Note: Descriptions are shown in the official language in which they were submitted.
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
METHOD AND APPARATUS FOR REPAIRING A TENDON OR LIGAMENT
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority as a continuation-in-part of U.S. non-
provisional patent Application No. 12/716,724 filed March 3,2010, the
disclosure of
which is incorporated herein fully be reference, which is a non-provisional of
provisional patent Application No. 61/304,003 filed Feb. 12, 2010, the
disclosure of
which is incorporated herein fully be reference. This application also claims
priority
to provisional patent Application No. 61/493,702 filed June 6, 2011,
provisional
patent Application No. 61/505,348 filed July 7, 2011, provisional patent
Application
No. 61/506,819 filed July 13, 2011, provisional patent Application No.
61/535,648
filed September, 16, 2011, the disclosures of each of which are hereby
incorporated herein fully by reference.
FIELD OF THE INVENTION
[0002]The invention pertains to methods and apparatus for repairing tendons,
ligaments, and the like. More particularly, the invention pertains to surgical
implants and techniques for repairing severed or injured tendons and
ligaments. It
is particularly well-suited for repairing tendons and ligaments of the
extremities with
minimal disruption of the surrounding tissues.
BACKGROUND OF THE INVENTION
[0003]The current standard of care for repairing severed tendons in the hand
is to
re-attach the two separated ends of the tendon with nothing but sutures. The
two
ends of the tendon are held together by the suture while the tendon heals.
Surgical repair of tendons and ligaments, particularly flexor tendons, has
been
accurately described as a technique-intensive surgical undertaking.
[0004]The repair must be of sufficient strength to prevent gapping at the
apposed
end faces of the repaired member to allow the member to reattach and heal as
well
as to permit post-repair application of rehabilitating manipulation of the
repaired
member. Considerable effort has been directed toward the development of
various
suturing techniques for this purpose. Two strand, four strand, and six strand
suturing techniques, primarily using locking stitches, have been widely used.
There
1
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
are a wide variety of suturing patterns which have been developed in an effort
to
attempt to increase the tensile strength across the surgical repair during the
healing process. A common suturing technique in recent times is known as the
Kessler repair, which involves the use of sutures that span, in a particular
configuration or pattern, across the opposed severed ends of the tendon (or
ligament). Evans and Thompson, "The Application of Force to the Healing
Tendon" The Journal of Hand Therapy, October-December, 1993, pages 266-282,
surveys the various suturing techniques that have been employed in surgical
tendon repair. Further, two articles by Strickland in the Journal of American
Academy of Orthopaedic Surgeons entitled "Flexor Tendon Injuries: I.
Foundations
of Treatment" and "Flexor Tendon Injuries: II. Operative Technique", Volume 3,
No. 1, January/February, 1995, pages 44-62, describe and illustrate various
suturing techniques.
[0005]Generally, the tensile strength of a tendon repair increases with
increased
complexity of the suturing scheme. As set forth in the Evans and Thompson
article, the loads at which failure occur across a sutured joint can vary
between
about 1,000 grams force to as much as about 8,000 grams force (or about 10 to
80
Newtons). There are at least two modes of potential failure, including
breakage of
the sutures or the sutures tearing out of the tendon. The Kessler and modified
Kessler repair techniques tend to exhibit failure toward the low end of the
range, for
example, between about 1,500 to 4,000 grams force (or about 15 to 40 Newtons),
which is much weaker than the original tendon and requires the patient to
exercise
extreme care during the healing process so as not to disrupt the tendon
repair.
[0006] For instance, normal flexing of the fingers of the hand without any
load
generates forces of about 40 Newtons (N) on the tendon. Flexing with force to
grasp something with the hand typically will place a force of about 60N-100N
on
the tendon. Finally, strong grasping of an object, such as might be involved
in an
athletic activity or in lifting of a heavy object, can place forces on the
tendons of the
hand on the order of 140N or more.
[0007]The various suturing techniques also are rather complex and, therefore,
difficult to reproduce and perfect as a technique, let alone perform it on the
small
tendons in the hand. Further, because they employ locking stitches, the two
tendon ends must be brought to and maintained in the correct position relative
to
2
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
each other (i.e., with the ends in contact) throughout the entire procedure
because
the locking stitches do not permit future adjustment of the repair (as did
some of
the earlier techniques that do not use locking stitches).
[0008]Another significant difficulty with repairing lacerated and avulsed
tendons in
the hand, and, particularly, in the fingers is the need to re-route the
severed tendon
(usually the proximal tendon stump) through the pulley system of the finger
joint.
Specifically, when a tendon is severed or avulsed, the proximal tendon stump
tends to recoil away from the laceration site toward the wrist. Accordingly,
it often
is necessary to make a longitudinal incision proximal to the laceration site
in order
to retrieve the proximal portion of the severed tendon and guide it through
the
pulley system of the finger back to the laceration site for reattachment to
the distal
tendon stump.
[0009]As reported in Evans and Thompson, at least one researcher has employed
a Mersilene mesh sleeve having a diameter slightly larger than the tendon that
is
subsequently sutured to the two apposed tendon ends. Experimental failure
loading as high as 10,000 grams force (100N) was reported using the sleeve.
However, Mersilene, which is a non-degradable polyester, a common material
used
for manufacturing sutures used in orthopedics, has the disadvantage that human
tissue will experience a local tissue response leading to adhesion of the
polyester
to tissue surrounding the repair site. This is undesirable in tendons and
ligaments
since the tendon must be able to glide freely relative to the surrounding
tissue,
such as the pulleys in the fingers. While a sleeve may be well suited for use
with
tendons and ligaments which are substantially cylindrical, it is less easily
employed
with tendons having a flat or ovaloid cross section. Moreover, any added bulk,
in
this case to the outside of the tendon, could be problematic as this repair
would
have to traverse the pulley system of the fingers.
[0010]U.S. Patent No. 6,102,947 discloses another method and apparatus for
repairing tendons that involves an implant that can be sutured to the tendon
and
which provides a splint running between the two tendon ends. The implant
essentially comprises a wire bearing a first pair of wedges on one side of the
midpoint of the wire with their pointed ends facing away from the midpoint and
a
second pair of wedges on the other side of the midpoint of the wire with their
pointed ends also facing away from the midpoint (i.e., facing oppositely to
the first
3
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
pair of wedges). The first pair of wedges is pushed (or pulled) into one of
the
severed ends of the tendon and the other pair is pushed (or pulled) into the
other
severed end of the tendon. The wedges are sutured to the tendon and are
retained within the tendon. This system provides high tensile strength to the
repair.
[0011]Further, Ortheon Medical of Winter Park, Florida, USA developed and
commercialized an implant for flexor tendon repair called the Teno Fix. The
Teno
Fix implant is substantially described in Su, B. et al, "A Device for Zone-II
Flexor
Tendon Repair: Surgical Technique", The Journal of Bone and Joint Surgery,
March 2006, Volume 88-A-Supplement 1, Part 1. The assembled implant
comprises two intratendonous, stainless-steel anchors (in the form of a coil
wrapped around a core) joined by a single multi-filament stainless steel
cable. The
implant is delivered to the surgeon unassembled, comprising a stainless steel
cable with a stop-bead affixed to one end of the cable, two separate anchors
with
through bores for passing the cable therethrough, and another stop-bead with a
through bore for passing the cable therethrough.
[0012] In practice, one of the anchors is advanced into a longitudinal
intratendonous split (tenotomy) made in the proximal tendon stump so that the
anchor sits within the longitudinal tenotomy and engages the tendon substance
by
capturing tendonous fibers between the core and the anchor. The other anchor
is
placed in the distal tendon stump in the same manner. Next, a straight needle
with
the stainless-steel cable attached thereto is threaded into the through-bore
of the
distal anchor from the small end of the anchor and is pulled through the
center of
the cut surface of the distal tendon stump until the stop-bead at the end of
the
cable opposite the needle contacts the distal anchor. The stainless-steel
cable
with the needle attached is then guided into the cut end of the proximal stump
and
through the through-bore of the anchor in the proximal stump from the large
end of
the anchor to the small end. The proximal stump of the tendon is then brought
into
contact with the distal stump by tensioning the cable, and the second stop-
bead is
placed over the stainless-steel cable at the proximal end of the proximal
anchor.
The second stop-bead is then crimped to lock it to the cable and the excess
cable
is cut so that the cable end is flush with the second stop-bead.
[0013]A disadvantage of the Teno Fix is the size of the tendon anchor, which
is
large and, thus, may add resistance to the tendon as it passes through the
pulley
4
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
system. Another disadvantage of the Teno Fix is the invasive nature of
implanting
the device wherein the entire track of skin over the tendon path must be
incised in
order to effect the implantation of the device. A third disadvantage is that
the
attachment of the anchor to the tendon is rather weak, reporting only about 46
Newtons of pull strength. These disadvantages are overcome by the subject and
method described in this invention.
[0014]A disadvantage of most, if not all, of the prior art techniques
discussed
above is a high infection rate.
5
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
SUMMARY OF THE INVENTION
[0015]The invention comprises methods and apparatus for reattaching anatomical
members, such as tendons, ligaments, or bone, during preparing and healing of
the member using a surgical repair device that can be securely attached to the
member and then safely guided through tortuous anatomy for reattachment and
repair. The repair device further includes structural means to secure opposed
ends
of the member against separation during healing. Devices for aiding in the
positioning of the surgical repair device also are provided, such as a crimp
connector holder tool for holding the crimp connector during threading
therethrough
of two sutures attached to two tendon stumps for bringing the two stumps into
abutment and crimping them in place.
6
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
DESCRIPTION OF THE DRAWINGS
[0016]Figure 1 shows the various components that may be used for repairing a
severed member, such as a tendon or ligament, in accordance with a first
embodiment of the apparatus of the invention.
[0019]Figure 2N is a close up view of the jaws of the crimp holder tool of
Figure
2M.
[0020]Figure 20 is a perspective view of an alternate crimp holder tool in
accordance with the principles of the present invention.
[0021]Figure 3 is a photograph of a completed tendon repair in accordance with
the first embodiment.
[0023]Figure 5 shows apparatus for reattaching a member in accordance with
another embodiment of the invention.
[0026]Figure 7 illustrates the pulley system of the finger.
[0028]Figure 8B illustrates the tendon repair device of Figure 9A as it is
preferably
delivered to the surgical site.
[0029]Figures 9A through 9C illustrate another embodiment of a tendon repair
7
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[0031] Figure 10B illustrates two of the devices of Figure 10A used to repair
a
tendon.
[0032]Figure 11A illustrates an alternative apparatus comprising a guidance
member in accordance with the invention.
[0033]Figures 11B-11D illustrate another alternate technique using the
apparatus
of Figure 11A.
[0034]Figure 11E illustrates an alternate embodiment of a guidance member in
accordance with the invention.
[0035]Figure 11F illustrates another alternate embodiment of a guidance member
in accordance with the invention.
[0036]Figure 11G illustrates yet another alternate embodiment of a guidance
member in accordance with the invention.
[0037]Figure 12A illustrates an alternative apparatus in accordance with the
invention.
[0038]Figures 12B-12C illustrate another alternate technique using the
apparatus
of Figure 12A.
[0039]Figure 13A is a perspective view of one embodiment of a unitary dilation
catheter in accordance with another embodiment.
[0040]Figure 13B is a perspective view of one embodiment of a multi-piece
dilation
catheter in accordance with another embodiment.
[0041]Figure 13C is a perspective view of one embodiment of a guide member for
the dilation catheters of Figures 13A and 13B.
[0042]Figures 14A-14G illustrate another alternate technique using the
apparatus
of Figure 13A or Figure 13B.
[0043]Figure 15 illustrates a tendon bearing a modified cruciate repair
stitch.
[0044]Figure 16 is a perspective view of a tendon holder in accordance with
another embodiment of the invention.
[0045]Figure 17A illustrates another alternate embodiment of a tendon repair
device in accordance with the principles of the invention.
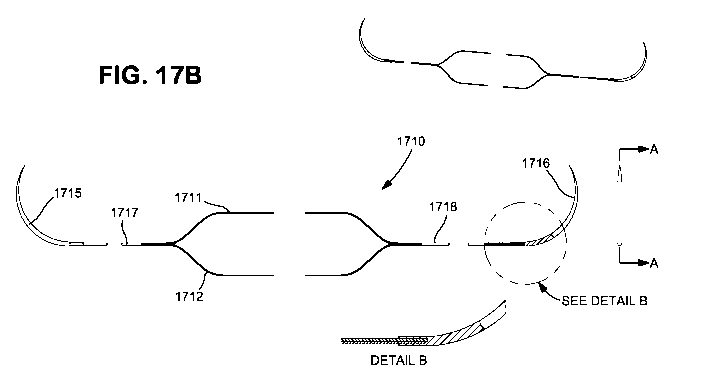
(00461 Figure 17B illustrates another alternate embodiment of a tendon repair
device in accordance with the principles of the invention.
[0047]Figures 18A-18H illustrate a stitching technique in accordance with the
principles of an embodiment of the invention.
8
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[0048]Figure 19 illustrates a bone repair using a repair device in accordance
with
the principles of a first embodiment of the invention used for bone fusing.
[0049]Figure 20 illustrates a bone repair using a repair device in accordance
with
the principles of a second embodiment of the invention for bone fusing.
[0050]Figure 21 illustrates a bone repair using a repair device in accordance
with
the principles of a third embodiment of the invention for bone fusing.
[0051]Figure 22 illustrates a bone repair using a repair device in accordance
with
the principles of a fourth embodiment of the invention for bone fusing.
[0052]Figure 23 illustrates a bone repair using a repair device in accordance
with
the principles of a fifth embodiment of the invention for bone fusing.
[0053]Figure 24 illustrates a bone repair using a repair device in accordance
with
the principles of a sixth embodiment of the invention for bone fusing.
[0054]Figure 25 is a perspective view of a drill guide in accordance with the
principles of the present invention.
[0055]Figure 26 is a side view of a guide pin in accordance with the
principles of
the present invention.
[0056]Figure 27 is a cross sectional side view of a bone anchor in accordance
with
the principles of the present invention.
[0057]Figure 28A-28L illustrate a portion of a bone fusion technique in
accordance
with the principles of the present invention.
DETAILED DESCRIPTION
[0058]In accordance with the present invention, a surgical implant and
associated
technique is disclosed for repairing tendons, ligaments, and the like
following
laceration, avulsion from the bone, or the like. The invention is particularly
adapted
for repairing a lacerated or avulsed flexor tendon, e.g., flexor digitorum
profundus
from the distal phalanx and/or the flexor digitorum superficialis from the
middle
phalanx.
First Set of Exemplary Embodiments
[0059] Figure 1 illustrates the components in accordance with a first
embodiment
of the invention. As will be described in detail below, not all of the
components
necessarily will be used in each surgical procedure. The components include a
9
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
pulley catheter 101 which will be used, if needed, to guide the tendon repair
device
of the present invention along with a severed tendon stump, ligament stump, or
similar anatomical feature through one or more anatomical restrictions to the
repair
site, e.g., through the pulley system of the finger. The components further
include
a flanged catheter 103, which will be used to guide a severed tendon stump
through anatomical restrictions to the repair site, if necessary. A catheter
connector 105 may be used to connect the pulley catheter 101 and the flanged
catheter 103 together end to end, as will be described in detail below. The
catheter
connector 105 may be a metal dowel. A tendon holder tool 107 may be used, as
necessary, to hold the tendon during the surgical repair procedure.
[0060]One or more of the tendon repair devices 109 are the actual devices that
will
affect the repair by reattaching two tendon stumps. Each tendon anchor 109
comprises a multi-filament stainless-steel cable 110. From one end 141 of the
cable to an intermediate point 143 of the cable, the individual filaments of
the cable
are wound in the normal fashion to form a single cable portion 144. A straight
needle 111 is attached to the first end 141 of the cable. From the
intermediate
point 143 in the direction opposite from end 141, the individual filaments of
the
cable are unwound so as to form a plurality of (in this particular embodiment,
seven) separate sutures 147a-147g. A needle, preferably a curved needle 114a-
114g, is attached to the end of each of the seven separate cable portions 147a-
147g. A fitting attached at the intermediate point 143 keeps the cable portion
144
from unwinding. The fitting, for instance, may be a sleeve 149. In one
preferred
embodiment of the invention, the stainless-steel cable is formed of 343
individual
strands wound in groups of seven. Thus, from the sleeve 149 to the first end
141,
the cable 144 comprises 343 individual strands making up seven intermediate
strands, and each of the intermediate strands comprised of seven smaller wound
strands of 49 filaments each, and each of those smaller strands comprised of
seven individual strands of seven filaments each. In the other direction from
the
sleeve 149, each of the seven individual strands 147a-147g comprises seven of
those smaller strands wound together (wherein each of those smaller strands
comprises seven individual strands wound together).
[0061] The afore-described embodiment of the tendon repair device 109 is
advantageous because it is particularly easy to fabricate from widely
available
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
materials. (e.g., 343 strand stainless steel suture cable and a crimp). The
materials can be chosen from the implantable family of metals and alloys
including
the stainless steels, cobalt chrome alloys, titanium and its alloys and nickel-
titanium
alloy (NiTinol). However, the tendon repair device 109 can be formed of other
materials, such as a polymer fiber, and assembled in other manners, such as
braiding, welding, or molding. For instance, it may be formed of individual
filaments, fibers or yarns welded together.
[0062]In the following discussion, in order to more clearly differentiate
them, the
single ended portion 144 of the tendon repair device 109 will be referred to
as
cable portion 144, whereas the strands 147a-147g will be referred to as
sutures.
However, it is to be understood that the use of these terms is not intended to
indicate that they are formed of different materials, since, for instance, in
the
exemplary embodiment described herein, all of the strands are formed of
stainless
steel wire.
[0063]A connector 112 is used to affix two tendon repair devices 109 to each
other
as will be described in detail below. The connector 112 in this illustrated
embodiment comprises a block of material, preferably a deformable metal such
as
stainless steel, having two side-by-side through bores 151, 152 having inner
diameters slightly larger than cable portion 144. As will be described in
greater
detail below, near the end of the tendon re-attachment procedure, each cable
portion 144 will be inserted in opposing directions through each through bore
151
and 152 of the connector 112 and the connector will be deformed (i.e.,
crimped) to
lock the cable portions 144 therein.
[0064]Finally, a bone anchor 400 or 450 can be used in procedures where the
tendon has avulsed from the bone or has been severed too close to the bone to
provide sufficient tendon length to retain a tendon repair device 109. In a
first
embodiment, the bone anchor 400 has a threaded distal end 401 for screwing
securely into bone. The proximal end 403 includes an eyelet 402 through which
sutures can be passed. As will be described in more detail hereinbelow, the
sutures can be tied in the eyelet. Alternately, the proximal end 403 can be
formed
of a deformable material, such as a thin-walled metal, so that the eyelet can
be
crushed by a crimping tool to capture the sutures therein. In a second
= embodiment, the bone anchor 450 may be manufactured with one or more
sutures
11
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
451 extending from the proximal end 455, such as four sutures 451a, 451b,
451c,
451d. The ends of the sutures are provided with needles 452a, 452b, 452c,
452d.
[0065]The tendon repair devices, surgical tools, and methods will be described
herein below in connection with the repair of a lacerated flexor digitorum
profundus
at the level of the middle phalanx. However, it should be understood that this
is
exemplary only. Various stages of the procedure are illustrated by Figures 2A-
2L.
(0066] First, if the proximal end of the divided tendon can be reached from
the
wound site, then it is gently retrieved through the wound to be held by the
tendon
holder 107.
[0067]The tendon holder 107 comprises a handle 201, a cross bar 203 at the
distal
end of the handle 201, and first and second needles 205 and 207, respectively,
extending distally from the cross bar 203. The needles 205 and 207 are
slidable
laterally within slots 209 and 211, respectively, in the cross bar 203.
Particularly,
the proximal ends of the needles comprise a stop shoulder 213, and an
internally
threaded bore running from the stop shoulder 213 to the proximal end of the
needle. A screw 217 can be threaded into the proximal end of each needle 205,
207 to trap the cross bar 203 between the head of the screw 217 and the stop
shoulder 213 of the needle 205, 207 to affix each needle in any given position
along its slot 209, 211.
[0068]Depending on the length of tendon extending outside of the wound
opening,
the surgeon may pierce the tendon with one or both of the needles 205, 207 of
the
tendon holder 107 to hold the tendon outside of the wound. See Figure 20, for
example, which illustrates the tendon holder 107 holding a tendon stump 153a.
The surgeon preferably pierces the tendon about 1 cm from the severed end.
[0069]However, if the tendon is not readily retrievable from the wound and
must be
accessed through another incision and brought back to the wound site, the
tendon
holder 107 still may be used, but first the tendon must be retrieved to the
wound
site. In such a case, the pulley catheter 101 and flanged catheter 103 will be
used
to retrieve the tendon. Specifically, the pulley catheter 101 is a hollow
plastic tube
formed of a biocompatible polymer of such composition and/or wall thickness so
that it is relatively rigid, but bendable. It might, for instance, have the
approximate
flexibility of a typical surgical vascular catheter. The relative rigidity of
the pulley
catheter will permit it to be pushed through narrow anatomical passages, such
as
12
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
the pulleys of the fingers. However, its flexibility will permit some bending
to
accommodate an overall curved path. Preferably, the pulley catheter is formed
of
a material having a low friction coefficient to allow the pulley catheter to
readily
pass through and around bodily tissues such as the tendon pulley system.
Suitable biocompatible polymers include homopolymers, copolymers and blends of
silicone, polyurethane, polyethylene, polypropylene, polyamide, polyaryl,
flouropolymer, or any other biocompatible polymer system that meets the
mechanical characteristics above. Various cross sections of the pulley
catheter
other than a simple tubular structure can also be used, such as a solid
structure,
multi-lumen, or complex geometry that would provide the mechanical
characteristics above. The coefficient of friction of the surfaces of the
pulley
catheter may be inherent to the materials used to construct the device or may
be
enhanced through a surface preparation such as a lubricious coating or
mechanical
modification of the surface such as longitudinal recesses.
[0070]The particular length, material, wall thickness, inner diameter, outer
diameter, and stiffness of the pulley catheter 101 may vary greatly depending
on
the particular tendon or ligament with which is it to be used. The length, of
course,
would be dictated by the longest length that it might be required to traverse.
The
inner diameter must be large enough to easily accommodate the cable portion
144
of the tendon repair device 109. The outer diameter must be small enough to
pass
through the anatomy that it may be called upon to pass through. The particular
material and cross sectional geometry (e.g., wall thickness) of the pulley
catheter
will largely dictate the stiffness of the catheter and, as noted above, should
be
selected to provide enough rigidity to allow it to be pushed through a narrow
path,
but flexible enough to bend to accommodate bends in the path. In the exemplary
case of the flexor digitorum profundus at the level of the middle phalanx, the
pulley
catheter may be formed of silicone and be 120 millimeters in length with a
wall
thickness of 0.5 mm, and an outer diameter of 2 mm. A silicone having a
durometer of 50-80 (Shore A) may be used for the pulley catheter.
[0071]The flanged catheter 103 also is a hollow tube formed of a biocompatible
material, preferably a polymer. However, the flanged catheter preferably is
softer
than the pulley catheter. The flanged catheter has a first end 157 having a
diameter that is approximately equal to the diameter of the pulley catheter
103 so
13
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
that it can be connected end-to-end with the pulley catheter, as described in
more
detail further below. It also has a flanged end 159 that is tapered so as to
essentially form a funnel for accepting the end of a tendon stump, also as
will be
described in more detail further below. As will become clear in the ensuing
discussion, while the flanged catheter will traverse essentially the same path
as the
pulley catheter, the pulley catheter will guide or pull the flanged catheter
into the
anatomical path along with the tendon repair device attached to the tendon
stump
inside the flanged portion 159 of the flanged catheter. Accordingly, the
flanged
catheter need not be rigid. Actually, the flanged catheter should be
relatively
flexible because it may need to be bent into a tortuous shape to accommodate
passage of the cable portion 144 of the tendon repair device 109. Furthermore,
the flange portion 159 of the flanged catheter 103 particularly should be
readily
collapsible in order to collapse around the tendon stump and pass through
narrow
anatomical passages, such as the pulleys of the fingers, with the tendon stump
and
tendon repair device enclosed therein as will be described in more detail
below.
[0072]The flanged catheter 103 should have a length, wall thickness, inner
diameter, outer diameter, and material composition suited to its purpose. Its
purpose is to allow the single-ended portion 144 of the tendon repair device
109 to
pass through it and to follow the pulley catheter through an anatomical path,
as will
be described more fully below. Accordingly, the flanged catheter has a narrow
end
157 and a wide end 158. The wide end terminates in a cone or flange 159 in
order
to make it easier to insert the straight needle 111 at the end of cable
portion 144 of
the tendon repair device 109 into it as well as contain the tendon stump. The
narrow end 157 of the flanged catheter 109 is narrow in order to be mated to
the
end of the pulley catheter.
[0073]The flanged catheter 103 also is preferably formed of a material having
a
low friction coefficient to allow the flanged catheter to readily pass through
and
around bodily tissues such as the tendon pulley system. Such biocompatible
polymers can be chosen from homopolymers, copolymers, and blends of silicone,
polyurethane, polyethylene, polypropylene, polyamide, polyaryl, flouropolymer,
or
any other biocompatible polymer system that meets the mechanical
characteristics
above. Various cross sections of the flanged catheter other than a simple
tubular
structure can also be used such as a solid structure, multi-lumen, or complex
14
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
geometry that would provide the mechanical characteristics above. The
coefficient
of friction of the surfaces of the flanged catheter may be inherent to the
materials
used to construct the device or may be enhanced through a surface preparation
such as a lubricious coating or mechanical modification of the surface such as
longitudinal recesses.
[0074]In the exemplary case of the flexor digitorum profundus at the level of
the
middle phalanx, the flanged catheter may be formed of silicone and be 120
millimeters in length with a wall thickness of 0.5 mm, and an outer diameter
of 2
mm. However, it is preferred that the flange portion 159 of the catheter be
fabricated of a thinner cross section material, for example, 0.25 mm or less,
that
will allow the flange portion 159 of the flanged catheter to envaginate the
tendon
stump and collapse as it tracks through the anatomical pathway for
repositioning of
the tendon stump, e.g., pulley system of the finger. A softer silicone, for
instance,
of 20 to 40 durometer (Shore A) is preferred for the flanged catheter.
[0075]Referring now to Figure 2A, in use, if the tendon has retracted and must
be
retrieved from a first incision 161 into a second incision (or the wound) 160,
as is
typical of tendon lacerations in the hand, an incision 161 is made, typically
in the
palm of the hand, where the tendon 153 can be retrieved. If, on the other
hand,
the proximal tendon stump is distal to the A2 pulley, then the tendon would be
exposed through an incision just distal to the A2 pulley. The pulley system of
the
pinky finger is shown in Figure 7 disembodied from the surrounding tissue for
sake
of clarity. It comprises five annular pulleys, termed Al through A5, and three
cruciate pulleys, termed Cl, C2, and C3 as shown. The pulley system is not
shown in most other Figures in order not to obfuscate the invention.
[0076]The pulley catheter 101 is passed into the wound or incision 160 at the
laceration site and slowly pushed proximally toward the new incision 161
beneath
the A3 pulley through the pulley system of the finger. If resistance is
encountered
such that the pulley catheter 101 cannot be pushed through proximally, then a%
cm to 1 cm incision (not shown) may be made midway between the skin creases of
the proximal interphalangeal joint of the finger and the crease at the base of
the
finger. This is at a level between the A2 pulley and the A3 pulley of the
finger. The
dissection is carried down gently to the flexor sheath where the pulley
catheter will
be found. The pulley catheter can then be pulled past the obstruction or
resistance
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
through this incision. Then the pulley catheter can continue to be advanced
proximally through the pulley system of the finger by pushing gently on it
until it
reaches the tendon retrieval incision 161 and is exposed proximally.
[0077]Next, as shown in Figure 2B, the narrow end 157 of the flanged catheter
103
is connected to the proximal end of the pulley catheter 101. If the components
are
sufficiently large and/or the surgeon is sufficiently dexterous, the narrow
end of the
flanged catheter may be inserted directly into the proximal end of the pulley
catheter. Otherwise, a metal dowel 105 or other form of catheter connector
(e.g., a
hook) may be used to make the connection. Particularly, the catheter connector
105 is rigid and the narrow end 157 of the flanged catheter 103 can be
inserted
over one end of the catheter connector. Then, the other end of the catheter
connector 105 can be inserted into a tight friction fit in the proximal end of
the
pulley catheter 101 to interconnect the pulley catheter 101 and the flanged
catheter
103.
[0078]Next, with reference to Figure 2C, the proximal stump 153a of the tendon
is
delivered through the incision 161 in the palm so that approximately 2 cm of
the
tendon is exposed outside of the incision 161. (If the proximal tendon stump
has
retracted only a short distance and is present at the level of the proximal
phalanx,
then the tendon can be delivered through an incision distal to the A2 pulley
or
between the Al and A2 pulleys, as the case may be). Preferably, a flexible
barrier
165 is placed under the tendon holder 107 and the proximal tendon stump 153a
to
create a working 'table' for practicing this technique. With the pulley
catheter 101
and the flanged catheter 103 attached, the pulley is pulled distally from
incision 160
to draw the flanged catheter 103 into and through the pulley system between
incisions 160 and 161. When the leading end 157 of the flanged catheter 103
exits
through incision 160 so that the flanged catheter 103 is running between the
two
incisions 160, 161, the pulley catheter 101 and connector 105 are removed, as
shown in Figure 2C.
[0079]Turning now to Figure 2D, the straight needle 111 at the end of cable
portion
144 of the tendon repair device 109 is then placed in the tendon stump 153a
approximately 1 cm from the end 168a of the stump 153a and the needle 111 is
directed out through cut end 168a of the tendon stump 153a. The needle 111 is
pulled through until the sleeve 149 is approximately 1/2 cm from the cut end
168a.
16
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
If the tendon exposure is too little, then the sleeve 149 may be positioned
somewhat closer to the cut end 168a.
[0080]Next, a small tenotomy is made in the tendon so that the crimp can be
buried within the tendon. The condition of the tendon and tendon repair device
at
this point of the procedure is shown in Figure 2D.
[0081]With the tendon repair device 109 in this position, the seven free
strands
147a-147g of the tendon repair device are used to stitch the tendon repair
device
109 to the tendon stump 153a. More particularly, two of the sutures, e.g.,
147a
and 147g, are pushed through the tendon using the curved needles 114a and 114g
and tied to each other in a knot 185. In a preferred embodiment, the two
sutures
are stitched to the tendon 153a using a locking cross stitch or cruciate
pattern. In
this instance, the loading will be spread amongst multiple points of fixation
along
the length of the repair. Also, due to the cruciate method, under tension, the
repaired tendon would tend to reduce in diameter which would facilitate
traversing
through the pulley system. The sutures 147a, 147g are cut at the far side of
the
knot to remove excess material beyond the knot. In order not to obfuscate the
invention, however, the stitches are shown in most of the drawings, including
Figures 2E-2J, representatively as Xs. Only in drawings that are of suitable
scale,
such as Figure 2L, or in which some significant discussion of the stitches is
given in
the corresponding text is the stitching represented more accurately.
[0082]Next, two more sutures, e.g., 147b and 147f, are stitched to the tendon
using the curved needles and 114b and 114f and tied together in another knot
187.
Preferably, the knot 187 is a crisscross locking stitch with the two limbs
traveling
proximally. The sutures are cut after the knot is tied. In a preferred
embodiment of
the invention, as shown in Figure 2E, the first knot 185 and the second knot
187
are tied at different levels along the length of the tendon stump 153a.
Finally, two
more sutures, e.g., 147c and 147e, are tied in a similar crisscross knot (not
seen)
on the other side of the tendon stump 153a and cut.
[0083]Finally, the single remaining suture 147d may be cut off or may be used
to
couple with any of the other free ends (prior to trimming) to form yet another
knot.
It is preferable that there be multiple points of fixation of the tendon
repair device to
the tendon stump.
17
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[0084]In one embodiment of the invention, the sutures can be of different
lengths,
organized in pairs, such that each of the two sutures forming a pair are the
same
length and each pair of sutures is of a different length. When stitching the
sutures
to the tendon, each pair of sutures of the same length are stitched to the
tendon
and knotted to each other. This embodiment is advantageous in that it provides
an
easy visual indication to the surgeon which pairs of sutures are to be tied to
each
other during the procedure (the sutures of the same length) thus simplifying
the
procedure.
[0085]Referring to Figure 2F, now that the tendon repair device 109 is
securely
fixed to the proximal tendon stump 153a, the tendon is removed from the tendon
holder and the straight needle 111 at the end of cable portion 144 is inserted
into
the flange 159 of the flanged catheter 103. Tendon repair device 109 is
advanced
through the flanged catheter until the end of the tendon stump 153a (which is
stitched to the back end of the tendon repair device 109) is in the flange
portion
159 of the flanged catheter 103. Cable portion 144 preferably is rigid enough
that
the cable can be pushed along with the flanged catheter through the pulley
system
of the finger and follow the flanged catheter 103 out of the wound 160. Now
the
surgeon can grasp the needle 111 through the flanged catheter 103 with a clamp
and pull the needle 111, cable portion 144, flanged catheter 103 and tendon
stump
153a (contained inside collapsible flange 159 of flanged catheter 103),
through the
pulley system of the finger and out of the wound 160. Alternately, if the
needle 111
protrudes from the distal end 157 of the flanged catheter, the surgeon can
grasp
the needle 111 or cable portion 144 directly by hand or with a clamp and pull
the
needle 111, cable portion 144, flanged catheter 103, and tendon stump 153a
(contained inside collapsible flange 159 of flanged catheter 103), through the
pulley
system of the finger and out of the wound 160. If any resistance is
encountered,
then the path through the pulley system can be inspected through a separate
incision.
[0086]The flange 159 of the flanged catheter 103 will collapse around the
tendon
stump as needed to pass through the pulley system of the fingers.
[0087]Referring to Figure 2G, once the tendon stump 153a has reached the wound
160, flanged catheter 103 can be removed from the tendon repair device 109 and
tendon stump 153a, thereby exposing the tendon repair device 109 and tendon
18
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
stump 153a through the wound 160. Needle 205 of tendon holder 107 can be
placed across the proximal tendon stump 153a to hold the tendon stump 153a in
a
stable position.
[0088]In Figure 2G and subsequent drawings, the length of the tendon stump(s)
may be exaggerated to help with the illustration of the repair. However, it
should
be understood that, once the tendon has been retrieved to or near the original
wound site (as in Figure 2G), there is little or no excess tendon to expose
outside
of the skin, especially if the finger is in an open (i.e., unflexed)
condition. In
actuality, if the finger is unflexed, the surgeon will probably be working on
the
tendon primarily within the skin. However, in some of the drawing figures, the
length(s) of the tendon stump(s) may be exaggerated in order not to obscure
the
illustration of the methods and apparatus being described in connection
therewith.
Furthermore, in some of the drawings in which the stitches are not
substantially
related to the features being discussed in connection therewith, the stitches
and/or
knots are represented by a simple criss-cross pattern in order not to overly
complicate the drawings. In other drawings in which the stitching or knots are
more
closely related to the features being the discussed, a more accurate
representation
of an appropriate knot/stitch is presented.
[0089]It also should be noted that other features, such as the diameters or
lengths
of the sutures, crimps, crimp connectors, and needles, are not necessarily
drawn to
scale in all of the figures.
[0090]Next, referring to Figure 2H, a very similar procedure is performed with
respect to the distal tendon stump. Particularly, the distal tendon stump 153b
is
delivered into the wound 160 in a similar fashion as described above in
connection
with the proximal tendon stump 153a. That is, if adequate exposure is not
possible
to retrieve the distal tendon stump 153b directly from the wound 160, a 1 cm
incision 174 may be made just distal to the crease at the distal
interphalangeal joint
and dissection carried down onto the distal extent of the A5 pulley so that
the distal
tendon stump 153b can be exposed through this new incision. The pullet
catheter
101 is guided between the incisions 160, and 174 and the flanged catheter 103
is
inserted into the distal end of the pulley catheter 101. The pulley catheter
101 is
then pulled through the pulley system with the flanged catheter 103 following
it until
the flanged catheter 103 is positioned through the pulley system and extending
at
19
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
opposite ends from incision 160 and 174, as shown in Figure 2H. Next, another
tendon repair device 109 is attached to the distal tendon stump 153b in the
same
manner as described above in connection with the proximal tendon stump. Figure
2H illustrates the procedure at this stage.
[0091]Referring next to Figure 21, the distal tendon stump is next guided to
the
original wound site 160 using pulley catheter 101 and the flanged catheter 103
as
described above in connection with the proximate tendon stump 153a. The second
needle 207 of the tendon holder 107 may be placed through the distal tendon
stump 153b, exposing approximately 1 cm of tendon as described above in
connection with the proximal tendon stump. This stage of the procedure is
illustrated in Figure 21.
[0092]Next, referring to Figure 2J, the connector 112 is brought to the site
and the
straight needles 111 at the ends of the cable portions 144 are inserted
through the
bores 151, 152 in the connector 112. More particularly, the straight needle
111 of
the tendon repair device 109 that is attached to the proximal tendon stump
153a is
passed through one of the bores 151 traveling in the proximal-to-distal
direction
and the straight needle 111 of the tendon repair device 109 that is attached
to the
distal tendon stump 153b is passed through the other through bore 152 in the
connector traveling in the opposite direction, i.e., from the distal-to-
proximal
direction.
[0093]Referring now to Figure 2K, the proximal and distal tendon stumps 153a,
153b are removed from their respective tendon holder needles (and the tendon
holder is put aside) and traction is applied to pull the distal tendon stump
153b
proximally and pull the proximal tendon stump 153a distally until there is
overlap of
the two tendon stumps of approximately 1 mm, with the connector 112
essentially
buried in tendon between the tendon ends 168a, 168b.
[0094]A crimping tool 113 is then used to crimp the connector 112, thereby
securely affixing the cable portions 144 of the two tendon repair devices
inside of
the connector 112. More particularly, with reference to Figure 2K, the tendon
stumps 153a, 153b can be folded back slightly to expose the connector 112 so
that
the crimping tool 113 can be placed over the crimp connector without
contacting or
damaging the tendon.
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[0095]Alternatively, if necessary, the tendon holder 107 can be used to help
bring
or hold the tendon stumps together by adjusting the positions of the two
needles
205, 207 in the slots 209, 211 of the tendon holder 107 towards the center so
that
they are very close to each other and piercing each tendon stump with one of
the
needles.
[0096]The extra lengths of cable portions 144 extending from the connector 112
are then cut as close to the edge of the crimp connector as possible and
discarded.
The connector 112 will then retract into the substance of the tendon when it
is
released and the tendon ends are unfolded and there will be excellent
cooptation
of the tendon ends, as illustrated in Figure 2L. Figure 2L represents four
cruciate
stitches 185, 187, 185', and 187' made using the tendon repair devices. While
cruciate stitches are believed to be particularly efficacious, other types of
stitches
can be used as well. If desired, one or more 6-0 nylon epitendonous stitches
183
can be placed around the tendon ends to assure good cooptation of the tendon
ends in order to 'tidy up' the edges of the repair.
[0097]Figure 2M shows a crimp holder tool that may be used to handle and
manipulate the connector 112 in connection with the bringing of the connector
112
to the repair site and the threading of the sutures through the connector.
Figure 2N
is a detailed view of the jaw portion 971 of the tool.
[0098]The connector 112 can be quite small, and thus quite difficult to handle
manually, depending on the size of the particular tendon or other anatomy
being
repaired. In the particular examples discussed herein, i.e., for repairing a
flexor
digitorum profundus in the finger, the connector 112 may be quite small and
difficult to handle. Furthermore, because the crimp connector 112 is soft and
deformable, handling it with a conventional surgical clamp or the like may
cause
the surgeon to inadvertently deform the connector if it is substantially
handled with
a clamp or other conventional surgical tool. Accordingly a crimp holding tool,
such
as tool 970 illustrated in Figures 2M and 2N is useful. Figure 2M is a
perspective
view of the tool 970 and Figure 2N is a detail view of the jaw portion 972 of
the tool
970. The tool 970 may be formed of a bar, wire, rod, dowel, or any elongated
form
971 of resilient material, such as a thin gauge stainless steel, titanium, or
polymer
so as to have spring-like properties as discussed hereinbelow. The elongated
material (hereinafter wire) is formed into a loop 971 as shown so as to take
on a
21
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
circular or oval shape with the opposing ends 971a, 971b of the wire
essentially
meeting to form a jaw 972 of the tool. More specifically, the two ends 971a,
971
cross over each other and then extend laterally from the loop 971 in parallel
so that
the resiliency of the wire biasing the loop 971 to straighten out at least
partially
biases the jaw 972 naturally closed (i.e., biases the two ends 971a and 971b
toward each other. The loop 971 preferably is sized to fit comfortably in a
human
hand. For instance, it may be an oval having a long axis of about 2 inches and
a
short axis of about 1 inch. The length of the ends 971a, 971b that form the
jaw will
at least partially be dictated by the size of the particular connector 112
with which it
will be used, but may be on the order of about 0.5 inches.
[0099]Two finger rests 973a, 973b may be welded or otherwise attached to the
loop 971 in opposing relationship to each other and preferably approximately
in the
middle of the length L of the loop 971 so that squeezing the two finger rests
973a,
073b toward each other opens the jaw 972. The finger rests 973a, 973b are
sized
and shaped to provide a pair of opposing planar surfaces of sufficient size to
provide stability of the tool in the surgeon's hand. They may be substantially
rectangular or oval in shape with a length in the long dimension 975b of the
loop
971 of about 0.5 inches and a width in the short dimension 975c of the loop of
about 0.25 inches. The outside opposing surfaces of the finger rests 973a,
973b
may be knurled or otherwise made to have high friction.
[00100] In an alternate embodiment 970' illustrated in Figure 20, finger rests
973a' and 973b' may be integrated directly in the loop material 971'.
[00101] In one embodiment, the jaw is shaped to provide a channel 974 in the
transverse direction 975a to the plane defined by the loop 971 in order to
maintain
the connector 112 oriented with its longitudinal axis in the aforementioned
transverse direction 975a. In connection with exemplary connector 112, the
channel is cylindrical to match the cylindrical shape of the connector 112.
The
cross-section of the channel 974 may be sized large enough to accept
connectors
of several different sizes. The channel 974 should be shaped and sized so
that,
even when the smallest sized connector is within the channel, it keeps the
jaws
from engaging each other (e.g., planar surface 976a, 976b do not meet) so that
the
resilient bias of the jaw will hold the smallest connector snugly in the
channel. The
___......_
22
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
spring force of the loop 971 should be sufficient to snugly hold the connector
in this
condition without deforming it.
[00102] A chamfer 979 at each end of the channel 974 provides easy access to
the crimp for loading the sutures therethough and for easy visualization.
[00103] The connector may be delivered to the surgeon pre-loaded into the
crimp holder. Alternately, the connector may be delivered separate from the
crimp
holder and the surgeon can grab the connector by hand or with a clamp or other
jawed instrument and then squeeze the two finger rests 973a, 973b toward each
other to open the jaw 972, place the connector 112 in the channel 972, and
then
release the pressure on the finger rests 973a, 973b to lock the connector 112
in
the jaw 972. The surgeon should grab the connector at its longitudinal ends so
as
not to crush it accidentally and also so that it can be placed within the
channel
without the clamp or the surgeon's fingers being within the jaw 972.
[00104] Then, the sutures are passed through the channels within the connector
as previously described. The mating shapes of the channel 974 and connector
prevents the connector from pitching, rolling, yawing, translating, or
otherwise
moving while the surgeon is trying to thread the sutures through the
connector.
Once the sutures are through the connector 112, the surgeon can release the
connector 112 from the tool 970 by again squeezing the finger rests 973a, 973b
toward each other to open the jaw. The connector 112 will be stably supported
by
the sutures passing through it. The surgeon then applies the desired traction
on
the sutures and crimps the connector with a crimping tool as previously
mentioned.
[00105] Figure 3 is a photograph of an actual tendon repair performed in
accordance with the first embodiment of the invention. The first and second
knots
185 and 187, respectively, can be seen in the proximal tendon stump 153a.
Similar knots 185' and 187' are seen in the distal tendon stump 153b. Four
epitendonous stitches 183 also can be seen.
[00106] The one or more skin wounds can be stitched closed as usual and the
procedure is ended.
[00107] While the procedure and apparatus has been described above in
connection with one particular procedure relating to the repair of a flexor
tendon
laceration, flexor digitorum profundus at the level of the middle phalanx,
this is
23
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
merely an exemplary application. The invention can be applied to reattach
other
types of tendons, ligaments, or other similar load-bearing soft tissues.
Second Set of Exemplary Embodiments
[00108] Figures 4A-4D illustrate another apparatus and procedure in accordance
with the principles of the present invention that can be used in situations
where the
tendon (or ligament) has avulsed or otherwise been separated from the bone,
rather than severed. The apparatus and procedure described in connection with
Figures 4A-4D also may be used in situations where the tendon or ligament has
been severed very close to the bone so that there is not enough tendon length
left
to effectively attach a tendon repair device 109 to that stump.
[00109] In these types of situations, a tendon repair device such as the afore-
described tendon repair device 109 is still used in the manner described above
in
connection with Figures 2A-2H in connection with the stump that has sufficient
length, e.g., at least 2 cm, (typically the proximal stump). However, with
respect to
the bone or short tendon stump, one or more cables are attached directly to a
bone
anchor 400 instead of using a second tendon repair device.
[00110] The bone anchor may be any bone anchor that can be attached to bone
at its distal end and to which a suture or cable can be attached to the
proximal end
thereof. Suitable bone anchors are disclosed, for instance, in PCT
International
Published Patent Application WO 2008/054814, which is incorporated herein by
reference. However, much simpler bone anchors can be used also.
[00111] In a simple embodiment of a suitable bone anchor, such as illustrated
in
Figure 1, the bone anchor may comprise a threaded distal portion 401 for
threading
into bone and an eyelet 402 for receiving the cable of the tendon repair
device
integrally formed in the proximal portion of the bone anchor main body. In
other
embodiments, the bone anchor may be prefabricated with one or more sutures
integrally formed therein and extending from the proximal end thereof.
[00112] A surgical procedure in accordance with this embodiment will now be
described in connection with an exemplary injury in which the flexor digitorum
profundus has been lacerated very close to the distal phalanx. However, it
should
be understood that variations of this procedure can generally be used in
connection
24
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
with any tendon or ligament that has avulsed from the bone or been severed
close
to the bone.
[00113] Figures 4A-4D illustrate various stages of an exemplary procedure for
effecting a four strand repair (i.e., the repair will have four suture strands
running
between the two tendon stumps). This embodiment utilizes a different tendon
repair device 1001 than the tendon repair device 109 illustrated in Figures 1-
2L.
This tendon anchor is illustrated in Figure 10A, which is discussed in more
detail
below in connection with another exemplary surgical procedure. With reference
to
Figure 10A, it comprises two strands or filaments 1047a, 1047b, with each
strand
having a needle at each end. In the illustrated embodiment, curved needles
1014a
and 1014b are provided at the first ends of the strands 1047a, 1047b,
respectively,
and straight needles 1011a, 1011b are provided at the second end of the
strands1047a, 1047b, respectively. The two strands comprising the tendon
repair
1001 device are joined intermediate their ends, such as by a fixed or slidable
crimp
1049. The crimp 1049 may initially be uncrimped so that it can slide along the
device and, if desired, crimped at a suitable stage of the procedure. As shown
in
Figure 10A, the tendon repair device 1001 may be delivered to the surgeon with
a
portion of the sutures and the straight needles 1011a, 1011b on end 1001a
enclosed in a sheath 1011 to ease the process of passing that end of the
tendon
repair device 1001 into the pulley catheter 101 and/or flanged catheter 103.
[00114] The long tendon stump 501 is operated upon essentially as described
above in connection with the first embodiment. Particularly, with reference to
Figure 4A, the tendon stump 501 is retrieved, if necessary, by making a
retrieval
incision 531 where needed, exposing the tendon stump 501, and stitching end
1001b of the tendon repair device 1001 to the tendon stump using the curved
needles. In this exemplary case, where there are only two sutures 1047a,
1047b,
one cruciate stitch is preferred. In embodiments using tendon repair devices
having more sutures, such as the tendon repair device 109 of Figures 1-2L
having
seven sutures, then the tendon repair device can be stitched to the tendon
stump
using multiple cruciate or other stitches, exactly as described above in
connection
with the embodiment of Figures 1-2L, for instance. Next, the pulley catheter
101,
flanged catheter 103, and catheter connector 105 (if needed) can be used as
previously described to guide the tendon repair device 1001 and tendon stump
501
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
back to the injury site 533. The narrow sheath 1011, if provided, will
facilitate
threading of the end 1001a of the tendon repair device 1001 into and through
the
catheters.
[00115] Then, the tendon stump 501 is placed in a tendon holder 107 while the
distal tendon stump is prepared. Figure 4A shows the condition of the surgical
site
after these steps have been performed, i.e., with the tendon 501 in a tendon
holder
107 with a tendon repair device 1001 stitched thereto.
[00116] Next, referring to Figure 4B, with respect to the bone 503 (and distal
stump 505, if any is present), an incision 532 (which may include original
injury
532) is made and dissection is carried down to expose the bone 503 of the
distal
phalanx. A bone anchor, such as bone anchor 450 shown in Figure 1, is then
affixed to this bone 503 by screwing it in securely.
[00117] Next, with reference to Figure 4C, since this exemplary embodiment is
a
four strand repair, two of the sutures 451c, 451d of the bone anchor 450 can
be cut
off at or as close to the bone anchor as possible. The other two sutures 451a,
451b are threaded through the distal stump 505. Now, referring to Figure 4D,
the
tendon stumps are brought together with a slight amount of overlap and the two
sutures 451a, 451b of the bone anchor 450 are stitched and knotted to the
proximal stump 501. Likewise, the tendon repair device 1001 that is already
stitched to the proximal tendon stump 501 at one end thereof is then stitched
to the
distal stump 505 at the other end. Figure 4D shows the completed repair in
accordance with this embodiment.
[00118] Of course, the number of strands on the bone anchor 450 and the
number of strands on the tendon repair device 1001 can be increased to provide
a
stronger repair, such as a six eight, ten, or even twelve strand repair, if
desired.
[00119] A tendon injury of the type illustrated in Figures 4A-4D, in which
there is
only a short distal tendon stump remaining (or none at all) also can be
repaired
using a tendon repair device 109 such as illustrated in Figures 2A-2L and the
other
bone anchor 400 shown in Figure 1, the long tendon stump 501 is operated upon
exactly as described above in connection with the first embodiment of Figures
2A-
2L. Particularly, the proximal tendon stump 501 is retrieved, if necessary, by
making a retrieval incision where needed, exposing the tendon stump 501,
attaching a tendon repair device 109 to the tendon stump, and using the pulley
26
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
catheter 101, flanged catheter 103, and catheter connector 105 (if needed) as
previously described to guide the tendon stump back to the injury site.
[00120] Next, an incision is made and the bone anchor 400 is affixed to the
bone
essentially as described above in connection with Figures 4A-4D, except that
it is
bone anchor 400, rather than bone anchor 450.
[00121] Next, if a distal stump of the flexor is still present, such as stump
505 in
Figures 4A-4D, then the needle 111 and cable 144 of tendon repair device 109
is
run through this stump 505 and into and through the eyelet 402 of the bone
anchor
400. Particularly, the straight needle 111 at the end of cable portion 144 is
brought
into the short distal tendon stump 505 through the severed end of the tendon
stump 505 and out through the side of the tendon stump near where the stump
505
is still attached to the bone 503 and then through the eyelet 402 in the bone
anchor
400.
[00122] Next, traction is applied to the cable 144 to draw the proximal tendon
stump 501 distally until there is a 1 mm overlap of the proximal tendon stump
501
with the distal tendon stump 505.
[00123] Then, the cable 144 is fixed to the eyelet of the bone anchor 503.
This
can be done by tying the suture or cable to the eyelet 402 of the bone anchor.
In a
more preferred embodiment, however, the proximal end of the bone anchor 503 is
crimped to crush the eyelet 402 of the bone anchor 400, thereby trapping the
cable
144 therein.
[00124] Finally, the procedure is completed essentially as described above in
connection with the embodiment of Figures 2A-2L or 4A-4D.
[00125] If, on the other hand, there is no or virtually no distal tendon stump
remaining to attach to, the proximal stump would instead be attached directly
to the
bone using the bone anchor. Preferably, the cable portion 144 of the tendon
repair
device attached to the tendon stump is directly attached to the bone anchor
without
the use of a second suture or cable 509 and the proximal tendon stump is
pulled
distally so that the stump envelopes the bone anchor and contacts the bone
around the bone anchor. As is often the case, the surgeon may roughen, counter
bore or tunnel the bone in the area around the bone anchor for the tendon to
attach to.
27
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00126] In another alternate embodiment, only the bone anchor 450 with
multiple strands (with needles at the ends of the strands) already extending
from
the bone anchor is used. No separate tendon repair device 109 or 1001 is used.
Rather, the sutures extending from the bone anchor 450 are stitched directly
to the
proximal tendon stump. This type of embodiment is most suited to an injury in
which (1) the proximal tendon stump has not retracted significantly and is,
therefore, present at the incision near the distal stump without the need to
be
retrieved through another incision and (2) there is no distal tendon stump to
include
in the repair. Particularly, with respect to the first point, if the proximal
tendon
stump needs to be retrieved, then it would likely be more practical to use the
technique described in connection with Figures 4A-4D. More specifically, if
the
proximal tendon stump must be retrieved, then a separate tendon repair device
probably will have to be attached to the proximal tendon stump for purposes of
retrieving the stump, in any event. In such a situation, it would be simpler
to attach
the tendon repair device that is already stitched to the proximal tendon stump
to
the bone anchor than to add another set of sutures.
[00127] With respect to the second point, if there is a distal tendon stump,
it
would be preferable to include sutures emanating from the proximal stump that
exert a force pulling the distal tendon stump toward the proximal tendon
stump. In
the absence of a proximal tendon repair device, no sutures exerting such a
force
would be present and, therefore, the distal tendon stump could conceivably
slide
away from the end to end contact of the two tendon stumps prior to healing of
the
tendon stumps.
[00128] In repairs in accordance with the bone anchor embodiment, the load on
the distal end is borne completely by the bone and bone anchor.
[00129] Preliminary testing has shown failure strengths of tendon
reattachments
performed in accordance with the principles of the present invention of
approximately 70-100 Newtons. Accordingly, a tendon and ligament repair in
accordance with the principles of the present invention results in a much
stronger
repair that the current standard of care.
[00130] In addition, the procedure is greatly simplified as compared to the
present standard of care.
28
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
Third Set of Exemplary Embodiments
[00131] Figure 5 illustrates another embodiment in accordance with the
principles of the present invention. Figure 5 is a close up of the proximal
tendon
stump 153a in accordance with this embodiment of the invention at a stage
after
the tendon repair device 109 has been stitched to the tendon stump. It is
essentially similar to the stage shown in Figure 2E, but illustrating a
different way to
finish off the stitches other than tying them in knots in pairs.
[00132] This embodiment involves a simpler procedure than in the
aforedescribed embodiment in so far as the surgeon will not be required to tie
any
knots. Rather, as shown in Figure 5, rather than tying knots in the sutures
147a-
147g after stitching them to the tendon, a crimp 603 can be advanced over each
suture against the stitch as far as it will go and then crimped with a
crimping tool to
lock the crimp to the suture, thus locking the stitch to the tendon. Depending
on
the particular configuration of the curved needles 114a-114g and the crimps
603,
the crimps may be slipped over and around the needles onto the sutures 147a-
147g. If this is not possible, then the needles 114a-114g can be cut off of
the
sutures 147a-147g after the corresponding stitch is tied to permit the crimp
to be
placed on the suture. In this embodiment, the surgeon is not required to tie
any
knots with the sutures, thus simplifying the procedure. The surgeon is free to
use
the sutures to create any stitches desired, but they do not need to be knotted
at the
end.
Fourth Set of Exemplary Embodiments
[00133] Figures 6A and 6B illustrate an alternative to the crimp connector 115
for attaching two tendon repair devices 109 (or a tendon repair device 109 and
a
bone anchor 115) to each other. In this embodiment, the connector 701
comprises
a connector main body 711 having two parallel, longitudinal through bores 713,
715. The main body 711 may be cylindrical, rectangular, or any other
reasonable
shape. Another bore 717 is provided in the main body 711 transverse to the
direction of longitudinal through bores 713, 715, this bore intersecting the
two
longitudinal through bores 713, 715. A pin in the form of a block 719 fits in
the
transverse bore 717. Accordingly, when the block is inserted in the transverse
bore
717 as shown in Figure 6b, it also transversely passes through portions of the
29
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
longitudinal through bores 713, 715. The dimensions of the block 719, the
transverse bore 717 as shown in Figure 6B, the longitudinal through bores 713,
715, and cable portions 144 (that will pass through the longitudinal through
bores
713, 715) are chosen so that the block 719, when inserted into the transverse
bore
717 will compress the cables in the longitudinal bores 713, 715 between the
side
wall of the block 719 and the side walls of the longitudinal bores 713, 715,
thereby
trapping the cables in the connector 701.
[00134] Thus, in this embodiment, rather than crushing the crimp connector
with
a crimping tool, a pliers or clamp type tool acts on the block 719 and the
connector
701 and pushes the block 719 into the connector 701 against the resistance of
the
cable portions 144 in the longitudinal through bores 713, 715, thereby
capturing the
cables as described above.
[00135] Some of the advantages of this embodiment of the connector include a
much lower force requirement for locking since the block 719 does not have to
be
plastically deformed. Rather, this mechanism relies on the wedging of cables
144
against the inner wall of connector 701 to effect the lock.
[00136] There are many possible alternative stitching techniques to the few
described above. The present invention can accommodate and permit the surgeon
to use any stitching technique desired. In alternate embodiments, the tendon
repair device may have only four sutures or, if it has more than four sutures,
the
surgeon may decide to cut off those sutures that he or she does not use. For
instance, two of the sutures of the tendon repair device 109 of Figures 1-2L,
e.g.
sutures 147a and 147g, may be stitched to the tendon using cross stitches and
are
knotted together as previously described in connection with the embodiment of
Figures 2A-2L, except that the remaining distal portions of the sutures 147a,
148g
extending from the knots are not cut off at this time. Next, another two
sutures,
e.g., 147b, 147f, are stitched to the tendons at a different level than the
first two
sutures and knotted, also as described in connection with the embodiment of
Figures 2A-2L. Then, sutures 147a and 147b are tied in a knot and sutures 147g
and 147f are tied in another knot. Now, the distal ends of each of sutures
147a,
147g, 147b, and 147f may be cut off. The other 3 sutures 147c, 147d, 147e, may
be cut off and not used or may be used to form other knots. The inter-
dependence
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
of the two pairs of sutures in this technique provides greater assurance that
the
sutures will not tear out of the tendon.
[00137] In yet other embodiments, the third pair of sutures also may be tied
together with the first two pairs of sutures. The various permutations of
stitching
techniques and tying together of the sutures are virtually endless.
Sixth Set of Exemplary Embodiments
[00138] Figures 8A illustrates an alternative embodiment of the tendon repair
device. This embodiment is particularly suited to, but not limited to,
surgical
procedures in which either one or none of the tendon stumps needs to be
retrieved
from a separate incision and be guided back to the wound site. This embodiment
also has the advantage of being capable of effecting a repair using only a
single
tendon repair device, if desired.
[00139] As can be seen in this embodiment, rather than having one side of
the
anchor comprised of multiple sutures and the other side comprised of one cable
as
was the case for the embodiments illustrated in Figures 1-2L and 4A-4E, this
tendon repair device has multiple sutures on both sides 901a, 901b of the
tendon
repair device 901. More particularly, this tendon repair device may be formed
of
four sutures 947a-947d attached together at one or more intermediate points
along
their lengths. In one embodiment that is particularly convenient to
manufacture,
the tendon repair device 901 comprises four sutures 947a-947d with at least
one
crimp 949 intermediate their lengths holding them together. The crimp may be
initially uncrimped so that it can slide along the lengths of the sutures
during the
procedure. It may be crimped to lock its position relative to the sutures at
any point
during the procedure. In some procedures, it may not be crimped at all.
[00140] In this embodiment, the tendon repair device 901 preferably is
delivered
to the surgical site in the condition illustrated in Figure 8B, i.e., with at
least one of
the side 901a contained in a narrow sheath 911 (e.g., a plastic tube) that can
be
easily passed through the flanged catheter. However, depending on the
diameters
of the needles, sutures, flanged catheter, the number of sutures in the
device, and
the material of the flanged catheter, a sheath may be unnecessary or may cover
only part of the end 901a (such as just the tips of the needles 913a-913d). In
this
embodiment, the needles 913a-913d attached to the ends of the sutures on side
31
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
901a of the crimp 949 that will be placed in the sheath 911 should be straight
needles in order to more readily fit into the sheath 911 and/or through the
catheters
101, 103.The needles attached to the other ends of the sutures 947a-947d may
be
curved needles 914a-914d to facilitate stitching. However, they also may be
straight needles.
[00141] The first half of the surgical procedure is essentially identical to
the
procedure described above in connection with the first embodiment illustrated
in
Figures 2A through 2L. More particularly, the procedure is essentially
identical to
that embodiment up to the stage illustrated in Figure 2F, the only difference
being
that, instead of a single cable 144 extending from the far side of the
intermediate
crimp 949, there are four individual sutures (or cables) contained in a sheath
911.
[00142] After the device has been stitched to one tendon stump, the sheath
911,
containing the four straight needles and sutures is traversed through the
pulley
system to the site of the wound as described previously. Next, the protective
sheath 911 is removed; thereby releasing the four sutures 947a-947d and
straight
needles 913a-914d.
[00143] In one embodiment, the sheath 911 is cut with a knife or scissor. In
another embodiment, the sheath can be torn by hand. In yet another embodiment,
and, particularly, the illustrated embodiment, the sheath 911 comprises an
integral
longitudinal strip 911a, such as a string embedded within the material of the
sheath, having a "tail" 911b extending beyond at least one end of the sheath
so
that it can be grasped by the surgeon and pulled to tear the sheath, thus
freeing
the needles for attachment to the tendon stump. Alternately, the strip may
comprise a weakened radial segment of the sheath running the full longitudinal
length of the sheath. The weakened segment may comprise a strip of the sheath
that is integrally formed with the rest of the sheath, but having a thinner
wall
thickness than the rest of the sheath.
[00144] The crimp 949 may be crimped at this stage of the procedure to lock
its
position on the device 901. For instance, it may be crimped immediately
adjacent
the end of the tendon stump 902a to which it has been stitched at this point.
[00145] When using this embodiment, the other tendon stump 902b preferably is
exposed at the wound site without the need to be retrieved. If, however, it
must be
retrieved through a different incision, it can be retrieved using any
reasonable
32
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
technique, including conventional techniques for tendon retrieval or using the
pulley
catheter and flanged catheter of the present specification as described above.
For
instance, a small suture can be stitched to the tendon temporarily and the
suture
can be advanced through the pulley system of the finger using the pulley
catheter
101 and flanged catheter 103 much as described above in connection with the
first
embodiment.
[00146] In any event, with the other tendon stump 902b exposed at the wound,
the two stumps 902a, 902b are positioned with their ends opposed to each other
and the second end 901a of the tendon repair device can be stitched to the
distal
tendon stump 902b much in the same way as described above in connection with
the first embodiment. Care should be taken to assure that the two tendon ends
902a, 902b appose each other, since it will be difficult, if not impossible,
to adjust
the relative positions of the ends of the tendon stumps after the first stitch
is
completed and locked. The tendon holder 107 can be used as previously
described to hold the tendon ends apposed to each other. The sutures may be
stitched to the tendon in pairs as previously described. The repair can be
completed with an epitendonous stitch between the two stumps as previously
noted.
[00147] This embodiment is advantageous in that it requires no crimp connector
or crimping tool and has fewer parts. For example, only one tendon repair
device
is involved in the procedure, that tendon repair device being double headed,
as
shown in Figure 8A.
Seventh Set of Exemplary Embodiments
[00148] Figures 9A-9C help illustrate yet another embodiment of a tendon
repair
device and technique particularly suited, but not limited, to repairs where
both
tendon stumps must be retrieved to the repair site by being tracked through
anatomy between two incisions. Figure 9A shows the tendon repair device 951 in
accordance with this embodiment. In this embodiment, two tendon repair devices
951 are used, each comprising two strands or filaments 953a, 953b, with each
strand having a needle at each end. In the illustrated embodiment, curved
needles
954 are provided at the first end and straight needles 955 are provided at the
second end of each strand. The two strands comprising a single tendon repair
33
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
device are joined intermediate their ends, such as by a slidable crimp 956 as
previously described in connection with other embodiments. The crimp 956 may
initially be uncrimped so that it can slide along the device and, if desired,
crimped
at a suitable stage of the procedure.
[00149] As shown in Figure 9B, one end 951a of each tendon repair device 951-
1, 951-2 is stitched to a respective tendon stump 961a, 961b using the two
strands
of that end. The other end 951b of each tendon repair device may be initially
encased within a sheath 968 similarly to the embodiment of Figures 8A and 8B
for
purposes of being passed through anatomy, such as the pulleys of the finger,
using
the pulley catheter and flanged catheter described above in connection with
other
embodiments. However, as noted above in connection with the embodiments of
Figures 8A and 8B, the sheath may not be necessary.
[00150] Next, the tendon repair devices and tendon stumps to which they are
stitched can be tracked through anatomy to the repair incision using the
pulley and
flanged catheters as previously described. The condition of the tendon repair
procedure at this point is illustrated in Figure 9B. Referring now to Figure
9C, the
two tendon stumps 961a, 961b are brought together. If desired, they can be
held
in position using the tendon holder 107, with one needle 205,207 in each of
the
tendon stumps 961a, 961b (not shown).
[00151] Next, the free ends 951b of the two strands of the first tendon repair
device 951-1 (the other ends 951a of which are already stitched to the first
tendon
stump 961a) are stitched to the second tendon stump 961b, preferably at a
different level than the stitches of the second tendon repair device 951-2.
Likewise, the free ends 951b of the two strands of the second tendon repair
device
951-2 (the other ends 951a of which are already stitched to the second tendon
stump 961b) are stitched to the first tendon stump 961b. The completed repair
is
shown in Figure 9D. The repair can be completed with an epitendonous stitch as
previously described, if desired.
[00152] Like the embodiment of Figures 8A-8B, this embodiment provides four
strands running between the two tendon stumps, and two stitches at different
levels
in each tendon stump, thereby providing a very sturdy repair.
Eighth Set of Exemplary Embodiments
34
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00153] Figure 10A illustrates a tendon repair device in accordance with yet
another embodiment of the invention. This device 1001 is essentially the same
device of Figure 9A, but with one side in a sheath, as will be described in
more
detail below. In these embodiments, two tendon repair devices will be used, as
in
the first embodiment as illustrated in Figures 1 and 2A-2L. However, both of
these
tendon repair devices 1001 have multiple strands at each end, as in the
embodiments illustrated in Figure 8A-8B and 9A-9D. More particularly, each
tendon repair device 1001 comprises two sutures 1047a, 1047b. The two sutures
may be coupled together intermediate their ends, such as by a crimp 1049 or
sliding sleeve. Alternately, the two sutures may be independent of each other.
[00154] Even further, the tendon repair device 1001 may comprise a
single
cable or suture over much of its length and be broken out into two sutures
only
near the opposite ends of the anchor. Again, such a tendon repair device may
be
formed of two sutures twisted together over much of their length and separated
near the opposite ends with a crimp, such as crimp 956, at each end of the
twisted
portion holding the twisted portion together. As in the embodiment of the
tendon
repair device illustrated in Figures 8A-8B and 9A-9D, straight needles 1013a,
1013b preferably are employed on at least one end 1001a of the device 1001 and
curved needles 1014a, 1014b are employed on the other end 1001b. As shown,
the tendon repair device may be delivered to the surgeon with the sutures and
straight needles 1011a, 1011b on end 1001a enclosed in a sheath 1011. The
procedures and apparatus for repairing a tendon using this embodiment of the
tendon repair device are rather similar to those described previously in
connection
with the first and second embodiments. Particularly, one or both of the tendon
stumps can be retrieved through the pulley system of the finger, as needed,
exactly
as described in connection with the first embodiment of the invention
illustrated in
Figures 1 and 2A-2L, except that only two sutures are stitched to each tendon
stump at one side 1001b of the tendon repair device 1001.
[00155] In this embodiment two of the tendon repair devices 1001-1 and 1001-2
are used. One side 1001a of each tendon repair device 1001-1 and 1001-2 is
stitched to one of the tendon stumps.
[00156] Figure 10B helps illustrate how two of these fixation devices 1001
could
be used to effect a repair by looping them around each other in accordance
with
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
this embodiment of the invention. Generally, one tendon repair device 1001-1
would be folded to form a loop 1091 and stitched to the first tendon stump
1087a
and the other tendon repair device 1001-2 would be folded to form another loop
1092 and embedded in the other tendon stump 1087b with the loops joined in the
middle as described in detail below.
[00157] Specifically, the two sutures 1047a, 1047b and curved needles 1014a,
1014b on one side 1001b of first tendon repair device 1001-1 would be stitched
to
the first tendon stump 1087a with the other side 1001a of the device sticking
out of
the end of the respective tendon stump, basically as described in connection
with
previous embodiments.
[00158] Next, with reference to Figure 10B, the other side 1001a of the first
tendon repair device 1001-1 is returned back into the tendon same stump
through
the end of the stump so that the tendon repair device 1001-1 forms a loop 1091
sticking out of the end of the tendon stump 1087a. This may be performed by
individually threading each of the two sutures and straight needles 1014a,
1014b
back through the end of the tendon stump 1087a and pulling them out through
the
side of the tendon stump. The suture(s) should be pulled through so that the
loop
1091 protrudes from the end of the tendon stump 1087a by 1 millimeter or less.
Preferably, the sutures are pulled through so that the loop 1091 does not
protrude
at all, but is essentially in the substance of the tendon stump 1087a. Then,
the two
sutures 1047a, 1047b are stitched to the tendon essentially as described above
in
connection with the previously described embodiments. At this point, both ends
1001a, 1001b of the tendon repair device 1001-1 are stitched to the tendon
stump
1087a and a loop 1091 is located at the severed end of the tendon stump 1087a.
[00159] Next, the second tendon repair device 1001-2 is attached to the second
tendon stump 1087b in essentially the same manner as the first tendon repair
device 1001-1 was attached to the first tendon stump 1001a, except that, after
the
first two needles 1013a, 1013b at the first end of the1001a anchor 1001-2 are
stitched to the tendon, the other two needles 1014a, 1014b and sutures 1047a,
1047b are guided through the loop 1091 formed by the first tendon repair
device
1001-1 to form a second loop 1092 before being stitched to the second tendon
stump 1087b. If the loop 1091 of the first tendon repair device 1001-1 is
within the
substance of the first tendon stump 1087, the substance of the first tendon
stump
36
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
may need to be retracted with a suitable retractor tool to expose the loop
momentarily for the second tendon repair device needles and sutures to be
passed
through the loop. Alternately, the surgeon may simply pierce the tendon
substance
with the second tendon repair device 1001-2 to access the loop 1091. Then the
two sutures and needles 1014a, 1014b at the second end 1001b of the second
tendon repair device 1001-2 are stitched to the second tendon stump. This
embodiment offers another technique for providing a four strand repair between
the
two tendon stumps.
Ninth Set of Exemplary Embodiments
[00160] Figures 11A-11E illustrate alternate embodiments and associated
techniques to be used therewith, which techniques can be used in conjunction
with
some or all of the features and aspects of many of the other embodiments of
both
the methods and apparatus disclosed herein. Figure 11A is a perspective view
of
the apparatus in accordance with this alternate embodiment. Particularly, in
this
embodiment the flanged catheter is replaced with a guidance member in the form
of a funnel 1101.
[00161] In a preferred embodiment, funnel 1101 is formed of a biocompatible
material, such as a biocompatible plastic, that is relatively rigid, so that
it is not
easily collapsible. The funnel 1101 comprises a small opening 1102 at one end
and a large opening at the other end 1103. Funnel 1101 defines a frustoconical
surface when in an unbiased condition, but is split along its entire length,
whereby
it can be radially spread apart at the split 1104 to resiliently deform the
funnel to
provide a lateral gap at the split 1104 through which a tendon, ligament or
the like
can be inserted into the funnel. Alternately, the funnel may overlap somewhat
at
the split as long as it can be spread apart radially to provide a lateral
opening.
[00162] The small opening 1102 should be smaller than the entrance to the
anatomical passage in connection with which it will be used for introducing a
tendon therethrough and the large opening 1103 is larger than the anatomical
passage. For instance, in the various embodiments of the invention discussed
above in connection with a repair of a finger tendon, the small opening should
be
sized to help facilitate entry into the pulleys of a finger. The large opening
at the
other end 1103 of the funnel 1101 should be sufficiently large to readily
accept the
37
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
end of a tendon stump with a tendon repair device stitched thereto. A handle
1197
can be provided extending from the side of the funnel 1101 to facilitate easy
manipulation by the surgeon.
[00163] Figures 11B-11D illustrate a surgical technique using the funnel
1101.
With reference to Figure 11B, a pulley catheter 101 is positioned through the
pulley
system of the finger between two incisions 1112, 1113, as previously
described,
and a tendon repair device 1114, which could be any of the tendon repair
devices
previously discussed herein, is attached to the end of the proximal tendon
stump
1116. Furthermore, the leading end 1114a of the tendon repair device 1114 is
passed into the pulley catheter 101 also essentially as previously described,
except
without the use of a flanged catheter 103, the function of which will
essentially be
replaced by the funnel 1101, as described in detail below.
[00164] In this embodiment, the leading end 1114a of the tendon repair device
1114 is pushed through the pulley catheter 101 to a point where the end of the
tendon stump 1116 is close to, but not touching the trailing end 101b of the
pulley
catheter 101. Next, the pulley catheter 101 and tendon repair device 1114 are
pulled distally through the pulley system of the finger from the distal
incision 1113
to a point where the trailing end 101b of the pulley catheter 101 passes the
entrance of the first pulley 1121 that must be traversed, but the tendon stump
1116
is near the entrance to the pulley 1121, but has not passed it yet.
Specifically, as
previously noted, the end of the tendon stump 1116 is deformed and enlarged
and
is unlikely to pass easily through the pulley 1121 without a structure to
compress it
and guide it in. In the previously discussed embodiments, that structure was
the
flanged catheter 103. In this embodiment, it will be the funnel 1101.
[00165] Thus, with reference to Figure 11C, funnel 1101 is spread apart and
slipped over the tendon stump 1116 with the small end 1102 of the funnel
facing
the entrance to the pulley 1121 and the large end 1103 facing away from the
entrance to the pulley. More particularly, the surgeon positions the funnel
1101 in
the entrance to the pulley 1121 in order to dilate the pulley 1121 and
facilitate the
tendon's entering into and passing through the pulley, as shown in Figure 11C.
Funnels of different sizes may be provided as part of a kit in order to
accommodate
different sized parts of the anatomy and/or different sized patients and to
facilitate
dilation of the pulley (or other anatomical feature).
38
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00166] With the funnel in the position shown in Figure 11C, the surgeon can
then pull on the leading end 1114a of the tendon repair device 1114 to draw
the
end of the tendon stump 1116 into and through the funnel 1101 and the pulley
1121.
[00167] It should be apparent that the primary issue addressed by the funnel
1101 (as well as the flanged portion 159 of the flanged catheter 103 disclosed
in
connection with previous embodiments) is that often, if not always, the end of
the
tendon stump with the trailing end of the tendon repair device attached
thereto
bunches up to become larger than the passageway through the pulley and
therefore difficult to insert into and through the pulley. The funnel (as well
as the
flanged portion 159 of the aforedescribed flanged catheter 103) contains the
end of
the tendon stump gradually to facilitate insertion into and passage through
the
pulley (or other narrow anatomical passage as the case may be). The funnel
1101
of this embodiment also serves to dilate the entrance to the pulley to even
further
facilitate passage.
[00168] Unlike the embodiment utilizing the flanged catheter 103, in this
embodiment, the funnel 1101 does not pass through the pulleys. It remains in
the
position shown in Figure 11C just inside the entrance to the pulley, while the
tendon stump 1116 slides through the funnel 1101 and through the pulley 1121.
Once the end of the tendon stump 1116 has passed through the pulley 1121, the
funnel 1101 is removed. Particularly, it can be spread apart and slipped off
the
tendon. Alternately, the funnel can be cut away. Figure 11D shows the repair
at
this point of the procedure.
[00169] If the tendon stump 1116 must be guided through a second or
subsequent pulley, the same process is essentially repeated with respect to
the
second pulley. For instance, if the tendon must pass through a second pulley,
then
another incision can be made above that pulley (in the corresponding crease of
the
finger) and the aforedescribed process can be repeated using the same or a
different funnel. However, the surgeon should first attempt to pull the tendon
through without using the funnel, as, often, the tendon might track through a
second or subsequent pulley without the help of the funnel.
[00170] U.S. provisional patent application No. 61/506.809, to which the
present
application claims priority and which is incorporated herein fully by
reference
39
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
discloses techniques for locating the pulleys and thus positioning any
incisions for
guiding the tendon through a pulley using, for instance, the funnels and other
guidance members disclosed herein. The contents of that application are
contained herein under the heading Appendix A.
[00171] The tendon stump can then be (1) attached to the distal tendon stump
directly, (2) attached to another tendon repair device attached to the distal
tendon
stump, or (3) be attached to a bone anchor, as the case may be, using any one
of
the aforedescribed tendon repair devices and/or techniques.
[00172] Figure 11E illustrates an alternate embodiment of the guidance
member. The guidance member 1140 in this embodiment is of a split hollow
frustoconical form having a smaller diameter end 1143 and a larger diameter
end
1144, with a portion of the frustoconical surface removed. The lateral opening
1142 defined by the removed portion of the surface should be sufficiently wide
to
permit easy insertion of the particular tendon, ligament, or other anatomical
feature
with which it is intended for use, but sufficiently narrow so as not to permit
the
tendon to slip out of the member 1140 accidentally. Thus, preferably, the
opening
is no more than 50% of the conical surface. The opening, for instance, may be
about 5%-35% of the conical surface with 1/3 being preferred. In this
embodiment,
since the guidance member 1140 need not deform to permit the tendon to be
inserted therein, it preferably is substantially rigid and not deformable
under normal
loads. It may be formed of a biocompatible metal, such as stainless steel or
titanium. Again, a handle 1198 may be provided to facilitate handling of the
guidance member 1140 by the surgeon.
[00173] The guidance member 1140 of this embodiment is used essentially
exactly as was described above in connection with the funnel 1101 of the
preceding embodiment, except that the member 1140 is not be spread apart in
order to insert the tendon therein. Rather, the tendon can simply be laid
inside the
member 1201 through the lateral opening 1142. As in the previous embodiment, a
handle 1198 may be provided to facilitate manipulation by the surgeon.
[00174] This embodiment is advantageous in that it is easier to insert a
tendon
in the member. Furthermore, the guidance member is rigid and, therefore,
provides more efficient dilation of the anatomy.
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00175] Figure 11F illustrates yet another alternate embodiment of the
guidance
member. Like the embodiment of Figure 11A, the guidance member 1150 in this
embodiment is a funnel 1151 with a small opening 1152 and a large opening
1153.
It is split along its entire length, whereby it can be radially spread apart
at the split
1154 to resiliently deform the funnel to provide a lateral gap at the split
1154
through which a tendon, ligament or the like can be inserted into the funnel.
[00176] A lip 1156 is provided at the large end to prevent the funnel from
being
inadvertently pulled through a pulley. A small handle 1157 provides a place
for the
surgeon to grasp the guidance member 1150. The use of a small handle or merely
a lip with no handle at all per se facilitates the ease with which a surgeon
may spin
the guidance member about its longitudinal axis. Specifically, spinning is
sometimes helpful in introducing the small end 1152 of the guidance member
into
the pulley. A longer handle might interfere with the ability to freely spin
the
guidance member because a longer handle is more likely to hit an obstruction,
such as another part of the patient's hand or another surgical instrument.
[00177] In still other embodiments, the guidance member could be formed as a
split cone with overlap at the split so as to have a spiral-like shape. The
overlap
should be relatively small, such as on the order of between about 5 and 90
of
radial overlap, and preferably about 70 of radial overlap when unstrained.
Particularly, too much radial overlap might make it difficult to spread the
guidance
member apart sufficiently to open the gap through which the tendon must pass.
[00178] Embodiments with overlapping at the split have several advantages.
First, the overlap would make it essentially impossible for the tendon to
accidentally
slip out of the gap in the guidance member once the expansion pressure to open
the gap is removed. Second, the overlap will permit some adjustability to the
radial
size of the guidance member. That is, by applying inward radial force on the
outer
wall of the guide member, the radius of the guide member can be decreased
temporarily to help fit the small opening of the guidance member into a pulley
should that be necessary. Conversely, the radius of the guidance member may be
temporarily increased should that be necessary to allow a tendon to pass
through
the opening at the small end of the guidance member, but without opening the
gap
in the side wall, which might allow the tendon stump to inadvertently escape
from
the guidance member through the gap). More specifically, if the tendon stump
41
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
being guided through the pulley system by the guidance member is smaller than
the opening at the small end of the guidance member, the pulling force on the
tendon repair device and tendon stump will simultaneously also apply a
radially
outward force on the inner wall of the guidance member. That outward radial
force
will force the guidance member to radially expand, which will cause the
opening at
the smaller end to increase in diameter and allow the tendon stump to pass
through.
[00179] Figure 11G illustrates yet another embodiment of a guidance member.
This guidance member 1160 may be considered a hybrid of the flanged catheter
103 of the embodiment of Figures 1-2L and the guidance members of Figures 11A,
11E, and 11F. Particularly, like the flanged catheter 103, guidance member
1160
is adapted to pass through the pulley system along with the tendon stump and
repair device. It protects the tendon, helps uniformly compress it as needed
to
pass through the pulleys, and presents a smooth surface to the pulleys so as
help
pass through. However, like the guidance members of Figures 11A, 11E, and 11F,
it is a relatively short, funnel-shaped member.
[00180] The guidance member 1160 is a frustoconically shaped element having
a smaller hole 1165 at its leading end 1171 and a larger opening at its
trailing end
1172. The leading end of the member should be smaller than the pulley catheter
into which it will be inserted and the trailing end should be larger. The
guidance
member 1160 may be split, as with the guidance members of Figures 11A, 11E,
and 11F, so that the tendon and repair device may be introduced into it
laterally.
However, the illustrated embodiment is not split, such that the tendon and
repair
device must be introduced into it longitudinally.
[00181] The leading approximately third 1161 of the member 1160 is solid. The
trailing end 1162 comprises a plurality of leaves 1166 (four in this
embodiment)
extending longitudinally rearward and separated by gaps 1168. Preferably, the
member 1160 is formed by starting with a completely solid frustoconical member
and them removing material (such as by cutting) so as to form the gaps 1168.
The
gaps may be substantially of V shape with a 15 angle. The gaps 1168 assist in
allowing the trailing end 1162 of the guidance member 1160, which is larger in
diameter than the pulley system and pulley catheter 101, to crumple into a
smaller
diameter to fit through the pulley system with the tendon stump contained
within it.
42
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
The design with the leaflets and gaps substantially prevents the leaflets 1166
from
overlapping with each other when the guidance member collapses on itself for
passing through the pulleys, thus allowing the trailing end 1162 of the
guidance
member 1160 to collapse to as small a diameter as possible. The front end of
the
gaps 1168 preferably are cut so as to form arcuate edges 1164 so as to help
prevent splitting of the member 1160. Particularly, if the gaps 1168b are cut
straight so as to end at a point, deformation of the member 1160 (for passing
through the pulley system) might cause the cuts to unintentionally extend
forwardly.
An arcuate surface at the front end of the cut will help prevent this from
happening.
[00182] The taper of the guidance member may be 15 . The outer diameter of
the hole 1165 should be smaller than the inner diameter of the pulley catheter
into
which it will be inserted (or other catheter into which it will be inserted,
such as
dilator catheter 1301 discussed in connection with another alternate
embodiment
further below) and expand in diameter rearwardly so that the rear opening is
larger
than the pulley catheter. In this manner, the front of the guidance member
1160
can be inserted into the trailing end of the pulley catheter 101 and also, the
hole
1165 is smaller than the tendon stump so that the tendon stump cannot pass
through it. Preferably, the rear opening is substantially larger than the
pulley
catheter and the tendon stump because at least part of the purpose of the rear
opening of the guidance member 1160 is to make it easy to insert the repair
device
and tendon stump into it. In one embodiment, the hole 1165 is about 1 mm in
diameter and the trailing end is about 8 mm in diameter. In one embodiment,
the
widest portion of the solid portion 1161 of the guidance member is smaller
than the
smallest pulley through which the system will be passed so that the solid
portion
1161 of the guidance member does not need to crumple or collapse. Only the
leaves 1166 collapse and fold inwardly. In one embodiment for use in flexor
digitorum profundus, the largest diameter of the solid portion (i.e., at its
rear) is
about 4 mm.
[00183] The guide member may be made of a thin film material that is flexible
so
that it can crumple in on itself to pass through a pulley system and compress
the
tendon stump contained within it, yet have a tendency to return to its
original shape
upon release of force. In one embodiment, the guidance member is formed of
43
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
polyethylene terepthalate (PET) film of 0.02 mm thickness. If the guide member
is
split, the tendon can be inserted into it in a lateral direction at any time.
However, if
it is not split, as in the illustrated embodiment, then the repair device and
tendon
stump must be inserted into the guidance member 1160 before or simultaneously
with the insertion of the repair device into the pulley catheter.
[00184] The guidance member 1160 is used similarly to the flanged catheter 103
of Figures 1-2L in that it is passed through the pulley system along with the
repair
device and tendon stump. Particularly, after the pulley catheter is in place
through
the pulley system and the repair device has been attached to the tendon stump
as
previously described, the leading end 1171 of the guidance member 1160 is
inserted into the proximal end of the pulley catheter 101. Next, the repair
device is
inserted longitudinally into the rear opening of the guidance member.1160 and
advanced through the guidance member 1160 and into the pulley catheter 101
until
the end of the tendon stump is in the guidance member 1160. The surgeon can
then pull the repair device, pulley catheter, guidance member, and tendon
stump
(contained within collapsible guidance member 1160) through the pulley system
substantially as described in connection with the procedure of Figures 2A-2L.
The
guidance member 1160 will collapse around the tendon stump and compress the
tendon stump as needed to pass through the pulley system of the fingers.
[00185] The rest of the surgery can then be completed in any of the manners
discussed herein.
Tenth Set of Exemplary Embodiments
[00186] While the invention has been described above in connection with
attaching two tendon stumps and/or one tendon stump directly to bone, it
should
be understood by those of skill in the related arts that it can also be
employed in
connection with repairs that use a tendon graft. In such situations, one end
of the
tendon graft is attached to one tendon stump and the other end of the tendon
graft
is attached to either another tendon stump or directly to bone using the above-
described apparatus and techniques. The tendon graft may be taken from another
part of the patient's body, such as the patient's foot, or may be an
allograft.
[00187] In accordance with another aspect of the invention, a thin walled tube
that functions as an adhesion barrier may be placed over the tendon at the
repair
44
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
site in order to facilitate the free gliding of the tendon through the pulley
system of
the finger. More particularly, as an injured tendon, ligament, or other
longitudinal
anatomical member heals, scar tissue forms around the repair site. During the
healing process, the scar tissue can interfere with the free movement of the
tendon
through the pulley system. Additional surgery may also be needed to remove
such
scar tissue.
[00188] In order to facilitate the free movement of the tendon through the
pulley
system, the repair site(s) may be encased in an adhesion barrier in the form
of a
thin walled tube. The adhesion barrier may comprise a thin walled tube 1201
such
as illustrated in Figure 12A. Figure 12B illustrates one particular embodiment
of
the adhesion barrier being used in connection with a tendon repair in which
two
tendon stumps are being reattached without an intervening graft. As shown, the
tube 1201 may be slipped over the end of one of the severed tendon stumps
1203a prior to the repair being performed and slid out of the way during the
repair
process. Then, referring to Figure 12C, after the repair is completed, the
tube
1201 may be slid along the repaired tendon to the repair site 1204 (including
the
stitches, the tendon repair device, and both tendon stumps 1203a, 1203b).
Preferably, the tube 1201 is stitched to the tendon at this point with at
least one
stitch 1221 and, preferably, with each at least one stitch 1221 at each end of
the
tube.
[00189] The tube will provide a barrier to allow healing to take place along
the
length of the tendon (inside the tube) rather than outwardly where such scar
tissue
might interfere with the free movement of the tendon through the pulley
system.
The tube may also provide guidance for growth on the outside of the tube
diameter
to bolster the structure that will ultimately provide the passageway for the
repaired
tissue inside the tube. The external and internal surfaces of the tube should
be
lubricious and have a low friction coefficient so that it (with the tendon
inside of it)
can slide freely through the pulley system and allow the tube to be removed
after
healing has occurred.
[00190] The wall thickness of the tube should be as thin as possible so as to
add
minimal bulk to the tissues being repaired. In the case of flexor tendon
repair, wall
thicknesses of less than 0.25 mm are contemplated. However, the best wall
thickness of the tube depends upon the surgical application of the repair and
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
should proportionally thin compared to the tissue being repaired. The length
and
diameter of the tube will, of course, be dictated primarily by the particular
repair.
Furthermore, the tube should be formed of a bio-inert material, such as a
material
chosen from the family of fluoropolymers of Teflon TM, PET, PTFE, and EPTFE or
the family of silicone polymers. Preferably, the tube is porous so as to allow
fluid
exchange therethrough in order to keep the tendon healthy. It may have holes
or
other openings to facilitate such fluid transfer. Preferably, the holes are
small
enough so as not to permit tissue ingrowth therethrough. It may also be coated
with a lubricant to facilitate sliding through the pulley system (or any other
anatomical restrictions). Passive motion of the finger during the healing
period of
the tendon will also prevent any scar tissue adherence of the tendon to the
surrounding tissues through the holes in the tube.
[00191] The tube should be long enough to completely cover the repair site. In
the case of a repair utilizing a graft, depending on the length of the graft,
accessibility and other factors, a single longer tube may be used to cover
both
ends of the graft or two separate, smaller tubes may be used.
[00192] The tube will remain in place for the duration of the healing process,
from several weeks to several months. At the end of the process, it may be
removed by making one or more small incisions in the patient near one end of
the
tube and then carefully pulling the tube out of the incision as the surgeon
cuts the
tube. In alternate embodiments, the tube may be formed of a bioabsorbable
material that will simply dissolve over time, provided that the bioabsorbable
material does not promote adhesions or a local tissue response as it absorbs.
An
example of a bioabsorbable material would be a crosslinked Hyaluronic Acid or
other bioinert polymer. In yet another embodiment, the adhesion barrier may be
provided with a longitudinal slit over its entire length so that no cutting
would be
necessary when it is removed, but rather, it would simply need to be spread
apart
to be removed from the tendon. Such an embodiment would also facilitate the
option of installing the adhesion barrier over the repair site by spreading it
apart
and slipping it over the tendon after the repair is completed, thereby
eliminating the
need to slide it longitudinally over the end of a tendon stump before the
repair and
then sliding it over the repair site after the repair is completed. This may
be
advantageous where the repair site is long and/or there is insufficient
available
46
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
length of the tendon stump to slide the adhesion barrier out of the way during
the
repair procedure.
Eleventh Set of Exemplary Embodiments
[00193] Figures 13A-13C, and 14A-14C illustrate further alternate embodiments
and associated techniques to be used therewith, which techniques can be used
in
conjunction with some or all of the features and aspects of many of the other
embodiments of both the methods and apparatus disclosed herein. Figure 13A is
a perspective view of one embodiment of a unitary dilation catheter in
accordance
with this set of embodiments. Figure 13B is a perspective view of one
embodiment
of a multi-piece dilation catheter in accordance with this set of embodiments.
The
dilation catheter is designed to fit through and dilate the passage or
passages (e.g.,
pulleys) through which the longitudinal anatomical member (e.g., tendon) must
be
pulled. As will be described in detail below, it will essentially be used like
and serve
the same functions as the pulley catheter 101 of the first set of embodiments
described in connection with Figures 1 and 2A-2L above. However it also will
serve to dilate the passage. Figure 13C is a perspective view of a guide
member
that may be used in conjunction with the dilation catheter to dilate the
passage.
Particularly, as will be described in detail below, it may serve essentially
as a
guidewire for inserting the dilation catheter through the pulley system.
However, it
is believed that the guide member will be unnecessary in the majority of
applications.
[00194] The dilation catheter 1301 comprises an elongated tube having a lumen
therein. The tube comprises a series of consecutive stepped diameter
longitudinal
segments 1302, 1303, 1304, 1305, each consecutive segment larger than the
previous. The use of four steps in the illustrated embodiment is merely
exemplary.
Any number of steps is possible. The outer diameters and number of different
diameter segments should be determined as a function of the size of the
anatomical passage or opening through which the dilation catheter 1301 will
pass.
We will continue to use the example of a severed tendon in the hand in the
following discussion. The smallest diameter segment should be smaller than the
diameter of the pulley system of the smallest hand size reasonable through
which it
must pass so that the smallest diameter segment can pass through any pulley
47
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
system relatively easily. Each larger diameter segment should be designed to
gently and in a gradual, stepped manner dilate the pulley system to a larger
size in
preparation for passing the tendon stump therethrough. The last, largest
diameter
segment of the dilation catheter should be at least as large as the largest
diameter
to which one would reasonably dilate the pulley system of the largest
reasonable
hand size.
[00195] As will be seen from the discussion below, according to one exemplary
technique, any segment having a diameter that is larger than needs to be
passed
through the pulley system of the particular patient simply will not be passed
through
the pulley. Therefore, the largest diameter segment of the dilation catheter
can be
virtually any diameter. In one embodiment adapted for use in passing tendons
through the pulleys of the fingers, the various segments of the dilation
catheter
range from a smallest diameter of about 10 French to a largest diameter of
about
18 French. In one embodiment, this is accomplished with nine segments of 10F,
11F, 12F, 13F, 14F, 15F, 16F, 17F, and 18F diameters. Each segment may be
about 10 cm in length.
[00196] In one embodiment such as illustrated in Figure 13A, the dilation
catheter 1301 can be unitary. If, prior to or during the surgical procedure,
the
surgeon determines that any of the smaller diameter segments are clearly
smaller
than will be needed and/or any of the larger diameter segments are larger than
will
be needed, the surgeon may simply cut them off prior to use or during the
procedure. Hence a single dilation catheter can be offered that can be used in
a
large number of different anatomical passages and with a large number of
different
sized patients, thus reducing the number of different versions of the dilation
catheter that need to be manufactured.
[00197] In another embodiment of the dilation catheter 1310 such as
illustrated
in Figure 13B, each diameter segment 1311, 1312, 1314, et seq. may be
separable
from each other. For instance, in one simple embodiment, each catheter segment
may have a neck portion 1315 near its proximal longitudinal end sized to mate
in
an interference fit with the distal end of the next smaller diameter segment.
Preferably, the necked down portion is sized to fit within the distal end of
the next
smaller segment so that the edges of the longitudinal ends of the various
segments
will not be exposed on the outside of the dilation catheter 1310.
48
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00198] Like the pulley catheter 101, the dilation catheter preferably is
formed of
a biocompatible, low friction material having a wall thickness sufficient to
make the
entire catheter sufficiently stiff to be pushed through the pulley system and
to serve
the purpose of dilating (holding open) the pulleys against their natural size,
yet soft
and resilient enough to track through curves in the anatomical passage through
which it must pass. It might, for instance, have the approximate flexibility
of a
typical surgical vascular catheter. The inner diameter of all of the segments
should
be large enough to easily accommodate the tendon repair device that will be
used
with the dilation catheter.
[00199] In yet other embodiments, the dilation catheter need not have discrete
segments of different diameter, but may be continuously tapered over its
entire
length. As in the segmented embodiments, any portion or portions of the
catheter
clearly not necessary for the surgery may be cut off before insertion and any
portion not necessary after insertion may be curt off after the dilation
catheter is in
place in the anatomical passage.
[00200] Figure 13C illustrates an optional guide member 1320. As shown, the
guide member comprises an elongate member having an outer diameter smaller
that the inner diameter of lumen in the smallest diameter segment of the
dilation
catheter so that the dilation catheter may pass over the guide member easily.
In
other words, the entirety of the lumen of the dilation catheter is of
sufficient size
and shape to accept the guide member 1320 therethrough. The guide member
1320 may be cannulated. Alternately, it may be solid (e.g., essentially a
guidewire). The guide member should be relatively stiff so that it can be
pushed
through the pulley system without kinking, yet sufficiently flexible to track
through
curves in the anatomical passages through which it will be passed in
accordance
with the techniques disclosed herein. The outer diameter of the guide member
should be substantially smaller than the anatomical passage through which it
must
pass.
[00201] In most practical embodiments, the guide member and dilation catheter
will both be cylindrical. However, a cylindrical cross-section is not
necessary, and,
depending on the particular anatomical passage through which the guide member
and dilation catheter will be passed, other shaped cross-sections may be
49
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
preferable. The term diameter is used in this application in a non-limiting
manner
and not to imply that the cross-section necessarily is cylindrical.
[00202] Preferably, each of the segments of different diameter of the dilation
catheter is long enough to individually traverse the entire length of the
anatomical
passage through which it will be passed and stick out sufficiently at each end
thereof to provide easy access thereto to the surgeon. Particularly, as will
be
discussed in detail further below, after the dilation catheter is placed
through the
relevant anatomical passage, all segments other than the largest segment that
fit
through the passage can be cut off or removed. For a human hand, 10 cm should
be sufficient.
[00203] The dilation catheter (and optional guide member) is used to dilate
the
pulley system so as to best assure that the tendon stump will be able to pass
through the pulley system without binding. Both the guide member and the
dilation
catheter are hollow tubes formed of a biocompatible polymer of such
composition
and/or wall thickness so that it is bendable, but sufficiently rigid to be
pushed
through a pulley system. The relative rigidity of the dilation catheter and
guide
member will permit it to be pushed through narrow anatomical passages, such as
the pulleys of the fingers. However, its flexibility will permit some bending
to
accommodate an overall curved path. Preferably, the dilation catheter is
formed of
a material having a low friction coefficient to allow the dilation catheter to
readily
pass through and around bodily tissues such as the tendon pulley system.
Suitable biocompatible polymers include homopolymers, copolymers and blends of
silicone, polyurethane, polyethylene, polypropylene, polyamide, polyaryl,
flouropolymer, or any other biocompatible polymer system that meets the
mechanical characteristics above (PELLETHANETm a DOW thermoplastic
polyurethane elastomers (TPU), which is commonly use in other dilating
catheters
is targeted for this device.)
[00204] The required low coefficient of friction of the surfaces of the
dilation
catheter may be inherent to the materials used to construct the device or may
be
enhanced through a surface preparation such as a lubricious coating or
mechanical
modification of the surface such as longitudinal recesses.
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00205] The particular length, material, wall thickness, inner diameter, outer
diameter, and stiffness of the dilation catheter will vary depending on the
particular
tendon or ligament with which is it to be used.
[00206] The inner diameter should be large enough to easily accommodate the
cable portion and straight needle of the tendon repair device. The particular
material and cross sectional geometry (e.g., wall thickness) of the dilation
catheter
will largely dictate the stiffness of the catheter and, as noted above, should
be
selected to provide enough rigidity to allow it to be pushed through a narrow
path,
but flexible enough to bend to accommodate bends in the path. In the exemplary
case of the flexor digitorum profundus at the level of the middle phalanx, the
pulley
catheter may be formed of silicone and be 120 millimeters in length with a
wall
thickness of 0.5 mm, and an outer diameter of 2 mm. A biocompatible elastomer
having a durometer of 50-90 (Shore A) may be used for the dilation catheter.
[00207] Similarly to the pulley catheter 101 of Figure 1, the dilation
catheter,
with or without the guide member, can be used in connection with a tendon or
other
repair using virtually any of the tendon repair devices and related
accoutrement
described herein and in conjunction with virtually any of the surgical
techniques
described herein.
[00208] Figures 14A-14G illustrate various stages in an exemplary surgical
procedure to reattach a severed tendon. If the tendon stump has retracted and
must be retrieved from a first incision into a second incision (or the wound),
as is
typical of tendon lacerations in the hand, first, an incision 1361 is made,
typically in
the palm of the hand, as illustrated, where the proximal tendon stump 1370 can
be
retrieved. If, on the other hand, the proximal tendon stump is distal to the
A2
pulley, then the tendon would be exposed through an incision just distal to
the A2
pulley. Referring first to Figure 14A, if a guide member is used, the guide
member
1320 is passed into the wound or incision 1360 at the laceration site and
slowly
pushed proximally toward the other incision 1361 beneath the A3 pulley through
the pulley system of the finger. If resistance is encountered such that the
pulley
catheter cannot be pushed through proximally, then a% cm to 1 cm incision (not
shown) may be made midway between the skin creases of the proximal
interphalangeal joint of the finger and the crease at the base of the finger.
This is
at a level between the A2 pulley and the A3 pulley of the finger. The
dissection is
51
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
carried down gently to the flexor sheath where the pulley will be found. The
dilation
catheter 1301 can then be pulled past the obstruction or resistance through
this
incision. The guide member 1320, if used, should be long enough to pass
entirely
through the pulley system and stick out at both ends. If the guide member is
substantially longer than the desired length, it may be cut to a suitable
length either
before it is inserted or after.
[00209] With reference to Figure 14B, once the guide member 1320 is in place,
the dilation catheter 1301 (or 1310) is slipped through the pulley system over
the
guide member 1320 working from distal to proximal. Particularly, the smallest
diameter portion 1302 is slipped over the guide member 1320 and pushed over
the
guide member through the pulley system until it exits the other incision. In
embodiments omitting the guide member 1320, the smallest diameter segment of
the dilation catheter 1301 is simply inserted through the pulley system just
as
described above for the guide member.
[00210] In either event, the smallest diameter segment of the dilation
catheter is
slid back-and-forth about 10 mm to enlarge the annular rings. Then the next
larger
catheter segment is pulled through and slid similarly. This continues for each
longitudinal segment of the dilation catheter until the surgeon determines
that the
annular rings in the pulley system are enlarged enough to accept passage of
the
tendon stump. Generally, this will be at about the 14, 16, or 18 French
diameters
for most hands. This largest fitting catheter size is centered between the two
surgical wounds 1360, 1361. In this example, segment 1304 is the largest
segment passed through the pulley system.
[00211] With reference to Figure 14C, once the dilation catheter 1301 is in
place, the guide member, if used, 1320 may be removed.
[00212] With reference to Figure 14D, at this point, all of the segments of
the
dilation catheter other than the one traversing the pulley system can be
removed.
As previously mentioned, if the dilation catheter is unitary, then the other
diameter
segments of the dilation catheter can be cut off. On the other hand, if the
dilation
catheter comprises multiple separable segments, then the other segments can
simply be pulled off. In addition, the surgeon also may cut off part of the
remaining
segment if it is longer than needed.
52
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00213] At this point, the surgical procedure to reattach the tendon stump can
be
performed essentially as described in accordance with any of the embodiments
discussed previously in this specification, with the tendon repair device and
tendon
stumps being passed through the dilation catheter rather than the pulley
catheter.
110dG/7 1 A/K/1
53
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00216] Whichever type of suture is used, it may be desirable to lodge at
least
the tips of the needles on the ends of the suture in a small diameter rod that
is
smaller than the inner diameter of the dilation catheter before passing them
through the dilation catheter 1301. This will help prevent the needles from
sticking
into the side of the lumen of the dilation catheter 1301 and getting stuck. In
one
embodiment, the rod may be a small, double lumen tube, and each needle 1351,
1352 may be inserted into one of the lumens. The lumens may be sized so that
the needles 1351, 1352 fit within the respective lumens in a friction fit.
Alternately,
the rod may be solid (i.e., not a hollow tube with a lumen) and made of a
material
soft enough to be punctured by the needles so that the needles could be pushed
into the end of the rod, like a pin cushion. Figure 14E illustrates yet
another
embodiment, in which a tube 1368 has a single lumen sized to accept both
needles
1351, 1352 together in a friction fit. The tube 1368 need be only long enough
to
accept the tips of the needles and provide a sufficient length over the
needles to
form a reasonable friction fit so that the tube does not fall of the needles.
[00217] In other embodiments, if one of the needles is a curved needle, the
needle can be cut off after stitching and the bare suture end can be inserted
into
the tube 1368 along with the needle at the other end of the suture. In yet
even
further embodiments, only one end of the suture may be passed through the
dilation catheter 1301. Thus, the other end of the suture may have a curved
needle that is simply cut off after stitching or no needle at all.
[00218] In any event, Figure 14F illustrates yet another possible
embodiment. In
this embodiment, the tube 1368 of Figure 14E is replaced with a much longer
tube
or rod 1380. Tube or rod 1380 is long enough to be passed through the dilation
catheter in the distal to proximal direction and extend from both ends of the
dilation
catheter. After the suture 1350 (or other tendon repair device) has been
stitched to
the tendon stub 1370, the needle(s) 1351, 1352 can be inserted into the
proximally
facing end 1380a of the tube 1380 and the surgeon can grasp the distally
facing
end 1380b of the tube or rod that is protruding from the distal end of the
dilation
catheter 1301 and pull the suture(s)/tendon repair device 1350 through the
dilation
catheter, rather than pushing it through. This embodiment is advantageous in
that
it allows other types of suture(s), such as nylon sutures, that may not have
sufficient stiffness to be pushed through the dilation catheter, to be used in
the
54
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
repair. Alternately, a short tube, rod, block or anything to which the needles
can be
temporarily affixed (e.g., by sticking, adhesive, tape etc.) may be attached
to the
end of any longitudinal member (e.g., another suture, a narrow surgical
instrument)
that is thin enough to fit within the dilation catheter in order to pull the
sutures
through the dilation catheter.
[00219] In any event, after the tendon repair device/suture 1350 is through
the
dilation catheter and extending from its distal end 1301a, if the stitched end
of the
tendon stump 1370 is sufficiently small to pass into the dilation catheter
itself, it can
be pulled just into the proximal end 1301b of the dilation catheter 1301 and
then
the dilation catheter 1301, tendon repair device/suture(s) 1350, and tendon
stump
1370 can be pulled through the pulley system as a unit as previously described
in
connection with the pulley catheter 101 of Figure 1.
[00220] However, with reference now to Figure 14G, most likely the tendon
stump 1370, because of its deformation and excess bulk due to the stitching,
will
not readily fit within the dilation catheter 1301. In such cases, the leading
end of
the tendon repair device 1350 is pushed or pulled through the dilation
catheter
1301 to a point where the end of the tendon stump 1370 is close to, but not
touching the trailing end 1301b of the dilation catheter 1301, as seen in
Figure
14G.
[00221] Next, the dilation catheter 1301, tendon repair device 1350, and
tendon
stump 1370 are pulled as a unit through the pulley system to a point where the
trailing end 1301b of the dilation catheter 1301 has passes the entrance of
the first
pulley 1321 that must be traversed with the end tendon stump 1370 is near the
entrance to the pulley 1321, as shown in Figure 14G. This may require the
making
of an additional incision 1333 adjacent an end of the pulley if the existing
incisions
are not already adjacent the pulley entrance. In fact, as will become clear,
such an
additional incision may be necessary for each separate pulley that must be
traversed. A funnel, such as 1140 of Figure 11E, is slipped over the tendon
stump
1370 with the small end 1143 of the funnel positioned slightly inside of the
entrance
to the pulley 1121 and the large end 1144 facing away from the entrance to the
pulley.
[00222] With the funnel 1140 in the position shown in Figure 14G, the surgeon
can then pull on the leading end of the tendon repair device 1350 and dilation
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
catheter 1301 to draw the end of the tendon stump 1370 into and through the
funnel 1140 and the pulley 1321.
[00223] The funnel 1140 contains the end of the tendon stump 1370 gradually to
facilitate insertion into and passage through the pulley 1321. The tendon
stump
1370 slides through the funnel 1140 and through the pulley 1321. Once the end
of
the tendon stump 1370 has passed through the pulley 1321, the funnel 1140 is
removed, as seen in Figure 14G.
[00224] The dilation catheter 1301 may be provided with mm markers on its
surface to assist in determining exactly where a hidden blockage is positioned
(and
a new incision must be made) when pulling the tendon through the pulley system
with the dilation catheter. Particularly, the specific mm mark at the skin in
the
incision is noted prior to pulling the tendon through the finger. If a
resistance is
encountered, then the mm marking at the same location of the skin is noted.
The
exact site of the blockage is calculated by determining the difference between
the
two observed markings and measuring the equivalent distance on the skin
surface
of the patient.
[00225] If the tendon stump 1370 must be guided through a second or
subsequent pulley, the same process using the funnel 1140 is repeated with
respect to the second or subsequent pulley.
[00226] If there is a distal tendon stump that has retracted and must be
passed
through a different portion of the pulley system in the opposite direction,
then that
can be done using the techniques and apparatus just described, but working in
the
opposite direction.
[00227] The tendon stump 1370 can then be (1) attached to the other, mating
tendon stump directly, (2) attached to another tendon repair device attached
to the
other, mating tendon stump, or (3) be attached to a bone anchor, as the case
may
be, using any one of the aforedescribed techniques. Particularly, the two
tendon
stumps are brought together in an abutting condition and the needle(s) and
suture(s) extending from the proximal tendon stump are stitched to the distal
tendon stump. A tendon holder may be used to help bring or hold the tendon
stumps together by adjusting the positions of the needles of the tendon holder
toward the center so that they are very close to each other and piercing each
tendon stump with one of the needle pairs. The needle(s) and suture(s), if
any,
56
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
previously attached to and extending from the distal tendon stump can also be
stitched to the proximal tendon stump to double the strength of the repair.
Again, a
modified cruciate stitch may be used.
[00228] Figure 15 illustrates the aforementioned modified cruciate repair
stitch
as used in the exemplary repair procedure of Figures 14A-14G. The numbers 1-14
in Figure 15 provided alongside some of the linear segments of the sutures and
near the knots help indicate the chronological order of the stitching steps.
The
dashed lines indicate that the suture is within the substance of the tendon
and the
solid lines indicate that the suture is on the surface of the tendon.
[00229] Chronologically, (1) the first suture 1350 is stitched to the
proximal
tendon stump 1370 using a modified cruciate stitch as shown (steps 1-3), (2) a
second suture 1380 is stitched to the distal tendon stump 1390 also using a
modified cruciate stitch as shown (steps 4-6), (3) after the two tendon stumps
are
brought together (a tendon holder may be used to help bring or hold the tendon
stumps together by adjusting the positions of the needles of the tendon holder
toward the center so that they are very close to each other and piercing each
tendon stump with one of the needles or needle sets), the first suture is then
stitched to the distal tendon stump using another modified cruciate stitch
(steps 7-
9), (4) the two ends of the first suture are tied together with a knot (steps
10), (5)
the second suture is stitched to the proximal tendon stump with another
modified
cruciate stitch (steps 11-13), and (6) the two ends of the second suture are
tied
together with a knot (steps 14). Finally, although not shown in Figure 15 in
order
not to obfuscate the illustration of the modified cruciate stitches, one or
more
epitendonous stitches (using 6-0 EthibondTM) may be applied circumferentially
at
the repair junction.
[00230] Figure 16 is a perspective view of another embodiment of a tendon
holder. In this embodiment, the tendon holder 807 still comprises a handle
801,
and a cross bar 803 at the distal end of the handle 801. In this embodiment,
the
cross bar holds a turnbuckle 812 (essentially a screw with oppositely directed
threads on each half 812a, 812b of its length) between two rotatable mounting
points 813, 814 on arms 816a, 816b. A knob is attached to at least one end of
the
turnbuckle to permit the surgeon to rotate the turnbuckle. A needle holder
block
815 is threadedly mounted on each half 812a, 812b. Thus, when the turnbuckle
57
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
812 is rotated in one direction, the two needle holder blocks 815 approximate
each
other (i.e., they move medially toward each other on the turnbuckle 812. When
the
turnbuckle 812 is rotated in the other direction, the two needle holder blocks
815
move laterally away from each other on the cross bar 803. An unthreaded larger
diameter cylindrical portion 821 of the turnbuckle 812 exactly in the middle
of the
turnbuckle may be provided to prevent the two needle holder blocks 815 from
hitting each other. A support block 822 may hold the unthreaded cylindrical
portion
821 rotatably therein to provide support for the turnbuckle 812 intermediate
its two
ends.
[00231] Each needle block can hold a number of different needles in different
configurations. Particularly, each needle block 815 includes a transverse
threaded
hole 825 for accepting a needle holder 823. The needle holder 823 comprises a
screw shank 826 with mating threads to the transverse threaded holes and a
head
827 at its proximal end for manually rotating the screw 826 into the
transverse hole
825 of the needle block 815. One or more needles 828 extend from the distal
end
of the screw 826 for holding tendons. Different needle holders with different
numbers and configurations of needles can be provided for addressing different
surgical conditions.
[00232] Each needle block 815 further comprises one or more additional holes
818 through which needles or K-wires may be inserted. The various holes 818
may be oriented at different angles in order to provide a plurality of choices
as to
the angle(s) at which the needle(s) or K-wire(s) extend from the block.
Particularly,
when the apparatus and techniques of the present invention are used to
reattach a
tendon or ligament that has avulsed from the bone, rather than been lacerated,
one
of the blocks can be used to attach the tendon holder to the bone, rather than
one
of the tendon stumps. Then, the tendon holder can be to approximate the tendon
stump to the bone. For instance, one or more a K-wire may be passed through
one or more of holes 818 of one of the blocks 816 and stuck into the bone to
which
the avulsed tendon stump is to be reattached (such as by any of the techniques
described above in connection with Figures 4A-4D). The needle(s) of the other
block 816 are stuck into the tendon stump and the turnbuckle is turned to
approximate the tendon stump to the bone.
58
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00233] In use, the turnbuckle can be turned to position the needles with a
desired spacing relative to each other before piercing the tendon stump(s)
with the
needles. Alternately, two tendon stumps that are to be rejoined can be pierced
with one of the needles (or plurality of needles) and then the turnbuckle can
be
turned to draw the needle blocks medially toward each other to bring the
stumps
into abutting contact.
[00234] A stabilizer bar 831 may be provided for use with the tendon holder,
into
which the tips of the needles 828 can be stuck both before and during surgery.
The stabilizer bar 831 may be a cylinder formed of a relatively soft cylinder
of
material 832 that the needles can penetrate relatively easily that is
partially
wrapped in a second annulus of harder material 833 with a gap 834 through
which
the soft inner material 832 is accessible for sticking the needles into it..
The harder
outer material 833 is much more difficult to penetrate with the needles, and
thus
will prevent the needles from poking all the way through the stabilizer bar
831 and
becoming exposed again. Alternately, the stabilizer bar may be formed
unitarily of
materials with two different hardnesses, such as by a dual extrusion process.
[00235] Both before and during surgery, the stabilizer bar 831 can serve
several
functions. First, it protects the needle tips, preventing the surgical
personnel from
inadvertently sticking themselves or anything else with the needles. Second,
it
braces the needles, creating a rectangular structure that helps prevent the
needles
from inadvertently being bent out of shape. Finally, during surgery, it can
prevent
the tendon stumps from becoming inadvertently disengaged from the needles.
Twelfth Set of Exemplary Embodiments
[00236] Figure 17A illustrates another particular embodiment of a repair
device
1400 comprising a single suture 1701 with a curved needle 1702, 1703 on each
end. The repair device may be stitched to a tendon stump using one or both
needles. The suture 1701 may be a stainless steel suture as previously
mentioned
with sufficient stiffness that if can be pushed into and/or through the
catheter, such
as any of catheters 1301, 1310 or 103, substantially as previously described
in
connection with other embodiments. As also mentioned in connection with the
previously described embodiments, curved needles often are preferable to
straight
needles because it can be easier to stitch with them. Also, as with the
previously
59
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
described embodiments, straight needles have the advantage of more easily
passing into and through the catheter. Although curved needles may be used at
both ends of the repair device in any of the embodiments, the present
embodiment
with one suture is particularly suitable for curved needles at both ends.
Specifically, assuming one of the procedures in which the suture stitched to
one of
the tendon stumps is not to be stitched to the other tendon stump, then curved
needles may be placed at both ends of the repair device 1400. This is because
the
needles will no longer be needed after stitching to the one tendon stump and,
thus,
can be cut off after the repair device is stitched to the first tendon stump
and before
it is passed into and through the catheter 1301, 1310, 103.
[00237] This repair device can be used in a procedure largely in any of the
manners previously described in connection with other embodiments discussed
hereinabove. For instance, two such devices 1700 can be attached to opposing
tendon stumps and then connected to each other using a crimp connector such as
connector 112 with the two stumps in abutment as previously described.
[00238] In one exemplary procedure, the repair device is stitched to the
tendon
such that both ends of the repair device extend out of the front of the tendon
stump
and both of the free ends are passed through the catheter simultaneously.
Thus,
four suture strands will pass through the connector 112 (i.e., the two ends of
the
suture of the first repair device in one direction and the two ends of the
second
repair device in the other direction. The connector 112 may have two bores,
such
as bores 151, 152 in exemplary connector 112 shown in Figure 1 with each bore
big enough to accept two suture strands therethrough. Alternately, the
connector
may have a single large bore for accepting all four suture strands
therethrough
simultaneously. In yet other embodiments, the connector may have four bores,
one for each suture.
[00239] Figure 17B illustrates yet another embodiment of a repair device 1710
comprising two sutures 1711, 1712 and two curved needles 1715, 1716 (one at
each end of the device 1710). More particularly, the first ends of both
sutures are
attached to one needle 1715 and the other ends of both sutures are attached to
another needle 1716. This embodiment is quite similar to the embodiment of
Figure 17A, except that it has greater repair strength because it has two
sutures
instead of one, but maintains similar flexibility. In this manner, eight
separate
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
suture strands connect the two tendon stumps to each other and run through the
crimp connector, providing a very strong repair. As previously mentioned, the
crimp connector may have one large bore for accepting all eight strands.
[00240] In one embodiment, the entire lengths of the sutures may be encased
within a sheath. However, in the illustrated embodiment, two short sheaths
1717,
1718 in the form of shrink wrap tubing, for instance, encase the two sutures
1711,
1712 only near their opposite ends adjacent the needles 1715, 1716. Most of
the
lengths of the sutures 1711, 1712 intermediate the opposing ends of the
sutures
are left unsheathed. The sheaths 1717, 1718 serve at least two functions.
First, it
simplifies the passing of the sutures through the catheter as well as through
the
crimp connector because it holds the ends of both sutures together, thus
allowing
both sutures to be passed through the catheter and/or crimp connector at once.
Furthermore, it keeps the ends of the sutures from unraveling or fraying after
the
needles are cut off. Hence, such sheaths also may be incorporated into the
single
strand embodiment of Figure 17A.
[00241] Figures 18A-18H illustrate another stitching technique that provides
very
strong attachment of the repair device to the tendon stump and that is
particularly
useful with repair devices such as illustrated in Figures 14 and 15 having
essentially one suture (or two sutures essentially acting as one for purposes
of
stitching) in which both ends of the suture will extend from the end of the
tendon
stump. This stitch technique will prevent tear out of the suture from the
tendon
under tensile stress of the tendon.
[00242] Referring to Figure 18A, the surgeon should first expose 0.5cm of
tendon 1801 on each side of the laceration. This may require pulling the
proximal
end out of the proximal pulley. The distal tendon may be delivered by flexing
the
distal joints. Using an appropriate marking implement, such as methylene blue
or a
marking pen, the surgeon may make as many as seven marks 1802, 1803, 1804,
1805, 1806, 1807, 1808 on the tendon stump for use as visual references for
stitching. Depending on surgeon preference, some of the marks may be omitted
(e.g., 1805, 1807, because other marks, e.g., 1804, 1808, are close enough
that
they can be used as references for those marks as well. Some surgeons may
prefer to use no marks at all. Although Figure 18A shows only one stump, this
should be performed on both tendon stumps.
61
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00243] Referring now to Figure 18B, make a first pass of the suture 1810
perpendicular to the longitudinal axis of the tendon 1801 closer to the
posterios
surface than the palmar (using the two rear-most marks 1802, 1808 as
references
for the entry point and exit point) and pull the suture through the tendon
1801 so
the ends of the suture (not shown) are even with each other.
[00244] Next, referring to Figure 18C, take one end of the suture 1810 and
cross
the suture over the tendon and place the needle (not shown) through the tendon
at
approximately marked point 1804, once again exactly perpendicular to the long
axis of the tendon, and pull it through. Take the other end of the suture and
cross
over the tendon so that the needle enters the tendon at approximately mark
1808,
but preferably about 1 mm further from the cut end 1801a than the other end of
the
suture. Mark 1808 may be made about 1 mm further away from the cut end 1801a
than mark 1802. Alternately, if marks 1802 and 1808 are made even along the
length of the tendon, the surgeon may instead enter the tendon about 1mm
further
away from the cut end 1801 than mark 1808. Pull the suture ends tight.
[00245] Next, with reference to Figure 18D, pass each of the two ends of the
suture back into the tendon at point 1805 and point 1807, respectively, and
back
out of the cut end of the tendon. One should try to exit the end 1801a of the
tendon 1801 as close as possible to the middle of the cut end, i.e., near mark
1806. Also note that re-entering the tendon at points 1805, 1807, which are
slightly
closer to the cut end 1801a than the exit points 1804, 1806, will help prevent
locking the stitch. The needles may be cut off of the ends of the sutures at
this
point, especially if the repair devices have curved needles that cannot be
passed
through the crimp connector. Otherwise, the needles may be cut off any time
after
the sutures are passed through the crimp.
[00246] Next, all of the steps described above should be repeated on the other
tendon stump 1821 with the suture 1820 of another repair device.
[00247] Next, with reference to Figure 18E, pass both ends of one of the
sutures
1810, 1820 through a crimp connector 1812 in one direction. Then, preferably
while holding those suture strands out of the way by, for instance, biasing
them
outwardly to increase space in the passage of the crimp connector, pass both
ends
of the other suture through the crimp connector 1812 in the opposite
direction.
62
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
[00248] Referring now to Figure 18F, place and tie the first epitendonous
stitch
1825 at the lateral edge of the repair at a point away from the surgeon. This
epitendonous stitch will control ideal rotation. It is advisable to leave the
epitendonous stitch long for later use in completing the stitch all the way
around the
tendon.
[00249] Next, pull the strands of sutures 1810 and 1820 in opposite directions
being sure to tension each individual suture strand in each direction as
equally as
possible to maximize the strength of the repair while rotating the tendon as
shown
in Figure 18G. This rotational orientation will allow a crimping tool 1830 to
come in
from the lateral angle rather from the anteroposterior angle in order to
flatten the
crimp. Any extra tendon edges may be trimmed at this time. Crimp with the
crimping tool 1830 for 10 seconds to assure proper deformation of the crimp
1812.
[00250] Finally, with reference to Figure 18H, complete the epitendonous
stitch
1825 across the palmar surface of the repair to bury the crimp 1812 within the
substance of the tendon. The surgeon may use either continuous stitching or a
few interrupted stitches on each side of the crimp and at the mid axis of the
tendon.
[00251] Epitendonous stitching of the posterior wall is optional and depends
on
the appearance. Epitendonous stitches in the posterior wall may interfere with
blood supply to the repair site and, if perfectly together, testing shows no
increase
in strength.
[00252] After the repair is completed as described above, the surgeon should
take the tendon through five or six excursions to ensure good motion through
the
pulleys. A small transverse incision proximal of the original incision may be
needed
to view the pulley.
Thirteenth Set of Exemplary Embodiments
[00253] As previously mentioned, one or more repair devices as disclosed
herein may be used to fuse bones together, including the repair of broken
bones or
fusing of bones for other purposes, such as (a) Scapholunate Ligament Repair,
including with a widened scapholunate gap, with a vertical scaphoid, and with
either static or dynamic instability (SLR), (b) other joints in the wrist, (c)
metacarpophalangeal (MP) joints, (d) thumbs and fingers, (e) volar plate, (f)
63
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
adjunct to fusions, (g) PIP and DIP distal and proximal interphalangeal)
joints, and
(h) reduction of fractures in the wrist and hand
[00254] Figure 19 shows a bone repair in the hand employing two of the bone
anchors such as bone anchors 400 and/or 450 shown in Figures 1 and 4B-4D and
one crimp connector such as crimp connector 112. Each bone anchor has one
suture 1903, 1904, respectively, extending therefrom. Specifically, one bone
anchor, e.g., anchor 400, may be implanted into each bone 1901, 1902 (or bone
fragment) and the suture or sutures 1905 extending from each bone anchor are
run
through and crimped into a connector 112 substantially as described above in
connection with Figures 2J-2L or 18E-18G. More specifically, after the
suture(s)
extending from the two bone anchors 400 are run through the connector 112 in
opposite directions, traction is applied in opposite directions until the
bones contact
each other. Then, the connector 112 may be crimped to permanently hold the two
bones in contact.
[00255] If the bones being joined together are anatomically restrained so that
they are not likely to fold relative to each other under the force of the
repair
apparatus, then a pair of joined bone anchors 400 may be placed on only one
side
of the bones 1901, 1902, such as illustrated in the SLR of Figure 19. However,
if
the bones might fold relative to each other (or move in any degree of freedom
relative to each other) when restrained by only one pair of bone anchors 400
joined
by a connector 112 on only one side of the bone, then one or more additional
pairs
of bone anchors joined by a connector 112 may be used to join the bones at
additional locations. For instance, in Figure 19, the scaphoid and lunate
bones are
joined by a two bone anchors implanted into the bones form the top (or volar)
side
of the hand joined by a single connector with the sutures running along the
top of
the bone. In order to assure that the force on the sutures does not cause the
two
bones to fold relative to each other, another pair of bone anchors could be
implanted into the two bones from the bottom (or dorsal) side so that the
sutures
running between those two bone anchors run along the dorsal side of the bones
and counterbalance the force of the sutures running along the volar side of
the
bones.
[00256] Referring to Figure 20, for repairs of fractured tubular bones, such
as
the ulna or the fibula, three pairs of bone anchors (the sutures 2002 of each
pair
64
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
joined by a connector 112) are shown disposed at 1200 intervals rotationally
around the longitudinal axis of the bone 2000 to effect a repair. Other
numbers of
pairs of bone anchors are possible also.
[00257] In yet other embodiments, such as illustrated in Figure 20, three or
more
bone anchors 400 can be joined by one or more sutures 2001 to effect a repair.
Each bone anchor may be implanted in a separate bone (or bone fragment) 2002,
2003, 2004, as shown. Further, a single suture 2005 may be run through the
eyelets of the three bone anchors 400 and the two ends of the single suture
run
through a connector 112 in opposite directions, also as shown. However, in
other
procedures, two or more of the bone anchors 400 may be implanted into a single
bone fragment and joined to a third or further bone anchor(s) in one or more
other
bones (or bone fragments). Virtually any combination of bone anchors,
connectors, and sutures may be employed to effect a repair. For example,
different sutures may be used to join different subsets of the bone anchors to
each
other, while different connectors 112.
[00258] Figure 22 illustrates an exemplary repair of a bone 2201 fractured in
two
pieces 2201a, 2201b at fracture 2202 using the principles of the present
invention.
In this example, one anchor 400 may be implanted in one bone fragment 2201a
and a single suture 2203 may be wrapped around the bone 2201 as shown and its
opposite ends 2203a, 2203b run through a connector 112 in opposite directions.
[00259] The repair illustrated in Figure 22 may be made in yet other ways such
as with two anchors 400, one crimp 112 and one suture 2203, as illustrated in
Figure 23. The second anchor 400 better controls the path of the suture 2203
in
that the suture passes through the eyelet of the second anchor and assures
that
the suture 2203 will not slide away from at least the position of the second
bone
anchor over time.
[00260] In accordance with another embodiment, as illustrated in Figure 24, a
similar repair can be made in which the connector 112 is eliminated entirely.
Particularly, a bone anchor 400 is implanted in one of the bone segments 2201a
and one suture 2203 is wrapped around the bone 2201 and its opposite ends
2203a, 2203b are passed back through the eyelet of the bone anchor 400. The
eyelet pin is deployed into the locked position to lock the two ends 2203a,
2203b of
the suture 2203 in the eyelet. Yet further, a second anchor 400 (illustrated
in
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
phantom in Figure 24) may be implanted in the second bone segment 2201b and
the suture 2203 may be passed through the eyelet of that anchor in order to
better
control the path of the suture 2203 and assure that the suture will not slip
out of
position over time.
[00261] Certain repairs, such as the scapholunate repair mentioned hereinabove
can be difficult to perform, particularly from the volar side, due to the
obstruction of
the field of vision of the bones by surrounding tissue. More particularly, in
repairs
where there is obstruction of the field of vision, it can be particularly
difficult to
insert the bone anchors into the pre-drilled bores in the bones. In
scapholunate
ligament repair, the scaphoid bone and the lunate bone are joined together
(even
though this is not the natural condition of these two bones). Using the
technique of
the present invention, it is advisable to implant and interconnect anchors on
both
the dorsal side and the lunate side of the bones in order to prevent folding
of the
bones relative to each other as previously mentioned.
[00262] Figures 25, 26, 27, and 28A-28L illustrate a procedure and related
apparatus for overcoming such difficulty. The invention will be described in
connection with a typical scapholunate fusion. However, it should be
understood
that this is merely exemplary and that the technique and apparatus is
applicable to
many other bone fusions and other repairs.
[00263] Briefly, a drill guide 2501 shown in Figure 25 is provided for
drilling two
(or more) bores for receiving the bone anchors in precise relationship to each
other
and to the bone or bones. Using the drill guide, two or more bores are drilled
into
and completely through the bone or bones so that each bore Is open on each end
and can receive either one or two anchors, namely, one in one end of the bore,
e.g., one anchor on the dorsal side of the bone, one anchor on the volar side
of the
bone, or one on each side. By drilling completely through the bone, each bore
can
be used for one bone anchor on the volar side and another anchor on the dorsal
side, thus cutting in half the number of bores that need to be drilled in the
bone in
some cases. A pin 2525 of sufficient length, shown in Figure 26, is inserted
into
each bore and completely through the bone so as to stand proud of the bone
from
both ends of the bore sufficiently beyond the obscuring tissue to be easily
visualized. The pins are sized on diameter to fit snuggly but slidably within
the
bores (e.g., they have the same nominal diameter as the drill bit). The pins
include
66
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
posts 2526 extending longitudinally from at least one of their ends having a
smaller
diameter than the diameter of the majority of the length of the pin.
[00264] The bone anchors 2505, shown mounted on the end of an insertion tool
2507 in Figure 27, have mating recesses 2515 at their distal ends for engaging
the
posts 2526. As can be seen, in addition to the distal recess 2525 for
receiving the
post on the pin, there are two parallel bores 2513, 2514 in the bone anchor
for
retaining the suture 2517 that will be used to interconnect the bone anchors
as
previously described.
[00265] Once the pins 2525 are in place, the surgeon can readily see the ends
of the pins (with the posts 2526 extending therefrom) standing proud of the
bone
as well as any other tissue. The surgeon then docks the bone anchor 2505 onto
the pin by inserting the distal recess 2515 of the anchor 2505 over the post
2526.
The surgeon can then push down on the insertion tool 2507 to force the anchor
2505 and pin 2525 downwardly through the bore until the tip of the bone anchor
is
in the bore, the pin having guided the bone anchor directly into the bore.
Then the
bone anchor 2505 is screwed into the bone using the insertion tool 2507. The
pin
2525 can then be pulled out of the bone from the other side of the bone.
[00266] The present invention is particularly well suited to the scapholunate
repair because (1) anchors usually will need to be implanted on both the volar
and
dorsal sides of the bones, (2) both sides of the bones can be made available
through opposing incisions on the dorsal and volar sides of the hand, and (3)
one
side, the dorsal side, is relatively obstruction-free compared to the other
side, the
volar side, which is very difficult to navigate due to obstructing tissue.
[00267] Figures 28A-28L illustrate the procedure. For clarity, a D in the
figure
indicates the dorsal side of the bones and a V indicates the volar side.
Further,
usually the bone anchors are pre-loaded with sutures prior to implantation.
However, in order not to obfuscate the invention, the sutures are omitted from
Figures 28A-28L.
[00268] With reference to Figure 28A, a drill guide 2501 designed specifically
for
the type of repair at hand is provided having guide holes 2502a and 2502b for
two
drill holes that must be drilled into the scaphoid 2520 and lunate 2522 at
precise
angles and positions relative to each other. For simplicity in describing the
basic
aspects of the invention, the illustrated drill guide 2501 has two parallel
drill guide
67
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
holes 2502a and 2502b. However, some scapholunate fusions may require three
of more anchors on one or both sides of the bones. Further, not all of those
bores
are necessarily parallel to each other.
[00269] In any event, in one embodiment, the bottom side 2503 of the drill
guide
2501 is shaped complementarily to the shape of the surface of the bone(s)
2520,
2522 for which it is intended to be used so that it can be placed directly on
the
bone(s) with the contours of the bottom side of the drill guide 2501 aligning
precisely with the mating complementary contours of the bone surfaces in a
semi-
locking engagement. The drill guide 2501 can, of course, simply be lifted off
of the
surface of the bone(s), but when pressed down onto the bone, it will lock with
the
bone laterally due to the complementary mating of contours, like a jigsaw
puzzle
piece. This will be possible only in connection with certain bones, as many
bones
vary too greatly from patient to patient in shape and/or size to be reasonably
subject to templating as noted above. However, the scaphoid and lunate bones
are
relatively uniform across a large segment of the population so that as few as
three
sizes of drill guides (for different sized patients) should be sufficient for
a very large
majority of the population.
[00270] In accordance with the first step of the process as illustrated in
Figure
28A, after incisions have been made on both the dorsal and volar sides of the
hand, the drill guide 2501 is placed over the bones on the dorsal side with
the
contours of the bottom side 2503 of the drill guide 2501 against the mating
contours of the dorsal side of the scaphoid and lunate bones 2520 and 2522,
respectively. An appropriate drill bit is then inserted through each of the
drill guide
holes 2502a and 2502b on the drill guide and used to drill completely into and
through the bones.
[00271] With reference to Figure 28B, after each hole 2530, 2531 is drilled, a
pin
2525 is inserted into the holes and completely through the bone so that the
pin
stands proud of the bone on both sides of the bone. The sides of the pins with
the
guide posts 2526 are inserted first, so that the ends of the pins with the
guide posts
will extend from the volar side of the bones. Figure 28C shows the condition
after
the pins 2525 have been inserted.
[00272] One or more of the pins may be inserted into the holes with the drill
guide still in place in order to simplify the navigation of the pin into the
hole(s)
68
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
which, otherwise, may be difficult to visualize due to obstructing tissue. The
drill
guide may be left in place and used as a guide for inserting the pins into the
bores
with respect to any two ore more of the drill guide holes that are parallel to
each
other. In this embodiment, the two guide holes 2502a, 2502b in the drill guide
2501 are parallel to each other, such that the both pins 2525 can be inserted
through those two guide holes and into the bores in the bone. However, in
other
drill guides, if any of the drill guide holes are not parallel to the other
drill guide
holes, then the drill guide should be removed before the pins are inserted
into non-
parallel bore holes since non-parallel pins in the drill guide will prevent
the drill
guide from being withdrawn without bending the pins.
[00273] In any event, next, as illustrated in Figure 28D, the hand is turned
over
to access the volar side. The surgeon may clear out some tissue around the pin
on
the volar side in order to (1) improve visibility, (2) prevent pinching or
catching of
tissue when the bone anchors are introduced, and (3) generally neaten up the
surgical site.
[00274] Next, referring to Figures 27 and 28E, the surgeon then prepares a
bone
anchor 2505 for screwing into one of the bores 2530. In one embodiment, the
bone anchor is delivered pre-loaded onto an implantation tool 2507 and with a
suture pre-loaded in the bone anchor. As best seen in Figure 27, the distal
end of
the bone anchor 2505 is bored out to form a recess 2515 for receiving the post
2526 on the end of the guide pin (not shown in Figure 27). The proximal end of
the
anchor 2505 includes a proximal bore 2509 for receiving a tip 2506 of the
insertion
tool 2507. The tip 2506 and the proximal bore 2509 are matingly contoured so
that
tool 2507 can be twisted in order to rotate the anchor 2505 about its
longitudinal
axis for screwing into the bone.
[00275] Since the pin 2526 is standing proud of the bone and surrounding
tissue
(not shown), the surgeon can easily see the pin 2525 and the post 2526 for
placement of the bone anchor 2505 on the post 2526. Hence, the surgeon inserts
the distal aperture 2515 of the bone anchor 2505 over and onto the post 2526
of
the pin 2525 to dock the bone anchor 2505 on the pin 2526.
[00276] Referring now to Figure 28F, at this point, the surgeon pushes down on
the insertion tool 2507 to force the pin 2525 back out of the dorsal side of
the bone
2520 as the anchor 2505 is guided by the pin into the bore 2530. The recess
2515
69
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
in the distal end of the bone anchor 2505 and the post 2526 on the end of the
pin
2525 may be matingly contoured (as are the proximal bore 2509 of the bone
anchor and the tip 2506 of the tool 2507) so that the twisting of the bone
anchor
2505 by the tool 2507 will also cause the pin 2525 to twist so that the
surgeon can
twist the tool, anchor, and pin as a unit to ease the advancement of the pin
2525
through the bore 2530. When the distal end of the bone anchor 2505 reaches the
bone 2530, the surgeon screws the bone anchor 2505 into the bore 2530 until
the
proximal end of the bone anchor is flush with the bone surface, as best seen
in
Figure 28G.
[00277] Referring to Figure 28H, the process is repeated for the other pin
2525
and bore 2531 by advancing the bone anchor 2505 and pin 2525 until the anchor
is
screwed into the bone 2532 with its top flush with the bone surface.
[00278] Referring to Figure 281 the hand is turned over again to provide
access
to the dorsal side of the bones.
[00279] Referring to Figure 28J, a first pin 2525 is pulled out of one of the
bores,
e.g., 2530, from the dorsal side. Referring to Figure 28K, another bone anchor
2505 is screwed into the dorsal side of the exposed bore 2530 using the
insertion
tool 2507 as previously described. On the dorsal side, there is much less
tissue
obstructing the view of the holes and the holes should be easily visualized
from the
dorsal side, even after the pin is removed. Nevertheless, in any event, if the
surgeon pulls out the pins and screws in the bone anchor one at a time (as
opposed to pulling out all pins before screwing in any of the anchors), the
remaining pin(s), which are easily visible can help the surgeon with
orientation and
therefore with finding the bore no longer containing a pin.
[00280] The process is repeated for the other bore 2531 so that each bore
2530,
2531, contains two bone anchors 2505, one at each end of the bore.
[00281] The bone anchors having been implanted, the procedure can then be
completed as previously described by running one or more sutures between the
various bone anchors on the dorsal side and one or more sutures between the
bone anchors on the volar side.
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
Conclusion
[00282] Preliminary testing has shown failure strengths of tendon
reattachments
performed in accordance with the principles of the present invention of
approximately 70-100 Newtons. Accordingly, a tendon and ligament repair in
accordance with the principles of the present invention results in a much
stronger
result that the current standard of care. In addition, the procedure is
greatly
simplified as compared to the present standard of care.
[00283] The present invention provides a safe, simple, easy, and strong repair
for tendons, ligaments, and the like. In preliminary tests, failure strengths
of up to
100 N have been observed.
[00284] It should be understood that the numbers of sutures/cables and needles
forming the various parts of the tendon repair devices described in
association with
the various embodiments herein are merely exemplary and that fewer or more
sutures/cables (and needles) may be provided depending on the desired strength
of the repair, the particular tissue that is being repaired, the strength of
the material
from which the tendon repair device is manufactured, and other factors.
[00285] Even though description of the utility of the various embodiments was
limited to the flexor tendons of the hand, it must be understood that many
soft
tissue repairs can be carried out by use of the device as described, either in
part of
in full. Examples of such anatomical structures include the tendons and
ligaments
of the body as well as any other structure require fixation in multiple
points,
[00286] Having thus described particular embodiments of the invention, various
alterations, modifications, and improvements will readily occur to those
skilled in
the art. Such alterations, modifications, and improvements as are made obvious
by this disclosure are intended to be part of this description though not
expressly
stated herein, and are intended to be within the spirit and scope of the
invention.
Accordingly, the foregoing description is by way of example only, and not
limiting.
The invention is limited only as defined in the following claims and
equivalents
thereto.
71
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
APPENDIX A
THE SURFACE ANATOMY OF THE PULLEY SYSTEM AND FLEXOR TENDONS
IN THE PALM AND FINGERS WITH REFERENCE TO SMALL TRANSVERSE
MINI-INCISIONS
INTRODUCTION
Verdan utilized mid-lateral incisions for flexor tendon repair and Bunnell
used a
mid-axial incision ( Verdan )(Boyes, Surgery of the Hand). In 1967, Bruner
described a
zigzag incision on the palmar surface of the finger and palm to provide easy
exposure
of the flexor tendons and pulley system (Bruner, Zig-zag volar-digital
incision 1967).
This extensile incision has gained wide popularity among hand surgeons for
flexor
tendon surgery and other procedures on the palmar surface of the finger and is
still
commonly used today. It does however require extensive dissection with skin
flaps
raised along the length of the finger.
The detailed structure and function of the pulley system of the fingers and
thumb has been well delineated.(Doy/e). When these extensile incisions are
used
there is wide exposure of the pulleys (Bruner). In order to use less extensile
incisions
and limit the amount of dissection, a detailed knowledge of the surface
anatomy of the
underlying structures is essential. We did many cadaver dissections and
measurements to delineate the tendon and pulley surface anatomy. In this paper
we
describe the relationship of the finger creases to the underlying structures
and relate
this surface anatomy to small transverse incisions that can accurately expose
specific
structures within the flexor tendon system while maintaining the integrity of
the pulley
system.
In recent years, surgeons in many disciplines have moved away from large
incisions and extensile exposure towards less invasive approaches. This has
led to
the development of the fields of arthroscopy and endoscopy, which in many
cases
have entirely eclipsed open surgery. The reduced morbidity, swelling, bleeding
and
quicker recovery has been well documented and clinical experience has borne
these benefits out. In the field of hand surgery endoscopic techniques have
been
applied to the radio-ulnar joint, carpo-metacarpal joint and carpal tunnel
release
(Vasiliadis).
We dissected 8 palms and 32 fingers in 8 cadaver hands. The palmar and
finger creases were marked with methylene blue before the dissection. A 2-
72
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
millimeter strip of each skin crease was retained in position, while the rest
of the
palmar skin, finger skin and subcutaneous tissue was removed down to the
flexor
tendon sheath. All of the pulleys were carefully protected.
Using a millimeter rule, the distance of the proximal and distal extent of
each
pulley was measured relative to the more proximal finger crease and recorded.
The anticipated position of the proximal end of the A-1 pulley was measured
according to the method of Fiorini. The proximal and distal edges of the Al,
and
the proximal end of the A2 pulley were measured from this point and recorded.
The distal end of A-2 and the proximal and distal end of the C1 pulley and the
proximal end of the A-3 pulley were recorded relative to the palmar-proximal
finger
crease. The distal edge of A3 and the proximal and distal end of A4 and the
proximal end of A-5 were measured relative to the middle finger crease.
Finally,
the distal edge of A5 and the proximal end of the metaphysis of the distal
phalanx
was measured from the distal finger crease.
The location of the decussation of the FDS tendon and the proximal and
distal extent of Camper's Chiasm was measured relative to the pulley system
and
recorded as was the location of the insertion of the FDS and the FDP tendons.
The measurements were taken along the central longitudinal axis of the finger.
While we did not use a quantitative or histological method to measure the
thickness or strength of each pulley, each pulley was observed and given a
rating
of flimsy, moderate or strong.
By using the proximal and distal position of each pulley, the position of
transverse incisions across the finger was strategically planned so that the
incision
would afford exposure of the flexor sheath between the annular pulleys. To
eliminate the variability of finger size, the position of these transverse
'mini-
incisions' was expressed as a percentage of distance from one palmar or finger
crease to the next.
Finally we analyzed the distances to provide distances to serve as a guide
for planning small incisions to expose specific sites within the flexor tendon
system.
RESULTS
Pulley Thickness:
In general the A-1, A-2, and A-4 pulleys were strong and complete. The A-1
pulley
73
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
often was divided into two or three segments. Because these function as a unit
they were measured as one. A segment was sometimes present between the A-1
and A-2 pulleys that was flimsy and could have been assessed as a separation
between the two or a flimsy distal segment of A-1 and in that case it was
measured
as part of A-1. The A-3 and A-5 were always present but often narrow and thin.
The C-1 was usually well developed but composed of one thicker bundle
and one more flimsy bundle and it usually originated approximately one
millimeter
proximal to the distal extent of the A-2 pulley. The 0-2 and C-3 were much
more
flimsy and in some specimens difficult to delineate.
Fascial Bands:
There were fascial bands crossing the tendons in the palm, 3 to 4 mm
proximal to A-1. These originated from the palmar fascia and in most cases are
directly under the distal palmar crease. They are not discreet pulleys but may
function as pulleys.
Surface Anatomy and Location of Pulleys:
Both the proximal and distal extent of the pulleys were recorded. The
distance was measured in the palm from the expected location of the proximal
extent of A-1 according to Fiorini. All the rest of the distances were
recorded in the
proximal middle and distal phalanges from the palmar-finger crease, the
proximal
finger crease and the distal finger crease. The averages for each pulley were
calculated for each finger. In order to correct for hand size the distances
were also
expressed as a percent of the distance from one crease to the next.
Decussation of FDS and Location of Camper's Chiasm:
FDS anatomy was consistent across all specimens and fingers. The
decussation of FDS occurred at the proximal end of A2. The proximal and distal
extent of Camper's Chiasm was consistently found at the distal end of A2 and
the
proximal end of A3, respectively.
Tendon Insertion:
We noted a consistent insertion for both FDP and FDS in all fingers, across
our eight specimens. FDS inserted consistently under the A4 pulley into the
middle
third of the middle phalanx. FDP inserted into the proximal and middle third
of the
distal phalanx.
74
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
Anatomic Safe Zones for Making Incisions:
There are 6 key measurements that allow placement of small transverse
incisions that do not interfere with the integrity of important pulleys. These
are:
1 - The proximal origin of the A-1 and A-2 pulleys
2 ¨ The proximal and distal extent of the C-1 pulley
3¨ The proximal and distal extent of the A-4 pulley
4 - The location of Camper's Chiasm and the split of the FDS tendon
5 - The area of insertion of the FDS into the middle phalanx
6 ¨ The position of the metaphysis of the distal phalanx and insertion of FDP
into
the distal phalanx
Based our results, we have constructed a diagram of safe-incision zones
that maintain the integrity of the A-1, A-2, A-3, A-4 and A-5 pulleys
CLINICAL APPLICATION AND MINI-INCISIONS
The surface anatomy of the tendons and the pulley and sheath system can
be used to provide a guide for the incisions for tendon surgery. The
relationship of
these structures to the finger creases is suggested to plan incisions and
easily
locate the underlying structures. We propose using this surface anatomy to
plan
the repair of flexor tendons as well as the passage of the tendon through the
pulley
system.
The surface anatomy is also essential to reliably locate the insertion of the
FDS into the middle phalanx for repair, for resection of one slip of the
tendon or to
expose Camper's Chiasm if, for example, the tendon gets hung up in this area.
For
this reason the surface anatomy of the point of division of the tendon into
two
tendon slips and the location of Camper's Chiasm related to the pulleys has
been
measured.
Resection of one slip of the FDS has been advocated as a method of
reducing friction in the A-1 and A-2 pulley regions. This also potentially
avoids the
narrow canal for the FDP to traverse as it runs through the split in the FDS
tendon.
We describe the exact location of this anatomy so that one slip of the FDS can
be
resected even through a small transverse incision.
To remove a slip it can be exposed at the distal insertion of the FDS at the
proximal extent of the A-4 pulley and deep to this pulley. It can be divided
distally
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
and then Camper's Chiasm can be divided just proximal to the proximal finger
crease between the A-2 pulley and A-3 under the C-1 pulley. The slip is then
delivered through a small transverse incision between A-1 and A-2 and finally
delivered and the slip resected from the rest of the FDS proximal to the
proximal
origin of A-1 in the palm.
Based on these measurements we worked out a guide for approaching the
flexor system and not injuring important structures, which we call the Rule of
Thirds
because it primarily involves breaking various distances within the palm and
digit
into thirds and making incisions in the safe thirds. More particularly, safe
incisions
within the palm and digit can be made according to the following rules.
With reference to Figure A below in which the blue zones indicate the
unsafe areas for incisions, the safe zone for making an incision in the distal
palm
between the Al and A2 pulleys is the area that is proximal of the palmar
digital
crease 113 by one third of the distance between the palmar digital crease 113
and
the proximal extent of the A-1 pulley of the digit 117. More specifically, if
the
distance between the palmar digital crease 113 and the proximal crease 107of a
digit is A, then the proximal end of the Al pulley is located proximal of the
palmar
digital crease this same distance A. Hence, it is not safe to make an incision
within
the region 115 that is within one third of A proximally of the palmar digital
crease
113 because that is where the A2 pulley resides.
For incisions between the A2 and A3 pulleys in the proximal phalanx, the
safe zone is the distal two thirds of the distance between the palmar digital
crease
113 and the proximal crease 107 of the digit. The proximal one third 109 of
that
distance is not safe, as it includes the A2 pulley.
In the middle phalanx, the proximal one third of the distance between the
proximal digit crease 107 and the distal digit crease 103 is safe for making
an
incision between the A3 and A4 pulleys, and the distal one third of that same
distance between the proximal digit crease 107 and the distal digit crease 103
is
safe for making incisions between the A4 and A5 pulleys. The middle one third
105 of the distance between the proximal digit crease 107 and the distal digit
crease 103 is not safe for incision as it contains the A4 pulley.
Finally, in the distal phalanx, the safe zone for incision is the distal two
thirds
of distance between the distal digit crease 103 and the end of the digit 111.
The
76
CA 02838509 2013-12-05
WO 2013/002981
PCT/US2012/041063
region 101 that is proximal the one third of the distance between the distal
digit
crease 103 and the end of the digit 111 is occupied by the A5 pulley and the
DIP
joint and is not safe.
DISCUSSION
Knowledge of the anatomy of the flexor system allows easy location all the
underlying structures. This allows for small transverse incisions or other
relatively
modest incisions which may limit the amount of dissection and exposure at
surgery.
We measured the proximal extent of the A-1 as described by Wilhelmi and
Fiorini. Wilihelmi and Fiornin in two independent studies demonstrated that
the
distance between the proximal finger crease and the PIP finger crease is the
same
distance as the proximal finger crease to the proximal edge of the A-1 pulley.
This
makes location of this pulley easy and reliable. From a practical standpoint a
small
transverse incision in this region will be close enough to the pulley origin
to expose
it easily as the difference is only 2 to 4mm.
The pulley location was found to vary by 2 to 4 mm in other locations as well
but the area of the A-1 A-2 A-3 and A-4 were consistent and as these are the
pulleys most important to protect the rule of thirds as depicted in fig --- is
found to
be helpful in the clinical situation
The separation between the A-1 and the A-2 pulleys may be distinct or
may be indistinct and difficult to discern. Previous studies indicate that 95
% of
specimens had no separation between Al and A2. We found that in ¨ of our ---
specimens that was the case. In that case we judge the end of A-1 and the
start of
A-2 as being one third of the distance between the start of A-1 and the palmar
digital crease based on the other specimens in which there was a separation
between the two pulleys.
We found fascial bands crossing the tendon proximal to the A-1 pulley in
some specimens. These fascial bands may hinder access to the entrance to theA-
1 pulley. (Fiorini) and may act as a pulley functionally
Fiorini JHS March 2011
Fiorini biblio no's 6,7,8,10, 12, 13.
77