Note: Descriptions are shown in the official language in which they were submitted.
CA 02860031 2014-06-20
WO 2013/091094
PCT/CA2012/050907
Organics and Nutrient Recovery from Anaerobic Digester Residues
[0001] This application claims the benefit of US provisional application
number
61/707,467 filed on September 28, 2012 and US provisional application number
61/578,703 filed on December 21, 2011. This application also claims priority
from
International Application Number PCT/CA2012/000144 filed on February 17, 2012.
FIELD
[0002] This specification relates to the recovery of organics and nutrients
from
waste, to anaerobic digestion alone or in combination with a wastewater
treatment plant,
to products such as fertilizer or compost made from anaerobic digester residue
device,
and to a method for removing ammonia from water, such as sludge dewatering
centrate.
BACKGROUND
[0003] The following discussion is not an admission that anything
discussed
below is common general knowledge or citable as prior art.
[0004] Various organic waste products contain nutrients that make the
waste
potentially valuable as fertilizer. For example, some animal manures and
organic sludges
or slurries could be applied directly to land. However, due for example to the
large
quantities of material involved relative to the nutrient content, and
potential problems with
odors, this practice is limited to selected appropriate operations located
near the source
of the waste. The manure, sludge or slurry might be treated to remove large
fibers,
physically dewatered, partially dried thermally, extruded into a solid
fertilizer product and
then further thermally dried. However such a product would not be stable and
would tend
to decompose or attract mold during storage because of its high biodegradable
organic
matter content. Alternatively, manures, sludges or slurries could be digested
in an
anaerobic digester to produce a biogas. The digested sludge could then be
applied to the
land as a fertilizer. While the biogas produced is useful as a fuel, use of
the digester
sludge as a fertilizer is still limited to selected appropriate operations
near the source of
the waste.
[0005] In an activated sludge wastewater treatment plant, ammonia is
removed
from the wastewater at least in jurisdictions with relevant discharge
regulations. In these
plants, waste activated sludge may be sent to an anaerobic digester. Sludge
from the
digester, comprising digestate, is typically de-watered before it is disposed
or treated
- 1 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
further. The liquid stream from the de-watering device, which may be called
reject water,
centrate or filtrate, is often returned to the main activated sludge process.
This centrate
contains ammonia, and there have been some attempts to remove ammonia from the
centrate before it is sent back to the main process. A paper by Tim
Constantine,
presented at the 2006 WEFTEC conference and entitled "North American
Experience
with Centrate Treatment Technologies for Ammonia and Nitrogen Removal",
provides a
summary of ammonia removal technologies that have been used in North American
facilities.
[0006] US Patent Application Publication Number 2007/0297953 to Kemp
et al.
describes a system in which ammonia is removed from water in a vacuum assisted
flash
stripping tower. The water is treated before stripping to remove solids
removal tank and
multivalent cations and increase its pH.
[0007] US Patent Number 7,416,644 to Bonde describes a fermenter with
a side
stream ammonia stripping step. Ammonia is stripped from fermented biomass in a
shunt.
Effluent from the fermenter passes through the shunt while water vapor is
injected into
the shunt.
INTRODUCTION
[0008] The following paragraphs are intended to introduce the reader
to the more
detailed description to follow, and not to limit or define any claimed
invention.
[0009] This specification describes, among other things, a fertilizer
product, a
method of making a fertilizer product, a method of treating anaerobic digester
sludge and
a waste treatment process including anaerobic digestion. In brief, sludge from
an
anaerobic digester is treated to produce a generally dry nitrogen rich
fertilizer product,
which may be called a pellet or a granule herein.
[0010] In a treatment plant and process described in further detail
below, solids
are separated from liquids in the sludge and dewatered, dried or both. The
liquids in the
sludge contain aqueous ammonia that is released in one or more gasses or
liquids
produced during dewatering or drying. These liquids or gases are collected and
then
treated in an ammonia recovery system to produce a concentrated acidified
ammonium
salt solution. This solution, relative to the liquid in the digester sludge,
has a higher
concentration of ammonia, reduced alkalinity and reduced pH. The acidic
ammonium
solution is reintroduced to the dried solids to produce a moist pellet. The
moist pellets are
then dried at ambient to moderate temperatures, for example by a flow of warm
air over
- 2 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
the pellets. After drying the pellets, ammonia from the recovered liquid
remains with the
pellets as an ammonium salt.
[0011] Anaerobic digester sludge is more stable than the undigested
feedstock
because it has a reduced concentration of biodegradable solids. Nevertheless,
anaerobic
digester sludge contains carbon and nitrogen, among other nutrients, in
mineralized and
organic forms that are useful as fertilizer. However, the nitrogen exists
primarily in
aqueous forms of ammonia. A typical digester sludge dewatering process would
therefore
lose much of the ammonia with removed water. Further, the liquid in the
digested sludge
also has a high pH and is heavily buffered with alkalinity. Heating the de
watered sludge
cake under typical sludge drying temperatures, given its high pH, would
convert the
ammonia remaining in the liquid in the cake primarily into ammonia gas,
resulting in more
loss of ammonia along with the evaporated water.
[0012] However, in a process and apparatus described herein, one or
more of the
liquids, vapors or gases produced by dewatering or drying the sludge, or both,
are
.. collected and processed in an ammonia recovery system. In the recovery
system, water
or vapor with an increased concentration of ammonia and reduced alkalinity is
created
and mixed with an acid. With reduced volume and alkalinity (relative to the
water in the
digester sludge), a reasonable amount of acid is able to produce an ammonia
containing
liquid with low pH. Alternatively, a concentrated ammonium hydroxide solution
can be
produced in the recovery system from the collected gases, optionally with
added water,
optionally after a de-aeration process to separate ammonia gas from water
vapour.
Further, the volume of liquid carrying the recovered ammonia is reduced to the
point
where it is feasible to reintroduce the concentrated ammonia liquid into the
dried solids in
a pellet making process. The produced pellets are moist and have a high
surface area
per unit volume, allowing drying by way of a flow of air at moderate
temperature to
produce a pellet dry enough, considering the stabilized nature of the solids,
for storage
and transport. With the moisture in the pellet at a low pH and drying at
moderate
temperatures, ammonia ions in the moist pellet tend to precipitate as salts
that remain
with the pellets rather than forming ammonia gas. In this way, the apparatus
and process
.. described herein produce a pellet with higher nitrogen content than would
be achieved
merely by dewatering, drying and pelletizing the anaerobic digester sludge.
[0013] This disclosure also describes a system and process to recover
fibers, or
solids or liquids with a high nutrient content, or both, from anaerobic
digester residues.
The fibers can be used in a plant growing medium. The solids, for example in a
granule
- 3 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
or flake form, or liquids can be used as a fertilizer. The fibers and solids
or liquids can
also be used in combination to produce a plant growing medium.
[0014] A device and process for removing ammonia from a liquid are
described
herein. The ammonia flows through a series of sequential stages. Bubbles, for
example
of air, are provided in the liquid in the stages. At the same time, air flows
across the
surface of the liquid in the stages. The flow rate of the surface flow is
greater than the
flow rate of the bubbles.
[0015] The device and process for removing ammonia can be used in the
system
and process to recover fibers, or solids or liquids with a high nutrient
content, or both,
from anaerobic digester residues. Alternatively, the device and process for
removing
ammonia can be used in other applications, for example removing ammonia from
municipal wastewater plant digester centrate or other waste streams with
ammonia. The
device and process for removing ammonia can be used in combination with a
commercial
acid or ammonia scrubber.
[0016] Elements of the various systems and process described herein may be
combined. For example, solids, liquids or vapors produced in a system and
process
described herein for making flakes can be used to make a pelletized fertilizer
as
described herein.
FIGURES
[0017] Figure 1 is a schematic process flow diagram for a plant for
treating sludge
to produce a fertilizer pellet, coupled with an anaerobic digester to produce
the sludge
from a waste stream.
[0018] Figure 2 is a schematic process flow diagram of a nutrients
recovery
system including an ammonia removal system.
[0019] Figure 3 is schematic representation of an optional ammonia
removal
system having reactors in series.
[0020] Figure 4 is a schematic process flow diagram of an alternative
ammonia
removal system using cavitation.
DETAILED DESCRIPTION
[0021] Figure 1 shows a plant for producing a solid fertilizer product,
pellets Q,
from sludge, particularly anaerobic digester residue or digestate B. This
plant is coupled
with an anaerobic digester 1, which produces the digestate B from a waste
stream or feed
stock A. Examples of suitable digester feedstock A that results in high
nutrient content
- 4 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
digestate B include animal manure, post consumer food waste, pre consumer food
processing waste, biofuels processing by products, agricultural waste, and
municipal
wastewater sludge, among others. The origin and nature of the feed stock A,
and possibly
the type of chemicals used in the fertilizer production process to be
described below, may
allow the fertilizer product to be labeled as "organic" or by another related
term in
accordance with applicable regulations. The product may be called a pellet or
a granule.
Either of these words may be used herein to denote a substantially dry product
in the
form of many small (for example 1 mm to 50 mm in the largest dimension)
pieces, but
without intending to limit the product to any particular size or shape of
product.
[0022] Solid fertilizer products typically have a higher value than raw
wastes or
liquid fertilizer products because a solid product facilitates transporting,
storing and using
the fertilizer with less cost and with reduced nuisance, particularly odors.
In general, it is
desirable for a solid fertilizer product to have a substantial concentration
of nutrients,
including nitrogen. The product should also have a low concentration of
pathogens and
be organically stable such that it does not decompose and grow mold readily in
storage. It
is also advantageous for the product to be sufficiently hard, uniformly sized
and flowable
through machinery so as to allow the product to be stored, transported and
broadcast
with conventional dry fertilizer application equipment used in agriculture and
horticulture.
The size and shape of a pellet Q can be made to satisfy the physical
requirements
described above. The steps involved in processing the digestate B, to be
described in
more detail below, are intended to avoid the loss of nutrients, particularly
nitrogen, that
might otherwise occur if digestate B were more simply dewatered, dried and
pelletized.
[0023] For comparison, some animal manures and organic sludges and
slurries
could be dewatered without anaerobic digestion and then thermally dried and
extruded,
after removing large fibers, to produce a pellet. Such a product would not be
stable and
instead would be prone to decompose and grow mold during storage. The product
would
also have a significantly reduced nitrogen content compared to the feedstock
since most
of the soluble nitrogen present in the raw waste would be lost during
dewatering and
drying and would not be incorporated into the pellets.
[0024] Alternatively, and for further comparison, a manure, sludge or
slurry could
be first digested in an anaerobic digester and then mechanically dewatered,
thermally
dried and pelletized. The use of an anaerobic digester allows a biogas to be
created and
collected that can be used as a fuel for power or heat generation, or both.
Anaerobic
digestion also reduces greenhouse gas emissions relative to allowing organic
waste to
decompose to methane in the soil. The resulting pelletized sludge would also
be more
- 5 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
stable than the dried pelletized raw waste discussed above since many of the
organic
compounds in the raw waste are mineralized in the anaerobic digestion process,
and in
particular the concentration of carbon in biodegradable forms is greatly
reduced. For
these reasons, digesting the waste stream would be an improvement over simply
pelletizing organic waste. However, the nitrogen content in the pellets would
still be low
since most of the organic nitrogen would be converted to ammonia that would
exist
primarily in the liquid fraction of the sludge. This ammonia would again be
lost, for
reasons that will be explained in more detail below, first with the liquid
fraction removed
during dewatering and then as ammonia gas during the thermal drying process.
[0025] The plant of Figure 1 uses digester residue to produce fertilizer
pellets but
differs from the alternative described above in that additional steps are
provided to retain
nitrogen contained in the liquid fraction of the digestate B. The result is a
stable fertilizer
pellet, but with increased nitrogen compared to simply pelletizing the
digestate solids The
fertilizer producing apparatus could be located separately from the anaerobic
digester.
However, when the fertilizer producing apparatus is co located with the
anaerobic
digester 1 as in Figure 1, the need to move digestate B or intermediate
products is
reduced, the biogas or waste heat from power generation can be used in the
fertilizer
manufacturing process, and waste liquid streams may be advantageously returned
to
anaerobic digester 1.
[0026] As mentioned above, the nutrients in organic waste are partially
mineralized, or converted into inorganic forms, in the anaerobic digestion
process.
Organic waste streams typically contain a combination of volatile and non
volatile, or
inert, solids. Volatile solids may comprise 70 to 90% of the solids fraction
in typical waste
streams amenable to anaerobic digestion. Depending on the nature of the
volatile solids
only a fraction, usually ranging from 40 to 80%, is anaerobically degradable
by bacteria in
digesters and is converted into methane, carbon dioxide and water. The solids
remaining
in the digestate still contain some carbon, and the loss in carbon has been
compensated
for by the production of biogas. The digestate also has generally unchanged
amounts of
other nutrients such as nitrogen, phosphorous and potassium. These nutrients
tend to be
mineralized during digestion and the inorganic forms of the nutrients may be
more useful
to plants than the organic forms. Therefore, in addition to being more stable
due to the
reduction in organic carbon, applying digestate to the land may provide more
nutrient
value to crops compared with the raw waste. However, some of the mineralized
nutrients
are aqueous or suspended. Since the goal is to produce a dry product, solids
need to be
separated from liquid in the digestate, This is typically done by mechanical
separation (de
- 6 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
watering) processes followed by thermal drying, meaning drying at a
significant
temperature for example 100 degrees C or more. The effect of these processes
on the
nutrients phosphorous, potassium and nitrogen is discussed further below.
[0027] Phosphorous, in manure for example, is present mostly as
organic
phosphorous associated with particulate organic matter and dissolved
unreactive
phosphorous comprising organic phosphorous and polyphosphates. A minor
proportion is
dissolved reactive phosphorus or orthophosphate. Substantially all of the
phosphorous
present in the manure will still be present in the digester sludge. During
anaerobic
digestion, organic phosphorous contained in volatile solids and biomass
solubilizes and
adds to the soluble organic phosphorous present in the waste. The soluble
organic
phosphorous mineralizes and becomes adsorbed to particulate bound solids.
Because
phosphorous does not easily form gasses, it tends to stay in the manure or
other
substrates through digestion Solids separation (de watering) operations
performed on
the sludge may partition up to 70% of phosphorous in a cake portion of the
sludge,
particularly if the solids separation process is augmented with coagulants. If
only
dewatering flocculants are used, then about 50% of the phosphorous may remain
in the
cake after de watering. The phosphorous contained in the liquid traction of
the cake will
be substantially retained as solids when water evaporates as the cake is
thermally dried.
[0028] Potassium is not highly reactive and is mostly present in a
soluble form in
manures and other organic slurries and sludge. Potassium remains essentially
unchanged during digestion and it does not become a gas on drying. During
sludge de
watering, some potassium will remain in the liquid fraction removed and some
will remain
in liquids that are part of the solids fraction, or cake. When the cake is
dried, potassium
contained in the liquid portion of the cake will remain as a solid when water
evaporates.
[0029] Nitrogen may be present in the feedstock as urea, amino acids,
protein,
and various other forms of particulate and soluble organic nitrogen. During
anaerobic
digestion, these organic forms undergo mineralization and are converted
primarily into
dissolved (aqueous) ammonia and ammonium. Passing through a digester has
little effect
on the total nitrogen content of the waste. A negligible amount of nitrogen
may be emitted
as NH3 (unionized ammonia gas), but the majority will be found as NH4 (ionized
ammonia or ammonium) or ammonia gas in solution in the liquid fraction of the
digester
sludge, and a minor proportion as organic nitrogen in undigested volatile
solids. The
ammonium content of digester sludge is usually higher than that of the raw
waste. The
relative presence of ammonia (NH3 gas) and ammonium (NH4+ ion in solution) in
the
liquid of the digestate is a function of pH and temperature. A larger fraction
is present as
- 7 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
unionized ammonia (NH3 gas) with increased temperature and with increased pH.
In the
mesophilic and thermophilic range of digesters, operating at 35 to 55 degrees
Celsius
and at a pH of between 7.5 and 8.2, most of the reduced nitrogen exists as
ammonium
ions. Total ammonia concentrations are typically not allowed to exceed about
5000 ppm
in mesophilic reactors and about 3000 ppm in thermopohilic reactors since the
unionized
ammonia fraction is toxic to methanogenic organisms. Therefore digesters for
manures
with high solids and high nitrogen content, such as digesters for poultry
manure, are
typically diluted.
[0030] During mechanical de watering of digestate, most of the
nitrogen will be
removed in the liquid fraction as soluble ammonium. The cake portion will only
contain
the ammonium dissolved in the liquid portion of the cake and organic nitrogen
contained
in undigested volatile solids. However, particularly in digestate containing a
high nitrogen
concentration that would be useful to produce fertilizer, the pH may be as
high as 81 and
the alkalinity can be as high as 8000 to 20000 mg/L as CaCO3. At this
relatively high pH,
when temperature increases during thermal drying of the cake, most of the
ammonium
contained in the cake moisture will shift to ammonia gas and will be driven
off the cake
along with the evaporated moisture. This further reduces the nitrogen content
in the dried
solids, and the nitrogen that remains will be mostly organic N that is not
readily available
to crops. Attempting to retain some of the nitrogen in the liquid by adding
acid to the cake
to reduce the pH would not likely be cost effective. The liquid in the cake is
well buffered
by the alkalinity and would require very large amounts of acid to be added to
the cake to
significantly reduce the pH.
[0031] In summary, for phosphorous and potassium, some but not
necessarily
most, of the nutrients are removed with water during mechanical digestate
dewatering but
remaining nutrients remain in the cake after thermal drying. In contrast, most
of the
nitrogen in digestate is removed with water during mechanical de watering, and
most of
the nitrogen that remains is driven off as a gas during thermal drying.
Accordingly, and
because nitrogen is arguably the most important nutrient, particular attention
is paid to
retaining nitrogen in the product fertilizer in the process that will be
described below.
[0032] The process and apparatus of Figure 1 produces a fertilizer pellet Q
from a
digestate B or, when coupled with an anaerobic digester 1, from a waste stream
or feed
stock A. The process recovers at least some, and preferably most, of the
mineralized
nitrogen present in the digestate B as an ammonium salt that is incorporated
into the
pellet Q. This increases the nitrogen content of the pellet Q, relative to
merely dewatering
and drying the digestate B, and provides the nitrogen in a from that is
readily available to
- 8 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
crops. Since the nitrogen in the ammonium salt was originally present in the
digestate B
and the feed stock A, the pellet 0 may qualify as a natural or organic
fertilizer depending
on applicable regulations.
[0033] In Figure 1, animal manure or other digester feedstock (A)
enters an
anaerobic digester (1). Digested sludge or digestate (B) goes to a mechanical
dewatering
device (2), which can be for example a centrifuge, screw press, belt press,
rotary press or
any other mechanical dewatering device. Optionally, a flocculant or polymer
(T) is added
to help flocculate digested solids and increase solids capture and cake
dryness. The
dewatering device produces a cake (C) with solids content ranging, for
example, from 14
to 30% or more depending on the digestate and the type of dewatering device
used. The
dewatering device (2) also produces a liquid stream (D) called centrate herein
although
filtrate or pressate or other words may be more appropriate depending on the
dewatering
device used The cake (C) goes to an indirect thermal dryer (3) that may use,
for
example, biogas, natural gas or electricity as an energy source (V) to
evaporate water
from the cake. The dryer (3) can be, for example, a hollow screw type dryer
with steam or
hot oil circulation, a disc type dryer or a press type dryer, etc. Dry cake
(K), though not
absolutely dry, may be referred to as a solids fraction on the digestate (B).
[0034] An indirect enclosed dryer is used such that gasses (E) from
the cake does
not mix with combustion air or other gases, or with dust that the solids may
produce in the
drying process. Gasses (E) emitted from the cake (C) in the dryer will include
water vapor
and ammonia gas that evolves from liquid in the cake (C) as a result of a
shift from
ammonium (ion in solution) to ammonia (gas in solution) as the liquid is
heated in the
dryer (3). The gases (E), comprising water vapor and ammonia, go to a
condenser (10),
for example an indirect condenser that uses open loop or re circulating
cooling water (A,
B). Here vapor becomes condensate (U) comprising liquid water and ammonia in
solution. It is desirable to maintain the condensate at a high temperature,
for example 90
degrees C or more. The condensate (U) and centrate (D) may be combined in a
storage
tank, which may be a separate tank or part of an ammonia recovery system (4).
The
relative high temperature of the condensate (U) increases the temperature of
the
combined liquid (D + U), for example to about 40 to 45 degrees Celcius, which
is useful
for a subsequent ammonia recovery step. The combined liquid (D+U) likely
contains
some solids from the centrate (D), but may be referred to as a liquid fraction
of the
digestate (B).
[0035] The combined liquid (D + U) is fed to an ammonia recovery
system (4).
The ammonia recovery system (4) can include, for example, a steam stripper, a
vacuum
- 9 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
and heat based stripping system, or an air stripper. In the ammonia recovery
system, the
combined liquid (D+U) is treated to release ammonia gas, typically with water
vapor. The
gas/vapor mix is collected and condensed as ammonium hydroxide (ammonia water)
with
an acid added to produce an acidic ammonium salt solution. The gas/vapor mix
may be
condensed before the acid is added, or the ammonia gas and water vapor mixture
can be
mixed without a distinct condensing step into an acidic solution. Optionally,
the
ammonium salt solution may then be further concentrated. Further optionally,
additional
acid may be added to the ammonium salt solution to further reduce its pH.
[0036] Although any form of ammonia recovery system might be used,
centrates
(D) with high ammonia content, which are thus suited for making fertilizer,
also tend to
have high alkalinities. For instance chicken manure digester centrate with a
concentration
of about 5000 mg/L ammonia N maintained by dilution in the digester (1), can
have 16000
to 20000 mg/L of alkalinity In the digester (1), carbon dioxide in the binges
reacts with
ammonium to form ammonium carbonate, a strong buffering system. Such centrates
(D)
are well buffered and would require a large amount of caustic to increase the
pH.
Therefore, some ammonia recovery systems which would use a caustic to drive
ammonia
gas out of the combined liquid (D+U) would not be as cost effective as other
systems due
to the high chemical cost. For example, one system produced by Envimac
Engineering
GmbH relies on raising the pH to over 9.4 to drive the ammonium in a liquid to
ammonia
gas, and then strips the ammonia gas using from falling liquid using counter
current air. A
packed media bed is used to increase the surface area of a top sprayed liquid
for
improved mass transfer with the air. The air stripping is followed by an acid
scrubber step.
However, as discussed above, a large amount of caustic would be required to
raise the
pH of the buffered combined liquid (D+U).
[0037] Another option for recovering ammonia is using steam stripping.
Ammonia
removal systems using steam are also available, for example, from Envimac
Engineering
GmbH. These methods use less chemicals than air stripping methods, but require
more
energy than air stripping methods. Steam stripping methods may be particularly
useful in
plants where the combined liquid (D+U) flow rate is low relative to waste
energy available
to produce steam. Waste energy may include, for example, energy available from
heat
recovery steam generators using the exhaust gases from internal combustion gas
engines operated with biogas in digestion plants, or low pressure steam
boilers operating
with biogas or other fuel that would not otherwise be used. A first steam
decarbonation
step may be used to drive carbon dioxide from the combined liquid (D+U) and
reduce its
buffering capacity. Some caustic is then added to raise the pH of the liquid,
and an
- 10 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
ammonia steam stripping step is applied wherein the liquid drops through a
column of
rising steam. Due to the heat of the stream, the ammonia can be driven out of
the
combined liquid (D+U) at a lower pH and so less caustic is required. The
ammonia gas
forms ammonium hydroxide (ammonia water) with the steam. An acid can then be
added
to the ammonium hydroxide to form a stable ammonium salt.
[0038] Another, and possibly preferred, option for an ammonia recovery
system
(4) uses a flash vacuum distillation systems in which ammonia gas is extracted
from the
combined liquid (D+U) using heat and vacuum to shift the ammonium to ammonia.
The
combined liquid (D+U) may be heated to about 80 degrees C and then sprayed as
a mist
into a column under a vacuum, which causes the ammonia to be released from the
liquid.
The ammonia gas and some water vapor are collected in flow of air rising
upwards
through the column towards an inlet to the vacuum source. In the RCAST system
produced by ThermoEnergy Corporation of Worscester, MA, the vacuum source is a
venturi nozzle through which water or an acidic solution is recirculated. When
an acidic
solution is recirculated, the collected gas/vapor mixture is drawn directly
into the acidic
solution to produce an ammonium salt solution generally simultaneously with
condensation.
[0039] In an ammonia recovery system (4) requiring heat, heat of
evaporation
introduced to the dryer (3) may be recovered in the condenser (10) as hot
water (AB),
and that heat may be sufficient to further increase the temperature of the
centrate and
condensate mix (D + U) to about 50 degrees Celcius or more, possibly to 70 or
80
degrees Celcius. The salt produced in the ammonia recovery system may be, for
example, ammonium sulfate, ammonium acetate or ammonium citrate, depending on
the
acid used. Ammonium sulfate in particular is accepted as a useful fertilizer.
[0040] The concentration of the ammonium salt solution initially produced
in the
recovery system (4) may be such that using all of the ammonium salt solution
would
introduce too much water to the mixer (6) and pelletizer (7) to be described
below.
Excess ammonium salt solution could be sold as a liquid fertilizer.
Alternatively, the
recovery system (4) may include an ammonium salt solution concentration step.
The
ammonium salt solution may be concentrated to, for example 35 to 45%, at which
concentration all of the ammonium salt solution may be used in the pellets.
The
concentration can be done, for example by thermal distillation, by using gas
permeable
membranes or by flash evaporation. By any of these methods, water vapor is
produced
and removed from the salt solution. One suitable system is the CAST system, a
modified
flash evaporation system, produced by ThermoEnergy Corporation.
- 11 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
[0041] In summary, depending on the ammonia recovery system (4) used,
ammonia is stripped from the combined liquid (D+U) by either increasing
temperature by
introducing heat or steam (S), and/or increasing pH adding a strong base such
as sodium
hydroxide (W) and/or reducing partial pressure by introducing vacuum. For
ammonia
recovery systems that rely on increasing the temperature of the liquid it is
desirable to use
the heat of evaporation recovered in the condenser (10), which is the heat of
evaporation
from the water removed in vapour (E) from wet cake in the dryer (3). In the
ammonia
recovery system (4), the ammonium is converted to ammonia gas, released from
the
combined liquid (D+U) and captured, typically in solution with water or water
vapor from
one or more of vapors from the combined liquid (D+U), stripping steam or
recirculating
liquid producing a vacuum. The ammonia gas released from the
condensate/centrate
stream (D+U) is reacted with an acid (F) to form a stable ammonium salt in
solution. The
acid added can be sulfuric, acetic, citric, or other The resulting ammonium
acid solution
is preferably further concentrated, for example to 35 to 45%, for example
using flash
distillation with vacuum, heat, a gas permeable membrane, or combinations of
these to
produce a concentrated ammonium salt solution (G). In the ammonium salt
concentration
process, excess acid is preferably added to produce a salt solution with a low
pH, for
example 5 or less, or 3.5 to 5.
[0042] After ammonia recovery, the remainder of the
centrate/condensate stream
(D + U) exits the recovery unit (4) as an effluent (H) with reduced ammonia
content.
Ammonia removal ratios ranging from 40 to 90% are possible. The effluent (H)
with
reduced ammonia can be discharged to sewer, further treated for discharge to
the
environment or recirculated to the digester (1). The effluent (H) contains
some
phosphorus and potassium that can thereby be reintroduced into the digester
(1). Further,
the effluent (H) can function as dilution water to reduce the solids and
ammonia content in
the digester. As an example, in digesters (1) treating chicken manure,
dilution water is
required to reduce the solids content of the manure and reduce the ammonia
concentration in the digestate to avoid the toxic effects of unionized ammonia
on methane
forming bacteria (methanogens). Returning the low ammonia effluent (H) to the
digester
(1) is useful to reduce the amount of new fresh dilution water required. When
using
ammonia recovery systems that require heat, the effluent (H) may exit at a
relatively high
temperature (for example 50 to 70 degrees C). Using this as dilution water for
the
digester (1), if required, also contributes heat useful for operating the
digester at preferred
temperatures (for example 35 to 55 degrees C) when returned for dilution of
the digester
feed (A).
- 12 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
[0043] Returning to the dryer (3), a dry solids stream (K) leaves the
dryer (3) with,
typically, 90 to 98% solids content. The dried solids (K) can exit as a flake,
granule, or an
aggregate depending on the dryer technology used. The dry solids (K) may be
cooled in a
discharge cooling conveyor before further processing. The dry solids (K) may
pass
through a crusher (5) to reduce the size of the granules, flakes or other
forms of solid
clumps coming out of the dryer (3). Crushed solids (L) go to a pin or other
type of mill or
mixer (6). In the mill (6), crushed solids and a first portion of the ammonium
salt solution
(I) are mixed. The resulting conditioned pelletizer feed (M) may have a solids
content of,
for example, 65 75% solids. The mill (6) homogenizes and conditions the
material to
introduce it to a granulator, for example a disc or pan pelletizer (7). In the
pelletizer (7) the
remaining ammonium salt solution (J), for example 10 30% of the ammonium salt
solution
(G), is introduced using spraying nozzles. The added liquid sprayed on the
granules
being formed by the rotation action of a pan pelletizer (7) acts as binder to
help form
granules. In an alternative process configuration, an extruder may be used
instead of the
disc granulator (7). The extruder uses a positive displacement pump or press
to drive the
conditioned material from the mill (6) through an extrusion die.
[0044] A granulated or extruded moist (or "green") pellet (N) is
produced with
approximately 60% to 70% solids content as a result of the moisture added with
the
ammonium salt solution (I + J). The ammonium salt becomes part of the moist
pellet (N),
increasing its nitrogen content and slightly reducing the pH of the moist
pellet (N). The
moist granule or pellet (N) goes to a dryer (8), for example a low temperature
belt dryer,
where air (AA) and heat (V) are introduced to dry the green pellet (N). The
dryer (8) may
operate at temperatures below 90 degrees C, for example between 60 and 80
degrees
Celsius. The low temperature in conjunction with the low pH, particularly if
the pH is
further reduced with an acidic ammonium salt solution (G), minimizes the shift
of
ammonium to ammonia gas on drying and so minimizes ammonia loss in the dryer
(8). As
a result most of the ammonia introduced with the ammonium salt solution (G)
remains as
solids in the dry pellet (0). The pellets (0) may have a small nominal
diameter, for
example 1 to 4 mm, to provide a large surface area to further enable efficient
direct drying
with heated air at low temperature. Moisture is removed and the green pellets
gain
strength. Dry pellets (0) exit with 90 to 92% solids content.
[0045] Optionally, a sizing screen (9) classifies the dry pellets (0)
within a
specified size range. The undersized and oversized pellets (P) may go to the
feed
crusher (5) where they are crushed and blended with the solids (K) out of the
dryer to
feed the conditioning mill (6). The pellets retained between the screens in a
specified
- 13 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
particle size range, for example retained between 1 mm and 4 mm screens, exit
the
screen classifier (9) as finished pellets (0). A transport device, such as a
bucket elevator,
may convey the finished pellets (Q) to a bagging unit (11) which may have, for
example, a
hopper, a filling head and a scale (11). The finished pellets (Q) may be
bagged, for
example, in 1 ton super sacks (R) for storage and distribution. Alternatively
the finished
pellets (Q) can be bagged in smaller bags, for example 5 to 50 pound bags, for
distribution to retail stores.
[0046] The air exiting the belt dryer (X) may contain dust and may be
directed to a
cyclone (12), where collected dust (Z) is removed and sent to the conditioning
mill (6).
Cleaned air (Y) exits to the atmosphere with a low content of particulates.
Optionally a
bag house may be used after the cyclone (12) in locations with more
restrictive particulate
emission limits.
[0047] As an example, applying the process described above to digested
poultry
manure would, based on calculations, produce pellets with over 8% nitrogen
concentration on a dry mass basis. In comparison, the raw manure in the
calculation has
a nitrogen concentration of 4%. For further comparison, simply de watering and
drying the
same digestate would result in pellets with no more than 3.5% nitrogen.
Although any
ammonia recovery would be beneficial, with many feedstocks it should be
possible to add
at least 2% to the nitrogen concentration of the pellets on a dry mass basis
by way of
ammonium salts precipitated from a solution containing ammonia recovered from
a liquid
fraction of the digestate.
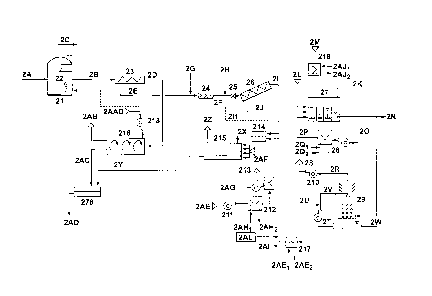
[0048] Referring to Figure 2, a digester feedstock (alternatively
called a
substrate), or combination of feedstocks, (2A) is fed to an anaerobic digester
(21), which
is stirred with a mixer (22). The digester (21) can be arranged in single or
multiple stages.
Depending on the substrate(s) (2A) the digestate (2B) may contain undigested
fibrous
material with lignocellulose. In addition to fibers, it may contain undigested
suspended
and colloidal organic matter particles that were not degraded by anaerobic
bacteria,
inorganic solids, and anaerobic bacteria that grow in the digester.
Additionally the
digestate (2B) is mixed in a sludge with water with ammonia, potassium and
phosphorous
in solution. The particulate solids may also have organic nitrogen and
phosphorous.
[0049] Digestate (2B) is directed to a solids separator (23). The
solids content of
the digestate sludge may vary, for example, from 3 to 9% depending on the
substrate and
type of digester (21) and mixing system. The separator (23) is preferably a
screw press
with openings larger than 400 microns but smaller than 1000 microns. The
separator (23)
produces a solids cake (2D) and a liquid fraction (2E), alternatively called a
reject, filtrate,
- 14 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
centrate or pressate. Other solids separation devices can be used, such as
screens or
roller presses. The cake (2D) contains fibers and large particles retained by
the press
screen and a small fraction of the small particles in the digestate (26),
including some
anaerobic bacteria, that independently would have passed the screen based on
size but
became trapped and entrained in the larger particle and fiber matrix. Cake
solids content
may range, for example, from 20 to 35%.
[0050] The solids separator (23) and steps treating the cake (2D) may
optionally
be omitted, particularly if there is not a significant concentration of
solids, particularly
fiberous solids, in the digestate (2B). A similar cake separation and related
step may
.. optionally be added to the system of Figure 1 if its digestate contains
fibrous solids.
[0051] The cake (2D) goes to composting, for example aerobic
thermophilic
composting. Figure 2 shows an enclosed rotary mechanical drum composter (216)
where
atmospheric air (2AA) is fed by a blower (218) The composter shown is rotary
drum type
but other types of in-vessel composting processes can be used, such as systems
with
modified shipping containers or plastic agricultural bags. Open windrow
composting can
also be used. In the composter, temperature increases as a result of bacterial
and fungal
activity. Bacteria decompose simple organic compounds and fungi process more
complex
substrates in the compost. High temperatures are beneficial for the
destruction of
pathogenic organisms and undesirable weed seeds that may have survived the
intestinal
tract of ruminants if the feedstock to the digester includes animal manure,
and also
survived the anaerobic digestion process. Optionally, other or supplemental
methods of
pathogen and/or vector destruction may be used. Decomposition is more rapid in
the
thermophilic temperature range of 135 to 160 Fahrenheit. Foul air (2AB) that
contains
ammonia, VOCs and some particulates exit the composter. This air may be
treated, for
example in a biofilter. The compost (2AC) may be cured and then used to
prepare, or as
part of, a plant growing medium or mix.
[0052] Synthetic nitrogen fertilizers may be added to the compost (2AC)
to
increase its nutrient content and value as a soil enhancing medium.
Alternatively, as
described in the example of a process shown in Figure 2, the addition of
synthetic
nitrogen is not required as nitrogen is recovered from the screw press (23)
filtrate (2E)
and incorporated into the blend as a high nutrient granule or flake (2Y). The
process to
produce this granule or flake is described below. A blender (278) blends
compost (2AC)
and high nutrient granules or flakes (2Y) to prepare a final soil medium
product (2AD) can
be done at the facility where the digester is located and the organics and
nutrient
recovery process takes place. Alternatively, the granules (2Y) and compost
(2AC) may be
- 15 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
shipped separately to a blending plant that also has bagging and packaging
facilities. Yet
a third option is to sell or use separately the compost (2AC) and the nutrient-
rich granules
or flakes (2Y). For example, the granules or flakes (2Y) can be used to
enhance compost
prepared by other processes. Alternatively, pellets or intermediate products
produced by
the system of Figure 1 may be added to compost on site or in a separate
facility.
[0053] The liquid fraction or filtrate (2E) out of the screw press (23)
may have, for
example, 2 to 5% total solids content depending on the digester feedstock (2A)
and the
size of the openings in the screw press (23) screen. The dissolved solids in
the filtrate
(2E) may be, for example, 1 to 1.5%. The rest of the solids content is
suspended solids.
Total solids removal in the press (23) varies depending on the digester
feedstock and the
screw press screen size but typical removal efficiencies are about 50% total
suspended
solids (TSS) and 35% total solids (TS). Associated with this separation, a
portion of the
nutrients in the digestate remain with the solids fraction out of the press
Typical portion
of the nutrients remaining in the cake may be about 25% of N, 50% of P, and 6%
of K.
[0054] Filtrate (2E) goes to a second step of solids separation. A
coagulant salt
(2G) such as ferric chloride or alum is added. A mixer (24) disperses the
coagulant in the
liquid stream. A dilute polymer (2H) is fed after to flocculate the microflocs
formed by
coagulant addition. A shear valve (25) enhances the dispersion of dilute
polymer. The
liquid dosed with coagulant and polymer enters a rotary screw dewaterer (26).
In this
dewaterer about 95% of the TSS and 65 to 70% of the TS is retained. Typical
rates of N,
P and K removal from the liquid are about 35% for N, 80% for P, and 8% for P.
These
nutrients remain in the cake (21) along with the TSS. The cake has typically
20% to 22%
solids content. The cake (21) goes to a blender mill (214), where it is
combined with
ammonium sulfate (217) recovered from the filtrate (2J). Introducing ammonium
sulfate
increases the nitrogen content of the product.
[0055] In some cases when high rate, short hydraulic retention time
anaerobic
digesters are operated, it is important to return bacterial biomass to the
digester. This
increases the solids retention time in the digester and improves process
stability. A large
fraction of the suspended solids captured by the dewaterer and concentrated
into cake
(21) is anaerobic bacteria. A portion of the cake (211) can be returned to the
digester if
needed or desirable for the operation of the digester (21). This can be done
using a
positive displacement pump. Optionally, a portion of filtrate (2E) may be
returned to the
digester (21). Both of streams (2E) and (21) are advantageously reduced in
liquid
content, which helps increase the solids content in the digester. Stream (21)
is preferred
- 16 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
as a means of solids recycle because of its higher solids content and reduced
ammonia
content relative to stream (2E).
[0056] The blend of cake and ammonium sulfate (2)() is fed to a thermal
dryer
(215). The dryer depicted in Figure 2 is a low temperature direct belt dryer.
Other dryers
can also be used, such as indirect hollow screw, disc, thin film, direct drum,
etc. The
dryer removes moisture and leaves the solids. However, depending on the pH of
the
mixture and the dryer temperature, a portion of the ammonia in the
cake/ammonium
sulfate blend may volatilize and escape the dryer along with the evaporated
water.
Ammonia loss is minimized by reducing the pH below 6, such that most of the
ammonia
.. exists as ammonium ion and not as unionized ammonia gas that volatilizes.
The pH
reduction to 6 results in less than 5% ammonia loss while drying at 105
degrees C.
Higher drying temperatures such as in direct drum dryers require reducing the
pH to 5 to
maintain the losses in the same range Sulfuric acid (2AL) can be dosed into
the
ammonium sulfate line (217), to make a more acidic ammonium sulfate solution.
.. Alternatively, ammonia may be recovered from dryer vapor as shown in Figure
1.
[0057] In the direct low temperature belt dryer shown in Figure 2,
atmospheric air
(2AE) is fed with a blower (211). The air is heated in a liquid to gas heat
exchanger using
waste heat as hot water (2AH1) in a closed loop from an internal combustion
engine
running on biogas (2C) or other source of waste heat. The return heating water
(2AH2)
goes in a closed loop to the waste heat source. If the available waste heat is
insufficient
to meet the requirements of the dryer, a fuel fired air heater (213) is used
in addition to
the waste heat air heater. The fuel (2AG) can be gaseous or liquid. The hot
air (2AF)
enters the belt dryer (215). Hot air (2Z) may go to air treatment to remove
particulates
and/or to a biofilter or thermal oxidizer depending on local emission
standards. Hot air
(2Z) can also be used as a heat source anywhere else in the process requiring
heat. The
granule or flake exiting the dryer (2Y) has a high content of nitrogen and
phosphorous.
The concentrations depend on the N and P content of the feedstock (2A).
[0058] The filtrate (2J) out of the dewatering device (26) goes to an
ammonia
stripping unit (27), optionally called a stripper. The ammonia stripping unit
may be
contained in an enclosed vessel, for example a rectangular box, or a
cylindrical vessel,
and can operate with a low liquid level, for example 1 m of depth or less,
usually about
0.6 meters of depth. The volume of the vessel is such that it provides about
30 to 40, or
even up to 70 minutes of hydraulic retention time based on filtrate
throughput. The
stripper receives subsurface diffused air (2M) through medium bubble
diffusers, and
.. optionally surface crossflow sweeping air (2L). The stripper operates at
above ambient
- 17 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
pressure, for example 50 degrees C or more, or about 70 degrees C or more. The
stripper is heated by recirculating stripper effluent (20) with a hot water
centrifugal pump
or circulator through a liquid/liquid heat exchanger (28). The heat exchanger
is part of a
hot water loop (2Q1 and 2Q2) and employs as a heat source waste heat from an
internal
combustion engine operating on biogas (2C) or another heat source. The heated
return
(2P) is directed to the inlet of the stripper (27). The stripper operates with
multiple stages,
for example 3 to 5 stages. The stages may be divided with perforated baffles
or by other
means such as a weir or piped connection with flow between the stages by
gravity.
Bubble diffusers are placed in each stage and connected to the diffused air
2M. After the
last stage the stripper has an overflow weir that controls the level of the
water in the
stripper. An internal reservoir at the end of the stripper allows the effluent
to de-aereate
such that it can be pumped for recirculation heating or directed as effluent
(2N) to a
storage tank Effluent (2N) can be used as dilution water for the digester, if
required, or
sent for disposal optionally after further treatment. In some cases mechanical
de-aeration
devices may be required in the circulation loop.
[0059] The stripper can remove ammonia without adding chemicals for pH
increase. At 70 degrees G, the diffused air drives carbon dioxide out of the
liquid. The
crossflow air is introduced optionally at the surface of the liquid further
reduces the
concentration of carbon dioxide in the headspace of the stripper. This enables
increased
CO2 stripping. The CO2 is in the filtrate as ammonium bicarbonate which
results from the
digestion process and is in equilibrium with the high CO2 content of the
biogas in the
digester headspace, usually 30 to 45%. Stripping raises the pH to 9.2 or
higher. At this
high pH and high temperature, the majority of the ammonia becomes unionized
ammonia
gas in the filtrate and is driven out of solution and into the stripper
headspace by the
subsurface diffused air.
[0060] The surface crossflow air reduces the ammonia concentration in
the
stripper headspace at the interface between water and air. This is an
equilibrium reaction.
The dilution of the headspace facilitates ammonia removal due to the higher
concentration gradient between the liquid and the air above it.
[0061] The combination of subsurface diffused air and crossflow air now
laden
with ammonia and CO2 (2K) is driven out of the stripper headspace by a slight
negative
pressure created by an induced draft fan (210), optionally part of a
downstream ammonia
acid scrubber. As a calculated example, a digestate filtrate flow of 170 gpm
containing
5000 mg/L of ammonia will require 8,000 scfm of diffused air and 12,000 cfm of
low
- 18 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
pressure crossflow air to reach 90% ammonia removal operating at 70 degrees C
and pH
9.3. The ammonia concentration in the air outlet stream (2K) is 8000 ppm by
volume.
[0062] Subsurface air is introduced by a blower. In cold climates the
subsurface
(2M) air can be heated prior to entering the stripper using a gas to liquid
heat exchanger
(219). This exchanger can be placed is series with the recirculation heat
exchanger (28)
such that the incoming hot water (2AJ1) is the outlet water (2Q2) of the
recirculation
exchanger (28). This enables more efficient use of the waste heat. Other
sources of heat
can also be used. The heat demand for the flow rate in the example above is
approximately 3 MW and a portion of it is used to make up for the heat of
evaporation, as
.. a small fraction of the water is lost to evaporation.
[0063] The crossflow air uses less energy per unit of flow than the
subsurface air.
The flow rate of the subsurface air is less than half of the flow rate of the
cross flow air.
For example, the subsurface air may be 15 to 45% of the total air flow The
headspace
may be restricted to a low height or volume, for example 1 m or less. Waste
heat, for
example from a turbine burning the biogas C, can be used to heat the air or
feed liquid.
A high temperature in the stripper helps prevent phosphate salts in the feed
liquid from
settling as the pH rises. Cooling the effluent (2N) after it exits the
stripper allows these
salts to be precipitated in a controlled location such as a storage tank.
[0064] The ammonia-laden air (2K) goes to an ammonia acid scrubber
(29). The
scrubber uses a counter flow column configuration with air circulating from
the bottom up
through a packed bed with plastic media to enhance gas/liquid mass transfer
surface
area. An acid shower with excess sulfuric acid (2V) flows from the top down
and reacts
with the ammonia gas in the air stream to form ammonium sulfate. Ammonium
sulfate is
stored in a sump at the bottom of the scrubber column. Ammonium sulfate (2T)
is
.. pumped for recirculation and sulfuric acid (2U) is added. Alternatively,
water can be
recirculated to form ammonium hydroxide for use in place of ammonium sulfate.
Sulfuric
acid addition is controlled automatically based on a pH set point. Excess
sulfuric acid can
be added to the recirculation stream to produce an acidic ammonium sulfate
solution to
reduce ammonia volatilization in the dryer. This is an alternative to sulfuric
acid injection
.. (2AL) to the product ammonium sulfate stream. The acid scrubber (29)
produces 28 to
30% ammonium sulfate solution when no excess sulfuric acid is dosed. The
ammonium
sulfate stream (W) goes to a mixer (14) to combine with solids cake (I) and
then to the
dryer (15). The scrubber exit air with low ammonia concentration (2R) is moved
by a fan
(210) and discharged to the atmosphere (2S). As an alternative to sending 30%
ammonium sulfate solution to the dryer, a concentration system (217) can be
used that
- 19 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
concentrates the solution to 68%. The concentrator uses waste heat (2AE1 and
2AE2)
and vacuum (2AI) and operates at about 70 degrees C. In some cases removing
moisture
from the ammonium sulfate solution is cost effective compared to removing this
moisture
in the dryer, mostly when dealing with indirect dryers that are more expensive
than direct
belt dryers. Alternatively, an ammonia stripping and recovery device described
in relation
to Figure 1 can be used.
[0065] Any suitable process steps or equipment from Figure 2 can be
used in
place of similar process steps of equipment in Figure 1. Any suitable process
steps or
equipment from Figure 1 can be used in place of similar process steps of
equipment in
Figure 2. Either system can be used to treat sludge from a wastewater
treatment plant.
Ammonia reduced liquid may be returned to the wastewater treatment plant.
Either of the
ammonia removal systems shown in Figure 3 and Figure 4 may be used in place of
the
ammonia removal systems described in relation to Figures 1 and 2
[0066] Figure 3 shows an alternative ammonia stripper 50. The stripper
50 has
one or more jacket reactors 52. If there are multiple reactors 52, they are
preferably
connected in series with the liquid effluent from one being fed to the next.
Stripper
effluent 55, which may be effluent 2N, is removed from the last reactor 52.
The reactors
52 may be located at decreasing elevations in the direction of flow such that
flow between
them may be by gravity. Alternatively, the liquid may be pumped between them.
The
jacket reactors 52 have jackets around their outer walls allowing them to be
heated, for
example to about 50 degrees C or more or about 70 degrees C or more. Feed
water (51)
which may be for example filtrate 2J, enters each reactor 52 through a porous
baffle 54
which breaks the feed flow up into multiple streams which fall through an air
gap before
reaching the surface of the liquid in the reactor. Air bubbles are produced in
the liquid
from a diffuser 56. Gas is collected in the head space of each reactor 52 and
removed by
connecting their gas outlets 60 to the suction side of a pump. The headspace
of a reactor
may be at a partial vaccum with the gas outlets 60 being the only gas
openings.
Optionally, a sweep gas may be added through one or more ports 58. In another
option,
the sweep gas may flow through one or more reactors in series by connecting
the gas
outlet 60 of an upstream reactor to the ports 58 of a downstream reactor. One
or more of
the outlets 60 are corrected to an outlet pipe 53 which may carry, for
example, ammonia
laden air 2K.
[0067] The anaerobic digestion of organic wastes, for example wastes
derived
from food processing, manure from farms, or sludge from wastewater treatment,
results in
several residues one of which is the digested sludge or digestate. After
leaving the
- 20 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
digester, digestate is commonly dewatered, and in some cases fiber is removed.
This
breaks up the digestate into two main parts: the solids fraction, commonly
referred to as
cake, and the liquid fraction which can be called centrate, filtrate, reject
water or
otherwise. The liquid fraction is often re-used in digestion processes, for
example as
water to dilute the digester feed, or in pre-processing organic wastes to
create a feed
slurry which can be directed to the digester. In other cases, the liquid
fraction may be
sent for additional treatment, such as treatment in a conventional activated
sludge plant
or otherwise. The liquid fraction contains ammonia. The ammonia concentration
is
preferably reduced before the liquid fraction is used in the processes
mentioned above to
avoid build up in the digester, or to reduce the cost of post treatment.
[0068] Bubbles can be used to strip ammonia from the liquid fraction.
These
bubbles can be induced by injecting air or steam into a reactor or,
alternatively, bubbles
can be created by cavitation Flashing is a similar process which will be
considered as a
type of cavitation in this document. Cavitation occurs when a liquid
experiences a
sudden change in pressure and can be induced by several methods. These methods
include passing the liquid through a flow restriction such as an orifice or
nozzle, flowing
the liquid across a restriction in piping or channels such as gates or valves,
inducing a
pressure change locally at propellers, causing a collision of liquid flows, by
ultrasonic
radiation or otherwise.
[0069] A cavitation based device can remove ammonia without adding
chemicals to increase the pH of the liquid fraction. At 70 degrees C, the
bubbles drive
carbon dioxide out of the liquid. The carbon dioxide is in the liquid fraction
as ammonium
bicarbonate, which results from the digestion process and is in equilibrium
with the high
carbon dioxide content of the biogas in the digester headspace, usually 30 to
45%.
Stripping carbon dioxide raises the pH to 9.2 or higher. At this high pH and
high
temperature the majority of the ammonia becomes unionized ammonia gas in the
liquid
fraction and is driven out of solution and into the stripper headspace by the
cavitation
bubbles.
[0070] Referring to Figure 4, an alternative ammonia removal system
100 has a
cavitation device 102 and a de-aerator 104. A de-aerator device is utilized to
separate
water and/or water vapour from the gas effluent which is carrying the ammonia
constituents. A feed liquid 106, such as the liquid fraction of digestate,
flows into the
cavitation device 102 and leaves as first effluent 108 with entrained bubbles
produced by
cavitation. The first effluent 108 flows into the de-aerator 104. The bubbles,
and possibly
some dissolved gas, are separated from the first effluent 108 in the de-
aerator 104 to
- 21 -
CA 02860031 2014-06-20
WO 2013/091094 PCT/CA2012/050907
produce a gas stream 110 including ammonia. A second effluent 112 with a
reduced
ammonia concentration is also drawn from the de-aerator 104. Optionally, the
system
100 may have multiple stages. For example, the feed liquid 106 may flow
through a first
cavitation device 102 and de-aerator 104 which primarily remove carbon
dioxide. An
effluent form the first stage may then flow through one or more further sets
of cavitation
devices and de-aerators for ammonia removal.
[0071] The cavitation device may be an enclosed device such as a
controlled
hydrodynamic cavitation device. One example of such a device is sold under the
VRTX
trade mark by HydroXS BV for use in cooling system descaling. Alternatively,
the
cavitation device may also draw in a supplemental gas 114 such as air. For
example, a
cavitation device as used with a cavitation air flotation unit may be adapted
for removing
ammonia. These devices use a rotating disc aerator which draws ambient air
from a
shaft to produce micro-bubbles in water
[0072] The de-aerator 104 comprises a vessel to receive the first
effluent 108 and
to provide a residence time sufficient for gas bubbles to be collected from
the surface of
the first effluent 108. The de-aerator preferably operates at ambient pressure
or under a
partial vacuum. The vacuum may be produced, for example, with an eductor or a
fan
type unit. Optionally, the first effluent 108 may be sprayed into the vessel
or flow over a
dispersing medium to help bring very small bubbles to a liquid surface.
Further optionally,
the vessel may have screens or other media to provide sites for small bubbles
to attach
and combine with other small bubbles, thus creating large bubbles that are
more easily
removed. Optionally, ammonia gas separated from water and/or water vapour in
the de-
aerator 104 may be used as aqueous ammonium hydroxide, optionally after mixing
with
water.
Example
[0073] Waste activated sludge was treated in an anaerobic digester at a
municipal
wastewater treatment plant. Sludge from the digester was dewatered with a
centrifuge to
produce a centrate. Ammonium bicarbonate was added to the centrate to reach
ammonia and alkalinity concentrations typical of a high solids anaerobic
digester used to
treat industrial and agricultural substrates. No other chemicals were added.
The
modified centrate was treated in an ammonia removal device and process as
described
above. Concentrations of ammonia and alkalinity were measured in the centrate
feed to
the ammonia stripping device and in the liquid effluent removed from the
ammonia
stripping device. The results of the experiment are shown in Table 1 below.
Process
- 22 -
WO 2013/091094
PCT/CA2012/050907
conditions and parameters and device (reactor) volumes during the experiment
are given
in Table 2 below.
Table 1
% Removal %
Removal
Sample Alkalinity pH NH3-N Ammonia-N
Alkalinity
mg/L as CaCO3 mg/L
Feed 23750 8.6 7400
Effluent 3750 9.2 1000 86% 84%
Table 2
Diffused Crossflow
Reactor Reactor Volume Reactor (bubbled)
(surface)
Volume No Air With Air Influent HRT
Temperature Air Air
gal gal gpm min Deg Celsius scfm Scfm
4.56 7.13 0.128 36 67 20 40
- 23 -
Date Recue/Date Received 2021-04-26