Note: Descriptions are shown in the official language in which they were submitted.
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
PHARMACEUTICAL PRODUCT AND METHOD OF ANALYSING LIGHT EXPOSURE OF A
PHARMACEUTICAL PRODUCT
Background
[0001] This patent is directed to a pharmaceutical product, and, in
particular, to a
pharmaceutical product with photosensitive properties, including a
photosensitive
device that defines the product in part and methods of using the
photosensitive device
in conjunction with the pharmaceutical product.
[0002] It has been recognized that when proteins are exposed to visible
and
ultraviolet (UV) radiation, the proteins may degrade. See, for example, Bruce
Kerwin
and Richard Remmele, Protect from light: Photode gradation and protein
biologics in
Journal of Pharmaceutical Sciences, vol. 96, June 2007, pp. 1468-80. This
degradation may be both chemical and physical, in the forms of photo-induced
oxidation, covalent aggregation, and deamidation of Asn residues. While
relatively
little is known about how this light exposure affects biopharmaceuticals, the
authors
suggest that it is foreseeable that photolysis of proteins in
biopharmaceuticals could
produce changes in the conformation leading to aggregation, with small
concentrations of aggregates acting as nucleating centers leading to visible
particulates. Other problems may include changes to the immunogenic potential
of
the protein or reduction in bioactivity.
[0003] As noted by Kerwin and Remmele, products may be exposed to
visible
and UV light sources during manufacturing, storage, and/or transportation, as
well as
during use in clinics and other healthcare facilities. During manufacturing,
for
example, the products may be exposed to light sources during fill and finish
operations, visual inspections, and packaging. During use, the products may be
exposed when removed from the protective packaging in which they are stored
prior
to delivery, and may be exposed even at the time of delivery when diluted into
solutions in clear IV bags as may occur when the product is delivered
intravenously.
[0004] The solutions proposed by Kerwin and Remmele, as well as others,
address the photodecompositions indirectly through packaging or directly
through the
use of excipients or inert atmospheric packaging. As to the former solution,
it relies
upon the creation of a barrier to block incoming light. As noted by the
authors, such a
solution necessarily relies on identifying all possible regions of the
container where
- 1 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
the product may be exposed to light (e.g., at an inspection window), and
establishing
barriers to exposure at each such region. As to the latter solution, while
existing
publications suggest promise for the use of excipients and inert atmospheric
packaging, the literature is limited and the possibility for unintended
consequences
exists.
[0005] As set forth in more detail below, the present disclosure sets
forth a
pharmaceutical product embodying advantageous alternatives to the conventional
devices discussed above.
Summary
[0006] In an aspect of the present disclosure, a pharmaceutical product
includes a
container having an exterior surface and an interior chamber, an active
ingredient
disposed in the interior chamber, the active ingredient having a
photosensitive
property that changes based on at least cumulative exposure to light having a
wavelength within the range between X and Y, and a layer of photosensitive
material
disposed on the exterior surface of the container and exposed to environmental
conditions contemporaneous with the active ingredient being disposed in the
interior
chamber. The photosensitive material is reactive to light having a wavelength
within
the range between X and Y to experience a property change at a threshold of
cumulative exposure to light received within the range between X and Y related
to the
change in the photosensitive property of the active ingredient.
[0007] In another aspect of the present disclosure, a pharmaceutical
product
includes a container having an interior chamber, the container constructed
from a
material that is photo-resistive, and a layer of photosensitive material
disposed in the
interior chamber of the container. The photosensitive material is reactive to
light
having a wavelength within the range between X and Y to experience a property
change at a threshold of cumulative exposure to light received within the
range
between X and Y related to the change in a photosensitive property of an
active
ingredient.
[0008] In a further aspect of the present disclosure, a pharmaceutical
product
includes a container having an exterior surface and an interior chamber, an
active
ingredient disposed in the interior chamber, the active ingredient having a
photosensitive property that changes based on at least cumulative exposure to
light
having a wavelength within the range between X and Y, and a label having a
first
- 2 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
region affixed to the exterior surface of the container with a layer of
photosensitive
material applied thereto and a second region removably attached to the
exterior
surface of the container with a layer of the photosensitive material applied
thereto, the
first and second regions exposed to environmental conditions contemporaneous
with
the active ingredient being disposed in the interior chamber. The
photosensitive
material is reactive to light having a wavelength within the range between X
and Y to
experience a property change at a threshold of cumulative exposure to light
received
within the range between X and Y related to the change in the photosensitive
property
of the active ingredient.
[0009] In a still further aspect of the present disclosure, a method of
confirming
correct handling of photo-sensitive material includes applying a layer of
photosensitive material to an exterior surface of a container having an active
ingredient disposed therein, the active ingredient having a photosensitive
property that
changes based on at least cumulative exposure to light having a wavelength
within the
range between X and Y and the photosensitive material reactive to light having
a
wavelength within the range between X and Y to experience a property change at
a
threshold of cumulative exposure to light received within the range between X
and Y
related to the change in the photosensitive property of the active ingredient.
The
method also includes delivering the container to a recipient, collecting the
container
from the recipient, examining the layer of photosensitive to determine if the
photosensitive material has experienced the property change, and identifying
the
container as mishandled if the photosensitive materials has experienced the
property
change.
[0010] In yet another aspect of the present disclosure, a method of
analyzing light
exposure of a pharmaceutical product includes identifying a path for the
pharmaceutical product within a facility comprising at least one space through
which
the pharmaceutical product passes, the pharmaceutical product comprising an
active
ingredient having a photosensitive property that changes based on at least
cumulative
exposure to light having a wavelength within the range between X and Y, and
disposing at least one photosensitive device along the path, the at least one
photosensitive device comprising a layer of photosensitive material reactive
to light
having a wavelength within the range between X and Y to experience a property
change at a threshold of cumulative exposure to light received within the
range
- 3 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
between X and Y. The method also includes reading the at least one
photosensitive
device after being disposed along the path for evidence of the property change
to the
photosensitive material of the photosensitive device, and changing the path
for the
pharmaceutical product through the facility if the property change occurs to
the
photosensitive material of the photosensitive device.
Brief Description of the Drawings
[0011] It is believed that the disclosure will be more fully understood
from the
following description taken in conjunction with the accompanying drawings.
Some of
the figures may have been simplified by the omission of selected elements for
the
purpose of more clearly showing other elements. Such omissions of elements in
some
figures are not necessarily indicative of the presence or absence of
particular elements
in any of the exemplary embodiments, except as may be explicitly delineated in
the
corresponding written description. None of the drawings are necessarily to
scale.
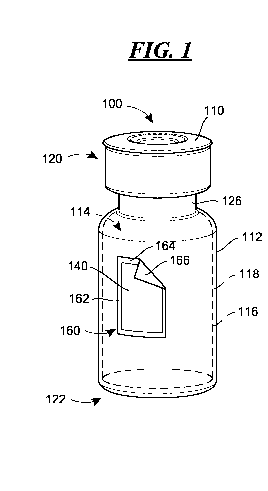
[0012] Fig. 1 is a perspective view of a pharmaceutical product
according to the
present disclosure having a container with a layer of photosensitive material
disposed
on an exterior surface of the container;
[0013] Fig. 2 is a perspective view of another pharmaceutical product
according
to the present disclosure having a container with a layer of photosensitive
material
disposed on an exterior surface of the container;
[0014] Fig. 3 is a graph illustrating the color change of a layer of
photosensitive
material, as registered using a colorimeter, with increasing UV exposure;
[0015] Fig. 4 is a graph illustrating the color change of a layer of
photosensitive
material, as registered using a colorimeter, with increasing visible light
exposure;
[0016] Fig. 5 is a graph illustrating one measure of the degradation of
a protein
exposed to visible light;
[0017] Fig. 6 is a graph illustrating a measure of the degradation (%
increase in
high molecular weight species) of a monoclonal antibody polypeptide exposed to
visible light, with reference to a color change of an associated layer of
photosensitive
material, as registered using a colorimeter;
[0018] Fig. 7 is a graph illustrating a measure of the degradation
(yellow index)
of a monoclonal antibody polypeptide exposed to visible light, with reference
to a
color change of an associated layer of photosensitive material, as registered
using a
colorimeter;
- 4 -
CA 02840637 2013-12-27
WO 2013/033600
PCT/US2012/053450
[0019] Fig. 8 is a graph illustrating a measure of the degradation
(increase in
basic peak for Cation Exchange-HPLC) of a monoclonal antibody polypeptide
exposed to visible light, with reference to a color change of an associated
layer of
photosensitive material, as registered using a colorimeter;
[0020] Fig. 9 is a graph illustrating a measure of the degradation
(increase in
acidic peak for Cation Exchange-HPLC) of a monoclonal antibody polypeptide
exposed to visible light, with reference to a color change of an associated
layer of
photosensitive material, as registered using a colorimeter;
[0021] Fig. 10 is a graph illustrating a measure of the degradation
(absorption
change) of a small molecule exposed to UV light, with reference to a color
change of
an associated layer of photosensitive material, as registered using a
colorimeter;
[0022] Fig. 11 is a graph illustrating a measure of the degradation
(yellow index)
of a small molecule exposed to UV light, with reference to a color change of
an
associated layer of photosensitive material, as registered using a
colorimeter;
[0023] Fig. 12 is a graph illustrating a measure of the degradation (%
increase
high molecular weight species) of a monoclonal antibody polypeptide exposed to
UV
light, with reference to a color change of an associated layer of
photosensitive
material, as registered using a colorimeter;
[0024] Fig. 13 is a graph illustrating a measure of the degradation (%
increase
low molecular weight species) of a monoclonal antibody polypeptide exposed to
UV
light, with reference to a color change of an associated layer of
photosensitive
material, as registered using a colorimeter;
[0025] Fig. 14 is a graph illustrating a measure of the degradation
(yellow index)
of an amino acid exposed to UV light, with reference to a color change of an
associated layer of photosensitive material, as registered using a
colorimeter;
[0026] Fig. 15 is a graph illustrating the color change of a layer of
photosensitive
material, as registered using a colorimeter, with increasing visible light
exposure over
a range of temperatures;
[0027] Fig. 16 is a perspective view of an alternative to the
pharmaceutical
product of Fig. 1;
[0028] Fig. 17 is a perspective view of a pharmaceutical product
including an
alternative label to that illustrated in Fig. 1, with a photo-resistive cover;
- 5 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
[0029] Fig. 18 is a perspective view of a pharmaceutical product
including an
alternative label to that illustrated in Fig. 1, with first and second
detachable regions;
[0030] Fig. 19 is a perspective of a system including a product
including the label
of Fig. 18 and a syringe;
[0031] Fig. 20 is a perspective view of a pharmaceutical product
including an
alternative label to that illustrated in Fig. 1, with first and second
detachable regions
and a cover over the second region;
[0032] Fig. 21 is a perspective of a system including a product
including the label
of Fig. 20 and a syringe;
[0033] Fig. 22 is a perspective view of a pharmaceutical product
including a
primary label similar to that illustrated in Fig. 1, and a secondary label for
determining changes in temperature in a first state;
[0034] Fig. 23 is the product of Fig. 22, with the secondary label in a
second state
wherein the label has changed at least one characteristic to highlight that
the product
has been exposed to high temperatures;
[0035] Fig. 24 is a perspective view of another pharmaceutical product
according
to the present disclosure having a container with a layer of photosensitive
material
disposed within the container;
[0036] Fig. 25 is a graph of illustrating the color change of a layer of
photosensitive material disposed within a container as illustrated in Fig. 23,
as
registered using a colorimeter, with increasing UV light exposure for a range
of
materials used in the fabrication of the container;
[0037] Fig. 26 is a schematic diagram of a manufacturing plant layout
wherein
phototracking units according to the present disclosure have been disposed
along a
path, P, along which the pharmaceutical product passes within the
manufacturing
plant;
[0038] Fig. 27 is a graph illustrating changes in color of different
sensitivities (in
increasing order of sensitivity: "sen 1", "sen2", "sen3", "sen4", "sen5") of
photosensitive material that may be used in the products, systems and methods
disclosed herein, with increasing UV exposure;
[0039] Fig. 28 is a graph illustrating the color change of a layer of
photosensitive
material, as registered using a colorimeter, stored at 4 C compared with a
control;
- 6 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
[0040] Fig. 29 is a graph illustrating the color change of a layer of
photosensitive
material, as registered using a colorimeter, stored at 25 C compared with a
control;
and
[0041] Fig. 30 is a graph illustrating the color change of a layer of
photosensitive
material disposed inside and outside containers for a range of materials used
in the
fabrication of the container.
Detailed Description of Various Embodiments
[0042] Although the following text sets forth a detailed description of
different
embodiments of the invention, it should be understood that the legal scope of
the
invention is defined by the words of the claims set forth at the end of this
patent. The
detailed description is to be construed as exemplary only and does not
describe every
possible embodiment of the invention because describing every possible
embodiment
would be impractical, if not impossible. Numerous alternative embodiments
could be
implemented, using either current technology or technology developed after the
filing
date of this patent, which would still fall within the scope of the claims
defining the
invention.
[0043] It should also be understood that, unless a term is expressly
defined in this
patent using the sentence "As used herein, the term ' ' is hereby defined
to
mean..." or a similar sentence, there is no intent to limit the meaning of
that term,
either expressly or by implication, beyond its plain or ordinary meaning, and
such
term should not be interpreted to be limited in scope based on any statement
made in
any section of this patent (other than the language of the claims). To the
extent that
any term recited in the claims at the end of this patent is referred to in
this patent in a
manner consistent with a single meaning, that is done for sake of clarity only
so as to
not confuse the reader, and it is not intended that such claim term be
limited, by
implication or otherwise, to that single meaning. Finally, unless a claim
element is
defined by reciting the word "means" and a function without the recital of any
structure, it is not intended that the scope of any claim element be
interpreted based
on the application of 35 U.S.C. 112, sixth paragraph.
[0044] As disclosed herein, a pharmaceutical product includes a
container having
an exterior surface and an interior chamber, which interior chamber may be
defined
by an interior surface. A material, such as a polypeptide, may be disposed
within the
interior chamber, which material may be photosensitive and may experience
- 7 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
degradation with exposure to light over a particular range of wavelengths. A
layer of
photosensitive material may be disposed either on the exterior surface of the
container
or in the interior chamber, and may be exposed to environmental conditions
contemporaneous with the polypeptide being disposed in the interior chamber,
according to certain embodiments. According to other embodiments, the exposure
of
the layer of photosensitive material to environmental conditions may be
delayed. The
photosensitive layer may be reactive to light of a particular wavelength so as
to
experience a property change at a threshold of cumulative exposure, which
property
change of the photosensitive layer may be related to the change in the
photosensitive
property of the polypeptide or other material. As one example, the property
change of
the material defining the photosensitive layer can be a color change that is
"colorimetrically detectable" (i.e., a color change that is detectable
visually or by the
use of colorimetric instrumentation).
[0045] As one example of such a product, a pharmaceutical product 100 is
illustrated in Fig. 1 that includes a container 110 having an exterior surface
112 and
an interior chamber 114, which chamber 114 may be defined by an interior
surface
116. The exterior surface 112 and the interior surface 116 may be defined by a
wall
118 made of a single layer, or the surfaces 112, 116 may be defined by
different
layers of a multi-layered structure, for example.
[0046] According to the illustrated exemplary embodiment of Fig. 1, the
container 110 may be a glass vial having an open end 120 and a closed end 122,
with
the open end 120 including an opening formed at the end of a neck region 126
of
reduced cross-section defined by a rim, which opening may be closed off by a
rubber
stopper held in place with a metal crimp ring or seal. While an exemplary
embodiment of the container 110 has been illustrated in Fig. 1, the present
disclosure
is not limited to the illustrated embodiment. For example, the container 110
may be
made of polymeric materials, such as polycarbonate, polypropylene or Teflon,
instead
of glass. Moreover, the container 110 may be larger or smaller than the
illustrated
example, and have a different shape than that illustrated in Fig. 1. For
example, the
container 110 may be a larger container used for storage and transportation,
such as a
carboy (see Fig. 2), or a smaller container used for a single-dose treatment,
such as a
single-dose vial similar in structure but smaller in size than the container
illustrated in
- 8 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
Fig. 1. The container also may have a non-rigid shape, such as in the form of
a plastic
bag, for example.
[0047] As noted above, the pharmaceutical product 100 may also include a
material disposed within the interior chamber 114 of the container 110. In
general
terms, the material disposed in the chamber 114 typically refers to (and thus
includes)
active ingredients, such as polypeptides, amino acids, and/or small molecules,
and/or
may also refer to (and thus include) an inactive ingredient or excipient in
addition to
the polypeptides, amino acids and/or small molecules. Active ingredients may
also
include viruses, which viruses may contain cDNA or RNA encoded by
glycoproteins
and lipid layers. The one or more active ingredients can be therapeutically
active
ingredients, stabilizing agents (e.g., amino acids), or diagnostic reagents,
or a
combination of therapeutic molecules, stabilizing agents, and/or diagnostic
reagents.
In particular, the material disposed within the interior chamber 114 may be a
material
that has sensitivity to light. When the material is exposed to light, the
light may
change the characteristics of the material in the container. In certain cases,
exposure
to light may cause the material to degrade, and be less useful or cease to be
useful for
its intended purpose.
[0048] As one non-limiting example, the material disposed in the
interior
chamber 114 of the container 110 may be a polypeptide. More particularly, the
polypeptide may be suspended in an aqueous medium, and may be fluid (a liquid
state) or frozen (a solid state). The polypeptide may have, for example, a
photosensitive property that changes based on at least cumulative exposure to
light
having a wavelength within the range between X and Y. According to other
embodiments, it may be desirable to monitor light exposure even when no
relationship has been previously established between change or degradation of
the
polypeptide or other material and light exposure.
[0049] In this regard, it should be noted that polypeptide and protein
are used
interchangeably herein and include a molecular chain of two or more amino
acids
linked covalently through peptide bonds. The terms do not refer to a specific
length
of the product. Thus, peptides and oligopeptides are included within the
definition of
polypeptide. The terms include post-translational modifications of the
polypeptide,
for example, glycosylations, acetylations, biotinylations, 4-pentynoylations,
PEGylations, phosphorylations and the like. In addition, protein fragments,
analogs,
- 9 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
mutated or variant proteins, fusion proteins and the like are included within
the
meaning of polypeptide. The terms also include molecules in which one or more
amino acid analogs or non-canonical or unnatural amino acids are included as
can be
expressed recombinantly using known protein engineering techniques.
[0050] Non-limiting examples of materials that may be disposed in the
interior
chamber 114 may include darbepoetin alfa (such as Aranesp0), epoetin alfa
(such as
Epogen0), anakinra (Kineret0), pegfilgrastim (such as Neulasta0), denosumab
(such
as Prolia0 or XGEVATM) and filgrastim (such as Neupogen0). Aranesp0, Epogen0,
Kineret0, Neulasta0, Prolia0, XGEVATM and Neupogen0 are manufactured by
Amgen Inc. of Thousand Oaks, California. In addition, products such as
etanercept
(such as Enbre10), adalimumab (such as Humira0), infliximab (such as
Remicade0),
certolizumab pegol (such as Cimzia0), golimumab (such as Simponi0), abatacept
(such as Orencia0), tocilizumab (such as Actemra0), panitumumab (such as
Vectibix0), cetuximab (such as Erbitux0), trastuzumab (such as Herceptin0),
bevacizumab (such as Avastin0), pegylated epoetin beta (such as Mircera 0),
peginesatide (such as HematideTM) and rituximab (such as Rituxan0) may be
disposed in the interior chamber 114. Still further non-limiting examples
include
epoetin beta, epoetin zeta, epoetin theta, mogamulizumab, omalizumab (such as
Xolair0), brodalumab, secukinumab, nimotuzumab, and ixekizumab.
[0051] In fact, the material disposed in the interior chamber 114 may
include
proteins with amino acid sequences identical to or substantially similar to
all or part of
one of the following proteins: a flt3 ligand (as described in International
Application
WO 94/28391, incorporated herein by reference), a CD40 ligand (as described in
US
Patent No. 6,087,329, incorporated herein by reference), erythropoeitin,
thrombopoeitin, calcitonin, leptin, IL-2, angiopoietin-2 (as described by
Maisonpierre
et al. (1997), Science 277(5322): 55-60, incorporated herein by reference),
Fas ligand,
ligand for receptor activator of NF-kappa B (RANKL, as described in
International
Application WO 01/36637, incorporated herein by reference), tumor necrosis
factor
(TNF)-related apoptosis-inducing ligand (TRAIL, as described in International
Application WO 97/01633, incorporated herein by reference), thymic stroma-
derived
lymphopoietin, granulocyte colony stimulating factor, granulocyte-macrophage
colony stimulating factor (GM-CSF, as described in Australian Patent No.
588819,
incorporated herein by reference), mast cell growth factor, stem cell growth
factor
- 10 -
CA 02840637 2013-12-27
WO 2013/033600
PCT/US2012/053450
(described in e.g. US Patent No.6,204,363, incorporated herein by reference),
epidermal growth factor, keratinocyte growth factor, megakaryote growth and
development factor, RANTES, human fibrinogen-like 2 protein (FGL2; NCBI
accession no. NM_00682; Riiegg and Pytela (1995), Gene 160: 257-62) growth
hormone, insulin, insulinotropin, insulin-like growth factors, parathyroid
hormone,
interferons including a interferons, y interferon, and consensus interferons
(such as
those described in US Patent Nos. 4,695,623 and 4,897471, both of which are
incorporated herein by reference), nerve growth factor, brain-derived
neurotrophic
factor, synaptotagmin-like proteins (SLP 1-5), neurotrophin-3, glucagon,
interleukins
1 through 18, colony stimulating factors, lymphotoxin-13, tumor necrosis
factor (TNF),
leukemia inhibitory factor, oncostatin M, and various ligands for cell surface
molecules ELK and Hek (such as the ligands for eph-related kinases or LERKS).
Other descriptions of proteins may be found in, for example, Human Cytokines:
Handbook for Basic and Clinical Research, Vol. II (Aggarwal and Gutterman,
eds.
Blackwell Sciences, Cambridge, MA, 1998); Growth Factors: A Practical Approach
(McKay and Leigh, eds., Oxford University Press Inc., New York, 1993); and The
Cytokine Handbook (A.W. Thompson, ed., Academic Press, San Diego, CA, 1991),
all of which are incorporated herein by reference.
[0052] Other
exemplary proteins may include proteins comprising all or part of
the amino acid sequence of a receptor for any of the above-mentioned proteins,
an
antagonist to such a receptor or any of the above-mentioned proteins, and/or
proteins
substantially similar to such receptors or antagonists. These receptors and
antagonists
include: both forms of tumor necrosis factor receptor (TNFR, referred to as
p55 and
p'75, as described in US Patent No. 5,395,760 and US Patent No. 5,610,279,
both of
which are incorporated herein by reference), Interleukin-1 (IL 1) receptors
(types I
and II; described in EP Patent No. 0 460 846, US Patent No. 4,968,607, and US
Patent No. 5,767,064, all of which are incorporated herein by reference), IL-1
receptor antagonists (such as those described in US Patent No. 6,337,072,
incorporated herein by reference), IL-1 antagonists or inhibitors (such as
those
described in US Patent Nos. 5,981,713, 6,096,728, and 5,075,222, all of which
are
incorporated herein by reference) IL-2 receptors, IL-4 receptors (as described
in EP
Patent No. 0 367 566 and US Patent No. 5,856,296, both of which are
incorporated by
reference), IL-15 receptors, IL-17 receptors, IL-18 receptors, Fc receptors,
- 11 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
granulocyte-macrophage colony stimulating factor receptor, granulocyte colony
stimulating factor receptor, receptors for oncostatin-M and leukemia
inhibitory factor,
receptor activator of NF-kappa B (RANK, described in WO 01/36637 and US Patent
No. 6,271,349, both of which are incorporated by reference), osteoprotegerin
(described in e.g. US. Patent No. 6,015,938, incorporated by reference),
receptors for
TRAIL (including TRAIL receptors 1, 2, 3, and 4), and receptors that comprise
death
domains, such as Fas or Apoptosis-Inducing Receptor (AIR).
[0053] Still further exemplary proteins may include proteins comprising
all or
part of the amino acid sequences of differentiation antigens (referred to as
CD
proteins) or their ligands or proteins substantially similar to either of
these. Such
antigens are disclosed in Leukocyte Typing VI (Proceedings of the VIth
International
Workshop and Conference, Kishimoto, Kikutani et al., eds., Kobe, Japan, 1996,
which
is incorporated by reference). Similar CD proteins are disclosed in subsequent
workshops. Examples of such antigens include CD22, CD27, CD30, CD39, CD40,
and ligands thereto (CD27 ligand, CD30 ligand, etc.). Several of the CD
antigens are
members of the TNF receptor family, which also includes 41BB and 0X40. The
ligands are often members of the TNF family, as are 41BB ligand and 0X40
ligand.
[0054] In addition, enzymatically active proteins or their ligands may
be included
as part of the product 100. Examples include proteins with all or part of one
of the
following proteins or their ligands or a protein substantially similar to one
of these:
metalloproteinase-disintegrin family members, various kinases,
glucocerebrosidase,
superoxide dismutase, tissue plasminogen activator, Factor VIII, Factor IX,
apolipoprotein E, apolipoprotein A-I, globins, an IL-2 antagonist, alpha-1
antitrypsin,
TNF-alpha Converting Enzyme, ligands for any of the above-mentioned enzymes,
and
numerous other enzymes and their ligands.
[0055] Still further, the product 100 may include antibodies or portions
thereof.
The term "antibody" includes reference to both glycosylated and non-
glycosylated
immunoglobulins of any isotype or subclass or to an antigen-binding region
thereof
that competes with the intact antibody for specific binding, unless otherwise
specified,
including human, humanized, chimeric, multi-specific, monoclonal, polyclonal,
and
oligomers or antigen binding fragments thereof. Antibodies can be any class of
immunoglobulin. Also included are proteins having an antigen binding fragment
or
region such as Fab, Fab', F(ab')2, Fv, diabodies, Fd, dAb, maxibodies, single
chain
- 12 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
antibody molecules, complementarity determining region (CDR) fragments, scFv,
diabodies, triabodies, tetrabodies and polypeptides that contain at least a
portion of an
immunoglobulin that is sufficient to confer specific antigen binding to a
target
polypeptide. The term "antibody" is inclusive of, but not limited to, those
that are
prepared, expressed, created or isolated by recombinant means, such as
antibodies
isolated from a host cell transfected to express the antibody.
[0056] Thus, the antibodies may include human antibodies, or portions
thereof,
as well as chimeric or humanized antibodies. Chimeric antibodies include human
constant antibody immunoglobulin domains coupled to one or more murine
variable
antibody immunoglobulin domain, fragments thereof, or substantially similar
proteins. Humanized antibodies include variable regions comprising framework
portions of human origin and CDR portion from a non-human source. The 100
product may also include conjugates of an antibody and a cytotoxic or
luminescent
substance. Such substances include: maytansine derivatives (such as DM1);
enterotoxins (such as a Staphlyococcal enterotoxin); iodine isotopes (such as
iodine-
125); technium isotopes (such as Tc-99m); cyanine fluorochromes (such as
Cy5.5.18);
and ribosome-inactivating proteins (such as bouganin, gelonin, or saporin-56).
The
product 100 may further include chimeric proteins selected in vitro to bind to
a
specific target protein and modify its activity such as those described in
International
Applications WO 01/83525 and WO 00/24782, both of which are incorporated by
reference.
[0057] Other examples of antibodies, in vitro-selected chimeric
proteins, or
antibody/cytotoxin or antibody/luminophore conjugates may include those that
recognize any one or a combination of proteins including, but not limited to,
the
above-mentioned proteins and/or the following antigens: CD2, CD3, CD4, CD8,
CD11a, CD14, CD18, CD20, CD22, CD23, CD25, CD33, CD40, CD44, CD52,
CD80 (B7.1), CD86 (B7.2), CD147, IL-la, IL-10, IL-2, IL-3, IL-7, IL-4, IL-5,
IL-8,
IL-10, IL-2 receptor, IL-4 receptor, IL-6 receptor, IL-13 receptor, IL-18
receptor
subunits, FGL2, PDGF-I3 and analogs thereof (such as those described in US
Patent
Nos. 5,272,064 and 5,149,792), VEGF, TGF, TGF-I32, TGF-I31, EGF receptor
(including those described in US Patent No. 6,235,883 Bl, incorporated by
reference)
VEGF receptor, hepatocyte growth factor, osteoprotegerin ligand, interferon
gamma,
B lymphocyte stimulator (BlyS, also known as BAFF, THANK, TALL-1, and
- 13 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
zTNF4; see Do and Chen-Kiang (2002), Cytokine Growth Factor Rev. 13(1): 19-
25),
C5 complement, IgE, tumor antigen CA125, tumor antigen MUC1, PEM antigen,
LCG (which is a gene product that is expressed in association with lung
cancer),
HER-2, a tumor-associated glycoprotein TAG-72, the SK-1 antigen, tumor-
associated
epitopes that are present in elevated levels in the sera of patients with
colon and/or
pancreatic cancer, cancer-associated epitopes or proteins expressed on breast,
colon,
squamous cell, prostate, pancreatic, lung, and/or kidney cancer cells and/or
on
melanoma, glioma, or neuroblastoma cells, the necrotic core of a tumor,
integrin alpha
4 beta 7, the integrin VLA-4, B2 integrins, TRAIL receptors 1, 2, 3, and 4,
RANK,
RANK ligand, TNF-a, the adhesion molecule VAP-1, epithelial cell adhesion
molecule (EpCAM), intercellular adhesion molecule 3 (ICAM-3), leukointegrin
adhesin, the platelet glycoprotein gp IIb/IIIa, cardiac myosin heavy chain,
parathyroid
hormone, rNAPc2 (which is an inhibitor of factor VIIa-tissue factor), MHC I,
carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), tumor necrosis factor
(TNF), CTLA-4 (which is a cytotoxic T lymphocyte-associated antigen), Fc-y-1
receptor, HLA-DR 10 beta, HLA-DR antigen, L-selectin, Respiratory Syncitial
Virus,
human immunodeficiency virus (HIV), hepatitis B virus (HBV), Streptococcus
mutans, and Staphlycoccus aureus.
[0058] Specific examples of known antibodies include, but are not
limited to,
adalimumab, bevacizumab, infliximab, abciximab, alemtuzumab, bapineuzumab,
basiliximab, belimumab, briakinumab, canakinumab, certolizumab pegol,
cetuximab,
conatumumab, denosumab, eculizumab, gemtuzumab ozogamicin, golimumab,
ibritumomab tiuxetan, labetuzumab, mapatumumab, matuzumab, mepolizumab,
mogamulizumab, motavizumab, muromonab-CD3, natalizumab, nimotuzumab,
ofatumumab, omalizumab, oregovomab, palivizumab, panitumumab, pemtumomab,
pertuzumab, ranibizumab, rituximab, rovelizumab, tocilizumab, tositumomab,
trastuzumab, ustekinumab, zalutumumab, and zanolimumab.
[0059] The product 100 may include an anti-idiotypic antibody or a
substantially
similar protein, including anti-idiotypic antibodies against: an antibody
targeted to
the tumor antigen gp72; an antibody against the ganglioside GD3; an antibody
against
the ganglioside GD2; or antibodies substantially similar to these.
[0060] The product 100 may include recombinant fusion proteins including
any
of the above-mentioned proteins. For example, recombinant fusion proteins
including
- 14 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
one of the above-mentioned proteins plus a multimerization domain, such as a
leucine
zipper, a coiled coil, an Fc portion of an antibody, or a substantially
similar protein,
can be produced using the methods of the invention. See e.g. W094/10308;
Lovejoy
et al. (1993), Science 259:1288-1293; Harbury et al. (1993), Science 262:1401-
05;
Harbury et al. (1994), Nature 371:80-83; Hakansson et al.(1999), Structure
7:255-64,
all of which are incorporated by reference. Specifically included among such
recombinant fusion proteins are proteins in which a portion of TNFR or RANK is
fused to an Fc portion of an antibody etanercept (a p75 TNFR:Fc), and
belatacept
(CTLA4:Fc). TNFR:Fc comprises the Fc portion of an antibody fused to an
extracellular domain of TNFR, which includes amino acid sequences
substantially
similar to amino acids 1-163, 1-185, or 1-235 of Figure 2A of US Patent No.
5,395,
760, which is incorporated by reference. RANK:Fc is described in International
Application WO 01/36637, which is incorporated by reference.
[0061] In addition, the pharmaceutical product 100 illustrated in Fig. 1
includes a
layer 140 of photosensitive material disposed on the exterior surface 112 of
the
container 110. The photosensitive material may be reactive to light having a
wavelength within the range between X and Y so as to experience a property
change.
According to certain embodiments, the color of the material may change, from
yellow
to green for example. A non-limiting example of a photosensitive material that
may
be used in the layer 140 is available from UV Process Supply, Inc. of Chicago,
Illinois
under the UV FastCheckTM brand.
[0062] Along these lines, Fig. 3 illustrates a graph prepared relative
to a
photosensitive material that experiences a change in color (as exhibited by a
change a
colorimeter reading) with increasing UV exposure, the UV exposure occurring
with a
peak wavelength of about 350 to 370 nm and over a range of wavelengths about
315
to 400 nm (UVA). Similarly, Fig. 4 illustrates a graph prepared relative to a
photosensitive material that experiences a change in color (as exhibited by a
change in
a colorimeter reading) with increasing visible light exposure, the visible
light
exposure occurring with a range of wavelengths from 401 to 750 nm. These
measurements were and similar measurements may be determined using a
colorimeter, such as the HunterLab UltraScan PRO colorimeter available from
Hunter
Associates Laboratory Inc. of Reston, Virginia, while a color reflection
- 15 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
spectrodensitometer, such as the X-rite Model 530 available from X-rite Inc.
of Grand
Rapids, Michigan, may be used according to other embodiments.
[0063] It will be recognized that the threshold of cumulative exposure
to light
received within the range between X and Y related to the property (e.g.,
color) change
of the material in photosensitive layer 140 may be related to the change in
the
photosensitive property of the polypeptide or other material or substance
disposed in
the container 110. For example, the change in the photosensitive property of
the
polypeptide may be measured quantitatively using separation techniques such as
chromatography, for example. In particular, size-exclusion (SEC), cation-
exchange
(CEX) and hydrophobic-interaction (HIC) chromatography may be used to
correlate
light exposure to product degradations. Fig. 5 shows the relationship between
readings generated using HIC with an exemplary polypeptide exposed to visible
light,
with increasing HIC readings reflecting an increase in inactive species of the
polypeptide with increased light exposure. Given these results and the known
performance of the material in the photosensitive layer 140, illustrated for
example in
Fig. 4, a correlation may be defined between the colorimeter readings and the
HIC
readings over a range of light exposures such that a particular colorimeter
reading
may be associated with a particular degree of inactivation or degradation of
the
polypeptide represented.
[0064] In further support of these correlations, the graphs of Figs. 6-
14 directly
illustrate the relationship between (i) various measures of the degradation of
monoclonal antibody polypeptides (as an example of a polypeptide), of quinine
monohydrochloride dihydrate (as an example of a small molecule), or of
tryptophan
(as an example of an amino acid) with increasing visible light or UV light
exposure
and (ii) colorimeter readings of the color change of an associated layer of
photosensitive material. Without attempting to limit the discussion of the
degradation
reflected in the results of this testing to a particular mechanism, it is
noted that
degradation may be both chemical and physical, and may be, for example, in the
form
of photo-induced oxidation, covalent aggregation, and/or deamidation of Asn
residues. In particular, Figs. 6-9, 12 and 13 relate to experiments performed
with a
monoclonal antibody, while Figs. 10 and 11 relate to experiments performed
with
quinine monohydrochloride dihydrate (2% solution) and Fig. 14 relates to
experiments performed with tryptophan (1 mM aqueous solution). Moreover, Figs.
6-
- 16 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
9 relate to experiments performed using visible light, while Figs. 10-14
relate to
experiments performed using UV light. These experiments were conducted with
light
having a range of wavelengths between 190 to 1100 nm, with the UV exposure
occurring with a peak wavelength of about 350 to 370 nm and principally over a
range of wavelengths from about 315 to 400 nm (UVA) and the visible light
exposure
occurring principally over a range of wavelengths from about 401 to 750 nm.
[0065] In regard to these experiments, all were performed at room
temperature
(25 C) and 40% relative humidity. A 3 cc glass vial was filled with a sample
of
monoclonal antibody ("mAb"), quinine monohydrochloride dihydrate ("quinine"),
or
tryptophan and the vial was capped. A layer of photosensitive material was
then
applied to the outside wall of an empty 3 cc glass vial. Three different
sensitivities of
photosensitive material were used in these experiments, as reflected in the
graphs of
Figs. 6-14, with the material associated with "senl" having the lowest light
sensitivity
and the material associated with "sen3" having the highest light sensitivity,
the
materials being commonly available from UV Process Supply Inc. of Chicago,
Illinois
as CON-TROL-CURE UV FastcheckTm Strips, part # NO10-002. The three
sensitivities ("sen 1", "sen2", and "sen3") are the same as the three
similarly named
sensitivities ("sen 1", "sen2" and "sen3") found in Fig. 27, discussed below.
The
filled vial and the empty vial were exposed to the same light source (a cool
white light
with a 10 klux intensity setting or a UV light with a 30 W/m2 intensity
setting).
[0066] In all experiments, the color of the layer of photosensitive
material was
measured using an X-Rite spectodensitometer (manufactured by X-Rite of Grand
Rapids, Michigan), which device is exemplary of the types of color-measuring
equipment that may be used in such an experimental set-up. The various
measurements related to the degradation of the monoclonal antibody or quinine
sample were generated using appropriate techniques. For example, Size
Exclusion-
HPLC was used to determine the measurements relating to % increase in high
molecular weight or low molecular weight species (Figs. 6, 12, 13), while
Cation
Exchange-HPLC was used to determine the measurements relating to changes in
basic
and acidic peak (Figs. 8, 9). In this regard, a high molecular weight species
is
typically in the form of aggregates that may lead to immunogenic reactions or
adverse
events upon administration, while a low molecular weight species is typically
in the
form of degradants. A Ultrascan PRO spectrophotometer (manufactured by Hunter
- 17 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
Associates Labs., Inc. of Reston, Virginia) was used to determine the
measurements
relating to the yellow index (Figs. 7, 11, 14). It will be recognized that
reference to
the yellow index refers to a single value calculated from spectrophotometric
data to
describe the change in color of a test sample from clear or white toward
yellow, which
value is commonly associated with general product degradation and is chiefly
used to
quantify this degradation, with the calculation being described, for example,
in the
application note on Yellowness Indices provided by Hunter Associates
Laboratory
Inc. of Reston, Virginia, vol. 8, no. 15 (2008), which application note is
incorporated
by reference herein in its entirety. A Beckman Coulter spectrophotometer was
used to
measure the light absorbance at 400 nm (Fig. 10).
[0067] In each of the graphs of Figs. 6-14, it will be recognized that
the color
change in the photosensitive material increases with increasing light
exposure.
Similarly, the measure of the degradation of the sample also increases with
increasing
light exposure. That is an increase in high or low molecular weight species is
reflective of degradation of the mAb polypeptide (Figs. 6, 12, 13), as would
be an
increase in the acidic or basic peak produced using Cation Exchange-HPLC
(Figs. 8,
9) representing a change in the charged species. In a similar fashion, an
increase in
the yellow index (Figs. 7, 11) is reflective of degradation of the mAb
polypeptide, the
small molecule (e.g., quinine), or the amino acid (e.g., tryptophan). Further,
a change
in the absorbance of the quinine is reflective of a change in the small
molecule. In
each case, while the color change between the photosensitive material and the
measure of the degradation of the sample may not be the same for all
characteristics
examined, it is true that there is a direct relationship between the change in
color of
the photosensitive material and the measure of the degradation of the active
ingredient, such that the photosensitive material may be used as a real-time
indicator
for changes occurring to an active ingredient in a container.
[0068] The use of a correlation between the degradation of the material
(e.g.,
polypeptide) disposed within the interior chamber 114 and the property (e.g.,
color)
change of the photosensitive layer 140 as a real-time indication and indicator
of the
changes and/or degradation of the material within the interior chamber 114
provides a
number of opportunities for optimization of the product 100 through selection
of the
photosensitive material for the layer 140. A number of factors may also need
to be
accounted for in providing a stabile and reliable indication and indicator.
Certain
- 18 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
examples of opportunities for optimization and factors to be accounted for are
provided herein; the list is not intended to be exhaustive.
[0069] For example, where the material (e.g., polypeptide) within the
interior
chamber 114 undergoes degradation in reaction to light of a particular
wavelength or
range of wavelengths, the photosensitive material may be selected to be
responsive
only to light of that wavelength or to range of wavelengths. In fact, the
sensitivity of
the material to the wavelength of interest may be selected so as to match or
relate a
more sensitive (reactive) material for the photosensitive layer 140 with a
more
sensitive (reactive) material (e.g., a more sensitive polypeptide) disposed
within the
container 110 relative to the wavelength under consideration.
[0070] Alternatively, the material (e.g., polypeptide) within the
interior chamber
114 may experience degradation as a consequence of a number of reactions, each
of
which is attributable to light of a different wavelength. It may be the case
that all of
these factors contribute to the reduced efficacy of the material for treatment
of a
particular medical condition, and no one factor predominates. Based on this
analysis,
a photosensitive material reactive to a wide range of wavelengths may be used
for the
layer 140.
[0071] It is also possible that while the material or substance (e.g.,
polypeptide)
in the interior chamber 114 may exhibit degradation when exposed to a light
over a
particular range of wavelengths, it may be possible that the environment will
selectively eliminate or provide only certain wavelengths within that range.
As a
consequence, rather than selecting a material based on the entire range of
wavelengths
known to cause degradation of the material disposed within the interior
chamber 114,
the photosensitive material may be selected according to the wavelengths known
to
exist within a given environment. As a consequence, the photosensitive
material
selected for a layer 140 applied to a container 110 that will predominantly be
used
within a manufacturing facility, such as a carboy, may differ from the
photosensitive
material selected for a layer 140 applied to a container 110 that will be
carried by
paramedics or the like for use in the field.
[0072] On the other hand, factors such as temperature may affect the
performance of the photosensitive material, and thus the stability or
robustness of the
correlation derived between the property change of the photosensitive material
and
the change of the material disposed in the container 110. Along such lines,
Fig. 15
- 19 -
CA 02840637 2013-12-27
WO 2013/033600
PCT/US2012/053450
illustrates the change in colorimeter readings for a photosensitive material
that may be
used in the layer 140 over a range of light exposures and a plurality of
temperatures.
It may be observed from Fig. 15 that the change in colorimeter reading may
vary less
dramatically at lower temperatures (e.g. 4 C) than at higher temperatures (37
C).
Consequently, not only may a material be selected for the layer 140 according
to the
reactivity of the material (e.g., polypeptide) in the container 110, the
material for the
layer 140 may also be selected according to the performance characteristics of
the
photosensitive material over the operational range of temperatures expected.
In the
alternative, the knowledge of variations in colorimeter readings based on
temperature
may be used to create corrections for correlations between the changes in the
material
of the layer 140 and the changes in the material in the container 110 that are
temperature dependent, which further corrections may enhance the stability and
reliability of the correlations.
[0073] While it
is believed that temperature may affect the rate of color change,
Figs. 28 and 29 illustrate that little reversibility is believed to exist in
the color change
of photosensitive material that has been exposed and then subsequently stored
in a
space without further light exposure (i.e., in the dark) over a wide range of
temperatures (e.g., 4 C (Fig. 28) or 25 C (Fig. 29)). That is, there is little
difference
in the color measurements for a control sample and a sample stored at the
specified
temperature in the dark for a prolonged period of time (e.g., approximately 48
hours)).
The samples were controlled for humidity, and the measurements were made using
an
X-Rite spectrodensitometer, which device is exemplary of the types of color-
measuring equipment that may be used in such an experimental set-up.
[0074] The size
and placement of the layer 140 may also be selected to improve
the stability of the readings. This in turn may affect the reliability of the
derived
correlation, as well as reliability of an assessment of the condition of the
material
(e.g., polypeptide) in the chamber 114 made in reliance on the correlation. In
particular, readings obtained from a layer 140 that is planar or appears
substantially
planar relative to the measuring device or equipment used to take the color
reading of
the layer 140 may be more consistent than readings obtained from a layer 140
with a
curved profile. As a consequence, when dealing with a generally cylindrical
object
(such as a vial or a carboy) having a particular length and diameter, wider
layers 140
are believed to appear to have a more curved profile relative to measurement
- 20 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
equipment, resulting in greater variation in readings that narrower layers
140. As a
consequence, this potential source of variation may be addressed by more
extensive
mapping of the potential curvature of the layer 140 as applied to the
container 110, or
by selecting the size and/or dimensions of the layer 140 to minimize the
effects of the
curvature of the layer 140 on the variation in the readings obtained (i.e.,
make the
layer 140 appear more planar relative to the equipment used to take the
reading).
[0075] As a consequence of these various considerations and factors,
while an
exemplary embodiment has been illustrated in Fig. 1 in which a single layer
140 of
material is disposed on the exterior surface 112 of the container 110, more
than one
layer of material may be disposed on the exterior surface 112 of the
container110, or
may be associated with the container 110, as explained in greater detail
below.
According to such an embodiment, each layer may be useful in determining
exposure
to a different wavelength of light, may have different sensitivity to
temperature, or
may be of different sizes and shapes. Of course, the presence of multiple
layers 140
on a single container 110 may lead to user confusion in certain circumstances,
so the
layers 140 may be disposed on portions of the exterior surface 112 of the
container
110 remote from each other, or in such a manner that at least one of the
layers 140
may be removed from the exterior surface 112, for example, once the
photosensitive
material in the layer no longer is capable of undergoing a property change,
e.g., a
colorimetrically detectable property change.
[0076] As mentioned above, the layer 140 may be exposed to environmental
conditions contemporaneous with the polypeptide being disposed in the interior
chamber 114. That is, the layer 140 may be disposed on the exterior surface
112 of
the container 110 and exposed to the same conditions the material (e.g.,
polypeptide)
is exposed to at the same time the material is disposed into the interior
chamber 114
of the container 110, or within some time period before or after the material
is
disposed into the chamber 114. The length of the time period considered to be
contemporaneous may be determined by the sensitivity of the material in the
chamber
114 and/or the sensitivity of the material used to define the layer 140, for
example.
[0077] As was mentioned above and will be explained in greater detail
below, the
layer 140 may be exposed to environmental conditions at some point in time
that is
not considered contemporaneous with the material (e.g., polypeptide) being
disposed
in the interior chamber 114. Where the reaction of material disposed within
the
-21 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
container 110 happens rapidly, even the passage of a limited period of time
may be
considered to be non-contemporaneous. However, in more general terms, this may
encompass a situation wherein the layer 140 is purposefully shielded from
exposure to
the same conditions as the material disposed in the chamber 114, for example
through
the use of a physical barrier or photoresistive material.
[0078] Returning again to the embodiment of Fig. 1, it will be
recognized that the
layer 140 is disposed on the exterior surface 112 through the use of a label
160. The
label 160 may include a substrate 162 having a first surface 164 and a second
surface
166, which surfaces 164, 166 are disposed on opposite sides of the substrate
162. The
size of the substrate 162 relative to the size of the layer 140 has been
purposely
exaggerated in Fig. 1 so that the substrate 162 may be more easily visualized
and
identified. According to a preferred embodiment, the layer 140 extends to the
edges
of the substrate 162; however, it is possible for the edges of the layer 140
to be spaced
from the edges of the substrate 162 as shown. Also, a corner of the substrate
162 is
turned back in Fig. 1 to expose the surface 166, although this corner would
typically
lie along the surface 112 as assembled.
[0079] The substrate 162 may be a paper product, but also may be made of
plastic or other polymer, for example. On one surface (surface 166, as
illustrated)
may be disposed an adhesive or other compound that may be used to affix,
adhere or
attach the label 160 to the surface 112. Of course, for that matter, the
substrate 162
may be attached to the exterior surface 112 by applying a material over the
surface
164 of the label, which material would be selected to be sufficiently
transparent such
that the layer 140 may be visualized or scanned and such that the operation of
the
layer 140 (i.e., the photosensitivity of the layer 140) would not be affected.
For
example, a clear, single-side tape product may be used to attach the substrate
162 (and
thus the label 160) to the surface 112. The application of additional layer
may not
only attach the substrate 162 to the surface 112, but may protect the layer
from
environmental conditions (e.g., humidity) as well; consequently, the
additional layer
may be present even when not used to attach the substrate 162 to the surface.
The use
of an adhesive-backed label 160 may also facilitate the assembly of the
product 100
including the label 160 during manufacturing.
[0080] As one alternative, Fig. 16 illustrates an embodiment wherein the
layer
140 of photosensitive material is applied directly to the exterior surface 112
of the
- 22 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
container 110. According to such an embodiment, the surface 112 may need to be
prepared prior to application of the layer 140 to the surface 112. The
preparation of
the surface 112 may require the application of other chemicals to the surface
112 to
permit a satisfactory joining or bonding to occur between the material of the
layer 140
and the material of the container 110. It will be recognized that while such
chemicals
may technically present an intermediate layer between the layer 140 and the
surface
112 of the container 110, the layer 140 may still be understood as being
applied to the
surface 112 of the container 110 for the purposes of this disclosure.
[0081] The label 160 may also include other elements beyond the layer
140. For
example, the label 160 may include indicia to identify the material (e.g.,
polypeptide)
disposed within the chamber 114, such as the name of the material, the name of
the
manufacturer, instructions relating to use of the material, etc. As a further
example,
the label 160 may include indicia relevant to the layer 140, such as a color
scale, so
that a user would know without further reference to additional materials how
to
interpret the layer 140 so as to know if the material (e.g., polypeptide)
within the
chamber 114 should be safe to administer or not. This color scale may be based
on
the correlations between the changes in the material of the layer 140 and in
the
material in the chamber 114 discussed above.
[0082] The label 160 may also include other structures that cooperate
with the
layer 140. For example, as alluded to previously, the layer 140 may be
shielded or
concealed from exposure to light. Consequently, Fig. 17 illustrates a label
160
including the substrate 162 on which the layer 140 is disposed, and a further
layer 180
of removable photo-resistive material applied over the layer 140 of
photosensitive
material. According to certain embodiments, the photo-resistive material of
the layer
180 may block all light exposure; according to other embodiments, the photo-
resistive
material of the layer 180 may block only a portion of the light exposure. In
the
particular embodiment illustrated, the layer 180 of removable photo-resistive
material
comprises a cover removably attached to the exterior surface 112 of the
container 110
over the layer 140 of photosensitive material to block light of all
wavelengths, and
may be made of coated paper, coated plastic, or metallic (e.g., aluminum) foil
having
a releasable adhesive applied to at least a portion of the surface 182 facing
the layer
140. This cover 180 may be removed as required. While the cover 180 may be
removably attached directly to the exterior surface 112 of the container 110
according
-23 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
to certain embodiments, according to the embodiment illustrated in Fig. 17,
the cover
180 is removably attached to the substrate 162, which is in turn disposed on
the
surface 112 of the container 110.
[0083] A further example of an alternative structure for a label 160 is
illustrated
in Fig. 18. According to this embodiment, the label 160 has a first region 190
disposed on the exterior surface 112 of the container 110 with a layer 191 of
photosensitive material applied thereto and a second region 192 removably
disposed
on the exterior surface 112 of the container 110 with a layer 193 of the
photosensitive
material applied thereto. The first region 190 and the second region 192 of
the label
may be integrally attached (i.e., formed as a single piece), but the boundary
between
the first and second regions 190, 192 may be defined by a perforation 194,
which may
facilitate the separation of the first and second regions 190, 192. According
to other
embodiments, the boundary between the first and second regions 190, 192 may
simply be defined by a marking on the label 160.
[0084] According to the illustrated embodiment, the first and second
regions 190,
192 may be exposed to environmental conditions contemporaneous with the
material
(e.g., polypeptide) being disposed in the interior chamber 114. As such, the
layer 191
and the layer 193 should experience roughly the same exposure to light as the
material
in the chamber 114. Therefore, the regions 190, 192 and the respective layers
191,
193 may be referred to in much the same manner to determine the overall
exposure
history of the material in the chamber 114.
[0085] As to how a label 160 such as the one illustrated in Fig. 18 may
be used,
consider that while the container 110 may be used for storage of the material,
it is
likely that the material will be removed from the container 110 and disposed
in a
delivery device before the material is administered to the patient. For
example, where
the container 110 is a single-dose vial, for example, the material disposed in
the
container 110 may be removed from the container using a syringe 200 with a
needle
(see Fig. 19) or a syringe with a luer tip and a vial adapter. In either case,
it may be
some time before the material in syringe is administered to the patient.
Because the
exposure to light will continue throughout this time, it may be advantageous
to
continue the monitoring of the light exposure by removing the second region
190 of
the label 160, and attaching the second region 192 to the syringe 200, such
that the
layer 193 may be observed. As a consequence, the monitoring of the light
exposure
- 24 -
CA 02840637 2013-12-27
WO 2013/033600
PCT/US2012/053450
of the material in the syringe 200 may continue even after the material is
removed
from the container 110 using such a system.
[0086] It will be further recognized that it is possible to incorporate
features from
the label illustrated in Figs. 18 and 19 with those of the label illustrated
in Fig. 17.
For example, a further embodiment of a label 160 is illustrated in Fig. 20.
According
to this embodiment, the label 160 includes a substrate 162 having a first
portion 190
and a second portion 192, each with its respective layer 191, 193 of
photosensitive
material. However, a cover 210 in Fig. 21 is disposed over the layer 193
arranged on
the second portion 192 of the substrate 162. This cover 210 may be similar in
nature
to the cover 182 discussed above in that the cover 210 may be defined by a
layer of
photo-resistive material removably attached to the exterior surface 112 of the
container 110 over the layer 193 of photosensitive material. As illustrated,
the cover
210 may be removably attached to the portion of the substrate 162 that defines
the
second portion 192 of the label, such that the second section 192 and the
cover 210
may be removed as a single item from the container 110.
[0087] The label illustrated in Fig. 20 may be used in a fashion similar
to that of
the label illustrated in Figs. 18 and 19. That is, when the material in the
chamber 114
of the container 110 is transferred to a syringe 220 (see Fig. 21), the second
section
192 and the cover 210 may be removed from the container 110 and applied to the
syringe 220. The cover 210 may then be removed from the second section 192 of
the
label 160 to expose the layer 193 of photosensitive material beneath the cover
210.
The monitoring of the light exposure of the material in the syringe 220 may
thus
continue with the layer 193. It will be recognized that a similar effect may
be
achieved if the cover 210 is first removed from the second section 192 of the
label
160 (and thus the layer 193) prior to application of the second section 192 to
the
syringe 220.
[0088] A label such as that illustrated in Figs. 20 and 21 may be
advantageous,
for example, when the material used to make the container 110 has a filtering
or a
blocking effect on the light to which the container 110 is exposed, but that
same
material is not used in the manufacture of the syringe 220. According to such
an
embodiment, it may be appropriate to use a layer 191 of photosensitive
material that
has been selected to more closely correlate the light exposure of the material
(e.g.
polypeptide) when disposed in the container 110, and to use a layer 193 of
- 25 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
photosensitive material that has been selected to more closely correlate the
light
exposure of the material (e.g., polypeptide) when disposed in the syringe 210.
To
prevent the layer 193 from providing an inaccurate representation of the light
exposure of the material in the syringe 220, the cover 210 is removed
contemporaneously with the transfer of the material from the container 110 to
the
syringe 220, rather than when the polypeptide or other substance is disposed
into the
chamber 114.
[0089] It is also possible to design a label or labeling system that
incorporates the
layer of photosensitive material in combination with a layer of material
reactive to
other environmental conditions, such as temperature or humidity. For example,
Figs.
22 and 23 illustrate a container 250 in which a polypeptide, for example, is
disposed.
The container 250 is used in conjunction with a label or labeling system that
permits
monitoring of light exposure and temperature, for example. Such a device may
be
useful where the polypeptide has a photosensitive property that changes based
on
exposure to light having a wavelength within the range between X and Y, and
also has
a temperature-sensitive property. Alternatively, the temperature-sensitive
layer may
be used to signal the user to use a different correlation for the
photosensitive layer 252
where the photosensitive material of the photosensitive layer 252 is
temperature-
sensitive (see Fig. 15, above).
[0090] To address the photosensitivity, or potential photosensitivity,
of the
material (e.g., polypeptide) in the container 250, a layer 252 of
photosensitive
material may be disposed on an exterior surface 254 of the container 250 and
exposed
to environmental conditions contemporaneous with the polypeptide being
disposed in
the interior chamber, for example. The photosensitive material may be reactive
to
light having a wavelength within the range between X and Y to experience a
property
change at a threshold of cumulative exposure to light received within the
range
between X and Y related to the change in the photosensitive property of the
polypeptide. It will be recognized that any of the variations in regard to the
layer of
photosensitive material illustrated and/or discussed above may be used in
conjunction
with the layer 252.
[0091] In addition, a label 256 including a layer 258 of temperature
sensitive
material may be disposed on the exterior surface 254 of the container 250. The
layer
258 may experience a property change (e.g., a colorimetrically detectable
property
- 26 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
change) at a threshold of temperature exposure related to a change in a
temperature-
sensitive property of the material in the container 250, i.e., the polypeptide
according
to the present embodiment. This layer 258 may be disposed on or against a
background 260 of varying colors to facilitate detection or visualization of
the change
of the layer 258 (compare Fig. 22 to Fig. 23, for example). In fact, a similar
mechanism may be used in combination with the layer of photosensitive
material, in
this or the other embodiments described herein, to facilitate detection or
visualization
of the property change of the photosensitive material wherein this property
change is
a color change.
[0092] Having thus described numerous embodiments of a product according
to
the present disclosure with reference to Figs. 1-23, a number of uses may now
be
described for these embodiments.
[0093] Initially, it will be recognized that one use that may be made of
the
product according to the present disclosure is to determine the status of a
material
disposed in the chamber 114 in real time. This determination may be made, for
example, once a correlation has been defined between the property change of
the
material in the layer 140 and the photosensitivity of the material in the
chamber 114.
Once known, the correlation may be used to define a scale (e.g., a color
scale) that
will permit inspection of the layer 140 to be used to determine that status of
the
material disposed in the chamber 114. The correlation may be determined, for
example, by disposing material in the chamber 114 contemporaneous with
disposing a
label 160 on the exterior surface 112 of the container 110, monitoring both
the
property change of the material in the layer 140 and the photosensitive
property of the
material in the chamber 114, for example, at a series of time increments over
a test
period, and collecting the data. The data collected for the material in the
layer 140
and for the material in the chamber 114 may be compared, and a correlation or
relationship may be defined. Based on the defined relationship, a scale may be
determined to permit a level of light exposure for the material in the chamber
114 to
be identified with changes in the property of the material in the layer 140
for real-time
assessment of the status of the substance in the chamber 114.
[0094] The monitoring and/or determination of the change in the property
of the
photosensitive material in the layer 140 will vary according to the material
used. For
example, if the photosensitive material in the layer 140 experiences a color
change,
-27 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
then the change in the property may be determined visually by the user or by
an
optical colorimetric sensing device, such described above. The optical sensing
device
may be coupled to a computerized system, which system may be programmed with
the correlation or relationship between the property change and the status of
the
material (e.g., polypeptide) in the chamber 114, and which system may be
further
programmed to remove a container from inventory if the determination of status
suggests that the material is no longer safe and/or effective to administer.
[0095] The pharmaceutical product thus described may also be used in a
method
of confirming correct handling of photosensitive material (e.g., polypeptide)
in the
container. According to such a method, the layer 140 of photosensitive
material
would be applied to the exterior surface 112 of the container 110 having the
polypeptide or other material disposed therein, the polypeptide or other
material
having a photosensitive property that changes based on at least cumulative
exposure
to light having a wavelength within the range between X and Y and the
photosensitive
material reactive to light having a wavelength within the range between X and
Y to
experience a property change (e.g., a colorimetric ally detectable property
change) at a
threshold of cumulative exposure to light received within the range between X
and Y
related to the change in the photosensitive property of the polypeptide. The
container
110 would then be delivered to and collected from a recipient within the
manufacturing or distribution chain of the pharmaceutical product, for
example, but
not limited to, a warehouse, a packaging or filling plant, a distributor, a
testing lab, a
pharmacy, a dispensary, a clinic, a hospital or other healthcare facility, a
healthcare
provider, or a patient. The layer of photosensitive material may be examined
to
determine if the photosensitive material has experienced the property change,
and the
container may be identified as mishandled if the photosensitive material has
experienced the property change.
[0096] For example, the product 100 may be packaged within a box or
wrapper
that is intended to be removed only when the material in the container 110 is
to be
administered to the patient. In such a setting, the box or packaging may be
used to
limit the light exposure of the material in the container 110. If, however,
the layer
140 has undergone a colorimetrically detectable property change, this may be
suggestive of the fact that the product 100 has been prematurely taken out of
the
protective packaging in contravention of the express instructions.
- 28 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
[0097] While all of the embodiments illustrated in Figs. 1-23 relate to
products
where in the photosensitive layer is disposed on the exterior surface of the
container,
it is also possible to dispose the photosensitive layer within the interior
chamber of
the container, as mentioned in the introductory paragraphs. According to the
embodiment illustrated in Fig. 24, a pharmaceutical product 300 is
illustrated, the
product 300 including a container 310 having an interior chamber 314, the
container
310 constructed from a material that is photo-resistive. As noted above, the
material
may filter or block light of one or more ranges of wavelengths. As such, the
exposure
of a material disposed in the chamber 314 of the container 310 may be
different than
if the material was exposed to environmental conditions.
[0098] The product may also include a layer 340 of photosensitive
material
disposed in the interior chamber 314 of the container 310. As illustrated, the
layer
340 may be disposed on monitoring card 360 including a substrate 362 having a
first
side 364 and a second side 366. According to other embodiments, the material
may
be applied to both sides 364, 366 of the substrate 362. As illustrated, the
substrate
362 may be made of a rigid material, such as a rigid cardstock or plastic,
which will
permit the substrate 362 to remain upright within the chamber 314 when the
container
310 is placed on a surface, thereby facilitating the readability of the layer
340.
According to other embodiments, the surface 366 may have an adhesive applied
thereto, and the card 360 may be attached at a particular location within the
chamber
314.
[0099] As was the case with the layer 140, the material of the layer 340
may be a
photosensitive material reactive to light having a wavelength within the range
between X and Y to experience a property change at a threshold of cumulative
exposure to light received within the range between X and Y related to the
change in a
photosensitive property of a polypeptide. While the polypeptide or other
material
may be disposed in the chamber 314 with the card 360, it may also be the case
that the
card 360 is disposed within the chamber 314 without the material also being
present
in the chamber 314.
[00100] For example, consider the situation where the photo-resistivity of
the
material used for the container 310 is unknown or not well known, or at least
where
the photo-resistivity of the material used for the container 310 is unknown or
not well
known relative to the photo-sensitivity of the material that will be disposed
within the
- 29 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
chamber 314 of the container 310. In such a setting, the card 360 may be
disposed in
the chamber 314 of the container 310, and then the container 310 may be
exposed to a
light source of interest. The card 360, and in particular the layer 340, may
be
monitored for changes as to the property change (e.g., a colorimetrically
detectable
property change) while exposed to the light. The correspondence of the
property
change of the layer 340 may be collected, for example, at a series of time
increments
over a test period.
[00101] The collected data may be used for a variety of uses. For
example, data
collected may be compared with data collected with the instance of the card
360
disposed in another container 310 made of another material for which the photo-
resistivity is known (perhaps, at least relative to the photo-sensitivity of
the material
that will be disposed in the chamber 314 is known). Based on the comparison, a
determination may be made either to use the material for the container 310 or
not to
use the material for the container 310.
[00102] Fig. 25 illustrates an example where a system similar to that
illustrated in
Fig. 24 was used to measure light exposure (as reflected in colorimeter
readings) for a
variety of materials: glass, polycarbonate, and Teflon. As illustrated, the
lowest plot
of data points corresponds to the card 360 disposed in a glass vial, while the
upper
two plots correspond to the cards disposed in containers of polycarbonate and
Teflon.
As a consequence, it appears that greater light exposure occurs in glass vials
rather
than in containers of polycarbonate or Teflon.
[00103] This conclusion is further supported by the graph of Fig. 30,
wherein the
UV intensity measurements from inside and outside of teflon, polycarbonate
("PC")
and glass containers have been plotted. In this testing, a 3UV-38 3UV Lamp
from
UVP LLC of Upland, California was used, and the measurements inside and
outside
the container were taken using PMA 2110 UVA detectors from Solar Light Co. of
Glenside, Pennsylvania. The inside and outside detectors were placed
approximately
47 to 48 cm from the lamp, with the inside detector placed inside the
container with
the cap or lid on. The smallest difference between the values measured inside
and
outside the container occurred with regard to the glass container, suggesting
that less
light is absorbed by the glass walls than by the walls of other containers,
such that
greater light exposure occurs within the glass vials as opposed to those made
of other
materials.
- 30 -
CA 02840637 2013-12-27
WO 2013/033600
PCT/US2012/053450
[00104] As another example of a use of the product illustrated in Fig.
24, the
material may be disposed in the chamber 314 at the same time as the card 360,
and
both the property change of the material in the layer 340 and the
photosensitive
property of the material in the chamber 314 may be monitored and collected,
for
example, at a series of time increments over a test period. The data collected
for the
material in the layer 340 and for the material in the chamber 314 may be
compared,
and a correlation or relationship may be defined. Based on the defined
relationship, a
scale may be determined to permit a level of light exposure for the material
in the
chamber 314 to be identified with changes in the property of the material in
the layer
340.
[00105] Still another system and use for the present technology may be
described
according to the schematic of a facility, such as a manufacturing facility for
example,
according to Fig. 26, and may involve a system and method for analyzing light
exposure of a pharmaceutical product passing through the facility. This system
and
use may be particularly helpful when and where it is not possible to limit the
light
exposure of the product passing through the facility because of regulatory
directives,
for example. For instance, European Council Directive 89/654/ECC (of Nov. 30,
1989) requires that workplaces must, as far as possible, receive sufficient
natural light
and be equipped with artificial lighting adequate for protections of workers'
safety
and health. As such, it may not be possible to limit light exposure to the
product, and
yet comply with the light requirements of the Council Directive relative to
the
workers' safety and health.
[00106] In particular, the manufacturing facility or plant 400 may
include at least
one space (according to the illustrated embodiment of Fig. 26, a plurality of
spaces)
through which a pharmaceutical product according to the present disclosure may
pass.
These spaces may be physically separated from each other through the presence
of
walls or other barriers; in other instances, the spaces may be demarked from
an
organizational standpoint, but there may no physical barrier demarking one
space,
area or region from the other. The product may include a label as according to
any of
the preceding embodiments. However, in addition to such a label affixed to the
pharmaceutical product, additional photosensitive devices 402, or
phototrackers, may
be disposed about the plant 400 once a path P along which the product passes
through
the plant has been identified. The information regarding light exposure
developed
-31 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
through the use of the devices 402 may be used in conjunction with information
from
the labels associated with the product, or may be used separately instead.
[00107] As illustrated, the plant 400 includes a first space 410, wherein
the
pharmaceutical product is prepared. For example, a polypeptide that represents
the
active component in the product may be combined within the space 410 with a
media
in which the polypeptide will be stored and administered to a patient. The
polypeptide may have a photosensitive property that changes based on at least
cumulative exposure to light having a wavelength within the range between X
and Y.
The active component and the media may be disposed in a container such as that
illustrated in Fig. 2 at this time. The active component and the media may
then pass
from the space 410 into space 412, wherein the active component and media is
filled
into a smaller containers, such as those illustrated in Fig. 1.
[00108] The filled containers may then pass into a transfer space 414
before
moving to an inspection space 416 or a storage (or warehouse) space 418. As
indicated by the arrows along the flow path, P, product may flow from the
filling
space 410 directly through the transfer space 414 to the inspection space 416,
or may
detour through the storage space 418. Additionally, before moving along the
path P
from the inspection space 416, the product may be returned to the warehouse
space
418.
[00109] Once the containers have passed inspection within the inspection
space
416, the product may be assembled with other items to define a system or a kit
within
assembly space 420. As illustrated, the product may arrive at assembly space
420
either from the inspection space 416 or the warehouse space 418. The product
may be
combined with a syringe, such as is illustrated in Figs. 19 or 21, to define a
system or
to be packaged as part of a kit, in an injection kit clam shell, for example.
Alternatively, the product may be assembled as part of system in the form of a
medical device, such as an autoinjector (as illustrated in Figs. 22 and 23),
microinfuser, or the like. Once assembly is complete, the system or kit may
pass
through a loading dock 422, prior to removal from the plant 400.
[00110] It will be observed that devices 402 are disposed along the path
P that the
product takes through the spaces 410, 412, 414, 416, 418, 420, 422. The
devices 402
may include a layer of photosensitive material reactive to light having a
wavelength
within the range between X and Y to experience a property change (e.g., a
- 32 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
colorimetrically detectable property change) at a threshold of cumulative
exposure to
light received within the range between X and Y. In many cases, such as in
spaces
410, 412, 418, 420, 422, the devices 402 are disposed on either side of the
path P. In
certain spaces, such as spaces 414, 416, the devices 402 are disposed only on
one side
of the path P. The number of devices may differ between spaces, such that
spaces
414, 416 have only a single device, while spaces 410, 412 have two devices,
space
420 has three devices, space 422 has four devices, and space 418 has six
devices. The
devices 402 may be attached through the use of an adhesive backing, for
example, to
particular structures, equipment or machinery disposed within the spaces 410-
422,
such as mixing tanks or syringe/cartridge filling machines for example.
[00111] It will be recognized that the placement and number of devices
used may
be dictated by a number of factors. For example, in certain rooms, certain
equipment
or other environmental light sources (lamps, overhead lighting, windows, etc.)
may be
disposed primarily to one side of the path or the other. Additionally, it may
be more
desirable to place additional devices in a larger room, or a room in which the
path has
multiple entry and exit points, or a more complex path. For example, space 418
might
qualify under all of these criteria for the inclusion of more devices 402 that
in other
spaces.
[00112] However, there is at least one significant advantage of using the
phototrackers or photosensitive devices along the path P. Disposing the
devices 402
along the path P permits identification and isolation of points along the path
P at
which light of a particular wavelength or intensity impacts the product.
Through the
use of the devices 402, this identification and isolation may be performed in
real time.
For example, the photosensitive devices 402 may be read for evidence of a
property
change using an optical sensing device proximate to the device 402, the
optical
sensing device coupled to a computing device that displays an indication of
light
exposure experienced by the at least one device 402. As a consequence, changes
may
be made to the equipment (e.g., using different mixing tanks, syringe fillers,
etc.),
environment surrounding the path P (e.g., erecting walls, screens, etc.), the
product
(e.g., changing the container used), or to the path P (e.g., modifying the
path P
between or within the various spaces 410, 412, 414, 416, 418, 420, 422) to
minimize
or eliminate light exposure at points along the path P.
-33 -
CA 02840637 2013-12-27
WO 2013/033600 PCT/US2012/053450
[00113] That is, a label affixed to a product may be used to determine
the
cumulative exposure of the individual product, and as a consequence whether
the
product should be used or discarded. However, the label will not provide a
history of
where the product has received the exposure that has been recorded through the
use of
the label on the product. Even if a label attached to a product were to be
inspected for
cumulative light exposure at various points along the path P, using the light
exposure
information obtained in this fashion may still make identification and
isolation of a
localized spaces, areas or regions of increased light exposure or light
exposure of
particular wavelengths difficult. This is particular true given that certain
spaces, such
as space 420, may have product passing through it after having passed through
any of
a number of preceding spaces, because of the manner in which product may be
transported between the transfer, inspection and warehouse spaces 414, 416,
418.
[00114] By contrast, through the use of the phototrackers or
photosensitive
devices 402 disposed along the path P, the light exposure of the product
within the
plant 400 may be analyzed separately from the product itself, and considered
without
having to account for the movement of the product along the path P. For
example,
readings obtained from the devices 402 disposed in the space 418 suggest that
product
moving along the path P are exposed to considerable amounts of light having a
wavelength or wavelengths of interest relative to that product in the portion
of the
space where the product is stored between the inspection and assembly spaces
416,
420. Additional devices 402 may then be disposed in the space 418 to further
identify
the source of the light, or along alternative paths within the space 418 to
determine the
viability of these alternative paths for the product prior to modification of
the path P
within the space 418. If the labels were used only on the product, the
identification of
such sources or alternative paths may only occur through the exposure of
additional
amounts of light exposure that may result in the degradation of the product
and the
need for its disposal.
[00115] This is not to suggest that information from photosensitive
labels might
not play a role in conjunction with the photosensitive devices 402, but it is
not
necessary to attempt to back track the exact travel of a particular instance
of the
product along the path P. For instance, while the devices 402 may be used to
identify
the level of exposure of product to light along the path P, the spacing of the
devices
402 may be such that it is not possible to capture every possible source of
undesirable
- 34 -
CA 02840637 2013-12-27
WO 2013/033600
PCT/US2012/053450
light exposure. Alternatively, conditions along the path P may change relative
to a
previously adequate distribution of devices 402 that makes the previous
distribution
inadequate to identify and isolate undesirable sources of light exposure. To
this end,
the label associated with the product may be inspected, and the results of
that
inspection compared against the readings determined at various points along
the path
P to determine, for example, if the distribution needs to be altered relative
to
placement or number of devices 402 used.
[00116] It will also be recognized that many of the comments made above
will be
of general usefulness in selecting the material used in the devices 402, as
well as the
shape and placement of the devices 402. For example, while the devices 402 may
provide a real time reading of the cumulative light exposure in a given
portion of the
plant, the devices 402 may not be monitored continuously. As a consequence, it
may
be desirable to select a less sensitive material for use in the devices 402,
as it is
intended that the device 402 be exposed to environmental lighting conditions
for long
periods of time between being monitored. Fig. 27 illustrates the range and
nature of
color change possible for five different sensitivities of a material that may
be used in
the devices 402 when exposed to varying amounts of visible light, the color
change
being measured according to the L*a*b index. It will also be recognized that
the use
of this system and method is not limited to a production facility, but might
be used in
other buildings or structures as well, such as a healthcare facility, testing
lab, hospital
or clinic.
[00117] As will be recognized, the devices according to the present
disclosure
may have one or more advantages relative to conventional technology, any one
or
more of which may be present in a particular embodiment in accordance with the
features of the present disclosure included in that embodiment. Other
advantages not
specifically listed herein may also be recognized as well.
- 35 -