Note: Descriptions are shown in the official language in which they were submitted.
CA 02833915 2013-10-22
OXIDATIVE DEHYDROGENATION OF METHANOL TO FORMALDEHYDE OVER SILVER-
CONTAINING KNITS
The present invention relates to a process for producing Cl-Cio aldehydes by
oxidative
dehydrogenation of C1-C10 alcohols over a shaped catalytst body which
comprises shaped silver-
containing fibers and/or threads, wherein the average diameter of these silver-
containing fibers
and/or threads is in the range from 30 pm to 200 pm.
The process for producing formaldehyde by oxidation/dehydrogenation of
methanol over a silver
catalyst is long known, see for example Ullmann's Encyclopedia of Industrial
Chemistry, 2005,
pages.1 if.
The silver catalyst used can be used in various forms. For example as granular
crystalline silver
but also in the form of silver nets or silver gauze.
US 4,076,754 (Du Pont) describes a two-stage process for the manufacture of
formaldehyde from
methanol, air and water. The catalyst used comprises 40 superposed sheets of
20 mesh silver
gauze (i.e., mesh size 1.25 mm) made from silver wire 0.014 inch (i.e., 350 pm
or 0.35 mm) in
diameter. The density or the void fraction of the silver gauze is not
disclosed in US 4,076,754.
DE 2829035 Al (Heraeus) describes a catalyst comprising catalytically active
metallic fibers which
consist of silver, platinum, rhodium, palladium or an alloy based on one
thereof, wherein the
metallic fibers are interconnected feltlike in the manner of a needle-bonded
composite. The
catalyst can be used for ammonia oxidation and the manufacture of hydrocyanic
acid or
formaldehyde. The cross section of a ribbon-shaped fiber can be rectangular
with the dimensions
of 100 pm and 50 pm, the length can be between 10 cm and 1 m.
The density or the void fraction of the interfelted metallic fiber body is not
described.
DE 3047193 Al (Johnson Matthey) describes a catalyst made of silver or a
silver alloy. The
catalyst body is produced by melt spun process or melt extraction process. For
example, a ribbon
= CA 02833915 2013-10-22
2
1 to 2 mm in width and 50 to 60 pm in thickness is processed by crimping and
cutting to yield
undulating catalyst bodies about 1 cm in length and hence rather short-
fibered.
DE 3047193 Al does not disclose a braid, knit, felt or the like formed from
these catalyst bodies.
Although the catalytic oxidation/dehydrogenation of alcohols to aldehydes,
more particularly
methanol to formaldehyde, is already long known, there is still room for
improvement, for example
an increase in catalytic activity, in formaldehyde selectivity, advantageously
for unchanged
catalytic activity, or in the pressure drop over the catalyst.
Applicant studies have shown, particularly with regard to the oxidative
dehydrogenation of
methanol to formaldehyde, that it is not a straightforward matter to use fiber-
or wire-containing
structures as catalysts. This is because the constitution of the shaped
catalyst body has to make it
possible to establish a stable ignited reaction zone under the operating
conditions of the industrial
process for example of the oxidative dehydrogenation of methanol to
formaldehyde. However, the
current state of the art does not disclose the requisite features of such a
shaped catalyst body.
The problem addressed by the present invention was that of improving the
aldehyde yield of the
oxidative dehydrogenation of alcohols to aldehydes, more particularly methanol
to formaldehyde,
by using a shaped catalyst body comprising shaped silver-containing fibers
and/or threads.
Useful C1-C10 alcohols include alcohols having 1 to 10 carbon atoms and one or
more, preferably
two to three, OH groups. The alcohols preferably have one or two OH groups.
The alcohols may
be aliphatic, linear, branched or cyclic, comprise one or more C-C double or
triple bonds in the
molecule, they may be aralyk or alkylaryl alcohols. They are preferably
primary alcohols or in the
case of polyhydric alcohols vicinal Ci-Cio diols.
Examples of the 01-010 alcohols mentioned are methanol, ethanol, 1-propanol,
isopropanol, n-
butanol, isobutanol, sec-butanol, tert-butanol, 1,2-ethanediol, 1,2-
propanediol, allyl alcohol,
prenol, isoprenol. Methanol is particularly preferred.
Useful C1-C10 aldehydes include the aldehydes obtainable from the
abovementioned Ci-Cio
alcohols by oxidative dehydrogenation. The aldehydes may have one or more
aldehyde groups in
CA 02833915 2013-10-22
3
the molecule, preferably they have one or two aldehyde groups in the molecule.
Examples of C1-
C10 aldehydes that are in accordance with the present invention are
formaldehyde (methanal),
glyoxal (HCO-CH0), prenal or isoprenal.
In a particularly preferred embodiment, the process of the present invention
is used for producing
formaldehyde (methanal) from methanol, as described in what follows. However,
this methanol
oxidation process can also be carried out in analogous form for the
abovementioned Cl-Clo
aldehydes.
Suitable starting materials for the methanol oxidation process are pure
methanol, technical grade
methanol, crude methanol produced by high or low pressure process, or
advantageously mixtures
thereof with water; the methanol concentration of the aqueous mixtures in the
starting material is
advantageously in the range from 60% to 95% by weight and preferably in the
range from 70% to
90% by weight. An advantageous embodiment uses crude methanol purified
according to the
processes described in DE-B-12 77834, DE-C-12 35 881 and DE-C11 36 318 by
removal of a
lower-boiling fraction or, respectively, by treatment with oxidizing agents
and/or alkalis.
The methanol is fed to the reactor space in vapor form, advantageously
admixture with water
vapor and optionally with an inert gas. Nitrogen is useful as an inert gas for
the process for
example.
The oxidizing agent used can be not only pure oxygen but also preferably
oxygen-containing
gases, more particularly air. Oxygen and methanol are advantageously used in a
molar ratio of
0.25 to 0.6 and more particularly 0.35 to 0.5 mol of oxygen per mol of
methanol. The total amount
of water vapor is preferably not more than 3.0, and advantageously 0.67 to
1.75 mol per mol of
methanol.
In the industrial operation of the formaldehyde process, the reaction mixture
described above is
generally introduced into the reactor at a temperature between 50 C and 200 C
and also typically
at an absolute pressure between 1 bar and 2 bar.
CA 02833915 2013-10-22
=
4
The starting materials mentioned are then typically passed into one or more
zones wherein the
shaped catalyst body of the present invention is situated.
The shaped catalyst body of the present invention is a three-dimensional
construct obtainable by
three-dimensional shaping and/or arranging in space of silver-containing
fibers or silver-containing
threads.
The silver-containing fibers or threads comprise silver in an amount ranging
from 50% by weight
to 100% by weight, preferably from 90% by weight to 100% by weight and more
preferably from
98% by weight to 100% by weight, and further metals of the 10th or 11th group
of the periodic
table, preferably metals selected from the group consisting of copper,
palladium, titanium in the
range from 0% by weight to 50% by weight, preferably from 0% by weight to 10%
by weight and
more preferably from 0% by weight to 2% by weight.
Highly suitable silver-containing fibers or threads comprise virtually 100% by
weight of silver.
Silver-containing fibers in accordance with the present invention are
generally from about 1 mm to
100 mm in length, while silver-containing threads which are in accordance with
the present
invention can be infinite in theory while in practice they generally have a
length in the range from a
few centimeters up to several kilometers.
The average diameter (in the case of an essentially round cross section) or
the average diagonal
length (in the case of an essentially rectangular or square cross section) of
the silver-containing
fibers or threads is in the range from 30 to 200 pm, preferably in the range
from 30 to 150 pm and
more preferably in the range from 30 to 70 pm.
The average diameter or the average diagonal length is determined using the
method of DIN ISO
4782 "Nominal Wire Diameters for Woven Screens".
Silver-containing fibers or threads are known to a person skilled in the art,
are commercially
available and are used for example as an electrical conductor material, in
high value textiles or in
corrosion-resistant sensory applications (pH determination for example).
CA 02833915 2013-10-22
The three-dimensional shaping and/or arranging of the silver-containing fibers
or threads in space
can be effected with or without order.
Shaping and/or arranging the silver-containing fibers which are in accordance
with the present
5 invention or preferably silver-containing threads which are in accordance
with the present
invention without order typically leads to so-called clews. They are
obtainable for example by
fibers or wires to form a statistically nonuniformly arranged clew and then
are further compressed,
using various pressures, to the desired clew density or the desired void
fraction in the clew.
In such clews, the silver-containing fibers or threads which are in accordance
with the present
invention are arranged in space without regularity, and can also be
interengaged with each other
feltlike and thereby acquire their particular mechanical stability for
example. Clews of this kind are
hereinafter also referred to as "inventive silver-containing clews".
The shaping and/or arranging of the silver-containing fibers or threads with
order leads to
essentially regular and ordered structures having periodically repeating unit
cells, for example
meshes or holes. Highly suitable processes for shaping and/or arranging the
silver-containing
fibers or preferably threads in space with order is knitting or weaving or the
like and subsequent
densifying.
Highly suitable ordered structures formed from silver-containing fibers or
preferably silver-
containing threads are so-called knits or nets, for example having a mesh size
in the range from
300 to 50 mesh (80 pm to 500 pm], preferably in the range from 300 to 100 mesh
(80 pm to
250 pm). These knits or nets are hereinafter also referred to as "inventive
silver-containing knits".
The density of inventive silver-containing knits or inventive silver-
containing clews is generally in
the range from 2 g/cm3 to 4 g/cm g/cm3 and preferably in the range from 3
g/cm3 to 4 g/cm3.
The density generally corresponds to a void fraction in the inventive silver-
containing knits or
inventive silver-containing clews ranging from 60% to 80% and preferably from
60% to 75%.
A void fraction of more than 80% is disadvantageous. The inventive void
fraction of the inventive
silver-containing knits or inventive silver-containing clews is also
advantageous to ensure an
= CA 02833915 2013-10-22
6
"ignition" of the catalytic oxidation/dehydrogenation of the methanol at very
low temperatures, for
example 350 C or less and advantageously in the range from 200 to 350 C.
Typically, the
inventive silver-containing knit or the inventive silver-containing clew is
preheated until the
reaction (oxidative dehydrogenation of methanol to formaldehyde) lights off.
Thereafter, the
reaction mentioned is generally self-sustaining under adiabatic conditions.
The abovementioned density and the void fraction of the inventive silver-
containing knits or
inventive silver-containing clews is determined with the method as follows: A
body of known
geometry is weighed. The ratio of its weight to the volume occupied by it
determines the density.
The ratio to the weight of a geometrically identical massive body composed of
the same material
defines the void fraction.
The shaped catalyst body of the present invention can be present in manifold
spatial form.
For example, the inventive silver-containing clews forming the shaped catalyst
body of the present
invention or preferably the inventive silver-containing knits can be present
as mats or disks, i.e.,
as sheetlike constructs whose lengths and widths are many times greater than
their heights.
Optionally, a plurality of shaped bodies can be present stacked on top of each
other or put
together in segment fashion.
For example, the inventive silver-containing clews forming the shaped catalyst
body of the present
invention or preferably the inventive silver-containing knits can also be
present as Raschig rings
and/or as helices.
The absolute measurements of the shaped catalyst bodies of the present
invention generally
depend on the dimensions of the reactor in which the shaped catalyst body is
used.
Exemplary dimensions for the shaped catalyst bodies of the present invention
range from 120 to
cm in length, from 50 to 10 cm in width and from 1 to 10 cm and preferably
from 2 to 4 cm in
30 height.
The geometric shape of the shaped catalyst body of the present invention is
generally variable.
= CA 02833915 2013-10-22
7
Preference is given to rectangular/cuboid or round/roundel-shaped or
cylindrical catalyst body of
the abovementioned dimensions, and the diameter of the round catalyst body is
for example in the
range from 2 cm to 300 cm, preferably in the range from 25 cm to 300 cm and
more preferably in
the range from 50 cm to 300 cm.
Typically, the shaped catalyst body is used in the reaction space, wherein the
abovementioned
starting materials, for example alcohol, such as methanol, oxygen-containing
gas, are reacted,
resting on a carrier device.
Such carrier devices are known, for example grids, baskets or perforate plates
or stable nets of
diverse materials, preferably of metals, for example stainless steel or
silver.
The shaped catalyst body of the present invention can be present as sole
catalytically active
constituent in the reaction zone in which the abovementioned starting
materials/streams
comprising methanol, oxygen and water are used. However, the shaped catalyst
body of the
present invention can also be present in the presence of granular silver
catalysts and/or other
catalysts for the oxidative dehydrogenation of the alcohols to aldehydes.
For example, a layered structure of shaped catalyst bodies according to the
present invention //
granular silver catalysts can be present.
It is also possible to use a plurality of reaction zones in which the
abovementioned starting
materials/streams, for example alcohol, such as methanol, oxygen-containing
gas are used and
which contain the catalyst of the present invention "connected in series".
This connection in series
can be actualized in one reactor or in a reactor cascade.
The process is otherwise carried out in a conventional manner by, for example,
passing a gas
mixture of methanol vapor, air, optionally inert gas and advantageously water
vapor in
aforementioned amounts at temperatures of about 550 to 750 C and more
particularly 595 to
710 C, through the reaction zone or zones containing the catalyst of the
present invention. The
process is generally carried out in a continuous operation at an absolute
pressure between 0.5
and 2 bar and preferably between 1.2 and 1.8 bar. It is advantageous here for
the reaction gases
=
CA 02833915 2013-10-22
8
leaving the catalyst zone to be cooled down, for example to temperatures of 50
to 350 C, within a
short time. After the gas mixture has cooled down, it is then advantageously
fed to an absorption
tower in which the formaldehyde is scrubbed with water out of the gas mixture,
advantageously
countercurrently.
The process is also more particularly described in Ullmann's Encyclopedia of
Industrial Chemistry,
2005, pages1 if.
The advantages of the process according to the present invention are in
particular:
An improved yield of C1-C10 aldehyde, especially formaldehyde, compared with
conventional
catalysts (higher selectivities to, for example, formaldehyde and, for
example, comparable
methanol conversions at lower catalyst mass).
An improved uniformity of the catalyst packing in respect of layer thickness
and material density.
The possibility of influencing the catalytic properties of the shaped catalyst
body of the present
invention via the specific adjustment of the geometry of the shaped body, more
particularly of the
wire/fiber structure, concerning the diameter, and the density of the shaped
body.
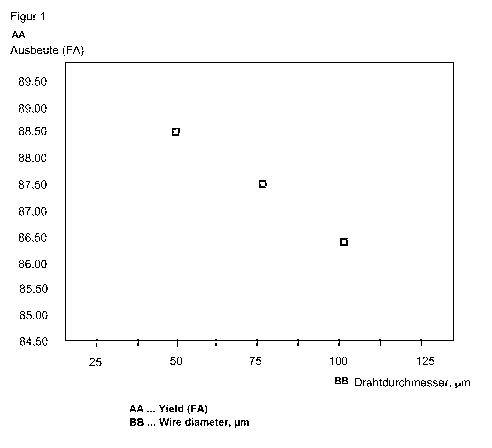
Surprisingly, relationships result between the geometric and structural
parameters of the shaped
catalyst bodies and the enhanced chemical productivity. It was observed in
this connection that in
the oxidation dehydration of methanol, for example, the formaldehyde yield in
a single pass
through the catalyst bed increases with decreasing wire diameter. Moreover, an
advantageous
ignition behavior of the shaped catalyst body correlates with the packing
density thereof.
Examples
Example 1
A gaseous water-methanol mixture having a molar ratio of water/methanol equal
to 1.0 was mixed
with air (140 NI/h) and nitrogen (50 NI/h) such that the molar ratio of
methanol to oxygen was 2.5.
This mixture was heated to 140 C in a preheater upstream of the reactor, and
subsequently
passed through a knitted silver catalyst. This catalyst consisted of a shaped
cylindrical body
CA 02833915 2013-10-22
9
having a height of 10 mm and a diameter of 20 mm. The shaped body consisting
of compressed
silver wool having a fiber diameter of 0.05 mm (density of shaped body: 3
g/cm3, void fraction:
75%). The runs were done adiabatically in a quartz glass reactor having an
internal diameter of 20
mm. The adiabacy of the reactor was achieved through passive insulation, and
completely
dispenses with any compensatory heating. To ensure an ignition of the
adiabatically performed
reaction on the silver catalyst, the methanol-water-air-nitrogen mixture was
heated to 300 C, at
which temperatures the molar ratio of methanol/oxygen was 7:1 and nitrogen was
metered at 300
NI/h. The adiabatic ignition ensued at 300 C. Then, the abovementioned
composition of
water/methanol/air/nitrogen was metered incrementally. On setting the metering
and the preheater
temperature as described above, the catalyst bed reached temperatures of 595 C
in the ignited
adiabatic reaction. A weight hourly space velocity of 95 000 h-1 over the
catalyst was achieved.
The product mixture emerging from the catalyst bed was cooled to 120 C in a
heat exchanger.
The composition of the product mixture was analyzed by gas chromatography.
Under the
conditions mentioned, a methanol conversion of 99% and a formaldehyde
selectivity of 90% were
achieved. A conventionally used electrolytically produced granular silver
catalyst (fraction size 0.5
to 2 mm) achieved a formaldehyde selectivity of 87% at a methanol conversion
of 99%.
Example 2
Example 1 was repeated with regard to reactant metering and catalyst ignition.
The catalyst used
was a three-dimensional shaped cylindrical body made of compressed nets of
silver. The
diameter of the silver wire was 0.076 mm. The height of the shaped catalyst
body was 20 mm and
the diameter was 20 mm. Under the conditions mentioned, a methanol conversion
of 98% and a
formaldehyde selectivity of 90% were achieved. A conventionally used
electrolytically produced
granular silver catalyst (fraction size 0.5 to 2 mm) achieved a formaldehyde
selectivity of 87% at a
methanol conversion of 98%.
CA 02833915 2013-10-22
Example 3
Example 1 was repeated with regard to reactant metering and catalyst ignition.
The catalyst used
was a three-dimensional shaped cylindrical body made of a knitted and
subsequently compressed
5 wire of silver. The diameter of the silver wire was 0.1 mm. The density
of the pressed knit was 3
g/cm3. The height of the shaped catalyst body was 10 mm, the diameter was 20
mm. Under the
conditions mentioned, a methanol conversion of 96% and a formaldehyde
selectivity of 91% were
achieved. A conventionally used electrolytically produced granular silver
catalyst (fraction size 0.5
to 2 mm) achieved a formaldehyde selectivity of 90% at a methanol conversion
of 96%.
Parameters of Examples 1 to 3 are depicted below in Figure 1.
Figure 1: dependence of catalyst performance (yield of formaldehyde, based on
methanol) on
diameter of wire used to form the shaped catalytic body. All the shaped bodies
have the same
volume and the same density. The reaction conditions are identical.