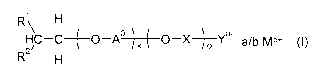Note: Descriptions are shown in the official language in which they were submitted.
= BASF SE
CA 02848961 2014-03-17 PF 0000072938 SE/PP
1
Process for producing mineral oil using surfactants based on a mixture of C28
Guerbet-, 030 Guerbet-, 032 Guerbet-containing hydrocarbyl alkoxylates
Description
This patent application claims the benefit of pending US provisional patent
application
Serial Number 61/550,459 filed October 24, 2011 incorporated in its entirety
herein by
reference.
The present invention relates to a surfactant mixture, to the use and
preparation
thereof, and to aqueous surfactant formulations comprising the mixtures, and
to
processes for producing mineral oil by means of Winsor type III microemulsion
flooding, in which the aqueous surfactant formulation is injected into a
mineral oil
deposit through injection wells and crude oil is withdrawn from the deposit
through
production wells.
In natural mineral oil deposits, mineral oil is present in the cavities of
porous reservoir
rocks which are sealed toward the surface of the earth by impervious top
layers. The
cavities may be very fine cavities, capillaries, pores or the like. Fine pore
necks may,
for example, have a diameter of only about 1 kim. As well as mineral oil,
including
fractions of natural gas, a deposit comprises water with a greater or lesser
salt content.
In mineral oil extraction, a distinction is generally drawn between primary,
secondary
and tertiary extraction. In primary extraction, the mineral oil flows, after
commencement
of drilling of the deposit, of its own accord through the borehole to the
surface owing to
the autogenous pressure of the deposit.
After primary extraction, secondary extraction is therefore used. In secondary
extraction, in addition to the boreholes which serve for the extraction of the
mineral oil,
the so-called production wells, further boreholes are drilled into the mineral
oil-bearing
formation. Water is injected into the deposit through these so-called
injection wells in
order to maintain the pressure or to increase it again. As a result of the
injection of the
water, the mineral oil is forced slowly through the cavities into the
formation,
proceeding from the injection well in the direction of the production well.
However, this
only works for as long as the cavities are completely filled with oil and the
more viscous
oil is pushed onward by the water. As soon as the mobile water breaks through
cavities, it flows on the path of least resistance from this time, i.e.
through the channel
formed, and no longer pushes the oil onward.
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
2
By means of primary and secondary extraction, generally only approx. 30 to 35%
of the
amount of mineral oil present in the deposit can be extracted.
It is known that the mineral oil yield can be enhanced further by measures for
tertiary
oil extraction. A review of tertiary oil extraction can be found, for example,
in "Journal of
Petroleum Science of Engineering 19 (1998)", pages 265 to 280. Tertiary oil
extraction
includes, for example, thermal methods in which hot water or steam is injected
into the
deposit. This lowers the viscosity of the oil. The flow medium used may
likewise be
gases such as CO2 or nitrogen.
Tertiary mineral oil extraction also includes methods in which suitable
chemicals are
used as assistants for oil extraction. These can be used to influence the
situation
toward the end of the water flow and as a result also to extract mineral oil
hitherto held
firmly within the rock formation.
Viscous and capillary forces act on the mineral oil which is trapped in the
pores of the
deposit rock toward the end of the secondary extraction, the ratio of these
two forces
relative to one another being determined by the microscopic oil separation. By
means
of a dimensionless parameter, the so-called capillary number, the action of
these
forces is described. It is the ratio of the viscosity forces (velocity x
viscosity of the
forcing phase) to the capillary forces (interfacial tension between oil and
water x
wetting of the rock):
N= ___________
a cos
In this formula, p is the viscosity of the fluid mobilizing mineral oil, v is
the Darcy
velocity (flow per unit area), (5 is the interfacial tension between liquid
mobilizing
mineral oil and mineral oil, and 0 is the contact angle between mineral oil
and the rock
(C. Melrose, C.F. Brandner, J. Canadian Petr. Techn. 58, Oct. ¨ Dec., 1974).
The
higher the capillary number, the greater the mobilization of the oil and hence
also the
degree of oil removal.
It is known that the capillary number toward the end of secondary mineral oil
extraction
is in the region of about 10-6 and that it is necessary to increase the
capillary number to
about 10-3 to 10-2 in order to be able to mobilize additional mineral oil.
For this purpose, it is possible to conduct a particular form of the flooding
method -
what is known as Winsor type III microemulsion flooding. In Winsor type III
microemulsion flooding, the injected surfactants should form a Winsor type III
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
3
microemulsion with the water phase and oil phase present in the deposit. A
Winsor
type III microemulsion is not an emulsion with particularly small droplets,
but rather a
thermodynamically stable, liquid mixture of water, oil and surfactants. The
three
advantages thereof are that
- a very low interfacial tension G between mineral oil and aqueous phase is
thus
achieved,
- it generally has a very low viscosity and as a result is not trapped in a
porous
matrix,
- it forms with even the smallest energy inputs and can remain stable over an
infinitely long period (conventional emulsions, in contrast, require high
shear
forces which predominantly do not occur in the reservoir, and are merely
kinetically stabilized).
The Winsor type III microemulsion is in an equilibrium with excess water and
excess
oil. Under these conditions of microemulsion formation, the surfactants cover
the oil-
water interface and lower the interfacial tension 6 more preferably to values
of
< 10-2 mN/m (ultra-low interfacial tension). In order to achieve an optimal
result, the
proportion of the microemulsion in the water-microemulsion-oil system, with a
defined
amount of surfactant, should by its nature be at a maximum, since this allows
lower
interfacial tensions to be achieved.
In this manner, it is possible to alter the form of the oil droplets
(interfacial tension
between oil and water is lowered to such a degree that the smallest interface
state is
no longer favored and the spherical form is no longer preferred), and they can
be
forced through the capillary openings by the flooding water.
When all oil-water interfaces are covered with surfactant, in the presence of
an excess
amount of surfactant, the Winsor type III microemulsion forms. It thus
constitutes a
reservoir for surfactants which cause a very low interfacial tension between
oil phase
and water phase. By virtue of the Winsor type III microemulsion being of low
viscosity,
it also migrates through the porous deposit rock in the flooding process
(emulsions, in
contrast, can become trapped in the porous matrix and block deposits). When
the
Winsor type III microemulsion meets an oil-water interface as yet uncovered
with
surfactant, the surfactant from the microemulsion can significantly lower the
interfacial
tension of this new interface, and lead to mobilization of the oil (for
example by
deformation of the oil droplets).
The oil droplets can subsequently combine to a continuous oil bank. This has
two
advantages:
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
4
Firstly, as the continuous oil bank advances through new porous rock, the oil
droplets
present there can coalesce with the bank.
Moreover, the combination of the oil droplets to give an oil bank
significantly reduces
the oil-water interface and hence surfactant no longer required is released
again.
Thereafter, the surfactant released, as described above, can mobilize oil
droplets
remaining in the formation.
Winsor type III microemulsion flooding is consequently an exceptionally
efficient
process, and requires much less surfactant compared to an emulsion flooding
process.
In microemulsion flooding, the surfactants are typically optionally injected
together with
cosolvents and/or basic salts (optionally in the presence of chelating
agents).
Subsequently, a solution of thickening polymer is injected for mobility
control. A further
variant is the injection of a mixture of thickening polymer and surfactants,
cosolvents
and/or basic salts (optionally with chelating agent), and then a solution of
thickening
polymer for mobility control. These solutions should generally be clear in
order to
prevent blockages of the reservoir.
The requirements on surfactants for tertiary mineral oil extraction differ
significantly
from requirements on surfactants for other applications: suitable surfactants
for tertiary
oil extraction should reduce the interfacial tension between water and oil
(typically
approx. 20 mN/m) to particularly low values of less than 10-2 mN/m in order to
enable
sufficient mobilization of the mineral oil. This has to be done at the
customary deposit
temperatures of approx. 15 C to 130 C and in the presence of water of high
salt
contents, more particularly also in the presence of high proportions of
calcium and/or
magnesium ions; the surfactants thus also have to be soluble in deposit water
with a
high salt content.
To fulfill these requirements, there have already been frequent proposals of
mixtures of
surfactants, especially mixtures of anionic and nonionic surfactants.
US 7,119,125 B1 describes a mixture of sulfated Guerbet alcohol alkoxylate and
of low
molecular weight sulfated alkyl alkoxylate in oil production. Particularly
good
emulsifying properties are attributed to the bimodal distribution. However,
these
emulsifying properties do not play a major role in Winsor type III
microemulsion
flooding. Too much surfactant would be required for the emulsification of oil,
and the
shear forces required are barely present in the flooding operation (apart from
the region
around the injector).
US-A 2008/217064 describes a drilling fluid solution comprising a nonionic
surfactant -
consisting of at least one branched alkyl ethoxylate and a capped alkyl
ethoxylate - and
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
a detergent builder and a viscosifier. The nonionic surfactant may be a 010
Guerbet
alcohol ethoxylate.
US-A 2009/270281 describes the use of a surfactant mixture for the production
of
mineral oil, which comprises at least one surfactant with an alkyl radical of
12 to 30
5 carbon atoms and a branched cosurfactant with an alkyl radical of 6 to 11
carbon
atoms. The degree of branching of the alkyl radical in the cosurfactant ranges
from 1 to
2.5, and may thus comprise Guerbet alcohols of the 2-ethylhexyl or 2-
propylheptyl
type. The cosurfactants may be alcohol ethoxylates or anionically modified
alcohol
ethoxylates (for example alkyl ether sulfate).
Further surfactant mixtures are described in WO 2011/037975 A2, W02011/110501
Al, WO 2011/110502 Al, W02011/110503 Al, and in international applications
PCT/EP2011/055884 and PCT/EP2011/056325.
The use parameters, for example type, concentration and mixing ratio of the
surfactants used relative to one another, are therefore adjusted by the person
skilled in
the art to the conditions prevailing in a given oil formation (for example
temperature
and salt content).
As described above, mineral oil production is proportional to the capillary
number. The
lower the interfacial tension between oil and water, the higher the capillary
number.
The higher the mean number of carbon atoms in the crude oil, the more
difficult low
interfacial tensions are to achieve. For low interfacial tensions, suitable
surfactants are
those which possess a long alkyl radical. The longer the alkyl radical, the
better the
reducibility of the interfacial tensions. However, the availability of such
compounds is
very limited.
It is therefore an object of the present invention to provide a particularly
efficient
surfactant or an efficient surfactant mixture for use for surfactant flooding,
and an
improved process for tertiary mineral oil production. It is further object of
the invention
to provide a process for preparing these surfactants or this surfactant
mixture.
The object is achieved by a surfactant mixture comprising at least three ionic
surfactants which differ in terms of the hydrocarbyl moiety (R1)(R2)-CH-CH2-
and are of
the general formula (I)
,1
HC C ( 0¨A0 ) 0X ) Ya- a/b Mb+ (I)
/
R2
where
B11/72938US
= BASF SE
CA 02848961 2014-03-17 PF 0000072938 SE/PP
6
R1 is a linear or branched, saturated or unsaturated aliphatic
hydrocarbyl radical
having 12 to 14 carbon atoms;
R2 is a linear or branched, saturated or unsaturated aliphatic
hydrocarbyl radical
having 14 to 16 carbon atoms;
each A is independently ethylene, propylene (preferably 1,2-propylene) or
butylene
(preferably 1,2-butylene);
is an integer from 1 to 99,
X is a branched or unbranched alkylene group which has 1 to 10
carbon atoms and
may be substituted by an OH group;
o is 0 or 1;
Mb+ is a cation;
Ya- is a sulfate group, sulfonate group, carboxylate group or phosphate group
(preferably a sulfate or carboxylate group, more preferably a sulfate group);
b is 1, 2 or 3 (preferably 1) and
a is 1 or 2 (preferably 1).
A further aspect of the present invention relates to an aqueous surfactant
formulation
comprising an inventive surfactant mixture, said surfactant formulation
preferably
having a total surfactant content of 0.05 to 5% by weight based on the total
amount of
the aqueous surfactant formulation.
A further aspect of the present invention relates to the use of an inventive
surfactant
mixture or of an inventive surfactant formulation in mineral oil production by
means of
Winsor type Ill microemulsion flooding.
A further aspect of the present invention relates to processes for producing
mineral oil
by means of Winsor type III microemulsion flooding, in which an inventive
aqueous
surfactant formulation is injected into a mineral oil deposit through at least
one injection
well for the purpose of lowering the interfacial tension between oil and water
to
<0.1 mN/m, and crude oil is withdrawn from the deposit through at least one
production well.
Accordingly, a mixture of at least 3 ionic surfactants which differ in terms
of the
hydrocarbyl moiety (R1)(R2)-CH-CH2- and a process for tertiary mineral oil
production
by means of Winsor type III microemulsion flooding are provided, in which an
aqueous
surfactant formulation comprising at least three ionic surfactants which
differ in terms of
the hydrocarbyl moiety (R1)(R2)-CH-CH2- is injected into a mineral oil deposit
through at
least one injection well, the interfacial tension between oil and water is
lowered to
values of <0.1 mN/m, preferably to values of <0.05 mN/m, more preferably to
values
B11/72938US
BASF SE CA 02848961 2014-03-17
PF 0000072938 SE/PP
=
7
of <0.01 mN/m, and crude oil is withdrawn from the deposit through at least
one
production well.
In a preferred embodiment, R1 is a linear or branched, saturated or
unsaturated
aliphatic hydrocarbyl radical having 12 or 14 carbon atoms; and R2 is a linear
or
branched, saturated or unsaturated aliphatic hydrocarbyl radical having 14 or
16
carbon atoms.
In a particularly preferred embodiment, R1 is a linear saturated or
unsaturated
(preferably saturated) aliphatic hydrocarbyl radical having 12 or 14 carbon
atoms; and
R2 is a linear saturated or unsaturated (preferably saturated) aliphatic
hydrocarbyl
radical having 14 or 16 carbon atoms, the result of which is especially the
presence of
at least 3 ionic surfactants of the general formula (I) with hydrocarbyl
radicals having 28
carbon atoms, 30 carbon atoms and 32 carbon atoms. When the molar sum is
formed
from these three surfactants, the 028 surfactant of the general formula (I) is
more
preferably within a range from 40% to 60%, the C30 surfactant of the general
formula (I)
within a range from 30% to 50% and the 032 surfactant of the general formula
(I) within
a range from 1% to 20%, based on the sum. It is additionally preferred that
the
proportion by weight of the 3 ionic surfactants based on the total weight of
the inventive
surfactant mixture is greater than 50% by weight, more preferably greater than
60% by
weight, even more preferably greater than 70% by weight, even more preferably
greater than 80% by weight, most preferably greater than 90% by weight.
Preferably, k is an integer in the range from 4 to 50.
Preferably, the (0X)0Ya- radical in formula (I) is OS(0)20", OCH2CH2S(0)20-,
OCH2CH(OH)CH2S(0)20-, 0(0H2)3S(0)20 , S(0)20 , CH2C(0)0- or CH2CH(R)C(0)0 ,
where R' is hydrogen or an alkyl radical having 1 to 4 carbon atoms (for
example
methyl).
The alkyleneoxy (AO) groups OA in formula (I), which occur k times, may be
the same
or different. If they are different, they may be arranged in random
distribution,
alternately or in blocks, i.e. in two, three, four or more blocks.
Accordingly, (0A)k in formula (I) may represent n butyleneoxy (BuO), m
propyleneoxy
(PO) and I ethyleneoxy (E0) groups, where n, m, I are natural numbers
including 0,
and: n+m+I=k.
Preferably, the n butyleneoxy, m propyleneoxy and I ethyleneoxy groups are at
least
partially arranged in blocks (in numerical terms, preferably to an extent of
at least 50%,
more preferably to an extent of at least 60%, even more preferably to an
extent of at
B11/72938US
BASF SE CA 02848961 2014-03-17
PF 0000072938 SE/PP
=
8
least 70%, more preferably to an extent of at least 80%, more preferably to an
extent of
at least 90%, especially completely).
In the context of the present invention, "arranged in blocks" means that at
least one AO
has a neighboring AO group which is chemically identical, such that these at
least two
AO form a block.
Preferably, the (R1)(R2)-CH-CH2- radical in formula (I) is followed,
representing (04,
by a butyleneoxy block with n butyleneoxy groups, followed by a propyleneoxy
block
with m propyleneoxy groups, and finally an ethyleneoxy block with I
ethyleneoxy
groups.
Preferably, m is an integer from 4 to 15 (more preferably 5 to 9) and/or I is
an integer
from 0 to 25 (more preferably 4 to 15) and/or n is an integer from 2 to 15
(more
preferably 5 to 9).
In a more preferred embodiment, the invention relates to a mixture of three
ionic
surfactants in terms of the hydrocarbyl moiety (R1)(R2)-CH-CH2- and to the use
thereof,
where m is a number from 4 to 15, n is a number from 0 to 15 and 11' is
selected from
the group of sulfate groups, sulfonate groups and carboxylate groups, where
the BuO,
PO and E0 groups are present to an extent of more than 80% in block form in
the
sequence BuO, PO, E0 commencing from (R1)(R2)-CH-CH2, and the sum of I + m + n
is in the range from 5 to 49.
A particularly preferred embodiment is when n is a number from 2 to 15, m is a
number
from 5 to 9, and r- is selected from the group of sulfate groups, sulfonate
groups and
carboxylate groups, where the A and B groups are present to an extent of more
than
80% in block form in the sequence BuO, PO and EO commencing from
(R1)(R2)-CH-CH2, the sum of I + m + n is in the range from 4 to 50 and the BuO
block
consists to an extent of more than 80% of 1,2-butylene oxide.
A preferred inventive surfactant mixture further comprises surfactants of the
formula
H H
I I
IR'C C _______________ 0¨A )k __________ Ya- a/b 1\413+ (II)
I I 0
H H
and of the formula
B11/72938US
BASF SE CA 02848961 2014-03-17
PF 0000072938 SE/PP
=
9
R2 ( 0k ________________ ya- a/b Mb+ (III)
0
where R1, R2, A , X, Ya-, Mb+, k, o, a and b are each as defined for formula
(I).
Preferably, the proportion of surfactants of the formula (I) in relation to
the sum of the
amounts of surfactants of the formulae (I), (II) and (III) is in the range
from 80% by
weight to 99% by weight.
In a particularly preferred embodiment of the invention, in general formula
(II), R1 is a
linear, saturated, aliphatic hydrocarbyl radical having 12 or 14 carbon atoms,
and R2 in
the general formula (III) is a linear, saturated, aliphatic hydrocarbyl
radical having 14 or
16 carbon atoms.
In the process according to the invention as described above for mineral oil
production
by means of Winsor type III microemulsion flooding, an aqueous surfactant
formulation
comprising at least three surfactants of the general formula (I) which differ
in terms of
the hydrocarbyl moiety (R1)(R2)-CH-CH2- is used. It may further comprise
further
surfactants and/or other components.
In the process according to the invention for tertiary mineral oil production
by means of
Winsor type III microemulsion flooding, the use of the inventive surfactant
mixture
lowers the interfacial tension between oil and water to values of < 0.1 mN/m,
preferably
to <0.05 mN/m, more preferably to <0.01 mN/m. The interfacial tension between
Oil
and water is thus lowered to values in the range from 0.1 mN/m to 0.0001 mN/m,
preferably to values in the range from 0.05 mN/m to 0.0001 mN/m, more
preferably to
values in the range from 0.01 mN/m to 0.0001 mN/m.
The three surfactants which differ in terms of the hydrocarbyl moiety (R1)(R2)-
CH-CH2-
can be encompassed by the general formula (I). The difference can arise
through the
number of carbon atoms, the number of unsaturated bonds, the branching
frequency
and/or the degree of branching. More particularly, the surfactants differ in
the chain
length for R1 and R2. By way of example, R1/R2 be hydrocarbyl chains having
12/14,
12/15, 12/16, 13/14, 13/15, 13/16, 14/14, 14/15, 14/16, preferably 12/14,
12/16, 14/14,
14/16, carbon atoms. As a result of the preparation, it is also possible for
more than
three different surfactants of the general formula to be present in the
surfactant
formulation. Preferably, the three surfactants with 28, 30 and 32 carbon atoms
in the
hydrocarbyl moiety (R1)(R2)-CH-CH2- constitute the main components of the
inventive
surfactant mixture. The proportion thereof is preferably at least 25% by
weight, based
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
on the total weight of the surfactant mixture, more preferably at least 30% by
weight,
more preferably at least 40% by weight, more preferably at least 50% by
weight.
The R1 radical is a linear or branched, saturated or unsaturated aliphatic
hydrocarbyl
5 radical having 12 to 14 carbon atoms. The R2 radical is a linear or
branched, saturated
or unsaturated aliphatic hydrocarbyl radical having 14 to 16 carbon atoms. R1
is either
identical to R2 or preferably has not more than two carbon atoms (more
preferably
exactly two carbon atoms) fewer than R2.
10 In the case of branched R1 or R2 radicals, the degree of branching for
R1 or R2 is
preferably in the range of 0.1 ¨5 (preferably of 0.1 ¨ 1.5). For the branched
aliphatic
hydrocarbyl radical (R1)(R2)-CHCH2, this gives rise to a degree of branching
of 1.2 to
11 (preferably 1.2 to 4).
The term "degree of branching" is defined here in a manner known in principle
as the
number of methyl groups in a molecule of the alcohol minus 1. The mean degree
of
branching is the statistical mean of the degrees of branching of all molecules
in one
sample.
However, a preferred embodiment is the use of linear saturated or unsaturated
R1
radicals having 12 or 14 carbon atoms, or R2 having 14 or 16 carbon atoms.
Particular
preference is given to the use of linear saturated R1 and R2 radicals. This
gives a
degree of branching of 1 for the aliphatic hydrocarbyl radical (R1)(R2)-CHCH2.
In the above-defined general formula, I, m and n are each natural numbers
including 0,
i.e. 0, 1, 2 etc. It is, however, clear to the person skilled in the art in
the field of
polyalkoxylates that this definition is the definition of a single surfactant
in each case. In
the case of presence of surfactant mixtures or surfactant formulations which
comprise
a plurality of surfactants of the general formula, the numbers I and m are
each mean
values over all molecules of the surfactants, since the alkoxylation of
alcohol with
ethylene oxide and/or propylene oxide and/or butylene oxide in each case
affords a
certain distribution of chain lengths. This distribution can be described in a
manner
known in principle by what is called the polydispersity D. D = NAJMn is the
quotient of
the weight-average molar mass and the number-average molar mass. The
polydispersity can be determined by means of the methods known to those
skilled in
the art, for example by means of gel permeation chromatography.
Preferably, I is a number from 0 to 99, preferably 1 to 40, more preferably 1
to 20.
Preferably, m is a number from 0 to 99, preferably 1 to 20, more preferably 4
to 15.
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
11
Preferably, n is a number from 0 to 99, preferably 1 to 20, more preferably 2
to 15.
According to the invention, the sum of I + m + n (=k) is a number which is in
the range
from 1 to 99, preferably in the range from 5 to 50, more preferably in the
range from 8
to 39.
In formula (I), X is a branched or unbranched alkylene group which has 1 to 10
and
preferably 2 to 4 carbon atoms and may be substituted by an OH group. The
alkylene
group is preferably a methylene, ethylene or propylene group. More
particularly, X is
preferably CH2CH2, CH2CH(OH)CH2, (CH2)3, CH2 or CH2CH(R), where FR' is
hydrogen
or an alkyl radical having 1 to 4 carbon atoms (for example methyl). X may be
present
(o = 1) or absent (o = 0).
In the above general formula, r- is a sulfonate, sulfate, carboxylate group or
phosphate group (preferably sulfonate, sulfate or carboxylate group,
especially sulfate
and carboxylate). a may thus have values of 1 or 2.
In the above formula, M+ is a cation, preferably a cation selected from the
group of Na;
K+, Li+, NH4+, H+, Mg2+ and Ca2+ (preferably Na+, K4 or NH4+). Overall, b may
have
values of 1, 2 or 3.
The alcohols (R1)(R2)-CH-CH2-0H which can serve as a starting compound for
preparation of the inventive surfactants are obtainable, for example, by the
dimerization
of alcohols of the R1CH2CH2OH and R2OH type with elimination of water.
Accordingly, a further aspect of the present invention is a process for
preparing an
inventive surfactant mixture, comprising the steps of:
(a) preparing Guerbet alcohols of the general formula (IV) (R1)(R2)-CH-
CH2OH (IV),
where R1 and R2 are each as defined above, by condensing a mixture of at least
two primary alcohols of the formula R-CH2-CH2-0H, where R is a linear or
branched, saturated or unsaturated aliphatic hydrocarbyl radical having 12 to
14
carbon atoms,
(b) alkoxylating the alcohols obtained in process step (a),
(c) reacting the alcohol alkoxylates obtained in step (b) with a 11'
group, optionally to
form a spacer group OX.
The preparation of the Guerbet alcohol of the general formula (IV) (R1)(R2)-CH-
CH2OH
in process step (a) is known to those skilled in the art.
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
12
In the course of the Guerbet reaction, primary alcohols are ultimately
dimerized to give
p-branched primary alcohols in the presence of suitable catalysts. The primary
products formed from the alcohol are aldehydes, which subsequently dimerize by
aldol
condensation with elimination of water and subsequent hydrogenation to give
saturated
alcohols. In addition to the main product, the Guerbet alcohol, it is also
possible for
various by-products to form, for example unsaturated 13-branched primary
alcohols if
the hydrogenation of the double bond is incomplete, saturated a-branched
aldehydes if
the hydrogenation to give the Guerbet alcohol was incomplete, or more
particularly
13-branched primary alcohols which have additional branches in the side chain
or main
chain.
The dimerization of the alcohols of the formula R-CH2CH2-0H may give rise to a
mixture of alcohols. This may include a C14C16 fatty alcohol mixture (linear,
saturated), a C14C16 mixture of Ziegler alcohols with 14 and 16 carbon atoms,
a
C14C16 fatty alcohol mixture (linear and partly unsaturated) or a mixture of
C14C16
oxo alcohol.
The dimerization of the alcohols of the formula R-CH2CH2-0H where R is a
linear or
branched, saturated or unsaturated aliphatic hydrocarbyl radical having 12 or
14
carbon atoms affords, in a preferred embodiment of the invention, Guerbet
alcohols
having 28, 30 and 32 carbon atoms.
In a particularly preferred embodiment, R is a linear saturated or unsaturated
(preferably saturated) aliphatic hydrocarbyl radical having 12 or 14 carbon
atoms.
To prepare the Guerbet alcohols in process step (a), mixtures of the alcohols
(II) are
condensed. Preferably, the proportion of alcohols where R = 12 is between 50 ¨
80
mol%, the proportion of alcohols where R = 14 between 20 ¨ 50 mol%. Particular
preference is given to reacting about 66 mol% of alcohols where R = 12 and 33
mol%
of alcohols where R = 14.
The condensation of alcohols (II) to give Guerbet alcohols is preferably
performed in
the presence of 0.5 to 10% by weight, based on the alcohol, of alkali metal or
alkaline
earth metal hydroxide, for example lithium hydroxide, sodium hydroxide, cesium
hydroxide or potassium hydroxide, preferably potassium hydroxide. With a view
to a
high reaction rate and a low proportion of secondary components, it will be
necessary
to use the alkali metal hydroxide or alkaline earth metal hydroxide in a
concentration of
3 to 6% by weight, based on the alcohol. The alkali metal hydroxide or
alkaline earth
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
13
metal hydroxide can be used in solid form (flakes, powder) or in the form of a
30 to
70%, preferably 50%, aqueous solution.
In a preferred embodiment, the alcohols of the formula (II) are condensed in
the
presence of NaOH and/or KOH.
Suitable catalyst(s) are the catalysts known from the prior art, for example
in
US3119880 (nickel, lead salts), in US35558716 (copper, lead, zinc, chromium,
molybdenum, tungsten und manganese oxides), in US 3979466 (palladium
complexes)
or else in US 3864407 (silver complexes). Preference is given to using ZnO as
a
catalyst for the dimerization.
The catalyst(s) preferably comprise(s) ZnO catalysts, which are generally
added to the
mixture from which the Guerbet alcohols are prepared.
The mixture of Guerbet alcohols can be prepared by the process known from DE
3901095 Al.
In a preferred embodiment of the invention, the Guerbet alcohols are
synthesized in
process step (a) at a temperature in the range from 150 to 320 C, preferably
at a
temperature in the range from 180 to 280 C, optionally in the presence of a
catalyst or
catalysts.
The surfactants of the general formula can be prepared in a manner known in
principle
by alkoxylating corresponding alcohols (R1)(R2)-CH-CH2-0H in process step (b).
The
performance of such alkoxylations is known in principle to those skilled in
the art. It is
likewise known to those skilled in the art that the molar mass distribution of
the
alkoxylates can be influenced through the reaction conditions, especially the
selection
of the catalyst.
The surfactants of the general formula can preferably be prepared in process
step (b)
by base-catalyzed alkoxylation. In this case, the alcohol (R1)(R2)-CH-CH2-0H
can be
admixed in a pressure reactor with alkali metal hydroxides, preferably
potassium
hydroxide, or with alkali metal alkoxides, for example sodium methoxide. Water
still
present in the mixture can be drawn off by means of reduced pressure (for
example
< 100 mbar) and/or increasing the temperature (30 to 150 C). Thereafter, the
alcohol is
present in the form of the corresponding alkoxide. This is followed by
inertization with
inert gas (for example nitrogen) and stepwise addition of the alkylene
oxide(s) at
temperatures of 60 to 180 C up to a maximum pressure of 10 bar. In a preferred
embodiment, the alkylene oxide is metered in initially at 130 C. In the course
of the
reaction, the temperature rises up to 170 C as a result of the heat of
reaction released.
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
14
In a further preferred embodiment of the invention, the butylene oxide is
first added at a
temperature in the range from 125 to 145 C, then the propylene oxide is added
at a
temperature in the range from 130 to 145 C, and then the ethylene oxide is
added at a
temperature in the range from 125 to 155 C. At the end of the reaction, the
catalyst can
be neutralized, for example by adding acid (for example acetic acid or
phosphoric
acid), and filtered off if required.
However, the alkoxylation of the alcohols (R1)(R2)-CH-CH2-0H can also be
undertaken
by means of other methods, for example by acid-catalyzed alkoxylation. In
addition, it is
possible to use, for example, double hydroxide clays, as described in DE
4325237 Al,
or it is possible to use double metal cyanide catalysts (DMC catalysts).
Suitable DMC
catalysts are disclosed, for example in DE 10243361 Al, especially in
paragraphs
[0029] to [0041] and the literature cited therein. For example, it is possible
to use
catalysts of the Zn-Co type. To perform the reaction, the alcohol (R1)(R2)-CH-
CH2-0H
can be admixed with the catalyst, and the mixture can be dewatered as
described
above and reacted with the alkylene oxides as described. Typically not more
than 1000
ppm of catalyst based on the mixture are used, and the catalyst can remain in
the
product owing to this small amount. The amount of catalyst may generally be
less than
1000 ppm, for example 250 ppm or less.
Process step (c) relates to the reaction of the alcohol alkoxylates obtained
in step (b)
with a Ya- group, optionally with formation of a spacer group OX.
For example, it is possible to introduce sulfate and phosphate groups by
reacting them
with the alcohol directly (optionally after activation). Sulfonate groups can
be introduced
by vinyl addition, substitution reaction or aldol reaction, optionally with
subsequent
hydrogenation, to obtain corresponding spacers OX. Alternatively, the alcohol
can also
be converted to a chloride beforehand, which is subsequently amenable to a
direct
sulfonation. Carboxylates can be obtained, for example, by reaction with
chloroacetate,
acrylate or substituted acrylates H2C:---(R)C(0)0-, where IR is H or an alkyl
radical
having 1 to 4 carbon atoms.
In principle, the anionic Ya- group is composed of the functional Ya- group,
which is a
sulfate, sulfonate, carboxylate or phosphate group, and the spacer OX, which
in the
simplest case may be a single bond (o = 0). In the case of a sulfate group, it
is
possible, for example, to employ the reaction with sulfuric acid,
chlorosulfonic acid or
sulfur trioxide in a falling-film reactor with subsequent neutralization. In
the case of a
sulfonate group, it is possible, for example, to employ the reaction with
propane sultone
and subsequent neutralization, with butane sultone and subsequent
neutralization, with
vinylsulfonic acid sodium salt or with 3-chloro-2-hydroxypropanesulfonic acid
sodium
salt. To prepare sulfonates, the terminal OH group can also be converted to a
chloride,
for example with phosgene or thionyl chloride, and then reacted, for example,
with
B11/72938US
= BASF SE
CA 02848961 2014-03-17 PF 0000072938 SE/PP
sulfite. In the case of a carboxylate group, it is possible, for example, to
employ the
oxidation of the alcohol with oxygen and subsequent neutralization, or the
reaction with
chloroacetic acid sodium salt. Carboxylates can also be obtained, for example,
by
Michael addition of (meth)acrylic acid or ester. Phosphates can be obtained,
for
5 example, by esterification reaction with phosphoric acid or phosphorus
pentachloride.
In addition to the surfactants of the general formulae (I), (II) and (III),
the formulation
may additionally optionally comprise further surfactants. These are, for
example,
anionic surfactants of the alkylarylsulfonate, petroleumsulfonate or
olefinsulfonate
10 (alpha-olefinsulfonate or internal olefinsulfonate) type and/or nonionic
surfactants of the
alkyl ethoxylate or alkyl polyglucoside type. It is also possible to use
betaine
surfactants. These further surfactants may especially also be oligomeric or
polymeric
surfactants. It is advantageous to use such polymeric cosurfactants to reduce
the
amount of surfactants needed to form a microemulsion. Such polymeric
cosurfactants
15 are therefore also referred to as "microemulsion boosters". Examples of
such polymeric
surfactants comprise amphiphilic block copolymers which comprise at least one
hydrophilic block and at least one hydrophobic block. Examples comprise
polypropylene oxide-polyethylene oxide block copolymers, polyisobutene-
polyethylene
oxide block copolymers, and comb polymers with polyethylene oxide side chains
and a
hydrophobic main chain, where the main chain preferably comprises essentially
olefins
or (meth)acrylates as monomers. The term "polyethylene oxide" here should in
each
case include polyethylene oxide blocks comprising propylene oxide units as
defined
above. Further details of such surfactants are disclosed in WO 2006/131541 Al.
In the process according to the invention for mineral oil production, a
suitable aqueous
formulation of the surfactants of the general formula is injected through at
least one
injection well into the mineral oil deposit, and crude oil is withdrawn from
the deposit
through at least one production well. The term "crude oil" in this context of
course does
not mean single-phase oil, but rather the usual crude oil-water emulsions. In
general, a
deposit is provided with several injection wells and with several production
wells.
The main effect of the surfactant lies in the reduction of the interfacial
tension between
water and oil ¨ desirably to values significantly < 0.1 mN/m. After the
injection of the
surfactant formulation, known as "surfactant flooding", or preferably the
Winsor type III
"microemulsion flooding", the pressure can be maintained by injecting water
into the
formation ("water flooding") or preferably a higher-viscosity aqueous solution
of a
polymer with strong thickening action ("polymer flooding"). Also known,
however, are
techniques by which the surfactants are first of all allowed to act on the
formation. A
further known technique is the injection of a solution of surfactants and
thickening
polymers, followed by a solution of thickening polymer. The person skilled in
the art is
aware of details of the industrial performance of "surfactant flooding",
"water flooding",
B11/72938US
BASF SE CA 02848961 2014-03-17
PF 0000072938 SE/PP
16
and "polymer flooding", and employs an appropriate technique according to the
type of
deposit.
For the process according to the invention, an aqueous formulation which
comprises
= 5 surfactants of the general formula (I) is used. In addition to
water, the formulations may
optionally also comprise water-miscible or at least water-dispersible organic
substances or other substances. Such additives serve especially to stabilize
the
surfactant solution during storage or transport to the oil field. The amount
of such
additional solvents should, however, generally not exceed 50% by weight,
preferably
20% by weight. In a particularly advantageous embodiment of the invention,
exclusively
water is used for formulation. Examples of water-miscible solvents include
especially
alcohols such as methanol, ethanol and propanol, butanol, sec-butanol,
pentanol, butyl
ethylene glycol, butyl diethylene glycol or butyl triethylene glycol.
In a preferred embodiment of the invention, the three surfactants of the
general formula
(I) which differ in terms of the hydrocarbyl moiety (R1)(R2)-CH-0H2- should
constitute
the main component among all surfactants in the formulation which is
ultimately
injected into the deposit. These are preferably at least 25% by weight, more
preferably
at least 30% by weight, even more preferably at least 40% by weight and even
more
preferably still at least 50% by weight of all surfactants used.
The mixture used in accordance with the invention can preferably be used for
surfactant flooding of deposits. It is especially suitable for Winsor type III
microemulsion
flooding (flooding in the Winsor Ill range or in the range of existence of the
bicontinuous microemulsion phase). The technique of microemulsion flooding has
already been described in detail at the outset.
In addition to the surfactants, the formulations may also comprise further
components,
for example C4 to 08 alcohols and/or basic salts (so-called "alkali surfactant
flooding").
Such additives can be used, for example, to reduce retention in the formation.
The ratio
of the alcohols based on the total amount of surfactant used is generally at
least 1:1 ¨
however, it is also possible to use a significant excess of alcohol. The
amount of basic
salts may typically range from 0.1% by weight to 5% by weight. It is
optionally possible
to add chelating agents (for example EDTA) to the basic salts ¨ typically
0.03% by
weight to 5% by weight.
The deposits in which the process is employed generally have a temperature of
at least
10 C, for example 10 to 150 C, preferably a temperature of at least 15 C to
120 C.
The total concentration of all surfactants together is 0.05 to 5% by weight,
based on the
total amount of the aqueous surfactant formulation, preferably 0.1 to 2.5% by
weight.
The person skilled in the art makes a suitable selection according to the
desired
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
17
properties, especially according to the conditions in the mineral oil
formation. It is clear
here to the person skilled in the art that the concentration of the
surfactants can change
after injection into the formation because the formulation can mix with
formation water,
or surfactants can also be absorbed on solid surfaces of the formation. It is
the great
advantage of the mixture used in accordance with the invention that the
surfactants
lead to a particularly good lowering of interfacial tension.
It is of course possible and also advisable first to prepare a concentrate
which is only
diluted on site to the desired concentration for injection into the formation.
In general,
the total concentration of the surfactants in such a concentrate is 10 to 45%
by weight.
Examples
Part I: Synthesis of the surfactants
General method 1: Preparation of the Guerbet alcohol
In a 1 I flask, the alcohol(s) (1 eq.) is/are initially charged and, if
necessary, melted at
50 C. KOH powder (0.24 eq.) and zinc oxide (5% by weight based on the starter
alcohol) are added while stirring. The reaction mixture is heated as quickly
as possible
to 180-230 C and the water of reaction which forms is distilled off via a
distillation
outlet. For the fastest possible removal of the water of reaction, the glass
flask is
optionally insulated with aluminum foil. The reaction mixture is stirred at
the given
temperature for a further 6 to 30 hours. The alcohol mixture formed is
analyzed by gas
chromatography and used for the subsequent alkoxylation without further
workup.
General method 2: Alkoxylation by means of KOH catalysis (relevant to use
of E0,
PO and/or 1,2-BuO)
In a 21 autoclave, the alcohol to be alkoxylated (1.0 eq) is optionally
admixed with an
aqueous KOH solution comprising 50% by weight of KOH. The amount of KOH is
0.2%
by weight of the product to be prepared. While stirring, the mixture is
dewatered at
100 C and 20 mbar for 2 h. This is followed by purging with N2 three times,
establishment of a supply pressure of approx. 1.3 bar of N2, and an increase
in the
temperature to 120 to 130 C. The alkylene oxide is metered in such that the
temperature remains between 125 C and 155 C (in the case of ethylene oxide) or
130
and 145 C (in the case of propylene oxide) or 125 and 145 C (in the case of
1,2-butylene oxide). This is followed by stirring at 125 to 145 C for a
further 5 h,
purging with N2, cooling to 70 C and emptying of the reactor. The basic crude
product
is neutralized with the aid of acetic acid. Alternatively, the neutralization
can also be
effected with commercial magnesium silicates, which are subsequently filtered
off. The
light-colored product is characterized with the aid of a 1H NMR spectrum in
CDCI3, gel
B11/72938US
BASF SE CA 02848961 2014-03-17
PF 0000072938 SE/PP
=
18
permeation chromatography and an OH number determination, and the yield is
determined.
General method 3: Alkoxylation by means of DMC catalysis
In a 2 I autoclave, the alcohol to be alkoxylated (1.0 eq) is mixed with a
double metal
cyanide catalyst (for example DMC catalyst of the Zn-Co type from BASF) at 80
C. To
activate the catalyst, approximately 20 mbar is applied at 80 C for 1 h. The
amount of
DMC is 0.1% by weight or less of the product to be prepared. This is followed
by
purging three times with N2, establishment of a supply pressure of approx. 1.3
bar of N2
and a temperature increase to 120 to 130 C. The alkylene oxide is metered in
such
that the temperature remains between 125 C and 135 C (in the case of ethylene
oxide)
or 130 and 140 C (in the case of propylene oxide) or 135 and 145 C (in the
case of
1,2-butylene oxide). This is followed by stirring at 125 to 145 C for a
further 5 h,
purging with N2, cooling to 70 C and emptying of the reactor. The light-
colored product
is characterized with the aid of a 1H NMR spectrum in CDCI3, gel permeation
chromatography and OH number determination, and the yield is determined.
General method 4: Sulfonation by means of chlorosulfonic acid
In a 1 I round-neck flask, the alkyl alkoxylate to be sulfonated (1.0 eq) is
dissolved in
1.5 times the amount of dichloromethane (based on percent by weight) and
cooled to 5
to 10 C. Thereafter, chlorosulfonic acid (1.1 eq) is added dropwise such that
the
temperature does not exceed 10 C. The mixture is allowed to warm up to room
temperature and is stirred under an N2 stream at this temperature for 4 h
before the
above reaction mixture is added dropwise to an aqueous NaOH solution of half
the
volume at max. 15 C. The amount of NaOH is calculated to give rise to a slight
excess
based on the chlorosulfonic acid used. The resulting pH is approx. pH 9 to 10.
The
dichloromethane is removed at max. 50 C on a rotary evaporator under gentle
vacuum.
The product is characterized by 1H NMR and the water content of the solution
is
determined (approx. 70%).
For the synthesis, the following alcohols are used.
Alcohol Description
014016 Commercially available fatty alcohol mixture consisting
of linear
C14H29-0H and C16H33-0H
C28-Guerbet commercial Guerbet alcohol 2-Dodecylhexadecan-1-ol
C32-Guerbet commercial Guerbet alcohol 2-Tetradecyloctadecan-1-ol
B11/72938US
BASF SE CA 02848961 2014-03-17
PF 0000072938 SE/PP
19
Performance tests
The surfactants obtained are used to conduct the following tests in order to
assess the
= 5 suitability thereof for tertiary mineral oil production.
Description of the test methods
Determination of SP*
a) Principle of the measurement:
The interfacial tension between water and oil is determined in a known manner
via the
measurement of the solubilization parameter SP*. The determination of the
interfacial
tension via the determination of the solubilization parameter SP* is a method
for
approximate determination of the interfacial tension which is accepted in the
technical
field. The solubilization parameter SP* indicates how many ml of oil are
dissolved per
ml of surfactant used in a microemulsion (Winsor type III). The interfacial
tension (IFT)
can be calculated therefrom via the approximate formula IFT 0.3/(SP*)2, if
equal
volumes of water and oil are used (C. Huh, J. Coll. Interf. Sc., Vol. 71, No.
2 (1979)).
b) Procedure
To determine the SP*, a 100 ml measuring cylinder with a magnetic stirrer bar
is filled
with 20 ml of oil and 20 ml of water. To this are added the concentrations of
the
particular surfactants. Subsequently, the temperature is increased stepwise
from 20 to
90 C, and the temperature window in which a microemulsion forms is observed.
The formation of the microemulsion can be assessed visually or else with the
aid of
conductivity measurements. A triphasic system forms (upper oil phase, middle
microemulsion phase, lower water phase). When the upper and lower phase are of
equal size and do not change over a period of 24 h, the optimal temperature
(T.,t) of
the microemulsion has been found. The volume of the middle phase is
determined. The
volume of surfactant added is subtracted from this volume. The value obtained
is then
divided by two. This volume is then divided by the volume of surfactant added.
The
result is noted as SP*.
The type of oil and water used to determine SP* is determined according to the
system
to be examined. It is possible either to use mineral oil itself or a model
oil, for example
decane. The water used may either be pure water or saline water, in order
better to
model the conditions in the mineral oil formation. The composition of the
aqueous
B11/72938US
= BASF SE
CA 02848961 2014-03-17 PF 0000072938 SE/PP
phase can be adjusted, for example, according to the composition of a
particular
deposit water. Alternatively, an aqueous NaCI solution can also be used.
For the purpose of comparability with known systems, the surfactants are
optionally
5 combined and tested with anionic cosurfactants and cosolvents.
A further possible test form is that of the determination of the interfacial
tension of
crude oil in the presence of the surfactant solution at an appropriate
temperature by the
spinning drop method on an SVT20 from DataPhysics. For this purpose, an oil
droplet
10 is injected into a capillary filled with saline surfactant solution and
the expansion of the
droplet at approx. 4500 revolutions per minute is observed until a constant
value is
established. This is typically the case after 2 h. The interfacial tension IFT
(or GO is
calculated - as described by Hans-Dieter Dorfler in "Grenzflachen und kolloid-
disperse
Systeme [Interfaces and colloidally disperse systems]" Springer Verlag Berlin
15 Heidelberg 2002 - by the following formula from the cylinder diameter
dz, the angular
speed co and the density difference (d1-d2):
-7: 0.25 = dz3 = 0)2 = (d1-d2)
For the spinning-drop-experiment in this case crude oil from a reservoir was
used at
20 20 C. Crude oil has 16 API. Reservoir temperature is around 20 C.
Formation water
contains salt. Artificial water is made out of NaCI und NaHCO3.
A surfactant solution is used, which could be injected into the formation.
Beside
surfactnts it also contains water and salt. As additional salt component 0.25%
Na2CO3
has been added. Surfactant solution contained 0.1% of a mixture out of alkyl
ether
sulfate of type Guerbetalky1-7Bu0-7P0-10E0-Sulfate und Petrostep S3B (internal
olefin sulfonate from Stepan) and 0,05% butyl diethylene glycol and 0.07%
Sokalan
PA 20 (polyacrylate sodium salt). As alkyl ether sulfates inventive and non-
inventive
und surfactants were used. In case of latter surfactants examples were marked
with 'V'.
Surfactant concentration and amount of Na2CO3 refers to active compound and
are
given in weight percent of the aqueous phase.
Test results of spinning-drop-experiment are shown in table 1.
Table 1 Tests with crude oil at 20 C
Exa Surfactant solution Na2CO3 NaCI + IFT
mpl NaHCO
3
V1 0,08% C28-Guerbet-7Bu0-7P0-10E0-Sulfat, 0.25% 1.2% + 0,0564
0,02% Petrostep S3B, 0,05% butyl diethylene 0.41% mN/m
B11/72938US
BASF SE CA 02848961 2014-03-17 PF
0000072938 SE/PP
21
glycole, 0.07% Sokalan PA 20 (rest water and
salt as shown on the right side)
V2 0,08% C32-Guerbet-7Bu0-7P0-10E0-Sulfat, 0.25% 1.2% + 0,0415
0,02% Petrostep S3B, 0,05% butyl diethylene 0.41% mN/m
glycole, 0.07% Sokalan PA 20 (rest water and
salt as shown on the right side)
3 0,08% C28C30C32-Guerbet-7Bu0-7P0-10E0- 0.25% 1.2% + 0,0079
Sulfat, 0,02% Petrostep S3B, 0,05% butyl 0.41% mN/m
diethylene glycole, 0.07% Sokalan PA 20 (rest
water and salt as shown on the right side)
As shown in example! V1 and V2 of table 1 non-inventive surfactants based on
only
one Guerbet alcohol (C28-Guerbert at V1 and C32-Guerbet at V2) give only
moderate
interfacial tension of 0.0564 mN/m and 0.0415 mN/m. If inventive surfactants
based on
a mixture of at least 3 guerbet alcohols (C28C30032-Guerbet) are used under
identical
conditions as shown in example, surprisingly ultralow interfacial tension of
<0.01 mN/m
( 0.0079 mN/m in example 3) were found. This is even more surprising as
mixture in
example 3 contains single surfactants out of V1 and V2.
B11/72938US