamber 141, with laser light, e.g., light having a wavelength of
from about 225 rim
to about 1600 nm. The optical configuration shown allows the monochromatic
light 143
generated by the laser 142 to be directed using mirrors 144 and 145 onto the
target after passing
though a lens 146 in the vacuum chamber 141. Typically, the pressure in the
vacuum chamber
is maintained at less than about le mm Hg. In some embodiments, infrared
radiation is used,
e.g., 1.06 micron radiation from a Nd-YAG laser. In such embodiments, a
infrared sensitive
dye can be combined with the cellulosic material to produce a cellulosic
target. The infrared
dye can enhance the heating of the cellulosic material. Laser treatment of
polymers is
described by Blanchet-Fincher et al. in U.S. Patent No. 5,942,649.
Referring again to FIG. 21, feedstock materials can be rapidly oxidized by
coating a
tungsten filament 150, together with an oxidant, such as a peroxide, with the
desired cellulosic
material while the material is housed in a vacuum chamber 151. To affect
oxidation, current is
passed through the filament, which causes a rapid heating of the filament for
a desired time.
Typically, the heating is continued for seconds before allowing the filament
to cool. In some
embodiments, the heating is performed a number of times to effect the desired
amount of
oxidation.
Referring again to FIG. 12, in some embodiments, feedstock materials can be
oxidized
with the aid of sound and/or cavitation. Generally, to effect oxidation, the
materials are
sonicated in an oxidizing environment, such as water saturated with oxygen or
another
chemical oxidant, such as hydrogen peroxide.
Referring again to FIGS. 9 and 10, in certain embodiments, ionizing radiation
is used to
aid in the oxidation of feedstock materials. Generally, to effect oxidation,
the materials are
irradiated in an oxidizing environment, such as air or oxygen. For example,
gamma radiation
and/or electron beam radiation can be employed to irradiate the materials.
81
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Other Processes
Steam explosion can be used alone without any of the processes described
herein, or in
combination with any of the processes described herein.
FIG 23 shows an overview of the entire process of converting a fiber source
400 into a
product 450, such as ethanol, by a process that includes shearing and steam
explosion to
produce a fibrous material 401, which is then hydrolyzed and converted, e.g.,
fermented, to
produce the product. The fiber source can be transformed into the fibrous
material 401 through
a number of possible methods, including at least one shearing process and at
least one steam
explosion process.
For example, one option includes shearing the fiber source, followed by
optional
screening step(s) and optional additional shearing step(s) to produce a
sheared fiber source 402,
which can then be steam exploded to produce the fibrous material 401. The
steam explosion
process is optionally followed by a fiber recovery process to remove liquids
or the "liquor"
404, resulting from the steam exploding process. The material resulting from
steam exploding
the sheared fiber source may be further sheared by optional additional
shearing step(s) and/or
optional screening step(s).
In another method, the fibrous material 401 is first steam exploded to produce
a steam
exploded fiber source 410. The resulting steam exploded fiber source is then
subjected to an
optional fiber recovery process to remove liquids, or the liquor. The
resulting steam exploded
fiber source can then be sheared to produce the fibrous material. The steam
exploded fiber
source can also be subject to one or more optional screening steps and/or one
or more optional
additional shearing steps. The process of shearing and steam exploding the
fiber source to
produce the sheared and steam exploded fibrous material will be further
discussed below.
The fiber source can be cut into pieces or strips of confetti material prior
to shearing or
steam explosion. The shearing processes can take place in a dry (e.g., having
less than 0.25
percent by weight absorbed water), hydrated, or even while the material is
partially or fully
submerged in a liquid, such as water or isopropanol. The process can also
optimally include
steps of drying the output after steam exploding or shearing to allow for
additional steps of dry
shearing or steam exploding. The steps of shearing, screening, and steam
explosion can take
place with or without the presence of various chemical solutions.
82
= CA 02823376 2013-08-07
''1983-7
=
=
In a steam explosion process, the fiber source or the sheared fiber source is
contacted
with steam under high pressure, and the steam diffuses into the structures of
the fiber source
(e.g., the lignocellulosic structures). The steam then condenses under high
pressure thereby.
"wetting" the fiber source. The moisture in the fiber source can hydrolyze any
acetyl groups in
the fiber source (e.g., the acetyl groups in the hemicellulose fractions),
forming organic acids
such as acetic and uronic acids. The acids, in turn, can catalyze the
depolymerization of
hemicellulose, releasing xylan and limited amounts of glucan. The "wet". fiber
source (or
sheared fiber source, etc.) is then "exploded" when the pressure is released.
The condensed
moisture instantaneously evaporates due to the sudden decrease in pressure and
the expansion
of the water vapor exerts a shear force upon the fiber source (or sheared
fiber source, etc.). A
sufficient shear force will cause the mechanical breakdown of the internal
structures (e.g., the
= the ligocellulosic struructures) of the fiber source.
The sheared and steam exploded fibrous material is then converted into a
useful
product, such as ethanol. In some embodiments, the fibrous material is
converted into a fuel..
One method of converting the fibrous material into a fuel is by hydrolysis to
produce
fermentable sugars, 412, which are then fermented to produce the product.
Other known and
unknown methods of converting fibrous materials into fuels may also be used.
In some embodiments, prior to combining the microorganism, the sheared and
steam
exploded fibrous material 401 is sterilized to kill any competing
microorganisms that may be
on the fibrous material. For example, the fibrous material can be sterilized
by exposing the
fibrous material to radiation, such as infrared radiation, ultraviolet
radiation, or an ionizing .
radiation, such as gamma radiation. The microorganisms can also be killed
using chemical -
sterilants, such as bleach (e.g., sodium hypochlorite), chlorhexidine, or
ethylene oxide.
One method to hydrolyze the sheared and steam exploded fibrous material is by
the use
of cellulases, 414. Celluloses are a group of enzymes that act synergistically
to hydrolyze cellulose. -
= Commercially available AccellcraseTM 1000, which contains a complex of
enzymes that
reduces lignocellulosic biomass into fermentable sugars can also be used.
According to current understanding, the components of cellulase include
endoglucanases, exoglucanases (cellobiohydrolases), and b-glucosidases
(cellobiases).
= Synergism between the cellulase components exists when hydrolysis by a
combination of two
=
or more components exceeds the sum of the activities expressed by the
individual components.
= 83
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
The generally accepted mechanism of a cellulase system (particularly of T.
longibrachiatum)
on crystalline cellulose is: endoglucanase hydrolyzes internall3-1,4-
glycosidic bonds of the
amorphous regions, thereby increasing the number of exposed non-reducing ends.
Exoglucanases then cleave off cellobiose units from the nonreducing ends,
which in turn are
hydrolyzed to individual glucose units by b-glucosidases. There are several
configurations of
both endo- and exo- glucanases differing in stereospecificities. In general,
the synergistic
action of the components in various configurations is required for optimum
cellulose
hydrolysis. Cellulases, however, are more inclined to hydrolyze the amorphous
regions of
cellulose. A linear relationship between crystallinity and hydrolysis rates
exists whereby higher
crystallinity indices correspond to slower enzyme hydrolysis rates. Amorphous
regions of
cellulose hydrolyze at twice the rate of crystalline regions. The hydrolysis
of the sheared and
steam exploded fibrous material may be performed by any hydrolyzing biomass
process.
Steam explosion of biomass sometimes causes the formation of by-products,
e.g.,
toxicants, that are inhibitory to microbial and enzymatic activities. The
process of converting
the sheared and steam exploded fibrous material into a fuel can therefore
optionally include an
overliming step prior to fermentation to precipitate some of the toxicants.
For example, the pH
of the sheared and steam exploded fibrous material may be raised to exceed the
pH of 10 by
adding calcium hydroxide (Ca(OH)2) followed by a step of lowering the pH to
about 5 by
adding H2SO4. The overlimed fibrous material may then be used as is without
the removal of
precipitates. As shown in FIG 23, the optional overliming step occurs just
prior to the step of
hydrolysis of the sheared and steam exploded fibrous material, but it is also
contemplated to
perform the overliming step after the hydrolysis step and prior to the
fermenting step.
FIG. 24 depicts an example of a steam explosion apparatus 460. The steam
explosion
apparatus 460 includes a reaction chamber 462, in which the fiber source
and/or the fibrous
material placed through a fiber source inlet 464. The reaction chamber is
sealed by closing
fiber source inlet valve 465. The reaction chamber further includes a
pressurized steam inlet
466 that includes a steam valve 467. The reaction chamber further includes an
explosive
depressurization outlet 468 that includes an outlet valve 469 in communication
with the
cyclone 470 through the connecting pipe 472. Once the reaction chamber
includes the fiber
source and/or sheared fiber source and is sealed by closing valves 465, 467
and 469, steam is
delivered into the reaction chamber 462 by opening the steam inlet valve 467
allowing steam to
84
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
travel through steam inlet 466. Once the reaction chamber reaches target
temperature, which
can take about 20 - 60 seconds, the holding time begins. The reaction
temperature is held at the
target temperature for the desired holding time, which typically lasts from
about 10 seconds to
minutes. At the end of the holding time period, outlet valve is open to allow
for explosive
depressurization to occur. The process of explosive depressurization propels
the contents of the
reaction chamber 462 out of the explosive depressurization outlet 468, through
the connecting
pipe 472, and into the cyclone 470. The steam exploded fiber source or fibrous
material then
exits the cyclone in a sludge form into the collection bin 474 as much of the
remaining steam
exits the cyclone into the atmosphere through vent 476. The steam explosion
apparatus further
includes wash outlet 478 with wash outlet valve 479 in communication with
connecting pipe
472. The wash outlet valve 479 is closed during the use of the steam explosion
apparatus 460
for steam explosion, but opened during the washing of the reaction chamber
462. The target
temperature of the reaction chamber 462 is preferably between 180 and 240
degrees Celsius or
between 200 and 220 degrees Celsius. The holding time is preferably between 10
seconds and
30 minutes, or between 30 seconds and 10 minutes, or between 1 minute and 5
minutes.
Because the steam explosion process results in a sludge of steam exploded
fibrous
material, the steam exploded fibrous material may optionally include a fiber
recovery process
where the "liquor" is separated from the steam exploded fibrous material. This
fiber recovery
step is helpful in that it enables further shearing and/or screening processes
and can allow for
the conversion of the fibrous material into fuel. The fiber recovery process
occurs through the
use of a mesh cloth to separate the fibers from the liquor. Further drying
processes can also be
included to prepare the fibrous material or steam exploded fiber source for
subsequent
processing.
Any processing technique described herein can be used at pressure above or
below
normal, earth-bound atmospheric pressure. For example, any process that
utilizes radiation,
sonication, oxidation, pyrolysis, steam explosion, or combinations of any of
these processes to
provide materials that include a carbohydrate can be performed under high
pressure, which, can
increase reaction rates. For example, any process or combination of processes
can be
performed at a pressure greater than about greater than 25 MPa, e.g., greater
than 50 MPa, 75
MPa, 100 MPa, 150 MPa, 200 MPa, 250 MPa, 350 MPa, 500 MPa, 750 MPa, 1,000 MPa,
or
greater than 1,500 MPa.
CA 02823376 2013-08-07
WO 2008/073186 PCMS2007/022719
Combinations of Irradiating. Sonicating, and Oxidizing Devices
=
In some embodiments, it may be advantageous to combine two or more separate
irradiation, sonication, pyrolization, and/or oxidation devices into a single
hybrid machine. For
such a hybrid machine, multiple processes may be performed in close
juxtaposition or even
simultaneously, with the benefit of increasing pretreatment throughput and
potential cost
savings.
For example, consider the electron beam irradiation and sonication processes.
Each
separate process is effective in lowering the mean molecular weight of
cellulosic material by an
order of magnitude or more, and by several orders of magnitude when performed
serially.
Both irradiation and sonication processes can be applied using a hybrid
electron
beam/sonication device as is illustrated in FIG. 25. Hybrid electron
beam/sonication device
2500 is pictured above a shallow pool (depth ¨ 3-5 cm) of a slurry of
cellulosic material 2550
dispersed in an aqueous, oxidant medium, such as hydrogen peroxide or
carbamide peroxide.
Hybrid device 2500 has an energy source 2510, which powers both electron beam
emitter 2540
and sonication horns 2530..
Electron beam emitter 2540 generates electron beams which pass though an
electron
beam aiming device 2545 to impact the slurry 2550 containing cellulosic
material. The
electron beam aiming device can be a scanner that sweeps a beam over a range
of up to about 6
feet in a direction approximately parallel to the surface of the slurry 2550.
On either side of the electron beam emitter 2540 are sonication horns 2530,
which
deliver ultrasonic wave energy to the slurry 2550. The sonication horns 2530
end in a
detachable endpiece 2535 that is in contact with the slurry 2550.
The sonication horns 2530 are at risk of damage from long-term residual
exposure to
the electron beam radiation. Thus, the horns can be protected with a standard
shield 2520, e.g.,
made of lead or a heavy-metal-containing alloy such as Lipowitz metal, which
is impervious to
electron beam radiation. Precautions must be taken, however, to ensure that
the ultrasonic
energy is not affected by the presence of the shield. The detachable endpieces
2535, are
constructed of the same material and attached to the horns 2530, are used to
be in contact with
the cellulosic material 2550 and are expected to be damaged. Accordingly, the
detachable
endpieces 2535 are constructed to be easily replaceable.
86
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
A further benefit of such a simultaneous electron beam and ultrasound process
is that
the two processes have complementary results. With electron beam irradiation
alone, an
insufficient dose may result in cross-linking of some of the polymers in the
cellulosic material,
which lowers the efficiency of the overall depolymerization process. Lower
doses of electron
beam irradiation and/or ultrasound radiation may also be used to achieve a
similar degree of
depolymerization as that achieved using electron beam irradiation and
sonication separately.
An electron beam device can also be combined with one or more of high-
frequency,
rotor-stator devices, which can be used as an alternative to ultrasonic energy
devices, and
performs a similar function.
Further combinations of devices are also possible. For example, an ionizing
radiation
device that produces gamma radiation emitted from, e.g., 60Co pellets, can be
combined with
an electron beam source and/or an ultrasonic wave source. Shielding
requirements may be
more stringent in this case.
The radiation devices for pretreating biomass discussed above can also be
combined
with one or more devices that perform one or more pyrolysis processing
sequences. Such a
combination may again have the advantage of higher throughput. Nevertheless,
caution must
be observed, as there may be conflicting requirements between some radiation
processes and
pyrolysis. For example, ultrasonic radiation devices may require the feedstock
be immersed in
a liquid oxidizing medium. On the other hand, as discussed previously, it may
be advantageous
for a sample of feedstock undergoing pyrolysis to be of a particular moisture
content. In this
case, the new systems automatically measure and monitor for a particular
moisture content and
regulate the same Further, some or all of the above devices, especially the
pyrolysis device, can
be combined with an oxidation device as discussed previously.
PRIMARY PROCESSES
Fermentation
Generally, various microorganisms can produce a number of useful products,
such as a
fuel, by operating on, e.g., fermenting the pretreated biomass materials. For
example, alcohols,
organic acids, hydrocarbons, hydrogen, proteins or mixtures of any of these
materials can be
produced by fermentation or other processes.
87
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
The microorganism can be a natural microorganism or an engineered
microorganism.
For example, the microorganism can be a bacterium, e.g., a cellulolytic
bacterium, a fungus,
e.g., a yeast, a plant or a protist, e.g., an algae, a protozoa or a fungus-
like protist, e.g., a slime
mold. When the organisms are compatible, mixtures of organisms can be
utilized.
To aid in the breakdown of the materials that include the cellulose, one or
more
=
enzymes, e.g., a cellulolytic enzyme can be utilized. In some embodiments, the
materials that
include the cellulose are first treated with the enzyme, e.g., by combining
the material and the
enzyme in an aqueous solution. This material can then be combined with the
microorganism.
In other embodiments, the materials that include the cellulose, the one or
more enzymes and the
microorganism are combined at the concurrently, e.g., by combining in an
aqueous solution.
Also, to aid in the breakdown of the materials that include the cellulose, the
materials
can be treated post irradiation with heat, a chemical (e.g., mineral acid,
base or a strong
oxidizer such as sodium hypochlorite), and/or an enzyme.
During the fermentation, sugars released from cellulolytic hydrolysis or the
saccharification step, are fermented to, e.g., ethanol, by a fermenting
microorganism such as
yeast.. Suitable fermenting microorganisms have the ability to convert
carbohydrates, such as
glucose, xylose, arabinose, mannose, galactose, oligosaccharides or
polysaccharides into
fermentation products. Fermenting microorganisms include strains of the genus
Sacchromyces
spp. e.g., Sacchromyces cerevisiae (baker's yeast), Saccharomyces distaticus,
Saccharomyces
uvarum; the genus Kluyveromyces, e.g., species Kluyveromyces marxianus,
Kluyveromyces
fragilis; the genus Candida, e.g., Candida pseudotropicalis, and Candida
brassicae, the genus
Clavispora, e.g., species Clavispora lusitaniae and Clavispora opuntiae the
genus Pachysolen,
e.g., species Pachysolen tannophilus, the genus Bretannomyces, e.g., species
Bretannomyces
clausenii (Philippidis, G. P., 1996, Cellulose bioconversion technology, in
Handbook on
Bioethanol: Production and Utilization, Wyman, C.E., ed., Taylor & Francis,
Washington, DC,
179-212).
Commercially available yeast include, for example, Red Stare/Lesaffre Ethanol
Red
(available from Red Star/Lesaffre, USA) FALI (available from Fleischmann's
Yeast, a
division of Burns Philip Food Inc., USA), SUPERSTART (available from
Alltech), GERT
STRAND (available from Gert Strand AB, Sweden) and FERMOL (available from
DSM
Specialties).
88
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Bacteria that can ferment bimoss to ethanol and other products include, e.g.,
Zymomonas mobilis and Clostridium thermocellum (Philippidis, 1996, supra).
Leschine et al.
(International Journal of Systematic and Evolutionaty Microbiology 2002, 52,
1155-1160)
isolated an anaerobic, mesophilic, cellulolytic bacterium from forest soil,
Clostridium
phytofermentans sp. nov., which converts cellulose to ethanol.
Fermentation of biomass to ethanol and other products may be carried out using
certain
types of thermophilic or genetically engineered microorganisms, such
Thermoanaerobacter
species, including T. mat hranii, and yeast species such as Pichia species. An
example of a
strain of T. mathranii is A3M4 described in Sonne-Hansen et al. (Applied
Microbiology and
Biotechnology 1993, 38, 537-541) or Airing et al. (Arch. Microbial. 1997, 168,
114-119).
Yeast and Zymomonas bacteria can be used for fermentation or conversion. The
optimum pH for yeast is from about pH 4 to 5, while the optimum pH for
Zymomonas is from
about pH 5 to 6. Typical fermentation times are about 24 to 96 hours with
temperatures in the
range of 26 C to 40 C, however thermophilic microorganisms prefer higher
temperatures.
Enzymes which break down biomass, such as cellulose, to lower molecular weight
carbohydrate-containing materials, such as glucose, during saccharification
are referred to as
cellulolytic enzymes or cellulase. These enzymes may be a complex of enzymes
that act
synergistically to degrade crystalline cellulose. Examples of cellulolytic
enzymes include:
endoglucanases, cellobiohydrolases, and cellobiases (13-glucosidases). A
cellulosic substrate is
initially hydrolyzed by endoglucanases at random locations producing
oligomeric
intermediates. These intermediates are then substrates for exo-splitting
glucanases such as
cellobiohydrolase to produce cellobiose from the ends of the cellulose
polymer. Cellobiose is a
water-soluble [3-1,4-linked dimer of glucose. Finally cellobiase cleaves
cellobiose to yield
glucose.
A cellulase is capable of degrading biomass and may be of fungal or bacterial
origin.
Suitable enzymes include cellulases from the genera Bacillus, Pseudomonas,
Humicola,
Fusarium, Thielavia, Acremonium, Chpysosporium and Trichoderma, and include
species of
Humicola, Coprinus, Thielavia, Fusarium, Myceliophthora, Acremonium,
Cephalosporium,
Scytalidium, Penicillium or Aspergillus (see, e.g., EP 458162), especially
those produced by a
strain selected from the species Humicola insolens (reclassified as
Scytalidium thermophilum,
see, e.g., U.S. Patent No. 4,435,307), Coprinus cinereus, Fusarium oxysporum,
89
=
CA 02823376 2013-08-07
'1983-7
= Myceliophthora therm ophila, Meripilus giganteus, Thielavia terrestris,
Acremonium sp.,
Acremonium persicinum, Acremonium acremonium, Acremonium brachypenium,
Acremonium
= diChromosporum, Acremonium obclavatum, Acremonium pinkertoniae,
Acremonium
roseogriseum, Acremonium incoloratum, and Acremonium furatum; preferably from
the species
Humicola insolens DSM 1800, Fusarium oxysporum DSM 2672, Myceliophthora therm
ophila
CBS 117.65, Cephalosporium sp. RYM-202, Acremonium sp. CBS 478.94, Acremonium
sp. -
CBS 265.95, Acremonium persicinum CBS 169.65, Acremonium acremonium AHU 9519,
Cephalosporium sp. CBS 535.71, Acremonium brachypenium CBS 866.73, Acremonium
= dichromosporum CBS 683.73, Acremonium obclavatum CBS 311.74, Acremonium
pinkertoniae CBS 157.70, Acremonium roseogrisewn CBS 134.56, Acremonium
incoloratum
CBS 146.62, and Acremonium furatum CBS 299.70H. Cellulolytic enzymes may also
be
obtained from Cluysosporium, preferably a strain of Cluysosporium lucknowense.
Additionally, Trichoderma (particularly Trichoderma viride, Trichoderma
reesei, and
= Trichoderma koningii), alkalophilic Bacillus (see, for example, U.S.
Patent No. 3,844,890 and
EP 458162), and Streptomyces (see, e.g., El' 458162) may be used.
=
= Anaerobic cellulolytic bacteria have also been isolated from soil,
e.g., a novel= =
cellulolytic species of Clostiridium, Clostridium phytofermentans sp. nov.
(see Leschine et
al, International Journal of Systematic and Evolutionary Microbiology (2002),
52, 1155-1160).
Cellulolytic enzymes using recombinant technology can also be used (see, e.g.,
WO -
2007/071818 and WO 2006/110891).
The cellulolytic enzymes used can be produced by fermentation of the above-
noted
microbial strains on a-nutrient medium containing suitable carbon and nitrogen
sources and
= inorganic salts, using procedures known in the art (see, e.g., Bennett,
J.W. and LaSure, L.
(eds.), More Gene Manipulations in Fungi, Academic Press, CA 1991). Suitable
media are
available from commercial suppliers or may be prepared according to published
compositions.
Temperature ranges and other _ _ _
=
= conditions suitable for growth and cellulase production are known in the
art (see, e.g., Bailey,
LE, and 011is, D.F., Biochemical Engineering Fundamentals, McGraw-Hill Book
Company, "
NY, 1986).
Treatment of cellulose with cellulase is usually carried out at temperatures
between 30
C and 65 C. Cellulases are active over a range of pH of about 3 to 7. A
saccharification step
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
may last up to 120 hours. The cellulase enzyme dosage achieves a sufficiently
high level of
cellulose conversion. For example, an appropriate cellulase dosage is
typically between 5.0
and 50 Filter Paper Units (FPU or IU) per gram of cellulose. The FPU is a
standard
measurement and is defined and measured according to Ghose (1987, Pure and
Appl. Chem.
59:257-268).
Gasification
In addition to using pyrolysis for pre-treatment of feedstock, pyrolysis can
also be used
to process pre-treated feedstock to extract useful materials. In particular, a
form of pyrolysis
known as gasification can be employed to generate fuel gases along with
various other gaseous,
liquid, and solid products. To perform gasification, the pre-treated feedstock
is introduced into
a pyrolysis chamber and heated to a high temperature, typically 700 C or
more. The
temperature used depends upon a number of factors, including the nature of the
feedstock and
the desired products.
Quantities of oxygen (e.g., as pure oxygen gas and/or as air) and steam (e.g.,
superheated steam) are also added to the pyrolysis chamber to facilitate
gasification. These
compounds react with carbon-containing feedstock material in a multiple-step
reaction to
generate a gas mixture called synthesis gas (or "syngas"). Essentially, during
gasification, a
limited amount of oxygen is introduced into the pyrolysis chamber to allow
some feedstock
material to combust to form carbon monoxide and generate process heat. The
process heat can
then be used to promote a second reaction that converts additional feedstock
material to
hydrogen and carbon monoxide.
In a first step of the overall reaction, heating the feedstock material
produces a char that
can include a wide variety of different hydrocarbon-based species. Certain
volatile materials
can be produced (e.g., certain gaseous hydrocarbon materials), resulting in a
reduction of the
overall weight of the feedstock material. Then, in a second step of the
reaction, some of the
volatile material that is produced in the first step reacts with oxygen in a
combustion reaction to
produce both carbon monoxide and carbon dioxide. The combustion reaction
releases heat,
which promotes the third step of the reaction. In the third step, carbon
dioxide and steam (e.g.,
water) react with the char generated in the first step to form carbon monoxide
and hydrogen
91
CA 02823376 2013-08-07
WO 2008/073186 PCMS2007/022719
gas. Carbon monoxide can also react with steam, in a water gas shift reaction,
to form carbon
dioxide and further hydrogen gas.
Gasification can be used as a primary process to generate products directly
from pre-
treated feedstock for subsequent transport and/or sale, for example.
Alternatively, or in
addition, gasification can be used as an auxiliary process for generating fuel
for an overall
processing system. The hydrogen-rich syngas that is generated via the
gasification process can
be burned, for example, to generate electricity and/or process heat that can
be directed for use
at other locations in the processing system. As a result, the overall
processing system can be at
least partially self-sustaining. A number of other products, including
pyrolysis oils and gaseous
hydrocarbon-based substances, can also be obtained during and/or following
gasification; these
can be separated and stored or transported as desired.
A variety of different pyrolysis chambers are suitable for gasification of pre-
treated
feedstock, including the pyrolysis chambers disclosed herein. In particular,
fluidized bed
reactor systems, in which the pre-treated feedstock is fluidized in steam and
oxygen/air,
provide relatively high throughput and straightforward recovery of products.
Solid char that
remains following gasification in a fluidized bed system (or in other
pyrolysis chambers) can be
burned to generate additional process heat to promote subsequent gasification
reactions.
POST-PROCESSING
Distillation
After fermentation, the resulting fluids can be distilled using, for example,
a "beer
column" to separate ethanol and other alcohols from the majority of water and
residual solids.
The vapor exiting the beer column can be 35% by weight ethanol and fed to a
rectification
column. A mixture of nearly azeotropic (92.5%) ethanol and water from the
rectification
column can be purified to pure (99.5%) ethanol using vapor-phase molecular
sieves. The beer
column bottoms can be sent to the first effect of a three-effect evaporator.
The rectification
column reflux condenser can provide heat for this first effect. After the
first effect, solids can
be separated using a centrifuge and dried in a rotary dryer. A portion (25%)
of the centrifuge
effluent can be recycled to fermentation and the rest sent to the second and
third evaporator
effects. Most of the evaporator condensate can be returned to the process as
fairly clean
92
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
condensate with a small portion split off to waste water treatment to prevent
build-up of low-
boiling compounds.
Waste water treatment
Wastewater treatment is used to minimize makeup water requirements of the
plant by
treating process water for reuse within the plant. Wastewater treatment can
also produce fuel
(e.g., sludge and biogas) that can be used to improve the overall efficiency
of the ethanol
production process. For example, as described in further detail below, sludge
and biogas can
be used to create steam and electricity that can be used in various plant
processes.
Wastewater is initially pumped through a screen (e.g., a bar screen) to remove
large
particles, which are collected in a hopper. In some embodiments, the large
particles are sent to
a landfill. Additionally or alternatively, the large particles are burned to
create steam and/or
electricity as described in further detail below. In general, the spacing on
the bar screen is
between 1/4 inch to 1 inch spacing (e.g., 1/2 inch spacing).
The wastewater then flows to an equalization tank, where the organic
concentration of
the wastewater is equalized during a retention time. In general, the retention
time is between 8
hours and 36 hours (e.g., 24 hours). A mixer is disposed within the tank to
stir the contents of
the tank. In some embodiments, a plurality of mixers disposed throughout the
tank are used to
stir the contents of the tank. In certain embodiments, the mixer substantially
mixes the contents
of the equalization tank such that conditions (e.g., wastewater concentration
and temperature)
throughout the tank are uniform.
A first pump moves water from the equalization tank through a liquid-to-liquid
heat
exchanger. The heat exchanger is controlled (e.g., by controlling the flow
rate of fluid through
the heat exchanger) such that wastewater exiting the heat exchanger is at a
desired temperature
for anaerobic treatment. For example, the desired temperature for anaerobic
treatment can be
between 40 C to 60 C.
After exiting the heat exchanger, the wastewater enters one or more anaerobic
reactors.
In some embodiments, the concentration of sludge in each anaerobic reactor is
the same as the
overall concentration of sludge in the wastewater. In other embodiments, the
anaerobic reactor
has a higher concentration of sludge than the overall concentration of sludge
in the wastewater.
93
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
A nutrient solution containing nitrogen and phosphorus is metered into each
anaerobic
reactor containing wastewater. The nutrient solution reacts with the sludge in
the anaerobic
reactor to produce biogas which can contain 50% methane and have a heating
value of
approximately 12,000 British thermal units, or Btu, per pound). The biogas
exits each
anaerobic reactor through a vent and flows into a manifold, where a plurality
of biogas streams
are combined into a single stream. A compressor moves the stream of biogas to
a boiler or a
combustion engine as described in further detail below. In some embodiments,
the compressor
also moves the single stream of biogas through a desulphurization catalyst.
Additionally or
alternatively, the compressor can move the single stream of biogas through a
sediment trap.
A second pump moves anaerobic effluent from the anaerobic reactors to one or
more
aerobic reactors' (e.g., activated sludge reactors). An aerator is disposed
within each aerobic
reactor to mix the anaerobic effluent, sludge, oxygen (e.g., oxygen contained
in air). Within
each aerobic reactor, oxidation of cellular material in the anaerobic effluent
produces carbon
dioxide, water, and ammonia.
Aerobic effluent moves (e.g.) via gravity) to a separator, where sludge is
separated from
treated water. Some of the sludge is returned to the one or more aerobic
reactors to create an
elevated sludge concentration in the aerobic reactors, thereby facilitating
the aerobic
breakdown of cellular material in the wastewater. A conveyor removes excess
sludge from the
separator. As described in further detail below, the excess sludge is used as
fuel to create steam
and/or electricity.
The treated water is pumped from the separator to a settling tank. Solids
dispersed
throughout the treated water settle to the bottom of the settling tank and are
subsequently
removed. After a settling period, treated water is pumped from the settling
tank through a fine
filter to remove any additional solids remaining in the water. In some
embodiments, chlorine is
added to the treated water to kill pathogenic bacteria. In some embodiments,
one or more
physical-chemical separation techniques are used to further purify the treated
water. For
example, treated water can be pumped through a carbon adsorption reactor. As
another
example, treated water can pumped through a reverse osmosis reactor.
94
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Waste combustion
The production of alcohol from biomass can result in the production of various
by-
product streams useful for generating steam and electricity to be used in
other parts of the plant.
For example, steam generated from burning by-product streams can be used in
the distillation
process. As another example, electricity generated from burning by-product
streams can be
used to power electron beam generators and ultrasonic transducers used in
pretreatment.
The by-products used to generate steam and electricity are derived from a
number of
sources throughout the process. For example, anaerobic digestion of wastewater
produces a
biogas high in methane and a small amount of waste biomass (sludge). As
another example,
= post-distillate solids (e.g., unconverted lignin, cellulose, and
hemicellulose remaining from the
pretreatment and primary processes) can be used as a fuel.
The biogas is diverted to a combustion engine connected to an electric
generator to
produce electricity. For example, the biogas can be used as a fuel source for
a spark-ignited
natural gas engine. As another example, the biogas can be used as a fuel
source for a direct-
injection natural gas engine. As another example, the biogas can be used as a
fuel source for a
combustion turbine. Additionally or alternatively, the combustion engine can
be configured in
a cogeneration configuration. For example, waste heat from the combustion
engines can be
used to provide hot water or steam throughout the plant.
The sludge, and post-distillate solids are burned to heat water flowing
through a heat
exchanger. In some embodiments, the water flowing through the heat exchanger
is evaporated
and superheated to steam. In certain embodiments, the steam is used in the
pretreatment rector
and in heat exchange in the distillation and evaporation processes.
Additionally or
alternatively, the steam expands to power a multi-stage steam turbine
connected to an electric
generator. Steam exiting the steam turbine is condensed with cooling water and
returned to the
heat exchanger for reheating to steam. In some embodiments, the flow rate of
water through
the heat exchanger is controlled to obtain a target electricity output from
the steam turbine
connected to an electric generator. For example, water can be added to the
heat exchanger to
ensure that the steam turbine is operating above a threshold condition (e.g.,
the turbine is
spinning fast enough to turn the electric generator).
While certain embodiments have been described, other embodiments are possible.
CA 02823376 2013-08-07
1983-7
As an example, while the biogas is described as being diverted to a combustion
engine
connected to an electric generator, in certain embodiments, the biogas can be
passed through a
fuel reformer to produce hydrogen. The hydrogen is then converted to
electricity through a fuel
cell.
As another example, while the biogas is described as being burned apart from
the
sludge and post-distillate solids, in certain embodiments, all of the waste by-
products can be
burned together to produce steam.
=
PRODUCTS / CO-PRODUCTS =
Alcohols
The alcohol produced can be a monohydroxy alcohol, e.g., ethanol, or a
polyhydroxy
alcohol, e.g., ethylene glycol or glycerin. Examples of alcohols that can be
produced include
= methanol, ethanol, propanol, isopropanol, butanol, e.g., n-, sec- or t-
butanol, ethylene glycol,
propylene glycol, 1, 4-butane diol, glycerin or mixtures of these alcohols.
= Each of the alcohols produced by the Plant have commercial value as
industrial -
feedstock. For example, ethanol can be used in the manufacture of varnishes
and perfume. As
another example, methanol can be used as a solvent used as a component in
windshield wiper
fluid.. As still another example, butanol can be used in plasticizers, resins,
lacquers, and brake.
fluids.
Bioethanol produced by the plant is valuable as an ingredient used in the food
and
beverage industry. For example, the ethanol produced by the plant can be
purified to food
grade alcohol and used as a primary ingredient in the alcoholic beverages.
Bioethanol produced by the plant also has commercial value as a transportation
fuel;.
The use of ethanol-as a transportation fuel can be implemented with relatively
little capital
investment from spark ignition engine manufacturers and owners (e.g., changes
to injection
. timing, fuel-to-air ratio, and components of the fuel injection system).
Many automotive
manufacturers currently Offer flex-fuel vehicles capable of operation on
ethanol/gas. ohne blends
up to 85% ethanol by volume (e.g., -standard equipment on a Chevy Taho.m4 x
4).
Bioethanol produced by this plant can be used as an engine fuel to improve
environmental and economic conditions beyond the location of the plant. For
example, ethanol
= produced by this plant and used as a fuel can reduce greenhouse gas
emissions from manmade
96
=
CA 02823376 2013-08-07
W020081073186 PCTTUS2007/022719
sources (e.g., transportation sources). As another example, ethanol produced
by this plant and
used as an engine fuel can also displace consumption of gasoline refined from
oil.
Bioethanol has a greater octane number than conventional gasoline and, thus,
can be
used to improve the performance (e.g., allow for higher compression ratios) of
spark ignition
engines. For example, small amounts (e.g., 10% by volume) of ethanol can be
blended with
gasoline to act as an octane enhancer for fuels used in spark ignition
engines. As another
example, larger amounts (e.g., 85% by volume) of ethanol can be blended with
gasoline to
further increase the fuel octane number and displace larger volumes of
gasoline.
Bioethanol strategies are discussed, e.g., by DiPardo in Journal of Outlook
for Biomass
Ethanol Production and Demand (EIA Forecasts), 2002; Sheehan in Biotechnology
Progress,
15:8179, 1999; Martin in Enzyme Microbes Technology, 31:274, 2002; Greer in
BioCycle, 61-
65, April 2005; Lynd in Microbiology and Molecular Biology Reviews, 66:3, 506-
577, 2002;
Ljungdahl et al. in U.S. Patent No. 4,292,406; and Bellamy in U.S. Patent No.
4,094,742.
Organic acids
The organic acids produced can include monocarboxylic acids or a
polycarboxylic
acids. Examples of organic acids include formic acid, acetic acid, propionic
acid, butyric acid,
valeric acid, caproic, palmitic acid, stearic acid, oxalic acid, malonic acid,
succinic acid,
glutaric acid, oleic acid, linoleic acid, glycolic acid, lactic acid, 7-
hydroxybutyric acid or
mixtures of these acids.
Foodstocks
In some embodiments, all or a portion of the fermentation process can be
interrupted
before the cellulosic material is completely converted to ethanol. The
intermediate
fermentation products include high concentrations of sugar and carbohydrates.
These
intermediate fermentation products can be used in preparation of food for
human or animal
consumption. In some embodiments, irradiation pretreatment of the cellulosic
material will
render the intermediate fermentation products sterile (e.g., fit for human
consumption). In
some embodiments, the intermediate fermentation products will require post-
processing prior
to use as food. For example, a dryer can be used to remove moisture from the
intermediate
fermentation products to facilitate storage, handling, and shelf-life.
Additionally or
97
CA 02823376 2013-08-07
4 WO 2008/073186
PCT/US2007/022719
, =
alternatively, the intermediate fermentation products can be ground to a fine
particle size in a
stainless-steel laboratory mill to produce a flour-like substance.
Animal feed
Distillers grains and solubles can be converted into a valuable byproduct of
the
distillation-dehydration process. After the distillation-dehydration process,
distillers grains and =
solubles can be dried to improve the ability to store and handle the material.
The resulting
dried distillers grains and solubles (DDGS) is low in starch, high in fat,
high in protein, high in
fiber, and=high in phosphorous. Thus, for example, DDGS can be valuable as a
source of
animal feed (e.g., as a feed source for dairy cattle). DDGS can be
subsequently combined with
nutritional additives to meet specific dietary requirements of specific
categories of animals
(e.g., balancing digestible lysine and phosphorus for swine diets).
Pharmaceuticals
The pretreatment processes discussed above can be applied to plants with
medicinal
properties. In some embodiments, sonication can stimulate bioactivity and/or
bioavailabilty of
the medicinal components of plants with medicinal properties. Additionally or
alternatively,
irradiation stimulates bioactivity and/or bioavailabilty of the medicinal
components of plants
with medicinal properties. For example, sonication and irradiation can be
combined in the
pretreatment of willow bark to stimulate the production of salicin.
Nutriceuticais
In some embodiments, intermediate fermentation products (e.g., products that
include
high concentrations of sugar and carbohydrates) can be supplemented to create
a nutriceutical.
For example, intermediate fermentation products can be supplemented with
calcium create a
nutriceutical that provides energy and helps improve or maintain bone
strength.
Co-Products
Lignin Residue
As described above, lignin containing residues from primary and pretreatment
processes
has value as a high/medium energy fuel and can be used to generate power and
steam for use in
plant processes. However, such lignin residues are a new type of solids fuel
and there is little
demand for it outside of the plant boundaries, and the costs of drying it for
transportation only
98
CA 02823376 2013-08-07
WO 2008/073186 PCT/1JS2007/022719
subtract from its potential value. In some cases, gasification of the lignin
residues can
converting it to a higher-value product with lower cost.
Other Co-Products
Cell matter, furfural, and acetic acid have been identified as potential co-
products of
biomass-to-fuel processing facilities. Interstitial cell matter could be
valuable, but might
require significant purification. Markets for furfural and acetic acid are in
place, although it is '
unlikely that they are large enough to consume the output of a fully
commercialized
lignocellulose-to-ethanol industry.
EXAMPLES
The following Examples are intended to illustrate, and do not limit the
teachings of this
disclosure.
Example 1 ¨ Preparation Of Fibrous Material From Polycoated Paper
A 1500 pound skid of virgin, half-gallon juice cartons made of un-printed
polycoated
white Kraft board having a bulk density of 20 lbift3 was obtained from
International Paper.
Each carton was folded fiat, and then fed into a 3 hp Flinch Baugh shredder at
a rate of
approximately 15 to 20 pounds per hour. The shredder was equipped with two 12
inch rotary
blades, two fixed blades and a 0.30 inch discharge screen. The gap between the
rotary and
fixed blades was adjusted to 0.10 inch. The output from the shredder resembled
confetti having
a width of between 0.1 inch and 0.5 inch, a length of between 0.25 inch and 1
inch and a
thickness equivalent to that of the starting material (about 0.075 inch).
The confetti-like material was fed to a Munson rotary knife cutter, Model
SC30. Model
SC30 is equipped with four rotary blades, four fixed blades, and a discharge
screen having 1/8
inch openings. The gap between the rotary and fixed blades was set to
approximately 0.020
inch. The rotary knife cutter sheared the confetti-like pieces across the
knife-edges, tearing the
pieces apart and releasing a fibrous material at a rate of about one pound per
hour. The fibrous
material had a BET surface area of 0.9748 m2/g +/- 0.0167 m2/g, a porosity of
89.0437 percent
and a bulk density (@0.53 psia) of 0.1260 g/mL. An average length of the
fibers was 1.141
mm and an average width of the fibers was 0.027 mm, giving an average LID of
42:1. A
scanning electron micrograph of the fibrous material is shown in FIG 26 at 25
X magnification.
99
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Example 2¨ Preparation Of Fibrous Material From Bleached Kraft Board
A 1500 pound skid of virgin bleached white Kraft board having a bulk density
of 30
lb/ft3 was obtained from International Paper. The material was folded flat,
and then fed into a 3
hp Flinch Baugh shredder at a rate of approximately 15 to 20 pounds per hour.
The shredder
was equipped with two 12 inch rotary blades, two fixed blades and a 0.30 inch
discharge
screen. The gap between the rotary and fixed blades was adjusted to 0.10 inch.
The output
from the shredder resembled confetti having a width of between 0.1 inch and
0.5 inch, a length
of between 0.25 inch and 1 inch and a thickness equivalent to that of the
starting material
(about 0.075 inch). The confetti-like material was fed to a Munson rotary
knife cutter, Model
SC30. The discharge screen had 1/8 inch openings. The gap between the rotary
and fixed
blades was set to approximately 0.020 inch. The rotary knife cutter sheared
the confetti-like
pieces, releasing a fibrous material at a rate of about one pound per hour.
The fibrous material
had a BET surface area of 1.1316 m2/g +/- 0.0103 m2/g, a porosity of 88.3285
percent and a
bulk density (@0.53 psia) of 0.1497 g/mL. An average length of the fibers was
1.063 mm and
an average width of the fibers was 0.0245 mm, giving an average L/D of 43:1. A
scanning
electron micrographs of the fibrous material is shown in FIG 27 at 25 X
magnification.
Example 3-. Preparation Of Twice Sheared Fibrous Material From Bleached Kraft
Board
A 1500 pound skid of virgin bleached white Kraft board having a bulk density
of 30
lb/ft3 was obtained from International Paper. The material was folded flat,
and then fed into a 3
hp Flinch Baugh shredder at a rate of approximately 15 to 20 pounds per hour.
The shredder
was equipped with two 12 inch rotary blades, two fixed blades and a 0.30 inch
discharge
screen. The gap between the rotary and fixed blades was adjusted to 0.10 inch.
The output
from the shredder resembled confetti (as above). The confetti-like material
was fed to a
Munson rotary knife cutter, Model SC30. The discharge screen had 1/16 inch
openings. The
gap between the rotary and fixed blades was set to approximately 0.020 inch.
The rotary knife
cutter the confetti-like pieces, releasing a fibrous material at a rate of
about one pound per hour.
The material resulting from the first shearing was fed backinto the same setup
described above
and sheared again. The resulting fibrous material had a BET surface area of
1.4408 m2/g +/-
0.0156 m2/g, a porosity of 90.8998 percent and a bulk density (@0.53 psia) of
0.1298 g/mL.
An average length of the fibers was 0.891 mm and an average width of the
fibers was 0.026
100
CA 02823376 2013-08-07
=
WO 2008/073186
PCT/US2007/022719
'
mm, giving an average LID of 34:1. A scanning electron micrograph of the
fibrous material is
shown in FIG 28 at 25 X magnification.
Example 4¨ Preparation Of Thrice Sheared Fibrous Material From Bleached Kraft
Board
A 1500 pound skid of virgin bleached white Kraft board having a bulk density
of 30
lb/ft3 was obtained from International Paper. The material was folded flat,
and then fed into a 3
hp Flinch Baugh shredder at a rate of approximately 15 to 20 pounds per hour.
The shredder
was equipped with two 12 inch rotary blades, two fixed blades and a 0.30 inch
discharge
screen. The gap between the rotary and fixed blades was adjusted to 0.10 inch.
The output
from the shredder resembled confetti (as above). The confetti-like material
was fed to a
Munson rotary knife cutter, Model SC30. The discharge screen had 1/8 inch
openings. The
gap between the rotary and fixed blades was set to approximately 0.020 inch.
The rotary knife
cutter sheared the confetti-like pieces across the knife-edges. The material
resulting from the
first shearing was fed back into the same setup and the screen was replaced
with a 1/16 inch
screen. This material was sheared. The material resulting from the second
shearing was fed
back into the same setup and the screen was replaced with a 1/32 inch screen.
This material
was sheared. The resulting fibrous material had a BET surface area of 1.6897
m2/g +1- 0.0155
m2/g, a porosity of 87.7163 percent and a bulk density (@0.53 psia) of 0.1448
g/mL. An
average length of the fibers was 0.824 mm and an average width of the fibers
was 0.0262 mm,
giving an average LTD of 32:1. A scanning electron micrograph of the fibrous
material is
shown in FIG 29 at 25 X magnification.
Example 5 ¨ Preparation Of Densified Fibrous Material From Bleached Kraft
Board Without
Added Binder
Fibrous material was prepared according to Example 2. Approximately 1 lb of
water
was sprayed onto each 10 lb of fibrous material. The fibrous material was
densified using a
California Pellet Mill 1100 operating at 75 C. Pellets were obtained having a
bulk density
ranging from about 7 lb/ft3to about 15 lb/113.
Example 6¨ Preparation Of Densified Fibrous Material From Bleached Kraft Board
With Binder
Fibrous material was prepared according to Example 2.
A 2 weight percent stock solution of POLY0X114 WSR N10 (polyethylene oxide)
was
prepared in water.
101
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Approximately 1 lb of the stock solution was sprayed onto each 10 lb of
fibrous
material. The fibrous material was densified using a California Pellet Mill
1100 operating at 75
C. Pellets were obtained having a bulk density ranging from about 15 lb/ft3 to
about 40 lb/&.
Example 7 - Reducing the Molecular Weight of Cellulose in Fibrous Kraft Paper
by Gamma
Radiation with Minimum Oxidation
Fibrous material is prepared according to Example 4. The fibrous Kraft paper
is
densified according to Example 5.
The densified pellets are placed in a glass ampoule having a maximum capacity
of 250
mL. The glass ampoule is evacuated under high vacuum (10 torr) for 30 minutes,
and then
back-filled with argon gas. The ampoule is sealed under argon. The pellets in
the ampoule are
= irradiated with gamma radiation for about 3 hours at a dose rate of about
1 Mrad per hour to
provide an irradiated material in which the cellulose has a lower molecular
weight than the
fibrous Kraft starting material.
Example 8 -Reducing the Molecular Weight of Cellulose in Fibrous Kraft Paper
by Gamma
Radiation with Maximum Oxidation
Fibrous material is prepared according to Example 4. The fibrous Kraft paper
is
densified according to Example 5.
The densified pellets are placed in a glass ampoule having a maximum capacity
of 250
mL. The glass ampoule is sealed under an atmosphere of air. The pellets in the
ampoule are
irradiated with gamma radiation for about 3 hours at a dose rate of about 1
Mrad per hour to
provide an irradiated material in which the cellulose has a lower molecular
weight than the
fibrous Kraft starting material.
Example 9 - Methods of Determining Molecular Weight of Cellulosic and
Lignocellulosic
Materials by Gel Permeation Chromatography
Cellulosic and lignocellulosic materials for analysis were treated according
to Example
4. Sample materials presented in the following tables include Kraft paper (P),
wheat straw
(WS), alfalfa (A), and switchgrass (SG). The number "132" of the Sample ID
refers to the
particle size of the material after shearing through a 1/32 inch screen. The
number after the
dash refers to the dosage of radiation (MRad) and "US" refers to ultrasonic
treatment. For
102
CA 02823376 2013-08-07
i.
WO 2008/073186
PCT/US2007/022719
example, a sample ID ÷P132-10" refers to Kraft paper that has been sheared to
a particle size of
132 mesh and has been irradiated with 10 MRad.
Table 1. Peak Average Molecular Weight of Irradiated Kraft Paper
Sample Dosage'
Sample ID Ultrasound:
Average MW *
Source (MRad Std Dev.
Kraft Paper P132 0 No 32853 10006
P132-10 10 .. 61398
2468**
P132-100 100 .. 8444 580
P132-181 181 46 6668 77
P132-US 0 Yes 3095 1013
**Low doses of radiation appear to increase the molecular weight of some
materials
'Dosage Rate = 1MRacl/hour
'Treatment for 30 minutes with 20kHz ultrasound using a 1000W horn under re-
circulating
conditions with the material dispersed in water.
Table 2. Peak Average Molecular Weight of Irradiated Materials
Peak Dosage'Average MW
Sample ID Ultrasound2
# (MRad) Std Dev.
WS132 1 0 No
1407411* 175191
2 .. 66 39145 * 3425
3 tl 66 2886 177
WS132-10* 1 10 lt 26040 3240
WS132-100* 1 100 64 23620 1 453
A132 1 0 46
1604886 151701
2 .. tt 37525 3751
3 44 La 2853 1 490
A132-10* 1 10 44 50853* 1665
66
2 .. 2461 17
A132-100* 1 100 46 38291 2235
66
2 .. 2487 15
SG132 1 0 "
1557360* 83693
2 it 44 42594 t 4414
3 66 64 3268 249
SG132-10* 1 10 .. 60888 * 9131
SG132-100* 1 100 44 22345 * 3797
SG132-10-US 1 10 Yes 86086
43518
2 64 64 2247 468
S0132-100-US 1 100 4696 1465
*Peaks coalesce after treatment
**Low doses of radiation appear to increase the molecular weight of some
materials
'Dosage Rate = 1MRad/hour
'Treatment for 30 minutes with 20kHz ultrasound using a 1000W horn under re-
circulating
conditions with the material dispersed in water.
Gel Permeation Chromatography (GPC) is used to determine the molecular weight
distribution of polymers. During GPC analysis, a solution of the polymer
sample is passed
103
CA 02823376 2013-08-07
WO 2008/073186 PCT/1JS2007/022719
through a column packed with a porous gel trapping small molecules. The sample
is separated
based on molecular size with larger molecules eluting sooner than smaller
molecules. The
retention time of each component is most often detected by refractive index
(RI), evaporative
light scattering (ELS), or ultraviolet (UV) and compared to a calibration
curve. The resulting
data is then used to calculate the molecular weight distribution for the
sample.
A distribution of molecular weights rather than a unique molecular weight is
used to
characterize synthetic polymers. To characterize this distribution,
statistical averages are
utilized. The most common of these averages are the "number average molecular
weight" (Ms)
and the "weight average molecular weight" (MO.
Mr, is similar to the standard arithmetic mean associated with a group of
numbers.
When applied to polymers, Mn refers to the average molecular weight of the
molecules in the
polymer. Mn is calculated affording the same amount of significance to each
molecule
regardless of its individual molecular weight. The average Mn is calculated by
the following
formula where I=1; is the number of molecules with a molar mass equal to Mi.
N M
Mn = __________________________________________
ENi
Mv, is another statistical descriptor of the molecular weight distribution
that places a
greater emphasis on larger molecules than smaller molecules in the
distribution. The formula
below shows the statistical calculation of the weight average molecular
weight.
NM. i .2
i
M= ____________________________________________
W
ENMi
104
CA 02823376 2013-08-07
-1983-7
=
The polydispersity index or PI is defined as the ratio of KIM. The larger the
PI, the
broader or more disperse the distribution. The lowest value that a PI can be
is 1. This
represents a monodisierse sample; that is, a polymer with all of the molecules
in the
distribution being the same molecular weight.
. The peak molecular weight value (Mp) is another descriptor defined as
the mode of the
molecular weight distribution. It signifies the molecular weight that is most
abundant in the
distribution. This value also gives insight to the molecular Weight
distribution.
Most GPC measurements are made relative to a different polymer standard. The
accuracy of the results.depends on how closely the characteristics of the
polymer being
analyzed match those of the standard used. The expected error in
reproducibility between
different series of determinations, calibrated separately, is ca. 5-10% and is
characteristic to
the limited precision of GPC determinations. Therefore, GPC results are most
useful when a
comparison between the molecular weight distribution of different samples is
made during the
same series of determinations.
The lignocellulosic samples required sample preparation prior to GPC analysis.
First, a =
saturated solution (8.4% by weight) of lithium chloride (LiC1).was prepared in
dimethyl
acetamide (DMAc). Approximately 100 mg of the sample was added to
approximately 10 g of
a freshly prepared saturated LiCl/DMAc solution, and the mixture was heated to
approximately
150 C-170 C with stirring for 1 hour. The resulting solutions were generally
light- to dark-
yellow in color. The temperature of the solutions were decreased to
approximately 100 C and
heated for an additional 2 hours. The temperature of the solutions were then
decreased to
approximately 50 C and the sample solution was heated for approximately 48 to
60 hours. Of
. note, samples irradiated at 100 MRad were more easily solubilized as
compared to their
untreated counterpart. Additionally, the sheared samples (denoted by the
number 132) had
=
slightly lower average molecular weights as compared with uncut samples.
The resulting sample solutions were diluted 1:1. using DMAc as solvent and
were =
filtered through a 0.45 gm PTFE filter. The filtered sample solutions were
then analyzed by
GPC. The peak average molecular weight (Mp) of the samples, as detennined by
Gel
Permeation Chromatography (GPC), are summarized in Tables 1 and 2.Each sample
was
prepared in duplicate and each preparation of the sample was analyzed in
duplicate (two
TIA
injections) for a total of four injections per sample. The EasiCal polystyrene
standards PS lA
105
CA 02823376 2013-08-07
'1983-7 .
=
and PS 1B were used to generate a calibration curve for the molecular weight
scale from about
580 to 7,500,00 Dalions.
= Table 3. GPG
Analysis Conditions =
Instrument: Waters Alliance' GPC 2000
Plgel 10p. Mixed-B
Columns (3):. S/N's: 10M-MB-148-83; 10M-MB-148-=84; 10M-
MB-
174-129
=
=
Mobile Phase (solvent): 0.5% LiCI in DMAc (1.0 mL/min.)
Column/Detector Temperature: .70 C
Injector Temperature: 70 C
Sample Loop Size: 323.5 pL
=
= =
Example 10- Determining Cyrstallinity of Irradiated Material by X-Ray
Diffraction
X-ray diffraction (XRD) is a method by which a crystalline sample is
irradiated with =
monoenergetic x-rays. The interaction of the lattice structure of the sample
with these x-rays is
recorded and provides information-about the crystalline structure being
irradiated. The
resulting characteristic "fingerprint" allows for the identification of the
crystalline compounds
present in the sample. Using a whole-pattern fitting analysis (the Rietvelt
Refinement), it is
possible to perform quantitative analyses on samples containing more than one
crystalline
compound.
Table 4.. XRD Data Including Domain Size and % Crystallinity
Sample
Domai(An Size
ID % Crystallinity =
)
P132 55 55
= P132-10 46 58
P132-100 50 55
P132-181 48 52 =
P132-US 26 40
A132 28 42
A132-10 26 40
A132-100 28 . 35
WS132 30 36
= WS132-10 27 37
=
WS132-100 30 41
SG132 29 40
SG132-10 28 38
SG132-100 28 37
SG132-10-US 25 42
SG132-100-US 21 34
106
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Each sample was placed on a zero background holder and placed in a Phillips
PW1800
diffractometer using Cu radiation. Scans were then run over the range of 50 to
50 with a step
size of 0.05 and a counting time of 2 hours each.
Once the diffraction patterns was obtained, the phases were identified with
the aid of
the Powder Diffraction File published by the International Centre for
Diffraction Data. In all
samples the crystalline phase identified was cellulose ¨ Ia, which has a
triclinic structure.
The distinguishing feature among the 20 samples is the peak breadth, which is
related to
the crystallite domain size. The experimental peak breadth was used to compute
the domain
size and percent crystallinity and are reported in Table 4.
Percent crystallinity (Xc %) is measured as a ratio of the crystalline area to
the total area
under the x-ray diffraction peaks,
A
X %= _______________________________ x100%
c
{/1õ + A}
c
where,
A, = Area of crystalline phase
=
Aa
= Area of amorphous phase
X, = Percent of crystallinity
To determine the percent crystallinity for each sample it was necessary to
first extract
the amount of the amorphous phase. This is done by estimating the area of each
diffraction
pattern that can be attributed to the crystalline phase (represented by the
sharper peaks) and the
non-crystalline phase (represented by the broad humps beneath the pattern and
centered at 22
and 38 ).
A systematic process was used to minimize error in these calculations due to
broad
crystalline peaks as well as high background intensity, First, a linear
background was applied
and then removed. Second, two Gaussian peaks centered at 22 and 38 with
widths of 10-12
107
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
each were fitted to the humps beneath the crystalline peaks. Third, the area
beneath the two
broad Gaussian peaks and the rest of the pattern were determined. Finally,
percent crystallinity
was calculated by dividing the area beneath the crystalline peak by the total
intensity (after
background subtraction). Domain size and % crystallinity of the samples as
determined by X-
ray diffraction (XRD) are presented in Table 4.
Example 11 - Porosimetry Analysis of Irradiated Materials
Mercury pore size and pore volume analysis (Table 5) is based on forcing
mercury (a
non-wetting liquid) into a porous structure under tightly controlled
pressures. Since mercury
does not wet most substances and will not spontaneously penetrate pores by
capillary action, it
must be forced into the voids of the sample by applying external pressure. The
pressure
required to fill the voids is inversely proportional to the size of the pores.
Only a small amount
of force or pressure is required to fill large voids, whereas much greater
pressure is required to
fill voids of very small pores.
Table 5. Pore Size and Volume Distribution by Mercury Porosimetry
Median Median Average Bulk
Total TotalApparent
Pore Pore Pore Density
Intrusion Pore(skeletal) Porosity
Sample ID
Volume Area Diameter Diameter Diameter @ 0.50
Density (%)
(nadig) (niz/g1 .(Volume) (Area) (4V/A) psia (gimp
(11m) qin9 (winL)
P132 6.0594 1.228 36.2250 13.7278 19.7415 0.1448 1.1785 87.7163
P132-10 5.5436 1.211 46.3463 4.5646 18.3106 0.1614 1.5355 89.4875
P132-100 5.3985 0.998 34.5235 18.2005 21.6422 0.1612 1.2413 87.0151
P132-181 3.2866 0.868 25.3448 12.2410 15.1509 0.2497 1.3916 82.0577
P132-US 6.0005 14.787 98.3459 0.0055 1.6231 0.1404 0.8894 84.2199
A132 2.0037 11.759 64.6308 0.0113 0.6816 0.3683 1.4058 73.7990
A132-10 1.9514 10.326 53.2706 0.0105 0.7560 0.3768 1.4231 73.5241
A132-100 1.9394 10.205 43.8966 0.0118 0.7602 0.3760 1.3889 72.9264
SG132 2.5267 8.265 57.6958 0.0141 1.2229 0.3119 1.4708 78.7961
SG132-10 2.1414 8.643 26.4666 0.0103 0.9910 0.3457 1.3315 74.0340
SG132-100 2.5142 10.766 32.7118 0.0098 0.9342 0.3077 1.3590 77.3593
SG132-10-US 4.4043 1.722 71.5734 1.1016 10.2319 0.1930 1.2883 85.0169
SG132-100-US 4.9665 7.358 24.8462 0.0089 2.6998 0.1695 1.0731 84.2010
WS132 2.9920 5.447 76.3675 0.0516 2.1971 0.2773 1.6279 82.9664
WS132-10 3.1138 2.901 57.4727 0.3630 4.2940 0.2763 1.9808 86.0484
WS132-100 3.2077 3.114 52.3284 0.2876 4.1199 0.2599
1.5611 83.3538
108
CA 02823376 2013-08-07
cL3983-7
The AutoPore 9520 can attain a maximum pressure of 414 MPa or 60,000 psia.
There
are four low pressure stations for sample preparation and collection of
macropore data from 0.2
psia to 50 psia. There aretwo high pressure chambers which collects data from
25 psia to
. 60,000 psia. The sample is placed in a bowl-like apparatus called a
penetrometer, which is
bonded to a glass capillary stem with a metal coating. As mercury invades the
voids in and
around the sample, it moves down the capillary stem. The loss of mercury from
the capillary
stem results in a change in the electrical capacitance. The change in
capacitance during the =
experiment is converted to volume of mercury by knowing the stem volume of the
penetrometer in use. A variety of penetrometers with different bowl (sample)
sizes and .
capillaries are available to accommodate most sample sizes and configurations.
Table 6 below
defines some of the key parameters calculated for each sample.
=
Table 6. Definition of Parameters
=
Parameter Description
The total .volume of mercury irauded during an experiment This
Total Intrusion Volume: can include interstitial filling between small
particles, porosity of
sample, and compression volume of sample.
The total intrusion volume converted to an area assuming
Total Pore Area: cylindrical shaped pores.
Median Pore Diameter
The size at the 50th percentile on the cumulative volume graph.
(volume):
Median Pore Diameter (area): The size at the 50th percentile on the cumulative
area graph.
Average Pore Diameter: The total pore volume divided by the total pore
area (4V/A).
The mass of the sample divided by the bulk Volume. Bulk volume
Bulk Density:
is determined at the filling pressure, typically 0.5 psia.
The mass of sample divided by the volume of sample measured at =
Apparent Density: =
highest pressure, typically 60,000 psia.
Porosity: (Bulk Density/ Apparent Density).x 100%
Example 12- Particle Size Analysis of Irradiated Materials
The technique of particle sizing by static light scattering is based on Mie
theory (which
also encompasses Fraunhofer theory). Mie theory predicts the intensity vs.
angle relationship
as a function of the size for spherical scattering particles provided that
other system variables
are known and held constant. These variables are the wavelength of incident
light and the
109
- = CA 02823376 2013-08-07
relative refractive index Of the sample material. Application of Mie theory
provides the
=
detailed particle size information. Table 7 summarizes particle size using
median diameter,
mean diameter, and modal diameter as parameters.
Table 7. Particle Size by Laser Light Scattering (Dry Sample Dispersion)
Median Diameter Mean Diameter Modal Diameter
Sample ID
(pm) (11m) 011n)
A132 380.695 -418.778 442.258
= A132-10 321.742 366.231
410.156
A132-100 301.786 348.633 444.169
SG132 369.400 411.790 455.508
= S0132-10 278.793 325.497
426.717
S0132-100 242.757 298.686 " 390.097
= WS132 407335 445.618
467.978
WS132-10 194.237 226.604 297.941
WS132-100 201.975 236.037 307.304
Particle size was determined by Laser Light Scattering (Dry Sample Dispersion)
using a .
7
Malvern Mastersize14r 2000 using the following conditions:
Feed Rate: 35%
-Disperser Pressure: 4 Bar
= - Optical Model: (2.610,
1.000i), 1.000
An appropriate amount of sample was introduced onto a vibratory tray. The feed
rate
and air pressure were adjusted to ensure that the particles were properly
dispersed. The key
component is selecting an air pressure that will break up agglomerations, but
does not
compromise the sample integrity. The amount of sample needed varies depending
on the size
of the particles. In general, samples with fine particles require less
material than samples with
coarse particles.
Example 13- Surface Area Analysis of Irradiated Materials
Table 8. Summary of Surface Area by Gas Adsorption
= Surface area of each sample was analyzed using a Micromeritic,s ASAP 2420
Accelerated Surface Area and Porosimetry System. The samples were prepared by
first
110
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
degassing for 16 hours at 40 C. Next, free space (both warm and cold) with
helium is
calculated and then the sample tube is evacuated again to remove the helium.
Data collection
begins after this second evacuation and consists of defining target pressures
which controls how
much gas is dosed onto the sample. At each target pressure, the quantity of
gas adsorbed and
the actual pressure are determined and recorded. The pressure inside the
sample tube is
measured with a pressure transducer. Additional doses of gas will continue
until the target
pressure is achieved and allowed to equilibrate. The quantity of gas adsorbed
is determined by
summing multiple doses onto the sample. The pressure and quantity define a gas
adsorption
isotherm and are used to calculate a number of parameters, including BET
surface area (Table
8).
Sample ID Single point surface area (m /g) BETeaS(unrlf/awee
132 @ P/Po= 0.250387771 1.5253
1.6897
P132-10 @ P/Po= 0.239496722 1.0212 1.2782
P132-100 P/Po= 0.240538100 1.0338 1.2622
P132-181 @ P/Po= 0.239166091 0.5102 0.6448
P132-US @ P/Po= 0.217359072 1.0983 1.6793
A132 @ P/Po= 0.240040610 0.5400
0.7614
A132-10 @ P/Po= 0.211218936 0.5383
0.7212
A132-100 @ P/Po= 0.238791097 0.4258 0.5538
SG132 @ P/Po= 0.237989353 0.6359
0.8350
SG132-10 @ P/Po= 0.238576905 0.6794 0.8689
SG132-100 @ P/Po= 0.241960361 0.5518 0.7034
SG132-10-US @ P/Po= 0.225692889 0.5693 0.7510
SG132-100-US @ P/Po= 0.225935246 1.0983 1.4963
WS132 @ P/Po= 0.237823664 0.6582
0.8663
WS132-10 @ P/Po= 0.238612476 0.6191 0.7912
WS132-100 @ P/Po= 0.238398832 0.6255 0.8143
The BET model for isotherms is a widely used theory for calculating the
specific
surface area. The analysis involves determining the monolayer capacity of the
sample surface
by calculating the amount required to cover the entire surface with a single
densely packed
layer of krypton. The monolayer capacity is multiplied by the cross sectional
area of a
molecule of probe gas to determine the total surface area. Specific surface
area is the surface
area of the sample aliquot divided by the mass of the sample.
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Example 14 - Fiber Length Determination of Irradiated Materials
Fiber length distribution testing was performed in triplicate on the samples
submitted
using the Techpap MorFi LB01 system. The average length and width are reported
in Table 9.
Table 9. Summary of Lignocellulosic Fiber Length and Width Data
Statistically
Arithmetic Average Length
Corrected Average Width
Sample ID Average Weighted in
Length Weighted in (micrometers)
(mm) Length (mm) (um)
Length (mm)
P132-10 0.484 0.615 0.773 24.7
P132-100 - 0.369 0.423 0.496 23.8
P132-181 0.312 0.342 0.392 24.4
A132-10 0.382 0.423 0.650 43.2
A132-100 0.362 0.435 0.592 29.9
SG132-10 0.328 0.363 0.521 44.0
SG132-100 0.325 0.351 0.466 43.8
WS132-10 0.353 0.381 0.565 44.7
WS132-100 0.354 0.371 0.536 45.4
Example 15- Ultrasonic Treatment of Irradiated and Un-irradiated Switchgrass
Switchgrass was sheared according to Example 4. The switchgrass was treated by
ultrasound alone or irradiation with 10 Mrad or 100 Mrad of gamma rays, and
then sonicated.
The resulting materials correspond to G132-BR (un-irradiated), G132-10-BR (10
Mrad and
sonication) and G132-100-BR (100 Mrad and sonication), as presented in Table
1. Sonication
was performed on each sample for 30 minutes using 20kHz ultrasound from a
1000W horn
under re-circulating conditions. Each sample was dispersed in water at a
concentration of
about 0.10 g/mL.
FIGS. 30 and 31 show the apparatus used for sonication. Apparatus 500 includes
a
converter 502 connected to booster 504 communicating with a horn 506
fabricated from
titanium or an alloy of titanium. The horn, which has a seal 510 made from
VITON about its
perimeter on its processing side, forms a liquid tight seal with a processing
cell 508. The
processing side of the horn is immersed in a liquid, such as water, that has
dispersed therein the
sample to be sonicated. Pressure in the cell is monitored with a pressure
gauge 512. In
operation, each' sample is moved by pump 517 from tank 516 through the
processing cell and is
sonicated. After, sonication, the sample is captured in tank 520. The process
can be reversed in
that the contents of tank 520 can be sent through the processing cell and
captured in tank 516.
112
CA 02823376 2013-08-07
WO 2008/073186 PCMS2007/022719
This process can be repeated a number of times until a desired level of
processing is delivered
to the sample.
Example 16 - Scanning Electron Micrographs of Un-irradiated Switchgrass in
Comparison to
Irradiated and Irradiated and Sonicated Switchorass
Switchgrass samples for the scanning electron micrographs were applied to
carbon tape
and gold sputter coated (70 seconds). Images were taken with a JEOL 6500 field
emission
scanning electron microscope.
FIG. 32 is a scanning electron micrograph at 1000 X magnification of a fibrous
material
produced from shearing switchgrass on a rotary knife cutter, and then passing
the sheared
material through a 1/32 inch screen.
FIGS. 33 and 34 are scanning electron micrographs of the fibrous material of
FIG. 32
after irradiation with 10 Mrad and 100 Mrad gamma rays, respectively, at 1000
X
magnification.
FIG. 35 is a scanning electron micrographs of the fibrous material of FIG. 32
after
irradiation with 10 Mrad and sonicaiton at 1000 X magnification.
FIG. 36 is a scanning electron micrographs of the fibrous material of FIG. 32
after
irradiation with 100 Mrad and sonicaiton at 1000 X magnification.
Example 17 - Infrared Spectrum of Irradiated Kraft Paper In Comparison to Un-
irradiated Kraft Paper
The FT-IR analysis was performed on a Nicolet/Impact 400. The results indicate
that
all samples reported in Table 1 are consistent with a cellulose-based
material.
FIG 37 is an infrared spectrum of Kraft board paper sheared according to
Example 4,
while FIG 38 is an infrared spectrum of the Kraft paper of FIG 38 after
irradiation with 100
Mrad of gamma radiation. The irradiated sample shows an additional peak in
region A
(centered about 1730 cm") that is not found in the un-irradiated material.
Example 18 - Combination of Electron Beam and Sonication Pretreatment
Switchgrass is used as the feedstock and is sheared with a Munson rotary knife
cutter
into a fibrous material. The fibrous material is then evenly distributed onto
an open tray
composed of tin with an area of greater than about 500 in2. The fibrous
material is distributed
so that it has a depth of about 1 ¨2 inches in the open tray. The fibrous
material may be
113
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
distributed in plastic bags at lower doses of irradiation (under 10 MRad), and
left uncovered on
the metal tray at higher doses of radiation.
Separate samples of the fibrous material are then exposed to successive doses
of
electron beam radiation to achieve a total dose of 1 Mrad, 2 Mrad, 3, Mrad, 5
Mrad, 10 Mrad,
50 Mrad, and 100 Mrad. Some samples are maintained under the same conditions
as the
remaining samples, but are not irradiated, to serve as controls. After
cooling, the irradiated
fibrous material is sent on for further processing through a sonication
device.
The sonication device includes a converter connected to booster communicating
with a
horn fabricated from titanium or an alloy of titanium. The horn, which has a
seal made from
VITONS about its perimeter on its processing side, forms a liquid tight seal
with a processing
cell. The processing side of the horn is immersed in a liquid, such as water,
into which the
irradiated fibrous material to be sonicated is immersed. Pressure in the cell
is monitored with a
pressure gauge. In operation, each sample is moved by pump through the
processing cell and is
sonicated.
To prepare the irradiated fibrous material for sonication, the irradiated
fibrous material
is removed from any container (e.g., plastic bags) and is dispersed in water
at a concentration
of about 0.10 g/mL. Sonication is performed on each sample for 30 minutes
using 20 kHz
ultrasound from a 1000 W horn under re-circulating conditions. After
sonication, the irradiated
fibrous material is captured in a tank. This process can be repeated a number
of times until a
desired level of processing is achieved based on monitoring the structural
changes in the
switchgrass. Again, some irradiated samples are kept under the same conditions
as the
remaining samples, but are not sonicated, to serve as controls. In addition,
some samples that
were not irradiated are sonicated, again to serve as controls. Thus, some
controls are not
processed, some are only irradiated, and some are only sonicated.
Example 19 ¨ Microbial Testing of Pretreated Biomass
Specific lignocellulosic materials pretreated as described herein are analyzed
for
toxicity to common strains of yeast and bacteria used in the biofuels industry
for the
fermentation step in ethanol production. Additionally, sugar content and
compatibility with
cellulase enzymes are examined to determine the viability of the treatment
process. Testing of
the pretreated materials is carried out in two phases as follows.
114
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
I. Toxicity and Sugar Content
Toxicity of the pretreated grasses and paper feedstocks is measured in yeast
Saccharomyces cerevisiae (wine yeast) and Pichia stipitis (ATCC 66278) as well
as the
bacteria Zymomonas mobilis (ATCC 31821) and Clostridium thermocellum (ATCC
31924). A
growth study is performed with each of the organisms to determine the optimal
time of
incubation and sampling.
Each of the feedstocks is then incubated, in duplicate, with S. cerevisiae, P.
stipitis, Z
mobilis, and C. thermocellum in a standard microbiological medium for each
organism. YM
broth is used for the two yeast strains, S. cerevisiae and P. stipitis. RM
medium is used for Z.
mobilis and CM4 medium for C. thermocellum. A positive control, with pure
sugar added, but
no feedstock, is used for comparison. During the incubation, a total of five
samples is taken
over a 12 hour period at time 0, 3, 6, 9, and 12 hours and analyzed for
viability (plate counts for
Z mobilis and direct counts for S. cerevisiae) and ethanol concentration.
Sugar content of the feedstocks is measured using High Performance Liquid
Chromatography (HPLC) equipped with either a Shodex TM sugar SP0810 or Biorad
Asninexe
HPX-87P column. Each of the feedstocks (approx. 5 g) is mixed with reverse
osmosis (RO)
water for 1 hour. The liquid portion of the mixture is removed and analyzed
for glucose,
galactose, xylose, mannose, arabinose, and cellobiose content. The analysis is
performed
according to National Bioenergy Center protocol Determination of Structural
Carbohydrates
and Lignin in Biomass.
II. Cellulase Compatibility
Feedstocks are tested, in duplicate, with commercially available AccelleraseTm
1000,
which contains a complex of enzymes that reduces lignocellulosic biomass into
fermentable
sugars."two different cello lase preparations, Trichoderma reesei and
Aspergillus nidulans at the
recommended temperature and concentration in an Erlenmeyer flask. The flasks
are incubated
with moderate shaking at around 200 rpm for 12 hours. During that time,
samples are taken
every three hours at time 0, 3, 6, 9, and 12 hours to determine the
concentration of reducing
sugars (Hope and Dean, Biotech j, 1974, 144:403) in the liquid portion of the
flasks.
115
CA 02823376 2013-08-07
=
WO 2008/073186
PCT/US2007/022719
Example 20 - Alcohol Production Using irradiation-Sonication Pretreatment
The optimum size for biomass conversion plants is affected by factors
including
economies of scale and the type and availability of biomass used as feedstock.
Increasing plant
size tends to increase economies of scale associated with plant processes.
However, increasing
plant size also tends to increase the costs (e.g., transportation costs) per
unit of biomass feedstock.
Studies analyzing these factors suggest that the appropriate size for biomass
conversion plants can
range from 2000 to 10,000 dried tons of biomass feedstock per day. The plant
described below is
sized to process 2000 tons of dry biomass feedstock per day.
FIG. 39 shows a process schematic of a biomass conversion system configured to
process
switchgrass. The feed preparation subsystem processes raw biomass feedstock to
remove foreign
objects and provide consistently sized particles for further processing. The
pretreatment
subsystem changes the molecular structure (e.g., reduces the average molecular
weight and the
crystallinity) of the biomass feedstock by irradiating the biomass feedstock,
mixing the irradiated
the biomass feedstock with water to form a slurry, and applying ultrasonic
energy to the slurry.
The irradiation and sonication convert the cellulosic and lignocellulosic
components of the
biomass feedstock into fermentable materials. The primary process subsystem
ferments the
glucose and other low weight sugars present after pretreatment to form
alcohols.
Feed preparation
The selected design feed rate for the plant is 2,000 dry tons per day of
switchgrass
biomass. The design feed is chopped and/or sheared switchgrass.
Biomass feedstock in the form of bales of switchgrass are received by the
plant on truck
trailers. As the trucks are received, they are weighed and unloaded by
forklifts. Some bales are
sent to on-site storage while others are taken directly to the conveyors. From
there, the bales
are conveyed to an automatic unwrapping system that cuts away the plastic
wrapping and/or net
surrounding the bales. The biomass feedstock is then conveyed past a magnetic
separator to
remove tramp metal, after which it is .introduced to shredder-shearer trains
where the material is
reduced in size. Finally, the biomass feedstock is conveyed to the
pretreatment subsystem.
In some cases, the switchgrass bales are wrapped with plastic net to ensure
they don't
break apart when handled, and may also be wrapped in plastic film to protect
the bale from
116
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
weather. The bales are either square or round. The bales are received at the
plant from off-site
storage on large truck trailers.
Since switchgrass is only seasonally available, long-term storage is required
to provide
feed to the plant year-round. Long-term storage will likely consist of 400-500
acres of
uncovered piled rows of bales at a location (or multiple locations) reasonably
close to the
ethanol plant. On-site short-term storage is provided equivalent to 72 hours
of production at an
outside storage area. Bales and surrounding access ways as well as the
transport conveyors will
be on a concrete slab. A concrete slab is used because of the volume of
traffic required to
deliver the large amount of biomass feedstock required. A concrete slab will
minimize the
amount of standing water in the storage area, as well as reduce the biomass
feedstock's
exposure to dirt. The stored material provides a short-term supply for
weekends, holidays, and
when normal direct delivery of material into the process is interrupted.
The bales are off-loaded by forklifts and are placed directly onto bale
transport
conveyors or in the short-term storage area. Bales are also reclaimed from
short-term storage
by forklifts and loaded onto the bale transport conveyors.
Bales travel to one of two bale unwrapping stations. Unwrapped bales are
broken up
using a spreader bar and then discharged onto a conveyor which passes a
magnetic separator to
remove metal prior to shredding. A tramp iron magnet is provided to catch
stray magnetic
metal and a scalping screen removes gross oversize and foreign material ahead
of multiple
shredder-shearer trains, which reduce the biomass feedstock to the proper size
for pretreatment.
The shredder-shearer trains include shredders and rotary knife cutters. The
shredders reduce
the size of the raw biomass feedstock and feed the resulting material to the
rotary knife cutters.
The rotary knife cutters concurrently shear the biomass feedstock and screen
the resulting
material.
Three storage silos are provided to limit overall system downtime due to
required
maintenance on and/or breakdowns of feed preparation subsystem equipment. Each
silo can
hold approximately 55,000 cubic feet of biomass feedstock (-3 hours of plant
operation).
Pretreatment.
A conveyor belt carries the biomass feedstock from the feed preparation
subsystem 110
to the pretreatment subsystem 114. As shown in FIG. 40, in the pretreatment
subsystem 114,
117
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
the biomass feedstock is irradiated using electron beam emitters, mixed with
water to form a
slurry, and subjected to the application of ultrasonic energy. As discussed
above, irradiation of
the biomass feedstock changes the molecular structure (e.g., reduces the
average molecular
weight and the crystallinity) of the biomass feedstock. Mixing the irradiated
biomass feedstock
into a slurry and applying ultrasonic energy to the slurry further changes the
molecular structure
of the biomass feedstock. Application of the radiation and sonication in
sequence may have
synergistic effects in that the combination of techniques appears to achieve
greater changes to
the molecular structure (e.g., reduces the average molecular weight and the
crystallinity) than
either technique can efficiently achieve on its own. Without wishing to be
bound by theory, in
addition to reducing the polymerization of the biomass feedstock by breaking
intrarnolecular
bonds between segments of cellulosic and lignocellulosic components of the
biomass
feedstock, the irradiation may make the overall physical structure of the
biomass feedstock
more brittle. After the brittle biomass feedstock is mixed into a slurry, the
application of
ultrasonic energy further changes the molecular structure (e.g., reduces the
average molecular
weight and the crystallinity) and also can reduce the size of biomass
feedstock particles.
Electron Beam Irradiation
The conveyor belt 491 carrying the biomass feedstock into the pretreatment
subsystem
distributes the biomass feedstock into multiple feed streams (e.g., 50 feed
streams) each leading
to separate electron beam emitters 492. In this embodiment, the biomass
feedstock is irradiated
while it is dry. Each feed stream is carried on a separate conveyor belt to an
associated electron
beam emitter. Each irradiation feed conveyor belt can be approximately one
meter wide.
Before reaching the electron beam emitter, a localized vibration is induced in
each conveyor
belt to evenly distribute the dry biomass feedstock over the cross-sectional
width of the
conveyor belt.
Electron beam emitter 492 (e.g., electron beam irradiation devices
commercially
available from Titan Corporation, San Diego, CA) are configured to .apply a
100 kilo-Gray dose
of electrons applied at a power of 300 kW. The electron beam emitters are
scanning beam
devices with a sweep width of 1 meter to correspond to the width of the
conveyor belt. In some
embodiments, electron beam emitters with large, fixed beam widths are used.
Factors
including belt/beam width, desired dose, biomass feedstock density, and power
applied govern
118
CA 02823376 2013-08-07
983-7
_
the number of electron beam emitters required for the plant to process 2,000
tons per day of
= dry feed.
=
Sonication
The irradiated biomass feedstock is mixed with water to form a slurry before
ultrasonic energy is applied. There can be a separate sonication system
associated with each
= electron beam feed stream or several electron beam streams can be
aggregated as feed for a single
sonication system.
In each sonication system, the irradiated biomass feedstock is fed into a
reservoir 14
through a first intake 32 and water is fed into the reservoir 14 through
second intake 34.
Appropriate valves (manual or automated) control the flow of biomass feedstock
and the flow of
water to produce a desired ratio of biomass feedstock to water (e.g., 10%
cellulosic material, weight
by volume). Each reservoir 14 includes a mixer 40 to agitate the contents of
volume 36 and disperse
biomass feedstock throughout the water.
In each sonication system, the slurry is pumped (e.g., using a recessed
impeller -
IS vortex pump 18) from reservoir 14 to and through a flow cell 24
including an ultrasonic transducer
26. In some embodiments, pump 18 is configured to agitate the slurry 16 such
that the mixture of
biomass feedstock and water is substantially uniform at inlet 20 of the flow
cell 24. For example,
= the pump 18 can agitate the shiny 16 to create a turbulent flow that
persists throughout the piping
between the first pump and inlet 20 of flow cell 24.
Within the flow cell 24, ultrasonic transducer 26 transmits ultrasonic energy
into
slurry 16 as the slurry flows through flow cell 24. Ultrasonic transducer 26
converts electrical energy
into high frequency mechanical energy (e.g., ultrasonic energy) which is then
delivered to the slurry
through booster 48. Ultrasonic transducers are commercially available (e.g.,
fromIlelscher USA, Inc.
of Ringwood, New Jersey) that are capable of delivering a continuous power of
16 kilowatts.
The ultrasonic energy traveling through booster 48 in reactor volume 44
creates a series
of compressions and rarefactions in process stream 16 with an intensity
sufficient to create cavitation in
process stream 16. Cavitation disaggregates components of the biomass
feedstock including, for
119
=3983-7 CA 02823376 2013-08-07
.:
=
example, cellulosic and lignocellulosic material dispersed in process stream
16 (e.g., slurry).
Cavitation also produces free radicals in the water of process stream 16
(e.g., slurry). These free
radicals act to further break down the cellulosic material in process stream
16. In general, about
250 MJ/m3 of ultrasonic energy is applied to process stream 16 containing
fragments of poplar chips.
Other levels of ultrasonic energy (between about 5 and about 4000 MJ/m3, e.g.,
10, 25, 50, 100, 250,
500, 750, 1000,2000, or 3000) can be applied to other biomass feedstocks.
After exposure to
ultrasonic energy in reactor volume 44, process stream 16 exits flow cell 24
through outlet 22.
Flow cell 24 also includes a heat exchanger 46 in thermal communication with
at
least a portion of reactor volume 44. Cooling fluid 48 (e.g., water) flows
into heat exchanger 46 and
absorbs heat generated when process stream 16 (e.g., slurry) is sonicated in
reactor volume 44. In
some embodiments, the flow of cooling fluid 48 into heat exchanger 46 is
controlled to maintain an
approximately constant temperature in reactor volume 44. In addition or in the
alternative, the
temperature of cooling fluid 48 flowing into heat exchanger 46 is controlled
to maintain an
approximately constant temperature in reactor volume 44.
The outlet 42 of flow cell 24 is arranged near the bottom of reservoir 14 to
induce a
gravity feed of process stream 16 (e.g., slurry) out of reservoir 14 towards
the inlet of a second pump
= 30 which pumps process stream 16 (e.g., slurry) towards the primary
process subsystem.
Sonication systems can include a single flow path (as described above) or
multiple
parallel flow paths each with an associated individual sonication units.
Multiple sonication units can
also be arranged to series to increase the amount of sonic energy applied to
the slurry.
Primary Processes
A vacuum rotary drum type filter removes solids from the slurry before
fermentation. Liquid from the filter is pumped cooled prior to entering the
fermentors. Filtered
solids are passed to the post-processing subsystem for further processing.
The fermentation tanks are large, low pressure, stainless steel vessels with
conical
bottoms and slow speed agitators. Multiple first stage fermentation tanks can
be arranged in series.
The temperature in the first stage fermentation tanks is controlled to 30
degrees
120
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
centigrade using external heat exchangers. Yeast is added to the first stage
fermentation tank at
the head of each series of tanks and carries through to the other tanks in the
series.
Second stage fermentation consists of two continuous fermentors in series.
Both
fermentors are continuously agitated with slow speed mechanical mixers.
Temperature is
controlled with chilled water in external exchangers with continuous
recirculation.
Recirculation pumps are of the progressive cavity type because of the high
solids concentration.
Off gas from the fermentation tanks and fermentors is combined and washed in a
counter-current water column before being vented to the atmosphere. The off
gas is washed to
recover ethanol rather than for air emissions control.
Post-Processing
Distillation
Distillation and molecular sieve adsorption are used to recover ethanol from
the raw
fermentation beer and produce 99.5% ethanol. Distillation is accomplished in
two columns¨
the first, called the beer column, removes the dissolved CO2 and most of the
water, and the
second concentrates the ethanol to a near azeotropic composition.
All the water from the nearly azeotropic mixture is removed by vapor phase
molecular
sieve adsorption. Regeneration of the adsorption columns requires that an
ethanol water
mixture be recycled to distillation for recovery.
Fermentation vents (containing mostly CO2, but also some ethanol) as well as
the beer
column vent are scrubbed in a water scrubber, recovering nearly all of the
ethanol. The
scrubber effluent is fed to the first distillation column along with the
fermentation beer.
The bottoms from the first distillation contain all the unconverted insoluble
and
dissolved solids. The insoluble solids are dewatered by a pressure filter and
sent to a
combustor. The liquid from the pressure filter that is not recycled is
concentrated in a multiple
effect evaporator using waste heat from the distillation. The concentrated
syrup from the
evaporator is mixed with the solids being sent to the combustor, and the
evaporated condensate
is used as relatively clean recycle water to the process.
Because the amount of stillage water that can be recycled is limited, an
evaporator is
included in the process. The total amount of the water from the pressure
filter that is directly
recycled is set at 25%. Organic salts like ammonium acetate or lactate, steep
liquor
121
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
components not utilized by the organism, or inorganic compounds in the biomass
end up in this
stream. Recycling too much of this material can result in levels of ionic
strength and osmotic
pressures that can be detrimental to the fermenting organism's efficiency. For
the water that is
not recycled, the evaporator concentrates the dissolved solids into a syrup
that can be sent to
the combustor, minimizing the load to wastewater treatment.
Wastewater Treatment
The wastewater treatment section treats process water for reuse to reduce
plant makeup
water requirements. Wastewater is initially screened to remove large
particles, which are
collected in a hopper and sent to a landfill. Screening is followed by
anaerobic digestion and
aerobic digestion to digest organic matter in the stream. Anaerobic digestion
produces a biogas
stream that is rich in methane that is fed to the combustor. Aerobic digestion
produces a
relatively clean water stream for reuse in the process as well as a sludge
that is primarily
composed of cell mass. The sludge is also burned in the combustor. This
screening / anaerobic
digestion / aerobic digestion scheme is standard within the current ethanol
industry and
facilities in the 1-5 million gallons per day range can be obtained as "off-
the-shelf' units from
vendors.
Combustor. Boiler. and Turbouenerator
The purpose of the combustor, boiler, and turbogenerator subsystem is to burn
various
by-product streams for steam and electricity generation. For example, some
lignin, cellulose,
and hemicellulose remains unconverted through the pretreatment and primary
processes. The
majority of wastewater from the process is concentrated to a syrup high in
soluble solids.
Anaerobic digestion of the remaining wastewater produces a biogas high in
methane. Aerobic
digestion produces a small amount of waste biomass (sludge). Burning these by-
product
streams to generate steam and electricity allows the plant to be self
sufficient in energy, reduces
solid waste disposal costs, and generates additional revenue through sales of
excess electricity.
Three primary fuel streams (post-distillate solids, biogas, and evaporator
syrup) are fed
to a circulating fluidized bed combustor. The small amount of waste biomass
(sludge) from
wastewater treatment is also sent to the combustor. A fan moves air into the
combustion
chamber. Treated water enters the heat exchanger circuit in the combustor and
is evaporated
and superheated to 510 C (950 F) and 86 atm (1265 psia) steam. Flue gas from
the combustor
122
CA 02823376 2013-08-07
,
= 983-7
preheats the entering combustion air then enters a baghouse. to remove
particulates, which are
landfilled. The gas is exhausted through a stack. =
A multistage turbine and generator are used to generate electricity. Steam is
extracted
from the turbine at three different conditions for injection into the
pretreatment reactor and heat
exchange in distillation and evaporation. The remaining steam is condensed
with cooling water
and returned to the boiler feedwater system along with condensate from the
various heat =
exchangers in the process. Treated well water is used as makeup to replace
steam used in direct
injection.
OTHER EMBODIMENTS
A number of embodiments of the invention have been described;
In some embodiments, relatively low doses of radiation, optionally, combined
with
acoustic energy, e.g., ultrasound, are utilized to crosslink, graft, or
otherwise increase the
molecular weight of a natural or synthetic carbohydrate-containing material,
such as any of -
those materials in any form (e.g., fibrous form) described herein, e.g.,
sheared or un-sheared
cellulosic or lignocellulosic materials; such as cellulose. The crosslinking,
grafting, or
. otherwise increasing the molecular weight of the natural or synthetic
carbohydrate-containing
material can be performed in a controlled and predetermined manner by
selecting the type or
types of radiation employed (e.g., e-beam and ultraviolet or e-beam and gamma)
and/or dose or
number of doses of radiation applied. Such a material having increased
molecular weight can
be useful in making a composite, such as a fiber-resin composite, having
improved mechanical
properties, such as abrasion resistance, compression strength, fracture
resistance, impact.
strength, bending strength, tensile modulus, flexural modulus and elongation
at break.
.CrosslinIcing, grafting, or otherwise increasing the molecular weight of a
selected material can
improve the thermal stability of the material relative to an un-treated
material. Increasing the
thermal stability of the selected material can allow it to be processed at
higher temperatures
without degradation. In addition, treating materials with radiation can
sterilize the materials,
which can reduce their tendency to rot, e.g., while in a composite. The
crosslinking, grafting,
or otherwise increasing the molecular weight of a natural or synthetic
carbohydrate-containing
123 =
CA 02823376 2013-08-07
WO 2008/073186 PCT/1JS2007/022719
material can be performed in a controlled and predetermined manner for a
particular application
to provide optimal properties, such as strength, by selecting the type or
types of radiation
employed and/or dose or doses of radiation applied.
When used, the combination of radiation, e.g., low dose radiation, and
acoustic energy,
e.g., sonic or ultrasonic energy, can improve material throughput and/or
minimize energy
usage.
The resin can be any thermoplastic, thermoset, elastomer, adhesive, or
mixtures of these
resins. Suitable resins include any resin, or mixture of resins described
herein.
In addition to the resin alone, the material having the increased molecular
weight can be
combined, blended, or added to other materials, such as metals, metal alloys,
ceramics (e.g.,
cement), lignin, elastomers, asphalts, glass, or mixtures of any of these
and/or resins. When
added to cement, fiber-reinforced cements can be produced having improved
mechanical
properties, such as the properties described herein, e.g., compression
strength and/or fracture
resistance.
Crosslinlcing, grafting, or otherwise increasing the molecular weight of a
natural or
synthetic carbohydrate-containing material utilizing radiation can provide
useful materials in
many forms and for many applications. For example, the carbohydrate-containing
material can
be in the form of a paper product, such as paper, paper pulp, or paper
effluent, particle board,
glued lumber laminates, e.g., veneer, or plywood, lumber, e.g., pine, poplar,
oak, or even balsa
wood lumber. Treating paper, particle board, laminates or lumber, can increase
their
mechanical properties, such as their strength. For example, treating pine
lumber with radiation
can make a high strength structural material.
When paper is made using radiation, radiation can be utilized at any point in
its
manufacture. For example, the pulp can be irradiated, a pressed fiber preform
can be irradiated,
or the finished paper itself can be irradiated. In some embodiments, radiation
is applied at
more than one point during the manufacturing process..
For example, a fibrous material that includes a first cellulosic and/or
lignocellulosic
material having a first molecular weight can be irradiated in a manner to
provide a second
cellulosic and/or lignocellulosic material having a second molecular weight
higher than the first
molecular weight. For example, if gamma radiation is utilized as the radiation
source, a dose of
from about 0.2 Mrad to about 10 Mrad, e.g., from about 0.5 Mrad to about 7.5
Mrad, or from
124
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
about 2.0 Mrad to about 5.0 Mrad, can be applied. If e-beam radiation is
utilized, a smaller
dose can be utilized (relative to gamma radiation), such as a dose of from
about 0.1 Mrad to
about 5 Mrad, e.g., between about 0.2 Mrad to about 3 Mrad, or between about
0.25 Mrad and
about 2.5 Mrad. After the relatively low dose of radiation, the second
cellulosic and/or
lignocellulosic material can be combined with a material, such as a resin, and
formed into a
composite, e.g., by compression molding, injection molding or extrusion.
Forming resin-fiber
composites is described in WO 2006/102543. Once composites are formed, they
can be
irradiated to further increase the molecular weight of the carbohydrate-
containing material
while in the composite.
Alternatively, a fibrous material that includes a first cellulosic and/or
lignocellulosic
material having a first molecular weight can be combined with a material, such
as a resin, to
provide a composite, and then the composite can be irradiated with a
relatively low dose of
radiation so as to provide a second cellulosic and/or lignocellulosic material
having a second
molecular weight higher than the first molecular weight. For example, if gamma
radiation is
utilized as the radiation source, a dose of from about 1 Mrad to about 10 Mrad
can be applied.
Using this approach increases the molecular weight of the material while it is
with a matrix,
such as a resin matrix. In some embodiments, the resin is a cross-linkable
resin, and, as such, it
crosslinks as the carbohydrate-containing material increases in molecular
weight, which can
provide a synergistic effect to provide maximum mechanical properties to a
composite. For
example, such composites can have excellent low temperature performance, e.g.,
having a
reduced tendency to break and/or crack at low temperatures, e.g., temperatures
below 0 C, e.g.,
below -10 C, -20 C, -40 C, -50 C, -60 C or even below -100 C, and/or
excellent
performance at high temperatures, e.g., capable of maintaining their
advantageous mechanical
properties at relatively high temperature, e.g., at temperatures above 100 C,
e.g., above 125 C,
150 C, 200 C, 250 C, 300 C, 400 C, or even above 500 C. In addition,
such composites
can have excellent chemical resistance, e.g., resistance to swelling in a
solvent, e.g., a
hydrocarbon solvent, resistance to chemical attack, e.g., by strong acids,
strong bases, strong
oxidants (e.g., chlorine or bleach) or reducing agents (e.g., active metals
such as sodium and
potassium).
In some embodiments, the resin, or other matrix material, does not crosslink
during
irradiation. In some embodiments, additional radiation is applied while the
carbohydrate-
125
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
containing material is within the matrix to further increase the molecular
weight of the
carbohydrate-containing material. In some embodiments, the radiation causes
bonds to form
between the matrix and the carbohydrate-containing material.
In some embodiments, the carbohydrate-containing material is in the form of
fibers. In
such embodiments, when the fibers are utilized in a composite, the fibers can
be randomly
oriented within the matrix. In other embodiments, the fibers can be
substantially oriented, such
as in one, two, three or four directions. If desired, the fibers can be
continuous or discrete.
Any of the following additives can added to the fibrous materials, densified
fibrous
materials a or any other materials and composites described herein. Additives,
e.g., in the form
of a solid, a liquid or a gas, can be added, e.g., to the combination of a
fibrous material and
resin. Additives include fillers such as calcium carbonate, graphite,
wollastonite, mica, glass,
fiber glass, silica, and talc; inorganic flame retardants such as alumina
trihydrate or
magnesium hydroxide; organic flame retardants such as chlorinated or
brominated organic
compounds; ground construction waste; ground tire rubber; carbon fibers; or
metal fibers or
powders (e.g., aluminum, stainless steel). These additives can reinforce,
extend, or change
electrical, mechanical or compatibility properties. Other additives include
lignin, fragrances,
coupling agents, compatibilizers, e.g., maleated polypropylene, processing
aids, lubricants, e.g.,
fluorinated polyethylene, plasticizers, antioxidants, opacifiers, heat
stabilizers, colorants,
foaming agents, impact modifiers, polymers, e.g., degradable polymers,
photostabilizers,
biocides, antistatic agents, e.g., stearates or ethoxylated fatty acid amines.
Suitable antistatic
compounds include conductive carbon blacks, carbon fibers, metal fillers,
cationic compounds,
e.g., quaternary ammonium compounds, e.g., N-(3-chloro-2-hydroxypropy1)-
trimethylammonium chloride, alkanolamides, and amines. Representative
degradable polymers
include polyhydroxy acids, e.g., polylactides, polyglycolides and copolymers
of lactic acid and
glycolic acid, poly(hydroxybutyric acid), poly(hydroxyvaleric acid),
poly[lactide-co-(e-
caprolactone)], poly[glycolide-co-(e-caprolactone)], polycarbonates,
poly(amino acids),
poly(hydroxyalkanoate)s, polyanhydrides, polyorthoesters and blends of these
polymers.
When described additives are included, they can be present in amounts,
calculated on a
dry weight basis, of from below 1 percent to as high as 80 percent, based on
total weight of the
fibrous material. More typically, amounts range from between about 0.5 percent
to about 50
percent by weight, e.g., 5 percent, 10 percent, 20 percent, 30, percent or
more, e.g., 40 percent.
126
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Any additives described herein can be encapsulated, e.g., spray dried or
microencapsulated, e.g., to protect the additives from heat or moisture during
handling.
The fibrous materials, densified fibrous materials, resins or additives may be
dyed. For
example, the fibrous material can be dyed before combining with the resin and
compounding to
form composites. In some embodiments, this dyeing can be helpful in masking or
hiding the
fibrous material, especially large agglomerations of the fibrous material, in
molded or extruded
parts, when this is desired. Such large agglomerations, when present in
relatively high
concentrations, can show up as speckles in the surfaces of the molded or
extruded parts.
For example, the desired fibrous material can be dyed using an acid dye,
direct dye or a
reactive dye. Such dyes are available from Spectra Dyes, Kearny, NJ or
Keystone Aniline
Corporation, Chicago, IL. Specific examples of dyes include SPECTRATm LIGHT
YELLOW
2G, SPECTRACIDTm YELLOW 4GL CONC 200, SPECTRANYLTm RHODAMINE 8,
SPECTRANYLTm NEUTRAL RED B, SPECTRAMINETm BENZOPERPURINE,
SPECTRADIAZOTm BLACK OB, SPECTRAMINETm TURQUOISE G, and
SPECTRAMINETm GREY LVL 200%, each being available from Spectra Dyes.
In some embodiments, resin color concentrates.containing pigments are blended
with
dyes. When such blends are then compounded with the desired fibrous material,
the fibrous
material may be dyed in-situ during the compounding. Color concentrates are
available from
Clariant.
It can be advantageous to add a scent or fragrance to the fibrous materials,
densified
fibrous or composites. For example, it can be advantageous for the composites
smell and/or
look like natural wood, e.g., cedarwood. For example, the fragrance, e.g.,
natural wood
fragrance, can be compounded into the resin used to make the composite. In
some
implementations, the fragrance is compounded directly into the resin as an
oil. For example,
the oil can be compounded into the resin using a roll mill, e.g., a Banbury
mixer or an
extruder, e.g., a twin-screw extruder with counter-rotating screws. An example
of a Banbury
mixer is the F-Series Banbury mixer, manufactured by Farrel. An example of a
twin-screw
extruder is the WP ZSK 50 MEGAcompunderTM, manufactured by Krupp Werner &
Pfleiderer.
After compounding, the scented resin can be added to the fibrous material and
extruded or
molded. Alternatively, master batches of fragrance-filled resins are available
commercially
from International Flavors and Fragrances, under the tradename PolyIffrm or
from the RTP
127
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
Company. In some embodiments, the amount of fragrance in the composite is
between about
0.005 % by weight and about 10 % by weight, e.g., between about 0.1 % and
about 5 % or 0.25
% and about 2.5 %.
Other natural wood fragrances include evergreen or redwood. Other fragrances
include
peppermint, cherry, strawberry, peach, lime, spearmint, cinnamon, anise,
basil, bergamot, black
pepper, camphor, chamomile, citronella, eucalyptus, pine, fir, geranium,
ginger, grapefruit,
jasmine, juniperberry, lavender, lemon, mandarin, marjoram, musk, myrhh,
orange, patchouli,
rose, rosemary, sage, sandalwood, tea tree, thyme, wintergreen, ylang ylang,
vanilla, new car or
mixtures of these fragrances. In some embodiments, the amount of fragrance in
the fibrous
material-fragrance combination is between about 0.005 % by weight and about 20
% by weight,
e.g., between about 0.1 % and about 5 % or 0.25 % and about 2.5 %.
While fibrous materials have been described, such as cellulosic and
lignocellulosic
fibrous materials, other fillers may be used for making the composites. For
example, inorganic
fillers such as calcium carbonate (e.g., precipitated calcium carbonate or
natural calcium
carbonate), aragonite clay, orthorhombic clays, calcite clay, rhombohedral
clays, kaolin, clay,
bentonite clay, dicalcium phosphate, tricalcium phosphate, calcium
pyrophosphate, insoluble
sodium metaphosphate, precipitated calcium carbonate, magnesium
orthophosphate,
trimagnesium phosphate, hydroxyapatites, synthetic apatites, alumina, silica
xerogel, metal
aluminosilicate complexes, sodium aluminum silicates, zirconium silicate,
silicon dioxide or
combinations of the inorganic additives may be used. The fillers can have,
e.g., a particle size
of greater than 1 micron, e.g., greater than 2 micron, 5 micron, 10 micron, 25
micron or even
greater than 35 microns.
Nanometer scale fillers can also be used alone, or in combination with fibrous
materials
of any size and/or shape. The fillers can be in the form of, e.g., a particle,
a plate or a fiber.
For example, nanometer sized clays, silicon and carbon nanotubes, and silicon
and carbon
nanowires can be used. The filler can have a transverse dimension less than
1000 urn, e.g., less
than 900 nm, 800 urn, 750 urn, 600 urn, 500 run, 350 urn, 300 urn, 250 urn,
200 urn, less than
100 urn, or even less than 50 urn.
In some embodiments, the nano-clay is a montmorillonite. Such clays are
available
from Nanocor, Inc. and Southern Clay products, and have been described in U.S.
Patent Nos.
6,849,680 and 6,737,464. The clays can be surface treated before mixing into,
e.g., a resin or a
128
CA 02823376 2013-08-07
WO 2008/073186 PCT/US2007/022719
fibrous material. For example, the clay can be surface is treated so that its
surface is ionic in
nature, e.g., cationic or anionic.
Aggregated or agglomerated nanometer scale fillers, or nanometer scale fillers
that are
assembled into supramolecular structures, e.g., self-assembled supramolecular
structures can
also be used. The aggregated or supramolecular fillers can be open or closed
in structure, and
can have a variety of shapes, e.g., cage, tube or spherical.
Accordingly, other embodiments are within the scope of the following claims.
129