Note: Descriptions are shown in the official language in which they were submitted.
ASSESSMENT OF CORONARY HEART DISEASE wrrn CARBON DIOXIDE
GOVERNMENT RIGHTS
The invention was made with government support under Grant No. HL091989
awarded by
the National Institutes of Health, The government has certain rights to the
invention.
FIELD OF INVENTION
The invention is directed to methods for detecting coronary heart disease
using carbon
dioxide (CO2) to induce hyperemia and monitor vascular reactivity.
BACKGROUND
The following description includes information that may be
useful in understanding the present invention. It is not an admission that any
of the
information provided herein is prior art or relevant to the presently claimed
invention, or that
any publication specifically or implicitly referenced is prior art.
Coronary artery disease (CAD) leads to narrowing of the small blood vessels
that supply
blood and oxygen to the heart. Typically, atherosclerosis is the cause of CAD.
As the
coronary arteries narrow, blood flow to the heart can slow down or stop,
causing, amongst
other symptoms, chest pain (stable angina), shortness of breath and/or
myocardial infarction.
Numerous tests help diagnose CAD. Such tests include coronary
angiography/arteriography,
CT angiography, echocardiogram, electrocardiogram (ECG), electron-beam
computed
tomography (EBCT), magnetic resonance angiography, nuclear scan and exercise
stress test.
Functional assessment of the myocardium (for example the assessment of
myocardium's
oxygen status) requires that a patient's heart is stressed either via
controlled exercise or
pharmacologically.
1
Date Recue/Date Received 2021-02-26
CA 02832851 2013-11-08
Assessment of vascular reactivity in the heart is the hallmark of stress
testing in cardiac
imaging aimed at understanding ischemic heart disease. This is routinely done
in Nuclear
Medicine with radionuclide injection (such as Thallium) in conjunction with
exercise to
identify territories of the heart muscle that are subtended by a suspected
narrowed coronary
artery. In patients who are contraindicated for exercise stress-testing, this
approach is
typically used in conjunction with hyperemia inducing drugs, for example via
adenosine
infusion. Reduced coronary narrowing is expected to reduce hyperemic response
and the
perfusion reserve. Since nuclear methods are hampered by the need for
radioactive tracers
combined with limited imaging resolution, other imaging methods, such as
ultrasound (using
adenosine along with microbubble contrast) and MRI (also using adenosine and
various
conjugates of gadolinium (Gd) (fast-pass perfusion) or alterations in oxygen
saturation in
response to hyperemia, also known as the Blood-Oxygen-Level-Dependent (BOLD)
effect)
are under clinical investigation. Nonetheless, in patients who are
contraindicated for exercise
stress-testing, currently all imaging approaches require adenosine to elicit
hyperemia.
However, adenosine has undesirable side effects (such as the feeling of
"impending doom",
bradycardia, arrhythmia, transient or prolonged episode of asystole,
ventricular fibrillation
(rarely), chest pain, headache, dyspnea, and nausea), making it less than
favorable for initial
or follow-up studies and many patients request that they do not undergo
repeated adenosine
stress testing. Nonetheless repeated stress testing is indicated in a
significant patient
population to assess the effectiveness of interventional or medical
therapeutic regimens. In
view of the side effects of hyperemia inducing drugs, there is a need for
alternatives, which
induce hyperemia in patients who are contraindicated for exercise stress-
testing but do not
cause the side effects caused by the existing hyperemia inducing drugs.
SUMMARY OF THE INVENTION
Applicants' invention is directed to the use of carbon dioxide to replace
adenosine to induce
hyperemia in subjects contra-indicated for exercise stress testing so as to
diagnose coronary
heart diseases but without the side effects of adenosine. In an embodiment,
the CO2 levels
are altered while the 02 levels are held constant. In another embodiment, the
CO2 levels are
2
139678543.2
CA 02832851 2013-11-08
controlled by administering a blend of air and a controlled amount of a gas
mixture
comprising 20% oxygen and 80% carbon dioxide.
The invention is directed to methods for diagnosing coronary heart disease in
a subject in
need thereof comprising administering an admixture comprising CO2 to a subject
to produce
a hyperemic response corresponding to at least one selected increase in a
subject's coronary
PaCO2, monitoring vascular reactivity in the subject and diagnosing the
presence or absence
of coronary heart disease in the subject. The presence of coronary disease can
be detected by
monitoring a parameter indicative of a disease-associated change in a
vasoreactive response
to the at least one increase in PaCO2 in at least one coronary blood vessel or
region of the
heart. The inventors have found that such a change can be captured by
monitoring the
quantum of change in a parameter affected by a change in PaCO2. from an first
PaCO2 level
to a PaCO2 second level, for example a parameter correlated with vasodilation
such as
increased blood flow.
An observation of a change in a vasodilatory response can be extended to
comparing
responses among different subjects, wherein a decreased vascular reactivity in
a subject in
need of a diagnosis compared to that of a control subject is indicative of
coronary heart
disease.
Thus, according to one embodiment, the invention also provides a method for
assessing
hyperemic response in a subject in need thereof comprising administering an
admixture
comprising CO2 to a subject to reach a predetermined PaCO2 in the subject to
induce
hyperemia, monitoring vascular reactivity in the subject and assessing
hyperemic response in
the subject, wherein decreased vascular reactivity in the subject compared to
a control subject
is indicative of poor hyperemic response, thereby assessing hyperemic response
in the
subject in need thereof.
The invention may be directed to assessing organ perfusion in a subject in
need thereof.
3
13567858.2
CA 02832851 2013-11-08
The invention may be directed to assessing vascular reactivity of an organ in
a subject in
need thereof.
The invention further provides methods of producing coronary vasodilation in a
subject in
need thereof comprising administering an admixture comprising CO2 to a subject
to reach a
predetermined PaCO2 in the subject so as to produce coronary vasodilation,
thereby
producing coronary vasodilation in the subject.
The invention also provides methods from increasing sensitivity and
specificity for BOLD
MRI. The method includes administering an admixture comprising CO2 to a
subject to reach
a predetermined PaCO2 in the subject to induce hyperemia and imaging the
myocardium
using MRI to assess a hypermic response in response to a predetermined
modulation in
PaCO2. Optionally, imaging the myocardium comprises (i) obtaining free-
breathing cardiac
phase resolved 3D myocardial BOLD images; (ii) registering and segmenting the
images to
obtain the myocardial dynamic volume; and (iii) identifying ischemic territory
and quantify
image volume.
The invention is also directed to the use a CO2 containing gas for inducing
hyperemia in a
subject in need of a diagnostic assessment of coronary heart disease, wherein
the CO2
containing gas is used to attain at least one increase in a subject's coronary
PaCO2 sufficient
for diagnosing coronary heart disease from imaging data, wherein the imaging
data is
indicative of a cardiovascular-disease-associated vasoreactive response to the
least one
increase in PaCO2 in. at least one coronary blood vessel or region of the
h.eart.
The invention also provides a method for inducing hyperemia in a subject in
need of a
diagnostic assessment of coronary heart disease comprising administering a CO2
containing
gas, attaining at least one increase in a subject's coronary PaCO2 sufficient
for diagnosing
coronary heart disease from imaging data and imaging the heart during a period
in which the
increase in PaCO2 is measurable, wherein the imaging data is indicative of a
cardiovascular
disease-associated vasoreactive response in at least one coronary blood vessel
or region of
the heart,
4
13967uss
CA 02832851 2013-11-08
Optionally, the at least one increase in the subject's PaCO2 is selected to
produce a coronary
vasoreactive response sufficient for replacing a hyperemia inducing drug in
assessing
coronary disease.
Optionally, the use/method comprises attaining a particular predetermined
PaCO2.
Optionally, the pre-determined PaCO2 is patient specific, for example an 8 to
20 mm Hg
increase relative a baseline steady level measured at the time of testing.
Optionally, the use/method comprises administering carbon dioxide in a
stepwise manner.
Optionally, the use/method comprises administering carbon dioxide in a block
manner.
Optionally, the CO2 is administered via inhalation.
Optionally, the disease-associated coronary vasoreactive response is assessed
relative to a
control subject.
Optionally, the PaCO2 is increased and decreased repeatedly.
Optionally, the at least one PaCO2 produces at least an 8%-12% increase in a
BOLD signal
intensity.
Optionally, the disease-associated vasoreactive response is a compromised
increase in blood
flow.
Optionally, the imaging data is indicative of the presence or absence of a two-
fold increase in
blood flow in a coronary artery.
5
1396785u
CA 02832851 2013-11-08
Optionally the imaging data are obtained by MR1 and the imaging method obtains
input of a
change in signal intensity of a BOLD MRI signal.
Optionally, the imaging method is PET or SPECT and the measure of a disease-
associated
vasoreactive response is the presence or absence of a threshold increase in
blood flow.
Optionally, the at least one increase in PaCO2produces at least a 10% increase
in intensity of
a BOLD IvIR1 signal.
Optionally, the at least one increase in PaCO2 produces a 10-20% increase in,
intensity of a
BOLD MRI signal.
Optionally, the use/method comprises: (i) imaging the myocardium to obtain
free-breathing
cardiac phase resolved 31) myocardial BOLD images. (ii) registering and
segmenting the
images to obtain the myocardial dynamic volume and (Hi) identifying isehemic
territory and
quantifmg image volume.
Optionally, the at least one PaCO2 is at least a 10 mm Hg increase from a
first level which is
determined to be between 30 and 55 ram Hg. Optionally, the first level is
first determined to
be between 35 and 45 mm Hg.
Optionally, the sufficiency of the increase in PaCO2 is determined by
increasing PaCO2 in a
stepwise manner.
Optionally, the yasoreactive response is sufficient for obtaining a disease-
associated change
in BOLD MR1 signal obtained by administering CO2 in a manner effective to
alternate
between two or more PaCO2 levels over a period of time and using repeated BOLD
MRI
measurements to statistically assess the hyperemic response.
6
139678582
CA 02832851 2013-11-08
Optionally, the coronary vasoreactive response corresponds to a vasodilatory
response
produced by administering a hyperemia inducing drug for a duration and in
amount per unit
of time effective to assess coronary disease.
Optionally, the hyperemia inducing drug is adenosine, wherein adenosine is
administered in a
regimen of 140 milligrams/litre per minute over 4 to 6 minutes.
Optionally, the use/method comprises admixing air with a selected amount of a
CO2
containing gas controlled to obtain a predetermined size increase in PaCO2
from a previous
value, for example a measured baseline value.
The CO2 containing gas may contain, for example, 75 to 100% CO2. Optionally
the CO2
containing gas comprises a percentage composition of oxygen in the 18-23%
range,
optionally about 20%.
In one embodiment the invention is directed to a method for diagnosing
coronary heart
disease in a subject in need thereof comprising:
(i) administering an admixture comprising CO2 to a subject in a stepwise or
block
manner to reach a predetermined PaCO2 in the subject to induce hyperemia;
(ii) monitoring vascular reactivity in the subject; and
(Hi) diagnosing the presence or absence of coronary heart disease in the
subject, wherein
decreased vascular reactivity in the subject compared to a control subject is
indicative of coronary heart disease,
thereby diagnosing coronary heart disease in the subject in need thereof.
As elaborated below, administering carbon dioxide to alter PaCO2 in block
manner, is
optionally repeated over time. Optionally carbon dioxide is administered so as
to alternate
between two or more levels of PaCO2 over a period of time.
Vascular reactivity may be monitored using any one or more of a variety of
advanced
imaging methods including positron emission tomography (PET), single photon
emission
7
139673582
CA 02832851 2013-11-08
computed tomography/computed tomography (SPECT), computed tomography (CT), and
magnetic resonance imaging (MRI), to name a few. Optionally, vascular
reactivity may be
measured using FFR.
A particularly advantageous admixture of CO2 and 02 for inducing hyperemia,
particularly
for blending a CO2 containing gas with air for inhalation is an admixture in
which 02 is
present in the range of 19-22%, for example about 20%. In this embodiment, CO2
may make
up the rest of the admixture (81-78% respectively) or there may be a third gas
in the
admixture.
BRIEF DESCRIPTION OF FIGURES
Figure 1 depicts, in accordance with an embodiment of the present invention,
the vascular
reactivity in dogs as measured by the BOLD-effect using medical-grade Carbogen
(5% CO2
and 95% 02) with and without coronary artery stenosis-
Figure 2 depicts myocardial BOLD MRI with CO2 in canines under normocarbic and
hypercarbic conditions under free breathing conditions.
Figure 3 depicts myocardial BOLD response to step-wise PaCO2 ramp up in
canines while
holding basal Pa02 constant.
Figure 4 depicts myocardial BOLD response to repeated (block) administration
CO2
response.
Figure 5 depicts the Doppler flow through the left anterior descending artery
in response to
PaCO2 modulation while Pa02 is held constant.
Figure 6 depicts the Doppler flow through the LAD, RCA and LCX arteries in,
response to
PaCO2 modulation while Pa02 is held constant.
8
139678S2.2
Figure 7 is a bar graph depicting the territorial myocardial BOLD response to
PaCO2
modulations in canines while Pa02 is held constant.
Figure 8 is a bar graph depicting the BOLD effect associated with PaCO2
modulation in
blood, muscle and air while Pa02 is held constant,
Figure 9 is a table summarizing the statistical BOLD data associated with the
PaCO2
modulation in myocardial territories, blood, muscle and air, while Pa02 is
held constant.
Figure 10 is a comparison of BOLD response to adenosine and PaCO2 (while Pa02
is held
constant).
Figure 11 depicts the early findings of BOLD response to PaCO2 in humans,
while Pa02 is
held constant.
Figure 12(a) depicts a simulated BOLD signal for a change in PaCO2 (red line)
with
definitions for noise variability ( =o=2- 0) and response. Figure 12(b)
depicts a relation between
BOLD response (y-axis) and the number of measurements (x-axis) required to
establish
statistical significance (color-coded p-values). For a given BOLD response,
the number of
repeated measurements (N) required for reliable assessment (p<0.05) of a
change from
baseline condition lies at the right of the white dotted line. For e.g., to
reliably detect a
BOLD response from a voxel with peak BOLD signal response of 10%, greater than
8
measurements are needed. The bar on the right gives the scale for p values
associated with
the statistical significance.
DETAILED DESCRIPTION OF THE INVENTION
Unless defined otherwise, technical and scientific terms used herein have the
same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Singleton Cr at., Dictionary of Microbiology and Molecular Biology rd
ed, J.
9
Date Recue/Date Received 2021-02-26
CA 02832851 2013-11-08
Wiley & Sons (New York, NY 2001); March, Advanced Organic Chemistry Reactions,
Mechanisms and Structure 5th ed, J. Wiley & Sons (New York, NY 2001); and
Sambrook
and Russel, Molecular Cloning: A Laboratory Manual 3rd ed., Cold Spring Harbor
Laboratory Press (Cold Spring Harbor, NY 2001), provide one skilled in the art
with a
general guide to many of the terms used in the present application.
One skilled in the art will recognize many methods and materials similar or
equivalent to
those described herein, which could be used in the practice of the present
invention. Indeed,
the present invention is in no way limited to the methods and materials
described. For
purposes of the present invention, the following terms are defined below.
"Beneficial results" may include, but are in no way limited to, lessening or
alleviating the
severity of the disease condition, preventing the disease condition from
worsening, curing the
disease condition, preventing the disease condition from developing, lowering
the chances of
a patient developing the disease condition and prolonging a patient's life or
life expectancy.
"Mammal" as used herein refers to any member of the class Mammalia, including,
without
limitation, humans and nonhuman primates such as chimpanzees and other apes
and monkey
species; farm animals such as cattle, sheep, pigs, goats and horses; domestic
mammals such
as dogs and cats; laboratory animals including rodents such as mice, rats and
guinea pigs,. and
the like. The term does not denote a particular age or sex. Thus, adult and
newborn subjects,
as well as fetuses, whether male or female, are intended to be included within
the scope of
this term.
"Treatment" and "treating," as used herein refer to both therapeutic treatment
and
prophylactic or preventative measures, wherein the object is to prevent or
slow down (lessen)
the targeted pathologic condition, prevent the pathologic condition, pursue or
obtain
beneficial results, or lower the chances of the individual developing the
condition even if the
treatment is ultimately unsuccessful. Those in need of treatment include those
already with
the condition as well as those prone to have the condition or those in whom
the condition is
to be prevented.
13467858.2
CA 02832851 2013-11-08
"Carbogen" as used herein is an admixture of carbon dioxide and oxygen. The
amounts of
carbon dioxide and oxygen in the admixture may be determined by one skilled in
the art.
Medical grade carbogen is typically 5% CO2 and 95% 02. In various other
embodiments,
carbon dioxide is used to induce hyperemia may be an admixture of ranges
including but not
limited to 94% 02 and 6% CO2, 93% 02 and 7% CO2, 92% 02 and 8% CO2, 91% 02 and
9%
CO2, 90% 02 and 10% CO2, 85% 02 and 15% CO2, 80% 02 and 20% CO2, 75% 02 and
25%
CO2 and/or 70% 02 and 30% CO2. Optionally, for blending with air, the CO2
containing gas
comprises 20% oxygen.
"BOLD" as used herein refers to blood-oxygen-level dependence.
A "vascular-disease-associated" coronary vasoreactive response means a type
and/or
quantum of vasoreactive response elicited by cardiac stress testing (e.g.
exercise or
administration of a hyperemic drug or a CO2 containing gas) as demonstrable in
an imaging
study using one or more diagnostic imaging parameters of the type suitable to
diagnose
coronary vascular disease. For example, with respect to PET and SPECT, a
normal response
would be considered a four to five fold increase in blood flow. With respect
to BOLD MRI
imaging, a 10-12+% increase in BOLD signal would be considered normal. Disease
associated responses are those which are not normal in varying significant
degrees among
which, as evidence of disease, benchmarks may be adopted to categorize
differences with
represent a clearer-cut diagnosis or a progression of disease that warrants
greater follow-up
or more proactive treatment, for example a less than two-fold increase in
blood flow as
measured by PET or SPECT (typically measured in ml. of blood/min/gm of
tissue).
Accordingly, a benchmark which represent a change from a value that clinicians
described as
"normal" which is at least statistically significant and optionally is also
comparable to a
standard for cardiac stress testing adopted by clinicians with respect to
inducing stress
represents a clear-cut benchmark for using CO2 as a vasoactive stress
stimulus.
A targeted increase in PaCO2 will be selected to cause a similar vasoreactive
response in
normal and diseased tissue. From the standpoint of statistical significance,
it will be
11
1396785182
CA 02832851 2013-11-08
appreciated that selection of a discriminatory increase in PaCO2 may depend on
whether or
not repeat measurements are made, for example, the number of repeat
measurements of a
BOLD signal intensity that are made at while at lower and increased PaCO2
levels,
Current methods for inducing hyperemia in subjects include the use of
compounds such as
adenosine, analogs thereof and/or functional equivalents thereof. However,
such compounds
(for example, adenosine) have adverse side effects including bradycardia,
arrhythmia,
transient or prolonged episode of asystole, ventricular fibrillation (rarely),
chest pain,
headache, dyspnea, and nausea, making it less than favorable for initial or
follow-up studies.
The invention described herein is directed to the use of CO2 instead of
hyperemia-inducing
drugs, in view of their side effects, to assess myocardial response and risk
of coronary artery
diseases. To date, however, it has not been possible to independently control
arterial CO2
and 02, hence direct association of the influence of partial pressure of CO2
(PaCO2) on
coronary vasodilation has been difficult to determine. With the development of
gas flow
controller devices designed to control gas concentrations in the lungs and
blood (for example,
RespirACT1m, Thornhill Research,W0/2013/0082703), it is now possible to
precisely
control the arterial CO2, while, in some embodiments, holding 01 constant.
With such
devices, the desired PaCO2 changes are rapid (1-2 breaths) and are independent
of minute
ventilation. The inventors are the first adopters of such devices for the
assessment of
myocardial response to CO2.
The claimed invention is believed to be the first to use modulation of CO2
levels to show that
the carbon dioxide has the same effect as the clinical dose of other hyperemia-
inducing drugs
such as adenosine but without the side effects. The inventors induce hyperemia
by
administering an admixture comprising a predetermined amount of CO2 to a
subject in need
thereof to assess myocardial response, evaluate coronary artery disease and
identify ischernic
heart disease. In an embodiment, hyperemia is induced by independently
altering the
12
134678582
CA 02832851 2013-11-08
administered CO2 level while holding oxygen (02) constant to assess myocardial
response,
evaluate coronary artery disease and identify ischemic heart disease. A
subject's myocardial
response after administration of CO2 may be monitored using various imaging
techniques
such as MRI.
Cardiac Stress Testing
When exercise stress testing is contra-indicated (in nearly 50% of patients),
every existing
imaging modality uses adenosine (or its analogues such as dipyridamole or
regadenoson) to
induce hyperemia. However, as described above, adenosine or analogs thereof or
functional
equivalents thereof, are well known for their adverse side effects such as
bradycazdia,
arrhythmia, transient or prolonged episode of asystole, ventricular
fibrillation (rarely), chest
pain, headache, dyspnea, and nausea, malting it less than favorable for
initial or follow-up
studies. Direct measures of ischemic burden may be determined on the basis of
single-
photon emission computed tomography (SPECT/SPET), positron emission tomography
(PET), myocardial contrast echocardiography (MCE), and first-pass perfusion
magnetic
resonance imaging (FPP-MRI). SPECT and PET use radiotracers as contrast
agents. While
SPECT and PET studies account for approximately 90% myocardial ischemia-
testing studies,
the sensitivity and specificity for both methods combined for the
determination of severe
ischemia is below 70%. Both MCE and FPP-MRI are relatively newer approaches
that
require the use of exogenous contrast media and intravenous pharmacological
stress agent
(adenosine), both carrying significant risks and side effects in certain
patient populations.
BOLD-MRI
An alternate method, BOLD (Blood-Oxygen-Level-Dependent) MRI, relies on
endogenous
contrast mechanisms (changes in blood oxygen saturation, %02) to identify
ischernic
territories. The potential benefits of BOLD MRI for detecting global or
regional myocardial
ischemia due to coronary artery disease (CAD) were demonstrated by the
inventors and
others at least a decade ago. Although a number of pilot clinical studies have
demonstrated
the feasibility of using BOLD MRI for identifying clinically significant
myocardial ischemia
13
13967858.2
CA 02832851 2013-11-08
due to CAD, the method is inherently limited by sensitivity and specificity
due to low BOLD
contrast-to-noise ratio (CNR). The repeatability of BOLD MRI using CO2
provides the
means to improve sensitivity and specificity, which is not possible using
adenosine or
analogs thereof
The invention provides a method for increasing the sensitivity and specificity
of BOLD MR'.
The method includes administering an admixture comprising of CO2 to the
subject in need
thereof to induce hyperemia and imaging the myocardium using MRI to assess a
hypermic
response in response to a predetermined modulation in PaCO2.
The proposed method utilizes (i) an individualized targeted change in arterial
partial pressure
of CO2 (PaCO2) as the non-invasive vasoactive stimulus, (ii) fast, high-
resolution, 4D BOLD
MRI at 3T and (iii) statistical models (for example, the generalized linear
model (GLM)
theory) to derive statistical parametric maps (SPM) to reliably detect and
quantify the
prognostic ally significant ischemic burden through repeated measurements
(i.e. in a data-
driven fashion).
The method for increasing the sensitivity and specificity of BOLD MRI
comprises (i)
obtaining free-breathing cardiac phase-resolved 3D myocardial BOLD images
(under
different PaCO2 states established via inhalation of an admixture of gases
comprising of
CO2) ; (ii) registering and segmenting the images to obtain the myocardial
dynamic volume
and (iii) identifying ischemic territory and quantify image volume_
Obtaining the images
The first step in increasing the sensitivity and specificity of BOLD MRI is to
obtain free-
breathing cardiac phase resolved 31) myocardial BOLD images. Subjects are
placed on the
MRI scanner table, ECG leads are placed, and necessary surface coils are
positioned.
Subsequently their hearts are localized and the cardiac shim protocol is
prescribed over the
whole heart. K-space lines, time stamped for trigger time are collected using
Gine SSFP
acquisition with image acceleration along the long axis. Central k-space lines
corresponding
14
13967850
CA 02832851 2013-11-08
to each cardiac phase will be used to derive the center of mass (COM) curves
along the z-
axis via 1-D fast Fourier transform (FFT). Based on the COM curves, the k-
space lines from
each cardiac phase will be sorted into 1-30 bins, each corresponding to a
respiratory state
with the first bin being the reference bin (end-expiration) and the last bin
corresponding to
end inspiration.
To minimize the artifacts from under sampling, the data will be processed with
a 3D filter,
followed by re-gridding the k-space lines, application of a spatial mask (to
restrict the
registration to region of the heart) and performing FFT to obtain the under
sampled 3D image
for each respiratory bin. Using the end-expiration image as the reference
image, images from
all bins (except bin 1) are registered using kits such as Insight Tool Kit
(freely available from
www.itk.org), or an equivalent software platform, in an iterative fashion and
the transform
parameters will be estimated for rotation, scaling, shearing, and translation
of heart between
the different respiratory bins. The k-space data will again be divided into 1
to 30 respiratory
bins, re-gridded, transformed to the reference image (3D affme transform),
summed together,
and the final 3D image will be reconstructed. Imaging parameters may be TR =
3.0 to 10 ms
and flip angle = 10 to 900. In this fashion, 31) eine data under controlled
PaCO2 values (hypo-
and hyper-carbic states) are collected.
Registration and Segmentation of Images
The next step in increasing the sensitivity and specificity of BOLD MRI is
registeration and
segmentation of the images to obtain the myocardial dynamic volume. The
pipeline utilizes
MATLAB and C4+ using the ITK framework or an equivalent software platform. The
myocardial MR images obtained with repeat CO2 stimulation blocks will be
loaded in
MATLAB (or an equivalent image processing platform) and arranged in a four-
dimensional
(4D) matrix, where the first 3 dimensions represent volume (voxels) and the
fourth
dimension is time (cardiac phase). Subsequently, each volume is resampled to
achieve
isotropic voxel size. End-systole (ES) are identified for each stack based on
our minimum
cross-correlation approach. A 4D non-linear registration algorithm is used to
find voxel-to-
voxel correspondences (deformation fields) across all cardiac phases. Using
the recovered
139785.2
CA 02832851 2013-11-08
deformation, all cardiac phases are wrapped to the space of ES, such that all
phases are
aligned to ES. Recover the transformations across all ES images from repeat
CO2 blocks and
bring them to the same space using a diffeomorphic volume registration tool,
such as ANTS.
Upon completion, all cardiac phases from all acquisitions will be spatially
aligned to the
space of ES of the first acquisition (used as reference) and all phase-to-
phase deformations
and acquisition-to-acquisition transformations will be known. An expert
delineation of the
myocardium in the ES of the first (reference) acquisition will then be
performed. Based on
the estimated deformation fields and tranaforinations, this segmentation is
propagated to all
phases and acquisitions, resulting in fully registered and segmented
myocardial dynamic
volumes.
Image analysis to identity and Quanti,b, Ischemic Territories
The final step needed for increasing the sensitivity and specificity of BOLD
MR1 is
identifying ischernic territory and quantify image volume. Since BOLD
responses are
optimally observed in systolic frames, only L systolic cardiac volumes
(centered at ES) are
retained from each fully registered and segmented 4D BOLD MR image set
obtained above.
Only those voxels contained in the myocardium are retained and the
corresponding R.PP
(rate-pressure-product) and PaCO2 are noted. Assuming N acquisitions per CO2
state
(hypocarbic or hypercarbic) and K. CO2 stimulation blocks, and each cardiac
volume consists
of nxmxp (x--. multiplication) isotropic voxels, build a concatenated fully
registered 4D
dataset consisting of mcmxpxt pixels, where xnultiplication and t = LxKxN, and
export this
dataset in NIFTI (or an equivalent) format using standard tools. The 41)
dataset is loaded
into a voxel-based statistical model fitting (such as FSL-FEAT developed for
fMRI), to fit
the model for each voxel. The statistical analysis outputs a P-statistic
volume, i.e., the SPM,
where for each voxel in the myocardium the p-value of the significance of the
correlation to
the model is reported. The statistical parametric maps (SPM) are thresholded
by identifying
the voxels that have p<0.05. Those voxels are identified as hyperemic for
responding to the
CO2 stimulation. The total number of hyperemic voxels (VII) are counted and
their relative
volume (Yu/ := \TR / total voxels in myocarditun) is determined. The voxels
that do not
respond to CO2 stimulation (on SPM) are identified as ischemic and used to
generate a binary
16
13967M2
CA 02832851 2013-11-08
3D map of ischemic voxels (3D-ISCH.p). In addition, total ischemic voxels (VI)
and the
relative ischemic volume (V51 --VI /total myocardial voxels) are determined.
The above methods provide ischemic volumes that can be reliably identified on
the basis of
statistical analysis applied to repeatedly acquire 4D BOLD images under
precisely targeted
changes in, PaCO2. These volumes are closely related to the clinical index of
fractional flow
reserve FFR.
FFR
An additional method, fractional flow reserve (FFR) is used in coronary
catheterization to
measure pressure differences across a coronary artery stenosis to determine
the likelihood
that the stenosis impedes oxygen delivery to the heart muscle (myocardial
ischemia).
Fractional flow reserve measures the pressure behind (distal to) a stenosis
relative to the
pressure before the stenosis, using adenosine or papaverine to induce
hyperemia. A cut-off
point of 0.75 to 0.80 has been used wherein higher values indicate a non-
significant stenosis
and lower values indicate a significant lesion. FFR, determined as the
relative pressure
differences across the stenotic coronary artery has emerged as the new
standard for
determining clinically significant ischenlia (FFR. -E0.75). However, it is
invasive, expensive,
and exposes the patient to ionizing radiation and the side-effects of the use
of adenosine. In
view of the side-effects of adenosine discussed above, Applicants propose
using carbon
dioxide instead of adenosine to induce hyperemia, by administering to a
subject an admixture
.. comprising CO2 to reach a predetermined PaCO2 in the subject to induce
hyperemia. In
some embodiments, the admixture comprises any one or more of carbon dioxide,
oxygen and
nitrogen; carbon dioxide and oxygen; carbon dioxide and nitrogen; or carbon
dioxide alone.
In one embodiment, the amounts of CO2 and 02 administered are both altered. In
another
embodiment, the amount of CO2 administered is altered to a predetermined level
while the
amount of 02 administered is held constant. In various embodiments, the
amounts of any
17
13967858.2
CA 02832851 2013-11-08
one or more of CO2, 02 or N2 in an admixture are changed or held constant as
would be
readily apparent to a person having ordinary skill in the art.
Methods of the invention
The invention is directed to methods for diagnosing coronary heart disease in
a subject in
need thereof comprising administering an admixture comprising CO2 to a subject
to reach a
predetermined PaCO2 in the subject to induce hyperemia, monitoring vascular
reactivity in
the subject and diagnosing the presence or absence of coronary heart disease
in the subject,
wherein decreased vascular reactivity in the subject compared to a control
subject is
indicative of coronary heart disease. In an embodiment, CO2 is administered
via inhalation.
In another embodiment, CO2 levels are altered while the 02 levels remain
unchanged so that
the PaCO2 is changed independently of the 02 level. In a further embodiment,
vascular
reactivity is monitored using imagining techniques deemed appropriate by one
skilled in the
art, including but not limited to any one or more of positron emission
tomography (PET),
single photon emission computed tomography/computed tomography (SPECT),
computed
tomography (CT), magnetic resonance imaging (MR1), functional magnetic
resonance
imaging (fMRI), single photon emission computed tomography/computed tomography
(SPEC/CT), positron emission tomography/computed tomography (PET/CT),
ultrasound,
electrocardiogram (ECG), Electron-beam computed tomography (EBCT),
echocardiogram
(ECHO), electron spin resonance (ESR) and/or any combination of the imaging
modalities
such as (PET/MR), PET/CT, and/or SPECT/MR. In an embodiment, vascular
reactivity is
monitored using free-breathing BOLD MR1. In some embodiments, the admixture
comprises
any one or more of carbon dioxide, oxygen and nitrogen; carbon dioxide and
oxygen; carbon
dioxide and nitrogen; or carbon dioxide alone. In one embodiment, the amounts
of CO2 and
02 administered are both altered. In another embodiment, the amount of CO2
administered is
altered to a predetermined level while the amount of 02 administered is held
constant. In
various embodiments, the amounts of any one or more of CO2, 02 or N2 in an
admixture are
changed or held constant as would be readily apparent to a person having
ordinary skill in the
art.
18
13967858.2
CA 02832851 2013-11-08
The invention also provides a method for assessing hyperemic response in a
subject in need
thereof comprising administering an admixture comprising CO2 to a subject to
reach a
predetermined PaCO2 in the subject to induce hyperemia, monitoring vascular
reactivity in
the subject and assessing hyperemic response in the subject, wherein decreased
vascular
reactivity in the subject compared to a control subject is indicative of poor
hyperemic
response, thereby assessing hyperemic response in the subject in need thereof.
This method
may also be used to assess organ perfusion and assess vascular reactivity. In
an embodiment,
CO2 is administered via inhalation. In another embodiment, CO2 levels are
altered while the
02 levels remain unchanged so that the PaCO2 is changed independently of the
02 level. In a
further embodiment, vascular reactivity is monitored using imagining
techniques deemed
appropriate by one skilled in the art, including but not limited to any one or
more of positron
emission tomography (PET), single photon emission computed tomography/computed
tomography (SPECT), computed tomography (CT), magnetic resonance imaging
(1VIRI),
functional magnetic resonance imaging (NRI), single photon emission computed
tomography/computed tomography (SPECT/CT), positron emission
tomography/computed
tomography (PET/CT), ultrasound, electrocardiogram (ECG), Electron-beam
computed
tomography (EBCT), echocardiogram (ECHO), electron spin resonance (ESR)
arid/or any
combination of the imaging modalities such as (PET/MR), PET/CT, and/or
SPECT/MR. In
an embodiment, vascular reactivity is monitored using free-breathing BOLD MM.
In some
embodiments, the admixture comprises any one or more of carbon dioxide, oxygen
and
nitrogen; carbon dioxide and oxygen; carbon dioxide and nitrogen; or carbon
dioxide alone.
In one embodiment, the amounts of CO2 and 02 administered are both altered. In
another
embodiment, the amount of CO2 administered is altered to a predetermined level
while the
amount of 02 administered is held constant. In various embodiments, the
amounts of any
one or more of CO2, 02 or N2 in an admixture are changed or held constant as
would be
readily apparent to a person having ordinary skill in the art.
The invention is further directed to methods for producing coronary
vasodilation in a subject
in need thereof comprising providing a composition comprising CO2 and
administering the
composition comprising CO2 to a subject to reach a predetermined PaCO2 in the
subject so as
to produce coronary vasodilation in the subject, thereby producing coronary
vasodilation in
19
13967858.2
CA 02832851 2013-11-08
the subject. In an embodiment, CO2 is administered via inhalation. In another
embodiment,
CO2 levels are altered while the 02 levels remain unchanged so that the PaCO2
is changed
independently of the 02 level, In a further embodiment, vascular reactivity is
monitored
using imagining techniques deemed appropriate by one skilled in the art,
including but not
limited to any one or more of positron emission tomography (PET), single
photon emission
computed tomography/computed tomography (SPECT), computed tomography (CT),
magnetic resonance imaging (MRI), functional magnetic resonance imaging
(fMRI), single
photon emission computed tomography/computed tomography (SPECT/CT), positron
emission tomography/computed tomography (PET/CT), ultrasound,
electrocardiogram
(ECG), Electron-beam computed tomography (EBCT), echocardiogram (ECHO),
electron
spin resonance (ESR) andlor any combination of the imaging modalities such as
(PET/MR),
PET/CT, and/or SPECT/MR. In an embodiment, vascular reactivity is monitored
using free-
breathing BOLD MRI. In some embodiments, the admixture comprises any one or
more of
carbon dioxide, oxygen and nitrogen; carbon dioxide and oxygen; carbon dioxide
and
nitrogen; or carbon dioxide alone. In one embodiment, the amounts of CO2 and
02
administered are both altered, In another embodiment, the amount of CO2
administered is
altered to a predetermined level while the amount of 02 administered is held
constant. In
various embodiments, the amounts of any one or more of CO2, 02 or N2 in an
admixture are
changed or held constant as would be readily apparent to a person having
ordinary skill in the
.. art.
The invention also provides a method for assessing tissue and/or organ
perfusion in a subject
in need thereof comprising administering an admixture comprising CO2 to a
subject to reach
a predetermined PaCO2 in the subject to induce hyperemia, monitoring vascular
reactivity in
the tissue and/or organ and assessing tissue and/or organ perfusion by
assessing the
hyperemic response in the subject, wherein decreased vascular reactivity in
the subject
compared to a control subject is indicative of poor hyperemic response and
therefore poor
tissue and/or organ perfusion. In an embodiment, CO2 is administered via
inhalation. In
another embodiment, CO2 levels are altered while the 02 levels remain
unchanged so that the
PaCO2 is changed independently of the 02 level. In a further embodiment,
vascular
reactivity is monitored using imagining techniques deemed appropriate by one
skilled in the
13967103a
CA 02832851 2013-11-08
art, including but not limited to any one or more of positron emission
tomography (PET),
single photon emission computed tomography/computed tomography (SPECT),
computed
tomography (CT), magnetic resonance imaging (MRI), functional magnetic
resonance
imaging (fMRI), single photon emission computed tomography/computed tomography
(SPECT/CT), positron emission tomography/computed tomography (PET/CT),
ultrasound,
electrocardiogram (ECG), Electron-beam computed tomography (EBCT),
echocardiogram
(ECHO), electron spin resonance (ESR) and/or any combination of the imaging
modalities
such as (PET/MR), PET/CT, and/or SPECT/MR. In an embodiment, vascular
reactivity is
monitored using free-breathing BOLD IvERI. In some embodiments, the admixture
comprises
.. any one or more of carbon dioxide, oxygen and nitrogen; carbon dioxide and
oxygen; carbon
dioxide and nitrogen; or carbon dioxide alone. In one embodiment, the amounts
of CO2 and
02 administered are both altered. In another embodiment, the amount of CO2
administered is
altered to a predetermined level while the amount of 02 administered is held
constant. In
various embodiments, the amounts of any one or more of CO2, 02 or N2 in an
admixture are
IS changed or held constant as would be readily apparent to a person having
ordinary skill in the
art.
In some embodiments, the admixture comprising CO2 is administered at high
doses for short
duration or at low doses for longer durations. Por example, administering the
admixture
comprising CO2 at high doses of CO2 for a short duration comprises
administering any one or
more of 40mmllg to 45mmHg, 45mmHg to 50mmHg, 50mmlig to 55mmHg, 55mmHg CO2
to 601orn. Hg CO2, 60mml-Ig CO2 to 65mm Hg CO2, 65mmlig CO2 to 70mm Hg CO2,
70mmHg CO2 to 75mm Hg CO2, 75mml-Ig CO2 to 80mm Hg CO2, 80mmHg CO2 to 85min
Hg CO2 or a combination thereof, for about 20 minutes, 15 minutes, 10 minutes,
9 minutes, 8
.. minutes, 7 minutes, 6 minutes, 5 minutes, 4 minutes, 3 minutes, 2 minutes,
1 minute or a
combination thereof. In various embodiments, the predetermined levels of CO2
are
administered so that the arterial level of CO2 reaches the PaCO2 of any one or
more of the
above ranges.
For example, administering low doses of predetermined amounts of CO2 for a
longer
duration comprises administering the predetermined amount of CO2 at any one or
more of
21
13967358.2
CA 02832851 2013-11-08
about 30nuntig CO2 to about 35rtunlig CO2, about 35mmlig CO2 to about 40mmHg
CO2,
about 40mmHg CO2 to about 45nunfig CO2or a combination thereof for any one or
more of
about 20 to 24 hours, about 15 to20 hours, about 10 to 15 hours, about 5 to 10
hours, about 4
to 5 hours, about 3 to 4 hours, about 2 to 3 hours, about 1 to 2 hours, or a
combination
thereof, before inducing hyperemia. In various embodiments, the predetermined
levels of
CO2 are administered so that the arterial level of CO2 reaches the PaCO2 of
any one or more
of the above ranges.
In one embodiment, CO2 is administered in a stepwise manner. In another
embodiment,
administering carbon dioxide in a stepwise manner includes administering
carbon dioxide in
5nmilig increments in the range of any one or more of lOmmHg to 100roralig
CO2,
20mmHg to 100mning CO2, 30mmHg to 100minHg CO2, 40mmHg to 100mmHg CO2,
50mnalig to 100mmHg CO2, 60mmHg to 100mmilg CO2, 10minHg to 90mrnHg CO2,
20mmlig to 90mmHg CO2, 30minHg to 90rmaillg CO2, 40mmHg to 90mrallg CO2,
50mmlIg to 90mmilg CO2, 60mrriHg to 90minHg CO2, 10mrnHg to 80mmilg CO2,
20mmHg to 80mmHg CO2, 30trunHg to 80rnialHg CO2, 40rnmElg to 80mmrig CO2,
50mml-Ig to 80mmI4g CO2, 60mmHg to 80mmllg CO2, 10nunHg to 70mmHg CO2,
20mrxitIg to 70=rdig CO2, 30inmHg to 70mmlig CO2, 40mmHg to 70mmHg CO2,
50mmllg to 70mml-Ig CO2, 60rainHg to 70rnmIlg CO2, lOnanalg to 60nunHg CO2,
20rnm.Hg to 70nainHg CO2, 30mnallg to 70inmflg CO2, 40minHg to 70mmlIg CO2,
50mnillg to 70mm11g CO2, 60mmlig to 70mm11g CO2, 10trimilg to 60ramHg CO2,
20mmHg to 60nunHg CO2, 30rrunflg to 60rattillg CO2, 40mmHg to 60mmlig CO2 or
50mmilg to 60mmilg CO2. In various embodiments, the predetermined levels of
CO2 are
administered so that the arterial level of CO2 reaches the PaCO2 of any one or
more of the
above ranges.
In another embodiment, administering carbon dioxide in a stepwise manner
includes
administering carbon dioxide in lOmmlig increments in the range of any one or
more of
1 mmHg to 100mm1-ig CO2, 20mmHg to 100nunHg CO2, 30mmHg to 100nunHg CO2,
40namHg to 100nunHg CO2, 50innalg to 100mmlig CO2, 60mmlig to 100mmHg CO2,
lOmmlig to 90mmHg CO2, 20mtnElg to 90mmHg CO2, 30mm11g to 90mm11g CO2,
22
1397S582
CA 02832851 2013-11-08
40mmHg to 90mmHg CO2, 50mm1EEg to 90=i-1g CO2, 6OrnmHg to 90mmIt CO2,
1 OmmIlg to 80minlig CO2, 20trunlig to 80mmHg CO2, 30rainHg to 80mmHg CO2,
40mm1Hg to 80mm11g CO2, 50mmHg to 80mmilg CO2, 60mmtig to 80mmlig CO2,
10=i-1g to 70mmHg CO2, 20mmElg to 70mmlig CO2, 30mmHg to 70rnmilg CO2,
40mmHg to 70mmHg CO2, 50mmHg to 70minfig CO2, 60nunlig to 70mmHg CO2,
1 OmmHg to 60nunlig CO2, 20mmlIg to 70nunHg CO2, 30mmilg to 70mmHg CO2,
40mmHg to 70mmilg CO2, 50nunHg to 70mmHg CO2, 60mraHg to 70mmHg CO2,
1 Omml-Ig to 60tninHg CO2, 20mralig to 60mmHg CO2, 30ramlig to 60mmHg CO2,
=40mmHg to 60mmHg CO2 or 50mmHg to 6OrnmHg CO2. In various embodiments, the
predetermined levels of CO2 are administered so that the arterial level of CO2
reaches the
PaCO2 of any one or more of the above ranges.
In a further embodiment, administering carbon dioxide in a stepwise manner
includes
administering carbon dioxide in 20mmilg increments in the range of any one or
more of
1 OmmHg to 100mmHg CO2, 20mmHg to 100rnmHg CO2, 30mrailg to 100nunHg CO2,
40mmHg to 100mmHg CO2, 50mmHg to 100mmllg CO2, 60mmHg to 100nunlig CO2.
lOmmlig to 9Ortunlig CO2, 20mmHg to 90nunkrg CO2, 30mtnlig to 90mmilg CO2,
40mmHg to 90mmHg CO2, 50nunHg to 90mmHg CO2, 60minHg to 90mmHg CO2,
1 mmHg to 80ml-11i-1g CO2, 20mmlig to 80nunHg CO2, 30tninHg to 80mmlig CO2,
40mmHg to 80mmHg CO2, 50mnilig to 80mmHg CO2,, 60mmHg to 80mmHg CO2,
lOmmHg to 70mmHg CO2, 20mra11g to 70mratig CO2, 30mmHg to 70mmilg CO2,
40mmHg to 70mmHg CO2, 50mmlig to 70mm11g CO2, 60mmHg to 70mmIig CO2,
lOmmHg to 60mmHg CO2, 20mmtig to 70mnifig CO2, 30mniHg to 70mmHg CO2,
40mmHg to 70mmHg CO2, 50mmHg to 70nunlIg CO2, 60trunEg to 70mmHg CO2,
lOmmlig to 60mmHg CO2, 20mmilg to 60mrolig CO2, 30mnaig to 60mmHg CO2,
40mmHg to 60mmHg CO2 or 50mnalig to 60mmHg CO2. In various embodiments, the
predetermined levels of CO2 are adrninistered so that the arterial level of
CO2 reaches the
PaCO2 of any one or more of the above ranges.
In a further embodiment, administering carbon dioxide in a stepwise manner
includes
administering carbon dioxide in 301runHg increments in the range of any one or
more of
23
139678582
CA 02 832 851 2 013-11- 08
1 01/1121Hg to 100minHg CO2, 20mmllg to 100nunHg CO2, 30rnm1lg to 100ramllg
CO2,
40mmHg to 100mmng CO2, 5Ornmilg to 100mmHg CO2, 60mmlig to 100mnal-Ig CO2,
lOrrunHg to 90mmHg CO2, 20=111g to 90mmllg CO2, 30minHg to 90mm1-Ig CO2,
40minHg to 90mmlig CO2, 50mm11g to 90mraHg CO2, 60mm11g to 90nunHg CO2,
10nunHg to 80nurdig CO2, 2OrnmHg to 80mmilg CO2, 30mmHg to 80mtntig CO2,
40mmHg to 80mml1g CO2, 50namHg to 80mm1Hg CO2, 60minlig to 80mmlig CO2,
lOmmHg to 70mmHg CO2, 20mm11g to 70mmHg CO2, 30mmlig to 70mmHg CO2,
40mmHg to 70mmHg CO2, 50mrnHg to 70mmlig CO2, 60mmHg to 70mmHg CO2.
10mmHg to 60mmHg CO2, 20mmHg to 70mmHg CO2, 30mmilg to 70mmHg CO2,
40mmHg to 7OrnmHg CO2, 50trimHg to 70.tumfig CO2, 60mm.Hg to 70mmHg CO2,
lOmmlig to 60mmHg CO2, 20mmHg to 60rrunHg CO2, 30mmHg to 60mmHg CO2,
40ranHg to 60mmHg CO2 or 50mrn.lig to 60mmHg CO2. In various embodiments, the
predetermined levels of CO2 are administered so that the arterial level of CO2
reaches the
PaCO2 of any one or more of the above ranges.
In a further embodiment, administering carbon dioxide in a stepwise manner
includes
administering carbon dioxide in 40mmHg increments in the range of any one or
more of
lOmmHg to 100mmHg CO2, 20mm1-Ig to 100mmHg CO2, 30mtnifg to 100mmHg CO2.
40mmHg to 100mmHg CO2, 50ramHg to 100mmHg CO2, 60mmHg to 100mmlig CO2,
lOrrunHg to 90mmlig CO2, 20mmilg to 90mmHg CO2, 30mmHg to 90mmHg CO2,
40mmHg to 90-minHg CO2, 50mtnHg to 90mm11g CO2, 60mmHg to 90nun11g CO2,
lOmmHg to 80mmHg CO2, 20mm14g to 80rrunHg CO2, 30mmllg to 80nunHg CO2,
40rtunllg to 80mmHg CO2, SOrrinaHg to 80mmiig CO2, 60mmHg to 80mmHg CO2,
lOmmHg to 70innfflg CO2, 20mmHg to 70mmHg CO2, 30mmlig to 70mmHg CO2,
40mmHg to 70mmlig CO2, 50nunHg to 70mmHg CO2, 60mmHg to 70mmHg CO2,
lOmmHg to 60mmHg CO2, 20mnillg to 70mmHg CO2, 30mmHg to 70mmHg c02,
40mmHg to 70mmHg CO2, 50mtnHg to 70mmHg CO2, 60mmHg to 70mmllg CO2,
lOmmilg to 60nunHg CO2, 20mml1g to 60mmllg CO2, 30mml1g to 60mmHg CO2,
4Ornmitg to 60nutHg CO2 or 50mmHg to 60mmHg CO2, In various embodiments, the
predetermined levels of CO2 are administered so that the arterial level of CO2
reaches the
PaCO2 of any one or more of the above ranges.
24
13967855.2
CA 02832851 2013-11-08
In a further embodiment, administering carbon dioxide in a stepwise manner
includes
administering carbon dioxide in 50nmi11g increments in the range of any one or
more of
lOmmHg to 100romHg CO2, 20mmilg to 100mmlig CO2, 30mmHg to 100mmHg CO2,
40mmHg to 100mmilg CO2, 50mmHg to 100mrnHg CO2, 60mmHg to 100mmlig CO2,
lOmmHg to 90mm11g CO2, 20mmHg to 90mmHe CO2, 30mmHg to 90mmlig CO2,
40minfig to 90mmHg CO2, 50mmHg to 90mmHg 002, 60mmHg to 90mmit CO2,
lOmmHg to 80mmHg CO2, 20mm11g to 80mmHg CO2, 30mmHg to 80mmHg CO2,
40mmHg to 80mmHg CO2, 50mmHg to 80inmHg CO2, 60minHg to 80nuntig CO2,
10mralig to 70namHg CO2, 20nunHg to 70mmHg CO2, 30mmHg to 70mmllg CO2,
40mmlig to 70mmHg CO2, 50mmHg to 70mmHg CO2, 60mmHg to 70mmHg CO2,
1 ()mmHg to 60mmlig CO2, 20mmHg to 70rnmHg 002, 30mmHg to 70mmHg CO2,
40mmlig to 70mmHg CO2, 50mmHg to 70mmHg 002, 60mmHg to 70mmHg 002,
1 OmmHg to 60mm11g CO2, 20nunHg to 60mmHg CO2, 30mmHg to 60nuntig CO2,
40mnallg to 60mmHg CO2 or 50irimHg to 60mmHg CO2. In various embodiments, the
predetermined levels of CO2 are administered so that the arterial level of CO2
reaches the
PaCO2 of any one or more of the above ranges.
Other increments of carbon dioxide to be administered in a stepwise manner
will a readily
apparent to a person having ordinary skill in the art.
In a further embodiment, predetermined amount of CO2 is administered in a
block manner.
Block administration of carbon dioxide comprises administering carbon dioxide
in
alternating amounts over a period of time. In alternating amounts of CO2
comprises
alternating between any of 20trunlig and 40mmHg, 30mmHg and 40mtnHg, 20rtutHg
and
50mmHg, 30mrnHg and 50mmHg, 40mmlig and 50imnHg, 20mmHg and 60mmHg,
30narnfig and 60mmHg, 40minHg and 60mmHg, or 50mmHg and 60mmHg. In various
embodiments, the predetermined levels of CO2 are administered so that the
arterial level of
CO2 reaches the PaCO2 of any one or more of the above ranges. Other amounts of
carbon
dioxide to be used in alternating amounts over a period of time will be
readily apparent to a
person having ordinary skill in the art.
1390858,2
CA 02832851 2013-11-08
In one embodiment, vascular reactivity may be measured by characterization of
Myocardial
Perfusion Reserve, which is defined as a ratio of Myocardial Perfusion at
Stress to
Myocardial Perfusion at Rest. In healthy subjects the ratio may vary from 5:1
to 6:1. The
ratio diminishes with disease. A decrease in this ratio to 2:1 from the
healthy level is
considered the clinically significant and indicative of poor vascular
reactivity.
In another embodiment, vascular reactivity may be measured via differential
absolute
perfusion, which may be obtained using imaging methods such as first pass
perfusion,
SPECT/PET, CT perfusion or echocardiography in units of ml/sec/g of tissue.
In an embodiment the CO2 gas is administered via inhalation. CO2 may be
administered
using, for example, RespirACTrm technology from Thornhill Research. In various
embodiments, CO2 is administered for 1-2 minutes, 24 minutes, 4-6 minutes, 6-8
minutes, 8-
10 minutes, 10-12 minutes, 12-14 minutes, 14-16 minutes, 16-18 minutes and/or
18-20
minutes. In a preferred embodiment, CO2 is silministered for 4-6 minutes. In
an additional
embodiment CO2 is administered for an amount of time it takes for the arterial
PaCO2 (partial
pressure of carbon dioxide) to reach 50-60 mmHg from the standard levels of 30
mmHg
during CO2-enhanced imaging.
In one embodiment, carbon dioxide used to induce hyperemia is medical-grade
carbogen
which is an admixture of 95% 02 and 5% CO2. In various other embodiments,
carbon
dioxide is used to induce hyperemia may be an admixture of ranges including
but not limited
to 94% 02 and 6% CO2, 93% 02 and 7% CO2, 92% 02 and 8% CO2, 91% 02 and 9% CO2,
90% 02 and 10% CO2, 85% 02 and 15% CO2, 80% 02 and 20% CO2, 75% 02 and 25% CO2
and/or 70% 02 and 30% CO2.
In another embodiment, vascular reactivity and/or vasodilation are monitored
using any one
or more of positron emission tomography (PET), single photon emission computed
tomography/computed tomography (SPECT), computed tomography (CT), magnetic
resonance imagine (MRI), functional magnetic resonance imaging (fMRI), single
photon
26
13967853.2
CA 02832851 2013-11-08
emission computed tomography/computed tomography (SPECT/CT), positron emission
tomography/computed tomography (PET/CT), ultrasound, electrocardiogram (ECG),
Electron-beam computed tomography (EBCT), echocardiogram (ECHO), electron spin
resonance (SR) and/or any combination of the imaging modalities such as
(PET/MR),
PET/CT, and/or SPECT/MR In an embodiment, vascular reactivity is monitored
using free-
breathing BOLD MRI.
Imaging techniques using carbon dioxide involve a free-breathing approach so
as to permit
entry of CO2 into the subject's system. In an embodiment, the subjects include
mammalian
subjects, including, human, monkey, ape, dog, cat, cow, horse, goat, pig,
rabbit, mouse and
rat. In a preferred embodiment, the subject is human.
ADVANTAGES OF THE INVENTION
.. The methods described herein to functionally assess the oxygen status of
the myocardium
include administering an effective amount of CO2 to the subject in need
thereof. In an
embodiment, the 02 level is held constant while the CO2 level is altered so as
to induce
hyperemia. Applicants herein show the vascular reactivity in subjects in
response to changes
in PaCO2. The existing methods use adenosine, dipyridamole, or regadenoson
which have
significant side-effects described above. As described in the Examples below,
CO2 is at least
just as effective as the existing methods (which use, for example, adenosine)
but without the
side effects.
The use of CO2 provides distinct advantages over the use of, for example,
adenosine,
Administering CO2 is truly non-invasive because it merely involves inhaling
physiologically
sound levels of CO2. The instant methods are simple, repeatable and fast and
most likely
have the best chance for reproducibility. Not even breath-holding is necessary
during
acquisition of images using the methods described herein. The instant methods
are also
highly cost-effective as no pharmacological stress agents are required,
cardiologists may not
need to be present during imaging and rapid imaging reduces scan times and
costs.
27
13967856,2
CA 02832851 2013-11-08
Further, the improved BOLD MRI technique described above provides a non-
invasive and
reliable determination of ischemic volume (no radiation, contrast-media, or
adenosine) and
other value-added imaging biomarkers from the same acquisition (Ejection
Fraction, Wall
Thickening). Additionally, the subject does not experience adenosine-related
adverse side
effects and thus greater patient tolerance for repeat ischemia testing. There
is a significant
cost-savings from abandoning exogenous contrast media and
adenosine/regadenoson.
Moreover, the proposed BOLD MRI paradigm will be accompanied by significant
technical
advances as well: (i) a fast, high-resolution, free-breathing 4D SSFP MRI at
31, that can
impact cardiac MRI in general; (ii) Repeated stimulations of the heart via
precisely targeted
changes in PaCO2; and (iii) adoption of sophisticated analytical methods
employed in the
brain to the heart.
EXAMPLES
All imaging studies were performed in instrumented animals with a Doppler flow
probe
attached to the LAD coronary arteries for measurement of flow changes in
response to CO2
and clinical dose of adenosine. In these studies, CO2 and 02 delivery were
tightly controlled
using Respiract. CO2 values were incremented in steps of 10 mmHg starting from
30 mmHg
to 60 mmHg and were ramped down in decrements of 10 mmHg. At each CO2 level,
free-
breathing and cardiac gated blood-oxygen-level-dependent (BOLD) acquisitions
were
prescribed at mid diastole and Doppler flow velocities were measured. Similar
acquisitions
were also performed with block sequences of CO2 levels; that is, CO2 levels
were alternated
between 40 and 50 mmHg and BOLD images (and corresponding Doppler flow
velocities)
were acquired at each CO2 level to assess the reproducibility of the signal
changes associated
with different CO2 levels. Each delivery of CO2 using Respirgtct were made in
conjunction
with arterial blood draw to determine the arterial blood CO2 levels. Imaging-
based
demonstration of myocardial hyperemic response to changes in PaCO2 was shown
in in
health human volunteers with informed consent.
Example 1
28
13957858.2
CA 02832851 2013-11-08
The inventor has shown that CO2 can increase myocardial perfusion by a similar
amount, as
does adenosine in canine models. The inventor has also shown that in the
setting of coronary
artery narrowing, it is possible to detect regional variations in hyperemic
response with the
use of MRI by detecting signal changes in the myocardium due to chpnges in
oxygen
saturation (also known as the BOLD effect) using a free-breathing BOLD MRI
approach.
As show in Figure 1, the inventor found a 20% BOLD signal increase (hyperemic
response)
with medical-grade carbogen breathing in the absence of stenosis in dogs. With
a severe
stenosis, the hyperemic response was significantly reduced in the LAD (left
anterior
descending) territory but the other regions showed an increase in signal
intensity (as
observed with adenosine). First-pass perfusion images acquired with adenosine
under severe
stenosis (in. the same slice position and trigger time) is also shown for
comparison. Heart
rate increase of around 5-10% and a drop in blood pressure (measured
invasively) by about
5% was also observed in this animal under carbogen. All acquisitions were
navigator gated
T2-prep 2D SSFP (steady-state free precession) and triggered at mid/end
diastole (acquisition
window of 50 ins). To date 10 dogs have been studied with medical-grade
carbogen and have
yielded highly reproducible results.
In detail, the color images (lower panel of figure 1) are color-coded to the
signal intensities
of grayscale images (above). The darker colors (blue/black) represent
territories of baseline
myocardial oxygenation and the brighter regions represent those regions that
are hyperemic.
On average the signal difference between a dark blue (low signal) and orange
color (high
signal) is about 20%. Note that in the absence of stenosis, as one goes from
100% 02 to
Carbogen, the BOLD signal intensity is elevated (second image from left)
suggesting CO2
based vasoreactivity of the myocardium. The dogs were instrumented with an
occluder over
the left-anterior descending (LAD) coronary artery. As the LAD is occluded,
note that the
region indicated by an arrow (i.e. approximately between 11 o'clock and 1-2
o'clock (region
supplied by the LAD)) becomes darker (3rd image from left), suggesting that
vasodilation is
no longer possible or is reduced. The first pass image (obtained with
adenosine stress
following BOLD images) at the same stenosis level also shows this territory
clearly. The
inventor has also been comparing the epicardial flow enhancements in response
to Carbogen
29
13961M2
CA 02832851 2013-11-08
(with ETCO2 reaching 48-50 mm Hg) against clinical dose of adenosine and the
responses
have been quite similar (-20% response).
Example 2
Co-relation between inhaled CO2 and oxygen saturation
Applicants assessed the difference between myocardial blood-oxygen-level
dependent
(BOLD) response under hypercarbia and nobnocarbia conditions in canines. The
BOLD
signal intensity is proportional to oxygen saturation.
Top panels of Figure 2 depict the myocardial response under hypercarbia (60mm
Hg) and
normocarbia (30minHg) conditions and show an increase in BOLD signal intensity
under
hypercarbia condition. The lower panel depicts the difference as obtained by
subtracting the
signal under rest from that under stress. The myocardial BOLD signal
difference between
the two is depicted in grey and shows the responsiveness of canines to
hypercarbia
conditions.
Applicants further assessed the myocardial BOLD response to stepwise CO2
increase (ramp-
up) in canines. As shown in Figure 3, as the amount of CO2 administered
increases, the
BOLD signal intensity increases which is indicative of an increase in
hyperemic response to
increased uptake of CO2 and oxygen saturation.
To further evaluate vascular reactivity and coronary response to CO2,
Applicants measured
the myocardial BOLD signal in response to block increases of CO2 in canines.
Specifically,
the myocardial BOLL) signal was measured as the amount of CO2 administered to
the canine
subjects alternated between 40mm1Hg CO2 and 50mmHg CO2. As shown in Figure 4,
an
increase in CO2 level from 40mmHg CO2 to 50nnnHg CO2 resulted in an increase
in BOLD
signal intensity and the subsequent decrease in CO2 level to 40mmilg resulted
in a decreased
BOLD signal. These results show a tight co-relation between administration of
CO2 and
vascular reactivity and coronary response.
13967858.2
CA 02832851 2013-11-08
Example 3
Co-relation between the amount of CO2 inhaled and Doppler flow
Doppler flow, an ultrasound-based approach which uses sound waves to measure
blood flow,
was used to show that administration of CO2 leads to vasodilation which
results in greater
blood flow, while Pa02 is held constant. The Doppler flow was measured in the
left anterior
descending (LAD) artery. As shown in Figure 5, as the amount of administered
CO2
increases the Doppler flow increases. The relative change in coronary flow
velocity is
directly proportional to the amount of CO2 administered.
Example 4
Each of the arteries which supply blood to the myocardium responds to the CO2
levels
The myocardium is supplied with blood by the left anterior descending (LAD)
artery, the
right coronary artery (RCA) and the left circumflex (LCX) artery. Applicants
measured the
blood flow through each of these arteries in response to increasing CO2
supply. As shown in
Figure 6 and summarized in Figure 7, in each of the three LAD, RCA and LCX
arteries, there
is a direct correlation between the amount of CO2 administered and the signal
intensity; as
the amount of administered CO2 increases, the signal intensity, signaling the
blood flow, in
each of the three arteries increases. Further, as shown in Figure 6 and
summarized in Figure
8, there is no response to CO2 modulation in control territories such as
blood, skeletal muscle
or air. As shown in Figure 9, the mean hyperemic response is approximately
16%.
Example 5
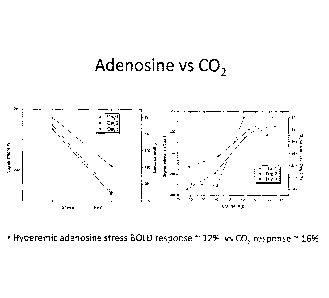
Vascular reactivity to CO2 comparable to adenosine
Vascular reactivity of three canines that were administered with adenosine was
compared
31
1.39678!2,2
with the vascular reactivity of canines that were administered with CO2. As
shown in Figure
10, the hyperemic adenosine stress BOLD response is approximately 12% compared
with
16% in response to CO2.
Further, as shown in Figure 11, early human data shows a hyperemic response of
approximately 11% for a partial pressure CO2 (pCO2) change of lOnm-.Mg, from
35mmHg to
45mmHg.
Example 6
To derive a theoretical understanding of how repeated measurements may affect
the BOLD
signal response, for a given BOLD response to PaCO2. Applicants performed
numerical
simulations of statistical fits assuming various peak hyperemic BOLD responses
to two
different PaCO2 levels (as in Fig.12a) along with known variability in BOLD
signals. The
results (Fig. 12b) showed that as the BOLD response decreases, the number of
measurements
required to establish statistical significance (p<0.05) associated with the
BOLD response
increases exponentially. This model provides the basis for developing a
statistical framework
for identifying ischemie volume on the basis of repeated measures.
Various embodiments of the invention are described above in the Detailed
Description.
While these descriptions directly describe the above embodiments, it is
understood that those
skilled in the art may conceive modifications and/or variations to the
specific embodiments
shown and described herein. Any such modifications or variations that fall
within the
purview of this description are intended to be included therein as well.
Unless specifically
noted, it is the intention of the inventors that the waft and phrases in the
specification
be given the ordinary and accustomed meanings to those of ordinary skill in
the
applicable= art(s).
The foregoing description of various embodiments of the invention known to the
applicant at
this time of filing the application has been presented and is intended for the
purposes of
illustration and description. The present description is not intended to be
exhaustive nor limit
32
Date Recue/Date Received 2021-02-26
CA 02832851 2013-11-08
the invention to the precise form disclosed and many modifications and
variations are
possible in the light of the above teachings. The embodiments described serve
to explain the
principles of the invention and its practical application and to enable others
skilled in the art
to utilize the invention in various embodiments and with various modifications
as are suited
to the particular use contemplated. Therefore, it is intended that the
invention not be limited
to the particular embodiments disclosed for carrying out the invention.
While particular embodiments of the present invention have been shown and
described, it
will be obvious to those skilled in the art that, based upon the teachings
herein, changes and
modifications may be made without departing from this invention and its
broader aspects. It
will be understood by those within the art that, in general, terms used herein
are generally
intended as "open" terms (e.g., the term "including" should be interpreted as
"including but
not limited to," the term "having" should be interpreted as "having at least,"
the term
"includes" should be interpreted as "includes but is not limited to," etc.).
33
13967858.2