Note: Descriptions are shown in the official language in which they were submitted.
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
DESTRUCTIBLE CONTAINERS FOR DOWNHOLE MATERIAL
AND CHEMICAL DELIVERY
Background
Drilling, cementing, stimulation, and various treatments, including workover
operations, of oil and gas wells frequently require using various chemical
additives. In
some situations it is necessary to deliver such chemical additives downhole
while
minimizing their interactions with wellbore fluids or the wellbore itself
An example of a need to deliver materials downhole without interaction with
the injection fluid is the use of slugs of fiber-containing slurries for
fracture isolation.
Treatments, for example of horizontal oil and gas wells as well as multi-
layered
formations, frequently require using diverting techniques in order to enable
treatment
redirection between different zones. Diverting methods include, but are not
limited to,
using mechanical isolation devices such as packers and wellbore plugs, setting
bridge
plugs, pumping ball sealers, pumping slurried benzoic acid flakes and pumping
removable and/or degradable particulates. Fracture isolation with fibers is
achieved by
initial bridging of fibers inside the fracture, which results in plug
formation by
accumulation of the rest of the fiber material (and other solids if present)
on the
bridge formed. Initiation of fiber bridging depends on the fiber
concentration, so
fracture isolation by this method is very sensitive to the degree of dilution
of the fiber
slurry with wellbore fluid during injection, which is difficult to control.
Similarly,
treatment diversion with particulates is typically based on bridging of
particles of the
diverting material behind the casing and forming a plug by accumulating the
rest of
the particles at the bridge formed. Two typical problems related to treatment
diversion
with particulate materials are that 1) there may be reduced bridging ability
of the
diverting slurry during pumping resulting from dilution of the slurry by the
wellbore
fluid (because of interface mixing), and 2) when mixtures of particle sizes
are used,
there may be particle separation by size or other parameters during pumping
which
results in the formation of more permeable plugs and poorer treatment
diversion.
Excessive volumes of diverting slurry typically must be pumped to minimize
these
effects, which increase costs and also result in a significant increase in the
risk of
wellbore plugging by the excess diverting material. A method of delivery of a
slurry
that minimizes slurry dilution or alteration during injection would be
valuable.
1
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
An example of the need to deliver chemicals downhole without their
interaction with the wellbore is delivery of acid for zonal stimulation.
Usually such
treatments carry a risk of casing damage because of the potential reaction of
the
casing with acid. To overcome this problem, acid retarders, which slow down
the
reaction of the acid with metal, are introduced into the pumping fluid. Such
retarders
are not always effective and full casing protection is difficult to achieve. A
method of
delivering acid to the bottom of a well without acid interaction with the
wellbore
would be desirable.
Summary
One embodiment is a method of treating a downhole region penetrated by a
wellbore with a treatment agent. The method includes delivering the treatment
agent
to the wellsite enclosed in one or more destructible containers, each having a
shell,
inserting the one or more destructible containers into the fluid being pumped
down
the well, and mechanically breaking the shell of the one or more destructible
containers in the wellbore or in the formation to release the treatment agent.
The one
or more destructible containers may be made of one or more materials. At least
one of
the materials making up the one or more destructible containers may be
degradable.
At least a portion of the treatment agent may also be degradable. The breaking
of the
destructible container may be promoted by at least partial dissolution of the
shell of
the container in the wellbore fluid. The treatment agent may be in the form of
a slurry
inside the one or more destructible containers. The one or more destructible
containers may include one or more fluid flow paths allowing entry of wellbore
fluid
into the container. When the wellbore is cased and the casing is perforated,
and the
smallest dimension of the one or more destructible containers is larger than
the
diameter of the perforations, the one or more destructible containers may be
mechanically destroyed by contact with one or more perforations. A restriction
may
be placed in the wellbore to break the one or more destructible containers at
a desired
location; the restriction has an opening smaller than the smallest dimension
of the one
or more destructible containers. Optionally, more than one restriction may be
placed
in the wellbore, said restrictions successively smaller the farther away from
the
surface. The container also may break when a fracture or wormhole in a
formation
2
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
becomes smaller than the container. The one or more destructible containers
may be
made by shrink wrapping one or more films around the treatment agent. The one
or
more destructible containers may have a hollow shell into which the treatment
agent
is placed. The shell of the one or more destructible containers may be made of
polyvinyl alcohol or gelatin. A plurality of destructible containers may be
used and
the destructible containers may vary in one or more of size, composition, or
contents.
Another embodiment is a method of treating a downhole region penetrated by
a wellbore with a special solid diverting material. The method includes
delivering the
special solid diverting material to the wellsite enclosed in one or more
containers,
each having a shell, inserting the one or more containers into fluid being
pumped
down the well, and allowing the one or more containers to release the special
solid
diverting material in the wellbore. The one or more containers may be made of
one or
more materials. At least one of the materials making up the one or more
containers is
generally at least partially degradable. At least a portion of the special
solid diverting
material may be degradable. At least a portion of the special solid diverting
material
may include a blend of particles having at least three distinct sizes. At
least a portion
of the special solid diverting material may include one or more of fibers,
fiber flocks,
fibrillated fibers, ribbons, flakes or platelets. The special solid diverting
material may
be in the form of a slurry inside the container. The one or more containers
may
include one or more fluid flow paths allowing entry of wellbore fluid into the
container. The release of the special solid diverting material may be by
mechanical
destruction of the container. The release of the special solid diverting
material may be
promoted by a chemical that reacts with the container. The release of the
special solid
diverting material may be promoted by dissolution of the container's shell in
the
wellbore fluid. The one or more containers may be made by shrink wrapping one
or
more films around the special solid diverting material. The one or more
containers
may include a hollow shell into which the special solid diverting material is
placed.
The shell of the one or more containers may be made of polyvinyl alcohol or
gelatin.
A plurality of containers may be used and the containers may vary in one or
more of
size, composition, or contents.
Yet another embodiment is a system for delivery of a special solid diverting
material to a downhole location without dilution or separation of special
solid
3
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
diverting material components; this system includes the special solid
diverting
material enclosed in one or more destructible containers. The one or more
destructible
containers may be made of one or more materials. At least one of the materials
making up the one or more containers may be degradable. The one or more
destructible containers may include one or more fluid flow paths. The special
solid
diverting material may be in the form of a slurry inside the one or more
destructible
containers. The one or more destructible containers may be mechanically
destructible.
At least a portion of the special solid diverting material may be degradable.
The one
or more destructible containers may be made by shrink wrapping one or more
films
around the special solid diverting material. The one or more destructible
containers
may include a hollow shell into which the special solid diverting material is
placed.
The one or more destructible containers may be made of a shell made of
polyvinyl
alcohol or gelatin. A plurality of destructible containers may be used and the
destructible containers may vary in one or more of size, composition, or
contents.
Brief Description of the Drawings
Figure 1 is a schematic of destruction of a destructible container at a
perforation.
Figure 2 is a schematic of destruction of a destructible container at a
restriction
apparatus placed in a wellbore.
Figure 3 is a schematic of the destruction of a container having a dissolvable
shell in
a wellbore.
Figure 4 is a schematic of one device for introducing destructible containers
into a
high pressure flow line.
Figure 5 shows a schematic of the experimental apparatus for studying the
release of
particulate materials from destructible containers at perforations.
Detailed Description
The description and examples are presented solely for the purpose of
illustrating some embodiments and should not be construed as a limitation to
the
scope and applicability. Although some of the following discussion emphasizes
4
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
diversion in fracturing, the destructible container and method may be used in
many
other wellbore operations. Some embodiments shall be described in terms of
treatment of horizontal wells, but are equally applicable to wells of any
orientation.
Some embodiments shall be described for hydrocarbon production wells, but it
is to
be understood that the they may be used for wells for production of other
fluids, such
as water or carbon dioxide, or, for example, for injection or storage wells.
It should
also be understood that throughout this specification, when a concentration or
amount
range is described as being useful, or suitable, or the like, it is intended
that any and
every concentration or amount within the range, including the end points, is
to be
considered as having been stated. Furthermore, each numerical value should be
read
once as modified by the term "about" (unless already expressly so modified)
and then
read again as not to be so modified unless otherwise stated in context. For
example,
"a range of from 1 to 10" is to be read as indicating each and every possible
number
along the continuum between about 1 and about 10. In other words, when a
certain
range is expressed, even if only a few specific data points are explicitly
identified or
referred to within the range, or even when no data points are referred to
within the
range, it is to be understood that the inventors appreciate and understand
that any and
all data points within the range are to be considered to have been specified,
and that
the inventors have possession of the entire range and all points within the
range.
We have devised destructible containers for chemicals and materials and
devised a method of using these destructible containers for downhole delivery
of
chemical and material treatment agents. The method comprises introducing the
destructible containers containing chemicals or materials into the pumping
line or into
the wellbore followed by bullheading the containers and their contents
downhole. The
destructible containers minimize the risk of interaction of the chemicals or
materials
with wellbore fluid or with the wellbore itself until destruction of the
containers,
which may occur, for example:
= at the surface during introduction into the pumping line or wellhead,
= along the wellbore during downhole delivery,
= at a specific location in the wellbore, and/or
= at bottomhole.
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
Note that in all these situations, destruction occurs before the containers
can enter
the formation surrounding the wellbore. The destructible container comprises a
shell
holding one or more treatment agents. The shell separates the treatment agent
or
agents from the outside environment until the shell is destroyed, although if,
desired a
small amount of an outside fluid may be allowed to enter the shell before
destruction
of the shell. The shell may also be defined as a chemical or physical barrier
or bond,
including some form of adhesion of particles and/or fibers due to interaction
of a coating on
the surface of the materials, holding chemicals and/or materials in a pre-
defined volume
until it is intended to expand the contents beyond the confines of the
designed
volume. The shell may have various mechanical properties and may be, for
example,
rigid, flexible, elastic, hard, fragile, or not fragile. Destruction of the
containers may
be brought about by mechanical destruction, chemical action, dissolution or
thermal
destruction. When the destruction is by a mechanical action, then it is
preferred that
the remains of the empty, or nearly empty, container be subsequently destroyed
in the
wellbore or in the near wellbore region by chemical action, dissolution or
thermal
destruction. The treatment agent may be any chemical or particulate materials
used in
wellbores and formations, such as, but not limited to, fluid loss additives,
pH
changing agents, lost circulation materials, scale dissolvers, cross linking
agents,
oxidizers, sealing agents, diverting agents, viscosifying agents, fibers,
fluid breakers
and viscosity reducing additives, clean-up additives, surfactants, rigid gels,
chemical
plugs, salts, and chemicals that react exothermically. Note that the treatment
agent in
the shell may be only part of a final chemical or physical process in the
well. For
example, the treatment agent may be a crosslinker that reacts with polymer
that was
not in the destructible container to form a gel that performs some treatment
in the
well. In addition, treatment agents inside the shell, including diverting
agents, may be
coated, for example with a resin, a polymer, a salt, an organic compound,
and/or an
inorganic compound.
We have in particular found a method of using these destructible downhole
containers and their contents for treatment diversion during stimulation
operations for
zonal isolation and/or for changing the injection profile of a treating fluid
into the
formation when several perforated zones are treated in one stage. For
diversion, the
destructible container consists of a destructible enclosure or shell filled
with a
diverting material, preferably a diverting material that is partially or
completely
6
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
degradable, soluble, reactible, meltable or otherwise destroyable other than
mechanically. Optionally, the degradation may be caused or enhanced by a
chemical
inside the destructible container, for example in addition to the main
treatment agent.
Optionally, shell destruction optionally may be initiated by penetration of
the
wellbore fluid into the container (as examples, a water-soluble enzyme breaker
placed
inside a container having a gelatin shell; citric acid placed inside a
container having
an acid-soluble or acid-destructible shell such as one made of borate cross-
linked guar
or cellulose; or solids such as NaOH, Ca(OH)2, CaCO3 or Mg(OH)2 included in
the
contents of a polylactic acid shell). In describing a diverting material as
being at least
partially destroyable or degradable we mean that the diverting material, or at
least one
component of the diverting material is sufficiently destroyable or degradable
that the
ability of the plug or seal to block fluid flow is reduced so that it no
longer blocks
fluid flow; in some cases this may be as little as about 5 percent of the
diverting
material being destroyable or degradable. The container, whose shell protects
the
contents from dilution with wellbore fluids as well as from particle size
and/or shape
separation during pumping, is introduced into the wellbore and pumped
downhole.
The container is destroyed, for example mechanically, causing the release of
the
diverting material which plugs or seals a stimulated interval and provides
treatment
diversion to another region. Several preferred mechanisms may be used for
enclosure
or shell destruction:
= shear and/or mechanical collisions in the wellbore,
= differential pressure attempting to force the container through
perforations,
and/or
= destruction by a downhole apparatus, for example a restriction, specially
set in
the wellbore for this purpose.
As is usually desired, removal of the diverting material as well as shell
components is preferably achieved by self-degradation under downhole
conditions; by
introduction of special chemical agents, such as solvents, especially under
dynamic
underbalanced conditions; or by specialized wellbore intervention (for
examples
hydrojet cleaning, solvent cleaning, and using a downhole heater).
7
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
Optionally, the container may be destroyed sufficiently by at least partial
dissolution (for example more than 5% of the shell being dissolved) in the
surrounding fluid (for example a polyvinyl alcohol shell), or by weakening of
the
shell by partial dissolution in the surrounding fluid followed by mechanical
destruction (for example a gelatin shell).
Using destructible containers for fiber and/or particle delivery for fracture
isolation allows maintenance of high fiber and/or particle concentrations at
the
downhole location by minimizing the risk of dilution of the fibers with
wellbore fluid.
The fiber and/or particles in the destructible containers may be dried or
slurried. The
liquid in which the solids are slurried may be, for example, an aqueous liquid
or a
linear or crosslinked aqueous polymer solution. In one specific embodiment the
solids
may be slurried in situ in a wellbore fluid which penetrates the container.
The liquid
phase of the slurry may also be non-aqueous, such as an alcohol (as examples
glycerol, ethanol, methanol, and isopropanol, ethylene glycol); and/or liquid
hydrocarbons such as diesel, hexane, or aromatic hydrocarbons such as benzene,
toluene etc. Delivery of acid in such containers with subsequent acid release
of the
acid downhole provides casing or wellbore wall protection during acid
treatments.
Other treatment agents may be delivered advantageously, for example bases such
as
sodium hydroxide.
Furthermore, using particle or fiber-holding destructible containers
significantly simplifies wellsite delivery of those or other container
contents.
Problems with existing methods of fiber and/or particle delivery based on
using screw
feeders include, but are not limited to, metering difficulties and plugging of
equipment. Wellsite delivery of special solid diverting materials, such as
fibers and/or
particles, in destructible containers solves these problems, because such
containers
may be introduced into the treating fluid with the same techniques as commonly
used
for proppant or any solid or particulate material. In one embodiment fibers
and/or
particulates are vacuum packed into small bundles (to maximize the
concentration)
and surrounded by a coating or put into an enclosure, for example shrink-
wrapped or
vacuum packed, that is engineered to have various degradation times or
destruction
degrees of difficulty. Alternatively, an additive is used at various
concentrations to
interact with the coating or container at varying rates. In one specific
example, where
8
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
the container's principal purposes are wellsite delivery and metering, and it
is not
necessary to minimize dilution or separation of the contents as they travel
downhole,
such a shell or coating may optionally quickly be degraded or destroyed after
introduction of fiber (or other shape) and/or particle packs into the treating
fluid so
that fiber (or other shape) and/or particle dispersion occurs in the mixing
and/or
pumping equipment or while traveling downhole. This method may still deliver
higher concentrations of special solid diverting materials to a location
downhole.
It should be noted that using small (for example from about 1 mm to about
100 mm, especially from about 1 mm to about 70 mm, and most especially from
about 1 mm to about 20 mm) containers for fibers or other shapes simplifies
fiber (or
other shapes) delivery. These containers may be introduced through a blender
in large
amounts and simple equipment may be used. Premature destruction of some of the
containers in the treating equipment is not critical as long as the majority
(for example
at least about 60 %) of the containers survive to enter the wellbore. Note
that if a
container is made of multiple layers, it is not considered destroyed until all
of the
layers have been destroyed to the point that the contents can be released. The
ultimate
fiber concentration that can reliably be obtained downhole is much higher than
if
fibers are fed directly into the fluid.
The following discussion of possible alternatives merely provides context
information related to the disclosure and may not constitute prior art. The
inventors
are not aware of any method of wellsite delivery of solid materials useful for
fluid
flow diversion that utilizes mechanically destructible containers which are
destroyed
in surface equipment and/or which are bullheaded downhole and destroyed in the
wellbore by any means before entering a formation. The inventors are not aware
of
any method of downhole delivery of any chemical agents or solid materials that
are
placed in destructible containers that are pre-formed empty and then filled,
or are
placed around the agents or materials, and then broken in surface equipment or
in the
wellbore by special apparatus previously placed in the wellbore or by
perforations to
release the contents into a formation or fracture, such as but not limited to,
bags or
hollow balls (that may optionally be rigid and may optionally dissolve,
degrade, etc.
after release of the contents). U.S. Pat. No. 7,049,272 discloses a method of
treating a
well with solids, liquids or apparatuses by 1) encasing said solids, liquids
or
9
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
apparatuses in a pre-formed water soluble shell such as a PVA cylinder, 2)
conveying
said encased solids, liquids or apparatuses to a predetermined location in the
well, and
3) allowing the water-soluble shell to dissolve in the aqueous phase in the
wellbore.
The shell is "resistant to diffusion in either direction" and "able to resist
substantial
physical and mechanical forces without breaking"; illustrative examples
include
placing encased soap at the bottom of the well for assisting in gas-lift, and
placing
corrosion inhibitors. No action is taken to destroy the shell and the shell
does not
release any material before it reaches the treatment location. There are
several
applications in the oil-field industry which are based on using encapsulated
chemicals
for delayed triggering of chemical reactions downhole. U. S. Pat. No.
6,794,340
discloses a method of removing drill cuttings from wellbores and drilling
fluids by
crosslinking drilling fluid with a crosslinker and a crosslinker activator
that is
encapsulated and released by destruction of the capsule as it passes through
the drill
bit or that is released by dissolution or melting of the encapsulation
material. All
encapsulated material and any remaining encapsulation material are returned to
the
surface. U. S. Pat. No. 4,614,599 discloses a lost circulation treatment
comprising
encapsulating lime in a reaction-preventive protective casing (such as a film
of wax)
in a circulating drilling fluid to prevent the lime from reacting with clays
in the
borehole until it is desired to breach the casing; if lost circulation occurs,
circulation is
slowed or stopped so that the temperature rises and the time of the fluid in
the lost
circulation pathway lengthens and the coating dissolves or melts and the lime
reacts
with clays in the drilling fluid and/or the formation to plug the lost
circulation
pathway. Using encapsulated liquids for formation treatments is disclosed in
U.S. Pat.
No. 6,761,220 in which contents of capsules "within the downhole region of a
well"
may be released by crushing, rupturing, dissolving, diffusion of fluid
through, or
melting of, the capsule. U. S. Pat. No. 6,924,253 discloses release of
encapsulated
ionic liquids for scale removal in the wellbore or near wellbore region.
Encapsulated
chemicals, other than diverting solids, for downhole or in-formation release
for
various treatments, such as gel breakers for hydraulic fracturing, are known;
breaker
release in the fracture after leaving the wellbore is activated by temperature
or by
crushing capsules during fracture closure. Capsules may also degrade in the
wellbore
or formation, or dissolve, or melt, or be ruptured by entrance of a fluid by
osmosis.
There are also downhole tools that can be controlled to release active
chemicals.
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
Some are integrated into the casing where transferring the internal fluid from
the
reservoir relies on the Venturi effect. Others are wireline or string conveyed
apparatuses; release of chemicals is activated from the surface after
positioning the
apparatuses at the desired location. Other diversion methods include but are
not
limited to diversion with 1) viscous fluids or fluids that become viscous,
such as the
so-called self-diverting fluids, 2) foams and emulsions, 3) ball sealers,
including
degradable and soluble ball sealers, 4) mechanical tools and well completion
tools, 5)
limited entry perforation diverting techniques, and 6) stress assisted
diversion.
In one embodiment, the destructible containers are made of a material that is
at
least partially degradable, soluble, reactible, meltable or otherwise
destroyable other
than mechanically, or are made of more than one component, at least one of
which is
destroyable other than mechanically, so that after destruction at least part
of the
container will disappear. In describing a container as at least partially
destroyable or
degradable we mean that at least 5 %, preferably at least 50 %, of the
container is
destroyable or degradable.
For wellsite delivery of the proposed destructible containers, existing or
modified delivery equipment may be used, depending on the mechanism of the
destruction of the containers and the purpose of their use. The location at
which the
destruction occurs may be determined by selection, adaptation, or special
design of
surface and/or downhole equipment.
Some embodiments include delivering diverting materials downhole in
destructible containers. The principle advantages include 1) delivering the
material to
a desired location downhole in concentrated form while eliminating or reducing
the
problems of dilution or size or density separation of the materials before
they arrive
at the desired downhole location, and 2) convenient delivery to the well site
and
convenient injection into the injection fluid without the problems associated
with
transporting and metering materials that may be difficult to handle, such as
fibers and
mixtures of different sizes of, or different shapes of, materials.
Embodiments may be described here in terms of solid diverting materials in
fracturing, but the destructible container can carry any inert or active
solids, fluids, or
combinations of solids and fluids to any desired downhole location for any
purpose.
The container is generally either a pre-formed container such as a hollow
sphere, for
11
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
example of polylactic acid (PLA), or a bag that is filled after it is made or
a similar
structure that is fashioned around the contained material; either way, it is
sealed after
it is filled. The container may be referred to as a shell, envelope, etc. The
container is
then introduced into the fluid being injected downhole, is carried to the
desired
location, and then is deliberately broken there to release the contents.
Except for
"special solid diverting material", by destructible "container" we do not mean
a
coating of another material that is put on by spray coating or polymerization
and the
like as is often meant in the literature when a material is described as
"encapsulated".
By special solid diverting material we mean, for example, fibers; other shapes
such as
flakes, platelets, ribbons, rods, precipitated material from chemical
reactions, grains,
pellets; mixtures of different sizes of approximately spherical materials; and
mixtures
of fibers or flakes or other shapes and one or more sizes of approximately
spherical
materials (for example having aspect ratios of less than about 5, preferably
less than
about 3). Non-limiting examples of approximately spherical materials include
plastic
beads, sand, ceramic beads, glass, wax beads, proppant, silica flour, alumina,
and
calcium carbonate. All such special solid diverting materials are designed to
plug
openings of a certain size, such as a wellbore, a vug, a fluid loss pathway, a
hydraulic
fracture, wormhole etc. Special solid diverting materials may be enclosed in
any way
and be within the scope of embodiments. Preferably the special solid diverting
material is degradable and/or removable under downhole conditions. Chemicals
or
materials other than special solid diverting materials enclosed in
mechanically
destructible containers are within the scope of embodiments.
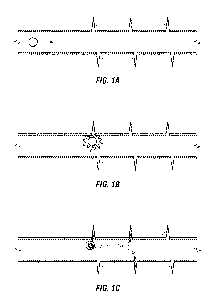
Figures 1, 2, and 3 show several methods of deliberately breaking destructible
containers downhole. In Figure 1, release of diverting material is caused by
destruction of downhole containers at perforations. The wellbore is shown as
horizontal but may be in any orientation. The container flows along the
wellbore (A)
and is pressed against the opening of the first perforation it encounters, by
the fluid
pressure, and the container breaks (B), which is believed to be due to
differential
pressure across the perforation. The perforations have dimensions smaller than
the
smallest dimension of the container. Some or all of the container contents
passes
through the perforation and into the formation; any material that does not
pass
through the perforation that broke the container is carried further along the
wellbore
and into one or more subsequently encountered perforations (C).
12
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
In Figure 2 the destruction is caused by a special restriction, having a cross
section smaller than that of the destructible container that has been placed
in the
wellbore; the restriction typically is placed upstream of the perforations and
has a
diameter smaller than the smallest dimension of the container. After the
container is
broken the contents enter downstream perforations. A variation of this system
is the
use of varying sizes of destructible containers and several progressively
smaller
restrictions along the perforated zone. Smaller containers pass through the
first,
larger, restrictions and do not break until they reach a restriction smaller
than the
container. This ensures that all the perforations receive treatment material;
this
scheme can also be used to deliver different materials to different regions of
the
perforated zone. Not shown is that in these cases the perforations may
optionally be
larger than at least some of the containers, or alternatively some of the
containers may
be broken by perforations.
In Figure 3 the destruction is caused by dissolution of the shell of the
destructible container as it passes down the wellbore. After the container
dissolves or
is broken by at least partial dissolution, the contents enter downstream
perforations.
The container is in the wellbore at [I], and destroyed by at least partial
dissolution of
the shell by the time it reaches location [2]. The contents [3] are released
and
displaced into the perforations at location [4].
Alternatively, destruction of the container may be caused by shear downhole
in the wellbore (for example at a change in direction or a narrowing of the
wellbore),
or by passing near or collision with other apparatus downhole such as a
perforating
gun. Deliberate mechanical destruction may be aided by partial chemical
destruction
or dissolution or thermal weakening of the shell or by a combination of such
processes. Destructible containers may also be sized to break at a certain
point inside
a fracture or wormhole.
Destructible containers may be tested, preferably in the laboratory, to ensure
that they break where desired. For example, if the destructible containers are
to be
broken by a downhole restriction or by perforations, they may be tested to
ensure that
they are not broken by high differential pressures encountered first or by
striking
surface line or wellbore walls (for example at bends). If necessary, the
strength of the
shell may be increased or holes or a leaf valve or leaf burst valve may be
used to
13
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
relieve differential pressure. Dissolvable container may be tested by
measuring the
time required for sufficient destruction of the shell by dissolution at
conditions
emulating the conditions during pumping (shear rate, temperature, pressure,
etc.). The
shell is considered to have been destroyed at the point at which, although the
shell
may not have been completely dissolved, the mechanical integrity has been
reduced
significantly enough that the contents can be released.
The mechanically destructible downhole container may be of any shape, but is
preferably spherical or has an aspect ratio of less than about 3. An
approximately
spherical shape is advantageous because: (1) if the container is not
approximately
spherical and one dimension is significantly longer than the others, then the
container
may become trapped in surface lines or connections if it is not correctly
oriented when
it enters a connection or pipe; (2) surface handling of spheres is easier than
surface
handling of non-spherical shapes and the orientation when feeding the
container into
the well is not an issue; and (3) for spheres, the same equipment,
calculations, and
considerations established for ball sealers may be used. Those correlations do
not
apply of container is not spherical The exact dimensions depend upon the
nature of
the wellbore, surface equipment, and downhole equipment, but typically, the
volume
of the destructible downhole containers varies from about 0.5 cm3 (which
corresponds
to a sphere having a diameter of about 1 cm), to about 24 L (which corresponds
to a
cylinder having a diameter of about 17.5 cm and a length of about 100 cm). The
preferred volume of the destructible downhole containers ranges from about 8
cm3 to
about 2.8 L (which corresponds to spheres having diameters from about 2.5 cm
to
about 17.5 cm). The most preferred volume of the destructible downhole
containers is
in the range of from about 20 cm3 to about 1 L (which corresponds to spheres
having
diameters from about 5 cm to about 12.5 cm). When the primary purpose of the
containers is wellsite delivery of fiber-based materials, the preferred volume
of the
containers is in the range of about 0.5 cm3 and about 2 cm3, which allows
pumping
such containers through typical surface equipment.
The outer enclosure or shell (or bag or envelope, etc.) of the destructible
container, which may be rigid or flexible, is made of a material which is
mechanically
destructible at downhole conditions. Examples of such materials include
plastics,
glass, ceramics, gelatin etc. The shell of the container in some embodiments
may also
14
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
be chemically degradable, dissolvable or meltable. Normally, degradation takes
place
after the shell is broken.
In one embodiment the shell may be degradable in, or soluble in, the wellbore
or formation fluids. This minimizes the risk of formation damage by the shell
material
and assists in wellbore and formation clean-up. Examples of degradable
materials
which may be used for making the shell of the destructible downhole container
are
polyesters (including PLA, PGA, esters of lactic acid, glycolic acid, other
hydroxyacids, and copolymers thereof; polyamides and copolymers thereof;
polyethers and copolymers thereof); polyurethanes, etc.
Nonlimiting examples of degradable materials that may be used include
certain polymer materials that are capable of generating acids upon
degradation.
These polymer materials may herein be referred to as "polymeric acid
precursors";
they can be used as destructible shell materials or as degradable diverting
materials,
depending on their properties. These materials are typically solids at room
temperature. The polymeric acid precursor materials include the polymers and
oligomers that hydrolyze or degrade in certain chemical environments under
known
and controllable conditions of temperature, time and pH to release organic
acid
molecules that may be referred to as "monomeric organic acids." As used
herein, the
expression "monomeric organic acid" or "monomeric acid" may also include
dimeric
acid or acid with a small number of linked monomer units that function
similarly to
monomer acids composed of only one monomer unit, in that they are fully in
solution
at room temperature.
Polymer materials may include those polyesters obtained by polymerization of
hydroxycarboxylic acids, such as the aliphatic polyesters of lactic acid,
referred to as
polylactic acid; of glycolic acid, referred to as polyglycolic acid; of 3-
hydroxbutyric
acid, referred to as polyhydroxybutyrate; of 2-hydroxyvaleric acid, referred
to as
polyhydroxyvalerate; of epsilon caprolactone, referred to as polyepsilon
caprolactone
or polycaprolactone; the polyesters obtained by esterification of hydroxyl
aminoacids
such as serine, threonine and tyrosine; and the copolymers obtained by
mixtures of the
monomers listed above. A general structure for the above-described
homopolyesters
is:
H- {0- [C(R1,R2)].- [C(R3,R4)]y-C=0 I z-OH
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
where R1, R2, R3, and R4 are either H, linear alkyl, such as CH3,
CH2CH3 (CH2).CH3, branched alkyl, aryl, alkylaryl, a functional alkyl
group (bearing carboxylic acid groups, amino groups, hydroxyl groups,
thiol groups, or others) or a functional aryl group (bearing carboxylic
acid groups, amino groups, hydroxyl groups, thiol groups, or others);
x is an integer between 1 and 11;
y is an integer between 0 and 10; and
z is an integer between 2 and 50,000.
Under appropriate conditions (pH, temperature, water content) polyesters such
as those described here may hydrolyze and degrade to yield hydroxycarboxylic
acids
and compounds such as those acids referred to in the foregoing as "monomeric
acids."
One example of a suitable degradable polymeric acid precursor, as mentioned
above, is the polymer of lactic acid, sometimes called polylactic acid, "PLA,"
polylactate or polylactide. Lactic acid is a chiral molecule and has two
optical
isomers. These are D-lactic acid and L-lactic acid. The poly(L-lactic acid)
and
poly(D-lactic acid) forms are generally crystalline in nature. Polymerization
of a
mixture of the L- and D-lactic acids to poly(DL-lactic acid) results in a
polymer that
is more amorphous in nature. The polymers described herein are essentially
linear.
The degree of polymerization of the linear polylactic acid can vary from as
few units
as necessary to make them solids under downhole conditions to several thousand
units
(e.g. 2000-5000). Cyclic structures may also be used. The degree of
polymerization
of these cyclic structures may be smaller than that of the linear polymers.
These
cyclic structures may include cyclic dimmers if they are solids under storage
and
wellsite ambient conditions.
Another example is the polymer of glycolic acid (hydroxyacetic acid), also
known as polyglycolic acid ("PGA"), or polyglycolide. Other materials suitable
as
polymeric acid precursors (destructible shell materials or degradable
diverting
materials, depending on their properties) are all those polymers of glycolic
acid with
itself or with other hydroxy-acid-containing moieties, for example as
described in
U.S. Patent Nos. 4,848,467; 4,957,165; and 4,986,355.
The polylactic acid and polyglycolic acid may each be used as homopolymers,
which may contain less than about 0.1% by weight of other comonomers. As used
16
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
with reference to polylactic acid, "homopolymer(s)" is meant to include
polymers of
D-lactic acid, L-lactic acid and/or mixtures or copolymers of pure D-lactic
acid and
pure L-lactic acid. Additionally, random copolymers of lactic acid and
glycolic acid
and block copolymers of polylactic acid and polyglycolic acid may be used.
Combinations of the described homopolymers and/or the above-described
copolymers
may also be used.
Other examples of polyesters of hydroxycarboxylic acids that may be used as
polymeric acid precursors are the polymers of hydroxyvaleric acid
(polyhydroxyvalerate), hydroxybutyric acid (polyhydroxybutyrate) and their
copolymers with other hydroxycarboxylic acids. Polyesters resulting from the
ring
opening polymerization of lactones such as epsilon caprolactone
(polyepsiloncaprolactone) or copolymers of hydroxyacids and lactones may also
be
used as polymeric acid precursors.
Polyesters obtained by esterification of other hydroxyl-containing acid-
containing monomers such as hydroxyaminoacids may be used as polymeric acid
precursors. Naturally occurring aminoacids are L-aminoacids. The three most
common aminoacids that contain hydroxyl groups are L-serine, L-threonine, and
L-
tyrosine. These aminoacids may be polymerized to yield polyesters at the
appropriate
temperature and using appropriate catalysts by reaction of their alcohol and
their
carboxylic acid groups. D-aminoacids are less common in nature, but their
polymers
and copolymers may also be used as polymeric acid precursors (destructible
shell or
degradable diverting materials, depending upon properties).NatureWorks, LLC,
Minnetonka, MN, USA, produces solid cyclic lactic acid dimer called "lactide"
and
from it produces lactic acid polymers, or polylactates, with varying molecular
weights
and degrees of crystallinity, under the generic trade name NATUREWORKSTm PLA.
The PLA's currently available from NatureWorks, LLC have number average
molecular weights (M.) of up to about 100,000 and weight averaged molecular
weights (Mw) of up to about 200,000, although any polylactide (made by any
process
by any manufacturer) may be used. Those available from NatureWorks, LLC
typically have crystalline melt temperatures of from about 120 to about 170
C, but
others are obtainable. Poly(d,l-lactide) of various molecular weights is
also
commercially available from Bio-Invigor, Beijing and Taiwan. Bio-Invigor also
17
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
supplies polyglycolic acid (also known as polyglycolide) and various
copolymers of
lactic acid and glycolic acid, often called "polygalactin" or poly(lactide-co-
glycolide).
The extent of the crystallinity can be controlled by the manufacturing method
for homopolymers and by the manufacturing method and the ratio and
distribution of
lactide and glycolide for the copolymers. Additionally, the chirality of the
lactic acid
used also affects the crystallinity of the polymer. Polyglycolide can be made
in a
porous form. Some of the polymers dissolve very slowly in water before they
hydrolyze.
Amorphous polymers may be useful in certain applications. An example of a
commercially available amorphous polymer is that available as NATUREWORKS
4060D PLA, available from NatureWorks, LLC, which is a poly(DL-lactic acid)
and
contains approximately 12% by weight of D-lactic acid and has a number average
molecular weight (Me) of approximately 98,000 g/mol and a weight average
molecular weight (Mw) of approximately 186,000 g/mol.
Other polymer materials that may be useful are the polyesters obtained by
polymerization of polycarboxylic acid derivatives, such as dicarboxylic acid
derivatives with polyhydroxy-contaning compounds, in particular dihydroxy
containing compounds. Polycarboxylic acid derivatives that may be used are
those of
dicarboxylic acids such as oxalic acid, propanedioic acid, malonic acid,
fumaric acid,
maleic acid, succinic acid, glutaric acid, pentanedioic acid, adipic acid,
phthalic acid,
isophthalic acid, terphthalic acid, aspartic acid, or glutamic acid;
polycarboxylic acid
derivatives are those such as of citric acid, poly and oligo acrylic acid and
methacrylic
acid copolymers; other materials that may be used if they are solids, or may
be used
as starting materials for polymerization if they are liquids, are dicarboxylic
acid
anhydrides, such as, maleic anhydride, succinic anhydride, pentanedioic acid
anhydride, adipic acid anhydride, phthalic acid anhydride; dicarboxylic acid
halides,
primarily dicarboxylic acid chlorides, such as propanedioic acyl chloride,
malonyl
chloride, fumaroyl chloride, maleyl chloride, succinyl chloride, glutaroyl
chloride,
adipoyl chloride, and phthaloyl chloride. Useful polyhydroxy containing
compounds
for making useful degradable polymers are those dihydroxy compounds such as
ethylene glycol, propylene glycol, 1,4 butanediol, 1,5 pentanediol, 1,6
hexanediol,
hydroquinone, resorcinol, bisphenols such as bisphenol acetone (bisphenol A)
or
18
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
bisphenol formaldehyde (bisphenol F); and polyols such as glycerol. When both
a
dicarboxylic acid derivative and a dihydroxy compound are used, a linear
polyester
results. It is understood that when one type of dicarboxylic acid is used, and
one type
of dihydroxy compound is used, a linear homopolyester is obtained. When
multiple
types of polycarboxylic acids and/or polyhydroxy containing monomers are used,
copolyesters are obtained. According to the Flory Stockmayer kinetics, the
"functionality" of the polycarboxylic acid monomers (number of acid groups per
monomer molecule) and the "functionality" of the polyhydroxy containing
monomers
(number of hydroxyl groups per monomer molecule) and their respective
concentrations, determine the configuration of the polymer (linear, branched,
star,
slightly crosslinked or fully crosslinked). All these configurations can be
hydrolyzed
or "degraded" to carboxylic acid monomers, and therefore can be considered as
polymeric acid precursors (solids that can be used as destructible shell
components or
as degradable diverting material components). As one non-limiting example, not
descriptive all the possible polyester structures that can be used, but
providing an
indication of the general structure of the most simple cases encountered, the
general
structure for the linear homopolyesters useful is:
H-10- R1-0-C=0 ¨ R2-C=O}-OH
where R1 and R2 are linear alkyl, branched alkyl, aryl, and alkylaryl groups;
and z is an integer between 2 and 50,000.
Other examples of suitable polymeric acid precursors are the polyesters
derived from phthalic acid derivatives such as polyethylene terephthalate
(PET),
polybutylene terephthalate (PBT), polyethylene naphthalate (PEN), and the
like.
Under the appropriate conditions (for example pH, temperature, and water
content) polyesters such as those described herein can "hydrolyze" and
"degrade" to
yield polycarboxylic acids and polyhydroxy compounds, regardless of the
original
polyester synthesized from any of the polycarboxylic acid derivatives listed
above.
The polycarboxylic acid compounds yielded by the polymer degradation process
are
also considered monomeric acids.
Other examples of polymer materials that may be used are those obtained by
the polymerization of sulfonic acid derivatives with polyhydroxy compounds,
such as
polysulphones or phosphoric acid derivatives with polyhydroxy compounds, such
as
19
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
polyphosphates.
Such solid polymeric acid precursor material may be capable of undergoing an
irreversible breakdown into fundamental acid products downhole. As referred to
herein, the term "irreversible" will be understood to mean that the solid
polymeric
acid precursor material, once broken downhole, does not reconstitute downhole,
e.g.,
the material breaks down in situ but does not reconstitute in situ. The term
"break
down" refers to both of the two extreme cases of hydrolytic degradation that
the solid
polymeric acid precursor material may undergo, e.g., bulk erosion and surface
erosion, and any stage of degradation in between these two. This degradation
can be a
result of, inter alia, a chemical reaction. The rate at which the chemical
reaction takes
place may depend on, inter alia, the chemicals added, temperature and time.
The
breakdown of solid polymeric acid precursor materials may or may not depend,
at
least in part, on their structure. For instance, the presence of hydrolyzable
and/or
oxidizable linkages in the backbone often yields a material that will break
down as
described herein. The rates at which such polymers break down are dependent on
factors such as, but not limited to, the type of repetitive unit, composition,
sequence,
length, molecular geometry, molecular weight, morphology (e.g., crystallinity,
size of
spherulites, and orientation), hydrophilicity, hydrophobicity, surface area,
and
additives. The manner in which the polymer breaks down also may be affected by
the
environment to which the polymer is exposed, e.g., temperature, presence of
moisture,
oxygen, microorganisms, enzymes, pH, and the like.
Some suitable examples of solid polymeric acid precursor materials that may be
used
include, but are not limited to, those described in the publication in
Advances in
Polymer Science, Vol. 157, entitled "Degradable Aliphatic Polyesters," edited
by A.
C. Albertsson, pages 1-138. Examples of polyesters that may be used include
homopolymers, and random, block, graft, and star- and hyper-branched aliphatic
polyesters.
Another class of suitable solid polymeric materials that may be used as
destructible containers and/or degradable diversion materials includes
polyamides and
polyimides. Such polymers may comprise hydrolyzable groups in the polymer
backbone that may hydrolyze under the conditions that exist downhole.
Nonlimiting
examples of suitable polyamides include proteins, polyaminoacids, nylon, and
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
poly(capro 1 actam). Another class of polymers that may be suitable for use is
those
polymers that may contain hydrolyzable groups, not in the polymer backbone,
but as
pendant groups. Hydrolysis of the pendant groups may generate a water-soluble
polymer and other byproducts. A nonlimiting example of such a polymer is
polyvinylacetate, which upon hydrolysis forms water-soluble polyvinylalcohol
and
acetate salts. Other suitable materials include polysaccharides, chitins,
chitosans,
orthoesters, polyanhydrides, polycarbonates, poly(orthoesters), poly(ethylene
oxdides), and polyphosphazenes.
The shell may be made of a material that will disintegrate into smaller pieces
at downhole conditions over a time which is much longer (for example at least
100
times longer, preferably at least 5 times longer, most preferably at least 3
times
longer) than the time it takes to pump the container to the release location.
It should
be noted that some materials that disintegrate also degrade by other
mechanisms and
vice versa. Materials that eventually disintegrate include plastics such as
polylactic
acid (PLA), polyamides and composite materials comprising degradable plastic
and
non-degradable fine solids. It should be mentioned that some degradable
materials
pass through a disintegration stage during the degradation process. An example
is
PLA, which turns into a fragile material before complete degradation.
Optionally, the shell of the destructible downhole container may also be
deformable and engineered to minimize the risk of premature destruction during
pumping through the surface equipment and the wellbore if desired. Optionally,
the
shell of the destructible downhole container may be engineered to be broken
during
pumping through the surface equipment and the wellbore if desired. The shell
may
also have perforations or holes in its surface to allow fluid, for example
wellbore
fluid, penetration inside the destructible container. Optionally, the holes
may be in
the form of check valves or one-way valves such as leaf valves or leaf burst
valves.
Such perforations may (a) equalize the hydraulic pressure outside and inside
the
container and thus minimize the risk of premature destructing of the container
by
hydraulic pressure, and/or (b) enable greater mobility of the material inside
the
container, which will assist in better release of the material, such as
special solid
diverting material, from the container after its destruction.
21
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
Many solid diverting materials are suitable for delivery by destructible
containers for the purpose of creating a fluid-diverting plug. Suitable solid
diverting
agents include, but are not limited to, rock salt, wax beads, oil-soluble
resins, benzoic
acid flakes, degradable polymer particles and fibers, cellophane flakes,
various
precipitates, nuts, and shells. Diversion may be used to enable treatment
redirection in
matrix stimulation operations below fracture pressure as well as in single or
multi-
stage hydraulic fracturing. In matrix stimulation the effect may be achieved
by
reducing the permeability of the formation because of solids penetration. The
mechanism for solid-assisted diversion during fracturing operations is more
complicated and is based on bridging of the fibers and/or particulates in the
fracture
with subsequent accumulation of additional solid material on the bridge,
creating a
plug. The advantage of diversion with solids over other treatment redirection
methods
is in lower cost and simplicity. However the amount of solid material required
for
effective diversion also needs to be designed properly, which is not always
technically
practicable, especially in multi-stage fracturing treatments. Introduction of
the
diverting material in a volume less than required may lead to poorer or no
diversion;
introduction of excess diverting material may result in its accumulation in
the
wellbore and possibly in a screen-out.
Diverting materials inside destructible downhole containers may be in many
forms, such as particulates, approximately spherical particles, particles
having aspect
ratios less than about 5 and preferably less than about 3; fibers, flakes,
viscous or
viscosifiable fluids, and mixtures thereof Such diverting materials may be
degradable, removable, soluble in wellbore or formation fluids, or meltable.
In the
case of mixtures of diverting materials, some components of such mixtures may
be
stable at downhole conditions and some may be degradable, removable, soluble
in
wellbore or formation fluids, or meltable. Diverting materials may also
disintegrate
into smaller pieces under downhole conditions after creating seals.
In one embodiment, one example of special solid diverting materials,
destructible downhole containers are filled with blends of particles designed
for
sealing narrow voids such as perforations, fractures, wormholes, etc. There
are many
such designs. In an example disclosed in U. S. Patent Application Publication
No.
2009/0025934, the diverting agent is a blend including a first amount of
particulates
22
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
having a first average particle size between about 2 mm and 2 cm and a second
amount of particulates having a second average size between about two and ten
times
smaller than the first average particle size or a second amount of flakes
having a
second average size up to ten times smaller than the first average particle
size. In
another example, the blend includes a first amount of particulates having a
first
average particle size between about 50 to 100 % of the perforation diameter
and a
second amount of particulates having a second average size between about 1.6
and 20
times smaller than the first average particle size, or a second amount of
flakes having
a second average size up to ten times smaller than the first average particle
size. Yet
another example is disclosed in U. S. Patent No. 7,784,541: a blend having an
amount
of particles having a first average particle size between about 200 and about
2000
microns, an amount of particles having a second average particle size between
about
three and about ten times smaller than the first average particle size, and an
amount of
particles having a third average particle size smaller than the second average
particle
size. Yet another example is disclosed in U. S. Patent No. 7,004,255: a blend
of
coarse particles having diameters from about 0.20 mm to about 2.35 mm, and a
quantity of smaller particles selected from medium particles, fine particles,
and
mixtures thereof; preferably the coarse particles have diameters from about
0.20 mm
to about 0.43 mm, the medium particles have diameters from about 0.10 mm to
about
0.20 mm, and the fine particles have diameters less than about 0.10 mm. In yet
another example, disclosed in U. S. Patent Application Publication No.
2010/0152070, the diverting material includes a mixture of coarse particles,
for
example having an average particle size of from 300 to 1200 rim, medium
particles,
for example having an average particle size of from 20 to 150 [tm and
optionally fine
particles, for example having an average particle size of from 5 to 15 [tm,
and a blend
of long fibers, for example having an average length of from 8 to 15 mm and
short
fibers, for example having an average length of from 1 to 8 mm; the long
fibers are
rigid and the short fibers are flexible; the long fibers form a tridimensional
mat or net,
for example in a lost-circulation pathway, that traps the mixture of particles
and short
flexible fibers to form a plug. Yet another example is disclosed in U. S.
Patent
Application Publication No. 2010/0298175: a blend of coarse, medium and
optional
fine particles, and a blend of two different rigid fibers that includes fibers
of different
lengths or different diameters or different compositions, in which at least a
portion of
23
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
the medium particles or coarse particles or both swells in the presence of
oil.
Additional blends that may be used as special solid diverting materials are
known or
may be developed. It is particularly important that such blends of special
solid
diverting materials be delivered to the diverting site with as little dilution
or size or
shape separation as possible; this is achieved by the destructible containers.
For enabling better release of the diverting material from the container after
its
destruction, the diverting material may optionally be placed inside the
container in
slurried form. In a slurry, there is less chance for solid/solid contacts to
form and to
resist mixing forces when the solids are subsequently exposed to the fluid
outside the
shell. The liquid phase acts as a lubricant, as well as a suspension agent,
and helps the
particles to be released rather than forming agglomerates that don't break
apart. In one
specific embodiment, diverting material is loaded into a destructible
container in a dry
form and then becomes slurred in wellbore fluid which penetrates the container
after
exposure of the container to the wellbore fluid.
The thickness of the container shell may range from about 0.01 mm to about 5
mm, preferably from about 0.05 mm to about 2 mm, and most preferably from
about
0.1 mm to about 1 mm. Optionally, the container may be made with several
layers,
for example up to about 10 layers, that may be the same or different. Multiple
layers
increases the mechanical stability of the container and/or allows control of
the
dissolution time of the shell. In one embodiment, the material, for example
special
solid diverting material, is placed into heat shrinkable plastic film (for
example a
polyvinyl alcohol film or fabric, embossed polyvinyl alcohol film,
polyethylene film,
other polyolefin films, PVC film, oriented films having at least one or two
oriented
layers, multilayer oriented films, shrinkable polyester films such as those
made of
polylactic acid, polyglycolic acids or other polyesters or copolymers thereof,
polysaccharide films such as starch films or cellulose films, etc.), sealed
in, heated to
cause shrinkage to form a container, and if desired for greater strength the
first
container is placed into a second heat shrinkable film, sealed in, and heated
to cause
shrinkage to form a stronger container. Additional films may also be used. The
films
may be the same or different. Such film or films may optionally be selected to
degrade at a desired rate. In another embodiment the material, for example
special
solid diverting material, is placed into a hollow plastic ball that is
initially in at least
24
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
two components that are then sealed together. In one specific example these
components are two half spheres. Optionally, the components may be made of a
gelatin, for example from a mixture including water, a water-soluble polymer
gelatin
material that may include, for example, agar or processed seaweed, non toxic
white
glue, and the like, plasticizers, and a preserving additive such as benzoic
acid, that is
formed and dried.
The tensile strength of the container shell, especially for mechanically
destructible containers, is preferably in the range of from about 1 MPa to
about 1000
MPa, more preferably from about 5 MPa to about 300 MPa, and most preferably
from
about 10 MPa to about 100 MPa. The Young's Modulus for the container shells is
preferably in the range of from about 0.01 GPa to about 200 GPa, more
preferably
from about 0.1 GPa to about 100 GPa, and most preferably from about 0.1 GPa to
about 10 GPa. For containers that dissolve, melt, react, disintegrate, etc.,
optionally in
addition to mechanical destruction, during pumping or downhole, the preferred
time
for this to occur is from about 1 second to about 1 hour, more preferably from
about
seconds to about 30 minutes, and most preferably from about 1 minute to about
15
minutes, at a preferred temperature range of from about 1 C to about 100 C,
more
preferably from about 10 C to about 50 C, and most preferably from about 10
C to
about 30 C.
It is preferable to use destructible containers (with their contents) having a
density similar to that of the injected fluid, although higher density
destructible
containers may be used at high pumping rates. The preferred density is from
about
0.5 to about 5 times the fluid density, more preferably from about 1 to about
2.5 times
the fluid density. The preferred density is from about 0.5 kg/L to about 5
kg/L, more
preferably from about 1 kg/L to about 2.5 kg/L.
One method of manufacturing a water soluble skin containing another material
is described in W01992/022355 which discloses making a water-soluble golf ball
"comprising a core, said core, formed of a first water soluble material, and
an external
skin formed from two skin halves or semi-spheres, said skin formed of a second
water
soluble material, when the two skin halves or semi-spheres and core are
adhered
together with a water soluble non toxic adhesive..." The skin is made for
example
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
from paper pulp or from material selected from gelatin, agar, processed
seaweed, and
non toxic glue.
Containers comprising various filling materials can be made by placing such
materials into hollow objects or chambers, preferably of spherical shape.
Methods of
making hollow plastic spheres are disclosed in Japanese Patents 56021836,
57066920,
and 61239936; and Japanese Patent Application 2005349678 also discloses a
plastic
ball containing a closed cell foam.
U. S. Patent No. 7,395,646 discloses an article packaging device and a method
for packing individual articles in a tubular thermoplastic sheet. U. S. Patent
No.
7,306,093 describes a method and apparatus of packing materials, including
fiber-
comprising bulk materials, into a sealed package shaped like a bale. U. S.
Patent No.
7,739,857 also discloses a method and apparatus for vacuum packing of fiber
and
other materials into one or more bales and packages. All these methods and
devices
may be adapted for use in some embodiments.
Containers with various fillers can also be prepared for use by surrounding
portions of the fillers with a polymer or thermoplastic material. In some
specific
examples, shrinkable films or stretch films can be used. Shrinkable films and
methods
of making such films are disclosed in U. S. Patent Nos. 7,846,517 (polylactic
acids),
6,340,532 (polyesters), 7,638,203 (polyesters), 7,744,806 (polyamides), and
6,340,532 (polyethylenes). A method of shrink-wrapping a material into a
shrinkable
plastic film with sample holes is disclosed in U. S. Patent No. 7,172,065
.A process of preparing water-soluble containers is disclosed in U. S. Patent
No. 6,898,921. The process comprises a) thermoforming a first poly(vinyl
alcohol)
film having a water content of less than 5% to produce a pocket; b) filling
the pocket
with a composition; c) placing a second film on the top of the pocket; and d)
sealing
the first film and the second film together. The process may be adapted for
use in
some embodiments.
There are also disclosures of methods of making paint balls which comprise
deformable mechanically destructible shells and liquidized filling
compositions (for
example U. S. Patent Nos. 5,254,379; 5,393,054; and 5,639,526). There are also
26
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
numerous disclosed methods of making golf balls, making multilayer golf balls,
and
finishing golf balls (for example U. S. Patent Nos. 5,122,046 and 6,887,135;
G. B.
Patent No. 2319481, and U. S. Patent Application No. 2004/092,335). These
methods
may be adapted to manufacture the containers filled with solids, liquids, or
gases.
Destructible containers are intended to be introduced into a wellbore and
pumped down to a target zone. For introducing such containers into the fluid
for
destruction in the wellbore, a standard or modified flow injector, for example
those
used for ball sealers may be placed in the high pressure line. Such devices
are
typically used for injection of large destructible containers (greater than
about 10 mm
in diameter) and the injector is commonly installed after the pumping units so
the
destructible containers are not subjected to forces that would break them in
the
surface equipment. A schematic is shown in Figure 4. Destructible containers
are
loaded into the accumulator, which is isolated from the main pumping line by
two
remotely operated valves. (Note that the same technique can be used to
increase the
concentration of destructible containers in the accumulator as is used to
increase the
concentration of particles in a fluid: use a mixture of a first size of
destructible
containers, and a second size of from about 7 to about 10 times smaller than
the first,
optionally a third size of from about 7 to about 10 times smaller than the
second, and
optionally additional sizes. This mixture of sizes can also be used for
selective
destruction (for example of selected amounts) at specific locations (for
example by
different-sized restrictions) and for selective delivery of different
materials (in
different sized destructible containers) at different locations.) Then the
accumulator is
closed, valves are opened and the containers are flushed from the accumulator
by
pumping fluid. A simple flow-through injection apparatus may also be used.
When it
is desired that the containers be destroyed at the surface, flow-through
blenders or
blenders equipped with dry additive systems can be used.
For some applications, such as treatment diversion, destructible downhole
containers may be used for setting temporary seals (plugs) formed by the
contents of
the containers. There are several methods that may be used, if desired, for
removal of
the seals formed:
= Self degradation. Some examples of degradable materials are polyesters,
including esters of lactic acid, glycolic acid, other hydroxy acids and
copolymers
27
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
thereof; polyamides and copolymers thereof; polyethers and copolymers thereof;
polyurethanes, etc. Other examples of degradable materials were described
above.
= Reaction with chemical agents. Some examples of materials that may be
removed by reacting with other agents are carbonates including calcium and
magnesium carbonates and mixtures thereof (reactive to acids and chelating
agents);
acid soluble cement (reactive to acids); polyesters including PGA, PLA, esters
of
lactic acid, glycolic acid, other hydroxy acids, and copolymers thereof (can
be
hydrolyzed with acids and bases); active metals such as magnesium, aluminum,
zinc
and their alloys (reactive to water, acids and bases), etc.
= Melting of at least one component of a sealing blend. When the seal
contains
a meltable component, its melting results in reduction of the mechanical
stability of
the plug. Examples of materials that melt under downhole conditions include
hydrocarbons having 30 or more carbon atoms; polycaprolactones; paraffins and
waxes; carboxylic acids such as benzoic acid and its derivatives; etc.
= Dissolution of at least one component of the sealing composition. Plug
removal is also achieved through physical dissolution of at least one of the
components of the diverting blend in the surrounding fluid. Solubility of the
component(s) may depend significantly on the temperature. In this case, post-
treatment temperature recovery in the sealed zone can trigger the removal of
the seal.
Materials that dissolve in water include water-soluble polymers, water-soluble
elastomers, carbonic acids, rock salt, amines, and inorganic salts. Materials
that
dissolve in oil include oil-soluble polymers, oil-soluble resins, oil-soluble
elastomers,
polyethylenes, carbonic acids, amines, and waxes.
Disintegration of at least one component of the sealing composition. Plug
removal is also achieved through disintegration of the seal into smaller
pieces that are
flushed away. Materials that can disintegrate include plastics such as PLA,
polyamides and composite materials comprising degradable plastics and non-
degradable fine solids. It should be noted that some degradable materials pass
through
a disintegration stage during the degradation process; an example is PLA,
which turns
into fragile materials before complete degradation.
28
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
Although the discussion above emphasizes delivery of materials in containers
that are deliberately mechanically destroyed in the wellbore, when the
contents of the
container comprise special solid diverting materials designed to divert fluid
flow, such
as fibers, fiber flocks, and other shapes designed to form plugs such as
flakes, ribbons,
platelets, rods, solid precipitates, grains, and pellets; mixtures of
different sizes of
approximately spherical materials; and mixtures of fibers and/or or other
shapes such
as flakes and one or more sizes of approximately spherical materials, then the
container may also be destroyed by self-degradation, chemical degradation,
osmotic
rupturing, dissolution, melting and other mechanisms known for release of
conventionally encapsulated materials delivered to a downhole location;
optionally
the container may be partially degraded by one of these mechanisms and then
the
release of the contents completed by a mechanical method. A container for
special
solid diverting materials may optionally be a coating that is engineered to
degrade at a
specific rate to release the enclosed material at a predetermined time, or
pressure, or
depth. In these cases, the container may optionally degrade before it reaches
the zone
to be treated, in which case it releases a concentrated aggregation of the
container
contents. This is a method of introducing slugs of an additive, for example
fibers,
flakes and/or or particle blends, without having to attempt to feed slugs at
the surface.
In another embodiment, destructible containers are used for wellsite delivery
of materials that may be difficult to handle, such as fibers, fiber flocks,
fibrillated
fibers, ribbons, platelets, flakes, etc. that may be difficult to transport to
a well site
and then to meter into a fluid. For example, fibers, fiber flocks, fibrillated
fibers,
ribbons, platelets, flakes, etc. may be tightly packed and enclosed in a
destructible
coating so that the size of the containers is, for example, in the range of
about 1 to
about 10 mm. Various mechanisms of coating destruction may be used, such as
dissolution in water, mechanical destruction, reaction with chemicals, or
combination
thereof In one specific embodiment, the coating is a film made of water, a
soluble
polymer such as polyvinyl alcohol, starch or a gelatin. Optionally, the
gelatin may be
made, for example, from a mixture including water, a water-soluble polymer
gelatin
material that may include, for example, agar or processed seaweed, non toxic
white
glue, and the like, plasticizers, and a preserving additive such as benzoic
acid which
quickly dissolves in water after introduction of the containers into a pumping
fluid.
Such containers, comprising packed fibers, fiber flocks, fibrillated fibers,
ribbons,
29
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
platelets, flakes, etc., can be introduced into the treating fluid on-the-fly
using the
surface equipment traditionally used for wellsite delivery of proppants and
other
particulate materials. Such equipment includes, but is not limited to, flow-
through
blenders, dry-additive systems, ball injectors etc. Upon introduction of such
containers into the treating fluid, the shell is destroyed, releasing the
material, which
is dispersed in the treating fluid. This approach has several advantages over
the
traditionally used methods of delivery as it enables better metering and
eliminates the
risk of plugging surface equipment with fibers, fiber flocks, fibrillated
fibers, ribbons,
flakes, etc.
Some embodiments may be understood further from the following example.
Example 1
The results of the release of particulate materials from destructible
containers
made by shrink-wrapping solid slurries in a 50 micron polyethylene shell are
shown.
The contents of the containers are shown in Table 1. The results demonstrated
that the
contents of the destructible container should have good fluidity to promote
reliable
release of the slurry into perforations upon destruction of the container.
Experiment Liquid Phase Solid Phase Total
Volume
1 0.5% guar 700 lam PLA particles (50% by 65 ml
solution
volume)
(50% by volume)
2 0.5% guar 700 lam PLA particles (36% by 65 ml
solution volume)
(40% by volume) 100 lam PLA particles (15% by
volume)
lam PLA particles (9% by volume)
Table 1.
It should be noted that the slurry used for filling the container in
experiment 2 was
designed according to the recommendations for designing high solids content
fluids
CA 02835130 2013-11-04
WO 2012/155045
PCT/US2012/037512
given in U. S. Patent Application Publication No. 2009/0025934. Such a slurry
is
characterized by its high loading of solid material and good fluidity
properties.
Figure 5 shows a schematic of the experimental apparatus used for studying
the release of particulate materials from destructible containers. The
apparatus
consisted of a 50 mm transparent pipe, equipped with an injector for
containers, and
having one 8.4 mm perforation hole. The destructible container had an
approximately
spherical shape with a volume of 60 ml. The pipe was connected to a water pump
having a maximum pumping capacity of 36 L/min. Before the experiment a
destructible container was placed in the apparatus through the injector and
the
removable plug on the injector was replaced. Then the pump was started and the
container was displaced to the perforation by water at the maximum pumping
rate.
The following results were obtained.
= Destruction of the container in experiment 1 resulted in bridging of the
particulate
material at the entry of the perforation hole without squeezing of the
container
through the perforation.
= Destruction of the container in experiment 2 resulted in complete
squeezing of the
particulate matter and the shell of the container through the perforation
hole.
This result demonstrated that the composition of the material in the
destructible container should have good fluidity properties to enable reliable
release
into perforations upon destruction of the container. Example 1 shows that this
approach significantly increased the reliability of delivery and release of
the diverting
material from a destructible container.
31