Note: Descriptions are shown in the official language in which they were submitted.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
1
PERMANENT CATHODE AND A METHOD FOR TREATING THE
SURFACE OF A PERMANENT CATHODE
FIELD OF THE INVENTION
The invention relates to a permanent cathode defined in the independent
claims, for use in the electrolytic recovery and electrowinning of metals.
Furthermore, the invention relates to a method for treating the surface of a
permanent cathode plate.
BACKGROUND OF THE INVENTION
When the intention is to manufacture pure metal such as copper,
hydrometallurgical methods such as electrolytic refining or recovery are
used. In electrolytic refining, impure copper anodes are dissolved
electrochemically, and the copper dissolved from them is reduced onto the
cathode. In electrolytic recovery, the copper is reduced directly from the
electrolytic solution, which is typically a copper sulphate solution. The rate
of
deposit of the metal, such as copper, on the cathode surfaces depends
mostly on the current density used. The cathodes used in the process can be
starter sheets made of the metal to be reduced, or permanent cathodes
made of steel, for example. A transition to the use of permanent cathodes
has been the prevailing trend at electrolytic plants for a long time, and in
practice, all new copper electrolysis processes are based on this technology.
A permanent cathode by itself is formed of a cathode plate and an attached
suspension bar using which the cathode is suspended in the electrolytic
bath. The copper can be mechanically stripped from the permanent
cathode's cathode plate, and the permanent cathodes can be reused.
Permanent cathodes can be used in both electrolytic refining and recovery of
metals. The mere corrosion resistance of the steel grade used as a
permanent cathode plate in the electrolyte is not enough to guarantee that
the properties required of the cathode are fulfilled. Substantial attention
must
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
2
be paid to the adhesion properties of the cathode plate surface. The surface
properties of a permanent cathode plate must be appropriate so that the
depositing metal does not spontaneously strip off from the surface during the
electrolytic process but adheres sufficiently, however not preventing the
deposited metal from being removed using a stripping machine, for example.
The most important properties required of a permanent cathode plate include
corrosion resistance, straightness and surface properties with regard to the
adhesion and removability of the deposit.
A prior art method is the manufacture of permanent cathode plates from
stainless steel. Stainless steel is an iron-based alloy containing more than
10.5% chromium and less than 1.2% carbon. The chromium forms a thin
oxide layer on the steel surface, known as the passive film, which
substantially improves the corrosion resistance of the steel. Other alloying
elements can also be used to influence the properties of the passive film and
thus corrosion resistance. For example, molybdenum improves the
endurance of the passive film against pitting caused by chlorides, in which
the protective passive film is damaged locally. Alloying elements are also
used to influence other properties, for example mechanical properties and
manufacturing properties such as weldability.
Stainless steels are widely used in applications requiring good corrosion
resistance, such as the process industry, the chemical industry and the pulp
and paper industry. Due to the large volume of use, stainless steels are
usually manufactured by hot rolling. After this, the rolling scale is removed
from the steel surface. When making thinner plates with tighter thickness
tolerances, cold rolling is used. Processing after cold rolling depends on the
desired surface quality. Standard SFS-EN 10088-2 defines, for example, that
a surface of type 2B shall be cold rolled, heat treated, pickled and skinpass-
rolled. 2B thus describes the manufacturing route of the material and
therefore only specifies the surface properties at a very general level, with
the basic parameters being surface smoothness and brightness.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
3
Surface roughness is typically used to describe surfaces. Surface roughness
can be defined in a myriad of different ways but, for example, the widely
used Ra index refers to the mean deviation of surface roughness. However, it
does not address the surface profile at all ¨ whether the surface roughness is
formed by peaks or valleys. In other words, surfaces of very different
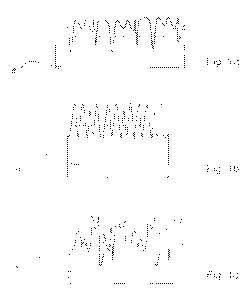
qualities may have exactly the same Ra index. This is illustrated in Figures
la, lb and 1 c.
According to patent publication US 7807028 B2, it is proposed that a
permanent cathode plate be made of an alloy at least partially comprised of
duplex steel. A duplex grade of steel refers to a steel containing 30% to 70%
austenite with the remainder being of ferritic structure. The desired
structure
can be created through appropriate alloying. According to the publication,
the roughness of the cathode plate surface is an essential factor for the
adherence of the metal deposit. The publication also presents structures to
be made on the cathode plate surface for ensuring the adherence of the
metal deposit. Such structures include, for example, various types of holes,
grooves and ledges.
According to patent publication US 7807029 B2, it is proposed that a
permanent cathode plate be made of Grade 304 steel. This grade is a
universal stainless steel having a composition very close to the grade known
as acid-proof steel and an austenitic structure. According to this
publication,
the roughness of the cathode plate surface is an essential factor for the
adherence of the metal deposit, and also this publication presents structures
to be made on the cathode plate surface for ensuring the adherence of the
metal deposit. It is further proposed that the steel be manufactured with 2B
finish in order to achieve appropriate adhesion of the metal deposit.
An optimal surface is typically defined using parameters such as the surface
roughness parameter Ra. A way of describing a surface with a certain finish
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
4
is AISI 316 2B, describing a certain grade of steel that has been skinpass-
rolled. The characteristic manufacturing route produces a smooth, semi-
bright but not mirroring surface. The publication US 7807028 B2 proposes
the parameter 2B for the cathode surface finish, meaning that the surface
has been processed by methods including cold rolling, heat treatment and
pickling. Material processing and the processing parameters are used to
influence the properties of the final surface. However, merely the above-
mentioned ways of defining the surface cannot be considered sufficient for
determining an optimal surface for a permanent cathode.
In electrodeposition of stiff metals such as nickel on a permanent cathode
several problems are faced. Adherence to the cathode plate should be very
strong, because the metal deposit easily starts to peel off from the plate. On
the other hand, if the adhesion is too strong, it is hard to strip the deposit
off,
because it is almost impossible to slip a knife between the deposit and the
cathode plate.
OBJECT OF THE INVENTION
It is an object of the invention to present a new type of permanent cathode
for electrolytic purification and recovery of metal, with usable properties
and
preference over prior art. It is a further object of the invention to define
the
surface finish parameters for an optimal permanent cathode plate, taking into
account the above problems with the use of permanent cathodes.
A further object of the invention is to provide an improved permanent
cathode for electrodeposition of stiff metals.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
SUMMARY OF THE INVENTION
The essential characteristics of the invention are evident from the attached
claims.
5
The invention relates to a permanent cathode to be used as an electrode in
the electrowinning of metals, including a permanent cathode plate at least
partially made of steel and providing the possibility of electrochemically
depositing metal from an electrolytic solution onto its surface. The grain
boundary dimensions of the permanent cathode plate surface have been
arranged to be suitable for the adhesion of deposited metal on the surface
and the stripping of metal from the surface at least in a part of the surface
that is in contact with the electrolyte.
According to an embodiment of the invention, the size of the grains in the
permanent cathode plate is 1 to 40 micrometres measured by the linear
intercept method. According to an embodiment of the invention, the average
grain boundary width W in the permanent cathode plate is 1 to 3
micrometres. The average grain boundary depth d in the permanent cathode
plate is less than 1 micrometre. According to the invention, an optimal
permanent cathode can be created by influencing the grain boundary
properties of the permanent cathode plate surface.
According to an embodiment of the invention, the permanent cathode plate is
at least partially ferritic steel. According to another embodiment of the
invention, the permanent cathode plate is at least partially austenitic steel.
According to an embodiment of the invention, the permanent cathode plate is
at least partially duplex steel. The permanent cathode plate material surface
properties according to the invention make it possible to use different grades
of steel for the electrowinning of metals.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
6
According to an embodiment of the invention, the permanent cathode plate
comprises a surface area provided with strong adhesion properties and a
surface area provided with weak adhesion properties, said adhesion
properties being dependent on the dimensions of the grain boundaries in
said surface area. Preferably, the surface area with weak adhesion
properties forms a part of the surface that is in contact with the electrolyte
and said surface area is located at a point where the stripping of metal
deposit is meant to start.
The invention also relates to an arrangement to be used for the
electrowinning of metals, said arrangement containing an electrolytic bath of
an electrolytic solution in which anodes and permanent cathodes are
alternately arranged, and said permanent cathodes being supported in the
bath by a support element, the permanent cathode according to the invention
thus being a part of the arrangement.
The invention also relates to a method for treating the surface of a
permanent cathode plate, in which the permanent cathode plate is formed at
least partially of steel plate. According to the method, the grain boundaries
of
the permanent cathode plate surface at least on a part of the surface that is
in contact with the electrolyte are treated chemically or electrochemically to
achieve the desired surface properties for the adhesion of deposited metal
on the surface and the stripping of metal from the surface.
According to a characteristic feature of the invention, the permanent cathode
plate surface is treated until the desired separating force is achieved, for
example by etching the surface of the permanent cathode plate.
According to an embodiment of the invention, different areas of the
permanent cathode plate surface that are in contact with the electrolyte are
treated differently to produce an area with strong adhesion and an area with
weak adhesion. Preferably, the area with weak adhesion is produced on a
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
7
part of the cathode plate surface where the stripping of metal deposit is
meant to start.
LIST OF FIGURES
The invention is described in more detail by reference to the drawings, where
Figures la, lb and lc illustrate the roughness of a permanent cathode plate
surface,
Figure 2 illustrates an arrangement according to the invention,
Figure 3a illustrates a permanent cathode,
Figure 3b illustrates the surface of the permanent cathode,
Figure 4 illustrates the surface of a sample piece from a permanent cathode
plate,
Figures 5a and 5b illustrate permanent cathodes with areas of different
adhesion properties,
Figure 6 illustrates stripping of a deposit from the permanent cathode,
Figure 7 illustrates the preferred fracture path between a deposit and the
cathode plate.
DETAILED DESCRIPTION OF THE INVENTION
Figures la, lb and lc illustrate different versions of the surface roughness
of
a cathode plate 4 in a permanent cathode 1. Figures la, lb and lc have the
same Ra index describing surface roughness even though they look different
in closer view, as schematically illustrated by the Figures. According to the
invention, the mere surface roughness index is not enough to achieve a
sufficiently optimal permanent cathode surface.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
8
The permanent cathode 1 according to the invention is illustrated in its
operating environment in Figure 2. The permanent cathode is intended to be
used for the electrowinning of metals. In this case the permanent cathode is
placed in an electrolytic solution in the electrolytic bath 3 alternately with
anodes 2 over the entire length of the bath, and the desired metal is
deposited from the electrolytic solution onto the surface of the cathode plate
4 in the permanent cathodes 1. The permanent cathode plate 4 is supported
in the bath using a support element 5.
Prior art has described permanent cathodes in which the surface roughness
constitutes a crucial factor for the adhesion of the metal deposit. However,
in
addition to surface roughness caused by the manufacturing process, the
metal surface also has grain boundaries that play an essential role in the
adhesion of copper onto the surface. Solid metal has a crystalline structure,
which means that the atoms are tightly packed in a regular array, and the
same array extends over a long distance compared to the interatomic
distance. These crystals are collectively referred to as grains. The grains
form irregular volume areas because their growth is limited by adjacent
grains growing at the same time. In multigranular metal, each grain is joined
with its neighbouring grains tightly across its surface at the grain boundary.
The grain boundary is an area of high surface energy in which the depositing
copper primarily nucleates. Therefore special attention must be paid to the
number and properties of grain boundaries.
Grain boundaries can be seen with an optical microscope or a scanning
electron microscope but examination of the dimensions of grain boundaries
requires an atomic force microscope (AFM). An AFM has a sharp probe
connected to a flexible support arm. When the probe is moved on the surface
of the sample under examination, interactions between the surface and the
probe are registered as bending of the support arm. The bending can be
measured with a laser beam, allowing the generation of a three-dimensional
image of the surface profile of the sample. An AFM can be used to measure
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
9
the dimensions, depth and width, of the grain boundary. The width and depth
of grain boundaries naturally vary to a certain extent. This variation can be
represented as a normal deviation, allowing statistical processing of the
dimensions.
The grain size of a material can be defined in several different ways. One of
the methods is the linear intercept method (Metals Handbook, Desk Edition,
ASM International, Metals Park, Ohio, USA, 1998 pp. 1405-1409), in which
the grain size I is
I= 1/NL
in which NL is the number of grain boundaries divided by the measurement
distance. According to the formula, grain size is inversely proportional to
the
number of grain boundaries per unit length.
Figures 3a and 3b illustrate the surface 6 of a permanent cathode plate 4 in a
permanent cathode 1 according to the invention, and the schematic drawing
presents the width W and depth d of the grain boundary between grains 8 in
the surface. The grain boundary width can be estimated from an image taken
using an optical microscope or a scanning electron microscope, or it can be
measured from AFM results. In accordance with the invention, at least a part
of the surface of the permanent cathode plate 6 that it is in contact with the
electrolyte is treated. The grain boundaries 7 between grains 8 in the
permanent cathode plate surface 6 are treated so as to be suitable for the
adhesion of deposited metal onto the surface and the stripping of metal from
it. An optimal surface for the growth of metal can be achieved in accordance
with the invention. In accordance with the invention, the dimensions of grain
boundaries 7 in the surface 6 are modified in order to achieve an optimal
permanent cathode surface. The grain size of grains 8 in the surface 6 of an
optimal permanent cathode plate 4, measured by the linear intercept method,
is 1 to 40 micrometres, the average grain boundary width W is 1 to 3
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
micrometres, and the grain boundary depth d is less than 1 micrometre. A
permanent cathode plate according to the invention can be manufactured of
austenitic steel, for example. In accordance with the invention, the
permanent cathode plate surface is treated by electroetching, for example,
5 until the desired separating force is achieved. The separating force
represents the separability of the deposited material from the surface. If the
separating force is too small, the metal deposit will be prematurely self-
stripped from the permanent cathode plate surface, while an excessively
great separating force makes it difficult to strip the metal deposit from the
10 permanent cathode plate surface.
Since the full deposit of stiff metal needs a strong adhesion on the cathode
surface in order to avoid peeling or self-stripping, it also makes the start
of
stripping more difficult. It may be difficult to insert a knife between the
cathode plate and deposit to strip the deposit from the plate. Flexing the
plate may be impossible because of the stiffness of the metal deposit. This
problem can be solved by arranging an area with less adhesion close to the
electrolyte level, that is, close to the level where deposition starts. This
area
of weak adhesion strips off easily and gives a good starting point for
stripping
of the deposit. Two or more areas of different adhesion properties can be
easily manufactured, for instance, by etching one area and by not etching
another area.
Figure 5a illustrates a permanent cathode provided with three surface areas
6a, 6b and 6c with different adhesion properties. Line L indicates the level
of
electrolytic solution when the permanent cathode plate 4 is immersed in an
electrolytic bath. The main part of the cathode plate surface, area 6a, is
etched in such a way that the desired relative dimensions of the grain
boundaries are achieved to improve the adhesion of metal deposit onto the
permanent cathode plate 4. The part of the permanent cathode plate 4 above
the electrolyte level L, area 6c, may be non-etched or gently etched.
Between the more strongly etched area 6a and the non-etched or gently
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
11
etched area 6c, below the electrolyte level L, there is a third area 6b which
is
non-etched or etched in such a way that grain boundary dimensions cause
only weak adhesion. The adhesion properties of the two non-etched or gently
etched areas 6b and 6c may be similar or different. It is important that the
.. permanent cathode plate 4 contains at least one area 6a with strong
adhesion and at least one area 6b with weak adhesion, the area 6b with
weak adhesion at least partly lying below the electrolyte level L.
Figure 5b shows an alternative embodiment where the area 6b with low
.. adhesion is located in the central area of the width of the cathode plate 4
and
the edges of the area below the electrolyte line L form a part of the more
strongly etched area 6a.
The embodiments of Figures 5a and 5b make it easy to start stripping when
.. the main part 6a of the permanent cathode plate 4 has strong deposit
adhesion. In the case of copper, stripping can be easily started by flexing
the
permanent cathode plate 4 in order to loosen the adhesion of the deposition
on the plate. However, if nickel is deposited as a thick deposit using so
called
full-deposit permanent cathodes, bending of the permanent cathode plate 4
.. may be difficult, because nickel is a stiff metal which does not deform
easily.
Good adhesion properties are preferably achieved by etching at least a part
of the cathode plate 4. In the embodiments of Figures 5a and 5b, the part 6b
of the cathode plate 4 located just below the electrolyte level L is kept non-
.. etched or it is etched just gently to obtain an area 6b with much weaker
adhesion properties than the major part 6a of the cathode plate 4.
Manufacturing of this kind of permanent cathode plate 4 is in principle easy.
The areas 6b, 6c that are not to be etched are, for instance, covered by a
tape, or even more simply, the plate is just immersed to a certain depth into
.. an etching solvent.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
12
Figure 6 illustrates the operation of a permanent cathode plate 4 according to
Figure 5a. In practice, metal deposits 11 are on both sides of the cathode
plate 4, but for the sake of simplicity, only one metal deposit 11 is shown in
Figure 6.
Stripping the metal deposit 11 off the permanent cathode plate 4 is started by
pushing a knife 10, or wedge, of a stripping machine between the permanent
cathode plate 4 and the metal deposit 11. The major part of the metal deposit
11 is strongly adhered to the surface 6a of the cathode plate 4 with strong
adhesion. In the upper part of the metal deposit 11, there is a deposit llb
having only a weak adhesion to the surface 6b of the cathode plate 4.
Consequently, in that area it is easy to push the knife 10 between the metal
deposit 10b and the plate 4. This makes a good starting point for stripping
off
the metal deposit 11.
The principle behind the functioning of the starting point of stripping can be
theoretically explained with basic fracture mechanics. The force required to
generate a fracture, that is, to remove the deposited metal 11 from the
permanent cathode surface 6a, 6b, can be approximated by the following
formula:
K1
F= ________________________________________ A
1.12 \ / c t
where F is the required force, A is the area to be stripped, K1 is the stress
intensity factor, and a is the initial crack size.
If the initial crack size a is very small, the force F required will
consequently
be very high. In contrast, when the value of a is increased, for instance by
generating an above described starting point for stripping, the force F can be
reduced substantially.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
13
Figure 7 illustrates the self-alignment of the preferred fracture path 13 into
the interface between the metal deposit 11 and the permanent cathode plate
4 when stripping in the presence of imperfections 12 in the upper end of the
deposit 11. Because the interface between the metal deposit 11 and the
cathode plate 4 is the weakest point, the fracture will preferentially occur
along the interface, even though the edges 12 of the metal deposit 11 were
"feather-like", as depicted in Figure 7.
In the following, the invention is illustrated with the help of examples.
Example 1
The permanent cathode plates used having materials with different grain
boundary properties. The materials were: AISI 316L (EN 1.4404) in delivery
state 2B (sample 1), AISI 316L (EN 1.4404) heavily etched (sample 2), LDX
2101 (EN 1.4162) in delivery state 2E (sample 3) ja AISI 444 (EN 1.4521) 2B
with two different degrees of etching (samples 4 and 5). Material AISI 316L
was etched to enlarge the grain boundaries, and material AISI 444 was
etched to open the grain boundaries. The etching method used was
electrolytic etching. Small samples were cut off the permanent cathode plate
materials and subjected to AFM inspection for determining the grain
boundary dimensions of the materials. The measured dimensions are
presented in Table 1. In the table, W refers to grain boundary width and d
refers to grain boundary depth.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
14
Table 1. Average grain boundary dimensions in the permanent cathode plate
materials.
Sample Permanent cathode plate
number material Grain boundary dimensions
Whim dhim W/c1 d/W
1 AISI 316L 2B 2.2 0.5 4.2 0.2
2 AISI 316L 2B, etched 4.1 1.4 2.8 0.4
3 LDX 2101 2E 2.8 0.7 3.7 0.3
4 444 2B, etched 1.5 0.4 3.7 0.3
444 2B, etched 2.2 1.1 2.1 0.5
5 Laboratory-scale electrolysis experiments were conducted to deposit
copper
onto these selected permanent cathode surfaces. The permanent cathode
surface was covered with a perforated plastic sheet so that it was possible to
deposit a total of four copper discs of 20 mm diameter onto each permanent
cathode during one electrolysis experiment. The anode used in the
experiments was a plate cut from copper cathode sheet. The distance
between the cathode and anode surfaces was 30 mm. After deposition, the
copper discs were separated from the permanent cathode plate using a
special stripping device that could measure the force required for separation.
The electrolysis equipment consisted of a 3-litre electrolytic cell and a 5-
litre
circulation tank. The electrolyte was pumped from the circulation tank into
the electrolytic cell, from which it returned back to the circulation tank by
overflow at a solution circulation rate of 7 litres per minute. The
circulation
tank was fitted with heating equipment and an agitator.
The electrolyte used for the experiments was made of copper sulphate and
sulphuric acid and contained 50 g/I of copper and 150 g/I of sulphuric acid.
Hydrochloric acid was also added to the electrolyte so that the electrolyte
had a chloride content of 50 mg/I. Bone glue and thiourea were used as
additives and were continuously fed into the circulation tank as an aqueous
solution. The electrolyte temperature in the electrolytic cell was maintained
at
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
65 QC by regulating the electrolyte temperature in the circulation tank. The
cathodic current density in the experiments was 30 mA/cm2, which
corresponds well to the current density used in production-scale electrolysis.
The electrolysis duration in each experiment was 20 hours. After electrolysis,
5 the mask plate was removed from the permanent cathode, and the copper
discs were separated from the permanent cathode after a fixed period of time
from the end of the experiment. The force required for separation was
measured, and the forces are presented in Table 2 as relative forces where
the reference is AISI 316L in delivery condition 2B. The choice of reference
10 is based on the fact that such permanent cathode material is generally
used
at copper electrolysis plants.
On the basis of the experimental results, the magnitude of the separating
force is clearly dependent on the grain boundary dimensions of the
15 permanent cathode material. Etching can be used to further open the
grain
boundaries of the materials in both the width and the depth dimension. The
duplex material LDX 2101 was not treated in any way before the
experiments, and also the separating force measured on that material is
greater than the separating force measured on the reference material.
Table 2. Separating forces measured on different permanent cathode
materials
Sample Permanent cathode plate
number material Relative separating force
1 AISI 316L 2B 1.0
2 AISI 316L 2B, etched 3.9
3 LDX 2101 2E 1.8
4 444 2B, etched 0.8
5 444 2B, etched 2.5
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
16
A comparison of the measured separating forces with the grain boundary
dimensions measured in AFM analysis (Table 1) shows that the wider and
deeper the grain boundaries, the greater separating force is required.
Particularly the relation between the width and depth of the grain boundaries
has a substantial effect on the required separating force.
The surface roughnesses (Ra indices) of the permanent cathode materials
chosen for the separation experiments were also measured, and the
measured values are presented in Table 3. It can be noted that etching
treatment, among other things, has changed the surface roughness to some
extent. However, no clear correlation can be found in a comparison of
surface roughnesses and the measurement results from the separation
experiments. The surface roughness index does not measure the grain
boundary dimensions. Therefore the roughness index alone cannot be
considered a sufficient criterion for achieving the desired adhesion and
separating force.
Table 3. Ra indices of the permanent cathode plate materials.
Sample Permanent cathode plate
number material Surface roughness, Ra/um
1 AISI 316L 2B 0.2
2 AISI 316L 2B, etched 0.8
3 LDX 2101 2E 2.8
4 444 2B, etched 0.1
5 444 2B, etched 0.8
Furthermore, average grain sizes of the different permanent cathode
materials were measured using a microscope and the linear intercept
method. The measurement results are presented in Table 4.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
17
Table 4. Grain sizes of the permanent cathode plate materials.
Sample Permanent cathode plate
number material Grain size/um
1 AISI 316L 2B 16
2 AISI 316L 2B, etched 24
3 LDX 2101 2E 8
4 444 2B, etched 19
444 2B, etched 22
5 Example 2
When the permanent cathodes were tested in production-scale copper
electrolysis, a phenomenon called self-stripping occurred immediately after
start-up. This means that the copper deposited on the permanent cathode
surface spontaneously strips off from the permanent cathode plate surface
either during the deposition process or when the permanent cathode is lifted
from the electrolytic bath. The phenomenon naturally causes problems at an
electrolytic plant, and such permanent cathodes cannot be used. A sample
piece was cut off the self-stripping permanent cathode (material AISI 316L)
for analysing its surface. The surface structure of the permanent cathode
plate is illustrated in Figure 4 as a scanning electronic microscopic image.
The surface structure of the permanent cathode plate immediately reveals
that the grain boundaries of the material have opened too much during
pickling, and no appropriate adhesion surface for copper can be found any
longer. The delivery state of the permanent cathode plate was 2B, and
according to measurements, the Ra index of its surface varied between 0.4
and 0.5 m. The grain boundary width of the sample, measured from a
scanning electronic microscopic image, was 8 to 10 m.
CA 02838877 2013-12-10
WO 2012/175803 PCT/F12012/050637
18
The occurrence of self-stripping on the cathode shows that the delivery state
and surface roughness indices of a permanent cathode plate are not
sufficient criteria for proper operation of the plate in copper electrolysis
but
that the grain boundary dimensions have to be managed.
It is obvious to a person skilled in the art that with the progress of
technology,
the basic idea of the invention can be implemented in several different ways.
Thus the invention and its embodiments are not restricted to the examples
described above but may vary within the scope of the claims.