Note: Descriptions are shown in the official language in which they were submitted.
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
HARVESTING FAT TISSUE USING TISSUE LIQUEFACTION
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of US provisional application
61/536,896,
filed September 20, 2011, which is incorporated herein by reference.
BACKGROUND
[0002] In certain circumstances, it may be desirable to harvest fat from
one location
of a patient's body and introduce the extracted fat into a second anatomic
location of the
patient. One common procedure for fat harvesting is the Coleman approach. In
the Coleman
approach, fat tissue is extracted from a source location (e.g., the buttocks)
using a syringe.
The tissue that is extracted is then centrifuged for a specified length of
time at particular
settings. After centrifuging, the high density portion is on the bottom and
the low density
portion is on top. The high density portion of the centrifuged matter is then
selected (e.g. by
skimming off the top one third or top one half and discarding the skimmed-off
portion). The
high density portion is then injected into the target site (e.g. a breast).
The Coleman approach
has a number of disadvantages, including the fact that it is difficult to
obtain a large volume
of tissue rapidly. Other possible sources of fat include fat that is obtained
by a conventional
liposuction technique e.g., Suction Assisted Lipoplasty ("SAL") or Vaser-
Ultrasonic Assisted
Lipoplasty ("V-UAL"). But the fat that is obtained using these liposuction
procedures is not
ideal for reintroduction to the patient's body due to low-viability issues and
other problems.
[0003] In other circumstances, it may be desirable to harvest adipose
stem cells from
a patient's body for subsequent use. This is sometimes referred to as stem
cell isolation. One
conventional approach for isolating stem cells is to start with a lipoaspirate
from a
1
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
conventional liposuction technique (e.g., SAL or V-UAL). The lipoaspirate is
first gravity-
separated into a supranatant (which contains mostly fat) and an infranatant
(which contains
mostly blood and fluids that were injected during the liposuction). The
supranatant is then
treated with the collagenase to separate the cells from each other. After the
collagenase
treatment, the supranatant is centrifuged, which separates the supranatant
into three layers: a
second generation supranatant on top, an infranatant beneath the supranatant,
and a stromal
vascular fraction ("SVF") beneath the infranatant. The SVF contains adipose
stem cells
which can then be used for all permitted purposes. But this approach is
problematic because
it requires collagenase, which can be difficult to remove, and can be very
dangerous.
SUMMARY
[0004] With the methods and apparatuses described herein, portions of
fatty tissue are
drawn into orifices in a cannula, and a heated solution is impinged against
those portions of
tissue. The heated solution liquefies or gellifies parts of the fatty tissue,
so they can be
removed from the patient's body more easily. The fat that is so removed is
better suited for
reintroduction into a patient's body as compared to fat that is harvested
using other
approaches. The fat that is removes using the methods and apparatuses
described herein can
also be used as a raw material for stem cell isolation, without relying on the
use of
collagenase.
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] FIG. 1 shows an embodiment of a tissue liquefaction system.
[0006] FIG. 2 is a detail of the distal end of the FIG. 1 embodiment.
2
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
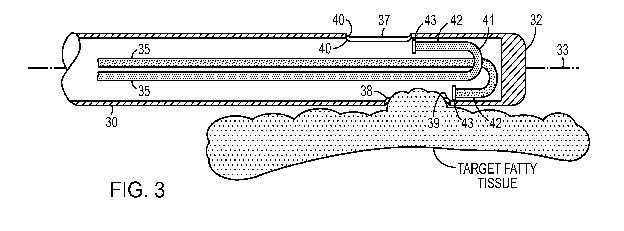
[0007] FIG. 3 is a section view of alternative configuration for the
distal end of the
FIG. 1 embodiment.
[0008] FIG. 4 is a detail of another alternative configuration for the
distal end of the
FIG. 1 embodiment.
[0009] FIGS. 5 and 5A show another embodiment of a tissue liquefaction
system,
which includes a forward-facing external tumescent spray applicator.
[0010] FIG. 6 shows some variations of the distal end of the cannula.
[0011] FIG. 7 shows how the cannula can be configured with external fluid-
supply
paths, in less preferred embodiments.
[0012] FIG. 8 shows how the cannula can be configured with the fluid
supply paths
internal to the suction path.
[0013] FIG. 9 shows a cannula with a single fluid supply tube internal to
the suction
path
[0014] FIG. 10 shows a cannula configuration with two internal fluid
supply tubes.
[0015] FIG. 11 shows a cannula having two fluid supply paths internal to
the suction
path.
[0016] FIG. 12 shows a cannula with six fluid supply paths internal to
the suction
path.
[0017] FIG. 13 shows an alternative cannula configuration with six
internal fluid
supply paths.
3
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
[0018] FIG. 14 is a block diagram of a suitable fluid heating and
pressurization
system.
[0019] FIG. 15 shows a high speed camera fluid supply image and pressure
rise
graph.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0020] The embodiments described below generally involve the delivery of
pressurized heated biocompatible fluid to heat targeted tissue and soften,
gellify, or liquefy
the target tissue for removal from a living body. The heated biocompatible
fluid is preferably
delivered as a series of pulses, but in alternative embodiments may be
delivered as a
continuous stream. After the tissue has been softened, gellified, or
liquefied, it is sucked
away out of the subject's body.
[0021] The interaction with the subject takes place at a cannula 30,
examples of
which are depicted in FIGS. 1-4. The distal end of cannula is preferably
smooth and rounded
for introduction into the subject's body, and the proximal end of the cannula
is configured to
mate with a handpiece 20. The cannula 30 has an interior cavity with one or
more orifice
ports 37 that open into the cavity. These orifices 37 are preferably located
near the distal
portion of the cannula 30. When a low pressure source is connected up to the
cavity via a
suitable fitting, suction is generated which draws target tissue into the
orifice ports 37.
[0022] The cannula also includes one or more fluid supply tubes 35 that
direct the
heated fluid onto the target tissue that has been drawn into the cavity. These
fluid supply
tubes are preferably arranged internally to the outside wall of the cannula
(as shown in FIG.
8), but in alternative embodiments may be external to the cannula for a
portion of the length
of the supply tube (as shown in FIG. 7). The heated fluid supply tubes 35
preferably
4
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
terminate within the outside wall of the cannula, in the vicinity of the
suction orifice ports 37.
The fluid supply tubes 35 are arranged to spray the fluid across the orifice
ports 37 so that the
fluid strikes the target tissue that has been drawn into the cavity. Delivery
of the tissue fluid
stream is preferably contained within the outer wall of the cannula.
[0023] The fluid delivery portion may be implemented using a fluid supply
reservoir
4, a heat source 8 that heats the fluid in the reservoir 4, and a temperature
regulator 9 that
controls the heat source 8 as required to maintain the desired temperature.
The heated fluid
from the fluid supply 4 is delivered under pressure by a suitable arrangement
such as a pump
system 19 with a pressure regulator 11. Optionally, a heated fluid metering
device 12 may
also be provided to measure the fluid that has been delivered.
[0024] Pump 19 pumps the heated fluid from the reservoir or fluid supply
source 4
down the fluid supply tubes 35 that run from the proximal end of the cannula
30 down to the
distal end of the cannula. Near the distal tip of the cannula, these fluid
supply tubes
preferably make a U-turn so as to face back towards the proximal end of the
cannula 30. As a
result, when the heated fluid exits the supply tube 35 at the supply tube's
delivery orifice 43,
the fluid is traveling in a substantially distal-to-proximal direction.
Preferably, the pump
delivers a pressurized, pulsating output of heated fluid down the supply tube
35 so that a
series of boluses of fluid are ejected from the delivery orifice 43, as
described in greater
detail below.
[0025] The vacuum source and the fluid source interface with the cannula
30 via a
handpiece 20. The heated solution supply is connected on the proximal side of
hand piece 20
with a suitable fitting, and a vacuum supply is also connected to the proximal
side of
handpiece 20 with a suitable fitting. Cannula 30 is connected to the distal
side of hand piece
20 with suitable fittings so that (a) the heated fluid from the fluid supply
is routed to the
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
supply tubes 35 in the cannula and (b) the vacuum is routed from the vacuum
source 14 to the
cavity in the cannula, to evacuate material from the cavity.
[0026] More specifically, the pressurized heated solution that is
discharged from
pump 19 is connected to the proximal end of the handle 20 via high pressure
flexible tubing,
and routed through the handpiece 20 to the cannula 30 with an interface made
using an
appropriate fitting. The vacuum source 14 is connected to an aspiration
collection canister
15, which in turn is connected to the proximal end of the handle via flexible
tubing 16 or
other fluid coupling, and then routed through the handpiece 20 to the cannula
30 with an
interface made using an appropriate fitting.
[0027] In the fat harvesting embodiments discussed below, the aspiration
collection
canister 15, and the flexible tubing 16 are preferably sterile, and optionally
disposable.
Optionally, a cooling system (not shown) may be added to cool the matter that
is suctioned
into the collection container in order to extend the life of the fat cells.
The cooling may take
place using any conventional approach while the aspirated material is in the
tubing on its way
into the collection canister 15, or alternatively in the collection canister
itself A wide variety
of cooling systems may be used, including but not limited to compressor /
evaporator based
systems, Peltier based systems, and ice or cold water-jacket based systems. In
situations
where the cooling takes place in the tubing 16, the degree of cooling is
preferably not so
severe so as to cause the aspirate to coagulate in the tubing.
[0028] The pressurized fluid supply line connection between the handle
and the
cannula 30 may be implemented using a high pressure quick disconnect fitting
located at the
distal end of the handle, and configured so that once the cannula is inserted
into the distal end
of the handle it aligns and connects with both the fluid supply and the vacuum
supply. The
cannula 30 may be held in place on the handle 20 by an attachment cap.
6
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
[0029] As best seen in FIG. 3, after the cannula 30 is inserted into the
body; vacuum
source 14 creates a low pressure region within cannula 30 such that the target
fatty tissue is
drawn into the cannula 30 through suction orifice 37. The geometry of the end
of the supply
tube 35 is configured so the trajectory of the boluses leaving the delivery
orifice will strike
the fatty tissue that has been drawn into the cannula 30 through suction
orifice 37. For that
purpose, the end of the supply tube preferably points in direction that is
substantially parallel
to that of the inside wall of the cannula 30 where it is affixed. Preferably,
it is oriented that
the stream flows across the orifice in a distal to proximal direction. This
placement of the tip
43 of the supply tube 35 advantageously maximizes the energy transfer (kinetic
and thermal)
to the fatty tissues, minimizes fluid loss, and helps prevent clogs by pushing
the heated fluid
and the liquefied/gellified/softened material in the same direction that it is
being pulled by the
vacuum source.
[0030] Once the targeted fatty tissue enters the suction orifice 37, it
is repeatedly
struck by the boluses of heated fluid that are exiting the supply tubes 35 via
the delivery
orifice 43. The target fatty tissue is heated by the impinging boluses of
fluid and is softened,
gellified, or liquefied. After that occurs, the loose material in the cavity
(i.e., the heated fluid
and the portions of tissue that were dislodged by the fluid) is drawn away
from the
surrounding tissue by the vacuum source 14, and is deposited into the canister
15 (shown in
FIG. 1).
[0031] Advantageously, fat is more readily softened, gellified, or
liquefied (as
compared to other types of tissue), so the process targets subcutaneous fat
more than other
types of tissue. Note that the distal-to-proximal direction of the boluses is
the same as the
direction that the liquefied/gellified tissue travels when it is being
suctioned out of the patient
via the cannula 30. By having the fluid stream flow in the distal to proximal
direction,
7
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
additional energy (vacuum, fluid thermal and kinetic) is transferred in the
same direction,
which aids in moving the aspirated tissues through the cannula. This further
contributes to
reducing clogs, which can reduce the time it takes to perform a procedure.
[0032] Notably, in the embodiments described herein, the majority of the
fluid stays
within the interior of the cannula during operation (although a small amount
of fluid may
escape into the subject's body through the suction orifices 37). This is
advantageous because
minimizing fluid leakage from the cannula into the tissue maximizes the energy
transfer
(thermal and kinetic) from the fluid stream to the tissue drawn into the
cannula for
liquefaction.
[0033] The fluid supply portion of the system will now be described with
additional
detail. FIG. 3 depicts a cut-away view of an embodiment of the cannula 30 that
has two
supply tubes 35. Each of the supply tubes 35 is provided for delivering the
heated fluid.
Supply tube 35 extends from the proximal portion of cannula 30 to the distal
tip 32 of
cannula 30. Supply tube 35 extends along the interior of cannula 35 and may be
a separate
structure secured to the interior of cannula 35 or lumen integrated into the
wall of cannula 30.
Supply tube 35 is configured to deliver heated biocompatible solution for
liquefying tissue.
The heated solution is delivered through hand piece 20 and into supply tube
35.
[0034] The supply tube 35 extends longitudinally along axis 33 from the
proximal
end 31 to the distal tip 32. Supply tube 35 includes U-bend 41, effectively
turning the run of
the supply tube 35 along the inner wall of the distal tip 32. Adjacent the
terminal end of u-
bend 41 is supply tube terminal portion 42, which includes delivery orifice
43. Delivery
orifice 43 is configured to direct heated solution exiting supply tube 35
across suction orifice
port 37. In this manner, supply tube 35 is configured to direct the fluid onto
a target tissue
that has entered the cannula 30 through the suction orifice port 37.
8
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
[0035] Heated solution supply tube 35 may be constructed of surgical
grade tubing.
Alternatively, in embodiments wherein the heated solution supply tube is
integral to the
construction of cannula 30, the supply tube 35 may be made of the same
material as cannula
30. The diameter of supply tube 35 may be dependent on the target tissue
volume
requirements for the heated solution and on the number of supply tubes
required to deliver
the heated solution across the one or more suction orifice ports 37. The
cannula 30 tube
diameters vary with the cannula outside diameters and those can range from 2-6
mm. The
fluid supply tube 35 diameters are dependent on the inside diameters of the
tubes. A
preferred range of supply tube 35 diameters is from about 0.008" to 0.032". In
one preferred
embodiment, the supply tube 35 is a 0.02" diameter for the length of the
cannula 30, with an
exit nozzle formed by reducing the diameter to 0.008" over the last 0.1". The
shape and size
of delivery orifice 43 may vary, including reduced diameter and flattened
configurations,
with the reduced diameter being preferred.
[0036] In alternative embodiments, the cannula 30 may have a different
number of
heated solution supply tubes 35, each corresponding to a respective suction
orifice port. For
example, a cannula 30 with three suction orifice ports 37 would preferably
include three
heated solution supply tubes 35. Additionally, heated solution supply tubes
may be added to
accommodate one or more suction orifice ports, e.g., when four suction orifice
ports are
provided, four heated solution supply tubes may be provided. In another
embodiment, a
supply tube 35 may branch into multiple tubes, each branch servicing a suction
orifice port.
In another embodiment, one or more supply tubes may deliver the heated fluid
to a single
orifice port. In yet another embodiment, supply tube 35 may be configured to
receive one or
more fluids in the proximal portion of cannula 30 and deliver the one or more
fluids though a
single delivery orifice 43. In another embodiment, the cannula may be attached
to an
9
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
endoscope or other imaging device. In yet another embodiment depicted in FIGS.
5 and 5A,
cannula 30 may include a forward-facing external fluid delivery applicator 45
in addition to
the distal-to-proximal fluid supply tube 35.
[0037] The heated fluid should be biocompatible, and may comprise a
sterile
physiological serum, saline solution, glucose solution, Ringer-lactate,
hydroxyl-ethyl-starch,
or a mixture of these solutions. The heated biocompatible solution may
comprise a
tumescent solution. The tumescent solution may comprise a mixture of one or
more products
producing different effects, such as a local anesthetic, a vasoconstrictor,
and a disaggregating
product. For example, the biocompatible solution may include xylocaine,
marcaine,
nesacaine, Novocain, diprivan, ketalar, or lidocaine as the anesthetic agent.
Epinephrine,
levorphonal, phenylephrine, athyl-adrianol, or ephedrine may be used as
vasoconstrictors.
The heated biocompatible fluid may also comprise saline or sterile water or
may be
comprised solely of saline or sterile water.
[0038] FIG. 14 depicts one example of a suitable way to heat the fluid
and deliver it
under pressure. The components in FIG. 14 operate using the following steps:
Room
temperature saline drains from the IV bag 51 into mixing storage reservoir 54.
Once the fluid
in the reservoir 54 reaches a fixed limit, the fixed speed peristaltic pump 55
of the heater
system 8 moves fluid from the reservoir 54 to the heater bladder 56. The fluid
is circulated
through the bladder and is heated by the electric panels 57 of the heater
system 8. The heated
fluid is returned back to the reservoir 54 and mixes with the other fluid in
the storage
container. The fixed speed peristaltic pump 55 continues to circulate fluid to
the heater unit
and back into the reservoir 54. The continuous circulation of fluid provides a
very stable and
uniform heated fluid volume supply. Temperature control may be implemented
using any
conventional technique, which will be readily apparent to persons skilled in
the relevant arts,
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
such as a thermostat or a temperature-sensing integrated circuit. The
temperature may be set
to a desired level by any suitable user interface, such as a dial or a digital
control, the design
of which will also be apparent to persons skilled in the relevant arts.
[0039] The pump 58 may be a piston-type pump that draws heated fluid from
the
fluid reservoir 54 into the pump chamber when the pump plunger travels in a
backstroke.
The fluid inlet to the pump has an in-line one-way check valve that allows
fluid to be
suctioned into the pump chamber, but will not allow fluid to flow out. Once
the pump
plunger backstroke is completed, the forward travel of the plunger starts to
pressurize the
fluid in the pump chamber. The pressure increase causes the one-way check
valve at the inlet
of the pump 58 to shut preventing flow from going out the pump inlet. As the
pump plunger
continues its forward travel the fluid in the pump chamber increases in
pressure. Once the
pressure reaches the preset pressure on the pump discharge pressure regulator
the discharge
valve opens. This creates a bolus of pressurized heated fluid that travels
from the pump 58
through cannula handle 20 and from there into the supply tube 35 in the
cannula 30. After
the pump plunger has completed its forward travel the fluid pressure decreases
and the
discharge valve shuts. These steps are then repeated to generate a series of
boluses. Suitable
repetition rates (i.e., pulse rates) are discussed below.
[0040] One example of a suitable approach for implementing the positive
displacement pump is to use an off-set cam on the pump motor that causes the
pump shaft to
travel in a linear motion. The pump shaft is loaded with an internal spring
that maintains
constant tension against the off-set cam. When the pump shaft travels
backwards towards the
off-set cam it creates a vacuum in the pump chamber and suctions heated saline
from the
heated fluid reservoir. A one-way check valve is located at the inlet port to
the pump
chamber, which allows fluid to flow into the chamber on the backstroke and
shuts once the
11
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
fluid is pressurized on the forward stroke. Multiple inlet ports can allow for
either heated or
cooled solutions to be used. Once the heated fluid has filled the pump chamber
at the end of
the pump shaft backwards travel, the off-set portion of the cam will start to
push the pump
shaft forward. The heated fluid is pressurized to a preset pressure (e.g. 1100
psi) in the pump
chamber, which causes the valve on the discharge port to open, discharging the
pressurized
contents of the pump chamber to fluid supply tubes 35. Once the pump plunger
completes its
full stroke based on the off-set of the cam, the pressure in the pump chamber
decreases and
the discharge valve closes. As the cam continues to turn the process is
repeated. The pump
shaft can be made with a cut relief, which will allow the user to vary the
boluses size. The
cut off on the shaft will allow for all the fluid in the pumping chamber to be
ported through
the discharge path to the supply tubes or a portion of the pressurized fluid
to be ported back
to the reservoir.
[0041] The heated biocompatible solution in a tissue liquefaction system
is preferably
delivered in a manner optimized for softening, gellifying, or liquefying the
target tissue.
Variable parameters include, without limitation, the temperature of the
solution, the pressure
of the solution, the pulse rate or frequency of the solution, and the duty
cycle of the pulses or
boluses within a stream. Additionally, the vacuum pressure applied to the
cannula through
vacuum source 14 may be optimized for the target tissue.
[0042] It has been found that for liposuction procedures targeting
subcutaneous fatty
deposits within the human body, the biocompatible heated solution should
preferably be
delivered to the target fatty tissue at a temperature between 75 and 250
degrees F, and more
preferably between 110 and 140 degrees F. A particular preferred operating
temperature for
the heated solution is about 120 degrees F, since this temperature appears
very effective and
safe. Also, for liquefaction of fatty deposits the pressure of the heated
solution is preferably
12
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
between about 200 and about 2500 psi, more preferably between about 600 and
about 1300
psi, and still more preferably between about 900 and about 1300 psi. A
particular preferred
operating pressure is about 1100 psi, which provides the desired kinetic
energy while
minimizing fluid flow. The pulse rate of the solution is preferably between 20
and 150 pulses
per second, more preferably between 25 and 60 pulses per second. In some
embodiments, a
pulse rate of about 40 pulses per second was used. And the heated solution may
have a duty
cycle (i.e., the duration of the pulses divided by the period at which the
pulses are delivered)
of between 1-100%. In preferred embodiments, the duty cycle may range between
30 and
60%, and more particularly between 30 and 50%.
[0043] In preferred embodiments, the rise rate (i.e., the speed with
which the fluid is
brought to the desired pressure) is about 1 millisecond or faster. This may be
accomplished
by having a standard relief valve that opens once the pressure in the pump
chamber reaches
the set point (which, for example, may be set to 1100 psi). As shown in FIG.
15, the pressure
increase is almost instantaneous, as evidenced by the spike representing the
rise rate in the
pressure rise graph (inset). FIG. 15 further illustrates how the fluid exits
the fluid supply
tubes during a very short time span.
[0044] Returning now to the suction subsystem, FIG. 3 depicts an expanded
cut-away
view of an embodiment that includes two suction orifices. As shown, the
cannula 30 has two
suction orifices 37 located near the distal region of the cannula 30 and
proximal to distal tip
32. Suction orifice ports 37 may be positioned in various configurations about
the perimeter
of the distal region of cannula 30. In the illustrated embodiment, the suction
orifice ports 37
are on opposite sides of tile cannula 30, but in alternative embodiments they
may be
positioned differently with respect to each other. Suction orifice ports 37
are configured to
allow fatty tissue to enter the orifices in response to low pressure within
the cannula shaft
13
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
created by vacuum supply 14. The material that is located in the cavity (i.e.,
tissue that has
been dislodged and the heated fluid that exited the supply tube 35) is then
suctioned away in
a proximal direction up through the cannula 30, the handpiece 20, the tubing
16, and into the
canister 15 (all shown in FIG. 1). A conventional vacuum pump (e.g., the AP-
III HK
Aspiration Pump from HK surgical) may be used for the vacuum source.
[0045] In some preferred embodiments, the aspiration vacuum that sucks
the
liquefied/gellified tissue back up through the cannula ranges from 0.33 ¨ 1
atmosphere (1
atmosphere = 760 mm Hg). Varying this parameter is not expected to effect any
significant
changes in system performance. Optionally, the vacuum level may be adjustable
by the
operator during the procedure. Because reduced aspiration vacuum is expected
to lower
blood loss, operator may prefer to work at the lower end of the vacuum range.
[0046] When the embodiments described herein are used for fat harvesting,
as
discussed below, the aspiration vacuum preferably ranges from 300-700 mm Hg.
Exceeding
700 mm Hg is not recommended during fat harvesting because it can have an
adverse impact
on the viability of the fat cells that are harvested.
[0047] Returning to FIGS. 1-4, the cannula 30 and handpiece 20 will now
be
described in greater detail. Hand piece 20 has a proximal end 21 and a distal
end 22, a fluid
supply connection 23 and a vacuum supply connection 24 preferably located at
the proximal
end, and a fluid supply fitting and a vacuum supply fitting at the distal end
(to interface with
the cannula). The hand piece 20 routes the heated fluid from the fluid supply
to the supply
tubes 35 in the cannula and routes the vacuum from the vacuum source 14 to the
cavity in the
cannula, to evacuate material from the cavity.
14
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
[0048] In some embodiments, a cooling fluid supply 6 may be used to
dampen the
heat effect of the heated fluid stream in the surgical field. In these
embodiments, the
handpiece also routes the cooling fluid into the cannula 35 using appropriate
fittings at each
end of the handpiece. In these embodiments, a cooling fluid metering device 13
may
optionally be included. The hand piece 20 may optionally include operational
and ergonomic
features such as a molded grip, vacuum supply on/off control, heat source
on/off control,
alternate cooling fluid on/off control, metering device on/off control, and
fluid pressure
control. Hand piece 20 may also optionally include operational indicators
including cannula
suction orifice location indicators, temperature and pressure indicators, as
well as indicators
for delivered fluid volume, aspirated fluid volume, and volume of tissue
removed.
Alternatively, one or more of the aforementioned controls may be placed on a
separate
control panel.
[0049] The distal end 22 of hand piece 20 is configured to mate with the
cannula 30.
Cannula 30 comprises a hollow tube of surgical grade material, such as
stainless steel, that
extends from a proximal end 31 and terminates in a rounded tip at a distal end
32. The
proximal end 31 of the cannula 30 attaches to the distal end 22 of hand piece
20. Attachment
may be by means of threaded screw fittings, snap fittings, quick-release
fittings, frictional
fittings, or any other attachment connection known in the art. It will be
appreciated that the
attachment connection should prevent dislocation of cannula 30 from hand piece
20 during
use, and in particular should prevent unnecessary movement between cannula 30
and hand
piece 20 as the surgeon moves the cannula hand piece assembly in a back and
forth motion
approximately parallel to the cannula longitudinal axis 33.
[0050] The cannula may include designs of various diameters, lengths,
curvatures,
and angulations to allow the surgeon anatomic accuracy based upon the part of
the body
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
being treated, the amount of fat extracted as well as the overall patient
shape and
morphology. This would include cannula diameters ranging from the sub
millimeter range
(0.25 mm) for delicate precise liposuction of small fatty deposits to cannulas
with diameters
up to 2 cm for large volume fat removal (i.e. abdomen, buttocks, hips, back,
thighs etc.), and
lengths from 2 cm for small areas (i.e. eyelids, cheeks, jowls, face etc.) up
to 50 cm in length
for larger areas and areas on the extremities (i.e. legs, arms, calves, back,
abdomen, buttocks,
thighs etc.). A myriad of designs include, without limitation, a C-shaped
curves of the distal
tip alone, S-shaped curves, step-off curves from the proximal or distal end as
well as other
linear and nonlinear designs. The cannula may be a solid cylindrical tube,
articulated, or
flexible.
[0051] Each of the suction orifice ports 37 includes a proximal end 38, a
distal end
39, and a suction orifice port perimeter 40. Although the illustrated suction
orifices are oval
or round, in alternative embodiments they may be made in other shapes (e.g.,
egg shaped,
diamond or polygonal shaped, or an amorphous shape). As depicted in FIG. 3,
the suction
orifice ports 37 may be arranged in a linear fashion on one or more sides of
cannula 30.
Alternatively, the suction orifice ports 37 may be provided in a multiple
linear arrangement,
as depicted in FIG. 4. Optionally, the dimensions or shape of each suction
orifice port may
change, for example, from the most distal suction orifice port to the most
proximal, as
illustrated in FIG. 4, where the diameter of each suction orifice port may
decrease in
succession from the distal port to the proximal port.
[0052] In some embodiments, the suction orifice perimeter edge 40 is
configured to
present a smooth, unsharpened edge to discourage shearing, tearing or cutting
of the target
fatty tissue. Because the target tissue is liquefied/gellified/softened; the
cannula 30 does not
need to shear tissue as much as found in traditional liposuction cannulas. In
these
16
CA 02849081 2014-03-18
WO 2013/043703
PCT/US2012/056088
embodiments, the perimeter edge 40 is duller and thicker than typically found
in prior-art
liposuction cannulas. In alternative embodiments, the cannula may use shearing
suction
orifices, or a combination of reduced-shearing and shearing suction orifice
ports. The suction
orifice port perimeter edge 40 of any individualized suction orifice port may
also be
configured to include a shearing surface or a combination of shearing and
reduced-shearing
surfaces, as appropriate for the particular application.
[0053] Using
between one and six suction orifices 37 is preferable, and using two or
three suction orifices is more preferable. The suction orifices may be made in
different
shapes, such as round or oblong. FIG. 6 shows some exemplary suction orifices
of different
size. Cross section F is shown with a standard shearing orifice port 37. Cross
section G has a
larger shearing orifice port 37, while cross section H has a perimeter with a
smooth and
unsharpened edge to discourage shearing. When oblong suction orifices are
used, the long
axis should preferably be oriented substantially parallel to the distal-to-
proximal axis. The
suction orifices should not be too large, because with smaller suction
orifices less fat is
suctioned into the cannula for a given bolus of energy. On the other hand they
should not be
too small, to permit the fatty tissue to enter. A suitable size range for
circular suction orifices
is between about 0.04" and 0.2". A suitable side for oblong suction orifices
is between about
0.2" x 0.05" and about 1/2" x 1/8". The size of the suction orifices can
further be varied for
different applications depending on the surgeon's requirements. More extensive
areas to be
suctioned may require larger orifices which require more shearing surface.
[0054] As
shown in FIGS. 7-13, the surface area of a unit length of the suction path
can be calculated by multiplying the total perimeter of the suction path by a
unit length. An
exemplary perimeter of the suction path is n(4.115 mm), which when multiplied
by 1 mm
length, gives a unit length area of 12.9 mm2. FIG. 7 shows the diameter of the
inside of the
17
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
suction path (which would then be multiplied by it to give the perimeter
length and then by a
unit length of 1 mm to give the surface area of 12.93). For the embodiment
shown in FIG. 7,
the resistance ratio of the suction path calculates to be 12.92 mm2/13.30 mm2
= 0.97. And
the resistance ratio of the fluid path (both tubes included) calculates to be:
5.10 mm2/1.04
mm2 = 4.90. Comparing resistive ratios, with the first passage being defined
as the suction
path, in the FIG. 7 embodiment, we see that the comparative resistance ratio
is 0.97/4.90 =
0.20.
[0055] For the embodiment shown in FIG. 8, the calculated resistance
ratio of the
suction path is 1.68 and the calculated resistance ratio of the fluid path
(both tubes included)
is 4.92. Accordingly, the comparative resistance ratio is 0.38. Similarly, in
FIG. 9, the
suction resistance ratio is 1.11 and the fluid resistance ratio 4.61, so the
comparative
resistance ratio is 0.24. In FIG. 10, the suction resistance ratio is 1.20 and
the fluid resistance
ratio 5.98, so the comparative resistance ratio equals 0.20. In FIG. 11, the
suction resistance
ratio is 1.31 and the fluid resistance ratio is 4.65, so the comparative
resistance ratio is 0.28.
In FIG. 12, the suction resistance ratio is 2.25 and the fluid resistance
ratio 7.88, so the
comparative resistance ratio is 0.29. In FIG. 13, the suction resistance ratio
is 1.23 and the
fluid resistance ratio is 10.23, so the comparative resistance ratio is 0.12.
[0056] The embodiments described above may also be used to selectively
harvest
viable fat cells (adipocytes) which can be extracted and processed for re-
injection into other
areas of the body (e.g., areas of fat deficiency). This would include, without
limitation, areas
around the face, brow, eyelids, tear troughs, smile lines, nasolabial folds,
labiomental folds,
cheeks, jaw line, chin, breast, chest abdomen, buttocks, arms, biceps,
triceps, forearms,
hands, flanks, hips, thighs, knees, calves, shin, feet, and back. A similar
method may be used
to address post liposuction depressions and/or concavities from over
aggressive liposuction.
18
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
Other procedures utilizing a similar method include; without limitation,
breast augmentation,
breast lifts, breast reconstruction, general plastic surgery reconstruction,
facial reconstruction,
reconstruction of the trunk and/or extremities.
[0057] It turns out that harvesting fat cells using the embodiments
described above
result in significant improvements in the cell viability in many respects as
compared to other
approaches for harvesting fat cells from a subject. Moreover, (1) the speed of
harvesting and
the quantity of fat cells that can be harvested is significantly better than
with other
approaches for harvesting fat cells; (2) the cells are in a state of cell
suspension in small
clumps with very little or no blood, which is advantageous for implantation;
(3) it is easy to
separate out a portion of the lipoaspirate that is rich in stem cells by
simply centrifuging it;
(4) the viability of the extracted fat cells is significantly better than with
other approaches;
and (5) the fact that the cells are in a state of cell suspension in small
clumps makes it easier
to inject the cells under lower pressure (and pressure during injection is
known to damage the
fat cells so that they do not "take" when injected). These benefits are
explained in the
paragraphs that follow.
[0058] Adipose tissue cell viability of four different fat harvesting
modalities was
compared by analyzing fresh tissue samples taken from one live human subject
using all four
different modalities. The four fat harvesting modalities were: (1) using the
embodiments and
methods described above (referred to herein as "Andrew" Lipoplasty, based on
the name of
the inventor of this application); (2) using a Coleman syringe ("CS"); (3)
using standard
Suction Assisted Lipoplasty ("SAL"); and (4) using Vaser-Ultrasonic Assisted
Lipoplasty
("V-UAL"). Four samples from the Andrew modality and one sample from each of
the other
modalities were analyzed, making a total of seven samples.
19
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
[0059] The testing was performed under expert guidance, directed by a
world
authority on adipose tissue cell biology. A total of four PhDs in cell biology
were present.
Tissue sample preparation of all four fat harvesting modalities was identical,
using standard
centrifugation and collagenase protocols. The steps that were implemented are
described
below.
[0060] The waste containers containing the fat aspirates were brought
from the third
floor operating suite to the first floor lab. By the time the waste containers
arrived in the lab,
the material in the containers was already settling into an obvious
supranatant layer (an upper
layer) consisting of mainly fat tissue, and an infranatant layer (a lower
layer) consisting
mainly of a fluidic mixture of blood and/or saline. The difference between the
Andrew
containers and all the other containers was obvious and marked: the Andrew
supranatant was
light yellow in color, was clearly a homogeneous liquid, was devoid of chunks
of connective
tissue ("CT") and clumps of fat tissue, and was devoid of blood ¨ there was no
hint of
redness whatsoever. The Andrew infranatant was a thin, light salmon/pink
colored liquid.
All other non-Andrew lipo waste containers looked similar: the supranatant was
reddish-
orange in color and clearly contained blood, the SAL and V-UAL supranatants
were not
homogeneous liquids and contained obvious chunks of CT tissue and clumps of
fat, the
Coleman supranatant appeared thick and clumpy and was not a homogeneous liquid
(but
definitive appearing chunks of connective tissue were not discernible), and
all the non-
Andrew infranatants appeared to be a dark red, thick, blood-like fluid. The
seven aspirate
samples arrived in the lab sequentially, at 15 -20 minute intervals from one
to the next. As
the samples arrived they were allowed to settle for a few minutes.
[0061] The first analysis that was done was to determine whether the
lipoasprirate
was in a state of cell suspension. To accomplish this, samples of the Coleman
and Andrew
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
supranatants (#1) were taken using a pipette and exposed to trypan blue stain.
The stained
samples were then placed on a hemocytometer cell counting slide and viewed
under the
microscope. Microscopically, the Andrew supranatant was observed to be in a
state of cell
suspension, and was observed to be almost a single cell suspension. (It was
believed by all
cell biologists present that the #1 Andrew sample could be gotten to a single
cell suspension
by diluting it.) The Coleman sample was in clumps and was not in a cell
suspension state.
Three of the cell biologists present observed that it was inconceivable that
the SAL and V-
UAL aspirates would be in a state of cell suspension, based on their obvious
chunky and
clumpy appearance, so they did not look at the fat tissues from the SAL and V-
UAL aspirates
under the microscope. The significance of the fact that the #1 Andrew sample
was in a cell
suspension state is discussed below.
[0062] Cell viability was then measured for all seven samples. A sample
from the
each supranatant was taken using a pipette and placed in a test tube and
labeled. Then a
smaller sample was taken using a pipette from the test tube and placed in a 2
ml centrifuge
tube. (Epindorf centrifuge.) The sample was spun at 800 rpm for 5 minutes.
Then a
collagenase digestion was performed on that post-spun sample in a 37 degree C
water bath,
using 1 mg/ml of collagenase (Worthington type 1) for 45 minutes. Then, post
digestion, the
sample was spun again in the centrifuge. Then a sample was taken using a
pipette from the
supranatant in the centrifuge tube and exposed to two fluorescent dyes for
approximately 10
minutes. Then a small sample from that post fluorescent dye stained sample was
placed onto
the Vision Cell Analyzer slide, the slide was placed into the automated cell
counter (a Vision
Cell Analyzer from Nexcelom, Inc. of Lawrence, Massachusetts) and it was read.
The
identical process and procedure was done to all seven aspirate samples.
21
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
[0063] The Vision Cell Analyzer distinguishes adipocytes from lipid
droplets; the
fluorescent dyes stain only cells and not lipid droplets. (When reading the
slides manually
through a microscope it is very difficult to distinguish a lipid droplet from
an adipocyte.) The
first dye stains all cells present, alive, and dead cells. The second dye
stains only dead cells.
The automated cell counter counts all cells present and can distinguish
between live and dead
cells. The software in the Vision Cell Analyzer does a subtraction and gives
you the
percentage of live cells present. Four separate fields are read and averaged.
The results for
the four different modalities are tabulated on Table 1 below. All the samples
were prepared
identically (i.e., all were post centrifugation and post collagenase
digestion). Note that four
different samples using the Andrew modality were tested (at various
temperature and
pressure settings and two different anatomical locations).
[0064] Looking at the images from the Vision Cell Analyzer on the laptop
screen
which showed the field of cells being read, one field at a time, one of the
cell biologists
present commented that in all fields "it is clear that the majority of cells
being read are
adipocytes; from what we know of adipose tissue cellular biology, the other
cells present are
progenitor cells, pre-adipocytes, endothelial cells and macrophages...".
22
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
Liposuction Vacuum Power Cannula Anatomical Viable
Modality Setting Setting Location Cell
%
Coleman N/A (Hand N/A 3 mm Posterior 85.5
Syringe) Coleman Flank
SAL 300 N/A 3 mm Posterior 82.7
mmHg 3 aperture Flank
V-UAL 300 70% 2-ring 3.7 Posterior 72.7
(Vaser) mmHg continuous mm probe Flank
For 3 mm
5-minutes 3 aperture
cannula
Andrew 1 300 37 C 3 mm Posterior 98.0
mmHg 600 psi 2 aperture Flank
Andrew 2 300 37 C 3 mm Abdomen 94.4
mmHg 600 psi 2 aperture
Andrew 3 300 45 C 3 mm Abdomen 99.2
mmHg 1100 psi 2 aperture
Andrew 4 660 53 C 3 mm Abdomen 94.7
mmHg 1300 psi 2 aperture
TABLE 1
[0065] A review of the data in Table 1 reveals that the Andrew Lipoplasty
modality
had the best cell viability determination. The four Andrew samples ranged from
94.4 % to
99.2 % cell viability, with an average of 96.6 %. The Andrew Lipoplasty system
evidenced
excellent cell viability at all machine settings, even at the highest
temperature and pressure
settings. The Coleman modality came in second, SAL third, and V-UAL fourth.
[0066] Note that in the cell viability procedure described above,
collagenase was used
to separate the cells from each other. This was done because the cell counter
machines can
only count cells when they are separated, and cell counter machines were
required to measure
cell viability. But in medical applications, when the fat is extracted and
then reintroduced to
a person's body, it is strongly preferably to avoid using collagenase in the
process. Since
collagenase will not be used, the configuration of cells in the matter that is
extracted from the
patient becomes very significant in determining how well the cells will take
in their
transplanted location. First of all, cells that are in a cell suspension are
preferable for
23
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
introduction in a patient as compared to cells that are not in a cell
suspension state. And
second of all, even within situations where the cells are in a cell suspension
state, the size of
the cell clumps in that suspension has a significant effect on how well the
cells will take in
their transplanted location. It turns out that the cells take better when the
cells are in smaller
clumps (as compared to cells that are in larger clumps). But the clumps should
also not be
too small. Some experts have indicated that a clump size is on the order of
200 cells per
clump is ideal, and the Andrew system advantageously yields a large amount of
clumps that
contain between 100 and 400 cells per clump, which is a relatively small clump
size that is
also not too small.
[0067] Base on the tests described above, it become apparent that the
Andrew
approach is superior to the other three approaches in many ways including: the
speed of
collection and the nature of the collected matter; the nature of the post-
collection processing
of lipoaspirate that must be done; and suitability for injection into a target
location.
Regarding speed, the Andrew, SAL, and V-UAL systems all remove tissue from a
patient's
body relatively quickly, but the Coleman approach is comparatively slow. As
for the nature
of the collected matter, the fat extracted using the Andrew system is in a
cell suspension state
with relatively small clump size; the fat extracted using the Coleman approach
ends up in
clumps of fat that are not in a cell suspension state; and the matter
extracted using SAL and
UAL was not in a cell suspension state at all. Fat that is in a cell
suspension state with
relatively small clump size is ideal for reintroduction into a target site in
the patient's body,
and the Andrew system is the only approach that provides rapid extraction of
fat tissue that is
in a cell suspension state with relatively small clump size. The Andrew
approach is therefore
superior to the other three approaches in this regard.
24
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
[0068] Another reason why the Andrew approach is superior to the other
three
approaches is because the cell viability is highest using the Andrew approach,
as shown in the
data presented above.
[0069] Yet another reason why the Andrew approach is superior to the
other three
approaches is because less processing of the lipoaspirate is required. The
Andrew
lipoaspirate gravity-separates relatively quickly and the supranatant appears
to be devoid of
blood. In contrast, the lipoaspirate from the UAL and SAL approaches contain a
significant
amount of blood in other undesirable components. As a result, the Andrew
lipoaspirate will
probably not need washing before it can be introduced into the patient's body
(or, at the very
least, will require less washing as compared to the other approaches).
[0070] Yet another reason why the Andrew approach is superior to the
other
approaches is its improved injectability. When fat is injected into a target
site, it is known
that squeezing the injection syringe too hard can kill or damage some of the
fat cells that are
being injected, which prevents them from taking in their new location. The
Andrew
lipoaspirate had a smoother consistency (possibly due to the fact that the
Andrew lipoaspirate
is in a cell suspension state with a relatively small clump size), and can
therefore be pushed
out of the injection syringe using lower pressure. In contrast, the fat cells
in the Coleman
approach was not as smooth (possibly due to the larger clump size) and would
require a
higher injection pressure to push out of the injection syringe. Since higher
pressure can
damage the fat being injected, the Andrew approach is superior in this regard
as well.
[0071] Overall cell viability for the Andrew approach is superior to the
other
approaches because the cells in the extracted matter start off having the
highest viability, as
explained with the data presented above. This high initial viability is then
compounded by
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
the fact that fewer fat cells are damaged during the injection process, which
means that the
percentage of fat cells that actually take in the target location will go up
even further.
[0072] For all these reasons, the Andrew Lipoplasty system described
herein (i.e., the
methods and embodiments described above) appears to be an ideal fat harvesting
modality.
The supranatant that is collected using the Andrew approach may be centrifuged
in a manner
that is similar to the centrifuging process described above in the background
section in
connection with the Coleman approach. The low density portion can be skimmed
away and
discarded and the remainder can be loaded into implantation syringes.
Alternatively, the high
density portion can be drained off the bottom into implantation syringes. The
higher density
portion, which contains viable fat cells and is also rich in adipose
progenitor cells (i.e., stem
cells), can then be used for implantation into the subject.
[0073] Preferably, the thermal and mechanical energy is just enough to
achieve the
desired effect on fat; the thermal energy is not high enough to liquefy non-
fat tissue or to
burn any tissue; and the mechanical energy not high enough to cut any tissue.
In some
preferred embodiments, the temperature of the fluid is between 100 and 131 F,
the pressure is
between 600 and 1300 psi, and the boluses contain between 20 and 50
microliters per pulse
and are generated at between 30 and 50 pulses per second. In one preferred
embodiment, the
temperature is 120 F, the pressure is 1100 psi, the boluses contain 25
microliters per pulse,
and the boluses are generated at about 40 pulses per second.
[0074] At the cellular level, liquefaction of fat tissue is apparently
achieved by cell
dispersion / cell disaggregation ¨ not by emulsification. Cell membranes are
not broken and
lipids do not spill out because the energy levels used are not high enough to
break cell
membranes. Possible explanations for the resulting cell diaggregation include
the following:
(1) the energized saline stream may acts as a "selective proteolytic agent,"
causing
26
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
proteolysis of some proteins but not others; (2) the energized saline stream
may act as a
"pseudo-protease"; and (3) the energized saline stream may causes a disabling
of some of the
adhesive glycoproteins located on the surface of adipose tissue cell membranes
and in the
extracellular matrix (ECM). These glycoproteins are disabled and the cells
"unstick" from
each other. The cells then disperse or disaggregate.
[0075] When the lipoaspirate was allowed to settle, a visual inspection
revealed that it
separated out into top and middle layers. Examination of these layers revealed
that the cells
clumps in the top layer were bigger (i.e., they had more cells) while the
cells clumps in the
middle layer were smaller (i.e., they had fewer cells). The inventor has
recognized that this
phenomenon may be relied on to obtain a desired clump size (e.g., in
situations where clumps
of a specific size are preferred) without using a centrifuge as described in
some of the
embodiments discussed above. To verify this, an experiment was performed in
which a live
human patient had liposuction done to the outer thighs, upper hips and
buttocks using the
approaches described herein using the HydraSolve Lipoplasty System (FDA
approved under
title Phaser Lipoplasty System). The machine was set to 113 F and 1100 psi,
and generated
40 pulses per second.
[0076] Lipoaspirates from those anatomic sites were collected in 3 liter
waste
canisters and were allowed to gravity separate for about 20 to 30 minutes. The
lipoaspirates
settled into a top portion containing fat tissue, called the supernatant, and
into a bottom
portion containing saline and a little blood, called the infranatant. The top
portion of the
supernatant was found to be visibly different in appearance from the bottom
portion, with the
division being visibly demarcated at almost the exact mid point of the overall
supernatant. In
particular, the top half of the supernatant appeared "grainier" and less
completely
homogeneous than the bottom half of the supernatant (which appeared very fine
and
27
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
homogeneous). In other words, the supernatant contained a plurality of layers,
each of which
had different properties.
[0077] A sample from the top half of the supernatant was pippetted onto a
hemacytometer grid cell counting slide and viewed under 40x magnification: a
localized, tiny
clump of adipocytes was observed, which appeared to have between 100 to 400
adipocytes.
(Note that it is difficult to determine the precise number of adipocytes in a
given clump
because the clumps were a number of cell layers deep, and the depth is hard to
see on a slide).
A total of 6 samples (two samples form each anatomic site) were obtained and
viewed in this
manner, and all six samples had similar clump sizes and appearance. The same
process was
done to the bottom half of the supernatant and it was found that the clump
sizes were
consistently smaller, with about half as many cells per clump (as compared to
the clumps in
the top half of the supernatant).
[0078] As explained above, some experts have indicated that a clump size
is on the
order of 200 cells per clump is ideal. Since the clumps in the top half of the
supernatant
appeared to have between 100 to 400 adipocytes per clump, gravity settling may
be used to
separate the best portion of the lipoaspirate for subsequent implantation by
simply waiting
20-30 minutes for the lipoaspirate to settle into a supernatant and an
infranatant, and then
using the top half of the supernatant as a source of fat cells that are well
suited for subsequent
reintroduction into another part of the patient's body. Alternatively, if fat
cells arranged in
smaller clumps are desired, the bottom half of the supernatant would be used
instead. In this
manner, the selection of a layer from within the gravity-settled supernatant
provides
selectivity for obtaining the desired clump size for a given use.
[0079] The fact that the Andrew supranatant is in a state of cell
suspension also
provides another major advantage: Since the supranatant automatically reaches
a state of
28
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
cellular suspension, it becomes possible to separate out the adipose
progenitor cells (i.e., stem
cells) from the rest of the fat using a centrifuge without using collagenase
or other similar
functioning enzymes or chemicals. Since adipose progenitor cells have the
ability to
differentiate into many different types of tissue, they can be very useful for
many purposes.
(Note that the G forces used to separate stem cells will be higher than the G
forces that are
used to separate the high density portion of the supranatant from the low
density portion.)
While the viability of the adipose stem cells was not tested separately, it is
safe to assume that
they are viable because adipose progenitor cells are hardier than adipocytes,
and the overall
viability was tested and found to be extremely high in the Andrew modality, as
seen in Table
1 above. The Andrew approach, used together with a centrifuge, is therefore an
excellent
way to obtain adipose progenitor cells.
[0080] Note that when a doctor intends to reintroduce the fat that is
being extracted
from the body into another location, the fluid pressure and vacuum settings
may be reduced
to make the process more gentle, in order not to traumatize the fat tissue. On
the other hand,
when the fat will be discarded, this is not a concern and higher pressure and
vacuum settings
may be used.
[0081] One aspect of the invention relates to a method of harvesting fat
tissue from a
first anatomic location of a subject using a cannula that has an interior
cavity and an orifice
configured to permit fat tissue to enter the interior cavity. This method
includes generating a
negative pressure in the interior cavity so that a portion of the fat tissue
is drawn into the
interior cavity via the orifice. Fluid is delivered, via a conduit, so that
the fluid exits the
conduit within the interior cavity and impinges against the portion of the fat
tissue that was
drawn into the interior cavity. The fluid is delivered at a pressure and
temperature that causes
the fat tissue to soften, liquefy, or gellify. Matter is suctioned matter out
of the interior
29
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
cavity, and the matter includes at least some of the delivered fluid and at
least some of the fat
tissue that has been softened, liquefied, or gellified. The matter that was
suctioned away is
collected and the operation of gravity, over time, separates the collected
matter into (a) a
supernatant that has a plurality of layers and (b) an infranatant. Fat that is
suitable for
implantation in the subject is extracted from a particular layer selected from
the plurality of
layers.
[0082] Optionally, the extracted fat is introduced into a second anatomic
location of
the subject. Optionally, the collected matter may be cooled. In some
embodiments, the fluid
is traveling in a substantially distal to proximal direction just before it
impinges against the
portion of the fat tissue that was drawn into the orifice.
[0083] Preferably, the fluid is delivered in pulses at a temperature
between 98 F and
140 F, and more preferably between 110 F and 120 F. Preferably, the fluid
is delivered at
a pressure between 600 and 1300 psi, and more preferably between 900 and 1300
psi.
Preferably, the matter is suctioned out of the interior cavity using a vacuum
pressure between
300 and 700 mm Hg, and between 450 and 550 mm Hg may be a sweet spot within
this
range.
[0084] Another aspect of the invention relates to a method of harvesting
fat tissue
from a first anatomic location of a subject using a cannula that has an
interior cavity and an
orifice configured to permit fat tissue to enter the interior cavity. This
method includes
generating a negative pressure in the interior cavity so that a portion of the
fat tissue is drawn
into the interior cavity via the orifice. Fluid is delivered, via a conduit,
so that the fluid exits
the conduit within the interior cavity and impinges against the portion of the
fat tissue that
was drawn into the interior cavity. The fluid is delivered at a pressure and
temperature that
causes the fat tissue to soften, liquefy, or gellify. Matter is suctioned
matter out of the
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
interior cavity, and the matter includes at least some of the delivered fluid
and at least some
of the fat tissue that has been softened, liquefied, or gellified. The matter
that was suctioned
away is collected and the operation of gravity, over time, separates the
collected matter into
(a) a supernatant that has a top half and a bottom half and (b) an
infranatant. Fat that is
suitable for implantation in the subject is extracted from the top half of the
supernatant.
[0085] Optionally, the extracted fat is introduced into a second anatomic
location of
the subject. Optionally, the collected matter may be cooled. In some
embodiments, the fluid
is traveling in a substantially distal to proximal direction just before it
impinges against the
portion of the fat tissue that was drawn into the orifice.
[0086] Preferably, the fluid is delivered in pulses at a temperature
between 98 F and
140 F, and more preferably between 110 F and 120 F. Preferably, the fluid
is delivered at
a pressure between 600 and 1300 psi, and more preferably between 900 and 1300
psi.
Preferably, the matter is suctioned out of the interior cavity using a vacuum
pressure between
300 and 700 mm Hg, and between 450 and 550 mm Hg may be a sweet spot within
this
range.
[0087] Another aspect of the invention relates to a method of harvesting
fat tissue
from a first anatomic location of a subject using a cannula that has an
interior cavity and an
orifice configured to permit fat tissue to enter the interior cavity. This
method includes
generating a negative pressure in the interior cavity so that a portion of the
fat tissue is drawn
into the interior cavity via the orifice. Fluid is delivered via a conduit, so
that the fluid exits
the conduit within the interior cavity and impinges against the portion of the
fat tissue that
was drawn into the interior cavity. The fluid is delivered in pulses at a
temperature between
98 F and 140 F and at a pressure between 600 and 1300 psi, and is traveling
in a
substantially distal to proximal direction just before it impinges against the
portion of the fat
31
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
tissue that was drawn into the orifice. At least some of the fat tissue that
was drawn into the
interior cavity is softened, liquefied, or gellified. Matter is suctioned out
of the interior
cavity, and the matter includes at least some of the delivered fluid and at
least some of the fat
tissue that has been softened, liquefied, or gellified. The matter that was
suctioned away is
collected and the operation of gravity, over time, separates the collected
matter into (a) a
supernatant that has a top half and a bottom half and (b) an infranatant. Fat
that is suitable for
implantation in the subject is extracted from the top half of the supernatant.
[0088] Optionally, the extracted fat is introduced into a second anatomic
location of
the subject. Optionally, the collected matter may be cooled. Preferably, the
fluid is delivered
at a temperature between 110 F and 140 F, and more preferably between 110 F
and 120
F. Preferably, the fluid is delivered at a pressure between 900 and 1300 psi.
Preferably, the
matter is suctioned out of the interior cavity using a vacuum pressure between
300 and 700
mm Hg, and between 450 and 550 mm Hg may be a sweet spot within this range.
[0089] The embodiments described above may be used in various liposuction
procedures including, without limitation, liposuction of the face, neck,
jowls, eyelids,
posterior neck (buffalo hump), back, shoulders, arms, triceps, biceps,
forearms, hands, chest,
breasts, abdomen, abdominal etching and sculpting, flanks, love handles, lower
back,
buttocks, banana roll, hips, saddle bags, anterior and posterior thighs, inner
thighs, mons
pubis, vulva, knees, calves, shin, pretibial area, ankles and feet. They may
also be used in
revisional liposuction surgery to precisely remove residual fatty tissues and
firm scar tissue
(areas of fibrosis) after previous liposuction.
[0090] The embodiments described above may also be used in conjunction
with other
plastic surgery procedures in which skin, fat, fascia and/or muscle flaps are
elevated and/or
removed as part of the surgical procedure. This would include, but is not
limited to facelift
32
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
surgery (rhytidectomy) with neck sculpting and submental fat removal, jowl
excision, and
cheek fat manipulation, eyelid surgery (blepharoplasty), brow surgery, breast
reduction,
breast lift, breast augmentation, breast reconstruction, abdominoplasty, body
contouring,
body lifts, thigh lifts, buttock lifts, arm lifts (brachioplasty), as well as
general reconstructive
surgery of the head, neck, breast abdomen and extremities. It will be further
appreciated that
the embodiments described above have numerous applications outside the field
of
liposuction.
[0091] The embodiments described above may be used in skin resurfacing of
areas of
the body with evidence of skin aging including but not limited to sun damage
(actinic
changes), wrinkle lines, smokers' lines, laugh lines, hyper pigmentation,
melasma, acne scars,
previous surgical scars, keratoses, as well as other skin proliferative
disorders.
[0092] The embodiments described above may target additional tissue types
including, without limitation, damaged skin with thickened outer layers of the
skin (keratin)
and thinning of the dermal components (collagen, elastin, hyaluronic acid)
creating abnormal,
aged skin. The cannula would extract, remove, and target the damaged outer
layers, leaving
behind the healthy deep layers (a process similar to traditional dermabrasion,
chemical peels
(trichloroacetic acid, phenol, croton oil, salicyclic acid, etc.) and ablative
laser resurfacing
(carbon dioxide, erbium, etc.) The heated stream would allow for deep tissue
stimulation,
lightening as well as collagen deposition creating tighter skin, with
improvement of overall
skin texture and/or skin tone with improvements in color variations. This
process would
offer increased precision with decreased collateral damage over traditional
methods utilizing
settings and delivery fluids which are selective to only the damaged target
tissue.
[0093] Other implementations include various distal tip designs and
lighter pressure
settings that may be used for tissue cleansing particularly in the face but
also applied to the
33
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
neck, chest and body for deep cleaning, exfoliation and overall skin hydration
and
miniaturization. Higher pressure settings may also be used for areas of
hyperkeratosis, callus
formation in the feet, hands knees, and elbows to soften, hydrate and
moisturize excessively
dry areas.
[0094] Additional uses include tissue removal in the spine or spinal
nucleotomy. The
cannula used in spinal nucleotomy procedures includes heated solution supply
tubes within
the cannula as described above. The cannula further includes a flexible tip
capable of moving
in multiple axes, for example, up, down, right and left. Because of the
flexible tip, a surgeon
may insert a cannula through an opening in the annulus fibrosis and into the
central area,
where the nucleus pulpous tissue is located. The surgeon can then direct the
cannula tip in
any direction. Using the cannula in this manner the surgeon is able to clean
out the nucleus
pulpous tissue while leaving the annulus fibrosis and nerve tissue intact and
unharmed.
[0095] In another implementation, the present design can be incorporated
in to an
endovascular catheter for removal of vascular thrombus and atheromatous
plaque, including
vulnerable plaque in the coronary arteries and other vasculature.
[0096] In another implementation, a cannula using the present design can
be used in
urologic applications that include, but are not limited to, trans-urethral
prostatectomy and
trans-urethral resection of bladder tumors.
[0097] In another implementation, the present design can be incorporated
into a
device or cannula used in endoscopic surgery. An example of one such
application is
chondral or cartilage resurfacing in arthroscopic surgery. The cannula can be
used to remove
irregular, damaged, or torn cartilage, scar tissue and other debris or
deposits to generate a
smoother articular surface. Another example is in gynecologic surgery and the
endoscopic
34
CA 02849081 2014-03-18
WO 2013/043703 PCT/US2012/056088
removal of endometrial tissue in proximity to the ovary, fallopian tubes or in
the peritoneal or
retroperitoneal cavities.
[0098] In yet a further implementation to treat chronic bronchitis and
emphysema
(COPD), the cannula can be modified to be used in the manner a bronchoscope is
used; the
inflamed lining of the bronchial tubes would be liquefied and aspirated,
thereby allowing
new, healthy bronchial tube tissue to take its place.
[0099] The various embodiments described each provide at least one of the
following
advantages: (1) differentiation between target tissue and non-target tissue;
(2) clog
resistance, since the liquid projected in a distal-to-proximal direction
across the suction
orifices, which generally prevents the suction orifice or the cannula from
clogging or
becoming obstructed; (3) a reduction in the level of suction compared to
traditional
liposuction, which mitigates damage to non-target tissue; (4) a significant
reduction in the
time of the procedure and the amount of cannula manipulation required; (5) a
significant
reduction in surgeon fatigue; (6) a reduction in blood loss to the patient;
and (7) improved
patient recovery time because there is less need for shearing of fatty tissue
during the
procedure.
[00100] Although the present invention has been described in detail with
reference to
certain implementations, other implementations are possible and contemplated
herein.
[00101] All the features disclosed in this specification may be replaced
by alternative
features serving the same, equivalent, or similar purpose, unless expressly
stated otherwise.
Thus, unless expressly stated otherwise, each feature disclosed is one example
only of a
generic series of equivalent or similar features.