Note: Descriptions are shown in the official language in which they were submitted.
1
POLYURETHANE HAVING IMPROVED INSULATING PROPERTIES
FIELD OF THE INVENTION
The invention relates to a polyurethane having improved insulating properties,
which
is preferably used in the insulation of hollow bodies, in particular in the
insulation of pipe
systems.
BACKGROUND
Hollow spaces are frequently filled with foams, preferably polyurethane foams,
which
have excellent thermal conductivity values in order to improve the insulating
properties of
the hollow space in this way.
A liquid reaction mixture is introduced into the hollow spaces by means of a
polyurethane metering machine, and this mixture has to become distributed in
still liquid
form in the hollow space before the reaction commences. Once the reaction
commences,
further distribution due to flow of the foam which steadily increases in
viscosity takes place
until the material has fully reacted. In order to insulate, in particular,
large hollow spaces,
the reaction profile of the reacting polyurethane foam has to be very slow.
This is based on
the fact that the foam has to achieve good predistribution in the hollow space
in the liquid,
i.e. still unreacted, state. Furthermore, in the case of large hollow space
dimensions,
sufficient time has to be available for the polyurethane material required to
be introduced
into the hollow space within the cream time. Here too, a long cream time is
required. The
cream time is the time which elapses before the liquid reaction mixture begins
to foam.
However, long cream times have been found to lead to relatively large cell
diameters in the
foam system produced in this way since many of the cells formed join to
produce larger
units when the reacting reaction mixture does not fix these by means of an
increase in
CA 2825836 2019-01-23
2
viscosity and reaching of the fiber time. Since the radiative thermal
conductivity is a function
of the square of the radius of the cells in the foam, a foam having a long
cream time
experiences a significant increase in the thermal conductivity compared to a
foam having a
short cream time, and is then once again not suitable for filling large hollow
spaces.
However, for economic and ecological reasons, a minimal thermal conductivity
of the foam
and thus a maximum energy saving is desired in the insulation of hollow
spaces.
This question is also particularly important in the case of pipe systems which
are
utilized for the transport of media which are hot or cold relative to the
ambient temperature,
particularly when they have a life of more than 30 years. Such pipe systems,
in particular
for district heating, are frequently made up of individual pipe segments. Pipe
lengths of, for
example, 6 m, 12 m or 16 m are normally used for this purpose. Longer lengths
required
are specially manufactured or cut to size from existing manufactured ware.
Pipes insulated
by polyurethane foams are known in the prior art and are described, for
example, in EP-A-
865 893 and DE-A-197 42 012. The individual pipe segments are welded together
and
after-insulated in the region of the welding seam using the existing muff
technology. The
time taken to produce a muff connection is a limited time factor in laying of
the pipes. In
addition, it is frequently a weak point in the insulating properties since it
is usually produced
under time pressure and weathering influences such as temperature, soiling and
moisture
on the building site. Furthermore, the number of muff connections represents a
large cost
factor in the installation of pipe systems.
It is therefore desirable in the pipe-processing industry to install the
fewest possible
muff connections per unit length of a line and at the same time to optimize
the quality, in
particular the thermal conductivity. This is achieved by use of relatively
long individual pipe
segments, but the production of these involves more demanding requirements and
frequently leads to technical problems. The larger the annular gap to be
filled with foam
CA 2825836 2019-01-23
3
between the outer and inner pipe, the more liquid reaction mixture has to be
introduced and
the slower does the reaction have to take place, with the abovementioned
disadvantages.
In providing the pipes with foam, a clear distinction has to be made between
two
terms. Firstly, in stage 1, reference is made to "predistribution" of the not
yet reacting
reaction mixture, and subsequently, in stage 2, to "flowing" of the foaming
reaction mixture.
Owing to the required technical properties, for example in respect of the
compressive
strength, polyurethane systems comprising high-functionality polyols having a
correspondingly high viscosity are used in the prior art. This results in
polyol mixtures
having high overall viscosities which, although they display good flow in
stage 2, give poor
predistribution in stage 1.
A uniform foam density distribution of the foam is important for the quality
of the
pipes, but this is not sufficiently good when the polyurethane systems known
hitherto are
used. A relatively low foam density is usually obtained at the ends and a
higher foam
density is obtained in the middle of the pipe. The longer the pipe, the
higher, for production
reasons, the required overall foam density of the foam in the annular gap
Furthermore, the flow of the foam is limited in systems of the prior art, so
that, after
the fiber time has been reached, the foam rolling forward leads to poor
properties (low
compressive strength, high water absorption, poor thermal conductivity, etc).
This foam
structure generally occurs at the pipe ends and is referred to as "push zone".
SUMMARY OF THE INVENTION
It is therefore an object of the invention to provide a reaction mixture for
large hollow
spaces which quickly becomes uniformly distributed in these hollow spaces, has
a long
cream time and nevertheless has a very small cell diameter in the resulting
rigid foam in
order to have, in particular, good insulating properties, also cures quickly
and has good flow
CA 2825836 2019-01-23
4
properties. A further object of the invention is to provide a polyurethane
system which can
be used advantageously in the production of insulated pipes and there leads to
a
polyurethane foam having a uniform foam density distribution. Furthermore, the
occurrence
of a "push zone" should be avoided.
This object is achieved by introducing polyurethane foam constituents into a
low-
viscosity polyurethane system, also referred to as reaction mixture, and
allowing this
mixture to cure, preferably in a mold, particularly preferably in a hollow
space.
The invention thus provides the mixture described, the rigid polyurethane foam
formed therefrom, articles produced using the rigid polyurethane foam and the
process for
producing shaped articles using this mixture or filling hollow bodies and also
the use of the
starting materials for a corresponding process.
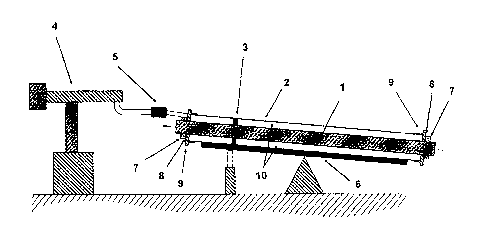
BRIEF DESCRIPTION OF THE DRAWING
Figure 1 illustrates a preferred process for producing a thermally insulating
pipe.
DETAILED DESCRIPTION
In the figure, the reference numerals have the following meanings:
1 Pipe for a medium, i.e. pipe for conveying liquids and/or gases
2 Outer pipe
3 Spacer
4 Metering machine for low-viscosity polyurethane system
Mixing head
6 Tiltable foaming table
7 Clamp
8 Seal
CA 2825836 2019-01-23
5
9 End cap with venting holes
Annular gap with mixture of low-viscosity polyurethane system and rigid
polyurethane
foam constituents which cure to form the rigid polyurethane foam.
In this preferred process, the pipe (1) for a medium is provided with spacers
(3) which
serve to center the inner pipe, pipe 1 for a medium, in the outer pipe (2).
The pipe (1) for a
medium is pushed into the outer pipe (2) so that a concentric annular gap (10)
is obtained
between the two pipes. This annular gap is filled with a mixture which is
composed of a low-
viscosity polyurethane system and rigid polyurethane foam constituents and
cures to form
the rigid polyurethane foam in such a way that the rigid polyurethane foam
constituents are
distributed uniformly in a polyurethane matrix formed from the low-viscosity
polyurethane
system. The mixture is produced in the mixing head (5) where the starting
materials for the
polyurethane matrix (= low-viscosity polyurethane system) are mixed uniformly
with the
polyurethane foam constituents.
Production of the polyurethane foam constituents
The polyurethane foam constituents are preferably produced on continuously
operating double belt units. For this purpose, the polyol component and
isocyanate
component are metered by means of a high-pressure machine and mixed in a
mixing head.
Catalysts and/or blowing agents are preferably introduced beforehand into the
polyol
mixture by means of separate pumps. The mixture of the starting components is
applied
continuously to a lower covering layer. The lower covering layer together with
the applied
material and the upper covering layer run into the double belt. Here, the
starting
components foam and cure. After leaving the double bond, the continuous strip
of foam is
comminuted to the desired dimensions, preferably by means of mechanical
comminution.
CA 2825836 2019-01-23
6
Mechanical comminution is preferably carried out using rollers, mills,
shredders or similar
devices. In other preferred embodiments, comminution is carried out by means
of
compressed air or a jet of water. In further preferred embodiments, the
polyurethane foam
constituents are produced thermally or by means of high-energy radiation,
preferably using
laser beams.
In further preferred embodiments, a thin polyurethane strip is extruded as
foam which
is then comminuted to give the desired small polyurethane foam constituents.
Here too, the
abovementioned comminution processes are preferably employed.
The starting components are preferably mixed at a temperature of from 15 C to
35 C,
more preferably from 20 C to 30 C. The mixed starting components are
preferably poured
by means of high- or low-pressure metering machines into closed support tools.
In other embodiments, polyurethane foam constituents are produced from work-up
and
reuse (recycling) of suitable foams. Suitable scrap is obtained, in
particular, in the
continuous manufacture of pipes or sandwich panels.
The rigid polyurethane foams of the invention are preferably used for thermal
insulation, more preferably of hollow spaces, preferably of hollow spaces in
refrigeration
appliances, containers, pipes or buildings, particularly preferably pipes
which are used for
the transport of hot and/or cold media, very particularly preferably district
heating pipes,
particularly preferably in accordance with EN 253:2009. The rigid polyurethane
foam is
likewise preferably used for the filling of muffs which, when two insulated
pipes for a
medium are connected, at least insulate the connection and also protect it
against
mechanical damage.
Polyurethane foams, in particular rigid polyurethane foams, have been known
for a
long time and are widely described in the literature. They are usually
produced by reacting
organic polyisocyanates (al) with compounds having at least two hydrogen atoms
which
CA 2825836 2019-01-23
7
are reactive toward isocyanate groups (bl), hereinafter also referred to as
compound (bl)
in the interests of simplicity, preferably polyols.ln preferred embodiments,
auxiliaries and/or
additives are added to the polyurethane foams.
As organic polyisocyanates (al), preference is given to using aromatic
polyfunctional
isocyanates.
Preferred isocyanates are tolylene 2,4- and 2,6-diisocyanate (TDI) and the
corresponding isomer mixtures, diphenylmethane 4,4'-, 2,4'- and 2,2'-
diisocyanate (MDI)
and the corresponding isomer mixtures, mixtures of diphenylmethane 4,4'- and
2,4'-diisocyanates, polyphenylpolymethylene polyisocyanates, mixtures of
diphenylmethane
4,4'-, 2,4'- and 2,2'-diisocyanates and polyphenylpolymethylene
polyisocyanates (crude
MDI) and mixtures of crude MDI and tolylene diisocyanates.
The organic diisocyanates and polyisocyanates can be used individually or in
the
form of mixtures.
Preference is likewise given to using polyfunctional isocyanates, i.e.
products which
are obtained by chemical reaction of organic diisocyanates and/or
polyisocyanates.
Examples which may be mentioned are preferably diisocyanates and/or
polyisocyanates
comprising isocyanurate and/or urethane groups. In preferred embodiments,
modified
polyisocyanates are mixed with one another or with unmodified organic
polyisocyanates
such as diphenylmethane 2,4'-, 4,4'-diisocyanate crude MDI, tolylene 2,4-
and/or
2,6-diisocyanate.
In one of the preferred embodiments, reaction products of polyfunctional
isocyanates
with polyfunctional polyols and mixtures thereof with other diisocyanates and
polyisocyanates are also used.
As organic polyisocyanate (al), particular preference is given to crude MDI
having an
NCO content of from 29% by weight to 33% by weight and a viscosity at 25 C in
the range
CA 2825836 2019-01-23
8
from 0.15 Pa*s to 1 Pa*s, determined in accordance with DIN 53019-1:2009
(9.2.5 ¨
cylindrical rotational viscometer).
As compounds (b1), use is made, in particular, of polyether alcohols and/or
polyester
alcohols having OH numbers in the range from 0.1 to 1.2 g KOH/g.
The polyester alcohols are usually prepared by condensation of polyfunctional
alcohols, preferably diols, having from 2 to 12 carbon atoms, preferably from
2 to 6 carbon
atoms, with polyfunctional carboxylic acids having from 2 to 12 carbon atoms,
preferably
succinic acid, glutaric acid, adipic acid, suberic acid, azelaic acid, sebacic
acid,
decanedicarboxylic acid, maleic acid, fumaric acid and more preferably
phthalic acid,
isophthalic acid, terephthalic acid and the isomeric naphthalenedicarboxylic
acids.
The compound (b1) having at least two hydrogen atoms which are reactive toward
isocyanate preferably has a functionality in the range from 2 to 8, in
particular from 3 to 7.
Preference is given to using polyether polyols which are prepared by known
methods,
for example by anionic polymerization of alkylene oxides in the presence of
catalysts,
preferably alkali metal hydroxides.
As alkylene oxides, preference is given to using ethylene oxide and/or
propylene
oxide, more preferably pure 1,2-propylene oxide.
As starter molecules for the compound (b1), preference is given to using
compounds
having at least 3, preferably from 4 to 8, hydroxyl groups or at least two
primary amino
groups per molecule.
The starter molecules having at least 3, preferably from 4 to 8, hydroxyl
groups in the
molecule are preferably selected from the following group: trimethylolpropane,
glycerol,
pentaerythritol, sugar compounds, preferably glucose, sorbitol, mannitol
and/or sucrose,
polyhydric phenols, resols, oligomeric condensation products of phenol and
formaldehyde,
Mannich condensates of phenols, formaldehyde and dialkanolamines and also
melamine.
CA 2825836 2019-01-23
9
As starter molecules having at least two primary amino groups in the molecule,
preference is given to using aromatic diamines and/or polyamines, preferably
phenylenediamines, 2,3-, 2,4-, 3,4- and 2,6-tolylenediamine and 4,4'-, 2,4'-
and
2,2'-diaminodiphenylmethane, and also aliphatic diamines and polyamines, such
as
ethylenediamine.
The polyether polyols have a functionality of preferably from 3 to 8 and
hydroxyl
numbers of preferably from 0.1 g KOH/g to 1.2 g KOH/g and in particular from
0.24 g
KOH/g to 0.57 g KOH/g.
The compounds having at least two hydrogen atoms which are reactive toward
isocyanate (b) also include the chain extenders and/or crosslinkers which may
optionally be
concomitantly used. Preference is given to using bifunctional chain extenders,
trifunctional
and higher-functional crosslinkers or optionally mixtures thereof for
modifying the
mechanical properties.
As chain extenders and/or crosslinkers, preference is given to using
alkanolamines
and in particular diols and/or triols having number average molecular weights
of less than
0.4 kmol, preferably from 0.06 kmol to 0.3 kmol.
Chain extenders, crosslinkers or mixtures thereof are advantageously used in
an
amount of from 1% by weight to 20% by weight, preferably from 2% by weight to
5% by
weight, based on the compound (131), preferably the polyol.
Further information on the polyether alcohols and polyester alcohols used and
also
their preparation may be found, for example, in Kunststoffhandbuch, volume 7
"Polyurethane", edited by GOnter Oertel, Carl-Hanser-Verlag Munich, 3rd
edition, 1993.
The polyurethane foams are preferably produced in the presence of blowing
agents,
catalysts and cell stabilizers and, if necessary, further auxiliaries and/or
additives.
CA 2825836 2019-01-23
10
A preferred blowing agent is water which reacts with isocyanate groups to
eliminate
carbon dioxide. In other preferred embodiments, physical blowing agents are
used in
combination with or in place of water. These are compounds which are inert
toward the
starting components, are usually liquid at room temperature and vaporize under
the
conditions of the urethane reaction. The boiling point of the physical blowing
agents is
preferably below 51 C. The physical blowing agents also include compounds
which are
gaseous at room temperature and are introduced under superatmospheric pressure
into the
starting components or dissolved therein; some preferred representatives are
carbon
dioxide, low-boiling alkanes and fluoroalkanes.
The physical blowing agents are preferably selected from the group consisting
of
alkanes and/or cycloalkanes having at least 4 carbon atoms, dialkyl ethers,
esters, ketones,
acetals, fluoroalkanes having from 1 to 8 carbon atoms and tetraalkylsilanes
having from 1
to 3 carbon atoms in the alkyl chain, in particular tetramethylsilane.
Further preferred physical blowing agents are propane, n-butane, isobutane and
cyclobutane, n-pentane, isopentane and cyclopentane, cyclohexane, dimethyl
ether, methyl
ethyl ether, methyl butyl ether, methyl formate, acetone and also
fluoroalkanes which are
degraded in the troposphere and therefore to not harm the ozone layer, e.g.
trifluoromethane, difluoromethane, 1,1,1,3,3-pentafluorobutane, 1,1,1,3,3-
pentafluoropropane, 1,1,1,2-tetrafluoroethane, difluoroethane and
heptafluoropropane.
The physical blowing agents mentioned can be used either alone or in any
combinations with one another.
Catalysts used are in particular compounds which strongly accelerate the
reaction of
the isocyanate groups with the compound (b1) which is reactive toward
isocyanate groups.
Such catalysts are strongly basic amines, e.g. secondary aliphatic amines,
imidazoles, amidines and also alkanolamines.
CA 2825836 2019-01-23
11
In some preferred embodiments, isocyanurate groups are built into the
polyurethane
foam. Specific isocyanurate catalysts are required for this purpose. As
isocyanurate
catalysts, preference is given to using, for example, metal carboxylates, in
particular
potassium acetate and solutions thereof.
The catalysts are, dependent on requirements, used either alone or in any
mixtures
with one another.
In preferred embodiments of the production of the polyurethane foams,
auxiliaries
and/or additives are used. These include, for example, surface-active
substances, foam
stabilizers, cell regulators, fillers, flame retardants, nucleating agents,
oxidation inhibitors,
stabilizers, lubricants and mold release agents, dyes and pigments.
Further details regarding the customary auxiliaries and additives may be found
in the
specialist literature, see, for example, Kunststoffhandbuch, volume VII,
edited by Vieweg
and Hochtlen, Carl Hanser Verlag, Munich 1966 (pages 103-113).
As flame retardants, preference is given to using organic phosphoric esters
and/or
phosphonic esters. Preference is given to using compounds which are not
reactive toward
isocyanate groups. Chlorine-comprising phosphoric esters are also among the
preferred
compounds.
Preferred representatives of this group of flame retardants are triethyl
phosphate,
diphenyl cresyl phosphate, tris(chloropropyl) phosphate and diethyl
ethanephosphonate.
Bromine-comprising flame retardants are also used in other preferred
embodiments.
As bromine-comprising flame retardants, preference is given to using compounds
having
groups which are reactive toward the isocyanate group. Such compounds are
preferably
esters of tetrabromophthalic acid with aliphatic diols and alkoxylation
products of
dibromobutenediol.
CA 2825836 2019-01-23
12
Compounds derived from brominated neopentyl compounds comprising OH groups
are also preferably employed.
To produce the rigid polyurethane foams, the organic polyisocyanates (al) and
the
compound (bl) are reacted in such amounts that the isocyanate index is in the
range from
100 to 220, preferably from 105 to 180, particularly preferably from 110 to
160. The
isocyanate index is calculated. It is the ratio of the number of isocyanate
groups available
for the reaction to the number of hydroxyl groups available. This ratio is
then multiplied by
100. In the case of values greater than 100, an excess of isocyanate is
present.
The rigid polyurethane foams are preferably produced either batchwise or
continuously by means of known mixing apparatuses.
The rigid polyurethane foams of the invention are preferably produced by the
two-
component process. In this process, the compounds having at least two hydrogen
atoms
which are reactive toward isocyanate groups (b), optionally together with at
least one flame
retardant, at least one blowing agent, optionally at least one catalyst and
optionally further
auxiliaries and/or additives are mixed to form the polyol component and this
is reacted with
the at least one polyisocyanate or mixtures of the polyisocyanates and
optionally blowing
agents, also referred to as isocyanate component.
Polyol component and isocyanate component are also referred to as starting
components. These starting components are usually mixed at a temperature of
from 15 C
to 35 C, preferably from 20 C to 30 C. The reaction mixture can be poured by
means of
high- or low-pressure metering machines into closed support tools. Sandwich
elements, for
example, are produced batchwise by means of this technology.
In addition, the reaction mixture can also be poured or sprayed freely onto
surfaces or
into hollow spaces. Roofs and complicated containers can be insulated in situ
by this
process.
CA 2825836 2019-01-23
13
Continuous mixing of the isocyanate component with the polyol component to
produce sandwich elements or insulation elements on double belt units is also
a preferred
embodiment. In the case of this technology, the catalysts and the blowing
agents are
preferably metered by means of further metering pumps into the polyol
component. Here,
the original components can be divided up into up to 8 individual components.
The foaming
formulations can be simply converted, on the basis of the two-component
process, to the
processing of multiple component systems.
The density of the rigid polyurethane foam constituents is preferably from 10
kg/m3 to
400 kg/m3, preferably from 20 kg/m3 to 200 kg/m3, more preferably from 30
kg/m3 to
80 kg/m3, very particularly preferably from 30 kg/m3 to 70 kg/m3.
The advantage is that the foams used have a lower foam density and/or a
smaller cell
diameter than the matrix. Both lead to a lower thermal conductivity of the
total foam in the
hollow space.
The foam cells in the rigid polyurethane foam constituents preferably have an
average cell diameter in the range from 0.1 mm to 0.5 mm, more preferably from
0.11 mm
to 0.3 mm, particularly preferably from 0.12 mm to 0.2 mm and very
particularly preferably
from 0.12 mm to 0.18 mm. The average cell diameter is determined in accordance
with EN
489:2009 (according to section 5.4.5.1).
At least 80% of the foam cells per unit volume more preferably have the stated
cell
diameter, even more preferably at least 90% and very particularly preferably
at least 95%.
The maximum dimension of the polyurethane foam constituents is in the range
from
0.06 cm to 1 cm, more preferably from 0.1 cm to 0.8 cm, particularly
preferably from 0.1 cm
to 0.5 cm.
In a preferred embodiment, polyurethane foam constituents have any shape as
they
are formed from larger foams in comminution operations. In another preferred
embodiment,
CA 2825836 2019-01-23
14
the polyurethane foam constituents have rounded corners and are preferably egg-
shaped
and/or round.
The polyurethane matrix is produced by reaction of at least one isocyanate
component (a2) with at least one compound (b2) which is reactive toward
isocyanate,
preferably a polyol, optionally using at least one catalyst (c2) and/or
optionally at least one
chemical blowing agent (d2). The mixture from which the polyurethane matrix is
produced is
also referred to as reaction mixture or low-viscosity polyurethane system.
The compound (b2), optionally comprising at least one catalyst (c2) and/or at
least
one chemical blowing agent (d2), preferably has a viscosity of less than 2
Pa*s and more
than 0.4 Pa*s, preferably less than 1.8 Pa*s and more than 0.5 Pa*s and
particularly
preferably less than 1.5 Pa*s and more than 0.5 Pa*s, measured in accordance
with DIN
53019-1:2008 (9.2.5 ¨ cylindrical rotational viscometer) at 20 C.
If a blowing agent is added in the production of the rigid foam, the
viscosities
indicated apply to the compound (b2) and also to those of the reaction
mixture, in each
case based on the viscosity with the blowing agent.
The reaction mixture preferably has a viscosity in the unreacted state at 20 C
in the
range from 0.3 Pa*s to 1.5 Pa*s, preferably from 0.35 Pa*s to 1.2 Pa*s,
particularly
preferably from 0.4 Pa*s to 1.0 Pa*s and very particularly preferably from 0.4
Pa*s to
0.8 Pa*s, determined in accordance with DIN 53019-1:2008 (9.2.5 ¨ cylindrical
rotational
viscometer). The viscosity of the polyurethane system is measured before
addition of the
polyurethane foam constituents when all constituents of the reaction mixture
have been
mixed. If a physical blowing agent is used, this has to be comprised before
the viscosity
measurement.
As isocyanate component (a2), use is made of the customary aliphatic,
cycloaliphatic
and in particular aromatic diisocyanates and/or polyisocyanates. Preference is
given to
CA 2825836 2019-01-23
15
using tolylene diisocyanate (TDI), diphenylmethane diisocyanate (MDI) and in
particular
mixtures of diphenylmethane diisocyanate and polyphenylenepolymethylene
polyisocyanates (crude MDI). The isocyanates can also be modified, for example
by
incorporation of uretdione, carbamate, isocyanurate, carbodiimide, allophanate
and in
particular urethane groups.
The isocyanate component (a2) can also be used in the form of polyisocyanate
prepolymers. These prepolymers are known in the prior art. They are prepared
in a known
manner by reacting above-described polyisocyanates (a2), for example at
temperatures of
about 80 C, with compounds having hydrogen atoms which are reactive
isocyanates (b2),
preferably with polyols, to form polyisocyanate prepolymers. The
polyol/polyisocyanate ratio
is generally selected so that the NCO content of the prepolymer is from 8% by
weight to
25% by weight, preferably from 10% by weight to 22% by weight, particularly
preferably
from 13% by weight to 20% by weight.
The polyurethane matrix is preferably produced using crude MDI.
In a preferred embodiment, the isocyanate component (a2) is selected so that
it has a
viscosity of less than 1300 mPas, preferably from 100 mPas to 800 mPas,
particularly
preferably from 120 mPas to 600 mPas, in particular from 180 mPas to 350 mPas,
measured in accordance with DIN 53019-1:2008 (9.2.5 ¨ cylinder rotational
viscometer) at
20 C.
In the prior art, it may be customary to incorporate isocyanurate groups into
the
polyisocyanate. Catalysts which preferentially form isocyanurate groups, for
example alkali
metal salts either alone or in combination with tertiary amines, are used for
this purpose.
Isocyanurate formation leads to flame-resistant polyisocyanurate (PIR) foams
which are
preferably used in industrial rigid foam, for example in building and
construction as
insulation board or sandwich elements.
CA 2825836 2019-01-23
16
For the purposes of the present invention, the polyurethane systems and
polyurethane foams according to the invention are preferably essentially free
of
isocyanurate groups. The ratio of isocyanurate group to urethane group in the
foam is
preferably less than 1:10, particularly preferably less than 1:100. In
particular, essentially no
isocyanurate groups are present in the rigid polyurethane foam of the
invention.
In a further preferred embodiment, a physical blowing agent is added to the
compound (b2). The addition of a physical blowing agent leads to a significant
decrease in
the viscosity.
Possible compounds (b2) are generally compounds having at least two groups
which
are reactive toward isocyanate, i.e. having at least two hydrogen atoms which
are reactive
toward isocyanate groups. Examples are compounds having OH groups, SH groups,
NH
groups and/or NH2 groups.
As polyols, preference is given to using compounds based on polyesters or
polyetherols. The functionality of the polyetherols and/or polyesterols is
preferably from 1.9
to 8, more preferably from 2.4 to 7, particularly preferably from 2.9 to 6.
The compound (b2) preferably has a hydroxyl number in the range from 200 mg
KOH/g to 1000 mg KOH/g, preferably from 250 mg KOH/g to 800 mg KOH/g,
particularly
preferably from 300 mg KOH/g to 600 mg KOH/g, very particularly preferably
from 300 mg
KOH/g to 500 mg KOH/g.
Compound (b2) preferably comprises polyether polyols prepared by known
methods,
for example from one or more alkylene oxides having from 2 to 4 carbon atoms
in the
alkylene radical by anionic polymerization using alkali metal hydroxides such
as sodium or
potassium hydroxide or alkali metal alkoxides such as sodium methoxide, sodium
or
potassium ethoxide or potassium isopropoxide as catalysts with addition of at
least one
starter molecule comprising from 2 to 8, preferably from 3 to 8, reactive
hydrogen atoms in
CA 2825836 2019-01-23
17
bound form or by cationic polymerization using Lewis acids such as antimony
pentachloride, boron fluoride etherate, etc., or bleaching earth as catalysts.
Suitable alkylene oxides are, for example, tetrahydrofuran, 1,3-propylene
oxide,
1,2- or 2,3-butylene oxide, styrene oxide and preferably ethylene oxide and
1,2-propylene
oxide. The alkylene oxides can be used individually, alternately in succession
or as
mixtures.
Possible starter molecules are alcohols such as glycerol, trimethylolpropane
(TMP),
pentaerythritol, sucrose, sorbitol and also amines such as methylamine,
ethylamine,
isopropylamine, butylamine, benzylamine, aniline, toluidine, toluenediamine,
naphthylamine, ethylenediamine, diethylenetriamine, 4,4"-methylenedianiline,
1,3-
propanediamine, 1,6-hexanediamine, ethanolamine, diethanolamine,
triethanolamine and
the like.
Further compounds which can be used as starter molecules are condensation
products of formaldehyde. phenol and diethanolamine or ethanolamine,
formaldehyde,
alkylphenols and diethanolamine or ethanolamine, formaldehyde, bisphenol A and
diethanolamine or ethanolamine, formaldehyde, aniline and diethanolamine or
ethanolamine, formaldehyde, cresol and diethanolamine or ethanolamine,
formaldehyde,
toluidine and diethanolamine or ethanolamine and also formaldehyde,
toluenediamine
(TDA) and diethanolamine or ethanolamine and the like.
Preference is given to using sucrose, glycerol and TDA as starter molecule.
The polyol mixture can optionally comprise, as constituent (c2), catalysts
which
accelerate the PU and/or PIR reaction. Catalysts (c2) used are usually
compounds which
accelerate the reaction of the hydroxyl-comprising compounds of compound (b2)
with the
isocyanate groups.
CA 2825836 2019-01-23
=
18
Possible catalysts are preferably organic tin compounds such as tin(II) salts
of
organic carboxylic acids and/or basic amine compounds, preferably tertiary
amines such as
triethylamine and/or 1,4¨diazabicyclo[2.2.2]octane and also
(2-hydroxypropyl)trimethylammonium 2-ethylhexanoate (CAS number 62314-22-1),
(2-
hydroxypropyl)trimethylammonium formate (CAS number 62314-25-4), 2-((2-
dimethylamino)ethyl)methylamino)ethanol (CAS number 2212-32-0) and/or N,N',N"-
tris(dimethylaminopropyl)hexahydrotriazine (CAS number 15875-13-5),
dimethylcyclohexylamine (CAS number 98-94-2).
As preferred compounds which catalyze the formation of isocyanurate structures
(PIR
catalysts), use is made of potassium acetate, potassium formate and/or
potassium octoate,
particularly preferably potassium acetate.
The catalysts are generally used in an amount of from 0.001 to 5% by weight,
in
particular from 0.05 to 3.0% by weight, of catalyst, based on the weight of
the component
(b).
In this text, the CAS number is the definitive chemical designation in case of
doubt.
Compound (b2) can optionally further comprise chemical blowing agents as
constituent (c3). As chemical blowing agents, preference is given to water or
carboxylic
acids, in particular formic acid. The chemical blowing agent is generally used
in an amount
of from 0.1 to 5% by weight, in particular from 1.0 to 3.0% by weight, based
on the weight of
the compound (b2).
As mentioned above, the compound (b2) can comprise physical blowing agents.
These are compounds which are dissolved or emulsified in the starting
materials for
polyurethane production and vaporize under the conditions of polyurethane
formation.
These are, for example, hydrocarbons, halogenated hydrocarbons and other
compounds,
for example perfluorinated alkanes such as perfluorohexane,
chlorofluorocarbons and also
CA 2825836 2019-01-23
19
ethers, esters, ketones and/or acetals. These are usually used in an amount of
from 1% by
weight to 25% by weight, preferably from 2% by weight to 20% by weight,
particularly
preferably from 3% by weight to 16% by weight, based on the total weight of
the component
(b2) comprising the blowing agent.
The compounds having at least two reactive hydrogen atoms which are reactive
toward isocyanate (b2) also include the chain extenders and/or crosslinkers
which may
optionally be concomitantly used. Preference is also given to using
bifunctional chain
extenders, trifunctional and higher-functional crosslinkers or optionally
mixtures thereof for
modifying the mechanical properties.
As chain extenders and/or crosslinkers, preference is given to using
alkanolamines
and in particular diols and/or triols having number-average molecular weights
of less than
0.4 kmol, preferably from 0.06 kmol to 0.3 kmol.
Chain extenders, crosslinkers or mixtures thereof are preferably used in an
amount of
from 1% by weight to 20% by weight, more preferably from 2% by weight to 5% by
weight,
based on the compound (b1), preferably the polyol comprising the chain
extenders,
crosslinkers or mixtures thereof but without physical blowing agent.
The crosslinkers are generally used in an amount of from 1% by weight to 10%
by
weight, preferably from 2% by weight to 6% by weight, based on the total
weight of the
compound (b2) comprising the chain extender, crosslinker or mixtures thereof
but without
physical blowing agent.
In a preferred embodiment, the compound (b2) comprises, as constituent (e5),
chain
extenders which serve to increase the crosslinking density. For the purposes
of the present
invention, chain extenders are compounds which have a molecular weight of from
0.06 kg/mol to < 0.4 kg/mol and have 2 hydrogen atoms which are reactive
toward
CA 2825836 2019-01-23
20
isocyanates. Preferred examples are butanediol, diethylene glycol, dipropylene
glycol and
ethylene glycol.
The chain extenders are generally used in an amount of from 2% by weight to
20% by
weight, preferably from 4 to 15% by weight, based on the total weight of the
compound
(b2). The amount of physical blowing agent is not taken into account here.
The crosslinkers (d2) and chain extenders (e2) can be used individually or in
combination in the compound (b2).
The compound (b2), preferably the at least one polyol, has a hydroxyl number
of
more than 70 mg KOH/g, preferably more than 100 mg KOH/g, more preferably more
than
150 mg KOH/g, with the hydroxyl number preferably being less than or equal to
1000 mg
KOH/g, more preferably less than 800 mg KOH/g and particularly preferably less
than
600 mg KOH/g.
In the reaction of the starting materials of the polyurethane matrix, the
polyisocyanates (a2) and the compound (b2), preferably polyols, are preferably
reacted in
such amounts that the isocyanate index of the matrix is from 90 to 250,
preferably from 100
to 180, particularly preferably from 110 to 159.
In a preferred embodiment, the components of the starting materials for the
polyurethane matrix are selected so that the resulting foam has a compressive
strength (at
a foam density of 60 kg/m3) of greater than 0.25 N/mm2, preferably greater
than 0.3 N/mm2,
particularly preferably greater than 0.35 N/mm2, measured in accordance with
DIN 53421.
Additives (f) can optionally also be incorporated into the isocyanate (al)
and/or (a2)
and/or the compound (bl) and/or (b2). For the purposes of the present
invention, additives
(f) are the customary auxiliaries and additives known in the prior art, but
without physical
blowing agents. Mention may be made by way of example of surface-active
substances,
CA 2825836 2019-01-23
21
foam stabilizers, cell regulators, fillers, dyes, pigments, flame retardants,
antistatics,
hydrolysis inhibitors and/or fungistatic and bacteriostatic substances.
The invention further provides an insulated pipe made up of
i) an inner pipe which serves for transport of a medium, also referred to
as pipe for a
medium,
ii) a layer of insulation material comprising the rigid polyurethane foam
of the invention
and
iii) an outer pipe within which the tube for a medium is located,
preferably concentrically.
The pipe (i) for a medium is generally a steel pipe having an external
diameter in the
range of 170 cm to 8 cm, preferably 1 cm to 120 cm, more preferably from 4 cm
to 110 cm,
and a length of from 1 m to 24 m, preferably from 6 m to 16 m.
A layer of insulating material (ii) comprising the rigid polyurethane foam of
the
invention is arranged on the outside of the pipe for a medium. This layer
generally has a
thickness of from 1 cm to 20 cm, preferably from 2 cm to 15 cm.
In a preferred embodiment, the layer of insulation material has an overall
foam
density of less than 110 kg/m3, preferably from 50 kg/m3 to 100 kg/m3,
particularly
preferably from 60 kg/m3 to 90 kg/m3. Here, the overall foam density is the
foam density
distribution over the cross section of the pipe and the length of the pipe.
In a further preferred embodiment, the layer of insulation material (ii)
comprising the
polyurethane foam of the invention has a thermal conductivity of less than 28
mW/mK,
preferably from 22 mW/mK to 27 mW/mK, particularly preferably from 22 mW/mK to
26.5
mW/mK, measured in accordance with EN ISO 8497 at an average temperature of 50
C.
The outer pipe (iii) surrounds the layer of insulation material and generally
comprises
plastic, preferably polyethylene, and usually has a wall thickness of from 1
mm to 30 mm,
CA 2825836 2019-01-23
22
preferably from 5 mm to 30 mm. The internal diameter of the outer pipe (iii)
is preferably
from 6 cm to 140 cm, particularly preferably from 10 cm to 120 cm.
The outer pipe (iii) can optionally comprise a plurality of layers which are
assembled
during the extrusion operation. An example is the introduction of multilayer
films between
rigid polyurethane foam and outer pipe, with the film comprising at least one
metallic layer
to improve the barrier action.
Suitable outer pipes of this type are described in EP-A-960 723.
In a particularly preferred embodiment, the insulated pipe is an insulated
composite
outer pipe for district heating networks laid in the ground, which meets the
requirements of
DIN EN 253.
Finally, the invention provides a process for producing insulated hollow
bodies,
preferably insulated pipes, which comprises the steps:
1) production of a hollow body, preferably a pipe for a medium and an outer
pipe, with
the pipe for a medium being arranged within the outer pipe in such a way that
a
preferably uniformly wide annular gap is formed in all places, preferably by
means of
spacers,
2) introduction of the mixture comprising rigid polyurethane foam
constituents and
starting materials for producing a polyurethane matrix into the hollow space,
preferably the annular gap between pipe for a medium and outer pipe,
3) curing of the polyurethane matrix.
In a preferred embodiment, insulated pipes are produced by means of the
batchwise
pipe-in-pipe production method. In this process, the inner pipe, which is
preferably made of
steel, is pushed, preferably concentrically into an outer pipe which is
referred to as
sheathing pipe, so that an annular gap is formed between the two pipes.
Spacers which
serve to center the inner pipe are preferably present between the inner pipe,
also referred
CA 2825836 2019-01-23
23
to as pipe for a medium, and the sheathing pipe. In principle, any sheath
which is stable
over the production time and serves to shape the rigid polyurethane foam is
suitable as
sheathing pipe. The sheathing pipe is, in a preferred embodiment, made of
polyethylene or
folded spiral-seam metal sheet.
In a further preferred embodiment, the inner pipe is laid in a reusable pipe
mold which
comprises two or more segments and allows the reaction mixture to react fully
in a tubular
shape. A sheathing pipe is subsequently applied on top of the PUR foam by
means of
extrusion. This process has the advantage that the number of spacers may be
able to be
reduced or such spacers may be able to be dispensed with entirely.
The unit comprising inner pipe for a medium and outer sheathing pipe is also
referred
to as double pipe having an intermediate annular gap. The annular gap is
filled with the
mixture comprising rigid polyurethane foam constituents and a reaction
mixture. For the
filling operation, the double pipe is provided with end caps (9) which close
up the annular
gap in both directions and are provided with venting holes so that gas can
escape from the
annular gap during filling and curing operations.
The double pipe is preferably filled with the mixture on a slightly inclined
foaming
table (6), with the pipe preferably being inclined at an angle of from 1 to
100, more
preferably from 1.50 to 70. The double pipe is preferably provided with end
caps (9) so that
the mixture can spread out only in the annular gap.
The liquid mixture is introduced into the annular gap by means of a
polyurethane
metering machine (4) and fills the annular gap while still in liquid form and
then cures.
In another preferred embodiment, the mixture is strongly compressed in the
hollow
space so that the end caps (9) would be pushed away without clamps (7).
Without the seal
8, material would be squeezed out between pipe (1) for a medium and end cap
(9). The
CA 2825836 2019-01-23
24
venting holes of the end caps are closed by means of stoppers or automatic
valves when
foam begins to come out.
In the process for producing insulated hollow spaces, preferably insulated
pipes, the
use of the mixture according to the invention leads to the following
advantages:
Simplified and/or quicker production of relatively large hollow spaces,
preferably relative
long pipe segments, reduced overall foam density in the hollow body, better
foam density
distribution, lower thermal conductivity, increased productivity due to
shorter curing times.
The insulated hollow spaces produced therefrom have corresponding advantages.
The invention further provides for the use of rigid foam constituents and/or
reaction
mixture which has not been fully cured having the above-described properties
for producing
rigid polyurethane foam comprising previously produced rigid foam
constituents.
All embodiments described in the application count as disclosed in each case
with
every other embodiment even when for reasons of simplicity not every
individual
combination of one embodiment with every other embodiment has been formulated.
CA 2825836 2019-01-23