Note: Descriptions are shown in the official language in which they were submitted.
CA 02841384 2014-01-09
PROCESS FOR PREPARING (METH)ACRYLIC ESTERS OF POLYOLS
Field of the Invention
The invention relates to a new process for preparing
(meth)acrylic esters of polyhydric alcohols (polyols).
Prior Art
(Meth)acrylic esters of polyhydric alcohols, more
particularly from the group of the dihydric to hexahydric
aliphatic saturated alcohols, and their oxalkylation
products, are finding increasing importance as high-
reactivity ingredients in radiation-curing systems. Such
polyfunctional (meth)acrylic esters can be used, for
example, as coatings raw materials for electron beam
curing or as an ingredient of UV-curing printing inks or
corresponding coating materials, filling compositions,
molding compositions or casting compositions, and also in
adhesives, more particularly those which cure
anaerobically. Their preparation, however, is not without
problems. The requirement in particular is for colorless
products with a low acid number and high storage
stability, which also have virtually no inherent odor.
Distillative purification of the (meth)acrylic esters of
the type in question here is generally ruled out by their
high molecular weight and their high reactivity. The
products are therefore to be obtained directly, as very
1
CA 02841384 2014-01-09
largely colorless reaction products, from the
esterification. Implementing the esterification reaction
requires the accompanying use of high-activity
inhibitors, which in turn do not trigger any unwanted
secondary reactions, such as discolorations, for example.
The predominant industrial production process for the
direct esterification of (meth)acrylic acid with hydroxy
compounds is based on the use of volatile organic
solvents as a liquid reaction medium, also known as
solvent operation. Suitable volatile organic solvents
are, for example, toluene, cyclohexane, methylcyclohexane
or n-heptane, which, moreover, are utilized as an
azeotropic entrainer for the continuous removal of the
resultant water of reaction from the reaction mixture and
are removed by distillation after the end of reaction.
Corresponding process descriptions are found in
US-6,838,515 and EP-A-127,766, for example. In spite of
the distillative removal of the volatile organic solvents
after the end of reaction, however, monomer and oligomer
(meth)acrylates prepared in this way always have residual
solvent traces, the amounts of which vary in the range of
50 - 10 000 ppm.
Since the use of organic solvents is being regulated to
increasing degrees, on environmental protection
considerations, the importance is gaining of an
alternative production process, in which the direct
esterification of (meth)acrylic acid with hydroxy
compounds is carried out in (meth)acrylic acid itself as
2
CA 02841384 2014-01-09
the liquid reaction medium, without use of volatile
organic solvents; this process is also known as solvent-
free operation. The water of reaction formed is removed
from the reaction mixture by distillation, in the form of
a water/(meth)acrylic acid mixture. Corresponding
processes are described in EP-B-449 919
and
EP-B-1 204 472, for example. In this way, completely
solvent-free monomer and oligomer (meth)acrylates can be
prepared, and are used in applications including those
where traces of volatile organic solvents may produce an
unwanted inherent odor, such as in the packaging sector,
for example.
Both production technologies have been subject to
continuous ongoing development over the course of the
years, with the focal points being on optimizing the
throughput and minimizing the use of raw materials. The
resulting purity of the monomer and oligomer
(meth)acrylates prepared in this way, in contrast, has
been paid no great attention to date. The purity, in the
relevant technical literature, is generally understood to
be the total amount of fully and partially
(meth)acrylated species of substance in the end product,
and is calculated from the residual amount of unreacted
hydroxy compounds and situated, accordingly, at usually
more than 97% (GC area-percent).
Strictly speaking, however, this widespread definition of
the concept of purity says nothing about the amount that
is actually present of the desired, fully (meth)acrylated
3
CA 02841384 2014-01-09
species of substance in the end product, and is therefore
unsatisfactory. In the context of the present invention,
therefore, "purity" means the amount of fully
(meth)acrylated species of substance in (meth)acrylic
esters. Fully (meth)acrylated species of substance are
understood to be those in which all of the OH groups of
the alcohol component of the (meth)acrylic ester are
present in esterified form.
This definition of the term purity, which applies
strictly in the context of the present invention, is
particularly important in view in particular of the fact
that, for polyfunctional monomer and oligomer
(meth)acrylates, the identification and classification of
substances under REACH (Registration, Evaluation,
Authorization and Restriction of Chemicals) is
exclusively via CAS numbers, with corresponding purity
requirements related to the respective fully
(meth)acrylated species of substance (see ECHA
publication "Guidance for Identification and Naming of
Substances under REACH - June 2007"). For instance, for
what REACH calls "mono-constituent substances", which
include, for example, 1,6-hexanediol diacrylate,
tripropylene glycol diacrylate and trimethylolpropane
triacrylate, for example, purities of at least 80% -
based on the desired, fully acrylated species of
substance - in the end product are required.
From the technical literature it is known that, for
example, polyfunctional monomer acrylates such as 1,6-
hexanediol diacrylate, tripropylene glycol diacrylate,
trimethylolpropane triacrylate and pentaerythritol
4
CA 02841384 2014-01-09
tetraacrylate constitute mixtures of species of substance
having different degrees of acrylation with other by-
products, in which the desired, fully acrylated species
of substance is only part of the mixture (cf. R.H. Hall,
F.P.B. Van Der Maeden, A.C.C.M. Willemsen, Spec. Chem.,
7, 56-64 (1987) and M. Matsunaga, Y.
Matsushima,
H. Ohtani, S. Tsuge, Anal. Sc, 17, 1295-1299 (2001)).
Even more complex compositions are a characteristic of
those polyfunctional monomer and oligomer (meth)acrylates
that are based on polyhydroxy compounds alkoxylated with
ethylene oxide and/or propylene oxide. As an inevitable
concomitant of the process, such products always have a
distribution curve with different degrees of alkoxylation
around a middle value, and so the number of possible
species of substance (meth)acrylated fully and partially
is not only dependent on the number of available hydroxyl
groups but is also connected, on a multiplicative basis,
with the number of differently alkoxylated polyhydroxy
species.
Corresponding investigations into the composition of such
polyfunctional, alkoxylated monomer and oligomer
acrylates, such as ethoxylated 1,6-hexanediol diacrylate,
propoxylated neopentylglycol diacrylate, ethoxylated
trimethylolpropane triacrylate, propoxylated glycerol
triacrylate and polypropylene glycol diacrylate, are
found in T. Marek, U. Grollman, DIC Technical Review, No.
5, 85-93 (1999), M. Matsunaga, Y. Matsushima, H. Yokoi,
H. Ohtani, S. Tsuge, Anal. Sci., 18, 277-281 (2002) and
5
CA 02841384 2014-01-09
S.J. Yoo, G.V. Pace, B.K. Khoo, J. Lech, T.G. Hartman,
RadTech Report, May/June, 60-68 (2004).
The acidically catalyzed, direct esterification of
(meth)acrylic acid with monohydroxy compounds can be
considered in a first approximation still to be a simple
phase equilibrium reaction, in which the phase
equilibrium can be shifted almost entirely to the product
side by the continuous removal of the water of reaction
from the reaction mixture, via the appropriate choice of
temperature, pressure and reaction time.
X1 X1
P, t, ,C, 1
R1-0H + 1-12c' C- H _____________________ C R + H20
ti
OH 0
X1=H,CH3
The end product generally contains the desired, fully
(meth)acrylated species of substance in purities of
greater than 97%.
For polyhydroxy compounds, in contrast, the profile of
the acidically catalyzed, direct esterification of
(meth)acrylic acid is substantially more complex, as
described in L.-D. Shiau, T.-R. Ling, D.-S. Tseng, Chem.
Eng. Comm., 179, 133-148 (2000), since different phase
equilibrium reactions, coupled with one another, take
place alongside one another, and may also result,
depending on reaction regime, in substantial amounts of
6
CA 02841384 2014-01-09
partially (meth)acrylated monomer or
oligomer
(meth)acrylates in the end product.
X'
T, p, t, H+ 2-0H
õC õO __
HO-R2-0H H2C-- ?" C R + H20
OH 0
X1 X1 X1
2,0H T, p, t, H+ õC,õ õ0õ 2-0,,
+ H20
H2c" R + H2C' - ______ H2C' R CH2
0 OH 0 0
X1= H, CH3
Furthermore, as set out by
R.H. Hall,
F.P.B. Van Der Maeden, A.C.C.M. Willemsen, Spec. Chem.,
7, 56-64 (1987), these partially (meth)acrylated monomer
or oligomer (meth)acrylates - and also unreacted
(meth)acrylic acid and unreacted polyhydroxy compounds -
may enter into secondary reactions such as Michael
additions with the fully (meth)acrylated species of
substance, and also with one another.
X1 XI
R` ,,OH
H2C-- C CH2 ""C
H H
0 0
X1 H, CH3
Michael adduct mono(meth)acrylate/(meth)acrylic acid
7
CA 02841384 2014-01-09
X1 X1 X1
CH2 C CH2
R ti
0 0 0
X1 = H, CH3
Michael adduct di (meth) acrylate/ (meth) acrylic acid
X1 X1
H2C". Rc= CH2
0 0
XI ===-- H, CH3
Michael adduct mono (meth) acrylate/mono (meth) acrylate
X1 X1
0, 2,0õCH, -0, 2,0õC,
H2C" R CH2 R CH2
0 0 0
= H, CH3
Michael adduct di (meth) acrylate/mono (meth) acrylate
HOõ 2,0H
R2 C -'GH2 R
0
X1 H, CH3
Michael adduct mono (meth) acrylate/diol
X1 X1
2-0õCH,
H2C,' R CH2 R2 OH
0 0
X1 = H, CH3
Michael adduct di (meth) acrylate/diol
8
CA 02841384 2014-01-09
The presence of partially (meth)acrylated monomer or
oligomer (meth)acrylates and of Michael adducts in the
end product leads inevitably to a considerable loss of
purity relative to the desired, fully (meth)acrylated
species of substance.
Although many known monomer acrylates such as 1,6-
hexanediol diacrylate, tripropylene glycol diacrylate,
trimethylolpropane triacrylate or pentaerythritol tetra-
acrylate contain more than 97% of acrylated species of
substance, the amount therein of the desired, fully
acrylated species of substance is nevertheless often
significantly below 80%. The unwanted by-products such as
Michael adducts or partially acrylated species of
substance in these products do not only cause a reduced
purity, but may also considerably influence the profile
of properties. For instance, partially (meth)acrylated
monomer and oligomer (meth)acrylates lead to higher
viscosities because of the formation of hydrogen bonds
via their free hydroxyl groups, and also to detractions
from the reactivity, owing to an absence of double bonds.
Michael adducts, on account of their high molecular
weight, likewise produce an increase in viscosity and
lower the density of double bonds, since double bonds are
consumed when the adducts are formed.
Description of the Invention
9
CA 02841384 2014-01-09
An object of the present invention, in light of the
circumstances outlined above, was to provide a new
process for preparing solvent-free (meth)acrylic esters
of polyhydric alcohols; the (meth)acrylic esters made
available by the new process ought to fulfill the
condition that at least 80 mol% of the OH groups present
in the polyols used are in esterified form, corresponding
to a purity of at least 80% in the sense of the
definition set out above.
Surprisingly it has been found that the addition of
(meth)acrylic acid in portions to polyols, with
subsequent or simultaneous removal of the water of
reaction formed in the esterification, results in a
purity of at least 80%, relative to the desired, fully
acrylated species of substance in the end product, it
being necessary for specific parameters to be observed.
The present invention provides a process for preparing
(meth)acrylic esters of polyols, the amount in these
esters of species in which all of the OH groups of the
polyols are esterified being 80 mol% or more, by reaction
of polyols with acrylic acid and/or methacrylic acid in
the presence of acidic esterification catalysts and in
the presence of polymerization inhibitors, operating with
reaction mixtures which are liquid at reaction
temperature and are free from nonreacting solvents and/or
azeotropic entrainers, the resultant water of
condensation being stripped from the gas phase of the
reaction space, characterized in that (meth)acrylic acid
CA 02841384 2014-01-09
is metered in in three or more portions, with the
following provisos:
= the amount of the individual (meth)acrylic acid
portions is set in each case in the range from 5 to
40 mol%, based on the entirety of the OH groups of
the polyols used,
= the number of (meth)acrylic acid portions,
multiplied by the amount of (meth)acrylic acid
portions used (in mol%), produces a figure of at
least 100 (mol%),
= the reaction temperature is set to a level in the
range from 70 to 150 C, and
= the water formed in the reaction is removed from the
reaction space under reduced pressure, the reduced
pressure being 600 hPa or less.
By "nonreacting solvents and/or azeotropic entrainers"
are meant those solvents and azeotropic entrainers,
respectively, which are chemically inert under the
reaction conditions of the process of the invention. The
above phrase "operating with reaction mixtures which are
liquid at reaction temperature and which are free from
nonreacting solvents and/or azeotropic entrainers" means,
therefore, that such solvents or azeotropic entrainers
are not used in the process of the invention.
As stated, the metering of (meth)acrylic acid takes place
in three or more portions. In this context it is also
possible to refer to successive process stages. The first
metered addition of (meth)acrylic acid is in this sense
11
CA 02841384 2014-01-09
the first process stage, the second metered addition of
(meth)acrylic acid then being the second process stage,
and so on.
In one embodiment, after addition of each (meth)acrylic
acid portion, a vacuum gradient is applied such that the
reaction mixture boils continuously.
In one embodiment, the water formed during the reaction
is removed continuously from the reaction space.
In one embodiment, each subsequent (meth)acrylic acid
portion is metered in only when the acid number of the
reaction mixture has dropped below a level of 100 mg
KOH/g. It is particularly preferred in this case for the
acid number of the reaction mixture in the subsequent
process stage to be equal to or higher than that in the
preceding process stage. In the last process stage, the
acid number is preferably set lower than the acid number
of the penultimate process stage; this has the advantage
that it shortens the downstream alkaline washing stages,
particularly since the acid number in the final end
product is adjusted preferably to a level of below 1 mg
KOH/g, so that the end product is stable in storage and
so that autocatalytic acidic hydrolysis of the acrylate
and an associated loss in product properties are avoided.
In one embodiment, the (meth)acrylic acid is metered in
in 4 to 16 portions. In this case it is particularly
preferred to meter the (meth)acrylic acid in in an equal
12
CA 02841384 2014-01-09
amount in each case, more particularly in amounts in each
case in the range from 5 to 25 mol% and very preferably
in the range from 10 to 20 mol%.
In one particularly preferred embodiment, the total
amount of the (meth)acrylic acid portions metered in is
105 to 160 mol%, based on the entirety of the OH groups
of the polyols used.
Polyols in the context of the present specification are
organic substances having two or more OH groups per
molecule. The individual OH groups of the polyols may, in
each case independently of one another, be primary,
secondary or tertiary OH groups. Examples of suitable
polyols include aliphatic, cycloaliphatic or aromatic
polyhydroxy compounds. Examples of suitable polyols are
for instance the following: glycerol, trimethylolpropane,
tripropylene glycol, dipropylene glycol, 1,4-
cyclohexanedimethanol, tricyclodecanedimethanol, neo-
pentylglycol, 3-methyl-1,5-pentanediol, 1,6-hexanediol,
1,10-decanediol, polyethylene glycols with different
molecular weights, polypropylene glycols with different
molecular weights, pentaerythritol, dipentaerythritol,
ditrimethylolpropane, diglycerol, triglycerol, poly-
glycerols with different molecular weights, and
correspondingly ethoxylated and/or
propoxylated
derivatives.
Besides the OH groups, the polyols may optionally also
contain further functional groups, more particularly
13
CA 02841384 2014-01-09
those which are inert under the reaction conditions,
examples being polyether polyols or polyurethane polyols.
Esterification catalysts used are strong organic or
inorganic acids having a pKa of 2.5 or less. Typical
examples of strong organic acids are, for instance,
methanesulfonic acid and p-toluenesulfonic acid; examples
of strong inorganic acids are, for instance, sulfuric
acid and phosphoric acid. It is also possible, though, to
use strongly acidic ion exchanger resins and zeolites.
As suitable polymerization inhibitors it is possible for
example to use quinones, alkylphenols, alkoxyphenols and
phenothiazines, with 4-methoxyphenol being particularly
preferred. Further examples of suitable polymerization
inhibitors can be found in WO-A-2009/106550.
As already stated, the reaction temperature is in the
range from 70 to 150 C. It is preferred to operate in the
range from 80 to 120 C.
As already stated, the water formed during the reaction
is removed under reduced pressure, by which is meant a
pressure of 600 hPa bar or less, from the reaction space.
It is preferred to operate at pressures of 400 hPa bar or
less.
A suitable vacuum gradient is set to remove the water of
reaction, formed in the esterification, rapidly and
effectively. The water of reaction in this case is
14
CA 02841384 2014-01-09
distilled off as a water/(meth)acrylic acid mixture, with
operation taking place preferably in reactors equipped
with dephlegmators or distillation columns. Depending on
the
separation performance and the ref lux ratio,
dephlegmators or distillation columns strip off a vapor
phase enriched in the lower-boiling water, while the
liquid ref lux is enriched in the higher-boiling
(meth)acrylic acid. In this way, the evaporation losses
of (meth)acrylic acid that occur in each case are
minimized, and the use of raw materials is optimized.
In one preferred embodiment, the polyols, the
polymerization inhibitor and the acidic catalyst are
charged to a reactor, through which air is passed, and
then the first portion of (meth)acrylic acid is metered
in, the total amount of (meth)acrylic acid added being
set preferably at a level in the range of 105-160 mol%.
After reduction of the pressure and heating to the
reaction temperature, a vacuum gradient (see figure 1) is
applied, and so the reaction mixture is held continuously
at boiling over a fixed time period, in order to remove
the water of reaction as far as possible completely from
the reaction mixture. Each subsequent portion of
(meth)acrylic acid is added - and hence the next process
stage initiated - preferably only when the acid number of
the reaction mixture has dropped to a level of 100 mg
KOH/g. Before the last process stage is initiated, the
acid number ought preferably to have dropped below a
level of 50 mg KOH/g.
15
CA 02841384 2014-01-09
The procedure outlined for the first process stage is
then repeated for the further process stages as well. In
one of these subsequent operating stages, optionally, not
only further acidic catalyst but also polymerization
inhibitor can be added.
The reaction mixture can be worked up, for example, by
neutralization, washing and filtration in accordance with
all of the methods that are known in this context to the
skilled person.
16
CA 02841384 2014-01-09
Examples
1. Substances used
Acrylic acid - BASF SE (CAS number 79-10-7, molecular
weight: 72.06 g/mol)
Tripropylene glycol - LyondellBasell (CAS number
24800-44-0, molecular weight: 192.26 g/mol)
Methanesulfonic acid (70% by weight) - BASF SE (CAS
number 75-75-2, molecular weight: 96.10 g/mol)
Sulfuric acid (95% by weight) - Quaron France (CAS number
7664-93-9, molecular weight: 98.08 g/mol)
Phosphinic acid (50% by weight) - Minakem S.A.S. (CAS
number 6303-21-5, molecular weight: 66.00 g/mol)
4-Methoxyphenol - Acros Chimica (CAS number 150-76-5,
molecular weight: 124.14 g/mol)
Sodium carbonate, anhydrous - Quaron France (CAS number
497-19-8, molecular weight: 105.99 g/mol)
Sodium carbonate decahydrate - Disachim S.A. (CAS number
6132-02-1, molecular weight: 286.14 g/mol)
Sodium sulfate, anhydrous - Brenntag N.V. (CAS number
7757-82-6, molecular weight: 142.04 g/mol)
Dicalite 4158 (natural
sodium/potassium/aluminum
silicate) - Dicalite Europe N.V. (CAS number 93763-70-3)
2. Measurement and test methods
= Acid number: standard NF EN ISO 660
= Water content: standard ISO 4317
= APHA color: standard ISO 6271
17
CA 02841384 2014-01-09
= Viscosity: standard ISO 2555
= Gas chromatography:
Gas chromatograph: 430-GC from Varian
Column: CP-Sil 8 CB (10 m length, 0.15 mm internal
diameter, film thickness 0.12 pm) from Agilent
Technologies
Carrier gas: helium
Injection volume: 5.0 pl
Split injection: 1:100
Detector: flame ionization
Injector temperature: 300 C
Detector temperature: 350 C
Temperature program: 120 C for 2 minutes, heating
120-300 C at 20 C per minute/300 C for 5 minutes
Sample preparation: none (direct injection)
Peak assignment, gas chromatography:
Indicated below are the retention times of certain
substances, together with their structure and the
chemical identification:
Retention time = 3.6 minutes
CH3 CH3
OH
0 CH3
Tripropylene glycol monoacrylate
18
CA 02841384 2014-01-09
Retention time = 5.2 minutes
CH3 CH3 0
H2C 0 0
0 CH3
Tripropylene glycol diacrylate
Retention time = 6.0 minutes
CH3 CH3
OH
0 0 CH3
Michael adduct tripropylene glycol monoacrylate/acrylic
acid
Retention time = 7.0 minutes
OH CH3 0 0
H2C 0 0 0
0 CH3
Michael adduct tripropylene glycol diacrylate/acrylic
acid
19
CA 02841384 2014-01-09
Retention time = 9.3 minutes
0 CH3 CH3 CH3
OH
0
CH3 CH3 0 CH3
Michael adduct tripropylene glycol monoacrylate/tri-
propylene glycol monoacrylate
Retention time = 10.0 minutes
0 CH CH3 CH3 0
0
CH3 CH3 0 CH3
Michael adduct tripropylene glycol diacrylate/tri-
propylene glycol monoacrylate
3. Working examples
Example 1 (inventive)
A 25 m3 reactor was charged at a reactor temperature of
45 C with 10 400 kg (54 093 mol) of tripropylene glycol,
with a constant stream of air being passed through the
reactor, and then a vacuum of 300 hPa was set.
In the first stage, in succession and with stirring,
15 kg (121 mol) of 4-methoxyphenol, 1197 kg (16 611 mol)
of acrylic acid, 188 kg (1424 mol) of 50% strength by
weight phosphinic acid and 188 kg (1821 mol) of 95%
CA 02841384 2014-01-09
strength by weight sulfuric acid were added. The
temperature of the reaction mixture was subsequently
raised to 90 C. Under these temperature/pressure
conditions, the direct esterification commenced, with a
mixture of water of reaction and acrylic acid being
removed continuously by distillation in line with the
composition of the boiling curve of the respective phase
equilibrium state. After 1 hour, the pressure was lowered
in steps, with the reaction mixture at a constant
temperature, initially to 150 hPa in 0.25 h and finally
to 100 hPa in 0.75 h. The pressure of 100 hPa was
maintained until the acid number of the reaction mixture
had dropped below 40 mg KOH/kg. The pressure was
subsequently raised again to 300 hPa.
In the second stage, 1197 kg (16 611 mol) of acrylic acid
were added, the pressure was held at 300 hPa for 0.75 h,
then lowered to 130 hPa over 0.15 h and finally run down
to 70 hPa over 1 h. The pressure of 70 hPa was maintained
until the acid number of the reaction mixture had dropped
below 50 mg KOH/kg. The pressure was subsequently raised
again to 300 hPa.
In the third stage, 1197 kg (16 611 mol) of acrylic acid
were added, the pressure was held at 300 hPa for 0.85 h,
then lowered to 200 hPa over 0.15 h and finally run down
to 60 hPa over 1 h. The pressure of 60 hPa was maintained
until the acid number of the reaction mixture had dropped
below 60 mg KOH/kg. The pressure was subsequently raised
again to 300 hPa.
21
CA 02841384 2014-01-09
In the fourth stage, 1197 kg (16 611 mol) of acrylic acid
were added, the pressure was held at 300 hPa for 0.85 h,
then lowered to 120 hPa over 0.15 h and finally run down
to 60 hPa over 1 h. The pressure of 60 hPa was maintained
until the acid number of the reaction mixture had dropped
below 60 mg KOH/kg. The pressure was subsequently raised
again to 300 hPa.
In the fifth stage, 1197 kg (16 611 mol) of acrylic acid
were added, the pressure was held at 300 hPa for 0.85 h,
then lowered to 120 hPa over 0.15 h and finally run down
to 90 hPa over 1 h. The pressure of 90 hPa was maintained
until the acid number of the reaction mixture had dropped
below 70 mg KOH/kg. The pressure was subsequently raised
again to 300 hPa.
In the sixth stage, 1197 kg (16 611 mol) of acrylic acid
and also 126 kg (918 mol) 70% strength by weight
methanesulfonic acid were added, the pressure was held at
300 hPa for 0.5 h, then lowered to 250 hPa over 0.35 h,
to 140 hPa over 0.15 h and finally run down to 80 hPa
over 1.5 h. The pressure of 80 hPa was maintained until
the acid number of the reaction mixture had dropped below
80 mg KOH/kg. The pressure was subsequently raised again
to 300 hPa.
In the seventh stage, 1197 kg (16 611 mol) of acrylic
acid were added, the pressure was held at 300 hPa for
0.1 h, then lowered to 140 hPa over 0.4 h and finally run
22
CA 02841384 2014-01-09
down to 80 hPa over 3 h. The pressure of 80 hPa was
maintained until the acid number of the reaction mixture
had dropped below 100 mg KOH/kg. The pressure was
subsequently raised again to 300 hPa.
In the eighth and last stage, 1197 kg (16 611 mol) of
acrylic acid were added, the pressure was held at 300 hPa
for 0.1 h, lowered to 130 hPa over 0.9 h, lowered to
80 hPa over 1.7 h, lowered to 50 hPa over 0.7 h, held for
1.5 h and finally run down to 10 hPa over 1.2 h. The
pressure of 10 hPa was maintained until the acid number
of the reaction mixture had dropped below 30 mg KOH/kg.
The pressure thereafter was set to atmospheric pressure.
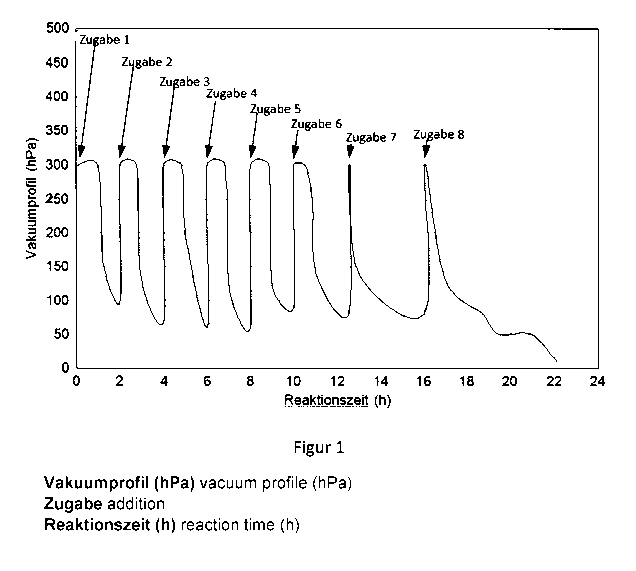
Figure 1 shows the pressure set over the reaction time,
with
Addition 1: 15.35 mol% acrylic acid
Addition 2: 15.35 mol% acrylic acid
Addition 3: 15.35 mol% acrylic acid
Addition 4: 15.35 mol% acrylic acid
Addition 5: 15.35 mol% acrylic acid
Addition 6: 15.35 mol% acrylic acid
Addition 7: 15.35 mol% acrylic acid
Addition 8: 15.35 mol% acrylic acid
Reaction temperature: 90 C
The 16 623 kg of crude product obtained in this way were
cooled to 55 C and then introduced with stirring into
3302 kg of a 15% strength by weight solution of anhydrous
sodium carbonate in demineralized water, which was set
23
CA 02841384 2014-01-09
likewise at 55 C. With stirring, 200 kg of sodium
carbonate decahydrate were slowly added, and stirring was
continued at 55 C for 2 hours. The mixture was then left
to stand at 55 C for 3 hours, without stirring, and the
aqueous phase was subsequently removed. The water content
of the remaining 15 312 kg of crude product was found to
be 2.3%. Thereafter the crude product was washed with
stirring at 55 C, with introduction of 3652 kg of an 8.4%
strength by weight solution of anhydrous sodium sulfate
in demineralized water, and stirring was continued at
this temperature for 0.75 hour. The mixture was then left
to stand at 55 C for 5 hours, without stirring, and the
aqueous phase was subsequently removed. The crude product
which remained was dried under vacuum with stirring, with
air being passed through, at 55 C, the pressure in the
first step being lowered from 1013 hPa to 170 hPa in
3.5 h, followed by 0.7 h at 90 hPa and finally by 0.9 h
at 10 hPa. For the
concluding purification and
filtration, the crude product was cooled to 40 C with
stirring, admixed with the filtration aid Dicalite 4158
and then passed through a filter press. This gave 15 000
kg of end product with the following analytical data:
Acid number -* 0.15 mg KOH/g
Water content -* 0.15% by weight
APHA color -* 36
Viscosity (25.0 C) -* 14 mPa.s
GC analysis of the end product (figure 2) gave the
following composition (GC area-percent):
24
CA 02841384 2014-01-09
Tripropylene glycol monoacrylate: 9.582%
Tripropylene glycol diacrylate: 80.873%
Michael adducts:
= Tripropylene glycol monoacrylate/acrylic acid:
0.379%
= Tripropylene glycol diacrylate/acrylic acid: 2.957%
= Tripropylene glycol monoacrylate/tripropylene glycol
monoacrylate: 0.508%
= Tripropylene glycol diacrylate/tripropylene glycol
monoacrylate: 4.160%
Remainder: 1.541%
Comparative example
A 25 m3 reactor was charged at a reactor temperature of
45 C with 10 400 kg (54 093 mol) of tripropylene glycol,
with a constant stream of air being passed through the
reactor, and the pressure was lowered to 400 hPa. Then,
in succession and with stirring, 15 kg (121 mol) of 4-
methoxyphenol, 7768 kg (107 799 mol) of acrylic acid,
188 kg (1424 mol) of 50% strength by weight phosphinic
acid and 188 kg (1821 mol) of 95% strength by weight
sulfuric acid were added. The temperature of the reaction
mixture was subsequently raised to 85 C and the pressure
was lowered to 370 hPa.
Under these temperature and pressure conditions, the
direct esterification commenced, with a mixture of water
of reaction and acrylic acid being removed continuously
by distillation in line with the composition of the
boiling curve of the respective phase equilibrium state.
CA 02841384 2014-01-09
After 0.5 h, the pressure, with the reaction mixture at
constant temperature, was reduced in steps, first to
250 hPa in 0.5 h, then to 215 hPa in 0.75 h and finally
to 100 hPa in 5.5 h. The pressure of 100 hPa was
maintained until the acid number of the reaction mixture
had dropped below 140 mg KOH/kg. The pressure was
subsequently raised to 370 hPa again and a further 904 kg
(12 545 mol) of acrylic acid and 126 kg (918 mol) of 70%
strength by weight methanesulfonic acid were added. The
pressure, subsequently, was lowered in steps again to
155 hPa in 0.75 h and then to 90 hPa in 2 h, this
pressure being maintained until the acid number of the
reaction mixture had dropped below 120 mg KOH/g. The
pressure was then raised again to 370 hPa and a further
904 kg (12 545 mol) of acrylic acid were added. To
finish, the pressure was lowered again in steps to
120 hPa in 1.1 h, to 80 hPa in 1.7 h, then to 30 hPa in
4 h and finally to 5 hPa in 2.45 h. The pressure of 5 hPa
was maintained until the acid number of the reaction
mixture had reached 30 mg KOH/g. The pressure thereafter
was set to atmospheric pressure.
Figure 3 shows the pressure set over the reaction time,
with
Addition 1: 99.64 mol% acrylic acid
Addition 2: 11.60 mol% acrylic acid
Addition 3: 11.60 mol% acrylic acid
Reaction temperature: 85 C
26
CA 02841384 2014-01-09
The 16 623 kg of crude product obtained in this way were
cooled to 55 C and then introduced with stirring into
3302 kg of a 15% strength by weight solution of anhydrous
sodium carbonate in demineralized water, which was set
likewise at 55 C, for neutralization. With stirring,
200 kg of sodium carbonate decahydrate were slowly added,
and stirring was continued at 55 C for 2 hours. The
mixture was then left to stand at 55 C for 3 hours,
without stirring, and the aqueous phase was subsequently
removed. The water content of the remaining 15 312 kg of
crude product was determined, and thereafter the crude
product was washed with stirring at 55 C, with
introduction of 3652 kg of an 8.4% strength by weight
solution of anhydrous sodium sulfate in demineralized
water, and stirring was continued at this temperature for
finally 0.75 hour. The mixture was then left to stand at
55 C for 5 hours, without stirring, and the aqueous phase
was subsequently removed. The crude product which
remained was dried under vacuum with stirring, with air
being passed through, at 55 C, the pressure in the first
step being lowered from 1013 hPa to 170 hPa in 3.5 h,
followed by 0.7 h at 90 hPa and finally by 0.9 h at
10 hPa. For filtration, the crude product was cooled to
40 C with stirring, admixed with the filtration aid
Dicalite 4158 and then placed in a filter press. This
gave 15 000 kg of end product with the following
analytical data:
Acid number -* 0.10 mg KOH/g
Water content -* 0.20% by weight
APHA color -4 8
27
CA 02841384 2014-01-09
Viscosity (25.0 C) -* 13 mPa.s
GC analysis of the end product (figure 4) gave the
following composition (GC area-percent):
Tripropylene glycol monoacrylate: 12.021%
Tripropylene glycol diacrylate: 76.092%
Michael adducts:
= Tripropylene glycol monoacrylate/acrylic acid:
0.461%
= Tripropylene glycol diacrylate/acrylic acid: 4.348%
= Tripropylene glycol monoacrylate/tripropylene glycol
monoacrylate: 0.637%
= Tripropylene glycol diacrylate/tripropylene glycol
monoacrylate: 4.435%
Remainder: 2.006%
28