Note: Descriptions are shown in the official language in which they were submitted.
CA 02848447 2014-04-07
1
CO2 CAPTURE FOR THERMAL IN SITU HYDROCARBON RECOVERY
OPERATIONS
TECHNICAL FIELD
The technical field relates to thermal in situ hydrocarbon recovery operations
and the
capture of CO2 generated in such operations.
BACKGROUND
Thermal in situ hydrocarbon recovery operations often include the generation
of steam
for injection into the hydrocarbon-bearing reservoir. Steam-Assisted Gravity
Drainage
(SAGD) is a thermal in situ hydrocarbon recovery method using a horizontal
well pair
that includes an overlying injection well and an underlying production well.
Steam is
generated at surface facilities and injected into the reservoir via the
injection well.
Production fluids that include condensate and mobilized hydrocarbons are
produced via
the production well, and are treated to separate hydrocarbons from produced
water. The
produced water is treated for reuse as steam.
Steam generators used in SAGD are typically Once-Through Steam Generators
(OTSGs), which burn natural gas in order to turn the incoming feed water into
wet steam
which is then separated into dry steam and a liquid component. The dry steam
is then
injected into the reservoir.
Cyclic Steam Stimulation (CSS) is another type of thermal in situ hydrocarbon
recovery
operation that used steam generation for injection to enhance hydrocarbon
recovery.
Steam generation also produces combustion products including CO2 gas.
Capturing CO2
gas can be challenging.
SUMMARY
In some aspects, there is provided a process for capturing CO2 from a CO2-
containing
gas generated from steam production in a thermal in situ hydrocarbon recovery
operation, comprising: supplying the CO2-containing gas to an absorber to
enable
contact of the gas with an absorption solution, to produce a CO2-depleted gas
and a
CO2-loaded solution; supplying the CO2-loaded solution to a desorber to
promote
CA 02848447 2014-04-07
2
release of CO2 from the 002-loaded solution, to produce a CO2 gas stream and a
regenerated solution; heating the absorber and/or the desorber with heat at
least partly
recovered from the thermal in situ hydrocarbon recovery operation.
In some aspects, the process includes recovering heat from a produced water
stream, a
produced hydrocarbon stream, a produced fluids stream, or a boiler flue gas
stream, for
heating the absorber and/or the desorber.
In some aspects, the process includes transferring heat from the thermal in
situ
hydrocarbon recovery operation to a heat transfer media, and then transferring
heat from
the heat transfer media to the absorber and/or the desorber.
In some aspects, the process includes supplying an in situ operation stream to
a first
heat exchanger to indirectly transfer heat to the heat transfer media, and
then supplying
the heat transfer media to a second heat exchanger to indirectly transfer heat
to a
stream use directly in the absorber and/or the desorber.
In some aspects, the second heat exchanger is a reboiler heat exchanger
configured to
transfer heat to the desorber.
In some aspects, the in situ operation stream comprises a flue gas stream.
In some aspects, the CO2-containing gas is derived from the flue gas stream.
In some aspects, the flue gas stream is generated from natural gas combustion
and
comprises water vapour, and wherein the heat recovered from the flue gas
stream
includes heat of condensation of at least a portion of the water vapour.
In some aspects, the process includes regulating the heating of the absorber
and/or the
desorber by adjusting a flow rate of the heat transfer media.
In some aspects, the flow rate of the heat transfer media is adjusted by
controlling a
valve and/or a pump associated with the flow of the heat transfer media.
In some aspects, the flow rate of the heat transfer media is adjusted in
accordance with
temperature measurements of the absorber and/or the desorber.
In some aspects, the heating comprises heating the desorber.
CA 02848447 2014-04-07
3
In some aspects, the thermal in situ hydrocarbon recovery operation is Steam-
Assisted
Gravity Drainage (SAGD).
In some aspects, the thermal in situ hydrocarbon recovery operation is Cyclic
Steam
Stimulation (CSS).
In some aspects, the CO2-containing gas generated from steam production is
generated
from a Once-Through Steam Generator (OTSG).
In some aspects, the process includes enzymatically enhancing the hydration of
the CO2
in the absorber.
In some aspects, carbonic anhydrase or an analog thereof is provided in the
absorption
solution.
In some aspects, the process includes enzymatically enhancing the release of
the CO2
from the CO2-loaded solution in the desorber.
In some aspects, carbonic anhydrase or an analog thereof is provided in the
CO2-loaded
solution.
In some aspects, the absorption solution comprises water and potassium
carbonate.
In some aspects, there is provided a system for capturing CO2 from a CO2-
containing
gas generated from steam production in a thermal in situ hydrocarbon recovery
operation, comprising:
an absorber comprising:
a gas inlet for receiving the CO2-containing gas;
a liquid inlet for receiving an absorption solution;
a reaction chamber allowing contact of the gas with an absorption
solution, to produce a CO2-depleted gas and a CO2-loaded solution;
a gas outlet for releasing the CO2-depleted gas; and
a liquid outlet for releasing the CO2-loaded solution;
CA 02848447 2014-04-07
4
a desorber comprising:
a liquid inlet for receiving the CO2-loaded solution;
a desorption chamber for promoting release of CO2 from the CO2-loaded
solution, to produce a CO2 gas and a regenerated solution;
a gas outlet for releasing the CO2 gas; and
a liquid outlet for releasing the regenerated solution; and
a heat transfer device for transferring heat from the thermal in situ
hydrocarbon
recovery operation to the absorber and/or the desorber.
In some aspects, the heat transfer device is configured to transfer heat from
a produced
water stream, a produced hydrocarbon stream, a produced fluids stream, or a
boiler flue
gas stream, to the absorber and/or the desorber.
In some aspects, the heat transfer device comprises a transfer line for
circulating a heat
transfer media between the thermal in situ hydrocarbon recovery operation and
the
absorber and/or the desorber, for indirectly transferring heat there-between.
In some aspects, the heat transfer device comprises a first heat exchanger
configured to
indirectly transfer heat from an in situ operation stream to the heat transfer
media, and a
second heat exchanger configured to indirectly transfer heat to a stream use
directly in
the absorber and/or the desorber.
In some aspects, the second heat exchanger is a reboiler heat exchanger
configured to
transfer heat to the desorber.
In some aspects, the in situ operation stream comprises a flue gas stream.
In some aspects, the CO2-containing gas is derived from the flue gas stream.
In some aspects, the flue gas stream is generated from natural gas combustion
and
comprises water vapour, and wherein the heat recovered from the flue gas
stream
includes heat of condensation of at least a portion of the water vapour.
CA 02848447 2014-04-07
In some aspects, the system includes a regulator coupled to the heat transfer
device and
configured to regulate the heating of the absorber and/or the desorber by
adjusting a
flow rate of the heat transfer media.
In some aspects, regulator comprises a valve and/or a pump associated with the
transfer
line for circulating the heat transfer media.
In some aspects, the regulator is coupled to a temperature measurement device
operatively connected to the absorber and/or the desorber, and is configured
to adjust
the flow rate of the heat transfer media in accordance with temperature
measurements
from the temperature measurement device.
In some aspects, the heat transfer device is configured for heating the
desorber.
In some aspects, the thermal in situ hydrocarbon recovery operation is Steam-
Assisted
Gravity Drainage (SAGD).
In some aspects, the thermal in situ hydrocarbon recovery operation is Cyclic
Steam
Stimulation (CSS).
In some aspects, the CO2-containing gas generated from steam production is
generated
from a Once-Through Steam Generator (OTSG).
In some aspects, the absorber is configured to accommodate enzymatically
enhanced
hydration of the CO2 in the reaction chamber.
In some aspects, carbonic anhydrase or an analog thereof is provided in the
absorption
solution.
In some aspects, carbonic anhydrase or an analog thereof is provided on or in
packing
within the reaction chamber.
In some aspects, the desorber is configured to accommodate enzymatically
enhanced
release of the CO2 from the CO2-loaded solution.
In some aspects, carbonic anhydrase or an analog thereof is provided in the
CO2-loaded
solution.
6
In some aspects, carbonic anhydrase or an analog thereof is provided on or in
packing
within the desorption chamber.
In some aspects, there is provided a process for capturing CO2 from a CO2-
containing
gas generated from steam production in a thermal in situ hydrocarbon recovery
operation,
comprising:
supplying the CO2-containing gas to an absorber to enable contact of the gas
with
an absorption solution comprising water and potassium carbonate, to produce a
CO2-depleted gas and a CO2-loaded solution;
supplying the CO2-loaded solution to a desorber to promote release of CO2 from
the CO2-loaded solution, to produce a CO2 gas stream and a regenerated
solution;
heating the desorber with heat at least partly recovered from the thermal in
situ
hydrocarbon recovery operation, the heat being derived from an in situ
operation
stream having a temperature from 30 C to 90 C; and
enzymatically enhancing hydration of the CO2 in the absorber, and
enzymatically
enhancing release of the CO2 from the CO2-loaded solution in the desorber.
In some aspects, there is also provided A system for capturing CO2 from a CO2-
containing
gas generated from steam production in a thermal in situ hydrocarbon recovery
operation,
comprising:
an absorber comprising:
a gas inlet for receiving the CO2-containing gas;
a liquid inlet for receiving an absorption solution comprising water and
potassium carbonate;
a reaction chamber allowing contact of the gas with the absorption solution,
to produce a CO2-depleted gas and a CO2-loaded solution;
a gas outlet for releasing the CO2-depleted gas; and
a liquid outlet for releasing the CO2-loaded solution;
Date Recue/Date Received 2021-07-16
6a
a desorber comprising:
a liquid inlet for receiving the CO2-loaded solution;
a desorption chamber for promoting release of CO2 from the CO2-loaded
solution, to produce a CO2 gas and a regenerated solution;
a gas outlet for releasing the CO2 gas; and
a liquid outlet for releasing the regenerated solution; and
a heat transfer device for transferring heat from the thermal in situ
hydrocarbon
recovery operation to the desorber, the heat being derived from an in situ
operation
stream having a temperature from 30 C to 90 C; and
wherein the absorber is configured to accommodate enzymatically enhanced
hydration of the CO2 in the reaction chamber, and the desorber is configured
to
accommodate enzymatically enhanced release of the CO2 from the CO2-loaded
solution.
BRIEF DESCRIPTION OF THE DRAWINGS
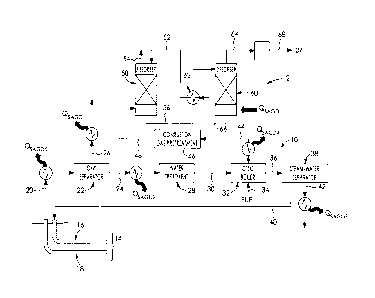
Fig 1 is a process flow diagram of SAGD and CO2 capture operations.
Fig 2 is a process flow diagram of a desorber.
Fig 3 is another process flow diagram of a desorber with a reboiler.
Fig 4 is a process flow diagram of a desorber and a heating circuit.
Fig 5 is a diagram of a heating circuit.
DETAILED DESCRIPTION
Thermal in situ hydrocarbon recovery operations, such as SAGD and CSS, produce
CO2-
containing gas that may be subjected to CO2 capture with effective use of heat
from SAGD
or CCS streams.
Date Recue/Date Received 2021-07-16
6b
SAGD and CO2 capture operations
While various scenarios are described in relation to SAGD, it should be noted
that similar
scenarios can also be used in CSS or other thermal in situ recovery
operations.
Referring to Fig 1, a SAGD operation 10 may be integrated with a CO2 capture
operation
12 for efficient use of heat and fluids. The SAGD operation 10 includes a SAGD
well pair
14 including an injection well 16 and a production well 18. Produced fluids 20
are
recovered via the production well 18 and are supplied to an oil/water
separator 22 to
produce a produced water stream 24 and a produced hydrocarbon stream 26.
The produced water stream 24 still includes contaminants and is thus subjected
to water
treatment 28 to produce treated water 30. The treated water 30 is supplied to
a steam
generator, such as an OTSG boiler 32. The OSTG boiler 32 also receives fuel,
such as
natural gas 34, and produces wet steam 36 that is typically about 80% steam.
The wet
steam 36 is supplied to a steam-water separator 38 to produce dry steam 40 and
a
separated water component 42. The dry steam 40 is then supplied to the
injection well
Date Recue/Date Received 2020-11-13
CA 02848447 2014-04-07
7
16 for injection into the reservoir to continue heating and mobilizing the in
situ
hydrocarbons in the SAGD process.
Still referring to Fig 1, the OTGS boiler 32 also produces combustion gas 44
that
includes CO2. The combustion gas 44 can be further treated in order to capture
the CO2.
Thus, the combustion gas 44 may be supplied to the CO2 capture operation 12,
which
will be described further below.
The combustion gas 44 may first be subjected to pre-treatments 46 to remove
unwanted
contaminants and/or reduce the temperature of the gas. The resulting gas 48 is
then
supplied to an absorber 50, which may be a packed tower. The absorber 50 also
received an absorption solution 52 which can flow counter-currently with
respect to the
gas. The absorber 50 enables capture of the CO2 gas present in the combustion
gas and
produces a CO2-depleted gas 54 and an ion-loaded solution 56 that are
withdrawn from
the absorber 50. The absorption step may be enhanced by providing
biocatalysts, which
may include an enzyme such as carbonic anhydrase, within the absorber and/or
within
the absorption solution 52. More regarding enzyme-enhanced CO2 absorption will
be
discussed further below.
The ion-loaded solution 56 is supplied to a desorber 60 and may be pre-heated
by a
desorption pre-heater 62. In the desorber 60, the conditions promote release
of CO2
from the solution in order to produce a CO2 stream 64 and a regenerated
solution 66.
This produced CO2 stream can be further compressed to produce compressed CO2
68
to conditions required for EOR, liquefaction, and/or pipeline specifications.
Favorable
conditions for CO2 release can be obtained via a temperature-swing operation
by
heating the liquid present in the desorber via a reboiler or a heat exchanger,
or by a
pressure-swing operation. The regenerated solution 66 is recycled back as at
least part
of the absorption solution 52, and may be cooled within the desorption pre-
heater 62 to
transfer heat to the ion-loaded solution 56 or in another heat exchanger.
Heat recovery from SAGD for use in CO2 capture
Referring still to Fig 1, the CO2 capture operation 12 has certain heat
requirements for
the absorption and desorption stages. In this regard, heat may be recovered
from the
SAGD operation 10 and reused in the CO2 capture operation 12. For instance, in
some
scenarios, heat may be recovered from various streams, such as the produced
water
CA 02848447 2014-04-07
8
stream 24 and/or the produced hydrocarbon stream 26. Heat can also be
recovered
from combustion gas exiting from the OTSG boiler (stream 44), from the
steam/water
separator (stream 42) or the compressed CO2 product (stream 68).
In addition, certain SAGD streams may be particularly suited for heat transfer
for certain
parts of the CO2 capture operation.
At least part of the CO2 stream 64 may be supplied to compression, storage,
transportation via pipeline, and/or various enhanced oil recovery methods.
Referring to Figs 2 and 3, hot SAGD streams may be used to recover heat that
can be
integrated in the desorber 60 of the CO2 capture plant. For example, a heat
transfer
stream 70 having SAGD heat (QsAGD) can be used via a direct exchange tube (72
in Fig
2) in the liquid phase of the desorber, or via a reboiler (74 in Fig 3).
Referring to Figs 4 and 5, a heating circuit 76 can be provided for
transferring heat from
a SAGD stream to the CO2 capture operation. The heating circuit 76 includes a
first heat
exchanger 78 for transferring heat from a SAGO stream 80 to a heat transfer
media
stream 82, which is supplied to a second heat exchanger 84 for transferring
heat from
the heated transfer media to the reboiler stream 86. Streams that may be used
as the
hot SAGD stream 80 can be selected based on having sufficient energy and/or
matching
temperatures, e.g. produced fluids 20, stack/flue gas off the boilers 44, oil
produced after
water/oil separation 26, compressed CO2 68 or compression train.
In some scenarios, the hot SAGD stream 80 can be selected based on an energy
content of 1 to 4 GJ/ton CO2 produced, or 2 to 3 GJ/ton CO2 produced. The hot
SAGD
stream 80 can be selected based on temperature levels from 30 C to 90 C,
optionally
from 50 C to 75 C or from 55 C to 65 C. All temperature ranges are also a
function of
the pressure level in the stripper. The hot SAGD stream may be able to
transfer heat of
condensation or heat of compression as part of the QsAGD transferred to the
CO2 capture
operation.
The heat transfer media 82 can be composed of various fluids, from thermal
process oil
to generic refrigerant and even ammonia.
Referring to Fig 5, the heating circuit 76 can also be equipped with a valve
system 88 to
control flow rate of the heat transfer media 82, and a pumping system 90 to
provide
CA 02848447 2014-04-07
9
hydraulic pressure to the heat transfer media 82. The pump system 90 and the
valve
system 88 can be operatively connected to a controller 92 that controls the
flow rate of
the heat transfer media 82, as well as other aspects of the heating circuit
76, in order to
provide a desired amount of heat to the reboiler stream 86, which may be based
on a
measured temperature of the desorber.
It should also be noted that the heating circuit 76 can include additional
heat exchangers
that progressively heat the heat transfer media 82 using multiple hot SAGD
streams.
It should also be noted that the SAGD scenarios described herein may be
adapted for
CSS or other thermal in situ hydrocarbon operations. Various in situ operation
streams
can thus be used to transfer heat to the CO2 capture operation.
Enzyme-enhanced CO2 capture
For enzyme-enhanced CO2 capture methods, various techniques can be used, some
of
which are described herein.
Biocatalysts employed in the process may be carbonic anhydrase or analogues
thereof.
It should also be noted that "carbonic anhydrase or analogues thereof" as used
herein
includes naturally occurring, modified, recombinant and/or synthetic enzymes
including
chemically modified enzymes, enzyme aggregates, cross-linked enzymes, enzyme
particles, enzyme-polymer complexes, polypeptide fragments, enzyme-like
chemicals
such as small molecules mimicking the active site of carbonic anhydrase
enzymes and
any other functional analogue of the enzyme carbonic anhydrase.
It should be understood that carbonic anhydrase is not just a single enzyme
form, but a
broad group of metalloproteins that exists in three genetically unrelated
families of
isoforms, a, 6 and v. Carbonic anhydrase (CA) is present in and may be derived
from
animals, plants, algae, bacteria, etc. The human variant CA II, located in red
blood cells,
is the most studied and has a high catalytic turnover number. The carbonic
anhydrase
includes any analogue, fraction and variant thereof and may be alpha, gamma or
beta
type from human, bacterial, fungal or other organism origins, having
thermostable or
other stability properties, as long as the carbonic anhydrase can be provided
to function
in the CO2 capture or desorption processes to enzymatically catalyse the
reaction.
CA 02848447 2014-04-07
Regarding delivery of the enzyme to the process, in one optional aspect the
enzyme is
provided directly as part of a formulation or solution. There may also be
enzyme
provided in a reactor to react with incoming solutions and gases; for
instance, the
enzyme may be fixed to a solid non-porous packing material, on or in a porous
packing
material, on or in particles or as aggregates flowing with the absorption
solution within a
packed tower or another type of reactor. The carbonic anhydrase may be in a
free or
soluble state in the formulation or immobilised on or in particles or as
aggregates,
chemically modified or stabilized, within the formulation. It should be noted
that enzyme
used in a free state may be in a pure form or may be in a mixture including
impurities or
additives such as other proteins, salts and other molecules coming from the
enzyme
production process. Immobilized enzyme free flowing in the solutions could be
entrapped inside or fixed to a porous coating material that is provided around
a support
that is porous or non-porous. The enzymes may be immobilised directly onto the
surface
of a support (porous or nonporous) or may be present as cross linked enzyme
aggregates (CLEAs) or cross linked enzyme crystals (CLECs). CLEA comprise
precipitated enzyme molecules forming aggregates that are then cross-linked
using
chemical agents. The CLEA may or may not have a 'support' or 'core' made of
another
material which may or may not be magnetic. CLEC comprise enzyme crystals and
cross
linking agent and may also be associated with a 'support' or 'core' made of
another
material. When a support is used, it may be made of polymer, ceramic,
metal(s), silica,
solgel, chitosan, nylon, alumina, cellulose, alginate, polyacrylamide,
magnetic particles,
titanium oxide, zirconium oxide and/or other materials known in the art to be
suitable for
immobilization or enzyme support. When the enzymes are immobilised or provided
on
particles, such as micro-particles, the particles are preferably sized and
provided in a
particle concentration such that they are pumpable with the solution
throughout the
process.
In some optional scenarios, the aqueous absorption solution may be a carbonate-
based
solution, such as sodium carbonate solution, potassium carbonate solution,
ammonium
carbonate solution, promoted sodium carbonate solutions, promoted potassium
carbonate solutions, or promoted ammonium carbonate; or any combination
thereof.
The absorption solution can include at least one of piperidine, piperazine,
derivatives of
piperidine or piperazine which are substituted by at least one alkanol group,
monoethanolamine (MEA), 2-amino-2-methyl-1-propanol (AMP), 2-
(2-
CA 02848447 2014-04-07
11
aminoethylamino)ethanol (AEE), 2-amino-2-hydroxymethy1-1,3-propanediol (Iris
also
known as AHPD), N-methyldiethanolamine (MDEA), dimethylmonoethanolamine
(DMMEA), diethylmonoethanolamine (DEMEA),
triisopropanolamine (TI PA),
triethanolamine (TEA), diethanolamine (DEA), diisopropylamine (Dl PA), methyl
monoethanolamine (MMEA), TIA, TBEE, HEP, hindered diamine (HDA), bis-
(tertiarybutylaminoethoxy)-ethane (BTEE),
ethoxyethoxyethanoltertiarybutylamine
(EEETB), bis-(tertiarybutylaminoethyl)ether, 1,2-bis-
(tertiarybutylaminoethoxy)ethane or
bis-(2-isopropylaminopropyl)ether, and the like, dialkylether of polyalkylene
glycols,
dialkylether or dimethylether of polyethylene glycol, amino acids comprising
glycine,
proline, arginine, histidine, lysine, aspartic acid, glutamic acid,
methionine, serine,
threonine, glutamine, cysteine, asparagine, valine, leucine, isoleucine,
alanine, tyrosine,
tryptophan, phenylalanine, and derivatives such as taurine, N,cyclohexyl 1,3-
propanediamine, N-secondary butyl glycine, N-methyl N-secondary butyl glycine,
diethylglycine, dimethylglycine, sarcosine, methyl taurine, methyl-a-
aminopropionic acid,
N-(6-ethoxy)taurine, N-(6-aminoethyptaurine, N-methyl alanine, 6-aminohexanoic
acid
and potassium or sodium salts of the amino acids, or mixtures thereof, sodium
carbonate, potassium carbonate and ammonium carbonate. The concentration of
the
compound in the absorption solution may vary from 0.2 to 8 M, for example.
In some scenarios, the absorption solution can include primary amines,
secondary
amines, tertiary amines, primary alkanolamines, secondary alkanolamines,
tertiary
alkanolamines, primary amino acids, secondary amino acids, tertiary amino
acids,
and/or carbonates.
It should be noted that in some applications various absorption compounds
described
herein can be used without enzymes, but rather alone or in combination with
various
catalysts or promoters.
In further optional scenarios, the ion-rich solution may contain from about
0.1 M to 10 M
of bicarbonate ions. The carbonate loading of the solution will depend on the
operating
conditions, reactor design and the chemical compounds that are added. For
instance,
when potassium or sodium bicarbonate compounds are used in the absorption
solution,
the ion rich solution may contain from about 0.2 M to 1.5 M of bicarbonate
ions. When
the ion rich solution is highly loaded with carbonate/bicarbonate ions, it may
become
much more viscous which can have a detrimental effect on mass transport within
the
12
solution. The presence of carbonic anhydrase flowing with the solution further
enhances
the mass transport along with the enzymatic reaction, thus improving the
overall CO2
capture, for instance by supersaturating the solution with bubbles of gaseous
CO2.
Various types of reactors may be used for the absorber and/or the desorber,
such as a
packed column, a bubble column, a fluidized bed, a spray reactor, a flow wire
reactor, or
another type or design, preferably for gas-liquid contact. Both of the
reactors may be, for
example, packed towers.
The processes disclosed herein can also be tailored and retrofitted according
to a specific
situation and application. For example, the CO2 capture vessels may be sized
and
operated in accordance with a desired CO2 capture level for an existing SAGD
operation,
or may be designed in conjunction with the design of a new SAGD operation.
The process may involve generating a biocatalyst-containing ion-loaded
solution that is
withdrawn from the CO2 absorber and is subjected to a separation step to
produce a
biocatalyst-enriched stream and a biocatalyst-depleted stream. The separation
step may
be performed using filtration, for example using a filtration membrane, and
may be
controlled to provide various advantages. The filtration membrane may be
provided so as
to be inert to the absorption solution, and having pores having smaller
diameter than a
diameter of the biocatalyst. The filtration membrane may also be used such
that the
retentate and filtrate have desired properties, such as fluidity to facilitate
handling of the
streams.
The filtration membrane may be based on polyethersulfone combined with a
separating
layer of sulfonated polyethersulfone, or based on a mixture of polyether
sulfone and
sulfonated polyether sulfone, polyethylene (PE), polypropylene (PP),
polysulfone (PS),
polyamide (PA), polyamide-imide (PAI), polyvinylidene difluoride (PVDF),
polyetheretherketone (PEEK), polyetherimide (PEI), polyvinylpyrrolydone and/or
polyimide (PI) hollow fiber (HF) membranes.
It should also be noted that various techniques may be used in connection with
the CO2
capture operation, such as processes and scenarios described in the following
patent
references: CA 2,291,785; CA 2,329,113, CA 2,393,016, CA 2,443,222, US
6,908,507;
EP 1 377 531, US 7,514,056, US 7,596,952; US 8,066,965, US 8,277,769, US
6,946,288,
US 7,740,689, _________________________________________________________
Date Recue/Date Received 2020-11-13
13
WO 2012/103653, US 2013/0203155, US 2011/0097781, US 2012/0129236,
US 8,846,377, US 8,722,391, US 2013/0052720, US 2007/0048856, WO 2014/012181,
and WO 2014/066999.
EXAMPLES
Recovery of the flue gas heat
For the scenario where flue gas from an OTSG is the heat source QsAGD, burning
methane
produces water vapour in the flue gas and the condensation of this water
vapour at or
around 100 C releases significant energy. Recovering energy from the
condensation
water vapour contained in the flue gas around this temperature can provide
efficient heat
integration with the CO2 capture operation. In the case of CO2 capture from
the burning
of natural gas, it may be assumed that the flue gas exits the boiler stack at
approximately
200 C. Cooling and eventual condensation of the water phase generates
2.256MJ/kg of
water produced in the combustion. This heat of condensation only represents
roughly half
the heat required in the reboiler to strip the CO2 from the ion-loaded
solution. Thus,
referring to Fig 1, heat of condensation from the flue gas 44 may be recovered
as QSAGD4,
which is supplied to a heating circuit 76 as at least part of the heat QsAGD
for heating heat
transfer media 82 used to heat the desorber 60 as shown in Fig 4.
Recovery of the heat of compression
A CO2 stream such as stream 64 illustrated in Fig 1 may be at partial vacuum
pressures
given the desorber operating conditions. The CO2 stream can then be compressed
to
suitable pressures for enhanced oil recovery (EOR). From partial vacuum to EOR
pressure levels, a compression ratio of 500 can be encountered. At such
compression
levels, 1.5MJ/kg of CO2 can theoretically be recovered using a configuration
as shown on
Fig 5, for example. The energy available is estimated using the following
formula:
P2 k-1
T2 = Ti *
P1
12 is the outlet polytropic compression temperature; Ti is the inlet
temperature; P2/P1 is
the compression ratio; and k is the isentropic constant. Using the heat
capacity for CO2
(889 J/kg-K), Ti of 313K, k of 1.3, a compression ratio of 500 (P1 of 0.3 and
P2 of 150
Date Recue/Date Received 2021-07-16
CA 02848447 2014-04-07
,
,
14
for example), and a calculated 12 of 2020K, one can obtain 1.5MJ/kg of CO2 as
the
available energy.
This heat of compression source of heat has the potential to cover about 30%
to 40% of
the heat required in certain implementations of the desorption/regeneration
process,
depending on the efficiency of the heat recovery circuit, type of compressors,
and
operating conditions of the desorber, for example.
It should be noted that various other streams in thermal in situ recovery
operations can
also be used as heat sources to contribute heat energy to the CO2 capture
operation.