Note: Descriptions are shown in the official language in which they were submitted.
CA 02833318 2013-10-16
=
Polychloroprene solid haying thixotropic properties
The invention relates to solid polychloroprene, to processes for obtaining and
isolating it,
and to the use thereof for production of rubber vulcanisates.
Polychloroprene production has been known for some time. By free-radical
emulsion
polymerization of chloroprene (2-chloro-1,3-butadiene), latices of
polychloroprene are
produced. Such latices are also referred to in the context of this application
as
"polychloroprene latices" or "polychloroprene dispersions".
In the production, the monomers are admixed in an emulsifier system in an
aqueous medium.
This emulsifier system is generally anionic in nature; in rare cases, nonionic
or cationic
systems are also used. The temperature range in which the polymerization is
performed
encompasses values from approx. 0 C to more than 80 C. Thus, the
polymerization can be
initiated by thermal free-radical initiators or by redox systems. In general,
molecular weight
regulators such as mercaptans or xanthogen disulphides are also used. In some
cases, the
molecular weight of the end product is also adjusted by copolymerization with
sulphur and
subsequent cleavage of the sulphidic bonds formed. The desired conversion is
established by
stopping the reaction with a suitable reagent.
Polychloroprene polymers are characterized by three essential criteria, namely
the
crystallization tendency, the polymer viscosity and, inter alia, the degree of
prior
crosslinking.
The prior art discloses polychloroprene polymers which have very low, low,
moderately high
and particularly high crystallization tendencies. Those with a particularly
high crystallization
tendency are used exclusively for application in the adhesives sector. The
others with a
lower crystallization tendency are employed in the manufacture of industrial
rubber
products, fabric rubberization, cables, hoses, moulded and injection-moulded
articles, and
foam rubber profiles.
For processing, the crystallization phenomena therefore play a very important
role.
Crystallization is understood to mean an increase in hardness as a function of
storage time,
the occurrence of which is enhanced particularly at low temperatures. The
hardening is a
reversible process and can be reversed as often as desired by heating or
dynamic stress on
the crystallized material.
The crystallization tendency can be adjusted through the choice of
polymerization
temperature during the polymerization. At polymerization temperatures of less
than 20 C,
CA 02833318 2013-10-16
- 2 -
polychloroprene polymers with high crystallization tendency are produced,
which are
particularly suitable for adhesives application. In the case of a
polymerization temperature
above 30 C, polychloroprene polymers which have a low crystallization tendency
and are
suitable for vulcanisates or rubber products are obtained.
In addition, the use of comonomers is also suitable for influencing the
crystallization
tendency of polychloroprene.
In the vast majority of cases, the dispersion of polychloroprene in water thus
obtained is
subsequently demonomerized by passing water vapour through. A portion of the
product
obtained finds direct industrial use as latex, but the majority is freed of
adhering water by
coagulation and sent to its final use as a solid product.
Solid polychloroprene (CR solid) and vulcanisates produced therefrom are
notable, given
appropriate blend formation, for high weathering and ozone stablility, for
flame retardancy,
very good ageing properties, moderate oil stability, and for considerable
resistance to many
chemicals. They have good mechanical properties, good elastic characteristics
and a high
wear resistance.
Vulcanisates formed from polychloroprene latices (CR latices) have values very
similar to
those of natural latex vulcanisates with regard to elasticity, tensile
strength, elongation at
break and modulus, and at the same time also display good solvent, chemical,
oil and fat
stability.
As mentioned above, the removal of the polychloroprene solids from the
dispersion is
typically accomplished by coagulation. For this purpose, a number of different
processes are
known. By mixing the polychloroprene latices with a coagulant, the emulsion is
broken. For
this purpose, it is possible to use any standard coagulant. For example, the
solids can be
coagulated out of CR latices which have been produced under alkaline
conditions by
acidification, for example with a mineral acid or an organic acid. In many
cases, mere
acidification is insufficient for complete coagulation of the polychloroprene,
and so strong
electrolytes (salts containing polyvalent cations such as Mg2', Ca2+ or AP+)
additionally have
to be added to the acid.
A disadvantage in this method is the large amount of acid or electrolytes to
achieve complete
precipitation of the solids. At the same time, relatively large amounts of
precipitant remain in
the product, which can lead to a deterioration in important product
properties. Therefore, the
coagulated solid is washed with relatively large amounts of water to remove
the precipitant,
which leads to economic and ecological problems. The polychloroprene is in
some cases
CA 02833318 2013-10-16
- 3 -
obtained in the form of large lumps, which in their interior contain either
still unprecipitated
CR latex or excess precipitant.
It is also known from the prior art that the coagulation can be enabled by the
action of higher
temperatures and/or elevated pressures, and by additional action of
electrolytes and shear
forces. Such a product is subjected to considerable thermal stress, which
leads to a
deterioration in the product properties.
Typically, polychloroprene is removed from aqueous dispersions by freezing.
This involves
cooling below the freezing point of the aqueous phase to freeze the CR latex.
In the course of
subsequent thawing under suitable conditions, the polychloroprene is present
as a coagulate
and can be separated from the aqueous phase.
In order to arrive at coagulation rates sufficiently high for industrial
purposes, the CR latex is
frozen in thin layers. For this purpose, coagulation rollers coolable from the
inside have been
developed, which are immersed into the CR latex while rotating and take on a
thin latex
layer as they rotate, which freezes on the surface (US-B 2,187,146). The thin
film of CR
coagulate and ice is removed from the roller with a scraper and passed on.
The prior art discloses further isolation processes. US 4,103,074 describes a
process for
coagulating a polymer latex using a screw extruder, wherein the polymer latex
is coagulated
during conveying in the screw channel.
US 3,926,877 describes a process for isolating a CR rubber, wherein the CR
latex is mixed
with an aqueous carbon black dispersion before being admixed with a coagulant.
The
coagulated product is separated from the aqueous phase.
DE 30 31 088 C2 discloses a process for producing a coagulated latex of a
synthetic
polymer, wherein a gaseous or liquid coagulant is applied to the polymer latex
droplets in the
form of a mist by means of a spray nozzle, and so polymer beads are
precipitated.
Isolated polychloroprene solids are stored intermediately for use as CR
vulcanisates. Even
though they have excellent long-term ageing stability, they do not have
unlimited stability
and storability. During storage, over the course of time, a change occurs in
the polymer
properties, more particularly an increase in flowability, which leads to a
considerable
impairment of processability. This impairment of processability is manifested,
more
particularly, in poorer kneadability, spreadability and sprayability of the
polychloroprenes,
and in less favourable characteristics in machine processing.
CA 02833318 2013-10-16
- 4 -
To avoid these disadvantages, the solid polychloroprene is masticated to
obtain low-viscosity
polychloroprene types as solid rubbers. As is well known, the mastication of
synthetic
rubbers does not proceed as easily as that of natural rubber, especially when
the rubbers have
electron-withdrawing substituents such as Zn, Cl. The prior art discloses
methods for
thermooxidative CR degradation. These involve using, for example,
dialkylxanthogen
disulphides as molecular weight regulators.
Low-viscosity CR types produced in such a way have the disadvantage of having
very high
amounts of regulator. Also known is thermooxidative degradation by means of
shear stress
by the use of extruders, by lowering the molecular weight. This so-called
mastication is very
time-consuming and can cause high processing costs under some circumstances.
The prior art includes numerous attempts to influence the product properties
of
polychloroprene. For example, addition of sulphur-containing organic chain
transfer agents,
for example mercaptans, controls the molecular weight of the polymer formed.
Also known
is the preparation of high-viscosity chloroprene polymers by adding the chain
transferer in
portions during the polymerization. The number and magnitude of the regulator
additions
are, however, dependent on the polymerization temperature, the degree of
conversion and
the desired polymer viscosity. The additions of regulators additionally have
to be made at
particular monomer conversions.
It is an object of the invention to provide solid polychloroprene which has
flow
characteristics which bring advantages for processability, and a process for
obtaining and
isolating it.
This object is achieved by provision of solid polychloroprene based on
polychloroprene
dispersion having thixotropic properties.
Thixotropy is generally understood to mean a change in viscosity as a function
of time at
constant shear. These effects are generally caused by superstructures which
change over the
measurement time. One observation in the case of the present invention is the
propensity of
liquid substances to be converted temporarily to a state of lower viscosity at
constant
temperature by mechanical action/shearing (for example stirring, shaking or
kneading). This
propensity depends on the duration of the mechanical action.
Surprisingly, an inventive solution of solid polychloroprene pretreated by
shearing has a
decrease in structural order and, at the same time, an increase in structural
order at rest. This
property has the advantage that the CR solids have excellent miscibility,
kneadability and
processability with other additives.
CA 02833318 2013-10-16
- 5 -
Preferably, the change in viscosity occurs over the course of time. It is thus
possible for the
processing characteristics of various CR types to be adjusted to the
requirements of the
rubber processing industry.
The thixotropic property is preferably determined by means of the Brookfield
viscosity
method. To determine the thixotropic property, the inventive solid
polychloroprene is
brought into solution, preferably in organic solvents, for example in benzene,
toluene,
cyclohexane. Then the solution of solid polychloroprene is subjected to
pretreatment by
shearing by means of a propeller stirrer, and the viscosity is measured
against time.
A further property of the inventive solid polychloroprene is the change in the
melt viscosity
after a pretreatment on a homogenization roller. The melt viscosity (Mooney
1+4 at 100 C,
ASTM D 1646) preferably decreases as a function of the frequency of
pretreatment on a
homogenization roller. It has also been found that the melt viscosity degrades
only slowly at
about 20 cycles on the homogenization roller, which is attributable to the
reduction in chain
length.
A homogenization roller is understood to mean a roller which is not suitable
for mastication
of a rubber. The homogenization roller differs from a mastication roller in
the lower specific
energy input thereof. A high energy input as is the case for the mastication
roller causes
destruction of the polymer chain and hence lowering of the melt viscosity.
It has been found that the inventive solid polychloroprene is preferably
obtained from a
polychloroprene dispersion which has been produced by means of an emulsion
polymerization at a polymerization temperature greater than 30 C, preferably
between 35 C
and 50 C.
The invention further relates to a process for isolating and obtaining
polychloroprene solids,
wherein an aqueous polychloroprene dispersion is contacted with water vapour
comprising
coagulant, such that the inventive solid polychloroprene coagulates.
It has been found that, surprisingly, the product property of the solid
polychloroprene has
been influenced and altered by the process according to the invention, even
though the
polychloroprene dispersion has been produced by conventional processes.
It preferably coagulates in strand form or in the form of crumbs.
The precipitated solid polychloroprene is subsequently separated from the
coagulation
suspension and then preferably dewatered in a dewatering apparatus. For
example, it is
CA 02833318 2013-10-16
- 6 -
possible here to use a Seiher screw or dewatering rollers. Other known
dewatering apparatus
is likewise conceivable.
Subsequently, the dewatered solid polychloroprene is dried by means of a
drying apparatus.
The drying apparatus is, for example, a twin-shaft extruder, a drying screw or
a drying
kneader. It is possible with preference to add additives and/or inerts in the
drying apparatus.
It is thus possible to optimally influence the product properties of the
inventive solid
polychloroprene for any requirement. Additives for influencing the product
properties are
preferably, for example, stabilizers, accelerators, emulsifiers, alkalis,
ageing stabilizers,
viscosity-influencing processing aids. It is possible to use all conventional
additives.
Inerts are, for example, nitrogen, argon, carbon dioxide, which are added to
influence
the polymer melting temperatures.
The inventive solid polychloroprene is preferably pelletized by means of
underwater
pelletization and cooled.
The polychloroprene dispersion is preferably a latex which has been produced
by means of
emulsion polymerization. The polymerization is effected at polymerization
temperature
greater than 30 C, preferably between 35 C and 50 C, more preferably between
35 C and
45 C, the polymerization conversion being between 50% and 80%. Excess monomer
is
removed by means of vacuum devolatilization to a range from 1000 ppm to 1 ppm.
Emulsion
polymerization processes are known from the prior art and can be used here.
For the polymerization, it is also possible with preference to add various
comonomers, for
instance 2,3-dichlorobutadiene, to chloroprene (2-chloro-1,3-butadiene) to
control the
crystallization.
The polychloroprene dispersion preferably has a solids content between 20 ¨
45% by weight
and a gel content between 0 ¨ 10% by weight. However, the gel content can also
be
increased in a controlled manner.
The water vapour comprising coagulant is formed by means of water vapour and
an aqueous
coagulant solution. The coagulant solution used is preferably an aqueous
solution of a
coagulant composed of inorganic salts, preferably of metals of the second and
third main
groups of the Periodic Table.
The coagulant used is preferably calcium chloride, magnesium chloride,
magnesium
sulphate, aluminium chloride and/or aluminium sulphate.
CA 02833318 2013-10-16
- 7 -
Preferably, the coagulant solution has a coagulant concentration between 1% by
weight and
60% by weight, preferably between 2% by weight and 55% by weight, more
preferably
between 10% by weight and 35% by weight, based on the coagulant solution.
Preference is given to diluting the polychloroprene dispersion prior to
contact with the water
vapour comprising coagulant.
In this case, the polychloroprene dispersion is preferably diluted to a solids
content of 38%
by weight to 45% by weight, preferably of 28% by weight to 35% by weight and
more
preferably of 20% by weight to 28% by weight, based on the polychloroprene
dispersion.
For the dilution, preference is given to using water, more preferably
demineralised water.
The dilution is important in that not only is the conglutination and blocking
of the
flow/coagulation apparatus prevented or reduced, but in that it is also
possible to ensure
optical coagulation, caused by the contact between the CR dispersion and the
water vapour
comprising coagulant.
Particular preference is given to using 80 to 1000 kg of water vapour per
tonne of solids of
the polychloroprene dispersion, preferably 80 to 300 kg of water vapour per
tonne of solids
of the polychloroprene dispersion.
In addition, 10 to 40 kg of coagulant are used per tonne of solids of the
polychloroprene
dispersion, preferably 10 to 25 kg of coagulant per tonne of solids of the
polychloroprene
dispersion.
For the coagulation, the aqueous polychloroprene dispersion is added in a
flow/coagulation
apparatus, said flow/coagulation apparatus having recesses through which the
water vapour
comprising coagulant can pass and encounters the polychloroprene dispersion in
the
flow/coagulation apparatus. This coagulates the inventive solid
polychloroprene.
The solid polychloroprene is preferably dewatered in the dewatering apparatus
down to a
residual moisture content of 10% by weight to 15% by weight, preferably 1.0%
by weight to
9% by weight, based on the solid polychloroprene.
In the drying apparatus, the dewatered solid polychloroprene is preferably
dried down to a
residual moisture content of 1% by weight to 1.5% by weight, preferably 0.5%
by weight to
1% by weight, more preferably 0.1% by weight to 0.5% by weight, based on the
dewatered
solid polychloroprene.
CA 02833318 2013-10-16
- 8 -
At the end of the drying phase in the drying apparatus, the solid
polychloroprene is present
as a rubber melt. The melt passes through a head plate and is processed with a
cutting
apparatus and cooled and transported in the underwater pelletization by water.
Preference is given to adding a separating agent to the water in the
underwater pelletization.
Examples of useful separating agents here include talc, metal stearates. Other
conventional
separating agents are likewise conceivable.
A further invention is the use of the inventive solid polychloroprene for
production of
vulcanisates. The invention likewise provides the vulcanisates comprising the
inventive solid
polychloroprene.
The invention is illustrated in detail hereinafter by examples and a drawing:
Examples
Production of a polychloroprene dispersion
A polychloroprene dispersion is produced using the base formulation below
(figures are in
parts by weight per 100 parts by weight of chloroprene used):
125 parts by wt. of water
100 parts by wt. of monomers
(2-chloro-1,3-butadiene or a mixture of 2-chloro-1,3-butadiene and 2,3-
dichlorobutadiene)
3 parts by wt. of sodium salt of disproportionated abietic acid
0.5 part by wt. of potassium hydroxide
0.2 part by wt. of n-dodecyl mercaptan
0.5 part by wt. of sodium salt of formaldehyde-condensed naphthalenesulphonic
acid
The polychloroprene dispersion is produced by free-radical emulsion
polymerization between 40 C
and 45 C from the above components by customary methods (e.g. Ullmanns
Encyclopaedia of
Industrial Chemistry, Vol 23A, p. 252-262). The polymerization is stopped at a
conversion between
50% and 70% and the dispersion is freed of residual monomers by vacuum
devolatilization.
Process for isolation and recovery of an inventive solid polychloroprene
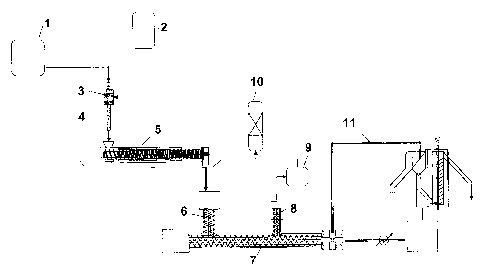
Fig. 1 shows a schematic structure of a process according to the invention.
CA 02833318 2013-10-16
- 9 -
The abovementioned polychloroprene dispersion is conveyed from a tank 1 into a
flow/coagulation
apparatus 3. Prior to introduction into the flow/coagulation apparatus 3, the
polychloroprene
dispersion can be diluted with water.
From a further tank 2, the aqueous coagulant, which has been mixed with water
vapour beforehand,
is supplied to the flow/coagulation apparatus 3 and contacted with the
polychloroprene dispersion
via the recesses therein. In the course of this, the polychloroprene
dispersion is quantitatively
precipitated in the flow/coagulation apparatus 3 and in the downstream
precipitation tube 4.
The precipitation tube 4 opens into the intake region of the dewatering
apparatus 5, wherein the
inventive precipitated solid polychloroprene is dewatered.
The dewatered solid polychloroprene is supplied to the drying apparatus 7
either as a strand or in
the form of crumbs and dried. In order to influence the product properties of
the inventive solid
polychloroprene, additives or inerts can be metered in in the feed screw 6 or
in the downstream
region of the drying apparatus 7.
Via domes 8 under reduced pressure, the vapours are drawn off and the
retention of rubber particles
is ensured with stuffing screws in the domes 8. Beyond the domes 8 are
separators 9 in which
entrained rubber particles are separated out and subsequently supplied to a
waste air scrubber 10.
The hot rubber melt from the drying apparatus 7 is cut into chips in the
underwater pelletization via
a head plate and cutting blades. The chips are cooled and transported by means
of a water stream
11 which may optionally be admixed with additives (e.g. separating agents).
The chips are first separated from the water by means of a sieving chute. The
residual energy of the
chips evaporates the water adhering on the surface. In addition, a warm air
stream can promote the
removal of the adhering water.
The inventive solid polychloroprene thus obtained is used for the further
property determinations.
Thixotropic property by means of the Brookfield viscosity method
For the determination of the thixotropic property, the inventive solid
polychloroprene and a
comparative example are dissolved in toluene.
The comparative example used is a solid polychloroprene which has been
obtained from the
abovementioned polychloroprene dispersion by means of a conventional freeze
coagulation with
subsequent drying in a nozzle belt dryer.
CA 02833318 2013-10-16
=
- 10 -
8.6 g of solid polychloroprene in each case were weighed with 91.4 g of
toluene into an Erlenmeyer
flask and stirred at room temperature by means of a magnetic stirrer bar until
complete solubility of the
solid polychloroprene.
For introduction of shear, both toluenic polychloroprene solutions are
pretreated by rapid stirring with
a propeller stirrer at 500 rpm for about 1 minute.
The viscosity is measured by means of a rotary viscometer of the Brookfield DV-
II+ brand
=
at 60 rpm, spindle 2 at 25 C.
Tab. 1: Viscosity profile after shear
Measurement time/mitt Viscosity /mPas
00:00 170
00:01 178
00:02 182
00:07 186
00:22 188
The viscosity profile indicated in Table 1 is found after the above-described
pretreatment with
subsequent viscosity measurement.
Tab. 2: Viscosity profile after storage
Measurement time/min Viscosity /mPas
00:00 230
00:01 202
00:02 198
00:05 193
00:10 191
The viscosity profile indicated in Table 2 is found after storage of the
polymer solution described in
Table 1 at 25 C without shear for 30 minutes.
The polymer solution produced with the inventive solid polychloroprene has
thixotropic behaviour.
The viscosity thereof is shear time-dependent and approaches an equilibrium in
an asymptotic manner
at constant shear. From the state of rest, the viscosity in a measurement
accordingly falls down to an
equilibrium. If additional shear energy is introduced into this solution by
means of the abovementioned
propeller stirrer and the viscosity is measured immediately, opposite
behaviour is observed; the
viscosity rises towards the same equilibrium viscosity.
CA 02833318 2013-10-16
- 11 -
A polymer solution produced with the comparative example does not exhibit this
behaviour. The
polymer solution has structurally viscous but not thixotropic behaviour. The
measurement after
storage and pretreatment always gives a constant value of 104 mPas for the
same measurement
time.
Melt viscosity profile after pretreatment on a homogenization roller
For the determination, the abovementioned inventive example and the
abovementioned comparative
example were compared. Shear was introduced at 25 C in a laboratory roller,
which has a roller width
of 30 cm and a roller gap of 1 mm and a rotation of 10 rpm/10 rpm (front/back
roller).
About 200 g of solid polychloroprene was used in each case. A Mooney viscosity
was measured to
ASTM D 1646 (Mooney 1+4 at 100 C).
Table 3: Melt viscosity profile
Type / pretreatment Inventive example
Comparative example
ML1+4 /ME ML1+4 /ME
Inventive solid polychloroprene
0 value
56 51
Inventive solid polychloroprene
10 cycles in roller
48 50
Inventive solid polychloroprene
cycles in roller 46 51
15 It has been found that, surprisingly, the inventive CR solid exhibits a
superstructure, i.e. the melt
viscosity (Mooney 1+4 at 100 C) depends on the pretreament of the polymer.
While the comparative
example, in spite of a pretreatment, has a constant viscosity, the viscosity
declines for the inventive
solid polychloroprene. The decrease is asymptotic towards an equilibrium
value.
Ageing properties of vulcanisates
20 It has been found that there is a change in the ageing properties of the
vulcanisates which have been
produced with the inventive solid polychloroprene, and that the hardening
thereof is reduced in the
course of storage under standard atmosphere at 100 C for 7 days.
For the measurement of the properties, the following blend formulation was
used:
CA 02833318 2013-10-16
- 12 -
Table 4: Blend formulation for production of the vulcanisates
Batch name: parts/phr
Polymer 100
Carbon black N772 30
Stearic acid 0.5
MgO 4
ETU 0.4
ZnO 5
The polymers used are the inventive solid polychloroprene and the comparative
example as described
above.
The carbon black used was a Cabot carbon black Regal SRF N772.
As a sulphur donor, ethylenethiourea from Rheinchemie "Rhenogran ETU-80".
"phr" means parts per hundred of rubber.
The blend was produced in a "Standard Internal Mixer" to ASTM D3182. The
vulcanization was
performed at 160 C for a period of 30 minutes. The specimens were produced to
ASTM D 3182.
Tensile strain tests were conducted before and after heat ageing at 100 C for
7 days (DIN 53508) on
an S2 tensile specimen (to DIN 53504) at room temperature.
The hardness was measured to DIN 53505 as the Shore A hardness at room
temperature.
Table 5: Initial values of stress-strain and hardness
Type and ageing temp.
for 7 d Comparative example Inventive example
Stress S10 MPa 0.5 0.5
Stress S25 MPa 0.9 1
Stress S 50 MPa 1.4 1.6
Stress S 100 MPa 2.4 2.7
Stress S 300 MPa 15.0 16.8
Elongation at break 370 377
Tensile strength MPa 20 23
Hardness Sh A 59 61
As evident from Table 5, the vulcanisates produced from the conventionally
produced solid
polychloroprene (comparative example) and from the inventive solid
polychloroprene exhibit virtually
the same vulcanisate properties in terms of tensile strain. They also exhibit
similar Shore hardness.
CA 02833318 2013-10-16
- 13 -
Table 6: Vulcanization properties after ageing at 100 C under standard
atmosphere for
7 days
Type and ageing temp. Comparative Inventive
for 7 d example 100 C example 100 C
Change in S10 80 60
Change in S25 100 50
Change in S 50 121 67
Change in S 100 154 77
Change in S 300
Change in elongation at
break -48 -37
Change in tensile
strength -27 -29
Change in ShA hardness Sh A 12 9
Table 6 describes the tensile strain properties of an S2 tensile specimen of
the comparative example
which has been stored under standard atmosphere at 100 C for 7 days. Various
ageing processes
result in hardening of the vulcanisate test specimen of the comparative
example, illustrated by the rise
in the stress values for a given strain and by the rise in the Shore hardness.
A lower degree of hardening is clearly evident in the vulcanisate test
specimen produced with the
inventive solid polychloroprene. This leads either to a higher sustained
operation temperature of the
vulcanisate or, at the same temperature, to an increased service life and
hence to a distinct
improvement in the vulcanisate.