Note: Descriptions are shown in the official language in which they were submitted.
CA 02826434 2013-09-06
ELECTRO-CATALYTIC HONEYCOMB FOR EXHAUST
EMISSIONS CONTROL
FIELD OF THE INVENTION
The present invention relates to an electro-catalytic
honeycomb, particularly to an electro-catalytic honeycomb for
controlling exhaust emissions to effectively decompose
nitrogen oxides (NOõ) and oxidize carbon monoxide (CO),
hydrocarbons (HCs) and particulate matter (PM) in exhaust
gas.
BACKGROUND OF THE INVENTION
Fresh and clean air is essential for human health. Science
and technology has promoted economical development.
However, the exhausts of vehicles and factories, especially
motor vehicles and heavy industry factories, seriously pollute
the air.
The emission standard of motor vehicles has been
increased persistently. However, the continuously increasing
motor vehicles still brings about more and more serious air
pollution. In a motor vehicle, the engine thereof burns fuel
and converts chemical energy into mechanical energy. The
burning process of fuel generates the polluting constituents,
including nitrogen oxides (NOõ), carbon monoxide (CO),
hydrocarbons (HCs), and particulate matter (PM), which
would form photochemical smog, deplete ozone, enhance the
greenhouse effect, cause acid rain, damage the ecological
CA 02826434 2013-09-06
environment and finally danger human health.
Carbon monoxide comes from incomplete combustion.
The capability of carbon monoxide to combine with
hemoglobin to form carboxyhemoglobin (COHb) is 300 times
higher than the capability of oxygen to combine with
hemoglobin to form oxyhemoglobin (Hb02). Therefore, too
high a concentration of carbon monoxide would degrade the
capability of hemoglobin to transport oxygen. Nitrogen oxides
are generated by the combination of nitrogen and oxygen and
mainly in form of nitrogen monoxide (NO) and nitrogen
dioxide (NO2). Reaction of nitrogen oxides and hydrocarbons
is induced by ultraviolet ray, generating poisonous
photochemical smog, which has a special odor, irritates eyes,
harm plants, and reduces the visibility of the ambient air.
Nitrogen oxides can react with water in the air to form nitric
acid and nitrous acid, which are the constituents of acid rain.
Hydrocarbons can irritate the respiratory system even at lower
concentration and will affect the central nervous system at
higher concentration. Particulate matter can danger human
health and can even cause cancer.
Therefore, many nations, including EU, USA, Japan and
Taiwan, have regulated stricter emission standards for
nitrogen oxides, carbon monoxide, hydrocarbons and
particulate matter, such as BINS of USA and EURO 6 of EU,
which not only regulate the emissions of the polluting
2
CA 02826434 2013-09-06
,
constituents but also encourage the manufacturers to develop,
fabricate or adopt the newest pollution control technologies
and apparatuses.
A U.S. Patent No. 5,401,372 disclosed an
"Electrochemical Catalytic Reduction Cell for the Reduction
of NO in an 02-Containing Exhaust Emission", which is
dedicated to removing nitrogen oxides, wherein an
electrochemical-catalytic reducing reaction and a vanadium
pentaoxide (V205) catalyst convert nitrogen oxides into
nitrogen. However, the prior art needs an electric source to
power an electrochemical cell. Therefore, the prior art
consumes power and cannot eliminate other polluting
constituents simultaneously.
A U.S. Patent Application No. 13/362,247 disclosed an
"Electrocatalytic Tube of Electrochemical-Catalytic Converter
for Exhaust Emissions Control", which can eliminate nitrogen
oxides (N0x), carbon monoxide (CO), hydrocarbons (HCs),
and particulate matter (PM) in the exhaust, and which
comprises the electrocatalytic tubes that can be assembled to
form a honeycomb monolith to be an advanced
electrochemical-catalytic converter, wherein the nitrogen
oxides are decomposed into nitrogen and oxygen, and wherein
carbon monoxide, hydrocarbons, and particulate matter are
oxidized into water and carbon dioxide. Therefore, the prior
art can eliminate multiple polluting constituents
3
CA 02826434 2013-09-06
simultaneously without consuming power or reducing gas.
However, the honeycomb monolith of the abovementioned
"Electrochemical-Catalytic Converter" needs one half of the
channels to be sealed to form the electrocatalytic tubes; this
decreases the treating area, in comparison to the conventional
honeycomb as automotive catalytic converter, and also
increases the fabrication cost. Therefore, the prior art has
room to be improved.
SUMMARY OF THE INVENTION
The primary objective of the present invention is to
overcome the problems of the honeycomb of the conventional
electrochemical-catalytic converter, including low treating
area and high fabrication cost.
To achieve the abovementioned objective, the present
invention proposes an electro-catalytic honeycomb for
controlling exhaust emissions, which adopts to purify a
lean-burn exhaust. The electro-catalytic honeycomb comprises
a honeycomb structural body, a solid-oxide layer and a
cathode layer. The honeycomb structural body comprises an
anode which is formed as a backbone of the honeycomb
structural body, a plurality of gas channels formed inside the
backbone for passing the lean-burn exhaust, and a shell
covering an outer surface of the anode. The anode is made of a
first porous material and has a reducing environment. The
shell is formed of a first dense structure. The solid-oxide layer
4
CA 02826434 2013-09-06
,
is adhered to an inner surface of the anode opposite to the
outer surface, which is formed of a second dense structure and
has a tube wall facing the gas channels. The solid-oxide layer
connects the shell so as to encapsulate the anode completely.
The cathode layer is adhered to the tube wall, which is made
of a second porous material and has an oxidizing environment.
The solid-oxide layer is disposed between the anode and the
cathode layer. The reducing environment and the oxidizing
environment facilitates an electromotive force to occur
between the anode and the cathode layer to promote a
decomposition of nitrogen oxides of the lean-burn exhaust
into nitrogen and oxygen on the cathode layer. The oxidizing
environment of the lean-burn exhaust over the cathode layer
can further enable the oxidation of carbon monoxide,
hydrocarbons, and particulate matter of the exhaust.
In the present invention, all of the channels of the
electro-catalytic honeycomb can be used for exhaust treatment.
Additionally, the electro-catalytic honeycomb is easier to be
fabricated than the honeycomb of the conventional
electrochemical-catalytic converter. Thus, the present
invention has larger areas for treatment and lower fabrication
cost than the honeycomb of the conventional
electrochemical-catalytic converter.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig.1 is a perspective view schematically showing an
5
CA 02826434 2013-09-06
electro-catalytic honeycomb according to a first embodiment
of the present invention.
Fig.2 is a sectional view schematically showing an
electro-catalytic honeycomb according to the first
embodiment of the present invention.
Fig.3 is a local sectional view schematically showing an
electro-catalytic honeycomb according to the first
embodiment of the present invention.
Fig.4 is a local sectional view schematically showing an
electro-catalytic honeycomb according to a second
embodiment of the present invention.
Fig.5 is a front view schematically showing an
electro-catalytic honeycomb according to a third embodiment
of the present invention.
DETAILED DESCRIPTION OF THE PREFERRED
EMBODIMENTS
The technical contents of the present invention are
described in detail in cooperation with drawings below.
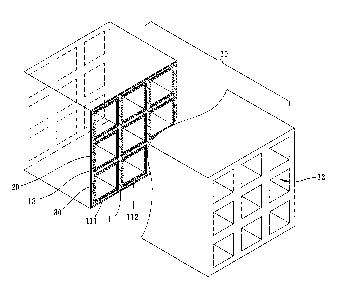
Refer to Fig.1, Fig.2 and Fig.3 which respectively show a
perspective view, a sectional view and a local sectional view
schematically of an electro-catalytic honeycomb according to
a first embodiment of the present invention. The
electro-catalytic honeycomb of the present invention adopts to
purify a lean-burn exhaust containing nitrogen oxides (N0),
carbon monoxide (CO), hydrocarbons (HCs), and particulate
6
CA 02826434 2013-09-06
matter (PM). The electro-catalytic honeycomb comprises a
honeycomb structural body 10, a solid-oxide layer 20 and a
cathode layer 30. The honeycomb structural body 10
comprises an anode 11, a plurality of gas channels 12, and a
shell 13. The anode 11 is formed as a backbone of the
honeycomb structural body 10. In this embodiment, the anode
11 is made of a first porous material having a plurality of
pores, such as a cermet of a metal and a fluorite metal oxide, a
fluorite metal oxide, a perovskite metal oxide, a metal-added
fluorite metal oxide or a metal-added perovskite metal oxide,
such as a cermet of nickel and YSZ (Yttria-Stabilized
Zirconia). The gas channels 12 are formed inside the backbone
and run through two opposite ends of the honeycomb
structural body 10 for passing the lean-burn exhaust. The shell
13 covers an outer surface 111 of the anode 11. And the shell
13 is formed of a first dense structure. The shell 13 is made of
a metal, a ceramics, or a glass, such as stainless steel, alumina,
or quartz glass.
The solid-oxide layer 20 is adhered onto an inner surface
112 of the anode 11 opposite to the outer surface 111 and
connects the shell 13 at two opposite ends of the anode 11 so
as to encapsulate the anode 11 completely, which has a
reducing environment formed therein. The solid-oxide layer
20 has a tube wall 21 facing the gas channels 12. And the
solid-oxide layer 20 is formed of a second dense structure and
7
CA 02826434 2013-09-06
. .
may have an oxygen-ion conductivity. The solid-oxide layer
20 is made of a fluorite metal oxide or a perovskite metal
oxide, such as YSZ, GDC (Gadolinia-Doped Ceria), or
lanthanum¨strontium¨gallium¨magnesium oxide (LSGM).
The cathode layer 30 is adhered onto the tube wall 21 of
the solid-oxide layer 20 such that the solid-oxide layer 20 is
interposed between the anode 11 and the cathode layer 30. In
this embodiment, the cathode layer 30 is made of a second
porous material having a plurality of pores, such as a
perovskite metal oxide, a fluorite metal oxide, a metal-added
perovskite metal oxide, or a metal-added fluorite metal oxide,
such as lanthanum¨strontium¨cobalt¨copper oxide,
lanthanum¨strontium¨manganese¨copper oxide, a
combination of lanthanum¨strontium¨cobalt¨copper oxide and
GDC, a combination of
lanthanum¨strontium¨manganese¨copper oxide and GDC,
silver-added lanthanum¨strontium¨cobalt¨copper oxide,
silver-added lanthanum¨strontium¨manganese¨copper oxide,
a combination of
silver-added
lanthanum¨strontium¨cobalt¨copper oxide and GDC, and a
combination of
silver-added
lanthanum¨strontium¨manganese¨copper oxide and GDC.
In the present invention, the anode 11 is formed from a
material containing a metal oxide initially. During fabrication,
the metal oxide is reduced to a metal by treating with a
8
CA 02826434 2013-09-06
reducing gas. For example, nickel oxide is reduced to nickel.
In one embodiment, the metal oxide may be reduced to an
oxygen-deficient metal oxide so as to form the reducing
environment of the anode 11. Alternatively, carbon monoxide
or hydrocarbons may be added into the anode 11 before the
solid-oxide layer 20 connects the shell 13 so as to encapsulate
the anode 11 completely. For example, methane, ethane,
propylene or propane is introduced into the anode 11 through
pore diffusion to form a carbon species adhering to the pores
that enhances the reducing environment of the anode 11. In
addition, the gas inside the pores of the anodes 11 can be
extracted to form a sub-atmospheric pressure or vacuum in the
anode 11 before the anode 11 is encapsulated completely,
whereby the honeycomb structural body 10 is exempted from
the structural damage caused by thermally-induced expansion
and contraction during the purification of the lean-burn
exhaust.
Refer to Fig.4 which shows a local sectional view of an
electro-catalytic honeycomb according to a second
embodiment of the present invention. In this embodiment, the
electro-catalytic honeycomb further comprises an interlayer
40 disposed between the cathode layer 30 and the solid-oxide
layer 20 such that the adherence between the cathode layer 30
and the solid-oxide layer 20 is improved. The interlayer 40
may be made of a fluorite metal oxide or a perovskite metal
9
CA 02826434 2013-09-06
. .
oxide, such as GDC.
In the second embodiment, the electro-catalytic
honeycomb may further include a catalytic oxidation layer 50
to assist in oxidation of a constituent of the exhaust which is
hard to oxidize on the cathode layer 30. The catalytic
oxidation layer 50 adheres to the cathode layer 30 and is made
of a metal, an alloy, a fluorite metal oxide, or a perovskite
metal oxide, such as palladium, GDC, or
lanthanum¨strontium¨manganese oxide.
Below is described the process of purifying the exhaust.
Firstly, place the electro-catalytic honeycomb of the present
invention in an environment of the exhaust. The exhaust is a
lean-burn exhaust and thus is oxygen-rich and can be further
enriched with oxygen via adding a secondary air. The working
temperature of the electro-catalytic honeycomb is from
ambient temperature to 800 C. The exhaust contains nitrogen
oxides, carbon monoxide, hydrocarbons, and particulate
matter. The purifying reactions undertaken by the present
invention include a decomposition reaction of removing
nitrogen oxides and an oxidation reaction of removing carbon
monoxide, hydrocarbons, and particulate matter.
Nitrogen oxides include nitrogen monoxide (NO) and
nitrogen dioxide (NO2). Nitrogen monoxide is decomposed
into nitrogen and oxygen on the cathode layer 30. The reaction
of NO decomposition is expressed by Formula (1):
CA 02826434 2013-09-06
2N0 ---> N2 + 02 ( 1 )
Nitrogen dioxide is decomposed into nitrogen monoxide
and oxygen on the cathode layer 30. The reaction of NO2
decomposition is expressed by Formula (2):
2NO2 ----> 2N0 +02 (2)
Then, nitrogen monoxide is further decomposed into
nitrogen and oxygen on the cathode layer 30.
The reducing environment of the anode 11 and the
oxidizing environment in the cathode layer 30 results in a
difference of the oxygen partial pressure between the anode 11
and the cathode layer 30 and thus generate an electromotive
force (emf) between the anode 11 and the cathode layer 30
according to Formula (3):
emf=[(RT)/(4F)]=In[(-P021Cathode)/(P021Anode)] (3)
wherein R is the gas constant, T the absolute temperature,
F the Faradic constant, and P02 the partial pressure of oxygen.
The metal or the oxygen-deficient metal oxide of the anode 11
or the carbon species adhering to the pores of the anode 11 is
a reducing compound which results in an environment
equivalent to a lower oxygen partial pressure over the anode
11 and thus results in the generation of larger electromotive
force. Different reducing compounds on the anode 11 result in
different oxygen partial pressures and thus generate different
electromotive forces. Different oxygen concentrations on the
cathode side also result in different oxygen partial pressures
11
CA 02826434 2013-09-06
and thus generate different electromotive forces. The higher
the oxygen concentration on the cathode side is, the larger the
electromotive force is generated and thus the greater
promotion of decomposing nitrogen oxides into oxygen and
nitrogen is resulted. Thus, although the lean-burn exhaust is at
an oxidizing environment which alone can generate an
electromotive force, adding the secondary air into the exhaust
results in a larger electromotive force. Within a given
temperature range, the lower the temperature is, the higher the
decomposition rate is resulted. The decomposition of nitrogen
oxides is as effective at ambient temperature as at higher
temperature.
As to eliminating carbon monoxide, hydrocarbons and
particulate matter of the exhaust, the exhaust is oxygen-rich or
can be further enriched with oxygen via adding the secondary
air. Then, the cathode layer 30 and the catalytic oxidation
layer 50 convert carbon monoxide, hydrocarbons, and
particulate matter into harmless gases. For example, carbon
monoxide is oxidized into carbon dioxide; hydrocarbons (HCs)
and particulate matter (carbon-containing) are oxidized into
carbon dioxide and water. The reactions thereof are expressed
by Formulae (4)-(6):
2C0 + 02 --> 2CO2 (4)
HCs + 02 ---> H2 0 + CO2 (5)
C + 02 ---> CO2 (6)
12
CA 02826434 2013-09-06
,
Via the abovementioned catalytic decomposition reactions
and catalytic oxidation reactions, the polluting constituents of
the exhaust are effectively removed.
Refer to Fig.5 which shows a front view of an
electro-catalytic honeycomb according to a third embodiment
of the present invention. In this embodiment, the cross section
of the honeycomb structural body 10 is formed as a hexagon
and the cross section of the gas channels 12 is formed as a
circle. However, the cross sections of the honeycomb
structural body 10 and the gas channels 12 are not limited to
the above shapes, but may be of any optimum shapes
depending on the requirement.
In conclusion, the present invention uses the difference in
the oxygen partial pressures between the anode side and the
cathode side to generate the electromotive force to promote
the catalytic decomposition reaction. Further, the present
invention has compact structure and lower fabrication cost.
Moreover, the present invention has a compact size able to be
installed underneath the passenger cars for eliminating the
polluting constituents of the exhaust and thus reducing air
pollution.
Therefore, the present invention possesses utility, novelty
and non-obviousness and meets the condition for a patent.
Thus, the Inventors file the application for a patent. It is
appreciated if the patent is approved fast.
13
CA 02826434 2015-01-14
The embodiments described above are only to exemplify the present invention.
Various modifications or variations will occur to the skilled person. The
scope of the
claims should not be limited by the preferred embodiments or the examples but
should
be given the broadest interpretation consistent with the description as a
whole.
14