Note: Descriptions are shown in the official language in which they were submitted.
CA 02836162 2013-12-10
1
MODULARIZATION FOR PRESCRIPTION FULFILLMENT AND ADHERENCE
RELATED APPLICATIONS
[0001] The present application is a continuation in part
application of U.S. Patent
Application Serial No. 14/097,850 filed December 5, 2013, titled "TECHNIQUES
TO
DELIVER MULTIPLE MEDICATIONS IN A SYNCHRONIZED MANNER," which claims
the benefit of U.S. Provisional Application No, 61/733,560 filed December 5,
2012, titled
"TECHNIQUES TO DELIVER MULTIPLE MEDICATIONS IN A SYNCHRONIZED
MANNER," each of which are incorporated by reference in their entirety.
BACKGROUND
100021 Patients frequently take multiple medications prescribed
by multiple physicians.
Often, there may be multiple pharmacies involved. Each of the prescriptions
has its own
instructions and schedule informing the patient of when and how to take each
medication. It can
be difficult and confusing for a patient to properly adhere to all the
schedules and instructions,
not to mention the effort required to obtain each individual prescription from
a pharmacy.
[0003] Coordinating multiple prescriptions within the context of
a complicated and dynamic
health care system involves additional challenges for pharmacies and other
health care
organizations. Recordkeeping, reporting requirements, and continually changing
patient needs
must be accommodated,
[0004] The present application is directed to these and other
considerations.
SUMMARY
[0005] The following presents a simplified summary in order to
provide a basic
understanding of some novel embodiments described herein, This summary is not
an extensive
overview, and it is not intended to identify key/critical elements or to
delineate the scope thereof.
Its sole purpose is to present some concepts in a simplified form as a prelude
to the more detailed
description that is presented later.
1
CA 02836162 2013-12-10
[0006] Various embodiments are generally directed to techniques to deliver
multiple
medications in a synchronized manner to improve patient adherence. Some
embodiments are
particularly directed to techniques to deliver multiple medications in a
synchronized manner to
individual patients in a coordinated and simplified packaging scheme. In one
embodiment, for
example, a system may comprise a patient customization engine operative on a
processor
component to receive a prescription order for a period of days comprised of
multiple
prescriptions for a single patient. Each prescription may be representative of
a medication and
include prescription data. The prescriptions may be aligned to allow for
filling the patient order
such that all prescriptions cover the same period of days. A customization
engine operative on
the processor component may determine a patient customized drug regime
schedule for all of the
prescriptions in the prescription order over the period of days. The
medications may be placed
into the appropriate medication packages of a single customized package based
on the PCDR
schedule. The single customized package houses all of the medications for all
of the eligible
prescriptions for a patient over a period of days and may inolude one or more
medication
packages for each day in that period of days. One or more medication packages
may be provided
for one or more dosing times for one or more days within the period of days,
10007] A patient intake engine operative on the processor component may
determine an
adherence assessment need for the patient. The adherence assessment may prompt
the patient to
respond to a set of questions. The questions may include how many pharmacies
the patient uses,
how many physicians prescribe medications to the patient, how many medications
a patient is
taking, the types of medications a patient is taking, disease state of the
patient, how many visits
to a hospital or emergency room that patient may have made over a recent time
period. The
patient responses to each question may be scored such that the greater the
number of pharmacies,
physicians, medications and hospital or emergency room visits result in a
higher score, The sum
of the scored responses may be compared to a threshold score in which a
patient score above the
threshold may indicate an adherence problem and/or a patient that may benefit
from adherence
assistance. In the alternative, certain patient responses to particular
questions may trigger the
system to notify the provider that this patient may benefit from the multi-med
adherence system.
Other embodiments are described and claimed.
2
CA 02836162 2013-12-10
=
(0008] To the accomplishment of the foregoing and related ends,
certain illustrative aspects
are described herein in connection with the following description and the
annexed drawings.
These aspects are indicative of the various ways in which the principles
disclosed herein can be
practiced and all aspects and equivalents thereof are intended to be within
the scope of the
claimed subject matter. Other advantages and novel features will become
apparent from the
following detailed description when considered in conjunction with the
drawings.
[0009] The present disclosure provides modularized coordination
and customization of drug
regimens, especially with respect to synchronization of multiple medication
prescription
fulfillment and long-term patient adherence.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] The present systems are illustrated by way of example,
and not by limitation, in the
figures of the accompanying drawings, wherein elements having the same
reference numeral
designations represent like elements throughout and wherein:
[0011] Figure 1 illustrates a network architecture diagram of an
embodiment of a system to
deliver multiple medications in a synchronized manner;
[0012] Figure 2 illustrates a block diagram of an embodiment of a
system to facilitate
delivery of multiple medications in a synchronized manner;
[0013] Figure 3 illustrates an embodiment of a logic flow to
deliver multiple medications in a
synchronized mariner;
[0014] Figure 4 illustrates an embodiment of a logic flow to
perform an adherence
assessment survey;
[0015] Figure 5 illustrates an embodiment of a computer screen
image for performing an
adherence assessment survey;
[0016] Figure 6 illustrates an embodiment of a logic flow to
reconcile the hours of
administration (140A) for multiple medications;
3
CA 02836162 2013-12-10
[0017] Figure 7 illustrates an example of a customized thirty (30) day
synchronized
packaging solution for multiple medications;
[0018] Figure 8 illustrates an embodiment of a computing architecture;
[0019) Figure 9 illustrates a sample calendar format;
[0020] Figure 10 is a high level diagram of a prescription fulfillment and
adherence system
in accordance with one or more embodiments;
[0021] Figure 11 is a high level diagram of a patient adherence system in
accordance with
one or more embodiments;
[0022] Figure 12 is a diagram of a method for medication coordination in
accordance with
one or more embodiments;
[0023] Figure 13 is a diagram of a method for patient adherence in
accordance with one or
more embodiments;
10024] = Figure 14(a)-(c) is a sample patient proscription order label;
[0025] Figure 15(a)-(c) is another sample patient prescription order label;
and
[0026] Figure 16 is a sample patient prescription calendar.
DETAILED DESCRIPTION
[0027] Various embodiments are generally directed to techniques to deliver
multiple
medications in a synchronized manner. Some embodiments are particularly
directed to
techniques to deliver multiple medications in a synchronized manner to
individual patients in a
coordinated and simplified packaging scheme.
[0028] Various embodiments may be implemented as part of a Multi-
Medication
Adherence Assurance (MMAA) system. The MMAA system is a customization engine
which
4
CA 02836162 2013-12-10
allows pharmacies and/or healthcare providers to create a customized
synchronized multi..
medication delivery and adherence program for customers (e.g., patients). The
MMAA system
may be categorized into multiple functions. There may be an adherence
assessment function, a
prescription synchronization function, a customization function, an inventory
function, a package
design function, customized labeling function, a prescription preparation
function, and/or a
prescription delivery function
[0029] The adherence assessment function generally refers to a survey
taken by
prospective participants in the MMAA program. The survey may be conducted on-
line or in
person using pen and paper at a participating pharmacy location. The survey is
designed to
assess whether the patient's current circumstances present an adherence
problem with respect to
taking their medications on the customized schedule that is developed based on
patient specific
activities of daily living. Activities of daily living include daily self-care
activities within an
individual place of residence, outdoor environment or both, such as, feeding,
bathing, dressing,
working, walking, leisure activities, behaviors, etc,
100301 The questions of the survey may be scored and compared to a
threshold score. If
the survey score indicates an adherence issue, the patient may be eligible for
participation in an
MMAA program. In that case, a pharmacy technician or heath care provider or
the system itself
may contact the patient's health care provider, physician and/or insurance
provider to seek
authorization for a new synchronized prescription plan or PCDR for the
patient. It is to be
understood that a patient may opt to participate in the program regardless of
the results of the
survey and that various methods of evaluating responses may be used besides
scoring. The
questions are filtered based on industry standards and evaluated by
pharmacist. Responses that
meet set criteria may be flagged for the pharmacist's attention as they may
indicate the patient
would benefit from the adherence program.
[0031] The synchronization function generally comprises coordinating all
of the patient's
current prescriptions and refills into a unified schedule such that the refill
date for every
prescription in the multi-med system is the same. Given the monthly calendar,
the period of days
that the prescriptions could he coordinated for may be 28, 29, 30, or 31 day
periods. In some
CA 02836162 2013-12-10
cases, a shorter or longer period of days is needed based on the patient's
particular medications
needs. In some cases, weekly alignment is best for a patient from an adherence
perspective and
in others 60 or 90 day periods are best for adherence. Individual
prescriptions may be re-written
such that re-fills and expirations are coordinated with other prescriptions in
order to align the
prescriptions within the selected period of days. Such alignment may assist
with adherence by
reducing the patient's number of trips to the pharmacy and allowing all
prescriptions to be filled
at once.
[0032] The prescription preparation function may be responsible for
filling and
packaging the patient's newly synchronized prescription plan. A single
pharmacy may create a
customized packaging solution that contains co-mingled (when permitted)
medications in one or
more medical containers on a patient customized administration schedule. The
one or more
medical containers may be pouches, blisters, bottles, trays, bags, or any
other such containers
suitable for holding medication, The prescription preparation function may
also include creating
a set of instructions for all the medications as well as determining a patient
customized drug
regimen that determines in which of the one or more medical container(s) the
various
medications may be packaged. The set of instructions may include one or more
of the following
a calendar, a table, digital forms, etc. and including information such as
drug indication, side
effects, patient friendly instructions, drug description, drug strength,
medication icons, symptom
icons, time of day icons, and/or complete list of all medication whether
contained within the
customized packaging or not. Figures 9 and 16 are sample formats for a
calendar. It is to be
understood a variety of formats could be used. The prescription delivery
function generally
comprises delivering the PCDR and customized packaging to a retail pharmacy,
patient, assisted
living, independent living, hospital discharges, hospital, skilled nursing
facility, and home
community based health care. A pharmacy technician or health care provider may
then contact
the patient for delivery or pick-up of the medications or in the case of
institutional settings
deliver the customized package and PCDR instructions to the patient, The
calendar may include
icons and/or words to convey information to the patient or caregiver, such as
time to take
medication, disease state that the medication is for (heart, lungs
stomach....) etc... The calendar
may use a variety of colors to assist in quick identification of medication
and/or dosage regimen
6
CA 02836162 2013-12-10
information such as the same color used for all morning pills, afternoon
pills, evening pills,
and/or bedtime pills. This color may also show up on the medication pouch or
package created
for that time period to further assist with patient adherence. The calendar
may include pictures
of the medication to assist the user in confirming the package has been filled
correctly and/or the
patient/caregiver that they are taking/providing the correct medications at
the correct time. The
calendar may also be barcoded such that additional information may be easily
reached by the
patient scanning with a smart device and/or so the calendar may be scanned to
confirm that it is
being provided with the correct patient package.
100331 With general reference to notations and nomenclature used herein,
the detailed
descriptions which follow may be presented in terms of program procedures
executed on a
computer or network of computers. These procedural descriptions and
representations are used
by those skilled in the art to most effectively convey the substance of their
work to others skilled
in the art.
[0034] A procedure is here, and generally, conceived to be a self-
consistent sequence of
operations leading to a desired result. These operations are those requiring
physical
manipulations of physical quantities. Usually, though not necessarily, these
quantities take the
form of electrical, magnetic or optical signals capable of being stored,
transferred, combined,
compared, and otherwise manipulated. It proves convenient at times,
principally for reasons of
common usage, to refer to these signals as bits, values, elements, symbols,
characters, terms,
numbers, or the like. It should be noted, however, that all of these and
similar terms are to be
associated with the appropriate physical quantities and are merely convenient
labels applied to
those quantities.
[0035] Further, the manipulations performed are often referred to in
terms, such as
adding or comparing, which are commonly associated with mental operations
performed by a
human operator. No such capability of a human operator is necessary, or
desirable in most cases,
in any of the operations described herein which form part of one or more
embodiments. Rather,
the operations are machine operations. Useful machines for performing
operations of various
embodiments include general purpose digital computers or similar devices,
7
CA 02836162 2013-12-10
[0036] Various embodiments also relate to apparatus or systems for
performing these
operations, This apparatus may be specially constructed for the required
purpose or it may
comprise a general purpose computer as selectively activated or reconfigured
by a computer
program stored in the computer. The procedures presented herein are not
inherently related to a
particular computer or other apparatus. Various general purpose machines may
be used with
programs written in accordance with the teachings herein, or it may prove
convenient to
construct more specialized apparatus to perform the required method steps. The
required
structure for a variety of these machines will appear from the description
given.
[00371 Reference is now made to the drawings, wherein like reference
numerals are used
to refer to like elements throughout. In the following description, for
purposes of explanation,
=numerous specific details are set forth in order to provide a thorough
understanding thereof. It
may be evident, however, that the novel embodiments can be practiced without
these specific
details, In other instances, well known structures and devices are shown in
block diagram form
in order to facilitate a description thereof. The intention is to cover all
modifications,
equivalents, and alternatives consistent with the claimed subject matter.
[0038] FIG. 1 illustrates a network architecture diagram 100 of an
embodiment of an
MMAA system to facilitate delivery of multiple medications in an adherence
friendly
synchronized manner. The MMAA system may be built upon a hub and spoke type of
logical
architecture in which a centralized hub pharmacy may be associated with
multiple spoke
pharmacies. The hub pharmacy may act as the central point of activity for the
MMAA system.
The spoke pharmacies, in turn, may have direct relationships with patients and
physicians. Thus,
a hub Rx server 110 may be representative of a computer system associated with
a hub pharmacy =
and an MMAA website for coordinating MMAA system activities. The hub Rx server
may be
coupled with a network 120 such as, for instance, the Internet. In addition,
the hub Rx server
110 may also be coupled with a hub Rx database server 115. The hub Rx database
server 115
may include multiple databases for storing data pertaining to the MMAA
program. Multiple
spoke pharmacy Rx servers 130 as well as one or more prescriber servers or
computers 150 may
be coupled with the network 120 to permit data exchanges with the hub Rx
server 110. Network
8
CA 02836162 2013-12-10
enabled patient computers and/or network enabled physician computers 150 may
also be
communicable with the spoke Rx servers 130 and/or the hub Rx server 110 over
network 120 if
so desired. Moreover, the hub pharmacy personnel may communicate with multiple
insurance
plans 160 and/or multiple physicians. It is to be understood that the Spoke
Pharmacies may be
linked to contact one or more insurance plan(s) 160.
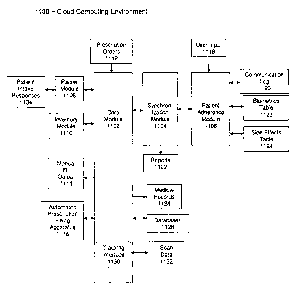
f00391 FIG. 2 illustrates a block diagram of an embodiment of an MMAA
system 200 to
facilitate delivery of multiple medications to patients in an adherence
friendly synchronized
manner. In one embodiment, the MMAA system 200 may comprise a computer-
implemented
system having one or more components. Although the MMAA system 200 shown in
FIG. 2 has
a limited number of elements in a certain topology, it may be appreciated that
the system 200
may include more or less elements in alternate topologies as desired for a
given implementation.
[0040] The MMAA system 200 may include a network device, such as the hub
Rx server
110. The hub Rx server 110 may be generally arranged to host and execute one
or more
additional MMAA system components. For instance, the hub Rx server 110 may
host an
MMAA website 210. The MMAA website 210 may be stored on the hub Rx server 110
and
operable via a processor component 205. The website 210 may be divided into
two parts. The
MMAA website 210 may include a public part and a protected part. The public
part of MMAA
website 210 may include general information that does not need to be specially
protected via
secure access techniques. Typically, the public part of the MMAA website 210
may not allow
access to any type of patient information (e.g., healthcare information),
account information
(e.g., user ID / password pairs), financial information (e.g., credit card
numbers), or specific
application information that is shared between an end-user (e.g., patient,
physician, pharmacy)
and the hub Rx server 110. Thus, when an end-user via a web browser seeks
access to the public
part of MMAA website 210, access may be granted over a connection such as, for
instance, the
Hypertext Transfer Protocol (1-r-rp).
[0041] HTTP is an application protocol for distributed communication among
networked
computers. HTTP is the protocol to exchange or transfer hypertext. HTTP
functions as a request-
response protocol in the client-server computing model. In this case, a web
browser, for example,
9
CA 02836162 2013-12-10
may be the client and an application running on processor component 205
hosting MMAA
website 210 may be the hub Rx server 110. The client submits an HTTP request
message to the
web server 110. The hub Rx server 110, which provides resources such as
Extensible Markup
Language (XML) files, Hypertext Markup Language (HTML) files and other
content, or
performs other functions on behalf of the client, returns a response message
to the client. The
response contains completion status information about the request and may also
contain
= requested content in its message body. Other protocols may be used as
well, and the
embodiments are not limited in this context.
[0042] The protected part of MMAA website 210 may include
information and
applications that are specific, unique, and private to individual end-users
(e. g, physicians,
patients, and pharmacies) of the MMAA website 210. Thus, when one of these
users accesses
the MMAA website 210, it can be done over a secure connection such as, for
instance, the
= Hypertext Transfer Protocol Secure (HTTPS) or other secure communications
protocol.
[0043] 111-1-PS is a communications protocol for secure
communication over a computer
network. HTTPS is widely deployed on the Internet. HTTPS is the result of
layering HTIT on
top of a secure socket layer (SSL) / transport layer security (TLS) protocol,
thus adding the
security capabilities of SSL/TLS to standard HTTP communications. HTTPS may
provide
authentication of the MMAA website 210 and associated web server 100 with
which a remote
computer is communicating over a network. HTTPS provides bidirectional
encryption of
communications between a client and web server 100, protecting against
eavesdropping and
tampering with and/or forging the contents of a communication. In the present
example,
HTTPS provides a reasonable guarantee that a remote computer is communicating
with the
intended MMAA website 210 and ensures the contents of communications between
the user and
MMAA website 210 cannot be read or forged by a third party.
= [0044] The hub Rx server 110 may be communicable over a network
120 such as, for
instance, the Internet. In turn, the network 120 may be communicable with
multiple network
enabled computers such as spoke Rx servers 130, patient computers 140, and
physician
computers 150. The connections between the network enabled computers 130, 150
and the web
CA 02836162 2013-12-10
server 110 over network 120 may be achieved using the aforementioned FITTP or
HTTPS
depending on the part of the MMAA website 210 with which a network enabled
computer 130,
150 wishes to communicate.
[0045] The protected part of MMAA website 210 may include multiple
components. The
multiple components may include, for instance, a website management component
215, a patient
intake engine 220, a synchronization engine 235, and a customization engine
240.
[0046] The website management component 215 may comprise a software
application
under control of the processor component 205. The website management component
215 may
be generally arranged to manage the interfaces between the MMAA website 210
and other
external components such as a network 120 (e.g., Internet) and multiple
databases. For example,
the MMAA website 210 may be communicable with a database server 115. The
database server
115 may be communicable with the hub Rx server 110 over a local network
connection and may
include a patient profile database 250 and an application database 260.
Communications with
the patient profile database 250 and an application database 260 may be
performed by, for
instance, a structured query language (SQL) interface. The embodiments are not
limited to these
examples.
[0047] The patient profile database 250 may store without limitation
information
pertaining to patient medical history, patient prescription data, patient
insurance data, physician
data, and adherence survey data. The application database 260 may store
without limitation
information pertaining to user registration such as login data, billing
information, and user
information such as contact data. The collection, storage, and management of
all health related
information shall be compliant with the Health Insurance Portability and
Accountability Act
(HIPAA). The embodiments are not limited to these examples. It is to be
understood alternate
database arrangements may be done and the patient and application data may be
stored in the
same database if so desired.
[0048] The website management component 215 may be further arranged to
manage the
MMAA website 210 accounts of end users and access by end users to the MMAA
website 210.
11
CA 02836162 2013-12-10
There may be two types or levels of MMAA website 210 users ¨ end-users and
website
administrators. MMAA website 210 administrators may control information and
services
provided to the end-users on the protected and public parts of the MMAA
website 210. End-
users may use the MMAA website 210 to access various services provided by the
MMAA
website 210. All communications between the MMAA website 210 and its end-users
may be
performed over HTTPS.
[0049] The website management component 215 may be further arranged to
manage the
end-user registration process. For example, end-users may register with the
MMAA website 210
using an appropriate secure socket layer (SSL) protected website form.
Registration may entail
creating a private user identifier (ID)/password pair using an SSL-protected
website form that
corresponds with the end-user. A registered end-user may login to the MMAA
website 210 by
providing their private user ID/password pair. If a User ID does not exist, a
generic error
message may be displayed such as, "Wrong login or password". An end-user's
User ID and
password may be stored in the application database 260.
[0050] The end-user's password may be hashed such that the end-user's
hashed password
may be compared with one stored in the application database 260. If it is
incorrect, the same
error message may be displayed. The MMAA website 210 may employ techniques
that make it
impossible to recover a forgotten password. Rather, a new password may be
assigned using a
standard access recovery and verification routine. The verification routine
may entail having the
end-user answer one or more pre-detennined personal questions the end-user
chose during the
registration process. The embodiments are not limited to these examples.
[0051] The patient intake engine 220 may comprise a software application
under control
of the processor component 205. The patient intake engine 220 may be generally
arranged to
guide and assist an end-user in populating the patient profile database with
the end-user's
personal and healthcare information relevant to the MMAA program. The patient
intake engine
220 may include a patient intake wizard 225 to perform this function, The
patient intake wizard
225 may be a software module that prompts the end-user for responses to
queries The patient
intake wizard 225 may ask the patient to answer questions pertaining to
eligibility in the MMAA
12
CA 02836162 2013-12-10
program. As an example, a patient may be eligible for the MMAA program only if
they approve
non-child resistant packaging, are not subject to mandatory mail order for
prescriptions, and have
a minimum number of qualifying medications, two (2) or more qualifying
medications that are
= currently being prescribed. Those eligibility requirements are by example
only, other factors
may be used to determine eligibility for the program. Utilizing the patient
intake wizard 225,
patient data, chart history, prescription data and insurance information may
be entered into the
patient's profile and stored in the patient profile database 250.
[0052] The patient intake wizard 225 software may be designed
as a web-based program
=
that each spoke pharmacy location may log into from their own spoke Rx server
130. Collected
patient intake data may be used to create a patient profile in which all of a
patient's prescriptions
may be linked to one file. Prescription data collected by patient intake
wizard 225 may be
forwarded to the synchronization engine 235 to be synchronized. The term
"synchronization"
may be used to define a process in which the hub Rx server 110 (e.g., a hub
pharmacy
technician) contacts the patient's insurance plan 160 to request the insurance
plan 160 authorize
all existing prescriptions in the patient's file based on an objectively
determined adherence
assessment and approve a synchronized prescription set for subsequent
adjudication by a spoke
pharmacy. In the alternative, it is to be understood that the hub and/or spoke
pharmacy may
obtain all new scripts of the patient's medications no adjudication is needed.
[0053] As part of the patient intake wizard 225, an adherence
assessment module 230
may be implemented to classify a patient's present adherence level pertaining
to their ability to
take all of their prescribed medications on the recommended administration
schedule and/or to
identify a patient's adherence risk. The adherence assessment module 230 may
determine a score
based on a number of factors used to determine the patient's need for an
adherence-based
packaging solution. The adherence score may be used as the basis to request an
insurance plan
160 to clear all previous prescriptions in a patient's file and allow for a
synchronization of new
prescriptions pursuant to the MMAA program. A patient's adherence need may be
determined
from the answers to the questions using a variety of determined acceptable
adherence standards.
The adherence standards may change based on the risk comfort level or needs of
the patient,
provider, pharmacy benefit manager and/or insurance plan. Answers to certain
questions may
13
CA 02836162 2013-12-10
automatically indicate the patient is a good candidate without any scoring
tool being used. In
addition, the patient's life-style or activities of daily living may qualify
them as an adherence
program candidate.
100541 The adherence assessment module 230 as administered by the patient
intake
wizard 225 may ask the patient to answer a series of questions. The questions
may include, but
are not limited to, the number of pharmacies the patient is currently
utilizing, the number of
physicians they have that are currently prescribing medications, the number of
medications,
admission into a skilled nursing facility, the number of hospital and/or
emergency room (ER)
visits within the last 30 days, how many medications the patient is taking,
the patient's health
condition/disease state, and whether the patient feels they would benefit from
an adherence
intervention or a support service such as reminder packaging. These questions
are by no means
all inclusive, and alternate questions may be used or added to determine
whether a patient would
benefit from an adherence program.
[0055] With respect to the first question, a score of 0 points may be
assigned if the
patient utilizes a single pharmacy. However, a score of 2.5 points may be
assigned if the patient
uses two pharmacies and a score of 5 points may be assigned if the patient
uses three (3) or more
pharmacies. With respect to the second question, a score of 0 points may be
assigned if the
patient sees a single physician. A score of 2.5 points may be assigned for two
physicians and a
score of 5 points may be assigned if the patient sees three (3) or more
physicians. With respect to
the third question, a score of 0 points may be assigned if the patient has not
visited a hospital/ER
in the last thirty (30) days, A score of 1 point may be assigned for one
hospital/ER visit. A score
of 5 points may be assigned for two hospital/ER visits and three (3) or more
hospital/ER visits
may have a score of 7.5 points assigned. With respect to the fourth question,
if the answer is yes,
a score of 5 points may be assigned. If no, 0 points are assigned. The
adherence assessment
module 230 may then calculate a total score for the patient based on the
answers provided. A
total score of less than 10 points may indicate the patient has only a
moderate adherence issue. A
score of greater than 10 points, however, may indicate the patient has a
severe adherence issue.
The embodiments are not limited to these examples. Different score values may
be assigned
based on answers and different questions may he used to solicit the answers.
The greater the
14
CA 02836162 2013-12-10
number of high risk responses the greater the need for adherence assistance.
In some situations,
a single high risk response may be sufficient to support a need for adherence
intervention.
[0056] The patient intake engine 220 may automatically provide the
patient's
prescription and insurance plan information to a hub pharmacy technician via a
user interface
and schedule a time for the hub pharmacy technician or health care provider to
contact the
patient's insurance plan 160 to request the insurance plan 160 authorize all
existing prescriptions
in the patient's file based on the assessed adherence need.
[0057] The hub pharmacy technician may receive the data from the patient
intake engine
220 and call the insurance plan 160. The hub pharmacy technician may request
the
synchronization of the patient's prescriptions. If the insurance plan 160
approves the request, a
spoke pharmacy technician may then successfully adjudicate the prescriptions.
If the insurance
plan 160 does not approve the request, the prescription(s) may be removed from
the first 30-day
supply (or whatever period of days are chosen for the first customized
package) and may be
added to the following cycle. The spoke pharmacy technician may obtain a
reduced quantity
prescription in order to provide the patient with a "reduced fill"
prescription in a vial for the
medication(s) that were not synchronized on the appropriate date.
[0058] The synchronization engine 235 may provide a scheduled time period
for a spoke
pharmacy technician to adjudicate the patient's prescriptions. Adjudication
may be necessary to
ensure coordination with the synchronization step in which the insurance plan
160 has approved
and replaced the patient's existing prescriptions with "synchronized" new
prescriptions. The
insurance provider of health plan may need to approve of the date for the
script to be filled. A
set production schedule may coordinate these time periods between the hub
pharmacy contacting
the insurance plan 160 and a spoke pharmacy adjudicating the prescriptions. It
is to be
understood, based on system preferences, that the spoke pharmacy may contact
the insurance
plan and that no adjudication may be necessary or that the hub pharmacy may
perform
adjudication for the spoke pharmacy.
[0059] The synchronization engine 235 may also calculate a synchronization
date based
on a hub pharmacy production schedule. Following the adjudication process, the
spoke pharmacy
may send the patient's prescriptions to the hub pharmacy in an electronic
format consistent with
CA 02836162 2013-12-10
that used for typical central fill facilities. These prescriptions may be
flagged to designate
fulfillment to be completed at the hub, mail order, spoke or central fill
pharmacy.
[00601 The customization engine 240 may automatically receive the
patient's
prescription orders from a spoke pharmacy. The prescriptions may be downloaded
from a secure
file transfer protocol site (sftp), virtual private network, or pharmacy
interface on a pre-
determined interval. The prescription orders may contain the physician's
instructions for taking
the medication and/or a product identification code. The product
identification code in this
situation may be the national drug code (NDC), the generic product identifier
(GPI), and/or the
generic code number (GCN). The physician instructions are assigned to the
particular
medications for each prescription. The customization engine 240 may also
obtain prescription
data from external sources (e.g., First Data Bank, Medi-Span, American
Geriatric Guidelines,
OBRA Guidelines), by using the product identification code, as well as a
proprietary file for each
specific prescription. It is to be understood that the customization engine
may also search
internal databases for this information. The product identification code that
it uses to search may
be the NDC code, the generic product identifier (GPI), and/or the generic code
number (GCN).
This second set of dosing instructions for each script is also assigned to the
respective
medication(s).
[0061] The prescription orders and the prescription data may be used to
determine a
combined dosing regimen that has the instructions for taking the individual
medications, Where
there is a conflict between the two sets of dosing instructions, the
customization engine may alert
the user, such that the user may contact the physician and confirm the proper
dosing instructions.
In the alternative, the customization engine may automatically contact the
prescribing physician
via email, text, fax, automated call, IM etc.... and alert him/her to the
conflicting prescribing
data and solicit his/her correction in that manner. Where there are no
prescribing instructions,
=the dosing instructions are set to a default setting. This default setting
may be morning, night,
afternoon, or any other chosen period suitable for the patient's schedule or
system preferenees,
100621 The dosing schedule is combined with personalized patient
information to create a
Patient Customized Drug Regimen (PCDR) schedule that determines which
medications, based
on the patient activities of daily living, need to go in which medical
container(s) of the
customized medication package. Only qualifying medications or medications that
can be
16
CA 02836162 2013-12-10
packaged into the medical containers by the robotic packaging machine 265 will
be placed into
the customized medication package, Extended packaged or reminder instructions
may be placed
on the outside of the package to remind the patient to take any non-qualifying
medication.
Qualifying medications are medications that the robotic machine is instructed
and capable of
packaging into the customized medication package, whether it be by using a
drop try or by
standard operation of the robotic machine, Non-qualifying medications may
include inhalers,
liquid medication, patches, topieals, sublinguals, troches, powders,
atomizers, infusions,
injections, sprays, drops, effervescents, capsules, suppositories, or
medications that should not be
combined or will not work with the robotic machine.
[00631 Personalized patient information may be their specific symptoms to
the drugs,
schedules (personal or professional or medical), and/or conflicts between
medications.
PCOR Assignment Process
[0064] The customization engine 240 may also be configured to execute a
medication
dosing assignment module or PCDR module 245 that determines how various
medications may
be placed into individual medication containers. A prescription order data set
that includes
multiple prescriptions for various medications may arrive from a spoke
pharmacy for filling. The
dosing assignment module 245 may parse the prescription data for each
prescription to determine
any specific physician assigned dosing instructions in the SIG (prescriber
instructions), If the
physician/prescriber has assigned specific dosing times in the SIG, then those
dosing instructions
are incorporated into a pera schedule. If not, the remaining prescription data
will flow to an
MMAA dosing assignment program implemented by the dosing assignment module
245. Each
medication's Product Identification Code (PIC) may be used to match codes
indicated in a
proprietary file developed from one or more internal database(s) and/or an
external source such
as the aforementioned First Data Bank, Medi-Span, Drug Manufacture Guidelines,
Red Book,
Gold Standard, American Geriatric Guidelines, 0I3RA Guidelines and/or any
other similar
sources. If a match for the PIC is found for a given prescription, that dosing
instruction may be
utilized for that prescription. If not, all remaining prescriptions may be
provided a default dosing
assignment.
17
CA 02836162 2013-12-10
[0065] The dosing assignment module 245 may then determine
whether a medication
from a prescription can be co-mingled and identify these medications as
requiring a separate
medication container according to that medication's GPI. The non co-mingled
medications may
then be packaged in separate medication containers with the first default
assignment and labeled
as "1 of total (e.g., "1 of 3", "2 of 3", and "3 of 3") for a dosing period.
In the alternative, a
medication in the robot machine inventory may be identified as being co-
mingled or not co-
mingled. Co- mingling may be an assignment done at the robotic packaging
machine.
[0066] Color coding may be used on the multi-med application
label(s) to indicate time
of day to take drugs contained in the multi-med package. Additionally other
symbols could be
used to indicate the time of day to take the medication in the package.
Furthermore, vials, blister
packs and other such medication containers that may or may not be a part of a
multi-tried
package could use color coding, icons, symbols or particular wording to
indicate the time of day
to take the drugs, the particular patient in a multi-person household, the
type of drug contained in
the package, and/or the strength of the drugs.
[0067] The first default assignment may be labeled the
"Morning/AM" period, based on
an assumption that patients will likely take their medications before starting
their daily activities.
The customization engine 240 may determine the number of medications that have
been assigned
to the "Morning/AM" pouch. The dosing assignment process may cap the number of
medications
for any given administration time at any number chosen by the program users.
For example, up
to four pills per administration time, up to two pills per administration
time, up to 6 pills per
administration time, or other such chosen number. This number may be assigned
based on
patient preference, patient abilities to swallow medication, patient age,
doctors/prescribers
preference, filling pharmacies preference, medication container size,
medication available
strength, SIG, care giver preference, pharmacist preference, federal rules and
regulations and /or
other such reason.
[0068] If the "Morning/AM" pouch has not been filled by the pre-
determined number of
medications, the next prescription in the file may be assigned to this
medication container. The
next prescription in the file may then be assigned to the current medication
container until that
= medication container reaches its maximum limit of four medications or
whatever the chosen
maximum number is If there are additional medications to be assigned, the
customization engine
18
CA 02836162 2013-12-10
240 may repeat the same process for a second dosing period, such as an
afternoon container,
mid-morning container, evening container etc... There may be multiple dosing
periods
throughout the day. For some patients two dosing periods are sufficient, for
others up to four, for
others up to 8, and so on. The system may have a set number of dosing periods
arranged in a
prioritized filling order such as ¨ morning dosing period always filled to
maximum first,
followed by evening dosing period, followed by afternoon dosing period, and so
on, or the
system may allow for customization of dosing period prioritization based on
patient, prescriber,
pharmacist, activities of daily living, insurance provider, care giver or
another such persons or
reasons preference. If there are still additional medications to be assigned,
then the
customization engine 240 may repeat the same process for a third dosing
period. The dosing
assignment process repeats until all prescriptions in the file have been
assigned to a specific
dosing period,
[0069] The
PCDR module 245 may be prograrruned to assume that all prescriptions taken
less frequently than daily will have a calendar date for administration listed
in the SIG. The
PCDR module 245 may further be programmed to assume that all medications to be
taken at
multiple administration times within a 24 hour period start with the
"Morning/AM" assignment.
The customization engine 240 may also be programmed to assume that
prescriptions with
alternating medication strengths will have the calendar date of administration
of the first dose in
the SIG. Any deviation from these assumptions or a lack of data for these
cases may be rejected
back to the spoke pharmacy to contact the prescribing physician for
clarification or to generate
an automated communication to the prescribing physician's office for
clarification. A hub
pharmacy technician may access the customization engine 240 via MMAA website
210 to
review any pertinent physician notes and match the patient's prescriptions to
the medications
currently available in the canisters contained within the drug packaging robot
265. The
customization engine prepares one or more set(s) of instructions for the
customized medication
package. This set of instructions may be a digital set of instructions that
can be programed into a
phone or device or emailed to a patient and/or pharmacist and/or doctor or it
may be a one-Page
BOA patient label, an adherence calendar, packaging instructions, packaging
labels, inventory
reports, and/or delivery reports. The set of instructions may contain the
patient's PCDR, drug
information, pill count, pharmacy information, prescriber information or other
such information.
19
CA 02836162 2013-12-10
[0070] The customization engine 240 may have a quality
assurance technician review
and check all reports before forwarding an order to a robot filler 265. The
customization engine
240 may then prepare the customized packaging and print out or send one or
more set of
instructions, which may be a patient label, adherence calendar, or any other
set of instructions
that complement the patient's customized packaging. The customized package may
contain
= individually labeled pouches, wherein the labels may be icons or
instructions to indicate dosing
time for the medications contained within the pouch, or other types of dosing
instructions.
[0071] The drug package design module 247 may assign the
appropriate dosing
instructions to the individual medication containers within the customized
package as well as one
or more of other patient specific and/or container specific information,
including but not limited
to patient name, day of the week, date to be taken, time to be taken, drug
name(s), drug code,
drug strength, number of tablets of each drug in the individual container,
12..x number, product
identifier code, expiration date of the product, manufacturer, manufacturer
lot number, name
= address and/or phone number of the pharmacy packaging and/or dispensing ,
medication
indication (indication for which the medication is prescribed), prescriber's
name, description of
the medication and/or imprint, package certification number, and/or the number
of containers to
be taken per dosing period. Based on manufacturing preferences, the dosing
instructions and or
other specific patient information may be contained within the set of
instructions created
separately and/or printed directly on the customized package itself. It is to
be understood that
icons may be used instead of wording. Once completed, the hub pharmacy
technician may place
the customized packaging and documentation into a hub checking stage.
Hub Filling Process
= [0072] A customization engine 240 identifies the
available canisters in inventory through
= its inventory canister management module 246 and may print or display a
Special Tablet System
(STS) report from the customization engine 240 for a store level batch. If
desired, the
customization engine may delay running a patient's customized package if there
is a medication
requiring the special tablet system. Medications that may require the special
tablet system may
be one or more of the following: half tablets, cyto-toxic medications,
medications without an
CA 02836162 2013-12-10
available canister, medications that are friable or have heat sensitive
coatings, or any medication
that requires special handling. Any medications that are not available in the
robot canisters may
be filled utilizing the STS process. When the STS report is being run, the
inventory canister
management module 246 is also checking the drug inventory within the robot
filler 265.
[0073] The inventory canister module 246 may track inventory
coming into the
pharmacy from multiple. places, assign origin information to the inventory and
customize the
canister(s) in the robotic filler to receive the inventory under one or more
of the following
identification codes, including but not limited to: customers name, patient's
name, drug name,
providers name, prescribers name, insurance providers name, drug number, drug
PIC, etc... The
identification code may he used to assist the robot filler in determining
which of the canisters to
use when filling an order. The customization engine 240 will facilitate
filling and refilling of
the canister through the use of identification codes, which may be associated
with a barcode.
The manufacturers medication may be associated with particular information
including but not
limited to drug name, image, strength, expiration date, lot number, pill size,
drug location, drug
linkage, pharmacy information, provider information, technician handling etc-
, All this
information may be stored within the inventory management module 246 to assist
the hub
pharmacy in tracking the medication used to fulfill scripts.
[0074] The inventory canister module 246 may also track and
alert the user regarding
= canisters that are not being utilized, This may prevent the drugs from
expiring, may promote
better storage for the medications, allows for more efficient usage of the
robotic machine such
that the canister may be re-purposed for a medication that is used more
frequently. The
inventory consider module 246, compares incoming orders against the available
canisters and
may sort the processing batches so they may be run most efficiently. For
example, the module
246 may identify which orders will need drugs not currently in available
canisters, and pushes
those runs towards the end so the user may reduce the number of times the
robotic machine
canisters need to be changed. All orders, whose medications are currently
within the machine,
will be processed in a row or a continuous batch if so desired. Once the first
set is done, the user
will be able to review the an inventory report from the inventory module 246
and switch or add
all the canisters needed to run the second batch or the batch of orders moved
to the end of the
run,
21
CA 02836162 2013-12-10
[0075] The customization engine 240 may print Or display an inventory
report after it
determines which canisters need to be re-filled prior to any batch runs. If
the medications are
available, the hub robot technician may refill the canisters, leaving the
filled canisters in the
staging area. If the medications are not available or in a "back-order"
status, the hub robot
technician may switch manufacturers for the product in the system, deactivate
the existing
canister in the robot, and then add the prescription to the STS report.
Patients having a dispense
as written (DAW) prescription that is not available due to a short supply, may
have that
prescription filled directly at a spoke pharmacy.
[00761 The hub pharmacist may check all of the re-filled canisters in the
staging area
prior to placing the canisters back into the robot. The hub robot technician
and the hub
pharmacist may then sign an electronic canister fill log in a customization
engine 240 inventory
management module 246. The hub robot technician may then ensure that the robot
filler has an
adequate supply of pouches and print ribbon for a batch run. The hub robot
technician may
review the final STS Report, scans their unique ID (bar code) and the STS
report, thereby
initiating a record which the customization engine 240 may write to the
patient profile database
250. The patient profile database 230 keeps a record of how their medication
package was
prepared, including but not limited to the technician who packaged it, the
technician who
checked the tray, the technician who quality checks the package, the
technician that packaged it
for shipping, the date run, the time scanned, the manifest is checked against
the staffing log, the
medications in the package, the set(s) of instructions, the instructions
printed on the medication
containers, the instructions sent with the medication package, the specific
patient's information,
the dispensing pharmacy information, the filling pharmacy information, the
tray number and slot
number for the STS system, It is to be understood that the foregoing could be
performed by a
pharmacist or a technician.
[0077] The hub robot technician may locate and brings all medications
required by the
STS report to the STS filling area. The trays may then be filled in the order
they will be staged
for the batch. The hub robot technician may leave the medication containers in
the area for
inspection and sign the electronic STS report. The hub pharmacist may scan
their unique ID (bar
code) and checks 100% of the STS trays against the original medication
containers for the batch.
The hub pharmacist may then electronically sign the STS report, thereby
completing the record
22
CA 02836162 2013-12-10
in the customization engine 240 program. The hub robot technician may return
all medications
required by the STS report to their original location.
100781 The hub robot technician may then initiate the batch
for the robot filler. When the
robot requests an STS tray, the hub robot technician may scan their ID and the
STS tray prior to
staging within the robot. The hub robot technician may monitor the filling
process for the batch,
repeating the STS tray staging as requested by the robot. If a canister issue
develops, the robot
display may identify the type of problem and the location of the canister. The
hub robot
technician may address any issue that is required and re-start the filling
process for the robot.
During the batch, the hub robot technician may be monitoring the packaged
medications as they
exit the robot for any obvious process or medication defects. If a problem
arises, the hub
pharmacy technician may pause the robot filler and diagnose the problem, and
may restart the
robot as necessary to complete the batch.
Quality Assurance Checks
00791 The hub quality assurance (QA) technician may retrieve
each medication strip
pack from the robot and place it in a QA inspection area. The hub quality
assurance technician
may scan their ID as well as the individual strip pack they are checking to
create a record in the
customization engine 240 program in the name of the QA technician conducting
the check. The
hub quality assurance technician may visually check each pouch for crushed or
broken tablets,
miss-drops, print quality issues, defective pouches, and the required number
of tablets per BOA,
Any defective pouches may be de-blistered and the patient order may be
repackaged to correct
the defect (repeat filling process). Once the batch check is completed, the
hub quality assurance
technician may scan their ID and electronically sign the manifest, and then
stage the pouches for
the hub pharmacist to review. If there is an error or an issue with a package,
the customization
= engine may create a label for that specific package that contains the
printed information that was
on the original package or adjusted information for the corrected package. The
technician can
then place the label onto the corrected package. In the alternative, an error
message may be
= placed on the package with corrected instructions ¨ don't take the extra
pill. The re-print or
23
CA 02836162 2013-12-10
adjusted instructions may be inputted by a pharmacist, prescriber, robot
machine operator,
technician and or any other qualified person.
[0080] The customization engine may use a drug package modification system
to allow
the pharmacist to access a batch that has been run, make a manual correction
within the package
run, print reports to show correction and/or labels to show correction, and
review any packaging
errors and corrections. The user may identify the batch by using one or more
of the following:
including but not limited to, patient name, batch number, bar code, patient
code, drug name,
prescriber name, spoke pharmacy identifier, technician etc... The corrected
information is added
to the patient's customized package report and the new order merged with the
existing order.
Instructions and/or labels may print automatically or may display
automatically. The pharmacist
will then be able to make and easy manual correcting within the container and
reseal the
package. This system allows for package correction without needing to run the
entire script
again, which decreases time and waste. In addition, should a recall issue,
this process allows for
manual correction with reduced packaging errors as the recalled drug may be
pulled and the
remaining drug left unaffected in the packages.
100811 The hub pharmacist may scan their ID and the individual strips of
pouches to
create a record in the customization engine 240 program of the name of the hub
pharmacist
conducting the check. The hub pharmacist may visually check the header pouch
of the strip
against a manifest, the actual ID of the medications against the prescription,
and ensure the 1-I0A
matches the physician's directions. Once a batch has been checked, the hub
pharmacist may scan
their ID and electronically sign the manifest. The hub quality assurance
technician or robotic
machine may roll or stack the individual medical containers or pouches and
prepare them for
placement into dispenser cartons or customized medication package. The
customized medication
package may then be placed in a staging area for shipping preparation.
[00821 In the alternative, software and/or mechanical means may be used
alone or in
combination with human technicians and or pharmacists to do optical character
recognition,
OCR, testing to confirm the medications contained in the packaging is correct.
A customized
medication package preview report may be prepared for each patient's
customized medication
package. The preview report may be used to compare against the final packaging
to confirm the
packages are filled correctly. The PCDR report may also be used alone or in
combination with
24
CA 02836162 2013-12-10
the preview report to confirm the customized medication package is prepared
correctly. In
addition to OCR, high speed videos and/or cameras may capture images of the
packaging and be
used to compare against the preview report and/or the PCDR report and/or the
drug image files.
Packages Prepared for Shipment
[0083] The hub quality assurance technician may scan the rolled pouches
for the store
level batch, The hub quality assurance technician may then match the patient's
calendar to the
corresponding package with attached patient label patient receipt, and patient
information leaflets
and the manifest, bar code scan each item, and then place the strip pouches
into the labeled
package. The customized packaging may then be closed and the patient calendar,
patient receipt,
and patient information leaflets are packed and bagged, affixed or inserted
into the carton. Each
completed patient order may then be staged in the batch order area. The hub
quality assurance
technician may scan each filled patient order prior to placement in a shipping
tote. The hub
pharmacist may rescan each individual patient as they are placed into the
individual spoke
pharmacy shipping tote, The scans may be checked against the spoke pharmacy
manifest as a
final quality check. The hub quality assurance technician may then secure the
spoke store
shipping container and pack the shipping manifest in the container. A copy of
the shipping
manifest may be retained at the hub pharmacy. The shipping tote and associated
shipping
documents may be given to a delivery service provider to ship the customized
packaging to the
spoke pharmacy location.
Medications Shipped and Dispensed to the Patient
[QOM The spoke pharmacy technician may receive the shipping tote and
scan in all
medications from the master shipping manifest. The spoke pharmacist may enter
their signature
of receipt into the spoke pharmacy system. This scan may release the
prescription for customer
pick up from the spoke pharmacy's system and send an electronic receipt back
to the hub
pharmacy. The spoke pharmacy technician may place finished packages in a
designated "will
call" storage area. A spoke pharmacy AVR (automated voice response) may call
the patient to
CA 02836162 2013-12-10
inform them that their medications are available for pick up. Alternatively,
the spoke pharmacy
and patient may arrange for delivery of the customized prescription packaging.
The patient may
arrive at the pharmacy to pick up their customized package of medications and
the spoke
pharmacist may discuss the medications, show the patient how their medications
are packaged,
how to open the containers, and discuss the information available via the
associated bar code or
= other databases, websites, sources. The spoke pharmacist may also perform
an MTM/CMR,
including explaining and discussing the importance of medication adherence and
bow it is
supported and enabled by the MIVIAA customized packaging. At the time the
medications are
dispensed, the spoke pharmacist may collect payment of the monthly MMAA fee
and co-pays
from the patient. Payment may also be made before the customized medication
package is
prepared.
Refills and Chanies to the Medication Routine
[0085] The spoke pharmacy central fill facility or call center
may confirm the patient's
next multiple prescription order via automated phone call or an on-line
reminder tool. The spoke
pharmacy AVR may ask the patient if they have any new prescriptions or changes
to the existing
medications and if so the call may be routed to the spoke pharmacy, the
healthcare provider, the
hub pharmacy as needed to discuss the changes in prescriptions. The spoke
pharmacist may
review any new prescriptions or changes and may consult with the prescribing
physician as
necessary to determine when to initiate the new routine (synchronizing the new
or changed
prescription). Any changes may be sent to the hub pharmacy for the next fill
cycle.
[0086] Included herein is a set of flow charts representative
of exemplary methodologies
for performing novel aspects of the disclosed architecture. While, for
purposes of simplicity of
explanation, the one or more methodologies shown herein, for example, in the
form of a flow
chart or flow diagram, are shown and described as a series of acts, it is to
be understood and
appreciated that the methodologies are not limited by the order of acts, as
some acts may, in
accordance therewith, occur in a different order and/or concurrently with
other acts from that
shown and described herein. For example, those skilled in the art will
understand and appreciate
26
CA 02836162 2013-12-10
that a methodology could alternatively be represented as a series of
interrelated states or events,
such as in a state diagram. Moreover, not all acts illustrated in a
methodology may be required
for a novel implementation.
[0087] FIG. 3 illustrates an embodiment of a logic flow 300 to deliver
multiple
medications in a synchronized manner. The logic flow 300 may be representative
of some or all
of the operations executed by one or more embodiments described herein.
[0088] In the illustrated embodiment shown in FIG. 3, the logic flow 300
may conduct a
patient adherence survey at block 305. For example, an adherence assessment
module 230 may
be implemented to classify a patient's present adherence level pertaining to
their ability to take
all of their prescribed medications on the recommended administration
schedule. The adherence
assessment module 226 may determine a score based on a number of factors used
to determine
the patient's need for an adherence-based packaging solution. The
administration of the patient
adherence survey may be more fully described below with reference to FIG. 4.
The
embodiments are not limited to this example.
[0089] In the illustrated embodiment shown in FIG. 3, the logic flow 300
may calculate a
prescription synchronization date at block 310. For example, collected patient
intake data may
be used to create a patient profile in which all of a patient's prescriptions
may be linked to one
file. Prescription data collected by patient intake wizard 225 may be
forwarded to the
synchronization engine 235 to be synchronized into a cohesive schedule for the
determined
period of days. The synchronization date may be based on the hub pharmacy's
production
schedule. The embodiments are not limited to this example.
[0090] In the illustrated embodiment shown in FIG. 3, the logic flow 300
may obtain
insurance plan approval at block 315. For example, a hub pharmacy technician
may contact the
patient's insurance plan(s) 160 to request authorization of all existing
prescriptions in a patient's
file to be approved on a modified synchronized determined period of day(s)
prescription
schedule. The request for approval may be based on a determined adherence
assessment need.
The embodiments are not limited to this example.
27
CA 02836162 2013-12-10
[0091] In the illustrated embodiment shown in FIG. 3, the logic flow 300
may adjudicate
prescriptions at block 320. For example, adjudication may be necessary to
ensure coordination
with the synchronization step in which the insurance plan 160 has approved and
replaced the
patient's existing prescriptions with "synchronized" new prescriptions. A set
production
schedule may coordinate these time periods between the hub pharmacy contacting
an insurance
plan 160 and a spoke pharmacy adjudicating the prescriptions. The embodiments
are not limited
to this example. Adjudication may be done at the spoke pharmacy or dispensing
pharmacy. In
the alternative, the hub pharmacy may adjudicate or an alternate location may
adjudicate. It is to
be understood that adjudication may not be necessary.
[0092] In the illustrated embodiment shown in FIG. 3, the logic flow 300
may send an
order to a hub pharmacy at block 325. For example, once a spoke pharmacy
technician has
adjudicated a new prescription schedule for a patient, an order setting out
the medications
associated with revised prescriptions may be sent to a hub pharmacy to be
filled. The
embodiments are not limited to this example.
[00931 In the illustrated embodiment shown in FIG. 3, the logic flow 300
may prepare an
order at block 330. For example, the hub pharmacy may prepare a patient's
synchronized order
by first determining an hours of administration (BOA) schedule for the blended
prescription
order. The FCD.R. schedule may be more fully described with reference to FIG.
6. Once the
PCDR schedule assignment process has completed, the hub pharmacy may utilize
an automated
robot filler process to collect and package the various medications into a
customized packaging
solution, The robot filler process may be monitored by hub pharmacy
technicians for quality
assurance (QA) purposes. The embodiments are not limited to this example.
100941 In the illustrated embodiment shown in FIG. 3, the logic flow 300
may ship an
order at block 335. For example, a filled and packaged order created at the
hub pharmacy may
be shipped to an appropriate spoke pharmacy. The spoke pharmacy, in turn, may
contact the
patient at block 340 to arrange for pick-up or delivery of the customized
medication package.
The embodiments are not limited to this example.
28
CA 02836162 2013-12-10
(00951 FIG. 4 illustrates an embodiment of a logic flow to perform an
adherence
assessment survey. Logic flow 400 may be representative of the steps taken in
block 305 of FIG.
3. For example, the adherence assessment module 230 as administered by the
patient intake
wizard 225 may ask the patient to answer a series of questions. The questions
may include, but
are not limited to, the number of pharmacies the patient is currently
utilizing, the number of
medications a patient is taking, the number of physicians they have that are
currently prescribing
medications, the number of hospital and/or emergency room (ER) visits within
the last 30 days,
and whether the patient feels if they would benefit from an adherence
intervention or a support
service such as reminder packaging. The logic flow 400 may be representative
of some or all of
the operations executed by one or more embodiments described herein. The
patient may be
prompted to put in the answers or the pharmacist or technician may input the
patient answers into
, the database.
[0096] In the illustrated embodiment shown in FIG. 4, the logic flow 400
may prompt a
patient to input the number of pharmacies they are currently using at block
405. For example,
the patient intake wizard 225 may prompt a prospective MMAA participant to
input the number
of pharmacies they are currently using. The response choices may include one,
two, or three or
more. Each response may be associated with a score. For instance, a single
pharmacy may be
scored at 0 points while two pharmacies may be scored at 2,5 points and three
or more
pharmacies may be scored at 5 points. The embodiments are not limited to this
example.
100971 In the illustrated embodiment shown in FIG. 4, the logic flow 400
may prompt a
patient to input the number of physicians that currently prescribe medications
at block 410. For
example, the patient intake wizard 225 may prompt a prospective MMAA
participant to input the
number of physicians they are currently seeing. The response choices may
include one, two, or
three or more. Each response may be associated with a score. For instance, a
single physician
may be scored at 0 points while two physicians may be scored at 2.5 points and
three or more
physicians may be scored at 5 points. The embodiments are not limited to this
example. In the
alternative, the survey could highlight the responses that meet high risk
scenarios and send these
responses to the physician or pharmacist for adherence evaluation or have a
default position if
you have 3 or more physicians your risk level warrants participation in the
program. The risk
29
CA 02836162 2013-12-10
comfort level may be determined based on input from the patient, the insurance
plans, the
physician etc.
[0098) In the illustrated embodiment shown in FIG. 4, the logic flow 400
may prompt a
patient to input the number of hospital and/or emergency room (ER) visits they
have made in the
last month at block 415. For example, the patient intake wizard 225 may prompt
a prospective
MMAA participant to input the number of hospital or emergency room visits they
have made in
the last thirty (30) days. The response choices may include zero, one, two, or
three or more.
Each response may be associated with a score. For instance, no visits may be
scored at 0 points,
a single visit may be scored at 1 point while two visits may be scored at 5
points and three or
more visits may be scored at 7.5 points. The embodiments are not limited to
this example.
[0099) In the illustrated embodiment shown in FIG. 4, the logic flow 400
may prompt a
patient to input a response to whether an adherence program would be
beneficial at block 420.
For example, a prospective participant may be given the option of deciding
whether an
adherence program would benefit them. If they select "yes", a score of 5
points may be added.
If they select "no", o points may be added to the score. The embodiments are
not limited to this
example.
[00100] In the illustrated embodiment shown in FIG. 4, the logic flow 400
may score the
patient's responses to the survey questions at block 425. For example, FIG. 5
illustrates an
embodiment of a computer screen image 500 for an adherence assessment survey.
In this
example, a prospective MMAA participant has answered the survey questions
described above,
The responses shown indicate that this person uses two pharmacies (2,5
points), sees three or
more physicians (5 points), and has made one hospital/ER visit in the last
thirty days (1 point),
Lastly, the prospective MMAA participant has indicated that an adherence
program would be
beneficial to them (5 points). The sum of the individual scores yields a total
score of 13.5 points.
The embodiments are not limited to this example.
[00101] In the illustrated embodiment shown in 600, the logic flow 400 may
provide an
adherence assessment program recommendation at block 430. For example, the
survey may be
designed such that a score of less than 10 points may indicate a moderate
adherence issue for the
prospective MMAA participant. A score of 10 or more points, however, may
indicate a severe
CA 02836162 2013-12-10
adherence issue for the prospective MMAA participant. Based on the score in
this example, the
adherence assessment module 230 may return a result indicating the prospective
MMAA
participant has a severe adherence issue and could benefit from participating
in an MMAA
program. The embodiments are not limited to this example.
[00102] FIG. 5 illustrates a sample adherence assessment survey. It is to
be understood
various formats could be used and various questions could be used,
[00103] FIG. 6 illustrates an embodiment of a logic flow to reconcile the
PCDR schedule
for multiple medications. The logic flow 600 may be representative of some or
all of the
operations executed by one or more embodiments described herein,
[00104] In the illustrated embodiment shown in FIG. 6, the logic flow 600
may parse
prescription order data received from a spoke Rx order at block 605. For
example, a spoke Rx
server 130 may forward an electronic data file containing the prescription
data for a particular
patient to the hub Rx server 110. A customization module 240 within hub Rx
server 110 may
parse the received prescription order data. The embodiments are not limited to
this example.
[00105] In the illustrated embodiment shown in FIG. 6, the logic flow 600
may determine
whether any physician SIG instructions (dosing instructions) are included with
the received order
at block 610. For example, the customization module 240 may determine whether
any physician
SIG instructions are included with the received order. If so, the logic flow
600 may place the
physician SIG data in an HOA assignment table at block 615. Otherwise, the
logic flow 600
may determine whether there are any data matches with known Product
Identification Code
(PIC) data at block 620, If a match is not found at block 625 the process
proceeds to block 630
to assign a default dosage time. The default dosing time may be in the
morning, afternoon or
evening. If a match is found at block 625 the process proceeds to block 635
and the information
is added to the PCDR schedule. The embodiments are not limited to this
example.
[00106] In the illustrated embodiment shown in FIG. 6, the logic flow 600
may default the
PCDR assignment for prescriptions without SIG or PIC associated data to an AM
scheduled
administration at block 630. Otherwise, the logic flow 600 may place the PIC
PCDR data in the
PCDR assignment table at block 635. The embodiments axe not limited to this
example.
31
CA 02836162 2013-12-10
[00107] In the illustrated embodiment shown in FIG. 6, the logic flow 600
may determine
whether a medication may be co-mingled with other medications at block 640. If
a medication
cannot be co-mingled then the logic flow may proceed to block 645. If the
medication can be
co-mingled then the logic flow may proceed to block 650. The embodiments are
not limited to
this example.
[001081 In the illustrated embodiment shown in FIG. 6, the logic flow 600
may flag each
medication that cannot be co-mingled as requiring a separate single medication
container in the
packaging at block 645. The embodiments are not limited to this example.
[00109] In the illustrated embodiment shown in FIG. 6, the logic flow 600
may determine
the number of medications in the order at block 650. For example, the MMAA
packaging may
be capped at 16 medications administered up to four times or up to 12 times
during a day. The
logic flow 600 may flag each medication as AM if there are between 1 and 4
medications in the
order at block 655. The logic flow 600 may flag medications 1-4 as AM and
medications 5-8 as
EVE if there are between 5 and 8 medications in the order at block 660. The
logic flow 600 may
flag medications 1-4 as AM, medications 5-8 as EVE, and medications 9-12 as
NOON if there
are between 9 and 12 medications in the order at block 665. The logic flow 600
may flag
medications 1-4 as SAM, medications 5-8 as 8PM, medications 9-12 as 12PM, and
medications
13-16 as 4PM if there are between 13 and 16 medications in the order at block
670. Once all of
the medications have been flagged by the PCDR assessment module 245 within
customization
engine 240 according to their hours of administration, a customized packaging
sheet may be
created. The customized packaging sheet may be filled by a hub pharmacy robot
filler according
the PCDP,. determination for each prescription in the order. The embodiments
are not limited to
this example. The PCDR schedule may be based on an analysis of the patient's
activities of
daily living, the prescription dosing instructions from the prescriber, the
PIC codes, and or the
default setting, and the number of medications assigned per dosing period.
Once the primary
dosing period is full, the program will assign the next set of medications to
a secondary dosing
period and so on. Preference may be given to the prescribers dosing
instructions and/or the PIC
code information.
32
CA 02836162 2013-12-10
[00110] FIG. 7 illustrates an example of a customized thirty (30) day
synchronized
packaging 700 solution for multiple medications, The customized packaging 700
may be
organized similar to a calendar. Each day of the month may be represented and
include one or
more pouches containing medications, The pouches may be of a blister pack
type. Each pouch
may be clearly labeled with an administration time to enhance adherence to a
prescription
regimen. In addition, the reverse side (not shown) of the customized packaging
may include
printed data pertaining to each medication as well as any particular physician
SIG instructions or
PIC data. The embodiments are not limited to this example.
[001111 In this example, twelve (12) different medications are taken by a
patient over the
course of the month. Eleven may be taken daily while one medication may be
taken weekly
(e.g., on Tuesdays). The 1-IGA schedule scheduling process determines that for
the daily
medications, four may be in the morning (AM), four may be taken in the evening
(EVE), and the
other three may be taken mid-day (noon). The schedule may be slightly altered
every Tuesday to
accommodate the once weekly medication. Moreover, the once weekly medication
is packaged
separately as it may have been labeled as "not to be co-mingled". To
accommodate the once
weekly medication, one of the morning medications may be shifted to mid-day
each Tuesday so
as not to exceed four medications in any given administration period in this
example.
[00112] The benefits of the customized packaging are numerous. The
customized
packaging coordinates all of a patient's medications in a single dispensing
system eliminating the
need for multiple medication bottles/blisters etc In addition, the entity that
creates the
customized packaging and places the medications into the medical containers
performs a patient
customized drug regime (PCDR) assessment to ensure that the medications are
placed into the
appropriate containers for ingestion at the proper time, Medications that
should not be co-
mingled are placed in their own separate containers and labeled accordingly.
The customized
packaging is designed to increase patient adherence to a recommended schedule
for all
prescriptions. This is accomplished by a packaging sheet or set of
instructions containing a
synchronized monthly supply of a patient's medications.
33
CA 02836162 2013-12-10
[00113] FIG. 8 illustrates an embodiment of an exemplary computing
architecture 800
suitable for implementing various embodiments as previously described. In one
embodiment,
the computing architecture 800 may comprise or be implemented as part of an
electronic device.
The embodiments are not limited in this context.
[00114] As used in this application, the terms "system" and "component" are
intended to
refer to a computer-related entity, either hardware, a combination of hardware
and software,
software, or software in execution, examples of which are provided by the
exemplary computing
architecture 800. For example, a component can be, but is not limited to
being, a process
running on a processor, a processor, a hard disk drive, multiple storage
drives (of optical and/or
magnetic storage medium), an object, an executable, a thread of execution, a
program, and/or a
computer. By way of illustration, both an application running on a server and
the server can be a
component. One or more components can reside within a process and/or thread of
execution,
and a component can be localized on one computer and/or distributed between
two or more
computers. Further, components may be communicatively coupled to each other by
various
types of communications media to coordinate operations. The coordination may
involve the uni-
directional or hi-directional exchange of information. For instance, the
components may
communicate information in the form of signals communicated over the
communications media.
The information can be implemented as signals allocated to various signal
lines. In such
allocations, each message is a signal. Further embodiments, however, may
alternatively employ
data messages. Such data messages may be sent across various connections.
Exemplary
connections include parallel interfaces, serial interfaces, and bus
interfaces. It is to be
understood that the system may be modified to protect the patient information
if so needed, by
eliminating wireless transfer of information.
[00115] The computing architecture 800 includes various common computing
elements,
such as one or more processors, multi-core processors, co-processors, memory
units, chipsets,
controllers, peripherals, interfaces, oscillators, timing devices, video
cards, audio cards,
multimedia input/output (1/0) components, power supplies, and so forth. The
embodiments,
however, are not limited to implementation by the computing architecture 800.
34
CA 02836162 2013-12-10
[00116] As shown in FIG. 8, the computing architecture 800
comprises a processing unit
804, a system memory 806 and a system bus 808. The processing unit 804 can be
any of various
commercially available processors, including without limitation an AMD Athlon
, Duran
and Opteron processors; ARM application, embedded and secure processors; IBM
and
Motorola DragonBall and PowerPC processors; IBM and Sony Cell processors;
Intel
Celeron , Core (2) Duo , Itaniume, Pentium , Xeon , and XScale processors;
and similar
processors. Dual microprocessors, multi-core processors, and other multi-
processor architectures
may also be employed as the processing unit 804.
100117] The system bus 808 provides an interface for system
components including, but
not limited to, the system memory 806 to the processing unit 804. The system
bus 808 can be
= any of several types of bus structure that may fbrther interconnect to a
memory bus (with or
without a memory controller), a peripheral bus, and a local bus using any of a
variety of
commercially available bus architectures. Interface adapters may connect to
the system bus 808
via a slot architecture. Example slot architectures may include without
limitation Accelerated
Graphics Port (AGP), Card Bus, (Extended) Industry Standard Architecture
((E)ISA), Micro
Channel Architecture (MCA), NuBus, Peripheral Component Interconnect
(Extended) (PCI(X)),
pci Express, Personal Computer Memory Card International Association (PCMCIA),
and the
like.
[00118] The computing architecture 800 may comprise or
implement various articles of
manufacture. An article of manufacture may comprise a computer-readable
storage medium to
store logic, Examples of a computer-readable storage medium may include any
tangible media
capable of storing electronic data, including volatile memory or non-volatile
memory, removable
or non-removable memory, erasable or non-erasable memory, writeable or re-
writeable memory,
and so forth. Examples of logic may include executable computer program
instructions
implemented using any suitable type of code, such as source code, compiled
code, interpreted
code, executable code, static code, dynamic code, object-oriented code, visual
code, and the like.
Embodiments may also be at least partly implemented as instructions contained
in or on a non-
CA 02836162 2013-12-10
=
transitory computer-readable medium, which may be read and executed by one or
more
processors to enable performance of the operations described herein.
[00119] The system memory 806 may include various types of
computer-readable storage
media in the form of one or more higher speed memory units, such as read-only
memory (ROM),
random-access memory (RAM), dynamic RAM (DRAM), Double-Data-Rate DRAM
(DDRAM), synchronous DRAM (SDRAM), static RAM (SRAM), programmable ROM
(PROM), erasable programmable ROM (EPROM), electrically erasable programmable
ROM
(EEPRO/vI), flash memory, polymer memory such as ferroelectric polymer memory,
ovonic
memory, phase change or ferroelectric memory, silicon-oxide-nitride-oxide-
silicon (SONOS)
= memory, magnetic or optical cards, an array of devices such as Redundant
Array of Independent
Disks (RAID) drives, solid state memory devices (e.g., USB memory, solid state
drives (SSD)
and any other type of storage media suitable for storing information. In the
illustrated
embodiment shown in FIG.. 8, the system memory 806 can include non-volatile
memory 810
and/or volatile memory 812. A basic input/output system (BIOS) can be stored
in the non-
volatile memory 810.
[00120] The computer 802 may include various types of computer-
readable storage media
in the form of one or more lower speed memory units, including an internal (or
external) hard
disk drive (HDD) 814, a magnetic floppy disk drive (FDD) $16 to read from or
write to a
removable magnetic disk 818, and an optical disk drive 820 to read from or
write to a removable
optical disk 822 (e.g., a CD-ROM or DVD), The HAD 814, FDD 816 and optical
disk drive 820
can be connected to the system bus 808 by a I-IDD interface $24, an FDD
interface 826 and an
optical drive interface 828, respectively. The I-IDD interface 824 for
external drive
implementations can include at least one or both of Universal Serial Bus (USB)
and IEEE 1394
interface technologies.
[00121] The drives and associated computer-readable media
provide volatile and/or
nonvolatile storage of data, data structures, computer-executable
instructions, and so forth. For
example, a number of program modules can be stored in the drives and memory
units 810, 812,
including an operating system 830, one or more application programs 832, other
program
36
CA 02836162 2013-12-10
modules 834, and program data 836. In one embodiment, the one or more
application programs
832, other program modules 834, and program data 836 can include, for example,
the various
applications and/or components of the system 100.
[00122] A user can enter commands and information into the computer 802
through one or
more wire/wireless input devices, for example, a keyboard 838 and a pointing
device, such as a
mouse 840. Other input devices may include microphones, infra-red (IR) remote
controls, radio-
frequency (RF) remote controls, game pads, stylus pens, card readers, dongles,
finger print
readers, gloves, graphics tablets, joysticks, keyboards, retina readers, touch
screens (e.g.,
capacitive, resistive, etc.), trackballs, trackpads, sensors, styluses, bar
code scanners, smart
phones, PDAs, and the like. These and other input devices are often connected
to the processing
unit 804 through an input device interface 842 that is coupled to the system
bus 808, but can be
connected by other interfaces such as a parallel port. IEEE 1394 serial port,
a game port, a IJSB
port, an IR interface, and so forth.
100123] A monitor 844 or other type of display device is also connected to
the system bus
808 via an interface, such as a video adaptor 846. The monitor 844 may be
internal or external
to the computer 802. In addition to the monitor 844, a computer typically
includes other
peripheral output devices, such as speakers, printers, and so forth.
[001241 The computer 802 may operate in a networked environment using
logical
connections via wire and/or wireless communications to One Or more remote
computers, such as
a remote computer 848. The remote computer 848 can be a workstation, a server
computer, a
router, a personal computer, portable computer, microprocessor-based
entertainment appliance, a
peer device or other common network node, and typically includes many or all
of the elements
described relative to the computer 802, although, for purposes of brevity,
only a memory/storage
device 850 is illustrated. The logical connections depicted include
wire/wireless connectivity to
a local area network (LAN) 852 and/or larger networks, for example, a wide
area network
(WAN) 854, Such LAN and WAN networking environments are commonplace in offices
and
companies, and facilitate enterprise-wide computer networks, such as
intranets, all of which may
connect to a global communications network, for example, the Internet.
37
CA 02836162 2013-12-10
[001251 When used in a LAN networking environment, the computer
802 is connected to
the LAN 852 through a wire and/er wireless communication network interface or
adaptor 856.
The adaptor 856 can facilitate wire and/or wireless communications to the LAN
852, which may
also include a wireless access point disposed thereon for communicating with
the wireless
functionality of the adaptor 856.
[00126] When used in a WAN networking environment, the computer
802 can include a
modem 858, or is connected to a communications server on the WAN 854, or has
other means
for establishing communications over the WAN 854, such as by way of the
Internet. The modem
858, which can be internal or external and a wire and/or wireless device,
connects to the system
= bus 808 via the input device interface 842. In a networked environment,
program modules
4: depicted relative to the computer 802, or portions thereof, can be
stored in the remote
memory/storage device 850. It will be appreciated that the network connections
shown are
exemplary and other means of establishing a communications link between the
computers can be
used.
[00127] The computer 802 is operable to communicate with wire
and wireless devices or
entities using the IEEE 802 family of standards, such as wireless devices
operatively disposed in
wireless communication (e.g., IEEE 802.15 over-the-air modulation techniques).
This includes
at least Wi-Fi (or Wireless Fidelity), WiMax, and BluetoothTM wireless
technologies, among
others. Thus, the communication can be a predefined structure as with a
conventional network
or simply an ad hoc communication between at least two devices. Wi-Fi networks
use radio
technologies called IEEE 802.15x (a, b, g, n, etc.) to provide secure,
reliable, fast wireless
connectivity. A Wi-Fi network can be used to connect computers to each other,
to the Internet,
and to wire networks (which use IEEE 802,3-related media and functions).
[00128] The invention described in the present application also
covers a system having a
processor component; and a patient customization engine on the processor
component
= wherein the patient customization engine receives one or more
prescription order(s) that covers
medication(s) to be taken over a period of days and during one or more
administration time(s),
the prescription order comprising one or more prescriptions for a single
patient, each prescription
38
CA 02836162 2013-12-10
representative of a medication and including prescription data comprised of
one or more
prescriber instructions and one or more product identification code(s);
determines a patient
customized drug regimen (POOR) schedule that assigns an administration time to
each of the
prescriptions in the prescription order over the period of days; and places
the qualifying
medications into the two or more medical container(s) of a customized package
based on the
PCDk schedule, the customized package housing all of the qualifying
medications for all of the
prescription orders for the period of days and comprising one or more medical
container(s) for
each of the one or more administration time(s) within the period of days. The
packages that
may be used with this system include one or more pouches, vials, blisters,
boxes, ampules, nebs
or a form-filled-seal-unit-dose packages, inhalers, pre-filled syringes, pens
and other such
= medication dosing containers.
1001291 A modular system for facilitating fulfillment and patient
adherence for multiple
prescriptions, in some embodiments, is shown in Figure 10. The modular system
is configured
as independently functioning units executing on a distributed computing
system. A distributed
computing system is any computing system in which processing functions and
data storage are
capable of being distributed among multiple units in communication with each
other. In some
embodiments, the distributed computing system is cloud computing environment
100% realized
on the internet. Cloud computing environments are well known in the art and
are not described
in detail in the present application.
1001301 In some embodiments, each module is an independently
executing software widget
configured to perform complementary tasks in a coordinated manner to achieve
specific
functions as described below. In some embodiments, some or all of the
functions described for
any two or more modules are combined into a single module configured to
perform the
combined functions. In some embodiments, at least one function described for a
given module is
performed by a different module.
[00131] In cases in which a module or function is analogous to a
component or function
described in the parent application of the present application, it is
understood that a module is
capable of being configured to perform at least the functions as previously
described. Module
functions described below therefore complement those previously described. In
other words,
39
CA 02836162 2013-12-10
these modules may work in a sequential and/or linear order or, the modules may
function in a
= stand-alone or independent fashion allowing the system user to pick and
choose which function
the user would like to run. This allows for the possibility of a customer to
use the entire system
or pick and choose which modules or functions the customer would like to use
in its pharmacy
system.
[00132] Similarly, in cases in which a feature is analogous to a
feature described in the parent
application of the present application, it is understood that such a feature
includes all of the
possible embodiments as previously described,
[00133] Core module 1102 is configured to receive one or more
prescription orders 1112. In
various embodiments, presciiption orders 1112 are received by any combination
of electronic
communications and manual input. Prescription orders cover one or more
medications to be
taken over a period of days and during one or more administration times. Each
prescription is
representative of a medication and includes prescription data. Prescription
data includes
prescriber instructions and one or more product identification codes, The one
or more
prescriptions may include oral solids, lotions, inhalers, liquids, device,
and/or any other form of
medication prescribed to a patient by a physician,
1001341 Core module 1102 is also configured to determine a
patient customized drug
regimen (PCDR) schedule that assigns an administration time to each
prescription in the
prescription orders over the period of days.
[00135] Depending on a hold status, core module 1102 is
configured to execute filling at
least one of the prescriptions for a patient. In various embodiments, a hold
status includes any
combination of a status level for the patient and a status level for each
medication represented by
a prescription. In some embodiments, a patient-level hold indicates that
filling is not executed
for any prescription for that patient. In some embodiments, a medication-level
hold indicates
that filling a prescription for a specific medication for that patient is not
executed. In some
embodiments, a medication-level hold applies to a single patient only. In some
embodiments, a
medication-level hold applies to more than one patient. It is to be understood
when there is a
medication is a patient's order that is put on hold a user may opt to fulfill
all of the medications
not on hold in a patients order. The same may apply to a single patient with a
hold. In other
CA 02836162 2013-12-10
words a user may run a batch of patients from a pharmacy excluding the one or
more patients
with a medication level hold or a patient-level hold.
1001361
In some embodiments, executing filling at least one of the
prescriptions includes
filling only those prescriptions for which no holds exist. For the case in
which no patient-level
hold exists, prescription filling for that patient is executed for any or all
of the prescriptions for
which no medication-level hold exists.
1001371
In various embodiments, executing filling at least one of the
prescriptions includes
any combination of manual and/or automatic methods. In various embodiments,
manual
methods include outputting data for manually filling a prescription 1114 to a
printer, a
computing device, a computing network, or any apparatus capable of
communicating
= prescription information to a technician, pharmacist, or other person.
[00138]
In some embodiments, automatic methods include communicating with at
least one
= prescription filling apparatus 1116. In various embodiments, prescription
filling apparatus 1116
= is a vial filling apparatus, a blister filler, a strip pack apparatus,
and/or any automated or robotic
prescription filling apparatus for blisters, vials, strip packs or any other
similar apparatus or
medication package type.
[001391
In some embodiments, communicating with prescription filling apparatus
1116
includes sending data to prescription filling apparatus 1116.
In some embodiments,
communicating with prescription filling apparatus 1116 also includes receiving
data from
prescription filling apparatus 1116.
[00140]
In some embodiments, data sent to prescription filling apparatus 1116
is formatted to
match an industry standard. In some embodiments, data sent to prescription
filling apparatus
1116 follows a customized format. In some embodiments, data sent to
prescription filling
apparatus 1116 includes label information, calendar information, patient
instructions, and/or
PCDR information.
100141)
In some embodiments, data received from prescription filling apparatus
1116
includes medication inventory information.
[001421
In some embodiments, synchronization module 1104 is configured to
receive patient
profile information. In various embodiments, patient profile information
includes any
combination of information related to patient medical history, prescription
data, insurance data,
41
CA 02836162 2013-12-10
= physician data, adherence data, patient-level hold information,
medication-level hold
= information, DNA and biometric data, patient-related input by an
operator, technician,
pharmacist, physician, or other caregiver, or any other patient-related
information.
[00143] In some embodiments, synchronization module 1104 is
configured to receive
information indicating changes to prescription orders. In various embodiments,
changes to
prescrip ion orders include any combination of new prescriptions, canceled
prescriptions,
temporary prescriptions, modifications to medication dosage levels,
administration times and
frequency, prescription payment status, and any other prescription-related
data.
[00144] In some embodiments, synchronization module 1104 is
configured to receive
inedi cation anomaly information. In various embodiments, medical anomaly
information
includes information related to medication availability, compatibility, side-
effects, prescription
fulfillment issues, and any other medication-related information. In various
embodiments,
prescription fulfillment issues include any combination of titration
requirements and special
= handling requirements, non-routine medications, creams, liquids,
inhalers, devices and any other
medication outliers.
[00145] In various embodiments, synchronization module 1104 is
configured to receive
information in any combination of continually, periodically, or
intermittently, based on an or
manually configured timing control. In various embodiments, synchronization
module 1104 is
= configured to receive information through any combination of electronic
communications, user
inputs, other modules, data storage devices, computer chips, and electronic
devices including
portable electronic devices and/or MIMI devices.
[00146] In some embodiments, synchronization module 1104 is
configured to determine a
hold status. In various embodiments, a hold status includes any combination of
a status level for
the patient and a status level for each medication represented by a
prescription. In some
embodiments, a patient-level hold indicates that filling is not executed for
any prescription for
= that patient. In some embodiments, a medication-level hold indicates that
filling a prescription
for a specific medication for that patient is not executed. In some
embodiments, a medication-
level hold applies to a single patient only. In some embodiments, a medication-
level hold
applies to more than one patient. In some embodiments a hold may be removed by
technician
approval, and/or pharmacist approval, and/or doctor approval.
42
CA 02836162 2013-12-10
(001471 In various embodiments, determining a hold status
includes evaluating any
combination of received information including patient profile information,
information
indicating changes to prescription orders, medication anomaly information,
information
indicating incompatibility between two or more medications, user inputs,
stored data, and any
other patient, prescription, or medication-related information from any
source.
[001481 In various embodiments, determining a hold status
includes receiving a hold status
indication from any of another module, user input, and received information
including patient
profile information, information indicating changes to prescription orders,
medication anomaly
information, user inputs, stored data, and any other patient, prescription,
device, or medication-
related information from any source,
[001491 In some embodiments, synchronization module 1104 is
configured to determine a
production date on which to start a drug regimen schedule. In some
embodiments,
synchronization module 1104 is configured to determine a start date based on
user input, In
some embodiments, synchronization module 1104 is configured to determine a
start date based
on an process. In some embodiments, synchronization module 1104 is configured
to determine a
start date based on a combination of user input and a process. In some
embodiments,
synchronization module 1104 is based off of an independently determined start
date entered by
the technician, pharmacist, doctor, patient or a system user.
(001501 In some embodiments, determining a start date is based in
part on any combination
of health and financial considerations including health risks, pharmacy costs,
patient
copay/lifestyle risks, generic/brand name drug preferences, or other similar
considerations,
[001511 In some embodiments, parser module 1106 is configured to
receive patient intake
responses 1134 to patient profile questions. In some embodiments, responses to
patient profile
= questions are received from a patient intake module. In some embodiments,
patient intake
questions are a fixed set of questions. In some embodiments, patient intake
questions are
branched, with follow-up questions determined based on one or more prior
responses. Patient
profile questions cover any combination of medication-related topics including
qualification for
multi-medication customization, lifestyle, medical history, demographic data,
child-resistant
medication opt-out status, and any other medication-related information.
43
CA 02836162 2013-12-10
1001521 In some embodiments, parser module 1106 is configured to extract
information from
patient intake responses 1134. In some embodiments, the extracted information
is PCDR-related
information. In various embodiments, parser module 1106 uses one or more
parsing engines to
extract formatted data from patient intake responses 1134. Patient intake
responses 1134 are
provided by patients, caregivers, family members, pharmacists, technicians,
physicians,
insurance representatives, or other interested parties.
[00153] In some embodiments, patient adherence module 1108 is configured to
provide a
plurality of patient adherence questions and receive responses to the
plurality of patient
adherence questions. In various embodiments, patient adherence module 1108 is
configured to
provide and receive responses through any combination of an embedded graphical
or textual user
interface, computing devices, web-based interfaces, mobile electronic devices,
kiosks, home
health care monitoring systems, and any other apparatus for direct or indirect
interfacing. In
various embodiments, patient adherence module 1108 is configured to provide
and receive
responses to and from any combination of patients, caregivers, family members,
pharmacists,
tecimicians, physicians, insurance representatives, and any other interested
parties.
[00154] In various embodiments, the plurality of patient adherence
questions includes open-
ended questions, closed ended-questions, medication-specific questions,
lifestyle questions, side
effect questions, questions related to medical history and physician and
emergency room visits,
accidents, sleeping habits, and any questions related to medications or
combinations of
medications. In some embodiments the questions are designed to be
conversationalist in nature.
For example, the system may prompt the user to answer one or more questions
regarding
specifics about an individual activity of daily living for the patient, such
as "what did you eat for
breakfast", versus asking for a patient's brief summary of events such as,
"are you eating."
Other types of conversation style questions may include, "how many hours did
you sleep last
night" or "what time did you fall asleep and what time did you wake up",
versus a summary
response - "are you sleeping well". The system is designed to prompt
additional follow-up
questions, based on whether any answers trigger the need for additional
information. For
example, if a patient says they slept three hours the prior night, the system
may ask how they
slept the day before to see if there is a pattern of sleep issues. In various
embodiments, the
system includes a regular monthly, weekly, bimonthly or other chosen set
period of time, to
44
CA 02836162 2013-12-10
solicit patient information in one or more follow-up call(s) or another form
of communication for
one or more patients in the system, The system provides the question prompts
for the user
whether via smart device or via a computer station or any other device. The
system is interactive
with the user responses and the user input 1118 as entered into the system (as
described
elsewhere herein) may be actively reviewed by the system during the call as
additional
information is provided. The system, using the parser module 1108, scans the
user input 1118
for keywords, such as health conditions or symptoms or side effects or any
other chosen keyword
type. The keywords identified may represent a medication issue. The system
than assesses the
keywords identified, compares the user input 1118 to known side effects or
interactions, which
may indicate potential medication incompatibility, and determines if
additional questions should
be asked, whether a medication-level hold or a patient-level hold should be
placed on an order,
and/or the severity of the type of hold to place on an order, aka what level
of user is able to
remove the hold.
100155] In some embodiments, patient adherence module 1108 is configured to
identify
medication issues from the received responses in the form of user input 118.
User input 1118
may be entered by the patient, care-giver, pharmacist, technician, doctor
and/or any other system
user through the computer terminal, smart device, telephone answers and manual
entry by a user
or any other device. In some embodiments, identifying medication issues is
based on
identification of a keyword of a side effect. In some embodiments,
identification of a keyword
of a side effect is based on a list or record of known side effects provided
by one or more drug
manufacturers, the Food and Drug Administration (FDA), or any similar such
organization. Side
effects are reported in data base files, such as and FDA database, Medi-Span,
first data bank and
or any other such medication side effect database. The system accesses one or
more such
databases, and may parse information within the database to determine a list
of known side
effects for a medication or combination of medications. In some embodiments,
identification of
a keyword of a side effect is based on prior responses received by patient
adherence module
1108 and new side-effects that the system has identified for particular
medications and/or
combinations of one or more medications as described later herein.
(001561 In some embodiments, patient adherence module 1108 is configured to
identify
medication issues based in part on patient DNA profile information. In some
embodiments,
CA 02836162 2013-12-10
identification of medication issues is based on combining patient DNA profile
information with
medication information. Any medication issues identified by the patient
adherence module
1108, based on any of the foregoing embodiments, may result in a hold on a
medication and/or a
patient order. Such hold may require input from the technician, pharmacist,
doctor and or
another system user before a medication and/or patient order may be processed.
The severity of
= the issue will determine what level of review is required to remove the
hold, with the less severe
issues resulting in a hold that may be release by a technician and as the
issue resulting in the hold
becomes more severe, a higher level of approval may be required to remove the
hold on the
medication and/or the patient order, escalating to a pharmacist or a doctor
for the most severe
issues resulting in a hold.
[00157] In some embodiments, patient adherence module 1108 is
configured to output
updated patient profile information. In various embodiments, updated patient
profile information
is output to any combination of core module 1102, synchronization module 1104,
databases
1128, or in the form of reports 1122. Reports may be generated on one or more
patients, for one
or more medications, for one or more pharmacies, for one or more disease
states, or for any
query factors entered into the system. Reporting also may generate labels for
multiple
prescriptions or single prescriptions, calendars, physician sheets, patient
education materials
and/or delivery information. A sample patient label is set forth in Figures
14(a)-(c). A sample
patient calendar is set forth in Figures 15. The one or more report(s) may
contain one or more
types of information such as the time of day to take the medication, the
disease state the
medication treats, medication information, patient information, dosage
information, potential
side effect information and or icons or pictures indicating any of the
forgoing. The one or more
reports may include icons and/or words to convey information to the patient or
caregiver, such as
time to take medication, disease state that the medication is for (heart,
lungs stomach....) etc...
The one or more reports may use one or more colors to assist in quick
identification of
medication and/or dosage regimen information such as the same color used for
all morning pills,
a different color used for all afternoon pills, a third color used for evening
pills, and/or a fourth
color used for all bedtime pills. The one or more color(s) may also show up on
the one or more
medication pouch or package created for that dosage period to further assist
with patient
46
CA 02836162 2013-12-10
adherence. The one or more reports may include pictures of the one or more
medications to
assist the user in confirming the package has been filled correctly and/or the
patient/caregiver
that they are taking/providing the correct medications at the correct time.
The one or more
reports may also be barcoded such that additional information may be easily
reached by the
patient scanning a bar code associated with the dosage period and/or
medication and/or
medication order with a smart device and/or so the one or more reports may be
scanned to
confirm that the one or more reports being provided/packaged with the correct
patient's
medication order.
1001581 In some embodiments, patient adherence module 1108 is configured to
maintain
various logs. In some embodiments, logs are file-based. In some embodiments,
logs are
database records. In some embodiments, logs include communications log 1120
based on
communications with patients, caregivers, family members, pharmacists,
technicians, physicians,
insurance representatives, and any other interested parties. In some
embodiments, logs include
biometrics table 1122 including medical record information, patient DNA
information, or any
other biornetric-related information. In some embodiments, logs include side
effects table 1124
including any combination of side effect information related to medications
and response to the
plurality of patient profile questions.
[00159] In some embodiments, patient adherence module 1108 is configured to
identify
medication issues based at least in part on the information in side effects
table 1124. In various
embodiments, patient adherence module 1108 is configured to identify
medication issues based
any combination a information in side effects table 1124 for a single patient,
multiple patients,
single medication, and combinations of medications.
[00160] In various embodiments, patient adherence module 1108 is configured
to identify
side effect issues based on any combination of number or percentage of
responses indicating a
possible side effect issue for one or more medications or one or more
medication combinations.
In various embodiments user input 1118 is entered into a log and new side
effects identified for
one or more medications and/or medication combinations once a pre-determined
number of
patients have reported similar side effects. The system includes these new
"learned" side effects
into the side effects table for one or more medications or combinations of one
or more
47
CA 02836162 2013-12-10
medications and once identified the system used in follow-up communication
with patients to
determine whether based on a patient's response(s) to the system's questions,
whether a hold
should be placed on one or more patient medications and/or one or more
medication order(s).
The number of patients having side effects or drug interaction symptoms
required to create a new
system recognized side effect or interaction may be up to 10 patients, 15
patients, 20 patients, 50
patients, 100 patients and/or any chosen number assigned to the system. The
system may alert
the user when a new side effect or drug interaction is identified for one or
more medication(s)
and/or medication combinations. The system may also alert or communicate with
one or more
internal or external side effect databases or drug interaction databases.
[00161] In some embodiments, identification of a medical issue is
used to determine a hold
status. In some embodiments, a user input is used to determine a hold status.
In various
embodiments, a hold status includes any combination of a status level for the
patient and a status
level for each medication represented by a prescription. =
[00162] In some embodiments, patient adherence module 1108 is
configured to initiate
communications to obtain updated patient profile information. Communications
are initiated in
= any combination of periodically and intermittently. In some embodiments,
patient adherence
module 1108 is configured to initiate communications monthly.
[00163] In some embodiments, core module 1102 includes tracking
interface 1130. In
various embodiments, tacking interface 1130 is configured to bilaterally
communicate scan data
1132 with tracking devices (not shown), In various embodiments, scan data
includes data
collected via any combination of bar code readers, biometric devices, RFID
transponders, and
any other device capable of collecting and communicating scan data.
[00164] In various embodiments, scan data includes any
combination of data used to identify
medications, labels, packaging, manifests, technicians, pharmacists, or any
other entity
associated with prescription fulfillment,
[00165] In some embodiments, core module 1102 is configured to
communicate bilaterally
with one or more databases 1128. In various embodiments, one or more data
bases 1128 are any
combination of local, server-based, and cloud-based database applications. In
some
embodiments, one or more databases 1128 are hosted on an HIFAA-compliant
server farm. In
various embodiments, on or more databases store any combination of patient
profile, patient
48
CA 02836162 2013-12-10
adherence, medication, medical record, biometric, DNA, side effect, pharmacy,
billing, or
physician information, scan data, and any other patient or medication-related
information, The
system may track a prescription order and each system user's interaction with
that order as the
order progresses through the system, by scanning when a user performs a task
or function at any
point during the prescription order process.
[00166] In
some embodiments, core module 1102 is configured to output medical records
1124. In various embodiments, medical records include any combination of
electronic medical
records, electronic health records, electronic medication administration
records, and any other
medical record format.
[00167] In
some embodiments, synchronization module 1104 is configured to output reports
1122. In various embodiments, reports 1122 includes any combination of report
topics including
adherence, outcomes, monthly fill data, calendars, labels, manifests,
physician reporting,
inventory reporting, and any other topic related to patient and medication
information.
[00168] In
some embodiments, inventory module 1110 is configured to communicate
bilaterally with core module 1102. In some embodiments, inventory module is
configured to
monitor medication availability.
[00169]
Referring to Figure 11, in some embodiments, patient adherence module 1208 is
configured to execute independently from other modules as a stand-alone
application. Patient
adherence module 1208 includes at least all of the features set forth above
for patient adherence
module 1108.
[00170] As
in the preceding description for patient module 1108, patient adherence module
1208 is implemented in, cloud computing environment 1200 equivalent to cloud
computing
environment 1100, and configured to receive user input 1218 equivalent to user
input 1118,
maintain communication log 1120, biometrics table 1122, and side effects table
1124, output
reports 1222 equivalent to reports 1122. In some embodiments, patient
adherence module 1208
is configured to communicate bilaterally with prescription service 1238. In
various
embodiments. Prescription service 1238 is any prescription medication service
system capable of
sending and receiving data related to patient adherence.
[00171]
Figure 12 is a flow chart of a method 1300 of medication coordination
implemented
on a distributed computing environment in accordance with one or more
embodiments such as
49
CA 02836162 2013-12-10
the system depicted in Figure 10. in various embodiments, any of the steps
represented in Figure
12 may be omitted or executed an order other than that depicted.
[001721 At step 1302, one or more prescription orders are received, a
prescription order
comprising one or more prescriptions for a single patient over a period of
days.
[00173] At step 1304, one or more of patient profile, prescription order
change, and
medication anomaly information is received. In various embodiments, patient
profile
information includes any combination of information related to patient medical
history,
prescription data, insurance data, physician data, adherence data, patient-
level hold information,
medication-level hold information, DNA and biometric data, patient-related
input by an operator,
technician, pharmacist, physician, or other caregiver, or any other patient-
related information.
[00174] In various embodiments, changes to prescription orders include any
combination of
new prescriptions, canceled prescriptions, temporary prescriptions,
modifications to medication
dosage levels, administration times and frequency, prescription payment
status, patient status,
patient discharge information, and any other prescription or patient-related
data.
1001751 In various embodiments, medical anomaly information includes
information related
to medication availability, compatibility, side-effects, prescription
fulfillment issues, and any
other medication-related information. In various embodiments, prescription
fulfillment issues
and any other medication or device related information include any combination
of titration
requirements and special handling information, non-routine medications,
creams, liquids,
inhalers, and any other medication outliers.
[001761 At step 1306, a PCDR schedule is determined that assigns an
administration time for
each of the prescriptions in the prescription order over the period of days.
In various
embodiments, medical anomaly information includes information related to
medication
availability, compatibility, side-effects, prescription fulfillment issues,
and any other medication-
related information. In various embodiments, prescription fulfillment issues
include any
combination of titration requirements, special handling requirements, special
dosing
requirements, of routine, non-routine medications or medication devices,
creams, liquids,
inhalers, specialty medications, compounded medications, over the counter
medications, and any
other medication outliers.
CA 02836162 2013-12-10
[00177] At step 1308, a production date is determined. On which to start or
stop a drug
regimen schedule. In some embodiments, determining a start or stop date is
based on user input
In some embodiments, determining a start or stop date is based on an automated
process. In
some embodiments, determining a start or stop date is based on a combination
of user input and
an automated process. In some cases a start of stop date is based on a
medication related or
healthcare related event otherwise reported from an external feed from an
electronic medical
record, electronic health record, prescribe service, or any other electronic
data feed that is
deemed appropriate for reporting said events. In some embodiments, a start
date is determined
independently by the user.
[00178] At step 1310, a hold status is determined. In various embodiments,
a hold status
includes any combination of a status level for the patient and a status level
for each medication
represented by a prescription.
[00179] At step 1312, depending on a hold status, filling at least one of
the prescriptions for
the single patient is executed. In various embodiments, executing filling a
prescription includes
any combination of outputting data for manual subscription fulfillment and
communicating with
at least one prescription filling apparatus.
[00180] At step 1314, prescription fulfillment functions are tracked. In
various
embodiments, tracking prescription fulfillment functions includes any
combination of sending
data to prompt scans by one or more scanning devices and receiving scan data
from one or more
scanning devices. In various embodiments, scan data includes any combination
of data used to
identify medications, labels, packaging, manifests, technicians, pharmacists,
or any other entity
associated with prescription fulfillment.
[00181] At step 1316, database records are updated. In various embodiments,
on or more
database records store any combination of patient profile, patient adherence,
medication, medical
record, biornetric, DNA, side effect, pharmacy, billing, or physician
information, scan data, and
any other patient or medication-related information.
[00182] At step 1318, medical records or pharmacy records are generated. In
various
embodiments, medical records or pharmacy records include any combination of
electronic
medical records, electronic health records, electronic medication
administration records, and any
other medical record format
51
CA 02836162 2013-12-10
[001831 At step 1320, reports are generated. In various embodiments,
reports includes any
combination of report topics including adherence, outcomes, monthly fill data,
calendars, labels,
manifests, physician reporting, inventory reporting, and any other topic
related to patient and
medication information.
[00184] At step 1322, Medication inventory is monitored.
[00185] Figure 13 is a flow chart of a method 1400 of medication adherence
implemented on
a distributed computing environment in accordance with one or more embodiments
such as the
module depicted in Figure 2. In various embodiments, any of the steps
represented in Figure 13
may be omitted or executed an order other than that depicted.
[001861 At step 1402, communication is initiated to obtain updated patient
profile
information. Communications are initiated in any combination of
periodically and
intermittently. In some embodiments, communications are initiated monthly.
1001871 At step 1404, a plurality of patient profile questions is provided,
and at step 1406,
responses to the plurality of patient profile questions are received. In
various embodiments,
providing and receiving responses is through any combination of an embedded
graphical or
textual user interface, computing devices, web-based interfaces, mobile
electronic devices,
kiosks, home health care monitoring systems, and any other apparatus for
direct or indirect
interfacing. In various embodiments, patient adherence module 1108 is
configured to provide
and receive responses to and from any combination of patients, caregivers,
family members,
pharmacists, technicians, physicians, insurance representatives, and any other
interested parties.
[00188] At step 1408, tables are maintained. In some embodiments, tables
include
communications logs based on communications with patients, caregivers, family
members,
pharmacists, technicians, physicians, insurance representatives, and any other
interested parties.
In some embodiments, tables include biometrics tables including medical record
information,
patient DNA information, or any other biornetric-related information. In some
embodiments,
tables include side effects tables including any combination of side effect
information related to
medications and response to the plurality of patient profile questions.
[00189] At step 1410, medication issues are identified from responses to
the plurality of
patient profile questions. In some embodiments, identifying medication issues
is based on
identification of a keyword of a side effect. In some embodiments,
identification of a keyword
52
CA 02836162 2013-12-10
of a side effect is based on a list or record of known side effects provided
by one or more drug
manufacturers, the Food and Drug Administration (FDA), or any similar such
organization. In
some embodiments, identification of a keyword of a side effect is based on
prior responses
received.
[00190] In some embodiments, medication issues are identified at least in
part on the
information in one or more side effects tables. In various embodiments,
medication issues are
identified based any combination of information in side effects tables for a
single patient,
multiple patients, single medication, and combinations of medications.
[00191] In various embodiments, patient adherence module 1108 is configured
to identify
side effect issues based on any combination of number or percentage of
responses indicating a
possible side effect issue.
[00192] In some embodiments, medication sensitivities, side effects, rate
of metabolization,
proper dosage, potential adverse drug events, and other issues are identified
in part on patient
DNA profile information. In some embodiments, identification of medication
sensitivities, side
effects, rate of rnetabolization, proper dosage, potential adverse drug
events, and other issues is
based on combining patient DNA profile information with medication and device
information.
[00193] At step 1412, a hold status is determined. In some embodiments,
identification of a
medication related event, adverse drug reaction, or medical issue is used to
determine a hold
status. In some embodiments, a user input is used to determine a hold status.
In various
embodiments, a hold status includes any combination of a status level for the
patient and a status
level for each medication represented by a prescription.
[00194] At step 1414, updated patient profile information is output. In
various embodiments,
updated patient profile information is output to any combination of systems
and databases or in
the form of reports,
[00195] Figures 14(a)-(c) show sample patient prescription order labels.
001961 Figures 15(a)-(c) show other sample patient prescription order labels.
[00197] Figure 16 shows a sample patient prescription calendar.
53
CA 02836162 2013-12-10
[00198] While the foregoing written description enables one of
ordinary skill to make and
use what is considered presently to be the best mode thereof, those of
ordinary skill will
understand and appreciate the existence of variations, combinations, and
equivalents of the
specific embodiment, method, and examples herein, The disclosure should
therefore not be
limited by the above described embodiment, method, and examples, but by all
embodiments and
methods within the scope and spirit of the disclosure,
[00199] Some embodiments may be described using the expression
"one embodiment" or
"an embodiment" along with their derivatives. These terms mean that a
particular feature,
=
structure, or characteristic described in connection with the embodiment is
included in at least
one embodiment. The appearances of the phrase "in one embodiment" in various
places in the
specification are not necessarily all referring to the same embodiment.
Further, some
embodiments may be described using the expression "coupled" and "connected"
along with their
derivatives. These terms are not necessarily intended as synonyms for each
other. For example,
some embodiments may be described using the terms "connected" and/or "coupled"
to indicate
that two or more elements are in direct physical or electrical contact with
each other. The term
= "coupled," however, may also mean that two or more elements are not in
direct contact with each
other, but yet still co-operate or interact with each other.
[00200] It is emphasized that the Abstract of the Disclosure
is provided to allow a reader
to quickly ascertain the nature of the technical disclosure, It is submitted
with the understanding
that it will not be used to interpret or limit the scope or meaning of the
claims. In addition, in the
foregoing Detailed Description, it can be seen that various features are
grouped together in a
single embodiment for the purpose of streamlining the disclosure. This method
of disclosure is
not to be interpreted as reflecting an intention that the claimed embodiments
require more
features than are expressly recited in each claim. Rather, as the following
claims reflect,
inventive subject matter lies in less than all features of a single disclosed
embodiment. Thus the
following claims are hereby incorporated into the Detailed Description, with
each claim standing
= on its own as a separate embodiment. In the appended claims, the terms
"including" and "in
which" are used as the plain-English equivalents of the respective terms
"comprising" and
54
CA 02836162 2013-12-10
"wherein," respectively. Moreover, the terms "first," "second," "third," and
so forth, are used
merely as labels, and are not intended to impose numerical requirements on
their objects.
[00201] What has been described above includes examples of the disclosed
architecture.
It is, of course, not possible to describe every conceivable combination of
components and/or
methodologies, but one of ordinary skill in the art may recognize that many
further combinations
and permutations are possible. Accordingly, the novel architecture is intended
to embrace all
such alterations, modifications and variations that fall within the spirit and
scope of the appended
claims.
=